Liquid-crystal display
The present invention relates to liquid-crystal (LC) media for use in LC displays of the PS (polymer stabilised) or PSA (polymer sustained alignment) type.
The liquid-crystal displays (LC displays) used at present are mostly those of the TN (twisted nematic) type. However, these have the disadvantage of a strong viewing-angle dependence of the contrast.
In addition, so-called VA (vertical alignment) displays are known which have a broader viewing angle. The LC cell of a VA display contains a layer of an LC medium between two transparent electrodes, where the LC medium usually has a negative value of the dielectric (DC) anisotropy. In the switched-off state, the molecules of the LC layer are aligned perpendicular to the electrode surfaces (homeotropically) or have a tilted homeo- tropic alignment. On application of an electrical voltage to the electrodes, a realignment of the LC molecules parallel to the electrode surfaces takes place.
Furthermore, OCB (optically compensated bend) displays are known which are based on a birefringence effect and have an LC layer with a so- called "bend" alignment and usually positive (DC) anisotropy. On application of an electrical voltage, a realignment of the LC molecules perpendi- cular to the electrode surfaces takes place. In addition, OCB displays normally contain one or more birefringent optical retardation films in order to prevent undesired transparency to light of the bend cell in the dark state. OCB displays have a broader viewing angle and shorter response times compared with TN displays.
Also known are IPS (in-plane switching) displays, which contain an LC layer between two substrates, but wherein the two electrodes are located only on one of the substrates, usually with comb-shaped, interdigital structures. When applying a voltage to the electrodes, an electric field which has a significant component parallel to the LC layer is thereby generated. This causes realignment of the LC molecules in the layer
plane. Furthermore, so-called FFS (fringe field switching) displays have been proposed (see, inter alia, S. H. Jung et al., Jpn. J. Appl. Phys., Volume 43, No. 3, 2004, 1028), which likewise contain two electrodes on the same substrate, but, in contrast to IPS displays, only one of these is in the form of a structured (comb-shaped) electrode, and the other electrode is unstructured. A strong, so-called "fringe field" is thereby generated, i.e. a strong electric field close to the edge of the electrodes, and, throughout the cell, an electric field which has both a strong vertical component and a strong horizontal component. Both IPS displays and also FFS displays have a low viewing-angle dependence of the contrast.
In VA displays of the more recent type, uniform alignment of the LC molecules is restricted to a plurality of relatively small domains within the LC cell. Disclinations can exist between these domains, also known as tilt domains. VA displays having tilt domains have, compared with conventional VA displays, a greater viewing-angle independence of the contrast and the grey shades. In addition, displays of this type are simpler to produce since additional treatment of the electrode surface for uniform alignment of the molecules in the switched-on state, such as, for example, by rubbing, is no longer necessary. Instead, the preferential direction of the tilt or pretilt angle is controlled by a special design of the electrodes. In so- called MVA (multidomain vertical alignment) displays, this is usually achieved by the electrodes having protrusions which cause a local pretilt. As a consequence, the LC molecules are aligned parallel to the electrode surfaces in different directions in different, defined regions of the cell on application of a voltage. "Controlled" switching is thereby achieved, and the formation of interfering disclination lines is prevented. Although this arrangement improves the viewing angle of the display, it results, however, in a reduction in its transparency to light. A further development of MVA uses protrusions on only one electrode side, while the opposite electrode has slits, which improves the transparency to light. The slitted electrodes generate an inhomogeneous electric field in the LC cell on application of a voltage, meaning that controlled switching is still achieved. For further improvement of the transparency to light, the separations between the slits and protrusions can be increased, but this in turn results in a lengthening of the response times. In the so-called PVA (patterned
VA)1 protrusions are rendered completely superfluous in that both electrodes are structured by means of slits on the opposite sides, which results in increased contrast and improved transparency to light, but is technologically difficult and makes the display more sensitive to mechani- cal influences (tapping, etc.). For many applications, such as, for example, monitors and especially TV screens, however, a shortening of the response times and an improvement in the contrast and luminance (transmission) of the display are desired.
A further development are the so-called PS (polymer stabilized) or PSA (polymer sustained alignment) displays. In these, a small amount (for example 0.3% by weight, typically < 1% by weight) of a polymerisable compound is added to the LC medium and, after introduction into the LC cell, is polymerised or crosslinked in situ, usually by UV photopolymerisa- tion, with an electrical voltage applied between the electrodes. The addition of polymerisable mesogenic or liquid-crystalline compounds, also known as "reactive mesogens" (RMs), to the LC mixture has proven particularly suitable.
In the meantime, the PS or PSA principle is being used in diverse classical LC displays. Thus, for example, PSA-VA, PSA-OCB, PS-IPS and PSTN displays are known. As can be demonstrated in test cells, the PSA method results in a pretilt in the cell. In the case of PSA-OCB displays, it is therefore possible for the bend structure to be stabilised so that an off- set voltage is unnecessary or can be reduced. In the case of PSA-VA displays, this pretilt has a positive effect on response times. For PSA-VA displays, a standard MVA or PVA pixel and electrode layout can be used. In addition, however, it is possible, for example, to manage with only one structured electrode side and no protrusions, which significantly simplifies production and at the same time results in very good contrast at the same time as very good transparency to light.
Unless indicated otherwise, the term 11PSA" is used to represent PS displays and PSA displays.
- A -
PSA-VA displays are described, for example, in JP 10-036847 A, EP 1 170 626 A2, , US 6,861 ,107, US 7,169,449, US 2004/0191428 A1 , US 2006/0066793 A1 and US 2006/0103804 A1. PSA-OCB displays are described, for example, in T.-J- Chen et al., Jpn. J. Appl. Phys. 45, 2006, 2702-2704 and S. H. Kim, L.-C- Chien, Jpn. J. Appl. Phys. 43, 2004,
7643-7647. PS-IPS displays are described, for example, in US 6,177,972 and Appl. Phys. Lett. 1999, 75(21 ), 3264. PS-TN displays are described, for example, in Optics Express 2004, 12(7), 1221.
In particular for monitor and especially TV applications, optimisation of the response times, but also of the contrast and luminance (i.e. also transmission) of the LC display, is still demanded. The PSA process still appears to provide crucial advantages here. In particular in the case of PSA-VA, a shortening of the response times, which correlate with a measurable pretilt in test cells, can be achieved without a significant adverse effects on other parameters.
However, it has been found that the LC mixtures and RMs known from the prior art still have some disadvantages on use in PSA displays. Thus, far from every desired soluble RM is suitable for PSA displays, and it often appears difficult to find more suitable selection criteria than just the direct PSA experiment with pretilt measurement. The choice becomes even smaller if polymerisation by means of UV light without the addition of photo- initiators is desired, which may be advantageous for certain applications.
In addition, the selected "material system" of LC mixture (also referred to below as "LC host mixture") + polymerisable component should have the best possible electrical properties, in particular the "voltage holding ratio" (HR or VHR). In connection with PSA-VA, a high HR after irradiation with (UV) light is, in particular, of central importance since this is an indispens- ible part of the process, but of course also occurs as "normal" stress in the finished display.
However, the problem arises that not every LC mixture + polymerisable component combination "functions" since, for example, an inadequate tilt
or none at all is established or since, for example, the HR is inadequate for TFT display applications.
Thus, there continues to be a great demand for PSA displays, in particular of the VA type, and for LC media and polymerisable compounds for use in such displays, which do not have the disadvantages described above or only do so to a small extent and have improved properties. In particular, there is a great demand for PSA displays or materials having high specific resistance at the same time as a large working-temperature range, short response times, even at low temperatures, and a low threshold voltage, which facilitate a large number of grey shades, high contrast and a wide viewing angle, and have high values for the voltage holding ratio (HR) after UV exposure. In PSA displays for mobile applications, it is especially desired to have available LC media that show low threshold voltage and high birefringence.
The invention was based on the object of providing PSA displays which do not have the disadvantages indicated above or only do so to a lesser extent, enable the setting of a pretilt angle and preferably at the same time have very high specific resistance values, low threshold voltages and short response times.
Surprisingly, it has now been found that this object can be achieved by using PSA displays according to the present invention which contain an LC medium as described hereinafter. In particular, it has been found, surprisingly, that when using LC mixtures wherein the unpolymerisable component (host component) is a nematic mixture consisting essentially of mesogenic or liquid crystalline compounds with one or more phenylene groups that are disubstituted in 2- and 3-position by F and/or Cl, preferably by F, it is possible to achieve a significantly lower threshold voltage and a higher birefringence compared to LC media and LC host components as diclosed in prior art. In addition, the LC media of the present invention have high specific resistance values and a good low temperature stability (LTS) against undesired spontaneous crystallization, and when used in PSA displays, exhibit adequate tilt angles, even without the use of a photoinitiator.
