WO1999065887A1 - Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents - Google Patents
Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents Download PDFInfo
- Publication number
- WO1999065887A1 WO1999065887A1 PCT/US1999/013397 US9913397W WO9965887A1 WO 1999065887 A1 WO1999065887 A1 WO 1999065887A1 US 9913397 W US9913397 W US 9913397W WO 9965887 A1 WO9965887 A1 WO 9965887A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- alkyl
- ethyl
- cyclohexylmethyl
- methyl
- Prior art date
Links
- 0 CCC(C*C)CC(*)NC(OC(C)(C)C)=O Chemical compound CCC(C*C)CC(*)NC(OC(C)(C)C)=O 0.000 description 4
- LFKDJXLFVYVEFG-UHFFFAOYSA-N CC(C)(C)OC(N)=O Chemical compound CC(C)(C)OC(N)=O LFKDJXLFVYVEFG-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/12—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms
- C07D295/125—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms with the ring nitrogen atoms and the substituent nitrogen atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings
- C07D295/13—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms with the ring nitrogen atoms and the substituent nitrogen atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings to an acyclic saturated chain
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/08—Drugs for disorders of the alimentary tract or the digestive system for nausea, cinetosis or vertigo; Antiemetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/08—Indoles; Hydrogenated indoles with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, directly attached to carbon atoms of the hetero ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/08—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms
- C07D211/18—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D211/20—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms with hydrocarbon radicals, substituted by singly bound oxygen or sulphur atoms
- C07D211/22—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms with hydrocarbon radicals, substituted by singly bound oxygen or sulphur atoms by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D319/00—Heterocyclic compounds containing six-membered rings having two oxygen atoms as the only ring hetero atoms
- C07D319/10—1,4-Dioxanes; Hydrogenated 1,4-dioxanes
- C07D319/14—1,4-Dioxanes; Hydrogenated 1,4-dioxanes condensed with carbocyclic rings or ring systems
- C07D319/16—1,4-Dioxanes; Hydrogenated 1,4-dioxanes condensed with carbocyclic rings or ring systems condensed with one six-membered ring
- C07D319/18—Ethylenedioxybenzenes, not substituted on the hetero ring
Definitions
- 5-HTIA agonists and antagonists may find use in the treatment of several diseases such as anxiety, depression, schizophrenia, cognitive deficits resulting from neurodegenerative diseases like Alzheimer's Disease, nausea and vomiting, and in the treatment of prostate cancer (for recent references, see: K. Rasmussen and V. P. Rocco, Recent Progress in Serotonin (5-HT) jA Receptor Modulators, in Annual Reports in Medicinal Chemistry, Volume 30, J. A. Bristol, ed., pp. 1-9 (1995)).
- X is selected from the group consisting of:
- n is selected from the integers 1 through 5;
- R 1 is C 6 -C ⁇ o-aryl or mono or bicyclic heteroaryl, optionally substituted by F, Cl, Br, I, -OH, -NH 2 , CO 2 H, -CO 2 -C ⁇ -C 6 alkyl, -CN, -NO 2 , - alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C ⁇ -C 6 perhaloalkyl, OR 4 , and -CO perhaloalkoxy, with a proviso that heteroaryl is not thiadiazole;
- R 2 is selected from the group consisting of H and C ⁇ -C 6 alkyl;
- R 3 is selected from the group consisting of H, COR 5 , COOR 5 , and CONR 5 R 6 ;
- R 4 is selected from the group consisting of H, C ⁇ -C 6 alkyl, C 2 -C alkenyl, C 2 -C 6 alkynyl, C 6 -C ⁇ o aryl, mono or bicyclic heteroaryl, C -Ci 4 aralkyl, and mono or bicyclic heteroaralkyl, where the aryl or heteroaryl group is optionally substituted with one to three substituents independently selected from the group consisting of F, Cl, Br, I, CN, -NH 2 , -NO 2 , -OH, alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C ⁇ -C 6 perhaloalkyl, C ⁇ -C 6 alkoxy, and C ⁇ -C 6 perhaloalkoxy;
- R 5 and R 6 are selected independently from the group consisting of H, C ⁇ -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 2 -C 6 cycloalkenyl, adamantyl, and noradamantyl or R 5 and R 6 taken together with the interposed nitrogen atom may form a 5-7 membered azacyclic ring, optionally containing an additional heteroatom selected from O, S, or NR 4 ; the optical isomers; and the pharmaceutically acceptable salts thereof.
