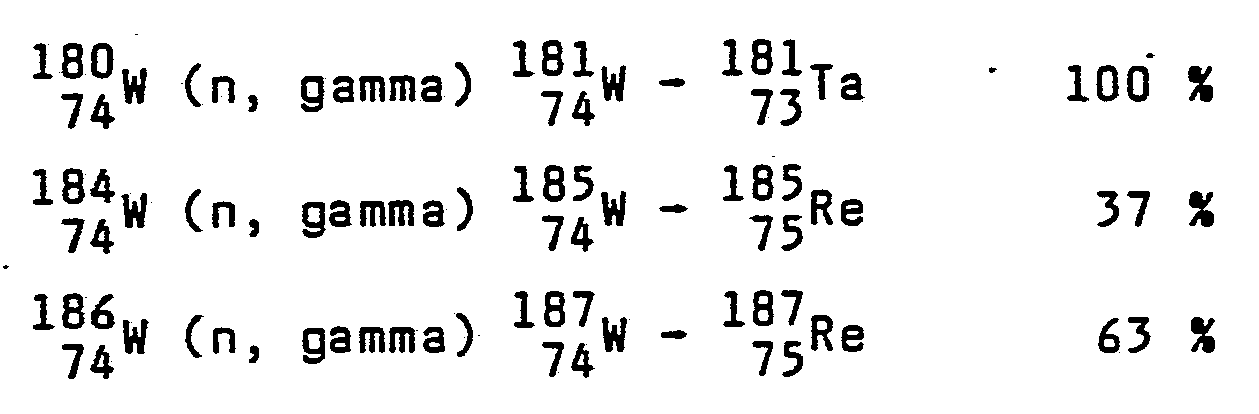WO1990006583A1 - METHOD OF UTILIZING THE (n, gamma) REACTION OF THERMAL NEUTRONS - Google Patents
METHOD OF UTILIZING THE (n, gamma) REACTION OF THERMAL NEUTRONS Download PDFInfo
- Publication number
- WO1990006583A1 WO1990006583A1 PCT/HU1989/000054 HU8900054W WO9006583A1 WO 1990006583 A1 WO1990006583 A1 WO 1990006583A1 HU 8900054 W HU8900054 W HU 8900054W WO 9006583 A1 WO9006583 A1 WO 9006583A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- target
- neutrons
- thermal neutrons
- gamma
- reaction
- Prior art date
Links
Classifications
-
- G—PHYSICS
- G21—NUCLEAR PHYSICS; NUCLEAR ENGINEERING
- G21G—CONVERSION OF CHEMICAL ELEMENTS; RADIOACTIVE SOURCES
- G21G1/00—Arrangements for converting chemical elements by electromagnetic radiation, corpuscular radiation or particle bombardment, e.g. producing radioactive isotopes
- G21G1/04—Arrangements for converting chemical elements by electromagnetic radiation, corpuscular radiation or particle bombardment, e.g. producing radioactive isotopes outside nuclear reactors or particle accelerators
- G21G1/06—Arrangements for converting chemical elements by electromagnetic radiation, corpuscular radiation or particle bombardment, e.g. producing radioactive isotopes outside nuclear reactors or particle accelerators by neutron irradiation
Definitions
- the present invention refers to a method of utilizing the (n, gamma) reaction of thermal neutrons, wherein a target is arranged before a source of thermal neutrons.
- the method of the invention results in possibility of making use of the thermal neutron flux of a nuclear reactor, with disregard to the kind of the reactor, whereby the economy of operating of the different reactors can be highly improved.
- the proposed method can be realised with reactors of diverse kinds, e.g. with experimental reactors, energetic or boiler reactors etc.
- the object of the present invention is to make use of the thermal neutron flux of a reactor for producing non radioactive materials, wherein no special security measures are to be taken.
- the invention is based on the recognition that ytterbium and tungsten can be transformed into a mixture of different elements showing no or very low level radioactivity by means of the thermal neutrons generated in each radioactive reactor.
- the invention proposes a method of utilizing the (n, gamma) reaction of thermal neutrons of a reactor, the method comprising the step of arranging a target directed with its front surface to a source of thermal neutrons, especially a reactor, wherein according to the invention the target is consisted of 70 Yb and/or 74 W. It is especially advantageous to apply before the target a plate shaped body for slowing down the quick and/or the reactor neutrons, consisted of 41 Nb for slowing down the reactorneutrons and/or 59 Pr for slowing down the quick neutrons.
- this moderator of neutrons can be made also of beryllium.
