WO2011139718A1 - Compositions and methods useful for reducing the viscosity of protein-containing formulations - Google Patents
Compositions and methods useful for reducing the viscosity of protein-containing formulations Download PDFInfo
- Publication number
- WO2011139718A1 WO2011139718A1 PCT/US2011/034001 US2011034001W WO2011139718A1 WO 2011139718 A1 WO2011139718 A1 WO 2011139718A1 US 2011034001 W US2011034001 W US 2011034001W WO 2011139718 A1 WO2011139718 A1 WO 2011139718A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- antibody
- protein
- arginine
- viscosity
- formulation
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39591—Stabilisation, fragmentation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
- A61K47/183—Amino acids, e.g. glycine, EDTA or aspartame
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/20—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing sulfur, e.g. dimethyl sulfoxide [DMSO], docusate, sodium lauryl sulfate or aminosulfonic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/08—Solutions
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2812—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD4
Definitions
- the invention relates to use of certain compounds including, for example, certain charged amino acids and structural analogs thereof, for reducing the viscosity of aqueous protein-containing formulations.
- Certain compounds including, for example, certain charged amino acids and structural analogs thereof, for reducing the viscosity of aqueous protein-containing formulations.
- Associated compositions of matter and methods of use are also contemplated within the present invention.
- Protein-based therapy (including antibody-based therapy) is usually administered on a regular basis and requires several mg/kg dosing by injection.
- Subcutaneous injection is a typical route of administration of these therapies. Because of the small volumes used for subcutaneous injection (usually 1.0 ml- 1.2 ml), for high dose antibody therapies, this route of administration requires the creation of high concentration protein formulations (e.g., 50 mg/ml - 300 mg/ml).
- the present invention is based upon the novel finding that certain molecules, including certain charged amino acids and derivitives, precursors or structural analogs thereof, are useful as additives to protein-containing formulations for the purpose of reducing the viscosity of those formulations in aqueous form.
- the invention relates to a composition of matter comprising a protein and a compound that is capable of reducing the viscosity of an aqueous formulation comprising said protein.
- the protein is an antibody.
- the compound that is capable of reducing the viscosity of an aqueous formulation comprising said protein is selected from the group consisting of arginine (either arginine-HCl or arginine in the presence of a succinate counterion, e.g., arginine succinate), arginine dipeptide, arginine tripeptide, polyarginine, homoarginine,
- Such compounds may be present in the formulation at a concentration which is at least 10 mM, preferably at least 20 mM, more preferably at least 50 mM, yet more preferably at least 100 mM, yet more preferably at a concentration between about 10 mM and 1 M.
- the composition may be in either aqueous or lyophilized form.
- the composition of matter may have a viscosity of no greater than about 150 cP, preferably no greater than about 120 cP, preferably no greater than about 100 cP, preferably no greater than about 90 cP, preferably no greater than about 80 cP, preferably no greater than about 70 cP, preferably no greater than about 60 cP, preferably no greater than about 50 cP, preferably no greater than about 40 cP.
- Total protein concentration present in the composition of matter is at least 50 mg/ml, preferably at least 75 mg/ml, more preferably at least 100 mg/ml, more preferably at least 150 mg/ml, more preferably at least 200 mg/ml, more preferably at least 250 mg/ml, more preferably at least 300 mg/ml.
- Another aspect of the present invention is directed to an article of manufacture comprising a container holding any of the herein described compositions of matter.
- a method for reducing the viscosity of a protein- containing formulation comprising the step of adding to the formulation a viscosity reducing amount of a compound that is capable of reducing the viscosity of an aqueous formulation comprising said protein.
- the protein is an antibody.
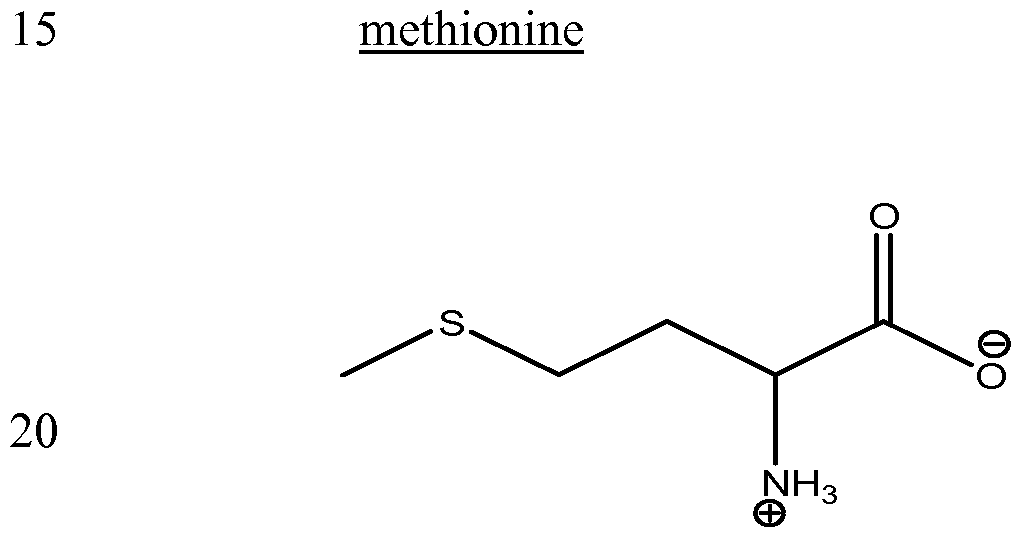
- the compound that is capable of reducing the viscosity of an aqueous formulation comprising said protein is selected from the group consisting of arginine (either arginine-HCl or arginine in the presence of a succinate counterion, e.g., arginine succinate), arginine dipeptide, arginine tripeptide, polyarginine, homoarginine, 2-amino-3-guanidino-propionic acid, guanidine, ornithine, agmatine, guanidobutyric acid, urea, citrulline, N-hydroxy-L-nor-arginine, nitroarginine methyl ester, argininamide, arginine methyl ester, arginine ethyl ester, lysine, lysinamide, lysine methyl ester, histidine, histidine methyl ester, histamine, alanine, alaninamide,
- Such compounds may be added to the formulation to reach a final concentration which is at least 10 mM, preferably at least 20 mM, more preferably at least 50 mM, yet more preferably at least 100 mM, yet more preferably at a concentration between about 10 mM and 1 M.
- the method further comprises the step of lyophilizing the formulation after the compound that is capable of reducing the viscosity of an aqueous formulation comprising said protein is added.
- the formulation may have a viscosity of no greater than about 150 cP, preferably no greater than about 120 cP, preferably no greater than about 100 cP, preferably no greater than about 90 cP, preferably no greater than about 80 cP, preferably no greater than about 70 cP, preferably no greater than about 60 cP, preferably no greater than about 50 cP, preferably no greater than about 40 cP.
- Total protein concentration present in the formulation is at least 50 mg/ml, preferably at least 75 mg/ml, more preferably at least 100 mg/ml, more preferably at least 150 mg/ml, more preferably at least 200 mg/ml, more preferably at least 250 mg/ml, more preferably at least 300 mg/ml.
- a method for preparing an aqueous protein- containing formulation, wherein the method comprises the step of adding to the formulation a viscosity reducing amount of a compound that is capable of reducing the viscosity of an aqueous formulation comprising said protein.
- the protein is an antibody.
- the compound that is capable of reducing the viscosity of an aqueous formulation comprising said protein is selected from the group consisting of arginine (either arginine-HCl or arginine in the presence of a succinate counterion, e.g., arginine succinate), arginine dipeptide, arginine tripeptide, polyarginine, homoarginine, 2-amino-3-guanidino-propionic acid, guanidine, ornithine, agmatine, guanidobutyric acid, urea, citrulline, N-hydroxy-L-nor-arginine, nitroarginine methyl ester, argininamide, arginine methyl ester, arginine ethyl ester, lysine, lysinamide, lysine methyl ester, histidine, histidine methyl ester, histamine, alanine, alaninamide,
- Such compounds may be added to the formulation to reach a final concentration which is at least 10 mM, preferably at least 20 mM, more preferably at least 50 mM, yet more preferably at least 100 mM, yet more preferably at a concentration between about 10 mM and 1 M.
- the formulation may have a viscosity of no greater than about 150 cP, preferably no greater than about 120 cP, preferably no greater than about 100 cP, preferably no greater than about 90 cP, preferably no greater than about 80 cP, preferably no greater than about 70 cP, preferably no greater than about 60 cP, preferably no greater than about 50 cP, preferably no greater than about 40 cP.
- Total protein concentration present in the formulation is at least 50 mg/ml, preferably at least 75 mg/ml, more preferably at least 100 mg/ml, more preferably at least 150 mg/ml, more preferably at least 200 mg/ml, more preferably at least 250 mg/ml, more preferably at least 300 mg/ml.
- the present invention is based upon the novel finding that certain compounds including, for example, certain charged amino acids and structural analogs thereof, for reducing the viscosity of aqueous protein-containing formulations. Accordingly, in one aspect, the present invention describes compositions of matter comprising a protein and a compound capable of reducing the viscosity of an aqueous formulation comprising the protein.
- compounds identified herein as being capable of reducing the viscosity of an aqueous formulation comprising a protein include, for example:
- the above described compounds may be employed singly as a viscosity reducing agent, or may be employed in combination with other viscosity reducing agents. Such compounds may be added to the protein-containing formulation to reach a final concentration (either singly or in combination) which is at least 10 mM, preferably at least 20 mM, more preferably at least 50 mM, yet more preferably at least 100 mM, yet more preferably at a concentration between about 10 mM and 1 M.
- the viscosity reducing agents of the present invention find use in reducing the viscosity of protein-containing formulations, wherein the protein concentration in the formulation is at least about 50 mg/ml, preferably at least 75 mg/ml, more preferably at least 100 mg/ml, more preferably at least 150 mg/ml, more preferably at least 200 mg/ml, more preferably at least 250 mg/ml, more preferably at least 300 mg/ml.
- the protein-containing formulation (after addition of the compound capable of reducing the viscosity of an aqueous protein-containing formulation) may have a viscosity of no greater than about 150 cP, preferably no greater than about 120 cP, preferably no greater than about 100 cP, preferably no greater than about 90 cP, preferably no greater than about 80 cP, preferably no greater than about 70 cP, preferably no greater than about 60 cP, preferably no greater than about 50 cP, preferably no greater than about 40 cP.
- polypeptide or “protein” is meant a sequence of amino acids for which the chain length is sufficient to produce the higher levels of tertiary and/or quaternary structure.
- proteins are distinguished from “peptides” which are also amino acid- based molecules that do not have such structure.
- a protein for use herein will have a molecular weight of at least about 5-20 kD, alternatively at least about 15-20 kD, preferably at least about 20 kD.
- Peptide is meant a sequence of amino acids that generally does not exhibit a higher level of tertiary and/or quaternary structure. Peptides generally have a molecular weight of less than about 5 kD.
- polypeptides encompassed within the definition herein include mammalian proteins, such as, e.g., renin; a growth hormone, including human growth hormone and bovine growth hormone; growth hormone releasing factor; parathyroid hormone; thyroid stimulating hormone; lipoproteins; alpha- 1 -antitrypsin; insulin A-chain; insulin B-chain; proinsulin; follicle stimulating hormone; calcitonin; luteinizing hormone; glucagon; clotting factors such as factor VIIIC, factor IX, tissue factor, and von Willebrands factor; anti-clotting factors such as Protein C; atrial natriuretic factor; lung surfactant; a plasminogen activator, such as urokinase or human urine or tissue-type plasminogen activator (t-PA); bombesin; thrombin; hemopoietic growth factor; tumor necrosis factor-alpha and -beta; enkephalinase; RANTES (regulated on activation normally
- BDNF neurotrophin-3, -4, -5, or -6
- a nerve growth factor such as NGF- ⁇
- platelet-derived growth factor (PDGF) fibroblast growth factor such as aFGF and bFGF
- EGF epidermal growth factor
- TGF transforming growth factor
- TGF-alpha and TGF-beta including TGF- ⁇ , TGF-p2, TGF- 3, TGF- 4, or TGF- 5
- insulin-like growth factor-I and -II IGF-I and IGF-I
- des(l-3)-IGF-I brain IGF-I
- insulin-like growth factor binding proteins IGFBPs
- CD proteins such as CD3, CD4, CD8, CD 19 and CD20; erythropoietin; osteoinductive factors; immunotoxins; a bone morphogenetic protein (BMP); an interferon such as interferon-alpha, -beta, and -
- the protein which is formulated is preferably essentially pure and desirably essentially homogeneous (i.e., free from contaminating proteins).
- Essentially pure protein means a composition comprising at least about 90% by weight of the protein, based on total weight of the composition, preferably at least about 95% by weight.
- Essentially homogeneous protein means a composition comprising at least about 99% by weight of protein, based on total weight of the composition.
- the protein is an antibody.
- the antibody herein is directed against an "antigen" of interest.
- the antigen is a biologically important protein and administration of the antibody to a mammal suffering from a disease or disorder can result in a therapeutic benefit in that mammal.
- antibodies directed against non- protein antigens are also contemplated.
- the antigen is a protein, it may be a transmembrane molecule (e.g., receptor) or ligand such as a growth factor.
- Exemplary antigens include those proteins discussed above.
- CD polypeptides such as CD3, CD4, CD8, CD19, CD20 and CD34; members of the HER receptor family such as the EGF receptor (HER1), HER2, HER3 or HER4 receptor; cell adhesion molecules such as LFA-1, Macl, pl50,95, VLA-4, ICAM-1, VCAM and av/b3 integrin including either a or b subunits thereof (e.g., anti-CD 11a, anti-CD 18 or anti-CD l ib antibodies); growth factors such as VEGF; IgE; blood group antigens; flk2/flt3 receptor; obesity (OB) receptor; mpl receptor; CTLA-4; polypeptide C etc.
- HER1 EGF receptor
- HER2 HER2
- HER3 or HER4 receptor cell adhesion molecules
- cell adhesion molecules such as LFA-1, Macl, pl50,95, VLA-4, ICAM-1, VCAM and av/b3 integrin including either a
- Soluble antigens or fragments thereof, optionally conjugated to other molecules, can be used as immunogens for generating antibodies.
- immunogens for transmembrane molecules, such as receptors, fragments of these ⁇ e.g., the extracellular domain of a receptor) can be used as the immunogen.
- transmembrane molecules such as receptors
- fragments of these ⁇ e.g., the extracellular domain of a receptor can be used as the immunogen.
- cells expressing the transmembrane molecule can be used as the immunogen.
- Such cells can be derived from a natural source ⁇ e.g., cancer cell lines) or may be cells which have been transformed by recombinant techniques to express the transmembrane molecule.
- antibodies to be purified herein include, but are not limited to: HER2 antibodies including trastuzumab (HERCEPTIN®) (Carter et al, Proc. Natl. Acad. Sci.
- VEGF or VEGF receptor antibodies including humanized and/or affinity matured VEGF antibodies such as the humanized VEGF antibody huA4.6.1 bevacizumab (AVASTIN®) and ranibizumab (LUCENTIS®) (Kim et al, Growth Factors, 7:53-64 (1992), International Publication No.
- CD25 or Tac antibodies such as CHI-621 (SIMULECT®) and ZENAPAX® (See US Patent No. 5,693,762 issued December 2, 1997); CD4 antibodies such as the cM-7412 antibody (Choy et al, Arthritis Rheum 39(l):52-56 (1996)); CD52 antibodies such as CAMPATH-1H (ILEX/Berlex) (Riechmann et al, Nature 332:323- 337 (1988)); Fc receptor antibodies such as the M22 antibody directed against FcyRI as in Graziano et al, J. Immunol. 155(10):4996-5002 (1995)); carcinoembryonic antigen (CEA) antibodies such as hMN-14 (Sharkey et al, Cancer Res. 55(23Suppl): 5935s-
- CD33 antibodies such as Hu M195 (Jurcic et al, Cancer Res 55(23 Suppl):5908s-5910s (1995)) and CMA-676 or CDP771; EpCAM antibodies such as 17-1 A (PANOREX®); GpIIb/IIIa antibodies such as abciximab or c7E3 Fab (REOPRO®); RSV antibodies such as MEDI- 493 (SYNAGIS®); CMV antibodies such as PROTOVIR®; HIV antibodies such as PR0542; hepatitis antibodies such as the Hep B antibody OSTAVIR®; CA125 antibody including anti-MUC16 (WO2007/001851; Yin, BWT and Lloyd, KO, J.
- chemokine receptor antibody such as a CCR2 antibody (e.g., MLN1202; Millieneum); anti-complement antibody, such as C5 antibody (e.g., eculizumab, 5G1.1; Alexion); oral formulation of human immunoglobulin (e.g., IgPO; Protein Therapeutics); IL-12 antibody such as ABT-874 (CAT/ Abbott); Teneliximab (BMS-224818; BMS); CD40 antibodies, including S2C6 and humanized variants thereof (WO00/75348) and TNX 100 (Chiron/Tanox); TNF-a antibodies including cA2 or infliximab (REMIC ADE® ) , CDP571, MAK-195, adalimumab (HUMIRATM), pegylated TNF-a antibody fragment such as CDP-870 (Celltech), D2E7 (Knoll), anti-TNF-a polyclonal antibody (
- CD20 antibodies include: “C2B8,” which is now called “rituximab” (“RITUXAN®”) (US Patent No. 5,736,137); the yttrium-[90]-labelled 2B8 murine antibody designated “Y2B8” or “Ibritumomab Tiuxetan” (ZEVALIN®) commercially available from IDEC Pharmaceuticals, Inc. (US Patent No. 5,736,137; 2B8 deposited with ATCC under accession no. HB11388 on June 22, 1993); murine IgG2a "Bl,” also called
- Tositumomab optionally labelled with I to generate the "131I-B1" or “iodine 1131 tositumomab” antibody (BEXXARTM) commercially available from Corixa (see, also, US Patent No. 5,595,721); murine monoclonal antibody “1F5" (Press et al., Blood 69(2):584- 591 (1987)) and variants thereof including "framework patched” or humanized 1F5 (WO 2003/002607, Leung, S.; ATCC deposit HB-96450); murine 2H7 and chimeric 2H7 antibody (US Patent No.
