WO2010018652A1 - Agent for promoting neuronal differentiation and method therefor - Google Patents
Agent for promoting neuronal differentiation and method therefor Download PDFInfo
- Publication number
- WO2010018652A1 WO2010018652A1 PCT/JP2009/003195 JP2009003195W WO2010018652A1 WO 2010018652 A1 WO2010018652 A1 WO 2010018652A1 JP 2009003195 W JP2009003195 W JP 2009003195W WO 2010018652 A1 WO2010018652 A1 WO 2010018652A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- coup
- protein
- inhibitor
- tfi
- tfii
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70567—Nuclear receptors, e.g. retinoic acid receptor [RAR], RXR, nuclear orphan receptors
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K67/00—Rearing or breeding animals, not otherwise provided for; New or modified breeds of animals
- A01K67/027—New or modified breeds of vertebrates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/43504—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates
- C07K14/43563—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates from insects
- C07K14/43577—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates from insects from flies
- C07K14/43581—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates from insects from flies from Drosophila
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2207/00—Modified animals
- A01K2207/05—Animals modified by non-integrating nucleic acids, e.g. antisense, RNAi, morpholino, episomal vector, for non-therapeutic purpose
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2207/00—Modified animals
- A01K2207/30—Animals modified by surgical methods
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2227/00—Animals characterised by species
- A01K2227/10—Mammal
- A01K2227/105—Murine
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2267/00—Animals characterised by purpose
- A01K2267/03—Animal model, e.g. for test or diseases
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/80—Fusion polypeptide containing a DNA binding domain, e.g. Lacl or Tet-repressor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/50—Physical structure
- C12N2310/53—Physical structure partially self-complementary or closed
- C12N2310/531—Stem-loop; Hairpin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2330/00—Production
- C12N2330/50—Biochemical production, i.e. in a transformed host cell
- C12N2330/51—Specially adapted vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2799/00—Uses of viruses
- C12N2799/02—Uses of viruses as vector
- C12N2799/021—Uses of viruses as vector for the expression of a heterologous nucleic acid
- C12N2799/027—Uses of viruses as vector for the expression of a heterologous nucleic acid where the vector is derived from a retrovirus
Definitions
- the present invention relates to agents for promoting neuronal differentiation of neural stem/progenitor cells, and methods therefor.
- NCPCs Neural stem/progenitor cells
- ES cells embryonic stem cells
- ES cells embryonic stem cells
- the NCPCs are capable of differentiating into both neurons and glial cells, and methods for inducing the differentiation into either neurons or glia have been developed.
- IL-6 family cytokine Koblar, S.A., et al. Proc.Natl.Acad.Sci.U.S.A. vol.95, pp.3178-3181 (1998)
- BMP2/4 Gross, R.E., et al. Neuron vol.17, pp.595-606 (1996)
- the present invention is intended to provide agents for promoting neuronal differentiation of NSPCs, and methods therefor.
- an agent for promoting neuronal differentiation of an NSPC includes an inhibitor of function of a COUP-TFI protein and/or a COUP-TFII protein.
- the inhibitor may inhibit expression(s) of (a) gene(s) encoding a COUP-TFI protein and/or a COUP-TFII protein.
- the inhibitor may be a nucleic acid capable of inhibiting expression of the gene.
- the nucleic acid may be an shRNA or an siRNA.
- the inhibitor may be a competitive inhibition mutant of a COUP-TFI protein or a COUP-TFII protein, and the competitive inhibition mutant may include a transcription activation domain and a DNA-binding domain of a COUP-TFI protein or a COUP-TFII protein.
- the NSPC may be derived from an embryonic stem cell or an induced pluripotent stem cell, and the NSPC may form a primary neurosphere.
- the neuron that differentiates by any agent described above may be an Isl-1-positive neuron, a DARPP-32-positive neuron or a Tbr1-positive neuron.
- the differentiated neuron may be a cholinergic neuron, a GABAergic neuron and a glutamatergic neuron.
- a method for promoting neuronal differentiation of an NSPC includes inhibiting function of a COUP-TFI protein and/or a COUP-TFII protein in the NSPC.
- the method may include inhibiting expression(s) of (a) gene(s) encoding a COUP-TFI protein and/or a COUP-TFII protein in the NSPC.
- a nucleic acid molecule capable of inhibiting expression of the gene may be introduced into the NSPC, and the nucleic acid molecule may be an shRNA or an siRNA.
- a competitive inhibition mutant of a COUP-TFI protein or a COUP-TFII protein may be introduced into the NSPC.
- the competitive inhibition mutant may include a transcription activation domain and a DNA-binding domain of a COUP-TFI protein or a COUP-TFII protein.
- the NSPC may be derived from an embryonic stem cell or an induced pluripotent stem cell, and the NSPC may form a primary neurosphere.
- the neuron that differentiates by any method described above may be an Isl-1-positive neuron, a DARPP-32-positive neuron and a Tbr1-positive neuron.
- the differentiated neuron may be a cholinergic neuron, a GABAergic neuron and a glutamatergic neuron.