The invention thus relates to a liquid-crystal (LC) medium comprising a polymerisable component comprising one or more polymerisable compounds, and a nematic component, characterized in that the nematic component contains from 90 to 100%, preferably >90 to 100%, by weight of one or more compounds, preferably selected from mesogenic or liquid crystalline compounds, which comprise one or more 1 ,4-phenylene groups that are substituted in 2- and 3-position by F and/or Cl
The invention further relates to an LC medium as described above and below, wherein the nematic component contains from 90 to 100%, preferably >90 to 100%, by weight of compounds selected from the group consisting of the following formulae:
in which the individual radicals have the following meanings:
denotes 1 or 2,
and — denote independently of each other
wherein at least one of and
R1 and R2 each, independently of one another, denote alkyl or alkenyl having 1 to 12 C atoms, in which, in addition, one or two non- adjacent CH2 groups may be replaced by -O-, -CH=CH-, -CO-, -OCO- or -COO- in such a way that O atoms are not linked directly to one another,
R5 and R6 each, independently of one another, have one of the meanings indicated above for R1,
Zx denotes -CH=CH-, -CH2O-, -OCH2-, -CF2O-, -OCF2-, -O-, -CH2-,
-CH2CH2- or a single bond, preferably a single bond,
1-4 each, independently of one another, denote F or Cl,
L5 and L6 each, independently of one another, denote F, Cl, OCF3, CF3, CH3, CH2F, CHF2.
The invention further relates to the use of the LC media as described above and below in LC displays, preferably in displays of the PS (polymer stabilised) or PSA (polymer sustained alignment) type.
The invention further relates to the use of the LC media as described above and below, wherein the polymerisable component is polymerised, in LC displays, preferably in displays of the PS (polymer stabilised) or PSA (polymer sustained alignment) type.
The invention further relates to an LC display comprising an LC medium as described above and below, which is preferably a display of the PS or PSA type, very preferably a PSA-VA or PSA-IPS display.
The invention further relates to an LC display comprising an LC medium as described above and below, which is preferably a display of the PS or PSA type, very preferably a PSA-VA or PSA-IPS display, wherein the polymerisable component is polymerised.
Preferably the PSA display contains a display cell comprising two substrates and two electrodes, wherein at least one substrate is transparent to light and at least one substrate has one or two electrodes provided thereon, and a layer of an LC medium comprising a polymerised component and a low-molecular-weight component located between the substrates, wherein the polymerised component is obtainable by polymerisation of one or more polymerisable compounds between the substrates of the display cell in the LC medium while applying a voltage to the electrodes, and wherein the low-molecular-weight component is a nematic component as described above and below.
The invention further relates to a method of producing a display as described above and below, by providing an LC medium comprising one or more polymerisable compounds and a nematic component as described above and below into a display cell comprising two substrates and two electrodes, wherein at least one substrate is transparent to light and at least one substrate has one or two electrodes provided thereon, and
polymerising one or more of the polymerisable compounds while applying a voltage to the electrodes.
The PS- and PSA-displays of the present invention contain two electrodes, preferably as transparent layers, wherein these two electrodes are provided on one or both of the two substrates forming the display cell. Thus, either one electrode is provided on each of the two substrates, for example in inventive displays of the VA type, or the two electrodes are both provided on one substrate and no electrode is provided on the other substrate, for example in inventive displays of the IPS or FFS type.
The LC media for use in the LC displays according to the invention contain one ore more polymerisable compounds and an LC mixture ("host mixture") comprising one or more, preferably two or more, low-molecular-weight (i.e. monomeric or unpolymerised) compounds, which are usually selected from mesogenic or liquid crystalline compounds. The latter are stable or unreactive to a polymerisation reaction under the conditions used for the polymerisation of the polymerisable compounds.
Preferably the LC media according to the present invention do essentially consist of one or more polymerisable compounds and a nematic component (or host LC mixture) as described above and below. However, the LC media may additionally comprise one or more further components or additives, for example selected from chiral dopants, polymerisation initiators, inhibitors, stabilizers, surfactants, nanoparticles etc.
The nematic component or LC host mixture is preferably a nematic LC mixture. The terms "nematic component" and "nematic LC mixture" here mean an LC mixture which has a nematic LC phase, but may in addition have other LC phases (like e.g. a smectic phase), but very preferably an LC mixture that has only a nematic LC phase and no other LC phases.
The LC host mixtures and LC media according to the present invention are advantageous as they show significantly lower threshold voltage and a higher birefringence compared to PSA displays comprising LC host
components as diclosed in prior art. They are therefore especially suitable for use in PSA displays for mobile applications.
The invention further relates to novel nematic components and LC host mixtures as described above and below (i.e. which do not contain a polymerisable compound, but do essentially consist of unpolymerisable or low-molecular-weight compounds ). These LC mixtures can be used in classic displays of the VA type, like VA- and MVA-displays. The invention further relates to LC displays, preferably VA and MVA displays containing such an LC mixture.
For the nematic component, especially preferred are compounds comprising one or more 1 ,4-phenylene groups that are disubstituted in 2- and 3-position by F. Further preferred are compounds of the formulae CY, PY and TY in which L1 , L2, L3 and L4 denote F.
In the formulae CY, PY and TY1 preferably both radicals L1 and L2 denote F, or one of the radicals L1 and L2 denotes F and the other denotes Cl, and preferably both radicals L3 and L4 denote F, or one of the radicals L3 and L4 denotes F and the other denotes Cl.
Especially preferred are LC mixtures containing at least one compound of formula CY. Further preferred are LC mixtures containing at least one compound of formula CY and at least one compound of formula PY. Further preferred are LC mixtures containing at least one compound of each of formulae CY, PY and TY.
Further preferred LC media and LC host mixtures are indicated below:
a) LC host mixture comprising one or more compounds selected from the following sub-formulae:
in which a denotes 1 or 2, alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, alkenyl denotes a straight-chain alkenyl radical having 2-6 C atoms, and (O) denotes an O atom or a single bond. Alkenyl preferably denotes CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2- CH=CH-, CH3-(CHa)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH-
(CHz)2-.
LC host mixture comprising one or more compounds selected from the following sub-formulae:
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, alkenyl denotes a straight-chain alkenyl radical having 2-6 C atoms, and (O) denotes an O atom or a single bond. Alkenyl preferably denotes CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2-CH=CH-, CH3-(CH2)2- CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH-(CH2)2-.
LC host mixture comprising one or more compounds selected from the following sub-formulae:
in which R denotes a straight-chain alkyl or alkoxy radical having 1-7 C atoms, R* denotes a straight-chain alkenyl radical having 2-7 C atoms, (O) denotes an O atom or a single bond, and m denotes an integer from 1 to 6. R* preferably denotes CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2-CH=CH-, CH3-(CH2)2- CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH-(CH2)2-.
R preferably denotes methyl, ethyl, propyl, butyl, pentyl, hexyl, meth- oxy, ethoxy, propoxy, butoxy or pentoxy.
LC host mixture which additionally comprises one or more compounds of the following formula:
in which the individual radicals have the following meanings:
f denotes 0 or 1 ,
R1 and R2 each, independently of one another, denote alkyl having 1 to 12 C atoms, in which, in addition, one or two non-adja- cent CH2 groups may be replaced by -O-, -CH=CH-, -CO-,
-OCO- or -COO-in such a way that O atoms are not linked directly to one another,
Z* and Zy each, independently of one another, denote -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, -COO-,
-OCO-, -C2F4-, -CF=CF-, -CH=CHCH2O- or a single bond, preferably a single bond,
L5 and L6 each, independently of one another, denote F, Cl, OCF3, CF3, CH3, CH2F, CHF2.
Preferably, both radicals L5 and L6 denote F or one of the radicals L5 and L6 denotes F and the other denotes Cl.
The compounds of the formula LY are preferably selected from the following sub-formulae:
in which R1 has the above-mentioned meaning, (O) denotes an O atom or a single bond, alkyl denotes a straight-chain alkyl radical having 1-6 C atoms, and v denotes an integer from 1 to 6. R1 preferably denotes straight-chain alkyl having 1-6 C atoms or straight-chain alkenyl having 2-6 C atoms, in particular CH3, C2H5, n- C3H7, n-C4H9, n-C5Hn, CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2-CH=CH-, CH3-(CHz)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH-(CH2)2-. The LC medium according to the invention preferably comprises one or more compounds of the above-mentioned formulae in amounts of > 0 to < 10% by weight.
LC host mixture which additionally comprises one or more compounds selected from the following formulae:
(O) .-alkyl Y14
in which R5 has one of the meanings indicated above for R1, alkyl denotes Ci-6-alkyl, d denotes 0 or 1 , and z and m each, independently of one another, denote an integer from 1 to 6. R5 in these compounds is particularly preferably Ci-6-alkyl or -alkoxy or C2-6-alkenyl, d is preferably 1. The LC medium according to the invention preferably comprises one or more compounds of the above-mentioned formulae in amounts of > 0 to < 10% by weight.
