WO2007029851A1 - Method for producing fatty acid alkyl esters and/or glycerin - Google Patents
Method for producing fatty acid alkyl esters and/or glycerin Download PDFInfo
- Publication number
- WO2007029851A1 WO2007029851A1 PCT/JP2006/317945 JP2006317945W WO2007029851A1 WO 2007029851 A1 WO2007029851 A1 WO 2007029851A1 JP 2006317945 W JP2006317945 W JP 2006317945W WO 2007029851 A1 WO2007029851 A1 WO 2007029851A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- reaction
- stage
- catalyst
- fatty acid
- glycerin
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11C—FATTY ACIDS FROM FATS, OILS OR WAXES; CANDLES; FATS, OILS OR FATTY ACIDS BY CHEMICAL MODIFICATION OF FATS, OILS, OR FATTY ACIDS OBTAINED THEREFROM
- C11C3/00—Fats, oils, or fatty acids by chemical modification of fats, oils, or fatty acids obtained therefrom
- C11C3/003—Fats, oils, or fatty acids by chemical modification of fats, oils, or fatty acids obtained therefrom by esterification of fatty acids with alcohols
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/03—Preparation of carboxylic acid esters by reacting an ester group with a hydroxy group
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/08—Preparation of carboxylic acid esters by reacting carboxylic acids or symmetrical anhydrides with the hydroxy or O-metal group of organic compounds
Definitions
- the present invention relates to a method for producing fatty acid alkyl esters and/or glycerin from fats or oils of animal or plant origin. More specifically, the present invention relates to a method for producing fatty acid alkyl esters and/or glycerin useful for fuel, food, cosmetics, pharmaceuticals and the like purposes.
- BACKGROUND ART Fatty acid alkyl esters derived from vegetable oils are used as cooking oil and, in addition, used in such fields as cosmetics and pharmaceuticals .
- fuels such as fossil diesel fuel.
- fatty acid alkyl esters are added to fossil diesel fuel in an amount of several percents as vegetable-derived biodiesel fuel for reducing emission of CO2.
- Glycerin is mainly used as a rawmaterial for producing nitroglycerin and is further used as a raw material for alkyd resins, or for pharmaceuticals, foods, printing inks, cosmetics and the like.
- This production method is an industrially very useful method because energy consumption in the production can be reduced and fatty acid alkyl esters or glycerin with high purity can be produced with advantage in terms of energy.
- a heterogeneous catalyst containing a metal oxide with a specific structure such as a zinc aluminum mixed oxide, a mixed oxide of aluminum and titanium, zirconium, or antimony, crystalline or amorphous titanosilicates, and a mixed oxide of zirconium oxide and titanium oxide
- a metal oxide with a specific structure such as a zinc aluminum mixed oxide, a mixed oxide of aluminum and titanium, zirconium, or antimony, crystalline or amorphous titanosilicates, and a mixed oxide of zirconium oxide and titanium oxide
- such a heterogeneous catalyst generally has activity lower than that of homogeneous alkali catalysts and therefore, the reaction generally needs to be performed under high temperature and high pressure conditions. Under such conditions, the reaction solution forms a uniform single phase. Therefore, such a method maybe disadvantageous in terms of theory of chemical equilibrium, as compared with the method using a homogeneous alkali catalyst, in which a glycerin phase is separated from an ester phase (liquid phase is divided into two phases) . That is, there is a problem in that fatty acid mono- and/or diglycerides intermediates often remains in fatty acid alkyl esters that are products.
- reaction solution is separated into two phases, that is, aphasemainly containing fatty acid alkyl esters
- esters phase and a phase mainly containing glycerin (glycerin phase) under mild temperature condition as in the method using a homogeneous alkali catalyst. Therefore, a concentration of glycerin components as one of products in the ester phase becomes extremely smaller (because the glycerin moves outside of the system) . Therefore, glycerin and fatty acid alkyl esters are easily obtained from monoglycerides and an alcohol in terms of chemical equilibrium (intermediate glycerides are reduced) .
- a basic metal oxide catalyst for example, referring to Japanese Kokai Publication Sho-61-254255 (pages land 2) and International Publication No .
- WO 98/56747 or an anion-exchange resin (for example, referring to Japanese Kokai Publication Sho-62-218495 (page 2)), which is a solid catalyst with activity under mild temperature condition, is used.
- the basic metal oxide catalyst has a short catalyst life because active components of the catalyst leached into the reaction solution.
- the fat or oil may be saponified to generate soaps, and therefore complicated steps such as the catalyst-separated and removed step, the washing step, and neutralization step are essentially performed.
- the anion-exchange resin is used, free fatty acids, intermediate glycerides or glycerin inhibits the reaction, failing in improvement in yield. Therefore, there is room for improvement in such production methods in order to produce fatty acid alkyl esters or glycerin with high purity more simply and with higher yield by improving these respects .
- a production method using such a method can be an industrially useful technique in this field.
- the method using an anion-exchange resin is certainly effective in that fatty acid alkyl esters and/or glycerin can be produced simply with high yield and high purity.
- it is industrially important that such a property can be maintained and exhibited for a prolonged period.
- Fatty acid alkyl esters or glycerin as industrial products may be greatly affected in terms of costs or quality because of the content of mono- and/or diglycerides, if the anion-exchange resin must be replaced in a short period of time, or reactivation of such an exchange resin takes time or costs, or production quality thereof is reduced in a short period. From such respects, there is room for improvement in production methods capable of eliminating all of the problems in conventional techniques.
- the present invention has been made in view of the above-mentioned state of the art.
- the present invention has an obj ect to provide amethod for effectively and simplyproducing fatty acid alkyl esters and/or glycerin suitable for applications such as fuel and food, at low costs and with high quality.
- the present inventors have made various investigations about methods for producing fatty acid alkyl esters and/or glycerin.
- the production method of the present invention includes the above specified matter (1) or (2), and the above specified matters (1) and (2) may be appropriately combined in the production method of the present invention.
- the production may be performed by the following method. That is, they have noted that if a reaction step of a fat or oil with an alcohol is performed in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst to sufficiently perform alcoholysis reaction of the fat or oil with the alcohol and then the obtained ester phase is matured under a condition in which glycerin to be produced is separated from the ester phase, transesterification reaction of the fat or oil with the alcohol can be almost completed and therefore, such a production method can be an industrially useful method.
- reaction step in the former stage is performed under high temperature and high pressure conditions in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, the complicated steps such as the catalyst-separated and removed step, and the washing step can be simplifiedor omitted, and also the esterphasehaving a reduced amount of components which may inhibit the reaction such as free fatty acids contained in the fat or oil, can be easily obtained.
- the reaction solution may not be necessarily separated.
- the reaction solution is generally separated into a phase mainly containing fatty acid alkyl esters (hereinafter, also referred to as “ester phase”) and a phase mainly containing glycerin (hereinafter, also referred to as “glycerin phase”) .
- esteer phase a phase mainly containing fatty acid alkyl esters
- glycerin phase a phase mainly containing glycerin
- the reaction is performed in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, the reaction is performed under high temperature and high pressure conditions for sufficient proceeding of the reaction. Thereby, free fatty acids contained in the fat or oil are simultaneously esterified to be fatty acid alkyl esters.
- reaction solution forms a single phase because of improved mutual solubility of the ester phase and the glycerin phase under high temperature and high pressure conditions, and thereby a glycerin concentration in the reaction system becomes higher, which is disadvantageous in theory of chemical equilibrium. Therefore, fatty acid monoglyceride intermediates may remain.
- all or part of the alcohol is evaporated if necessary, and then the reaction solution is cooled to separate into the ester phase and the glycerin phase.
- the purification of fatty acid alkyl esters may be insufficient even by distilation, and therefore purification step which may be subsequently performed may be complicated, because almost all of the glycerides as an intermediate is distributed into the ester phase, and some glycerides have a boiling point close to that of the fatty acid alkyl esters.
- the reaction of a fat or oil with an alcohol is performed in the presence of the solidbase catalyst such as anion-exchange resin, the reaction proceeds even under mild condition. Therefore, the mutual solubility of the ester phase and the glycerin phase becomes lower, which is advantageous in theory of chemical equilibrium.
- free fatty acids or glycerides at a high concentration inhibit the catalyst, and therefore the reaction is not completed.
- the present inventors have found that the content of the substances causing the reaction inhibition can be reduced if a fat or oil is previously subjected to alcoholysis reaction under high temperature and high pressure conditions, in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, and found that the residual intermediate fatty acid monoglycerids and the like after the reaction under high temperature and high pressure conditions are matured under mild condition in the presence of a solid base catalyst to be converted into fatty acid alkyl esters or glycerin, and thereby fatty acid alkyl esters with high impurity can be produced by easy purification process, and also equipment costs or utility costs can be suppressed.
- the present inventors have found that the content of the intermediate glycerides can be reduced even if a large excess of alcohol is not used as in a conventional solid catalysis method, and therefore costs on recovery of the alcohol can be suppressed enough. Thereby, the above-mentioned problems have been admirably solved.
- the present invention is a method for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol; a reaction step in n stage is an aging step; and the method comprises a free fatty acids-treated step of reducing a free fatty acid amount between the reaction step in the (n-1) stage and the reaction step in the n stage.
- This production method essentially comprises a step of reducing the content of the free fatty acid in the ester phase before the aging step.
- the aging step may be performed under the condition that the reaction solution is separated into phases, but not necessarily performedunder such a condition. That is, the reaction solution is not separated into phases in some cases, depending on kind of an alcohol to be used in the reaction step.
- Preferable embodiments of the above-mentioned production method include the following (1) to (4) embodiments or combinations thereof, and combinations of the above-mentioned production method and preferable embodiments described in the present description. That is, mentioned are (1) the aging step in the n stage is performed in the presence of a base catalyst; (2) the reaction step in the (n-1) stage is a step of esterifying a free fatty acid contained in a reaction solution obtained in (n-2) stage/ (3) a reaction temperature in the aging step in the n stage is 180°C or less; and (4) an acid value of an ester phase used in the aging step in the n stage is controlled to 4.0 or less (r ⁇ g-KOH/g-ester phase).
- the present invention also is a method for producing.fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol inn stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and a reaction step in n stage is an aging step in the presence of a solid base catalyst.
- n stages representing an integer of 2 or more
- a catalyst used in the reaction step in at least one stage from the first stage to the (n-1) stage and a catalyst used in the reaction step in the n stage are noted in order to solve conventional problems in the production method of fatty acid alkyl esters and/or glycerin.
- This production method specifies whether or not a catalyst is used and what catalyst is used if used in each of the reaction steps.
- Preferable embodiments of the above-mentioned production method include the following (1) to (5) embodiments or combinations thereof, and combinations of the above-mentioned production method and preferable embodiments described in the present description.
- the method comprises a free fatty acids-treated step of reducing a free fatty acid amount between the reaction step in the (n-1) stage and the reaction step in the n stage;
- the insoluble solid catalyst is a metal oxide catalyst;
- the reaction step in the (n-1) stage is a step of esterifying the free fatty acid contained in a reaction solution obtained in (n-2) stage;
- a reaction temperature in the aging step in the n stage is 180°C or less;
- an acid value of an ester phase used in the aging step in the n stage is controlled to 4.0 or less (mg-KOH/g-ester phase) .
- the following production method is part of the present invention.
- the present invention is also a method for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol at 180°C or more in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and a reaction step in n stage is an aging step at 180°C or less.
- reaction step in at least one stage from the first stage and the (n-1) stage is a step of reacting the fat or oil with the alcohol at 180°C or more in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, as mentioned above, is because 180°C is a boundary that determines whether or not the reaction solution is separated into two phases.
- the present invention also includes amethod for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and a reaction step in n stage is a step of aging an ester phase obtained in the reaction step in the (n-1) stage under the condition that all or part of glycerin to be generated is separated from the ester phase.
- n stages representing an integer of 2 or more
- a free fatty acids-treated step may be performed after the transesterification reaction of the fat or oil with the alcohol . That is, an embodiment in which this production method comprises a free fatty acids-treated step is one of preferable embodiments of the present invention.
- reaction solution may not be necessarily separated into phases in the aging step, as mentioned above.
- the reaction step in the (n-1) stage and the reactionstep (agingstep) in then stage shouldbe distinguished.
- a step of bringing a fat or oil into contact with an alcohol is performed in n stages (n representing an integer of 2 or more) .
- n stages representing an integer of 2 or more
- to perform the step of bringing a fat or oil into contact with an alcohol (hereinafter, also referred to as "contacting step") in two or more stages means that the reaction of the fat or oil with the alcohol is performed in two or more stages using two or more reactors serially connected.
- the alcoholysis step from the first stage to (n-1) stage and the aging step in the n stage are performed.
- the alcoholysis step means a step in which transesterification reaction of glycerides with an alcohol and esterification reaction of free fatty acids with an alcohol simultaneously proceed.
- the aging step means a step in which transesterification reaction of residual intermediate glycerides (mainly, monoglycerides) after the alcoholysis step with an alcohol mainly proceed.
- the reaction step in the (n-1) stage is a step of esterifying the free fatty acid contained in a reaction solution obtained in (n-2) stage.
- the free fatty acid contained in the reaction solution obtained in the (n-2) stage is esterified in the reaction step in the (n-1) stage, and thereby catalyst life in the ageing step and production efficiency and purity of products can be improved.
- the reaction solution out of the reactor is separated into a phase mainly containing fatty acid alkyl esters (ester phase) and a phase mainly containing glycerin (glycerin phase) in the alcoholysis step, andthen the esterphase is chargedas one of reactionrawmaterials, into a next reactor with an alcohol. That is, it is preferable that the ester phase is separated from the reaction solution after the reaction in the former stage and then recovered, and the reaction in the latter stage is performed using the ester phase and an alcohol as raw materials.
- ester phase is separated from the reaction solution after the reaction in the former stage and then recovered, and the reaction in the latter stage is performed using the ester phase and an alcohol as raw materials.
- the reaction in the former stage means a reaction firstly performed and the reaction in the latter stage means a reaction performed after the first reaction, if continuous reactions in two stages are focused. That is, in reaction in two stages, the first stage is a reaction in the former stage, and the second stage is a reaction in the latter stage. In reaction in three stages, the first stage is a reaction in the former stage and the second stage is a reaction inthe latter stage, if the reactions in the first and second stages are focused. And the second stage is a reaction in the former stage and the third stage is a reaction in the latter stage, if the reactions in the second and third stages are focused.
- the substances causing the reaction inhibition can be reduced through the alcoholysis step.
- the aging reaction can be preferably performed in the aging step under mild condition in the presence of a base catalyst (more preferably, a solid base catalyst) which is preferably used, as mentioned below.
- a base catalyst more preferably, a solid base catalyst
- the alcohol is evaporated from the reaction solution after the reaction of the fat or oil with the alcohol in the absence of a catalyst before the reaction solution is separated into the ester phase and glycerin phase in the alcoholysis step .
- the productionmethod may have an embodiment in which the reaction solution is separated into the ester phase and the glycerin phase without evaporating the alcohol, and then the ester phase is used in a subsequent reaction.
- substantially in the absence or “the absence of a catalyst” means an embodiment in which the reaction solution contains no catalyst or hardly contains a catalyst, that is, an embodiment in which it is not evaluated that the reaction is performed in the presence of a catalyst.
- a total concentration of active metal components leached from the catalyst into the reaction solution is preferably 1000 ppm or less.
- active metal component leached means metal components derived from the insoluble solid catalyst leached into the reaction solution and capable of serving as a homogeneous catalyst with catalytic activity in the transesterification and/or or the esterification under operation conditions.
- the leached active metal component having a concentration of more than 1000 ppm fails to suppress the reverse reaction sufficiently in the above-mentioned alcohol-distilled step . Therefore, load of utility in the production may be insufficiently reduced.
- the concentration thereof is preferably 800 ppm or less, and more preferably 600 ppm or less, and still more preferably 300 ppm or less.
- the reaction solution substantially contains no active metal components.
- the leaching amount of the above-mentioned active metal components of the catalyst in the reaction solution can be identified by subjecting the reaction solution as it is to fluorescent X-ray spectroscopy (XRF) analysis. Smaller leaching amount is preferably identified by inductively coupled plasma (ICP) emission spectrometry.
- the operational temperature in the above-mentioned alcohol-evaporated step is 300 0 C or less. If the operational temperature is more than 300 0 C, it may become impossible to evaporate the alcohol sufficiently while sufficiently inhibiting the desired product from being evaporated.
- the operational temperature is more preferably 280°C or less and still more preferably 25O 0 C or less.
- reduced pressure conditions or ordinarypressure conditions are generally employed. Increased pressure conditions also may be employed. In view of reduction in energy consumption of the production system, reducedpressure conditions are preferred.
- the evaporated alcohol is recovered after the above-mentioned alcohol-evaporated step, and then at least part of the recovered alcohol is used as a raw material if the reaction is continuously performed. Thereby, production costs can be more sufficiently reduced. It is economically more preferable that all of the recovered alcohol is used as a raw material. It is also preferable that the recovered alcohol is purified by removing impurities therefrom by a method such as distilation and then the purified alcohol is used in the reaction . If the alcohol reused as a raw material contains moisture, yield of the fatty acid alkyl esters may be insufficient due to the progress of hydrolysis of the fatty acid alkyl esters in the reaction step.
- the moisture concentration in the alcohol is preferably 5% by weight (% by mass or mass %) or less. If the moisture concentration is more than 5% by weight, the amount of hydrolysis of the fatty acid alkyl esters into the free fatty acids increases and not only the yield becomes insufficient but also a step of removing the free fatty acids becomes necessary, hence the method may not be carried out in a satisfactorily advantageous manner from the industrial viewpoint.
- the moisture concentration is more preferably 3% by weight or less and still more preferably 1.5% by weight or less.
- the operation can be permitted at a temperature lower than that in the above-mentioned alcohol-evaporated step.
- the operational temperature is preferably 25O 0 C or less.
- the operational temperature is more preferably 220°C or less, and still more preferably 210°C or less.
- the operational pressure is the same as in the above-mentioned alcohol-evaporated step.
- the reaction step in at least one stage in the alcoholysis step is a step of reacting a fat or oil with an alcohol under high temperature and high pressure conditions in which a reaction solution forms a single phase, in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst.
- the insoluble solid catalyst is not especially limited as long as it shows insolubility in the fat or oil and the alcohol as raw materials and in the products (the fatty acid alkyl esters and the glycerin) .
- the insolubility means that the reaction solution contains leached active components of the catalyst of 1000 ppm or less, and preferably 800 ppm or less, and more preferably 600 ppm or less, and still more preferably 300 ppm or less . Most preferably, the reaction solution substantially contains no active metal components. The reason why the catalyst is not limited is because the catalyst never changes chemical equilibrium.
- the insoluble solid catalyst is a catalyst capable of catalyzing the esterification of the free fatty acid contained in the fat or oil, namely one having activity in both the transesterification of the glycerides contained in the fat or oil and the esterification in which the fatty acid alkyl esters are producedby reaction of the free fatty acids with the alcohol .
- Examples of the above-mentioned insoluble solid catalyst include a zinc aluminum mixed oxide, mixed oxides of aluminum and titanium, zirconium, or antimony, crystalline or amorphous titanosilicates, mixed oxides of zirconium oxide and titanium oxide rutile type titanium oxides, anatase type titanium oxides, metal oxides having an ilmenite structure, and metal oxides having a slyrankite structure.
- Another catalyst other than the above-mentioned insoluble solid catalyst may be used in combination.
- One or two or more species of the above-mentioned catalysts may be used.
- the catalyst may contain impurities and other components generated during the catalyst-prepared step unless effects of the present invention are sacrificed.
- the insoluble solid catalyst is a metal oxide catalyst .
- metal oxide catalysts containing zirconium and at least one metallic element selected fromthe group consisting ofmetallic elements belonging to the Groups 4, 5 and 8 as essential components (2) metal oxide catalysts having an ilmenite structure and/or or a slyrankite structure; and (3) metal oxide catalysts containing an anatase type titanium oxide and/or or a rutile type titanium oxide as essential components.
- These metal oxide catalysts (1) to (3) are catalysts having an ability of catalyzing the esterification and the transesterification simultaneously, and are unaffected by a mineral acid or a metal component contained in the fat or oil, and further avoid decomposition of the alcohol .
- the above-mentioned metal oxide catalyst (1) is not especially limited as long as it contains the above-mentioned essential components, and may be, for example, a mixture of a single oxide or a mixed oxide, or contain an active component carried or immobilized on a carrier. And the metal oxide may be also in the form in which one of the above-mentioned essential components is used as the carrier and other components are carried or immobilized as the active component.
