WO2007110415A2 - Use of diacetyl trimers and cosmetic or therapeutic formulations containing these compounds - Google Patents
Use of diacetyl trimers and cosmetic or therapeutic formulations containing these compounds Download PDFInfo
- Publication number
- WO2007110415A2 WO2007110415A2 PCT/EP2007/052883 EP2007052883W WO2007110415A2 WO 2007110415 A2 WO2007110415 A2 WO 2007110415A2 EP 2007052883 W EP2007052883 W EP 2007052883W WO 2007110415 A2 WO2007110415 A2 WO 2007110415A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acid
- agents
- atoms
- hydroxy
- derivatives
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4973—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with oxygen as the only hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/02—Preparations for care of the skin for chemically bleaching or whitening the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D493/00—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system
- C07D493/12—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system in which the condensed system contains three hetero rings
- C07D493/18—Bridged systems
Definitions
- the invention concerns the use of 1 , 3,6,7, 9-pentamethyl-2, 8, 10,11 -tetraoxa- tricyclo[5.2.1.1 39 ]undecan-4-ones (diacetyl trimers) to lighten the skin and/or hair, cosmetic or therapeutic formulations containing these compounds, processes for skin and/or hair lightening, novel diacetyl trimers and diacetyl trimers for use as drug products.
- Skin-lightening active ingredients intervene in one form or another in melanin metabolism or catabolism.
- Melanin pigments which are normally brown to black in colour, are formed in the melanocytes of the skin, transferred to the keratinocytes and give the skin or hair its colour.
- the brown-black eumelanins are primarily formed from hydroxy-substituted aromatic amino acids such as L-tyrosine and L-DOPA, the yellow to red pheomelanins additionally from sulfur-containing molecules ⁇ Cosmetics & Toiletries 1996 , 111 (5), 43-51 ).
- L-DOPA L-3,4-dihydroxyphenylalanine
- Skin-lightening agents are used for various reasons: if for some reason the melanin-forming melanocytes in human skin are not evenly distributed, pigment spots occur which are either lighter or darker than the surrounding skin area. To overcome this problem, skin and hair lightening agents are sold which at least partially help to balance out such pigment spots. In addition, many people have a need to lighten their naturally dark skin colour or to prevent skin pigmentation. This requires very safe and effective skin and hair lightening agents. Many skin and hair lightening agents contain more or less powerful tyrosinase inhibitors. This is only one possible route towards skin and hair lightening, however.
- UV-absorbing substances are also used to protect against the increase in skin pigmentation caused by UV light. This is a purely physically induced effect, however, and must be distinguished from the biological action of skin-lightening agents on cellular melanin formation, which can also be detected in the absence of UV light. Moreover, UV absorbers do not bring about a true lightening of the skin but merely inhibit the increase in skin pigmentation caused by UV light.
- Hydroquinone hydroquinone derivatives such as e.g. arbutin, vitamin C, derivatives of ascorbic acid such as e.g. ascorbyl palmitate, kojic acid and derivatives of kojic acid such as e.g. kojic acid dipalmitate, are used in particular in commercial cosmetic or therapeutic skin and hair lightening formulations.
- hydroquinone derivatives such as e.g. arbutin, vitamin C, derivatives of ascorbic acid such as e.g. ascorbyl palmitate, kojic acid and derivatives of kojic acid such as e.g. kojic acid dipalmitate, are used in particular in commercial cosmetic or therapeutic skin and hair lightening formulations.
- hydroquinone One of the most commonly used skin and hair lighteners is hydroquinone.
- this compound has a cytotoxic effect on melanocytes and is irritating to the skin. For that reason such preparations are no longer authorised for cosmetic applications in Europe, Japan and South Africa, for example.
- hydroquinone is very sensitive to oxidation and can be stabilised only with difficulty in cosmetic formulations.
- Arbutin is a hydroquinone glucoside, which hydrolyses in situ to form hydroquinone and is therefore just as questionable in toxicological terms as hydroquinone.
- Vitamin C and ascorbic acid derivatives have only an inadequate effect on the skin. Furthermore, they do not act directly as tyrosinase inhibitors but instead reduce the coloured intermediate stages of melanin biosynthesis.
- Kojic acid (5-hydroxy-2-hydroxymethyl-4-pyranone) is a tyrosinase inhibitor which inhibits its catalytic action by chelating the copper atoms in the enzyme; it is used in commercial skin and hair lightening agents but has a high sensitising potential and causes contact allergies.
- the object of the present invention was to remedy the disadvantages of the prior art and in particular to provide highly effective skin lighteners which preferably inhibit tyrosinase or other cellular mechanisms of pigmentation.
- the stated object is achieved through the (cosmetic or therapeutic) use of one or more compounds having formula (I) (hereinafter also referred to as diacetyl trimers)
- R H and water (also in the presence of animal charcoal), petroleum ether 40-60, alcohol, ether, chloroform, acetone, benzene and glacial acetic acid; also the absence of a reaction with Fehling's solution, KMnO 4 solution and silver solution and resistance to alkalis (e.g. KOH solution) and instability towards mineral acid (oxalic acid is formed with concentrated HNO 3 );
- the present invention also concerns processes for cosmetic or therapeutic skin and/or hair lightening in which one or more compounds having formula (I) are used. All of the above statements regarding the choice of substituent naturally apply in this context too.
- a further aspect of the invention concerns (preferably topical) cosmetic or therapeutic formulations, in particular topical cosmetic formulations, which contain an amount having a lightening effect on skin and/or hair of one or more compounds having formula (I) and optionally other (conventional) formulation components. All of the above statements regarding the choice of substituent naturally apply in this respect too. Preferred formulations according to the invention are defined in the claims.
- Formulations according to the invention preferably contain 0.001 to 30 wt.%, preferably 0.01 wt.% to 20 wt.%, and particularly preferably 0.01 wt.% to 5 wt.%, based on the total weight of the formulation, of the compounds according to the invention or for use according to the invention having formula (I), such as e.g. the compound having formula (II).
- the cosmetic or therapeutic formulations according to the invention are produced by conventional processes known per se, such that the diacetyl trimers having formula (I) are incorporated into cosmetic or dermatological formulations which have a conventional composition and which in addition to the skin and hair lightening effect can also be used for the treatment, care and cleansing of the skin or hair.
- the formulations according to the invention are preferably in the form of an emulsion, e.g. VWO (water-in-oil), O/W (oil-in-water), W/O/W (water-in-oil-in- water), 0/W/O (oil-in-water-in-oil) emulsion, PIT emulsion, Pickering emulsion, emulsion with a low oil content, micro- or nanoemulsion, a solution, e.g.
- a gel including hydrogel, hydrodispersion gel, oleogel
- spray e.g. pump spray or spray with propellant
- a foam or an impregnating solution for cosmetic wipes e.g. soap, synthetic detergent, liquid washing, shower and bath preparation, bath product (capsule, oil, tablet, salt, bath salt, soap, etc.), effervescent preparation, a skin care product such as e.g.
- an emulsion as described above, ointment, paste, gel (as described above), oil, toner, balsam, serum, powder (e.g. face powder, body powder), a mask, a pencil, stick, roll-on, pump, aerosol (foaming, non-foaming or post-foaming), a deodorant and/or antiperspirant, mouthwash and mouth rinse, a foot care product (including keratolytic, deodorant), an insect repellent, a sunscreen, a self-tanning agent and/or aftersun preparation, a skin toner, a shaving product, aftershave balm, pre- and aftershave lotion, a depilatory agent, a hair care product such as e.g.
- shampoo including 2-in-1 shampoo, anti- dandruff shampoo, baby shampoo, shampoo for dry scalps, concentrated shampoo
- conditioner hair tonic, hair water, hair rinse, styling creme, pomade, perm and setting lotion
- hair spray e.g. gel or wax
- hair smoothing agent detangling agent, relaxer
- hair dye such as e.g. temporary direct-dyeing hair dye, semi-permanent hair dye, permanent hair dye, hair tint, hair lightener, hair conditioner, hair mousse, eye care product, make-up, make-up remover or baby product.
- the formulations according to the invention are particularly preferably in the form of an emulsion, in particular in the form of a W/O, O/W, W/O/W, 0/W/O emulsion, PIT emulsion, Pickering emulsion, emulsion with a low oil content, micro- or nanoemulsion, a gel (including hydrogel, hydrodispersion gel, oleogel), a solution e.g. in oil (fatty oils or fatty acid esters, in particular C6-C3 2 fatty acid C 2 -C30 esters)) or silicone oil, or a spray (e.g. pump spray or spray with propellant).
- a gel including hydrogel, hydrodispersion gel, oleogel
- a solution e.g. in oil (fatty oils or fatty acid esters, in particular C6-C3 2 fatty acid C 2 -C30 esters)) or silicone oil
- a spray e.g. pump spray or spray with propellant.
- the cosmetic or therapeutic (especially topical) formulations according to the invention can preferably contain cosmetic and/or dermatological auxiliary substances and additives such as are conventionally used in such formulations, e.g. cooling agents, sunscreens, (in particular UV filters and/or UV-filtering pigments), dyes, pigments having a colouring effect, antioxidants, preservatives, anti-irritants, softening, moisturising (moisture-releasing) and/or moisture-retaining substances (moisture regulators, e.g. glycerol or urea), osmolytes, anti-microbial agents (e.g.
- cosmetic and/or dermatological auxiliary substances and additives such as are conventionally used in such formulations, e.g. cooling agents, sunscreens, (in particular UV filters and/or UV-filtering pigments), dyes, pigments having a colouring effect, antioxidants, preservatives, anti-irritants, softening, moisturising (moisture-releasing) and/or moisture-retaining substances
- antibacterial agents bactericides, fungicides
- virucides deodorants (e.g. perspiration-inhibiting agents), surface-active agents (surfactants), emulsifiers, insect repellents (e.g. DEET, IR 3225, Dragorepel), plant extracts, anti-inflammatory substanaces (anti- inflammatory agents), substances to accelerate wound healing (e.g. chitin or chitosan and derivatives thereof), gel-forming agents, film-forming substances (film formers, e.g.
- polyvinyl pyrrolidones or chitosan or derivatives thereof fixatives, skin-smoothing agents, wrinkle-reducing agents such as beta-glucane from oats or blackberry leaf extract or soya extract, vitamins (e.g. vitamin C and derivatives, tocopherols and derivatives, vitamin A and derivatives), 2-hydroxycarboxylic acids (e.g. citric acid, malic acid, L-, D- or DL-lactic acid), skin colouring agents (e.g. walnut extracts or dihydroxyacetone), skin care and skin repair agents (e.g.
- vitamins e.g. vitamin C and derivatives, tocopherols and derivatives, vitamin A and derivatives
- 2-hydroxycarboxylic acids e.g. citric acid, malic acid, L-, D- or DL-lactic acid
- skin colouring agents e.g. walnut extracts or dihydroxyacetone
- skin care and skin repair agents e.g.
- linoleic acid alpha-linolenic acid, gamma-linolenic acid or arachidonic acid and the natural or synthetic esters thereof
- phospholipids e.g., phospholipids, waxes or other conventional constituents of a cosmetic or dermatological formulation
- alcohols alkanediols, polyols, polymers, electrolytes, organic solvents, silicones, silicone derivatives or chelating agents (e.g. ethylene diamine tetraacetic acid and derivatives), anti- dandruff agents (anti-dandruff substances, e.g.
- Auxiliary substances and additives can be included in quantities of 5 to 99 wt.%, preferably 10 to 80 wt.%, based on the total weight of the formulation.
- the amounts of cosmetic or dermatological auxiliary agents and additives and perfume to be used in each case can easily be determined by the person skilled in the art by simple trial and error, depending on the nature of the particular product.
- the formulations can also contain water in a quantity of up to 99.99 wt.%, preferably 5 to 80 wt.%, based on the total weight of the formulation.
- the formulations according to the invention can preferably also contain other active ingredients for skin and hair lightening which are suitable for cosmetic (e.g. dermatological) and/or therapeutic applications.
- active ingredients for skin and hair lightening are suitable for cosmetic (e.g. dermatological) and/or therapeutic applications.
- a more rapid skin and hair lightening action based in part on synergistic effects can be achieved in this way.
- Advantageous skin and hair lightening active ingredients in this respect are kojic acid (5-hydroxy-2-hydroxymethyl-4-pyranone), kojic acid derivatives e.g. kojic acid dipalmitate, arbutin, ascorbic acid, ascorbic acid derivatives, hydroquinone, hydroquinone derivatives, resorcinol, sulfur-containing molecules such as e.g. glutathione or cysteine, alpha-hydroxy acids (e.g.
- citric acid citric acid, lactic acid, malic acid) and derivatives thereof, N-acetyl tyrosine and derivatives, undecenoyl phenylalanine, gluconic acid, 4-alkyl resorcinols, 4-(1 -phenylethyl)-1 ,3- dihydroxybenzene, chromone derivatives such as aloesin, flavonoids, thymol derivatives, 1 -aminoethyl phosphinic acid, thiourea derivatives, ellagic acid, nicotinamide (niacinamide), zinc salts such as e.g.
- thujaplicin and derivatives such as maslinic acid, sterols such as ergosterol, benzofuranones such as senkyunolide, vinyl and ethyl guiacol, dionic acids such as octodecene dionic acid and azelaic acid, inhibitors of nitrogen oxide synthesis, such as e.g. L-nitroarginine and derivatives thereof, 2,7- dinitroindazole or thiocitrulline, metal chelators (e.g.
- alpha-hydroxy fatty acids palmitic acid, phytic acid, lactoferrin, humic acid, bile acid, bile extracts, bilirubin, biliverdin, EDTA, EGTA and derivatives thereof), retinoids, soya milk and extract, serine protease inhibitors or lipoic acid or other synthetic or natural active ingredients for skin and hair lightening, the latter also being used in the form of an extract from plants, such as e.g.
- bearberry extract such as glabridin or licochalcone A, artocarpus extract, extract of rumex and ramulus species, extracts of pine species (pinus) and extracts of vitis species or stilbene derivatives concentrated therefrom, extract of saxifrage, mulberry, scutelleria or/and grapes.
- the amount of the aforementioned examples of additional active ingredients for skin and hair lightening (one or more compounds) in the formulations according to the invention is then preferably 0.01 to 30 wt.%, preferably 0.01 to 20 wt.%, particularly preferably 0.01 to 5 wt.%, based on the total weight of the preparation.
- Cosmetic or therapeutic formulations according to the invention are preferably such formulations which are chosen from the group consisting of emulsion, solution, dispersion, suspension, creme, lotion, milk, gel, spray, foam, impregnating solution for cosmetic wipes, detergent, soap, synthetic detergent, washing preparation, shower preparation, bath preparation, bath product, effervescent preparation, skin care product, ointment, paste, oil, toner, balsam, serum, powder, mask, pencil, stick, roll-on, pump, aerosol, deodorant, antiperspirant, mouthwash, mouth rinse, foot care product, insect repellent, sunscreen, self-tanning agent, aftersun preparation, skin toner, shaving product, aftershave balm, preshave lotion, aftershave lotion, depilatory agent, hair care product, shampoo, conditioner, hair tonic, hair water, hair rinse, styling creme, pomade, perm lotion, setting lotion, hair spray, styling aid, hair-smoothing agent, blonding agent, hair dye, hair tint, hair lightener
- auxiliary substances and additives selected from the group consisting of abrasives, anti-dandruff agents, anti-inflammatory agents, antioxidants, perspiration-inhibiting agents, binders, buffers, chelating agents, depilatory agents, surface-active substances, emulsifiers, enzymes, essential oils, plant extracts, fibres, film formers, fixatives, foaming agents, foam stabilisers, substances to prevent foaming, foam boosters, gel-forming agents, hair care products, hair-shaping agents, hair-smoothing agents, moisture-releasing agents, moisture-retaining substances, insect repellents, optical brightening agents, lubricants, glossing agents, polymers, proteins, nourishing agents, skin-calming agents, skin-smoothing agents, wrinkle-reducing agents, sunscreens, vitamins, oils, waxes, fats, phospholipids, saturated fatty acids and salts thereof, mono- or polyunsaturated fatty acids and salts thereof, alpha-hydroxy acids, polyhydroxy fatty acids,
- cooling agents preferred for use in the context of the present invention are listed below.
- the person skilled in the art can extend the following list by a multiplicity of further cooling agents; the cooling agents listed can also be used in combination with one another: L-menthol, D-menthol, racemic menthol, menthone glycerol acetal (trade name: Frescolat ® MGA), menthyl lactate (trade name: Frescolat ® ML, the menthyl lactate is preferably L-menthyl lactate, in particular L-menthyl-L-lactate), substituted menthyl-3-carboxylic acid amides (e.g.
- menthyl-3-carboxylic acid-N-ethylamide 2-isopropyl-N-2,3-trimethyl butanamide, substituted cyclohexane carboxylic acid amides, 3-menthoxypropane-1 ,2-diol, 2- hydroxyethyl menthyl carbonate, 2-hydroxypropyl menthyl carbonate, N-acetyl glycine menthyl ester, isopulegol, menthyl hydroxycarboxylic acid esters (e.g.
- menthyl-3-hydroxybutyrate monomenthyl succinate
- 2-mercaptocyclodecanone menthyl-2-pyrrolidin-5-one carboxylate
- 2,3-dihydroxy-p-menthane 3,3,5- trimethyl cyclohexanone glycerol ketal
- 3-menthyl-3,6-di- and -trioxaalkanoates 3-menthyl methoxyacetate, icilin.
- Preferred cooling agents are: L-menthol, D-menthol, racemic menthol, menthone glycerol acetal (trade name: Frescolat ® MGA), menthyl lactate (preferably L- menthyl lactate, in particular L-menthyl-l-lactate, trade name: Frescolat ® ML), substituted menthyl-3-carboxylic acid amides (e.g.
- menthyl-3-carboxylic acid-N- ethylamide 2-isopropyl-N-2,3-trimethyl butanamide, substituted cyclohexane carboxylic acid amides, 3-menthoxypropane-1 ,2-diol, 2-hydroxyethyl menthyl carbonate, 2-hydroxypropyl menthyl carbonate, isopulegol.
- cooling agents are: L-menthol, racemic menthol, menthone glycerol acetal (trade name: Frescolat ® MGA), menthyl lactate (preferably L- menthyl lactate, in particular L-menthyl-L-lactate, trade name: Frescolat ® ML), 3- menthoxypropane-1 ,2-diol, 2-hydroxyethyl menthyl carbonate, 2-hydroxypropyl menthyl carbonate.
- cooling agents are: L-menthol, menthone glycerol acetal (trade name: Frescolat ® MGA), menthyl lactate (preferably L-menthyl lactate, in particular L-menthyl-L-lactate, trade name: Frescolat ® ML).
- the usage concentration of the cooling agents to be used is preferably in the concentration range from 0.01 to 20 wt.% and particularly preferably in the concentration range from 0.1 to 5 wt.%, based on the total mass of the finished (ready-for-use), preferably topical, cosmetic or therapeutic (pharmaceutical) formulation.
- the formulations containing compounds according to the invention or for use according to the invention having formula (I) are generally applied to the skin and/or hair in an adequate amount in the conventional manner for cosmetics and dermatological products.
- Particular advantages are offered in this regard by such cosmetic, dermatological and/or therapeutic formulations according to the invention which additionally include one or more sunscreen filters (UV absorbers, UV filters) and which thus act as both a hair or skin lightening or age spot reducing agent and a sunscreen.
- sunscreen filters UV absorbers, UV filters
- UV filters can improve the stability of the compounds having formula (I) in formulations according to the invention.
- UV filters can prevent or slow down a discoloration of the compounds having formula (I) caused by sunlight or other light. Both are important in cosmetic formulations in particular.
- UV filters are therefore used to stabilise the compounds having formula (I), in particular by using one or more UV filters in a formulation according to the invention in an adequate amount to stabilise the compounds having formula (I), preferably using the (preferred) UV filters cited below.
- a further aspect of the invention concerns the cosmetic or therapeutic use of one or more compounds having formula (I) for lightening skin and/or hair in the presence of an amount of one or more UV filters to stabilise the compound or compounds having formula (I), wherein all of the above statements regarding the choice of substituent naturally apply in this context too.
- the ratio of the total percentage by weight of UV filters to the total percentage by weight of the compounds according to the invention or for use according to the invention having formula (I) is preferably in the range from 100:1 to 1 :100, particularly preferably in the range from 10:1 to 1 :10, most particularly preferably in the range from 5:1 to 1 :5.
- Formulations according to the invention containing one or more UV filters preferably have a total proportion of UV filters in the range from 0.1 to 30 wt.%, particularly preferably in the range from 0.2 to 20 wt. %, most particularly preferably in the range from 0.5 to 15 wt.%, based on the total weight of the formulation.
- a formulation according to the invention contains a total amount of UV filters which is capable of bringing about a stabilisation of the compounds having formula (I) in a formulation according to the invention and thus of preventing a discoloration of the formulation according to the invention.
- the total amount of UV filters is preferably in the range from 0.1 to 2 wt.%, in particular 0.2 to 1 wt.%, based on the total weight of the formulation.
- the compounds according to the invention or for use according to the invention having formula (I) are particularly preferably combined with water-soluble UV filters, in a preferred embodiment with phenylene bis-benzimidazyl tetrasulfonic acid disodium salt (Neo Heliopan®AP) and/or 2-phenylbenzimidazole sulfonic acid (Neo Heliopan®Hydro).
- a formulation according to the invention contains a total amount of sunscreen agents, i.e. in particular UV filters and/or inorganic pigments (UV filtering pigments) such that the formulation according to the invention has a light protection factor of greater than or equal to 2 (preferably greater than or equal to 5).
- sunscreen agents i.e. in particular UV filters and/or inorganic pigments (UV filtering pigments)
- UV filtering pigments such that the formulation according to the invention has a light protection factor of greater than or equal to 2 (preferably greater than or equal to 5).
- Formulations according to the invention additionally containing one or more sunscreen filters can be in various forms, such as are conventionally used for sunscreen formulations, for example. Thus they can be in the form of an emulsion of the oil-in-water type (O/W), a gel, a hydrodispersion or an aerosol, for example.
