USRE44186E1 - Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method - Google Patents
Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method Download PDFInfo
- Publication number
- USRE44186E1 USRE44186E1 US13/308,658 US201113308658A USRE44186E US RE44186 E1 USRE44186 E1 US RE44186E1 US 201113308658 A US201113308658 A US 201113308658A US RE44186 E USRE44186 E US RE44186E
- Authority
- US
- United States
- Prior art keywords
- compound
- inhibitor
- agent
- mmol
- pharmaceutically acceptable
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime, expires
Links
- 239000003112 inhibitor Substances 0.000 title claims abstract description 80
- 238000000034 method Methods 0.000 title claims abstract description 48
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 title claims description 12
- 102000016622 Dipeptidyl Peptidase 4 Human genes 0.000 title abstract description 14
- 101000930822 Giardia intestinalis Dipeptidyl-peptidase 4 Proteins 0.000 title abstract description 14
- 150000001875 compounds Chemical class 0.000 claims abstract description 271
- 239000003472 antidiabetic agent Substances 0.000 claims abstract description 37
- 229940125708 antidiabetic agent Drugs 0.000 claims abstract description 33
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 18
- 201000010099 disease Diseases 0.000 claims abstract description 17
- 206010012601 diabetes mellitus Diseases 0.000 claims abstract description 16
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 claims abstract description 14
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 10
- XZWYZXLIPXDOLR-UHFFFAOYSA-N metformin Chemical compound CN(C)C(=N)NC(N)=N XZWYZXLIPXDOLR-UHFFFAOYSA-N 0.000 claims abstract description 10
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims abstract description 10
- 229960003105 metformin Drugs 0.000 claims abstract description 9
- YASAKCUCGLMORW-UHFFFAOYSA-N Rosiglitazone Chemical compound C=1C=CC=NC=1N(C)CCOC(C=C1)=CC=C1CC1SC(=O)NC1=O YASAKCUCGLMORW-UHFFFAOYSA-N 0.000 claims abstract description 8
- 239000000883 anti-obesity agent Substances 0.000 claims abstract description 8
- 229940125710 antiobesity agent Drugs 0.000 claims abstract description 8
- HYAFETHFCAUJAY-UHFFFAOYSA-N pioglitazone Chemical compound N1=CC(CC)=CC=C1CCOC(C=C1)=CC=C1CC1C(=O)NC(=O)S1 HYAFETHFCAUJAY-UHFFFAOYSA-N 0.000 claims abstract description 8
- 108090001061 Insulin Proteins 0.000 claims abstract description 7
- 229940125396 insulin Drugs 0.000 claims abstract description 7
- 229960004580 glibenclamide Drugs 0.000 claims abstract description 6
- ZNNLBTZKUZBEKO-UHFFFAOYSA-N glyburide Chemical compound COC1=CC=C(Cl)C=C1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCCCC2)C=C1 ZNNLBTZKUZBEKO-UHFFFAOYSA-N 0.000 claims abstract description 6
- 229960005095 pioglitazone Drugs 0.000 claims abstract description 4
- 229960004586 rosiglitazone Drugs 0.000 claims abstract description 4
- GXPHKUHSUJUWKP-UHFFFAOYSA-N troglitazone Chemical compound C1CC=2C(C)=C(O)C(C)=C(C)C=2OC1(C)COC(C=C1)=CC=C1CC1SC(=O)NC1=O GXPHKUHSUJUWKP-UHFFFAOYSA-N 0.000 claims abstract description 4
- 229960001641 troglitazone Drugs 0.000 claims abstract description 4
- GXPHKUHSUJUWKP-NTKDMRAZSA-N troglitazone Natural products C([C@@]1(OC=2C(C)=C(C(=C(C)C=2CC1)O)C)C)OC(C=C1)=CC=C1C[C@H]1SC(=O)NC1=O GXPHKUHSUJUWKP-NTKDMRAZSA-N 0.000 claims abstract description 3
- 230000002401 inhibitory effect Effects 0.000 claims abstract 2
- -1 arylalkylthioalkyl Chemical group 0.000 claims description 99
- 239000000203 mixture Substances 0.000 claims description 91
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical class OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 claims description 62
- 125000000217 alkyl group Chemical group 0.000 claims description 41
- 239000003795 chemical substances by application Substances 0.000 claims description 40
- 125000003118 aryl group Chemical group 0.000 claims description 29
- 102100038129 Transcription factor Dp family member 3 Human genes 0.000 claims description 28
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 28
- 125000001072 heteroaryl group Chemical group 0.000 claims description 18
- 125000003342 alkenyl group Chemical group 0.000 claims description 17
- 125000003545 alkoxy group Chemical group 0.000 claims description 16
- 125000000304 alkynyl group Chemical group 0.000 claims description 16
- 125000003710 aryl alkyl group Chemical group 0.000 claims description 16
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 13
- 150000003839 salts Chemical class 0.000 claims description 13
- 125000004414 alkyl thio group Chemical group 0.000 claims description 11
- 125000004658 aryl carbonyl amino group Chemical group 0.000 claims description 11
- 125000005843 halogen group Chemical group 0.000 claims description 11
- 102100031545 Microsomal triglyceride transfer protein large subunit Human genes 0.000 claims description 10
- 125000001316 cycloalkyl alkyl group Chemical group 0.000 claims description 10
- 125000004446 heteroarylalkyl group Chemical group 0.000 claims description 10
- 208000011580 syndromic disease Diseases 0.000 claims description 10
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims description 9
- 150000001602 bicycloalkyls Chemical group 0.000 claims description 9
- 125000004432 carbon atom Chemical group C* 0.000 claims description 9
- 150000002148 esters Chemical class 0.000 claims description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims description 9
- 125000005119 alkyl cycloalkyl group Chemical group 0.000 claims description 8
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 8
- 125000000392 cycloalkenyl group Chemical group 0.000 claims description 8
- 230000000694 effects Effects 0.000 claims description 8
- 125000005350 hydroxycycloalkyl group Chemical group 0.000 claims description 8
- 229940123518 Sodium/glucose cotransporter 2 inhibitor Drugs 0.000 claims description 7
- 229940100389 Sulfonylurea Drugs 0.000 claims description 7
- 125000003806 alkyl carbonyl amino group Chemical group 0.000 claims description 7
- 125000005842 heteroatom Chemical group 0.000 claims description 7
- 239000004059 squalene synthase inhibitor Substances 0.000 claims description 7
- 201000001320 Atherosclerosis Diseases 0.000 claims description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 6
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 claims description 6
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 6
- 102000004877 Insulin Human genes 0.000 claims description 6
- 208000008589 Obesity Diseases 0.000 claims description 6
- 102000023984 PPAR alpha Human genes 0.000 claims description 6
- 108010028924 PPAR alpha Proteins 0.000 claims description 6
- 102000000536 PPAR gamma Human genes 0.000 claims description 6
- 108010016731 PPAR gamma Proteins 0.000 claims description 6
- 125000002252 acyl group Chemical group 0.000 claims description 6
- 125000004466 alkoxycarbonylamino group Chemical group 0.000 claims description 6
- 125000004949 alkyl amino carbonyl amino group Chemical group 0.000 claims description 6
- 239000003937 drug carrier Substances 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 6
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 claims description 6
- 235000020824 obesity Nutrition 0.000 claims description 6
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 claims description 6
- ZGGHKIMDNBDHJB-NRFPMOEYSA-M (3R,5S)-fluvastatin sodium Chemical compound [Na+].C12=CC=CC=C2N(C(C)C)C(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)=C1C1=CC=C(F)C=C1 ZGGHKIMDNBDHJB-NRFPMOEYSA-M 0.000 claims description 5
- 208000030507 AIDS Diseases 0.000 claims description 5
- XUKUURHRXDUEBC-KAYWLYCHSA-N Atorvastatin Chemical compound C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CC[C@@H](O)C[C@@H](O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-KAYWLYCHSA-N 0.000 claims description 5
- XUKUURHRXDUEBC-UHFFFAOYSA-N Atorvastatin Natural products C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CCC(O)CC(O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-UHFFFAOYSA-N 0.000 claims description 5
- 208000023275 Autoimmune disease Diseases 0.000 claims description 5
- 229940123208 Biguanide Drugs 0.000 claims description 5
- 208000002249 Diabetes Complications Diseases 0.000 claims description 5
- 206010012655 Diabetic complications Diseases 0.000 claims description 5
- 208000036119 Frailty Diseases 0.000 claims description 5
- 102000004366 Glucosidases Human genes 0.000 claims description 5
- 108010056771 Glucosidases Proteins 0.000 claims description 5
- 206010060378 Hyperinsulinaemia Diseases 0.000 claims description 5
- 229940122355 Insulin sensitizer Drugs 0.000 claims description 5
- 208000001145 Metabolic Syndrome Diseases 0.000 claims description 5
- TUZYXOIXSAXUGO-UHFFFAOYSA-N Pravastatin Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(O)C=C21 TUZYXOIXSAXUGO-UHFFFAOYSA-N 0.000 claims description 5
- RYMZZMVNJRMUDD-UHFFFAOYSA-N SJ000286063 Natural products C12C(OC(=O)C(C)(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 RYMZZMVNJRMUDD-UHFFFAOYSA-N 0.000 claims description 5
- 206010003246 arthritis Diseases 0.000 claims description 5
- 206010003549 asthenia Diseases 0.000 claims description 5
- 229960005370 atorvastatin Drugs 0.000 claims description 5
- 229960005110 cerivastatin Drugs 0.000 claims description 5
- SEERZIQQUAZTOL-ANMDKAQQSA-N cerivastatin Chemical compound COCC1=C(C(C)C)N=C(C(C)C)C(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)=C1C1=CC=C(F)C=C1 SEERZIQQUAZTOL-ANMDKAQQSA-N 0.000 claims description 5
- 229960003765 fluvastatin Drugs 0.000 claims description 5
- 208000037824 growth disorder Diseases 0.000 claims description 5
- 230000003451 hyperinsulinaemic effect Effects 0.000 claims description 5
- 201000008980 hyperinsulinism Diseases 0.000 claims description 5
- 230000002519 immonomodulatory effect Effects 0.000 claims description 5
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 claims description 5
- AHLBNYSZXLDEJQ-FWEHEUNISA-N orlistat Chemical group CCCCCCCCCCC[C@H](OC(=O)[C@H](CC(C)C)NC=O)C[C@@H]1OC(=O)[C@H]1CCCCCC AHLBNYSZXLDEJQ-FWEHEUNISA-N 0.000 claims description 5
- 229960001243 orlistat Drugs 0.000 claims description 5
- 201000010065 polycystic ovary syndrome Diseases 0.000 claims description 5
- 229960002965 pravastatin Drugs 0.000 claims description 5
- TUZYXOIXSAXUGO-PZAWKZKUSA-N pravastatin Chemical compound C1=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC(O)=O)[C@H]2[C@@H](OC(=O)[C@@H](C)CC)C[C@H](O)C=C21 TUZYXOIXSAXUGO-PZAWKZKUSA-N 0.000 claims description 5
- 229960002855 simvastatin Drugs 0.000 claims description 5
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 claims description 5
- 229910052717 sulfur Inorganic materials 0.000 claims description 5
- 238000002054 transplantation Methods 0.000 claims description 5
- SWLAMJPTOQZTAE-UHFFFAOYSA-N 4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]benzoic acid Chemical compound COC1=CC=C(Cl)C=C1C(=O)NCCC1=CC=C(C(O)=O)C=C1 SWLAMJPTOQZTAE-UHFFFAOYSA-N 0.000 claims description 4
- 208000002705 Glucose Intolerance Diseases 0.000 claims description 4
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 claims description 4
- 125000003282 alkyl amino group Chemical group 0.000 claims description 4
- 125000005162 aryl oxy carbonyl amino group Chemical group 0.000 claims description 4
- 125000005161 aryl oxy carbonyl group Chemical group 0.000 claims description 4
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 claims description 4
- ZJJXGWJIGJFDTL-UHFFFAOYSA-N glipizide Chemical compound C1=NC(C)=CN=C1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCCCC2)C=C1 ZJJXGWJIGJFDTL-UHFFFAOYSA-N 0.000 claims description 4
- 229960001381 glipizide Drugs 0.000 claims description 4
- 201000001421 hyperglycemia Diseases 0.000 claims description 4
- 229960004844 lovastatin Drugs 0.000 claims description 4
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 claims description 4
- 229950004994 meglitinide Drugs 0.000 claims description 4
- 230000003647 oxidation Effects 0.000 claims description 4
- 238000007254 oxidation reaction Methods 0.000 claims description 4
- 229910052760 oxygen Inorganic materials 0.000 claims description 4
- DHHVAGZRUROJKS-UHFFFAOYSA-N phentermine Chemical compound CC(C)(N)CC1=CC=CC=C1 DHHVAGZRUROJKS-UHFFFAOYSA-N 0.000 claims description 4
- 201000009104 prediabetes syndrome Diseases 0.000 claims description 4
- YROXIXLRRCOBKF-UHFFFAOYSA-N sulfonylurea Chemical compound OC(=N)N=S(=O)=O YROXIXLRRCOBKF-UHFFFAOYSA-N 0.000 claims description 4
- 102000004217 thyroid hormone receptors Human genes 0.000 claims description 4
- 108090000721 thyroid hormone receptors Proteins 0.000 claims description 4
- XUFXOAAUWZOOIT-SXARVLRPSA-N (2R,3R,4R,5S,6R)-5-[[(2R,3R,4R,5S,6R)-5-[[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)-1-cyclohex-2-enyl]amino]-2-oxanyl]oxy]-3,4-dihydroxy-6-(hydroxymethyl)-2-oxanyl]oxy]-6-(hydroxymethyl)oxane-2,3,4-triol Chemical compound O([C@H]1O[C@H](CO)[C@H]([C@@H]([C@H]1O)O)O[C@H]1O[C@@H]([C@H]([C@H](O)[C@H]1O)N[C@@H]1[C@@H]([C@@H](O)[C@H](O)C(CO)=C1)O)C)[C@@H]1[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]1O XUFXOAAUWZOOIT-SXARVLRPSA-N 0.000 claims description 3
- KWTSXDURSIMDCE-QMMMGPOBSA-N (S)-amphetamine Chemical compound C[C@H](N)CC1=CC=CC=C1 KWTSXDURSIMDCE-QMMMGPOBSA-N 0.000 claims description 3
- BOVGTQGAOIONJV-BETUJISGSA-N 1-[(3ar,6as)-3,3a,4,5,6,6a-hexahydro-1h-cyclopenta[c]pyrrol-2-yl]-3-(4-methylphenyl)sulfonylurea Chemical compound C1=CC(C)=CC=C1S(=O)(=O)NC(=O)NN1C[C@H]2CCC[C@H]2C1 BOVGTQGAOIONJV-BETUJISGSA-N 0.000 claims description 3
- ROJNYKZWTOHRNU-UHFFFAOYSA-N 2-chloro-4,5-difluoro-n-[[2-methoxy-5-(methylcarbamoylamino)phenyl]carbamoyl]benzamide Chemical compound CNC(=O)NC1=CC=C(OC)C(NC(=O)NC(=O)C=2C(=CC(F)=C(F)C=2)Cl)=C1 ROJNYKZWTOHRNU-UHFFFAOYSA-N 0.000 claims description 3
- ILPUOPPYSQEBNJ-UHFFFAOYSA-N 2-methyl-2-phenoxypropanoic acid Chemical class OC(=O)C(C)(C)OC1=CC=CC=C1 ILPUOPPYSQEBNJ-UHFFFAOYSA-N 0.000 claims description 3
- 102000004146 ATP citrate synthases Human genes 0.000 claims description 3
- 108090000662 ATP citrate synthases Proteins 0.000 claims description 3
- 208000000103 Anorexia Nervosa Diseases 0.000 claims description 3
- XNCOSPRUTUOJCJ-UHFFFAOYSA-N Biguanide Chemical compound NC(N)=NC(N)=N XNCOSPRUTUOJCJ-UHFFFAOYSA-N 0.000 claims description 3
- RKWGIWYCVPQPMF-UHFFFAOYSA-N Chloropropamide Chemical compound CCCNC(=O)NS(=O)(=O)C1=CC=C(Cl)C=C1 RKWGIWYCVPQPMF-UHFFFAOYSA-N 0.000 claims description 3
- HEMJJKBWTPKOJG-UHFFFAOYSA-N Gemfibrozil Chemical compound CC1=CC=C(C)C(OCCCC(C)(C)C(O)=O)=C1 HEMJJKBWTPKOJG-UHFFFAOYSA-N 0.000 claims description 3
- FAEKWTJYAYMJKF-QHCPKHFHSA-N GlucoNorm Chemical compound C1=C(C(O)=O)C(OCC)=CC(CC(=O)N[C@@H](CC(C)C)C=2C(=CC=CC=2)N2CCCCC2)=C1 FAEKWTJYAYMJKF-QHCPKHFHSA-N 0.000 claims description 3
- 206010022489 Insulin Resistance Diseases 0.000 claims description 3
- 229940086609 Lipase inhibitor Drugs 0.000 claims description 3
- IBAQFPQHRJAVAV-ULAWRXDQSA-N Miglitol Chemical compound OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO IBAQFPQHRJAVAV-ULAWRXDQSA-N 0.000 claims description 3
- 208000001132 Osteoporosis Diseases 0.000 claims description 3
- 229940080774 Peroxisome proliferator-activated receptor gamma agonist Drugs 0.000 claims description 3
- 229940123495 Squalene synthetase inhibitor Drugs 0.000 claims description 3
- KJADKKWYZYXHBB-XBWDGYHZSA-N Topiramic acid Chemical compound C1O[C@@]2(COS(N)(=O)=O)OC(C)(C)O[C@H]2[C@@H]2OC(C)(C)O[C@@H]21 KJADKKWYZYXHBB-XBWDGYHZSA-N 0.000 claims description 3
- 229960002632 acarbose Drugs 0.000 claims description 3
- XUFXOAAUWZOOIT-UHFFFAOYSA-N acarviostatin I01 Natural products OC1C(O)C(NC2C(C(O)C(O)C(CO)=C2)O)C(C)OC1OC(C(C1O)O)C(CO)OC1OC1C(CO)OC(O)C(O)C1O XUFXOAAUWZOOIT-UHFFFAOYSA-N 0.000 claims description 3
- 239000000048 adrenergic agonist Substances 0.000 claims description 3
- 125000004457 alkyl amino carbonyl group Chemical group 0.000 claims description 3
- 125000004448 alkyl carbonyl group Chemical group 0.000 claims description 3
- 125000005196 alkyl carbonyloxy group Chemical group 0.000 claims description 3
- 229940125709 anorectic agent Drugs 0.000 claims description 3
- 239000002830 appetite depressant Substances 0.000 claims description 3
- 125000001769 aryl amino group Chemical group 0.000 claims description 3
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 3
- 229960001761 chlorpropamide Drugs 0.000 claims description 3
- 230000001684 chronic effect Effects 0.000 claims description 3
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 3
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 3
- 229940125542 dual agonist Drugs 0.000 claims description 3
- 239000000194 fatty acid Substances 0.000 claims description 3
- 229930195729 fatty acid Natural products 0.000 claims description 3
- 150000004665 fatty acids Chemical class 0.000 claims description 3
- 229940125753 fibrate Drugs 0.000 claims description 3
- 229960003627 gemfibrozil Drugs 0.000 claims description 3
- 229960000346 gliclazide Drugs 0.000 claims description 3
- 229960004346 glimepiride Drugs 0.000 claims description 3
- WIGIZIANZCJQQY-RUCARUNLSA-N glimepiride Chemical compound O=C1C(CC)=C(C)CN1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)N[C@@H]2CC[C@@H](C)CC2)C=C1 WIGIZIANZCJQQY-RUCARUNLSA-N 0.000 claims description 3
- 230000014101 glucose homeostasis Effects 0.000 claims description 3
- 125000004438 haloalkoxy group Chemical group 0.000 claims description 3
- 230000001771 impaired effect Effects 0.000 claims description 3
- 208000000509 infertility Diseases 0.000 claims description 3
- 230000036512 infertility Effects 0.000 claims description 3
- 231100000535 infertility Toxicity 0.000 claims description 3
- 150000002632 lipids Chemical class 0.000 claims description 3
- 239000002697 lyase inhibitor Substances 0.000 claims description 3
- 229960001110 miglitol Drugs 0.000 claims description 3
- PKWDZWYVIHVNKS-UHFFFAOYSA-N netoglitazone Chemical compound FC1=CC=CC=C1COC1=CC=C(C=C(CC2C(NC(=O)S2)=O)C=C2)C2=C1 PKWDZWYVIHVNKS-UHFFFAOYSA-N 0.000 claims description 3
- 125000006684 polyhaloalkyl group Polymers 0.000 claims description 3
- GCYXWQUSHADNBF-AAEALURTSA-N preproglucagon 78-108 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 GCYXWQUSHADNBF-AAEALURTSA-N 0.000 claims description 3
- 229960002354 repaglinide Drugs 0.000 claims description 3
- 229940076279 serotonin Drugs 0.000 claims description 3
- 229960004425 sibutramine Drugs 0.000 claims description 3
- UNAANXDKBXWMLN-UHFFFAOYSA-N sibutramine Chemical compound C=1C=C(Cl)C=CC=1C1(C(N(C)C)CC(C)C)CCC1 UNAANXDKBXWMLN-UHFFFAOYSA-N 0.000 claims description 3
- 229960004394 topiramate Drugs 0.000 claims description 3
- AMNXBQPRODZJQR-DITALETJSA-N (2s)-2-cyclopentyl-2-[3-[(2,4-dimethylpyrido[2,3-b]indol-9-yl)methyl]phenyl]-n-[(1r)-2-hydroxy-1-phenylethyl]acetamide Chemical compound C1([C@@H](C=2C=CC=C(C=2)CN2C3=CC=CC=C3C3=C(C)C=C(N=C32)C)C(=O)N[C@@H](CO)C=2C=CC=CC=2)CCCC1 AMNXBQPRODZJQR-DITALETJSA-N 0.000 claims description 2
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 claims description 2
- QBQLYIISSRXYKL-UHFFFAOYSA-N 4-[[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]methyl]-1,2-oxazolidine-3,5-dione Chemical compound CC=1OC(C=2C=CC=CC=2)=NC=1CCOC(C=C1)=CC=C1CC1C(=O)NOC1=O QBQLYIISSRXYKL-UHFFFAOYSA-N 0.000 claims description 2
- NFFXEUUOMTXWCX-UHFFFAOYSA-N 5-[(2,4-dioxo-1,3-thiazolidin-5-yl)methyl]-2-methoxy-n-[[4-(trifluoromethyl)phenyl]methyl]benzamide Chemical compound C1=C(C(=O)NCC=2C=CC(=CC=2)C(F)(F)F)C(OC)=CC=C1CC1SC(=O)NC1=O NFFXEUUOMTXWCX-UHFFFAOYSA-N 0.000 claims description 2
- IETKPTYAGKZLKY-UHFFFAOYSA-N 5-[[4-[(3-methyl-4-oxoquinazolin-2-yl)methoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione Chemical compound N=1C2=CC=CC=C2C(=O)N(C)C=1COC(C=C1)=CC=C1CC1SC(=O)NC1=O IETKPTYAGKZLKY-UHFFFAOYSA-N 0.000 claims description 2
- PTQXTEKSNBVPQJ-UHFFFAOYSA-N Avasimibe Chemical compound CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1CC(=O)NS(=O)(=O)OC1=C(C(C)C)C=CC=C1C(C)C PTQXTEKSNBVPQJ-UHFFFAOYSA-N 0.000 claims description 2
- HTQBXNHDCUEHJF-XWLPCZSASA-N Exenatide Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 HTQBXNHDCUEHJF-XWLPCZSASA-N 0.000 claims description 2
- 108010011459 Exenatide Proteins 0.000 claims description 2
- GGUVRMBIEPYOKL-WMVCGJOFSA-N GW 409544 Chemical compound C([C@H](NC(/C)=C\C(=O)C=1C=CC=CC=1)C(O)=O)C(C=C1)=CC=C1OCCC(=C(O1)C)N=C1C1=CC=CC=C1 GGUVRMBIEPYOKL-WMVCGJOFSA-N 0.000 claims description 2
- 102000000853 LDL receptors Human genes 0.000 claims description 2
- 108010001831 LDL receptors Proteins 0.000 claims description 2
- 239000000867 Lipoxygenase Inhibitor Substances 0.000 claims description 2
- 108010019598 Liraglutide Proteins 0.000 claims description 2
- 229940122014 Lyase inhibitor Drugs 0.000 claims description 2
- ZPXSCAKFGYXMGA-UHFFFAOYSA-N Mazindol Chemical compound N12CCN=C2C2=CC=CC=C2C1(O)C1=CC=C(Cl)C=C1 ZPXSCAKFGYXMGA-UHFFFAOYSA-N 0.000 claims description 2
- 125000005089 alkenylaminocarbonyl group Chemical group 0.000 claims description 2
- 125000004644 alkyl sulfinyl group Chemical group 0.000 claims description 2
- 125000004390 alkyl sulfonyl group Chemical group 0.000 claims description 2
- 125000004656 alkyl sulfonylamino group Chemical group 0.000 claims description 2
- 125000006350 alkyl thio alkyl group Chemical group 0.000 claims description 2
- 125000005095 alkynylaminocarbonyl group Chemical group 0.000 claims description 2
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 claims description 2
- 230000003262 anti-osteoporosis Effects 0.000 claims description 2
- 229950010046 avasimibe Drugs 0.000 claims description 2
- 108010014210 axokine Proteins 0.000 claims description 2
- MVCQKIKWYUURMU-UHFFFAOYSA-N cetilistat Chemical compound C1=C(C)C=C2C(=O)OC(OCCCCCCCCCCCCCCCC)=NC2=C1 MVCQKIKWYUURMU-UHFFFAOYSA-N 0.000 claims description 2
- 229950002397 cetilistat Drugs 0.000 claims description 2
- JUFFVKRROAPVBI-PVOYSMBESA-N chembl1210015 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(=O)N[C@H]1[C@@H]([C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO[C@]3(O[C@@H](C[C@H](O)[C@H](O)CO)[C@H](NC(C)=O)[C@@H](O)C3)C(O)=O)O2)O)[C@@H](CO)O1)NC(C)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 JUFFVKRROAPVBI-PVOYSMBESA-N 0.000 claims description 2
- 239000003354 cholesterol ester transfer protein inhibitor Substances 0.000 claims description 2
- 229960001214 clofibrate Drugs 0.000 claims description 2
- KNHUKKLJHYUCFP-UHFFFAOYSA-N clofibrate Chemical compound CCOC(=O)C(C)(C)OC1=CC=C(Cl)C=C1 KNHUKKLJHYUCFP-UHFFFAOYSA-N 0.000 claims description 2
- 125000004663 dialkyl amino group Chemical group 0.000 claims description 2
- DLNKOYKMWOXYQA-UHFFFAOYSA-N dl-pseudophenylpropanolamine Natural products CC(N)C(O)C1=CC=CC=C1 DLNKOYKMWOXYQA-UHFFFAOYSA-N 0.000 claims description 2
- 229960003638 dopamine Drugs 0.000 claims description 2
- 229960001519 exenatide Drugs 0.000 claims description 2
- 229960002297 fenofibrate Drugs 0.000 claims description 2
- YMTINGFKWWXKFG-UHFFFAOYSA-N fenofibrate Chemical compound C1=CC(OC(C)(C)C(=O)OC(C)C)=CC=C1C(=O)C1=CC=C(Cl)C=C1 YMTINGFKWWXKFG-UHFFFAOYSA-N 0.000 claims description 2
- 235000011187 glycerol Nutrition 0.000 claims description 2
- 125000005241 heteroarylamino group Chemical group 0.000 claims description 2
- 229950005809 implitapide Drugs 0.000 claims description 2
- 230000002757 inflammatory effect Effects 0.000 claims description 2
- 208000028774 intestinal disease Diseases 0.000 claims description 2
- 229960002701 liraglutide Drugs 0.000 claims description 2
- 229960000299 mazindol Drugs 0.000 claims description 2
- 229960000698 nateglinide Drugs 0.000 claims description 2
- OELFLUMRDSZNSF-BRWVUGGUSA-N nateglinide Chemical compound C1C[C@@H](C(C)C)CC[C@@H]1C(=O)N[C@@H](C(O)=O)CC1=CC=CC=C1 OELFLUMRDSZNSF-BRWVUGGUSA-N 0.000 claims description 2
- 229960003562 phentermine Drugs 0.000 claims description 2
- DLNKOYKMWOXYQA-APPZFPTMSA-N phenylpropanolamine Chemical compound C[C@@H](N)[C@H](O)C1=CC=CC=C1 DLNKOYKMWOXYQA-APPZFPTMSA-N 0.000 claims description 2
- 229960000395 phenylpropanolamine Drugs 0.000 claims description 2
- 229960002797 pitavastatin Drugs 0.000 claims description 2
- RHGYHLPFVJEAOC-FFNUKLMVSA-L pitavastatin calcium Chemical compound [Ca+2].[O-]C(=O)C[C@H](O)C[C@H](O)\C=C\C1=C(C2CC2)N=C2C=CC=CC2=C1C1=CC=C(F)C=C1.[O-]C(=O)C[C@H](O)C[C@H](O)\C=C\C1=C(C2CC2)N=C2C=CC=CC2=C1C1=CC=C(F)C=C1 RHGYHLPFVJEAOC-FFNUKLMVSA-L 0.000 claims description 2
- 125000005592 polycycloalkyl group Polymers 0.000 claims description 2
- 229940002612 prodrug Drugs 0.000 claims description 2
- 239000000651 prodrug Substances 0.000 claims description 2
- 241000894007 species Species 0.000 claims description 2
- 125000005420 sulfonamido group Chemical group S(=O)(=O)(N*)* 0.000 claims description 2
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 claims description 2
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 claims 4
- 241000124008 Mammalia Species 0.000 claims 4
- 125000004356 hydroxy functional group Chemical group O* 0.000 claims 3
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims 2
- 125000004076 pyridyl group Chemical group 0.000 claims 2
- 229940122142 Lipoxygenase inhibitor Drugs 0.000 claims 1
- 125000001091 aminosulfinyl group Chemical group [H]N([H])S(*)=O 0.000 claims 1
- 230000036765 blood level Effects 0.000 claims 1
- 229940125881 cholesteryl ester transfer protein inhibitor Drugs 0.000 claims 1
- 235000021588 free fatty acids Nutrition 0.000 claims 1
- 208000006575 hypertriglyceridemia Diseases 0.000 claims 1
- 125000003396 thiol group Chemical class [H]S* 0.000 claims 1
- 239000003524 antilipemic agent Substances 0.000 abstract description 15
- 239000003814 drug Substances 0.000 abstract description 14
- 229940124597 therapeutic agent Drugs 0.000 abstract description 7
- 102100023915 Insulin Human genes 0.000 abstract 1
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 194
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 189
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 118
- 239000000243 solution Substances 0.000 description 95
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 84
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 81
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 76
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 70
- 238000007429 general method Methods 0.000 description 64
- 235000019439 ethyl acetate Nutrition 0.000 description 58
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 57
- 239000011541 reaction mixture Substances 0.000 description 56
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 42
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 42
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 39
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 39
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 39
- 238000006243 chemical reaction Methods 0.000 description 39
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 38
- 238000003818 flash chromatography Methods 0.000 description 38
- 239000000047 product Substances 0.000 description 37
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 36
- 239000000741 silica gel Substances 0.000 description 36
- 229910002027 silica gel Inorganic materials 0.000 description 36
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 33
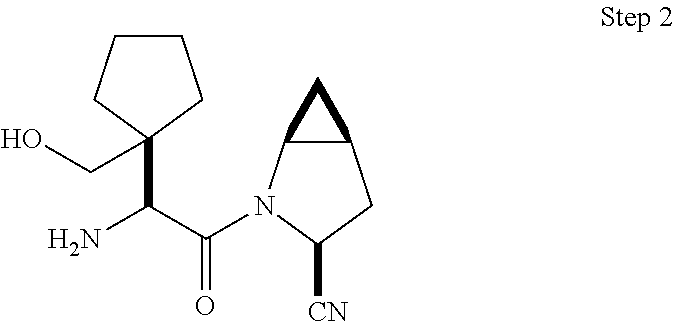
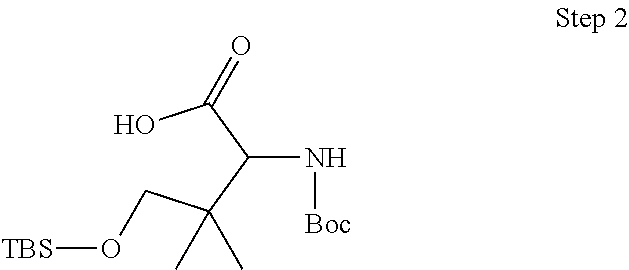
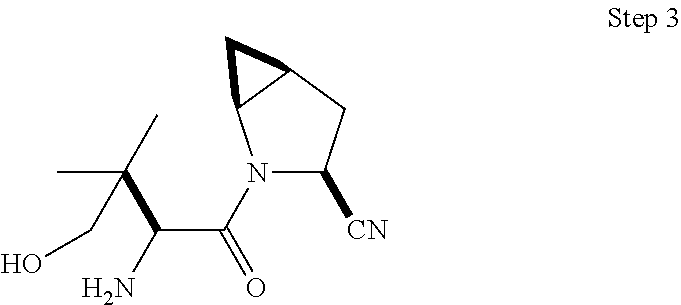
- 0 [1*]C([2*])(C(=O)N1CC2CC2CC1C)C([4*])N([3*])[H] Chemical compound [1*]C([2*])(C(=O)N1CC2CC2CC1C)C([4*])N([3*])[H] 0.000 description 32
- 229940024606 amino acid Drugs 0.000 description 31
- 239000007787 solid Substances 0.000 description 31
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 29
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 28
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 28
- 239000012267 brine Substances 0.000 description 27
- 239000003921 oil Substances 0.000 description 27
- 238000000746 purification Methods 0.000 description 27
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 26
- 230000002829 reductive effect Effects 0.000 description 26
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 25
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 24
- 229910052757 nitrogen Inorganic materials 0.000 description 24
- 150000001408 amides Chemical group 0.000 description 23
- 238000010511 deprotection reaction Methods 0.000 description 23
- 238000003756 stirring Methods 0.000 description 23
- 238000005897 peptide coupling reaction Methods 0.000 description 22
- 230000018044 dehydration Effects 0.000 description 21
- 238000006297 dehydration reaction Methods 0.000 description 21
- 239000010410 layer Substances 0.000 description 21
- 150000001413 amino acids Chemical class 0.000 description 20
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 20
- 229910052786 argon Inorganic materials 0.000 description 19
- 150000002500 ions Chemical class 0.000 description 19
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 18
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 18
- 239000002904 solvent Substances 0.000 description 18
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 description 16
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 15
- 238000002390 rotary evaporation Methods 0.000 description 15
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 14
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 14
- BGTOWKSIORTVQH-UHFFFAOYSA-N cyclopentanone Chemical compound O=C1CCCC1 BGTOWKSIORTVQH-UHFFFAOYSA-N 0.000 description 14
- DYHSDKLCOJIUFX-UHFFFAOYSA-N tert-butoxycarbonyl anhydride Chemical compound CC(C)(C)OC(=O)OC(=O)OC(C)(C)C DYHSDKLCOJIUFX-UHFFFAOYSA-N 0.000 description 14
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 13
- 229910019213 POCl3 Inorganic materials 0.000 description 13
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 13
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 13
- 125000001424 substituent group Chemical group 0.000 description 13
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 12
- 229910004373 HOAc Inorganic materials 0.000 description 12
- 102000001494 Sterol O-Acyltransferase Human genes 0.000 description 12
- 108010054082 Sterol O-acyltransferase Proteins 0.000 description 12
- 239000002002 slurry Substances 0.000 description 12
- 102000004190 Enzymes Human genes 0.000 description 11
- 108090000790 Enzymes Proteins 0.000 description 11
- 101800004295 Glucagon-like peptide 1(7-36) Proteins 0.000 description 11
- 102400000325 Glucagon-like peptide 1(7-36) Human genes 0.000 description 11
- 239000012230 colorless oil Substances 0.000 description 11
- 239000000706 filtrate Substances 0.000 description 11
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 11
- 239000002253 acid Substances 0.000 description 10
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 10
- 239000000284 extract Substances 0.000 description 10
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 10
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 9
- 150000002825 nitriles Chemical class 0.000 description 9
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 150000001412 amines Chemical class 0.000 description 8
- 150000001793 charged compounds Chemical class 0.000 description 8
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 8
- 150000002460 imidazoles Chemical class 0.000 description 8
- 230000005764 inhibitory process Effects 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 239000006186 oral dosage form Substances 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 7
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 7
- 238000007059 Strecker synthesis reaction Methods 0.000 description 7
- 230000008878 coupling Effects 0.000 description 7
- 238000010168 coupling process Methods 0.000 description 7
- 238000005859 coupling reaction Methods 0.000 description 7
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 7
- 238000001727 in vivo Methods 0.000 description 7
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 7
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 7
- 238000010992 reflux Methods 0.000 description 7
- 229920006395 saturated elastomer Polymers 0.000 description 7
- 239000000377 silicon dioxide Substances 0.000 description 7
- 239000006188 syrup Substances 0.000 description 7
- 235000020357 syrup Nutrition 0.000 description 7
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 7
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 6
- RGSFGYAAUTVSQA-UHFFFAOYSA-N Cyclopentane Chemical compound C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 description 6
- XXROGKLTLUQVRX-UHFFFAOYSA-N allyl alcohol Chemical compound OCC=C XXROGKLTLUQVRX-UHFFFAOYSA-N 0.000 description 6
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 238000010828 elution Methods 0.000 description 6
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 6
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 6
- 239000012071 phase Substances 0.000 description 6
- CHKVPAROMQMJNQ-UHFFFAOYSA-M potassium bisulfate Chemical compound [K+].OS([O-])(=O)=O CHKVPAROMQMJNQ-UHFFFAOYSA-M 0.000 description 6
- 229910000343 potassium bisulfate Inorganic materials 0.000 description 6
- 239000002244 precipitate Substances 0.000 description 6
- 238000002953 preparative HPLC Methods 0.000 description 6
- 238000000039 preparative column chromatography Methods 0.000 description 6
- 229960002429 proline Drugs 0.000 description 6
- 125000006239 protecting group Chemical group 0.000 description 6
- 230000002441 reversible effect Effects 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 6
- 150000003573 thiols Chemical class 0.000 description 6
- AQRLNPVMDITEJU-UHFFFAOYSA-N triethylsilane Chemical compound CC[SiH](CC)CC AQRLNPVMDITEJU-UHFFFAOYSA-N 0.000 description 6
- QAEDZJGFFMLHHQ-UHFFFAOYSA-N trifluoroacetic anhydride Chemical compound FC(F)(F)C(=O)OC(=O)C(F)(F)F QAEDZJGFFMLHHQ-UHFFFAOYSA-N 0.000 description 6
- ONDSBJMLAHVLMI-UHFFFAOYSA-N trimethylsilyldiazomethane Chemical compound C[Si](C)(C)[CH-][N+]#N ONDSBJMLAHVLMI-UHFFFAOYSA-N 0.000 description 6
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 5
- FPIRBHDGWMWJEP-UHFFFAOYSA-N 1-hydroxy-7-azabenzotriazole Chemical compound C1=CN=C2N(O)N=NC2=C1 FPIRBHDGWMWJEP-UHFFFAOYSA-N 0.000 description 5
- OISVCGZHLKNMSJ-UHFFFAOYSA-N 2,6-dimethylpyridine Chemical compound CC1=CC=CC(C)=N1 OISVCGZHLKNMSJ-UHFFFAOYSA-N 0.000 description 5
- YEDUAINPPJYDJZ-UHFFFAOYSA-N 2-hydroxybenzothiazole Chemical compound C1=CC=C2SC(O)=NC2=C1 YEDUAINPPJYDJZ-UHFFFAOYSA-N 0.000 description 5
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 5
- 102000009515 Arachidonate 15-Lipoxygenase Human genes 0.000 description 5
- 108010048907 Arachidonate 15-lipoxygenase Proteins 0.000 description 5
- OPGMZBQAJHOUTC-UHFFFAOYSA-N CC(C)(C)C1(CO)CCCC1 Chemical compound CC(C)(C)C1(CO)CCCC1 OPGMZBQAJHOUTC-UHFFFAOYSA-N 0.000 description 5
- 101800000224 Glucagon-like peptide 1 Proteins 0.000 description 5
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 5
- 102100040918 Pro-glucagon Human genes 0.000 description 5
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical class [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 5
- 229940123464 Thiazolidinedione Drugs 0.000 description 5
- 230000002378 acidificating effect Effects 0.000 description 5
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 5
- 239000002775 capsule Substances 0.000 description 5
- 239000000460 chlorine Substances 0.000 description 5
- 229910052801 chlorine Inorganic materials 0.000 description 5
- 239000012043 crude product Substances 0.000 description 5
- SHQSVMDWKBRBGB-UHFFFAOYSA-N cyclobutanone Chemical compound O=C1CCC1 SHQSVMDWKBRBGB-UHFFFAOYSA-N 0.000 description 5
- 239000002552 dosage form Substances 0.000 description 5
- 229910052731 fluorine Inorganic materials 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 125000001188 haloalkyl group Chemical group 0.000 description 5
- 230000014759 maintenance of location Effects 0.000 description 5
- MZRVEZGGRBJDDB-UHFFFAOYSA-N n-Butyllithium Substances [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 5
- 239000012279 sodium borohydride Substances 0.000 description 5
- 229910000033 sodium borohydride Inorganic materials 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- GGUBFICZYGKNTD-UHFFFAOYSA-N triethyl phosphonoacetate Chemical compound CCOC(=O)CP(=O)(OCC)OCC GGUBFICZYGKNTD-UHFFFAOYSA-N 0.000 description 5
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 5
- 238000001665 trituration Methods 0.000 description 5
