WO2011112516A1 - Treating and preventing hepatitis c virus infection using c-raf kinase antisense oligonucleotides - Google Patents
Treating and preventing hepatitis c virus infection using c-raf kinase antisense oligonucleotides Download PDFInfo
- Publication number
- WO2011112516A1 WO2011112516A1 PCT/US2011/027412 US2011027412W WO2011112516A1 WO 2011112516 A1 WO2011112516 A1 WO 2011112516A1 US 2011027412 W US2011027412 W US 2011027412W WO 2011112516 A1 WO2011112516 A1 WO 2011112516A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- hcv
- oligonucleotide
- agent
- interferon
- raf
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1137—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/322—2'-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
- C12N2310/335—Modified T or U
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/341—Gapmers, i.e. of the type ===---===
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/346—Spatial arrangement of the modifications having a combination of backbone and sugar modifications
Definitions
- This invention relates to compositions and methods for modulating expression of raf genes, using antisense oligonucleotides directed to raf mRNAs.
- This invention is also directed to methods for inhibiting viral replication, and related methods of treating and preventing viral infection, particularly HCV infection, using raf antisense oligonucleotides, optionally in combination with other anti-viral, e.g., anti-HCV, drugs.
- HCV hepatitis C virus
- HCV is a member of the Flaviviridae family of RNA viruses that affect animals and humans.
- the genome is a single, about 9.6-kilobase strand of RNA, and consists of one open reading frame that encodes for a polyprotein of about 3000 amino acids flanked by untranslated regions at both 5' and 3' ends (5'- and 3'-UTR).
- the polyprotein serves as the precursor to at least 10 separate viral proteins critical for replication and assembly of progeny viral particles. Because the replicative cycle of HCV does not involve any DNA intermediate and the virus is not integrated into the host genome, HCV infection can theoretically be cured.
- Standard treatment for chronic HCV includes interferon alpha (IFN- alpha) or pegylated IFN-alpha in combination with ribavirin, often involving at least six (6) months of treatment.
- Interferon alpha IFN- alpha
- Treatment of HCV with interferon has frequently been associated with adverse side effects such as fatigue, fever, chills, headache, myalgias, arthralgias, mild alopecia, psychiatric effects, autoimmune phenomena and thyroid dysfunction.
- Ribavirin an inhibitor of inosine 5'-monophosphate dehydrogenase (IMPDH)
- IMPDH inosine 5'-monophosphate dehydrogenase
- ribavirin causes significant hemolysis in 10-20% of patients treated at currently recommended doses, and the drug is both teratogenic and embryotoxic. Even with recent improvements, a substantial fraction of patients do not respond to conventional HCV treatment with a sustained reduction in viral load.
- a number of alternative approaches are being pursued to combat HCV. These include, for example, application of antisense oligonucleotides or ribozymes for inhibiting HCV replication.
- low-molecular weight compounds that directly inhibit HCV proteins and interfere with viral replication are considered as attractive strategies to control HCV infection.
- the NS3/4a protease/helicase and the NS5b RNA-dependent RNA polymerase are examples of viral targets against which new drugs are being developed.
- antiviral activity can also be achieved by targeting host cell proteins that are necessary for viral replication, such as cyclophilin inhibitors and TLR7 agonists.
- the present invention relates generally to compositions and methods for modulating HCV viral replication, and treating HCV infection in a patient, using raf antisense oligonucleotides, optionally in combination with one or more additional anti-HCV agents.
- oligonucleotide which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
- the oligonucleotide is 8 to 50 nucleotides in length.
- the oligonucleotide is a modified oligonucleotide comprising or consisting of a sequence 100% complementary to a region of mRNA encoding human c-raf (SEQ ID NO: 28).
- the oligonucleotide targeted to mRNA encoding human c-raf is a modified oligonucleotide comprising or consisting of a sequence 100%
- the modified oligonucleotide comprises: a gap segment consisting of linked deoxynucleosides; a 5' wing segment consisting of linked nucleosides; and a 3' wing segment consisting of linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; and wherein each nucleoside of each wing segment comprises a modified sugar.
- the modified oligonucleotide consists of 20 nucleobases.
- the modified oligonucleotide comprises: a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-O-methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine.
- the oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29).
- the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence, TCCCGCCTGTGACATGCATT (SEQ ID NO:8), with 2'-O-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
- the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence,
- the method further comprises a step of administering an anti- HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV.
- an anti- HCV agent such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV.
- the raf antisense oligonucleotide and the anti-HCV agent are administered concomitantly or are co-administered.
- the anti-HCV agent is an immunomodulatory agent selected from the group consisting of a-interferon, ⁇ - interferon, ⁇ -interferon, o-interferon, Intron® A, Roferon® A, Canferon®-A300, Advaferon®, Infergen®., Humoferon®., Sumiferon® MP, Alfaferone®, IFN- ⁇ ®, Feron®, polyethylene glycol derivatized (pegylated) interferon compounds, PEG interferon-a-2a (Pegasys®), PEG interferon-a-2b (PEG-lntron®) and pegylated IFN-a-con1.
- the anti-HCV agent is an antiviral agent selected from the group consisting of ribavirin, amantadine, viramidine, nitazoxanide, telbivudine; NOV-205, taribavirin; VX-950, VX-497, VX-148, and VX- 944.
- the anti-HCV agent is an HCV polymerase inhibitor selected from the group consisting of NM283 (valopicitabine), R803, JTK-109, JTK-003, HCV-371 , HCV-086, HCV-796 and R-1479.
- the anti-HCV agent is an HCV protease inhibitor selected from the group consisting of BILN-2061 , VX-950 (Telaprevir), GS-9132 (ACH-806), SCH-503034 (Boceprevir) and SCH-6.
- the anti-HCV agent is an HCV life cycle protein inhibitor selected from the group consisting of a NS3 helicase inhibitor, a metallo-protease inhibitor, and an alpha glucosidase inhibitors.
- the anti-HCV agent is selected from the group consisting of an antisense oligonucleotide inhibitor, a siRNA inhibitor, a short hairpin RNA (shRNA) inhibitor, and a ribozyme inhibitor
- the anti-HCV agent is selected from the group consisting of a-interferon, pegylated a-interferon, ribavirin, or a combination thereof.
- a pharmaceutical composition comprising a physiologically acceptable carrier, a therapeutically effective amount of at least one raf antisense
- oligonucleotide as described herein, and at least anti-HCV agent, as described herein.
- the present invention provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, for use, or prepared for use, in treating or preventing HCV infection in a patient, e.g., by administering to the patient or cells thereof a therapeutically effective amount of the oligonucleotide.
- the oligonucleotide is 8 to 50 nucleotides in length.
- the present invention further provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, for use, or prepared for use, in treating or preventing HCV infection in a patient in combination with an anti-HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV, e.g., by an anti-HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV, e.g., by an anti-HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent
- the oligonucleotide and anti-HCV agent are administered to the patient or cells thereof therapeutically effective amounts of the oligonucleotide and anti-HCV agent.
- the oligonucleotide and anti-HCV agent are administered to the patient or cells thereof therapeutically effective amounts of the oligonucleotide and anti-HCV agent.
- oligonucleotide is 8 to 50 nucleotides in length.
- the present invention provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, for use in the manufacture of a medicament for treating or preventing HCV infection in a patient, e.g., by administering to the patient or cells thereof a therapeutically effective amount of the oligonucleotide.
- the oligonucleotide is 8 to 50 nucleotides in length.
- the present invention further provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, for use in the manufacture of a medicament for treating or preventing HCV infection in a patient in combination with an anti-HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV, e.g., by administering to the patient or cells thereof therapeutically effective amounts of the oligonucleotide and anti-HCV agent.
- an anti-HCV agent such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV, e.g., by administering to the patient or cells thereof therapeutically effective amounts of the
- oligonucleotide is 8 to 50 nucleotides in length.
- the oligonucleotide is a modified oligonucleotide comprising or consisting of a sequence 100% complementary to a region of mRNA encoding human c-raf (SEQ ID NO: 28).
- the oligonucleotide targeted to mRNA encoding human c-raf is a modified oligonucleotide comprising or consisting of a sequence 100% complementary to nucleobases 2771 to 2790 of SEQ ID NO:28.
- the modified oligonucleotide comprises: a gap segment consisting of linked deoxynucleosides; a 5' wing segment consisting of linked nucleosides; and a 3' wing segment consisting of linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; and wherein each nucleoside of each wing segment comprises a modified sugar.
- the modified oligonucleotide consists of 20
- the modified oligonucleotide comprises: a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-0-methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine.
- the oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29).
- the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence, TCCCGCCTGTGACATGCATT (SEQ ID NO:8), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
- the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence,
- Figure 1 provides a graph showing the HCV titer (RNA copies/ml) measured in supernatants from cells transfected with the indicated amounts of c- raf antisense oligonucleotide at the indicated times post transfection, which demonstrates that treatment with raf antisense oligonucleotides resulted in a dose dependent reduction in virus titers (RNA copies/ml) in HCV-infected cells.
- Figure 2 provides a graph showing the amount of c-Raf protein
- the present invention is based, in part, on the identification that antisense oligonucleotides targeting a raf gene, e.g., a c-raf gene, are effective in inhibiting viral replication, including HCV replication.
- the present invention is further based, in part, on the identification of novel combinations of therapeutic agents that exhibit an enhanced ability to treat, prevent, inhibit, or reduce infection by viruses, including HCV. These combinations include at least one raf antisense oligonucleotide, which modulates expression of a raf gene, as well as one or more additional therapeutic agents having activity against HCV.
- the present invention contemplates the use of a raf antisense oligonucleotide to treat, prevent, inhibit, or reduce infection by viruses, including HCV. Definitions
- NCBI Biotechnology Information
- 2'-0-methoxyethyl refers to an O-methoxy-ethyl modification of the 2' position of a furosyl ring.
- a 2'-0-methoxyethyl modified sugar is a modified sugar.
- 2'-0-methoxyethyl nucleoside means a nucleoside comprising a 2'- O-methoxyethyl modified sugar moiety.
- 3' target site refers to the nucleotide of a target nucleic acid which is complementary to the 3'-most nucleotide of a particular antisense compound.
- 5' target site refers to the nucleotide of a target nucleic acid which is complementary to the 5'-most nucleotide of a particular antisense compound.
- 5-methylcytosine means a cytosine modified with a methyl group attached to the 5' position.
- a 5-methylcytosine is a modified nucleobase.
- administering refers to the co-administration of two agents in any manner in which the pharmacological effects of both are manifest in the patient. Concomitant administration does not require that both agents be administered in a single pharmaceutical composition, in the same dosage form, at the same time or by the same route of administration.
- administering means providing a pharmaceutical agent to an individual, and includes, but is not limited to, administering by a medical professional and self-administering.
- “Ameliorate” means to make better or improve the symptoms of a condition or disease in a subject.
- Animal refers to human or non-human animals, including, but not limited to, mice, rats, rabbits, dogs, cats, pigs, horses and non-human primates, including, but not limited to, monkeys and chimpanzees.
- Antisense compound means an oligomeric compound that is capable of undergoing hybridization to a target nucleic acid through hydrogen bonding.
- Antisense inhibition means reduction of target nucleic acid or protein levels in the presence of an antisense compound complementary to a target nucleic acid compared to target nucleic acid or protein levels in the absence of the antisense compound.
- Antisense oligonucleotide means a single-stranded oligonucleotide having a nucleobase sequence that permits hybridization to a complementary region or segment of a target nucleic acid.
- Bicyclic sugar means a furosyl ring modified by the bridging of two non-geminal ring atoms.
- a bicyclic sugar is a modified sugar moiety.
- Cap structure or "terminal cap moiety” means a chemical modification, which has been incorporated at a terminus of an antisense compound.
- An antisense compound can have both termini “capped”.
- Chimeric antisense compounds means antisense compounds that have at least 2 chemically distinct regions, each region includes a plurality of subunits.
- Co-administration means administration of two or more agents to an individual.
- the two or more agents can be in a single pharmaceutical composition, or can be in separate pharmaceutical compositions.
- Each of the two or more agents can be administered through the same or different routes of administration.
- Co-administration encompasses administration in parallel or sequentially.
- “Complementarity” means the capacity for pairing between nucleobases of a first nucleic acid and a second nucleic acid.
- Contiguous nucleobases means nucleobases immediately adjacent to each other.
- Cross-reactive means an oligomeric compound targeting one nucleic acid sequence can hybridize to a different nucleic acid sequence.
- an antisense oligonucleotide targeting human c-raf can cross-react with a murine c-raf.
- Whether an oligomeric compound cross- reacts with a nucleic acid sequence other than its designated target depends on the degree of complementarity the compound has to the nucleic acid sequence. The higher the complementarity between the oligomeric compound and the non- target nucleic acid, the more likely the oligomeric compound will cross-react with the nucleic acid.
- “Cure” means a method that restores health or a prescribed treatment for an illness.
- Deoxyribonucleotide means a nucleotide having a hydrogen atom at the 2' position of the sugar portion of the nucleotide. Deoxyribonucleotides can be modified with any of a variety of substituents.
- Designing or “Designed to” refer to the process of designing an oligomeric compound that specifically hybridizes with a selected nucleic acid molecule or portion thereof.
- “Diluent” means an ingredient in a composition that lacks pharmacological activity, but is pharmaceutically necessary or desirable.
- the diluent can be a liquid, e.g. saline solution.
- Dose means a specified quantity of a pharmaceutical agent provided in a single administration, or in a specified time period.
- a dose can be administered in two or more boluses, tablets, or injections.
- the desired dose may require a volume not easily accommodated by a single injection.
- two or more injections can be used to achieve the desired dose.
- a dose can be administered in two or more injections to minimize injection site reaction in an individual. Doses can be stated as the amount of pharmaceutical agent per hour, day, week or month.
- Dosage unit or “unit dosage form” means a form in which a pharmaceutical agent is provided, e.g., pill, tablet, or other dosage unit known in the art.
- a dosage unit is a vial containing lyophilized antisense oligonucleotide.
- a dosage unit is a vial containing reconstituted antisense oligonucleotide.
- Duration means the period of time during which an activity or event continues. In certain embodiments, the duration of treatment is the period of time during which doses of a pharmaceutical agent are administered.
- Effective amount in the context of modulating an activity or of treating, preventing, or inhibiting a condition means the administration of that amount of active ingredient to a subject in need of such modulation, treatment or prophylaxis, either in a single dose or as part of a series, that is effective for modulation of that effect, or for treatment or prophylaxis or improvement of that condition.
- the effective amount will vary depending upon the health and physical condition of the subject to be treated, the taxonomic group of subjects to be treated, the formulation of the composition, the assessment of the medical situation, and other relevant factors. In addition, the effective amount may vary when used in combination with another active ingredient.
- the effect amount of one or both of the raf antisense oligonucleotide or anti-HCV agent may be reduced.
- “Therapeutically effective amount” or “effective amount” also includes an amount of a pharmaceutical agent that provides a therapeutic benefit to an individual.
- “Efficacy” means the ability to produce a desired effect. “Expression” includes all the functions by which a gene's coded information is converted into structures present and operating in a cell. Such structures include, but are not limited to, the products of transcription and translation.
- First agent or “first therapeutic agent” means an agent that can be used in combination with a “second agent”.
- the first agent is any antisense compound, oligonucleotide or composition that inhibits c-raf described herein.
- “Fully complementary” or “100% complementary” means each nucleobase of a first nucleic acid has a complementary nucleobase in a second nucleic acid.
- a first nucleic acid is an antisense compound and a target nucleic acid is a second nucleic acid.
- an antisense oligonucleotide is a first nucleic acid and a target nucleic acid is a second nucleic acid.
- Gapmer means an antisense compound in which an internal position having a plurality of nucleotides that supports RNaseH cleavage is positioned between external regions having one or more nucleotides that are chemically distinct from the nucleosides of the internal region.
- a "gap segment” means the plurality of nucleotides that make up the internal region of a gapmer.
- a “wing segment” means the external region of a gapmer.
- Gap-widened means an antisense compound has a gap segment of
- Hybridization means the annealing of complementary nucleic acid molecules.
- complementary nucleic acid molecules include, but are not limited to, an antisense compound and a nucleic acid target.
- complementary nucleic acid molecules include, but are not limited to, an antisense oligonucleotide and a nucleic acid target.
- Immediately adjacent means there are no intervening nucleotides between the immediately adjacent elements. For example, between regions, segments, nucleotides and/or nucleosides.
- an amount effective to inhibit the activity or expression of c-raf means that the level of activity or expression of c-raf in a treated sample will differ from the level of c-raf activity or expression in untreated cells. Such terms are applied to, for example, levels of expression, and levels of activity.
- “Inhibiting the expression or activity” refers to a reduction, blockade of the expression or activity of the target and does not necessarily indicate a total elimination of expression or activity.
- Internucleoside linkage refers to the chemical bond between nucleosides.
- Linked nucleosides means adjacent nucleosides which are bonded together.
- mis refers to a non-complementary nucleobase within a complementary oligomeric compound.
- Modified internucleoside linkage refers to a substitution and/or any change from a naturally occurring internucleoside bond (i.e. a phosphodiester internucleoside bond).
- Modified nucleobase means any nucleobase other than adenine, cytosine, guanine, thymidine, or uracil.
- An "unmodified nucleobase” means the purine bases, adenine (A) and guanine (G), and the pyrimidine bases, thymine (T), cytosine (C) and uracil (U).
- Modified oligonucleotide means an oligonucleotide comprising a modified internucleoside linkage, a modified sugar, and/or a modified nucleobase.
- a modified oligonucleotide can also have a nucleoside mimetic or nucleotide mimetic.
- Modified sugar refers to a substitution and/or any change from a natural sugar.
- Modulation means a perturbation of function, for example, one associated with either an increase (stimulation or induction) or a decrease
- “Monomer” refers to a single unit of an oligomer. Monomers include, but are not limited to, nucleosides and nucleotides, whether naturally occuring or modified. "Motif means the pattern of unmodified and modified nucleosides in an antisense compound.
- Naturally occurring internucleoside linkage means a 3' to 5' phosphodiester linkage.
- Natural sugar means a sugar found in DNA (2'-H) or RNA (2'-OH).
- Nucleic acid refers to molecules composed of monomeric nucleotides.
- a nucleic acid includes, but is not limited to, ribonucleic acids (RNA), deoxyribonucleic acids (DNA), single-stranded nucleic acids, double-stranded nucleic acids, small interfering ribonucleic acids (siRNA), and microRNAs
- Nucleobase means a heterocyclic moiety capable of pairing with a base of another nucleic acid.
- Nucleobase complementarity refers to a nucleobase that is capable of base pairing with another nucleobase.
- adenine (A) is complementary to thymine (T).
- T thymine
- adenine (A) is
- complementary nucleobase refers to a nucleobase of an antisense compound that is capable of base pairing with a nucleobase of its target nucleic acid. For example, if a nucleobase at a certain position of an antisense compound is capable of hydrogen bonding with a nucleobase at a certain position of a target nucleic acid, then the position of hydrogen bonding between the oligonucleotide and the target nucleic acid is considered to be complementary at that nucleobase pair.
- Nucleobase sequence means the order of contiguous nucleobases independent of any sugar, linkage, and/or nucleobase modification.
- Nucleoside means a nucleobase linked to a sugar.
- Nucleotide means a nucleoside having a phosphate group covalently linked to the sugar portion of the nucleoside.
- Nucleoside mimetic includes those structures used to replace the sugar or the sugar and the base and not necessarily the linkage at one or more positions of an oligome c compound such as, for example, nucleoside mimetics having morpholino, cyclohexenyl, cyclohexyl, bicyclo or tricyclo sugar mimetics, e.g. non furanose sugar units.
- Oligomeric compound means a polymer of linked monomeric subunits which is capable of hybridizing to at least a region of a nucleic acid molecule.
- Oligonucleotide means an oligomer or polymer of linked nucleoside or nucleotide monomers each of which can be modified or unmodified,
- oligonucleotide includes oligomers comprising non-naturally occurring monomers, or portions thereof. Such modified or substituted oligonucleotides are often preferred over native forms because of properties such as, for example, enhanced cellular uptake and increased stability in the presence of nucleases.
- Parenteral administration means administration by a manner other than through the digestive tract, e.g., through topical administration, injection or infusion.
- Parenteral administration includes, but is not limited to, subcutaneous administration, intravenous administration, and intramuscular administration.
- “Pharmaceutically acceptable carrier” or “Pharmaceutically acceptable diluent” means a carrier or diluent that does not interfere with the structure of the oligonucleotide. Certain of such carries enable pharmaceutical compositions to be formulated as, for example, tablets, pills, dragees, capsules, liquids, gels, syrups, slurries, suspension and lozenges for the oral ingestion by a subject.
- a pharmaceutically acceptable carrier can be a sterile aqueous solution.
- “Pharmaceutically acceptable salts” means physiologically and pharmaceutically acceptable salts of antisense compounds, i.e., salts that retain the desired biological activity of the parent oligonucleotide and do not impart undesired toxicological effects thereto.
- composition means a mixture of substances suitable for administering to an animal.
- a composition can comprise one or more antisense oligonucleotides and a sterile aqueous solution.
- Phosphorothioate internucleoside linkage or "phosphorothioate linkage” means a linkage between nucleosides where the phosphodiester bond is modified by replacing one of the non-bridging oxygen atoms with a sulfur atom.
- a phosphorothioate linkage is a modified internucleoside linkage.
- Portion means a defined number of contiguous (i.e., linked) nucleobases of a nucleic acid. In certain embodiments, a portion is a defined number of contiguous nucleobases of a target nucleic acid. In certain
- a portion is a defined number of contiguous nucleobases of an antisense compound.
- Prevention or “preventing” refers to inhibiting, delaying or
- a condition or disease e.g., HCV infection
- forestalling the onset or development of a condition or disease for a period of time from hours to days, preferably weeks to months to years or permanently. It can also mean reducing the likelihood that a condition or disease will occur during a period of time.
- Prodrug means a therapeutic agent that is prepared in an inactive form that is converted to an active form (i.e., drug) within the body or cells thereof by the action of endogenous or non-endogenous enzymes or other chemicals and/or conditions.
- Random is defined as a portion of the target nucleic acid having at least one identifiable structure, function, or characteristic.
- “Ribonucleotide” means a nucleotide having a hydroxy at the 2' position of the sugar portion of the nucleotide. Ribonucleotides can be modified with any of a variety of substituents.
- Salts mean physiologically and pharmaceutically acceptable salts of antisense compounds, i.e., salts that retain the desired biological activity of the parent oligonucleotide and do not impart undesired toxicological effects thereto.
- a second therapeutic agent means an agent that can be used in combination with a "first agent”.
- a second therapeutic agent can be any agent that inhibits or prevents HCV replication.
- a second therapeutic agent can include, but is not limited to, an siRNA or antisense oligonucleotide.
- “Segments” are defined as smaller, sub-portions of regions within a target nucleic acid. “Shortened” or “truncated” versions of antisense
- oligonucleotides or target nucleic acids taught herein have one, two or more nucleosides deleted.
- Side effects mean physiological responses attributable to a treatment other than desired effects.
- side effects include, without limitation, injection site reactions, liver function test abnormalities, renal function abnormalities, liver toxicity, renal toxicity, central nervous system abnormalities, and myopathies.
- increased aminotransferase levels in serum can indicate liver toxicity or liver function abnormality.
- increased bilirubin can indicate liver toxicity or liver function abnormality.
- Single-stranded oligonucleotide means an oligonucleotide which is not hybridized to a complementary strand.
- oligonucleotide means a modified oligonucleotide which is not hybridized to a complementary strand.
- siRNA is defined as a double-stranded compound having a first and second strand and comprises a central complementary portion between said first and second strands and terminal portions that are optionally complementary between said first and second strands or with a target mRNA.
- the first strand of the siRNA is antisense to the target nucleic acid, while the second strand is complementary to the first strand.
- the antisense strand is designed to target a particular nucleic acid target, the sense strand of the siRNA can then be designed and synthesized as the complement of the antisense strand and either strand can contain modifications or additions to either terminus.
- Sites are defined as unique nucleobase positions within a target nucleic acid.
- Rapid progression means a decrease in the development of a disease, condition or symptom.
- Specifically hybridizable means an antisense compound that hybridizes to a target nucleic acid to induce a desired effect, while exhibiting minimal or no effects on non-target nucleic acids.
- Subject means a human or non-human animal selected for treatment or therapy.
- Targeted to means having a nucleobase sequence that will allow specific hybridization of an antisense compound to a target nucleic acid to induce a desired effect.
- Target nucleic acid means a nucleic acid capable of being targeted by antisense compounds.
- Targeting means the process of design and selection of an antisense compound that will specifically hybridize to a target nucleic acid and induce a desired effect.
- c-raf nucleic acid means any nucleic acid encoding c-Raf.
- a c-raf nucleic acid includes, without limitation, a DNA sequence encoding c-Raf, an RNA sequence transcribed from DNA encoding c-Raf, and an mRNA sequence encoding c-Raf.
- c-raf mRNA means an mRNA encoding a c-Raf protein.
- “Therapeutically effective amount” or “effective amount” means an amount of a pharmaceutical agent that provides a therapeutic benefit to an individual.
- Effective amount in the context of modulating an activity or of treating or preventing a condition means the administration of that amount of active ingredient to a subject in need of such inhibition, treatment or prophylaxis, either in a single dose or as part of a series of doses, that is effective for inhibition of that effect, or for treatment or prophylaxis or improvement of that condition.
- the effective amount will vary depending upon the health and physical condition of the subject to be treated, the taxonomic group of subjects to be treated, the formulation of the composition, the assessment of the medical situation, and other relevant factors.
- Treatment refers to administering a composition of the invention to effect an alteration or improvement of a disease, condition or symptom.
- Unmodified nucleotide means a nucleotide composed of naturally occuring nucleobases, sugar moieties and internucleoside linkages.
- an unmodified nucleotide is an RNA nucleotide (i.e., ⁇ -D- ribonucleosides) or a DNA nucleotide (i.e., ⁇ -D-deoxyribonucleoside).
- Wild segment means a plurality of nucleosides modified to impart to an oligonucleotide properties such as enhanced inhibitory activity, increased binding affinity for a target nucleic acid, or resistance to degradation by in vivo nucleases.
- the present invention provides methods for treating, preventing or inhibiting viral infection, e.g,. hepatitis C virus (HCV) infection using
- oligonucleotides that target c-raf may be used to inhibit viral replication and thereby treating, preventing, or inhibiting viral infection in a subject or patient.
- the methods and compositions of the present invention may be used to treat infection from any viral-mediated disease or disorder that benefits from reduced raf expression.
- certain particular embodiments of the present invention described herein refer to the treatment, prevention or inhibition of HCV infection, it is understood that they could be also be used to treat, prevent or inhibit other viral infections.
- the present invention includes a method of treating, preventing, or inhibiting HCV infection, comprising administering to a subject, or cells thereof, a therapeutically effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
- the present invention includes a method of treating, preventing, or inhibiting the replication of HCV in a subject, comprising administering to a subject, or cells thereof, a therapeutically effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
- the present invention includes a method of inhibiting or reducing the replication of HCV in cells, e.g., in vitro, comprising administering to the cells an effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
- methods of the present invention comprise administering a c-raf antisense oligonucleotide (e.g., an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf) in combination with one or more additional anti-HCV agents, including but not limited to any of those described herein.
- a c-raf antisense oligonucleotide e.g., an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf
- additional anti-HCV agents including but not limited to any of those described herein.
- the raf antisense oligonucleotide reduces raf expression in a subject or cell, e.g., a subject or cell infected with HCV. Therefore, in particular embodiments, methods of the present invention
- a raf antisense oligonucleotide in combination with an anti-HCV agent that directly targets HCV, thereby inhibiting the HCV by inhibiting both cellular processes involved in HCV replication and viral processes involved in HCV replication.
- the raf antisense oligonucleotide and the additional anti-HCV agent act synergistically to inhibit HCV.
- the present invention includes a method of treating, preventing, or inhibiting HCV infection, comprising co-administering to a subject, or cells thereof, both a therapeutically effective amount of an
- the present invention includes a method of treating, preventing, or inhibiting the replication of HCV in a subject, comprising coadministering to a subject, or cells thereof, a therapeutically effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, and a therapeutically effective amount of an anti-HCV agent.
- the present invention includes a method of inhibiting or reducing the replication of HCV in cells, e.g., in vitro, comprising co- administering to the cells an effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, and a therapeutically effective amount of an anti-HCV agent.
- an anti-HCV agent is selected from the group consisting of an HCV polymerase inhibitor, an HCV protease inhibitor, an inhibitor of another target in the HCV life cycle, an immunomodulatory agent, an antiviral agent, or a combination thereof.
- Raf antisense oligoncleotides may be delivered to any subject in need thereof, including a subject determined to be infected with HCV or a subject considered to be at risk of developing an HCV infection.
- Subjects may be any animal, including a mammal, such as a human.
- the present invention employs oligonucleotides targeted to nucleic acids encoding a raf family member, which modulate raf gene expression.
- the oligonucleotides target c-raf (raf-1 ); however, compositions and methods for modulating expression of other forms of raf are also contemplated.
- the oligonucleotides target human c-raf.
- the oligonucleotides target human c-raf.
- oligonucleotide comprises a sequence complementary to a raf mRNA sequence, e.g., a human c-raf mRNA sequence.
- Oligonucleotides that target a nucleic acid encoding a raf family member include any and all polynucleotide complexes, polynucleotides, and oligonucleotides comprising one or more sequences capable of hybridizing to a raf nucleic acid and modulating raf gene expression.
- the oligonucleotides may comprise various structures, including, e.g., a traditional single-stranded antisense oligonucleotide structures and various RNA interference (RNAi) agents, such as small interfering RNA (siRNA), short hairpin RNA (shRNA), and dicer substrates.
- RNAi RNA interference
- oligonucletoides that target a raf mRNA or gene may be, e.g., either single- stranded or double-stranded, or comprise both single-stranded and double- stranded regions, and they may, e.g., comprise DNA, RNA or both.
- oligonucleotides used according to the present invention comprise RNA, DNA, or peptide nucleic acids, or a combination of any or all of these types of molecules.
- the oligonucleotides may comprise modified nucleic acids, or derivatives or analogs of nucleic acids.
- nucleic acid modifications include, but are not limited to, biotin labeling, fluorescent labeling, amino modifiers introducing a primary amine into the polynucleotide, phosphate groups, deoxyuridine, halogenated nucleosides, phosphorothioates, 2'-0-Methyl RNA analogs, chimeric RNA analogs, wobble groups, universal bases, and deoxyinosine.
- the oligonucleotides of the present invention are single-stranded.
- the oligonucleotides comprise one or more double-stranded regions.
- An oligonucleotide comprising a double- stranded region may consist of one, two or more individual strands, each having a 5' and 3' end.
- an oligonucleotide comprising a double-stranded region may consist of a single oligonucleotide with a self-complementary region, which hybridizes to itself, and which may form a stem loop structure.
- an oligonucleotide comprising a double-stranded region may comprise two strands with regions complementary to each other, which hybridize to form a double-stranded structure.
- An oligonucleotide comprising a double- stranded region may be entirely double-stranded or partially double stranded.
- an oligonucleotide may be entirely double-stranded and have two blunt ends.
- an oligonucleotide may include one or more double- stranded regions and have one or more 5' or 3' single-stranded overhangs, e.g., of two to four nucleotides.
- the oligonucleotides in accordance with this invention are from about 8 to about 50 nucleotides in length, whether single- stranded or comprising a double-stranded region. In the context of this invention, it is understood that this encompasses non-naturally occurring oligomers as herein before described, having 8 to 50 monomers.