- TRU 015 (Trubion).
- antibody as used herein includes monoclonal antibodies (including full length antibodies which have an immunoglobulin Fc region), antibody compositions with polyepitopic specificity, multispecific antibodies ⁇ e.g., bispecific antibodies), diabodies, peptibodies, and single-chain molecules, as well as antibody fragments ⁇ e.g., Fab, F(ab') 2 , and Fv), any of which may optionally be conjugated to another component, e.g., a toxin.
- immunoglobulin Ig
- Ig immunoglobulin
- the basic 4-chain antibody unit is a heterotetrameric glycoprotein composed of two identical light (L) chains and two identical heavy (H) chains.
- An IgM antibody consists of 5 of the basic heterotetramer unit along with an additional polypeptide called a
- IgA antibodies comprise from 2-5 of the basic 4-chain units which can polymerize to form polyvalent assemblages in combination with the J chain.
- the 4-chain unit is generally about 150,000 daltons.
- Each L chain is linked to an H chain by one covalent disulfide bond, while the two H chains are linked to each other by one or more disulfide bonds depending on the H chain isotype.
- Each H and L chain also has regularly spaced intrachain disulfide bridges.
- Each H chain has at the N-terminus, a variable domain (V H ) followed by three constant domains (C H ) for each of the a and ⁇ chains and four C H domains for ⁇ and ⁇ isotypes.
- Each L chain has at the N-terminus, a variable domain (V L ) followed by a constant domain at its other end.
- the V L is aligned with the V H and the C L is aligned with the first constant domain of the heavy chain (C H I).
- Particular amino acid residues are believed to form an interface between the light chain and heavy chain variable domains.
- the pairing of a V H and V L together forms a single antigen-binding site.
- immunoglobulins can be assigned to different classes or isotypes. There are five classes of immunoglobulins: IgA, IgD, IgE, IgG and IgM, having heavy chains designated ⁇ , ⁇ , ⁇ , ⁇ . and ⁇ , respectively.
- the ⁇ and a classes are further divided into subclasses on the basis of relatively minor differences in the CH sequence and function, e.g., humans express the following subclasses: IgGl, IgG2, IgG3, IgG4, IgAl and IgA2.
- variable refers to the fact that certain segments of the variable domains differ extensively in sequence among antibodies.
- the V domain mediates antigen binding and defines the specificity of a particular antibody for its particular antigen.
- variability is not evenly distributed across the entire span of the variable domains. Instead, the V regions consist of relatively invariant stretches called framework regions (FRs) of about 15-30 amino acid residues separated by shorter regions of extreme variability called “hypervariable regions” or sometimes “complementarity determining regions” (CDRs) that are each approximately 9-12 amino acid residues in length.
- FRs framework regions
- hypervariable regions or sometimes “complementarity determining regions”
- variable domains of native heavy and light chains each comprise four FRs, largely adopting a ⁇ -sheet configuration, connected by three hypervariable regions, which form loops connecting, and in some cases forming part of, the ⁇ -sheet structure.
- the hypervariable regions in each chain are held together in close proximity by the FRs and, with the hypervariable regions from the other chain, contribute to the formation of the antigen binding site of antibodies (see Kabat et ah, Sequences of Proteins of Immunological Interest, 5th Ed. Public Health Service, National Institutes of Health, Bethesda, Md. (1991).
- the constant domains are not involved directly in binding an antibody to an antigen, but exhibit various effector functions, such as participation of the antibody dependent cellular cytotoxicity (ADCC).
- ADCC antibody dependent cellular cytotoxicity
- hypervariable region also known as “complementarity determining regions” or CDRs
- CDRs complementarity determining regions
- two residue identification techniques define regions of overlapping, but not identical regions, they can be combined to define a hybrid CDR.
- monoclonal antibody refers to an antibody obtained from a population of substantially homogeneous antibodies, i.e., the individual antibodies comprising the population are identical except for possible naturally occurring mutations and/or post-translation modifications (e.g., isomerizations, amidations) that may be present in minor amounts. Monoclonal antibodies are highly specific, being directed against a single antigenic site. Furthermore, in contrast to conventional (polyclonal) antibody preparations which typically include different antibodies directed against different determinants (epitopes), each monoclonal antibody is directed against a single determinant on the antigen.
- the monoclonal antibodies are advantageous in that they are synthesized by the hybridoma culture, uncontaminated by other immunoglobulins.
- the modifier "monoclonal” indicates the character of the antibody as being obtained from a substantially homogeneous population of antibodies, and is not to be construed as requiring production of the antibody by any particular method.
- the monoclonal antibodies to be used in accordance with the present invention may be made by the hybridoma method first described by Kohler et al., Nature, 256: 495 (1975), or may be made by recombinant DNA methods (see, e.g., U.S. Pat. No. 4,816,567).
- the "monoclonal antibodies” may also be isolated from phage antibody libraries using the techniques described in Clackson et al., Nature, 352:624-628 (1991) and Marks et al., J. Mol. Biol., 222:581-597 (1991), for example.
- the monoclonal antibodies herein specifically include "chimeric" antibodies (immunoglobulins) in which a portion of the heavy and/or light chain is identical with or homologous to corresponding sequences in antibodies derived from a particular species or belonging to a particular antibody class or subclass, while the remainder of the chain(s) is (are) identical with or homologous to corresponding sequences in antibodies derived from another species or belonging to another antibody class or subclass, as well as fragments of such antibodies, so long as they exhibit the desired biological activity (U.S. Pat. No. 4,816,567; Morrison et al, Proc. Natl. Acad. Sci. USA, 81 :6851 -6855 (1984)).
- Chimeric antibodies of interest herein include "primitized" antibodies comprising variable domain antigen-binding sequences derived from a non-human primate ⁇ e.g., Old World Monkey, Ape etc.) and human content region sequences.
- an “intact” antibody is one which comprises an antigen-binding site as well as a CL and at least the heavy chain domains, C H I , C H 2 and C H 3.
- the constant domains may be native sequence constant domains ⁇ e.g., human native sequence constant domains) or amino acid sequence variants thereof.
- the intact antibody has one or more effector functions.
- antibody fragment comprises a portion of an intact antibody, preferably the antigen binding and/or the variable region of the intact antibody.
- antibody fragments include Fab, Fab', F(ab') 2 and Fv fragments; diabodies; linear antibodies (see U.S. Pat. No. 5,641 ,870, Example 2; Zapata et al, Protein Eng. 8(10): 1057- 1062 [ 1995]); single-chain antibody molecules and multispecific antibodies formed from antibody fragments.
- Papain digestion of antibodies produced two identical antigen-binding fragments, called “Fab” fragments, and a residual "Fc” fragment, a designation reflecting the ability to crystallize readily.
- the Fab fragment consists of an entire L chain along with the variable region domain of the H chain (V H ), and the first constant domain of one heavy chain (C H I).
- V H variable region domain of the H chain
- C H I first constant domain of one heavy chain
- Each Fab fragment is monovalent with respect to antigen binding, i.e., it has a single antigen-binding site.
- Pepsin treatment of an antibody yields a single large F(ab') 2 fragment which roughly corresponds to two disulfide linked Fab fragments having different antigen-binding activity and is still capable of cross-linking antigen.
- Fab' fragments differ from Fab fragments by having a few additional residues at the carboxy terminus of the C H I domain including one or more cysteines from the antibody hinge region.
- Fab'-SH is the designation herein for Fab' in which the cysteine residue(s) of the constant domains bear a free thiol group.
- F(ab') 2 antibody fragments originally were produced as pairs of Fab' fragments which have hinge cysteines between them. Other chemical couplings of antibody fragments are also known.
- the Fc fragment comprises the carboxy-terminal portions of both H chains held together by disulfides.
- the effector functions of antibodies are determined by sequences in the Fc region, the region which is also recognized by Fc receptors (FcR) found on certain types of cells.
- Fv is the minimum antibody fragment which contains a complete antigen- recognition and -binding site. This fragment consists of a dimer of one heavy- and one light-chain variable region domain in tight, non-covalent association. From the folding of these two domains emanate six hypervarible loops (3 loops each from the H and L chain) that contribute the amino acid residues for antigen binding and confer antigen binding specificity to the antibody. However, even a single variable domain (or half of an Fv comprising only three CDRs specific for an antigen) has the ability to recognize and bind antigen, although at a lower affinity than the entire binding site.
- Single-chain Fv also abbreviated as “sFv” or “scFv” are antibody fragments that comprise the VH and VL antibody domains connected into a single polypeptide chain.
- the sFv polypeptide further comprises a polypeptide linker between the V H and V L domains which enables the sFv to form the desired structure for antigen binding.
- diabodies refers to small antibody fragments prepared by constructing sFv fragments (see preceding paragraph) with short linkers (about 5-10) residues) between the V H and V L domains such that inter-chain but not intra-chain pairing of the V domains is achieved, thereby resulting in a bivalent fragment, i.e., a fragment having two antigen-binding sites.
- Bispecific diabodies are heterodimers of two "crossover" sFv fragments in which the V H and V L domains of the two antibodies are present on different polypeptide chains.
- Diabodies are described in greater detail in, for example, EP 404,097; WO 93/11161; Hollinger et al, Proc. Natl. Acad. Sci. USA 90: 6444-6448 (1993).
- the antibodies of the invention may further comprise humanized antibodies or human antibodies.
- Humanized forms of non-human (e.g., murine) antibodies are chimeric immunoglobulins, immunoglobulin chains or fragments thereof (such as Fv, Fab, Fab', F(ab')2 or other antigen-binding subsequences of antibodies) which contain minimal sequence derived from non-human immunoglobulin.
- Humanized antibodies include human immunoglobulins (recipient antibody) in which residues from a complementary determining region (CDR) of the recipient are replaced by residues from a CDR of a non-human species (donor antibody) such as mouse, rat or rabbit having the desired specificity, affinity and capacity.
- CDR complementary determining region
- Fv framework residues of the human immunoglobulin are replaced by corresponding non-human residues.
- Humanized antibodies may also comprise residues which are found neither in the recipient antibody nor in the imported CDR or framework sequences.
- the humanized antibody will comprise substantially all of at least one, and typically two, variable domains, in which all or substantially all of the CDR regions correspond to those of a non-human immunoglobulin and all or substantially all of the FR regions are those of a human immunoglobulin consensus sequence.
- the humanized antibody optimally also will comprise at least a portion of an immunoglobulin constant region (Fc), typically that of a human immunoglobulin [Jones et al., Nature, 321 :522-525 (1986); Riechmann et al., Nature, 332:323-329 (1988); and Presta, Curr. Op. Struct. Biol, 2:593-596 (1992)].
- Fc immunoglobulin constant region
- a humanized antibody has one or more amino acid residues introduced into it from a source which is non-human. These non-human amino acid residues are often referred to as "import" residues, which are typically taken from an "import” variable domain. Humanization can be essentially performed following the method of Winter and co-workers [Jones et al, Nature, 321 :522-525 (1986); Riechmann et al, Nature, 332:323- 327 (1988); Verhoeyen et al., Science, 239: 1534-1536 (1988)], by substituting rodent CDRs or CDR sequences for the corresponding sequences of a human antibody.
- humanized antibodies are chimeric antibodies (U.S. Patent No. 4,816,567), wherein substantially less than an intact human variable domain has been substituted by the corresponding sequence from a non-human species.
- humanized antibodies are typically human antibodies in which some CDR residues and possibly some FR residues are substituted by residues from analogous sites in rodent antibodies.
- variable domains both light and heavy
- HAMA response human anti-mouse antibody
- the sequence of the variable domain of a rodent antibody is screened against the entire library of known human variable domain sequences.
- the human V domain sequence which is closest to that of the rodent is identified and the human framework region (FR) within it accepted for the humanized antibody (Sims et al, J. Immunol. 151 :2296 (1993); Chothia et al, J. Mol. Biol, 196:901 (1987)).
- Another method uses a particular framework region derived from the consensus sequence of all human antibodies of a particular subgroup of light or heavy chains.
- the same framework may be used for several different humanized antibodies (Carter et al., Proc. Natl. Acad. Sci. USA, 89:4285 (1992); Presta et al, J. Immunol. 151 :2623 (1993)).
- humanized antibodies are prepared by a process of analysis of the parental sequences and various conceptual humanized products using three-dimensional models of the parental and humanized sequences.
- Three-dimensional immunoglobulin models are commonly available and are familiar to those skilled in the art.
- Computer programs are available which illustrate and display probable three- dimensional conformational structures of selected candidate immunoglobulin sequences. Inspection of these displays permits analysis of the likely role of the residues in the functioning of the candidate immunoglobulin sequence, i.e., the analysis of residues that influence the ability of the candidate immunoglobulin to bind its antigen.
- FR residues can be selected and combined from the recipient and import sequences so that the desired antibody characteristic, such as increased affinity for the target antigen(s), is achieved.
- the hypervariable region residues are directly and most substantially involved in influencing antigen binding.
- the humanized antibody may be an antibody fragment, such as a Fab, which is optionally conjugated with one or more cytotoxic agent(s) in order to generate an immunoconjugate.
- the humanized antibody may be an intact antibody, such as an intact IgGl antibody.
- human antibodies can be generated.
- transgenic animals e.g., mice
- transgenic animals e.g., mice
- JH antibody heavy-chain joining region
- transfer of the human germ- line immunoglobulin gene array into such germ- line mutant mice will result in the production of human antibodies upon antigen challenge. See, e.g., Jakobovits et al, Proc. Natl. Acad. Sci.
- phage display technology (McCafferty et al, Nature 348:552-553 [1990]) can be used to produce human antibodies and antibody fragments in vitro, from immunoglobulin variable (V) domain gene repertoires from unimmunized donors.
- V immunoglobulin variable
- antibody V domain genes are cloned in-frame into either a major or minor coat protein gene of a filamentous bacteriophage, such as Ml 3 or fd, and displayed as functional antibody fragments on the surface of the phage particle.
- a filamentous bacteriophage such as Ml 3 or fd
- the filamentous particle contains a single-stranded DNA copy of the phage genome
- selections based on the functional properties of the antibody also result in selection of the gene encoding the antibody exhibiting those properties.
- the phage mimics some of the properties of the B-cell.
- Phage display can be performed in a variety of formats, reviewed in, e.g., Johnson, Kevin S. and Chiswell, David J., Current Opinion in Structural Biology 3:564-571 (1993).
- V-gene segments can be used for phage display.
- Clackson et al. Nature, 352:624-628 (1991) isolated a diverse array of anti- oxazolone antibodies from a small random combinatorial library of V genes derived from the spleens of immunized mice.
- a repertoire of V genes from unimmunized human donors can be constructed and antibodies to a diverse array of antigens (including self- antigens) can be isolated essentially following the techniques described by Marks et al, J. Mol. Biol. 222:581-597 (1991), or Griffith et al, EMBO J. 12:725-734 (1993). See, also, also,
- Human antibodies may also be generated by in vitro activated B cells (see U.S. Patents 5,567,610 and 5,229,275).
- Bispecific antibodies are antibodies that have binding specificities for at least two different epitopes.
- Exemplary bispecific antibodies may bind to two different epitopes of a protein as described herein.
- Other such antibodies may combine a protein binding site with a binding site for another protein.
- an anti-protein arm may be combined with an arm which binds to a triggering molecule on a leukocyte such as a T- cell receptor molecule (e.g. CD3) (see, e.g., Baeuerle, et al, Curr. Opin. Mol. Ther. l l(l):22-30 (2009)), or Fc receptors for IgG (FcyR), such as FcyRI (CD64), FcyRII
- Bispecific antibodies may also be used to localize cytotoxic agents to cells which express a target protein. These antibodies possess a protein-binding arm and an arm which binds the cytotoxic agent (e.g., saporin, anti-interferon-a, vinca alkaloid, ricin A chain, methotrexate or radioactive isotope hapten). Bispecific antibodies can be prepared as full length antibodies or antibody fragments (e.g., F(ab')2 bispecific antibodies).
- WO 96/16673 describes a bispecific anti-ErbB2/anti-FcYRIII antibody and U.S.
- Patent No. 5,837,234 discloses a bispecific anti-ErbB2/anti-FcyRI antibody.
- a bispecific anti-ErbB2/Fca antibody is shown in WO98/02463.
- U.S. Patent Nos. 5,821,337 and 6,407,213 teach bispecific anti-ErbB2/anti-CD3 antibodies. Additional bispecific antibodies that bind an epitope on the CD3 antigen and a second epitope have been described. See, for example, U.S. Patent Nos. 5,078,998 (anti-CD3/tumor cell antigen);
- bispecific antibodies are known in the art. Traditional production of full length bispecific antibodies is based on the co-expression of two immunoglobulin heavy chain-light chain pairs, where the two chains have different specificities (Millstein et al, Nature 305:537-539 (1983)). Because of the random assortment of immunoglobulin heavy and light chains, these hybridomas (quadromas) produce a potential mixture of 10 different antibody molecules, of which only one has the correct bispecific structure. Purification of the correct molecule, which is usually done by affinity chromatography steps, is rather cumbersome, and the product yields are low. Similar procedures are disclosed in WO 93/08829, and in Traunecker et al, EMBO J. 10:3655-3659 (1991).
- antibody variable domains with the desired binding specificities are fused to immunoglobulin constant domain sequences.
- the fusion is with an Ig heavy chain constant domain, comprising at least part of the hinge, CH2, and CH3 regions. It is preferred to have the first heavy-chain constant region (CHI) containing the site necessary for light chain bonding, present in at least one of the fusions.
- DNAs encoding the immunoglobulin heavy chain fusions and, if desired, the immunoglobulin light chain are inserted into separate expression vectors, and are co-transfected into a suitable host cell.
