WO1996038125A1 - COSMETIC OR DERMATOLOGICAL COMPOSITIONS CONTAINING OLIGOMERS OR POLYMERS OF α-HYDROXYCARBOXYLIC ACIDS - Google Patents
COSMETIC OR DERMATOLOGICAL COMPOSITIONS CONTAINING OLIGOMERS OR POLYMERS OF α-HYDROXYCARBOXYLIC ACIDS Download PDFInfo
- Publication number
- WO1996038125A1 WO1996038125A1 PCT/EP1996/002354 EP9602354W WO9638125A1 WO 1996038125 A1 WO1996038125 A1 WO 1996038125A1 EP 9602354 W EP9602354 W EP 9602354W WO 9638125 A1 WO9638125 A1 WO 9638125A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- groups
- oligomers
- polymers
- unbranched
- branched
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/74—Synthetic polymeric materials
- A61K31/765—Polymers containing oxygen
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
- A61K8/365—Hydroxycarboxylic acids; Ketocarboxylic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/85—Polyesters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/74—Biological properties of particular ingredients
- A61K2800/75—Anti-irritant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/08—Anti-ageing preparations
Definitions
- Cosmetic or dermatological preparations containing oligomers or polymers of ⁇ -hydroxycarboxylic acids containing oligomers or polymers of ⁇ -hydroxycarboxylic acids
- the present invention relates to preparations in the field of cosmetic and dermatological skin and hair care, in particular those preparations with low irritation potential or cosmetic or dermatological preparations for prophylaxis, amelioration, elimination or avoidance of skin irritation.
- the present invention relates to cosmetic and dermatological preparations for the prophylaxis and treatment of cosmetic or dermatological skin changes such as e.g. skin aging.
- the present invention relates to preparations against the appearance of dry or rough skin.
- the present invention relates to active substances and preparations containing such active substances for cosmetic and dermatological treatment or prophylaxis of erythematous, inflammatory, allergic or autoimmune-reactive symptoms, in particular dermatoses. Furthermore, the invention relates to the use of such active substances and preparations containing such active substances for immunostimulation of the skin, advantageously also for immunostimulation in the sense of treating injured skin, in particular for treating wounds.
- the invention relates to preparations with extremely low so-called "stinging potential”.
- Skin care is primarily understood to mean that the natural function of the skin as a barrier against environmental influences (e.g. dirt, chemicals, microorganisms) and against the loss of the body's own substances (e.g. water, natural fats, electrolytes) is strengthened or restored. If this function is disturbed, there may be an increased absorption of toxic or allergenic substances or an infestation of microorganisms and, as a result, toxic or allergic skin reactions.
- environmental influences e.g. dirt, chemicals, microorganisms
- the loss of the body's own substances e.g. water, natural fats, electrolytes
- the aim of skin care is also to compensate for the loss of fat and water in the skin caused by daily washing. This is especially important when the natural regeneration ability is insufficient.
- skin care products are intended to protect against environmental influences, especially sun and wind, and to delay the signs of aging.
- Medical topical compositions generally contain one or more medicaments in an effective concentration.
- medicaments in an effective concentration.
- the epidermis is richly equipped with nerves and nerve end devices such as Father Pacini lamellar bodies, Merkel cell neurite complexes and free nerve endings for pain, cold, heat sensation and itching.
- nerves and nerve end devices such as Father Pacini lamellar bodies, Merkel cell neurite complexes and free nerve endings for pain, cold, heat sensation and itching.
- This "sensitive skin” differs fundamentally from “dry skin” with thickened and hardened horny layers.
- Typical reactions of "stinging" to sensitive skin are reddening, tension and burning of the skin as well as itching.
- “Stinging 'phenomena” can be regarded as disorders to be treated cosmetically.
- severe itching in particular severe itching in the case of atopy, can also be described as a more serious dermatological disorder.
- Typical disturbing neurosensory phenomena associated with the terms “stinging” or “sensitive skin” are reddening of the skin, tingling, tingling, tension and burning of the skin and itching. They can be caused by stimulating environmental conditions such as massage, tenside effects, weather influences such as sun, cold, dryness, but also moist heat, heat radiation and UV radiation, such as the sun.
- citric acid is used to buffer cosmetic and / or dermatological preparations, but also as a synergist for antioxidants in skin and hair cosmetics.
- DE-OS 42 04 321 describes the use of longer-chain ⁇ -hydroxycarboxylic acids as active ingredients for cosmetic deodorants.
- US Pat. No. 4,363,815 describes the use of ⁇ -hydroxycarboxylic acids for the treatment of inflammatory skin symptoms.
- US Pat. No. 4,380,549 describes the use of ⁇ -hydroxycarboxylic acids to relieve the symptoms of dry skin.
- Other topical fields of application of the ⁇ -hydroxycarboxylic acids are also known, but these few examples already show that the ⁇ -hydroxycarboxylic acids are valuable substances effective on the skin, whether they are cosmetic or pharmaceutical applications.
- Erythematous skin symptoms also appear as side effects in certain skin diseases or irregularities. For example, the typical rash in the appearance of acne is usually more or less reddened.
- EP-OS 447 318 describes compositions for the cosmetic or pharmaceutical care of the upper skin layers, which contain polymeric, biodegradable nanoparticles in a suitable carrier, which coat an oily active substance.
- the polymeric coating materials described also include poly (L) lactic acid, poly (DL) lactic acid, polyglycolic acid and copolymers of lactic and glycolic acid.
- cosmetic or dermatological preparations according to the invention in particular when the oligomers, polymers, co-oligomers and / or copolymers according to the invention are incorporated into an oil phase, are stable against depolymerization (or de-oligomerization) and their advantageous First have an effect on human skin. Since the oligomers, polymers, co-oligomers and / or copolymers according to the invention gradually release the monomers on which they are based, they are particularly suitable as depot systems or for sustained release.
- the oligomers, polymers, co-oligomers and / or co-polymers according to the invention also have the advantage, surprisingly, even if their hydrolysis to the underlying monomers has not yet taken place or has not yet taken place completely, to increase the skin moisture.
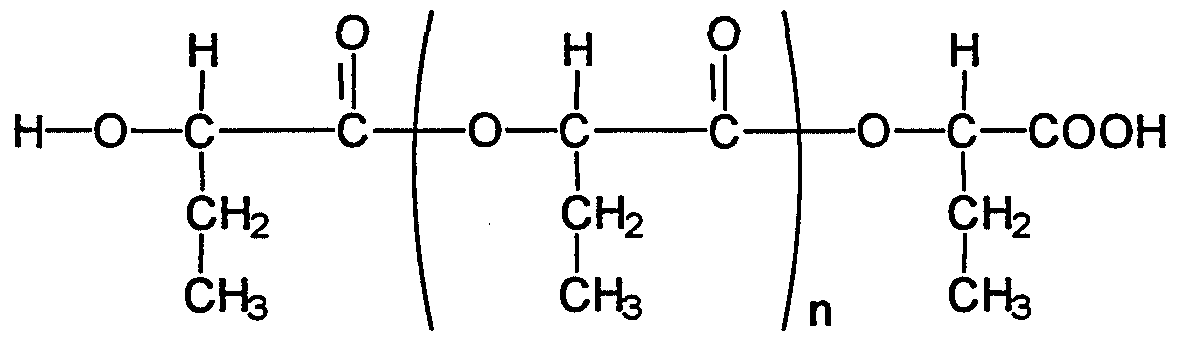
- the invention also relates to a process for stabilizing an effective amount of one or more oligomers and / or polymers and / or co-oligomers and / or co-polymers of one or more ⁇ -hydroxycarboxylic acids of the general formula
- ⁇ -hydroxycarboxylic acids on which the oligomers and / or polymers and / or co-oligomers and / or co-polymers are based are advantageously selected from the group of ⁇ -hydroxyalkanoic acids, which in turn is particularly advantageous from the group of C2- _-Alkyl carboxylic acids can be selected.
- oligomers and / or polymers and / or co-oligomers and / or copolymers of glycolic acid, lactic acid, ⁇ -hydroxybutyric acid and / or ⁇ -hydroxyvaleric acid are particularly preferred.
- oligo- or polyglycolides are characterized by their structure
- Co-oligo- or copolymers of lactic acid and glycolic acid are characterized, for example, by a structure according to the sum of the numbers k and j corresponds to the number n. It is clear to the person skilled in the art that the arrangement of the co-monomers in a co-oligomer or co-polymer can be carried out in blocks, alternating, randomly distributed or according to other arrangement principles. All of these types of arrangement can be used advantageously according to the invention.
- Oligo- or poly-2-hydroxybutyrates are characterized by the structure
- Oligo- or poly-2-hydroxyvalerates are characterized by the structure
- ⁇ -hydroxycarboxylic acids present as enantiomers as basic constituents of the oligomers or polymers which are more beneficial to skin care than their optical antipodes, for example L-lactic acid.
- the number n for at least 40% by weight of the oligomer and / or polymer and / or co-oligomer and / or co-polymer used is advantageously at least 8, preferably 10.
- the upper limit for n can in principle remain open, but nevertheless values for n of 60 to 1,000, in particular 200, have proven to be advantageous.
- polymers which consist of at least 40% by weight of approximately 60,000 monomer units and those which consist of at least 40% by weight of approximately 100,000 monomer units can also be used advantageously.
- Oligo (DL-lactate-co-glycolate) (50:50), average value for n * 8; j * k ⁇ ⁇
- the content of the oligomers, co-oligomers, polymers and / or copolymers in the cosmetic or pharmaceutical preparations is advantageously 0.01-20% by weight, preferably 0.5-5% by weight, particularly preferably 1 , 5 - 2.5 wt .-%, each based on the total weight of the cosmetic or pharmaceutical preparations.
- the oligomer or co-oligomers, polymers and / or copolymers in dissolved form into cosmetic formulations.
- the person skilled in the art can make a non-inventive selection from a large number of known solvents which are generally known for the given substance class. It was surprising, however, that partially or completely esterified polybasic carboxylic acids would prove to be particularly advantageous solvents for the oligomers, co-oligomers, polymers and / or co-polymers according to the invention.
- AOOC-X-COOB where X is selected from the group consisting of up to four carboxylic acid groups and up to four carboxylic acid alkyl ester groups -COOD, -COOE, -COOF and up to four hydroxyl groups, branched and or unbranched alkylene groups with up to six C. -Atoms and the group of the types substituted with up to four carboxylic acid groups and up to four carboxylic acid alkyl ester groups -COOD, -COOE, -COOF and up to four hydroxyl groups
- A, B, D, E and F represent branched or unbranched alkyl groups with up to five carbon atoms.
- esters are triethyl citrate
- esters are not only good solvents for the oligomers, polymers, co-oligomers and / or copolymers according to the invention, but also stabilize them against depolymerization or deoligomerization.
- esters preferably the citric acid esters, act synergistically with the oligomers, polymers according to the invention in relation to the cosmetic or dermatological action of the ⁇ -hydroxycarboxylic acids, in particular in relation to the caring effect, especially in relation to the action against skin aging.
- these esters act synergistically with the oligomers, polymers according to the invention in relation to the cosmetic or dermatological action of the ⁇ -hydroxycarboxylic acids, in particular in relation to the caring effect, especially in relation to the action against skin aging.
- Co-oligomers and / or copolymers together.
- the weight ratio of the oligomers, polymers, co-oligomers and / or copolymers on the one hand to the above-described stabilizing esters on the other hand is advantageously in the range from about 1:10 to 10: 1, preferably about 1: 4 to 2 : 1, particularly preferably about 3: 7.
- DE-GbM 88 04 423 It is known, for example from DE-GbM 88 04 423, to use corresponding citric acid esters as plasticizers for polyiactides / glycolides and similar polymers.
- the cosmetic or pharmaceutical use according to the invention was neither suggested nor anticipated by DE-GbM 88 04 423, which relates to surgical materials.
- DE-GbM 88 04 423 recommends acetone as the solvent, which is neither cosmetically nor pharmaceutically acceptable.
- silicone oils such as dimethicone as solvents for the oligomers, polymers, co-oligomers and / or copolymers according to the invention.
- the oligomers, polymers, co-oligomers or copolymers according to the invention are remarkably stable in cosmetic and pharmaceutical preparations and largely decompose only on human skin to form the underlying oligomers, which then become available to the skin with all its advantageous properties. It is also possible and possibly advantageous to use suspensions of the oligomers, polymers, co-oligomers and / or co-polymers according to the invention.
- micronization Before the solution or suspension process, it can be advantageous to precede a micronization process, it being preferred to prefer the micronization with cooling, for example at temperatures below -78 ° C., in particular at temperatures below -150 ° C. at approx. -190 ° C.
- the dissolving process is preferably carried out by means of strong agitation, for example in an ultra-turrax. It can also be advantageous to add a wetting agent or an antistatic agent during the solution or suspension process.
- Preparations according to the invention are produced in accordance with the usual rules familiar to those skilled in the art.
- the preparations according to the invention are advantageously in the form of emulsions, preferably O / W emulsions.
- emulsions preferably O / W emulsions.
- W / O emulsions hydrodispersions, gels, oils, multiple emulsions, for example in the form of W / O / W or OW / O emulsions, anhydrous ointments or ointment bases etc.
- finely dispersed droplets of the second phase water droplets in W / O or lipid vesicles in O / W emulsions
- finely dispersed droplets of the first phase are emulsified in such droplets.
- These droplets can also have finer disperse droplets (third degree multiple emulsion) and so on.
- W / O or O / W emulsions water-in-oil or oil-in-water
- W / O / W water-in-oil or oil-in-water
- the second degree multiple emulsions are sometimes referred to as "bimultiple systems", such third degrees as “trimultiple systems” etc. (W.Seifriz, Studies in Emulsions, J.Phys.Chem., 29 (1925) 738-749).
- Processes for producing multiple emulsions are known per se to the person skilled in the art. There are two-pot processes in which a simple emulsion (e.g. a W / O emulsion) is introduced and, by adding another phase (e.g. a water phase) with a corresponding emulsifier (e.g. an O / W emulsifier) into a multiple emulsion (e.g. a W / O / W emulsion) is transferred.
- a simple emulsion e.g. a W / O emulsion
- another phase e.g. a water phase
- a corresponding emulsifier e.g. an O / W emulsifier
- a multiple emulsion e.g. a W / O / W emulsion
- a second method consists in converting emulsifier mixtures with an oil phase and a water phase into a multiple W / O / W emulsion in a one-pot process.
- the emulsifiers are dissolved in the oil phase and combined with the water phase.
- Hydrodispersions are dispersions of a liquid, semi-solid or solid inner (discontinuous) lipid phase in an outer aqueous (continuous) phase.
- hydrodispersions are essentially free of emulsifiers. Like other emulsions, hydro dispersions represent metastable systems and are inclined to change into a state of two discrete phases that are coherent. In emulsions, the choice of a suitable emulsifier prevents phase separation.
- the stability of such a system can be ensured, for example, by building a gel structure in the aqueous phase in which the lipid droplets are stably suspended.
- Such preparations have proven to be extremely advantageous and particularly stable.
- Advantageous thickeners for this purpose are carbopols, polysaccharide-based thickeners and bentonites.
- Particularly advantageous preparations are also obtained if the active compounds according to the invention are combined with antioxidants and / or other substances which counteract cell aging.
- the preparations advantageously contain one or more antioxidants. All of the antioxidants suitable or customary for cosmetic and / or dermatological applications are used as inexpensive, but nevertheless optional anti-oxidants.
- Amino acids e.g. glycine, histidine, tyrosine, tryptophan
- imidazoles e.g. urocanic acid
- peptides such as D, L-carnosine, D-carnosine, L-camosine and their derivatives (e.g. anserine)
- carotenoids e.g. ⁇ -carotene, ß-carotene, lycopene
- lipoic acid and their derivatives e.g. dihydroliponic acid
- aurothioglucose propylthiouracil and other thiols
- thioredoxin glutathione, cysteine, cystine, cystamine and their glycosyl, N-acetyl -, methyl, ethyl, propyl, amyl, butyl and lauryl, palmitoyl, oleyl, ⁇ -linoleyl, cholesteryl and glyceryl esters) and their salts, dilaurylthiodipropionate, distearylthiodipropionate, thiodipropionic acid and their derivatives (esters, Ethers, peptides, lipids, nucleotides, nucleosides and salts) and sulfoximine compounds (e.g.
- buthionine sulfoximines homocysteine sulfoximine, buthionine sulfones, penta-, hexa-, heptahionine sulfoximine) in very low tolerable dosages (e.g. pmol to ⁇ mol / kg), also (metal) -Chela gates (e.g. ⁇ -hydroxy fatty acids, palmitic acid, phytic acid, lactoferrin), humic acid, bile acid, bile extracts, bilirubin, biliverdin, EDTA, EGTA and their derivatives, unsaturated fatty acids and their derivatives (e.g.
- the amount of the antioxidants (one or more compounds) in the preparations is preferably 0.001 to 30% by weight, particularly preferably 0.05-20% by weight, in particular 1-10% by weight, based on the total weight of the preparation.
- vitamin E and / or its derivatives represent the antioxidant (s)
- vitamin A or vitamin A derivatives or carotenes or their derivatives represent the antioxidant or antioxidants, it is advantageous to use their respective concentrations in the range from 0.001-10% by weight, based on the total weight of the formulation, to choose.
