USRE39634E1 - Bisarylimidazoly fatty acid amide hydrolase inhibitors - Google Patents
Bisarylimidazoly fatty acid amide hydrolase inhibitors Download PDFInfo
- Publication number
- USRE39634E1 USRE39634E1 US10/883,195 US88319504A USRE39634E US RE39634 E1 USRE39634 E1 US RE39634E1 US 88319504 A US88319504 A US 88319504A US RE39634 E USRE39634 E US RE39634E
- Authority
- US
- United States
- Prior art keywords
- phenyl
- methyl
- imidazol
- diphenyl
- carbamic acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 [1*]C.[2*]C.[3*]C1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1*[2H]C Chemical compound [1*]C.[2*]C.[3*]C1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1*[2H]C 0.000 description 28
- LHAHTHILLRCRBM-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=CC=C1F Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=CC=C1F LHAHTHILLRCRBM-UHFFFAOYSA-N 0.000 description 5
- NBASSYYNACEAGJ-UHFFFAOYSA-N CC1=CC=CC=C1OC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CC1=CC=CC=C1OC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 NBASSYYNACEAGJ-UHFFFAOYSA-N 0.000 description 2
- IJTDDFBTIDHGEJ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCBr Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCBr IJTDDFBTIDHGEJ-UHFFFAOYSA-N 0.000 description 2
- UQTYOKFXJYYFQI-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)C(=O)O Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)C(=O)O UQTYOKFXJYYFQI-UHFFFAOYSA-N 0.000 description 2
- HMJCRVRGZFQOMD-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(NC(=O)OC1=CC=CC=C1F)C(C)C Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(NC(=O)OC1=CC=CC=C1F)C(C)C HMJCRVRGZFQOMD-UHFFFAOYSA-N 0.000 description 2
- QFKRYSDMIXOOCH-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(C(=O)O)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(C(=O)O)C=C1 QFKRYSDMIXOOCH-UHFFFAOYSA-N 0.000 description 2
- RFLKXRLXXACOCO-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC(Cl)=CC=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC(Cl)=CC=C2)C=C1 RFLKXRLXXACOCO-UHFFFAOYSA-N 0.000 description 2
- WIHGSFJBAYXSNS-UHFFFAOYSA-N CCOC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound CCOC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 WIHGSFJBAYXSNS-UHFFFAOYSA-N 0.000 description 2
- ABKJPGCDYPXWKQ-UHFFFAOYSA-N COC1=CC=C(OC(=O)NCCCCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound COC1=CC=C(OC(=O)NCCCCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 ABKJPGCDYPXWKQ-UHFFFAOYSA-N 0.000 description 2
- SDPQMYWYNNWTLU-UHFFFAOYSA-N COC1=CC=CC=C1OC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC1=CC=CC=C1OC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 SDPQMYWYNNWTLU-UHFFFAOYSA-N 0.000 description 2
- FZGDFPYGTTVJHF-UHFFFAOYSA-N COC1=CC=CC=C1OC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC1=CC=CC=C1OC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 FZGDFPYGTTVJHF-UHFFFAOYSA-N 0.000 description 2
- ZJAOXVIHLCXOEQ-UHFFFAOYSA-N CC(=O)CCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CC(=O)CCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 ZJAOXVIHLCXOEQ-UHFFFAOYSA-N 0.000 description 1
- KCHYCLFYQLLRPM-UHFFFAOYSA-N CC(=O)CCCCCCN1C(C)=NC(C2=CC=C(F)C=C2)=C1C1=CC=C(F)C=C1 Chemical compound CC(=O)CCCCCCN1C(C)=NC(C2=CC=C(F)C=C2)=C1C1=CC=C(F)C=C1 KCHYCLFYQLLRPM-UHFFFAOYSA-N 0.000 description 1
- ODYSEFBEROHLIL-UHFFFAOYSA-N CC(=O)CCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CC(=O)CCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 ODYSEFBEROHLIL-UHFFFAOYSA-N 0.000 description 1
- PENLQZRNXJROQI-UHFFFAOYSA-N CC(=O)CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CC(=O)CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 PENLQZRNXJROQI-UHFFFAOYSA-N 0.000 description 1
- FJZBGEHKFHAJNK-UHFFFAOYSA-N CC(C)=NOC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CC(C)=NOC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 FJZBGEHKFHAJNK-UHFFFAOYSA-N 0.000 description 1
- AHDIMISIDXKJLT-UHFFFAOYSA-N CC1=CC=C(OCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound CC1=CC=C(OCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 AHDIMISIDXKJLT-UHFFFAOYSA-N 0.000 description 1
- VLNJXAQTUCJUTR-UHFFFAOYSA-N CC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound CC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 VLNJXAQTUCJUTR-UHFFFAOYSA-N 0.000 description 1
- XMNBKBJKPYCATP-UHFFFAOYSA-N CC1=CC=CC=C1OC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound CC1=CC=CC=C1OC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 XMNBKBJKPYCATP-UHFFFAOYSA-N 0.000 description 1
- IBVGJCKUNHQTJG-UHFFFAOYSA-N CC1=CC=CC=C1OC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CC1=CC=CC=C1OC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 IBVGJCKUNHQTJG-UHFFFAOYSA-N 0.000 description 1
- UXFHDVNGNFTBIX-UHFFFAOYSA-N CC1=CC=CC=C1OC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CC1=CC=CC=C1OC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 UXFHDVNGNFTBIX-UHFFFAOYSA-N 0.000 description 1
- DRBDPIAOSYZKQD-UHFFFAOYSA-N CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1 Chemical compound CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1 DRBDPIAOSYZKQD-UHFFFAOYSA-N 0.000 description 1
- QTUSOWXMSBHOMD-UHFFFAOYSA-N CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1CCCCCCC(=O)O Chemical compound CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1CCCCCCC(=O)O QTUSOWXMSBHOMD-UHFFFAOYSA-N 0.000 description 1
- FROPPAHABGJTLA-UHFFFAOYSA-N CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1CCCCCCNC(=O)OC1=C(F)C=CC=C1 Chemical compound CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1CCCCCCNC(=O)OC1=C(F)C=CC=C1 FROPPAHABGJTLA-UHFFFAOYSA-N 0.000 description 1
- FTFMPYWXZDLNAM-UHFFFAOYSA-N CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1CCCCCCNC(=O)OC1=C(F)C=CC=C1F Chemical compound CC1=NC(C2=CC=C(F)C=C2)=C(C2=CC=C(F)C=C2)N1CCCCCCNC(=O)OC1=C(F)C=CC=C1F FTFMPYWXZDLNAM-UHFFFAOYSA-N 0.000 description 1
- WBDSXISQIHMTGL-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1 WBDSXISQIHMTGL-UHFFFAOYSA-N 0.000 description 1
- WAWFIZWARJFZNB-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCBr Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCBr WAWFIZWARJFZNB-UHFFFAOYSA-N 0.000 description 1
- NVTHULYBHMPHQJ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(=O)O Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(=O)O NVTHULYBHMPHQJ-UHFFFAOYSA-N 0.000 description 1
- XPJWOMMTPWCGRU-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C(=O)O)(C(=O)O)C1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C(=O)O)(C(=O)O)C1=CC=CC=C1 XPJWOMMTPWCGRU-UHFFFAOYSA-N 0.000 description 1
- QYBNPFICEBDYAQ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C(=O)O)C(C)C Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C(=O)O)C(C)C QYBNPFICEBDYAQ-UHFFFAOYSA-N 0.000 description 1
- JRHDWMZXBJHQPC-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C(=O)O)C1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C(=O)O)C1=CC=CC=C1 JRHDWMZXBJHQPC-UHFFFAOYSA-N 0.000 description 1
- GPGHRPQJRXQXJN-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)(C)C1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)(C)C1=CC=CC=C1 GPGHRPQJRXQXJN-UHFFFAOYSA-N 0.000 description 1
- PMBBYWXXAFBHSE-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)C Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)C PMBBYWXXAFBHSE-UHFFFAOYSA-N 0.000 description 1
- OAAICIAKHJPGDB-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)C(C)C Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)C(C)C OAAICIAKHJPGDB-UHFFFAOYSA-N 0.000 description 1
- LXGNTFODLNMYKQ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)NC(=O)OC1=CC=CC=C1F Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCC(C)NC(=O)OC1=CC=CC=C1F LXGNTFODLNMYKQ-UHFFFAOYSA-N 0.000 description 1
- DCQWOKVSKCZSCU-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCBr Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCBr DCQWOKVSKCZSCU-UHFFFAOYSA-N 0.000 description 1
- WCOYQHBPQYYEBN-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCC(=O)O Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCC(=O)O WCOYQHBPQYYEBN-UHFFFAOYSA-N 0.000 description 1
- MKBWLFNKDKSMMC-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCC(=O)O Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCC(=O)O MKBWLFNKDKSMMC-UHFFFAOYSA-N 0.000 description 1
- WCFPXERLDSROKC-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(C#N)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(C#N)C=C1 WCFPXERLDSROKC-UHFFFAOYSA-N 0.000 description 1
- WJLTUBGHNUKNRQ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(Cl)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(Cl)C=C1 WJLTUBGHNUKNRQ-UHFFFAOYSA-N 0.000 description 1
- YEYYSMBWLXKXSJ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(F)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(F)C=C1 YEYYSMBWLXKXSJ-UHFFFAOYSA-N 0.000 description 1
- ZFCUKLAHWGJHDA-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(F)C=C1F Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=C(F)C=C1F ZFCUKLAHWGJHDA-UHFFFAOYSA-N 0.000 description 1
- UWKODOYNVJUKMA-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=CC=C1 UWKODOYNVJUKMA-UHFFFAOYSA-N 0.000 description 1
- KVVFDLOMTIYNNM-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=CC=C1F Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCCNC(=O)OC1=CC=CC=C1F KVVFDLOMTIYNNM-UHFFFAOYSA-N 0.000 description 1
- AQGCGQZAGYNHMV-WEMUVCOSSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=C([N+](=O)[O-])C=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=C([N+](=O)[O-])C=CC=C1 AQGCGQZAGYNHMV-WEMUVCOSSA-N 0.000 description 1
- HVWDTGYGEZTXSE-WEMUVCOSSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC([N+](=O)[O-])=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC([N+](=O)[O-])=CC=C1 HVWDTGYGEZTXSE-WEMUVCOSSA-N 0.000 description 1
- JNGAIEHLWZGYSY-STKMKYKTSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=C(F)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=C(F)C=C1 JNGAIEHLWZGYSY-STKMKYKTSA-N 0.000 description 1
- HICAUSZTPHPJQV-WEMUVCOSSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=C([N+](=O)[O-])C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=C([N+](=O)[O-])C=C1 HICAUSZTPHPJQV-WEMUVCOSSA-N 0.000 description 1
- HIZMGDVERFPBNM-AWSUPERCSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=CC=C1 HIZMGDVERFPBNM-AWSUPERCSA-N 0.000 description 1
- QEJUQEIJZLCLCZ-WEMUVCOSSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=CN=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)O/N=C/C1=CC=CN=C1 QEJUQEIJZLCLCZ-WEMUVCOSSA-N 0.000 description 1
- DWNVRDYRAQCINB-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC(C)C Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC(C)C DWNVRDYRAQCINB-UHFFFAOYSA-N 0.000 description 1
- DNURSCPDSPXAJU-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC(F)=C(F)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC(F)=C(F)C=C1 DNURSCPDSPXAJU-UHFFFAOYSA-N 0.000 description 1
- LNRLTYJRJMLPES-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(C#N)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(C#N)C=C1 LNRLTYJRJMLPES-UHFFFAOYSA-N 0.000 description 1
- XJAKUYMMKREWNM-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(Cl)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(Cl)C=C1 XJAKUYMMKREWNM-UHFFFAOYSA-N 0.000 description 1
- CSFBUGVVQNTZGS-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(F)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(F)C=C1 CSFBUGVVQNTZGS-UHFFFAOYSA-N 0.000 description 1
- NSWZAERQBNOYAM-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(F)C=C1F Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=C(F)C=C1F NSWZAERQBNOYAM-UHFFFAOYSA-N 0.000 description 1
- KCHOVCICRIAYSX-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1=CC=CC=C1 KCHOVCICRIAYSX-UHFFFAOYSA-N 0.000 description 1
- JCUFQSXXINWVIU-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1CCCCC1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC1CCCCC1 JCUFQSXXINWVIU-UHFFFAOYSA-N 0.000 description 1
- YWDYYCUYUHBNGL-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OCC1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OCC1=CC=CC=C1 YWDYYCUYUHBNGL-UHFFFAOYSA-N 0.000 description 1
- JQCVZSZSAVHKDK-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(C#N)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(C#N)C=C1 JQCVZSZSAVHKDK-UHFFFAOYSA-N 0.000 description 1
- UGSXGFMGLUGUAU-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(Cl)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(Cl)C=C1 UGSXGFMGLUGUAU-UHFFFAOYSA-N 0.000 description 1
- LJVAEURIXMPCHM-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(F)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(F)C=C1 LJVAEURIXMPCHM-UHFFFAOYSA-N 0.000 description 1
- QMIKRYNXYTZTKA-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(F)C=C1F Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=C(F)C=C1F QMIKRYNXYTZTKA-UHFFFAOYSA-N 0.000 description 1
- VNIZFYRBACXAQP-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=CC=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=CC=C1 VNIZFYRBACXAQP-UHFFFAOYSA-N 0.000 description 1
- BWPRPBUIQPVVFD-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=CC=C1F Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OC1=CC=CC=C1F BWPRPBUIQPVVFD-UHFFFAOYSA-N 0.000 description 1
- RUFUOXCZFVHSGF-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=C(F)C=CC=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=C(F)C=CC=C2)C=C1 RUFUOXCZFVHSGF-UHFFFAOYSA-N 0.000 description 1
- JYGJXLXLTFXCAQ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=C(F)C=CC=C2F)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=C(F)C=CC=C2F)C=C1 JYGJXLXLTFXCAQ-UHFFFAOYSA-N 0.000 description 1
- CYCGLDOPUFZCSV-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC(F)=C(F)C=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC(F)=C(F)C=C2)C=C1 CYCGLDOPUFZCSV-UHFFFAOYSA-N 0.000 description 1
- QLVDMDDQLWVPLM-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC=C(Cl)C=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC=C(Cl)C=C2)C=C1 QLVDMDDQLWVPLM-UHFFFAOYSA-N 0.000 description 1
- JKCXQDIHXXYILY-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC=CC=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCOC1=CC=C(NC(=O)OC2=CC=CC=C2)C=C1 JKCXQDIHXXYILY-UHFFFAOYSA-N 0.000 description 1
- WMAATRDUOAZYRH-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(C(=O)O)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(C(=O)O)C=C1 WMAATRDUOAZYRH-UHFFFAOYSA-N 0.000 description 1
- WOLFTIJTUYIRFQ-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=C(Cl)C=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=C(Cl)C=C2)C=C1 WOLFTIJTUYIRFQ-UHFFFAOYSA-N 0.000 description 1
- IDONYIIJPCLJKI-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=C(F)C(F)=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=C(F)C(F)=C2)C=C1 IDONYIIJPCLJKI-UHFFFAOYSA-N 0.000 description 1
- SHTWNZPEBIGSSW-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=C(F)C=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=C(F)C=C2)C=C1 SHTWNZPEBIGSSW-UHFFFAOYSA-N 0.000 description 1
