WO2006031455A2 - Lithography technique using silicone molds - Google Patents
Lithography technique using silicone molds Download PDFInfo
- Publication number
- WO2006031455A2 WO2006031455A2 PCT/US2005/031150 US2005031150W WO2006031455A2 WO 2006031455 A2 WO2006031455 A2 WO 2006031455A2 US 2005031150 W US2005031150 W US 2005031150W WO 2006031455 A2 WO2006031455 A2 WO 2006031455A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acrylate
- methacrylate
- meth
- curable
- component
- Prior art date
Links
Classifications
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/0002—Lithographic processes using patterning methods other than those involving the exposure to radiation, e.g. by stamping
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C59/00—Surface shaping of articles, e.g. embossing; Apparatus therefor
- B29C59/02—Surface shaping of articles, e.g. embossing; Apparatus therefor by mechanical means, e.g. pressing
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y10/00—Nanotechnology for information processing, storage or transmission, e.g. quantum computing or single electron logic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
- C08F220/16—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms
- C08F220/18—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms with acrylic or methacrylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
- C08F222/102—Esters of polyhydric alcohols or polyhydric phenols of dialcohols, e.g. ethylene glycol di(meth)acrylate or 1,4-butanediol dimethacrylate
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/0046—Photosensitive materials with perfluoro compounds, e.g. for dry lithography
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/027—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
- C08F220/16—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms
- C08F220/18—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms with acrylic or methacrylic acids
- C08F220/1804—C4-(meth)acrylate, e.g. butyl (meth)acrylate, isobutyl (meth)acrylate or tert-butyl (meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
- C08F220/16—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms
- C08F220/18—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms with acrylic or methacrylic acids
- C08F220/1811—C10or C11-(Meth)acrylate, e.g. isodecyl (meth)acrylate, isobornyl (meth)acrylate or 2-naphthyl (meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/22—Esters containing halogen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
- C08F222/103—Esters of polyhydric alcohols or polyhydric phenols of trialcohols, e.g. trimethylolpropane tri(meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
- C08F222/104—Esters of polyhydric alcohols or polyhydric phenols of tetraalcohols, e.g. pentaerythritol tetra(meth)acrylate
Definitions
- This invention relates to a method using a curable (meth)acrylate formulation with a silicone mold.
- the method finds use in various lithography techniques.
- Curable (meth)acrylate formulations may be cured with high resolution of the mold pattern by using a UV cure mechanism or a combination of UV and thermal cure mechanisms. Mold release may be improved by using a fluorofunctional (meth)acrylate.
- This invention relates to method a comprising:
- (Meth)acrylate means a reactant that does not contain silicon atoms and that does contain at least one group of the formula:
- Curable (Meth)acrylate Formulation [0010]
- the curable (meth)acrylate formulation suitable for use in this invention is curable by exposure to UV radiation, heat, or combinations thereof.
- the viscosity of the curable (meth)acrylate formulation may be selected depending on the desired feature size to be formed by the method of this invention. For example, when viscosity is greater than 200 cP, resolution may be 100 micrometers or more. When viscosity is 200 cP or less, resolution may be up 30 micrometers.
- the curable (meth)acrylate formulation comprises: (a) a fluorofunctional (meth)acrylate or a combination of a fluorofunctional (meth)acrylate and a (meth)acrylate and (b) a photoinitiator.
- the curable (meth)acrylate formulation comprises: (a) a (meth)acrylate, a fluorofunctional (meth)acrylate, or a combination thereof and (b) a photoinitiator.
- the curable (meth)acrylate formulation may further comprise one or more optional components selected from the group consisting of (c) an antioxidant, (d) a fluorescent dye, (e) a reactive diluent, (f) a light stabilizer, (g) a photosensitizer, (h) a wetting agent, (i) a silane, and Q) a UV absorber.
- fluorofunctional (meth)acrylates do not self associate to the extent that polar molecules do; therefore, a fluorofunctional (meth)acrylate may help retain low viscosity of the curable (meth)acrylate formulation when the fluorofunctional (meth)acrylate is added to the curable (meth)acrylate formulation. Fluorofunctional (meth)acrylates may also facilitate mold release.
- the (meth)acrylate may be monofunctional or multifunctional, or a combination thereof.
- Component (a) may comprise a monofunctional (meth)acrylate, a difunctional (meth)acrylate, a trifunctional (meth)acrylate, a tetrafunctional (meth)acrylate, a pentafunctional (meth)acrylate, or a combination thereof.
- component (a) may comprise a monofunctional (meth)acrylate, a difunctional (meth)acrylate, a trifunctional (meth)acrylate, or a combination thereof.
- the (meth)acrylate is free of fluorine atoms.
- the fluorofunctional (meth)acrylate may be monofunctional or multifunctional, or a combination thereof.
- the fluorofunctional (meth)acrylate comprises at least one fluorine atom.
- the fluorofunctional (meth)acrylate may comprise a monofunctional fluorofunctional
- Component (a) may comprise at least one fluorofunctional (meth)acrylate.
- Component (a) may comprise one or more components having the general formula:
- Q is a hydrogen atom or an organic group
- each R is independently a hydrogen atom or a methyl group
- the subscript n represents the degree of functionality. For example, when n is 1, Q is monofunctional. When n is 2, Q is difunctional. When n is 3, Q is trifunctional. When n is 4, Q is tetrafunctional. When n is 5, Q is pentafunctional. When n is 6, Q is hexafunctional.
- Q is a hydrogen atom or an organic group free of fluorine atoms
- the component is a (meth)acrylate.
- Q is an organic group containing at least one fluorine atom
- the component is a fluorofunctional (meth)acrylate.
- Monofunctional (meth)acrylates may have the general formula: O
- R where R is a hydrogen atom or a methyl group and R 1 is a monovalent organic group free of fluorine atoms.
- Monovalent organic groups for R 1 may be linear, branched, or cyclic. Examples of monovalent organic groups for R 1 include, but are not limited to, monovalent hydrocarbon groups.
- Monovalent hydrocarbon groups include, but are not limited to, alkyl groups exemplified by methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, and ethylhexyl; alkenyl groups exemplified by vinyl and allyl; cyclic hydrocarbon groups exemplified by cyclopentyl, cyclohexyl, and isobornyl.
- Examples of monovalent organic groups for R 1 further include, but are not limited to, monovalent hydrocarbonoxy functional organic groups such as alkoxy groups exemplified by methoxy, ethoxy, propoxy, and butoxy; alkoxyalkyl such as methoxymethyl, ethoxymethyl, methoxyethyl, and ethoxyethyl; alkoxyalkoxyalkyl such as methoxymethoxymethyl, ethoxyethoxymethyl, methoxymethoxyethyl, and ethoxy ethoxy ethyl.