- a pharmaceutical composition in another embodiment, includes an inhibitor of function of a COUP-TFI protein and/or a COUP-TFII protein.
- the inhibitor may inhibit expression(s) of (a) gene(s) encoding a COUP-TFI protein and/or a COUP-TFII protein.
- the inhibitor may be a nucleic acid capable of inhibiting expression of the gene.
- the nucleic acid may be an shRNA or an siRNA.
- the inhibitor may be a competitive inhibition mutant of a COUP-TFI protein or a COUP-TFII protein, and the competitive inhibition mutant may include a transcription activation domain and a DNA-binding domain of a COUP-TFI protein or a COUP-TFII protein.
- the inhibitor may inhibit the function of a COUP-TFI protein and/or a COUP-TFII protein in an NSPC.
- the NSPC may be derived from an embryonic stem cell or an induced pluripotent stem cell, and the NSPC may form a primary neurosphere.
- the neuron that differentiates by any pharmaceutical composition described above may be an Isl-1-positive neuron, a DARPP-32-positive neuron and a Tbr1-positive neuron.
- the differentiated neuron may be a cholinergic neuron, a GABAergic neuron and a glutamatergic neuron.
- This pharmaceutical composition may be administered to a patient with brain ischemia, traumatic brain injury, Huntington's disease and Alzheimer's disease.
- a method for treating a neurological disorder includes inhibiting function of a COUP-TFI protein and/or a COUP-TFII protein in a NSPC.
- the method may include inhibiting expression(s) of (a) gene(s) encoding a COUP-TFI protein and/or a COUP-TFII protein in the NSPC.
- a nucleic acid molecule capable of inhibiting expression of the gene may be introduced into the NSPC, and the nucleic acid molecule may be an shRNA or an siRNA.
- a competitive inhibition mutant of a COUP-TFI protein or a COUP-TFII protein may be introduced into the NSPC.
- the competitive inhibition mutant may include a transcription activation domain and a DNA-binding domain of a COUP-TFI protein or a COUP-TFII protein.
- the NSPC may be derived from an embryonic stem cell or an induced pluripotent stem cell, and the NSPC may form a primary neurosphere.
- the neuron that differentiates by any method described above may be an Isl-1-positive neuron, a DARPP-32-positive neuron and a Tbr1-positive neuron.
- the differentiated neuron may be a cholinergic neuron, a GABAergic neuron and a glutamatergic neuron.
- the neurological disorder may be brain ischemia, traumatic brain injury, Huntington's disease and Alzheimer's disease.
- Fig.1 is a graph showing the inhibition of the expression of the COUP-TF protein by knockdown of the Coup-tfI gene and/or Coup-tfII gene in an example according to the present invention.
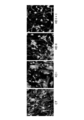
- Fig.2 is photographs showing the morphology of NSPCs in which the Coup-tfI gene and/or the Coup-tfII gene has been knocked down in an example according to the present invention.
- Fig.3 is a graph showing the ratios of neurons, astrocytes and oligodendrocytes which appeared when differentiation of the NSPCs in which the Coup-tfI gene and/or the Coup-tfII gene had been knocked down was induced in an example according to the present invention.
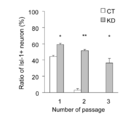
- Fig.4 is a graph showing the ratio of Isl-1 positive neurons that appeared when the NSPCs in which the Coup-tfI gene and the Coup-tfII gene had been knocked down were passaged and their differentiation was induced in an example according to the present invention.
- Fig.5 is a graph showing the ratios of neurons, oligodendrocyte progenitor cells (OPCs) and other cells (Others) which differentiated from the brain cells, into which shRNA KDI+II had been introduced at E10.5, in the mouse brain at E16.5 in an example according to the present invention.
- OPCs oligodendrocyte progenitor cells
- Others other cells
- Fig.6 is a graph showing the ratios of neurons, astrocytes and other cells (Others) which differentiated from the brain cells, into which shRNA KDI+II had been introduced at E12.5, in the mouse brain at P20 in an Example according to the present invention.
- Fig.7 is graphs showing the ratios of Isl-1 positive neurons, DARPP-32 positive neurons and Tbr1 positive neurons, which appeared when the Coup-tfI gene and the Coup-tfII gene had been knocked down in vivo in an example according to the present invention.
- Fig.8 is a schematic diagram showing the structures of the fusion proteins to be used in the experiment of the competitive inhibition of the DNA binding function of the COUP-TF protein in an example according to the present invention.
- Fig.9 is a graph showing the ratios of neurons that appeared when the NSPCs, in which the DNA binding function of the COUP-TF protein was competitively inhibited, were induced to differentiate in an example according to the present invention.
- Embodiments of the present invention accomplished based on the above-described findings are hereinafter described in detail by giving Examples. Where there is no particular explanations in embodiments or Examples, methods described in standard sets of protocols such as J. Sambrook, E. F. Fritsch & T. Maniatis (Ed.) Molecular cloning, a laboratory manual (3rd edition) Cold Spring Harbor Press, Cold Spring Harbor, New York (2001); F. M. Ausubel, R. Brent, R. E. Scientific, D. D. Moore, J.G. Seidman, J. A. Smith, K. Struhl (Ed.) Current Protocols in Molecular Biology, John Wiley & Sons Ltd., or their modified/changed methods are used. When using a commercial reagent kit and/or a measuring apparatus, protocols attached to them are used unless otherwise explained.