- C 6 -C ⁇ o aryl includes phenyl and naphthyl.
- Monocyclic heteroaryl means a 5-6 membered heteroaryl group having from 1-3 heteroatoms selected independently from N, O, and S, such as pyridine, pyrrole, thiophene, furan, imidazole, oxazole, pyrimidine, pyridazine, pyrazine, thiazole and oxathiazole.
- Bicyclic heteroaryl includes phenyl fused to a monocyclic 5-6 membered heteroaryl group or a 5-6 membered heteroaryl group fused to another 5-6 membered heteroaryl group, including, but not limited to indole, quinoline, isoquinoline, benzofuran, benzodioxan, benzothiophene, benzimidazole, naphthyridine, and imidazopyridine.
- C -Ci 4 aralkyl means a C1-C 4 alkyl group having a phenyl or naphthyl group as a substituent
- heteroaralkyl means a C 1 -C 4 alkyl group having a mono or bicyclic heteroaryl group as defined above as a substituent
- Optical isomers of the invention compounds can be selectively synthesized or separated using conventional procedures known to those skilled in the art of organic synthesis.
- the pharmaceutically acceptable salts of the invention compounds include the conventional acid addition salts which are formed from an invention compound and a pharmaceutically acceptable organic or inorganic acid.
- the acid addition salts include, but is not limited to, the acetate, adipate, alginate, aspartate, benzoate, benzene- sulfonate, bisulfate, butyrate, citrate, camphorate, camphorsulfonate, dodecylsulfate, ethanesulfonate, fumarate, glycerophosphate, phosphate, hemisulfate, hydrochloride, hydrobromide, hydroiodide, lactate, maleate, methanesulfonate, nicotinate, oxalate, pamoate, pectinate, pivalate, propionate, succinate, tartrate, and tosylate.
- the basic nitrogen-containing groups may be quatemized with such agents as lower alkyl halides, dialkyl sulfates, long chain halides such as lauryl bromide, aralkyl halides like benzyl and phenethyl bromides.
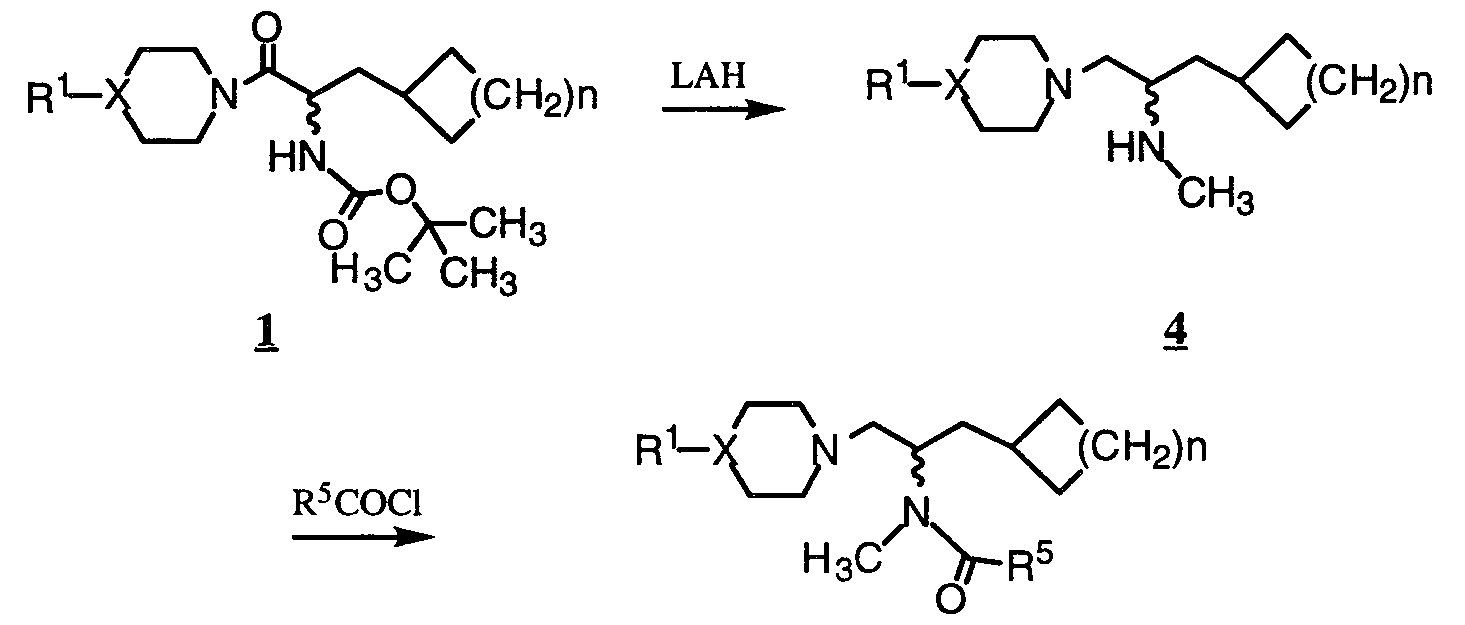
- Carbamates and ureas can be prepared from the intermediate amines 2, 4, and 6 either by treatment with an appropriate isocyanate or by reacting the amine with a phosgene equivalent such as trichloromethylchloroformate or triphosgene followed by treatment with an appropriate alcohol or amine.