- 239000003039 volatile agent Substances 0.000 description 5
- QJCNLJWUIOIMMF-YUMQZZPRSA-N (2s,3s)-3-methyl-2-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)OC(C)(C)C QJCNLJWUIOIMMF-YUMQZZPRSA-N 0.000 description 4
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 4
- ZOBPZXTWZATXDG-UHFFFAOYSA-N 1,3-thiazolidine-2,4-dione Chemical compound O=C1CSC(=O)N1 ZOBPZXTWZATXDG-UHFFFAOYSA-N 0.000 description 4
- YOETUEMZNOLGDB-UHFFFAOYSA-N 2-methylpropyl carbonochloridate Chemical compound CC(C)COC(Cl)=O YOETUEMZNOLGDB-UHFFFAOYSA-N 0.000 description 4
- TYMLOMAKGOJONV-UHFFFAOYSA-N 4-nitroaniline Chemical compound NC1=CC=C([N+]([O-])=O)C=C1 TYMLOMAKGOJONV-UHFFFAOYSA-N 0.000 description 4
- ZHWCECJLIMXSCD-UHFFFAOYSA-N CC(C)(C)C1(C)CCCCC1 Chemical compound CC(C)(C)C1(C)CCCCC1 ZHWCECJLIMXSCD-UHFFFAOYSA-N 0.000 description 4
- MJSPZDMZMZEFNH-TVHXTYDWSA-N [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 Chemical compound [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 MJSPZDMZMZEFNH-TVHXTYDWSA-N 0.000 description 4
- 239000000556 agonist Substances 0.000 description 4
- 150000001299 aldehydes Chemical class 0.000 description 4
- 125000005236 alkanoylamino group Chemical group 0.000 description 4
- 125000003277 amino group Chemical group 0.000 description 4
- 239000008346 aqueous phase Substances 0.000 description 4
- 125000004104 aryloxy group Chemical group 0.000 description 4
- 229910052794 bromium Inorganic materials 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- CSJLBAMHHLJAAS-UHFFFAOYSA-N diethylaminosulfur trifluoride Chemical compound CCN(CC)S(F)(F)F CSJLBAMHHLJAAS-UHFFFAOYSA-N 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 239000006260 foam Substances 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 238000004949 mass spectrometry Methods 0.000 description 4
- 230000001404 mediated effect Effects 0.000 description 4
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 4
- 239000012044 organic layer Substances 0.000 description 4
- 238000005949 ozonolysis reaction Methods 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- JQWHASGSAFIOCM-UHFFFAOYSA-M sodium periodate Substances [Na+].[O-]I(=O)(=O)=O JQWHASGSAFIOCM-UHFFFAOYSA-M 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- LTMRRSWNXVJMBA-UHFFFAOYSA-L 2,2-diethylpropanedioate Chemical compound CCC(CC)(C([O-])=O)C([O-])=O LTMRRSWNXVJMBA-UHFFFAOYSA-L 0.000 description 3
- PYTMYKVIJXPNBD-OQKDUQJOSA-N 2-[4-[(z)-2-chloro-1,2-diphenylethenyl]phenoxy]-n,n-diethylethanamine;hydron;2-hydroxypropane-1,2,3-tricarboxylate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C1=CC(OCCN(CC)CC)=CC=C1C(\C=1C=CC=CC=1)=C(/Cl)C1=CC=CC=C1 PYTMYKVIJXPNBD-OQKDUQJOSA-N 0.000 description 3
- FRRRFCLRZQETEG-UHFFFAOYSA-N 2-cyclopentylideneethyl 2-[(2-methylpropan-2-yl)oxycarbonylamino]acetate Chemical compound CC(C)(C)OC(=O)NCC(=O)OCC=C1CCCC1 FRRRFCLRZQETEG-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 3
- 238000006969 Curtius rearrangement reaction Methods 0.000 description 3
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 108010000817 Leuprolide Proteins 0.000 description 3
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 3
- OBFPUWUWFMMVNI-SDYISJDRSA-N [C-]#[N+][C@@H]1[C@H]2C[C@H]2CN1C(=O)[C@@H](C)C(C)CC.[C-]#[N+][C@H]1[C@@H]2C[C@@H]2CN1C(=O)[C@@H](C)C(C)CC Chemical compound [C-]#[N+][C@@H]1[C@H]2C[C@H]2CN1C(=O)[C@@H](C)C(C)CC.[C-]#[N+][C@H]1[C@@H]2C[C@@H]2CN1C(=O)[C@@H](C)C(C)CC OBFPUWUWFMMVNI-SDYISJDRSA-N 0.000 description 3
- 150000001336 alkenes Chemical class 0.000 description 3
- 230000000879 anti-atherosclerotic effect Effects 0.000 description 3
- 239000012223 aqueous fraction Substances 0.000 description 3
- 125000005110 aryl thio group Chemical group 0.000 description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 230000003197 catalytic effect Effects 0.000 description 3
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 3
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 3
- WHBIGIKBNXZKFE-UHFFFAOYSA-N delavirdine mesylate Natural products CC(C)NC1=CC=CN=C1N1CCN(C(=O)C=2NC3=CC=C(NS(C)(=O)=O)C=C3C=2)CC1 WHBIGIKBNXZKFE-UHFFFAOYSA-N 0.000 description 3
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 3
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 3
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 3
- 229910000397 disodium phosphate Inorganic materials 0.000 description 3
- 239000003480 eluent Substances 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- KZNQNBZMBZJQJO-YFKPBYRVSA-N glyclproline Chemical compound NCC(=O)N1CCC[C@H]1C(O)=O KZNQNBZMBZJQJO-YFKPBYRVSA-N 0.000 description 3
- 108010077515 glycylproline Proteins 0.000 description 3
- DMEGYFMYUHOHGS-UHFFFAOYSA-N heptamethylene Natural products C1CCCCCC1 DMEGYFMYUHOHGS-UHFFFAOYSA-N 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 238000007327 hydrogenolysis reaction Methods 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 229910000069 nitrogen hydride Inorganic materials 0.000 description 3
- 238000005580 one pot reaction Methods 0.000 description 3
- 239000003538 oral antidiabetic agent Substances 0.000 description 3
- 229910052762 osmium Inorganic materials 0.000 description 3
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 3
- 229910000489 osmium tetroxide Inorganic materials 0.000 description 3
- NXJCBFBQEVOTOW-UHFFFAOYSA-L palladium(2+);dihydroxide Chemical compound O[Pd]O NXJCBFBQEVOTOW-UHFFFAOYSA-L 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 150000003254 radicals Chemical class 0.000 description 3
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 235000017557 sodium bicarbonate Nutrition 0.000 description 3
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- XJDNKRIXUMDJCW-UHFFFAOYSA-J titanium tetrachloride Chemical compound Cl[Ti](Cl)(Cl)Cl XJDNKRIXUMDJCW-UHFFFAOYSA-J 0.000 description 3
- 229920002554 vinyl polymer Polymers 0.000 description 3
- 239000011592 zinc chloride Substances 0.000 description 3
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 3
- HSINOMROUCMIEA-FGVHQWLLSA-N (2s,4r)-4-[(3r,5s,6r,7r,8s,9s,10s,13r,14s,17r)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-methylpentanoic acid Chemical compound C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2CC[C@]2(C)[C@@H]([C@H](C)C[C@H](C)C(O)=O)CC[C@H]21 HSINOMROUCMIEA-FGVHQWLLSA-N 0.000 description 2
- IJXJGQCXFSSHNL-QMMMGPOBSA-N (R)-(-)-2-Phenylglycinol Chemical compound OC[C@H](N)C1=CC=CC=C1 IJXJGQCXFSSHNL-QMMMGPOBSA-N 0.000 description 2
- YWWWGFSJHCFVOW-QMMMGPOBSA-N 1-o-tert-butyl 2-o-ethyl (2s)-5-oxopyrrolidine-1,2-dicarboxylate Chemical compound CCOC(=O)[C@@H]1CCC(=O)N1C(=O)OC(C)(C)C YWWWGFSJHCFVOW-QMMMGPOBSA-N 0.000 description 2
- 125000004214 1-pyrrolidinyl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- OZNLMEISNSNKIX-UHFFFAOYSA-N 2-[(1-ethenylcyclopentyl)-[(2-methylpropan-2-yl)oxycarbonyl]amino]acetic acid Chemical compound CC(C)(C)OC(=O)N(CC(O)=O)C1(C=C)CCCC1 OZNLMEISNSNKIX-UHFFFAOYSA-N 0.000 description 2
- VRPJIFMKZZEXLR-UHFFFAOYSA-N 2-[(2-methylpropan-2-yl)oxycarbonylamino]acetic acid Chemical compound CC(C)(C)OC(=O)NCC(O)=O VRPJIFMKZZEXLR-UHFFFAOYSA-N 0.000 description 2
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 2
- FVTWJYOGVFLUNJ-UHFFFAOYSA-N 3,3-dimethyl-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)(C)OC(=O)N1CCC(C)(C)C1C(O)=O FVTWJYOGVFLUNJ-UHFFFAOYSA-N 0.000 description 2
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 2
- SEPQTYODOKLVSB-UHFFFAOYSA-N 3-methylbut-2-enal Chemical compound CC(C)=CC=O SEPQTYODOKLVSB-UHFFFAOYSA-N 0.000 description 2
- MVDXXGIBARMXSA-PYUWXLGESA-N 5-[[(2r)-2-benzyl-3,4-dihydro-2h-chromen-6-yl]methyl]-1,3-thiazolidine-2,4-dione Chemical compound S1C(=O)NC(=O)C1CC1=CC=C(O[C@@H](CC=2C=CC=CC=2)CC2)C2=C1 MVDXXGIBARMXSA-PYUWXLGESA-N 0.000 description 2
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 2
- OGSPWJRAVKPPFI-UHFFFAOYSA-N Alendronic Acid Chemical compound NCCCC(O)(P(O)(O)=O)P(O)(O)=O OGSPWJRAVKPPFI-UHFFFAOYSA-N 0.000 description 2
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 2
- RCRITELOOGNISN-UHFFFAOYSA-N C=CC1(C(C)C(=O)O)CCCC1 Chemical compound C=CC1(C(C)C(=O)O)CCCC1 RCRITELOOGNISN-UHFFFAOYSA-N 0.000 description 2
- XRSXFIGDFPLGOG-VHGBLZLWSA-N C=CC1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 Chemical compound C=CC1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 XRSXFIGDFPLGOG-VHGBLZLWSA-N 0.000 description 2
- HQGWKULPCCYPTC-URJDPWSZSA-N CC(C)(CO)[C@H](N)C(=O)N1C2C[C@H]2C[C@H]1C#N Chemical compound CC(C)(CO)[C@H](N)C(=O)N1C2C[C@H]2C[C@H]1C#N HQGWKULPCCYPTC-URJDPWSZSA-N 0.000 description 2
- NNVMGIFWFVGAFC-VHGBLZLWSA-N CC1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCCC1 Chemical compound CC1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCCC1 NNVMGIFWFVGAFC-VHGBLZLWSA-N 0.000 description 2
- KXDHJXZQYSOELW-UHFFFAOYSA-N Carbamic acid Chemical compound NC(O)=O KXDHJXZQYSOELW-UHFFFAOYSA-N 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- 102000012336 Cholesterol Ester Transfer Proteins Human genes 0.000 description 2
- 108010061846 Cholesterol Ester Transfer Proteins Proteins 0.000 description 2
- 238000005821 Claisen rearrangement reaction Methods 0.000 description 2
- 102000034534 Cotransporters Human genes 0.000 description 2
- 108020003264 Cotransporters Proteins 0.000 description 2
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 2
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 2
- 108010036949 Cyclosporine Proteins 0.000 description 2
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 2
- BXZVVICBKDXVGW-NKWVEPMBSA-N Didanosine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(NC=NC2=O)=C2N=C1 BXZVVICBKDXVGW-NKWVEPMBSA-N 0.000 description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 description 2
- WTDHULULXKLSOZ-UHFFFAOYSA-N Hydroxylamine hydrochloride Chemical compound Cl.ON WTDHULULXKLSOZ-UHFFFAOYSA-N 0.000 description 2
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 2
- 239000007836 KH2PO4 Substances 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 2
- AHLBNYSZXLDEJQ-UHFFFAOYSA-N N-formyl-L-leucylester Natural products CCCCCCCCCCCC(OC(=O)C(CC(C)C)NC=O)CC1OC(=O)C1CCCCCC AHLBNYSZXLDEJQ-UHFFFAOYSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- BLXXJMDCKKHMKV-UHFFFAOYSA-N Nabumetone Chemical compound C1=C(CCC(C)=O)C=CC2=CC(OC)=CC=C21 BLXXJMDCKKHMKV-UHFFFAOYSA-N 0.000 description 2
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 2
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 2
- 101000857870 Squalus acanthias Gonadoliberin Proteins 0.000 description 2
- XNKLLVCARDGLGL-JGVFFNPUSA-N Stavudine Chemical compound O=C1NC(=O)C(C)=CN1[C@H]1C=C[C@@H](CO)O1 XNKLLVCARDGLGL-JGVFFNPUSA-N 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 2
- DKJJVAGXPKPDRL-UHFFFAOYSA-N Tiludronic acid Chemical compound OP(O)(=O)C(P(O)(O)=O)SC1=CC=C(Cl)C=C1 DKJJVAGXPKPDRL-UHFFFAOYSA-N 0.000 description 2
- WREGKURFCTUGRC-POYBYMJQSA-N Zalcitabine Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](CO)CC1 WREGKURFCTUGRC-POYBYMJQSA-N 0.000 description 2
- ATZKAUGGNMSCCY-VVFNRDJMSA-N [(1r,2r,3r)-2-[(3e)-4,8-dimethylnona-3,7-dienyl]-2-methyl-3-[(1e,5e)-2,6,10-trimethylundeca-1,5,9-trienyl]cyclopropyl]methyl phosphono hydrogen phosphate Chemical compound CC(C)=CCC\C(C)=C\CC\C(C)=C\[C@@H]1[C@@H](COP(O)(=O)OP(O)(O)=O)[C@]1(C)CC\C=C(/C)CCC=C(C)C ATZKAUGGNMSCCY-VVFNRDJMSA-N 0.000 description 2
- KCKSGMQTZZMFFW-CTWIGJCISA-N [C-]#[N+][C@@H]1CC2CC2N1C(=O)[C@@H](C)C(C)CC Chemical compound [C-]#[N+][C@@H]1CC2CC2N1C(=O)[C@@H](C)C(C)CC KCKSGMQTZZMFFW-CTWIGJCISA-N 0.000 description 2
- DQEUTALJSMITIY-YIYTYNFBSA-N [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(C)C(C)(C)C Chemical compound [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(C)C(C)(C)C DQEUTALJSMITIY-YIYTYNFBSA-N 0.000 description 2
- OBFPUWUWFMMVNI-DXRABYRISA-N [C-]#[N+][C@@H]1[C@@H]2C[C@@H]2CN1C(=O)[C@@H](C)C(C)CC.[C-]#[N+][C@H]1[C@H]2C[C@H]2CN1C(=O)[C@@H](C)C(C)CC Chemical compound [C-]#[N+][C@@H]1[C@@H]2C[C@@H]2CN1C(=O)[C@@H](C)C(C)CC.[C-]#[N+][C@H]1[C@H]2C[C@H]2CN1C(=O)[C@@H](C)C(C)CC OBFPUWUWFMMVNI-DXRABYRISA-N 0.000 description 2
- 229960001138 acetylsalicylic acid Drugs 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- JIMXXGFJRDUSRO-UHFFFAOYSA-N adamantane-1-carboxylic acid Chemical compound C1C(C2)CC3CC2CC1(C(=O)O)C3 JIMXXGFJRDUSRO-UHFFFAOYSA-N 0.000 description 2
- 125000004450 alkenylene group Chemical group 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- 125000006242 amine protecting group Chemical group 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 2
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 2
- 235000011130 ammonium sulphate Nutrition 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 239000002259 anti human immunodeficiency virus agent Substances 0.000 description 2
- 230000002058 anti-hyperglycaemic effect Effects 0.000 description 2
- 239000003529 anticholesteremic agent Substances 0.000 description 2
- 125000004659 aryl alkyl thio group Chemical group 0.000 description 2
- 125000002102 aryl alkyloxo group Chemical group 0.000 description 2
- 125000005160 aryl oxy alkyl group Chemical group 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 2
- 229960002170 azathioprine Drugs 0.000 description 2
- 235000019445 benzyl alcohol Nutrition 0.000 description 2
- PUJDIJCNWFYVJX-UHFFFAOYSA-N benzyl carbamate Chemical compound NC(=O)OCC1=CC=CC=C1 PUJDIJCNWFYVJX-UHFFFAOYSA-N 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- 150000001576 beta-amino acids Chemical class 0.000 description 2
- JESWDXIHOJGWBP-UHFFFAOYSA-N bicyclo[2.2.1]heptane-3-carboxylic acid Chemical compound C1CC2C(C(=O)O)CC1C2 JESWDXIHOJGWBP-UHFFFAOYSA-N 0.000 description 2
- 150000004283 biguanides Chemical class 0.000 description 2
- 239000003613 bile acid Substances 0.000 description 2
- 229920000080 bile acid sequestrant Polymers 0.000 description 2
- 229940096699 bile acid sequestrants Drugs 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 239000006227 byproduct Substances 0.000 description 2
- 125000001589 carboacyl group Chemical group 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 238000009903 catalytic hydrogenation reaction Methods 0.000 description 2
- 229940047495 celebrex Drugs 0.000 description 2
- DCFKHNIGBAHNSS-UHFFFAOYSA-N chloro(triethyl)silane Chemical compound CC[Si](Cl)(CC)CC DCFKHNIGBAHNSS-UHFFFAOYSA-N 0.000 description 2
- PJGJQVRXEUVAFT-UHFFFAOYSA-N chloroiodomethane Chemical compound ClCI PJGJQVRXEUVAFT-UHFFFAOYSA-N 0.000 description 2
- 229940125904 compound 1 Drugs 0.000 description 2
- 229940125773 compound 10 Drugs 0.000 description 2
- 229940125890 compound Ia Drugs 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 239000013058 crude material Substances 0.000 description 2
- 150000001924 cycloalkanes Chemical class 0.000 description 2
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 238000005888 cyclopropanation reaction Methods 0.000 description 2
- POZRVZJJTULAOH-LHZXLZLDSA-N danazol Chemical compound C1[C@]2(C)[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=CC2=C1C=NO2 POZRVZJJTULAOH-LHZXLZLDSA-N 0.000 description 2
- 229960000766 danazol Drugs 0.000 description 2
- QQKNSPHAFATFNQ-UHFFFAOYSA-N darglitazone Chemical compound CC=1OC(C=2C=CC=CC=2)=NC=1CCC(=O)C(C=C1)=CC=C1CC1SC(=O)NC1=O QQKNSPHAFATFNQ-UHFFFAOYSA-N 0.000 description 2
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 229960002656 didanosine Drugs 0.000 description 2
- 150000005690 diesters Chemical class 0.000 description 2
- ISOLMABRZPQKOV-UHFFFAOYSA-N diethyl 2-acetamidopropanedioate Chemical compound CCOC(=O)C(NC(C)=O)C(=O)OCC ISOLMABRZPQKOV-UHFFFAOYSA-N 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- QYJOOVQLTTVTJY-YFKPBYRVSA-N ethyl (2s)-5-oxopyrrolidine-2-carboxylate Chemical compound CCOC(=O)[C@@H]1CCC(=O)N1 QYJOOVQLTTVTJY-YFKPBYRVSA-N 0.000 description 2
- TXXWSOKUXUMWRZ-UHFFFAOYSA-N ethyl 2-cyclopentylideneacetate Chemical compound CCOC(=O)C=C1CCCC1 TXXWSOKUXUMWRZ-UHFFFAOYSA-N 0.000 description 2
- 239000002024 ethyl acetate extract Substances 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 239000003862 glucocorticoid Substances 0.000 description 2
- XLXSAKCOAKORKW-AQJXLSMYSA-N gonadorelin Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 XLXSAKCOAKORKW-AQJXLSMYSA-N 0.000 description 2
- 229940035638 gonadotropin-releasing hormone Drugs 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 125000004447 heteroarylalkenyl group Chemical group 0.000 description 2
- 125000005553 heteroaryloxy group Chemical group 0.000 description 2
- 150000002431 hydrogen Chemical class 0.000 description 2
- 230000000055 hyoplipidemic effect Effects 0.000 description 2
- 229960001680 ibuprofen Drugs 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 2
- 229940125699 infertility agent Drugs 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 2
- BWHLPLXXIDYSNW-UHFFFAOYSA-N ketorolac tromethamine Chemical compound OCC(N)(CO)CO.OC(=O)C1CCN2C1=CC=C2C(=O)C1=CC=CC=C1 BWHLPLXXIDYSNW-UHFFFAOYSA-N 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- RGLRXNKKBLIBQS-XNHQSDQCSA-N leuprolide acetate Chemical compound CC(O)=O.CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 RGLRXNKKBLIBQS-XNHQSDQCSA-N 0.000 description 2
- 125000005647 linker group Chemical group 0.000 description 2
- ZCSHNCUQKCANBX-UHFFFAOYSA-N lithium diisopropylamide Chemical compound [Li+].CC(C)[N-]C(C)C ZCSHNCUQKCANBX-UHFFFAOYSA-N 0.000 description 2
- GLXDVVHUTZTUQK-UHFFFAOYSA-M lithium;hydroxide;hydrate Chemical compound [Li+].O.[OH-] GLXDVVHUTZTUQK-UHFFFAOYSA-M 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 229940087857 lupron Drugs 0.000 description 2
- 206010025135 lupus erythematosus Diseases 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000003760 magnetic stirring Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- KBOPZPXVLCULAV-UHFFFAOYSA-N mesalamine Chemical compound NC1=CC=C(O)C(C(O)=O)=C1 KBOPZPXVLCULAV-UHFFFAOYSA-N 0.000 description 2
- 229910000402 monopotassium phosphate Inorganic materials 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- QAGYKUNXZHXKMR-HKWSIXNMSA-N nelfinavir Chemical compound CC1=C(O)C=CC=C1C(=O)N[C@H]([C@H](O)CN1[C@@H](C[C@@H]2CCCC[C@@H]2C1)C(=O)NC(C)(C)C)CSC1=CC=CC=C1 QAGYKUNXZHXKMR-HKWSIXNMSA-N 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- 238000002414 normal-phase solid-phase extraction Methods 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 239000012285 osmium tetroxide Substances 0.000 description 2
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 2
- 238000007248 oxidative elimination reaction Methods 0.000 description 2
- 125000004043 oxo group Chemical group O=* 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- 229940000596 parlodel Drugs 0.000 description 2
- FDPIMTJIUBPUKL-UHFFFAOYSA-N pentan-3-one Chemical compound CCC(=O)CC FDPIMTJIUBPUKL-UHFFFAOYSA-N 0.000 description 2
- KHIWWQKSHDUIBK-UHFFFAOYSA-N periodic acid Chemical compound OI(=O)(=O)=O KHIWWQKSHDUIBK-UHFFFAOYSA-N 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 125000000587 piperidin-1-yl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- QYSPLQLAKJAUJT-UHFFFAOYSA-N piroxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=CC=CC=N1 QYSPLQLAKJAUJT-UHFFFAOYSA-N 0.000 description 2
- 229940096701 plain lipid modifying drug hmg coa reductase inhibitors Drugs 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- NNFCIKHAZHQZJG-UHFFFAOYSA-N potassium cyanide Chemical compound [K+].N#[C-] NNFCIKHAZHQZJG-UHFFFAOYSA-N 0.000 description 2
- GNSKLFRGEWLPPA-UHFFFAOYSA-M potassium dihydrogen phosphate Chemical compound [K+].OP(O)([O-])=O GNSKLFRGEWLPPA-UHFFFAOYSA-M 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 229960003912 probucol Drugs 0.000 description 2
- FYPMFJGVHOHGLL-UHFFFAOYSA-N probucol Chemical compound C=1C(C(C)(C)C)=C(O)C(C(C)(C)C)=CC=1SC(C)(C)SC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 FYPMFJGVHOHGLL-UHFFFAOYSA-N 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000006722 reduction reaction Methods 0.000 description 2
- NCDNCNXCDXHOMX-XGKFQTDJSA-N ritonavir Chemical compound N([C@@H](C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1SC=NC=1)CC=1C=CC=CC=1)C(=O)N(C)CC1=CSC(C(C)C)=N1 NCDNCNXCDXHOMX-XGKFQTDJSA-N 0.000 description 2
- QWAXKHKRTORLEM-UGJKXSETSA-N saquinavir Chemical compound C([C@@H]([C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)C=1N=C2C=CC=CC2=CC=1)C1=CC=CC=C1 QWAXKHKRTORLEM-UGJKXSETSA-N 0.000 description 2
- 239000000849 selective androgen receptor modulator Substances 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- MNWBNISUBARLIT-UHFFFAOYSA-N sodium cyanide Chemical compound [Na+].N#[C-] MNWBNISUBARLIT-UHFFFAOYSA-N 0.000 description 2
- 239000002594 sorbent Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000011593 sulfur Substances 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 229940037128 systemic glucocorticoids Drugs 0.000 description 2
- XBXCNNQPRYLIDE-UHFFFAOYSA-N tert-butylcarbamic acid Chemical compound CC(C)(C)NC(O)=O XBXCNNQPRYLIDE-UHFFFAOYSA-N 0.000 description 2
- BCNZYOJHNLTNEZ-UHFFFAOYSA-N tert-butyldimethylsilyl chloride Chemical compound CC(C)(C)[Si](C)(C)Cl BCNZYOJHNLTNEZ-UHFFFAOYSA-N 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 2
- 150000003672 ureas Chemical class 0.000 description 2
- 229960004295 valine Drugs 0.000 description 2
- 229940087652 vioxx Drugs 0.000 description 2
- 238000010792 warming Methods 0.000 description 2
- 229960000523 zalcitabine Drugs 0.000 description 2
- HBOMLICNUCNMMY-XLPZGREQSA-N zidovudine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](N=[N+]=[N-])C1 HBOMLICNUCNMMY-XLPZGREQSA-N 0.000 description 2
- IPSRAFUHLHIWAR-UHFFFAOYSA-N zinc;ethane Chemical compound [Zn+2].[CH2-]C.[CH2-]C IPSRAFUHLHIWAR-UHFFFAOYSA-N 0.000 description 2
- UAOUIVVJBYDFKD-XKCDOFEDSA-N (1R,9R,10S,11R,12R,15S,18S,21R)-10,11,21-trihydroxy-8,8-dimethyl-14-methylidene-4-(prop-2-enylamino)-20-oxa-5-thia-3-azahexacyclo[9.7.2.112,15.01,9.02,6.012,18]henicosa-2(6),3-dien-13-one Chemical compound C([C@@H]1[C@@H](O)[C@@]23C(C1=C)=O)C[C@H]2[C@]12C(N=C(NCC=C)S4)=C4CC(C)(C)[C@H]1[C@H](O)[C@]3(O)OC2 UAOUIVVJBYDFKD-XKCDOFEDSA-N 0.000 description 1
- YIWUAPISITUDBY-UHFFFAOYSA-N (2,3,4-trifluorophenyl)methanamine Chemical compound NCC1=CC=C(F)C(F)=C1F YIWUAPISITUDBY-UHFFFAOYSA-N 0.000 description 1
- VXEVKSKAINMPFG-QWUNSSNDSA-N (2S)-5-[[(5S)-5-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S,3R)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-(1H-imidazol-4-yl)propanoyl]amino]propanoyl]amino]-4-carboxybutanoyl]amino]acetyl]amino]-3-hydroxybutanoyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-methylpentanoyl]amino]-4-carboxybutanoyl]amino]acetyl]amino]-5-oxopentanoyl]amino]propanoyl]amino]propanoyl]amino]-6-[[(2S)-4-carboxy-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-(carboxymethylamino)-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxobutan-2-yl]amino]-6-oxohexyl]amino]-2-(hexadecanoylamino)-5-hydroxypentanoic acid Chemical compound CCCCCCCCCCCCCCCC(=O)N[C@@H](CCC(NCCCC[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(=O)N)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC4=CC=C(C=C4)O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC5=CC=CC=C5)NC(=O)[C@H]([C@@H](C)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](CC6=CNC=N6)N)O)C(=O)O VXEVKSKAINMPFG-QWUNSSNDSA-N 0.000 description 1
- OGARKMDCQCLMCS-QMMMGPOBSA-N (2r)-3-tert-butylsulfanyl-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid Chemical compound CC(C)(C)OC(=O)N[C@H](C(O)=O)CSC(C)(C)C OGARKMDCQCLMCS-QMMMGPOBSA-N 0.000 description 1
- NPDBDJFLKKQMCM-SCSAIBSYSA-N (2s)-2-amino-3,3-dimethylbutanoic acid Chemical compound CC(C)(C)[C@H](N)C(O)=O NPDBDJFLKKQMCM-SCSAIBSYSA-N 0.000 description 1
- WAMWSIDTKSNDCU-ZETCQYMHSA-N (2s)-2-azaniumyl-2-cyclohexylacetate Chemical compound OC(=O)[C@@H](N)C1CCCCC1 WAMWSIDTKSNDCU-ZETCQYMHSA-N 0.000 description 1
- LRFZIPCTFBPFLX-SSDOTTSWSA-N (2s)-3,3-dimethyl-2-[(2-methylpropan-2-yl)oxycarbonylamino]butanoic acid Chemical compound CC(C)(C)OC(=O)N[C@H](C(O)=O)C(C)(C)C LRFZIPCTFBPFLX-SSDOTTSWSA-N 0.000 description 1
- PZEMWPDUXBZKJN-LURJTMIESA-N (2s)-4-hydroxy-2-[(2-methylpropan-2-yl)oxycarbonylamino]butanoic acid Chemical compound CC(C)(C)OC(=O)N[C@H](C(O)=O)CCO PZEMWPDUXBZKJN-LURJTMIESA-N 0.000 description 1
- ZMGMYCHEWPVBEL-OLQVQODUSA-N (3r,4s)-3,4-dimethylcyclopentan-1-one Chemical compound C[C@H]1CC(=O)C[C@H]1C ZMGMYCHEWPVBEL-OLQVQODUSA-N 0.000 description 1
- MCEWPPMUTVLMJG-YCXLAJEKSA-N (3s)-2-azabicyclo[3.1.0]hexane-3-carboxamide Chemical compound N1[C@H](C(=O)N)CC2CC21 MCEWPPMUTVLMJG-YCXLAJEKSA-N 0.000 description 1
- RWIUTHWKQHRQNP-ZDVGBALWSA-N (9e,12e)-n-(1-phenylethyl)octadeca-9,12-dienamide Chemical compound CCCCC\C=C\C\C=C\CCCCCCCC(=O)NC(C)C1=CC=CC=C1 RWIUTHWKQHRQNP-ZDVGBALWSA-N 0.000 description 1
- DTCCTIQRPGSLPT-ONEGZZNKSA-N (E)-2-pentenal Chemical compound CC\C=C\C=O DTCCTIQRPGSLPT-ONEGZZNKSA-N 0.000 description 1
- COLOHWPRNRVWPI-UHFFFAOYSA-N 1,1,1-trifluoroethane Chemical compound [CH2]C(F)(F)F COLOHWPRNRVWPI-UHFFFAOYSA-N 0.000 description 1
- JLHMJWHSBYZWJJ-UHFFFAOYSA-N 1,2-thiazole 1-oxide Chemical class O=S1C=CC=N1 JLHMJWHSBYZWJJ-UHFFFAOYSA-N 0.000 description 1
- NUBQKPWHXMGDLP-UHFFFAOYSA-N 1-[4-benzyl-2-hydroxy-5-[(2-hydroxy-2,3-dihydro-1h-inden-1-yl)amino]-5-oxopentyl]-n-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide;sulfuric acid Chemical compound OS(O)(=O)=O.C1CN(CC(O)CC(CC=2C=CC=CC=2)C(=O)NC2C3=CC=CC=C3CC2O)C(C(=O)NC(C)(C)C)CN1CC1=CC=CN=C1 NUBQKPWHXMGDLP-UHFFFAOYSA-N 0.000 description 1
- PJUPKRYGDFTMTM-UHFFFAOYSA-N 1-hydroxybenzotriazole;hydrate Chemical compound O.C1=CC=C2N(O)N=NC2=C1 PJUPKRYGDFTMTM-UHFFFAOYSA-N 0.000 description 1
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 1
- 229940123153 15 Lipoxygenase inhibitor Drugs 0.000 description 1
- JDIIGWSSTNUWGK-UHFFFAOYSA-N 1h-imidazol-3-ium;chloride Chemical compound [Cl-].[NH2+]1C=CN=C1 JDIIGWSSTNUWGK-UHFFFAOYSA-N 0.000 description 1
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 1
- LZKJTKZMASYYML-UHFFFAOYSA-N 2-[(1-ethylcyclopentyl)-[(2-methylpropan-2-yl)oxycarbonyl]amino]acetic acid Chemical compound CC(C)(C)OC(=O)N(CC(O)=O)C1(CC)CCCC1 LZKJTKZMASYYML-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000000069 2-butynyl group Chemical group [H]C([H])([H])C#CC([H])([H])* 0.000 description 1
- QVJQOGBUMUORJZ-UHFFFAOYSA-N 2-cyclopentylideneethanol Chemical compound OCC=C1CCCC1 QVJQOGBUMUORJZ-UHFFFAOYSA-N 0.000 description 1
- HRTOQFBQOFIFEE-UHFFFAOYSA-N 2-dehydropantolactone Chemical compound CC1(C)COC(=O)C1=O HRTOQFBQOFIFEE-UHFFFAOYSA-N 0.000 description 1
- 125000006040 2-hexenyl group Chemical group 0.000 description 1
- XWKFPIODWVPXLX-UHFFFAOYSA-N 2-methyl-5-methylpyridine Natural products CC1=CC=C(C)N=C1 XWKFPIODWVPXLX-UHFFFAOYSA-N 0.000 description 1
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- VWFJDQUYCIWHTN-YFVJMOTDSA-N 2-trans,6-trans-farnesyl diphosphate Chemical class CC(C)=CCC\C(C)=C\CC\C(C)=C\CO[P@](O)(=O)OP(O)(O)=O VWFJDQUYCIWHTN-YFVJMOTDSA-N 0.000 description 1
- CZMRCDWAGMRECN-UHFFFAOYSA-N 2-{[3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound OCC1OC(CO)(OC2OC(CO)C(O)C(O)C2O)C(O)C1O CZMRCDWAGMRECN-UHFFFAOYSA-N 0.000 description 1
- MCFYQEDDIWDFPJ-UHFFFAOYSA-N 2h-pyran-4-carbaldehyde Chemical compound O=CC1=CCOC=C1 MCFYQEDDIWDFPJ-UHFFFAOYSA-N 0.000 description 1
- LRFZIPCTFBPFLX-UHFFFAOYSA-N 3,3-dimethyl-2-[(2-methylpropan-2-yl)oxycarbonylamino]butanoic acid Chemical compound CC(C)(C)OC(=O)NC(C(O)=O)C(C)(C)C LRFZIPCTFBPFLX-UHFFFAOYSA-N 0.000 description 1
- AJFFONDFZKSGAZ-UHFFFAOYSA-N 3,3-dimethylpyrrolidine-2-carboxylic acid;hydrochloride Chemical compound Cl.CC1(C)CCNC1C(O)=O AJFFONDFZKSGAZ-UHFFFAOYSA-N 0.000 description 1
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 1
- QBWKPGNFQQJGFY-QLFBSQMISA-N 3-[(1r)-1-[(2r,6s)-2,6-dimethylmorpholin-4-yl]ethyl]-n-[6-methyl-3-(1h-pyrazol-4-yl)imidazo[1,2-a]pyrazin-8-yl]-1,2-thiazol-5-amine Chemical compound N1([C@H](C)C2=NSC(NC=3C4=NC=C(N4C=C(C)N=3)C3=CNN=C3)=C2)C[C@H](C)O[C@H](C)C1 QBWKPGNFQQJGFY-QLFBSQMISA-N 0.000 description 1
- NBYATBIMYLFITE-UHFFFAOYSA-N 3-[decyl(dimethyl)silyl]-n-[2-(4-methylphenyl)-1-phenylethyl]propanamide Chemical compound C=1C=CC=CC=1C(NC(=O)CC[Si](C)(C)CCCCCCCCCC)CC1=CC=C(C)C=C1 NBYATBIMYLFITE-UHFFFAOYSA-N 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- 125000006041 3-hexenyl group Chemical group 0.000 description 1
- LHCOVOKZWQYODM-CPEOKENHSA-N 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one;1-[(2r,4s,5s)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1.O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](N=[N+]=[N-])C1 LHCOVOKZWQYODM-CPEOKENHSA-N 0.000 description 1
- FUSNOPLQVRUIIM-UHFFFAOYSA-N 4-amino-2-(4,4-dimethyl-2-oxoimidazolidin-1-yl)-n-[3-(trifluoromethyl)phenyl]pyrimidine-5-carboxamide Chemical compound O=C1NC(C)(C)CN1C(N=C1N)=NC=C1C(=O)NC1=CC=CC(C(F)(F)F)=C1 FUSNOPLQVRUIIM-UHFFFAOYSA-N 0.000 description 1
- MXNQOXJLUJUCGE-UHFFFAOYSA-N 4-amino-2-(4,4-dimethyl-2-oxoimidazolidin-1-yl)-n-[3-(trifluoromethyl)phenyl]pyrimidine-5-carboxamide;hydrochloride Chemical compound Cl.O=C1NC(C)(C)CN1C(N=C1N)=NC=C1C(=O)NC1=CC=CC(C(F)(F)F)=C1 MXNQOXJLUJUCGE-UHFFFAOYSA-N 0.000 description 1
- WUBBRNOQWQTFEX-UHFFFAOYSA-N 4-aminosalicylic acid Chemical compound NC1=CC=C(C(O)=O)C(O)=C1 WUBBRNOQWQTFEX-UHFFFAOYSA-N 0.000 description 1
- CNPURSDMOWDNOQ-UHFFFAOYSA-N 4-methoxy-7h-pyrrolo[2,3-d]pyrimidin-2-amine Chemical compound COC1=NC(N)=NC2=C1C=CN2 CNPURSDMOWDNOQ-UHFFFAOYSA-N 0.000 description 1
- RIWPMNBTULNXOH-ARJAWSKDSA-N 4-methyl-2-pentenal Chemical compound CC(C)\C=C/C=O RIWPMNBTULNXOH-ARJAWSKDSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- ZCLFPKBADABTMG-UHFFFAOYSA-N 9-[4-[4-[[2-(2,2,2-trifluoroethoxy)benzoyl]amino]piperidin-1-yl]butyl]-n-(2,2,2-trifluoroethyl)fluorene-9-carboxamide Chemical group C12=CC=CC=C2C2=CC=CC=C2C1(C(=O)NCC(F)(F)F)CCCCN(CC1)CCC1NC(=O)C1=CC=CC=C1OCC(F)(F)F ZCLFPKBADABTMG-UHFFFAOYSA-N 0.000 description 1
- OZAIFHULBGXAKX-VAWYXSNFSA-N AIBN Substances N#CC(C)(C)\N=N\C(C)(C)C#N OZAIFHULBGXAKX-VAWYXSNFSA-N 0.000 description 1
- DJQOOSBJCLSSEY-UHFFFAOYSA-N Acipimox Chemical compound CC1=CN=C(C(O)=O)C=[N+]1[O-] DJQOOSBJCLSSEY-UHFFFAOYSA-N 0.000 description 1
- DHMQDGOQFOQNFH-UHFFFAOYSA-M Aminoacetate Chemical compound NCC([O-])=O DHMQDGOQFOQNFH-UHFFFAOYSA-M 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- 102100033367 Appetite-regulating hormone Human genes 0.000 description 1
- 101710111255 Appetite-regulating hormone Proteins 0.000 description 1
- GFHVXFVGAGSORS-UHFFFAOYSA-N C.C.C.C.C.C1=CC2=C(C=C1)CNCC2.C1=CC2=C(C=C1)OCCO2.C1CC2CC2N1.C1CC2OC1C1CNCC21.C1CCCNCC1.C1CCCOCC1.C1CCNCC1.C1CCOCC1.C1CNCCN1.C1COCCN1.C1COCOC1.C1COCSC1.C1CSCCN1.C1NCC2CC12.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.O=CN1CCNCC1 Chemical compound C.C.C.C.C.C1=CC2=C(C=C1)CNCC2.C1=CC2=C(C=C1)OCCO2.C1CC2CC2N1.C1CC2OC1C1CNCC21.C1CCCNCC1.C1CCCOCC1.C1CCNCC1.C1CCOCC1.C1CNCCN1.C1COCCN1.C1COCOC1.C1COCSC1.C1CSCCN1.C1NCC2CC12.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.O=CN1CCNCC1 GFHVXFVGAGSORS-UHFFFAOYSA-N 0.000 description 1
- JDSICHHKIVOLLX-UHFFFAOYSA-N C1=CC2=C(C=C1)CCC2.C1=CC2=C(C=C1)N=NC2.C1=CC2=C(C=C1)NC=N2.C1=CC2=C(C=C1)NN=C2.C1=CC2=C(C=C1)OC=C2.C1=CC2=C(C=C1)OC=N2.C1=CC2=C(C=C1)OCC2.C1=CC2=C(C=C1)OCN2.C1=CC2=C(C=C1)OCO2.C1=CC2=C(C=C1)SC=N2.C1=CN=C2C=CC=CC2=C1.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.O=C1C=CC2=CC=CC=C2O1.O=C1NC2=C(C=CC=C2)O1 Chemical compound C1=CC2=C(C=C1)CCC2.C1=CC2=C(C=C1)N=NC2.C1=CC2=C(C=C1)NC=N2.C1=CC2=C(C=C1)NN=C2.C1=CC2=C(C=C1)OC=C2.C1=CC2=C(C=C1)OC=N2.C1=CC2=C(C=C1)OCC2.C1=CC2=C(C=C1)OCN2.C1=CC2=C(C=C1)OCO2.C1=CC2=C(C=C1)SC=N2.C1=CN=C2C=CC=CC2=C1.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.O=C1C=CC2=CC=CC=C2O1.O=C1NC2=C(C=CC=C2)O1 JDSICHHKIVOLLX-UHFFFAOYSA-N 0.000 description 1
- XXIWQILMTXRQSM-UHFFFAOYSA-N C1=CC2=C(C=C1)OCC2.C1=CC2=NON=C2C=C1.C1=CC=C2NC=CC2=C1.C1=CC=C2OCOC2=C1.C1=CC=C2SC=NC2=C1.C1=CC=NC=C1.C1=CN=CC=N1.C1=CN=CN=C1.C1=CN=NC=N1.C1=CNC=C1.C1=CNC=C1.C1=COC=C1.C1=COC=N1.C1=CSC=C1.C1=CSC=N1.C1=NC=NO1.C1=NN=CO1.C1=NN=CS1.C1=NNN=N1.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC(C)C1=NC=NC=N1.CN1C=NN=C1 Chemical compound C1=CC2=C(C=C1)OCC2.C1=CC2=NON=C2C=C1.C1=CC=C2NC=CC2=C1.C1=CC=C2OCOC2=C1.C1=CC=C2SC=NC2=C1.C1=CC=NC=C1.C1=CN=CC=N1.C1=CN=CN=C1.C1=CN=NC=N1.C1=CNC=C1.C1=CNC=C1.C1=COC=C1.C1=COC=N1.C1=CSC=C1.C1=CSC=N1.C1=NC=NO1.C1=NN=CO1.C1=NN=CS1.C1=NNN=N1.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC(C)C1=NC=NC=N1.CN1C=NN=C1 XXIWQILMTXRQSM-UHFFFAOYSA-N 0.000 description 1
- ACBVPCWSPVVJQS-GEGKGCMMSA-N C1=C\C2CCCC(C/1)CCC2.C1C2CC3CC1CC(C2)C3.C1CC1.C1CC2CCC1C2.C1CC2CCC1CC2.C1CC2CCCC(C1)CCC2.C1CCC2CCCCC2C1.CC1(C)C2CCC1CC2 Chemical compound C1=C\C2CCCC(C/1)CCC2.C1C2CC3CC1CC(C2)C3.C1CC1.C1CC2CCC1C2.C1CC2CCC1CC2.C1CC2CCCC(C1)CCC2.C1CCC2CCCCC2C1.CC1(C)C2CCC1CC2 ACBVPCWSPVVJQS-GEGKGCMMSA-N 0.000 description 1
- LVZWSLJZHVFIQJ-UHFFFAOYSA-N C1CC1 Chemical compound C1CC1 LVZWSLJZHVFIQJ-UHFFFAOYSA-N 0.000 description 1
- BTHHAHWQFHWIMH-UHFFFAOYSA-N C1CCCCC1.CC.CC.CC1CC1C Chemical compound C1CCCCC1.CC.CC.CC1CC1C BTHHAHWQFHWIMH-UHFFFAOYSA-N 0.000 description 1
- 102100024881 C3 and PZP-like alpha-2-macroglobulin domain-containing protein 8 Human genes 0.000 description 1
- UFWIKSXTQWXSFV-CHWGNKNJSA-N C=C(C)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCC1.C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1 Chemical compound C=C(C)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCC1.C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1 UFWIKSXTQWXSFV-CHWGNKNJSA-N 0.000 description 1
- NCUYIMZHHAQBCJ-NIVGQHHOSA-N C=C(C)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCCC1.C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 Chemical compound C=C(C)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCCC1.C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 NCUYIMZHHAQBCJ-NIVGQHHOSA-N 0.000 description 1
- WUTYLSQMWFNYMB-MROQNXINSA-N C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1 Chemical compound C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1 WUTYLSQMWFNYMB-MROQNXINSA-N 0.000 description 1
- DNSHVVAWUNKDAD-ZOBORPQBSA-N C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 Chemical compound C=C(C)C1([C@H](N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 DNSHVVAWUNKDAD-ZOBORPQBSA-N 0.000 description 1
- VFMWYELGTJWGDQ-FEZOTEKNSA-N C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1 Chemical compound C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1 VFMWYELGTJWGDQ-FEZOTEKNSA-N 0.000 description 1
- XWZIHUCUFAVKAU-AGZWWOCSSA-N C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1.C=C(F)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCC1 Chemical compound C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCC1.C=C(F)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCC1 XWZIHUCUFAVKAU-AGZWWOCSSA-N 0.000 description 1
- MIJLRCUAILZBET-VHGBLZLWSA-N C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 Chemical compound C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 MIJLRCUAILZBET-VHGBLZLWSA-N 0.000 description 1
- RSTPIGDSAOLESE-QBFIPYABSA-N C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1.C=C(F)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCCC1 Chemical compound C=C(F)C1(C(N)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1.C=C(F)C1(C(NC(=O)OC(C)(C)C)C(=O)O)CCCC1 RSTPIGDSAOLESE-QBFIPYABSA-N 0.000 description 1
- ASBKETIFTJBUSU-PIVWYWHXSA-N C=C(F)C1([C@H](N)C(=O)N2C3C[C@H]3C[C@H]2C)CCCC1.C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CCC(CC1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCCCC1.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 Chemical compound C=C(F)C1([C@H](N)C(=O)N2C3C[C@H]3C[C@H]2C)CCCC1.C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CCC(CC1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCCCC1.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 ASBKETIFTJBUSU-PIVWYWHXSA-N 0.000 description 1
- KCKRALDFWKXDBQ-OXGYUJOESA-N C=C(F)C1([C@H](N)C(=O)N2[C@H]3C[C@H]3C[C@H]2C)CCCC1.C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1(CO)CCC1.C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1(CO)CCCC1.N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CCC(CC1)C2.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)C(N)C1(CO)CCCCC1.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CC3CC(CC(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 Chemical compound C=C(F)C1([C@H](N)C(=O)N2[C@H]3C[C@H]3C[C@H]2C)CCCC1.C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1(CO)CCC1.C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1(CO)CCCC1.N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CCC(CC1)C2.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)C(N)C1(CO)CCCCC1.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CC3CC(CC(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 KCKRALDFWKXDBQ-OXGYUJOESA-N 0.000 description 1