- Cttronic acid, salicylic acid, other ⁇ -hydroxycarboxylic acids, biotin and the like can advantageously be used as other substances which counteract cell aging.
- the cosmetic preparations according to the invention can contain cosmetic auxiliaries as are usually used in such preparations, e.g. Preservatives, bactericides, deodorizing substances, irritant substances, antiperspirants, insect repellents, vitamins, anti-foaming agents, dyes, pigments with a coloring effect, thickeners, softening substances, moisturizing and / or moisturizing substances, fats, Oils, waxes or other common components of a cosmetic formulation such as alcohols, polyols, polymers, foam stabilizers, electrolytes, organic solvents or silicone derivatives.
- cosmetic auxiliaries e.g. Preservatives, bactericides, deodorizing substances, irritant substances, antiperspirants, insect repellents, vitamins, anti-foaming agents, dyes, pigments with a coloring effect, thickeners, softening substances, moisturizing and / or moisturizing substances, fats, Oils, waxes or other common components of a cosmetic formulation such as alcohols,
- the preparations according to the invention can also advantageously contain substances which absorb UV radiation in the UVB range, the total amount of the filter substances being, for example, 0.1% by weight to 30% by weight, preferably 0.5 to 10% by weight. %, in particular 1 to 6% by weight, based on the total weight of the preparation, in order to provide cosmetic preparations which protect the skin from the entire range of ultraviolet radiation, and in which the stinging of the ⁇ -hydroxycarboxylic acids or ⁇ -keto carboxylic acids are prevented or drastically reduced.
- the pH of the preparations according to the invention can advantageously be set in the acidic range, the pH range from 3.5 to 7 being preferred, particularly preferably from 4 to 6, very particularly about 5.5.
- the stability of the oligomers, polymers, co-oligomers and / or copolymers according to the invention is additionally increased by buffering.
- Preparations according to the invention can also advantageously serve as sunscreens.
- Emulsions according to the invention e.g. in the form of a sun protection cream or sun protection milk are advantageous and contain e.g. the fats, oils, waxes and other fat bodies mentioned, as well as water and an emulsifier, as is usually used for such a type of formulation.
- Oils such as triglycerides of capric or caprylic acid, but preferably castor oil; Fats, waxes and other natural and synthetic fat bodies, preferably esters of fatty acids with alcohols of low C number, e.g. with isopropanol, propylene glycol or glycerin, or esters of fatty alcohols with low C number alkanoic acids or with fatty acids; Alkyl benzoates;
- Silicone oils such as dimethylpolysiloxanes, diethylpolysiloxanes, diphenylpolysiloxanes and mixed forms thereof.
- Alcohols, diols or polyols of low C number, and their ethers preferably ethanol, isopropanol, propylene glycol, glycerol, ethylene glycol, ethylene glycol monoethyl or monobutyl ether, propylene glycol monomethyl, monoethyl or monobutyl ether, diethylene glycol monomethyl or monoethyl ether and analogous products, furthermore alcohols with a low C number, for example ethanol, isopropanol, 1, 2-propanediol, glycerol and in particular one or more thickeners, which one or more can advantageously be selected from the group consisting of silicon dioxide, aluminum silicates, polysaccharides or their derivatives, for example hyaluronic acid, xanthan gum, hydroxypropyl methyl cellulose, particularly advantageously from the group of polyacrylates, are preferred a polyacrylate from the group of the so-called carbopoles, for example carbopoles of types
- the cosmetic or dermatological sunscreen preparations advantageously contain inorganic pigments, in particular micropigments, e.g. in amounts of 0.1% by weight to 30% by weight, preferably in amounts of 0.5% by weight to 10% by weight, but in particular 1% by weight to 6% by weight on the total weight of the preparations.
- the light protection formulations according to the invention can advantageously contain substances which absorb UV radiation in the UVB range, the total amount of the filter substances e.g. 0.1% by weight to 30% by weight, preferably 0.5 to 10% by weight, in particular 1 to 6% by weight, based on the total weight of the preparations, in order to provide cosmetic preparations places that protect the skin from the entire range of ultraviolet radiation. They can also serve as sunscreens.
- the UVB filters can be oil-soluble or water-soluble.
- Advantageous oil-soluble UVB filter substances include:
- 3-benzylidene camphor derivatives preferably 3- (4-methylbenzylidene) camphor, 3-benzylidene camphor;
- 4-aminobenzoic acid derivatives preferably 4- (dimethylamino) benzoic acid (2-ethylhexyl ester, 4- (dimethylamino) benzoic acid amyl ester;
- esters of cinnamic acid preferably 4-methoxycinnamic acid (2-ethylhexyl) ester, 4-methoxycinnamic acid isopentyl ester;
- benzophenone preferably 2-hydroxy-4-methoxybenzophenone, 2-hydroxy-4-methoxy-4'-methylbenzophenone, 2,2'-dihydroxy-4-methoxybenzophenone;
- Esters of benzalmalonic acid preferably 4-methoxybenzalmalonic acid di (2-ethylhexyl) ester;
- Advantageous water-soluble UVB filter substances include: - Salts of 2-phenylbenzimidazole-5-sulfonic acid such as its sodium, potassium or triethanammonium salt, and the sulfonic acid itself;
- UVB filters which can be used in combination with the active compound combinations according to the invention, is of course not intended to be limiting.
- UVA filters which have hitherto usually been contained in cosmetic preparations.
- These substances are preferably derivatives of dibenzoylmethane, in particular 1- (4'-tert-butylphenyl) -3- (4'-methoxyphenyl) propane-1,3-dione and 1-phenyI- 3- (4'-isopropylphenyl) propane-1,3-dione.
- These combinations or preparations containing these combinations are also the subject of the invention.
- the quantities used for the UVB combination can be used.
- the total amount of UVA filter substances can advantageously be 0.1% by weight to 30% by weight, preferably 0.5 to 10% by weight, in particular 1 to 6% by weight, based on the total weight of the preparation, in order to provide cosmetic preparations which protect the skin from the entire range of ultraviolet radiation and in which the stinging of the ⁇ -hydroxycarboxylic acids or ⁇ -ketocarboxylic acids is prevented or drastically reduced.
- Cosmetic and dermatological preparations which are in the form of a sunscreen, a pre-soleil or apres-soleil product are also advantageous. These advantageously also contain at least one UVA filter and / or at least one UVB filter.
- cosmetic and dermatological preparations which are in the form of a sunscreen, a pre-soleil or apres-soleil product are also particularly advantageous. product and in addition to the UVA filter (s) and / or the UVB filter (s)
- Filters contain one or more antioxidants.
- Cosmetic and dermatological preparations according to the invention preferably contain inorganic pigments based on metal oxides and / or other metal compounds which are sparingly soluble or insoluble in water, in particular the oxides of titanium T ⁇ O2), zinc (ZnO), iron (eg F ⁇ 2 ⁇ 3), zirconium (Zr ⁇ 2), Silicon (Si ⁇ 2), manganese (e.g. MnO), aluminum (AI2O3), cerium (e.g. C ⁇ 2 ⁇ 3), mixed oxides of the corresponding metals and mixtures of such oxides. It is particularly preferred to use pigments based on TiO 2
- a prerequisite for the use of inorganic pigments for the purposes of the invention is, of course, the cosmetic or dermatological harmlessness of the underlying substances.
- the particle diameter of the pigments used is advantageous to choose the particle diameter of the pigments used to be less than 100 nm.
- the inorganic pigments are in hydrophobic form, that is to say that they have been surface-treated to be water-repellent.
- This surface treatment can consist in that the pigments are provided with a thin hydrophobic layer by methods known per se.
- One such method consists, for example, in that the hydrophobic surface layer according to a reaction
- n and m are stoichiometric parameters to be used at will, R and R 'are the desired organic radicals.
- hydrophobized pigments shown in analogy to DE-OS 33 14 742 are advantageous.
- Advantageous TiO 2 pigments are available, for example, under the trade names T 805 (DEGUSSA) or M 262 (KEMIRA) or M 160 (KEMIRA) or MT 100 T (TAYCA).
- Advantageous SiO 2 pigments can be selected from the range of hydrophobic pigments sold under the trade names AEROSIL (DEGUSSA), for example AEROSIL R 812 or AEROSIL R 972.
- Preparations according to the invention are advantageously characterized by a content of 0.1 to 10% by weight, in particular 0.5 to 5.0% by weight, of hydrophobic inorganic pigments, in each case based on the total weight of the composition.
- Cetyl stearyl isononanoate PEG-20 cetyl stearyl ether, cetyl stearyl alcohol, glyceryl stearate, glycerol, cetyl palmitate, PEG-12 cetyl stearyl ether, benzoic acid 15.00
- Cetyl stearyl isononanoate PEG-20 cetyl stearyl ether, cetyl stearyl alcohol, glyceryl stearate, glycerol, cetyl palmitate, PEG-12 cetyl stearyl ether, benzoic acid 15.00
- Resomer 104 (30% by weight) in triethyl citrate 6.60
- Resomer 104 (30% by weight in triethyl citrate) 6.60
- Resomer 104 (30% by weight in tributyl citrate) 6.60
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Birds (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Emergency Medicine (AREA)
- Dermatology (AREA)
- Cosmetics (AREA)
Abstract
Cosmetic or dermatological compositions that contain an effective amount of one or several dissolved or suspended oligomers, and/or polymers, and/or co-oligomers and/or co-polymers of one or several α-hydroxycarboxylic acids having the general formula (I) and linked to each other according to the scheme (II) are useful to administer the monomer α-hydroxycarboxylic acids upon which they are based in cosmetic or dermatological compositions.
Description
Beschreibung description
Kosmetische oder dermatologische Zubereitungen, enthaltend Oligomere oder Polymere von α-HvdroxycarbonsäurenCosmetic or dermatological preparations containing oligomers or polymers of α-hydroxycarboxylic acids
Die vorliegende Erfindung betrifft Zubereitungen auf dem Gebiete der kosmetischen und dermatologischen Haut- und Haarpflege, insbesondere solche Zubereitungen mit niedrigem Reizpotential bzw. kosmetische oder dermatologische Zubereitungen zur Prophylaxe, Lin¬ derung, Beseitigung oder Vermeidung von Hautreizung. In einer bevorzugten Ausfüh¬ rungsform betrifft die vorliegende Erfindung kosmetische und dermatologische Zube¬ reitungen zur Prophylaxe und Behandlung kosmetischer oder dermatologischer Hautverän¬ derungen wie z.B. der Hautalterung. Weiterhin betrifft die vorliegende Erfindung Zuberei¬ tungen gegen das Erscheinungsbild der trockenen bzw. rauhen Haut.The present invention relates to preparations in the field of cosmetic and dermatological skin and hair care, in particular those preparations with low irritation potential or cosmetic or dermatological preparations for prophylaxis, amelioration, elimination or avoidance of skin irritation. In a preferred embodiment, the present invention relates to cosmetic and dermatological preparations for the prophylaxis and treatment of cosmetic or dermatological skin changes such as e.g. skin aging. Furthermore, the present invention relates to preparations against the appearance of dry or rough skin.
Weiterhin betrifft die vorliegende Erfindung Wirkstoffe und Zubereitungen, solche Wirk¬ stoffe enthaltend, zur kosmetischen und dermatologischen Behandlung oder Prophylaxe erythematöser, entzündlicher, allergischer oder autoimmunreaktiver Erscheinungen, insbe¬ sondere Dermatosen. Ferner betrifft die Erfindung die Verwendung solcher Wirkstoffe und Zubereitungen, solche Wirkstoffe enthaltend, zur Immunstimulation der Haut, dabei vor¬ teilhaft auch zur Immunstimulation im Sinne einer Behandlung der verletzten Haut, ins¬ besondere zur Behandlung von Wunden.Furthermore, the present invention relates to active substances and preparations containing such active substances for cosmetic and dermatological treatment or prophylaxis of erythematous, inflammatory, allergic or autoimmune-reactive symptoms, in particular dermatoses. Furthermore, the invention relates to the use of such active substances and preparations containing such active substances for immunostimulation of the skin, advantageously also for immunostimulation in the sense of treating injured skin, in particular for treating wounds.
Schließlich betrifft die Erfindung Zubereitungen mit extrem niedrigem sogenanntem "Stin- ging Potential".Finally, the invention relates to preparations with extremely low so-called "stinging potential".
Unter Hautpflege ist in erster Linie zu verstehen, daß die natürliche Funktion der Haut als Barriere gegen Umwelteinflüsse (z.B. Schmutz, Chemikalien, Mikroorganismen) und gegen den Verlust von körpereigenen Stoffen (z.B. Wasser, natürliche Fette, Elektrolyte) gestärkt oder wiederhergestellt wird.
Wird diese Funktion gestört, kann es zu verstärkter Resorption toxischer oder allergener Stoffe oder zum Befall von Mikroorganismen und als Folge zu toxischen oder allergischen Hautreaktionen kommen.Skin care is primarily understood to mean that the natural function of the skin as a barrier against environmental influences (e.g. dirt, chemicals, microorganisms) and against the loss of the body's own substances (e.g. water, natural fats, electrolytes) is strengthened or restored. If this function is disturbed, there may be an increased absorption of toxic or allergenic substances or an infestation of microorganisms and, as a result, toxic or allergic skin reactions.
Ziel der Hautpflege ist es ferner, den durch tägliches Waschen verursachten Fett- und Was¬ serverlust der Haut auszugleichen. Dies ist gerade dann wichtig, wenn das natürliche Regenerationsvermögen nicht ausreicht. Außerdem sollen Hautpflegeprodukte vor Umwelteinflüssen, insbesondere vor Sonne und Wind, schützen und die Zeichen der Hautalterung verzögern.The aim of skin care is also to compensate for the loss of fat and water in the skin caused by daily washing. This is especially important when the natural regeneration ability is insufficient. In addition, skin care products are intended to protect against environmental influences, especially sun and wind, and to delay the signs of aging.
Medizinische topische Zusammensetzungen enthalten in der Regel ein oder mehrere Me¬ dikamente in wirksamer Konzentration. Der Einfachheit halber wird zur sauberen Unterschei¬ dung zwischen kosmetischer und medizinischer Anwendung und entsprechenden Produkten auf die gesetzlichen Bestimmungen der Bundesrepublik Deutschland verwiesen (z.B. Kosmetikverordnung, Lebensmittel- und Arzneimittelgesetz).Medical topical compositions generally contain one or more medicaments in an effective concentration. For the sake of simplicity, for a clear distinction between cosmetic and medical use and corresponding products, reference is made to the legal provisions of the Federal Republic of Germany (e.g. cosmetics regulation, food and drug law).
Die Epidermis ist reich mit Nerven und Nervenendapparaten wie Vater-Pacini-Lamellen- körpern, Merkel-Zell-Neuritenkomplexen und freien Nervenenden für Schmerz-, Kälte-, Wärmeempfindung und Juckreiz ausgestattet.The epidermis is richly equipped with nerves and nerve end devices such as Father Pacini lamellar bodies, Merkel cell neurite complexes and free nerve endings for pain, cold, heat sensation and itching.
Bei Menschen mit sensibler, empfindlicher oder verletzlicher Haut kann ein mit "Stinging" (<engl.> "to sting" = verletzen, brennen, schmerzen) bezeichnetes neurosensorisches Phänomen beobachtet werden. Diese "sensible Haut" unterscheidet sich grundsätzlich von "trockener Haut" mit verdickten und verhärteten Hornschichten.In people with sensitive, sensitive or vulnerable skin, a neurosensory phenomenon called "stinging" (<engl.> "To sting" = injure, burn, pain) can be observed. This "sensitive skin" differs fundamentally from "dry skin" with thickened and hardened horny layers.
Typische Reaktionen des "Stinging" bei sensibler Haut sind Rötung, Spannen und Brennen der Haut sowie Juckreiz.Typical reactions of "stinging" to sensitive skin are reddening, tension and burning of the skin as well as itching.
Als neurosensorisches Phänomen ist der Juckreiz bei atopischer Haut anzusehen, sowie Juckreiz bei Hauterkrankungen.Itching in atopic skin and itching in skin diseases are to be regarded as a neurosensory phenomenon.
"Stinging'-Phänomene können als kosmetisch zu behandelnde Störungen angesehen wer¬ den. Starker Juckreiz dagegen, insbesondere bei Atopie auftretendes starkes Hautjucken, kann auch als schwerwiegendere dermatologische Störung bezeichnet werden.
Typische, mit den Begriffen "Stinging" oder "empfindlicher Haut" in Verbindung gebrachte, störende neurosensorische Phänomene sind Hautrötung, Kribbeln, Prickeln, Spannen und Brennen der Haut und Juckreiz. Sie können durch stimulierende Umgebungsbedingungen z.B. Massage, Tensideinwirkung, Wettereinfluß wie Sonne, Kälte, Trockenheit, aber auch feuchte Wärme, Wärmestrahlung und UV-Strahlung, z.B. der Sonne, hervorgerufen werden."Stinging 'phenomena can be regarded as disorders to be treated cosmetically. In contrast, severe itching, in particular severe itching in the case of atopy, can also be described as a more serious dermatological disorder. Typical disturbing neurosensory phenomena associated with the terms "stinging" or "sensitive skin" are reddening of the skin, tingling, tingling, tension and burning of the skin and itching. They can be caused by stimulating environmental conditions such as massage, tenside effects, weather influences such as sun, cold, dryness, but also moist heat, heat radiation and UV radiation, such as the sun.