- the oligonucleotides comprise from about 8 to about 30 nucleobases (i.e., from about 8 to about 30 linked nucleosides).
- raf antisense oligonucleotides are used to reduce or inhibit the expression of the targeted raf family member.
- Raf antisense oligonucleotides are single-stranded oligonucleotide having a nucleobase sequence that permits hybridization to a complementary region or segment of a raf nucleic acid.
- Exemplary raf antisense oligonucleotides that may be used according to the present invention are described herein and also in U.S. Patent Nos. 5,563,255, 5,952,229, 6,358,932, 5,656,612, 5,919,773, 6,410,518, and 6,806,258, the contents of which are incorporated herein by reference in their entireties.
- oligonucleotide refers to an oligomer or polymer of nucleotide or nucleoside monomers consisting of naturally occurring bases, sugars and intersugar (backbone) linkages.
- oligonucleotide also includes oligomers comprising non-naturally occurring monomers, or portions thereof, which function similarly. Such modified or substituted oligonucleotides are often preferred over native forms because of properties such as, for example, enhanced cellular uptake and increased stability in the presence of nucleases.
- a "subunit" of a polynucleotide or oligonucleotide refers to one nucleotide (or nucleotide analog) unit.
- the term may refer to the nucleotide unit with or without the attached intersubunit linkage, although, when referring to a "charged subunit", the charge typically resides within the intersubunit linkage (e.g., a phosphate or phosphorothioate linkage or a cationic linkage).
- a given raf antisense oligonucleotide may utilize one or more different types of subunits and/or intersubunit linkages, mainly to alter its stability, Tm, RNase sensitivity, or other characteristics, as desired. For instance, certain embodiments may employ RNA subunits with one or more 2'-0-methyl RNA subunits.
- oligonucleotides of this invention are chimeric
- oligonucleotides are oligonucleotides which contain two or more chemically distinct regions, each made up of at least one nucleotide. These oligonucleotides typically contain at least one region of modified nucleotides that confers one or more beneficial properties (such as, for example, increased nuclease resistance, increased uptake into cells, increased binding affinity for the RNA target) and a region that is a substrate for RNase H cleavage.
- a chimeric oligonucleotide comprises at least one region modified to increase target binding affinity, and, usually, a region that acts as a substrate for RNAse H.
- Affinity of an oligonucleotide for its target is routinely determined by measuring the Tm of an oligonucleotide/target pair, which is the temperature at which the oligonucleotide and target dissociate; dissociation is detected spectrophotometrically. The higher the Tm, the greater the affinity of the oligonucleotide for the target.
- oligonucleotide The relationship between an oligonucleotide and its complementary nucleic acid target to which it hybridizes is commonly referred to as "antisense.”
- “Targeting” an oligonucleotide to a chosen nucleic acid target is a multistep process. The process usually begins with identifying a nucleic acid sequence whose function is to be modulated. This may be, as examples, a cellular gene (or mRNA made from the gene) whose expression is associated with a particular disease state, or a foreign nucleic acid from an infectious agent.
- the target is a nucleic acid encoding raf; in other words, the raf gene or mRNA expressed from the raf gene.
- the targeting process also includes determination of a site or sites within the nucleic acid sequence for the oligonucleotide interaction to occur such that the desired effect- modulation of gene expression-will result. Once the target site or sites have been identified, oligonucleotides are chosen which are sufficiently complementary to the target, i.e., hybridize sufficiently well and with sufficient specificity, to give the desired modulation.
- Raf antisense oligonucleotides inhibit or reduce expression of the raf cDNA, mRNA, and protein. Inhibition of raf expression can be measured in ways which are routine in the art, for example by Northern blot assay of mRNA expression or Western blot assay of protein expression using methods well known in the art.
- Hybridization in the context of this invention, means hydrogen bonding, also known as Watson-Crick base pairing, between complementary bases, usually on opposite nucleic acid strands or two regions of a nucleic acid strand. Guanine and cytosine are examples of complementary bases which are known to form three hydrogen bonds between them. Adenine and thymine are examples of complementary bases which form two hydrogen bonds between them.
- oligonucleotide and “complementary” are terms which are used to indicate a sufficient degree of complementarity such that stable and specific binding occurs between the DNA or RNA target and the oligonucleotide. It is understood that an oligonucleotide need not be 100% complementary to its target nucleic acid sequence to be specifically hybridizable.
- An oligonucleotide is specifically hybridizable when binding of the oligonucleotide to the target interferes with the normal function of the target molecule to cause a loss of utility, and there is a sufficient degree of complementarity to avoid non-specific binding of the oligonucleotide to non-target sequences under conditions in which specific binding is desired, i.e., under physiological conditions in the case of in vivo assays or therapeutic treatment, or, in the case of in vitro assays, under conditions in which the assays are conducted.
- oligonucleotides are provided which are targeted to mRNA encoding c-raf, B-raf or A-raf.
- mRNA includes not only the coding region which carries the information to encode a protein using the three letter genetic code, but also associated
- oligonucleotides which form a region known to such persons as the 5'-untranslated region, the 3'-untranslated region, the 5' cap region, intron regions and intron/exon or splice junction ribonucleotides.
- oligonucleotides may be formulated in accordance with this invention that are targeted wholly or in part to these associated ribonucleotides as well as to the coding ribonucleotides.
- the oligonucleotide is targeted to a translation initiation site (AUG codon) or sequences in the 5'- or 3'-untranslated region of the human c-raf mRNA.
- messenger RNA to be interfered with include all vital functions such as translocation of the RNA to the site for protein translation, actual translation of protein from the RNA, splicing or maturation of the RNA and possibly even independent catalytic activity which may be engaged in by the RNA.
- the overall effect of such interference with the RNA function is to cause interference with raf protein expression.
- an oligonucleotide of the present invention targets a human c-raf polynucleotide or mRNA, e.g., the oligonucleotide is fully complementary to a region of a human c-raf mRNA.
- the sequence of an exemplary human c-raf mRNA is provided in SEQ ID NO:28, which corresponds to the sequence provided at GenBank Accession No. NM_002880.3.
- an oligonucleotide of the present invention comprises one or more modifications. It is not necessary for all positions in a given compound to be uniformly modified, and in fact more than one of the modifications described infra may be incorporated in a single compound or even at a single nucleoside within an oligonucleotide.
- Certain oligonucleotides of this invention are chimeric oligonucleotides. "Chimeric oligonucleotides" or “chimeras”, in the context of this invention, are oligonucleotides which contain two or more chemically distinct regions, each made up of at least one nucleotide.
- oligonucleotides typically contain at least one region of modified nucleotides that confers one or more beneficial properties (such as, for example, increased nuclease resistance, increased uptake into cells, increased binding affinity for the RNA target) and a region that is a substrate for RNase H cleavage.
- a chimeric oligonucleotide comprises at least one region modified to increase target binding affinity, and, usually, a region that acts as a substrate for RNAse H.
- Affinity of an oligonucleotide for its target is routinely determined by measuring the Tm of an oligonucleotide/target pair, which is the temperature at which the oligonucleotide and target dissociate; dissociation is detected spectrophotometrically.
- Tm the temperature at which the oligonucleotide and target dissociate
- dissociation is detected spectrophotometrically. The higher the Tm, the greater the affinity of the oligonucleotide for the target.
- the oligonucleotides e.g., raf antisense oligonucleotides, are modified to increase RNA binding affinity.
- the region of the oligonucleotide which is modified to increase raf mRNA binding affinity comprises at least one nucleotide modified at the 2' position of the sugar, e.g., a 2'-0-alkyl, 2'-0-alkyl-0-alkyl or 2'-fluoro-modified nucleotide.
- modifications are routinely incorporated into oligonucleotides and these oligonucleotides have been shown to have a higher Tm (i.e., higher target binding affinity) than 2'-deoxyoligonucleotides against a given target. The effect of such increased affinity is to greatly enhance antisense oligonucleotide inhibition of raf gene expression.
- RNAse H is a cellular endonuclease that cleaves the RNA strand of RNA:DNA duplexes; activation of this enzyme therefore results in cleavage of the RNA target, and thus can greatly enhance the efficiency of antisense inhibition. Cleavage of the RNA target can be routinely demonstrated by gel electrophoresis.
- the oligonucleotides e.g., raf antisense oligonucleotides
- Cells contain a variety of exo- and endo-nucleases which can degrade nucleic acids. A number of nucleotide and nucleoside modifications have been shown to make the
- oligonucleotide into which they are incorporated more resistant to nuclease digestion than the native oligodeoxynucleotide. Nuclease resistance is routinely measured by incubating oligonucleotides with cellular extracts or isolated nuclease solutions and measuring the extent of intact oligonucleotide remaining over time, usually by gel electrophoresis. Oligonucleotides which have been modified to enhance their nuclease resistance survive intact for a longer time than unmodified oligonucleotides. A variety of oligonucleotide modifications have been demonstrated to enhance or confer nuclease resistance. In one embodiment, the oligonucleotide contains at least one phosphorothioate modification. In one embodiment, the oligonucleotide is fully phosphorothioate modified. In some cases, oligonucleotide modifications which enhance target binding affinity are also, independently, able to enhance nuclease resistance.
- the oligonucleotides in accordance with this invention are from about 8 to about 50 nucleotides in length. In the context of this invention it is understood that this encompasses non-naturally occurring oligomers as herein before described, having 8 to 50 monomers. In further embodiments, the oligonucleotides comprise from about 8 to about 30
- nucleobases i.e., from about 8 to about 30 linked nucleosides.
- nucleoside is a base-sugar combination.
- the base portion of the nucleoside is normally a heterocyclic base.
- the two most common classes of such heterocyclic bases are the purines and the pyrimidines.
- Nucleotides are nucleosides that further include a phosphate group covalently linked to the sugar portion of the nucleoside.
- the phosphate group can be linked to either the 2', 3' or 5' hydroxyl moiety of the sugar.
- the phosphate groups covalently link adjacent nucleosides to one another to form a linear polymeric compound.
- this linear polymeric structure can be further joined to form a circular structure; however, open linear structures are generally preferred.
- the phosphate groups are commonly referred to as forming the internucleoside backbone of the oligonucleotide.
- the normal linkage or backbone of RNA and DNA is a 3' to 5' phosphodiester linkage.
- oligonucleotides e.g., raf antisense oligonucleotides
- useful in this invention include oligonucleotides containing modified backbones or non-natural internucleoside linkages.
- oligonucleotides having modified backbones include those that retain a phosphorus atom in the backbone and those that do not have a phosphorus atom in the backbone.
- modified oligonucleotides that do not have a phosphorus atom in their internucleoside backbone can also be considered to be
- Modified oligonucleotide backbones include, for example,
- phosphonates including 3'-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates including 3'-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, and boranophosphates having normal 3'-5' linkages, 2'-5' linked analogs of these, and those having inverted polarity wherein the adjacent pairs of nucleoside units are linked 3'-5' to 5'-3' or 2'-5' to 5'-2'.
- Various salts, mixed salts and free acid forms are also included.
- modified oligonucleotide backbones that do not include a phosphorus atom therein have backbones that are formed by short chain alkyl or cycloalkyl internucleoside linkages, mixed heteroatom and alkyl or cycloalkyl internucleoside linkages, or one or more short chain heteroatomic or heterocyclic internucleoside linkages.
- morpholino linkages formed in part from the sugar portion of a nucleoside
- siloxane backbones sulfide, sulfoxide and sulfone backbones
- formacetyl and thioformacetyl backbones methylene formacetyl and thioformacetyl backbones
- both the sugar and the internucleoside linkage, i.e., the backbone, of the nucleotide units are replaced with novel groups.
- the base units are maintained for hybridization with an appropriate nucleic acid target compound.
- an oligonucleotide mimetic that has been shown to have excellent hybridization properties, is referred to as a peptide nucleic acid (PNA).
- PNA peptide nucleic acid
- the sugar-backbone of an oligonucleotide is replaced with an amide containing backbone, in particular an aminoethylglycine backbone.
- the nucleobases are retained and are bound directly or indirectly to aza nitrogen atoms of the amide portion of the backbone.
- Representative United States patents that teach the preparation of PNA compounds include, but are not limited to, U.S. Pat. Nos.
- oligonucleotides e.g., raf antisense oligonucleotides, with phosphorothioate backbones and
- oligonucleosides with heteroatom backbones and in particular -CH 2 -NH-O-CH 2 - -, -CH 2 -N(CH 3 ) -O-CH 2 - [known as a methylene (methylimino) or MMI backbone], -CH 2 -0-N(CH 3 ) -CH 2 -, -CH2-N(CH 3 )-N(CH 3 ) -CH 2 - and -O- N(CH 3 ) -CH 2 -CH 2 - [wherein the native phosphodiester backbone is represented as -O-P-O-CH 2 -] of the above referenced U.S. Pat. No.
- Modified oligonucleotides may contain one or more substituted sugar moieties.
- Certain oligonucleotides comprise one of the following at the 2' position: OH; F; O-, S-, or N-alkyl, O-alkyl-O-alkyl, O-, S-, or N-alkenyl, or O-, S- or N-alkynyl, wherein the alkyl, alkenyl and alkynyl may be substituted or unsubstituted Ci to C 10 alkyl or C 2 to C 10 alkenyl and alkynyl.
- oligonucleotides comprise one of the following at the 2' position: Ci to C 10 lower alkyl, substituted lower alkyl, alkaryl, aralkyl, O-alkaryl or O-aralkyl, SH, SCH 3 , OCN, CI, Br, CN, CF 3 , OCF 3 , SOCH 3 , SO 2 CH 3 , ONO 2 , NO 2 , N 3 , NH 2 , heterocycloalkyl, heterocycloalkaryl,
- aminoalkylamino aminoalkylamino, polyalkylamino, substituted silyl, an RNA cleaving group, a reporter group, an intercalator, a group for improving the pharmacokinetic properties of an oligonucleotide, or a group for improving the pharmacodynamic properties of an oligonucleotide, and other substituents having similar properties.
- 2'-dimethylaminooxyethoxy i.e., a O(CH 2 ) 2 ON(CH 3 ) 2 group, also known as 2'- DMAOE, and 2'-dimethylaminoethoxyethoxy (2'-DMAEOE) as described in examples herein below.
- Other modifications include 2'-methoxy (2'-O ⁇ CH 3 ), 2'- aminopropoxy (2'-OCH 2 CH 2 CH 2 NH 2 ) and 2'-fluoro (2'-F).
- oligonucleotides e.g., raf antisense oligonucleotides
- sugar mimetics such as cyclobutyl moieties in place of the pentofuranosyl sugar.
- Representative United States patents that teach the preparation of such modified sugars structures include, but are not limited to, U.S. Pat. Nos. 4,981 ,957;
- oligonucleotides may also include nucleobase (often referred to in the art simply as “base”) modifications or substitutions.
- nucleobases include the purine bases adenine (A) and guanine (G), and the pyrimidine bases thymine (T), cytosine (C) and uracil (U).
- Modified nucleobases include other synthetic and natural nucleobases such as 5-methylcytosine (5-me-C or m5c), 5-hydroxymethyl cytosine, xanthine, hypoxanthine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine and guanine, 2-propyl and other alkyl derivatives of adenine and guanine, 2-thiouracil, 2-thiothymine and 2-thiocytosine, 5-halouracil and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and thymine, 5- uracil (pseudouracil), 4-thiouracil, 8-halo, 8-amino, 8-thiol, 8-thioalkyl, 8-hydroxyl and other 8-substituted adenines and guanines, 5-halo particularly 5-bromo, 5- trifluoromethyl and
- nucleobases include those disclosed in U.S. Pat. No. 3,687,808, those disclosed in the Concise Encyclopedia Of Polymer Science And Engineering 1990, pages 858-859, Kroschwitz, J. I., ed. John Wiley & Sons, those disclosed by Englisch et al.
- nucleobases are particularly useful for increasing the binding affinity of the oligomeric compounds of the invention. These include 5-substituted pyrimidines, 6-azapyrimidines and N-2, N-6 and 0-6 substituted purines, including 2- aminopropyladenine, 5-propynyluracil and 5-propynylcytosine. 5-methylcytosine substitutions have been shown to increase nucleic acid duplex stability by 0.6- 1.2.
- 5-methylcytosine substitutions are present in combination with 2'-0-methoxyethyl sugar modifications.
- Oligonucleotides provided herein may comprise one or more peptide nucleic acid (PNAs) subunits.
- PNAs Peptide nucleic acids
- PNAs are analogs of DNA in which the backbone is structurally homomorphous with a deoxyribose backbone, consisting of N-(2-aminoethyl) glycine units to which pyrimidine or purine bases are attached.
- PNAs containing natural pyrimidine and purine bases hybridize to complementary oligonucleotides obeying Watson-Crick base-pairing rules, and mimic DNA in terms of base pair recognition (Egholm, Buchardt et al. 1993).
- the backbone of PNAs is formed by peptide bonds rather than phosphodiester bonds, making them well-suited for antisense applications (see structure below).
- a backbone made entirely of PNAs is uncharged, resulting in PNA/DNA or PNA/RNA duplexes that exhibit greater than normal thermal stability. PNAs are not recognized by nucleases or proteases.
- PNAs may be produced synthetically using any technique known in the art.
- PNA is a DNA analog in which a polyamide backbone replaces the traditional phosphate ribose ring of DNA. Despite a radical structural change to the natural structure, PNA is capable of sequence-specific binding in a helix form to DNA or RNA. Characteristics of PNA include a high binding affinity to
- PanageneTM has developed its proprietary Bts PNA monomers (Bts;
- PNA compounds include, but are not limited to, U.S. Pat. Nos. 5,539,082; 5,714,331 ; and 5,719,262, each of which is herein incorporated by reference. Further teaching of PNA compounds can be found in Nielsen et al., Science, 1991 , 254, 1497.
- LNAs locked nucleic acid subunits
- the structures of LNAs are known in the art: For example, Wengel, et al., Chemical Communications (1998) 455; Tetrahedron (1998) 54, 3607, and Accounts of Chem. Research (1999) 32, 301 ); Obika, et al., Tetrahedron Letters (1997) 38, 8735; (1998) 39, 5401 , and Bioorganic Medicinal Chemistry (2008)16, 9230.
- Polynucleotides and oligonucleotides may incorporate one or more LNAs; in some cases, the compounds may be entirely composed of LNAs.
- Typical intersubunit linkers include phosphodiester and phosphorothioate moieties; alternatively, non-phosphorous containing linkers may be employed.
- One embodiment includes an LNA containing compound where each LNA subunit is separated by a RNA or a DNA subunit (i.e., a deoxyribose nucleotide). Further exemplary compounds may be composed of alternating LNA and RNA or DNA subunits where the intersubunit linker is phosphorothioate.
- Certain polynucleotides or oligonucleotides may comprise morpholino-based subunits bearing base-pairing moieties, joined by uncharged or substantially uncharged linkages.
- morpholino oligomer or "PMO” (phosphoramidate- or phosphorodiamidate morpholino oligomer) refer to an oligonucleotide analog composed of morpholino subunit structures, where (i) the structures are linked together by phosphorus-containing linkages, one to three atoms long, preferably two atoms long, and preferably uncharged or cationic, joining the morpholino nitrogen of one subunit to a 5' exocyclic carbon of an adjacent subunit, and (ii) each morpholino ring bears a purine or pyrimidine or an equivalent base-pairing moiety effective to bind, by base specific hydrogen bonding, to a base in a polynucleotide.
- the oxygen attached to phosphorus may be substituted with sulfur (thiophosphorodiamidate).
- the 5' oxygen may be substituted with amino or lower alkyl substituted amino.
- the pendant nitrogen attached to phosphorus may be unsubstituted, monosubstituted, or disubstituted with (optionally substituted) lower alkyl.
- the purine or pyrimidine base pairing moiety is typically adenine, cytosine, guanine, uracil, thymine or inosine.
- the oligonucleotides e.g., raf antisense oligonucleotides, comprises a cap structure" or "terminal cap moiety.
- oligonucleotides e.g., raf antisense oligonucleotides
- chemically linking to the oligonucleotide one or more moieties or conjugates which enhance the activity, cellular distribution or cellular uptake of the oligonucleotide include but are not limited to lipid moieties such as a cholesterol moiety (Letsinger et al., Proc. Natl. Acad. Sci. USA 1989, 86, 6553-6556), cholic acid (Manoharan et al., Bioorg. Med. Chem. Lett.
- a thioether e.g., hexyl-S-tritylthiol (Manoharan et al., Ann. N.Y. Acad. Sci. 1992, 660, 306-309; Manoharan et al., Bioorg. Med. Chem. Let. 1993, 3, 2765-2770), a thiocholesterol (Oberhauser et al., Nucl. Acids Res. 1992, 20, 533-538), an aliphatic chain, e.g., dodecandiol or undecyl residues (Saison-Behmoaras et al., EMBO J.
- a phospholipid e.g., di-hexadecyl-rac-glycerol or triethylammonium 1 ,2-di-O- hexadecyl-rac-glycero-3-H-phosphonate (Manoharan et al., Tetrahedron Lett. 1995, 36, 3651 -3654; Shea et al., Nucl. Acids Res.
- an oligonucleotide used according to the present invention has a structure as described in PCT Application Publication No. WO2009/102427, PCT Application Publication No. WO2010/001 1346, or PCT Application Publication No. WO2010/033246.
- WO2009/102427 describes, e.g., double-stranded RNA (dsRNA) constructs of 12- 49 (preferably 19-49) nucleotides in length, for inhibiting expression of a target gene, said dsRNA comprising: (1 ) a sense strand having a 5'-end and a 3'-end, wherein one or more nucleotides at each of said 5'- and 3'-ends of said sense strand have 2'-modified ribose sugars, and, (2) an antisense strand having a 5'-end and a 3'-end, which hybridizes to said sense strand and to mRNA of said target gene, wherein said antisense strand includes a 2'-modified ribose sugar at the 2nd nucleotide from the 5'-end of the antisense strand, wherein (a) said dsRNA is resistant to cleavage by Dicer, (b) the antisense strand associates with RISC, and
- WO2010/01 1346 describes, e.g., a polynucleotide construct comprising two identical single-stranded polynucleotides, each of the single-stranded polynucleotide comprising a 5 '-stem sequence having a 5 '-end, a 3 '-stem sequence having a 3 '-end, and a linker sequence linking the 5'-stem sequence and the 3'-stem sequence, wherein: (1 ) the 5'-stem sequence of a first single-stranded polynucleotide hybridize with the 3 '- stem sequence of a second single- stranded polynucleotide to form a first double- stranded stem region; (2) the 5'-stem sequence of the second single-stranded polynucleotide hybridize with the 3 '-stem sequence of the first single-stranded polynucleotide to form a second double-stranded stem region; and, (3) the
- WO2010/033246 describes, e.g., a double stranded nucleic acid molecule comprising a guide strand, with a minimal length of 16 nucleotides, and a passenger strand forming a double stranded nucleic acid, having a double stranded region and a single stranded region, the double stranded region having 8-15 nucleotides in length, the single stranded region having 4-12 nucleotides in length, wherein position 1 of the guide strand is 5' phosphorylated or has a 2' O-methyl modification, wherein the passenger strand is linked to a lipophilic group, wherein at least 40% of the nucleotides of the double stranded nucleic acid are modified, and wherein the double stranded nucleic acid molecule has one end that is blunt or includes a one nucleotide overhang.
- RNAi RNA interference
- RNA duplexes have a structure as described in this article.
- oligonucleotides used in accordance with this invention may be conveniently and routinely made through the well-known technique of solid phase synthesis. Equipment for such synthesis is sold by several vendors including Applied Biosystems. Any other means for such synthesis may also be employed; the actual synthesis of the oligonucleotides is well within the talents of the routineer. It is also well known to use similar techniques to prepare other oligonucleotides such as the phosphorothioates and alkylated derivatives.
- CPG controlled-pore glass
- oligonucleotides e.g., raf antisense oligonucleotides, of the present invention modulate raf gene expression. In certain embodiments, they inhibit c-raf gene expression. In particular embodiments, they target a
- polynucleotide having a sequence set forth in SEQ ID NO: 28 (GENBANK
- selection of a sequence region complementary to a target gene is based upon analysis of the chosen target sequence and determination of secondary structure, T m , binding energy, and relative stability and cell specificity. Such sequences may be selected based upon their relative inability to form dimers, hairpins, or other secondary structures that would reduce structural integrity of the polynucleotide or prohibit specific binding to the target gene in a host cell.
- target regions of the target gene or mRNA may include those regions at or near the AUG translation initiation codon and those sequences that are substantially complementary to 5' regions of the gene or mRNA.
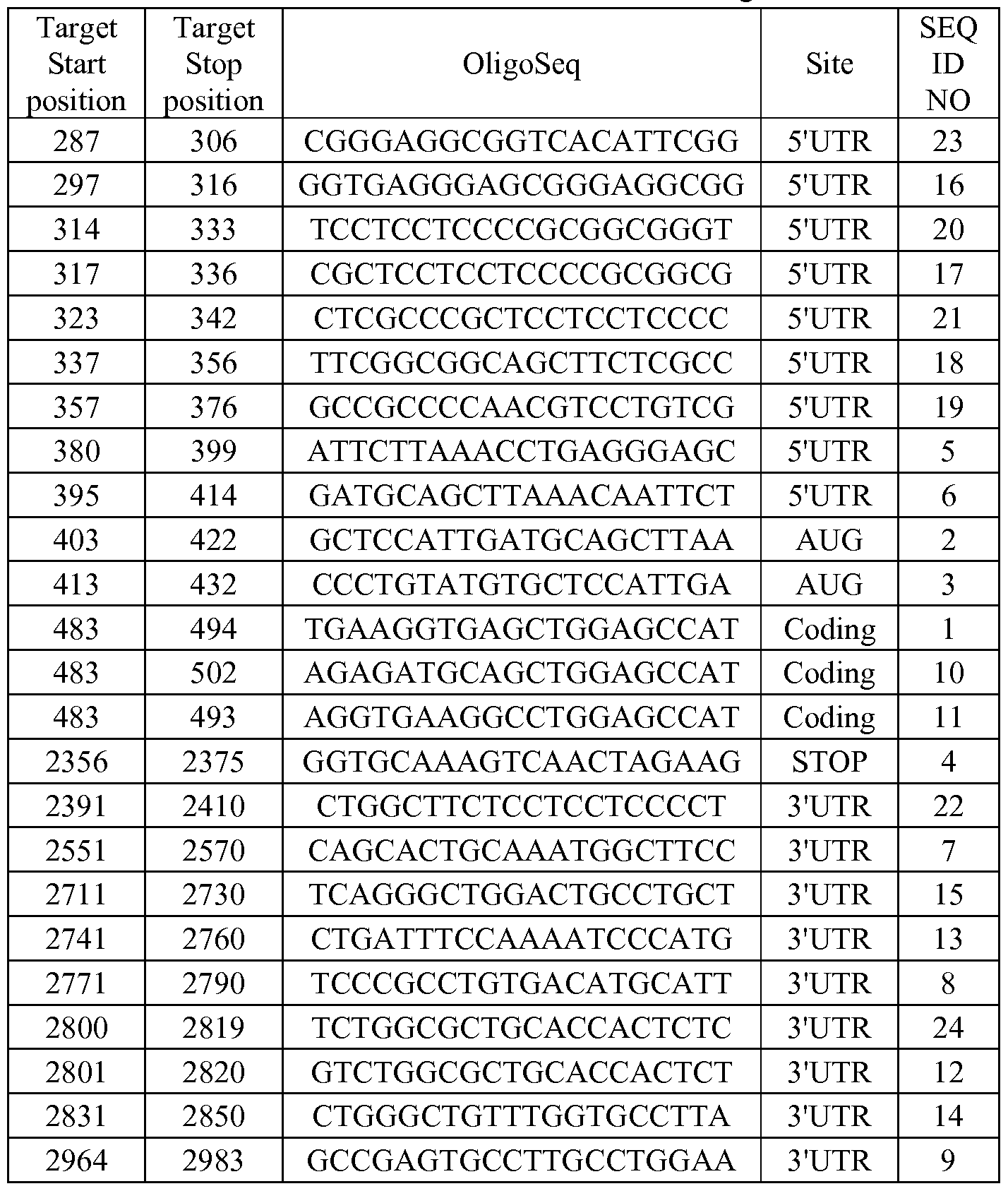
- oligonucleotides that may be used according to the methods and compositions of the present invention are shown in Tables 1 -4.
- the antisense oligonucleotides listed in the tables target human c-raf of SEQ ID NO: 28 (GENBANK Accession No. NM_002880).
- Target start position' indicates the 5'-most nucleotide to which the antisense oligonucleotide is targeted.
- Target stop position' indicates the 3'- most nucleotide to which the antisense oligonucleotide is targeted. Sequences are shown 5' - 3'.
- Table 2. Uniformly 2' Sugar- modified c-raf Oligonucleotides
- Exemplary chimeric oligonucleotides having SEQ ID NO: 8 and having central "gap" regions of 6, 8, or 10 deoxynucleotides flanked by two regions of 2'-O-methyl modified nucleotides are shown in Table 3.
- Backbones may be uniformly phosphorothioate.
- Additional chimeric oligonucleotides having one or more regions of 2'-O-methyl modification and uniform phosphorothioate backbones are shown in Table 3. All are phosphorothioates; bold regions indicate 2'-O- methyl modified regions.
- Table 3. Chimeric 2'-0-methyl P S c-raf oligonucleotides
- oligonucleotides described herein may include one or more uridine residues instead of one or more thymidine residues.
- 2'-methoxyethyl-5-methyluridine (2'MOE Me U) nucleosides are also sometimes designated as 2'-methoxyethylribothymidine (2'-MOE T).
- a raf antisense oligonucleotide of the present invention has a "gapmer” or “gap-widened” structure, which is an antisense compound in which an internal sequence (or region) having a plurality of nucleotides that supports RNaseH cleavage is positioned between external sequences (or regions) having one or more nucleotides that are chemically distinct from the nucleosides of the internal region.
- a “gap segment” means the plurality of nucleotides that make up the internal region of a gapmer.
- a “wing segment” means the external region of a gapmer.
- a gap segment includes 4, 5, 6, 7, 8, 9, 10, 1 1 , or 12 nucleotides.
- an oligonucleotide comprises a gap segment of 10 nucleotides flanked by two wing segments of 5 nucleotides each. In certain embodiments, an oligonucleotide comprises a gap segment of 8 nucleotides flanked by two wing segments of 6 nucleotides each.
- a c-raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence
- a c-raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy, which may also be depicted as 5'-
- methods of the present invention utilize a modified c-raf antisense oligonucleotide comprising or consisting of a sequence 100% complementary to nucleobases 2771 to 2790 of SEQ ID NO:28.
- the modified oligonucleotide comprises or consists of the nucleobase sequence of SEQ ID NO:29.
- the modified oligonucleotide comprises: a gap segment consisting of linked deoxynucleosides; a 5' wing segment consisting of linked nucleosides; and a 3' wing segment consisting of linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; and wherein each nucleoside of each wing segment comprises a modified sugar.
- the modified oligonucleotide consists of 20 nucleobases.
- the modified oligonucleotide comprises: a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-0-methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine.
- the c-raf antisense oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29).
- the oligonucleotide is a full phosphorothioate analog.
- this oligonucleotide comprises one or more 2'- O-methoxyethyl substitutions.
- the oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), wherein the oligonucleotide is a full phosphorothioate analog, wherein the oligonucleotide comprises 2'-0-methoxyethyl substitutions at positions 1 -6 and 15- 20, and wherein residues 7-14 are unmodified 2'-deoxy.
- the present invention further includes salt forms of the raf antisense oligonucleotides, such as, e.g., nonadecasodium salts.
