WO2008067531A2 - Fiber reinforced composite material - Google Patents
Fiber reinforced composite material Download PDFInfo
- Publication number
- WO2008067531A2 WO2008067531A2 PCT/US2007/086067 US2007086067W WO2008067531A2 WO 2008067531 A2 WO2008067531 A2 WO 2008067531A2 US 2007086067 W US2007086067 W US 2007086067W WO 2008067531 A2 WO2008067531 A2 WO 2008067531A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composite
- composite material
- fiber
- mpa
- matrix
- Prior art date
Links
- 239000000463 material Substances 0.000 title claims abstract description 70
- 239000003733 fiber-reinforced composite Substances 0.000 title claims abstract description 18
- 239000002131 composite material Substances 0.000 claims abstract description 117
- 239000011159 matrix material Substances 0.000 claims abstract description 87
- 239000002657 fibrous material Substances 0.000 claims abstract description 38
- 239000000203 mixture Substances 0.000 claims abstract description 31
- 229920001432 poly(L-lactide) Polymers 0.000 claims abstract description 24
- JVTAAEKCZFNVCJ-REOHCLBHSA-N L-lactic acid Chemical compound C[C@H](O)C(O)=O JVTAAEKCZFNVCJ-REOHCLBHSA-N 0.000 claims abstract description 20
- 229910052729 chemical element Inorganic materials 0.000 claims abstract description 14
- 230000015556 catabolic process Effects 0.000 claims description 54
- 238000006731 degradation reaction Methods 0.000 claims description 53
- 239000000835 fiber Substances 0.000 claims description 52
- 229920000642 polymer Polymers 0.000 claims description 49
- 239000003795 chemical substances by application Substances 0.000 claims description 43
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 claims description 40
- 239000003365 glass fiber Substances 0.000 claims description 32
- 235000010216 calcium carbonate Nutrition 0.000 claims description 20
- 229910000019 calcium carbonate Inorganic materials 0.000 claims description 20
- 229920001577 copolymer Polymers 0.000 claims description 17
- 229920002959 polymer blend Polymers 0.000 claims description 17
- 235000002639 sodium chloride Nutrition 0.000 claims description 15
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical group [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 12
- 239000000872 buffer Substances 0.000 claims description 12
- 239000011780 sodium chloride Substances 0.000 claims description 12
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical class [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 claims description 11
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims description 10
- 230000002209 hydrophobic effect Effects 0.000 claims description 7
- 239000001506 calcium phosphate Substances 0.000 claims description 6
- 235000011010 calcium phosphates Nutrition 0.000 claims description 6
- 235000019739 Dicalciumphosphate Nutrition 0.000 claims description 5
- NKWPZUCBCARRDP-UHFFFAOYSA-L calcium bicarbonate Chemical class [Ca+2].OC([O-])=O.OC([O-])=O NKWPZUCBCARRDP-UHFFFAOYSA-L 0.000 claims description 5
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical class [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 claims description 5
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 claims description 5
- 239000001095 magnesium carbonate Substances 0.000 claims description 5
- 229910000021 magnesium carbonate Inorganic materials 0.000 claims description 5
- 235000014380 magnesium carbonate Nutrition 0.000 claims description 5
- 229910000029 sodium carbonate Inorganic materials 0.000 claims description 5
- 235000017550 sodium carbonate Nutrition 0.000 claims description 5
- 235000019731 tricalcium phosphate Nutrition 0.000 claims description 5
- 239000000047 product Substances 0.000 description 17
- 208000010392 Bone Fractures Diseases 0.000 description 12
- 206010017076 Fracture Diseases 0.000 description 12
- -1 but not limited to Chemical class 0.000 description 12
- 238000000034 method Methods 0.000 description 9
- 238000012545 processing Methods 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 229920000954 Polyglycolide Polymers 0.000 description 6
- 230000003139 buffering effect Effects 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 229920005594 polymer fiber Polymers 0.000 description 6
- 239000012783 reinforcing fiber Substances 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 239000007943 implant Substances 0.000 description 5
- 229920000747 poly(lactic acid) Polymers 0.000 description 5
- 239000004633 polyglycolic acid Substances 0.000 description 5
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 230000002411 adverse Effects 0.000 description 4
- 230000035876 healing Effects 0.000 description 4
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 239000008055 phosphate buffer solution Substances 0.000 description 4
- 229920001244 Poly(D,L-lactide) Polymers 0.000 description 3
- 238000001125 extrusion Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 230000014759 maintenance of location Effects 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- 238000013001 point bending Methods 0.000 description 3
- 229920001610 polycaprolactone Polymers 0.000 description 3
- 239000004632 polycaprolactone Substances 0.000 description 3
- CHWRSCGUEQEHOH-UHFFFAOYSA-N potassium oxide Chemical compound [O-2].[K+].[K+] CHWRSCGUEQEHOH-UHFFFAOYSA-N 0.000 description 3
- 229910001950 potassium oxide Inorganic materials 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- ODINCKMPIJJUCX-UHFFFAOYSA-N Calcium oxide Chemical compound [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 description 2
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 2
- 229920002430 Fibre-reinforced plastic Polymers 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000032683 aging Effects 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229920002988 biodegradable polymer Polymers 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 230000001010 compromised effect Effects 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- 239000011151 fibre-reinforced plastic Substances 0.000 description 2
- 239000003517 fume Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 2
- 230000002452 interceptive effect Effects 0.000 description 2
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 2
- 239000004310 lactic acid Substances 0.000 description 2
- 235000014655 lactic acid Nutrition 0.000 description 2
- 150000002596 lactones Chemical class 0.000 description 2
- 229920000052 poly(p-xylylene) Polymers 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- DCKVNWZUADLDEH-UHFFFAOYSA-N sec-butyl acetate Chemical group CCC(C)OC(C)=O DCKVNWZUADLDEH-UHFFFAOYSA-N 0.000 description 2
- KKCBUQHMOMHUOY-UHFFFAOYSA-N sodium oxide Chemical compound [O-2].[Na+].[Na+] KKCBUQHMOMHUOY-UHFFFAOYSA-N 0.000 description 2
- 210000004872 soft tissue Anatomy 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 238000005303 weighing Methods 0.000 description 2
- SJZRECIVHVDYJC-UHFFFAOYSA-M 4-hydroxybutyrate Chemical compound OCCCC([O-])=O SJZRECIVHVDYJC-UHFFFAOYSA-M 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- 229920001273 Polyhydroxy acid Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 239000004411 aluminium Substances 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 210000003423 ankle Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229910052790 beryllium Inorganic materials 0.000 description 1
- ATBAMAFKBVZNFJ-UHFFFAOYSA-N beryllium atom Chemical compound [Be] ATBAMAFKBVZNFJ-UHFFFAOYSA-N 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000000292 calcium oxide Substances 0.000 description 1
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000007596 consolidation process Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 239000002178 crystalline material Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 229920006240 drawn fiber Polymers 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 210000003041 ligament Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 229920001434 poly(D-lactide) Polymers 0.000 description 1
- 229920000117 poly(dioxanone) Polymers 0.000 description 1
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 1
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920002643 polyglutamic acid Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 235000011176 polyphosphates Nutrition 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 230000035882 stress Effects 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- 230000008542 thermal sensitivity Effects 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
- 210000000707 wrist Anatomy 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/12—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A61L31/125—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix
- A61L31/128—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix containing other specific inorganic fillers not covered by A61L31/126 or A61L31/127
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K7/00—Use of ingredients characterised by shape
- C08K7/02—Fibres or whiskers
- C08K7/04—Fibres or whiskers inorganic
- C08K7/14—Glass
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/12—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A61L31/125—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix
- A61L31/129—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix containing macromolecular fillers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/148—Materials at least partially resorbable by the body
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/04—Reinforcing macromolecular compounds with loose or coherent fibrous material
- C08J5/0405—Reinforcing macromolecular compounds with loose or coherent fibrous material with inorganic fibres
- C08J5/043—Reinforcing macromolecular compounds with loose or coherent fibrous material with inorganic fibres with glass fibres
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/04—Reinforcing macromolecular compounds with loose or coherent fibrous material
- C08J5/046—Reinforcing macromolecular compounds with loose or coherent fibrous material with synthetic macromolecular fibrous material
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/24—Acids; Salts thereof
- C08K3/26—Carbonates; Bicarbonates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K9/00—Use of pretreated ingredients
- C08K9/02—Ingredients treated with inorganic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B2017/00004—(bio)absorbable, (bio)resorbable or resorptive
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/24—Acids; Salts thereof
- C08K3/26—Carbonates; Bicarbonates
- C08K2003/265—Calcium, strontium or barium carbonate
Definitions
- the present disclosure relates to bioresorbable composites and more specifically to a fiber reinforced polymer composite material that is used to make bioresorbable products, RELATED ART
- the currently marketed bioresorbable products include those products manufactured from injection molded polymers, polymer blends, and co-polymers. These products have been utilized in the areas of craniomaxilofacial implants and non-load bearing fracture fixation implants, such as pins and screws, for wrist and ankle applications and for reattaching soft tissues, such as ligaments and tendons, to bone. In addition, there are also some spinal products available that make use of the compressive properties of these polymers. Products including these materials are easy to process, but are limited by the mechanical properties of the materials. These materials have a tensile strength in the range of between about 50 MPa to about 100 MPa. Depending on the choice of polymer or co-polymer, products in this category retain the majority of their strength for less than about 12 weeks. Therefore, these materials are not suitable for fracture fixation applications beyond simple non-loaded pins and screws.
- bioresorbable products include self reinforced products that have improved strength due to orientation of the polymer during processing of the product. Even though these products have improved strength, their flexural strength is still only around 250 MPa. This limits the uses of this technology for fracture fixation to screws and pins.
- composites have been made where the matrix was a polymer with the same chemical composition as the fiber or where the matrix was a blend with the majority of the blend being a polymer with the same chemical composition as the matrix. These composites have an initial flexural strength of between 120 to 140 MPa, with most of this strength lost within about 12 weeks of use.
- a fiber polymer composite contains at least 50% of fiber by volume, it would be anticipated that a calcium carbonate-containing matrix would interfere adversely with the interface between the polymer matrix and reinforcing fibers. This could result in the fiber-reinforced composite substantially weakening or even falling apart before complete healing of a fracture.
- the fiber and matrix material have certain requirements.
- the fiber needs to have both a high initial tensile strength, and the ability to retain the majority of this strength, for the fracture to heal.
- the fibers need to be highly orientated and be present at about 40% by volume of the composite.
- the fibers should also have some crystallinity, as this imparts stability against relaxation of the orientation in the fiber.
- the matrix material also needs to be able to retain the majority of its strength for a suitable time, approximately between about 6 to about 12 weeks, for the fracture to heal.
- the matrix should have a sufficiently high initial molecular weight.
- Additives such as calcium carbonate or other buffering materials, can be added to the matrix to control the degradation rate.
- the amount of the buffering material should be around 30% by weight of the matrix without adversely interfering with the interface between the polymer matrix and the reinforcing fibers.
- the matrix material needs to be processable at a temperature which is low enough to not significantly affect the strength of the fiber and adhere well enough to the fiber to allow stress transfer from the matrix to the fiber.
- both semi- crystalline and amorphous co-polymers can be used.
- Semi-crystalline co-polymers are typically composed of lactic acid and one or more additional monomer units whose function is to lower the melting point of the co-polymer matrix to a point where the strength of the fiber is not affected during the consolidation step.
- Amorphous or non- crystalline materials, such as poly (D- lactide) acid polymers, are suitable for processing with the fiber, as they soften at relatively low temperatures. However, these materials do not have a long strength retention time.
- This strength retention can be improved by incorporating a buffering material, such as calcium carbonate, into the matrix material.
- a buffering material such as calcium carbonate
- the calcium carbonate acts as both a buffer and also reduces the thermal sensitivity of the polymer to breakdown during processing.
- the affect of the calcium carbonate is to both slow the rate of degradation of the polymer and help preserve the molecular weight during processing, without adversely interfering with the interface between the polymer matrix and the reinforcing fibers.
- the present disclosure incorporates these requirements to produce a bioresorbable material which has a high initial strength and retains a significant proportion of this strength for a useful time.
