WO2013061911A1 - 測定装置、測定方法および測定プログラムを格納した記録媒体 - Google Patents
測定装置、測定方法および測定プログラムを格納した記録媒体 Download PDFInfo
- Publication number
- WO2013061911A1 WO2013061911A1 PCT/JP2012/077213 JP2012077213W WO2013061911A1 WO 2013061911 A1 WO2013061911 A1 WO 2013061911A1 JP 2012077213 W JP2012077213 W JP 2012077213W WO 2013061911 A1 WO2013061911 A1 WO 2013061911A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- aneurysm
- frequency
- pulse wave
- measurement
- phase
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/02—Detecting, measuring or recording pulse, heart rate, blood pressure or blood flow; Combined pulse/heart-rate/blood pressure determination; Evaluating a cardiovascular condition not otherwise provided for, e.g. using combinations of techniques provided for in this group with electrocardiography or electroauscultation; Heart catheters for measuring blood pressure
- A61B5/02007—Evaluating blood vessel condition, e.g. elasticity, compliance
- A61B5/02014—Determining aneurysm
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/02—Detecting, measuring or recording pulse, heart rate, blood pressure or blood flow; Combined pulse/heart-rate/blood pressure determination; Evaluating a cardiovascular condition not otherwise provided for, e.g. using combinations of techniques provided for in this group with electrocardiography or electroauscultation; Heart catheters for measuring blood pressure
- A61B5/021—Measuring pressure in heart or blood vessels
- A61B5/02108—Measuring pressure in heart or blood vessels from analysis of pulse wave characteristics
- A61B5/02125—Measuring pressure in heart or blood vessels from analysis of pulse wave characteristics of pulse wave propagation time
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/02—Detecting, measuring or recording pulse, heart rate, blood pressure or blood flow; Combined pulse/heart-rate/blood pressure determination; Evaluating a cardiovascular condition not otherwise provided for, e.g. using combinations of techniques provided for in this group with electrocardiography or electroauscultation; Heart catheters for measuring blood pressure
- A61B5/021—Measuring pressure in heart or blood vessels
- A61B5/022—Measuring pressure in heart or blood vessels by applying pressure to close blood vessels, e.g. against the skin; Ophthalmodynamometers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/02—Detecting, measuring or recording pulse, heart rate, blood pressure or blood flow; Combined pulse/heart-rate/blood pressure determination; Evaluating a cardiovascular condition not otherwise provided for, e.g. using combinations of techniques provided for in this group with electrocardiography or electroauscultation; Heart catheters for measuring blood pressure
- A61B5/024—Detecting, measuring or recording pulse rate or heart rate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/72—Signal processing specially adapted for physiological signals or for diagnostic purposes
- A61B5/7235—Details of waveform analysis
- A61B5/7246—Details of waveform analysis using correlation, e.g. template matching or determination of similarity
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/72—Signal processing specially adapted for physiological signals or for diagnostic purposes
- A61B5/7271—Specific aspects of physiological measurement analysis
- A61B5/7282—Event detection, e.g. detecting unique waveforms indicative of a medical condition
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/6813—Specially adapted to be attached to a specific body part
- A61B5/6828—Leg
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/72—Signal processing specially adapted for physiological signals or for diagnostic purposes
- A61B5/7235—Details of waveform analysis
- A61B5/7253—Details of waveform analysis characterised by using transforms
- A61B5/7257—Details of waveform analysis characterised by using transforms using Fourier transforms
Definitions
- the present invention relates to a measuring apparatus, a measuring method, and a recording medium storing a measuring program for evaluating an aneurysm that can occur in a blood vessel path.
- An aneurysm is one of the lesions that occur on the vascular pathway.
- an aortic aneurysm that occurs in the abdomen has no initial symptoms, magnetic resonance tomography (MRI), computed tomography (CT), ultrasound diagnosis.
- MRI magnetic resonance tomography
- CT computed tomography
- ultrasound diagnosis In many cases, it is discovered in a situation just before rupture, such as pulsation of the abdomen. For this reason, it is desired to be detected early by a test that is easily performed at an opportunity such as a health checkup.
- Patent Document 1 discloses a device that makes a diagnosis using ultrasonic waves.
- Patent Document 2 discloses a method of detecting a lesion as a singular part by comparing a plurality of image data by X-ray CT, MRI, or the like.
- Patent Document 1 the ultrasonic diagnostic apparatus disclosed in Patent Document 1
- the aorta must be sequentially measured with the possibility that an aneurysm exists.
- the method disclosed in Patent Document 2 is based on the premise that a whole-body blood vessel image is captured by X-ray CT, MRI, or the like. According to such a conventional technique, an apparatus and an inspection content for inspecting an aneurysm become large. For this reason, it is not realistic to use aneurysm inspection as a test item for regular medical examinations due to time and cost, and as a result, it is easy to find an aortic aneurysm before a serious situation occurs. is not.
- the present invention has been made in view of such a problem, and is a measuring apparatus, a measuring method, and a measuring method that can evaluate the presence and / or size of an aneurysm occurring in a blood vessel path with a relatively simple configuration and procedure.
- An object is to provide a recording medium storing a program.
- a measuring apparatus includes a first measurement position of a blood vessel path through a portion where an aneurysm is expected to be generated from the subject's heart, and a portion where an aneurysm is expected to be generated from the subject's heart
- a first measurement position of a blood vessel path through a portion where an aneurysm is expected to be generated from the subject's heart
- a portion where an aneurysm is expected to be generated from the subject's heart In order to calculate the comparison result by comparing the frequency characteristic between the detection means for detecting each pulse wave signal and the pulse wave signal at the second measurement position of the blood vessel path through a different part from Comparing means and determining means for determining at least one of the presence or absence and size of an aneurysm based on a predetermined feature amount for the frequency included in the comparison result.
- the comparison means includes means for calculating a transfer function between the first measurement position and the second measurement position.
- the determination means determines the presence or absence of an aneurysm based on the degree of phase variation in the phase difference characteristic of the transfer function.
- the comparing means includes means for calculating a phase delay time for each frequency of the pulse wave signal, and the determining means is a phase calculated based on an average of the calculated phase delay times for each frequency. Based on the number of times the phase difference characteristic of the transfer function intersects the corner, at least one of the presence and size of the aneurysm is determined.
- the determining means determines the size of the aneurysm based on the frequency interval at which the phase difference characteristic of the transfer function intersects the phase angle calculated based on the calculated average of the phase delay times for each frequency.
- the comparing means includes means for calculating a phase delay time for each frequency of the pulse wave signal, and the determining means is a phase angle calculated based on an average of the calculated phase delay times for each frequency.
- the determining means is a phase angle calculated based on an average of the calculated phase delay times for each frequency.
- the determining means determines at least one of the presence and size of an aneurysm based on a frequency interval at which an extreme value occurs in the gain characteristic of the transfer function.
- the comparison means includes means for calculating a frequency characteristic regarding a pulse wave propagation speed between pulse wave signals.
- the determination means determines the presence or absence of an aneurysm based on the degree of variation of the pulse wave propagation speed in the frequency characteristics of the pulse wave propagation speed.
- the determining means determines at least one of the presence and the size of an aneurysm based on a frequency interval of fluctuations that occur in the pulse wave velocity in the frequency characteristics of the pulse wave velocity.
- the first measurement position of the blood vessel path through the portion where the aneurysm is expected to be generated from the subject's heart, and the occurrence of the aneurysm from the subject's heart are expected.
- a step of detecting each pulse wave signal at a second measurement position of the blood vessel path that has passed through a portion different from the portion, a step of calculating a comparison result by comparing frequency characteristics between the pulse wave signals, and comparison Determining at least one of the presence and size of an aneurysm based on a predetermined feature amount for the frequency included in the result.
- a recording medium storing a measurement program When a recording medium storing a measurement program according to another aspect of the present invention is executed by a computer, the computer measures the first measurement position of the blood vessel path through a portion where an aneurysm is expected from the heart of the subject. And a step of detecting each pulse wave signal at a second measurement position of the blood vessel path that has passed through a portion different from the portion where an aneurysm is expected to occur from the heart of the subject, and a frequency between the pulse wave signals A step of calculating a comparison result by comparing the characteristics and a step of determining at least one of the presence and size of an aneurysm based on a predetermined feature amount for the frequency included in the comparison result are executed.
- FIG. 17 is a gain diagram and a phase diagram of the transfer function P A / P B when the inner radius R of the abdominal artery of segment number 65 is changed.
- FIG. 6 is a gain diagram and a phase diagram of transfer functions P A / P B when the inner radius of the aneurysm is fixed to 50 mm and the length is sequentially increased.
- FIG. 6 is a gain diagram and a phase diagram of a transfer function P A / P B when the entire Young's modulus is changed with the inner radius of the aneurysm fixed at 50 mm.
- It is a schematic structure figure of a measuring device concerning an embodiment of the invention. 6 is a schematic diagram showing functional blocks that realize processing for detecting an aneurysm according to Embodiment 1.
- FIG. It is a figure which shows the phase diagram calculated from the measurement signal actually acquired from the some test subject.
- FIG. 5 is a flowchart showing a processing procedure related to determination of an aneurysm according to Embodiment 1.
- FIG. 10 is a schematic diagram showing functional blocks that realize processing for detecting an aneurysm according to Embodiment 2.
- 6 is a diagram for explaining a phase angle (reference phase difference characteristic) set by a phase angle setting unit according to Embodiment 2.
- FIG. 10 is a flowchart showing a processing procedure related to determination of an aneurysm according to the second embodiment.
- FIG. 10 is a schematic diagram showing functional blocks that realize processing for detecting an aneurysm according to Embodiment 3.
- FIG. 10 is a diagram for explaining determination processing in a determination unit according to Embodiment 3.
- FIG. 10 is a flowchart showing a processing procedure related to determination of an aneurysm according to Embodiment 3.
- FIG. 10 is a schematic diagram showing functional blocks that realize processing for detecting an aneurysm according to a fourth embodiment. It is a figure which shows the pulse wave velocity (PWV) calculated from the measurement signal actually acquired from the some test subject.
- PWV pulse wave velocity
- the measurement method according to the present embodiment focuses on the loss of transmitted pulse waves as if an aneurysm was regarded as a partial expansion of an elastic duct.
- an aneurysm model By applying such an aneurysm model to a pulse wave propagation model of a systemic artery, the presence / absence and / or size (inner radius and length) of the aneurysm is evaluated from the change in frequency characteristics.
- Such frequency characteristics include, for example, a lower limb (measurement position of a blood vessel path through a portion where an aneurysm is expected to be generated from the subject's heart) and an upper limb (a portion where an aneurysm is expected to be generated from the subject's heart).
