WO2004009717A1 - Superprimer - Google Patents
Superprimer Download PDFInfo
- Publication number
- WO2004009717A1 WO2004009717A1 PCT/US2003/023055 US0323055W WO2004009717A1 WO 2004009717 A1 WO2004009717 A1 WO 2004009717A1 US 0323055 W US0323055 W US 0323055W WO 2004009717 A1 WO2004009717 A1 WO 2004009717A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition
- percent
- water soluble
- silane
- bis
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D183/00—Coating compositions based on macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon, with or without sulfur, nitrogen, oxygen, or carbon only; Coating compositions based on derivatives of such polymers
- C09D183/14—Coating compositions based on macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon, with or without sulfur, nitrogen, oxygen, or carbon only; Coating compositions based on derivatives of such polymers in which at least two but not all the silicon atoms are connected by linkages other than oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/08—Anti-corrosive paints
- C09D5/082—Anti-corrosive paints characterised by the anti-corrosive pigment
- C09D5/086—Organic or non-macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/01—Use of inorganic substances as compounding ingredients characterized by their specific function
- C08K3/013—Fillers, pigments or reinforcing additives
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L63/00—Compositions of epoxy resins; Compositions of derivatives of epoxy resins
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L67/00—Compositions of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Compositions of derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L75/00—Compositions of polyureas or polyurethanes; Compositions of derivatives of such polymers
- C08L75/04—Polyurethanes
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C2222/00—Aspects relating to chemical surface treatment of metallic material by reaction of the surface with a reactive medium
- C23C2222/20—Use of solutions containing silanes
Definitions
- the present inventions relates to corrosion protection and increased adhesion between substrates and a subsequent bonded material. More specifically, the present invention is related to primers, manufactured from at least one organofunctional bis-silane, having increased film thickness, chemical and scratch resistance, as well as being substantially chromate-free and comprising little to no VOCs.
- organofunctional silanes have been shown to be powerful systems for protecting a wide range of metals against corrosion when applied as primers.
- the adhesion of paint is drastically improved when organofunctional silanes are applied as a primer pretreatment.
- Adhesion and adhesion durability of metals to rubber compounds and structural adhesives have also been objectives of prior art organofunctional silanes systems.
- prior art silane films When used as a corrosion protection treatment without paint, prior art silane films have limitations in that the film thickness is not more than 0.3 ⁇ m. Such films provide remarkable protection so long as they are not scratched or otherwise damaged. Also, it has been very difficult to apply a thin silane film without pmholes or other defects appearing in the film. In addition, prior art silane films have been transparent and colorless, thereby providing little visual detection as to defects in the film. Consequently, prior art silane films applied alone provided only temporary protection of metals and, therefore, there is a need for metal treatment systems that meet or exceed the following criteria* which prior art silane films cannot meet entirely:
- the film thickness should range from 1 to 20 ⁇ m
- the coating should cure in air or thermally at slightly elevated temperatures
- the coating should adhere very well to metals and should be paintable by all common paint systems such as epoxies, polyesters, acrylates, polyurethanes and the like;
- the coating should have a high UV resistance so that it can be used externally without overcoating
- the compounds used in the coating should all be water soluble or dispersible; it should be a low NOC system (Volatile Organic Compound);
- the coating should be applied by dipping, wiping, spraying brushing, and other well known applications methods;
- the coating should not be completely transparent but opaque and have a color, so that the metal can still be observed but the film can be detected visually;
- the coating should have a high thermal stability (it should be stable to at least 250°C for one hour).
- the present invention provides a superprimer that can be used in a wide range of environments, on all metals of engineering interest, as a standalone process or as a primer for a paint application process.
- the superprimer generally comprises: a) at least one organofunctional silane; b) a low-molecular weight water soluble or dispersible polymer or coplymer; c) a pigment; d) a water soluble inhibitor; and e) additional components such as emulsifiers, surfactants, film builders, UV absorbers or reflectors, thickeners, or toughening agents such as end-functionalized silicones.
- Fig. 1 is a graphical representation of small angle X-ray scattering (SAXS) from tetramethylorthosilicate (TMOS) polymerized at three pH values;
- SAXS small angle X-ray scattering
- TMOS tetramethylorthosilicate
- Fig. 2 is a dynamic mechanical analysis (DMA) relative to two-phase films with and without organosilane treatment
- Fig. 3 is a chart listing metals and forms of corrosion where bis-silane films have been demonstrated to be very effective
- Fig. 4 is a set of polarization curves in 3.5% aerated NaCl of AA 2024-T3 alloy coated with silanes and resin or nanoparticle-modified silanes in accordance with the present invention
- Fig. 5 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained with exemplary embodiments of the present invention
- Fig. 6 is a chart reflecting the compositions and parts relative to one another evaluated in Experiment 1;
- Fig. 7 is a chart listing exemplary coating compositions evaluated in accordance with the present invention in Experiment 1;
- Fig. 8 is a graphical representation of direct current polarization data obtained from exemplary embodiments of the present invention in Experiment 1;
- Fig. 9 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained with exemplary embodiments of the present invention in Experiment 1;
- Fig. 10 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained with exemplary embodiments of the present invention in Experiment 1;
- Fig. 11 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained with exemplary embodiments of the present invention in Experiment 1;
- Fig. 12 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation #1, and experimental formulation #2 of the present invention in Experiment 2;
- EIS electrochemical impedance spectroscopy
- Fig. 13 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation #3, and experimental formulation #4 of the present invention in Experiment 2;
- EIS electrochemical impedance spectroscopy
- Fig. 14 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation #5, and experimental formulation #6 of the present invention in Experiment 2;
- EIS electrochemical impedance spectroscopy
- Fig. 15 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation #7, and experimental formulation #8 of the present invention in Experiment 2;
- EIS electrochemical impedance spectroscopy
- Fig. 16 is a graphical representation of electrochemical impedance spectroscopy (EIS) data in a Bode plot obtained from the control formulation, experimental formulation #1, and experimental formulation #2 of the present invention in Experiment 2;
- Fig. 17 is a graphical representation of electrochemical impedance spectroscopy (EIS) data in a Bode plot obtained from the control formulation, experimental formulation #3, and experimental formulation #4 of the present invention in Experiment 2;
- Fig. 18 is a graphical representation of electrochemical impedance spectroscopy (EIS) data in a Bode plot obtained from the control formulation, experimental formulation #5, and experimental formulation #6 of the present invention in Experiment 2;
- EIS electrochemical impedance spectroscopy
- Fig. 19 is a graphical representation of electrochemical impedance spectroscopy (EIS) data in a Bode plot obtained from the control formulation, experimental formulation #7, and experimental formulation #8 of the present invention in Experiment 2;
- EIS electrochemical impedance spectroscopy
