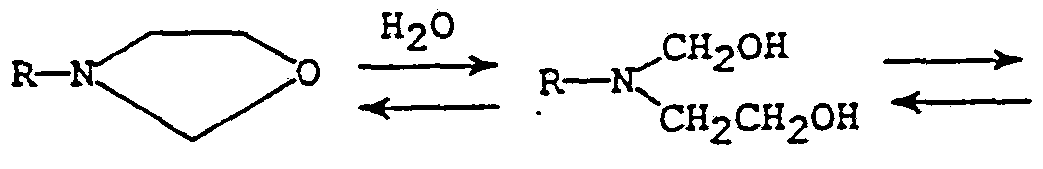WO1992013907A1 - Thermally stable curable hindered isocyanate-oxazolidine composition - Google Patents
Thermally stable curable hindered isocyanate-oxazolidine composition Download PDFInfo
- Publication number
- WO1992013907A1 WO1992013907A1 PCT/US1992/000176 US9200176W WO9213907A1 WO 1992013907 A1 WO1992013907 A1 WO 1992013907A1 US 9200176 W US9200176 W US 9200176W WO 9213907 A1 WO9213907 A1 WO 9213907A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- oxazolidine
- group
- compound
- composition
- groups
- Prior art date
Links
- 0 *NC1COCC1 Chemical compound *NC1COCC1 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/08—Processes
- C08G18/10—Prepolymer processes involving reaction of isocyanates or isothiocyanates with compounds having active hydrogen in a first reaction step
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/62—Polymers of compounds having carbon-to-carbon double bonds
- C08G18/6283—Polymers of nitrogen containing compounds having carbon-to-carbon double bonds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/74—Polyisocyanates or polyisothiocyanates cyclic
- C08G18/76—Polyisocyanates or polyisothiocyanates cyclic aromatic
- C08G18/7614—Polyisocyanates or polyisothiocyanates cyclic aromatic containing only one aromatic ring
- C08G18/7628—Polyisocyanates or polyisothiocyanates cyclic aromatic containing only one aromatic ring containing at least one isocyanate or isothiocyanate group linked to the aromatic ring by means of an aliphatic group
- C08G18/765—Polyisocyanates or polyisothiocyanates cyclic aromatic containing only one aromatic ring containing at least one isocyanate or isothiocyanate group linked to the aromatic ring by means of an aliphatic group alpha, alpha, alpha', alpha', -tetraalkylxylylene diisocyanate or homologues substituted on the aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J175/00—Adhesives based on polyureas or polyurethanes; Adhesives based on derivatives of such polymers
- C09J175/04—Polyurethanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2170/00—Compositions for adhesives
- C08G2170/20—Compositions for hot melt adhesives
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2190/00—Compositions for sealing or packing joints
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2666/00—Composition of polymers characterized by a further compound in the blend, being organic macromolecular compounds, natural resins, waxes or and bituminous materials, non-macromolecular organic substances, inorganic substances or characterized by their function in the composition
- C08L2666/02—Organic macromolecular compounds, natural resins, waxes or and bituminous materials
Definitions
- the invention relates to improved oxazolidine- isocyanate compositions that can be cured in the presence of moisture under hot melt conditions to form chain extended and/or crosslinked materials.
- the compositions can be used as adhesives, sealants, and in other end uses where hydrocurable or moisture curing hot melt compositions can be cured into a functional layer or coating.
- the isocyanate compounds are typically used in small molecule or monomer forms or in the form of an isocyanate-terminated (-NC0) prepolymer having at least two available -NCO groups.
- the ring of the oxazolidine compound can open when combined with water to lose one mole of a carbonyl compound according to the following generalized reaction (I): RNHCH 2 CH 2 OH + CH 2 (O)
- the reactants are typically combined before using, isolated from any activating conditions, and then later activated, by moisture from the atmosphere or from an intentionally added moisture source.
- oxazolidines have a high rate of physical property development because the rate of reaction between water and the oxazolidine is faster than the rate of reaction between water and the isocyanate, thereby resulting in strong bonds being formed between the resultant active hydrogens on the -OH and -NH groups and the isocyanate groups; (2) have reduced carbon dioxide bubble formation because of the preferential reaction of water with oxazolidine over an -NCO group; and (3) have lower levels of free isocyanate but achieve the same level of crosslinking and ultimate physical strength, particularly if an oxazolidine with reactive pendant functional groups is employed.
- This trimerization reaction in the oxazolidine-isocyanate systems is a particular problem because of the presence of one or more tertiary amine groups in the oxazolidines.
- a tertiary amine group is an excellent catalyst for the trimerization reaction and promotes rapid formation of the isocyanurate structure.
- isocyanurates are particularly undesirable because the "pot life", i.e., stability, of the oxazolidine-isocyanate compositions at elevated temperatures is substantially reduced with isocyanurate formation. Furthermore, the cured isocyanate adhesive bond, coating, caulk, or other structure is weakened due to the network formation and decreased elastomeric properties resulting from isocyanurate formation. Also, fewer isocyanate groups are available for crosslinking with the amino and hydroxy moieties formed upon the water-driven ring opening of the oxazolidine compounds.
- aromatic isocyanates which trimerize rapidly in the presence of a tertiary amine, have been found to be commercially unsuitable for use in combination with such tertiary amines as oxazolidine compounds to make hydrocurable systems. While the trimerization reaction of aliphatic isocyanates in the presence of tertiary amines is slower than the rate of aromatic isocyanates, the use of aliphatic isocyanates in combination with oxazolidines is also precluded at the temperatures of typical hot melt systems because the trimerization reaction is enhanced at such temperatures.
- Polyurethane hydrocurable systems containing certain oxazolidine compositions and polyfunctional aliphatic or aromatic isocyanates suitable for coatings, adhesives, and other applications are well known and disclosed in, for example, U.S. Patent Nos. 3,661,923; 3,743,626; 3,912,691; 4,024,117; 4,032,686; 4,101,527; 4,118,376; 4,138,545; and 4,471,102. Certain of these systems are disclosed as having poor thermal stability; relatively poor flexibility, weatherability, and abrasion resistance; and objectionable color, especially when used as coatings.
- U.S. Pat. No. 4,879,365 is disclosed in U.S. Pat. No. 4,879,365 as providing improved coatings having good color, abrasion resistance, and weatherability.
- the composition includes at least one oxazolidine ring covalently bonded through an alkylene-urethane linking group to a prepolymer radical containing tetramethylxylenediisocyanate (TMXDI) .
- TXDI tetramethylxylenediisocyanate
- the oxazolidine- isocyanate compound is produced from a hydroxy oxazolidine compound and a polycarbonate, polyester, or polyether tetramethylxylene diisocyanate prepolymer containing at least two isocyanate groups, in the presence of a solvent.
- One aspect of the invention is a "one pot" hydrocurable, hot melt, composition
- a "one pot" hydrocurable, hot melt, composition comprising an oxazolidine compound and a sterically hindered aliphatic polyfunctional isocyanate compound or an isocyanate- terminated prepolymer made from the sterically hindered polyfunctional isocyanate compound.
- a second aspect of the invention comprises a one component moisture curing material containing the hindered isocyanate compound or prepolymer, the oxazolidine compound, and a thermoplastic resin along with other optional ingredients including tackifiers, plasticizers, etc.
- a third aspect of the invention is a method of using moisture curing material containing a hindered isocyanate-terminated prepolymer or isocyanate monomer and the oxazolidine compound at elevated temperatures in hot melt formulations used as an adhesive or sealant.
- Another aspect of the invention comprises a vinyl unsaturated monomer having a hindered isocyanate end group bonded to an oxazolidine group, polymers made from monomers with such pendant groups, and curable systems using a curable polymer with a pendant isocyanate group, an epoxy group, or mixtures thereof.
- the thermally stable, hydrocurable, hot melt compositions of the invention comprise an oxazolidine compound, that can react with water to release an active hydrogen compound, and a sterically hindered isocyanate compound, typically in the form of a small molecule hindered isocyanate or an isocyanate-terminated prepolymer composition.
- a sterically hindered isocyanate compound typically in the form of a small molecule hindered isocyanate or an isocyanate-terminated prepolymer composition.
- Such hydrocurable compositions can take the form of adhesives, sealants, and other typical end uses for hydrocurable, hot melt materials. Adhesives and sealants are the preferred end uses, however.
- the present invention provides for an efficient production of oxazolidine- isocyanate polymeric materials that are substantially free of tri er isocyanurate impurities.
- the oxazolidine-isocyanurate polymeric materials are produced by the process of the invention such that there is less than about 1 wt-% of the isocyanurate compound present.
- Oxazolidines are typically defined as a class of five- or six-membered heterocyclic compounds containing nitrogen and oxygen functionality.
- the term "oxazolidine” includes five- membered ring oxazolidines, six-membered ring tetrahydro- oxazines, and cyclic, bicyclic, polycyclic, etc., compounds.
- the oxazolidine ring Upon exposure to water, at a bond site where curing is desirable, the oxazolidine ring reacts with water and the ring opens to provide reactive (active hydrogen containing) amino and hydroxy moieties. Thereby, an active hydrogen atom is provided for reaction with a sterically hindered isocyanate compound.
- oxazolidine compounds contain at least a secondary amino nitrogen and typically contain a tertiary amino nitrogen. Tertiary amino nitrogens are particularly troublesome, as was explained above, because they can catalyze trimerization of the isocyanates into undesirable isocyanurates, particularly at the temperatures of the typical hot melt process.
- this problem has been solved by the use of sterically hindered isocyanate compositions of the present invention.
- oxazolidines can be used in the compositions of the invention. This includes, for example, aliphatic and aromatic monocyclic and bicyclic oxazolidines, monofunctional ester oxazolidines, polyfunctional polyol ester oxazolidines, polyfunctional polycarboxylic ester oxazolidines, polymers and copolymers of oxazolidinylalkyl acrylates and methacrylates, and polyfunctional oxazolidine-terminated polyesters as disclosed in U.S. Patent No. 4,138,545.