- NSPCs Neuronal stem/progenitor cells
- the agent according to the present invention is used for promoting neuronal differentiation of NSPCs and contains an inhibitor of function of COUP-TFI protein and/or COUP-TFII protein.
- promotion of neuronal differentiation of NSPCs means a phenomenon in which the ratio of glia decreased and the ratio of neurons increased in differentiation of the NSPCs by administering the agent for promoting neuronal differentiation.
- the NSPCs may be either cells present in vivo or in vitro.
- the agent for promoting neuronal differentiation may be administered to an individual animal to promote neuronal differentiation of the NSPCs in vivo.
- the NSPCs in culture may be prepared by isolating and culturing the NSPCs in vivo. Alternatively, they may be prepared by differentiating embryonic stem cells (ES cells) or induced pluripotent stem cells (iPS cells) into the NSPCs.
- ES cells embryonic stem cells
- iPS cells induced pluripotent stem cells
- the method for inducing differentiation of ES cells is disclosed in Japanese Patent Application Laid-open Publication No.2002-291469 in detail, which is herein incorporated by reference. This method can be applied to iPS cells as well.
- ES cells or iPS cells to form embryoid bodies (EBs) in advance, differentiate them into the NSPCs and then administer the agent to the NSPCs under the culturing condition.
- the formation of the EBs can be achieved by, for example, culturing ES cells or iPS cells in the presence of retinoic acid or Noggin protein.
- the retinoic acid may be added at a low level (10 -9 M to 10 -6 M) to the medium.
- the Xenopus noggin gene may be introduced into cultured mammalian cells to express the Noggin protein transiently and then the culture supernatant may be recovered and used as it is (1 to 50% (v/v)), or alternatively, recombinant Noggin protein (final concentration of approx. 1 ug/ml) may be used.
- the NSPCs can differentiate and grow in a form of neurospheres.
- FGF-2 10 to 100 ng/ml
- sonic hedgehog protein 1 to 20 nM
- the addition of sonic hedgehog protein can improve the efficiency of the induction of differentiation from the NSPCs into motoneuron precursor cells as well as the efficiency of growth, and indeed a subsequent culture allows the cells to differentiate into motoneurons as well as into GABAergic neurons.
- the serum-free medium it is preferable to use DMEM medium supplemented with glucose, glutamine, insulin, transferrin, progesterone, putrescine, selenium chloride, heparin and the like in addition to the above-mentioned supplements, and more preferable to use DMEM:F12 medium.
- the cells are preferably cultured under the condition of 5% CO 2 at 35 to 40 O C for 7 to 9 days.
- the neurospheres directly derived from EBs are referred to as primary neurospheres.
- the primary neurospheres can be dissociated and cultured under the same condition to form the neurospheres again, which are referred to as secondary neurospheres.
- neurospheres that underwent at least one passage under culturing condition are referred to as secondary neurospheres.
- cholinergic neurons including motoneurons and GABAergic neurons are induced to differentiate.
- the medium for inducing differentiation it is preferable to use the DMEM:F12 medium supplemented with glucose, glutamine, insulin, transferrin, progesterone, putrescine and selenium chloride, which corresponds to the medium for growing neural stem cells from which FGF and heparin are omitted.
- Sonic hedgehog protein may or may not be added in the medium. It is preferable that the cells are cultured under the condition of 5% CO 2 at 35 to 40 O C for 5 to 7 days. If secondary neurospheres are cultured under the same condition, they differentiate not only into neurons that are mainly cholinergic neurons including motoneurons and GABAergic neurons but also into glial cells.
- COUP-TF protein (Coup-tfI and Coup-tfII are herein collectively referred to as Coup-tf in either case of a gene or a protein) may be derived from a vertebrate such as human and mouse. Although it has preferably the same origin as the NSPCs to be targeted, their origins may be different as long as the inhibitor can inhibit the function of the COUP-TF protein in the cell to be targeted.
- the inhibitor may inhibit the function of the COUP-TF protein in any mechanism as long as the inhibition is achieved; it may reduce the activity of the COUP-TF protein directly, or it may inhibit the expression of the COUP-TF protein in a cell.
- the inhibitor to be used is not particularly limited as long as it is a substance capable of inhibiting the function of the COUP-TF protein; it may be a low molecular compound, or a high molecular compound such as a nucleic acid or a protein.
- inhibitor nucleic acid examples include siRNAs, shRNAs and antisense RNAs capable of inhibiting the expression of the gene encoding the COUP-TF protein. It may be designed in any technique known to those skilled in the art, and its sequence, type of the nucleic acid (for example, it may include DNA or inosine), and modification are not particularly limited as long as it can function as an inhibitor. Further, it may inhibit the expression of the COUP-TF protein at either transcription level or translation level.
- the inhibitor protein examples include a mutant COUP-TF protein that inhibits function of the COUP-TF wild-type protein competitively.