- Fig. 20 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation of AMME cured for 10 minutes, experimental formulation of AMVS cured for 10 minutes, and experimental formulation of AMVS + Resin cured for 10 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 21 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation of AMME cured for 30 minutes, experimental formulation of AMVS cured for 30 minutes, and experimental formulation of AMVS + Resin cured for 30 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 22 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation of AMME cured for 60 minutes, experimental formulation of AMVS cured for 60 minutes, and experimental formulation of AMVS + Resin cured for 60 minutes in accordance with the present invention in Experiment 3;
- Fig. 23 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation of AMME cured for 10 minutes, experimental formulation of AMME cured for 30 minutes, and experimental formulation of AMME cured for 60 minutes in accordance with the present invention in Experiment 3;
- Fig. 24 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation of AMVS cured for 10 minutes, experimental formulation of AMVS cured for 30 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 25 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation of AMVS + Resin cured for 10 minutes, experimental formulation of AMVS + Resin cured for 30 minutes, and experimental formulation of AMVS +Resin cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 26 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS cured for 10 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 27 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS cured for 30 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- Fig. 28 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS cured for 60 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- Fig. 29 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS + Resin cured for 10 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 30 is a graphical representation of electrochemical impedance spectroscopy (EIS) data obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS + Resin cured for 30 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 31 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation of AMME cured for 10 minutes, experimental formulation of AMVS cured for 10 minutes, and experimental formulation of AMVS + Resin cured for 10 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 32 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation of AMME cured for 30 minutes, experimental formulation of AMVS cured for 30 minutes, and experimental formulation of AMVS + Resin cured for 30 minutes in accordance with the present invention in Experiment 3;
- Fig. 33 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation of AMME cured for 60 minutes, experimental formulation of AMNS cured for 60 minutes, and experimental formulation of AMNS + Resin cured for 60 minutes in accordance with the present invention in Experiment 3;
- Fig. 34 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation of AMME cured for 10 minutes, experimental formulation of AMME cured for 30 minutes, and experimental formulation of AMME cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 35 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation of AMVS cured for 10 minutes, experimental formulation of AMVS cured for 30 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 36 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation of AMVS + Resin cured for 10 minutes, experimental formulation of AMVS + Resin cured for 30 minutes, and experimental formulation of AMVS + Resin cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 37 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS cured for 10 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- Fig. 38 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS cured for 30 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- Fig. 39 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS + Resin cured for 10 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Fig. 41 is a graphical representation of electrochemical impedance spectroscopy (EIS) data a Bode plot obtained from the control formulation, experimental formulation with Resin cured for 60 minutes, experimental formulation of AMVS + Resin cured for 30 minutes, and experimental formulation of AMVS cured for 60 minutes in accordance with the present invention in Experiment 3;
- EIS electrochemical impedance spectroscopy
- Organofunctional silanes have been shown to be powerful systems for protecting a wide range of metals against corrosion.
- a coating which: a) adheres very well to the metal and is paintable by common paint systems such as, for example, epoxies, polyesters, acrylates, and polyurethanes, as well as improving adhesion of both soft (e.g.
- the present invention which, in the exemplary embodiment, provides a coating meeting the aforementioned goals, comprises at least one organofunctional silane, an organic resin and a nanoparticle filler.
- the novel primer dubbed the "superprimer” is amenable to dipping, spraying, wiping or brushing onto any clean metal surface. No conversion coating, either phosphate or chromate, is required.
- One of the principles underlying the present invention is the hydrophobicity transition exhibited by organosilanes. While the unpolymerized silanes are . hydrophilic and water soluble, they become highly hydrophobic on deposition, resulting in extremely low water-transmission rates.
- silane films involve a number of seemingly contradictory requirements. It is desirable that the film precursors are hydrophilic to permit water-borne deposition. On the other hand, it is desirable that the film itself be very hydrophobic to assure superior protection. Also, it is desirable that the silane films be thin enough to be pore free yet thick enough to provide an adequate barrier to water penetration. Thin organosilane prior art films have been dense and have shown the required hydrophobicity, but lacked toughness. Thick films, on the other hand, were porous and susceptible to cracking and typically had reduced hydrophobicity.
- any practical coating system support a variety of deposition methods such as dip coating, spraying and brushing. It is well known, however, that the nature of the final film in a reactive-evaporative system depends on the physics and chemistry active during the deposition process. The transient gel-like state that exists at the drying front may ultimately determine the density of the film.
- the gel network will resist collapse, rendering the film porous.
- dense hydrophobic films are required. To achieve such films, management of the condensation reactions and evaporation rate assures that rigidity sets in only after the solvent has evaporated.
- the level of corrosion performance correlates with the formation of an interfacial reaction product between the silane film and the metal oxide.
- effective bis- silanes are actually conversion coatings that form a complex silicate layer with a high ohmic resistivity and low electrolyte permeability.
- These silicate layers differ from conventional sol- gel coatings, which are subject to facile hydrolysis. Therefore, the superior performance of bis- silane films is likely related to the nature of the interfacial conversion product.
- the present invention is directed to a coating with a greatly enhanced film builds generally from 0.1 ⁇ m to 20 ⁇ m.
- the increased film build will in part be the result of the incorporation of nanoparticles, which will interact strongly with the silane molecules.
- the present invention is a superprimer generally comprising the following components:
- One or more of an organofunctional silane preferably a bis-silane.
- An exemplary group of bis-silanes shown to be effective in the present invention are:
- This polymer or copolymer is generally selected from the classes of: epoxy, polyester, polyurethane or acrylate.
- a pigment comprising nanoparticles generally having a size on the order of 0.01-500 nm.
- the particles may be: metal oxides that adsorbs silanes such as zinc oxide, aluminum oxide, iron oxide, magnesium oxide and silica; phthalocyanines; sulfides; silicone oils such as xanthene and anthraquinone dyes; vat dyes such as 3-hydroxyindole (indoxyl), 7,8,7,8- dibenzothioindigo, pyranthrone and indanthrene brilliant orange.
- the pigment may be dispersed into the coating by sol-gel methods or by high-shear blending.
- This component is variable in that it is selected on the basis of the substrate.
- Exemplary inhibitors include: salt of trivalent cerium (Ce); salt of trivalent lanthanum (Le); salts of yttrium (Y); molybdates; phosphates; phosphonates; phosphomolybdates; vanadates; borates; amines; glycolates; sulfenamides, tungstates, and various mixtures of the above.
- concentration of this inhibitor will generally less than 1.0% of the resultant superprimer.