- the present disclosure relates to a fiber reinforced composite material including a PLLA fiber material, such as a continuous PLLA fiber material, and a matrix material that does not have the same chemical element composition as the fiber material.
- the composite further includes a degradation controlling agent dispersed in the matrix material.
- the degradation controlling agent includes a buffer material selected from a group including calcium carbonate, calcium hydrogen carbonates, calcium phosphates, tricalcium phosphates, dicalcium phosphates, magnesium carbonate, and sodium carbonate.
- the degradation controlling agent includes a common salt.
- the degradation controlling agent is selected from a group including a buffer material, a common salt, and combinations thereof.
- the degradation controlling agent is between about 0.1% to about 40% by weight of the matrix material.
- the composite further includes an accelerant dispersed in the fiber or matrix material.
- the PLLA fiber material is about 50% by volume of the composite.
- the fiber material, which is bioabsorbable has a tensile strength of between about 500 MPa to about 2000 MPa and a molecular weight of between about 290,000 g/mol and about 516,000 g/mol.
- the matrix material is bioresorbable and is selected from a group including a polymer, a copolymer, and a polymer blend.
- the blend when a polymer blend is used as the matrix, the blend includes at least two polymers and at least one of the polymers has a chemical element composition that is different to that of the fiber.
- the polymer having a chemical element composition that is different to that of the fiber comprises at least 50% of the polymer blend.
- the polymer having a chemical element composition that is different to that of the fiber comprises more than 50% of the polymer blend.
- the matrix material is bioabsorbable.
- the composite has an initial tensile strength of at least 250 MPa and retains at least 75% of the initial tensile strength for at least 8 weeks.
- the composite material includes a flexural strength of about 200 MPa and a shear strength of at least 140 MPa.
- the present disclosure includes a fiber reinforced composite material having a matrix material, a glass fiber material, and a degradation controlling agent.
- the matrix material is selected from a group including a polymer, a copolymer, and a polymer blend.
- the matrix material is bioabsorbable.
- the glass fiber material is bioabsorbable.
- the glass fiber material includes a tensile strength between about 300 MPa and about 1200 MPa.
- the glass fiber material includes a hydrophobic material.
- the glass fiber material is about 50% by volume of the composite.
- the degradation controlling agent is dispersed in the matrix material.
- the degradation controlling agent is coated on a surface of the fiber material.
- the degradation controlling agent is between about 0.1% to about 40% by weight of the matrix material.
- the degradation controlling agent includes a buffer material selected from a group including calcium carbonate, calcium hydrogen carbonates, calcium phosphates, tricalcium phosphates, dicalcium phosphates, magnesium carbonate, and sodium carbonate.
- the degradation controlling agent includes a common salt, hi an embodiment, the degradation controlling agent is selected from a group including a buffer material, a common salt, and combinations thereof.
- the composite has an initial tensile strength of at least 250 MPa and retains the initial tensile strength for at least 8 weeks.
- the composite includes an initial flexural strength of between about 250 MPa and about 400 MPa.
- the composite includes an initial flexural modulus of between about 20-30 GPa.
- the composite retains about 98% of an initial mass for at least 2 weeks.
- the present disclosure includes a fiber reinforced composite material having a matrix material, a fiber material, and a degradation controlling agent.
- the present disclosure includes a fiber reinforced composite material having a matrix material and a glass fiber material, wherein the glass fiber material includes a tensile strength of between about 300 MPa and about 1200 MPa.
- the present disclosure includes a fiber reinforced composite material having a PLLA fiber material and a matrix material, wherein the fiber material includes a molecular weight of between about 290,000 g/mol and about 516,000 g/mol.
- the present disclosure relates to a fiber-reinforced composite material having a PLLA fiber material and a matrix material that does not have the same chemical element composition as the fiber material.
- a continuous PLLA fiber is extruded and drawn to provide the fiber with a tensile strength of between about 500 MPa to about 2000 MPa and a molecular weight of between about 290,000 g/mol to about 516,000 g/mol.
- the extrusion and drawing process used to make the fiber may be any extrusion and drawing process known to one of ordinary skill in the art.
- the PLLA fiber material is about 50% by volume of the composite and is bioabsorbable.
- the matrix material which is bioabsorbable and selected from a group that includes a polymer, a copolymer, and a polymer blend, is then made.
- a matrix material that does not have the same chemical element composition as the fiber material is defined as the following: If the matrix material is a polymer, then the polymer may not be a pure polylactide material. If the matrix material is a copolymer, then at least one of the monomeric species is not a lactone monomer. If the matrix material is a polymer blend, then at least one of the polymers has a chemical element composition that is different to that of the fiber.
- the polymer that has a chemical element composition different to that of the fiber comprises at least 50% or more of the polymer blend.
- a matrix material that has the same chemical element composition as the fiber material which is also within the scope of this disclosure, is defined as the following: If the matrix material is a polymer, then the polymer is a pure polylactide material. If the matrix material is a copolymer, then both monomeric species are lactone monomers. If the matrix material is a polymer blend, then both polymers are pure polylactide materials.
- the composite may further include a degradation controlling agent.
- the degradation controlling agent may include a buffer material, a common salt, and combinations thereof.
- the buffer material is selected from a group including, but not limited to, calcium carbonate, calcium hydrogen carbonates, calcium phosphates, tricalcium phosphates, dicalcium phosphates, magnesium carbonate, and sodium carbonate.
- the common salt is water soluble and may be organic or inorganic.
- the salt may be based on, without limitation, one of the following: a Group I metal, including but not limited to, lithium, sodium, and potassium; a Group II metal, including but not limited to, beryllium, magnesium, calcium, strontium, and barium; transition metals, including but not limited to, copper, zinc, silver, gold, iron, and titanium; a Group III metal, including but not limited to, aluminum and boron.
- the salt may include, without limitation, a carbonate, a hydrogen carbonate, a phosphate, a hydrogen phosphate, silicates, polyphosphates, and polysilicates.
- the salt may be a single element, a compound, or a mixture thereof.
- the degradation controlling agent is dispersed in the matrix material and is used as a buffer agent and to slow the degradation of the composite.
- the degradation controlling agent is between about 0.1% to about 40% by weight of the matrix material.
- the composite may further include an accelerant, such as the tertiary butyl ester of lauric acid or the ditertiary butyl ester of fumaric acid, dispersed in the matrix material or fiber material. Other accelerants known to those of ordinary skill in the art may be used. Use of these accelerants accelerates the degradation rate of the fiber or matrix.
- the composite material has an initial tensile strength of at least 250 MPa and retains at least 75% of this initial tensile strength for at least 8 weeks. For the purposes of this disclosure, an initial tensile strength is taken to mean the tensile strength of the composite material prior to degradation.
- the composite has a fiexural strength of about 200 MPa and a shear strength of at least 140 MPa.
- the present disclosure relates to a fiber-reinforced composite material including a matrix material, a glass fiber material, and a degradation controlling agent.
- the matrix material may be any biodegradable polymer, polymer blend, copolymer, or other biodegradable material known to those skilled in the art.
- biodegradable polymers include alpha-polyhydroxy acids, polyglycolide (PGA), poly(L-lactide), poly(D,L-lactide), poly(.epsilon.-caprolactone), poly(trimethylene carbonate), poly(ethylene oxide) (PEO), poly(.beta.hydroxybutyrate) (PHB), poly(.beta.-hydroxyvalerate) (PHVA), poly(p- dioxanone) (PDS), poly(ortho esters), tyrosine-derived polycarbonates, polypeptides, polyurethane, and combinations thereof.
- the glass fiber material is bioabsorbable and represents about 50% by volume of the composite.
- the glass fiber material may be extruded and drawn by any extrusion and drawing process known to one of ordinary skill in the art.
- the fiber includes a tensile strength of between about 300 MPa and about 1200 MPa.
- the fiber material may include a hydrophobic material to slow down the degradation of the glass fiber material.
- the hydrophobic material may be a component of the composition of the glass fiber material or coated on a surface of the glass fiber material. Examples of hydrophobic materials include, without limitation, polycaprolactone, poly-para-xylylene (e.g. Parylene), isomers and co-polymers of polylactide, polypeptide, ceramic materials (i.e.
- the glass fibers include about 50 mol % potassium oxide (P2O 5 ), about 30 mol % calcium oxide (CaO), about 15 mol % sodium oxide (Na 2 O), and 5 mol % iron oxide (Fe 2 O 3) .
- P2O 5 potassium oxide
- CaO calcium oxide
- Na 2 O sodium oxide
- Fe 2 O 3 iron oxide
- glass fibers of different compositions may be used.
- the degradation controlling agent may be of the same type as the degradation controlling agents described above and may be dispersed in the matrix material or coated on a surface of the fiber material.
- the agent acts as a means to control the degradation of the composite and/or the glass fiber. Specifically, with regards to the glass fibers, it is believed that the common salt substantially reduces the release of ions from the fibers.
- the degradation controlling agent is dispersed in the matrix material, the agent represents between about 0.1% to about 40% by weight of the matrix material.
- the composite has an initial tensile strength of at least 250 MPa and is able to retain this initial tensile strength for at least 8 weeks.
- the composite includes an initial flexural strength of between about 250 MPa and about 400 MPa.
- the composite retains about 98% of an initial mass for at least 2 weeks when it is placed in in-vivo conditions.
- the reinforcing fibers of both composites preferably have mechanical properties that are not substantially compromised when tested in a physiological (aqueous, 37° C.) environment.
- the fibers are preferably insoluble in the solvent used to dissolve the matrix polymer.
- the degradation controlling agent of both composites must be one that reacts with the acid by-products that are generated during the degradation of the polymer fiber or matrix or the glass fiber, including, without limitation, lactic acid, glycolic acid, caproic acid, and different forms of phosphoric acid.
- the particles may have a number of sizes, ranging from about 1 mm to about 10 ran, and geometries, such as needle, cubic, platelet, fibers, spheres, and other geometries known to one of ordinary skill in the art. It is important, but not required, that the particles have a shape that enhances the mechanical properties of the particles.
- Biological agents such as cells, growth factors, antibiotics, anti-microbials, or other such factors may be added to one or more components of the composites to promote healing of the fracture.
- PLLA fiber was first made by taking PLLA granules with a nominal intrinsic viscosity of 3.8 and extruding the granules into a fiber.
- a single screw extruder fitted with a gear pump and a 2 mm spinneret die was used. The extruder also had a provision for air cooling.
- the extruded fiber was batched on spools for the next processing step. Subsequently, the fiber was progressively stretched at elevated temperatures to produce a final diameter of ca. 100 microns and a draw ratio between about 8 and about 15.
- the final molecular weight of the drawn fiber was between about 290,000 g/mol "1 to about 516,000 gmol "1 .
- the resultant fiber had an average tensile strength of greater than about 800 MPa.
- Composites were then made using an 85:15 co-polymer of PDLLA and PGA with a 35 % weight addition of calcium carbonate (CaCO 3 ) as the matrix material.
- the drawn poly (L-lactide) fibers were then wound around a support frame of parallel bars that were held a constant distance apart. For each sample the fiber was wrapped 75 times around the support frame, resulting in 150 fibers in each composite.
- the matrix was dissolved in a solvent, methyl acetate, at 10% wt/vol of solvent.
- the solvent/polymer mixture was then coated onto the fibers.
- the composite was then placed in a vacuum oven at 40 0 C for 12 hours to remove the solvent.
- the composite was then placed in a cylindrical mold and heated to 165°C. This temperature is used to melt the matrix material to allow it to flow and consolidate the composite. Once thermal equilibrium was reached, slight tension was applied to the fibers to align them in the mold. The mold was then closed completely to consolidate the fibers and the matrix. The closed mold was then maintained at 165°C for up to 5 minutes and then removed from the heated press and placed between cool metal blocks to cool the composite down to room temperature to allow tension to be released from the fibers.
- Composites were made that included poly-L-lactic acid (PLLA) fibers and a copolymer matrix of poly-L-lactic acid (PLLA) and polyglycolic acid (PGA) (PLGA 85: 15) using the method described in example 1.
- the composite did not include calcium carbonate or other degradation controlling agents.