- the raf antisense oligonucleotide is a nonadecasodium salt of a c-raf antisense
- oligonucleotide that is a full phosphorothioate analog consisting of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy, which has a molecular formula of C234H 3 io 680i26 i9Si 9 Nai9.
- oligonucleotides may be prepared by methods known in the art. In one embodiment, they are synthesized by a multi-step process that may be divided into two distinct operations: solid phase synthesis and downstream processing. In the solid-phase synthesis operation, the nucleoside sequence is assembled by a computer controlled solid-phase synthesizer. The downstream process includes deprotection, reverse-phase chromatographic purification, isolation, and lyophilization.
- the present invention contemplates the use of any and all raf antisense oligonucleotides comprising or consisting of one or more of these exemplified sequences, in addition to any other raf antisense oligonucleotides or other oligonucletoides capable of modulating raf gene expression.
- a raf antisense oligonucleotide or other oligonucleotide of the present invention selectively modulates expression of one raf gene, while in other embodiments, a raf antisense oligonucleotide of the present invention modulates expression of two or more raf genes.
- raf antisense oligonucleotides as described above are used in combination with one or more other anti-HCV agents.
- the present invention contemplates that essentially any type of agent or treatment having a desired activity against HCV may be used in combination with the raf antisense oligonucleotides provided herein.
- agents may include any of a variety of different types of molecules, including, but not limited to, peptides, polypeptides, polynucleotides, antisense oligonucleotides, siRNA's, short hairpin RNA (shRNA), DNAzymes, ribozymes, antibodies, small organic compounds, metals, small inorganic molecules, radionuclides and the like.
- an anti-HCV agent is selected from the group consisting of an HCV polymerase inhibitor, an HCV protease inhibitor, an inhibitor of another target in the HCV life cycle, an immunomodulatory agent, an antiviral agent, or a combination thereof.
- immunomodulatory agents include, but are not limited to; natural and recombinant interferon isoform compounds, a-interferon, ⁇ -interferon, ⁇ -interferon, o-interferon, Intron® A, Roferon® A, Canferon®-A300, Advaferon®, Infergen®, Humoferon®, Sumiferon® MP, Alfaferone®, IFN- ⁇ ®, Feron®, polyethylene glycol derivatized (pegylated) interferon compounds, PEG interferon- a-2a (Pegasys®), PEG interferon-a-2b (PEG-lntron®), and pegylated IFN-a-con1 and the like; long acting formulations and derivatizations of interferon compounds such as the albumin-fused interferon albuferon-a and the like; and compounds that stimulate the synthesis of interferon in cells, such as resiquimod and the like.
- immunomodulatory agents can include, for example, interleukins; compounds that enhance the development of type 1 helper T cell response, such as SCV-07 and the like; other cytokines; CpG
- oligonucleotides such as CpG-10101 (actilon), isatoribine and the like; thymosin a-1 ; ANA-245; ANA-246; histamine
- antiviral agents include, but are not limited to, ribavirin, amantadine, viramidine, nitazoxanide, telbivudine, NOV-205, taribavirin, inhibitors of internal ribosome entry, broad-spectrum viral inhibitors, such as IMPDH inhibitors (e.g., compounds of U.S. Pat. No.
- HCV polymerase inhibitors useful according to the present invention include, but are not limited to, NM283 (valopicitabine), R803, JTK-109, JTK-003, HCV-371 , HCV-086, HCV-796 and R-1479.
- Illustrative inhibitors of HCV proteases include, but are not limited to, compounds described in WO02/18369.
- Other illustrative agents include BILN-2061 , VX-950, GS-9132 (ACH-806), SCH-503034, and SCH-6.
- Still other illustrative agents include those disclosed in WO98/17679, WO00/056331 , WO 98/22496, WO 99/07734, WO 2005/073216 and WO 2005073195.
- Exemplary inhibitors HCV life cycle targets can include NS3 helicase inhibitors, metallo-protease inhibitors, alpha glucosidase and the like.
- the anti-HCV agent used in combination with a raf antisense oligonucleotide is a-interferon, pegylated a-interferon, ribavirin, or a combination thereof.
- the present invention further provides pharmaceutical compositions and formulations comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, in combination with another anti-HCV agent.
- an oligonucleotide that targets a raf nucleic acid e.g., a raf antisense oligonucleotide
- another anti-HCV agent e.g., a raf antisense oligonucleotide
- raf antisense oligonucleotides of the present invention are used in combination with one or more anti-HCV agents to provide increased or synergistic improvement in treating HCV infection based, for example, on mechanism of action and non-overlapping toxicity profiles.
- oligonucleotide(s) that targets a raf nucleic acid may be administered prior to, subsequent to, or simultaneously with one or more anti-HCV agents.
- a raf antisense oligonucleotide may be administered prior to, subsequent to, or simultaneously with one or more anti-HCV agents.
- oligonucleotide(s) that targets a raf nucleic acid e.g., a raf antisense
- oligonucleotide may be delivered in a separate formulation or in the same formulation as the additional anti-HCV agent(s). Furthermore, where
- oligonucleotides of the present invention are administered in a drug delivery device, including those described herein, the second therapeutic agent may be delivered independently or may also be included in the drug delivery device.
- Oligonucleotides that target a raf nucleic acid e.g., raf antisense oligonucleotides, and anti-HCV agents of the invention may be formulated as pharmaceutical compositions suitable for delivery to a subject.
- compositions will often further comprise one or more buffers ⁇ e.g., neutral buffered saline or phosphate buffered saline), carbohydrates (e.g., glucose, mannose, sucrose, dextrose or dextrans), mannitol, proteins, polypeptides or amino acids such as glycine, antioxidants, bacteriostats, chelating agents such as EDTA or glutathione, adjuvants (e.g., aluminum hydroxide), solutes that render the formulation isotonic, hypotonic or weakly hypertonic with the blood of a recipient, suspending agents, thickening agents, preservatives and the like.
- buffers e.g., neutral buffered saline or phosphate buffered saline
- carbohydrates e.g., glucose, mannose, sucrose, dextrose or dextrans
- mannitol proteins
- proteins polypeptides or amino acids
- proteins e.glycine
- antioxidants
- compositions for use in the present invention can be found, e.g., in Remington's Pharmaceutical Sciences, Mack Publishing Company, Philadelphia, PA, 17 th Ed. (1985).
- intravenous compositions will comprise a solution of the therapeutic agent in an acceptable carrier, such as an aqueous carrier.
- an aqueous carrier e.g., water, buffered water, 0.4% saline, 0.9% isotonic saline, 0.3% glycine, 5% dextrose, and the like, and may include glycoproteins for enhanced stability, such as albumin, lipoprotein, globulin, etc.
- compositions can be sterilized by conventional sterilization techniques, such as filtration.
- the resulting aqueous solutions may be packaged for use or filtered under aseptic conditions and lyophilized, the lyophilized preparation being combined with a sterile aqueous solution prior to administration.
- the compositions may also contain pharmaceutically acceptable auxiliary substances as required to approximate physiological conditions, such as pH adjusting and buffering agents, tonicity adjusting agents and the like, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, etc.
- compositions and formulations of the invention may be administered by essentially any suitable means, illustrative examples of which include parenteral, intravenous, systemic, local, oral, intratumoral, intramuscular, intraocular, eye drops, subcutaneous, intraperitoneal, inhalation, or any other suitable method of delivery.
- the therapeutic agents of the present invention are administered parenterally, i.e., intraarticular ⁇ , intravenously, intraperitoneal ⁇ , subcutaneously, or intramuscularly. In a more specific embodiment, they are administered by intravenous infusion or intraperitoneally by a bolus injection.
- a patient is given an intravenous infusion of one or more raf antisense oligonucleotides and/or anti-HCV agents through a running intravenous line over, e.g., 5-10 minutes, 15-20 minutes, 30 minutes, 60 minutes, 90 minutes, or longer. In one embodiment, a 60 minute infusion is used. In other embodiments, an infusion ranging from 6-10 or 15-20 minutes is used.
- Such infusions can be given periodically, e.g., once every 1 , 3, 5, 7, 10, 14, 21 , or 28 days or longer.
- the therapeutic agents of the present invention are administered subcutaneously, e.g., on a weekly basis. They may alternatively be administered subcutaneously more or less frequently, e.g., once every 3-5 days, once every two weeks, or once every four weeks for a period of time.
- both agents may be administered subcutaneously, e.g., once a week, once every 3-5 days, once every two weeks, or once every four weeks for a period of time,
- Formulations for topical administration may include, for example, ointments, lotions, creams, gels, drops, suppositories, sprays, liquids and powders.
- Conventional pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the like may be necessary or desirable.
- Formulations for oral administration may include, for example, powders or granules, suspensions or solutions in water or non-aqueous media, capsules, sachets, or tablets. Thickeners, flavorings, diluents, emulsifiers, dispersing aids or binders may also be desirable.
- Formulations for parenteral administration may include, for example, sterile aqueous solutions which may also contain buffers, diluents and other suitable additives.
- cationic lipids or other agents may be included in the formulations to facilitate oligonucleotide uptake.
- LIPOFECTIN a 1 :1 liposome formulation of the cationic lipid N-[1 -(2,3-dioleyloxy)propyl]-N,N,N- trimethylammonium chloride (DOTMA) and dioleoyl phosphatidylethanolamine (DOPE)) (BRL, Bethesda Md.).
- Dosing is generally dependent on severity and responsiveness of the condition to be treated, with a typical course of treatment lasting from several days to several months or until a cure is effected or a diminution of disease state is achieved.
- Optimal dosing schedules can be calculated from measurements of drug accumulation in the body or at a localized site or based upon a patient's response. Persons of ordinary skill can easily determine optimum dosages, dosing methodologies, and repetition rates. Optimum dosages may vary depending on the relative potency of individual oligonucleotides, and can generally be estimated based on EC50's in in vitro and in vivo animal studies.
- anti-HCV agents used according to the present invention may be administered using conventional doses and routes of administration known to those having skill in the art.
- oligonucleotide in accordance with the invention, commonly in a pharmaceutically acceptable carrier, in amounts and for periods which will vary depending upon the nature of the particular disease, its severity and the patient's overall condition.
- compositions and formulations of the present invention can be used in methods for inhibiting the replication of HCV and thereby treating or preventing HCV infection in a patient.
- the methods may also be used to treat infection from any viral-mediated disease or disorder that benefits from reduced raf expression.
- compositions comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and/or one or more of the additional HCV therapeutic agents are administered to a patient so as to achieve systemic delivery of the agents.
- pharmaceutical compositions comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and/or one or more of the additional HCV therapeutic agents are administered to a patient so as to achieve local delivery of the agents.
- compositions comprising oligonucleotides that targets a raf nucleic acid, e.g., raf antisense oligonucleotides, and/or one or more of the additional HCV therapeutic agents are provided to a patient in a drug delivery device, typically to achieve prolonged delivery over a relatively long time period.
- this time period is at least one week, at least two weeks, at least three weeks, at least one month, at least two months, at least three months, at least four months, at least six months, at least nine months, or at least one year.
- oligonucleotides targeted to portions of the c-raf mRNA are particularly useful for inhibiting raf expression and for interfering with HCV replication.
- tissues or cells are contacted with the compositions and formulations of the invention.
- to "contact" tissues or cells means to add the oligonucleotide(s) and/or the anti-HCV agent(s), usually in a liquid carrier, pharmaceutical composition, and/or drug delivery device, to a cell suspension or tissue sample, either in vitro or ex vivo, or to administer the oligonucleotide(s) and/or the anti-HCV agent(s) to cells or tissues within an animal, again, typically in a pharmaceutical formulation or drug delivery device.
- the oligonucleotide that targets a raf nucleic acid is delivered prior to, concurrently with, or after delivery of the one or more additional anti-HCV agents used in the combination therapy.
- One or more of the oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and one or more additional therapeutic HCV agents may be delivered using a single drug delivery devices, or, alternatively, they may be delivered in separate drug delivery devices. Of course, in particular embodiments, none, one or some of the therapeutic agents are delivered using a drug delivery device.
- kits comprising one or more pharmaceutical compositions of the present invention.
- a kit of the present invention comprises a pharmaceutical composition comprising a raf antisense oligonucleotide and a pharmaceutical composition comprising an additional therapeutic agent, such as an anti-HCV agent described above.
- the present invention further includes drug delivery devices comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, optionally further comprising one or more additional anti-HCV agents, as described herein.
- the drug delivery devices of the present invention are particularly well-suited for the administration and controlled release of therapeutic agents over a prolonged time-course.
- Drug delivery devices according to the present invention are typically biocompatible.
- Drug delivery devices include microcapsules.
- an oligonucleotide of the present invention is entrapped in a microcapsule prepared, for example, by coacervation techniques or by interfacial polymerization, for example, hydroxymethylcellulose or gelatin- microcapsules and poly-(methylmethacylate) microcapsules, respectively; in colloidal drug delivery systems (for example, liposomes, niosomes, albumin microspheres, microemulsions, nano-particles and nanocapsules) or in macroemulsions.
- colloidal drug delivery systems for example, liposomes, niosomes, albumin microspheres, microemulsions, nano-particles and nanocapsules
- Other microcapsules include, e.g., nanoparticles and microparticles.
- a drug delivery device of the present invention comprises a sustained-release preparation of an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, optionally further comprising one or more other anti-HCV agents.
- sustained-release preparations include semipermeable matrices of solid hydrophobic polymers containing the antagonist, which matrices are in the form of shaped articles, e.g. films, or microcapsules.
- sustained-release matrices include polyesters, hydrogels (for example, poly(2-hydroxyethyl-methacrylate), or poly(vinylalcohol)), polylactides (U.S. Pat. No. 3,773,919), copolymers of L- glutamic acid and y ethyl-L-glutamate, non-degradable ethylene-vinyl acetate, degradable lactic acid-glycolic acid copolymers such as the LUPRON DEPOT.TM. (injectable microspheres composed of lactic acid-glycolic acid copolymer and leuprolide acetate), biocompatible polymers, and poly-D-(-)-3-hydroxybutyric acid.
- polyesters for example, poly(2-hydroxyethyl-methacrylate), or poly(vinylalcohol)
- polylactides U.S. Pat. No. 3,773,919
- drug delivery devices according to the present invention comprise a biocompatible matrix.
- Biocompatible matrices of the present invention may comprise, e.g., collagen, metal, hydroxyapatite, bioglass, aluminate, bioceramic materials, and purified proteins.
- the drug delivery device comprises AtrigelTM (QLT, Inc., Vancouver, B.C.).
- the Atrigel ® drug delivery system consists of biodegradable polymers dissolved in biocompatible carriers. Pharmaceuticals may be blended into this liquid delivery system at the time of manufacturing or, depending upon the product, may be added later by the physician at the time of use.
- the liquid product is injected into the subcutaneous space through a small gauge needle or placed into accessible tissue sites through a cannula, water in the tissue fluids causes the polymer to precipitate and trap the drug in a solid implant.
- the drug encapsulated within the implant is then released in a controlled manner as the polymer matrix biodegrades with time.
- the present invention provides a drug delivery device suitable for ocular administration of an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, alone or in combination with one or more additional therapeutic agents.
- the drug delivery device comprises an erodable polylacticglycolic acid (PLGA) matrix, such as that used in the Posurdex® system (Allergan, Irvine, CA).
- PLGA polylacticglycolic acid
- Posurdex® is a bioerodable extended release implant that delivers dexamethasone to the targeted disease site at the posterior-segment of the eye.
- a drug delivery device of the present invention comprises a polymer.
- Polymers such as poly(lactic acid) or poly(glycolic acid) undergo hydrolytic degradation in the body and become monomers of lactic acid or glycolic acid. These monomers can be metabolized and eliminated from the tissues. It is possible to incorporate drugs in the matrix of these polymers. The polymer containing the drug releases the drug for a sustained period and undergoes degradation simultaneously. These polymers have been used as materials of absorbable surgical sutures for many years and proved to be safe and biocompatible.
- Particularly suitable drug delivery devices of the present invention provide prolonged delivery of the oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and/or one or more additional therapeutic agents, as compared to the duration of delivery in the absence of the drug delivery device.
- a drug delivery device increases the pharmacokinetics, e.g., half-life, of the oligonucleotide by at least two-fold, at least five-fold, or at least ten-fold as compared to in the absence of the drug delivery device.
- human hepatocyte cells HuH7.5 were transfected with varying concentrations (0-500 nM) of an illustrative raf antisense
- the raf antisense oligonucleotide compound used in the experiments was a full phosphorothioate analog consisting of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0- methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
- the U nucleosides are also sometimes designated as T.
- HuH7.5 cells were cultured in complete medium in the absence of antibiotics for one week. The cells were then plated on a 12 well plate at 20% density, one day prior to transfection, to achieve 30-40% density on the day of transfection. Prior to transfection, the complete medium was removed and replaced by OptiMEM medium (Gibco, Invitrogen Corp., Carlsbad, CA, USA;
- oligofectamin (Invitrogen Corp., Carlsbad, CA, USA) per 100 ⁇ volume was used regardless of the amount of oligonucleotide being transfected.
- transfection with a control sequence 200 nM
- transfection with a fluorescent oligonucleotide 200 nM, Trackit; Gibco, Invitrogen Corp., Carlsbad, CA, USA
- DMEM fetal calfected cells
- the cells were infected with HCV on Day 0 for four hours and further cultured in DMEM containing 10% FBS. Cell viability was assessed daily using a conventional microscope. Medium was collected at given days for determining HCV viral production until Day 6.
- HCV titers were determined by quantitative RT-PCR.
- RNA was extracted from the cell culture medium by using a Roche High Pure Viral nucleic acid kit according to the instructions provided with the kit (Roche Applied Science, Indianapolis, IN, USA).
- cDNA was then produced from the RNA template using Superscript III reverse transcriptase (Invitrogen Corp., Carlsbad, CA, USA), according to the instructions provided.
- An HCV specific primer (5'- AGGTTTAGGATTCGTGCTCAT) was used.
- Quantitative PCR was then performed using an Applied Biosystems/Taqman Real Time PCR system (Life Technologies Corp., Carlsbad, CA, USA) and Taqman chemistry, with all measurements determined in duplicate. 6-FAM-
- CACCCTATCAGGCAGTACCACAAGGCC-TAMRA was used as the HCV specific detection probe, and a primer set detecting the conserved 5'UTR region of HCV (5'-TGCGGAACCGGTGAGTACA, 5'-AGGTTTAGGATTCGTGCTCAT).
- pCV-H77c a standard curve of known dilutions of a plasmid containing the sequence for HCV variant H77c
- treatment with the raf antisense oligonucleotide compound resulted in a dose dependent reduction in HCV titers measured in the cells, with a moderate reduction in viral titers at 200 nM (60%) and 80-95% at higher concentrations (300-500 nM), in cells transfected the oligonucleotide, compared to cells transfected with a control antisense molecule.
- the suppressing effect was short-lived at lower concentrations, but sustained for up to 8 days post infection at higher concentrations (single dose). The maximum effect was observed at four to 6 days post treatment.
- HCV core protein showed a similar trend as HCV titers, and NS3 protein levels were less affected by iCo-007 treatment (data not shown).
- oligonucleotide were reduced by up to 95%, for up to 5 days post transfection. The maximum effect was seen at four days post treatment. Inhibition at a higher concentration resulted in a longer suppression of c-Raf expression. C-Raf suppression did not result in reduced cell growth, and at lower concentrations (100-200 nM), it appeared to have a slightly stimulating effect on cell growth.
Abstract
The present invention relates generally to compositions and methods comprising raf antisense oligonucleotides for the treatment or prevention of HCV infection in a patient. In certain embodiments, the raf antisense oligonucleotides are used in combination with other agents or treatments having activity against HCV.
Description
TREATING AND PREVENTING HEPATITIS C VIRUS INFECTION USING C-RAF KINASE ANTISENSE OLIGONUCLEOTIDES
CROSS-REFERENCES TO RELATED APPLICATIONS
This application claims the benefit under 35 U.S.C. § 1 19(e) of U.S. Provisional Patent Application No. 61/31 1 ,710 filed March 8, 2010; U.S.
Provisional Patent Application No. 61/332,582 filed May 7, 2010; and U.S.
Provisional Patent Application No. 61/353,997 filed June 1 1 , 2010, where these (three) provisional application are incorporated herein by reference in their entireties. STATEMENT REGARDING SEQUENCE LISTING
The Sequence Listing associated with this application is provided in text format in lieu of a paper copy, and is hereby incorporated by reference into the specification. The name of the text file containing the Sequence Listing is
480231_409PC_SEQUENCE_LISTING.txt. The text file is 10 KB, was created on March 7, 201 1 and is being submitted electronically via EFS-Web.
BACKGROUND OF THE INVENTION
Field of the Invention
This invention relates to compositions and methods for modulating expression of raf genes, using antisense oligonucleotides directed to raf mRNAs. This invention is also directed to methods for inhibiting viral replication, and related methods of treating and preventing viral infection, particularly HCV infection, using raf antisense oligonucleotides, optionally in combination with other anti-viral, e.g., anti-HCV, drugs.
Description of the Related Art
Chronic infection with hepatitis C virus (HCV) is a major health problem associated with liver cirrhosis, hepatocellular carcinoma, and liver failure. Infection by HCV is insidious in a high proportion of chronically infected (and infectious) carriers who may not experience clinical symptoms for many years. Liver cirrhosis can ultimately lead to liver failure, and liver failure resulting from chronic HCV infection is now recognized as a leading cause of liver
transplantation.
HCV is a member of the Flaviviridae family of RNA viruses that affect animals and humans. The genome is a single, about 9.6-kilobase strand of RNA, and consists of one open reading frame that encodes for a polyprotein of about 3000 amino acids flanked by untranslated regions at both 5' and 3' ends (5'- and 3'-UTR). The polyprotein serves as the precursor to at least 10 separate viral proteins critical for replication and assembly of progeny viral particles. Because the replicative cycle of HCV does not involve any DNA intermediate and the virus is not integrated into the host genome, HCV infection can theoretically be cured.
Standard treatment for chronic HCV includes interferon alpha (IFN- alpha) or pegylated IFN-alpha in combination with ribavirin, often involving at least six (6) months of treatment. Treatment of HCV with interferon has frequently been associated with adverse side effects such as fatigue, fever, chills, headache, myalgias, arthralgias, mild alopecia, psychiatric effects, autoimmune phenomena and thyroid dysfunction. Ribavirin, an inhibitor of inosine 5'-monophosphate dehydrogenase (IMPDH), enhances the efficacy of IFN-alpha in the treatment of HCV. However, a number of patients still have significant side effects due to ribavirin. For example, ribavirin causes significant hemolysis in 10-20% of patients treated at currently recommended doses, and the drug is both teratogenic and embryotoxic. Even with recent improvements, a substantial fraction of patients do not respond to conventional HCV treatment with a sustained reduction in viral load.
A number of alternative approaches are being pursued to combat HCV. These include, for example, application of antisense oligonucleotides or ribozymes for inhibiting HCV replication. In addition, low-molecular weight compounds that directly inhibit HCV proteins and interfere with viral replication are considered as attractive strategies to control HCV infection. Among the viral targets, the NS3/4a protease/helicase and the NS5b RNA-dependent RNA polymerase are examples of viral targets against which new drugs are being developed. Besides targeting viral genes and their transcription and translation products, antiviral activity can also be achieved by targeting host cell proteins that are necessary for viral replication, such as cyclophilin inhibitors and TLR7 agonists.
Despite these developments, there remains an important unmet need for improved compositions and methods for treating and preventing HCV infection, as well as improved delivery devices and methods relating to the same. The present invention meets these needs and offers other related advantages.
BRIEF SUMMARY OF THE INVENTION
The present invention relates generally to compositions and methods for modulating HCV viral replication, and treating HCV infection in a patient, using raf antisense oligonucleotides, optionally in combination with one or more additional anti-HCV agents.
Therefore, according to one aspect of the present invention, there is provided a method of treating or preventing HCV infection comprising
administering to a patient or cells thereof a therapeutically effective amount of an oligonucleotide, which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf. In certain embodiments, the oligonucleotide is 8 to 50 nucleotides in length.
In particular embodiments, the oligonucleotide is a modified oligonucleotide comprising or consisting of a sequence 100% complementary to a
region of mRNA encoding human c-raf (SEQ ID NO: 28). In particular
embodiments, the oligonucleotide targeted to mRNA encoding human c-raf is a modified oligonucleotide comprising or consisting of a sequence 100%
complementary to nucleobases 2771 to 2790 of SEQ ID NO:28. In certain embodiments, the modified oligonucleotide comprises: a gap segment consisting of linked deoxynucleosides; a 5' wing segment consisting of linked nucleosides; and a 3' wing segment consisting of linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; and wherein each nucleoside of each wing segment comprises a modified sugar. In particular embodiments, the modified oligonucleotide consists of 20 nucleobases.
In certain embodiments, the modified oligonucleotide comprises: a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-O-methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine. In certain embodiments, the oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29).
In one specific embodiment of this aspect of the invention, the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence, TCCCGCCTGTGACATGCATT (SEQ ID NO:8), with 2'-O-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy. In a highly related embodiment, the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence,
UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-O-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
In certain embodiments, in addition to administering raf antisense oligonucleotides, the method further comprises a step of administering an anti- HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV. In particular embodiments, the raf antisense oligonucleotide and the anti-HCV agent are administered concomitantly or are co-administered.
In one illustrative embodiment, the anti-HCV agent is an immunomodulatory agent selected from the group consisting of a-interferon, β- interferon, γ-interferon, o-interferon, Intron® A, Roferon® A, Canferon®-A300, Advaferon®, Infergen®., Humoferon®., Sumiferon® MP, Alfaferone®, IFN-β®, Feron®, polyethylene glycol derivatized (pegylated) interferon compounds, PEG interferon-a-2a (Pegasys®), PEG interferon-a-2b (PEG-lntron®) and pegylated IFN-a-con1.
In another illustrative embodiment, the anti-HCV agent is an antiviral agent selected from the group consisting of ribavirin, amantadine, viramidine, nitazoxanide, telbivudine; NOV-205, taribavirin; VX-950, VX-497, VX-148, and VX- 944.
In yet another illustrative embodiment, the anti-HCV agent is an HCV polymerase inhibitor selected from the group consisting of NM283 (valopicitabine), R803, JTK-109, JTK-003, HCV-371 , HCV-086, HCV-796 and R-1479.
In still another illustrative embodiment, the anti-HCV agent is an HCV protease inhibitor selected from the group consisting of BILN-2061 , VX-950 (Telaprevir), GS-9132 (ACH-806), SCH-503034 (Boceprevir) and SCH-6.
In another illustrative embodiment, the anti-HCV agent is an HCV life cycle protein inhibitor selected from the group consisting of a NS3 helicase inhibitor, a metallo-protease inhibitor, and an alpha glucosidase inhibitors.
In another illustrative embodiment, the anti-HCV agent is selected from the group consisting of an antisense oligonucleotide inhibitor, a siRNA inhibitor, a short hairpin RNA (shRNA) inhibitor, and a ribozyme inhibitor
In a more specific embodiment of the invention, the anti-HCV agent is selected from the group consisting of a-interferon, pegylated a-interferon, ribavirin, or a combination thereof.
According to another aspect of the present invention, there is provided a pharmaceutical composition comprising a physiologically acceptable carrier, a therapeutically effective amount of at least one raf antisense
oligonucleotide, as described herein, and at least anti-HCV agent, as described herein.
In another aspect, the present invention provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, for use, or prepared for use, in treating or preventing HCV infection in a patient, e.g., by administering to the patient or cells thereof a therapeutically effective amount of the oligonucleotide. In particular embodiments, the oligonucleotide is 8 to 50 nucleotides in length.
In a related aspect, the present invention further provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, for use, or prepared for use, in treating or preventing HCV infection in a patient in combination with an anti-HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV, e.g., by
administering to the patient or cells thereof therapeutically effective amounts of the oligonucleotide and anti-HCV agent. In particular embodiments, the
oligonucleotide is 8 to 50 nucleotides in length.
In another aspect, the present invention provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said
oligonucleotide inhibits expression of human c-raf, for use in the manufacture of a medicament for treating or preventing HCV infection in a patient, e.g., by administering to the patient or cells thereof a therapeutically effective amount of the oligonucleotide. In particular embodiments, the oligonucleotide is 8 to 50 nucleotides in length.
In a related aspect, the present invention further provides an oligonucleotide targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, for use in the manufacture of a medicament for treating or preventing HCV infection in a patient in combination with an anti-HCV agent, such as an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent, an antiviral agent, or any other agent having activity against HCV, e.g., by administering to the patient or cells thereof therapeutically effective amounts of the oligonucleotide and anti-HCV agent. In particular embodiments, the
oligonucleotide is 8 to 50 nucleotides in length.
In particular embodiments of the above aspects of the invention, the oligonucleotide is a modified oligonucleotide comprising or consisting of a sequence 100% complementary to a region of mRNA encoding human c-raf (SEQ ID NO: 28). In particular embodiments, the oligonucleotide targeted to mRNA encoding human c-raf is a modified oligonucleotide comprising or consisting of a sequence 100% complementary to nucleobases 2771 to 2790 of SEQ ID NO:28. In certain embodiments, the modified oligonucleotide comprises: a gap segment consisting of linked deoxynucleosides; a 5' wing segment consisting of linked nucleosides; and a 3' wing segment consisting of linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; and wherein each nucleoside of each wing segment comprises a modified sugar. In particular embodiments, the modified oligonucleotide consists of 20
nucleobases.
In certain embodiments, the modified oligonucleotide comprises: a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-0-methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine. In certain embodiments, the oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29).
In specific embodimens of these aspects of the invention, the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence, TCCCGCCTGTGACATGCATT (SEQ ID NO:8), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy. In highly related embodiments, the raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence,
UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 provides a graph showing the HCV titer (RNA copies/ml) measured in supernatants from cells transfected with the indicated amounts of c- raf antisense oligonucleotide at the indicated times post transfection, which demonstrates that treatment with raf antisense oligonucleotides resulted in a dose dependent reduction in virus titers (RNA copies/ml) in HCV-infected cells.
Figure 2 provides a graph showing the amount of c-Raf protein
(normalized to actin protein) measured in cells transfected with the indicated amounts of c-raf antisense oligonucleotide at the indicated times post transfection,
which demonstrates that treatment with raf antisense oligonucleotides resulted in a dose dependent reduction in c-Raf expression.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is based, in part, on the identification that antisense oligonucleotides targeting a raf gene, e.g., a c-raf gene, are effective in inhibiting viral replication, including HCV replication. The present invention is further based, in part, on the identification of novel combinations of therapeutic agents that exhibit an enhanced ability to treat, prevent, inhibit, or reduce infection by viruses, including HCV. These combinations include at least one raf antisense oligonucleotide, which modulates expression of a raf gene, as well as one or more additional therapeutic agents having activity against HCV. In addition, the present invention contemplates the use of a raf antisense oligonucleotide to treat, prevent, inhibit, or reduce infection by viruses, including HCV. Definitions
Unless specific definitions are provided, the nomenclature utilized in connection with, and the procedures and techniques of, analytical chemistry, synthetic organic chemistry, and medicinal and pharmaceutical chemistry described herein are those well known and commonly used in the art. Standard techniques can be used for chemical synthesis, and chemical analysis. To the extent permitted, all patents, applications, published applications and other publications, GENBANK Accession Numbers and associated sequence
information obtainable through databases such as National Center for
Biotechnology Information (NCBI) and other data referred to herein are hereby incorporated by reference in their entirety.
Unless otherwise indicated, the following terms have the following meanings:
"2'-0-methoxyethyl" (also 2'-MOE, 2'-0-(2-methoxyethyl) and 2'- 0(CH2)2-OCH3) refers to an O-methoxy-ethyl modification of the 2' position of a furosyl ring. A 2'-0-methoxyethyl modified sugar is a modified sugar.