- the carrier on which the active component is carried or immobilized examples include silica, alumina, silica-alumina, various zeolites, activated carbon, kieselguhr, zirconium oxide, rutile type titanium oxide, tin oxide, and lead oxide.
- the above-mentioned metal oxide catalyst (1) is preferably in the form in which zirconium oxide is used as the carrier and a single or mixed oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 5 and 8 is used as the active component, and the active component is carried or immobilized on the carrier .
- zirconium oxide is used as the carrier, the catalyst has high activity.
- the above-mentioned zirconium oxide preferably has a monoclinic structure. Such monoclinic zirconium oxide further improves catalytic activity, and suppresses leaching of the above-mentionedmetallic element which is the activemetal component.
- the above-mentioned at least one metallic element selected form the group consisting of metallic elements belonging to the Groups 4, 5 and 8 is preferably a single or mixed oxide as mentioned above.
- the mixed oxide may be a mixed oxide of the at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 5 and 8, and other metallic elements.
- the single or mixed oxide more preferably contains at least one metallic element selected from the group consisting of Ti, V, and Fe. Among them, it is more preferable that Ti is essentially contained.
- titanium-containing oxide examples include titanium oxides such as anatase type TiO 2 and rutile type TiO 2 , titania silica, titania zirconia, titania magnesia, titania calcia, titania yttria, titaniaboria, titania-tin oxide; titaniumvanadiummixed oxides such as TiVO 4 ; vanadium oxides; iron vanadiummixed oxides such as FeVO 4 ; cobalt vanadium mixed oxides such as Co 2 V 2 O 7 ; cerium vanadiummixed oxides such as CeVO 4 ; zinc vanadiummixed oxides; nickel vanadium mixed oxides; copper vanadium mixed oxide, scandium vanadium mixed oxides ; yttrium vanadium mixed oxides ; lanthanum vanadiummixed oxides; tin vanadiummixed oxides; lead vanadium mixed oxides; antimony vanadium mixed oxides; bismuth vana
- One or two or more species of them may be used. Among them, anatase type TiO 2 , rutile type TiO 2 , and iron vanadiummixed oxides are preferred. Rutile type TiO 2 and triclinic FeVO 4 are more preferred.
- the mixed oxide may be in any form, and the following forms may be mentioned: a form in which a first atom and a second atom are bonded by covalent bond with an oxygen atom therebetween; a form in which a first atom and a second atom are bonded, and the bonded atoms and an oxygen atom are bonded by covalent bond; and a composite of oxides of a first atom and a second atom and solid solution thereof.
- the above-mentioned metal oxide catalyst (1) is a mixed oxide of zirconium and the above-mentioned metallic element, the mixed oxide is preferably Zro.5Tio.5O2 (slyrankite) .
- the slyrankite structure is mentioned below in the above metal oxide catalyst (2) .
- the above-mentioned metal oxide catalyst (1) preferably contains a metal oxide having a triclinic structure as an active component .
- the triclinic structure means a crystal systemwhich has three crystallographic axes obliquely crossing with one another, the lengths of the crystallographic axes being different from one another, and has a triclinic lattice.
- the metal oxide is preferably an oxide containing iron, vanadium, and zirconium, in which triclinic FeVCU mixed oxide is carried or immobilized on zirconium oxide.
- the above-mentioned oxide containing iron, vanadium, and zirconium may be, other than the above oxide, an oxide in which iron vanadium mixed oxide that is not triclinic is carried or immobilized on zirconium oxide, or a ternary mixed oxide containing iron, vanadium, and zirconium. These are one of the preferable embodiments of the present invention. Use of the oxide containing iron, vanadium, and further zirconium suppresses leaching of the active component as compared with common oxides containing iron and vanadium, and thereby the catalyst life can be more improved.
- the content of the zirconium in the above-mentioned metal oxide catalyst (1) has a lower limit of 1% by weight, and an upper limit of 80% by weight as a metal, relative to 100% by weight of a total amount of the catalyst of the present invention . If the content is less than 1% by weight, the metal oxide may neither exhibit high activity nor suppress leaching to the raw material or the product enough. If the content is more than 80%byweight, high activity may not be exhibited any more. More preferably, the lower limit is 2% by weight and the upper limit is 75% by weight.
- the content of the at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 5, and 8 has a lower limit of 1% by weight and an upper limit of 80% by weight as a metal, relative to 100% by weight of a total amount of the catalyst of the present invention. If the content is less than 1% by weight, the catalyst activity is insufficient, which possibly fails to improve the reaction efficiency. If it exceeds 80% by weight, the above-mentioned oxide of the metallic element agglutinates on the carrier, and thereby the metal oxide catalyst may not exhibit high activity and functional effects attributed to the presence of zirconium may be insufficiently exhibited. More preferably, the lower limit is 2% by weight and the upper limit is 75% by weight.
- the above-mentioned content of the metallic element can be identified by X-ray fluorescence (XRF) analysis.
- the above-mentioned metal oxide catalyst (2) is not especially limited as long as it has an ilmenite structure and/or a slyrankite structure, and may be, for example, a mixture of a single oxide or a mixed oxide, or contain an active component (forexample, a single ormixed oxide ofmetallic element) carried or immobilized on a carrier.
- an active component forexample, a single ormixed oxide ofmetallic element
- the carrier on which the active component is carried or immobilized include silica, alumina, silica-alumina, various zeolites, activated carbon, kieselguhr, zirconium oxide, rutile type titanium oxide, tin oxide, and lead oxide.
- the above-mentioned ilmenite structure is expressed by the formula ABX 3 (A and B being cations and X being an anion) , and means a rhombohedral lattice which is a slightly distorted hexagonal closest packing of X with the octahedral holes occupied by A and B regularly arranging as 6 coordination.
- a mixed oxide represented by FeTiO 3 has a structure in which a position of Al of ⁇ -alumina (corundum type) is replaced with Fe and Ti regularly.
- the catalyst has such a structure, the catalyst has sufficient insolubility to any of the fat or oil and the alcohol which are rawmaterials of the present invention, and the products (the fatty acid alkyl esters, glycerin and the like) , and the catalyst life is remarkably improved. Therefore, in the production method of the present invention, recycling efficiency of the catalyst is more improved and the catalyst-separated and removed step canbe remarkably simplified or omitted. Therefore, utility costs or equipment costs are sufficiently reduced.
- the metal oxide has such an ilmenite structure in which at least one of A and B is titanium in the above formula.
- the catalyst preferably contains a metal oxide having an ilmenite structure containing titanium and at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 7, 8, 9, and 10. In this case, elution of the active component is more sufficiently suppressed, which permits sufficient exhibition of functional effects of the present invention. More preferably, the above-mentioned metal oxide is MnTiO 3 , FeTiO 3 , CoTiO 3 , or NiTiO 3 .
- the above-mentioned slyrankite structure means a mixed oxide and/or solid solution, mainly composed of titanium and zirconium, whichhas the same structure as inorthorhombic ⁇ -Pb ⁇ 2, represented by Ti 0 .67Zr 0 . 33 ⁇ 2 discovered by A. Willgallis et al in 1983. That is, the slyrankite structure characteristically has a structure in which Ti 4+ and Zr 4+ are randomly arranged in place of Pb 4+ of W-PbO 2 . Specifically, the slyrankite structure is a mixed oxide and/or solid solution in which atomic ratio of a total atomic number of Ti 4+ and Zr 4+ to oxygen is 1:2.
- the atomic ratio of Ti to Zr is preferably (1 to 0.2) : ( 0 to 0.8 ) , more preferably (0.8 to 0.3): (0.2 to 0.7), and still more preferably (0.7 to 0.4): (0.3 to 0.6).
- the above-mentioned catalyst (2) containing such a metal oxide exhibits activity higher than that of titanium oxide, silica-carried titanium oxide or the like. Therefore, according to the production method of the present invention, recycling efficiency of this catalyst is more improved and therebyutility costs or equipment costs are sufficientlyreduced, which permits production of fatty acid alkyl esters and/or glycerin with high yield and high selectivity. It can be identified using powder X-ray diffraction measurement (XRD) whether the above-mentioned catalyst has an ilmenite structure or a slyrankite structure.
- XRD powder X-ray diffraction measurement
- the above-mentioned metal oxide catalyst (3) is not especially limited as long as it contains the above-mentioned essential components.
- the titanium content preferably has a lower limit of 0.5% by weight, and an upper limit of 60% by weight, relative to 100% by weight of the metal oxide catalyst.
- the lower limit is more preferably 1% by weight and still more preferably 2% by weight.
- the upper limit is more preferably 45% by weight, and still more preferably 30% by weight.
- anatase structure and "rutile structure” mean crystal structures belonging to the tetragonal system and formed by a compound represented by AB 2 (A: positive atom, B: negative atom) .
- a atom is octahedrally coordinatedbyB atom.
- resulting octahedrons form a skeletal structure as a result of each octahedron sharing 4 edges with eachneighboring octahedron .
- resulting octahedrons form a skeletal structure as a result of each octahedron sharing 2 edges with each neighboring octahedron.
- Such compounds having the rutile structure can be obtained by calcining compounds having the anatase structure.
- Powder X-ray diffraction measurement can show whether the catalyst contains the anatase type titanium oxide and/or the rutile type titanium oxide.
- the above-mentioned metal oxide catalyst (3) may contain a single oxide or amixedoxide other than the anatase type titanium oxide and/or the rutile type titanium oxide. And the catalyst also may contain the above titanium oxide as a carrier or an active component, the active component being carried or immobilized on the carrier.
- the carrier include silica, alumina, silica-alumina, various zeolites, activated carbon, kieselguhr, zirconium oxide, tin oxide, and lead oxide, other than the above-mentioned titanium oxide.
- the above-mentioned metal oxide catalyst (3) further contains an oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14.
- the catalyst is a mixture of the oxide and the above-mentioned titanium oxide, or contains the oxide or the above-mentioned titanium oxide as a carrier or an active component, the active component being carried on the carrier.
- the catalyst contains an oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 as a carrier, and contains anatase type titanium oxide and/or rutile type titanium oxide as an active component.
- Use of such a catalyst can sufficiently suppress leaching of the titanium component which is the active component and improves the catalyst activity and the catalyst life. Therefore, such a catalyst may be most preferably used for the production method of the present invention.
- the above-mentioned oxide containing at least onemetallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 is preferably a single oxide of one of . these metallic elements, a mixture thereof, or a mixed oxide of these metallic elements.
- the mixed oxide may be a mixed oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14, and other metallic elements .
- the single or mixed oxide of the metallic elements more preferably contains at least one metallic element selected from the group consisting of Si, Zr, and Al .
- silica, alumina, silica-alumina, and zirconium oxide may be mentioned.
- the above-mentioned oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 may contain amorphous titanium oxide, or a mixed oxide consisted of titanium and a metal other than titanium, but the oxide does not contain anatase type titanium oxide and/or rutile type titanium oxide.
- the content (not including titanium content constituting anatase type titanium oxide and/or rutile type titanium oxide) of the at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 has a lower limit of 1% by weight and an upper limit of 80% by weight as a metal, relative to 100% by weight of a total amount of the catalyst.
- Use of the catalyst (3) containing a metal element within such a range can further improve the catalyst activity and the reaction efficiency, which allows sufficient exhibition of functional effects of the present invention . More preferably, the lower limit is 2% by weight and the upper limit is 75% by weight .
- the metallic element content can be identified by X-ray fluorescence (XRF) analysis.
- the above-mentioned metal oxide catalyst (3) contains a sulfur component of 700 ppm or less. If the catalyst contains a sulfur component of more than 700 ppm, the catalyst activity and the catalyst life may be insufficiently improved. Therefore, functional effects of the invention, in which the catalyst-separated, removed, and recovered steps can be remarkably simplified or omitted and the catalyst can be repeatedly used for continuous reactions, may be insufficiently exhibited.
- the sulfur component content is preferably 500 ppm or less, and more preferably 200 ppm or less .
- the sulfur component content in the catalyst can be identified by fluorescence X rays (XRF) analysis or high frequency induction plasma (ICP) emission spectrometry.
- the component can be identified by measuring the catalyst powder as it is or by a glass bead method.
- the component can be identified by measuring a catalyst powder after being dissolved in a hydrofluoric acid aqueous solution.
- the sulfur component is preferably identified by a glass bead method of XRF, in view of measurement easiness .
- the catalyst may be prepared using no sulfate as a raw material, or using sufficiently reduced amount of sulfate, or washed with sufficient amount of a solvent such as water.
- a calcined metal oxide catalyst may be used as the above-mentioned metal oxide catalysts (1) to (3) .
- the calcination temperature is preferably determined in consideration of the catalyst surface area and the crystal structure, and preferably has a lower limit of 280°C and an upper limit of 1300°C, for example. If the temperature is less than 280°C, suppression of the leaching maybe insufficient. If it is more than 1300°C, sufficient catalyst surface area may not be obtained, which fails to produce fatty acid alkyl esters and/or glycerin withhigh efficiency. More preferably, the lower limit is 400°C, and the upper limit is 1200°C.
- the calcination time preferably has a lower limit of 30 minutes and an upper limit of 24 hours. More preferably, the lower limit is 1 hour, and the upper limit is 12 hours.
- Gas phase atmosphere during the calcination is preferably air, nitrogen, argon, oxygen, and hydrogen atmosphere, and also mixed gas thereof. More preferably, the calcination is carried out under air or nitrogen atmosphere .
- the catalyst in which the active component consisted of the single or mixed oxide of the metallic element is carried or immobilized on the carrier is produced, as mentioned above, the catalyst is preferably produced by mixing and carrying the active component on a compoundused as a carrierby an impregnation method or a kneading method and the like, and then by calcinating the compound under the above-mentioned calcination conditions .
- the catalyst can disperse the active component sufficiently over the carrier surface to function as a solid catalyst .
- the catalyst amount preferably has a lower limit of 0.5% by weight and an upper limit of 20% by weight, relative to 100% by weight of a total fed amount of the fat or oil, the alcohol and the catalyst, for example, in a batch method. If the catalyst amount is less than 0.5% by weight, the reaction rate may be insufficiently improved. If the upper limit is more than 20% by weight, the catalyst costs may be insufficiently reduced. The lower limit is more preferably 1.5% by weight, and the upper limit is more preferably 10% by weight.
- a liquid hourly space velocity (LHSV) calculated by the following formula preferably has a lower limit of 0.1 hr “1 and an upper limit of 20 hr “1 . More preferably, the lower limit is 0.2 hr "1 and the upper limit is 10 hr "1 .
- the reaction temperature in the above-mentioned alcoholysis step is preferably 18O 0 C or more, and 300°C or less, for example. Particularly if the insoluble solid catalyst is used, it is preferable that the reaction temperature is 18O 0 C or more and 300 0 C or less. If the reaction temperature is less than 18O 0 C, the reaction rate may be insufficiently improved. If the reaction temperature is more than 300 0 C, suppression of a side reaction such as alcohol decompositionmaybe insufficient .
- the reaction temperature is more preferably 185°C or more and 29O 0 C or less, and still more preferably 19O 0 C or more and 280 0 C or less.
- the catalyst used in the above-mentioned contacting step preferably contains anactivemetal component not leached at the reaction temperatures within the above range. Thereby, the activity of the catalyst can be maintained enough even at high reaction temperatures, and the reaction can be performed well.
- the reaction pressure is preferably 0.1 MPa or more and 10 MPa or less, for example. If the reaction pressure is less than 0.1 MPa, the reaction rate may be insufficient. If the reaction pressure is more than 10 MPa, a side reactionmay proceed easily. In addition, a special apparatus durable for high pressures may be needed, hence utility costs or equipment costs may not be reduced enough.
- the reaction pressure is more preferably 0.2 MPa or more and 9 MPa or less, and still more preferably 0.3 MPa or more and 8 MPa or less.
- the above-mentioned “substantially in the absence of a catalyst” means an embodiment in which the catalyst is not contained at all or the catalyst is hardly contained. That is, the reaction is performed in an embodiment in which it is not evaluated that the reaction is performed in the presence of the catalyst. In this case, it is preferable that a fat or oil and an alcohol are reacted in supercritical state of the alcohol.
- supercritical state means a region over a specific critical temperature and critical pressure of a substance itself. If methanol is used as the alcohol, the state means a condition in which the temperature is 239°C or more and the pressure is 8.0 MPa or more .
- the lower limit is 250°C and the upper limit is 350 0 C. More preferably, the lower limit is 260 0 C and the upper limit is 330°C. Still more preferably, the lower limit is 27O 0 C and the upper limit is320°C.
- the lower limit is 8.0 MPa, and the upper limit is 15.0 MPa. If the insoluble solid catalyst is used in the above-mentioned reaction step, the reaction may be performed in such supercritical state. The catalyst may or may not used in the alcoholysis step, using catalyst is prefelable in view of lowering the reaction temperature.
- the alcoholysis step is preferably performed by a batch method (batch-wise) or continuous flow method.
- a fixed bed flow method is preferably employed because the step of separating and recovering the catalyst is unnecessary.
- a reactor to be used mentioned may be tubular reactors, stirring tank type reactors, and reaction kettle type reactors, for example.
- a particularly preferable embodiment of the above-mentionedproductionmethod is an embodiment inwhich fatty acid alkyl esters and/or glycerin are/is continuously produced by reacting a fat or oil with an alcohol using a fixed bed reactor filled with the catalyst.
- a preferable embodiment in the above-mentioned batch method is an embodiment in which the catalyst is added into a mixed system of a fat or oil and an alcohol .
- the reaction time is preferably l ⁇ minutes ormore and 30 hours or less, although it varies depending on the amount of the catalyst to be used and the reaction temperature to be employed.
- the reaction time is more preferably 30 minutes or more and 24 hours or less.
- the reaction step in at least one stage from the first stage to the (n-1) stage is a step of reacting a fat or oil with an alcohol ; and the reaction step in the n stage is an aging step.
- transesterification of the fat or oil with the alcohol is generally performed in the reaction in at least one stage from the first stage to the (n-1) stage.
- the transesterification is almost completed in the reaction in the n stage.
- the aging step in the n stage may become unnecessary if a catalyst that is extremely effective in the former transesterification step is found.
- the reaction is completed through such an aging step, and thereby products with high quality can be effectively obtained.
- the aging step in the n stage in the production method of the present invention is not necessarily a step of reacting the ester phase under the condition that glycerin to be produced is separated from the ester phase.
- the reaction is performed under the condition that the phase separation occurs or does not occur, depending on an alcohol to be used, for example. If ethanol is used, for example, the reaction solution is not separated into phases and forms a single phase at 50°C. Therefore, in the aging step in the n stage, the ester phase may be or may not be reacted under the condition that glycerin to be produced is separated from the ester phase.
- the aging step in the n stage is a step of reacting the ester phase under the condition that glycerin to be produced is separated from the ester phase, that is, if the ester phase obtained in the alcoholysis step is matured under the condition that glycerin to be produced is separated from the ester phase, the reaction solution is sufficiently separated into two phases, that is, the ester phase and the glycerin phase, and glycerin is extracted enough from the ester phase. Therefore, chemical equilibrium appears to function advantageously. Thereby, in such a case, reaction intermediates such as fatty acid monoglycerides that can be contained in the reaction solution obtained through the steps up to the aging step, can be significantly reduced.
- fatty acid alkyl esters with high purity can be produced, while the yield is improved and a purification step that can be subsequently performed is simplified.
- the content of the intermediate glycerides can be reducedwithoutuseof suchalargeexcessof alcohol. Therefore, costs on recovery of the alcohol can be suppressed enough.
- the n step means a substantial final reaction stage . That is, if the reaction of the fat or oil with the alcohol is substantially completed, the reaction in the n stage is performed and then the step is performed under the condition different from that in the n stage in order to adjust physical properties substantially uninvolved in the reaction.
- the above-mentioned “under the condition that glycerin to be generated is separated from the ester phase” means ⁇ 'under the condition that the reaction solution is separated into two phases, that is, the ester phase and the glycerin phase.
- the reaction temperature is 180°C or less. It is preferable that the reaction temperature in the aging step in the n stage is 180 0 C or less . If the reaction temperature is more than 180°C, the reaction solution forms a single phase. Thereby, effects of the present invention under the reaction condition that the reaction solution is separated from two phases, that is, the ester phase and the glycerin phase, can be insufficiently exhibited.
- the reaction temperature is preferably 10°C ormore.
- the reactionrate maybe insufficiently improved if the reaction temperature is less than 1O 0 C.
- the reaction temperature is more preferably 20°C or more and 170 0 C or less. Still more preferably, the reaction is performed at around 65°C. Utility costs and equipment costs can be reduced enough if the reaction is performed at around 65°C.