- the oxazolidines are monocyclic and bicyclic, and more preferably, they are bicyclic.
- Monofunctional oxazolidine compounds are generally represented by the following formula:
- R is an organic radical
- R 1 is a hydrogen atom, a phenyl group, a benzyl group, or a C-. 12 alkyl group
- R 2 is a hydrogen atom or a C ⁇ alkyl group
- R 1 and R 2 when taken together with the attached carbon atom, can be representative of a saturated cyclic carbon ring having five or more carbon atoms
- Y represents the group:
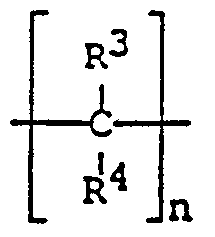
- R 3 and R 4 are independently hydrogen atoms, C-_ X2 alkyl groups, C 6 . 10 aryl groups, or C-_ 12 alkyl substituted a yl groups or aryl substituted alkyl groups, and n is an integer of 2 or 3.
- bicyclic oxazolidine compounds useful in the compositions of the invention are generally characterized by a fused-ring system having a single nitrogen atom common to both rings. They can be represented by the following formula:
- R 5 is the same as described for R 1 and R 2 above, Y is similarly described as above, and R 6 can be hydrogen, alkyl, hydroxyalkyl, aminoalkyl, etc.
- R 6 can be hydrogen, alkyl, hydroxyalkyl, aminoalkyl, etc.
- These compounds are generally formed from a primary amine containing at least two C-hydroxyalkyl groups and a carbonyl group.
- Known carbonyl precursors include, for example, formaldehyde, acetaldehyde, butyraldehyde, and others as disclosed in U.S. Patent No. 4,101,527.
- Oxazolidine compounds that contain no pendant reactive functional groups prior to the ring-opening reaction are preferred for certain compositions of the invention. These include groups such as halogen substituents, alkoxy substituents, nitro groups, and others. These compounds typically function as reactive diluents. Examples of such compounds are those bicyclic oxazolidines prepared from primary amines
- Oxazolidines having no available active hydrogens prior to reaction with water can be used to advantage because such compositions generally have greater stability or "pot life", especially at elevated temperatures when compared to oxazolidines with active hydrogen groups.
- the oxazolidine compounds can contain active hydrogen- containing substituents for certain other advantageous applications of the invention.
- active hydrogen we mean hydrogen atoms present in groups such as primary and secondary amino groups, carboxylic acid groups, hydroxy groups, and sulfhydryl groups, which readily undergo reaction with electrophilic reagents such as isocyanates . These groups can be used to link the oxazolidine nucleus to form prepolymers, monomers, or other active materials containing the oxazolidine nucleus.
- An example of an oxazolidine containing a reactive pendant group is one that results from the reaction product of diethanolamine and a carbonyl compound.
- oxazolidine compounds are those generally prepared by the reaction of a ⁇ - aminoalcohol and a carbonyl compound, such as a ketone or aldehyde. Because of facile trimerization of aliphatic isocyanates, oxazolidines prepared from diethanolamine are a particularly preferred group of compounds. These compounds can be generally represented by the following formula:
- each R' is independently a hydrogen, lower alkyl (i.e., C-_ 6 alkyl), substituted lower alkyl of 1 to 12 carbon atoms, cycloalkyl, and phenyl or substituted phenyl.
- the substitution of a 3-hydroxyalkyl group for an alkyl group on an amine nitrogen is known to result in a decrease in the base strength of the amine by approximately 1 pK a unit. Therefore, the substitution of a .-hydroxyalkyl group for an alkyl group on the nitrogen atom of the oxazolidine decreases the base strength of the amine which decreases the rate of the trimerization reaction of the isocyanates.
- Bicyclic oxazolidines are particularly preferred over monocyclic oxazolidines. This is because, at least in part, the bicyclic nature of these oxazolidines provides, minimally, a functionality of three with the high likelihood of additional reactive sites leading to extensive crosslinking in the final polymer formed. Furthermore, bicyclic oxazolidines are preferred over monocyclic oxazolidines because they are readily incorporated into the system by simple mixing or blending. There is a wide range of stoichiometries possible between the isocyanate terminated prepolymer and the bicyclic oxazolidine. The bicyclic oxazolidine are relatively weak bases and are stable in the presence of isocyanate terminated prepolymers.
- aliphatic isocyanate compound means that the -NCO group is attached to an aliphatic carbon.
- the sterically hindered organic isocyanate compounds of the present invention include organic compounds containing one or more isocyanate groups (-NC0) wherein the nitrogen atom of the isocyanate compound is directly attached to a tertiary aliphatic carbon atom.
- the tertiary carbon atom can be a carbon atom in a branched or unbranched tertiary aliphatic group and can also be an aliphatic ring carbon atom. These aliphatic carbon atoms can also be substituents on aromatic ring systems. Examples of such a tertiary bonded isocyanate group are shown in the following formulas:
- R is a C-_ 12 alkyl, aryl, etc., group.
- the above formulas show, respectively, the isocyanate group attached to a tertiary alkyl carbon, and within a ring system having a tertiary carbon (the unfilled valences representing the ring) .
- the tertiary carbon or ring carbon can be a portion of an inorganic compound having virtually any structure or substituent.
- Hindered isocyanate compounds for use in this invention also include compounds wherein the tertiary carbon of the hindered isocyanate substituent is attached to an aliphatic or aromatic ring structure represented by the following formulas:
- R is a C-_ 12 alkyl, aryl, etc., group
- the preferred compounds are isocyanate compounds formed on aromatic ring structures wherein the hindered isocyanate group can be substituted meta, ortho, or para to a vinyl group or to a second hindered isocyanate group.
- Such compounds include hindered aromatic diisocyanate compounds and vinyl-substituted hindered onoisocyanate aromatic monomers.
- Representative examples of the most preferred isocyanate compounds comprises m-TMI and m-TMXDI [known as 3-isopropenyl( ⁇ , ⁇ -dimethylbenzyl)isocyanate and l,3-bis( ⁇ - isocyanatoisopropyl)benzene, respectively] .
- the preparation of these compounds is disclosed in U.S. Patent No. 4,377,530.
- the isocyanate compound referred to as - TMXDI is also known as tetramethylxylenediisocyanate.
- TMXDI tetramethylxylenediisocyanate.
- para isomers of these compounds i.e., 4-isopropenyl( ⁇ , ⁇ - dimethylbenzyl)isocyanate and l,4-bis-( ⁇ - isocyanatoisopropyl)benzene, respectively.
- Most preferred are the hindered aromatic diisocyanate compounds.
- compositions of the invention comprising an oxazolidine compound and a
- ET sterically hindered polyfunctional isocyanate small molecule or an isocyanate-terminated prepolymer made from the sterically hindered polyfunctional isocyanate compound the preferred isocyanate is one having at least two -NCO groups. This is similarly so for the one component moisture curing material containing the hindered isocyanate compound or prepolymer, the oxazolidine compound, and a thermoplastic resin along with other optional ingredients including tackifiers, plasticizers, etc.
- the preferred isocyanate is an aromatic compound with the hindered isocyanate group substituted meta, ortho, or para to a vinyl group.
- Prepolymers useful in manufacturing the oxazolidine isocyanate compounds of the invention often are prepolymers formed in the reaction of a polyol compound and the sterically hindered isocyanate compound.
- Polyols useful in making the prepolymers of the invention are organic compounds having a hydroxy functionality of 2 to 4, preferably 2 to 3, which are prepared by well known preparatory techniques.
- polyol compounds useful in the invention are polyoxyalkylene polyol compounds including polyoxyethylene glycols, polyoxypropylene glycols, etc. , polyester polyol compounds prepared by methods well known in the art including polymerization of ring-like "tones" such as caprolactone, butyrolactone, or valerolactone or by reacting a dibasic acid such as phthlatic acid or 1,6- hexane dicarboxylic acid with a diol such as ethylene diol or 1,4-butanediol. Polyester and polyether polyols of the invention usually have a molecular weight of between about
- the reaction mixture used to prepare the isocyanate prepolymer for use in the hydrocurable compounds of the invention typically comprises an excess of isocyanate group for reaction with the hydroxy functionality of the polyester or polyether polyol.
- sufficient isocyanate concentration is used to react with essentially all available hydroxy functionality on the polyol forming the isocyanate prepolymer.
- Equivalent weight ratio of diisocyanate to the polyol includes greater than about 1.0 to 3.5 parts of isocyanate to polyol, preferably from about 1.5 to 3 parts isocyanate per part of polyol and is preferably about 2 parts isocyanate per part of polyol.
- Suitable reaction temperatures are usually between about 75°C and 150°C.
- the reaction time is usually between about 1 hour and 8 hours.
- a catalyst such as dibutyltin dilaurate, or the like, is present in the reaction mixture to reduce the time for preparing the prepolymer.
- the isocyanate and oxazolidines are combined in 100% solids systems under typical hot melt conditions.
- the reaction temperature is generally within the range of about 37°C to 175°C, preferably within about 60°C to 80°C.
- the ratio of isocyanate to oxazolidine in the compositions of the invention is not critical and can be varied greatly to influence the nature and properties of the polymeric material which will be formed. In general, the ratio of molar equivalents of isocyanate to oxazolidine in the compositions is from about 1:0.5 to about 1:2, with a preferred ratio of about 1:0.75.
- the composition When an oxazolidine-isocyanate composition of the invention is employed as a use locus, the composition typically interacts with atmospheric moisture which initiates ring opening of the oxazolidine ring and leads to the reaction of the thus formed active hydrogen compound and the isocyanate groups of the prepolymer. Trace amounts of moisture derived from the atmosphere are typically all that is necessary to initiate reaction and cause curing.
- the relative humidity of the atmosphere to which the composition is exposed is, however, desirable to be at least about 10%, and preferably about 40% or higher.