- a lower limb measurement position of a blood vessel path through a portion where an aneurysm is expected to be generated from the subject's heart
- an upper limb a portion where an aneurysm is expected to be generated from the subject's heart
- the transfer function indicating the blood vessel path is calculated by dividing the blood vessel path of the subject into a plurality of sections and modeling each section with a one-dimensional linear distribution constant model. This transfer function is analytically calculated from a dynamic model in which the pulse wave propagates through the blood vessel.
- An aneurysm is a lesion whose inner diameter increases in the middle of a vascular pathway (artery).
- the characteristic impedance of the vascular route changes. This phenomenon is similar to the phenomenon that occurs in a reactance silencer for sound waves, and the propagating pulse wave is reduced.
- an aneurysm is detected using this phenomenon.
- the blood vessel path is an elastic pipe line, it differs from the reactance silencer in that not only the characteristic impedance but also the pulse wave velocity changes due to a change in the inner diameter.
- the loss (transmission loss) when the pulse wave propagating through the range where the aneurysm exists is mainly focused.
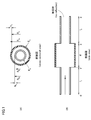
- FIG. 1 is a diagram showing a model of an aneurysm adopted in the embodiment of the present invention.
- 1A shows a cross-sectional view
- FIG. 1B shows a side view.
- a blood vessel route having three sections is present, and an aneurysm is generated at an intermediate portion thereof. That is, it is assumed that an aneurysm has occurred in the (1-2) section, and the (0-1) section and (2-3) section on both sides thereof (hereinafter, both are also referred to as “peripheral portions”) Suppose that it is a healthy vascular route.
- This (1-2) section is a model as an elastic pipe with an expanded inner diameter.
- the length (d), Young's modulus is E A
- the inner radius is R A
- the outer radius is R Ao
- the wall thickness is h A for the section (1-2) that is the middle part.
- the lengths are l 1 and l 2
- Young's modulus is E A
- the inner radius is R B
- the outer radius is R Bo
- the wall the thickness and h B.
- Point 0 and point 3 are non-reflective ends. In the following analysis, the position in each section is indicated by using the distance x from the point 0 (the right direction on the paper is positive).
- the pressure p 01 (x, t) in the (0-1) section is obtained by using the traveling wave pressure p f1 and the backward wave pressure p r1 at the point 0, the distance x from the point 0, and the propagation constant ⁇ B in the peripheral portion. It can be expressed by the following formula (1).
- volume flow rate q 01 (x, t) can be expressed by the following equation (2) using the characteristic impedance Z 0B of the peripheral portion.
- the pressure p 12 (x, t) and the volume flow rate q 12 (x, t) in the section (1-2) are the traveling wave pressure p f2 and the backward wave pressure p r2 , the characteristic impedance Z 0A at the center, Using the distance x from the point 0 and the intermediate propagation constant ⁇ A , they can be expressed by the following equations (3) and (4), respectively.
- the pressure p 23 (x, t) and the volume flow rate q 23 (x, t) in the section (2-3) are determined by the traveling wave pressure p f3 , the distance x from the point 0, and the propagation constant ⁇ B in the peripheral portion. And can be expressed by the following equations (5) and (6), respectively. It should be noted that since no backward wave pressure exists under the condition that the point 3 is a non-reflective end, only the traveling wave pressure pf3 needs to be considered.
- Equation (8) is established.
- Equation (10) is established.
- Expression (15) shows the relationship between the traveling wave pressure pf1 in the (0-1) section and the traveling wave pressure pf3 in the (2-3) section. From this relational expression, the point 0 to the point 3 is expressed.
- the transmission loss can be calculated. That is, the transmission loss T from point 0 to point 3 can be expressed by the following equation (16) depending on the energy ratio of the traveling wave at each point.
- pulse wave propagation velocity c in the blood vessel path reference 1 (Hirsunori Sato, Yuji Izeki, Hideo Utsuno, Hiroshi Matsuhisa, Keisuke Yamada, Katsu Sawada, Identification of pulse wave propagation characteristics in elastic ducts (Identification of Pulse Wave Propagation Characteristics in Viscoelastic Tube), The Japan Society of Mechanical Engineers Proceedings (Part B), Volume 76, Issue 766 (2010), pp.961-969).
- the equivalent bulk modulus formula with the wall is introduced. Substituting this bulk modulus equation, the pulse wave velocity c in the blood vessel path is given by the following formula (18).
- the pulse wave propagation velocity in the intermediate section (1-2) is c A
- the pulse wave propagation velocity in the peripheral sections (0-1) and (2-3) is c B.
- the propagation constant ⁇ A in the intermediate portion and the propagation constant ⁇ B in the peripheral portion can be expressed by the following equations (21) and (22) with respect to the angular frequency ⁇ .
- the characteristic impedance Z 0 related to the volume flow rate can be expressed by the following equation (23) when the cross-sectional area is S.
- ⁇ B3 Relationship between shape of intermediate portion and transmission loss>
- the effect of the change in the shape (inner radius and length) of the intermediate part simulating an aneurysm will be examined. As described later, this means that the relationship between the size of the aneurysm and the actually observed pulse wave is evaluated on the model.
- the influence on the transmission loss when the inner radius R A , Young's modulus E A , and length d of the intermediate portion are changed will be examined.
- the pulse wave Propagation speed is also equal.
- the equation (24) can be transformed into the following equation (27) by the equation (26).
- the transmission loss T takes the maximum value T max when the following equation (30) is satisfied for the integer m.
- the maximum value T max of the transmission loss T is expressed by the following equation (31).
- the maximum value of the transmission loss T increases as the ratio ⁇ R of the inner radius R A of the intermediate portion to the inner radius R B of the peripheral portion increases.
- the transmission loss T takes a maximum value for each angular frequency ⁇ f satisfying the equation (32).
- a frequency interval at which the transmission loss T takes a maximum value is referred to as a “basic frequency”. This frequency interval (fundamental frequency) is determined by the ratio between the pulse wave propagation velocity c A and the length d.
- the pulse wave propagation velocity c A at the intermediate portion can be expressed as the following equation (35).
- the characteristic impedance Z 0A of the intermediate portion can also be expressed by the following equation (36) from the equation (24).
- the square root of the ratio of the Young's modulus of the middle part to the Young's modulus of the peripheral part is equal to the ratio of the peripheral part to the middle part in terms of pulse wave propagation velocity and characteristic impedance.
- 2 and 3 are diagrams showing evaluation results of the influence of the inner radius in the intermediate portion calculated according to the transmission loss calculation formula according to the present embodiment.
- FIG. 2 shows changes in transmission loss (frequency characteristics) when the inner radius of the intermediate portion is changed while keeping other parameters and wall thickness constant.
- FIG. 3 is a diagram showing a change in transmission loss according to the above equation (31), with the ratio ⁇ R of the inner radius of the intermediate portion to the inner radius of the peripheral portion as a parameter.
- the transmission loss T increases as the ratio ⁇ R of the inner radius of the intermediate portion to the inner radius of the peripheral portion increases.
- the ratio ⁇ R does not affect the fundamental frequency of the transmission loss T.
- 4 and 5 are diagrams showing the evaluation results of the influence of the length in the intermediate part calculated according to the transmission loss calculation formula according to the present embodiment.
- the inner radius RA of the intermediate portion is fixed to 20 mm.
- FIG. 4 shows the transmission loss of pressures p 0 and p 3 between points 0 and 3 when the inner radius RA of the intermediate portion is fixed to 20 mm and the length d of the intermediate portion is changed. Changes in (frequency characteristics) are shown.
- FIG. 5 shows the relationship between the length d of the intermediate portion and the frequency interval (fundamental frequency) at which the minimum value is obtained according to the above equation (32).
- FIG. 6 and FIG. 7 are diagrams showing evaluation results of the influence of Young's modulus in the intermediate portion calculated according to the transmission loss calculation formula according to the present embodiment.
- the inner radius RA of the intermediate portion is fixed to 20 mm.
- Figure 6 is, fix the inner radius R A of the intermediate portion to 20 mm, transmission of the pressure p 0, p 3 between the point 0 and the point 3 when the Young's modulus E A of the intermediate portion is changed Indicates the change in loss (frequency characteristics). 7, according to the above equation (38) shows the relationship between the frequency spacing to take Young's modulus E A and the minimum value of the intermediate portion (the fundamental frequency).
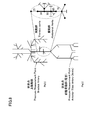
- FIG. 8 is a schematic diagram showing a pulse wave propagation model of a systemic artery.
- the pulse propagation model of the systemic artery shown in FIG. 8 is document 2 (Avolio AP, “Multi-branched model of human arterial system”, Medical and Biological Engineering and Computing, Vol. 18 (1980), pp. 709-718) , And Reference 3 (Hironori Sato, Yuji Iseki, Hideo Utsuno, Hiroshi Matsuhisa, Keisuke Yamada, Katsu Sawada, refinement of the multi-branched model of human arteries), Japan Society of Mechanical Engineers Proceedings ( C), 77, 779 (2011), pp. 2695-2710).
- abdominal aortic aneurysms with different lengths d were assumed. More specifically, as shown in the following Table 2, three types of aortic aneurysms were assumed.
- the segment number in Table 2 indicates the number where an aortic aneurysm exists. For example, in the case where the segment numbers are “50” and “65”, an aortic aneurysm exists in the whole body artery pulse wave propagation model shown in FIG. 8 over the segment numbers “50” and “65”. Means that.
- the transfer function P A / P B of the pulse wave between the upper limb and the lower limb was calculated by changing the inner radius R, the length d, and the overall Young's modulus.
- This transfer function P A / P B is obtained by converting Fourier signals Pa (f) and Pb (f) obtained by Fourier transform (frequency conversion) of measurement signals Pa (t) and Pb (t), which are pulse wave signals, respectively. And can be defined as the following equation (39).
- the pulse waves in the upper limb and the lower limb can typically be obtained by attaching arbitrary detection devices to the brachial artery and the anterior tibial artery, respectively. That is, the pulse wave in the lower limb is detected at the measurement position of the blood vessel path through the portion where the aneurysm is expected to be generated from the subject's heart, and the pulse wave in the upper limb is expected to be generated from the heart of the subject. It is detected at the measurement position of the blood vessel route that has passed through a part different from the part.
- 9 to 11 are diagrams showing the results of transfer functions obtained by simulation using a pulse wave propagation model of the whole-body artery.
- FIG. 9 shows a gain diagram and a phase diagram of the transfer function P A / P B when the inner radius R of the abdominal artery of segment number 65 is changed.
- “Base” means that the inner radius R of the abdominal artery is set to 5.7 mm defined in the pulse wave propagation model of the systemic artery. That is, this corresponds to a state in which there is no aortic aneurysm.
