WO2005016255A2 - Substituted tetrahydroquinolines, phenylacetic acids and benzoic acids as hepatocyte nuclear factor 4 (hnf-4 ) modulator compounds - Google Patents
Substituted tetrahydroquinolines, phenylacetic acids and benzoic acids as hepatocyte nuclear factor 4 (hnf-4 ) modulator compounds Download PDFInfo
- Publication number
- WO2005016255A2 WO2005016255A2 PCT/US2004/023093 US2004023093W WO2005016255A2 WO 2005016255 A2 WO2005016255 A2 WO 2005016255A2 US 2004023093 W US2004023093 W US 2004023093W WO 2005016255 A2 WO2005016255 A2 WO 2005016255A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- optionally substituted
- halogens
- compound
- patient
- certain embodiments
- Prior art date
Links
- VCFHWGKSAFBOSN-XUJQIHCRSA-N OC(Cc1ccc(C(C2[C@@H]3OCC2)Nc2c3cccc2)cc1)=O Chemical compound OC(Cc1ccc(C(C2[C@@H]3OCC2)Nc2c3cccc2)cc1)=O VCFHWGKSAFBOSN-XUJQIHCRSA-N 0.000 description 1
- KDKJGRQVAAUSOP-CGTJXYLNSA-N OC(c1ccc([C@H]2Nc3ccccc3[C@H]3OCCC[C@@H]23)cc1)=O Chemical compound OC(c1ccc([C@H]2Nc3ccccc3[C@H]3OCCC[C@@H]23)cc1)=O KDKJGRQVAAUSOP-CGTJXYLNSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/04—Ortho-condensed systems
- C07D491/044—Ortho-condensed systems with only one oxygen atom as ring hetero atom in the oxygen-containing ring
- C07D491/052—Ortho-condensed systems with only one oxygen atom as ring hetero atom in the oxygen-containing ring the oxygen-containing ring being six-membered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
Definitions
- This invention relates to compounds that bind to and/or modulate hepatocyte nuclear factor 4 ⁇ receptors and to methods for making and using such compounds.
- Hepatocyte nuclear factor 4 ⁇ (HNF-4 ⁇ ) has been described as a member of the steroid/thyroid superfamily of transcription factors that is expressed in liver, kidney, intestine and pancreas. Sladek et al, (1990 Genes Dev. 4, 2353-2365; Miquerol et al, (1994) J. Biol. Chem. 269, 8944-8951. No ligand has been identified at present and therefore HNF-4 ⁇ is referred to as an orphan member of the intracellular receptor family (3-5). Tsai & O'Malley (1994) Annu. Rev. Biochem.
- HNF-4 ⁇ has been described as being capable of activating transcription in tissue culture cells under certain conditions.
- HNF-4 ⁇ plays a role in one or more metabolic pathways, including glucose and lipid homeostasis. Ladias et al, (1992) J. Biol. Chem. 267: 15849-15860; Montgomery-Snyder et al, (1992) Mol. Cell. Biol. 12: 1708-1718; Metzger et al,. (1993) J. Biol. Chem. 268: 16831-16838; Yamagata et al, (1996) Nature 384:458-460; Stoffel & Duncan(1997) Eroc. Natl. Acad. Sci. U.S.A. 94:13209-13214. [005] Certain mutations of HNF-4 ⁇ result in defective function of the endocrine
- HNF-4 ⁇ plays a role in metabolic gene regulation.
- Yamagata et al (1996) Nature 384:458-460.
- Liver-specific knockouts demonstrate that HNF-4 plays a role in liver development and function. Li et al, (2000) Genes & Dev. 14:464-474; Hayhurst et al, (2001) Mol. Cell. Biol. 21: 1393-1403; Fraser et al, (1998) Nuc. Acids Res. 26:2702-2707.
- the present invention provides compounds of formula I:
- R 1 is selected from H, a halogen, and a methyl optionally substituted with one or more fluorines
- R 2 , R 3 , R 4 , and R 5 are each independently selected from H, a halogen, an amide, a sulfonamide, a C 1 -C 5 alkyl optionally substituted with one or more halogens, a -C 5 alkenyl optionally substituted with one or more halogens, a C 2 -C 5 alkynyl optionally substituted with one or more halogens, a C ⁇ -C alkoxy optionally substituted with one or more halogens a C ⁇ -C 4 thioalkyl optionally substituted with one or more halogens, a C 2 - C 5 thioalkenyl optionally substituted with one or more halogens, and a C 2 -C 5 thioalkenyl optionally substituted with one or more halogens, and a C 2
- R 2 and R 3 taken together form a 3 to 8 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines; or
- R and R 4 taken together form a 5 to 6 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines;
- R 4 and R 5 taken together form a 5 to 6 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines;
- R 6 is selected from H a -C 5 alkyl optionally substituted with one or more halogens, a -C5 alkenyl optionally substituted with one or more halogens, and a C 2 -C 5 alkynyl optionally substituted with one or more halogens;
- R 7 is selected from CH 2 OH, CHO, COOH and a group of formula (a):
- the invention provides a pharmaceutical agentcomprising a pharmaceutically acceptable carrier and a compound of Formula I.
- the invention provides a method of treating a patient comprising administering to said patient a pharmaceutical agent comprising a pharmaceutically acceptable carrier and a pharmaceutically effective amount of a compound of Formula I. [009] In certain embodiments, the invention provides a selective HNF-4 ⁇ modulator of Formula I.
- Standard chemical symbols are used interchangeably with the full names represented by such symbols. Thus, for example, the terms "hydrogen” and “H” are understood to have identical meaning.
- Standard techniques may be used for chemical syntheses, chemical analyses, pharmaceutical preparation, formulation, and delivery, and treatment of patients. Standard techniques may be used for recombinant DNA metholodolgy, oligonucleotide synthesis, tissue culture and transformation (e.g., electroporation, lipofection).
- Reactions and purification techniques may be performed e.g., using kits according to manufacturer's specifications, as commonly accomplished in the art or as described herein.
- the foregoing techniques and procedures may be generally performed according to conventional methods well known in the art and as described in various general or more specific references that are cited and discussed throughout the present specification. See e.g., Sambrook et al. Molecular Cloning: A Laboratory Manual (2d ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (1989)), which is incorporated herein by reference for any purpose.
- selective binding compound refers to a compound that selectively binds to any portion of one or more target receptors.
- selective HNF-4 ⁇ receptor binding compound refers to a compound that selectively binds to any portion of an HNF-4 ⁇ receptor.
- selectively binds refers to the ability of a selective binding compound to bind to a target receptor with greater affinity than it binds to a non-target receptor.
- selective binding refers to binding to a target with an affinity that is at least 10, 50, 100, 250, 500, or 1000 times greater than the affinity for a non-target.
- target receptor refers to a receptor or a portion of a receptor capable of being bound by a selective binding compound.
- a target receptor is an HNF-4 ⁇ receptor.
- modulator refers to a compound that alters or elicits an activity of a molecule. For example, a modulator may cause an increase or decrease in the magnitude of a certain activity of a molecule compared to the magnitude of the activity in the absence of the modulator.
- a modulator is an inhibitor, which decreases the magnitude of one or more activities of a molecule. In certain embodiments, an inhibitor completely prevents one or more activities of a molecule. In certain embodiments, a modulator is an activator, which increases the magnitude of at least one activity of a molecule. In certain embodiments the presence of a modulator results in an activity that does not occur in the absence of the modulator. [019] The term “selective modulator” refers to a compound that selectively modulates a target activity. [020] The term “selective HNF-4 ⁇ receptor modulator” refers to a compound that selectively modulates at least one activity associated with an HNF-4a receptor.
- target activity refers to a biological activity capable of being modulated by a selective modulator. Certain exemplary target activities include, but are not limited to, changes in binding affinity, signal transduction, enzymatic activity, transcription of one or more genes, tumor growth, changes in blood glucose concentration, and inflammation or inflammation-related processes.
- receptor-mediated activity refers to any biological activity that results, either directly or indirectly, from binding of a ligand to a receptor.
- agonist refers to a compound, the presence of which results in a biological activity of a receptor that is the same as the biological activity resulting from the presence of a naturally occurring ligand for the receptor.
- partial agonist refers to a compound the presence of which results in a biological activity of a receptor that is of the same type as that resulting from the presence of a naturally occurring ligand for the receptor, but of a lower magnitude.
- antagonist refers to a compound, the presence of which results in a decrease in the magnitude of a biological activity of a receptor. In certain embodiments, the presence of an antagonist results in complete inhibition of a biological activity of a receptor.
- alkyl refers to an optionally substituted straight-chain or branched-chain alkyl radical having from 1 to about 12 carbon atoms.
- the term also includes substituted straight-chain or branched-chain alkyl radicals having from 1 to about 6 carbon atoms as well as those having from 1 to about 4 carbon atoms.
- alkyl radicals include methyl, ethyl, n-propyl, isopropyl, n- butyl, isobutyl, sec-butyl, tert-butyl, tert-amyl, pentyl, hexyl, heptyl, octyl and the like.
- alkenyl refers to an optionally substituted straight-chain or branched-chain hydrocarbon radical having one or more carbon-carbon double-bonds and having from 2 to about 18 carbon atoms.
- the term also includes substituted straight-chain or branched-chain alkyl radicals having one or more carbon-carbon double bonds and having from 2 to about 6 carbon atoms as well as those having from 2 to about 4 carbon atoms.
- alkenyl radicals include ethenyl, propenyl, 1 ,4-butadienyl and the like.
- alkynyl refers to an optionally substituted straight-chain or branched-chain hydrocarbon radical having one or more carbon-carbon triple-bonds and having from 2 to about 12 carbon atoms.
- the term also includes substituted straight-chain or branched-chain alkyl radicals having one or more carbon-carbon tyriple bonds and having from 2 to about 6 carbon atoms as well as those having from 2 to about 4 carbon atoms.
- alkynyl radicals include ethynyl, propynyl, butynyl and the like.
- an alkyl comprises 1 to 20 carbon atoms (whenever it appears herein, a numerical range such as “1 to 20” refers to each integer in the given range; e.g., "1 to 20 carbon atoms” means that an alkyl group may comprise only 1 carbon atom, 2 carbon atoms, 3 carbon atoms, etc., up to and including 20 carbon atoms, although the term “alkyl” also includes instances where no numerical range of carbon atoms is designated).
- the term “lower alkyl” refers to an alkyl comprising 1 to 6 carbon atoms.
- the term “medium alkyl” refers to an alkyl comprising 7 to 12 carbon atoms.
- An alkyl may be designated as "C ⁇ -C 4 alkyl” or similar designations.
- “C ⁇ -C 4 alkyl”, “C ⁇ -C 4 alkenyl” and"C ⁇ -C 4 alkynyl” indicate a radical having one, two, three, or four carbon atoms (e.g., methyl, ethyl, propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, t-butyl, ethenyl, propenyl, butenyl, ethynyl, propynyl, and butynyl).
- haloalkyl refers to an alkyl in which at least one hydrogen atom is replaced with a halogen atom. In certain of the embodiments in which two or more hydrogen atom are replaced with halogen atoms, the halogen atoms are all the same as each other. In certain of such embodiments, the halogen atoms are not all the same as each other.
- heteroalkyl refers to a group comprising an alkyl and one or more heteroatoms. Certain heteroalkyls are acylalkyls, in which the one or more heteroatoms are outside an alkyl chain.
- thioalkyl refers to a heteroalkyl comprising at least one sulfur atom.
- heterohaloalkyl refers to a heteroalkyl in which at least one hydrogen atom is replaced with a halogen atom.
- the term "carbocycle” refers to a group comprising a covalently closed ring, wherein each of the atoms forming the ring is a carbon atom. Carbocylic rings may be formed by three, four, five, six, seven, eight, nine, or more than nine carbon atoms. Carbocycles may be optionally substituted. [037]
- the term “heterocycle” refers to a group comprising a covalently closed ring wherein at least one atom forming the ring is a heteroatom. Heterocyclic rings may be formed by three, four, five, six, seven, eight, nine, or more than nine atoms.
- heterocyclic rings may comprise one, two, three, four, five, six, seven, eight, nine, or more than nine heteroatoms.
- those two or more heteroatoms may be the same as or different from each other.
- Heterocycles may be optionally substituted. Binding to a heterocycle can be at a heteroatom or via a carbon atom. For example, binding for benzo-fused derivatives, may be via a carbon of the benzenoid ring.
- heterocycles include, but are not limited to, the following:
- D, E, F, and G each independently represent a heteroatom.
- D, E, F, and G may be the same as or different from each other.
- heteroatom refers to an atom other than carbon or hydrogen. Heteroatoms are typically independently selected from oxygen, sulfur, nitrogen, and phosphorus, but are not limited to those atoms. In embodiments in which two or more heteroatoms are present, the two or more heteroatoms may all be the same, or some or all of the two or more heteroatoms may each be different from the others.
- aromatic refers to a group comprising a covalently closed ring having a delocalized ⁇ -electron system. Aromatic rings may be formed by five, six, seven, eight, nine, or more than nine atoms. Aromatics may be optionally substituted.
- aromatic groups include, but are not limited to phenyl, naphthalenyl, phenanthrenyl, anthracenyl, tetralinyl, fluorenyl, indenyl, and indanyl.
- aromatic includes, for example, benzenoid groups, connected via one of the ring-forming carbon atoms, and optionally carrying one or more substituents selected from an aryl, a heteroaryl, a cycloalkyl, a non-aromatic heterocycle, a halo, a hydroxy, an amino, a cyano, a nitro, an alkylamido, an acyl, a C ⁇ - 6 alkoxy, a C ⁇ - 6 alkyl, a C ⁇ -6 hydroxyalkyl, a C ⁇ - 6 aminoalkyl, a C ⁇ - 6 alkylamino, an alkylsulfenyl, an alkylsulfinyl, an alkylsulfon
- an aromatic group is substituted at one or more of the para, meta, and/or ortho positions.
- aromatic groups comprising substitutions include, but are not limited to, phenyl, 3- halophenyl, 4-halophenyl, 3-hydroxyphenyl, 4-hydroxyphenyl, 3-aminophenyl, 4- aminophenyl, 3-methylphenyl, 4-methylphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 4- trifluoromethoxyphenyl, 3-cyanophenyl, 4-cyanophenyl, dimethylphenyl, naphthyl, hydroxynaphthyl, hydroxymethylphenyl, (trifluoromethyl)phenyl, alkoxyphenyl, 4- mo holin-4-ylphenyl, 4-pyrrolidin-l-ylphenyl, 4-pyrazolylphenyl, 4-triazolylphenyl, and 4-(2-oxopyrrolidin- 1 -yl)phenyl
- aryl refers to an aromatic group wherein each of the atoms forming the ring is a carbon atom.
- Aryl rings may be formed by five, six, seven, eight, nine, or more than nine carbon atoms.
- Aryl groups may be optionally substituted.
- heteroaryl refers to an aromatic group wherein at least one atom forming the aromatic ring is a heteroatom. Heteroaryl rings may be formed by three, four, five, six, seven, eight, nine, or more than nine atoms. Heteroaryl groups may be optionally substituted. Examples of heteroaryl groups include, but are not limited to, aromatic C .
- heterocyclic groups comprising one oxygen or sulfur atom or up to four nitrogen atoms, or a combination of one oxygen or sulfur atom and up to two nitrogen atoms, and their substituted as well as benzo- and pyrido-fused derivatives, for example, connected via one of the ring-forming carbon atoms.
- heteroaryl groups are optionally substituted with one or more substituents, independently selected from halo, hydroxy, amino, cyano, nitro, alkylamido, acyl, C ⁇ -C 6 alkoxy, C ⁇ -C 6 alkyl, Ci-C ⁇ hydroxyalkyl, C ⁇ -C 6 aminoalkyl, Ci-C ⁇ -alkylamino, alkylsulfenyl, alkylsulfinyl, alkylsulfonyl, sulfamoyl, and trifluoromethyl.
- substituents independently selected from halo, hydroxy, amino, cyano, nitro, alkylamido, acyl, C ⁇ -C 6 alkoxy, C ⁇ -C 6 alkyl, Ci-C ⁇ hydroxyalkyl, C ⁇ -C 6 aminoalkyl, Ci-C ⁇ -alkylamino, alkylsulfenyl, alkylsulfinyl, alkyls
- heteroaryl groups include, but are not limited to, unsubstituted and mono- or di-substituted derivatives of furan, benzofiiran, thiophene, benzothiophene, py ⁇ ole, pyridine, indole, oxazole, benzoxazole, isoxazole, benzisoxazole, thiazole, benzothiazole, isothiazole, imidazole, benzimidazole, pyrazole, indazole, tetrazole, quinoline, isoquinoline, pyridazine, pyrimidine, purine and pyrazine, furazan, 1,2,3-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, triazole, benzotriazole, pteridine, phenoxazole, oxadiazole, benzopyrazole, quinolizine, cinnoline,
- the substituents are halo, hydroxy, cyano, O-C ⁇ -6 alkyl, C ⁇ -C 6 alkyl, hydroxy-C ⁇ -C 6 alkyl, or amino-Ci-C ⁇ alkyl.
- non-aromatic ring refers to a group comprising a covalently closed ring that does not have a delocalized ⁇ -electron system.
- cycloalkyl alone or in combination, refers to a monocyclic, bicyclic or tricyclic alkyl radical wherein each cyclic moiety has from 3 to about 8 carbon atoms.
- cycloalkyl radicals include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and the like.
- Cycloalkyl rings may be formed by three, four, five, six, seven, eight, nine, or more than nine carbon atoms. Cycloalkyls may be optionally substituted.
- non-aromatic heterocycle refers to a group comprising a non- aromatic ring wherein one or more atoms forming the ring is a heteroatom.
- Non- aromatic heterocyclic rings may be formed by three, four, five, six, seven, eight, nine, or more than nine atoms.
- Non-aromatic heterocycles may be optionally substituted.
- non-aromatic heterocycles comprise one or more carbonyl or thiocarbonyl groups such as, for example, oxo- and thio-containing groups.
- non-aromatic heterocycles include, but are not limited to, lactams, lactones, cyclic imides, cyclic thioimides, cyclic carbamates, tetrahydrothiopyran, 4H-pyran, tetrahydropyran, piperidine, 1,3-dioxin, 1,3-dioxane, 1,4-dioxin, 1,4-dioxane, piperazine, 1,3-oxathiane, 1 ,4-oxathiin, 1,4-oxathiane, tetrahydro-l,4-thiazine, 2H-l,2-oxazine, maleimide, succinimide, barbituric acid, thiobarbituric acid, dioxopiperazine,
- arylalkyl refers to a group comprising an aryl group bound to an alkyl group.
- carbocyclic cycloalkyl ring refers to a group comprising a carbocyclic cycloalkyl ring. Carbocycloalkyl rings may be formed by three, four, five, six, seven, eight, nine, or more than nine carbon atoms. Carbocycloalkyl groups may be optionally substituted.
- ring refers to any covalently closed structure.
- Rings include, for example, carbocycles (e.g., aryls and cycloalkyls), heterocycles (e.g., heteroaryls and non-aromatic heterocycles), aromatics (e.g., aryls and heteroaryls), and non-aromatics (e.g., cycloalkyls and non-aromatic heterocycles). Rings may be optionally substituted. Rings may form part of a ring system. [048] The term “ring system” refers to two or more rings, wherein two or more of the rings are fused. The term “fused" refers to structures in which two or more rings share one or more bonds.
- the substituent "R” appearing by itself and without a number designation refers to a substituent selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heteroaryl (bonded through a ring carbon) and non-aromatic heterocycle (bonded through a ring carbon).
- the term "null” refers to a group being absent from a structure.
- R' x' R" example, in the structure ⁇ - ⁇ , if X is C, then both R' and R" exist, but if X is N, then one of those R groups is null, meaning that only three groups are bound to the N.
- cyano refers to a group of formula -CN.
- isocyanato refers to a group of formula -NCO.
- thiocyanato refers to a group of formula -CNS.
- isothiocyanato refers to a group of formula -NCS.
- esters refers to a chemical moiety with formula -(R) ⁇ -COOR', where R and R' are independently selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heteroaryl (bonded through a ring carbon) and non- aromatic heterocycle (bonded through a ring carbon), where n is 0 or 1.
- amide refers to a chemical moiety with formula -(R) n -C(O)NHR' or -(R) n -NHC(O)R', where R and R' are independently selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heteroaryl (bonded through a ring carbon) and heteroalicyclic (bonded through a ring carbon), where n is 0 or 1.
- an amide may be an amino acid or a peptide.
- alkoxy refers to an alkyl ether radical.
- alkoxy examples include, but are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, iso- butoxy, sec-butoxy, tert-butoxy and the like.
- formyl includes aldehydes attached to a compound via an alkyl, aryl, heteroaryl, arylalkyl or heteroarylalkyl group (e.g., -alkyl-CHO, -aryl-CHO, arylalkyl-CHO or -heteroarylalkyl-CHO, etc.).
- oxime refers to a group of formula:
- hydrazone refers to a group of formula:
- hydroxylamine refers to a group of formula:
- sulfonamide refers to a group of formula:
- halogen includes F, Cl, Br and I.
- amine “hydroxy,” and “carboxyl” include such groups that have been esterified or amidified. Procedures and specific groups used to achieve esterification and amidification are known to those of skill in the art and can readily be found in reference sources such as Greene and Wuts, Protective Groups in Organic Synthesis, 3rd Ed., John Wiley & Sons, New York, NY, 1999, which is incorporated by reference herein in its entirety.
- the term “optionally substituted,” refers to a group in which none, one, or more than one of the hydrogen atoms has been replaced with one or more group(s) individually and independently selected from: alkyl, alkenyl, alkynyl, heteroalkyl, heteroalkenyl, heteroalkynyl, haloalkyl, haloalkenyl, haloalkynyl, heterohaloalkyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, heteroaryl, non-aromatic heterocycle, hydroxy, alkoxy, aryloxy, mercapto, alkylthio, arylthio, alkenylthio, alkynylthio, cyano, halo, carbonyl, thiocarbonyl, O- carbamyl, N
- protective derivatives and protecting groups that may form such protective derivatives
- the substituent groups may together form a ring.
- carrier refers to a compound that facilitates the incorporation of another compound into cells or tissues.
- DMSO dimethyl sulfoxide
- pharmaceutical agent refers to a chemical compound or composition capable of inducing a desired therapeutic effect in a patient.
- a pharmaceutical agent comprises an active agent, which is the agent that induces the desired therapeutic effect.
- a pharmaceutical agent comprises a prodrug.
- a pharmaceutical agent comprises inactive ingredients such as carriers, excipients, and the like.