LC host mixture which additionally comprises one or more compounds of the following formula:
in which
R9 denotes H, CH3, C2H5 or n-C3H7, (F) denotes an optional fluoro substituent, q denotes 1 , 2 or 3, and R7 has one of the meanings indicated for R1, preferably in amounts of > 3% by weight, in particular > 5% by weight and very particularly preferably 5-30% by weight.
Particularly preferred compounds of the formula Fl are selected from the following sub-formulae:
in which R
7 preferably denotes straight-chain alkyl having 1-6 C atoms, and R
9 denotes CH
3, C
2H
5 or
Particular preferen given to the compounds of the formulae FH , FI2 and FI3.
g) LC host mixture which additionally comprises one or more compounds of the following formulae:
in which R8 has the meaning indicated for R1, and alkyl denotes a straight-chain alkyl radical having 1-6 C atoms.
h) LC host mixture which additionally comprises one or more compounds which contain a tetrahydronaphthyl or naphthyl unit, such as, for example, the compounds selected from the following formulae:
in which R10 and R11 each, independently of one another, have one of the meanings indicated for R1, preferably denote straight-chain alkyl or straight-chain alkoxy having 1-6 C atoms or straight-chain alkenyl having 2-6 C atoms, and Z1 and Z2 each, independently of one another, denote -C2H4-, -CH=CH-, -(CH2)4-, -(CH2)3O-, -O(CH2)3- , -CH=CHCH2CH2-, -CH2CH2CH=CH-, -CH2O-, -OCH2-, -COO-, -OCO-, -C2F4-, -CF=CF-, -CF=CH-, -CH=CF-, -CH2- or a single bond.
LC host mixture which additionally comprises one or more difluoro- dibenzochromans and/or chromans of the following formulae:
in which R
11 and R
12 each, independently of one another, have the above-mentioned meaning, and c denotes 0 or 1 , preferably in amounts of 3 to 20% by weight, in particular in amounts of 3 to 15% by weight.
Particularly preferred compounds of the formulae BC and CR are selected from the following sub-formulae:
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl and alkenyl* each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl and alkenyl* preferably denote CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2-
CH=CH-, CH3-(CH2)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH- (CH2J2-.
Very particular preference is given to mixtures comprising one, two or three compounds of the formula BC-2.
LC host mixture which additionally comprises one or more fluorinated phenanthrenes or dibenzofurans of the following formulae:
in which R11 and R12 each, independently of one another, have the above-mentioned meanings, b denotes 0 or 1 , L denotes F, and r denotes 1 , 2 or 3.
Particularly preferred compounds of the formulae PH and BF are selected from the following sub-formulae:
in which R and R1 each, independently of one another, denote a straight-chain alkyl or alkoxy radical having 1-7 C atoms.
I) LC host mixture or nematic component in which the proportion of compounds containing one or more 1 ,4-phenylene groups substituted in 2- and 3-position by F and/or Cl, related to the host mixture (or nematic component) as a whole, is > 90%, preferably > 95%, very preferably > 98%, most preferably 100% by weight.
m) LC host mixture or nematic component which comprises one or more, preferably from 3 to 20 compounds of the formulae CY, PY and/or TY. The proportion of these compounds in the host mixture as a whole is preferably from > 90 to 100%, very preferably > 95%, most preferably 100% by weight. The content of these individual compounds is preferably in each case from 2 to 30% by weight.
n) LC host mixture or nematic component wherein the compounds of formulae CY, PY and TY are selected from the group consisting of formulae CY1 , CY2, CY9, CY10, PY1 , PY2, PY9, PY10, TY1 and TY2, very preferably from the group consisting of formulae CY1 , CY2, CY9, CY10, PY9, PY10 and TY1.
o) LC medium which, apart from the polymerisable compounds as described above and below, comprises no compounds which contain a terminal vinyl or vinyloxy group (-CH=CH2, -0-CH=CH2).
p) LC medium which comprises 1 to 5, preferably 1 , 2 or 3 polymer- isable compounds.
q) LC medium in which the proportion of polymerisable compounds in the medium as a whole is 0.05 to 5%, preferably 0.1 to 1 %.
r) LC medium which comprises in addition one or more, preferably low- molecular-weight and/or unpolymerisable, chiral dopants, very preferably selected from Table B, preferably in the concentration ranges given for Table B.
The LC host mixture may also contain from > 0 to < 10% of compounds without phenylene rings that are disubstituted in 2- and 3-position by F and/or Cl. Such compounds, if present, are preferably selected from the following embodiments:
1 ) LC host mixture which comprises one or more compounds of the following formula:
in which the individual radicals have the following meanings:
denotes
R
3 and R
4 each, independently of one another, denote alkyl having 1 to 12 C atoms, in which, in addition, one or two non-adjacent CH
2 groups may be replaced by -O-, -CH=CH-, -CO-, -OCO- or -COO- in such a way that O atoms are not linked directly to one another,
Zy denotes -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O-,
-OCH2-, -COO-, -OCO-, -C2F4-, -CF=CF-, -CH=CHCH2O- or a single bond, preferably a single bond.
The compounds of the formula ZK are preferably selected from the following sub-formulae:
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl denotes a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl preferably denotes CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3- CH2-CH=CH-, CH3-(CH2)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3- CH=CH-(CH2)2-.
2) LC host mixture which additionally comprises one or more compounds of the following formula:
in which the individual radicals have on each occurrence, identically or differently, the following meanings:
R5 and R6 each, independently of one another, have one of the meanings indicated above for R1,
and
denotes 1 or 2.
The compounds of the formula DK are preferably selected from the following sub-formulae:
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl and alkenyl* each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl and alkenyl* preferably denote CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2- CH=CH-, CH3-(CH2)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH- (CH2)2-.
3) LC host mixture which additionally comprises one or more compounds selected from the following formulae:
alkyl G1
alkyl G2
in which alkyl denotes Ci-6-alkyl, Lx denotes H or F, and X denotes F, Cl, OCF3, OCHF2 or OCH=CF2. Particular preference is given to compounds of the formula G1 in which X denotes F.
LC host mixture which additionally comprises one or more biphenyl compounds of the following formulae:
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl and alkenyl* each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl and alkenyl* preferably denote CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2- CH=CH-, CH3-(CH2)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH- (CH2)2-.
The proportion of the biphenyls of the formulae B1 to B3 in the LC mixture is preferably at least 3% by weight, in particular > 5% by weight.
The compounds of the formula B2 are particularly preferred.
The compounds of the formulae B1 to B3 are preferably selected from the following sub-formulae:
in which alkyl* denotes an alkyl radical having 1-6 C atoms. The medium according to the invention particularly preferably comprises one or more compounds of the formulae B1a and/or B2c.
5) LC host mixture which additionally comprises one or more terphenyl compounds of the following formula:
in which R
5 and R
6 each, independently of one another, have one of the meanings indicated above for R
1, and and each, independently of
one another, denote
in which L5 denotes F or Cl, preferably F, and L6 denotes F, Cl, OCF3, CF3, CH3, CH2F or CHF2, preferably F.
The compounds of the formula T are preferably selected from the following sub-formulae:
in which R denotes a straight-chain alkyl or alkoxy radical having 1-7 C atoms, R
* denotes a straight-chain alkenyl radical having 2-7 C atoms, (O) denotes an O atom or a single bond, and m denotes an integer from 1 to 6. R
* preferably denotes CH
2=CH-, CH
2=CHCH
2CH
2-, CH
3-CH=CH-, CH
3-CH
2-CH=CH-, CH
3-(CH
2)
2- CH=CH-, CH
3-(CH
2)
3-CH=CH- or CH
3-CH=CH-(CH
2)
2-.
R preferably denotes methyl, ethyl, propyl, butyl, pentyl, hexyl, meth- oxy, ethoxy, propoxy, butoxy or pentoxy.
6) LC host mixture which additionally comprises one or more compounds of the following formulae:
in which R1 and R2 have the above-mentioned meanings and preferably each, independently of one another, denote straight-chain alkyl or alkenyl.
Preferred mixtures comprise one or more compounds selected from the formulae 01 , 03 and 04.
The combination of compounds of the preferred embodiments mentioned above with the polymerised compounds described above and below effects low threshold voltages and very good low-temperature stabilities with maintenance of high clearing points and high HR values in the LC media according to the invention and allows a pretilt angle to be set in PSA displays. In particular, the LC media exhibit significantly shortened response times, in particular also the grey-shade response times, in PSA displays compared with the media from the prior art.
The LC host mixture preferably has a nematic phase range of at least 80 K, particularly preferably at least 100 K, and a rotational viscosity of not greater than 450 mPa-s, preferably not greater than 350 mPa-s, at 200C.
The LC host mixture preferably has a negative dielectric anisotropy Δε, preferably of about -0.5 to -7.5, in particular of about -2.5 to -6.0, at 200C and 1 kHz.
The LC host mixture preferably has a birefringence Δn > 0.06, very preferably > 0.09, most preferably > 0.12, and preferably has a birefringence Δn < 0.20, very preferably < 0.18, most preferably < 0.16.