- a beryllium plated can be applied also for covering the rear side of the target - this ensures reflection of the neutrons back to the target.
- about 30 % of the amount of ytterbium can be transformed into lutetion and the same amount of tungsten into rhenium. Above that about 20 % of tungsten transform into osmium.
- the metals received, i.e. lutetium, rhenium and osmium are much more expensive than the input metal of the process and can be separated therefrom by simple thermal processing because of considerable differences in the respective melting points.
- FIG. 1 shows the cross-section of a target applied in realising the present invention.
- the target 2 In the vicinity of a reactor 1 limited by a wall 7 a target 2 is arranged in an appropriate place.
- the target 2 consists of a front layer 3 forming a moderating body, a metal plate 4 including ytterbium and/or tungsten to be transformed and a rear reflecting layer 5.
- the front layer 3 is made of 41 Nb and/or 59 Pr. If necessary, 4 Be can be applied to.
- the mentioned metals slow down the flux of the neutrons leaving the interior of the reactor 2.
- the reflecting layer 5 covering the rear surface of the metal plate 4 reflects the neutrons back to the metal plate 4.
- the target 2 is arranged to be irradiated by a thermal neutron flux 6 and the front layer 3 receives the neutrons before entering the metal plate 4.
- the neutron flux 6 can be directed to the target 2 through the wall 7 of the raactor 1 in a known way, e.g. by the means of a window prepared in the wall 7.
- the metal plate 4 is made of ytterbium and/or tungsten.
- the irradiation of this plate carried out by the thermal neutrons generated by the reactor 1 or produced by the front layer in a (n, 2n) reaction should result in an alloy like mixture consisting of the following metals (the composition is given with approxinate data):
- the metal When taking ytterbium, the metal includes the following isotopes:
- the percentage values means the proportion of the given stable isotope in the metal mentioned.
- the metal When taking tungsten, the metal includes the following isotopes: v
- rhenium Re can be also activated and in decay processes (e-, gamma, K) it can be transformed partly into tungsten, partly into osmium: a dominate part, however, remains unchanged in form of rhenium.
- the gamma radiation coming into being is a low energy, low intensity weak radiation.
- the metallic mixtures prepared by the invention require at least 1/2 year storage before further processing. During this time the radiation level of the mixture falls under a maximum level allowed by the rules.
- the target 2 in the proximity of the active zone of the reactor, but under the condition that the target can not be the object of radiation comprising charged particles and fission products. If these factors are excluded the only disturbing effects follow from the gamma radiation of the reactor and the flux of quick neutrons emitted from the reactor. In both cases the loss of neutrons by the nucleus can follow in (gamma, n) and (n, 2n) reactions, however, these are low probability processes Therefore the only requirement is to moderate the quick neutrons, because the reactions with loss of neutron constitute a part of the reactions which hardly play important rule.
- the reactor neutrons show a wide spectrum with average energy 0.72 MeV (the flux may contain also neutrons with energy 20 MeV), therefore it is advantageous to slow down (moderate) the reactor neutrons and the quick neutrons by the means of (n, 2n) reactions whereby the yield of neutrons can be increased.
- the beryllium moderator is in this case a further element after that applied for slowing down the reactor and quick neutrons.
- the reactions of the reactor neutrons are characterized by small cross-section. Hence, they can be slowed down by means of the reaction 93
- the target 2 includes advantageously a rear reflecting layer 5 for reflecting back the neutrons.
- This layer can be made of beryllium ( 4 Be).
- the plate 4 of the target 2 is arranged preferably so that the neutron flux of the reactor falls under right angle (90°) on its surface.
- the method of the invention should be realised with a target 2 including after the reactor a layer made of ⁇ and/or b, a moderator (of 4 Be), the metal plate 4 made of 74 W and/or 70 Yb and a mirror layer (rear reflecting layer 5, made of 4 Be) .
- the beryllium can be preferred because it is a neutron source under influence of the gamma radiation emitted by the reactor, with the following reactions:
- the process of the invention can be applied for preparing catalyzer substances - this improves the economy of operating a reactor. No specific security means or expenses are necessary.
- the metal mixtures can be separated into components according to the known thermal techniques or applied as alloys.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- General Engineering & Computer Science (AREA)
- High Energy & Nuclear Physics (AREA)
- Particle Accelerators (AREA)
Abstract
The invention refers to a method of utilizing the (n, gamma) reaction of thermal neutrons, comprising the steps of arranging a target (2) before a source (1) of thermal neutrons, the target (2) having a front surface directed to the source (1) of the thermal neutrons and a rear surface lying behind the front surface, preparing the target (2) with a basic metal body (4) made of 70Yb and/or 74W, producing by the means of the thermal neutrons a metallic mixture including the basic metal(s) and at least one of the pairs of metals 71Lu + 72Hf and 75Re + 76Os and storing the metallic mixture for reducing its nuclear activity.