- FIG. 10 shows a gain diagram and a phase diagram of the transfer function P A / P B when the inner radius of the aneurysm is fixed to 50 mm and the length is sequentially increased. More specifically, as shown in cases (1) to (3) shown in Table 2, the range in which the aneurysm exists from the state where the aneurysm exists only in the abdominal artery of segment number 65 The length d of the central portion (expansion portion) was changed by sequentially adding to the abdominal artery No. 50 and the thoracic artery (Thoracic aorta) No. 34. In FIG. 10, “Base” means a case where the length d of the central portion (extended portion) is “0”. That is, this corresponds to a state in which there is no aortic aneurysm.
- the fundamental frequency is lower (narrower) in proportion to the length d of the central portion (expanded portion), while there is no change in transmission loss. It can also be seen from the phase diagram that the phase changes by 180 degrees for each fundamental frequency.
- FIG. 11 shows a gain diagram and a phase diagram of the transfer function P A / P B when the whole radius of elasticity is changed with the inner radius of the aneurysm fixed at 50 mm. More specifically, the inner radii of the abdominal arteries of segment numbers 50 and 65 were both fixed to 50 mm, and the overall Young's modulus was changed to 1, 2, and 3 times, respectively.
- “Base” corresponds to a state where no aortic aneurysm exists.
- the transmission loss T increases as the ratio ⁇ R between the inner radius of the peripheral portion and the inner radius of the intermediate portion increases.
- the fundamental frequency which is the frequency interval at which extreme values (maximum value / minimum value) occur, is inversely proportional to the length d of the aneurysm.
- the fundamental frequency is determined by the ratio between the pulse wave velocity and the length of the aneurysm. Therefore, the presence of the aneurysm can be estimated from the size of the transmission loss T and the fundamental frequency, and the size (inner diameter and length) of the aneurysm can be estimated by measuring the entire Young's modulus.
- pulse wave signals are detected at two measurement points on the subject's artery, and frequency characteristics between these detected pulse wave signals are detected.
- frequency characteristics between these detected pulse wave signals are detected.
- Each pulse wave signal is estimated from the measurement position of the blood vessel path (typically the anterior tibial artery) through the part where an aneurysm is expected from the subject's heart and the aneurysm from the subject's heart. It is preferable that detection is performed at a measurement position (typically, the brachial artery) of a blood vessel route that has passed through a portion different from the portion to be applied. This is to more accurately detect the presence / absence and size of the aneurysm by preventing one of the detected pulse wave signals from being affected by the aneurysm. By using such a pulse wave signal, it is particularly effective for detecting an abdominal aortic aneurysm or a thoracic aortic aneurysm.
- a transfer function is calculated from each pulse wave signal, and the presence / absence and size of an aneurysm is detected from the following viewpoint based on the calculated transfer function. To do.
- the inner diameter of the aneurysm is estimated.
- the length of the aneurysm is estimated based on the frequency interval (fundamental frequency) at which the gain (amplitude) of the transfer function takes the extreme value (maximum value / minimum value). At this time, the phase change of the transfer function corresponds to the fundamental frequency.
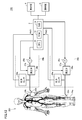
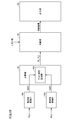
- FIG. 12 is a schematic configuration diagram of the measuring apparatus 100 according to the embodiment of the present invention.
- measurement apparatus 100 includes a processing unit 2, a display unit 4, an operation unit 6, and measurement units 20a and 20b.
- the processing unit 2 is a device that controls the entire measuring apparatus 100, and typically includes a CPU (Central Processing Unit) 10, a ROM (Read Only Memory) 12, and a RAM (Random Access Memory) 14. Consists of.
- CPU Central Processing Unit
- ROM Read Only Memory
- RAM Random Access Memory
- the CPU 10 corresponds to an arithmetic processing unit, reads a program stored in advance in the ROM 12, and executes the program while using the RAM 14 as a work memory.
- the display unit 4 and the operation unit 6 are connected to the processing unit 2.
- the display unit 4 prompts the user to input various settings and displays the calculation result from the processing unit 2.
- the user operates the operation unit 6 while confirming the content displayed on the display unit 4 to perform a desired setting input or operation.
- the display part 4 consists of LED (Light Emitting Diode), LCD (Liquid Crystal Display) etc. as an example.
- the processing unit 2 gives measurement commands to the measurement units 20a and 20b and receives measurement signals Pa (t) and Pb (t) measured in response to the measurement commands. Based on the measurement signals Pa (t) and Pb (t), processing according to the present embodiment as described later is executed.
- the measurement units 20a and 20b pressurize the internal pressure (hereinafter referred to as “cuff pressure”) of the pressure cuffs (air bags) 24a and 24b attached to the predetermined measurement sites of the subject 200, and the pulse waves at the respective measurement sites. Measure the time waveform. That is, the measurement signals Pa (t) and Pb (t) are pulse wave signals at positions where the press cuffs 24a and 24b are mounted, respectively. As will be described later, the processing unit 2 performs processing using the frequency characteristic between the measurement signal Pa (t) and the measurement signal Pb (t), and therefore the processing units 2 measure the measurement units 20a and 20b. Measurement commands are given simultaneously so that the measurement signals can be measured in synchronization with each other.
- cuff pressure the internal pressure of the pressure cuffs (air bags) 24a and 24b attached to the predetermined measurement sites of the subject 200, and the pulse waves at the respective measurement sites. Measure the time waveform. That is, the measurement signals Pa (t) and Pb (t) are pulse wave
- the press cuffs 24a and 24b are attached to the ankle part (preferably around the anterior tibial artery) and the upper arm part (preferably around the brachial artery) of the subject 200, respectively, and the piping 22a and Pressure is applied by air supplied from the measurement units 20a and 20b via 22b.
- the press cuffs 24a and 24b are pressed to the corresponding measurement site, and the pressure change corresponding to the pulse wave at the measurement site is transmitted to the measurement units 20a and 20b via the pipes 22a and 22b, respectively.
- the measuring units 20a and 20b measure the time waveform of the pulse wave at the measurement site by detecting the transmitted pressure change. Since it is preferable to perform arithmetic processing on predetermined frequency components (for example, 0 to 20 [Hz]) of the measurement signals Pa (t) and Pb (t), the measurement signals Pa (t) and Pb ( The measurement period (sampling period) of t) is preferably shorter than the time interval (for example, 25 msec) corresponding to this frequency component.
- the measurement unit 20a includes a pressure sensor 28a, a pressure regulating valve 26a, a pressure pump 25a, and a pipe 27a.
- the pressure sensor 28a is a detection part for detecting pressure fluctuation transmitted through the pipe 22a.
- the pressure sensor 28a includes a plurality of sensor elements arranged at predetermined intervals on a semiconductor chip such as single crystal silicon.
- the pressure regulating valve 26a is inserted between the pressure pump 25a and the pressure cuff 24a, and maintains the pressure used for pressurizing the pressure cuff 24a within a predetermined range during measurement.
- the pressure pump 25a operates according to a measurement command from the processing unit 2 and supplies pressurized air for pressurizing the pressing cuff 24a.
- the measurement unit 20b includes a pressure sensor 28b, a pressure regulating valve 26b, a pressure pump 25b, and a pipe 27b. About the structure of each part, it is the same as that of the measurement part 20a.
- a pulse wave signal which is a biological signal
- a pressure change caused by the pulse wave using a pressure cuff While flowing an electric current, the voltage change which arises by the change of the impedance (bioimpedance) produced according to propagation of a pulse wave may be acquired as a pulse wave signal.
- Embodiment 1 [D. Aneurysm Judgment Logic (Embodiment 1)] ⁇ D1: Overview> As Embodiment 1, from each detected pulse wave signal, a measurement position (measurement position of a blood vessel path through a portion where an aneurysm is expected to be generated from the heart of the subject) and a pressure cuff are detected. The transfer function of the pressure between the measurement position (the measurement position of the blood vessel path passing through a part different from the part where the aneurysm is expected to be generated from the subject's heart) is calculated, and the calculated transfer function A configuration for detecting the presence and / or size of an aneurysm will be described with reference to FIG.
- the presence / absence of an aneurysm is detected based on the degree of variation in phase difference characteristics related to the transfer function.
- the phase changes by 180 degrees for each fundamental frequency. If the fundamental frequency is low (narrow), this phase will occur more frequently. Further, since the fundamental frequency is inversely proportional to the length of the aneurysm, it can be said that the probability that an aneurysm exists is high when the fundamental frequency is relatively low (narrow). Therefore, it can be determined that the greater the degree of variation, the higher the probability that an aneurysm exists.
- each of the measurement signals Pa (t) and Pb (t) is frequency-converted to calculate a phase characteristic, and further, a phase difference is calculated for each frequency, thereby transmitting the signal.
- the phase difference characteristic ( ⁇ (P A / P B )) of the function P A / P B is calculated.
- phase characteristics ⁇ a (f) and ⁇ b (f) that can be defined as the following equation (40) using Fourier signals Pa (f) and Pb (f), respectively.
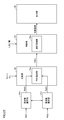
- FIG. 13 is a schematic diagram illustrating functional blocks that implement processing for detecting an aneurysm according to the first embodiment.
- Each functional block illustrated in FIG. 13 is typically realized by the CPU 10 (FIG. 12) of the processing unit 2 executing arithmetic processing according to a program stored in advance in the ROM 12 or the like.
- processing unit 2 includes frequency conversion units 30 a and 30 b, comparison unit 40, determination unit 50, and output unit 60.
- the frequency conversion units 30a and 30b receive the measurement signals Pa (t) and Pb (t), which are time waveforms, over a predetermined period, respectively, and receive the received measurement signals Pa (t) and Pb (t) in the frequency domain. Convert to Typically, the frequency conversion units 30a and 30b perform frequency conversion using fast Fourier transform (FFT). Any conversion algorithm may be used as long as it converts a time-domain signal into a frequency-domain signal such as a Fourier series.
- FFT fast Fourier transform
- the frequency conversion units 30a and 30b output phase characteristics ⁇ a (f) and ⁇ b (f) as frequency domain information. More specifically, the frequency conversion unit 30a calculates a phase characteristic ⁇ a (f) indicating a phase for each frequency component of the measurement signal Pa (t), and sends the calculated phase characteristic ⁇ a (f) to the comparison unit 40. Output. Similarly, the frequency conversion unit 30b calculates a phase characteristic ⁇ b (f) indicating a phase for each frequency component of the measurement signal Pb (t), and outputs the calculated phase characteristic ⁇ b (f) to the comparison unit 40.
- the comparison unit 40 compares the frequency characteristics (in the first embodiment, the phase difference characteristics) between the measurement signals Pa (t) and Pb (t), which are pulse wave signals, to obtain the phase difference at each frequency. Calculated as a comparison result. More specifically, the comparison unit 40 includes a phase difference characteristic calculation unit 40a. The phase difference characteristic calculating unit 40a calculates a difference between the phase characteristic ⁇ a (f) and the phase characteristic ⁇ b (f) for each frequency, thereby indicating a phase difference characteristic (phase characteristic ⁇ a (f ) -Phase characteristic ⁇ b (f)) is calculated.