- AWVQTTGVJJPGQC-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=CC(Cl)=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=CC(Cl)=C2)C=C1 AWVQTTGVJJPGQC-UHFFFAOYSA-N 0.000 description 1
- RNYLSGGTAIRFDR-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=CC=C2)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=CC=C2)C=C1 RNYLSGGTAIRFDR-UHFFFAOYSA-N 0.000 description 1
- HVBXMFCCTUBMJH-UHFFFAOYSA-N CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=CC=C2F)C=C1 Chemical compound CC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCOC1=CC=C(NC(=O)OC2=CC=CC=C2F)C=C1 HVBXMFCCTUBMJH-UHFFFAOYSA-N 0.000 description 1
- PFRSLNYQKWVOQF-UHFFFAOYSA-N CCC(C)CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CCC(C)CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 PFRSLNYQKWVOQF-UHFFFAOYSA-N 0.000 description 1
- XMKGLPANGRKGMU-UHFFFAOYSA-N CCC(CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1)C(=O)O Chemical compound CCC(CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1)C(=O)O XMKGLPANGRKGMU-UHFFFAOYSA-N 0.000 description 1
- BDZLAFXHYNNBSK-UHFFFAOYSA-N CCC(CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1)NC(=O)OC1=CC=CC=C1F Chemical compound CCC(CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1)NC(=O)OC1=CC=CC=C1F BDZLAFXHYNNBSK-UHFFFAOYSA-N 0.000 description 1
- JFGCBMQXGWFESU-UHFFFAOYSA-N CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1 Chemical compound CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1 JFGCBMQXGWFESU-UHFFFAOYSA-N 0.000 description 1
- DCTHHOLKFVDFIY-UHFFFAOYSA-N CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCC(=O)O Chemical compound CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCC(=O)O DCTHHOLKFVDFIY-UHFFFAOYSA-N 0.000 description 1
- JUHSEWAZJYAHHI-UHFFFAOYSA-N CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCC(C)=O Chemical compound CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCC(C)=O JUHSEWAZJYAHHI-UHFFFAOYSA-N 0.000 description 1
- VLTNNAFYFSVVGS-UHFFFAOYSA-N CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC(C)(C)C Chemical compound CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC(C)(C)C VLTNNAFYFSVVGS-UHFFFAOYSA-N 0.000 description 1
- NGMMWUXERKZVJI-UHFFFAOYSA-N CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC(C)CC Chemical compound CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCCNC(=O)OC(C)CC NGMMWUXERKZVJI-UHFFFAOYSA-N 0.000 description 1
- YLGFHSSURRSZBJ-UHFFFAOYSA-N CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OCCCCCl Chemical compound CCC1=NC(C2=CC=CC=C2)=C(C2=CC=CC=C2)N1CCCCCNC(=O)OCCCCCl YLGFHSSURRSZBJ-UHFFFAOYSA-N 0.000 description 1
- VJHMAYPKFOEEMF-UHFFFAOYSA-N CCOC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CCOC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 VJHMAYPKFOEEMF-UHFFFAOYSA-N 0.000 description 1
- GSVJWQNGCOHAMM-UHFFFAOYSA-N CCOC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CCOC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 GSVJWQNGCOHAMM-UHFFFAOYSA-N 0.000 description 1
- VZSXUVQCPYUMAW-UHFFFAOYSA-N CCOC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound CCOC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 VZSXUVQCPYUMAW-UHFFFAOYSA-N 0.000 description 1
- MVTNIYDFOXZYNZ-UHFFFAOYSA-N CCOC(CCCCCCC[n]1c(-c2ccccc2)c(-c2ccccc2)nc1C)=O Chemical compound CCOC(CCCCCCC[n]1c(-c2ccccc2)c(-c2ccccc2)nc1C)=O MVTNIYDFOXZYNZ-UHFFFAOYSA-N 0.000 description 1
- BZNKOJZOSNRABX-UHFFFAOYSA-N CCOC(CCCCCC[n]1c(-c(cc2)ccc2F)c(-c(cc2)ccc2F)nc1C)=O Chemical compound CCOC(CCCCCC[n]1c(-c(cc2)ccc2F)c(-c(cc2)ccc2F)nc1C)=O BZNKOJZOSNRABX-UHFFFAOYSA-N 0.000 description 1
- MHJQSDYZIKKEPE-UHFFFAOYSA-N COC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound COC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 MHJQSDYZIKKEPE-UHFFFAOYSA-N 0.000 description 1
- OFSWZLPOOKDXDP-UHFFFAOYSA-N COC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 OFSWZLPOOKDXDP-UHFFFAOYSA-N 0.000 description 1
- CRYHRFPPPYGXLU-UHFFFAOYSA-N COC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 CRYHRFPPPYGXLU-UHFFFAOYSA-N 0.000 description 1
- WUTDHSLCSCRJJE-UHFFFAOYSA-N COC1=C(OC(=O)NC2=CC=C(OCCCN3C(C)=NC(C4=CC=CC=C4)=C3C3=CC=CC=C3)C=C2)C=CC=C1 Chemical compound COC1=C(OC(=O)NC2=CC=C(OCCCN3C(C)=NC(C4=CC=CC=C4)=C3C3=CC=CC=C3)C=C2)C=CC=C1 WUTDHSLCSCRJJE-UHFFFAOYSA-N 0.000 description 1
- CTCPSLZBROEIJK-UHFFFAOYSA-N COC1=CC=C(OC(=O)NC2=CC=C(OCCCN3C(C)=NC(C4=CC=CC=C4)=C3C3=CC=CC=C3)C=C2)C=C1 Chemical compound COC1=CC=C(OC(=O)NC2=CC=C(OCCCN3C(C)=NC(C4=CC=CC=C4)=C3C3=CC=CC=C3)C=C2)C=C1 CTCPSLZBROEIJK-UHFFFAOYSA-N 0.000 description 1
- DBTOBDWFVHSNGN-UHFFFAOYSA-N COC1=CC=C(OC(=O)NC2=CC=C(OCCN3C(C)=NC(C4=CC=CC=C4)=C3C3=CC=CC=C3)C=C2)C=C1 Chemical compound COC1=CC=C(OC(=O)NC2=CC=C(OCCN3C(C)=NC(C4=CC=CC=C4)=C3C3=CC=CC=C3)C=C2)C=C1 DBTOBDWFVHSNGN-UHFFFAOYSA-N 0.000 description 1
- VLPBIVLQDLAAMM-UHFFFAOYSA-N COC1=CC=C(OC(=O)NCCCCCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound COC1=CC=C(OC(=O)NCCCCCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 VLPBIVLQDLAAMM-UHFFFAOYSA-N 0.000 description 1
- ZCXQKBFXBDHCMV-UHFFFAOYSA-N COC1=CC=C(OC(=O)NCCCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound COC1=CC=C(OC(=O)NCCCCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 ZCXQKBFXBDHCMV-UHFFFAOYSA-N 0.000 description 1
- VHHYCPSXRJPUPU-UHFFFAOYSA-N COC1=CC=CC(OC)=C1OC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC1=CC=CC(OC)=C1OC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 VHHYCPSXRJPUPU-UHFFFAOYSA-N 0.000 description 1
- TXTYAAMKKBDEHL-UHFFFAOYSA-N COC1=CC=CC(OC)=C1OC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC1=CC=CC(OC)=C1OC(=O)NCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 TXTYAAMKKBDEHL-UHFFFAOYSA-N 0.000 description 1
- AYBJAAXONTZTRT-UHFFFAOYSA-N COC1=CC=CC(OC)=C1OC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC1=CC=CC(OC)=C1OC(=O)NCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 AYBJAAXONTZTRT-UHFFFAOYSA-N 0.000 description 1
- MLFASIRABYZUIU-UHFFFAOYSA-N COC1=CC=CC=C1OC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 Chemical compound COC1=CC=CC=C1OC(=O)NC1=CC=C(OCCN2C(C)=NC(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1 MLFASIRABYZUIU-UHFFFAOYSA-N 0.000 description 1
- IUNZJOWJONPLGO-UHFFFAOYSA-N COC1=CC=CC=C1OC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 Chemical compound COC1=CC=CC=C1OC(=O)NCCCCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1 IUNZJOWJONPLGO-UHFFFAOYSA-N 0.000 description 1
- LVGPJSBRMDHIRR-UHFFFAOYSA-N [H]N(C(=O)OC1=CC=CC=C1F)C(CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1)C1=CC=CC=C1 Chemical compound [H]N(C(=O)OC1=CC=CC=C1F)C(CCCCCN1C(C)=NC(C2=CC=CC=C2)=C1C1=CC=CC=C1)C1=CC=CC=C1 LVGPJSBRMDHIRR-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/56—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, attached to ring carbon atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/08—Drugs for disorders of the alimentary tract or the digestive system for nausea, cinetosis or vertigo; Antiemetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/14—Prodigestives, e.g. acids, enzymes, appetite stimulants, antidyspeptics, tonics, antiflatulents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
- A61P25/12—Antiepileptics; Anticonvulsants for grand-mal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
Definitions
- the present invention relates to bisarylimidazolyl derivatives and pharmaceutical compositions comprising said derivatives which inhibit fatty acid amide hydrolase and are useful for the treatment of conditions affected by inhibiting fatty acid amide hydrolase.
- Neuropathic pain is caused by injury to nerves as the result of many factors including physical damage (e.g., trauma, surgery), drugs such as Zidovudine (AZT), Carmustine (BCNU) and disease (e.g., diabetes, herpes zoster).
- drugs such as Zidovudine (AZT), Carmustine (BCNU) and disease (e.g., diabetes, herpes zoster).
- ZCT Zidovudine
- BCNU Carmustine
- diabetes herpes zoster
- the prevalence in the United States of neuropathies associated with diabetes, herpes and amputation is estimated at 1.5 million.
- the worldwide prevalence of diabetic neuropathy alone is expected to reach 12 million by 2007.
- Nerve injury can result in both allodynia and hyperalgesia.
- NSAIDs non-steroidal anti-inflammatory drugs
- analgesics such as aspirin and acetaminophen
- anticonvalsants e.g., carbamazepine, gabapentin
- tricyclic antidepressants e.g., amitryptiline
- cannabinoids The analgesic properties of cannabinoids have been known for many years and to many cultures. Cannabinoids are active in many pre-clinical models of pain, including neuropathic pain. Within the last few years, several endogenous cannabinoids, including the fatty acid amides arachidonylethanolamide (anandamide), and arachidonyl amino acids such as N-arachidonylglycine, homo- ⁇ -linolenyl-ethanolamide and docosatetraenyl-ethanolamide, as well as 2-arachidonyl-glycerol, have been shown to induce analgesia in laboratory animals (De Vane, W.A. et.
- arachidonyl amino acids such as N-arachidonylglycine, homo- ⁇ -linolenyl-ethanolamide and docosatetraenyl-ethanolamide, as well as 2-arachidonyl-glycerol
- FAAH fatty acid amide hydrolase
- R 1 are R 2 are each independently H, C 1-3 alkyl or halo;
- R 3 is C 1 -C 3 alkyl or C 3-7 cycloalkyl
- A is C 1-12 alkylene or L
- G′ is H, C 1-5 alkyl or C 1-5 haloalkyl
- compounds of Formula (I) according to the first embodiment of the first aspect wherein G is phenyl or —C 1-2 alkylene-phenyl, said phenyl or phenyl of said —C 1-2 alkylene-phenyl are optionally substituted with the same or different substituents selected from the group consisting of halo, CN, —C(O)O—C 1-3 alkyl, C 1-3 alkyl and C 1-3 alkoxy.
- compounds of Formula (I) according to the first embodiment of the first aspect wherein G is phenyl or —C 1-2 alkylene-phenyl, said phenyl or phenyl of said —C 1-2 alkylene-phenyl are substituted with halo, —C(O)O—C 1-3 -alkyl, C 1-3 alkyl or C 1-3 alkoxy.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 7-10 alkylene, D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 1-5 alkylene, D is X(O)O and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 7-10 alkylene, D is X(O)N(G′) and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 1-5 alkylene, D is X(O)N(G′) and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 7-10 alkylene, D is HYC(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 1-5 alkylene, D is HYC(O)O and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 7-10 alkylene, D is HYC(O)ON ⁇ C(G′) and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- R 1 and R 2 are each H, R 3 is C 1-3 alkyl, A is C 1-5 alkylene, D is HYC(O)ON ⁇ C(G′) and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene.
- compositions comprising compounds of Formula (I) as defined herein.
- a third aspect of the present invention are provided methods of treating conditions the treatment of which can be effected by the inhibition of FAAH by the administration of pharmaceutical compositions comprising compounds of Formula (I) as defined herein.
- compositions comprising compounds of Formula (I) as defined herein.
- a method of treating pain, more particularly chronic pain, acute pain and neuropathic pain by the administration of a pharmaceutical composition comprising or salt or solvate thereof.
- a method of treating neuropathic pain by the administration of a pharmaceutical composition comprising or salt or solvate thereof.
- a method of providing neuroprotection and contraception and yet further methods of psychomotor disorder, hypertension, cardiovascular disease, eating disorder, nausea, AIDS-related complex, glaucoma, inflammation, psoriasis and multiple sclerosis by the administration of pharmaceutical compositions comprising compounds of Formula (I) as defined herein.
- Raphael Mechoulam “Looking Back at Cannabis Research,” Current Pharmaceutical Design, 2000, Vol. 6, No. 13, pp. 1313-1322 (p. 1319); Sumner H. Burstein, “Ajulemic Acid (CT3): A Potent Analog of the Acid Metabolites of THC,” Current Pharmaceutical Design, 2000, Vol. 6, No. 13, pp. 1339-1345 (p.
- embodiments of the present invention may comprise a suitable combination of two or more of embodiments and/or aspects disclosed herein.
- FIG. 1A illustrates results from a rat formalin model used for testing acute chemo-induced pain.
- the single asterisk (*) applies when p is less than 0.05.
- FIG. 1B illustrates results from a rat formalin model used for testing chronic chemo-induced pain.
- the single asterisk (*) applies when p is less than 0.05.
- FIG. 2 illustrates results from the Hargreaves Test used for measuring acute thermal pain.
- the single asterisk (*) applies when p is less than 0.05 whereas the double asterisk (**) applies when p is less than 0.01.
- n 6.
- FIG. 3 illustrates results from the Chung Model used for measuring neuropathic pain.
- halo or halogen includes fluoro, chloro, bromo and iodo.
- alkyl or “alkylene” includes straight or branched chain configurations.
- the compounds of this invention can exist in the form of pharmaceutically acceptable salts.
- Such salts include addition salts with inorganic acids such as, for example, hydrochloric acid and sulfuric acid, and with organic acids such as, for example, acetic acid, citric acid, methanesulfonic acid, toluenesulfonic acid, tartaric acid and maleic acid.
- the acidic group can exist in the form of alkali metal salts such as, for example, a potassium salt and a sodium salt; alkaline earth metal salts such as, for example, a magnesium salt and a calcium salt; and salts with organic bases such as a triethylammonium salt and an arginine salt.
- the compounds of the present invention may be hydrated or non-hydrated.
- the compounds of this invention can be administered in such oral dosage forms as tablets, capsules (each of which includes sustained release or timed release formulations), pills, powders, granules, elixirs, tinctures, suspensions, syrups and emulsions.
- the compounds of this invention may also be administered intravenously, intraperitoneally, subcutaneously, or intramuscularly, all using dosage forms well known to those skilled in the pharmaceutical arts.
- the compounds can be administered alone, but generally will be administered with a pharmaceutical carrier selected upon the basis of the chosen route of administration and standard pharmaceutical practice.
- Compounds of this invention can also be administered in intranasal form by topical use of suitable intranasal vehicles, or by transdermal routes, using transdermal skin patches. When compounds of this invention are administered transdermally the dosage will be continuous throughout the dosage regimen.
- the dosage and dosage regimen and scheduling of a compounds of the present invention must in each case be carefully adjusted, utilizing sound professional judgment and considering the age, weight and condition of the recipient, the route of administration and the nature and extent of the disease condition. In accordance with good clinical practice, it is preferred to administer the instant compounds at a concentration level which will produce effective beneficial effects without causing any harmful or untoward side effects.