- a phosgene equivalent such as trichloromethylchloroformate or triphosgene followed by treatment with an appropriate alcohol or amine.
- Other synthetic procedures may be apparent to those skilled in the art of organic synthesis.
- the compounds of this invention are prepared by conventional methods which are well known to one skilled in the art of chemistry using chemicals that are either commercially available or readily prepared following standard literature procedures.
- the reaction mixture was allowed to stir under nitrogen at 0°C for one hour, and was then concentrated on a rotary evaporator, diluted with ethyl acetate and washed with saturated aqueous NaHCO3 and brine.
- reaction mixture was allowed to stir under nitrogen overnight at ambient temperature, and was then concentrated on a rotary evaporator, diluted with ethyl acetate and washed with H 2 O and brine.
- the organic phase was dried over anhydrous sodium sulfate, filtered and concentrated on a rotary evaporator to yield the crude product, which was purified by flash chromatography on silica gel (ethyl acetate/hexanes) and then converted to the hydrochloride • hemihydrate salt of the title compound with ethereal HCl to yield 0.82 g
- Example 11 1-Methyl-cyclohexanecarboxylic acid ⁇ (lR)-l-cyclohexylmethyl-2-[4-(2- methoxyphenyl)-piperidin-l-yl]-ethyl ⁇ -amide
- Example 13 1-Methyl-cyclohexanecarboxylic acid ⁇ (lR)-l-cyclohexylmethyl-2-[4-(2- methoxyphenyl)-piperidin-l-yl]-ethyl ⁇ -methyl-amide
- the aqueous phase was extracted with three additional portions of dichloromethane.
- the combined organic phases were dried over anhydrous sodium sulfate, filtered and concentrated on a rotary evaporator to yield the crude product, which was purified by flash chromatography on silica gel (dichloromethane/methanol) and converted to the hydrochloride • 0.75 hydrate salt of the title compound with isopropanolic HCl to yield
- the reaction mixture was allowed to stir under nitrogen at 0°C for one hour, and was then concentrated on a rotary evaporator, diluted with ethyl acetate and washed with saturated aqueous NaHCO3 and brine.
- Affinity for the serotonin 5-HTIA receptor was established by assaying the test compound's ability to displace [ 3 H] 8-OHDPAT from its binding site on the receptor complex in CHO cells stably transfected with the human 5-HTIA receptor following the procedure described by J. Dunlop, Y. Zhang, D. Smith and L. Schechter (Eur. J. Pharmacol., submitted; variation of a procedure described by J. Zgombick et al., Naunyn-Schmiedeberg's Arch. Pharmacol., 354, 226-236 (1996)).
- the compounds of this invention displayed high affinity for the 5-HTIA receptor, as described in Table 1.
- Example 16 2.75 nM - 45.5 nM
- Some of the compounds of this invention displayed 5-HT ⁇ A partial agonist activity, as assessed by the test compound's ability to stimulate the binding of [ 35 S]-GTP ⁇ S to the 5-HTIA receptor-G protein complex in CHO cells stably transfected with the human 5- HT ⁇ A receptor following a variation of the procedure described by Lazareno and Birdsall [Br. J. Pharmacol., 109, 1120 (1993)].
- Selected compounds of this invention which demonstrated agonist activity in this assay are shown in Table 1.