- phase difference characteristic calculation unit 40a corrects such discontinuous points in the phase diagram in units corresponding to one or two or more periods (n ⁇ 360 °), and then performs actual measurement. Calculate the phase difference characteristic.
- Phase difference characteristic calculation unit 40a is the difference between the phase characteristics .PHI.a (f) and phase characteristics .PHI.b (f), is plotted on the phase line phase difference A i corresponding to the frequency f i.
- the frequency f i is the i-th frequency component counted from the low frequency side.
- the phase difference characteristic calculation unit 40a calculates a regression line using the phase difference A i plotted on the phase diagram.
- the slope of the regression line ([deg / Hz]) corresponds to the phase line characteristic.
- the phase difference characteristic calculation unit 40a outputs the calculated comparison result (phase difference characteristic) to the determination unit 50. That is, the comparison unit 40 (phase difference characteristic calculation unit 40a) is configured to transfer the pressure between the measurement position where the pressing cuff 24a is mounted and the measurement position where the pressing cuff 24b is mounted (at least the phase difference characteristic). Will be calculated.
- the determination unit 50 determines the presence or absence of an aneurysm based on a predetermined feature amount for the frequency included in the comparison result calculated by the comparison unit 40. More specifically, the determination unit 50 determines the presence or absence of an aneurysm based on the degree of phase variation in the phase difference characteristic related to the calculated transfer function.
- FIG. 14 is a diagram showing a phase diagram calculated from measurement signals actually obtained from a plurality of subjects. That is, the comparator 40 outputs phase difference characteristics as shown in FIGS.
- the determination unit 50 evaluates the degree of phase variation for each of the phase difference characteristics shown in FIGS. As an example, the determination unit 50 sets regression lines determined by a known method, and calculates a deviation amount from the set regression line as a degree of variation. Then, the determination unit 50 determines whether or not the calculated degree of variation exceeds a predetermined threshold value. When the calculated degree of variation exceeds a predetermined threshold value, it is determined that an aneurysm that cannot be ignored exists on the blood vessel path to be examined.
- the regression lines shown in FIGS. 14A to 14C may be calculated by statistically processing the phase for each measured frequency.
- the determination unit 50 includes a statistical processing unit 50a.
- the statistical processing unit 50a determines a regression line for evaluating the degree of phase variation, and calculates the degree of phase variation based on the determined regression line. Or you may determine a regression line using methods, such as a least squares method, with respect to a phase difference characteristic.
- the pulse wave velocity is measured at a healthy site (eg, between the heart and the carotid artery) where no aneurysm is present, and the intrinsic phase difference characteristics are determined from the measured pulse wave velocity.
- a regression line may be determined from the calculated intrinsic phase difference characteristic.
- the degree of variation may be calculated by integrating the amount of deviation from the regression line for each frequency, or may be calculated from the total area between the regression line and the phase difference characteristic. Further, the degree of variation may be calculated by a known method such as standard deviation.
- output unit 60 causes display unit 4 (FIG. 12) to display the determination result in determination unit 50.
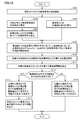
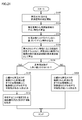
- FIG. 15 is a flowchart showing a processing procedure related to determination of an aneurysm according to the first embodiment.
- CPU 10 in response to a user operation on operation unit 6 (FIG. 12) or the like, CPU 10 gives a measurement command to measurement units 20a and 20b, and measurement units 20a and 20b are attached to subject 200. Measurement of pulse wave signals in the pressed cuffs 24a, 24b is started (step S100).
- the CPU 10 calculates the phase characteristics ⁇ a (f) and ⁇ b (f) in the frequency domain from the measurement signals Pa (t) and Pb (t), which are time waveforms measured by the measurement units 20a and 20b (step). S102). Then, the CPU 10 calculates the phase difference characteristic based on the phase difference at each frequency between the phase characteristic ⁇ a (f) and the phase characteristic ⁇ b (f) (step S104).
- the CPU 10 sets a regression line for the calculated phase difference characteristic (step S106), and calculates the degree of variation of the phase difference characteristic based on the set regression line (step S108).
- the CPU 10 compares the calculated degree of variation of the phase difference characteristic with a predetermined threshold value, and determines whether or not the degree of variation is larger than the threshold value (step S110).
- the CPU 10 detects an aneurysm or the like on the blood vessel path from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached. Assuming that there is a possibility that a predetermined lesion exists, the evaluation result is output to the display unit 4 (step S112). Then, the measurement process ends.
- the degree of variation is smaller than the threshold value (NO in step S110)
- the CPU 10 is on the blood vessel path from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached. The evaluation result is output to the display unit 4 (step S114). Then, the measurement process ends.
- the presence or absence of a lesion such as an aneurysm can be determined only by measuring the pulse wave signal from the lower limb and upper limb of the subject. Therefore, it is possible to diagnose an aneurysm with a simpler configuration and with a simpler procedure. Further, according to the present embodiment, the presence / absence of a lesion such as an aneurysm is determined using the phase signal of the pulse wave signal that can be detected with relatively high accuracy, so that the determination accuracy can be improved.
- the reference phase difference characteristic is calculated based on the phase delay time for each frequency of the pulse wave signal. More specifically, the phase angle in the phase diagram is calculated based on the average of the phase delay times for each frequency of the pulse wave signal. Then, the presence / absence and size of the aneurysm are detected based on the number of times or the frequency interval at which the reference phase difference characteristic and the phase difference characteristic related to the transfer function intersect. As described above, in the phase diagram of the transfer function, the phase changes by 180 degrees for each fundamental frequency. If the fundamental frequency is low (narrow), this phase will occur more frequently.
- the fundamental frequency is inversely proportional to the length of the aneurysm, it can be said that the probability that an aneurysm exists is high when the fundamental frequency is relatively low (narrow). Therefore, the presence and size (inner diameter and length) of the aneurysm can be estimated based on the deviation from the characteristic characteristics of the reference, based on the phase delay that will be caused by the state of the blood vessel path of the subject.
- the second embodiment is different from the first embodiment mainly in the process of calculating the reference phase difference characteristic and the process of evaluating the deviation from the reference characteristic. Therefore, in the following description, such differences are mainly described.
- FIG. 16 is a schematic diagram illustrating functional blocks that implement processing for detecting an aneurysm according to the second embodiment.
- Each functional block shown in FIG. 16 is typically realized by the CPU 10 (FIG. 12) of the processing unit 2 executing arithmetic processing according to a program stored in advance in the ROM 12 or the like.
- processing unit 2 includes frequency conversion units 30 a and 30 b, frequency selection units 31 a and 31 b, comparison unit 41, determination unit 51, and output unit 60. . Since frequency conversion units 30a and 30b and output unit 60 have been described with reference to FIG. 13, detailed description thereof will not be repeated.
- the frequency selectors 31a and 31b extract and compare only specific frequency components included in the measurement signals Pa (t) and Pb (t), respectively, in order to calculate the phase delay time for each frequency of the pulse wave signal. Output to the unit 41.
- the frequency selectors 31a and 31b function as bandpass filters. Here, since it is necessary for the frequency selection units 31a and 31b to extract the same frequency components, the frequencies to be extracted are synchronized with each other.
- the comparison unit 41 includes a phase delay time calculation unit 41a in addition to the phase difference characteristic calculation unit 40a described in the first embodiment.
- the phase delay time calculation unit 41a compares specific frequency components included in the measurement signals Pa (t) and Pb (t) extracted from the frequency selection units 31a and 31b with each other, so that the pulse wave signal is changed for each frequency. The phase delay time is calculated.
- the determination unit 51 includes a phase angle setting unit 51a in addition to the statistical processing unit 50a described in the first embodiment.
- the phase angle setting unit 51a calculates the phase angle based on the average of the phase delay times for each frequency calculated by the phase delay time calculation unit 41a. This phase angle indicates the phase lag that would result from the condition of the subject's vascular pathway.
- FIG. 17 is a diagram for explaining a phase angle (reference phase difference characteristic) set by the phase angle setting unit 51a according to the second embodiment.
- the reference phase difference characteristic is set based on the phase angle ⁇ calculated by the phase angle setting unit 51a. This phase angle ⁇ indicates the inclination in the phase diagram.
- the determination unit 51 Assess the presence and / or size of the aneurysm.
- the phase changes by 180 degrees for each fundamental frequency. If the fundamental frequency is low (narrow), this phase will occur more frequently. Further, since the fundamental frequency is inversely proportional to the length of the aneurysm, it can be said that the probability that an aneurysm exists is high when the fundamental frequency is relatively low (narrow). Accordingly, it can be determined that the probability that an aneurysm exists is higher as the number of times the phase difference characteristic of the pulse wave signal intersects the reference phase difference characteristic. Since the fundamental frequency is inversely proportional to the length of the aneurysm, it can be estimated that a longer aneurysm exists when the fundamental frequency is relatively low (narrow).
- the frequency interval at which the reference phase difference characteristic and the phase difference characteristic for the pulse wave signal intersect is the above-described fundamental frequency itself, the presence / absence and / or size of an aneurysm is determined based on the intersecting frequency interval. It can also be estimated.
- the determination unit 51 determines whether or not an aneurysm exists based on the number of times the phase difference characteristic of the transfer function intersects the phase angle calculated based on the average of the phase delay times for each frequency. And / or determine the size. Alternatively, the determination unit 51 determines the presence / absence and / or size of an aneurysm based on the frequency interval at which the phase difference characteristic of the transfer function intersects the phase angle calculated based on the average of the phase delay times for each frequency. Judging.
- phase difference characteristic calculated from the measurement signals Pa (t) and Pb (t) may include an error
- a specific frequency component is used or an interpolation process is performed on the phase difference characteristic. For example, the influence of the error may be reduced.
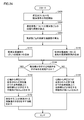
- FIG. 18 is a flowchart showing a processing procedure related to determination of an aneurysm according to the second embodiment. In the flowchart shown in FIG. 18, the same steps as those in the flowchart shown in FIG.
- CPU 10 in response to a user operation on operation unit 6 (FIG. 12) or the like, CPU 10 gives a measurement command to measurement units 20a and 20b, and measurement units 20a and 20b are attached to subject 200. Measurement of pulse wave signals in the pressed cuffs 24a, 24b is started (step S100).