- the effects of the present invention can be sufficiently obtained if the liquid composition changes with proceeding of the reaction and thereby the reaction solution is separated into two phases, even if the reaction solution forms a single phase at the beginning of the reaction.
- the state of the liquid phase under high temperature and high pressure conditions can be identified using a glass autoclave container and the like.
- the present inventors have noted that the above-mentioned equilibrium functions advantageously just by lowering the aging temperature.
- the equilibrium functions advantageously in the aging step even if a large amount of intermediate glycerides is contained in the reaction solution in the (n-1) step used in the aging step. Therefore, the amount of the intermediate glycerides can be effectively reduced. Therefore, the alcohol amount in the reaction step in at least one stage from the first stage to the (n-1) stage can be reduced, which makes it possible to sufficiently suppress costs on recovery of the alcohol.
- the alcohol amount in the reaction step in the first stage can be more reduced than usual, and the alcohol amount in the reaction step in the (n-1) stage can be also reduced.
- the alcohol amount in the reaction step in the first stage can be more reduced than usual and the alcohol amount in the reaction step in the second stage can be also reduced, if the aging temperature is lowered in the aging step in the n stage such that the above-mentioned equilibrium functions advantageously, for example, in the cases where the following reaction steps are sequentially performed in the following order: the reaction step in the first stage in which the transesterification reaction of the fat or oil with the alcohol is performed; the alcohol-evaporated step of evaporating the alcohol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and then extracting and removing the glycerin phase; the esterification step of supplying the alcohol and esterifying the free fatty acids into fatty acid alkyl esters; the alcohol-evaporated step of evaporating the alcohol; andthephase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and then extracting and
- the reaction temperature in the aging step in the n step is 180°C or less.
- the aging step in the n stage is not necessarily a step of reacting the ester phase under the condition that glycerin to be produced is separated from the ester phase. If the reaction temperature is more than 180°C, the above-mentioned functional effects may be insufficiently exhibited.
- the reaction temperature is preferably 0°C or more.
- the reaction rate maybe insufficiently improved if the reaction temperature is less than 0 0 C.
- the reaction temperature is more preferably 5°C or more and 170 0 C or less, and still more preferably 1O 0 C or more and 70 0 C or less. Utility costs and equipment costs can be more sufficiently reduced as mentioned above if the aging reaction is performed at 1O 0 C or more and 17O 0 C or less.
- the catalyst used in the above-mentioned aging step is preferably a catalyst containing an active component not leached, and the like, if used at the reaction temperature within the above-mentioned range. Thereby, the activity of the catalyst can be maintained enough and the reaction can be performed well .
- the reaction pressure may be appropriately determined depending on the alcohol to be used and the reaction temperature to be employed.
- the reaction temperature is preferably 3 MPa or less and more preferably 1.5 MPa or less, for example. Still more preferably, the reaction is performed at around ordinary pressure. Utility costs and equipment costs can be more sufficiently reduced if the aging reaction is performed at around ordinary pressure.
- the molar ratio of the fat or oil to the alcohol is 1:3 to 50, generally.
- the molar ratio of the fat or oil to the alcohol is more preferably 1:5 to 40.
- the molar ratio of the fat or oil to the alcohol can be 1 : 5 to 30 if, as mentioned above, the aging temperature is lowered such that the equilibrium functions advantageously, that is, if the reaction temperature in the aging step in the n stage is 180°C or less. Further, the molar ratio of the fat or oil to the alcohol is 1:5 to 30.
- the method comprises a free fatty acids-treated step of reducing a free fatty acid amount between the reaction step in the (n-1) stage and the reaction step in the n stage.
- an esterification step of esterifying the free fatty acids into fatty acid alkyl esters is performed between the reaction step in the (n-1) stage and the reaction step in the n step, as the above-mentioned the free fatty acid-treated step.
- the free fatty acid can be treated by sequentially performing the following reaction steps in the following order : the alcohol-evaporated step of evaporating the alcohol (for example, methanol) (alcohol cut step, methanol cut step) ; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and extracting and removing the glycerin phase; and the esterification step of supplying the alcohol (for example, methanol) and esterifying the free fatty acids into fatty acid alkyl esters (Fig.2).
- the alcohol-evaporated step of evaporating the alcohol for example, methanol
- alcohol cut step for example, methanol cut step
- phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and extracting and removing the glycerin phase
- the esterification step of supplying the alcohol (for example, methanol) and esterifying the free fatty acids into
- an esterification step of producing fatty acid alkyl esters and monoglycerides from the free fatty acids, the alcohol (for example, methanol) , and the glycerin is performed between the reaction step in the (n-1) stage and the reaction step in the n-step.
- the free fatty acid can be treated, for example, by sequentially performing the following reaction steps in the following order: this esterification step; the alcohol-evaporated step of evaporating the alcohol (for example, methanol) (alcohol cut step, methanol cut step) ; and the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase (Fig.3) .
- the preferable embodiments of the present invention include these embodiments. It is preferable that the above-mentioned esterification step is performed in the presence of an acid catalyst.
- the esterification step is more preferably performed in the presence of a solid acid catalyst and still more preferably in the presence of a cation-exchange resin.
- Homogeneous catalysts such as p-toluene sulfonic acid may be used.
- the above-mentioned cation-exchange resin has a functional group such as a sulfonyl group, and form of particles of the resin is not especially limited.
- the cation-exchange resin is preferably aH-type ion exchange resin inview of reaction activity and prevention of generation of soap components. Any ion exchange resins among weakly to strongly acidic ion exchange resins may be used.
- an acid value of the ester phase used in the aging step in the n stage is controlled to 4.0 or less (mg-KOH/g-ester phase) . That is, it is preferable that the acid value of the ester phase obtained in the alcoholysis step (the acidvalue of the esterphase obtained in the reaction step in the (n-1) stage and used in the aging step in the n step) is 4.0 or less (mg-KOH/g-ester phase) (an amount of the free fatty acid contained in the ester phase used in the aging step is preferably 4.0 or less (mg-KOH/g-esterphase) as an acid value) .
- the acid value means the number of "mg" of potassium hydroxide needed for neutralization of free fatty acids, resin acids, and the like, contained in 1 g of a sample.
- the acid value can be measured by the method according to Japanese Industrial Standards (JIS) .
- JIS Japanese Industrial Standards
- the above-mentioned acid value is more preferably 3.5 or less (mg-KOH/g-ester phase) and still more preferably 3.0 or less (mg-KOH/g-ester phase) .
- the following free fatty acid-removed step maybe performed: the free fatty acid contained in the ester phase is converted into soaps and the soaps are washed; the free fatty acids are esterified with an alcohol to be converted into fatty acid alkyl esters or glycerides; or the free fatty acids are adsorbed on an anion exchanger, activated carbon, silica-alumina, or the like.
- the following operations in the alcoholysis step up to the aging step can reduce the free fatty acid: the alcoholysis step is performed in multi-stages; the reaction is performed at a higher temperature; the use amount of the alcohol is increased; and the contacting time of the reaction raw materials to the catalyst is prolonged. It is preferable that the acid value of the ester phase used in the aging step in the n stage is measured immediately before the aging step.
- the acid value of the ester phase is measured between the esterification step of esterifying the free fatty acids into the fatty acid alkyl esters and the aging step, for example, if the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in the n step: the alcohol-evaporated step of evaporating the alcohol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase; and the esterification step of supplying the alcohol and esterifying the free fatty acids into fatty acid alkyl esters.
- the acid value of the ester phase is measured between the phase separation step of extracting and removing the glycerin phase and the aging step, for example if the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in the n step: the esterification step of generating fatty acid alkyl esters and monoglycerides fromthe free fatty acids; the alcohol-evaporated step of evaporating the alcohol; and the phase separation step of separating,the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase.
- the acid value of the ester phase used in the aging step can be reduced also if the free fatty acids contained in the fat or oil used as the raw material in the alcoholysis step are esterified with a polyol to be converted into glycerides, and then the glycerides are used in the alcoholysis step.
- the preferable embodiments of the present invention include an embodiment in which the free fatty acids contained in the fat or oil are converted into glycerides and then the glycerides are used in the alcoholysis step .
- the polyol to be used is preferably glycerin. The same effect can be obtained if an alcohol is used in place of the polyol and the free fatty acids are converted into alkyl esters and then used in the alcoholysis step. In this case, it is preferable that the same alcohol as used in the alcoholysis step is used. Further, load to the catalyst in the alcoholysis step can be reduced if the free fatty acids are converted into glycerides or alkyl esters.
- the ester phase separated from the reaction solution without evaporating the alcohol from the reaction solution after the alcoholysis step may be used as it is as the above-mentioned ester phase used in the aging step in the n stage .
- the aging reaction canbe performedwithout addition of an alcohol because the alcohol remains in the ester phase.
- An alcohol may be addeddepending onthe residual amount of the alcohol .
- the ester phase separated from the glycerin phase after evaporating the alcohol from the reaction solution obtained in the reaction step in the (n-1) stage may be used.
- the amount of the alcohol used in the aging step in the n stage is preferably 50% by weight or less, relative to a total fed amount of the ester phase and the alcohol. If the amount of the alcohol is more than 50% by weight, costs may be increased because the recovery or recycle amount of the alcohol increases .
- the amount of the alcohol is more preferably 40% by weight or less, and still more preferably 30% by weight or less.
- the aging step in then stage is performed in the presence of abase catalyst.
- a base catalyst is preferably used also in the aging step in the n stage under the condition that glycerin to be produced is separated from the ester phase.
- the preferable embodiments of the present invention include an embodiment in which the aging step in the n stage is performed in the presence of a base catalyst.
- the reaction step in the n stage is an aging step in the presence of a solid base catalyst.
- the above-mentionedbase catalyst is preferably an insoluble solid catalyst, andmore preferably an anion exchanger, and still morepreferably ananion-exchange resin.
- Homogeneous catalysts such as sodium alkoxide and potassium alkoxide may be used.
- Hydrotalcites, IXE (tradename, product of TOAGOSEI CO., LTD.), and anion-exchange resins may be mentioned as the above-mentioned anion exchanger.
- the above-mentioned anion-exchange resin has a functional group such as an amine group, and the particle form of the resin is not especially limited.
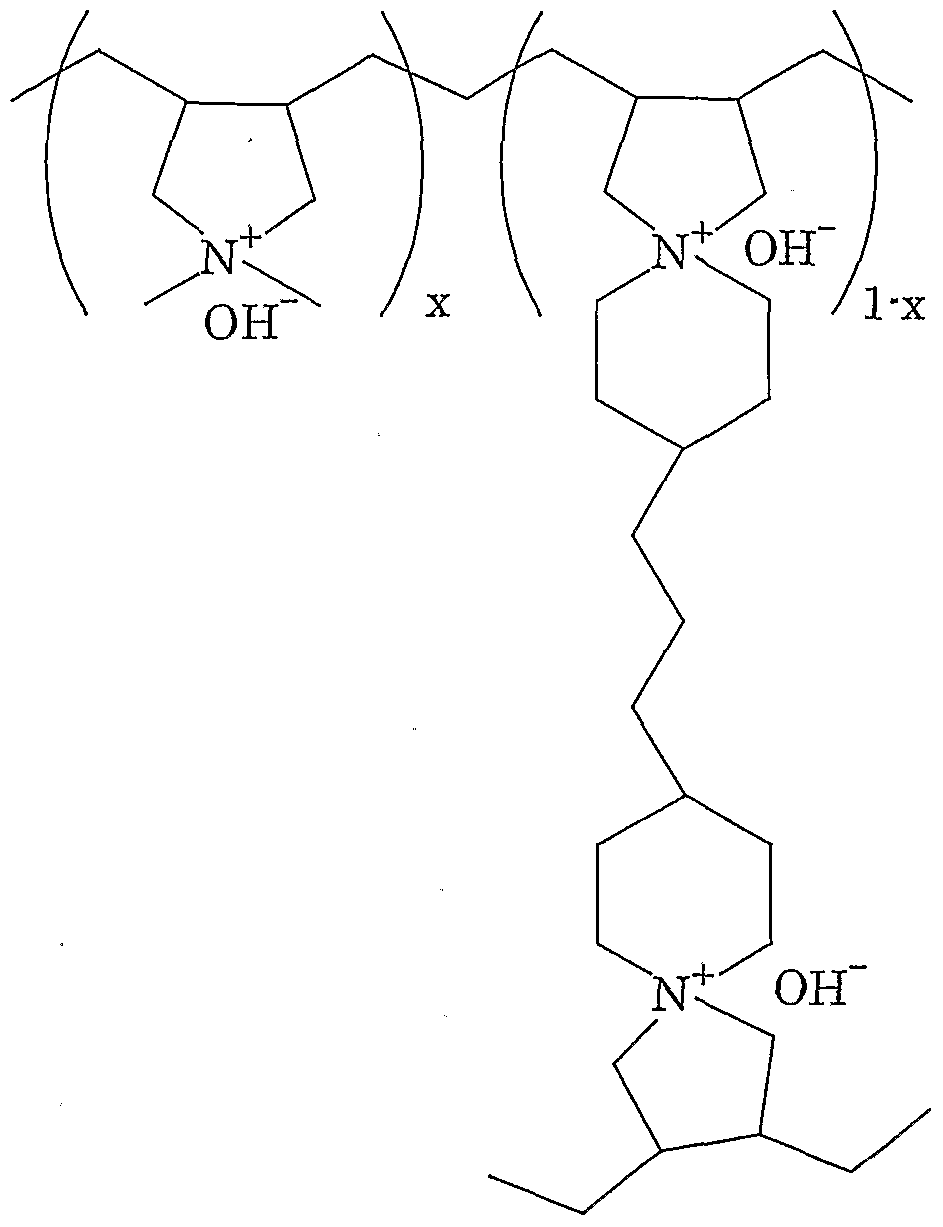
- OMe-type or OH-type ion exchange resins are preferable in view of prevention of mixing of impurity ions to the reaction system and reaction activity. Any ion exchange resins among weakly to strongly basic ion exchange resins may be used. Specific examples of such an ion exchange resin include an ion exchange resin represented by the following formula (1) disclosed in Japanese Kokai Publication No.
- the preferable embodiments of the present invention include an embodiment in which a deteriorated anion exchanger is reproduced and then used again in the aging step in the n stage.
- the following methods may be adopted as a method of the reproduction: the deteriorated anion exchanger is washed with
- NaOH aqueous solution/ and the deteriorated anion exchanger is washedwith dilute hydrochloric acidand/orNaCl aqueous solution and then washed with NaOH aqueous solution.
- the amount of the above-mentioned catalyst preferably has a lower limit of 0.5% by volume, and an upper limit of 60% by volume, relative to 100% by volume of a total fed amount of the fat or oil, the alcohol, and the catalyst, in a batch method. If the amount of the catalyst is less than 0.5% by volume, the reaction rate may be insufficiently improved. If the amount of the catalyst is more than 60% by volume, costs on the catalyst maybe insufficiently reduced. More preferably, the lower limit is 1.5% by volume, and the upper limit is 50% by volume.
- a liquid hourly space velocity (LHSV) calculated by the following formula preferably has a lower limit of 0.1 hr "1 and an upper limit of 20 hr "1 .
- the lower limit is 0.3 hr "1 and the upper limit is 10 hr “1 .
- LHSV (hr ⁇ 1 ) ⁇ (flow rate of a fat or oil per hour (HiL-IIr “1 ))+ (flow rate of an alcohol per hour (mL-hr "1 ) ) ⁇ / (volume of a catalyst (mL))
- the form of the above-mentioned aging step may be appropriately determined depending on the phase state of the reaction solution, and is preferably a batch method (batch-wise method) or a continuous flow method. If the reaction solution is separated into two phases, a continuous-flow fluidized bed reactor is more preferred in view of the contacting efficiency between the reaction solution and the catalyst . If the reaction solution is not separated into two phases, a continuous-flow fixed bed reactor is more preferred in view of suppression of deterioration in physical properties such as breakage of the catalyst and easiness of the catalyst separation.
- Two or more reactors may be used in the above-mentioned aging step.
- the reactors may be connected in parallel or serially. If the reactors are connected inparallel, the ester phase in a large amount can be treated simultaneously, and the aging reaction can be performed in one reactor while the catalyst is reproduced in other reactors. If the reactors are connected serially, effects such as further reduction in the intermediate glyceride amount can be expected.
- the number of stages M n" of the step of bringing a fat or oil into contact with an alcohol is 2 or more, and preferably 5 or less in view of equipment costs, manufacturing efficiency, and the like. More preferably, the number of stages is 2. That is, more preferred is a method in which the step of reacting a fat or oil with an alcohol in the presence of the solid catalyst or substantially in the absence of the catalyst is performed in one stage, and then the aging step in the presence of the base catalyst performed in one stage.
- the methanol is evaporated from the reaction solution obtained after the aging step in the n stage and then the reaction solutionis separated into the esterphase and the glycerinphase, or the reaction solution obtained after the aging step in the n stage is separated into the ester phase and the glycerin phase and then the methanol is evaporated from the reaction solution.
- a fatty acid methyl ester and glycerin can be produced.
- fatty acid methyl ester and/or glycerin may be subjectedto apurification step suchas distilation, filtration, and washing, depending on specification of the intended use.
- the fat or oil used in the above-mentioned production method contains fatty acid esters of glycerin and may be any species capable of serving as a raw material for fatty acid alkyl esters and/or glycerin together with an alcohol.
- fatty acid esters of glycerin may be any species capable of serving as a raw material for fatty acid alkyl esters and/or glycerin together with an alcohol.
- those generally called “fats and oils” may be used.
- Fatty acid esters of glycerin such as triolein also may be used.
- fats and oils are vegetable oils such as rapeseed oil, canola oil, sesame oil, soybean oil, corn oil, sunflower oil, palm oil, palm kernel oil, coconut oil, safflower oil, linseed oil, flaxseed oil, cottonseed oil, tung oil, jatropha oil, castor oil, hemp oil, mustard oil, peanut oil, and jojoba oil; animal fats and oils such as beef fat, lard, fish oil and whale oil/ and various used edible fats and oils (waste cooking oil) . One or two or more species of them may be used.
- the fat or oil contains phospholipids, proteins or the like as impurities
- the fat or oil is preferably used after subjected to a degumming step of adding a mineral acid such as sulfuric acid, nitric acid, phosphoric acid or boric acid to the fat or oil for removing the impurities therefrom.
- the alcohol is preferably an alcohol containing 1 to 6 carbon atoms and more preferably an alcohol containing 1 to 3 carbon atoms, for easy evaporation of the alcohol in the n stage and the like.
- the alcohol containing 1 to 6 carbon atoms are, for example, methanol, ethanol, propanol, isopropyl alcohol, 1-butanol, 2-butanol, t-butyl alcohol, 1-pentanol, 3-pentanol, 1-hexanol, and 2-hexanol.
- methanol is preferred. These may be used singly or in combination of two or more species.
- Apolyol ispreferablyusedas the above-mentioned alcohol .
- the above-mentioned polyol may be preferably ethylene glycol, propylene glycol, glycerin, pentaerythritol, sorbitol or the like. Among them, glycerin is more preferred. These may be used singly or in combination of two or more species .
- the fatty acid alkyl esters obtained in the productionmethod of thepresent invention means esters of a fatty acids contained in the fat or oil with the alcohol.
- R may be the same or different, and each represent an alkyl group containing 6 to 22 carbon atoms or an alkenyl group containing 6 to 22 carbon atoms and having one or more unsaturated bond.
- glycerin is produced together with fatty acid alkyl esters in the transesterification reaction, as shown by the above formula.
- purified glycerin can be industrially easily produced, as mentioned above.
- Such glycerin is preferably used as a chemical material in various applications .
- the fatty acid alkyl esters produced by the method of the present invention are suitably used for various uses as, for example, industrial raw materials, raw materials of pharmaceuticals and fuels.
- diesel fuel containing the fatty acid alkyl esters produced from a vegetable oil, or waste cooking oil by the above productionmethod can sufficiently reduce utility costs or equipment costs in the production step.
- diesel fuel can sufficiently contribute to environmental preservation in the production step, and suitably used for fuel in various applications .
- Such diesel fuel containing the fatty acid alkyl esters produced by the above-mentioned production method is also a preferable embodiment of the present invention .
- Fig. 1 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which the production is performed using: methanol as the alcohol; a flow fixed bed reactor as the reactor in the first stage reaction; a metal oxide catalyst as the catalyst in the first stage reaction; a continuous stirred tank reactor (hereinafter, also referred to as ⁇ CSTR") as the reactor in the second reaction; and an anion-exchange resin as the catalyst in the second stage reaction.