- sunscreen filters UV filters, UV absorbers
- formulations according to the invention advantageously contain at least one UV-A filter and/or at least one UV-B filter and/or a broadband filter and/or at least one inorganic pigment.
- Formulations according to the invention preferably contain at least one UV-B filter or a broadband filter, more particularly preferably at least one UV-A filter and at least one UV-B filter.
- Suitable UV filters are, for example, organic UV absorbers from the class comprising 4-aminobenzoic acid and derivatives, salicylic acid derivatives, benzophenone derivatives, dibenzoylmethane derivatives, diphenyl acrylates, 3- imidazol-4-yl acrylic acid and esters thereof, benzofuran derivatives, benzylidene malonate derivatives, polymeric UV absorbers containing one or more organosilicon radicals, cinnamic acid derivatives, camphor derivatives, trianilino-s- triazine derivatives, 2-hydroxyphenylbenzotriazole derivatives, phenylbenzimidazole sulfonic acid derivatives and salts thereof, anthranilic acid menthyl esters, benzotriazole derivatives, indole derivatives.
- organic UV absorbers from the class comprising 4-aminobenzoic acid and derivatives, salicylic acid derivatives, benzophenone derivatives, dibenzoylmethane
- UV filters cited below which can be used within the context of the present invention are preferred but naturally are not limiting.
- UV-B filters such as e.g.:
- Broadband filters such as e.g.
- UV-A filters such as e.g.
- UV filters which are particularly suitable for combining are
- menthyl anthranilate (Neo Heliopan ® MA)
- particulate UV filters or inorganic pigments which can optionally be hydrophobed, can be used, such as the oxides of titanium (TiO 2 ), zinc (ZnO), iron (Fe 2 Os), zirconium (ZrO 2 ), silicon (SiO 2 ), manganese (e.g. MnO), aluminium (AI 2 O 3 ), cerium (e.g. Ce 2 O 3 ) and/or mixtures thereof.
- Formulations according to the invention can also advantageously contain dyes and/or coloured pigments, particularly if they are intended for use in the area of decorative cosmetics.
- the dyes and coloured pigments can be selected from the corresponding positive list in the German cosmetics ordinance or the EU list of cosmetic colorants. In most cases they are identical to the dyes approved for foodstuffs.
- Advantageous coloured pigments are for example titanium dioxide, mica, iron oxides (e.g. Fe 2 O 3 Fe 3 O 4 , FeO(OH)) and/or tin oxide.
- Advantageous dyes are for example carmine, Berlin blue, chromium oxide green, ultramarine blue and/or manganese violet.
- the dermatological formulations according to the invention are intended for use in the facial area, it is convenient to choose as the dye one or more substances from the following group: 2,4-dihydroxyazobenzol, 1-(2'-chloro-4'-nitro-1 '- phenylazo)-2-hydroxynaphthalene, Ceres red, 2-(4-sulfo-1-naphthylazo)-1- naphthol-4-sulfonic acid, calcium salt of 2-hydroxy-1 ,2'-azonaphthalene-1 '- sulfonic acid, calcium and barium salts of 1 -(2-sulfo-4-methyl-1-phenylazo)-2- naphthyl carboxylic acid, calcium salt of 1 -(2-sulfo-1-naphthylazo)-2- hydroxynaphthalene-3-carboxylic acid, aluminium salt of 1-(4-sulfo-1-phenylazo)- 2-naphthol-6-sulfonic
- oil-soluble natural dyes such as e.g. paprika extracts, ⁇ - carotene or cochineal.
- Natural pearlescent pigments such as e.g. - "pearl essence” (guanine/hypoxanthine mixed crystals obtained from fish scales) and
- Monocrystalline pearlescent pigments such as e.g. bismuth oxychloride (BiOCI) 3.
- Layered substrate pigments e.g. mica / metal oxide
- pearlescent pigments for example by powdered pigments or castor oil dispersions of bismuth oxychloride and/or titanium dioxide and bismuth oxychloride and/or titanium dioxide on mica.
- the lustre pigment listed under CIN 77163, for example, is particularly advantageous.
- pearlescent pigments within the meaning of the present invention are obtainable in many ways known per se.
- substrates other than mica can be coated with other metal oxides, such as e.g. silica and the like.
- Iron pearlescent pigments which are produced without the use of mica, are particularly preferred. Such pigments are available from BASF, for example, under the trade name Sicopearl Copper 1000.
- glitter particles are mixed with various auxiliary substances and dyes (for example the dyes with CIN 19140, 77007, 77289, 77491 ).
- the dyes and pigments can be present both individually and mixed together and coated with one another, wherein different colour effects can generally be obtained by means of varying coating thicknesses.
- the total amount of dyes and colouring pigments is advantageously chosen from the range from e.g. 0.1 wt.% to 30 wt.%, preferably 0.5 to 15 wt.%, in particular 1.0 to 10 wt.%, based in each case on the total weight of the (cosmetic) formulations.
- the formulations according to the invention can also contain (additional) antioxidants or preservatives. All antioxidants which are suitable or commonly used for cosmetic (e.g. dermatological) and/or therapeutic applications can be used as antioxidants or preservatives.
- Antioxidants within the meaning of the invention are all substances which lower the amount of free radicals in cells and tissue.
- Antioxidants are advantageously chosen from the group comprising amino acids (e.g. glycine, histidine, tyrosine, tryptophane) and derivatives thereof, imidazoles (e.g. urocanic acid) and derivatives thereof, peptides such as D,L-carnosine, D-carnosine, L-carnosine and derivatives thereof (e.g. anserine), carotenoids, carotenes (e.g. alpha- carotene, beta-carotene, lycopene) and derivatives thereof, lipoic acid and derivatives thereof (e.g.
- thioredoxine glutathione, cysteine, cystine, cystamine and glycosyl, N-acetyl, methyl, ethyl, propyl, amyl, butyl and lauryl, palmitoyl, oleyl, gamma-linoleyl, cholesteryl, glyceryl and oligoglyceryl esters thereof
- salts thereof dilauryl thiodipropionate, distearyl thiodipropionate, thiodipropionic acid and derivatives thereof (esters, ethers, peptides, lipids, nucleotides, nucleosides and salts) and sulfoximine compounds (e.g.
- buthionine sulfoximines in very small tolerated doses (e.g. pmol to ⁇ mol/kg), also (metal) chelators (e.g. alpha-hydroxy fatty acids, palmitic acid, phytic acid, lactoferrin, alpha-hydroxy acids (e.g.
- citric acid lactic acid, malic acid
- humic acid bile acid, bile extracts, tannins, bilirubin, biliverdin, EDTA, EGTA and derivatives thereof
- unsaturated fatty acids and derivatives thereof e.g. gamma-linolenic acid, linoleic acid, oleic acid
- folic acid and derivatives thereof ubiquinone and ubiquinol and derivatives thereof
- vitamin C and derivatives e.g. ascorbyl palmitate, Mg ascorbyl phosphate, ascorbyl acetate, ascorbyl glucoside
- tocopherols and derivatives e.g.
- vitamin E acetate
- vitamin A and derivatives vitamin A palmitate
- coniferyl benzoate of benzoic resin rutinic acid and derivatives thereof
- flavonoids and glycosylated precursors thereof in particular quercetin and derivatives thereof, e.g.
- coenzymes such as e.g. coenzyme Q10, plastoquinone, menaquinone, ubiquinols 1 -10, ubiquinones 1 -10 or derivatives of these substances.
- the amount of antioxidants (one or more compounds) in the formulations according to the invention is preferably 0.01 to 20 wt.%, particularly preferably 0.05 to 10 wt.%, in particular 0.2 to 5 wt.%, based on the total weight of the formulation.
- vitamin E and/or derivatives thereof are used as the antioxidant(s), it is advantageous to choose their concentrations from the range from 0.001 to 10 wt.%, based on the total weight of the formulation.
- vitamin A or vitamin A derivatives or carotenes or derivatives thereof are used as the antioxidant(s), it is advantageous to choose their concentrations from the range from 0.001 to 10 wt.%, based on the total weight of the formulation.
- Formulations according to the invention can also contain preservatives.
- preservatives can be used as preservatives: all antioxidants which are suitable or commonly used for cosmetic (e.g. dermatological) and/or therapeutic applications, traditional preservatives (e.g. formaldehyde, glutardialdehyde, parabens (e.g. methyl, ethyl, propyl and butyl paraben), dibromodicyanobutane, imidazolidinyl ureas (“Germall”), isothiazolinones (“Kathon”), methyl chlorothiazolidine, methyl thiazolidine, organic acids (e.g.
- lantadin A e.g. lantadin A, caryophyllene, hesperidin, diosmin, phellandrene, pigenin, quercetin, hypericin, aucubin, diosgenin, plumbagin, corlilagin, etc.
- anti-irritants in the formulations according to the invention can also be advantageous.
- Anti-irritants in this connection can be all anti-inflammatory active ingredients or active ingredients to relieve reddening and itching which are suitable for or commonly used in cosmetic (e.g. dermatological) and/or therapeutic applications. All substances which reduce the amount of cytokines, interleukins, prostaglandins and/or leukotrienes in cells and tissue are preferred.
- Steroidal anti-inflammatory substances of the corticosteroid type such as e.g. hydrocortisone, dexamethasone, dexamethasone phosphate, methyl prednisolone or cortisone, are advantageously used as anti-inflammatory active ingredients or active ingredients to relieve reddening and itching, the list of which can be extended by the addition of other steroidal anti-inflammatories. Nonsteroidal anti-inflammatories can also be used.
- oxicams such as piroxicam or tenoxicam
- salicylates such as aspirin, disalcid, solprin or fendosal
- acetic acid derivatives such as diclofenac, fenclofenac, indomethacin, sulindac, tolmetin or clindanac
- fenamates such as mefenamic, meclofenamic, flufenamic or niflumic
- propionic acid derivatives such as ibuprofen, naproxen, benoxaprofen or pyrazoles such as phenylbutazone, oxyphenylbutazone, febrazone or azapropazone.
- Plant extracts special highly active plant extract fractions and highly pure active substances isolated from plant extracts can be used. Particularly preferred are extracts, fractions and active substances from camomile, aloe vera, commiphora species, rubia species, echinacea species, willow, willowherb, oats, black and green tea, gingko, coffee, pepper, blackcurrant, tomato, vanilla, almonds, as well as pure substances such as inter alia bisabolol, apigenin-7- glucoside, boswellic acid, phytosterols, glycyrrhizinic acid, glabridin or licochalcone A.
- the amount of anti-irritants (one or more compounds) in the formulations according to the invention is preferably 0.01 to 20 wt.%, particularly preferably 0.03 to 10 wt.%, in particular 0.05 to 5 wt.%, based on the total weight of the formulation.
- the formulations according to the invention can also contain moisture regulators and osmolytes.
- moisture regulators can be used as moisture regulators (moisturisers): sodium lactate, urea, alcohols (in particular 1 ,2-pentanediol, 1 ,2-hexanediol, 1 ,2- octanediol, 1 ,2-decanediol and mixtures thereof), sorbitol, glycerol, propylene glycol, collagen, elastin or hyaluronic acid, diacyl adipates, petroleum jelly, ectoine, urocanic acid, lecithin, pantheol, phytanetriol, lycopene, algal extract, ceramides, cholesterol, glycolipids, chitosan, chondroitin sulfate, polyamino acids and sugars, lanolin, lanolin esters, amino acids, alpha-hydroxy acids (e.g.
- citric acid lactic acid, malic acid
- sugars e.g. inositol
- alpha-hydroxy fatty acids e.g. 1,3-bis(trimethyl)
- phytosterols e.g. 1,3-bis(trimethyl)
- triterpene acids such as betulinic acid or ursolic acid, algal extracts.
- osmolytes for example: sugar alcohols (myo-inositol, mannitol, sorbitol), quaternary amines such as taurine, choline, betaine, betaine glycine, ectoine, diglycerol phosphate, phosphorylcholine, glycerophosphorylcholines, amino acids such as glutamine, glycine, alanine, glutamate, aspartate or proline, phosphatidylcholine, phosphatidylinositol, inorganic phosphates, and polymers of the cited compounds such as proteins, peptides, polyamino acids and polyols.
- sugar alcohols myo-inositol, mannitol, sorbitol
- quaternary amines such as taurine, choline, betaine, betaine glycine, ectoine, diglycerol phosphate, phosphorylcholine, glycerophospho
- formulations according to the invention also advantageously contain antimicrobial active ingredients.
- antimicrobial active ingredients examples which can be cited are:
- Mono- and oligoglycerides (up to 4 glycerol units) of aryl- or aryloxy-substituted unbranched or monoalkyl- and polyalkyl-branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds) fatty alcohols (mono- and oligoglycerol monoalkyl ethers), fatty acids (mono- and oligoglycerol monoalkyl esters), alkanediols (mono- and oligoglycerol monoalkyl ethers; bis(mono-/oligoglyceryl)alkyl diethers) and dicarboxylic acids (mono- and oligoglycerol monoalkyl esters; bis(mono- /oligoglyceryl) alkyl diesters) having chain lengths of C 2 to C 40 .
- Plant and animal fatty acid cuts containing unbranched or monoalkyl- and polyalkyl-branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds) fatty alcohols, fatty aldehydes and fatty acids having chain lengths of C 2 to C 40 (e.g. coconut fatty acids, palm kernel fatty acids, wool wax acids).
- Mono- and oligoglycerides of lanolin, of lanolin alcohols and lanolic acids e.g. glyceryl lanolate, neocerite), glycyrrhetic acid and derivatives (e.g. glycyrrhetinyl stearate), natural and synthetic cardenolides (e.g. digitoxin, dogoxin, digoxygenin, gitoxygenin, strophanthin and strophanthidin), natural and synthetic bufadienolides (e.g. scillaren A, scillarenin and bufotalin), sapogenins and steroid sapogenins (e.g.
- amyrins oleanolic acid, digitonin, gitogenin, tigogenin and diosgenin
- steroid alkaloids of plant and animal origin e.g. tomatidin, solanin, solanidin, conessin, batrachotoxin and homobatrachotoxin.
- Mono- and polyhalogenated nitriles, dinitriles, trinitriles or tetranitriles Mono- and oligohydroxy fatty acids having chain lengths of C 2 to C 24 (e.g. lactic acid, 2-hydroxypalmitic acid), oligomers and/or polymers thereof and plant and animal raw materials containing these.
- Acyclic terpenes terpene hydrocarbons (e.g. ocimene, myrcene), terpene alcohols (e.g. geraniol, linalool, citronellol), terpene aldehydes and ketones (e.g. citral, pseudoionone, beta-ionone); monocyclic terpenes: terpene hydrocarbons (e.g. terpinene, terpinolene, limonene), terpene alcohols (e.g. terpineol, thymol, menthol), terpene ketones (e.g.
- bicyclic terpenes terpene hydrocarbons (e.g. carane, pinane, bornane), terpene alcohols (e.g. borneol, isoborneol), terpene ketones (e.g. camphor); sesquiterpenes: acyclic sesquiterpenes (e.g. farnesol, nerolidol), monocyclic sesquiterpenes (e.g. bisabolol), bicyclic sesquiterpenes (e.g. cadinene, selinene, vetivazulene, guajazulene), tricyclic sesquiterpenes (e.g.
- santalene diterpenes (e.g. phytol), tricyclic diterpenes (e.g. abietic acid), triterpenes (squalenoids; e.g. squalene), tetraterpenes.
- diterpenes e.g. phytol
- tricyclic diterpenes e.g. abietic acid
- triterpenes squalenoids; e.g. squalene
- tetraterpenes tetraterpenes.
- Antimicrobial peptides and proteins having an amino acid value from 4 to 200 e.g. Skin Antimicrobial Peptides (SAPs), Lingual Antimicrobial Peptides (LAPs), human beta-defensins (in particular h-BD1 and h-BD2), lactoferrins and hydrolysates thereof and peptides obtained therefrom, Bactericidal/Permeability Increasing Proteins [BPIs], Cationic Microbial Proteins [CAPs], lysozyme.
- sugars are compounds containing sugars and substituted sugars or sugar groups.
- the sugars include in particular also the deoxy and dideoxy forms, N-acetyl galactosamine-, N-acetyl glucosamine- and sialic acid-substituted derivatives as well as sugar esters and ethers.
- Amylose, amylopectin, xanthan, alpha-, beta- and gamma-dextrin are particularly suitable.
- the polysaccharides can consist of e.g. 4 to 1 ,000,000, in particular 10 to 100,000, monosaccharides. Chain lengths are preferably chosen in each case which ensure that the active ingredient is soluble in or can be incorporated into the particular formulation.
- Sphingolipids such as sphingosine; N-monoalkylated sphingosines; N 1 N- dialkylated sphingosines; sphingosine-1 -phosphate; sphingosine-1 -sulfate; psychosine (sphingosine-beta-D-galactopyranoside); sphingosyl phosphoryl cholin; lysosulfatides (sphingosyl galactosyl sulfate; lysocerebroside sulfate); lecithin; sphingomyelin; sphinganine.
- Sphingolipids such as sphingosine; N-monoalkylated sphingosines; N 1 N- dialkylated sphingosines; sphingosine-1 -phosphate; sphingosine-1 -sulfate; psychosine (sphingosine-beta
- oils having an antibacterial action are, for example, oils of aniseed, lemon, orange, rosemary, wintergreen, clove, thyme, lavender, hops, citronella, wheat, lemongrass, cedarwood, cinnamon, geranium, sandalwood, violet, eucalyptus, peppermint, gum benzoin, basil, fennel, menthol and Ocmea origanum, Hydastis carradensis, Berberidaceae daceae, Ratanhiae or Curcuma longa.
- Important substances having an antimicrobial action which can be found in essential oils are for example anethol, catechol, camphene, carvacrol, eugenol, eucalyptol, ferulic acid, farnesol, hinokitiol, tropolone, limonene, menthol, methyl salicylate, thymol, terpineol, verbenone, berberine, curcumin, caryophyllene oxide, nerolodol, geraniol.
- anethol catechol, camphene, carvacrol, eugenol, eucalyptol, ferulic acid, farnesol, hinokitiol, tropolone, limonene, menthol, methyl salicylate, thymol, terpineol, verbenone, berberine, curcumin, caryophyllene oxide, nerolodol, geraniol.
- the amount of antimicrobial active ingredients in the formulations is preferably 0.01 to 20 wt. %, based on the total weight of the formulations, particularly preferably 0.05 to 10 wt.%.
- the formulations according to the invention can contain deodorants, i.e. active ingredients having a deodorising and perspiration-inhibiting action.
- deodorants i.e. active ingredients having a deodorising and perspiration-inhibiting action.
- active ingredients i.e. active ingredients having a deodorising and perspiration-inhibiting action.
- odour maskers such as the common perfume constituents, antiperspirants based on aluminium, zirconium or zinc salts
- odour absorbers for example the layered silicates described in the laid-open patent specification DE-P 40 09 347, in particular montmorillonite, kaolinite, nontronite, saponite, hectorite, bentonite, smectite, and also zinc salts of ricinoleic acid, for example.
- bactericidal or bacteriostatic deodorising substances such as e.g. hexachlorophene, 2,4,4'-trichloro-2'-hydroxydiphenyl ether (Irgasan), 1 ,6-di- (4-chlorophenylbiguanido)hexane (chlorhexidine), 3,4,4'-trichlorocarbanilide, and the active agents described in the laid-open patent specifications DE-37 40 186, DE-39 38 140, DE-42 04 321 , DE-42 29 707, DE-42 29 737, DE-42 37 081 , DE- 43 09 372, DE-43 24 219 and containing cation-active substances, such as e.g.
- quaternary ammonium salts and odour absorbers such as e.g. Grillocin® (combination of zinc ricinoleate and various additives) or triethyl citrate, optionally in combination with ion-exchange resins.
- the amount of deodorising and/or antiperspirant active ingredients in the formulations is preferably 0.01 to 20 wt.%, based on the total weight of the formulations, particularly preferably 0.05 to 10 wt.%.
- formulations in particular cosmetic formulations
- Anionic surfactants generally have carboxylate, sulfate or sulfonate groups as functional groups. In aqueous solution they form negatively charged organic ions in the acid or neutral environment. Cationic surfactants are almost exclusively characterised by the presence of a quaternary ammonium group. In aqueous solution they form positively charged organic ions in the acid or neutral environment. Amphoteric surfactants contain both anionic and cationic groups and therefore behave in aqueous solution in the same way as anionic or cationic surfactants, depending on the pH. They have a positive charge in a strongly acid environment and a negative charge in an alkaline environment. In the neutral pH range, by contrast, they are zwitterionic. Polyether chains are typical of non-ionic surfactants. Non-ionic surfactants do not form ions in the aqueous medium.
- Anionic surfactants which can advantageously be used are acyl amino acids (and salts thereof), such as
- acyl glutamates for example sodium acyl glutamate, di-TEA-palmitoyl aspartate and sodium caprylic/capric glutamate,
- acyl peptides for example palmitoyl-hydrolysed milk protein, sodium cocoyl- hydrolysed soya protein and sodium/potassium cocoyl-hydrolysed collagen,
- sarcosinates for example myristoyl sarcosin, TEA-lauroyl sarcosinate, sodium lauroyl sarcosinate and sodium cocoyl sarcosinate,
- taurates for example sodium lauroyl taurate and sodium methyl cocoyl taurate
- carboxylic acids and derivatives such as for example lauric acid, aluminium stearate, magnesium alkanolate and zinc undecylenate,
- ester carboxylic acids for example calcium stearoyl lactylate, laureth-6 citrate and sodium PEG-4 lauramide carboxylate,
- phosphoric acid esters and salts such as e.g. DEA-oleth-10-phosphate and dilaureth-4 phosphate,
- acyl isothionates e.g. sodium / ammonium cocoyl isothionate
- alkyl sulfonates for example sodium cocomonoglyceride sulfate, sodium C- 12 - 14 olefin sulfonate, sodium lauryl sulfoacetate and magnesium PEG-3 cocamide sulfate,
- sulfosuccinates for example dioctyl sodium sulfosuccinate, disodium laureth sulfosuccinate, disodium lauryl sulfosuccinate and disodium undecylenamido MEA sulfosuccinate
- sulfuric acid esters such as
- alkyl ether sulfate for example sodium, ammonium, magnesium, MIPA, TIPA laureth sulfate, sodium myreth sulfate and sodium C 12 - 1 3 pareth sulfate,
- alkyl sulfates for example sodium, ammonium and TEA lauryl sulfate.