- therapeutically effective amount refers to an amount of a pharmaceutical agent sufficient to achieve a desired therapeutic effect.
- prodrug refers to a pharmaceutical agent that is converted from a less active form into a co ⁇ esponding more active form in vivo.
- pharmaceutically acceptable refers to a formulation of a compound that does not significantly abrogate biological activity, a pharmacological activity and/or other properties of the compound when the formulated compound is administered to a patient. In certain embodiments, a pharmaceutically acceptable formulation does not cause significant irritation to a patient.
- co-administer refers to administering more than one pharmaceutical agent to a patient. In certain embodiments, co-administered pharmaceutical agents are administered together in a single dosage unit. In certain embodiments, co-administered pharmaceutical agents are administered separately. In certain embodiments, co-administered pharmaceutical agents are administered at the same time.
- co-administered pharmaceutical agents are administered at different times.
- the term "patient” includes human and animal subjects.
- the term "substantially pure” means an object species (e.g., compound) is the predominant species present (i.e., on a molar basis it is more abundant than any other individual species in the composition).
- a substantially pure composition is a composition wherein the object species comprises at least about 50 percent (on a molar basis) of all species present.
- a substantially pure composition is a composition wherein the object species comprises more than about 80%, 85%, 90%), 95%), or 99% of all species present in the composition.
- tissue-selective refers to the ability of a compound to modulate a biological activity in one tissue to a greater or lesser degree than it modulates a biological activity in another tissue.
- the biological activities modulated in the different tissues may be the same or they may be different.
- the biological activities modulated in the different tissues may be mediated by the same type of target receptor.
- a tissue-selective compound may modulate an HNF-4 ⁇ receptor-mediated biological activity in one tissue and fail to modulate, or
- an HNF-4 ⁇ receptor-mediated biological activity in another tissue type modulate to a lesser degree, an HNF-4 ⁇ receptor-mediated biological activity in another tissue type.
- the term "monitoring" refers to observing an effect or absence of any effect.
- cells are monitored after contacting those cells with a compound of the present invention. Examples of effects that may be monitored include, but are not limited to, changes in cell phenotype, cell proliferation, an HNF-4 ⁇ receptor
- cell phenotype refers to physical or biological characteristics. Examples of characteristics that constitute phenotype included, but are not limited to, cell size, cell proliferation, cell differentiation, cell survival, apoptosis (cell death), or the utilization of a metabolic nutrient (e.g., glucose uptake). Certain changes or the absence of changes in cell phenotype are readily monitored using techniques known in the art.
- cell proliferation refers to the rate at which cells divide.
- the number of cells growing in a vessel can be quantified by a person skilled in the art (e.g., by counting cells in a defined area using a light microscope, or by using laboratory apparatus that measure the density of cells in an appropriate medium).
- One skilled in that art can calculate cell proliferation by determining the number of cells in a sample at two or more times.
- the term "contacting" refers to bringing two or more materials into close enough proximity that they may interact. In certain embodiments, contacting can be accomplished in a vessel such as a test tube, a petri dish, or the like. In certain embodiments, contacting may be performed in the presence of additional materials. In certain embodiments, contacting may be performed in the presence of cells. In certain of such embodiments, one or more of the materials that are being contacted may be inside a cell. Cells may be alive or may dead. Cells may or may not be intact.
- Certain compounds that bind to HNF-4 ⁇ receptors and/or certain compounds that modulate an activity of such receptors play a role in health (e.g., normal growth, development, and/or absence of disease).
- compounds of the present invention are useful for treating any of a variety of diseases or conditions.
- Certain compounds have been previously described as receptor modulators. See e.g., U. S. Patent Nos. 6,462,038, 5,693,646; 6,380,207; 6,506,766;
- cyclothiocarbamate analogues have been described as progesterone receptor modulators (e.g., US 6,436,929 and US 6,509,334). Certain cyclocarbamate analogues have been described as progesterone receptor antagonists (e.g., U.S. Pat. Nos. 6,306,851, 6,380,178, 6,441,019, 6,444,668, 6,509,334, and 6,566,358; Zhang, et al. J. Med. Chem. 45:4379 (2002)). [095] In certain embodiments, the invention provides a compound of formula I:
- R 1 is selected from H, a halogen, a C ⁇ -C 6 alkyl optionally substituted with one or more halogens, a C 2 -C 6 alkenyl optionally substituted with one or more halogens, a C 2 -C 6 alkynyl optionally substituted with one or more halogens, an optionally substituted C ⁇ -C 6 heteroalkyl, an optionally substituted C 2 -C 6 heteroalkenyl, an optionally substituted C 2 -C 6 heteroalkynyl, an optionally substituted
- C ⁇ -C 6 haloalkyl an optionally substituted C 2 -C 6 alkenyl, an optionally substituted C 2 -C 6 alkynyl,, an optionally substituted C ⁇ -C 6 heterohaloalkyl, an optionally substituted C 2 -C 6 alkenyl, an optionally substituted C 2 -C 6 alkynyl, an optionally substituted C 3 -C 8 cycloalkyl, an optionally substituted a C 3 -C 8 cycloalkenyl, an optionally substituted C - C cycloalkynyl, an optionally substituted C 3 -C 8 heterocycle, an optionally substituted C -C aryl, an optionally substituted C -C 8 heteroaryl, an optionally substituted C ⁇ -C 2 alkoxy, an optionally substituted sulfonamide, an optionally substituted C 1 -C2 thioalkyl, an optionally substituted C ⁇ -C ⁇ thioalky
- R 1 is an optionally substituted C ⁇ -C 8 alkyl, an optionally substituted C 2 -C 8 alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C heteroalkyl, an optionally substituted C 2 -C 8 heteroalkenyl, an optionally substituted C 2 -C 8 heteroalkynyl, an optionally substituted C 3 -C 8 cycloalkyl, an optionally substituted C -C 8 cycloalkenyl, or an optionally substituted C 3 -C 8 cycloalkynyl.
- R 1 is an optionally substituted Cj-C 8 alkyl, an optionally substituted C 2 -C 8 alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C 8 heteroalkyl, an optionally substituted C 2 -C 8 heteroalkenyl, an optionally substituted C 2 -C 8 heteroalkynyl, an optionally substituted C -C 8 cycloalkyl, an optionally substituted C 3 -C 8 cycloalkenyl, an optionally substituted C 3 -C 8 cycloalkynyl, that is not fully saturated.
- R 1 is selected from an optionally substituted C 2 -C 8 alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C heteroalkenyl, an optionally substituted C 2 -C heteroalkynyl, an optionally substituted C 3 -C 8 cycloalkenyl, and an optionally substituted C 3 -C 8 cycloalkynyl.
- R 1 is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl.
- R 1 is F or Cl.
- R 2 , R 3 , R 4 , and R 5 are each independently selected from H, a halogen, an amide, a sulfonamide, a C1-C 5 alkyl optionally substituted with one or more halogens, a C 2 -C 6 alkenyl optionally substituted with one or more halogens, C 2 -C 6 alkynyl optionally substituted with one or more halogens, a C ⁇ -C 4 alkoxy optionally substituted with one or more halogens, a C ⁇ -C 4 thioalkyl optionally substituted with one or more halogens, a C 2 -C 4 thioalkenyl optionally substituted with one or more halogens, aC 2 -C 4 thioalkynyl optionally substituted with one or more halogens, an optionally substituted C ⁇ -C 6 alkyl, an optionally substituted C 2 -C 6 alken
- R 2 , R 3 , R 4 , and/or R 5 is an optionally substituted C ⁇ -C 8 alkyl, an optionally substituted C 2 -C 8 alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C 8 heteroalkyl, an optionally substituted C 2 -C 8 heteroalkenyl, an optionally substituted C 2 - C 8 heteroalkynyl, an optionally substituted C -C 8 cycloalkyl, an optionally substituted C 3 -C cycloalkenyl, or an optionally substituted C 3 -C 8 cycloalkynyl, that is fully saturated.
- R 2 , R 3 , R 4 , and/or R 5 is an optionally substituted Ci- C 8 alkyl, an optionally substituted C 2 -C alkenyl, an optionally substituted C 2 - alkyn> an optionally substituted C 2 -C 8 heteroalkyl, an optionally substituted C 2 -C 8 heteroalkenyl, an optionally substituted C 2 -C 8 heteroalkynyl or an optionally substitute C 3 -C 8 cycloalkyl, an optionally substituted C 3 -C 8 cycloalkenyl, or an optionally substituted C 3 -C cycloalkynyl.
- R 2 , R 3 , R 4 , and or R 5 i selected from an optionally substituted C 2 - alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C 3 heteroalkenyl, an optionally substituted C 2 -C 3 heteroalkynyl, an optionally substituted C 3 -C 8 cycloalkenyl, and an optionally substitute C 3 -C 8 cycloalkynyl.
- R 2 , R 3 , R 4 , and/or R 5 is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl. Ir certain of the embodiments where R 2 , R 3 , R 4 , and/or R 5 is a halogen, R 2 , R 3 , R 4 , and/or R 5 is F or Cl. [098] In certain embodiments, R and R taken together form a 3 to 8 membere carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines.
- Thu the ring formed by R and R in such embodiments would share a bond with the ring to which R 2 and R 3 are both bound.
- R 3 and R 4 taken together form a 5 to 6 membere carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines. Thu the ring formed by R 3 and R 4 in such embodiments would share a bond with the ring to which R 3 and R 4 are both bound.
- R 4 and R 5 taken together form a 5 to 6 membere carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines.
- R 6 is selected from H and a C 1 -C5 alkyl optionally substituted with one or more halogens, C2-C5 alkenyl optionally substituted with one or more halogens, C 2 -Cs alkynyl optionally substituted with one or more halogens.
- R 6 is an optionally substituted C ⁇ -C 8 alkyl, an optionally substituted C 2 -C 8 alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C 8 heteroalkyl, an optionally substituted C 2 -C 8 heteroalkenyl, an optionally substituted C 2 -C 8 heteroalkynyl, an optionally substituted C -C 8 cycloalkyl an optionally substituted C 3 -C 8 cycloalkenyl, or an optionally substituted C -C 8 cycloalkynyl.
- R 6 is selected from an optionally substituted C 2 -C 8 alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C heteroalkenyl, an optionally substituted C 2 -C 3 heteroalkynyl, an optionally substituted C 3 -C 8 cycloalkenyl, and an optionally substituted C 3 -C 8 cycloalkynyl.
- R is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl.
- R 7 is selected from CH 2 OH, CHO, COOH and a group of formula (a): (a); [0103] In certain embodiments, R and R are each independently selected from
- R 10 is selected from H and a Cp C 5 alkyl optionally substituted with one or more halogens, a C 2 -C 5 alkenyl optionally substituted with one or more halogens, a C 2 -C 5 alkynyl optionally substituted with one or more halogens.
- R 10 is an optionally substituted C ⁇ -C 8 alkyl, an optionally substituted C 2 -C 8 ⁇ alkenyl, an optionally substituted C 2 -C 8 alkynyl, an optionally substituted C 2 -C 8 heteroalkyl, an optionally substituted C 2 -C 8 heteroalkenyl, an optionally substituted C 2 -C 8 heteroalkynyl, an optionally substituted C -C 8 cycloalkyl, an optionally substituted C 3 -C 8 cycloalkenyl, or an optionally substituted C 3 - C 8 cycloalkynyl.
- R 10 is selected from an optionally substituted C 2 -C 8 alkenyl, an optionally substituted C 2 -C alkynyl, an optionally substituted C 2 -C 3 heteroalkenyl, an optionally substituted C 2 -C 3 heteroalkynyl, an optionally substituted C 3 -C 8 cycloalkenyl, and an optionally substituted C 3 -C 8 cycloalkynyl.
- R 10 is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl.
- stereoisomer refers to a compound made up of the same atoms bonded by the same bonds but having different three-dimensional structures which are not interchangeable. The three-dimensional structures are called configurations.
- enantiomer refers to two stereoisomers whose molecules are nonsuperimposable mirror images of one another.
- chiral center refers to a carbon atom to which four different groups are attached.
- diastereomers refers to stereoisomers which are not enantiomers.
- epimers two diastereomers which have a different configuration at only one chiral center are referred to herein as “epimers.”
- racemate racemic mixture” or “racemic modification” refer to a mixture of equal parts of enantiomers.
- the compounds of the present invention may be chiral, and it is intended that any enantiomers, as separated, pure or partially purified enantiomers or racemic mixtures thereof are included within the scope of the invention. Furthermore, when a double bond or a fully or partially saturated ring system or more than one center of asymmetry or a bond with restricted rotatability is present in the molecule diastereomers may be formed. It is intended that any diastereomers, as separated, pure or partially purified diastereomers or mixtures thereof are included within the scope of the invention.
- R rectus
- S sinister
- the priority of groups is based upon their atomic number (in order of decreasing atomic number). A partial list of priorities and a discussion of stereochemistry is contained in "Nomenclature of Organic Compounds: Principles and Practice", (J.H. Fletcher, et al., eds., 1974) at pages 103-120.
- the designation " ⁇ " refers to a bond that protrudes forward out of the plane of the page.
- the designation " " refers to a bond that protrudes backward out of the plane of the page.
- the designation " "" ⁇ " refers to a bond wherein the stereochemistry is not defined.
- the compounds of Formula I when existing as a diastereomeric mixture, may be separated into diastereomeric pairs of enantiomers by, for example, fractional crystallization from a suitable solvent, for example methanol or ethyl acetate or a mixture thereof.
- the pair of enantiomers thus obtained may be separated into individual stereoisomers by conventional means, for example by the use of an optically active acid as a resolving agent.
- any enantiomer of a compound of Formula I may be obtained by stereospecific synthesis using optically pure starting materials or reagents of known configuration or through enantioselective synthesis.
- enantiomeric enrichment refers to the increase in the amount of one enantiomer as compared to the other.
- the ee with respect to the first enantiomer is 40%.
- the ee with respect to the first enantiomer is 80%.
- An ee of greater than 90% is prefe ⁇ ed, an ee of greater than 95% is most prefe ⁇ ed and an ee of greater than 99% is most especially preferred.
- Enantiomeric enrichment is readily determined by one of ordinary skill in the art using standard techniques and procedures, such as gas or high performance liquid chromatography with a chiral column.
- the invention provides compounds selected from:
- a compound of Formula I is a selective HNF-4 ⁇ receptor modulator.
- a compound of Formula I is a selective HNF-4 ⁇ receptor agonist.
- a compound of Formula I is a selective HNF-4 ⁇ receptor antagonist.
- a compound of Formula I is a selective HNF-4 ⁇ receptor partial agonist.
- a compound of Formula I is a tissue-specific selective HNF-4 ⁇ receptor modulator.
- a compound of Formula I is a gene-specific selective HNF-4 ⁇ receptor modulator.
- a compound of Formula I is a selective HNF-4 ⁇ receptor binding compound.
- the present invention provides selective HNF-4 receptor modulators.
- the invention provides selective HNF-4 ⁇ receptor binding agents.
- the invention provides methods of making and methods of using selective HNF-4 ⁇ receptor modulators and/or selective HNF-4 ⁇ binding agents.
- selective HNF-4 ⁇ modulators are agonists, partial agonists, and/or antagonists for the HNF-4 ⁇ receptor.
- the invention provides compounds that are selective for an HNF-4 ⁇ receptor relative to a retinoic X receptor (RXR).
- RXR retinoic X receptor
- the invention provides compounds that are selective for an HNF-4 ⁇ receptor relative to an RXR by at least 8 times.
- the invention provides a salt co ⁇ esponding to any of the compounds provided herein. In certain embodiments, the invention provides a salt corresponding to a selective HNF-4 ⁇ modulator. In certain embodiments, the
- a salt co ⁇ esponding to a selective HNF-4 ⁇ receptor binding agent.
- a salt is obtained by reacting a compound with an inorganic acid, such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, and the like.
- a salt is obtained by reacting a compound with a base to form a salt such as an ammonium salt, an alkali metal salt, such as a sodium or a potassium salt, an alkaline earth metal salt, such as a calcium or a magnesium salt, a salt of organic bases such as dicyclohexylamine, N-methyl-D-glucamine, tris(hydroxymethyl)methylamine, and salts with amino acids such as arginine, lysine, and the like.
- a salt such as an ammonium salt, an alkali metal salt, such as a sodium or a potassium salt, an alkaline earth metal salt, such as a calcium or a magnesium salt, a salt of organic bases such as dicyclohexylamine, N-methyl-D-glucamine, tris(hydroxymethyl)methylamine, and salts with amino acids such as arginine, lysine, and the like.
- a salt such as an ammonium salt, an alkali metal salt, such as
- compounds of the present invention comprising one or more silicon atoms possess certain desired properties, including, but not limited to, greater stability and/or longer half-life in a patient, when compared to the same compound in which none of the carbon atoms have been replaced with a silicon atom.
- Protecting groups that may be used in the present invention include those that are commonly known to those skilled in the art, such groups include, but are not limited to TBDMS, TBS and Benzyl.
- compounds of the present invention are capable of modulating activity of HNF-4 ⁇ receptors in a "co-transfection” assay (also called a “cis-trans” assay), which has been discussed previously. See e.g., Evans et al., Science, 240:889-95 (1988); U.S. Patent Nos. 4,981,784 and 5,071,773; Pathirana et al., Mol. Pharm. 47:630-35 (1995)). Modulating activity in a co-transfection assay has been shown to co ⁇ elate with in vivo modulating activity. Thus, in certain embodiments, such assays are predictive of in vivo activity.
- co-transfection assays two different co-transfection plasmids are prepared.
- cloned cDNA encoding an intracellular receptor e.g., HNF-4 ⁇ receptor
- a constitutive promoter e.g., the SV 40 promoter
- cDNA encoding a reporter protein such as firefly luciferase (LUC)
- LEC firefly luciferase
- Both co-transfection plasmids are co-transfected into the same cells.
- Expression of the first co-transfection plasmid results in production of the intracellular receptor protein.
- Activation of that intracellular receptor protein results in production of a receptor- dependant activation factor for the promoter of the second co-transfection plasmid.
- That receptor-dependant activation factor in turn results in expression of the reporter protein encoded on the second co-transfection plasmid.
- reporter protein expression is linked to activation of the receptor.
- that reporter activity can be conveniently measured (e.g., as increased luciferase production).
- Certain co-transfection assays can be used to identify agonists, partial agonists, and/or antagonists of intracellular receptors.
- to identify agonists co-transfected cells are exposed to a test compound. If the test compound is an agonist or partial agonist, reporter activity is expected to be higher compared to co-transfected cells in the absence of the test compound.
- to identify antagonists the cells are exposed to a known agonist (e.g., the natural ligand for the receptor) in the presence and absence of a test compound. If the test compound is an antagonist, reporter activity is expected to be lower than that of cells exposed only to the known agonist.
- compounds of the invention are used to detect the presence, quantity and/or state of receptors in a sample.
- samples are obtained from a patient.
- compounds are radio- or isotopically-labeled.
- compounds of the present invention that selectively bind HNF-4 ⁇ receptors may be used to determine the presence or amount of such receptors in a sample, such as cell homogenates and lysates.
- the present invention provides for use of both CARLA and mammalian-2-hybrid assays, to characterize the in vitro profile of compounds of the invention on a HNF-4 ⁇ receptor.
- At least one selective HNF-4 ⁇ receptor modulator, or pharmaceutically acceptable salt, ester, amide, and/or prodrug thereof, either alone or combined with one or more pharmaceutically acceptable carriers forms a pharmaceutical agent.
- Techniques for formulation and administration of compounds of the present invention may be found for example, in "Remington's Pharmaceutical Sciences,” Mack Publishing Co., Easton, PA, 18th edition, 1990.
- a pharmaceutical agent comprising one or more compounds of the present invention is prepared using known techniques, including, but not limited to mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or tabletting processes.
- a pharmaceutical agent comprising one or more compounds of the present invention is a liquid (e.g., a suspension, elixir and/or solution).
- a liquid pharmaceutical agent comprising one or more compounds of the present invention is prepared using ingredients known in the art, including, but not limited to, water, glycols, oils, alcohols, flavoring agents, preservatives, and coloring agents.
- a pharmaceutical agent comprising one or more compounds of the present invention is a solid (e.g., a powder, tablet, and/or capsule).
- a solid pharmaceutical agent comprising one or more compounds of the present invention is prepared using ingredients known in the art, including, but not limited to, starches, sugars, diluents, granulating agents, lubricants, binders, and disintegrating agents.
- a pharmaceutical agent comprising one or more compounds of the present invention is formulated as a depot preparation. Certain of such depot preparations are typically longer acting than non-depot preparations. In certain embodiments, such preparations are administered by implantation (for example subcutaneously or intramuscularly) or by intramuscular injection.
- depot preparations are prepared using suitable polymeric or hydrophobic materials (for example an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives, for example, as a sparingly soluble salt.
- a pharmaceutical agent comprising one or more compounds of the present invention comprises a delivery system.
- delivery systems include, but are not limited to, liposomes and emulsions.
- Certain delivery systems are useful for preparing certain pharmaceutical agents including those comprising hydrophobic compounds.
- certain organic solvents such as dimethylsulfoxide are used.
- a pharmaceutical agent comprising one or more compounds of the present invention comprises one or more tissue-specific delivery molecules designed to deliver the pharmaceutical agent to specific tissues or cell types.
- pharmaceutical agents include liposomes coated with a tissue-specific antibody.
- a pharmaceutical agent comprising one or more compounds of the present invention comprises a co-solvent system.
- co-solvent systems comprise, for example, benzyl alcohol, a nonpolar surfactant, a water- miscible organic polymer, and an aqueous phase.
- co- solvent systems are used for hydrophobic compounds.
- VPD co-solvent system is a solution of absolute ethanol comprising 3% w/v benzyl alcohol, 8% w/v of the nonpolar surfactant Polysorbate 80TM , and 65% w/v polyethylene glycol 300.
- the proportions of such co-solvent systems may be varied considerably without significantly altering their solubility and toxicity characteristics.
- a pharmaceutical agent comprising one or more compounds of the present invention comprises a sustained-release system.
- a non- limiting example of such a sustained-release system is a semi-permeable matrix of solid hydrophobic polymers.
- sustained-release systems may, depending on their chemical nature, release compounds over a period of hours, days, weeks or months.
- compositions used in pharmaceutical agent of the present invention may be provided as pharmaceutically acceptable salts with pharmaceutically compatible counterions.
- Pharmaceutically compatible salts may be formed with many acids, including but not limited to hydrochloric, sulfuric, acetic, lactic, tartaric, malic, succinic, etc.
- a pharmaceutical agent comprising one or more compounds of the present invention comprises an active ingredient in a therapeutically effective amount.
