WO2000061162A1 - Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa - Google Patents
Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa Download PDFInfo
- Publication number
- WO2000061162A1 WO2000061162A1 PCT/US2000/008711 US0008711W WO0061162A1 WO 2000061162 A1 WO2000061162 A1 WO 2000061162A1 US 0008711 W US0008711 W US 0008711W WO 0061162 A1 WO0061162 A1 WO 0061162A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- mixture
- curcumin
- tetrahydrocurcuminoids
- curcuminoids
- linking
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q17/00—Barrier preparations; Preparations brought into direct contact with the skin for affording protection against external influences, e.g. sunlight, X-rays or other harmful rays, corrosive materials, bacteria or insect stings
- A61Q17/04—Topical preparations for affording protection against sunlight or other radiation; Topical sun tanning preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/12—Ketones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K41/00—Medicinal preparations obtained by treating materials with wave energy or particle radiation ; Therapies using these preparations
- A61K41/0057—Photodynamic therapy with a photosensitizer, i.e. agent able to produce reactive oxygen species upon exposure to light or radiation, e.g. UV or visible light; photocleavage of nucleic acids with an agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/35—Ketones, e.g. benzophenone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/02—Preparations for care of the skin for chemically bleaching or whitening the skin
Definitions
- a body cell owes its functioning in large part to its cytoskeleton, the structural
- the cytoskeleton is formed by components in the cytoplasm
- cytoskeleton includes several filamentous structures like microtubules,
- MMS proteins
- the electric potential of a cell is an aspect of cellular physiology important to
- the electric potential of a cell is maintained by a complex balance between negatively and positively charged
- pathological conditions e.g. physical or chemical assaults, a disease process
- Optimal resting potential is conductive to the
- transglutaminases One of the well known transglutaminases in the body is
- Transglutaminases are responsible for facilitating a physiological process of
- the cell is able to fulfill its purpose, e.g. mucus secreting cell, phagocytic cell,
- Melanoma is a rapidly spreading cancer
- inducers are retinoic acid and methylxantines. It appears that the therapeutic
- retinoids in melanoma is related to their ability to activate intracellular
- Cross-linking of intracellular proteins is, however, not always a positive event.
- lipofuscin nicknamed “the aging pigment” or “liver spots”, which occurs in the epidermis and mucous
- Lipofuscin has been shown to result from the oxidative degeneration of mitochondria
- Lipofuscin is postulated to be a result of a metabolic
- lipofuscin formation is in part a manifestation of the disrupted
- transglutaminases are responsible for facilitating
- pathology for example malignancy, e.g. melanoma.
- transglutaminase inducers like retinoids and methylxantines in
- melanoma is related to their ability to activate intracellular transglutaminases. This
- Curcumin derivatives including tetrahydrocurcumin and curcuminoids are well
- Curcuminoid book Majeed et al., U.S. Patent No. 5,861 ,415 established that the combination of naturally occurring curcuminoids isolated from turmeric root, i.e.
- curcumin, demethoxy curcumin and bisdemethoxy curcumin produce a broad range
- THC tetrahydrocurcumin(oids)
- curcumin(oids) (Majeed et a ⁇ .,Tumeric and the Healing Curcuminoids, Keats
- curcuminoids that they are not as highly colored curcuminoids and thus can be applied topically
- curcumin on the advanced cross-linking of collagen was more pronounced than its
- curcumin to prevent and to some degree treat excessive cross-linking of
- the mechanism of the invention is in preserving homeostasis and functioning
- curcumin and its derivatives is also unrelated to the well researched and described
- CLM or crossregulin indicates that a composition of the
- present invention exerts a positive influence on the post-translational protein
- the CLM effect of the invention is accomplished by using a specific
- the mechanism also occurs with modifications of the THC molecule, for example
- THC would not interfere with the above exemplified
- THC differentiation of cells, e.g. pre-malignant states or malignancy
- THC would prevent
- the invention also has a supportive mechanism in preventing random cross-
- THC specifically on the invention's ability to scavenge free-radicals. It is
- crossregulin and antioxidant action is particularly applicable in preventing
- THC molecule(s) can rotate more
- the invention is relatively inactive in
- Tetrahydro curcuminoids of the invention are products of the chemical
- curcuminoids obtained from the rhizomes of Curcuma longa, commonly
- turmeric or any other suitable plant from the botanical family
- THC tetrahydrocurcumin
- TLBDC tetrahydrobisdemethoxy curcumin
- the living cell the living cell.