- the bispecific antibodies are composed of a hybrid immunoglobulin heavy chain with a first binding specificity in one arm, and a hybrid immunoglobulin heavy chain-light chain pair (providing a second binding specificity) in the other arm. It was found that this asymmetric structure facilitates the separation of the desired bispecific compound from unwanted immunoglobulin chain combinations, as the presence of an immunoglobulin light chain in only one half of the bispecific molecule provides for a facile way of separation. This approach is disclosed in WO 94/04690. For further details of generating bispecific antibodies see, for example, Suresh et al, Methods in Enzymology 121 :210 (1986).
- the interface between a pair of antibody molecules can be engineered to maximize the percentage of heterodimers which are recovered from recombinant cell culture.
- the preferred interface comprises at least a part of the CH3 domain.
- one or more small amino acid side chains from the interface of the first antibody molecule are replaced with larger side chains (e.g., tyrosine or tryptophan).
- Compensatory "cavities" of identical or similar size to the large side chain(s) are created on the interface of the second antibody molecule by replacing large amino acid side chains with smaller ones (e.g., alanine or threonine). This provides a mechanism for increasing the yield of the heterodimer over other unwanted end-products such as homodimers.
- Bispecific antibodies include cross-linked or "heteroconjugate" antibodies.
- one of the antibodies in the heteroconjugate can be coupled to avidin, the other to biotin.
- Such antibodies have, for example, been proposed to target immune system cells to unwanted cells (U.S. Patent No. 4,676,980), and for treatment of HIV infection (WO 91/00360, WO 92/200373, and EP 03089).
- Heteroconjugate antibodies may be made using any convenient cross-linking methods. Suitable cross-linking agents are well known in the art, and are disclosed in U.S. Patent No. 4,676,980, along with a number of cross-linking techniques. Techniques for generating bispecific antibodies from antibody fragments have also been described in the literature.
- bispecific antibodies can be prepared using chemical linkage.
- Brennan et al, Science 229:81 (1985) describe a procedure wherein intact antibodies are proteolytically cleaved to generate F(ab')2 fragments. These fragments are reduced in the presence of the dithiol complexing agent, sodium arsenite, to stabilize vicinal dithiols and prevent intermolecular disulfide formation.
- the Fab' fragments generated are then converted to thionitrobenzoate (TNB) derivatives.
- One of the Fab'-TNB derivatives is then reconverted to the Fab'-thiol by reduction with mercaptoethylamine and is mixed with an equimolar amount of the other Fab'-TNB derivative to form the bispecific antibody.
- the bispecific antibodies produced can be used as agents for the selective immobilization of enzymes.
- bispecific antibodies have been produced using leucine zippers.
- the leucine zipper peptides from the Fos and Jun proteins were linked to the Fab' portions of two different antibodies by gene fusion.
- the antibody homodimers were reduced at the hinge region to form monomers and then re-oxidized to form the antibody heterodimers. This method can also be utilized for the production of antibody homodimers.
- the fragments comprise a VH connected to a VL by a linker which is too short to allow pairing between the two domains on the same chain. Accordingly, the VH and VL domains of one fragment are forced to pair with the complementary VL and VH domains of another fragment, thereby forming two antigen-binding sites.
- Another strategy for making bispecific antibody fragments by the use of single-chain Fv (sFv) dimers has also been reported. See Gruber et al, J. Immunol, 152:5368 (1994).
- Antibodies with more than two valencies are contemplated.
- trispecific antibodies can be prepared. Tutt et al., J. Immunol. 147:60 (1991).
- Heteroconjugate antibodies are also within the scope of the present invention.
- Heteroconjugate antibodies are composed of two covalently joined antibodies. Such antibodies have, for example, been proposed to target immune system cells to unwanted cells [U.S. Patent No. 4,676,980], and for treatment of HIV infection [WO 91/00360; WO 92/200373; EP 03089]. It is contemplated that the antibodies may be prepared in vitro using known methods in synthetic protein chemistry, including those involving crosslinking agents. For example, immunotoxins may be constructed using a disulfide exchange reaction or by forming a thioether bond. Examples of suitable reagents for this purpose include iminothiolate and methyl-4-mercaptobutyrimidate and those disclosed, for example, in U.S. Patent No. 4,676,980.
- a multivalent antibody may be internalized (and/or catabolized) faster than a bivalent antibody by a cell expressing an antigen to which the antibodies bind.
- the antibodies of the present invention can be multivalent antibodies (which are other than of the IgM class) with three or more antigen binding sites (e.g. tetravalent antibodies), which can be readily produced by recombinant expression of nucleic acid encoding the polypeptide chains of the antibody.
- the multivalent antibody can comprise a dimerization domain and three or more antigen binding sites.
- the preferred dimerization domain comprises (or consists of) an Fc region or a hinge region. In this scenario, the antibody will comprise an Fc region and three or more antigen binding sites amino- terminal to the Fc region.
- the preferred multivalent antibody herein comprises (or consists of) three to about eight, but preferably four, antigen binding sites.
- the multivalent antibody comprises at least one polypeptide chain (and preferably two polypeptide chains), wherein the polypeptide chain(s) comprise two or more variable domains.
- the polypeptide chain(s) may comprise VDl-(Xl)n-VD2-(X2)n- Fc, wherein VD1 is a first variable domain, VD2 is a second variable domain, Fc is one polypeptide chain of an Fc region, XI and X2 represent an amino acid or polypeptide, and n is 0 or 1.
- the polypeptide chain(s) may comprise: VH-CH1 -flexible linker-VH-CHl-Fc region chain; or VH-CHl-VH-CHl-Fc region chain.
- the multivalent antibody herein preferably further comprises at least two (and preferably four) light chain variable domain polypeptides.
- the multivalent antibody herein may, for instance, comprise from about two to about eight light chain variable domain polypeptides.
- the light chain variable domain polypeptides contemplated here comprise a light chain variable domain and, optionally, further comprise a CL domain.
- An antibody that "specifically binds to” or is “specific for” a particular polypeptide or an epitope on a particular polypeptide is one that binds to that particular polypeptide or epitope on a particular polypeptide without substantially binding to any other polypeptide or polypeptide epitope.
- solid phase describes a non-aqueous matrix to which the antibody of the present invention can adhere.
- solid phases encompassed herein include those formed partially or entirely of glass (e.g., controlled pore glass), polysaccharides (e.g., agarose), polyacrylamides, polystyrene, polyvinyl alcohol and silicones.
- the solid phase can comprise the well of an assay plate; in others it is a purification column (e.g., an affinity chromotography column).
- This term also includes a discontinuous solid phase of discrete particles, such as those described in U.S. Pat. No. 4,275,149.
- a "species-dependent antibody”, e.g., a mammalian anti-human IgE antibody, is an antibody which has a stronger binding affinity for an antigen from a first mammalian species than it has for a homologue of that antigen from a second mammalian species.
- the species-dependent antibody "bind specifically" to a human antigen (i.e., has a binding affinity (Kd) value of no more than about lxl 0 "7 M, alternatively no more than about 1x10 - " 8 M, alternatively no more than about 1x10 - " 9 M) but has a binding affinity for a homologue of the antigen from a second non-human mammalian species which is at least about 50 fold, at least about 500 fold, or at least about 1000 fold, weaker than its binding affinity for the non-human antigen.
- the species-dependent antibody can be of any of the various types of antibodies as defined above, but preferably is a humanized or human antibody.
- Antibody effector functions refer to those biological activities attributable to the Fc region (a native sequence Fc region or amino acid sequence variant Fc region) of an antibody, and vary with the antibody isotype. Examples of antibody effector functions include: Clq binding and complement dependent cytotoxicity; Fc receptor binding; antibody-dependent cell-mediated cytotoxicity (ADCC); phagocytosis; down regulation of cell surface receptors (e.g. , B cell receptors); and B cell activation.
- ADCC antibody-dependent cell-mediated cytotoxicity
- FcRs Fc receptors
- cytotoxic cells e.g., natural killer (NK) cells, neutrophils and macrophages
- NK cells natural killer cells
- monocytes express FcyRI, FcyRII and FcyRIII.
- Pat. No. 5,500,362 or 5,821,337 may be performed.
- Useful effector cells for such assays include peripheral blood mononuclear cells (PBMC) and natural killer (NK) cells.
- PBMC peripheral blood mononuclear cells
- NK natural killer cells
- ADCC activity of the molecule of interest may be assessed in vivo, e.g., in an animal model such as that disclosed in Clynes et ah, PNAS USA 95:652-656 (1998).
- Fc receptor or “FcR” describes a receptor that binds to the Fc region of an antibody.
- the preferred FcR is a native sequence human FcR.
- a preferred FcR is one which binds an IgG antibody (a gamma receptor) and includes receptors of the FcyRI, FcyRII, and FcyRIII subclasses, including allelic variants and alternatively spliced forms of these receptors, FcyRII receptors include FcyRIIA (an "activating receptor”) and
- FcyRIIB (an “inhibiting receptor”), which have similar amino acid sequences that differ primarily in the cytoplasmic domains thereof.
- Activating receptor FcyRIIA contains an immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic domain.
- Inhibiting receptor FcyRIIB contains an immunoreceptor tyrosine-based inhibition motif (ITIM) in its cytoplasmic domain, (see M. Daeron, Annu. Rev. Immunol. 15:203-234
- FcRs are reviewed in Ravetch and Kinet, Annu. Rev. Immunol. 9: 457-92 (1991); Capel et ah, Immunomethods 4: 25-34 (1994); and de Haas et ah, J. Lab. Clin. Med. 126: 330-41 (1995).
- Other FcRs including those to be identified in the future, are encompassed by the term "FcR” herein.
- the term also includes the neonatal receptor, FcRn, which is responsible for the transfer of maternal IgGs to the fetus. Guyer et ah, J.
- Human effector cells are leukocytes which express one or more FcRs and perform effector functions. Preferably, the cells express at least FcyRIII and perform ADCC effector function. Examples of human leukocytes which mediate ADCC include peripheral blood mononuclear cells (PBMC), natural killer (NK) cells, monocytes, cytotoxic T cells and neutrophils, with PBMCs and MNK cells being preferred.
- PBMC peripheral blood mononuclear cells
- NK natural killer cells
- monocytes cytotoxic T cells and neutrophils
- the effector cells may be isolated from a native source, e.g., blood.
- “Complement dependent cytotoxicity” of “CDC” refers to the lysis of a target cell in the presence of complement. Activation of the classical complement pathway is initiated by the binding of the first component of the complement system (Clq) to antibodies (of the appropriate subclass) which are bound to their cognate antigen.
- a CDC assay e.g., as described in Gazzano-Santoro et ah, J. Immunol. Methods 202: 163 (1996), may be performed.
- isolated when used to describe the various polypeptides and antibodies disclosed herein, means a polypeptide or antibody that has been identified, separated and/or recovered from a component of its production environment.
- the isolated polypeptide is free of association with all other components from its production environment.
- Contaminant components of its production environment such as that resulting from recombinant transfected cells, are materials that would typically interfere with diagnostic or therapeutic uses for the polypeptide, and may include enzymes, hormones, and other proteinaceous or non-proteinaceous solutes.
- the polypeptide will be purified (1) to a degree sufficient to obtain at least 15 residues of N-terminal or internal amino acid sequence by use of a spinning cup sequenator, or (2) to homogeneity by SDS-PAGE under non-reducing or reducing conditions using Coomassie blue or, preferably, silver stain.
- an isolated polypeptide or antibody will be prepared by at least one purification step.
- an "isolated" nucleic acid molecule encoding the polypeptides and antibodies herein is a nucleic acid molecule that is identified and separated from at least one contaminant nucleic acid molecule with which it is ordinarily associated in the environment in which it was produced. Preferably, the isolated nucleic acid is free of association with all components associated with the production environment.
- the isolated nucleic acid molecules encoding the polypeptides and antibodies herein is in a form other than in the form or setting in which it is found in nature. Isolated nucleic acid molecules therefore are distinguished from nucleic acid encoding the polypeptides and antibodies herein existing naturally in cells.
- control sequences refers to DNA sequences necessary for the expression of an operably linked coding sequence in a particular host organism.
- the control sequences that are suitable for prokaryotes include a promoter, optionally an operator sequence, and a ribosome binding site.
- Eukaryotic cells are known to utilize promoters, polyadenylation signals, and enhancers.
- Nucleic acid is "operably linked" when it is placed into a functional relationship with another nucleic acid sequence.
- DNA for a presequence or secretory leader is operably linked to DNA for a polypeptide if it is expressed as a preprotein that participates in the secretion of the polypeptide;
- a promoter or enhancer is operably linked to a coding sequence if it affects the transcription of the sequence; or
- a ribosome binding site is operably linked to a coding sequence if it is positioned so as to facilitate translation.
- "operably linked” means that the DNA sequences being linked are contiguous, and, in the case of a secretory leader, contiguous and in reading phase. However, enhancers do not have to be contiguous. Linking is accomplished by ligation at convenient restriction sites. If such sites do not exist, the synthetic oligonucleotide adaptors or linkers are used in accordance with conventional practice.
- epitope tagged when used herein refers to a chimeric polypeptide comprising a polypeptide or antibody described herein fused to a "tag polypeptide".
- the tag polypeptide has enough residues to provide an epitope against which an antibody can be made, yet is short enough such that it does not interfere with activity of the polypeptide to which it is fused.
- the tag polypeptide preferably also is fairly unique so that the antibody does not substantially cross-react with other epitopes.
- Suitable tag polypeptides generally have at least six amino acid residues and usually between about 8 and 50 amino acid residues (preferably, between about 10 and 20 amino acid residues).
- immunoadhesin designates antibody-like molecules which combine the binding specificity of a heterologous protein (an “adhesin”) with the effector functions of immunoglobulin constant domains.
- the immunoadhesins comprise a fusion of an amino acid sequence with the desired binding specificity which is other than the antigen recognition and binding site of an antibody (i.e., is “heterologous"), and an immunoglobulin constant domain sequence.
- the adhesin part of an immunoadhesin molecule typically is a contiguous amino acid sequence comprising at least the binding site of a receptor or a ligand.
- the immunoglobulin constant domain sequence in the immunoadhesin may be obtained from any immunoglobulin, such as IgG-1, IgG-2, IgG-3, or IgG-4 subtypes, IgA (including IgA-1 and IgA-2), IgE, IgD or IgM.
- the Ig fusions preferably include the substitution of a domain of a polypeptide or antibody described herein in the place of at least one variable region within an Ig molecule.
- the immunoglobulin fusion includes the hinge, CH2 and CH3, or the hinge, CHI, CH2 and
- pharmaceutical formulation refers to a preparation which is in such form as to permit the biological activity of the active ingredient to be effective, and which contains no additional components which are unacceptably toxic to a subject to which the formulation would be administered.
- An antibody possesses "biological activity" in a pharmaceutical formulation, if the biological activity of the antibody at a given time is within about 10% (within the errors of the assay) of the biological activity exhibited at the time the pharmaceutical formulation was prepared, as determined by the ability of the antibody in vitro or in vivo to bind to antigen and result in a measurable biological response.
- a “stable” or “stabilized” formulation is one in which the protein therein essentially retains its physical and/or chemical stability upon storage. Stability can be measured at a selected temperature for a selected time period. Preferably, the formulation is stable at room temperature ( ⁇ 30°C) or at 40°C for at least 1 month and/or stable at about 2-8°C for at least 1 year and preferably for at least 2 years. For example, the extent of aggregation during storage can be used as an indicator of protein stability. Thus, a “stable” formulation may be one wherein less than about 10% and preferably less than about 5% of the protein is present as an aggregate in the formulation.
- aqueous solution refers to a solution in which water is the dissolving medium or solvent.
- a substance dissolves in a liquid, the mixture is termed a solution.
- the dissolved substance is the solute, and the liquid that does the dissolving (in this case water) is the solvent.
- stabilizing agent or “stabilizer” as used herein is a chemical or compound that is added to a solution or mixture or suspension or composition or therapeutic composition to maintain it in a stable or unchanging state; or is one which is used because it produces a reaction involving changes in atoms or molecules leading to a more stable or unchanging state.
- a "viscosity reducing amount" of a compound that is capable of reducing viscosity of an aqueous protein-containing formulation is the amount that measurably reduces the viscosity of the formulation after addition thereto.
- An "isotonic" formulation is one which has essentially the same osmotic pressure as human blood. Isotonic formulations will generally have an osmotic pressure from about 250 to 350 mOsm.
- the term “hypotonic” describes a formulation with an osmotic pressure below that of human blood.
- the term “hypertonic” is used to describe a formulation with an osmotic pressure above that of human blood. Isotonicity can be measured using a vapor pressure or ice-freezing type osmometer, for example.
- a "reconstituted" formulation is one which has been prepared by dissolving a lyophilized protein or antibody formulation in a diluent such that the protein is dispersed in the reconstituted formulation.
- the reconstituted formulation is suitable for administration (e.g., parenteral administration) to a patient to be treated with the protein of interest and, in certain embodiments of the invention, may be one which is suitable for subcutaneous administration.
- “Surfactants” are surface active agents that can exert their effect at surfaces of solid-solid, solid-liquid, liquid-liquid, and liquid-air because of their chemical composition, containing both hydrophilic and hydrophobic groups. These materials reduce the concentration of proteins in dilute solutions at the air- water and/or water-solid interfaces where proteins can be adsorbed and potentially aggregated. Surfactants can bind to hydrophobic interfaces in protein formulations. Proteins on the surface of water will aggregate, particularly when agitated, because of unfolding and subsequent aggregation of the protein monolayer.
- “Surfactants” can denature proteins, but can also stabilize them against surface denaturation. Generally, ionic surfactants can denature proteins. However, nonionic surfactants usually do not denature proteins even at relatively high concentrations (1% w/v). Most parentally acceptable nonionic surfactants come from either the polysorbate or polyether groups. Polysorbate 20 and 80 are contemporary surfactant stabilizers in marketed protein formulations. However, other surfactants used in protein formulations include Pluronic F-68 and members of the "Brij" class. Non-ionic surfactants can be sugar based. Sugar based surfactants can be alkyl glycosides.