- additional sources of moisture in terms of artificially generated humidity, steam, etc. can be utilized to promote rapid curing.
- the hydrocuring reaction can also be carried out in the presence of a catalyst.
- a catalyst such as dibutyl tin dilaurate can be advantageously employed.
- the catalyst is 1,8- diazobicyclo[5.4.0]Undec-7-ene (DBU) .
- DBU 1,8- diazobicyclo[5.4.0]Undec-7-ene
- the catalyst will generally be present in an amount of from about 0.005 to about 1.5% based on the total system weight.
- the catalyst is used within a ratio of 0.01 to 1%.
- compositions of the invention can also contain other additives to advantage.
- additives can include, for example, tackifiers and plasticizers.
- Tackifiers can be used if desired for improving tack, and to impart pressure sensitive qualities to the adhesive.
- the tackifier can be a rosin ester, an aromatic resin, or mixtures thereof.
- Representative examples of rosin esters that are useful in the present invention include glycerol rosin ester, pentaerythritol rosin ester, and hydrogenated versions of these components.
- Representative examples of aromatic resins include alphamethyl styrene resin, styrene monomer, polystyrene, cuomorone-indene, and vinyl toluene.
- Other useable tackifiers include polymerized petroleum hydrocarbons, polymerized terbenes and ph.en--.l-formaldehyde condensates.
- Plasticizers can be any of a variety of compositions that do not interfere with the efficacy of the other components and that facilitates processing and increases toughness and flexibility of the adhesive composition.
- Representative plasticizers include liquid aromatic ester plasticizers including dioctyl phthalate esters; solid plasticizers including dicyclohexyl phthalate, cyclohexane dimethanol dibenzoate; and other plasticizers such as paraffinic oils, petrolatum and tritricresyl phosphate.
- various other compounds can be added to the hot melt composition. These compounds include dyes, inhibitors, antioxidants, UV absorbers, waxes, adhesion promoters, and other conventional additives. Typically, these additives are used in hot melt adhesive formulations.
- the general procedure for the preparation of such a material is as follows. Into a suitable sized reactor, charge the tackifying resin and heat to molten typically 170°F. or more without causing decomposition. Charge the additional polymers and heat to 170°F or molten. Reduce pressure to 1-5 torr for sufficient time (about 15 to 45 minutes) to dry the molten system. Back flush the reaction with nitrogen and charge the polyurethane prepolymer and the oxazolidine. The reaction contents are mixed for 15 to 45 minutes at 175°C under nitrogen and are discharged into containers that seal the adhesive from contact with moisture.
- compositions of the invention can be used to advantage as adhesives for both natural and synthetic substrates such as paper, textiles, wood, plastics, metal, leather, and also as binders for nonwoven fabrics. Further, the compositions of the invention can be used in forming films, fibers used in paints, lacquers, varnishes, seamless flooring caulks, sealants, coatings, impregnates, etc. Adhesives and sealants are preferred end uses, however.
- compositions of the invention provide an improved combination of increased pot life at elevated temperatures and increased curing speed with respect to those compositions containing nonsterically hindered isocyanates
- compositions of the invention exhibit a number of desirable and advantageous properties.
- the compositions can be sealed in a single package with moisture excluded. Undesirable thickening or gelling will not occur during storage over 3 to 9 months.
- the compositions of the invention can be sold as two part systems wherein the oxazolidine material is separated from the isocyanate compound or isocyanate prepolymer until mixed as the use locus.
- the following examples illustrate the preparation of certain oxazolidine compounds, the preparation of sterically hindered isocyanate prepolymers of the invention, the fully formed hydrocurable adhesive compositions of the invention, and the vinyl monomers of the invention containing a hindered isocyanate and an oxazolidine functionality.
- the examples are illustrative of the invention and contain a best mode. Further, comparative examples are included illustrating the failure of nonsterically hindered materials due to their instability during hot melt processing.
- Example 1 Preparation of 3-(2-Hydroxyethyl)oxazolidine A 250 ml, 3-necked, round-bottomed flask was fitted with a reflux condenser, a N 2 inlet, and a Dean-Stark water separator trap. The flask was charged with diethanolamine (35.0 g, 0.33 mol), toluene (150 ml), and 91% paraformaldehyde (11.0 g, 0.33 mol). The heterogeneous mixture was warmed to reflux while under an atmosphere of N 2 . After approximately one hour of refluxing conditions, 6.5 ml of water was collected in the Dean-Stark trap.
- the reaction mixture was cooled and the toluene was removed using a rotary evaporator.
- the residual oil was vacuum distilled.
- the fractions collected during distillation between 58°C and 61°C (0.50-0.60 torr) were combined.
- the yield was 36.5 g (94.3%).
- the toluene was removed using a rotary evaporator.
- the residual oil was vacuum distilled.
- the fractions collected during distillation between 95°C and 98°C (0.30-0.75 torr) were combined.
- the yield was 168.1 g (90.7%).
- a 250 ml, 3-necked, round-bottomed flask was fitted with a thermometer, a N 2 inlet, a magnetic spin bar, and a pressure-equalizing addition funnel.
- the flask was charged with 4 drops of Dabco T-12 (DBTDL available from Air Products Corp., 0.10 g, 0.16 mol), ethylacetate (100 ml), and 3-(2-hydro yethyl)oxazolidine (18.5 g, 0.10 mol).
- a solution of m-TMI (20.1 g, 0.10 mol) in 50 ml ethylacetate was added dropwise over a 30 minute interval to the oxazolidine solution at 25-30°C. The mixture was stirred overnight.
- polyester polyol TerathaneTM 2000 (282.21 g, 0.28 equivalents, available from duPont) and TerathaneTM 650 (130.58 g, 0.4 equivalents, available from duPont) were dried under vacuum (28 inches Hg) in a 1000 ml flask at 95 to 115°C for approximately 35 minutes. The mixture was then allowed to cool to 65°C while under N 2 . l,3-bis( ⁇ - isocyanatoisopropyl)benzene (m-TMXDI, 187.21 g, 3.97 equivalents, available from Am Cyanamide) was added to the mixture. The temperature was increased to 70-80°C and maintained at that temperature until the m-TMXDI was completely melted.
- the polyol TerathaneTM 1000 (429.72 g, 1.0 equivalents, available from duPont) was dried under vacuum (28.5 inches Hg) in a 1000 ml flask at 95-105°C for approximately 40 minutes.
- m-TMXDI (220.28 g, 2.06 equivalents) was quickly added to the polyol. The temperature was maintained at 65- 75°C under a positive N 2 atmosphere for approximately 20 minutes.
- the oxazolidine prepared according to Example 3 (220.28 g, 1.19 equivalents) was added to this hot melt moisture cure prepolymer at a rate of about 5 ml/min. The temperature was maintained at 70-75°C for 30 minutes under N 2 . The reaction was not exothermic. The reaction product was clear and of relatively low viscosity.
- the polyol TerathaneTM 2000 (1320.99 g, 1.3 equivalents, polytetramethylene glycol available from duPont) was dried under vacuum (29 inches Hg) in a 2000 ml flask at approximately 110°C for 30 minutes.
- m-TMXDI (479.01 g, 4.07 equivalents) was quickly added to the polyol.
- the temperature was maintained at 90°C under a positive N 2 atmosphere for approximately 10 minutes, at which time 0.05% dibutyltin dilaurate was added. The reaction was exothermic, but it maximized at about 120°C.
- the bicyclic oxazolidine available as ZoldineTM EBA from Angus Chemical Co.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Polyurethanes Or Polyureas (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
Abstract
Thermally stable compositions containing a polyfunctional aliphatic hindered isocyanate compound and an oxazolidine compound are disclosed. The materials are stable under hot melt conditions at elevated temperatures and can cure in the presence of moisture to afford polymeric or crosslinked materials. Due to the presence of the hindered isocyanate, the cured materials are substantially free of isocyanurate products formed through the trimerization reaction of the isocyanate compounds. The absence of trimerization products produce significantly improved hot melt materials particularly useful as adhesives and sealants. The cooperation between the hindered isocyanate compound and the oxazolidine agent result in a significant improvement in stability and performance.
Description
THERMALLY STABLE CURABLE HINDERED ISOCYANATE-OXAZOLIDINE COMPOSITION
Field of the Invention The invention relates to improved oxazolidine- isocyanate compositions that can be cured in the presence of moisture under hot melt conditions to form chain extended and/or crosslinked materials. The compositions can be used as adhesives, sealants, and in other end uses where hydrocurable or moisture curing hot melt compositions can be cured into a functional layer or coating.
Background of the Invention The reaction of an isocyanate compound with an active hydrogen-containing compound such as a carboxylic acid- an amine, water, an alcohol, etc., to form a polyurea or a polyurethane is well known. This general class of curable systems has been found to possess many useful properties with numerous applications, especially in the field of coating chemistry. One system that has been studied combines polyfunctional isocyanate compounds with monocyclic or bicyclic oxazolidines, which are heterocyclic ring systems containing nitrogen and oxygen functionality. The oxazolidine compounds are used as a source of the compound with two or more active hydrogens for curing. The isocyanate compounds are typically used in small molecule or monomer forms or in the form of an isocyanate-terminated (-NC0) prepolymer having at least two available -NCO groups. The ring of the oxazolidine compound can open when combined with water to lose one mole of a carbonyl compound according to the following generalized reaction (I):
RNHCH2CH2OH + CH2 (O)
to generate an active hydrogen compound that can further react with a free isocyanate group. In this type of system, known as a "one pot" composition, the reactants are typically combined before using, isolated from any activating conditions, and then later activated, by moisture from the atmosphere or from an intentionally added moisture source.