- LLFHCOGPDCJMKY-UHFFFAOYSA-N C=CC(C)(C)C(C)C Chemical compound C=CC(C)(C)C(C)C LLFHCOGPDCJMKY-UHFFFAOYSA-N 0.000 description 1
- MNFNPSLAPLLPSF-UHFFFAOYSA-N C=CC(CC)(CC)C(C)C Chemical compound C=CC(CC)(CC)C(C)C MNFNPSLAPLLPSF-UHFFFAOYSA-N 0.000 description 1
- QIIQSIOBBBJNHV-FNHRNIBMSA-N C=CC1(C(C)C(=O)N2[C@H](C(N)=O)C[C@@H]3C[C@@H]32)CCCC1 Chemical compound C=CC1(C(C)C(=O)N2[C@H](C(N)=O)C[C@@H]3C[C@@H]32)CCCC1 QIIQSIOBBBJNHV-FNHRNIBMSA-N 0.000 description 1
- QWGBASYFFAVZHU-UHFFFAOYSA-N C=CC1(C(C)C(=O)O)CCCC1.CCC1(C(C)C(=O)O)CCCC1 Chemical compound C=CC1(C(C)C(=O)O)CCCC1.CCC1(C(C)C(=O)O)CCCC1 QWGBASYFFAVZHU-UHFFFAOYSA-N 0.000 description 1
- MROHARWWTUQNRC-AYAARRBOSA-N C=CC1(C(C)C(=O)O)CCCC1.[H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)C(C)C1(C=C)CCCC1.[H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)C(C)C1(C=C)CCCC1.[H][C@@]1(C(N)=O)C[C@]2([H])C[C@]2([H])N1C(=O)C(C)C1(C=C)CCCC1.[H][C@]12C[C@@]([H])(C(N)=O)C[C@@]1([H])C2 Chemical compound C=CC1(C(C)C(=O)O)CCCC1.[H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)C(C)C1(C=C)CCCC1.[H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)C(C)C1(C=C)CCCC1.[H][C@@]1(C(N)=O)C[C@]2([H])C[C@]2([H])N1C(=O)C(C)C1(C=C)CCCC1.[H][C@]12C[C@@]([H])(C(N)=O)C[C@@]1([H])C2 MROHARWWTUQNRC-AYAARRBOSA-N 0.000 description 1
- KYNOYZYUSZQLRG-UHFFFAOYSA-N C=CC1(C(C)C)CC1 Chemical compound C=CC1(C(C)C)CC1 KYNOYZYUSZQLRG-UHFFFAOYSA-N 0.000 description 1
- BAYJHOIJEPURCK-UHFFFAOYSA-N C=CC1(C(C)C)CCC1 Chemical compound C=CC1(C(C)C)CCC1 BAYJHOIJEPURCK-UHFFFAOYSA-N 0.000 description 1
- VEJUEMCSCXRBEU-UHFFFAOYSA-N C=CC1(C(C)C)CCCC1 Chemical compound C=CC1(C(C)C)CCCC1 VEJUEMCSCXRBEU-UHFFFAOYSA-N 0.000 description 1
- BQMVPJYUPFSATI-UHFFFAOYSA-N C=CC1(C(C)C)CCCCC1 Chemical compound C=CC1(C(C)C)CCCCC1 BQMVPJYUPFSATI-UHFFFAOYSA-N 0.000 description 1
- CFXCVZWPUVYBJM-UHFFFAOYSA-N C=CC1(C(C)C)CCCCCC1 Chemical compound C=CC1(C(C)C)CCCCCC1 CFXCVZWPUVYBJM-UHFFFAOYSA-N 0.000 description 1
- BIPMNXCXCANATM-UHFFFAOYSA-N C=CC1(C(C)C)CCCCCCC1 Chemical compound C=CC1(C(C)C)CCCCCCC1 BIPMNXCXCANATM-UHFFFAOYSA-N 0.000 description 1
- GFBWAYSRAMYCFT-UHFFFAOYSA-N C=CC1(C(C)C)CCOCC1 Chemical compound C=CC1(C(C)C)CCOCC1 GFBWAYSRAMYCFT-UHFFFAOYSA-N 0.000 description 1
- OOQRAMNDSNBWJQ-UHFFFAOYSA-N C=CC1(C(NC(=O)OC(C)(C)C)C(=O)O)CCCC1.CC(C)(C)OC(=O)NCC(=O)OCC=C1CCCC1.CCOC(=O)C=C1CCCC1.O=C1CCCC1.OCC=C1CCCC1 Chemical compound C=CC1(C(NC(=O)OC(C)(C)C)C(=O)O)CCCC1.CC(C)(C)OC(=O)NCC(=O)OCC=C1CCCC1.CCOC(=O)C=C1CCCC1.O=C1CCCC1.OCC=C1CCCC1 OOQRAMNDSNBWJQ-UHFFFAOYSA-N 0.000 description 1
- LQLIBSUHOLQFMA-QVBJCFTNSA-N C=CC1(CCCC1)C(C(N(CC1[C@H]2C1)C2C#N)=O)N Chemical compound C=CC1(CCCC1)C(C(N(CC1[C@H]2C1)C2C#N)=O)N LQLIBSUHOLQFMA-QVBJCFTNSA-N 0.000 description 1
- UDJAFHKVOHLAKV-XFLICZMTSA-N C=CC1([C@H](C)C(=O)N2C3C[C@H]3C[C@H]2C#N)CCCC1.C[C@H](C(=O)N1C2C[C@H]2C[C@H]1C#N)C1(CO)CCCC1.C[C@H](C(=O)N1C2C[C@H]2C[C@H]1C#N)C1(CO)CCCC1 Chemical compound C=CC1([C@H](C)C(=O)N2C3C[C@H]3C[C@H]2C#N)CCCC1.C[C@H](C(=O)N1C2C[C@H]2C[C@H]1C#N)C1(CO)CCCC1.C[C@H](C(=O)N1C2C[C@H]2C[C@H]1C#N)C1(CO)CCCC1 UDJAFHKVOHLAKV-XFLICZMTSA-N 0.000 description 1
- DJBDZAFMSXYDOD-DDQLXMPWSA-N C=CC1([C@H](C)C(=O)N2[C@H](C#N)CC3C[C@@H]32)CCC1.C[C@H](C(=O)N1[C@H](C#N)CC2C[C@@H]21)C1(CO)CCC1.C[C@H](C(=O)N1[C@H](C#N)CC2C[C@@H]21)C1(CO)CCC1 Chemical compound C=CC1([C@H](C)C(=O)N2[C@H](C#N)CC3C[C@@H]32)CCC1.C[C@H](C(=O)N1[C@H](C#N)CC2C[C@@H]21)C1(CO)CCC1.C[C@H](C(=O)N1[C@H](C#N)CC2C[C@@H]21)C1(CO)CCC1 DJBDZAFMSXYDOD-DDQLXMPWSA-N 0.000 description 1
- LTLFRPCXJCRWQM-MQYQWHSLSA-N C=CC1([C@H](C)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 Chemical compound C=CC1([C@H](C)C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)CCCC1 LTLFRPCXJCRWQM-MQYQWHSLSA-N 0.000 description 1
- LQLIBSUHOLQFMA-KIGPFUIMSA-N C=CC1([C@H](N)C(=O)N2CC3C[C@H]3[C@H]2C#N)CCCC1 Chemical compound C=CC1([C@H](N)C(=O)N2CC3C[C@H]3[C@H]2C#N)CCCC1 LQLIBSUHOLQFMA-KIGPFUIMSA-N 0.000 description 1
- XNOXWQJDOLDBNR-ZSBIGDGJSA-N C=C[C@]1(C(C)C)C[C@H](C)[C@H](C)C1 Chemical compound C=C[C@]1(C(C)C)C[C@H](C)[C@H](C)C1 XNOXWQJDOLDBNR-ZSBIGDGJSA-N 0.000 description 1
- WSFSSNUMVMOOMR-UHFFFAOYSA-N C=O Chemical compound C=O WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 1
- NWIYAJUEIYBLKL-UHFFFAOYSA-N CB(O)N1CCC(C)(C)C1C(=O)O Chemical compound CB(O)N1CCC(C)(C)C1C(=O)O NWIYAJUEIYBLKL-UHFFFAOYSA-N 0.000 description 1
- QJTJDZYWLXIGPT-GVHYBUMESA-N CB(O)N1CC[C@H](C(C)C)C1C(=O)O Chemical compound CB(O)N1CC[C@H](C(C)C)C1C(=O)O QJTJDZYWLXIGPT-GVHYBUMESA-N 0.000 description 1
- ZTSGBSJGMKLQIM-UHFFFAOYSA-N CC(=O)C(C(=O)O)C1(C)CCCCC1 Chemical compound CC(=O)C(C(=O)O)C1(C)CCCCC1 ZTSGBSJGMKLQIM-UHFFFAOYSA-N 0.000 description 1
- QONCXQCIHCTQQX-UHFFFAOYSA-N CC(=O)C(C(=O)O)C1(C)CCCCC1.CC(=O)C(NC(=O)OC(C)(C)C)C1(C)CCCCC1.CC(C)(C)OC(=O)NC(C(=O)O)C1(C)CCCCC1.CCOC(=O)C(C(C)=O)=C1CCCCC1.CCOC(=O)C(C(C)=O)C1(C)CCCCC1.CNC(C(C)=O)C1(C)CCCCC1.O=C1CCCCC1 Chemical compound CC(=O)C(C(=O)O)C1(C)CCCCC1.CC(=O)C(NC(=O)OC(C)(C)C)C1(C)CCCCC1.CC(C)(C)OC(=O)NC(C(=O)O)C1(C)CCCCC1.CCOC(=O)C(C(C)=O)=C1CCCCC1.CCOC(=O)C(C(C)=O)C1(C)CCCCC1.CNC(C(C)=O)C1(C)CCCCC1.O=C1CCCCC1 QONCXQCIHCTQQX-UHFFFAOYSA-N 0.000 description 1
- PMDGMSWPMPBRPG-UHFFFAOYSA-N CC(=O)C(NC(=O)OC(C)(C)C)C1(C)CCCCC1 Chemical compound CC(=O)C(NC(=O)OC(C)(C)C)C1(C)CCCCC1 PMDGMSWPMPBRPG-UHFFFAOYSA-N 0.000 description 1
- IGGIMIVHMNPOPW-LRHRNSLUSA-N CC(C(=O)N1C2C[C@H]2C[C@H]1C(N)=O)C(C)(C)C Chemical compound CC(C(=O)N1C2C[C@H]2C[C@H]1C(N)=O)C(C)(C)C IGGIMIVHMNPOPW-LRHRNSLUSA-N 0.000 description 1
- AXSNOQIZWWPTHN-JUIFTKJUSA-N CC(C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21)C1(CO)CCCC1 Chemical compound CC(C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21)C1(CO)CCCC1 AXSNOQIZWWPTHN-JUIFTKJUSA-N 0.000 description 1
- DNSCARKXPISLTG-GMWNIWNXSA-N CC(C(=O)O)C(C)(C)C1=CC=CC=C1.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(N)C(C)(C)C1=CC=CC=C1 Chemical compound CC(C(=O)O)C(C)(C)C1=CC=CC=C1.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(N)C(C)(C)C1=CC=CC=C1 DNSCARKXPISLTG-GMWNIWNXSA-N 0.000 description 1
- BISNOGHLLUACPA-UHFFFAOYSA-N CC(C(=O)O)C1(C2=CC=CC=C2)CCCC1.OCC1(C2=CC=CC=C2)CCCC1.[H]C(=O)C1(C2=CC=CC=C2)CCCC1 Chemical compound CC(C(=O)O)C1(C2=CC=CC=C2)CCCC1.OCC1(C2=CC=CC=C2)CCCC1.[H]C(=O)C1(C2=CC=CC=C2)CCCC1 BISNOGHLLUACPA-UHFFFAOYSA-N 0.000 description 1
- OMLULQVTABFBLQ-MHGCJGGUSA-N CC(C(=O)O)C1CCOCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1CCOCC1 Chemical compound CC(C(=O)O)C1CCOCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1CCOCC1 OMLULQVTABFBLQ-MHGCJGGUSA-N 0.000 description 1
- ATYSCBFJSQGWLO-FVKBGGDRSA-N CC(C)(C)C(C(N(CC1[C@H]2C1)C2C#N)=O)N Chemical compound CC(C)(C)C(C(N(CC1[C@H]2C1)C2C#N)=O)N ATYSCBFJSQGWLO-FVKBGGDRSA-N 0.000 description 1
- SPQFNPAKAARNPO-UHFFFAOYSA-N CC(C)(C)OC(=O)CC(C(=O)O)C1(C2=CC=CC=C2)CCCC1 Chemical compound CC(C)(C)OC(=O)CC(C(=O)O)C1(C2=CC=CC=C2)CCCC1 SPQFNPAKAARNPO-UHFFFAOYSA-N 0.000 description 1
- BTYAVQVNQJIICM-XWDCQGHASA-N CC(C)(C)OC(=O)CC(C(=O)O)C1(C2=CC=CC=C2)CCCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1(C2=CC=CC=C2)CCCC1 Chemical compound CC(C)(C)OC(=O)CC(C(=O)O)C1(C2=CC=CC=C2)CCCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1(C2=CC=CC=C2)CCCC1 BTYAVQVNQJIICM-XWDCQGHASA-N 0.000 description 1
- WYSZMKLICSTOEQ-KKSQLVLISA-N CC(C)(C)OC(=O)C[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)N1C2C[C@@H]2C[C@H]1C#N Chemical compound CC(C)(C)OC(=O)C[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)N1C2C[C@@H]2C[C@H]1C#N WYSZMKLICSTOEQ-KKSQLVLISA-N 0.000 description 1
- QAWHRYBSZOSWPP-YKNYVSNTSA-N CC(C)(C)OC(=O)C[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)N1C2C[C@@H]2C[C@H]1C(N)=O Chemical compound CC(C)(C)OC(=O)C[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)N1C2C[C@@H]2C[C@H]1C(N)=O QAWHRYBSZOSWPP-YKNYVSNTSA-N 0.000 description 1
- ACYIOXZTRHWTHC-ZCTILFKHSA-N CC(C)(C)OC(=O)C[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)O.N#C[C@@H]1CC2C[C@H]2N1C(=O)[C@H](N)CCO Chemical compound CC(C)(C)OC(=O)C[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)O.N#C[C@@H]1CC2C[C@H]2N1C(=O)[C@H](N)CCO ACYIOXZTRHWTHC-ZCTILFKHSA-N 0.000 description 1
- SWHZCUSSNBJSMI-PNNOBFLRSA-N CC(C)(C)OC(=O)N1C2C[C@H]2C[C@H]1C(=O)O.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(C)C(C)(C)C Chemical compound CC(C)(C)OC(=O)N1C2C[C@H]2C[C@H]1C(=O)O.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(C)C(C)(C)C SWHZCUSSNBJSMI-PNNOBFLRSA-N 0.000 description 1
- CIRFYAMRXKSFCA-WBQMQQTJSA-N CC(C)(C)OC(=O)N1C2C[C@H]2C[C@H]1C(=O)O.[H][C@@]1(C(=O)N2C3C[C@H]3C[C@H]2[N+]#[C-])C[C@@H]2CC2N1 Chemical compound CC(C)(C)OC(=O)N1C2C[C@H]2C[C@H]1C(=O)O.[H][C@@]1(C(=O)N2C3C[C@H]3C[C@H]2[N+]#[C-])C[C@@H]2CC2N1 CIRFYAMRXKSFCA-WBQMQQTJSA-N 0.000 description 1
- VLAGXRRGXCNITB-PPSBICQBSA-N CC(C)(C)OC(=O)N1C2C[C@H]2C[C@H]1C(N)=O Chemical compound CC(C)(C)OC(=O)N1C2C[C@H]2C[C@H]1C(N)=O VLAGXRRGXCNITB-PPSBICQBSA-N 0.000 description 1
- XDTQZWWMVMFMRK-NLZKTAQOSA-N CC(C)(C)OC(=O)NC(C(=O)N1C2C[C@H]2C[C@H]1C#N)C1(CO)CCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1(CO)CCC1 Chemical compound CC(C)(C)OC(=O)NC(C(=O)N1C2C[C@H]2C[C@H]1C#N)C1(CO)CCC1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1(CO)CCC1 XDTQZWWMVMFMRK-NLZKTAQOSA-N 0.000 description 1
- ODFLRFYPQFCPNQ-UHFFFAOYSA-N CC(C)(C)OC(=O)NC(C(=O)O)C(C)(C)CO[Si](C)(C)C(C)(C)C Chemical compound CC(C)(C)OC(=O)NC(C(=O)O)C(C)(C)CO[Si](C)(C)C(C)(C)C ODFLRFYPQFCPNQ-UHFFFAOYSA-N 0.000 description 1
- PLAQGKKUVFMRFR-UHFFFAOYSA-N CC(C)(C)OC(=O)NC(C(=O)O)C1(C)CCCCC1 Chemical compound CC(C)(C)OC(=O)NC(C(=O)O)C1(C)CCCCC1 PLAQGKKUVFMRFR-UHFFFAOYSA-N 0.000 description 1
- WBSJQVRMQOLSAT-UHFFFAOYSA-N CC(C)(C)OC(=O)NC(C(=O)O)C1CCCC1 Chemical compound CC(C)(C)OC(=O)NC(C(=O)O)C1CCCC1 WBSJQVRMQOLSAT-UHFFFAOYSA-N 0.000 description 1
- MIVFZPXRTAVOLZ-VBJONLRCSA-N CC(C)(C)OC(=O)NC(CC1=CC=C(O)C=C1)C(=O)N1C2C[C@H]2C[C@H]1C#N Chemical compound CC(C)(C)OC(=O)NC(CC1=CC=C(O)C=C1)C(=O)N1C2C[C@H]2C[C@H]1C#N MIVFZPXRTAVOLZ-VBJONLRCSA-N 0.000 description 1
- VRRKAPDDLISBKN-JKLRMWSDSA-N CC(C)(C)OC(=O)NC(CC1=CC=C(OCC2=CC=CC=C2)C=C1)C(=O)N1C2C[C@H]2C[C@H]1C#N Chemical compound CC(C)(C)OC(=O)NC(CC1=CC=C(OCC2=CC=CC=C2)C=C1)C(=O)N1C2C[C@H]2C[C@H]1C#N VRRKAPDDLISBKN-JKLRMWSDSA-N 0.000 description 1
- KWWBYHRCPQBVHQ-QLFUNNCKSA-N CC(C)(C)OC(=O)NC1C(=O)OCC1(C)C.CC(C)(CO)[C@H](N)C(=O)N1C2C[C@H]2C[C@H]1C#N Chemical compound CC(C)(C)OC(=O)NC1C(=O)OCC1(C)C.CC(C)(CO)[C@H](N)C(=O)N1C2C[C@H]2C[C@H]1C#N KWWBYHRCPQBVHQ-QLFUNNCKSA-N 0.000 description 1
- APUITWXVDQDYKI-VMHBLCJISA-N CC(C)(C)OC(=O)N[C@@H](CS(=O)(=O)C(C)(C)C)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21.CC(C)(C)S(=O)(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 Chemical compound CC(C)(C)OC(=O)N[C@@H](CS(=O)(=O)C(C)(C)C)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21.CC(C)(C)S(=O)(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 APUITWXVDQDYKI-VMHBLCJISA-N 0.000 description 1
- KINHAWTWEXJNJR-DTWWHVGOSA-N CC(C)(C)OC(=O)N[C@@H](CS(=O)C(C)(C)C)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 Chemical compound CC(C)(C)OC(=O)N[C@@H](CS(=O)C(C)(C)C)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 KINHAWTWEXJNJR-DTWWHVGOSA-N 0.000 description 1
- WYCJESSLHHJCQD-CJSYELHBSA-N CC(C)(C)OC(=O)N[C@@H](CSC(C)(C)C)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21.CC(C)(C)S(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 Chemical compound CC(C)(C)OC(=O)N[C@@H](CSC(C)(C)C)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21.CC(C)(C)S(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 WYCJESSLHHJCQD-CJSYELHBSA-N 0.000 description 1
- IYQPLXVDGMNQKO-UHFFFAOYSA-N CC(C)(C)OC(NC(C1(CCCC1)C=C)C(O)=O)=O Chemical compound CC(C)(C)OC(NC(C1(CCCC1)C=C)C(O)=O)=O IYQPLXVDGMNQKO-UHFFFAOYSA-N 0.000 description 1
- WXCJXKIURJIMGX-RCWTZXSCSA-N CC(C)(C)S(=O)(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 Chemical compound CC(C)(C)S(=O)(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 WXCJXKIURJIMGX-RCWTZXSCSA-N 0.000 description 1
- HVIDGQBVVOQXSA-TYJZHKNSSA-N CC(C)(C)S(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 Chemical compound CC(C)(C)S(=O)C[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 HVIDGQBVVOQXSA-TYJZHKNSSA-N 0.000 description 1
- ATYSCBFJSQGWLO-NPTNVHDASA-N CC(C)(C)[C@H](N)C(=O)N1CC2C[C@H]2[C@H]1C#N Chemical compound CC(C)(C)[C@H](N)C(=O)N1CC2C[C@H]2[C@H]1C#N ATYSCBFJSQGWLO-NPTNVHDASA-N 0.000 description 1
- IYQQJOMTNUWLKJ-GPCQJSLASA-N CC(C)(O)[C@H](N)C(=O)N1C2CC2C[C@H]1C#N Chemical compound CC(C)(O)[C@H](N)C(=O)N1C2CC2C[C@H]1C#N IYQQJOMTNUWLKJ-GPCQJSLASA-N 0.000 description 1
- HRDWHBMAXHWZBF-UHFFFAOYSA-N CC(C)C(=O)C(N)C(C)C Chemical compound CC(C)C(=O)C(N)C(C)C HRDWHBMAXHWZBF-UHFFFAOYSA-N 0.000 description 1
- MKSSZBVUSBKULT-UHFFFAOYSA-N CC(C)C(=O)C(N)C1=CC=CC=C1 Chemical compound CC(C)C(=O)C(N)C1=CC=CC=C1 MKSSZBVUSBKULT-UHFFFAOYSA-N 0.000 description 1
- HOZWDEAVDXQKRF-UHFFFAOYSA-N CC(C)C(=O)C(N)CC1=CC=C(O)C=C1 Chemical compound CC(C)C(=O)C(N)CC1=CC=C(O)C=C1 HOZWDEAVDXQKRF-UHFFFAOYSA-N 0.000 description 1
- RXHBYGUHPWXAKY-UHFFFAOYSA-N CC(C)C(=O)C(N)CC1=CC=CC=C1 Chemical compound CC(C)C(=O)C(N)CC1=CC=CC=C1 RXHBYGUHPWXAKY-UHFFFAOYSA-N 0.000 description 1
- IPWJEOSEVPXGNB-UHFFFAOYSA-N CC(C)C(=O)C(N)CC1=CNC2=C1C=CC=C2 Chemical compound CC(C)C(=O)C(N)CC1=CNC2=C1C=CC=C2 IPWJEOSEVPXGNB-UHFFFAOYSA-N 0.000 description 1
- WAUMZVIWAOBGCC-UHFFFAOYSA-N CC(C)C(=O)C(N)CC1CCCCC1 Chemical compound CC(C)C(=O)C(N)CC1CCCCC1 WAUMZVIWAOBGCC-UHFFFAOYSA-N 0.000 description 1
- HWPPEPGHYNQQAT-UHFFFAOYSA-N CC(C)C(=O)C(N)CCC1=CC=CC=C1 Chemical compound CC(C)C(=O)C(N)CCC1=CC=CC=C1 HWPPEPGHYNQQAT-UHFFFAOYSA-N 0.000 description 1
- WWSBPYPNQCYAKZ-UHFFFAOYSA-N CC(C)C(=O)C(N)CSC(C)(C)C Chemical compound CC(C)C(=O)C(N)CSC(C)(C)C WWSBPYPNQCYAKZ-UHFFFAOYSA-N 0.000 description 1
- LGSJHWYSWYALJZ-UHFFFAOYSA-N CC(C)C(=O)C(N)CSCC1=CC=CC=C1 Chemical compound CC(C)C(=O)C(N)CSCC1=CC=CC=C1 LGSJHWYSWYALJZ-UHFFFAOYSA-N 0.000 description 1
- BXKUHKFASTUTCD-LBPRGKRZSA-N CC(C)C([C@H](Cc1cc(C#N)ccc1)N)=O Chemical compound CC(C)C([C@H](Cc1cc(C#N)ccc1)N)=O BXKUHKFASTUTCD-LBPRGKRZSA-N 0.000 description 1
- WUOGNYKYOBVTIY-KBHBFKLGSA-N CC(C)C1(C(N)C(=O)N2C3C[C@H]3C[C@H]2C#N)CCCC1 Chemical compound CC(C)C1(C(N)C(=O)N2C3C[C@H]3C[C@H]2C#N)CCCC1 WUOGNYKYOBVTIY-KBHBFKLGSA-N 0.000 description 1
- AEURWFNHWVWEER-UHFFFAOYSA-N CC(C)C1(CO)CCC1 Chemical compound CC(C)C1(CO)CCC1 AEURWFNHWVWEER-UHFFFAOYSA-N 0.000 description 1
- XPUKBZPFJLXWJU-UHFFFAOYSA-N CC(C)C1(CO)CCCC1 Chemical compound CC(C)C1(CO)CCCC1 XPUKBZPFJLXWJU-UHFFFAOYSA-N 0.000 description 1
- WDFYSCXYKARVAN-UHFFFAOYSA-N CC(C)C1(CO)CCCCC1 Chemical compound CC(C)C1(CO)CCCCC1 WDFYSCXYKARVAN-UHFFFAOYSA-N 0.000 description 1
- HEGMIMMSZBAFJC-UHFFFAOYSA-N CC(C)C1(CO)CCCCCC1 Chemical compound CC(C)C1(CO)CCCCCC1 HEGMIMMSZBAFJC-UHFFFAOYSA-N 0.000 description 1
- XMYDFAHOFRRNKF-UHFFFAOYSA-N CC(C)CC(N)C(=O)C(C)C Chemical compound CC(C)CC(N)C(=O)C(C)C XMYDFAHOFRRNKF-UHFFFAOYSA-N 0.000 description 1
- ZMOLCTZDIVONIV-JGPRNRPPSA-N CC(C)[C@@]1(CO)C[C@H](C)[C@H](C)C1 Chemical compound CC(C)[C@@]1(CO)C[C@H](C)[C@H](C)C1 ZMOLCTZDIVONIV-JGPRNRPPSA-N 0.000 description 1
- IGRUXQWGUWXGOQ-BDBFLJFWSA-N CC(C)[C@H](N)C(=O)N1CC2C[C@H]2[C@H]1C#N Chemical compound CC(C)[C@H](N)C(=O)N1CC2C[C@H]2[C@H]1C#N IGRUXQWGUWXGOQ-BDBFLJFWSA-N 0.000 description 1
- MUTKETLQEPQKEP-ULUSZKPHSA-N CC(C)[C@H]1CCNC1C(=O)O.Cl Chemical compound CC(C)[C@H]1CCNC1C(=O)O.Cl MUTKETLQEPQKEP-ULUSZKPHSA-N 0.000 description 1
- XLHCPOXKXPHGFJ-UHFFFAOYSA-N CC(C1(CCCC1)C(C(N1C(C2)(C3)C23CC1C#N)=O)N)=C Chemical compound CC(C1(CCCC1)C(C(N1C(C2)(C3)C23CC1C#N)=O)N)=C XLHCPOXKXPHGFJ-UHFFFAOYSA-N 0.000 description 1
- JENOMBHQGDXBPV-UHFFFAOYSA-N CC.CC#N Chemical compound CC.CC#N JENOMBHQGDXBPV-UHFFFAOYSA-N 0.000 description 1
- JQFLYFRHDIHZFZ-UHFFFAOYSA-N CC1(C)CCNC1C(=O)O.Cl Chemical compound CC1(C)CCNC1C(=O)O.Cl JQFLYFRHDIHZFZ-UHFFFAOYSA-N 0.000 description 1
- XUJIATXWPUGLOH-NCSKGSJWSA-N CC12CC3CC(C1)CC([C@H](NBC=O)C(=O)N1C4C[C@H]4C[C@H]1C(N)=O)(C3)C2 Chemical compound CC12CC3CC(C1)CC([C@H](NBC=O)C(=O)N1C4C[C@H]4C[C@H]1C(N)=O)(C3)C2 XUJIATXWPUGLOH-NCSKGSJWSA-N 0.000 description 1
- GPGLBISHLQFVPQ-JCXPFGTASA-N CC1=CC=C(CC(C)C(=O)N2C3C[C@H]3C[C@H]2C(N)=O)C=C1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)CC1=CC=C(O)C=C1 Chemical compound CC1=CC=C(CC(C)C(=O)N2C3C[C@H]3C[C@H]2C(N)=O)C=C1.N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)CC1=CC=C(O)C=C1 GPGLBISHLQFVPQ-JCXPFGTASA-N 0.000 description 1
- HYUIONHGQIXGAI-UHFFFAOYSA-N CC1CC2CC2CN1C Chemical compound CC1CC2CC2CN1C HYUIONHGQIXGAI-UHFFFAOYSA-N 0.000 description 1
- ZBFQHICJKKQHEE-IHXHZNKUSA-N CCC(C)C(C(N(C(C1)C1C1)[C@@H]1C(OCC)=O)=O)NC(OC(C)(C)C)=O Chemical compound CCC(C)C(C(N(C(C1)C1C1)[C@@H]1C(OCC)=O)=O)NC(OC(C)(C)C)=O ZBFQHICJKKQHEE-IHXHZNKUSA-N 0.000 description 1
- SCGZRIGHFZPTGP-KNHGFYQXSA-N CCC(C)[C@H](C)C(=O)N1C2CC2C[C@H]1C#N Chemical compound CCC(C)[C@H](C)C(=O)N1C2CC2C[C@H]1C#N SCGZRIGHFZPTGP-KNHGFYQXSA-N 0.000 description 1
- ADTGNTBRKPFWPV-FNNWFYBWSA-N CCC(C)[C@H](C)C(=O)N1C2CC2C[C@H]1C(=O)O Chemical compound CCC(C)[C@H](C)C(=O)N1C2CC2C[C@H]1C(=O)O ADTGNTBRKPFWPV-FNNWFYBWSA-N 0.000 description 1
- DSKYQFUFNKULFO-FNNWFYBWSA-N CCC(C)[C@H](C)C(=O)N1C2CC2C[C@H]1C(N)=O Chemical compound CCC(C)[C@H](C)C(=O)N1C2CC2C[C@H]1C(N)=O DSKYQFUFNKULFO-FNNWFYBWSA-N 0.000 description 1
- MOHOUBGPPZQTST-KIGPFUIMSA-N CCC1([C@H](N)C(=O)N2CC3C[C@H]3[C@H]2C#N)CCCC1 Chemical compound CCC1([C@H](N)C(=O)N2CC3C[C@H]3[C@H]2C#N)CCCC1 MOHOUBGPPZQTST-KIGPFUIMSA-N 0.000 description 1
- POAGYKSAQACBKY-UHFFFAOYSA-N CCCCC(N)C(=O)C(C)C Chemical compound CCCCC(N)C(=O)C(C)C POAGYKSAQACBKY-UHFFFAOYSA-N 0.000 description 1
- SQHAAFSBCPFSSI-DNFJNHEJSA-N CCOC(=O)C(C(C)=O)=C1CCCC1.N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1CCCC1 Chemical compound CCOC(=O)C(C(C)=O)=C1CCCC1.N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1CCCC1 SQHAAFSBCPFSSI-DNFJNHEJSA-N 0.000 description 1
- UEYYJOUBHCJMDN-UHFFFAOYSA-N CCOC(=O)C(C(C)=O)=C1CCCCC1 Chemical compound CCOC(=O)C(C(C)=O)=C1CCCCC1 UEYYJOUBHCJMDN-UHFFFAOYSA-N 0.000 description 1
- FISOOUYYGOAMQQ-UHFFFAOYSA-N CCOC(=O)C(C(C)=O)C1(C)CCCCC1 Chemical compound CCOC(=O)C(C(C)=O)C1(C)CCCCC1 FISOOUYYGOAMQQ-UHFFFAOYSA-N 0.000 description 1
- CRISDWSRLFVDOU-UHFFFAOYSA-N CCOC(=O)C(C(C)=O)C1CCCC1 Chemical compound CCOC(=O)C(C(C)=O)C1CCCC1 CRISDWSRLFVDOU-UHFFFAOYSA-N 0.000 description 1
- VXMSULZWQQQITH-PUIFHPDHSA-N CCOC(=O)C1(C(=O)OCC)[C@@H](C(C)C)CC(O)N1C(C)=O.[H][C@]1(C(=O)N2C3C[C@H]3C[C@H]2C#N)NCC[C@@H]1C(C)C Chemical compound CCOC(=O)C1(C(=O)OCC)[C@@H](C(C)C)CC(O)N1C(C)=O.[H][C@]1(C(=O)N2C3C[C@H]3C[C@H]2C#N)NCC[C@@H]1C(C)C VXMSULZWQQQITH-PUIFHPDHSA-N 0.000 description 1
- BNVFJVRXXJLZKO-GFCCVEGCSA-N CCOC(=O)C1(C(=O)OCC)[C@@H](C(C)C)CCN1C(C)=O Chemical compound CCOC(=O)C1(C(=O)OCC)[C@@H](C(C)C)CCN1C(C)=O BNVFJVRXXJLZKO-GFCCVEGCSA-N 0.000 description 1
- JQQRIVWUMGGDBK-DFPVQWTFSA-N CCOC(=O)C1(C(=O)OCC)[C@@H](CC)CC(O)N1C(C)=O.[H][C@]1(C(=O)N2C3C[C@H]3C[C@H]2C#N)NCC[C@@H]1CC Chemical compound CCOC(=O)C1(C(=O)OCC)[C@@H](CC)CC(O)N1C(C)=O.[H][C@]1(C(=O)N2C3C[C@H]3C[C@H]2C#N)NCC[C@@H]1CC JQQRIVWUMGGDBK-DFPVQWTFSA-N 0.000 description 1
- XBXLKRFKEWSJDU-NSHDSACASA-N CCOC(=O)C1(C(=O)OCC)[C@@H](CC)CCN1C(C)=O Chemical compound CCOC(=O)C1(C(=O)OCC)[C@@H](CC)CCN1C(C)=O XBXLKRFKEWSJDU-NSHDSACASA-N 0.000 description 1
- KJGNDKYUUHJMKE-UHFFFAOYSA-N CCOC(=O)C1CC2CC2C1 Chemical compound CCOC(=O)C1CC2CC2C1 KJGNDKYUUHJMKE-UHFFFAOYSA-N 0.000 description 1
- YMPDYKDVOTXICJ-RZGYODBHSA-N CCOC(=O)C1CC2CC2C1.[C-]#[N+][C@@H]1CC2CC2N1C(=O)[C@@H](C)C(C)CC Chemical compound CCOC(=O)C1CC2CC2C1.[C-]#[N+][C@@H]1CC2CC2N1C(=O)[C@@H](C)C(C)CC YMPDYKDVOTXICJ-RZGYODBHSA-N 0.000 description 1
- MNBKWHCSVMYLFN-GLNDQIJQSA-N CCOC(=O)C1CC2CC2C1.[C-]#[N+][C@@H]1CC2CC2N1C(=O)[C@@H](N)C(C)CC Chemical compound CCOC(=O)C1CC2CC2C1.[C-]#[N+][C@@H]1CC2CC2N1C(=O)[C@@H](N)C(C)CC MNBKWHCSVMYLFN-GLNDQIJQSA-N 0.000 description 1
- YMFKBJGIACDORR-AUURQCFLSA-N CCOC(=O)[C@@H]1CC2CC2N1C(=O)[C@@H](C)C(C)CC Chemical compound CCOC(=O)[C@@H]1CC2CC2N1C(=O)[C@@H](C)C(C)CC YMFKBJGIACDORR-AUURQCFLSA-N 0.000 description 1
- BNVFJVRXXJLZKO-UHFFFAOYSA-N CCOC(C(C(CC1)C(C)C)(C(OCC)=O)N1C(C)=O)=O Chemical compound CCOC(C(C(CC1)C(C)C)(C(OCC)=O)N1C(C)=O)=O BNVFJVRXXJLZKO-UHFFFAOYSA-N 0.000 description 1
- RNXPJDNPMPERPI-GVHYBUMESA-N CC[C@@H](C)C(N)C(=O)C(C)C Chemical compound CC[C@@H](C)C(N)C(=O)C(C)C RNXPJDNPMPERPI-GVHYBUMESA-N 0.000 description 1
- DJZSYOVDOCJIGD-ACJTYDJDSA-N CC[C@H](C)C(C(N1C[C@H](C2)[C@H]2C1)=O)NC(OC(C)(C)C)=O Chemical compound CC[C@H](C)C(C(N1C[C@H](C2)[C@H]2C1)=O)NC(OC(C)(C)C)=O DJZSYOVDOCJIGD-ACJTYDJDSA-N 0.000 description 1
- GUMDGHVFROVPCD-PKPIPKONSA-N CC[C@H]1CCN(B(C)O)C1C(=O)O Chemical compound CC[C@H]1CCN(B(C)O)C1C(=O)O GUMDGHVFROVPCD-PKPIPKONSA-N 0.000 description 1
- UGOPTYHSSLZCIG-ZBHICJROSA-N CC[C@H]1CCNC1C(=O)O.Cl Chemical compound CC[C@H]1CCNC1C(=O)O.Cl UGOPTYHSSLZCIG-ZBHICJROSA-N 0.000 description 1
- KOSAELQBJOUYGE-ICIXOUAASA-N CN1CC2CC2C[C@H]1C#N.CN1CC2C[C@H]2C[C@H]1C#N.C[C@@H]1C[C@@H]2CC2CN1C Chemical compound CN1CC2CC2C[C@H]1C#N.CN1CC2C[C@H]2C[C@H]1C#N.C[C@@H]1C[C@@H]2CC2CN1C KOSAELQBJOUYGE-ICIXOUAASA-N 0.000 description 1
- PXGWOSHJDOPJHY-UHFFFAOYSA-N CNC(C(=O)C(C)C)C(C)C Chemical compound CNC(C(=O)C(C)C)C(C)C PXGWOSHJDOPJHY-UHFFFAOYSA-N 0.000 description 1
- YABMIJVQOMLMFB-UHFFFAOYSA-N CNC(C(C)=O)C1(C)CCCCC1 Chemical compound CNC(C(C)=O)C1(C)CCCCC1 YABMIJVQOMLMFB-UHFFFAOYSA-N 0.000 description 1
- PRCTXEMAQDYUNN-UHFFFAOYSA-N COC1(C2=CC=CC=C2)CCCC1 Chemical compound COC1(C2=CC=CC=C2)CCCC1 PRCTXEMAQDYUNN-UHFFFAOYSA-N 0.000 description 1
- VLBPHZFRABGLFH-UHFFFAOYSA-N COC12CC3CC(CC(C3)C1)C2 Chemical compound COC12CC3CC(CC(C3)C1)C2 VLBPHZFRABGLFH-UHFFFAOYSA-N 0.000 description 1
- UTHHPMCQWUPFIA-XTHGAFRISA-N COC12CC3CC(CC(C3)C1)C2.Cl.Cl.N#C[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2.N[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2.O=C(O)[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2.O=CBN[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2.O=COCC12CC3CC(CC(C3)C1)C2.OCC12CC3CC(CC(C3)C1)C2 Chemical compound COC12CC3CC(CC(C3)C1)C2.Cl.Cl.N#C[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2.N[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2.O=C(O)[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2.O=CBN[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2.O=COCC12CC3CC(CC(C3)C1)C2.OCC12CC3CC(CC(C3)C1)C2 UTHHPMCQWUPFIA-XTHGAFRISA-N 0.000 description 1
- QAGYKUNXZHXKMR-UHFFFAOYSA-N CPD000469186 Natural products CC1=C(O)C=CC=C1C(=O)NC(C(O)CN1C(CC2CCCCC2C1)C(=O)NC(C)(C)C)CSC1=CC=CC=C1 QAGYKUNXZHXKMR-UHFFFAOYSA-N 0.000 description 1
- QBKJELGKEFALKS-UHFFFAOYSA-N CSCCC(N)C(=O)C(C)C Chemical compound CSCCC(N)C(=O)C(C)C QBKJELGKEFALKS-UHFFFAOYSA-N 0.000 description 1
- GHJCTCKKOQPAQC-XFPHUMHMSA-N C[C@H](C(=O)O)C1C2CC3CC(C2)CC1C3.O=CC(F)(F)F.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1C2CC3CC1CC(F)(C3)C2 Chemical compound C[C@H](C(=O)O)C1C2CC3CC(C2)CC1C3.O=CC(F)(F)F.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1C2CC3CC1CC(F)(C3)C2 GHJCTCKKOQPAQC-XFPHUMHMSA-N 0.000 description 1
- IUWIHPMKFXTZSM-SZVWITLZSA-N C[C@H](N)C(=O)N1C2C[C@H]2C[C@H]1C#N Chemical compound C[C@H](N)C(=O)N1C2C[C@H]2C[C@H]1C#N IUWIHPMKFXTZSM-SZVWITLZSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 229940122502 Cholesterol absorption inhibitor Drugs 0.000 description 1
- 229920001268 Cholestyramine Polymers 0.000 description 1
- KPSRODZRAIWAKH-JTQLQIEISA-N Ciprofibrate Natural products C1=CC(OC(C)(C)C(O)=O)=CC=C1[C@H]1C(Cl)(Cl)C1 KPSRODZRAIWAKH-JTQLQIEISA-N 0.000 description 1
- NJRFVURYVWPLKB-PZLWRYNDSA-N Cl.N[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2 Chemical compound Cl.N[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2 NJRFVURYVWPLKB-PZLWRYNDSA-N 0.000 description 1
- AJMYNFGVKPOATE-SNXNFCLHSA-N Cl.O=C(O)[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2 Chemical compound Cl.O=C(O)[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2 AJMYNFGVKPOATE-SNXNFCLHSA-N 0.000 description 1
- BMOVQUBVGICXQN-UHFFFAOYSA-N Clinofibrate Chemical compound C1=CC(OC(C)(CC)C(O)=O)=CC=C1C1(C=2C=CC(OC(C)(CC)C(O)=O)=CC=2)CCCCC1 BMOVQUBVGICXQN-UHFFFAOYSA-N 0.000 description 1
- 208000015943 Coeliac disease Diseases 0.000 description 1
- 229920002911 Colestipol Polymers 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 229910021591 Copper(I) chloride Inorganic materials 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- VMQMZMRVKUZKQL-UHFFFAOYSA-N Cu+ Chemical compound [Cu+] VMQMZMRVKUZKQL-UHFFFAOYSA-N 0.000 description 1
- XZMCDFZZKTWFGF-UHFFFAOYSA-N Cyanamide Chemical compound NC#N XZMCDFZZKTWFGF-UHFFFAOYSA-N 0.000 description 1
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 1
- PMPVIKIVABFJJI-UHFFFAOYSA-N Cyclobutane Chemical compound C1CCC1 PMPVIKIVABFJJI-UHFFFAOYSA-N 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- 229930105110 Cyclosporin A Natural products 0.000 description 1
- HPJYKMSFRBJOSW-JHSUYXJUSA-N Damsin Chemical compound C[C@H]1CC[C@H]2C(=C)C(=O)O[C@H]2[C@]2(C)C(=O)CC[C@@H]12 HPJYKMSFRBJOSW-JHSUYXJUSA-N 0.000 description 1
- 241000350052 Daniellia ogea Species 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- CYQFCXCEBYINGO-DLBZAZTESA-N Dronabinol Natural products C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@H]21 CYQFCXCEBYINGO-DLBZAZTESA-N 0.000 description 1
- DBVJJBKOTRCVKF-UHFFFAOYSA-N Etidronic acid Chemical compound OP(=O)(O)C(O)(C)P(O)(O)=O DBVJJBKOTRCVKF-UHFFFAOYSA-N 0.000 description 1
- 102000051325 Glucagon Human genes 0.000 description 1
- 108060003199 Glucagon Proteins 0.000 description 1
- 102400000326 Glucagon-like peptide 2 Human genes 0.000 description 1
- 101800000221 Glucagon-like peptide 2 Proteins 0.000 description 1
- 102000007390 Glycogen Phosphorylase Human genes 0.000 description 1
- 108010046163 Glycogen Phosphorylase Proteins 0.000 description 1
- 108010051696 Growth Hormone Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 238000006546 Horner-Wadsworth-Emmons reaction Methods 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 229940122199 Insulin secretagogue Drugs 0.000 description 1
- 206010067745 Intestinal mucosal atrophy Diseases 0.000 description 1
- 102000036770 Islet Amyloid Polypeptide Human genes 0.000 description 1
- 108010041872 Islet Amyloid Polypeptide Proteins 0.000 description 1
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 1
- PMRVFZXOCRHXFE-FMEJWYFOSA-L Kad 1229 Chemical compound [Ca+2].C([C@@H](CC(=O)N1C[C@@H]2CCCC[C@@H]2C1)C(=O)[O-])C1=CC=CC=C1.C([C@@H](CC(=O)N1C[C@@H]2CCCC[C@@H]2C1)C(=O)[O-])C1=CC=CC=C1 PMRVFZXOCRHXFE-FMEJWYFOSA-L 0.000 description 1
- 238000006000 Knoevenagel condensation reaction Methods 0.000 description 1
- 150000008575 L-amino acids Chemical class 0.000 description 1
- ZUKPVRWZDMRIEO-VKHMYHEASA-N L-cysteinylglycine Chemical compound SC[C@H]([NH3+])C(=O)NCC([O-])=O ZUKPVRWZDMRIEO-VKHMYHEASA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 102000007330 LDL Lipoproteins Human genes 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 239000002841 Lewis acid Substances 0.000 description 1
- 229910010084 LiAlH4 Inorganic materials 0.000 description 1
- 229910013596 LiOH—H2O Inorganic materials 0.000 description 1
- 238000005684 Liebig rearrangement reaction Methods 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 101710151321 Melanostatin Proteins 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 238000006845 Michael addition reaction Methods 0.000 description 1
- 208000026940 Microvillus inclusion disease Diseases 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- CHSGRFSLIUGLTF-DYSSGHCBSA-N N#CC1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CCC(CC1)C2.O=CC(F)(F)F Chemical compound N#CC1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CCC(CC1)C2.O=CC(F)(F)F CHSGRFSLIUGLTF-DYSSGHCBSA-N 0.000 description 1
- BRLUMYAYSUWXEL-HESWJOIASA-N N#C[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2 Chemical compound N#C[C@@H](N[C@@H](CO)C1=CC=CC=C1)C12CC3CC(CC(C3)C1)C2 BRLUMYAYSUWXEL-HESWJOIASA-N 0.000 description 1
- NAPBGFQFQGYHOI-FDNDLASISA-N N#C[C@@H]1CC2CC2N1C(=O)[C@H](N)CCO Chemical compound N#C[C@@H]1CC2CC2N1C(=O)[C@H](N)CCO NAPBGFQFQGYHOI-FDNDLASISA-N 0.000 description 1
- QSKQRLCATLZUSY-QKEWWQLBSA-N N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1CCOCC1 Chemical compound N#C[C@@H]1C[C@@H]2CC2N1C(=O)C(N)C1CCOCC1 QSKQRLCATLZUSY-QKEWWQLBSA-N 0.000 description 1
- YYBDDABMXURRRU-NMBWSBHNSA-N N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(C2=CC=CC=C2)CCCC1 Chemical compound N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(C2=CC=CC=C2)CCCC1 YYBDDABMXURRRU-NMBWSBHNSA-N 0.000 description 1
- CMKYSPWIUMHMJR-KJLIAQIISA-N N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCC1 Chemical compound N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1(CO)CCC1 CMKYSPWIUMHMJR-KJLIAQIISA-N 0.000 description 1
- WMHHYWDIABVJJD-OXZFGWJOSA-N N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CCC(CC1)C2.O=CC(F)(F)F.O=COCC12CCC(CC1)C2 Chemical compound N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CCC(CC1)C2.O=CC(F)(F)F.O=COCC12CCC(CC1)C2 WMHHYWDIABVJJD-OXZFGWJOSA-N 0.000 description 1
- DQFBIHLORBWGBL-ZGIDMGGYSA-N N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)CC1=CC=C(O)C=C1 Chemical compound N#C[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)CC1=CC=C(O)C=C1 DQFBIHLORBWGBL-ZGIDMGGYSA-N 0.000 description 1
- WJQRXWVSKOAFPJ-FEZOTEKNSA-N N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)C(N)C1(CO)CCCC1 Chemical compound N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)C(N)C1(CO)CCCC1 WJQRXWVSKOAFPJ-FEZOTEKNSA-N 0.000 description 1
- BUOKTADOTMIIFF-YMAVYUHTSA-N N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1(C(O)CO)CCCC1 Chemical compound N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1(C(O)CO)CCCC1 BUOKTADOTMIIFF-YMAVYUHTSA-N 0.000 description 1
- YXAYKXKMMGMPEY-RHYQMDGZSA-N N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1CCCC1 Chemical compound N#C[C@@H]1C[C@@H]2C[C@@H]2N1C(=O)[C@@H](N)C1CCCC1 YXAYKXKMMGMPEY-RHYQMDGZSA-N 0.000 description 1
- GPTNAFLCFIWAHF-HYWTVENDSA-N N#C[C@@H]1[C@@H]2CC2CN1C(=O)[C@@H](N)C1CCCCC1 Chemical compound N#C[C@@H]1[C@@H]2CC2CN1C(=O)[C@@H](N)C1CCCCC1 GPTNAFLCFIWAHF-HYWTVENDSA-N 0.000 description 1
- LFTLOKWAGJYHHR-UHFFFAOYSA-N N-methylmorpholine N-oxide Chemical compound CN1(=O)CCOCC1 LFTLOKWAGJYHHR-UHFFFAOYSA-N 0.000 description 1
- 150000001204 N-oxides Chemical class 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- AJRHWDKPEHVVMS-CQQQDSTESA-N N=Cl1CC2CC2C1.[H][C@@]12CN(C(=O)[C@@H](ClN)C(C)CC)C[C@]1([H])C2 Chemical compound N=Cl1CC2CC2C1.[H][C@@]12CN(C(=O)[C@@H](ClN)C(C)CC)C[C@]1([H])C2 AJRHWDKPEHVVMS-CQQQDSTESA-N 0.000 description 1
- IONMTHXOQDTHKK-DSDZBIDZSA-N NC(=O)[C@@H]1C[C@@H]2CC2N1C(=O)C(F)(F)F Chemical compound NC(=O)[C@@H]1C[C@@H]2CC2N1C(=O)C(F)(F)F IONMTHXOQDTHKK-DSDZBIDZSA-N 0.000 description 1
- RBVPXVJJMMTTGO-AMLPAPDGSA-N NC(=O)[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(F)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2 Chemical compound NC(=O)[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(F)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2 RBVPXVJJMMTTGO-AMLPAPDGSA-N 0.000 description 1
- IRVKLVYFYTZBMA-FVGPBMPESA-N NC(=O)[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(O)(C3)C1)C2 Chemical compound NC(=O)[C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(O)(C3)C1)C2 IRVKLVYFYTZBMA-FVGPBMPESA-N 0.000 description 1
- FNXPJLPPIAOEPI-HCWXCVPCSA-N NC(=O)[C@H]1CC[C@H]2C[C@H]21 Chemical compound NC(=O)[C@H]1CC[C@H]2C[C@H]21 FNXPJLPPIAOEPI-HCWXCVPCSA-N 0.000 description 1
- BUOKTADOTMIIFF-UHFFFAOYSA-N NC(C1(CCCC1)C(CO)O)C(N(C(C1)C1C1)C1C#N)=O Chemical compound NC(C1(CCCC1)C(CO)O)C(N(C(C1)C1C1)C1C#N)=O BUOKTADOTMIIFF-UHFFFAOYSA-N 0.000 description 1
- CMKYSPWIUMHMJR-SEQHWMEXSA-N NC(C1(CO)CCC1)C(N([C@@H](C1)C1C1)[C@@H]1C#N)=O Chemical compound NC(C1(CO)CCC1)C(N([C@@H](C1)C1C1)[C@@H]1C#N)=O CMKYSPWIUMHMJR-SEQHWMEXSA-N 0.000 description 1
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 229940122313 Nucleoside reverse transcriptase inhibitor Drugs 0.000 description 1
- MBBCVAKAJPKAKM-UHFFFAOYSA-N O=C(NC1CCN(CCCCC2(C(=O)NCC(F)(F)F)C3=C(C=CC=C3)C3=C\C=C/C=C\32)CC1)C1=CC=CC=C1C1=CC=C(C(F)(F)F)C=C1 Chemical compound O=C(NC1CCN(CCCCC2(C(=O)NCC(F)(F)F)C3=C(C=CC=C3)C3=C\C=C/C=C\32)CC1)C1=CC=CC=C1C1=CC=C(C(F)(F)F)C=C1 MBBCVAKAJPKAKM-UHFFFAOYSA-N 0.000 description 1
- AAHPSDCMWOLKRL-VBRVDACPSA-N O=CBN[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2 Chemical compound O=CBN[C@H](C(=O)O)C12CC3CC(CC(C3)C1)C2 AAHPSDCMWOLKRL-VBRVDACPSA-N 0.000 description 1
- BNKRHOLVNZKQGX-OMZAYAJMSA-N O=CBN[C@H](C(=O)O)C12CC3CC(CC(O)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 Chemical compound O=CBN[C@H](C(=O)O)C12CC3CC(CC(O)(C3)C1)C2.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(O)(C3)C1)C2 BNKRHOLVNZKQGX-OMZAYAJMSA-N 0.000 description 1
- VKBWLYPOTXWFRN-CCNFQMFXSA-N O=CBN[C@H](C(=O)O)C12CCC(CC1)C2 Chemical compound O=CBN[C@H](C(=O)O)C12CCC(CC1)C2 VKBWLYPOTXWFRN-CCNFQMFXSA-N 0.000 description 1
- SHMMXJGWEHTPFH-FNQJDLDUSA-N O=CC(F)(F)F.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2 Chemical compound O=CC(F)(F)F.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(F)(C3)C1)C2 SHMMXJGWEHTPFH-FNQJDLDUSA-N 0.000 description 1
- KTUVCGZPZKAKAZ-RYIGUBAASA-N O=CC(F)(F)F.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1C2CC3CC(C2)CC1C3 Chemical compound O=CC(F)(F)F.[C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C1C2CC3CC(C2)CC1C3 KTUVCGZPZKAKAZ-RYIGUBAASA-N 0.000 description 1
- TVEPXHHCOSKFBZ-UHFFFAOYSA-N O=COCC12CC3CC(CC(C3)C1)C2 Chemical compound O=COCC12CC3CC(CC(C3)C1)C2 TVEPXHHCOSKFBZ-UHFFFAOYSA-N 0.000 description 1
- VWKGSQHGSUHREM-UHFFFAOYSA-N O=COCC12CCC(CC1)C2 Chemical compound O=COCC12CCC(CC1)C2 VWKGSQHGSUHREM-UHFFFAOYSA-N 0.000 description 1
- JNUJHQSLSMMEKE-UHFFFAOYSA-N O=COCC12CCC(CC1Br)C2 Chemical compound O=COCC12CCC(CC1Br)C2 JNUJHQSLSMMEKE-UHFFFAOYSA-N 0.000 description 1
- WJMWSPUOZRYMJD-UHFFFAOYSA-N OCC1(C2=CC=CC=C2)CCCC1 Chemical compound OCC1(C2=CC=CC=C2)CCCC1 WJMWSPUOZRYMJD-UHFFFAOYSA-N 0.000 description 1
- MDVGOOIANLZFCP-UHFFFAOYSA-N OCC12CC3CC(CC(C3)C1)C2 Chemical compound OCC12CC3CC(CC(C3)C1)C2 MDVGOOIANLZFCP-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000003728 Peroxisome Proliferator-Activated Receptors Human genes 0.000 description 1
- 108090000029 Peroxisome Proliferator-Activated Receptors Proteins 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 102100028427 Pro-neuropeptide Y Human genes 0.000 description 1
- 102000035554 Proglucagon Human genes 0.000 description 1
- 108010058003 Proglucagon Proteins 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 229910019020 PtO2 Inorganic materials 0.000 description 1
- CVQUWLDCFXOXEN-UHFFFAOYSA-N Pyran-4-one Chemical compound O=C1C=COC=C1 CVQUWLDCFXOXEN-UHFFFAOYSA-N 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 208000017442 Retinal disease Diseases 0.000 description 1
- 206010038923 Retinopathy Diseases 0.000 description 1
- NCDNCNXCDXHOMX-UHFFFAOYSA-N Ritonavir Natural products C=1C=CC=CC=1CC(NC(=O)OCC=1SC=NC=1)C(O)CC(CC=1C=CC=CC=1)NC(=O)C(C(C)C)NC(=O)N(C)CC1=CSC(C(C)C)=N1 NCDNCNXCDXHOMX-UHFFFAOYSA-N 0.000 description 1
- AJLFOPYRIVGYMJ-UHFFFAOYSA-N SJ000287055 Natural products C12C(OC(=O)C(C)CC)CCC=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 AJLFOPYRIVGYMJ-UHFFFAOYSA-N 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- 229920005654 Sephadex Polymers 0.000 description 1
- 239000012507 Sephadex™ Substances 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 102100022831 Somatoliberin Human genes 0.000 description 1
- 101710142969 Somatoliberin Proteins 0.000 description 1
- 102100038803 Somatotropin Human genes 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 238000006859 Swern oxidation reaction Methods 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- CYQFCXCEBYINGO-UHFFFAOYSA-N THC Natural products C1=C(C)CCC2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3C21 CYQFCXCEBYINGO-UHFFFAOYSA-N 0.000 description 1
- ACWQBUSCFPJUPN-UHFFFAOYSA-N Tiglaldehyde Natural products CC=C(C)C=O ACWQBUSCFPJUPN-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical group CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 108010003205 Vasoactive Intestinal Peptide Proteins 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- AOGVIRLQNRZJGM-UHFFFAOYSA-N [C-]#[N+]C1=CC(CC(N)C(=O)C(C)C)=CC=C1 Chemical compound [C-]#[N+]C1=CC(CC(N)C(=O)C(C)C)=CC=C1 AOGVIRLQNRZJGM-UHFFFAOYSA-N 0.000 description 1
- IFBJJYIILXRLLX-UHFFFAOYSA-N [C-]#[N+]C1=CC=C(CC(N)C(=O)C(C)C)C=C1 Chemical compound [C-]#[N+]C1=CC=C(CC(N)C(=O)C(C)C)C=C1 IFBJJYIILXRLLX-UHFFFAOYSA-N 0.000 description 1