- reaction mixture was diluted with diethyl ether (30 mL), washed by water, and then was dried over MgSO 4 . After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO 2 : EtOAc/Hexanes).
- 6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexanoic acid (Scheme 1, (C)) Prepared as described for the example above.
- 1 H NMR DMSO ⁇ 1.17 (m, 2H), 1.33 (m, 2H), 1.51 (m, 2H), 2.09 (t, 2H), 2.76 (s, 3H), 4.03 (t, 2H), 7.38 (m, 5H), 7.49 (m, 2H), 7.65 (m, 3H).
- reaction solution was cooled to ⁇ 78° C., and 2-Iodopropane (499.97 ul, 849.95 mg, 5 mmole) was added in dropwise.
- the reaction was let stirred at room temperature for 1 hr, then at 50° C. for 1 hr. Analysis by TLC indicated consumption of starting material.
- the reaction was worked-up using the procedures as described above. Crude material was purified by flash column chromatography using Hexane/Ethyl Acetate (4:1) to give product (80 mg, 18.5%).
- the reaction mixture was let stirred at room temperature for 10 minutes under Nitrogen, then at 108° C. for 90 minutes.
- the reaction was let cooled to room temperature, to which 2-Fluorophenol (0.03 ml, 0.038 g, 0.338 mmole) was added.
- the reaction mixture was let stirred at room temperature for 30 minutes, then at 100° C. for 18 hrs.
- analysis by TLC (Dichloromethane/Ethyl Acetate 3:1) indicated consumption of starting material.
- the reaction was let cooled to room temperature, where the solvent was removed by rotorvap.
- the crude material was purified by flash column chromatography using Dichloromethane/Ethyl Acetate (6:1 to 3:1). Product was obtained (110 mg, 71%).
- the reaction mixture was let stirred at room temperature for 10 minutes under Nitrogen, then at 108° C. for 90 minutes.
- the reaction was let cooled to room temperature, to which 2-Fluorophenol (0.03 ml, 0.038 g, 0.338 mmole) was added.
- the reaction mixture was let stirred at room temperature for 10 minutes, then at 100° C. for 18 hrs.
- analysis by TLC (Dichloromethane/Ethyl Acetate 3:1) indicated consumption of starting material.
- the reaction was let cooled to room temperature, where the solvent was removed by rotorvap.
- the crude material was purified by flash column chromatography using dichloromethane/Ethyl Acetate (6:1 to 3:1). Product was obtained (58 mg, 33.1%).
- H4-FAAH cells Homogenates of crude membranes were prepared from H4 cells that express transfected human FAAH (H4-FAAH cells). Briefly, cells were grown in DMEM supplemented with 10% FBS and Geneticin at a final concentration of 500 ⁇ g/ml (Gibco BRL, Rockville, Md.). Confluent cultures of H4-FAAH cells were rinsed twice with phosphate-buffered saline [138 mM NaCl, 4.1 mM KCl, 5.1 mM Na 2 PO 4 , 1.5 mM KH 2 PO 4 (pH 7.5), 37° C.] and incubated for 5-10 min. at 4° C. in lysis buffer [1 mM sodium bicarbonate].
- example 1 was active in phase I (acute phase) and phase II (chronic phase) of the rat formalin test.
- phase I acute phase
- phase II chronic phase
- example 1 was active in animals that received 25 mg/kg, i.v, of Example 1, the number of paw flinches was reduced by nearly 40% in the first 10 minutes after administration of formalin. Paw flinches were reduced by approximately 30% over the following 50 minutes.
- the effect of example 1 was similar to that seen with a 3 mg/kg, i.p. dose of morphine.
- Example 1 was examined in the Chung model of neuropathic pain where animals exhibit a pain response (paw withdrawal) to a normally innocuous stimulus (light touch), In animals with a neuropathic injury, the threshold for withdrawal of the injured paw was increased (toward normal) in a dose-dependent fashion by Example 1.
- the anti-neuropathic effect observed with 20 mg/kg Example 1 exhibited earlier onset of action compared to 100 mg/kg gabapentin (reference compound) with similar peak efficacy.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Immunology (AREA)
- Virology (AREA)
- Pain & Pain Management (AREA)
- Heart & Thoracic Surgery (AREA)
- Ophthalmology & Optometry (AREA)
- Cardiology (AREA)
- AIDS & HIV (AREA)
- Rheumatology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Oncology (AREA)
- Molecular Biology (AREA)
- Psychology (AREA)
- Communicable Diseases (AREA)
- Dermatology (AREA)
- Nutrition Science (AREA)
- Hospice & Palliative Care (AREA)
- Otolaryngology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
The present invention relates to bisarylimidazolyl derivatives and pharmaceutical compositions comprising said compounds inhibiting fatty acid amide hydrolase and useful for the treatment of pain, particularly neuropathic pain, psychomotor disorder, hypertension, cardiovascular disease, eating disorder, nausea, AIDS-related complex, glaucoma, inflammation, psoriasis or multiple sclerosis, and other conditions the treatment of which can be effected by inhibiting fatty acid amide hydrolase.
Description
This non-provisional application claims priority from provisional application U.S. Ser. No. 60/286,827 filed Apr. 27, 2001.
This application is a reissue application of U.S. Pat. No. 6,562,846, issued May 13, 2003, on non-provisional application No. 10/128,480, filed Apr. 23, 2002 which claims priority from provisional application USSN 60/286,827 filed Apr. 27, 2001.
The present invention relates to bisarylimidazolyl derivatives and pharmaceutical compositions comprising said derivatives which inhibit fatty acid amide hydrolase and are useful for the treatment of conditions affected by inhibiting fatty acid amide hydrolase.
Neuropathic pain is caused by injury to nerves as the result of many factors including physical damage (e.g., trauma, surgery), drugs such as Zidovudine (AZT), Carmustine (BCNU) and disease (e.g., diabetes, herpes zoster). The prevalence in the United States of neuropathies associated with diabetes, herpes and amputation is estimated at 1.5 million. The worldwide prevalence of diabetic neuropathy alone is expected to reach 12 million by 2007. Nerve injury can result in both allodynia and hyperalgesia.
Current treatment of neuropathic pain involves the use of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and acetaminophen) and other analgesics as well as anticonvalsants (e.g., carbamazepine, gabapentin) and tricyclic antidepressants (e.g., amitryptiline). Effective treatment of pain with current therapies is limited by adverse effects and a lack of efficacy against all components of pain.
Current research is aimed at understanding the molecular and physiological components of pain processing to develop more effective analgesics (Levin, J. D., New Directions in Pain Research: Meeting Report Molecules to Maladies, Neuron 20: 649-654, 1998; Pastemak, G. W., The Central Questions in Pain Perception May Be Peripheral, PNAS 95:10354-10355, 1998).
The analgesic properties of cannabinoids have been known for many years and to many cultures. Cannabinoids are active in many pre-clinical models of pain, including neuropathic pain. Within the last few years, several endogenous cannabinoids, including the fatty acid amides arachidonylethanolamide (anandamide), and arachidonyl amino acids such as N-arachidonylglycine, homo-γ-linolenyl-ethanolamide and docosatetraenyl-ethanolamide, as well as 2-arachidonyl-glycerol, have been shown to induce analgesia in laboratory animals (De Vane, W.A. et. al., Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptors, Science 258: 1946-1949, 1992; Hanus, L. et. al., Two New Unsaturated Fatty Acid Ethanolamides in Brain that Bind to the Cannabinoid Receptor, J. Med. Chem. 36: 3032-3034, 1993; Machoulam, R. et. al., Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds To Cannabinoid Receptors, Biochem. Pharmacol. 50: 83-90, 1995; Vogel, Z. et. al., Cannabinomimetic Behavioral Effects of and Adenylate Cyclase Inhibition By Two New Endogenous Anandamides, Eur. J. Pharmacol. 287: 145-152, 1995; Hargreaves, K. M. et al., Cannabinoids Reduce Hyperalgesia and Inflammation Via Interaction With Peripheral CB1 Receptors, Pain 75: 111-119, 1998; Rice,A. S. C., et. al., The Anti-Hyperalgesic Actions of the Cannabinoid Anandamide and the Putative CB2 Receptor Agonist Palmitoylethanolamide in Visceral and Somatic Inflammatory Pain, Pain 76: 189-199, 1998; Huang, S. M., et al., Identification of a New Class of Molecules, the Arachidonyl Amino Acids, and Characterization of One Member That Inhibits Pain, J. Biological Chemistry, 276: 46, 42639-42644, 2001). The ability of cannabinoid receptor antagonists and cannabinoid receptor antisense to induce hyperalgesia in animals suggests that endogenous cannabinoids regulate the nociceptive threshold (Hargreaves, K. M. et al., Hypoactivity of the Spinal Cannabinoid System Results in NMDA-Dependent Hyperalgesia, J. Neurosci. 18: 451-457, 1998; Piomelli, D. et. al., Control of Pain Initiation By Endogenous Cannabinoids, Nature 394: 277-281, 1998; Fields, H. L. et. al., An Analgesia Circuit Activated By Cannabinoids, Nature 395: 381-383, 1998). Elevation of levels of neuroactive fatty acid amides such as anandamide may provide a unique mechanism to achieve analgesia. The mechanisms by which endogenous cannabinoids are synthesized are not well understood; therefore, target for drugs aimed at increasing the synthesis of these compounds are slow to be identified.
Anandamide and the other identified endogenous cannabinoids are inactivated through a cleavage mechanism by a membrane-bound enzyme, fatty acid amide hydrolase (FAAH). FAAH, therefore, provides an important target for regulating the activity of endogenous cannabinoids. The inhibition of FAAH may elevate levels of anandamide or other endogenous cannabinoids to increase the nociceptive threshold. Furthermore, the inhibition of FAAH would also extend the therapeutic benefits of other cannabinoid agoinsts in the treatment of emesis, anxiety, feeding, behaviors, movement disorders, glaucoma, neuroprotection and cardiovascular disease.
Thus according to a first embodiment of the first aspect of the present invention are provided compounds of Formula (I)
and pharmaceutically acceptable salts and solvates thereof wherein
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
-
-
- L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
- provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
- D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
- X is C and is attached to A;
- Y is N and is attached to A;
- G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substituents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy;
-
G′ is H, C1-5alkyl or C1-5haloalkyl;
-
- wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
- wherein
- Z is O or S and is attached to A;
- J is CH and is attached to A, D and J′;
- J′ is C1-4alkyl or phenyl; and
- provided that
- if A—D is interrpted with —Z-phenyl-, then A is C1-5alkylene;
- if A—D is not interrpted with —Z-phenyl-, then A is C5-12alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2are each H.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each halo.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each fluoro.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R3 is methyl.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R3is ethyl.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is L.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C3-10alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C7-10alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C4-8alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C5-7alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C8-9alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C9alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C6alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A is C1-4alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein D is X(O)O.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein D is X(O)N(G′).
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein D is HYC(O)O.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein D is HYC(O)ON═C(G′).
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein G is C1-5alkyl.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein G is C3-7cycloalkyl.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein G is —C1-2alkylene-phenyl.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are optionally substituted with the same or different substituents selected from the group consisting of halo, CN, —C(O)O—C1-3alkyl, C1-3alkyl and C1-3alkoxy.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are substituted with halo, —C(O)O—C1-3-alkyl, C1-3alkyl or C1-3alkoxy.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are substituted with fluoro.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are substituted with cyano.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A—D are not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A—D are interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A—D is interrupted with J—J′.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A—D is interrupted with —Z-phenyl- .
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein A—D is interrupted with —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3 is C1-3alkyl, A is C7-10alkylene, D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3 is C1-3alkyl, A is C1-5alkylene, D is X(O)O and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3 is C1-3alkyl, A is C7-10alkylene, D is X(O)N(G′) and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3is C1-3alkyl, A is C1-5alkylene, D is X(O)N(G′) and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3 is C1-3alkyl, A is C7-10alkylene, D is HYC(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3 is C1-3alkyl, A is C1-5alkylene, D is HYC(O)O and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3 is C1-3alkyl, A is C7-10alkylene, D is HYC(O)ON═C(G′) and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided compounds of Formula (I) according to the first embodiment of the first aspect wherein R1 and R2 are each H, R3 is C1-3alkyl, A is C1-5alkylene, D is HYC(O)ON═C(G′) and A—D is interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene.
According to another embodiment of the first aspect of the present invention are provided
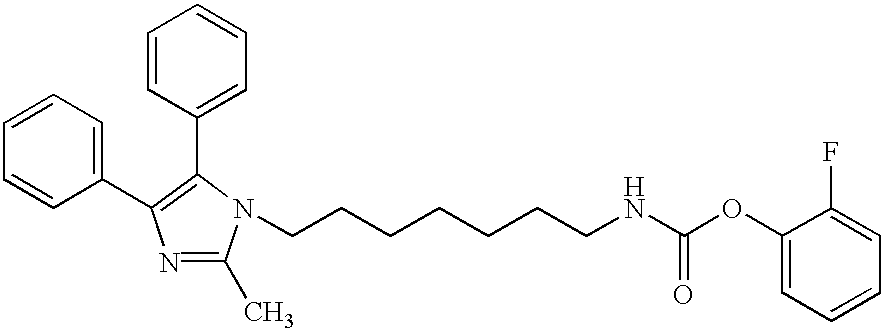
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- [6-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid tert-butyl ester;
- [6-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid sec-butyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid benzyl ester;
- 2-Propanone,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 2-Propanone,O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid methyl ester;
- 6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;
- [6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-methoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-cyano-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-methoxy-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl-carbamic acid methyl ester;
- [7-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-heptyl]-carbamic acid methyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid ethyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-fluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-methoxy-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid o-tolyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-methoxy-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid ethyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-fluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-fluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-chloro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-methoxy-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid o-tolyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-cyano-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-methoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-
carbamic acid 3,4-difluoro-phenyl ester; - {6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester;
- {6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2,6-difluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid ethyl ester;
- Benzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- Benzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 4-Fluorobenzaldehyde,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 4-Fluorobenzaldehyde,O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 2-Nitrobenzaldehye,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 2-Nitrobenzaldehye, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 3-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 3-Nitrobenzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 4-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 4-Nitrobenzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 3-Pyridinecarboxaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 3-Pyridinecarboxaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- {4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-
carbamic acid 3,4-difluoro-phenyl ester; - {4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-
carbamic acid 3,4-difluoro-phenyl ester; - {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-methoxy-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-methoxy-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3-chloro-phenyl ester;
- {4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid phenyl ester;
- {4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 2-fluoro-phenyl ester;
- {4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-fluoro-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid phenyl ester;
- {4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl)}-carbamic acid 4-methoxy-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-fluoro-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2,6-difluoro-phenyl ester;
- {4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid ethyl ester;
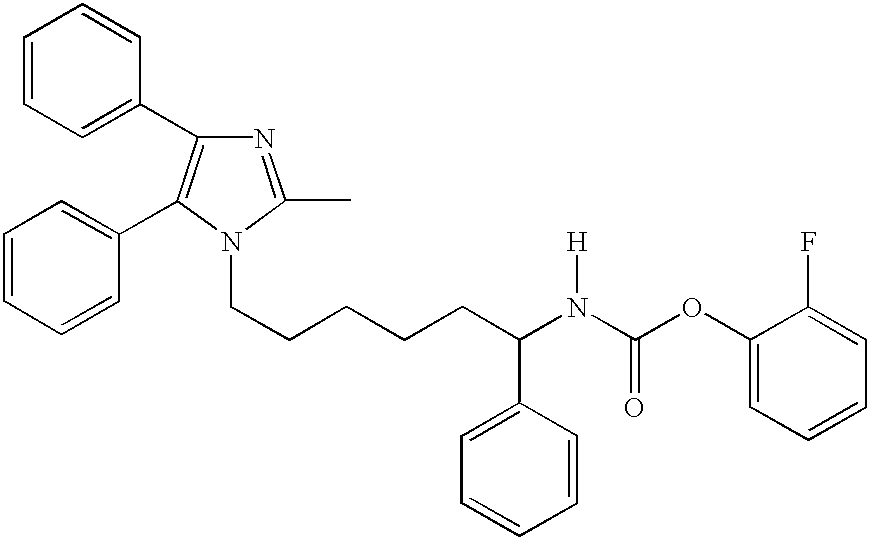
- [1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- [1-Ethyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- [1-Isopropyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester or
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-1-phenyl-hexyl]-carbamic acid 2-fluoro-phenyl ester.