The advantages of employing oxazolidines in isocyanate hydrocurable systems are that oxazolidines: (1) have a high rate of physical property development because the rate of reaction between water and the oxazolidine is faster than the rate of reaction between water and the isocyanate, thereby resulting in strong bonds being formed between the resultant active hydrogens on the -OH and -NH groups and the isocyanate groups; (2) have reduced carbon dioxide bubble formation because of the preferential reaction of water with oxazolidine over an -NCO group; and (3) have lower levels of free isocyanate but achieve the same level of crosslinking and ultimate physical strength, particularly if an oxazolidine with reactive pendant functional groups is employed.
However, significant problems occur when such oxazolidine-isocyanate compositions are heated, melted, and applied to materials under typical hot melt processing conditions, i.e., in the absence of solvent at temperatures between about 37°C and 175°C or higher. For example, the isocyanate materials typically used in the preparation of hydrocurable adhesives tend to trimerize forming isocyanurate compounds, which are highly functional and
typically give rise to highly crosslinked and brittle polymers. The trimerization occurs according to the following reaction (II):
This trimerization reaction in the oxazolidine-isocyanate systems is a particular problem because of the presence of one or more tertiary amine groups in the oxazolidines. A tertiary amine group is an excellent catalyst for the trimerization reaction and promotes rapid formation of the isocyanurate structure.
The formation of isocyanurates is particularly undesirable because the "pot life", i.e., stability, of the oxazolidine-isocyanate compositions at elevated temperatures is substantially reduced with isocyanurate formation. Furthermore, the cured isocyanate adhesive bond, coating, caulk, or other structure is weakened due to the network formation and decreased elastomeric properties resulting from isocyanurate formation. Also, fewer isocyanate groups are available for crosslinking with the amino and hydroxy moieties formed upon the water-driven ring opening of the oxazolidine compounds.
Because of the trimerization reaction, aromatic isocyanates, which trimerize rapidly in the presence of a tertiary amine, have been found to be commercially unsuitable for use in combination with such tertiary amines as oxazolidine compounds to make hydrocurable systems. While the trimerization reaction of aliphatic isocyanates in the presence of tertiary amines is slower than the rate of aromatic isocyanates, the use of aliphatic isocyanates
in combination with oxazolidines is also precluded at the temperatures of typical hot melt systems because the trimerization reaction is enhanced at such temperatures. Polyurethane hydrocurable systems containing certain oxazolidine compositions and polyfunctional aliphatic or aromatic isocyanates suitable for coatings, adhesives, and other applications are well known and disclosed in, for example, U.S. Patent Nos. 3,661,923; 3,743,626; 3,912,691; 4,024,117; 4,032,686; 4,101,527; 4,118,376; 4,138,545; and 4,471,102. Certain of these systems are disclosed as having poor thermal stability; relatively poor flexibility, weatherability, and abrasion resistance; and objectionable color, especially when used as coatings. One such system is disclosed in U.S. Pat. No. 4,879,365 as providing improved coatings having good color, abrasion resistance, and weatherability. It consists of a one-component air curable urethane solvent-based coating composition. The composition includes at least one oxazolidine ring covalently bonded through an alkylene-urethane linking group to a prepolymer radical containing tetramethylxylenediisocyanate (TMXDI) . The oxazolidine- isocyanate compound is produced from a hydroxy oxazolidine compound and a polycarbonate, polyester, or polyether tetramethylxylene diisocyanate prepolymer containing at least two isocyanate groups, in the presence of a solvent. Because of the problems associated with isocyanates and isocyanurate formation and because it has become increasingly desirable to avoid the use of solvents in the formation of polymeric materials, these systams are not completely suitable or acceptable for a commercial setting. Accordingly, a substantial need exists for moisture curing, i.e., hydrocurable, systems containing oxazolidine compounds and isocyanate compounds that are relatively stable and resistant to trimerization under hot melt
conditions, but rapidly react to form high strength, resilient, cohesive, adhesive bonds and stable sealants, etc.
Summary of the Invention
We have found that high quality hydrocurable systems can be manufactured and used under hot melt conditions, in the absence of solvent, by combining a hindered polyfunctional isocyanate with an oxazolidine compound. The use of such compositions substantially reduces the amount of isocyanurates formed, which can significantly reduce the value of an adhesive system, but does not hinder reaction between the isocyanate compound and curing agents derived from the oxazolidine. As a result, the blended materials are stable at elevated hot melt temperatures without measurable trimerization of the isocyanate. Therefore, as a result of our developments in this technology, the first hydrocurable, hot melt, solvent-free isocyanate-oxazolidine compositions are now a commercial reality.
One aspect of the invention is a "one pot" hydrocurable, hot melt, composition comprising an oxazolidine compound and a sterically hindered aliphatic polyfunctional isocyanate compound or an isocyanate- terminated prepolymer made from the sterically hindered polyfunctional isocyanate compound. A second aspect of the invention comprises a one component moisture curing material containing the hindered isocyanate compound or prepolymer, the oxazolidine compound, and a thermoplastic resin along with other optional ingredients including tackifiers, plasticizers, etc. A third aspect of the invention is a method of using moisture curing material containing a hindered isocyanate-terminated prepolymer or isocyanate monomer and the oxazolidine compound at elevated temperatures in hot melt formulations used as an adhesive
or sealant. Another aspect of the invention comprises a vinyl unsaturated monomer having a hindered isocyanate end group bonded to an oxazolidine group, polymers made from monomers with such pendant groups, and curable systems using a curable polymer with a pendant isocyanate group, an epoxy group, or mixtures thereof.
Detailed Description of the Invention - The thermally stable, hydrocurable, hot melt compositions of the invention comprise an oxazolidine compound, that can react with water to release an active hydrogen compound, and a sterically hindered isocyanate compound, typically in the form of a small molecule hindered isocyanate or an isocyanate-terminated prepolymer composition. Such hydrocurable compositions can take the form of adhesives, sealants, and other typical end uses for hydrocurable, hot melt materials. Adhesives and sealants are the preferred end uses, however. The present invention provides for an efficient production of oxazolidine- isocyanate polymeric materials that are substantially free of tri er isocyanurate impurities. Preferably the oxazolidine-isocyanurate polymeric materials are produced by the process of the invention such that there is less than about 1 wt-% of the isocyanurate compound present. Oxazolidines are typically defined as a class of five- or six-membered heterocyclic compounds containing nitrogen and oxygen functionality. In describing the compositions of the invention, the term "oxazolidine" includes five- membered ring oxazolidines, six-membered ring tetrahydro- oxazines, and cyclic, bicyclic, polycyclic, etc., compounds.
Upon exposure to water, at a bond site where curing is desirable, the oxazolidine ring reacts with water and the ring opens to provide reactive (active hydrogen containing) amino and hydroxy moieties. Thereby, an active hydrogen
atom is provided for reaction with a sterically hindered isocyanate compound. Such oxazolidine compounds contain at least a secondary amino nitrogen and typically contain a tertiary amino nitrogen. Tertiary amino nitrogens are particularly troublesome, as was explained above, because they can catalyze trimerization of the isocyanates into undesirable isocyanurates, particularly at the temperatures of the typical hot melt process. However, this problem has been solved by the use of sterically hindered isocyanate compositions of the present invention.
A wide variety of well-known oxazolidines can be used in the compositions of the invention. This includes, for example, aliphatic and aromatic monocyclic and bicyclic oxazolidines, monofunctional ester oxazolidines, polyfunctional polyol ester oxazolidines, polyfunctional polycarboxylic ester oxazolidines, polymers and copolymers of oxazolidinylalkyl acrylates and methacrylates, and polyfunctional oxazolidine-terminated polyesters as disclosed in U.S. Patent No. 4,138,545. Preferably the oxazolidines are monocyclic and bicyclic, and more preferably, they are bicyclic.
Monofunctional oxazolidine compounds are generally represented by the following formula:
wherein R is an organic radical; R1 is a hydrogen atom, a phenyl group, a benzyl group, or a C-.12 alkyl group; R2 is a hydrogen atom or a C^ alkyl group; R1 and R2, when taken together with the attached carbon atom, can be representative of a saturated cyclic carbon ring having five or more carbon atoms; and Y represents the group:
wherein R3 and R4 are independently hydrogen atoms, C-_X2 alkyl groups, C6.10 aryl groups, or C-_12 alkyl substituted a yl groups or aryl substituted alkyl groups, and n is an integer of 2 or 3.
The bicyclic oxazolidine compounds useful in the compositions of the invention are generally characterized by a fused-ring system having a single nitrogen atom common to both rings. They can be represented by the following formula:
wherein R5 is the same as described for R1 and R2 above, Y is similarly described as above, and R6 can be hydrogen, alkyl, hydroxyalkyl, aminoalkyl, etc. These compounds are generally formed from a primary amine containing at least two C-hydroxyalkyl groups and a carbonyl group. Known carbonyl precursors include, for example, formaldehyde, acetaldehyde, butyraldehyde, and others as disclosed in U.S. Patent No. 4,101,527. Oxazolidine compounds that contain no pendant reactive functional groups prior to the ring-opening reaction are preferred for certain compositions of the invention. These include groups such as halogen substituents, alkoxy substituents, nitro groups, and others. These compounds typically function as reactive diluents. Examples of such
compounds are those bicyclic oxazolidines prepared from primary amines containing C-hydroxyalkyl groups as represented by the following reaction (III):
Oxazolidines having no available active hydrogens prior to reaction with water can be used to advantage because such compositions generally have greater stability or "pot life", especially at elevated temperatures when compared to oxazolidines with active hydrogen groups.
Although oxazolidines without pendant reactive functional groups are advantageous in certain compositions, the oxazolidine compounds can contain active hydrogen- containing substituents for certain other advantageous applications of the invention. By "active hydrogen" atoms, we mean hydrogen atoms present in groups such as primary and secondary amino groups, carboxylic acid groups, hydroxy groups, and sulfhydryl groups, which readily undergo reaction with electrophilic reagents such as isocyanates . These groups can be used to link the oxazolidine nucleus to form prepolymers, monomers, or other active materials containing the oxazolidine nucleus. An example of an oxazolidine containing a reactive pendant group is one that results from the reaction product of diethanolamine and a carbonyl compound.