- the general structure of the alkyl glycoside is Ri-0-(CH 2 )x-R , where R is independently CH 3 or cyclohexyl (CeHn) and Ri is independently glucose or maltose.
- exemplary alkyl glycosides include those in which Ri is glucose, R is CH 3 , and x is 5 (n-hexyl-P-D-glucopyranoside), x is 6 (n-heptyl- ⁇ -D-glucopyranoside), x is 7 (n-octyl-P-D-glucopyranoside), x is 8 (n-nonyl- ⁇ - ⁇ - glucopyranoside), x is 9 (n-decyl-P-D-glucopyranoside), and x is 11 (n-dodecyl- ⁇ - ⁇ - glucopyranoside).
- glucopyranosides are called glucosides.
- Exemplary alkyl glycosides additionally include those in which Ri is maltose, R is CH , and x is 5 (n- hexyl-P-D-maltopyranoside), x is 7 (n-octyl-P-D-maltopyranoside), x is 8 (n-nonyl- ⁇ - ⁇ - maltopyranoside), x is 9 (n-decyl-P-D-maltopyranoside), x is 10 (n-undecyl- ⁇ - ⁇ - maltopyranoside), x is 11 (n-dodecyl-P-D-maltopyranoside), x is 12 (n-tridecyl-P-D- maltopyranoside), x is 13 (n-tetradecyl-P-D-maltopyranoside), and x is 15 (n-hexadecyl- ⁇ -
- maltopyranosides are called maltosides.
- exemplary alkyl glycosides further include those in which Ri is glucose, x is 3, and R is cyclohexyl (3 -cyclohexyl- 1 -propyl- ⁇ -D-glucoside); and in which Ri is maltose, x is 4, and R is cyclohexyl (4-cyclohexyl-l -butyl- ⁇ -D-maltoside).
- a “pharmaceutically acceptable acid” includes inorganic and organic acids which are non toxic at the concentration and manner in which they are formulated.
- suitable inorganic acids include hydrochloric, perchloric, hydrobromic, hydroiodic, nitric, sulfuric, sulfonic, sulfinic, sulfanilic, phosphoric, carbonic, etc.
- Suitable organic acids include straight and branched-chain alkyl, aromatic, cyclic, cyloaliphatic, arylaliphatic, heterocyclic, saturated, unsaturated, mono, di- and tri-carboxylic, including for example, formic, acetic, 2-hydroxyacetic, trifluoroacetic, phenylacetic, trimethylacetic, t-butyl acetic, anthranilic, propanoic, 2-hydroxypropanoic, 2-oxopropanoic, propandioic, cyclopentanepropionic, cyclopentane propionic, 3-phenylpropionic, butanoic, butandioic, benzoic, 3-(4-hydroxybenzoyl)benzoic, 2-acetoxy-benzoic, ascorbic, cinnamic, lauryl sulfuric, stearic, muconic, mandelic, succinic, embonic, fumaric, malic, maleic, hydroxymaleic
- “Pharmaceutically-acceptable bases” include inorganic and organic bases which are non-toxic at the concentration and manner in which they are formulated.
- suitable bases include those formed from inorganic base forming metals such as lithium, sodium, potassium, magnesium, calcium, ammonium, iron, zinc, copper, manganese, aluminum, N-methylglucamine, morpholine, piperidine and organic nontoxic bases including, primary, secondary and tertiary amine, substituted amines, cyclic amines and basic ion exchange resins, [e.g., N(R') 4 + (where R' is independently H or Ci_ 4 alkyl, e.g., ammonium, Tris)], for example, isopropylamine, trimethylamine, diethylamine, triethylamine, tripropylamine, ethanolamine, 2-diethylaminoethanol, trimethamine, dicyclohexylamine, lysine, arginine, histidine, caffeine, proca
- Additional pharmaceutically acceptable acids and bases useable with the present invention include those which are derived from the amino acids, for example, histidine, glycine, phenylalanine, aspartic acid, glutamic acid, lysine and asparagine.
- “Pharmaceutically acceptable” buffers and salts include those derived from both acid and base addition salts of the above indicated acids and bases. Specific buffers and/or salts include histidine, succinate and acetate.
- a “lyoprotectant” is a molecule which, when combined with a protein of interest, significantly prevents or reduces physicochemical instability of the protein upon lyophilization and subsequent storage.
- exemplary lyoprotectants include sugars and their corresponding sugar alcohols; an amino acid such as monosodium glutamate or histidine; a methylamine such as betaine; a lyotropic salt such as magnesium sulfate; a polyol such as trihydric or higher molecular weight sugar alcohols, e.g.
- glycerin dextran, erythritol, glycerol, arabitol, xylitol, sorbitol, and mannitol; propylene glycol; polyethylene glycol; Pluronics®; and combinations thereof.
- Additional exemplary lyoprotectants include glycerin and gelatin, and the sugars mellibiose, melezitose, raffinose, mannotriose and stachyose.
- reducing sugars include glucose, maltose, lactose, maltulose, iso- maltulose and lactulose.
- non-reducing sugars include non-reducing glycosides of polyhydroxy compounds selected from sugar alcohols and other straight chain polyalcohols.
- Preferred sugar alcohols are monoglycosides, especially those compounds obtained by reduction of disaccharides such as lactose, maltose, lactulose and maltulose.
- the glycosidic side group can be either glucosidic or galactosidic.
- Additional examples of sugar alcohols are glucitol, maltitol, lactitol and iso-maltulose.
- the preferred lyoprotectant are the non-reducing sugars trehalose or sucrose.
- the lyoprotectant is added to the pre-lyophilized formulation in a "lyoprotecting amount" which means that, following lyophilization of the protein in the presence of the lyoprotecting amount of the lyoprotectant, the protein essentially retains its physicochemical stability upon lyophilization and storage.
- a “pharmaceutically acceptable sugar” is a molecule which, when combined with a protein of interest, significantly prevents or reduces physicochemical instability of the protein upon storage.
- “pharmaceutically acceptable sugars” may also be known as a “lyoprotectant”.
- Exemplary sugars and their corresponding sugar alcohols includes: an amino acid such as monosodium glutamate or histidine; a methylamine such as betaine; a lyotropic salt such as magnesium sulfate; a polyol such as trihydric or higher molecular weight sugar alcohols, e.g., glycerin, dextran, erythritol, glycerol, arabitol, xylitol, sorbitol, and mannitol; propylene glycol; polyethylene glycol; Pluronics®; and combinations thereof.
- an amino acid such as monosodium glutamate or histidine
- a methylamine such as betaine
- a lyotropic salt such as magnesium sulfate
- a polyol such as trihydric or higher molecular weight sugar alcohols, e.g., glycerin, dextran, erythritol, glycerol, arabitol
- Additional exemplary lyoprotectants include glycerin and gelatin, and the sugars mellibiose, melezitose, raffinose, mannotriose and stachyose.
- reducing sugars include glucose, maltose, lactose, maltulose, iso-maltulose and lactulose.
- non-reducing sugars include non-reducing glycosides of polyhydroxy compounds selected from sugar alcohols and other straight chain polyalcohols.
- Preferred sugar alcohols are monoglycosides, especially those compounds obtained by reduction of disaccharides such as lactose, maltose, lactulose and maltulose.
- glycosidic side group can be either glucosidic or galactosidic.
- sugar alcohols are glucitol, maltitol, lactitol and iso-maltulose.
- the preferred pharmaceutically-acceptable sugars are the non-reducing sugars trehalose or sucrose.
- diluents are added to the formulation in a "protecting amount" ⁇ e.g., pre-lyophilization) which means that the protein essentially retains its physicochemical stability during storage ⁇ e.g., after reconstitution and storage).
- the "diluent" of interest herein is one which is pharmaceutically acceptable (safe and non-toxic for administration to a human) and is useful for the preparation of a liquid formulation, such as a formulation reconstituted after lyophilization.
- Exemplary diluents include sterile water, bacteriostatic water for injection (BWFI), a pH buffered solution (e-g-, phosphate-buffered saline), sterile saline solution, Ringer's solution or dextrose solution.
- diluents can include aqueous solutions of salts and/or buffers.
- a "preservative” is a compound which can be added to the formulations herein to reduce bacterial activity.
- the addition of a preservative may, for example, facilitate the production of a multi-use (multiple-dose) formulation.
- potential preservatives include octadecyldimethylbenzyl ammonium chloride, hexamethonium chloride, benzalkonium chloride (a mixture of alkylbenzyldimethylammonium chlorides in which the alkyl groups are long-chain compounds), and benzethonium chloride.
- preservatives include aromatic alcohols such as phenol, butyl and benzyl alcohol, alkyl parabens such as methyl or propyl paraben, catechol, resorcinol, cyclohexanol, 3- pentanol, and m-cresol.
- aromatic alcohols such as phenol, butyl and benzyl alcohol
- alkyl parabens such as methyl or propyl paraben
- catechol resorcinol
- cyclohexanol 3- pentanol
- m-cresol m-cresol
- Treatment refers to both therapeutic treatment and prophylactic or preventative measures. Those in need of treatment include those already with the disorder as well as those in which the disorder is to be prevented.
- “Mammal” for purposes of treatment refers to any animal classified as a mammal, including humans, domestic and farm animals, and zoo, sports, or pet animals, such as dogs, horses, rabbits, cattle, pigs, hamsters, gerbils, mice, ferrets, rats, cats, etc.
- the mammal is human.
- a “disorder” is any condition that would benefit from treatment with the protein. This includes chronic and acute disorders or diseases including those pathological conditions which predispose the mammal to the disorder in question.
- disorders to be treated herein include carcinomas and inflammations.
- a “therapeutically effective amount” is at least the minimum concentration required to effect a measurable improvement or prevention of a particular disorder.
- Therapeutically effective amounts of known proteins are well known in the art, while the effective amounts of proteins hereinafter discovered may be determined by standard techniques which are well within the skill of a skilled artisan, such as an ordinary physician.
- “Viscosity,” as used herein, may be “absolute viscosity” or “kinematic viscosity.”
- “Absolute viscosity,” sometimes called dynamic or simple viscosity is a quantity that describes a fluid's resistance to flow.
- Kininematic viscosity is the quotient of absolute viscosity and fluid density.
- Kinematic viscosity is frequently reported when characterizing the resistive flow of a fluid using a capillary viscometer.
- a viscous fluid takes longer than a less viscous fluid to flow through the capillary. If one fluid takes 200 seconds to complete its flow and another fluid takes 400 seconds, the second fluid is twice as viscous as the first on a kinematic viscosity scale. If both fluids have equal density, the second fluid is twice as viscous as the first on an absolute viscosity scale.
- the dimensions of kinematic viscosity are L 2 /T where L represents length and T represents time.
- the SI units of kinematic viscosity are m /s. Commonly, kinematic viscosity is expressed in centistokes, cSt, which is equivalent to mm /s.
- the dimensions of absolute viscosity are M/L/T, where M represents mass and L and T represent length and time, respectively.
- the SI units of absolute viscosity are Pa » s, which is equivalent to kg/m/s.
- the absolute viscosity is commonly expressed in units of centiPoise, cP, which is equivalent to milliPascal-second, mPa » s.
- Antibodies and other proteins may be formulated in accordance with the present invention in either aqueous or lyophilized form, the latter being capable if being reconstituted into an aqueous form.
- the formulations described herein may be prepared as reconstituted lyophilized formulations.
- the proteins or antibodies described herein are lyophilized and then reconstituted to produce the liquid formulations of the invention.
- a "pre- lyophilized formulation" is produced after preparation of the protein of interest as described above.
- the amount of protein present in the pre- lyophilized formulation is determined taking into account the desired dose volumes, mode(s) of administration etc.
- the starting concentration of an intact antibody can be from about 2 mg/ml to about 50 mg/ml, preferably from about 5 mg/ml to about 40 mg/ml and most preferably from about 20-30 mg/ml.
- the protein to be formulated is generally present in solution.
- the protein in the liquid formulations of the invention, may be present in a pH-buffered solution at a pH from about 4-8, and preferably from about 5-7.
- the buffer concentration can be from about 1 mM to about 200 mM, alternatively from about 1 mM to about 100 mM, alternatively from about 1 mM to about 50 mM, alternatively from about 3 mM to about
- Exemplary buffers and/or salts are those which are pharmaceutically acceptable and may be created from suitable acids, bases and salts thereof, such as those which are defined under "pharmaceutically acceptable” acids, bases or buffers.
- a lyoprotectant is added to the pre-lyophilized formulation.
- the amount of lyoprotectant in the pre-lyophilized formulation is generally such that, upon reconstitution, the resulting formulation will be isotonic. However, hypertonic reconstituted formulations may also be suitable.
- the amount of lyoprotectant must not be too low such that an unacceptable amount of degradation/aggregation of the protein occurs upon lyophilization.
- exemplary lyoprotectant concentrations in the pre-lyophilized formulation are from about 10 mM to about 400 mM, alternatively from about 30 mM to about 300 mM, alternatively from about 50 mM to about 100 mM.
- Exemplary lyoprotectants include sugars and sugar alcohols such as sucrose, mannose, trehalose, glucose, sorbitol, mannitol. However, under particular circumstances, certain lyoprotectants may also contribute to an increase in viscosity of the formulation. As such, care should be taken so as to select particular lyoprotectants which minimize or neutralize this effect. Additional lyoprotectants are described above under the definition of "lyoprotectants”, also referred herein as "pharmaceutically-acceptable sugars”.
- the ratio of protein to lyoprotectant can vary for each particular protein or antibody and lyoprotectant combination.
- the molar ratio of lyoprotectant to antibody may be from about 100 to about 1500 moles lyoprotectant to 1 mole antibody, and preferably from about 200 to about 1000 moles of lyoprotectant to 1 mole antibody, for example from about 200 to about 600 moles of lyoprotectant to 1 mole antibody.
- a mixture of the lyoprotectant (such as sucrose or trehalose) and a bulking agent (e.g., mannitol or glycine) may be used in the preparation of the pre-lyophilization formulation.
- the bulking agent may allow for the production of a uniform lyophilized cake without excessive pockets therein etc.
- Other pharmaceutically acceptable carriers, excipients or stabilizers such as those described in Remington 's Pharmaceutical Sciences
- Acceptable carriers, excipients or stabilizers are nontoxic to recipients at the dosages and concentrations employed and include; additional buffering agents; preservatives; co- solvents; antioxidants including ascorbic acid and methionine; chelating agents such as EDTA; metal complexes ⁇ e.g., Zn-protein complexes); biodegradable polymers such as polyesters; and/or salt-forming counterions such as sodium.
- the formulation herein may also contain more than one protein as necessary for the particular indication being treated, preferably those with complementary activities that do not adversely affect the other protein.
- Such proteins are suitably present in combination in amounts that are effective for the purpose intended.
- the formulations to be used for in vivo administration must be sterile. This is readily accomplished by filtration through sterile filtration membranes, prior to, or following, lyophilization and reconstitution. Alternatively, sterility of the entire mixture may be accomplished by autoclaving the ingredients, except for protein, at about 120°C for about 30 minutes, for example.
- the formulation is lyophilized.
- freeze-dryers are available for this purpose such as Hull50TM (Hull, USA) or GT20TM (Leybold-Heraeus, Germany) freeze-dryers. Freeze-drying is accomplished by freezing the formulation and subsequently subliming ice from the frozen content at a temperature suitable for primary drying. Under this condition, the product temperature is below the eutectic point or the collapse temperature of the formulation.
- the shelf temperature for the primary drying will range from about -30 to 25°C (provided the product remains frozen during primary drying) at a suitable pressure, ranging typically from about 50 to 250 mTorr.
- the formulation, size and type of the container holding the sample (e.g., glass vial) and the volume of liquid will mainly dictate the time required for drying, which can range from a few hours to several days (e.g., 40-60 hrs).
- a secondary drying stage may also be performed depending upon the desired residual moisture level in the product.
- the temperature at which the secondary drying is carried out ranges from about 0-40°C, depending primarily on the type and size of container and the type of protein employed.
- the shelf temperature throughout the entire water removal phase of lyophilization may be from about 15-30°C (e.g., about 20°C).
- the time and pressure required for secondary drying will be that which produces a suitable lyophilized cake, dependent, e.g., on the temperature and other parameters.
- the secondary drying time is dictated by the desired residual moisture level in the product and typically takes at least about 5 hours (e.g., 10-15 hours).
- the pressure may be the same as that employed during the primary drying step. Freeze-drying conditions can be varied depending on the formulation and vial size.
- the lyophilized formulation Prior to administration to the patient, the lyophilized formulation is reconstituted with a pharmaceutically acceptable diluent such that the protein concentration in the reconstituted formulation is at least about 50 mg/ml, for example from about 50 mg/ml to about 400 mg/ml, alternatively from about 80 mg/ml to about 300 mg/ml, alternatively from about 90 mg/ml to about 150 mg/ml.
- a pharmaceutically acceptable diluent such that the protein concentration in the reconstituted formulation is at least about 50 mg/ml, for example from about 50 mg/ml to about 400 mg/ml, alternatively from about 80 mg/ml to about 300 mg/ml, alternatively from about 90 mg/ml to about 150 mg/ml.
- Such high protein concentrations in the reconstituted formulation are considered to be particularly useful where subcutaneous delivery of the reconstituted formulation is intended.
- lower concentrations of the protein in the reconstituted formulation may be desired (for example from about 5-50 mg/ml, or from about
- the protein concentration in the reconstituted formulation is significantly higher than that in the pre-lyophilized formulation.
- the protein concentration in the reconstituted formulation may be about 2-40 times, alternatively 3-10 times, alternatively 3-6 times (e.g., at least three fold or at least four fold) that of the pre- lyophilized formulation.
- Reconstitution generally takes place at a temperature of about 25°C to ensure complete hydration, although other temperatures may be employed as desired.
- the time required for reconstitution will depend, e.g., on the type of diluent, amount of excipient(s) and protein.