According to another embodiment of the first aspect of the present invention are provided
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- 2-Propanone,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 2-Propanone,O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid cyclohexyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid methyl ester;
- 6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;
- [6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-methoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-cyano-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-methoxy-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid methyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid ethyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-fluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-methoxy-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid o-tolyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-methoxy-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid ethyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-fluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-fluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-chloro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-methoxy-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid o-tolyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-cyano-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-methoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-
carbamic acid 3,4-difluoro-phenyl ester; - [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid isopropyl ester;
- {6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester;
- {6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2,6-difluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid ethyl ester;
- Benzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- Benzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 4-Fluorobenzaldehyde,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 4-Fluorobenzaldehyde,O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 2-Nitrobenzaldehye,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 2-Nitrobenzaldehye, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 3-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 3-Nitrobenzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 4-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 4-Nitrobenzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 3-Pryridinecarboxaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 3-Pyridinecarboxaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-
carbamic acid 3,4-difluoro-phenyl ester; - {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-methoxy-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-methoxy-phenyl ester;
- {4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3-chloro-phenyl ester;
- [1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- [1-Ethyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- [1-Isopropyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- or
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-1-phenyl-hexyl]-carbamic acid 2-fluoro-phenyl ester.
According to another embodiment of the first aspect of the present invention are provided
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- 2-Propanone,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 2-Propanone,O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;
- 6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-methoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-cyano-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid ethyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-fluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-methoxy-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid o-tolyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-methoxy-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-fluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-fluoro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-chloro-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-methoxy-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-cyano-phenyl ester;
- [5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-
carbamic acid 3,4-difluoro-phenyl ester; - {6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester;
- {6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2,6-difluoro-phenyl ester;
- Benzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- Benzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 4-Fluorobenzaldehyde,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 4-Fluorobenzaldehyde,O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 2-Nitrobenzaldehye,O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 2-Nitrobenzaldehye, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 3-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 3-Nitrobenzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 4-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 4-Nitrobenzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- 3-Pyridinecarboxaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;
- 3-Pyridinecarboxaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
- [1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester; or
- [1-Ethyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester.
According to another embodiment of the first aspect of the present invention are provided
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
- 6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;
- [6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
- [7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
- [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-
carbamic acid 3,4-difluoro-phenyl ester; - {6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester;
- or
- [1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester.
According to various embodiments of a second aspect of the present invention are provided pharmaceutical formulations comprising compounds of Formula (I) as defined herein.
According to various embodiments of a third aspect of the present invention are provided methods of treating conditions the treatment of which can be effected by the inhibition of FAAH by the administration of pharmaceutical compositions comprising compounds of Formula (I) as defined herein.
According to another embodiment of the third aspect of the present invention is provided a method of treating pain, more particularly chronic pain, acute pain and neuropathic pain by the administration of pharmaceutical compositions comprising compounds of Formula (I) as defined herein.
According to another embodiment of the third aspect of the present invention is provided a method of treating pain, more particularly chronic pain, acute pain and neuropathic pain by the administration of a pharmaceutical composition comprising
or salt or solvate thereof.
or salt or solvate thereof.
According to another embodiment of the third aspect of the present invention is provided a method of treating neuropathic pain by the administration of a pharmaceutical composition comprising
or salt or solvate thereof.
or salt or solvate thereof.
According to another embodiment of the third aspect of the present invention is provided a method of providing neuroprotection and contraception and yet further methods of psychomotor disorder, hypertension, cardiovascular disease, eating disorder, nausea, AIDS-related complex, glaucoma, inflammation, psoriasis and multiple sclerosis by the administration of pharmaceutical compositions comprising compounds of Formula (I) as defined herein. See Raphael Mechoulam, “Looking Back at Cannabis Research,” Current Pharmaceutical Design, 2000, Vol. 6, No. 13, pp. 1313-1322 (p. 1319); Sumner H. Burstein, “Ajulemic Acid (CT3): A Potent Analog of the Acid Metabolites of THC,” Current Pharmaceutical Design, 2000, Vol. 6, No. 13, pp. 1339-1345 (p. 1340); Vincenzo Di Marzo, et al., “Endocannabinoids: New Targets for Drug Development,” Current Pharmaceutical Design, 2000, Vol. 6, No. 13, pp. 1361-1380 (p. 1362); and Sonya L. Palmer, et al., “Natural and Synthetic Endocannabinoids and Their Structure-Activity Relationships,” Current Pharmaceutical Design, 2000, Vol. 6, No. 13, pp. 1381-1397 (p. 1386).
Other embodiments of the present invention may comprise a suitable combination of two or more of embodiments and/or aspects disclosed herein.
Yet other embodiments and aspects of the invention will be apparent according to the description provided below.
The description of the invention herein should be construed in congruity with the laws and principles of chemical bonding. For example, when a moiety is optionally substituted and said substitution requires the removal of a hydrogen atom from the moiety to be substituted, the description of the moiety should be read to include the moiety with or without said hydrogen atom. As another example, if a variable is defined as a particular moiety or atom and is further defined to have value of 0 or some integer, the bond(s) attaching said moiety should be suitably removed in the event the variable equals 0. An embodiment or aspect which depends from another embodiment or aspect, will describe only the variables having values and provisos that differ from the embodiment or aspect from which it depends. It is to be understood that the present invention may include any and all possible stereoisomers, geometric isomers, diastereoisomers, enantiomers, anomers and optical isomers, unless a particular description specifies otherwise. As used herein, “halo” or “halogen” includes fluoro, chloro, bromo and iodo. As used herein, “alkyl” or “alkylene” includes straight or branched chain configurations.
The compounds of this invention can exist in the form of pharmaceutically acceptable salts. Such salts include addition salts with inorganic acids such as, for example, hydrochloric acid and sulfuric acid, and with organic acids such as, for example, acetic acid, citric acid, methanesulfonic acid, toluenesulfonic acid, tartaric acid and maleic acid. Further, in case the compounds of this invention contain an acidic group, the acidic group can exist in the form of alkali metal salts such as, for example, a potassium salt and a sodium salt; alkaline earth metal salts such as, for example, a magnesium salt and a calcium salt; and salts with organic bases such as a triethylammonium salt and an arginine salt. The compounds of the present invention may be hydrated or non-hydrated.
The compounds of this invention can be administered in such oral dosage forms as tablets, capsules (each of which includes sustained release or timed release formulations), pills, powders, granules, elixirs, tinctures, suspensions, syrups and emulsions. The compounds of this invention may also be administered intravenously, intraperitoneally, subcutaneously, or intramuscularly, all using dosage forms well known to those skilled in the pharmaceutical arts. The compounds can be administered alone, but generally will be administered with a pharmaceutical carrier selected upon the basis of the chosen route of administration and standard pharmaceutical practice. Compounds of this invention can also be administered in intranasal form by topical use of suitable intranasal vehicles, or by transdermal routes, using transdermal skin patches. When compounds of this invention are administered transdermally the dosage will be continuous throughout the dosage regimen.
The dosage and dosage regimen and scheduling of a compounds of the present invention must in each case be carefully adjusted, utilizing sound professional judgment and considering the age, weight and condition of the recipient, the route of administration and the nature and extent of the disease condition. In accordance with good clinical practice, it is preferred to administer the instant compounds at a concentration level which will produce effective beneficial effects without causing any harmful or untoward side effects.
Compounds of the present invention may be synthesized according to the description provided below. Variables provided in the schema below are defined in accordance with the description of compounds of Formula (I) unless otherwise specified.
The following Intermediates 1-13 may be used to synthesize Examples 1-51.
2-Methyl-4,5-diphenyl-1H-imidazole: (Scheme 1, (A)) To a solution of benzil (3.0 g, 14 mmol) in glacial acetic acid (100 mL) was added ammonium acetate (22.2 g, 284 mmol) followed with acetaldehyde (1.26 g, 28 mmol). The resultant suspension was stirred at 100° C. for 2.5 hours. After removal of most of solvent, the residue was dissolved in EtOAc. The precipitate ammonium acetate was filtered off. The filtrate was washed with 2N NaOH, H2O, and then was dried over MgSO4. After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO2: EtOAc/Hexanes). This compound was obtained as a white solid (0.96 g, 4.1 mmol, 29% yield): mp 232-235° C.; MS m/e 235.0 (MH+); 1H NMR (DMSO-d6) δ7.27 (br m, 10H), 2.33 (s, 3H); 13C NMR (DMSO-d6) δ144.3, 128.7, 128.3, 127.8, 127.3, 126.3, and 14.0. Anal. Calcd for C16H14N2.0.12 H2O: C, 81.26; H, 6.07; N, 11.85. Found:C, 81.20; H, 6.03; N, 11.89.
2-Ethyl-4,5-diphenyl-1H-imidazole: (Scheme 1, (A)) Prepared as described for the example above. 1H NMR (DMSO): δ1.30 (t, 3H), 2.72 (q, 2H), 7.44 (b, 10H), 12.02 (b, 1H); Mass Spec: 249.26 (MH+).
4,5-Bis-(4-fluoro-phenyl)-2-methyl-1H-imidazole: (Scheme 1, (A)) Prepared as described for the example above. 1H NMR (DMSO): δ2.32 (s, 3H), 7.13 (t, 2H), 7.27 (t, 2H), 7.47 (m, 4H), 12.15 (b, 1H).
7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester: (Scheme 1, (B)) To a solution of 2-methyl-4,5-diphenyl-1H-imidazole (0.20 g, 0.85 mmol) in DMF (6 mL) was added NaH (60% in mineral oil, 0.038 g, 0.94 mmol). The resulting mixture was stirred at rt for 10 min. The stirring continued for 2 hours after addition of ethyl 7-bromoheptanoate (0.21 g, 0.90 mmol). The reaction mixture was diluted with diethyl ether (30 mL), washed by water, and then was dried over MgSO4. After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO2: EtOAc/Hexanes). This compound was obtained as a colorless oil (0.24 g, 0.61 mmol, 72% yield): 1H NMR (DMSO-d6) δ1.08 (m, 4H), 1.15 (t, J=7.2 Hz, 3H), 1.33 (m, 4H), 2.16 (t, J=6.6 Hz, 2H), 2.40 (s, 3H), 3.68 (t, J=7.8 Hz, 2H), 4.03 (q, J=4.5 Hz, 2H), 7.05)m, 1H), 7.13 (m, 2H), 7.34 (m, 4H), 7.48 (m, 3H); 13C NMR (DMSO-d6) δ13.4, 14.4, 24.3, 25.7, 27.8, 29.6, 33.5, 43.3, 59.9, 125.8, 126.0, 128.1, 128.3, 128.8, 129.3, 131.1, 131.8, 135.2, 135.3, and 144.0. Anal. Calcd for C25H30N2O2: C, 76.89; H, 7.74; N, 7.17. Found: C, 76.33; H, 7.67, N, 6.85.
8-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-octanoic acid ethyl ester: (Scheme 1, (B)) Prepared as described for the example above. 1H NMR (DMSO): δ1.06 (b, 6H), 1.20 (t, 3H), 1.42 (m, 4H), 2.22 (t, 2H), 2.49 (s, 3H), 3.70 (t, 2H), 4.06 (q, 2H), 7.16 (m, 3H), 7.35 (m, 4H), 7.51 (m, 3H). Mass Spec: 405.32 (MH+).
6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexanoic acid ethyl ester: (Scheme 1, (B)) Prepared as described for the example above. 1H NMR (DMSO): δ1.13 (m, 2H), 1.17 (t, 3H), 1.43 (m, 4H), 2.15 (t, 2H), 2.40 (s, 3H), 3.70 (t, 2H), 4.04 (q, 2H), 7.13 (m, 3H), 7.47 (m, 4H), 7.54 (m, 3H). Mass Spec: 377.26 (MH+).
8-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester: (Scheme 1, (B)) Prepared as described for the example above. 1H NMR (DMSO-d6) δ1.08 (m, 4H), 1.15 (t, J=7.2 Hz, 3H), 1.33 (m, 7H), 2.16 (t, J=6.6 Hz, 2H), 2.71 (q, J=7.5 Hz), 3.68 (t, J=7.8 Hz, 2H), 4.03 (q, J=4.5 Hz, 2H), 7.05 (m, 1H), 7.13 (m, 2H), 7.34 (m, 4H), 7.48 (m, 3H). Anal. Calcd for C26H32N2O2: C, 77.19; H, 7.97; N, 6.92. Found: C, 77.06; H, 8.13; N, 6.89. Mass Spec: 405.2 (MH+).
7-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-heptanoic acid ethyl ester: (Scheme 1, (B)) Prepared as described for the example above. 1H NMR (DMSO): δ1.09 (m, 4H), 1.17 (t, 3H), 1.38 (m, 4H), 2.19 (t, 2H), 2.39 (s, 3H), 3.6 (t, 2H), 4.05 (q, 2H), 7.03 (t, 2H), 7.36 (m, 4H), 7.41 (m, 2H). Mass Spec: 427.49 (MH+).
7-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-heptanoic acid: (Scheme 1, (C)) To a solution of 7-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-heptanoic acid ethyl ester (1.9 g, 4.4 mmol) in EtOH (10 mL) was added NaOH (10 N, 2 mL, 20 mmol). The resulting mixture was stirred at rt for 1 hour, diluted with EtOAc (100 mL), washed by HCl (0.5 N), and then was dried over MgSO4. After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO2: MeOH/CH2Cl2). This compound was obtained as a white solid in HCl salt form (1.9 g, 4.3 mmol, 98% yield): 1H NMR (DMSO): δ1.15 (m, 4H), 1.37 (t, 2H), 1.47 (t, 2H), 2.13 (t, 2H), 2.73 (s, 3H), 4.03 (t, 2H), 7.35 (t,2H), 7.45 (m, 4H), 7.57 (m, 2H), 12.1 (b, 1H).
8-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid: (Scheme 1, (C)) Prepared as described for the example above. 1H NMR (DMSO-d6) δ11.95 (br s, 1H), 7.56 (m, 3H), 7.46 (m, 2H), 7.38 (m, 2H), 7.28 (m, 3H), 3.83 (t, 2H, J=7.5 Hz), 2.67 (s, 3H), 2.09 (t, 2H, J=7.5 Hz), 1.38 (m, 2H), 1.25 (m, 2H), and 1.09 (m, 4H), 13C NMR (DMSO-d6) δ174.5, 144.4, 131.3, 130.1, 129.6, 128,9, 128.8, 128.4, 128.1, 126.8, 44.3, 33.8, 29.4, 28.9, 25.6, 22.3 and 11.7. Anal. Calcd for C23H26N2O2.0.95 HCl.0.32 C6H14: C, 70.48; H, 7.46; N, 6.60. Found: C, 70.82; H, 7.08, N, 6.64.