- ⁇ CSTR continuous stirred tank reactor
- methanol is evaporated froma reaction solution after reacted in a reaction column filled with a metal oxide catalyst and the reaction solution from which the methanol is evaporated is kept standing inside a settler, thereby being separated into an ester phase and a glycerin phase.
- Methanol is further added into the ester phase obtained by separated from the glycerin phase, and a reaction in the second stage is performed in a CSTR in which an anion-exchange resin is dispersed. Then, the methanol is evaporated from the reaction solution, and then the reaction solution is kept standing in the settler, therebybeing separated into an ester phase and a glycerin phase .
- a fatty acid methyl ester and glycerin can be obtained.
- Fig. 2 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which methanol is used as the alcohol, and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step (aging step) in the n step: the methanol cut step of evaporating the methanol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and extracting and removing the glycerin phase; and the esterification step of supplying the methanol and esterifying the free fatty acids into fatty acid alkyl esters .
- Fig. 3 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which methanol is used as the alcohol, and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in the n step: the esterification step of generating fatty acid alkyl esters and monoglycerides from free fatty acids; the methanol cut step of evaporating the methanol; and the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and extracting and removing the glycerin phase .
- the reaction step in the (n-1) stage may be the reaction step in the (n-2) stage.
- the reaction step in the (n-1) stage may be the esterification step of supplying the methanol and esterifying the free fatty acids into the fatty acid alkyl esters or the esterification step of generating the fatty acid alkyl esters and the monoglycerides from the free fatty acids.
- it is important that the free fatty acid-treated step is performed between the esterification step which is performed before the aging step and the aging step.
- the preferable embodiments of the present invention include an embodiment in which the reaction step in the (n-2) stage, the reaction step in the (n-1) stage, and the reaction step in the n stage (aging step) are performed in this order and the reaction step in the (n-1) stage is the free fatty acid-treated step.
- the yield of fatty acid alkyl esters so referred to herein means degree of conversion of effective fatty acid components in the raw material fat or oil and can be calculated from the following formula.
- Yield of fatty acid alkyl esters (total molar flow rate of fatty acid alkyl esters at a reactor outlet/total molar flow rate of effective fatty acid components at a reactor inlet) x 100 (%)
- the term "effective fatty acid components” means those components capable of producing fatty acid alkyl esters by the method of the present invention, and specifically means fatty acid triglycerides, diglycerides, monoglycerides, free fatty acids and fatty acid alkyl esters contained in the fat or oil as well as in the reused raw materials.
- the molar flow rate of the effective fatty acids at the reactor inlet canbe calculated by the following formula.
- Total molar flow rate of effective fatty acids (mol/h) [flow rate of raw materials at the reactor inlet (g/h) x saponification value of raw materials at reactor inlet/56100] .
- FIG. 1 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which the production is performed using methanol as the alcohol; a flow fixed bed reactor as the reactor in the first stage reaction; a metal oxide catalyst as the catalyst in the first stage reaction; a continuous stirred tank reactor as the reactor in the second reaction; and an anion-exchange resin as the catalyst in the second stage reaction.
- Fig. 2 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which methanol is used as the alcohol and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in then step: themethanol cut step of evaporating the methanol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase; and the esterification step of supplying the methanol and esterifying free fatty acids into fatty acid alkyl esters.
- Fig. 3 is a view schematically showing a preferable embodiment of the present invention according to the present invention, in which methanol is used as the alcohol, and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in the n step: the esterification step of generating fatty acid alkyl esters and monoglycerides from free fatty acids; the methanol cut step of evaporating the methanol; and the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase .
- the symbols in the figures are as follows. P : Pump
- Alcoholysis reaction of Palm oil with methanol by using continuous-flow fixed bed reaction system was performed using MnTi ⁇ 3 catalyst as the reaction in the first stage.
- a straight tubular reactor made of SUS-316 stainless steel in 10 mm inside diameter and 210 mm length was filled with MnTiO 3 catalyst 15 mL.
- XRD analysis of the MnTiO 3 catalyst used in this Example showed that the catalyst had an ilmenite structure .
- an automatic backpressure regulator was equipped at the reactor outlet.
- the reactor was heated from the outside using heating oven until the temperature reached 200°C.
- the methanol was evaporated from the obtained reaction solution which was obtained at 50 hours later after stabilization of the temperature and the pressure. Then, an ester phase was obtained by phase separation from a glycerin phase .
- the content of each component in the ester phase was as follows: fatty acid methyl esters 91.8%; monoglycerides 3.6%; diglycerides 1.2%; triglycerides 2.9%; free fatty acids 0.4%; and glycerin 0.1%.
- the acid value of the ester phase was 0.74 (mg-KOH/g-ester phase) .
- esterification reaction of free fatty acids in the ester phase by using continuous-flow fixed bed reaction system was performed as the reaction in the second stage, using a cation-exchange resin, Dowex50W ⁇ 8.
- a cation-exchange resin Dowex50W ⁇ 8.
- 15 mL of H-type Dowex50W ⁇ 8 after sufficiently washed with methanol to remove water therefrom was charged into a reaction tube made of Teflon (registered trademark) in 10 mm inside diameter and 210 mm length.
- Teflon registered trademark
- an automatic backpressure regulator was equipped at the reactor outlet .
- the reactor was placed into an oil bath for temperature control.
- the ester phase obtained in the first stage and methanol were feeding to the reactor at flow rates satisfying a ratio by weight of the ester phase/the methanol of 3.7, and LHSV of 1.0 hr "1 , using a precision pump.
- the pressure inside the reactor was set to 0.1 MPa, and the temperature of the oil bath was set to 65 0 C.
- the methanol was evaporated from the obtained reaction solutionwhichwas obtained at 24 hours later after stabilization of the temperature and the pressure.
- the content of each component on the ester phase was as follows: fatty acid methyl esters 92.0%; monoglycerides 3.8%; diglycerides 1.2%; triglycerides 2.9%; free fatty acids 0.05%; and glycerin 0.05% .
- the acid value of the reaction solution was 0.10 (mg-KOH/g-ester phase) .
- each component of the ester phase was as follows: fatty acid methyl esters 49.0 g; monoglycerides 8.1 g; diglycerides 1.8 g; triglycerides 0.4 g; free fatty acids 1.1 g; and glycerin 0.2 g (fatty acid methyl esters 80.9 %; monoglycerides 13.4 %; diglycerides 3.0 %; triglycerides 0.7 %; free fatty acids 1.8 %; and glycerin 0.3 %) , and the acid value was 3.6 (mg-KOH/g-ester phase).
- fatty acid methyl esters 57.8 g monoglycerides 0.9 g; diglycerides 0.1 g; triglycerides 0.4 g; free fatty acids 0.1 g; and glycerin 2.3 g (fatty acid methyl esters 93.8 %; monoglycerides 1.5 %; diglycerides 0.2 %; triglycerides 0.6 %; free fatty acids 0.2 %; and glycerin 3.7 %) .
- the reaction solution was separated into two phases.
- the acid value was 0.8 (mg-KOH/g-ester phase) .
- aging reaction was performed in the same manner as in Example 2 except that the ester phase (37.0 g) , the methanol (13.O g), and the HiMAC901 (6.OmL) were used.
- fatty acid methyl esters 38.7 g free fatty acids 0.02 g; monoglycerides 0.19 g; diglycerides 0.02 g; triglycerides 0.01 g/ and glycerin 0.33 g (fatty acid methyl esters 98.6 %; monoglycerides 0.48 %; diglycerides 0.05 %; triglycerides 0.03 %; free fatty acids 0.05 %; and glycerin 0.84 %) .
- the content of intermediate glycerides after the aging reaction was 0.6%. After completion of the aging reaction, the reaction solution was separated into two phases .
- reaction solution was separated into two phases .
- HiMAC901 was used in the reaction as reproduced HiMAC901. Reactions were performed in the same manner as in Example 2 except that the reproduced H1MAC901 was used as the catalyst in the aging step and an ester phase (30 g) and methanol (10 g) were used in the aging step.
- fatty acid methyl esters 27.1 g monoglycerides 0.8 g; diglycerides 0.8 g; triglycerides 0.2 g; free fatty acids -0.03 g; and glycerin 1.0 g (fatty acid methyl esters 90.5 %; monoglycerides 2.7 %; diglycerides 2.7 %; triglycerides 0.7 %; free fatty acids 0.1 %; and glycerin 3.3 %) .
- the reaction solution was separated into two phases.
- triolein 60 g
- methanol 2Og
- OH-type HiMAC 901 8.9mL
- the reaction was allowed to proceed at a reaction temperature of 100°C for 24 hours with internal stirring.
- the yield of methyl oleate was 34%
- the yield of glycerin was 26%.
- the method for producing fatty acid alkyl esters and/or glycerin of the present invention has the above-mentioned configuration.
- the production method is an industrially very useful method because the method can effectively and simply produce fatty acid alkyl esters and/or glycerin suitable for applications such as food and fuel, with low costs and high quality.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
Abstract
To provide a method capable of effectively and easily producing fatty acid alkyl esters and/or glycerin suitable in various applications such as fuel and food, at low costs. A method for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more), wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol; a reaction step in n stage is an aging step; and the method comprises a free fatty acids-treated step of reducing a free fatty acid amount between the reaction step in the (n-1) stage and the reaction step in the n stage.
Description
DESCRIPTION
METHOD FOR PRODUCING FATTY ACID ALKYL ESTERS AND/OR GLYCERIN
TECHNICAL FIELD
The present invention relates to a method for producing fatty acid alkyl esters and/or glycerin from fats or oils of animal or plant origin. More specifically, the present invention relates to a method for producing fatty acid alkyl esters and/or glycerin useful for fuel, food, cosmetics, pharmaceuticals and the like purposes.
BACKGROUND ART Fatty acid alkyl esters derived from vegetable oils are used as cooking oil and, in addition, used in such fields as cosmetics and pharmaceuticals . In recent years, attention has been paid to uses as additives to fuels such as fossil diesel fuel. For example, fatty acid alkyl esters are added to fossil diesel fuel in an amount of several percents as vegetable-derived biodiesel fuel for reducing emission of CO2. Glycerin is mainly used as a rawmaterial for producing nitroglycerin and is further used as a raw material for alkyd resins, or for pharmaceuticals, foods, printing inks, cosmetics and the like.
As amethod of producing such fatty acid alkyl esters and/or glycerin, it is known transesterification of triglycerides, which is a main component of fat or oil, with an alcohol in the presence of a homogeneous alkali catalyst, utilizing chemical equilibrium shown in the following formula.
ers
fat or oil(triglycerides) diglycerides
rs
However, such a method using a homogeneous alkali catalyst needs complicated steps in order to separate and remove the catalyst. Also, the alkali catalyst causes saponification with free fatty acids contained in the fat or oil. Therefore, soaps are produced as byproduct, which needs a step of washing the produced soaps with large amounts of water. In addition, emulsification of the soaps would lead to yield decrease of fatty acid alkyl esters and make a subsequent glycerin purification process complicated. Therefore, such a method has room for improvement in these respects.
Disclosed is amethod for producing fatty acid alkyl esters or glycerin effectively and simply by reacting a fat or oil with an alcohol in the presence of an insoluble solid catalyst, as a method for producing fatty acid alkyl esters or glycerin in which the complicated steps such as the catalyst-separated and removed step and the washing step are simplified (for example, referring to International Publication No. WO 2005/021697) . This production method is an industrially very useful method because energy consumption in the production can be reduced and fatty acid alkyl esters or glycerin with high purity can be produced with advantage in terms of energy. Also disclosed is a method using a heterogeneous catalyst containing a metal oxide with a specific structure, such as a zinc aluminum mixed oxide, a mixed oxide of aluminum and titanium, zirconium, or antimony, crystalline or amorphous titanosilicates, and a mixed oxide of zirconium oxide and titanium oxide (for example, referring to U.S. Patent No. 5908946 (pages 4, 5, and 52), European Patent Publication No. 1505048 (pages 2 and 9), Japanese Kokai Publication No . Hei-07-173103 (pages2and3) and Japanese Kokai Publication No. 2005-177722 (pages 2 and 3) ) . However, such a heterogeneous catalyst generally has activity lower than that of homogeneous alkali catalysts and therefore, the reaction generally needs to be performed under high temperature and high pressure conditions. Under such conditions, the reaction solution forms a uniform single phase. Therefore, such a method maybe disadvantageous in terms of theory of chemical equilibrium, as compared with the method using a homogeneous alkali catalyst, in which a glycerin phase is separated from an ester phase (liquid phase is divided into two phases) . That is, there is a problem in that fatty acid mono- and/or diglycerides intermediates often remains in fatty acid alkyl esters that are products. If mono-and/or diglycerides remain in products, there is a problem when it is used in various uses such as fuels or foods . Therefore, various measures for making the production method to be more industrially useful by reducing a content of intermediate
glycerides have been investigated. The following methods have been adopted as such measure: (1) a method in which a large excess of alcohol is used (for example, referring to Japanese Kokai Publication No. 2000-204392 (page 2)); (2) a method in which, from a reaction solution after reaction of a fat or oil with an alcohol in a fixed bed reactor filled with a heterogeneous catalyst under high temperature and high pressure conditions, the alcohol is evaporated and the reaction solution is separated into phases and glycerin is removed, and then, monoglycerides contained in the obtained ester phase are transesterified with coexistent fatty acid alkyl esters to be converted into diglycerides or triglycerides, and then the triglycerides and the diglycerides are recovered through a distillation step and then reused as raw materials (for example, referring to European Patent No. 924185) ; (3) a method in which a fat or oil and an alcohol are reacted in a first fixed bed reactor filled with aheterogeneous catalyst underhightemperature andhighpressure conditions, and the alcohol is evaporated from the reaction solution and the reaction solution is separated into phases and glycerin is removed, andthe obtained crude ester phase is reacted again with an alcohol in a second fixed bed reactor under high temperature andhighpressure conditions (for example, referring to European Patent Publication No.1460124) . Such methods need a large excess of alcohol, high temperature condition, a distilation column, or the like. Therefore, such methods insufficiently satisfy the demand for making the production method to be industrially more useful.
In contrast, if a large excess of alcohol is not used, it is known that the reaction solution is separated into two phases, that is, aphasemainly containing fatty acid alkyl esters
(ester phase) and a phase mainly containing glycerin (glycerin phase) under mild temperature condition as in the method using a homogeneous alkali catalyst. Therefore, a concentration of glycerin components as one of products in the ester phase becomes extremely smaller (because the glycerin moves outside of the
system) . Therefore, glycerin and fatty acid alkyl esters are easily obtained from monoglycerides and an alcohol in terms of chemical equilibrium (intermediate glycerides are reduced) . However, there are the following problems if a basic metal oxide catalyst (for example, referring to Japanese Kokai Publication Sho-61-254255 (pages land 2) and International Publication No . WO 98/56747) or an anion-exchange resin (for example, referring to Japanese Kokai Publication Sho-62-218495 (page 2)), which is a solid catalyst with activity under mild temperature condition, is used. The basic metal oxide catalyst has a short catalyst life because active components of the catalyst leached into the reaction solution. Also, if the basic metal oxide catalyst is used, the fat or oil may be saponified to generate soaps, and therefore complicated steps such as the catalyst-separated and removed step, the washing step, and neutralization step are essentially performed. If the anion-exchange resin is used, free fatty acids, intermediate glycerides or glycerin inhibits the reaction, failing in improvement in yield. Therefore, there is room for improvement in such production methods in order to produce fatty acid alkyl esters or glycerin with high purity more simply and with higher yield by improving these respects .
As mentioned above, improvement in the content of mono- and/or diglycerides and catalyst life is mentioned as a present challenge in production technique of fatty acid alkyl esters or glycerin. Therefore, it has been desired to find out a method of prolonging the catalyst life itself, or a method of reactivation the catalyst. A production method using such a method can be an industrially useful technique in this field. For example, the method using an anion-exchange resin is certainly effective in that fatty acid alkyl esters and/or glycerin can be produced simply with high yield and high purity. However, it is industrially important that such a property can be maintained and exhibited for a prolonged period. Fatty acid alkyl esters or glycerin as industrial products may be greatly
affected in terms of costs or quality because of the content of mono- and/or diglycerides, if the anion-exchange resin must be replaced in a short period of time, or reactivation of such an exchange resin takes time or costs, or production quality thereof is reduced in a short period. From such respects, there is room for improvement in production methods capable of eliminating all of the problems in conventional techniques.
SUMMARY OF THE INVENTION The present invention has been made in view of the above-mentioned state of the art. The present invention has an obj ect to provide amethod for effectively and simplyproducing fatty acid alkyl esters and/or glycerin suitable for applications such as fuel and food, at low costs and with high quality. The present inventors have made various investigations about methods for producing fatty acid alkyl esters and/or glycerin. They have noted that (1) if a step of reacting a fat or oil with an alcohol is performed in n stages (so-called multistage step) ; and a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol; and a reaction step in n stage is an aging step, transesterification reaction of the fat or oil with the alcohol can be almost completed and such a method can be an industrially useful method. They have found that in this case, if a free fatty acids-treated step of reducing a free fatty acid amount is performed between the reaction step in the (n-1) stage and the reaction step in the n stage, catalyst life is improved, which can eliminate all of the above-mentioned problems in conventional solid catalysis techniques. Also, they have found that the above-mentionedproblems canbe effectively eliminated, if (2) the reaction step in at least one stage from the first stage to the (n-1) stage is a step of reacting the fat or oil with the alcohol in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and the reaction step in the n stage is an aging step in the presence of a solid
base catalyst. As mentioned above, the production method of the present invention includes the above specified matter (1) or (2), and the above specified matters (1) and (2) may be appropriately combined in the production method of the present invention.
The present inventors have found that in addition to or instead of the above-rαentionedproductionmethod, the production may be performed by the following method. That is, they have noted that if a reaction step of a fat or oil with an alcohol is performed in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst to sufficiently perform alcoholysis reaction of the fat or oil with the alcohol and then the obtained ester phase is matured under a condition in which glycerin to be produced is separated from the ester phase, transesterification reaction of the fat or oil with the alcohol can be almost completed and therefore, such a production method can be an industrially useful method. They have found that if the reaction step in the former stage is performed under high temperature and high pressure conditions in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, the complicated steps such as the catalyst-separated and removed step, and the washing step can be simplifiedor omitted, and also the esterphasehaving a reduced amount of components which may inhibit the reaction such as free fatty acids contained in the fat or oil, can be easily obtained. Also, they have found that if the aging step in the latter stage is performed under mild reaction temperature condition in which all or part of glycerin to be produced is separated from the ester phase, such a reaction becomes advantageous in theory of chemical equilibrium, and therefore, alcoholysis of residual fatty acid monoglycerides in the reaction step in the former stage can further proceed and the aging step can proceed without reaction inhibition, and therefore fatty acid alkyl esters or glycerin with high purity can be effectively and easily produced at low costs and with high quality. In the aging step in this
production method, the reaction solution may not be necessarily separated.
That is, if the reaction of a fat or oil with an alcohol proceeds, the reaction solution is generally separated into a phase mainly containing fatty acid alkyl esters (hereinafter, also referred to as "ester phase") and a phase mainly containing glycerin (hereinafter, also referred to as "glycerin phase") . If the reaction is performed in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, the reaction is performed under high temperature and high pressure conditions for sufficient proceeding of the reaction. Thereby, free fatty acids contained in the fat or oil are simultaneously esterified to be fatty acid alkyl esters. However, the reaction solution forms a single phase because of improved mutual solubility of the ester phase and the glycerin phase under high temperature and high pressure conditions, and thereby a glycerin concentration in the reaction system becomes higher, which is disadvantageous in theory of chemical equilibrium. Therefore, fatty acid monoglyceride intermediates may remain. In this case, all or part of the alcohol is evaporated if necessary, and then the reaction solution is cooled to separate into the ester phase and the glycerin phase. However, the purification of fatty acid alkyl esters may be insufficient even by distilation, and therefore purification step which may be subsequently performed may be complicated, because almost all of the glycerides as an intermediate is distributed into the ester phase, and some glycerides have a boiling point close to that of the fatty acid alkyl esters. In contrast, if the reaction of a fat or oil with an alcohol is performed in the presence of the solidbase catalyst such as anion-exchange resin, the reaction proceeds even under mild condition. Therefore, the mutual solubility of the ester phase and the glycerin phase becomes lower, which is advantageous in theory of chemical equilibrium. However, free fatty acids or glycerides at a high concentration inhibit the catalyst, and
therefore the reaction is not completed.