- monovalent hydrocarbonoxy functional organic groups such as alkoxy groups exemplified by methoxy, ethoxy, propoxy, and butoxy
- alkoxyalkyl such as methoxymethyl, ethoxymethyl, methoxyethyl, and ethoxyethyl
- alkoxyalkoxyalkyl such as methoxymethoxymethyl, ethoxyethoxymethyl, methoxyme
- Examples of monofunctional (meth)acrylates include, but are not limited to, 2(2- ethoxyethoxy)ethyl acrylate, 2-acryloylethyl-2-hydroxyethyl-o-phthalate, 2- ethoxyethoxyethyl acrylate, 2-ethoxyethyl acrylate, 2-ethoxyethylmethacrylate, 2-ethylhexyl methacrylate, 2-hydroxyethyl acrylate, 2-hydroxyethyl methacrylate, 2-hydroxypropyl acrylate, 2-hydroxypropyl methacrylate, 2-methoxyethyl acrylate, 2-phenoxyethyl acrylate, 4-hydroxybutyl acrylate, acrylic acid, alkoxylated lauryl acrylate, alkoxylated phenol acrylate, alkoxylated tetrahydrofurfuryl acrylate, allyl methacrylate, benzyl acrylate, benzyl methacrylate
- Difunctional (meth)acrylates may have the general formula: O O
- each R is independently a hydrogen atom or a methyl group and R 2 is a divalent organic group free of fluorine atoms.
- divalent organic groups for R 2 include, but are not limited to, divalent hydrocarbon groups such as alkylene groups exemplified by methylene, ethylene, propylene, butylene, pentylene, hexylene, heptylene, and ethylhexylene.
- divalent organic groups for R 2 further include, but are not limited to, divalent hydrocarbonoxy functional organic groups such as groups of the formula:
- R', R" and R'" are each independently a divalent hydrocarbon group such as those described above.
- difunctional (meth)acrylates include, but are not limited to, 1,12- dodecandiol dimethacrylate, 1,3-butandiol dimethacrylate, 1,3-butylene glycol diacrylate, 1,3-butylene glycol dimethacrylate, 1 ,4-butanediol diacrylate, 1,4 butanediol dimethacrylate, 1,6-hexanediol diacrylate, 1,6-hexanediol dimethacrylate, alkoxylated aliphatic diacrylate, aliphatic dimethacrylate, bisphenol A diacrylate, bisphenol A ethoxylate dimethacrylate, butanediol dimethacrylate, diethylene glycol diacrylate, diethylene glycol dimethacrylate, dipropylene glycol diacrylate, dipropylene glycol dimethacrylate, ethoxylated bisphenol-A diacrylate, ethylene glycol diacrylate,
- Trifunctional (meth)acrylates may have the general formula: , where each R is independently a hydrogen atom or a methyl group and R 3 is a trivalent organic group free of fluorine atoms.
- Examples of trivalent organic groups for R 3 include, but are not limited to, trivalent hydrocarbon groups such as ethylyne, propylyne, and butylyne.
- Examples of trivalent organic groups for R 3 further include, but are not limited to, hydrocarbonoxy functional groups such as R 1 -C-[R' a -O-(R"b-O) c -R'" d ]- 3 , where R 1 , R', R", R'", a, b, c, and d are as described above.
- trifunctional (meth)acrylates include, but are not limited to, ethoxylated trimethylol propane triacrylate, glycelyl propoxy triacrylate, pentaerythritol triacrylate, propoxylated glycerol triacrylate, propoxylated trimethylolpropane triacrylate, triacrylate ester, trimethacrylate ester, trimethylol propane triacrylate, trimethylol propane trimethacrylate, trimethylolpropane ethoxy triacrylate, and combinations thereof.
- Other multifunctional (meth)acrylates having more than 3 (meth)acrylate containing groups may be used.
- multifunctional (meth)acrylates include, but are not limited to, tetrafunctional acrylate, acrylate ester of pentaerythritol, pentaerythritol tetraacrylate, dipentaerythritol pentaacrylate, ditrimethylolpropane tetraacrylate, ethoxylated pentaerythritol tetraacrylate, caprolactone modified dipentaerythritol hexaacrylate, caprolactone modified dipentaerythritol hexamethacrylate, and combinations thereof.
- Monofunctional, fluorofunctional (meth)acrylates may have the general formula:
- R is a monovalent organic group containing at least one fluorine atom.
- suitable monovalent organic groups for R 11 include, but are not limited to, fluorinated monovalent hydrocarbon groups such as fluorinated alkyl groups exemplified by heptadecafluorodecyl, heptafluoropentyl, nonafluorohexyl, octafluoropentyl, pentafluorobutyl, tetrafluopropyl, trifluoroethyl, and trifluoropropyl.
- R 11 may be octafluoropentyl or trifluoroethyl.
- Suitable monofunctional, fluorofunctional (meth)acrylates include, but are not limited to, heptadecafluorodecyl acrylate, octafluoropentyl acrylate, octafluoropentyl methacrylate, tetrafluopropyl acrylate, trifluoroethyl acrylate, trifluoroethyl methacrylate, and combinations thereof.
- Difunctional, fluorofunctional (meth)acrylates may have the general formula: Q O
- R R where each R is independently a hydrogen atom or a methyl group and R is a divalent organic group containing at least one fluorine atom.
- suitable divalent organic groups for R include, but are not limited to, fluorinated divalent hydrocarbon groups such as fluorinated alkylene groups exemplified by heptadecafluorodecylene, heptafluoropentylene, nonafluorohexylene, octafluoropentylenee, pentafluorobutylene, tetrafluopropylene, trifluoroethylene, and trifluoropropylene.
- Trifunctional, fluorofunctional (meth)acrylates may have the general formula:
- R 31 is a trivalent organic group containing at least one fluorine atom.
- suitable trivalent organic groups for R include, but are not limited to, fluorinated trivalent hydrocarbon groups such as fluorinated alkylyne groups exemplified by heptadecafluorodecylyne, heptafluoropentylyne, nonafluorohexylyne, octafluoropentylyne, pentafluorobutylyne, tetrafluopropylyne, trifluoroethylyne, and trifluoropropylyne.
- Suitable fluorofunctional (meth)acrylates and (meth)acrylates for component (a) are known in the art and commercially available from, for example, Osaka Organic Chemical Industry LTD; Rohm Monomers of Europe; Sartomer Company, Inc., of Lancaster, Pennsylvania, U.S.A.; and The UCB Group of Belgium.
- the amount of component (a) may range from 90 to 99.5% based on the weight of the curable (meth)acrylate formulation.
- the amount of (meth)acrylate may range from 0 to 75%, based on the weight of the curable (meth)acrylate formulation.
- the amount of fluorofunctional (meth)acrylate may range from 0 to 99.5%, alternatively 25 to 99.5%, alternatively 20 to 90%, based on the weight of the curable (meth)acrylate formulation.
- the amount of fluorofunctional (meth)acrylate in the curable (meth)acrylate formulation may be sufficient to provide at least 0.5% fluorine at the surface of a feature prepared by molding the curable (meth)acrylate formulation.
- Component (b) is a photoinitiator.
- the amount of component (b) is sufficient to promote cure of the curable (meth)acrylate formulation and depends on the type of photoinitiator selected and the ingredients in component (a). However, the amount of component (b) may range from 0.5 to 10% based on the weight of the curable (meth)acrylate formulation. When a free radical photoinitiator is used, the amount may range from 0.01 to 5%, alternatively 0.1 to 2%, based on the total weight of the curable (meth)acrylate formulation.