6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexanoic acid: (Scheme 1, (C)) Prepared as described for the example above. 1H NMR DMSO): δ1.17 (m, 2H), 1.33 (m, 2H), 1.51 (m, 2H), 2.09 (t, 2H), 2.76 (s, 3H), 4.03 (t, 2H), 7.38 (m, 5H), 7.49 (m, 2H), 7.65 (m, 3H).
8-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-octanoic acid: (Scheme 1, (C)) Prepared as described for the example above. 1H NMR (DMSO): δ1.07 (b, 6H), 1.39 (m, 2H), 1.48 (m, 2H), 2.15 (t, 2H), 2.72 (s, 3H), 3.92 (t, 2H), 7.35 (s, 5H), 7.52 (m, 2H), 7.606 (m, 3H), 12.1 (b, 1H). Anal. Calcd. for C24H28N2O2.0.982HCl. 0.59H2O: C, 68.16; H, 7.19; N, 6.62. Found: C, 68.00; H, 7.09; N, 6.81.
8-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid: (Scheme 1, (C)) Prepared as described for the example above. 1H NMR (DMSO-d6) δ11.95 (br s, 1H), 7.56 (m, 3H), 7.46 (m, 2H), 7.38 (m, 2H), 7.28 (m, 3H), 3.83 (t, 2H, J=7.5 Hz), 3.13 (q, J=7.8 Hz, 2H), 2.09 (t, 2H, J=7.5 Hz), 1.38 (m, 5H), 1.25 (m, 2H), and 1.09 (m, 4H). Anal. Calcd for C24H28N2O2.1.00HCl.0.44 C6H14: C, 68.44; H, 7.15; N, 6.65. Found: C, 68.43; H, 6.98; N, 6.53.
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester: (Scheme 1 (D)) To a suspension of 8-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid (11.3 g, 28.3 mmol) in a mixture of Et3N (10 g, 99 mmol) and toluene (200 mL) was added azide (11.0 g, 39.7 mmol). The resultant mixture was stirred at r.t. for 10 min. and then at 108° C. under N2 for 90 min. After the mixture was cooled to r.t., 2-fluorophenol (3.8 g, 37 mmol) was added. The reaction mixture was stirred at r.t. for 10 min and then at 80° C. for 1 h. The mixture was diluted with EtOAc, washed with H2O, and then was dried over MgSO4. After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO2: EtOAc/Hexanes). This compound was obtained as a white solid (7.3 g, 15.5 mmol, 55% yield): mp 129-131° C.; 1H NMR (DMSO-d6) δ7.85 (br s, 1H), 7.50 (m, 3H), 7.33 (m, 5H), 7.30-7.05 (m, 6H), 3.69 (t, 2H, J=4.8 Hz), 2.95 (dd, 2H, J=4.8, 3.6 Hz), 2.4 (s, 3H), 1.4 (m, 2H), 1.3 (m, 2H), 1.09 (m, 4H). Anal. Calcd for C29H30FN3O2: C, 73.86; H, 6.41; N, 8.91. Found: C, 73.63; H, 6.45; N, 8.81. Mass Spec: 472.2 (MH+).
[6-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid tert-butyl ester (Scheme 1, (D)) Prepared as described for the example above. 1H NMR (DMSO): δ1.04 (m, 4H), 1.28 (m, 7H), 1.35 (s, 9H), 2.79 (m, 4H), 3.68 (t, 2H), 7.08 (t, 1H), 7.16 (t, 2H), 7.36 (m, 4H), 7.51 (m, 3H). Anal. Calcd. for C28H37N3O2. 0.196 CH2Cl2. 0.4 C6H14: C, 73.68; H, 8.69; N, 8.43. Found: C, 73.81; H, 8.38; N, 8.19. Mass Spec: 448.2 (MH+).
6-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid sec-butyl ester: (Scheme 1, (D)) Prepared as described for the example above. 1H NMR (DMSO): δ0.84 (t, 3H), 1.03 (bs, 4H), 1.11 (d, J=6.27 Hz, 3H), 1.36 (t, 2H), 1.48 (m, 7H), 2.76 (q, 2H), 2.84 (q, 2H), 3.71 (t, 2H), 4.55 (m, 1H), 6.8 (t, 1H), 7.05 (m, 1H), 7.16 (t, 2H), 7.36 (m, 4H), 7.50 (m, 3H). Anal. Calcd. for C28H37N3O2. 0.17 CH2Cl2. 0.245 C6H14: C, 73.66; H, 8.50; N, 8.70. Found: C, 73.73; H, 8.19; N, 8.69. Mass Spec: 448.2 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-butyl ester: (Scheme 1, (D)) Prepared as described for the example above. Analytical HPLC 1.46 min (89%). Mass Spec: 454.3 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid benzyl ester: (Scheme 1 (D)) Prepared as described for the example above. This compound was purified by preparative HPLC (YMC 30×100 mm (5 uM packing), 10% MeOH/90% water/01% TFA as mobile phase A, 90% MeOH/10%water/0.1% TFA as mobile phase B). 1H NMR (DMSO): δ1.067 (bs, 4H), 1.26 (t, 2H), 1.47 (t, 2H), 2.73 (s, 3H), 2.91 (q, 2H), 3.94 (t, 2H), 5.01 (s, 2H), 7.20 (m, 3H), 7.35 (m, 8H), 7.49 (m, 2H), 7.59 (d, J=6.69 mHz, 3H). Mass Spec: 468.17 (MH+).
2-Propanone, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino)carbonyl]oxime:2-Propanone,O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime: (Scheme 1 (D)) Prepared as described for the example above. 1H NMR (DMSO): δ1.11 (m, 4H), 1.30 (m, 2H), 1.50 (m, 2H), 1.92 (d, J=9.25 mHz, 6H), 2.74 (s, 3H), 2.96 (m, 2H), 3.94 (t, 2H), 7.29 (t, 1H), 7.39 (m, 2H), 7.41 (m, 3H), 7.49 (m, 2H), 7.61 (m, 2H). Mass Spec: 433.31 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid cyclohexyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.64 min (85%). Mass spec: 460.21 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid methyl ester: Prepared as described for the example above. Analytical HPLC 1.33 min. (80%). Mass spec: 392.12 (MH+).
6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.50 min (83%). Mass Spec: 454.15 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.52 min (97%). Mass Spec: 472.09 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.54 min (96%). Mass Spec. 490.06 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical 1.61 min (95%). Mass Spec: 488.02 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-methoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.51 min (96%). Mass Spec: 484.11 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester: (Scheme 1 (D)) Prepared as described for the example above. H Analytical HPLC 1.54 min (92%). Mass Spec: 468.11 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-cyano-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.46 min (94%). Mass Spec: 479.08 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,6-dimethoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.43 min (94%). Mass Spec: 514.10 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-methoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.48 min (99%). Mass Spec: 484.12 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid methyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.41 min (98%). Mass Spec: 406.32 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid ethyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.49 min (95%). Mass Spec: 420.35 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.58 min (99%). Mass Spec: 468.32 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-fluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.60 min (98%). Mass Spec: 486.30 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.58 min (96%). Mass Spec: 486.31 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,4-difluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.61 min (90%). Mass Spec: 504.31 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.68 min (90%). Mass Spec: 502.29 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-methoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.59 min (90%). Mass Spec: 498.33 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid o-tolyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.63 min (90%). Mass Spec: 482.33 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.53 min (90%). Mass Spec: 493.31 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,6-dimethoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.53 min (96%). Mass Spec: 528.37 (MH+).
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-methoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.57 min (90%). Mass Spec: 498.33 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid ethyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.36 min (98%). Mass Spec: 392.35 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.48 min (97%). Mass Spec: 440.36 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-fluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.51 min (97%). Mass Spec: 458.33 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,4-difluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.52 min (95%). Mass Spec: 476.32 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-fluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.48 min (98%). Mass Spec: 458.33 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-chloro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.62 min (98%). Mass Spec: 474.29 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-methoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.49 min (99%). Mass Spec: 470.35 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid o-tolyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.56 min (95%). Mass Spec: 454.36 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-cyano-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.44 min (99%). Mass Spec: 465.32 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,6-dimethoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.44 min (99%). Mass Spec: 500.38 (MH+).
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-methoxy-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.46 min (97%). Mass Spec: 470.34 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 3,4-difluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.55 min (84%). Mass Spec: 490.32 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid isopropyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.47 min (83%). Mass Spec: 420.17 (MH+).
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.50 min (95%). Mass Spec: 508.29 (MH+).
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2,6-difluoro-phenyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.50 min (85%). Mass Spec: 526.31 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid ethyl ester: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.40 min (82%). Mass Spec: 406.15 (MH+).
Benzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime:Benzaldehyde, O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.48 min (89%). Mass Spec: 481.26 (MH+).
4-Fluorobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime:4-Fluorobenzaldehyde,O-[ 6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.58 min (87%). Mass Spec: 499.32 (MH+).
2-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime:2-Nitrobenzaldehye, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.56 min (95%). Mass Spec: 526.3 (MH+).
3-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime:3-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.57 min (83%). Mass Spec: 526.32 (MH+).
4-Nitrobenzaldehyde, O-{6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime:4-Nitrobenzaldehyde, O-{6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.58 min (94%). Mass Spec: 526.29 (MH+).
3-Pyridinecarboxaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime:3-Pyridinecarboxaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime: (Scheme 1 (D)) Prepared as described for the example above. Analytical HPLC 1.24 min (94%). Mass Spec: 482.26 (MH+).
The following Intermediates 14-20 may be used to synthesize Examples 52-74.
1-(5-Bromo-pentyl)-2-methyl-4,5-diphenyl-1H-imidazole: (Scheme 2 (E)) To a solution of 2-Methyl-4,5-diphenyl-1H-imidazole (2.0 g, 8.5 mmol) and 1,5-dibromopentane (3.01 g, 12.7 mmol) in DMF (100 mL) was added NaH (60% in mineral oil, 0.50 g, 12.7 mmol). The resulting mixture was stirred at rt for 1 hour, quenched by addition of water, extracted by CH2Cl2, washed by water, and then was dried over MgSO4. After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO2: EtOAc/Hexanes). This compound was obtained as a pale yellow oil (2.2 g, 5.7 mmol, 67% yield): 1H NMR (DMSO): δ1.20 (m, 2H), 1.47 (m, 2H), 1.64 (m, 2H), 2.41 (s, 3H), 3.45 (t, 2H), 3.72 (t, 2H), 7.16 (m, 3H), 7.31 (m, 4H), 7.55 (t, 3H). Mass Spec: 384.57 (MH+).
1-(6-Bromo-hexyl)-2-methyl-4,5-diphenyl-1H-imidazole: (Scheme 2 (E)) Prepared as described for the example above. 1H NMR (DMSO): δ1.2 (m, 4H), 1.5 (m, 2H), 1.75 (m, 2H), 2.5 (s, 3H), 3.4 (t, 2H), 3.69 (t, 2H), 7.14 (m, 3H), 7.36 (m, 4H), 7.516 (m, 3H). Mass Spec: 399.14 (MH+).
1-(3-Bromo-propyl)-2-methyl-4,5-diphenyl-1H-imidazole: (Scheme 2 (E)) Prepared as described for the example above. 1H NMR (DMSO): δ1.99 (m, 2H), 2.43 (s, 3H), 3.39 (t, 2H), 3.88 (t, 2H), 7.17 (m, 3H), 7.35 (m, 4H), 7.53 (m, 3H), Mass Spec: 356.59 (MH+).
4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-benzoic acid ethyl ester: (Scheme 2 (H)) To a solution of 1-(3-Bromo-propyl)-2-methyl-4,5-diphenyl-1H-imidazole (0.80 g, 2.2 mmol) and ethyl 4-hydroxybenzoate (1.20 g, 7.2 mmol) in DMF (30 mL) was added K2CO3 (0.40 g, 2.9 mmol). The resulting mixture was stirred at 55° C. for 1 hour, quenched by addition of water, extracted by EtOAc, washed by water, and then was dried over MgSO4. After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO2: EtOAc/Hexanes). This compound was obtained as a pale yellow gum (0.92 g, 2.0 mmol, 94% yield): 1H NMR (DMSO): δ1.32 (t, 3H), 1.85 (m, 2H), 2.402 (s, 3H), 3.87 (m, 4H), 4.28 (q, 2H), 6.89 (d, J=8.82 Hz, 2H), 7.143 (m, 3H), 7.36 (m, 4H), 7.46 (m, 3H), 7.82 (d, J=8.85 mHz, 2H). Mass spec: 441.28 (MH+).
4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-benzoic acid ethyl ester: (Scheme 2 (H)) Prepared as described for the example above. 1H NMR (DMSO): δ1.31 (t, 3H), 2.5 (s, 3H), 4.07 (m, 2H), 4.15 (m, 2H), 4.26 (q, 2H), 6.91 (d, J=8.88 mHz, 2H), 7.16 (m, 3H), 7.33 (d, J=7.56 mHz, 2H), 7.41 (m, 2H), 7.53 (m, 3H), 7.85 (d, J=8.85 mHz, 2H).
4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-benzoic acid: (Scheme 2 (I)) To a solution of 4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-benzoic acid ethyl ester (0.80 g, 1.8 mmol) in EtOH (20 mL) was added NaOH (10 N, 4.0 mL, 40.0 mmol). The resulting mixture was stirred at rt for 3 hours, diluted with water, acidified to pH˜1 using 1N HCl, extracted by CH2Cl2, and then was dried over MgSO4. After filtration and concentration in vacuo, the residue was purified by flash chromatography (SiO2: MeOH/CH2Cl2). This compound was obtained as a white dry foam in HCl salt form (0.80 g, 1.8 mmol, 99% yield): 1H NMR (DMSO): δ1.93 (m, 2H), 2.67 (s, 3H), 3.97 (t, 2H), 4.13 (t, 2H), 6.85 (d, J=8.82 mHz, 2H), 7.33 (s, 5H), 7.43 (m, 2H), 7.55 (m, 3H), 7.86 (d, J=8.82 mHz, 2H), 12.64 (b, 1H).
4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxyl-benzoic acid: (Scheme 2 (I)) Prepared as described for the example above. 1H NMR (DMSO): δ2.82 (s, 3H), 4.19 (m, 2H), 4.39 (m, 2H), 6.93 (d, J=8.85 mHz, 2H), 7.359 (m, 5H), 7.54 (m, 2H), 7.61 (m, 3H), 7.86 (d, J=8.76 mHz, 2H), 12.685 (b, 1H).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 3,4-difluoro-phenyl ester: (Scheme 2 (J)) To a suspension of 4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-benzoic acid (0.10 g, 0.23 mmol) in a mixture of Et3N (0.09 g, 0.88 mmol) and toluene (2 mL) was added azide (0.1 g, 0.35 mmol). The resultant mixture was stirred at r.t. for 10 min. and then at 108° C. under N2 for 45 min. After the mixture was cooled to r.t., 3,4-difluorophenol (0.10 g, 1.0 mmol) was added. The reaction mixture was stirred at r.t. for 10 min and then at 80° C. for 1 h. The mixture was diluted with EtOAc, washed with H2O. After filtration and concentration in vacuo, the residue was purified by preparative HPLC (YMC 30×100 mm (5 uM packing), 10% MeOH/90% water/01% TFA as mobile phase A, 90% MeOH/10% water/0.1% TFA as mobile phase B). This compound was obtained as a white solid (0.082 g, 0.13 mmol, 55% yield): 1H NMR (DMSO): δ2.83 (s, 3H), 4.06 (t, 2H), 4.39 (t, 2H), 6.82 (d, J=7.05 mHz, 2H), 7.29 (m, 2H), 7.32 (m, 2H), 7.45 (m, 4H), 7.52 (m, 4H), 7.61 (m, 3H). Mass Spec: 526.22 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. 1H NMR (DMSO): δ2.83 (s, 3H), 4.07 (t, 2H), 4.39 (t, 2H), 6.83 (d, J=10.3 mHz, 2H), 7.24 (d, J=10.3 mHz, 2H), 7.30 (m, 2H), 7.36 (m, 5H), 7.46 (d, J=12.6 mHz, 2H), 7.59 (m, 2H), 7.61 (m, 3H). Mass Spec: 524.18 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid methyl ester: (Scheme 2 (J)) Prepared as described for the example above. 1H NMR (DMSO): δ2.82 (s, 3H), 3.62 (s, 3H), 4.05 (t, 2H), 4.38 (t, 2H), 6.77 (d, J=7 mHZ, 2H), 7.28 (m, 1H), 7.31 (m, 3H), 7.36 (m, 3H), 7.53 (m, 2H), 7.61 (m, 3H), 9.45 (b, 1H). Mass Spec: 428.24 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3,4-difluoro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.66 min (95%). Mass Spec: 540.25 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-methoxy-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.52 min (98%). Mass Spec: 534.35 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.62 min (81%). Mass Spec: 538.22 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-methoxy-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.49 min (95%). Mass Spec: 534.43 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3-chloro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.71 min (90%). Mass Spec: 538.23 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.58 min (84%). Mass Spec: 490.25 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 2-fluoro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.49 min (92%). Mass Spec: 508.23 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-fluoro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.53 min (92%). Mass Spec: 508.23 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol1-yl)-propoxy]-phenyl}-carbamic acid phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.53 min (95%). Mass Spec: 504.39 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-methoxy-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.50 min (94%). Mass Spec: 520.24 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid o-tolyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.54 min (92%). Mass Spec: 504.25 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-fluoro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.52 min (95%). Mass Spec: 522.32 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 2-methoxy-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.46 min (97%). Mass Spec: 520.25 (MH+).