In "Journal of the Society of Cosmetic Chemists" 28, S.197 - 209 (Mai 1977) beschreiben P.J.Frosch und A.M.Kligman eine Methode zur Abschätzung des "Stinging-Potentials" topisch verabreichter Substanzen. Als positive Substanzen werden hier z.B. Milchsäure und Brenztraubensäure eingesetzt.In "Journal of the Society of Cosmetic Chemists" 28, pp. 197-209 (May 1977) P.J. Frosch and A.M. Kligman describe a method for estimating the "stinging potential" of topically administered substances. As positive substances, e.g. Lactic acid and pyruvic acid are used.
Nach bisherigen Erkenntnissen tritt eine derartige Empfindlichkeit gegenüber ganz be¬ stimmten Substanzen individuell unterschiedlich auf. Dies bedeutet, eine Person, die bei Kontakt mit einer Substanz, beispielweise Milchsäure "Stingingeffekte" erlebt, wird sie mit hoher Wahrscheinlichkeit bei jedem weiteren Kontakt wiederholt erleben. Der Kontakt mit anderen "Stingem" kann aber auch bei ein und derselben Person auch ohne jede Reaktion verlaufen.According to previous knowledge, such a sensitivity to very specific substances occurs individually in different ways. This means that a person who experiences "stinging effects" when in contact with a substance, for example lactic acid, is likely to experience it repeatedly with each further contact. The contact with other "Stingem" can also take place with one and the same person without any reaction.
Die kosmetische und dermatologische Verwendung von α-Hydroxycarbonsäuren ist an sich bekannt. Beispielsweise wird Citronensäure zur Pufferung kosmetischer und/oder dermato¬ logischer Zubereitungen, aber auch als Synergist für Antioxidantien in der Haut- und Haarkosmetik verwendet.The cosmetic and dermatological use of α-hydroxycarboxylic acids is known per se. For example, citric acid is used to buffer cosmetic and / or dermatological preparations, but also as a synergist for antioxidants in skin and hair cosmetics.
DE-OS 42 04 321 beschreibt die Verwendung längerkettiger α-Hydroxycarbonsäuren als Wirkstoffe für kosmetische Desodorantien. Die US-PS 4,363,815 beschreibt die Verwen¬ dung von α-Hydroxycarbonsäuren zur Behandlung entzündlicher Hauterscheinungen. Die US-PS 4,380,549 beschreibt die Verwendung von α-Hydroxycarbonsäuren zur Erleichterung der Symptome der trockenen Haut. Auch weitere topische Anwendungsgebiete der α- Hydroxycarbonsäuren sind bekannt, diese wenigen Beispiele zeigen aber bereits, daß die α- Hydroxycarbonsäuren wertvolle hautwirksame Substanzen darstellen, handele es sich nun um kosmetische oder pharmazeutische Anwendung.DE-OS 42 04 321 describes the use of longer-chain α-hydroxycarboxylic acids as active ingredients for cosmetic deodorants. US Pat. No. 4,363,815 describes the use of α-hydroxycarboxylic acids for the treatment of inflammatory skin symptoms. US Pat. No. 4,380,549 describes the use of α-hydroxycarboxylic acids to relieve the symptoms of dry skin. Other topical fields of application of the α-hydroxycarboxylic acids are also known, but these few examples already show that the α-hydroxycarboxylic acids are valuable substances effective on the skin, whether they are cosmetic or pharmaceutical applications.
Es mangelte dem Stande der Technik allerdings an Darreichungsformen für α-Hydroxycar¬ bonsäuren. Es war somit eine Aufgabe der vorliegenden Erfindung, den Stand "der Technik
in dieser Hinsicht zu bereichern und neue Darreichungsformen für α-Hydroxycarbonsäuren zu entwickeln. Eine wertere Aufgabe war, solche Darreichungsformen zu entwickeln, in welchen die α-Hydroxycarbonsäuren nicht mit anderen Substanzen der kosmetischen bzw. dermatologischen Formulierungen, in welche sie eingearbeitet vorliegen, wechselwirken, sondern ihre Wirkung erst auf der Haut entfalten.However, the prior art lacked dosage forms for α-hydroxycarboxylic acids. It was therefore an object of the present invention, the subject "of the art to enrich in this regard and to develop new dosage forms for α-hydroxycarboxylic acids. Another task was to develop administration forms in which the α-hydroxycarboxylic acids do not interact with other substances of the cosmetic or dermatological formulations in which they are incorporated, but rather only develop their effect on the skin.
Nachteilig ist jedoch, daß die sichere Einsatzmenge der α-Hydroxycarbonsäuren im Einzelfalle begrenzt sein kann, da bei empfindlichen Personen bereits bei Konzentrationen unterhalb von 0,5 Gew.-% das vorab beschriebene "Stinging" auftreten kann.It is disadvantageous, however, that the safe amount of α-hydroxycarboxylic acids used can be limited in individual cases, since the "stinging" described above can occur in sensitive people even at concentrations below 0.5% by weight.
Da es aber wünschenswert ist, auch empfindlichen Personen die kosmetische oder der¬ matologische Verabreichung von α-Hydroxycarbonsäuren zu ermöglichen, war es eine Aufgabe der vorliegenden Erfindung, kosmetische oder dermatologische Zubereitungen zu entwickeln, welche zwar α-Hydroxycarbonsäuren enthalten oder freisetzen, sich aber durch ein extrem niedriges "Stinging-Potential" auszeichnen, günstigenfalls praktisch frei von "Stingingeffekten" sein sollten.However, since it is desirable to enable even sensitive people to be given the cosmetic or dermatological administration of α-hydroxycarboxylic acids, it was an object of the present invention to develop cosmetic or dermatological preparations which contain or release α-hydroxycarboxylic acids, but which are released an extremely low "stinging potential", ideally should be practically free of "stinging effects".
Erythematöse Hauterscheinungen treten auch als Begleiterscheinungen bei gewissen Haut¬ erkrankungen oder -Unregelmäßigkeiten auf. Beispielsweise ist der typische Hautausschlag beim Erscheinungsbild der Akne regelmäßig mehr oder weniger stark gerötet.Erythematous skin symptoms also appear as side effects in certain skin diseases or irregularities. For example, the typical rash in the appearance of acne is usually more or less reddened.
Aufgabe war also, den Nachteilen des Standes der Technik Abhilfe zu schaffen. Insbeson¬ dere war Aufgabe der vorliegenden Erfindung, kosmetische oder dermatologische Zube¬ reitungen zur Verfügung zu stellen, in welche einesteils die vorteilhaften Eigenschaften der α-Hydroxycarbonsäuren genutzt werden können, ohne daß aber der Nachteil etwaiger Unverträglichkeiten, etwa des Stingings auftreten würde. Weiterhin sollten Wirkstoffe und Zubereitungen, solche Wirkstoffe enthaltend, zur kosmetischen und dermatologischen Be¬ handlung und/oder Prophylaxe erythematöser, entzündlicher, allergischer oder autoimmun- reaktiver Erscheinungen, insbesondere Dermatosen zur Verfügung gestellt werden.The task was therefore to remedy the disadvantages of the prior art. In particular, it was an object of the present invention to provide cosmetic or dermatological preparations in which the advantageous properties of the α-hydroxycarboxylic acids can be used to some extent, but without the disadvantage of any incompatibilities, such as stinging. Furthermore, active substances and preparations containing such active substances should be made available for cosmetic and dermatological treatment and / or prophylaxis of erythematous, inflammatory, allergic or autoimmune-reactive symptoms, in particular dermatoses.
Polymere vieler α-Hydroxycarbonsäuren, wobei die Verknüpfung der Monomeren unterein¬ ander über Esterbindung der α-Hydroxygruppen mit den Carboxylgruppen erfolgt, sind an sich bekannt. Hauptsächlich solche auf der Basis von Milchsäure und/oder Glycolsäure, werden in der Chirurgie als resorbierbares Naht- oder Implantatmaterial eingesetzt. Sie
werden auch in der Kosmetik verwendet, dort allerdings ausschließlich als Hüllmaterial. Ihr Nachteil ist, daß sie in den meisten kosmetischen und pharmazeutisch unbedenklichen Lösemitteln nicht oder nur unzureichend löslich sind, weswegen eine andere kosmetische oder pharmazeutische Verwendung als als Hüllmaterial bislang ausgeschlossen werden mußte. Eine weitere Aufgabe der vorliegenden Erfindung war, diesem Mangel abzuhelfen.Polymers of many .alpha.-hydroxycarboxylic acids, the linking of the monomers with one another via ester linkage of the .alpha.-hydroxy groups with the carboxyl groups, are known per se. Mainly those based on lactic acid and / or glycolic acid are used in surgery as resorbable sutures or implants. she are also used in cosmetics, but only there as a covering material. Their disadvantage is that they are not or only insufficiently soluble in most cosmetic and pharmaceutically acceptable solvents, which is why cosmetic or pharmaceutical use other than shell material has had to be ruled out until now. Another object of the present invention was to remedy this deficiency.
Erstaunlicherweise hat sich herausgestellt, und darin liegt die Lösung all dieser Aufgaben begründet, daß kosmetische oder dermatologische Zubereitungen, enthaltend eine wirksame Menge eines oder mehrerer in gelöster oder suspendierter Form vorliegender Oli- gomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere einer oder mehrerer α-Hydroxycarbonsäuren der allgemeinen FormelSurprisingly, it has been found, and this is the solution to all of these problems, that cosmetic or dermatological preparations containing an effective amount of one or more oligomers and / or polymers and / or co-oligomers and / or present in dissolved or suspended form Copolymers of one or more α-hydroxycarboxylic acids of the general formula
R"R "
I R'-C— COOHI R'-C- COOH
I OH welche gemäß dem SchemaI OH which according to the scheme
untereinander verknüpft sind, wobei jeweils R' und R" unabhängig voneinander gewählt werden aus der Gruppe are linked to one another, R 'and R "being selected independently of one another from the group
(a1) H- ,(a1) H-,
(a2) verzweigtes oder unverzweigtes C-j_25-AlkyI-,(a2) branched or unbranched C-j_25-alkyl-,
(a3) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Al¬ dehydgruppen und/oder Oxogruppen (Ketogruppen) substituiertes verzweigtes oder unverzweigtes Cι_25"Alkyl-(a3) with one or more carboxyl groups and / or hydroxyl groups and / or aldehyde groups and / or oxo groups (keto groups) branched or unbranched C 1-25 "alkyl
(a4) Phenyl-,(a4) phenyl,
(a5) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder ver¬ zweigten und/oder unverzweigten Cι_25-Alkylgruppen substituiertes Phenyl-, oder wobei das α-Kohlenstoffatom der α-Hydroxycarbonsäure mit R' und R" zusammen eine(a5) Phenyl- substituted with one or more carboxyl groups and / or hydroxyl groups and / or branched and / or unbranched C 25 -alkyl groups, or wherein the α-carbon atom of the α-hydroxycarboxylic acid with R 'and R "together form a
(a6) unsubstituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen oder eine(a6) unsubstituted cycloalkyl group with 3 to 7 ring atoms or one
(a7) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Oxo¬ gruppen (Ketogruppen) und/oder verzweigten und/oder unverzweigten C-|_25- Alkylgruppen substituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen
ausbildet, wobei die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 4 beträgt, und wobei R' und R" der einzelnen α-Hydroxycarbonsäureeinheiten innerhalb eines Co-Oli- gomers bzw. Co-Polymers zu R' bzw. R" einer oder mehrerer α-Hydroxycarbonsäureeinhei¬ ten desselben Co-Oligomers bzw. Co-Polymers identisch oder auch verschieden sein kann, in gelöster oder suspendierter Form, den Nachteilen des Standes der Technik Abhilfe schaf¬ fen.(a7) with one or more carboxyl groups and / or hydroxyl groups and / or oxo groups (keto groups) and / or branched and / or unbranched C- | _25- Alkyl groups substituted cycloalkyl groups with 3 to 7 ring atoms forms, the number n for at least 40 wt .-% of the oligomers and / or polymers and / or co-oligomers and / or copolymers used is at least 4, and wherein R 'and R "of the individual α-hydroxycarboxylic acid units within a co-oligomer or co-polymer to R 'or R "of one or more α-hydroxycarboxylic acid units of the same co-oligomer or co-polymer may be identical or different, in dissolved or suspended form, the Remedy disadvantages of the prior art.
Erfindungsgemäß ist auch die Verwendung einer wirksamen Menge eines oder mehrerer Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere einer oder mehrerer α-Hydroxycarbonsäuren der allgemeinen FormelThe use of an effective amount of one or more oligomers and / or polymers and / or co-oligomers and / or co-polymers of one or more α-hydroxycarboxylic acids of the general formula is also according to the invention
R" I R'-C— COOH I OH welche gemäß dem SchemaR "I R'-C- COOH I OH which according to the scheme
untereinander verknüpft sind, wobei jeweils R' und R" unabhängig voneinander gewählt werden aus der Gruppe are linked to one another, R 'and R "being selected independently of one another from the group
(a1) H- ,(a1) H-,
(a2) verzweigtes oder unverzweigtes Cι_25-Alkyl-,(a2) branched or unbranched C 25 25 alkyl,
(a3) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Al¬ dehydgruppen und/oder Oxogruppen (Ketogruppen) substituiertes verzweigtes oder unverzweigtes Cι_25-Alkyl-(a3) with one or more carboxyl groups and / or hydroxyl groups and / or aldehyde groups and / or oxo groups (keto groups) branched or unbranched C 25 alkyl
(a4) Phenyl-,(a4) phenyl,
(a5) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder ver¬ zweigten und/oder unverzweigten C-|_25-Alkylgruppen substituiertes Phenyl-, oder wobei das α-Kohlenstoffatom der α-Hydroxycarbonsäure mit R' und R" zusammen eine(a5) Phenyl- substituted with one or more carboxyl groups and / or hydroxyl groups and / or branched and / or unbranched C- | _25-alkyl groups, or wherein the α-carbon atom of the α-hydroxycarboxylic acid together with R 'and R "together is one
(a6) unsubstituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen oder eine(a6) unsubstituted cycloalkyl group with 3 to 7 ring atoms or one
(a7) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Oxo¬ gruppen (Ketogruppen) und/oder verzweigten und/oder unverzweigten C1.25- Alkylgruppen substituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen
ausbildet, wobei die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 4 beträgt, und wobei R' und R" der einzelnen α-Hydroxycarbonsäureeinheiten innerhalb eines Co-Oli- gomers bzw. Co-Polymers zu R' bzw. R" einer oder mehrerer α-Hydroxycarbonsäureeinhei¬ ten desselben Co-Oligomers bzw. Co-Polymers identisch oder auch verschieden sein kann, zur Darreichung der zugrundeliegenden monomeren α-Hydroxycarbonsäuren in kosmeti¬ schen oder dermatologischen Zubereitungen.(a7) with one or more carboxyl groups and / or hydroxyl groups and / or oxo groups (keto groups) and / or branched and / or unbranched C1.25-alkyl groups substituted cycloalkyl group with 3 to 7 ring atoms forms, the number n for at least 40 wt .-% of the oligomers and / or polymers and / or co-oligomers and / or copolymers used is at least 4, and wherein R 'and R "of the individual α-hydroxycarboxylic acid units within a co-oligomer or co-polymer to R 'or R "of one or more α-hydroxycarboxylic acid units of the same co-oligomer or co-polymer may be identical or different, to provide the underlying monomeric α- Hydroxycarboxylic acids in cosmetic or dermatological preparations.
Es war insbesondere nicht vorherzusehen gewesen, daß die erfindungsgemäße Verwen¬ dung nicht nur das "Stinging-Potential" der α-Hydroxycarbonsäuren selbst für empfindliche Personen praktisch auf Null reduziert werden, sondern daß darüberhinaus die volle Aktivität der α-Hydroxycarbonsäuren erhalten bleiben würde.In particular, it could not have been foreseen that the use according to the invention would not only reduce the "stinging potential" of the α-hydroxycarboxylic acids to practically zero even for sensitive people, but that the full activity of the α-hydroxycarboxylic acids would also be retained.
Ferner war erstaunlich, daß die vorliegende Erfindung die Möglichkeit eröffnet, stabile und zuverlässig den Wirkstoff freisetzende Darreichungsformen für α-Hydroxycarbonsäuren herzustellen.It was also surprising that the present invention opens up the possibility of producing stable and reliable dosage forms for α-hydroxycarboxylic acids which release the active ingredient.