- the present invention provides a new, more efficient method of recovering
- THCs This process prevents the loss of TBDMC and TDMC, and provides a
- creamy white powder having a melting point of 92-94°C.
- This process utilizes a novel method of saturation of two olefinic bonds in
- curcuminoids in the catalytic hydrogen transfer reaction known as a hydride transfer
- curcuminoids were treated
- THC can be administered topically in a suitable vehicle or with an application
- a transdermal patch in a concentration ranging from 0.025% to 5%; in
- THC is
- routes of administration include but are not limited to: aerosol or other
- compositions of tetrahydrocurcuminoids for topical administration should be
- composition contains no color or such a small amount of color that the composition
- color removed curcumin refers to the tetrahydrocurcuminoid
- THC molecule acting as an "anchor" for amino acids of proteins, to initiate protein crosslinks. This mechanism would contribute to the physiological cross-links.
- THC molecule acting as a buffer in preventing random cross-links in UN precipitated cross-links. This mechanism would contribute to die prevention of pathological crosslinks.
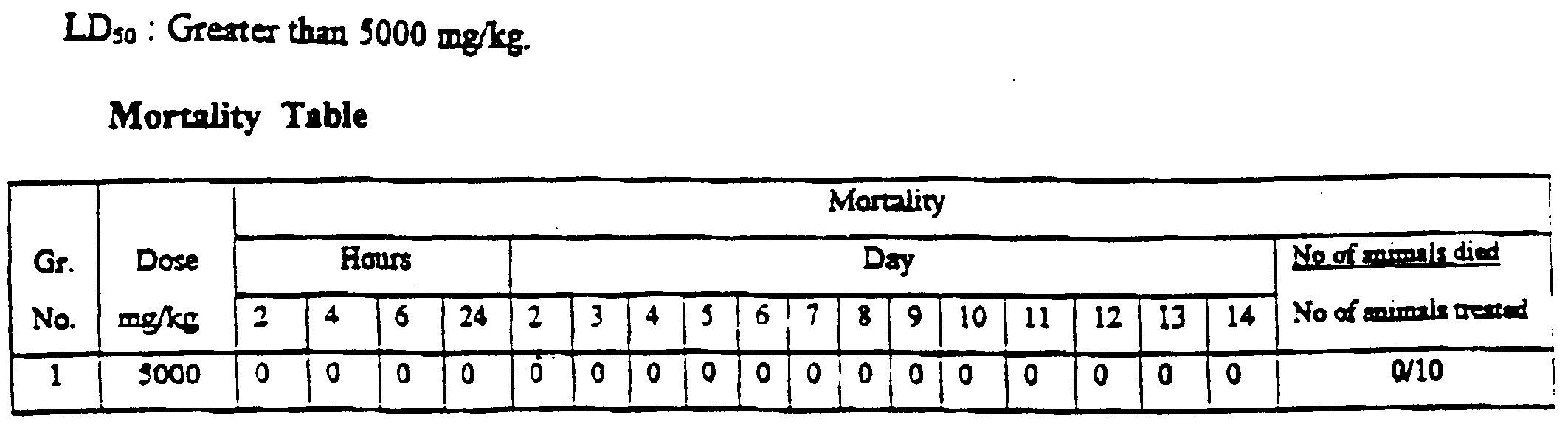
- the test substance suspended in co oil was administered by oral route to rats.
- the rats were observed for 14 days after treatment for the product related symptoms.