Description
METHOD OF UTILIZING THE (n, gamma) REACTION
OF THERMAL NEUTRONS
FIELD OF THE INVENTION
The present invention refers to a method of utilizing the (n, gamma) reaction of thermal neutrons, wherein a target is arranged before a source of thermal neutrons. The method of the invention results in possibility of making use of the thermal neutron flux of a nuclear reactor, with disregard to the kind of the reactor, whereby the economy of operating of the different reactors can be highly improved. The proposed method can be realised with reactors of diverse kinds, e.g. with experimental reactors, energetic or boiler reactors etc.
BACKGROUND OF THE INVENTION
It is known from the literature that the (n, gamma) reaction can be applied for producing some isotopes. For example, the reaction
is the basis of generating the very important isotope of cobalt having mass numer 60 which is widely used in the medicine and industry. In this process the end product is a substance showing high level of radioactivity (gamma-activity). This process may not be ralised without special security measures.
The theory of atomic nuclei recites lots of theoretical and practical reactions for transforming chemical elements, i.e. atomic nuclei. In the handbooks e.g. the process
can be found for producing gold, wherein the half-period of decay of the intermediate platinum isotope is relatively short, about 20 hours. This way of producing gold is very expensive and inconvenient: the substance at the beginning of the process is twice so expensive than the gold received. Another disadvantage of this process is that the platinum isotope with mass number 196 amounts about 25.3 % of the whole platinum mass and therefore a separate process is necessary for yielding the gold.
SUMMARY OF THE INVENTION
The object of the present invention is to make use of the thermal neutron flux of a reactor for producing non radioactive materials, wherein no special security measures are to be taken.
The invention is based on the recognition that ytterbium and tungsten can be transformed into a mixture of different elements showing no or very low level radioactivity by means of the thermal neutrons generated in each radioactive reactor.
Hence, the invention proposes a method of utilizing the (n, gamma) reaction of thermal neutrons of a reactor, the method comprising the step of arranging a target directed with its front surface to a source of thermal neutrons, especially a reactor, wherein according to the invention the target is consisted of 70Yb and/or 74W. It is especially advantageous to apply before the target a plate shaped body for slowing down the quick and/or the reactor neutrons, consisted of 41Nb for slowing down the reactorneutrons and/or 59Pr for slowing down the quick neutrons. Of course, this moderator of neutrons can be made also of beryllium. A beryllium plated can be applied also for covering the rear side of the target - this ensures reflection of the neutrons back to the target.
By the means of the method proposed by the invention about 30 % of the amount of ytterbium can be transformed into lutetion and the same amount of tungsten into rhenium. Above that about 20 % of tungsten transform into osmium. The metals received, i.e. lutetium, rhenium and osmium are much more expensive than the input metal of the process and can be separated therefrom by simple thermal processing because of considerable differences in the respective melting points.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be further disclosed in more detail by way of example and with reference to the attached drawings. In the drawings
FIG. 1 shows the cross-section of a target applied in realising the present invention.
DETAILLED DESCRIPTION OF THE PREFERRED EMBODIMENTS
In the vicinity of a reactor 1 limited by a wall 7 a target 2 is arranged in an appropriate place. The target 2 consists of a front layer 3 forming a moderating body, a metal plate 4 including ytterbium and/or tungsten to be transformed and a rear reflecting layer 5. The front layer 3 is made of 41Nb and/or 59Pr. If necessary, 4Be can be applied to. The mentioned metals slow down the flux of the neutrons leaving the interior of the reactor 2. The reflecting layer 5 covering the rear surface of the metal plate 4 reflects the neutrons back to the metal plate 4. The target 2 is arranged to be irradiated by a thermal neutron flux 6 and the front layer 3 receives the neutrons before entering the metal plate 4.
The neutron flux 6 can be directed to the target 2 through the wall 7 of the raactor 1 in a known way, e.g.
by the means of a window prepared in the wall 7.