- Cationic surfactants which can advantageously be used are - alkyl amines,
- Quaternary surfactants contain at least one N atom, which is covalently bonded to 4 alkyl or aryl groups. This leads to a positive charge, regardless of the pH.
- Alkyl betaine, alkyl amidopropyl betaine and alkyl amidopropyl hydroxysulfaine are advantageous.
- the cationic surfactants used can also preferably be chosen from the group of quaternary ammonium compounds, in particular benzyl trialkyl ammonium chlorides or bromides, such as benzyl dimethylstearyl ammonium chloride for example, also alkyl trialkyl ammonium salts, for example cetyl trimethyl ammonium chloride or bromide, alkyl dimethyl hydroxyethyl ammonium chlorides or bromides, dialkyl dimethyl ammonium chlorides or bromides, alkyl amide ethyl trimethyl ammonium ether sulfates, alkyl pyridinium salts, for example lauryl or cetyl pyrimidinium chloride, imidazoline derivatives and compounds having a cationic character such as amine oxides, for example alkyl dimethyl amine oxides or alkyl aminoethyl dimethyl amine oxides. Cetyl trimethyl ammonium salts are particularly advantageously used.
- acyl/dialkyl ethylene diamine for example sodium acyl amphoacetate, disodium acyl amphodipropionate, disodium alkyl amphodiacetate, sodium acyl amphohydroxypropyl sulfonate, disodium acyl amphodiacetate and sodium acyl amphopropionate,
- N-alkyl amino acids for example aminopropyl alkyl glutamide, alkyl aminopropionic acid, sodium alkyl imidodipropionate and lauroamphocarboxyglycinate.
- amine oxides such as cocamidopropylamine oxide
- ethers for example ethoxylated/propoxylated alcohols, ethoxylated/ propoxylated esters, ethoxylated/propoxylated glycerol esters, ethoxylated/propoxylated cholesterols, ethoxylated/propoxylated triglyceride esters, ethoxylated/propoxylated lanolin, ethoxylated/propoxylated polysiloxanes, propoxylated POE ethers and alkyl polyglycosides such as lauryl glucoside, decyl glycoside and cocoglycoside,
- polyglycerol esters diglycerol esters, monoglycerol esters,
- anionic and/or amphoteric surfactants with one or more non-ionic surfactants is also advantageous.
- the surface-active substance (surfactant) or the combination of surface-active substances can be present in the formulations according to the invention in a concentration of between 1 and 98 wt.%, based on the total weight of the formulations.
- Cosmetic (e.g. dermatological) or therapeutic formulations according to the invention containing the compounds according to the invention or for use according to the invention having formula (I) can also take the form of emulsions.
- oil phase (lipid phase) in the formulations according to the invention can advantageously be selected from the following group of substances:
- mineral oils (advantageously paraffin oil), mineral waxes
- esters of fatty acids with low C-number alcohols for example with isopropanol, propylene glycol or glycerol, or esters of fatty alcohols with low C-number alkanoic acids or with fatty acids;
- - alkyl benzoates e.g. mixtures of n-dodecyl, n-tridecyl, n-tetradecyl or n-pentadecyl benzoate
- cyclic or linear silicone oils such as dimethyl polysiloxanes, diethyl polysiloxanes, diphenyl polysiloxanes and mixed forms thereof.
- esters are advantageously used, in particular (a) esters of saturated and/or unsaturated branched and/or unbranched alkane carboxylic acids having a chain length of 3 to 30 C atoms and saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 3 to 30 C atoms,
- esters of aromatic carboxylic acids and saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 3 to 30 C atoms Preferred ester oils are isopropyl myristate, isopropyl palmitate, isopropyl stearate, isopropyl oleate, n-butyl stearate, n-hexyl laurate, n-decyl laurate, n-decyl oleate, isooctyl stearate, isononyl stearate, isononyl isononanoate, 3,5,5-trimethylhexyl-3,5,5- trimethylhexanoate, 2-ethylhexyl isononanoate, 2-ethylhexyl-3,5,5- trimethylhexanoate, 2-ethylhexyl-2-ethylhexanoate, cetearyl-2
- the oil phase can also advantageously be chosen from the group consisting of branched and unbranched hydrocarbons and hydrocarbon waxes, silicone oils, dialkyl ethers, the group consisting of saturated or unsaturated, branched or unbranched alcohols, and of fatty acid triglycerides, in particular the triglycerol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms.
- the fatty acid triglycerides can advantageously be selected from the group of synthetic, semisynthetic and natural oils, e.g.
- capric or caprylic acid apricot kernel oil, avocado oil, cottonseed oil, borage seed oil, thistle oil, groundnut oil, gamma-oryzanol, rosehip seed oil, hemp oil, hazelnut oil, blackcurrant seed oil, coconut oil, cherry kernel oil, salmon oil, flax oil, maize oil, macadamia nut oil, almond oil, evening primrose oil, mink oil, olive oil, palm oil, palm kernel oil, pecan nut oil, peach kernel oil, pistachio nut oil, rapeseed oil, rice bran oil, castor oil, safflower oil, sesame oil, soya oil, sunflower oil, teatree oil, grape seed oil or wheat germ oil, and the like.
- apricot kernel oil avocado oil, cottonseed oil, borage seed oil, thistle oil, groundnut oil, gamma-oryzanol, rosehip seed oil, hemp oil, hazelnut oil, blackcurrant seed oil, coconut oil, cherry kernel oil
- any blends of such oil and wax components can also advantageously be used.
- waxes for example cetyl palmitate
- the oil phase advantageously being chosen from the group consisting of 2-ethylhexyl isostearate, octyl dodecanol, isotridecyl isononanoate, isoeicosane, 2-ethylhexyl cocoate, Ci 2- i5-alkyl benzoate, caprylic- capric acid triglyceride and dicaprylyl ether.
- Ci 2 -i5-alkyl benzoate and 2-ethylhexyl isostearate Mixtures of Ci 2 -i5-alkyl benzoate and 2-ethylhexyl isostearate, mixtures of Ci 2- i5-alkyl benzoate and isotridecyl isononanoate and mixtures of Ci 2 -i5-alkyl benzoate, 2-ethylhexyl isostearate and isotridecyl isononanoate are particularly advantageous.
- the hydrocarbons paraffin oil, squalane and squalene can also advantageously be used.
- the oil phase can advantageously also have a content of cyclic or linear silicone oils or consist entirely of such oils, it being preferable, however, to use an additional content of other oil phase components along with the silicone oil or silicone oils.
- Cyclomethicone e.g. decamethyl cyclopentasiloxane
- silicone oil can advantageously be used as the silicone oil.
- Other silicone oils can also advantageously be used, however, for example undecamethyl cyclotrisiloxane, polydimethyl siloxane and poly(methylphenyl siloxane).
- Mixtures of cyclomethicone and isotridecyl isononanoate and of cyclomethicone and 2-ethylhexyl isostearate are also particularly advantageous.
- the aqueous phase of formulations according to the invention in the form of an emulsion can advantageously include: alcohols, diols or polyols having a low C number, and ethers thereof, preferably ethanol, isopropanol, propylene glycol, glycerol, ethylene glycol, ethylene glycol monoethyl or monobutyl ether, propylene glycol monomethyl, monoethyl or monobutyl ether, diethylene glycol monomethyl or monoethyl ether and analogous products, also alcohols having a low C number, e.g.
- ethanol isopropanol, 1 ,2- propanediol, glycerol and in particular one or more thickeners, which can advantageously be chosen from the group comprising silicon dioxide, aluminium silicates such as e.g. bentonites, polysaccharides or derivatives thereof, e.g.
- hyaluronic acid, guar gum, xanthan gum, hydroxypropyl methyl cellulose, or allulose derivatives particularly advantageously from the group of polyacrylates, preferably a polyacrylate from the group of so-called carbopols, for example type 980, 981 , 1382, 2984, 5984 carbopols, either individually or in combination, or from the group of polyurethanes, also alpha- or beta-hydroxy acids, preferably lactic acid, citric acid or salicylic acid, also emulsifiers, which can advantageously be selected from the group of ionic, non-ionic, polymeric, phosphate-containing and zwitterionic emulsifiers.
- carbopols for example type 980, 981 , 1382, 2984, 5984 carbopols, either individually or in combination, or from the group of polyurethanes, also alpha- or beta-hydroxy acids, preferably lactic acid, citric acid or salicylic acid, also e
- Formulations according to the invention in the form of an emulsion advantageously include one or more emulsifiers.
- O/W emulsifiers for example, can advantageously be chosen from the group of polyethoxylated or polypropoxylated or polyethoxylated and polypropoxylated products, e.g.:
- the polyethoxylated or polypropoxylated or polyethoxylated and polypropoxylated 0/W emulsifiers used are chosen from the group of substances having HLB values of 11 to 18, most particularly advantageously having HLB values of 14.5 to 15.5, if the O/W emulsifiers have saturated R and R' radicals. If the O/W emulsifiers have unsaturated R and/or R' radicals, or if isoalkyl derivatives are present, the preferred HLB value of such emulsifiers can also be lower or higher.
- fatty alcohol ethoxylates from the group of ethoxylated stearyl alcohols, cetyl alcohols, cetyl stearyl alcohols (cetearyl alcohols). Particularly preferred are:
- polyethylene glycol (12) isolauryl ether (isolaureth-12).
- Sodium laureth-11 carboxylate can advantageously be used as the ethoxylated alkyl ether carboxylic acid or its salt.
- Sodium laureth 1-4 sulfate can advantageously be used as the alkyl ether sulfate.
- Polyethylene glycol (30) cholesteryl ether can advantageously be used as the ethoxylated cholesterol derivative.
- Polyethylene glycol (25) soya sterol has also proved itself.
- Polyethylene glycol (60) evening primrose glycerides can advantageously be used as ethoxylated triglycerides.
- sorbitan esters from the group comprising polyethylene glycol (20) sorbitan monolaurate, polyethylene glycol (20) sorbitan monostearate, polyethylene glycol (20) sorbitan monoisostearate, polyethylene glycol (20) sorbitan monopalmitate, polyethylene glycol (20) sorbitan monooleate.
- W/O emulsifiers fatty alcohols having 8 to 30 carbon atoms, monoglycerol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms, diglycerol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms, monoglycerol ethers of saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 8 to 24, in particular 12 to 18 C atoms, diglycerol ethers of saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 8 to 24, in particular 12 to 18 C atoms, propylene glycol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of
- W/O emulsifiers are glyceryl monostearate, glyceryl monoisostearate, glyceryl monomyristate, glyceryl monooleate, diglyceryl monostearate, diglyceryl monoisostearate, propylene glycol monostearate, propylene glycol monoisostearate, propylene glycol monocaprylate, propylene glycol monolaurate, sorbitan monoisostearate, sorbitan monolaurate, sorbitan monocaprylate, sorbitan monoisooleate, sucrose distearate, cetyl alcohol, stearyl alcohol, arachidyl alcohol, behenyl alcohol, isobehenyl alcohol, selachyl alcohol, chimyl alcohol, polyethylene glycol (2) stearyl ether (steareth-2), glyceryl monolaurate, glyceryl monocaprinate, glyceryl monocaprylate.
- the amount of active ingredients in the formulations according to the invention is preferably 0.01 to 20 wt.%, based on the total weight of the formulation, particularly preferably 0.05 to 10 wt.%.
- topical formulations according to the invention in particular formulations for skin and hair lightening, are applied to the skin and/or hair in an adequate amount in the conventional manner for cosmetics.
- a further aspect of the invention concerns novel compounds having formula (I)
- R denotes an alkyl group having 1 to 32 C atoms or an acyl group having 1 C atom or 3 to 20 C atoms.
- R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, for use as a drug product, in particular for therapeutic skin lightening.
- hyperpigmentations e.g. scar hyperpigmentations, post-traumatic drug-induced hyperpigmentations, postinflammatory hyperpigmentations induced by phototoxic reactions, ephelides.
- a final aspect of the compound therefore concerns the use of a compound having formula (I)
- R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, for the production of a drug product for therapeutic skin lightening.
- Example 1 “Water-in-oil” emulsion with UVA/B broadband protection
- Example 2 “Oil-in-water” emulsion with UVA/B broadband protection
- Example 3 Skin-lightening "oil-in-water” emulsion with UVA/B broadband protection
- Example 4 Skin -lightening oil-free sun spray with UVA/B broadband protection
- Example 5 Skin-lightening balm with UVA/UVB protection
- Example 6 Skin-lightening aerosol foam with UVB/UVA protection
- Example 7 Skin-lightening non-aerosol foam
- Example 8 Shampoo with hair-lightening properties
- Example 9 Hair-lightening hair conditioner with UVB/UVA protection
- Example 10 Skin-lightening moisture cream O/W
- Example 11 Skin-lightening face cream O/W
- Table 1 Compositions of formulations according to the invention (Examples 1-11)
- B16V mouse melanoma cells are disseminated in a 96-well microtitre plate in a concentration of 2 x 10 4 cells/well. After cultivation for 24 h at 37°C and 5 % CO 2 in RPMI medium, enriched with 10% foetal calf serum, the medium is drawn off.
- test substances dissolved in fresh medium enriched with 5% foetal calf serum (samples) are added and incubated as above for a further 48 h.
- cells are incubated with sodium lauryl sulfate in concentrations of 0.01 mM, 0.1 mM, 1 mM and 10 mM as standards. Only fresh medium enriched with 5% foetal calf serum is added to the controls. After incubation the medium is drawn off and the cells are incubated for 2 h with MTT
- Viability (%) [(Atestcompound/Acontrol) X 100]
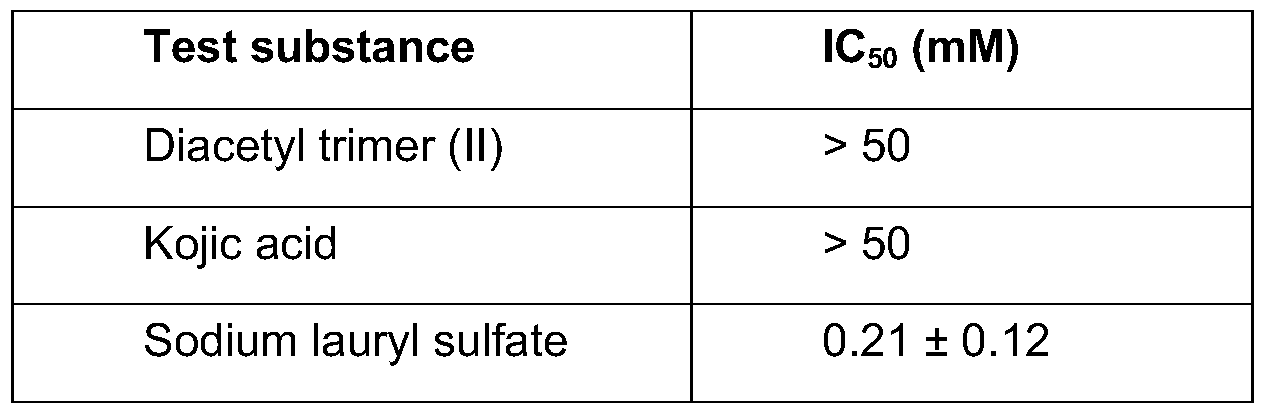
- the diacetyl trimer having formula (II) is extremely cell-compatible (IC 5 O MTT > 10 mM).
- B16V mouse melanoma cells are disseminated in a 96-well microtitre plate in a concentration of 5 x 10 3 cells/well. After cultivation for 24 h at 37°C and 5% CO 2 in RPMI medium, enriched with 10% foetal calf serum, various concentrations of the test substances and 0.3 mM tyrosine and 10 nM ⁇ -MSH ( ⁇ -melanocyte stimulating hormone) are added and incubated for a further 96 h. The maximum concentration of the test substances used corresponds to 0.1 times the value of the IC 2 0 value of the cytotoxicity assay.
- the IC 50 for each test compound is calculated. This is the concentration of a test compound at which pigmentation is inhibited by 50%.
- diacetyl trimer having formula (II) has a more than 40 times stronger depigmenting effect on B16V melanoma cells than kojic acid.
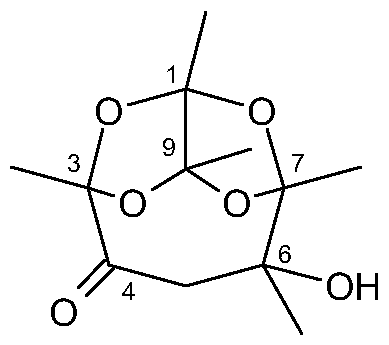
- Example 14 Production of the diacetyl trimer having formula Il (6-hydroxy- 1 ,3,6,7,9-pentamethyl-2,8,10,11 -tetraoxatricvclor5.2.1.1 39 lundecan-4-one)
- Diacetyl (butane-2,4-dione, 50 g, 0.581 mol) is mixed with concentrated hydrochloric acid (20 ml) and stirred for 5 h while being cooled in an ice bath. The mixture is stored overnight at 5°C and the resulting brown, crystalline mass is filtered off. The deposit is rinsed with a little iced water and recrystallised out of water (150 ml).
Abstract
The invention relates to a cosmetic or therapeutic use of one or more compounds having formula (I) wherein R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, for the lightening of skin and/or hair.
Description
Use of diacetyl trimers and cosmetic or therapeutic formulations containing these compounds
The invention concerns the use of 1 , 3,6,7, 9-pentamethyl-2, 8, 10,11 -tetraoxa- tricyclo[5.2.1.139]undecan-4-ones (diacetyl trimers) to lighten the skin and/or hair, cosmetic or therapeutic formulations containing these compounds, processes for skin and/or hair lightening, novel diacetyl trimers and diacetyl trimers for use as drug products.
Skin-lightening active ingredients intervene in one form or another in melanin metabolism or catabolism. Melanin pigments, which are normally brown to black in colour, are formed in the melanocytes of the skin, transferred to the keratinocytes and give the skin or hair its colour. In mammals, the brown-black eumelanins are primarily formed from hydroxy-substituted aromatic amino acids such as L-tyrosine and L-DOPA, the yellow to red pheomelanins additionally from sulfur-containing molecules {Cosmetics & Toiletries 1996 , 111 (5), 43-51 ). Starting from L-tyrosine, L-3,4-dihydroxyphenylalanine (L-DOPA) is formed by the copper-containing key enzyme tyrosinase and is in turn converted by tyrosinase
to dopachrome. By a series of steps catalysed by various enzymes, the latter is oxidised to form melanin.
Skin-lightening agents are used for various reasons: if for some reason the melanin-forming melanocytes in human skin are not evenly distributed, pigment spots occur which are either lighter or darker than the surrounding skin area. To overcome this problem, skin and hair lightening agents are sold which at least partially help to balance out such pigment spots. In addition, many people have a need to lighten their naturally dark skin colour or to prevent skin pigmentation. This requires very safe and effective skin and hair lightening agents. Many skin and hair lightening agents contain more or less powerful tyrosinase inhibitors. This is only one possible route towards skin and hair lightening, however.
Furthermore, UV-absorbing substances are also used to protect against the increase in skin pigmentation caused by UV light. This is a purely physically induced effect, however, and must be distinguished from the biological action of skin-lightening agents on cellular melanin formation, which can also be detected in the absence of UV light. Moreover, UV absorbers do not bring about a true lightening of the skin but merely inhibit the increase in skin pigmentation caused by UV light.
Hydroquinone, hydroquinone derivatives such as e.g. arbutin, vitamin C, derivatives of ascorbic acid such as e.g. ascorbyl palmitate, kojic acid and derivatives of kojic acid such as e.g. kojic acid dipalmitate, are used in particular in commercial cosmetic or therapeutic skin and hair lightening formulations.
One of the most commonly used skin and hair lighteners is hydroquinone. However, this compound has a cytotoxic effect on melanocytes and is irritating to the skin. For that reason such preparations are no longer authorised for cosmetic applications in Europe, Japan and South Africa, for example. In addition, hydroquinone is very sensitive to oxidation and can be stabilised only with difficulty in cosmetic formulations.
Arbutin is a hydroquinone glucoside, which hydrolyses in situ to form hydroquinone and is therefore just as questionable in toxicological terms as hydroquinone.
Vitamin C and ascorbic acid derivatives have only an inadequate effect on the skin. Furthermore, they do not act directly as tyrosinase inhibitors but instead reduce the coloured intermediate stages of melanin biosynthesis.
Kojic acid (5-hydroxy-2-hydroxymethyl-4-pyranone) is a tyrosinase inhibitor which inhibits its catalytic action by chelating the copper atoms in the enzyme; it is used in commercial skin and hair lightening agents but has a high sensitising potential and causes contact allergies.
The object of the present invention was to remedy the disadvantages of the prior art and in particular to provide highly effective skin lighteners which preferably inhibit tyrosinase or other cellular mechanisms of pigmentation.
In accordance with a first aspect of the present invention, the stated object is achieved through the (cosmetic or therapeutic) use of one or more compounds having formula (I) (hereinafter also referred to as diacetyl trimers)
(I) wherein
R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms (i.e. R = H, CrC32 alkyl or Ci-C2o acyl),
for the lightening of skin and/or hair.
Compounds having formula (I) are preferred wherein
- A -
R denotes hydrogen, an alkyl group having 1 to 6 C atoms or an acyl group having 1 to 16 C atoms (i.e. R = H, CrC6 alkyl or Ci-Ci6 acyl).
A compound having formula (I) wherein R denotes hydrogen (i.e. R = H) is particularly preferred.
The compound having formula (I) wherein R = H (6-hydroxy-1 ,3,6,7,9- pentamethyl-2,8,10,11 -tetraoxatricyclo[5.2.1.139]undecan-4-one) can be produced by acid-catalysed oligomerisation of diacetyl (butane-2,3-dione), as described in Chem. Ber. 1902, 35, 3290-3299, Tetrahedron 1985, 41, 1985-1987, Tetrahedron 1999 , 55, 5867-5874, Tetrahedron 2000 , 56, 10005-10009 and Tetrahedron Lett. 1993, 34, 1517-1520. These documents are primarily concerned with the production and the structural clarification of different structural isomers of various diacetyl dimers and trimers. A use of these compounds is not mentioned.