- the test snbstance caused salivation all animals and polyurea in two animals with onset at 2 hours after the treatment.
- AH *t n_. survived through the study period of 14 days and were free of intoxicating signs 24 hours after the treatment.
- Randomization Randomly selected in groups of five of like sex at the time of initiating the study.
- the rats were housed S each, of the same sex in polypropylene cages provided with bedding of husk.
- the temperature was maintained between 20 &-24 °C and relative humidity between 50 and 60%; 12 hours each of dark and light cycle was maintained.
- test substance 10 ml/kg The test substance, suspended in coin oil was administered once each by oral gxvage in rats of both saxes. The rats were starved for 16 hours before and 2 hours after the administration of the test substance. The animals woe observed for 14 days after the dosing for the product related toxic symptoms and mortality. Necropsy was carried out animals died during the observation period. At the end of the observation period the surviving test animals were sacrificed, dissected and examined for gross pathological abnormality, if any.
- Group 1 Salivation in all animals and polyurea in two animals were observed with onset at 2 hours after the treatment. All animals survived through the study period of 14 days and were free of intoxicating signs 24 hours after the treatment.
- Necropsy findings No significant changes could be seen with naked eye.
- Solubility Soluble in methanol
- Randomization Randomly selected in groups of three of like sex at the time of initiating the study.
- the rabbits were housed individually each, in stainless steel cages provided with stainless steel mesh bottom.
- the temperature was maintained between 20 & 23 "fiend relative humidity between 50 and 60 ; 12 hours each of dark and light cycle was maintained.
- Severe edema (raised more than 1 mm and extending beyond area of exposure) 4
- mice Female SKH-1 mice (8-9 weeks old) were treated topically with 100 ⁇ l acetone, or test compound in 100 ⁇ l acetone. Five minutes later, the mice were irradiated with a ⁇ lngl ⁇ dose of UVB (30 mJ/cm2). The mice were killed one hour later, and the skin samples were stored in a 10% formalin-phosphate buffer for thymine dim ⁇ rs assay.
- mice Female Sencar mice were irradiated with UVB (80 mJ/cn.2), The mice were treated topically with 100 ⁇ l acetone or inhibitor in 100 ⁇ l aoetone at 5 min before the UVB irradiation. Eight hours later, the mice were killed and skin samples were removed and kept in 10 % formalin phosphate buffer for sunburn cells assay.
- UVB 80 mJ/cn.2
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Birds (AREA)
- Emergency Medicine (AREA)
- Molecular Biology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Steroid Compounds (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DK00921582T DK1171144T3 (en) | 1999-04-09 | 2000-04-07 | Use of tetrahydrocurcuminoids for the regulation of physiological and pathological conditions in the skin and mucous membranes |
| EP00921582A EP1171144B1 (en) | 1999-04-09 | 2000-04-07 | Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa |
| DE60033383T DE60033383T2 (en) | 1999-04-09 | 2000-04-07 | USE OF TETRAHYDROCURCUMINOIDS FOR THE REGULATION OF PHYSIOLOGICAL AND PATHOLOGICAL CASES IN THE SKIN AND MUCOSA |
| JP2000610495A JP2002541205A (en) | 1999-04-09 | 2000-04-07 | Methods of using tetrahydrocurcuminoids to control physiological and pathological events involving skin and mucous membranes and methods of making the same |
| CA002369381A CA2369381A1 (en) | 1999-04-09 | 2000-04-07 | Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa |
| NZ514884A NZ514884A (en) | 1999-04-09 | 2000-04-07 | Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa |
| AU41879/00A AU4187900A (en) | 1999-04-09 | 2000-04-07 | Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa |
| US09/972,150 US6653327B2 (en) | 1999-04-09 | 2001-10-09 | Cross-regulin composition of tumeric-derived tetrahydrocurcuminoids for skin lightening and protection against UVB rays |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12854099P | 1999-04-09 | 1999-04-09 | |
| US60/128,540 | 1999-04-09 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/972,150 Continuation-In-Part US6653327B2 (en) | 1999-04-09 | 2001-10-09 | Cross-regulin composition of tumeric-derived tetrahydrocurcuminoids for skin lightening and protection against UVB rays |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2000061162A1 true WO2000061162A1 (en) | 2000-10-19 |
| WO2000061162A9 WO2000061162A9 (en) | 2001-11-29 |
Family
ID=22435823
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2000/008711 WO2000061162A1 (en) | 1999-04-09 | 2000-04-07 | Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa |
Country Status (11)
| Country | Link |
|---|---|
| EP (1) | EP1171144B1 (en) |
| JP (1) | JP2002541205A (en) |
| AT (1) | ATE353662T1 (en) |
| AU (1) | AU4187900A (en) |
| CA (1) | CA2369381A1 (en) |
| DE (1) | DE60033383T2 (en) |
| DK (1) | DK1171144T3 (en) |
| ES (1) | ES2282101T3 (en) |
| NZ (1) | NZ514884A (en) |
| PT (1) | PT1171144E (en) |
| WO (1) | WO2000061162A1 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1108419A1 (en) * | 1999-12-14 | 2001-06-20 | Avon Products, Inc. | Cosmetic composition and methods of use |
| WO2002032415A2 (en) * | 2000-10-19 | 2002-04-25 | Sabinsa Corporation | Process of making and method of use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa cells |
| DE10121090A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | New cosmetic or dermatological compositions comprise a synergistic combination of sericoside and tetrahydrocurcumin or its derivatives, useful for treating e.g. skin inflammation and aging symptoms |
| DE10121069A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Use of tetrahydrocurcumin, tetrahydrodemethoxycurcumin and/or tetrahydrobisdemethoxycurcumin to prepare a cosmetic or dermatological composition for treating or preventing skin aging |
| DE10121089A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Cosmetic or dermatological compositions, useful against, e.g. skin inflammation, containing synergistic combination of nitrogen monoxide synthase inhibitor and tetrahydrocurcumin or derivative |
| DE10121093A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Tetrahydrocurcumin, tetrahydrodemethoxycurcumin and/or tetrahydrobisdemethoxycurcumin is/are used in production of cosmetic or dermatological skin barrier preparations |
| DE10121067A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Cosmetic or dermatological preparations acting against skin pigmentation or tanning contain synergistic combinations of L-tyrosine sulfate or tyrosine O-sulfate ester with curcuminoids |
| DE10121070A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Use of tetrahydrocurcumin, tetrahydrodemethoxycurcumin and/or tetrahydrobisdemethoxycurcumin to prepare a cosmetic or dermatological composition for stimulating skin ceramide biosynthesis |
| US20110077275A1 (en) * | 2008-11-26 | 2011-03-31 | Jan Zielinski | Antioxidant compositions for soft oral tissue and methods of formulation and use thereof |
| WO2013049507A1 (en) | 2011-09-30 | 2013-04-04 | Perio Sciences, Llc | Antioxidant compositions for treatment of inflammation or oxidative damage |
| FR2983402A1 (en) * | 2011-12-05 | 2013-06-07 | Oreal | CAPILLARY COLORING COMPOSITION COMPRISING A TETRAHYDROCURCUMINOID, A CAPILLARY COLOR, AND A LIQUID MONOALCOHOL |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106266988A (en) * | 2016-09-12 | 2017-01-04 | 四川聚豪生物科技有限公司 | A kind of for Chinese medicine composition treating goiter due to disorder of QI and preparation method thereof |