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 3-chloro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.61 min (97%). Mass Spec: 524.18 (MH+).
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2,6-difluoro-phenyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.58 min (90%). Mass Spec: 540.25 (MH+). Notebook number:
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid ethyl ester: (Scheme 2 (J)) Prepared as described for the example above. Analytical HPLC 1.45 min (72%). Mass Spec: 442.25 (MH+).
The following Intermediates 21-24 may be used to synthesize Examples 75-77.
2-Methyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester: (Scheme 3 (O)) Sodium bis (trimethylsilyl)amide (1M in THF) (3.0 ml, 3.0 mmole) was added dropwise to a solution of starting material 7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester (1 g, 2.5 mmole) in anhydrous THF (10 ml) at −78° C. under Nitrogen. After addition, the reaction was let stirred 2 minutes, then Iodomethane (0.06 ml, 0.96 mmole) was added slowly at −78° under Nitrogen. The reaction mixture was let stirred at −78° C. for 1 hr, then warming up to room temperature and let stirred for 18 hrs. The next day, analysis by TLC indicated consumption of starting material. The reaction was quenched with aqueous Ammonium Chloride (10 ml). The aqueous layer was extracted with Ethyl Acetate (3×25 ml). The organic layers obtained were combined, dried over Sodium Sulfate and filtered. The resultant filtrate was concentrated in vacuo. Purification by flash column chromatography using Hexane/Ethyl Acetate (4:1) gave rise to product (150 mg, 45%). 1H NMR (CDCl3): δ1.09 (d, J=6.95 mHz, 6H), 1.15 (t, 3H), 1.51 (m, 2H), 2.32 (m, 1H), 2.50 (s, 3H), 3.70 (t, 2H), 4.11 (q, 2H), 7.25 (m, 3H), 7.32 (m, 2H), 7.45 (m, 5H), 13C NMR (CDCl3): δ13.7, 14.3, 17.1, 26.4, 26.6, 30.3, 33.4, 39.4, 43.8, 60.2, 125.9, 126.5, 128.0, 128.4, 128.5, 129.0, 131.1, 131.8, 134.8, 136.3, 144.0, 176.6. Anal. Calcd. for C26H32N2O20.25 H2O: C, 76.34; H, 8.01; N, 6.85. Found: C, 76.38; H, 8.13; N, 6.83. Mass Spec: 405.29 (MH+).
2-Ethyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester: (Scheme 3 (O)) This compound was obtained using the procedures as described above. The following scales and reagents were used: 7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester (390 mg, 1 mmole), Sodium bis(trimethylsilyl)amide (1M in THF) (1.2 ml, 1.2 mmole), Iodoethane (0.4 ml, 779.8 mg, 5 mmole), anhydrous THF (10 ml). Product was obtained (100 mg, 24%). 1H NMR (CDCl3): δ0.88 (t, 3H), 1.23 (m, 4H), 1.33 (t, 3H), 1.54 (m, 4H), 1.68 (b, 1H), 2.04 (s, 1H), 2.17 (m, 1H), 2.49 (s, 3H), 3.709 (t, 2H), 4.15 (m, 2H), 7.19 (m, 3H), 7.42 (m, 3H), 7.46 (m, 4H). 13C NMR (CDCl3): δ12.0, 13.9, 14.6, 25.7, 26.6, 27.0, 30.5, 31.9, 44.0, 47.3, 60.2, 126.1, 126.7, 128.2, 128.7, 129.2, 131.3. Anal. Calcd. for C27H34N2O2: C, 77.48; H, 8.19; N, 6.69. Found: C, 77.34; N, 8.01; N, 6.56. Mass Spec: 419.32 (MH+).
2-Isopropy-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester: (Scheme 3 (O)) Sodium bis (trimethylsilyl)amide (1M in THF) (1.2 ml, 1.2 mmole) was added dropwise to a solution 7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester (390 mg, 1 mmole) in anhydrous THF (10 ml) at −78° C. under Nitrogen. The reaction solution was let stirred warming up to room temperature during a period of 3 hrs. The reaction solution was cooled to −78° C., and 2-Iodopropane (499.97 ul, 849.95 mg, 5 mmole) was added in dropwise. The reaction was let stirred at room temperature for 1 hr, then at 50° C. for 1 hr. Analysis by TLC indicated consumption of starting material. The reaction was worked-up using the procedures as described above. Crude material was purified by flash column chromatography using Hexane/Ethyl Acetate (4:1) to give product (80 mg, 18.5%). 1H NMR CDCl3): δ0.89 (t, 6H), 1.14 (m, 4H), 1.249 (t, 3H), 1.49 (m, 3H), 1.81 (m, 2H), 1.989 (m, 1H), 2.49 (s, 3H), 3.70 (t, 2H), 4.1 (m, 2H), 7.15 (m, 1H), 7.31 (t, 2H), 7.40 (m, 2H), 7.445 (m, 5H). 13C NMR (CDCl3): δ13.7, 14.4, 20.2, 20.4, 26.5, 27.2, 29.3, 30.3, 30.7, 43.8, 52.6, 59.9, 125.9, 126.5, 128.0, 128.3, 128.5, 129.0, 131.0, 131.8, 134.8, 136.3, 144.0, 175.6. Anal. Calcd. for C28H36N2O2.0.21 H2O: C, 77.07, H, 8.41; N, 6.42. Found: C, 77.08; H, 8.84; N, 6.22. Mass Spec: 433.2 (MH+).
2-Methyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid: (Scheme 3 (P)) A solution of starting material 2-Methyl-7-(2-methyl-4,5-diphenyl-imidazol-2-yl)-heptanoic acid ethyl ester (130 mg, 0.32 mmole) in Methanol (5 ml) and Sodium Hydroxide (64 mg, 1.61 mmole) was let stirred under reflux for 18 hrs. The next day, the reaction was let cooled to room temperature and concentrated in vacuo. The residue was diluted with water, and acidified with Hydrochloric Acid (3N). The aqueous layer was extracted with Dichloromethane (3×10 ml). The organic layers were combined, dried over Sodium Sulfate and filtered. The resultant filtrate was concentrated in vacuo to afford product as a white solid (120 mg, 99%). Mass Spec: 377 (MH+).
2-Ethyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid: (Scheme 3 (P)) This compound was obtained using the procedures as described above. The following scales and reagents were used: 2-Ethyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester (100 mg, 0.24 mmole), Sodium hydroxide (2N, 0.5 mL, 1.0 mmole), Methanol (5 mL). Product was obtained as white solid (90 mg, 96%). Mass Spec: 391.25 (MH+).
2-Ethyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid: (Scheme 3 (P)) This compound was obtained using the procedures as described above. The following scales and reagents were used: 2-Isopropyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid ethyl ester (80 mg, 0.18 mmole), Sodium hydroxide (2N, 0.5 mL, 1.0 mmole), Methanol (5 mL). Product was obtained as white solid (46 mg, 64%). Mass Spec: 405.37 (MH+).
[1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester: (Scheme 3 (Q)) Diphenylphosphoryl Azide (0.083 ml, 0.38 mmole) was added to a suspension of starting material 2-methyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid (120 mg, 0.32 mmole) and Triethylamine (0.14 ml, 1.005 mmole) in Toluene (5 ml) at room temperature. The reaction mixture was let stirred at room temperature for 10 minutes under Nitrogen, then at 108° C. for 90 minutes. The reaction was let cooled to room temperature, to which 2-Fluorophenol (0.03 ml, 0.038 g, 0.338 mmole) was added. The reaction mixture was let stirred at room temperature for 30 minutes, then at 100° C. for 18 hrs. The next day, analysis by TLC (Dichloromethane/Ethyl Acetate 3:1) indicated consumption of starting material. The reaction was let cooled to room temperature, where the solvent was removed by rotorvap. The crude material was purified by flash column chromatography using Dichloromethane/Ethyl Acetate (6:1 to 3:1). Product was obtained (110 mg, 71%). 1H NMR (CDCl3): δ1.21 (d, J=8.75 mHz, 6H), 1.35 (m, 2H), 1.51 (m, 2H), 2.50 (s, 3H), 3.71 (m, 3H), 4.93 (b, 1H), 7.18 (m, 6H), 7.32 (m, 2H), 7.46 (m, 6H). 13C NMR (CDCl3): 813.7, 21.1, 25.2, 30.3, 36.7, 43.7, 47.5, 116.5, 116.650, 124.132, 124.325, 125.943, 126.520, 128.027, 128.347, 128.509, 129.018, 131.101, 131.796, 134.8, 136.4, 138.6, 144.1, 153.0, 153.7, 155.6. Anal. Calcd. for C30H32FN3O20.42 H2O: C, 73.06; H, 6.71; N, 8.52. Found: C, 73.24; H, 6.82; N, 8.29. Mass Spec: 486.27 (MH+).
[1-Ethyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester: (Scheme 3 (Q)) This compound was prepared using the procedures as described above. The following scales and reagents were used: Starting material 2-ethyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid (90 mg, 0.23 mmole). Diphenylphosphoryl Azide (0.060 ml, 0.276 mmole), Triethylamine (0.14 ml, 1.005 mmole), and 2-Fluorophenol (30 ul, 0.338 mmole). After purification by flash column chromatography, product was obtained (90 mg, 18%). 1H NMR (CDCl3): δ0.96 (t, 3H), 1.24 (m, 5H), 1.51 (m, 2H), 1.54 (m, 3H), 2.50 (s, 3H), 3.72 (t, 2H), 4.77 (d, J=9.28 mHz, 1H), 7.16 (m, 7H), 7.342 (m, 2H), 7.45 (m, 5H). 13C NMR (CDCl3): δ10.2, 13.7, 25.1, 26.3, 28.2, 30.3, 34.7, 43.7, 53.076, 116.466, 116.650, 124.108, 124.276, 125.937, 126.527, 128.015, 128.348, 128.483, 129.001, 131.1, 131.8, 134.8, 136.3, 144.1, 153.5. Anal. Calcd. for C31H34FN3O2: C, 74.52; H, 6.86; N, 8.41. Found: C, 74.43; H, 6.98; N, 8.32. Mass Spec: 500.34 (MH+).
[1-Isopropyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester: (Scheme 3 (Q)) This compound was obtained using the procedures as described above. The following scales and reagents were used: Starting material 2-isopropyl-7-(2-methyl-4,5-diphenyl-imidazol-1-yl)-heptanoic acid (45.8 mg, 0.113 mmole), Diphenylphosphoryl Azide (0.029 ml, 0.135 mmole), Triethylamine (0.049 ml, 0.351 mmole), and 2-Fluorophenol (0.012 ml, 0.135 mmole). After purification by flash column chromatography, product was obtained (27.5 mg, 47%). 1H NMR (CDCl3): δ0.95 (m, 6H), 1.27 (m, 5H), 1.53 (m, 2H), 1.73 (m, 2H), 2.50 (s, 3H), 3.56 (m, 1H), 3.73 (t, 2H), 4.75 (d, J=10 mHz, 1H), 7.19 (m, 7H), 7.33 (m, 2H), 7.45 (m, 5H). 13C NMR (CDCl3): δ13.7, 17.5, 19.3, 25.5, 26.3, 30.3, 32.2, 32.3, 43.7, 56.7, 116.5, 116.6, 124.1, 124.3, 124.3, 125.9, 126.4, 126.5, 128.0, 128.3, 128.5, 128.8, 131.1, 131.8, 134.8, 136.4, 144.1, 153.7, Anal. Calcd. for C32H36FN3O2.0.59 H2O: C, 73.31; H, 7.15; N, 8.01. Found: C, 73.45; 7.20; N, 7.61. Mass Spec: 514.2 (MH+).
The following Intermediates 27-29 may be used to synthesize Example 78.
2-[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-2-phenyl]-malonic acid diethyl ester: (Scheme 4 (R)) A solution of star[]ting material 1-(5-bromo-pentyl)-2-methyl-4,5-diphenyl-1H-imidazole (0.5 g, 1.3 mmole) in DMF (5 ml) was added to a suspension of Sodium Hydride (63 mg, 1.56 mmole) in DMF (5 ml) at room temperature under Nitrogen. The reaction suspension was let stirred for 30 minutes at room temperature. Diethyl Phenyl Malonate (0.29 ml, 313.5 mg, 1.3 mmole) was added to the reaction suspension dropwise at room temperature under nitrogen. The reaction mixture was let stirred at 45-50° C. for 48 hrs. Analysis by TLC indicated only a trace of starting material remained. The reaction was let cooled to room temperature, then poured into saturated Sodium Chloride solution. The aqueous layer was extracted with Ethyl Acetate (3×25 ml). The organic layers were combined and washed with water (1×30 ml). The organic layer was separated, dried over Sodium Sulfate and filtered. The filtrate was concentrated in vacuo. The crude material was purified by flash column chromatography using Ethyl Acetate/Toluene (2.5:7.5). Product was obtained as a colorless oil (650 mg, 93%). 1H NMR (CDCl3): δ1.12 (m, 4H), 1.20 (t, 6H), 1.47 (m, 2H), 2.16 (t, 2H), 2.46 (s, 3H), 3.66 (t, 2H), 4.22 (m, 4H), 7.15 (m, 1H), 7.26 (m, 4H), 7.32 (m, 5H), 7.43 (m, 5H). 13C NMR (CDCl3): δ13.7, 14.0, 24.2, 26.8, 30.2, 35.7, 43.8, 61.5, 62.5, 125.9, 126.5, 127.5, 127.9, 128.1, 128.2, 128.3, 128.5, 129.0, 131.0, 144.1, 170.6. Anal. Calcd. for C34H38N2O4.0.34 Toluene: C, 76.77; H, 7.20; N, 4.91. Found: C, 76.64; H, 7.27; N, 4.78. Mass Spec: 539.29 (MH+).