However, the present inventors have found that the content of the substances causing the reaction inhibition can be reduced if a fat or oil is previously subjected to alcoholysis reaction under high temperature and high pressure conditions, in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, and found that the residual intermediate fatty acid monoglycerids and the like after the reaction under high temperature and high pressure conditions are matured under mild condition in the presence of a solid base catalyst to be converted into fatty acid alkyl esters or glycerin, and thereby fatty acid alkyl esters with high impurity can be produced by easy purification process, and also equipment costs or utility costs can be suppressed. Also, the present inventors have found that the content of the intermediate glycerides can be reduced even if a large excess of alcohol is not used as in a conventional solid catalysis method, and therefore costs on recovery of the alcohol can be suppressed enough. Thereby, the above-mentioned problems have been admirably solved. That is, the present invention is a method for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol; a reaction step in n stage is an aging step; and the method comprises a free fatty acids-treated step of reducing a free fatty acid amount between the reaction step in the (n-1) stage and the reaction step in the n stage.
In this production method of the present invention, it is noted that the content of free fatty acids in the ester phase is reduced before the aging step in view of catalyst life, in order to solve conventional problems in the production method of fatty acid alkyl esters and/or glycerin. This production method essentially comprises a step of reducing the content of the free fatty acid in the ester phase before the aging step.
The aging step may be performed under the condition that the reaction solution is separated into phases, but not necessarily performedunder such a condition. That is, the reaction solution is not separated into phases in some cases, depending on kind of an alcohol to be used in the reaction step.
In the cases where an insoluble solid catalyst showing activity in both of the transesterification reaction and the esterification reaction is used in at least one stage of the reaction step from the first stage to the (n-1) stage, free fatty acids maybe generatedbymoisture contained in the rawmaterials . Further, even if fats and oils used as the raw material contain free fatty acids, the esterification reaction proceeds simultaneously, and therefore the amount of the free fatty acids decreases. However, the amount is not sufficiently reduced in some cases. In such cases, if a free fatty acids-treated step of reducing the free fatty acid amount is performed between the reaction step in the (n-1) stage and the aging step in the n stage, the life of the solid base catalyst used in the n stage can be improved. Thereby, the present inventors have found that the above-mentioned problems can be effectively eliminated.
Preferable embodiments of the above-mentioned production method include the following (1) to (4) embodiments or combinations thereof, and combinations of the above-mentioned production method and preferable embodiments described in the present description. That is, mentioned are (1) the aging step in the n stage is performed in the presence of a base catalyst; (2) the reaction step in the (n-1) stage is a step of esterifying a free fatty acid contained in a reaction solution obtained in (n-2) stage/ (3) a reaction temperature in the aging step in the n stage is 180°C or less; and (4) an acid value of an ester phase used in the aging step in the n stage is controlled to 4.0 or less (rαg-KOH/g-ester phase).
The present invention also is a method for producing.fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol inn stages (n representing an integer
of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and a reaction step in n stage is an aging step in the presence of a solid base catalyst.
In this production method of the present invention, a catalyst used in the reaction step in at least one stage from the first stage to the (n-1) stage and a catalyst used in the reaction step in the n stage are noted in order to solve conventional problems in the production method of fatty acid alkyl esters and/or glycerin. This production method specifies whether or not a catalyst is used and what catalyst is used if used in each of the reaction steps. Preferable embodiments of the above-mentioned production method include the following (1) to (5) embodiments or combinations thereof, and combinations of the above-mentioned production method and preferable embodiments described in the present description. That is, mentioned are (1) the method comprises a free fatty acids-treated step of reducing a free fatty acid amount between the reaction step in the (n-1) stage and the reaction step in the n stage; (2) the insoluble solid catalyst is a metal oxide catalyst; (3) the reaction step in the (n-1) stage is a step of esterifying the free fatty acid contained in a reaction solution obtained in (n-2) stage; (4) a reaction temperature in the aging step in the n stage is 180°C or less; and (5) an acid value of an ester phase used in the aging step in the n stage is controlled to 4.0 or less (mg-KOH/g-ester phase) . The following production method is part of the present invention. That is, the present invention is also a method for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step
of reacting the fat or oil with the alcohol at 180°C or more in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and a reaction step in n stage is an aging step at 180°C or less. The reason why the reaction step in at least one stage from the first stage and the (n-1) stage is a step of reacting the fat or oil with the alcohol at 180°C or more in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst, as mentioned above, is because 180°C is a boundary that determines whether or not the reaction solution is separated into two phases.
The following productionmethod is alsopart of the present invention. That is, the present invention also includes amethod for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and a reaction step in n stage is a step of aging an ester phase obtained in the reaction step in the (n-1) stage under the condition that all or part of glycerin to be generated is separated from the ester phase. In this production method, a free fatty acids-treated step may be performed after the transesterification reaction of the fat or oil with the alcohol . That is, an embodiment in which this production method comprises a free fatty acids-treated step is one of preferable embodiments of the present invention.
Also in this production method, the reaction solution may not be necessarily separated into phases in the aging step, as mentioned above. The reaction step in the (n-1) stage and the reactionstep (agingstep) in then stage shouldbe distinguished.
DETAILED DESCRIPTION OF THE INVENTION The present invention is described in more detail below.
In the method for producing fatty acid alkyl esters and/or glycerin of the present invention, a step of bringing a fat or oil into contact with an alcohol is performed in n stages (n representing an integer of 2 or more) . Herein, to perform the step of bringing a fat or oil into contact with an alcohol (hereinafter, also referred to as "contacting step") in two or more stages means that the reaction of the fat or oil with the alcohol is performed in two or more stages using two or more reactors serially connected. In the above-mentioned production method, the alcoholysis step from the first stage to (n-1) stage and the aging step in the n stage are performed. The alcoholysis step means a step in which transesterification reaction of glycerides with an alcohol and esterification reaction of free fatty acids with an alcohol simultaneously proceed. The aging step means a step in which transesterification reaction of residual intermediate glycerides (mainly, monoglycerides) after the alcoholysis step with an alcohol mainly proceed.
In the production method, it is also preferable that the reaction step in the (n-1) stage is a step of esterifying the free fatty acid contained in a reaction solution obtained in (n-2) stage. In this case, the free fatty acid contained in the reaction solution obtained in the (n-2) stage is esterified in the reaction step in the (n-1) stage, and thereby catalyst life in the ageing step and production efficiency and purity of products can be improved.
For example, it is preferable that the reaction solution out of the reactor is separated into a phase mainly containing fatty acid alkyl esters (ester phase) and a phase mainly containing glycerin (glycerin phase) in the alcoholysis step, andthen the esterphase is chargedas one of reactionrawmaterials, into a next reactor with an alcohol. That is, it is preferable that the ester phase is separated from the reaction solution after the reaction in the former stage and then recovered, and the reaction in the latter stage is performed using the ester
phase and an alcohol as raw materials.
The reaction in the former stage means a reaction firstly performed and the reaction in the latter stage means a reaction performed after the first reaction, if continuous reactions in two stages are focused. That is, in reaction in two stages, the first stage is a reaction in the former stage, and the second stage is a reaction in the latter stage. In reaction in three stages, the first stage is a reaction in the former stage and the second stage is a reaction inthe latter stage, if the reactions in the first and second stages are focused. And the second stage is a reaction in the former stage and the third stage is a reaction in the latter stage, if the reactions in the second and third stages are focused.
As mentioned above, the substances causing the reaction inhibition can be reduced through the alcoholysis step.
Therefore, the aging reaction can be preferably performed in the aging step under mild condition in the presence of a base catalyst (more preferably, a solid base catalyst) which is preferably used, as mentioned below. In the above-mentioned production method, it is also preferable that the alcohol is evaporated from the reaction solution after the reaction of the fat or oil with the alcohol in the absence of a catalyst before the reaction solution is separated into the ester phase and glycerin phase in the alcoholysis step . Thereby, mutual solubility of the upper layer mainly containing fatty acid alkyl esters and the lower layer mainly containing glycerin lowers, and thereby separation of the fatty acid alkyl esters from the glycerin can be improved, resulting in improvement in yield. If active metal components of the catalyst leached into the reaction solution, a reverse reaction proceeds when the alcohol is evaporated from the reaction solution because the transesterification reaction is a reversible reaction. Thereby, yield of the fatty acid alkyl esters may be insufficient. However, if the alcohol is evaporated from the reaction solution in the absence of a catalyst
and then, the reaction solution is separated into phases, purification can be easily performed in the method for producing fatty acid alkyl esters and/or glycerin. Thereby, yield can be improved enough. The productionmethodmay have an embodiment in which the reaction solution is separated into the ester phase and the glycerin phase without evaporating the alcohol, and then the ester phase is used in a subsequent reaction.
The above-mentioned "substantially in the absence" or "the absence of a catalyst" means an embodiment in which the reaction solution contains no catalyst or hardly contains a catalyst, that is, an embodiment in which it is not evaluated that the reaction is performed in the presence of a catalyst.
In the present invention, it is preferable that a total concentration of active metal components leached from the catalyst into the reaction solution is preferably 1000 ppm or less. The term "active metal component leached" means metal components derived from the insoluble solid catalyst leached into the reaction solution and capable of serving as a homogeneous catalyst with catalytic activity in the transesterification and/or or the esterification under operation conditions. The leached active metal component having a concentration of more than 1000 ppm fails to suppress the reverse reaction sufficiently in the above-mentioned alcohol-distilled step . Therefore, load of utility in the production may be insufficiently reduced. The concentration thereof is preferably 800 ppm or less, and more preferably 600 ppm or less, and still more preferably 300 ppm or less. Most preferably, the reaction solution substantially contains no active metal components.
The leaching amount of the above-mentioned active metal components of the catalyst in the reaction solution can be identified by subjecting the reaction solution as it is to fluorescent X-ray spectroscopy (XRF) analysis. Smaller leaching amount is preferably identified by inductively coupled plasma (ICP) emission spectrometry. It is preferable that the operational temperature in the
above-mentioned alcohol-evaporated step is 3000C or less. If the operational temperature is more than 3000C, it may become impossible to evaporate the alcohol sufficiently while sufficiently inhibiting the desired product from being evaporated. The operational temperature is more preferably 280°C or less and still more preferably 25O0C or less. As for the operational pressure, reduced pressure conditions or ordinarypressure conditions are generally employed. Increased pressure conditions also may be employed. In view of reduction in energy consumption of the production system, reducedpressure conditions are preferred.
It is preferable that the evaporated alcohol is recovered after the above-mentioned alcohol-evaporated step, and then at least part of the recovered alcohol is used as a raw material if the reaction is continuously performed. Thereby, production costs can be more sufficiently reduced. It is economically more preferable that all of the recovered alcohol is used as a raw material. It is also preferable that the recovered alcohol is purified by removing impurities therefrom by a method such as distilation and then the purified alcohol is used in the reaction . If the alcohol reused as a raw material contains moisture, yield of the fatty acid alkyl esters may be insufficient due to the progress of hydrolysis of the fatty acid alkyl esters in the reaction step. The moisture concentration in the alcohol is preferably 5% by weight (% by mass or mass %) or less. If the moisture concentration is more than 5% by weight, the amount of hydrolysis of the fatty acid alkyl esters into the free fatty acids increases and not only the yield becomes insufficient but also a step of removing the free fatty acids becomes necessary, hence the method may not be carried out in a satisfactorily advantageous manner from the industrial viewpoint. The moisture concentration is more preferably 3% by weight or less and still more preferably 1.5% by weight or less.
In the above-mentioned alcohol-purified and recovered step, the operation can be permitted at a temperature lower than
that in the above-mentioned alcohol-evaporated step. The operational temperature is preferably 25O0C or less. The operational temperature is more preferably 220°C or less, and still more preferably 210°C or less. The operational pressure is the same as in the above-mentioned alcohol-evaporated step. In the production method of the present invention, it is preferable that the reaction step in at least one stage in the alcoholysis step is a step of reacting a fat or oil with an alcohol under high temperature and high pressure conditions in which a reaction solution forms a single phase, in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst. If a base catalyst is used, free fatty acids contained in the fat or oil andthe like cause reaction inhibition. However, the aging reaction proceeds without the reaction inhibition even if the base catalyst is used in the aging step in the n stage, because the content of the components such as the free fatty acids contained in the fat or oil is reduced in the reaction step from the first stage to the (n-1) stage. Therefore, fatty acid alkyl esters or glycerin with high purity can be effectively and easily produced at low costs.
In such a reaction step, the insoluble solid catalyst is not especially limited as long as it shows insolubility in the fat or oil and the alcohol as raw materials and in the products (the fatty acid alkyl esters and the glycerin) . The insolubility means that the reaction solution contains leached active components of the catalyst of 1000 ppm or less, and preferably 800 ppm or less, and more preferably 600 ppm or less, and still more preferably 300 ppm or less . Most preferably, the reaction solution substantially contains no active metal components. The reason why the catalyst is not limited is because the catalyst never changes chemical equilibrium. Further, it is preferable that the insoluble solid catalyst is a catalyst capable of catalyzing the esterification of the free fatty acid contained in the fat or oil, namely one having activity in both the transesterification of the glycerides contained in the fat or
oil and the esterification in which the fatty acid alkyl esters are producedby reaction of the free fatty acids with the alcohol . By employing such an embodiment, it becomes possible to cause the transesterification reaction and the esterification reaction to proceed even if the fat or oil as the raw material contains a free fatty acid, hence it becomes possible to improve the yield of the fatty acid alkyl esters without providing any esterification reaction step separately from the transesterification reaction step. Examples of the above-mentioned insoluble solid catalyst include a zinc aluminum mixed oxide, mixed oxides of aluminum and titanium, zirconium, or antimony, crystalline or amorphous titanosilicates, mixed oxides of zirconium oxide and titanium oxide rutile type titanium oxides, anatase type titanium oxides, metal oxides having an ilmenite structure, and metal oxides having a slyrankite structure.
Another catalyst other than the above-mentioned insoluble solid catalyst may be used in combination. One or two or more species of the above-mentioned catalysts may be used. The catalyst may contain impurities and other components generated during the catalyst-prepared step unless effects of the present invention are sacrificed.
It is preferable that the insoluble solid catalyst is a metal oxide catalyst . Among them, preferred are (1) metal oxide catalysts containing zirconium and at least one metallic element selected fromthe group consisting ofmetallic elements belonging to the Groups 4, 5 and 8 as essential components; (2) metal oxide catalysts having an ilmenite structure and/or or a slyrankite structure; and (3) metal oxide catalysts containing an anatase type titanium oxide and/or or a rutile type titanium oxide as essential components. These metal oxide catalysts (1) to (3) are catalysts having an ability of catalyzing the esterification and the transesterification simultaneously, and are unaffected by a mineral acid or a metal component contained in the fat or oil, and further avoid decomposition of the alcohol . Therefore,
use of such catalysts can simplify or omit the complicated steps such as the catalyst-recovered step, as comparedwithhomogeneous catalysts used in conventional methods. Thereby, fatty acid alkyl esters and/or glycerin canbe producedwith high efficiency and high selectivity.
The above-mentioned metal oxide catalyst (1) is not especially limited as long as it contains the above-mentioned essential components, and may be, for example, a mixture of a single oxide or a mixed oxide, or contain an active component carried or immobilized on a carrier. And the metal oxide may be also in the form in which one of the above-mentioned essential components is used as the carrier and other components are carried or immobilized as the active component.
Examples of the carrier on which the active component is carried or immobilized include silica, alumina, silica-alumina, various zeolites, activated carbon, kieselguhr, zirconium oxide, rutile type titanium oxide, tin oxide, and lead oxide. Among them, the above-mentioned metal oxide catalyst (1) is preferably in the form in which zirconium oxide is used as the carrier and a single or mixed oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 5 and 8 is used as the active component, and the active component is carried or immobilized on the carrier . As mentioned above, if zirconium oxide is used as the carrier, the catalyst has high activity. And it can be possible to extend catalyst life of a vanadium catalyst generally having a short catalyst life. Therefore, recycling efficiency of the above-mentioned catalyst is improved and thereby utility costs or equipment costs are sufficiently reduced, which permits production of glycerin and/or or fatty acid alkyl esters with high yield and high selectivity. The above-mentioned zirconium oxide preferably has a monoclinic structure. Such monoclinic zirconium oxide further improves catalytic activity, and suppresses leaching of the above-mentionedmetallic element which is the activemetal component.
In the above-mentioned metal oxide catalyst (1), the above-mentioned at least one metallic element selected form the group consisting of metallic elements belonging to the Groups 4, 5 and 8 is preferably a single or mixed oxide as mentioned above. The mixed oxide may be a mixed oxide of the at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 5 and 8, and other metallic elements. The single or mixed oxide more preferably contains at least one metallic element selected from the group consisting of Ti, V, and Fe. Among them, it is more preferable that Ti is essentially contained. Specific examples of titanium-containing oxide include titanium oxides such as anatase type TiO2 and rutile type TiO2, titania silica, titania zirconia, titania magnesia, titania calcia, titania yttria, titaniaboria, titania-tin oxide; titaniumvanadiummixed oxides such as TiVO4; vanadium oxides; iron vanadiummixed oxides such as FeVO4; cobalt vanadium mixed oxides such as Co2V2O7; cerium vanadiummixed oxides such as CeVO4; zinc vanadiummixed oxides; nickel vanadium mixed oxides; copper vanadium mixed oxide, scandium vanadium mixed oxides ; yttrium vanadium mixed oxides ; lanthanum vanadiummixed oxides; tin vanadiummixed oxides; lead vanadium mixed oxides; antimony vanadium mixed oxides; bismuth vanadiummixed oxides; seleniumvanadiummixed oxides; tellurium vanadium mixed oxides; and iron oxides. One or two or more species of them may be used. Among them, anatase type TiO2, rutile type TiO2, and iron vanadiummixed oxides are preferred. Rutile type TiO2 and triclinic FeVO4 are more preferred.
The mixed oxide may be in any form, and the following forms may be mentioned: a form in which a first atom and a second atom are bonded by covalent bond with an oxygen atom therebetween; a form in which a first atom and a second atom are bonded, and the bonded atoms and an oxygen atom are bonded by covalent bond; and a composite of oxides of a first atom and a second atom and solid solution thereof. If the above-mentioned metal oxide catalyst (1) is a mixed
oxide of zirconium and the above-mentioned metallic element, the mixed oxide is preferably Zro.5Tio.5O2 (slyrankite) . The slyrankite structure is mentioned below in the above metal oxide catalyst (2) . The above-mentioned metal oxide catalyst (1) preferably contains a metal oxide having a triclinic structure as an active component . The triclinic structure means a crystal systemwhich has three crystallographic axes obliquely crossing with one another, the lengths of the crystallographic axes being different from one another, and has a triclinic lattice. Among them, the metal oxide is preferably an oxide containing iron, vanadium, and zirconium, in which triclinic FeVCU mixed oxide is carried or immobilized on zirconium oxide.
The above-mentioned oxide containing iron, vanadium, and zirconium may be, other than the above oxide, an oxide in which iron vanadium mixed oxide that is not triclinic is carried or immobilized on zirconium oxide, or a ternary mixed oxide containing iron, vanadium, and zirconium. These are one of the preferable embodiments of the present invention. Use of the oxide containing iron, vanadium, and further zirconium suppresses leaching of the active component as compared with common oxides containing iron and vanadium, and thereby the catalyst life can be more improved.
The content of the zirconium in the above-mentioned metal oxide catalyst (1) has a lower limit of 1% by weight, and an upper limit of 80% by weight as a metal, relative to 100% by weight of a total amount of the catalyst of the present invention . If the content is less than 1% by weight, the metal oxide may neither exhibit high activity nor suppress leaching to the raw material or the product enough. If the content is more than 80%byweight, high activity may not be exhibited any more. More preferably, the lower limit is 2% by weight and the upper limit is 75% by weight.
The content of the at least one metallic element selected from the group consisting of metallic elements belonging to the
Groups 4, 5, and 8 has a lower limit of 1% by weight and an upper limit of 80% by weight as a metal, relative to 100% by weight of a total amount of the catalyst of the present invention. If the content is less than 1% by weight, the catalyst activity is insufficient, which possibly fails to improve the reaction efficiency. If it exceeds 80% by weight, the above-mentioned oxide of the metallic element agglutinates on the carrier, and thereby the metal oxide catalyst may not exhibit high activity and functional effects attributed to the presence of zirconium may be insufficiently exhibited. More preferably, the lower limit is 2% by weight and the upper limit is 75% by weight. The above-mentioned content of the metallic element can be identified by X-ray fluorescence (XRF) analysis.
The above-mentioned metal oxide catalyst (2) is not especially limited as long as it has an ilmenite structure and/or a slyrankite structure, and may be, for example, a mixture of a single oxide or a mixed oxide, or contain an active component (forexample, a single ormixed oxide ofmetallic element) carried or immobilized on a carrier. Examples of the carrier on which the active component is carried or immobilized include silica, alumina, silica-alumina, various zeolites, activated carbon, kieselguhr, zirconium oxide, rutile type titanium oxide, tin oxide, and lead oxide.