- Component (b) may comprise a free radical photoinitiator exemplified by benzoins (e.g., benzoin alkyl ethers), benzophenone and its derivatives (e.g., 4,4'-dimethyl-amino- benzophenone), acetophenones (e.g., dialkoxyacetophenones, dichloroacetophenones, and trichloroacetophenones), benzils (e.g., benzil ketals), quinones, and O-acylated-.alpha.- oximinoketones.
- benzoins e.g., benzoin alkyl ethers
- benzophenone and its derivatives e.g., 4,4'-dimethyl-amino- benzophenone
- acetophenones e.g., dialkoxyacetophenones, dichloroacetophenones, and trichloroacetophenones
- the free radical photoinitiator may comprise a compound represented by the following structural formula: , where R4 is a hydrogen atom, an alkoxy group, a substituted alkoxy group, or a halogen atom; R5 is a hydroxyl group, an alkoxy group, a substituted alkoxy group, or a halogen atom; and R6 is a hydrogen atom, an alkyl group, a substituted alkyl group, an aryl group, a substituted aryl group, or a halogen atom.
- R4 may be a methyl group
- R5 may be a hydroxyl group
- R6 may be a methyl group or a phenyl group.
- R4 is a hydrogen atom
- R5 is an alkoxy group
- R6 is a phenyl group.
- R4 and R5 are each independently an alkoxy group and R6 is a hydrogen atom.
- R4 and R5 are each a chlorine atom and R6 is a chlorine atom or a hydrogen atom.
- Suitable photoinitiators are known in the art and are commercially available.
- photoinitiator examples include, but are not limited to, alpha-hydroxy ketone; phenylglyoxylate; benzildimethyl-ketal; alpha-aminoketone; mono acyl phosphine; bis acyl phosphine; benzoin ether; benzoin isobutyl ether; benzoin isopropyl ether; benzophenone; benzoylbenzoic acid; methyl benzoylbenzoate; 4-benzoyl-4'-methyldiphenyl sulfide; benzylmethylketal; 2-n-butoxyethyl-4-dimethylaminobenzoate; 2-chlorothioxanthone; 2,4- diethylthioxanthanone; 1-hydroxy-cyclohexyl-phenyl-ketone (Ciba® IRGACURE® 184 from Ciba Specialty Chemicals, Inc.
- Ciba® IRGACURE® 184 from Ciba Special
- Ciba® IRGACURE® 819 also from Ciba Specialty Chemicals, Inc.
- a combination of bis(2,6- dimethoxybenzoyl)-2,4,4-trimethyl-pentylphosphineoxide and 1-hydroxy-cyclohexyl-phenyl- ketone (Ciba® IRGACURE® 1800 also from Ciba Specialty Chemicals, Inc.); 2-hydroxy-2- methyl-1-phenyl-propan-l-one (Ciba® DAROCUR® 1173 also from Ciba Specialty Chemicals, Inc.); 2-benzyl-2-dimethylamino-l-(4-morpholinophenyl)-butanone-l (Ciba® IRGACURE® 369 also from Ciba Specialty
- the curable (meth)acrylate formulation may further comprise an optional component.
- optional components include, but are not limited to, (c) an antioxidant, (d) a fluorescent dye, (e) a reactive diluent, (f) a light stabilizer, (g) a photosensitizer, (h) a wetting agent, (i) a silane, and (j) a UV absorber.
- Component (c) Antioxidant [0032] Component (c) is an antioxidant that may be optionally added to the curable (meth)acrylate formulation. The amount of component (c) may be up to 1% based on the weight of the curable (meth)acrylate formulation. Suitable antioxidants are known in the art and commercially available.
- Suitable antioxidants include phenolic antioxidants and combinations of phenolic antioxidants with stabilizers.
- Phenolic antioxidants include fully sterically hindered phenols and partially hindered phenols.
- Stabilizers include organophosphorous derivatives such as trivalent organophosphorous compound, phosphites, phosphonates, and a combination thereof; thiosynergists such as organosulfur compounds including sulfides, dialkyldithiocarbamate, dithiodipropionates, and a combination thereof; and sterically hindered amines such as tetramethyl-piperidine derivatives.
- Suitable antioxidants and stabilizers are disclosed in Zweifel, Hans, "Effect of Stabilization of Polypropylene During Processing and Its Influence on Long-Term Behavior under Thermal Stress," Polymer Durability, Ciba-Geigy AG, Additives Division, CH-4002, Basel, Switzerland, American Chemical Society, vol. 25, pp. 375-396, 1996.
- Suitable phenolic antioxidants include vitamin E and IRGANOX® 1010 also from Ciba Specialty Chemicals, Inc.
- IRGANOX® 1010 comprises pentaerythriol tetrakis(3-(3,5- di-t-butyl-4-hydroxyphenyl)propionate) .
- the curable (meth)acrylate formulation may comprise: 90 to 99.5% component (a), 0.5 to 10% component (b), and 0 to 1% component (c).
- Component (d) is a fluorescent dye that may optionally be added to the curable (meth)acrylate formulation.
- fluorescent dyes include but are not limited to rhodamine 6G, 2,2'-(2,5 thio ⁇ henediyl)bis-[(tert)butylbenzoxazole] UVITEX OB from Ciba Specialty Chemicals, Inc. of Tarrytown, New York 10591, U.S.A.
- the amount of component (d) used may be 0 to 1% based on the total amount of curable (meth)acrylate formulation.
- Component (e) is a reactive diluent that does not contain a (meth)acrylate.
- component (e) is governed by many factors such as the solubility and miscibility of the components in the curable (meth)acrylate formulation, the method of using the curable (meth)acrylate formulation, and safety and environmental regulations.
- suitable reactive diluents include, but are not limited to, maleic anhydrides, vinyl acetates, vinyl ester, vinyl ethers, fluoro alkyl vinyl ethers, vinyl pyrrolidones such as N-vinyl pyrrolidone, styrene, and combinations thereof.
- Suitable vinyl ethers include, but are not limited to butanediol divinyl ether, cyclohexanedimethanol divinyl ether, cyclohexanedimethanol monovinyl ether, cyclohexyl vinyl ether, diethyleneglycol divinyl ether, diethyleneglycol monovinyl ether, dodecyl vinyl ether, ethyl vinyl ether, hydroxybutyl vinyl ether, isobutyl vinyl ether, isopropyl vinyl ether, n-butyl vinyl ether, n-propyl vinyl ether, octadecyl vinyl ether, triethyleneglycol divinyl ether, and combinations thereof.
- Vinyl ethers are known in the art and commercially available from BASF AG of Germany.
- the amount of component (e) used may be 0 to 1% based on the total amount of curable (meth)acrylate formulation.
- Component (f) is known in the art
- Component (f) is a light stabilizer that may optionally be added to the curable (meth)acrylate formulation.
- suitable light stabilizers include, but are not limited to, decanedioic acid, bis(2,2,6,6-tetramethyl-l-(octyloxy)-4-piperidinyl) ester, reaction products with 1,1-dimethylethylhydroperoxide and octane, which is commercially available as Ciba® TINUVTN® 123 from Ciba Specialty Chemicals, Inc. of Tarrytown, New York 10591, U.S.A.
- component (f) used may be 0 to 1% based on the total amount of curable (meth)acrylate formulation.