- BTTOCCGWVPTGPI-HQSMGLLHSA-N [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(N)C(C)(C)C1=CC=CC=C1 Chemical compound [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)C(N)C(C)(C)C1=CC=CC=C1 BTTOCCGWVPTGPI-HQSMGLLHSA-N 0.000 description 1
- QGKWMFYAUJERKB-XTWPEQDISA-N [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(C3)C1)C2 Chemical compound [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](N)C12CC3CC(CC(C3)C1)C2 QGKWMFYAUJERKB-XTWPEQDISA-N 0.000 description 1
- HCTUSKCCPZGEHV-VAIXNTTOSA-N [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(C)(C3)C1)C2 Chemical compound [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(C)(C3)C1)C2 HCTUSKCCPZGEHV-VAIXNTTOSA-N 0.000 description 1
- HDEWVFOKCBHLLR-LEYTYUIDSA-N [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(F)(C3)C1)C2 Chemical compound [C-]#[N+][C@@H]1C[C@@H]2CC2N1C(=O)[C@@H](NBC=O)C12CC3CC(CC(F)(C3)C1)C2 HDEWVFOKCBHLLR-LEYTYUIDSA-N 0.000 description 1
- KCHTXUATCPXNHD-VOULRHJBSA-N [C-]#[N+][C@@H]1NC[C@@H]2C[C@H]12.[C-]#[N+][C@@H]1[C@@H]2C[C@@H]2CN1C(=O)[C@@H](C)C(C)CC.[C-]#[N+][C@H]1NC[C@H]2C[C@H]21.[C-]#[N+][C@H]1[C@H]2C[C@H]2CN1C(=O)[C@@H](C)C(C)CC Chemical compound [C-]#[N+][C@@H]1NC[C@@H]2C[C@H]12.[C-]#[N+][C@@H]1[C@@H]2C[C@@H]2CN1C(=O)[C@@H](C)C(C)CC.[C-]#[N+][C@H]1NC[C@H]2C[C@H]21.[C-]#[N+][C@H]1[C@H]2C[C@H]2CN1C(=O)[C@@H](C)C(C)CC KCHTXUATCPXNHD-VOULRHJBSA-N 0.000 description 1
- XXQSDCAGWUVNLK-BFSCJLJSSA-N [C-]#[N+][C@@H]1NC[C@H]2C[C@H]21.[C-]#[N+][C@H]1NC[C@@H]2C[C@@H]21 Chemical compound [C-]#[N+][C@@H]1NC[C@H]2C[C@H]21.[C-]#[N+][C@H]1NC[C@@H]2C[C@@H]21 XXQSDCAGWUVNLK-BFSCJLJSSA-N 0.000 description 1
- RNXPJDNPMPERPI-MQWKRIRWSA-N [H][C@@](N)(C(=O)C(C)C)C(C)CC Chemical compound [H][C@@](N)(C(=O)C(C)C)C(C)CC RNXPJDNPMPERPI-MQWKRIRWSA-N 0.000 description 1
- RNXPJDNPMPERPI-SFYZADRCSA-N [H][C@@](N)(C(=O)C(C)C)[C@H](C)CC Chemical compound [H][C@@](N)(C(=O)C(C)C)[C@H](C)CC RNXPJDNPMPERPI-SFYZADRCSA-N 0.000 description 1
- NHOQWCLNXVRHFW-HHEYZYDJSA-N [H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)[C@@H](C)C(C)(C)C.[H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)[C@@H](C)C(C)(C)C.[H][C@@]1(C(=O)OCC)C[C@]2([H])C[C@]2([H])N1C(=O)OC(C)(C)C.[H][C@@]1(C(N)=O)C[C@]2([H])C[C@]2([H])N1C(=O)OC(C)(C)C.[H][C@@]1(C(N)=O)C[C@]2([H])C[C@]2([H])N1C(=O)[C@@H](C)C(C)(C)C.[H][C@]12C[C@@]([H])(C(N)=O)C[C@@]1([H])C2 Chemical compound [H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)[C@@H](C)C(C)(C)C.[H][C@@]1(C#N)C[C@]2([H])C[C@]2([H])N1C(=O)[C@@H](C)C(C)(C)C.[H][C@@]1(C(=O)OCC)C[C@]2([H])C[C@]2([H])N1C(=O)OC(C)(C)C.[H][C@@]1(C(N)=O)C[C@]2([H])C[C@]2([H])N1C(=O)OC(C)(C)C.[H][C@@]1(C(N)=O)C[C@]2([H])C[C@]2([H])N1C(=O)[C@@H](C)C(C)(C)C.[H][C@]12C[C@@]([H])(C(N)=O)C[C@@]1([H])C2 NHOQWCLNXVRHFW-HHEYZYDJSA-N 0.000 description 1
- YQDFHMVXGVIJQP-QMMMGPOBSA-N [H][C@@]1(C(=O)C(C)C)CCCCN1 Chemical compound [H][C@@]1(C(=O)C(C)C)CCCCN1 YQDFHMVXGVIJQP-QMMMGPOBSA-N 0.000 description 1
- PBQZSVNJVABPPR-ZETCQYMHSA-N [H][C@@]1(C(=O)C(C)C)CCCN1 Chemical compound [H][C@@]1(C(=O)C(C)C)CCCN1 PBQZSVNJVABPPR-ZETCQYMHSA-N 0.000 description 1
- GCFVAIRPUWJIAJ-IYXWEFQISA-N [H][C@@]1(C(=O)N2C3C[C@H]3C[C@H]2[N+]#[C-])C[C@@H]2CC2N1 Chemical compound [H][C@@]1(C(=O)N2C3C[C@H]3C[C@H]2[N+]#[C-])C[C@@H]2CC2N1 GCFVAIRPUWJIAJ-IYXWEFQISA-N 0.000 description 1
- OBRNTVHUFYDQAR-OFLUOSHYSA-N [H][C@@]12CN(C(=O)C(C)[C@@H](C)CC)C[C@]1([H])C2 Chemical compound [H][C@@]12CN(C(=O)C(C)[C@@H](C)CC)C[C@]1([H])C2 OBRNTVHUFYDQAR-OFLUOSHYSA-N 0.000 description 1
- UOQHNBYSMQYWMO-UPBARMNASA-N [H][C@@]12CN(C(=O)[C@@H](ClN)C(C)CC)C[C@]1([H])C2 Chemical compound [H][C@@]12CN(C(=O)[C@@H](ClN)C(C)CC)C[C@]1([H])C2 UOQHNBYSMQYWMO-UPBARMNASA-N 0.000 description 1
- DZLFEATUGWDDCH-YUMQZZPRSA-N [H][C@]1(C(=O)C(C)C)NCC[C@@H]1C Chemical compound [H][C@]1(C(=O)C(C)C)NCC[C@@H]1C DZLFEATUGWDDCH-YUMQZZPRSA-N 0.000 description 1
- DNKHDIRQNJCPRL-ISNSYZFTSA-N [H][C@]1(C(=O)N2C3CC3C[C@H]2C#N)NCC[C@@H]1CC Chemical compound [H][C@]1(C(=O)N2C3CC3C[C@H]2C#N)NCC[C@@H]1CC DNKHDIRQNJCPRL-ISNSYZFTSA-N 0.000 description 1
- RRLVNFXQXWNDBE-KJLIAQIISA-N [H][C@]1(C(=O)N2C3C[C@H]3C[C@H]2C#N)NCCC1(C)C Chemical compound [H][C@]1(C(=O)N2C3C[C@H]3C[C@H]2C#N)NCCC1(C)C RRLVNFXQXWNDBE-KJLIAQIISA-N 0.000 description 1
- NLQWRGFIIOKQOS-DMGDTADUSA-N [H][C@]1(C(=O)N2[C@H](C#N)CC3C[C@@H]32)NCC[C@@H]1C(C)C Chemical compound [H][C@]1(C(=O)N2[C@H](C#N)CC3C[C@@H]32)NCC[C@@H]1C(C)C NLQWRGFIIOKQOS-DMGDTADUSA-N 0.000 description 1
- RRLVNFXQXWNDBE-VPOLOUISSA-N [H][C@]1(C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)NCCC1(C)C Chemical compound [H][C@]1(C(=O)N2[C@H](C#N)C[C@@H]3C[C@@H]32)NCCC1(C)C RRLVNFXQXWNDBE-VPOLOUISSA-N 0.000 description 1
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- DFDGRKNOFOJBAJ-UHFFFAOYSA-N acifran Chemical compound C=1C=CC=CC=1C1(C)OC(C(O)=O)=CC1=O DFDGRKNOFOJBAJ-UHFFFAOYSA-N 0.000 description 1
- 229950000146 acifran Drugs 0.000 description 1
- 229960003526 acipimox Drugs 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- VGBBJZYOAOCLLS-UHFFFAOYSA-N adamantane-2-carbaldehyde Chemical compound C1C(C2)CC3CC1C(C=O)C2C3 VGBBJZYOAOCLLS-UHFFFAOYSA-N 0.000 description 1
- YKIOKAURTKXMSB-UHFFFAOYSA-N adams's catalyst Chemical compound O=[Pt]=O YKIOKAURTKXMSB-UHFFFAOYSA-N 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 229960004343 alendronic acid Drugs 0.000 description 1
- 229910001413 alkali metal ion Inorganic materials 0.000 description 1
- 229910001420 alkaline earth metal ion Inorganic materials 0.000 description 1
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 1
- 125000004419 alkynylene group Chemical group 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229960004909 aminosalicylic acid Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000002924 anti-infective effect Effects 0.000 description 1
- 229940127226 anticholesterol agent Drugs 0.000 description 1
- 229940058303 antinematodal benzimidazole derivative Drugs 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 125000003435 aroyl group Chemical group 0.000 description 1
- 125000005018 aryl alkenyl group Chemical group 0.000 description 1
- 125000001691 aryl alkyl amino group Chemical group 0.000 description 1
- 125000005015 aryl alkynyl group Chemical group 0.000 description 1
- 125000005100 aryl amino carbonyl group Chemical group 0.000 description 1
- 125000005129 aryl carbonyl group Chemical group 0.000 description 1
- 125000005199 aryl carbonyloxy group Chemical group 0.000 description 1
- 125000005135 aryl sulfinyl group Chemical group 0.000 description 1
- 125000004657 aryl sulfonyl amino group Chemical group 0.000 description 1
- 125000005164 aryl thioalkyl group Chemical group 0.000 description 1
- 125000005325 aryloxy aryl group Chemical group 0.000 description 1
- 229940072224 asacol Drugs 0.000 description 1
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 125000003785 benzimidazolyl group Chemical class N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- UTZLYIFQYGQUPA-UHFFFAOYSA-N benzyl 2,5-dioxopyrrolidine-1-carboxylate Chemical compound O=C1CCC(=O)N1C(=O)OCC1=CC=CC=C1 UTZLYIFQYGQUPA-UHFFFAOYSA-N 0.000 description 1
- DTCCTIQRPGSLPT-UHFFFAOYSA-N beta-Aethyl-acrolein Natural products CCC=CC=O DTCCTIQRPGSLPT-UHFFFAOYSA-N 0.000 description 1
- 229960000516 bezafibrate Drugs 0.000 description 1
- IIBYAHWJQTYFKB-UHFFFAOYSA-N bezafibrate Chemical compound C1=CC(OC(C)(C)C(O)=O)=CC=C1CCNC(=O)C1=CC=C(Cl)C=C1 IIBYAHWJQTYFKB-UHFFFAOYSA-N 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- 239000012455 biphasic mixture Substances 0.000 description 1
- OZVBMTJYIDMWIL-AYFBDAFISA-N bromocriptine Chemical compound C1=CC(C=2[C@H](N(C)C[C@@H](C=2)C(=O)N[C@]2(C(=O)N3[C@H](C(N4CCC[C@H]4[C@]3(O)O2)=O)CC(C)C)C(C)C)C2)=C3C2=C(Br)NC3=C1 OZVBMTJYIDMWIL-AYFBDAFISA-N 0.000 description 1
- 229960002802 bromocriptine Drugs 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 125000005392 carboxamide group Chemical group NC(=O)* 0.000 description 1
- 238000010531 catalytic reduction reaction Methods 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 230000001906 cholesterol absorption Effects 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- 229960002174 ciprofibrate Drugs 0.000 description 1
- KPSRODZRAIWAKH-UHFFFAOYSA-N ciprofibrate Chemical compound C1=CC(OC(C)(C)C(O)=O)=CC=C1C1C(Cl)(Cl)C1 KPSRODZRAIWAKH-UHFFFAOYSA-N 0.000 description 1
- 229950003072 clinofibrate Drugs 0.000 description 1
- 229940046989 clomiphene citrate Drugs 0.000 description 1
- 229910052681 coesite Inorganic materials 0.000 description 1
- 229960002604 colestipol Drugs 0.000 description 1
- GMRWGQCZJGVHKL-UHFFFAOYSA-N colestipol Chemical compound ClCC1CO1.NCCNCCNCCNCCN GMRWGQCZJGVHKL-UHFFFAOYSA-N 0.000 description 1
- 230000006957 competitive inhibition Effects 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 229940125810 compound 20 Drugs 0.000 description 1
- 229940125846 compound 25 Drugs 0.000 description 1
- 238000010924 continuous production Methods 0.000 description 1
- 239000013256 coordination polymer Substances 0.000 description 1
- OXBLHERUFWYNTN-UHFFFAOYSA-M copper(I) chloride Chemical compound [Cu]Cl OXBLHERUFWYNTN-UHFFFAOYSA-M 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 229910052906 cristobalite Inorganic materials 0.000 description 1
- 229940088900 crixivan Drugs 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 229940045803 cuprous chloride Drugs 0.000 description 1
- MGNCLNQXLYJVJD-UHFFFAOYSA-N cyanuric chloride Chemical compound ClC1=NC(Cl)=NC(Cl)=N1 MGNCLNQXLYJVJD-UHFFFAOYSA-N 0.000 description 1
- 125000005112 cycloalkylalkoxy group Chemical group 0.000 description 1
- 125000002993 cycloalkylene group Chemical group 0.000 description 1
- CGZZMOTZOONQIA-UHFFFAOYSA-N cycloheptanone Chemical compound O=C1CCCCCC1 CGZZMOTZOONQIA-UHFFFAOYSA-N 0.000 description 1
- 125000001162 cycloheptenyl group Chemical group C1(=CCCCCC1)* 0.000 description 1
- 125000003678 cyclohexadienyl group Chemical group C1(=CC=CCC1)* 0.000 description 1
- IIRFCWANHMSDCG-UHFFFAOYSA-N cyclooctanone Chemical compound O=C1CCCCCCC1 IIRFCWANHMSDCG-UHFFFAOYSA-N 0.000 description 1
- 125000000522 cyclooctenyl group Chemical group C1(=CCCCCCC1)* 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 150000001942 cyclopropanes Chemical class 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 229950006689 darglitazone Drugs 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 229960000475 delavirdine mesylate Drugs 0.000 description 1
- MEPNHSOMXMALDZ-UHFFFAOYSA-N delavirdine mesylate Chemical compound CS(O)(=O)=O.CC(C)NC1=CC=CN=C1N1CCN(C(=O)C=2NC3=CC=C(NS(C)(=O)=O)C=C3C=2)CC1 MEPNHSOMXMALDZ-UHFFFAOYSA-N 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000005595 deprotonation Effects 0.000 description 1
- 238000010537 deprotonation reaction Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- MHDVGSVTJDSBDK-UHFFFAOYSA-N dibenzyl ether Chemical compound C=1C=CC=CC=1COCC1=CC=CC=C1 MHDVGSVTJDSBDK-UHFFFAOYSA-N 0.000 description 1
- 229960001193 diclofenac sodium Drugs 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- RKUPWPSMWSKJQP-UHFFFAOYSA-N diethyl 2-(phenylmethoxycarbonylamino)propanedioate Chemical compound CCOC(=O)C(C(=O)OCC)NC(=O)OCC1=CC=CC=C1 RKUPWPSMWSKJQP-UHFFFAOYSA-N 0.000 description 1
- GLFVNTDRBTZJIY-UHFFFAOYSA-N diethyl 2-aminopropanedioate;hydron;chloride Chemical compound Cl.CCOC(=O)C(N)C(=O)OCC GLFVNTDRBTZJIY-UHFFFAOYSA-N 0.000 description 1
- WGSPBTQXEQZIMR-UHFFFAOYSA-N diethyl 3,3-dimethylpyrrolidine-2,2-dicarboxylate Chemical compound CCOC(=O)C1(C(=O)OCC)NCCC1(C)C WGSPBTQXEQZIMR-UHFFFAOYSA-N 0.000 description 1
- 229940043279 diisopropylamine Drugs 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- HPYNZHMRTTWQTB-UHFFFAOYSA-N dimethylpyridine Natural products CC1=CC=CN=C1C HPYNZHMRTTWQTB-UHFFFAOYSA-N 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- MKRTXPORKIRPDG-UHFFFAOYSA-N diphenylphosphoryl azide Chemical compound C=1C=CC=CC=1P(=O)(N=[N+]=[N-])C1=CC=CC=C1 MKRTXPORKIRPDG-UHFFFAOYSA-N 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229960004242 dronabinol Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 229950002375 englitazone Drugs 0.000 description 1
- 238000010931 ester hydrolysis Methods 0.000 description 1
- 229940083571 etidronate disodium Drugs 0.000 description 1
- GWBBVOVXJZATQQ-UHFFFAOYSA-L etidronate disodium Chemical compound [Na+].[Na+].OP(=O)([O-])C(O)(C)P(O)([O-])=O GWBBVOVXJZATQQ-UHFFFAOYSA-L 0.000 description 1
- 229940085363 evista Drugs 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 229940065410 feldene Drugs 0.000 description 1
- 239000002871 fertility agent Substances 0.000 description 1
- 229940001490 fosamax Drugs 0.000 description 1
- 238000001640 fractional crystallisation Methods 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 230000030136 gastric emptying Effects 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 1
- 229960004666 glucagon Drugs 0.000 description 1
- 108010063245 glucagon-like peptide 1 (7-36)amide Proteins 0.000 description 1
- TWSALRJGPBVBQU-PKQQPRCHSA-N glucagon-like peptide 2 Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](C)CC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)CC)C1=CC=CC=C1 TWSALRJGPBVBQU-PKQQPRCHSA-N 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- XLXSAKCOAKORKW-UHFFFAOYSA-N gonadorelin Chemical class C1CCC(C(=O)NCC(N)=O)N1C(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 XLXSAKCOAKORKW-UHFFFAOYSA-N 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 150000004795 grignard reagents Chemical class 0.000 description 1
- 239000000122 growth hormone Substances 0.000 description 1
- 239000003324 growth hormone secretagogue Substances 0.000 description 1
- JAXFJECJQZDFJS-XHEPKHHKSA-N gtpl8555 Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@H](B1O[C@@]2(C)[C@H]3C[C@H](C3(C)C)C[C@H]2O1)CCC1=CC=C(F)C=C1 JAXFJECJQZDFJS-XHEPKHHKSA-N 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005114 heteroarylalkoxy group Chemical group 0.000 description 1
- 125000005368 heteroarylthio group Chemical group 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- MSYBLBLAMDYKKZ-UHFFFAOYSA-N hydron;pyridine-3-carbonyl chloride;chloride Chemical compound Cl.ClC(=O)C1=CC=CN=C1 MSYBLBLAMDYKKZ-UHFFFAOYSA-N 0.000 description 1
- XXSMGPRMXLTPCZ-UHFFFAOYSA-N hydroxychloroquine Chemical compound ClC1=CC=C2C(NC(C)CCCN(CCO)CC)=CC=NC2=C1 XXSMGPRMXLTPCZ-UHFFFAOYSA-N 0.000 description 1
- 229960004171 hydroxychloroquine Drugs 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 230000000871 hypocholesterolemic effect Effects 0.000 description 1
- 229950010293 imanixil Drugs 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- NUBQKPWHXMGDLP-BDEHJDMKSA-N indinavir sulfate Chemical compound OS(O)(=O)=O.C([C@H](N(CC1)C[C@@H](O)C[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H]2C3=CC=CC=C3C[C@H]2O)C(=O)NC(C)(C)C)N1CC1=CC=CN=C1 NUBQKPWHXMGDLP-BDEHJDMKSA-N 0.000 description 1
- 229960004243 indinavir sulfate Drugs 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 229960000905 indomethacin Drugs 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 208000037817 intestinal injury Diseases 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 229940088976 invirase Drugs 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 125000004491 isohexyl group Chemical group C(CCC(C)C)* 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- TVSBRLGQVHJIKT-UHFFFAOYSA-N isopropyl cyclopentane Natural products CC(C)C1CCCC1 TVSBRLGQVHJIKT-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229960004384 ketorolac tromethamine Drugs 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229960001627 lamivudine Drugs 0.000 description 1
- JTEGQNOMFQHVDC-NKWVEPMBSA-N lamivudine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1 JTEGQNOMFQHVDC-NKWVEPMBSA-N 0.000 description 1
- 229940033984 lamivudine / zidovudine Drugs 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 150000007517 lewis acids Chemical class 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000037356 lipid metabolism Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000012280 lithium aluminium hydride Substances 0.000 description 1
- DLEDOFVPSDKWEF-UHFFFAOYSA-N lithium butane Chemical compound [Li+].CCC[CH2-] DLEDOFVPSDKWEF-UHFFFAOYSA-N 0.000 description 1
- 229910001416 lithium ion Inorganic materials 0.000 description 1
- OVEHNNQXLPJPPL-UHFFFAOYSA-N lithium;n-propan-2-ylpropan-2-amine Chemical compound [Li].CC(C)NC(C)C OVEHNNQXLPJPPL-UHFFFAOYSA-N 0.000 description 1
- 239000013541 low molecular weight contaminant Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- NXPHGHWWQRMDIA-UHFFFAOYSA-M magnesium;carbanide;bromide Chemical compound [CH3-].[Mg+2].[Br-] NXPHGHWWQRMDIA-UHFFFAOYSA-M 0.000 description 1
- VXWPONVCMVLXBW-UHFFFAOYSA-M magnesium;carbanide;iodide Chemical compound [CH3-].[Mg+2].[I-] VXWPONVCMVLXBW-UHFFFAOYSA-M 0.000 description 1
- 150000002690 malonic acid derivatives Chemical class 0.000 description 1
- 229940099262 marinol Drugs 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 229940090004 megace Drugs 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- 229950008446 melinamide Drugs 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229960004963 mesalazine Drugs 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- HOVAGTYPODGVJG-VEIUFWFVSA-N methyl alpha-D-mannoside Chemical compound CO[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O HOVAGTYPODGVJG-VEIUFWFVSA-N 0.000 description 1
- AJLFOPYRIVGYMJ-INTXDZFKSA-N mevastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=CCC[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 AJLFOPYRIVGYMJ-INTXDZFKSA-N 0.000 description 1
- 229950009116 mevastatin Drugs 0.000 description 1
- BOZILQFLQYBIIY-UHFFFAOYSA-N mevastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CCC=C21 BOZILQFLQYBIIY-UHFFFAOYSA-N 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 229960003365 mitiglinide Drugs 0.000 description 1
- 125000002950 monocyclic group Chemical group 0.000 description 1
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 1
- PSHKMPUSSFXUIA-UHFFFAOYSA-N n,n-dimethylpyridin-2-amine Chemical compound CN(C)C1=CC=CC=N1 PSHKMPUSSFXUIA-UHFFFAOYSA-N 0.000 description 1
- WGESLFUSXZBFQF-UHFFFAOYSA-N n-methyl-n-prop-2-enylprop-2-en-1-amine Chemical class C=CCN(C)CC=C WGESLFUSXZBFQF-UHFFFAOYSA-N 0.000 description 1
- 229960004270 nabumetone Drugs 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 229960002009 naproxen Drugs 0.000 description 1
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 1
- 208000037931 necrotizing enteritis Diseases 0.000 description 1
- 230000023187 negative regulation of glucagon secretion Effects 0.000 description 1
- 229960000884 nelfinavir Drugs 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 201000001119 neuropathy Diseases 0.000 description 1
- 230000007823 neuropathy Effects 0.000 description 1
- 229960003512 nicotinic acid Drugs 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- YCWSUKQGVSGXJO-NTUHNPAUSA-N nifuroxazide Chemical group C1=CC(O)=CC=C1C(=O)N\N=C\C1=CC=C([N+]([O-])=O)O1 YCWSUKQGVSGXJO-NTUHNPAUSA-N 0.000 description 1
- 125000002560 nitrile group Chemical group 0.000 description 1
- 230000006959 non-competitive inhibition Effects 0.000 description 1
- 229940042402 non-nucleoside reverse transcriptase inhibitor Drugs 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 239000002726 nonnucleoside reverse transcriptase inhibitor Substances 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940072250 norvir Drugs 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 229940127234 oral contraceptive Drugs 0.000 description 1
- 239000003539 oral contraceptive agent Substances 0.000 description 1
- MUMZUERVLWJKNR-UHFFFAOYSA-N oxoplatinum Chemical compound [Pt]=O MUMZUERVLWJKNR-UHFFFAOYSA-N 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 229940083256 peripheral vasodilators nicotinic acid and derivative Drugs 0.000 description 1
- 210000002824 peroxisome Anatomy 0.000 description 1
- 239000008024 pharmaceutical diluent Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- ICFJFFQQTFMIBG-UHFFFAOYSA-N phenformin Chemical compound NC(=N)NC(=N)NCCC1=CC=CC=C1 ICFJFFQQTFMIBG-UHFFFAOYSA-N 0.000 description 1
- 229960003243 phenformin Drugs 0.000 description 1
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 1
- FAIAAWCVCHQXDN-UHFFFAOYSA-N phosphorus trichloride Chemical compound ClP(Cl)Cl FAIAAWCVCHQXDN-UHFFFAOYSA-N 0.000 description 1
- 230000008288 physiological mechanism Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229960002702 piroxicam Drugs 0.000 description 1
- 229910003446 platinum oxide Inorganic materials 0.000 description 1
- 229920000371 poly(diallyldimethylammonium chloride) polymer Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 230000029537 positive regulation of insulin secretion Effects 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000012286 potassium permanganate Substances 0.000 description 1
- LJCNRYVRMXRIQR-OLXYHTOASA-L potassium sodium L-tartrate Chemical compound [Na+].[K+].[O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O LJCNRYVRMXRIQR-OLXYHTOASA-L 0.000 description 1
- 150000003858 primary carboxamides Chemical class 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000583 progesterone congener Substances 0.000 description 1
- 229940095055 progestogen systemic hormonal contraceptives Drugs 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 150000003235 pyrrolidines Chemical class 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 229940087462 relafen Drugs 0.000 description 1
- 229940063627 rescriptor Drugs 0.000 description 1
- 229940064914 retrovir Drugs 0.000 description 1
- 229960000311 ritonavir Drugs 0.000 description 1
- 239000003419 rna directed dna polymerase inhibitor Substances 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- 150000003873 salicylate salts Chemical class 0.000 description 1
- 229940063122 sandimmune Drugs 0.000 description 1
- 229960001852 saquinavir Drugs 0.000 description 1
- 230000036186 satiety Effects 0.000 description 1
- 235000019627 satiety Nutrition 0.000 description 1
- IMNTVVOUWFPRSB-JWQCQUIFSA-N sch-48461 Chemical compound C1=CC(OC)=CC=C1[C@H]1N(C=2C=CC(OC)=CC=2)C(=O)[C@@H]1CCCC1=CC=CC=C1 IMNTVVOUWFPRSB-JWQCQUIFSA-N 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000010898 silica gel chromatography Methods 0.000 description 1
- 229940112726 skelid Drugs 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 235000011006 sodium potassium tartrate Nutrition 0.000 description 1
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- JGMJQSFLQWGYMQ-UHFFFAOYSA-M sodium;2,6-dichloro-n-phenylaniline;acetate Chemical compound [Na+].CC([O-])=O.ClC1=CC=CC(Cl)=C1NC1=CC=CC=C1 JGMJQSFLQWGYMQ-UHFFFAOYSA-M 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 229960001203 stavudine Drugs 0.000 description 1
- 229910052682 stishovite Inorganic materials 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- NCEXYHBECQHGNR-QZQOTICOSA-N sulfasalazine Chemical compound C1=C(O)C(C(=O)O)=CC(\N=N\C=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-QZQOTICOSA-N 0.000 description 1
- 229960001940 sulfasalazine Drugs 0.000 description 1
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- CPDDZSSEAVLMRY-FEQFWAPWSA-N tegaserod maleate Chemical compound [H+].[H+].[O-]C(=O)\C=C/C([O-])=O.C1=C(OC)C=C2C(/C=N/NC(=N)NCCCCC)=CNC2=C1 CPDDZSSEAVLMRY-FEQFWAPWSA-N 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 150000001467 thiazolidinediones Chemical class 0.000 description 1
- 125000004001 thioalkyl group Chemical group 0.000 description 1
- 230000036964 tight binding Effects 0.000 description 1
- 229940019375 tiludronate Drugs 0.000 description 1
- QGUALMNFRILWRA-UHFFFAOYSA-M tolmetin sodium Chemical compound [Na+].C1=CC(C)=CC=C1C(=O)C1=CC=C(CC([O-])=O)N1C QGUALMNFRILWRA-UHFFFAOYSA-M 0.000 description 1
- 229960002044 tolmetin sodium Drugs 0.000 description 1
- 229940019127 toradol Drugs 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- DBGVGMSCBYYSLD-UHFFFAOYSA-N tributylstannane Chemical compound CCCC[SnH](CCCC)CCCC DBGVGMSCBYYSLD-UHFFFAOYSA-N 0.000 description 1
- 229910052905 tridymite Inorganic materials 0.000 description 1
- STMPXDBGVJZCEX-UHFFFAOYSA-N triethylsilyl trifluoromethanesulfonate Chemical compound CC[Si](CC)(CC)OS(=O)(=O)C(F)(F)F STMPXDBGVJZCEX-UHFFFAOYSA-N 0.000 description 1
- 125000004385 trihaloalkyl group Chemical group 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- CSRZQMIRAZTJOY-UHFFFAOYSA-N trimethylsilyl iodide Substances C[Si](C)(C)I CSRZQMIRAZTJOY-UHFFFAOYSA-N 0.000 description 1
- 230000006967 uncompetitive inhibition Effects 0.000 description 1
- 125000002948 undecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000003828 vacuum filtration Methods 0.000 description 1
- 239000004474 valine Chemical group 0.000 description 1
- 208000019553 vascular disease Diseases 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 229940023080 viracept Drugs 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
- 229940087450 zerit Drugs 0.000 description 1
- 229960002555 zidovudine Drugs 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 235000005074 zinc chloride Nutrition 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/10—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/22—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of hydrocarbon radicals substituted by carboxyl groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/08—Drugs for genital or sexual disorders; Contraceptives for gonadal disorders or for enhancing fertility, e.g. inducers of ovulation or of spermatogenesis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/06—Drugs for disorders of the endocrine system of the anterior pituitary hormones, e.g. TSH, ACTH, FSH, LH, PRL, GH
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
- A61P5/50—Drugs for disorders of the endocrine system of the pancreatic hormones for increasing or potentiating the activity of insulin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/52—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring condensed with a ring other than six-membered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/56—Ring systems containing bridged rings
- C07C2603/58—Ring systems containing bridged rings containing three rings
- C07C2603/70—Ring systems containing bridged rings containing three rings containing only six-membered rings
- C07C2603/74—Adamantanes
Definitions
- the present invention relates to cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV (DP-4), and to a method for treating diabetes, especially Type II diabetes, as well as hyperglycemia, Syndrome X, diabetic complications, hyperinsulinemia, obesity, atherosclerosis and related diseases, as well as various immunomodulatory diseases and chronic inflammatory bowel disease, employing such cyclopropyl-fused pyrrolidines alone or in combination with another type antidiabetic agent and/or other type therapeutic agent.
- diabetes especially Type II diabetes, as well as hyperglycemia, Syndrome X, diabetic complications, hyperinsulinemia, obesity, atherosclerosis and related diseases, as well as various immunomodulatory diseases and chronic inflammatory bowel disease
- Depeptidyl peptidase IV is a membrane bound non-classical serine aminodipeptidase which is located in a variety of tissues (intestine, liver, lung, kidney) as well as on circulating T-lymphocytes (where the enzyme is known as CD-26). It is responsible for the metabolic cleavage of certain endogenous peptides (GLP-1(7-36), glucagon) in vivo and has demonstrated proteolytic activity against a variety of other peptides (GHRH, NPY, GLP-2, VIP) in vitro.
- GLP-1(7-36) is a 29 amino-acid peptide derived by post-translational processing of proglucagon in the small intestine.
- GLP-1(7-36) has multiple actions in vivo including the stimulation of insulin secretion, inhibition of glucagon secretion, the promotion of satiety, and the slowing of gastric emptying. Based on its physiological profile, the actions of GLP-1(7-36) are expected to be beneficial in the prevention and treatment of type II diabetes and potentially obesity.
- exogenous administration of GLP-1(7-36) continuously infusion in diabetic patients has demonstrated efficacy in this patient population.
- GLP-1(7-36) is degraded rapidly in vivo and has been shown to have a short half-life in vivo (t1/2 ⁇ 1.5 min). Based on a study of genetically bred DP-4 KO mice and on in vivo/in vitro studies with selective DP-4 inhibitors, DP-4 has been shown to be the primary degrading enzyme of GLP-1(7-36) in vivo. GLP-1(7-36) is degraded by DP-4 efficiently to GLP-1(9-36), which has been speculated to act as a physiological antagonist to GLP-1(7-36). Thus, inhibition of DP-4 in vivo should potentiate endogenous levels of GLP-1(7-36) and attenuate formation of its antagonist GLP-1(9-36) and thus serve to ameliorate the diabetic condition.
- cyclopropyl-fused pyrrolidine-based compounds which inhibit DP-4 and have the structure
- a method for treating diabetes especially Type II diabetes, as well as impaired glucose homeostasis, impaired glucose tolerance, infertility, polycystic ovary syndrome, growth disorders, frailty, arthritis, allograft rejection in transplantation, autoimmune diseases (such as scleroderma and multiple sclerosis), various immunomodulatory diseases (such as lupus erythematosis or psoriasis), AIDS, intestinal diseases (such as necrotizing enteritis, microvillus inclusion disease or celiac disease), inflammatory bowel syndrome, chemotherapy-induced intestinal mucosal atrophy or injury, anorexia nervosa, osteoporosis, Syndrome X, dysmetabolic syndrome, diabetic complications, hyperinsulinemia, obesity, atherosclerosis and related diseases, as well as inflammatory bowel disease (such as Crohn's disease and ulcerative colitis), wherein a therapeutically effective amount of a compound of structure I (which inhibits DP 4)
- a method for treating diabetes and related diseases as defined above and hereinafter as well as any of the other disease states mentioned above, wherein a therapeutically effective amount of a combination of a compound of structure I and one, two, three or more of other types of antidiabetic agent(s) (which may be employed to treat diabetes and related diseases) and/or one, two or three or more other types of therapeutic agent(s) is administered to a human patient in need of treatment.
- diabetes and related diseases refers to Type II diabetes, Type I diabetes, impaired glucose tolerance, obesity, hyperglycemia, Syndrome X, dysmetabolic syndrome, diabetic complications, dysmetabolic syndrome, and hyperinsulinemia.
- diabetes complications include retinopathy, neuropathy and nephropathy, and other known complications of diabetes.
- other type(s) of therapeutic agents refers to one or more antidiabetic agents (other than DP4 inhibitors of formula I), one or more anti-obesity agents, and/or one or more lipid-modulating agents (including anti-atherosclerosis agents), and/or one or more infertility agents, one or more agents for treating polycystic ovary syndrome, one or more agents for treating growth disorders, one or more agents for treating frailty, one or more agents for treating arthritis, one or more agents for preventing allograft rejection in transplantation, one or more agents for treating autoimmune diseases, one or more anti-AIDS agents, one or more anti-osteoporosis agents, one or more agents for treating immunomodulatory diseases, one or more agents for treating chronic inflammatory bowel disease or syndrome and/or one or more agents for treating anorexia nervosa.
- lipid-modulating agent refers to agents which lower LDL and/or raise HDL and/or lower triglycerides and/or lower total cholesterol and/or other known mechanisms for therapeutically treating lipid disorders.
- the compound of structure I will be employed in a weight ratio to the antidiabetic agent or other type therapeutic agent (depending upon its mode of operation) within the range from about 0.01:1 to about 500:1, preferably from about 0.1:1 to about 100:1, more preferably from about 0.2:1 to about 10:1.
- R 3 is H or alkyl
- R 1 is H, alkyl, cycloalkyl, bicycloalkyl, tricycloalkyl, alkylcyclo alkyl, hydroxyalkyl, hydroxytricyclo alkyl, hydroxycycloalkyl, hydroxybicycloalkyl, or hydroxyalkylcycloalkyl
- R 2 is H or alkyl
- n is 0,
- X is CN
- x is 0 or 1
- y is 0 or 1.
- R 1 is alkyl, cycloalkyl, bicycloalkyl, tricycloalkyl, alkylcycloalkyl, hydroxyalkyl, hydroxycycloalkyl, hydroxyalkylcycloalkyl, hydroxybicycloalkyl or hydroxytricycloalkyl;
- R 1 is alkyl, cycloalkyl, bicycloalkyl, tricycloalkyl, hydroxybicycloalkyl, hydroxytricycloalkyl, alkylcycloalkyl, hydroxyalkyl, hydroxycycloalkyl or hydroxyalkylcycloalkyl as well as the following:
- compound 1 where PG 1 is a common amine protecting group such as Boc, Cbz, or FMOC and X 1 is H or CO 2 R 9 as set out below, may be generated by methods as described herein or in the literature (for example see Sagnard et al, Tet-Lett., 1995, 36, pp. 3148-3152, Tverezovsky et al, Tetrahedron, 1997, 53, pp. 14773-14792, Hanessian et al, Bioorg. Med. Chem. Lett., 1998, 8, p. 2123-2128). Removal of the PG 1 group by conventional methods (e.g.
- the ester may be hydrolyzed under a variety of conditions, for example with aqueous NaOH in a suitable solvent such as methanol, THF, or dioxane, to provide the acid 5.
- aqueous NaOH in a suitable solvent such as methanol, THF, or dioxane
- Conversion of the acid group to the primary carboxamide, affording 6, may be effected by activation of the acid group (e.g. employing i-BuOCOCl/TEA or EDAC) followed by treatment with NH 3 or an ammonia equivalent in a solvent such as dioxane, ether, or methanol.
- the amide functionality may be converted to the nitrile group by a variety of standard conditions (e.g. POCl 3 /pyridine/imidazole or cyanuric chloride/DMF or trifluoroacetic anhydride, THF, pyridine) to give 7.
- POCl 3 /pyridine/imidazole or cyanuric chloride/DMF or trifluoroacetic anhydride, THF, pyridine trifluoroacetic anhydride
- compound 1 where X 1 is CO 2 R 9 may be saponified to the acid and subsequently amidated as described above to give amide 8. Removal of the PG 1 group followed by peptide coupling to 3 affords compound 6, an intermediate in the synthesis of Ib.
- the carboxamide group in 8 may be converted to the nitrile as described above to give compound 9.
- Deprotection of PG 1 affords 10 which may be subject to standard peptide coupling conditions to afford 7, an intermediate in the synthesis of Ib.
- Compound 10 may also be generated by oxidation of the amine 2 (e.g. NCS) followed by hydrolysis and subsequent cyanide treatment.
- Compound 10 may be obtained as a mixture of stereoisomers or a single isomer/diastereomer which may be epimerized (employing conventional procedures) to afford a mixture of stereoisomers.
- ⁇ -amino acids such as
- lower alkyl as employed herein alone or as part of another group includes both straight and branched chain hydrocarbons, containing 1 to 20 carbons, preferably 1 to 10 carbons, more preferably 1 to 8 carbons, in the normal chain, such as methyl, ethyl, propyl, isopropyl, butyl, t-butyl, isobutyl, pentyl, hexyl, isohexyl, heptyl, 4,4-dimethylpentyl, octyl, 2,2,4-trimethyl-pentyl, nonyl, decyl, undecyl, dodecyl, the various branched chain isomers thereof, and the like as well as such groups including 1 to 4 substituents such as halo, for example F, Br, Cl or I or CF 3 , alkyl, alkoxy, aryl, aryloxy, ary
- cycloalkyl as employed herein alone or as part of another group includes saturated or partially unsaturated (containing 1 or 2 double bonds) cyclic hydrocarbon groups containing 1 to 3 rings, including monocyclic alkyl, bicyclic alkyl (or bicycloalkyl) and tricyclic alkyl (tricycloalkyl), containing a total of 3 to 20 carbons forming the ring, preferably 3 to 10 carbons, forming the ring and which may be fused to 1 or 2 aromatic rings as described for aryl, which includes cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclodecyl and cyclododecyl, cyclohexenyl, adamantyl,
- any of which groups may be optionally subsituted with 1 to 4 substituents such as halogen, alkyl, alkoxy, hydroxy, aryl, aryloxy, arylalkyl, cycloalkyl, hydroxyalkyl, alkylamido, alkanoylamino, oxo, acyl, arylcarbonylamino, amino, nitro, cyano, thiol and/or alkylthio and/or any of the substituents for alkyl.
- substituents such as halogen, alkyl, alkoxy, hydroxy, aryl, aryloxy, arylalkyl, cycloalkyl, hydroxyalkyl, alkylamido, alkanoylamino, oxo, acyl, arylcarbonylamino, amino, nitro, cyano, thiol and/or alkylthio and/or any of the substituents for alkyl.