The compound having formula (I) wherein R = acetyl (6-acetoxy-1 ,3,6,7,9- pentamethyl^.δ.i O.H -tetraoxatricyclofδ^.i .^^undecan^-one) can be produced as described in Chem. Ber. 1902, 35, 3290-3299 or Tetrahedron 1985, 41, 1985-1987 by acetylating the compound having formula (I) wherein R = H with acetic anhydride.
Chem. Ber. 1902, 35, 3290-3299 describes mixtures comprising the compound
having formula (I) wherein R = H and water (also in the presence of animal charcoal), petroleum ether 40-60, alcohol, ether, chloroform, acetone, benzene and glacial acetic acid; also the absence of a reaction with Fehling's solution, KMnO4 solution and silver solution and resistance to alkalis (e.g. KOH solution) and instability towards mineral acid (oxalic acid is formed with concentrated HNO3);
having formula (I) wherein R = acetyl and alcohol and water, also in the presence of animal charcoal.
The aforementioned text passages from Tetrahedron, Tetrahedron Lett, and Chem. Ber. and all mixtures containing a compound having formula (I) disclosed therein form part of the present text as references.
Compounds having formula (I) wherein R is not equal to H can be produced by reacting the compound having formula (I) wherein R = H by acylation or alkylation methods known per se. Reference is made in this regard to the methods known from the literature (M. B. Smith, J. March in "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure", 5th edition, Wiley, 2001 , or H.G.O. Becker, W. Berger, G. Domschke, "Organikum", 22nd edition, Wiley-VCH, 2004).
Surprisingly it was found in our own investigations that the compounds having formula (I) for use according to the invention inhibit the pigmentation of melanocytes particularly efficiently.
In particular, the compound having formula (II) (6-hydroxy-1 ,3,6,7, 9-pentamethyl- 2,8,10,11 -tetraoxatricyclo[5.2.1.139]undecan-4-one), which is the compound having formula (I) wherein R = H,
(H)
is substantially more effective than kojic acid. It is therefore outstandingly suitable for use as an active ingredient in cosmetic (including dermatological) and therapeutic skin and hair lightening agents.
Correspondingly the present invention also concerns processes for cosmetic or therapeutic skin and/or hair lightening in which one or more compounds having formula (I) are used. All of the above statements regarding the choice of
substituent naturally apply in this context too. The compound having formula (II) (compound having formula (I) wherein R = H) is therefore preferably used.
A further aspect of the invention concerns (preferably topical) cosmetic or therapeutic formulations, in particular topical cosmetic formulations, which contain an amount having a lightening effect on skin and/or hair of one or more compounds having formula (I) and optionally other (conventional) formulation components. All of the above statements regarding the choice of substituent naturally apply in this respect too. Preferred formulations according to the invention are defined in the claims. The compound having formula (II) (compound having formula (I) wherein R = H) is therefore particularly preferably used.
Formulations according to the invention preferably contain 0.001 to 30 wt.%, preferably 0.01 wt.% to 20 wt.%, and particularly preferably 0.01 wt.% to 5 wt.%, based on the total weight of the formulation, of the compounds according to the invention or for use according to the invention having formula (I), such as e.g. the compound having formula (II).
No mention is made in the prior art of a cosmetic or therapeutic use or a depigmenting action of compounds having formula (I) or of their use in skin and hair lightening agents.
The cosmetic or therapeutic formulations according to the invention are produced by conventional processes known per se, such that the diacetyl trimers having formula (I) are incorporated into cosmetic or dermatological formulations which have a conventional composition and which in addition to the skin and hair lightening effect can also be used for the treatment, care and cleansing of the skin or hair.
The formulations according to the invention are preferably in the form of an emulsion, e.g. VWO (water-in-oil), O/W (oil-in-water), W/O/W (water-in-oil-in- water), 0/W/O (oil-in-water-in-oil) emulsion, PIT emulsion, Pickering emulsion, emulsion with a low oil content, micro- or nanoemulsion, a solution, e.g. in oil (fatty oils or fatty acid esters, in particular C6-C32 fatty acid C2-C30 esters) or silicone oil, dispersion, suspension, creme, lotion or milk, depending on the
production method and ingredients, a gel (including hydrogel, hydrodispersion gel, oleogel), spray (e.g. pump spray or spray with propellant) or a foam or an impregnating solution for cosmetic wipes, a detergent, e.g. soap, synthetic detergent, liquid washing, shower and bath preparation, bath product (capsule, oil, tablet, salt, bath salt, soap, etc.), effervescent preparation, a skin care product such as e.g. an emulsion (as described above), ointment, paste, gel (as described above), oil, toner, balsam, serum, powder (e.g. face powder, body powder), a mask, a pencil, stick, roll-on, pump, aerosol (foaming, non-foaming or post-foaming), a deodorant and/or antiperspirant, mouthwash and mouth rinse, a foot care product (including keratolytic, deodorant), an insect repellent, a sunscreen, a self-tanning agent and/or aftersun preparation, a skin toner, a shaving product, aftershave balm, pre- and aftershave lotion, a depilatory agent, a hair care product such as e.g. shampoo (including 2-in-1 shampoo, anti- dandruff shampoo, baby shampoo, shampoo for dry scalps, concentrated shampoo), conditioner, hair tonic, hair water, hair rinse, styling creme, pomade, perm and setting lotion, hair spray, styling aid (e.g. gel or wax), hair smoothing agent (detangling agent, relaxer), a blonding agent, hair dye such as e.g. temporary direct-dyeing hair dye, semi-permanent hair dye, permanent hair dye, hair tint, hair lightener, hair conditioner, hair mousse, eye care product, make-up, make-up remover or baby product.
It is also advantageous to administer the compounds having formula (I) in encapsulated form, e.g. in gelatine, wax materials, liposomes or cellulose capsules.
The formulations according to the invention are particularly preferably in the form of an emulsion, in particular in the form of a W/O, O/W, W/O/W, 0/W/O emulsion, PIT emulsion, Pickering emulsion, emulsion with a low oil content, micro- or nanoemulsion, a gel (including hydrogel, hydrodispersion gel, oleogel), a solution e.g. in oil (fatty oils or fatty acid esters, in particular C6-C32 fatty acid C2-C30 esters)) or silicone oil, or a spray (e.g. pump spray or spray with propellant).
The cosmetic or therapeutic (especially topical) formulations according to the invention, in particular skin and hair lightening agents, can preferably contain
cosmetic and/or dermatological auxiliary substances and additives such as are conventionally used in such formulations, e.g. cooling agents, sunscreens, (in particular UV filters and/or UV-filtering pigments), dyes, pigments having a colouring effect, antioxidants, preservatives, anti-irritants, softening, moisturising (moisture-releasing) and/or moisture-retaining substances (moisture regulators, e.g. glycerol or urea), osmolytes, anti-microbial agents (e.g. antibacterial agents, bactericides, fungicides), virucides, deodorants (e.g. perspiration-inhibiting agents), surface-active agents (surfactants), emulsifiers, insect repellents (e.g. DEET, IR 3225, Dragorepel), plant extracts, anti-inflammatory substanaces (anti- inflammatory agents), substances to accelerate wound healing (e.g. chitin or chitosan and derivatives thereof), gel-forming agents, film-forming substances (film formers, e.g. polyvinyl pyrrolidones or chitosan or derivatives thereof), fixatives, skin-smoothing agents, wrinkle-reducing agents such as beta-glucane from oats or blackberry leaf extract or soya extract, vitamins (e.g. vitamin C and derivatives, tocopherols and derivatives, vitamin A and derivatives), 2-hydroxycarboxylic acids (e.g. citric acid, malic acid, L-, D- or DL-lactic acid), skin colouring agents (e.g. walnut extracts or dihydroxyacetone), skin care and skin repair agents (e.g. cholesterol, ceramides, pseudoceramides, creatine and creatine ester), skin-soothing agents, nourishing agents, optical brightening agents, lubricants, glossing agents, fats, oils, saturated fatty acids and salts thereof, monounsaturated or polyunsaturated fatty acids and salts thereof, alpha- hydroxy acids, polyhydroxy fatty acids or derivatives thereof (e.g. linoleic acid, alpha-linolenic acid, gamma-linolenic acid or arachidonic acid and the natural or synthetic esters thereof), phospholipids, waxes or other conventional constituents of a cosmetic or dermatological formulation such as alcohols, alkanediols, polyols, polymers, electrolytes, organic solvents, silicones, silicone derivatives or chelating agents (e.g. ethylene diamine tetraacetic acid and derivatives), anti- dandruff agents (anti-dandruff substances, e.g. climbazole, ketoconazole, piroctone olamine, zinc pyrithione), hair care products, hair-shaping agents, hair- smoothing agents, depilatory agents, perfumes, essential oils, foaming agents, foam stabilisers, foam boosters, substances to prevent foaming, thickeners, binders, plant parts (e.g. fibres) and plant extracts (e.g. arnica, aloe, beard lichen, ivy, stinging nettle, ginseng, henna, camomile, marigold, rosemary, sage, horsetail or thyme), animal extracts such as e.g. royal jelly, propolis, proteins,
protein hydrolysates, yeast extracts, hop and wheat extracts, peptides or thymus extracts, abrasives (abrasive substances), buffers, enzymes.
Constituents (auxiliary substances and additives) with which the compounds having formula (I) can be combined are particularly preferred:
Abrasives, anti-dandruff agents, anti-inflammatory agents, antioxidants, perspiration-inhibiting agents, binders, buffers, chelating agents, depilatory agents, surface-active substances, emulsifiers, enzymes, essential oils, plant extracts, fibres, film formers, fixatives, foaming agents, foam stabilisers, substances to prevent foaming, foam boosters, gel-forming agents, hair care products, hair-shaping agents, hair-smoothing agents, moisture-releasing agents, moisture-retaining substances, insect repellents, optical brightening agents, lubricants, glossing agents, polymers, proteins, nourishing agents, skin-calming agents, skin-smoothing agents, wrinkle-reducing agents, sunscreens, vitamins, oils, waxes, fats, phospholipids, saturated fatty acids and salts thereof, mono- or polyunsaturated fatty acids and salts thereof, alpha-hydroxy acids, polyhydroxy fatty acids, polyols, alkanediols, silicones or silicone derivatives.
Auxiliary substances and additives can be included in quantities of 5 to 99 wt.%, preferably 10 to 80 wt.%, based on the total weight of the formulation. The amounts of cosmetic or dermatological auxiliary agents and additives and perfume to be used in each case can easily be determined by the person skilled in the art by simple trial and error, depending on the nature of the particular product.
The formulations can also contain water in a quantity of up to 99.99 wt.%, preferably 5 to 80 wt.%, based on the total weight of the formulation.
The formulations according to the invention can preferably also contain other active ingredients for skin and hair lightening which are suitable for cosmetic (e.g. dermatological) and/or therapeutic applications. A more rapid skin and hair lightening action based in part on synergistic effects can be achieved in this way. Advantageous skin and hair lightening active ingredients in this respect are kojic acid (5-hydroxy-2-hydroxymethyl-4-pyranone), kojic acid derivatives e.g. kojic
acid dipalmitate, arbutin, ascorbic acid, ascorbic acid derivatives, hydroquinone, hydroquinone derivatives, resorcinol, sulfur-containing molecules such as e.g. glutathione or cysteine, alpha-hydroxy acids (e.g. citric acid, lactic acid, malic acid) and derivatives thereof, N-acetyl tyrosine and derivatives, undecenoyl phenylalanine, gluconic acid, 4-alkyl resorcinols, 4-(1 -phenylethyl)-1 ,3- dihydroxybenzene, chromone derivatives such as aloesin, flavonoids, thymol derivatives, 1 -aminoethyl phosphinic acid, thiourea derivatives, ellagic acid, nicotinamide (niacinamide), zinc salts such as e.g. zinc chloride or gluconate, thujaplicin and derivatives, triterpenes such as maslinic acid, sterols such as ergosterol, benzofuranones such as senkyunolide, vinyl and ethyl guiacol, dionic acids such as octodecene dionic acid and azelaic acid, inhibitors of nitrogen oxide synthesis, such as e.g. L-nitroarginine and derivatives thereof, 2,7- dinitroindazole or thiocitrulline, metal chelators (e.g. alpha-hydroxy fatty acids, palmitic acid, phytic acid, lactoferrin, humic acid, bile acid, bile extracts, bilirubin, biliverdin, EDTA, EGTA and derivatives thereof), retinoids, soya milk and extract, serine protease inhibitors or lipoic acid or other synthetic or natural active ingredients for skin and hair lightening, the latter also being used in the form of an extract from plants, such as e.g. bearberry extract, rice extract, papaya extract, liquorice root extract or constituents concentrated therefrom, such as glabridin or licochalcone A, artocarpus extract, extract of rumex and ramulus species, extracts of pine species (pinus) and extracts of vitis species or stilbene derivatives concentrated therefrom, extract of saxifrage, mulberry, scutelleria or/and grapes.
The amount of the aforementioned examples of additional active ingredients for skin and hair lightening (one or more compounds) in the formulations according to the invention is then preferably 0.01 to 30 wt.%, preferably 0.01 to 20 wt.%, particularly preferably 0.01 to 5 wt.%, based on the total weight of the preparation.
Cosmetic or therapeutic formulations according to the invention are preferably such formulations
which are chosen from the group consisting of emulsion, solution, dispersion, suspension, creme, lotion, milk, gel, spray, foam, impregnating solution for cosmetic wipes, detergent, soap, synthetic detergent, washing preparation, shower preparation, bath preparation, bath product, effervescent preparation, skin care product, ointment, paste, oil, toner, balsam, serum, powder, mask, pencil, stick, roll-on, pump, aerosol, deodorant, antiperspirant, mouthwash, mouth rinse, foot care product, insect repellent, sunscreen, self-tanning agent, aftersun preparation, skin toner, shaving product, aftershave balm, preshave lotion, aftershave lotion, depilatory agent, hair care product, shampoo, conditioner, hair tonic, hair water, hair rinse, styling creme, pomade, perm lotion, setting lotion, hair spray, styling aid, hair-smoothing agent, blonding agent, hair dye, hair tint, hair lightener, hair conditioner, hair mousse, eye care product, make-up, make-up remover and baby product, and/or
which contain one or more auxiliary substances and additives selected from the group consisting of abrasives, anti-dandruff agents, anti-inflammatory agents, antioxidants, perspiration-inhibiting agents, binders, buffers, chelating agents, depilatory agents, surface-active substances, emulsifiers, enzymes, essential oils, plant extracts, fibres, film formers, fixatives, foaming agents, foam stabilisers, substances to prevent foaming, foam boosters, gel-forming agents, hair care products, hair-shaping agents, hair-smoothing agents, moisture-releasing agents, moisture-retaining substances, insect repellents, optical brightening agents, lubricants, glossing agents, polymers, proteins, nourishing agents, skin-calming agents, skin-smoothing agents, wrinkle-reducing agents, sunscreens, vitamins, oils, waxes, fats, phospholipids, saturated fatty acids and salts thereof, mono- or polyunsaturated fatty acids and salts thereof, alpha-hydroxy acids, polyhydroxy fatty acids, polyols, alkanediols, silicones and silicone derivatives, and/or
which contain an amount having a lightening effect on skin or hair of one or more further active ingredients for skin and hair lightening which are not compounds having formula (I), and/or
which are intended for application on the hair and/or skin.
The mixtures disclosed in the aforementioned publications in Chem. Ber., Tetrahedron and Tetrahedron Lett, are not cosmetic or therapeutic formulations according to the invention.
Individual cooling agents preferred for use in the context of the present invention are listed below. The person skilled in the art can extend the following list by a multiplicity of further cooling agents; the cooling agents listed can also be used in combination with one another: L-menthol, D-menthol, racemic menthol, menthone glycerol acetal (trade name: Frescolat®MGA), menthyl lactate (trade name: Frescolat®ML, the menthyl lactate is preferably L-menthyl lactate, in particular L-menthyl-L-lactate), substituted menthyl-3-carboxylic acid amides (e.g. menthyl-3-carboxylic acid-N-ethylamide), 2-isopropyl-N-2,3-trimethyl butanamide, substituted cyclohexane carboxylic acid amides, 3-menthoxypropane-1 ,2-diol, 2- hydroxyethyl menthyl carbonate, 2-hydroxypropyl menthyl carbonate, N-acetyl glycine menthyl ester, isopulegol, menthyl hydroxycarboxylic acid esters (e.g. menthyl-3-hydroxybutyrate), monomenthyl succinate, 2-mercaptocyclodecanone, menthyl-2-pyrrolidin-5-one carboxylate, 2,3-dihydroxy-p-menthane, 3,3,5- trimethyl cyclohexanone glycerol ketal, 3-menthyl-3,6-di- and -trioxaalkanoates, 3-menthyl methoxyacetate, icilin.
Preferred cooling agents are: L-menthol, D-menthol, racemic menthol, menthone glycerol acetal (trade name: Frescolat®MGA), menthyl lactate (preferably L- menthyl lactate, in particular L-menthyl-l-lactate, trade name: Frescolat®ML), substituted menthyl-3-carboxylic acid amides (e.g. menthyl-3-carboxylic acid-N- ethylamide), 2-isopropyl-N-2,3-trimethyl butanamide, substituted cyclohexane carboxylic acid amides, 3-menthoxypropane-1 ,2-diol, 2-hydroxyethyl menthyl carbonate, 2-hydroxypropyl menthyl carbonate, isopulegol.
Particularly preferred cooling agents are: L-menthol, racemic menthol, menthone glycerol acetal (trade name: Frescolat®MGA), menthyl lactate (preferably L- menthyl lactate, in particular L-menthyl-L-lactate, trade name: Frescolat®ML), 3- menthoxypropane-1 ,2-diol, 2-hydroxyethyl menthyl carbonate, 2-hydroxypropyl menthyl carbonate.
Most particularly preferred cooling agents are: L-menthol, menthone glycerol acetal (trade name: Frescolat®MGA), menthyl lactate (preferably L-menthyl lactate, in particular L-menthyl-L-lactate, trade name: Frescolat®ML).
The usage concentration of the cooling agents to be used, depending on the substance, is preferably in the concentration range from 0.01 to 20 wt.% and particularly preferably in the concentration range from 0.1 to 5 wt.%, based on the total mass of the finished (ready-for-use), preferably topical, cosmetic or therapeutic (pharmaceutical) formulation.
For use, the formulations containing compounds according to the invention or for use according to the invention having formula (I) are generally applied to the skin and/or hair in an adequate amount in the conventional manner for cosmetics and dermatological products. Particular advantages are offered in this regard by such cosmetic, dermatological and/or therapeutic formulations according to the invention which additionally include one or more sunscreen filters (UV absorbers, UV filters) and which thus act as both a hair or skin lightening or age spot reducing agent and a sunscreen.
In rare cases, discolorations and/or instabilities can arise in formulations containing compounds according to the invention or for use according to the invention having formula (I), especially if they are in aqueous-alcoholic or purely alcoholic solutions. Surprisingly it was found that UV filters can improve the stability of the compounds having formula (I) in formulations according to the invention. In particular, UV filters can prevent or slow down a discoloration of the compounds having formula (I) caused by sunlight or other light. Both are important in cosmetic formulations in particular. According to the invention UV filters are therefore used to stabilise the compounds having formula (I), in particular by using one or more UV filters in a formulation according to the invention in an adequate amount to stabilise the compounds having formula (I), preferably using the (preferred) UV filters cited below. In this connection a further aspect of the invention concerns the cosmetic or therapeutic use of one or more compounds having formula (I) for lightening skin and/or hair in the presence of an amount of one or more UV filters to stabilise the compound or compounds having
formula (I), wherein all of the above statements regarding the choice of substituent naturally apply in this context too.
The ratio of the total percentage by weight of UV filters to the total percentage by weight of the compounds according to the invention or for use according to the invention having formula (I) is preferably in the range from 100:1 to 1 :100, particularly preferably in the range from 10:1 to 1 :10, most particularly preferably in the range from 5:1 to 1 :5.
Formulations according to the invention containing one or more UV filters (sunscreen filters, UV absorbers) preferably have a total proportion of UV filters in the range from 0.1 to 30 wt.%, particularly preferably in the range from 0.2 to 20 wt. %, most particularly preferably in the range from 0.5 to 15 wt.%, based on the total weight of the formulation.
In a further preferred embodiment, a formulation according to the invention contains a total amount of UV filters which is capable of bringing about a stabilisation of the compounds having formula (I) in a formulation according to the invention and thus of preventing a discoloration of the formulation according to the invention. For the purposes of stabilisation the total amount of UV filters is preferably in the range from 0.1 to 2 wt.%, in particular 0.2 to 1 wt.%, based on the total weight of the formulation.
The compounds according to the invention or for use according to the invention having formula (I) are particularly preferably combined with water-soluble UV filters, in a preferred embodiment with phenylene bis-benzimidazyl tetrasulfonic acid disodium salt (Neo Heliopan®AP) and/or 2-phenylbenzimidazole sulfonic acid (Neo Heliopan®Hydro).
In a further preferred embodiment a formulation according to the invention contains a total amount of sunscreen agents, i.e. in particular UV filters and/or inorganic pigments (UV filtering pigments) such that the formulation according to the invention has a light protection factor of greater than or equal to 2 (preferably
greater than or equal to 5). Such formulations according to the invention are particularly suitable for protecting the skin and hair.
Formulations according to the invention additionally containing one or more sunscreen filters (UV filters, UV absorbers) can be in various forms, such as are conventionally used for sunscreen formulations, for example. Thus they can be in the form of an emulsion of the oil-in-water type (O/W), a gel, a hydrodispersion or an aerosol, for example.
The formulations according to the invention advantageously contain at least one UV-A filter and/or at least one UV-B filter and/or a broadband filter and/or at least one inorganic pigment. Formulations according to the invention preferably contain at least one UV-B filter or a broadband filter, more particularly preferably at least one UV-A filter and at least one UV-B filter.