- Exemplary diluents include sterile water, bacteriostatic water for injection (BWF), a pH buffered solution (e.g., phosphate-buffered saline), sterile saline solution, Ringer's solution or dextrose solution.
- BWF bacteriostatic water for injection
- the diluent optionally contains a preservative. Exemplary preservatives have been described above, with aromatic alcohols such as benzyl or phenol alcohol being the preferred preservatives.
- the amount of preservative employed is determined by assessing different preservative concentrations for compatibility with the protein and preservative efficacy testing. For example, if the preservative is an aromatic alcohol (such as benzyl alcohol), it can be present in an amount from about 0.1-2.0% and preferably from about 0.5-1.5%, but most preferably about 1.0-1.2%.
- aromatic alcohol such as benzyl alcohol
- the reconstituted formulation has less than 6000 particles per vial which are >10 ⁇ in size.
- Therapeutic formulations are prepared for storage by mixing the active ingredient having the desired degree of purity with optional pharmaceutically acceptable carriers, excipients or stabilizers (Remington's Pharmaceutical Sciences 18th edition, Mack Publishing Co., Easton, Pa. 18042 [1990]).
- Acceptable carriers, excipients, or stabilizers are nontoxic to recipients at the dosages and concentrations employed, and include buffers, antioxidants including ascorbic acid, methionine, Vitamin E, sodium metabisulfite, preservatives, isotonicifiers, stabilizers, metal complexes (e.g., Zn-protein complexes), and/or chelating agents such as EDTA.
- the therapeutic agent is an antibody fragment
- the smallest fragment which specifically binds to the binding domain of the target protein is preferred.
- antibody fragments or even peptide molecules can be designed which retain the ability to bind the target protein sequence.
- Such peptides can be synthesized chemically and/or produced by recombinant DNA technology (see, e.g., Marasco et al, Proc. Natl. Acad. Sci. USA 90: 7889-7893
- Buffers are used to control the pH in a range which optimizes the therapeutic effectiveness, especially if stability is pH dependent. Buffers are preferably present at concentrations ranging from about 1 mM to about 200 mM, alternatively from about 1 mM to about 100 mM, alternatively from about 1 mM to about 50 mM, alternatively from about 3 mM to about 15 mM.
- Suitable buffering agents for use with the present invention include both organic and inorganic acids and salts thereof. For example, citrate, phosphate, succinate, tartrate, fumarate, gluconate, oxalate, lactate, acetate. Additionally, buffers may be comprised of histidine and trimethylamine salts such as Tris.
- Preservatives are added to retard microbial growth, and are typically present in a range from 0.2%- 1.0% (w/v).
- Suitable preservatives for use with the present invention include octadecyldimethylbenzyl ammonium chloride; hexamethonium chloride; benzalkonium halides (e.g., chloride, bromide, iodide), benzethonium chloride; thimerosal, phenol, butyl or benzyl alcohol; alkyl parabens such as methyl or propyl paraben; catechol; resorcinol; cyclohexanol, 3-pentanol, and m-cresol.
- octadecyldimethylbenzyl ammonium chloride hexamethonium chloride
- benzalkonium halides e.g., chloride, bromide, iodide
- Tonicity agents sometimes known as “stabilizers” are present to adjust or maintain the tonicity of a liquid composition. When used with large, charged biomolecules such as proteins and antibodies, they are often termed “stabilizers” because they can interact with the charged groups of the amino acid side chains, thereby lessening the potential for inter and intra-molecular interactions.
- Tonicity agents can be present in any amount between 0.1% to 25% by weight, preferably 1 to 5%, taking into account the relative amounts of the other ingredients.
- Preferred tonicity agents include polyhydric sugar alcohols, preferably trihydric or higher sugar alcohols, such as glycerin, erythritol, arabitol, xylitol, sorbitol and mannitol.
- excipients include agents which can serve as one or more of the following: (1) bulking agents, (2) solubility enhancers, (3) stabilizers and (4) and agents preventing denaturation or adherence to the container wall.
- excipients include: polyhydric sugar alcohols (enumerated above); amino acids such as alanine, glycine, glutamine, asparagine, histidine, arginine, lysine, ornithine, leucine, 2-phenylalanine, glutamic acid, threonine, etc.; organic sugars or sugar alcohols such as sucrose, lactose, lactitol, trehalose, stachyose, mannose, sorbose, xylose, ribose, ribitol, myoinisitose, myoinisitol, galactose, galactitol, glycerol, cyclitols (e.g., inositol),
- the formulations In order for the formulations to be used for in vivo administration, they must be sterile.
- the formulation may be rendered sterile by filtration through sterile filtration membranes.
- the therapeutic compositions herein generally are placed into a container having a sterile access port, for example, an intravenous solution bag or vial having a stopper pierceable by a hypodermic injection needle.
- the route of administration is in accordance with known and accepted methods, such as by single or multiple bolus or infusion over a long period of time in a suitable manner, e.g., injection or infusion by subcutaneous, intravenous, intraperitoneal, intramuscular, intraarterial, intralesional or intraarticular routes, topical administration, inhalation or by sustained release or extended-release means.
- the formulation herein may also contain more than one active compound as necessary for the particular indication being treated, preferably those with complementary activities that do not adversely affect each other.
- the composition may comprise a cytotoxic agent, cytokine or growth inhibitory agent.
- cytotoxic agent cytokine or growth inhibitory agent.
- Such molecules are suitably present in combination in amounts that are effective for the purpose intended.
- the active ingredients may also be entrapped in microcapsules prepared, for example, by coacervation techniques or by interfacial polymerization, for example, hydroxymethylcellulose or gelatin-microcapsules and poly-(methylmethacylate) microcapsules, respectively, in colloidal drug delivery systems (for example, liposomes, albumin microspheres, microemulsions, nano-p articles and nanocapsules) or in macroemulsions.
- colloidal drug delivery systems for example, liposomes, albumin microspheres, microemulsions, nano-p articles and nanocapsules
- Sustained-release preparations may be prepared. Suitable examples of sustained- release preparations include semipermeable matrices of solid hydrophobic polymers containing the antibody, which matrices are in the form of shaped articles, e.g., films, or microcapsules. Examples of sustained-release matrices include polyesters, hydrogels (for example, poly(2-hydroxyethyl-methacrylate), or poly(vinylalcohol)), polylactides (U.S. Pat. No.
- the sustained-release formulations of these proteins may be developed using poly lactic-coglycolic acid (PLGA) polymer due to its biocompatibility and wide range of biodegradable properties.
- PLGA poly lactic-coglycolic acid
- the degradation products of PLGA, lactic and glycolic acids, can be cleared quickly within the human body.
- the degradability of this polymer can be adjusted from months to years depending on its molecular weight and composition. Lewis, “Controlled release of bioactive agents from lactide/glycolide polymer", in Biodegradable Polymers as Drug Delivery Systems (Marcel Dekker; New York, 1990), M. Chasin and R. Langer (Eds.) pp. 1-41.
- stabilization may be achieved by modifying sulfhydryl residues, lyophilizing from acidic solutions, controlling moisture content, using appropriate additives, and developing specific polymer matrix compositions.
- Liposomal or proteinoid compositions may also be used to formulate the proteins or antibodies disclosed herein. See U.S. Pat. Nos. 4,925,673 and 5,013,556.
- Example anions that can form water soluble salts with the above polyvalent metal cations include those formed from inorganic acids and/or organic acids. Such water-soluble salts have a solubility in water (at 20°C) of at least about 20 mg/ml, alternatively at least about 100 mg/ml, alternative at least about 200 mg/ml.
- Suitable inorganic acids that can be used to form the "water soluble polyvalent metal salts" include hydrochloric, sulfuric, nitric, thiocyanic and phosphoric acid.
- Suitable organic acids that can be used include aliphatic carboxylic acid and aromatic acids. Aliphatic acids within this definition may be defined as saturated or unsaturated C2-9 carboxylic acids (e.g., aliphatic mono-, di- and tri-carboxylic acids).
- exemplary monocarboxylic acids within this definition include the saturated C2-9 monocarboxylic acids acetic, proprionic, butyric, valeric, caproic, enanthic, caprylic pelargonic and capryonic, and the unsaturated C2-9 monocarboxylic acids acrylic, propriolic methacrylic, crotonic and isocrotonic acids.
- exemplary dicarboxylic acids include the saturated C2-9 dicarboxylic acids malonic, succinic, glutaric, adipic and pimelic, while unsaturated C2-9 dicarboxylic acids include maleic, fumaric, citraconic and mesaconic acids.
- Exemplary tricarboxylic acids include the saturated C2-9 tricarboxylic acids tricarballylic and 1 ,2,3-butanetricarboxylic acid. Additionally, the carboxylic acids of this definition may also contain one or two hydroxyl groups to form hydroxy carboxylic acids. Exemplary hydroxy carboxylic acids include glycolic, lactic, glyceric, tartronic, malic, tartaric and citric acid. Aromatic acids within this definition include benzoic and salicylic acid.
- Commonly employed water soluble polyvalent metal salts which may be used to help stabilize the encapsulated polypeptides of this invention include, for example: (1) the inorganic acid metal salts of halides (e.g., zinc chloride, calcium chloride), sulfates, nitrates, phosphates and thiocyanates; (2) the aliphatic carboxylic acid metal salts (e.g., calcium acetate, zinc acetate, calcium proprionate, zinc glycolate, calcium lactate, zinc lactate and zinc tartrate); and (3) the aromatic carboxylic acid metal salts of benzoates (e.g., zinc benzoate) and salicylates.
- halides e.g., zinc chloride, calcium chloride
- sulfates e.g., nitrates, phosphates and thiocyanates
- aliphatic carboxylic acid metal salts e.g., calcium acetate, zinc acetate, calcium proprionate, zinc glycolate, calcium lac
- an active agent for the prevention or treatment of disease, the appropriate dosage of an active agent will depend on the type of disease to be treated, as defined above, the severity and course of the disease, whether the agent is administered for preventive or therapeutic purposes, previous therapy, the patient's clinical history and response to the agent, and the discretion of the attending physician.
- the agent is suitably administered to the patient at one time or over a series of treatments.
- the method of the invention can be combined with known methods of treatment for a disorder, either as combined or additional treatments steps or as additional components of a therapeutic formulation.
- Dosages and desired drug concentration of pharmaceutical compositions of the present invention may vary depending on the particular use envisioned. The determination of the appropriate dosage or route of administration is well within the skill of an ordinary artisan. Animal experiments provide reliable guidance for the determination of effective doses for human therapy. Interspecies scaling of effective doses can be performed following the principles laid down by Mordenti, J. and Chappell, W. "The Use of Interspecies Scaling in Toxicokinetics," In Toxicokinetics and New Drug Development, Yacobi et ah, Eds, Pergamon Press, New York 1989, pp. 42-46.
- normal dosage amounts may vary from about 10 ng/kg up to about 100 mg/kg of mammal body weight or more per day, preferably about 1 mg/kg/day to 10 mg/kg/day, depending upon the route of administration.
- Guidance as to particular dosages and methods of delivery is provided in the literature; see, for example, U.S. Pat. No. 4,657,760; 5,206,344; or 5,225,212. It is within the scope of the invention that different formulations will be effective for different treatments and different disorders, and that administration intended to treat a specific organ or tissue may necessitate delivery in a manner different from that to another organ or tissue.
- dosages may be administered by one or more separate administrations, or by continuous infusion. For repeated administrations over several days or longer, depending on the condition, the treatment is sustained until a desired suppression of disease symptoms occurs.
- other dosage regimens may be useful. The progress of this therapy is easily monitored by conventional techniques and assays.
- the formulations of the present invention are administered to a mammal in need of treatment with the protein, preferably a human, in accord with known methods, such as intravenous administration as a bolus or by continuous infusion over a period of time, by intramuscular, intraperitoneal, intracerebrospinal, subcutaneous, intra-articular, intrasynovial, intrathecal, oral, topical, or inhalation routes.
- the formulations are administered to the mammal by subcutaneous ⁇ i.e., beneath the skin) administration.
- the formulation may be injected using a syringe.
- other devices for administration of the formulation are available such as injection devices ⁇ e.g., the Inject-easeTM and GenjectTM devices); injector pens (such as the GenPenTM); auto-injector devices, needleless devices (e.g., MediJectorTM and BioJectorTM); and subcutaneous patch delivery systems.
- kits for a single dose-administration unit comprise a container of an aqueous formulation of therapeutic protein or antibody, including both single or multi-chambered pre-filled syringes.
- exemplary pre-filled syringes are available from Vetter GmbH, Ravensburg, Germany.
- the appropriate dosage (therapeutically effective amount) of the protein will depend, for example, on the condition to be treated, the severity and course of the condition, whether the protein is administered for preventive or therapeutic purposes, previous therapy, the patient's clinical history and response to the protein, the type of protein used, and the discretion of the attending physician.
- the protein is suitably administered to the patient at one time or over a series of treatments and may be administered to the patient at any time from diagnosis onwards.
- the protein may be administered as the sole treatment or in conjunction with other drugs or therapies useful in treating the condition in question.
- the protein of choice is an antibody
- from about 0.1-20 mg/kg is an initial candidate dosage for administration to the patient, whether, for example, by one or more separate administrations.
- other dosage regimens may be useful. The progress of this therapy is easily monitored by conventional techniques.
- an article of manufacture which contains the formulation and preferably provides instructions for its use.
- the article of manufacture comprises a container.
- Suitable containers include, for example, bottles, vials (e.g., dual chamber vials), syringes (such as single or dual chamber syringes) and test tubes.
- the container may be formed from a variety of materials such as glass or plastic.
- the label, which is on, or associated with, the container holding the formulation may indicate directions for reconstitution and/or use. The label may further indicate that the formulation is useful or intended for subcutaneous administration.
- the container holding the formulation may be a multi-use vial, which allows for repeat administrations ( e -g- > from 2-6 administrations) of the reconstituted formulation.
- the article of manufacture may further comprise a second container comprising a suitable diluent (e.g., BWFI).
- a suitable diluent e.g., BWFI
- the final protein concentration in the reconstituted formulation will generally be at least 50 mg/ml.
- the article of manufacture may further include other materials desirable from a commercial and user standpoint, including other buffers, diluents, filters, needles, syringes, and package inserts with instructions for use.
- This example illustrates measurements of viscosity of various antibody-containing formulations.
- This example illustrates how arginine-HCl and arginine succinate (arginine-S) effect the viscosity of an aqueous monoclonal antibody-containing formulation.
- Example 2 illustrates how various arginine derivatives, precursors and structural analogs effect the viscosity of an aqueous monoclonal antibody-containing formulation. Given that the data in Example 2 demonstrated that arginine-HCl and arginine succinate have a beneficial effect on reducing the viscosity of high concentration antibody-containing formulations, we next sought to determine the effect that various different arginine derivatives, precursors and structural analogs would have on such protein-containing formulations.
- buffered solutions containing various concentrations of anti-CD4 monoclonal antibody (20 mM Histidine- succinate, pH 6.3) were prepared in combination with various concentrations of different derivatives, precursors or analogs of arginine and the viscosity of the resulting solution was determined using a standard cone and plate rheometer as described above. More specifically, viscosity was measured using a standard cone-and-plate rheometer (TA Instruments AR-G2 stress rheometer using a 20 mm diameter, 1 degree cone, and water solvent trap) at a temperature of 25°C and a shear rate of 1000 1/s.
- TA Instruments AR-G2 stress rheometer using a 20 mm diameter, 1 degree cone, and water solvent trap
- each sample was allowed to equilibrate for 2 minutes at 25°C prior to the start of data collection. Data was collected for a minimum of 2 minutes to ensure steady state was reached. Solutions were prepared by dialysis and/or addition of the dry excipient into a concentrated protein solution to achieve the desired final excipient concentration. Samples were stored at 2-8°C until being brought to room temperature prior to sample loading. Protein concentration measurements of each sample were made using UV absorbance spectroscopy by gravimetric dilution.