- cycloalkenyl as employed herein alone or as part of another group refers to cyclic hydrocarbons containing 3 to 12 carbons, preferably 5 to 10 carbons and 1 or 2 double bonds.
- exemplary cycloalkenyl groups include cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl, cyclohexadienyl, and cycloheptadienyl, which may be optionally substituted as defined for cycloalkyl.
- cycloalkylene refers to a “cycloalkyl” group which includes free bonds and thus is a linking group such as
- alkanoyl as used herein alone or as part of another group refers to alkyl linked to a carbonyl group.
- lower alkenyl or “alkenyl” as used herein by itself or as part of another group refers to straight or branched chain radicals of 2 to 20 carbons, preferably 2 to 12 carbons, and more preferably 1 to 8 carbons in the normal chain, which include one to six double bonds in the normal chain, such as vinyl, 2-propenyl, 3-butenyl, 2-butenyl, 4-pentenyl, 3-pentenyl, 2-hexenyl, 3-hexenyl, 2-heptenyl, 3-heptenyl, 4-heptenyl, 3-octenyl, 3-nonenyl, 4-decenyl, 3-undecenyl, 4-dodecenyl, 4,8,12-tetradecatrienyl, and the like, and which may be optionally substituted with 1 to 4 substituents, namely, halogen, haloalkyl, alkyl, alkoxy, alkenyl, alkyn
- lower alkynyl or “alkynyl” as used herein by itself or as part of another group refers to straight or branched chain radicals of 2 to 20 carbons, preferably 2 to 12 carbons and more preferably 2 to 8 carbons in the normal chain, which include one triple bond in the normal chain, such as 2-propynyl, 3-butynyl, 2-butynyl, 4-pentynyl, 3-pentynyl, 2-hexynyl, 3-hexynyl, 2-heptynyl, 3-heptynyl, 4-heptynyl, 3-octenyl, 3-nonenyl, 4-decenyl,3-undecenyl, 4-dodecenyl and the like, and which may be optionally substituted with 1 to 4 substituents, namely, halogen, haloalkyl, alkyl, alkoxy, alkenyl, alkynyl,
- arylalkenyl and arylalkynyl as used alone or as part of another group refer to alkenyl and alkynyl groups as described above having an aryl substituent.
- alkyl groups as defined above have single bonds for attachment to other groups at two different carbon atoms, they are termed “alkylene” groups and may optionally be substituted as defined above for “alkyl”.
- alkenyl groups as defined above and alkynyl groups as defined above, respectively, have single bonds for attachment at two different carbon atoms, they are termed “alkenylene groups” and “alkynylene groups”, respectively, and may optionally be substituted as defined above for “alkenyl” and “alkynyl”.
- halogen or “halo” as used herein alone or as part of another group refers to chlorine, bromine, fluorine, and iodine as well as CF 3 , with chlorine or fluorine being preferred.
- metal ion refers to alkali metal ions such as sodium, potassium or lithium and alkaline earth metal ions such as magnesium and calcium, as well as zinc and aluminum.
- aryl refers to monocyclic and bicyclic aromatic groups containing 6 to 10 carbons in the ring portion (such as phenyl or naphthyl including 1-naphthyl and 2-naphthyl) and may optionally include one to three additional rings fused to a carbocyclic ring or a heterocyclic ring (such as aryl, cycloalkyl, heteroaryl or cycloheteroalkyl rings for example
- lower alkoxy as employed herein alone or as part of another group includes any of the above alkyl, aralkyl or aryl groups linked to an oxygen atom.
- substituted amino refers to amino substituted with one or two substituents, which may be the same or different, such as alkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, cycloheteroalkyl, cycloheteroalkylalkyl, cycloalkyl, cycloalkylalkyl haloalkyl, hydroxyalkyl, alkoxyalkyl or thioalkyl. These substituents may be further substituted with any of the R 1 groups or substituents for R 1 as set out above.
- amino substituents may be taken together with the nitrogen atom to which they are attached to form 1-pyrrolidinyl, 1-piperidinyl, 1-azepinyl, 4-morpholinyl, 4-thiamorpholinyl, 1-piperazinyl, 4-alkyl-1-piperazinyl, 4-arylalkyl-1-piperazinyl, 4-diarylalkyl-1-piperazinyl, 1-pyrrolidinyl, 1-piperidinyl, or 1-azepinyl, optionally substituted with alkyl, alkoxy, alkylthio, halo, trifluoromethyl or hydroxy.
- lower alkylthio as employed herein alone or as part of another group includes any of the above alkyl, aralkyl or aryl groups linked to a sulfur atom.
- lower alkylamino as employed herein alone or as part of another group includes any of the above alkyl, aryl or arylalkyl groups linked to a nitrogen atom.
- acyl as employed herein by itself or part of another group, as defined herein, refers to an organic radical linked to a carbonyl
- acyl groups include any of the R 1 groups attached to a carbonyl, such as alkanoyl, alkenoyl, aroyl, aralkanoyl, heteroaroyl, cycloalkanoyl, cycloheteroalkanoyl and the like.
- cycloheteroalkyl refers to a 5-, 6- or 7-membered saturated or partially unsaturated ring which includes 1 to 2 hetero atoms such as nitrogen, oxygen and/or sulfur, linked through a carbon atom or a heteroatom, where possible, optionally via the linker (CH 2 ) r (where r is 1, 2 or 3), such as:
- any of the cycloheteroalkyl rings can be fused to a cycloalkyl, aryl, heteroaryl or cycloheteroalkyl ring.
- heteroaryl refers to a 5- or 6-membered aromatic ring which includes 1, 2, 3 or 4 hetero atoms such as nitrogen, oxygen or sulfur, and such rings fused to an aryl, cycloalkyl, heteroaryl or cycloheteroalkyl ring (e.g. benzothiophenyl, indolyl), and includes possible N-oxides.
- the heteroaryl group may optionally include 1 to 4 substituents such as any of the substituents set out above for alkyl. Examples of heteroaryl groups include the following:
- cycloheteroalkylalkyl as used herein alone or as part of another group refers to cycloheteroalkyl groups as defined above linked through a C atom or heteroatom to a (CH 2 ) r chain.
- heteroarylalkyl or “heteroarylalkenyl” as used herein alone or as part of another group refers to a heteroaryl group as defined above linked through a C atom or heteroatom to a —(CH 2 ) r — chain, alkylene or alkenylene as defined above.
- polyhaloalkyl refers to an “alkyl” group as defined above which includes from 2 to 9, preferably from 2 to 5, halo substituents, such as F or Cl, preferably F, such as CF 3 CH 2 , CF 3 or CF 3 CF 2 CH 2 .
- polyhaloalkoxy refers to an “alkoxy” or “alkyloxy” group as defined above which includes from 2 to 9, preferably from 2 to 5, halo substituents, such as F or Cl, preferably F, such as CF 3 CH 2 O, CF 3 O or CF 3 CF 2 CH 2 O.
- All stereoisomers of the compounds of the instant invention are contemplated, either in admixture or in pure or substantially pure form.
- the compounds of the present invention can have asymmetric centers at any of the carbon atoms including any one or the R substituents. Consequently, compounds of formula I can exist in enantiomeric or diastereomeric forms or in mixtures thereof.
- the processes for preparation can utilize racemates, enantiomers or diastereomers as starting materials. When diastereomeric or enantiomeric products are prepared, they can be separated by conventional methods for example, chromatographic or fractional crystallization.
- the compounds of structure I may be used in combination with one or more other types of antidiabetic agents (employed to treat diabetes and related diseases) and/or one or more other types of therapeutic agents which may be administered orally in the same dosage form, in a separate oral dosage form or by injection.
- the other type of antidiabetic agent which may be optionally employed in combination with the DP4 inhibitor of formula I may be 1,2,3 or more antidiabetic agents or antihyperglycemic agents including insulin secretagogues or insulin sensitizers, or other antidiabetic agents preferably having a mechanism of action different from DP4 inhibition and may include biguanides, sulfonyl ureas, glucosidase inhibitors, PPAR ⁇ agonists, such as thiazolidinediones, SGLT2 inhibitors, PPAR ⁇ / ⁇ dual agonists, aP2 inhibitors, glycogen phosphorylase inhibitors, advanced glycosylation end (AGE) products inhibitors, and/or meglitinides, as well as insulin, and/or glucagon-like peptide-1 (GLP-1) or mimetics thereof.
- biguanides such as thiazolidinediones, SGLT2 inhibitors, PPAR ⁇ / ⁇ dual
- the other antidiabetic agent may be an oral antihyperglycemic agent preferably a biguanide such as metformin or phenformin or salts thereof, preferably metformin HCl.
- the compounds of structure I will be employed in a weight ratio to biguanide within the range from about 0.01:1 to about 100:1, preferably from about 0.1:1 to about 5:1.
- the other antidiabetic agent may also preferably be a sulfonyl urea such as glyburide (also known as glibenclamide), glimepiride (disclosed in U.S. Pat. No. 4,379,785), glipizide, gliclazide or chlorpropamide, other known sulfonylureas or other antihyperglycemic agents which act on the ATP-dependent channel of the ⁇ -cells, with glyburide and glipizide being preferred, which may be administered in the same or in separate oral dosage forms.
- glyburide also known as glibenclamide
- glimepiride also known as glimepiride
- glipizide also known as gliclazide
- chlorpropamide other known sulfonylureas or other antihyperglycemic agents which act on the ATP-dependent channel of the ⁇ -cells
- glyburide and glipizide
- the compounds of structure I will be employed in a weight ratio to the sulfonyl urea in the range from about 0.01:1 to about 100:1, preferably from about 0.05:1 to about 5:1.
- the oral antidiabetic agent may also be a glucosidase inhibitor such as acarbose (disclosed in U.S. Pat. No. 4,904,769) or miglitol (disclosed in U.S. Pat. No. 4,639,436), which may be administered in the same or in a separate oral dosage forms.
- acarbose disclosed in U.S. Pat. No. 4,904,769
- miglitol disclosed in U.S. Pat. No. 4,639,436
- the compounds of structure I will be employed in a weight ratio to the glucosidase inhibitor within the range from about 0.01:1 to about 100:1, preferably from about 0.2:1 to about 50:1.
- the compounds of structure I may be employed in combination with a PPAR ⁇ agonist such as a thiazolidinedione oral anti-diabetic agent or other insulin sensitizers (which has an insulin sensitivity effect in NIDDM patients) such as troglitazone (Warner-Lambert's Rezulin®, disclosed in U.S. Pat. No. 4,572,912), rosiglitazone (en), pioglitazone (Takeda), Mitsubishi MCC-555 (disclosed in U.S. Pat. No.
- a PPAR ⁇ agonist such as a thiazolidinedione oral anti-diabetic agent or other insulin sensitizers (which has an insulin sensitivity effect in NIDDM patients) such as troglitazone (Warner-Lambert's Rezulin®, disclosed in U.S. Pat. No. 4,572,912), rosiglitazone (en), pioglitazone (Takeda), Mitsubishi MCC
- Glaxo-Wellcome's GL-262570 englitazone (CP-68722, Pfizer) or darglitazone (CP-86325, Pfizer, isaglitazone (MIT/J&J), JTT-501 (JPNT/P&U), L-895645 (Merck), R-119702 (Sankyo/WL), NN-2344 (Dr. Reddy/NN), or YM-440 (Yamanouchi), preferably rosiglitazone and pioglitazone.
- the compounds of structure I will be employed in a weight ratio to the thiazolidinedione in an amount within the range from about 0.01:1 to about 100:1, preferably from about 0.1:1 to about 10:1.
- the sulfonyl urea and thiazolidinedione in amounts of less than about 150 mg oral antidiabetic agent may be incorporated in a single tablet with the compounds of structure I.
- the compounds of structure I may also be employed in combination with a antihyperglycemic agent such as insulin or with glucagon-like peptide-1 (GLP-1) such as GLP-1(1-36) amide, GLP-1(7-36) amide, GLP-1(7-36) (as disclosed in U.S. Pat. No. 5,614,492 to Habener, disclosure of which is incorporated herein by reference), or a GLP-1 mimic such as AC2993 or Exendin-4 (Amylin) and LY-315902 or LY-307167 (Lilly) and NN2211 (Novo-Nordisk), which may be administered via injection, intranasal, or by transdermal or buccal devices.
- GLP-1 glucagon-like peptide-1
- GLP-1 mimic such as AC2993 or Exendin-4 (Amylin) and LY-315902 or LY-307167 (Lilly) and NN2211 (Novo-Nordisk
- metformin the sulfonyl ureas, such as glyburide, glimepiride, glipyride, glipizide, chlorpropamide and gliclazide and the glucosidase inhibitors acarbose or miglitol or insulin (injectable, pulmonary, buccal, or oral) may be employed in formulations as described above and in amounts and dosing as indicated in the Physician's Desk Reference (PDR).
- PDR Physician's Desk Reference
- metformin or salt thereof may be employed in amounts within the range from about 500 to about 2000 mg per day which may be administered in single or divided doses one to four times daily.
- the thiazolidinedione anti-diabetic agent may be employed in amounts within the range from about 0.01 to about 2000 mg/day which may be administered in single or divided doses one to four times per day.
- insulin may be employed in formulations, amounts and dosing as indicated by the Physician's Desk Reference.
- GLP-1 peptides may be administered in oral buccal formulations, by nasal administration (for example inhalation spray) or parenterally as described in U.S. Pat. Nos. 5,346,701 (TheraTech), 5,614,492 and 5,631,224 which are incorporated herein by reference.
- the other antidiabetic agent may also be a PPAR ⁇ / ⁇ dual agonist such as AR-HO39242 (Astra/Zeneca), GW-409544 (Glaxo-Wellcome), KRP297 (Kyorin Merck) as well as those disclosed by Murakami et al, “A Novel Insulin Sensitizer Acts As a Coligand for Peroxisome Proliferation—Activated Receptor Alpha (PPAR alpha) and PPAR gamma. Effect on PPAR alpha Activation on Abnormal Lipid Metabolism in Liver of Zucker Fatty Rats”, Diabetes 47, 1841-1847 (1998), and in U.S. application Ser. No. 09/664,598, filed Sep. 18, 2000, (attorney file LA29NP) the disclosure of which is incorporated herein by reference, employing dosages as set out therein, which compounds designated as preferred are preferred for use herein.
- AR-HO39242 Astra/Zeneca
- GW-409544
- the other antidiabetic agent may be an SGLT2 inhibitor such as disclosed in U.S. application Ser. No. 09/679,027, filed Oct. 4, 2000 (attorney file LA49NP), which is incorporated herein by reference, employing dosages as set out herein. Preferred are the compounds designated as preferred in the above application.
- the other antidiabetic agent which may be optionally employed in combination with the DP4 inhibitor of formula I may be an aP2 inhibitor such as disclosed in U.S. application Ser. No. 09/391,053, filed Sep. 7, 1999, and U.S. application Ser. No. 09/519,079, filed Mar. 6, 2000 (attorney file LA27NP), which is incorporated herein by reference, employing dosages as set out herein.
- the other antidiabetic agent which may be optionally employed in combination with the DP4 inhibitor of formula I may be a glycogen phosphorylase inhibitor such as disclosed in WO 96/39384, WO 96/39385, EP 978279, WO 2000/47206, WO 99/43663, and U.S. Pat. Nos. 5,952,322 and 5,998,463, WO 99/26659 and EP 1041068.
- the meglitinide which may optionally be employed in combination with the compound of formula I of the invention may be repaglinide, nateglinide (Novartis) or KAD1229 (PF/Kissei), with repaglinide being preferred.
- the DP4 inhibitor of formula I will be employed in a weight ratio to the meglitinide, PPAR ⁇ agonist, PPAR ⁇ / ⁇ dual agonist, SGLT2 inhibitor, aP2 inhibitor, or glycogen phosphorylase inhibitor within the range from about 0.01:1 to about 100:1, preferably from about 0.1:1 to about 10:1.
- the hypolipidemic agent or lipid-modulating agent which may be optionally employed in combination with the compounds of formula I of the invention may include 1,2,3 or more MTP inhibitors, HMG CoA reductase inhibitors, squalene synthetase inhibitors, fibric acid derivatives, ACAT inhibitors, lipoxygenase inhibitors, cholesterol absorption inhibitors, ileal Na + /bile acid cotransporter inhibitors, upregulators of LDL receptor activity, ATP citrate lyase inhibitors, cholesteryl ester transfer protein inhibitors, bile acid sequestrants, and/or nicotinic acid and derivatives thereof.
- MTP inhibitors employed herein include MTP inhibitors disclosed in U.S. Pat. No. 5,595,872, U.S. Pat. No. 5,739,135, U.S. Pat. No. 5,712,279, U.S. Pat. No. 5,760,246, U.S. Pat. No. 5,827,875, U.S. Pat. No. 5,885,983 and U.S. application Ser. No. 09/175,180 filed Oct. 20, 1998, now U.S. Pat. No. 5,962,440. Preferred are each of the preferred MTP inhibitors disclosed in each of the above patents and applications.
- MTP inhibitors to be employed in accordance with the present invention include preferred MTP inhibitors as set out in U.S. Pat. Nos. 5,739,135 and 5,712,279, and U.S. Pat. No. 5,760,246 as well as implitapide (Bayer).
- MTP inhibitor 9-[4-[4-[[2-(2,2,2-Trifluoroethoxy)benzoyl]amino]-1-piperidinyl] butyl]-N-(2,2,2-trifluoroethyl)-9H-fluorene-9-carboxamide
- the hypolipidemic agent may be an HMG CoA reductase inhibitor which includes, but is not limited to, mevastatin and related compounds as disclosed in U.S. Pat. No. 3,983,140, lovastatin (mevinolin) and related compounds as disclosed in U.S. Pat. No. 4,231,938, pravastatin and related compounds such as disclosed in U.S. Pat. No. 4,346,227, simvastatin and related compounds as disclosed in U.S. Pat. Nos. 4,448,784 and 4,450,171.
- HMG CoA reductase inhibitors which may be employed herein include, but are not limited to, fluvastatin, disclosed in U.S. Pat. No.
- the squalene synthetase inhibitors suitable for use herein include, but are not limited to, a-phosphono-sulfonates disclosed in U.S. Pat. No. 5,712,396, those disclosed by Biller et al, J. Med. Chem., 1988, Vol. 11, No. 10, pp 1869-1871, including isoprenoid (phosphinyl-methyl) phosphonates as well as other known squalene synthetase inhibitors, for example, as disclosed in U.S. Pat. Nos. 4,871,721 and 4,924,024 and in Biller, S. A., Neuenschwander, K., Ponpipom, M. M., and Poulter, C. D., Current Pharmaceutical Design, 2, 1-40 (1996).
- squalene synthetase inhibitors suitable for use herein include the terpenoid pyrophosphates disclosed by P. Ortiz de Montellano et al, J. Med. Chem., 1977, 20, 243-249, the farnesyl diphosphate analog A and presqualene pyrophosphate (PSQ-PP) analogs as disclosed by Corey and Volante, J. Am. Chem. Soc., 1976, 98, 1291-1293, phosphinylphosphonates reported by McClard, R. W. et al, J.A.C.S., 1987, 10, 5544 and cyclopropanes reported by Capson, T. L., PhD dissertation, June, 1987, Dept. Med. Chem. U of Utah, Abstracts Table of Contents, pp 16, 17, 40-43, 48-51, Summary.
- hypolipidemic agents suitable for use herein include, but are not limited to, fibric acid derivatives, such as fenofibrate, gemfibrozil, clofibrate, bezafibrate, ciprofibrate, clinofibrate and the like, probucol, and related compounds as disclosed in U.S. Pat. No.
- bile acid sequestrants such as cholestyramine, colestipol and DEAE-Sephadex (Secholex®, policexide®), as well as lipostabil (Rhone-Poulenc), Eisai E-5050 (an N-substituted ethanolamine derivative), imanixil (HOE-402), tetrahydrolipstatin (THL), istigmastanylphos-phorylcholine (SPC, Roche), aminocyclodextrin (Tanabe Seiyoku), Ajinomoto AJ-814 (azulene derivative), melinamide (Sumitomo), Sandoz 58-035, American Cyanamid CL-277,082 and CL-283,546 (disubstituted urea derivatives), nicotinic acid, acipimox, acifran, neomycin, p-aminosalicylic acid, aspirin
- the other hypolipidemic agent may be an ACAT inhibitor such as disclosed in, Drugs of the Future 24, 9-15 (1999), (Avasimibe); “The ACAT inhibitor, Cl-1011 is effective in the prevention and regression of aortic fatty streak area in hamsters”, Nicolosi et al, Atherosclerosis (Shannon, Irel). (1998), 137(1), 77-85; “The pharmacological profile of FCE 27677: a novel ACAT inhibitor with potent hypolipidemic activity mediated by selective suppression of the hepatic secretion of ApoB100-containing lipoprotein”, Ghiselli, Giancarlo, Cardiovasc. Drug Rev.
- the hypolipidemic agent may be an upregulator of LD2 receptor activity such as MD-700 (Taisho Pharmaceutical Co. Ltd) and LY295427 (Eli Lilly).
- the hypolipidemic agent may be a cholesterol absorption inhibitor preferably Schering-Plough's SCH48461 as well as those disclosed in Atherosclerosis 115, 45-63 (1995) and J. Med. Chem. 41, 973 (1998).
- the hypolipidemic agent may be an ileal Na + /bile acid cotransporter inhibitor such as disclosed in Drugs of the Future, 24, 425-430 (1999).
- the lipid-modulating agent may be a cholesteryl ester transfer protein (CETP) inhibitor such as Pfizer's CP 529, 414 (WO/0038722 and EP 818448) and Pharmacia's SC-744 and SC-795.
- CETP cholesteryl ester transfer protein
- the ATP citrate lyase inhibitor which may be employed in the combination of the invention may include, for example, those disclosed in U.S. Pat. No. 5,447,954.
- Preferred hypolipidemic agents are pravastatin, lovastatin, simvastatin, atorvastatin, fluvastatin, cerivastatin, atavastatin and ZD-4522.
- the compounds of formula I of the invention will be employed in a weight ratio to the hypolipidemic agent (were present), within the range from about 500:1 to about 1:500, preferably from about 100:1 to about 1:100.
- the dose administered must be carefully adjusted according to age, weight and condition of the patient, as well as the route of administration, dosage form and regimen and the desired result.
- the dosages and formulations for the hypolipidemic agent will be as disclosed in the various patents and applications discussed above.
- the MTP inhibitor for oral administration, a satisfactory result may be obtained employing the MTP inhibitor in an amount within the range of from about 0.01 mg/kg to about 500 mg and preferably from about 0.1 mg to about 100 mg, one to four times daily.
- a preferred oral dosage form such as tablets or capsules, will contain the MTP inhibitor in an amount of from about 1 to about 500 mg, preferably from about 2 to about 400 mg, and more preferably from about 5 to about 250 mg, one to four times daily.
- an HMG CoA reductase inhibitor for example, pravastatin, lovastatin, simvastatin, atorvastatin, fluvastatin or cerivastatin in dosages employed as indicated in the Physician's Desk Reference, such as in an amount within the range of from about 1 to 2000 mg, and preferably from about 4 to about 200 mg.
- the squalene synthetase inhibitor may be employed in dosages in an amount within the range of from about 10 mg to about 2000 mg and preferably from about 25 mg to about 200 mg.
- a preferred oral dosage form such as tablets or capsules, will contain the HMG CoA reductase inhibitor in an amount from about 0.1 to about 100 mg, preferably from about 5 to about 80 mg, and more preferably from about 10 to about 40 mg.
- a preferred oral dosage form such as tablets or capsules will contain the squalene synthetase inhibitor in an amount of from about 10 to about 500 mg, preferably from about 25 to about 200 mg.
- the other hypolipidemic agent may also be a lipoxygevase inhibitor including a 15-lipoxygenase (15-LO) inhibitor such as benzimidazole derivatives as disclosed in WO 97/12615, 15-LO inhibitors as disclosed in WO 97/12613, isothiazolones as disclosed in WO 96/38144, and 15-LO inhibitors as disclosed by Sendobry et al “Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties”, Brit. J. Pharmacology (1997) 120, 1199-1206, and Cornicelli et al, “15-Lipoxygenase and its Inhibition: A Novel Therapeutic Target for Vascular Disease”, Current Pharmaceutical Design, 1999, 5, 11-20.
- 15-LO 15-lipoxygenase
- the compounds of formula I and the hypolipidemic agent may be employed together in the same oral dosage form or in separate oral dosage forms taken at the same time.
- compositions described above may be administered in the dosage forms as described above in single or divided doses of one to four times daily. It may be advisable to start a patient on a low dose combination and work up gradually to a high dose combination.
- the preferred hypolipidemic agent is pravastatin, simvastatin, lovastatin, atorvastatin, fluvastatin or cerivastatin.
- the other type of therapeutic agent which may be optionally employed with the DP4 inhibitor of formula I may be 1, 2, 3 or more of an anti-obesity agent including a beta 3 adrenergic agonist, a lipase inhibitor, a serotonin (and dopamine) reuptake inhibitor, a thyroid receptor beta drug, an anorectic agent and/or a fatty acid oxidation upregulator.
- an anti-obesity agent including a beta 3 adrenergic agonist, a lipase inhibitor, a serotonin (and dopamine) reuptake inhibitor, a thyroid receptor beta drug, an anorectic agent and/or a fatty acid oxidation upregulator.
- the beta 3 adrenergic agonist which may be optionally employed in combination with a compound of formula I may be AJ9677 (Takeda/Dainippon), L750355 (Merck), or CP331648 (Pfizer) or other known beta 3 agonists as disclosed in U.S. Pat. Nos. 5,541,204, 5,770,615, 5,491,134, 5,776,983 and 5,488,064, with AJ9677, L750,355 and CP331648 being preferred.
- the lipase inhibitor which may be optionally employed in combination with a compound of formula I may be orlistat or ATL-962 (Alizyme), with orlistat being preferred.
- the serotonin (and dopoamine) reuptake inhibitor which may be optionally employed in combination with a compound of formula I may be sibutramine, topiramate (Johnson & Johnson) or axokine (Regeneron), with sibutramine and topiramate being preferred.
- the thyroid receptor beta compound which may be optionally employed in combination with a compound of formula I may be a thyroid receptor ligand as disclosed in WO97/21993 (U. Cal SF), WO099/00353 (KaroBio) and GB98/284425 (KaroBio), with compounds of the KaroBio applications being preferred.
- the anorectic agent which may be optionally employed in combination with a compound of formula I may be dexamphetamine, phentermine, phenylpropanolamine or mazindol, with dexamphetamine being preferred.
- the fatty acid oxidation upregulator which may be optionally employed in combination with the compound of formula I can be famoxin (Genset).
- anti-obesity agents described above may be employed in the same dosage form with the compound of formula I or in different dosage forms, in dosages and regimens as generally known in the art or in the PDR.
- the infertility agent which may be optionally employed in combination with the DP4 inhibitor of the invention may be 1, 2, or more of clomiphene citrate (Clomid®, Aventis), bromocriptine mesylate (Parlodel®, Novartis),LHRH analogs, Lupron (TAP Pharm.), danazol, Danocrine (Sanofi), progestogens or glucocorticoids, which may be employed in amounts specified in the PDR.
- the agent for polycystic ovary syndrome which may be optionally employed in combination with the DP4 inhibitor of the invention may be 1, 2, or more of gonadotropin releasing hormone (GnRH), leuprolide (Lupron®), Clomid®, Parlodel®, oral contraceptives or insulin sensitizers such as PPAR agonists, or other conventional agents for such use which may be employed in amounts specified in the PDR.
- GnRH gonadotropin releasing hormone
- Lupron® leuprolide
- Clomid® Parlodel®
- oral contraceptives or insulin sensitizers such as PPAR agonists, or other conventional agents for such use which may be employed in amounts specified in the PDR.
- the agent for treating growth disorders and/or frailty which may be optionally employed in combination with the DP4 inhibitor of the invention may be 1, 2, or more of a growth hormone or growth hormone secretagogue such as MK-677 (Merck), CP-424,391 (Pfizer), and compounds disclosed in U.S. Ser. No. 09/506,749 filed Feb. 18, 2000 (attorney docket LA26), as well as selective androgen receptor modulators (SARMs), which is incorporated herein by reference, which may be employed in amounts specified in the PDR, where applicable.
- a growth hormone or growth hormone secretagogue such as MK-677 (Merck), CP-424,391 (Pfizer), and compounds disclosed in U.S. Ser. No. 09/506,749 filed Feb. 18, 2000 (attorney docket LA26), as well as selective androgen receptor modulators (SARMs), which is incorporated herein by reference, which may be employed in amounts specified in the PDR, where applicable.
- the agent for treating arthritis which may be optionally employed in combination with the DP4 inhibitor of the invention may be 1, 2, or more of aspirin, indomethacin, ibuprofen, diclofenac sodium, naproxen, nabumetone (Relafen®, SmithKline Beecham), tolmetin sodium (Tolectin®, Ortho-McNeil), piroxicam (Feldene®, Pfizer), ketorolac tromethamine (Toradol®, Roche), celecoxib (Celebrex®, Searle), rofecoxib (Vioxx®, Merck) and the like, which may be employed in amounts specified in the PDR.
- DP4 inhibitor of the invention Conventional agents for preventing allograft rejection in transplantation such as cyclosporin, Sandimmune (Novartis), azathioprine, Immuran (Faro) or methotrexate may be optionally employed in combination with the DP4 inhibitor of the invention, which may be employed in amounts specified in the PDR.
- autoimmune diseases such as multiple sclerosis and immunomodulatory diseases such as lupus erythematosis, psoriasis, for example, azathioprine, Immuran, cyclophosphamide, NSAIDS such as ibuprofen, cox 2 inhibitors such as Vioxx and Celebrex, glucocorticoids and hydroxychloroquine, may be optionally employed in combination with the DP4 inhibitor of the invention, which may be employed in amounts specified in the PDR.
- the AIDS agent which may be optionally employed in combination with the DP4 inhibitor of the invention may be a non-nucleoside reverse transcriptase inhibitor, a nucleoside reverse transcriptase inhibitor, a protease inhibitor and/or an AIDS adjunct anti-infective and may be 1, 2, or more of dronabinol (Marinol®, Roxane Labs), didanosine (Videx®, Bristol-Myers Squibb), megestrol acetate (Megace®, Bristol-Myers Squibb), stavudine (Zerit®, Bristol-Myers Squibb), delavirdine mesylate (Rescriptor®, Pharmacia), lamivudine/zidovudine (CombivirTM, Glaxo), lamivudine (EpivirTM, Glaxo), zalcitabine (Hivid®, Roche), zidovudine (Retrovir®, Glaxo), indinavir sulfate
- the above anti-AIDS agents may be employed in amounts specified in the PDR.
- the agent for treating inflammatory bowel disease or syndrome which may be optionally employed in combination with the DP4 inhibitor of the invention may be 1, 2, or more of sulfasalazine, salicylates, mesalamine (Asacol®, P&G) or Zelmac®, (Bristol-Myers Squibb), which may be employed in amounts specified in the PDR or otherwise known in the art.
- the agent for treating osteoporosis which may be optionally employed in combination with the DP4 inhibitor of the invention may be 1, 2, or more of alendronate sodium (Fosamax®, Merck, tiludronate (Skelid®, Sanofi), etidronate disodium (Didronel®, P&G), raloxifene HCl (Evista®, Lilly), which may be employed in amounts specified in the PDR.
- a pharmaceutical composition will be employed containing the compounds of structure I, with or without another antidiabetic agent and/or other type therapeutic agent, in association with a pharmaceutical vehicle or diluent.
- the pharmaceutical composition can be formulated employing conventional solid or liquid vehicles or diluents and pharmaceutical additives of a type appropriate to the mode of desired administration.
- the compounds can be administered to mammalian species including humans, monkeys, dogs, etc. by an oral route, for example, in the form of tablets, capsules, granules or powders, or they can be administered by a parenteral route in the form of injectable preparations.
- the dose for adults is preferably between 10 and 1,000 mg per day, which can be administered in a single dose or in the form of individual doses from 1-4 times per day.
- a typical capsule for oral administration contains compounds of structure I (250 mg), lactose (75 mg) and magnesium stearate (15 mg). The mixture is passed through a 60 mesh sieve and packed into a No. 1 gelatin capsule.
- a typical injectable preparation is produced by aseptically placing 250 mg of compounds of structure I into a vial, aseptically freeze-drying and sealing. For use, the contents of the vial are mixed with 2 mL of physiological saline, to produce an injectable preparation.
- DP4 inhibitor activity of the compounds of the invention may be determined by use of an in vitro assay system which measures the potentiation of inhibition of DP4.
- Inhibition constants (Ki values) for the DP4 inhibitors of the invention may be determined by the method described below.
- Porcine enzyme was purified as previously described (1), with several modifications. Kidneys from 15-20 animals were obtained, and the cortex was dissected away and frozen at ⁇ 80° C. Frozen tissue (2000 -2500 g) was homogenized in 12 L of 0.25 M sucrose in a Waring blender. The homogenate then was left at 37° C. for 18 hours to facilitate cleavage of DP-4 from cell membranes. After the cleavage step, the homogenate was clarified by centrifugation at 7000 ⁇ g for 20 min at 4° C., and the supernatant was collected. Solid ammonium sulfate was added to 60% saturation, and the precipitate was collected by centrifugation at 10,000 ⁇ g and was discarded. Additional ammonium sulfate was added to the supernatant to 80% saturation, and the 80% pellet was collected and dissolved in 20 mM Na 2 HPO 4 , pH 7.4.
- the preparation was clarified by centrifugation at 10,000 ⁇ g. The clarified preparation then was applied to 300 mL of ConA Sepharose that had been equilibrated in the same buffer. After washing with buffer to a constant A 280 , the column was eluted with 5% (w/v) methyl ⁇ -D-mannopyranoside. Active fractions were pooled, concentrated, and dialyzed against 5 mM sodium acetate, pH 5.0. Dialyzed material then was flowed through a 100 mL Pharmacia Resource S column equilibrated in the same buffer. The flow through material was collected and contained most of the enzyme activity.
- Active material again was concentrated and dialyzed into 20 mM Na 2 HPO 4 , pH 7.4. Lastly, the concentrated enzyme was chromatographed on a Pharmacia S-200 gel filtration column to removed low molecular weight contaminants. Purity of column fractions was analyzed by reducing SDS-PAGE, and the purest fractions were pooled and concentrated. Purified enzyme was stored in 20% glycerol at ⁇ 80° C.
- Enzyme was assayed under steady-state conditions as previously described (2) with gly-pro-p-nitroanilide as substrate, with the following modifications. Reactions contained, in a final volume of 100 ⁇ l, 100 mM Aces, 52 mM TRIS, 52 mM ethanolamine, 500 ⁇ M gly-pro-p-nitroanilide, 0.2 % DMSO, and 4.5 nM enzyme at 25° C., pH 7.4. For single assays at 10 ⁇ M test compound, buffer, compound, and enzyme were added to wells of a 96 well microtiter plate, and were incubated at room temperature for 5 min.
- Step 1 title compound was synthesized by following the literature procedure [Stephen Hanessian, Ulrich Reinhold, Michel Saulnier, and Stephen Claridge; Bioorganic & Medicinal Chemistry Letters 8 (1998) 2123-2128] or with the following modifications.
- L-pyroglutamic acid ethyl ester was N-protected as the t-butylcarbamate (Boc 20 , DMAP or NaH) and then dehydrated to the 4,5-dehydroproline ethyl ester in one pot by carbonyl reduction (triethylborohydride, toluene, ⁇ 78° C.) followed by dehydration (TFAA, lutidine).
- L-pyroglutamic acid ethyl ester (200 g, 1.27 mol) was dissolved in 1.2 liters of methylene chloride and treated sequentially with di-tert-butyldicarbonate (297 g, 1.36 mol) and a catalytic DMAP (1.55 g, 0.013 mol) at ambient temperature. After 6 h, the mixture was quenched with saturated brine and the organic phase was dried (Na 2 SO 4 ) and filtered through a short silica gel column to give 323 g (100%) of N-Boc- L-pyroglutamic acid ethyl ester.
- N-Boc-L-pyroglutamic acid ethyl ester 160 g, 0.62 mol was dissolved in 1 liter of toluene, cooled to ⁇ 78° C. and treated with lithium triethylborohydride (666 mL of a 1.0 M soln in THF) and added dropwise over 90 minutes. After 3 h, 2,6-lutidine (423 mL, 3.73 mol) was added dropwise followed by DMAP (0.2 g, 0.0016 mol). To this mixture was added TFAA (157 g, 0.74 mol) and the reaction was allowed to come to ambient temperature over 2 h.
- 4,5-Dehydro-L-proline ethyl ester (35.0 g, 0.145 mol) was added to a solution of neat Et 2 Zn (35.8 g, 0.209 mol) in 1 liter of 1,2-dichloroethane at ⁇ 15° C. To this mixture was added a dropwise addition of ClCH 2 I (102 g, 0.58 mol) over 1 h and the mixture stirred at ⁇ 15° C. for 18 h.
- reaction was quenched with saturated aqueous bicarbonate and the solvent was evaporated and the reaction was taken up in EtOAc, washed with brine and purified by silica gel chromatography using a stepwise gradient of from 20% EtOAc/hexanes to 50% EtOAc/hexanes to give 17.5 g (50%) of diastereomerically pure step 1 title compound.
- Step 1 compound (411 mg, 1.61 mmol) in CH 2 Cl 2 (1.5 mL) at rt was added TFA (1.5 mL). The reaction mixture was stirred at rt for 2 h and evaporated. The residue was diluted with CH 2 Cl 2 and then evaporated and re-evaporated three times to give the title compound as a colorless oil, 433 mg, 100% yield,
- Step 3 compound 530 mg, 1.44 mmol
- MeOH MeOH
- H 2 O 4 mL
- LiOH—H 2 O 91 mg, 2.16 mmol
- the reaction mixture was stirred at rt overnight and evaporated.
- Water (10 mL) was added to the residue and extracted with Et 2 O (2 ⁇ 10 mL).
- the aqueous layer was acidified to ⁇ pH 4 by adding 4% KHSO 4 dropwise.
- the milky solution was extracted with EtOAc (15 mL ⁇ 3). Combined EtOAc layers were washed with brine, dried over Na 2 SO 4 and evaporated to give the title compound as a white solid, 440 mg, 90% yield.
- Step 4 compound 300 mg, 0.88 mmol
- THF 6 mL
- 4-methylmorpholine 0.12 mL, 1.06 mmol
- isobutyl chloroformate 0.13 mL, 0.97 mmol
- White precipitate was formed.
- the reaction mixture was stirred at ⁇ 15° C. under nitrogen for 25 min and a solution of NH 3 in dioxane (8.8 mL, 4.4 mmol) was added.
- the reaction mixture was stirred at ⁇ 15° C. for 30 min, warmed to rt and stirred at rt overnight.
- Step 5 compound (248 mg, 1.38 mmol) and imidazole (94 mg, 1.38 mmol) in dry pyridine (12 mL) at ⁇ 35° C. under nitrogen was added POCl 3 (0.26 mL, 2.76 mmol) dropwise.
- POCl 3 (0.26 mL, 2.76 mmol
- the reaction mixture was stirred between ⁇ 35° C. to ⁇ 20° C. for 1 h and evaporated.
- CH 2 Cl 2 (10 mL) was added and white precipitates were formed. After filtration, the filtrate was concentrated and purified by flash chromatography (2:5 EtOAc/hexane) to give the title compound as a colorless oil, 196 mg, 88% yield.
- Step 6 compound 130 mg, 0.4 mmol
- CH 2 Cl 2 2 mL
- TFA 2 mL
- the reaction mixture was stirred at rt for 2 h.
- the reaction mixture was added slowly to a pre-cooled slurry of NaHCO 3 (3.8 g) in H 2 O (3 mL).
- Step 1 title compound was synthesized by following the literature procedure. [Stephen Hanessian, Ulrich Reinhold, Michel Saulnier, and Stephen Claridge; Bioorganic & Medicinal Chemistry Letters 8 (1998) 2123-2128.]
- Step 1 title compound was prepared by following the literature procedure. [Willy D. Kollmeyer, U.S. Pat. No. 4,183,857.].
- Step 1 title compound, as a 1:1 ratio of enantiomers, was prepared by following the literature procedure. [Willy D. Kollmeyer, U.S. Pat. No. 4,183,857.]
- Example 4 To a solution of Example 4, Step 1 compound (150 mg, 1.39 mmol) in 2-propanol (0.8 mL), was added NaCN (40 mg, 1.0 mmol). The reaction mixture was heated to reflux for 3 h. After cooling to rt, the reaction mixture was evaporated and then slurried in Et 2 O (5 mL). After filtration, the filtrate was evaporated to give Example 4 Step 1 compounds and Example 5 Step 1 compounds (140 mg, 93%) as a 2:1 mixture of diastereomers, each as a racemic mixture.
- Example 1 Step 1 compound (1.40 g, 5.49 mmol) in 40 mL of a 1:1 methanol:water solution at rt was added lithium hydroxide (0.20 g, 8.30 mmol). The reaction mixture was stirred at rt for 18 h and then heated to 50° C. for 2 h. The mixture was diluted with equal volumes of ether and water (50 mL) and then acidified with KHSO 4 to pH 3. The milky solution was extracted with ether (3 ⁇ 20 mL). The combined ether layers were dried over Na 2 SO 4 and evaporated. The residue was stripped from toluene (2 ⁇ 10 mL) and dried under reduced pressure to give the title compound as a thick syrup, 1.20 g, 96%.
- Step 1 compound (1.20 g, 5.28 mmol) in THF (20 mL) at ⁇ 15° C. under nitrogen was added 4-methylmorpholine (0.71 mL, 6.50 mmol) and then isobutyl chloroformate (0.78 mL, 6.00 mmol) over 5 min.
- the reaction was stirred at ⁇ 15° C. for 30 min, cooled to ⁇ 30° C. and treated with a solution of NH 3 in dioxane (50 mL, 25 mmol).
- the reaction mixture was stirred at ⁇ 30° C. for 30 min, warmed to rt and stirred overnight.
- Step 2 compound (0.90 g, 4.00 mmol) in CH 2 Cl 2 (3 mL) at 0° C. was added TFA (3 mL). The reaction mixture was stirred at 0° C. for 18 h. The reaction mixture was concentrated under reduced pressure to produce title compound in the form of a thick oil, 0.98 g, 100%. The oil gradually solidified upon prolonged standing.
- Step 3 compound 56 mg, 0.22 mmol
- N-tert-butoxycarbonyl-(L)-tert-leucine 53 mg, 0.23 mmol
- dimethylaminopyridine 0.11 g, 0.88 mmol
- CH 2 Cl 2 4 mL
- the tube was sealed under nitrogen atmosphere and treated with 1-[(3-(dimethypamino)propyl]-3-ethylcarbodiimide (84 mg, 0.44 mmol). The mixture was placed in a shaker and vortexed overnight.
- the product was purified by solid phase extraction using a United Technology SCX column (2 g of sorbent in a 6 mL column) by loading the material on a SCX ion exchange column and successively washing with CH 2 Cl 2 (5 mL), 30% methanol in CH 2 Cl 2 (5 mL), 50% methanol in CH 2 Cl 2 (5 mL) and methanol (10 mL).
- the product containing fractions were concentrated under reduced pressure to give the desired amide.
- Further purification by reverse phase preparative column chromatography on a YMC S5 ODS 20 ⁇ 250 mm column gave the title compound, 50 mg (68% yield).
- Purification conditions Gradient elution from 30% methanol/water/0.1 TFA to 90% methanol/water/0.1 TFA over 15 min. 5 min hold at 90% methanol/water/0.1 TFA. Flow rate: 20 mL/min Detection wavelength: 220. Retention Time: 14 min.
- Step 4 compound 50 mg, 0.15 mmol
- imidazole 31 mg, 0.46 mmol
- pyridine 1 mL
- the tube was sealed under nitrogen atmosphere and cooled to ⁇ 30° C.
- Slow addition of POCl 3 141 mg, 88 uL, 0.92 mmol gave after mixing a thick slurry.
- the tube was mixed at ⁇ 30° C. for 3 h and the volatiles evaporated.
- the product was purified by solid phase extraction using a United Technology silica extraction column (2 g of sorbent in a 6 mL column) by loading the material on a silica column and successively washing with CH 2 Cl 2 (5 mL), 5% methanol in CH 2 Cl 2 (5 mL), 7% methanol in CH 2 Cl 2 (5 mL) and 12% methanol in CH 2 Cl 2 (10 mL).
- the product containing fractions were pooled and concentrated under reduced pressure to give the title compound, 46 mg, 96%.
- Step 5 compound (0.45 mg, 0.14 mmol), CH 2 Cl 2 (1 mL), and TFA (1 mL).
- the reaction mixture was vortexed for 40 min at rt, diluted with toluene (4 mL) and concentrated under reduced pressure to a thick oil.
- the product was purified by reverse phase preparative column chromatography on a YMC S5 ODS 20 ⁇ 250 mm column to give the Example 6 compound, 14 mg, 35%. Purification conditions: gradient elution from 10% methanol/water/0.1 TFA to 90% methanol/water/0.1 TFA over 18 min; 5 min hold at 90% methanol/water/0.1 TFA. Flow rate: 20 mL/min Detection wavelength: 220. Retention Time: 10 min.
- Examples 7-27 were prepared from amino acids available from commercial sources according to the procedure in Example 6.
- Step 1 amide was dehydrated to the nitrile using the general method C (which follows Example 29) (FAB MH+462).
- Step 2 benzyl ether was cleaved by catalytic hydrogenolysis using 10% palladium on carbon and 1 atmosphere hydrogen gas in MeOH at rt for 1.5 h.
- the reaction was filtered through celite and concentrated to an oil and taken on without further purification (FAB MH+372).
- Step 3 N-[N-Boc-L-Tyrosine-]-(2S,4S,5S)-2-cyano-4,5-methano-L-prolylamide was dissolved in CH 2 Cl 2 and TFA was added at rt. The reaction stirred for 1 h and was evaporated and purified by preparative HPLC as described in general method B (set out following Example 29) to afford the title compound (FAB MH+272).
- the title compound was prepared by coupling (2S,4S,5S)-4,5-methano-L-proline carboxylamide, TFA salt described in Example 6 Step 3 compound with N-(tert-butyloxy-carbonylhydroxyvaline. After hydroxyl protection with triethylsilyl chloride and dehydration of the amide with POCl 3 /imidazole in pyridine and deprotection (N-terminal nitrogen and valine hydroxyl) with TFA using general method C (FAB MH+224), the title compound was obtained.
- Step 1 compound 333 mg, 1.0 mmol
- 6 mL of CH 2 Cl 2 1-[3-(dimethylamino)-propyl]-3-ethylcarbodiimide hydrochloride (256 mg, 1.32 mmol).
- the solution was then stirred at rt for 30 min, followed by addition with Example 6 Step 3 amine TFA salt (160 mg, 0.66 mmol) and 4-(dimethylamino)pyridine (244 mg, 2.0 mmol).
- the solution was then stirred at rt overnight.
- Step 2 compound 350 mg, 0.79 mmol
- imidazole 108 mg, 1.58 mmol
- pyridine 3 mL
- the flask under argon was cooled to ⁇ 30° C.
- Slow addition of POCl 3 (0.30 mL, 3.16 mmol) gave after mixing a thick slurry.
- the slurry was mixed at ⁇ 30° C. for 3 h and the volatiles evaporated.
- Dichloromethane (5 mL) was then added and the insoluble solid was removed by filtration.