- Component (g) [0038] Component (g) photosensitizer that may optionally be added to the curable (meth)acrylate formulation in addition to or instead of component (b). Component (g) changes the wavelength of radiation required to cure the curable (meth)acrylate formulation.
- One skilled in the art would be able to select appropriate photosensitizers without undue experimentation based on the specific (meth)acrylates and fluorofunctional (meth)acrylates selected for component (a).
- Component (g) may comprise a ketone, coumarin dye, xanthene dye, acridine dye, thiazole dye, thiazine dye, oxazine dye, azine dye, aminoketone dye, porphyrin, aromatic polycyclic hydrocarbon, p-substituted aminostyryl ketone compound, aminotriaryl methane, merocyanine, squarylium dye, pyridinium dye, or combination thereof.
- component (g) examples include, but are not limited to rose bengal, camphorquinone, glyoxal, biacetyl, 3,3,6,6-tetramethylcyclohexanedione, 3,3,7,7-tetramethyl-l,2- cycloheptanedione, S ⁇ j ⁇ j ⁇ -tetramethyl-l ⁇ -cyclooctanedione, 3,3,18,18-tetramethyl-l,2- cyclooctadecanedione, dipivaloyl, benzil, furil, hydroxybenzil, 2,3-butanedione, 2,3- pentanedione, 2,3-hexanedione, 3,4-hexanedione, 2,3-heptanedione, 3,4-heptanedione, 2,3- octanedione, 4,5-octanedione, 1,2-cyclohexanedione
- component (g) may comprise 2- isopropylthioxanthone or benzophenone or a combination thereof.
- the amount of component (g) used may be 0 to 2%, alternatively 0.01 to 2%, and alternatively 0.05 to 0.5% based on the total amount of curable (meth)acrylate formulation.
- Component (h) is a wetting agent that may optionally be added to the curable (meth)acrylate formulation.
- component (h) include, but are not limited to silicone diacrylate, which is commercially available as EBECRYL® 350 from UCB Chemicals of Belgium; silicone hexaacrylate, which is commercially available as EBECRYL® 1360 also from UCB Chemicals; polyether modified polydimethylsiloxanes, which are commercially available as BYK®-307, BYK®-UV 3510, and BYK®-333 from BYK-Chemie GmbH of Germany; polyether modified acryl functional polydimethylsiloxane, which is commercially available as BYK®-UV 3500, also from BYK-Chemie GmbH; and polyacrylic copolymer, which is commercially available as BYK®-381 also from BYK- Chemie GmbH; crosslinkable silicone acrylates, which are commercially available as Rad 2100, Rad 2500, Rad 2600
- Component (i) is an silane that may optionally be added to the curable (meth)acrylate formulation.
- component (i) include, but are not limited to alkoxysilanes such as glycidoxypropyltriethoxysilane, glycidoxypropyltrimethoxysilane, methacryloxypropyltriethoxysilane, methacryloxypropyltrimethoxysilane, tetraethoxysilane, tetramethoxysilane, vinyltriethoxysilane, vinyltrimethoxysilane, and combinations thereof.
- alkoxysilanes such as glycidoxypropyltriethoxysilane, glycidoxypropyltrimethoxysilane, methacryloxypropyltriethoxysilane, methacryloxypropyltrimethoxysilane, tetraethoxysilane, tetramethoxysilane, vinyltrieth
- the amount of component (i) used may be 0 to 2% based on the total amount of curable
- Component (j) is a UV absorber that may optionally be added to the curable
- component (j) examples include, but are not limited to l-methoxy-2-propanol and 1,3-benzenediol, 4-[4,6-bis(2,4- dimethylphenyl) - l,3,5-triazin-2-yl]- reaction products with [(dodecyloxy)methyl]oxirane and oxirane mono[(C10-16 alkyloxy)methyl derivatives, which is commercially available as TINUVrN® 400 from Ciba Specialty Chemicals, Inc. of Tarrytown, New York 10591,
- the amount of component (j) used may be 0 to 1 % based on the total amount of curable (meth)acrylate formulation.
- This invention relates to a molding method.
- This invention may be used in various lithography techniques, such as soft lithography techniques.
- soft lithography a mold may be prepared by replica molding, in which a curable silicone composition is cast against a master that has a patterned relief structure on its surface.
- An example of a curable silicone composition suitable for this purpose is SYLGARD® 184, which is commercially available from Dow Corning Corporation of Midland, Michigan, U.S.A.
- the curable silicone composition is then cured and removed from the master.
- the resulting product is a silicone mold having a patterned surface.
- the method of this invention comprises:
- the method may optionally further comprise: I) casting a curable silicone composition against a master,
- Step A) may be performed by various methods.
- step A) may be performed by contacting the patterned surface of the silicone mold with a substrate, such that patterned structures in the patterned surface form a network of empty channels.
- the curable (meth)acrylate formulation may be placed at open ends of the network, capillary action fills the channels with the curable (meth)acrylate formulation.
- the curable (meth)acrylate formulation may be applied to the patterned surface before contacting the patterned surface with a substrate.
- the curable (meth)acrylate formulation may be applied to a surface of a substrate before the patterned surface is contacted with the substrate.
- the mold may be sprayed with some or all of the fluorofunctional (meth)acrylate before the remaining components of the curable (meth)acrylate formulation are combined and filled in the silicone mold.
- the mold may be sprayed with a fluorofunctional surfactant before the curable (meth)acrylate formulation is filled in the silicone mold.
- Step B) may be performed by exposing the curable (meth)acrylate formulation to UV radiation, by heating the curable (meth)acrylate formulation, or a combination thereof.
- the exposure dose depends on the specific curable (meth)acrylate formulation selected and the configuration of the mold, however, exposure may be 100 milliJoule to 4000 milliJoule.
- the temperature to which the composition is heated also depends on the specific (meth)acrylate formulation selected, however the temperature may be 50 0 C to 200 0 C, alternatively 100 0 C to 120 0 C.
- Step C) may be performed by any convenient means such as removing the silicone mold from the patterned feature by, for example, manually peeling the silicone mold off the patterned feature or automatically using, for example, a micromolding tool from SUSS MicroTec, Inc. of Indianapolis, Indiana 46204, U.S.A.
- Step D) may be performed by techniques known in the art, for example, reactive ion etching or wet etching. In some lithography techniques, such as imprint molding, solid may form on a substrate in undesired areas during step B). Etching may be used to remove this excess solid, or to remove layers under the excess solid, or both.
- This invention may be used in various lithography techniques.
- lithography techniques include, but are not limited to, imprint molding, step and flash imprint molding, solvent assisted micromolding (SAMBVI), microtransfer molding, and micromolding in capillaries (MIMIC).
- SAMBVI solvent assisted micromolding
- MIMIC micromolding in capillaries
- This invention may be used for imprint molding.
- the curable (meth)acrylate formulation is applied on a surface of a substrate.
- the patterned surface of the silicone mold is brought into contact with the surface of the substrate, thereby distributing the curable (meth)acrylate formulation in the silicone mold.
- the curable (meth)acrylate formulation is then cured to a solid, and the silicone mold is removed.
- Imprint molding may be used to prepare, for example, photodetectors and quantum-wire, quantum- dot, and ring transistors.