- Some of the compounds of this invention demonstrated 5-HT ⁇ A antagonist activity, as measured by the test compound's ability to inhibit forskolin-stimulated cAMP turnover in CHO cells stably transfected with the human 5-HTIA receptor using a procedure described by J. Dunlop, Y. Zhang, D. Smith and L. Schechter [Eur. J. Pharmacol., submitted; variation of a procedure described by J. Zgombick et al., Naunyn-Schmiedeberg's Arch. Pharmacol., 354, 226-236 (1996)]. Selected compounds of this invention which demonstrated 5-HTIA antagonist activity in this are shown in Table 1.
- PHARMACEUTICAL COMPOSITION Applicable solid carriers can include one or more substances which may also act as flavoring agents, lubricants, solubilizers, suspending agents, fillers, glidants, compression aids, binders or tablet-disintegrating agents or encapsulating materials.
- the carrier is a finely divided solid which is in admixture with the finely divided active ingredient.
- the active ingredient is mixed with a carrier having the necessary compression properties in suitable proportions and compacted in the shape and size desired.
- the powders and tablets preferably contain up to 99% of the active ingredient.
- Suitable solid carriers include, for example, calcium phosphate, magnesium stearate, talc, sugars, lactose, dextrin, starch, gelatin, cellulose, methyl cellulose, sodium carboxymethyl cellulose, polyvinylpyrrolidine, low melting waxes and ion exchange resins.
- Liquid carriers may be used in preparing solutions, suspensions, emulsions, syrups and elixirs.
- the active ingredient in this invention can be dissolved or suspended in a pharmaceutically acceptable liquid carrier such as water, an organic solvent, a mixture of both or pharmaceutically acceptable oils or fat.
- the liquid carrier can contain other suitable pharmaceutical additives such as solubilizers, emulsifiers, buffers, preservatives, sweeteners, flavoring agents, suspending agents, thickening agents, colors, viscosity regulators, stabilizers or osmo-regulators.
- suitable examples of liquid carriers for oral and parenteral administration include water (particularly containing additives as above, e.g.
- cellulose derivatives preferable sodium carboxymethyl cellulose solution
- alcohols including monohydric alcohols and polyhydric alcohols, e.g. glycols
- oils e.g. fractionated coconut oil and arachis oil
- the carrier can also be an oily ester such as ethyl oleate and isopropyl myristate.
- Sterile liquid carriers are used in sterile liquid form compositions for parenteral administration.
- Liquid pharmaceutical compositions which are sterile solutions or suspensions can be utilized by, for example, intramuscular, intraperitoneal or subcutaneous injection. Sterile solutions can also be administered intravenously. Oral administration may be either liquid or solid composition form.
- the pharmaceutical composition is in unit dosage form, e.g. as tablets or capsules.
- the composition is sub-divided in unit dose containing appropriate quantities of the active ingredient;
- the unit dosage forms can be packaged compositions, for example packeted powders, vials, ampoules, prefilled syringes or sachets containing liquids.
- the unit dosage form can be, for example, a capsule or tablet itself, or it can be the appropriate number of any such compositions in package form.
- the dosage to be used in the treatment of a specific disease must be subjectively determined by the attending physician.
- the variables involved include the specific disease state and the size, age and response pattern of the patient.