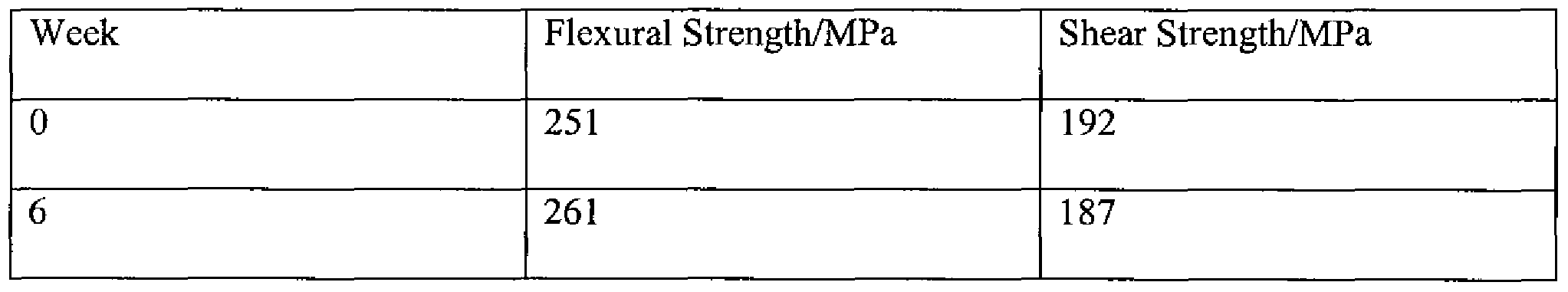
- the flexural and shear properties of the resultant pins were tested, via a 3-point bending test, after aging in PBS at 37°C. The results are given in Table 3. TABLE 3
- the coated fiber strips were cut into 120 mm lengths and compression molded at 16O 0 C to produce composite bars with nominal measurements of 10 x 3 x 120 mm. The bars were accurately measured and weighed to calculate their compositions.
- the flexural mechanical properties of the composites were tested using a 3 point bend test method. The length/distance ratio of the composites was 32 and the test speed was 4.74 mrn/min. The moduli were determined from 3 measurements and the strength/strain to failure from 1 specimen.
- the compositions and mechanical properties results are shown in Table 6.
- the table shows that the glass fiber composites have substantially similar flexural strengths to the polymer fiber composites in Table 2.
- the modulus is a quantity that expresses the degree to which a substance possesses a property, such as elasticity.
- the composites were compression moulded in an aluminium mould with a cavity measuring 120 x 3 x 10 mm.
- the mould was lined with a strip of PTFE impregnated glass cloth to allow the product to be removed more easily.
- the moulding was done at 160 0 C under 100 kN pressure.
- the mould was pre-heated and then strips were loaded into the cavity by hand one or two at a time. Once the mould was full, the pressure was applied for a few seconds, the mould was then re-opened, and further strips added. This was repeated until no further strips could be forced into the mould.
- the mould was then cooled to room temperature under pressure.
- the composite bars were trimmed and then capped with a layer of filled matrix to seal the ends.
- the weights and compositions of the fibers are shown in Table 7. TABLE 7
- the polymer fiber composite material of the present disclosure includes a polylactic acid fiber of high strength and a matrix material that is suitable for working with this fiber.
- the matrix allows for a good interfacial strength between the fiber and the matrix, which provides the composite with a high mechanical strength and a decreased degradation rate.
- polymer and glass fiber composite materials having a concentration of buffering material that has been shown to not adversely interfere with the interface between the polymer matrices and the fiber materials. Rather, the testing results show that the buffering material works to provide the composite with the ability to retain a majority of its initial strength over a longer period of time by slowing the rate of degradation of the polymer matrix and, in the glass fiber composite, the degradation rate of the glass fiber.
- a composite material containing a matrix material and a mixture of the above- described glass and polymer fibers, with or without a degradation controlling agent, is also within the scope of this disclosure.
- the matrix and the glass and polymer fibers may be of the same type and made by the same processes as the above-described matrices and polymer/glass fibers.
- the degradation contolling agents may be of the same type as described above.
- the processing conditions for making the composite may be the same as the processing conditions for making the above-described polymer fiber composites.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Materials Engineering (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Polymers & Plastics (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Animal Behavior & Ethology (AREA)
- Vascular Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Surgery (AREA)
- Epidemiology (AREA)
- Composite Materials (AREA)
- Manufacturing & Machinery (AREA)
- Inorganic Chemistry (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Reinforced Plastic Materials (AREA)
- Materials For Medical Uses (AREA)
- Laminated Bodies (AREA)
Abstract
Description
Claims
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE602007011671T DE602007011671D1 (en) | 2006-11-30 | 2007-11-30 | FIBER REINFORCED COMPOSITE MATERIAL |
| CN2007800438419A CN101594831B (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
| EP07864978A EP2120745B1 (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
| AU2007325001A AU2007325001B2 (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
| CA2679365A CA2679365C (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
| JP2009539508A JP2010511751A (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
| US12/516,573 US8722783B2 (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
| AT07864978T ATE493081T1 (en) | 2006-11-30 | 2007-11-30 | FIBER REINFORCED COMPOSITE MATERIAL |
| US14/262,018 US20140235754A1 (en) | 2006-11-30 | 2014-04-25 | Fiber reinforced composite material |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US86797806P | 2006-11-30 | 2006-11-30 | |
| US60/867,978 | 2006-11-30 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/516,573 A-371-Of-International US8722783B2 (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
| US14/262,018 Division US20140235754A1 (en) | 2006-11-30 | 2014-04-25 | Fiber reinforced composite material |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2008067531A2 true WO2008067531A2 (en) | 2008-06-05 |
| WO2008067531A3 WO2008067531A3 (en) | 2008-10-02 |
Family
ID=39432887
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2007/086067 WO2008067531A2 (en) | 2006-11-30 | 2007-11-30 | Fiber reinforced composite material |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US8722783B2 (en) |
| EP (1) | EP2120745B1 (en) |
| JP (3) | JP2010511751A (en) |
| CN (2) | CN102274552B (en) |
| AT (1) | ATE493081T1 (en) |
| AU (2) | AU2007325001B2 (en) |
| CA (1) | CA2679365C (en) |
| DE (1) | DE602007011671D1 (en) |
| ES (1) | ES2361360T3 (en) |
| WO (1) | WO2008067531A2 (en) |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010122098A2 (en) | 2009-04-23 | 2010-10-28 | Vivoxid Oy | Biocompatible composite and its use |
| CN102677304A (en) * | 2012-05-29 | 2012-09-19 | 蔡紫林 | Yarn-dyed fabric |
| CN104857577A (en) * | 2015-05-28 | 2015-08-26 | 上海益生源药业有限公司 | Absorbable bone fixation material and preparation method thereof |
| US9120919B2 (en) | 2003-12-23 | 2015-09-01 | Smith & Nephew, Inc. | Tunable segmented polyacetal |
| US9770534B2 (en) | 2007-04-19 | 2017-09-26 | Smith & Nephew, Inc. | Graft fixation |
| US9815240B2 (en) | 2007-04-18 | 2017-11-14 | Smith & Nephew, Inc. | Expansion moulding of shape memory polymers |
| US10028776B2 (en) | 2010-10-20 | 2018-07-24 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| US10525169B2 (en) | 2010-10-20 | 2020-01-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US10525168B2 (en) | 2010-10-20 | 2020-01-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US10857261B2 (en) | 2010-10-20 | 2020-12-08 | 206 Ortho, Inc. | Implantable polymer for bone and vascular lesions |
| EP3470097B1 (en) | 2017-10-16 | 2021-03-31 | Arctic Biomaterials Oy | Orthopedic bioabsorbable implants |
| US11058796B2 (en) | 2010-10-20 | 2021-07-13 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11207109B2 (en) | 2010-10-20 | 2021-12-28 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11291483B2 (en) | 2010-10-20 | 2022-04-05 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| US11351261B2 (en) | 2010-10-20 | 2022-06-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| US11352491B2 (en) | 2016-09-08 | 2022-06-07 | Schaefer Kalk Gmbh & Co. Kg | Calcium-salt-containing composite powder having microstructured particles |
| US11441008B2 (en) | 2016-09-08 | 2022-09-13 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles |
| US11484627B2 (en) | 2010-10-20 | 2022-11-01 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11548998B2 (en) | 2016-09-08 | 2023-01-10 | Schaefer Kalk Gmbh & Co. Kg | Inhibiting calcium carbonate additive |
| US11760874B2 (en) | 2016-09-08 | 2023-09-19 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles having inhibiting calcium carbonate |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9849216B2 (en) | 2006-03-03 | 2017-12-26 | Smith & Nephew, Inc. | Systems and methods for delivering a medicament |
| US8852625B2 (en) | 2006-04-26 | 2014-10-07 | Micell Technologies, Inc. | Coatings containing multiple drugs |
| AU2008242737B2 (en) | 2007-04-19 | 2013-09-26 | Smith & Nephew, Inc. | Multi-modal shape memory polymers |
| CN102481195B (en) | 2009-04-01 | 2015-03-25 | 米歇尔技术公司 | Drug delivery medical device |
| JP5633291B2 (en) * | 2010-10-05 | 2014-12-03 | 東洋製罐株式会社 | Biodegradable resin composition |
| US9415440B2 (en) | 2010-11-17 | 2016-08-16 | Alcoa Inc. | Methods of making a reinforced composite and reinforced composite products |
| GB201102468D0 (en) * | 2011-02-11 | 2011-03-30 | Univ Manchester | Biocompatible composite materials |
| CN102247622A (en) * | 2011-06-10 | 2011-11-23 | 东华大学 | Degradable fiber-enhanced polycaprolactone degradable bone nail and preparation method thereof through solution method |
| CN103796618A (en) * | 2011-07-15 | 2014-05-14 | 史密夫和内修有限公司 | Fiber-reinforced composite orthopaedic device having embedded electronics |
| KR101269127B1 (en) * | 2011-10-18 | 2013-05-29 | 포항공과대학교 산학협력단 | Membrane type scaffold and fabrication method thereof |
| ES2706149T3 (en) * | 2012-02-08 | 2019-03-27 | Toray Industries | Material sensitive to stimuli and medical material that comprises |
| WO2014165264A1 (en) | 2013-03-12 | 2014-10-09 | Micell Technologies, Inc. | Bioabsorbable biomedical implants |
| KR102079613B1 (en) * | 2013-05-15 | 2020-02-20 | 미셀 테크놀로지즈, 인코포레이티드 | Bioabsorbable biomedical implants |
| CN103611198B (en) * | 2013-12-03 | 2016-09-28 | 中国科学院长春应用化学研究所 | A kind of absorbable medical perforated membrane and preparation method thereof |
| US10869954B2 (en) * | 2016-03-07 | 2020-12-22 | Ossio, Ltd. | Surface treated biocomposite material, medical implants comprising same and methods of treatment thereof |
| CN105818492B (en) * | 2016-03-29 | 2018-02-16 | 中材科技股份有限公司 | A kind of bioactivity phosphate base continuous glass fibre composite material for weaving and application thereof |
| CN106039424B (en) * | 2016-05-25 | 2019-04-05 | 南京凤源新材料科技有限公司 | A kind of novel polylactic acid glass fiber composite material for skeletal fixation |