The LC media may also comprise further additives known to the person skilled in the art and described in the literature, like for example polymeirsation initiators, inhibitors, stabilizers, surface active compounds or chiral dopants. These additives can be polymerisable or unpolymerisable. Accordingly, polymerisable additives will belong to the polymerisable component, and unpolymerisable additives will belong to the nematic component of the LC media.
The LC media can for example contain one or more chiral dopants, which are preferably selected from the group consisting of compounds from Table B below.
For example, 0 to 15% by weight of pleochroic dyes may be added, furthermore nanoparticles, conductive salts, preferably ethyldimethyldodecylammonium 4-hexoxybenzoate, tetrabutylammonium tetraphenylborate or complex salts of crown ethers (cf., for example, Haller et al., MoI. Cryst. Liq. Cryst. 24, 249-258 (1973)), may be added in order to improve the conductivity, or substances may be added in order to modify the dielectric anisotropy, the viscosity and/or the alignment of the nematic phases. Substances of this type are described, for example, in DE-A 22 09 127, 22 40 864, 23 21 632, 23 38 281 , 24 50 088, 26 37 430 and 28 53 728.
The individual components of the preferred embodiments of the LC media according to the invention are either known or the ways in which they are prepared can readily be derived from the prior art by the person skilled in the relevant art since they are based on standard methods described in the literature. Corresponding compounds of the formula CY are described,
for example, in EP-A-O 364 538. Corresponding compounds of the formula ZK are described, for example, in DE-A-26 36 684 and DE-A-33 21 373.
Preference is furthermore given to LC media comprising one, two or three polymerisable compounds as described above and below.
Preference is furthermore given to achiral polymerisable compounds and LC media comprising, preferably consisting exclusively of, achiral com- pounds.
Preference is furthermore given to PSA displays and LC media in which the polymerisable component comprises one or more polymerisable compounds containing a polymerisable group (monoreactive) and one or more polymerisable compounds containing two or more, preferably two, polymerisable groups (di- or multireactive).
Preference is furthermore given to PSA displays and LC media in which the polymerisable component consists exclusively of polymerisable compounds containing two polymerisable groups (direactive).
The polymerisable compounds can be added individually to the LC media, but it is also possible to use mixtures comprising two or more polymerisable compounds according to the invention. Copolymers are formed on polymerisation of such mixtures. The invention furthermore relates to the polymerisable mixtures mentioned above and below. The polymerisable compounds are mesogenic or non-mesogenic, preferably mesogenic or liquid-crystalline.
The proportion of the polymerisable component in the LC media is preferably < 5%, especially < 1%, very preferably < 0.5 %.
The proportion of the nematic component in the LC media is preferably > 95%, very preferably > 99%.
In a preferred embodiment of the invention, the polymerisable compounds are selected from formula I
Ra-A1-(Z1-A2)m1-Rb I
in which the individual radicals have the following meanings:
Ra and Rb each, independently of one another, denote P-Sp-, H, halogen, SF5, NU2, a carbon group or hydrocarbon group
P on each occurrence, identically or differently, denotes a polymerisable group,
Sp on each occurrence, identically or differently, denotes a spacer group or a single bond,
A1 and A2 each, independently of one another, denote an aromatic, heteroaromatic, alicyclic or heterocyclic group, preferably having 4 to 25 ring atoms, which may also contain fused rings, and which is optionally mono- or polysubstituted by L,
Z1 on each occurrence, identically or differently, denotes -O-,
-S-, -CO-, -CO-O-, -OCO-, -O-CO-O-, -OCH2-, -CH2O-, -SCH2-, -CH2S-, -CF2O-, -OCF2-, -CF2S-, -SCF2-, -(CH2)n1-, -CF2CH2-, -CH2CF2-, -(CF2)nr, -CH=CH-, -CF=CF-, -C≡C-,
-CH=CH-COO-, -OCO-CH=CH-, CR0R00 or a single bond,
L denotes P-Sp-, H, OH, CH2OH, halogen, SF5, NO2, a carbon group or hydrocarbon group,
R0 and R00 each, independently of one another, denote H or alkyl having 1 to 12 C atoms,
ml denotes O, 1 , 2, 3 or 4,
n1 denotes 1 , 2, 3 or 4,
wherein at least one of the radicals Ra, R and L denotes P-Sp-,
Particularly preferred compounds of the formula I are those in which
A1 and A2 each, independently of one another, denote 1 ,4-phenylene, naphthalene-1 ,4-diyl or naphthalene-2,6-diyl, in which, in addition, one or more CH groups in these groups may be replaced by N, cyclohexane-1 ,4-diyl, in which, in addition, one or more non-adjacent CH2 groups may be replaced by O and/or S, 1 ,4-cyclohexenylene, bicyclo[1.1.1]pentane-1 ,3- diyl, bicyclo[2.2.2]octane-1 ,4-diyl, spiro[3.3]heptane-2,6-diyl, piperidine-1 ,4-diyl, decahydronaphthalene-2,6-diyl, 1 ,2,3,4- tetrahydronaphthalene-2,6-diyl, indane-2,5-diyl, octahydro- 4,7-methanoindane-2,5-diyl, or phenanthrene-2,7-diyl, where all these groups may be unsubstituted or mono- or polysubstituted by L,
L denotes P-Sp-, OH1 CH2OH, F, Cl, Br, I, -CN, -NO2, -NCO, -NCS, -OCN1 -SCN, -C(=O)N(RX)2, -C(=O)Y1, -C(=O)RX,
-N(RX)2, optionally substituted silyl, optionally substituted aryl having 6 to 20 C atoms, straight-chain or branched alkyl or alkoxy having 1 to 25 C atoms, or straight-chain or branched alkenyl, alkinyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy or alkoxycarbonyloxy having 2 to 25 C atoms, wherein in all of these groups, in addition, one or more H atoms may be replaced by F, Cl or P-Sp-,
Y1 denotes halogen,
Rx denotes P-Sp-, H, halogen, straight-chain, branched or cyclic alkyl having 1 to 25 C atoms, in which, in addition, one or more non-adjacent CH2 groups may be replaced by -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by
F1 Cl or P-Sp-, an optionally substituted aryl or aryloxy group having 6 to 40 C atoms, or an optionally substituted hetero- aryl or heteroaryloxy group having 2 to 40 C atoms,
Ra and Rb each, independently of one another, denote P-Sp-, H, L as defined above, or straight-chain or branched alkyl having 1 to 25 C atoms, in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(RX)=C(RX)-, -C≡C-, -N(RX)-, -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by F, Cl, Br1 I, CN or P-Sp-,
where at least one of the radicals Ra, Rb and L denotes P-Sp-.
Particular preference is given to compounds of the formula I in which one or both of the radicals Ra and Rb denote P-Sp-.
Particularly preferred compounds of the formula I are selected from the following sub-formulae:
in which P, Sp, L and Z1 on each occurrence, identically or differently, have one of the above-mentioned meanings,
R has one of the meanings indicated for Rx and preferably denotes P-Sp- or straight-chain or branched alkyl or alkoxy having 1 to 12 C atoms,
L is as defined above and preferably denotes F or CH3,
Z1 preferably denotes -COO-, -OCO- or a single bond,
Zx denotes -O-, -CO- or CRyRz,
Ry and Rz denote independently of one another H, F, CH3 or CF3, m2 and m3 each, independently of one another, denote an integer from 1 to 8,
o denotes 0 or 1 , r denotes 0, 1 , 2, 3 or 4, s denotes 0, 1 , 2 or 3, t denotes 0, 1 or 2, x denotes 0 or 1.
In a further preferred embodiment of the invention, the polymerisable compounds are chiral compounds selected from formula II:
(R*-(A1-Z1)m1)k-Q Il
in which A1, Z1 and ml have on each occurrence, identically or differently, one of the meanings indicated in formula I,
R* on each occurrence, identically or differently, has one of the meanings indicated for Ra in formula I,
Q denotes a k-valent chiral group, which is optionally mono- or polysub- stituted by L,
k is 1 , 2, 3, 4, 5 or 6,
where the compounds contain at least one radical R* or L which denotes or contains a group P-Sp- as defined above.
Particularly preferred compounds of the formula Il contain a monovalent group Q of the formula III
in which L and r have on each occurrence, identically or differently, the mmeeaanniinnαgss iinnddiiccaatteedd aabboovvee.,
A* and B* each, independently of one another, denote fused benzene, cyclohexane or cyclohexene,
on each occurrence, identically or differently, denotes 0, 1 or 2, and
u on each occurrence, identically or differently, denotes 0, 1 or
2.
Particular preference is given to groups of the formula III in which x denotes 1 or 2.