- This invention may also be used in SAMEVI.
- the curable (meth)acrylate formulation is applied on a surface of a substrate.
- a patterned surface of a silicone mold is wetted with a solvent and is brought into contact with the surface of the curable (meth)acrylate formulation.
- the choice of solvent depends on various factors including the specific silicone mold and curable (meth)acrylate formulation selected; the solvent should rapidly dissolve or swell the surface of the curable (meth)acrylate formulation but not swell the silicone mold.
- the curable (meth)acrylate formulation is then cured to a solid, and the silicone mold is removed.
- This invention may be used in microtransfer molding, in which a curable (meth)acrylate formulation described above is applied to the patterned surface of the silicone mold. If any excess curable (meth)acrylate formulation is present, it may be removed, for example, by scraping with a flat block or by blowing with stream of inert gas. The resulting filled mold may be contacted with a substrate. The curable (meth)acrylate formulation is then cured by heating, exposure to UV radiation, or a combination thereof. When the curable (meth)acrylate formulation has cured to a solid, the mold may be peeled away to leave a patterned feature on the substrate.
- Microtransfer molding may be used to fabricate, for example, optical waveguides, couplers, and interferometers.
- This invention may also be used for MIMIC.
- the patterned surface of the silicone mold is contacted with a surface of a substrate.
- the patterned structures in the silicone mold form a network of empty channels.
- capillary action fills the channels with the curable (meth)acrylate formulation.
- the curable (meth)acrylate formulation is then cured to a solid, and the silicone mold is removed.
- the method may be used to prepare a resist layer or a permanent layer in a lithography technique selected from the group consisting of imprint molding, step and flash imprint molding, solvent assisted micromolding, microtransfer molding, and micromolding in capillaries.
- This invention may be used during fabrication of various devices, including but not limited to light emitting diodes, including but not limited to organic light emitting diodes; transistors such as organic field effect transistors and thin film transistors; display devices such as plasma displays and liquid crystal displays, photodetectors, optical waveguides, couplers, and interferometers. Examples
- the formulations are mixed in a Hauschild mixer by adding the amounts of components as defined in the examples below.
- Viscosity is measured with Cannon-Fenske routine (Ubbelohde) viscometer tubes from International Research Glassware, Kenilworth, New Jersey, 07033 U.S.A.
- the method for viscosity measurement is according to ASTM D 445 and ISO 3104. Specifications conform to ASTM D 446 and ISO 3105.
- the cure studies on thick films are performed on a Fusion curing processor (300 or 600 Watt lamps).
- a coating of the formulation is applied to one of the following substrates: glass slide, silicon wafer, glass wafer, or plastic such as acrylic substrate.
- the coating is applied manually or by using a roll coater.
- the substrate is conveyed through the Fusion curing processor at a fixed line speed, and adjusting belt speeds controlled cure energy.
- An IL 1350 radiometer/photometer (from International Lights) is used to monitor the UV light flux at the sample.
- the extent of cure is measured by observing surface tack (dry to touch) immediately after UV light curing. Through cure is evaluated by removing the cured film from the substrate and evaluating tack at the bottom.
- UV cure studies are performed as per the following procedures.
- the formulation can be cured both in air (under PDMS mold) and in Argon atmosphere (either under PDMS mold or without PDMS mold) to ensure absence of any oxygen inhibition effects.
- the formulation and substrates are transported in an Ar glove box first.
- the formulation is dispersed on a substrate by spin coating.
- a spin speed of 500 - 2000 rpm is used to spread the formulation.
- the resulting film is transferred into a container and sealed under vacuum for taking to the UV cure tool, either with a PDMS or without a PDMS mold on top of the film.
- the UV exposure tool has N 2 knife edge for help purging O 2 .
- the film surface is covered with a cover glass to prevent contamination with particles.
- the UV exposure is set around 500 mJ/cm 2 .
- the film is sent back to the Argon glove box for further thermal cure at 120 0 C for two minute to increase cross-linking density.
- the PDMS mold is released from the cured acrylate film surface.
- a pattern transfer from the PDMS mold onto the cured acrylate film surface is observed using visual inspection, optical microscopy, and electron microscopy. Air Atmosphere
- the formulation is dispensed on a substrate by spin coating or doctor blade drawdown technique.
- spin coating a spin speed of 500 - 2000 rpm is used to spread the formulation into a film.
- a SYLGARD® 184 PDMS mold is placed on top of the film.
- the film with the mold is sent to the UV cure tool for curing.
- UV cure the mold is released from the cured film.
- a pattern transfer is accomplished from the mold surface to the film surface.
- the film under the PDMS mold is cured and the area not under PDMS mold was not cured.
- a pattern transfer from the PDMS mold onto the cured film surface is observed using visual inspection, optical microscopy, and electron microscopy.
- Comparative Example 1 A curable (meth)acrylate formulation is prepared by mixing the following components.
- a curable (meth)acrylate formulation is prepared by mixing the following components.
- the formulation is cured under UV exposure. However, the cured film sticks to a
- a curable (meth)acrylate formulation is prepared by mixing the following components
- a curable (meth)acrylate formulation is prepared by mixing the following components
- a curable (meth)acrylate formulation is prepared by mixing the following components.
- a curable (meth)acrylate formulation is prepared by mixing the following components. ,
- a curable (meth)acrylate formulation is prepared by mixing the following components.
- a curable (meth)acrylate formulation is prepared by mixing the following components.
- a curable (meth)acrylate formulation is prepared by mixing the following components.
- a curable (meth)acrylate formulation is prepared by mixing the following components.
- Curable (meth)acrylate formulations are prepared by mixing the components in the amounts shown in the table.
- Amounts in the table of 1,4-butanediol diacrylate, dipropyleneglycol diacrylate, isobomyl acrylate, ethoxyethoxyethylacrylate, trimethylolpropane triacrylate, tetraethoxysilane, and methacryloxypropyltrimethoxysilane are mixed for 30 minutes.
- Acrylic acid in the amount in the table is added, and the resulting composition is mixed for another 30 minutes.
- Water in the amount in the table is added, and the resulting composition is mixed for 60 minutes.
- the resulting composition is stripped at 70 0 C under reduced pressure to produce a composition containing resin formed in situ.