One class of more preferred oxazolidine compounds are those generally prepared by the reaction of a β- aminoalcohol and a carbonyl compound, such as a ketone or aldehyde. Because of facile trimerization of aliphatic isocyanates, oxazolidines prepared from diethanolamine are
a particularly preferred group of compounds. These compounds can be generally represented by the following formula:
wherein each R' is independently a hydrogen, lower alkyl (i.e., C-_6 alkyl), substituted lower alkyl of 1 to 12 carbon atoms, cycloalkyl, and phenyl or substituted phenyl. The substitution of a 3-hydroxyalkyl group for an alkyl group on an amine nitrogen is known to result in a decrease in the base strength of the amine by approximately 1 pKa unit. Therefore, the substitution of a .-hydroxyalkyl group for an alkyl group on the nitrogen atom of the oxazolidine decreases the base strength of the amine which decreases the rate of the trimerization reaction of the isocyanates. The following list of amines and their base strengths, in pKa units, illustrate this point: triethylamine 11.0 diethyl-aminoethanol 9.9 et yl-diethano1amine 8.9 triethanolamine 7.8 An additional advantage of the combination of hindered isocyanate compounds and oxazolidines according to the present invention results because of the wider variety of oxazolidines that can be employed in useful hydrocuring systems. That is, the use of oxazolidines containing two 3-hydroxyalkyl groups bonded to the nitrogen atom was one of the only known methods for reducing the occurrence of the trimerization reaction and increasing the stability of aliphatic isocyanate-oxazolidine hydrocurable compositions prior to the present invention. Therefore, the variety of types of oxazolidines that could be used in hydrocuring aliphatic isocyanate composition was severely limited prior
to the present invention. Although monocyclic oxazolidines prepared from diethanolamine are particularly preferred, there is no requirement that the invention is limited thereby.
Both monocyclic and bicyclic oxazolidines in which the hydroxyalkyl group is pendant to a carbon atom instead of the nitrogen atom can be used. Examples of these types of oxazolidines are shown in the following reactions (IV) and (V):
Bicyclic oxazolidines are particularly preferred over monocyclic oxazolidines. This is because, at least in part, the bicyclic nature of these oxazolidines provides, minimally, a functionality of three with the high likelihood of additional reactive sites leading to extensive crosslinking in the final polymer formed. Furthermore, bicyclic oxazolidines are preferred over monocyclic oxazolidines because they are readily incorporated into the system by simple mixing or blending. There is a wide range of stoichiometries possible between the isocyanate terminated prepolymer and the bicyclic oxazolidine. The bicyclic oxazolidine are relatively weak bases and are stable in the presence of isocyanate terminated prepolymers.
The term "aliphatic isocyanate compound" means that the -NCO group is attached to an aliphatic carbon. The sterically hindered organic isocyanate compounds of the present invention include organic compounds containing one or more isocyanate groups (-NC0) wherein the nitrogen atom of the isocyanate compound is directly attached to a tertiary aliphatic carbon atom. The tertiary carbon atom can be a carbon atom in a branched or unbranched tertiary aliphatic group and can also be an aliphatic ring carbon atom. These aliphatic carbon atoms can also be substituents on aromatic ring systems. Examples of such a tertiary bonded isocyanate group are shown in the following formulas:
R
I
R - C - NCO
I R
R
! RING - C - NCO
!
RING
wherein R is a C-_12 alkyl, aryl, etc., group. The above formulas show, respectively, the isocyanate group attached to a tertiary alkyl carbon, and within a ring system having a tertiary carbon (the unfilled valences representing the ring) . The tertiary carbon or ring carbon can be a portion of an inorganic compound having virtually any structure or substituent.
Hindered isocyanate compounds for use in this invention also include compounds wherein the tertiary carbon of the hindered isocyanate substituent is attached to an aliphatic
or aromatic ring structure represented by the following formulas:
wherein R is a C-_12 alkyl, aryl, etc., group, the aromatic ring is merely a representation of a substituted ring or multi-ring system, and preferably n = 1-3.
The preferred compounds are isocyanate compounds formed on aromatic ring structures wherein the hindered isocyanate group can be substituted meta, ortho, or para to a vinyl group or to a second hindered isocyanate group. Such compounds include hindered aromatic diisocyanate compounds and vinyl-substituted hindered onoisocyanate aromatic monomers. Representative examples of the most preferred isocyanate compounds comprises m-TMI and m-TMXDI [known as 3-isopropenyl(α,α-dimethylbenzyl)isocyanate and l,3-bis(α- isocyanatoisopropyl)benzene, respectively] . The preparation of these compounds is disclosed in U.S. Patent No. 4,377,530. The isocyanate compound referred to as - TMXDI is also known as tetramethylxylenediisocyanate. Also beneficial and preferred would be the para isomers of these compounds, i.e., 4-isopropenyl(α,α- dimethylbenzyl)isocyanate and l,4-bis-(α- isocyanatoisopropyl)benzene, respectively. Most preferred are the hindered aromatic diisocyanate compounds.
For the "one pot" hydrocurable, hot melt, compositions of the invention comprising an oxazolidine compound and a
ET
sterically hindered polyfunctional isocyanate small molecule or an isocyanate-terminated prepolymer made from the sterically hindered polyfunctional isocyanate compound, the preferred isocyanate is one having at least two -NCO groups. This is similarly so for the one component moisture curing material containing the hindered isocyanate compound or prepolymer, the oxazolidine compound, and a thermoplastic resin along with other optional ingredients including tackifiers, plasticizers, etc. For the vinyl unsaturated monomer having a hindered isocyanate end group bonded to an oxazolidine group, however, the preferred isocyanate is an aromatic compound with the hindered isocyanate group substituted meta, ortho, or para to a vinyl group. Prepolymers useful in manufacturing the oxazolidine isocyanate compounds of the invention often are prepolymers formed in the reaction of a polyol compound and the sterically hindered isocyanate compound. Polyols useful in making the prepolymers of the invention are organic compounds having a hydroxy functionality of 2 to 4, preferably 2 to 3, which are prepared by well known preparatory techniques. Included within the scope of polyol compounds useful in the invention are polyoxyalkylene polyol compounds including polyoxyethylene glycols, polyoxypropylene glycols, etc. , polyester polyol compounds prepared by methods well known in the art including polymerization of ring-like "tones" such as caprolactone, butyrolactone, or valerolactone or by reacting a dibasic acid such as phthlatic acid or 1,6- hexane dicarboxylic acid with a diol such as ethylene diol or 1,4-butanediol. Polyester and polyether polyols of the invention usually have a molecular weight of between about
300 and 5,000, and preferably between about 400 and 4,000.
The reaction mixture used to prepare the isocyanate prepolymer for use in the hydrocurable compounds of the
invention typically comprises an excess of isocyanate group for reaction with the hydroxy functionality of the polyester or polyether polyol. Preferably, sufficient isocyanate concentration is used to react with essentially all available hydroxy functionality on the polyol forming the isocyanate prepolymer. Equivalent weight ratio of diisocyanate to the polyol includes greater than about 1.0 to 3.5 parts of isocyanate to polyol, preferably from about 1.5 to 3 parts isocyanate per part of polyol and is preferably about 2 parts isocyanate per part of polyol. Suitable reaction temperatures are usually between about 75°C and 150°C. The reaction time is usually between about 1 hour and 8 hours. Optionally, a catalyst such as dibutyltin dilaurate, or the like, is present in the reaction mixture to reduce the time for preparing the prepolymer.
The isocyanate and oxazolidines are combined in 100% solids systems under typical hot melt conditions. The reaction temperature is generally within the range of about 37°C to 175°C, preferably within about 60°C to 80°C. The ratio of isocyanate to oxazolidine in the compositions of the invention is not critical and can be varied greatly to influence the nature and properties of the polymeric material which will be formed. In general, the ratio of molar equivalents of isocyanate to oxazolidine in the compositions is from about 1:0.5 to about 1:2, with a preferred ratio of about 1:0.75.
When an oxazolidine-isocyanate composition of the invention is employed as a use locus, the composition typically interacts with atmospheric moisture which initiates ring opening of the oxazolidine ring and leads to the reaction of the thus formed active hydrogen compound and the isocyanate groups of the prepolymer. Trace amounts of moisture derived from the atmosphere are typically all that is necessary to initiate reaction and cause curing.
HEET
The relative humidity of the atmosphere to which the composition is exposed is, however, desirable to be at least about 10%, and preferably about 40% or higher. During times of the year where atmospheric moisture is limited, additional sources of moisture in terms of artificially generated humidity, steam, etc., can be utilized to promote rapid curing.
The hydrocuring reaction can also be carried out in the presence of a catalyst. Under certain conditions, a catalyst such as dibutyl tin dilaurate can be advantageously employed. Preferably, the catalyst is 1,8- diazobicyclo[5.4.0]Undec-7-ene (DBU) . The catalyst will generally be present in an amount of from about 0.005 to about 1.5% based on the total system weight. Preferably, the catalyst is used within a ratio of 0.01 to 1%.
The compositions of the invention can also contain other additives to advantage. These additives can include, for example, tackifiers and plasticizers. Tackifiers can be used if desired for improving tack, and to impart pressure sensitive qualities to the adhesive. The tackifier can be a rosin ester, an aromatic resin, or mixtures thereof. Representative examples of rosin esters that are useful in the present invention include glycerol rosin ester, pentaerythritol rosin ester, and hydrogenated versions of these components. Representative examples of aromatic resins include alphamethyl styrene resin, styrene monomer, polystyrene, cuomorone-indene, and vinyl toluene. Other useable tackifiers include polymerized petroleum hydrocarbons, polymerized terbenes and ph.en--.l-formaldehyde condensates.