Es war zwar bekannt, Polymere bzw. Copolymere von Milchsäure und/oder Glycolsäure in Kosmetika, nämlich als Hüllmaterial für wirkstoffhaltige Mikrokapseln, einzusetzen. So be¬ schreibt die EP-OS 447 318 Zusammensetzungen für die kosmetische bzw. pharmazeu¬ tische Pflege der oberen Hautschichten, welche in einem geeigneten Träger polymere, bio¬ logisch abbaubare Nanopartikel enthalten, welche eine ölige aktive Substanz umhüllen. Unter den a.a.O. beschriebenen polymeren Hüllmaterialien finden sich auch die Poly- (L)Milchsäure die Poly-(DL)Milchsäure, die Poly-Glycolsäure sowie Copolymere aus Milch- und Glycolsäure. Ein Hinweis, der in die Richtung der vorliegenden Erfindung hätte weisen können, findet sich in jener Schrift jedoch nicht.It was known to use polymers or copolymers of lactic acid and / or glycolic acid in cosmetics, namely as a covering material for active ingredient-containing microcapsules. For example, EP-OS 447 318 describes compositions for the cosmetic or pharmaceutical care of the upper skin layers, which contain polymeric, biodegradable nanoparticles in a suitable carrier, which coat an oily active substance. Among the above-mentioned The polymeric coating materials described also include poly (L) lactic acid, poly (DL) lactic acid, polyglycolic acid and copolymers of lactic and glycolic acid. However, there is no indication in the direction of the present invention in that document.
Besonders erstaunlich war, daß erfindungsgemäße kosmetische oder dermatologische Zubereitungen, insbesondere dann, wenn die erfindungsgemäßen Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere in eine Ölphase eingearbeitet sind, gegen Depolymeri- sation (bzw. Deoligomerisation) stabil sind und ihre vorteilhafte Wirkung erst auf der menschlichen Haut entfalten.
Da die erfindungsgemäßen Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere die ihnen zugrundeliegenden Monomere allmählich freisetzen, eigenen sie sich in vorzüglicher Weise als Depotsysteme bzw. zur retardierten Freisetzung.It was particularly surprising that cosmetic or dermatological preparations according to the invention, in particular when the oligomers, polymers, co-oligomers and / or copolymers according to the invention are incorporated into an oil phase, are stable against depolymerization (or de-oligomerization) and their advantageous First have an effect on human skin. Since the oligomers, polymers, co-oligomers and / or copolymers according to the invention gradually release the monomers on which they are based, they are particularly suitable as depot systems or for sustained release.
Die erfindungsgemäßen Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere haben darüberhinaus in überraschender Weise den Vorteil, auch wenn ihre Hydrolyse zu den zugrundeliegenden Monomeren noch nicht oder noch nicht vollständig erfolgt ist, die Hautfeuchtigkeit zu steigern.The oligomers, polymers, co-oligomers and / or co-polymers according to the invention also have the advantage, surprisingly, even if their hydrolysis to the underlying monomers has not yet taken place or has not yet taken place completely, to increase the skin moisture.
Erfindungsgemäß ist dementsprechend auch ein Verfahren zu Stabilisierung einer wirk¬ samen Menge eines oder mehrerer Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere einer oder mehrerer α-Hydroxycarbonsäuren der allgemeinen FormelAccordingly, the invention also relates to a process for stabilizing an effective amount of one or more oligomers and / or polymers and / or co-oligomers and / or co-polymers of one or more α-hydroxycarboxylic acids of the general formula
R" I R'-C— COOH I OH welche gemäß dem SchemaR "I R'-C- COOH I OH which according to the scheme
untereinander verknüpft sind, wobei jeweils R' und R" unabhängig voneinander gewählt werden aus der Gruppe are linked to one another, R 'and R "being selected independently of one another from the group
(a1) H- ,(a1) H-,
(a2) verzweigtes oder unverzweigtes Cι_25-Alkyl-,(a2) branched or unbranched C 25 25 alkyl,
(a3) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Al¬ dehydgruppen und/oder Oxogruppen (Ketogruppen) substituiertes verzweigtes oder unverzweigtes C-|_25-Alkyl-(a3) branched or unbranched C- | substituted with one or more carboxyl groups and / or hydroxyl groups and / or aldehyde groups and / or oxo groups (keto groups) _25-alkyl
(a4) Phenyl-,(a4) phenyl,
(a5) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder ver¬ zweigten und/oder unverzweigten Cι_25-Alkylgruppen substituiertes Phenyl-, oder wobei das α-Kohlenstoffatom der α-Hydroxycarbonsäure mit R' und R" zusammen eine(a5) Phenyl- substituted with one or more carboxyl groups and / or hydroxyl groups and / or branched and / or unbranched C 25 -alkyl groups, or wherein the α-carbon atom of the α-hydroxycarboxylic acid with R 'and R "together form a
(a6) unsubstituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen oder eine
(a7) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Oxo- gruppen (Ketogruppen) und/oder verzweigten und/oder unverzweigten C-|_25- Alkylgruppen substituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen ausbildet, wobei die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 4 beträgt, und wobei R' und R" der einzelnen α-Hydroxycarbonsäureeinheiten innerhalb eines Co-Oli- gomers bzw. Co-Polymers zu R' bzw. R" einer oder mehrerer α-Hydroxycarbonsäureeinhei¬ ten desselben Co-Oligomers bzw. Co-Polymers identisch oder auch verschieden sein kann, in kosmetischen oder dermatologischen Zubereitungen gegen Depolymerisation bzw. Deoligomerisation durch Hydrolyse, dadurch gekennzeichnet, daß die eingesetzten Oligo¬ mere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere in eine Ölphase eingearbeitet werden.(a6) unsubstituted cycloalkyl group with 3 to 7 ring atoms or one (a7) with one or more carboxyl groups and / or hydroxyl groups and / or oxo groups (keto groups) and / or branched and / or unbranched C- | _25- Alkyl group-substituted cycloalkyl group with 3 to 7 ring atoms, the number n being at least 4 for at least 40% by weight of the oligomer and / or polymer and / or co-oligomer and / or co-polymer used, and wherein R 'and R "of the individual α-hydroxycarboxylic acid units within a co-oligomer or co-polymer to form R' or R" of one or more α-hydroxycarboxylic acid units of the same co-oligomer or co-polymer are identical or different can be in cosmetic or dermatological preparations against depolymerization or deoligomerization by hydrolysis, characterized in that the oligomers and / or polymers and / or co-oligomers and / or copolymers used are incorporated into an oil phase.
Die den erfindungsgmäßen Oligomeren und/oder Polymeren und/oder Co-Oligomeren und/oder Co-Polymeren zugrundeliegenden α-Hydroxycarbonsäuren werden vorteilhaft ge¬ wählt aus der Gruppe der α-Hydroxyalkansäuren, wobei diese wiederum besonders vor¬ teilhaft aus der Gruppe der C2-_-Alkylcarbonsäuren gewählt werden.The α-hydroxycarboxylic acids on which the oligomers and / or polymers and / or co-oligomers and / or co-polymers are based are advantageously selected from the group of α-hydroxyalkanoic acids, which in turn is particularly advantageous from the group of C2- _-Alkyl carboxylic acids can be selected.
Besonders bevorzugt ist, Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere der Glycolsäure, der Milchsäure, der α-Hydroxybuttersäure und/oder der α-Hy- droxyvaleriansäure zu wählen.It is particularly preferred to choose oligomers and / or polymers and / or co-oligomers and / or copolymers of glycolic acid, lactic acid, α-hydroxybutyric acid and / or α-hydroxyvaleric acid.
Beispielsweise zeichnen sich Oligo- bzw. Polyglycolide durch die StrukturFor example, oligo- or polyglycolides are characterized by their structure
Oligo- bzw. Polylactide zeichnen sich durch die StrukturOligo- or polylactides are characterized by their structure
Co-Oligo- bzw. Copolymere von Milchsäure und Glycolsäure zeichnen sich beispielsweise durch eine Struktur gemäß
die Summe der Zahlen k und j der Zahl n entspricht. Es ist dem Fachmanne dabei klar, daß die Anordnung der Co-Monomere in einem Co-Oligomer bzw. Co-Polymer blockweise, alternierend, statistisch verteilt oder nach anderen Anordnungsprinzipien erfolgen kann. All diese Anordnungsarten sind erfindungsgemäß vorteilhaft zu verwenden.Co-oligo- or copolymers of lactic acid and glycolic acid are characterized, for example, by a structure according to the sum of the numbers k and j corresponds to the number n. It is clear to the person skilled in the art that the arrangement of the co-monomers in a co-oligomer or co-polymer can be carried out in blocks, alternating, randomly distributed or according to other arrangement principles. All of these types of arrangement can be used advantageously according to the invention.
Oligo- bzw. Poly-2-hydroxybutyrate zeichnen sich durch die StrukturOligo- or poly-2-hydroxybutyrates are characterized by the structure
Oligo- bzw. Poly-2-hydroxyvalerate zeichnen sich durch die StrukturOligo- or poly-2-hydroxyvalerates are characterized by the structure
Erfindungsgemäß ist vorteilhaft, diejenigen als Enantiomeren vorliegenden α-Hydroxycar¬ bonsäuren als Grundbestandteile der Oligo- bzw. Polymere zu wählen, die der Hautpflege zuträglicher sind als ihre optischen Antipoden, also beispielsweise die L-Milchsäure.
According to the invention, it is advantageous to choose those α-hydroxycarboxylic acids present as enantiomers as basic constituents of the oligomers or polymers which are more beneficial to skin care than their optical antipodes, for example L-lactic acid.
R-Milchsäure (≡ D-Milchsäure) S-Milchsäure (≡ L-Milchsäure)R-lactic acid (≡ D-lactic acid) S-lactic acid (≡ L-lactic acid)
Es kann allerdings auch aus anderen Gründen, beispielsweise der schieren Verfügbarkeit der Einsatzstoffe, vorteilhafter sein, eine freie Auswahl aus zwei Enantiomeren zu treffen, ein beliebiges Enantiomerengemisch oder auch ein Racemat zu wählenHowever, it can also be more advantageous for other reasons, for example the sheer availability of the starting materials, to make a free choice from two enantiomers, to choose any desired enantiomer mixture or also a racemate
Vorteilhaft beträgt die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligo¬ mere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 8, bevorzugt 10. Die Obergrenze für n kann grundsätzlich offenbleiben, dennoch haben sich Werte für n von 60 bis 1.000, insbesondere 200 als vorteilhaft erwiesen. Aber auch Polymere, die zu mindestens 40 Gew.-% aus ca. 60.000 Monomereinheiten und solche, die zu mindestens 40 Gew.-% aus ca. 100.000 Monomereinheiten bestehen, sind vorteilhaft zu verwenden.The number n for at least 40% by weight of the oligomer and / or polymer and / or co-oligomer and / or co-polymer used is advantageously at least 8, preferably 10. The upper limit for n can in principle remain open, but nevertheless values for n of 60 to 1,000, in particular 200, have proven to be advantageous. However, polymers which consist of at least 40% by weight of approximately 60,000 monomer units and those which consist of at least 40% by weight of approximately 100,000 monomer units can also be used advantageously.
Vorteilhafte Handelsprodukte sind beispielsweise:Examples of advantageous commercial products are:
Resomer R 104/Boehringer Ingelheim, Oligo-(D,L)-Milchsäure (Hauptkomponente: n = 12, nmax » 50. Durchschnittlicher Wert für n «■ 20.Resomer R 104 / Boehringer Ingelheim, oligo- (D, L) -lactic acid (main component: n = 12, n max »50. Average value for n« ■ 20.
Resomer L 104 Oligo(L)-Milchsäure, ähnliche Werte wie R 104Resomer L 104 oligo (L) -lactic acid, values similar to R 104
Resomer R 207 Oligo(D,L)-Milchsäure Durchschnittlicher Wert für n « 60.000Resomer R 207 oligo (D, L) -lactic acid Average value for n «60,000
Oligo(D.L-lactat-co-glycolat) (50:50), Durchschnittlicher Wert für n * 8 ; j * k ■■
Vorteilhaft beträgt der Gehalt an den erfindungsgemäßen Oiigomeren, Co-Oligomeren, Polymeren und/oder Co-Polymeren in den kosmetischen oder pharmazeutischen Zubereitungen 0,01 - 20 Gew.-%, bevorzugt 0,5 - 5 Gew.-%, insbesondere bevorzugt 1,5 - 2,5 Gew.-%, jeweils bezogen auf das Gesamtgewicht der kosmetischen oder pharmazeutischen Zubereitungen.Oligo (DL-lactate-co-glycolate) (50:50), average value for n * 8; j * k ■ ■ The content of the oligomers, co-oligomers, polymers and / or copolymers in the cosmetic or pharmaceutical preparations is advantageously 0.01-20% by weight, preferably 0.5-5% by weight, particularly preferably 1 , 5 - 2.5 wt .-%, each based on the total weight of the cosmetic or pharmaceutical preparations.
Erfindungsgemäß wird bevorzugt, das oder die Oligomere, Co-Oligomere, Polymere und/oder Co-Polymere in gelöster Form in kosmetische Formulierungen einzuarbeiten. Dazu kann der Fachmann aus einer Vielzahl bekannter, und auch für die gegebene Stoffklasse im allgemeinen bekannter Lösemittel eine nicht-erfinderische Auswahl treffen. Es war allerdings erstaunlich, daß partiell oder vollständig veresterte mehrbasige Carbonsäuren sich als besonders vorteilhafte Lösemittel für die erfindungsgemäßen Oligomere, Co-Oligomere, Polymere und/oder Co-Polymere erweisen würden.According to the invention, it is preferred to incorporate the oligomer or co-oligomers, polymers and / or copolymers in dissolved form into cosmetic formulations. To this end, the person skilled in the art can make a non-inventive selection from a large number of known solvents which are generally known for the given substance class. It was surprising, however, that partially or completely esterified polybasic carboxylic acids would prove to be particularly advantageous solvents for the oligomers, co-oligomers, polymers and / or co-polymers according to the invention.
Solche Ester werden vorteilhaft gewählt aus der Gruppe der kosmetisch oder pharmazeu¬ tisch unbedenklichen Substanzen der allgemeinen StrukturformelSuch esters are advantageously chosen from the group of cosmetically or pharmaceutically acceptable substances of the general structural formula
AOOC-X-COOB, wobei X gewählt wird aus der Gruppe der mit bis zu vier Carbonsäuregruppen und bis zu vier Carbonsäurealkylestergruppen -COOD, -COOE, -COOF und bis zu vier Hydroxy¬ gruppen substituierten verzweigten und oder unverzweigten Alkylengruppen mit bis zu sechs C-Atomen und der Gruppe der mit bis zu vier Carbonsäuregruppen und bis zu vier Carbonsäurealkylestergruppen -COOD, -COOE, -COOF und bis zu vier Hydroxygruppen substituierten Arylengruppen der TypenAOOC-X-COOB, where X is selected from the group consisting of up to four carboxylic acid groups and up to four carboxylic acid alkyl ester groups -COOD, -COOE, -COOF and up to four hydroxyl groups, branched and or unbranched alkylene groups with up to six C. -Atoms and the group of the types substituted with up to four carboxylic acid groups and up to four carboxylic acid alkyl ester groups -COOD, -COOE, -COOF and up to four hydroxyl groups
wobei A, B, D, E und F verzweigte oder unverzweigte Alkylgruppen mit bis zu fünf C-Atomen darstellen. where A, B, D, E and F represent branched or unbranched alkyl groups with up to five carbon atoms.
Bevorzugte Ester sind das TriethylcitratPreferred esters are triethyl citrate
CH -COO— Et I HO— C— COO— Et I CH2-COO— Et
sowie das Diethylphthalat.CH -COO- Et I HO- C- COO- Et I CH 2 -COO- Et as well as the diethyl phthalate.
Es hat sich werterhin in überraschender Weise herausgestellt, daß diese Ester nicht nur gute Lösungsmittel für die erfindungsgemäßen Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere sind, sondern diese zusätzlich gegen Depolymerisation bzw. Deoligomerisation stabilisieren.It has surprisingly been found that these esters are not only good solvents for the oligomers, polymers, co-oligomers and / or copolymers according to the invention, but also stabilize them against depolymerization or deoligomerization.
Darüberhinaus wirken diese Ester, bevorzugt die Citronensäureester, in bezug auf die kos¬ metische oder dermatologische Wirkung der α-Hydroxycarbonsäuren, insbesondere in bezug auf die pflegende Wirkung, vor allem in bezug auf die Wirkung gegen Hautalterung, synergistisch mit den erfindungsgemäßen Oligomeren, Polymeren, Co-Oligomeren und/oder Co-Polymeren zusammen.In addition, these esters, preferably the citric acid esters, act synergistically with the oligomers, polymers according to the invention in relation to the cosmetic or dermatological action of the α-hydroxycarboxylic acids, in particular in relation to the caring effect, especially in relation to the action against skin aging. Co-oligomers and / or copolymers together.
Vorteilhaft liegt das Gewichtsverhältnis von den erfindungsgemäßen Oligomeren, Polyme¬ ren, Co-Oligomeren und/oder Co-Polymeren einerseits zu den vorbeschriebenen sta¬ bilisierenden Estern andererseits im Bereich von etwa 1 : 10 bis 10 : 1, bevorzugt etwa 1 : 4 bis 2 : 1 , insbesondere bevorzugt etwa 3 : 7.The weight ratio of the oligomers, polymers, co-oligomers and / or copolymers on the one hand to the above-described stabilizing esters on the other hand is advantageously in the range from about 1:10 to 10: 1, preferably about 1: 4 to 2 : 1, particularly preferably about 3: 7.