- the COUP-TF protein is a transcription regulator, forced expression of a recombinant mutant COUP-TF protein in cells, which has its DNA-binding domain and lacks its transcription inhibition domain, will inhibit binding of the endogenous COUP-TF protein to DNA competitively, resulting in inhibiting the function of the COUP-TF protein.
- a DNA-binding domain of mouse COUP-TFI protein is the region at position 86 to 149 of the amino acid sequence of SEQ ID NO.1
- that of COUP-TFII protein is the region at position 80 to 144 of the amino acid sequence of SEQ ID NO.2; these regions may be used to construct a competitive inhibition mutant.
- this competitive inhibition mutant may include a transcription activation domain.
- the transcription activation domain is not limited as long as it functions as an independent activation domain when bound with a DNA-binding domain of a heterologous protein, and any of known activation domains such as GAL4, Bicoid, c-Fos, B42 and VP16 may be used.
- the inhibitor of the function of the COUP-TF protein may be formulated as a neuronal differentiation promoting agent using any of pharmaceutically acceptable carrier, diluent, or excipient known to those skilled in the art.
- This agent may be used as an experimental reagent in vitro or a medical drug in vivo.
- the agent inhibits neuronal differentiation of the NSPCs in various ways: if the inhibitor is a small molecule, it may be added to culture medium; if it is a nucleic acid, it may be used for transfection; and if it is a protein, an expression vector to express a gene encoding the protein can be transfected, or it can be made as a PTD fusion protein by using TAT domain etc., which is added to culture medium etc.
- the inhibitor is preferably introduced into the cells at the stage of the primary neurosphere.
- the disease to be targeted by the drug is not particularly limited as long as it is a defect requiring promotion of neuronal differentiation of the NSPCs for treatment in a human or non-human vertebrate, and examples of such defect include neurologic diseases and nerve injuries in central nervous system such as a cerebral infarction and a traumatic brain injury, brain ischemia, , Huntington's disease and Alzheimer's disease as well as functional disorder by aging.
- the administration method and the dosage amount of the neuronal differentiation promoting agent for a patient can be appropriately chosen by those skilled in the art based on the administration purpose, the dosage form, the state of the patient etc.
- the inhibitor may be used solely by itself or in a combination of multiple forms.
- an inhibitor for COUP-TFI protein and an inhibitor for COUP-TFII protein may be used in a combination, or an inhibitor nucleic acid and an inhibitor protein for COUP-TF protein may be used in a combination.
- an inhibitor which inhibits the functions of both the COUP-TFI protein and the COUP-TFII protein may be used.
- Such inhibitor may be designed by utilizing a conserved nucleotide sequence between the COUP-TF genes.
- Example 1 ⁇ Knockdown of Coup-tf gene in NSPCs>
- a Coup-tf gene is knocked down in NSPCs, the cells preferentially differentiate into neurons when they are placed in a differentiation condition.
- EB3 cells which were obtained by inserting a Blasticidin-resistance gene at Oct3/4 locus of an ES cell line E14tg2a derived from a mouse strain 129/Ola to enable selection of undifferentiated ES cells, were cultured using Glasgow minimum essential medium (GMEM) supplemented with 10% FCS, non-essential amino acids, 1 mM sodium pyruvate, 0.1 mM 2-mercaptoehtanol and 1000 U/mL leukemia inhibitory factor (LIF) under the condition of 5% CO 2 , 37 O C.
- GMEM Glasgow minimum essential medium
- FCS non-essential amino acids
- LIF leukemia inhibitory factor
- the ES cells were washed with PBS, dissociated by 0.25% trypsin-1mM EDTA treatment, seeded in bacterial culture dishes at a concentration of 1 x 10 5 cell/mL, and cultured in suspension for 4 to 8 days to allow formation of EBs using the culture supernatant as medium, which was obtained by transiently expressing the Xenopus Noggin gene in COS7 cells.
- NSPCs Isolation of NSPCs from EBs by selective culture
- the EBs thus formed were treated with 0.25% trypsin-1mM EDTA solution and were dispersed into cells.
- the dispersed cells were seeded in bacterial culture dishes at a concentration of 5 x 10 4 cells/mL in MHM medium, which is DMEM:F12(1:1) supplemented with glucose (0.6%), glutamate (2mM), insulin (25ug/mL), transferrin (100ug/mL), progesterone (20nM), putrescine (60uM), selenium chloride (30nM), FGF-2 (20ng/mL) and heparin (2ug/mL) and further added with mouse sonic hedgehog1 protein (5nM), and cultured in suspension for 7 to 9 days to allow formation of primary neurospheres.
- MHM medium which is DMEM:F12(1:1) supplemented with glucose (0.6%), glutamate (2mM), insulin (25u
- KDI for Coup-tfI
- GATGCTGCCCACATCGAAATTCAAGAGATTTCGATGTGGGCAGCATC SEQ ID NO.3
- KDII for Coup-tfII KD
- GTCCCAGTGTGCTTTGGAATTCAAGAGATTGGAAAGCACACTGGGAC SEQ ID NO.4
- KDI+II for Coup-tf KD
- GTCGAGCGGCAAGCACTACTTCAAGAGAGTAGTGCTTGCCGCTCGAC SEQ ID NO.5
- 2.1-U6 ACTACCGTTGTTATAGGTGTTCAAGAGACACCTATAACAACGGTAGT (SEQ ID NO.6)
- shRNAs under the control of histone H1 promoter, their complementary sequences were synthesized and their double strand DNAs were formed by annealing, each of which was inserted between BglII site and XbaI site of an Entry Vector for shRNA pENTR4-H1.