2-[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-2-phenyl-malonic acid: (Scheme 4 (S)) A solution of starting material 2-[5-(2-methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-2-phenyl-malonic acid diethyl ester (630 mg, 1.17 mmole) in THF (18 ml) and Sodium hydroxide (2N) (8 ml) was let stirred at 80° C. for 18 hrs. The next day, analysis by TLC indicated no significant change in the reaction. The THF solvent was removed by rotorvap. The residue was diluted in Methanol (20 ml). The reaction solution was let stirred under reflux for 2 hrs. Analysis by TLC indicated consumption of starting material. The organic solvent was removed by rotorvap. The residue was diluted with water (20 ml). The aqueous layer was extracted Diethyl Ether (2×20 ml). The organic layers were combined and extracted with Sodium Hydroxide (10%) (2×10 ml). The basic aqueous layers were combined and acidified with Hydrochloric acid (3N) to pH=1, then extracted with dichloromethane (2×20 ml). The organic layers were combined, dried over Sodium Sulfate and filtered. The resultant filtrate was concentrated in vacuo to provide product as a white solid (580 mg, quantitative yield). Mass Spec: 483.54 (MH+).
7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-2-phenyl-heptanoic acid: (Scheme 4 (T)) A solution of starting material 2-[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-2-phenyl-malonic acid (580 mg, 1.32 mmole)) in glacial Acetic Acid (20 ml) was let stirred under reflux for 18 hrs. The next day, the reaction was let cooled to room temperature and concentrated in vacuo. Product was obtained 398.6 mg, 77.7%). 1H NMR (CDCl3): δ1.11 (b, 4H), 1.42 (bd, 2H), 1.59 (m, 1H), 1.96 (m, 1H), 2.55 (s, 3H), 3.43 (t, 1H), 3.96 (t, 2H), 7.199 (m, 3H), 7.27 (m, 7H), 7.39 (m, 2H), 7.45 (m, 3H), 11.857 (b, 1H), 13C NMR (CDCl3): δ6 12.0, 21.4, 26.1, 26.8, 29.5, 29.7, 33.1, 44.2, 52.2, 127.0, 127.2, 127.4, 128.0, 128.4, 128.4, 128.5, 129.1, 129.3, 129.5, 130.6, 131.0, 133.0, 139.8, 144.1, 175.8, 177.7. Mass Spec: 439.24 (MH+).
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-1-phenyl-hexyl]-carbamic acid 2-fluoro-phenyl ester: (Scheme 4 (U)) Diphenylphosphoryl Azide (0.083 ml, 0.38 mmole) was added to a suspension of starting material 7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-2-phenyl-heptanoic acid (140 mg, 0.32 mmole) and Triethylamine (0.14 ml, 1.005 mmole) in Toluene (5 ml) at room temperature. The reaction mixture was let stirred at room temperature for 10 minutes under Nitrogen, then at 108° C. for 90 minutes. The reaction was let cooled to room temperature, to which 2-Fluorophenol (0.03 ml, 0.038 g, 0.338 mmole) was added. The reaction mixture was let stirred at room temperature for 10 minutes, then at 100° C. for 18 hrs. The next day, analysis by TLC (Dichloromethane/Ethyl Acetate 3:1) indicated consumption of starting material. The reaction was let cooled to room temperature, where the solvent was removed by rotorvap. The crude material was purified by flash column chromatography using dichloromethane/Ethyl Acetate (6:1 to 3:1). Product was obtained (58 mg, 33.1%). 1H NMR (CDCl3): δ1.18 (t, 3H), 1.27 (t, 2H), 1.497 (t, 2H), 2.47 (s, 3H), 3.70 (t, 2H), 4.63 (q, 1H), 5.38 (d, J=8.16 mHz, 1H), 7.18 (m, 7H), 7.37 (m, 7H), 7.44 (m, 5H). 13C NMR (CDCl3): δ13.6, 25.4, 26.1, 30.2, 36.0, 43.6, 55.7, 116.5, 116.7, 124.1, 124.3, 124.3, 126.0, 126.4, 126.5, 127.7, 128.0, 128.3, 128.5, 128.8, 129.0, 131.0, 131.7, 134.7, 136.3, 141.6, 144.1, 153.0. Anal. Calcd. for C35H34FN3O2.0.42 H2O: C, 75.71; H, 6.32; N, 7.57. Found: C, 75.75; H, 6.49; N, 7.50. Mass Spec: 548.27 (MH+).
Determination of FAAH Activity
Homogenates of crude membranes were prepared from H4 cells that express transfected human FAAH (H4-FAAH cells). Briefly, cells were grown in DMEM supplemented with 10% FBS and Geneticin at a final concentration of 500 μg/ml (Gibco BRL, Rockville, Md.). Confluent cultures of H4-FAAH cells were rinsed twice with phosphate-buffered saline [138 mM NaCl, 4.1 mM KCl, 5.1 mM Na2PO4, 1.5 mM KH2PO4 (pH 7.5), 37° C.] and incubated for 5-10 min. at 4° C. in lysis buffer [1 mM sodium bicarbonate]. Cells were transferred from plates to polypropylene tubes (16×100 mm), homogenized and centrifuged at 32,000×g for 30 min. Pellets were resuspended by homogenization in lysis buffer and centrifuged at 32,000×g for 30 min. Pellets were resuspended in lysis buffer (15-20 μg protein/ml) then stored at −80° C. until needed. On the day of an experiment, membranes were diluted to 2.67 μg protein/ml in 125 mM Tris-Cl, pH 9.0
Activity of FAAH was measured using a modification of the method described by Omeir et al., 1995 (Life Sci 56:1999, 1995). Membrane homogenates (240 ng protein) were incubated at room temperature for one hour with 1.67 nM anandamide [ethanolamine 1-3H] (American Radiolabeled Chemical Inc., St Louis, Mo.) and 10 μM anandamide (Sigma/RBI, St. Louis, Mo.) in the absence and presence of inhibitors. The reaction was stopped by the addition of 1 volume of a solution of methanol and dichloroethane (1:1). The mixture was shaken and then centrifuged at 1000×g for 15 min. to separate the aqueous and organic phases. An aliquot of the aqueous phase, containing [3H]-ethanolamine was withdrawn and counted by scintillation spectroscopy. Data were expressed as the percentage of [3H]-ethanolamine formed versus vehicle, after subtraction of the background radioactivity determined in the presence of 10 μM arachidonyl trifluoromethyl ketone (ATFMK), an inhibitor of FAAH. IC50 values were determined using a four-parameter logistic equation for dose-response curves. Compounds for which IC50 values are not provided herein showed no FAAH inhibition or marginal FAAH inhibition in preliminary tests.
* A<10 nM; B 10 nM<100 nM; C 100 nM<1,000 nM; D 1,000 nM<10,000 nM
| Example No. | IC50 (nM) | ||
| 1 | A | ||
| 2 | D | ||
| 3 | D | ||
| 5 | D | ||
| 6 | B | ||
| 8 | C | ||
| 9 | A | ||
| 10 | A | ||
| 11 | A | ||
| 12 | A | ||
| 13 | B | ||
| 14 | A | ||
| 15 | B | ||
| 16 | C | ||
| 17 | C | ||
| 18 | C | ||
| 19 | B | ||
| 20 | A | ||
| 21 | B | ||
| 22 | A | ||
| 23 | B | ||
| 24 | A | ||
| 25 | B | ||
| 26 | B | ||
| 27 | A | ||
| 28 | C | ||
| 29 | B | ||
| 30 | C | ||
| 31 | B | ||
| 32 | B | ||
| 33 | B | ||
| 34 | B | ||
| 35 | B | ||
| 36 | B | ||
| 37 | C | ||
| 38 | B | ||
| 39 | D | ||
| 40 | C | ||
| 41 | A | ||
| 43 | A | ||
| 44 | B | ||
| 45 | C | ||
| 46 | B | ||
| 47 | B | ||
| 48 | B | ||
| 49 | B | ||
| 50 | B | ||
| 51 | B | ||
| 52 | D | ||
| 53 | D | ||
| 55 | C | ||
| 56 | C | ||
| 57 | C | ||
| 59 | C | ||
| 60 | D | ||
| 61 | D | ||
| 62 | D | ||
| 63 | D | ||
| 64 | D | ||
| 66 | D | ||
| 69 | D | ||
| 70 | D | ||
| 71 | A | ||
| 72 | B | ||
| 73 | C | ||
| 74 | C | ||
In vivo results
In FIG. 1. , example 1, was active in phase I (acute phase) and phase II (chronic phase) of the rat formalin test. In animals that received 25 mg/kg, i.v, of Example 1, the number of paw flinches was reduced by nearly 40% in the first 10 minutes after administration of formalin. Paw flinches were reduced by approximately 30% over the following 50 minutes. The effect of example 1 was similar to that seen with a 3 mg/kg, i.p. dose of morphine.
In FIG. 2. , animals that received Example 1, the latency to paw withdrawal was increased significantly. The present results confirm, the activity of Example 1 against acute pain.
In FIG. 3. , Example 1 was examined in the Chung model of neuropathic pain where animals exhibit a pain response (paw withdrawal) to a normally innocuous stimulus (light touch), In animals with a neuropathic injury, the threshold for withdrawal of the injured paw was increased (toward normal) in a dose-dependent fashion by Example 1. The anti-neuropathic effect observed with 20 mg/kg Example 1 exhibited earlier onset of action compared to 100 mg/kg gabapentin (reference compound) with similar peak efficacy.
Claims (31)
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3 alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is HYC(O)O;
X is C and is attached to A;
Y is N and is attached to A;
G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H; C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is C3-7cycloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is —C1-2alkylene-phenyl said —C1-2alkylene-phenyl is optionally substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are optionally substituted with the same or different substitutents selected from the group consisting of halo, CN, —C(O)O—C1-3-alkyl, C1-3alkyl and C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are substituted with halo, —C(O)O—C1-3-alkyl, C1-3alkyl or C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are substituted with fluoro;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each independently H, C1-3alkyl or halo;
R3 is C1-C3alkyl or C3-7cycloalkyl;
A is C1-12alkylene or L;
L is -phenyl-O—C1-4alkylene wherein said C1-4alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C1-3 alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is phenyl or —C1-2alkylene-phenyl, said phenyl or phenyl of said —C1-2alkylene-phenyl are substituted with cyano;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each H;
R3 is C1-C3alkyl;
A is C7-10alkylene;
D is HYC(O)O;
Y is N and is attached to A;
G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy; and
G′ is H, C1-5alkyl or C1-5haloalkyl.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each H;
R3 is C1-C3alkyl;
A is C1-5alkylene;
D is HYC(O)O;
Y is N and is attached to A;
G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each H;
R3 is C1-C3alkyl;
A is C7-10alkylene;
D is HYC(O)ON═C(G′);
Y is N and is attached to A;
G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy; and
G′ is H, C1-5alkyl or C1-5haloalkyl.
and pharmaceutically acceptable salts and solvates thereof wherein
R1 are R2 are each H;
R3 is C1-C3alkyl;
A is C1-5alkylene;
D is HYC(O)ON═C(G′);
Y is N and is attached to A;
G is H, C1-5alkyl, C1-5haloalkyl, C3-7cycloalkyl, phenyl, —C1-2alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C1-2alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substitutents selected from the group consisting of halo, NO2, CN, —C(O)O—C1-3-alkyl, C1-3alkyl, hydroxy and C1-3alkoxy;
G′ is H, C1-5alkyl or C1-5haloalkyl; and
wherein A—D is interrupted with J—J′, —Z-phenyl- or —Z—C1-3alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C1-4alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C1-5alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C5-12 alkylene.
14. [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
[6-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid tert-butyl ester;
6-(2-Ethyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid sec-butyl ester;[6-( 2 -Ethyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid sec-butyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid benzyl ester;
2-Propanone, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;2-Propanone, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid methyl ester;
6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;[6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-methoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-cyano-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-methoxy-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid methyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid ethyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-fluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,4-difluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-methoxy-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid o-tolyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-methoxy-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid ethyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-fluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,4-difluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-fluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-chloro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-methoxy-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid o-tolyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-cyano-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-methoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 3,4-difluoro-phenyl ester;
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester;
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2,6-difluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid ethyl ester;
Benzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;Benzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
4-Fluorobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;4-Fluorobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
2-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;2-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
3-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;3-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
4-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;4-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
3-Pyridinecarboxaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;3-Pyridinecarboxaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 3,4-difluoro-phenyl ester;
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3,4-difluoro-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-methoxy-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-methoxy-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3-chloro-phenyl ester;
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid phenyl ester;
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 2-fluoro-phenyl ester;
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-fluoro-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid phenyl ester;
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid 4-methoxy-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-fluoro-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2,6-difluoro-phenyl ester;
{4-[2-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-ethoxy]-phenyl}-carbamic acid ethyl ester;
[1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
[1-Ethyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
[1-Isopropyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester; or
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-1-phenyl-hexyl]-carbamic acid 2-fluoro-phenyl ester or a pharmaceutically acceptable salt or solvate thereof.
15. [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
2-Propanone, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;2-Propanone, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid cyclohexyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid methyl ester;
6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;[6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-methoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-cyano-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-methoxy-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid methyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid ethyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-fluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,4-difluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-methoxy-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid o-tolyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-methoxy-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid ethyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-fluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,4-difluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-fluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-chloro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-methoxy-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid o-tolyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-cyano-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-methoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 3,4-difluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid isopropyl ester;
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester;
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2,6-difluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid ethyl ester;
Benzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;Benzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
4-Fluorobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;4-Fluorobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
2-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;2-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
3-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;3-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
4-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;4-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
3-Pyridinecarboxaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;3-Pyridinecarboxaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3,4-difluoro-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-methoxy-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 4-chloro-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 2-methoxy-phenyl ester;
{4-[3-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-propoxy]-phenyl}-carbamic acid 3-chloro-phenyl ester;
[1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
[1-Ethyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
[1-Isopropyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester; or
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-1-phenyl-hexyl]-carbamic acid 2-fluoro-phenyl ester or a pharmaceutically acceptable salt or solvate thereof.
16. [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
2-Propanone, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;2-Propanone, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;[6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-methoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-cyano-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid ethyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-fluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2,4-difluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-methoxy-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid o-tolyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-methoxy-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-fluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,4-difluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2-fluoro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-chloro-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-methoxy-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 4-cyano-phenyl ester;
[5-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-pentyl]-carbamic acid 2,6-dimethoxy-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 3,4-difluoro-phenyl ester;
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester;
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2,6-difluoro-phenyl ester;
Benzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;Benzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
4-Fluorobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;4-Fluorobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
2-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;2-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
3-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;3-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
4-Nitrobenzaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;4-Nitrobenzaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
3-Pyridinecarboxaldehyde, O-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]amino]carbonyl]oxime;3-Pyridinecarboxaldehyde, O-[6 -( 2 -methyl- 4,5 -diphenyl- 1H-imidazol- 1 -yl)hexylaminocarbonyl]oxime;
[1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester; or
[1-Ethyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester or a pharmaceutically acceptable salt or solvate thereof.
17. [6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester;
6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid phenyl ester;[6-( 2 -Methyl- 4,5 -diphenyl-imidazol- 1 -yl)-hexyl]-carbamic acid phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-fluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2,4-difluoro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 4-chloro-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid o-tolyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 2-fluoro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-chloro-phenyl ester;
[7-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-heptyl]-carbamic acid 4-cyano-phenyl ester;
[6-(2-Methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 3,4-difluoro-phenyl ester;
{6-[4,5-Bis-(4-fluoro-phenyl)-2-methyl-imidazol-1-yl]-hexyl}-carbamic acid 2-fluoro-phenyl ester; or
[1-Methyl-6-(2-methyl-4,5-diphenyl-imidazol-1-yl)-hexyl]-carbamic acid 2-fluoro-phenyl ester or a pharmaceutically acceptable salt or solvate thereof.