Landscapes
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
- Pain & Pain Management (AREA)
- Urology & Nephrology (AREA)
- Otolaryngology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Hydrogenated Pyridines (AREA)
- Indole Compounds (AREA)
- Heterocyclic Compounds That Contain Two Or More Ring Oxygen Atoms (AREA)
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| BR9911280-9A BR9911280A (en) | 1998-06-15 | 1999-06-14 | Aryl piperazines, piperidines and tetrahydro pyridines substituted with an alkyl cycle as serotonergic agents |
| AU45663/99A AU4566399A (en) | 1998-06-15 | 1999-06-14 | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
| JP2000554713A JP2002518382A (en) | 1998-06-15 | 1999-06-14 | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonin agonists |
| EP99928648A EP1087954A1 (en) | 1998-06-15 | 1999-06-14 | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
| CA002334254A CA2334254A1 (en) | 1998-06-15 | 1999-06-14 | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US9746398A | 1998-06-15 | 1998-06-15 | |
| US09/097,463 | 1998-06-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1999065887A1 true WO1999065887A1 (en) | 1999-12-23 |
Family
ID=22263490
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1999/013397 WO1999065887A1 (en) | 1998-06-15 | 1999-06-14 | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP1087954A1 (en) |
| JP (1) | JP2002518382A (en) |
| CN (1) | CN1312802A (en) |
| AU (1) | AU4566399A (en) |
| BR (1) | BR9911280A (en) |
| CA (1) | CA2334254A1 (en) |
| WO (1) | WO1999065887A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6376494B1 (en) * | 1998-06-15 | 2002-04-23 | American Home Products Corporation | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
| WO2002085871A2 (en) * | 2001-04-04 | 2002-10-31 | Wyeth | Serotonergic agents with long-acting in vivo effects |
| US7067518B2 (en) | 2002-09-05 | 2006-06-27 | Wyeth | Pyridinyl-methyl-ethyl cyclohexanecarboxamides as serotonergic agents |
| EP2338873A1 (en) | 2009-12-22 | 2011-06-29 | Gmeiner, Peter | New aminotetraline derivatives |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016079168A (en) * | 2014-10-17 | 2016-05-16 | 塩野義製薬株式会社 | 9 membered condensed-ring derivative |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0395313A2 (en) * | 1989-04-22 | 1990-10-31 | American Home Products Corporation | Tertiary alkyl functionalized piperazine derivatives |
| EP0434561A2 (en) * | 1989-12-20 | 1991-06-26 | Adir Et Compagnie | 1-Naphthyl-piperazine derivatives, process for their preparation and pharmaceutical compositions containing them |
| EP0506468A1 (en) * | 1991-03-29 | 1992-09-30 | Eli Lilly And Company | N-substituted 4-phenyl-piperidine opioid-antagonists |
| EP0512755A2 (en) * | 1991-05-02 | 1992-11-11 | JOHN WYETH & BROTHER LIMITED | Piperazine derivatives |
| WO1993003016A1 (en) * | 1991-07-30 | 1993-02-18 | Boehringer Ingelheim Italia S.P.A. | Benzimidazolone derivatives as 5-ht1a and 5-ht2 antagonists |
| WO1993011122A1 (en) * | 1991-12-05 | 1993-06-10 | John Wyeth & Brother Limited | Piperazine derivatives as 5-ht1a antagonists |
| WO1994021610A1 (en) * | 1993-03-24 | 1994-09-29 | John Wyeth & Brother Limited | Piperazine derivatives as 5-ht1a ligands |
| WO1995033743A1 (en) * | 1994-06-03 | 1995-12-14 | John Wyeth & Brother Limited | Piperazine derivatives as 5ht1a antagonists |
| WO1995033729A1 (en) * | 1994-06-08 | 1995-12-14 | H. Lundbeck A/S | Serotonin 5-ht1a and dopamin d2 receptor ligands |
| WO1996001656A1 (en) * | 1994-07-08 | 1996-01-25 | John Wyeth & Brother Limited | 5-ht1a ligands |
| WO1996016949A1 (en) * | 1994-12-02 | 1996-06-06 | Pierre Fabre Medicament | Novel 3,5-dioxo-(2h,4h)-1,2,4-triazine derivatives, their preparation and use as drugs |
| EP0737678A1 (en) * | 1995-04-10 | 1996-10-16 | American Home Products Corporation | 4-Indolylpiperazinyl derivatives |
| WO1997040038A1 (en) * | 1996-04-18 | 1997-10-30 | Merck Patent Gmbh | Piperidines and pyrrolidines |