The above-mentioned ilmenite structure is expressed by the formula ABX3 (A and B being cations and X being an anion) , and means a rhombohedral lattice which is a slightly distorted hexagonal closest packing of X with the octahedral holes occupied by A and B regularly arranging as 6 coordination. For example, a mixed oxide represented by FeTiO3 has a structure in which a position of Al of α-alumina (corundum type) is replaced with Fe and Ti regularly. If the catalyst has such a structure, the catalyst has sufficient insolubility to any of the fat or oil and the alcohol which are rawmaterials of the present invention, and the products (the fatty acid alkyl esters, glycerin and the like) , and the catalyst life is remarkably improved. Therefore,
in the production method of the present invention, recycling efficiency of the catalyst is more improved and the catalyst-separated and removed step canbe remarkably simplified or omitted. Therefore, utility costs or equipment costs are sufficiently reduced.
It is preferable that the metal oxide has such an ilmenite structure in which at least one of A and B is titanium in the above formula. For example, MnTiO3, FeTiO3, CoTiO3, NiTiO3 and ZnTiO3 are mentioned. Among them, the catalyst preferably contains a metal oxide having an ilmenite structure containing titanium and at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 7, 8, 9, and 10. In this case, elution of the active component is more sufficiently suppressed, which permits sufficient exhibition of functional effects of the present invention. More preferably, the above-mentioned metal oxide is MnTiO3, FeTiO3, CoTiO3, or NiTiO3.
The above-mentioned slyrankite structure means a mixed oxide and/or solid solution, mainly composed of titanium and zirconium, whichhas the same structure as inorthorhombicα-Pbθ2, represented by Ti0.67Zr0.33θ2 discovered by A. Willgallis et al in 1983. That is, the slyrankite structure characteristically has a structure in which Ti4+ and Zr4+ are randomly arranged in place of Pb4+ of W-PbO2. Specifically, the slyrankite structure is a mixed oxide and/or solid solution in which atomic ratio of a total atomic number of Ti4+ and Zr4+ to oxygen is 1:2. In the present invention, the atomic ratio of Ti to Zr is preferably (1 to 0.2) : ( 0 to 0.8 ) , more preferably (0.8 to 0.3): (0.2 to 0.7), and still more preferably (0.7 to 0.4): (0.3 to 0.6). The above-mentioned catalyst (2) containing such a metal oxide exhibits activity higher than that of titanium oxide, silica-carried titanium oxide or the like. Therefore, according to the production method of the present invention, recycling efficiency of this catalyst is more improved and therebyutility costs or equipment costs are sufficientlyreduced,
which permits production of fatty acid alkyl esters and/or glycerin with high yield and high selectivity. It can be identified using powder X-ray diffraction measurement (XRD) whether the above-mentioned catalyst has an ilmenite structure or a slyrankite structure.
The above-mentioned metal oxide catalyst (3) is not especially limited as long as it contains the above-mentioned essential components. The titanium content preferably has a lower limit of 0.5% by weight, and an upper limit of 60% by weight, relative to 100% by weight of the metal oxide catalyst. The lower limit is more preferably 1% by weight and still more preferably 2% by weight. The upper limit is more preferably 45% by weight, and still more preferably 30% by weight.
The above-mentioned "anatase structure" and "rutile structure" mean crystal structures belonging to the tetragonal system and formed by a compound represented by AB2 (A: positive atom, B: negative atom) . In such a crystal structure, A atom is octahedrally coordinatedbyB atom. In the anatase structure, resulting octahedrons form a skeletal structure as a result of each octahedron sharing 4 edges with eachneighboring octahedron . In the rutile structure, resulting octahedrons form a skeletal structure as a result of each octahedron sharing 2 edges with each neighboring octahedron. Such compounds having the rutile structure can be obtained by calcining compounds having the anatase structure.
Powder X-ray diffraction measurement (XRD) can show whether the catalyst contains the anatase type titanium oxide and/or the rutile type titanium oxide.
The above-mentioned metal oxide catalyst (3) may contain a single oxide or amixedoxide other than the anatase type titanium oxide and/or the rutile type titanium oxide. And the catalyst also may contain the above titanium oxide as a carrier or an active component, the active component being carried or immobilized on the carrier. Examples of the carrier include silica, alumina, silica-alumina, various zeolites, activated
carbon, kieselguhr, zirconium oxide, tin oxide, and lead oxide, other than the above-mentioned titanium oxide.
It is preferable that the above-mentioned metal oxide catalyst (3) further contains an oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14. For example, it is preferable that the catalyst is a mixture of the oxide and the above-mentioned titanium oxide, or contains the oxide or the above-mentioned titanium oxide as a carrier or an active component, the active component being carried on the carrier. It is most preferable that the catalyst contains an oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 as a carrier, and contains anatase type titanium oxide and/or rutile type titanium oxide as an active component. Use of such a catalyst can sufficiently suppress leaching of the titanium component which is the active component and improves the catalyst activity and the catalyst life. Therefore, such a catalyst may be most preferably used for the production method of the present invention.
The above-mentioned oxide containing at least onemetallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 is preferably a single oxide of one of. these metallic elements, a mixture thereof, or a mixed oxide of these metallic elements. The mixed oxide may be a mixed oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14, and other metallic elements . The single or mixed oxide of the metallic elements more preferably contains at least one metallic element selected from the group consisting of Si, Zr, and Al . For example, silica, alumina, silica-alumina, and zirconium oxide may be mentioned. The above-mentioned oxide of at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 may contain amorphous titanium oxide, or a mixed oxide
consisted of titanium and a metal other than titanium, but the oxide does not contain anatase type titanium oxide and/or rutile type titanium oxide.
In the above-mentioned metal oxide catalyst (3), the content (not including titanium content constituting anatase type titanium oxide and/or rutile type titanium oxide) of the at least one metallic element selected from the group consisting of metallic elements belonging to the Groups 4, 13, and 14 has a lower limit of 1% by weight and an upper limit of 80% by weight as a metal, relative to 100% by weight of a total amount of the catalyst. Use of the catalyst (3) containing a metal element within such a range can further improve the catalyst activity and the reaction efficiency, which allows sufficient exhibition of functional effects of the present invention . More preferably, the lower limit is 2% by weight and the upper limit is 75% by weight . The metallic element content can be identified by X-ray fluorescence (XRF) analysis.
It is preferable that the above-mentioned metal oxide catalyst (3) contains a sulfur component of 700 ppm or less. If the catalyst contains a sulfur component of more than 700 ppm, the catalyst activity and the catalyst life may be insufficiently improved. Therefore, functional effects of the invention, in which the catalyst-separated, removed, and recovered steps can be remarkably simplified or omitted and the catalyst can be repeatedly used for continuous reactions, may be insufficiently exhibited. The sulfur component content is preferably 500 ppm or less, and more preferably 200 ppm or less . The sulfur component content in the catalyst can be identified by fluorescence X rays (XRF) analysis or high frequency induction plasma (ICP) emission spectrometry. According to XRF analysis, the component can be identified by measuring the catalyst powder as it is or by a glass bead method. According to ICP emission spectrometry, the component can be identified by measuring a catalyst powder after being dissolved in a hydrofluoric acid aqueous solution. However, the sulfur component is preferably
identified by a glass bead method of XRF, in view of measurement easiness .
For reducing the sulfur components contained in the catalyst, for example, the catalyst may be prepared using no sulfate as a raw material, or using sufficiently reduced amount of sulfate, or washed with sufficient amount of a solvent such as water.
A calcined metal oxide catalyst may be used as the above-mentioned metal oxide catalysts (1) to (3) . Thereby, leaching of the active metal components can be more suppressed. The calcination temperature is preferably determined in consideration of the catalyst surface area and the crystal structure, and preferably has a lower limit of 280°C and an upper limit of 1300°C, for example. If the temperature is less than 280°C, suppression of the leaching maybe insufficient. If it is more than 1300°C, sufficient catalyst surface area may not be obtained, which fails to produce fatty acid alkyl esters and/or glycerin withhigh efficiency. More preferably, the lower limit is 400°C, and the upper limit is 1200°C. The calcination time preferably has a lower limit of 30 minutes and an upper limit of 24 hours. More preferably, the lower limit is 1 hour, and the upper limit is 12 hours. Gas phase atmosphere during the calcination is preferably air, nitrogen, argon, oxygen, and hydrogen atmosphere, and also mixed gas thereof. More preferably, the calcination is carried out under air or nitrogen atmosphere .
If the catalyst in which the active component consisted of the single or mixed oxide of the metallic element is carried or immobilized on the carrier is produced, as mentioned above, the catalyst is preferably produced by mixing and carrying the active component on a compoundused as a carrierby an impregnation method or a kneading method and the like, and then by calcinating the compound under the above-mentioned calcination conditions . Thus-produced catalyst can disperse the active component sufficiently over the carrier surface to function as a solid
catalyst .
If the insoluble solid catalyst is used in the above-mentioned alcoholysis step, the catalyst amount preferably has a lower limit of 0.5% by weight and an upper limit of 20% by weight, relative to 100% by weight of a total fed amount of the fat or oil, the alcohol and the catalyst, for example, in a batch method. If the catalyst amount is less than 0.5% by weight, the reaction rate may be insufficiently improved. If the upper limit is more than 20% by weight, the catalyst costs may be insufficiently reduced. The lower limit is more preferably 1.5% by weight, and the upper limit is more preferably 10% by weight. In a fixed bed flow method, a liquid hourly space velocity (LHSV) calculated by the following formula preferably has a lower limit of 0.1 hr"1 and an upper limit of 20 hr"1. More preferably, the lower limit is 0.2 hr"1 and the upper limit is 10 hr"1.
LHSV (hr"1)^! (flow rate of a fat or oil per hour (mL-hr"1))+ (flow rate of an alcohol per hour (mL-hr"1) ) } / (volume of a catalyst
(ml.)) The reaction temperature in the above-mentioned alcoholysis step is preferably 18O0C or more, and 300°C or less, for example. Particularly if the insoluble solid catalyst is used, it is preferable that the reaction temperature is 18O0C or more and 3000C or less. If the reaction temperature is less than 18O0C, the reaction rate may be insufficiently improved. If the reaction temperature is more than 3000C, suppression of a side reaction such as alcohol decompositionmaybe insufficient . The reaction temperature is more preferably 185°C or more and 29O0C or less, and still more preferably 19O0C or more and 2800C or less.
The catalyst used in the above-mentioned contacting step (alcoholysis step) preferably contains anactivemetal component not leached at the reaction temperatures within the above range. Thereby, the activity of the catalyst can be maintained enough even at high reaction temperatures, and the reaction can be
performed well.
The reaction pressure is preferably 0.1 MPa or more and 10 MPa or less, for example. If the reaction pressure is less than 0.1 MPa, the reaction rate may be insufficient. If the reaction pressure is more than 10 MPa, a side reactionmay proceed easily. In addition, a special apparatus durable for high pressures may be needed, hence utility costs or equipment costs may not be reduced enough. The reaction pressure is more preferably 0.2 MPa or more and 9 MPa or less, and still more preferably 0.3 MPa or more and 8 MPa or less.
In the above-mentioned reaction step, the above-mentioned "substantially in the absence of a catalyst" means an embodiment in which the catalyst is not contained at all or the catalyst is hardly contained. That is, the reaction is performed in an embodiment in which it is not evaluated that the reaction is performed in the presence of the catalyst. In this case, it is preferable that a fat or oil and an alcohol are reacted in supercritical state of the alcohol. The term "supercritical state" means a region over a specific critical temperature and critical pressure of a substance itself. If methanol is used as the alcohol, the state means a condition in which the temperature is 239°C or more and the pressure is 8.0 MPa or more . As a preferable temperature range, the lower limit is 250°C and the upper limit is 3500C. More preferably, the lower limit is 2600C and the upper limit is 330°C. Still more preferably, the lower limit is 27O0C and the upper limit is320°C. As a preferable pressure range, the lower limit is 8.0 MPa, and the upper limit is 15.0 MPa. If the insoluble solid catalyst is used in the above-mentioned reaction step, the reaction may be performed in such supercritical state. The catalyst may or may not used in the alcoholysis step, using catalyst is prefelable in view of lowering the reaction temperature.
In the above-mentionedproductionmethod, the alcoholysis step is preferably performed by a batch method (batch-wise) or continuous flow method. Among them, a fixed bed flow method
is preferably employed because the step of separating and recovering the catalyst is unnecessary. As a reactor to be used, mentioned may be tubular reactors, stirring tank type reactors, and reaction kettle type reactors, for example. A particularly preferable embodiment of the above-mentionedproductionmethodis an embodiment inwhich fatty acid alkyl esters and/or glycerin are/is continuously produced by reacting a fat or oil with an alcohol using a fixed bed reactor filled with the catalyst. Thereby, the step of separating and recovering the catalyst becomes unnecessary, which makes it possible to produce fatty acid alkyl esters and/or glycerin more industrially. A preferable embodiment in the above-mentioned batch method is an embodiment in which the catalyst is added into a mixed system of a fat or oil and an alcohol . The reaction time is preferably lδminutes ormore and 30 hours or less, although it varies depending on the amount of the catalyst to be used and the reaction temperature to be employed. The reaction time is more preferably 30 minutes or more and 24 hours or less.
In the above-mentionedproductionmethod, it is preferable that the reaction step in at least one stage from the first stage to the (n-1) stage is a step of reacting a fat or oil with an alcohol ; and the reaction step in the n stage is an aging step. In this case, transesterification of the fat or oil with the alcohol is generally performed in the reaction in at least one stage from the first stage to the (n-1) stage. The transesterification is almost completed in the reaction in the n stage. The aging step in the n stage may become unnecessary if a catalyst that is extremely effective in the former transesterification step is found. However, in the present condition, the reaction is completed through such an aging step, and thereby products with high quality can be effectively obtained.
The aging step in the n stage in the production method of the present invention is not necessarily a step of reacting the ester phase under the condition that glycerin to be produced
is separated from the ester phase. In the present invention, the reaction is performed under the condition that the phase separation occurs or does not occur, depending on an alcohol to be used, for example. If ethanol is used, for example, the reaction solution is not separated into phases and forms a single phase at 50°C. Therefore, in the aging step in the n stage, the ester phase may be or may not be reacted under the condition that glycerin to be produced is separated from the ester phase.
If the aging step in the n stage is a step of reacting the ester phase under the condition that glycerin to be produced is separated from the ester phase, that is, if the ester phase obtained in the alcoholysis step is matured under the condition that glycerin to be produced is separated from the ester phase, the reaction solution is sufficiently separated into two phases, that is, the ester phase and the glycerin phase, and glycerin is extracted enough from the ester phase. Therefore, chemical equilibrium appears to function advantageously. Thereby, in such a case, reaction intermediates such as fatty acid monoglycerides that can be contained in the reaction solution obtained through the steps up to the aging step, can be significantly reduced. Thereby, fatty acid alkyl esters with high purity can be produced, while the yield is improved and a purification step that can be subsequently performed is simplified. The content of the intermediate glycerides can be reducedwithoutuseof suchalargeexcessof alcohol. Therefore, costs on recovery of the alcohol can be suppressed enough.
The n step means a substantial final reaction stage . That is, if the reaction of the fat or oil with the alcohol is substantially completed, the reaction in the n stage is performed and then the step is performed under the condition different from that in the n stage in order to adjust physical properties substantially uninvolved in the reaction.
Herein, the above-mentioned "under the condition that glycerin to be generated is separated from the ester phase" means λ'under the condition that the reaction solution is separated
into two phases, that is, the ester phase and the glycerin phase. " For example, it is preferable that the reaction temperature is 180°C or less. It is preferable that the reaction temperature in the aging step in the n stage is 1800C or less . If the reaction temperature is more than 180°C, the reaction solution forms a single phase. Thereby, effects of the present invention under the reaction condition that the reaction solution is separated from two phases, that is, the ester phase and the glycerin phase, can be insufficiently exhibited. The reaction temperature is preferably 10°C ormore. The reactionratemaybe insufficiently improved if the reaction temperature is less than 1O0C. The reaction temperature is more preferably 20°C or more and 1700C or less. Still more preferably, the reaction is performed at around 65°C. Utility costs and equipment costs can be reduced enough if the reaction is performed at around 65°C. The effects of the present invention can be sufficiently obtained if the liquid composition changes with proceeding of the reaction and thereby the reaction solution is separated into two phases, even if the reaction solution forms a single phase at the beginning of the reaction. The state of the liquid phase under high temperature and high pressure conditions can be identified using a glass autoclave container and the like.
In contrast, the present inventors have noted that the above-mentioned equilibrium functions advantageously just by lowering the aging temperature. In this case, the equilibrium functions advantageously in the aging step even if a large amount of intermediate glycerides is contained in the reaction solution in the (n-1) step used in the aging step. Therefore, the amount of the intermediate glycerides can be effectively reduced. Therefore, the alcohol amount in the reaction step in at least one stage from the first stage to the (n-1) stage can be reduced, which makes it possible to sufficiently suppress costs on recovery of the alcohol. Particularly, the alcohol amount in the reaction step in the first stage can be more reduced than usual, and the alcohol amount in the reaction step in the (n-1)
stage can be also reduced. The alcohol amount in the reaction step in the first stage can be more reduced than usual and the alcohol amount in the reaction step in the second stage can be also reduced, if the aging temperature is lowered in the aging step in the n stage such that the above-mentioned equilibrium functions advantageously, for example, in the cases where the following reaction steps are sequentially performed in the following order: the reaction step in the first stage in which the transesterification reaction of the fat or oil with the alcohol is performed; the alcohol-evaporated step of evaporating the alcohol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and then extracting and removing the glycerin phase; the esterification step of supplying the alcohol and esterifying the free fatty acids into fatty acid alkyl esters; the alcohol-evaporated step of evaporating the alcohol; andthephase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and then extracting and removing the glycerin phase. It is preferable that the reaction temperature in the aging step in the n step is 180°C or less. In this embodiment, as mentioned above, the aging step in the n stage is not necessarily a step of reacting the ester phase under the condition that glycerin to be produced is separated from the ester phase. If the reaction temperature is more than 180°C, the above-mentioned functional effects may be insufficiently exhibited. The reaction temperature is preferably 0°C or more. The reaction rate maybe insufficiently improved if the reaction temperature is less than 00C. The reaction temperature is more preferably 5°C or more and 1700C or less, and still more preferably 1O0C or more and 700C or less. Utility costs and equipment costs can be more sufficiently reduced as mentioned above if the aging reaction is performed at 1O0C or more and 17O0C or less.
The catalyst used in the above-mentioned aging step is preferably a catalyst containing an active component not leached,
and the like, if used at the reaction temperature within the above-mentioned range. Thereby, the activity of the catalyst can be maintained enough and the reaction can be performed well .
The reaction pressure may be appropriately determined depending on the alcohol to be used and the reaction temperature to be employed. The reaction temperature is preferably 3 MPa or less and more preferably 1.5 MPa or less, for example. Still more preferably, the reaction is performed at around ordinary pressure. Utility costs and equipment costs can be more sufficiently reduced if the aging reaction is performed at around ordinary pressure.
With respect to a preferable range of the alcohol amount in the stages from the first stage and the (n-1) stage, it is preferable that the molar ratio of the fat or oil to the alcohol is 1:3 to 50, generally. The molar ratio of the fat or oil to the alcohol is more preferably 1:5 to 40.
The molar ratio of the fat or oil to the alcohol can be 1 : 5 to 30 if, as mentioned above, the aging temperature is lowered such that the equilibrium functions advantageously, that is, if the reaction temperature in the aging step in the n stage is 180°C or less. Further, the molar ratio of the fat or oil to the alcohol is 1:5 to 30. These embodiments are part of the preferable embodiments of the present invention . In such cases , load of the process of recovering the methanol excessively supplied is reduced as mentioned above, and thereby such a process can be industrially advantageous process.
In the present invention, it is preferable that the method comprises a free fatty acids-treated step of reducing a free fatty acid amount between the reaction step in the (n-1) stage and the reaction step in the n stage.
It is preferable that an esterification step of esterifying the free fatty acids into fatty acid alkyl esters is performed between the reaction step in the (n-1) stage and the reaction step in the n step, as the above-mentioned the free fatty acid-treated step. The free fatty acid can be treated by
sequentially performing the following reaction steps in the following order : the alcohol-evaporated step of evaporating the alcohol (for example, methanol) (alcohol cut step, methanol cut step) ; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and extracting and removing the glycerin phase; and the esterification step of supplying the alcohol (for example, methanol) and esterifying the free fatty acids into fatty acid alkyl esters (Fig.2). It is also preferable -that an esterification step of producing fatty acid alkyl esters and monoglycerides from the free fatty acids, the alcohol (for example, methanol) , and the glycerin is performed between the reaction step in the (n-1) stage and the reaction step in the n-step. In this case, the free fatty acid can be treated, for example, by sequentially performing the following reaction steps in the following order: this esterification step; the alcohol-evaporated step of evaporating the alcohol (for example, methanol) (alcohol cut step, methanol cut step) ; and the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase (Fig.3) .