| DE102016116387A1 (en) * | 2016-09-01 | 2018-03-01 | Karl Leibinger Medizintechnik Gmbh & Co. Kg | Fiber-reinforced bioresorbable implant and method for its production |
| CN110144064B (en) * | 2019-05-28 | 2021-08-13 | 广东工业大学 | Bio-based reinforcing material, bio-based composite material and preparation method thereof |
| CN112679760B (en) * | 2020-11-19 | 2021-12-21 | 宁波宝亭生物科技有限公司 | Preparation method of glass fiber reinforced biodegradable polymer composite material |
| CN116036386B (en) * | 2023-02-22 | 2024-04-30 | 天津纳博特医疗器械有限公司 | Absorbable glass fiber reinforced polylactic acid composite material and craniomaxillofacial nail plate system |
Family Cites Families (343)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL216498A (en) | 1955-11-30 | |||
| US3531561A (en) | 1965-04-20 | 1970-09-29 | Ethicon Inc | Suture preparation |
| BE758156R (en) | 1970-05-13 | 1971-04-28 | Ethicon Inc | ABSORBABLE SUTURE ELEMENT AND ITS |
| US3797499A (en) | 1970-05-13 | 1974-03-19 | Ethicon Inc | Polylactide fabric graphs for surgical implantation |
| US3736646A (en) | 1971-10-18 | 1973-06-05 | American Cyanamid Co | Method of attaching surgical needles to multifilament polyglycolic acid absorbable sutures |
| DE2817778A1 (en) * | 1977-05-09 | 1978-11-23 | Firestone Tire & Rubber Co | FIBERGLASS REINFORCED POLYAMIDE COMPOSITIONS |
| US4137921A (en) | 1977-06-24 | 1979-02-06 | Ethicon, Inc. | Addition copolymers of lactide and glycolide and method of preparation |
| US4181983A (en) | 1977-08-29 | 1980-01-08 | Kulkarni R K | Assimilable hydrophilic prosthesis |
| DE2947985A1 (en) * | 1979-11-28 | 1981-09-17 | Vsesojuznyj naučno-issledovatel'skij i ispytatel'nyj institut medicinskoj techniki, Moskva | Matrix material for fixing bone fractures - consisting of a copolymer of hydrophilic and hydrophobic monomers reinforced with resorbable non-non-toxic fibres |
| JPS56164842A (en) * | 1980-05-23 | 1981-12-18 | Toray Industries | Carbon fiber reinforced thermoplastic resin molding |
| JPS5798556A (en) * | 1980-10-20 | 1982-06-18 | American Cyanamid Co | Refomation of polyglycolic acid obtaining variable vital body physical properties |
| US5110852A (en) | 1982-07-16 | 1992-05-05 | Rijksuniversiteit Te Groningen | Filament material polylactide mixtures |
| US4700704A (en) | 1982-10-01 | 1987-10-20 | Ethicon, Inc. | Surgical articles of copolymers of glycolide and ε-caprolactone and methods of producing the same |
| US4523591A (en) | 1982-10-22 | 1985-06-18 | Kaplan Donald S | Polymers for injection molding of absorbable surgical devices |
| US4539981A (en) | 1982-11-08 | 1985-09-10 | Johnson & Johnson Products, Inc. | Absorbable bone fixation device |
| US4438253A (en) | 1982-11-12 | 1984-03-20 | American Cyanamid Company | Poly(glycolic acid)/poly(alkylene glycol) block copolymers and method of manufacturing the same |
| JPS6017118A (en) * | 1983-07-06 | 1985-01-29 | Mitsubishi Mining & Cement Co Ltd | Calcium phosphate fiber |
| US4636215A (en) | 1984-01-11 | 1987-01-13 | Rei, Inc. | Combination tray and condylar prosthesis for mandibular reconstruction and the like |
| US4990161A (en) | 1984-03-16 | 1991-02-05 | Kampner Stanley L | Implant with resorbable stem |
| US4559945A (en) | 1984-09-21 | 1985-12-24 | Ethicon, Inc. | Absorbable crystalline alkylene malonate copolyesters and surgical devices therefrom |
| US4604097A (en) * | 1985-02-19 | 1986-08-05 | University Of Dayton | Bioabsorbable glass fibers for use in the reinforcement of bioabsorbable polymers for bone fixation devices and artificial ligaments |
| FI75493C (en) | 1985-05-08 | 1988-07-11 | Materials Consultants Oy | SJAELVARMERAT ABSORBERBART PURCHASING SYNTHESIS. |
| US6005161A (en) | 1986-01-28 | 1999-12-21 | Thm Biomedical, Inc. | Method and device for reconstruction of articular cartilage |
| FI80605C (en) * | 1986-11-03 | 1990-07-10 | Biocon Oy | Bone surgical biocomposite material |
| FI81498C (en) * | 1987-01-13 | 1990-11-12 | Biocon Oy | SURGICAL MATERIAL OCH INSTRUMENT. |
| US4756307A (en) | 1987-02-09 | 1988-07-12 | Zimmer, Inc. | Nail device |
| JPH0781204B2 (en) * | 1987-04-21 | 1995-08-30 | 株式会社バイオマテリアルユニバ−ス | Polylactic acid fiber |
| US5527337A (en) | 1987-06-25 | 1996-06-18 | Duke University | Bioabsorbable stent and method of making the same |
| DE8716607U1 (en) | 1987-12-14 | 1989-01-12 | Mecron Medizinische Produkte GmbH, 12277 Berlin | Implantable prosthesis |
| US4916207A (en) | 1987-12-17 | 1990-04-10 | Allied-Signal, Inc. | Polycarbonate homopolymer-based fiber compositions and method of melt-spinning same and device |
| JP2561853B2 (en) | 1988-01-28 | 1996-12-11 | 株式会社ジェイ・エム・エス | Shaped memory molded article and method of using the same |
| GB2215209B (en) | 1988-03-14 | 1992-08-26 | Osmed Inc | Method and apparatus for biodegradable, osteogenic, bone graft substitute device |
| US5444113A (en) | 1988-08-08 | 1995-08-22 | Ecopol, Llc | End use applications of biodegradable polymers |
| US5502158A (en) | 1988-08-08 | 1996-03-26 | Ecopol, Llc | Degradable polymer composition |
| US5250584A (en) | 1988-08-31 | 1993-10-05 | G-C Dental Industrial Corp. | Periodontium-regenerative materials |
| JPH0739506B2 (en) | 1988-09-30 | 1995-05-01 | 三菱重工業株式会社 | Shape memory polymer foam |
| US4938763B1 (en) | 1988-10-03 | 1995-07-04 | Atrix Lab Inc | Biodegradable in-situ forming implants and method of producing the same |
| US5633002A (en) | 1988-10-04 | 1997-05-27 | Boehringer Ingelheim Gmbh | Implantable, biodegradable system for releasing active substance |
| DE3936188A1 (en) | 1988-11-01 | 1990-05-03 | Boehringer Ingelheim Kg | Continuous prodn. of bio:absorbable polyester(s) - by polymerisation in temp.-controlled extruder |
| FI85223C (en) | 1988-11-10 | 1992-03-25 | Biocon Oy | BIODEGRADERANDE SURGICAL IMPLANT OCH MEDEL. |
| US5037178A (en) | 1988-12-22 | 1991-08-06 | Kingston Technologies, L.P. | Amorphous memory polymer alignment device |
| FR2641692A1 (en) | 1989-01-17 | 1990-07-20 | Nippon Zeon Co | Plug for closing an opening for a medical application, and device for the closure plug making use thereof |
| US5108755A (en) | 1989-04-27 | 1992-04-28 | Sri International | Biodegradable composites for internal medical use |
| DK0401844T3 (en) | 1989-06-09 | 1996-02-19 | Aesculap Ag | Resorbable moldings and processes for making them |
| US5294395A (en) | 1989-09-01 | 1994-03-15 | Ethicon, Inc. | Thermal treatment of theraplastic filaments for the preparation of surgical sutures |
| DE58908155D1 (en) | 1989-09-15 | 1994-09-08 | N Proizv Ob Edinenie Kompleksn | ENDOPROTHESIS OF THE HIP JOINT. |
| US5053035A (en) | 1990-05-24 | 1991-10-01 | Mclaren Alexander C | Flexible intramedullary fixation rod |
| US7208013B1 (en) | 1990-06-28 | 2007-04-24 | Bonutti Ip, Llc | Composite surgical devices |
| IL94910A (en) | 1990-06-29 | 1994-04-12 | Technion Research Dev Foundati | Biomedical adhesive compositions |
| US5047035A (en) | 1990-08-10 | 1991-09-10 | Mikhail Michael W E | System for performing hip prosthesis revision surgery |
| ATE139126T1 (en) | 1990-09-10 | 1996-06-15 | Synthes Ag | MEMBRANE FOR BONE REGENERATION |
| CA2062012C (en) | 1991-03-05 | 2003-04-29 | Randall D. Ross | Bioabsorbable interference bone fixation screw |
| DE4110316A1 (en) | 1991-03-28 | 1992-10-01 | Uwe Storch | USE OF A MIXTURE FOR THE PRODUCTION OF MEDICAL IMPLANTS |
| EP0520177B1 (en) | 1991-05-24 | 1995-12-13 | Synthes AG, Chur | Resorbable tendon and bone augmentation device |
| EP0523926A3 (en) | 1991-07-15 | 1993-12-01 | Smith & Nephew Richards Inc | Prosthetic implants with bioabsorbable coating |
| DE4226465C2 (en) | 1991-08-10 | 2003-12-04 | Gunze Kk | Jaw bone reproductive material |
| US5275601A (en) | 1991-09-03 | 1994-01-04 | Synthes (U.S.A) | Self-locking resorbable screws and plates for internal fixation of bone fractures and tendon-to-bone attachment |
| US5500013A (en) | 1991-10-04 | 1996-03-19 | Scimed Life Systems, Inc. | Biodegradable drug delivery vascular stent |
| US5360448A (en) | 1991-10-07 | 1994-11-01 | Thramann Jeffrey J | Porous-coated bone screw for securing prosthesis |
| US5383931A (en) | 1992-01-03 | 1995-01-24 | Synthes (U.S.A.) | Resorbable implantable device for the reconstruction of the orbit of the human skull |
| FI95537C (en) | 1992-01-24 | 1996-02-26 | Biocon Oy | Surgical implant |
| ES2125329T3 (en) | 1992-02-14 | 1999-03-01 | Smith & Nephew Inc | SCREWS OF POLYMER MATERIALS AND COATINGS FOR SURGICAL USES. |
| US5333624A (en) | 1992-02-24 | 1994-08-02 | United States Surgical Corporation | Surgical attaching apparatus |
| US5571193A (en) | 1992-03-12 | 1996-11-05 | Kampner; Stanley L. | Implant with reinforced resorbable stem |
| US5407445A (en) | 1992-05-20 | 1995-04-18 | Cytrx Corporation | Gel composition for implant prosthesis and method of use |
| DE4220216C1 (en) | 1992-06-20 | 1994-01-13 | S & G Implants Gmbh | Endoprosthesis - has bio-resorbable distance rings to set gap between prosthesis and bone |
| US5319003A (en) * | 1992-09-30 | 1994-06-07 | Union Carbide Chemicals & Plastics Technology Corporation | Method for improving the mechanical performance of composite articles |
| WO1994008078A1 (en) | 1992-10-02 | 1994-04-14 | Cargill, Incorporated | A melt-stable lactide polymer fabric and process for manufacture thereof |
| US5376120A (en) | 1992-10-21 | 1994-12-27 | Biomet, Inc. | Biocompatible implant and method of using same |
| US5437918A (en) | 1992-11-11 | 1995-08-01 | Mitsui Toatsu Chemicals, Inc. | Degradable non-woven fabric and preparation process thereof |
| US5441515A (en) | 1993-04-23 | 1995-08-15 | Advanced Cardiovascular Systems, Inc. | Ratcheting stent |
| US5716410A (en) | 1993-04-30 | 1998-02-10 | Scimed Life Systems, Inc. | Temporary stent and method of use |
| FR2707477A1 (en) | 1993-07-02 | 1995-01-20 | Cahlix Marc Andre | Obturator for bone cavities |
| CA2127636C (en) | 1993-07-21 | 2009-10-20 | Cheng-Kung Liu | Plasticizers for fibers used to form surgical devices |
| US6315788B1 (en) | 1994-02-10 | 2001-11-13 | United States Surgical Corporation | Composite materials and surgical articles made therefrom |
| US5417712A (en) | 1994-02-17 | 1995-05-23 | Mitek Surgical Products, Inc. | Bone anchor |
| US5569250A (en) | 1994-03-01 | 1996-10-29 | Sarver; David R. | Method and apparatus for securing adjacent bone portions |