- the CPU 10 calculates the phase characteristics ⁇ a (f) and ⁇ b (f) in the frequency domain from the measurement signals Pa (t) and Pb (t), which are time waveforms measured by the measurement units 20a and 20b (step). S102). Then, the CPU 10 calculates the phase difference characteristic based on the phase difference at each frequency between the phase characteristic ⁇ a (f) and the phase characteristic ⁇ b (f) (step S104). In parallel with or in succession to the processes of steps S102 and S104, the CPU 10 calculates a phase delay time for each frequency for the measurement signals Pa (t) and Pb (t) (step S105).
- the CPU 10 calculates the phase angle in the phase diagram based on the average of the phase delay times for each frequency calculated in step S105, and sets a reference phase difference characteristic with respect to the calculated phase difference characteristic. (Step S107). Then, the CPU 10 searches for a position where the reference phase difference characteristic and the phase difference characteristic related to the transfer function intersect (step S109), and finds the number of intersecting positions (number of intersections) and / or between the intersecting positions. (Interval frequency interval) is calculated (step S111).
- the CPU 10 determines whether or not a condition indicating the possibility that an aneurysm exists is satisfied based on the information calculated in step S111 (step S113). More specifically, the CPU 10 determines whether or not the number of intersections calculated in step S111 exceeds a predetermined threshold number, or whether or not the frequency interval of intersections calculated in step S111 is lower than a predetermined threshold frequency. Determine whether.
- the CPU 10 If the condition indicating the possibility of the presence of an aneurysm is satisfied (YES in step S113), the CPU 10 is on the blood vessel path from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached. Assuming that there is a possibility that a predetermined lesion such as an aneurysm exists, the evaluation result is output to the display unit 4 (step S115). Further, the CPU 10 causes the display unit 4 to output information indicating the size of the aneurysm estimated to exist based on the value of the intersecting frequency interval calculated in step S111 (step S117). Ends.
- step S110 when the condition indicating the possibility of the presence of an aneurysm is not satisfied (NO in step S110), the CPU 10 moves from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached. Assuming that there is a low possibility that a predetermined lesion such as an aneurysm is present on the blood vessel route, the evaluation result is output to the display unit 4 (step S119). Then, the measurement process ends.
- the presence or absence of a lesion such as an aneurysm can be determined only by measuring the pulse wave signal from the lower limb and upper limb of the subject. Therefore, it is possible to diagnose an aneurysm with a simpler configuration and with a simpler procedure. Further, according to the present embodiment, since the size of an aneurysm can be estimated in addition to the presence or absence of an aneurysm, the progress of the aneurysm whose presence is estimated can also be estimated.
- the presence / absence and / or size of an aneurysm is detected based on a frequency interval at which an extreme value occurs in the gain characteristic of the transfer function.
- the frequency interval at which the extreme value (maximum value / minimum value) occurs is the fundamental frequency, and this fundamental frequency is inversely proportional to the length of the aneurysm. If it is relatively low (narrow), the probability that an aneurysm is present is high.
- the measurement signals Pa (t) and Pb (t) are frequency-converted to calculate gain characteristics, and further, the gain ratio is calculated for each frequency, so that the transfer function P A / gain characteristic of P B (
- gain characteristics Ga (f) and Gb (f) that can be defined as the following equation (41) using Fourier signals Pa (f) and Pb (f), respectively.
- an aneurysm exists in the target blood vessel path based on the frequency interval at which the extreme value (maximum value / minimum value) occurs in the gain characteristic of the calculated transfer function.
- the size (length) of the aneurysm is estimated.
- FIG. 19 is a schematic diagram illustrating functional blocks that implement processing for detecting an aneurysm according to the third embodiment.
- Each functional block shown in FIG. 19 is typically realized by the CPU 10 (FIG. 12) of the processing unit 2 executing arithmetic processing according to a program stored in advance in the ROM 12 or the like.
- processing unit 2 includes frequency conversion units 32 a and 32 b, comparison unit 42, determination unit 52, and output unit 60.
- the frequency converters 32a and 32b receive the measurement signals Pa (t) and Pb (t), which are time waveforms, over a predetermined period, respectively, and receive the received measurement signals Pa (t) and Pb (t) in the frequency domain. Convert to Typically, the frequency conversion units 32a and 32b perform frequency conversion using fast Fourier transform (FFT). Any conversion algorithm may be used as long as it converts a time-domain signal into a frequency-domain signal such as a Fourier series.
- FFT fast Fourier transform
- the frequency converters 32a and 32b output gain characteristics Ga (f) and Gb (f) as frequency domain information. More specifically, the frequency conversion unit 32a calculates a gain characteristic Ga (f) indicating a gain for each frequency component of the measurement signal Pa (t), and sends the calculated gain characteristic Ga (f) to the comparison unit 42. Output. Similarly, the frequency conversion unit 32b calculates a gain characteristic Gb (f) indicating a gain for each frequency component of the measurement signal Pb (t), and outputs the calculated gain characteristic Gb (f) to the comparison unit 42.
- the comparison unit 42 compares the gain characteristics at each frequency by comparing the frequency characteristics (gain characteristics in the third embodiment) between the measurement signals Pa (t) and Pb (t), which are pulse wave signals. Calculate as a result. More specifically, the comparison unit 42 includes a gain characteristic calculation unit 42a. The gain characteristic calculation unit 42a calculates a gain characteristic indicating a gain ratio for each frequency by calculating a ratio (or difference) between the gain characteristic Ga (f) and the gain characteristic Gb (f) for each frequency. . The comparison result (gain characteristic) calculated by the comparison unit 42 is output to the determination unit 52. That is, the comparison unit 42 calculates a pressure transfer function (at least a gain characteristic) between the measurement position where the pressing cuff 24a is mounted and the measurement position where the pressing cuff 24b is mounted.
- a pressure transfer function at least a gain characteristic
- the determination unit 52 determines the presence or absence of an aneurysm based on a predetermined feature amount for the frequency included in the comparison result calculated by the comparison unit 42. More specifically, the determination unit 52 determines the presence and / or size of an aneurysm based on a frequency interval at which an extreme value (maximum value / minimum value) occurs in the gain characteristic of the calculated transfer function.
- FIG. 20 is a diagram for explaining determination processing in the determination unit 52 according to the third embodiment.
- the comparator 42 outputs gain characteristics as shown in FIG.
- the determination unit 52 searches for extreme values (maximum value and minimum value) in the gain characteristics as shown in FIG. As a method for searching for such extreme values, a known method can be employed. And the judgment part 52 calculates the frequency interval between the adjacent extreme values searched in this way as a fundamental frequency. Note that the frequency interval may vary depending on the position where the extreme value exists due to some measurement error. In such a case, an average of a plurality of frequency intervals may be set as the fundamental frequency.
- the determination unit 52 determines whether there is an aneurysm that cannot be ignored on the blood vessel path to be examined based on the calculated magnitude of the fundamental frequency, and the aneurysm exists. If it is determined that the possibility is high, the size is estimated from the fundamental frequency. Typically, the determination unit 52 determines that there is a high possibility that an aneurysm is present when the calculated fundamental frequency is lower than the predetermined threshold value compared with the predetermined threshold value. To do.
- FIG. 21 is a flowchart showing a processing procedure related to determination of an aneurysm according to the third embodiment. In the flowchart shown in FIG. 21, the same steps as those in the flowchart shown in FIG.
- CPU 10 in response to a user operation on operation unit 6 (FIG. 12) or the like, CPU 10 gives a measurement command to measurement units 20a and 20b, and measurement units 20a and 20b are attached to subject 200. Measurement of pulse wave signals in the pressed cuffs 24a, 24b is started (step S100).
- the CPU 10 calculates gain characteristics Ga (f) and Gb (f) in the frequency domain from the measurement signals Pa (t) and Pb (t), which are time waveforms measured by the measuring units 20a and 20b (step). S122). Then, the CPU 10 calculates the gain characteristic based on the gain ratio at each frequency between the gain characteristic Ga (f) and the gain characteristic Gb (f) (step S124).
- the CPU 10 searches for extreme values (maximum value and minimum value) generated in the calculated gain characteristic, and calculates a frequency interval between the searched adjacent extreme values as a basic frequency (step S126).
- the CPU 10 compares the calculated fundamental frequency with a predetermined threshold value, and determines whether or not the fundamental frequency is smaller than the threshold value (step S128).
- the CPU 10 detects an aneurysm or the like on the blood vessel path from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached. Assuming that there is a possibility that a predetermined lesion exists, the evaluation result is output to the display unit 4 (step S130). Further, the CPU 10 causes the display unit 4 to output information indicating the size of the aneurysm that is estimated to exist based on the size of the fundamental frequency (step S132). Then, the measurement process ends.
- the CPU 10 is on the blood vessel path from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached.
- the evaluation result is output to the display unit 4 (step S134). Then, the measurement process ends.
- the presence or absence of a lesion such as an aneurysm can be determined only by measuring the pulse wave signal from the lower limb and upper limb of the subject. Therefore, it is possible to diagnose an aneurysm with a simpler configuration and with a simpler procedure. Further, according to the present embodiment, since the fundamental frequency can be directly calculated, the size of the aneurysm can be estimated with higher accuracy.
- the pulse wave velocity indicates a feature amount corresponding to the phase difference characteristic used in the first embodiment, and also indicates a feature amount corresponding to the phase delay time used in the second embodiment. Therefore, the pulse wave propagation velocity indicates a feature quantity for the same frequency as the phase difference characteristic and the phase delay time.
- the presence / absence and / or size of an aneurysm is detected from the frequency characteristics of the pulse wave velocity using the same determination method as in the first or second embodiment.
- FIG. 22 is a schematic diagram illustrating functional blocks that implement processing for detecting an aneurysm according to the fourth embodiment.
- Each functional block shown in FIG. 22 is typically realized by the CPU 10 (FIG. 12) of the processing unit 2 executing arithmetic processing according to a program stored in advance in the ROM 12 or the like.
- processing unit 2 includes frequency selection units 31 a and 31 b, comparison unit 43, determination unit 53, and output unit 60.
- the frequency selectors 31a and 31b use only specific frequency components included in the measurement signals Pa (t) and Pb (t), respectively. Extracted and output to the comparison unit 43.
- the frequency selectors 31a and 31b function as bandpass filters.
- the frequency selection units 31a and 31b since it is necessary for the frequency selection units 31a and 31b to extract the same frequency component, the extracted frequencies are synchronized with each other to synchronize.
- the comparison unit 43 calculates a frequency characteristic of the pulse wave propagation speed between the measurement signals Pa (t) and Pb (t), which are pulse wave signals, as a comparison result.
- the comparison unit 40 includes a PWV calculation unit 43a.
- the PWV calculation unit 43a receives the measurement signals Pa (t) and Pb (t) over a predetermined period, and calculates the appearance time difference of each pulse wave waveform appearing on the time axis for each frequency as the propagation time difference Td i . That is, the frequency selection units 31a and 31b output the time waveform corresponding to the selected frequency component of the measurement signals Pa (t) and Pb (t) to the PWV calculation unit 43a. Is the propagation time difference Td i for each frequency.