The preferable embodiments of the present invention include these embodiments. It is preferable that the above-mentioned esterification step is performed in the presence of an acid catalyst. The esterification step is more preferably performed in the presence of a solid acid catalyst and still more preferably in the presence of a cation-exchange resin. Homogeneous catalysts such as p-toluene sulfonic acid may be used.
The above-mentioned cation-exchange resin has a functional group such as a sulfonyl group, and form of particles of the resin is not especially limited. The cation-exchange resin is preferably aH-type ion exchange resin inview of reaction activity and prevention of generation of soap components. Any
ion exchange resins among weakly to strongly acidic ion exchange resins may be used.
In the present invention, it is preferable that an acid value of the ester phase used in the aging step in the n stage is controlled to 4.0 or less (mg-KOH/g-ester phase) . That is, it is preferable that the acid value of the ester phase obtained in the alcoholysis step (the acidvalue of the esterphase obtained in the reaction step in the (n-1) stage and used in the aging step in the n step) is 4.0 or less (mg-KOH/g-ester phase) (an amount of the free fatty acid contained in the ester phase used in the aging step is preferably 4.0 or less (mg-KOH/g-esterphase) as an acid value) . If the acid value is more than 4.0, the base catalyst used in the aging reaction is inhibited, possibly resulting in insufficient proceeding of the reaction. The acid value means the number of "mg" of potassium hydroxide needed for neutralization of free fatty acids, resin acids, and the like, contained in 1 g of a sample. The acid value can be measured by the method according to Japanese Industrial Standards (JIS) . The above-mentioned acid value is more preferably 3.5 or less (mg-KOH/g-ester phase) and still more preferably 3.0 or less (mg-KOH/g-ester phase) .
If the acid value of the ester phase obtained in the alcoholysis step is more than 4.0, the following free fatty acid-removed stepmaybe performed: the free fatty acid contained in the ester phase is converted into soaps and the soaps are washed; the free fatty acids are esterified with an alcohol to be converted into fatty acid alkyl esters or glycerides; or the free fatty acids are adsorbed on an anion exchanger, activated carbon, silica-alumina, or the like. The following operations in the alcoholysis step up to the aging step can reduce the free fatty acid: the alcoholysis step is performed in multi-stages; the reaction is performed at a higher temperature; the use amount of the alcohol is increased; and the contacting time of the reaction raw materials to the catalyst is prolonged. It is preferable that the acid value of the ester phase
used in the aging step in the n stage is measured immediately before the aging step. It is preferable that the acid value of the ester phase is measured between the esterification step of esterifying the free fatty acids into the fatty acid alkyl esters and the aging step, for example, if the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in the n step: the alcohol-evaporated step of evaporating the alcohol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase; and the esterification step of supplying the alcohol and esterifying the free fatty acids into fatty acid alkyl esters.
It is preferable that the acid value of the ester phase is measured between the phase separation step of extracting and removing the glycerin phase and the aging step, for example if the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in the n step: the esterification step of generating fatty acid alkyl esters and monoglycerides fromthe free fatty acids; the alcohol-evaporated step of evaporating the alcohol; and the phase separation step of separating,the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase.
The acid value of the ester phase used in the aging step can be reduced also if the free fatty acids contained in the fat or oil used as the raw material in the alcoholysis step are esterified with a polyol to be converted into glycerides, and then the glycerides are used in the alcoholysis step.
That is, the preferable embodiments of the present invention include an embodiment in which the free fatty acids contained in the fat or oil are converted into glycerides and then the glycerides are used in the alcoholysis step . The polyol to be used is preferably glycerin. The same effect can be
obtained if an alcohol is used in place of the polyol and the free fatty acids are converted into alkyl esters and then used in the alcoholysis step. In this case, it is preferable that the same alcohol as used in the alcoholysis step is used. Further, load to the catalyst in the alcoholysis step can be reduced if the free fatty acids are converted into glycerides or alkyl esters.
The ester phase separated from the reaction solution without evaporating the alcohol from the reaction solution after the alcoholysis step may be used as it is as the above-mentioned ester phase used in the aging step in the n stage . In this case, the aging reaction canbe performedwithout addition of an alcohol because the alcohol remains in the ester phase. An alcohol may be addeddepending onthe residual amount of the alcohol . Further, the ester phase separated from the glycerin phase after evaporating the alcohol from the reaction solution obtained in the reaction step in the (n-1) stage may be used. Thereby, the mutual solubility of the ester phase and the glycerin phase decreases, and thereby the separation of the fatty acid alkyl esters from the glycerin can be improved, which permits improvement in the yield. Therefore, such an embodiment is one of the preferable embodiments . In this case, the aging reaction cannot be performed without addition of alcohol.
The amount of the alcohol used in the aging step in the n stage is preferably 50% by weight or less, relative to a total fed amount of the ester phase and the alcohol. If the amount of the alcohol is more than 50% by weight, costs may be increased because the recovery or recycle amount of the alcohol increases . The amount of the alcohol is more preferably 40% by weight or less, and still more preferably 30% by weight or less.
In the present invention, it is preferable that the aging step in then stage is performed in the presence of abase catalyst. A base catalyst is preferably used also in the aging step in the n stage under the condition that glycerin to be produced is separated from the ester phase. As mentioned above, the
preferable embodiments of the present invention include an embodiment in which the aging step in the n stage is performed in the presence of a base catalyst. Thereby, alkyl esters or glycerin with high purity can be easily produced with high effectiveness, because the aging reaction rate in the n stage is improved and the amount of intermediate glycerides can be sufficiently reduced.
It is preferable that the reaction step in the n stage is an aging step in the presence of a solid base catalyst. That is, the above-mentionedbase catalyst is preferably an insoluble solid catalyst, andmore preferably an anion exchanger, and still morepreferably ananion-exchange resin. Homogeneous catalysts such as sodium alkoxide and potassium alkoxide may be used.
Hydrotalcites, IXE (tradename, product of TOAGOSEI CO., LTD.), and anion-exchange resins may be mentioned as the above-mentioned anion exchanger.
The above-mentioned anion-exchange resin has a functional group such as an amine group, and the particle form of the resin is not especially limited. OMe-type or OH-type ion exchange resins are preferable in view of prevention of mixing of impurity ions to the reaction system and reaction activity. Any ion exchange resins among weakly to strongly basic ion exchange resins may be used. Specific examples of such an ion exchange resin include an ion exchange resin represented by the following formula (1) disclosed in Japanese Kokai Publication No.
2002-105138 (applicant : NIPPON SHOKUBAI Co ., Ltd. ) (hereinafter, also referred to as λλHiMAC") ; DAIAION TSA1200, DAIAION SAlOA, DAIAION PA308, DAIAION HPA25 and DAIAION NSAlOO (each of them is trade name, product of Mitsubishi Chemical Corp.); Dowex SBR-P-C (trade name, product of the Dow Chemical Co.); and AMBERLITE IRA400J, andAMBERLITE IRA402BL (each of them is trade name, product of Organo Corp.) . Among them, HiMAC and DAIAION TSA1200 are preferred in view of the heat resistance.
The preferable embodiments of the present invention include an embodiment in which a deteriorated anion exchanger is reproduced and then used again in the aging step in the n stage.
The following methods may be adopted as a method of the reproduction: the deteriorated anion exchanger is washed with
NaOH aqueous solution/ and the deteriorated anion exchanger is washedwith dilute hydrochloric acidand/orNaCl aqueous solution and then washed with NaOH aqueous solution.
The amount of the above-mentioned catalyst preferably has a lower limit of 0.5% by volume, and an upper limit of 60% by volume, relative to 100% by volume of a total fed amount of the fat or oil, the alcohol, and the catalyst, in a batch method. If the amount of the catalyst is less than 0.5% by volume, the reaction rate may be insufficiently improved. If the amount of the catalyst is more than 60% by volume, costs on the catalyst maybe insufficiently reduced. More preferably, the lower limit is 1.5% by volume, and the upper limit is 50% by volume. In
a fixed bed flow method, a liquid hourly space velocity (LHSV) calculated by the following formula preferably has a lower limit of 0.1 hr"1 and an upper limit of 20 hr"1. More preferably, the lower limit is 0.3 hr"1 and the upper limit is 10 hr"1. LHSV (hr~1)={ (flow rate of a fat or oil per hour (HiL-IIr"1))+ (flow rate of an alcohol per hour (mL-hr"1) ) } / (volume of a catalyst (mL))
The form of the above-mentioned aging step may be appropriately determined depending on the phase state of the reaction solution, and is preferably a batch method (batch-wise method) or a continuous flow method. If the reaction solution is separated into two phases, a continuous-flow fluidized bed reactor is more preferred in view of the contacting efficiency between the reaction solution and the catalyst . If the reaction solution is not separated into two phases, a continuous-flow fixed bed reactor is more preferred in view of suppression of deterioration in physical properties such as breakage of the catalyst and easiness of the catalyst separation.
Two or more reactors may be used in the above-mentioned aging step. In this case, the reactors may be connected in parallel or serially. If the reactors are connected inparallel, the ester phase in a large amount can be treated simultaneously, and the aging reaction can be performed in one reactor while the catalyst is reproduced in other reactors. If the reactors are connected serially, effects such as further reduction in the intermediate glyceride amount can be expected.
In the above-mentioned production method, the number of stages Mn" of the step of bringing a fat or oil into contact with an alcohol is 2 or more, and preferably 5 or less in view of equipment costs, manufacturing efficiency, and the like. More preferably, the number of stages is 2. That is, more preferred is a method in which the step of reacting a fat or oil with an alcohol in the presence of the solid catalyst or substantially in the absence of the catalyst is performed in one stage, and then the aging step in the presence of the base
catalyst performed in one stage.
The methanol is evaporated from the reaction solution obtained after the aging step in the n stage and then the reaction solutionis separated into the esterphase and the glycerinphase, or the reaction solution obtained after the aging step in the n stage is separated into the ester phase and the glycerin phase and then the methanol is evaporated from the reaction solution. Thereby, a fatty acid methyl ester and glycerin can be produced. Thus-produced fatty acid methyl ester and/or glycerin may be subjectedto apurification step suchas distilation, filtration, and washing, depending on specification of the intended use.
With respect to the raw materials used in the above-mentioned production method, the fat or oil used in the above-mentioned production method contains fatty acid esters of glycerin and may be any species capable of serving as a raw material for fatty acid alkyl esters and/or glycerin together with an alcohol. Thus, those generally called "fats and oils" may be used. Generally preferred are fats and oils containing triglycerides (triesters of higher fatty acids with glycerin) as amain component and containing small amounts of diglycerides, monoglycerides and other minor components. Fatty acid esters of glycerin such as triolein also may be used.
Usable as the above-mentioned fats and oils are vegetable oils such as rapeseed oil, canola oil, sesame oil, soybean oil, corn oil, sunflower oil, palm oil, palm kernel oil, coconut oil, safflower oil, linseed oil, flaxseed oil, cottonseed oil, tung oil, jatropha oil, castor oil, hemp oil, mustard oil, peanut oil, and jojoba oil; animal fats and oils such as beef fat, lard, fish oil and whale oil/ and various used edible fats and oils (waste cooking oil) . One or two or more species of them may be used.
If the above-mentioned fat or oil contains phospholipids, proteins or the like as impurities, the fat or oil is preferably used after subjected to a degumming step of adding a mineral acid such as sulfuric acid, nitric acid, phosphoric acid or boric
acid to the fat or oil for removing the impurities therefrom.
With respect to the raw materials used in the above-mentioned production method, the alcohol is preferably an alcohol containing 1 to 6 carbon atoms and more preferably an alcohol containing 1 to 3 carbon atoms, for easy evaporation of the alcohol in the n stage and the like. Employable as the alcohol containing 1 to 6 carbon atoms are, for example, methanol, ethanol, propanol, isopropyl alcohol, 1-butanol, 2-butanol, t-butyl alcohol, 1-pentanol, 3-pentanol, 1-hexanol, and 2-hexanol. Among them, methanol is preferred. These may be used singly or in combination of two or more species.
Apolyol ispreferablyusedas the above-mentioned alcohol . The above-mentioned polyol may be preferably ethylene glycol, propylene glycol, glycerin, pentaerythritol, sorbitol or the like. Among them, glycerin is more preferred. These may be used singly or in combination of two or more species . The fatty acid alkyl esters obtained in the productionmethod of thepresent invention means esters of a fatty acids contained in the fat or oil with the alcohol. If the fat or oil is reacted with the alcohol in the above-mentioned production method, transesterification reaction of a triglyceride and methanol finally gives a fatty acidmethyl ester and glycerin, as shownby the following formula. In the formula, R may be the same or different, and each represent an alkyl group containing 6 to 22 carbon atoms or an alkenyl group containing 6 to 22 carbon atoms and having one or more unsaturated bond.
In the above-mentioned production method, glycerin is produced together with fatty acid alkyl esters in the transesterification reaction, as shown by the above formula. In the present invention, purified glycerin can be industrially easily produced, as mentioned above. Such glycerin is preferably used as a chemical material in various applications . The fatty acid alkyl esters produced by the method of the present invention are suitably used for various uses as, for example, industrial raw materials, raw materials of pharmaceuticals and fuels. Among them, diesel fuel containing the fatty acid alkyl esters produced from a vegetable oil, or waste cooking oil by the above productionmethod can sufficiently reduce utility costs or equipment costs in the production step. And no need of the catalyst-separated and recovered step permits a repeating use of the catalyst. Therefore, thus-produced diesel fuel can sufficiently contribute to environmental preservation in the production step, and suitably used for fuel in various applications . Such diesel fuel containing the fatty acid alkyl esters produced by the above-mentioned production method is also a preferable embodiment of the present invention .
The preferable embodiments of the present invention are described below with reference to Figs. 1 to 3. The present invention is not limited to these embodiments. Fig. 1 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which the production is performed using: methanol
as the alcohol; a flow fixed bed reactor as the reactor in the first stage reaction; a metal oxide catalyst as the catalyst in the first stage reaction; a continuous stirred tank reactor (hereinafter, also referred to as ΛλCSTR") as the reactor in the second reaction; and an anion-exchange resin as the catalyst in the second stage reaction. In such an embodiment, methanol is evaporated froma reaction solution after reacted in a reaction column filled with a metal oxide catalyst and the reaction solution from which the methanol is evaporated is kept standing inside a settler, thereby being separated into an ester phase and a glycerin phase. Methanol is further added into the ester phase obtained by separated from the glycerin phase, and a reaction in the second stage is performed in a CSTR in which an anion-exchange resin is dispersed. Then, the methanol is evaporated from the reaction solution, and then the reaction solution is kept standing in the settler, therebybeing separated into an ester phase and a glycerin phase . Thereby, a fatty acid methyl ester and glycerin can be obtained.
Fig. 2 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which methanol is used as the alcohol, and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step (aging step) in the n step: the methanol cut step of evaporating the methanol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and extracting and removing the glycerin phase; and the esterification step of supplying the methanol and esterifying the free fatty acids into fatty acid alkyl esters .
Fig. 3 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which methanol is used as the alcohol, and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step
in the (n-1) stage and the reaction step in the n step: the esterification step of generating fatty acid alkyl esters and monoglycerides from free fatty acids; the methanol cut step of evaporating the methanol; and the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerin phase and extracting and removing the glycerin phase .
In these embodiments, the reaction step in the (n-1) stage may be the reaction step in the (n-2) stage. In such a case, the reaction step in the (n-1) stage may be the esterification step of supplying the methanol and esterifying the free fatty acids into the fatty acid alkyl esters or the esterification step of generating the fatty acid alkyl esters and the monoglycerides from the free fatty acids. In the present invention, it is important that the free fatty acid-treated step is performed between the esterification step which is performed before the aging step and the aging step. That is, the preferable embodiments of the present invention include an embodiment in which the reaction step in the (n-2) stage, the reaction step in the (n-1) stage, and the reaction step in the n stage (aging step) are performed in this order and the reaction step in the (n-1) stage is the free fatty acid-treated step.
The yield of fatty acid alkyl esters so referred to herein means degree of conversion of effective fatty acid components in the raw material fat or oil and can be calculated from the following formula.
Yield of fatty acid alkyl esters (%) = (total molar flow rate of fatty acid alkyl esters at a reactor outlet/total molar flow rate of effective fatty acid components at a reactor inlet) x 100 (%)
The term "effective fatty acid components" means those components capable of producing fatty acid alkyl esters by the method of the present invention, and specifically means fatty acid triglycerides, diglycerides, monoglycerides, free fatty acids and fatty acid alkyl esters contained in the fat or oil
as well as in the reused raw materials. The molar flow rate of the effective fatty acids at the reactor inlet canbe calculated by the following formula. Total molar flow rate of effective fatty acids (mol/h) = [flow rate of raw materials at the reactor inlet (g/h) x saponification value of raw materials at reactor inlet/56100] .
BRIEF DESCRIPTION OF THE DRAWINGS Fig. 1 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which the production is performed using methanol as the alcohol; a flow fixed bed reactor as the reactor in the first stage reaction; a metal oxide catalyst as the catalyst in the first stage reaction; a continuous stirred tank reactor as the reactor in the second reaction; and an anion-exchange resin as the catalyst in the second stage reaction.
Fig. 2 is a view schematically showing a preferable embodiment of the production method according to the present invention, in which methanol is used as the alcohol and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in then step: themethanol cut step of evaporating the methanol; the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase; and the esterification step of supplying the methanol and esterifying free fatty acids into fatty acid alkyl esters.
Fig. 3 is a view schematically showing a preferable embodiment of the present invention according to the present invention, in which methanol is used as the alcohol, and the following reaction steps are performed in the following order as the free fatty acid-treated step between the reaction step in the (n-1) stage and the reaction step in the n step: the esterification step of generating fatty acid alkyl esters and monoglycerides from free fatty acids; the methanol cut step of
evaporating the methanol; and the phase separation step of separating the reaction solution into two phases, i.e., ester phase and glycerinphase and extracting and removing the glycerin phase . In addition, the symbols in the figures are as follows. P : Pump
1 : Former reaction 2: Metal oxide catalyst 3: Latter reaction 4: Anion-exchange resin 5: n-1 step
6: Methanol-evaporated step 7 : Phase separation step
8: Esterification step (FFA→FAME) 9: Catalyst
10: Aging step
11: n-1 or n-2 step
12: Esterification. step (FFA + glycerin + methanol → FAME + monoglycerides)
BEST MODE FOR CARRYING OUT THE INVENTION The present invention will, hereinafter, be described in more detail with reference to Examples, but the present invention is not limited only to these Examples. The term "%" represents ΛΛ% by weight" unless otherwise specified.
Example 1
Alcoholysis reaction of Palm oil with methanol by using continuous-flow fixed bed reaction system was performed using MnTiθ3 catalyst as the reaction in the first stage. Specifically, a straight tubular reactor made of SUS-316 stainless steel in 10 mm inside diameter and 210 mm length was filled with MnTiO3 catalyst 15 mL. XRD analysis of the MnTiO3 catalyst used in this Example showed that the catalyst had an ilmenite structure . To control reaction pressure, an automatic backpressure
regulator was equipped at the reactor outlet. The pressure inside the reactor was set at 5.0 MPa with the back pressure regulator, while feedingbothpalmoil andmethanol to the reactor at flow rate of 0.105 g/rαin (molar ratio=27, LHSV=LO hr"1) , using a precision and high-pressure metering pump . The reactor was heated from the outside using heating oven until the temperature reached 200°C. The methanol was evaporated from the obtained reaction solution which was obtained at 50 hours later after stabilization of the temperature and the pressure. Then, an ester phase was obtained by phase separation from a glycerin phase . The content of each component in the ester phase was as follows: fatty acid methyl esters 91.8%; monoglycerides 3.6%; diglycerides 1.2%; triglycerides 2.9%; free fatty acids 0.4%; and glycerin 0.1%. The acid value of the ester phase was 0.74 (mg-KOH/g-ester phase) .