| AU689846B2 (en) | 1994-03-29 | 1998-04-09 | Zimmer Gmbh | Screw made of biodegradable material for bone surgery purposes, and screwdriver suitable therefor |
| US5626861A (en) | 1994-04-01 | 1997-05-06 | Massachusetts Institute Of Technology | Polymeric-hydroxyapatite bone composite |
| US5947893A (en) | 1994-04-27 | 1999-09-07 | Board Of Regents, The University Of Texas System | Method of making a porous prothesis with biodegradable coatings |
| US6001101A (en) | 1994-07-05 | 1999-12-14 | Depuy France | Screw device with threaded head for permitting the coaptation of two bone fragments |
| DE4424883A1 (en) | 1994-07-14 | 1996-01-18 | Merck Patent Gmbh | Femoral prosthesis |
| EP0696605B1 (en) | 1994-08-10 | 2000-09-20 | Peter Neuenschwander | Biocompatible block copolymer |
| US5837276A (en) | 1994-09-02 | 1998-11-17 | Delab | Apparatus for the delivery of elongate solid drug compositions |
| FR2725617B1 (en) | 1994-10-12 | 1997-09-19 | Prost Didier | FEMALE ROD FOR HIP PROSTHESIS |
| US5690671A (en) | 1994-12-13 | 1997-11-25 | Micro Interventional Systems, Inc. | Embolic elements and methods and apparatus for their delivery |
| US5741329A (en) | 1994-12-21 | 1998-04-21 | Board Of Regents, The University Of Texas System | Method of controlling the pH in the vicinity of biodegradable implants |
| US5634936A (en) | 1995-02-06 | 1997-06-03 | Scimed Life Systems, Inc. | Device for closing a septal defect |
| ES2140828T3 (en) * | 1995-03-13 | 2000-03-01 | Rue De Int Ltd | SECURITY ROLE. |
| US6027742A (en) * | 1995-05-19 | 2000-02-22 | Etex Corporation | Bioresorbable ceramic composites |
| US5641502A (en) | 1995-06-07 | 1997-06-24 | United States Surgical Corporation | Biodegradable moldable surgical material |
| US5633343A (en) | 1995-06-30 | 1997-05-27 | Ethicon, Inc. | High strength, fast absorbing, melt processable, gycolide-rich, poly(glycolide-co-p-dioxanone) copolymers |
| FI98136C (en) | 1995-09-27 | 1997-04-25 | Biocon Oy | A tissue-soluble material and process for its manufacture |
| US6113624A (en) | 1995-10-02 | 2000-09-05 | Ethicon, Inc. | Absorbable elastomeric polymer |
| US5716413A (en) | 1995-10-11 | 1998-02-10 | Osteobiologics, Inc. | Moldable, hand-shapable biodegradable implant material |
| US6902584B2 (en) | 1995-10-16 | 2005-06-07 | Depuy Spine, Inc. | Bone grafting matrix |
| US6419945B1 (en) * | 1996-01-17 | 2002-07-16 | Cambridge Scientific, Inc. | Buffered resorbable internal fixation devices and methods for making material therefore |
| US5817328A (en) | 1996-01-17 | 1998-10-06 | Cambridge Scientific, Inc. | Material for buffered resorbable internal fixation devices and method for making same |
| US5902599A (en) | 1996-02-20 | 1999-05-11 | Massachusetts Institute Of Technology | Biodegradable polymer networks for use in orthopedic and dental applications |
| US5856288A (en) | 1996-04-26 | 1999-01-05 | Nippon Shokubai Co., Ltd. | Polyalkylene glycol-polyglyoxylate block copolymer, its production process and use |
| JP3731838B2 (en) | 1996-04-30 | 2006-01-05 | 株式会社クレハ | Polyglycolic acid oriented film and method for producing the same |
| EP0806283B1 (en) | 1996-05-09 | 2003-10-01 | Kureha Kagaku Kogyo Kabushiki Kaisha | Stretch blow molded container and production process thereof |
| US6143948A (en) | 1996-05-10 | 2000-11-07 | Isotis B.V. | Device for incorporation and release of biologically active agents |
| US5670161A (en) | 1996-05-28 | 1997-09-23 | Healy; Kevin E. | Biodegradable stent |
| CA2252860C (en) | 1996-05-28 | 2011-03-22 | 1218122 Ontario Inc. | Resorbable implant biomaterial made of condensed calcium phosphate particles |
| US5935172A (en) | 1996-06-28 | 1999-08-10 | Johnson & Johnson Professional, Inc. | Prosthesis with variable fit and strain distribution |
| US5824413A (en) * | 1996-07-15 | 1998-10-20 | Ppg Industries, Inc. | Secondary coating for fiber strands, coated strand reinforcements, reinforced polymeric composites and a method of reinforcing a polymeric material |
| US5756651A (en) | 1996-07-17 | 1998-05-26 | Chronopol, Inc. | Impact modified polylactide |
| US5904658A (en) | 1996-08-23 | 1999-05-18 | Osteobiologics, Inc. | Hand-held materials tester |
| US7351421B2 (en) | 1996-11-05 | 2008-04-01 | Hsing-Wen Sung | Drug-eluting stent having collagen drug carrier chemically treated with genipin |
| US5893850A (en) | 1996-11-12 | 1999-04-13 | Cachia; Victor V. | Bone fixation device |
| US6139963A (en) * | 1996-11-28 | 2000-10-31 | Kuraray Co., Ltd. | Polyvinyl alcohol hydrogel and process for producing the same |
| DE69732721T2 (en) | 1996-12-03 | 2006-05-18 | Osteobiologics, Inc., San Antonio | BIODEGRADABLE ARTIFICIAL FILMS |
| US5733330A (en) | 1997-01-13 | 1998-03-31 | Advanced Cardiovascular Systems, Inc. | Balloon-expandable, crush-resistant locking stent |
| SE512050C2 (en) | 1997-01-21 | 2000-01-17 | Nobel Biocare Ab | Rotationally symmetrical leg anchoring element |
| US5997580A (en) | 1997-03-27 | 1999-12-07 | Johnson & Johnson Professional, Inc. | Cement restrictor including shape memory material |
| US5977204A (en) | 1997-04-11 | 1999-11-02 | Osteobiologics, Inc. | Biodegradable implant material comprising bioactive ceramic |
| US6071982A (en) | 1997-04-18 | 2000-06-06 | Cambridge Scientific, Inc. | Bioerodible polymeric semi-interpenetrating network alloys for surgical plates and bone cements, and method for making same |
| JPH10298435A (en) * | 1997-04-24 | 1998-11-10 | Dainippon Ink & Chem Inc | Biodegradable molding, biodegradable material and their production |
| JP3503045B2 (en) | 1997-05-13 | 2004-03-02 | タキロン株式会社 | Shape memory biodegradable absorbent material |
| WO1998053768A1 (en) | 1997-05-30 | 1998-12-03 | Osteobiologics, Inc. | Fiber-reinforced, porous, biodegradable implant device |
| US7524335B2 (en) | 1997-05-30 | 2009-04-28 | Smith & Nephew, Inc. | Fiber-reinforced, porous, biodegradable implant device |
| US5980564A (en) | 1997-08-01 | 1999-11-09 | Schneider (Usa) Inc. | Bioabsorbable implantable endoprosthesis with reservoir |
| US6001100A (en) | 1997-08-19 | 1999-12-14 | Bionx Implants Oy | Bone block fixation implant |
| GB9717433D0 (en) | 1997-08-19 | 1997-10-22 | Univ Nottingham | Biodegradable composites |
| US7541049B1 (en) | 1997-09-02 | 2009-06-02 | Linvatec Biomaterials Oy | Bioactive and biodegradable composites of polymers and ceramics or glasses and method to manufacture such composites |
| US7985415B2 (en) | 1997-09-10 | 2011-07-26 | Rutgers, The State University Of New Jersey | Medical devices employing novel polymers |
| SE510868C2 (en) | 1997-11-03 | 1999-07-05 | Artimplant Dev Artdev Ab | Molds for use as implants in human medicine and a method for making such molds |
| US6168570B1 (en) | 1997-12-05 | 2001-01-02 | Micrus Corporation | Micro-strand cable with enhanced radiopacity |
| CA2314963A1 (en) * | 1998-01-06 | 1999-07-15 | Bioamide, Inc. | Bioabsorbable fibers and reinforced composites produced therefrom |
| US6150497A (en) | 1998-01-14 | 2000-11-21 | Sherwood Services Ag | Method for the production of polyglycolic acid |
| WO1999040865A1 (en) | 1998-02-13 | 1999-08-19 | Chugai Seiyaku Kabushikikaisha | Bone fixing pin |
| US6160084A (en) | 1998-02-23 | 2000-12-12 | Massachusetts Institute Of Technology | Biodegradable shape memory polymers |
| CA2316945A1 (en) | 1998-02-23 | 1999-08-26 | Mnemoscience Gmbh | Shape memory polymers |
| BR9908806A (en) | 1998-03-11 | 2001-12-18 | Dow Chemical Co | Structures and articles manufactured having format memory made of "alpha" -olefin / vinyl or aromatic vinylidene interpolymers and / or hindered vinyl or aliphatic vinyl |
| US5997582A (en) | 1998-05-01 | 1999-12-07 | Weiss; James M. | Hip replacement methods and apparatus |
| KR100569179B1 (en) | 1998-05-28 | 2006-04-07 | 군제 가부시키가이샤 | Lactide-containing polymer and medical material |
| US5939453A (en) | 1998-06-04 | 1999-08-17 | Advanced Polymer Systems, Inc. | PEG-POE, PEG-POE-PEG, and POE-PEG-POE block copolymers |
| US20020022588A1 (en) | 1998-06-23 | 2002-02-21 | James Wilkie | Methods and compositions for sealing tissue leaks |
| EP0968690A1 (en) | 1998-07-02 | 2000-01-05 | Sulzer Orthopädie AG | Plug system for the medullary canal of a tubular bone |
| US6248430B1 (en) | 1998-08-11 | 2001-06-19 | Dainippon Ink And Chemicals, Inc. | Lactic acid-based polymer laminated product and molded product |
| SE515572C2 (en) | 1998-09-09 | 2001-09-03 | Lanka Ltd | Implants, ways of making it and using it |
| JP2000085054A (en) | 1998-09-14 | 2000-03-28 | Daicel Chem Ind Ltd | Collapsible laminate and manufacture thereof |
| US6248108B1 (en) | 1998-09-30 | 2001-06-19 | Bionx Implants Oy | Bioabsorbable surgical screw and washer system |
| US6162225A (en) | 1998-10-26 | 2000-12-19 | Musculoskeletal Transplant Foundation | Allograft bone fixation screw method and apparatus |
| US6255408B1 (en) | 1998-11-06 | 2001-07-03 | Poly-Med, Inc. | Copolyesters with minimized hydrolytic instability and crystalline absorbable copolymers thereof |
| EP1000958B1 (en) | 1998-11-12 | 2004-03-17 | Takiron Co. Ltd. | Shape-memory, biodegradable and absorbable material |
| US6283973B1 (en) | 1998-12-30 | 2001-09-04 | Depuy Orthopaedics, Inc. | Strength fixation device |
| US6147135A (en) | 1998-12-31 | 2000-11-14 | Ethicon, Inc. | Fabrication of biocompatible polymeric composites |
| US6293950B1 (en) | 1999-01-15 | 2001-09-25 | Luitpold Pharmaceuticals, Inc. | Resorbable pin systems |
| AU757391B2 (en) | 1999-02-04 | 2003-02-20 | Synthes Gmbh | Bone screw |
| US6299448B1 (en) | 1999-02-17 | 2001-10-09 | Ivanka J. Zdrahala | Surgical implant system for restoration and repair of body function |
| US6206883B1 (en) | 1999-03-05 | 2001-03-27 | Stryker Technologies Corporation | Bioabsorbable materials and medical devices made therefrom |
| AU6406700A (en) | 1999-03-16 | 2000-10-04 | Regeneration Technologies, Inc. | Molded implants for orthopedic applications |
| EP1277482A3 (en) | 1999-03-19 | 2005-05-11 | The Regents of The University of Michigan | Mineralization and cellular patterning on biomaterial surfaces |
| EP1867348B1 (en) | 1999-03-25 | 2012-05-16 | Metabolix, Inc. | Medical devices and applications of polyhydroxyalkanoate polymers |
| US6296645B1 (en) | 1999-04-09 | 2001-10-02 | Depuy Orthopaedics, Inc. | Intramedullary nail with non-metal spacers |