Zwar ist, beispielsweise aus DE-GbM 88 04 423, bekannt, entsprechende Citronensäu¬ reester als Weichmacher für Polyiactide/glycolide und ähnliche Polymere einzusetzen. Die erfindungsgemäße kosmetische bzw. pharmazeutische Verwendung wurde durch DE-GbM 88 04 423, welches chirurgische Materialien betrifft, weder nahegelegt noch vorweggenom¬ men. Zudem wird gerade in DE-GbM 88 04 423 als Lösemittel Aceton empfohlen, welches weder kosmetisch noch pharmazeutisch akzeptabel ist.It is known, for example from DE-GbM 88 04 423, to use corresponding citric acid esters as plasticizers for polyiactides / glycolides and similar polymers. The cosmetic or pharmaceutical use according to the invention was neither suggested nor anticipated by DE-GbM 88 04 423, which relates to surgical materials. In addition, DE-GbM 88 04 423 recommends acetone as the solvent, which is neither cosmetically nor pharmaceutically acceptable.
Es ist aber auch vorteilhaft, Siliconöle wie Dimethicon als Lösemittel für die erfindungsgemä¬ ßen Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere zu verwenden.However, it is also advantageous to use silicone oils such as dimethicone as solvents for the oligomers, polymers, co-oligomers and / or copolymers according to the invention.
Die erfindungsgemäßen Oligomere, Polymere, Co-Oligomere bzw. Co-Polymere sind in kosmetischen und pharmazeutischen Zubereitungen bemerkenswert stabil und zersetzen sich weitestgehend erst auf der menschlichen Haut zu den zugrundeliegenden Oligomeren, welche dann für die Haut mit all ihren vorteilhaften Eigenschaften verfügbar werden.
Es ist auch möglich und gegebenenfalls vorteilhaft, Suspensionen der erfindungsgemäßen Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere zu verwenden.The oligomers, polymers, co-oligomers or copolymers according to the invention are remarkably stable in cosmetic and pharmaceutical preparations and largely decompose only on human skin to form the underlying oligomers, which then become available to the skin with all its advantageous properties. It is also possible and possibly advantageous to use suspensions of the oligomers, polymers, co-oligomers and / or co-polymers according to the invention.
Vor dem Lösungs bzw. Suspensionsvorgang kann es vorteilhaft sein, einen Mikronisierungs- vorgang vorzuschalten, wobei es bevorzugt wird, die Mikronisierung unter Kühlung, bei¬ spielsweise bei Temperaturen kleiner als -78° C, insbesondere bei Temperaturen kleiner als -150° C, bevorzugt bei ca. -190° C, durchzuführen.Before the solution or suspension process, it can be advantageous to precede a micronization process, it being preferred to prefer the micronization with cooling, for example at temperatures below -78 ° C., in particular at temperatures below -150 ° C. at approx. -190 ° C.
Der Lösungsvorgang ist bevorzugt mittels starker Agitation, beispielsweise in einem Ultra- turrax, vorzunehmen. Es kann auch vorteilhaft sein, beim Lösungs- bzw. Suspensionsvor¬ gang ein Netzmittel oder ain antistatisches Mittel zuzugeben.The dissolving process is preferably carried out by means of strong agitation, for example in an ultra-turrax. It can also be advantageous to add a wetting agent or an antistatic agent during the solution or suspension process.
Die Herstellung erfindungsgemäßer Zubereitungen geschieht nach den üblichen, dem Fach¬ manne geläufigen Regeln. Vorteilhaft liegen die erfindungsgemäßen Zubereitungen in Form von Emulsionen, bevorzugt O/W-Emulsionen vor. Es ist aber auch möglich und erfindungsgemäß gegebenenfalls vorteilhaft, andere Formulierungsarten zu wählen, beispielsweise W/O-Emulsionen, Hydrodispersionen, Gele, Öle, multiple Emulsionen, bei¬ spielsweise in Form von W/O/W- oder O W/O-Emulsionen, wasserfreie Salben bzw. Salben¬ grundlagen usw.Preparations according to the invention are produced in accordance with the usual rules familiar to those skilled in the art. The preparations according to the invention are advantageously in the form of emulsions, preferably O / W emulsions. However, it is also possible and advantageous according to the invention to select other types of formulation, for example W / O emulsions, hydrodispersions, gels, oils, multiple emulsions, for example in the form of W / O / W or OW / O emulsions, anhydrous ointments or ointment bases etc.
In einfachen Emulsionen liegen in der einen Phase feindisperse, von einer Emulgatorhülle umschlossene Tröpfchen der zweiten Phase (Wassertröpfchen in W/O- oder Lipidvesikel in O/W-Emulsionen) vor. In einer multiplen Emulsion (zweiten Grades) hingegen sind in solchen Tröpfchen feiner disperse Tröpfchen der ersten Phase emulgiert. Auch in diesen Tröpfchen wiederum können noch feiner disperse Tröpfchen vorliegen (multiple Emulsion dritten Grades) und so fort.In simple emulsions, finely dispersed droplets of the second phase (water droplets in W / O or lipid vesicles in O / W emulsions) are contained in one phase and enclosed by an emulsifier shell. In contrast, in a multiple emulsion (second degree), finely dispersed droplets of the first phase are emulsified in such droplets. These droplets can also have finer disperse droplets (third degree multiple emulsion) and so on.
So wie man bei den einfachen Emulsionen von W/O- oder O/W-Emulsionen spricht (Wasser-in-Oel oder Oel-in-Wasser), gibt es bei multiplen Emulsionen W/O/W-, O/W/O-, O/W/O/W-, W/O /V/O-Emulsionen und so fort.Just as the simple emulsions are called W / O or O / W emulsions (water-in-oil or oil-in-water), there are W / O / W, O / W / O in multiple emulsions -, O / W / O / W, W / O / V / O emulsions and so on.
Multiple Emulsionen, bei welchen die jeweiligen inneren und äußeren Wasserphasen oder inneren und äußeren Ölphasen unterschiedlich geartet sind (also z.B. W/O/W- und O/W/O' -Emulsionen), sind der Präparation durch Zweitopfverfahren zugängig. Solche Emulsionen,
in welchen die inneren und äußeren Wasser- bzw. Ölphasen nicht unterschiedlich geartet sind, sind sowohl durch Ein- als auch durch Zweitopfverfahren erhältlich.Multiple emulsions in which the respective inner and outer water phases or inner and outer oil phases are of different types (for example W / O / W and O / W / O 'emulsions) are accessible to the preparation by two-pot processes. Such emulsions, in which the inner and outer water or oil phases are not different, can be obtained both by one and two-pot processes.
Die multiplen Emulsionen zweiten Grades werden gelegentlich als "bimultiple Systeme", solche dritten Grades als "trimultiple Systeme" usw., bezeichnet (W.Seifriz, Studies in Emulsions, J.Phys.Chem., 29 (1925) 738 - 749).The second degree multiple emulsions are sometimes referred to as "bimultiple systems", such third degrees as "trimultiple systems" etc. (W.Seifriz, Studies in Emulsions, J.Phys.Chem., 29 (1925) 738-749).
Verfahren zur Herstellung multipler Emulsionen sind dem Fachmann an sich geläufig. So gibt es Zweitopfverfahren, in welchen eine einfache Emulsion (z.B. eine W/O-Emulsion) vorgelegt und durch Zugabe einer weiteren Phase (z.B. eine Wasserphase) mit einem entsprechenden Emulgator (z.B. ein O/W-Emulgator) in eine multiple Emulsion (z.B. eine W/O/W- Emulsion) überführt wird.Processes for producing multiple emulsions are known per se to the person skilled in the art. There are two-pot processes in which a simple emulsion (e.g. a W / O emulsion) is introduced and, by adding another phase (e.g. a water phase) with a corresponding emulsifier (e.g. an O / W emulsifier) into a multiple emulsion (e.g. a W / O / W emulsion) is transferred.
Ein zweites Verfahren besteht darin, Emulgatorgemische mit einer Ölphase und einer Wasserphase in einem Eintopfverfahren in eine multiple W/O/W-Emulsion zu überführen. Die Emulgatoren werden in der Ölphase gelöst und mit der Wasserphase vereinigt. Voraussetzung für ein solches Verfahren ist, daß die HLB-Werte (HLB = Hydrophil-Lipophil- Balance) der eingesetzten einzelnen Emulgatoren sich deutlich voneinander unterscheiden.A second method consists in converting emulsifier mixtures with an oil phase and a water phase into a multiple W / O / W emulsion in a one-pot process. The emulsifiers are dissolved in the oil phase and combined with the water phase. A prerequisite for such a process is that the HLB values (HLB = hydrophilic-lipophilic balance) of the individual emulsifiers used differ significantly from one another.
Hydrodispersionen stellen Dispersionen einer flüssigen, halbfesten oder festen inneren (diskontinuierlichen) Lipidphase in einer äußeren wäßrigen (kontinuierlichen) Phase dar.Hydrodispersions are dispersions of a liquid, semi-solid or solid inner (discontinuous) lipid phase in an outer aqueous (continuous) phase.
Im Gegensatze zu O/W-Emulsionen, die sich durch eine ähnliche Phasenanordnung aus¬ zeichnen, sind Hydrodispersionen aber im wesentlichen frei von Emulgatoren. Hydro¬ dispersionen stellen, wie im übrigen auch Emulsionen metastabile Systeme dar, und sind geneigt, in einen Zustand zweier in sich zusammenhängender diskreter Phasen überzu¬ gehen. In Emulsionen verhindert die Wahl eines geeigneten Emulgators die Phasentrennung.In contrast to O / W emulsions, which are characterized by a similar phase arrangement, hydrodispersions are essentially free of emulsifiers. Like other emulsions, hydro dispersions represent metastable systems and are inclined to change into a state of two discrete phases that are coherent. In emulsions, the choice of a suitable emulsifier prevents phase separation.
Bei Hydrodispersionen einer flüssigen Lipidphase in einer äußeren wäßrigen Phase kann die die Stabilität eines solchen Systems beispielsweise dadurch gewährleistet werden, daß in der wäßrigen Phase ein Gelgerüst aufgebaut wird, in welchem die Lipidtröpfchen stabil suspendiert sind. Solche Zubereitungen haben sich als äußerst vorteilhaft und besonders stabil erwiesen. Vorteilhafte Verdicker für diese Zwecke sind Carbopole, Verdicker auf Polysaccharidbasis und Bentonite.
Besonders vorteilhafte Zubereitungen werden ferner erhalten, wenn die erfindungsgemäßen Wirkstoffe mit Antioxidantien und/oder anderen der Zellalterung entgegenwirkenden Sub¬ stanzen kombiniert werden. Erfindungsgemäß enthalten die Zubereitungen vorteilhaft eines oder mehrere Antioxidantien. Als günstige, aber dennoch fakultativ zu verwendende Anti¬ oxidantien alle für kosmetische und/oder dermatologische Anwendungen geeigneten oder gebräuchlichen Antioxidantien verwendet werden.In the case of hydrodispersions of a liquid lipid phase in an outer aqueous phase, the stability of such a system can be ensured, for example, by building a gel structure in the aqueous phase in which the lipid droplets are stably suspended. Such preparations have proven to be extremely advantageous and particularly stable. Advantageous thickeners for this purpose are carbopols, polysaccharide-based thickeners and bentonites. Particularly advantageous preparations are also obtained if the active compounds according to the invention are combined with antioxidants and / or other substances which counteract cell aging. According to the invention, the preparations advantageously contain one or more antioxidants. All of the antioxidants suitable or customary for cosmetic and / or dermatological applications are used as inexpensive, but nevertheless optional anti-oxidants.
Besonders vorteilhaft werden die Antioxidantien gewählt aus der Gruppe bestehend ausThe antioxidants are particularly advantageously selected from the group consisting of
Aminosäuren (z.B. Glycin, Histidin, Tyrosin, Tryptophan) und deren Derivate, Imidazole (z.B. Urocaninsäure) und deren Derivate, Peptide wie D,L-Carnosin, D-Carnosin, L-Camosin und deren Derivate (z.B. Anserin), Carotinoide, Carotine (z.B. α-Carotin, ß-Carotin, Lycopin) und deren Derivate, Liponsäure und deren Derivate (z.B. Dihydroliponsäure), Aurothioglucose, Propylthiouracil und andere Thiole (z.B. Thioredoxin, Glutathion, Cystein, Cystin, Cystamin und deren Glycosyl-, N-Acetyl-, Methyl-, Ethyl-, Propyl-, Amyl-, Butyl- und Lauryl-, Palmitoyl- Oleyl-, γ-Linoleyl-, Cholesteryl - und Glycerylester) sowie deren Salze, Dilaurylthiodipropionat, Distearylthiodipropionat, Thiodipropionsäure und deren Derivate (Ester, Ether, Peptide, Lipide, Nukleotide, Nukleoside und Salze) sowie Sulfoximin- verbindungen (z.B. Buthioninsulfoximine, Homocysteinsulfoximin, Buthioninsulfone, Penta-, Hexa-, Heptahioninsulfoximin) in sehr geringen verträglichen Dosierungen (z.B. pmol bis μmol/kg), ferner (Metall)-Chelatoren (z.B. α-Hydroxyfettsäuren, Palmitinsäure, Phytinsäure, Lactoferrin), Huminsäure, Gallensäure, Gallenextrakte, Bilirubin, Biliverdin, EDTA, EGTA und deren Derivate, ungesättigte Fettsäuren und deren Derivate (z.B. γ-Linolensäure, Linolsäure, Ölsäure), Folsäure und deren Derivate, Ubichinon und Ubichinol deren Derivate, Vitamin C und dessen Derivate (z.B. Ascorbylpalmitate, Mg - Ascorbylphosphate, Ascorbyl- acetate), Tocopherole und Derivate (z.B. Vitamin E - acetat), Vitamin A und Derivate (Vita¬ min A - palmitat) sowie Konyferylbenzoat des Benzoeharzes, Rutinsäure und deren Deriva¬ te, Ferulasäure und deren Derivate, Butylhydroxytoluol, Butylhydroxyanisol, Furalglucttol, Nordihydroguajakharzsäure, Nordihydroguajaretsäure, Trihydroxybutyrophenon, Harnsäure und deren Derivate, Mannose und deren Derivate, Zink und dessen Derivate (z.B. ZnO, ZnSθ ) Selen und dessen Derivate (z.B. Selenmethionin), Stilbene und deren Derivate (z.B. Stilbenoxid, Trans-Stilbenoxid) und die erfindungsgemäß geeigneten Derivate (Salze, Ester, Ether, Zucker, Nukleotide, Nukleoside, Peptide und Lipide) dieser genannten Wirkstoffe.
Besonders vorteilhaft im Sinne der vorliegenden Erfindung können öllösliche Antioxidantien eingesetzt werden.Amino acids (e.g. glycine, histidine, tyrosine, tryptophan) and their derivatives, imidazoles (e.g. urocanic acid) and their derivatives, peptides such as D, L-carnosine, D-carnosine, L-camosine and their derivatives (e.g. anserine), carotenoids, carotenes (e.g. α-carotene, ß-carotene, lycopene) and their derivatives, lipoic acid and their derivatives (e.g. dihydroliponic acid), aurothioglucose, propylthiouracil and other thiols (e.g. thioredoxin, glutathione, cysteine, cystine, cystamine and their glycosyl, N-acetyl -, methyl, ethyl, propyl, amyl, butyl and lauryl, palmitoyl, oleyl, γ-linoleyl, cholesteryl and glyceryl esters) and their salts, dilaurylthiodipropionate, distearylthiodipropionate, thiodipropionic acid and their derivatives (esters, Ethers, peptides, lipids, nucleotides, nucleosides and salts) and sulfoximine compounds (e.g. buthionine sulfoximines, homocysteine sulfoximine, buthionine sulfones, penta-, hexa-, heptahionine sulfoximine) in very low tolerable dosages (e.g. pmol to μmol / kg), also (metal) -Chela gates (e.g. α-hydroxy fatty acids, palmitic acid, phytic acid, lactoferrin), humic acid, bile acid, bile extracts, bilirubin, biliverdin, EDTA, EGTA and their derivatives, unsaturated fatty acids and their derivatives (e.g. γ-linolenic acid, linoleic acid, oleic acid), folic acid and their Derivatives, ubiquinone and ubiquinol, their derivatives, vitamin C and its derivatives (eg ascorbyl palmitate, Mg - ascorbyl phosphate, ascorbyl acetate), tocopherols and derivatives (eg vitamin E acetate), vitamin A and derivatives (vitamin A palmitate) and Konyferyl benzoate of benzoin, rutinic acid and its derivatives, ferulic acid and its derivatives, butylated hydroxytoluene, butylated hydroxyanisole, fural gluctol, nordihydroguajak resinic acid, nordihydroguajaretic acid, trihydroxybutyrophenone, uric acid and its derivatives, eg zinc and its derivatives, mannose and its derivatives, mannose and its derivatives, mannose and its derivatives, znose and its derivatives, mannose and its derivatives, z its derivatives (eg selenium methionine), stilbenes and their derivatives (eg stilbene oxide, trans-stilbene oxide) and the invent derivatives according to the invention (salts, esters, ethers, sugars, nucleotides, nucleosides, peptides and lipids) of these active ingredients. Oil-soluble antioxidants can be used particularly advantageously for the purposes of the present invention.