Suitable UV filters are, for example, organic UV absorbers from the class comprising 4-aminobenzoic acid and derivatives, salicylic acid derivatives, benzophenone derivatives, dibenzoylmethane derivatives, diphenyl acrylates, 3- imidazol-4-yl acrylic acid and esters thereof, benzofuran derivatives, benzylidene malonate derivatives, polymeric UV absorbers containing one or more organosilicon radicals, cinnamic acid derivatives, camphor derivatives, trianilino-s- triazine derivatives, 2-hydroxyphenylbenzotriazole derivatives, phenylbenzimidazole sulfonic acid derivatives and salts thereof, anthranilic acid menthyl esters, benzotriazole derivatives, indole derivatives.
The UV filters cited below which can be used within the context of the present invention are preferred but naturally are not limiting.
Advantageous UV filters are
UV-B filters such as e.g.:
• p-aminobenzoic acid
• p-aminobenzoic acid ethyl ester (25 mol) ethoxylated (INCI name: PEG-25 PABA)
• p-dimethylaminobenzoic acid-2-ethylhexyl ester
• p-aminobenzoic acid ethyl ester (2 mol) N-propoxylated
• p-aminobenzoic acid glycerol ester
• salicylic acid homomenthyl ester (homosalates) (Neo Heliopan®HMS)
• salicylic acid-2-ethylhexyl ester (Neo Heliopan®OS)
• triethanolamine salicylate
• 4-isopropyl benzyl salicylate
• anthranilic acid menthyl ester (Neo Heliopan®MA)
• diisopropyl cinnamic acid ethyl ester
• p-methoxycinnamic acid-2-ethylhexyl ester (Neo Heliopan®AV)
• diisopropyl cinnamic acid methyl ester
• p-methoxycinnamic acid isoamyl ester (Neo Heliopan®E 1000)
• p-methoxycinnamic acid diethanolamine salt
• p-methoxycinnamic acid isopropyl ester
• 2-phenylbenzimidazole sulfonic acid and salts (Neo Heliopan®Hydro)
• 3-(4'-trimethylammonium) benzylidene bornan-2-one methyl sulfate
• beta-imidazole-4(5)-acrylic acid (urocanic acid)
• 3-(4'-sulfo)benzylidene bornan-2-one and salts
• 3-(4'-methyl benzylidene)-D,L-camphor (Neo Heliopan®MBC)
• 3-benzylidene-D,L-camphor
• N-[(2 and 4)-[2-(oxoborn-3-ylidene) methyl]benzyl] acrylamide polymer
• 4,4'-[(6-[4-(1 ,1-dimethyl)aminocarbonyl) phenylamino]-1 ,3,5-tιϊazine-2,4- diyl)diimino]-bis-(benzoic acid-2-ethylhexyl ester) (Uvasorb®HEB)
• benzylidene malonate polysiloxane (Parsol®SLX)
• glyceryl ethylhexanoate dimethoxycinnamate
• dipropylene glycol salicylate
• tris(2-ethylhexyl)-4,4',4"-(1 ,3,5-triazine-2,4,6-triyltriimino)tribenzoate (= 2,4,6-trianilino-(p-carbo-2'-ethylhexyl-1 '-oxy)-1 ,3,5-triazine) (Uvinul®T150)
Broadband filters such as e.g.
• 2-ethylhexyl-2-cyano-3,3-diphenyl acrylate (Neo Heliopan®303)
• ethyl-2-cyano-3,3'-diphenyl acrylate
• 2-hydroxy-4-methoxybenzophenone (Neo Heliopan®BB)
• 2-hydroxy-4-methoxybenzophenone-5-sulfonic acid
• dihydroxy-4-methoxybenzophenone
• 2,4-dihydroxybenzophenone
• tetrahydroxybenzophenone
• 2,2'-dihydroxy-4,4'-dimethoxybenzophenone
• 2-hydroxy-4-n-octoxybenzophenone
• 2-hydroxy-4-methoxy-4'-methyl benzophenone
• sodium hydroxymethoxybenzophenone sulfonate
• disodium-2,2'-dihydroxy-4,4'-dimethoxy-5,5'-disulfobenzophenone
• phenol, 2-(2H-benzotriazol-2-yl)-4-methyl-6-(2-methyl-3(1 ,3,3,3- tetramethyl-1 -(trimethylsilyl)oxy)disiloxyanyl) propyl) (Mexoryl®XL)
• 2,2'-methylene bis-(6-(2H-benzotriazol-2-yl)-4-1 ,1 ,3,3-tetramethylbutyl) phenol) (Tinosorb®M)
• 2,4-bis-[4-(2-ethylhexyloxy)-2-hydroxyphenyl]-1 ,3,5-triazine
• 2,4-bis-[{(4-(2-ethylhexyloxy)-2-hydroxy}phenyl]-6-(4-methoxyphenyl)- 1 ,3,5-triazine (Tinosorb®S)
• 2,4-bis-[{(4-(3-sulfonato)-2-hydroxypropyloxy)-2-hydroxy}phenyl]-6-(4- methoxyphenyl)-1 ,3,5-triazine sodium salt
• 2,4-bis-[{(3-(2-propyloxy)-2-hydroxypropyloxy)-2-hydroxy}phenyl]-6-(4- methoxyphenyl)-1 ,3,5-triazine
• 2,4-bis-[{4-(2-ethylhexyloxy)-2-hydroxy}phenyl]-6-[4-(2-methoxyethyl carbonyl) phenylamino]-1 ,3,5-triazine
• 2,4-bis-[{4-(3-(2-propyloxy)-2-hydroxypropyloxy)-2-hydroxy}phenyl]-6-[4- (2-ethylcarboxyl) phenylamino]-1 ,3,5-triazine
• 2,4-bis-[{4-(2-ethylhexyloxy)-2-hydroxy}phenyl]-6-(1 -methylpyrrol-2-yl)- 1 ,3,5-triazine
• 2,4-bis-[{4-tris-(trimethylsiloxysilylpropyloxy)-2-hydroxy}phenyl]-6-(4- methoxyphenyl)-1 ,3,5-triazine
• 2,4-bis-[{4-(2"-methylpropenyloxy)-2-hydroxy}phenyl]-6-(4- methoxyphenyl)-1 ,3,5-triazine
• 2,4-bis-[{4-(1 ', 1 ', 1 ',3',5',5',5'-heptamethylsiloxy-2"-methylpropyloxy)-2- hydroxy}phenyl]-6-(4-methoxyphenyl)-1 ,3,5-triazine
UV-A filters such as e.g.
• 4-isopropyl dibenzoyl methane
• terephthalylidene dibornane sulfonic acid and salts (Mexoryl®SX)
• 4-t-butyl-4'-methoxydibenzoyl methane (avobenzone) / (Neo Heliopan®357)
• phenylene bis-benzimidazyl tetrasulfonic acid disodium salt (Neo Heliopan®AP)
• 2,2'-(1 ,4-phenylene)-bis-(1 H-benzimidazole-4,6-disulfonic acid), monosodium salt
• 2-(4-diethylamino-2-hydroxybenzoyl) benzoic acid hexyl ester (Uvinul® A Plus)
• indanylidene compounds in accordance with DE 100 55 940 (= WO 02/38537)
UV filters which are particularly suitable for combining are
• p-aminobenzoic acid
• 3-(4'-trimethylammonium) benzylidene bornan-2-one methyl sulfate
• salicylic acid homomenthyl ester (Neo Heliopan®HMS)
• 2-hydroxy-4-methoxybenzophenone (Neo Heliopan®BB)
• 2-phenylbenzimidazole sulfonic acid (Neo Heliopan®Hydro)
• terephthalylidene dibornane sulfonic acid and salts (Mexoryl®SX)
• 4-tert-butyl-4'-methoxydibenzoyl methane (Neo Heliopan®357)
• 3-(4'-sulfo)benzylidene bornan-2-one and salts
• 2-ethylhexyl-2-cyano-3,3-diphenyl acrylate (Neo Heliopan®303)
• N-[(2 and 4)-[2-(oxoborn-3-ylidene) methyl]benzyl] acrylamide polymer
• p-methoxycinnamic acid-2-ethylhexyl ester (Neo Heliopan®AV)
• p-aminobenzoic acid ethyl ester (25 mol) ethoxylated (INCI name: PEG-25 PABA)
• p-methoxycinnamic acid isoamyl ester (Neo Heliopan®E1000)
• 2,4,6-trianilino-(p-carbo-2'-ethylhexyl-1 '-oxy)-1 ,3,5-triazine (Uvinul®T150)
• phenol, 2-(2H-benzotriazol-2-yl)-4-methyl-6-(2-methyl-3(1 ,3,3,3- tetramethyl-i-(trimethylsilyl)oxy)disiloxyanyl) propyl) (Mexoryl®XL)
• 4,4'-[(6-[4-(1 ,1-dimethyl)aminocarbonyl) phenylamino]-1 ,3,5-triazine-2,4- diyl)diimino]-bis-(benzoic acid-2-ethylhexyl ester) (Uvasorb HEB)
• 3-(4'-methyl benzylidene)-D,L-camphor (Neo Heliopan®MBC)
• 3-benzylidene camphor
• salicylic acid-2-ethylhexyl ester (Neo Heliopan®OS)
• 4-dimethylaminobenzoic acid-2-ethylhexyl ester (Padimate O)
• hydroxy^-methoxybenzophenone-δ-sulfonic acid and Na salt
• 2,2'-methylene bis-(6-(2H-benzotriazol-2-yl)-4-1 ,1 ,3,3-tetramethylbutyl) phenol) (Tinosorb®M)
• phenylene bis-benzimidazyl tetrasulfonic acid disodium salt (Neo Heliopan®AP)
• 2,4-bis-[{(4-(2-ethylhexyloxy)-2-hydroxy}phenyl]-6-(4-methoxyphenyl)- 1 ,3,5-triazine (Tinosorb®S)
benzylidene malonate polysiloxane (Parsol®SLX)
menthyl anthranilate (Neo Heliopan®MA)
• 2-(4-diethylamino-2-hydroxybenzoyl) benzoic acid hexyl ester (Uvinul A Plus)
• indanylidene compounds in accordance with DE 100 55 940 (= WO 02/38537)
Furthermore, particulate UV filters or inorganic pigments, which can optionally be hydrophobed, can be used, such as the oxides of titanium (TiO2), zinc (ZnO), iron (Fe2Os), zirconium (ZrO2), silicon (SiO2), manganese (e.g. MnO), aluminium (AI2O3), cerium (e.g. Ce2O3) and/or mixtures thereof.
Formulations according to the invention, in particular dermatological formulations, can also advantageously contain dyes and/or coloured pigments, particularly if they are intended for use in the area of decorative cosmetics. The dyes and coloured pigments can be selected from the corresponding positive list in the German cosmetics ordinance or the EU list of cosmetic colorants. In most cases they are identical to the dyes approved for foodstuffs. Advantageous coloured pigments are for example titanium dioxide, mica, iron oxides (e.g. Fe2O3 Fe3O4, FeO(OH)) and/or tin oxide. Advantageous dyes are for example carmine, Berlin blue, chromium oxide green, ultramarine blue and/or manganese violet.
If the dermatological formulations according to the invention are intended for use in the facial area, it is convenient to choose as the dye one or more substances from the following group: 2,4-dihydroxyazobenzol, 1-(2'-chloro-4'-nitro-1 '- phenylazo)-2-hydroxynaphthalene, Ceres red, 2-(4-sulfo-1-naphthylazo)-1- naphthol-4-sulfonic acid, calcium salt of 2-hydroxy-1 ,2'-azonaphthalene-1 '- sulfonic acid, calcium and barium salts of 1 -(2-sulfo-4-methyl-1-phenylazo)-2- naphthyl carboxylic acid, calcium salt of 1 -(2-sulfo-1-naphthylazo)-2- hydroxynaphthalene-3-carboxylic acid, aluminium salt of 1-(4-sulfo-1-phenylazo)- 2-naphthol-6-sulfonic acid, aluminium salt of 1 -(4-sulfo-1-naphthylazo)-2-
naphthol-3,6-disulfonic acid, 1-(4-sulfo-1-naphthylazo)-2-naphthol-6,8-disulfonic acid, aluminium salt of 4-(4-sulfo-1-phenylazo)-1-(4-sulfophenyl)-5- hydroxypyrazolone-3-carboxylic acid, aluminium and zirconium salts of 4,5- dibromofluorescein, aluminium and zirconium salts of 2,4,5,7- tetrabromofluorescein, 3',4',5',6'-tetrachloro-2,4,5,7-tetrabromofluorescein and its aluminium salt, aluminium salt of 2,4,5,7-tetraiodofluorescein, aluminium salt of quinophthalone disulfonic acid, aluminium salt of indigo disulfonic acid, red and black iron oxide (Colour Index Number (CIN): 77491 (red) and 77499 (black)), iron oxide hydrate (CIN: 77492), manganese ammonium diphosphate and titanium dioxide.
Also advantageous are oil-soluble natural dyes, such as e.g. paprika extracts, β- carotene or cochineal.
Also advantageous within the meaning of the present invention are dermatological formulations containing pearlescent pigments. The types of pearlescent pigment listed below are particularly preferred:
1. Natural pearlescent pigments, such as e.g. - "pearl essence" (guanine/hypoxanthine mixed crystals obtained from fish scales) and
- "mother of pearl" (ground mussel shells)
2. Monocrystalline pearlescent pigments such as e.g. bismuth oxychloride (BiOCI) 3. Layered substrate pigments: e.g. mica / metal oxide
The basis for pearlescent pigments is formed for example by powdered pigments or castor oil dispersions of bismuth oxychloride and/or titanium dioxide and bismuth oxychloride and/or titanium dioxide on mica. The lustre pigment listed under CIN 77163, for example, is particularly advantageous.
The list of cited pearlescent pigments is naturally not intended to be limiting. Advantageous pearlescent pigments within the meaning of the present invention are obtainable in many ways known per se. For example, substrates other than
mica can be coated with other metal oxides, such as e.g. silica and the like. Siθ2 particles coated with TiO2 and Fe2O3 ("Ronaspheres"), for example, which are sold by Merck and are particularly suitable for the optical reduction of fine lines, are advantageous.
It can also be advantageous to dispense altogether with a substrate such as mica. Iron pearlescent pigments, which are produced without the use of mica, are particularly preferred. Such pigments are available from BASF, for example, under the trade name Sicopearl Copper 1000.
Particularly advantageous also are special effect pigments, which are available from Flora Tech under the trade name Metasomes Standard / Glitter in various colours (yellow, red, green, blue). Here the glitter particles are mixed with various auxiliary substances and dyes (for example the dyes with CIN 19140, 77007, 77289, 77491 ).
The dyes and pigments can be present both individually and mixed together and coated with one another, wherein different colour effects can generally be obtained by means of varying coating thicknesses. The total amount of dyes and colouring pigments is advantageously chosen from the range from e.g. 0.1 wt.% to 30 wt.%, preferably 0.5 to 15 wt.%, in particular 1.0 to 10 wt.%, based in each case on the total weight of the (cosmetic) formulations.
The formulations according to the invention can also contain (additional) antioxidants or preservatives. All antioxidants which are suitable or commonly used for cosmetic (e.g. dermatological) and/or therapeutic applications can be used as antioxidants or preservatives.
Antioxidants within the meaning of the invention are all substances which lower the amount of free radicals in cells and tissue. Antioxidants are advantageously chosen from the group comprising amino acids (e.g. glycine, histidine, tyrosine, tryptophane) and derivatives thereof, imidazoles (e.g. urocanic acid) and derivatives thereof, peptides such as D,L-carnosine, D-carnosine, L-carnosine and derivatives thereof (e.g. anserine), carotenoids, carotenes (e.g. alpha-
carotene, beta-carotene, lycopene) and derivatives thereof, lipoic acid and derivatives thereof (e.g. dihydrolipoic acid), aurothioglucose, propyl thiouracil and other thiols (e.g. thioredoxine, glutathione, cysteine, cystine, cystamine and glycosyl, N-acetyl, methyl, ethyl, propyl, amyl, butyl and lauryl, palmitoyl, oleyl, gamma-linoleyl, cholesteryl, glyceryl and oligoglyceryl esters thereof) and salts thereof, dilauryl thiodipropionate, distearyl thiodipropionate, thiodipropionic acid and derivatives thereof (esters, ethers, peptides, lipids, nucleotides, nucleosides and salts) and sulfoximine compounds (e.g. buthionine sulfoximines, homocysteine sulfoximine, buthionine sulfones, penta-, hexa-, heptathionine sulfoximine) in very small tolerated doses (e.g. pmol to μmol/kg), also (metal) chelators (e.g. alpha-hydroxy fatty acids, palmitic acid, phytic acid, lactoferrin, alpha-hydroxy acids (e.g. citric acid, lactic acid, malic acid), humic acid, bile acid, bile extracts, tannins, bilirubin, biliverdin, EDTA, EGTA and derivatives thereof), unsaturated fatty acids and derivatives thereof (e.g. gamma-linolenic acid, linoleic acid, oleic acid), folic acid and derivatives thereof, ubiquinone and ubiquinol and derivatives thereof, vitamin C and derivatives (e.g. ascorbyl palmitate, Mg ascorbyl phosphate, ascorbyl acetate, ascorbyl glucoside), tocopherols and derivatives (e.g. vitamin E acetate), vitamin A and derivatives (vitamin A palmitate) and coniferyl benzoate of benzoic resin, rutinic acid and derivatives thereof, flavonoids and glycosylated precursors thereof, in particular quercetin and derivatives thereof, e.g. alpha-glucosyl rutin, rosmarinic acid, carnosol, carnosolic acid, resveratrol, caffeic acid and derivatives thereof, sinapic acid and derivatives thereof, ferulic acid and derivatives thereof, curcuminoids, chlorogenic acid and derivatives thereof, retinoids, ursolic acid, levulinic acid, butyl hydroxytoluene, butyl hydroxyanisole, nordihydroguaiac acid, nordihydroguaiaretic acid, trihydroxybutyrophenone, uric acid and derivatives thereof, mannose and derivatives thereof, zinc and derivatives thereof (e.g. ZnO, ZnSO4), selenium and derivatives thereof (e.g. selenium methionine), superoxide dismutase, stilbenes and derivatives thereof (e.g. stilbene oxide, trans-stilbene oxide) and the derivatives (salts, esters, ethers, sugars, nucleotides, nucleosides, peptides and lipids) of these cited active ingredients which are suitable according to the invention or extracts or fractions of plants having an antioxidant effect, such as e.g. green tea, rooibos, honeybush, grape, rosemary, sage, melissa, thyme, lavender, olive, oats, cocoa, ginkgo, ginseng, liquorice, honeysuckle,
sophora, pueraria, pinus, citrus, Phyllanthus emblica or St. John's wort, grape seeds, wheat germ, Phyllanthus emblica.
Also suitable are coenzymes, such as e.g. coenzyme Q10, plastoquinone, menaquinone, ubiquinols 1 -10, ubiquinones 1 -10 or derivatives of these substances.
The amount of antioxidants (one or more compounds) in the formulations according to the invention is preferably 0.01 to 20 wt.%, particularly preferably 0.05 to 10 wt.%, in particular 0.2 to 5 wt.%, based on the total weight of the formulation.
If vitamin E and/or derivatives thereof are used as the antioxidant(s), it is advantageous to choose their concentrations from the range from 0.001 to 10 wt.%, based on the total weight of the formulation.
If vitamin A or vitamin A derivatives or carotenes or derivatives thereof are used as the antioxidant(s), it is advantageous to choose their concentrations from the range from 0.001 to 10 wt.%, based on the total weight of the formulation.
Formulations according to the invention can also contain preservatives. The following can be used as preservatives: all antioxidants which are suitable or commonly used for cosmetic (e.g. dermatological) and/or therapeutic applications, traditional preservatives (e.g. formaldehyde, glutardialdehyde, parabens (e.g. methyl, ethyl, propyl and butyl paraben), dibromodicyanobutane, imidazolidinyl ureas ("Germall"), isothiazolinones ("Kathon"), methyl chlorothiazolidine, methyl thiazolidine, organic acids (e.g. benzoic acid, sorbic acid, salicylic acid) and salts and esters thereof, propionic acid and formic acid and salts thereof, glycols (e.g. propylene glycol, 1 ,2-dihydroxyalkanes), plant- based preservative aids such as e.g. lantadin A, caryophyllene, hesperidin, diosmin, phellandrene, pigenin, quercetin, hypericin, aucubin, diosgenin, plumbagin, corlilagin, etc.
The use of anti-irritants in the formulations according to the invention can also be advantageous. Anti-irritants in this connection can be all anti-inflammatory active ingredients or active ingredients to relieve reddening and itching which are suitable for or commonly used in cosmetic (e.g. dermatological) and/or therapeutic applications. All substances which reduce the amount of cytokines, interleukins, prostaglandins and/or leukotrienes in cells and tissue are preferred.
Steroidal anti-inflammatory substances of the corticosteroid type, such as e.g. hydrocortisone, dexamethasone, dexamethasone phosphate, methyl prednisolone or cortisone, are advantageously used as anti-inflammatory active ingredients or active ingredients to relieve reddening and itching, the list of which can be extended by the addition of other steroidal anti-inflammatories. Nonsteroidal anti-inflammatories can also be used. Examples which can be cited here are oxicams such as piroxicam or tenoxicam; salicylates such as aspirin, disalcid, solprin or fendosal; acetic acid derivatives such as diclofenac, fenclofenac, indomethacin, sulindac, tolmetin or clindanac; fenamates such as mefenamic, meclofenamic, flufenamic or niflumic; propionic acid derivatives such as ibuprofen, naproxen, benoxaprofen or pyrazoles such as phenylbutazone, oxyphenylbutazone, febrazone or azapropazone. Alternatively, natural anti- inflammatory substances or substances to relieve reddening and itching can be used. Plant extracts, special highly active plant extract fractions and highly pure active substances isolated from plant extracts can be used. Particularly preferred are extracts, fractions and active substances from camomile, aloe vera, commiphora species, rubia species, echinacea species, willow, willowherb, oats, black and green tea, gingko, coffee, pepper, blackcurrant, tomato, vanilla, almonds, as well as pure substances such as inter alia bisabolol, apigenin-7- glucoside, boswellic acid, phytosterols, glycyrrhizinic acid, glabridin or licochalcone A.