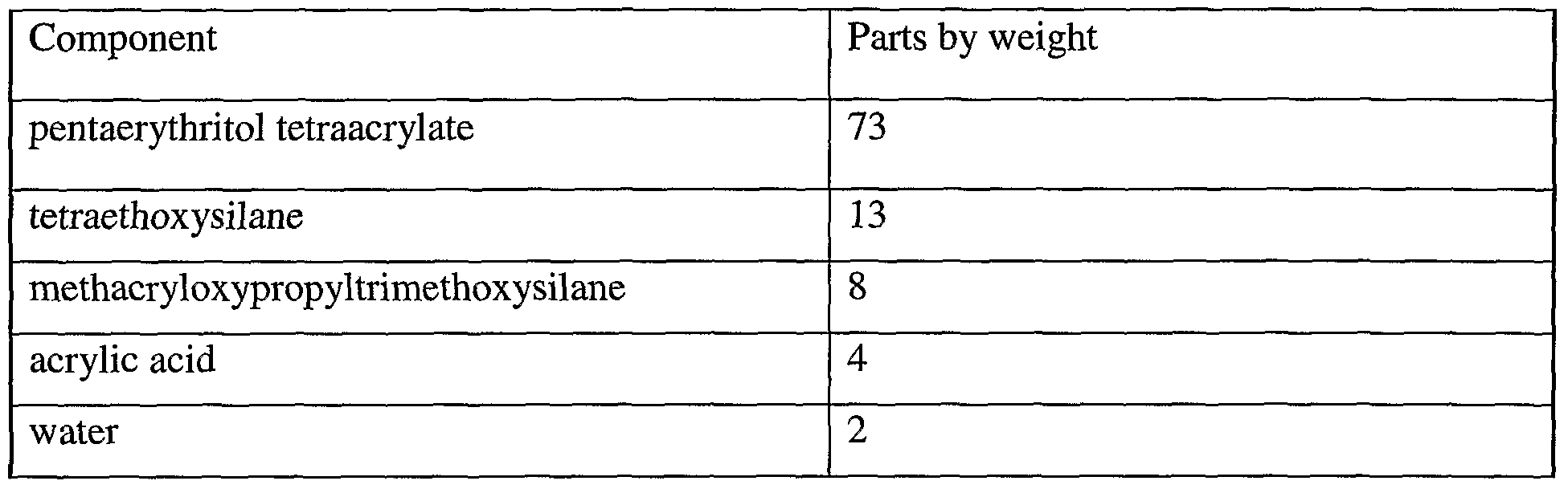
- Curable (meth)acrylate formulations are prepared by mixing the components in the amounts shown in the table below.
- Amounts in the table of pentaerythritol tetraacrylate and acrylic acid are mixed for 30 minutes. Water in the amount in the table is added, and the resulting composition is mixed for 60 minutes. The resulting composition is stripped at 70 0 C under reduced pressure to produce a composition containing resin formed in situ.
- Curable (meth)acrylate formulations are prepared by mixing the components in the amounts shown in the table below.
- curable (meth)acrylate formulations used in these examples demonstrate pattern resolution and mold release properties. Without wishing to be bound by theory, it is thought that transfer of monomers from the curable (meth)acrylate formulation to the mold is minimized by the presence of the fluorofunctional (meth)acrylate, and this increases mold life by decreasing mold fouling and swelling of the mold. This process may provide a lower cost alternative to photolithographic methods for providing a patterned coating or resist by increasing throughput, decreasing process time, or both.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Nanotechnology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Theoretical Computer Science (AREA)
- Mathematical Physics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Manufacturing & Machinery (AREA)
- Mechanical Engineering (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Moulds For Moulding Plastics Or The Like (AREA)
- Casting Or Compression Moulding Of Plastics Or The Like (AREA)
- Macromonomer-Based Addition Polymer (AREA)
- Polymerisation Methods In General (AREA)
- Shaping Of Tube Ends By Bending Or Straightening (AREA)
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05793402A EP1803033A2 (en) | 2004-09-13 | 2005-08-31 | Lithography technique using silicone molds |
| US11/659,989 US20070269747A1 (en) | 2004-09-13 | 2005-08-31 | Lithography Technique Using Silicone Molds |
| CN2005800306251A CN101019074B (en) | 2004-09-13 | 2005-08-31 | Lithography technique using silicone molds |
| KR1020077005858A KR101237766B1 (en) | 2004-09-13 | 2005-08-31 | Lithography technique using silicone molds |
| JP2007531232A JP2008512281A (en) | 2004-09-13 | 2005-08-31 | Lithographic techniques using silicone molds |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US60942504P | 2004-09-13 | 2004-09-13 | |
| US60/609,425 | 2004-09-13 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2006031455A2 true WO2006031455A2 (en) | 2006-03-23 |
| WO2006031455A3 WO2006031455A3 (en) | 2006-10-26 |
Family
ID=35539401
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2005/031150 WO2006031455A2 (en) | 2004-09-13 | 2005-08-31 | Lithography technique using silicone molds |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20070269747A1 (en) |
| EP (1) | EP1803033A2 (en) |
| JP (2) | JP2008512281A (en) |
| KR (1) | KR101237766B1 (en) |
| CN (1) | CN101019074B (en) |
| WO (1) | WO2006031455A2 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008155344A (en) * | 2006-12-26 | 2008-07-10 | Hitachi Chem Co Ltd | Liquid photopolymerizable composition, nano-structure with same and manufacturing method therefor |
| WO2008106245A2 (en) * | 2007-02-12 | 2008-09-04 | Dow Corning Corporation | Method of forming soft lithographic molds with fluorine modified elastomers |
| JP2010258026A (en) * | 2009-04-21 | 2010-11-11 | Jsr Corp | Photo-curing composition for nanoimprint lithography, and nanoimprinting method |
| JP2011511722A (en) * | 2008-01-29 | 2011-04-14 | エルジー・ケム・リミテッド | Manufacturing method of viewing angle limiting film |
| US8025833B2 (en) | 2008-02-12 | 2011-09-27 | Fujifilm Corporation | Curable composition for nanoimprint, and patterning method |
| CN102393600A (en) * | 2011-10-27 | 2012-03-28 | 南京大学 | Preparation method of nano-imprinting composite template |
| US8173225B2 (en) * | 2006-11-30 | 2012-05-08 | Lg Display Co., Ltd. | Photocurable organic material and method of fabricating array substrate for liquid crystal display device using the same |
| US8735481B2 (en) | 2008-05-01 | 2014-05-27 | Roller Bearing Company Of America, Inc. | Self-lubricating surface coating composition for low friction or soft substrate applications |
| US8741996B2 (en) | 2008-05-01 | 2014-06-03 | Roller Bearing Company Of America, Inc. | Self-lubricating surface coating composition |
| US8822125B2 (en) | 2006-05-24 | 2014-09-02 | Lg Display Co., Ltd. | Composition for forming pattern and in-plane printing method using the same |
| WO2016043263A1 (en) * | 2014-09-19 | 2016-03-24 | 横浜ゴム株式会社 | Ultraviolet light curable resin composition and laminate using same |
Families Citing this family (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9040090B2 (en) | 2003-12-19 | 2015-05-26 | The University Of North Carolina At Chapel Hill | Isolated and fixed micro and nano structures and methods thereof |
| EP1704585B1 (en) | 2003-12-19 | 2017-03-15 | The University Of North Carolina At Chapel Hill | Methods for fabricating isolated micro- and nano- structures using soft or imprint lithography |
| JP5264113B2 (en) * | 2007-07-13 | 2013-08-14 | 旭化成イーマテリアルズ株式会社 | Photocurable resin composition, molded article, and method for producing molded article |
| US7891636B2 (en) * | 2007-08-27 | 2011-02-22 | 3M Innovative Properties Company | Silicone mold and use thereof |
| KR100929381B1 (en) * | 2007-11-22 | 2009-12-02 | 주식회사 미뉴타텍 | Mold sheet composition and mold sheet manufacturing method using the same |
| KR101401488B1 (en) * | 2008-01-10 | 2014-06-03 | 동우 화인켐 주식회사 | Colored photosensitive resin composition, and color filter and liquid crystal display device prepared by using the same |
| CN102037407B (en) | 2008-04-25 | 2014-01-01 | 西北大学 | Polymer pen lithography |
| JP5306903B2 (en) * | 2008-07-02 | 2013-10-02 | 富士フイルム株式会社 | Curable composition for imprint, cured product using the same, method for producing the same, and member for liquid crystal display device |
| JP2010113170A (en) * | 2008-11-07 | 2010-05-20 | Fujifilm Corp | Curable composition for optical imprint, cured product using the same, method for producing cured product, and member for liquid crystal display |