As mentioned, the metal plate 4 is made of ytterbium and/or tungsten. The irradiation of this plate carried out by the thermal neutrons generated by the reactor 1 or produced by the front layer in a (n, 2n) reaction should result in an alloy like mixture consisting of the following metals (the composition is given with approxinate data):
a) on the basis of ytterbium:
37 112 101.4 127
70Yb + 71Lu + 72Hf (+ 69Tm)
60 % 30 % 10 % 0.1 %
b) on the basis of tungsten:
19.2 86 15.3 21
74w + 75Re + 76Os (+ 73Ta)
50 % 30 % 20 % 0.1 %
The line over the signs of the elements give the value of the cross-section for the process expressed in barns.
When taking ytterbium, the metal includes the following isotopes:
From this table it follows that about 55.3 % of all (n, gamma) reactions do not result in any change of the atomic number. These reactions are:
The following reactions result in transformation of elements:
The percentage values means the proportion of the given stable isotope in the metal mentioned.
ed by the half-period 6.75 days.
When taking tungsten, the metal includes the following isotopes: v
From this table it follows that about 40.8 % of all (n, gamma) reactions do not result in any change of the atomic number. These reactions are:
The following reactions result in transformation of elements :
In normal circumstances, 7 e is transformed into
characterised by half-period about 5.1010 years by weak e- radiation. In a (n, gamma) reaction, however another process dominates:
The half-period of rhenium is 18 hours, the isomeric osmium nucleus shows half-period 26 days. In these conditions the rhenium
Re can be also activated and in decay processes (e-, gamma, K) it can be transformed partly into tungsten, partly into osmium: a dominate part, however, remains unchanged in form of rhenium.
In both series of reactions, the gamma radiation coming into being is a low energy, low intensity weak radiation.
The metallic mixtures prepared by the invention require at least 1/2 year storage before further processing. During this time the radiation level of the mixture falls under a maximum level allowed by the rules.
When considering the basic metal and the metallic components produced bv the method of the invention it can be stated that they are capable of bearing high thermal load and the alloy received in the process is stable. The melting points of the metals in the mixtures mentioned are the following:
70Yb + 71Lu + 72Hf + 69Tm
824 ºc 1652 ºC 2222 ºC 1545 °C
74W + 75Re + 7 6Os + 73Ta
3410 ºC 3180 ºC 2700 °C 2996 °C
In realising the method of the invention it is advantageous to arrange the target 2 in the proximity of the active zone of the reactor, but under the condition that the target can not be the object of radiation comprising charged particles and fission products. If these factors are excluded the only disturbing effects follow from the gamma radiation of the reactor and the flux of quick neutrons emitted from the reactor. In both cases the loss of neutrons by the nucleus can follow in (gamma, n) and (n, 2n) reactions, however, these are low probability processes Therefore the only requirement is to moderate the quick neutrons, because the reactions with loss of neutron constitute a part of the reactions which hardly play important rule.
The reactor neutrons show a wide spectrum with average energy 0.72 MeV (the flux may contain also neutrons with energy 20 MeV), therefore it is advantageous to slow down (moderate) the reactor neutrons and the quick neutrons by the means of (n, 2n) reactions whereby the yield of neutrons can be increased. The beryllium moderator is in this case a further element after that applied for slowing down the reactor and quick neutrons.
The reactions of the reactor neutrons are characterized by small cross-section. Hence, they can be slowed down by means of the reaction 93
effective reaction for slowing down the quick neutrons having energy in the range about 14 to 15 MeV is based on praesodymium: (n, 2n) . The processes mentioned
result in increased yield of neutrons. The advantageous character of these reaction can be seen from the following
scheme of reactions:
10 days, decay e-, gamma, K) - (n, 2n)
Other reaction scheme are possible with low probability, because of the short half-period.
The target 2 includes advantageously a rear reflecting layer 5 for reflecting back the neutrons. This layer can be made of beryllium (4Be).
The plate 4 of the target 2 is arranged preferably so that the neutron flux of the reactor falls under right angle (90°) on its surface.
Summarizing, the method of the invention should be realised with a target 2 including after the reactor a layer made of
^ and/or
b, a moderator (of 4Be), the metal plate 4 made of 74W and/or 70Yb and a mirror layer (rear reflecting layer 5, made of 4Be) . The beryllium can be preferred because it is a neutron source under influence of the gamma radiation emitted by the reactor, with the following reactions:
wherein the neutrons at the output have energy 110 keV.
The process of the invention can be applied for preparing catalyzer substances - this improves the economy of operating a reactor. No specific security means or expenses are necessary. The metal mixtures can be separated into components according to the known thermal techniques or applied as alloys.