The amount of anti-irritants (one or more compounds) in the formulations according to the invention is preferably 0.01 to 20 wt.%, particularly preferably 0.03 to 10 wt.%, in particular 0.05 to 5 wt.%, based on the total weight of the formulation.
The formulations according to the invention (in particular topical cosmetic formulations) can also contain moisture regulators and osmolytes. The following substances, for example, can be used as moisture regulators (moisturisers): sodium lactate, urea, alcohols (in particular 1 ,2-pentanediol, 1 ,2-hexanediol, 1 ,2- octanediol, 1 ,2-decanediol and mixtures thereof), sorbitol, glycerol, propylene glycol, collagen, elastin or hyaluronic acid, diacyl adipates, petroleum jelly, ectoine, urocanic acid, lecithin, pantheol, phytanetriol, lycopene, algal extract, ceramides, cholesterol, glycolipids, chitosan, chondroitin sulfate, polyamino acids and sugars, lanolin, lanolin esters, amino acids, alpha-hydroxy acids (e.g. citric acid, lactic acid, malic acid) and derivatives thereof, sugars (e.g. inositol), alpha-hydroxy fatty acids, phytosterols, triterpene acids such as betulinic acid or ursolic acid, algal extracts. The following can be used as osmolytes, for example: sugar alcohols (myo-inositol, mannitol, sorbitol), quaternary amines such as taurine, choline, betaine, betaine glycine, ectoine, diglycerol phosphate, phosphorylcholine, glycerophosphorylcholines, amino acids such as glutamine, glycine, alanine, glutamate, aspartate or proline, phosphatidylcholine, phosphatidylinositol, inorganic phosphates, and polymers of the cited compounds such as proteins, peptides, polyamino acids and polyols.
The formulations according to the invention (e.g. topical cosmetic formulations) also advantageously contain antimicrobial active ingredients. Examples which can be cited are:
Aryl- or aryloxy-substituted, unbranched or monoalkyl- and polyalkyl-branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds) fatty alcohols, fatty aldehydes and fatty acids having chain lengths of C2 to C40.
Aryl- or aryloxy-substituted, unbranched or monoalkyl- and polyalkyl-branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds) alkane diols, dialdehydes and dicarboxylic acids having chain lengths of C2 to C4o, particularly preferably chain lengths of C4 to Ci2.
Mono- and oligoglycerides (up to 4 glycerol units) of aryl- or aryloxy-substituted unbranched or monoalkyl- and polyalkyl-branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds) fatty alcohols (mono- and oligoglycerol monoalkyl ethers), fatty acids (mono- and oligoglycerol monoalkyl esters), alkanediols (mono- and oligoglycerol monoalkyl ethers; bis(mono-/oligoglyceryl)alkyl diethers) and dicarboxylic acids (mono- and oligoglycerol monoalkyl esters; bis(mono- /oligoglyceryl) alkyl diesters) having chain lengths of C2 to C40.
Fatty acid esters of unbranched or monoalkyl- and polyalkyl-branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds), optionally also aryl- or aryloxy-substituted, carboxylic acids having chain lengths of C2 to C40 with unbranched or monoalkyl- and polyalkyl- branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds), optionally also aryl- or aryloxy- substituted, monohydric to hexahydric fatty alcohols having chain lengths of C2 to
Plant and animal fatty acid cuts, containing unbranched or monoalkyl- and polyalkyl-branched saturated or mono- to pentaunsaturated (up to five double or triple bonds, also mixed ene/ine compounds) fatty alcohols, fatty aldehydes and fatty acids having chain lengths of C2 to C40 (e.g. coconut fatty acids, palm kernel fatty acids, wool wax acids).
Mono- and oligoglycerides of lanolin, of lanolin alcohols and lanolic acids (e.g. glyceryl lanolate, neocerite), glycyrrhetic acid and derivatives (e.g. glycyrrhetinyl stearate), natural and synthetic cardenolides (e.g. digitoxin, dogoxin, digoxygenin, gitoxygenin, strophanthin and strophanthidin), natural and synthetic bufadienolides (e.g. scillaren A, scillarenin and bufotalin), sapogenins and steroid sapogenins (e.g. amyrins, oleanolic acid, digitonin, gitogenin, tigogenin and diosgenin), steroid alkaloids of plant and animal origin (e.g. tomatidin, solanin, solanidin, conessin, batrachotoxin and homobatrachotoxin).
Mono- and polyhalogenated nitriles, dinitriles, trinitriles or tetranitriles.
Mono- and oligohydroxy fatty acids having chain lengths of C2 to C24 (e.g. lactic acid, 2-hydroxypalmitic acid), oligomers and/or polymers thereof and plant and animal raw materials containing these.
Acyclic terpenes: terpene hydrocarbons (e.g. ocimene, myrcene), terpene alcohols (e.g. geraniol, linalool, citronellol), terpene aldehydes and ketones (e.g. citral, pseudoionone, beta-ionone); monocyclic terpenes: terpene hydrocarbons (e.g. terpinene, terpinolene, limonene), terpene alcohols (e.g. terpineol, thymol, menthol), terpene ketones (e.g. pulegone, carvone); bicyclic terpenes: terpene hydrocarbons (e.g. carane, pinane, bornane), terpene alcohols (e.g. borneol, isoborneol), terpene ketones (e.g. camphor); sesquiterpenes: acyclic sesquiterpenes (e.g. farnesol, nerolidol), monocyclic sesquiterpenes (e.g. bisabolol), bicyclic sesquiterpenes (e.g. cadinene, selinene, vetivazulene, guajazulene), tricyclic sesquiterpenes (e.g. santalene), diterpenes (e.g. phytol), tricyclic diterpenes (e.g. abietic acid), triterpenes (squalenoids; e.g. squalene), tetraterpenes.
Ethoxylated, propoxylated or mixed ethoxylated/propoxylated cosmetic fatty alcohols, fatty acids and fatty acid esters having chain lengths of C2 to C40 with 1 to 150 E/O and/or P/O units.
Antimicrobial peptides and proteins having an amino acid value from 4 to 200, e.g. Skin Antimicrobial Peptides (SAPs), Lingual Antimicrobial Peptides (LAPs), human beta-defensins (in particular h-BD1 and h-BD2), lactoferrins and hydrolysates thereof and peptides obtained therefrom, Bactericidal/Permeability Increasing Proteins [BPIs], Cationic Microbial Proteins [CAPs], lysozyme.
Very suitable carbohydrates or "carbohydrate derivatives", which in the interests of brevity can also be included under the term "carbohydrates", are compounds containing sugars and substituted sugars or sugar groups. The sugars include in particular also the deoxy and dideoxy forms, N-acetyl galactosamine-, N-acetyl glucosamine- and sialic acid-substituted derivatives as well as sugar esters and ethers. Preference is given to
a) monosaccharides, including in particular pentoses and hexoses, b) disaccharides, including in particular sucrose, maltose, lactobiose, c) oligosaccharides, including in particular the tri- and tetrasaccharides, and d) polysaccharides, including in particular starch, glycogen, cellulose, dextran, tunicin, inulin, chitin, in particular chitosans, chitin hydrolysates, alginic acid and alginates, plant gums, body mucosa, pectins, mannans, galactans, xylans, araban, polyoses, chondroitin sulfates, heparin, hyaluronic acid and glycosaminoglycanes, hemicelluloses, substituted cellulose and substituted starch, in particular the hydroxyalkyl-substituted polysaccharides in each case.
Amylose, amylopectin, xanthan, alpha-, beta- and gamma-dextrin are particularly suitable. The polysaccharides can consist of e.g. 4 to 1 ,000,000, in particular 10 to 100,000, monosaccharides. Chain lengths are preferably chosen in each case which ensure that the active ingredient is soluble in or can be incorporated into the particular formulation.
Sphingolipids such as sphingosine; N-monoalkylated sphingosines; N1N- dialkylated sphingosines; sphingosine-1 -phosphate; sphingosine-1 -sulfate; psychosine (sphingosine-beta-D-galactopyranoside); sphingosyl phosphoryl cholin; lysosulfatides (sphingosyl galactosyl sulfate; lysocerebroside sulfate); lecithin; sphingomyelin; sphinganine.
So-called "natural" antibacterial active ingredients can also be used, most of which are essential oils. Typical oils having an antibacterial action are, for example, oils of aniseed, lemon, orange, rosemary, wintergreen, clove, thyme, lavender, hops, citronella, wheat, lemongrass, cedarwood, cinnamon, geranium, sandalwood, violet, eucalyptus, peppermint, gum benzoin, basil, fennel, menthol and Ocmea origanum, Hydastis carradensis, Berberidaceae daceae, Ratanhiae or Curcuma longa.
Important substances having an antimicrobial action which can be found in essential oils are for example anethol, catechol, camphene, carvacrol, eugenol, eucalyptol, ferulic acid, farnesol, hinokitiol, tropolone, limonene, menthol, methyl
salicylate, thymol, terpineol, verbenone, berberine, curcumin, caryophyllene oxide, nerolodol, geraniol.
Mixtures of the cited active systems or active ingredients and active ingredient combinations containing these active ingredients can also be used.
The amount of antimicrobial active ingredients in the formulations is preferably 0.01 to 20 wt. %, based on the total weight of the formulations, particularly preferably 0.05 to 10 wt.%.
The formulations according to the invention (in particular cosmetic, including dermatological formulations) can contain deodorants, i.e. active ingredients having a deodorising and perspiration-inhibiting action. These include, for example, odour maskers, such as the common perfume constituents, antiperspirants based on aluminium, zirconium or zinc salts, odour absorbers, for example the layered silicates described in the laid-open patent specification DE-P 40 09 347, in particular montmorillonite, kaolinite, nontronite, saponite, hectorite, bentonite, smectite, and also zinc salts of ricinoleic acid, for example. They also include bactericidal or bacteriostatic deodorising substances, such as e.g. hexachlorophene, 2,4,4'-trichloro-2'-hydroxydiphenyl ether (Irgasan), 1 ,6-di- (4-chlorophenylbiguanido)hexane (chlorhexidine), 3,4,4'-trichlorocarbanilide, and the active agents described in the laid-open patent specifications DE-37 40 186, DE-39 38 140, DE-42 04 321 , DE-42 29 707, DE-42 29 737, DE-42 37 081 , DE- 43 09 372, DE-43 24 219 and containing cation-active substances, such as e.g. quaternary ammonium salts and odour absorbers such as e.g. Grillocin® (combination of zinc ricinoleate and various additives) or triethyl citrate, optionally in combination with ion-exchange resins.
The amount of deodorising and/or antiperspirant active ingredients in the formulations is preferably 0.01 to 20 wt.%, based on the total weight of the formulations, particularly preferably 0.05 to 10 wt.%.
The formulations (in particular cosmetic formulations) according to the invention can also contain anionic, cationic, non-ionic and/or amphoteric surfactants,
especially if crystalline or microcrystalline solids, for example inorganic micropigments, are to be incorporated into the formulations.
Anionic surfactants generally have carboxylate, sulfate or sulfonate groups as functional groups. In aqueous solution they form negatively charged organic ions in the acid or neutral environment. Cationic surfactants are almost exclusively characterised by the presence of a quaternary ammonium group. In aqueous solution they form positively charged organic ions in the acid or neutral environment. Amphoteric surfactants contain both anionic and cationic groups and therefore behave in aqueous solution in the same way as anionic or cationic surfactants, depending on the pH. They have a positive charge in a strongly acid environment and a negative charge in an alkaline environment. In the neutral pH range, by contrast, they are zwitterionic. Polyether chains are typical of non-ionic surfactants. Non-ionic surfactants do not form ions in the aqueous medium.
A. Anionic surfactants
Anionic surfactants which can advantageously be used are acyl amino acids (and salts thereof), such as
acyl glutamates, for example sodium acyl glutamate, di-TEA-palmitoyl aspartate and sodium caprylic/capric glutamate,
acyl peptides, for example palmitoyl-hydrolysed milk protein, sodium cocoyl- hydrolysed soya protein and sodium/potassium cocoyl-hydrolysed collagen,
sarcosinates, for example myristoyl sarcosin, TEA-lauroyl sarcosinate, sodium lauroyl sarcosinate and sodium cocoyl sarcosinate,
taurates, for example sodium lauroyl taurate and sodium methyl cocoyl taurate,
- acyl lactylates, lauroyl lactylate, caproyl lactylate
alaninates
carboxylic acids and derivatives, such as
for example lauric acid, aluminium stearate, magnesium alkanolate and zinc undecylenate,
ester carboxylic acids, for example calcium stearoyl lactylate, laureth-6 citrate and sodium PEG-4 lauramide carboxylate,
- ether carboxylic acids, for example sodium laureth-13 carboxylate and sodium PEG-6 cocamide carboxylate,
phosphoric acid esters and salts, such as e.g. DEA-oleth-10-phosphate and dilaureth-4 phosphate,
sulfonic acids and salts, such as
- acyl isothionates, e.g. sodium / ammonium cocoyl isothionate,
alkyl aryl sulfonates,
alkyl sulfonates, for example sodium cocomonoglyceride sulfate, sodium C-12-14 olefin sulfonate, sodium lauryl sulfoacetate and magnesium PEG-3 cocamide sulfate,
- sulfosuccinates, for example dioctyl sodium sulfosuccinate, disodium laureth sulfosuccinate, disodium lauryl sulfosuccinate and disodium undecylenamido MEA sulfosuccinate
and
sulfuric acid esters, such as
- alkyl ether sulfate, for example sodium, ammonium, magnesium, MIPA, TIPA laureth sulfate, sodium myreth sulfate and sodium C12-13 pareth sulfate,
alkyl sulfates, for example sodium, ammonium and TEA lauryl sulfate.
B. Cationic surfactants
Cationic surfactants which can advantageously be used are
- alkyl amines,
- alkyl imidazoles,
- ethoxylated amines and
- quaternary surfactants.
RNH2CH2CH2COO" (where pH=7)
RNHCH2CH2COO- B+ (where pH=12) B+ = any cation, e.g. Na+
- esterquats
Quaternary surfactants contain at least one N atom, which is covalently bonded to 4 alkyl or aryl groups. This leads to a positive charge, regardless of the pH. Alkyl betaine, alkyl amidopropyl betaine and alkyl amidopropyl hydroxysulfaine are advantageous. The cationic surfactants used can also preferably be chosen from the group of quaternary ammonium compounds, in particular benzyl trialkyl ammonium chlorides or bromides, such as benzyl dimethylstearyl ammonium chloride for example, also alkyl trialkyl ammonium salts, for example cetyl trimethyl ammonium chloride or bromide, alkyl dimethyl hydroxyethyl ammonium chlorides or bromides, dialkyl dimethyl ammonium chlorides or bromides, alkyl amide ethyl trimethyl ammonium ether sulfates, alkyl pyridinium salts, for example lauryl or cetyl pyrimidinium chloride, imidazoline derivatives and compounds having a cationic character such as amine oxides, for example alkyl dimethyl amine oxides or alkyl aminoethyl dimethyl amine oxides. Cetyl trimethyl ammonium salts are particularly advantageously used.
C. Amphoteric surfactants
Amphoteric surfactants which can advantageously be used are
- acyl/dialkyl ethylene diamine, for example sodium acyl amphoacetate, disodium acyl amphodipropionate, disodium alkyl amphodiacetate, sodium
acyl amphohydroxypropyl sulfonate, disodium acyl amphodiacetate and sodium acyl amphopropionate,
N-alkyl amino acids, for example aminopropyl alkyl glutamide, alkyl aminopropionic acid, sodium alkyl imidodipropionate and lauroamphocarboxyglycinate.
D. Non-ionic surfactants
Non-ionic surfactants which can advantageously be used are
alcohols,
- alkanolamides, such as cocamides MEA/DEA/MIPA,
amine oxides, such as cocamidopropylamine oxide,
esters produced by esterification of carboxylic acids with ethylene oxide, glycerol, sorbitan or other alcohols,
ethers, for example ethoxylated/propoxylated alcohols, ethoxylated/ propoxylated esters, ethoxylated/propoxylated glycerol esters, ethoxylated/propoxylated cholesterols, ethoxylated/propoxylated triglyceride esters, ethoxylated/propoxylated lanolin, ethoxylated/propoxylated polysiloxanes, propoxylated POE ethers and alkyl polyglycosides such as lauryl glucoside, decyl glycoside and cocoglycoside,
- sucrose esters, ethers,
polyglycerol esters, diglycerol esters, monoglycerol esters,
methyl glucose esters, esters of hydroxy acids.
The use of a combination of anionic and/or amphoteric surfactants with one or more non-ionic surfactants is also advantageous.
The surface-active substance (surfactant) or the combination of surface-active substances can be present in the formulations according to the invention in a
concentration of between 1 and 98 wt.%, based on the total weight of the formulations.
Cosmetic (e.g. dermatological) or therapeutic formulations according to the invention containing the compounds according to the invention or for use according to the invention having formula (I) can also take the form of emulsions.
The oil phase (lipid phase) in the formulations according to the invention (in particular topical cosmetic formulations) can advantageously be selected from the following group of substances:
mineral oils (advantageously paraffin oil), mineral waxes
- fatty oils, fats, waxes and other natural and synthetic fat bodies, preferably esters of fatty acids with low C-number alcohols, for example with isopropanol, propylene glycol or glycerol, or esters of fatty alcohols with low C-number alkanoic acids or with fatty acids;
- alkyl benzoates (e.g. mixtures of n-dodecyl, n-tridecyl, n-tetradecyl or n-pentadecyl benzoate);
cyclic or linear silicone oils such as dimethyl polysiloxanes, diethyl polysiloxanes, diphenyl polysiloxanes and mixed forms thereof.
(Natural or synthetic) esters are advantageously used, in particular (a) esters of saturated and/or unsaturated branched and/or unbranched alkane carboxylic acids having a chain length of 3 to 30 C atoms and saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 3 to 30 C atoms,
(b) esters of aromatic carboxylic acids and saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 3 to 30 C atoms. Preferred ester oils are isopropyl myristate, isopropyl palmitate, isopropyl stearate, isopropyl oleate, n-butyl stearate, n-hexyl laurate, n-decyl laurate, n-decyl oleate, isooctyl stearate, isononyl stearate, isononyl isononanoate, 3,5,5-trimethylhexyl-3,5,5- trimethylhexanoate, 2-ethylhexyl isononanoate, 2-ethylhexyl-3,5,5- trimethylhexanoate, 2-ethylhexyl-2-ethylhexanoate, cetearyl-2-ethylhexanoate, 2- ethylhexyl palmitate, 2-ethylhexyl laurate, 2-hexyldecyl stearate, 2-octyldecyl
palmitate, 2-octyldodecyl palmitate, oleyl oleate, oleyl erucate, erucyl oleate, erucyl erucate and synthetic, semisynthetic and natural mixtures of such esters, e.g. jojoba oil.
The oil phase can also advantageously be chosen from the group consisting of branched and unbranched hydrocarbons and hydrocarbon waxes, silicone oils, dialkyl ethers, the group consisting of saturated or unsaturated, branched or unbranched alcohols, and of fatty acid triglycerides, in particular the triglycerol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms. The fatty acid triglycerides can advantageously be selected from the group of synthetic, semisynthetic and natural oils, e.g. triglycerides of capric or caprylic acid, apricot kernel oil, avocado oil, cottonseed oil, borage seed oil, thistle oil, groundnut oil, gamma-oryzanol, rosehip seed oil, hemp oil, hazelnut oil, blackcurrant seed oil, coconut oil, cherry kernel oil, salmon oil, flax oil, maize oil, macadamia nut oil, almond oil, evening primrose oil, mink oil, olive oil, palm oil, palm kernel oil, pecan nut oil, peach kernel oil, pistachio nut oil, rapeseed oil, rice bran oil, castor oil, safflower oil, sesame oil, soya oil, sunflower oil, teatree oil, grape seed oil or wheat germ oil, and the like. Any blends of such oil and wax components can also advantageously be used. In some cases it is also advantageous to use waxes, for example cetyl palmitate, as the sole lipid component of the oil phase, the oil phase advantageously being chosen from the group consisting of 2-ethylhexyl isostearate, octyl dodecanol, isotridecyl isononanoate, isoeicosane, 2-ethylhexyl cocoate, Ci2-i5-alkyl benzoate, caprylic- capric acid triglyceride and dicaprylyl ether. Mixtures of Ci2-i5-alkyl benzoate and 2-ethylhexyl isostearate, mixtures of Ci2-i5-alkyl benzoate and isotridecyl isononanoate and mixtures of Ci2-i5-alkyl benzoate, 2-ethylhexyl isostearate and isotridecyl isononanoate are particularly advantageous. The hydrocarbons paraffin oil, squalane and squalene can also advantageously be used. The oil phase can advantageously also have a content of cyclic or linear silicone oils or consist entirely of such oils, it being preferable, however, to use an additional content of other oil phase components along with the silicone oil or silicone oils. Cyclomethicone (e.g. decamethyl cyclopentasiloxane) can advantageously be used as the silicone oil. Other silicone oils can also advantageously be used,
however, for example undecamethyl cyclotrisiloxane, polydimethyl siloxane and poly(methylphenyl siloxane). Mixtures of cyclomethicone and isotridecyl isononanoate and of cyclomethicone and 2-ethylhexyl isostearate are also particularly advantageous.