| US7462162B2 (en) | 2001-09-04 | 2008-12-09 | Broncus Technologies, Inc. | Antiproliferative devices for maintaining patency of surgically created channels in a body organ |
| US20050177144A1 (en) | 1999-08-05 | 2005-08-11 | Broncus Technologies, Inc. | Methods and devices for maintaining patency of surgically created channels in a body organ |
| US20050137715A1 (en) | 1999-08-05 | 2005-06-23 | Broncus Technologies, Inc. | Methods and devices for maintaining patency of surgically created channels in a body organ |
| US7033603B2 (en) * | 1999-08-06 | 2006-04-25 | Board Of Regents The University Of Texas | Drug releasing biodegradable fiber for delivery of therapeutics |
| CA2319969A1 (en) | 1999-09-24 | 2001-03-24 | Isotis B.V. | Composites |
| DE59901812D1 (en) | 1999-10-21 | 2002-07-25 | Storz Karl Gmbh & Co Kg | interference screw |
| US6579533B1 (en) | 1999-11-30 | 2003-06-17 | Bioasborbable Concepts, Ltd. | Bioabsorbable drug delivery system for local treatment and prevention of infections |
| CN1215231C (en) * | 1999-12-17 | 2005-08-17 | 三井化学株式会社 | Road reinforcing sheet, structure of asphalt reinforced pavement and method for paving road |
| EP1110510B1 (en) | 1999-12-23 | 2002-03-27 | Karl Storz GmbH & Co. KG | Screw driven non-centrally |
| US6630153B2 (en) | 2001-02-23 | 2003-10-07 | Smith & Nephew, Inc. | Manufacture of bone graft substitutes |
| US6425923B1 (en) | 2000-03-07 | 2002-07-30 | Zimmer, Inc. | Contourable polymer filled implant |
| US20040052992A1 (en) | 2000-03-16 | 2004-03-18 | Adele Boone | Biodegradeable shrink wrap |
| AU2815001A (en) | 2000-03-24 | 2001-09-27 | Ethicon Inc. | Thermoforming of absorbable medical devices |
| US6468277B1 (en) | 2000-04-04 | 2002-10-22 | Ethicon, Inc. | Orthopedic screw and method |
| JP2001303387A (en) * | 2000-04-26 | 2001-10-31 | Kyowa Co Ltd | Sheet for construction work having biodegradability |
| US6869445B1 (en) | 2000-05-04 | 2005-03-22 | Phillips Plastics Corp. | Packable ceramic beads for bone repair |
| CA2410637C (en) | 2000-05-31 | 2007-04-10 | Mnemoscience Gmbh | Shape memory polymers seeded with dissociated cells for tissue engineering |
| US6447515B1 (en) | 2000-06-21 | 2002-09-10 | Russell Meldrum | Bioresorbable implant for fracture fixation |
| CA2771263A1 (en) | 2000-07-27 | 2002-02-07 | Rutgers, The State University | Therapeutic polyesters and polyamides |
| CA2418380A1 (en) | 2000-08-17 | 2002-02-21 | Tyco Healthcare Group Lp | Sutures and coatings made from therapeutic absorbable glass |
| ATE377048T1 (en) | 2000-09-06 | 2007-11-15 | Ap Pharma Inc | DEGRADABLE POLYACETAL POLYMERS |
| JP2002078790A (en) * | 2000-09-06 | 2002-03-19 | Gunze Ltd | Medical material for osteosynthesis and method of manufacturing for the same |
| US6605090B1 (en) | 2000-10-25 | 2003-08-12 | Sdgi Holdings, Inc. | Non-metallic implant devices and intra-operative methods for assembly and fixation |
| US6613089B1 (en) | 2000-10-25 | 2003-09-02 | Sdgi Holdings, Inc. | Laterally expanding intervertebral fusion device |
| AU2002243270B2 (en) | 2000-10-25 | 2006-03-09 | Warsaw Orthopedic, Inc. | Vertically expanding intervertebral body fusion device |
| US7776310B2 (en) | 2000-11-16 | 2010-08-17 | Microspherix Llc | Flexible and/or elastic brachytherapy seed or strand |
| US6599323B2 (en) | 2000-12-21 | 2003-07-29 | Ethicon, Inc. | Reinforced tissue implants and methods of manufacture and use |
| JP2004517187A (en) * | 2000-12-29 | 2004-06-10 | チバ スペシャルティ ケミカルズ ホールディング インコーポレーテッド | Polyester composition with low residual aldehyde content |
| US6719935B2 (en) | 2001-01-05 | 2004-04-13 | Howmedica Osteonics Corp. | Process for forming bioabsorbable implants |
| US6623487B1 (en) | 2001-02-13 | 2003-09-23 | Biomet, Inc. | Temperature sensitive surgical fastener |
| US6827743B2 (en) | 2001-02-28 | 2004-12-07 | Sdgi Holdings, Inc. | Woven orthopedic implants |
| JP4412901B2 (en) | 2001-03-02 | 2010-02-10 | ウッドウェルディング・アクチェンゲゼルシャフト | Implants for making connections to tissue parts, in particular skeletal parts, and devices and methods for implantation of implants |
| US6913765B2 (en) | 2001-03-21 | 2005-07-05 | Scimed Life Systems, Inc. | Controlling resorption of bioresorbable medical implant material |
| AUPR408001A0 (en) | 2001-03-29 | 2001-04-26 | Cochlear Limited | Laminated electrode for a cochlear implant |
| US20040265385A1 (en) | 2001-04-12 | 2004-12-30 | Therics, Inc. | Porous biostructure partially occupied by interpenetrant and method for making same |
| US6726696B1 (en) | 2001-04-24 | 2004-04-27 | Advanced Catheter Engineering, Inc. | Patches and collars for medical applications and methods of use |
| TWI267378B (en) * | 2001-06-08 | 2006-12-01 | Wyeth Corp | Calcium phosphate delivery vehicles for osteoinductive proteins |
| GB0115320D0 (en) | 2001-06-22 | 2001-08-15 | Univ Nottingham | Matrix |
| GB0116341D0 (en) * | 2001-07-04 | 2001-08-29 | Smith & Nephew | Biodegradable polymer systems |
| AUPR626401A0 (en) | 2001-07-10 | 2001-08-02 | Australian Surgical Design And Manufacture Pty Limited | Surgical fixation device |
| US6494916B1 (en) | 2001-07-30 | 2002-12-17 | Biomed Solutions, Llc | Apparatus for replacing musculo-skeletal parts |
| US6749639B2 (en) | 2001-08-27 | 2004-06-15 | Mayo Foundation For Medical Education And Research | Coated prosthetic implant |
| US6841111B2 (en) | 2001-08-31 | 2005-01-11 | Basf Corporation | Method for making a polyurea-polyurethane composite structure substantially free of volatile organic compounds |
| US20050137611A1 (en) | 2001-09-04 | 2005-06-23 | Broncus Technologies, Inc. | Methods and devices for maintaining surgically created channels in a body organ |
| US7708712B2 (en) | 2001-09-04 | 2010-05-04 | Broncus Technologies, Inc. | Methods and devices for maintaining patency of surgically created channels in a body organ |
| US6916321B2 (en) | 2001-09-28 | 2005-07-12 | Ethicon, Inc. | Self-tapping resorbable two-piece bone screw |
| JP2003126238A (en) * | 2001-10-22 | 2003-05-07 | Gunze Ltd | Base material for regenerating bone and osteochondro- bone |
| US20030125745A1 (en) | 2001-11-05 | 2003-07-03 | Bio One Tech Inc. | Bone-fixing device |
| WO2003045460A1 (en) | 2001-11-27 | 2003-06-05 | Takiron Co., Ltd. | Implant material and process for producing the same |
| JP3631994B2 (en) * | 2001-11-29 | 2005-03-23 | 旭ファイバーグラス株式会社 | Long fiber reinforced thermoplastic resin sheet and composite molded body reinforced by the sheet |
| EP1448166A1 (en) | 2001-11-30 | 2004-08-25 | Pfizer Inc. | Controlled release polymeric compositions of bone growth promoting compounds |
| US7713272B2 (en) | 2001-12-20 | 2010-05-11 | Ethicon, Inc. | Bioabsorbable coatings of surgical devices |
| ATE337760T1 (en) | 2001-12-21 | 2006-09-15 | Smith & Nephew Inc | ROTATING JOINT SYSTEM |
| SE524709C2 (en) | 2002-01-11 | 2004-09-21 | Edwards Lifesciences Ag | Device for delayed reshaping of a heart vessel and a heart valve |
| EP2181670A3 (en) | 2001-12-28 | 2011-05-25 | Edwards Lifesciences AG | Device for reshaping a cardiac valve |
| GB0202233D0 (en) | 2002-01-31 | 2002-03-20 | Smith & Nephew | Bioresorbable polymers |
| WO2003065996A2 (en) | 2002-02-05 | 2003-08-14 | Cambridge Scientific, Inc. | Bioresorbable osteoconductive compositions for bone regeneration |
| US20030153971A1 (en) | 2002-02-14 | 2003-08-14 | Chandru Chandrasekaran | Metal reinforced biodegradable intraluminal stents |
| US20030153972A1 (en) | 2002-02-14 | 2003-08-14 | Michael Helmus | Biodegradable implantable or insertable medical devices with controlled change of physical properties leading to biomechanical compatibility |
| US6758862B2 (en) | 2002-03-21 | 2004-07-06 | Sdgi Holdings, Inc. | Vertebral body and disc space replacement devices |
| US6843799B2 (en) | 2002-03-25 | 2005-01-18 | Edwin C. Bartlett | Suture anchor system and associated method |
| WO2003080119A1 (en) | 2002-03-26 | 2003-10-02 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Responsive biomedical composites |
| EP1501424B1 (en) | 2002-04-18 | 2018-06-06 | Helmholtz-Zentrum Geesthacht Zentrum für Material- und Küstenforschung GmbH | Biodegradable shape memory polymeric sutures |
| DE10217350C1 (en) | 2002-04-18 | 2003-12-18 | Mnemoscience Gmbh | polyesterurethanes |
| US7261734B2 (en) | 2002-04-23 | 2007-08-28 | Boston Scientific Scimed, Inc. | Resorption-controllable medical implants |
| US6830575B2 (en) | 2002-05-08 | 2004-12-14 | Scimed Life Systems, Inc. | Method and device for providing full protection to a stent |
| US6869985B2 (en) * | 2002-05-10 | 2005-03-22 | Awi Licensing Company | Environmentally friendly polylactide-based composite formulations |
| US7166133B2 (en) | 2002-06-13 | 2007-01-23 | Kensey Nash Corporation | Devices and methods for treating defects in the tissue of a living being |
| US7192448B2 (en) | 2002-06-27 | 2007-03-20 | Ferree Bret A | Arthroplasty devices with resorbable component |
| US20040002770A1 (en) | 2002-06-28 | 2004-01-01 | King Richard S. | Polymer-bioceramic composite for orthopaedic applications and method of manufacture thereof |
| US20050019404A1 (en) | 2003-06-30 | 2005-01-27 | Hsing-Wen Sung | Drug-eluting biodegradable stent |
| ES2388623T3 (en) | 2002-09-05 | 2012-10-17 | Catherine G. Ambrose | Antibiotic microspheres for the treatment of infections and osteomyelitis |
| AU2003270802A1 (en) | 2002-09-20 | 2004-04-08 | The Children's Hospital Of Philadelphia | Engineering of material surfaces |
| EP1549359A2 (en) | 2002-10-08 | 2005-07-06 | Osteotech, Inc. | Coupling agents for orthopedic biomaterials |
| AU2003300377B2 (en) | 2002-10-11 | 2009-04-02 | University Of Connecticut | Blends of amorphous and semicrystalline polymers having shape memory properties |
| US20040078090A1 (en) * | 2002-10-18 | 2004-04-22 | Francois Binette | Biocompatible scaffolds with tissue fragments |
| JP4467059B2 (en) | 2002-11-12 | 2010-05-26 | カーモン ベン−ジオン | Expansion device and method for tissue expansion, regeneration and fixation |
| JP2006509539A (en) | 2002-12-12 | 2006-03-23 | オステオテック,インコーポレイテッド | Formable and curable polymer bone composite and method for producing the same |
| US20050251267A1 (en) * | 2004-05-04 | 2005-11-10 | John Winterbottom | Cell permeable structural implant |