Plasticizers can be any of a variety of compositions that do not interfere with the efficacy of the other components and that facilitates processing and increases toughness and flexibility of the adhesive composition. Representative plasticizers include liquid aromatic ester
plasticizers including dioctyl phthalate esters; solid plasticizers including dicyclohexyl phthalate, cyclohexane dimethanol dibenzoate; and other plasticizers such as paraffinic oils, petrolatum and tritricresyl phosphate. In addition to the above indicated optional components, various other compounds can be added to the hot melt composition. These compounds include dyes, inhibitors, antioxidants, UV absorbers, waxes, adhesion promoters, and other conventional additives. Typically, these additives are used in hot melt adhesive formulations. The general procedure for the preparation of such a material is as follows. Into a suitable sized reactor, charge the tackifying resin and heat to molten typically 170°F. or more without causing decomposition. Charge the additional polymers and heat to 170°F or molten. Reduce pressure to 1-5 torr for sufficient time (about 15 to 45 minutes) to dry the molten system. Back flush the reaction with nitrogen and charge the polyurethane prepolymer and the oxazolidine. The reaction contents are mixed for 15 to 45 minutes at 175°C under nitrogen and are discharged into containers that seal the adhesive from contact with moisture.
The compositions of the invention can be used to advantage as adhesives for both natural and synthetic substrates such as paper, textiles, wood, plastics, metal, leather, and also as binders for nonwoven fabrics. Further, the compositions of the invention can be used in forming films, fibers used in paints, lacquers, varnishes, seamless flooring caulks, sealants, coatings, impregnates, etc. Adhesives and sealants are preferred end uses, however.
The compositions of the invention provide an improved combination of increased pot life at elevated temperatures and increased curing speed with respect to those compositions containing nonsterically hindered isocyanates
EET
and oxazolidines. Various embodiments of the compositions of the invention and the materials formed therefrom exhibit a number of desirable and advantageous properties. The compositions can be sealed in a single package with moisture excluded. Undesirable thickening or gelling will not occur during storage over 3 to 9 months. The compositions of the invention can be sold as two part systems wherein the oxazolidine material is separated from the isocyanate compound or isocyanate prepolymer until mixed as the use locus.
The following examples illustrate the preparation of certain oxazolidine compounds, the preparation of sterically hindered isocyanate prepolymers of the invention, the fully formed hydrocurable adhesive compositions of the invention, and the vinyl monomers of the invention containing a hindered isocyanate and an oxazolidine functionality. The examples are illustrative of the invention and contain a best mode. Further, comparative examples are included illustrating the failure of nonsterically hindered materials due to their instability during hot melt processing.
Example 1 Preparation of 3-(2-Hydroxyethyl)oxazolidine A 250 ml, 3-necked, round-bottomed flask was fitted with a reflux condenser, a N2 inlet, and a Dean-Stark water separator trap. The flask was charged with diethanolamine (35.0 g, 0.33 mol), toluene (150 ml), and 91% paraformaldehyde (11.0 g, 0.33 mol). The heterogeneous mixture was warmed to reflux while under an atmosphere of N2. After approximately one hour of refluxing conditions, 6.5 ml of water was collected in the Dean-Stark trap. The reaction mixture was cooled and the toluene was removed using a rotary evaporator. The residual oil was vacuum distilled. The fractions collected during distillation
between 58°C and 61°C (0.50-0.60 torr) were combined. The yield was 36.5 g (94.3%).
Example 2
Alternative Preparation of 3-(2-Hydroxyethyl)oxazolidine The procedure outlined in Example 1 was followed using diethanolamine (106.1 g, 1.0 mol), hexanes (150 ml), and
91% paraformaldehyde (33.0 g, 1.0 mol). The fractions collected during distillation between 64°C and 73°C
(between 0.25 and 1.25 torr) were combined. The yield was 111.6 g (95.2%) .
Example 3
Preparation of 3-(2-hydroxyethyl)-2, 2-pentamethylene Oxazolidine A 300 ml, 3-necked, round-bottomed flask was fitted with a reflux condenser, a N2 inlet, a magnetic spin bar, and a Dean-Stark water separator. The flask was charged with diethanolamine (106.1 g, 1.0 mol), toluene (150 ml), and cyclohexanone (98.2 g, 1.0 mol). The mixture was warmed to reflux under an atmosphere of N2 and allowed to reflux for approximately six hours, at which time 18.0 ml of water was collected in the Dean-Stark trap. The reaction mixture was allowed to cool to 25°C and filtered. The toluene was removed using a rotary evaporator. The residual oil was vacuum distilled. The fractions collected during distillation between 95°C and 98°C (0.30-0.75 torr) were combined. The yield was 168.1 g (90.7%).
Example 4
Preparation of a Vinyl Substituted Oxazolidine N-(2-oxazolidinylethyl)-N^α,α-dimethyl-
3-isopropenyl benzyl) urea
A 250 ml, 3-necked, round-bottomed flask was fitted with a thermometer, a N2 inlet, a magnetic spin bar, and a pressure-equalizing addition funnel. The flask was charged with 4 drops of Dabco T-12 (DBTDL available from Air Products Corp., 0.10 g, 0.16 mol), ethylacetate (100 ml),
and 3-(2-hydro yethyl)oxazolidine (18.5 g, 0.10 mol). A solution of m-TMI (20.1 g, 0.10 mol) in 50 ml ethylacetate was added dropwise over a 30 minute interval to the oxazolidine solution at 25-30°C. The mixture was stirred overnight. A colorless oil resulted. Thin layer chromatography (ethylacetate/methanol, 23:2) indicated a trace of residual isocyanate, although the IR spectrum showed no evidence of any -NCO groups. The ethylacetate was removed on a rotary evaporator affording 44 g of a colorless oil. The reaction is shown below:
Example 5
Alternative Preparation of a Vinyl Substituted Oxazolidine N-(2-oxazolidinylethyl)-N -g,α-dimethyl- 3-isopropenyl benzyl) urea
A 250 ml, 3-necked, round-bottomed flask was fitted with a thermometer, a N2 inlet, a magnetic spin bar, and a pressure-equalizing addition funnel. The flask was charged with DBTDL (4 drops), m-TMI (21.1 g, 0.105 mol), and hexanes (75 ml). A solution of 3-(2- hydroxyethyl)oxazolidine (11.2 g, 0.10 mol) in ethylacetate (25 ml) and hexanes (25 ml) . The reaction mixture was stirred overnight at 25°C, filtered, and the solvents removed using a rotary evaporator affording 33.4 g of a
colorless oil. The infrared spectrum showed evidence of a residual amount of -NCO groups.
Example 6
Reaction of an Oxazolidine and a Hot Melt Moisture Cure Isocyanate Prepolymer
The polyester polyol Terathane™ 2000 (282.21 g, 0.28 equivalents, available from duPont) and Terathane™ 650 (130.58 g, 0.4 equivalents, available from duPont) were dried under vacuum (28 inches Hg) in a 1000 ml flask at 95 to 115°C for approximately 35 minutes. The mixture was then allowed to cool to 65°C while under N2. l,3-bis(α- isocyanatoisopropyl)benzene (m-TMXDI, 187.21 g, 3.97 equivalents, available from Am Cyanamide) was added to the mixture. The temperature was increased to 70-80°C and maintained at that temperature until the m-TMXDI was completely melted. 3-(2-Hydroxyethyl)oxazolidine (33.4 g) , prepared according to Example 2, was added to this hot melt moisture cure prepolymer with constant stirring, while the temperature was maintained below 80°C. The reaction product was clear, of relatively low viscosity and contained 4% free -NCO.
Example 7 Preparation of a Hot Melt Adhesive Composition A 1000 ml glass reactor was charged with Kristalex™
3100 (240 g) , a x-methyl styrene tackifying resin available from Hercules, Inc.) and melted at approximately 150°C. Vynathene™ EY904-25 (60 g, an ethylene-vinyl acetate copolymer available from Quantum Chemical Corp.) was added to the reaction vessel and melted at approximately 190°C. A high molecular weight polyester elastomer (60 gm) , which had been dried in a vacuum oven for approximately 60 minutes to remove excess water, was added to the reaction vessel and melted. This mixture was exposed to a vacuum (28 inches Hg) at 190°C for 30 minutes, and then allowed to
cool to 135°C while under N2. The oxazolidine/prepolymer (24 g) prepared according to Example 6, was added to the reaction mixture. There was no evidence of an exothermic reaction. The temperature of the mixture was maintained at 125-140°C for approximately 30 minutes. There were gel¬ like particles in the mixture initially, but with time these particles melted and the moisture curing hot melt composition was homogeneous.
Example 8 Incorporation of an Oxazolidine into a
Hot melt Moisture Cure Prepolymer
The polyol Terathane™ 1000 (429.72 g, 1.0 equivalents, available from duPont) was dried under vacuum (28.5 inches Hg) in a 1000 ml flask at 95-105°C for approximately 40 minutes. m-TMXDI (220.28 g, 2.06 equivalents) was quickly added to the polyol. The temperature was maintained at 65- 75°C under a positive N2 atmosphere for approximately 20 minutes. The oxazolidine prepared according to Example 3 (220.28 g, 1.19 equivalents) was added to this hot melt moisture cure prepolymer at a rate of about 5 ml/min. The temperature was maintained at 70-75°C for 30 minutes under N2. The reaction was not exothermic. The reaction product was clear and of relatively low viscosity. Example 9
Preparation of a Hot Melt Adhesive Composition A 1000 ml glass reactor was charged with Kristalex™ 3100 (260 g) and melted at approximately 177°C over a period of 45 minutes. Vynathene EY904-25 (65 g) was added to the reaction vessel and melted using slow agitation over a period of 35 minutes at approximately 191°C. A high molecular weight polyester elastomer (65 gm) which had been vacuum dried, was added to the reaction vessel and melted using slow agitation over a period of one hour at 204- 218°C. This mixture was exposed to a vacuum (28 inches Hg)
at 216°C for 30 minutes, and then allowed to cool to 154°C while under N2. The oxazolidine/prepolymer (260 g) prepared according to Example 8, was added to the reaction mixture. There was no evidence of an exothermic reaction. The temperature of the mixture was maintained at 125-150°C for approximately 30 minutes. The final product was clear and of relatively low viscosity.