- Additional components such as emulsifiers, surfactants, film builders, UV absorbers or reflectors, thickeners, or toughening agents such as end-functionalized silicones. These components are present in very low concentrations on the order of 0.5% solids. Examples of such UV absorbers and reflectors include zinc oxide (ZnO) and titanium dioxide (TiO 2 ).
- the functional group in the silane is selected such that it reacts with the functional group in the polymer backbone.
- the hydroxide groups (-OH) in epoxies will react with the secondary amine groups in the silane, bis-[triethoxysilylpropyl]amine or bis- [triethoxysilylpropyljethylenediamine. It has been shown that mixtures of two silanes may be markedly more effective than individual silanes alone.
- the silanes not only cross-link with the polymer, but also cross-link with themselves and form a three-dimensional siloxane network. This network will be interpenetrated with the cross-linked polymer.
- the cross-linked polymer or copolymer is generally a low-molecular weight, approximately 500 grams on the low side, that is water soluble polymer and may include or be supplanted by a higher molecular weight polymer having been end-functionalized so as to become water soluble or dispersible.
- This polymer or copolymer may be an epoxy, a polyester, a polyurethane, an acrylate or a modified polymer such as polyvinylidene fluoride.
- the result of such a composition is a much thicker and denser film than one produced using a silane alone or a polymer film alone. Since the siloxane network is very hydrophobic, the film will have an extremely low permeability to water. The silane film alone would be brittle in high thicknesses, but the presence of the interpenetrated polymer will result in a much more pliable and formable material. One could argue that the polymer acts as a toughener of the silane film.
- a dense, uniform silane film as provided for in the present invention can be achieved by a balance of forces. This balance is, however, even more critical when one of the components is undergoing a hydrophobicity transition. At some point the components of the system will be thermodynamically incompatible unless specific steps are taken to assure compatibility.
- the domain size of hybrid systems of the present invention are controlled by a balance of entropic and enthalpic forces.
- the former are largely determined by the matrix cross-link density and the latter can be managed through interface-active agents.
- systems may be chosen that are amenable to formation of cross-links between the organic and inorganic phases. Inter-phase coupling acts like an attractive force and imparts compatibility to otherwise incompatible polymers.
- the inter-phase cross-linking should be managed, however, since the growth of molecular weight in the early stages of the cross-linking reaction can actually induce phase separation.
- Prior art reinforced polymers often show a sharp decline in the storage modulus with strain due to break-up of a percolated filler networks.
- Fig.2 demonstrates a dynamic mechanical analysis (DMA) relative to two-phase films with and without organosilane treatment.
- DMA dynamic mechanical analysis
- the figure shows the dynamic mechanical response of a freestanding s ⁇ yrene- butadiene elastomer film reinforced with nanophase silica.
- the upper curve shows a high modulus and a very large Payne effect (reduction of the modulus with strain).
- both the modulus and the Payne effect are dramatically reduced (lower curve). This reduction is obtained because the silane treatment reduces the polymer-filler interfacial energy and therefore eliminates the tendency of the filler to "phase separate" and form the percolated network.
- the bis-silane of the present invention is clearly playing multiple roles, which presumably account for the effectiveness of these films.
- Linkage to the polymer (through the functional groups) provides toughening, and cross-linking of the bis-silane with itself leads to a hydrophobic network with extremely low water permeability.
- the silane anchors the film to the metal substrate by formation of covalent bonds.
- the present invention is therefore covalently linked to the metal by the coupling agent through the formation of a cross-linked interfacial layer of a siloxane that also contains metal ions.
- the present invention underlies that while other factors, such as porosity, oxide bonding, and corrosion inhibition are important, hydrophobicity is of primary concern.
- Hydrolyzed silanes are very hydrophilic due to the silanol groups. As a result, they readily adsorb on hydrophilic surfaces, such as metals, glass or metal oxide powders. After adsorption and curing they become hydrophobic, as they lose water and cross-link to form Si-O-Si units. The transition from hydrophilic to hydrophobic is what makes silanes, and therefore, the present invention so unique. No other surface treatment or coupling agent currently known shows this behavior.
- any coating system should completely encapsulate (or dissolve) any hydrophilic resin molecules, which are necessarily somewhat hydrophilic to assure dispersion in the carrier fluid.
- a transition upon curing must take place that renders the entire film highly hydrophobic.
- Figure 3 presents an overview of metals and forms of corrosion where bis-silane films have been demonstrated to be very effective.
- the present invention is formulated by bis-silanes of the generic type (XO) 3 -Si-(CH ) 3 - R-(CH 2 ) 3 -Si-(OX) 3 which have been shown to perform much better than films of the well- known mono-silanes of the type (XO) 3 -Si-(CH 2 ) 3 -R. While mono-silanes may provide adhesion to polymers, e.g., paints, such adhesion does not result in adequate corrosion resistance of the painted metal. As an example of the corrosion inhibiting performance, it has been shown that AA2024-T3 (Aluminum alloy) can survive 2 weeks of ASTM B-l 17 salt spray after treatment with the present invention.
- AA2024-T3 Alluminum alloy
- nanoparticle pigment which has a very high specific surface area. These particles are bonded to themselves and to the polymer by the silane.
- Some exemplary nanoparticles, having sizes generally between 0.01 nmto 500 nm, that have been shown to be effective in the present invention include: Al 2 O 3 , TiO 2 , clay, zeolite, MgO, ZnO and ZrO . In a more specific range, the nanoparticles have sizes generally between 50 mn to 100 nm.
- the nanoparticles also improve the scratch resistance of the coating and lower its permeability to electrolyte. In fact, the nanoparticles may also accelerate the cure of the coating by catalytic effects.
- Fig. 4 shows polarization curves in 3.5% aerated NaCl of AA2024-T3 alloy coated with silanes and resin or nanoparticle-modified silanes; (1) untreated control; (2) coated with a water- based silane mixture of 2% concentration; (3) coated with the same silane mixture but now also containing 4% water soluble acrylic resin; (4) as (3), but now also containing 5% colloidal silica (exemplary embodiment of the present invention).
- Fig. 5 shows EIS Bode plots of exemplary water soluble silane films on hot-dipped galvanized steel (HDG) in aerated 3.5%NaCl, showing a higher modulus after addition of an acrylic-sryrene resin to the films (3 vs. 1) and the increased water resistance of the resin- containing system (4 vs. 2); 2 and 4 were obtained after 3 days of continuous immersion.