"2'-0-methoxyethyl nucleoside" means a nucleoside comprising a 2'- O-methoxyethyl modified sugar moiety.
"3' target site" refers to the nucleotide of a target nucleic acid which is complementary to the 3'-most nucleotide of a particular antisense compound.
"5' target site" refers to the nucleotide of a target nucleic acid which is complementary to the 5'-most nucleotide of a particular antisense compound.
"5-methylcytosine" means a cytosine modified with a methyl group attached to the 5' position. A 5-methylcytosine is a modified nucleobase.
"Administered concomitantly" refers to the co-administration of two agents in any manner in which the pharmacological effects of both are manifest in the patient. Concomitant administration does not require that both agents be administered in a single pharmaceutical composition, in the same dosage form, at the same time or by the same route of administration.
"Administering" means providing a pharmaceutical agent to an individual, and includes, but is not limited to, administering by a medical professional and self-administering.
"Ameliorate" means to make better or improve the symptoms of a condition or disease in a subject.
"Animal" refers to human or non-human animals, including, but not limited to, mice, rats, rabbits, dogs, cats, pigs, horses and non-human primates, including, but not limited to, monkeys and chimpanzees.
"Antisense compound" means an oligomeric compound that is capable of undergoing hybridization to a target nucleic acid through hydrogen bonding.
"Antisense inhibition" means reduction of target nucleic acid or protein levels in the presence of an antisense compound complementary to a
target nucleic acid compared to target nucleic acid or protein levels in the absence of the antisense compound.
"Antisense oligonucleotide" means a single-stranded oligonucleotide having a nucleobase sequence that permits hybridization to a complementary region or segment of a target nucleic acid.
"Bicyclic sugar" means a furosyl ring modified by the bridging of two non-geminal ring atoms. A bicyclic sugar is a modified sugar moiety.
"Cap structure" or "terminal cap moiety" means a chemical modification, which has been incorporated at a terminus of an antisense compound. An antisense compound can have both termini "capped".
"Chimeric antisense compounds" means antisense compounds that have at least 2 chemically distinct regions, each region includes a plurality of subunits.
"Co-administration" means administration of two or more agents to an individual. The two or more agents can be in a single pharmaceutical composition, or can be in separate pharmaceutical compositions. Each of the two or more agents can be administered through the same or different routes of administration. Co-administration encompasses administration in parallel or sequentially.
"Complementarity" means the capacity for pairing between nucleobases of a first nucleic acid and a second nucleic acid.
"Comprise," "comprises" and "comprising" are to be understood to imply the inclusion of a stated step or element or group of steps or elements but not the exclusion of any other step or element or group of steps or elements.
"Contiguous nucleobases" means nucleobases immediately adjacent to each other.
"Cross-reactive" means an oligomeric compound targeting one nucleic acid sequence can hybridize to a different nucleic acid sequence. For example, in some instances an antisense oligonucleotide targeting human c-raf
can cross-react with a murine c-raf. Whether an oligomeric compound cross- reacts with a nucleic acid sequence other than its designated target depends on the degree of complementarity the compound has to the nucleic acid sequence. The higher the complementarity between the oligomeric compound and the non- target nucleic acid, the more likely the oligomeric compound will cross-react with the nucleic acid.
"Cure" means a method that restores health or a prescribed treatment for an illness.
"Deoxyribonucleotide" means a nucleotide having a hydrogen atom at the 2' position of the sugar portion of the nucleotide. Deoxyribonucleotides can be modified with any of a variety of substituents.
"Designing" or "Designed to" refer to the process of designing an oligomeric compound that specifically hybridizes with a selected nucleic acid molecule or portion thereof.
"Diluent" means an ingredient in a composition that lacks pharmacological activity, but is pharmaceutically necessary or desirable. For example, in drugs that are injected, the diluent can be a liquid, e.g. saline solution.
"Dose" means a specified quantity of a pharmaceutical agent provided in a single administration, or in a specified time period. In certain embodiments, a dose can be administered in two or more boluses, tablets, or injections. For example, in certain embodiments, where ocular administration is desired, the desired dose may require a volume not easily accommodated by a single injection. In such embodiments, two or more injections can be used to achieve the desired dose. In certain embodiments, a dose can be administered in two or more injections to minimize injection site reaction in an individual. Doses can be stated as the amount of pharmaceutical agent per hour, day, week or month.
"Dosage unit" or "unit dosage form" means a form in which a pharmaceutical agent is provided, e.g., pill, tablet, or other dosage unit known in
the art. In certain embodiments, a dosage unit is a vial containing lyophilized antisense oligonucleotide. In certain embodiments, a dosage unit is a vial containing reconstituted antisense oligonucleotide.
"Duration" means the period of time during which an activity or event continues. In certain embodiments, the duration of treatment is the period of time during which doses of a pharmaceutical agent are administered.
"Effective amount" in the context of modulating an activity or of treating, preventing, or inhibiting a condition means the administration of that amount of active ingredient to a subject in need of such modulation, treatment or prophylaxis, either in a single dose or as part of a series, that is effective for modulation of that effect, or for treatment or prophylaxis or improvement of that condition. The effective amount will vary depending upon the health and physical condition of the subject to be treated, the taxonomic group of subjects to be treated, the formulation of the composition, the assessment of the medical situation, and other relevant factors. In addition, the effective amount may vary when used in combination with another active ingredient. For example, when an raf antisense oligonucleotide is used in combination with another anti-HCV agent, the effect amount of one or both of the raf antisense oligonucleotide or anti-HCV agent may be reduced. "Therapeutically effective amount" or "effective amount" also includes an amount of a pharmaceutical agent that provides a therapeutic benefit to an individual.
"Efficacy" means the ability to produce a desired effect. "Expression" includes all the functions by which a gene's coded information is converted into structures present and operating in a cell. Such structures include, but are not limited to, the products of transcription and translation.
"First agent" or "first therapeutic agent" means an agent that can be used in combination with a "second agent". In certain embodiments, the first agent
is any antisense compound, oligonucleotide or composition that inhibits c-raf described herein.
"Fully complementary" or "100% complementary" means each nucleobase of a first nucleic acid has a complementary nucleobase in a second nucleic acid. In certain embodiments, a first nucleic acid is an antisense compound and a target nucleic acid is a second nucleic acid. In certain such embodiments, an antisense oligonucleotide is a first nucleic acid and a target nucleic acid is a second nucleic acid.
"Gapmer" means an antisense compound in which an internal position having a plurality of nucleotides that supports RNaseH cleavage is positioned between external regions having one or more nucleotides that are chemically distinct from the nucleosides of the internal region. A "gap segment" means the plurality of nucleotides that make up the internal region of a gapmer. A "wing segment" means the external region of a gapmer.
"Gap-widened" means an antisense compound has a gap segment of
12 or more contiguous 2'-deoxyribonucleotides positioned between and
immediately adjacent to 5' and 3' wing segments of from one to six nucleotides having modified sugar moieties.
"Hybridization" means the annealing of complementary nucleic acid molecules. In certain embodiments, complementary nucleic acid molecules include, but are not limited to, an antisense compound and a nucleic acid target. In certain embodiments, complementary nucleic acid molecules include, but are not limited to, an antisense oligonucleotide and a nucleic acid target.
"Immediately adjacent" means there are no intervening nucleotides between the immediately adjacent elements. For example, between regions, segments, nucleotides and/or nucleosides.
"Induce", "inhibit", "potentiate", "elevate", "increase", "decrease" or the like, e.g., denote quantitative differences between two states. For example, "an amount effective to inhibit the activity or expression of c-raf means that the level of
activity or expression of c-raf in a treated sample will differ from the level of c-raf activity or expression in untreated cells. Such terms are applied to, for example, levels of expression, and levels of activity. "Inhibiting the expression or activity" refers to a reduction, blockade of the expression or activity of the target and does not necessarily indicate a total elimination of expression or activity.
"Internucleoside linkage" refers to the chemical bond between nucleosides.
"Linked nucleosides" means adjacent nucleosides which are bonded together. "Mismatch" refers to a non-complementary nucleobase within a complementary oligomeric compound.
"Modified internucleoside linkage" refers to a substitution and/or any change from a naturally occurring internucleoside bond (i.e. a phosphodiester internucleoside bond).
"Modified nucleobase" means any nucleobase other than adenine, cytosine, guanine, thymidine, or uracil. An "unmodified nucleobase" means the purine bases, adenine (A) and guanine (G), and the pyrimidine bases, thymine (T), cytosine (C) and uracil (U).
"Modified oligonucleotide" means an oligonucleotide comprising a modified internucleoside linkage, a modified sugar, and/or a modified nucleobase. A modified oligonucleotide can also have a nucleoside mimetic or nucleotide mimetic.
"Modified sugar" refers to a substitution and/or any change from a natural sugar.
"Modulation" means a perturbation of function, for example, one associated with either an increase (stimulation or induction) or a decrease
(inhibition or reduction) in expression.
"Monomer" refers to a single unit of an oligomer. Monomers include, but are not limited to, nucleosides and nucleotides, whether naturally occuring or modified.
"Motif means the pattern of unmodified and modified nucleosides in an antisense compound.
"Naturally occurring internucleoside linkage" means a 3' to 5' phosphodiester linkage.
"Natural sugar" means a sugar found in DNA (2'-H) or RNA (2'-OH).
"Nucleic acid" refers to molecules composed of monomeric nucleotides. A nucleic acid includes, but is not limited to, ribonucleic acids (RNA), deoxyribonucleic acids (DNA), single-stranded nucleic acids, double-stranded nucleic acids, small interfering ribonucleic acids (siRNA), and microRNAs
(miRNA).
"Nucleobase" means a heterocyclic moiety capable of pairing with a base of another nucleic acid.
"Nucleobase complementarity" refers to a nucleobase that is capable of base pairing with another nucleobase. For example, in DNA, adenine (A) is complementary to thymine (T). For example, in RNA, adenine (A) is
complementary to uracil (U). In certain embodiments, complementary nucleobase refers to a nucleobase of an antisense compound that is capable of base pairing with a nucleobase of its target nucleic acid. For example, if a nucleobase at a certain position of an antisense compound is capable of hydrogen bonding with a nucleobase at a certain position of a target nucleic acid, then the position of hydrogen bonding between the oligonucleotide and the target nucleic acid is considered to be complementary at that nucleobase pair.
"Nucleobase sequence" means the order of contiguous nucleobases independent of any sugar, linkage, and/or nucleobase modification.
"Nucleoside" means a nucleobase linked to a sugar.
"Nucleotide" means a nucleoside having a phosphate group covalently linked to the sugar portion of the nucleoside.
"Nucleoside mimetic" includes those structures used to replace the sugar or the sugar and the base and not necessarily the linkage at one or more
positions of an oligome c compound such as, for example, nucleoside mimetics having morpholino, cyclohexenyl, cyclohexyl, bicyclo or tricyclo sugar mimetics, e.g. non furanose sugar units.
"Nucleotide mimetic" includes those structures used to replace the nucleoside and the linkage at one or more positions of an oligomeric compound such as for example peptide nucleic acids or morpholinos (morpholinos linked by - N(H)-C(=0)-0- or other non-phosphodiester linkage).
Oligomeric compound" means a polymer of linked monomeric subunits which is capable of hybridizing to at least a region of a nucleic acid molecule.
Oligonucleotide" means an oligomer or polymer of linked nucleoside or nucleotide monomers each of which can be modified or unmodified,
independent one from another. The term "oligonucleotide" includes oligomers comprising non-naturally occurring monomers, or portions thereof. Such modified or substituted oligonucleotides are often preferred over native forms because of properties such as, for example, enhanced cellular uptake and increased stability in the presence of nucleases.
"Parenteral administration," means administration by a manner other than through the digestive tract, e.g., through topical administration, injection or infusion. Parenteral administration includes, but is not limited to, subcutaneous administration, intravenous administration, and intramuscular administration.
"Pharmaceutically acceptable carrier" or "Pharmaceutically acceptable diluent" means a carrier or diluent that does not interfere with the structure of the oligonucleotide. Certain of such carries enable pharmaceutical compositions to be formulated as, for example, tablets, pills, dragees, capsules, liquids, gels, syrups, slurries, suspension and lozenges for the oral ingestion by a subject. For example, a pharmaceutically acceptable carrier can be a sterile aqueous solution.
"Pharmaceutically acceptable salts" means physiologically and pharmaceutically acceptable salts of antisense compounds, i.e., salts that retain the desired biological activity of the parent oligonucleotide and do not impart undesired toxicological effects thereto.
"Pharmaceutical composition" or "composition" means a mixture of substances suitable for administering to an animal. For example, a composition can comprise one or more antisense oligonucleotides and a sterile aqueous solution.
"Phosphorothioate internucleoside linkage" or "phosphorothioate linkage" means a linkage between nucleosides where the phosphodiester bond is modified by replacing one of the non-bridging oxygen atoms with a sulfur atom. A phosphorothioate linkage is a modified internucleoside linkage.
"Portion" means a defined number of contiguous (i.e., linked) nucleobases of a nucleic acid. In certain embodiments, a portion is a defined number of contiguous nucleobases of a target nucleic acid. In certain
embodiments, a portion is a defined number of contiguous nucleobases of an antisense compound.
"Prevention" or "preventing" refers to inhibiting, delaying or
forestalling the onset or development of a condition or disease, e.g., HCV infection, for a period of time from hours to days, preferably weeks to months to years or permanently. It can also mean reducing the likelihood that a condition or disease will occur during a period of time.
"Prodrug" means a therapeutic agent that is prepared in an inactive form that is converted to an active form (i.e., drug) within the body or cells thereof by the action of endogenous or non-endogenous enzymes or other chemicals and/or conditions.
"Region" is defined as a portion of the target nucleic acid having at least one identifiable structure, function, or characteristic.
"Ribonucleotide" means a nucleotide having a hydroxy at the 2' position of the sugar portion of the nucleotide. Ribonucleotides can be modified with any of a variety of substituents.
"Salts" mean physiologically and pharmaceutically acceptable salts of antisense compounds, i.e., salts that retain the desired biological activity of the parent oligonucleotide and do not impart undesired toxicological effects thereto.
"Second agent" or "second therapeutice agent" means an agent that can be used in combination with a "first agent". In certain embodiments, a second therapeutic agent can be any agent that inhibits or prevents HCV replication. A second therapeutic agent can include, but is not limited to, an siRNA or antisense oligonucleotide.
"Segments" are defined as smaller, sub-portions of regions within a target nucleic acid. "Shortened" or "truncated" versions of antisense
oligonucleotides or target nucleic acids taught herein have one, two or more nucleosides deleted.
"Side effects" mean physiological responses attributable to a treatment other than desired effects. In certain embodiments, side effects include, without limitation, injection site reactions, liver function test abnormalities, renal function abnormalities, liver toxicity, renal toxicity, central nervous system abnormalities, and myopathies. For example, increased aminotransferase levels in serum can indicate liver toxicity or liver function abnormality. For example, increased bilirubin can indicate liver toxicity or liver function abnormality.
"Single-stranded oligonucleotide" means an oligonucleotide which is not hybridized to a complementary strand. "Single-stranded modified
oligonucleotide" means a modified oligonucleotide which is not hybridized to a complementary strand.
"siRNA" is defined as a double-stranded compound having a first and second strand and comprises a central complementary portion between said first and second strands and terminal portions that are optionally complementary
between said first and second strands or with a target mRNA. In one non-limiting example, the first strand of the siRNA is antisense to the target nucleic acid, while the second strand is complementary to the first strand. Once the antisense strand is designed to target a particular nucleic acid target, the sense strand of the siRNA can then be designed and synthesized as the complement of the antisense strand and either strand can contain modifications or additions to either terminus.
"Sites," as used herein, are defined as unique nucleobase positions within a target nucleic acid.
"Slows progression" means a decrease in the development of a disease, condition or symptom.
"Specifically hybridizable" means an antisense compound that hybridizes to a target nucleic acid to induce a desired effect, while exhibiting minimal or no effects on non-target nucleic acids.
"Subject" means a human or non-human animal selected for treatment or therapy.
"Targeted" or "targeted to" means having a nucleobase sequence that will allow specific hybridization of an antisense compound to a target nucleic acid to induce a desired effect.
"Target nucleic acid," "target RNA," "target RNA transcript" and "nucleic acid target" all mean a nucleic acid capable of being targeted by antisense compounds.
"Targeting" means the process of design and selection of an antisense compound that will specifically hybridize to a target nucleic acid and induce a desired effect.
"c-raf nucleic acid" means any nucleic acid encoding c-Raf. For example, in certain embodiments, a c-raf nucleic acid includes, without limitation, a DNA sequence encoding c-Raf, an RNA sequence transcribed from DNA encoding c-Raf, and an mRNA sequence encoding c-Raf. "c-raf mRNA" means an mRNA encoding a c-Raf protein.
"Therapeutically effective amount" or "effective amount" means an amount of a pharmaceutical agent that provides a therapeutic benefit to an individual. "Effective amount" in the context of modulating an activity or of treating or preventing a condition means the administration of that amount of active ingredient to a subject in need of such inhibition, treatment or prophylaxis, either in a single dose or as part of a series of doses, that is effective for inhibition of that effect, or for treatment or prophylaxis or improvement of that condition. The effective amount will vary depending upon the health and physical condition of the subject to be treated, the taxonomic group of subjects to be treated, the formulation of the composition, the assessment of the medical situation, and other relevant factors.
"Treatment" refers to administering a composition of the invention to effect an alteration or improvement of a disease, condition or symptom.
"Unmodified nucleotide" means a nucleotide composed of naturally occuring nucleobases, sugar moieties and internucleoside linkages. In certain embodiments, an unmodified nucleotide is an RNA nucleotide (i.e., β-D- ribonucleosides) or a DNA nucleotide (i.e., β-D-deoxyribonucleoside).
"Wing segment" means a plurality of nucleosides modified to impart to an oligonucleotide properties such as enhanced inhibitory activity, increased binding affinity for a target nucleic acid, or resistance to degradation by in vivo nucleases.
Methods of Treating and Inhibiting Viral Infection
The present invention provides methods for treating, preventing or inhibiting viral infection, e.g,. hepatitis C virus (HCV) infection using
oligonucleotides that target c-raf. As described herein, these methods may be used to inhibit viral replication and thereby treating, preventing, or inhibiting viral infection in a subject or patient. The methods and compositions of the present invention may be used to treat infection from any viral-mediated disease or
disorder that benefits from reduced raf expression. Although certain particular embodiments of the present invention described herein refer to the treatment, prevention or inhibition of HCV infection, it is understood that they could be also be used to treat, prevent or inhibit other viral infections.
Thus, in one embodiment, the present invention includes a method of treating, preventing, or inhibiting HCV infection, comprising administering to a subject, or cells thereof, a therapeutically effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
In a related embodiment, the present invention includes a method of treating, preventing, or inhibiting the replication of HCV in a subject, comprising administering to a subject, or cells thereof, a therapeutically effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
In a related embodiment, the present invention includes a method of inhibiting or reducing the replication of HCV in cells, e.g., in vitro, comprising administering to the cells an effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
In certain embodiments, methods of the present invention comprise administering a c-raf antisense oligonucleotide (e.g., an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf) in combination with one or more additional anti-HCV agents, including but not limited to any of those described herein.
According to the present invention, the raf antisense oligonucleotide reduces raf expression in a subject or cell, e.g., a subject or cell infected with HCV. Therefore, in particular embodiments, methods of the present invention
contemplate contacting a subject or cell with a raf antisense oligonucleotide in
combination with an anti-HCV agent that directly targets HCV, thereby inhibiting the HCV by inhibiting both cellular processes involved in HCV replication and viral processes involved in HCV replication. In particular embodiments, the raf antisense oligonucleotide and the additional anti-HCV agent act synergistically to inhibit HCV.
Thus, in one embodiment, the present invention includes a method of treating, preventing, or inhibiting HCV infection, comprising co-administering to a subject, or cells thereof, both a therapeutically effective amount of an
oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, and a therapeutically effective amount of an anti-HCV agent.
In a related embodiment, the present invention includes a method of treating, preventing, or inhibiting the replication of HCV in a subject, comprising coadministering to a subject, or cells thereof, a therapeutically effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, and a therapeutically effective amount of an anti-HCV agent.
In a related embodiment, the present invention includes a method of inhibiting or reducing the replication of HCV in cells, e.g., in vitro, comprising co- administering to the cells an effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, and a therapeutically effective amount of an anti-HCV agent.
In certain embodiments, an anti-HCV agent is selected from the group consisting of an HCV polymerase inhibitor, an HCV protease inhibitor, an inhibitor of another target in the HCV life cycle, an immunomodulatory agent, an antiviral agent, or a combination thereof.
Raf antisense oligoncleotides, alone or in combination with another anti-HCV agent, may be delivered to any subject in need thereof, including a
subject determined to be infected with HCV or a subject considered to be at risk of developing an HCV infection. Subjects may be any animal, including a mammal, such as a human.
Raf Antisense Oligonucleotides
In certain embodiments, the present invention employs oligonucleotides targeted to nucleic acids encoding a raf family member, which modulate raf gene expression. In various embodiments, the oligonucleotides target c-raf (raf-1 ); however, compositions and methods for modulating expression of other forms of raf are also contemplated. In particular embodiments, the oligonucleotides target human c-raf. In particular embodiments, the
oligonucleotide comprises a sequence complementary to a raf mRNA sequence, e.g., a human c-raf mRNA sequence.
Oligonucleotides that target a nucleic acid encoding a raf family member include any and all polynucleotide complexes, polynucleotides, and oligonucleotides comprising one or more sequences capable of hybridizing to a raf nucleic acid and modulating raf gene expression. The oligonucleotides may comprise various structures, including, e.g., a traditional single-stranded antisense oligonucleotide structures and various RNA interference (RNAi) agents, such as small interfering RNA (siRNA), short hairpin RNA (shRNA), and dicer substrates. Thus, oligonucletoides that target a raf mRNA or gene may be, e.g., either single- stranded or double-stranded, or comprise both single-stranded and double- stranded regions, and they may, e.g., comprise DNA, RNA or both.
In various embodiments, oligonucleotides used according to the present invention comprise RNA, DNA, or peptide nucleic acids, or a combination of any or all of these types of molecules. In addition, the oligonucleotides may comprise modified nucleic acids, or derivatives or analogs of nucleic acids.
General examples of nucleic acid modifications include, but are not limited to, biotin labeling, fluorescent labeling, amino modifiers introducing a primary amine
into the polynucleotide, phosphate groups, deoxyuridine, halogenated nucleosides, phosphorothioates, 2'-0-Methyl RNA analogs, chimeric RNA analogs, wobble groups, universal bases, and deoxyinosine.
In certain embodiments, the oligonucleotides of the present invention are single-stranded. In particular embodiments, the oligonucleotides comprise one or more double-stranded regions. An oligonucleotide comprising a double- stranded region may consist of one, two or more individual strands, each having a 5' and 3' end. For example, an oligonucleotide comprising a double-stranded region may consist of a single oligonucleotide with a self-complementary region, which hybridizes to itself, and which may form a stem loop structure. In other embodiments, an oligonucleotide comprising a double-stranded region may comprise two strands with regions complementary to each other, which hybridize to form a double-stranded structure. An oligonucleotide comprising a double- stranded region may be entirely double-stranded or partially double stranded. For example, an oligonucleotide may be entirely double-stranded and have two blunt ends. In another example, an oligonucleotide may include one or more double- stranded regions and have one or more 5' or 3' single-stranded overhangs, e.g., of two to four nucleotides.
In particular embodiments, the oligonucleotides in accordance with this invention are from about 8 to about 50 nucleotides in length, whether single- stranded or comprising a double-stranded region. In the context of this invention, it is understood that this encompasses non-naturally occurring oligomers as herein before described, having 8 to 50 monomers. In further embodiments, the oligonucleotides comprise from about 8 to about 30 nucleobases (i.e., from about 8 to about 30 linked nucleosides).
In particular embodiments, raf antisense oligonucleotides are used to reduce or inhibit the expression of the targeted raf family member. Raf antisense oligonucleotides are single-stranded oligonucleotide having a nucleobase sequence that permits hybridization to a complementary region or segment of a raf
nucleic acid. Exemplary raf antisense oligonucleotides that may be used according to the present invention are described herein and also in U.S. Patent Nos. 5,563,255, 5,952,229, 6,358,932, 5,656,612, 5,919,773, 6,410,518, and 6,806,258, the contents of which are incorporated herein by reference in their entireties.
In the context of this invention, the term "oligonucleotide" refers to an oligomer or polymer of nucleotide or nucleoside monomers consisting of naturally occurring bases, sugars and intersugar (backbone) linkages. The term
"oligonucleotide" also includes oligomers comprising non-naturally occurring monomers, or portions thereof, which function similarly. Such modified or substituted oligonucleotides are often preferred over native forms because of properties such as, for example, enhanced cellular uptake and increased stability in the presence of nucleases.
A "subunit" of a polynucleotide or oligonucleotide refers to one nucleotide (or nucleotide analog) unit. The term may refer to the nucleotide unit with or without the attached intersubunit linkage, although, when referring to a "charged subunit", the charge typically resides within the intersubunit linkage (e.g., a phosphate or phosphorothioate linkage or a cationic linkage). A given raf antisense oligonucleotide may utilize one or more different types of subunits and/or intersubunit linkages, mainly to alter its stability, Tm, RNase sensitivity, or other characteristics, as desired. For instance, certain embodiments may employ RNA subunits with one or more 2'-0-methyl RNA subunits.
It is not necessary for all positions in a given compound to be uniformly modified, and in fact more than one of the aforementioned modifications may be incorporated in a single compound or even at a single nucleoside within an oligonucleotide. Certain oligonucleotides of this invention are chimeric
oligonucleotides. "Chimeric oligonucleotides" or "chimeras", in the context of this invention, are oligonucleotides which contain two or more chemically distinct regions, each made up of at least one nucleotide. These oligonucleotides typically
contain at least one region of modified nucleotides that confers one or more beneficial properties (such as, for example, increased nuclease resistance, increased uptake into cells, increased binding affinity for the RNA target) and a region that is a substrate for RNase H cleavage. In one embodiment, a chimeric oligonucleotide comprises at least one region modified to increase target binding affinity, and, usually, a region that acts as a substrate for RNAse H. Affinity of an oligonucleotide for its target (in this case a nucleic acid encoding raf) is routinely determined by measuring the Tm of an oligonucleotide/target pair, which is the temperature at which the oligonucleotide and target dissociate; dissociation is detected spectrophotometrically. The higher the Tm, the greater the affinity of the oligonucleotide for the target.
The relationship between an oligonucleotide and its complementary nucleic acid target to which it hybridizes is commonly referred to as "antisense." "Targeting" an oligonucleotide to a chosen nucleic acid target, in the context of this invention, is a multistep process. The process usually begins with identifying a nucleic acid sequence whose function is to be modulated. This may be, as examples, a cellular gene (or mRNA made from the gene) whose expression is associated with a particular disease state, or a foreign nucleic acid from an infectious agent. In the present invention, the target is a nucleic acid encoding raf; in other words, the raf gene or mRNA expressed from the raf gene. The targeting process also includes determination of a site or sites within the nucleic acid sequence for the oligonucleotide interaction to occur such that the desired effect- modulation of gene expression-will result. Once the target site or sites have been identified, oligonucleotides are chosen which are sufficiently complementary to the target, i.e., hybridize sufficiently well and with sufficient specificity, to give the desired modulation.
Raf antisense oligonucleotides inhibit or reduce expression of the raf cDNA, mRNA, and protein. Inhibition of raf expression can be measured in ways which are routine in the art, for example by Northern blot assay of mRNA
expression or Western blot assay of protein expression using methods well known in the art.
"Hybridization," in the context of this invention, means hydrogen bonding, also known as Watson-Crick base pairing, between complementary bases, usually on opposite nucleic acid strands or two regions of a nucleic acid strand. Guanine and cytosine are examples of complementary bases which are known to form three hydrogen bonds between them. Adenine and thymine are examples of complementary bases which form two hydrogen bonds between them.
"Specifically hybridizable" and "complementary" are terms which are used to indicate a sufficient degree of complementarity such that stable and specific binding occurs between the DNA or RNA target and the oligonucleotide. It is understood that an oligonucleotide need not be 100% complementary to its target nucleic acid sequence to be specifically hybridizable. An oligonucleotide is specifically hybridizable when binding of the oligonucleotide to the target interferes with the normal function of the target molecule to cause a loss of utility, and there is a sufficient degree of complementarity to avoid non-specific binding of the oligonucleotide to non-target sequences under conditions in which specific binding is desired, i.e., under physiological conditions in the case of in vivo assays or therapeutic treatment, or, in the case of in vitro assays, under conditions in which the assays are conducted.
In certain embodiments of this invention, oligonucleotides are provided which are targeted to mRNA encoding c-raf, B-raf or A-raf. In
accordance with this invention, persons of ordinary skill in the art will understand that mRNA includes not only the coding region which carries the information to encode a protein using the three letter genetic code, but also associated
ribonucleotides which form a region known to such persons as the 5'-untranslated region, the 3'-untranslated region, the 5' cap region, intron regions and intron/exon or splice junction ribonucleotides. Thus, oligonucleotides may be formulated in accordance with this invention that are targeted wholly or in part to these
associated ribonucleotides as well as to the coding ribonucleotides. In certain embodiments, the oligonucleotide is targeted to a translation initiation site (AUG codon) or sequences in the 5'- or 3'-untranslated region of the human c-raf mRNA. The functions of messenger RNA to be interfered with include all vital functions such as translocation of the RNA to the site for protein translation, actual translation of protein from the RNA, splicing or maturation of the RNA and possibly even independent catalytic activity which may be engaged in by the RNA. The overall effect of such interference with the RNA function is to cause interference with raf protein expression.
In particular embodiments, an oligonucleotide of the present invention targets a human c-raf polynucleotide or mRNA, e.g., the oligonucleotide is fully complementary to a region of a human c-raf mRNA. The sequence of an exemplary human c-raf mRNA is provided in SEQ ID NO:28, which corresponds to the sequence provided at GenBank Accession No. NM_002880.3.
In particular embodiments, an oligonucleotide of the present invention comprises one or more modifications. It is not necessary for all positions in a given compound to be uniformly modified, and in fact more than one of the modifications described infra may be incorporated in a single compound or even at a single nucleoside within an oligonucleotide. Certain oligonucleotides of this invention are chimeric oligonucleotides. "Chimeric oligonucleotides" or "chimeras", in the context of this invention, are oligonucleotides which contain two or more chemically distinct regions, each made up of at least one nucleotide. These oligonucleotides typically contain at least one region of modified nucleotides that confers one or more beneficial properties (such as, for example, increased nuclease resistance, increased uptake into cells, increased binding affinity for the RNA target) and a region that is a substrate for RNase H cleavage. In one embodiment, a chimeric oligonucleotide comprises at least one region modified to increase target binding affinity, and, usually, a region that acts as a substrate for RNAse H. Affinity of an oligonucleotide for its target (in this case a nucleic acid
encoding raf) is routinely determined by measuring the Tm of an oligonucleotide/target pair, which is the temperature at which the oligonucleotide and target dissociate; dissociation is detected spectrophotometrically. The higher the Tm, the greater the affinity of the oligonucleotide for the target.