- the PWV calculation unit 43a obtains the distances La and Lb of the respective blood vessel paths from the heart of the subject 200 to the measurement site to which the pressing cuffs 24a and 24b are attached, or the difference ⁇ L between the distances.
- the pulse wave propagation velocity (PWV i ) for each frequency is calculated by dividing the difference ⁇ L by the propagation time difference Td i .
- the PWV calculation unit 43 a outputs the calculated pulse wave velocity (PWV i ) for each frequency to the determination unit 53.
- the comparison unit 43 calculates the frequency characteristic of the pulse wave velocity between the pulse wave signals.
- the determination unit 53 determines the presence / absence and / or size of an aneurysm based on a predetermined feature amount for the frequency included in the frequency characteristics of the pulse wave velocity between the pulse wave signals calculated by the comparison unit 43. to decide. More specifically, the determination unit 53 is based on the degree of variation in the pulse wave propagation speed and / or the frequency interval of the fluctuation occurring in the pulse wave propagation speed in the calculated frequency characteristic of the pulse wave propagation speed. Determine the presence and / or size of an aneurysm.
- FIG. 23 is a diagram illustrating a pulse wave velocity (PWV) calculated from measurement signals actually acquired from a plurality of subjects. That is, the comparison unit 43 outputs frequency characteristics regarding the pulse wave velocity as shown in FIGS. 23 (A) to (C). The determination unit 53 evaluates the variation degree of the pulse wave propagation speed and / or the frequency interval of the fluctuation occurring in the pulse wave propagation speed for each of the phase difference characteristics shown in FIGS. 23 (A) to (C). .
- PWV pulse wave velocity
- the determination unit 53 sets a regression line determined by a known method, and calculates a deviation amount from the set regression line as a degree of variation.
- FIG. 23A shows an example of the set regression line. Then, the determination unit 53 determines whether or not the calculated degree of variation exceeds a predetermined threshold value. When the calculated degree of variation exceeds a predetermined threshold value, it is determined that an aneurysm that cannot be ignored exists on the blood vessel path to be examined.
- the determination unit 53 may determine the presence and / or size of an aneurysm based on the frequency interval of fluctuations that occur in the pulse wave velocity.
- periodic fluctuations occur in the pulse wave propagation velocity.
- the determination unit 53 calculates the fluctuation period, that is, the frequency interval of the fluctuation, for such a periodic fluctuation of the pulse wave propagation velocity.
- the frequency interval of the fluctuation corresponds to the above-described fundamental frequency, and the presence and / or length of the aneurysm can be evaluated based on the magnitude of the fundamental frequency.
- the determination unit 53 determines whether there is an aneurysm that cannot be ignored on the vascular path to be examined based on the calculated frequency interval of fluctuation, and there is a high possibility that the aneurysm exists. Is determined from the frequency interval of the fluctuation.
- FIG. 24 is a flowchart showing a processing procedure related to determination of an aneurysm according to the fourth embodiment.
- the same step numbers are assigned to the same processes as those in the flowchart shown in FIG.
- CPU 10 in response to a user operation on operation unit 6 (FIG. 12) or the like, CPU 10 gives a measurement command to measurement units 20a and 20b, and measurement units 20a and 20b are attached to subject 200. Measurement of pulse wave signals in the pressed cuffs 24a, 24b is started (step S100).
- the CPU 10 calculates a propagation time difference for each frequency for the measurement signals Pa (t) and Pb (t) (step S140), and from the subject's heart to the measurement site to which the press cuffs 24a and 24b are attached.
- the pulse wave velocity for each frequency is calculated by dividing the difference between the respective blood vessel paths by the difference in propagation time (step S142).
- the CPU 10 calculates the variation degree of the pulse wave propagation speed with respect to the frequency characteristics of the pulse wave propagation speed calculated from the pulse wave propagation speed for each frequency calculated in step S142 (step S144).
- the CPU 10 applies pulse wave propagation to the frequency characteristics of the pulse wave propagation velocity calculated from the pulse wave propagation velocity for each frequency calculated in step S142.
- a frequency interval of fluctuations occurring in the speed is calculated (step S146). Note that only one of steps S144 and S146 may be performed.
- the CPU 10 determines whether or not a condition indicating the possibility that an aneurysm exists is satisfied based on the information calculated in step S144 and / or S146 (step S148).
- the CPU 10 determines whether or not the variation degree of the pulse wave velocity calculated in step S144 exceeds a predetermined threshold level, or the frequency interval of the fluctuation generated in the pulse wave velocity calculated in step S146. Is less than a predetermined threshold frequency.
- the CPU 10 When the condition indicating the possibility of the presence of an aneurysm is satisfied (YES in step S148), the CPU 10 is on the blood vessel path from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached. Assuming that there is a possibility that a predetermined lesion such as an aneurysm exists, the evaluation result is output to the display unit 4 (step S150). Further, the CPU 10 causes the display unit 4 to output information indicating the size of the aneurysm that is estimated to exist based on the value of the frequency interval of the fluctuation generated in the pulse wave velocity calculated in step S146 (step S146). S152) Then, the measurement process ends.
- step S148 if the condition indicating the possibility of the presence of an aneurysm is not satisfied (NO in step S148), the CPU 10 moves from the heart of the subject 200 to the measurement position where the press cuffs 24a and 24b are attached. Assuming that there is a low possibility that a predetermined lesion such as an aneurysm is present on the vascular route, the evaluation result is output to the display unit 4 (step S154). Then, the measurement process ends.
- the presence or absence of a lesion such as an aneurysm can be determined only by measuring the pulse wave signal from the lower limb and upper limb of the subject. Therefore, it is possible to diagnose an aneurysm with a simpler configuration and with a simpler procedure.
- the pulse wave velocity (PWV) that has been conventionally used for diagnosis can be used, harmony with existing diagnosis methods is easy.
- a measurement method for evaluating an aneurysm that may occur in a vascular route may be realized by executing a program.
- a program is stored in a computer-readable recording medium such as a flexible disk attached to the computer, a CD-ROM (Compact Disk-Read Only Memory), a ROM, a RAM, and a memory card, and provided as a program product. You can also.
- the program can be provided by being stored in a recording medium such as a hard disk built in the computer.
- a program can also be provided by downloading via a network.
- the program according to the present embodiment is a program module that is provided as a part of a computer operating system (OS) and calls necessary modules in a predetermined arrangement at a predetermined timing to execute processing. There may be. In that case, the program itself does not include the module, and the process is executed in cooperation with the OS. A program that does not include such a module can also be included in the program according to the present embodiment.