- the therapeutically effective amount is sufficient to prevent, alleviate or ameliorate symptoms of a disease or to prolong the survival of the subject being treated. Determination of a therapeutically effective amount is well within the capability of those skilled in the art.
- a pharmaceutical agent comprising one or more compounds of the present invention is formulated as a prodrug.
- prodrugs are useful because they are easier to administer than the co ⁇ esponding active form.
- a prodrug may be more bioavailable (e.g., through oral administration) than is the co ⁇ esponding active form.
- a prodrug may have improved solubility compared to the co ⁇ esponding active form.
- a prodrug is an ester.
- such prodrugs are less water soluble than the co ⁇ esponding active form.
- such prodrugs possess superior transmittal across cell membranes, where water solubility is detrimental to mobility.
- the ester in such prodrugs is metabolically hydrolyzed to carboxylic acid.
- the carboxylic acid containing compound is the co ⁇ esponding active form.
- a prodrug comprises a short peptide (polyaminoacid) bound to an acid group.
- the peptide is metabolized to form the co ⁇ esponding active form.
- a pharmaceutical agent comprising one or more compounds of the present invention is useful for treating a conditions or disorder in a mammalian, and particularly in a human patient.
- Suitable administration routes include, but are not limited to, oral, rectal, transmucosal, intestinal, enteral, topical, suppository, through inhalation, intrathecal, intraventricular, intraperitoneal, intranasal, intraocular and parenteral (e.g., intravenous, intramuscular, intramedullary, and subcutaneous).
- pharmaceutical intrathecals are administered to achieve local rather than systemic exposures.
- pharmaceutical agents may be injected directly in the area of desired effect (e.g., in the renal or cardiac area).
- a pharmaceutical agent comprising one or more compounds of the present invention is administered in the form of a dosage unit (e.g., tablet, capsule, bolus, etc.).
- such dosage units comprise a selective a HNF-4 ⁇ receptor modulator in a dose from about 1 ⁇ g/kg of body weight to about 50 mg/kg of body weight. In certain embodiments, such dosage units comprise a selective a HNF-4 ⁇ receptor modulator in a dose from about 2 ⁇ g/kg of body weight to about 25 mg/kg of body weight. In certain embodiments, such dosage units comprise a selective a HNF-4 ⁇ receptor modulator in a dose from about 10 ⁇ g/kg of body weight to about 5 mg/kg of body weight. In certain embodiments, pharmaceutical agents are administered as needed, once per day, twice per day, three times per day, or four or more times per day.
- a pharmaceutical agent comprising a compound of the present invention is prepared for oral administration.
- a pharmaceutical agent is formulated by combining one or more compounds of the present invention with one or more pharmaceutically acceptable carriers. Certain of such carriers enable compounds of the invention to be formulated as tablets, pills, dragees, capsules, liquids, gels, syrups, slurries, suspensions and the like, for oral ingestion by a patient.
- pharmaceutical agents for oral use are obtained by mixing one or more compounds of the present invention and one or more solid excipient.
- Suitable excipients include, but are not limited to, fillers, such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such as, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methyl cellulose, hydroxypropylmethyl-cellulose, sodium carboxymethylcellulose, and/or polyvinylpy ⁇ olidone (PVP).
- such a mixture is optionally ground and auxiliaries are optionally added.
- pharmaceutical agents are formed to obtain tablets or dragee cores.
- disintegrating agents e.g., cross-linked polyvinyl py ⁇ olidone, agar, or alginic acid or a salt thereof, such as sodium alginate
- dragee cores are provided with coatings.
- concentrated sugar solutions may be used, which may optionally contain gum arabic, talc, polyvinyl py ⁇ olidone, carbopol gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions, and suitable organic solvents or solvent mixtures.
- Dyestuffs or pigments may be added to tablets or dragee coatings.
- pharmaceutical agents for oral administration are push-fit capsules made of gelatin.
- Such push-fit capsules comprise one or more compounds of the present invention in admixture with one or more filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers.
- pharmaceutical agents for oral administration are soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol.
- one or more compounds of the present invention are be dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols.
- stabilizers may be added.
- pharmaceutical agents are prepared for buccal administration.
- a pharmaceutical agent is prepared for administration by injection (e.g., intravenous, subcutaneous, intramuscular, etc.).
- a pharmaceutical agent comprises a carrier and is formulated in aqueous solution, such as water or physiologically compatible buffers such as Hanks's solution, Ringer's solution, or physiological saline buffer.
- other ingredients are included (e.g., ingredients that aid in solubility or serve as preservatives).
- injectable suspensions are prepared using appropriate liquid carriers, suspending agents and the like.
- Certain pharmaceutical agents for injection are presented in unit dosage form, e.g., in ampoules or in multi-dose containers.
- Certain pharmaceutical agents for injection are suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- Certain solvents suitable for use in pharmaceutical agents for injection include, but are not limited to, lipophilic solvents and fatty oils, such as sesame oil, synthetic fatty acid esters, such as ethyl oleate or triglycerides, and liposomes.
- Aqueous injection suspensions may contain substances that increase the viscosity of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran.
- such suspensions may also contain suitable stabilizers or agents that increase the solubility of the compounds to allow for the preparation of highly concentrated solutions.
- a pharmaceutical agent is prepared for transmucosal administration.
- penetrants appropriate to the barrier to be permeated are used in the formulation. Such penetrants are generally known in the art.
- a pharmaceutical agent is prepared for administration by inhalation. Certain of such pharmaceutical agents for inhalation are prepared in the form of an aerosol spray in a pressurized pack or a nebulizer.
- Such pharmaceutical agents comprise a propellant, e.g., dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas.
- the dosage unit may be determined with a valve that delivers a metered amount.
- capsules and cartridges for use in an inhaler or insufflator may be formulated.
- Certain of such formulations comprise a powder mixture of a compound of the invention and a suitable powder base such as lactose or starch.
- a pharmaceutical agent is prepared for rectal administration, such as a suppositories or retention enema.
- a pharmaceutical agent comprises known ingredients, such as cocoa butter and/or other glycerides.
- a pharmaceutical agent is prepared for topical administration.
- Certain of such pharmaceutical agents comprise bland moisturizing bases, such as ointments or creams.
- Exemplary suitable ointment bases include, but are not limited to, petrolatum, petrolatum plus volatile silicones, lanolin and water in oil emulsions such as EucerinTM, available from Beiersdorf (Cincinnati, Ohio).
- Exemplary suitable ointment bases include, but are not limited to, petrolatum, petrolatum plus volatile silicones, lanolin and water in oil emulsions such as EucerinTM, available from Beiersdorf (Cincinnati, Ohio).
- suitable cream bases include, but are not limited to, NiveaTM Cream, available from
- the formulation, route of administration and dosage for a pharmaceutical agent of the present invention can be chosen in view of a particular patient's condition. (See e.g., Fingl et al. 1975, in "The Pharmacological Basis of Therapeutics", Ch. 1 p. 1).
- a pharmaceutical agent is administered as a single dose.
- a pharmaceutical agent is administered as a series of two or more doses administered over one or more days.
- a pharmaceutical agent of the present invention is administered to a patient between about 0.1% and 500%, more preferably between about
- a daily dosage regimen for a patient comprises an oral dose of between 0.1 mg and 2000 mg of a compound of the present invention.
- a daily dosage regimen is administered as a single daily dose.
- a daily dosage regimen is administered as two, three, four, or more than four doses.
- a pharmaceutical agent of the present invention is administered by continuous intravenous infusion.
- a pharmaceutical agent of the invention is administered for a period of continuous therapy.
- a pharmaceutical agent of the present invention may be administered over a period of days, weeks, months, or years.
- Dosage amount, interval between doses, and duration of treatment may be adjusted to achieve a desired effect.
- dosage amount and interval between doses are adjusted to maintain a desired concentration on compound in a patient.
- dosage amount and interval between doses are adjusted to provide plasma concentration of a compound of the present invention at an amount sufficient to achieve a desired effect.
- the plasma concentration is maintained above the minimal effective concentration (MEC).
- pharmaceutical agents of the present invention are administered with a dosage regimen designed to maintain a concentration above the MEC for 10-90%) of the time, between 30-90%> of the time, or between 50-90%) of the time.
- the dosage regimen is adjusted to achieve a desired local concentration of a compound of the present invention.
- a pharmaceutical agent may be presented in a pack or dispenser device which may contain one or more unit dosage forms containing the active ingredient.
- the pack may for example comprise metal or plastic foil, such as a blister pack.
- the pack or dispenser device may be accompanied by instructions for administration.
- the pack or dispenser may also be accompanied with a notice associated with the container in form prescribed by a governmental agency regulating the manufacture, use, or sale of pharmaceuticals, which notice is reflective of approval by the agency of the form of the drug for human or veterinary administration.
- a notice for example, may be the labeling approved by the U.S. Food and Drug Administration for prescription drugs, or the approved product insert.
- Compositions comprising a compound of the invention formulated in a compatible pharmaceutical carrier may also be prepared, placed in an appropriate container, and labeled for treatment of an indicated condition.
- a pharmaceutical agent is in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
- one or more pharmaceutical agents of the present invention are co-administered with one or more other pharmaceutical agents.
- such one or more other pharmaceutical agents are designed to treat the same disease or condition as the one or more pharmaceutical agents of the present invention.
- such one or more other pharmaceutical agents are designed to treat a different disease or condition as the one or more pharmaceutical agents of the present invention.
- such one or more other pharmaceutical agents are designed to treat an undesired effect of one or more pharmaceutical agents of the present invention.
- one or more pharmaceutical agents of the present invention is co-administered with another pharmaceutical agent to treat an undesired effect of that other pharmaceutical agent.
- one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are administered at the same time. In certain embodiments, one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are administered at the different times. In certain embodiments, one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are prepared together in a single formulation. In certain embodiments, one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are prepared separately.
- Examples of pharmaceutical agents that may be co-administered with a pharmaceutical agent of the present invention include, but are not limited to, analgesics (e.g., acetaminophen); anti-inflammatory agents, including, but not limited to non- steroidal anti-inflammatory drugs (e.g., ibuprofen, COX-1 inhibitors, and COX-2, inhibitors); salicylates; antibiotics; antivirals; antifungal agents; antidiabetic agents (e.g., biguanides, glucosidase inhibitors, insulins, sulfonylureas, and thiazolidenediones); adrenergic modifiers; diuretics; hormones (e.g., anabolic steroids, androgen, estrogen, calcitonin, progestin, somatostatin, and thyroid hormones); immunomodulators; muscle relaxants; antihistamines; osteoporosis agents (e.g., biphosphonates,
- the invention provides methods of treating a patient comprising administering one or more compounds of the present invention.
- Compounds of the present invention including, but not limited to, pharmaceutically acceptable salts, solvates and hydrates, are expected to be effective in treating diseases or conditions that are mediated by HNF-4 ⁇ . Therefore, in certain embodiments, compounds of the invention are effective in treating conditions that are mediated by HNF-4 ⁇ , including, but not limited to, syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia.
- a patient is treated prophylactically to reduce or prevent the occu ⁇ ence of a condition.
- the present invention provides a method of lowering blood glucose levels in a mammal by administering to the patient a pharmaceutically effective amount of at least one compound of the present invention.
- the patient is a mammal.
- the patient is a human.
- the present invention provides a method of lowering plasma triglycerides levels in a patient by administering to the mammal a pharmaceutically effective amount of at least one compound of the present invention.
- the patient is a mammal.
- the patient is a human.
- the present invention provides a method of increasing insulin levels in a patient by administering to the mammal a pharmaceutically effective amount of at least one compound of the present invention.
- the patient is a mammal.
- the patient is a human.
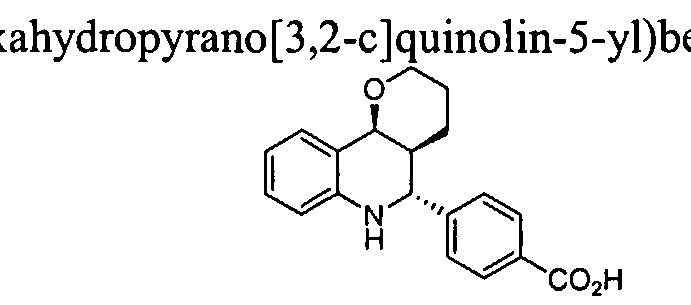
- Methyl 4-phenylimino benzoate (2.0 g, 8.4 mmol) was suspended in 40 mL of CH CN and the mixture was cooled to 0°C before addition of 3,4-dihydro-2H-pyran (1.15 mL, 12.6 mmol) and ytterbium triflate monohydrate (0.52 g, 0.84 mmol). The mixture was sti ⁇ ed at 0°C for 30 min, then at room temperature for 2 h. A white precipitate was isolated by filtration, providing 1.55 g of the desired product methyl 4-(2,3,4a,5,6,10b-Hexahydropyrano[3,2-c]quinolin-5-yl)benzoate.
- Lithium hydroxide (0.005 g,0.2 mmol) is added and is heated for 2 hours.
- the reaction is acidified with 1 N ⁇ C1 and is extracted with ethyl acetate.
- the reaction is dried with brine and dried over sodium sulfate, evaporation yields the title compound, mass spectrum : 322 (M-l)
- Lithium hydroxide (0.019g, 0.785 mmol) dissolved in water (2 mL), are combined an heated at 50° C for 1 hour. Additional Lithium hydroxide (0.070g, 2.9 mmol) is addec and the reaction is heated at 60° C for 1 hour. The reaction is acidified with 1 N ⁇ C1 a extracted with ethyl acetate. The organic layer is washed with brine and dried over sodium sulfate. The organic layer is filtered and concentrated under reduced pressure give the title compound, mass spectrum (m/e) :310.0 ( M+l) Example 26
- CV-1 cells African green monkey kidney fibroblasts
- DMEM Dulbecco's Modified Eagle Medium
- CH-FBS charcoal resin-stripped fetal bovine serum
- the CV-1 cells were transiently transfected by FuGENE 6 transfection reagent in 175 cm2 flask with the following plasmids: pCMX- HNF-4 ⁇ DF (3 ⁇ g/flask), apoAl-LUC reporter (l ⁇ g/flask), and filler DNA (pcDNA; 3 ⁇ g/flask).
- the receptor plasmid, pCMX-HNF-4 ⁇ DF contains the rat HNF-4 ⁇ l under constitutive control of the CMV promoter, as more fully described in J.D.
- the reporter plasmid contains the cDNA for firefly luciferase (LUC) under control of a multimerized HNF-4 ⁇ response element (the A site from the apo Al promoter) linked to the TK minimal promoter. See e.g., Fraser et al. supra. Twenty four hours after transfection the cells were harvested and plated in 96 well plates at 10,000 cells/well. Media containing one of the modulator compounds of
- LGO 100695 4-[5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-3-ethyloxy- 2-naphthalenyl]benzoyl benzoic acid (LGO 100695), which had previously been found to have agonist activity on HNF-4 ⁇ , were included as a reference agonist.
- LGO 100695 has the following structure:
- Antagonist activity was determined by testing the amount of LUC expression in the asbsence of exogenous compound (presence only of any endogenous ligand) as HNF-4 ⁇ receptor agonist. The concentration of a test compound that inhibited 50% of LUC expression was quantified (IC50). In addition, the efficacy of antagonists was determined as a function (%) of maximal inhibition. Data for 5 compounds of the present invention are provided in Table 1.
- HNF-4 ⁇ receptor modulator compounds of present invention Agonist, partial agonist, antagonist activity of HNF-4 ⁇ receptor modulator compounds of present invention. Efficacy (%) for HNF-4 ⁇ agonist was determined by comparing activity (e.g., luciferase production) of putative agonist to that LGO 100695. Efficacy (%) for HNF-4 ⁇ antagonist was determined by the percentage amount by which the luciferase production was reduced (maximum concentration of antagonist) from the luciferase production without compound.
- the present invention includes any combination of the various species and subgeneric groupings falling within the generic disclosure. This invention therefore includes the generic description of the invention with a proviso or negative limitation removing any subject matter from the genus, regardless of whether or not the excised material is specifically recited herein.
- description of the various embodiments and processing conditions have been provided, the scope of the invention is not to be limited thereto or thereby. Modifications and alterations of the present invention will be apparent to those skilled in the art without departing from the scope and spirit of the present invention. [0218] Therefore, it will be appreciated that the scope of this invention is to be defined by the appended claims, rather than by the specific examples which have been presented merely to illustrate certain embodiments of the present invention.
Abstract
This invention relates to substituted tetrahydroquinoline phenylacetic acids and benzoic acids that bind to and/or modulate hepatocyte factor 4α receptors and to methods for making and using such compounds.
Description
SUBSTITUTED TETRAHYDROQUINOLINES. PHENYLACETIC ACIDS AND BENZOIC ACIDS AS HEPATOCYTE NUCLEAR FACTOR 4α (HNF-4α) MODULATOR COMPOUNDS
RELATED APPLICATIONS
[001 ] This application claims the benefit of priority of U.S. Provisional Application Ser. No. 60/488,071 filed July 16, 2003, the entire disclosure of which is incorporated herein by reference. TECHNICAL FIELD
[002] This invention relates to compounds that bind to and/or modulate hepatocyte nuclear factor 4α receptors and to methods for making and using such compounds. BACKGROUND
[003] Hepatocyte nuclear factor 4α (HNF-4α) has been described as a member of the steroid/thyroid superfamily of transcription factors that is expressed in liver, kidney, intestine and pancreas. Sladek et al, (1990 Genes Dev. 4, 2353-2365; Miquerol et al, (1994) J. Biol. Chem. 269, 8944-8951. No ligand has been identified at present and therefore HNF-4α is referred to as an orphan member of the intracellular receptor family (3-5). Tsai & O'Malley (1994) Annu. Rev. Biochem. 63, 451-486; Mangelsdorf & Evans(1995) Cell 83:841-850; Kastner et al, (1995) Cell 83:859-869. [004] HNF-4α has been described as being capable of activating transcription in tissue culture cells under certain conditions. Kou et al, (1992) Nature 355: 457-461; Ladias et al,. (1992) J. Biol. Chem. 267: 15849-15860; Mietus-Snyder et al, (1992) Mol.
Cell. Biol. 12: 1708-1718; Metzger et al,. (1993) J. Biol. Chem. 268: 16831-16838. It has been suggested that HNF-4α plays a role in one or more metabolic pathways, including glucose and lipid homeostasis. Ladias et al, (1992) J. Biol. Chem. 267: 15849-15860; Mietus-Snyder et al, (1992) Mol. Cell. Biol. 12: 1708-1718; Metzger et al,. (1993) J. Biol. Chem. 268: 16831-16838; Yamagata et al, (1996) Nature 384:458-460; Stoffel & Duncan(1997) Eroc. Natl. Acad. Sci. U.S.A. 94:13209-13214. [005] Certain mutations of HNF-4α result in defective function of the endocrine
pancreas and maturity-onset diabetes of the young (MODY1), suggesting that HNF-4 α plays a role in metabolic gene regulation. Yamagata et al, (1996) Nature 384:458-460. Liver-specific knockouts demonstrate that HNF-4 plays a role in liver development and function. Li et al, (2000) Genes & Dev. 14:464-474; Hayhurst et al, (2001) Mol. Cell. Biol. 21: 1393-1403; Fraser et al, (1998) Nuc. Acids Res. 26:2702-2707.
SUMMARY OF THE INVENTION
[006] In certain embodiments, the present invention provides compounds of formula I:
I and a pharmaceutically acceptable salts, esters, amides, and prodrug thereof, wherein:
R1 is selected from H, a halogen, and a methyl optionally substituted with one or more fluorines; R2, R3, R4, and R5 are each independently selected from H, a halogen, an amide, a sulfonamide, a C1-C5 alkyl optionally substituted with one or more halogens, a -C5 alkenyl optionally substituted with one or more halogens, a C2-C5 alkynyl optionally substituted with one or more halogens, a Cι-C alkoxy optionally substituted with one or more halogens a Cι-C4 thioalkyl optionally substituted with one or more halogens, a C2- C5 thioalkenyl optionally substituted with one or more halogens, and a C2-C5 thioalkynyl optionally substituted with one or more halogens; or
R2 and R3 taken together form a 3 to 8 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines; or
R and R4 taken together form a 5 to 6 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines; or
R4 and R5 taken together form a 5 to 6 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines;
R6 is selected from H a -C5 alkyl optionally substituted with one or more halogens, a -C5 alkenyl optionally substituted with one or more halogens, and a C2-C5 alkynyl optionally substituted with one or more halogens;
R7 is selected from CH2OH, CHO, COOH and a group of formula (a):
/^C02H Re R9 (a) ;
wherein R8 and R9 are each independently selected from H, OH and a methyl optionally substituted with one or more fluorines; n is 1, 2 or 3; X is O, NR10 or S; and R10 is selected from H a Ci- C5 alkyl optionally substituted with one or more halogens, a C2-C5 alkenyl optionally substituted with one or more halogens, and a C2-C5 alkynyl optionally substituted with one or more halogens. [007] In certain embodiments, the invention provides a pharmaceutical agentcomprising a pharmaceutically acceptable carrier and a compound of Formula I. [008] In certain embodiments, the invention provides a method of treating a patient comprising administering to said patient a pharmaceutical agent comprising a pharmaceutically acceptable carrier and a pharmaceutically effective amount of a compound of Formula I. [009] In certain embodiments, the invention provides a selective HNF-4α modulator of Formula I.
DETAILED DESCRIPTION OF THE INVENTION
[010] It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory only and are not restrictive of the invention claimed. As used herein, the singular includes the plural unless specifically stated otherwise. As used herein, "or" means "and/or" unless stated otherwise. Furthermore, use of the term "including" as well as other forms, such as "includes," and "included," is not limiting.
[Oi l] The section headings used herein are for organizational purposes only and are not to be construed as limiting the subject matter described. All documents, or portions of documents, cited in the application including, but not limited to, patents, patent applications, articles, books, manuals, and treatises are hereby expressly incorporated by reference in their entirety for any purpose. Definitions
[012] Unless specific definitions are provided, the nomenclatures utilized in connection with, and the laboratory procedures and techniques of, analytical chemistry, synthetic organic chemistry, medicinal chemistry and pharmaceutical chemistry described herein are those known in the art. Standard chemical symbols are used interchangeably with the full names represented by such symbols. Thus, for example, the terms "hydrogen" and "H" are understood to have identical meaning. Standard techniques may be used for chemical syntheses, chemical analyses, pharmaceutical preparation, formulation, and delivery, and treatment of patients. Standard techniques may be used for recombinant DNA metholodolgy, oligonucleotide synthesis, tissue culture and transformation (e.g., electroporation, lipofection). Reactions and purification techniques may be performed e.g., using kits according to manufacturer's specifications, as commonly accomplished in the art or as described herein. The foregoing techniques and procedures may be generally performed according to conventional methods well known in the art and as described in various general or more specific references that are cited and discussed throughout the present specification. See e.g., Sambrook et al. Molecular Cloning: A Laboratory Manual (2d ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (1989)), which is incorporated herein by reference for any purpose.