The aqueous phase of formulations according to the invention (in particular topical cosmetic formulations) in the form of an emulsion can advantageously include: alcohols, diols or polyols having a low C number, and ethers thereof, preferably ethanol, isopropanol, propylene glycol, glycerol, ethylene glycol, ethylene glycol monoethyl or monobutyl ether, propylene glycol monomethyl, monoethyl or monobutyl ether, diethylene glycol monomethyl or monoethyl ether and analogous products, also alcohols having a low C number, e.g. ethanol, isopropanol, 1 ,2- propanediol, glycerol and in particular one or more thickeners, which can advantageously be chosen from the group comprising silicon dioxide, aluminium silicates such as e.g. bentonites, polysaccharides or derivatives thereof, e.g. hyaluronic acid, guar gum, xanthan gum, hydroxypropyl methyl cellulose, or allulose derivatives, particularly advantageously from the group of polyacrylates, preferably a polyacrylate from the group of so-called carbopols, for example type 980, 981 , 1382, 2984, 5984 carbopols, either individually or in combination, or from the group of polyurethanes, also alpha- or beta-hydroxy acids, preferably lactic acid, citric acid or salicylic acid, also emulsifiers, which can advantageously be selected from the group of ionic, non-ionic, polymeric, phosphate-containing and zwitterionic emulsifiers.
Formulations according to the invention in the form of an emulsion advantageously include one or more emulsifiers. O/W emulsifiers, for example, can advantageously be chosen from the group of polyethoxylated or polypropoxylated or polyethoxylated and polypropoxylated products, e.g.:
- fatty alcohol ethoxylates,
- ethoxylated wool wax alcohols,
- polyethylene glycol ethers having the general formula R-O-(-CH2-CH2-O-)n-R',
- fatty acid ethoxylates having the general formula R-COO-(-CH2-CH2-O-)n-H,
- etherified fatty acid ethoxylates having the general formula
R-COO-(-CH2-CH2-O-)n-R',
- esterified fatty acid ethoxylates having the general formula
R-COO-(-CH2-CH2-O-)n-C(O)-R',
- polyethylene glycol glycerol fatty acid esters,
- ethoxylated sorbitan esters,
- cholesterol ethoxylates,
- ethoxylated triglycerides,
- alkyl ether carboxylic acids having the general formula
R-COO-(-CH2-CH2-O-)n-OOH, where n represents a number from 5 to 30,
- polyoxyethylene sorbitol fatty acid esters,
- alkyl ether sulfates having the general formula R-O-(-CH2-CH2-O-)n-SO3-H,
- fatty alcohol propoxylates having the general formula R-O-(-CH2-CH(CH3)-O-)n-H,
- polypropylene glycol ethers having the general formula
R-O-(-CH2-CH(CH3)-O-)n-R',
- propoxylated wool wax alcohols,
- etherified fatty acid propoxylates R-COO-(-CH2-CH(CH3)-O-)n-R',
- esterified fatty acid propoxylates having the general formula
R-COO-(-CH2-CH(CH3)-O-)n-C(O)-R',
- fatty acid propoxylates having the general formula
R-COO-(-CH2-CH(CH3)-O-)n-H,
- polypropylene glycol glycerol fatty acid esters,
- propoxylated sorbitan esters,
- cholesterol propoxylates,
- propoxylated triglycerides,
- alkyl ether carboxylic acids having the general formula
R-O-(-CH2-CH(CH3)-O-)n-CH2-COOH,
- alkyl ether sulfates or the acids on which these sulfates are based
having the general formula R-O-(-CH2-CH(CH3)-O-)n-Sθ3-H,
- fatty alcohol ethoxylates/propoxylates having the general formula R-O-Xn-Ym-H,
- polypropylene glycol ethers having the general formula R-O-Xn-Ym-R',
- etherified fatty acid propoxylates having the general formula R-COO-Xn-Ym-R\
- fatty acid ethoxylates/propoxylates having the general formula R-COO-Xn-Ym-H.
Particularly advantageously according to the invention the polyethoxylated or polypropoxylated or polyethoxylated and polypropoxylated 0/W emulsifiers used are chosen from the group of substances having HLB values of 11 to 18, most particularly advantageously having HLB values of 14.5 to 15.5, if the O/W emulsifiers have saturated R and R' radicals. If the O/W emulsifiers have unsaturated R and/or R' radicals, or if isoalkyl derivatives are present, the preferred HLB value of such emulsifiers can also be lower or higher.
It is advantageous to choose the fatty alcohol ethoxylates from the group of ethoxylated stearyl alcohols, cetyl alcohols, cetyl stearyl alcohols (cetearyl alcohols). Particularly preferred are:
polyethylene glycol (n) stearyl ether (steareth-n) where n = 13-20,
polyethylene glycol (n) cetyl ether (ceteth-n) where n = 13-20,
polyethylene glycol (n) isocetyl ether (isoceteth-n) where n = 13-20,
polyethylene glycol (n) cetyl stearyl ether (ceteareth-n) where n = 13-20,
polyethylene glycol (m) isostearyl ether (isosteareth-m) where m = 12-20,
polyethylene glycol (k) oleyl ether (oleth-k) where k = 12-15,
polyethylene glycol (12) lauryl ether (laureth-12),
polyethylene glycol (12) isolauryl ether (isolaureth-12).
It is also advantageous to choose the fatty acid ethoxylates from the following group:
polyethylene glycol (n) stearate where n = 20-25,
polyethylene glycol (m) isostearate where m = 12-25,
polyethylene glycol (k) oleate where k = 12-20.
Sodium laureth-11 carboxylate can advantageously be used as the ethoxylated alkyl ether carboxylic acid or its salt. Sodium laureth 1-4 sulfate can advantageously be used as the alkyl ether sulfate. Polyethylene glycol (30) cholesteryl ether can advantageously be used as the ethoxylated cholesterol derivative. Polyethylene glycol (25) soya sterol has also proved itself.
Polyethylene glycol (60) evening primrose glycerides can advantageously be used as ethoxylated triglycerides.
It is also advantageous to choose the polyethylene glycol glycerol fatty acid esters from the group comprising polyethylene glycol (n) glyceryl laurate where n = 20-23, polyethylene glycol (6) glyceryl caprate/caprinate, polyethylene glycol (20) glyceryl oleate, polyethylene glycol (20) glyceryl isostearate, polyethylene glycol (18) glyceryl oleate/cocoate.
It is likewise beneficial to choose the sorbitan esters from the group comprising polyethylene glycol (20) sorbitan monolaurate, polyethylene glycol (20) sorbitan monostearate, polyethylene glycol (20) sorbitan monoisostearate, polyethylene glycol (20) sorbitan monopalmitate, polyethylene glycol (20) sorbitan monooleate.
The following can be used as advantageous W/O emulsifiers: fatty alcohols having 8 to 30 carbon atoms, monoglycerol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms, diglycerol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms, monoglycerol ethers of saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 8 to 24, in particular 12 to 18 C atoms, diglycerol ethers of saturated and/or unsaturated, branched and/or unbranched alcohols having a chain length of 8 to 24, in particular 12 to 18 C atoms, propylene glycol esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms, and sorbitan esters of saturated and/or unsaturated, branched and/or unbranched alkane carboxylic acids having a chain length of 8 to 24, in particular 12 to 18 C atoms.
Particularly advantageous W/O emulsifiers are glyceryl monostearate, glyceryl monoisostearate, glyceryl monomyristate, glyceryl monooleate, diglyceryl monostearate, diglyceryl monoisostearate, propylene glycol monostearate, propylene glycol monoisostearate, propylene glycol monocaprylate, propylene glycol monolaurate, sorbitan monoisostearate, sorbitan monolaurate, sorbitan monocaprylate, sorbitan monoisooleate, sucrose distearate, cetyl alcohol, stearyl alcohol, arachidyl alcohol, behenyl alcohol, isobehenyl alcohol, selachyl alcohol, chimyl alcohol, polyethylene glycol (2) stearyl ether (steareth-2), glyceryl monolaurate, glyceryl monocaprinate, glyceryl monocaprylate.
Mixtures of the cited active systems can also be used.
The amount of active ingredients in the formulations according to the invention is preferably 0.01 to 20 wt.%, based on the total weight of the formulation, particularly preferably 0.05 to 10 wt.%.
For use, topical formulations according to the invention, in particular formulations for skin and hair lightening, are applied to the skin and/or hair in an adequate amount in the conventional manner for cosmetics.
A further aspect of the invention concerns novel compounds having formula (I)
(I) wherein R denotes an alkyl group having 1 to 32 C atoms or an acyl group having 1 C atom or 3 to 20 C atoms.
As stated above, no mention is made in the prior art of a cosmetic or therapeutic use or a depigmenting action of compounds having formula (I) or of their use in skin and hair lightening agents. A further aspect of the invention therefore concerns a compound having formula (I)
(I) wherein R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, for use as a drug product, in particular for therapeutic skin lightening.
One area of application in this regard is the therapeutic treatment of melamin- induced pigmentation disorders such as hyperpigmentations (e.g. scar
hyperpigmentations, post-traumatic drug-induced hyperpigmentations, postinflammatory hyperpigmentations induced by phototoxic reactions, ephelides).
A final aspect of the compound therefore concerns the use of a compound having formula (I)
R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, for the production of a drug product for therapeutic skin lightening.
Other preferred embodiments of the invention follow from the examples below and the appended claims. Unless otherwise specified, all figures stated relate to the weight.
Examples 1-1 1 : Formulations according to the invention with compositions according to Table 1
Example 1 : "Water-in-oil" emulsion with UVA/B broadband protection Example 2: "Oil-in-water" emulsion with UVA/B broadband protection Example 3: Skin-lightening "oil-in-water" emulsion with UVA/B broadband protection Example 4: Skin -lightening oil-free sun spray with UVA/B broadband protection
Example 5: Skin-lightening balm with UVA/UVB protection Example 6: Skin-lightening aerosol foam with UVB/UVA protection Example 7: Skin-lightening non-aerosol foam Example 8: Shampoo with hair-lightening properties Example 9: Hair-lightening hair conditioner with UVB/UVA protection
Example 10: Skin-lightening moisture cream O/W Example 11 : Skin-lightening face cream O/W
Table 1: Compositions of formulations according to the invention (Examples 1-11)
Example 12 (cytotoxicity determination)
B16V mouse melanoma cells are disseminated in a 96-well microtitre plate in a concentration of 2 x 104 cells/well. After cultivation for 24 h at 37°C and 5 % CO2 in RPMI medium, enriched with 10% foetal calf serum, the medium is drawn off.
Various concentrations of the test substances, dissolved in fresh medium enriched with 5% foetal calf serum (samples), are added and incubated as above for a further 48 h. In parallel, cells are incubated with sodium lauryl sulfate in concentrations of 0.01 mM, 0.1 mM, 1 mM and 10 mM as standards. Only fresh medium enriched with 5% foetal calf serum is added to the controls. After incubation the medium is drawn off and the cells are incubated for 2 h with MTT
(3-[4,5-dimethylthiazol-2-yl]2,5-diphenyl tetrazolium bromide) (0.5 mg/ml in RPMI medium). Blanks contain the MTT solution but no cells. After extraction of the dye (MTT) with pure isopropanol (10 min), the absorption (A) is measured at 570 nm.
The mean and standard deviation of the absorption of the controls, standards, blanks and samples are calculated. The mean of the blanks is subtracted from the means of the controls, standards and samples. The viability of the cells (proportion of living cells) is stated as a percentage relative to the controls (100%):
Viability (%) = [(Atestcompound/Acontrol) X 100]
From the viability (%) in a series of concentrations of test compounds, the IC5O (mean inhibitory concentration) for each test compound is calculated. This indicates the test substance concentration at which only 50% of the cells are still vital (living).
Table 1
As can be seen from Table 1 , the diacetyl trimer having formula (II) is extremely cell-compatible (IC5O MTT > 10 mM).
Example 13 (depigmenting effect)
B16V mouse melanoma cells are disseminated in a 96-well microtitre plate in a concentration of 5 x 103 cells/well. After cultivation for 24 h at 37°C and 5% CO2 in RPMI medium, enriched with 10% foetal calf serum, various concentrations of the test substances and 0.3 mM tyrosine and 10 nM α-MSH (α-melanocyte stimulating hormone) are added and incubated for a further 96 h. The maximum concentration of the test substances used corresponds to 0.1 times the value of the IC20 value of the cytotoxicity assay. Standards are incubated with kojic acid in concentrations of 0.01 mM, 0.1 mM and 1 mM in addition to tyrosine and α-MSH. Only tyrosine and α-MSH are added to the controls. After incubation, sodium
lauryl sulfate and sodium hydroxide solution (final concentrations: 1 mM and 1 M respectively) are added to the culture medium and the absorption (A) is measured after 3 h at 400 nm.
The inhibition of pigmentation in the presence of the test compounds or kojic acid was calculated using the following equation:
Inhibition of pigmentation (%) = 100- [(Atestcompound/Acontroi) x 100]
From the inhibition of pigmentation (%) in a series of dilutions of test compounds, the IC50 for each test compound is calculated. This is the concentration of a test compound at which pigmentation is inhibited by 50%.
Table 2
This data shows that diacetyl trimer having formula (II) has a more than 40 times stronger depigmenting effect on B16V melanoma cells than kojic acid.
Example 14: Production of the diacetyl trimer having formula Il (6-hydroxy- 1 ,3,6,7,9-pentamethyl-2,8,10,11 -tetraoxatricvclor5.2.1.139lundecan-4-one)
(II)
Diacetyl (butane-2,4-dione, 50 g, 0.581 mol) is mixed with concentrated hydrochloric acid (20 ml) and stirred for 5 h while being cooled in an ice bath. The mixture is stored overnight at 5°C and the resulting brown, crystalline mass is
filtered off. The deposit is rinsed with a little iced water and recrystallised out of water (150 ml).
Yield: 15.3 g (GC: 99.2 % purity).
HPLC-MS (APCI-): m/z = 257 [M-H]"
1H-NMR (400 MHz, CDCI3): δ = 3.36 (1 H, d, J = 10.7 Hz, H-5a), 2.52 (1 H, s, OH), 2.44 (1 H, d, J = 10.7 Hz, H-5b), 1.59 (3H, s, CH3), 1.56 (3H, s, CH3), 1.44 (3H, s, CH3), 1.39 (3H, s, CH3), 1.29 (3H, s, CH3) ppm.
13C-NMR (100 MHz, CDCI3): δ = 212.28 (C, C-4), 1 12.39 (C, C-3), 1 11.91 (C, C- 1 ), 1 1 1.54 (C, C-9), 105.88 (C, C-7), 81.3 (C, C-6), 46.83 (CH2, C-5), 23.86 (CH3), 22.00 (CH3), 21.40 (CH3), 20.62 (CH3), 20.00 (CH3) ppm.
Claims
1. Cosmetic or therapeutic use of one or more compounds having formula (I)
R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, for the lightening of skin and/or hair.
2. Cosmetic or therapeutic use according to claim 1 , wherein
R denotes hydrogen, an alkyl group having 1 to 6 C atoms or an acyl group having 1 to 16 C atoms.
3. Cosmetic or therapeutic use according to claim 1 , wherein R denotes hydrogen.
4. Process for the cosmetic or therapeutic lightening of skin and/or hair, comprising the following step:
Application of a cosmetically or therapeutically effective amount of a compound having formula (I)
(I) wherein
R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, to the hair and/or skin.
5. Process according to claim 4, wherein R denotes hydrogen, an alkyl group having 1 to 6 C atoms or an acyl group having 1 to 16 C atoms.
6. Process according to claim 4, wherein R denotes hydrogen.
7. Cosmetic or therapeutic formulation containing an amount having a lightening effect on skin or hair of one or more compounds having formula (I)
R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms.
8. Formulation according to claim 7, wherein
R denotes an alkyl group having 1 to 32 C atoms, preferably 1 to 6 C atoms, or an acyl group having 1 C atom or 3 to 20 C atoms, preferably 1 C atom or 3 to 16 C atoms.
9. Formulation according to claim 7, wherein
R denotes hydrogen, an alkyl group having 1 to 6 C atoms or an acyl group having 1 to 16 C atoms.
10. Formulation according to claim 7, wherein R denotes hydrogen.
1 1. Formulation according to one of claims 7 to 10, containing an amount having a lightening effect on skin or hair of one or more further active ingredients for skin and hair lightening which are not compounds having formula (I).
12. Formulation according to claim 1 1 , containing one or more active ingredients for skin and hair lightening selected from the group consisting of:
kojic acid, kojic acid derivatives, arbutin, ascorbic acid, ascorbic acid derivatives, hydroquinone, hydroquinone derivatives, resorcinol, sulfur-containing molecules, alpha-hydroxy acids, derivatives of alpha-hydroxy acids, N-acetyl tyrosine, N- acetyl tyrosine derivatives, undecenoyl phenylalanine, gluconic acid, 4-alkyl resorcinols, 4-(1 -phenylethyl)-1 ,3-dihydroxybenzene, chromone derivatives, flavonoids, thymol derivatives, 1 -aminoethyl phosphinic acid, thiourea derivatives, ellagic acid, nicotinamide, zinc salts, thujaplicin, thujaplicin derivatives, triterpenes, sterols, benzofuranones, vinyl guiacol, ethyl guiacol, dionic acids, inhibitors of nitrogen oxide synthesis, metal chelators, retinoids, soya milk and extract, serine protease inhibitors, lipoic acid, plant extracts and constituents concentrated from plant extracts.
13. Formulation according to one of claims 7 to 12 in a form selected from the group consisting of:
VWO emulsion, O/W emulsion, W/O/W emulsion, 0/W/O emulsion, PIT emulsion, Pickering emulsion, emulsion with a low oil content, microemulsion, nanoemulsion, gel, solution and spray.
14. Formulation according to one of claims 7 to 13, containing one or more substances selected from the group consisting of:
Abrasives, anti-dandruff agents, anti-inflammatory agents, antioxidants, perspiration-inhibiting agents, binders, buffers, chelating agents, depilatory agents, surface-active substances, emulsifiers, enzymes, essential oils, plant extracts, fibres, film formers, fixatives, foaming agents, foam stabilisers, substances to prevent foaming, foam boosters, gel-forming agents, hair care products, hair-shaping agents, hair-smoothing agents, moisture-releasing agents, moisture-retaining substances, insect repellents, optical brightening agents, lubricants, glossing agents, polymers, proteins, nourishing agents, skin-calming agents, skin-smoothing agents, wrinkle-reducing agents, sunscreens, vitamins, oils, waxes, fats, phospholipids, saturated fatty acids, salts of saturated fatty acids, mono- or polyunsaturated fatty acids, salts of mono- or polyunsaturated fatty acids, alpha-hydroxy acids, polyhydroxy fatty acids, polyols, alkanediols, silicones and silicone derivatives.
15. Formulation according to claim 14, containing one or more sunscreens, wherein the sunscreen(s) are one or more UV filters.
16. Formulation according to claim 15, containing one or more UV filters selected from the group consisting of: p-aminobenzoic acid; p-aminobenzoic acid ethyl ester (25 mol) ethoxylated; p- dimethylaminobenzoic acid-2-ethylhexyl ester; p-aminobenzoic acid ethyl ester (2 mol) N-propoxylated; p-aminobenzoic acid glycerol ester; salicylic acid homomenthyl ester; salicylic acid-2-ethylhexyl ester; triethanolamine salicylate; 4- isopropyl benzyl salicylate; anthranilic acid menthyl ester; diisopropyl cinnamic acid ethyl ester; p-methoxycinnamic acid-2-ethylhexyl ester; diisopropyl cinnamic acid methyl ester; p-methoxycinnamic acid isoamyl ester; p-methoxycinnamic acid diethanolamine salt; p-methoxycinnamic acid isopropyl ester; 2-phenyl benzimidazole sulfonic acid and salts; 3-(4'-trimethyl ammonium) benzylidene bornan-2-one methyl sulfate; beta-imidazole-4(5)-acrylic acid; 3-(4'- sulfo)benzylidene bornan-2-one; salts of 3-(4'-sulfo)benzylidene bornan-2-one; 3- (4'-methyl benzylidene)-D,L-camphor; 3-benzylidene-D,L-camphor; N-[(2 and 4)- [2-(oxoborn-3-ylidene)methyl]benzyl] acrylamide polymer; 4,4'-[(6-[4-(1 ,1- dimethyl)aminocarbonyl) phenylamino]-1 ,3,5-triazine-2,4-diyl)diimino]-bis- (benzoic acid-2-ethylhexyl ester); benzylidene malonate polysiloxane; glyceryl ethyl hexanoate dimethoxycinnamate; dipropylene glycol salicylate; tris(2- ethylhexyl)-4,4',4"-(1 ,3,5-triazine-2,4,6-triyltriimino) tribenzoate; 2-ethylhexyl-2- cyano-3,3-diphenyl acrylate; ethyl-2-cyano-3,3'-diphenyl acrylate; 2-hydroxy-4- methoxybenzophenone; 2-hydroxy-4-methoxybenzophenone-5-sulfonic acid; dihydroxy-4-methoxybenzophenone; 2,4-dihydroxybenzophenone; tetrahydroxybenzophenone; 2,2'-dihydroxy-4,4'-dimethoxybenzophenone; 2- hydroxy-4-n-octoxybenzophenone; 2-hydroxy-4-methoxy-4'- methylbenzophenone; sodium hydroxymethoxybenzophenone sulfonate; disodium-2,2'-dihydroxy-4,4'-dimethoxy-5,5'-disulfobenzophenone; phenol, 2-(2H- benzotriazol-2-yl)-4-methyl-6-(2-methyl-3(1 ,3,3,3-tetramethyl-1 -
(trimethylsilyl)oxy)disiloxyanyl)propyl); 2,2'-methylene-bis-(6-(2H-benzotriazol-2- yl)-4-1 ,1 ,3,3-tetramethylbutyl)phenol); 2,4-bis-[4-(2-ethylhexyloxy)-2- hydroxyphenyl]-1 ,3,5-triazine; 2,4-bis-[{(4-(2-ethylhexyloxy)-2-hydroxy}-phenyl]- 6-(4-methoxyphenyl)-1 ,3,5-triazine; 2,4-bis-[{(4-(3-sulfonato)-2- hydroxypropyloxy)-2-hydroxy}-phenyl]-6-(4-methoxyphenyl)-1 ,3,5-triazine sodium salt; 2,4-bis-[{(3-(2-propyloxy)-2-hydroxypropyloxy)-2-hydroxy}-phenyl]-6-(4- methoxy-phenyl)-1 ,3,5-triazine; 2,4-bis-[{4-(2-ethylhexyloxy)-2-hydroxy}-phenyl]- 6-[4-(2-methoxyethyl carbonyl)-phenylamino]-1 ,3,5-triazine; 2,4-bis-[{4-(3-(2-propyloxy)-2-hydroxypropyloxy)-2-hydroxy}phenyl]-6-[4-(2- ethylcarboxyl)-phenylamino]-1 ,3,5-tιϊazine; 2,4-bis-[{4-(2-ethylhexyloxy)-2- hydroxy}-phenyl]-6-(1 -methyl-pyrrol-2-yl-)-1 ,3,5-triazine; 2,4-bis-[{4-tris-
(trimethylsiloxy-silylpropyloxy)-2-hydroxy}-phenyl]-6-(4-methoxyphenyl)-1 ,3,5- triazine; 2,4-bis-[{4-(2"-methylpropenyloxy)-2-hydroxy}-phenyl]-6-(4- methoxyphenyl)-1 ,3,5-triazine; 2,4-bis-[{4-(1 ',1 ',1 ',3',5',5',5'-heptamethylsiloxy-2"- methylpropyloxy)-2-hydroxy}-phenyl]-6-(4-methoxyphenyl)-1 ,3,5-triazine; 4- isopropyldibenzoylmethane; terephthalylidene dibornane sulfonic acid; salts of terephthalylidene dibornane sulfonic acid; 4-t-butyl-4'-methoxy dibenzoylmethane; phenylene-bis-benzimidazyl tetrasulfonic acid disodium salt; 2,2'-(1 ,4-phenylene)-bis-(1 H-benzimidazole-4,6-disulfonic acid), monosodium salt; 2-(4-diethylamino-2-hydroxybenzoyl) benzoic acid hexyl ester and indanylidene compounds.