- the organic layer was washed with H 2 O, 10% citric acid, brine and dried over Na 2 SO 4 . Removal of solvent gave crude desired nitrile (330 mg) (LC/Mass, + ion): 424 (M+H).
- Trifluoroacetic acid (3.3 mL) was added to a stirred solution of Step 3 compound (330 mg, 0.58 mmol) in 3.3 mL CH 2 Cl 2 .
- the solution was then stirred at rt for 30 min, a few drops of water were added and the mixture mixture stirred for 0.5 h.
- the mixture was diluted with CH 2 Cl 2 (5 mL) and concentrated under reduced pressure to a thick oil.
- the product was purified by reverse phase preparative column chromatography on a YMC S5 ODS 20 ⁇ 100 mm column to give the title compound, 59 mg, 17%.
- Step 2 To a flame-dried 500-mL round-bottomed flask containing N-(tert-butyloxycarbonyl)glycine (13.45 g, 76.75 mmol) in 100 mL CH 2 Cl 2 at rt was added Step 2 compound 8.61 g, 76.75 mmol, 1.00 equiv) in 20 mL CH 2 Cl 2 , followed by dicyclohexylcarbodiimide (16.63 g, mmol, 1.05 equiv) in 80 mL CH 2 Cl 2 . To this reaction mixture was then added 4-dimethylaminopyridine (0.94 mg, mmol, 0.10 equiv), and the mixture was allowed to stir overnight.
- 4-dimethylaminopyridine (0.94 mg, mmol, 0.10 equiv
- reaction mixture was then filtered through a medium sintered-glass funnel, rinsing with 100 mL CH 2 Cl 2 , and concentrated under reduced pressure.
- the crude product was then purified by flash chromatography (silica gel, hexanes/EtOAc, 20:1 to 1:1 gradient) to give 19.43 g (94%) of the desired glycinyl ester as a colorless oil.
- the TFA salt of amide 13 was coupled to a variety of racemic quaternary protected amino acids using HOBT/EDC in DMF at rt to give a D/L mixture of diastereomers at the N-terminal amino acid.
- the desired L diastereomer was chromatographically isolated either as the amide 21 or as the nitrile 22.
- Nitrile 22 was obtained by treatment of the amide with POCl 3 /imidazole in pyridine at ⁇ 20° C.
- the final target 23 was obtained by deprotection under acidic conditions using TFA in CH 2 Cl 2 .
- Example 6 Step 3 compound (877 mg, 3.65 mmol) and N-Boc cyclopentylvinylamino acid, described in Step 4 of general method B (1.13 g, 4.20 mmol) were dissolved in 20 mL anhydrous DMF, cooled to 0° C. and to this mixture was added EDAC (1.62 g, 8.4 mmol), HOBT hydrate (2.54 g, 12.6 mmol, and TEA (1.27 g, 12.6 mmol) and the reaction was allowed to warm to rt and stirred for 24 h.
- Step 1 compound (1.38 g, 3.65 mmol) and imidazole (497 mg, 7.30 mmol) were dried by toluene azeotrope (5 mL ⁇ 2), dissolved in 10 mL anhydrous pyridine, cooled to ⁇ 30° C. under nitrogen gas and POCl 3 (2.23 g, 14.60 mmol) was added by syringe.
- the reaction was complete after 1 h and was evaporated to dryness and the remainder purified by two sequential flash column chromatographies over silica gel. The first column (100% EtOAc) was used to isolate the mixture of diastereomers (1.15 g, 88%) from the by-products of the reaction.
- the second column gradient of 25% EtOAC/hexanes to 50% EtOAc/hexanes was run to resolve the mixture of diastereomers and provided 504 mg of the desired Step 2 nitrile (MH+360).
- Step 2 compound (32 mg, 0.09 mmol) was dissolved in 1 mL of CH 2 Cl 2 and 1 mL of TFA was added and the reaction stirred for 30 min at rt and was evaporated to dryness.
- the product was purified by reverse phase preparative column chromatography on a YMC S5 ODS 20 ⁇ 250 mm column to give 12 mg of the TFA salt (lyophilized from water or isolated after evaporation of eluent and trituration with ether) the title compound.
- Purification conditions gradient elution from 10% methanol/water/0.1 TFA to 90% methanol/water/0.1 TFA over 18 min; 5 min. hold at 90% water/0.1 trifluoroacetic acid. Flow rate: 20 mL/min. Detection wavelength: 220.
- Examples 30-39 were prepared by the methods outlined in General Method B and General Method C starting from cyclopentanone, cyclobutanone, cyclohexanone, cycloheptanone, cyclooctanone, cis-3,4-dimethylcyclopentanone, and 4-pyranone, cyclopropaneethylhemiacetal, acetone, and 3-pentanone respectively.
- Step 1 compound was prepared employing general method B starting from cyclopentanone and 2-fluorotriethylphos-phonoacetate instead of triethylphosphonoacetate.
- Step 1 compound was prepared employing general method B starting from cyclobutanone and 2-fluorotriethylphos-phonoacetate instead of triethylphosphonoacetate.
- Step 1 compound was prepared employing general method B starting from cyclopentanone and triethylphosphono propionate instead of triethylphosphonoacetate.
- Step 1 compound was prepared employing general method B starting from cyclobutanone and triethylphosphono propionate instead of triethylphosphonoacetate.
- N-Boc protected cyclobutylvinyl compound (Example 31, prepared by general method C) (0.16 g, 0.46 mmol) was dissolved in 10 mL of a 1:1 mixture of THF:water and treated with OsO4 (12 mg, catalyst) and NaIO 4 (0.59 g, 2.76 mmol, 6 equiv). After 2 h, the reaction mixture was diluted with 50 mL of ether and 10 mL of water. The layers were equilibrated and the organic fraction was washed one time with NaHCO 3 solution, dried over MgSO 4 and concentrated to give a dark oil. The oil was diluted with 10 mL of methanol and treated with NaBH 4 (0.08 g, 2.0 mmol).
- Part B A 200-mL round bottomed flask was charged with Part A solid (@ 39 mmol) and diluted with 80 mL of ethanol and 39 mL of 2N HCl (78 mmol). The mixture was treated with 1.0 g of 5% Pd/carbon and the mixture degassed. The flask was placed under an atmosphere of H 2 for 8 h. The mixture was filtered through celite and the filtrate concentrated to an off white solid.
- Part C A 250-mL round bottomed flask was charged with Part B solid and diluted with THF (50 mL) and water (15 mL). The mixture was treated with di-tert-butyldicarbonate (12.7 g, 117 mmol) and sodium bicarbonate (10.0 g, 117 mmol). After 4 h of stirring the mixture was diluted with 50 mL of ether and 50 mL of water. The layers were separated and the organic fraction dried over MgSO 4 and concentrated. The residue was purified by flash column chromatography on silica gel with 30% EtOAc in hexanes to give 2.00 g (22% overall) of Step 1 compound as a white solid.
- Step 1 compound (1.00 g, 3.80 mmol) in THF (20 mL) at rt under nitrogen was added LiOH hydrate (0.16 g, 3.80 mmol) and then water (5 mL). The reaction was stirred at 40° C. for 0.5 h and then cooled to rt. The mixture was concentrated to dryness and the remainder was stripped from THF (2 ⁇ ), toluene (2 ⁇ ) and THF (1 ⁇ ). The remaining glass was diluted with 5 mL of THF and treated with imidazole (0.63 g, 9.19 mmol) followed by t-butyl-dimethylsilyl chloride (1.26 g, 8.36 mmol).
- Step 2 The reaction was stirred overnight and quenched with 10 mL of methanol. After 1 h of stirring the mixture was concentrated. An additional portion of methanol was added and the mixture concentrated. The oil was diluted with ether and 0.1 N HCl (pH 2). The layers were equilibrated and aqueous drawn off. The organic fraction was dried over MgSO 4 and concentrated to give 1.25 g (83%) of Step 2 compound as a colorless glass.
- Examples 50-56 were prepared by the peptide coupling of amino acids (where the vinyl substituent has been hydrogenated according to general method F) followed by dehydration and deprotection as described in general method C.
- Example 57 The title compound in Example 57 was prepared by the peptide coupling of the isopropyl cyclobutane amino acid (where the olefin substituent has been hydrogenated according to general method F) followed by dehydration and deprotection as described in general method C.
- Example 58 The title compound in Example 58 was prepared by the peptide coupling of the isopropyl cyclopentane amino acid (where the olefin substituent has been hydrogenated according to general method F) followed by dehydration and deprotection as described in general method C. MS (M+H) 276
- Step 1 compound (10.7 g, 0.055 mmol, 1 equiv) was dissolved in anhydrous THF (150 mL) under argon and was treated with a solution of LiAlH 4 (1 M in THF, 69 mL, 69 mmol, 1.25 equiv). After stirring at rt for 1.5 h, the reaction was cooled to 0° C. and quenched sequentially with H 2 O (5.1 mL), 15% aq NaOH (5.1 mL), and H 2 O (10.2 mL). After stirring at rt for 15 min, the slurry was vacuum filtered, and the solids washed with EtOAc (2 ⁇ 100 mL).
- Step 3 compound (9.40 g, 57 mmol, 1 equiv) was suspended in H 2 O (145 mL) and cooled to 0° C. The mixture was treated with NaHSO 3 (5.95 g, 57 mmol, 1 equiv), KCN (4.0 g, 59 mmol, 1.04 equiv), and a solution of (R)-( ⁇ )-phenylglycinol (8.01 g, 57 mmol, 1 equiv) in MeOH (55 mL). The resulting mixture was stirred at rt for 2 h, then refluxed for 16 h. The mixture was cooled to rt, and 200 mL of EtOAc added. After mixing for 15 min the layers were separated.
- Step 4 nitrile (5.65 g, 18 mmol) was heated in conc. HCl (120 mL) and HOAc (30 mL) at 80° C. for 18 h, at which time the reaction was cooled in an ice bath. Vacuum filtration of the resulting precipitate afforded the desired product as a white solid (5.21 g, 14 mmol, 78%). MS m/e 330 (m+H) + .
- Step 6 compound (5.21 g, 14 mmol) was dissolved in MeOH (50 mL) and HOAc (10 mL), and hydrogenated with H 2 (50 psi) and Pearlman's catalyst (20% Pd(OH) 2 , 1.04 g, 20% w/w) for 18 h.
- the reaction was filtered through a PTFE membrane filter and the catalyst washed with MeOH (3 ⁇ 25 mL). The filtrate was concentrated by rotary evaporation to afford a white solid.
- the product was used in Step 7 without further purification.
- Step 6 compound (@ 14 mmol) was dissolved in anhydrous DMF (50 mL) under argon and treated with K 2 CO 3 (5.90 g, 42 mmol, 3 equiv) and di-tert-butyldicarbonate (3.14 g, 14 mmol, 1 equiv) under argon at rt. After 19 h, the DMF was removed by rotary evaporation (pump) and the residue dried further under reduced pressure. The residue was mixed with H 2 O (100 mL) and Et 2 O (100 mL), the layers separated, and the alkaline aqueous with Et 2 O (2 ⁇ 100 mL) to remove the by-product from the hydrogenolysis step.
- K 2 CO 3 5.90 g, 42 mmol, 3 equiv
- di-tert-butyldicarbonate 3.14 g, 14 mmol, 1 equiv
- the aqueous was cooled to 0° C., diluted with EtOAc (200 mL), and stirred vigorously while care fully acidifying the aqueous to pH 3 with 1N aq HCl.
- the layers separated and the aqueous extracted with EtOAc (100 mL).
- the combined EtOAc extracts were washed with brine (50 mL), dried (Na 2 SO 4 ), filtered and the filtrate concentrated by rotary evaporation.
- the residue was purified by SiO 2 flash column (5 ⁇ 12 cm) with 5% MeOH/CH 2 Cl 2 +0.5% HOAc.
- the product was chased with hexanes to afford the product as a white foam (4.07 g, 13 mmol, 92%): MS m/e 310 (m+H) + .
- Example 59 The title compound in Example 59 was prepared by the peptide coupling of the Step 7 compound in general method G followed by dehydration and deprotection as described in general method C.MS m/e 300 (m+H) + .
- Step 1 compound (404 mg, 1.24 mmol, 1 equiv) was dissolved in anhydrous DMF (10 mL) under argon and cooled to 0° C. The following were added in order: Example 6 Step 3 salt (328 mg, 1.37 mmol, 1.1 equiv), HOBT (520 mg, 3.85 mmol, 3.1 equiv), EDAC (510 mg, 2.61 mmol, 2.1 equiv), and TEA (0.54 mL, 3.85 mmol, 3.1 equiv). The reaction mixture was allowed to warm to rt overnight and the DMF removed by rotary evaporation (pump). The remainder was dried further under vacuum.
- Step 2 compound (95 mg, 0.22 mmol, 1 equiv) was dissolved in anhydrous CH 2 Cl 2 (2.5 mL) under argon and cooled to ⁇ 78° C.
- the mixture was treated with diisopropylethylamine (65 ⁇ L, 0.37 mmol, 1.7 equiv), and triethylsilyl triflate (75 ⁇ L, 0.33 mmol, 1.5 equiv), and stirred at 0° C. for 1.5 h.
- the reaction was mixed with MeOH (0.5 mL), silica gel (200 mg) and H 2 O (2 drops) and stirred at rt for 18 h.
- Step 3 compound (90 mg, 0.16 mmol, 1 equiv) was dissolved in anhydrous pyridine (2 mL) under argon and cooled to ⁇ 30° C.
- the product was purified by flash column chromatography on silica gel (2.5 ⁇ 10 cm) with 7% EtOAc/CH 2 Cl 2 to afford the product as a white foam (76 mg, 87%): MS m/e 530 (m+H) +
- Step 4 compound (76 mg, 0.14 mmol) was dissolved in anhydrous CH 2 Cl 2 (1 mL) and cooled to 0° C. and treated with TFA (1 mL) and H 2 O (2 drops) and stirred for 1.5 hr at 0° C. The solvents were removed by rotary evaporation and the residue was chased with toluene (5 mL) and dried under reduced pressure. Trituration with Et 2 O afforded the title compound as a white solid (54 mg, 88%): MS m/e 316 (m+H) + .
- the fluorinated amide from Step 1 (161 mg, 0.37 mmol, 1 equiv) was dissolved in anhydrous pyridine (4 mL) under argon and cooled to ⁇ 30° C. The mixture was treated with imidazole (54 mg, 0.77 mmol, 2.1 equiv) and phosphorous oxychloride (143 ⁇ L, 1.52 mmol, 4.1 equiv) and stirred at ⁇ 30° C. for 40 min. The solvent was removed by rotary evaporation and dried further under reduced pressure.
- Step 2 compound 125 mg, 0.30 mmol was dissolved in TFA/CH 2 Cl 2 (1:1 v/v, 2 mL), and stirred at rt. After 30 min, the solvents were removed by rotary evaporation, the remainder was chased with toluene (2 ⁇ 5 mL), and the solid dried under reduced pressure. Trituration with Et 2 O afforded the title compound as a white solid (93 mg, 0.21 mmol, 72%): MS m/e 318 (m+H) + .
- Step 1 compound was prepared beginning with 2-adamantanal and elaborated to the homochiral Boc-amino acid by an asymmetric Strecker synthesis according to general method G.
- Example 62 The title compound in Example 62 was prepared by the peptide coupling of the 2-adamantyl amino acid described in Step 1 followed by dehydration and deprotection as described in general method C.MS (M+H) 300.
- the mixture was washed with 10% aq NaHS0 3 (50 mL), H 2 O (2 ⁇ 50 mL), and brine (25 mL).
- the ether fraction was dried (Na 2 SO 4 ), filtered and concentrated by rotary evaporation.
- the product was purified by flash column chromatography on silica gel (5 ⁇ 15 cm) with 2% to 4% MeOH/CH 2 Cl 2 +0.5% HOAc.
- the product was chased with hexanes to remove residual HOAc.
- the isolated material consists of two inseparable materials (4.7 g), which was used without further purification in the next step.
- Methyl exo-2-bromonorbornane-1-carboxylate (2.0 g, 8.58 mmol, 1 equiv) was dissolved in anhydrous THF (50 mL) in an oven-dried 3-neck flask equipped with a condenser, and purged with argon. The mixture was treated with AIBN (288 mg, 1.71 mmol, 0.2 equiv) and tributyltin hydride (3.6 mL, 12.87 mmol, 1.5 equiv), and then heated to reflux for 2 h. The flask was cooled to rt, and the THF was removed by rotary evaporation to give the crude product. The product was purified by flash column chromatography on silica gel(5 ⁇ 10 cm) with 5% EtOAc/hexanes. The resulting material was used in the next step without further purification.
- Step 1 compound was prepared beginning with 1-norbonyl methyl carboxylate and elaborated to the homochiral Boc amino acid by an asymmetric Strecker synthesis according to general method G.
- Example 63 The title compound in Example 63 was prepared by the peptide coupling of the 1-norbonyl amino acid described in Step 2, followed by dehydration and deprotection as described in general method C. MS (M+H) 260.
- Step 1 compound was prepared beginning with 4-formylpyran and elaborated to the homochiral Boc amino acid by an asymmetric Strecker synthesis according to general method G.
- Example 64 The title compound in Example 64 was prepared by the peptide coupling of the 4-pyranyl amino acid described in Step 2, followed by dehydration and deprotection as described in general method C. MS (M+H) 250.
- Step 1 compound (0.80 g, 4.50 mmol) in 15 mL of CH 2 Cl 2 at rt was added celite (5 g) followed by PCC (1.95 g, 5.00 mmol). After stirring for 3 h the reaction mixture was diluted with 40 mL of CH 2 Cl 2 and filtered through celite. The filtrate was filtered an additional time through silica gel resulting in a colorless filtrate. The CH 2 Cl 2 fraction was evaporated to give 0.72 g (91%) of the aldehyde as a colorless oil.
- Step 2 compound (0.72 g, 4.20 mmol) in 8 mL of water at rt was added NaCN (0.20 g, 4.20 mmol) followed by NH 4 Cl (0.20 g, 5.00 mmol).
- methanol 8 mL
- the reaction mixture was then extracted with ether (2 ⁇ 15 mL), dried (MgSO 4 ) and concentrated under reduced pressure to give the crude Strecker product.
- Step 1 compound The synthesis of the Step 1 compound was described in general method H for the Strecker synthesis of racemic amino acids.
- Example 65 The title compound in Example 65 was prepared by the peptide coupling of the cyclopentylphenyl amino acid described in Step 1 and general method H followed by dehydration and deprotection as described in general method C. MS (M+H) 310.
- Step 1 compound was prepared using racemic Strecker synthesis according to general method H starting from 2,2-dimethyl-phenylacetic acid.
- Example 66 The title compound in Example 66 was prepared by the peptide coupling of the dimethylphenyl amino acid described in step 1 followed by dehydration and deprotection as described in general method C. MS (M+H) 284.
- N-(Benzyloxycarbonyl)succinimide (5.6 g, 22.4 mmol) was dissolved in CH 2 Cl 2 (25 mL) and the solution was added to a cooled (0° C.) and stirred solution of diethyl aminomalonate hydrochloride (5.0 g, 23.6 mmol) and triethylamine (13.4 mL, 95 mmol) in CH 2 Cl 2 (125 ml). The resulting solution was stirred at 0° C. for 10 min and then at rt for 1 h.
- Step 1 compound (6.18 g, 20 mmol) was dissolved in dry ethanol (30 mL) and added to a solution of sodium ethoxide (2.85 g, 8.8 m mol; 21% w/w solution in ethanol (6 mL).
- Acetic acid (0.56 mL) was then added the solution hydrogenated at 50 psi for 24 h using 10% Pd/C (2.0 g) as catalyst.
- Step 2 compound (173 mg, 0.97 mmol) was dissolved in DMF (3 mL)/water (3 mL). To this clear solution was added triethylamine (0.46 mL, 3.18 mmol) and di-t-butyl dicarbonate (0.23 g, 1.06 mmol), and the reaction mixture was stirred at rt for 5 h. The solution was evaporated and the residue chromatographed on silica column using CH 2 Cl 2 /methanol (9:1) as eluent to yield t-butyloxy-carbonyl-3,3-dimethyl-dl-proline (200 mg) as an oil (LC/Mass, + ion): 244 (M+H).
- Example 67 The title compound in Example 67 was prepared by the peptide coupling of the t-butyloxycarbonyl-3,3-dimethyl-dl-proline amino acid described in Step 3 followed by dehydration and deprotection as described in general method C. MS (M+H) 220.
- Step 1 compound (2.87 g, 9.5 mmol) and triethylsilane (2.28 mL, 14.3 mmol) in CH 2 Cl 2 (30 mL) under argon was added TFA (7.35 mL, 95.3 mmol) dropwise with stirring while maintaining the internal temperature at 25° C. by means of an ice bath. After stirring for 4 h at rt, the solution was concentrated. The residue was diluted with CH 2 Cl 2 (100 mL) , then treated with H 2 O (50 mL) and solid Na 2 CO 3 with vigorous stirring until the mixture was basic. The organic layer was separated, dried (Na 2 SO 4 ), filtered, then concentrated to give the Step 2 compound as a yellow oil which was used without further purification (LC/Mass: + ions, 308 M+Na).
- Step 2 compound (3.73 g, 9.5 mmol) was suspended in 6 N HCl (20 mL) and HOAc (5 mL) and heated at reflux for 20 h. The reaction mixture was then cooled, washed with EtOAc (20 mL), then concentrated to give an oil which crystallized upon trituration with ether to give the title compound (1.2 g, 70.6%) (LC/Mass, + ion): 144 (M+H).
- Step 3 compound (692 mg, 3.76 mmol) was dissolved in acetone (12 mL)/ water (12 mL). To this clear solution was added triethylamine (1.9 mL, 12.8 mmol) and di-t-butyl dicarbonate (928 mg, 4.24 mmol). The reaction mixture was stirred at rt for 18 h. The solvents were evaporated and the residue chromatographed on silica with 1:9 methanol:CH 2 Cl 2 to give the Step 4 compound as an oil (LC/Mass: + ions, 266 M+Na).
- Example 68 compound was prepared by peptide coupling of Step 4 amino acid followed by dehydration and deprotection as described in general method C (MS (M+H) 234).
- Step 1 compound (3.0g, 9.5 mmol) and triethylsilane (2.28 mL, 14.3 mmol) in CH 2 Cl 2 (30 mL) under argon was added TFA (7.35 mL, 95.3 mmol) dropwise with stirring while maintaining the internal temperature at 25° C., by means of an ice bath. After stirring for 4 h at rt, the solution was concentrated, the residue diluted with CH 2 Cl 2 (100 mL), then treated with H 2 O (50 mL) and solid Na 2 CO 3 with vigorous stirring until the mixture was basic. The organic layer was separated, dried (Na 2 SO 4 ), filtered, then concentrated to give the title compound as an oil which was used without further purification (LC/Mass:+ ions, 300 M+H).
- Step 2 compound (3.8 g, 9.5 mmol) was suspended in 6 N HCl (20 mL) and HOAc (5 mL) and heated at reflux for 20 h. The reaction mixture was cooled, washed with EtOAc (20 mL), then concentrated to give an oil which crystallized upon trituration with ether to give the step 3 compound (1.4 g, 76.0%).
- Step 3 compound (728 mg, 3.76 mmol) was dissolved in a 1:1 acetone/water solution (24 mL). To this clear solution was added triethylamine (1.9 mL, 12.8 mmol) and di-t-butyl dicarbonate (928 mg, 4.24 mmol). The reaction mixture was stirred at rt for 18 h. The solution was evaporated and the residue chromatographed on silica column using CH 2 Cl 2 /methanol (9:1) as eluent to give the title compound as an oil (LC/Mass, + ion): 258 (M+H).
- Example 69 compound was prepared by peptide coupling of Step 4 amino acid followed by dehydration and deprotection as described in general method C (MS (M+H) 248).
- Step 1 compound was prepared by the procedure described in General Method C starting from N-Boc-S-t-butylcysteine.
- Step 1 compound (78 mg, 0.21 mmol) and chloroform (3 mL).
- the mixture was cooled to 0° C. and treated with m-chloroperoxybenzoic acid (85 mg, 0.44 mmol) in CHCl 3 (2 mL).
- the solution was diluted with CHCl 3 (7 mL), washed with 5% NaHCO 3 (2 ⁇ 5 mL), H 2 O and dried over Na 2 SO 4 . Removal of solvent gave crude sulfoxide (100 mg), which was used without further purification (LC/Mass, + ions): 384 (M+H).
- Trifluoroacetic acid (1.5 mL) was added to a cooled (0° C.) solution of Step 2 compound (100 mg, 0.26 mmol) in 5 mL CH 2 Cl 2 . The solution was then stirred at 0° C. for 1.5 h, diluted with CH 2 Cl 2 (5 mL) and concentrated under reduced pressure to a thick oil. The product was purified by reverse phase preparative column chromatography on a YMC S5 ODS 20 ⁇ 100 mm column to give the title compound of Example 70 , 17 mg, 16%. Purification conditions: gradient elution from 10% methanol/water/0.1 TFA to 90% methanol/water/0.1 TFA over 15 min 5 min hold at 90% methanol/water/0.1 TFA. Flow rate: 20 mL/min. Detection wavelength: 220. Retention Time 10 Min (LC/Mass, + ion): 284 (M+H).
- Trifluoroacetic acid (1.5 mL) was added to a cooled (0° C.) and stirred solution of Step 1 compound (100 mg, 0.26 mmol) in 5 mL CH 2 Cl 2 . The solution was stirred at 0° C. for 30 min, diluted with CH 2 Cl 2 (5 mL) and concentrated under reduced pressure to a thick oil. The product was purified by reverse phase preparative column chromatography on a YMC S5 ODS 20 ⁇ 100 mm column to give the title compound, 14 mg, 17%. Purification conditions: gradient elution from 10% methanol/water/0.1 TFA to 90% methanol/water/0.1 TFA over 15 min 5 min hold at 90% methanol/water/0.1 TFA. Flow rate: 20 mL/min. Detection wavelength: 220. Retention Time 10 Min. (LC/Mass, + ion): 300 (M+H).
- the title compound was prepared following a published procedure (Sasaki et al, Tetrahedron Lett. 1995, 36, 3149, Sasaki et al. Tetrahedron 1994, 50, 7093) used to synthesize (2S,3R,4S)-N-Boc-3,4-methano-L-proline carboxylate.
- the corresponding amide was prepared by general method A and deprotected with TFA to give the TFA salt also as described in general method A.
- the title compound was prepared by coupling (2S,3R,4S)-3,4-methano-L-proline carboxamide-N-trifluoroacetate described in Example 72 with L-cyclohexylglycine and then dehydrated to the amide with POCl 3 /imidazole and deprotected (N-terminal nitrogen) with TFA using general C (FAB MH+248).
- the title compound was prepared by coupling (2S,3R,4S)-3,4-methano-L-proline carboxamide-N-trifluoroacetate described in Example 72 with L-tert-butylglycine and then dehydrated to the amide with POCl 3 /imidazole and deprotected (N-terminal nitrogen) with TFA using general C (FAB MH+222).
- the title compound was prepared by coupling (2S,3R,4S)-3,4-methano-L-proline carboxamide-N-trifluoroacetate described in Example 72 with N-(tert-butyloxycarbonyl)-(1′ethylcyclopentyl)glycine described in General Method B and then dehydrated to the amide with POCl 3 /imidazole and deprotected (N-terminal nitrogen) with TFA using general C (FAB MH+262).
- the title compound was prepared by coupling (2S,3R,4S)-3,4-methano-L-proline carboxamide-N-trifluoroacetate described in Example 72 with N-(tert-butyloxycarbonyl)-(1′vinylcyclopentyl)glycine described in General Method B and then dehydrated to the amide with POCl 3 /imidazole and deprotected (N-terminal nitrogen) with TFA using General Method C (FAB MH+260).
- Step 2 N-[((S)-cyclopentylvinyl)-N-tert-butoxycarbonylglycinyl]-(2S,4S,5S)-2-cyano-4,5-methano-L-prolylamide (70 mg, 0.19 mmol) described in General Method C, Step 2 was dissolved in a mixture of 2 mL t-BuOH/3 mL THF and N-methylmorpholine-N-oxide (33mg, 0.28 mmol) was added followed by osmium tetroxide (0.1 mmol, 50 mol %).
- Cyclohexanone and diethylmalonate underwent Knoevenagel condensation mediated by titanium tetrachloride in THF and CCl 4 to give 40.
- Copper (I) mediated Grignard addition of methylmagnesium bromide gave 41 which was selectively saponified to 42.
- Curtius rearrangement with trapping by benzyl alcohol gave 43 which was converted to 44 by a standard deprotection-protection protocol. Ester 44 was saponified to give the quaternary amino acid 45.
- reaction mixture was then treated with a solution of dry pyridine (32 mL, 0.40 mole) in dry THF (60 mL), stirred at 0° C. for 1.0 h, then at rt for 72 h.
- the reaction mixture was quenched with water (100 mL), stirred for 5 min then extracted with ether (2 ⁇ 200 mL).
- the combined organic extracts were washed with saturated sodium chloride (100 mL), saturated sodium bicarbonate (100 mL) and brine (100 mL), dried over anhydrous magnesium sulfate, filtered and concentrated. Flash chromatography using 5% EtOAc in hexane gave step 1 compound as a light yellow oil. Yield: 5.25 g (22%). MS (M+Na) 263.
- Step 2 compound (1.09 g, 4.03 mmol) in a mixture of methanol (5.4 mL) and water (2.7 mL) was treated with 1N sodium hydroxide (4.84 mL, 4.84 mmol or 1.2 equiv) and stirred at rt for 6 days.
- the reaction mixture still showed the presence of starting material, so THF (4.0 mL) was added and the entire mixture stirred for another 2 days.
- the solution was evaporated to dryness and the resulting syrup partitioned between water (8.0 mL) and ether (15 mL).
- the aqueous phase was acidified with 1N hydrochloric acid (4.8 mL) to pH 2-3 and extracted with EtOAc (3 ⁇ 25 mL).
- step 3 compound as a thick syrup. Yield: 875 mg, (95.1%). MS (M+H) 229.
- solutions of the diester in a mixture of ethanol, THF, dioxane and water or mixtures thereof may be hydrolyzed with sodium hydroxide.
- Step 3 compound 0.875 g, 3.83 mmol
- dry benzene 4.0 mL
- triethylamine 0.52 mL, 3.83 mmol
- diphenylphosphoryl azide 0.85 mL, 3.83 mmol
- the solution was treated with benzyl alcohol (0.60 mL, 5.75 mmol or 1.5 equiv), refluxed for 17 h, cooled then diluted with ether (40 mL).
- Step 4 compound (1.15 g, 3.46 mmol) in EtOAc (60 mL) was treated with palladium hydroxide on carbon (298 mg) and hydrogenated at rt for 20 h. The mixture was filtered through a celite pad and then washing the pad well with EtOAc (3 ⁇ 25 mL) then the filtrate was concentrated to give the free amine.
- a solution of the amine in tetrahydrofuran (12 mL) and water (12 mL) was treated with di-t-butyl dicarbonate (1.0 g, 4.58 mmol or 1.48 equiv) and potassium carbonate (854 mg, 6.18 mmol or 2.0 equiv), then stirred at rt for 20 h.
- Solutions of the benzylcarbamate in methanol may be subjected to hydrogenolysis in the present of di-t-butyldicarbonate to give the BOC-protected amine in a “one-pot” manner.
- Step 5 compound (1.18 g, 3.09 mmol) in dioxane (8.0 mL) was treated with 1N sodium hydroxide (9.1 mL, 9.1 mmol or 3.0 equiv) and stirred at 60° C. (oil bath) for 28 h.
- the reaction mixture was concentrated to a syrup which was dissolved in water (15 mL) and extracted with ether (25 mL).
- the aqueous phase was acidified to pH 2-3 with 1N hydrochloric acid (9.2 mL) then extracted with EtOAc (3 ⁇ 50 mL).
- the combined organic extracts were washed with saturated sodium chloride (10 mL), dried (MgSO 4 ), filtered, and concentrated to give Step 6 compound as an off-white solid. Yield: 808 mg (96%).
- Compounds 90-100 were prepared by General Method I and General Method C starting from cyclohexanone, cyclopentanone and cyclobutanone, and employing methyl-, ethyl-, allyl- and propylmagnesium halides as Grignard reagents.
- Example 79 A mixture of dry carbon tetrachloride (50 mL) was cooled to 0° C. (ice-salt bath) and treated with titanium tetrachloride (11.0 mL, 0.1 mol). The resulting yellow suspension was stirred at 0° C. for 5 min, treated sequentially with cyclopentanone (4.42 mL, 0.05 mol) and distilled diethylmalonate (7.6 mL, 0.05 mol) then stirred at 0° C. for 30 min The reaction mixture was then treated with a solution of dry pyridine (16 mL, 0.20 mol) in dry THF (30 mL), stirred at 0° C.
- Step 1 compound (1.00 g 4.42 mmol) in methanol (50 mL) was treated with 10% Pd/C (0.20 g, 10 mol %) and hydrogenated (balloon pressure) at rt for 20 h.
- the mixture was diluted with methanol and filtered through a pad of celite.
- the filtrate was concentrated and purified by flash column chromatography on silica gel with 7% EtOAc in hexanes to give 0.84 g (91%) of Step 2 compound.
- Step 3 compound was prepared by the process outlined in General Method H, where the ester underwent hydrolysis, Curtius Rearrangement, protecting group exchange, and again final ester hydrolysis.
- Examples 86 and 87 were prepared by the procedures used for Example 85 starting from cyclohexanone and cyclobutanone respectively
- Step 1 compound was prepared in Example 6 Step 1.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Diabetes (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Immunology (AREA)
- Endocrinology (AREA)
- Physical Education & Sports Medicine (AREA)
- Rheumatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Emergency Medicine (AREA)
- Reproductive Health (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Child & Adolescent Psychology (AREA)
- Virology (AREA)
- Oncology (AREA)
- Psychiatry (AREA)
- AIDS & HIV (AREA)
- Tropical Medicine & Parasitology (AREA)
- Molecular Biology (AREA)
- Neurosurgery (AREA)
- Communicable Diseases (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
Abstract
-
- x is 0 or 1 and y is 0 or 1 (provided that
- x=1 when y=0 and x=0 when y=1);
- n is 0 or 1; X is H or CN;
- and wherein R1, R2, R3 and R4 are as described herein.
Description
-
- x is 0 or 1 and y is 0 or 1 (provided that
- x=1 when y=0 and
- x=0 when y=1);
- n is 0 or 1;
- X is H or CN (that is cyano);
- R1, R2, R3 and R4are the same or different and are independently selected from H, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, bicycloalkyl, tricycloalkyl, alkylcycloalkyl, hydroxyalkyl, hydroxyalkylcycloalkyl, hydroxycycloalkyl, hydroxybicycloalkyl, hydroxytricycloalkyl, bicycloalkylalkyl, alkylthioalkyl, arylalkylthioalkyl, cycloalkenyl, aryl, aralkyl, heteroaryl, heteroarylalkyl, cycloheteroalkyl and cycloheteroalkylalkyl, all optionally substituted through available carbon atoms with 1, 2, 3, 4 or 5 groups selected from hydrogen, halo, alkyl, polyhaloalkyl, alkoxy, haloalkoxy, polyhaloalkoxy, alkoxycarbonyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, polycycloalkyl, heteroarylamino, arylamino, cycloheteroalkyl, cycloheteroalkylalkyl, hydroxy, hydroxyalkyl, nitro, cyano, amino, substituted amino, alkylamino, dialkylamino, thiol, alkylthio, alkylcarbonyl, acyl, alkoxycarbonyl, aminocarbonyl, alkynylaminocarbonyl, alkylaminocarbonyl, alkenylaminocarbonyl, alkylcarbonyloxy, alkylcarbonylamino, arylcarbonylamino, alkylsulfonylamino, alkylaminocarbonylamino, alkoxycarbonylamino, alkylsulfonyl, aminosulfonyl, alkylsulfinyl, sulfonamido or sulfonyl;
- and R1 and R3 may optionally be taken together to form —(CR5R6)m— where m is 2 to 6, and R5 and R6 are the same or different and are independently selected from hydroxy, alkoxy, cyano, H, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, cycloheteroalkyl, halo, amino, substituted amino, cycloheteroalkylalkyl, alkylcarbonylamino, arylcarbonylamino, alkoxycarbonylamino, aryloxycarbonylamino, alkoxycarbonyl, aryloxycarbonyl, or alkylaminocarbonylamino, or R1 and R4 may optionally be taken together to form —(CR7R8)p— where p is 2 to 6, and R7 and R8 are the same or different and are independently selected from hydroxy, alkoxy, cyano, H, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, cycloheteroalkyl, halo, amino, substituted amino, cycloheteroalkylalkyl, alkylcarbonylamino, arylcarbonylamino, alkoxycarbonylamino, aryloxycarbonylamino, alkoxycarbonyl, aryloxycarbonyl, or alkylaminocarbonylamino, or optionally R1 and R3 together with
-
- form a 5 to 7 membered ring containing a total of 2 to 4 heteroatoms selected from N, O, S, SO, or SO2;
- or optionally R1 and R3 together with
-
- form a 4 to 8 membered cycloheteroalkyl ring wherein the cycloheteroalkyl ring has an optional aryl ring fused thereto or an optional 3 to 7 membered cycloalkyl ring fused thereto;
- and including pharmaceutically acceptable salts thereof, and prodrug esters thereof, and all stereoisomers thereof.
wherein R1 is alkyl, cycloalkyl, bicycloalkyl, tricycloalkyl, alkylcycloalkyl, hydroxyalkyl, hydroxycycloalkyl, hydroxyalkylcycloalkyl, hydroxybicycloalkyl or hydroxytricycloalkyl;
B)
wherein R1 is alkyl, cycloalkyl, bicycloalkyl, tricycloalkyl, hydroxybicycloalkyl, hydroxytricycloalkyl, alkylcycloalkyl, hydroxyalkyl, hydroxycycloalkyl or hydroxyalkylcycloalkyl as well as the following:
may be coupled with 2, the free amine of 8, or 10 to give the corresponding amides which may be converted to the β-amino acid derivatives of compound Ia or Ib following the same chemistry.
any of which groups may be optionally subsituted with 1 to 4 substituents such as halogen, alkyl, alkoxy, hydroxy, aryl, aryloxy, arylalkyl, cycloalkyl, hydroxyalkyl, alkylamido, alkanoylamino, oxo, acyl, arylcarbonylamino, amino, nitro, cyano, thiol and/or alkylthio and/or any of the substituents for alkyl.
and may be optionally substituted through available carbon atoms with 1, 2, or 3 groups selected from hydrogen, halo, haloalkyl, alkyl, haloalkyl, alkoxy, haloalkoxy, alkenyl, trifluoromethyl, trifluoromethoxy, alkynyl, cycloalkylalkyl, cycloheteroalkyl, cycloheteroalkylalkyl, aryl, heteroaryl, arylalkyl, aryloxy, aryloxyalkyl, arylalkoxy, arylthio, arylazo, heteroarylalkyl, heteroarylalkenyl, heteroarylheteroaryl, heteroaryloxy, hydroxy, nitro, cyano, amino, substituted amino wherein the amino includes 1 or 2 substituents (which are alkyl, aryl or any of the other aryl compounds mentioned in the definitions), thiol, alkylthio, arylthio, heteroarylthio, arylthioalkyl, alkoxyarylthio, alkylcarbonyl, arylcarbonyl, alkylaminocarbonyl, arylaminocarbonyl, alkoxycarbonyl, aminocarbonyl, alkylcarbonyloxy, arylcarbonyloxy, alkylcarbonylamino, arylcarbonylamino, arylsulfinyl, arylsulfinylalkyl, arylsulfonylamino or arylsulfon-aminocarbonyl and/or any of the alkyl substituents set out herein.
group; examples of acyl groups include any of the R1 groups attached to a carbonyl, such as alkanoyl, alkenoyl, aroyl, aralkanoyl, heteroaroyl, cycloalkanoyl, cycloheteroalkanoyl and the like.
and the like. The above groups may include 1 to 4 substituents such as alkyl, halo, oxo and/or any of the alkyl substituents set out herein. In addition, any of the cycloheteroalkyl rings can be fused to a cycloalkyl, aryl, heteroaryl or cycloheteroalkyl ring.