Example 10
Incorporation of a Bicyclic Oxazolidine into a Hot melt Moisture Cure Isocyanate-Ter inated Prepolymer
The polyol Terathane™ 2000 (1320.99 g, 1.3 equivalents, polytetramethylene glycol available from duPont) was dried under vacuum (29 inches Hg) in a 2000 ml flask at approximately 110°C for 30 minutes. m-TMXDI (479.01 g, 4.07 equivalents) was quickly added to the polyol. The temperature was maintained at 90°C under a positive N2 atmosphere for approximately 10 minutes, at which time 0.05% dibutyltin dilaurate was added. The reaction was exothermic, but it maximized at about 120°C. The bicyclic oxazolidine available as Zoldine™ EBA from Angus Chemical Co. was added to this hot melt moisture cure prepolymer at a rate of about 5 ml/min. The temperature was maintained at 70-75°C for 30 minutes under N2. The reaction was not exothermic. Two separate experiments were performed, one in which an equivalent ratio of 1:1 Zoldine™ EBA:NCO functionality was used, and one in which the ratio was 2:1. The reaction products for each showed no evidence of -NCO functionality in the infrared spectra after three days at ambient temperature and humidity.
Comparative Example 1 The polyether polyols Terathane™ 2000 (128.09 g, 0.128 equivalents) and Terathane™ 650 (44.45 g, 0.136 equivalents) were dried under vacuum (28 inches Hg) in a 1000 ml flask at 80-105°C for approximately 40 minutes.
The mixture was then allowed to cool to 60°C while under N,. Mondur M ( ,4'-diphenylmethane diisocyanate, 77.96 g, 0.63 equivalents) and 3-(2-hydroxyethyl)oxazolidine (22.04 g) , prepared according to Example 2, were added to this mixture with constant stirring, while the temperature was maintained below 80°C. The reaction was highly exothermic. After two hours, the material was observed to be very viscous and to contain evidence of gelled material resulting from the trimerization of the Mondur M. Therefore, 22.01 g of 2-butanone was added. A film of this material was drawn out on teflon sheets. After two days of curing under atmospheric conditions, a large number of bubbles appeared making the film unusable. The experiment was repeated without the addition of 2-butanone and with a curing time of two weeks. The film produced also contained entrained bubbles.
Comparative Example 2 The polyether polyols Terathane™ 2000 (116.57 g, 0.116 equivalents) and Terathane™ 650 (53.94 g, 0.166 equivalents) were dried under vacuum (28 inches Hg) in a 1000 ml flask at 95-115°C for approximately 35 minutes. The mixture was then allowed to cool to 65°C while under N2. Mondur M (MDI, 79.49 g, 0.64 equivalents) was added to the mixture and the temperature raised to 70-80°C and maintained until the MDI was completely melted. 3-(2- hydroxyethyl)oxazolidine (22.04 g) , prepared according to Example 2, was added to this mixture with constant stirring, while the temperature was maintained below 80°C. It was necessary to immerse the vessel in a cold water bath in order to maintain this temperature because of the excess heat produced during the reaction with the oxazolidine compound. After 1.25 hours, gelling was observed.
The above specification, examples, comparative examples and data provide a detailed discussion of the current
embodiments of the claimed invention. However, many embodiments of the invention can be made without departing from the spirit and scope of the invention. Accordingly, the invention resides in the claims hereinafter appended.
T
Claims
1. A solvent-free, hot melt, hydrocurable composition comprising:
(a) about 5 to 99.9 wt-% of an isocyanate terminated polymer comprising the reaction product of:
(i) a sterically hindered isocyanate compound having at least two isocyanate groups; and
(ii) a polymeric polyol compound having at least two hydroxy groups, wherein there are about
1.05 to 5 equivalent of -NCO per equivalent of - OH; and
(b) about 0.1 to 20 wt-% of an oxazolidine compound capable of generating an -NCO reactive compound.
2. The composition of claim 1 wherein the hindered diisocyanate compound comprises l,3-bis-(α- isocyanatoisopropyl)benzene and l,4-bis-(α- isocyanatoisopropyl)benzene.
3. The composition of claim 1 wherein the polyol comprises a polyether polyol.
4. The composition of claim 1 wherein the polyol comprises a polyester polyol.
5. The composition of claim 4 wherein the polyol comprises a reaction product of a terephthalic acid and a diol or triol.
6. The composition of claim 1 wherein the oxazolidine compound is a bicyclic oxazolidine.
7. The composition of claim 1 wherein the oxazolidine compound contains no pendant reactive functional group prior to activation by water.
8. The composition of claim 1 wherein the oxazolidine is a compound of the formula
wherein R is an organic radical; R1 is a hydrogen atom, a phenyl group, a benzyl group, or a C-..-2 alkyl group; R2 is a hydrogen atom or a C-_4 alkyl group; R1 and R2, when taken together with the attached carbon atom, can be representative of a saturated cyclic carbon ring having five or more carbon atoms; and Y represents the group:
wherein R3 and R4 are independently hydrogen atoms, C-.12 alkyl groups, C6.10 aryl groups, or C-.1Z alkyl substituted aryl groups or aryl substituted alkyl groups, and n is an integer of 2 or 3.
9. The composition of claim 1 wherein the oxazolidine is a compond of the formula:
wherein R' is independently hydrogen lower alkyl, substituted lower alkyl, cycloalkyl, phenyl or substituted phenyl.
EET
10. The composition of claim 1 wherein the oxazolidine is a compund of the formula:
R6
wherein R5 and R6 are independently hydrogen atoms, a phenyl group, a benzyl group, or a C-_-2 alkyl group, when taken together with an R5 and the attached carbon atom, can be representative of a saturated cyclic carbon ring having five or more carbon atoms; and Y represents the group:
wherein R3 and R4 are independently hydrogen atoms, C1.12 alkyl groups, C6.10 aryl groups, or Cx_12 alkyl substituted aryl groups or aryl substituted alkyl groups, and n is an integer of 2 or 3.
11. A solvent-free, hot melt adhesive composition comprising:
(a) an isocyanate prepolymer comprising the reaction product of:
(i)a sterically hindered isocyanate compound having at least two isocyanate groups; and
(ii) a polymeric polyol compound having at least two hydroxy groups;
(b) an oxazolidine compound;
(c) a tackifier; and
(d) a plasticizer.
12. The composition of claim 11 wherein the hindered diisocyanate compound comprises l,3-bis-(α- isocyanatoisopropyl)benzene and l,4-bis-(α- isocyanatoisopropyl)benzene.
13. The composition of claim 11 wherein the oxazolidine is a bicyclic oxazolidine.
14. The composition of claim 11 wherein the oxazolidine contains no pendant reactive functional group prior to activation by water.
15. The composition of claim 11 wherein the oxazolidine is a compound of the formula:
wherein R is an organic radical; R1 is a hydrogen atom, a phenyl group, a benzyl group, or a C-.12 alkyl group; R2 is a hydrogen atom or a C^ alkyl group; R1 and R2, when taken together with the attached carbon atom, can be representative of a saturated cyclic carbon ring having five or more carbon atoms; and Y represents the group:
wherein R and RA are independently hydrogen atoms, C-_-2 alkyl groups, C6.10 aryl groups, or C-_12 alkyl substituted aryl groups or aryl substituted alkyl groups, and n is an integer of 2 or 3.
BSTITUTE SHEET
16. The composition of claim 11 wherein the oxazolidine is a compound of the formula:
wherein R1 is independently hydrogen lower alkyl, substituted lower alkyl, cycloalkyl, phenyl or substituted phenyl.
17. The composition of claim 11 wherein the oxazolidine is a compound of the formula:
wherein R5 and R6 are independently hydrogen atoms, a phenyl group, a benzyl group, or a C1.12 alkyl group, R5, when taken together with an R5 and the attached carbon atom, can be representative of a saturated cyclic carbon ring having five or more carbon atoms; and Y represents the group:
wherein R3 and R4 are independently hydrogen atoms, C-_ι:> alkyl groups, C6_1Q aryl groups, or C-_12 alkyl substituted aryl groups or aryl substituted alkyl groups, and n is an integer of 2 or 3.
18. A monomer comprising
wherein R6 is an organic radical; R1 is a hydrogen atom, a phenyl group, a benzyl group, or a
alkyl group; R2 is a hydrogen atom or a Cw alkyl group; R1 and R2, when taken together with the attached carbon atom, can be representative of a saturated cyclic carbon ring having five or more carbon atoms; and Y represents the group:
or
wherein R" and R" are independently hydrogen atoms, C-_12 alkyl groups, C6.10 aryl groups, or C-.12 alkyl substituted aryl groups or aryl substituted alkyl groups, and n is an
integer of 2 or 3.