- HDG hot-dipped galvanized steel
- the test data pertaining to the present shows: a) a coating having a high resistance to solvents and other chemicals; b) a film thickness being variable and ranging from 1 ⁇ m to 20 ⁇ m; c) a coating curing thermally at or near room temperature; d) a coating withstanding mechanical deformation such as deep drawing, i.e., the coating is flexible; e) a coating that is UV resistant without overcoating; f) a coating that can be applied by dipping, wiping, spraying or brushing; g) a coating that is translucent allowing direct inspection of both the film and substrate; h) a coating that is thermally stable to at least 250° C for one hour; and, i) a coating that is very hydrophobic (surface energy typically that of silanes, i.e., ⁇ 25 mJ/m 2 ).
- the present invention is also compatible with conventional corrosion inhibition strategies.
- the function of a conventional inhibitor is to provide corrosion protection from nicks and scratches in the coating. Since the film produced by the present invention is densely cross- linked, a water soluble inhibitor may be added to the coating that leaches out very slowly due to the extreme hydrophobicity of the film.
- Some exemplary inhibitors that may be utilized in the present invention include: organophosphonates, useful for steel substrates; amines useful for steel and zinc substrates; benzothiazoles, useful on zinc substrates; cobalt ions, useful on zinc substrates; thioglycolates, useful on zinc substrates; tolyltriazole, benzocarboxytriazole and cerium ions, Ce(III), useful on aluminum alloy substrates; tobacco extract, useful on aluminum substrates; benzocarboxytriazole and tolytriazole, useful on aluminum alloy substrates.
- the present invention provides flexibility when choosing the inhibitor based on the target substrate. It is also a consideration to choose an inhibitor showing minimal chemical reactivity with either the silane or the resin.
- the inhibitor may also replace the defect healing capabilities of chromates used in conventional metal primers.
- a UV absorber zinc oxide
- silanes are known to adsorb on zinc oxide.
- nanoparticles of various types SiO 2 , Fe 2 O 3 , CuO
- Ti0 2 Ti0 2 as the UV scatterer in those cases where ZnO might lead to excessive heating of the coating.
- These components comprising the present invention may be stored individually, optionally with the polymer being in an aqueous solution. Prior to use, these components in pure chemical form are mixed together, diluted with water, and then dispersed, using a high-shear blender in order to break down the agglomerated pigment particles. The resulting mixture is then applied to a clean metal object by dipping, spraying, wiping or brushing. The primer film is cured at room temperature for 24 hours or, preferable, by heating at around 100°C for 1 hour.
- This preferred concentrations of the components of the mixture are: at least one silane comprising 30-40 volume %; a low-molecular weight polymer comprising 30-40 volume %; a nanoparticle pigment 20-30 volume %; additives comprising less than 1 volume %.
- All coating solutions are made by direct addition of the various components almost simultaneously and immediate high shear mixing for approximately 5 to 30 seconds. Extended mixing is known to heat the solution, which can induce premature polymerization.
- the total volume of the coating solutions produced is 100 ml.
- Silane mixture An bis-ureidoproplytrialkoxysilane(Y 15445)/ vinyltriacetoxysilane (VTAS), bis-trimethoxysilylpropylamine (Al 170)/ vinyltriacetoxysilane (VTAS), or an bis-ureidoproplytrialkoxysilane (Y15445)/ ureidoproplytrialkoxysilane (UPS)/ vinyltriacetoxysilane (VTAS) mixture is prepared before the coating addition.
- a pure silane oil mixture at a 4: 1 or a 1/5/1 ratio respectively has been used. The mixture is allowed to react for at least 15 minutes before the silane is added.
- Substrates Metal panels, hot-dipped galvanized G70 (HDG G70), were cleaned in a 7% KOH solution at 60-70°C for 3-7 minutes and rinsed in deionized water before being coated.
- Coatings were applied by "drawn-down bar” technique consistent with normal paint/coating procedures. A # 28 bar was used, but most of the coatings displayed a low viscosity that might utilize a lower bar # for optimum application. Surfactant was added to reduce spotting. The coated panels were cured for one hour to a tack-free state, typically at 110°C, or 130°C for Rhoplex K-3.
- Fig.9 continues to highlight the effect of composition as both H6 and B6 are similar except for the silane mixture and the overall ratio used.
- Fig. 10 shows the eventual failure of B6 after 5 days as it begins to absorb significant amounts of water, reflected in the downward shift of the impedance slope to a more capacitance value.
- Fig. 11 shows the superior properties B6 has over the pure silane itself.
- All coating solutions are made by direct addition of the various components almost simultaneously and immediate high shear mixing for approximately 5 to 30 seconds. Extended mixing is known to heat the solution, which can induce premature polymerization.
- the total volume of the coating solutions produced is 100 ml.
- Silane solution- Table 1 lists the components utilized in the present data set. The components included: AMME comprising 65-75% aminopropylsilsesquioxane and 25-35% methylsilsequioxane; AMVS comprising 60-65% aminopropylsilseequioxane and 35-40% vinylsilsesequioxane; deionized water.
- the A 19201 Chemat Silane includes nanoparticles and the PU 402 A is a polyurethane resin.
- Substrates Cold rolled steel panels were ultrasonically cleaned in acetone for 5 minutes. Thereafter the surface of each panel was wiped to remove any residue. The acetone cleaned panels were then subjected to an alkaline cleaner (7% KOH) at 60°C and thereafter rinsed with deionized water and immediately dried.
- alkaline cleaner 7% KOH
- Coatings were applied by "drawn-down bar” technique consistent with normal paint/coating procedures. A #14 bar was used to apply the coatings. The coated panels were placed in a horizontal orientation and cured for 2 hours at 120°C to a tack-free state.
- All coating solutions are made by direct addition of the various components almost simultaneously and immediate high shear mixing for approximately 5 to 30 seconds. Extended mixing is known to heat the solution, which can induce premature polymerization.
- the total volume of the coating solutions produced is 100 ml.
- Silane solution- Table 1 lists the components utilized in the present data set. The components included: AMME comprising 65-75% aminopropylsilsesquioxane and 25-35% methylsilsequioxane; AMVS comprising 60-65% aminopropylsilseequioxane and 35-40% vinylsilsesequioxane; and PU 402 A which is a polyurethane resin.