- Each of the inserted shRNAs and histone H1 promoter were recombined into pCS-RfA-EG, a lentivirus vector for expressing shRNA, by using Gateway system (Invitrogen).
- the recombinant lentivirus vectors were introduced into 293T cells along with pCMV-VSV-G-RSV-rev and pCAG-HIVgp by using FuGENE6 (Roche), and the resulting culture supernatants were recovered, from which recombinant lentiviruses were purified at high concentration by ultracentrifugation. It should be noted that the lentivirus vectors contain the GFP gene to be expressed constitutively in order to enable to identify infected cells.
- the recovered lentiviruses were infected to the neurospheres at MOI25. Infected neurospheres were dispersed into single cells, and cultured in GMEM for 7 days to form secondary neurospheres. The neurospheres were subsequently passaged every 6 days.
- the lentivirus vector expressing respective shRNA was introduced to Coup-tf expressing 293T cells to which the Coup-tf genes had been stably introduced to express both of the COUP-TF proteins and Western blotting was conducted on cell extracts of the shRNA-expressing cells.
- anti-COUP-TFI antibody mouse IgG, RRMX PP-H8132-00, 100-fold dilution
- anti-COUP-TFII antibody mouse IgG, RRMX PP-H7147-00, 100-fold dilution
- each shRNA of KDI, KDII and KDI+II was capable of inhibiting the expression of the COUP-TFI protein, the COUP-TFII protein and both of them, respectively.
- the cells were immunostained with anti-beta III tubulin antibody used as a marker for neurons (mouse IgG, Sigma T8660, 1000-fold dilution), anti-GFAP antibody as a marker for astrocytes (rabbit IgG, DAKO Z0334, 400-fold dilution) and anti-O4 antibody as a marker for oligodendrocytes (mouse IgM, Chemicon MAB345, 8000-fold dilution) to observe their cellular morphology and the stained cell types by using fluorescent microscopy (Fig.2). In each sample, cell numbers of neurons, astrocytes and oligodendrocytes were counted and ratios of each cell type were plotted on a graph (Fig.3).
- the cells have come to differentiate into neurons preferentially when placed in the differentiating condition.
- Isl-1 a marker for early differentiating neurons in forebrain, hindbrain and spinal cord
- the cells were induced to differentiate and stained with anti-Isl-1 antibody (mouse IgG, DSHB 40.2D6, 200-fold dilution) to count the cell numbers of each cell type. Then the ratios of Isl-1 positive neurons against the number of total virus-infected neurons were calculated and plotted on a graph as shown in Fig.4.
- CT untreated cells
- the expression of Isl-1 have been almost lost by the third passage, whereas in the cells to which shRNA KDI+II were introduced, the expression of Isl-1 was maintained at almost the same level even after the third passage.
- the change of the neuronal type due to the induction of differentiation can be suppressed by the inhibition of the function of the COUP-TF protein.
- Example 2 ⁇ Knockdown of the Coup-tf gene in living mice> In this Example, it is shown that during differentiation of NSPCs in a living mouse, knockdown of the Coup-tf gene induces neuronal differentiation.
- Example 2 Mice and viral infection
- the shRNA-containing lentivirus as described in Example 1 was microinjected into cerebral ventricles of mouse embryos in uteri of ICR mice at 10.5 days or 12.5 days after fertilization.
- VS40 and Vevo660 VisualSonics
- anti-NeuN antibody was used as a marker for neurons; anti-Sox antibody and anti-Olig2 antibody were used as markers for oligodendrocyte precursor cells (OPCs), and double-positive cells were accounted as OPCs.
- OPCs oligodendrocyte precursor cells
- Non-neurons other than OPCs were classified as "Others (other cells)".
- the ratio of the OPCs in the cells to which shRNA KDI+II had been introduced decreased from 11% to 3.4% and the ratio of neurons increased from 73.8% to 80.4%, as compared with the negative control (CT) to which shRNA 2.1-U6 had been introduced.
- the anti-NeuN antibody was used as a marker for neurons
- the anti-GFAP antibody was used as a marker for astrocytes
- non-neurons which were negative for the anti-GFAP antibody were classified as "Others”.
- the ratio of astrocytes in the cells to which shRNA KDI+II had been introduced decreased from 12.2% to 1.3% and the ratio of neurons increased from 42.2% to 86.5%, as compared with the negative control (CT) to which shRNA 2.1-U6 had been introduced.
- Isl-1 positive neurons and DARPP-32 positive neurons increased in striatum from 29.5 % to 56.4 % and from 11.5% to 27.7% as compared with the negative control (CT), respectively.