[013] As used herein, the following terms are defined with the following meanings: [014] The term "selective binding compound" refers to a compound that selectively binds to any portion of one or more target receptors. [015] The term "selective HNF-4α receptor binding compound" refers to a compound that selectively binds to any portion of an HNF-4α receptor. [016] The term "selectively binds" refers to the ability of a selective binding compound to bind to a target receptor with greater affinity than it binds to a non-target receptor. In certain embodiments, selective binding refers to binding to a target with an affinity that is at least 10, 50, 100, 250, 500, or 1000 times greater than the affinity for a non-target. [017] The term "target receptor" refers to a receptor or a portion of a receptor capable of being bound by a selective binding compound. In certain embodiments, a target receptor is an HNF-4α receptor. [018] The term "modulator" refers to a compound that alters or elicits an activity of a molecule. For example, a modulator may cause an increase or decrease in the magnitude of a certain activity of a molecule compared to the magnitude of the activity in the absence of the modulator. In certain embodiments, a modulator is an inhibitor, which decreases the magnitude of one or more activities of a molecule. In certain embodiments, an inhibitor completely prevents one or more activities of a molecule. In certain embodiments, a modulator is an activator, which increases the magnitude of at least one activity of a molecule. In certain embodiments the presence of a modulator results in an activity that does not occur in the absence of the modulator.
[019] The term "selective modulator" refers to a compound that selectively modulates a target activity. [020] The term "selective HNF-4 α receptor modulator" refers to a compound that selectively modulates at least one activity associated with an HNF-4a receptor. [021] The term "selectively modulates" refers to the ability of a selective modulator to modulate a target activity to a greater extent than it modulates a non-target activity. [022] The term "target activity" refers to a biological activity capable of being modulated by a selective modulator. Certain exemplary target activities include, but are not limited to, changes in binding affinity, signal transduction, enzymatic activity, transcription of one or more genes, tumor growth, changes in blood glucose concentration, and inflammation or inflammation-related processes. [023] The term "receptor-mediated activity" refers to any biological activity that results, either directly or indirectly, from binding of a ligand to a receptor. [024] The term "agonist" refers to a compound, the presence of which results in a biological activity of a receptor that is the same as the biological activity resulting from the presence of a naturally occurring ligand for the receptor. [025] The term "partial agonist" refers to a compound the presence of which results in a biological activity of a receptor that is of the same type as that resulting from the presence of a naturally occurring ligand for the receptor, but of a lower magnitude. [026] The term "antagonist" refers to a compound, the presence of which results in a decrease in the magnitude of a biological activity of a receptor. In certain embodiments, the presence of an antagonist results in complete inhibition of a biological activity of a receptor.
[027] The term "alkyl," alone or in combination, refers to an optionally substituted straight-chain or branched-chain alkyl radical having from 1 to about 12 carbon atoms. The term also includes substituted straight-chain or branched-chain alkyl radicals having from 1 to about 6 carbon atoms as well as those having from 1 to about 4 carbon atoms. Examples of alkyl radicals include methyl, ethyl, n-propyl, isopropyl, n- butyl, isobutyl, sec-butyl, tert-butyl, tert-amyl, pentyl, hexyl, heptyl, octyl and the like. [028] The term "alkenyl," alone or in combination, refers to an optionally substituted straight-chain or branched-chain hydrocarbon radical having one or more carbon-carbon double-bonds and having from 2 to about 18 carbon atoms. The term also includes substituted straight-chain or branched-chain alkyl radicals having one or more carbon-carbon double bonds and having from 2 to about 6 carbon atoms as well as those having from 2 to about 4 carbon atoms. Examples of alkenyl radicals include ethenyl, propenyl, 1 ,4-butadienyl and the like. [029] The term "alkynyl," alone or in combination, refers to an optionally substituted straight-chain or branched-chain hydrocarbon radical having one or more carbon-carbon triple-bonds and having from 2 to about 12 carbon atoms. The term also includes substituted straight-chain or branched-chain alkyl radicals having one or more carbon-carbon tyriple bonds and having from 2 to about 6 carbon atoms as well as those having from 2 to about 4 carbon atoms. Examples of alkynyl radicals include ethynyl, propynyl, butynyl and the like. [030] In certain embodiments, an alkyl comprises 1 to 20 carbon atoms (whenever it appears herein, a numerical range such as "1 to 20" refers to each integer in the given range; e.g., "1 to 20 carbon atoms" means that an alkyl group may comprise only 1 carbon atom, 2 carbon atoms, 3 carbon atoms, etc., up to and including 20 carbon
atoms, although the term "alkyl" also includes instances where no numerical range of carbon atoms is designated). [031] The term "lower alkyl" refers to an alkyl comprising 1 to 6 carbon atoms. The term "medium alkyl" refers to an alkyl comprising 7 to 12 carbon atoms. An alkyl may be designated as "Cι-C4 alkyl" or similar designations. By way of example only, "Cι-C4 alkyl", "Cι-C4 alkenyl" and"Cι-C4 alkynyl" indicate a radical having one, two, three, or four carbon atoms (e.g., methyl, ethyl, propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, t-butyl, ethenyl, propenyl, butenyl, ethynyl, propynyl, and butynyl). [032] The term "haloalkyl" refers to an alkyl in which at least one hydrogen atom is replaced with a halogen atom. In certain of the embodiments in which two or more hydrogen atom are replaced with halogen atoms, the halogen atoms are all the same as each other. In certain of such embodiments, the halogen atoms are not all the same as each other. [033] The term "heteroalkyl" refers to a group comprising an alkyl and one or more heteroatoms. Certain heteroalkyls are acylalkyls, in which the one or more heteroatoms are outside an alkyl chain. Examples of heteroalkyls, heteroalkenyl, and heteroalkynyls include, but are not limited to, CH3C(=O)CH2-, CH3C(=O)CH2CH2-, CH3CH2C(=O)CH2CH2-, CH3C(=O)CH2CH2CH2-, CH3OCH2CH2-, CH3NHCH2-, and the like. [034] The term "thioalkyl" refers to a heteroalkyl comprising at least one sulfur atom. [035] The term "heterohaloalkyl" refers to a heteroalkyl in which at least one hydrogen atom is replaced with a halogen atom.
[036] The term "carbocycle" refers to a group comprising a covalently closed ring, wherein each of the atoms forming the ring is a carbon atom. Carbocylic rings may be formed by three, four, five, six, seven, eight, nine, or more than nine carbon atoms. Carbocycles may be optionally substituted. [037] The term "heterocycle" refers to a group comprising a covalently closed ring wherein at least one atom forming the ring is a heteroatom. Heterocyclic rings may be formed by three, four, five, six, seven, eight, nine, or more than nine atoms. Any number of those atoms may be heteroatoms (i.e., a heterocyclic ring may comprise one, two, three, four, five, six, seven, eight, nine, or more than nine heteroatoms). In heterocyclic rings comprising two or more heteroatoms, those two or more heteroatoms may be the same as or different from each other. Heterocycles may be optionally substituted. Binding to a heterocycle can be at a heteroatom or via a carbon atom. For example, binding for benzo-fused derivatives, may be via a carbon of the benzenoid ring. Examples of heterocycles include, but are not limited to, the following:
wherein D, E, F, and G each independently represent a heteroatom. Each of D, E, F, and G may be the same as or different from each other.
[038] The term "heteroatom" refers to an atom other than carbon or hydrogen. Heteroatoms are typically independently selected from oxygen, sulfur, nitrogen, and phosphorus, but are not limited to those atoms. In embodiments in which two or more heteroatoms are present, the two or more heteroatoms may all be the same, or some or all of the two or more heteroatoms may each be different from the others. [039] The term "aromatic" refers to a group comprising a covalently closed ring having a delocalized π-electron system. Aromatic rings may be formed by five, six, seven, eight, nine, or more than nine atoms. Aromatics may be optionally substituted. Examples of aromatic groups include, but are not limited to phenyl, naphthalenyl, phenanthrenyl, anthracenyl, tetralinyl, fluorenyl, indenyl, and indanyl. The term aromatic includes, for example, benzenoid groups, connected via one of the ring-forming carbon atoms, and optionally carrying one or more substituents selected from an aryl, a
heteroaryl, a cycloalkyl, a non-aromatic heterocycle, a halo, a hydroxy, an amino, a cyano, a nitro, an alkylamido, an acyl, a Cι-6 alkoxy, a Cι-6 alkyl, a Cι-6 hydroxyalkyl, a Cι-6 aminoalkyl, a Cι-6 alkylamino, an alkylsulfenyl, an alkylsulfinyl, an alkylsulfonyl, an sulfamoyl, and a trifluoromethyl. In certain embodiments, an aromatic group is substituted at one or more of the para, meta, and/or ortho positions. Examples of aromatic groups comprising substitutions include, but are not limited to, phenyl, 3- halophenyl, 4-halophenyl, 3-hydroxyphenyl, 4-hydroxyphenyl, 3-aminophenyl, 4- aminophenyl, 3-methylphenyl, 4-methylphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 4- trifluoromethoxyphenyl, 3-cyanophenyl, 4-cyanophenyl, dimethylphenyl, naphthyl, hydroxynaphthyl, hydroxymethylphenyl, (trifluoromethyl)phenyl, alkoxyphenyl, 4- mo holin-4-ylphenyl, 4-pyrrolidin-l-ylphenyl, 4-pyrazolylphenyl, 4-triazolylphenyl, and 4-(2-oxopyrrolidin- 1 -yl)phenyl . [040] The term "aryl" refers to an aromatic group wherein each of the atoms forming the ring is a carbon atom. Aryl rings may be formed by five, six, seven, eight, nine, or more than nine carbon atoms. Aryl groups may be optionally substituted. [041] The term "heteroaryl" refers to an aromatic group wherein at least one atom forming the aromatic ring is a heteroatom. Heteroaryl rings may be formed by three, four, five, six, seven, eight, nine, or more than nine atoms. Heteroaryl groups may be optionally substituted. Examples of heteroaryl groups include, but are not limited to, aromatic C . heterocyclic groups comprising one oxygen or sulfur atom or up to four nitrogen atoms, or a combination of one oxygen or sulfur atom and up to two nitrogen atoms, and their substituted as well as benzo- and pyrido-fused derivatives, for example, connected via one of the ring-forming carbon atoms. In certain embodiments, heteroaryl groups are optionally substituted with one or more substituents, independently selected
from halo, hydroxy, amino, cyano, nitro, alkylamido, acyl, Cι-C6 alkoxy, Cι-C6 alkyl, Ci-Cό hydroxyalkyl, Cι-C6 aminoalkyl, Ci-Cδ-alkylamino, alkylsulfenyl, alkylsulfinyl, alkylsulfonyl, sulfamoyl, and trifluoromethyl. Examples of heteroaryl groups include, but are not limited to, unsubstituted and mono- or di-substituted derivatives of furan, benzofiiran, thiophene, benzothiophene, pyπole, pyridine, indole, oxazole, benzoxazole, isoxazole, benzisoxazole, thiazole, benzothiazole, isothiazole, imidazole, benzimidazole, pyrazole, indazole, tetrazole, quinoline, isoquinoline, pyridazine, pyrimidine, purine and pyrazine, furazan, 1,2,3-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, triazole, benzotriazole, pteridine, phenoxazole, oxadiazole, benzopyrazole, quinolizine, cinnoline, phthalazine, quinazoline, and quinoxaline. In some embodiments, the substituents are halo, hydroxy, cyano, O-Cι-6 alkyl, Cι-C6 alkyl, hydroxy-Cι-C6 alkyl, or amino-Ci-Cβ alkyl. [042] The term "non-aromatic ring" refers to a group comprising a covalently closed ring that does not have a delocalized π-electron system. [043] The term "cycloalkyl", alone or in combination, refers to a monocyclic, bicyclic or tricyclic alkyl radical wherein each cyclic moiety has from 3 to about 8 carbon atoms. Examples of cycloalkyl radicals include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and the like.Cycloalkyl rings may be formed by three, four, five, six, seven, eight, nine, or more than nine carbon atoms. Cycloalkyls may be optionally substituted. = [044] The term "non-aromatic heterocycle" refers to a group comprising a non- aromatic ring wherein one or more atoms forming the ring is a heteroatom. Non- aromatic heterocyclic rings may be formed by three, four, five, six, seven, eight, nine, or more than nine atoms. Non-aromatic heterocycles may be optionally substituted. In
certain embodiments, non-aromatic heterocycles comprise one or more carbonyl or thiocarbonyl groups such as, for example, oxo- and thio-containing groups. Examples of non-aromatic heterocycles include, but are not limited to, lactams, lactones, cyclic imides, cyclic thioimides, cyclic carbamates, tetrahydrothiopyran, 4H-pyran, tetrahydropyran, piperidine, 1,3-dioxin, 1,3-dioxane, 1,4-dioxin, 1,4-dioxane, piperazine, 1,3-oxathiane, 1 ,4-oxathiin, 1,4-oxathiane, tetrahydro-l,4-thiazine, 2H-l,2-oxazine, maleimide, succinimide, barbituric acid, thiobarbituric acid, dioxopiperazine, hydantoin, dihydrouracil, morpholine, trioxane, hexahydro-l,3,5-triazine, tetrahydrothiophene, tetrahydrofuran, pyrroline, pyπolidine, pyπolidone, pyrrolidione, pyrazoline, pyrazolidine, imidazoline, imidazolidine, 1,3-dioxole, 1,3-dioxolane, 1,3-dithiole, 1,3- dithiolane, isoxazoline, isoxazolidine, oxazoline, oxazolidine, oxazolidinone, thiazoline, thiazolidine, and 1,3-oxathiolane. [045] The term "arylalkyl" refers to a group comprising an aryl group bound to an alkyl group. [046] The term "carbocycloalkyl" refers to a group comprising a carbocyclic cycloalkyl ring. Carbocycloalkyl rings may be formed by three, four, five, six, seven, eight, nine, or more than nine carbon atoms. Carbocycloalkyl groups may be optionally substituted. [047] The term "ring" refers to any covalently closed structure. Rings include, for example, carbocycles (e.g., aryls and cycloalkyls), heterocycles (e.g., heteroaryls and non-aromatic heterocycles), aromatics (e.g., aryls and heteroaryls), and non-aromatics (e.g., cycloalkyls and non-aromatic heterocycles). Rings may be optionally substituted. Rings may form part of a ring system.
[048] The term "ring system" refers to two or more rings, wherein two or more of the rings are fused. The term "fused" refers to structures in which two or more rings share one or more bonds. [049] The substituent "R" appearing by itself and without a number designation refers to a substituent selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heteroaryl (bonded through a ring carbon) and non-aromatic heterocycle (bonded through a ring carbon). [050] The term "null" refers to a group being absent from a structure. For
R' x'R" example, in the structure ^- ^ , if X is C, then both R' and R" exist, but if X is N, then one of those R groups is null, meaning that only three groups are bound to the N. [051 ] The term "O-carboxy" refers to a group of formula RC(=0)0-. [052] The term "C-carboxy" refers to a group of formula -C(=0)OR. [053] The term "acetyl" refers to a group of formula -C(=O)CH . [054] The term "trihalomethanesulfonyl" refers to a group of formula X3CS(=O)2- where X is a halogen. [055] The term "cyano" refers to a group of formula -CN. [056] The term "isocyanato" refers to a group of formula -NCO. [057] The term "thiocyanato" refers to a group of formula -CNS. [058] The term "isothiocyanato" refers to a group of formula -NCS. [059] The term "sulfonyl" refers to a group of formula -S(=O)-R. [060] The term "S-sulfonamido" refers to a group of formula -S(=0)2NR. [061] The term "N-sulfonamido" refers to a group of formula RS(=O)2NH-.
[062] The term "trihalomethanesulfonamido" refers to a group of formula X3CS(=O)2NR-. [063] The term "O-carbamyl" refers to a group of formula -OC(=O)-NR. [064] The term "N-carbamyl" refers to a group of formula ROC(=O)NH-. [065] The term "O-thiocarbamyl" refers to a group of formula -OC(=S)-NR. [066] The term "N-thiocarbamyl" refers to a group of formula ROC(=S)NH-. [067] The term "C-amido" refers to a group of formula -C(=O)-NR2. [068] The term "N-amido" refers to a group of formula RC(=O)NH-. [069] The term "ester" refers to a chemical moiety with formula -(R)π-COOR', where R and R' are independently selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heteroaryl (bonded through a ring carbon) and non- aromatic heterocycle (bonded through a ring carbon), where n is 0 or 1. [070] The term "amide" refers to a chemical moiety with formula -(R)n-C(O)NHR' or -(R)n-NHC(O)R', where R and R' are independently selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heteroaryl (bonded through a ring carbon) and heteroalicyclic (bonded through a ring carbon), where n is 0 or 1. In certain embodiments, an amide may be an amino acid or a peptide. [071 ] The term "alkoxy," refers to an alkyl ether radical. Examples of alkoxy include, but are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, iso- butoxy, sec-butoxy, tert-butoxy and the like. [072] The term "formyl" includes aldehydes attached to a compound via an alkyl, aryl, heteroaryl, arylalkyl or heteroarylalkyl group (e.g., -alkyl-CHO, -aryl-CHO, arylalkyl-CHO or -heteroarylalkyl-CHO, etc.).