Die Menge der Antioxidantien (eine oder mehrere Verbindungen) in den Zubereitungen be¬ trägt vorzugsweise 0,001 bis 30 Gew.-%, besonders bevorzugt 0,05 - 20 Gew.-%, insbeson¬ dere 1 - 10 Gew.-%, bezogen auf das Gesamtgewicht der Zubereitung.The amount of the antioxidants (one or more compounds) in the preparations is preferably 0.001 to 30% by weight, particularly preferably 0.05-20% by weight, in particular 1-10% by weight, based on the total weight of the preparation.
Sofern Vitamin E und/oder dessen Derivate das oder die Antioxidantien darstellen, ist vor¬ teilhaft, deren jeweilige Konzentrationen aus dem Bereich von 0,001 - 10 Gew.-%, bezogen auf das Gesamtgewicht der Formulierung, zu wählen.If vitamin E and / or its derivatives represent the antioxidant (s), it is advantageous to choose their respective concentrations from the range of 0.001-10% by weight, based on the total weight of the formulation.
Sofern Vitamin A, bzw. Vitamin-A-Derivate, bzw. Carotine bzw. deren Derivate das oder die Antioxidantien darstellen, ist vorteilhaft, deren jeweilige Konzentrationen aus dem Bereich von 0,001 - 10 Gew.-%, bezogen auf das Gesamtgewicht der Formulierung, zu wählen.If vitamin A or vitamin A derivatives or carotenes or their derivatives represent the antioxidant or antioxidants, it is advantageous to use their respective concentrations in the range from 0.001-10% by weight, based on the total weight of the formulation, to choose.
Als wertere der Zellalterung entgegenwirkende Substanzen können vorteilhaft Cttronensäu- re, Salicylsäure, andere ß-Hydroxycarbonsäuren, Biotin und dergleichen verwendet werden.Cttronic acid, salicylic acid, other β-hydroxycarboxylic acids, biotin and the like can advantageously be used as other substances which counteract cell aging.
Die erfindungsgemäßen kosmetischen Zubereitungen können kosmetische Hilfsstoffe ent¬ halten, wie sie üblicherweise in solchen Zubereitungen verwendet werden, z.B. Konservie¬ rungsmittel, Bakterizide, desodorierend wirkende Substanzen, reizmindernde Stoffe, Anti- transpirantien, Insektenrepellentien, Vitamine, Mittel zum Verhindern des Schäumens, Farb¬ stoffe, Pigmente mit färbender Wirkung, Verdickungsmittel, weichmachende Substanzen, anfeuchtende und/oder feuchhaltende Substanzen, Fette, Öle, Wachse oder andere übliche Bestandteile einer kosmetischen Formulierung wie Alkohole, Polyole, Polymere, Schaumstabilisatoren, Elektrolyte, organische Lösemittel oder Silikonderivate.The cosmetic preparations according to the invention can contain cosmetic auxiliaries as are usually used in such preparations, e.g. Preservatives, bactericides, deodorizing substances, irritant substances, antiperspirants, insect repellents, vitamins, anti-foaming agents, dyes, pigments with a coloring effect, thickeners, softening substances, moisturizing and / or moisturizing substances, fats, Oils, waxes or other common components of a cosmetic formulation such as alcohols, polyols, polymers, foam stabilizers, electrolytes, organic solvents or silicone derivatives.
Vorteilhaft können die erfindungsgemäßen Zubereitungen außerdem Substanzen enthalten, die UV-Strahlung im UVB-Bereich absorbieren, wobei die Gesamtmenge der Filtersub¬ stanzen z.B. 0,1 Gew.-% bis 30 Gew.-%, vorzugsweise 0,5 bis 10 Gew.-%, insbesondere 1 bis 6 Gew.-% beträgt, bezogen auf das Gesamtgewicht der Zubereitung, um kosmetische Zubereitungen zur Verfügung zu stellen, die die Haut vor dem gesamten Bereich der ultravioletten Strahlung schützen, und in denen das Stinging der α-Hydroxycarbonsäuren bzw. α-Ketocarbonsäuren verhindert oder drastisch vermindert ist.
Der pH-Wert der erfindungsgemäßen Zubereitungen kann vorteilhaft im sauren Bereich ein¬ gestellt werden, wobei der pH-Bereich von 3,5 - 7 bevorzugt wird, besonders bevorzugt von 4 - 6, ganz besonders etwa 5,5. Ersatunlicherweise erfährt die Stabilität der erfin¬ dungsgemäßen Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere durch Puf¬ ferung eine zusätzliche Steigerung.The preparations according to the invention can also advantageously contain substances which absorb UV radiation in the UVB range, the total amount of the filter substances being, for example, 0.1% by weight to 30% by weight, preferably 0.5 to 10% by weight. %, in particular 1 to 6% by weight, based on the total weight of the preparation, in order to provide cosmetic preparations which protect the skin from the entire range of ultraviolet radiation, and in which the stinging of the α-hydroxycarboxylic acids or α-keto carboxylic acids are prevented or drastically reduced. The pH of the preparations according to the invention can advantageously be set in the acidic range, the pH range from 3.5 to 7 being preferred, particularly preferably from 4 to 6, very particularly about 5.5. The stability of the oligomers, polymers, co-oligomers and / or copolymers according to the invention is additionally increased by buffering.
Erfindungsgemäße Zubereitungen können auch vorteilhaft als Sonnenschutzmittel dienen.Preparations according to the invention can also advantageously serve as sunscreens.
Emulsionen gemäß der Erfindung z.B. in Form einer Sonnenschutzcreme oder einer Son¬ nenschutzmilch sind vorteilhaft und enthalten z.B. die genannten Fette, Öle, Wachse und anderen Fettkörper, sowie Wasser und einen Emulgator, wie er üblicherweise für einen solchen Typ der Formulierung verwendet wird.Emulsions according to the invention e.g. in the form of a sun protection cream or sun protection milk are advantageous and contain e.g. the fats, oils, waxes and other fat bodies mentioned, as well as water and an emulsifier, as is usually used for such a type of formulation.
Die Lipidphase kann vorteilhaft gewählt werden aus folgender Substanzgruppe:The lipid phase can advantageously be selected from the following group of substances:
Öle, wie Triglyceride der Caprin- oder der Caprylsäure, vorzugsweise aber Rizinusöl; Fette, Wachse und andere natürliche und synthetische Fettkörper, vorzugsweise Ester von Fettsäuren mit Alkoholen niedriger C-Zahl, z.B. mit Isopropanol, Propylen- glykol oder Glycerin, oder Ester von Fettalkoholen mit Alkansäuren niedriger C-Zahl oder mit Fettsäuren; Alkylbenzoate;Oils, such as triglycerides of capric or caprylic acid, but preferably castor oil; Fats, waxes and other natural and synthetic fat bodies, preferably esters of fatty acids with alcohols of low C number, e.g. with isopropanol, propylene glycol or glycerin, or esters of fatty alcohols with low C number alkanoic acids or with fatty acids; Alkyl benzoates;
Silikonöle wie Dimethylpolysiloxane, Diethylpolysiloxane, Diphenylpolysiloxane sowie Mischformen daraus.Silicone oils such as dimethylpolysiloxanes, diethylpolysiloxanes, diphenylpolysiloxanes and mixed forms thereof.
Die wäßrige Phase der erfindungsgemäßen Zubereitungen enthält gegebenenfalls vorteil¬ haftThe aqueous phase of the preparations according to the invention may advantageously contain
Alkohole, Diole oder Polyole niedriger C-Zahl, sowie deren Ether, vorzugsweise Ethanol, Isopropanol, Propylenglykol, Glycerin, Ethylenglykol, Ethylenglykolmono- ethyl- oder -monobutylether, Propylenglykolmonomethyl, -monoethyl- oder -monobu- tylether, Diethylenglykolmonomethyl- oder -monoethylether und analoge Produkte, ferner Alkohole niedriger C-Zahl, z.B. Ethanol, Isopropanol, 1 ,2-Propandiol, Glycerin sowie insbesondere ein oder mehrere Verdickungsmittel, welches oder welche vor¬ teilhaft gewählt werden können aus der Gruppe Siliciumdioxid, Aluminiumsilikate, Po- lysaccharide bzw. deren Derivate, z.B. Hyaluronsäure, Xanthangummi, Hydroxypro- pylmethylcellulose, besonders vorteilhaft aus der Gruppe der Polyacrylate, bevorzugt
ein Polyacrylat aus der Gruppe der sogenannten Carbopole, beispielsweise Car¬ bopole der Typen 980, 981, 1382, 2984, 5984, jeweils einzeln oder in Kombination.Alcohols, diols or polyols of low C number, and their ethers, preferably ethanol, isopropanol, propylene glycol, glycerol, ethylene glycol, ethylene glycol monoethyl or monobutyl ether, propylene glycol monomethyl, monoethyl or monobutyl ether, diethylene glycol monomethyl or monoethyl ether and analogous products, furthermore alcohols with a low C number, for example ethanol, isopropanol, 1, 2-propanediol, glycerol and in particular one or more thickeners, which one or more can advantageously be selected from the group consisting of silicon dioxide, aluminum silicates, polysaccharides or their derivatives, for example hyaluronic acid, xanthan gum, hydroxypropyl methyl cellulose, particularly advantageously from the group of polyacrylates, are preferred a polyacrylate from the group of the so-called carbopoles, for example carbopoles of types 980, 981, 1382, 2984, 5984, each individually or in combination.
Die kosmetischen oder dermatologischen Lichtschutzzubereitungen enthalten vorteilhaft an¬ organische Pigmente, insbesondere Mikropigmente, z.B. in Mengen von 0,1 Gew.-% bis 30 Gew.-%, vorzugsweise in Mengen von 0,5 Gew.-% bis 10 Gew.-%, insbesondere aber 1 Gew.-% bis 6 Gew.-%, bezogen auf das Gesamtgewicht der Zubereitungen.The cosmetic or dermatological sunscreen preparations advantageously contain inorganic pigments, in particular micropigments, e.g. in amounts of 0.1% by weight to 30% by weight, preferably in amounts of 0.5% by weight to 10% by weight, but in particular 1% by weight to 6% by weight on the total weight of the preparations.
Es ist erfindungsgemäß vorteilhaft, außer den erfindungsgemäßen Kombinationen öllösliche UVA-Filter und/oder UVB-Filter in der Lipidphase und/oder wasserlösliche UVA-Filter und/oder UVB-Filter in der wäßrigen Phase einzusetzen.In addition to the combinations according to the invention, it is advantageous according to the invention to use oil-soluble UVA filters and / or UVB filters in the lipid phase and / or water-soluble UVA filters and / or UVB filters in the aqueous phase.
Vorteilhaft können die erfindungsgemäßen Lichtschutzformulierungen Substanzen enthalten, die UV-Strahlung im UVB-Bereich absorbieren, wobei die Gesamtmenge der Filtersubstanzen z.B. 0,1 Gew.-% bis 30 Gew.-%, vorzugsweise 0,5 bis 10 Gew.-%, insbe¬ sondere 1 bis 6 Gew.-% beträgt, bezogen auf das Gesamtgewicht der Zubereitungen, um kosmetische Zubereitungen zur Verfügung zu stellen, die die Haut vor dem gesamten Bereich der ultravioletten Strahlung schützen. Sie können auch als Sonnenschutzmittel dienen.The light protection formulations according to the invention can advantageously contain substances which absorb UV radiation in the UVB range, the total amount of the filter substances e.g. 0.1% by weight to 30% by weight, preferably 0.5 to 10% by weight, in particular 1 to 6% by weight, based on the total weight of the preparations, in order to provide cosmetic preparations places that protect the skin from the entire range of ultraviolet radiation. They can also serve as sunscreens.
Die UVB-Filter können öllöslich oder wasserlöslich sein. Vorteilhafte öllösliche UVB- Filtersubstanzen sind z.B.:The UVB filters can be oil-soluble or water-soluble. Advantageous oil-soluble UVB filter substances include:
- 3-Benzylidencampher-Derivate, vorzugsweise 3-(4-Methylbenzyliden)campher, 3-Benzyli- dencampher;- 3-benzylidene camphor derivatives, preferably 3- (4-methylbenzylidene) camphor, 3-benzylidene camphor;
- 4-Aminobenzoesäure-Derivate, vorzugsweise 4-(Dimethylamino)-benzoesäure(2-ethyIhe- xyhester, 4-(Dimethylamino)benzoesäureamylester;- 4-aminobenzoic acid derivatives, preferably 4- (dimethylamino) benzoic acid (2-ethylhexyl ester, 4- (dimethylamino) benzoic acid amyl ester;
- Ester der Zimtsäure, vorzugsweise 4-Methoxyzimtsäure(2-ethylhexyl)ester, 4-Methoxy- zimtsäureisopentylester;- Esters of cinnamic acid, preferably 4-methoxycinnamic acid (2-ethylhexyl) ester, 4-methoxycinnamic acid isopentyl ester;
- Derivate des Benzophenons, vorzugsweise 2-Hydroxy-4-methoxybenzophenon, 2-Hy- droxy-4-methoxy-4'-methylbenzophenon, 2,2'-Dihydroxy-4-methoxybenzophenon;- Derivatives of benzophenone, preferably 2-hydroxy-4-methoxybenzophenone, 2-hydroxy-4-methoxy-4'-methylbenzophenone, 2,2'-dihydroxy-4-methoxybenzophenone;
- Ester der Benzalmalonsäure, vorzugsweise 4-Methoxybenzalmalonsäuredi(2-ethylhexyl)- ester;Esters of benzalmalonic acid, preferably 4-methoxybenzalmalonic acid di (2-ethylhexyl) ester;
- 2,4,6-Trianilino-(p-carbo-2'-ethyl-1'-hexyloxy)-1 ,3,5-triazin.- 2,4,6-trianilino- (p-carbo-2'-ethyl-1'-hexyloxy) -1, 3,5-triazine.
Vorteilhafte wasserlösliche UVB-Filtersubstanzen sind z.B.:
- Salze der 2-Phenylbenzimidazol-5-sulfonsäure wie ihr Natrium-, Kalium- oder ihr Trietha- noIammonium-Salz, sowie die Sulfonsäure selbst;Advantageous water-soluble UVB filter substances include: - Salts of 2-phenylbenzimidazole-5-sulfonic acid such as its sodium, potassium or triethanammonium salt, and the sulfonic acid itself;
- Sulfonsäure-Derivate von Benzophenonen, vorzugsweise 2-Hydroxy-4-methoxybenzo- phenon-5-sulfonsäure und deren Salze;- Sulfonic acid derivatives of benzophenones, preferably 2-hydroxy-4-methoxybenzophenone-5-sulfonic acid and its salts;
- Sulfonsäure-Derivate des 3-Benzylidencamphers, wie z.B. 4-(2-Oxo-3-bornylidenmethyl)- benzolsulfonsäure,2-Methyl-5-(2-oxo-3-bomylidenmethyl)sulfonsäure und deren Salze.- Sulfonic acid derivatives of 3-benzylidene camphor, e.g. 4- (2-oxo-3-bornylidenemethyl) benzenesulfonic acid, 2-methyl-5- (2-oxo-3-bomylidenemethyl) sulfonic acid and their salts.
Die Liste der genannten UVB-Filter, die in Kombination mit den erfindungsgemäßen Wirk- stoffkombinationen verwendet werden können, soll selbstverständlich nicht limitierend sein.The list of the UVB filters mentioned, which can be used in combination with the active compound combinations according to the invention, is of course not intended to be limiting.
Es kann auch von Vorteil sein, die erfindungsgemäßen Kombinationen mit UVA-Filtern zu kombinieren, die bisher üblicherweise in kosmetischen Zubereitungen enthalten sind. Bei diesen Substanzen handelt es sich vorzugsweise um Derivate des Dibenzoylmethans, ins¬ besondere um 1-(4'-tert.Butylphenyl)-3-(4'-methoxyphenyl)propan-1,3-dion und um 1-Phe- nyI-3-(4'-isopropylphenyl)propan-1,3-dion. Auch diese Kombinationen bzw. Zubereitungen, die diese Kombinationen enthalten, sind Gegenstand der Erfindung. Es können die für die UVB-Kombination verwendeten Mengen eingesetzt werden.It can also be advantageous to combine the combinations according to the invention with UVA filters which have hitherto usually been contained in cosmetic preparations. These substances are preferably derivatives of dibenzoylmethane, in particular 1- (4'-tert-butylphenyl) -3- (4'-methoxyphenyl) propane-1,3-dione and 1-phenyI- 3- (4'-isopropylphenyl) propane-1,3-dione. These combinations or preparations containing these combinations are also the subject of the invention. The quantities used for the UVB combination can be used.
Ferner ist vorteilhaft, die erfindungsgemäßen Wirkstoffkombinationen mit werteren UVA- und/oder UVB-Filtern zu kombinieren.It is also advantageous to combine the active ingredient combinations according to the invention with other UVA and / or UVB filters.