| EP1433489A1 (en) | 2002-12-23 | 2004-06-30 | Degradable Solutions AG | Biodegradable porous bone implant with a barrier membrane sealed thereto |
| US20040143221A1 (en) | 2002-12-27 | 2004-07-22 | Shadduck John H. | Biomedical implant for sustained agent release |
| US20050013793A1 (en) | 2003-01-16 | 2005-01-20 | Beckman Eric J. | Biodegradable polyurethanes and use thereof |
| EP1596765A2 (en) | 2003-02-10 | 2005-11-23 | Smith & Nephew, Inc. | Resorbable devices |
| US20040156878A1 (en) | 2003-02-11 | 2004-08-12 | Alireza Rezania | Implantable medical device seeded with mammalian cells and methods of treatment |
| US20070043376A1 (en) | 2003-02-21 | 2007-02-22 | Osteobiologics, Inc. | Bone and cartilage implant delivery device |
| US20040193154A1 (en) | 2003-02-21 | 2004-09-30 | Osteobiologics, Inc. | Bone and cartilage implant delivery device |
| US7314480B2 (en) | 2003-02-27 | 2008-01-01 | Boston Scientific Scimed, Inc. | Rotating balloon expandable sheath bifurcation delivery |
| US20070065652A1 (en) | 2003-03-13 | 2007-03-22 | Willaim Marsh Rice University | Composite injectable and pre-fabricated bone replacement material and method for the production of such bone replacement material |
| GB0307011D0 (en) | 2003-03-27 | 2003-04-30 | Regentec Ltd | Porous matrix |
| US7012106B2 (en) | 2003-03-28 | 2006-03-14 | Ethicon, Inc. | Reinforced implantable medical devices |
| NZ544074A (en) | 2003-06-12 | 2007-10-26 | Synthes Gmbh | Intramedullary surgical pin with transverse hole and insert |
| CA2527976C (en) | 2003-06-13 | 2011-11-22 | Mnemoscience Gmbh | Stents |
| US6974862B2 (en) | 2003-06-20 | 2005-12-13 | Kensey Nash Corporation | High density fibrous polymers suitable for implant |
| US7300439B2 (en) | 2003-06-24 | 2007-11-27 | Depuy Mitek, Inc. | Porous resorbable graft fixation pin |
| GB0317192D0 (en) | 2003-07-19 | 2003-08-27 | Smith & Nephew | High strength bioresorbable co-polymers |
| US7794476B2 (en) | 2003-08-08 | 2010-09-14 | Warsaw Orthopedic, Inc. | Implants formed of shape memory polymeric material for spinal fixation |
| FI120333B (en) | 2003-08-20 | 2009-09-30 | Bioretec Oy | A porous medical device and a method of making it |
| DE10340392A1 (en) | 2003-09-02 | 2005-04-07 | Mnemoscience Gmbh | Amorphous polyester urethane networks with shape-memory properties |
| CA2539751C (en) * | 2003-09-05 | 2016-04-26 | Norian Corporation | Bone cement compositions having fiber-reinforcement and/or increased flowability |
| US7648504B2 (en) | 2003-09-09 | 2010-01-19 | Bioretec Ltd | Bioabsorbable band system |
| JP4251061B2 (en) | 2003-10-03 | 2009-04-08 | ブリヂストンスポーツ株式会社 | Golf club head |
| US20050085814A1 (en) * | 2003-10-21 | 2005-04-21 | Sherman Michael C. | Dynamizable orthopedic implants and their use in treating bone defects |
| US7699879B2 (en) | 2003-10-21 | 2010-04-20 | Warsaw Orthopedic, Inc. | Apparatus and method for providing dynamizable translations to orthopedic implants |
| US7645292B2 (en) | 2003-10-27 | 2010-01-12 | Boston Scientific Scimed, Inc. | Vaso-occlusive devices with in-situ stiffening elements |
| US7689260B2 (en) | 2003-11-06 | 2010-03-30 | The Regents Of The University Of Colorado | Shape-memory polymer coated electrodes |
| US8157855B2 (en) | 2003-12-05 | 2012-04-17 | Boston Scientific Scimed, Inc. | Detachable segment stent |
| US20050136764A1 (en) | 2003-12-18 | 2005-06-23 | Sherman Michael C. | Designed composite degradation for spinal implants |
| GB0329654D0 (en) * | 2003-12-23 | 2004-01-28 | Smith & Nephew | Tunable segmented polyacetal |
| WO2005069884A2 (en) | 2004-01-16 | 2005-08-04 | Osteobiologics, Inc. | Bone-tendon-bone implant |
| US20050177245A1 (en) | 2004-02-05 | 2005-08-11 | Leatherbury Neil C. | Absorbable orthopedic implants |
| US7378144B2 (en) | 2004-02-17 | 2008-05-27 | Kensey Nash Corporation | Oriented polymer implantable device and process for making same |
| US8882786B2 (en) | 2004-02-17 | 2014-11-11 | Lawrence Livermore National Security, Llc. | System for closure of a physical anomaly |
| US7744619B2 (en) | 2004-02-24 | 2010-06-29 | Boston Scientific Scimed, Inc. | Rotatable catheter assembly |
| US8729202B2 (en) | 2004-03-03 | 2014-05-20 | Polynovo Biomaterials Pty Limited | Biocompatible polymer compositions for dual or multi staged curing |
| CN1942498A (en) | 2004-03-03 | 2007-04-04 | 联邦科学和工业研究组织 | Polymer compositions for dual or multi staged curing |
| CA2555586A1 (en) | 2004-03-09 | 2005-09-22 | Osteobiologics, Inc. | Implant scaffold combined with autologous or allogenic tissue |
| CN1263519C (en) * | 2004-03-17 | 2006-07-12 | 武汉理工大学 | Method for preparing artificial head bones made from composite material and for modifying surface |
| JP5496457B2 (en) | 2004-03-24 | 2014-05-21 | ポリィノボ バイオマテリアルズ ピーティワイ リミテッド | Biodegradable polyurethane and polyurethaneurea |
| CN100471912C (en) * | 2004-04-06 | 2009-03-25 | 石宗利 | Phosphate fibrous reinforced polylactic composite material with controllable degradable absorbing biological activity and preparation thereof |
| US7285130B2 (en) | 2004-04-27 | 2007-10-23 | Boston Scientific Scimed, Inc. | Stent delivery system |
| ATE481044T1 (en) | 2004-05-21 | 2010-10-15 | Myers Surgical Solutions Llc | FRACTURE FIXATION AND SITUS STABILIZATION SYSTEM |
| US7824434B2 (en) | 2004-06-07 | 2010-11-02 | Degima Gmbh | Self foreshortening fastener |
| US20080249633A1 (en) | 2006-08-22 | 2008-10-09 | Tim Wu | Biodegradable Materials and Methods of Use |
| EP1604693A1 (en) | 2004-06-09 | 2005-12-14 | Scil Technology GmbH | In situ forming scaffold, its manufacturing and use |
| US7285087B2 (en) | 2004-07-15 | 2007-10-23 | Micardia Corporation | Shape memory devices and methods for reshaping heart anatomy |
| CA2574933C (en) | 2004-07-26 | 2015-05-19 | Synthes (U.S.A.) | Biocompatible, biodegradable polyurethane materials with controlled hydrophobic to hydrophilic ratio |
| CA2576007A1 (en) | 2004-07-30 | 2006-02-09 | University Of Nebraska | Bioresorbable composites and method of formation thereof |
| CN101018512B (en) * | 2004-08-13 | 2011-05-18 | 马斯特生物外科股份公司 | Surgical prosthesis having biodegradable and nonbiodegradable regions |
| US20060067971A1 (en) | 2004-09-27 | 2006-03-30 | Story Brooks J | Bone void filler |
| US20060120994A1 (en) * | 2004-10-29 | 2006-06-08 | Cotton Nicholas J | Bioabsorbable polymers |
| US20080267963A1 (en) * | 2004-11-02 | 2008-10-30 | Biomedical Research Model, Inc. | Methods of Cancer Treatment/Prevention Using Cancer Cell-Specific Surface Antigens |
| WO2006055261A2 (en) | 2004-11-05 | 2006-05-26 | Carnegie Mellon University | Degradable polyurethane foams |
| FI122108B (en) | 2004-11-17 | 2011-08-31 | Jvs Polymers Oy | Crosslinking biopolymer |
| JP2008521560A (en) | 2004-11-30 | 2008-06-26 | オステオバイオロジクス・インコーポレーテッド | Implant and its delivery system for treating joint surface defects |
| US20060121087A1 (en) | 2004-12-06 | 2006-06-08 | Williams Michael S | Polymeric endoprostheses with modified erosion rates and methods of manufacture |
| EP1819375A2 (en) | 2004-12-08 | 2007-08-22 | Interpore Spine Ltd. | Continuous phase composite for musculoskeletal repair |
| US7772352B2 (en) | 2005-01-28 | 2010-08-10 | Bezwada Biomedical Llc | Bioabsorbable and biocompatible polyurethanes and polyamides for medical devices |
| AU2006210847A1 (en) | 2005-02-01 | 2006-08-10 | Osteobiologics, Inc. | Method and device for selective addition of a bioactive agent to a multi-phase implant |
| US20060177480A1 (en) | 2005-02-10 | 2006-08-10 | Hsing-Wen Sung | Drug-eluting biodegradable stent |
| US20060200150A1 (en) * | 2005-03-01 | 2006-09-07 | Jouko Ilomaki | Bone screw and driver system |
| CA2603081C (en) | 2005-04-04 | 2013-09-03 | Sinexus, Inc. | Device and methods for treating paranasal sinus conditions |
| FI20055194A (en) * | 2005-04-27 | 2006-10-28 | Bioretec Oy | Bioabsorbent and bioactive composite material and process for manufacturing composite |
| US7963287B2 (en) | 2005-04-28 | 2011-06-21 | Boston Scientific Scimed, Inc. | Tissue-treatment methods |
| CN100400114C (en) | 2005-04-30 | 2008-07-09 | 中国科学院金属研究所 | Biomedicine implant material with controllable degrading rate and its application |
| US7824433B2 (en) | 2005-05-03 | 2010-11-02 | Williams Lytton A | Bone anchored surgical mesh |
| WO2006130796A2 (en) | 2005-06-02 | 2006-12-07 | Zimmer Spine, Inc. | Interbody fusion ring and method of using the same |
| US20070014831A1 (en) | 2005-07-12 | 2007-01-18 | Hsing-Wen Sung | Biodegradable occlusive device with moisture memory |
| FI122342B (en) | 2005-07-18 | 2011-12-15 | Bioretec Oy | Bioabsorbable tape system, bioabsorbable tape and method of forming a bioabsorbable tape. |
| CA2619552A1 (en) | 2005-08-18 | 2007-02-22 | Smith & Nephew, Plc | Multimodal high strength devices and composites |
| JP2009504929A (en) | 2005-08-18 | 2009-02-05 | スミス アンド ネフュー ピーエルシー | High-strength devices and composite materials |
| US20070048383A1 (en) | 2005-08-25 | 2007-03-01 | Helmus Michael N | Self-assembled endovascular structures |
| GB0517499D0 (en) | 2005-08-26 | 2005-10-05 | West Hertfordshire Hospitals N | Surgical scaffold |
| US20070050018A1 (en) | 2005-09-01 | 2007-03-01 | John Wainwright | Biodegradable stents |
| US20070067043A1 (en) | 2005-09-19 | 2007-03-22 | Dericks Gerard H | "Cement and bone graft absorbable & implantable detachable sac," a delivery system |
| JP4499013B2 (en) * | 2005-09-30 | 2010-07-07 | トヨタ紡織株式会社 | Manufacturing method of wood-based fiber molded body |
| US20070100449A1 (en) | 2005-10-31 | 2007-05-03 | O'neil Michael | Injectable soft tissue fixation technique |
| US7858078B2 (en) | 2005-12-06 | 2010-12-28 | Tyco Healthcare Group Lp | Bioabsorbable surgical composition |
| EP1957695B1 (en) | 2005-12-07 | 2011-02-09 | Ramot at Tel-Aviv University Ltd. | Drug-delivering composite structures |
| BRPI0620047A2 (en) | 2005-12-21 | 2011-11-01 | Synthes Gmbh | bone plate set |
| EP1976460A4 (en) | 2006-01-19 | 2012-06-20 | Warsaw Orthopedic Inc | Injectable and moldable bone substitute materials |
| AU2007212501B2 (en) | 2006-02-07 | 2011-03-31 | Tepha, Inc. | Polymeric, degradable drug-eluting stents and coatings |
| JP5538881B2 (en) * | 2006-04-25 | 2014-07-02 | テレフレックス・メディカル・インコーポレイテッド | Calcium phosphate polymer composites and methods |
| US20070260324A1 (en) | 2006-05-05 | 2007-11-08 | Joshi Ashok V | Fully or Partially Bioresorbable Orthopedic Implant |