- This example illustrates the effect of varying excipient concentration on the viscosity of an aqueous monoclonal antibody-containing formulation.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
Claims
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2011800320023A CN102958538A (en) | 2010-05-03 | 2011-04-26 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
| CA 2794864 CA2794864A1 (en) | 2010-05-03 | 2011-04-26 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
| BR112012027828A BR112012027828A2 (en) | 2010-05-03 | 2011-04-26 | matter composition, article of manufacture and method of reducing the viscosity of a protein containing formulation and preparing an aqueous protein containing formulation |
| EP20110720633 EP2566510A1 (en) | 2010-05-03 | 2011-04-26 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
| KR20127031426A KR20130060227A (en) | 2010-05-03 | 2011-04-26 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
| JP2013509113A JP2013525484A (en) | 2010-05-03 | 2011-04-26 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
| MX2012012743A MX2012012743A (en) | 2010-05-03 | 2011-04-26 | Compositions and methods useful for reducing the viscosity of protein-containing formulations. |
| RU2012151500/15A RU2012151500A (en) | 2010-05-03 | 2011-04-26 | COMPOSITIONS AND METHODS SUITABLE FOR REDUCING VISCOSITY OF PROTEIN-CONTAINING COMPOSITIONS |
| US13/666,990 US20130058958A1 (en) | 2010-05-03 | 2012-11-02 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US33068910P | 2010-05-03 | 2010-05-03 | |
| US61/330,689 | 2010-05-03 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/666,990 Continuation US20130058958A1 (en) | 2010-05-03 | 2012-11-02 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011139718A1 true WO2011139718A1 (en) | 2011-11-10 |
Family
ID=44263176
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/034001 WO2011139718A1 (en) | 2010-05-03 | 2011-04-26 | Compositions and methods useful for reducing the viscosity of protein-containing formulations |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20130058958A1 (en) |
| EP (1) | EP2566510A1 (en) |
| JP (1) | JP2013525484A (en) |
| KR (1) | KR20130060227A (en) |
| CN (1) | CN102958538A (en) |
| BR (1) | BR112012027828A2 (en) |
| CA (1) | CA2794864A1 (en) |
| MX (1) | MX2012012743A (en) |
| RU (1) | RU2012151500A (en) |
| WO (1) | WO2011139718A1 (en) |
Cited By (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013165732A1 (en) | 2012-05-01 | 2013-11-07 | Pfenex Inc. | Process for purifying recombinant plasmodium falciparum circumsporozoite protein |
| EP2694708A2 (en) * | 2011-04-07 | 2014-02-12 | Glaxosmithkline LLC | Formulations with reduced viscosity |
| WO2014023816A1 (en) | 2012-08-10 | 2014-02-13 | Adocia | Method for lowering the viscosity of high-concentration protein solutions |
| JPWO2013012022A1 (en) * | 2011-07-19 | 2015-02-23 | 中外製薬株式会社 | A stable protein-containing preparation containing arginine amide or a similar compound |
| US9457089B2 (en) | 2012-09-10 | 2016-10-04 | Adocia | Highly concentrated aqueous protein solution with reduced viscosity |
| US9605051B2 (en) | 2014-06-20 | 2017-03-28 | Reform Biologics, Llc | Viscosity-reducing excipient compounds for protein formulations |
| US9695233B2 (en) | 2012-07-13 | 2017-07-04 | Roche Glycart Ag | Bispecific anti-VEGF/anti-ANG-2 antibodies and their use in the treatment of ocular vascular diseases |
| US9827217B2 (en) | 2015-08-25 | 2017-11-28 | Rgenix, Inc. | Pharmaceutically acceptable salts of B-guanidinopropionic acid with improved properties and uses thereof |
| US9833513B2 (en) | 2013-09-11 | 2017-12-05 | Eagle Biologics, Inc. | Liquid protein formulations for injection comprising 1-butyl-3-methylimidazolium methanesulfonate and uses thereof |
| US9884813B1 (en) | 2017-03-01 | 2018-02-06 | Rgenix, Inc. | Pharmaceutically acceptable salts of B-guanidinopropionic acid with improved properties and uses thereof |
| US10000562B2 (en) | 2011-10-31 | 2018-06-19 | Genentech, Inc. | Antibody formulations |
| WO2018201064A1 (en) * | 2017-04-28 | 2018-11-01 | Amgen Inc. | N-acetylated and non-acetylated dipeptides containing arginine to reduce the viscosity of viscous compositions of therapeutic polypeptides |
| WO2018200533A1 (en) | 2017-04-28 | 2018-11-01 | Amgen Inc. | Excipients to reduce the viscosity of antibody formulations and formulation compositions |
| WO2019050780A1 (en) | 2017-09-05 | 2019-03-14 | Merck Sharp & Dohme Corp. | Compounds for reducing the viscosity of biological formulations |
| EP3383435A4 (en) * | 2015-11-30 | 2019-07-10 | Medimmune, LLC | Optimized ratios of amino acids and sugars as amorphous stabilizing compounds in pharmaceutical compositions containing high concentrations of protein-based therapeutic agents |
| WO2019201904A1 (en) * | 2018-04-16 | 2019-10-24 | Merck Patent Gmbh | Viscosity reduction of highly concentrated protein formulations |
| US10478498B2 (en) | 2014-06-20 | 2019-11-19 | Reform Biologics, Llc | Excipient compounds for biopolymer formulations |
| US10646569B2 (en) | 2017-05-16 | 2020-05-12 | Bhami's Research Laboratory, Pvt. Ltd. | High concentration protein formulations with reduced viscosity |
| WO2021053001A1 (en) * | 2019-09-17 | 2021-03-25 | Merck Patent Gmbh | Camphorsulfonic acid and combinations thereof with cationic excipients as viscosity reducing agents in high concentrated protein formulations |
| US11103552B2 (en) | 2018-05-10 | 2021-08-31 | Regeneron Pharmaceuticals, Inc. | High concentration VEGF receptor fusion protein containing formulations |
| WO2022013171A1 (en) * | 2020-07-13 | 2022-01-20 | Merck Patent Gmbh | Viscosity reducing excipients and combinations thereof for highly concentrated protein formulations |
| US11357857B2 (en) | 2014-06-20 | 2022-06-14 | Comera Life Sciences, Inc. | Excipient compounds for protein processing |
| US11471479B2 (en) | 2014-10-01 | 2022-10-18 | Eagle Biologics, Inc. | Polysaccharide and nucleic acid formulations containing viscosity-lowering agents |
| US11633476B2 (en) | 2017-05-02 | 2023-04-25 | Merck Sharp & Dohme Llc | Stable formulations of programmed death receptor 1 (PD-1) antibodies and methods of use thereof |
| WO2023075700A1 (en) * | 2021-10-29 | 2023-05-04 | Aslan Pharmaceuticals Pte Ltd | Anti-il-13r antibody formulation |
| WO2023075702A1 (en) * | 2021-10-29 | 2023-05-04 | Aslan Pharmaceuticals Pte Ltd | Anti-il-13r antibody formulation |
| WO2023079086A1 (en) * | 2021-11-05 | 2023-05-11 | Astrazeneca Uk Limited | Composition for treatment and prevention of covid-19 |
| US11813328B2 (en) | 2014-10-23 | 2023-11-14 | Amgen Inc. | Methods for reducing the viscosity of liquid pharmaceutical formulations comprising therapeutic proteins |
| US11845798B2 (en) | 2017-05-02 | 2023-12-19 | Merck Sharp & Dohme Llc | Formulations of anti-LAG3 antibodies and co-formulations of anti-LAG3 antibodies and anti-PD-1 antibodies |
| US12011427B2 (en) | 2019-12-11 | 2024-06-18 | Inspirna, Inc. | Methods of treating cancer |
| US12030927B2 (en) | 2022-02-18 | 2024-07-09 | Rq Biotechnology Limited | Antibodies capable of binding to the spike protein of coronavirus SARS-CoV-2 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MA37777B1 (en) * | 2012-06-21 | 2017-07-31 | Ucb Pharma Sa | Pharmaceutical preparation |
| JP6179939B2 (en) * | 2013-07-09 | 2017-08-16 | 国立大学法人 筑波大学 | Method for reducing viscosity of high-concentration gamma globulin preparation |
| CN104740630A (en) * | 2013-12-25 | 2015-07-01 | 浙江海正药业股份有限公司 | Medicine composition containing Campath |
| MA41629A (en) | 2015-03-04 | 2018-01-09 | Center For Human Reproduction | COMPOSITIONS AND METHODS OF USE OF ANTI-MÜLLERIAN HORMONE FOR THE TREATMENT OF INFERTILITY |
| WO2018213151A1 (en) * | 2017-05-18 | 2018-11-22 | Merck Sharp & Dohme Corp. | Pharmaceutical formulation comprising incretin-insulin conjugates |
| GB201900728D0 (en) * | 2019-01-18 | 2019-03-06 | Univ Birmingham | Drug delivery system |
Citations (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| EP0003089A1 (en) | 1978-01-06 | 1979-07-25 | Bernard David | Drier for silkscreen printed sheets |
| US4275149A (en) | 1978-11-24 | 1981-06-23 | Syva Company | Macromolecular environment control in specific receptor assays |
| US4515893A (en) | 1979-04-26 | 1985-05-07 | Ortho Pharmaceutical Corporation | Hybrid cell line for producing complement-fixing monoclonal antibody to human T cells |
| US4657760A (en) | 1979-03-20 | 1987-04-14 | Ortho Pharmaceutical Corporation | Methods and compositions using monoclonal antibody to human T cells |
| US4676980A (en) | 1985-09-23 | 1987-06-30 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Target specific cross-linked heteroantibodies |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4925673A (en) | 1986-08-18 | 1990-05-15 | Clinical Technologies Associates, Inc. | Delivery systems for pharmacological agents encapsulated with proteinoids |
| EP0404097A2 (en) | 1989-06-22 | 1990-12-27 | BEHRINGWERKE Aktiengesellschaft | Bispecific and oligospecific, mono- and oligovalent receptors, production and applications thereof |
| WO1991000360A1 (en) | 1989-06-29 | 1991-01-10 | Medarex, Inc. | Bispecific reagents for aids therapy |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5078998A (en) | 1985-08-02 | 1992-01-07 | Bevan Michael J | Hybrid ligand directed to activation of cytotoxic effector T lymphocytes and target associated antigen |
| US5091178A (en) | 1986-02-21 | 1992-02-25 | Oncogen | Tumor therapy with biologically active anti-tumor antibodies |
| US5091313A (en) | 1988-08-05 | 1992-02-25 | Tanox Biosystems, Inc. | Antigenic epitopes of IgE present on B cell but not basophil surface |
| WO1992020373A1 (en) | 1991-05-14 | 1992-11-26 | Repligen Corporation | Heteroconjugate antibodies for treatment of hiv infection |
| WO1993004173A1 (en) | 1991-08-14 | 1993-03-04 | Genentech, Inc. | Immunoglobulin variants for specific fc epsilon receptors |
| US5206344A (en) | 1985-06-26 | 1993-04-27 | Cetus Oncology Corporation | Interleukin-2 muteins and polymer conjugation thereof |
| WO1993008829A1 (en) | 1991-11-04 | 1993-05-13 | The Regents Of The University Of California | Compositions that mediate killing of hiv-infected cells |
| WO1993011161A1 (en) | 1991-11-25 | 1993-06-10 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5225212A (en) | 1989-10-20 | 1993-07-06 | Liposome Technology, Inc. | Microreservoir liposome composition and method |
| US5229275A (en) | 1990-04-26 | 1993-07-20 | Akzo N.V. | In-vitro method for producing antigen-specific human monoclonal antibodies |
| WO1994004690A1 (en) | 1992-08-17 | 1994-03-03 | Genentech, Inc. | Bispecific immunoadhesins |
| EP0420937B1 (en) | 1988-06-21 | 1994-11-09 | Genentech, Inc. | Therapeutic compositions for the treatment of myocardial infarction |
| US5428130A (en) | 1989-02-23 | 1995-06-27 | Genentech, Inc. | Hybrid immunoglobulins |
| WO1995019181A1 (en) | 1994-01-18 | 1995-07-20 | Genentech, Inc. | A METHOD OF TREATMENT OF PARASITIC INFECTION USING IgE ANTAGONISTS |
| WO1995023865A1 (en) | 1994-03-03 | 1995-09-08 | Genentech, Inc. | Anti-il-8 monoclonal antibodies for treatment of inflammatory disorders |
| WO1996007399A1 (en) | 1994-09-09 | 1996-03-14 | Takeda Chemical Industries, Ltd. | Sustained release preparation containing metal salt of a peptide |
| US5500362A (en) | 1987-01-08 | 1996-03-19 | Xoma Corporation | Chimeric antibody with specificity to human B cell surface antigen |
| WO1996016673A1 (en) | 1994-12-02 | 1996-06-06 | Chiron Corporation | Method of promoting an immune response with a bispecific antibody |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5545807A (en) | 1988-10-12 | 1996-08-13 | The Babraham Institute | Production of antibodies from transgenic animals |
| WO1996030046A1 (en) | 1995-03-30 | 1996-10-03 | Genentech, Inc. | Vascular endothelial cell growth factor antagonists |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| US5567610A (en) | 1986-09-04 | 1996-10-22 | Bioinvent International Ab | Method of producing human monoclonal antibodies and kit therefor |
| US5569825A (en) | 1990-08-29 | 1996-10-29 | Genpharm International | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5573905A (en) | 1992-03-30 | 1996-11-12 | The Scripps Research Institute | Encoded combinatorial chemical libraries |
| WO1996040072A2 (en) | 1995-06-07 | 1996-12-19 | Alkermes Controlled Therapeutics, Inc. | Composition for sustained release of human growth hormone |
| WO1996040210A1 (en) | 1995-06-07 | 1996-12-19 | Imclone Systems Incorporated | Antibody and antibody fragments for inhibiting the growth of tumors |
| US5591669A (en) | 1988-12-05 | 1997-01-07 | Genpharm International, Inc. | Transgenic mice depleted in a mature lymphocytic cell-type |
| US5595721A (en) | 1993-09-16 | 1997-01-21 | Coulter Pharmaceutical, Inc. | Radioimmunotherapy of lymphoma using anti-CD20 |
| WO1997003692A1 (en) | 1995-07-14 | 1997-02-06 | Novo Nordisk A/S | A stabilized pharmaceutical formulation comprising a growth hormone pre-treated with zinc and optionally lysine or calcium ions |
| US5622700A (en) | 1992-08-21 | 1997-04-22 | Genentech, Inc. | Method for treating a LFA-1-mediated disorder |
| WO1997017852A1 (en) | 1995-11-15 | 1997-05-22 | Hoechst Schering Agrevo Gmbh | Synergetic herbicidal mixtures |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| WO1997026912A2 (en) | 1996-01-23 | 1997-07-31 | Genentech, Inc. | Anti-cd18 antibodies for use against stroke |
| US5654010A (en) | 1992-12-02 | 1997-08-05 | Alkermes, Inc. | Composition for sustained release of human growth hormone |
| US5693762A (en) | 1988-12-28 | 1997-12-02 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| WO1998002463A1 (en) | 1996-07-11 | 1998-01-22 | Medarex, Inc. | THERAPEUTIC MULTISPECIFIC COMPOUNDS COMPRISED OF ANTI-FCα RECEPTOR ANTIBODIES |
| US5714338A (en) | 1993-12-10 | 1998-02-03 | Genentech, Inc. | Methods for diagnosis of allergy |
| WO1998006248A2 (en) | 1996-08-15 | 1998-02-19 | Leukosite, Inc. | HUMANIZED IMMUNOGLOBULIN REACTIVE WITH α4β7 INTEGRIN |
| US5725856A (en) | 1988-01-12 | 1998-03-10 | Genentech, Inc. | Monoclonal antibodies directed to the HER2 receptor |
| US5731168A (en) | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| US5736137A (en) | 1992-11-13 | 1998-04-07 | Idec Pharmaceuticals Corporation | Therapeutic application of chimeric and radiolabeled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma |
| WO1998023761A1 (en) | 1996-11-27 | 1998-06-04 | Genentech, Inc. | HUMANIZED ANTI-CD11a ANTIBODIES |
| US5821337A (en) | 1991-06-14 | 1998-10-13 | Genentech, Inc. | Immunoglobulin variants |
| WO1998045331A2 (en) | 1997-04-07 | 1998-10-15 | Genentech, Inc. | Anti-vegf antibodies |
| US5837234A (en) | 1995-06-07 | 1998-11-17 | Cytotherapeutics, Inc. | Bioartificial organ containing cells encapsulated in a permselective polyether suflfone membrane |
| WO1998051793A1 (en) | 1997-05-15 | 1998-11-19 | Genentech, Inc. | Apo-2 RECEPTOR |
| US6037454A (en) | 1996-11-27 | 2000-03-14 | Genentech, Inc. | Humanized anti-CD11a antibodies |
| WO2000075348A1 (en) | 1999-06-08 | 2000-12-14 | Seattle Genetics, Inc. | Recombinant anti-cd40 antibody and uses thereof |
| WO2001000245A2 (en) | 1999-06-25 | 2001-01-04 | Genentech, Inc. | HUMANIZED ANTI-ErbB2 ANTIBODIES AND TREATMENT WITH ANTI-ErbB2 ANTIBODIES |
| WO2001040309A2 (en) | 1999-10-29 | 2001-06-07 | Genentech, Inc. | Anti-prostate stem cell antigen (psca) antibody compositions and methods of use |
| WO2002024909A2 (en) | 2000-09-18 | 2002-03-28 | Biogen, Inc. | Receptor nucleic acids and polypeptides |