[073] The term "oxime" refers to a group of formula:
[074] The term "hydrazone" refers to a group of formula:
[075] The term "hydroxylamine" refers to a group of formula:
R R" v
[076] The term sulfonamide refers to a group of formula:
.SO, HN
[077] The term "halogen" includes F, Cl, Br and I. [078] The terms "amine," "hydroxy," and "carboxyl" include such groups that have been esterified or amidified. Procedures and specific groups used to achieve esterification and amidification are known to those of skill in the art and can readily be found in reference sources such as Greene and Wuts, Protective Groups in Organic
Synthesis, 3rd Ed., John Wiley & Sons, New York, NY, 1999, which is incorporated by reference herein in its entirety. [079] Unless otherwise indicated, the term "optionally substituted," refers to a group in which none, one, or more than one of the hydrogen atoms has been replaced with one or more group(s) individually and independently selected from: alkyl, alkenyl, alkynyl, heteroalkyl, heteroalkenyl, heteroalkynyl, haloalkyl, haloalkenyl, haloalkynyl, heterohaloalkyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, heteroaryl, non-aromatic heterocycle, hydroxy, alkoxy, aryloxy, mercapto, alkylthio, arylthio, alkenylthio, alkynylthio, cyano, halo, carbonyl, thiocarbonyl, O- carbamyl, N-carbamyl, O-thiocarbamyl, N-thiocarbamyl, C-amido, N-amido, S- sulfonamido, N-sulfonamido, C-carboxy, O-carboxy, isocyanato, thiocyanato, isothiocyanato, nitro, silyl, trihalomethanesulfonyl, and amino, including mono- and di-substiruted amino groups, and the protected derivatives of amino groups. Such protective derivatives (and protecting groups that may form such protective derivatives) are known to those of skill in the art and may be found in references such as Greene and Wuts, above. In embodiments in which two or more hydrogen atoms have been substituted, the substituent groups may together form a ring. [080] The term "carrier" refers to a compound that facilitates the incorporation of another compound into cells or tissues. For example, dimethyl sulfoxide (DMSO) is a commonly used carrier for improving incorporation of certain organic compounds into cells or tissues. [081] The term "pharmaceutical agent" refers to a chemical compound or composition capable of inducing a desired therapeutic effect in a patient. In certain embodiments, a pharmaceutical agent comprises an active agent, which is the agent that
induces the desired therapeutic effect. In certain embodiments, a pharmaceutical agent comprises a prodrug. In certain embodiments, a pharmaceutical agent comprises inactive ingredients such as carriers, excipients, and the like. [082] The term "therapeutically effective amount" refers to an amount of a pharmaceutical agent sufficient to achieve a desired therapeutic effect. [083] The term "prodrug" refers to a pharmaceutical agent that is converted from a less active form into a coπesponding more active form in vivo. [084] The term "pharmaceutically acceptable" refers to a formulation of a compound that does not significantly abrogate biological activity, a pharmacological activity and/or other properties of the compound when the formulated compound is administered to a patient. In certain embodiments, a pharmaceutically acceptable formulation does not cause significant irritation to a patient. [085] The term "co-administer" refers to administering more than one pharmaceutical agent to a patient. In certain embodiments, co-administered pharmaceutical agents are administered together in a single dosage unit. In certain embodiments, co-administered pharmaceutical agents are administered separately. In certain embodiments, co-administered pharmaceutical agents are administered at the same time. In certain embodiments, co-administered pharmaceutical agents are administered at different times. [086] The term "patient" includes human and animal subjects. [087] The term "substantially pure" means an object species (e.g., compound) is the predominant species present (i.e., on a molar basis it is more abundant than any other individual species in the composition). In certain embodiments, a substantially pure composition is a composition wherein the object species comprises at least about 50
percent (on a molar basis) of all species present. In certain embodiments, a substantially pure composition is a composition wherein the object species comprises more than about 80%, 85%, 90%), 95%), or 99% of all species present in the composition. In certain embodiments, a substantially pure object species is purified to essential homogeneity (contaminant species cannot be detected in the composition by conventional detection methods) wherein the composition consists essentially of the single object species. [088] The term "tissue-selective" refers to the ability of a compound to modulate a biological activity in one tissue to a greater or lesser degree than it modulates a biological activity in another tissue. The biological activities modulated in the different tissues may be the same or they may be different. The biological activities modulated in the different tissues may be mediated by the same type of target receptor. For example, in certain embodiments, a tissue-selective compound may modulate an HNF-4α receptor-mediated biological activity in one tissue and fail to modulate, or
modulate to a lesser degree, an HNF-4α receptor-mediated biological activity in another tissue type. [089] The term "monitoring" refers to observing an effect or absence of any effect. In certain embodiments, cells are monitored after contacting those cells with a compound of the present invention. Examples of effects that may be monitored include, but are not limited to, changes in cell phenotype, cell proliferation, an HNF-4α receptor
activity, or the interaction between an HNF-4 receptor and a natural binding partner. [090] The term "cell phenotype" refers to physical or biological characteristics. Examples of characteristics that constitute phenotype included, but are not limited to, cell size, cell proliferation, cell differentiation, cell survival, apoptosis (cell death), or the
utilization of a metabolic nutrient (e.g., glucose uptake). Certain changes or the absence of changes in cell phenotype are readily monitored using techniques known in the art. [091] The term "cell proliferation" refers to the rate at which cells divide. The number of cells growing in a vessel can be quantified by a person skilled in the art (e.g., by counting cells in a defined area using a light microscope, or by using laboratory apparatus that measure the density of cells in an appropriate medium). One skilled in that art can calculate cell proliferation by determining the number of cells in a sample at two or more times. [092] The term "contacting" refers to bringing two or more materials into close enough proximity that they may interact. In certain embodiments, contacting can be accomplished in a vessel such as a test tube, a petri dish, or the like. In certain embodiments, contacting may be performed in the presence of additional materials. In certain embodiments, contacting may be performed in the presence of cells. In certain of such embodiments, one or more of the materials that are being contacted may be inside a cell. Cells may be alive or may dead. Cells may or may not be intact. Certain compounds
[093] Certain compounds that bind to HNF-4α receptors and/or certain compounds that modulate an activity of such receptors play a role in health (e.g., normal growth, development, and/or absence of disease). In certain embodiments, compounds of the present invention are useful for treating any of a variety of diseases or conditions. [094] Certain compounds have been previously described as receptor modulators. See e.g., U. S. Patent Nos. 6,462,038, 5,693,646; 6,380,207; 6,506,766;
5,688,810; 5,696,133; 6,569,896, 6,673,799; 4,636,505; 4,097,578; 3,847,988; U.S. Pat
Application No. 10/209,461 (Pub. No. US 2003/0055094); International Patent
Application Nos. WO 01/27086& WO 02/22585; Zhi, et al. Bioorganic & Med. Chem. Lett. (2000) 10: 415-418; Pooley, et al., J. Med. Chem. (1998) 41:3461; Hamann, et al. J. Med. Chem. (1998) 41: 623; and Yin, et al, Molecular Pharmacology, 2003, 63 (1), 211-223 the entire disclosures of which are incorporated by reference herein in their entirety. Certain cyclothiocarbamate analogues have been described as progesterone receptor modulators (e.g., US 6,436,929 and US 6,509,334). Certain cyclocarbamate analogues have been described as progesterone receptor antagonists (e.g., U.S. Pat. Nos. 6,306,851, 6,380,178, 6,441,019, 6,444,668, 6,509,334, and 6,566,358; Zhang, et al. J. Med. Chem. 45:4379 (2002)). [095] In certain embodiments, the invention provides a compound of formula I:
I and a pharmaceutically acceptable salts, esters, amides, and prodrugs thereof. [096] In certain embodiments, R1 is selected from H, a halogen, a Cι-C6 alkyl optionally substituted with one or more halogens, a C2-C6 alkenyl optionally substituted with one or more halogens, a C2-C6 alkynyl optionally substituted with one or more halogens, an optionally substituted Cι-C6 heteroalkyl, an optionally substituted C2-C6 heteroalkenyl, an optionally substituted C2-C6 heteroalkynyl, an optionally substituted
Cι-C6 haloalkyl, an optionally substituted C2-C6 alkenyl, an optionally substituted C2-C6 alkynyl,, an optionally substituted Cι-C6 heterohaloalkyl, an optionally substituted C2-C6 alkenyl, an optionally substituted C2-C6 alkynyl, an optionally substituted C3-C8 cycloalkyl, an optionally substituted a C3-C8 cycloalkenyl, an optionally substituted C -
C cycloalkynyl, an optionally substituted C3-C8 heterocycle, an optionally substituted C -C aryl, an optionally substituted C -C8 heteroaryl, an optionally substituted Cι-C2 alkoxy, an optionally substituted sulfonamide, an optionally substituted C1-C2 thioalkyl, an optionally substituted C∑-Cβ thioalkynyl, an optionally substituted C2 thioalkenyl, an optionally substituted nitro, an optionally substituted formyl, an optionally substituted acyl, and an optionally substituted hydroxylamine. In certain embodiments, R1 is an optionally substituted Cι-C8 alkyl, an optionally substituted C2-C8 alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C heteroalkyl, an optionally substituted C2-C8 heteroalkenyl, an optionally substituted C2-C8 heteroalkynyl, an optionally substituted C3-C8 cycloalkyl, an optionally substituted C -C8 cycloalkenyl, or an optionally substituted C3-C8 cycloalkynyl. In certain embodiments, R1 is an optionally substituted Cj-C8 alkyl, an optionally substituted C2-C8 alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C8 heteroalkyl, an optionally substituted C2-C8 heteroalkenyl, an optionally substituted C2-C8 heteroalkynyl, an optionally substituted C -C8 cycloalkyl, an optionally substituted C3-C8 cycloalkenyl, an optionally substituted C3-C8 cycloalkynyl, that is not fully saturated. In certain of such embodiments, R1 is selected from an optionally substituted C2-C8 alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C heteroalkenyl, an optionally substituted C2-C heteroalkynyl, an optionally substituted C3-C8 cycloalkenyl, and an optionally substituted C3-C8 cycloalkynyl. In certain embodiments, R1 is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl. In certain of the embodiments where R1 is a halogen, R1 is F or Cl. [097] In certain embodiments, R2, R3, R4, and R5 are each independently selected from H, a halogen, an amide, a sulfonamide, a C1-C5 alkyl optionally substituted
with one or more halogens, a C2-C6 alkenyl optionally substituted with one or more halogens, C2-C6 alkynyl optionally substituted with one or more halogens, a Cι-C4 alkoxy optionally substituted with one or more halogens, a Cι-C4 thioalkyl optionally substituted with one or more halogens, a C2-C4 thioalkenyl optionally substituted with one or more halogens, aC2-C4 thioalkynyl optionally substituted with one or more halogens, an optionally substituted Cι-C6 alkyl, an optionally substituted C2-C6 alkenyl, an optionally substituted C2-C6 alkynyl, an optionally substituted Cι-C6 heteroalkyl, an optionally substituted C2-C6 heteroalkenyl, an optionally substituted C2-C6 heteroalkynyl, an optionally substituted Cι-C6 haloalkyl, an optionally substituted C2-C6 alkenyl, an optionally substituted C2-C6 alkynyl, an optionally substituted Cι-C6 heterohaloalkyl, an optionally substituted C2-C6 heteroalkenyl, an optionally substituted C2-C6 heteroalkynyl, an optionally substituted C3-C8 cycloalkyl, an optionally substituted C3-C8 cycloalkenyl, an optionally substituted C -C8 cycloalkynyl, an optionally substituted C -C8 heterocycle, an optionally substituted C3-C8 aryl, an optionally substituted C -C8 heteroaryl, an optionally substituted Cι-C2 alkoxy, an optionally substituted sulfonamide, an optionally substituted C1-C2 thioalkyl, an optionally substituted C2 thioalkenyl, an optionally substituted C2 thioalkynyl, an optionally substituted nitro, an optionally substituted formyl, an optionally substituted acyl, and an optionally substituted hydroxy lamine. In certain embodiments, R2, R3, R4, and/or R5 is an optionally substituted Cι-C8 alkyl, an optionally substituted C2-C8 alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C8 heteroalkyl, an optionally substituted C2-C8 heteroalkenyl, an optionally substituted C2- C8 heteroalkynyl, an optionally substituted C -C8 cycloalkyl, an optionally substituted C3-C cycloalkenyl, or an optionally substituted C3-C8 cycloalkynyl, that is fully
saturated. In certain embodiments, R2, R3, R4, and/or R5 is an optionally substituted Ci- C8 alkyl, an optionally substituted C2-C alkenyl, an optionally substituted C2- alkyn> an optionally substituted C2-C8 heteroalkyl, an optionally substituted C2-C8 heteroalkenyl, an optionally substituted C2-C8 heteroalkynyl or an optionally substitute C3-C8 cycloalkyl, an optionally substituted C3-C8 cycloalkenyl, or an optionally substituted C3-C cycloalkynyl. In certain of such embodiments, R2, R3, R4, and or R5 i selected from an optionally substituted C2- alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C3 heteroalkenyl, an optionally substituted C2-C3 heteroalkynyl, an optionally substituted C3-C8 cycloalkenyl, and an optionally substitute C3-C8 cycloalkynyl. In certain embodiments, R2, R3, R4, and/or R5 is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl. Ir certain of the embodiments where R2, R3, R4, and/or R5 is a halogen, R2, R3, R4, and/or R5 is F or Cl. [098] In certain embodiments, R and R taken together form a 3 to 8 membere carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines. Thu the ring formed by R and R in such embodiments would share a bond with the ring to which R2 and R3 are both bound. [099] In certain embodiments, R3 and R4 taken together form a 5 to 6 membere carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines. Thu the ring formed by R3 and R4 in such embodiments would share a bond with the ring to which R3 and R4 are both bound. [0100] In certain embodiments, R4 and R5 taken together form a 5 to 6 membere carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines. Thu
the ring formed by R4 and R5 in such embodiments would share a bond with the ring to which R4 and R5 are both bound. [0101] In certain embodiments, R6 is selected from H and a C1-C5 alkyl optionally substituted with one or more halogens, C2-C5 alkenyl optionally substituted with one or more halogens, C2-Cs alkynyl optionally substituted with one or more halogens. In certain embodiments, R6 is an optionally substituted Cι-C8 alkyl, an optionally substituted C2-C8 alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C8 heteroalkyl, an optionally substituted C2-C8 heteroalkenyl, an optionally substituted C2-C8 heteroalkynyl, an optionally substituted C -C8 cycloalkyl an optionally substituted C3-C8 cycloalkenyl, or an optionally substituted C -C8 cycloalkynyl. In certain of such embodiments, R6 is selected from an optionally substituted C2-C8 alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C heteroalkenyl, an optionally substituted C2-C3 heteroalkynyl, an optionally substituted C3-C8 cycloalkenyl, and an optionally substituted C3-C8 cycloalkynyl. In certain embodiments, R is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl. [0102] In certain embodiments, R7 is selected from CH2OH, CHO, COOH and a group of formula (a):
(a); [0103] In certain embodiments, R and R are each independently selected from
H, OH and a methyl optionally substituted with one or more fluorines; [0104] In certain embodiments, n is 1, 2 or 3; [0105] In certain embodiments X is O, NR10 or S.
[0106] In certain embodiments, R10 is selected from H and a Cp C5 alkyl optionally substituted with one or more halogens, a C2-C5 alkenyl optionally substituted with one or more halogens, a C2-C5 alkynyl optionally substituted with one or more halogens. In certain embodiments, R10 is an optionally substituted Cι-C8 alkyl, an optionally substituted C2-C8ι alkenyl, an optionally substituted C2-C8 alkynyl, an optionally substituted C2-C8 heteroalkyl, an optionally substituted C2-C8 heteroalkenyl, an optionally substituted C2-C8 heteroalkynyl, an optionally substituted C -C8 cycloalkyl, an optionally substituted C3-C8 cycloalkenyl, or an optionally substituted C3- C8 cycloalkynyl. In certain of such embodiments, R10 is selected from an optionally substituted C2-C8 alkenyl, an optionally substituted C2-C alkynyl, an optionally substituted C2-C3 heteroalkenyl, an optionally substituted C2-C3 heteroalkynyl, an optionally substituted C3-C8 cycloalkenyl, and an optionally substituted C3-C8 cycloalkynyl. In certain embodiments, R10 is selected from an optionally substituted methyl, ethyl propyl isopropyl, butyl, sec-butyl, and tert-butyl. [0107] Certain compounds of the present inventions may exist as stereoisomers including, but not limited to, optical isomers. The present disclosure is intended to include all stereoisomers and both the racemic mixtures of such stereoisomers as well as the individual enantiomers that may be separated according to methods that are known in the art or that may be excluded by synthesis schemes known in the art designed to yield predominantly one enantomer relative to another. [0108] As used herein, the term "stereoisomer" refers to a compound made up of the same atoms bonded by the same bonds but having different three-dimensional structures which are not interchangeable. The three-dimensional structures are called configurations. As used herein, the term "enantiomer" refers to two stereoisomers whose
molecules are nonsuperimposable mirror images of one another. The term "chiral center" refers to a carbon atom to which four different groups are attached. As used herein, the term "diastereomers" refers to stereoisomers which are not enantiomers. In addition, two diastereomers which have a different configuration at only one chiral center are referred to herein as "epimers." The terms "racemate," "racemic mixture" or "racemic modification" refer to a mixture of equal parts of enantiomers. [0109] The compounds of the present invention may be chiral, and it is intended that any enantiomers, as separated, pure or partially purified enantiomers or racemic mixtures thereof are included within the scope of the invention. Furthermore, when a double bond or a fully or partially saturated ring system or more than one center of asymmetry or a bond with restricted rotatability is present in the molecule diastereomers may be formed. It is intended that any diastereomers, as separated, pure or partially purified diastereomers or mixtures thereof are included within the scope of the invention. Furthermore, some of the compounds of the present invention may exist in different tautomeric forms and it is intended that any tautomeric forms, which the compounds are able to form, are included within the scope of the present invention. Thus, as one skilled in the art knows, certain aryls may exist in tautomeric forms. The invention also includes tautomers, enantiomers and other stereoisomers of the compounds of Formula I. Such variations are contemplated to be within the scope of the invention. [0110] The terms "R" and "S" are used herein as commonly used in organic chemistry to denote specific configuration of a chiral center. The term "R" (rectus) refers to that configuration of a chiral center with a clockwise relationship of group priorities (highest to second lowest) when viewed along the bond toward the lowest priority group. The term "S" (sinister) refers to that configuration of a chiral center with
a counterclockwise relationship of group priorities (highest to second lowest) when viewed along the bond toward the lowest priority group. The priority of groups is based upon their atomic number (in order of decreasing atomic number). A partial list of priorities and a discussion of stereochemistry is contained in "Nomenclature of Organic Compounds: Principles and Practice", (J.H. Fletcher, et al., eds., 1974) at pages 103-120. [0111] The designation " ~~ " refers to a bond that protrudes forward out of the plane of the page. [0112] The designation " " refers to a bond that protrudes backward out of the plane of the page. [0113] The designation " ""^ " refers to a bond wherein the stereochemistry is not defined. [0114] The compounds of Formula I, when existing as a diastereomeric mixture, may be separated into diastereomeric pairs of enantiomers by, for example, fractional crystallization from a suitable solvent, for example methanol or ethyl acetate or a mixture thereof. The pair of enantiomers thus obtained may be separated into individual stereoisomers by conventional means, for example by the use of an optically active acid as a resolving agent. Alternatively, any enantiomer of a compound of Formula I may be obtained by stereospecific synthesis using optically pure starting materials or reagents of known configuration or through enantioselective synthesis.
[0115] The term "enantiomeric enrichment" as used herein refers to the increase in the amount of one enantiomer as compared to the other. A convenient method of expressing the enantiomeric enrichment achieved is the concept of enantiomeric excess, or "ee," which is found using the following equation: ee = E' - E2 X 100 E' + E2 [0116] wherein E1 is the amount of the first enantiomer and E2 is the amount of the second enantiomer. Thus, if the initial ratio of the two enantiomers is 50:50, such as is present in a racemic mixture, and an enantiomeric enrichment sufficient to produce a final ratio of 70:30 is achieved, the ee with respect to the first enantiomer is 40%. However, if the final ratio is 90:10, the ee with respect to the first enantiomer is 80%. An ee of greater than 90% is prefeπed, an ee of greater than 95% is most prefeπed and an ee of greater than 99% is most especially preferred. Enantiomeric enrichment is readily determined by one of ordinary skill in the art using standard techniques and procedures, such as gas or high performance liquid chromatography with a chiral column. Choice of the appropriate chiral column, eluent and conditions necessary to effect separation of the enantiomeric pair is well within the knowledge of one of ordinary skill in the art. In addition, the specific stereoisomers and enantiomers of compounds of Formula I can be prepared by one of ordinary skill in the art utilizing well known techniques and processes, such as those disclosed by J. Jacques, et al., "Enantiomers, Racemates, and Resolutions," John Wiley and Sons, Inc., 1981, and E.L. Eliel and S.H. Wilen," Stereochemistry of Organic Compounds." (Wiley-Interscience 1994), and European Patent Application No. EP-A-838448, published April 29, 1998. Examples of resolutions include recrystallization techniques or chiral chromatography.
[0117] The following table provides examples of certain variables from various Markush groups in this application. One of ordinary skill in the art will recognize that the variables may selected in any combination. [0118]
In certain embodiments, the invention provides compounds selected from:
4-(2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid (Compounds 1 and
4-(2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)phenylacetic acid (Compounds 3 and 4)
4-(8,9-Dimethyl-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid (Compound 5)
4-(8,9-Cyclopentano-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5- yl)benzoic acid (Compound 6)
4-(2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)phenylacetic acid (Compound 8)
4-(9,10-Benzo-2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)benzoic acid (Compound 9)
4-(9,10-benzo-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid (Compound 10)
and pharmaceutically acceptable salts, esters, amides, and/or prodrugs of any of those compounds.
[0119] In certain embodiments, a compound of Formula I is a selective HNF-4α receptor modulator. In certain embodiments, a compound of Formula I is a selective HNF-4α receptor agonist. In certain embodiments, a compound of Formula I is a selective HNF-4α receptor antagonist. In certain embodiments, a compound of Formula I is a selective HNF-4α receptor partial agonist. In certain embodiments, a compound of Formula I is a tissue-specific selective HNF-4α receptor modulator. In certain embodiments, a compound of Formula I is a gene-specific selective HNF-4α receptor modulator. In certain embodiments, a compound of Formula I is a selective HNF-4α receptor binding compound. [0120] In certain embodiments, the present invention provides selective HNF-4 receptor modulators. In certain embodiments, the invention provides selective HNF-4α receptor binding agents. In certain embodiments, the invention provides methods of making and methods of using selective HNF-4α receptor modulators and/or selective HNF-4α binding agents. In certain embodiments, selective HNF-4α modulators are agonists, partial agonists, and/or antagonists for the HNF-4α receptor. In certain embodiments, the invention provides compounds that are selective for an HNF-4α receptor relative to a retinoic X receptor (RXR). In certain embodiments, the invention provides compounds that are selective for an HNF-4α receptor relative to an RXR by at least 8 times.
Certain Synthesis Methods Compounds of formula I may be synthesized using the procedure described in scheme 1.
SCHEME I
Wherein the definitions of Ri through R7 are identical to those described in Formula I.
[0121] In certain embodiments, the invention provides a salt coπesponding to any of the compounds provided herein. In certain embodiments, the invention provides a salt corresponding to a selective HNF-4α modulator. In certain embodiments, the
invention provides a salt coπesponding to a selective HNF-4α receptor binding agent. In certain embodiments, a salt is obtained by reacting a compound with an inorganic acid, such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, and the like. In certain embodiments, a salt is obtained by reacting a compound with a base to form a salt such as an ammonium salt, an alkali metal salt, such as a sodium or a potassium salt, an alkaline earth metal salt, such as a calcium or a magnesium salt, a salt of organic bases such as dicyclohexylamine, N-methyl-D-glucamine, tris(hydroxymethyl)methylamine, and salts with amino acids such as arginine, lysine, and the like.
[0122] In certain embodiments, one or more carbon atoms of a compound of the present invention is replaced with silicon. See e.g., WO 03/037905A1; Tacke and Zilch, Endeavour, New Series, 10, 191-197 (1986); Bains and Tacke, Curr. Opin. Drug Discov Devel. Jul:6(4):526-43(2003). In certain embodiments, compounds of the present invention comprising one or more silicon atoms possess certain desired properties, including, but not limited to, greater stability and/or longer half-life in a patient, when compared to the same compound in which none of the carbon atoms have been replaced with a silicon atom. [0123] Protecting groups that may be used in the present invention include those that are commonly known to those skilled in the art, such groups include, but are not limited to TBDMS, TBS and Benzyl. Certain Assays
[0124] In certain embodiments, compounds of the present invention are capable of modulating activity of HNF-4α receptors in a "co-transfection" assay (also called a "cis-trans" assay), which has been discussed previously. See e.g., Evans et al., Science, 240:889-95 (1988); U.S. Patent Nos. 4,981,784 and 5,071,773; Pathirana et al., Mol. Pharm. 47:630-35 (1995)). Modulating activity in a co-transfection assay has been shown to coπelate with in vivo modulating activity. Thus, in certain embodiments, such assays are predictive of in vivo activity. See, e.g., Berger et al., J. Steroid Biochem. Molec. Biol. 41:773 (1992). [0125] In certain co-transfection assays, two different co-transfection plasmids are prepared. In the first co-transfection plasmid, cloned cDNA encoding an intracellular receptor (e.g., HNF-4α receptor) is operatively linked to a constitutive promoter (e.g., the SV 40 promoter). In the second co-transfection plasmid, cDNA encoding a reporter
protein, such as firefly luciferase (LUC), is operatively linked to a promoter that is activated by a receptor-dependant activation factor. Both co-transfection plasmids are co-transfected into the same cells. Expression of the first co-transfection plasmid results in production of the intracellular receptor protein. Activation of that intracellular receptor protein (e.g., by binding of an agonist) results in production of a receptor- dependant activation factor for the promoter of the second co-transfection plasmid. That receptor-dependant activation factor in turn results in expression of the reporter protein encoded on the second co-transfection plasmid. Thus, reporter protein expression is linked to activation of the receptor. Typically, that reporter activity can be conveniently measured (e.g., as increased luciferase production). [0126] Certain co-transfection assays can be used to identify agonists, partial agonists, and/or antagonists of intracellular receptors. In certain embodiments, to identify agonists, co-transfected cells are exposed to a test compound. If the test compound is an agonist or partial agonist, reporter activity is expected to be higher compared to co-transfected cells in the absence of the test compound. In certain embodiments, to identify antagonists, the cells are exposed to a known agonist (e.g., the natural ligand for the receptor) in the presence and absence of a test compound. If the test compound is an antagonist, reporter activity is expected to be lower than that of cells exposed only to the known agonist. [0127] In certain embodiments, compounds of the invention are used to detect the presence, quantity and/or state of receptors in a sample. In certain of such embodiments, samples are obtained from a patient. In certain embodiments, compounds are radio- or isotopically-labeled. For example, compounds of the present invention that
selectively bind HNF-4α receptors may be used to determine the presence or amount of such receptors in a sample, such as cell homogenates and lysates. [0128] In certain embodiments, the present invention provides for use of both CARLA and mammalian-2-hybrid assays, to characterize the in vitro profile of compounds of the invention on a HNF-4α receptor.