Claims
1. Method of utilizing the (n, gamma) reaction of thermal neutrons, comprising the step of arranging a target before a source of thermal neutrons, the target having a front surface directed to the source of the thermal neutrons and a rear surface behind the front surface,
characterized in the further steps of - preparing the target with a basic metal body made of at least one metal selected from the group including 70Yb and 74W,
- producing by the means of thermal neutrons a metallic mixture including the basic metal(s) and at least one pair of metals selected from the group including 71Lu +
+ 72Hf and 75Re + 76Os and
- storing the metallic mixture for reducing its activity.
2. The method as set forth in claim 1, characterized in preparing the basic metal body in the form of a plate and arranging it perpendicularly to the flux of the thermal neutrons.
3. The method as set forth in claim 1 or 2, characterized in the step of arranging on the front surface of the basic metal body a layer for slowing down fast and reactor neutrons by the means of (n, 2n) reactions, the layer consisting of at least one metal selected from the group including 41Nb and 59Pr.
4. The method as set forth in any of claims 1 to 3, characterized in the step of arranging at least one beryllium moderating layer on at least one of the front and rear surfaces of the target.
5. The method as set forth in any of claims 1 to 4, characterized in the further step of carrying out thermal decomposition of the metallic mixture after the storing period.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| HU607788 | 1988-11-28 | ||
| HU6077/88 | 1988-11-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1990006583A1 true WO1990006583A1 (en) | 1990-06-14 |
Family
ID=10971176
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/HU1989/000054 WO1990006583A1 (en) | 1988-11-28 | 1989-11-20 | METHOD OF UTILIZING THE (n, gamma) REACTION OF THERMAL NEUTRONS |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0400122A1 (en) |
| AU (1) | AU4528389A (en) |
| CA (1) | CA2003671A1 (en) |
| WO (1) | WO1990006583A1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998059347A1 (en) * | 1997-06-19 | 1998-12-30 | European Organization For Nuclear Research | Neutron-driven element transmuter |
| WO2011144954A3 (en) * | 2010-05-20 | 2012-01-12 | Teleki Peter | Method of utilizing nuclear reactions of neutrons to produce primarily lanthanides and/or platinum metals |
| DE10037439B4 (en) * | 2000-07-25 | 2012-06-28 | Helmholtz-Zentrum Dresden - Rossendorf E.V. | Method and device for activating the radioactivity of atomic nuclei, in particular for activating short-lived radioactive isotopes for medical purposes |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB440023A (en) * | 1934-03-12 | 1935-12-18 | Leo Szilard | Improvements in or relating to the transmutation of chemical elements |
| GB974622A (en) * | 1960-08-30 | 1964-11-11 | Atomic Energy Authority Uk | Improvements in or relating to targets for neutron generators |
| GB1075411A (en) * | 1963-10-04 | 1967-07-12 | Nra Inc | Solid tritium and deuterium targets for neutron generators |
| DE1908144A1 (en) * | 1968-02-20 | 1969-09-11 | Stark Donald Sutherland | Neutron target |
| US4055686A (en) * | 1976-02-20 | 1977-10-25 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | Method of forming metal hydride films |
| DE2941096A1 (en) * | 1978-10-13 | 1980-04-30 | Philips Nv | NEUTRON GENERATOR WITH A MEETING PLATE |
-
1989
- 1989-11-20 EP EP19890912826 patent/EP0400122A1/en not_active Withdrawn
- 1989-11-20 AU AU45283/89A patent/AU4528389A/en not_active Abandoned
- 1989-11-20 WO PCT/HU1989/000054 patent/WO1990006583A1/en not_active Application Discontinuation
- 1989-11-23 CA CA 2003671 patent/CA2003671A1/en not_active Abandoned
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB440023A (en) * | 1934-03-12 | 1935-12-18 | Leo Szilard | Improvements in or relating to the transmutation of chemical elements |
| GB974622A (en) * | 1960-08-30 | 1964-11-11 | Atomic Energy Authority Uk | Improvements in or relating to targets for neutron generators |
| GB1075411A (en) * | 1963-10-04 | 1967-07-12 | Nra Inc | Solid tritium and deuterium targets for neutron generators |
| DE1908144A1 (en) * | 1968-02-20 | 1969-09-11 | Stark Donald Sutherland | Neutron target |
| GB1243262A (en) * | 1968-02-20 | 1971-08-18 | Nat Res Dev | Improvements in or relating to neutron targets |