In certain embodiments, the oligonucleotides, e.g., raf antisense oligonucleotides, are modified to increase RNA binding affinity. In one
embodiment, the region of the oligonucleotide which is modified to increase raf mRNA binding affinity comprises at least one nucleotide modified at the 2' position of the sugar, e.g., a 2'-0-alkyl, 2'-0-alkyl-0-alkyl or 2'-fluoro-modified nucleotide. Such modifications are routinely incorporated into oligonucleotides and these oligonucleotides have been shown to have a higher Tm (i.e., higher target binding affinity) than 2'-deoxyoligonucleotides against a given target. The effect of such increased affinity is to greatly enhance antisense oligonucleotide inhibition of raf gene expression. RNAse H is a cellular endonuclease that cleaves the RNA strand of RNA:DNA duplexes; activation of this enzyme therefore results in cleavage of the RNA target, and thus can greatly enhance the efficiency of antisense inhibition. Cleavage of the RNA target can be routinely demonstrated by gel electrophoresis.
In other embodiments, the oligonucleotides, e.g., raf antisense oligonucleotides, are modified to enhance nuclease resistance. Cells contain a variety of exo- and endo-nucleases which can degrade nucleic acids. A number of nucleotide and nucleoside modifications have been shown to make the
oligonucleotide into which they are incorporated more resistant to nuclease digestion than the native oligodeoxynucleotide. Nuclease resistance is routinely measured by incubating oligonucleotides with cellular extracts or isolated nuclease solutions and measuring the extent of intact oligonucleotide remaining over time, usually by gel electrophoresis. Oligonucleotides which have been modified to enhance their nuclease resistance survive intact for a longer time than unmodified oligonucleotides. A variety of oligonucleotide modifications have been
demonstrated to enhance or confer nuclease resistance. In one embodiment, the oligonucleotide contains at least one phosphorothioate modification. In one embodiment, the oligonucleotide is fully phosphorothioate modified. In some cases, oligonucleotide modifications which enhance target binding affinity are also, independently, able to enhance nuclease resistance.
In particular embodiments, the oligonucleotides in accordance with this invention are from about 8 to about 50 nucleotides in length. In the context of this invention it is understood that this encompasses non-naturally occurring oligomers as herein before described, having 8 to 50 monomers. In further embodiments, the oligonucleotides comprise from about 8 to about 30
nucleobases (i.e., from about 8 to about 30 linked nucleosides).
As is known in the art, a nucleoside is a base-sugar combination. The base portion of the nucleoside is normally a heterocyclic base. The two most common classes of such heterocyclic bases are the purines and the pyrimidines. Nucleotides are nucleosides that further include a phosphate group covalently linked to the sugar portion of the nucleoside. For those nucleosides that include a pentofuranosyl sugar, the phosphate group can be linked to either the 2', 3' or 5' hydroxyl moiety of the sugar. In forming oligonucleotides, the phosphate groups covalently link adjacent nucleosides to one another to form a linear polymeric compound. In turn the respective ends of this linear polymeric structure can be further joined to form a circular structure; however, open linear structures are generally preferred. Within the oligonucleotide structure, the phosphate groups are commonly referred to as forming the internucleoside backbone of the oligonucleotide. The normal linkage or backbone of RNA and DNA is a 3' to 5' phosphodiester linkage.
Specific examples of oligonucleotides, e.g., raf antisense oligonucleotides, useful in this invention include oligonucleotides containing modified backbones or non-natural internucleoside linkages. As defined in this specification, oligonucleotides having modified backbones include those that retain
a phosphorus atom in the backbone and those that do not have a phosphorus atom in the backbone. For the purposes of this specification, and as sometimes referenced in the art, modified oligonucleotides that do not have a phosphorus atom in their internucleoside backbone can also be considered to be
oligonucleosides.
Modified oligonucleotide backbones include, for example,
phosphorothioates, chiral phosphorothioates, phosphorodithioates,
phosphotriesters, aminoalkylphosphotri-esters, methyl and other alkyl
phosphonates including 3'-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates including 3'-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, and boranophosphates having normal 3'-5' linkages, 2'-5' linked analogs of these, and those having inverted polarity wherein the adjacent pairs of nucleoside units are linked 3'-5' to 5'-3' or 2'-5' to 5'-2'. Various salts, mixed salts and free acid forms are also included.
Representative United States patents that teach the preparation of the above phosphorus-containing linkages include, but are not limited to, U.S. Pat. Nos. 3,687,808; 4,469,863; 4,476,301 ; 5,023,243; 5,177,196; 5,188,897;
5,264,423; 5,276,019; 5,278,302; 5,286,717; 5,321 ,131 ; 5,399,676; 5,405,939; 5,453,496; 5,455,233; 5,466,677; 5,476,925; 5,519,126; 5,536,821 ; 5,541 ,306; 5,550, 1 1 1 ; 5,563,253; 5,571 ,799; 5,587,361 ; and 5,625,050, the contents of which are incorporated herein by reference.
Certain modified oligonucleotide backbones that do not include a phosphorus atom therein have backbones that are formed by short chain alkyl or cycloalkyl internucleoside linkages, mixed heteroatom and alkyl or cycloalkyl internucleoside linkages, or one or more short chain heteroatomic or heterocyclic internucleoside linkages. These include those having morpholino linkages (formed in part from the sugar portion of a nucleoside); siloxane backbones; sulfide, sulfoxide and sulfone backbones; formacetyl and thioformacetyl backbones;
methylene formacetyl and thioformacetyl backbones; alkene containing
backbones; sulfamate backbones; methyleneimino and methylenehydrazino backbones; sulfonate and sulfonamide backbones; amide backbones; and others having mixed N, O, S and CH.sub.2 component parts.
Representative United States patents that teach the preparation of the above oligonucleosides include, but are not limited to, U.S. Pat. Nos.
5,034,506; 5,166,315; 5,185,444; 5,214,134; 5,216,141 ; 5,235,033; 5,264,562; 5,264,564; 5,405,938; 5,434,257; 5,466,677; 5,470,967; 5,489,677; 5,541 ,307; 5,561 ,225; 5,596,086; 5,602,240; 5,610,289; 5,602,240; 5,608,046; 5,610,289; 5,618,704; 5,623,070; 5,663,312; 5,633,360; 5,677,437; and 5,677,439, the contents of which are incorporated herein by reference.
In other oligonucleotide mimetics, both the sugar and the internucleoside linkage, i.e., the backbone, of the nucleotide units are replaced with novel groups. The base units are maintained for hybridization with an appropriate nucleic acid target compound. One such oligomeric compound, an oligonucleotide mimetic that has been shown to have excellent hybridization properties, is referred to as a peptide nucleic acid (PNA). In PNA compounds, the sugar-backbone of an oligonucleotide is replaced with an amide containing backbone, in particular an aminoethylglycine backbone. The nucleobases are retained and are bound directly or indirectly to aza nitrogen atoms of the amide portion of the backbone. Representative United States patents that teach the preparation of PNA compounds include, but are not limited to, U.S. Pat. Nos.
5,539,082; 5,714,331 ; and 5,719,262, the contents of which are incorporated herein by reference. Further teaching of PNA compounds can be found in Nielsen et al. (Science, 1991 , 254, 1497-1500).
Certain embodiments of the invention are oligonucleotides, e.g., raf antisense oligonucleotides, with phosphorothioate backbones and
oligonucleosides with heteroatom backbones, and in particular -CH2-NH-O-CH2- -, -CH2-N(CH3) -O-CH2- [known as a methylene (methylimino) or MMI
backbone], -CH2-0-N(CH3) -CH2-, -CH2-N(CH3)-N(CH3) -CH2- and -O- N(CH3) -CH2-CH2- [wherein the native phosphodiester backbone is represented as -O-P-O-CH2-] of the above referenced U.S. Pat. No. 5,489,677, and the amide backbones of the above referenced U.S. Pat. No. 5,602,240. Other embodiments are oligonucleotides having morpholino backbone structures of the above-referenced U.S. Pat. No. 5,034,506.
Modified oligonucleotides, e.g., raf antisense oligonucleotides, may contain one or more substituted sugar moieties. Certain oligonucleotides comprise one of the following at the 2' position: OH; F; O-, S-, or N-alkyl, O-alkyl-O-alkyl, O-, S-, or N-alkenyl, or O-, S- or N-alkynyl, wherein the alkyl, alkenyl and alkynyl may be substituted or unsubstituted Ci to C10 alkyl or C2 to C10 alkenyl and alkynyl. Particularly preferred are O[(CH2)nO]mCH3, O(CH2)nOCH3, O(CH2)2ON(CH3)2, O(CH2)nNH2, O(CH2)nCH3, O(CH2)nONH2, and O(CH2)nON[(CH2)nCH3)]2, where n and m are from 1 to about 10. Other oligonucleotides comprise one of the following at the 2' position: Ci to C10 lower alkyl, substituted lower alkyl, alkaryl, aralkyl, O-alkaryl or O-aralkyl, SH, SCH3, OCN, CI, Br, CN, CF3, OCF3, SOCH3, SO2CH3, ONO2, NO2, N3, NH2, heterocycloalkyl, heterocycloalkaryl,
aminoalkylamino, polyalkylamino, substituted silyl, an RNA cleaving group, a reporter group, an intercalator, a group for improving the pharmacokinetic properties of an oligonucleotide, or a group for improving the pharmacodynamic properties of an oligonucleotide, and other substituents having similar properties.
One modification is 2'-methoxyethoxy (2'-O-CH2CH2OCH3, also known as 2'-O-(2-methoxyethyl) or 2'-MOE) (Martin et ai, Helv. Chim. Acta 1995, 78, 486-504) i.e., an alkoxyalkoxy group. Other modifications include
2'-dimethylaminooxyethoxy, i.e., a O(CH2)2ON(CH3)2 group, also known as 2'- DMAOE, and 2'-dimethylaminoethoxyethoxy (2'-DMAEOE) as described in examples herein below. Other modifications include 2'-methoxy (2'-O~CH3), 2'- aminopropoxy (2'-OCH2CH2CH2NH2) and 2'-fluoro (2'-F). Similar modifications may also be made at other positions on the oligonucleotide, particularly the 3'
position of the sugar on the 3' terminal nucleotide or in 2'-5' linked oligonucleotides and the 5' position of 5' terminal nucleotide.
The oligonucleotides, e.g., raf antisense oligonucleotides, may have sugar mimetics such as cyclobutyl moieties in place of the pentofuranosyl sugar. Representative United States patents that teach the preparation of such modified sugars structures include, but are not limited to, U.S. Pat. Nos. 4,981 ,957;
5,1 18,800; 5,319,080; 5,359,044; 5,393,878; 5,446,137; 5,466,786; 5,514,785; 5,519,134; 5,567,81 1 ; 5,576,427; 5,591 ,722; 5,597,909; 5,610,300; 5,627,053; 5,639,873; 5,646,265; 5,658,873; 5,670,633; and 5,700,920, the contents of which are incorporated herein by reference.
The oligonucleotides, e.g., raf antisense oligonucleotides, may also include nucleobase (often referred to in the art simply as "base") modifications or substitutions. As used herein, "unmodified" or "natural" nucleobases include the purine bases adenine (A) and guanine (G), and the pyrimidine bases thymine (T), cytosine (C) and uracil (U). Modified nucleobases include other synthetic and natural nucleobases such as 5-methylcytosine (5-me-C or m5c), 5-hydroxymethyl cytosine, xanthine, hypoxanthine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine and guanine, 2-propyl and other alkyl derivatives of adenine and guanine, 2-thiouracil, 2-thiothymine and 2-thiocytosine, 5-halouracil and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and thymine, 5- uracil (pseudouracil), 4-thiouracil, 8-halo, 8-amino, 8-thiol, 8-thioalkyl, 8-hydroxyl and other 8-substituted adenines and guanines, 5-halo particularly 5-bromo, 5- trifluoromethyl and other 5-substituted uracils and cytosines, 7-methylguanine and 7-methyladenine, 8-azaguanine and 8-azaadenine, 7-deazaguanine and 7- deazaadenine and 3-deazaguanine and 3-deazaadenine. Further nucleobases include those disclosed in U.S. Pat. No. 3,687,808, those disclosed in the Concise Encyclopedia Of Polymer Science And Engineering 1990, pages 858-859, Kroschwitz, J. I., ed. John Wiley & Sons, those disclosed by Englisch et al.
(Angewandte Chemie, International Edition 1991 , 30, 613-722), and those
disclosed by Sanghvi, Y. S., Chapter 15, Antisense Research and Applications 1993, pages 289-302, Crooke, S. T. and Lebleu, B., ed., CRC Press. Certain of these nucleobases are particularly useful for increasing the binding affinity of the oligomeric compounds of the invention. These include 5-substituted pyrimidines, 6-azapyrimidines and N-2, N-6 and 0-6 substituted purines, including 2- aminopropyladenine, 5-propynyluracil and 5-propynylcytosine. 5-methylcytosine substitutions have been shown to increase nucleic acid duplex stability by 0.6- 1.2. degree. C. (Sanghvi, Y. S., Crooke, S. T. and Lebleu, B., eds., Antisense Research and Applications 1993, CRC Press, Boca Raton, pages 276-278). In certain embodiments, 5-methylcytosine substitutions are present in combination with 2'-0-methoxyethyl sugar modifications.
Representative United States patents that teach the preparation of certain of the above noted modified nucleobases as well as other modified nucleobases include, but are not limited to, U.S. Pat. Nos. 3,687,808, 4,845,205; 5,130,302; 5,134,066; 5,175,273; 5,367,066; 5,432,272; 5,457,187; 5,459,255; 5,484,908; 5,502,177; 5,525,71 1 ; 5,552,540; 5,587,469; 5,594,121 , 5,596,091 ; 5,614,617; and 5,681 ,941 , the contents of which are incorporated herein by reference.
Oligonucleotides provided herein may comprise one or more peptide nucleic acid (PNAs) subunits. Peptide nucleic acids (PNAs) are analogs of DNA in which the backbone is structurally homomorphous with a deoxyribose backbone, consisting of N-(2-aminoethyl) glycine units to which pyrimidine or purine bases are attached. PNAs containing natural pyrimidine and purine bases hybridize to complementary oligonucleotides obeying Watson-Crick base-pairing rules, and mimic DNA in terms of base pair recognition (Egholm, Buchardt et al. 1993). The backbone of PNAs is formed by peptide bonds rather than phosphodiester bonds, making them well-suited for antisense applications (see structure below). A backbone made entirely of PNAs is uncharged, resulting in PNA/DNA or PNA/RNA
duplexes that exhibit greater than normal thermal stability. PNAs are not recognized by nucleases or proteases.
PNAs may be produced synthetically using any technique known in the art. PNA is a DNA analog in which a polyamide backbone replaces the traditional phosphate ribose ring of DNA. Despite a radical structural change to the natural structure, PNA is capable of sequence-specific binding in a helix form to DNA or RNA. Characteristics of PNA include a high binding affinity to
complementary DNA or RNA, a destabilizing effect caused by single-base mismatch, resistance to nucleases and proteases, hybridization with DNA or RNA independent of salt concentration and triplex formation with homopurine DNA. Panagene™ has developed its proprietary Bts PNA monomers (Bts;
benzothiazole-2-sulfonyl group) and proprietary oligomerisation process. The PNA oligomerisation using Bts PNA monomers is composed of repetitive cycles of deprotection, coupling and capping. Panagene's patents to this technology include US 6969766, US 721 1668, US 7022851 , US 7125994, US 7145006 and US
7179896. Representative United States patents that teach the preparation of PNA compounds include, but are not limited to, U.S. Pat. Nos. 5,539,082; 5,714,331 ; and 5,719,262, each of which is herein incorporated by reference. Further teaching of PNA compounds can be found in Nielsen et al., Science, 1991 , 254, 1497.
Also included are "locked nucleic acid" subunits (LNAs). The structures of LNAs are known in the art: For example, Wengel, et al., Chemical Communications (1998) 455; Tetrahedron (1998) 54, 3607, and Accounts of Chem. Research (1999) 32, 301 ); Obika, et al., Tetrahedron Letters (1997) 38, 8735; (1998) 39, 5401 , and Bioorganic Medicinal Chemistry (2008)16, 9230.
Polynucleotides and oligonucleotides may incorporate one or more LNAs; in some cases, the compounds may be entirely composed of LNAs.
Methods for the synthesis of individual LNA nucleoside subunits and their incorporation into oligonucleotides are known in the art: U.S. Patents 7,572,582;
7,569,575; 7,084,125; 7,060,809; 7,053,207; 7,034,133; 6,794,499; and 6,670,461. Typical intersubunit linkers include phosphodiester and phosphorothioate moieties; alternatively, non-phosphorous containing linkers may be employed. One embodiment includes an LNA containing compound where each LNA subunit is separated by a RNA or a DNA subunit (i.e., a deoxyribose nucleotide). Further exemplary compounds may be composed of alternating LNA and RNA or DNA subunits where the intersubunit linker is phosphorothioate.
Certain polynucleotides or oligonucleotides may comprise morpholino-based subunits bearing base-pairing moieties, joined by uncharged or substantially uncharged linkages. The terms "morpholino oligomer" or "PMO" (phosphoramidate- or phosphorodiamidate morpholino oligomer) refer to an oligonucleotide analog composed of morpholino subunit structures, where (i) the structures are linked together by phosphorus-containing linkages, one to three atoms long, preferably two atoms long, and preferably uncharged or cationic, joining the morpholino nitrogen of one subunit to a 5' exocyclic carbon of an adjacent subunit, and (ii) each morpholino ring bears a purine or pyrimidine or an equivalent base-pairing moiety effective to bind, by base specific hydrogen bonding, to a base in a polynucleotide.
Variations can be made to this linkage as long as they do not interfere with binding or activity. For example, the oxygen attached to phosphorus may be substituted with sulfur (thiophosphorodiamidate). The 5' oxygen may be substituted with amino or lower alkyl substituted amino. The pendant nitrogen attached to phosphorus may be unsubstituted, monosubstituted, or disubstituted with (optionally substituted) lower alkyl. The purine or pyrimidine base pairing moiety is typically adenine, cytosine, guanine, uracil, thymine or inosine. The synthesis, structures, and binding characteristics of morpholino oligomers are detailed in U.S. Patent Nos. 5,698,685, 5,217,866, 5,142,047, 5,034,506,
5,166,315, 5,521 ,063, and 5,506,337, and PCT Appn. Nos. PCT/US07/1 1435
(cationic linkages) and US08/012804 (improved synthesis), all of which are incorporated herein by reference.
In certain embodiments, the oligonucleotides, e.g., raf antisense oligonucleotides, comprises a cap structure" or "terminal cap moiety.
Another optional modification of the oligonucleotides, e.g., raf antisense oligonucleotides, involves chemically linking to the oligonucleotide one or more moieties or conjugates which enhance the activity, cellular distribution or cellular uptake of the oligonucleotide. Such moieties include but are not limited to lipid moieties such as a cholesterol moiety (Letsinger et al., Proc. Natl. Acad. Sci. USA 1989, 86, 6553-6556), cholic acid (Manoharan et al., Bioorg. Med. Chem. Lett. 1994, 4, 1053-1059), a thioether, e.g., hexyl-S-tritylthiol (Manoharan et al., Ann. N.Y. Acad. Sci. 1992, 660, 306-309; Manoharan et al., Bioorg. Med. Chem. Let. 1993, 3, 2765-2770), a thiocholesterol (Oberhauser et al., Nucl. Acids Res. 1992, 20, 533-538), an aliphatic chain, e.g., dodecandiol or undecyl residues (Saison-Behmoaras et al., EMBO J. 1991 , 10, 1 1 1 1 -1 1 18; Kabanov et al., FEBS Lett. 1990, 259, 327-330; Svinarchuk et al., Biochimie 1993, 75, 49-54), a phospholipid, e.g., di-hexadecyl-rac-glycerol or triethylammonium 1 ,2-di-O- hexadecyl-rac-glycero-3-H-phosphonate (Manoharan et al., Tetrahedron Lett. 1995, 36, 3651 -3654; Shea et al., Nucl. Acids Res. 1990, 18, 3777-3783), a polyamine or a polyethylene glycol chain (Manoharan et al., Nucleosides & Nucleotides 1995, 14, 969-973), or adamantane acetic acid (Manoharan et al., Tetrahedron Lett. 1995, 36, 3651 -3654), a palmityl moiety (Mishra et al., Biochim. Biophys. Acta 1995, 1264, 229-237), or an octadecylamine or hexylamino- carbonyl-oxycholesterol moiety (Crooke et al., J. Pharmacol. Exp. Ther., 1996, 277, 923-937).
Representative United States patents that teach the preparation of such oligonucleotide conjugates include, but are not limited to, U.S. Pat. Nos. 4,828,979; 4,948,882; 5,218,105; 5,525,465; 5,541 ,313; 5,545,730; 5,552,538; 5,578,717, 5,580,731 ; 5,580,731 ; 5,591 ,584; 5,109,124; 5,1 18,802; 5,138,045;
5,414,077; 5,486,603; 5,512,439; 5,578,718; 5,608,046; 4,587,044; 4,605,735; 4,667,025; 4,762,779; 4,789,737; 4,824,941 ; 4,835,263; 4,876,335; 4,904,582; 4,958,013; 5,082,830; 5,1 12,963; 5,214,136; 5,082,830; 5,1 12,963; 5,214,136; 5,245,022; 5,254,469; 5,258,506; 5,262,536; 5,272,250; 5,292,873; 5,317,098; 5,371 ,241 , 5,391 ,723; 5,416,203, 5,451 ,463; 5,510,475; 5,512,667; 5,514,785; 5,565,552; 5,567,810; 5,574,142; 5,585,481 ; 5,587,371 ; 5,595,726; 5,597,696; 5,599,923; 5,599,928 and 5,688,941 , the contents of which are incorporated herein by reference.
In certain embodiments, an oligonucleotide used according to the present invention has a structure as described in PCT Application Publication No. WO2009/102427, PCT Application Publication No. WO2010/001 1346, or PCT Application Publication No. WO2010/033246. WO2009/102427 describes, e.g., double-stranded RNA (dsRNA) constructs of 12- 49 (preferably 19-49) nucleotides in length, for inhibiting expression of a target gene, said dsRNA comprising: (1 ) a sense strand having a 5'-end and a 3'-end, wherein one or more nucleotides at each of said 5'- and 3'-ends of said sense strand have 2'-modified ribose sugars, and, (2) an antisense strand having a 5'-end and a 3'-end, which hybridizes to said sense strand and to mRNA of said target gene, wherein said antisense strand includes a 2'-modified ribose sugar at the 2nd nucleotide from the 5'-end of the antisense strand, wherein (a) said dsRNA is resistant to cleavage by Dicer, (b) the antisense strand associates with RISC, and (c) the dsRNA inhibits expression of the target gene in a sequence-dependent manner. WO2010/01 1346 describes, e.g., a polynucleotide construct comprising two identical single-stranded polynucleotides, each of the single-stranded polynucleotide comprising a 5 '-stem sequence having a 5 '-end, a 3 '-stem sequence having a 3 '-end, and a linker sequence linking the 5'-stem sequence and the 3'-stem sequence, wherein: (1 ) the 5'-stem sequence of a first single-stranded polynucleotide hybridize with the 3 '- stem sequence of a second single- stranded polynucleotide to form a first double- stranded stem region; (2) the 5'-stem sequence of the second single-stranded
polynucleotide hybridize with the 3 '-stem sequence of the first single-stranded polynucleotide to form a second double-stranded stem region; and, (3) the linker sequences of the first and the second single-stranded polynucleotides form a loop or bulge connecting the first and the second double-stranded stem regions, wherein the 5 '-stem sequence and at least a portion of the linker sequence form a guide sequence complementary to a transcript of a target gene, wherein the polynucleotide construct mediates sequence-dependent gene silencing of expression of the target gene. WO2010/033246 describes, e.g., a double stranded nucleic acid molecule comprising a guide strand, with a minimal length of 16 nucleotides, and a passenger strand forming a double stranded nucleic acid, having a double stranded region and a single stranded region, the double stranded region having 8-15 nucleotides in length, the single stranded region having 4-12 nucleotides in length, wherein position 1 of the guide strand is 5' phosphorylated or has a 2' O-methyl modification, wherein the passenger strand is linked to a lipophilic group, wherein at least 40% of the nucleotides of the double stranded nucleic acid are modified, and wherein the double stranded nucleic acid molecule has one end that is blunt or includes a one nucleotide overhang.
In certain embodiments, an oligonucletoide used according to the present invention is a Dicer substrate. In other embodiments, it is not processed by Dicer but is incorporated into RISC. Chemical modification of RNA duplexes can provide practical advantages for RNA interference (RNAi) triggering molecules including increased stability, safety and specificity. It is known that dsRNAs >/=25 bp are processed by Dicer to create classic 19-bp siRNAs with 3'-end overhangs. The impact of nucleotide modifications on Dicer processing, RISC loading and RNAi-mediated mRNA cleavage were investigated with duplexes >/=25 bp in length by Salomon, W. and Bulock, et al. They demonstrated that the presence of minimal modification configurations on longer RNA duplexes can block Dicer processing and result in the loading of the full-length guide strand into RISC with resultant mRNA cleavage at a defined site. These longer, modified duplexes can
be highly potent gene silencers, with EC50s in the picomolar concentration range, demonstrating that Dicer processing is not required for incorporation into RISC or potent target silencing. (Salomon, W. et al. (2010). "Modified dsRNAs that are not processed by Dicer maintain potency and are incorporated into the RISC." Nucleic Acids Res. 2010, 1 -9). In particular embodiments, an oligonucleotide used according to the present invention has a structure as described in this article.
The oligonucleotides used in accordance with this invention may be conveniently and routinely made through the well-known technique of solid phase synthesis. Equipment for such synthesis is sold by several vendors including Applied Biosystems. Any other means for such synthesis may also be employed; the actual synthesis of the oligonucleotides is well within the talents of the routineer. It is also well known to use similar techniques to prepare other oligonucleotides such as the phosphorothioates and alkylated derivatives. It is also well known to use similar techniques and commercially available modified amidites and controlled-pore glass (CPG) products such as biotin, fluorescein, acridine or psoralen-modified amidites and/or CPG (available from Glen Research, Sterling, VA) to synthesize fluorescently labeled, biotinylated or other modified oligonucleotides such as cholesterol-modified oligonucleotides.
The oligonucleotides, e.g., raf antisense oligonucleotides, of the present invention modulate raf gene expression. In certain embodiments, they inhibit c-raf gene expression. In particular embodiments, they target a
polynucleotide having a sequence set forth in SEQ ID NO: 28 (GENBANK
Accession No. NM_002880).
In certain embodiments, selection of a sequence region complementary to a target gene (or gene) is based upon analysis of the chosen target sequence and determination of secondary structure, Tm, binding energy, and relative stability and cell specificity. Such sequences may be selected based upon their relative inability to form dimers, hairpins, or other secondary structures that would reduce structural integrity of the polynucleotide or prohibit specific binding to
the target gene in a host cell. For example, target regions of the target gene or mRNA may include those regions at or near the AUG translation initiation codon and those sequences that are substantially complementary to 5' regions of the gene or mRNA. These secondary structure analyses and target site selection considerations can be performed, for example, using v.4 of the OLIGO primer analysis software and/or the BLASTN 2.0.5 algorithm software (Altschul et al., Nucleic Acids Res. 1997, 25(17):3389-402) or Oligoengine Workstation 2.0, as well as other algorithms available in the art to identify target sequences within an mRNA.
The sequences of exemplary c-raf antisense oligonucleotides that may be used according to the methods and compositions of the present invention are shown in Table 1. The sequences of exemplary c-raf antisense
oligonucleotides that may be used according to the methods and compositions of the present invention are shown in Tables 1 -4. The antisense oligonucleotides listed in the tables target human c-raf of SEQ ID NO: 28 (GENBANK Accession No. NM_002880). Target start position' indicates the 5'-most nucleotide to which the antisense oligonucleotide is targeted. Target stop position' indicates the 3'- most nucleotide to which the antisense oligonucleotide is targeted. Sequences are shown 5' - 3'.
Table 1. Human c-raf Kinase Antisense Oligonucleotides
Exemplary 2'-modified c-raf antisense oligonucleotides comprising either phosphodiester (P=O) or phosphorothioate (P=S) backbones and uniformly substituted at the 2' position of the sugar with either a 2'-O-methyl, 2'-O-propyl, or 2'-fluoro group are shown in Table 2.
Table 2. Uniformly 2' Sugar- modified c-raf Oligonucleotides
Exemplary chimeric oligonucleotides having SEQ ID NO: 8 and having central "gap" regions of 6, 8, or 10 deoxynucleotides flanked by two regions of 2'-O-methyl modified nucleotides are shown in Table 3. Backbones may be uniformly phosphorothioate. Additional chimeric oligonucleotides having one or more regions of 2'-O-methyl modification and uniform phosphorothioate backbones are shown in Table 3. All are phosphorothioates; bold regions indicate 2'-O- methyl modified regions.
Table 3. Chimeric 2'-0-methyl P = S c-raf oligonucleotides
Additional exemplary chimeric oligonucleotides with various 2' modifications are shown in Table 4. All are phosphorothioates; bold regions indicate 2'-modified regions.
Table 4. Chimeric 2'-modified P = S c-raf oligonucleotides
Exemplary chimeric oligonucleotides with 2'-0-propyl sugar modifications and chimeric P=0/P=S backbones are shown in Table 5, in which italic regions indicate regions which are both 2'-modified and have phosphodiester backbones.
Table 5. Chimeric 2'-modified P=S/P=0 c-raf oligonucleotides
It is understood that for any of the oligonucleotides described herein may include one or more uridine residues instead of one or more thymidine residues. In addition, 2'-methoxyethyl-5-methyluridine (2'MOEMeU) nucleosides are also sometimes designated as 2'-methoxyethylribothymidine (2'-MOE T).
In particular embodiments, a raf antisense oligonucleotide of the present invention has a "gapmer" or "gap-widened" structure, which is an antisense compound in which an internal sequence (or region) having a plurality of nucleotides that supports RNaseH cleavage is positioned between external sequences (or regions) having one or more nucleotides that are chemically distinct from the nucleosides of the internal region. A "gap segment" means the plurality of nucleotides that make up the internal region of a gapmer. A "wing segment" means the external region of a gapmer. In particular embodiments, a gap segment includes 4, 5, 6, 7, 8, 9, 10, 1 1 , or 12 nucleotides. In certain embodiments, an oligonucleotide comprises a gap segment of 10 nucleotides flanked by two wing segments of 5 nucleotides each. In certain embodiments, an oligonucleotide comprises a gap segment of 8 nucleotides flanked by two wing segments of 6 nucleotides each.
In one embodiment, a c-raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence,
TCCCGCCTGTGACATGCATT (SEQ ID NO:8), with 2'-0-methoxyethyl
substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy. In a highly related embodiment, a c-raf antisense oligonucleotide is a full phosphorothioate analog consisting of the sequence,
UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy, which may also be depicted as 5'-
MeUMeCMeCMeCGMeCCTGTGACAMeUGMeCAMeUMeU-3' (SEQ ID NO:29), wherein the underlined residues are 2'MOE nucleosides.
In certain embodiments, methods of the present invention utilize a modified c-raf antisense oligonucleotide comprising or consisting of a sequence 100% complementary to nucleobases 2771 to 2790 of SEQ ID NO:28. In one embodiment, the modified oligonucleotide comprises or consists of the nucleobase sequence of SEQ ID NO:29.
In certain embodiments, the modified oligonucleotide comprises: a gap segment consisting of linked deoxynucleosides; a 5' wing segment consisting of linked nucleosides; and a 3' wing segment consisting of linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; and wherein each nucleoside of each wing segment comprises a modified sugar. In particular embodiments, the modified oligonucleotide consists of 20 nucleobases. In certain embodiments, the modified oligonucleotide comprises: a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-0-methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine.