Certain Pharmaceutical Agents [0129] In certain embodiments, at least one selective HNF-4α receptor modulator, or pharmaceutically acceptable salt, ester, amide, and/or prodrug thereof, either alone or combined with one or more pharmaceutically acceptable carriers, forms a pharmaceutical agent. Techniques for formulation and administration of compounds of the present invention may be found for example, in "Remington's Pharmaceutical Sciences," Mack Publishing Co., Easton, PA, 18th edition, 1990. [0130] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention is prepared using known techniques, including, but not limited to mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or tabletting processes. [0131] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention is a liquid (e.g., a suspension, elixir and/or solution). In certain of such embodiments, a liquid pharmaceutical agent comprising one or more compounds of the present invention is prepared using ingredients known in the art, including, but not limited to, water, glycols, oils, alcohols, flavoring agents, preservatives, and coloring agents.
[0132] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention is a solid (e.g., a powder, tablet, and/or capsule). In certain of such embodiments, a solid pharmaceutical agent comprising one or more compounds of the present invention is prepared using ingredients known in the art, including, but not limited to, starches, sugars, diluents, granulating agents, lubricants, binders, and disintegrating agents. [0133] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention is formulated as a depot preparation. Certain of such depot preparations are typically longer acting than non-depot preparations. In certain embodiments, such preparations are administered by implantation (for example subcutaneously or intramuscularly) or by intramuscular injection. In certain embodiments, depot preparations are prepared using suitable polymeric or hydrophobic materials (for example an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives, for example, as a sparingly soluble salt. [0134] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention comprises a delivery system. Examples of delivery systems include, but are not limited to, liposomes and emulsions. Certain delivery systems are useful for preparing certain pharmaceutical agents including those comprising hydrophobic compounds. In certain embodiments, certain organic solvents such as dimethylsulfoxide are used. [0135] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention comprises one or more tissue-specific delivery molecules designed to deliver the pharmaceutical agent to specific tissues or cell types.
For example, in certain embodiments, pharmaceutical agents include liposomes coated with a tissue-specific antibody. [0136] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention comprises a co-solvent system. Certain of such co- solvent systems comprise, for example, benzyl alcohol, a nonpolar surfactant, a water- miscible organic polymer, and an aqueous phase. In certain embodiments, such co- solvent systems are used for hydrophobic compounds. A non-limiting example of such a co-solvent system is the VPD co-solvent system, which is a solution of absolute ethanol comprising 3% w/v benzyl alcohol, 8% w/v of the nonpolar surfactant Polysorbate 80™ , and 65% w/v polyethylene glycol 300. The proportions of such co-solvent systems may be varied considerably without significantly altering their solubility and toxicity characteristics. Furthermore, the identity of co-solvent components may be varied: for example, other surfactants may be used instead of Polysorbate 80™; the fraction size of polyethylene glycol may be varied; other biocompatible polymers may replace polyethylene glycol, e.g., polyvinyl pyπolidone; and other sugars or polysaccharides may substitute for dextrose. [0137] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention comprises a sustained-release system. A non- limiting example of such a sustained-release system is a semi-permeable matrix of solid hydrophobic polymers. In certain embodiments, sustained-release systems may, depending on their chemical nature, release compounds over a period of hours, days, weeks or months.
[0138] Certain compounds used in pharmaceutical agent of the present invention may be provided as pharmaceutically acceptable salts with pharmaceutically compatible counterions. Pharmaceutically compatible salts may be formed with many acids, including but not limited to hydrochloric, sulfuric, acetic, lactic, tartaric, malic, succinic, etc. [0139] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention comprises an active ingredient in a therapeutically effective amount. In certain embodiments, the therapeutically effective amount is sufficient to prevent, alleviate or ameliorate symptoms of a disease or to prolong the survival of the subject being treated. Determination of a therapeutically effective amount is well within the capability of those skilled in the art. [0140] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention is formulated as a prodrug. In certain embodiments, prodrugs are useful because they are easier to administer than the coπesponding active form. For example, in certain instances, a prodrug may be more bioavailable (e.g., through oral administration) than is the coπesponding active form. In certain instances, a prodrug may have improved solubility compared to the coπesponding active form. In certain embodiments, a prodrug is an ester. In certain embodiments, such prodrugs are less water soluble than the coπesponding active form. In certain instances, such prodrugs possess superior transmittal across cell membranes, where water solubility is detrimental to mobility. In certain embodiments, the ester in such prodrugs is metabolically hydrolyzed to carboxylic acid. In certain instances the carboxylic acid containing compound is the coπesponding active form. In certain embodiments, a
prodrug comprises a short peptide (polyaminoacid) bound to an acid group. In certain of such embodiments, the peptide is metabolized to form the coπesponding active form. [0141] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention is useful for treating a conditions or disorder in a mammalian, and particularly in a human patient. Suitable administration routes include, but are not limited to, oral, rectal, transmucosal, intestinal, enteral, topical, suppository, through inhalation, intrathecal, intraventricular, intraperitoneal, intranasal, intraocular and parenteral (e.g., intravenous, intramuscular, intramedullary, and subcutaneous). In certain embodiments, pharmaceutical intrathecals are administered to achieve local rather than systemic exposures. For example, pharmaceutical agents may be injected directly in the area of desired effect (e.g., in the renal or cardiac area). [0142] In certain embodiments, a pharmaceutical agent comprising one or more compounds of the present invention is administered in the form of a dosage unit (e.g., tablet, capsule, bolus, etc.). In certain embodiments, such dosage units comprise a selective a HNF-4α receptor modulator in a dose from about 1 μg/kg of body weight to about 50 mg/kg of body weight. In certain embodiments, such dosage units comprise a selective a HNF-4α receptor modulator in a dose from about 2 μg/kg of body weight to about 25 mg/kg of body weight. In certain embodiments, such dosage units comprise a selective a HNF-4α receptor modulator in a dose from about 10 μg/kg of body weight to about 5 mg/kg of body weight. In certain embodiments, pharmaceutical agents are administered as needed, once per day, twice per day, three times per day, or four or more times per day. It is recognized by those skilled in the art that the particular dose, frequency, and duration of administration depends on a number of factors, including,
without limitation, the biological activity desired, the condition of the patient, and tolerance for the pharmaceutical agent. [0143] In certain embodiments, a pharmaceutical agent comprising a compound of the present invention is prepared for oral administration. In certain of such embodiments, a pharmaceutical agent is formulated by combining one or more compounds of the present invention with one or more pharmaceutically acceptable carriers. Certain of such carriers enable compounds of the invention to be formulated as tablets, pills, dragees, capsules, liquids, gels, syrups, slurries, suspensions and the like, for oral ingestion by a patient. In certain embodiments, pharmaceutical agents for oral use are obtained by mixing one or more compounds of the present invention and one or more solid excipient. Suitable excipients include, but are not limited to, fillers, such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such as, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methyl cellulose, hydroxypropylmethyl-cellulose, sodium carboxymethylcellulose, and/or polyvinylpyπolidone (PVP). In certain embodiments, such a mixture is optionally ground and auxiliaries are optionally added. In certain embodiments, pharmaceutical agents are formed to obtain tablets or dragee cores. In certain embodiments, disintegrating agents (e.g., cross-linked polyvinyl pyπolidone, agar, or alginic acid or a salt thereof, such as sodium alginate) are added. [0144] In certain embodiments, dragee cores are provided with coatings. In certain of such embodiments, concentrated sugar solutions may be used, which may optionally contain gum arabic, talc, polyvinyl pyπolidone, carbopol gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions, and suitable organic solvents or solvent mixtures. Dyestuffs or pigments may be added to tablets or dragee coatings.
[0145] In certain embodiments, pharmaceutical agents for oral administration are push-fit capsules made of gelatin. Certain of such push-fit capsules comprise one or more compounds of the present invention in admixture with one or more filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers. In certain embodiments, pharmaceutical agents for oral administration are soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol. In certain soft capsules, one or more compounds of the present invention are be dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols. In addition, stabilizers may be added. [0146] In certain embodiments, pharmaceutical agents are prepared for buccal administration. Certain of such pharmaceutical agents are tablets or lozenges formulated in conventional manner. [0147] In certain embodiments, a pharmaceutical agent is prepared for administration by injection (e.g., intravenous, subcutaneous, intramuscular, etc.). In certain of such embodiments, a pharmaceutical agent comprises a carrier and is formulated in aqueous solution, such as water or physiologically compatible buffers such as Hanks's solution, Ringer's solution, or physiological saline buffer. In certain embodiments, other ingredients are included (e.g., ingredients that aid in solubility or serve as preservatives). In certain embodiments, injectable suspensions are prepared using appropriate liquid carriers, suspending agents and the like. Certain pharmaceutical agents for injection are presented in unit dosage form, e.g., in ampoules or in multi-dose containers. Certain pharmaceutical agents for injection are suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents. Certain solvents suitable for use in
pharmaceutical agents for injection include, but are not limited to, lipophilic solvents and fatty oils, such as sesame oil, synthetic fatty acid esters, such as ethyl oleate or triglycerides, and liposomes. Aqueous injection suspensions may contain substances that increase the viscosity of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran. Optionally, such suspensions may also contain suitable stabilizers or agents that increase the solubility of the compounds to allow for the preparation of highly concentrated solutions. [0148] In certain embodiments, a pharmaceutical agent is prepared for transmucosal administration. In certain of such embodiments penetrants appropriate to the barrier to be permeated are used in the formulation. Such penetrants are generally known in the art. [0149] In certain embodiments, a pharmaceutical agent is prepared for administration by inhalation. Certain of such pharmaceutical agents for inhalation are prepared in the form of an aerosol spray in a pressurized pack or a nebulizer. Certain of such pharmaceutical agents comprise a propellant, e.g., dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas. In certain embodiments using a pressurized aerosol, the dosage unit may be determined with a valve that delivers a metered amount. In certain embodiments, capsules and cartridges for use in an inhaler or insufflator may be formulated. Certain of such formulations comprise a powder mixture of a compound of the invention and a suitable powder base such as lactose or starch.
[0150] In certain embodiments, a pharmaceutical agent is prepared for rectal administration, such as a suppositories or retention enema. Certain of such pharmaceutical agents comprise known ingredients, such as cocoa butter and/or other glycerides. [0151] In certain embodiments, a pharmaceutical agent is prepared for topical administration. Certain of such pharmaceutical agents comprise bland moisturizing bases, such as ointments or creams. Exemplary suitable ointment bases include, but are not limited to, petrolatum, petrolatum plus volatile silicones, lanolin and water in oil emulsions such as Eucerin™, available from Beiersdorf (Cincinnati, Ohio). Exemplary
suitable cream bases include, but are not limited to, Nivea™ Cream, available from
Beiersdorf (Cincinnati, Ohio), cold cream (USP), Purpose Cream™, available from Johnson & Johnson (New Brunswick, New Jersey), hydrophilic ointment (USP) and Lubriderm™, available from Pfizer (Morris Plains, New Jersey). [0152] In certain embodiments, the formulation, route of administration and dosage for a pharmaceutical agent of the present invention can be chosen in view of a particular patient's condition. (See e.g., Fingl et al. 1975, in "The Pharmacological Basis of Therapeutics", Ch. 1 p. 1). In certain embodiments, a pharmaceutical agent is administered as a single dose. In certain embodiments, a pharmaceutical agent is administered as a series of two or more doses administered over one or more days. [0153] In certain embodiments, a pharmaceutical agent of the present invention is administered to a patient between about 0.1% and 500%, more preferably between about
25%) and 75% of an established human dosage. Where no human dosage is established, a suitable human dosage may be infeπed from ED5o or ID50 values, or other appropriate values derived from in vitro or in vivo studies.
[0154] In certain embodiments, a daily dosage regimen for a patient comprises an oral dose of between 0.1 mg and 2000 mg of a compound of the present invention. In certain embodiments, a daily dosage regimen is administered as a single daily dose. In certain embodiments, a daily dosage regimen is administered as two, three, four, or more than four doses. [0155] In certain embodiments, a pharmaceutical agent of the present invention is administered by continuous intravenous infusion. In certain of such embodiments, from 0.1 mg to 500 mg of a composition of the present invention is administered per day. [0156] In certain embodiments, a pharmaceutical agent of the invention is administered for a period of continuous therapy. For example, a pharmaceutical agent of the present invention may be administered over a period of days, weeks, months, or years. [0157] Dosage amount, interval between doses, and duration of treatment may be adjusted to achieve a desired effect. In certain embodiments, dosage amount and interval between doses are adjusted to maintain a desired concentration on compound in a patient. For example, in certain embodiments, dosage amount and interval between doses are adjusted to provide plasma concentration of a compound of the present invention at an amount sufficient to achieve a desired effect. In certain of such embodiments the plasma concentration is maintained above the minimal effective concentration (MEC). In certain embodiments, pharmaceutical agents of the present invention are administered with a dosage regimen designed to maintain a concentration above the MEC for 10-90%) of the time, between 30-90%> of the time, or between 50-90%) of the time.
[0158] In certain embodiments in which a pharmaceutical agent is administered locally, the dosage regimen is adjusted to achieve a desired local concentration of a compound of the present invention. [0159] In certain embodiments, a pharmaceutical agent may be presented in a pack or dispenser device which may contain one or more unit dosage forms containing the active ingredient. The pack may for example comprise metal or plastic foil, such as a blister pack. The pack or dispenser device may be accompanied by instructions for administration. The pack or dispenser may also be accompanied with a notice associated with the container in form prescribed by a governmental agency regulating the manufacture, use, or sale of pharmaceuticals, which notice is reflective of approval by the agency of the form of the drug for human or veterinary administration. Such notice, for example, may be the labeling approved by the U.S. Food and Drug Administration for prescription drugs, or the approved product insert. Compositions comprising a compound of the invention formulated in a compatible pharmaceutical carrier may also be prepared, placed in an appropriate container, and labeled for treatment of an indicated condition. [0160] In certain embodiments, a pharmaceutical agent is in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
Certain Combination Therapies [0161] In certain embodiments, one or more pharmaceutical agents of the present invention are co-administered with one or more other pharmaceutical agents. In certain embodiments, such one or more other pharmaceutical agents are designed to treat the same disease or condition as the one or more pharmaceutical agents of the present invention. In certain embodiments, such one or more other pharmaceutical agents are designed to treat a different disease or condition as the one or more pharmaceutical agents of the present invention. In certain embodiments, such one or more other pharmaceutical agents are designed to treat an undesired effect of one or more pharmaceutical agents of the present invention. In certain embodiments, one or more pharmaceutical agents of the present invention is co-administered with another pharmaceutical agent to treat an undesired effect of that other pharmaceutical agent. In certain embodiments, one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are administered at the same time. In certain embodiments, one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are administered at the different times. In certain embodiments, one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are prepared together in a single formulation. In certain embodiments, one or more pharmaceutical agents of the present invention and one or more other pharmaceutical agents are prepared separately. [0162] Examples of pharmaceutical agents that may be co-administered with a pharmaceutical agent of the present invention include, but are not limited to, analgesics (e.g., acetaminophen); anti-inflammatory agents, including, but not limited to non- steroidal anti-inflammatory drugs (e.g., ibuprofen, COX-1 inhibitors, and COX-2,
inhibitors); salicylates; antibiotics; antivirals; antifungal agents; antidiabetic agents (e.g., biguanides, glucosidase inhibitors, insulins, sulfonylureas, and thiazolidenediones); adrenergic modifiers; diuretics; hormones (e.g., anabolic steroids, androgen, estrogen, calcitonin, progestin, somatostatin, and thyroid hormones); immunomodulators; muscle relaxants; antihistamines; osteoporosis agents (e.g., biphosphonates, calcitonin, and estrogens); prostaglandins, antineoplastic agents; psychotherapeutic agents; sedatives; poison oak or poison sumac products; antibodies; and vaccines. Certain Indications [0163] In certain embodiments, the invention provides methods of treating a patient comprising administering one or more compounds of the present invention. Compounds of the present invention, including, but not limited to, pharmaceutically acceptable salts, solvates and hydrates, are expected to be effective in treating diseases or conditions that are mediated by HNF-4α. Therefore, in certain embodiments, compounds of the invention are effective in treating conditions that are mediated by HNF-4α, including, but not limited to, syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia. In certain embodiments, a patient is treated prophylactically to reduce or prevent the occuπence of a condition. [0164] In certain embodiments, the present invention provides a method of lowering blood glucose levels in a mammal by administering to the patient a pharmaceutically effective amount of at least one compound of the present invention. In certain embodiments, the patient is a mammal. In certain embodiments, the patient is a human.
[0165] In certain embodiments, the present invention provides a method of lowering plasma triglycerides levels in a patient by administering to the mammal a pharmaceutically effective amount of at least one compound of the present invention. In certain embodiments, the patient is a mammal. In certain embodiments, the patient is a human. [0166] In certain embodiments, the present invention provides a method of increasing insulin levels in a patient by administering to the mammal a pharmaceutically effective amount of at least one compound of the present invention. In certain embodiments, the patient is a mammal. In certain embodiments, the patient is a human. EXAMPLES [0167] The following examples, including experiments and results achieved, are provided for illustrative purposes only and are not to be construed as limiting the present invention. Example 1
Methyl 4-(2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoate (Compound 1)
[0168] A mixture of aniline (1.4 mL, 15 mmol) and methyl 4-formylbenzoate (2.6 g, 16 mmol) in 10 mL of EtOH was heated at reflux for 2 h, then allowed to cool. A white precipitate was isolated by filtration and dried under vacuum to leave 3.52 g (98%) of methyl 4-phenylimino benzoate. Methyl 4-phenylimino benzoate (2.0 g, 8.4 mmol)
was suspended in 40 mL of CH CN and the mixture was cooled to 0°C before addition of 3,4-dihydro-2H-pyran (1.15 mL, 12.6 mmol) and ytterbium triflate monohydrate (0.52 g, 0.84 mmol). The mixture was stiπed at 0°C for 30 min, then at room temperature for 2 h. A white precipitate was isolated by filtration, providing 1.55 g of the desired product methyl 4-(2,3,4a,5,6,10b-Hexahydropyrano[3,2-c]quinolin-5-yl)benzoate. The filtrate was diluted with 75 mL of EtOAc. This solution was washed with 75 mL of aqueous saturated NaHCO solution and 75 mL of saturated aqueous NaCI solution, then dried and concentrated to leave a yellow oil. This crude product was purified by silica gel chromatography (10% followed by 20% EtOAc/hexane) to afford an additional 0.59 g of product. This material was combined with the aforementioned precipitate to yield 2.14 g (79%) of product as a mixture of two diastereomers (1:1 cis/trans). Although this mixture was caπied on to the next step, it was found during a separate run that the diastereomers are separable by silica gel chromatography (10%> followed by 25% EtOAc/hexane). Cis isomer, Η NMR (500 MHz, CDC13) δ: 8.06 (d, J = 8.2 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 7.6 Hz, 1H), 7.12 (dd, J = 7.3, 7.9 Hz, 1H), 6.83 (dd, J = 7.3, 7.6, 1H), 6.64 (d, J = 7.9, 1H), 5.35 (d, J = 5.5 Hz, 1H), 4.76 (d, J = 2.4 Hz, 1H), 3.94 (s, 3H), 3.59 (m, 1H), 3.44 (ddd, J = 2.4, 11.3, 11.6, 1H), 2.20 (m, 1H), 1.4-1.6 (m, 4H), 1.24 (m, 1H). Trans isomer, Η NMR (500 MHz, CDC13) δ: 8.05 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 8.2 Hz, 2H), 7.24 (dd, J = 1.5, 7.6 Hz, 1H), 7.12 (ddd, J = 1.5, 7.6, 7.9 Hz, 1H), 6.74 (ddd, J = 0.9, 7.3, 7.6 Hz, 1H), 6.57 (d, J = 7.9 Hz, 1H), 4.79 (d, J = 10.7 Hz, 1H), 4.40 (d, J = 2.1 Hz, 1H), 4.09 (m, 1H), 3.94 (s, 3H), 3.73 (ddd, J = 2.4, 11.3, 11.3 Hz, 1H), 2.10 (m, 1H), 1.85 (m, 1H), 1.68 (m, 1H), 1.4 (m, 2H).
Example 2
4-(2,3,4a,5,6,10b-hex enzoic acid (Compound 2)
[0169] To a suspension of methyl 4-(2,3,4a,5,6,10b-hexahydropyrano[3,2- c]quinolin-5-yl)benzoate (2.05 mg, 6.3 mmol, 1:1 cis/trans mixture) in 15 mL of THF was added 8 mL of water and lithium hydroxide monohydrate (0.57 g, 14 mmol). The mixture was stiπed and heated at 50° C before treatment with 20 mL of IM aqueous HCI solution and extraction with EtOAc (2 x 50 mL). The organic layer was washed with 20 mL of saturated aqueous NaCI solution, dried and concentrated to leave an off-white solid that was triturated with CH CN to leave 1.87 g (96%) of the coπesponding carboxylic acid 4-(2,3,4a,5,6,10b-Hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid as a mixture of two diastereomers (1:1 cis/trans). These diastereomers were separated by reverse-phase HPLC (2" C18 column, 80 mL/min 80:20:0.1 MeOH/H2O/trifluoroacetic acid). Cis isomer, Η NMR (500 MHz, D6-DMSO) δ: 7.94 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 8.2 Hz, 2H), 7.17 (d, J = 7.6 Hz, IH), 6.98 (dd, J = 7.3, 7.9 Hz, IH), 6.68 (d, J = 7.9, IH), 6.63 (ddd, J = 0.9, 7.6, 8 Hz, IH), 6.03 (s, IH), 5.23 (d, J = 5.5 Hz, IH), 4.71 (d, J = 2.2, IH), 3.47 (d, J = 11.9 Hz, IH), 3.23 (m, IH), 2.05 (m, IH), 1.35 (m, 3H), 1.02 (m, IH).
Trans isomer, Η NMR (500 MHz, CDC13) δ: 7.95 (d, J = 7.8, 2H), 7.56 (d, J = 8.3 Hz, 2H), 7.05 (d, J = 7 Hz, IH), 7.00 (dd, J = 7.3, 7.8 Hz, IH), 6.60 (d, J = 7.8 Hz, IH), 6.52 (dd, J = 7.3, 7.3 Hz, IH), 6.22 (s, IH), 4.60 (d, J = 10.3 Hz, IH), 4.29 (d, J = 2.9 Hz, IH),
3.89 (d, J = 11.2 Hz, 1H), 3.61 (ddd, J = 2.4, 11.2, 11.2 Hz, IH), 1.95 (m, IH), 1.76 (m,
IH), 1.63 (m, lH), 1.26 (m, 2H).