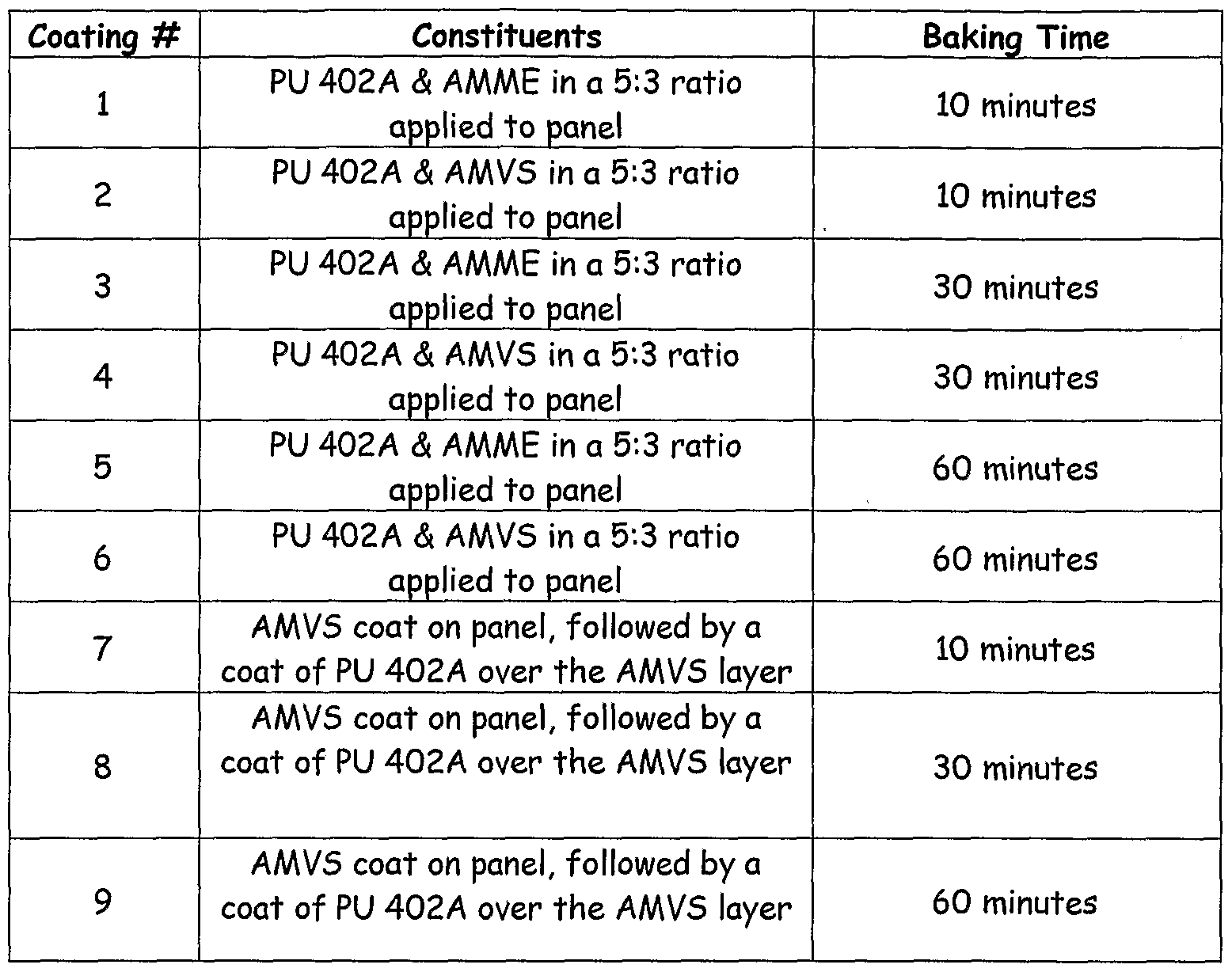
- Coatings were applied by "drawn-down bar” technique consistent with normal paint/coating procedures. A #14 bar was used to apply the coatings. Panels were coated with the solutions as shown in Table 3, along with some panels having a first coat of AMVS silane and a top coat of PU 402 A. The coated panels were placed in a horizontal orientation and cured at 120°C at the various times shown in Table 4. Thereafter the panels were removed and allowed to cool for 24 hours at room temperature to arrive at a tack-free state.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Paints Or Removers (AREA)
Abstract
Description
Claims
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA2492936A CA2492936C (en) | 2002-07-24 | 2003-07-24 | Superprimer |
| MXPA05000879A MXPA05000879A (en) | 2002-07-24 | 2003-07-24 | Superprimer. |
| EP03765986A EP1523530A1 (en) | 2002-07-24 | 2003-07-24 | Superprimer |
| AU2003259219A AU2003259219A1 (en) | 2002-07-24 | 2003-07-24 | Superprimer |
| US11/041,352 US7482421B2 (en) | 2002-07-24 | 2005-01-24 | Superprimer |
| US12/316,680 US7732016B2 (en) | 2002-07-24 | 2008-12-17 | Superprimer |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US39824702P | 2002-07-24 | 2002-07-24 | |
| US60/398,247 | 2002-07-24 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/041,352 Continuation US7482421B2 (en) | 2002-07-24 | 2005-01-24 | Superprimer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2004009717A1 true WO2004009717A1 (en) | 2004-01-29 |
Family
ID=30771204
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2003/023055 WO2004009717A1 (en) | 2002-07-24 | 2003-07-24 | Superprimer |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US7482421B2 (en) |
| EP (1) | EP1523530A1 (en) |
| AU (1) | AU2003259219A1 (en) |
| CA (1) | CA2492936C (en) |
| MX (1) | MXPA05000879A (en) |
| WO (1) | WO2004009717A1 (en) |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006069376A2 (en) * | 2004-12-22 | 2006-06-29 | University Of Cincinnati | Improved superprimer |
| WO2007032923A2 (en) * | 2005-09-09 | 2007-03-22 | The University Of Cincinnati | Silane coating compositions and methods of use thereof |
| WO2008016873A2 (en) * | 2006-07-31 | 2008-02-07 | Ecosil Technologies Llc | Addition of silanes to coating compositions |
| CN100439564C (en) * | 2005-04-08 | 2008-12-03 | 中国科学院金属研究所 | Nano self-assembling granular membrane surface treatment liquid and method for preparing same |
| WO2008151028A2 (en) * | 2007-05-30 | 2008-12-11 | Inhibitrol Inc. | Coatings including tobacco products as corrosion inhibitors |
| US20090023850A1 (en) * | 2006-09-07 | 2009-01-22 | Yong Yang | Self-Priming Color Foundation Finishes |
| US7482421B2 (en) | 2002-07-24 | 2009-01-27 | The University Of Cincinnati | Superprimer |
| WO2009114573A2 (en) | 2008-03-14 | 2009-09-17 | Ecosil Technologies Llc | Method of applying silanes to metal in an oil bath containing a controlled amount of water |
| WO2010025567A1 (en) * | 2008-09-05 | 2010-03-11 | National Research Council Of Canada | Corrosion inhibitor for mg and mg-alloys |
| US7704563B2 (en) | 2005-09-09 | 2010-04-27 | The University Of Cincinnati | Method of applying silane coating to metal composition |
| US8012374B2 (en) | 2004-11-04 | 2011-09-06 | The University Of Cincinnati | Slow-release inhibitor for corrosion control of metals |
| EP2500377A3 (en) * | 2005-08-26 | 2012-11-28 | PPG Industries Ohio, Inc. | Coating compositions exhibiting corrosion resistance properties, related coated substrates, and methods |
| US8383204B2 (en) | 2006-11-17 | 2013-02-26 | Ecosil Technologies, Llc | Siloxane oligomer treatment for metals |
| US8597482B2 (en) | 2010-09-14 | 2013-12-03 | Ecosil Technologies Llc | Process for depositing rinsable silsesquioxane films on metals |
| CN104311969A (en) * | 2014-11-10 | 2015-01-28 | 青岛科技大学 | Triethanolamine N-coco acy-L-glutamate volatile corrosion inhibitor (VCI) resin, as well as preparation method and application thereof |
| CN104311970A (en) * | 2014-11-10 | 2015-01-28 | 青岛鑫盈鑫包装材料有限公司 | 2-phenyl ethyl-1-boric acid diethanol amine ester gas-phase anti-rusting master batch as well as preparation method and application thereof |
| WO2015026465A1 (en) * | 2013-08-21 | 2015-02-26 | General Electric Company | Coating systems and fluorescent lamps provided therewith |
| EP1871924B1 (en) * | 2005-04-07 | 2015-05-06 | Momentive Performance Materials Inc. | No-rinse pretreatment methods and compositions for metal surfaces |
| CN107429086A (en) * | 2015-04-03 | 2017-12-01 | 莫克斯泰克公司 | Hydrophobicity phosphonate ester and silane chemistries material |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2849445B1 (en) * | 2002-12-26 | 2006-07-28 | Rhodia Chimie Sa | ANTI-SOIL SILICONE VARNISH, METHOD FOR APPLYING THE VARNISH TO A SUPPORT, AND SUPPORT THUS PROCESSED |
| EP1871824B1 (en) | 2005-03-24 | 2017-03-01 | Bridgestone Corporation | Compounding silica-reinforced rubber with low volatile organic compound (voc) emission |
| US8231970B2 (en) * | 2005-08-26 | 2012-07-31 | Ppg Industries Ohio, Inc | Coating compositions exhibiting corrosion resistance properties and related coated substrates |
| US7915368B2 (en) * | 2007-05-23 | 2011-03-29 | Bridgestone Corporation | Method for making alkoxy-modified silsesquioxanes |