Further preferred compounds of the formula Il contain a monovalent group Q or one or more groups R* of the formula IV
-Q1-CH-Q2 ,w
Q3
in which
Q1 denotes alkylene or alkyleneoxy having 1 to 9 C atoms or a single bond,
Q denotes optionally fluorinated alkyl or alkoxy having 1 to 10 C atoms, in which, in addition, one or two non-adjacent CH2 groups may be replaced by -O-, -S-, -CH=CH-, -CO-, -OCO-, -COO-, -O-COO-, -S- CO-, -CO-S- or -C≡C- in such a way that O and/or S atoms are not linked directly to one another,
Q3 denotes F, Cl, CN or alkyl or alkoxy as defined for Q2, but different from Q2.
Preferred groups of the formula IV are, for example, 2-butyl (= 1-methyl- propyl), 2-methylbutyl, 2-methylpentyl, 3-methylpentyl, 2-ethylhexyl, 2- propylpentyl, in particular 2-methylbutyl, 2-methylbutoxy, 2-methylpentoxy, 3-methylpentoxy, 2-ethylhexoxy, 1 -methyl hexoxy, 2-octyloxy, 2-oxa-3- methylbutyl, 3-oxa-4-methylpentyl, 4-methylhexyl, 2-hexyl, 2-octyl, 2-nonyl, 2-decyl, 2-dodecyl, 6-methoxyoctoxy, 6-methyloctoxy, 6-methyloctanoyl- oxy, 5-methylheptyloxycarbonyl, 2-methylbutyryloxy, 3-methylvaleroyloxy, 4-methylhexanoyloxy, 2-chloropropionyloxy, 2-chloro-3-methylbutyryloxy, 2-chloro-4-methylvaleryloxy, 2-chloro-3-methylvaleryloxy, 2-methyl-3-oxa- pentyl, 2-methyl-3-oxahexyl, 1-methoxypropyl-2-oxy, 1 -ethoxypropyl-2-oxy, i-propoxypropyl-2-oxy, 1-butoxypropyl-2-oxy, 2-fluorooctyloxy, 2- fluorodecyloxy, 1 ,1 ,1-trifluoro-2-octyloxy, 1 ,1 ,1-trifluoro-2-octyl, 2-fluoro- methyloctyloxy.
Further preferred compounds of the formula Il contain a divalent group Q of the formula V
in which L, r, t, A* and B* have the meanings indicated above.
Further preferred compounds of the formula Il contain a divalent group Q selected from the following formulae:
in which Phe denotes phenyl, which is optionally mono- or polysubstituted by L, and Rx denotes F or optionally fluohnated alkyl having 1 to 4 C atoms.
Particularly preferred compounds of the formula Il are selected from the following sub-formulae:
30
in which L, P, Sp, ml , r and t have the meanings indicated above, Z and A have on each occurrence, identically or differently, one of the meanings indicated for Z1 and A1 respectively, and t1 on each occurrence, identically or differently, denotes 0 or 1.
The chiral compounds of formula Il can be employed either in optically active form, i.e. as pure enantiomers, or as any desired mixture of the two enantiomers, or as the racemate thereof. The use of the racemates is preferred. The use of the racemates has some advantages over the use of pure enantiomers, such as, for example, significantly more straightforward synthesis and lower material costs.
Above and below, the following meanings apply:
The term "mesogenic group" is known to the person skilled in the art and is described in the literature, and denotes a group which, due to the ani- sotropy of its attracting and repelling interactions, essentially contributes to causing a liquid-crystal (LC) phase in low-molecular-weight or polymeric
substances. Compounds containing mesogenic groups ("mesogenic compounds") do not necessarily have to have an LC phase themselves. It is also possible for mesogenic compounds to exhibit LC phase behaviour only after mixing with other compounds and/or after polymerisation. Typi- cal mesogenic groups are, for example, rigid rod- or disc-shaped units. An overview of the terms and definitions used in connection with mesogenic or LC compounds is given in Pure Appl. Chem. 73(5), 888 (2001 ) and C. Tschierske, G. PeIzI, S. Diele, Angew. Chem. 2004, 116, 6340-6368.
The term "spacer group", also referred to as "Sp" above and below, is known to the person skilled in the art and is described in the literature, see, for example, Pure Appl. Chem. 73(5), 888 (2001 ) and C. Tschierske, G. PeIzI, S. Diele, Angew. Chem. 2004, 116, 6340-6368. Unless indicated otherwise, the term "spacer group" or "spacer" above and below denotes a flexible group which connects the mesogenic group and the polymer- isable group(s) to one another in a polymerisable mesogenic compound ("RM").
The term "reactive mesogen" or "RM" denotes a compound containing a mesogenic group and one or more functional groups which are suitable for polymerisation (also known as polymerisable group or group P).
The terms "low-molecular-weight compound" and "unpolymerisable compound" denote compounds, usually monomeric, which do not contain any functional group which is suitable for polymerisation under the usual conditions known to the person skilled in the art, in particular under the conditions used for the polymerisation of the RMs.
The term "organic group" denotes a carbon or hydrocarbon group.
The term "carbon group" denotes a mono- or polyvalent organic group containing at least one carbon atom which either contains no further atoms (such as, for example, -C≡C-) or optionally contains one or more further atoms, such as, for example, N, O, S, P, Si1 Se, As, Te or Ge (for example carbonyl, etc.). The term "hydrocarbon group" denotes a carbon group which additionally contains one or more H atoms and optionally one
or more heteroatoms, such as, for example, N, O, S, P, Si, Se, As, Te or Ge.
"Halogen" denotes F, Cl, Br or I.
A carbon or hydrocarbon group can be a saturated or unsaturated group. Unsaturated groups are, for example, aryl, alkenyl or alkynyl groups. A carbon or hydrocarbon radical having more than 3 C atoms can be straight-chain, branched and/or cyclic and may also have spiro links or condensed rings.
The terms "alkyl", "aryl", "heteroaryl", etc., also encompass polyvalent groups, for example alkylene, arylene, heteroarylene, etc.
The term "aryl" denotes an aromatic carbon group or a group derived therefrom. The term "heteroaryl" denotes "aryl" in accordance with the above definition containing one or more heteroatoms.
Preferred carbon and hydrocarbon groups are optionally substituted alkyl, alkenyl, alkynyl, alkoxy, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy and alkoxycarbonyloxy having 1 to 40, preferably 1 to 25, particularly preferably 1 to 18 C atoms, optionally substituted aryl or aryloxy having 6 to 40, preferably 6 to 25 C atoms, or optionally substituted alkylaryl, arylalkyl, alkylaryloxy, arylalkyloxy, arylcarbonyl, aryloxycarbonyl, arylcarbonyloxy and aryloxycarbonyloxy having 6 to 40, preferably 6 to 25 C atoms.
Further preferred carbon and hydrocarbon groups are Ci-C4O alkyl, C2-C40 alkenyl, C2-C4O alkynyl, C3-C4O allyl. C4-C4O alkyldienyl, C4-C4O polyenyl, C6- C40 aryl, C6-C40 alkylaryl, C6-C40 arylalkyl, C6-C40 alkylaryloxy, C6-C40 aryl- alkyloxy, C2-C40 heteroaryl, C4-C40 cycloalkyl, C4-C40 cycloalkenyl, etc. Particular preference is given to C1-C22 alkyl, C2-C22 alkenyl, C2-C22 alkynyl, C3-C22 allyl, C4-C22 alkyldienyl, C6-Ci2 aryl, C6-C20 arylalkyl and C2-C20 heteroaryl.
Further preferred carbon and hydrocarbon groups are straight-chain, branched or cyclic alkyl radicals having 1 to 40, preferably 1 to 25 C
atoms, which are unsubstituted or mono- or polysubstituted by F, Cl, Br, I or CN and in which one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(RX)=C(RX)-, -C≡C-, -N(RX)-, -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another.
Rx preferably denotes H1 halogen, a straight-chain, branched or cyclic alkyl chain having 1 to 25 C atoms, in which, in addition, one or more non- adjacent C atoms may be replaced by -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, and in which one or more H atoms may be replaced by fluorine, an optionally substituted aryl or aryloxy group having 6 to 40 C atoms or an optionally substituted heteroaryl or heteroaryloxy group having 2 to 40 C atoms.
Preferred alkyl groups are, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, s-butyl, t-butyl, 2-methylbutyl, n-pentyl, s-pentyl, cyclo- pentyl, n-hexyl, cyclohexyl, 2-ethylhexyl, n-heptyl, cycloheptyl, n-octyl, cyclooctyl, n-nonyl, n-decyl, n-undecyl, n-dodecyl, dodecanyl, trifluoro- methyl, perfluoro-n-butyl, 2,2,2-thfluoroethyl, perfluorooctyl, perfluoro- hexyl, etc.
Preferred alkenyl groups are, for example, ethenyl, propenyl, butenyl, pentenyl, cyclopentenyl, hexenyl, cyclohexenyl, heptenyl, cycloheptenyl, octenyl, cyclooctenyl, etc.
Preferred alkynyl groups are, for example, ethynyl, propynyl, butynyl, pentynyl, hexynyl, octynyl, etc.