Die Gesamtmenge der UVA-Filtersubstanzen kann vorteilhaft 0,1 Gew.-% bis 30 Gew.-%, vorzugsweise 0,5 bis 10 Gew.-%, insbesondere 1 bis 6 Gew.-% betragen, bezogen auf das Gesamtgewicht der Zubereitung, um kosmetische Zubereitungen zur Verfügung zu stellen, die die Haut vor dem gesamten Bereich der ultravioletten Strahlung schützen, und in denen das Stinging der α-Hydroxycarbonsäuren bzw. α-Ketocarbonsäuren verhindert oder dra¬ stisch vermindert ist.The total amount of UVA filter substances can advantageously be 0.1% by weight to 30% by weight, preferably 0.5 to 10% by weight, in particular 1 to 6% by weight, based on the total weight of the preparation, in order to provide cosmetic preparations which protect the skin from the entire range of ultraviolet radiation and in which the stinging of the α-hydroxycarboxylic acids or α-ketocarboxylic acids is prevented or drastically reduced.
Vorteilhaft sind auch solche kosmetische und dermatologische Zubereitungen, die in der Form eines Sonnenschutzmittels, eines Pre-Soleil- oder Apres-Soleil-Produktes vorliegen. Vorteilhaft enthalten diese zusätzlich mindestens einen UVA-Filter und/oder mindestens einen UVB-Filter.Cosmetic and dermatological preparations which are in the form of a sunscreen, a pre-soleil or apres-soleil product are also advantageous. These advantageously also contain at least one UVA filter and / or at least one UVB filter.
Ferner sind auch solche kosmetische und dermatologische Zubereitungen besonders vor¬ teilhaft, die in der Form eines Sonnenschutzmittels, eines Pre-Soleil- oder Apres-Soleil-Pro-
duktes vorliegen und zusätzlich zu dem oder den UVA-Filtern und/oder dem oder den UVB-Furthermore, cosmetic and dermatological preparations which are in the form of a sunscreen, a pre-soleil or apres-soleil product are also particularly advantageous. product and in addition to the UVA filter (s) and / or the UVB filter (s)
Filtern ein oder mehrere Antioxidantien enthalten.Filters contain one or more antioxidants.
Erfindungsgemäße kosmetische und dermatologische Zubereitungen enthalten bevorzugt anorganische Pigmente auf Basis von Metalloxiden und/oder anderen in Wasser schwer¬ löslichen oder unlöslichen Metaliverbindungen, insbesondere der Oxide des Titans TΪO2), Zinks (ZnO), Eisens (z.B. Fβ2θ3), Zirkoniums (Zrθ2), Siliciums (Siθ2), Mangans (z.B. MnO), Aluminiums (AI2O3), Cers (z.B. Cβ2θ3), Mischoxiden der entsprechenden Metalle sowie Abmischungen aus solchen Oxiden. Besonders bevorzugt handelt es sich um Pig¬ mente auf der Basis von Tiθ2-Cosmetic and dermatological preparations according to the invention preferably contain inorganic pigments based on metal oxides and / or other metal compounds which are sparingly soluble or insoluble in water, in particular the oxides of titanium TΪO2), zinc (ZnO), iron (eg Fβ2θ3), zirconium (Zrθ2), Silicon (Siθ2), manganese (e.g. MnO), aluminum (AI2O3), cerium (e.g. Cβ2θ3), mixed oxides of the corresponding metals and mixtures of such oxides. It is particularly preferred to use pigments based on TiO 2
Voraussetzung für die Verwendbarkeit anorganischer Pigmente für die erfindungsgemäßen Zwecke ist natürlich die kosmetische bzw. dermatologische Unbedenklichkeit der zugrun¬ deliegenden Substanzen.A prerequisite for the use of inorganic pigments for the purposes of the invention is, of course, the cosmetic or dermatological harmlessness of the underlying substances.
Vorteilhaft ist, den Partikeldurchmesser der verwendeten Pigmente kleiner als 100 nm zu wählen.It is advantageous to choose the particle diameter of the pigments used to be less than 100 nm.
Erfindungsgemäß liegen die anorganischen Pigmente in hydrophober Form vor, d.h., daß sie oberflächlich wasserabweisend behandelt sind. Diese Oberflächenbehandlung kann darin bestehen, daß die Pigmente nach an sich bekannten Verfahren mit einer dünnen hydrophoben Schicht versehen werden.According to the invention, the inorganic pigments are in hydrophobic form, that is to say that they have been surface-treated to be water-repellent. This surface treatment can consist in that the pigments are provided with a thin hydrophobic layer by methods known per se.
Eines solcher Verfahren besteht beispielsweise darin, daß die hydrophobe Oberflächen¬ schicht nach einer Reaktion gemäßOne such method consists, for example, in that the hydrophobic surface layer according to a reaction
n Tiθ2 + m (RO)3Si-R' -> n ΗO2 (oberfl.)n Tiθ2 + m (RO) 3Si-R '-> n ΗO2 (surface)
erzeugt wird, n und m sind dabei nach Belieben einzusetzende stöchiometrische Parameter, R und R' die gewünschten organischen Reste. Beispielsweise in Analogie zu DE-OS 33 14 742 dargestellte hydrophobisierte Pigmente sind von Vorteil.is generated, n and m are stoichiometric parameters to be used at will, R and R 'are the desired organic radicals. For example, hydrophobized pigments shown in analogy to DE-OS 33 14 742 are advantageous.
Vorteilhafte Tiθ2-Pigmente sind beispielsweise unter den Handelsbezeichnungen T 805 (DEGUSSA) oder M 262 (KEMIRA) oder M 160 (KEMIRA) oder MT 100 T (TAYCA) erhält¬ lich.
Vorteilhafte Siθ2-Pigmente können aus der Reihe der unter den Handelsbezeichnungen AEROSIL (DEGUSSA) vertriebenen hydrophoben Pigmente, beispielsweise AEROSIL R 812 oder AEROSIL R 972, gewählt werden.Advantageous TiO 2 pigments are available, for example, under the trade names T 805 (DEGUSSA) or M 262 (KEMIRA) or M 160 (KEMIRA) or MT 100 T (TAYCA). Advantageous SiO 2 pigments can be selected from the range of hydrophobic pigments sold under the trade names AEROSIL (DEGUSSA), for example AEROSIL R 812 or AEROSIL R 972.
Erfindungsgemäße Zubereitungen sind vorteilhaft durch einen Gehalt von 0,1 bis 10 Gew.- %, insbesondere 0,5 - 5,0 Gew.-%, an hydrophoben anorganischen Pigmenten ge¬ kennzeichnet, jeweils bezogen auf das Gesamtgewicht der Zusammensetzung.Preparations according to the invention are advantageously characterized by a content of 0.1 to 10% by weight, in particular 0.5 to 5.0% by weight, of hydrophobic inorganic pigments, in each case based on the total weight of the composition.
In den folgenden Beispielen werden vorteilhafte Verkörperungen der vorliegenden Erfindung aufgeführt.
In the following examples, advantageous embodiments of the present invention are listed.
Beispiel 1example 1
Gew.-%% By weight
Cetylstearylisononanoat, PEG-20-Cetyl-Stearylether, Cetylstearylalkohol, Glycerylstearat, Glycerin, Cetyl- palmitat, PEG-12-Cetyl-Stearylether, Benzoesäure 15,00Cetyl stearyl isononanoate, PEG-20 cetyl stearyl ether, cetyl stearyl alcohol, glyceryl stearate, glycerol, cetyl palmitate, PEG-12 cetyl stearyl ether, benzoic acid 15.00
Cetylstearylisononanoat 4,00Cetyl stearyl isononanoate 4.00
Dicaprylether 7,00Dicapryl ether 7.00
Glycerin 3,00Glycerin 3.00
Carbopol 2984 0,40Carbopol 2984 0.40
Resomer 104 (30 Gew.-%) in Dimethicon-Copolyol 6,60Resomer 104 (30% by weight) in dimethicone copolyol 6.60
NaOH (!0-Gew.% in H2O) 1,60NaOH (! 0 wt% in H 2 O) 1.60
Parfüm, Konservierungsmittel, Antixidantien q.s.Perfume, preservatives, anti-oxidants q.s.
Wasser ad 100,00Water ad 100.00
Beispiel 2Example 2
Gew.-%% By weight
Cetylstearylisononanoat, PEG-20-Cetyl-Stearylether, Cetylstearylalkohol, Glycerylstearat, Glycerin, Cetyl- palmitat, PEG-12-Cetyl-Stearylether, Benzoesäure 15,00Cetyl stearyl isononanoate, PEG-20 cetyl stearyl ether, cetyl stearyl alcohol, glyceryl stearate, glycerol, cetyl palmitate, PEG-12 cetyl stearyl ether, benzoic acid 15.00
Cetylstearylisononanoat 4,00Cetyl stearyl isononanoate 4.00
Dicaprylether 7,00Dicapryl ether 7.00
Glycerin 3,00Glycerin 3.00
Carbopol 2984 0,40Carbopol 2984 0.40
Resomer 104 (30 Gew.-%) in Triethylcitrat 6,60Resomer 104 (30% by weight) in triethyl citrate 6.60
NaOH (!0-Gew.% in H2O) 1 ,60NaOH (! 0 wt.% In H 2 O) 1.60
Parfüm, Konservierungsmittel, Antixidantien q.s.Perfume, preservatives, anti-oxidants q.s.
Wasser ad 100,00
Beispiel 3Water ad 100.00 Example 3
Gew.-%% By weight
Polyglycerin Methyl Glucose Distearat 4,50Polyglycerol methyl glucose distearate 4.50
Glycerinmonolaurat 0,75Glycerol monolaurate 0.75
Octan-/Decansäuretriglyceride 1,00Octanoic / decanoic acid triglycerides 1.00
Octyldodecanol 2,00Octyldodecanol 2.00
Isopropylstearat 2,00Isopropyl stearate 2.00
Glycerin 3,00Glycerin 3.00
Myristylalkohol 1.75Myristyl alcohol 1.75
Cetylalkohol 4,50Cetyl alcohol 4.50
Carbomerpol 2984 0,10Carbomerpol 2984 0.10
Resomer 104Resomer 104
(30 Gew.-% in Triethylcitrat/Dimethicon Copolyol 1:1) 6,60(30% by weight in triethyl citrate / dimethicone copolyol 1: 1) 6.60
NaOH 0,09NaOH 0.09
Parfüm, Konservierungsmrttel, Antixidantien q.s.Perfume, preservatives, antioxidants q.s.
Wasser ad 100,00Water ad 100.00
Beispiel 4Example 4
Gew.-%% By weight
PEG-40-Stearat 1,00PEG-40 stearate 1.00
Glycerinmonostearat 2,00Glycerol monostearate 2.00
Octan-/Decansäuretriglyceride 4,30Octanoic / decanoic acid triglycerides 4.30
Octyldodecanol 1,00Octyldodecanol 1.00
Ci2-Ci5-Alkylbenzoate 1 ,00Ci 2 -Ci 5 alkyl benzoates 1, 00
Glycerin 6,00Glycerin 6.00
Myristylalkohol 1,75Myristyl alcohol 1.75
Cetylalkohol 3,00Cetyl alcohol 3.00
NaCI 2,40NaCI 2.40
Resomer 104 (30 Gew.-% in Triethylcitrat) 6,60Resomer 104 (30% by weight in triethyl citrate) 6.60
Cyclomethicon 3,00Cyclomethicone 3.00
Parfüm, Konservierungsmittel, Antixidantien q.s.Perfume, preservatives, anti-oxidants q.s.
Wasser ad 100,00
Beispiel 5Water ad 100.00 Example 5
Gew.-%% By weight
Wollwachsalkohol 0,10Wool wax alcohol 0.10
Octan-/Decansäuretriglyceride 1 ,00Octanoic / decanoic acid triglycerides 1, 00
Octyldodecanol 1 ,00Octyldodecanol 1.00
Ci2-Ci5-Alkylbenzoate 2,00Ci 2 -Ci 5 alkyl benzoates 2.00
Butylenglycol 2,00Butylene glycol 2.00
Cetylstearylalkohol 1,00Cetylstearyl alcohol 1.00
Diglycerylmonocaprat 0,10Diglyceryl monocaprate 0.10
Glycin 0,35Glycine 0.35
Resomer 104 (30 Gew.-% in Tributylcitrat) 6,60Resomer 104 (30% by weight in tributyl citrate) 6.60
Panthenol 0,15Panthenol 0.15
NaOH 0,27NaOH 0.27
Ethanol 5,00Ethanol 5.00
Parfüm, Konservierungsmittel, Antixidantien q.s.Perfume, preservatives, anti-oxidants q.s.
Wasser ad 100,00
Water ad 100.00
Claims
1. Kosmetische oder dermatologische Zubereitungen, enthaltend eine wirksame Menge eines oder mehrerer , in gelöster oder suspendierter Form voriiegender Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere einer oder mehrerer α-Hydroxycar¬ bonsäuren der allgemeinen Formel1. Cosmetic or dermatological preparations containing an effective amount of one or more oligomers and / or polymers and / or co-oligomers and / or co-polymers of one or more α-hydroxycarboxylic acids of the general formula present in dissolved or suspended form
untereinander verknüpft sind, wobei jeweils R' und R" unabhängig voneinander gewählt werden aus der Gruppe (a1) H- , are linked to one another, R 'and R "being selected independently of one another from the group (a1) H-,
(a2) verzweigtes oder unverzweigtes C-|_25-Alkyl-,(a2) branched or unbranched C- | _25-alkyl,
(a3) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Al¬ dehydgruppen und/oder Oxogruppen (Ketogruppen) substituiertes verzweigtes oder unverzweigtes C-|_25-Alkyl- (a4) Phenyl-,(a3) branched or unbranched C- | _25-alkyl- (a4) phenyl-, substituted with one or more carboxyl groups and / or hydroxyl groups and / or aldehyde groups and / or oxo groups (keto groups),
(a5) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder ver¬ zweigten und/oder unverzweigten C-|_25-Alkylgruppen substituiertes Phenyl-, oder wobei das α-Kohlenstoffatom der α-Hydroxycarbonsäure mit R* und R" zusammen eine (a6) unsubstituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen oder eine (a7) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Oxo¬ gruppen (Ketogruppen) und/oder verzweigten und/oder unverzweigten C1.25- Alkylgruppen substituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen ausbildet, wobei die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 4 beträgt, und wobei R' und R" der einzelnen α-Hydroxycarbonsäureeinheiten innerhalb eines Co-Oli- gomers bzw. Co-Polymers zu R' bzw. R" einer oder mehrerer α-Hydroxycarbonsäureeinhei¬ ten desselben Co-Oligomers bzw. Co-Polymers identisch oder auch verschieden sein kann. (a5) with one or more carboxyl groups and / or hydroxyl groups and / or branched and / or unbranched C- | _25-alkyl groups substituted phenyl, or wherein the α-carbon atom of the α-hydroxycarboxylic acid with R * and R "together is an (a6) unsubstituted cycloalkyl group with 3 to 7 ring atoms or (a7) with one or more carboxyl groups and / or hydroxyl groups and / or oxo groups (keto groups) and / or branched and / or unbranched C1.25-alkyl groups which form substituted cycloalkyl groups with 3 to 7 ring atoms, the number n representing at least 40% by weight of the oligomers and / or polymers used and / or co-oligomers and / or co-polymers is at least 4, and wherein R 'and R "of the individual α-hydroxycarboxylic acid units within a co-oligomer or co-polymer to R' or R" are one or more α-Hydroxycarboxylic acid units of the same co-oligomer or co-polymer may be identical or different.
2. Verwendung einer wirksamen Menge eines oder mehrerer Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere einer oder mehrerer α-Hydroxycarbonsäuren der allgemeinen Formel2. Use of an effective amount of one or more oligomers and / or polymers and / or co-oligomers and / or co-polymers of one or more α-hydroxycarboxylic acids of the general formula
R" I R'-C— COOH I OH welche gemäß dem SchemaR "I R'-C- COOH I OH which according to the scheme
untereinander verknüpft sind, wobei jeweils R' und R" unabhängig voneinander gewählt werden aus der Gruppe (a1) H- , are linked to one another, R 'and R "being selected independently of one another from the group (a1) H-,
(a2) verzweigtes oder unverzweigtes C-|_25-Alkyl-,(a2) branched or unbranched C- | _25-alkyl,
(a3) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Al¬ dehydgruppen und/oder Oxogruppen (Ketogruppen) substituiertes verzweigtes oder unverzweigtes C-j_25-Alkyl- (a4) Phenyl-,(a3) with one or more carboxyl groups and / or hydroxyl groups and / or Al¬ dehydgruppen and / or oxo groups (keto groups) substituted branched or unbranched C j _25-alkyl (a4) phenyl,
(a5) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder ver¬ zweigten und/oder unverzweigten C-|_25-Alkylgruppen substituiertes Phenyl-, oder wobei das α-Kohlenstoffatom der α-Hydroxycarbonsäure mit R' und R" zusammen eine (a6) unsubstituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen oder eine (a7) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Oxo¬ gruppen (Ketogruppen) und/oder verzweigten und/oder unverzweigten C-|_25- Alkylgruppen substituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen ausbildet, wobei die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 4 beträgt, und wobei R' und R" der einzelnen α-Hydroxycarbonsäureeinheiten innerhalb eines Co-Oli- gomers bzw. Co-Polymers zu R' bzw. R" einer oder mehrerer α-Hydroxycarbonsäureeinhei¬ ten desselben Co-Oligomers bzw. Co-Polymers identisch oder auch verschieden sein kann, zur Darreichung der zugrundeliegenden monomeren α-Hydroxycarbonsäuren in kosmeti¬ schen oder dermatologischen Zubereitungen. (a5) with one or more carboxyl groups and / or hydroxyl groups and / or branched and / or unbranched C- | _25-alkyl groups substituted phenyl, or where the α-carbon atom of the α-hydroxycarboxylic acid with R 'and R "together is an (a6) unsubstituted cycloalkyl group with 3 to 7 ring atoms or one (a7) with one or more carboxyl groups and / or hydroxyl groups and / or oxo groups (keto groups) and / or branched and / or unbranched C 1-25 alkyl groups, substituted cycloalkyl groups with 3 to 7 ring atoms, the number n representing at least 40% by weight of the oligomer (s) used and / or Polymers and / or co-oligomers and / or co-polymers is at least 4, and where R 'and R "of the individual α-hydroxycarboxylic acid units within a co-oligomer or co-polymer to R' or R" is one or of several α-hydroxycarboxylic acid units of the same co-oligomer or co-polymer may be identical or different, for the purpose of providing the underlying monomeric α-hydroxycarboxylic acids in cosmetic or dermatological preparations.