| FI119177B (en) | 2006-05-05 | 2008-08-29 | Bioretec Oy | Bioabsorbable, deformable fixation material and implants |
| JP2007302718A (en) * | 2006-05-08 | 2007-11-22 | Osaka Univ | Fiber-reinforced composite material |
| US8221468B2 (en) | 2006-05-11 | 2012-07-17 | Gaines Jr Robert W | Use of bioabsorbable materials for anterior extradiscal correction of thoracolumbar pathologies |
| US7914806B2 (en) | 2006-06-01 | 2011-03-29 | Boston Scientific Scimed, Inc. | Medical devices having improved performance |
| FI20065385L (en) | 2006-06-06 | 2007-12-27 | Bioretec Oy | Bone fixation device |
| US20090171064A1 (en) | 2006-06-28 | 2009-07-02 | Gunze Limited | Bio-degradable/ absorbable polymer having reduced metal catalyst content, and process for production thereof |
| FI124017B (en) | 2006-06-30 | 2014-01-31 | Stick Tech Oy | Curing Fiber Reinforced Composites and Methods for Making Application Oriented Fiber Reinforced Composites |
| US20080015578A1 (en) | 2006-07-12 | 2008-01-17 | Dave Erickson | Orthopedic implants comprising bioabsorbable metal |
| WO2008039476A1 (en) | 2006-09-27 | 2008-04-03 | Osman Said G | Biologic intramedullary fixation device and methods of use |
| US8828419B2 (en) | 2006-10-06 | 2014-09-09 | Cordis Corporation | Bioabsorbable device having encapsulated additives for accelerating degradation |
| US8394488B2 (en) | 2006-10-06 | 2013-03-12 | Cordis Corporation | Bioabsorbable device having composite structure for accelerating degradation |
| US7771476B2 (en) | 2006-12-21 | 2010-08-10 | Warsaw Orthopedic Inc. | Curable orthopedic implant devices configured to harden after placement in vivo by application of a cure-initiating energy before insertion |
| US8480718B2 (en) | 2006-12-21 | 2013-07-09 | Warsaw Orthopedic, Inc. | Curable orthopedic implant devices configured to be hardened after placement in vivo |
| US8870871B2 (en) | 2007-01-17 | 2014-10-28 | University Of Massachusetts Lowell | Biodegradable bone plates and bonding systems |
| US20080206297A1 (en) | 2007-02-28 | 2008-08-28 | Roeder Ryan K | Porous composite biomaterials and related methods |
| US20080234762A1 (en) | 2007-03-06 | 2008-09-25 | Zimmer Technology, Inc. | Self-tapping screw with resorbable tip |
| CA2681940A1 (en) | 2007-03-27 | 2008-10-02 | University Of Southern California | Device which enhances the biological activity of locally applied growth factors with particular emphasis on those used for bone repair |
| AU2008242737B2 (en) | 2007-04-19 | 2013-09-26 | Smith & Nephew, Inc. | Multi-modal shape memory polymers |
| ZA200905442B (en) | 2007-04-27 | 2010-10-27 | Synthes Gmbh | Implant devices constructed with metallic and polymeric components |
| EP2195361B1 (en) | 2007-10-03 | 2014-11-26 | Polynovo Biomaterials Limited | High modulus polyurethane and polyurethane/urea compositions |
| US8323322B2 (en) | 2007-10-05 | 2012-12-04 | Zimmer Spine, Inc. | Medical implant formed from porous metal and method |
| FI124190B (en) | 2007-12-05 | 2014-04-30 | Bioretec Oy | Medical agent and preparation thereof |
| US8507614B2 (en) | 2008-02-07 | 2013-08-13 | Poly-Med, Inc. | Multiphasic absorbable compositions of segmented l-lactide copolymers |
-
2007
- 2007-11-30 WO PCT/US2007/086067 patent/WO2008067531A2/en active Application Filing
- 2007-11-30 JP JP2009539508A patent/JP2010511751A/en active Pending
- 2007-11-30 CA CA2679365A patent/CA2679365C/en not_active Expired - Fee Related
- 2007-11-30 AU AU2007325001A patent/AU2007325001B2/en not_active Ceased
- 2007-11-30 AT AT07864978T patent/ATE493081T1/en not_active IP Right Cessation
- 2007-11-30 CN CN201110118592.3A patent/CN102274552B/en not_active Expired - Fee Related
- 2007-11-30 CN CN2007800438419A patent/CN101594831B/en not_active Expired - Fee Related
- 2007-11-30 ES ES07864978T patent/ES2361360T3/en active Active
- 2007-11-30 DE DE602007011671T patent/DE602007011671D1/en active Active
- 2007-11-30 EP EP07864978A patent/EP2120745B1/en not_active Not-in-force
- 2007-11-30 US US12/516,573 patent/US8722783B2/en not_active Expired - Fee Related
-
2014
- 2014-04-25 US US14/262,018 patent/US20140235754A1/en not_active Abandoned
- 2014-07-02 AU AU2014203617A patent/AU2014203617A1/en not_active Abandoned
- 2014-10-02 JP JP2014203910A patent/JP2015006514A/en not_active Ceased
-
2017
- 2017-07-18 JP JP2017139051A patent/JP2017190462A/en not_active Ceased
Non-Patent Citations (1)
| Title |
|---|
| None |
Cited By (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9120919B2 (en) | 2003-12-23 | 2015-09-01 | Smith & Nephew, Inc. | Tunable segmented polyacetal |
| US9815240B2 (en) | 2007-04-18 | 2017-11-14 | Smith & Nephew, Inc. | Expansion moulding of shape memory polymers |
| US9770534B2 (en) | 2007-04-19 | 2017-09-26 | Smith & Nephew, Inc. | Graft fixation |
| WO2010122098A2 (en) | 2009-04-23 | 2010-10-28 | Vivoxid Oy | Biocompatible composite and its use |
| US10857261B2 (en) | 2010-10-20 | 2020-12-08 | 206 Ortho, Inc. | Implantable polymer for bone and vascular lesions |
| US11207109B2 (en) | 2010-10-20 | 2021-12-28 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US10028776B2 (en) | 2010-10-20 | 2018-07-24 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| US10517654B2 (en) | 2010-10-20 | 2019-12-31 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| US10525169B2 (en) | 2010-10-20 | 2020-01-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US10525168B2 (en) | 2010-10-20 | 2020-01-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11850323B2 (en) | 2010-10-20 | 2023-12-26 | 206 Ortho, Inc. | Implantable polymer for bone and vascular lesions |
| US11484627B2 (en) | 2010-10-20 | 2022-11-01 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11058796B2 (en) | 2010-10-20 | 2021-07-13 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11351261B2 (en) | 2010-10-20 | 2022-06-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| US11291483B2 (en) | 2010-10-20 | 2022-04-05 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| CN102677304A (en) * | 2012-05-29 | 2012-09-19 | 蔡紫林 | Yarn-dyed fabric |
| CN104857577A (en) * | 2015-05-28 | 2015-08-26 | 上海益生源药业有限公司 | Absorbable bone fixation material and preparation method thereof |
| US11352491B2 (en) | 2016-09-08 | 2022-06-07 | Schaefer Kalk Gmbh & Co. Kg | Calcium-salt-containing composite powder having microstructured particles |
| US11441008B2 (en) | 2016-09-08 | 2022-09-13 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles |
| US11548998B2 (en) | 2016-09-08 | 2023-01-10 | Schaefer Kalk Gmbh & Co. Kg | Inhibiting calcium carbonate additive |
| US11760874B2 (en) | 2016-09-08 | 2023-09-19 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles having inhibiting calcium carbonate |
| EP3470097B1 (en) | 2017-10-16 | 2021-03-31 | Arctic Biomaterials Oy | Orthopedic bioabsorbable implants |
| US11813007B2 (en) | 2017-10-16 | 2023-11-14 | Arctic Biomaterials Oy | Orthopedic bioabsorbable implants |
Also Published As
| Publication number | Publication date |
|---|---|
| DE602007011671D1 (en) | 2011-02-10 |
| EP2120745B1 (en) | 2010-12-29 |
| JP2010511751A (en) | 2010-04-15 |
| CN102274552A (en) | 2011-12-14 |
| AU2007325001B2 (en) | 2014-04-10 |
| JP2015006514A (en) | 2015-01-15 |
| US20140235754A1 (en) | 2014-08-21 |
| US8722783B2 (en) | 2014-05-13 |
| ATE493081T1 (en) | 2011-01-15 |
| EP2120745A2 (en) | 2009-11-25 |
| AU2014203617A1 (en) | 2014-07-17 |
| CN101594831A (en) | 2009-12-02 |
| CA2679365A1 (en) | 2008-06-05 |
| ES2361360T3 (en) | 2011-06-16 |
| WO2008067531A3 (en) | 2008-10-02 |
| JP2017190462A (en) | 2017-10-19 |
| AU2007325001A1 (en) | 2008-06-05 |
| CA2679365C (en) | 2016-05-03 |
| CN101594831B (en) | 2011-09-14 |
| US20100137491A1 (en) | 2010-06-03 |
| CN102274552B (en) | 2017-03-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8722783B2 (en) | Fiber reinforced composite material | |
| CA2342894C (en) | Bioactive, bioabsorbable surgical composites and devices | |
| KR101742017B1 (en) | Resorbable and biocompatible fibre glass compositions and their uses | |
| Cheung et al. | A potential material for tissue engineering: Silkworm silk/PLA biocomposite | |
| US20030206928A1 (en) | Bioactive, bioabsorbable surgical polyethylene glycol and polybutylene terephtalate copolymer composites and devices | |
| JPH0763504B2 (en) | Material for bone grafting device and method for manufacturing the same | |
| Slivka et al. | Fiber‐matrix interface studies on bioabsorbable composite materials for internal fixation of bone fractures. I. Raw material evaluation and measurement of fiber—matrix interfacial adhesion | |
| Leonor et al. | Novel starch thermoplastic/Bioglass® composites: Mechanical properties, degradation behavior and in-vitro bioactivity | |
| KR20210024109A (en) | Biodegradable polymer blends for making medical devices | |
| Niemelä et al. | Self-reinforced composites of bioabsorbable polymer and bioactive glass with different bioactive glass contents. Part II: In vitro degradation | |
| WO2007110611A1 (en) | Composite material | |
| Rönkkö | Preparation of Porous Composite Scaffolds for Bone Tissue Engineering | |
| WO2023156544A1 (en) | Biocompatible and resorbable composite material and method for obtaining such | |
| JP3141088B2 (en) | Method for producing biodegradable and absorbable surgical materials | |
| Vannaladsaysy et al. | Tensile deformation behavior of collagen/PCL hybrid scaffolds for ligament tissue engineering | |
| Ahmed et al. | Biodegradable composite materials as bone regenerative implants | |
| BIOCOMPOSITE | STUDY ON A SILKWORM SILK FIBER | |
| Steckel | Physio-mechanical properties of absorbable composites: CSM short fiber reinforced PDS and PGA |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200780043841.9 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007325001 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 3250/DELNP/2009 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2679365 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2009539508 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2007325001 Country of ref document: AU Date of ref document: 20071130 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007864978 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12516573 Country of ref document: US |