In certain embodiments, the c-raf antisense oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29). In particular embodiments, the oligonucleotide is a full phosphorothioate analog. In additional embodiments, this oligonucleotide comprises one or more 2'- O-methoxyethyl substitutions. In one embodiment, the oligonucleotide comprises or consists of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29),
wherein the oligonucleotide is a full phosphorothioate analog, wherein the oligonucleotide comprises 2'-0-methoxyethyl substitutions at positions 1 -6 and 15- 20, and wherein residues 7-14 are unmodified 2'-deoxy.
The present invention further includes salt forms of the raf antisense oligonucleotides, such as, e.g., nonadecasodium salts. In one embodiment, the raf antisense oligonucleotide is a nonadecasodium salt of a c-raf antisense
oligonucleotide that is a full phosphorothioate analog consisting of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy, which has a molecular formula of C234H3io 680i26 i9Si9Nai9.
The above oligonucleotides may be prepared by methods known in the art. In one embodiment, they are synthesized by a multi-step process that may be divided into two distinct operations: solid phase synthesis and downstream processing. In the solid-phase synthesis operation, the nucleoside sequence is assembled by a computer controlled solid-phase synthesizer. The downstream process includes deprotection, reverse-phase chromatographic purification, isolation, and lyophilization.
The present invention contemplates the use of any and all raf antisense oligonucleotides comprising or consisting of one or more of these exemplified sequences, in addition to any other raf antisense oligonucleotides or other oligonucletoides capable of modulating raf gene expression. In particular embodiments, a raf antisense oligonucleotide or other oligonucleotide of the present invention selectively modulates expression of one raf gene, while in other embodiments, a raf antisense oligonucleotide of the present invention modulates expression of two or more raf genes.
Anti-HCV Agents
In certain aspects, raf antisense oligonucleotides as described above are used in combination with one or more other anti-HCV agents. In fact, the
present invention contemplates that essentially any type of agent or treatment having a desired activity against HCV may be used in combination with the raf antisense oligonucleotides provided herein. Such agents may include any of a variety of different types of molecules, including, but not limited to, peptides, polypeptides, polynucleotides, antisense oligonucleotides, siRNA's, short hairpin RNA (shRNA), DNAzymes, ribozymes, antibodies, small organic compounds, metals, small inorganic molecules, radionuclides and the like.
In certain embodiments, an anti-HCV agent is selected from the group consisting of an HCV polymerase inhibitor, an HCV protease inhibitor, an inhibitor of another target in the HCV life cycle, an immunomodulatory agent, an antiviral agent, or a combination thereof.
Exemplary immunomodulatory agents include, but are not limited to; natural and recombinant interferon isoform compounds, a-interferon, β-interferon, γ-interferon, o-interferon, Intron® A, Roferon® A, Canferon®-A300, Advaferon®, Infergen®, Humoferon®, Sumiferon® MP, Alfaferone®, IFN-β®, Feron®, polyethylene glycol derivatized (pegylated) interferon compounds, PEG interferon- a-2a (Pegasys®), PEG interferon-a-2b (PEG-lntron®), and pegylated IFN-a-con1 and the like; long acting formulations and derivatizations of interferon compounds such as the albumin-fused interferon albuferon-a and the like; and compounds that stimulate the synthesis of interferon in cells, such as resiquimod and the like.
Other exemplary immunomodulatory agents can include, for example, interleukins; compounds that enhance the development of type 1 helper T cell response, such as SCV-07 and the like; other cytokines; CpG
oligonucleotides; TOLL-like receptor agonists such as CpG-10101 (actilon), isatoribine and the like; thymosin a-1 ; ANA-245; ANA-246; histamine
dihydrochloride; propagermanium; tetrachlorodecaoxide; ampligen; IMP-321 ; KRN- 7000; antibodies, such as civacir, XTL-6865 and the like; and prophylactic and therapeutic vaccines such as InnoVac C, HCV E1 E2/MF59 and the like.
Illustrative antiviral agents include, but are not limited to, ribavirin, amantadine, viramidine, nitazoxanide, telbivudine, NOV-205, taribavirin, inhibitors of internal ribosome entry, broad-spectrum viral inhibitors, such as IMPDH inhibitors (e.g., compounds of U.S. Pat. No. 5,807,876, U.S. Pat. No. 6,498, 178, U.S. Pat. No. 6,344,465, U.S. Pat. No. 6,054,472, WO97/40028, WO98/40381 , WO00/56331 , and mycophenolic acid and derivatives thereof, and including, but not limited to VX-950, merimepodib (VX-497), VX-148, and/or VX-944); or combinations of any of the above.
Illustrative HCV polymerase inhibitors useful according to the present invention include, but are not limited to, NM283 (valopicitabine), R803, JTK-109, JTK-003, HCV-371 , HCV-086, HCV-796 and R-1479.
Illustrative inhibitors of HCV proteases (e.g., NS2-NS3 inhibitors and NS3-NS4A inhibitors) include, but are not limited to, compounds described in WO02/18369. Other illustrative agents include BILN-2061 , VX-950, GS-9132 (ACH-806), SCH-503034, and SCH-6. Still other illustrative agents include those disclosed in WO98/17679, WO00/056331 , WO 98/22496, WO 99/07734, WO 2005/073216 and WO 2005073195.
Exemplary inhibitors HCV life cycle targets can include NS3 helicase inhibitors, metallo-protease inhibitors, alpha glucosidase and the like.
In one specific embodiment of the invention, the anti-HCV agent used in combination with a raf antisense oligonucleotide is a-interferon, pegylated a-interferon, ribavirin, or a combination thereof.
Pharmaceutical Compositions
The present invention further provides pharmaceutical compositions and formulations comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, in combination with another anti-HCV agent. For example, in one illustrative embodiment, raf antisense oligonucleotides of the present invention are used in combination with one or more anti-HCV agents to
provide increased or synergistic improvement in treating HCV infection based, for example, on mechanism of action and non-overlapping toxicity profiles.
The oligonucleotide(s) that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, may be administered prior to, subsequent to, or simultaneously with one or more anti-HCV agents. In addition, the
oligonucleotide(s) that targets a raf nucleic acid, e.g., a raf antisense
oligonucleotide, may be delivered in a separate formulation or in the same formulation as the additional anti-HCV agent(s). Furthermore, where
oligonucleotides of the present invention are administered in a drug delivery device, including those described herein, the second therapeutic agent may be delivered independently or may also be included in the drug delivery device.
Oligonucleotides that target a raf nucleic acid, e.g., raf antisense oligonucleotides, and anti-HCV agents of the invention may be formulated as pharmaceutical compositions suitable for delivery to a subject. The
pharmaceutical compositions will often further comprise one or more buffers {e.g., neutral buffered saline or phosphate buffered saline), carbohydrates (e.g., glucose, mannose, sucrose, dextrose or dextrans), mannitol, proteins, polypeptides or amino acids such as glycine, antioxidants, bacteriostats, chelating agents such as EDTA or glutathione, adjuvants (e.g., aluminum hydroxide), solutes that render the formulation isotonic, hypotonic or weakly hypertonic with the blood of a recipient, suspending agents, thickening agents, preservatives and the like. Alternatively, compositions of the present invention may be formulated as a lyophilizate. Of course, it will be understood that the pharmaceutical compositions may further comprise any additional agents necessary or desired for any of a variety of reasons, including increasing efficacy, reducing undesirable side effects, etc.
Suitable formulations for use in the present invention can be found, e.g., in Remington's Pharmaceutical Sciences, Mack Publishing Company, Philadelphia, PA, 17th Ed. (1985). Often, intravenous compositions will comprise a solution of the therapeutic agent in an acceptable carrier, such as an aqueous
carrier. Any of a variety of aqueous carriers can be used, e.g., water, buffered water, 0.4% saline, 0.9% isotonic saline, 0.3% glycine, 5% dextrose, and the like, and may include glycoproteins for enhanced stability, such as albumin, lipoprotein, globulin, etc. Often, normal buffered saline (135-150 mM NaCI) or 5% dextrose will be used. These compositions can be sterilized by conventional sterilization techniques, such as filtration. The resulting aqueous solutions may be packaged for use or filtered under aseptic conditions and lyophilized, the lyophilized preparation being combined with a sterile aqueous solution prior to administration. The compositions may also contain pharmaceutically acceptable auxiliary substances as required to approximate physiological conditions, such as pH adjusting and buffering agents, tonicity adjusting agents and the like, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, etc.
The compositions and formulations of the invention may be administered by essentially any suitable means, illustrative examples of which include parenteral, intravenous, systemic, local, oral, intratumoral, intramuscular, intraocular, eye drops, subcutaneous, intraperitoneal, inhalation, or any other suitable method of delivery.
In one embodiment, the therapeutic agents of the present invention are administered parenterally, i.e., intraarticular^, intravenously, intraperitoneal^, subcutaneously, or intramuscularly. In a more specific embodiment, they are administered by intravenous infusion or intraperitoneally by a bolus injection. For example, in one embodiment, a patient is given an intravenous infusion of one or more raf antisense oligonucleotides and/or anti-HCV agents through a running intravenous line over, e.g., 5-10 minutes, 15-20 minutes, 30 minutes, 60 minutes, 90 minutes, or longer. In one embodiment, a 60 minute infusion is used. In other embodiments, an infusion ranging from 6-10 or 15-20 minutes is used. Such infusions can be given periodically, e.g., once every 1 , 3, 5, 7, 10, 14, 21 , or 28 days or longer.
In another more specific embodiment, the therapeutic agents of the present invention are administered subcutaneously, e.g., on a weekly basis. They may alternatively be administered subcutaneously more or less frequently, e.g., once every 3-5 days, once every two weeks, or once every four weeks for a period of time. In particular embodiments where a therapeutic agent of the present invention is used in combination with another anti-HCV agent, both agents may be administered subcutaneously, e.g., once a week, once every 3-5 days, once every two weeks, or once every four weeks for a period of time,
Formulations for topical administration may include, for example, ointments, lotions, creams, gels, drops, suppositories, sprays, liquids and powders. Conventional pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the like may be necessary or desirable.
Formulations for oral administration may include, for example, powders or granules, suspensions or solutions in water or non-aqueous media, capsules, sachets, or tablets. Thickeners, flavorings, diluents, emulsifiers, dispersing aids or binders may also be desirable.
Formulations for parenteral administration may include, for example, sterile aqueous solutions which may also contain buffers, diluents and other suitable additives.
In addition to such pharmaceutical carriers, cationic lipids or other agents may be included in the formulations to facilitate oligonucleotide uptake. One such composition shown to facilitate uptake is LIPOFECTIN (a 1 :1 liposome formulation of the cationic lipid N-[1 -(2,3-dioleyloxy)propyl]-N,N,N- trimethylammonium chloride (DOTMA) and dioleoyl phosphatidylethanolamine (DOPE)) (BRL, Bethesda Md.).
Dosing is generally dependent on severity and responsiveness of the condition to be treated, with a typical course of treatment lasting from several days to several months or until a cure is effected or a diminution of disease state is achieved. Optimal dosing schedules can be calculated from measurements of
drug accumulation in the body or at a localized site or based upon a patient's response. Persons of ordinary skill can easily determine optimum dosages, dosing methodologies, and repetition rates. Optimum dosages may vary depending on the relative potency of individual oligonucleotides, and can generally be estimated based on EC50's in in vitro and in vivo animal studies.
Routes of administration and dosages can be readily determined by the skilled artisan upon considering the particular disease and, if present, drug delivery device used. For example, anti-HCV agents used according to the present invention may be administered using conventional doses and routes of administration known to those having skill in the art.
The formulation of therapeutic compositions and their subsequent administration is within the skill in the art. In general, for therapeutics, a patient suspected of needing such therapy is given an oligonucleotide in accordance with the invention, commonly in a pharmaceutically acceptable carrier, in amounts and for periods which will vary depending upon the nature of the particular disease, its severity and the patient's overall condition.
As note above, the pharmaceutical compositions and formulations of the present invention can be used in methods for inhibiting the replication of HCV and thereby treating or preventing HCV infection in a patient. The methods may also be used to treat infection from any viral-mediated disease or disorder that benefits from reduced raf expression.
In certain embodiments of the present invention, pharmaceutical compositions comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and/or one or more of the additional HCV therapeutic agents are administered to a patient so as to achieve systemic delivery of the agents. In certain embodiments of the present invention, pharmaceutical compositions comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and/or one or more of the additional HCV
therapeutic agents are administered to a patient so as to achieve local delivery of the agents.
In certain other embodiments, pharmaceutical compositions comprising oligonucleotides that targets a raf nucleic acid, e.g., raf antisense oligonucleotides, and/or one or more of the additional HCV therapeutic agents are provided to a patient in a drug delivery device, typically to achieve prolonged delivery over a relatively long time period. In certain embodiments, this time period is at least one week, at least two weeks, at least three weeks, at least one month, at least two months, at least three months, at least four months, at least six months, at least nine months, or at least one year.
As described herein, oligonucleotides targeted to portions of the c-raf mRNA are particularly useful for inhibiting raf expression and for interfering with HCV replication. In certain embodiments of the methods of the invention, tissues or cells are contacted with the compositions and formulations of the invention. In the context of this invention, to "contact" tissues or cells means to add the oligonucleotide(s) and/or the anti-HCV agent(s), usually in a liquid carrier, pharmaceutical composition, and/or drug delivery device, to a cell suspension or tissue sample, either in vitro or ex vivo, or to administer the oligonucleotide(s) and/or the anti-HCV agent(s) to cells or tissues within an animal, again, typically in a pharmaceutical formulation or drug delivery device.
According to various embodiments of the methods of the present invention, the oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, is delivered prior to, concurrently with, or after delivery of the one or more additional anti-HCV agents used in the combination therapy. One or more of the oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and one or more additional therapeutic HCV agents may be delivered using a single drug delivery devices, or, alternatively, they may be delivered in separate drug delivery devices. Of course, in particular embodiments,
none, one or some of the therapeutic agents are delivered using a drug delivery device.
The present invention further includes kits comprising one or more pharmaceutical compositions of the present invention. In particular embodiments, a kit of the present invention comprises a pharmaceutical composition comprising a raf antisense oligonucleotide and a pharmaceutical composition comprising an additional therapeutic agent, such as an anti-HCV agent described above.
Delivery Devices
The present invention further includes drug delivery devices comprising an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, optionally further comprising one or more additional anti-HCV agents, as described herein. The drug delivery devices of the present invention are particularly well-suited for the administration and controlled release of therapeutic agents over a prolonged time-course. Accordingly, they offer significant advantages over other methods of administration, such as, e.g., systemic intravenous delivery, since they require only a single injection or surgical implantation of the drug delivery device comprising a raf antisense oligonucleotide, as compared to the multiple intraocular or periocular injections of raf antisense oligonucleotides or multiple intravenous administrations required when the raf antisense oligonucleotide is not in a drug delivery device. Drug delivery devices according to the present invention are typically biocompatible.
Drug delivery devices according to the present invention include microcapsules. In certain embodiment, an oligonucleotide of the present invention is entrapped in a microcapsule prepared, for example, by coacervation techniques or by interfacial polymerization, for example, hydroxymethylcellulose or gelatin- microcapsules and poly-(methylmethacylate) microcapsules, respectively; in colloidal drug delivery systems (for example, liposomes, niosomes, albumin microspheres, microemulsions, nano-particles and nanocapsules) or in
macroemulsions. Such techniques are disclosed in Remington's Pharmaceutical Sciences 16th edition, Osol, A. Ed. (1980). Other microcapsules include, e.g., nanoparticles and microparticles.
In one embodiment, a drug delivery device of the present invention comprises a sustained-release preparation of an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, optionally further comprising one or more other anti-HCV agents. Suitable examples of sustained-release preparations include semipermeable matrices of solid hydrophobic polymers containing the antagonist, which matrices are in the form of shaped articles, e.g. films, or microcapsules. Examples of sustained-release matrices include polyesters, hydrogels (for example, poly(2-hydroxyethyl-methacrylate), or poly(vinylalcohol)), polylactides (U.S. Pat. No. 3,773,919), copolymers of L- glutamic acid and y ethyl-L-glutamate, non-degradable ethylene-vinyl acetate, degradable lactic acid-glycolic acid copolymers such as the LUPRON DEPOT.TM. (injectable microspheres composed of lactic acid-glycolic acid copolymer and leuprolide acetate), biocompatible polymers, and poly-D-(-)-3-hydroxybutyric acid.
In additional embodiments, drug delivery devices according to the present invention comprise a biocompatible matrix. Biocompatible matrices of the present invention may comprise, e.g., collagen, metal, hydroxyapatite, bioglass, aluminate, bioceramic materials, and purified proteins.
In one particular embodiment, the drug delivery device comprises Atrigel™ (QLT, Inc., Vancouver, B.C.). The Atrigel® drug delivery system consists of biodegradable polymers dissolved in biocompatible carriers. Pharmaceuticals may be blended into this liquid delivery system at the time of manufacturing or, depending upon the product, may be added later by the physician at the time of use. When the liquid product is injected into the subcutaneous space through a small gauge needle or placed into accessible tissue sites through a cannula, water in the tissue fluids causes the polymer to precipitate and trap the drug in a solid
implant. The drug encapsulated within the implant is then released in a controlled manner as the polymer matrix biodegrades with time.
In certain embodiments, the present invention provides a drug delivery device suitable for ocular administration of an oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, alone or in combination with one or more additional therapeutic agents. In one embodiment, the drug delivery device comprises an erodable polylacticglycolic acid (PLGA) matrix, such as that used in the Posurdex® system (Allergan, Irvine, CA). Posurdex® is a bioerodable extended release implant that delivers dexamethasone to the targeted disease site at the posterior-segment of the eye.
In another embodiment, a drug delivery device of the present invention comprises a polymer. Polymers such as poly(lactic acid) or poly(glycolic acid) undergo hydrolytic degradation in the body and become monomers of lactic acid or glycolic acid. These monomers can be metabolized and eliminated from the tissues. It is possible to incorporate drugs in the matrix of these polymers. The polymer containing the drug releases the drug for a sustained period and undergoes degradation simultaneously. These polymers have been used as materials of absorbable surgical sutures for many years and proved to be safe and biocompatible.
Particularly suitable drug delivery devices of the present invention provide prolonged delivery of the oligonucleotide that targets a raf nucleic acid, e.g., a raf antisense oligonucleotide, and/or one or more additional therapeutic agents, as compared to the duration of delivery in the absence of the drug delivery device. In preferred embodiments, a drug delivery device increases the pharmacokinetics, e.g., half-life, of the oligonucleotide by at least two-fold, at least five-fold, or at least ten-fold as compared to in the absence of the drug delivery device.
EXAMPLES
EXAMPLE 1
REDUCTION OF HCV TITERS BY TREATMENT WITH RAF ANTISENSE
In order to demonstrate that c-raf antisense oligonucleotides can inhibit HCV viral replication, human hepatocyte cells (HuH7.5) were transfected with varying concentrations (0-500 nM) of an illustrative raf antisense
oligonucleotide compound of the invention. The raf antisense oligonucleotide compound used in the experiments was a full phosphorothioate analog consisting of the sequence, UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0- methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy. The U nucleosides are also sometimes designated as T.
HuH7.5 cells were cultured in complete medium in the absence of antibiotics for one week. The cells were then plated on a 12 well plate at 20% density, one day prior to transfection, to achieve 30-40% density on the day of transfection. Prior to transfection, the complete medium was removed and replaced by OptiMEM medium (Gibco, Invitrogen Corp., Carlsbad, CA, USA;
reduced serum medium). Transfection (day -1 ) was performed according to instructions provided by the manufacturer, with the exception that 1.5 μΙ
oligofectamin (Invitrogen Corp., Carlsbad, CA, USA) per 100 μΙ volume was used regardless of the amount of oligonucleotide being transfected. In each experiment, transfection with a control sequence (200 nM) and transfection with a fluorescent oligonucleotide (200 nM, Trackit; Gibco, Invitrogen Corp., Carlsbad, CA, USA) was performed to assess transfection efficiency. After four hours, an equal volume of DMEM containing 20% serum was added, and the cells were further incubated overnight. The following day, the cells were washed and transfection efficiencies were estimated under the fluorescence microscope. Following about 16 hours of exposure to the compound, the cells were infected with HCV on Day 0 for four hours and further cultured in DMEM containing 10% FBS. Cell viability was
assessed daily using a conventional microscope. Medium was collected at given days for determining HCV viral production until Day 6.
HCV titers were determined by quantitative RT-PCR. First, RNA was extracted from the cell culture medium by using a Roche High Pure Viral nucleic acid kit according to the instructions provided with the kit (Roche Applied Science, Indianapolis, IN, USA). cDNA was then produced from the RNA template using Superscript III reverse transcriptase (Invitrogen Corp., Carlsbad, CA, USA), according to the instructions provided. An HCV specific primer (5'- AGGTTTAGGATTCGTGCTCAT) was used. Quantitative PCR was then performed using an Applied Biosystems/Taqman Real Time PCR system (Life Technologies Corp., Carlsbad, CA, USA) and Taqman chemistry, with all measurements determined in duplicate. 6-FAM-
CACCCTATCAGGCAGTACCACAAGGCC-TAMRA was used as the HCV specific detection probe, and a primer set detecting the conserved 5'UTR region of HCV (5'-TGCGGAACCGGTGAGTACA, 5'-AGGTTTAGGATTCGTGCTCAT). For absolute quantitation, a standard curve of known dilutions of a plasmid containing the sequence for HCV variant H77c (pCV-H77c) was created.
Cell lysates obtainined on given days were used for determination of cRaf and actin levels by western blot. Total protein levels were determined by Lowry protein assay.
As shown in Figure 1 , treatment with the raf antisense oligonucleotide compound resulted in a dose dependent reduction in HCV titers measured in the cells, with a moderate reduction in viral titers at 200 nM (60%) and 80-95% at higher concentrations (300-500 nM), in cells transfected the oligonucleotide, compared to cells transfected with a control antisense molecule. The suppressing effect was short-lived at lower concentrations, but sustained for up to 8 days post infection at higher concentrations (single dose). The maximum effect was observed at four to 6 days post treatment. HCV core protein showed a
similar trend as HCV titers, and NS3 protein levels were less affected by iCo-007 treatment (data not shown).
Transfection efficiency was typically >90%. As shown in Figure 2, c- Raf protein levels in cells transfected with a single dose of 200-500 nM
oligonucleotide were reduced by up to 95%, for up to 5 days post transfection. The maximum effect was seen at four days post treatment. Inhibition at a higher concentration resulted in a longer suppression of c-Raf expression. C-Raf suppression did not result in reduced cell growth, and at lower concentrations (100-200 nM), it appeared to have a slightly stimulating effect on cell growth.
These data demonstrate that raf antisense oligonucleotides efficiently inhibit viral production in a tissue culture model of HCV infection, supporting their use to treat HCV infection in patients.
All of the above U.S. patents, U.S. patent application publications, U.S. patent applications, foreign patents, foreign patent applications and nonpatent publications referred to in this specification and/or listed in the Application Data Sheet, are incorporated herein by reference, in their entirety.
From the foregoing it will be appreciated that, although specific embodiments of the invention have been described herein for purposes of illustration, various modifications may be made without deviating from the spirit and scope of the invention. Accordingly, the invention is not limited except as by the appended claims.
Claims
1 . A method of treating or preventing HCV infection comprising administering to a subject, or cells thereof, a therapeutically effective amount of an oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf.
2. The method of claim 1 , wherein the oligonucleotide is a modified oligonucleotide 100% complementary to nucleobases 2771 to 2790 of SEQ ID NO:28.
3. The method of claim 2, wherein the modified oligonucleotide comprises:
a gap segment consisting of linked deoxynucleosides;
a 5' wing segment consisting of linked nucleosides; and
a 3' wing segment consisting of linked nucleosides;
wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment, and wherein each nucleoside of each wing segment comprises a modified sugar.
4. The method of claim 3, wherein the modified oligonucleotide consists of 20 nucleobases.
5. The method of claim 4, wherein the modified oligonucleotide comprises:
a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides; wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-0- methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine.
6. The method of any one of claims 1 -5, wherein the oligonucleotide comprises the nucleobase sequence of SEQ ID NO:8 or SEQ ID NO:29.
7. The method of claim 6, wherein the oligonucleotide is a full phosphorothioate analog consisting of the sequence, TCCCGCCTGTGACATGCATT (SEQ ID NO:8) or UCCCGCCTGTGACAUGCAUU (SEQ ID NO:29), with 2'-0- methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
8. The method of any one of claims 1 -7, further comprising a step of administering an anti-HCV agent to the subject.
9. The method of claim 8, wherein the anti-HCV agent is selected from the group consisting of an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent and an antiviral agent.
10. The method of claim 3, wherein the anti-HCV agent is an immunomodulatory agent selected from the group consisting of a-interferon, β- interferon, .γ-interferon, o -interferon, Intron® A, Roferon® A, Canferon®-A300, Advaferon®, Infergen®., Humoferon®., Sumiferon® MP, Alfaferone®., IFN-β®., Feron®, polyethylene glycol derivatized (pegylated) interferon compounds, PEG interferon-a-2a (Pegasys®), PEG interferon-a-2b (PEG-lntron®) and pegylated IFN-a- conl
1 1. The method of claim 8, wherein the anti-HCV agent is an antiviral agent selected from the group consisting of ribavirin, amantadine, viramidine, nitazoxanide; telbivudine; NOV-205; taribavirin; VX-950, VX-497, VX-148, and VX-944.
12. The method of claim 8, wherein the anti-HCV agent is an HCV polymerase inhibitor selected from the group consisting of NM283 (valopicitabine), R803, JTK-109, JTK-003, HCV-371 , HCV-086, HCV-796 and R-1479.
13. The method of claim 8, wherein the anti-HCV agent is an HCV protease inhibitor selected from the group consisting of BILN-2061 , VX-950, GS-9132 (ACH-806), SCH-503034, and SCH-6.
14. The method of claim 8, wherein the anti-HCV agent is an HCV life cycle protein inhibitor selected from the group consisting of a NS3 helicase inhibitor; a metallo-protease inhibitor and an alpha glucosidase inhibitors.
15. The method of claim 8, wherein the anti-HCV agent is selected from the group consisting of an antisense oligonucleotide inhibitor, an siRNA inhibitor, a short hairpin RNA (shRNA) inhibitor and a ribozyme inhibitor
16. The method of claim 8, wherein the anti-HCV agent is selected from the group consisting of a-interferon, pegylated a-interferon, ribavirin, or a combination thereof.
17. A pharmaceutical composition comprising a physiologically acceptable carrier, a therapeutically effective amount of at least one oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, and at least one additional anti-HCV agent.
18. The pharmaceutical composition of claim 17, wherein the oligonucleotide is a modified oligonucleotide 100% complementary to nucleobases 2771 to 2790 of SEQ ID NO:28.
19. The pharmaceutical composition of claim 18, wherein the modified oligonucleotide comprises:
a gap segment consisting of linked deoxynucleosides;
a 5' wing segment consisting of linked nucleosides; and
a 3' wing segment consisting of linked nucleosides;
wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment, and wherein each nucleoside of each wing segment comprises a modified sugar.
20. The pharmaceutical composition of claim 19, wherein the modified oligonucleotide consists of 20 nucleobases.
21. The pharmaceutical composition of claim 20, wherein the modified oligonucleotide comprises:
a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and a 3' wing segment consisting of six linked nucleosides;
wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-0- methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine.
22. The pharmaceutical composition of any one of claims 17-21 , wherein the oligonucleotide comprises the nucleobase sequence of SEQ ID NO:29.
23. The pharmaceutical composition of claim 17, wherein the oligonucleotide is a full phosphorothioate analog consisting of the sequence,
TCCCGCCTGTGACATGCATT (SEQ ID NO:8), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
24. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is selected from the group consisting of an HCV
polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent and an antiviral agent.
25. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is an immunomodulatory agent selected from the group consisting of a-interferon, β-interferon, γ-interferon, o-interferon, Intron® A, Roferon® A, Canferon®-A300, Advaferon®, Infergen®., Humoferon®., Sumiferon® MP,
Alfaferone®., IFN-β®., Feron®, polyethylene glycol derivatized (pegylated) interferon compounds, PEG interferon-a-2a (Pegasys®), PEG interferon-a-2b (PEG-lntron®) and pegylated IFN-a-con1.
26. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is an antiviral agent selected from the group consisting of ribavirin, amantadine, viramidine, nitazoxanide; telbivudine; NOV-205; taribavirin; VX- 950, VX-497, VX-148, and VX-944.
27. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is an HCV polymerase inhibitor selected from the group consisting of NM283 (valopicitabine), R803, JTK-109, JTK-003, HCV-371 , HCV-086, HCV-796 and R-1479.
28. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is an HCV protease inhibitor selected from the group consisting of BILN-2061 , VX-950, GS-9132 (ACH-806), SCH-503034, and SCH-6.
29. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is an HCV life cycle protein inhibitor selected from the group consisting of a NS3 helicase inhibitor; a metallo-protease inhibitor and an alpha glucosidase inhibitors.
30. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is selected from the group consisting of an antisense oligonucleotide inhibitor, an siRNA inhibitor, a short hairpin RNA (shRNA) inhibitor and a ribozyme inhibitor
31. The pharmaceutical composition of any one of claims 17-23, wherein the anti-HCV agent is selected from the group consisting of a-interferon, pegylated a-interferon, ribavirin, or a combination thereof.
32. A kit comprising a first pharmaceutical composition comprising a physiologically acceptable carrier and a therapeutically effective amount of at least one oligonucleotide 8 to 50 nucleotides in length which is targeted to mRNA encoding human c-raf (SEQ ID NO: 28), wherein said oligonucleotide inhibits expression of human c-raf, and a second pharmaceutical composition comprising a physiologically acceptable carrier and a therapeutically effective amount of at least one additional anti- HCV agent.
33. The kit of claim 32, wherein the oligonucleotide is a modified oligonucleotide 100% complementary to nucleobases 2771 to 2790 of SEQ ID NO:28.
34. The kit of claim 33, wherein the modified oligonucleotide comprises: a gap segment consisting of linked deoxynucleosides;
a 5' wing segment consisting of linked nucleosides; and
a 3' wing segment consisting of linked nucleosides;
wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment, and wherein each nucleoside of each wing segment comprises a modified sugar.
35. The kit of claim 34, wherein the modified oligonucleotide consists of 20 nucleobases.
36. The kit of claim 35, wherein the modified oligonucleotide comprises: a gap segment consisting of eight linked deoxynucleosides; a 5' wing segment consisting of six linked nucleosides; and
a 3' wing segment consisting of six linked nucleosides;
wherein the gap segment is positioned between the 5' wing segment and the 3' wing segment; wherein each nucleoside of each wing segment comprises a 2'-0- methoxyethyl sugar; wherein each internucleoside linkage is a phosphorothioate linkage; and wherein each cytosine is a 5-methylcytosine.
37. The kit of any one of claims 32-36, wherein the oligonucleotide comprises the nucleobase sequence of SEQ ID NO:29.
38. The kit of claim 32, wherein the oligonucleotide is a full phosphorothioate analog consisting of the sequence, TCCCGCCTGTGACATGCATT (SEQ ID NO:8), with 2'-0-methoxyethyl substitutions at positions 1 -6 and 15-20, and wherein residues 7-14 are unmodified 2'-deoxy.
39. The kit of any one of claims 32-38, wherein the anti-HCV agent is selected from the group consisting of an HCV polymerase inhibitor, an HCV protease inhibitor, an HCV life cycle protein inhibitor, an immunomodulatory agent and an antiviral agent.