- CT negative control
- the result of the double-staining with anti-BrdU antibody indicates that about half of the Isl-1 positive neurons and about one-third of the DARPP-32 positive neurons were born around E15.5.
- Tbr1 positive neurons also increased in cortex from 17.0% to 52.6% and about half of the Tbr1 positive neurons were born around E15.5 although they are normally barely generated after E15.5.
- Brn2 positive neurons decreased from 48.1% to 19.2% in cortex, which might be sacrificed to the increase of Tbr1 positive neurons.
- Isl-1 positive neurons in striatum are common precursors of cholinergic and GABAergic neurons and differentiated cholinergic neurons including motoneurons
- the DARPP-32 positive neurons in striatum are GABAergic neurons
- the Tbr1 positive neurons in cortex are glutamatergic neurons. This differentiation pattern is consistent with the fact that neurospheres are induced to differentiate mainly into cholinergic neurons including motoneurons and GABAergic neurons in a general differentiation medium in vitro.
- the neurons that have differentiated by inhibiting the function of the COUP-TF protein in the NSPCs are at least partly Isl-1-positive, DARPP-32-positive or Tbr1-positive neurons, which should be cholinergic neurons, GABAergic neurons or glutamatergic neurons.
- Example 3 Comparative inhibition of DNA binding function of the COUP-TF protein in NSPCs>
- neuronal differentiation can be promoted by competitive inhibition of the DNA binding function of the COUP-TF protein in NSPCs.
- fusion proteins As for the fusion proteins to be used in this Example, either one of the DNA-binding domain at the N-terminal of the COUP-TFI protein (the amino acid sequence at position 2 to 403 of SEQ ID NO.1) or the DNA-binding domain at the N-terminal of the COUP-TFII protein (the amino acid sequence at position 2 to 394 of SEQ ID NO.2) was fused with either one of the transcription activation domain of VP16 (the amino acid sequence at position 413 to 490 of SEQ ID NO.7 ⁇ P06492>) or the transcription repression domain of Drosophila Engrailed (En) protein (the amino acid sequence at position 2 to 295 of SEQ ID NO.8 ⁇ NM 078976>) to construct either a dominant positive mutant (hereinafter referred to as VP-1 or VP-II) or a dominant negative mutant (hereinafter referred to as EnI or EnII) and used as respective mixtures (the mixed proteins are hereinafter referred to as VP-I
- COUP-TFI/II a mixture of wild-type COUP-TFI and COUP-TFII proteins as well as F-I/II (a mixture of wild-type COUP-TFI and wild-type COUP-TFII, both of which are bound with a flag-tag (DYKDDDDK: SEQ ID NO.9)) were used.
- VP-1, VP-II, EnI and EnII have a structure represented as Met-FLAG-GlySer-VP/En-LysLeuArgSer-COUP
- F-I and F-II have a structure represented by Met-FLAG-GlySer-COUP
- VP/EN indicates either one of VP-1, VP-II, EnI or EnII
- COUP indicates the DNA-binding domain of COUP-TFI or COUP-TFII
- Recombinant DNAs encoding these fusion proteins were inserted instead of the shRNA in Example 1 into the lentivirus vector, and recombinant lentiviruses were produced by using 293T cells.
- Example 2 Expression and analyses of the fusion proteins in NSPCs Like Example 1, by using the NSPCs derived from ES cells, primary neurospheres were infected with lentiviruses having the recombinant genes encoding the fusion proteins, and the cells were allowed to differentiate after the third passage, and ratios of neurons in the total cells were counted. The results were plotted on a graph as shown in Fig.9.
- the present invention enables to provide agents for promoting neuronal differentiation of NSPCs and methods therefor.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biochemistry (AREA)
- Insects & Arthropods (AREA)
- Biophysics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Wood Science & Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Neurosurgery (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Neurology (AREA)
- Physics & Mathematics (AREA)
- Environmental Sciences (AREA)
- Tropical Medicine & Parasitology (AREA)
- High Energy & Nuclear Physics (AREA)
- Cell Biology (AREA)
- Immunology (AREA)
- Biodiversity & Conservation Biology (AREA)
- Epidemiology (AREA)
- Animal Husbandry (AREA)
- Microbiology (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2009280769A AU2009280769B2 (en) | 2008-08-13 | 2009-07-08 | Agent for promoting neuronal differentiation and method therefor |
| CA2733775A CA2733775A1 (en) | 2008-08-13 | 2009-07-08 | Agent for promoting neuronal differentiation and method therefor |
| US13/058,621 US20110183912A1 (en) | 2008-08-13 | 2009-07-08 | Agent for promoting neuronal differentiation and method therefor |
| EP09806548.5A EP2326333B1 (en) | 2008-08-13 | 2009-07-08 | Agent for promoting neuronal differentiation and method therefor |