- OS computer operating system
- the program according to the present embodiment may be provided by being incorporated in a part of another program. Even in this case, the program itself does not include the module included in the other program, and the process is executed in cooperation with the other program. Such a program incorporated in another program can also be included in the program according to the present embodiment.
- the provided program product is installed in a program storage unit such as a hard disk and executed.
- the program product includes the program itself and a recording medium in which the program is stored.
- This embodiment can be used to screen aneurysms and estimate the size with a relatively simple configuration and procedure, and can be used to detect abdominal aortic aneurysms and thoracic aortic aneurysms. It has industrial applicability such as.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Cardiology (AREA)
- Engineering & Computer Science (AREA)
- Vascular Medicine (AREA)
- Public Health (AREA)
- Molecular Biology (AREA)
- Physics & Mathematics (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Pathology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Physiology (AREA)
- Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Signal Processing (AREA)
- Psychiatry (AREA)
- Computer Vision & Pattern Recognition (AREA)
- Artificial Intelligence (AREA)
- Neurosurgery (AREA)
- Ophthalmology & Optometry (AREA)
- Measuring Pulse, Heart Rate, Blood Pressure Or Blood Flow (AREA)
Abstract
Description
本実施の形態に係る測定方法は、動脈瘤を、弾性管路の部分的な拡張に見立てて、透過する脈波の損失に着目したものである。このような動脈瘤のモデルを、全身動脈の脈波伝播モデルに適用することで、周波数特性の変化から、動脈瘤の有無および/または大きさ(内半径および長さ)を評価する。
<b1:モデル化>
本実施の形態においては、被験者の血管経路を複数区間に区切り、各区間を一次元線形分布定数モデル化することで、血管経路を示す伝達関数を算出する。この伝達関数は、脈波が血管を伝播する力学的なモデルから解析的に算出される。
次に、図1に示すようなモデルにおいて、心臓から送出される脈波の伝播に係る力学的および物理的な観点から、数学的な解析を行なう。
次に、動脈瘤を模した中間部の形状(内半径および長さ)が変化することによる影響について検討する。これは、後述するように、動脈瘤の大きさと実際に観測される脈波との関係をモデル上において評価することを意味する。以下では、中間部の内半径RA、ヤング率EA、長さdがそれぞれ変化した場合の透過損失への影響を検討する。
まず、内半径RAの変化について検討する。ここで、式変形および評価の便宜上、周辺部((0-1)区間および(2-3)区間)の内半径RBに対する中間部((1-2)区間)の内半径RAの比αRを導入する。すなわち、比αRは、次の式(26)のように表わすことができる。
(1)透過損失Tの極大値は、周辺部の内半径RBに対する中間部の内半径RAの比αRの増加に伴って大きくなる。
次に、動脈瘤を模した中間部の長さdの変化について検討する。長さdは、上述の式(21)より中間部の伝播定数γAに影響を与えず、かつ、上述の式(24)より中間部の特性インピーダンスZ0Aにも影響を与えない。したがって、式(30)に式(22)を代入して、角周波数ωfについて解くと、次の式(32)を導出できる。
次に、ヤング率の変化について検討する。ここで、式変形および評価の便宜上、周辺部((0-1)区間および(2-3)区間)のヤング率に対する中間部((1-2)区間)のヤング率の比αEを導入する。すなわち、比αEは、次の式(33)のように表わすことができる。
(i)透過損失のシミュレーション
次に、上述した透過損失Tを示す式に基づいて、脈波が動脈瘤を通過することにより生じる透過損失の変化について検討する。上述の式(17)より、点0と点3との間の圧力p0,p3の透過損失を計算して、脈波伝播への影響を求める。ここでは、腹部に生じる大動脈瘤を想定し、腹動脈(Abdominal aorta)に近い値として、次の表1に示す諸元を採用した。
次に、全身動脈の脈波伝播モデルを用いて、上述の伝達関数に現れる特徴について検討する。より具体的には、血管壁および血液の粘性の影響、血管径のテーパの影響、血管の分岐および末梢の影響、伝達関数測定点の影響を含めた場合でも、動脈瘤が検出可能であることを確認する。
以上のような解析的アプローチによれば、動脈瘤を検出するために、以下のような点に着目すればよいと言える。
したがって、動脈瘤の存在は、透過損失Tの大きさおよび基本周波数により推定でき、動脈瘤の大きさ(内径および長さ)は、全体のヤング率の計測により推定できる。
2.伝達関数のゲイン(振幅)が極値(極大値/極小値)をとる周波数間隔(基本周波数)に基づいて、動脈瘤の長さを推定する。このとき、伝達関数の位相変化は、基本周波数に対応する。
次に、上述したような解析的な検討に基づいて、被験者の動脈瘤の有無および/または大きさを検出するための具体的な装置構成について説明する。
図12を参照して、測定装置100は、処理部2と、表示部4と、操作部6と、測定部20a,20bとを含む。
<d1:概要>
実施の形態1として、それぞれ検出された脈波信号から、押圧カフ24aが装着された計測位置(被験者の心臓から動脈瘤の発生が予想される部分を経た血管経路の計測位置)と、押圧カフ24bが装着された計測位置(被験者の心臓から動脈瘤の発生が予想される部分とは異なる部分を経た血管経路の計測位置)との間の圧力の伝達関数を算出し、この算出した伝達関数を用いて、動脈瘤の有無および/または大きさを検出する構成について説明する。実施の形態1においては、伝達関数に関する位相差特性のばらつき度合いに基づいて、動脈瘤の有無を検出する。上述したように、伝達関数の位相線図では、基本周波数ごとに位相が180度変化することになる。基本周波数が低い(狭い)場合には、より多くの頻度でこの位相が生じることになる。また、基本周波数は、動脈瘤の長さに反比例するので、基本周波数が相対的に低い(狭い)場合には、動脈瘤が存在する確率が高いといえる。したがって、ばらつき度合いが大きいほど、動脈瘤が存在する確率が高いと判断できる。
図13は、実施の形態1に係る動脈瘤を検出するための処理を実現する機能ブロックを示す模式図である。図13に示す各機能ブロックは、典型的には、処理部2のCPU10(図12)がROM12などに予め格納されているプログラムに従って演算処理を実行することで実現される。
次に、実施の形態1に係る動脈瘤の判断に係る処理手順について説明する。
本実施の形態によれば、被験者の下肢および上肢からそれぞれ脈波信号を測定するだけで、動脈瘤などの病変の有無を判断することができる。そのため、より簡素な構成で、かつ、より簡単な手順で、動脈瘤についての診断を行なうことができる。また、本実施の形態によれば、比較的高い精度で検出できる脈波信号の位相信号を用いて、動脈瘤などの病変の有無を判断するので、判断精度を高めることができる。
<e1:概要>
実施の形態2においては、上述の実施の形態1と同様に、それぞれ検出された脈波信号から、押圧カフ24aが装着された計測位置(被験者の心臓から動脈瘤の発生が予想される部分を経た血管経路の計測位置)と、押圧カフ24bが装着された計測位置(被験者の心臓から動脈瘤の発生が予想される部分とは異なる部分を経た血管経路の計測位置)との間の圧力の伝達関数を算出し、この算出した伝達関数を用いて、動脈瘤の有無および/または大きさを検出する構成について説明する。実施の形態2においては、脈波信号についての周波数ごとの位相遅れ時間に基づいて、基準の位相差特性を算出する。より具体的には、脈波信号についての周波数ごとの位相遅れ時間の平均に基づいて、位相線図における位相角を算出する。そして、この基準の位相差特性と伝達関数に関する位相差特性とが交差する回数、または、交差する周波数間隔に基づいて、動脈瘤の有無および大きさを検出する。上述したように、伝達関数の位相線図では、基本周波数ごとに位相が180度変化することになる。基本周波数が低い(狭い)場合には、より多くの頻度でこの位相が生じることになる。また、基本周波数は、動脈瘤の長さに反比例するので、基本周波数が相対的に低い(狭い)場合には、動脈瘤が存在する確率が高いといえる。したがって、被験者の血管経路の状態から生じるであろう位相遅れを基準として、この基準の特性特性からのずれに基づいて、動脈瘤の存在およびその大きさ(内径および長さ)を推定できる。
図16は、実施の形態2に係る動脈瘤を検出するための処理を実現する機能ブロックを示す模式図である。図16に示す各機能ブロックは、典型的には、処理部2のCPU10(図12)がROM12などに予め格納されているプログラムに従って演算処理を実行することで実現される。
次に、実施の形態2に係る動脈瘤の判断に係る処理手順について説明する。
本実施の形態によれば、被験者の下肢および上肢からそれぞれ脈波信号を測定するだけで、動脈瘤などの病変の有無を判断することができる。そのため、より簡素な構成で、かつ、より簡単な手順で、動脈瘤についての診断を行なうことができる。また、本実施の形態によれば、動脈瘤の有無に加えて、その大きさについても推定できるので、存在が推定される動脈瘤の進行状況についても推定することができる。
<f1:概要>
実施の形態3においても、上述の実施の形態1および2と同様に、それぞれ検出された脈波信号から、押圧カフ24aが装着された計測位置(被験者の心臓から動脈瘤の発生が予想される部分を経た血管経路の計測位置)と、押圧カフ24bが装着された計測位置(被験者の心臓から動脈瘤の発生が予想される部分とは異なる部分を経た血管経路の計測位置)との間の圧力の伝達関数を算出し、この算出した伝達関数を用いて、動脈瘤の有無および/または大きさを検出する構成について説明する。実施の形態3においては、伝達関数のゲイン特性において極値が生じる周波数間隔に基づいて、動脈瘤の有無および/または大きさを検出する。上述したように、伝達関数のゲイン線図において、極値(極大値/極小値)が生じる周波数間隔が基本周波数であり、この基本周波数は、動脈瘤の長さに反比例するので、基本周波数が相対的に低い(狭い)場合には、動脈瘤が存在する確率が高いといえる。
図19は、実施の形態3に係る動脈瘤を検出するための処理を実現する機能ブロックを示す模式図である。図19に示す各機能ブロックは、典型的には、処理部2のCPU10(図12)がROM12などに予め格納されているプログラムに従って演算処理を実行することで実現される。
次に、実施の形態3に係る動脈瘤の判断に係る処理手順について説明する。
本実施の形態によれば、被験者の下肢および上肢からそれぞれ脈波信号を測定するだけで、動脈瘤などの病変の有無を判断することができる。そのため、より簡素な構成で、かつ、より簡単な手順で、動脈瘤についての診断を行なうことができる。また、本実施の形態によれば、基本周波数を直接的に算出できるので、動脈瘤の大きさの推定をより高い精度で行なうことができる。
<g1:概要>
実施の形態4においては、それぞれ検出された脈波信号の間の脈波伝播速度についての周波数特性を算出し、この算出した脈波伝播速度についての周波数特性における、脈波伝播速度のばらつき、および/または、脈波伝播速度に生じる変動の周波数間隔に基づいて、動脈瘤の有無および/または大きさを検出する。
図22は、実施の形態4に係る動脈瘤を検出するための処理を実現する機能ブロックを示す模式図である。図22に示す各機能ブロックは、典型的には、処理部2のCPU10(図12)がROM12などに予め格納されているプログラムに従って演算処理を実行することで実現される。
次に、実施の形態4に係る動脈瘤の判断に係る処理手順について説明する。
本実施の形態によれば、被験者の下肢および上肢からそれぞれ脈波信号を測定するだけで、動脈瘤などの病変の有無を判断することができる。そのため、より簡素な構成で、かつ、より簡単な手順で、動脈瘤についての診断を行なうことができる。また、本実施の形態によれば、従来から診断に利用されている脈波伝播速度(PWV)を利用できるので、既存の診断手法との調和が容易である。
本実施の形態に係る血管経路に発生し得る動脈瘤を評価するための測定方法を、プログラムの実行によって実現してもよい。このようなプログラムは、コンピュータに付属するフレキシブルディスク、CD-ROM(Compact Disk-Read Only Memory)、ROM、RAMおよびメモリカードなどのコンピュータ読取可能な記録媒体に格納されて、プログラム製品として提供することもできる。あるいは、コンピュータに内蔵するハードディスクなどの記録媒体に格納して、プログラムを提供することもできる。また、ネットワークを介したダウンロードによって、プログラムを提供することもできる。
Claims (12)
- 被験者の心臓から動脈瘤の発生が予想される部分を経た血管経路の第1の計測位置、および、前記被験者の心臓から前記動脈瘤の発生が予想される部分とは異なる部分を経た血管経路の第2の計測位置において、それぞれの脈波信号を検出するための検出手段と、
前記脈波信号の間の周波数特性を比較することで比較結果を算出するための比較手段と、
前記比較結果に含まれる周波数についての所定の特徴量に基づいて、動脈瘤の有無および大きさの少なくとも一方を判断するための判断手段とを備える、測定装置。 - 前記比較手段は、前記第1の計測位置と前記第2の計測位置との間の伝達関数を算出するための手段を含む、請求項1に記載の測定装置。
- 前記判断手段は、前記伝達関数の位相差特性における位相のばらつき度合いに基づいて、動脈瘤の有無を判断する、請求項2に記載の測定装置。
- 前記比較手段は、前記脈波信号について周波数ごとの位相遅れ時間を算出するための手段を含み、
前記判断手段は、算出された周波数ごとの位相遅れ時間の平均に基づいて算出される位相角に対して、前記伝達関数の位相差特性が交差する回数に基づいて、動脈瘤の有無および大きさの少なくとも一方を判断する、請求項2に記載の測定装置。 - 前記判断手段は、算出された周波数ごとの位相遅れ時間の平均に基づいて算出される位相角に対して、前記伝達関数の位相差特性が交差する周波数間隔に基づいて、動脈瘤の大きさを判断する、請求項4に記載の測定装置。
- 前記比較手段は、前記脈波信号について周波数ごとの位相遅れ時間を算出するための手段を含み、
前記判断手段は、算出された周波数ごとの位相遅れ時間の平均に基づいて算出される位相角に対して、前記伝達関数の位相差特性が交差する周波数間隔に基づいて、動脈瘤の有無および大きさの少なくとも一方を判断する、請求項2に記載の測定装置。 - 前記判断手段は、前記伝達関数のゲイン特性において極値が生じる周波数間隔に基づいて、動脈瘤の有無および大きさの少なくとも一方を判断する、請求項2に記載の測定装置。
- 前記比較手段は、前記脈波信号の間の脈波伝播速度についての周波数特性を算出するための手段を含む、請求項1に記載の測定装置。
- 前記判断手段は、前記脈波伝播速度についての周波数特性における脈波伝播速度のばらつき度合いに基づいて、動脈瘤の有無を判断する、請求項8に記載の測定装置。
- 前記判断手段は、前記脈波伝播速度についての周波数特性における脈波伝播速度に生じる変動の周波数間隔に基づいて、動脈瘤の有無および大きさの少なくとも一方を判断する、請求項8に記載の測定装置。
- 被験者の心臓から動脈瘤の発生が予想される部分を経た血管経路の第1の計測位置、および、前記被験者の心臓から前記動脈瘤の発生が予想される部分とは異なる部分を経た血管経路の第2の計測位置において、それぞれの脈波信号を検出するステップと、
前記脈波信号の間の周波数特性を比較することで比較結果を算出するステップと、
前記比較結果に含まれる周波数についての所定の特徴量に基づいて、動脈瘤の有無および大きさの少なくとも一方を判断するステップとを含む、測定方法。 - 測定プログラムを格納した記録媒体であって、コンピュータで実行されると、コンピュータに、
被験者の心臓から動脈瘤の発生が予想される部分を経た血管経路の第1の計測位置、および、前記被験者の心臓から前記動脈瘤の発生が予想される部分とは異なる部分を経た血管経路の第2の計測位置において、それぞれの脈波信号を検出するステップと、