-
- Ph=phenyl
- Bn=benzyl
- i-Bu=iso-butyl
- Me=methyl
- Et=ethyl
- Pr=propyl
- Bu=butyl
- TMS=trimethylsilyl
- FMOC=fluorenylmethoxycarbonyl
- Boc or BOC=tert-butoxycarbonyl
- Cbz=carbobenzyloxy or carbobenzoxy or benzyloxycarbonyl
- HOAc or AcOH=acetic acid
- DMF=N,N-dimethylformamide
- EtOAc=ethyl acetate
- THF=tetrahydrofuran
- TFA=trifluoroacetic acid
- Et2NH=diethylamine
- NMM=N-methyl morpholine
- n-BuLi=n-butyllithium
- Pd/C=palladium on carbon
- PtO2=platinum oxide
- TEA=triethylamine
- EDAC=3-ethyl-3′-(dimethylamino)propyl-carbodiimide hydrochloride (or 1-[(3-(dimethyl)amino)propyl])-3-ethylcarbodiimide hydrochloride)
- HOBT or HOBT.H2O=1-hydroxybenzotriazole hydrate
- HOAT=1-hydroxy-7-azabenzotriazole
- PyBOP reagent=benzotriazol-1-yloxy-tripyrrolidino phosphonium hexafluorophosphate
- min=minute(s)
- h or hr=hour(s)
- L=liter
- mL=milliliter
- μL=microliter
- g=gram(s)
- mg=milligram(s)
- mol=mole(s)
- mmol=millimole(s)
- meq=milliequivalent
- rt=room temperature
- sat or sat'd=saturated
- aq.=aqueous
- TLC=thin layer chromatography
- HPLC=high performance liquid chromatography
- LC/MS=high performance liquid chromatography/mass spectrometry
- MS or Mass Spec=mass spectrometry
- NMR=nuclear magnetic resonance
- mp=melting point
| TABLE 1 |
|
|
| Example | R | [M + H] | ||
| 7 |
|
302 | ||
| 8 |
|
295 | ||
| 9 |
|
240 | ||
| 10 |
|
222 | ||
| 11 |
|
222 | ||
| 12 |
|
222 | ||
| 13 |
|
208 | ||
| 14 |
|
270 | ||
| 15 |
|
222 | ||
| 16 |
|
206 | ||
| 17 |
|
256 | ||
| 18 |
|
268 | ||
| 19 |
|
220 | ||
| 20 |
|
220 | ||
| 21 |
|
210 | ||
| 22 |
|
262 | ||
| 23 |
|
242 | ||
| 24 |
|
210 | ||
| 25 |
|
281 | ||
| 26 |
|
281 | ||
| 27 |
|
272 | ||
| TABLE 2 |
|
|
| MS | ||
| Example | R | [M + H] |
| 30 |
|
260 |
| 31 |
|
246 |
| 32 |
|
274 |
| 33 |
|
288 |
| 34 |
|
302 |
| 35 |
|
288 |
| 36 |
|
276 |
| 37* |
|
232 |
| 38 |
|
234 |
| 39 |
|
262 |
| *Step 3 compound was prepared by the method described in Tetrahedron Letters 1986, 1281-1284. | ||
| TABLE 3 |
|
|
| Method of | |||
| Example | R | Preparation | [M + H] |
| 44 |
|
Ozonolysis/borohydride | 264 |
| 45 |
|
Osmium/periodate/ borohydride | 250 |
| 46 |
|
Ozonolysis/borohydride | 278 |
| 47 |
|
Osmium/periodate/ borohydride | 292 |
| 48 |
|
Ozonolysis/borohydride | 292 |
| TABLE 4 |
|
|
| MS | ||
| Example | R1, R2 | [M + H] |
| 50 | Cyclopentyl | 262 |
| 51 | cyclobutyl | 248 |
| 52 | cycloheptyl | 290 |
| 53 | 4-pyranyl | 278 |
| 54 | methyl, methyl | 236 |
| 55 | ethyl, ethyl | 264 |
| 56 | methyl, ethyl | 250 |
| TABLE 5 |
|
|
| MS Data | |||||
| Example # | Cycloalkane | R | M + H | ||
| 79 | cyclohexane | Methyl | 262 | ||
| 80 | cyclohexane | Ethyl | 276 | ||
| 81 | cyclopentane | Methyl | 248 | ||
| 82 | cyclopentane | Allyl | 274 | ||
| 83 | cyclopentane | Propyl | 276 | ||
| 84 | cyclobutane | Methyl | 234 | ||
|
|
| Ex. # | n | x | y | R1 | R2 | R3 | R4 |
| 90 | 0 | 0 | 1 | t-Bu | H | H | — |
| 91 | 0 | 0 | 1 | adamantyl | H | H | — |
| 92 | 0 | 0 | 1 |
|
H | H | — |
| 93 | 0 | 0 | 1 |
|
H | Me | — |
| 94 | 0 | 1 | 0 | t-Bu | H | H | — |
| 95 | 0 | 1 | 0 | adamantyl | H | H | — |
| 96 | 0 | 1 | 0 |
|
H | H | — |
| 97 | 0 | 1 | 0 |
|
H | Me | — |
| 98 | 1 | 0 | 1 | H | H | H | t-Bu |
| 99 | 1 | 1 | 0 | Me | H | H | t-Bu |
Claims (43)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/308,658 USRE44186E1 (en) | 2000-03-10 | 2011-12-01 | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18855500P | 2000-03-10 | 2000-03-10 | |
| US09/788,173 US6395767B2 (en) | 2000-03-10 | 2001-02-16 | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
| US13/308,658 USRE44186E1 (en) | 2000-03-10 | 2011-12-01 | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/788,173 Reissue US6395767B2 (en) | 2000-03-10 | 2001-02-16 | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| USRE44186E1 true USRE44186E1 (en) | 2013-04-30 |
Family
ID=22693638
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/788,173 Ceased US6395767B2 (en) | 2000-03-10 | 2001-02-16 | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
| US13/308,658 Expired - Lifetime USRE44186E1 (en) | 2000-03-10 | 2011-12-01 | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/788,173 Ceased US6395767B2 (en) | 2000-03-10 | 2001-02-16 | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
Country Status (37)
| Country | Link |
|---|---|
| US (2) | US6395767B2 (en) |
| EP (4) | EP2995615B1 (en) |
| JP (5) | JP4460205B2 (en) |
| KR (2) | KR100754089B1 (en) |
| CN (2) | CN1213028C (en) |
| AR (1) | AR027634A1 (en) |
| AT (1) | ATE396176T1 (en) |
| AU (2) | AU2001245466B2 (en) |
| BE (2) | BE2010C008I2 (en) |
| BR (1) | BRPI0109115B8 (en) |
| CA (1) | CA2402894C (en) |
| CO (1) | CO5280198A1 (en) |
| CY (3) | CY1108273T1 (en) |
| CZ (3) | CZ307784B6 (en) |
| DE (3) | DE60134122D1 (en) |
| DK (1) | DK1261586T3 (en) |
| EG (1) | EG25854A (en) |
| ES (4) | ES2553573T3 (en) |
| FR (2) | FR10C0010I2 (en) |
| HK (1) | HK1049330B (en) |
| HU (5) | HU230347B1 (en) |
| IL (4) | IL151372A0 (en) |
| LU (2) | LU91650I2 (en) |
| MX (1) | MXPA02008837A (en) |
| MY (1) | MY124512A (en) |
| NL (1) | NL300436I1 (en) |
| NO (3) | NO324227B1 (en) |
| NZ (1) | NZ520821A (en) |
| PE (1) | PE20020771A1 (en) |
| PL (1) | PL207041B1 (en) |
| PT (1) | PT1261586E (en) |
| RU (1) | RU2286986C2 (en) |
| SG (1) | SG152030A1 (en) |
| TW (2) | TWI258468B (en) |
| UY (2) | UY26613A1 (en) |
| WO (1) | WO2001068603A2 (en) |
| ZA (1) | ZA200206816B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016016770A1 (en) | 2014-07-26 | 2016-02-04 | Wockhardt Limited | A novel modified release pharmaceutical composition of sitagliptin or pharmaceutically acceptable salt thereof |
Families Citing this family (313)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0487425A (en) * | 1990-07-31 | 1992-03-19 | Fujitsu Ltd | Circuit switching device |
| US6414002B1 (en) * | 1999-09-22 | 2002-07-02 | Bristol-Myers Squibb Company | Substituted acid derivatives useful as antidiabetic and antiobesity agents and method |
| US6395767B2 (en) * | 2000-03-10 | 2002-05-28 | Bristol-Myers Squibb Company | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
| US7622503B2 (en) | 2000-08-24 | 2009-11-24 | University Of Tennessee Research Foundation | Selective androgen receptor modulators and methods of use thereof |
| US6573287B2 (en) * | 2001-04-12 | 2003-06-03 | Bristo-Myers Squibb Company | 2,1-oxazoline and 1,2-pyrazoline-based inhibitors of dipeptidyl peptidase IV and method |
| US7196201B2 (en) * | 2001-06-27 | 2007-03-27 | Smithkline Beecham Corporation | Pyrrolidines as dipeptidyl peptidase inhibitors |
| ATE374181T1 (en) * | 2001-06-27 | 2007-10-15 | Smithkline Beecham Corp | FLUORPYRROLIDINES AS DIPEPTIDYLPEPTIDASE INHIBITORS |
| DE60221983T2 (en) * | 2001-06-27 | 2008-05-15 | Smithkline Beecham Corp. | FLUORPYRROLIDINES AS DIPEPTIDYL-PEPTIDASE INHIBITORS |
| EP1443919A4 (en) * | 2001-11-16 | 2006-03-22 | Bristol Myers Squibb Co | Dual inhibitors of adipocyte fatty acid binding protein and keratinocyte fatty acid binding protein |
| US8853266B2 (en) | 2001-12-06 | 2014-10-07 | University Of Tennessee Research Foundation | Selective androgen receptor modulators for treating diabetes |
| US20070066568A1 (en) | 2005-08-31 | 2007-03-22 | Dalton James T | Treating renal disease, burns, wounds and spinal cord injury with selective androgen receptor modulators |
| US7772433B2 (en) | 2002-02-28 | 2010-08-10 | University Of Tennessee Research Foundation | SARMS and method of use thereof |
| GB0205170D0 (en) | 2002-03-06 | 2002-04-17 | Astrazeneca Ab | Chemical compounds |
| GB0205175D0 (en) | 2002-03-06 | 2002-04-17 | Astrazeneca Ab | Chemical compounds |
| GB0205176D0 (en) | 2002-03-06 | 2002-04-17 | Astrazeneca Ab | Chemical compounds |
| GB0205166D0 (en) | 2002-03-06 | 2002-04-17 | Astrazeneca Ab | Chemical compounds |
| GB0205165D0 (en) | 2002-03-06 | 2002-04-17 | Astrazeneca Ab | Chemical compounds |
| GB0205162D0 (en) | 2002-03-06 | 2002-04-17 | Astrazeneca Ab | Chemical compounds |
| WO2003097038A1 (en) * | 2002-05-14 | 2003-11-27 | Ralph Ryback | Method for treating dermatoses and tissue damage |
| US7405234B2 (en) * | 2002-05-17 | 2008-07-29 | Bristol-Myers Squibb Company | Bicyclic modulators of androgen receptor function |
| US7378385B2 (en) * | 2002-08-08 | 2008-05-27 | University Of Cincinnati | Role for GLP-1 to mediate responses to disparate stressors |
| US7407955B2 (en) | 2002-08-21 | 2008-08-05 | Boehringer Ingelheim Pharma Gmbh & Co., Kg | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| US7238724B2 (en) * | 2002-09-19 | 2007-07-03 | Abbott Laboratories | Pharmaceutical compositions as inhibitors of dipeptidyl peptidase-IV (DPP-IV) |
| US20040121964A1 (en) * | 2002-09-19 | 2004-06-24 | Madar David J. | Pharmaceutical compositions as inhibitors of dipeptidyl peptidase-IV (DPP-IV) |
| US7262207B2 (en) * | 2002-09-19 | 2007-08-28 | Abbott Laboratories | Pharmaceutical compositions as inhibitors of dipeptidyl peptidase-IV (DPP-IV) |
| US7632858B2 (en) * | 2002-11-15 | 2009-12-15 | Bristol-Myers Squibb Company | Open chain prolyl urea-related modulators of androgen receptor function |
| US8309603B2 (en) | 2004-06-07 | 2012-11-13 | University Of Tennessee Research Foundation | SARMs and method of use thereof |
| US7420079B2 (en) * | 2002-12-09 | 2008-09-02 | Bristol-Myers Squibb Company | Methods and compounds for producing dipeptidyl peptidase IV inhibitors and intermediates thereof |
| EP1583534A4 (en) * | 2002-12-20 | 2007-08-29 | Merck & Co Inc | ACIDIC 3-AMINO-4-PHENYLBUTANOIC DERIVATIVES AS INHIBITORS OF DIPEPTIDYL PEPTIDASE FOR THE TREATMENT OR PREVENTION OF DIABETES |
| KR20050122220A (en) * | 2003-03-25 | 2005-12-28 | 다케다 샌디에고, 인코포레이티드 | Dipeptidyl peptidase inhibitors |
| EP2206496B1 (en) | 2003-05-05 | 2014-09-17 | Probiodrug AG | Screening of inhibitors of formation of pyroglutamic acid in amyloid beta peptide |
| AU2004237408C9 (en) | 2003-05-05 | 2010-04-15 | Probiodrug Ag | Medical use of inhibitors of glutaminyl and glutamate cyclases |
| ZA200508439B (en) | 2003-05-05 | 2007-03-28 | Probiodrug Ag | Medical use of inhibitors of glutaminyl and glutamate cyclases |
| JP2007511467A (en) | 2003-05-14 | 2007-05-10 | タケダ サン ディエゴ インコーポレイテッド | Dipeptidyl peptidase inhibitor |
| US7459474B2 (en) | 2003-06-11 | 2008-12-02 | Bristol-Myers Squibb Company | Modulators of the glucocorticoid receptor and method |
| US6995183B2 (en) * | 2003-08-01 | 2006-02-07 | Bristol Myers Squibb Company | Adamantylglycine-based inhibitors of dipeptidyl peptidase IV and methods |
| CA2535619A1 (en) * | 2003-08-13 | 2005-02-24 | Takeda Pharmaceutical Company Limited | 4-pyrimidone derivatives and their use as peptidyl peptidase inhibitors |
| US7169926B1 (en) | 2003-08-13 | 2007-01-30 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| US7678909B1 (en) | 2003-08-13 | 2010-03-16 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| US7205409B2 (en) * | 2003-09-04 | 2007-04-17 | Abbott Laboratories | Pharmaceutical compositions as inhibitors of dipeptidyl peptidase-IV (DPP-IV) |
| EP1697342A2 (en) * | 2003-09-08 | 2006-09-06 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| US7790734B2 (en) * | 2003-09-08 | 2010-09-07 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| US7371759B2 (en) * | 2003-09-25 | 2008-05-13 | Bristol-Myers Squibb Company | HMG-CoA reductase inhibitors and method |
| EA010108B1 (en) | 2003-10-15 | 2008-06-30 | Пробиодруг Аг | Use of effectors of glutaminyl and glutamate cyclases |
| NZ546887A (en) | 2003-11-03 | 2009-04-30 | Probiodrug Ag | Combinations useful for the treatment of neuronal disorders |
| US7317109B2 (en) * | 2003-11-12 | 2008-01-08 | Phenomix Corporation | Pyrrolidine compounds and methods for selective inhibition of dipeptidyl peptidase-IV |
| US7767828B2 (en) * | 2003-11-12 | 2010-08-03 | Phenomix Corporation | Methyl and ethyl substituted pyrrolidine compounds and methods for selective inhibition of dipeptidyl peptidase-IV |
| US7576121B2 (en) * | 2003-11-12 | 2009-08-18 | Phenomix Corporation | Pyrrolidine compounds and methods for selective inhibition of dipeptidyl peptidase-IV |
| SI1689757T1 (en) * | 2003-11-12 | 2015-01-30 | Sino-Med International Alliance, Inc. | Heterocyclic boronic acid compounds |
| KR20170005163A (en) | 2003-11-17 | 2017-01-11 | 노파르티스 아게 | Use of dipeptidyl peptidase iv inhibitors |
| US20070149451A1 (en) * | 2003-11-17 | 2007-06-28 | Holmes David G | Combination of a dpp IV inhibitor and an antiobesity or appetite regulating agent |
| US7420059B2 (en) * | 2003-11-20 | 2008-09-02 | Bristol-Myers Squibb Company | HMG-CoA reductase inhibitors and method |
| RS57561B1 (en) | 2004-01-20 | 2018-10-31 | Novartis Ag | Direct compression formulation and process |
| US7470810B2 (en) * | 2004-01-21 | 2008-12-30 | Bristol-Myers Squibb Company | Alkyl and aryl-thiotrifluoroacetates and process |
| WO2005073186A1 (en) * | 2004-01-29 | 2005-08-11 | Ono Pharmaceutical Co., Ltd. | Pyrrolidine derivatives |
| AU2005210004B2 (en) | 2004-02-05 | 2010-10-28 | Probiodrug Ag | Novel inhibitors of glutaminyl cyclase |
| AU2005210285B2 (en) * | 2004-02-05 | 2008-01-24 | Kyorin Pharmaceutical Co., Ltd. | Bicycloester derivative |
| US7501426B2 (en) | 2004-02-18 | 2009-03-10 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, their preparation and their use as pharmaceutical compositions |
| EP1717225A4 (en) * | 2004-02-18 | 2009-10-21 | Kyorin Seiyaku Kk | BICYCLIC AMIDE DERIVATIVES |
| EP1719757B1 (en) * | 2004-02-27 | 2013-10-09 | Kyorin Pharmaceutical Co., Ltd. | Bicyclo derivative |
| US20070238753A1 (en) * | 2004-02-27 | 2007-10-11 | Madar David J | Pharmaceutical Compositions as Inhibitors of Dipeptidyl Peptidase-IV (DPP-IV) |
| US7732446B1 (en) | 2004-03-11 | 2010-06-08 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| CA2559302C (en) * | 2004-03-15 | 2012-06-19 | Takeda Pharmaceutical Company Limited | 6-amino-1h-pyrimidine-2,4-dione derivatives as dipeptidyl peptidase inhibitors |
| TW200534879A (en) * | 2004-03-25 | 2005-11-01 | Bristol Myers Squibb Co | Coated tablet formulation and method |
| TW200538122A (en) * | 2004-03-31 | 2005-12-01 | Bristol Myers Squibb Co | Process for preparing a dipeptidyl peptidase Ⅳ inhibitor and intermediates employed therein |
| US7741082B2 (en) | 2004-04-14 | 2010-06-22 | Bristol-Myers Squibb Company | Process for preparing dipeptidyl peptidase IV inhibitors and intermediates therefor |
| US7829720B2 (en) * | 2004-05-04 | 2010-11-09 | Bristol-Myers Squibb Company | Process for preparing atazanavir bisulfate and novel forms |
| US20050256314A1 (en) * | 2004-05-04 | 2005-11-17 | Soojin Kim | Process employing controlled crystallization in forming crystals of a pharmaceutical |
| TW200536827A (en) * | 2004-05-04 | 2005-11-16 | Bristol Myers Squibb Co | Enzymatic ammonolysis process for the preparation of intermediates for DPP IV inhibitors |
| US7214702B2 (en) * | 2004-05-25 | 2007-05-08 | Bristol-Myers Squibb Company | Process for producing a dipeptidyl peptidase IV inhibitor |
| TWI415635B (en) * | 2004-05-28 | 2013-11-21 | 必治妥施貴寶公司 | Coated tablet formulation and method |
| US7687638B2 (en) * | 2004-06-04 | 2010-03-30 | Takeda San Diego, Inc. | Dipeptidyl peptidase inhibitors |
| US9884038B2 (en) | 2004-06-07 | 2018-02-06 | University Of Tennessee Research Foundation | Selective androgen receptor modulator and methods of use thereof |
| US9889110B2 (en) | 2004-06-07 | 2018-02-13 | University Of Tennessee Research Foundation | Selective androgen receptor modulator for treating hormone-related conditions |
| US7572805B2 (en) | 2004-07-14 | 2009-08-11 | Bristol-Myers Squibb Company | Pyrrolo(oxo)isoquinolines as 5HT ligands |
| WO2006019965A2 (en) | 2004-07-16 | 2006-02-23 | Takeda San Diego, Inc. | Dipeptidyl peptidase inhibitors |
| AU2005267093B2 (en) * | 2004-07-23 | 2009-10-01 | Nuada Llc | Peptidase inhibitors |
| TW200608967A (en) | 2004-07-29 | 2006-03-16 | Sankyo Co | Pharmaceutical compositions containing with diabetic agent |
| US20060035954A1 (en) * | 2004-08-11 | 2006-02-16 | Sharma Padam N | Ammonolysis process for the preparation of intermediates for DPP IV inhibitors |
| AR051446A1 (en) * | 2004-09-23 | 2007-01-17 | Bristol Myers Squibb Co | C-ARYL GLUCOSIDS AS SELECTIVE INHIBITORS OF GLUCOSE CONVEYORS (SGLT2) |
| DE102004054054A1 (en) | 2004-11-05 | 2006-05-11 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Process for preparing chiral 8- (3-amino-piperidin-1-yl) -xanthines |
| WO2006068978A2 (en) * | 2004-12-21 | 2006-06-29 | Takeda Pharmaceutial Company Limited | Dipeptidyl peptidase inhibitors |
| US7635699B2 (en) * | 2004-12-29 | 2009-12-22 | Bristol-Myers Squibb Company | Azolopyrimidine-based inhibitors of dipeptidyl peptidase IV and methods |
| US7589088B2 (en) * | 2004-12-29 | 2009-09-15 | Bristol-Myers Squibb Company | Pyrimidine-based inhibitors of dipeptidyl peptidase IV and methods |
| DOP2006000008A (en) | 2005-01-10 | 2006-08-31 | Arena Pharm Inc | COMBINED THERAPY FOR THE TREATMENT OF DIABETES AND RELATED AFFECTIONS AND FOR THE TREATMENT OF AFFECTIONS THAT IMPROVE THROUGH AN INCREASE IN THE BLOOD CONCENTRATION OF GLP-1 |
| JP2008024592A (en) * | 2005-01-28 | 2008-02-07 | Taisho Pharmaceut Co Ltd | Cyanopyrrolidine derivative-containing composition for solid preparation, solid preparation containing the same, and method for producing the same |
| ATE421518T1 (en) * | 2005-02-10 | 2009-02-15 | Bristol Myers Squibb Co | DIHYDROQUINAZOLINONES AS 5HT MODULATORS |
| JP2008115080A (en) * | 2005-04-22 | 2008-05-22 | Taisho Pharmaceutical Co Ltd | Concomitant medication |
| US7521557B2 (en) | 2005-05-20 | 2009-04-21 | Bristol-Myers Squibb Company | Pyrrolopyridine-based inhibitors of dipeptidyl peptidase IV and methods |
| US20060264433A1 (en) * | 2005-05-23 | 2006-11-23 | Backes Bradley J | Pharmaceutical compositions as inhibitors of dipeptidyl peptidase-IV (DPP-IV) |
| US7825139B2 (en) * | 2005-05-25 | 2010-11-02 | Forest Laboratories Holdings Limited (BM) | Compounds and methods for selective inhibition of dipeptidyl peptidase-IV |
| MY152185A (en) | 2005-06-10 | 2014-08-29 | Novartis Ag | Modified release 1-[(3-hydroxy-adamant-1-ylamino)-acetyl]-pyrrolidine-2(s)-carbonitrile formulation |
| EP1910361A2 (en) * | 2005-07-28 | 2008-04-16 | Brystol-Myers Squibb Company | Substituted tetrahydro-1h-pyrido[4,3,b]indoles as serotonin receptor agonists and antagonists |
| DE102005035891A1 (en) | 2005-07-30 | 2007-02-08 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | 8- (3-amino-piperidin-1-yl) -xanthines, their preparation and their use as pharmaceuticals |
| TW200738245A (en) * | 2005-08-22 | 2007-10-16 | Sankyo Co | Pharmaceutical composition containing FBPase inhibitor |
| US7795436B2 (en) * | 2005-08-24 | 2010-09-14 | Bristol-Myers Squibb Company | Substituted tricyclic heterocycles as serotonin receptor agonists and antagonists |
| ES2376351T5 (en) * | 2005-09-14 | 2014-07-15 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors for the treatment of diabetes |
| US20070060529A1 (en) * | 2005-09-14 | 2007-03-15 | Christopher Ronald J | Administration of dipeptidyl peptidase inhibitors |
| PT1931350E (en) * | 2005-09-14 | 2014-02-12 | Takeda Pharmaceutical | ADMINISTRATION OF DIPEPTIDIL PEPTIDASE INHIBITORS |
| TW200745079A (en) * | 2005-09-16 | 2007-12-16 | Takeda Pharmaceuticals Co | Polymorphs of benzoate salt of 2-[[6-[(3R)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]-benzonitrile and methods of use therefor |
| TW200745080A (en) * | 2005-09-16 | 2007-12-16 | Takeda Pharmaceuticals Co | Polymorphs of tartrate salt of 2-[2-(3-(R)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6H-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor |
| CN102675221A (en) | 2005-09-16 | 2012-09-19 | 武田药品工业株式会社 | Intermediate in method for preparing pyrimidinedione derivative |
| TW200738266A (en) * | 2005-09-29 | 2007-10-16 | Sankyo Co | Pharmaceutical agent containing insulin resistance improving agent |
| WO2007053819A2 (en) | 2005-10-31 | 2007-05-10 | Bristol-Myers Squibb Company | Pyrrolidinyl beta-amino amide-based inhibitors of dipeptidyl peptidase iv and methods |
| WO2007054577A1 (en) * | 2005-11-14 | 2007-05-18 | Probiodrug Ag | Cyclopropyl-fused pyrrolidine derivatives as dipeptidyl peptidase iv inhibitors |
| GB0526291D0 (en) * | 2005-12-23 | 2006-02-01 | Prosidion Ltd | Therapeutic method |
| AU2007224066B2 (en) * | 2006-03-08 | 2011-10-27 | Kyorin Pharmaceutical Co., Ltd. | Method for producing aminoacetylpyrrolidinecarbonitrile derivative and production intermediate thereof |
| RU2465264C2 (en) * | 2006-03-16 | 2012-10-27 | Вертекс Фармасьютикалз Инкорпорейтед | Deuterated hepatitis c protease inhibitors |
| WO2007112347A1 (en) | 2006-03-28 | 2007-10-04 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| JP2009531456A (en) * | 2006-03-28 | 2009-09-03 | 武田薬品工業株式会社 | Preparation of (R) -3-aminopiperidine dihydrochloride |
| US20070238770A1 (en) * | 2006-04-05 | 2007-10-11 | Bristol-Myers Squibb Company | Process for preparing novel crystalline forms of peliglitazar, novel stable forms produced therein and formulations |
| EP2402750A1 (en) | 2006-04-11 | 2012-01-04 | Arena Pharmaceuticals, Inc. | Methods of using GPR119 receptor to identify compounds useful for increasing bone mass in an individual |
| PE20071221A1 (en) | 2006-04-11 | 2007-12-14 | Arena Pharm Inc | GPR119 RECEPTOR AGONISTS IN METHODS TO INCREASE BONE MASS AND TO TREAT OSTEOPOROSIS AND OTHER CONDITIONS CHARACTERIZED BY LOW BONE MASS, AND COMBINED THERAPY RELATED TO THESE AGONISTS |
| NZ571761A (en) | 2006-04-12 | 2010-07-30 | Probiodrug Ag | 5-phenylimidazoles |
| WO2007122744A1 (en) * | 2006-04-17 | 2007-11-01 | Sumitomo Chemical Company, Limited | Process for producing polycyclic proline derivative or acid adduct salt thereof |
| EP1852108A1 (en) | 2006-05-04 | 2007-11-07 | Boehringer Ingelheim Pharma GmbH & Co.KG | DPP IV inhibitor formulations |
| PE20080251A1 (en) | 2006-05-04 | 2008-04-25 | Boehringer Ingelheim Int | USES OF DPP IV INHIBITORS |
| WO2007128721A1 (en) | 2006-05-04 | 2007-11-15 | Boehringer Ingelheim Internationalgmbh | Polymorphs |
| US7919598B2 (en) | 2006-06-28 | 2011-04-05 | Bristol-Myers Squibb Company | Crystal structures of SGLT2 inhibitors and processes for preparing same |
| EA031888B1 (en) | 2006-07-12 | 2019-03-29 | Юниверсити Оф Теннесси Рисерч Фаундейшн | Methods of use of substituted acylanilides |
| US9730908B2 (en) | 2006-08-24 | 2017-08-15 | University Of Tennessee Research Foundation | SARMs and method of use thereof |
| US9844528B2 (en) | 2006-08-24 | 2017-12-19 | University Of Tennessee Research Foundation | SARMs and method of use thereof |
| EA200900392A1 (en) | 2006-09-07 | 2010-06-30 | Никомед Гмбх | COMBINED TREATMENT OF DIABETES MELLITUS |
| PE20121528A1 (en) * | 2006-09-13 | 2012-12-12 | Smithkline Beecham Corp | METHODS FOR ADMINISTERING LONG-LIVED HYPOGLYCEMIANT AGENTS |
| JP5791228B2 (en) * | 2006-09-13 | 2015-10-07 | 武田薬品工業株式会社 | Use of 2-6- (3-amino-piperidin-1-yl) -3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl-4-fluoro-benzonitrile |
| US8324383B2 (en) | 2006-09-13 | 2012-12-04 | Takeda Pharmaceutical Company Limited | Methods of making polymorphs of benzoate salt of 2-[[6-[(3R)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]-benzonitrile |
| JP2010503710A (en) | 2006-09-18 | 2010-02-04 | ラプトール ファーマシューティカル インコーポレイテッド | Treatment of liver damage by administration of receptor-related protein (RAP) conjugates |
| JP5379692B2 (en) | 2006-11-09 | 2013-12-25 | プロビオドルグ エージー | 3-Hydroxy-1,5-dihydro-pyrrol-2-one derivatives as inhibitors of glutaminyl cyclase for the treatment of ulcers, cancer and other diseases |
| US8217025B2 (en) * | 2006-11-17 | 2012-07-10 | Harbor Therapeutics, Inc. | Drug screening and treatment methods |
| TW200838536A (en) * | 2006-11-29 | 2008-10-01 | Takeda Pharmaceutical | Polymorphs of succinate salt of 2-[6-(3-amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethy]-4-fluor-benzonitrile and methods of use therefor |
| SI2091948T1 (en) | 2006-11-30 | 2012-07-31 | Probiodrug Ag | Novel inhibitors of glutaminyl cyclase |
| KR101638035B1 (en) | 2007-01-22 | 2016-07-11 | 지티엑스, 인코포레이티드 | Nuclear receptor binding agents |
| US9623021B2 (en) | 2007-01-22 | 2017-04-18 | Gtx, Inc. | Nuclear receptor binding agents |
| US9604931B2 (en) | 2007-01-22 | 2017-03-28 | Gtx, Inc. | Nuclear receptor binding agents |
| CN101230058A (en) * | 2007-01-23 | 2008-07-30 | 上海恒瑞医药有限公司 | Bicycle aza alkyl derivative, preparation method and use in medicine thereof |
| WO2008093882A1 (en) | 2007-02-01 | 2008-08-07 | Takeda Pharmaceutical Company Limited | Solid preparation comprising alogliptin and pioglitazone |
| US20110020797A1 (en) * | 2007-02-09 | 2011-01-27 | Bristol-Myers Squibb Company | Methods For Identifying Patients With An Increased Likelihood Of Responding To DPP-IV Inhibitors |
| US8093236B2 (en) | 2007-03-13 | 2012-01-10 | Takeda Pharmaceuticals Company Limited | Weekly administration of dipeptidyl peptidase inhibitors |
| US20080064701A1 (en) * | 2007-04-24 | 2008-03-13 | Ramesh Sesha | Anti-diabetic combinations |
| JPWO2008114857A1 (en) * | 2007-03-22 | 2010-07-08 | 杏林製薬株式会社 | Process for producing aminoacetylpyrrolidinecarbonitrile derivative |
| PE20090185A1 (en) | 2007-03-22 | 2009-02-28 | Bristol Myers Squibb Co | PHARMACEUTICAL FORMULATIONS CONTAINING AN SGLT2 INHIBITOR |
| ES2529149T3 (en) | 2007-04-03 | 2015-02-17 | Mitsubishi Tanabe Pharma Corporation | A combination of dipeptidyl peptidase IV inhibitor and sweetener for use in the treatment of obesity |
| JP5667440B2 (en) | 2007-04-18 | 2015-02-12 | プロビオドルグ エージー | Thiourea derivatives as glutaminyl cyclase inhibitors |
| PE20090696A1 (en) * | 2007-04-20 | 2009-06-20 | Bristol Myers Squibb Co | CRYSTALLINE FORMS OF SAXAGLIPTIN AND PROCESSES FOR PREPARING THEM |
| JP2010528023A (en) * | 2007-05-18 | 2010-08-19 | ブリストル−マイヤーズ スクイブ カンパニー | Crystal structure of SGLT2 inhibitor and method for producing the same |
| CN101318925A (en) * | 2007-06-04 | 2008-12-10 | 上海恒瑞医药有限公司 | Pyrrolidine-tetratomic ring derivants, preparation method and medical uses thereof |
| MX2009013354A (en) | 2007-06-04 | 2010-07-06 | Univ Ben Gurion | TRIARILO COMPOUNDS AND COMPOSITIONS THAT CONTAIN THEM. |
| JP2010530892A (en) * | 2007-06-22 | 2010-09-16 | ブリストル−マイヤーズ スクイブ カンパニー | Tablet composition containing atazanavir |
| MX2009013499A (en) * | 2007-06-22 | 2010-01-18 | Bristol Myers Squibb Co | Tableted compositions containing atazanavir. |
| WO2009002823A2 (en) * | 2007-06-22 | 2008-12-31 | Bristol-Myers Squibb Company | Tableted compositions containing atazanavir |
| DK2170292T3 (en) * | 2007-06-22 | 2014-04-07 | Bristol Myers Squibb Holdings Ireland | Tablet-containing Atazanavir Compositions |
| CL2008002427A1 (en) | 2007-08-16 | 2009-09-11 | Boehringer Ingelheim Int | Pharmaceutical composition comprising 1-chloro-4- (bd-glucopyranos-1-yl) -2- [4 - ((s) -tetrahydrofuran-3-yloxy) benzyl] -benzene combined with 1 - [(4-methylquinazolin- 2-yl) methyl] -3-methyl-7- (2-butyn-1-yl) -8- (3- (r) -aminopiperidin-1-yl) xanthine; and its use to treat type 2 diabetes mellitus. |
| KR101610005B1 (en) * | 2007-08-17 | 2016-04-08 | 베링거 인겔하임 인터내셔날 게엠베하 | Purin derivatives for use in the treatment of FAB-related diseases |
| US7968603B2 (en) | 2007-09-11 | 2011-06-28 | University Of Tennessee Research Foundation | Solid forms of selective androgen receptor modulators |
| US20090076118A1 (en) * | 2007-09-13 | 2009-03-19 | Protia, Llc | Deuterium-enriched saxagliptin |
| US8338450B2 (en) * | 2007-09-21 | 2012-12-25 | Lupin Limited | Compounds as dipeptidyl peptidase IV (DPP IV) inhibitors |
| CL2008003653A1 (en) | 2008-01-17 | 2010-03-05 | Mitsubishi Tanabe Pharma Corp | Use of a glucopyranosyl-derived sglt inhibitor and a selected dppiv inhibitor to treat diabetes; and pharmaceutical composition. |
| US8551524B2 (en) * | 2008-03-14 | 2013-10-08 | Iycus, Llc | Anti-diabetic combinations |
| PE20091730A1 (en) | 2008-04-03 | 2009-12-10 | Boehringer Ingelheim Int | FORMULATIONS INVOLVING A DPP4 INHIBITOR |
| EP2146210A1 (en) | 2008-04-07 | 2010-01-20 | Arena Pharmaceuticals, Inc. | Methods of using A G protein-coupled receptor to identify peptide YY (PYY) secretagogues and compounds useful in the treatment of conditions modulated by PYY |
| EP2993239A1 (en) | 2008-05-16 | 2016-03-09 | AstraZeneca AB | Methods for identifying subjects with an increased likelihood of responding to dpp-iv inhibitors |
| PE20100156A1 (en) * | 2008-06-03 | 2010-02-23 | Boehringer Ingelheim Int | NAFLD TREATMENT |
| WO2009155481A1 (en) | 2008-06-20 | 2009-12-23 | Gtx, Inc. | Metabolites of selective androgen receptor modulators and methods of use thereof |
| BRPI0916997A2 (en) | 2008-08-06 | 2020-12-15 | Boehringer Ingelheim International Gmbh | DPP-4 INHIBITOR AND ITS USE |
| UY32030A (en) | 2008-08-06 | 2010-03-26 | Boehringer Ingelheim Int | "TREATMENT FOR DIABETES IN INAPPROPRIATE PATIENTS FOR THERAPY WITH METFORMIN" |
| EP2322499A4 (en) * | 2008-08-07 | 2011-12-21 | Kyorin Seiyaku Kk | PROCESS FOR PRODUCING A BICYCLOÝ2.2.2¨OCTYLAMINE DERIVATIVE |
| RU2011108420A (en) * | 2008-08-14 | 2012-09-20 | Киорин Фармасьютикал Ко., Лтд. (Jp) | STABILIZED PHARMACEUTICAL COMPOSITION |
| NZ604091A (en) | 2008-08-15 | 2014-08-29 | Boehringer Ingelheim Int | Purin derivatives for use in the treatment of fab-related diseases |
| JP2012502081A (en) | 2008-09-10 | 2012-01-26 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Combination therapy for the treatment of diabetes and related symptoms |
| US20200155558A1 (en) | 2018-11-20 | 2020-05-21 | Boehringer Ingelheim International Gmbh | Treatment for diabetes in patients with insufficient glycemic control despite therapy with an oral antidiabetic drug |
| ES2772731T3 (en) | 2008-10-17 | 2020-07-08 | Sanofi Aventis Deutschland | Combination of an insulin and a GLP-1 agonist |
| US8889618B2 (en) | 2008-11-07 | 2014-11-18 | The General Hospital Corporation | C-terminal fragments of glucagon-like peptide-1 (GLP-1) |
| JP2012509277A (en) * | 2008-11-19 | 2012-04-19 | オースペックス・ファーマシューティカルズ・インコーポレイテッド | Hydroxyadamantyl inhibitor of dipeptidyl peptidase IV |
| US20100144140A1 (en) * | 2008-12-10 | 2010-06-10 | Novellus Systems, Inc. | Methods for depositing tungsten films having low resistivity for gapfill applications |
| DE102008062136B4 (en) | 2008-12-16 | 2012-05-03 | Kamamed Ug | Pharmaceutical composition based on peptide of camel milk |
| CN102256976A (en) | 2008-12-23 | 2011-11-23 | 贝林格尔.英格海姆国际有限公司 | Salt Forms of Organic Compounds |
| TW201036975A (en) | 2009-01-07 | 2010-10-16 | Boehringer Ingelheim Int | Treatment for diabetes in patients with inadequate glycemic control despite metformin therapy |
| US8706650B2 (en) * | 2009-01-14 | 2014-04-22 | Integral Analytics, Inc. | Optimization of microgrid energy use and distribution |
| TWI466672B (en) | 2009-01-29 | 2015-01-01 | Boehringer Ingelheim Int | Treatment for diabetes in paediatric patients |
| HUE050287T2 (en) | 2009-02-13 | 2020-11-30 | Boehringer Ingelheim Int | Pharmaceutical composition comprisng a sglt2 inhibitor, a dpp-iv inhibitor and optionally a further antidiabetic agent and uses thereof |
| KR20110115582A (en) | 2009-02-13 | 2011-10-21 | 베링거 인겔하임 인터내셔날 게엠베하 | Antidiabetic agents comprising a DPP-4 inhibitor (linagliptin), optionally in combination with other antidiabetics |
| CA2756428A1 (en) | 2009-03-27 | 2010-09-30 | Mamoru Fukuda | Matrix-type sustained release preparation containing basic additive |
| EP2411005A1 (en) * | 2009-03-27 | 2012-02-01 | Bristol-Myers Squibb Company | Methods for preventing major adverse cardiovascular events with dpp-iv inhibitors |
| KR101292379B1 (en) * | 2009-04-08 | 2013-08-09 | 브리스톨-마이어스 스큅 컴퍼니 | A genetically stable plasmid expressing pdh and fdh enzymes |
| RU2539590C2 (en) | 2009-04-09 | 2015-01-20 | Сандоз Аг | Crystalline forms of saxagliptin |
| US8748457B2 (en) | 2009-06-18 | 2014-06-10 | Lupin Limited | 2-amino-2- [8-(dimethyl carbamoyl)- 8-aza- bicyclo [3.2.1] oct-3-yl]-exo- ethanoyl derivatives as potent DPP-IV inhibitors |
| AR077642A1 (en) | 2009-07-09 | 2011-09-14 | Arena Pharm Inc | METABOLISM MODULATORS AND THE TREATMENT OF DISORDERS RELATED TO THE SAME |
| ES2548913T3 (en) | 2009-09-11 | 2015-10-21 | Probiodrug Ag | Heterocyclic derivatives such as glutaminyl cyclase inhibitors |
| EP2308847B1 (en) | 2009-10-09 | 2014-04-02 | EMC microcollections GmbH | Substituted pyridines as inhibitors of dipeptidyl peptidase IV and their application for the treatment of diabetes and related diseases |
| CN105193761B (en) | 2009-11-13 | 2019-12-06 | 阿斯利康(瑞典)有限公司 | Bilayer tablet |
| AR078973A1 (en) | 2009-11-13 | 2011-12-14 | Sanofi Aventis Deutschland | PHARMACEUTICAL COMPOSITION THAT INCLUDES AN GLP-1 AND METIONINE AGONIST |
| MX2012005365A (en) | 2009-11-13 | 2012-05-29 | Bristol Myers Squibb Co | IMMEDIATE RELEASE TABLET FORMULATIONS. |
| MY180661A (en) | 2009-11-13 | 2020-12-04 | Sanofi Aventis Deutschland | Pharmaceutical composition comprising a glp-1 agonist, an insulin and methionine |
| EP2504002B1 (en) | 2009-11-27 | 2019-10-09 | Boehringer Ingelheim International GmbH | Treatment of genotyped diabetic patients with dpp-iv inhibitors such as linagliptin |
| TWI562775B (en) | 2010-03-02 | 2016-12-21 | Lexicon Pharmaceuticals Inc | Methods of using inhibitors of sodium-glucose cotransporters 1 and 2 |
| US9181233B2 (en) | 2010-03-03 | 2015-11-10 | Probiodrug Ag | Inhibitors of glutaminyl cyclase |
| EP2545047B9 (en) | 2010-03-10 | 2015-06-10 | Probiodrug AG | Heterocyclic inhibitors of glutaminyl cyclase (qc, ec 2.3.2.5) |
| EP2547339A1 (en) | 2010-03-18 | 2013-01-23 | Boehringer Ingelheim International GmbH | Combination of a gpr119 agonist and the dpp-iv inhibitor linagliptin for use in the treatment of diabetes and related conditions |
| EP2368874A1 (en) | 2010-03-26 | 2011-09-28 | Sandoz AG | Racemisation of (R)-N-Boc-3-hydroxyadamant-1-yl glycine |
| PH12012501974A1 (en) * | 2010-04-05 | 2013-01-07 | Cadila Pharmaceuticals Ltd | Novel hypoglycemic compounds |
| JP2013523819A (en) | 2010-04-06 | 2013-06-17 | アリーナ ファーマシューティカルズ, インコーポレイテッド | GPR119 receptor modulators and treatment of disorders related thereto |
| JP5945532B2 (en) | 2010-04-21 | 2016-07-05 | プロビオドルグ エージー | Benzimidazole derivatives as inhibitors of glutaminyl cyclase |
| US9186392B2 (en) | 2010-05-05 | 2015-11-17 | Boehringer Ingelheim International Gmbh | Combination therapy |
| WO2011140328A1 (en) | 2010-05-05 | 2011-11-10 | Teva Pharmaceutical Industries Ltd. | Saxagliptin intermediates, saxagliptin polymorphs, and processes for preparation thereof |
| BR112012032579B1 (en) | 2010-06-24 | 2021-05-11 | Boehringer Ingelheim International Gmbh | use of linagliptin and pharmaceutical composition comprising linagliptin and long-acting basal insulin |
| WO2012017029A1 (en) | 2010-08-06 | 2012-02-09 | Sandoz Ag | Novel salts of saxagrliptin with organic di-acids |
| WO2012017028A1 (en) | 2010-08-06 | 2012-02-09 | Sandoz Ag | A novel crystalline compound comprising saxagliptin and phosphoric acid |
| HUE031181T2 (en) | 2010-08-30 | 2017-06-28 | Sanofi Aventis Deutschland | Use of ave0010 for the manufacture of a medicament for the treatment of diabetes mellitus type 2 |
| KR20130137624A (en) | 2010-09-03 | 2013-12-17 | 브리스톨-마이어스 스큅 컴퍼니 | Drug formulations using water soluble antioxidants |
| EP2611770A1 (en) | 2010-09-03 | 2013-07-10 | Sandoz AG | Process for the reductive amination of -keto carboxylic acids |
| US9616097B2 (en) | 2010-09-15 | 2017-04-11 | Synergy Pharmaceuticals, Inc. | Formulations of guanylate cyclase C agonists and methods of use |
| CN103221410B (en) | 2010-09-22 | 2017-09-15 | 艾尼纳制药公司 | GPR119 receptor modulators and treatment of disorders associated therewith |
| US20120077778A1 (en) | 2010-09-29 | 2012-03-29 | Andrea Bourdelais | Ladder-Frame Polyether Conjugates |
| WO2012047871A1 (en) | 2010-10-04 | 2012-04-12 | Assia Chemical Industries Ltd | Polymorphs of saxagliptin hydrochloride and processes for preparing them |
| WO2012061466A2 (en) | 2010-11-02 | 2012-05-10 | The General Hospital Corporation | Methods for treating steatotic disease |
| US9034883B2 (en) | 2010-11-15 | 2015-05-19 | Boehringer Ingelheim International Gmbh | Vasoprotective and cardioprotective antidiabetic therapy |
| TWI631963B (en) | 2011-01-05 | 2018-08-11 | 雷西肯製藥股份有限公司 | Composition and application method comprising inhibitors of sodium-glucose co-transporters 1 and 2 |
| EP3009136A1 (en) | 2011-01-31 | 2016-04-20 | Cadila Healthcare Limited | Treatment for lipodystrophy |
| MX2013008372A (en) | 2011-02-01 | 2013-08-12 | Astrazeneca Uk Ltd | Pharmaceutical formulations including an amine compound. |
| US8530670B2 (en) | 2011-03-16 | 2013-09-10 | Probiodrug Ag | Inhibitors |
| WO2012135570A1 (en) | 2011-04-01 | 2012-10-04 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012145361A1 (en) | 2011-04-19 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| US20140051714A1 (en) | 2011-04-22 | 2014-02-20 | Arena Pharmaceuticals, Inc. | Modulators Of The GPR119 Receptor And The Treatment Of Disorders Related Thereto |
| WO2012145603A1 (en) | 2011-04-22 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| US9821032B2 (en) | 2011-05-13 | 2017-11-21 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical combination for improving glycemic control as add-on therapy to basal insulin |
| WO2012170702A1 (en) | 2011-06-08 | 2012-12-13 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2013006692A2 (en) | 2011-07-06 | 2013-01-10 | The General Hospital Corporation | Methods of treatment using a pentapeptide derived from the c-terminus of glucagon-like peptide 1 (glp-1) |
| PL2731947T3 (en) | 2011-07-15 | 2019-07-31 | Boehringer Ingelheim International Gmbh | Substituted dimeric quinazoline derivative, its preparation and its use in pharmaceutical compositions for the treatment of type i and ii diabetes |
| CN103917241A (en) | 2011-08-29 | 2014-07-09 | 赛诺菲-安万特德国有限公司 | Drug combinations for glycemic control in patients with type 2 diabetes |
| AR087744A1 (en) | 2011-09-01 | 2014-04-16 | Sanofi Aventis Deutschland | PHARMACEUTICAL COMPOSITION FOR USE IN THE TREATMENT OF A NEURODEGENERATIVE DISEASE |
| US20140302150A1 (en) | 2011-09-07 | 2014-10-09 | Umit Cifter | Dpp-iv inhibitor formulations |
| EP2578208B1 (en) | 2011-10-06 | 2014-05-21 | Sanovel Ilac Sanayi ve Ticaret A.S. | DPP-IV inhibitor solid dosage formulations |
| WO2013055910A1 (en) | 2011-10-12 | 2013-04-18 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2013081100A1 (en) * | 2011-11-30 | 2013-06-06 | 積水メディカル株式会社 | Adamantyl hydantoin compound |
| US9115082B2 (en) | 2012-01-18 | 2015-08-25 | Catherine Yang | Dipeptidyl-peptidase-IV inhibitors for treatment of type 2 diabetes complex with hypertension |
| US9555001B2 (en) | 2012-03-07 | 2017-01-31 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition and uses thereof |
| US20150087686A1 (en) | 2012-04-25 | 2015-03-26 | Enantia, S.L. | Crystalline forms of saxagliptin |
| EP2849755A1 (en) | 2012-05-14 | 2015-03-25 | Boehringer Ingelheim International GmbH | A xanthine derivative as dpp -4 inhibitor for use in the treatment of podocytes related disorders and/or nephrotic syndrome |
| EP4151218A1 (en) | 2012-05-14 | 2023-03-22 | Boehringer Ingelheim International GmbH | Linagliptin, a xanthine derivative as dpp-4 inhibitor, for use in the treatment of sirs and/or sepsis |
| US8664443B2 (en) | 2012-05-23 | 2014-03-04 | Divi's Laboratories Ltd. | Process for the preparation of (1S, 3S, 5S)-2-[2(S)-2-amino-2-(3-hydroxy-1-adamantan-1-yl) acetyl]-2-azabicyclo [3.1.0] hexane-3-carbonitrile |
| ES2690321T3 (en) | 2012-05-24 | 2018-11-20 | Apotex Inc. | Saxagliptin salts with organic acids |
| WO2013174767A1 (en) | 2012-05-24 | 2013-11-28 | Boehringer Ingelheim International Gmbh | A xanthine derivative as dpp -4 inhibitor for use in modifying food intake and regulating food preference |
| CA2879824A1 (en) | 2012-07-02 | 2014-01-09 | Ranbaxy Laboratories Limited | Saxagliptin salts |
| CN103539724B (en) * | 2012-07-12 | 2017-09-26 | 博瑞生物医药(苏州)股份有限公司 | The novel crystal forms and purification process of BMS-477118 single stereoisomers |
| PT2872482T (en) | 2012-07-13 | 2020-09-22 | Oncternal Therapeutics Inc | A method of treating androgen receptor (ar)-positive breast cancers with selective androgen receptor modulator (sarms) |