7.43 (d, J= 8.1 Hz, 2H), 7.33 (d, j = 8.1 Hz, 2H), 7.28 (d, J = 7.7 Hz, IH), 7.15 (t, J =
7.3 Hz, IH), 6.81 (t, j = 7.3, IH), 6.62 (d, J = 7.7 Hz, IH), 4.76 (d, j = 11.2 Hz, IH),
4.46 (d, J = 2.4, IH), 4.14 (d, J = 10.7 Hz, IH), 3.75 (m, 3H), 2.20 (m, IH), 1.81 (m,
IH), 1.65 (m, IH), 1.52 (m, IH), 1.40 (m, IH). Examples 3 and 4
4-(2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)phenylacetic acid (Compounds 3 and 4)
[0170] 4-(2,3,4a,5,6,10b- hexahydropyrano[3,2-c]quinolin-5-yl)phenylacetic acid was synthesized according to the procedure described in Examples 1 and 2 using 4-ethyl-
4-formylphenyl acetate, aniline and 3,4-dihydro-2H-pyran. Cis isomer: Η NMR (500
MHz, CDC13) δ: 7.42 (d, J = 10.9 Hz, IH), 7.39 (d, J = 8.1 Hz, 2H), 7.30 (d, J = 8.1 Hz,
2H), 7.10 (t, J = 7.5 Hz, IH), 6.80 (t, J = 7.5 Hz, IH), 6.60 (d, j = 7.9 Hz, IH), 5.33 (d, j
= 5.5 Hz, IH), 4.69 (d, j = 2.0 Hz, IH), 3.68 (s, 2H), 3.59 (m, IH), 3.48 (m, IH), 2.12
(m, IH), 1.4-1.6 (m, 4H), 1.24 (m, IH).
Trans isomer: Η NMR (500 MHz, CDC13) δ: 7.43 (d, J= 8.1 Hz, 2H), 7.33 (d, J = 8.1
Hz, 2H), 7.28 (d, j = 1.1 Hz, IH), 7.15 (t, j = 7.3 Hz, IH), 6.81 (t, = 7.3, IH), 6.62 (d, j
= IH Hz, IH), 4.76 (d, J = 11.2 Hz, IH), 4.46 (d, j = 2.4, IH), 4.14 (d, j = 10.7 Hz, IH),
3.75 (m, 3H), 2.20 (m, IH), 1.81 (m, IH), 1.65 (m, IH), 1.52 (m, IH), 1.40 (m, IH).
Example 5
4-(8,9-dimethyl-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid (Compound 5)
[0171] 4-(8,9-dimethyl-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5- yl)benzoic acid was synthesized according to the procedure described for Examples 1 and 2 using methyl-4-formyl benzoate, 3,4-dimethylaniline and 3,4-dihydro-2H-pyran. Η NMR (400 MHz, D6-DMSO) δ: 7.94 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 8.1 Hz, 2H), 6.95 (s, IH), 6.50 (s, IH), 5.74 (s, IH), 5.19 (d, J = 5.1, IH), 4.64 (s, IH), 3.46 (d, J = 10.5 Hz, IH), 3.25 (m, 2H), 2.09 (s, 6H), 2.01 (m, 4H). Example 6
4-(8,9-Cyclopentano-2,3,4a,5,6,10b-hexahydropyrano[3,2-c] quinolin-5-yl)benzoic acid (Compound 6)
[0172] Synthesis of 4-(8,9-Cyclopentano-2,3,4a,5,6,10b-hexahydropyrano[3,2-c] quinolin-5-yl)benzoic acid was synthesized according to the procedure described in Examples 1 and 2 using methyl-4-formyl benzoate, 3,4-cyclopentaneaniline and 3,4- dihydro-2H-pyran. Η NMR (400 MHz, D6-DMSO) δ: 7.94 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 8.2 Hz, 2H), 6.89 (s, IH), 6.47 (s, IH), 5.98 (s, IH), 4.57 (d, J = 10.1, IH), 4.24 (s,
IH), 3.82 ( , 2H), 3.58 (m, 2H), 2.69 (m, 4H), 1.95 (m, 2H), 1.85 (m, IH), 1.61 (m, IH), 1.11 (m, 2H). Example 7
4-(2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)benzoic acid (Compound 7)
[0173] 4-(2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)benzoic acid was synthesized according to the procedure described in Examples 1 and 2 using methyl-4- formyl benzoate, aniline and 3,4-dihydro-2H-furan. Η NMR (500 MHz, D6-DMSO) δ: 7.96 (d, J = 8.2 Hz, 2H), 7.60 (d, J = 8.2 Hz, 2H), 7.19 (d, J = 7.3 Hz, IH), 7.02 (dd, J = 7.3, 7.8 Hz, IH), 6.72 (d, J = 7.8, IH), 6.62 (ddd, J = 0.9, 7.6, 7.9 Hz, IH), 6.27 (s, IH), 4.44 (d, J = 4.9 Hz, IH), 3.89 (m, IH), 3.71 (d, J = 10.8 Hz, IH), 3.65 (m, IH), 2.25 (m, IH), 1.95 (m, IH), 1.53 (m, IH). Example 8
4-(2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)phenylacetic acid (Compound 8)
[0174] 4-(2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)phenylacetic acid was synthesized according to the procedure described in Examples 1 and 2 using methyl-4- formyl phenyl acetate, aniline and 3,4-dihydro-2H-furan. Η NMR (500 MHz, D6-
DMSO) δ: 7.42 (d, J = 8.0 Hz, IH), 7.40 (d, J = 8.1 Hz, 2H), 7.30 (d, J = 8.1 Hz, 2H),
7.11 (t J = 8.0 Hz, IH), 6.80 (d, J = 8.0 Hz, IH), 6.62 (d, J = 8.0 Hz, IH), 4.61 (d, J = 5.0 Hz, IH), 4.02 (m, IH), 3.85 (m, IH), 3.80 (d, J = 11.0 Hz, IH), 3.69 (s, 2H), 1.93 (m, IH), 1.73 (m, IH).
Example 9
4-(8,9-Benzo-2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)benzoic acid (Compound 9)
[0175] 4-(8,9-Benzo-2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)benzoic acid was synthesized according to the procedure described in Examples 1 and 2 using methyl-4-formyl benzoate, 2-naphthaniline and 3,4-dihydro-2H-furan. Η NMR (400 MHz, D6-DMSO) δ: 12.9 (broad s, IH), 8.19 (d, J = 8.2 Hz, 2H), 7.97 (d, J = 8.2 Hz, 2H), 7.88 (m, IH), 7.62 (m, IH), 7.22 (m, IH), 7.19 (m, IH), 7.09 (m, IH), 6.68 (s, IH), 4.57 (d, J = 5.2 Hz, IH, cis isomer), 4.35 (d, J = 10.3 Hz, IH, trans isomer), 3.85 (m, IH), 2.22 (m, IH), 1.91 (m, IH), 1.62(m, IH). Example 10
4-(9,10-benzo-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid (Compound 10)
[0176] 4-(9,10-benzo-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5- yl)benzoic acid was synthesized according to the procedure described in Examples 1 and 2 using methyl-4-formyl benzoate, 2-naphthaniline and 3,4-dihydro-2H-pyran. Η NMR (400 MHz, D6-DMSO) δ: 12.9 (broad s, IH), 8.11 (s, IH), 7.98 (d, J = 8.1 Hz, 2H), 7.84 (m, IH), 7.63 (m, IH), 7.21 (m, IH), 7.15 (m, IH), 7.10 (m, IH), 7.05 (d, J = 8.1 Hz, 2H), 6.60 (s, IH), 4.89 (d, J = 2.4 Hz, IH, cis isomer), 4.75 (d, J = 11.6 Hz, IH, trans isomer), 3.92 (m, IH), 3.08 (m, IH), 2.10 (m, IH), 1.82 (m, IH), 1.41 (m, IH). Example 11
4-(6-Methyl - 3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester
[0177] 4-(3,4,4a,5,6, 10b-Ηexahydro-2H-pyrano[3,2-c]quinolin-5-yl)- benzoic acid methyl ester is dissolved in DMF (2mL). To this is added triethylamine
(0.059 g, 0.59 mmol) followed by iodomethane (0.045g, 0.586 mmol) . The reaction is heated at 50° C overnight .The reaction is partitioned between 1 N ΗC1 and ethyl acetate. The combined organic layers are washed with brine and dried over sodium sulfate and concentrated under reduced pressure to give a solid. The solid is triturated with hexane and diethyl ether to give a solid. Filtration gives the title compound. (0.015 g (15 % yield ) : mass spectrum (m/e):338.1(M+l)
Example 12
4-(6-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0178] 4-(6-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester ( 0.013 g, 0.04 mmol) is dissolved in TΗF (6mL) and lithium hydroxide (0.003 g, 0.08 mmol) is added and the reaction is heated at 60° C for 3 hours. TLC is performed using hexane: ethyl acetate (2:1) showing incomplete reaction.
Lithium hydroxide (0.005 g,0.2 mmol) is added and is heated for 2 hours. The reaction is acidified with 1 N ΗC1 and is extracted with ethyl acetate. The reaction is dried with brine and dried over sodium sulfate, evaporation yields the title compound, mass spectrum : 322 (M-l)
Example 13
4-(6-Allyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester
[0179] 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester and allyl bromide (0.106 g, 0.88mmol) and triethylamine (0.09 g, 123 mmol) are dissolved in DMF (1 mL). The reaction is heated at 50° C for 6 hrs. The reaction is partitioned between ethyl acetate and water. The organic layers are washed with brine and dried with sodium sulfate. The reaction is concentrated under reduced pressure to give a residue. The residue is purified with flash chromatography with hexane :ethyl acetate (95:5 ) and the polarity is gradually increased with hexane:ethyl acetate (85: 15) to give the title compound (0.017 g, 48 % yield), mass spectrum (m/e)
364.0 (M+l) Example 14
6-Allyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinoline-5 carboxylic acid
[0180] 4-(6-Allyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)- benzoic acid methyl ester (0.017 g, 0.05 mmol) is dissolved in TΗF (2 mL). Lithium hydroxide (0.003 g, 0.14 mmol) is added and the reaction is heated for 3 hours. The
reaction is acidified with 1 N HCI and extracted with ethyl acetate. The combined organic layer is washed with brine and dried over sodium sulfate, filtered and concentrated under reduced pressure to give the title compound, mass spectrum (m/e) 349.9 (M+l) Example 15 [4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-phenyl]-methanol
[0181] 4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester (0.090 g, 0.291 mmol) is dissolved in TΗF (2 ml) and is cooled in an ice bath. Lithium borohydride (0.006 g, 0.3 mmol) is added and the ice bath is removed after 40 minutes. The reaction is quenched with ethyl acetate then acidified with 1 N ΗC1. The reaction is extracted with ethyl acetate and the combined organic layers are washed with brine and dried over sodium sulfate. Filtration and concentration under reduced pressure gives a crude residue. The residue is purified with flash chromatography eluting with hexane : ethyl acetate (3:1) increasing the polarity with hexane : ethyl acetate (2:1), to give the title compound (0.018 g, 21 % yield), mass spectrum (m/e) 296.2 ( M+l)
Example 16
4-(6-Benzyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester
[0182] 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester (0.15 g, 0.46 mmol) is dissolved in DMF (1.5 mL). Benzyl bromide (0.24 g, 01.39 mmol) and triethylamine (0.14 g, 1.39 mmol) are added and the reaction is heated at 50° C for 9 hours. To the reaction is added IN ΗC1, and then extracted with ethyl acetate. The organic layers are combined and washed with brine and dried over sodium sulfate. The solvent is removed under vacuum and the residue is purified with flash chromatography using hexane : ethyl acetate (3: 1) to give the title compound (0.030 g, 16 % yield), mass spectrum (m/e) 414.0 (M+l) Example 17
4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester
50mmol), 3,4-Dihydro-2Η-pyran (6.31g,75mmol), and scandium triflate ( 2.41 g,
4.9mmol) are added to acetonitrile (380mL) and stiπed for 16 hours. The white solid (5.83 g) is filtered off to give a 50:50 mixture of the two diastereomers and the filtrate is diluted with ethyl acetate (240 mL) and is washed with saturated sodium bicarbonate (240mL) in water, and the organic layer is then washed with saturated brine (240mL). The organic layer is dried over sodium sulfate, filtered, and concentrated under reduced pressure yields a 50:50 mixture (12 g). A portion of the white solid (3.5 g) is purified by flash chromatography using hexane : ethyl acetate (95:5), gradually increasing the polarity with hexane : ethyl acetate(85:15) to give the all cis title compound (1.7g). mass spectrum (m/e)324.0 (M +1) Example 18
4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester, Isomer 1 , and Isomer 2
Chiral
[0184] 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester (1.7 g, 5.2 mmol) of the racemate is separated on a chiral OD column using methanol : DMEA (99.8:0.2) as eluent . Isomer 1 is 95.6 % ee. Isomer 2 is 97.8 % pure by ΗPLC.
Example 19
4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benzoic acid
[0185] 4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benzoic acid methyl ester (0.660g, 2.04 mmol) is dissolved in TΗF (15 mL), and Lithium hydroxide (0.145 g, 6.09 mmol) dissolved in water (7.5 mL) is heated at 50° C for 2 hours. The reaction is acidified with 1 N ΗC1 and is extracted with ethyl acetate. The organic layer is washed with brine and dried over sodium sulfate. The reaction is filtered and concentrated under reduced pressure gives the title compound (0.497g, 80 % yield).
(1 Η NMR, 400 MHz, CDC13) : δ7.95 (d, J=8.3 Hz,2 H), δ 7.39 (d, J=7 Hz,2 H), δ
7.30(d ,J=7 Hz,2 H), δ 7.30(d, J=7.5 hz,l H), δ 6.98 (t, J=9.1 Hz), δ 6.56 (d, J=8.3
Hz,lH), δ 5.20 (d, J=8.3 Hz,l H), δ 5.20 (d, J=4.2 Hz,l H), δ 4.54(d, J=2.7 Hz,lH), δ
3.47 (d, J=13.8 Hz), δ 3.31 (t, J=l 1.1 Hz,l H), δ 2.07 (b,lH), δ 1.49-1.27 (m,3H), δ 1.26
(b,lH) Example 20
4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benzoic acid, Isomer 1
[0186] 4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano (3, 2-c)quinolin-5-yl)-benzoic acid methyl ester (0.522 g, 1.61 mmol) is dissolved in TΗF(12 ml ), and Lithium hydroxide (0.116 mmol, 4.83 mmol) dissolved in water (lmL) is added and the reaction is heated at 60° C for 16 hours. The reaction is acidified with 1 N HCI and extracted with ethyl acetate. The organic layer is washed with brine and dried over sodium sulfate. The organic layer is filtered and evaporated under reduced pressure to give the title compound (0.310 g, 63 %) (1 H NMR, 400 MHz, CDC13) : δ7.95 (d, J=8.3 Hz,2 H), δ 7.39 (d, J=7 Hz,2 H), δ 7.30(d ,J=7 Hz,2 H), δ 7.30(d, J=7.5 hz,l H), δ 6.98 (t, J=9.1 Hz), δ 6.56 (d, J=8.3 Hz,lH), δ 5.20 (d, J=8.3 Hz,l H), δ 5.20 (d, J=4.2 Hz,l H), δ 4.54(d, J=2.7 Hz,lH), δ 3.47 (d, J=13.8 Hz), δ 3.31 (t, J=l 1.1 Hz,l H), δ 2.07 (b,lH), δ 1.49- 1.27 (m,3H), δ 1.26 (b,lH) Example 21 4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benzoic acid, Isomer 2
[0187] 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano (3, 2-c)quinolin-5-yl)- benzoic acid methyl ester (0.553g, 1.71 mmol) and Lithium hydroxide (0.122 g,5.13 mmol) is dissolved in TΗF (12 mL) and is heated at 55° C overnight. The reaction is acidified with 1 N ΗC1 and extracted with ethyl acetate. The organic layer is washed with brine and dried over sodium sulfate. The organic layer is filtered and evaporated under reduced pressure to give the title compound (0.310 g, 60 %) (1 Η NMR, 400 MHz,
CDC13) : δ7.95 (d, J=8.3 Hz,2 H), δ 7.39 (d, J=7 Hz,2 H), δ 7.30(d ,J=7 Hz,2 H), δ
7.30(d, J=7.5 hz,l H), δ 6.98 (t, J=9.1 Hz), δ 6.56 (d, J=8.3 Hz,lH), δ 5.20 (d, J=8.3 1 H), δ 5.20 (d, J=4.2 Hz,l H), δ 4.54(d, J=2J Hz,lH), δ 3.47 (d, J=13.8 Hz), δ 3.31 (t J=l 1.1 Hz,l H), δ 2.07 (b,lH), δ 1.49-1.27 (m,3H), δ 1.26 (b,lH) Example 22
4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benzoic acid metfo ester
[0188] 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-ben acid methyl ester (6-Formyl-benzoic acid-methyl ester (8.2 g, 50mmol), aniline (4.9C
50mmol), 3,4-Dihydro-2Η-pyran (6.31g,75mmol), and scandium triflate( 2.41 g,
4.9mmol) are added to acetonitrile and the reaction is stiπed for 16 hours. The acetonitrile is removed under reduced pressure to give a residue. A portion of the res is purified by flash chromatography using hexane : ethyl acetate (85:15) to give a fraction (1.67 g) containing the title compound. The fraction is concentrated under reduced pressure to give a solid. The solid is recrystalized with ethyl acetate to give t title compound (0.296 g). (1 H NMR ,400 MHz, CDC13) : 8.08 (d,H=10.7 HZ,2H), 7
(d, J=10.7 Hz,2H),7.23 (d, J=8.6 Hz,l H), 7.10 (t, J=8.7 Hz, 1H),6.74 (t,J=6.9 Hz,l I
6.55 (d, J=8. 6 Hz,l H), 4.78 (d, J=10.3 Hz,l H), 4.39 (d, J=3.4 Hz), 4.10 (b, 1H),3.<
(s, 3H), 3.72 (t, J=12 Hz,lH) 2.09 (b, IH) ,1.83 (b,lH) 1.67 (b,lH), 1.45-1.32 (b ,2 H
Example 23
4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benzoic acid methy ester, Isomer 1 and Isomer 2
Chiral
[0189] Racemic 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano(3, 2- c)quinolin-5-yl)-benzoic acid methyl ester (0.296g, 0.916 mmol) is resolved with a cr
OD column using methanol :DMEA (99.8:0.2) as eluent. The resolution gives the titl< compounds (Isomer 1 : 0.084 g, 0.262 mmol; Isomer 2 : 0.084g, 0.262 mmol) Example 24 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano(3, 2-c)quinolin-5-yl-benzoic acid hydrochloride
[0190] 4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benz acid methyl ester, (0.40g, 0.123 mmol) is dissolved in TΗF (3.5 mL) and 1 N NaOΗ solution (lmL) is added and stiπed for 16 hours. The reaction is heated to 55° C for 1 hour whereupon an additional amount of 1 N NaOΗ solution (0.4 mL) is added. The reaction is partitioned between ether and water. The water layer is acidified with 1 N and is extracted with ethyl acetate. The organic layer is washed with brine and dried c
sodium sulfate. The reaction is filtered and concentration under reduced pressure give the title compound, mass spectrum (m/e) : 310. 0 (M+l) Example 25 4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl-benzoic acid, Isome
[0191] 4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benz acid methyl ester (Isomer 1, 0.084g, 0.262 mmol) is dissolved in TΗF (6 mL), and
Lithium hydroxide (0.019g, 0.785 mmol) dissolved in water (2 mL), are combined an heated at 50° C for 1 hour. Additional Lithium hydroxide (0.070g, 2.9 mmol) is addec and the reaction is heated at 60° C for 1 hour. The reaction is acidified with 1 N ΗC1 a extracted with ethyl acetate. The organic layer is washed with brine and dried over sodium sulfate. The organic layer is filtered and concentrated under reduced pressure give the title compound, mass spectrum (m/e) :310.0 ( M+l) Example 26
4-(3, 4, 4a, 5, 6, 10b-Ηexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benzoic acid, Isom
[0192] 4-(3, 4, 4a, 5, 6, 10b-Hexahydro-2H-pyrano(3, 2-c)quinolin-5-yl)-benz acid methyl ester (Isomer2, 0.085g, 0.262 mmol) is dissolved in TΗF (6 mL) and
Lithium hydroxide (0.018g, 0.785 mmol) dissolved in water (2 mL) is added and the reaction is heated at 6 hours. The reaction is acidified with 1 N HCI and extracted with ethyl acetate. The organic layer is washed with brine and dried over sodium sulfate. The organic layer is filtered and concentrated under reduced pressure to give the title compound, mass spectrum (m/e) : 309.9 (M+l) Example 27
4-(9-Methoxy-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester, Isomer 1 and Isomer 2
[0193] Methyl 4-formylbenzoate (400mg, 2.4 mmol) is dissolved in acetonitrile (20ml). To this is added p-anisidine (300mg, 2.4mmol) and this is stiπed at room temperature for 5 min. After this time 3,4-dihydro-2H-pyrane (0.331ml, 3.6mmol) is added along with scandium triflate (lOOmg, 0.24mmol). After stirring for 18hrs the reaction is diluted with ethyl acetate and washed with water and IN HCI. The organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel chromatography (30% ethyl acetate in hexanes) to give 92mg of the cis isomer (10.7%) (Isomer 1, the first eluting isomer), and 108.7mg of the trans isomer (12.6%) (Isomer 2, the second eluting isomer). MS = 354 (M+H+).
Example 28
4-(9-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester, Isomer 1 and Isomer 2
(20ml). To this is added p-toluidine (261mg, 2.4mmol) and this is stiπed at room temperature for 5 min. After this time 3,4-dihydro-2H-pyrane (0.331ml, 3.6mmol) is added along with scandium triflate (lOOmg, 0.24mmol). After stiπing for 18 hrs the reaction is diluted with ethyl acetate and washed with water and IN HCI. The organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel chromatography (30% ethyl acetate in hexanes) to give 257.5mg of the cis isomer
(31.3%) (Isomer 1, the first eluting isomer) and 276.3 mg of the trans isomer (33.6%)
(Isomer 2, the second eluting isomer). MS = 338 (M+H ). Example 29
4-(9-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester, Isomer 1 and Isomer 2
[0195] Methyl 4-formylbenzoate (400mg, 2.4 mmol) is dissolved in acetonitrile (20ml). To this is added 4-chloro-phenylamine (31 lmg, 2.4mmol) and this is stiπed at room temperature for 5 min. After this time 3,4-dihydro-2H-pyrane (0.331ml, 3.6mmol) is added along with scandium triflate (lOOmg, 0.24mmol). After stirring for 18hrs the reaction is diluted with ethyl acetate and washed with water and IN HCI. The organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (30% ethyl acetate in hexanes) to give 110.3mg of the cis isomer (12.7%)(Isomer 1, the first eluting isomer), and 272.4 mg of the trans isomer (31.2%) (Isomer 2, the second eluting isomer). MS = 358 (M+H+).