17. Formulation according to one of claims 15 or 16, wherein the ratio of the total percentage by weight of UV filters to the total percentage by weight of compounds having formula (I) is in the range from 100:1 to 1 :100.
18. Formulation according to one of claims 15 to 17, wherein the total proportion of UV filters is in the range from 0.1 to 30 wt.%, based on the total weight of the formulation.
19. Formulation according to one of claims 15 to 18, which contains an adequate total amount of UV filters to stabilise the compounds having formula (I) and to prevent the discoloration of the formulation.
20. Compound having formula (I)
(I) wherein
R denotes an alkyl group having 1 to 32 C atoms or an acyl group having 1 C atom or 3 to 20 C atoms.
21. Compound having formula (I)
(I) wherein
R denotes hydrogen, an alkyl group having 1 to 32 C atoms or an acyl group having 1 to 20 C atoms, for use as a drug product.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US78600306P | 2006-03-27 | 2006-03-27 | |
| US60/786,003 | 2006-03-27 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007110415A2 true WO2007110415A2 (en) | 2007-10-04 |
| WO2007110415A3 WO2007110415A3 (en) | 2009-06-04 |
Family
ID=38255437
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2007/052883 WO2007110415A2 (en) | 2006-03-27 | 2007-03-26 | Use of diacetyl trimers and cosmetic or therapeutic formulations containing these compounds |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2007110415A2 (en) |
Cited By (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009087242A2 (en) | 2009-04-09 | 2009-07-16 | Symrise Gmbh & Co. Kg | Compositions comprising trans-tert-butyl cyclohexanol as skin irritation-reducing agent |
| EP2158896A2 (en) | 2009-11-02 | 2010-03-03 | Symrise GmbH & Co. KG | Materials containing aromas incorporating neopentyl glycol isononanoate |
| EP2168570A2 (en) | 2008-09-30 | 2010-03-31 | Symrise GmbH & Co. KG | Extracts of isochrysis sp. |
| EP2174645A2 (en) | 2009-10-19 | 2010-04-14 | Symrise GmbH & Co. KG | Materials containing aromas incorporating cetyl nonanoate and/or stearyl nonanoate |
| EP2184339A1 (en) | 2009-10-19 | 2010-05-12 | Symrise GmbH & Co. KG | Perfume mixtures containing isolongifolanol and musk |
| EP2193785A2 (en) | 2008-12-05 | 2010-06-09 | Symrise GmbH & Co. KG | Extracts of Tetraselmis sp. for cosmetic and therapeutic purposes |
| WO2010070152A2 (en) | 2010-04-08 | 2010-06-24 | Symrise Gmbh & Co. Kg | Use of dihydrodehydrodiisoeugenol and preparations comprising dihydrodehydrodiisoeugenol |
| EP2286908A2 (en) | 2010-11-19 | 2011-02-23 | Symrise AG | Solubilizer for cosmetic preparations |
| EP2295031A2 (en) | 2009-08-05 | 2011-03-16 | Symrise AG | Use of pterocarpans as anti-cellulite agents |
| WO2011089247A1 (en) | 2010-01-22 | 2011-07-28 | Symrise Ag | Solubilization agent for solubilizing polyphenols, flavonoids and/or diterpenoid glucosides |
| EP2356977A1 (en) | 2011-02-02 | 2011-08-17 | Symrise AG | Preparations with wood extracts of gleditsia |
| EP2389922A1 (en) | 2010-05-25 | 2011-11-30 | Symrise AG | Cyclohexyl carbamate compounds as anti-ageing actives |
| DE102011082464A1 (en) | 2011-09-09 | 2011-12-01 | Symrise Ag | Fragrance mixture, useful for preparing a perfumed product, comprises (2S,5S,8R)-6-isopropyl-8-methoxy-2,10,10-trimethylspiro(4.5)dec-6-ene and (2S,5S,8S)-6-isopropyl-8-methoxy-2,10,10-trimethylspiro(4.5)dec-6-ene, and musk fragrances |
| EP2457554A1 (en) | 2010-11-24 | 2012-05-30 | Symrise AG | Mixture containing menthol |
| EP2474301A1 (en) | 2011-12-14 | 2012-07-11 | Symrise AG | Perfume mixtures containing Cyclopent-2-Enyl-ethyl acetate |
| WO2012143576A2 (en) | 2012-08-23 | 2012-10-26 | Symrise Ag | Compounds for preventing, reducing and/or alleviating itchy skin condition(s) |
| EP2524959A1 (en) | 2011-05-17 | 2012-11-21 | Symrise AG | Dioxolanes containing olfactory and/or aromatic substances |
| WO2013041130A1 (en) | 2011-09-20 | 2013-03-28 | Symrise Ag | Acetals and ketals as fragrances and flavors |
| WO2013112040A1 (en) | 2012-01-27 | 2013-08-01 | Biotropics Malaysia Berhad | Use of certain trioxygenated benzene derivatives in body fat management |
| US8563754B2 (en) | 2010-01-28 | 2013-10-22 | New York University | Compounds, compositions and methods for preventing skin darkening |
| US8623340B2 (en) | 2009-04-28 | 2014-01-07 | Symrise Ag | Omega-cyclohexylalkan-1-oles and use thereof as antimicrobial actives to combat body odor |
| EP2789369A1 (en) | 2013-04-14 | 2014-10-15 | Symrise AG | A composition for lightening skin and hair |
| EP2853254A1 (en) | 2013-09-26 | 2015-04-01 | Symrise AG | A cosmetic composition for lightening skin and/or hair |
| WO2017140336A1 (en) | 2016-02-15 | 2017-08-24 | Symrise Ag | Fragrant mixtures containing esters and ketones |
| WO2018041354A1 (en) | 2016-09-01 | 2018-03-08 | Symrise Ag | Product comprising a plastic container and a substance composition |
| WO2018154145A2 (en) | 2018-03-29 | 2018-08-30 | Symrise Ag | Compounds for skin improvement / treatment |
| WO2018171871A1 (en) | 2017-03-21 | 2018-09-27 | Symrise Ag | 2,3,6-trimethylcyclohexanol as a scenting and/or flavoring agent |
| WO2018171865A1 (en) | 2017-03-21 | 2018-09-27 | Symrise Ag | 5-bicyclo[2.2.1]hept-2-enyl-acetate as a scenting and/or flavoring agent |
| WO2018171872A1 (en) | 2017-03-21 | 2018-09-27 | Symrise Ag | Fragrance mixtures containing tricyclo[5.2.1.0]-decane-8-ethyl ether |
| WO2018192665A1 (en) | 2017-04-21 | 2018-10-25 | Symrise Ag | 4-ethyl-octene-2/3-nitrile as a fragrance |
| WO2018192652A1 (en) | 2017-04-19 | 2018-10-25 | Symrise Ag | 1,1-dimeth/ethoxynon-3-in as a fragrance |
| WO2018196988A1 (en) | 2017-04-28 | 2018-11-01 | Symrise Ag | Ethyl-2-acetyl-2,4-dimethyl-pent-4-enoate as a fragrance |
| US10272026B2 (en) | 2017-07-31 | 2019-04-30 | L'oreal | Water-in-oil emulsion compositions suitable for altering the color of hair |
| WO2019114969A1 (en) | 2017-12-14 | 2019-06-20 | Symrise Ag | Aromatic substance mixtures containing 8,8-dimethyl-6,10-dioxaspiro[4,5]decane |
| WO2020020434A1 (en) | 2018-07-23 | 2020-01-30 | Symrise Ag | Novel odorants having rose odour |
| WO2020233778A1 (en) | 2019-05-17 | 2020-11-26 | Symrise Ag | Fragrance or flavouring agent mixture |
| WO2021008696A1 (en) | 2019-07-16 | 2021-01-21 | Symrise Ag | Fragrance mixture containing 1,3-propanediol |
| WO2021204380A1 (en) | 2020-04-08 | 2021-10-14 | Symrise Ag | Esters as fragrance compounds |
| EP3944853A1 (en) | 2020-07-31 | 2022-02-02 | Basf Se | Liquid l-menthol composition |
| WO2022128164A1 (en) | 2020-12-14 | 2022-06-23 | Symrise Ag | Extracts of isochrysis species / tisochrysis species |
| WO2023001385A1 (en) | 2021-07-23 | 2023-01-26 | Symrise Ag | Compositions of dicaffeoylquinic acids with tocopherol |
| WO2023036439A1 (en) | 2021-09-13 | 2023-03-16 | Symrise Ag | Cyclopropanated fragrance compounds |
-
2007
- 2007-03-26 WO PCT/EP2007/052883 patent/WO2007110415A2/en active Application Filing
Non-Patent Citations (5)
| Title |
|---|
| ALEXANDROPOULOU, IOANNA ET AL: "Acid-promoted reactions of 2,3-butanedione" TETRAHEDRON , 55(18), 5867-5874 CODEN: TETRAB; ISSN: 0040-4020, 1999, XP002517649 cited in the application * |
| BALDENIUS, KAI ET AL: "Formation of biacetyl trimers in acidic media" TETRAHEDRON LETTERS , 34(9), 1517-20 CODEN: TELEAY; ISSN: 0040-4039, 1993, XP002517650 cited in the application * |
| DIELS, OTTO ET AL: "Preparation of diacetyl and a polymerisation product of diacetyl" BERICHTE DER DEUTSCHEN CHEMISCHEN GESELLSCHAFT , 35, 3290-9 FROM: J. CHEM. SOC., ABSTR. 82, I, 744 1902 CODEN: BDCGAS, 1902, XP002517651 cited in the application * |
| PATEL, A. V. ET AL: "Stereochemistry of 11-Hydroxy-1,3,5,7,11-pentamethyl-2,4,6,8- tetraoxatricyclo[3.3.3.0(3,7)]undecan-9-on e and Related Diols" TETRAHEDRON , 56(51), 10005-10009 CODEN: TETRAB; ISSN: 0040-4020, 2000, XP002517648 cited in the application * |
| POJE M ET AL: "THE STEREOSTRUCTURE OF TWO TRIMERS OF BIACETYL" TETRAHEDRON, ELSEVIER SCIENCE PUBLISHERS, AMSTERDAM, NL, vol. 41, no. 10, 1 January 1985 (1985-01-01), pages 1985-1987, XP009066360 ISSN: 0040-4020 cited in the application * |
Cited By (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10463891B2 (en) | 2008-09-30 | 2019-11-05 | Symrise Ag | Extracts of Isochrysis sp. |
| EP2168570A2 (en) | 2008-09-30 | 2010-03-31 | Symrise GmbH & Co. KG | Extracts of isochrysis sp. |
| EP2193785A2 (en) | 2008-12-05 | 2010-06-09 | Symrise GmbH & Co. KG | Extracts of Tetraselmis sp. for cosmetic and therapeutic purposes |
| EP3348255A1 (en) | 2008-12-05 | 2018-07-18 | Symrise AG | Extracts of tetraselmis sp. for cosmetic and therapeutic purposes |
| US9445988B2 (en) | 2008-12-05 | 2016-09-20 | Symrise Ag | Extracts of Tetraselmis sp |
| WO2009087242A2 (en) | 2009-04-09 | 2009-07-16 | Symrise Gmbh & Co. Kg | Compositions comprising trans-tert-butyl cyclohexanol as skin irritation-reducing agent |
| US8623340B2 (en) | 2009-04-28 | 2014-01-07 | Symrise Ag | Omega-cyclohexylalkan-1-oles and use thereof as antimicrobial actives to combat body odor |
| US8633203B2 (en) | 2009-08-05 | 2014-01-21 | Symrise Ag | Use of pterocarpans as active anti-cellulite ingredients |
| EP2295031A2 (en) | 2009-08-05 | 2011-03-16 | Symrise AG | Use of pterocarpans as anti-cellulite agents |
| EP2184339A1 (en) | 2009-10-19 | 2010-05-12 | Symrise GmbH & Co. KG | Perfume mixtures containing isolongifolanol and musk |
| US9050261B2 (en) | 2009-10-19 | 2015-06-09 | Symrise Ag | Compositions comprising fragrance substances and comprising cetyl nonanoate and/or stearyl nonanoate |
| EP2174645A2 (en) | 2009-10-19 | 2010-04-14 | Symrise GmbH & Co. KG | Materials containing aromas incorporating cetyl nonanoate and/or stearyl nonanoate |
| EP2158896A2 (en) | 2009-11-02 | 2010-03-03 | Symrise GmbH & Co. KG | Materials containing aromas incorporating neopentyl glycol isononanoate |
| EP2359702A1 (en) | 2010-01-22 | 2011-08-24 | Symrise AG | Solubilization agent for solubilizing polyphenols, flavonoids and/or diterpenoid glucosides |
| WO2011089247A1 (en) | 2010-01-22 | 2011-07-28 | Symrise Ag | Solubilization agent for solubilizing polyphenols, flavonoids and/or diterpenoid glucosides |
| US8563754B2 (en) | 2010-01-28 | 2013-10-22 | New York University | Compounds, compositions and methods for preventing skin darkening |
| WO2010070152A2 (en) | 2010-04-08 | 2010-06-24 | Symrise Gmbh & Co. Kg | Use of dihydrodehydrodiisoeugenol and preparations comprising dihydrodehydrodiisoeugenol |
| EP2389922A1 (en) | 2010-05-25 | 2011-11-30 | Symrise AG | Cyclohexyl carbamate compounds as anti-ageing actives |
| EP2455157A1 (en) | 2010-11-19 | 2012-05-23 | Symrise AG | Solubilizer for cosmetic preparations |
| EP2286908A2 (en) | 2010-11-19 | 2011-02-23 | Symrise AG | Solubilizer for cosmetic preparations |
| EP2457555A2 (en) | 2010-11-24 | 2012-05-30 | Symrise AG | Mixture containing menthol |
| EP2457554A1 (en) | 2010-11-24 | 2012-05-30 | Symrise AG | Mixture containing menthol |
| EP2356977A1 (en) | 2011-02-02 | 2011-08-17 | Symrise AG | Preparations with wood extracts of gleditsia |
| EP2524959A1 (en) | 2011-05-17 | 2012-11-21 | Symrise AG | Dioxolanes containing olfactory and/or aromatic substances |
| EP2568035A1 (en) | 2011-09-09 | 2013-03-13 | Symrise AG | Perfume mixtures containing certain isolongifolenyl methyl ethers |
| DE102011082464A1 (en) | 2011-09-09 | 2011-12-01 | Symrise Ag | Fragrance mixture, useful for preparing a perfumed product, comprises (2S,5S,8R)-6-isopropyl-8-methoxy-2,10,10-trimethylspiro(4.5)dec-6-ene and (2S,5S,8S)-6-isopropyl-8-methoxy-2,10,10-trimethylspiro(4.5)dec-6-ene, and musk fragrances |
| WO2013041130A1 (en) | 2011-09-20 | 2013-03-28 | Symrise Ag | Acetals and ketals as fragrances and flavors |
| EP2474301A1 (en) | 2011-12-14 | 2012-07-11 | Symrise AG | Perfume mixtures containing Cyclopent-2-Enyl-ethyl acetate |
| WO2013112040A1 (en) | 2012-01-27 | 2013-08-01 | Biotropics Malaysia Berhad | Use of certain trioxygenated benzene derivatives in body fat management |
| WO2012143576A2 (en) | 2012-08-23 | 2012-10-26 | Symrise Ag | Compounds for preventing, reducing and/or alleviating itchy skin condition(s) |
| EP2789369A1 (en) | 2013-04-14 | 2014-10-15 | Symrise AG | A composition for lightening skin and hair |
| EP2853254A1 (en) | 2013-09-26 | 2015-04-01 | Symrise AG | A cosmetic composition for lightening skin and/or hair |
| WO2015044390A1 (en) | 2013-09-26 | 2015-04-02 | Symrise Ag | A cosmetic composition for lightening skin and hair |
| WO2017140336A1 (en) | 2016-02-15 | 2017-08-24 | Symrise Ag | Fragrant mixtures containing esters and ketones |
| WO2018041354A1 (en) | 2016-09-01 | 2018-03-08 | Symrise Ag | Product comprising a plastic container and a substance composition |
| WO2018171871A1 (en) | 2017-03-21 | 2018-09-27 | Symrise Ag | 2,3,6-trimethylcyclohexanol as a scenting and/or flavoring agent |
| WO2018171865A1 (en) | 2017-03-21 | 2018-09-27 | Symrise Ag | 5-bicyclo[2.2.1]hept-2-enyl-acetate as a scenting and/or flavoring agent |
| WO2018171872A1 (en) | 2017-03-21 | 2018-09-27 | Symrise Ag | Fragrance mixtures containing tricyclo[5.2.1.0]-decane-8-ethyl ether |
| WO2018171917A1 (en) | 2017-03-21 | 2018-09-27 | Symrise Ag | 2,3,6-trimethylcyclohexanol as a scenting and/or flavoring agent |
| WO2018192652A1 (en) | 2017-04-19 | 2018-10-25 | Symrise Ag | 1,1-dimeth/ethoxynon-3-in as a fragrance |
| WO2018192665A1 (en) | 2017-04-21 | 2018-10-25 | Symrise Ag | 4-ethyl-octene-2/3-nitrile as a fragrance |
| WO2018196988A1 (en) | 2017-04-28 | 2018-11-01 | Symrise Ag | Ethyl-2-acetyl-2,4-dimethyl-pent-4-enoate as a fragrance |
| US10272026B2 (en) | 2017-07-31 | 2019-04-30 | L'oreal | Water-in-oil emulsion compositions suitable for altering the color of hair |
| WO2019114969A1 (en) | 2017-12-14 | 2019-06-20 | Symrise Ag | Aromatic substance mixtures containing 8,8-dimethyl-6,10-dioxaspiro[4,5]decane |
| WO2019185923A1 (en) | 2018-03-29 | 2019-10-03 | Symrise Ag | Retinol replacement in skin treatment |
| WO2018154145A2 (en) | 2018-03-29 | 2018-08-30 | Symrise Ag | Compounds for skin improvement / treatment |
| WO2020020434A1 (en) | 2018-07-23 | 2020-01-30 | Symrise Ag | Novel odorants having rose odour |
| US11634659B2 (en) | 2018-07-23 | 2023-04-25 | Symrise Ag | Fragrances with rose scent |
| WO2020233778A1 (en) | 2019-05-17 | 2020-11-26 | Symrise Ag | Fragrance or flavouring agent mixture |
| WO2021008696A1 (en) | 2019-07-16 | 2021-01-21 | Symrise Ag | Fragrance mixture containing 1,3-propanediol |
| WO2021009315A1 (en) | 2019-07-16 | 2021-01-21 | Symrise Ag | Fragrance mixture containing 1,3-propanediol |
| WO2021204380A1 (en) | 2020-04-08 | 2021-10-14 | Symrise Ag | Esters as fragrance compounds |
| EP3944853A1 (en) | 2020-07-31 | 2022-02-02 | Basf Se | Liquid l-menthol composition |
| WO2022128164A1 (en) | 2020-12-14 | 2022-06-23 | Symrise Ag | Extracts of isochrysis species / tisochrysis species |
| WO2023001385A1 (en) | 2021-07-23 | 2023-01-26 | Symrise Ag | Compositions of dicaffeoylquinic acids with tocopherol |
| WO2023036439A1 (en) | 2021-09-13 | 2023-03-16 | Symrise Ag | Cyclopropanated fragrance compounds |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007110415A3 (en) | 2009-06-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007110415A2 (en) | Use of diacetyl trimers and cosmetic or therapeutic formulations containing these compounds | |
| US9060949B2 (en) | Menthyl carbamate compounds as skin and/or hair lightening actives | |
| US9072676B2 (en) | Cyclohexyl carbamate compounds as skin and/or hair lightening actives | |
| EP2023910B1 (en) | Ah receptor antagonists | |
| US20090028804A1 (en) | AhR mediators | |
| JP2009522336A (en) | Stabilized formulation containing phenolic compound and benzophenone | |
| US20110294876A1 (en) | Cyclohexyl carbamate compounds as anti-ageing actives | |
| JP2009522338A (en) | Low oil content formulations containing diphenylmethane derivatives | |
| EP2887929A2 (en) | Compounds for preventing, reducing and/or alleviating itchy skin condition(s) | |
| WO2006040349A1 (en) | Process for strengthening the barrier function of undamaged skin | |
| JP2007523127A (en) | Use of (2-hydroxyphenyl) alcohols and cosmetic or therapeutic formulations containing these compounds | |
| EP2389922A1 (en) | Cyclohexyl carbamate compounds as anti-ageing actives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07727356 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07727356 Country of ref document: EP Kind code of ref document: A2 |