40. The kit of any one of claims 32-38, wherein the anti-HCV agent is an immunomodulatory agent selected from the group consisting of a-interferon, β- interferon, γ-interferon, o-interferon, Intron® A, Roferon® A, Canferon®-A300,
Advaferon®, Infergen®., Humoferon®., Sumiferon® MP, Alfaferone®., IFN-β®., Feron®, polyethylene glycol derivatized (pegylated) interferon compounds, PEG interferon-a-2a (Pegasys®), PEG interferon-a-2b (PEG-lntron®) and pegylated IFN-a- conl
41. The kit of any one of claims 32-38, wherein the anti-HCV agent is an antiviral agent selected from the group consisting of ribavirin, amantadine, viramidine, nitazoxanide; telbivudine; NOV-205; taribavirin; VX-950, VX-497, VX-148, and VX-944.
42. The kit of any one of claims 32-38, wherein the anti-HCV agent is an HCV polymerase inhibitor selected from the group consisting of NM283
(valopicitabine), R803, JTK-109, JTK-003, HCV-371 , HCV-086, HCV-796 and R-1479.
43. The kit of any one of claims 32-38, wherein the anti-HCV agent is an HCV protease inhibitor selected from the group consisting of BILN-2061 , VX-950, GS-9132 (ACH-806), SCH-503034, and SCH-6.
44. The kit of any one of claims 32-38, wherein the anti-HCV agent is an HCV life cycle protein inhibitor selected from the group consisting of a NS3 helicase inhibitor; a metallo-protease inhibitor and an alpha glucosidase inhibitors.
45. The kit of any one of claims 32-38, wherein the anti-HCV agent is selected from the group consisting of an antisense oligonucleotide inhibitor, a siRNA inhibitor, a short hairpin RNA (shRNA) inhibitor and a ribozyme inhibitor
46. The kit of any one of claims 32-38, wherein the anti-HCV agent is selected from the group consisting of α-interferon, pegylated α-interferon, ribavirin, or a combination thereof.
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US31171010P | 2010-03-08 | 2010-03-08 | |
| US61/311,710 | 2010-03-08 | ||
| US33258210P | 2010-05-07 | 2010-05-07 | |
| US61/332,582 | 2010-05-07 | ||
| US35399710P | 2010-06-11 | 2010-06-11 | |
| US61/353,997 | 2010-06-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011112516A1 true WO2011112516A1 (en) | 2011-09-15 |
Family
ID=43920161
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/027412 WO2011112516A1 (en) | 2010-03-08 | 2011-03-07 | Treating and preventing hepatitis c virus infection using c-raf kinase antisense oligonucleotides |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011112516A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013024158A1 (en) * | 2011-08-17 | 2013-02-21 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Combinations of protein kinase inhibitors and interferons or of protein kinase inhibitors and direct acting antivirals for the treatment and the prevention of hcv infection |
| EP2994140A4 (en) * | 2013-05-07 | 2017-05-03 | Inhibikase Therapeutics, Inc. | Methods for treating hcv infection |
Citations (162)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1280408A (en) | 1916-10-09 | 1918-10-01 | John Adolphus Cox | Tire-chain lock. |
| US3687808A (en) | 1969-08-14 | 1972-08-29 | Univ Leland Stanford Junior | Synthetic polynucleotides |
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| US4469863A (en) | 1980-11-12 | 1984-09-04 | Ts O Paul O P | Nonionic nucleic acid alkyl and aryl phosphonates and processes for manufacture and use thereof |
| US4476301A (en) | 1982-04-29 | 1984-10-09 | Centre National De La Recherche Scientifique | Oligonucleotides, a process for preparing the same and their application as mediators of the action of interferon |
| US4587044A (en) | 1983-09-01 | 1986-05-06 | The Johns Hopkins University | Linkage of proteins to nucleic acids |
| US4605735A (en) | 1983-02-14 | 1986-08-12 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US4667025A (en) | 1982-08-09 | 1987-05-19 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US4762779A (en) | 1985-06-13 | 1988-08-09 | Amgen Inc. | Compositions and methods for functionalizing nucleic acids |
| US4824941A (en) | 1983-03-10 | 1989-04-25 | Julian Gordon | Specific antibody to the native form of 2'5'-oligonucleotides, the method of preparation and the use as reagents in immunoassays or for binding 2'5'-oligonucleotides in biological systems |
| US4828979A (en) | 1984-11-08 | 1989-05-09 | Life Technologies, Inc. | Nucleotide analogs for nucleic acid labeling and detection |
| US4835263A (en) | 1983-01-27 | 1989-05-30 | Centre National De La Recherche Scientifique | Novel compounds containing an oligonucleotide sequence bonded to an intercalating agent, a process for their synthesis and their use |
| US4845205A (en) | 1985-01-08 | 1989-07-04 | Institut Pasteur | 2,N6 -disubstituted and 2,N6 -trisubstituted adenosine-3'-phosphoramidites |
| US4876335A (en) | 1986-06-30 | 1989-10-24 | Wakunaga Seiyaku Kabushiki Kaisha | Poly-labelled oligonucleotide derivative |
| US4904582A (en) | 1987-06-11 | 1990-02-27 | Synthetic Genetics | Novel amphiphilic nucleic acid conjugates |
| US4948882A (en) | 1983-02-22 | 1990-08-14 | Syngene, Inc. | Single-stranded labelled oligonucleotides, reactive monomers and methods of synthesis |
| US4958013A (en) | 1989-06-06 | 1990-09-18 | Northwestern University | Cholesteryl modified oligonucleotides |
| US4981957A (en) | 1984-07-19 | 1991-01-01 | Centre National De La Recherche Scientifique | Oligonucleotides with modified phosphate and modified carbohydrate moieties at the respective chain termini |
| US5023243A (en) | 1981-10-23 | 1991-06-11 | Molecular Biosystems, Inc. | Oligonucleotide therapeutic agent and method of making same |
| US5034506A (en) | 1985-03-15 | 1991-07-23 | Anti-Gene Development Group | Uncharged morpholino-based polymers having achiral intersubunit linkages |
| US5082830A (en) | 1988-02-26 | 1992-01-21 | Enzo Biochem, Inc. | End labeled nucleotide probe |
| US5109124A (en) | 1988-06-01 | 1992-04-28 | Biogen, Inc. | Nucleic acid probe linked to a label having a terminal cysteine |
| US5112963A (en) | 1987-11-12 | 1992-05-12 | Max-Planck-Gesellschaft Zur Foerderung Der Wissenschaften E.V. | Modified oligonucleotides |
| US5118802A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | DNA-reporter conjugates linked via the 2' or 5'-primary amino group of the 5'-terminal nucleoside |
| US5118800A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | Oligonucleotides possessing a primary amino group in the terminal nucleotide |
| US5130302A (en) | 1989-12-20 | 1992-07-14 | Boron Bilogicals, Inc. | Boronated nucleoside, nucleotide and oligonucleotide compounds, compositions and methods for using same |
| US5134066A (en) | 1989-08-29 | 1992-07-28 | Monsanto Company | Improved probes using nucleosides containing 3-dezauracil analogs |
| US5138045A (en) | 1990-07-27 | 1992-08-11 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5142047A (en) | 1985-03-15 | 1992-08-25 | Anti-Gene Development Group | Uncharged polynucleotide-binding polymers |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5175273A (en) | 1988-07-01 | 1992-12-29 | Genentech, Inc. | Nucleic acid intercalating agents |
| US5177196A (en) | 1990-08-16 | 1993-01-05 | Microprobe Corporation | Oligo (α-arabinofuranosyl nucleotides) and α-arabinofuranosyl precursors thereof |
| US5185444A (en) | 1985-03-15 | 1993-02-09 | Anti-Gene Deveopment Group | Uncharged morpolino-based polymers having phosphorous containing chiral intersubunit linkages |
| US5188897A (en) | 1987-10-22 | 1993-02-23 | Temple University Of The Commonwealth System Of Higher Education | Encapsulated 2',5'-phosphorothioate oligoadenylates |
| US5214136A (en) | 1990-02-20 | 1993-05-25 | Gilead Sciences, Inc. | Anthraquinone-derivatives oligonucleotides |
| US5214134A (en) | 1990-09-12 | 1993-05-25 | Sterling Winthrop Inc. | Process of linking nucleosides with a siloxane bridge |
| US5216141A (en) | 1988-06-06 | 1993-06-01 | Benner Steven A | Oligonucleotide analogs containing sulfur linkages |
| US5218105A (en) | 1990-07-27 | 1993-06-08 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5217866A (en) | 1985-03-15 | 1993-06-08 | Anti-Gene Development Group | Polynucleotide assay reagent and method |
| US5235033A (en) | 1985-03-15 | 1993-08-10 | Anti-Gene Development Group | Alpha-morpholino ribonucleoside derivatives and polymers thereof |
| US5245022A (en) | 1990-08-03 | 1993-09-14 | Sterling Drug, Inc. | Exonuclease resistant terminally substituted oligonucleotides |
| US5254469A (en) | 1989-09-12 | 1993-10-19 | Eastman Kodak Company | Oligonucleotide-enzyme conjugate that can be used as a probe in hybridization assays and polymerase chain reaction procedures |
| US5258506A (en) | 1984-10-16 | 1993-11-02 | Chiron Corporation | Photolabile reagents for incorporation into oligonucleotide chains |
| US5262536A (en) | 1988-09-15 | 1993-11-16 | E. I. Du Pont De Nemours And Company | Reagents for the preparation of 5'-tagged oligonucleotides |
| US5264423A (en) | 1987-03-25 | 1993-11-23 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US5264562A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences, Inc. | Oligonucleotide analogs with novel linkages |
| US5264564A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences | Oligonucleotide analogs with novel linkages |
| US5272250A (en) | 1992-07-10 | 1993-12-21 | Spielvogel Bernard F | Boronated phosphoramidate compounds |
| US5276019A (en) | 1987-03-25 | 1994-01-04 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US5278302A (en) | 1988-05-26 | 1994-01-11 | University Patents, Inc. | Polynucleotide phosphorodithioates |
| US5292873A (en) | 1989-11-29 | 1994-03-08 | The Research Foundation Of State University Of New York | Nucleic acids labeled with naphthoquinone probe |
| US5317098A (en) | 1986-03-17 | 1994-05-31 | Hiroaki Shizuya | Non-radioisotope tagging of fragments |
| US5319080A (en) | 1991-10-17 | 1994-06-07 | Ciba-Geigy Corporation | Bicyclic nucleosides, oligonucleotides, process for their preparation and intermediates |
| US5321131A (en) | 1990-03-08 | 1994-06-14 | Hybridon, Inc. | Site-specific functionalization of oligodeoxynucleotides for non-radioactive labelling |
| US5359044A (en) | 1991-12-13 | 1994-10-25 | Isis Pharmaceuticals | Cyclobutyl oligonucleotide surrogates |
| US5367066A (en) | 1984-10-16 | 1994-11-22 | Chiron Corporation | Oligonucleotides with selectably cleavable and/or abasic sites |
| US5371241A (en) | 1991-07-19 | 1994-12-06 | Pharmacia P-L Biochemicals Inc. | Fluorescein labelled phosphoramidites |
| US5391723A (en) | 1989-05-31 | 1995-02-21 | Neorx Corporation | Oligonucleotide conjugates |
| US5399676A (en) | 1989-10-23 | 1995-03-21 | Gilead Sciences | Oligonucleotides with inverted polarity |
| US5405938A (en) | 1989-12-20 | 1995-04-11 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5405939A (en) | 1987-10-22 | 1995-04-11 | Temple University Of The Commonwealth System Of Higher Education | 2',5'-phosphorothioate oligoadenylates and their covalent conjugates with polylysine |
| US5414077A (en) | 1990-02-20 | 1995-05-09 | Gilead Sciences | Non-nucleoside linkers for convenient attachment of labels to oligonucleotides using standard synthetic methods |
| US5432272A (en) | 1990-10-09 | 1995-07-11 | Benner; Steven A. | Method for incorporating into a DNA or RNA oligonucleotide using nucleotides bearing heterocyclic bases |
| US5434257A (en) | 1992-06-01 | 1995-07-18 | Gilead Sciences, Inc. | Binding compentent oligomers containing unsaturated 3',5' and 2',5' linkages |
| US5446137A (en) | 1993-12-09 | 1995-08-29 | Syntex (U.S.A.) Inc. | Oligonucleotides containing 4'-substituted nucleotides |
| US5451463A (en) | 1989-08-28 | 1995-09-19 | Clontech Laboratories, Inc. | Non-nucleoside 1,3-diol reagents for labeling synthetic oligonucleotides |
| US5455233A (en) | 1989-11-30 | 1995-10-03 | University Of North Carolina | Oligoribonucleoside and oligodeoxyribonucleoside boranophosphates |
| US5457187A (en) | 1993-12-08 | 1995-10-10 | Board Of Regents University Of Nebraska | Oligonucleotides containing 5-fluorouracil |
| US5459255A (en) | 1990-01-11 | 1995-10-17 | Isis Pharmaceuticals, Inc. | N-2 substituted purines |
| US5466677A (en) | 1993-03-06 | 1995-11-14 | Ciba-Geigy Corporation | Dinucleoside phosphinates and their pharmaceutical compositions |
| US5466786A (en) | 1989-10-24 | 1995-11-14 | Gilead Sciences | 2'modified nucleoside and nucleotide compounds |
| US5470967A (en) | 1990-04-10 | 1995-11-28 | The Dupont Merck Pharmaceutical Company | Oligonucleotide analogs with sulfamate linkages |
| US5476925A (en) | 1993-02-01 | 1995-12-19 | Northwestern University | Oligodeoxyribonucleotides including 3'-aminonucleoside-phosphoramidate linkages and terminal 3'-amino groups |
| US5484908A (en) | 1991-11-26 | 1996-01-16 | Gilead Sciences, Inc. | Oligonucleotides containing 5-propynyl pyrimidines |
| US5486603A (en) | 1990-01-08 | 1996-01-23 | Gilead Sciences, Inc. | Oligonucleotide having enhanced binding affinity |
| US5489677A (en) | 1990-07-27 | 1996-02-06 | Isis Pharmaceuticals, Inc. | Oligonucleoside linkages containing adjacent oxygen and nitrogen atoms |
| US5502177A (en) | 1993-09-17 | 1996-03-26 | Gilead Sciences, Inc. | Pyrimidine derivatives for labeled binding partners |
| US5506337A (en) | 1985-03-15 | 1996-04-09 | Antivirals Inc. | Morpholino-subunit combinatorial library and method |
| US5510475A (en) | 1990-11-08 | 1996-04-23 | Hybridon, Inc. | Oligonucleotide multiple reporter precursors |
| US5512667A (en) | 1990-08-28 | 1996-04-30 | Reed; Michael W. | Trifunctional intermediates for preparing 3'-tailed oligonucleotides |
| US5512439A (en) | 1988-11-21 | 1996-04-30 | Dynal As | Oligonucleotide-linked magnetic particles and uses thereof |
| US5514785A (en) | 1990-05-11 | 1996-05-07 | Becton Dickinson And Company | Solid supports for nucleic acid hybridization assays |
| US5519134A (en) | 1994-01-11 | 1996-05-21 | Isis Pharmaceuticals, Inc. | Pyrrolidine-containing monomers and oligomers |
| US5519126A (en) | 1988-03-25 | 1996-05-21 | University Of Virginia Alumni Patents Foundation | Oligonucleotide N-alkylphosphoramidates |
| US5521063A (en) | 1985-03-15 | 1996-05-28 | Antivirals Inc. | Polynucleotide reagent containing chiral subunits and methods of use |
| US5525465A (en) | 1987-10-28 | 1996-06-11 | Howard Florey Institute Of Experimental Physiology And Medicine | Oligonucleotide-polyamide conjugates and methods of production and applications of the same |
| US5525711A (en) | 1994-05-18 | 1996-06-11 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Pteridine nucleotide analogs as fluorescent DNA probes |
| US5539082A (en) | 1993-04-26 | 1996-07-23 | Nielsen; Peter E. | Peptide nucleic acids |
| US5541307A (en) | 1990-07-27 | 1996-07-30 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogs and solid phase synthesis thereof |
| US5545730A (en) | 1984-10-16 | 1996-08-13 | Chiron Corporation | Multifunctional nucleic acid monomer |
| US5550111A (en) | 1984-07-11 | 1996-08-27 | Temple University-Of The Commonwealth System Of Higher Education | Dual action 2',5'-oligoadenylate antiviral derivatives and uses thereof |
| US5552540A (en) | 1987-06-24 | 1996-09-03 | Howard Florey Institute Of Experimental Physiology And Medicine | Nucleoside derivatives |
| US5561225A (en) | 1990-09-19 | 1996-10-01 | Southern Research Institute | Polynucleotide analogs containing sulfonate and sulfonamide internucleoside linkages |
| US5563255A (en) | 1994-05-31 | 1996-10-08 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US5565552A (en) | 1992-01-21 | 1996-10-15 | Pharmacyclics, Inc. | Method of expanded porphyrin-oligonucleotide conjugate synthesis |
| US5567811A (en) | 1990-05-03 | 1996-10-22 | Amersham International Plc | Phosphoramidite derivatives, their preparation and the use thereof in the incorporation of reporter groups on synthetic oligonucleotides |
| US5571799A (en) | 1991-08-12 | 1996-11-05 | Basco, Ltd. | (2'-5') oligoadenylate analogues useful as inhibitors of host-v5.-graft response |
| US5574142A (en) | 1992-12-15 | 1996-11-12 | Microprobe Corporation | Peptide linkers for improved oligonucleotide delivery |
| US5576427A (en) | 1993-03-30 | 1996-11-19 | Sterling Winthrop, Inc. | Acyclic nucleoside analogs and oligonucleotide sequences containing them |
| US5578718A (en) | 1990-01-11 | 1996-11-26 | Isis Pharmaceuticals, Inc. | Thiol-derivatized nucleosides |
| US5580731A (en) | 1994-08-25 | 1996-12-03 | Chiron Corporation | N-4 modified pyrimidine deoxynucleotides and oligonucleotide probes synthesized therewith |
| US5585481A (en) | 1987-09-21 | 1996-12-17 | Gen-Probe Incorporated | Linking reagents for nucleotide probes |
| US5587361A (en) | 1991-10-15 | 1996-12-24 | Isis Pharmaceuticals, Inc. | Oligonucleotides having phosphorothioate linkages of high chiral purity |
| US5587371A (en) | 1992-01-21 | 1996-12-24 | Pharmacyclics, Inc. | Texaphyrin-oligonucleotide conjugates |
| US5591722A (en) | 1989-09-15 | 1997-01-07 | Southern Research Institute | 2'-deoxy-4'-thioribonucleosides and their antiviral activity |
| US5594121A (en) | 1991-11-07 | 1997-01-14 | Gilead Sciences, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified purines |
| US5596086A (en) | 1990-09-20 | 1997-01-21 | Gilead Sciences, Inc. | Modified internucleoside linkages having one nitrogen and two carbon atoms |
| US5595726A (en) | 1992-01-21 | 1997-01-21 | Pharmacyclics, Inc. | Chromophore probe for detection of nucleic acid |
| US5596091A (en) | 1994-03-18 | 1997-01-21 | The Regents Of The University Of California | Antisense oligonucleotides comprising 5-aminoalkyl pyrimidine nucleotides |
| US5597909A (en) | 1994-08-25 | 1997-01-28 | Chiron Corporation | Polynucleotide reagents containing modified deoxyribose moieties, and associated methods of synthesis and use |
| US5597696A (en) | 1994-07-18 | 1997-01-28 | Becton Dickinson And Company | Covalent cyanine dye oligonucleotide conjugates |
| US5599928A (en) | 1994-02-15 | 1997-02-04 | Pharmacyclics, Inc. | Texaphyrin compounds having improved functionalization |
| US5602240A (en) | 1990-07-27 | 1997-02-11 | Ciba Geigy Ag. | Backbone modified oligonucleotide analogs |
| US5608046A (en) | 1990-07-27 | 1997-03-04 | Isis Pharmaceuticals, Inc. | Conjugated 4'-desmethyl nucleoside analog compounds |
| US5610289A (en) | 1990-07-27 | 1997-03-11 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogues |
| US5610300A (en) | 1992-07-01 | 1997-03-11 | Ciba-Geigy Corporation | Carbocyclic nucleosides containing bicyclic rings, oligonucleotides therefrom, process for their preparation, their use and intermediates |
| US5614617A (en) | 1990-07-27 | 1997-03-25 | Isis Pharmaceuticals, Inc. | Nuclease resistant, pyrimidine modified oligonucleotides that detect and modulate gene expression |
| US5618704A (en) | 1990-07-27 | 1997-04-08 | Isis Pharmacueticals, Inc. | Backbone-modified oligonucleotide analogs and preparation thereof through radical coupling |
| US5623070A (en) | 1990-07-27 | 1997-04-22 | Isis Pharmaceuticals, Inc. | Heteroatomic oligonucleoside linkages |
| US5625050A (en) | 1994-03-31 | 1997-04-29 | Amgen Inc. | Modified oligonucleotides and intermediates useful in nucleic acid therapeutics |
| US5627053A (en) | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| US5633360A (en) | 1992-04-14 | 1997-05-27 | Gilead Sciences, Inc. | Oligonucleotide analogs capable of passive cell membrane permeation |
| US5639873A (en) | 1992-02-05 | 1997-06-17 | Centre National De La Recherche Scientifique (Cnrs) | Oligothionucleotides |
| US5646265A (en) | 1990-01-11 | 1997-07-08 | Isis Pharmceuticals, Inc. | Process for the preparation of 2'-O-alkyl purine phosphoramidites |
| US5656612A (en) | 1994-05-31 | 1997-08-12 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US5658873A (en) | 1993-04-10 | 1997-08-19 | Degussa Aktiengesellschaft | Coated sodium percarbonate particles, a process for their production and detergent, cleaning and bleaching compositions containing them |
| US5663312A (en) | 1993-03-31 | 1997-09-02 | Sanofi | Oligonucleotide dimers with amide linkages replacing phosphodiester linkages |
| US5670633A (en) | 1990-01-11 | 1997-09-23 | Isis Pharmaceuticals, Inc. | Sugar modified oligonucleotides that detect and modulate gene expression |
| US5677439A (en) | 1990-08-03 | 1997-10-14 | Sanofi | Oligonucleotide analogues containing phosphate diester linkage substitutes, compositions thereof, and precursor dinucleotide analogues |
| US5677437A (en) | 1990-07-27 | 1997-10-14 | Isis Pharmaceuticals, Inc. | Heteroatomic oligonucleoside linkages |
| US5681941A (en) | 1990-01-11 | 1997-10-28 | Isis Pharmaceuticals, Inc. | Substituted purines and oligonucleotide cross-linking |
| WO1997040028A1 (en) | 1996-04-23 | 1997-10-30 | Vertex Pharmaceuticals Incorporated | Urea derivatives as inhibitors of impdh enzyme |
| US5688941A (en) | 1990-07-27 | 1997-11-18 | Isis Pharmaceuticals, Inc. | Methods of making conjugated 4' desmethyl nucleoside analog compounds |
| US5714331A (en) | 1991-05-24 | 1998-02-03 | Buchardt, Deceased; Ole | Peptide nucleic acids having enhanced binding affinity, sequence specificity and solubility |
| US5719262A (en) | 1993-11-22 | 1998-02-17 | Buchardt, Deceased; Ole | Peptide nucleic acids having amino acid side chains |
| WO1998017679A1 (en) | 1996-10-18 | 1998-04-30 | Vertex Pharmaceuticals Incorporated | Inhibitors of serine proteases, particularly hepatitis c virus ns3 protease |
| WO1998022496A2 (en) | 1996-11-18 | 1998-05-28 | F. Hoffmann-La Roche Ag | Antiviral peptide derivatives |
| US5807876A (en) | 1996-04-23 | 1998-09-15 | Vertex Pharmaceuticals Incorporated | Inhibitors of IMPDH enzyme |
| WO1998040381A1 (en) | 1997-03-14 | 1998-09-17 | Vertex Pharmaceuticals Incorporated | Inhibitors of impdh enzyme |
| WO1999007734A2 (en) | 1997-08-11 | 1999-02-18 | Boehringer Ingelheim (Canada) Ltd. | Hepatitis c inhibitor peptide analogues |
| US5919773A (en) | 1994-05-31 | 1999-07-06 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US5952229A (en) | 1994-05-31 | 1999-09-14 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US6054472A (en) | 1996-04-23 | 2000-04-25 | Vertex Pharmaceuticals, Incorporated | Inhibitors of IMPDH enzyme |
| WO2002018369A2 (en) | 2000-08-31 | 2002-03-07 | Eli Lilly And Company | Peptidomimetic protease inhibitors |
| US6358932B1 (en) | 1994-05-31 | 2002-03-19 | Isis Pharmaceticals, Inc. | Antisense oligonucleotide inhibition of raf gene expression |
| US6410518B1 (en) | 1994-05-31 | 2002-06-25 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide inhibition of raf gene expression |
| US6451991B1 (en) * | 1996-02-14 | 2002-09-17 | Isis Pharmaceuticals, Inc. | Sugar-modified gapped oligonucleotides |
| US6498178B2 (en) | 1999-03-19 | 2002-12-24 | Vertex Pharmaceuticals Incorporated | Inhibitors of IMPDH enzyme |
| US6670461B1 (en) | 1997-09-12 | 2003-12-30 | Exiqon A/S | Oligonucleotide analogues |
| WO2005073216A2 (en) | 2004-01-30 | 2005-08-11 | Medivir Ab | Hcv ns-3 serine protease inhibitors |
| US6969766B2 (en) | 2002-04-26 | 2005-11-29 | Panagene, Inc. | PNA monomer and precursor |
| US7022851B2 (en) | 2002-01-24 | 2006-04-04 | Panagene, Inc. | PNA monomer and precursor |
| US7053207B2 (en) | 1999-05-04 | 2006-05-30 | Exiqon A/S | L-ribo-LNA analogues |
| US7060809B2 (en) | 2001-09-04 | 2006-06-13 | Exiqon A/S | LNA compositions and uses thereof |
| US7084125B2 (en) | 1999-03-18 | 2006-08-01 | Exiqon A/S | Xylo-LNA analogues |
| US7211668B2 (en) | 2003-07-28 | 2007-05-01 | Panagene, Inc. | PNA monomer and precursor |
| US7569575B2 (en) | 2002-05-08 | 2009-08-04 | Santaris Pharma A/S | Synthesis of locked nucleic acid derivatives |
| US7572582B2 (en) | 1997-09-12 | 2009-08-11 | Exiqon A/S | Oligonucleotide analogues |
| WO2009102427A2 (en) | 2008-02-11 | 2009-08-20 | Rxi Pharmaceuticals Corp. | Modified rnai polynucleotides and uses thereof |
| WO2010001134A2 (en) | 2008-07-02 | 2010-01-07 | Asterion Limited | Insulin fusion polypeptides |
| WO2010011346A1 (en) | 2008-07-24 | 2010-01-28 | Rxi Pharmaceuticals Corporation | Rnai constructs and uses therof |
| WO2010033246A1 (en) | 2008-09-22 | 2010-03-25 | Rxi Pharmaceuticals Corporation | Rna interference in skin indications |
-
2011
- 2011-03-07 WO PCT/US2011/027412 patent/WO2011112516A1/en active Application Filing
Patent Citations (189)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1280408A (en) | 1916-10-09 | 1918-10-01 | John Adolphus Cox | Tire-chain lock. |
| US3687808A (en) | 1969-08-14 | 1972-08-29 | Univ Leland Stanford Junior | Synthetic polynucleotides |
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| US4469863A (en) | 1980-11-12 | 1984-09-04 | Ts O Paul O P | Nonionic nucleic acid alkyl and aryl phosphonates and processes for manufacture and use thereof |
| US5023243A (en) | 1981-10-23 | 1991-06-11 | Molecular Biosystems, Inc. | Oligonucleotide therapeutic agent and method of making same |
| US4476301A (en) | 1982-04-29 | 1984-10-09 | Centre National De La Recherche Scientifique | Oligonucleotides, a process for preparing the same and their application as mediators of the action of interferon |
| US4789737A (en) | 1982-08-09 | 1988-12-06 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives and production thereof |
| US4667025A (en) | 1982-08-09 | 1987-05-19 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US4835263A (en) | 1983-01-27 | 1989-05-30 | Centre National De La Recherche Scientifique | Novel compounds containing an oligonucleotide sequence bonded to an intercalating agent, a process for their synthesis and their use |
| US4605735A (en) | 1983-02-14 | 1986-08-12 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US4948882A (en) | 1983-02-22 | 1990-08-14 | Syngene, Inc. | Single-stranded labelled oligonucleotides, reactive monomers and methods of synthesis |
| US5541313A (en) | 1983-02-22 | 1996-07-30 | Molecular Biosystems, Inc. | Single-stranded labelled oligonucleotides of preselected sequence |
| US4824941A (en) | 1983-03-10 | 1989-04-25 | Julian Gordon | Specific antibody to the native form of 2'5'-oligonucleotides, the method of preparation and the use as reagents in immunoassays or for binding 2'5'-oligonucleotides in biological systems |
| US4587044A (en) | 1983-09-01 | 1986-05-06 | The Johns Hopkins University | Linkage of proteins to nucleic acids |
| US5118800A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | Oligonucleotides possessing a primary amino group in the terminal nucleotide |
| US5118802A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | DNA-reporter conjugates linked via the 2' or 5'-primary amino group of the 5'-terminal nucleoside |
| US5550111A (en) | 1984-07-11 | 1996-08-27 | Temple University-Of The Commonwealth System Of Higher Education | Dual action 2',5'-oligoadenylate antiviral derivatives and uses thereof |
| US4981957A (en) | 1984-07-19 | 1991-01-01 | Centre National De La Recherche Scientifique | Oligonucleotides with modified phosphate and modified carbohydrate moieties at the respective chain termini |
| US5578717A (en) | 1984-10-16 | 1996-11-26 | Chiron Corporation | Nucleotides for introducing selectably cleavable and/or abasic sites into oligonucleotides |
| US5367066A (en) | 1984-10-16 | 1994-11-22 | Chiron Corporation | Oligonucleotides with selectably cleavable and/or abasic sites |
| US5552538A (en) | 1984-10-16 | 1996-09-03 | Chiron Corporation | Oligonucleotides with cleavable sites |
| US5545730A (en) | 1984-10-16 | 1996-08-13 | Chiron Corporation | Multifunctional nucleic acid monomer |
| US5258506A (en) | 1984-10-16 | 1993-11-02 | Chiron Corporation | Photolabile reagents for incorporation into oligonucleotide chains |
| US4828979A (en) | 1984-11-08 | 1989-05-09 | Life Technologies, Inc. | Nucleotide analogs for nucleic acid labeling and detection |