| JP2021023147A (en) * | 2019-07-31 | 2021-02-22 | ハウス食品株式会社 | COMPOSITION FOR RESTRAINING GLUCOSE OXIDE PRODUCTION, COMPOSITION FOR RESTRAINING AGEs PRODUCTION, AND COMPOSITION FOR CUTTING CROSSLINKING BETWEEN PROTEIN MOLECULES THROUGH AGEs |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5266344A (en) * | 1988-08-12 | 1993-11-30 | Kabushiki Kaisha Kobe Seiko Sho | Method for making tetrahydrocurcumin and a substance containing the antioxidative substance tetrahydrocurcumin |
| JPH06128133A (en) * | 1992-10-20 | 1994-05-10 | Kobe Steel Ltd | External agent for preventing ultraviolet hazard |
| WO1995018606A1 (en) * | 1994-01-06 | 1995-07-13 | Research Development Foundation | Curcumin, analogues of curcumin and novel uses thereof |
| WO1997003674A1 (en) * | 1995-07-14 | 1997-02-06 | Sabinsa Corporation | Bioprotectant composition, method of use and extraction process of curcuminoids |
-
2000
- 2000-04-07 JP JP2000610495A patent/JP2002541205A/en not_active Withdrawn
- 2000-04-07 AT AT00921582T patent/ATE353662T1/en not_active IP Right Cessation
- 2000-04-07 ES ES00921582T patent/ES2282101T3/en not_active Expired - Lifetime
- 2000-04-07 PT PT00921582T patent/PT1171144E/en unknown
- 2000-04-07 DK DK00921582T patent/DK1171144T3/en active
- 2000-04-07 AU AU41879/00A patent/AU4187900A/en not_active Abandoned
- 2000-04-07 CA CA002369381A patent/CA2369381A1/en not_active Abandoned
- 2000-04-07 EP EP00921582A patent/EP1171144B1/en not_active Expired - Lifetime
- 2000-04-07 NZ NZ514884A patent/NZ514884A/en not_active IP Right Cessation
- 2000-04-07 DE DE60033383T patent/DE60033383T2/en not_active Expired - Lifetime
- 2000-04-07 WO PCT/US2000/008711 patent/WO2000061162A1/en active Search and Examination
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5266344A (en) * | 1988-08-12 | 1993-11-30 | Kabushiki Kaisha Kobe Seiko Sho | Method for making tetrahydrocurcumin and a substance containing the antioxidative substance tetrahydrocurcumin |
| JPH06128133A (en) * | 1992-10-20 | 1994-05-10 | Kobe Steel Ltd | External agent for preventing ultraviolet hazard |
| WO1995018606A1 (en) * | 1994-01-06 | 1995-07-13 | Research Development Foundation | Curcumin, analogues of curcumin and novel uses thereof |
| WO1997003674A1 (en) * | 1995-07-14 | 1997-02-06 | Sabinsa Corporation | Bioprotectant composition, method of use and extraction process of curcuminoids |
Non-Patent Citations (8)
| Title |
|---|
| MENON L G ET AL: "Anti-metastatic activity of curcumin and catechin.", CANCER LETTERS, (1999 JUL 1) 141 (1-2) 159-65., XP000933941 * |
| MENON L G ET AL: "Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds.", CANCER LETTERS, (1995 AUG 16) 95 (1-2) 221-5., XP000934170 * |
| MULKY, N. ET AL: "Antimutagenicity of curcumins and related compounds: the structural requirement for the antimutagenicity of curcumins", INDIAN DRUGS (1987), 25(3), 91-5, XP000934197 * |
| NAGABHUSHAN, M. ET AL: "Curcumins as inhibitors of nitrosation in vitro", MUTAT. RES. (1988), 202(1), 163-9, XP000933930 * |
| OSAWA, TOSHIHIKO ET AL: "Antioxidative activity of tetrahydrocurcumin", INT. CONGR. SER. - EXCERPTA MED. (1992), 998(OXYGEN RADICALS), 801-4, XP000933940 * |
| RUBY, A. J. ET AL: "Antitumor and antioxidant activity of natural curcuminoids", CANCER LETT. (SHANNON, IREL.) (1995), 94(1), 79-83, XP000933937 * |
| SHARMA, O. P.: "Antioxidant activity of curcumin and related compounds", BIOCHEM. PHARMACOL. (1976), 25(15), 1811-12, XP000933938 * |