| WO2003002607A1 (en) | 2001-06-27 | 2003-01-09 | Shawn Shui-On Leung | Reducing immunogenicities of immunoglobulins by framework-patching |
| WO2003033658A2 (en) | 2001-10-17 | 2003-04-24 | Human Genome Sciences, Inc. | Neutrokine-alpha and neutrokine-alpha splice variant |
| US20030219433A1 (en) | 2002-02-14 | 2003-11-27 | Immunomedics, Inc. | Anti-CD20 antibodies and fusion proteins thereof and methods of use |
| WO2004035607A2 (en) | 2002-10-17 | 2004-04-29 | Genmab A/S | Human monoclonal antibodies against cd20 |
| US20040093621A1 (en) | 2001-12-25 | 2004-05-13 | Kyowa Hakko Kogyo Co., Ltd | Antibody composition which specifically binds to CD20 |
| WO2004056312A2 (en) | 2002-12-16 | 2004-07-08 | Genentech, Inc. | Immunoglobulin variants and uses thereof |
| WO2004091658A1 (en) * | 2003-04-04 | 2004-10-28 | Genentech, Inc. | High concentration antibody and protein formulations |
| WO2004103404A1 (en) | 2003-05-20 | 2004-12-02 | Applied Molecular Evolution, Inc. | Cd20 binding molecules |
| WO2005000901A2 (en) | 2003-05-09 | 2005-01-06 | Duke University | Cd20-specific antibodies and methods of employing same |
| WO2005014618A2 (en) | 2003-08-08 | 2005-02-17 | Immunomedics, Inc. | Bispecific antibodies for inducing apoptosis of tumor and diseased cells |
| WO2005016969A2 (en) | 2003-08-14 | 2005-02-24 | Merck Patent Gmbh | Cd20-binding polypeptide compositions |
| US6875432B2 (en) | 2000-10-12 | 2005-04-05 | Genentech, Inc. | Reduced-viscosity concentrated protein formulations |
| WO2006065746A2 (en) * | 2004-12-16 | 2006-06-22 | Genentech, Inc. | Methods of treating ige-mediated disorders comprising the administration of high concentration anti-ige antibody formulations |
| EP1688432A1 (en) * | 2003-10-09 | 2006-08-09 | Chugai Seiyaku Kabushiki Kaisha | Igm high concentration stabilized solution |
| WO2007001851A2 (en) | 2005-06-20 | 2007-01-04 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| WO2007076062A2 (en) * | 2005-12-21 | 2007-07-05 | Wyeth | Protein formulations with reduced viscosity and uses thereof |
| EP1977763A1 (en) * | 2005-12-28 | 2008-10-08 | Chugai Seiyaku Kabushiki Kaisha | Antibody-containing stabilizing preparation |
| US9813410B2 (en) | 2014-06-26 | 2017-11-07 | Rakuten, Inc. | Information processing apparatus, information processing method, and information processing program |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2332531B1 (en) * | 2001-11-13 | 2019-07-10 | Genentech, Inc. | Methods of purifying Apo2-Ligand/TRAIL |
-
2011
- 2011-04-26 BR BR112012027828A patent/BR112012027828A2/en not_active IP Right Cessation
- 2011-04-26 RU RU2012151500/15A patent/RU2012151500A/en not_active Application Discontinuation
- 2011-04-26 KR KR20127031426A patent/KR20130060227A/en not_active Application Discontinuation
- 2011-04-26 WO PCT/US2011/034001 patent/WO2011139718A1/en active Application Filing
- 2011-04-26 EP EP20110720633 patent/EP2566510A1/en not_active Withdrawn
- 2011-04-26 CN CN2011800320023A patent/CN102958538A/en active Pending
- 2011-04-26 MX MX2012012743A patent/MX2012012743A/en not_active Application Discontinuation
- 2011-04-26 CA CA 2794864 patent/CA2794864A1/en active Pending
- 2011-04-26 JP JP2013509113A patent/JP2013525484A/en active Pending
-
2012
- 2012-11-02 US US13/666,990 patent/US20130058958A1/en not_active Abandoned
Patent Citations (86)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| EP0003089A1 (en) | 1978-01-06 | 1979-07-25 | Bernard David | Drier for silkscreen printed sheets |
| US4275149A (en) | 1978-11-24 | 1981-06-23 | Syva Company | Macromolecular environment control in specific receptor assays |
| US4657760A (en) | 1979-03-20 | 1987-04-14 | Ortho Pharmaceutical Corporation | Methods and compositions using monoclonal antibody to human T cells |
| US4515893A (en) | 1979-04-26 | 1985-05-07 | Ortho Pharmaceutical Corporation | Hybrid cell line for producing complement-fixing monoclonal antibody to human T cells |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5206344A (en) | 1985-06-26 | 1993-04-27 | Cetus Oncology Corporation | Interleukin-2 muteins and polymer conjugation thereof |
| US5078998A (en) | 1985-08-02 | 1992-01-07 | Bevan Michael J | Hybrid ligand directed to activation of cytotoxic effector T lymphocytes and target associated antigen |
| US4676980A (en) | 1985-09-23 | 1987-06-30 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Target specific cross-linked heteroantibodies |
| US5091178A (en) | 1986-02-21 | 1992-02-25 | Oncogen | Tumor therapy with biologically active anti-tumor antibodies |
| US4925673A (en) | 1986-08-18 | 1990-05-15 | Clinical Technologies Associates, Inc. | Delivery systems for pharmacological agents encapsulated with proteinoids |
| US5567610A (en) | 1986-09-04 | 1996-10-22 | Bioinvent International Ab | Method of producing human monoclonal antibodies and kit therefor |
| US5500362A (en) | 1987-01-08 | 1996-03-19 | Xoma Corporation | Chimeric antibody with specificity to human B cell surface antigen |
| US5677180A (en) | 1987-01-08 | 1997-10-14 | Xoma Corporation | Chimeric antibody with specificity to human B cell surface antigen |
| US5725856A (en) | 1988-01-12 | 1998-03-10 | Genentech, Inc. | Monoclonal antibodies directed to the HER2 receptor |
| EP0420937B1 (en) | 1988-06-21 | 1994-11-09 | Genentech, Inc. | Therapeutic compositions for the treatment of myocardial infarction |
| US5091313A (en) | 1988-08-05 | 1992-02-25 | Tanox Biosystems, Inc. | Antigenic epitopes of IgE present on B cell but not basophil surface |
| US5545807A (en) | 1988-10-12 | 1996-08-13 | The Babraham Institute | Production of antibodies from transgenic animals |
| US5591669A (en) | 1988-12-05 | 1997-01-07 | Genpharm International, Inc. | Transgenic mice depleted in a mature lymphocytic cell-type |
| US5693762A (en) | 1988-12-28 | 1997-12-02 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5428130A (en) | 1989-02-23 | 1995-06-27 | Genentech, Inc. | Hybrid immunoglobulins |
| EP0404097A2 (en) | 1989-06-22 | 1990-12-27 | BEHRINGWERKE Aktiengesellschaft | Bispecific and oligospecific, mono- and oligovalent receptors, production and applications thereof |
| WO1991000360A1 (en) | 1989-06-29 | 1991-01-10 | Medarex, Inc. | Bispecific reagents for aids therapy |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5225212A (en) | 1989-10-20 | 1993-07-06 | Liposome Technology, Inc. | Microreservoir liposome composition and method |
| US5229275A (en) | 1990-04-26 | 1993-07-20 | Akzo N.V. | In-vitro method for producing antigen-specific human monoclonal antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5569825A (en) | 1990-08-29 | 1996-10-29 | Genpharm International | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| WO1992020373A1 (en) | 1991-05-14 | 1992-11-26 | Repligen Corporation | Heteroconjugate antibodies for treatment of hiv infection |
| US6407213B1 (en) | 1991-06-14 | 2002-06-18 | Genentech, Inc. | Method for making humanized antibodies |
| US5821337A (en) | 1991-06-14 | 1998-10-13 | Genentech, Inc. | Immunoglobulin variants |
| WO1993004173A1 (en) | 1991-08-14 | 1993-03-04 | Genentech, Inc. | Immunoglobulin variants for specific fc epsilon receptors |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| WO1993008829A1 (en) | 1991-11-04 | 1993-05-13 | The Regents Of The University Of California | Compositions that mediate killing of hiv-infected cells |
| WO1993011161A1 (en) | 1991-11-25 | 1993-06-10 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5573905A (en) | 1992-03-30 | 1996-11-12 | The Scripps Research Institute | Encoded combinatorial chemical libraries |
| WO1994004690A1 (en) | 1992-08-17 | 1994-03-03 | Genentech, Inc. | Bispecific immunoadhesins |
| US5622700A (en) | 1992-08-21 | 1997-04-22 | Genentech, Inc. | Method for treating a LFA-1-mediated disorder |
| US5736137A (en) | 1992-11-13 | 1998-04-07 | Idec Pharmaceuticals Corporation | Therapeutic application of chimeric and radiolabeled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma |
| US5654010A (en) | 1992-12-02 | 1997-08-05 | Alkermes, Inc. | Composition for sustained release of human growth hormone |
| US5595721A (en) | 1993-09-16 | 1997-01-21 | Coulter Pharmaceutical, Inc. | Radioimmunotherapy of lymphoma using anti-CD20 |
| US5714338A (en) | 1993-12-10 | 1998-02-03 | Genentech, Inc. | Methods for diagnosis of allergy |
| WO1995019181A1 (en) | 1994-01-18 | 1995-07-20 | Genentech, Inc. | A METHOD OF TREATMENT OF PARASITIC INFECTION USING IgE ANTAGONISTS |
| WO1995023865A1 (en) | 1994-03-03 | 1995-09-08 | Genentech, Inc. | Anti-il-8 monoclonal antibodies for treatment of inflammatory disorders |
| WO1996007399A1 (en) | 1994-09-09 | 1996-03-14 | Takeda Chemical Industries, Ltd. | Sustained release preparation containing metal salt of a peptide |
| WO1996016673A1 (en) | 1994-12-02 | 1996-06-06 | Chiron Corporation | Method of promoting an immune response with a bispecific antibody |
| US5731168A (en) | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| WO1996030046A1 (en) | 1995-03-30 | 1996-10-03 | Genentech, Inc. | Vascular endothelial cell growth factor antagonists |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| US5837234A (en) | 1995-06-07 | 1998-11-17 | Cytotherapeutics, Inc. | Bioartificial organ containing cells encapsulated in a permselective polyether suflfone membrane |
| WO1996040210A1 (en) | 1995-06-07 | 1996-12-19 | Imclone Systems Incorporated | Antibody and antibody fragments for inhibiting the growth of tumors |
| WO1996040072A2 (en) | 1995-06-07 | 1996-12-19 | Alkermes Controlled Therapeutics, Inc. | Composition for sustained release of human growth hormone |
| WO1997003692A1 (en) | 1995-07-14 | 1997-02-06 | Novo Nordisk A/S | A stabilized pharmaceutical formulation comprising a growth hormone pre-treated with zinc and optionally lysine or calcium ions |
| WO1997017852A1 (en) | 1995-11-15 | 1997-05-22 | Hoechst Schering Agrevo Gmbh | Synergetic herbicidal mixtures |
| WO1997026912A2 (en) | 1996-01-23 | 1997-07-31 | Genentech, Inc. | Anti-cd18 antibodies for use against stroke |
| WO1998002463A1 (en) | 1996-07-11 | 1998-01-22 | Medarex, Inc. | THERAPEUTIC MULTISPECIFIC COMPOUNDS COMPRISED OF ANTI-FCα RECEPTOR ANTIBODIES |
| WO1998006248A2 (en) | 1996-08-15 | 1998-02-19 | Leukosite, Inc. | HUMANIZED IMMUNOGLOBULIN REACTIVE WITH α4β7 INTEGRIN |
| WO1998023761A1 (en) | 1996-11-27 | 1998-06-04 | Genentech, Inc. | HUMANIZED ANTI-CD11a ANTIBODIES |
| US6037454A (en) | 1996-11-27 | 2000-03-14 | Genentech, Inc. | Humanized anti-CD11a antibodies |
| WO1998045331A2 (en) | 1997-04-07 | 1998-10-15 | Genentech, Inc. | Anti-vegf antibodies |
| WO1998051793A1 (en) | 1997-05-15 | 1998-11-19 | Genentech, Inc. | Apo-2 RECEPTOR |
| WO2000075348A1 (en) | 1999-06-08 | 2000-12-14 | Seattle Genetics, Inc. | Recombinant anti-cd40 antibody and uses thereof |
| WO2001000245A2 (en) | 1999-06-25 | 2001-01-04 | Genentech, Inc. | HUMANIZED ANTI-ErbB2 ANTIBODIES AND TREATMENT WITH ANTI-ErbB2 ANTIBODIES |
| WO2001040309A2 (en) | 1999-10-29 | 2001-06-07 | Genentech, Inc. | Anti-prostate stem cell antigen (psca) antibody compositions and methods of use |
| WO2002024909A2 (en) | 2000-09-18 | 2002-03-28 | Biogen, Inc. | Receptor nucleic acids and polypeptides |
| US6875432B2 (en) | 2000-10-12 | 2005-04-05 | Genentech, Inc. | Reduced-viscosity concentrated protein formulations |
| WO2003002607A1 (en) | 2001-06-27 | 2003-01-09 | Shawn Shui-On Leung | Reducing immunogenicities of immunoglobulins by framework-patching |
| WO2003033658A2 (en) | 2001-10-17 | 2003-04-24 | Human Genome Sciences, Inc. | Neutrokine-alpha and neutrokine-alpha splice variant |
| US20040093621A1 (en) | 2001-12-25 | 2004-05-13 | Kyowa Hakko Kogyo Co., Ltd | Antibody composition which specifically binds to CD20 |
| US20030219433A1 (en) | 2002-02-14 | 2003-11-27 | Immunomedics, Inc. | Anti-CD20 antibodies and fusion proteins thereof and methods of use |
| US20040167319A1 (en) | 2002-10-17 | 2004-08-26 | Jessica Teeling | Human monoclonal antibodies against CD20 |
| WO2004035607A2 (en) | 2002-10-17 | 2004-04-29 | Genmab A/S | Human monoclonal antibodies against cd20 |
| WO2004056312A2 (en) | 2002-12-16 | 2004-07-08 | Genentech, Inc. | Immunoglobulin variants and uses thereof |
| WO2004091658A1 (en) * | 2003-04-04 | 2004-10-28 | Genentech, Inc. | High concentration antibody and protein formulations |
| WO2005000901A2 (en) | 2003-05-09 | 2005-01-06 | Duke University | Cd20-specific antibodies and methods of employing same |
| WO2004103404A1 (en) | 2003-05-20 | 2004-12-02 | Applied Molecular Evolution, Inc. | Cd20 binding molecules |
| US20050025764A1 (en) | 2003-05-20 | 2005-02-03 | Watkins Jeffry D. | CD20 binding molecules |
| WO2005014618A2 (en) | 2003-08-08 | 2005-02-17 | Immunomedics, Inc. | Bispecific antibodies for inducing apoptosis of tumor and diseased cells |
| US20050069545A1 (en) | 2003-08-14 | 2005-03-31 | Carr Francis Joseph | CD20-Binding polypeptide compositions and methods |
| WO2005016969A2 (en) | 2003-08-14 | 2005-02-24 | Merck Patent Gmbh | Cd20-binding polypeptide compositions |
| EP1688432A1 (en) * | 2003-10-09 | 2006-08-09 | Chugai Seiyaku Kabushiki Kaisha | Igm high concentration stabilized solution |
| WO2006065746A2 (en) * | 2004-12-16 | 2006-06-22 | Genentech, Inc. | Methods of treating ige-mediated disorders comprising the administration of high concentration anti-ige antibody formulations |
| WO2007001851A2 (en) | 2005-06-20 | 2007-01-04 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| WO2007076062A2 (en) * | 2005-12-21 | 2007-07-05 | Wyeth | Protein formulations with reduced viscosity and uses thereof |
| EP1977763A1 (en) * | 2005-12-28 | 2008-10-08 | Chugai Seiyaku Kabushiki Kaisha | Antibody-containing stabilizing preparation |
| US9813410B2 (en) | 2014-06-26 | 2017-11-07 | Rakuten, Inc. | Information processing apparatus, information processing method, and information processing program |
Non-Patent Citations (76)
| Title |
|---|
| "Remington's Phannaceutical Sciences 18th edition,", 1990, MACK PUBLISHING CO. |
| BAEUERLE ET AL., CURR. OPIN. MOL. THER., vol. 11, no. 1, 2009, pages 22 - 30 |
| BRENNAN ET AL., SCIENCE, vol. 229, 1985, pages 81 |
| BRUGGCMANN, YCAR IN IMMUNO., vol. 7, 1993, pages 33 |
| CAPEL ET AL., IMMUNOMETHODS, vol. 4, 1994, pages 25 - 34 |
| CARTER ET AL., PROC. NATL. ACAD. SCI. USA, vol. 89, 1992, pages 4285 |
| CARTER ET AL., PROC. NATL. ACAD. SCI. USA, vol. 89, 1992, pages 4285 - 4289 |
| CERIANI ET AL., CANCER RES., vol. 55, no. 23, 1995, pages 5852S - 5856S |
| CHOTHIA ET AL., J. MOL. BIOL., vol. 196, 1987, pages 901 |
| CHOTHIA, C. ET AL., J. MOL. BIOL., vol. 196, 1987, pages 901 - 917 |
| CHOY ET AL., ARTHRITIS RHEUM, vol. 39, no. 1, 1996, pages 52 - 56 |
| CLACKSON ET AL., NATURE, vol. 352, 1991, pages 624 - 628 |
| CLELAND: "Vaccine Design: The Subunit and Adjuvant Approach", 1995, PLENUM PRESS, article "Design and Production of Single Immunization Vaccines Using Polylactide Polyglycolide Microsphere Systems", pages: 439 - 462 |
| CLYNES ET AL., PNAS USA, vol. 95, 1998, pages 652 - 656 |
| CRAGG ET AL., BLOOD, vol. 101, 2003, pages 1045 - 1052 |
| DANIEL P. STIES, ABBA I. TERR AND TRISTRAM G. PARSOLW: "Basic and Clinical Immunology, 8th Edition,", 1994, APPLETON & LANGE, pages: 71 |
| ELLIS ET AL., J. IMMUNOL., vol. 155, no. 2, 1995, pages 925 - 937 |
| GAZZANO-SANTORO ET AL., J. IMMUNOL. METHODS, vol. 202, 1996, pages 163 |
| GRAZIANO ET AL., J. IMMUNOL., vol. 155, no. 10, 1995, pages 4996 - 5002 |
| GRIFFITH ET AL., EMBO J., vol. 12, 1993, pages 725 - 734 |
| GRUBER ET AL., J. IMMUNOL., vol. 152, 1994, pages 5368 |
| GUYER ET AL., J. IMMUNOL., vol. 117, 1976, pages 587 |
| HAAS ET AL., J. LAB. CLIN. MED., vol. 126, 1995, pages 330 - 41 |