Preferred alkoxy groups are, for example, methoxy, ethoxy, 2-methoxy- ethoxy, n-propoxy, i-propoxy, n-butoxy, i-butoxy, s-butoxy, t-butoxy, 2- methylbutoxy, n-pentoxy, n-hexoxy, n-heptyloxy, n-octyloxy, n-nonyloxy, n-decyloxy, n-undecyloxy, n-dodecyloxy, etc.
Preferred amino groups are, for example, dimethylamino, methylamino, methylphenylamino, phenylamino, etc.
Aryl and heteroaryl groups can be monocyclic or polycyclic, i.e. they can have one ring (such as, for example, phenyl) or two or more rings, which may also be fused (such as, for example, naphthyl) or covalently linked (such as, for example, biphenyl), or contain a combination of fused and linked rings. Heteroaryl groups contain one or more heteroatoms, preferably selected from O, N1 S and Se.
Particular preference is given to mono-, bi- or tricyclic aryl groups having 6 to 25 C atoms and mono-, bi- or tricyclic heteroaryl groups having 2 to 25 C atoms, which optionally contain fused rings and are optionally substituted. Preference is furthermore given to 5-, 6- or 7-membered aryl and heteroaryl groups, in which, in addition, one or more CH groups may be replaced by N, S or O in such a way that O atoms and/or S atoms are not linked directly to one another.
Preferred aryl groups are, for example, phenyl, biphenyl, terphenyl, [1 ,1I:3I,1 "]terphenyl-2l-ylI naphthyl, anthracene, binaphthyl, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, tetracene, pentacene, benzo- pyrene, fluorene, indene, indenofluorene, spirobifluorene, etc.
Preferred heteroaryl groups are, for example, 5-membered rings, such as pyrrole, pyrazole, imidazole, 1 ,2,3-triazole, 1 ,2,4-triazole, tetrazole, furan, thiophene, selenophene, oxazole, isoxazole, 1 ,2-thiazole, 1 ,3-thiazole, 1 ,2,3-oxadiazole, 1 ,2,4-oxadiazole, 1 ,2,5-oxadiazole, 1 ,3,4-oxadiazole, 1 ,2,3-thiadiazole, 1 ,2,4-thiadiazole, 1 ,2,5-thiadiazole, 1 ,3,4-thiadiazole, 6-membered rings, such as pyridine, pyridazine, pyrimidine, pyrazine, 1 ,3,5-triazine, 1 ,2,4-thazine, 1 ,2,3-triazine, 1 ,2,4,5-tetrazine, 1 ,2,3,4- tetrazine, 1 ,2,3,5-tetrazine, or condensed groups, such as indole, iso- indole, indolizine, indazole, benzimidazole, benzotriazole, purine, naphth- imidazole, phenanthrimidazole, pyridimidazole, pyrazinimidazole, quinoxa- linimidazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxa- zole, isoxazole, benzothiazole, benzofuran, isobenzofuran, dibenzofuran, quinoline, isoquinoline, pteridine, benzo-5,6-quinoline, benzo-6,7-quino- line, benzo-7,8-quinoline, benzoisoquinoline, acridine, phenothiazine, phenoxazine, benzopyhdazine, benzopyhmidine, quinoxaline, phenazine, naphthyhdine, azacarbazole, benzocarboline, phenanthridine, phenan-
throline, thieno[2,3b]thiophene, thieno[3,2b]thiophene, dithienothiophene, isobenzothiophene, dibenzothiophene, benzothiadiazothiophene, or combinations of these groups. The heteroaryl groups may also be substituted by alkyl, alkoxy, thioalkyl, fluorine, fluoroalkyl or further aryl or heteroaryl groups.
The (non-aromatic) alicyclic and heterocyclic groups encompass both saturated rings, i.e. those which contain exclusively single bonds, and also partially unsaturated rings, i.e. those which may also contain multiple bonds. Heterocyclic rings contain one or more heteroatoms, preferably selected from Si, O, N, S and Se.
The (non-aromatic) alicyclic and heterocyclic groups can be monocyclic, i.e. contain only one ring (such as, for example, cyclohexane), or poly- cyclic, i.e. contain a plurality of rings (such as, for example, decahydro- naphthalene or bicyclooctane). Particular preference is given to saturated groups. Preference is furthermore given to mono-, bi- or tricyclic groups having 3 to 25 C atoms, which optionally contain fused rings and are optionally substituted. Preference is furthermore given to 5-, 6-, 7- or 8- membered carbocyclic groups in which, in addition, one or more C atoms may be replaced by Si and/or one or more CH groups may be replaced by N and/or one or more non-adjacent CH2 groups may be replaced by -O- and/or -S-.
Preferred alicyclic and heterocyclic groups are, for example, 5-membered groups, such as cyclopentane, tetrahydrofuran, tetrahydrothiofuran, pyrrolidine, 6-membered groups, such as cyclohexane, silinane, cyclohexene, tetrahydropyran, tetrahydrothiopyran, 1 ,3-dioxane, 1 ,3-dithiane, piperidine, 7-membered groups, such as cycloheptane, and fused groups, such as tetrahydronaphthalene, decahydronaphthalene, indane, bicyclo[1.1.1]- pentane-1 ,3-diyl, bicyclo[2.2.2]octane-1 ,4-diyl, spiro[3.3]heptane-2,6-diyl, octahydro-4,7-methanoindane-2,5-diyl.
The aryl, heteroaryl, carbon and hydrocarbon radicals optionally have one or more substituents, which are preferably selected from the group comprising silyl, sulfo, sulfonyl, formyl, amine, imine, nitrile, mercapto, nitro,
halogen, Ci-12 alkyl, Cβ-12 aryl, Ci-12 alkoxy, hydroxyl, or combinations of these groups.
Preferred substituents are, for example, solubility-promoting groups, such as alkyl or alkoxy, electron-withdrawing groups, such as fluorine, nitro or nitrile, or substituents for increasing the glass transition temperature (Tg) in the polymer, in particular bulky groups, such as, for example, t-butyl or optionally substituted aryl groups.
Preferred substituents, also referred to as "L" below, are, for example, F, Cl, Br, I, -CN, -NO2, -NCO, -NCS, -OCN, -SCN, -C(=O)N(RX)2) -C(=O)Y1, -C(=O)RX, -N(RX)2, in which Rx has the above-mentioned meaning, and Y1 denotes halogen, optionally substituted silyl or aryl having 6 to 40, preferably 6 to 20 C atoms, and straight-chain or branched alkyl, alkoxy, alkyl- carbonyl, alkoxycarbonyl, alkylcarbonyloxy or alkoxycarbonyloxy having 1 to 25 C atoms, in which one or more H atoms may optionally be replaced by F or Cl.
"Substituted silyl or aryl" preferably means substituted by halogen, -CN, R0, -OR0, -CO-R0, -CO-O-R0, -O-CO-R0 or -O-CO-O-R0, in which R0 has the above-mentioned meaning.
Particularly preferred substituents L are, for example, F, Cl, CN, NO2, CH3, C2H5, OCH3, OC2H5, COCH3, COC2H5, COOCH3, COOC2H5, CF3, OCF3, OCHF2, OC2F5, furthermore phenyl.
in which L has one of the above-mentioned meanings.
The polymerisable group P is a group which is suitable for a polymerisation reaction, such as, for example, free-radical or ionic chain polymerisation, polyaddition or polycondensation, or for a polymer-analogous reac- tion, for example addition or condensation onto a main polymer chain. Particular preference is given to groups for chain polymerisation, in par-
ticular those containing a C=C double bond or C≡C triple bond, and groups which are suitable for polymerisation with ring opening, such as, for example, oxetane or epoxide groups.
Preferred polymerisable groups are selected from the group consisting of
, , CH
2=CW
2-(O)
k3-, CW
1=CH-CO-(O)
k3-,
CW1=CH-CO-NH-, CH2=CW1-CO-NH-, CH3-CH=CH-O-, (CH2=CH)2CH- OCO-, (CH2=CH-CH2)2CH-OCO-, (CH2=CH)2CH-O-, (CH2=CH-CH2)2N-, (CH2=CH-CH2)2N-CO-, HO-CW2W3-, HS-CW2W3-, HW2N-, HO-CW2W3- NH-, CH2=CW1-CO-NH-, CH2=CH-(COO)krPhe-(O)k2-, CH2=CH-(CO)k1- Phe-(O)k2-, Phe-CH=CH-, HOOC-, OCN- and W4W5W6Si-, in which W1 denotes H, F, Cl, CN, CF3, phenyl or alkyl having 1 to 5 C atoms, in particular H, F, Cl or CH3, W2 and W3 each, independently of one another, denote H or alkyl having 1 to 5 C atoms, in particular H, methyl, ethyl or n-propyl, W4, W5 and W6 each, independently of one another, denote Cl, oxaalkyl or oxacarbonylalkyl having 1 to 5 C atoms, W7 and W8 each, independently of one another, denote H, Cl or alkyl having 1 to 5 C atoms, Phe denotes 1 ,4-phenylene, which is optionally substituted by one or more radicals L as being defined above but being different from P-Sp, and ki, k2 and k3 each, independently of one another, denote O or 1 , k3 preferably denotes 1 , and k4 is an integer from 1 to 10.