前記脈波信号の間の周波数特性を比較することで比較結果を算出するステップと、
前記比較結果に含まれる周波数についての所定の特徴量に基づいて、動脈瘤の有無および大きさの少なくとも一方を判断するステップとを実行させる、測定プログラムを格納した記録媒体。
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE112012004474.6T DE112012004474B4 (de) | 2011-10-28 | 2012-10-22 | Messvorrichtung, Messverfahren und Messprogramm speicherndes Aufzeichnungsmedium |
| US14/353,475 US10085650B2 (en) | 2011-10-28 | 2012-10-22 | Measurement device, measurement method, and recording medium storing a measurement program |
| CN201280052202.XA CN103957780B (zh) | 2011-10-28 | 2012-10-22 | 测定装置 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-237583 | 2011-10-28 | ||
| JP2011237583A JP5842539B2 (ja) | 2011-10-28 | 2011-10-28 | 測定装置、測定装置の作動方法および測定プログラム |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013061911A1 true WO2013061911A1 (ja) | 2013-05-02 |
Family
ID=48167743
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/077213 WO2013061911A1 (ja) | 2011-10-28 | 2012-10-22 | 測定装置、測定方法および測定プログラムを格納した記録媒体 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US10085650B2 (ja) |
| JP (1) | JP5842539B2 (ja) |
| CN (1) | CN103957780B (ja) |
| DE (1) | DE112012004474B4 (ja) |
| WO (1) | WO2013061911A1 (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018123194A1 (ja) * | 2016-12-27 | 2018-07-05 | オムロンヘルスケア株式会社 | 上下肢の脈波波形から腹部大動脈瘤(aaa)を検出する原理 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6248735B2 (ja) * | 2014-03-24 | 2017-12-20 | オムロンヘルスケア株式会社 | 血管指標値算出装置、血管指標値算出方法、および、血管指標値算出プログラム |
| JP6240581B2 (ja) * | 2014-09-24 | 2017-11-29 | 株式会社アドバンテスト | 脈波センサユニット |
| DE112019004512T5 (de) * | 2018-09-10 | 2021-06-24 | Mitsubishi Electric Corporation | Informationsverarbeitungseinrichtung, programm undinformationsverarbeitungsverfahren |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4008711A (en) * | 1975-06-30 | 1977-02-22 | Charles P. Olinger | Method and apparatus for non-invasive detection of intracranial aneurysms |
| JP2004049331A (ja) * | 2002-07-17 | 2004-02-19 | Nippon Colin Co Ltd | 動脈硬化診断装置 |
| JP2004261319A (ja) * | 2003-02-28 | 2004-09-24 | Colin Medical Technology Corp | 動脈狭窄診断装置 |
| JP2008246010A (ja) * | 2007-03-30 | 2008-10-16 | Kyoto Univ | 血管状態評価装置、血管状態評価方法および血管状態評価プログラム |
| JP2009112428A (ja) * | 2007-11-02 | 2009-05-28 | A & D Co Ltd | 脈波検出用圧迫帯、およびそれを備えた自動血圧測定装置、血管柔軟度測定装置、脈波伝播速度測定装置。 |
| JP2010284517A (ja) * | 2009-05-14 | 2010-12-24 | Delta Tooling Co Ltd | 腹部大動脈瘤検出装置 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3105952B2 (ja) | 1991-07-25 | 2000-11-06 | 日機装株式会社 | 超音波診断装置 |
| US6331162B1 (en) * | 1999-02-01 | 2001-12-18 | Gary F. Mitchell | Pulse wave velocity measuring device |
| DE102006008509A1 (de) | 2006-02-23 | 2007-08-02 | Siemens Ag | Verfahren und Vorrichtung zur verbesserten automatischen Detektion von Auffälligkeiten in medizinischen Bilddaten |
| WO2008004159A2 (en) * | 2006-07-05 | 2008-01-10 | Philips Intellectual Property & Standards Gmbh | Wearable blood pressure monitoring system |
| US8047998B2 (en) * | 2007-04-17 | 2011-11-01 | General Electric Company | Non-invasive blood pressure determination method |
| US8352021B2 (en) * | 2008-04-15 | 2013-01-08 | Christopher Scheib | Method and system for jointly monitoring physiological conditions |
| JP5615073B2 (ja) * | 2010-07-20 | 2014-10-29 | オムロンヘルスケア株式会社 | 測定装置 |
-
2011
- 2011-10-28 JP JP2011237583A patent/JP5842539B2/ja active Active
-
2012
- 2012-10-22 DE DE112012004474.6T patent/DE112012004474B4/de active Active
- 2012-10-22 US US14/353,475 patent/US10085650B2/en active Active
- 2012-10-22 WO PCT/JP2012/077213 patent/WO2013061911A1/ja active Application Filing
- 2012-10-22 CN CN201280052202.XA patent/CN103957780B/zh active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4008711A (en) * | 1975-06-30 | 1977-02-22 | Charles P. Olinger | Method and apparatus for non-invasive detection of intracranial aneurysms |
| JP2004049331A (ja) * | 2002-07-17 | 2004-02-19 | Nippon Colin Co Ltd | 動脈硬化診断装置 |
| JP2004261319A (ja) * | 2003-02-28 | 2004-09-24 | Colin Medical Technology Corp | 動脈狭窄診断装置 |
| JP2008246010A (ja) * | 2007-03-30 | 2008-10-16 | Kyoto Univ | 血管状態評価装置、血管状態評価方法および血管状態評価プログラム |
| JP2009112428A (ja) * | 2007-11-02 | 2009-05-28 | A & D Co Ltd | 脈波検出用圧迫帯、およびそれを備えた自動血圧測定装置、血管柔軟度測定装置、脈波伝播速度測定装置。 |
| JP2010284517A (ja) * | 2009-05-14 | 2010-12-24 | Delta Tooling Co Ltd | 腹部大動脈瘤検出装置 |
Non-Patent Citations (1)
| Title |
|---|
| HIRONORI SATO ET AL.: "An Estimation Method of Arterial Pathology by Pulse Wave Propagation Measurements (Seitai Keisoku - Hyoka II)", SYMPOSIUM ON SPORTS ENGINEERING : SYMPOSIUM ON HUMAN DYNAMICS, 2 November 2010 (2010-11-02), pages 425 - 428 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018123194A1 (ja) * | 2016-12-27 | 2018-07-05 | オムロンヘルスケア株式会社 | 上下肢の脈波波形から腹部大動脈瘤(aaa)を検出する原理 |
Also Published As
| Publication number | Publication date |
|---|---|
| DE112012004474T5 (de) | 2014-07-10 |
| DE112012004474B4 (de) | 2024-10-17 |
| JP5842539B2 (ja) | 2016-01-13 |
| JP2013094264A (ja) | 2013-05-20 |
| CN103957780A (zh) | 2014-07-30 |
| US20140257117A1 (en) | 2014-09-11 |
| US10085650B2 (en) | 2018-10-02 |
| CN103957780B (zh) | 2015-11-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5615073B2 (ja) | 測定装置 | |
| JP5109027B2 (ja) | 血管状態評価装置、血管状態評価方法および血管状態評価プログラム | |
| Vardoulis et al. | Validation of a novel and existing algorithms for the estimation of pulse transit time: advancing the accuracy in pulse wave velocity measurement | |
| US20220160328A1 (en) | Fluid Flow Analysis | |
| US10349838B2 (en) | Methods and apparatus for determining arterial pulse wave velocity | |
| Grabovskis et al. | Effect of probe contact pressure on the photoplethysmographic assessment of conduit artery stiffness | |
| Borlotti et al. | Experimental evaluation of local wave speed in the presence of reflected waves | |
| JP5842539B2 (ja) | 測定装置、測定装置の作動方法および測定プログラム | |
| WO2021250142A1 (en) | Systems and methods for obtaining a pulse wave velocity measurement | |
| Migliavacca et al. | Calculating blood flow from Doppler measurements in the systemic-to-pulmonary artery shunt after the Norwood operation: a method based on computational fluid dynamics | |
| JP5773281B2 (ja) | 血管疾患を判定するためのプログラム、媒体および装置 | |
| US11730381B1 (en) | Systems, apparatuses, and methods for locating blood flow turbulence in the cardiovascular system | |
| Zhang et al. | Pulse transit time-based blood pressure estimation using hilbert-huang transform | |
| JP6484787B2 (ja) | 診断支援装置及びコンピュータプログラム | |
| Liu et al. | The underlying mechanism of intersite discrepancies in ejection time measurements from arterial waveforms and its validation in the Framingham Heart Study | |
| Joseph et al. | Non-invasive estimation of arterial compliance | |
| US20200345323A1 (en) | Fluid flow analysis | |
| JP5958806B2 (ja) | 超音波診断装置および血流量推定プログラム | |
| Gerónimo et al. | Computational modelling and application of mechanical waves to detect arterial network anomalies: Diagnosis of common carotid stenosis | |
| Zhao et al. | A Comparison of Ultrasound-Based QA and ln (D) U Methods for Measurement Local Pulse Wave Velocity | |
| Lopez et al. | Study on continuous blood pressure estimation by pulse transit time | |
| Lancaster et al. | Continuous, Real-Time, Noninvasive Hemodynamic Cardiac Doppler Monitoring With a Novel Hands-Free Device: A Feasibility Study Compared to Standard Echo | |
| Reuderink et al. | Fluid flow through distensible models of the carotid artery bifurcation | |
| Li | Propagation and reflection of pulse waves in flexible tubes and relation to wall properties |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12843305 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE2 | Request for preliminary examination filed before expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 14353475 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 112012004474 Country of ref document: DE Ref document number: 1120120044746 Country of ref document: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12843305 Country of ref document: EP Kind code of ref document: A1 |