| US8501895B2 (en) * | 2007-05-23 | 2013-08-06 | Bridgestone Corporation | Method for making alkoxy-modified silsesquioxanes and amino alkoxy-modified silsesquioxanes |
| US8962746B2 (en) | 2007-12-27 | 2015-02-24 | Bridgestone Corporation | Methods of making blocked-mercapto alkoxy-modified silsesquioxane compounds |
| US8513371B2 (en) * | 2007-12-31 | 2013-08-20 | Bridgestone Corporation | Amino alkoxy-modified silsesquioxanes and method of preparation |
| US8794282B2 (en) | 2007-12-31 | 2014-08-05 | Bridgestone Corporation | Amino alkoxy-modified silsesquioxane adhesives for improved metal adhesion and metal adhesion retention to cured rubber |
| US8642691B2 (en) | 2009-12-28 | 2014-02-04 | Bridgestone Corporation | Amino alkoxy-modified silsesquioxane adhesives for improved metal adhesion and metal adhesion retention to cured rubber |
| RU2480499C2 (en) * | 2011-07-04 | 2013-04-27 | Российская Федерация в лице Министерства промышленности и торговли Российской Федерации (Минпромторг России) | Protective coating composition |
| US9545301B2 (en) | 2013-03-15 | 2017-01-17 | Covidien Lp | Coated medical devices and methods of making and using same |
| US9668890B2 (en) | 2013-11-22 | 2017-06-06 | Covidien Lp | Anti-thrombogenic medical devices and methods |
| CN104213112B (en) * | 2014-09-09 | 2016-04-06 | 重庆大学 | The preparation method of a kind of metallic surface automatically cleaning, high protection rete |
| WO2016109625A1 (en) | 2014-12-31 | 2016-07-07 | Bridgestone Corporation | Amino alkoxy-modified silsesquioxane adhesives for adhering steel alloy to rubber |
| CN108129967A (en) * | 2017-12-11 | 2018-06-08 | 郭文军 | A kind of aqueous polyurethane anticorrosive paint |
| CN110116083A (en) * | 2019-03-18 | 2019-08-13 | 中国科学院大学 | A kind of application of selfreparing hydrophobic coating and anticorrosion material and preparation method thereof |
| CN110670054B (en) * | 2019-10-11 | 2021-06-29 | 青海民族大学 | Magnesium alloy surface cerate conversion repair film and preparation method thereof |
| US11988060B2 (en) | 2022-03-31 | 2024-05-21 | Saudi Arabian Oil Company | Systems and methods in which polyacrylamide gel is used to resist corrosion of a wellhead component in a well cellar |
| US11891564B2 (en) | 2022-03-31 | 2024-02-06 | Saudi Arabian Oil Company | Systems and methods in which colloidal silica gel is used to resist corrosion of a wellhead component in a well cellar |
| US11680201B1 (en) | 2022-03-31 | 2023-06-20 | Saudi Arabian Oil Company | Systems and methods in which colloidal silica gel is used to seal a leak in or near a packer disposed in a tubing-casing annulus |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999014277A1 (en) * | 1997-09-12 | 1999-03-25 | Cytec Technology Corp. | Water based primer compositions |
| US6071566A (en) * | 1999-02-05 | 2000-06-06 | Brent International Plc | Method of treating metals using vinyl silanes and multi-silyl-functional silanes in admixture |
| EP1130131A2 (en) * | 2000-02-29 | 2001-09-05 | Nippon Paint Co., Ltd. | Nonchromate metallic surface-treating agent, method for surface treatment, and treated steel material |
| WO2002024344A2 (en) * | 2000-09-25 | 2002-03-28 | Chemetall Gmbh | Method for pretreating and coating metal surfaces, prior to forming, with a paint-like coating and use of substrates so coated |
| WO2002031064A1 (en) * | 2000-10-11 | 2002-04-18 | Chemetall Gmbh | Method for pretreating and/or coating metallic surfaces with a paint-like coating prior to forming and use of substrates coated in this way |
| WO2002031063A1 (en) * | 2000-10-11 | 2002-04-18 | Chemetall Gmbh | Method for coating metal surfaces with an aqueous, polymer-containing composition, said aqueous composition and the use of the coated substrates |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1562651A (en) * | 1976-07-20 | 1980-03-12 | Kansai Paint Co Ltd | Surface treatment of metals |
| US5252660A (en) | 1990-12-17 | 1993-10-12 | E. I. Du Pont De Nemours And Company | Coating comprising solution organosilane polymer and silane functional dispersed polymer |
| US5244959A (en) | 1990-12-17 | 1993-09-14 | E. I. Du Pont De Nemours And Company | Coatings comprising an organosilane solution polymer and a crosslink functional dispersed polymer |
| DE4200354A1 (en) | 1992-01-09 | 1993-07-15 | Degussa | (METH) ACRYLATE RESINS WITH REDUCED GAIN |
| JP2002507246A (en) * | 1998-04-24 | 2002-03-05 | シーケイ ウィトコ コーポレイション | Powder paint or adhesive using silane or silane-treated filler |
| US6251973B1 (en) | 1998-11-23 | 2001-06-26 | Akzo Nobel N.V. | Coatings and coating compositions of a reactive group-containing polymer, a hydrazide and a silane |
| US6416869B1 (en) * | 1999-07-19 | 2002-07-09 | University Of Cincinnati | Silane coatings for bonding rubber to metals |
| US6132808A (en) | 1999-02-05 | 2000-10-17 | Brent International Plc | Method of treating metals using amino silanes and multi-silyl-functional silanes in admixture |
| US6106901A (en) | 1999-02-05 | 2000-08-22 | Brent International Plc | Method of treating metals using ureido silanes and multi-silyl-functional silanes in admixture |
| DE10084461T1 (en) * | 1999-04-14 | 2002-03-21 | Univ Cincinnati Cincinnati | Silane treatments for corrosion resistance and adhesion |
| JP2001081392A (en) | 1999-09-13 | 2001-03-27 | Nippon Parkerizing Co Ltd | Treating agent for water-based coating substrate having excellent adhesion, production of metal material and metal material |