Particularly preferred groups P are CH2=CH-COO-, CH2=C(CH3)-COO-, CH2=CF-COO-, CH2=CH-, CH2=CH-O-, (CH2=CH)2CH-OCO-,
(CH
2=CH)
2CH-O-, W
2HC CH - and
in particular vinyloxy, acrylate, methacrylate, fluoroacrylate, chloroacrylate, oxetane and epoxide.
In a further preferred embodiment of the invention, the polymerisable compounds of the formulae I and Il and sub-formulae thereof contain, instead of one or more radicals P-Sp-, one or more branched radicals containing two or more polymerisable groups P (multifunctional polymerisable radicals). Suitable radicals of this type, and polymerisable compounds containing them, are described, for example, in US 7,060,200 B1 or US 2006/0172090 A1. Particular preference is given to multifunctional polymerisable radicals selected from the following formulae:
-X-alkyl-CHP1-CH2-CH2P2 l*a
-X-alkyl-C(CH2P1 )(CH2P2)-CH2P3 l*b
-X-alkyl-CHP1CHP2-CH2P3 l*c
-X-alkyl-C(CH2P1)(CH2P2)-CaaH2aa+i l*d
-X-alkyl-CHP1-CH2P2 l*e
-X-alkyl-CHP1P2 l*f
-X-alkyl-CP1P2-CaaH2aa+1 l*g
-X-alkyl-C(CH2P1)(CH2P2)-CH2OCH2-C(CH2P3)(CH2P4)CH2P5 l*h
-X-alkyl-CH((CH2)aaP1 )((CH2)bbP2) l*i
-X-alkyl-CHP1CHP2-CaaH2aa+i l*k
in which
alkyl denotes a single bond or straight-chain or branched alkylene having 1 to 12 C atoms, in which one or more non-adjacent CH2 groups may each be replaced, independently of one another, by
-C(RX)=C(RX)-, -C≡C-, -N(RX)-, -O-, -S-, -CO-, -CO-O-, -O-CO-,
-O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by F, Cl or CN1 where Rx has the above- mentioned meaning and preferably denotes R0 as defined above,
aa and bb each, independently of one another, denote O1 1 , 2, 3, 4, 5 or 6,
X has one of the meanings indicated for X', and
pP11-"55 each, independently of one another, have one of the meanings indicated above for P.
Preferred spacer groups Sp are selected from the formula Sp'-X1, so that the radical "P-Sp-" conforms to the formula "P-Sp'-X1-", where
Sp1 denotes alkylene having 1 to 20, preferably 1 to 12 C atoms, which is optionally mono- or polysubstituted by F, Cl, Br, I or CN and in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -O-, -S-, -NH-, -NR0-,
-SiR0R00-, -CO-, -COO-, -OCO-, -OCO-O-, -S-CO-, -CO-S-, -NR°-CO-O-, -O-CO-NR0-, -NR°-CO-NR0-, -CH=CH- or -C≡C- in such a way that O and/or S atoms are not linked directly to one another,
X' denotes -O-, -S-, -CO-, -COO-, -OCO-, -O-COO-, -CO-NR0-,
-NR°-CO-, -NR°-CO-NR0-, -OCH2-, -CH2O-, -SCH2-, -CH2S-, -CF2O-, -OCF2-, -CF2S-, -SCF2-, -CF2CH2-, -CH2CF2-, -CF2CF2-, -CH=N-, -N=CH-, -N=N-, -CH=CR0-, -CY2=CY3-, -C≡C-, -CH=CH-COO-, -OCO-CH=CH- or a single bond,
R0 and R00 each, independently of one another, denote H or alkyl having 1 to 12 C atoms, and
Y2 and Y3 each, independently of one another, denote H, F, Cl or CN.
X1 is preferably -O-, -S-, -CO-, -COO-, -OCO-, -O-COO-, -CO-NR0-, -NR°-CO-, -NR°-CO-NR°- or a single bond.
Typical spacer groups Sp1 are, for example, -(CH2)pi-, -(CH2CH2O)q1- CH2CH2-, -CH2CH2-S-CH2CH2-, -CH2CH2-NH-CH2CH2- or -(SiR°R00-O)p1-, in which p1 is an integer from 1 to 12, q1 is an integer from 1 to 3, and R0 and R00 have the above-mentioned meanings.
Particularly preferred groups -X'-Sp1- are -(CH2)p1-, -O-(CH2)pr, -OCO- (CH2)pr, -OCOO-(CH2)p1-
Particularly preferred groups Sp1 are, for example, in each case straight- chain ethylene, propylene, butylene, pentylene, hexylene, heptylene, octylene, nonylene, decylene, undecylene, dodecylene, octadecylene, ethyleneoxyethylene, methyleneoxybutylene, ethylenethioethylene, ethyl- ene-N-methyliminoethylene, 1-methylalkylene, ethenylene, propenylene and butenylene.
The polymerisable compounds are prepared analogously to processes known to the person skilled in the art and described in standard works of organic chemistry, such as, for example, in Houben-Weyl, Methoden der organischen Chemie [Methods of Organic Chemistry], Thieme-Verlag, Stuttgart. The synthesis of polymerisable acrylates and methacrylates of the formula I can be carried out analogously to the methods described in US 5,723,066. Further, particularly preferred methods are given in the examples.
In the simplest case, the synthesis is carried out by esterification or etheri- fication of commercially available diols of the general formula HO-A1-(Z1- A2)mi-OH, in which A1, A2, Z1 and m have the above-mentioned meanings, such as, for example, 2,6-dihydroxynaphthalene (naphthalene-2,6-diol), or 1-(4-hydroxyphenyl)phenyl-4-ol, using corresponding acids, acid derivatives, or halogenated compounds containing a group P, such as, for example, methacryloyl chloride or methacrylic acid, in the presence of a dehydrating reagent, such as, for example, DCC (dicyclohexylcarbodiimide).
The polymerisable compounds are polymerised or crosslinked (if a compound contains two or more polymerisable groups) by in-situ polymerisation in the LC medium between the substrates of the LC display with application of a voltage. Suitable and preferred polymerisation methods are, for example, thermal or photopolymerisation, preferably photopoly- merisation, in particular UV photopolymerisation. If necessary, one or more initiators may also be added here. Suitable conditions for the polymerisation, and suitable types and amounts of initiators, are known to the person skilled in the art and are described in the literature. Suitable for free-radical polymerisation are, for example, the commercially available photoinitiators Irgacure651®, Irgacure184®, lrgacure907®, Irgacure369® or Darocure1173® (Ciba AG). If an initiator is employed, its proportion in the mixture as a whole is preferably 0.001 to 5% by weight, particularly preferably 0.001 to 1 % by weight. However, the polymerisation can also take place without addition of an initiator. In a further preferred embodiment, the LC medium does not comprise a polymerisation initiator.
The polymerisable component or the LC medium may also comprise one or more stabilisers in order to prevent undesired spontaneous polymerisation of the RMs, for example during storage or transport. Suitable types and amounts of stabilisers are known to the person skilled in the art and are described in the literature. Particularly suitable are, for example, the commercially available stabilisers of the Irganox® series (Ciba AG). If stabilisers are employed, their proportion, based on the total amount of
RMs or polymerisable component A), is preferably 10 - 5000 ppm, particularly preferably 50 - 500 ppm.
The polymerisable compounds according to the invention are also suitable for polymerisation without initiator, which is associated with considerable advantages, such as, for example, lower material costs and in particular less contamination of the LC medium by possible residual amounts of the initiator or degradation products thereof.
The LC media according to the invention preferably comprise < 5%, particularly preferably < 1 %, very particularly preferably < 0.5%, of polymer-
isable compounds, in particular polymerisable compounds of the above- mentioned formulae.
The polymerisable compounds according to the invention can be added individually to the LC media, but it is also possible to use mixtures comprising two or more polymerisable compounds. On polymerisation of mixtures of this type, copolymers are formed. The invention furthermore relates to the polymerisable mixtures mentioned above and below.
The LC media which can be used in accordance with the invention are prepared in a manner conventional per se, for example by mixing one or more of the above-mentioned compounds with one or more polymerisable compounds as defined above and optionally with further liquid-crystalline compounds and/or additives. In general, the desired amount of the com- ponents used in lesser amount is dissolved in the components making up the principal constituent, advantageously at elevated temperature. It is also possible to mix solutions of the components in an organic solvent, for example in acetone, chloroform or methanol, and to remove the solvent again, for example by distillation, after thorough mixing. The invention furthermore relates to the process for the preparation of the LC media according to the invention.
It goes without saying to the person skilled in the art that the LC media according to the invention may also comprise compounds in which, for ex- ample, H, N, O, Cl, F have been replaced by the corresponding isotopes.