| US4845205A (en) | 1985-01-08 | 1989-07-04 | Institut Pasteur | 2,N6 -disubstituted and 2,N6 -trisubstituted adenosine-3'-phosphoramidites |
| US5506337A (en) | 1985-03-15 | 1996-04-09 | Antivirals Inc. | Morpholino-subunit combinatorial library and method |
| US5698685A (en) | 1985-03-15 | 1997-12-16 | Antivirals Inc. | Morpholino-subunit combinatorial library and method |
| US5235033A (en) | 1985-03-15 | 1993-08-10 | Anti-Gene Development Group | Alpha-morpholino ribonucleoside derivatives and polymers thereof |
| US5521063A (en) | 1985-03-15 | 1996-05-28 | Antivirals Inc. | Polynucleotide reagent containing chiral subunits and methods of use |
| US5142047A (en) | 1985-03-15 | 1992-08-25 | Anti-Gene Development Group | Uncharged polynucleotide-binding polymers |
| US5034506A (en) | 1985-03-15 | 1991-07-23 | Anti-Gene Development Group | Uncharged morpholino-based polymers having achiral intersubunit linkages |
| US5185444A (en) | 1985-03-15 | 1993-02-09 | Anti-Gene Deveopment Group | Uncharged morpolino-based polymers having phosphorous containing chiral intersubunit linkages |
| US5217866A (en) | 1985-03-15 | 1993-06-08 | Anti-Gene Development Group | Polynucleotide assay reagent and method |
| US4762779A (en) | 1985-06-13 | 1988-08-09 | Amgen Inc. | Compositions and methods for functionalizing nucleic acids |
| US5317098A (en) | 1986-03-17 | 1994-05-31 | Hiroaki Shizuya | Non-radioisotope tagging of fragments |
| US4876335A (en) | 1986-06-30 | 1989-10-24 | Wakunaga Seiyaku Kabushiki Kaisha | Poly-labelled oligonucleotide derivative |
| US5264423A (en) | 1987-03-25 | 1993-11-23 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US5286717A (en) | 1987-03-25 | 1994-02-15 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US5276019A (en) | 1987-03-25 | 1994-01-04 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US4904582A (en) | 1987-06-11 | 1990-02-27 | Synthetic Genetics | Novel amphiphilic nucleic acid conjugates |
| US5552540A (en) | 1987-06-24 | 1996-09-03 | Howard Florey Institute Of Experimental Physiology And Medicine | Nucleoside derivatives |
| US5585481A (en) | 1987-09-21 | 1996-12-17 | Gen-Probe Incorporated | Linking reagents for nucleotide probes |
| US5405939A (en) | 1987-10-22 | 1995-04-11 | Temple University Of The Commonwealth System Of Higher Education | 2',5'-phosphorothioate oligoadenylates and their covalent conjugates with polylysine |
| US5188897A (en) | 1987-10-22 | 1993-02-23 | Temple University Of The Commonwealth System Of Higher Education | Encapsulated 2',5'-phosphorothioate oligoadenylates |
| US5525465A (en) | 1987-10-28 | 1996-06-11 | Howard Florey Institute Of Experimental Physiology And Medicine | Oligonucleotide-polyamide conjugates and methods of production and applications of the same |
| US5112963A (en) | 1987-11-12 | 1992-05-12 | Max-Planck-Gesellschaft Zur Foerderung Der Wissenschaften E.V. | Modified oligonucleotides |
| US5082830A (en) | 1988-02-26 | 1992-01-21 | Enzo Biochem, Inc. | End labeled nucleotide probe |
| US5519126A (en) | 1988-03-25 | 1996-05-21 | University Of Virginia Alumni Patents Foundation | Oligonucleotide N-alkylphosphoramidates |
| US5453496A (en) | 1988-05-26 | 1995-09-26 | University Patents, Inc. | Polynucleotide phosphorodithioate |
| US5278302A (en) | 1988-05-26 | 1994-01-11 | University Patents, Inc. | Polynucleotide phosphorodithioates |
| US5109124A (en) | 1988-06-01 | 1992-04-28 | Biogen, Inc. | Nucleic acid probe linked to a label having a terminal cysteine |
| US5216141A (en) | 1988-06-06 | 1993-06-01 | Benner Steven A | Oligonucleotide analogs containing sulfur linkages |
| US5175273A (en) | 1988-07-01 | 1992-12-29 | Genentech, Inc. | Nucleic acid intercalating agents |
| US5262536A (en) | 1988-09-15 | 1993-11-16 | E. I. Du Pont De Nemours And Company | Reagents for the preparation of 5'-tagged oligonucleotides |
| US5512439A (en) | 1988-11-21 | 1996-04-30 | Dynal As | Oligonucleotide-linked magnetic particles and uses thereof |
| US5599923A (en) | 1989-03-06 | 1997-02-04 | Board Of Regents, University Of Tx | Texaphyrin metal complexes having improved functionalization |
| US5391723A (en) | 1989-05-31 | 1995-02-21 | Neorx Corporation | Oligonucleotide conjugates |
| US5416203A (en) | 1989-06-06 | 1995-05-16 | Northwestern University | Steroid modified oligonucleotides |
| US4958013A (en) | 1989-06-06 | 1990-09-18 | Northwestern University | Cholesteryl modified oligonucleotides |
| US5451463A (en) | 1989-08-28 | 1995-09-19 | Clontech Laboratories, Inc. | Non-nucleoside 1,3-diol reagents for labeling synthetic oligonucleotides |
| US5134066A (en) | 1989-08-29 | 1992-07-28 | Monsanto Company | Improved probes using nucleosides containing 3-dezauracil analogs |
| US5254469A (en) | 1989-09-12 | 1993-10-19 | Eastman Kodak Company | Oligonucleotide-enzyme conjugate that can be used as a probe in hybridization assays and polymerase chain reaction procedures |
| US5591722A (en) | 1989-09-15 | 1997-01-07 | Southern Research Institute | 2'-deoxy-4'-thioribonucleosides and their antiviral activity |
| US5399676A (en) | 1989-10-23 | 1995-03-21 | Gilead Sciences | Oligonucleotides with inverted polarity |
| US5264562A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences, Inc. | Oligonucleotide analogs with novel linkages |
| US5466786B1 (en) | 1989-10-24 | 1998-04-07 | Gilead Sciences | 2' Modified nucleoside and nucleotide compounds |
| US5264564A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences | Oligonucleotide analogs with novel linkages |
| US5466786A (en) | 1989-10-24 | 1995-11-14 | Gilead Sciences | 2'modified nucleoside and nucleotide compounds |
| US5292873A (en) | 1989-11-29 | 1994-03-08 | The Research Foundation Of State University Of New York | Nucleic acids labeled with naphthoquinone probe |
| US5455233A (en) | 1989-11-30 | 1995-10-03 | University Of North Carolina | Oligoribonucleoside and oligodeoxyribonucleoside boranophosphates |
| US5130302A (en) | 1989-12-20 | 1992-07-14 | Boron Bilogicals, Inc. | Boronated nucleoside, nucleotide and oligonucleotide compounds, compositions and methods for using same |
| US5405938A (en) | 1989-12-20 | 1995-04-11 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5486603A (en) | 1990-01-08 | 1996-01-23 | Gilead Sciences, Inc. | Oligonucleotide having enhanced binding affinity |
| US5646265A (en) | 1990-01-11 | 1997-07-08 | Isis Pharmceuticals, Inc. | Process for the preparation of 2'-O-alkyl purine phosphoramidites |
| US5578718A (en) | 1990-01-11 | 1996-11-26 | Isis Pharmaceuticals, Inc. | Thiol-derivatized nucleosides |
| US5587469A (en) | 1990-01-11 | 1996-12-24 | Isis Pharmaceuticals, Inc. | Oligonucleotides containing N-2 substituted purines |
| US5459255A (en) | 1990-01-11 | 1995-10-17 | Isis Pharmaceuticals, Inc. | N-2 substituted purines |
| US5670633A (en) | 1990-01-11 | 1997-09-23 | Isis Pharmaceuticals, Inc. | Sugar modified oligonucleotides that detect and modulate gene expression |
| US5681941A (en) | 1990-01-11 | 1997-10-28 | Isis Pharmaceuticals, Inc. | Substituted purines and oligonucleotide cross-linking |
| US5214136A (en) | 1990-02-20 | 1993-05-25 | Gilead Sciences, Inc. | Anthraquinone-derivatives oligonucleotides |
| US5414077A (en) | 1990-02-20 | 1995-05-09 | Gilead Sciences | Non-nucleoside linkers for convenient attachment of labels to oligonucleotides using standard synthetic methods |
| US5563253A (en) | 1990-03-08 | 1996-10-08 | Worcester Foundation For Biomedical Research | Linear aminoalkylphosphoramidate oligonucleotide derivatives |
| US5541306A (en) | 1990-03-08 | 1996-07-30 | Worcester Foundation For Biomedical Research | Aminoalkylphosphotriester oligonucleotide derivatives |
| US5536821A (en) | 1990-03-08 | 1996-07-16 | Worcester Foundation For Biomedical Research | Aminoalkylphosphorothioamidate oligonucleotide deratives |
| US5321131A (en) | 1990-03-08 | 1994-06-14 | Hybridon, Inc. | Site-specific functionalization of oligodeoxynucleotides for non-radioactive labelling |
| US5470967A (en) | 1990-04-10 | 1995-11-28 | The Dupont Merck Pharmaceutical Company | Oligonucleotide analogs with sulfamate linkages |
| US5567811A (en) | 1990-05-03 | 1996-10-22 | Amersham International Plc | Phosphoramidite derivatives, their preparation and the use thereof in the incorporation of reporter groups on synthetic oligonucleotides |
| US5514785A (en) | 1990-05-11 | 1996-05-07 | Becton Dickinson And Company | Solid supports for nucleic acid hybridization assays |
| US5541307A (en) | 1990-07-27 | 1996-07-30 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogs and solid phase synthesis thereof |
| US5218105A (en) | 1990-07-27 | 1993-06-08 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5138045A (en) | 1990-07-27 | 1992-08-11 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5608046A (en) | 1990-07-27 | 1997-03-04 | Isis Pharmaceuticals, Inc. | Conjugated 4'-desmethyl nucleoside analog compounds |
| US5610289A (en) | 1990-07-27 | 1997-03-11 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogues |
| US5614617A (en) | 1990-07-27 | 1997-03-25 | Isis Pharmaceuticals, Inc. | Nuclease resistant, pyrimidine modified oligonucleotides that detect and modulate gene expression |
| US5618704A (en) | 1990-07-27 | 1997-04-08 | Isis Pharmacueticals, Inc. | Backbone-modified oligonucleotide analogs and preparation thereof through radical coupling |
| US5489677A (en) | 1990-07-27 | 1996-02-06 | Isis Pharmaceuticals, Inc. | Oligonucleoside linkages containing adjacent oxygen and nitrogen atoms |
| US5623070A (en) | 1990-07-27 | 1997-04-22 | Isis Pharmaceuticals, Inc. | Heteroatomic oligonucleoside linkages |
| US5688941A (en) | 1990-07-27 | 1997-11-18 | Isis Pharmaceuticals, Inc. | Methods of making conjugated 4' desmethyl nucleoside analog compounds |
| US5602240A (en) | 1990-07-27 | 1997-02-11 | Ciba Geigy Ag. | Backbone modified oligonucleotide analogs |
| US5677437A (en) | 1990-07-27 | 1997-10-14 | Isis Pharmaceuticals, Inc. | Heteroatomic oligonucleoside linkages |
| US5245022A (en) | 1990-08-03 | 1993-09-14 | Sterling Drug, Inc. | Exonuclease resistant terminally substituted oligonucleotides |
| US5567810A (en) | 1990-08-03 | 1996-10-22 | Sterling Drug, Inc. | Nuclease resistant compounds |
| US5677439A (en) | 1990-08-03 | 1997-10-14 | Sanofi | Oligonucleotide analogues containing phosphate diester linkage substitutes, compositions thereof, and precursor dinucleotide analogues |
| US5177196A (en) | 1990-08-16 | 1993-01-05 | Microprobe Corporation | Oligo (α-arabinofuranosyl nucleotides) and α-arabinofuranosyl precursors thereof |
| US5512667A (en) | 1990-08-28 | 1996-04-30 | Reed; Michael W. | Trifunctional intermediates for preparing 3'-tailed oligonucleotides |
| US5214134A (en) | 1990-09-12 | 1993-05-25 | Sterling Winthrop Inc. | Process of linking nucleosides with a siloxane bridge |
| US5561225A (en) | 1990-09-19 | 1996-10-01 | Southern Research Institute | Polynucleotide analogs containing sulfonate and sulfonamide internucleoside linkages |
| US5596086A (en) | 1990-09-20 | 1997-01-21 | Gilead Sciences, Inc. | Modified internucleoside linkages having one nitrogen and two carbon atoms |
| US5432272A (en) | 1990-10-09 | 1995-07-11 | Benner; Steven A. | Method for incorporating into a DNA or RNA oligonucleotide using nucleotides bearing heterocyclic bases |
| US5510475A (en) | 1990-11-08 | 1996-04-23 | Hybridon, Inc. | Oligonucleotide multiple reporter precursors |
| US5714331A (en) | 1991-05-24 | 1998-02-03 | Buchardt, Deceased; Ole | Peptide nucleic acids having enhanced binding affinity, sequence specificity and solubility |
| US5371241A (en) | 1991-07-19 | 1994-12-06 | Pharmacia P-L Biochemicals Inc. | Fluorescein labelled phosphoramidites |
| US5571799A (en) | 1991-08-12 | 1996-11-05 | Basco, Ltd. | (2'-5') oligoadenylate analogues useful as inhibitors of host-v5.-graft response |
| US5587361A (en) | 1991-10-15 | 1996-12-24 | Isis Pharmaceuticals, Inc. | Oligonucleotides having phosphorothioate linkages of high chiral purity |
| US5319080A (en) | 1991-10-17 | 1994-06-07 | Ciba-Geigy Corporation | Bicyclic nucleosides, oligonucleotides, process for their preparation and intermediates |
| US5393878A (en) | 1991-10-17 | 1995-02-28 | Ciba-Geigy Corporation | Bicyclic nucleosides, oligonucleotides, process for their preparation and intermediates |
| US5594121A (en) | 1991-11-07 | 1997-01-14 | Gilead Sciences, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified purines |
| US5484908A (en) | 1991-11-26 | 1996-01-16 | Gilead Sciences, Inc. | Oligonucleotides containing 5-propynyl pyrimidines |
| US5359044A (en) | 1991-12-13 | 1994-10-25 | Isis Pharmaceuticals | Cyclobutyl oligonucleotide surrogates |
| US5587371A (en) | 1992-01-21 | 1996-12-24 | Pharmacyclics, Inc. | Texaphyrin-oligonucleotide conjugates |
| US5595726A (en) | 1992-01-21 | 1997-01-21 | Pharmacyclics, Inc. | Chromophore probe for detection of nucleic acid |
| US5565552A (en) | 1992-01-21 | 1996-10-15 | Pharmacyclics, Inc. | Method of expanded porphyrin-oligonucleotide conjugate synthesis |
| US5639873A (en) | 1992-02-05 | 1997-06-17 | Centre National De La Recherche Scientifique (Cnrs) | Oligothionucleotides |
| US5633360A (en) | 1992-04-14 | 1997-05-27 | Gilead Sciences, Inc. | Oligonucleotide analogs capable of passive cell membrane permeation |
| US5434257A (en) | 1992-06-01 | 1995-07-18 | Gilead Sciences, Inc. | Binding compentent oligomers containing unsaturated 3',5' and 2',5' linkages |
| US5700920A (en) | 1992-07-01 | 1997-12-23 | Novartis Corporation | Carbocyclic nucleosides containing bicyclic rings, oligonucleotides therefrom, process for their preparation, their use and intermediates |
| US5610300A (en) | 1992-07-01 | 1997-03-11 | Ciba-Geigy Corporation | Carbocyclic nucleosides containing bicyclic rings, oligonucleotides therefrom, process for their preparation, their use and intermediates |
| US5272250A (en) | 1992-07-10 | 1993-12-21 | Spielvogel Bernard F | Boronated phosphoramidate compounds |
| US5574142A (en) | 1992-12-15 | 1996-11-12 | Microprobe Corporation | Peptide linkers for improved oligonucleotide delivery |
| US5476925A (en) | 1993-02-01 | 1995-12-19 | Northwestern University | Oligodeoxyribonucleotides including 3'-aminonucleoside-phosphoramidate linkages and terminal 3'-amino groups |
| US5466677A (en) | 1993-03-06 | 1995-11-14 | Ciba-Geigy Corporation | Dinucleoside phosphinates and their pharmaceutical compositions |
| US5576427A (en) | 1993-03-30 | 1996-11-19 | Sterling Winthrop, Inc. | Acyclic nucleoside analogs and oligonucleotide sequences containing them |
| US5663312A (en) | 1993-03-31 | 1997-09-02 | Sanofi | Oligonucleotide dimers with amide linkages replacing phosphodiester linkages |
| US5658873A (en) | 1993-04-10 | 1997-08-19 | Degussa Aktiengesellschaft | Coated sodium percarbonate particles, a process for their production and detergent, cleaning and bleaching compositions containing them |
| US5539082A (en) | 1993-04-26 | 1996-07-23 | Nielsen; Peter E. | Peptide nucleic acids |
| US5502177A (en) | 1993-09-17 | 1996-03-26 | Gilead Sciences, Inc. | Pyrimidine derivatives for labeled binding partners |
| US5719262A (en) | 1993-11-22 | 1998-02-17 | Buchardt, Deceased; Ole | Peptide nucleic acids having amino acid side chains |
| US5457187A (en) | 1993-12-08 | 1995-10-10 | Board Of Regents University Of Nebraska | Oligonucleotides containing 5-fluorouracil |
| US5446137A (en) | 1993-12-09 | 1995-08-29 | Syntex (U.S.A.) Inc. | Oligonucleotides containing 4'-substituted nucleotides |
| US5446137B1 (en) | 1993-12-09 | 1998-10-06 | Behringwerke Ag | Oligonucleotides containing 4'-substituted nucleotides |
| US5519134A (en) | 1994-01-11 | 1996-05-21 | Isis Pharmaceuticals, Inc. | Pyrrolidine-containing monomers and oligomers |
| US5599928A (en) | 1994-02-15 | 1997-02-04 | Pharmacyclics, Inc. | Texaphyrin compounds having improved functionalization |
| US5596091A (en) | 1994-03-18 | 1997-01-21 | The Regents Of The University Of California | Antisense oligonucleotides comprising 5-aminoalkyl pyrimidine nucleotides |
| US5627053A (en) | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| US5625050A (en) | 1994-03-31 | 1997-04-29 | Amgen Inc. | Modified oligonucleotides and intermediates useful in nucleic acid therapeutics |
| US5525711A (en) | 1994-05-18 | 1996-06-11 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Pteridine nucleotide analogs as fluorescent DNA probes |
| US5563255A (en) | 1994-05-31 | 1996-10-08 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US6806258B2 (en) | 1994-05-31 | 2004-10-19 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US5919773A (en) | 1994-05-31 | 1999-07-06 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US5656612A (en) | 1994-05-31 | 1997-08-12 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US5952229A (en) | 1994-05-31 | 1999-09-14 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of raf gene expression |
| US6358932B1 (en) | 1994-05-31 | 2002-03-19 | Isis Pharmaceticals, Inc. | Antisense oligonucleotide inhibition of raf gene expression |
| US6410518B1 (en) | 1994-05-31 | 2002-06-25 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide inhibition of raf gene expression |
| US5597696A (en) | 1994-07-18 | 1997-01-28 | Becton Dickinson And Company | Covalent cyanine dye oligonucleotide conjugates |
| US5597909A (en) | 1994-08-25 | 1997-01-28 | Chiron Corporation | Polynucleotide reagents containing modified deoxyribose moieties, and associated methods of synthesis and use |
| US5591584A (en) | 1994-08-25 | 1997-01-07 | Chiron Corporation | N-4 modified pyrimidine deoxynucleotides and oligonucleotide probes synthesized therewith |
| US5580731A (en) | 1994-08-25 | 1996-12-03 | Chiron Corporation | N-4 modified pyrimidine deoxynucleotides and oligonucleotide probes synthesized therewith |
| US6451991B1 (en) * | 1996-02-14 | 2002-09-17 | Isis Pharmaceuticals, Inc. | Sugar-modified gapped oligonucleotides |
| US5807876A (en) | 1996-04-23 | 1998-09-15 | Vertex Pharmaceuticals Incorporated | Inhibitors of IMPDH enzyme |
| WO1997040028A1 (en) | 1996-04-23 | 1997-10-30 | Vertex Pharmaceuticals Incorporated | Urea derivatives as inhibitors of impdh enzyme |
| US6054472A (en) | 1996-04-23 | 2000-04-25 | Vertex Pharmaceuticals, Incorporated | Inhibitors of IMPDH enzyme |
| US6344465B1 (en) | 1996-04-23 | 2002-02-05 | Vertex Pharmaceuticals, Incorporated | Inhibitors of IMPDH enzyme |
| WO1998017679A1 (en) | 1996-10-18 | 1998-04-30 | Vertex Pharmaceuticals Incorporated | Inhibitors of serine proteases, particularly hepatitis c virus ns3 protease |
| WO1998022496A2 (en) | 1996-11-18 | 1998-05-28 | F. Hoffmann-La Roche Ag | Antiviral peptide derivatives |
| WO1998040381A1 (en) | 1997-03-14 | 1998-09-17 | Vertex Pharmaceuticals Incorporated | Inhibitors of impdh enzyme |
| WO1999007734A2 (en) | 1997-08-11 | 1999-02-18 | Boehringer Ingelheim (Canada) Ltd. | Hepatitis c inhibitor peptide analogues |
| US6670461B1 (en) | 1997-09-12 | 2003-12-30 | Exiqon A/S | Oligonucleotide analogues |
| US6794499B2 (en) | 1997-09-12 | 2004-09-21 | Exiqon A/S | Oligonucleotide analogues |
| US7572582B2 (en) | 1997-09-12 | 2009-08-11 | Exiqon A/S | Oligonucleotide analogues |
| US7034133B2 (en) | 1997-09-12 | 2006-04-25 | Exiqon A/S | Oligonucleotide analogues |
| US7084125B2 (en) | 1999-03-18 | 2006-08-01 | Exiqon A/S | Xylo-LNA analogues |
| US6498178B2 (en) | 1999-03-19 | 2002-12-24 | Vertex Pharmaceuticals Incorporated | Inhibitors of IMPDH enzyme |
| US7053207B2 (en) | 1999-05-04 | 2006-05-30 | Exiqon A/S | L-ribo-LNA analogues |
| WO2002018369A2 (en) | 2000-08-31 | 2002-03-07 | Eli Lilly And Company | Peptidomimetic protease inhibitors |
| US7060809B2 (en) | 2001-09-04 | 2006-06-13 | Exiqon A/S | LNA compositions and uses thereof |
| US7022851B2 (en) | 2002-01-24 | 2006-04-04 | Panagene, Inc. | PNA monomer and precursor |
| US6969766B2 (en) | 2002-04-26 | 2005-11-29 | Panagene, Inc. | PNA monomer and precursor |
| US7125994B2 (en) | 2002-04-26 | 2006-10-24 | Panagene, Inc. | PNA monomer and precursor |
| US7145006B2 (en) | 2002-04-26 | 2006-12-05 | Panagene, Inc. | PNA monomer and precursor |
| US7179896B2 (en) | 2002-04-26 | 2007-02-20 | Panagene, Inc. | Method of making PNA oligomers |
| US7569575B2 (en) | 2002-05-08 | 2009-08-04 | Santaris Pharma A/S | Synthesis of locked nucleic acid derivatives |
| US7211668B2 (en) | 2003-07-28 | 2007-05-01 | Panagene, Inc. | PNA monomer and precursor |
| WO2005073195A2 (en) | 2004-01-30 | 2005-08-11 | Medivir Ab | Hcv ns-3 serine protease inhibitors |
| WO2005073216A2 (en) | 2004-01-30 | 2005-08-11 | Medivir Ab | Hcv ns-3 serine protease inhibitors |
| WO2009102427A2 (en) | 2008-02-11 | 2009-08-20 | Rxi Pharmaceuticals Corp. | Modified rnai polynucleotides and uses thereof |
| WO2010001134A2 (en) | 2008-07-02 | 2010-01-07 | Asterion Limited | Insulin fusion polypeptides |
| WO2010011346A1 (en) | 2008-07-24 | 2010-01-28 | Rxi Pharmaceuticals Corporation | Rnai constructs and uses therof |
| WO2010033246A1 (en) | 2008-09-22 | 2010-03-25 | Rxi Pharmaceuticals Corporation | Rna interference in skin indications |
Non-Patent Citations (31)
| Title |
|---|
| "Antisense Research and Applications", 1993, CRC PRESS, pages: 276 - 278 |
| "Concise Encyclopedia Of Polymer Science And Engineering", 1990, JOHN WILEY & SONS, pages: 858 - 859 |
| "Remington's Pharmaceutical Sciences", 1980 |
| "Remington's Pharmaceutical Sciences", 1985, MACK PUBLISHING COMPANY |
| ALEXEI A. KOSHKIN, ET AL.: "LNA (Locked Nucleic Acids): Synthesis of the Adenine, Cytosine, Guanine, 5-Methylcytosine, Thymine and Uracil Bicyclonucleoside Monomers, Oligomerisation, and Unprecedented Nucleic Acid Recognition", TETRAHEDRON, vol. 54, 1998, pages 3607 - 3630, XP002268966, DOI: doi:10.1016/S0040-4020(98)00094-5 |
| ALTSCHUL ET AL., NUCLEIC ACIDS RES., vol. 25, no. 17, 1997, pages 3389 - 3402 |
| ANGEWANDTE CHEMIE, INTERNATIONAL EDITION, vol. 30, - 1991, pages 613 - 722 |
| BURCKSTUMMER T ET AL: "Raf-1 kinase associates with Hepatitis C virus NS5A and regulates viral replication", FEBS LETTERS, ELSEVIER, AMSTERDAM, NL, vol. 580, no. 2, 23 January 2006 (2006-01-23), pages 575 - 580, XP025170804, ISSN: 0014-5793, [retrieved on 20060123], DOI: DOI:10.1016/J.FEBSLET.2005.12.071 * |
| CROOKE ET AL., J. PHARMACOL. EXP. THER., vol. 277, 1996, pages 923 - 937 |
| HIMMELSBACH K ET AL: "New aspects of an anti-tumour drug: sorafenib efficiently inhibits HCV replication", GUT, BRITISH MEDICAL ASSOCIATION, LONDON, UK, vol. 58, no. 12, 1 December 2009 (2009-12-01), pages 1644 - 1653, XP008136282, ISSN: 0017-5749 * |
| KABANOVETAL., FEBS LETT., vol. 259, 1990, pages 327 - 330 |
| LETSINGER ET AL., PROC. NATL. ACAD. SCI. USA, vol. 86, 1989, pages 6553 - 6556 |
| MANOHARAN ET AL., ANN. N.Y. ACAD. SCI., vol. 660, 1992, pages 306 - 309 |
| MANOHARAN ET AL., BIOORG. MED. CHEM. LET., vol. 3, 1993, pages 2765 - 2770 |
| MANOHARAN ET AL., BIOORG. MED. CHEM., vol. 4, 1994, pages 1053 - 1059 |
| MANOHARAN ET AL., NUCLEOSIDES & NUCLEOTIDES, vol. 14, 1995, pages 969 - 973 |
| MANOHARAN ET AL., TETRAHEDRON LETT., vol. 36, 1995, pages 3651 - 3654 |
| MARTIN ET AL., HELV. CHIM. ACTA, vol. 78, 1995, pages 486 - 504 |
| MISHRA ET AL., BIOCHIM. BIOPHYS. ACTA, vol. 1264, 1995, pages 229 - 237 |
| NIELSEN P E; ET AL: "SEQUENCE-SELECTIVE RECOGNITION OF DNA BY STRAND DISPLACEMENT WITH ATHYMINE-SUBSTITUTED POLYAMIDE", SCIENCE, vol. 254, 1991, pages 1497 - 1500, XP000405940, DOI: doi:10.1126/science.1962210 |
| OBERHAUSER ET AL., NUCL. ACIDS RES., vol. 20, 1992, pages 533 - 538 |
| OBIKA ET AL.: "Synthesis of 2'-O,4'-C-Methyleneuridine and -cytidine. Novel Bicyclic Nucleosides Having a Fixed C a ,-endo Sugar Puckering", TETRAHEDRON LETTERS, vol. 38, no. 50, 1997, pages 8735 - 8738, XP004097164, DOI: doi:10.1016/S0040-4039(97)10322-7 |
| RANDALL G ET AL: "Cellular cofactors affecting hepatitis C virus infection and replication", PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES (PNAS), NATIONAL ACADEMY OF SCIENCE, US, vol. 104, no. 31, 31 July 2007 (2007-07-31), pages 12884 - 12889, XP002532652, ISSN: 0027-8424, DOI: DOI:10.1073/PNAS.0704894104 * |
| SAISON-BEHMOARAS ET AL., EMBO J., vol. 10, 1991, pages 1111 - 1118 |
| SALOMON, W. ET AL.: "Modified dsRNAs that are not processed by Dicer maintain potency and are incorporated into the RISC", NUCLEIC ACIDS RES., 2010, pages 1 - 9 |
| SANGHVI, Y. S.: "Antisense Research and Applications", 1993, CRC PRESS, pages: 289 - 302 |
| SANJAY K. SINGH, ET AL.: "LNA (locked nucleic acids): synthesis and high-affinity nucleic acid recognition", CHEMICAL COMMUNICATIONS, 1998, pages 455 - 456 |
| SATOSHI OBIKA, ET AL.: "Synthesis and properties of 30-amino- 20,40-BNA, a bridged nucleic acid with a N30-P50 phosphoramidate linkage", BIOORGANIC MEDICINAL CHEMISTRY, vol. 16, no. 20, 2008, pages 9230 - 9237 |
| SHEA ET AL., NUCL. ACIDS RES., vol. 18, 1990, pages 3777 - 3783 |
| SVINARCHUK ET AL., BIOCHIMIE, vol. 75, 1993, pages 49 - 54 |
| WENGEL J: "Synthesis of 3'-C- and 4'-C-Branched Oligodeoxynucleotides and the Development of Locked Nucleic Acid (LNA)", ACCOUNTS OF CHEM. RESEARCH, vol. 32, no. 4, 1999, pages 301 - 310 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013024158A1 (en) * | 2011-08-17 | 2013-02-21 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Combinations of protein kinase inhibitors and interferons or of protein kinase inhibitors and direct acting antivirals for the treatment and the prevention of hcv infection |
| EP2994140A4 (en) * | 2013-05-07 | 2017-05-03 | Inhibikase Therapeutics, Inc. | Methods for treating hcv infection |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7059242B2 (en) | Compounds and Methods for Regulating the Expression of Myotonic Dystrophy Protein Kinase (DMPK) | |
| EP1490490B1 (en) | Antisense iap nucleobase oligomers and uses thereof | |
| JP2020183379A (en) | Modulation of dystrophia myotonica-protein kinase (dmpk) expression | |
| JP7110485B2 (en) | Modulators of PNPLA3 expression | |
| CA3140917A1 (en) | Treatment of angiopoietin like 7 (angptl7) related diseases | |
| CN105452461A (en) | Modulators of growth hormone receptor | |
| US20230295629A1 (en) | Anti-c9orf72 oligonucleotides and related methods | |
| JP2023519246A (en) | RNAi agents for inhibiting expression of PNPLA3, pharmaceutical compositions thereof, and methods of use | |
| JP2021514657A (en) | Regulator of IRF4 expression | |
| JP2023075283A (en) | Modulators of APOL1 expression | |
| WO2011112516A1 (en) | Treating and preventing hepatitis c virus infection using c-raf kinase antisense oligonucleotides | |
| WO2007139943A2 (en) | Therapeutic drug combinations and delivery systems comprising c-raf kinase antisense polynucleotides for treating ocular diseases and disorders | |
| US20220282249A1 (en) | Compounds and methods useful for modulating gene splicing | |
| TW202329987A (en) | Composition and method for inhibiting expression of hepatitis b virus (HBV) protein | |
| WO2023193004A2 (en) | Methods for the treatment of neurodegenerative disorders | |
| TW202321452A (en) | Compositions and methods for inhibiting expression of angiopoietin-like 3 (angptl3) protein | |
| TW202346586A (en) | Compositions and methods for inhibiting expression of prekallikrein (PKK) protein | |
| TW202404615A (en) | Compositions and methods for inhibiting xanthine dehydrogenase (XDH) | |
| WO2011105901A2 (en) | Antagonists of complement component 9 (c9) and uses thereof | |
| US20080293653A1 (en) | Method of treating autoimmune diseases |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11708376 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11708376 Country of ref document: EP Kind code of ref document: A1 |