| WALKER, M. J. ET AL: "Curcumin in a chemoprevention model of melanoma.", PROCEEDINGS OF THE AMERICAN ASSOCIATION FOR CANCER RESEARCH ANNUAL MEETING, (MARCH, 1998) VOL. 39, PP. 19. MEETING INFO.: 89TH ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR CANCER RESEARCH NEW ORLEANS, LOUISIANA, USA MARCH 28-APRIL 1, 1998 AMERICAN, XP000929727 * |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1108419A1 (en) * | 1999-12-14 | 2001-06-20 | Avon Products, Inc. | Cosmetic composition and methods of use |
| US6521668B2 (en) | 1999-12-14 | 2003-02-18 | Avon Products, Inc. | Cosmetic composition and methods of use |
| WO2002032415A2 (en) * | 2000-10-19 | 2002-04-25 | Sabinsa Corporation | Process of making and method of use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa cells |
| WO2002032415A3 (en) * | 2000-10-19 | 2003-04-17 | Sabinsa Corp | Process of making and method of use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa cells |
| DE10121089A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Cosmetic or dermatological compositions, useful against, e.g. skin inflammation, containing synergistic combination of nitrogen monoxide synthase inhibitor and tetrahydrocurcumin or derivative |
| DE10121093A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Tetrahydrocurcumin, tetrahydrodemethoxycurcumin and/or tetrahydrobisdemethoxycurcumin is/are used in production of cosmetic or dermatological skin barrier preparations |
| DE10121067A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Cosmetic or dermatological preparations acting against skin pigmentation or tanning contain synergistic combinations of L-tyrosine sulfate or tyrosine O-sulfate ester with curcuminoids |
| DE10121070A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Use of tetrahydrocurcumin, tetrahydrodemethoxycurcumin and/or tetrahydrobisdemethoxycurcumin to prepare a cosmetic or dermatological composition for stimulating skin ceramide biosynthesis |
| DE10121069A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | Use of tetrahydrocurcumin, tetrahydrodemethoxycurcumin and/or tetrahydrobisdemethoxycurcumin to prepare a cosmetic or dermatological composition for treating or preventing skin aging |
| DE10121090A1 (en) * | 2001-04-26 | 2002-10-31 | Beiersdorf Ag | New cosmetic or dermatological compositions comprise a synergistic combination of sericoside and tetrahydrocurcumin or its derivatives, useful for treating e.g. skin inflammation and aging symptoms |
| US20110077275A1 (en) * | 2008-11-26 | 2011-03-31 | Jan Zielinski | Antioxidant compositions for soft oral tissue and methods of formulation and use thereof |
| WO2013049507A1 (en) | 2011-09-30 | 2013-04-04 | Perio Sciences, Llc | Antioxidant compositions for treatment of inflammation or oxidative damage |
| US9421180B2 (en) | 2011-09-30 | 2016-08-23 | Perio Sciences, Llc | Antioxidant compositions for treatment of inflammation or oxidative damage |
| US10918613B2 (en) | 2011-09-30 | 2021-02-16 | Perio Sciences, Llc | Antioxidant compositions for treatment of inflammation or oxidative damage |
| FR2983402A1 (en) * | 2011-12-05 | 2013-06-07 | Oreal | CAPILLARY COLORING COMPOSITION COMPRISING A TETRAHYDROCURCUMINOID, A CAPILLARY COLOR, AND A LIQUID MONOALCOHOL |
| WO2013083388A1 (en) | 2011-12-05 | 2013-06-13 | L'oreal | Hair dyeing composition comprising a tetrahydrocurcuminoid, a hair dye and a liquid monoalcohol |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2282101T3 (en) | 2007-10-16 |
| DE60033383D1 (en) | 2007-03-29 |
| DK1171144T3 (en) | 2007-06-11 |
| EP1171144B1 (en) | 2007-02-14 |
| ATE353662T1 (en) | 2007-03-15 |
| CA2369381A1 (en) | 2000-10-19 |
| JP2002541205A (en) | 2002-12-03 |
| AU4187900A (en) | 2000-11-14 |
| NZ514884A (en) | 2008-05-30 |
| PT1171144E (en) | 2007-04-30 |
| WO2000061162A9 (en) | 2001-11-29 |
| DE60033383T2 (en) | 2007-05-31 |