Then, esterification reaction of free fatty acids in the ester phase by using continuous-flow fixed bed reaction system was performed as the reaction in the second stage, using a cation-exchange resin, Dowex50Wχ8. Specifically, 15 mL of H-type Dowex50Wχ8 after sufficiently washed with methanol to remove water therefrom was charged into a reaction tube made of Teflon (registered trademark) in 10 mm inside diameter and 210 mm length. To control reaction pressure, an automatic backpressure regulator was equipped at the reactor outlet . The reactor was placed into an oil bath for temperature control. The ester phase obtained in the first stage and methanol were feeding to the reactor at flow rates satisfying a ratio by weight of the ester phase/the methanol of 3.7, and LHSV of 1.0 hr"1, using a precision pump. The pressure inside the reactor was set to 0.1 MPa, and the temperature of the oil bath was set to 650C. The methanol was evaporated from the obtained reaction solutionwhichwas obtained at 24 hours later after stabilization of the temperature and the pressure. The content of each component on the ester phase was as follows: fatty acid methyl esters 92.0%; monoglycerides 3.8%; diglycerides 1.2%;
triglycerides 2.9%; free fatty acids 0.05%; and glycerin 0.05% . The acid value of the reaction solution was 0.10 (mg-KOH/g-ester phase) .
Finally, the aging reaction by using continuous-flow fixed bed reaction system was performed as the aging step, using an anion-exchange resin, HiMAC901. Into a reactormade of Teflon
(registered trademark) in 5 mm diameter and 255 mm length was charged 5 mL of OMe-type HiMAC901 after sufficiently washed with methanol to remove water . The ester phase obtained in the second stage andiαethanol were fedto the reactor at flow rates satisfying a ratio by weight of the ester phase/the methanol of 3.0, and LHSV of 1.6 hr"1, using a precision and high-pressure metering pumpunder normal pressure at 20°C . The content of each component in the reaction solution obtained 24 hours later after the beginning of the flow: fatty acid methyl esters 95.9%; rαonoglycerides 0.5%; diglycerides 0.6%; triglycerides 2.7%; and glycerin 0.3%. No free fatty acid was detected. A reaction solution equal to the liquid obtained 24 hours later was obtained 48 hours later.
Example 2
A 200 mL autoclave was filled with palm oil (61.5 g) , methanol (20.0 g) and FeTiO3 catalyst (product of Aldrich Co.) (2.5 g), as the reaction in the first stage. XRD analysis showed that the FeTiO3 catalyst used in this Example had an ilmenite structure. After nitrogen replacement, the reaction was allowed to proceed at a reaction temperature of 200°C for 24 hours with internal stirring . After completion of the reaction, the methanol was evaporated and a glycerin phase was separated from an ester phase, thereby obtaining the ester phase. The content of each component of the ester phase was as follows: fatty acid methyl esters 49.0 g; monoglycerides 8.1 g; diglycerides 1.8 g; triglycerides 0.4 g; free fatty acids 1.1 g; and glycerin 0.2 g (fatty acid methyl esters 80.9 %; monoglycerides 13.4 %; diglycerides 3.0 %; triglycerides 0.7 %;
free fatty acids 1.8 %; and glycerin 0.3 %) , and the acid value was 3.6 (mg-KOH/g-ester phase). As the aging reaction in the second stage, into a separable flask equipped with a stirrer, a condenser, and a thermometer, were charged the ester phase obtained in the reaction in the first stage (60.5 g) and methanol (20.0 g) and OH-type HiMAC901 after sufficiently washed with methanol to remove water (the content of the crosslinked HiMA was 10 mol%) (8.9 mL) . The reaction was allowed to proceed at a reaction temperature of 65°C for 24 hours with internal stirring. Thereby, the following substances were obtained: fatty acid methyl esters 57.8 g; monoglycerides 0.9 g; diglycerides 0.1 g; triglycerides 0.4 g; free fatty acids 0.1 g; and glycerin 2.3 g (fatty acid methyl esters 93.8 %; monoglycerides 1.5 %; diglycerides 0.2 %; triglycerides 0.6 %; free fatty acids 0.2 %; and glycerin 3.7 %) . After completion of the reaction, the reaction solution was separated into two phases.
Example 3
Reactions and treatments were performed in the same manner as in the reaction in the first stage in Example 2 except that palm oil (40.0 g) and methanol (40.0 g) were used, thereby obtaining an ester phase. The content of each component in the obtained ester phase was as follows: fatty acid methyl esters 37.5 g; monoglycerides 1.3 g; diglycerides 0.03 g; triglycerides 0.07 g; free fatty acids 0.13 g; and glycerin 0.03 g (fatty acid methyl esters 96.0 %; monoglycerides 3.3 %; diglycerides 0.1 %; triglycerides 0.2 %; free fatty acids 0.3 %; and glycerin 0.1 %) . The acid value was 0.8 (mg-KOH/g-ester phase) . As the aging reaction in the second stage, aging reaction was performed in the same manner as in Example 2 except that the ester phase (37.0 g) , the methanol (13.O g), and the HiMAC901 (6.OmL) were used. As a result, the following substances were obtained: fatty acid methyl esters 38.7 g; free fatty acids 0.02 g; monoglycerides 0.19 g; diglycerides 0.02 g; triglycerides 0.01 g/ and glycerin 0.33 g (fatty acid methyl esters 98.6 %; monoglycerides 0.48 %;
diglycerides 0.05 %; triglycerides 0.03 %; free fatty acids 0.05 %; and glycerin 0.84 %) . The content of intermediate glycerides after the aging reaction was 0.6%. After completion of the aging reaction, the reaction solution was separated into two phases .
Example 4
Reactions were performed in the same manner as in Example
3 except that the anion-exchange resin in the aging step was replaced with DAIAION TSA1200. After the aging reaction, the following substances were obtained: fatty acid methyl esters
38.6 g; free fatty acids 0.02 g; monoglycerides 0.22 g; diglycerides 0 g; triglycerides 0.05 g; and glycerin 0.32 g ( fatty acid methyl esters 98.4 %; monoglycerides 0.56 %; diglycerides 0 %; triglycerides 0.13 %; free fatty acids 0.05 %; and glycerin
0.82 %) . After completion of the aging reaction, the reaction solution was separated into two phases .
Example 5 DeterioratedHiMAC901 after repeatedly used formany times in the aging reaction was reactivated by the following procedures: Deteriorated HiMAC901 (after the same reactions as in Example 2 were performed, the content of each component in the reaction solution after the aging reaction were as follows: fatty acid methyl esters 50.9 g; monoglycerides 6.2 g; diglycerides 1.7 g; triglycerides 0.6 g; free fatty acids 0.31 g; and glycerin 0.7 g (fatty acid methyl esters 84.3 %; monoglycerides 10.3 %; diglycerides 2.8 %; triglycerides 1.0 %; free fatty acids 0.5 %; and glycerin 1.2 %) .) 4.5 mL was sufficiently washed with methanol, and charged into a column with a glass filter; thereinto, 2N-NaOH 750 mL was feeding at SV=70 to reproduce HiMac901; and then the HiMAC901 was sufficiently washed with ion-exchange water until waste water became neutral pH; and the HiMAC901 was washed enough with methanol. Thus-obtained HiMAC901 was used in the reaction as
reproduced HiMAC901. Reactions were performed in the same manner as in Example 2 except that the reproduced H1MAC901 was used as the catalyst in the aging step and an ester phase (30 g) and methanol (10 g) were used in the aging step. After the aging reaction, the following substances were obtained: fatty acid methyl esters 27.1 g; monoglycerides 0.8 g; diglycerides 0.8 g; triglycerides 0.2 g; free fatty acids -0.03 g; and glycerin 1.0 g (fatty acid methyl esters 90.5 %; monoglycerides 2.7 %; diglycerides 2.7 %; triglycerides 0.7 %; free fatty acids 0.1 %; and glycerin 3.3 %) . After completion of the aging reaction, the reaction solution was separated into two phases.
Comparative Example 1
As the reaction in the second stage, into a 200 mL autoclave were charged an ester phase (60.5 g) obtained after the reaction in the first stage performed in the same manner as in Example 2, methanol (20.0 g) , and FeTiO3 catalyst having an ilmenite structure (2.5 g) . After nitrogen replacement, the reaction was allowed to proceed at a reaction temperature of 2000C for 24 hours with internal stirring. The following substances were obtained: fatty acid methyl esters 55.3 g; monoglycerides 2.7 g; diglycerides 0.3 g; triglycerides 0.1 g; free fatty acids 0.1 g; and glycerin 1.8 g (fatty acid methyl esters 91.7 %; monoglycerides 4.5 %; diglycerides 0.5 %; triglycerides 0.2 %; free fatty acids 0.2 %; and glycerin 3.0 %) .
Comparative Example 2
Into a 200 mL autoclave were charged triolein (60 g) , methanol (2Og) and OH-type HiMAC 901 (8.9mL) after sufficiently washed with methanol to remove water. After nitrogen replacement, the reaction was allowed to proceed at a reaction temperature of 100°C for 24 hours with internal stirring. The yield of methyl oleate was 34%, and the yield of glycerin was 26%.
INDUSTRIAL APPLICABILITY
The method for producing fatty acid alkyl esters and/or glycerin of the present invention has the above-mentioned configuration. The production method is an industrially very useful method because the method can effectively and simply produce fatty acid alkyl esters and/or glycerin suitable for applications such as food and fuel, with low costs and high quality.
This present application claims priority on Patent Application No. 2005-259946, "METHOD FOR PRODUCING FATTYACID ALKYL ESTERS AND/OR GLYCERIN" filed in Japan on September 7, 2005, the entire contents of which are hereby incorporated by reference .
Claims
1. A method for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol; a reaction step in n stage is an aging step; and the method comprises a free fatty acids-treated step of reducing free fatty acids amount between the reaction step in the (n-1) stage and the reaction step in the n stage.
2. The method for producing the fatty acid alkyl esters and/or glycerin according to Claim 1, wherein the aging step in the n stage is performed in the presence of a base catalyst.
3. A method for producing fatty acid alkyl esters and/or glycerin comprising a step of reacting a fat or oil with an alcohol in n stages (n representing an integer of 2 or more) , wherein a reaction step in at least one stage from first stage to (n-1) stage is a step of reacting the fat or oil with the alcohol in the presence of an insoluble solid catalyst or substantially in the absence of a catalyst; and a reaction step in n stage is an aging step in the presence of a solid base catalyst.
4. The method for producing the fatty acid alkyl esters and/or glycerin according to Claim 3, wherein the method comprises a free fatty acids-treated step of reducing free fatty acids amount between the reaction step in the (n-1) stage and the reaction step in the n stage.
5. The method for producing the fatty acid alkyl esters and/or glycerin according to Claim 3 or 4, wherein the insoluble solid catalyst is a metal oxide catalyst.
6. The method for producing the fatty acid alkyl esters and/or glycerin according to any of Claims 1 to 5, wherein the reaction step in the (n-1) stage is a step of esterifying the free fatty acids contained in a reaction solution obtained in (n-2) stage.
7. The method for producing the fatty acid alkyl esters and/or glycerin according to any of Claims 1 to 6, wherein a reaction temperature in the aging step in the n stage is 180°C or less.
8. The method for producing the fatty acid alkyl esters and/or glycerin according to any of Claims 1 to 1, wherein an acid value of an ester phase used in the aging step in the n stage is controlled to 4.0 or less (mg-KOH/g-ester phase) .
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2005259946 | 2005-09-07 | ||
| JP2005-259946 | 2005-09-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2007029851A1 true WO2007029851A1 (en) | 2007-03-15 |
Family
ID=37835962
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2006/317945 WO2007029851A1 (en) | 2005-09-07 | 2006-09-05 | Method for producing fatty acid alkyl esters and/or glycerin |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2007029851A1 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010118891A1 (en) * | 2009-04-17 | 2010-10-21 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Lubrication liquid and method for producing the same |
| US20100325942A1 (en) * | 2008-02-14 | 2010-12-30 | Katal I Sverige Ab | Biofuel |
| US7897798B2 (en) | 2006-08-04 | 2011-03-01 | Mcneff Research Consultants, Inc. | Methods and apparatus for producing alkyl esters from lipid feed stocks and systems including same |
| US7943791B2 (en) | 2007-09-28 | 2011-05-17 | Mcneff Research Consultants, Inc. | Methods and compositions for refining lipid feed stocks |
| US8017796B2 (en) | 2007-02-13 | 2011-09-13 | Mcneff Research Consultants, Inc. | Systems for selective removal of contaminants from a composition and methods of regenerating the same |
| US8361174B2 (en) | 2008-10-07 | 2013-01-29 | Sartec Corporation | Catalysts, systems, and methods for producing fuels and fuel additives from polyols |
| US8445709B2 (en) | 2006-08-04 | 2013-05-21 | Mcneff Research Consultants, Inc. | Systems and methods for refining alkyl ester compositions |
| US8585976B2 (en) | 2007-02-13 | 2013-11-19 | Mcneff Research Consultants, Inc. | Devices for selective removal of contaminants from a composition |
| WO2014041553A1 (en) * | 2012-09-12 | 2014-03-20 | Savita Oil Technologies Limited | Mustard oil based insulating fluid composition and process for preparation thereof |
| US9102877B2 (en) | 2008-11-12 | 2015-08-11 | Sartec Corporation | Systems and methods for producing fuels from biomass |
| WO2016054491A1 (en) | 2014-10-03 | 2016-04-07 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9518133B2 (en) | 2009-02-06 | 2016-12-13 | Nippon Shokubai Co., Ltd. | Hydrophilic polyacrylic acid (salt) resin and manufacturing method thereof |
| US10239812B2 (en) | 2017-04-27 | 2019-03-26 | Sartec Corporation | Systems and methods for synthesis of phenolics and ketones |
| US10544381B2 (en) | 2018-02-07 | 2020-01-28 | Sartec Corporation | Methods and apparatus for producing alkyl esters from a reaction mixture containing acidified soap stock, alcohol feedstock, and acid |
| US10696923B2 (en) | 2018-02-07 | 2020-06-30 | Sartec Corporation | Methods and apparatus for producing alkyl esters from lipid feed stocks, alcohol feedstocks, and acids |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4695411A (en) * | 1985-02-15 | 1987-09-22 | Institut Francais Du Petrol | Process for manufacturing a composition of fatty acid esters useful as gas oil substitute motor fuel with hydrated ethyl alcohol and the resultant esters composition |
| JP2005206575A (en) * | 2003-08-29 | 2005-08-04 | Nippon Shokubai Co Ltd | Production method for fatty acid alkyl ester and/or glycerol and fatty acid alkyl ester-containing composition |
-
2006
- 2006-09-05 WO PCT/JP2006/317945 patent/WO2007029851A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4695411A (en) * | 1985-02-15 | 1987-09-22 | Institut Francais Du Petrol | Process for manufacturing a composition of fatty acid esters useful as gas oil substitute motor fuel with hydrated ethyl alcohol and the resultant esters composition |
| JP2005206575A (en) * | 2003-08-29 | 2005-08-04 | Nippon Shokubai Co Ltd | Production method for fatty acid alkyl ester and/or glycerol and fatty acid alkyl ester-containing composition |
Non-Patent Citations (1)
| Title |
|---|
| JOSEPH ET AL.: "Capillary Column Gas Chromatographic Method for Analysis of Encapsulated Fish Oils and Fish Oil Ethyl Esters: Collaborative Study", J. AOAC INTERNAT., vol. 75, no. 3, 1992, pages 488 - 506, XP003009729 * |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8445709B2 (en) | 2006-08-04 | 2013-05-21 | Mcneff Research Consultants, Inc. | Systems and methods for refining alkyl ester compositions |
| US8686171B2 (en) | 2006-08-04 | 2014-04-01 | Mcneff Research Consultants, Inc. | Methods and apparatus for producing alkyl esters from lipid feed stocks and systems including same |
| US7897798B2 (en) | 2006-08-04 | 2011-03-01 | Mcneff Research Consultants, Inc. | Methods and apparatus for producing alkyl esters from lipid feed stocks and systems including same |
| US8585976B2 (en) | 2007-02-13 | 2013-11-19 | Mcneff Research Consultants, Inc. | Devices for selective removal of contaminants from a composition |
| US8017796B2 (en) | 2007-02-13 | 2011-09-13 | Mcneff Research Consultants, Inc. | Systems for selective removal of contaminants from a composition and methods of regenerating the same |
| US8466305B2 (en) | 2007-09-28 | 2013-06-18 | Mcneff Research Consultants, Inc. | Methods and compositions for refining lipid feed stocks |
| US7943791B2 (en) | 2007-09-28 | 2011-05-17 | Mcneff Research Consultants, Inc. | Methods and compositions for refining lipid feed stocks |
| US8465558B2 (en) * | 2008-02-14 | 2013-06-18 | Ecotraffic Erd3 Ab | Biofuel |
| US20100325942A1 (en) * | 2008-02-14 | 2010-12-30 | Katal I Sverige Ab | Biofuel |
| US8361174B2 (en) | 2008-10-07 | 2013-01-29 | Sartec Corporation | Catalysts, systems, and methods for producing fuels and fuel additives from polyols |
| US9102877B2 (en) | 2008-11-12 | 2015-08-11 | Sartec Corporation | Systems and methods for producing fuels from biomass |
| US9518133B2 (en) | 2009-02-06 | 2016-12-13 | Nippon Shokubai Co., Ltd. | Hydrophilic polyacrylic acid (salt) resin and manufacturing method thereof |
| WO2010118891A1 (en) * | 2009-04-17 | 2010-10-21 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Lubrication liquid and method for producing the same |
| WO2014041553A1 (en) * | 2012-09-12 | 2014-03-20 | Savita Oil Technologies Limited | Mustard oil based insulating fluid composition and process for preparation thereof |
| WO2016054491A1 (en) | 2014-10-03 | 2016-04-07 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10239812B2 (en) | 2017-04-27 | 2019-03-26 | Sartec Corporation | Systems and methods for synthesis of phenolics and ketones |
| US10544381B2 (en) | 2018-02-07 | 2020-01-28 | Sartec Corporation | Methods and apparatus for producing alkyl esters from a reaction mixture containing acidified soap stock, alcohol feedstock, and acid |
| US10696923B2 (en) | 2018-02-07 | 2020-06-30 | Sartec Corporation | Methods and apparatus for producing alkyl esters from lipid feed stocks, alcohol feedstocks, and acids |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007029851A1 (en) | Method for producing fatty acid alkyl esters and/or glycerin | |
| US20090069586A1 (en) | Method for producing fatty acid alkyl esters and/or glycerin | |
| US7605281B2 (en) | Method of production of fatty acid alkyl esters and/or glycerine and fatty acid alkyl ester-containing composition | |
| US8093416B2 (en) | Method for producing fatty acid alkyl esters and/or glycerin using fat or oil | |
| Ribeiro et al. | Synthesis of methyl esters and triacetin from macaw oil (Acrocomia aculeata) and methyl acetate over γ-alumina | |
| JP2008111085A (en) | Manufacturing method of fatty acid alkyl ester and/or glycerol | |
| JP3941876B2 (en) | Process for producing fatty acid alkyl ester and / or glycerin | |
| WO2006070661A1 (en) | Method for producing ester by transesterification | |
| WO2009158379A2 (en) | Process of manufacturing of fatty acid alkyl esters | |
| JP2005206575A (en) | Production method for fatty acid alkyl ester and/or glycerol and fatty acid alkyl ester-containing composition | |
| EP2376383B1 (en) | Solid, heterogeneous catalysts and methods of use | |
| JP2007254305A (en) | Method for producing fatty acid alkyl ester and/or glycerol | |
| EP2071016B1 (en) | Process for the production of biodiesel | |
| JP5186083B2 (en) | Process for producing fatty acid alkyl ester and / or glycerin from fats and oils | |
| JP2008156576A (en) | Manufacturing process for high purity aliphatic acid alkyl ester | |
| JP2006225352A (en) | Method for producing fatty acid alkyl ester and/or glycerin | |
| JP2009108266A (en) | Method for producing fatty acid alkyl ester and/or glycerin | |
| JP2006225578A (en) | Method for producing glycerol and/or fatty acid alkyl ester | |
| JP2013072071A (en) | Method for producing fatty acid alkyl ester and/or glycerol from oil and fat | |
| JP5313482B2 (en) | Process for producing fatty acid alkyl ester and / or glycerin | |
| JPWO2008133189A1 (en) | Process for producing fatty acid alkyl ester and / or glycerin from fats and oils | |
| JP2006225353A (en) | Method for producing glycerin and/or fatty acid alkyl ester | |
| JP2008260870A (en) | Method for producing fatty acid alkyl ester and/or glycerine | |
| EP2303827B1 (en) | Process of manufacturing of fatty acid alkyl esters | |
| JP2008094657A (en) | Ilmenite formed body, its producing method and catalyst for producing biodiesel fuel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 06797771 Country of ref document: EP Kind code of ref document: A1 |