-
1999
- 1999-06-14 BR BR9911280-9A patent/BR9911280A/en not_active IP Right Cessation
- 1999-06-14 CN CN99809567A patent/CN1312802A/en active Pending
- 1999-06-14 WO PCT/US1999/013397 patent/WO1999065887A1/en not_active Application Discontinuation
- 1999-06-14 CA CA002334254A patent/CA2334254A1/en not_active Abandoned
- 1999-06-14 JP JP2000554713A patent/JP2002518382A/en active Pending
- 1999-06-14 AU AU45663/99A patent/AU4566399A/en not_active Abandoned
- 1999-06-14 EP EP99928648A patent/EP1087954A1/en not_active Withdrawn
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0395313A2 (en) * | 1989-04-22 | 1990-10-31 | American Home Products Corporation | Tertiary alkyl functionalized piperazine derivatives |
| EP0434561A2 (en) * | 1989-12-20 | 1991-06-26 | Adir Et Compagnie | 1-Naphthyl-piperazine derivatives, process for their preparation and pharmaceutical compositions containing them |
| EP0506468A1 (en) * | 1991-03-29 | 1992-09-30 | Eli Lilly And Company | N-substituted 4-phenyl-piperidine opioid-antagonists |
| EP0512755A2 (en) * | 1991-05-02 | 1992-11-11 | JOHN WYETH & BROTHER LIMITED | Piperazine derivatives |
| WO1993003016A1 (en) * | 1991-07-30 | 1993-02-18 | Boehringer Ingelheim Italia S.P.A. | Benzimidazolone derivatives as 5-ht1a and 5-ht2 antagonists |
| WO1993011122A1 (en) * | 1991-12-05 | 1993-06-10 | John Wyeth & Brother Limited | Piperazine derivatives as 5-ht1a antagonists |
| WO1994021610A1 (en) * | 1993-03-24 | 1994-09-29 | John Wyeth & Brother Limited | Piperazine derivatives as 5-ht1a ligands |
| WO1995033743A1 (en) * | 1994-06-03 | 1995-12-14 | John Wyeth & Brother Limited | Piperazine derivatives as 5ht1a antagonists |
| WO1995033729A1 (en) * | 1994-06-08 | 1995-12-14 | H. Lundbeck A/S | Serotonin 5-ht1a and dopamin d2 receptor ligands |
| WO1996001656A1 (en) * | 1994-07-08 | 1996-01-25 | John Wyeth & Brother Limited | 5-ht1a ligands |
| WO1996016949A1 (en) * | 1994-12-02 | 1996-06-06 | Pierre Fabre Medicament | Novel 3,5-dioxo-(2h,4h)-1,2,4-triazine derivatives, their preparation and use as drugs |
| EP0737678A1 (en) * | 1995-04-10 | 1996-10-16 | American Home Products Corporation | 4-Indolylpiperazinyl derivatives |
| WO1997040038A1 (en) * | 1996-04-18 | 1997-10-30 | Merck Patent Gmbh | Piperidines and pyrrolidines |
Non-Patent Citations (6)
| Title |
|---|
| D.L. NELSON, PHARMACOLOGY BIOCHEMISTRY & BEHAVIOR, vol. 40, 1991, pages 1041 - 1051, XP002114414 * |
| GLENNON R A ET AL: "N-(PHTHALIMIDOALKYL) DERIVATIVES OF SEROTONERGIC AGENTS: A COMMON INTERACTION AT 5-HT1A SEROTONIN BINDING SITES?", JOURNAL OF MEDICINAL CHEMISTRY, vol. 32, no. 8, 1 August 1989 (1989-08-01), pages 1921 - 1926, XP000571455, ISSN: 0022-2623 * |
| GLENNON R A ET AL: "STIMULUS PROPERTIES OF ARYLPIPERAZINES: NAN-190, A POTENTIAL 5 -HT1A SEROTONIN ANTAGONIST", DRUG DEVELOPMENT RESEARCH, vol. 16, no. 2/04, 1 January 1989 (1989-01-01), pages 335 - 343, XP000575594, ISSN: 0272-4391 * |
| GLENNON R A: "CONCEPTS FOR THE DESIGN OF 5-HT1A SEROTONIN AGONISTS AND ANTAGONISTS", DRUG DEVELOPMENT RESEARCH, vol. 26, no. 3, 1 January 1992 (1992-01-01), pages 251 - 274, XP000562325, ISSN: 0272-4391 * |
| I.A. CLIFFE ET AL., DRUGS OF THE FUTURE, vol. 18, no. 7, 1993, pages 631 - 642, XP002114577 * |
| SLEIGHT A J ET AL: "IDENTIFICATION OF 5-HYDROXYTRYPTAMINE1A RECEPTOR AGENTS USING A COMPOSITE PHARMACOPHORE ANALYSIS AND CHEMICAL DATABASE SCREENING", NAUNYN-SCHMIEDEBERG'S ARCHIVES OF PHARMACOLOGY, vol. 343, 1 January 1991 (1991-01-01), pages 109 - 116, XP000650292 * |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6376494B1 (en) * | 1998-06-15 | 2002-04-23 | American Home Products Corporation | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
| US6518272B2 (en) * | 1998-06-15 | 2003-02-11 | Wyeth | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
| US7049330B2 (en) | 1998-06-15 | 2006-05-23 | Wyeth | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents |
| WO2002085871A2 (en) * | 2001-04-04 | 2002-10-31 | Wyeth | Serotonergic agents with long-acting in vivo effects |
| WO2002085871A3 (en) * | 2001-04-04 | 2003-02-20 | Wyeth Corp | Serotonergic agents with long-acting in vivo effects |
| US6696450B2 (en) | 2001-04-04 | 2004-02-24 | Wyeth | Serotonergic agents with long-acting in vivo effects |
| US6894053B2 (en) | 2001-04-04 | 2005-05-17 | Wyeth | Serotonergic agents with long-acting in vivo effects |
| US7067518B2 (en) | 2002-09-05 | 2006-06-27 | Wyeth | Pyridinyl-methyl-ethyl cyclohexanecarboxamides as serotonergic agents |
| EP2338873A1 (en) | 2009-12-22 | 2011-06-29 | Gmeiner, Peter | New aminotetraline derivatives |
| WO2011076708A1 (en) | 2009-12-22 | 2011-06-30 | Peter Gmeiner | New aminotetraline derivatives |
| US8586603B2 (en) | 2009-12-22 | 2013-11-19 | Peter Gmeiner | Aminotetraline derivatives |
| US8691839B2 (en) | 2009-12-22 | 2014-04-08 | Peter Gmeiner | Aminotetraline derivatives |
Also Published As
| Publication number | Publication date |
|---|---|
| AU4566399A (en) | 2000-01-05 |
| EP1087954A1 (en) | 2001-04-04 |
| CA2334254A1 (en) | 1999-12-23 |
| JP2002518382A (en) | 2002-06-25 |
| BR9911280A (en) | 2001-03-13 |
| CN1312802A (en) | 2001-09-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU701452B2 (en) | Benzylpiperidines and piperazines as muscarinic antagonists | |
| KR100359393B1 (en) | Piperazine derivatives as 5-HT1A antagonists and preparation method thereof | |
| US6294555B1 (en) | 1-[(1-Substituted-4-piperidinyl)methyl]-4-piperidine derivative, process for producing the same, medicinal compositions containing the same and intermediates of these compounds | |
| US4835157A (en) | Thieno- and furopyrimidine-2,4-dione piperidine derivatives as serotonin antagonists and alpha adrenergic blocking agents | |
| JP6173431B2 (en) | Morphinan derivatives | |
| AU645707B2 (en) | Piperidine derivatives | |
| EP0711291B1 (en) | N-(piperidinyl-1-alkyl)-substituted cyclohexane carboxylic acid amides as 5-ht1a receptor antagonists | |
| US5486518A (en) | 4-indolylpiperazinyl derivatives | |
| CA2131381A1 (en) | Nitrogen containing heterocyclic compounds useful as pharmaceuticals | |
| US6518272B2 (en) | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents | |
| US5519025A (en) | 4-indolylpiperazinyl derivatives | |
| CA2713412A1 (en) | Amide derivative and pharmaceutical composition containing the same | |
| WO1999065887A1 (en) | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents | |
| CA2317515A1 (en) | Oxazole derivatives as serotonin-1a receptor agonists | |
| EP1073651B1 (en) | Indolyl derivatives as serotonergic agents | |
| US6057340A (en) | Oxazole derivatives as serotonin-1A receptor agonists | |
| AU692917B2 (en) | Bicyclic carboxamides as 5-HT-1A antagonists | |
| EP0481742B1 (en) | Piperazine derivatives | |
| MXPA00012479A (en) | Cycloalkyl-substituted aryl-piperazines, piperidines and tetrahydropyridines as serotonergic agents | |
| US7067518B2 (en) | Pyridinyl-methyl-ethyl cyclohexanecarboxamides as serotonergic agents | |
| CA2330577A1 (en) | Serotonergic agents | |
| US6489342B2 (en) | Aryloxy piperidinyl indoles for treating depression |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 99809567.2 Country of ref document: CN |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AL AM AT AU AZ BA BB BG BR BY CA CH CN CU CZ DE DK EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT UA UG UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SL SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1999928648 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2334254 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2000 554713 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2000/012479 Country of ref document: MX |
|
| WWP | Wipo information: published in national office |
Ref document number: 1999928648 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1999928648 Country of ref document: EP |