| DE19947233A1 (en) | 1999-09-30 | 2001-11-22 | Chemetall Gmbh | Process for producing a polymeric film on a metal surface, and concentrate and treatment liquid therefor |
| JP3993729B2 (en) | 2000-02-28 | 2007-10-17 | 日本パーカライジング株式会社 | Metal plate material excellent in corrosion resistance, paintability, fingerprint resistance and workability, and manufacturing method thereof |
| US6416817B1 (en) * | 2000-03-03 | 2002-07-09 | Dow Corning Sa | Barrier coatings having bis-silanes |
| US6939451B2 (en) | 2000-09-19 | 2005-09-06 | Aclara Biosciences, Inc. | Microfluidic chip having integrated electrodes |
| DE10146446B4 (en) | 2000-09-25 | 2006-05-18 | Chemetall Gmbh | Coating metal strip for use in automobile, aircraft or aerospace industry, including formation of flexible, adherent lacquer layer using aqueous dispersion of UV-crosslinkable resin, wax and corrosion inhibitor |
| CA2492936C (en) | 2002-07-24 | 2011-02-15 | University Of Cincinnati | Superprimer |
| CA2517059C (en) | 2003-02-25 | 2012-10-23 | Chemetall Gmbh | Process for coating metallic surfaces with a composition that is rich in polymer |
-
2003
- 2003-07-24 CA CA2492936A patent/CA2492936C/en not_active Expired - Fee Related
- 2003-07-24 EP EP03765986A patent/EP1523530A1/en not_active Withdrawn
- 2003-07-24 MX MXPA05000879A patent/MXPA05000879A/en unknown
- 2003-07-24 AU AU2003259219A patent/AU2003259219A1/en not_active Abandoned
- 2003-07-24 WO PCT/US2003/023055 patent/WO2004009717A1/en not_active Application Discontinuation
-
2005
- 2005-01-24 US US11/041,352 patent/US7482421B2/en not_active Expired - Fee Related
-
2008
- 2008-12-17 US US12/316,680 patent/US7732016B2/en not_active Expired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999014277A1 (en) * | 1997-09-12 | 1999-03-25 | Cytec Technology Corp. | Water based primer compositions |
| US6071566A (en) * | 1999-02-05 | 2000-06-06 | Brent International Plc | Method of treating metals using vinyl silanes and multi-silyl-functional silanes in admixture |
| EP1130131A2 (en) * | 2000-02-29 | 2001-09-05 | Nippon Paint Co., Ltd. | Nonchromate metallic surface-treating agent, method for surface treatment, and treated steel material |
| WO2002024344A2 (en) * | 2000-09-25 | 2002-03-28 | Chemetall Gmbh | Method for pretreating and coating metal surfaces, prior to forming, with a paint-like coating and use of substrates so coated |
| WO2002031064A1 (en) * | 2000-10-11 | 2002-04-18 | Chemetall Gmbh | Method for pretreating and/or coating metallic surfaces with a paint-like coating prior to forming and use of substrates coated in this way |
| WO2002031063A1 (en) * | 2000-10-11 | 2002-04-18 | Chemetall Gmbh | Method for coating metal surfaces with an aqueous, polymer-containing composition, said aqueous composition and the use of the coated substrates |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7732016B2 (en) | 2002-07-24 | 2010-06-08 | The University Of Cincinnati | Superprimer |
| US7482421B2 (en) | 2002-07-24 | 2009-01-27 | The University Of Cincinnati | Superprimer |
| US8012374B2 (en) | 2004-11-04 | 2011-09-06 | The University Of Cincinnati | Slow-release inhibitor for corrosion control of metals |
| WO2006069376A3 (en) * | 2004-12-22 | 2006-10-26 | Univ Cincinnati | Improved superprimer |
| WO2006069376A2 (en) * | 2004-12-22 | 2006-06-29 | University Of Cincinnati | Improved superprimer |
| EP1871924B1 (en) * | 2005-04-07 | 2015-05-06 | Momentive Performance Materials Inc. | No-rinse pretreatment methods and compositions for metal surfaces |
| CN100439564C (en) * | 2005-04-08 | 2008-12-03 | 中国科学院金属研究所 | Nano self-assembling granular membrane surface treatment liquid and method for preparing same |
| US8541486B2 (en) | 2005-08-26 | 2013-09-24 | Prc-Desoto International, Inc. | Coating compositions exhibiting corrosion resistance properties, related coated substrates, and methods |
| EP1924653B1 (en) * | 2005-08-26 | 2015-12-09 | PPG Industries Ohio, Inc. | Coating compositions exhibiting corrosion resistance properties, related coated substrates, and methods |
| EP2500377A3 (en) * | 2005-08-26 | 2012-11-28 | PPG Industries Ohio, Inc. | Coating compositions exhibiting corrosion resistance properties, related coated substrates, and methods |
| US7964286B2 (en) | 2005-09-09 | 2011-06-21 | University of Cinicnnati | Coating composition of oil and organofunctional silane, and tire cord coated therewith |
| US7994249B2 (en) | 2005-09-09 | 2011-08-09 | The University Of Cincinnati | Silane coating compositions and methods of use thereof |
| WO2007032923A3 (en) * | 2005-09-09 | 2008-04-03 | Univ Cincinnati | Silane coating compositions and methods of use thereof |
| US7704563B2 (en) | 2005-09-09 | 2010-04-27 | The University Of Cincinnati | Method of applying silane coating to metal composition |
| WO2007032923A2 (en) * | 2005-09-09 | 2007-03-22 | The University Of Cincinnati | Silane coating compositions and methods of use thereof |
| WO2008016873A3 (en) * | 2006-07-31 | 2008-11-13 | Ecosil Technologies Llc | Addition of silanes to coating compositions |
| WO2008016873A2 (en) * | 2006-07-31 | 2008-02-07 | Ecosil Technologies Llc | Addition of silanes to coating compositions |
| US20090023850A1 (en) * | 2006-09-07 | 2009-01-22 | Yong Yang | Self-Priming Color Foundation Finishes |