| US9969683B2 (en) | 2012-07-13 | 2018-05-15 | Gtx, Inc. | Method of treating estrogen receptor (ER)-positive breast cancers with selective androgen receptor modulator (SARMS) |
| US9744149B2 (en) | 2012-07-13 | 2017-08-29 | Gtx, Inc. | Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs) |
| US10987334B2 (en) | 2012-07-13 | 2021-04-27 | University Of Tennessee Research Foundation | Method of treating ER mutant expressing breast cancers with selective androgen receptor modulators (SARMs) |
| US10314807B2 (en) | 2012-07-13 | 2019-06-11 | Gtx, Inc. | Method of treating HER2-positive breast cancers with selective androgen receptor modulators (SARMS) |
| US9622992B2 (en) | 2012-07-13 | 2017-04-18 | Gtx, Inc. | Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs) |
| US10258596B2 (en) | 2012-07-13 | 2019-04-16 | Gtx, Inc. | Method of treating HER2-positive breast cancers with selective androgen receptor modulators (SARMS) |
| WO2014030051A1 (en) | 2012-08-23 | 2014-02-27 | Aurobindo Pharma Limited | Stable pharmaceutical compositions comprising saxagliptin |
| WO2014057495A1 (en) | 2012-10-11 | 2014-04-17 | Lee Pharma Limited | A process for industrial preparation of [(s)-n-tert butoxycarbonyl-3-hydroxy]adamantylglycine |
| TWI500613B (en) | 2012-10-17 | 2015-09-21 | Cadila Healthcare Ltd | Novel heterocyclic compounds |
| WO2014074668A1 (en) | 2012-11-08 | 2014-05-15 | Arena Pharmaceuticals, Inc. | Modulators of gpr119 and the treatment of disorders related thereto |
| WO2014081660A1 (en) | 2012-11-20 | 2014-05-30 | Lexicon Pharmaceuticals, Inc. | Inhibitors of sodium glucose cotransporter 1 |
| US20150250734A1 (en) | 2012-12-21 | 2015-09-10 | Wockhardt Limited | Stable pharmaceutical compositions of saxagliptin or salts thereof |
| WO2014096983A1 (en) | 2012-12-21 | 2014-06-26 | Wockhardt Limited | Stable pharmaceutical compositions of saxagliptin or salts thereof |
| WO2014108830A1 (en) | 2013-01-10 | 2014-07-17 | Wockhardt Limited | A process for preparing pharmaceutically acceptable salt of saxagliptin |
| ITMI20130132A1 (en) | 2013-01-30 | 2014-07-31 | Laboratorio Chimico Int Spa | PROCEDURE FOR THE PREPARATION OF INTERMEDIATES OF SAXAGLIPTINA SYNTHESIS AND NEW COMPOUNDS |
| TWI641381B (en) | 2013-02-04 | 2018-11-21 | 法商賽諾菲公司 | Stabilized pharmaceutical formulations of insulin analogs and/or insulin derivatives |
| US8652527B1 (en) | 2013-03-13 | 2014-02-18 | Upsher-Smith Laboratories, Inc | Extended-release topiramate capsules |
| US9101545B2 (en) | 2013-03-15 | 2015-08-11 | Upsher-Smith Laboratories, Inc. | Extended-release topiramate capsules |
| CN104059068B (en) * | 2013-03-20 | 2017-02-08 | 中国科学院上海药物研究所 | Beta-amino-carbonyl compound, preparation method, its pharmaceutical composition and application thereof |
| CN104098481B (en) * | 2013-04-10 | 2016-05-11 | 浙江九洲药物科技有限公司 | A kind of preparation method of BMS-477118 intermediate |
| US20160166539A1 (en) | 2013-04-22 | 2016-06-16 | Cadila Healthcare Limited | A novel composition for nonalcoholic fatty liver disease (nafld) |
| WO2014193528A1 (en) * | 2013-04-29 | 2014-12-04 | Anovel Pharmaceuticals, Llc | Amorphous dosage forms and methods |
| WO2014195967A2 (en) | 2013-05-30 | 2014-12-11 | Cadila Healthcare Limited | A process for preparation of pyrroles having hypolipidemic hypocholesteremic activities |
| WO2014197720A2 (en) | 2013-06-05 | 2014-12-11 | Synergy Pharmaceuticals, Inc. | Ultra-pure agonists of guanylate cyclase c, method of making and using same |
| TW201636015A (en) | 2013-07-05 | 2016-10-16 | 卡地拉保健有限公司 | Synergistic compositions |
| IN2013MU02470A (en) | 2013-07-25 | 2015-06-26 | Cadila Healthcare Ltd | |
| EP2832723B1 (en) | 2013-07-29 | 2017-02-15 | Zentiva, a.s. | Stabilised amorphous forms of Saxagliptin |
| IN2013MU02813A (en) * | 2013-08-28 | 2015-07-03 | Amneal Pharmaceuticals Llc | |
| US10112898B2 (en) | 2013-09-06 | 2018-10-30 | Cadila Healthcare Limited | Process for the preparation of saroglitazar pharmaceutical salts |
| ITMI20131677A1 (en) * | 2013-10-10 | 2015-04-11 | Olon Spa | PROCEDURE FOR THE PREPARATION OF SAXAGLIPTINA |
| CA2928725A1 (en) | 2013-11-05 | 2015-05-14 | Esther Priel | Compounds for the treatment of diabetes and disease complications arising from same |
| WO2015067223A1 (en) | 2013-11-06 | 2015-05-14 | Zentiva, K., S. | L-tartrate salt of (1s,3s,5s)-2-[(2s)-2-amino-2-(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl) acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile and process for preparation thereof |
| WO2015071887A1 (en) | 2013-11-18 | 2015-05-21 | Ranbaxy Laboratories Limited | Oral pharmaceutical compositions of saxagliptin |
| WO2015071889A1 (en) | 2013-11-18 | 2015-05-21 | Ranbaxy Laboratories Limited | Oral compositions of saxagliptin |
| WO2015087262A1 (en) | 2013-12-11 | 2015-06-18 | Ranbaxy Laboratories Limited | Process for the preparation of saxagliptin and its intermediates |
| KR20160101195A (en) | 2014-01-09 | 2016-08-24 | 사노피 | Stabilized pharmaceutical formulations of insulin aspart |
| EP3091965A1 (en) | 2014-01-09 | 2016-11-16 | Sanofi | Stabilized glycerol free pharmaceutical formulations of insulin analogues and/or insulin derivatives |
| WO2015104314A1 (en) | 2014-01-09 | 2015-07-16 | Sanofi | Stabilized pharmaceutical formulations of insulin analogues and/or insulin derivatives |
| JP6615109B2 (en) | 2014-02-28 | 2019-12-04 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Medical use of DPP-4 inhibitors |
| CZ2014177A3 (en) * | 2014-03-24 | 2015-10-07 | Zentiva, K.S. | Process for preparing saxagliptin |
| AU2015265571A1 (en) | 2014-05-30 | 2016-11-17 | Pfizer Inc. | Carbonitrile derivatives as selective androgen receptor modulators |
| GB201415598D0 (en) | 2014-09-03 | 2014-10-15 | Univ Birmingham | Elavated Itercranial Pressure Treatment |
| CN104557667A (en) * | 2014-12-12 | 2015-04-29 | 山东省药学科学院 | 2-cyano pyrrolidine compound, and preparation method and application thereof |
| ES2949095T3 (en) | 2014-12-12 | 2023-09-25 | Sanofi Aventis Deutschland | Insulin glargine/lixisenatide fixed ratio formulation |
| WO2016144862A1 (en) | 2015-03-09 | 2016-09-15 | Intekrin Therapeutics, Inc. | Methods for the treatment of nonalcoholic fatty liver disease and/or lipodystrophy |
| TWI748945B (en) | 2015-03-13 | 2021-12-11 | 德商賽諾菲阿凡提斯德意志有限公司 | Treatment type 2 diabetes mellitus patients |
| TW201705975A (en) | 2015-03-18 | 2017-02-16 | 賽諾菲阿凡提斯德意志有限公司 | Treatment of type 2 diabetes mellitus patients |
| CN105037245B (en) * | 2015-08-03 | 2017-04-12 | 沧州那瑞化学科技有限公司 | Saxagliptin midbody preparing method |
| WO2017064635A2 (en) | 2015-10-14 | 2017-04-20 | Cadila Healthcare Limited | Pyrrole compound, compositions and process for preparation thereof |
| KR101715682B1 (en) | 2015-10-29 | 2017-03-13 | 경동제약 주식회사 | Novel intermediates for preparing saxagliptin, preparing methods thereof and preparing methods of saxagliptin using the same |
| CN109310697A (en) | 2016-06-10 | 2019-02-05 | 勃林格殷格翰国际有限公司 | Combination of linagliptin and metformin |
| EP3509604A4 (en) | 2016-09-07 | 2020-08-26 | Trustees of Tufts College | COMBINATION THERAPIES WITH IMMUNO DASH INHIBITORS AND PGE2 ANTAGONISTS |
| ES2894261T3 (en) | 2016-12-09 | 2022-02-14 | Cadila Healthcare Ltd | Treatment of primary biliary cholangitis |
| WO2018162722A1 (en) | 2017-03-09 | 2018-09-13 | Deutsches Institut Für Ernährungsforschung Potsdam-Rehbrücke | Dpp-4 inhibitors for use in treating bone fractures |
| KR20200036808A (en) | 2017-04-03 | 2020-04-07 | 코히러스 바이오사이언시스, 인크. | PPARγ agonist for the treatment of advanced nuclear paralysis |
| ES2812698T3 (en) | 2017-09-29 | 2021-03-18 | Probiodrug Ag | Glutaminyl cyclase inhibitors |
| CN109970620B (en) * | 2017-12-27 | 2022-07-12 | 江苏威凯尔医药科技有限公司 | Method for preparing saxagliptin intermediate |
| EP3807894A1 (en) | 2018-06-14 | 2021-04-21 | AstraZeneca UK Limited | Methods for lowering blood sugar with a dipeptidyl peptidase-4 inhibitor pharmaceutical composition |
| PE20210644A1 (en) | 2018-07-19 | 2021-03-23 | Astrazeneca Ab | METHODS OF TREATMENT OF HFpEF USING DAPAGLIFLOZIN AND COMPOSITIONS INCLUDING THE SAME |
| CN113039176A (en) | 2018-09-26 | 2021-06-25 | 莱西肯医药有限公司 | Crystalline forms of N- (1- ((2- (dimethylamino) ethyl) amino) -2-methyl-1-oxoprop-2-yl) -4- (4- (2-methyl-5- ((2S,3R,4R,5S,6R) -3,4, 5-trihydroxy-6- (methylthio) tetrahydro-2H-pyran-2-yl) benzyl) phenyl) butanamide and methods of synthesis thereof |
| RU2712097C1 (en) * | 2018-09-28 | 2020-01-24 | Общество с ограниченной ответственностью "Необиотек" | Dipeptidyl peptidase-4 inhibitor for treating type 2 diabetes mellitus, compounds (versions) |
| RU2727898C1 (en) * | 2020-02-25 | 2020-07-24 | Общество с ограниченной ответственностью «Необиотек» | Pharmaceutical composition based on an active substance, an inhibitor of dipeptidyl peptidase-4, for preventing the development and treatment of type 2 diabetes mellitus |
| BR112021008094A2 (en) | 2020-07-27 | 2022-02-22 | Astrazeneca Ab | Methods of treating chronic kidney disease with dapagliflozin |
| TW202220672A (en) | 2020-07-27 | 2022-06-01 | 瑞典商阿斯特捷利康公司 | Methods of treating chronic kidney disease with dapagliflozin |
| WO2023275715A1 (en) | 2021-06-30 | 2023-01-05 | Pfizer Inc. | Metabolites of selective androgen receptor modulators |
| CN113666846B (en) * | 2021-08-31 | 2023-06-27 | 济南立德医药技术有限公司 | Synthesis method of saxagliptin intermediate |
| US20250108065A1 (en) | 2022-01-26 | 2025-04-03 | Astrazeneca Ab | Methods of treating prediabetes or reducing the risk of developing type 2 diabetes with dapagliflozin |
| WO2025090919A1 (en) * | 2023-10-25 | 2025-05-01 | Piton Therapeutics, Inc. | Compositions and methods for treating inflammatory bowel disease |
Citations (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3325478A (en) | 1964-11-17 | 1967-06-13 | Du Pont | alpha-amino-1-adamantylmethyl penicillins |
| DE2449840A1 (en) | 1973-10-19 | 1975-04-24 | Kao Corp | ADAMANTYLAMIDINE AND THE PROCESS FOR THEIR MANUFACTURE |
| DE2521895A1 (en) | 1974-05-16 | 1976-04-08 | Pliva Pharm & Chem Works | Alpha-Amino-2-adamantylacetic acid prepn - from 2-adamantylacetic acid via alpha-bromo-2-adamantylacetic acid |
| EP0007652A1 (en) | 1978-06-27 | 1980-02-06 | Shell Internationale Researchmaatschappij B.V. | Derivatives of 3-azabicyclo(3.1.0)hexane and a process for their preparation |
| US4379785A (en) | 1979-12-19 | 1983-04-12 | Hoechst Aktiengesellschaft | Heterocyclic substituted sulfonyl ureas, and their use |
| DE3324263A1 (en) | 1983-07-06 | 1985-01-17 | Hoechst Ag, 6230 Frankfurt | DERIVATIVES OF 2-AZABICYCLO (3.1.0) HEXAN-3-CARBONIC ACID, METHOD FOR THE PRODUCTION THEREOF, THEIR SUBSTANCES AND THE USE THEREOF, AND 2-AZABICYCLO (3.1.0) HEXANE DERIVATIVES AS INTERMEDIATE PRODUCTS AND PROCESS PRODUCTS |
| EP0219782A2 (en) | 1985-10-15 | 1987-04-29 | Hoechst Aktiengesellschaft | Use of angiotensin-converting enzyme inhibitors in the treatment of atherosclerosis, thrombosis and peripheral vascular disease |
| DE3926606A1 (en) | 1989-08-11 | 1991-02-14 | Hoechst Ag | METHOD FOR THE TREATMENT OF CARDIALS AND VASCULAR HYPERTROPHY AND HYPERPLASIA |
| US5462928A (en) | 1990-04-14 | 1995-10-31 | New England Medical Center Hospitals, Inc. | Inhibitors of dipeptidyl-aminopeptidase type IV |
| EP0686642A2 (en) | 1994-06-10 | 1995-12-13 | Bristol-Myers Squibb Company | Modified guanidino and amidino thrombin inhibitors |
| WO1997015576A1 (en) | 1995-10-25 | 1997-05-01 | E.I. Du Pont De Nemours And Company | Herbicidal sulfonamides |
| WO1997040832A1 (en) | 1996-04-25 | 1997-11-06 | Probiodrug Gesellschaft für Arzneimittelforschung mbH | Use of dipeptidyl peptidase iv effectors for lowering the blood glucose level in mammals |
| WO1999026659A1 (en) | 1997-11-21 | 1999-06-03 | Pfizer Products Inc. | Combination of an aldose reductase inhibitor and a glycogen phosphorylase inhibitor |
| WO1999038501A2 (en) | 1998-02-02 | 1999-08-05 | Trustees Of Tufts College | Method of regulating glucose metabolism, and reagents related thereto |
| US5939560A (en) | 1993-12-03 | 1999-08-17 | Ferring B.V. | Inhibitors of DP-mediated processes, compositions and therapeutic methods thereof |
| WO1999047545A2 (en) | 1998-03-19 | 1999-09-23 | Vertex Pharmaceuticals Incorporated | Inhibitors of caspases |
| US5998463A (en) | 1998-02-27 | 1999-12-07 | Pfizer Inc | Glycogen phosphorylase inhibitors |
| WO1999067279A1 (en) | 1998-06-24 | 1999-12-29 | Probiodrug Gesellschaft für Arzneimittelforschung mbH | Compounds of unstable dipeptidyl peptidase iv inhibitors |
| US6011155A (en) | 1996-11-07 | 2000-01-04 | Novartis Ag | N-(substituted glycyl)-2-cyanopyrrolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV |
| WO2000010549A1 (en) | 1998-08-21 | 2000-03-02 | Point Therapeutics, Inc. | Regulation of substrate activity |
| WO2000047207A1 (en) | 1999-02-09 | 2000-08-17 | Bristol-Myers Squibb Company | LACTAM INHIBITORS OF FXa AND METHOD |
| US6110949A (en) | 1999-06-24 | 2000-08-29 | Novartis Ag | N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV |
| WO2000053171A1 (en) | 1999-03-05 | 2000-09-14 | Molteni L. E C. Dei Fratelli Alitti Societa' Di Esercizio S.P.A. | Use of metformin in the preparation of pharmaceutical compositions capable of inhibiting the enzyme dipeptidyl peptidase iv |
| WO2000056297A2 (en) | 1999-03-23 | 2000-09-28 | Ferring B.V. | Pharmaceutical compositions comprising dipeptidy peptidase iv inhibitors for the promotion of growth |
| WO2000056296A2 (en) | 1999-03-23 | 2000-09-28 | Ferring Bv | Compositions for improving fertility |
| EP1050540A2 (en) | 1991-10-22 | 2000-11-08 | New England Medical Center Hospitals, Inc. | Inhibitors of dipeptidyl-aminopeptidase type IV |
| WO2000069868A1 (en) | 1999-05-17 | 2000-11-23 | Zaidan Hojin Biseibutsu Kagaku Kenkyu Kai | Sulphostin analogues and processes for the preparation of sulphostin and analogues thereof |
| US6166063A (en) | 1998-12-10 | 2000-12-26 | Novartis Ag | N-(substituted glycyl)-2-cyanopyrrolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV |
| WO2002060894A2 (en) | 2001-01-30 | 2002-08-08 | Bristol-Myers Squibb Company | Sulfonamide lactam inhibitors of factor xa |
| WO2003004241A1 (en) | 2001-07-07 | 2003-01-16 | Trench Germany Gmbh | Method and device for producing an electric insulator made from plastic |
| US20060287317A1 (en) | 2005-06-17 | 2006-12-21 | Apogee Biotechnology Corporation | Sphingosine kinase inhibitors |
| US7205432B2 (en) | 2005-05-31 | 2007-04-17 | Kemfine Oy | Process for the preparation of adamantane derivatives |
| US7250529B2 (en) | 2004-09-17 | 2007-07-31 | Albemarle Corporation | Synthesis process for 2-(3-hydroxy-1-adamantyl)-2-oxoacetic acid |
Family Cites Families (57)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3674836A (en) | 1968-05-21 | 1972-07-04 | Parke Davis & Co | 2,2-dimethyl-{11 -aryloxy-alkanoic acids and salts and esters thereof |
| US4027009A (en) | 1973-06-11 | 1977-05-31 | Merck & Co., Inc. | Compositions and methods for depressing blood serum cholesterol |
| JPS5612114B2 (en) | 1974-06-07 | 1981-03-18 | ||
| US4183857A (en) | 1978-07-06 | 1980-01-15 | Shell Oil Company | 3-Benzyl-3-azabicyclo(3.1.0)hexane-2,4-dione |
| NO154918C (en) | 1977-08-27 | 1987-01-14 | Bayer Ag | ANALOGUE PROCEDURE FOR THE PREPARATION OF THERAPEUTIC ACTIVE DERIVATIVES OF 3,4,5-TRIHYDROXYPIPERIDINE. |
| US4231938A (en) | 1979-06-15 | 1980-11-04 | Merck & Co., Inc. | Hypocholesteremic fermentation products and process of preparation |
| MX7065E (en) | 1980-06-06 | 1987-04-10 | Sankyo Co | A MICROBIOLOGICAL PROCEDURE FOR PREPARING DERIVATIVES OF ML-236B |
| US4450171A (en) | 1980-08-05 | 1984-05-22 | Merck & Co., Inc. | Antihypercholesterolemic compounds |
| US4448784A (en) | 1982-04-12 | 1984-05-15 | Hoechst-Roussel Pharmaceuticals, Inc. | 1-(Aminoalkylphenyl and aminoalkylbenzyl)-indoles and indolines and analgesic method of use thereof |
| US5354772A (en) | 1982-11-22 | 1994-10-11 | Sandoz Pharm. Corp. | Indole analogs of mevalonolactone and derivatives thereof |
| JPS6051189A (en) | 1983-08-30 | 1985-03-22 | Sankyo Co Ltd | Thiazolidine derivative and its preparation |
| DE3543999A1 (en) | 1985-12-13 | 1987-06-19 | Bayer Ag | HIGH PURITY ACARBOSE |
| US5614492A (en) | 1986-05-05 | 1997-03-25 | The General Hospital Corporation | Insulinotropic hormone GLP-1 (7-36) and uses thereof |
| US4681893A (en) | 1986-05-30 | 1987-07-21 | Warner-Lambert Company | Trans-6-[2-(3- or 4-carboxamido-substituted pyrrol-1-yl)alkyl]-4-hydroxypyran-2-one inhibitors of cholesterol synthesis |
| US4759923A (en) | 1987-06-25 | 1988-07-26 | Hercules Incorporated | Process for lowering serum cholesterol using poly(diallylmethylamine) derivatives |
| JP2569746B2 (en) | 1987-08-20 | 1997-01-08 | 日産化学工業株式会社 | Quinoline mevalonolactones |
| US4871721A (en) | 1988-01-11 | 1989-10-03 | E. R. Squibb & Sons, Inc. | Phosphorus-containing squalene synthetase inhibitors |
| US4924024A (en) | 1988-01-11 | 1990-05-08 | E. R. Squibb & Sons, Inc. | Phosphorus-containing squalene synthetase inhibitors, new intermediates and method |
| NO177005C (en) | 1988-01-20 | 1995-07-05 | Bayer Ag | Analogous process for the preparation of substituted pyridines, as well as intermediates for use in the preparation |
| FI94339C (en) | 1989-07-21 | 1995-08-25 | Warner Lambert Co | Process for the preparation of pharmaceutically acceptable [R- (R *, R *)] - 2- (4-fluorophenyl) -, - dihydroxy-5- (1-methylethyl) -3-phenyl-4 - [(phenylamino) carbonyl] -1H- for the preparation of pyrrole-1-heptanoic acid and its pharmaceutically acceptable salts |
| US5177080A (en) | 1990-12-14 | 1993-01-05 | Bayer Aktiengesellschaft | Substituted pyridyl-dihydroxy-heptenoic acid and its salts |
| JP2648897B2 (en) | 1991-07-01 | 1997-09-03 | 塩野義製薬株式会社 | Pyrimidine derivatives |
| US5595872A (en) | 1992-03-06 | 1997-01-21 | Bristol-Myers Squibb Company | Nucleic acids encoding microsomal trigyceride transfer protein |
| DK36392D0 (en) | 1992-03-19 | 1992-03-19 | Novo Nordisk As | USE OF CHEMICAL COMPOUND |
| GB9209628D0 (en) | 1992-05-05 | 1992-06-17 | Smithkline Beecham Plc | Compounds |
| US5470845A (en) | 1992-10-28 | 1995-11-28 | Bristol-Myers Squibb Company | Methods of using α-phosphonosulfonate squalene synthetase inhibitors including the treatment of atherosclerosis and hypercholesterolemia |
| US5594016A (en) | 1992-12-28 | 1997-01-14 | Mitsubishi Chemical Corporation | Naphthalene derivatives |
| WO1994016693A1 (en) | 1993-01-19 | 1994-08-04 | Warner-Lambert Company | Stable oral ci-981 formulation and process of preparing same |
| US5346701A (en) | 1993-02-22 | 1994-09-13 | Theratech, Inc. | Transmucosal delivery of macromolecular drugs |
| US5739135A (en) | 1993-09-03 | 1998-04-14 | Bristol-Myers Squibb Company | Inhibitors of microsomal triglyceride transfer protein and method |
| US5484944A (en) * | 1993-10-27 | 1996-01-16 | Neurogen Corporation | Certain fused pyrrolecarboxanilides and their use as GABA brain receptor ligands |
| US5776983A (en) | 1993-12-21 | 1998-07-07 | Bristol-Myers Squibb Company | Catecholamine surrogates useful as β3 agonists |
| US5488064A (en) | 1994-05-02 | 1996-01-30 | Bristol-Myers Squibb Company | Benzo 1,3 dioxole derivatives |
| US5385929A (en) | 1994-05-04 | 1995-01-31 | Warner-Lambert Company | [(Hydroxyphenylamino) carbonyl] pyrroles |
| US5491134A (en) | 1994-09-16 | 1996-02-13 | Bristol-Myers Squibb Company | Sulfonic, phosphonic or phosphiniic acid β3 agonist derivatives |
| US5541204A (en) | 1994-12-02 | 1996-07-30 | Bristol-Myers Squibb Company | Aryloxypropanolamine β 3 adrenergic agonists |
| US5620997A (en) | 1995-05-31 | 1997-04-15 | Warner-Lambert Company | Isothiazolones |
| JP3314938B2 (en) | 1995-06-06 | 2002-08-19 | ファイザー・インコーポレーテッド | Substituted N- (indole-2-carbonyl) -glycinamides and derivatives as glycogen phosphorylase inhibitors |
| EP0832066B1 (en) | 1995-06-06 | 2001-09-12 | Pfizer Inc. | Substituted n-(indole-2-carbonyl-) amides and derivatives as glycogen phosphorylase inhibitors |
| WO1997012613A1 (en) | 1995-10-05 | 1997-04-10 | Warner-Lambert Company | Method for treating and preventing inflammation and atherosclerosis |
| WO1997021993A2 (en) | 1995-12-13 | 1997-06-19 | The Regents Of The University Of California | Nuclear receptor ligands and ligand binding domains |
| US5770615A (en) | 1996-04-04 | 1998-06-23 | Bristol-Myers Squibb Company | Catecholamine surrogates useful as β3 agonists |
| US5962440A (en) | 1996-05-09 | 1999-10-05 | Bristol-Myers Squibb Company | Cyclic phosphonate ester inhibitors of microsomal triglyceride transfer protein and method |
| US5827875A (en) | 1996-05-10 | 1998-10-27 | Bristol-Myers Squibb Company | Inhibitors of microsomal triglyceride transfer protein and method |
| US5885983A (en) | 1996-05-10 | 1999-03-23 | Bristol-Myers Squibb Company | Inhibitors of microsomal triglyceride transfer protein and method |
| HRP970330B1 (en) | 1996-07-08 | 2004-06-30 | Bayer Ag | Cycloalkano pyridines |
| TW492957B (en) * | 1996-11-07 | 2002-07-01 | Novartis Ag | N-substituted 2-cyanopyrrolidnes |
| US5952322A (en) | 1996-12-05 | 1999-09-14 | Pfizer Inc. | Method of reducing tissue damage associated with non-cardiac ischemia using glycogen phosphorylase inhibitors |
| US5760246A (en) | 1996-12-17 | 1998-06-02 | Biller; Scott A. | Conformationally restricted aromatic inhibitors of microsomal triglyceride transfer protein and method |
| GB9713739D0 (en) | 1997-06-27 | 1997-09-03 | Karobio Ab | Thyroid receptor ligands |
| DE19742601A1 (en) * | 1997-09-26 | 1999-04-29 | Siemens Ag | Method and device for generating frames around video images |
| NZ504769A (en) | 1998-02-27 | 2005-04-29 | Pfizer Prod Inc | N-[(substituted five-membered di- or triaza diunsaturated ring) carbonyl] guanidine derivatives for the treatment of ischemia |
| EP0978279A1 (en) | 1998-08-07 | 2000-02-09 | Pfizer Products Inc. | Inhibitors of human glycogen phosphorylase |
| WO2000038722A1 (en) | 1998-12-23 | 2000-07-06 | G.D. Searle & Co. | COMBINATIONS OF CHOLESTERYL ESTER TRANSFER PROTEIN INHIBITORS AND HMG CoA REDUCTASE INHIBITORS FOR CARDIOVASCULAR INDICATIONS |
| EP1150674A1 (en) | 1999-02-12 | 2001-11-07 | Novo Nordisk A/S | Use of pyrrolidine derivatives for the manufacture of a pharmaceutical composition for the treatment or prophylaxis of obesity or appetite regulation |
| DK1041068T3 (en) | 1999-04-01 | 2004-05-10 | Pfizer Prod Inc | Compounds for treating and preventing diabetic complications |
| US6395767B2 (en) * | 2000-03-10 | 2002-05-28 | Bristol-Myers Squibb Company | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
-
2001
- 2001-02-16 US US09/788,173 patent/US6395767B2/en not_active Ceased
- 2001-02-28 EG EG2001020212A patent/EG25854A/en active
- 2001-03-05 DE DE60134122T patent/DE60134122D1/en not_active Expired - Lifetime
- 2001-03-05 HU HU1200545A patent/HU230347B1/en unknown
- 2001-03-05 HU HU1200546A patent/HU230380B1/en unknown
- 2001-03-05 AU AU2001245466A patent/AU2001245466B2/en active Active
- 2001-03-05 EP EP15186007.9A patent/EP2995615B1/en not_active Expired - Lifetime
- 2001-03-05 HU HU0302792A patent/HU228110B1/en active Protection Beyond IP Right Term
- 2001-03-05 TW TW090104965A patent/TWI258468B/en not_active IP Right Cessation
- 2001-03-05 NZ NZ520821A patent/NZ520821A/en not_active IP Right Cessation
- 2001-03-05 CN CNB018063152A patent/CN1213028C/en not_active Expired - Lifetime
- 2001-03-05 CZ CZ2014-33A patent/CZ307784B6/en not_active IP Right Cessation
- 2001-03-05 ES ES10178907.1T patent/ES2553573T3/en not_active Expired - Lifetime
- 2001-03-05 CZ CZ2015493A patent/CZ307821B6/cs not_active IP Right Cessation
- 2001-03-05 CN CNA2005100785188A patent/CN1698601A/en active Pending
- 2001-03-05 CA CA2402894A patent/CA2402894C/en not_active Expired - Lifetime
- 2001-03-05 ES ES01918383T patent/ES2305062T3/en not_active Expired - Lifetime
- 2001-03-05 RU RU2002125491/04A patent/RU2286986C2/en active Protection Beyond IP Right Term
- 2001-03-05 JP JP2001567699A patent/JP4460205B2/en not_active Expired - Lifetime
- 2001-03-05 PL PL365520A patent/PL207041B1/en unknown
- 2001-03-05 KR KR1020027011806A patent/KR100754089B1/en not_active Expired - Lifetime
- 2001-03-05 AT AT01918383T patent/ATE396176T1/en active
- 2001-03-05 SG SG200405783-2A patent/SG152030A1/en unknown
- 2001-03-05 CZ CZ2002-2974A patent/CZ304355B6/en unknown
- 2001-03-05 ES ES15186007T patent/ES2768961T3/en not_active Expired - Lifetime
- 2001-03-05 IL IL15137201A patent/IL151372A0/en unknown
- 2001-03-05 TW TW095105942A patent/TW200624420A/en unknown
- 2001-03-05 WO PCT/US2001/007151 patent/WO2001068603A2/en not_active Ceased
- 2001-03-05 DE DE122010000008C patent/DE122010000008I1/en active Pending
- 2001-03-05 HK HK03101079.9A patent/HK1049330B/en unknown
- 2001-03-05 MX MXPA02008837A patent/MXPA02008837A/en active IP Right Grant
- 2001-03-05 EP EP01918383A patent/EP1261586B1/en not_active Expired - Lifetime
- 2001-03-05 PT PT01918383T patent/PT1261586E/en unknown
- 2001-03-05 EP EP10178907.1A patent/EP2272825B1/en not_active Expired - Lifetime
- 2001-03-05 BR BRPI0109115A patent/BRPI0109115B8/en not_active IP Right Cessation
- 2001-03-05 DK DK01918383T patent/DK1261586T3/en active
- 2001-03-05 AU AU4546601A patent/AU4546601A/en active Pending
- 2001-03-05 ES ES05005368.5T patent/ES2456667T3/en not_active Expired - Lifetime
- 2001-03-05 EP EP05005368.5A patent/EP1559710B1/en not_active Expired - Lifetime
- 2001-03-05 KR KR1020067004515A patent/KR100758407B1/en not_active Expired - Lifetime
- 2001-03-08 CO CO01018857A patent/CO5280198A1/en active IP Right Grant
- 2001-03-09 MY MYPI20011103A patent/MY124512A/en unknown
- 2001-03-09 AR ARP010101122A patent/AR027634A1/en active IP Right Grant
- 2001-03-12 UY UY26613A patent/UY26613A1/en not_active IP Right Cessation
- 2001-06-12 PE PE2001000554A patent/PE20020771A1/en not_active Application Discontinuation
-
2002
- 2002-08-21 IL IL151372A patent/IL151372A/en active Protection Beyond IP Right Term
- 2002-08-26 ZA ZA200206816A patent/ZA200206816B/en unknown
- 2002-09-09 NO NO20024295A patent/NO324227B1/en not_active IP Right Cessation
-
2006
- 2006-07-23 IL IL177019A patent/IL177019A/en active IP Right Grant
- 2006-07-23 IL IL177018A patent/IL177018A0/en active IP Right Grant
-
2008
- 2008-08-20 CY CY20081100885T patent/CY1108273T1/en unknown
-
2010
- 2010-01-14 JP JP2010006181A patent/JP5427047B2/en not_active Expired - Lifetime
- 2010-02-11 BE BE2010C008C patent/BE2010C008I2/fr unknown
- 2010-02-17 NL NL300436C patent/NL300436I1/en unknown
- 2010-02-17 LU LU91650C patent/LU91650I2/en unknown
- 2010-02-22 FR FR10C0010C patent/FR10C0010I2/en active Active
- 2010-03-16 NO NO2010006C patent/NO2010006I2/en unknown
- 2010-03-30 CY CY2010005C patent/CY2010005I2/en unknown
-
2011
- 2011-12-01 US US13/308,658 patent/USRE44186E1/en not_active Expired - Lifetime
-
2012
- 2012-04-18 BE BE2012C016C patent/BE2012C016I2/fr unknown
- 2012-04-23 FR FR12C0028C patent/FR12C0028I2/en active Active
- 2012-04-23 DE DE201212000023 patent/DE122012000023I1/en active Pending
- 2012-04-24 CY CY2012009C patent/CY2012009I2/en unknown
- 2012-04-25 LU LU91985C patent/LU91985I2/en unknown
- 2012-05-21 NO NO2012009C patent/NO2012009I2/en unknown
- 2012-11-30 JP JP2012263345A patent/JP2013040219A/en active Pending
- 2012-12-14 HU HUS1200031C patent/HUS1200031I1/en unknown
- 2012-12-14 HU HUS1200030C patent/HUS1200030I1/en unknown
-
2013
- 2013-03-20 UY UY0001034691A patent/UY34691A/en unknown
- 2013-12-02 JP JP2013249334A patent/JP5953292B2/en not_active Expired - Lifetime
-
2015
- 2015-04-28 JP JP2015092196A patent/JP5951843B2/en not_active Expired - Lifetime
Patent Citations (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3325478A (en) | 1964-11-17 | 1967-06-13 | Du Pont | alpha-amino-1-adamantylmethyl penicillins |
| DE2449840A1 (en) | 1973-10-19 | 1975-04-24 | Kao Corp | ADAMANTYLAMIDINE AND THE PROCESS FOR THEIR MANUFACTURE |
| US3906044A (en) | 1973-10-19 | 1975-09-16 | Kao Corp | Adamantylamidines and processes for making them |
| DE2521895A1 (en) | 1974-05-16 | 1976-04-08 | Pliva Pharm & Chem Works | Alpha-Amino-2-adamantylacetic acid prepn - from 2-adamantylacetic acid via alpha-bromo-2-adamantylacetic acid |
| EP0007652A1 (en) | 1978-06-27 | 1980-02-06 | Shell Internationale Researchmaatschappij B.V. | Derivatives of 3-azabicyclo(3.1.0)hexane and a process for their preparation |
| US4254057A (en) | 1978-06-27 | 1981-03-03 | Shell Oil Company | 2-Aminomethylcyclopropyl-1,1-dialkylacetals |
| US4255334A (en) | 1978-06-27 | 1981-03-10 | Shell Oil Company | Process for preparing 3-azabicyclo(3.1.0)hexane-2-carboxylic acid |
| US4379785A (en) | 1979-12-19 | 1983-04-12 | Hoechst Aktiengesellschaft | Heterocyclic substituted sulfonyl ureas, and their use |
| DE3324263A1 (en) | 1983-07-06 | 1985-01-17 | Hoechst Ag, 6230 Frankfurt | DERIVATIVES OF 2-AZABICYCLO (3.1.0) HEXAN-3-CARBONIC ACID, METHOD FOR THE PRODUCTION THEREOF, THEIR SUBSTANCES AND THE USE THEREOF, AND 2-AZABICYCLO (3.1.0) HEXANE DERIVATIVES AS INTERMEDIATE PRODUCTS AND PROCESS PRODUCTS |
| EP0219782A2 (en) | 1985-10-15 | 1987-04-29 | Hoechst Aktiengesellschaft | Use of angiotensin-converting enzyme inhibitors in the treatment of atherosclerosis, thrombosis and peripheral vascular disease |
| DE3926606A1 (en) | 1989-08-11 | 1991-02-14 | Hoechst Ag | METHOD FOR THE TREATMENT OF CARDIALS AND VASCULAR HYPERTROPHY AND HYPERPLASIA |
| US5462928A (en) | 1990-04-14 | 1995-10-31 | New England Medical Center Hospitals, Inc. | Inhibitors of dipeptidyl-aminopeptidase type IV |
| EP1050540A2 (en) | 1991-10-22 | 2000-11-08 | New England Medical Center Hospitals, Inc. | Inhibitors of dipeptidyl-aminopeptidase type IV |
| US5939560A (en) | 1993-12-03 | 1999-08-17 | Ferring B.V. | Inhibitors of DP-mediated processes, compositions and therapeutic methods thereof |
| EP0686642A2 (en) | 1994-06-10 | 1995-12-13 | Bristol-Myers Squibb Company | Modified guanidino and amidino thrombin inhibitors |
| US5561146A (en) | 1994-06-10 | 1996-10-01 | Bristol-Myers Squibb Company | Modified guanidino and amidino thrombin inhibitors |
| WO1997015576A1 (en) | 1995-10-25 | 1997-05-01 | E.I. Du Pont De Nemours And Company | Herbicidal sulfonamides |
| US6060432A (en) | 1995-10-25 | 2000-05-09 | E. I. Du Pont De Nemours And Company | Herbicidal sulfonamides |
| WO1997040832A1 (en) | 1996-04-25 | 1997-11-06 | Probiodrug Gesellschaft für Arzneimittelforschung mbH | Use of dipeptidyl peptidase iv effectors for lowering the blood glucose level in mammals |
| US6011155A (en) | 1996-11-07 | 2000-01-04 | Novartis Ag | N-(substituted glycyl)-2-cyanopyrrolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV |
| WO1999026659A1 (en) | 1997-11-21 | 1999-06-03 | Pfizer Products Inc. | Combination of an aldose reductase inhibitor and a glycogen phosphorylase inhibitor |
| US6803357B1 (en) | 1998-02-02 | 2004-10-12 | New England Medical Center Hospitals, Inc. | Method of regulating glucose metabolism, and reagents related thereto |
| WO1999038501A2 (en) | 1998-02-02 | 1999-08-05 | Trustees Of Tufts College | Method of regulating glucose metabolism, and reagents related thereto |
| US6890898B2 (en) | 1998-02-02 | 2005-05-10 | Trustees Of Tufts College | Method of regulating glucose metabolism, and reagents related thereto |
| US7078381B2 (en) | 1998-02-02 | 2006-07-18 | Trustees Of Tufts College | Method of regulating glucose metabolism, and reagents related thereto |
| US5998463A (en) | 1998-02-27 | 1999-12-07 | Pfizer Inc | Glycogen phosphorylase inhibitors |
| WO1999047545A2 (en) | 1998-03-19 | 1999-09-23 | Vertex Pharmaceuticals Incorporated | Inhibitors of caspases |
| WO1999067279A1 (en) | 1998-06-24 | 1999-12-29 | Probiodrug Gesellschaft für Arzneimittelforschung mbH | Compounds of unstable dipeptidyl peptidase iv inhibitors |
| WO2000010549A1 (en) | 1998-08-21 | 2000-03-02 | Point Therapeutics, Inc. | Regulation of substrate activity |
| US6166063A (en) | 1998-12-10 | 2000-12-26 | Novartis Ag | N-(substituted glycyl)-2-cyanopyrrolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV |
| WO2000047207A1 (en) | 1999-02-09 | 2000-08-17 | Bristol-Myers Squibb Company | LACTAM INHIBITORS OF FXa AND METHOD |
| US6297233B1 (en) | 1999-02-09 | 2001-10-02 | Bristol-Myers Squibb Company | Lactam inhibitors of FXa and method |
| WO2000053171A1 (en) | 1999-03-05 | 2000-09-14 | Molteni L. E C. Dei Fratelli Alitti Societa' Di Esercizio S.P.A. | Use of metformin in the preparation of pharmaceutical compositions capable of inhibiting the enzyme dipeptidyl peptidase iv |
| WO2000056296A2 (en) | 1999-03-23 | 2000-09-28 | Ferring Bv | Compositions for improving fertility |
| WO2000056297A2 (en) | 1999-03-23 | 2000-09-28 | Ferring B.V. | Pharmaceutical compositions comprising dipeptidy peptidase iv inhibitors for the promotion of growth |
| WO2000069868A1 (en) | 1999-05-17 | 2000-11-23 | Zaidan Hojin Biseibutsu Kagaku Kenkyu Kai | Sulphostin analogues and processes for the preparation of sulphostin and analogues thereof |
| US6110949A (en) | 1999-06-24 | 2000-08-29 | Novartis Ag | N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV |
| WO2002060894A2 (en) | 2001-01-30 | 2002-08-08 | Bristol-Myers Squibb Company | Sulfonamide lactam inhibitors of factor xa |
| US6555542B1 (en) | 2001-01-30 | 2003-04-29 | Bristol-Myers Squibb Company | Sulfonamide lactam inhibitors of FXa and method |
| WO2003004241A1 (en) | 2001-07-07 | 2003-01-16 | Trench Germany Gmbh | Method and device for producing an electric insulator made from plastic |
| US7250529B2 (en) | 2004-09-17 | 2007-07-31 | Albemarle Corporation | Synthesis process for 2-(3-hydroxy-1-adamantyl)-2-oxoacetic acid |
| US7205432B2 (en) | 2005-05-31 | 2007-04-17 | Kemfine Oy | Process for the preparation of adamantane derivatives |
| US20060287317A1 (en) | 2005-06-17 | 2006-12-21 | Apogee Biotechnology Corporation | Sphingosine kinase inhibitors |
Non-Patent Citations (28)
| Title |
|---|
| Asai, Y. et al, The Journal of Antibiotics, vol. 50, No. 8, pp. 653-657, Aug. 1997. |
| Ashworth, D.M. et al, Bioorganic & Medicinal Chemistry Letter, vol. 6, No. 10, pp. 1163-1166, 1996. |
| Ashworth, D.M. et al, Bioorganic & Medicinal Chemistry Letter, vol. 6, No. 22, pp. 2745-2748, 1996. |
| Augustyns, KJL et al, Eur. J. Med. Chem. 32, 301-309, (1997). |
| Belyaev, A. et al, J. Med. Chem., 42, 1041-1052, 1999. |
| David J. Augeri et al., "Discovery and Preclinical Profile of Saxagliptin (BMS-477118): A Highly Potent, Long-Acting, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes," J. Med. Chem. 2005, 48, 5025-5037. |
| David R. Magnin et al. "Synthesis of Novel Potent Dipeptidyl Peptidase IV Inhibitors with Enhanced Chemical Stability: Interplay Between the N-Terminal Amino Acid Alkyl Side Chain and the Cyclopropyl Group of alpha-Aminoacyl-L-cis-4,5-methanoloprolinenitrile-Based Inhibitors," J. Med. Chem. 2004, 47, 2587-2598. |
| David R. Magnin et al. "Synthesis of Novel Potent Dipeptidyl Peptidase IV Inhibitors with Enhanced Chemical Stability: Interplay Between the N-Terminal Amino Acid Alkyl Side Chain and the Cyclopropyl Group of α-Aminoacyl-L-cis-4,5-methanoloprolinenitrile-Based Inhibitors," J. Med. Chem. 2004, 47, 2587-2598. |
| Demuth, H.-U. et al, Diabetes, 2000, vol. 49, suppl. 1,A102. |
| Demuth, H.-U. et al, FEBS Letters, vol. 320, No. 1, pp. 23-27, Mar. 1993. |
| Hanessian, S. et al, Bioorganic & Medicinal Chem. Letters, vol. 8, No. 16, pp. 2123-2128, Aug. 18, 1998. |
| Hermann Stetter and Elli Rauscher, Zur Kenntnis der Adamantan-carbonsaure-(1) Chemische Berichte, 1960, vol. 93, No. 5, pp. 1161-1166. |
| Hiltmann, Arzneim.-Forsch. 24 (4) 548-600 1974 Abstract only. * |
| Hughes, T.E. et al, Biochemistry, 28, 11597-11603, 19993. |
| Lambeir, A.-M., et al, Biochimica et Biophysica Acts, 1290, pp. 76-82 (1996). |
| Li, J. et al, Archives of Biochemistry and Biophysics, vol. 323, No. 1, pp. 148-154, Oct. 20, 1995. |
| Lin, J. et al, Proc. Natl. Acad. Sci, USA, vol. 95, pp. 14020-14024, Nov. 1998. |
| Ohnuki, T. et al, Drugs of the Future, 24(6):665-670, 1999. |
| Peter Beak et al.,"Intramolecular Cyclizations of alpha-Lithioamine Synthetic Equivalents: Convenient Synthesis of 3-, 5-, and 6-Membered Ring Heterocyclic Nitrogen Compounds and Elaborations of 3-Mimbered Ring Systems," J. Org. Chem. vol. 59, No. 2. 1994, pp. 276-277. |
| Rotherberg, P. et al, Diabetes, 2000, vol. 49, Suppl. 1, A39. |
| Sagnard, I. et al, Tetrahedron Letters, vol. 36, No. 18, pp. 3149-3152, 1995. |
| Stockel, A. et al, Peptides: Chemistry, Structure and Biology, pp. 709-710, 1996. |
| Tanaka, S. et al, Immunopharmacology 40, 21-26 (1998). |
| Tverezovsky, V. V. et al., Tetrahedron, vol. 53, No. 43, pp. 14773-14792, 1997. |
| Von R. Hiltmann et al., "2-Acylaminopyridin-Derivate mit morphinagonistischer und antagonisterischer Wirksamkeit, Arzneimittel-Forschung," 1974, vol. 24, No. 4a, pp. 584-600. |
| Yamada, M. et al, Bioorganic & Medicinal Chemistry Letter 8, 1537-1540 (1998). |
| Yamada, M. et al, Bioorganic & Medicinal Chemistry Letters 8, 1537-1540 (1998). |
| Yoshimoto, T. et al, Agric. Biol. Chem., 55(4), pp. 1135-1136, 1991. |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016016770A1 (en) | 2014-07-26 | 2016-02-04 | Wockhardt Limited | A novel modified release pharmaceutical composition of sitagliptin or pharmaceutically acceptable salt thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| USRE44186E1 (en) | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method | |
| US6573287B2 (en) | 2,1-oxazoline and 1,2-pyrazoline-based inhibitors of dipeptidyl peptidase IV and method | |
| AU2001245466A1 (en) | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl IV, processes for their preparation, and their use | |
| AU2002254557A1 (en) | 2,1-Oxazoline and 1,2-pyrazoline-based inhibitors of dipeptidyl peptidase IV and method | |
| HK1152516B (en) | Protected amino hydroxy adamantane carboxylic acid and process for its preparation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| CC | Certificate of correction | ||
| FPAY | Fee payment |
Year of fee payment: 12 |
|
| AS | Assignment |
Owner name: ASTRAZENECA AB, SWEDEN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BRISTOL-MYERS SQUIBB COMPANY;REEL/FRAME:032541/0698 Effective date: 20140201 |
|
| AS | Assignment |
Owner name: ASTRAZENECA AB, SWEDEN Free format text: CORRECTIVE ASSIGNMENT TO CORRECT THE ADDRESS PREVIOUSLY RECORDED ON REEL 032541 FRAME 0698. ASSIGNOR(S) HEREBY CONFIRMS THE ADDRESS OF THE ASSIGNEE SHOULD BE SE-151 85, SODERTALJE, SWEDEN;ASSIGNOR:BRISTOL-MYERS SQUIBB COMPANY;REEL/FRAME:032858/0978 Effective date: 20140201 |
|
| IPR | Aia trial proceeding filed before the patent and appeal board: inter partes review |
Free format text: TRIAL NO: IPR2015-01340 Opponent name: MYLAN PHARMACEUTICALS INC. Effective date: 20150604 |
|
| IPR | Aia trial proceeding filed before the patent and appeal board: inter partes review |
Free format text: TRIAL NO: IPR2016-01029 Opponent name: WOCKHARDT BIO AG,WOCKHARDT LIMITED,WOCKHARDT USA L Effective date: 20160511 |
|
| IPR | Aia trial proceeding filed before the patent and appeal board: inter partes review |
Free format text: TRIAL NO: IPR2016-01122 Opponent name: TEVA PHARMACEUTICALS USA INC. ANDTEVA PHARMACEUTIC Effective date: 20160601 Free format text: TRIAL NO: IPR2016-01104 Opponent name: AMNEAL PHARMACEUTICALS LLC,AMNEAL PHARMACEUTICALS Effective date: 20160601 Free format text: TRIAL NO: IPR2016-01117 Opponent name: AUROBINDO PHARMA U.S.A., INC. ANDAUROBINDO PHARMA, Effective date: 20160602 |
|
| IPRC | Trial and appeal board: inter partes review certificate |
Kind code of ref document: K1 Free format text: INTER PARTES REVIEW CERTIFICATE; TRIAL NO. IPR2015-01340, JUN. 4, 2015; TRIAL NO. IPR2016-01029, MAY 11, 2016; TRIAL NO. IPR2016-01122, JUN. 1, 2016; TRIAL NO. IPR2016-01117, JUN. 2, 2016; TRIAL NO. IPR2016-01104, JUN. 3, 2016 INTER PARTES REVIEW CERTIFICATE FOR PATENT RE44,186, ISSUED APR. 30, 2013, APPL. NO. 13/308,658, DEC. 1, 2011 INTER PARTES REVIEW CERTIFICATE ISSUED JUN. 21, 2019 Effective date: 20190621 |
|
| IPRC | Trial and appeal board: inter partes review certificate |
Kind code of ref document: K1 Free format text: INTER PARTES REVIEW CERTIFICATE; TRIAL NO. IPR2015-01340, JUN. 4, 2015; TRIAL NO. IPR2016-01029, MAY 11, 2016; TRIAL NO. IPR2016-01122, JUN. 1, 2016; TRIAL NO. IPR2016-01117, JUN. 2, 2016; TRIAL NO. IPR2016-01104, JUN. 3, 2016 INTER PARTES REVIEW CERTIFICATE FOR PATENT RE44,186, ISSUED APR. 30, 2013, APPL. NO. 13/308,658, DEC. 1, 2011 INTER PARTES REVIEW CERTIFICATE ISSUED JUN. 21, 2019 Effective date: 20190621 |