| JP2011506509A JP5794693B2 (ja) | 2008-08-13 | 2009-07-08 | 神経分化促進剤 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US8852108P | 2008-08-13 | 2008-08-13 | |
| US61/088,521 | 2008-08-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010018652A1 true WO2010018652A1 (en) | 2010-02-18 |
Family
ID=41668806
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2009/003195 Ceased WO2010018652A1 (en) | 2008-08-13 | 2009-07-08 | Agent for promoting neuronal differentiation and method therefor |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20110183912A1 (enExample) |
| EP (1) | EP2326333B1 (enExample) |
| JP (1) | JP5794693B2 (enExample) |
| AU (1) | AU2009280769B2 (enExample) |
| CA (1) | CA2733775A1 (enExample) |
| WO (1) | WO2010018652A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012112737A3 (en) * | 2011-02-16 | 2012-11-08 | The Regents Of The University Of California | Alzheimer's disease cellular model for diagnostic and therapeutic development |
| EP2843049A4 (en) * | 2012-04-27 | 2015-04-22 | Univ Keio | PROMOTER FOR NEURAL DIFFERENTIATION |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11332719B2 (en) * | 2013-03-15 | 2022-05-17 | The Broad Institute, Inc. | Recombinant virus and preparations thereof |
| WO2019222134A1 (en) * | 2018-05-14 | 2019-11-21 | Baylor College Of Medicine | Coup-tfii receptor inhibitors and methods using same |
| WO2023201361A1 (en) | 2022-04-15 | 2023-10-19 | Aspen Neuroscience, Inc. | Methods of classifying the differentiation state of cells and related compositions of differentiated cells |
| CN116162657A (zh) * | 2022-11-21 | 2023-05-26 | 中国人民解放军军事科学院军事医学研究院 | 一种促进神经干细胞增殖和向神经元分化的方法及应用 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002291469A (ja) | 2001-03-30 | 2002-10-08 | Japan Science & Technology Corp | 胚性幹細胞からの神経幹細胞、運動ニューロン及びgaba作動性ニューロンの製造法 |
| WO2005017112A2 (en) * | 2003-08-05 | 2005-02-24 | Immusol Incorporated | Methods of inhibiting cancer growth by binding to nuclear receptors |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU8760298A (en) * | 1997-08-01 | 1999-02-22 | Baylor College Of Medicine | Coup-tfi directed therapies in the treatment of disease |
| US6395546B1 (en) * | 2000-02-01 | 2002-05-28 | Neurogeneration, Inc. | Generation of dopaminergic neurons from human nervous system stem cells |
| WO2003046141A2 (en) * | 2001-11-26 | 2003-06-05 | Advanced Cell Technology, Inc. | Methods for making and using reprogrammed human somatic cell nuclei and autologous and isogenic human stem cells |
| US20070184459A1 (en) * | 2006-02-03 | 2007-08-09 | Immusol Incorporated | Methods of inhibiting cancer growth by binding to nuclear receptors |
-
2009
- 2009-07-08 US US13/058,621 patent/US20110183912A1/en not_active Abandoned
- 2009-07-08 WO PCT/JP2009/003195 patent/WO2010018652A1/en not_active Ceased
- 2009-07-08 AU AU2009280769A patent/AU2009280769B2/en not_active Ceased
- 2009-07-08 EP EP09806548.5A patent/EP2326333B1/en not_active Not-in-force
- 2009-07-08 CA CA2733775A patent/CA2733775A1/en not_active Abandoned
- 2009-07-08 JP JP2011506509A patent/JP5794693B2/ja not_active Expired - Fee Related
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002291469A (ja) | 2001-03-30 | 2002-10-08 | Japan Science & Technology Corp | 胚性幹細胞からの神経幹細胞、運動ニューロン及びgaba作動性ニューロンの製造法 |
| WO2005017112A2 (en) * | 2003-08-05 | 2005-02-24 | Immusol Incorporated | Methods of inhibiting cancer growth by binding to nuclear receptors |
Non-Patent Citations (13)
| Title |
|---|
| "Current Protocols in Molecular Biology", JOHN WILEY & SONS LTD. |
| "Molecular cloning, a laboratory manual", 2001, COLD SPRING HARBOR PRESS, COLD SPRING HARBOR |
| FAEDO A ET AL.: "COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling.", CEREBRAL CORTEX, vol. 18, 28 December 2007 (2007-12-28), pages 2117 - 2131, XP008143925 * |
| GROSS, R.E. ET AL., NEURON, vol. 17, 1996, pages 595 - 606 |
| KANAI M I ET AL.: "seven-up controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts.", DEVELOPMENTAL CELL, vol. 8, 2005, pages 203 - 213, XP008143838 * |
| KOBLAR, S.A. ET AL., PROC.NATL.ACAD.SCI.U.S.A., vol. 95, 1998, pages 3178 - 3181 |
| NAKA H ET AL.: "Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development.", NAT. NEUROSCI., vol. 11, 24 August 2008 (2008-08-24), pages 1014 - 1023, XP008144820 * |
| OKADA Y ET AL.: "Spatiotemporal recapitulation of central nervous system development by murine embryonic stem cell-derived neural stem/progenitor cells.", STEM CELLS, vol. 26, 28 August 2008 (2008-08-28), pages 3086 - 3098, XP008143940 * |
| POULSEN C B ET AL.: "Brain response to traumatic brain injury in wild-type and interleukin-6 knockout mice: a microarray analysis.", J. NEUROCHEM., vol. 92, 2005, pages 417 - 432, XP008143920 * |