19. A vinyl polymer comprising about 0.1 to 99 mole-% of random units of the monomer of claim 18.
20. A hydrocurable adhesive comprising about 0.1 to 20 wt-% of the vinyl polymer of claim 19 and about 5 to 99.9 wt-% of an isocyanate terminated polymer comprising the reaction product of a sterically hindered aliphatic isocyanate compound having at least two isocyanate groups; and a polymeric polyol compound having at least two hydroxy groups, wherein there are about 1.05 to 5 equivalent of - NCO per equivalent of -OH.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US65196291A | 1991-02-07 | 1991-02-07 | |
| US651,962 | 1991-02-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1992013907A1 true WO1992013907A1 (en) | 1992-08-20 |
Family
ID=24614967
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1992/000176 WO1992013907A1 (en) | 1991-02-07 | 1992-01-10 | Thermally stable curable hindered isocyanate-oxazolidine composition |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO1992013907A1 (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996021688A1 (en) * | 1995-01-13 | 1996-07-18 | Essex Specialty Products, Inc. | Two-part moisture curable polyurethane adhesive |
| EP0830933A1 (en) * | 1996-09-24 | 1998-03-25 | Sollac S.A. | Method of fabrication of a core for a composite plate |
| US5852103A (en) * | 1996-05-08 | 1998-12-22 | Essex Specialty Products, Inc. | Two-part moisture curable polyurethane adhesive |
| US5977285A (en) * | 1997-08-07 | 1999-11-02 | Akzo Nobel N.V. | Sprayable coating compositions comprising oxazolidines, isocyanates and hydroxyl or amine functional resins |
| WO2000073262A1 (en) * | 1999-05-29 | 2000-12-07 | Bayer Aktiengesellschaft | Polymerizable, olefinically unsaturated monomers |
| US6271334B1 (en) | 1997-08-07 | 2001-08-07 | Akzo Nobel Nv | Sprayable coating compositions comprising oxazolidines and isocyanates |
| FR2842818A1 (en) * | 2002-07-26 | 2004-01-30 | Centre Nat Rech Scient | ADHESIVE COMPOSITION FOR WET MATERIAL |
| WO2007024836A2 (en) * | 2005-08-23 | 2007-03-01 | E. I. Du Pont De Nemours And Company | Preparation of tetrahydro-1,8-dioxa-4a-aza-naphthalenes and their use in coating applications |
| WO2010068736A2 (en) | 2008-12-12 | 2010-06-17 | Henkel Corporation | Reactive hot melt adhesive |
| US8362125B2 (en) | 2007-04-03 | 2013-01-29 | Henkel Ag & Co. Kgaa | Hot melt adhesive |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3661861A (en) * | 1970-05-12 | 1972-05-09 | Commercial Solvents Corp | Protective coating which is produced by reacting moisture with a prepolymer made by reacting an oxazoline with a diisocyanate |
| US4024117A (en) * | 1973-12-14 | 1977-05-17 | Rohm And Haas Company | Hydrocurable compositions of hydroxy(polyalkylenecarbonyloxy)-alkyleneoxazolidine and an isocyanate |
| US4101527A (en) * | 1977-02-18 | 1978-07-18 | The Sherwin-Williams Company | Moisture curing polymers prepared from polyfunctional isocyanates and a dioxazabicyclo octane |
| EP0141025A1 (en) * | 1983-11-04 | 1985-05-15 | Reichhold Chemicals, Inc. | Storage stable one component urethane compounds and method for making and using same |
| EP0372264A1 (en) * | 1988-11-14 | 1990-06-13 | Reichhold Chemicals, Inc. | High performance one-component urethane compositions with excellent weathering properties and method for making and using same |
-
1992
- 1992-01-10 WO PCT/US1992/000176 patent/WO1992013907A1/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3661861A (en) * | 1970-05-12 | 1972-05-09 | Commercial Solvents Corp | Protective coating which is produced by reacting moisture with a prepolymer made by reacting an oxazoline with a diisocyanate |
| US4024117A (en) * | 1973-12-14 | 1977-05-17 | Rohm And Haas Company | Hydrocurable compositions of hydroxy(polyalkylenecarbonyloxy)-alkyleneoxazolidine and an isocyanate |
| US4101527A (en) * | 1977-02-18 | 1978-07-18 | The Sherwin-Williams Company | Moisture curing polymers prepared from polyfunctional isocyanates and a dioxazabicyclo octane |
| EP0141025A1 (en) * | 1983-11-04 | 1985-05-15 | Reichhold Chemicals, Inc. | Storage stable one component urethane compounds and method for making and using same |
| EP0372264A1 (en) * | 1988-11-14 | 1990-06-13 | Reichhold Chemicals, Inc. | High performance one-component urethane compositions with excellent weathering properties and method for making and using same |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1070875C (en) * | 1995-01-13 | 2001-09-12 | 爱赛克斯特种产品公司 | Two-part moisture curable polyurethane adhesive |
| US5603798A (en) * | 1995-01-13 | 1997-02-18 | The Dow Chemical Company | Two-part moisture curable polyurethane adhesive |
| US5672652A (en) * | 1995-01-13 | 1997-09-30 | Essex Specialty Products Inc. | Two-part moisture curable polyurethane adhesive |
| WO1996021688A1 (en) * | 1995-01-13 | 1996-07-18 | Essex Specialty Products, Inc. | Two-part moisture curable polyurethane adhesive |
| US5852103A (en) * | 1996-05-08 | 1998-12-22 | Essex Specialty Products, Inc. | Two-part moisture curable polyurethane adhesive |
| EP0830933A1 (en) * | 1996-09-24 | 1998-03-25 | Sollac S.A. | Method of fabrication of a core for a composite plate |
| FR2753652A1 (en) * | 1996-09-24 | 1998-03-27 | Lorraine Laminage | METHOD FOR MANUFACTURING SOFT TABLE AND COMPOSITE PANEL |
| US6545117B1 (en) | 1997-08-07 | 2003-04-08 | Akzo Noble N.V. | Sprayable coating compositions comprising an oxazolidine functional compound, an isocyanate functional compound, and a compound selected from a mercapto and a sulfonic acid functional compound |
| US6271334B1 (en) | 1997-08-07 | 2001-08-07 | Akzo Nobel Nv | Sprayable coating compositions comprising oxazolidines and isocyanates |
| US5977285A (en) * | 1997-08-07 | 1999-11-02 | Akzo Nobel N.V. | Sprayable coating compositions comprising oxazolidines, isocyanates and hydroxyl or amine functional resins |
| WO2000073262A1 (en) * | 1999-05-29 | 2000-12-07 | Bayer Aktiengesellschaft | Polymerizable, olefinically unsaturated monomers |
| FR2842818A1 (en) * | 2002-07-26 | 2004-01-30 | Centre Nat Rech Scient | ADHESIVE COMPOSITION FOR WET MATERIAL |
| WO2004011520A1 (en) * | 2002-07-26 | 2004-02-05 | Centre National De La Recherche Scientifique (C.N.R.S) | Adhesive composition for moist material |
| WO2007024836A3 (en) * | 2005-08-23 | 2007-05-31 | Du Pont | Preparation of tetrahydro-1,8-dioxa-4a-aza-naphthalenes and their use in coating applications |
| WO2007024836A2 (en) * | 2005-08-23 | 2007-03-01 | E. I. Du Pont De Nemours And Company | Preparation of tetrahydro-1,8-dioxa-4a-aza-naphthalenes and their use in coating applications |
| US7812173B2 (en) | 2005-08-23 | 2010-10-12 | E.I. Du Pont De Nemours And Company | Tetrahydro-1,8-dioxa-4a-aza-naphthalenes in coating applications |
| US8362125B2 (en) | 2007-04-03 | 2013-01-29 | Henkel Ag & Co. Kgaa | Hot melt adhesive |
| WO2010068736A2 (en) | 2008-12-12 | 2010-06-17 | Henkel Corporation | Reactive hot melt adhesive |
| EP2356189A2 (en) * | 2008-12-12 | 2011-08-17 | Henkel Corporation | Reactive hot melt adhesive |
| US8277601B2 (en) | 2008-12-12 | 2012-10-02 | Henkel Corporation | Reactive hot melt adhesive |
| EP2356189A4 (en) * | 2008-12-12 | 2012-10-17 | Henkel Corp | Reactive hot melt adhesive |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US3743626A (en) | Hydrocurable oxazolidine-isocyanate compositions | |
| RU2272818C2 (en) | Adhesion enhancer for monomer-free reactive polyurethanes | |
| US4874805A (en) | Novel oxazolidines | |
| US4381388A (en) | Storage stable one component urethanes and method for using same | |
| US4396681A (en) | Process for coating one pot moisture curable coating composition onto non-porous substrate and article | |
| EP0141025B1 (en) | Storage stable one component urethane compounds and method for making and using same | |
| JPH07506362A (en) | Carbodiimides and their production method | |
| JPH02221287A (en) | Silane group-containing oxazolidine | |
| US4046744A (en) | Thermosetting coatings based on ketoxime-blocked isocyanates and oxazolidines | |
| GB2038811A (en) | Blocked isocyanate diols preparation thereof and polyurethanes prepared therefrom | |
| US5015321A (en) | Adhesive and its use for producing adhesive bonds | |
| JP2728304B2 (en) | Polyaldimine derivative | |
| US4879365A (en) | High preformance one-component urethane compositions with excellent weathering properties and method for making and using same | |
| US4032686A (en) | Articles coated with a cured composition of hydroxy(polyalkylenecarbonyloxy)alkyleneoxazolidine and a polyisocyanate | |
| US4193832A (en) | Method for making bonds | |
| WO1992013907A1 (en) | Thermally stable curable hindered isocyanate-oxazolidine composition | |
| US4443590A (en) | Composition for polyurethane resins and production of the same | |
| US4024117A (en) | Hydrocurable compositions of hydroxy(polyalkylenecarbonyloxy)-alkyleneoxazolidine and an isocyanate | |
| US3912691A (en) | Hydrocurable compositions of hydroxy (polyalkylenecarbonyloxy)-alkyleneoxazolidine and an isocyanate | |
| CN110698622A (en) | Ketimine latent curing agent, reactive polyurethane hot melt adhesive with ketimine latent curing agent and application of reactive polyurethane hot melt adhesive | |
| CA1197941A (en) | Powder coating composition | |
| JPH0255715A (en) | Storage-stable, hydraulic, one-pack oxazolidine-isocyanate composition | |
| US4719244A (en) | Polyisocyanate preparations and their use for the production of polyurethane plastics | |
| KR900007513B1 (en) | B-amino-b-propiolactamderivatives and moisture-curable polyurethane composition prepared therefrom | |
| JPH0424394B2 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): JP |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB GR IT LU MC NL SE |