The construction of the LC displays according to the invention corresponds to the conventional geometry for PSA displays, as described in the prior art cited at the outset. Geometries without protrusions are preferred, in particular those in which, in addition, the electrode on the colour filter side is unstructured and only the electrode on the TFT side has slits. Particularly suitable and preferred electrode structures for PSA-VA displays are described, for example, in US 2006/0066793 A1.
Unless the context clearly indicates otherwise, as used herein plural forms of the terms herein are to be construed as including the singular form and vice versa.
Throughout the description and claims of this specification, the words
"comprise" and "contain" and variations of the words, for example "comprising" and "comprises", mean "including but not limited to", and are not intended to (and do not) exclude other components.
It will be appreciated that variations to the foregoing embodiments of the invention can be made while still falling within the scope of the invention. Each feature disclosed in this specification, unless stated otherwise, may be replaced by alternative features serving the same, equivalent or similar purpose. Thus, unless stated otherwise, each feature disclosed is one example only of a generic series of equivalent or similar features.
All of the features disclosed in this specification may be combined in any combination, except combinations where at least some of such features and/or steps are mutually exclusive. In particular, the preferred features of the invention are applicable to all aspects of the invention and may be used in any combination. Likewise, features described in non-essential combinations may be used separately (not in combination).
The following examples explain the present invention without limiting it. However, they show the person skilled in the art preferred mixture concepts with compounds preferably to be employed and the respective concentrations thereof and combinations thereof with one another. In addition, the examples illustrate which properties and property combinations are accessible.
In the tables below the following abbreviations are used: (n, m, z: each, independently of one another, 1 , 2, 3, 4, 5 or 6)
Table A
CC-n-mV PP-n-m
CY-n-Om CY-n-m
CEY-V-m PY-n-(O)m
CQY-n-(O)m CQIY-n-(O)m
CCQY-n-(O)m CCQIY-n-(O)m
CLY-n-(O)m CYLI-n-m
CYYC-n-m
CCYY-n-m CPYG-n-(O)m
CENap-n-Om CTNap-n-Om
CETNap-n-Om CK-n-F
DFDBC-n(O)-(O)m C-DFDBF-n-(O)m
In a preferred embodiment of the present invention, the LC media according to the invention comprise one or more compounds selected from the group consisting of compounds from Table A.
Table B
Table B indicates possible dopants which can be added to the LC media according to the invention.
C 15 CB 15
CM 44 CM 45
R/S-4011 R/S-5011
The LC media preferably comprise O to 10% by weight, in particular 0.01 to 5% by weight and particularly preferably 0.1 to 3% by weight, of dopants. The LC media preferably comprise one or more dopants selected from the group consisting of compounds from Table B.
Table C
Table C indicates possible stabilisers which can be added to the LC media according to the invention.
(n here denotes an integer from 1 to 12)
35
The LC media preferably comprise 0 to 10% by weight, in particular 0.01 to 5% by weight and particularly preferably 0.1 to 3% by weight, of stabilisers. The LC media preferably comprise one or more stabilisers selected from the group consisting of compounds from Table C.
In addition, the following abbreviations and symbols are used:
V0 denotes threshold voltage, capacitive [V] at 200C, ne denotes extraordinary refractive index at 200C and 589 nm, n0 denotes ordinary refractive index at 200C and 589 nm,
Δn denotes optical anisotropy at 200C and 589 nm,
εi denotes the dielectric permittivity perpendicular to the director at 200C and 1 kHz, ε|| denotes dielectric permittivity parallel to the director at 200C and 1 kHz, Δε denotes dielectric anisotropy at 200C and 1 kHz, cl.p., T(N1I) denotes clearing point [0C], γi denotes rotational viscosity at 20°C [mPa s],
Ki denotes elastic constant, "splay" deformation at 200C [pN],
K2 denotes elastic constant, "twist" deformation at 200C [pN], K3 denotes elastic constant, "bend" deformation at 200C [pN],
LTS denotes low-temperature stability (phase), determined in test cells,
HR20 denotes voltage holding ratio at 20°C [%], and
HR100 denotes voltage holding ratio at 100°C [%].
Unless explicitly noted otherwise, all concentrations in the present application are indicated in per cent by weight and relate to the corresponding mixture or mixture component, unless explicitly indicated otherwise.
Unless explicitly noted otherwise, all temperature values indicated in the present application, such as, for example, the melting point T(C1N), the transition from the smectic (S) to the nematic (N) phase T(S1N) and the clearing point T(N1I), are indicated in degrees Celsius (0C).
All physical properties are and have been determined in accordance with "Merck Liquid Crystals, Physical Properties of Liquid Crystals", Status Nov. 1997, Merck KGaA1 Germany, and apply for a temperature of 200C, and Δn is determined at 589 nm and Δε is determined at 1 kHz, unless explicitly indicated otherwise in each case.
For the present invention, the term "threshold voltage" relates to the capa- citive threshold (Vo), also known as the Freedericksz threshold, unless explicitly indicated otherwise. In the examples, as is generally usual, the optical threshold for 10% relative contrast (V-io) may also be indicated.
The display used for measurement of the capacitive threshold voltage has two plane-parallel outer plates at a separation of 4 μm and electrode layers with overlying alignment layers of rubbed polyimide on the insides of the outer plates, which cause a homeotropic edge alignment of the liquid- crystal molecules.
The polymerisable compounds are polymerised in the display by UV irradiation for a p re-determined time, with a voltage simultaneously being applied to the display (usually 10 V to 30 V alternating current, 1 kHz). In the examples, unless indicated otherwise, a 28 mW/cm2 mercury vapour lamp was used, the intensity was measured using a standard UV meter (model Ushio UNI meter) fitted with a 365 nm band-pass filter.
The tilt angle is determined by a rotational crystal experiment (Autronic- Melchers TBA-105). A small value (i.e. a large deviation from a 90° angle) corresponds to a large tilt here.
Example 1
An LC medium consisting of the following components a)-c) a) 99.00 % of the nematic LC host mixture N1 having the composition shown below,
CY-3-O4 24,00 % Cl.p. + 88,2
CY-5-O4 10,00 % Δn 0,1443
CCY-3-O2 6,00 % Δε - 5,9
CCY-3-O3 6,00 % εll 4,2
CCY-4-O2 6,00 % 1 ,09
CCY-5-O2 4,00 % Yi 331
CPY-2-O2 10,00 % V0 1.7
CPY-3-O2 10,00 %
PYP-2-3 14,00 %
PYP-2-4 10,00 %
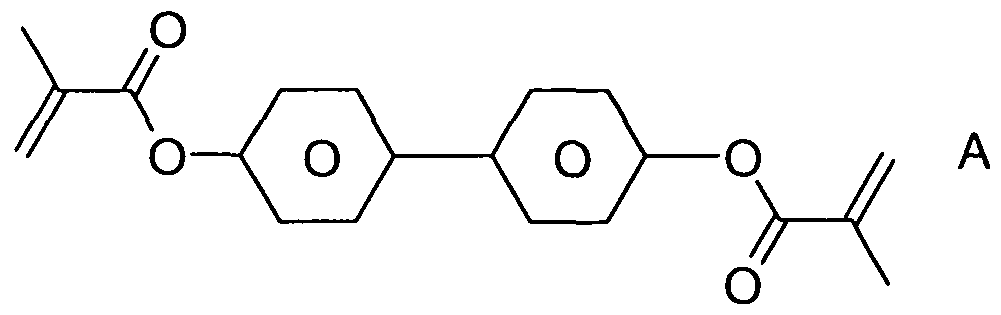
b) 0.25 % of the polymerisable monomeric compound A shown below,
and
c) 0.75 % of the chiral dopant S-4011 ,
10 is suitable for use in a PS-VA display.
Example 2
An LC medium consisting of 99.50 % of the nematic LC host mixture N1
1 S
(see Example 1 ) and 0.50 % of the polymerisable monomeric compound
A (see Example 1 ) is suitable for use in a PS-VA display.
Example 2
Of)
An LC medium consisting of 99.50 % of the nematic LC host mixture N1 (see Example 1 ), 0.25 % of the polymerisable monomeric compound A (see Example 1 ), and 0.25 % of the chiral dopant S-5011 is suitable for use in a PS-VA display.
25 Example 3
An LC medium consisting of 99.00 % of the nematic LC host mixture N1 (see Example 1 ), 0.25 % of the polymerisable monomeric compound B shown below, 30
35
and 0.75 % of the chiral dopant S-4011 is suitable for use in a PS-VA display.
Example 4
An LC medium consisting of 99.50 % of the nematic LC host mixture N1 (see Example 1 ) and 0.50 % of the polymerisable monomeric compound C shown below
is suitable for use in a PS-VA display.
Example 5
An LC medium consisting of 99.00 % of the nematic LC host mixture N1 (see Example 1 ), 0.25 % of the polymerisable monomeric compound D shown below,
and 0.75 % of the chiral dopant S-2011 is suitable for use in a PS-VA display.