| US4055686A (en) * | 1976-02-20 | 1977-10-25 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | Method of forming metal hydride films |
| DE2941096A1 (en) * | 1978-10-13 | 1980-04-30 | Philips Nv | NEUTRON GENERATOR WITH A MEETING PLATE |
Non-Patent Citations (2)
| Title |
|---|
| K.H. HOCKER, K. WEIMER, "Lexikon der Kern- und Reaktortechnik", published 1959, Franckh'sche Verlagshandlung W. Keller, Stuttgart, see page 635. * |
| W. EPPRECHT, "Werkstoffkunde der Kerntechnik", published 1961, Birkhaeuser Verlag, Basel und Stuttgart, see pages 221,323. * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998059347A1 (en) * | 1997-06-19 | 1998-12-30 | European Organization For Nuclear Research | Neutron-driven element transmuter |
| CZ298765B6 (en) * | 1997-06-19 | 2008-01-23 | European Organization For Nuclear Research | Method of exposing material to neutron flux, method of producing useful isotope comprising such exposing method and method of transmuting at least one long-lived isotope comprising such exposing method |
| DE10037439B4 (en) * | 2000-07-25 | 2012-06-28 | Helmholtz-Zentrum Dresden - Rossendorf E.V. | Method and device for activating the radioactivity of atomic nuclei, in particular for activating short-lived radioactive isotopes for medical purposes |
| WO2011144954A3 (en) * | 2010-05-20 | 2012-01-12 | Teleki Peter | Method of utilizing nuclear reactions of neutrons to produce primarily lanthanides and/or platinum metals |
| US9431139B2 (en) | 2010-05-20 | 2016-08-30 | Péter Teleki | Method of utilizing nuclear reactions of neutrons to produce primarily lanthanides and/or platinum metals |
Also Published As
| Publication number | Publication date |
|---|---|
| AU4528389A (en) | 1990-06-26 |
| CA2003671A1 (en) | 1990-05-28 |
| EP0400122A1 (en) | 1990-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Vandenbosch et al. | Spallation-Fission Competition in Heaviest Elements; Helium-Ion-Induced Reactions in Uranium Isotopes | |
| Slack et al. | Radiations from radioactive atoms in frequent use | |
| Kulcinski et al. | Comparison of Displacement and Gas Pro | |
| WO1990006583A1 (en) | METHOD OF UTILIZING THE (n, gamma) REACTION OF THERMAL NEUTRONS | |
| Moser et al. | Residual nuclei after antiproton annihilation in Mo and Ho | |
| Csikai | Utilization of intense neutron generators in science and technology | |
| Mukhrjee et al. | Pre-equilibrium analysis of the excitation functions of (α, xn) reactions on silver and holmium | |
| Debs et al. | Yields of Photonuclear Reactions with 320-Mev X-Rays. I. Experimental Results | |
| Hansen et al. | Predictions for neutron transport in air, based on integral measurements in nitrogen and oxygen at 14 MeV | |
| Quang et al. | Absolute measurements of the fast neutron capture cross section of 238U | |
| Balestrini et al. | Independent fission yields of Rb and Cs from U 238 induced by fission-spectrum neutrons | |
| Carter et al. | Monte Carlo Code Development in Los Alamos | |
| Khan et al. | Nuclear charge dispersion of light-mass fission products in the fission of 235U and 238U by medium-energy protons | |
| WO1991015857A1 (en) | Method of utilizing the k capture process by the means of high energy electrons | |
| Croft et al. | Mössbauer effect in 231Pa | |
| Abe et al. | Feasibility study of nuclear transmutation by negative muon capture reaction using the PHITS code | |
| Seaborg | The synthetic actinides-from discovery to manufacture | |
| Pollard | Masses of Stable Nuclei from Ne 20 to Fe 57 | |
| Heiman | Variation of gamma radiation rates for different elements following an underwater nuclear detonation | |
| Depuydt et al. | Average cross section of the 32S (n, p) 32P and 27AI (n, α) 24Na reactions for fission neutrons | |
| Poenitz | Fast neutron capture and activation cross sections of niobium isotopes | |
| Ullmaier | 2. 1 (n, alpha)-reactions: 3.2 Production processes and rates | |
| Zisin et al. | The cross section of the reaction 232Th (n, 2n) 231Th at 14· 7 MeV | |
| Kramer | SUMMARY OF THE RESEARCH PROGRESS MEETING OF SEPT. 7, 1950 | |
| Semat et al. | Fission and Fusion of Nuclei |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AU SU US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE ES FR GB IT LU NL SE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1989912826 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1989912826 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1989912826 Country of ref document: EP |