| EP1171144A1 (en) | 2002-01-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2019112422A (en) | Melanin modification compositions and methods of use | |
| EP1328263B1 (en) | Process of making and method of use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa cells | |
| EP1171144B1 (en) | Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa | |
| DE2616479C2 (en) | Substituted fluoroacylresorcinols, processes for their preparation and pharmaceuticals and cosmetics containing them | |
| US20100273725A1 (en) | Novel compounds useful in therapeutic and cosmetic methods | |
| KR102156731B1 (en) | Bakuchiol compositions for treatment of post inflammatory hyperpigmentation | |
| DE2817133C2 (en) | ||
| EP2762131B1 (en) | Use of Salvia Haenkei extracts in compositions for antisenescence | |
| VON et al. | PATHOLOGICAL EVENTS IN THE SKIN AND MUCOSA | |
| EP0526502B1 (en) | Phenolic amine depigmenting and antimelanoma agents | |
| US9668961B2 (en) | Screening method and substances for contrasting aging | |
| AU2006235807B2 (en) | Use of tetrahydrocurcuminoids to regulate physiological and pathological events in the skin and mucosa | |
| US20210113523A1 (en) | Compositions For Treating And/Or Preventing Photodynamic Therapy Side Effects | |
| KR20010079933A (en) | Use of a boldo extract in a cosmetic or dermatological product | |
| DE2616478A1 (en) | MEDICINAL PRODUCTS AND COSMETICS CONTAINING FLUORACYL RESORCINES | |
| ZUR et al. | REGULATE PHYSIOLOGICAL AND PATHOLOGICAL EVENTS IN THE SKIN AND MUCOSA CELLS | |
| KR100975078B1 (en) | A Cosmetic composition containing Azolla imbricata extract | |
| KR100449228B1 (en) | EGCG derivatives, Preparation method thereof and cosmetic composition containing thereof | |
| RomiszewskA et al. | The use of 5-aminolevulinic acid and its derivatives in photodynamic therapy and diagnosis | |
| EP1475097A2 (en) | Dermatological compositions comprising an extract of achyrocline sp (marcela), uses and process for the preparation thereof | |
| WO2021133307A1 (en) | Cosmetic formulation containing the smoke tree extract | |
| JPH08231368A (en) | Light-aging preventing agent and skin cosmetic containing the agent | |
| KR19990075041A (en) | Whitening cosmetic composition containing ascorbic acid-aminopropanol phosphate diester | |
| WO2003030814A2 (en) | Cross-regulin composition of turmeric-derived tetrahydrocurcuminoids for skin lightening and protection against uvb rays |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY CA CH CN CR CU CZ DE DK DM DZ EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2369381 Country of ref document: CA Ref country code: JP Ref document number: 2000 610495 Kind code of ref document: A Format of ref document f/p: F Ref country code: CA Ref document number: 2369381 Kind code of ref document: A Format of ref document f/p: F |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 514884 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2000921582 Country of ref document: EP |
|
| AK | Designated states |
Kind code of ref document: C2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY CA CH CN CR CU CZ DE DK DM DZ EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: C2 Designated state(s): GH GM KE LS MW SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| COP | Corrected version of pamphlet |
Free format text: PAGES 1-24, DESCRIPTION, REPLACED BY NEW PAGES 1-24; DUE TO LATE TRANSMITTAL BY THE RECEIVING OFFICE |
|
| WWP | Wipo information: published in national office |
Ref document number: 2000921582 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2000921582 Country of ref document: EP |
|
| DPE2 | Request for preliminary examination filed before expiration of 19th month from priority date (pct application filed from 20040101) |