3. Zubereitungen nach Anspruch 1 oder Verwendung nach Anspruch 2, dadurch gekenn¬ zeichnet, daß die den Oligomeren, Polymeren, Co-Oligomeren und/oder Co-Polymeren zugrundeliegenden α-Hydroxycarbonsäuren gewählt werden aus der Gruppe der α- Hydroxyalkansäuren.3. Preparations according to claim 1 or use according to claim 2, characterized gekenn¬ characterized in that the oligomers, polymers, co-oligomers and / or copolymers underlying α-hydroxycarboxylic acids are selected from the group of α-hydroxyalkanoic acids.
4. Zubereitungen nach Anspruch 1 oder Verwendung nach Anspruch 2, dadurch ge¬ kennzeichnet, daß die den Oligomeren, Polymeren, Co-Oligomeren und/oder Co-Polymeren zugrundeliegenden α-Hydroxycarbonsäuren gewählt werden aus der Gruppe Glycolsäure, Milchsäure, α-Hydroxybuttersäure und α-Hydroxyvaleriansäure.4. Preparations according to claim 1 or use according to claim 2, characterized ge indicates that the oligomers, polymers, co-oligomers and / or copolymers underlying α-hydroxycarboxylic acids are selected from the group glycolic acid, lactic acid, α-hydroxybutyric acid and α-hydroxyvaleric acid.
5. Zubereitungen nach Anspruch 1 oder Verwendung nach Anspruch 2, dadurch ge¬ kennzeichnet, daß die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligo¬ mere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 8, bevorzugt 10 beträgt.5. Preparations according to claim 1 or use according to claim 2, characterized ge indicates that the number n for at least 40 wt .-% of the oligo or mers used and / or polymers and / or co-oligomers and / or co- Polymer is at least 8, preferably 10.
6. Zubereitungen oder Verwendung nach Anspruch 5, dadurch gekennzeichnet, daß n maximal 100.000 beträgt.6. Preparations or use according to claim 5, characterized in that n is a maximum of 100,000.
7. Zubereitungen nach Anspruch 1 oder Verwendung nach Anspruch 2, dadurch ge¬ kennzeichnet, daß die Oligomere, Polymere, Co-Oligomere und/oder Co-Polymere in Kom¬ bination mit einer oder mehreren kosmetisch oder pharmazeutisch unbedenklichen Substanzen der allgemeinen Strukturformel7. Preparations according to claim 1 or use according to claim 2, characterized ge indicates that the oligomers, polymers, co-oligomers and / or copolymers in combination with one or more cosmetically or pharmaceutically acceptable substances of the general structural formula
AOOC-X-COOB vorliegen, wobei X gewählt wird aus der Gruppe der mit bis zu vier Carbonsäuregruppen und bis zu vier Carbonsäurealkylestergruppen -COOD, -COOE, -COOF und bis zu vier Hy¬ droxygruppen substituierten verzweigten und oder unverzweigten Alkylengruppen mit bis zu sechs C-Atomen und der Gruppe der mit bis zu vier Carbonsäuregruppen und bis zu vier Carbonsäurealkylestergruppen -COOD, -COOE, -COOF und bis zu vier Hydroxygruppen substituierten Arylengruppen der TypenAOOC-X-COOB are present, where X is selected from the group consisting of up to four carboxylic acid groups and up to four carboxylic acid alkyl ester groups -COOD, -COOE, -COOF and up to four hydroxy groups, branched and or unbranched alkylene groups with up to six C-atoms and the group of the types substituted with up to four carboxylic acid groups and up to four carboxylic acid alkyl ester groups -COOD, -COOE, -COOF and up to four hydroxyl groups
8. Zubereitungen oder Verwendung nach Anspruch 7, dadurch gekennzeichnet, daß als Substanz der allgemeinen Strukturformel AOOC-X-COOB das Triethylcitrat gewählt wird.8. Preparations or use according to claim 7, characterized in that the triethyl citrate is chosen as the substance of the general structural formula AOOC-X-COOB.
9. Verfahren zu Stabilisierung einer wirksamen Menge eines oder mehrerer Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere einer oder mehrerer α- Hydroxycarbonsäuren der allgemeinen Formel9. Process for stabilizing an effective amount of one or more oligomers and / or polymers and / or co-oligomers and / or co-polymers of one or more α-hydroxycarboxylic acids of the general formula
untereinander verknüpft sind, wobei jeweils R' und R" unabhängig voneinander gewählt werden aus der Gruppe are linked to one another, R 'and R "being selected independently of one another from the group
(a1) H- ,(a1) H-,
(a2) verzweigtes oder unverzweigtes C-ι_25-Alkyl-,(a2) branched or unbranched C 1-25 alkyl,
(a3) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Al¬ dehydgruppen und/oder Oxogruppen (Ketogruppen) substituiertes verzweigtes oder unverzweigtes C-j_25-Alkyl-dehydgruppen (a3) with one or more carboxyl groups and / or hydroxyl groups and / or Al¬ and / or oxo groups (keto groups) substituted branched or unbranched C j _25 alkyl
(a4) Phenyl-,(a4) phenyl,
(a5) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder ver¬ zweigten und/oder unverzweigten C-j_25-Alkylgruppen substituiertes Phenyl-, oder wobei das α-Kohlenstoffatom der α-Hydroxycarbonsäure mit R' und R" zusammen eine(a5) with one or more carboxyl groups and / or hydroxyl groups and / or ver¬ branched and / or unbranched C j _25-alkyl substituted phenyl, or wherein the α-carbon atom of the α-hydroxycarboxylic acid having R 'and R "together form a
(a6) unsubst'rtuierte Cycloalkylgruppe mit 3 bis 7 Ringatomen oder eine(a6) unsubst 'rtuierte cycloalkyl group having 3 to 7 ring atoms or a
(a7) mit einer oder mehreren Carboxylgruppen und/oder Hydroxygruppen und/oder Oxo¬ gruppen (Ketogruppen) und/oder verzweigten und/oder unverzweigten C-j.25- Alkylgruppen substituierte Cycloalkylgruppe mit 3 bis 7 Ringatomen ausbildet, wobei die Zahl n für mindestens 40 Gew.-% des oder der eingesetzten Oligomere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere mindestens 4 beträgt, und wobei R' und R" der einzelnen α-Hydroxycarbonsäureeinheiten innerhalb eines Co-Oli- gomers bzw. Co-Polymers zu R' bzw. R" einer oder mehrerer α-Hydroxycarbonsäureeinhei¬ ten desselben Co-Oligomers bzw. Co-Polymers identisch oder auch verschieden sein kann, in kosmetischen oder dermatologischen Zubereitungen gegen Depolymerisation bzw. Deoligomerisation durch Hydrolyse, dadurch gekennzeichnet, daß die eingesetzten Oligo¬ mere und/oder Polymere und/oder Co-Oligomere und/oder Co-Polymere in eine Ölphase eingearbeitet werden. (a7) with one or more carboxyl groups and / or hydroxyl groups and / or oxo groups (keto groups) and / or branched and / or unbranched Cj.25-alkyl groups substituted cycloalkyl group with 3 to 7 ring atoms, the number n being at least 40 % By weight of the oligomer and / or polymer and / or co-oligomer and / or co-polymer used is at least 4, and where R 'and R "of the individual α-hydroxycarboxylic acid units within a co-oligomer or co-polymer are identical to R' or R" of one or more α-hydroxycarboxylic acid units of the same co-oligomer or co-polymer or can also be different, in cosmetic or dermatological preparations against depolymerization or deoligomerization by hydrolysis, characterized in that the oligomers and / or polymers and / or co-oligomers and / or copolymers used are incorporated into an oil phase.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19520237.6 | 1995-06-02 | ||
| DE1995120237 DE19520237A1 (en) | 1995-06-02 | 1995-06-02 | Cosmetic or dermatological preparations containing oligomers or polymers of alpha-hydroxycarboxylic acids |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1996038125A1 true WO1996038125A1 (en) | 1996-12-05 |
Family
ID=7763491
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP1996/002354 WO1996038125A1 (en) | 1995-06-02 | 1996-05-31 | COSMETIC OR DERMATOLOGICAL COMPOSITIONS CONTAINING OLIGOMERS OR POLYMERS OF α-HYDROXYCARBOXYLIC ACIDS |
Country Status (2)
| Country | Link |
|---|---|
| DE (1) | DE19520237A1 (en) |
| WO (1) | WO1996038125A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0831767A1 (en) * | 1995-06-07 | 1998-04-01 | Ruey J. Dr. Yu | Alpha hydroxyacid esters for skin aging |
| EP0835651A2 (en) * | 1996-10-11 | 1998-04-15 | L'oreal | Oil in water cosmetic emulsion with high electrolyte content |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19711244A1 (en) * | 1997-03-18 | 1998-10-08 | Beiersdorf Ag | Light protection agent combination forming stable emulsion, especially oil-in-water emulsion |
| DE19713776A1 (en) * | 1997-04-03 | 1998-10-08 | Beiersdorf Ag | Light protection preparations with a content of basic amino acids and water-soluble UV filter substances |
| DE19714765A1 (en) * | 1997-04-10 | 1998-10-15 | Merck Patent Gmbh | Use of low molecular weight, oligomeric esters of alpha-hydroxy acids and / or aromatic o-hydroxy acids in cosmetic formulations |
| PT1216021E (en) * | 1999-09-15 | 2005-07-29 | Bayer Consumer Care Ag | PHARMACEUTICAL AND / OR COSMETIC COMPOSITIONS |
| WO2001022926A1 (en) * | 1999-09-30 | 2001-04-05 | L'oreal | Cosmetic composition comprising nanoparticles containing alumina |
| US20070027105A1 (en) | 2005-07-26 | 2007-02-01 | Alza Corporation | Peroxide removal from drug delivery vehicle |
| WO2008066642A2 (en) | 2006-11-03 | 2008-06-05 | Durect Corporation | Transdermal delivery systems comprising bupivacaine |
| CA3167217A1 (en) | 2020-01-13 | 2021-07-22 | Durect Corporation | Sustained release drug delivery systems with reduced impurities and related methods |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4363815A (en) * | 1975-07-23 | 1982-12-14 | Yu Ruey J | Alpha hydroxyacids, alpha ketoacids and their use in treating skin conditions |
| EP0413528A1 (en) * | 1989-08-15 | 1991-02-20 | Ruey J. Dr. Yu | Amphoteric compositions and polymeric forms of alpha hydroxyacids, and their therapeutic use |
| EP0447318A1 (en) * | 1990-03-16 | 1991-09-18 | L'oreal | Composition for the cosmetic and/or pharmaceutical treatment of the surface epidermal layers by topical application on the skin and the relative production process |
| EP0514067A1 (en) * | 1991-05-07 | 1992-11-19 | Unilever Plc | Cosmetic composition |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TW247878B (en) * | 1991-07-02 | 1995-05-21 | Takeda Pharm Industry Co Ltd | |
| EP0637450A3 (en) * | 1993-08-04 | 1995-04-05 | Collagen Corp | Composition for revitalizing scar tissue. |
-
1995
- 1995-06-02 DE DE1995120237 patent/DE19520237A1/en not_active Withdrawn
-
1996
- 1996-05-31 WO PCT/EP1996/002354 patent/WO1996038125A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4363815A (en) * | 1975-07-23 | 1982-12-14 | Yu Ruey J | Alpha hydroxyacids, alpha ketoacids and their use in treating skin conditions |
| EP0413528A1 (en) * | 1989-08-15 | 1991-02-20 | Ruey J. Dr. Yu | Amphoteric compositions and polymeric forms of alpha hydroxyacids, and their therapeutic use |
| EP0447318A1 (en) * | 1990-03-16 | 1991-09-18 | L'oreal | Composition for the cosmetic and/or pharmaceutical treatment of the surface epidermal layers by topical application on the skin and the relative production process |
| EP0514067A1 (en) * | 1991-05-07 | 1992-11-19 | Unilever Plc | Cosmetic composition |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0831767A1 (en) * | 1995-06-07 | 1998-04-01 | Ruey J. Dr. Yu | Alpha hydroxyacid esters for skin aging |
| EP0831767A4 (en) * | 1995-06-07 | 2000-05-17 | Yu Ruey J | Alpha hydroxyacid esters for skin aging |
| EP0835651A2 (en) * | 1996-10-11 | 1998-04-15 | L'oreal | Oil in water cosmetic emulsion with high electrolyte content |
| EP0835651A3 (en) * | 1996-10-11 | 2005-04-13 | L'oreal | Oil in water cosmetic emulsion with high electrolyte content |
Also Published As
| Publication number | Publication date |
|---|---|
| DE19520237A1 (en) | 1996-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE69110070T2 (en) | Composition for the cosmetic and / or pharmaceutical treatment of the uppermost epidermis layer by topical application on the skin and the corresponding manufacturing process. | |
| EP0695161B1 (en) | Cosmetic and medicinal topical preparations | |
| DE60303429T2 (en) | Cosmetic and / or dermatological use of a composition containing at least one oxidation-sensitive, hydrophilic active ingredient stabilized by at least one maleic anhydride copolymer | |
| EP0633017B1 (en) | Cosmetic and dermatologic compositions containing delta-aminolevulinic acid | |
| EP0802783B1 (en) | Topical preparations containing l-arginine | |
| EP0610926A1 (en) | Topical compositions for protection against stinging animals | |
| EP0801946A2 (en) | Use of salicin as agent against irritation in cosmetic and topical dermatological compositions | |
| EP0615748A1 (en) | Galenical matrices preferably in microsphere form | |
| WO1995015147A1 (en) | Use of l-arginine, l-ornithine or l-citrulline and topical preparations with these substances | |
| WO1996002223A1 (en) | Emulsifier-free, finely dispersed cosmetic or dermatological oil-in-water type preparations | |
| WO1998015255A1 (en) | Cosmetic or dermatological microemulsions | |
| EP0716847A1 (en) | Cosmetic or dermatologic compositions containing cinnamic acid derivatives and a flavonoid | |
| EP0687467A2 (en) | Cosmetic and dermatological combinations containing glycerylalkylethers as active ingredients | |
| EP0770379A2 (en) | Water in oil cosmetic or dermatological emulsifier-free finely dispersed composition | |
| EP0701814A2 (en) | Cosmetic or dermatologic composition containing hydrophobised inorganic pigment to maintain the amount of the skin urocanic acid | |
| DE4423450C2 (en) | Use of inorganic pigments in cosmetic or dermatological preparations containing alpha-hydroxycarboxylic acids and / or alpha-ketocarboxylic acids | |
| EP1049454B1 (en) | Cosmetic and dermatological preparations containing higher electrolyte concentrations | |
| WO1996038125A1 (en) | COSMETIC OR DERMATOLOGICAL COMPOSITIONS CONTAINING OLIGOMERS OR POLYMERS OF α-HYDROXYCARBOXYLIC ACIDS | |
| WO1995017160A2 (en) | Cosmetic or dermatological oil-in-water emulsions containing amino acids and inorganic pigments | |
| EP0850056A1 (en) | Topical anti-skin-cancer preparations | |
| DE10128910A1 (en) | Combination of arginine and ascorbic acid is used in the production of cosmetic or dermatological compositions for tightening and/or strengthening the skin, especially in cellulite treatment | |
| EP0801945A2 (en) | Use of ferulic acid glucosides as agents against irritation in cosmetic or topical dermatological compositions | |
| WO2002066007A1 (en) | Gel emulsions in the form of o/w emulsions having a hydrocolloid content | |
| DE60035613T2 (en) | Topical, cosmetic and pharmaceutical compositions containing specific alkoxylated diesters of fumaric acid | |
| EP0955038B1 (en) | Cosmetic and dermatologic compositions containing ceramids |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): JP US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LU MC NL PT SE |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| 122 | Ep: pct application non-entry in european phase |