| US8383204B2 (en) | 2006-11-17 | 2013-02-26 | Ecosil Technologies, Llc | Siloxane oligomer treatment for metals |
| WO2008151028A3 (en) * | 2007-05-30 | 2009-01-29 | Inhibitrol Inc | Coatings including tobacco products as corrosion inhibitors |
| WO2008151028A2 (en) * | 2007-05-30 | 2008-12-11 | Inhibitrol Inc. | Coatings including tobacco products as corrosion inhibitors |
| US7972659B2 (en) | 2008-03-14 | 2011-07-05 | Ecosil Technologies Llc | Method of applying silanes to metal in an oil bath containing a controlled amount of water |
| WO2009114573A2 (en) | 2008-03-14 | 2009-09-17 | Ecosil Technologies Llc | Method of applying silanes to metal in an oil bath containing a controlled amount of water |
| WO2010025567A1 (en) * | 2008-09-05 | 2010-03-11 | National Research Council Of Canada | Corrosion inhibitor for mg and mg-alloys |
| US8597482B2 (en) | 2010-09-14 | 2013-12-03 | Ecosil Technologies Llc | Process for depositing rinsable silsesquioxane films on metals |
| WO2015026465A1 (en) * | 2013-08-21 | 2015-02-26 | General Electric Company | Coating systems and fluorescent lamps provided therewith |
| US9404034B2 (en) | 2013-08-21 | 2016-08-02 | General Electric Company | Coating systems and fluorescent lamps provided therewith |
| CN104311969A (en) * | 2014-11-10 | 2015-01-28 | 青岛科技大学 | Triethanolamine N-coco acy-L-glutamate volatile corrosion inhibitor (VCI) resin, as well as preparation method and application thereof |
| CN104311970A (en) * | 2014-11-10 | 2015-01-28 | 青岛鑫盈鑫包装材料有限公司 | 2-phenyl ethyl-1-boric acid diethanol amine ester gas-phase anti-rusting master batch as well as preparation method and application thereof |
| CN104311969B (en) * | 2014-11-10 | 2016-06-08 | 青岛科技大学 | Cocoyl glutamic acid triethanolamine salt gas-phase anti-rust master batch and its preparation method and application |
| CN104311970B (en) * | 2014-11-10 | 2016-06-22 | 青岛鑫盈鑫包装材料有限公司 | 2-phenylethyl-1-boric acid diethanolamine ester gas-phase anti-rust master batch and its preparation method and application |
| CN107429086A (en) * | 2015-04-03 | 2017-12-01 | 莫克斯泰克公司 | Hydrophobicity phosphonate ester and silane chemistries material |
| EP3237208A4 (en) * | 2015-04-03 | 2018-06-13 | Moxtek, Inc. | Hydrophobic phosphonate and silane chemistry |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2492936C (en) | 2011-02-15 |
| US20050179011A1 (en) | 2005-08-18 |
| US7732016B2 (en) | 2010-06-08 |
| EP1523530A1 (en) | 2005-04-20 |
| CA2492936A1 (en) | 2004-01-29 |
| US20090155473A1 (en) | 2009-06-18 |
| MXPA05000879A (en) | 2005-10-19 |
| US7482421B2 (en) | 2009-01-27 |
| AU2003259219A1 (en) | 2004-02-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7732016B2 (en) | Superprimer | |
| CN107964351B (en) | Water-based composite zinc-aluminum anticorrosive paint | |
| EP1629136B1 (en) | Composition for coating metals to protect against corrosion | |
| Balgude et al. | Sol–gel derived hybrid coatings as an environment friendly surface treatment for corrosion protection of metals and their alloys | |
| KR101471948B1 (en) | Zinc-based metal coated steel sheet | |
| US7527872B2 (en) | Treated aluminum article and method for making same | |
| CN101717930B (en) | Environment-friendly nano water-based silane treatment agent capable of improving anti-corrosion performance of metal surface | |
| JP5579269B2 (en) | Aqueous silane systems for bare and metal corrosion protection | |
| EP2294152B1 (en) | A superhydrophobic aerogel that does not require per-fluoro compounds or contain any fluorine | |
| US9334407B2 (en) | Sol-gel coating method and composition | |
| KR101020526B1 (en) | Corrosion protection on metals | |
| KR101079778B1 (en) | Aqueous rust-resisting paint composition | |
| JP6502905B2 (en) | Corrosion prevention coating | |
| US20090264574A1 (en) | Superprimer | |
| JP6315750B2 (en) | Aqueous metal surface treatment agent | |
| KR20010034676A (en) | Method for protecting a metallic substrate against corrosion | |
| KR20010041809A (en) | Surface treatment composition and surface treatment method for metallic materials | |
| KR20080111029A (en) | Composition for metal surface treatment, metal surface treatment method, and metal material | |
| JP2009533308A (en) | Use of nanostructured materials as protective coatings on metal surfaces | |
| EP3801928A1 (en) | Invisible fingerprint coatings and process for forming same | |
| US6749945B2 (en) | Advanced composite ormosil coatings | |
| Pehkonen et al. | General background of sol-gel coatings for corrosion mitigation | |
| WO2015049696A1 (en) | A chromium-free water based coating for treating a galvannealed or galvanized steel surface | |
| JP2003113346A (en) | Rustproof coating material, galvanized steel sheet having rustproof film, and method for producing the same | |
| KR100491951B1 (en) | Non-chromic aqueous organic/inorganic sol-gel compositions for corrosion-resistance and anti-finger on metal and preparation method of thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2492936 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/A/2005/000879 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11041352 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2003765986 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2003765986 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: JP |