Example 30
4-(7-Methyl-3, 4, 4a, 5, 6,10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester, Isomer 1 and Isomer 2
[0196] Methyl 4-formylbenzoate (400mg, 2.4 mmol) is dissolved in acetonitrile (20ml). To this is added 2-methyl-phenylamine (261mg, 2.4mmol) and this is stiπed at room temperature for 5 min. After this time 3,4-dihydro-2H-pyrane (0.331ml, 3.6mmol) is added along with scandium triflate (lOOmg, 0.24mmol). After stiπing for 18hrs the reaction is diluted with ethyl acetate and washed with water and IN HCI. The organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel
chromatography (30% ethyl acetate in hexanes) to give 163.7mg of the cis isomer (20.0%) (Isomer 1, the first eluting isomer), and 352.7mg of the trans isomer (42.9%) (Isomer 2, the second eluting isomer). MS = 338 (M+H ). Example 31
4-(7-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester, Isomer 1 and Isomer 2
[0197] Methyl 4-formylbenzoate (400mg, 2.4 mmol) is dissolved in acetonitrile (20ml). To this is added 2-chloro-phenylamine (31 lmg, 2.4mmol) and this is stiπed at room temperature for 5 min. After this time 3,4-dihydro-2H-pyrane (0.331ml, 3.6mmol) is added along with scandium triflate (lOOmg, 0.24mmol). After stirring for 18 hrs the reaction is diluted with ethyl acetate and washed with water and IN HCI. The organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (30% ethyl acetate in hexanes) to give 56.6mg of the cis isomer (6.5%) (Isomer 1, the first eluting isomer), and 374.8mg of the trans isomer (43.0%) (Isomer 2, the second eluting isomer). MS = 358 (M+H+).
Example 32
4-(9-Fluoro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid methyl ester, Isomer 1 and Isomer 2
[0198] Methyl 4-formylbenzoate (200mg, 1.2 mmol) is dissolved in acetonitrile (20ml). To this is added 4-fluoro-phenylamine (0.115ml, 1.2mmol) and this is stiπed at room temperature for 5 min. After this time 3,4-dihydro-2H-pyrane (0.166ml, l.δmmol) is added along with scandium triflate (50mg, 0.12mmol). After stirring for lδhrs the reaction is diluted with ethyl acetate and washed with water and IN HCI. The organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel chromatography (30% ethyl acetate in hexanes) to give 162.6mg of the cis isomer (39.1%) (Isomer 1, the first eluting isomer), and 243.3mg of the trans isomer (58.5%) (Isomer 2, the second eluting isomer). MS = 342 (M+lX).
Example 33
4-(9-Methoxy-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0199] 4-(9-Methoxy-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 2 (25mg, 0.07 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (12mg, 0.28mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 11.7mg of 4-(9-Methoxy-3, 4, 4a, 5, 6, lOb-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (48.8%) as a white solid. MS = 338 (M-H"
Example 34
4-(9-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0200] 4-(9-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 2 (50mg, 0.15 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (25mg, 0.59mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 16.7mg of 4-(9-Methyl-3, 4, 4a, 5, 6, lOb-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (34.9%) as a white solid. MS = 322 (M-H'
)•
Example 35
4-(9-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0201] 4-(9-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 2 (50mg, 0.14 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (23mg, 0.56mmol) and this reaction is stiπed at room temperature. After stirring for 18 hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 20.5mg of 4-(9-Chloro-3, 4, 4a, 5, 6, 10b- hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (42.7%) as a white solid. MS = 342 (M-H").
Example 36
4-(7-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0202] 4-(7-Methyl-3, 4, 4a, 5, 6,10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 2 (50mg, 0.15 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (25mg, 0.60mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 42.0mg of 4-(7-Methyl-3, 4, 4a, 5, 6, lOb-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (87.7%) as a white solid. MS = 322 (M-H"
)•
Example 37
4-(7-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0203] 4-(7-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 2 (50mg, 0.14 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (23mg, 0.56mmol) and this reaction is stiπed at room temperature. After stiπing for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 47.1mg of 4-(7-Chloro-3, 4, 4a, 5, 6, lOb-hexahydro-
2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (98.1%) as a white solid. MS = 342 (M-H"
)• Example 38
4-(9-Fluoro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0204] 4-(9-Fluoro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 2 (50mg, 0.15 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (25mg, 0.60mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 9.2mg of example 12 (19.2%) as a white solid. MS = 326 (M-H"). Example 39 4-(9-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0205] 4-(9-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 1 (50mg, 0.15 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (25mg, 0.59mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 30.2mg of 4-(9-Methyl-3, 4, 4a, 5, 6, lOb-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (63.0%) as a white solid. MS = 322 (M-H"
)• Example 40
4-(9-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0206] 4-(9-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 1 (50mg, 0.14 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (23mg, 0.56mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 23.7mg of 4-(9-Chloro-3, 4, 4a, 5, 6, lOb-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (49.4%) as a white solid. MS = 342 (M-H"
Example 41
4-(7-Methyl-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0207] 4-(7-Methyl-3, 4, 4a, 5, 6,10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 1 (50mg, 0.15 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (25mg, 0.60mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgS04 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 40.0mg of 4-(7-Methyl-3, 4, 4a, 5, 6, 1 Ob-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (83.5%) as a white solid. MS = 322 (M-H"
Example 42
4-(7-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0208] 4-(7-Chloro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 1 (56mg, 0.16 mmol) is dissolved in THF (4ml)
and water (1ml). To this is added lithium hydroxide (25mg, 0.64mmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgSO4 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 52.8mg of 4-(7-Chloro-3, 4, 4a, 5, 6, lOb-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (98.1%) as a white solid. MS = 342 (M-H" )•
Example 43
4-(9-Fluoro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid
[0209] 4-(9-Fluoro-3, 4, 4a, 5, 6, 10b-hexahydro-2H-pyrano[3, 2-c]quinolin-5- yl)-benzoic acid methyl ester, Isomer 1 (50mg, 0.15 mmol) is dissolved in THF (4ml) and water (1ml). To this is added lithium hydroxide (25mg, O.όOmmol) and this reaction is stiπed at room temperature. After stirring for 18hrs the reaction brought to pH=6 with IN HCI. The reaction is then extracted with ethyl acetate and the organic layer is dried over MgS0 and evaporated. Purify the residue via silica gel chromatography (80% ethyl acetate in hexanes) to give 12.6mg of 4-(9-Fluoro-3, 4, 4a, 5, 6, 1 Ob-hexahydro- 2H-pyrano[3, 2-c]quinolin-5-yl)-benzoic acid (26.3%) as a white solid. MS = 326 (M-H"
EXAMPLE 44 Binding Assays [0210] Compounds of the invention were separately incubated with HNF-4α at varying concentrations in the presence of varying concentrations of radiolabeled methyl 4-[5,6,7,8-tetrahydro-5, 5, 8, 8-tetramethyl-3-ethyloxy-2-naphthalenyl]benzoyl benzoate, which had been previously found to bind to HNF-4α, to determine the compound's binding affinity for HNF-4α. Binding affinity (K,) for 5 compounds is provided in Table 1. Co-transfection assay
[0211] CV-1 cells (African green monkey kidney fibroblasts) were cultured in the presence of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% charcoal resin-stripped fetal bovine serum (CH-FBS) then transfeπed to 96-well microtiter plates one day prior to transfection. [0212] To determine HNF-4α receptor agonist and antagonist activity of the compounds of the present invention, the CV-1 cells were transiently transfected by FuGENE 6 transfection reagent in 175 cm2 flask with the following plasmids: pCMX- HNF-4αDF (3μg/flask), apoAl-LUC reporter (l μg/flask), and filler DNA (pcDNA; 3μg/flask). The receptor plasmid, pCMX-HNF-4αDF, contains the rat HNF-4αl under constitutive control of the CMV promoter, as more fully described in J.D. Fraser et al., "DNA binding and transcription activation specificity of hepatocyte nuclear factor 4" NAR, 26: 2702-2707 (1998). [0213] The reporter plasmid, apoAl-LUC, contains the cDNA for firefly luciferase (LUC) under control of a multimerized HNF-4α response element (the A site
from the apo Al promoter) linked to the TK minimal promoter. See e.g., Fraser et al. supra. Twenty four hours after transfection the cells were harvested and plated in 96 well plates at 10,000 cells/well. Media containing one of the modulator compounds of
the present invention in concentrations ranging from 10" 10 to 10"^ M were added to the cells. Three to four replicates were used for each sample. Transfections and subsequent procedures were performed on a Biomek 1000 automated laboratory work station. [0214] Samples containing 4-[5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-3-ethyloxy- 2-naphthalenyl]benzoyl benzoic acid (LGO 100695), which had previously been found to have agonist activity on HNF-4α, were included as a reference agonist. LGO 100695 has the following structure:
' _> ^ "CO:H
[0215] After 24 hours, the cells were washed with PBS, lysed with a Triton X- 100-based buffer and assayed for LUC activity using a NORTHSTAR HTS workstation. The mean and standard eπor of the mean (SEM) of the luciferase response were calculated. Data were plotted as the response of the compound compared to the reference compounds over the range of the dose-response curve. For agonist experiments, the effective concentration that produced 50% of the maximum response (EC50) was quantified. Agonist efficacy was a function (%) of LUC expression relative to the maximum LUC production by the reference agonist LGO 100695. Antagonist activity was determined by testing the amount of LUC expression in the asbsence of exogenous compound (presence only of any endogenous ligand) as HNF-4α receptor agonist. The concentration of a test compound that inhibited 50% of LUC expression was quantified (IC50). In addition, the efficacy of antagonists was determined as a
function (%) of maximal inhibition. Data for 5 compounds of the present invention are provided in Table 1.
Table 1: Agonist, partial agonist, antagonist activity of HNF-4α receptor modulator compounds of present invention. Efficacy (%) for HNF-4α agonist was determined by comparing activity (e.g., luciferase production) of putative agonist to that LGO 100695. Efficacy (%) for HNF-4α antagonist was determined by the percentage amount by which the luciferase production was reduced (maximum concentration of antagonist) from the luciferase production without compound.
[0216] The present invention includes any combination of the various species and subgeneric groupings falling within the generic disclosure. This invention therefore includes the generic description of the invention with a proviso or negative limitation removing any subject matter from the genus, regardless of whether or not the excised material is specifically recited herein.
[0217] While in accordance with the patent statutes, description of the various embodiments and processing conditions have been provided, the scope of the invention is not to be limited thereto or thereby. Modifications and alterations of the present invention will be apparent to those skilled in the art without departing from the scope and spirit of the present invention. [0218] Therefore, it will be appreciated that the scope of this invention is to be defined by the appended claims, rather than by the specific examples which have been presented merely to illustrate certain embodiments of the present invention.
Claims
1. A compound of formula I:
I or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof, wherein: R1 is selected from H, a halogen, and a methyl optionally substituted with one or more fluorines; R2, R3, R4, and R5 are each independently selected from H, a halogen, an amide, a sulfonamide, a C1-C5 alkyl optionally substituted with one or more halogens, a C2-C5 alkenyl optionally substituted with one or more halogens, a C2-C5 alkynyl optionally substituted with one or more halogens, a Cι-C4 alkoxy optionally substituted with one or more halogens and a Cι-C4 thioalkyl optionally substituted with one or more halogens, a C2-C4 thioalkenyl optionally substituted with one or more halogens, and a C2-C4 thioalkynyl optionally substituted with one or more halogens; or R2 and R3 taken together form a 3 to 8 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines; or
R3 and R4 taken together form a 5 to 6 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines; or
R4 and R5 taken together form a 5 to 6 membered carbocyclic or heterocyclic ring, optionally substituted with one or more fluorines; R6 is selected from H, a C1-C5 alkyl optionally substituted with one or more halogens, a C2-C5 alkenyl optionally substituted with one or more halogens, and a C2-C5 alkynyl optionally substituted with one or more halogens;
R7 is selected from CH2OH, CHO, COOH and a group of formula (a):
(a) ; wherein R8 and R9 are each independently selected from H, OH and a methyl optionally substituted with one or more fluorines; n is 1, 2 or 3; X is O, NR10 or S; and R10 is selected from H, a Ci- C5 alkyl optionally substituted with one or more halogens, a C2-C5 alkenyl optionally substituted with one or more halogens, and a C2-C5 alkynyl optionally substituted with one or more halogens.
2. A compound according to claim 1 , wherein: R is selected from H and a Cι-C4 alkyl optionally substituted with one or more halogens;
R6 is selected from H and a Cι-C3 alkyl group optionally substituted with one or more halogen;
R2, R3, R and R5 are each independently selected from H, a halogen, a Cι-C3 alkyl optionally substituted with one or more halogens, a C2-C alkenyl optionally substituted with one or more halogens, a C2-C3 alkynyl optionally substituted with one or more halogens, a Cι-C alkoxy optionally substituted with one or more halogens,a Cι-C3 thioalkyl optionally substituted with one or more halogens, a C2-C thioalkenyl optionally substituted with one or more halogens, and a C2-C thioalkynyl, optionally substituted with one or more halogens; or
R2 and R3 taken together form a 4 to 7 membered carbocyclic or heterocyclic ring optionally substituted with one or more halogens; or
R3 and R4 taken together form a 5 to 6 membered carbocyclic or heterocyclic ring optionally substituted with one or more halogens.
3. A compound according to claim 2, wherein: R2, R3, R4 and R5 each independently is selected from the group H, Cι-C2 alkyl optionally substituted with one or more halogens, a C2 alkenyl group optionally substituted with one or more halogens, an Cι-C2 alkoxy group optionally substituted with one or more halogens, and a Cι-C2 thioalkyl group optionally substituted with one or more halogens; or
R2 and R3 taken together form a 5 to 6 membered carbocyclic or ring optionally substituted with one or more fluorines; or
R3 and R4 taken together form a 5 to 6 membered carbocyclic ring optionally substituted with one or more fluorines; or
R and R taken together form a 5 to 6 membered carbocyclic ring optionally substituted with one or more fluorines.
4. A compound according to claim 1, wherein: R2 and R3 are each independently selected from H and methyl; or R2 and R3 taken together from a 3 to 8 membered carbocyclic ring.
5. A compound according to claim 1 , wherein: R3 and R4 are each independently methyl; or
R3 and R4 taken together from a 3 to 8 membered carboxylic ring.
6. A compound according to claim 1, wherein R1, R2, R3, and R4 are each independently selected from H and a methyl optionally substituted with one or more fluorines.
7. A compound according to any one of claims 2, 3, 4 and 6, wherein R5 and R6 are each
H.
8. A compound according to claim 7, wherein: R7 is C(R8)(R9)(OOH), wherein R and R are each independently selected from H and a methyl optionally substituted with one or more fluorines.
9. A compound selected from: 4-(2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5- yl)benzoic acid, 4-(2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)phenylacetic acid, 4-(8,9-dimethyl-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid,
4-(8,9-cyclopentano-2,3,4a,5,6,10b-hexahydropyrano[3,2-c]quinolin-5-yl)benzoic acid,
4-(9, 10-benzo-2,3,4a,5,6, 10b-hexahydropyrano[3 ,2-c]quinolin-5-yl)benzoic acid, 4-
(2,3,3a,4,5,9b-hexahydrofuro[3,2-c]quinolin-4-yl)benzoic acid, 4-(2,3,3a,4,5,9b- hexahydrofuro[3,2-c]quinolin-4-yl)phenylacetic acid, and 4-(9,10-benzo-2,3,3a,4,5,9b- hexahydrofuro[3,2-c]quinolin-4-yl)benzoic acid.
10. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 1.
11. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 2.
12. A pharmaceutical agent comprising a pharmaceutically acceptable caπier and a compound of claim 3.
13. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 4.
14. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 5.
15. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 6.
16. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 7.
17. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 8.
18. A pharmaceutical agent comprising a pharmaceutically acceptable carrier and a compound of claim 9.
19. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 1.
20. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 2.
21. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 3.
22. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 4.
23. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 5.
24. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 6.
25. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 7.
26. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 8.
27. A method of treating a patient having a disease or condition selected from the group of syndrome X, non-insulin dependent diabetes mellitus, cancer, obesity, cardiovascular disease and dyslipidemia comprising administering to said patient a pharmaceutically effective amount of a compound of claim 9.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US48807103P | 2003-07-16 | 2003-07-16 | |
| US60/488,071 | 2003-07-16 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2005016255A2 true WO2005016255A2 (en) | 2005-02-24 |
| WO2005016255A3 WO2005016255A3 (en) | 2005-06-16 |
Family
ID=34193072
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2004/023093 WO2005016255A2 (en) | 2003-07-16 | 2004-07-16 | Substituted tetrahydroquinolines, phenylacetic acids and benzoic acids as hepatocyte nuclear factor 4 (hnf-4 ) modulator compounds |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2005016255A2 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006002726A1 (en) * | 2004-06-30 | 2006-01-12 | Merck Patent Gmbh | Tetrahydroquinolines |
| WO2007054138A1 (en) * | 2005-06-13 | 2007-05-18 | Merck Patent Gmbh | Substituted tetrahydroquinolines |
| US7250423B2 (en) * | 2001-09-24 | 2007-07-31 | Chao-Jun Li | Methods for synthesizing heterocycles and therapeutic use of the heterocycles for cancers |
| EP2647628A1 (en) * | 2012-04-02 | 2013-10-09 | Almirall, S.A. | Substituted tricyclic compounds with activity towards ep1 receptors |
| EP2647638A1 (en) * | 2012-04-02 | 2013-10-09 | Almirall, S.A. | Substituted tricyclic compounds with activity towards ep1 receptors |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3631050A (en) * | 1968-11-13 | 1971-12-28 | Parke Davis & Co | Hexahydro-9b-methylfuro(3 2-c) quinoline compounds |
| WO1998027093A1 (en) * | 1996-12-18 | 1998-06-25 | Eli Lilly And Company | Combinatorial process for preparing hydrofuroquinoline libraries |
-
2004
- 2004-07-16 WO PCT/US2004/023093 patent/WO2005016255A2/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3631050A (en) * | 1968-11-13 | 1971-12-28 | Parke Davis & Co | Hexahydro-9b-methylfuro(3 2-c) quinoline compounds |
| WO1998027093A1 (en) * | 1996-12-18 | 1998-06-25 | Eli Lilly And Company | Combinatorial process for preparing hydrofuroquinoline libraries |
Non-Patent Citations (1)
| Title |
|---|
| REDDY ET AL.: 'Bismuth (III) chloride catalyzed aza-Diels-Adler reaction' SYNTHETIC COMMUNICATIONS vol. 31, no. 7, 2001, pages 1075 - 1080, XP008045206 * |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7250423B2 (en) * | 2001-09-24 | 2007-07-31 | Chao-Jun Li | Methods for synthesizing heterocycles and therapeutic use of the heterocycles for cancers |
| WO2006002726A1 (en) * | 2004-06-30 | 2006-01-12 | Merck Patent Gmbh | Tetrahydroquinolines |
| US7915416B2 (en) | 2004-06-30 | 2011-03-29 | Merck Patent Gmbh | Tetrahydroquinolines |
| WO2007054138A1 (en) * | 2005-06-13 | 2007-05-18 | Merck Patent Gmbh | Substituted tetrahydroquinolines |
| JP2008545803A (en) * | 2005-06-13 | 2008-12-18 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフトング | Substituted tetrahydroquinoline |
| US7893082B2 (en) | 2005-06-13 | 2011-02-22 | Merck Patent Gmbh | Substituted tetrahydroquinolines |
| AU2006312800B2 (en) * | 2005-06-13 | 2011-08-25 | Merck Patent Gmbh | Substituted tetrahydroquinolines |
| EP2647628A1 (en) * | 2012-04-02 | 2013-10-09 | Almirall, S.A. | Substituted tricyclic compounds with activity towards ep1 receptors |
| EP2647638A1 (en) * | 2012-04-02 | 2013-10-09 | Almirall, S.A. | Substituted tricyclic compounds with activity towards ep1 receptors |
| WO2013149996A1 (en) * | 2012-04-02 | 2013-10-10 | Almirall, S.A. | Substituted tricyclic compounds with activity towards ep1 receptors |
| WO2013149997A1 (en) * | 2012-04-02 | 2013-10-10 | Almirall, S.A. | Substituted tricyclic compounds with activity towards ep1 receptors |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2005016255A3 (en) | 2005-06-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9359285B2 (en) | Androgen receptor modulator compounds and methods | |
| CA2598216C (en) | Androgen receptor modulator compounds and methods | |
| JP6219882B2 (en) | IRE-1α inhibitor | |
| JP2010195799A (en) | 6-cycloamino-2-quinolinone derivative as androgen receptor modulator compound | |
| KR20080080305A (en) | Thrombopoietin activity modulating compounds and methods | |
| US7442696B2 (en) | Mineralocorticoid receptor modulator compounds, processes for their preparation, and their uses | |
| RU2296759C2 (en) | N-substituted 1h-indole-5-propionic acids, pharmaceutical composition containing these compounds and their using (variants) | |
| WO2007075884A2 (en) | Androgen receptor modulator compounds and methods | |
| WO2005009104A2 (en) | Benzoic and phenyl acetic acid derivatives as hnf-4 modulators | |
| WO2005016255A2 (en) | Substituted tetrahydroquinolines, phenylacetic acids and benzoic acids as hepatocyte nuclear factor 4 (hnf-4 ) modulator compounds | |
| WO2019149089A1 (en) | Nitrogen-containing benzoheterocycle compound comprising carboxylic acid group, preparation method and use thereof | |
| WO2005010202A2 (en) | PHENYL ACETIC ACID DERIVATIVES AS HEPATOCYTE NUCLEAR FACTOR 4α (HNF-4α) MODULATOR COMPOUNDS | |
| WO2005017185A2 (en) | HEPATOCYTE NUCLEAR FACTOR 4α MODULATOR COMPOUNDS | |
| MX2007016215A (en) | Alpha-(aryl-or heteroaryl-methyl)-beta-piperidinopropanoic acid compounds as orl1-receptor antagonists. | |
| US7435733B2 (en) | Mineralocorticoid receptor modulator compounds and methods, and pharmaceutical compositions containing these compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| 122 | Ep: pct application non-entry in european phase |