| HAISRNA ET AL., BLOOD, vol. 92, 1998, pages 184 |
| HOLLINGER ET AL., PROC. NATL. ACAD. SCI. USA, vol. 90, 1993, pages 6444 - 6448 |
| HORA ET AL., BIOLTECHNOLOGY, vol. 8, 1990, pages 755 - 758 |
| HOURMANT ET AL., TRANSPLANTATION, vol. 58, 1994, pages 377 - 380 |
| JAKOBOVITS ET AL., PROC. NATL. ACAD. SCI. USA, vol. 90, 1993, pages 2551 |
| JAKOBOVITS, NATURE, vol. 362, 1993, pages 255 - 258 |
| JOHNSON ET AL., NAT. MED., vol. 2, 1996, pages 795 - 799 |
| JOHNSON, KEVIN S., CHISWELL, DAVID J., CURRENT OPINION IN STRUCTURAL BIOLOGY, vol. 3, 1993, pages 564 - 571 |
| JONES ET AL., NATURE, vol. 321, 1986, pages 522 - 525 |
| JONES, A., ADV. DRUG DELIVERY REV., vol. 10, 1993, pages 29 - 90 |
| JONES, NATURE, vol. 321, 1986, pages 522 - 525 |
| JURCIC ET AL., CANCER RES, vol. 55, no. 23, 1995, pages 5908S - 5910S |
| KABAT ET AL.: "Sequences of Proteins of Immunological Interest, 5th Ed.", 1991, NATIONAL INSTITUTES OF HEALTH |
| KABAT ET AL.: "Sequences of' Proteins of' Innnunological Interest", 1991, NATIONAL INSTITUTE OF HEALTH |
| KIM ET AL., GROWTH FACTORS, vol. 7, 1992, pages 53 - 64 |
| KIM ET AL., J. IMMUNOL., vol. 24, 1994, pages 249 |
| KOHLER ET AL., NATURE, vol. 256, 1975, pages 495 |
| KOSTELNY ET AL., J. IMMUNOL., vol. 148, no. 5, 1992, pages 1547 - 1553 |
| LEWIS: "Biodegradable Polymers as Drug Delivery Systems", 1990, MARCEL DEKKER, article "Controlled release of bioactive agents from lactide/glycolide polymer", pages: 1 - 41 |
| LITTON ET AL., EUR J. IMMUNOL., vol. 26, no. 1, 1996, pages 1 - 9 |
| M. DAERON, ANNU. REV. IMMUNOL., vol. 15, 1997, pages 203 - 234 |
| MARASCO ET AL., PROC. NATL. ACAD. SCI. USA, vol. 90, 1993, pages 7889 - 7893 |
| MARKS ET AL., J. MOL. BIOL., vol. 222, 1991, pages 581 - 597 |
| MCCAFFERTY ET AL., NATURE, vol. 348, 1990, pages 552 - 553 |
| MILLSTEIN ET AL., NATURE, vol. 305, 1983, pages 537 - 539 |
| MORDENTI, J., CHAPPELL, W. ET AL.: "Toxicokinetics and New Drug Development", 1989, PERGAMON PRESS, article "The Use of Interspecies Scaling in Toxicokinetics", pages: 42 - 46 |
| MORRISON ET AL., PROC. NATL. ACAD. SCI. USA, vol. 81, 1984, pages 6851 - 6855 |
| OSOL, A.: "Remington's Pharmaceutical Sciences, 16th edition,", 1980 |
| PLUCKTHUN: "The Pharmacology of Monoclonal Antibodies", vol. 113, 1994, SPRINGCR-VCRLAG, pages: 269 - 315 |
| PRESS ET AL., BLOOD, vol. 69, no. 2, 1987, pages 584 - 591 |
| PRESTA ET AL., J. IMMUNOL., vol. 151, 1993, pages 2623 |
| PRESTA ET AL., J. IMMUNOL., vol. 151, 1993, pages 2623 - 2632 |
| PRESTA, CURR. OP. STRUCT. BIOL., vol. 2, 1992, pages 593 - 596 |
| RAVETCH, KINET, ANNU. REV. IMMUNOL., vol. 9, 1991, pages 457 - 92 |
| RICHMAN ET AL., CANCER RES., vol. 55, no. 23, 1995, pages 5916S - 5920S |
| RIECHMANN ET AL., NATURE, vol. 332, 1988, pages 323 - 327 |
| RIECHMANN ET AL., NATURE, vol. 332, 1988, pages 323 - 329 |
| RIECHMANN ET AL., NATURE, vol. 332, 1988, pages 323 - 337 |
| SHALABY ET AL., J. EXP. MED., vol. 175, 1992, pages 217 - 225 |
| SHARKEY ET AL., CANCER RES., vol. 55, no. 23, 1995, pages 5935S - 5945S |
| SIMS ET AL., J. IMMUNOL., vol. 151, 1993, pages 2296 |
| ST JOHN ET AL., CHEST, vol. 103, 1993, pages 932 |
| STOPPA ET AL., TRANSPLANT INTL., vol. 4, 1991, pages 3 - 7 |
| SURESH ET AL., METHODS IN ENZYMOLOGY, vol. 121, 1986, pages 210 |
| TRAUNECKER ET AL., EMBO J., vol. 10, 1991, pages 3655 - 3659 |
| TUTT ET AL., J. IMMUNOL., vol. 147, 1991, pages 60 |
| VALENTINE ET AL.: "Leukocyte Typing III", 1987, OXFORD UNIVERSITY PRESS, pages: 440 |
| VAN DE WINKEL, DRUG DISCOVERY TODAY, vol. 8, 2003, pages 503 - 510 |
| VERHOEYEN ET AL., SCIENCE, vol. 239, 1988, pages 1534 - 1536 |
| VINCENT LEE: "Peptide and Protein Drug Delivery", 1991, MARCEL DEKKER, INC., pages: 247 - 301 |
| YASUDA ET AL., BIOMED. THER., vol. 27, 1993, pages 1221 - 1223 |
| YIN, BWT, LLOYD, KO, J. BIOL. CHERN., vol. 276, 2001, pages 27371 - 27375 |
| ZAPATA ET AL., PROTEIN ENG., vol. 8, no. 10, 1995, pages 1057 - 1062 |
Cited By (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2694708A2 (en) * | 2011-04-07 | 2014-02-12 | Glaxosmithkline LLC | Formulations with reduced viscosity |
| EP2694708A4 (en) * | 2011-04-07 | 2014-10-01 | Glaxosmithkline Llc | Formulations with reduced viscosity |
| US9574005B2 (en) | 2011-07-19 | 2017-02-21 | Chugai Seiyaku Kabushiki Kaisha | Stable Protein-containing preparation containing argininamide or analogous compound thereof |
| JPWO2013012022A1 (en) * | 2011-07-19 | 2015-02-23 | 中外製薬株式会社 | A stable protein-containing preparation containing arginine amide or a similar compound |
| US10898572B2 (en) | 2011-07-19 | 2021-01-26 | Chugai Seiyaku Kabushiki Kaisha | Stable protein-containing preparation containing argininamide or analogous compound thereof |
| JP2017222654A (en) * | 2011-07-19 | 2017-12-21 | 中外製薬株式会社 | Stable protein-containing formulation containing arginine amide or analogue thereof |
| US10947307B2 (en) | 2011-10-31 | 2021-03-16 | Genentech, Inc | Antibody formulations |
| US10000562B2 (en) | 2011-10-31 | 2018-06-19 | Genentech, Inc. | Antibody formulations |
| US9849177B2 (en) | 2012-05-01 | 2017-12-26 | Pfenex Inc. | Process for purifying recombinant plasmodium falciparum circumsporozoite protein |
| CN104379596B (en) * | 2012-05-01 | 2018-05-29 | 菲尼克斯公司 | For purifying the method for recombinant plasmodium falciparum circumsporozoite protein |
| JP2015517464A (en) * | 2012-05-01 | 2015-06-22 | フェニックス インク. | Process for purification of recombinant P. falciparum sporozoite surrounding protein |
| KR102087461B1 (en) | 2012-05-01 | 2020-03-10 | 피페넥스 인크. | Process for purifying recombinant plasmodium falciparum circumsporozoite protein |
| US9169304B2 (en) | 2012-05-01 | 2015-10-27 | Pfenex Inc. | Process for purifying recombinant Plasmodium falciparum circumsporozoite protein |
| KR20150027743A (en) * | 2012-05-01 | 2015-03-12 | 피페넥스 인크. | Process for purifying recombinant plasmodium falciparum circumsporozoite protein |
| CN104379596A (en) * | 2012-05-01 | 2015-02-25 | 菲尼克斯公司 | Process for purifying recombinant plasmodium falciparum circumsporozoite protein |
| WO2013165732A1 (en) | 2012-05-01 | 2013-11-07 | Pfenex Inc. | Process for purifying recombinant plasmodium falciparum circumsporozoite protein |
| US9695233B2 (en) | 2012-07-13 | 2017-07-04 | Roche Glycart Ag | Bispecific anti-VEGF/anti-ANG-2 antibodies and their use in the treatment of ocular vascular diseases |
| US9278131B2 (en) | 2012-08-10 | 2016-03-08 | Adocia | Process for lowering the viscosity of highly concentrated protein solutions |
| WO2014023816A1 (en) | 2012-08-10 | 2014-02-13 | Adocia | Method for lowering the viscosity of high-concentration protein solutions |
| FR2994390A1 (en) * | 2012-08-10 | 2014-02-14 | Adocia | METHOD FOR LOWERING THE VISCOSITY OF HIGH CONCENTRATION PROTEIN SOLUTIONS |
| US9457089B2 (en) | 2012-09-10 | 2016-10-04 | Adocia | Highly concentrated aqueous protein solution with reduced viscosity |
| US9833513B2 (en) | 2013-09-11 | 2017-12-05 | Eagle Biologics, Inc. | Liquid protein formulations for injection comprising 1-butyl-3-methylimidazolium methanesulfonate and uses thereof |
| US10646571B2 (en) | 2013-09-11 | 2020-05-12 | Eagle Biologics, Inc. | Liquid protein formulations containing cimetidine |
| US9913905B2 (en) | 2013-09-11 | 2018-03-13 | Eagle Biologics, Inc. | Liquid pharmaceutical formulations for injection comprising thiamine pyrophosphate 1-(3-aminopropyl)-2-methyl-1H-imidazole and uses thereof |
| US10849977B2 (en) | 2013-09-11 | 2020-12-01 | Eagle Biologics, Inc. | Liquid Protein Formulations Containing Thiamine |
| US10821183B2 (en) | 2013-09-11 | 2020-11-03 | Eagle Biologics, Inc. | Liquid protein formulations containing 4-(3-butyl-1-imidazolio)-1-butane sulfonate (BIM) |
| US10179172B2 (en) | 2013-09-11 | 2019-01-15 | Eagle Biologics, Inc. | Liquid pharmaceutical formulations for injection comprising yellow 5 or orange G and uses thereof |
| US10821184B2 (en) | 2013-09-11 | 2020-11-03 | Eagle Biologics, Inc. | Liquid protein formulations containing thiamine pyrophosphate (TPP) |
| US9925263B2 (en) | 2013-09-11 | 2018-03-27 | Eagle Biologics, Inc. | Liquid pharmaceutical formulations for injection comprising procaine and uses thereof |
| US11819550B2 (en) | 2013-09-11 | 2023-11-21 | Eagle Biologics, Inc. | Liquid protein formulations containing cyclic adenosine monophosphate (cAMP) or adenosine triphosphate (ATP) |
| US11986526B2 (en) | 2013-09-11 | 2024-05-21 | Eagle Biologics, Inc. | Liquid protein formulations containing 4-ethyl-4-methylmorpholinium methylcarbonate (EMMC) |
| EP3791862A1 (en) * | 2013-09-11 | 2021-03-17 | Eagle Biologics, Inc. | Liquid protein formulations containing viscosity-lowering agents |
| US11357857B2 (en) | 2014-06-20 | 2022-06-14 | Comera Life Sciences, Inc. | Excipient compounds for protein processing |
| US10478498B2 (en) | 2014-06-20 | 2019-11-19 | Reform Biologics, Llc | Excipient compounds for biopolymer formulations |
| US11660343B2 (en) | 2014-06-20 | 2023-05-30 | Comera Life Sciences, Inc. | Viscosity-reducing excipient compounds for protein formulations |
| US9605051B2 (en) | 2014-06-20 | 2017-03-28 | Reform Biologics, Llc | Viscosity-reducing excipient compounds for protein formulations |
| US11672865B2 (en) | 2014-06-20 | 2023-06-13 | Comera Life Sciences, Inc. | Viscosity-reducing excipient compounds for protein formulations |
| US11696951B2 (en) | 2014-06-20 | 2023-07-11 | Comera Life Sciences, Inc. | Viscosity-reducing compounds for protein formulations |
| US11806399B2 (en) | 2014-06-20 | 2023-11-07 | Comera Life Sciences, Inc. | Excipient compounds for biopolymer formulations |
| US11471479B2 (en) | 2014-10-01 | 2022-10-18 | Eagle Biologics, Inc. | Polysaccharide and nucleic acid formulations containing viscosity-lowering agents |
| US11813328B2 (en) | 2014-10-23 | 2023-11-14 | Amgen Inc. | Methods for reducing the viscosity of liquid pharmaceutical formulations comprising therapeutic proteins |
| US9827217B2 (en) | 2015-08-25 | 2017-11-28 | Rgenix, Inc. | Pharmaceutically acceptable salts of B-guanidinopropionic acid with improved properties and uses thereof |
| US10512623B2 (en) | 2015-08-25 | 2019-12-24 | Rgenix, Inc. | Pharmaceutically acceptable salts of B-Guanidinopropionic acid with improved properties and uses thereof |
| EP3383435A4 (en) * | 2015-11-30 | 2019-07-10 | Medimmune, LLC | Optimized ratios of amino acids and sugars as amorphous stabilizing compounds in pharmaceutical compositions containing high concentrations of protein-based therapeutic agents |
| US9884813B1 (en) | 2017-03-01 | 2018-02-06 | Rgenix, Inc. | Pharmaceutically acceptable salts of B-guanidinopropionic acid with improved properties and uses thereof |
| US20200048367A1 (en) * | 2017-04-28 | 2020-02-13 | Amgen Inc. | N-acetylated and non-acetylated dipeptides containing arginine to reduce the viscosity of viscous compositions of therapeutic polypeptides |
| WO2018201064A1 (en) * | 2017-04-28 | 2018-11-01 | Amgen Inc. | N-acetylated and non-acetylated dipeptides containing arginine to reduce the viscosity of viscous compositions of therapeutic polypeptides |
| US12103979B2 (en) * | 2017-04-28 | 2024-10-01 | Amgen Inc. | N-acetylated and non-acetylated dipeptides containing arginine to reduce the viscosity of viscous compositions of therapeutic polypeptides |
| WO2018200533A1 (en) | 2017-04-28 | 2018-11-01 | Amgen Inc. | Excipients to reduce the viscosity of antibody formulations and formulation compositions |
| US11845798B2 (en) | 2017-05-02 | 2023-12-19 | Merck Sharp & Dohme Llc | Formulations of anti-LAG3 antibodies and co-formulations of anti-LAG3 antibodies and anti-PD-1 antibodies |
| US11633476B2 (en) | 2017-05-02 | 2023-04-25 | Merck Sharp & Dohme Llc | Stable formulations of programmed death receptor 1 (PD-1) antibodies and methods of use thereof |
| US10646569B2 (en) | 2017-05-16 | 2020-05-12 | Bhami's Research Laboratory, Pvt. Ltd. | High concentration protein formulations with reduced viscosity |
| US11738082B2 (en) | 2017-05-16 | 2023-08-29 | Bhami's Research Laboratory, Pvt. Ltd. | High concentration protein formulations with reduced viscosity |
| WO2019050780A1 (en) | 2017-09-05 | 2019-03-14 | Merck Sharp & Dohme Corp. | Compounds for reducing the viscosity of biological formulations |
| WO2019201904A1 (en) * | 2018-04-16 | 2019-10-24 | Merck Patent Gmbh | Viscosity reduction of highly concentrated protein formulations |
| EP3928765A1 (en) * | 2018-04-16 | 2021-12-29 | Merck Patent GmbH | Viscosity reduction of highly concentrated protein formulations |
| US11207412B2 (en) | 2018-04-16 | 2021-12-28 | Merck Patent Gmbh | Viscosity reduction of highly concentrated protein formulations |
| US11103552B2 (en) | 2018-05-10 | 2021-08-31 | Regeneron Pharmaceuticals, Inc. | High concentration VEGF receptor fusion protein containing formulations |
| CN114423414A (en) * | 2019-09-17 | 2022-04-29 | 默克专利股份有限公司 | Camphorsulfonic acids and combinations thereof with cationic excipients as viscosity reducers in highly concentrated protein formulations |
| WO2021053001A1 (en) * | 2019-09-17 | 2021-03-25 | Merck Patent Gmbh | Camphorsulfonic acid and combinations thereof with cationic excipients as viscosity reducing agents in high concentrated protein formulations |
| US12011427B2 (en) | 2019-12-11 | 2024-06-18 | Inspirna, Inc. | Methods of treating cancer |
| WO2022013171A1 (en) * | 2020-07-13 | 2022-01-20 | Merck Patent Gmbh | Viscosity reducing excipients and combinations thereof for highly concentrated protein formulations |
| WO2023075702A1 (en) * | 2021-10-29 | 2023-05-04 | Aslan Pharmaceuticals Pte Ltd | Anti-il-13r antibody formulation |
| WO2023075700A1 (en) * | 2021-10-29 | 2023-05-04 | Aslan Pharmaceuticals Pte Ltd | Anti-il-13r antibody formulation |
| WO2023079086A1 (en) * | 2021-11-05 | 2023-05-11 | Astrazeneca Uk Limited | Composition for treatment and prevention of covid-19 |
| US12030927B2 (en) | 2022-02-18 | 2024-07-09 | Rq Biotechnology Limited | Antibodies capable of binding to the spike protein of coronavirus SARS-CoV-2 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20130058958A1 (en) | 2013-03-07 |
| KR20130060227A (en) | 2013-06-07 |
| MX2012012743A (en) | 2012-11-23 |
| RU2012151500A (en) | 2014-06-10 |
| JP2013525484A (en) | 2013-06-20 |
| CA2794864A1 (en) | 2011-11-10 |
| BR112012027828A2 (en) | 2016-08-09 |
| EP2566510A1 (en) | 2013-03-13 |
| CN102958538A (en) | 2013-03-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20130058958A1 (en) | Compositions and methods useful for reducing the viscosity of protein-containing formulations | |
| US20200023063A1 (en) | Compositions and methods useful for stabilizing protein-containing formulations | |
| US11938189B2 (en) | Compositions and methods for stabilizing protein-containing formulations | |
| US20220125928A1 (en) | Compositions and methods for stabilizing protein-containing formulations |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180032002.3 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11720633 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2794864 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011720633 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 9285/CHENP/2012 Country of ref document: IN Ref document number: MX/A/2012/012743 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 2013509113 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20127031426 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2012151500 Country of ref document: RU Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112012027828 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112012027828 Country of ref document: BR Kind code of ref document: A2 Effective date: 20121030 |