| REYNOLDS, B.A.; WEISS, S., SCIENCE, vol. 255, 1992, pages 1707 - 1710 |
| See also references of EP2326333A4 * |
| YAMAGUCHI H ET AL.: "The nuclear orphan receptor COUP-TFI is important for differentiation of oligodendrocytes.", DEV. BIOL., vol. 266, 2004, pages 238 - 251, XP008144819 * |
| ZHUANG Y ET AL.: "Overexpression of COUP-TF1 in murine embryonic stem cells reduces retinoic acid-associated growth arrest and increases extraembryonic endoderm gene expression.", DIFFERENTIATION, vol. 76, 3 January 2008 (2008-01-03), pages 760 - 771, XP026772229 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012112737A3 (en) * | 2011-02-16 | 2012-11-08 | The Regents Of The University Of California | Alzheimer's disease cellular model for diagnostic and therapeutic development |
| EP2843049A4 (en) * | 2012-04-27 | 2015-04-22 | Univ Keio | PROMOTER FOR NEURAL DIFFERENTIATION |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2009280769B2 (en) | 2014-09-18 |
| EP2326333A4 (en) | 2013-01-23 |
| EP2326333B1 (en) | 2017-05-31 |
| CA2733775A1 (en) | 2010-02-18 |
| AU2009280769A1 (en) | 2010-02-18 |
| EP2326333A1 (en) | 2011-06-01 |
| AU2009280769A2 (en) | 2011-03-31 |
| JP2011530484A (ja) | 2011-12-22 |
| US20110183912A1 (en) | 2011-07-28 |
| JP5794693B2 (ja) | 2015-10-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Shimazaki et al. | A role for the POU‐III transcription factor Brn‐4 in the regulation of striatal neuron precursor differentiation | |
| Braga et al. | Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: potential role of follistatin in mediating testosterone action | |
| JP4179562B2 (ja) | ハンチンチン遺伝子の発現抑制 | |
| US8470594B2 (en) | Methods for identifying agents that affect the survival of motor neurons | |
| Bernard et al. | Role of transmembrane semaphorin Sema6A in oligodendrocyte differentiation and myelination | |
| EP2326333B1 (en) | Agent for promoting neuronal differentiation and method therefor | |
| Zhou et al. | Retroviral lineage analysis of fibroblast growth factor receptor signaling in FGF2 inhibition of oligodendrocyte progenitor differentiation | |
| Yan et al. | Neurotrophin-3 promotes proliferation and cholinergic neuronal differentiation of bone marrow-derived neural stem cells via notch signaling pathway | |
| Sandner et al. | Bone morphogenetic proteins prevent bone marrow stromal cell-mediated oligodendroglial differentiation of transplanted adult neural progenitor cells in the injured spinal cord | |
| Honnorat et al. | POP66, a paraneoplastic encephalomyelitis-related antigen, is a marker of adult oligodendrocytes | |
| Zhan et al. | A DEAD‐box RNA helicase Ddx54 protein in oligodendrocytes is indispensable for myelination in the central nervous system | |
| WO2008071960A2 (en) | Methods of increasing neurogenesis | |
| Merrick et al. | A role for Insulin-like growth factor 2 in specification of the fast skeletal muscle fibre | |
| JP6194517B2 (ja) | 神経分化促進剤 | |
| Semi et al. | Nuclear factor I/A coordinates the timing of oligodendrocyte differentiation/maturation via olig1 promoter methylation | |
| Liu et al. | PBX1 Improves Cognition and Reduces Amyloid‐β Pathology in APP/PS1 Mice by Transcriptionally Activating the CRTC2–CREB Pathway | |
| Chen et al. | Spontaneous differentiation across cell lineages of separate germ layer origin during progenitor cell-mediated regeneration of the central nervous system | |
| Domingues Pedro | Generation of oligodendrocytes and characterisation of their role in axonal support | |
| Pedro | Generation of Oligodendrocytes and Characterisation of Their Role in Axonal Support | |
| JP2017057173A (ja) | Tim−3をターゲットとする脳損傷疾患治療用組成物及びこのスクリーニング方法 | |
| Xu et al. | Cell-Astrocyte Integration and Inhibits Glial Scar Formation Ability | |
| WO2022216876A1 (en) | Cancer treatment by targeting proteins or interactions of ephrinb-rgs3-kif20a-sept7 axis | |
| KR101206927B1 (ko) | S100a9의 발현을 억제하는 shRNA를 포함하는 치매 치료용 약학 조성물 | |
| US20180327778A1 (en) | Animal models for brain inflammation and white matter degeneration | |
| Zander | Conserved Molecular Factors: Role of the Snail Family of Transcription Factors and the Fat Family of Atypical Cadherins in Cortical Development |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09806548 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2733775 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2011506509 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009280769 Country of ref document: AU |
|
| REEP | Request for entry into the european phase |
Ref document number: 2009806548 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009806548 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2009280769 Country of ref document: AU Date of ref document: 20090708 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13058621 Country of ref document: US |