| JP2010118434A (en) * | 2008-11-12 | 2010-05-27 | Fujifilm Corp | Curable composition for optical nano-imprint, and cured material and manufacturing method for the same |
| JP5968933B2 (en) * | 2008-12-03 | 2016-08-10 | 富士フイルム株式会社 | Curable composition for imprint, pattern forming method and pattern |
| JP2010157706A (en) * | 2008-12-03 | 2010-07-15 | Fujifilm Corp | Curable composition for optical imprint and method of manufacturing hardened material using same |
| US9978479B2 (en) | 2009-02-26 | 2018-05-22 | Corning Incorporated | Electrically isolating polymer composition |
| US20100215968A1 (en) * | 2009-02-26 | 2010-08-26 | Fields Lenwood L | Electrically isolating polymer composition |
| JP5263560B2 (en) * | 2009-08-25 | 2013-08-14 | 日産化学工業株式会社 | High hardness imprint material |
| JP5397941B2 (en) * | 2009-08-31 | 2014-01-22 | 日油株式会社 | Fluorescent resist composition and use thereof |
| US8753813B2 (en) | 2009-12-07 | 2014-06-17 | Northwestern University | Generation of combinatorial patterns by deliberate tilting of a polymer-pen array |
| US20110160321A1 (en) | 2009-12-30 | 2011-06-30 | Steven Ray Merrigan | Reduction of unpolymerized monomers in high internal phase emulsion foam |
| JP5762245B2 (en) * | 2010-10-20 | 2015-08-12 | 株式会社トクヤマ | Composition for photo-curable nanoimprint, pattern formation method using the composition, and replica mold for nanoimprint having a cured product of the composition |
| BRPI1005182A2 (en) * | 2010-12-10 | 2013-04-02 | 3M Innovative Properties Co | process for producing one adhesive and process for joining two parts per adhesive |
| EP2500009A1 (en) | 2011-03-17 | 2012-09-19 | 3M Innovative Properties Company | Dental ceramic article, process of production and use thereof |
| JP5679445B2 (en) * | 2011-06-14 | 2015-03-04 | 信越化学工業株式会社 | Concave and convex pattern forming method |
| JP6008628B2 (en) * | 2011-07-19 | 2016-10-19 | 株式会社トクヤマ | Pattern production method using photocurable nanoimprinting composition |
| CN103159889B (en) * | 2011-12-17 | 2015-11-25 | 清华大学 | Interpenetrating net polymer and preparation method thereof |
| WO2014104074A1 (en) | 2012-12-28 | 2014-07-03 | 東洋合成工業株式会社 | Curable resin composition, resin mold for imprinting, light imprinting method, production method for semiconductor integrated circuit, and production method for micro-optical element |
| JP6534347B2 (en) * | 2013-03-04 | 2019-06-26 | 東洋合成工業株式会社 | Composition, resin mold, optical imprint method, method of manufacturing optical element, and method of manufacturing electronic element |
| US9470395B2 (en) | 2013-03-15 | 2016-10-18 | Abl Ip Holding Llc | Optic for a light source |
| US10807329B2 (en) | 2013-05-10 | 2020-10-20 | Abl Ip Holding Llc | Silicone optics |
| CN103246164A (en) * | 2013-06-04 | 2013-08-14 | 苏州太速雷电子科技有限公司 | Photosensitive resin for stereo lithography forming and preparation method thereof |
| CN103578353A (en) * | 2013-11-13 | 2014-02-12 | 无锡英普林纳米科技有限公司 | Method for manufacturing gradient-gradual-change double-layer-system material and application in anti-counterfeiting identification |
| EP3136483B1 (en) * | 2014-04-21 | 2018-08-01 | FUJIFILM Wako Pure Chemical Corporation | Binder for lithium cell |
| JP6352742B2 (en) * | 2014-09-11 | 2018-07-04 | 東芝メモリ株式会社 | Photosensitive composition, imprint method and interlayer |
| TWI645252B (en) * | 2014-12-25 | 2018-12-21 | 日商富士軟片股份有限公司 | Photocurable composition for imprint, pattern forming method, and element manufacturing method |
| JP6669432B2 (en) * | 2015-02-05 | 2020-03-18 | 旭化成株式会社 | Alignment method, imprint method, and imprint apparatus |
| US10459337B2 (en) | 2015-12-14 | 2019-10-29 | University Of Maryland, College Park | Multicolor photolithography materials and methods |
| GB201715588D0 (en) * | 2017-09-26 | 2017-11-08 | Belron Int Ltd | Curing repair resin |
| EP4119588A4 (en) * | 2020-03-10 | 2024-04-24 | Toyo Gosei Co., Ltd. | Photocurable resin composition for imprint molding, resin mold, method for forming pattern using said resin mold, composite material having said resin mold, method for producing said composite material, and method for producing optical member |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5292927A (en) * | 1992-02-26 | 1994-03-08 | The United States Of America As Represented By The Secretary Of The Navy | Fluorinated resins with low dielectric constant |
| EP0832936A1 (en) * | 1996-09-25 | 1998-04-01 | Shin-Etsu Chemical Co., Ltd. | Photo-curable liquid silicone rubber compositions for templating mother molds |
| US20010034458A1 (en) * | 1998-01-20 | 2001-10-25 | Alliedsignal Inc. | Polymerizable halogenated vinyl ethers |
| WO2003072625A1 (en) * | 2002-02-28 | 2003-09-04 | Luvantix Co., Ltd. | Photocurable resin composition for optical waveguide and optical waveguide made of the same |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2727424B1 (en) * | 1994-11-30 | 1996-12-20 | Atochem Elf Sa | PHOTORETICULABLE COMPOSITIONS BASED ON TRIFLUOROETHYL METHACRYLATE AND PROCESSES FOR THEIR PREPARATION |
| US6262214B1 (en) * | 1996-10-29 | 2001-07-17 | Mitsubishi Rayon Co., Ltd. | Lowly birefringent polymer, process for the production thereof, and optical pickup lens |
| JP2002184719A (en) * | 2000-12-19 | 2002-06-28 | Matsushita Electric Ind Co Ltd | Method of forming pattern |
| CN1490400A (en) * | 2002-10-17 | 2004-04-21 | 中国科学院力学研究所 | Micro-carpillary tube covering method for controlling cell special distribution in shape and size, and use thereof |
| US8268446B2 (en) * | 2003-09-23 | 2012-09-18 | The University Of North Carolina At Chapel Hill | Photocurable perfluoropolyethers for use as novel materials in microfluidic devices |
| JP2005235625A (en) * | 2004-02-20 | 2005-09-02 | Shin Etsu Chem Co Ltd | Manufacturing method of liquid curing resin composition for electrolyte film and electrolyte film, and manufacturing method of electrolyte film/electrode assembly |
| JP4704434B2 (en) * | 2004-10-08 | 2011-06-15 | ダウ・コーニング・コーポレイション | Lithographic processes and patterns using phase change compositions |
| TWI432904B (en) * | 2006-01-25 | 2014-04-01 | Dow Corning | Epoxy formulations for use in lithography techniques |
-
2005
- 2005-08-31 KR KR1020077005858A patent/KR101237766B1/en not_active IP Right Cessation
- 2005-08-31 EP EP05793402A patent/EP1803033A2/en not_active Withdrawn
- 2005-08-31 CN CN2005800306251A patent/CN101019074B/en not_active Expired - Fee Related
- 2005-08-31 WO PCT/US2005/031150 patent/WO2006031455A2/en active Application Filing
- 2005-08-31 JP JP2007531232A patent/JP2008512281A/en active Pending
- 2005-08-31 US US11/659,989 patent/US20070269747A1/en not_active Abandoned
-
2011
- 2011-11-07 JP JP2011243140A patent/JP5551142B2/en not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5292927A (en) * | 1992-02-26 | 1994-03-08 | The United States Of America As Represented By The Secretary Of The Navy | Fluorinated resins with low dielectric constant |
| EP0832936A1 (en) * | 1996-09-25 | 1998-04-01 | Shin-Etsu Chemical Co., Ltd. | Photo-curable liquid silicone rubber compositions for templating mother molds |
| US20010034458A1 (en) * | 1998-01-20 | 2001-10-25 | Alliedsignal Inc. | Polymerizable halogenated vinyl ethers |
| WO2003072625A1 (en) * | 2002-02-28 | 2003-09-04 | Luvantix Co., Ltd. | Photocurable resin composition for optical waveguide and optical waveguide made of the same |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102007023581B4 (en) | 2006-05-24 | 2022-03-10 | Lg Display Co., Ltd. | Compositions for forming a pattern and in-plane printing methods using the same |
| US9000064B2 (en) | 2006-05-24 | 2015-04-07 | Lg Display Co., Ltd. | Composition for forming pattern and in-plane printing method using the same |
| US8822125B2 (en) | 2006-05-24 | 2014-09-02 | Lg Display Co., Ltd. | Composition for forming pattern and in-plane printing method using the same |
| KR101370969B1 (en) * | 2006-11-30 | 2014-03-10 | 엘지디스플레이 주식회사 | Photocurable organic material |
| US8173225B2 (en) * | 2006-11-30 | 2012-05-08 | Lg Display Co., Ltd. | Photocurable organic material and method of fabricating array substrate for liquid crystal display device using the same |
| JP2008155344A (en) * | 2006-12-26 | 2008-07-10 | Hitachi Chem Co Ltd | Liquid photopolymerizable composition, nano-structure with same and manufacturing method therefor |
| WO2008106245A2 (en) * | 2007-02-12 | 2008-09-04 | Dow Corning Corporation | Method of forming soft lithographic molds with fluorine modified elastomers |
| WO2008106245A3 (en) * | 2007-02-12 | 2008-12-24 | Dow Corning | Method of forming soft lithographic molds with fluorine modified elastomers |
| JP2011511722A (en) * | 2008-01-29 | 2011-04-14 | エルジー・ケム・リミテッド | Manufacturing method of viewing angle limiting film |
| US8444885B2 (en) | 2008-01-29 | 2013-05-21 | Lg Chem, Ltd. | Method for making privacy film |
| US8025833B2 (en) | 2008-02-12 | 2011-09-27 | Fujifilm Corporation | Curable composition for nanoimprint, and patterning method |
| US8735481B2 (en) | 2008-05-01 | 2014-05-27 | Roller Bearing Company Of America, Inc. | Self-lubricating surface coating composition for low friction or soft substrate applications |
| US8741996B2 (en) | 2008-05-01 | 2014-06-03 | Roller Bearing Company Of America, Inc. | Self-lubricating surface coating composition |
| JP2010258026A (en) * | 2009-04-21 | 2010-11-11 | Jsr Corp | Photo-curing composition for nanoimprint lithography, and nanoimprinting method |
| CN102393600A (en) * | 2011-10-27 | 2012-03-28 | 南京大学 | Preparation method of nano-imprinting composite template |
| WO2016043263A1 (en) * | 2014-09-19 | 2016-03-24 | 横浜ゴム株式会社 | Ultraviolet light curable resin composition and laminate using same |
Also Published As
| Publication number | Publication date |
|---|---|
| US20070269747A1 (en) | 2007-11-22 |
| EP1803033A2 (en) | 2007-07-04 |
| CN101019074B (en) | 2011-12-21 |
| CN101019074A (en) | 2007-08-15 |
| JP2008512281A (en) | 2008-04-24 |
| JP2012096542A (en) | 2012-05-24 |
| KR101237766B1 (en) | 2013-02-28 |
| KR20070052305A (en) | 2007-05-21 |
| WO2006031455A3 (en) | 2006-10-26 |
| JP5551142B2 (en) | 2014-07-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20070269747A1 (en) | Lithography Technique Using Silicone Molds | |
| JP5039717B2 (en) | Epoxy formulations for use in lithographic techniques | |
| TWI438235B (en) | Curable composition for imprint, curing object using the same, manufacturing method thereof and member for liquid crystal display device | |
| CN101000462B (en) | Photocurable resin composition and a method for forming a pattern | |
| US20130099423A1 (en) | Photocurable composition for imprint and method for formation of pattern using the composition | |
| JP6799070B2 (en) | Photo-curable silicone composition and its cured product | |
| WO2012096071A1 (en) | Resin composition for photoimprinting, patterning method and etching mask | |
| JP5453062B2 (en) | Photosensitive resin composition for imprinting and method for forming organic film on substrate | |
| JP2010006870A (en) | Curable composition for nanoimprinting, cured product and method for producing the same | |
| TWI427113B (en) | Curable resins and articles made therefrom | |
| JP2012079782A (en) | Photosensitive resin composition for uv nanoimprint, method for manufacturing resist substrate using the photosensitive resin composition, and method for manufacturing copying template | |
| CN109988540B (en) | LED light-cured adhesive composition, transfer-printed curing adhesive and preparation method | |
| KR20100126728A (en) | Curable composition for nanoimprint, cured product using the same, method for producing the cured product, and member for liquid crystal display device | |
| JP2011082347A (en) | Curable composition for imprint, method of manufacturing cured product, and the cured product | |
| JP2010070586A (en) | Curable composition, cured product, and method for producing the same | |
| JP6083178B2 (en) | Resist substrate manufacturing method, replica template manufacturing method, and nanoimprint lithography method | |
| CN112442147B (en) | Low-viscosity 3D fluororesin and preparation method and application thereof | |
| KR20110125177A (en) | Curable composition and method for manufacturing cured product using the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU LV MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 11659989 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020077005858 Country of ref document: KR Ref document number: 2007531232 Country of ref document: JP Ref document number: 200580030625.1 Country of ref document: CN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005793402 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005793402 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 11659989 Country of ref document: US |