18. A method of treating chronic pain, acute pain or neuropathic pain in a mammal in need thereof by the administration of an effective amount of a pharmaceutical composition comprising a compound according to claims 1, 2, 3 or 4.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each independently H, C
1-3
alkyl or halo;
R
3
is C
1
-C
3
alkyl or C
3-7
cycloalkyl;
A is C
1-12
alkylene or L;
L is -phenyl-O—C 1-4 alkylene wherein said C 1-4 alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is phenyl or —C 1-2 alkylene-phenyl, said phenyl or phenyl of said —C 1-2 alkylene-phenyl are substituted with fluoro;
G′ is H, C
1-5
alkyl or C
1-5
haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C 1-4 alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C 1-5 alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C 5-12 alkylene.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each independently H, C
1-3
alkyl or halo;
R
3
is C
1
-C
3
alkyl or C
3-7
cycloalkyl;
A is C
1-12
alkylene or L;
L is -phenyl-O—C 1-4 alkylene wherein said C 1-4 alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is phenyl or —C 1-2 alkylene-phenyl, said phenyl or phenyl of said —C 1-2 alkylene-phenyl are substituted with cyano;
G′ is H, C
1-5
alkyl or C
1-5
haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C 1-4 alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C 1-5 alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C 5-12 alkylene.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each independently H, C
1-3
alkyl or halo;
R
3
is C
1
-C
3
alkyl or C
3-7
cycloalkyl;
A is L;
L is -phenyl-O—C 1-4 alkylene wherein said C 1-4 alkylene is attached to D;
D is X(O)O;
X is C and is attached to A; and
G is H, C 1-5 alkyl, C 1-5 haloalkyl, C 3-7 cycloalkyl, phenyl, —C 1-2 alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C 1-2 alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substituents selected from the group consisting of halo, NO 2 , CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, hydroxy and C 1-3 alkoxy.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each independently H, C
1-3
alkyl or halo;
R
3
is C
1
-C
3
alkyl or C
3-7
cycloalkyl;
A is C
1-12
alkylene;
D is HYC(O)ON═C(G′);
Y is N and is attached to A;
G is H, C 1-5 alkyl, C 1-5 haloalkyl, C 3-7 cycloalkyl, phenyl, —C 1-2 alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C 1-2 alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substituents selected from the group consisting of halo, NO 2 , CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, hydroxy and C 1-3 alkoxy; and
G′ is H, C
1-5
alkyl or C
1-5
haloalkyl.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each independently H, C
1-3
alkyl or halo;
R
3
is C
1
-C
3
alkyl or C
3-7
cycloalkyl;
A is C
1-12
alkylene or L;
L is -phenyl-O—C 1-4 alkylene wherein said C 1-4 alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is —C 1-2 alkylene-phenyl, said —C 1-2 alkylene-phenyl is optionally substituted with one or more of the same or different substituents selected from the group consisting of halo, NO 2 , CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, hydroxy and C 1-3 alkoxy;
G′ is H, C
1-5
alkyl or C
1-5
haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C 1-4 alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C 1-5 alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C 5-12 alkylene.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each independently H, C
1-3
alkyl or halo;
R
3
is C
1
-C
3
alkyl or C
3-7
cycloalkyl;
A is C
1-12
alkylene or L;
L is -phenyl-O—C 1-4 alkylene wherein said C 1-4 alkylene is attached to D;
provided that if A is L, then D is X(O)O and A—D is not interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
D is X(O)O, X(O)N(G′), HYC(O)O or HYC(O)ON═C(G′);
X is C and is attached to A;
Y is N and is attached to A;
G is phenyl or —C 1-2 alkylene-phenyl, said phenyl or phenyl of said —C 1-2 alkylene-phenyl are optionally substituted with one or more of the same or different substituents selected from the group consisting of halo, CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, and C 1-3 alkoxy;
G′ is H, C
1-5
alkyl or C
1-5
haloalkyl; and
wherein A—D is optionally interrupted with J—J′, —Z-phenyl- or —Z—C 1-3 alkylene;
wherein
Z is O or S and is attached to A;
J is CH and is attached to A, D and J′;
J′ is C 1-4 alkyl or phenyl;
provided that
if A—D is interrupted with —Z-phenyl-, then A is C 1-5 alkylene;
if A—D is not interrupted with —Z-phenyl-, then A is C 5-12 alkylene.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each H;
R
3
is C
1
-C
3
alkyl;
A is C
7-10
alkylene;
D is HYC(O)O;
Y is N and is attached to A;
G is H, C 1-5 alkyl, C 1-5 haloalkyl, C 3-7 cycloalkyl, phenyl, —C 1-2 alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C 1-2 alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substituents selected from the group consisting of halo, NO 2 , CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, hydroxy and C 1-3 alkoxy.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each H;
R
3
is C
1
-C
3
alkyl;
A is C
1-5
alkylene;
D is HYC(O)O;
Y is N and is attached to A;
G is H, C 1-5 alkyl, C 1-5 haloalkyl, C 3-7 cycloalkyl, phenyl, —C 1-2 alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C 1-2 alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substituents selected from the group consisting of halo, NO 2 , CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, hydroxyl and C 1-3 alkoxy; and
wherein A—D is interrupted with —Z-phenyl-;
wherein Z is O or S and is attached to A.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each independently H;
R
3
is C
1
-C
3
alkyl;
A is C
7-10
alkylene;
D is HYC(O)ON═C(G′);
Y is N and is attached to A;
G is H, C 1-5 alkyl, C 1-5 haloalkyl, C 3-7 cycloalkyl, phenyl, —C 1-2 alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C 1-2 alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substituents selected from the group consisting of halo, NO 2 , CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, hydroxyl and C 1-3 alkoxy; and
G′ is H, C
1-5
alkyl or C
1-5
haloalkyl.
or a pharmaceutically acceptable salt or solvate thereof wherein
R
1
and R
2
are each H;
R
3
is C
1
-C
3
alkyl;
A is C
1-5
alkylene;
D is HYC(O)ON═C(G′);
Y is N and is attached to A;
G is H, C 1-5 alkyl, C 1-5 haloalkyl, C 3-7 cycloalkyl, phenyl, —C 1-2 alkylene-phenyl, C-pyridyl or N-pyridyl, said phenyl or —C 1-2 alkylene-phenyl are each optionally and independently substituted with one or more of the same or different substituents selected from the group consisting of halo, NO 2 , CN, —C(O)O—C 1-3-alkyl, C 1-3 alkyl, hydroxy and C 1-3 alkoxy;
G′ is H, C
1-5
alkyl or C
1-5
haloalkyl; and
wherein A—D is interrupted with —Z-phenyl-;
wherein Z is O or S and is attached to A.
31. A method of treating chronic pain, acute pain or neuropathic pain in a mammal in need thereof by the administration of an effective amount of a pharmaceutical composition comprising a compound according to claims 21, 22 or 23-30.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/883,195 USRE39634E1 (en) | 2001-04-27 | 2004-07-01 | Bisarylimidazoly fatty acid amide hydrolase inhibitors |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US28682701P | 2001-04-27 | 2001-04-27 | |
| US10/128,480 US6562846B2 (en) | 2001-04-27 | 2002-04-23 | Bisarylimidazolyl fatty acid amide hydrolase inhibitors |
| US10/883,195 USRE39634E1 (en) | 2001-04-27 | 2004-07-01 | Bisarylimidazoly fatty acid amide hydrolase inhibitors |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/128,480 Reissue US6562846B2 (en) | 2001-04-27 | 2002-04-23 | Bisarylimidazolyl fatty acid amide hydrolase inhibitors |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| USRE39634E1 true USRE39634E1 (en) | 2007-05-15 |
Family
ID=23100337
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/128,480 Ceased US6562846B2 (en) | 2001-04-27 | 2002-04-23 | Bisarylimidazolyl fatty acid amide hydrolase inhibitors |
| US10/883,195 Expired - Lifetime USRE39634E1 (en) | 2001-04-27 | 2004-07-01 | Bisarylimidazoly fatty acid amide hydrolase inhibitors |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/128,480 Ceased US6562846B2 (en) | 2001-04-27 | 2002-04-23 | Bisarylimidazolyl fatty acid amide hydrolase inhibitors |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US6562846B2 (en) |
| EP (1) | EP1389107A4 (en) |
| JP (1) | JP2004532229A (en) |
| CA (1) | CA2445294A1 (en) |
| IL (1) | IL158300A0 (en) |
| MX (1) | MXPA03009850A (en) |
| WO (1) | WO2002087569A1 (en) |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1383465B1 (en) * | 2001-03-29 | 2011-08-10 | Michael Davis | Acute pharmacologic augmentation of psychotherapy with d-cycloserine |
| AU2003210824A1 (en) * | 2002-02-08 | 2003-09-02 | Bristol-Myers Squibb Company | (oxime)carbamoyl fatty acid amide hydrolase inhibitors |
| EA010267B1 (en) | 2002-10-07 | 2008-06-30 | Те Риджентс Оф Те Юниверсити Оф Калифорния | Modulation of anxiety through blocade of anandamide hydrolysis |
| FR2850377B1 (en) * | 2003-01-23 | 2009-02-20 | Sanofi Synthelabo | ARYLALKYLCARBAMATE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC APPLICATION |
| FR2865205B1 (en) | 2004-01-16 | 2006-02-24 | Sanofi Synthelabo | ARYLOXYALKYLCARBAMATE DERIVATIVES, THEIR PREPARATION AND THERAPEUTIC USE THEREOF |
| FR2866886B1 (en) * | 2004-02-26 | 2007-08-31 | Sanofi Synthelabo | ARYL-AND HETEROARYL-AKYLCARBAMATES DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| FR2866884B1 (en) * | 2004-02-26 | 2007-08-31 | Sanofi Synthelabo | ARYL-AND HETEROARYL-PIPERIDINECARBOXYLATE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| FR2866885B1 (en) | 2004-02-26 | 2007-08-31 | Sanofi Synthelabo | PIPERIDINYLALKYLCARBAMATES DERIVATIVES, THEIR PREPARATION AND THERAPEUTIC USE THEREOF |
| US20080103209A1 (en) * | 2004-04-23 | 2008-05-01 | The Regents Of The University Of California | Compounds And Methods For Treating Non-Inflammatory Pain Using Ppar Alpha Agonists |
| WO2006044617A1 (en) | 2004-10-15 | 2006-04-27 | The Scripps Research Institute | Oxadiazole ketone inhibitors of fatty acid amide hydrolase |
| US20060084659A1 (en) * | 2004-10-19 | 2006-04-20 | Michael Davis | Augmentation of psychotherapy with cannabinoid reuptake inhibitors |
| TW200633990A (en) | 2004-11-18 | 2006-10-01 | Takeda Pharmaceuticals Co | Amide compound |
| EP2937341B1 (en) | 2004-12-30 | 2017-07-05 | Janssen Pharmaceutica N.V. | 4-(benzyl)-piperazine-1-carboxylic acid phenylamide derivatives and related compounds as modulators of fatty acid amide hydrolase (faah) for the treatment of anxiety, pain and other conditions |
| KR20130136010A (en) * | 2005-04-13 | 2013-12-11 | 네우렉슨 인코포레이티드 | Substituted indole compounds having nos inhibitory activity |
| US20090082435A1 (en) * | 2005-04-28 | 2009-03-26 | The Regents Of The University Of California | Methods, Compositions, And Compounds For Modulation Of Monoacylglycerol Lipase, Pain, And Stress-Related Disorders |
| AU2007217813A1 (en) | 2006-02-17 | 2007-08-30 | The Scripps Research Institute | Oxazole ketones as modulators of fatty acid amide hydrolase |
| KR100753240B1 (en) | 2006-03-10 | 2007-08-30 | 한국지질자원연구원 | Production method for alloy nano powders |
| EP2708262A3 (en) | 2007-10-12 | 2014-04-23 | The Government of the U.S.A. as represented by The Secretary of the dept. of Health & Human Services | Therapeutic applications of fatty acid amide hydrolase inhibitors |
| EP2062578A1 (en) * | 2007-11-12 | 2009-05-27 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Novel use of chemical compounds for the treatment of AIDS |
| NZ586082A (en) | 2007-11-16 | 2013-03-28 | Neuraxon Inc | Indole compounds and methods for treating visceral pain |
| US8598202B2 (en) | 2008-02-19 | 2013-12-03 | Janssen Pharmaceutica Nv | Aryl-hydroxyethylamino-pyrimidines and triazines as modulators of fatty acid amide hydrolase |
| US8207226B1 (en) | 2008-06-03 | 2012-06-26 | Alcon Research, Ltd. | Use of FAAH antagonists for treating dry eye and ocular pain |
| US8461159B2 (en) | 2008-11-25 | 2013-06-11 | Jannsen Pharmaceutica BV | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| WO2010068452A1 (en) | 2008-11-25 | 2010-06-17 | Janssen Pharmaceutica Nv | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| US8901111B2 (en) | 2009-06-05 | 2014-12-02 | Janssen Pharmaceutica Nv | Aryl-substituted heterocyclic urea modulators of fatty acid amide hydrolase |
| WO2010141817A1 (en) | 2009-06-05 | 2010-12-09 | Janssen Pharmaceutica Nv | Heteroaryl-substituted spirocyclic diamine urea modulators of fatty acid amide hydrolase |
| WO2011022348A1 (en) | 2009-08-18 | 2011-02-24 | Janssen Pharmaceutica Nv | Ethylene diamine modulators of fatty acid amide hydrolase |
| WO2011030798A1 (en) | 2009-09-09 | 2011-03-17 | 大日本住友製薬株式会社 | 8-oxodihydropurine derivative |
| US20120225097A1 (en) | 2009-11-12 | 2012-09-06 | Hawryluk Natalie A | Piperazinecarboxamide derivative useful as a modulator of fatty acid amide hydrolase (faah) |
| UA108233C2 (en) | 2010-05-03 | 2015-04-10 | Fatty acid amide hydrolysis activity modulators | |
| DE102012018115A1 (en) | 2012-09-13 | 2014-03-13 | Matthias Lehr | New aryl-N-(arylalkyl) carbamate compounds, used to prepare pharmaceutical composition for prophylactic and/or therapeutic treatment of diseases including e.g. acute and chronic neurogenic pain, and neurological and psychiatric diseases |
| PT2931291T (en) | 2012-12-11 | 2021-12-03 | Mclean Hospital Corp | Xenon and/or argon treatment as an adjunct to psychotherapy for psychiatric disorders |
| DE102013016573A1 (en) | 2013-10-04 | 2015-04-09 | Matthias Lehr | 1-Tetrazolylpropan-2-ones as inhibitors of cytosolic phospholipase A2 and fatty acid amide hydrolase, in particular suitable for topical application |
| US10414721B1 (en) | 2018-06-04 | 2019-09-17 | University Of Bern | Inhibitor of endocannabinoid cellular reuptake |
| CN111269083B (en) * | 2020-02-24 | 2023-08-25 | 珠海市柏瑞医药科技有限公司 | Synthesis method of guerbet acid |
| US11999703B1 (en) | 2023-10-25 | 2024-06-04 | King Faisal University | 5-(2,4,5-tris(4-chlorophenyl)-1H-imidazol-1-yl)pentanoic acid as an antimicrobial compound |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5648373A (en) | 1992-02-11 | 1997-07-15 | Smithkline Beecham Corporation | CoA-IT and PAF inhibitors |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU628862B2 (en) * | 1988-10-03 | 1992-09-24 | Glaxo Group Limited | Substituted n-vinyl imidazole derivatives |
| US5700826A (en) * | 1995-06-07 | 1997-12-23 | Ontogen Corporation | 1,2,4,5-tetra substituted imidazoles as modulators of multi-drug resistance |
-
2002
- 2002-04-23 IL IL15830002A patent/IL158300A0/en unknown
- 2002-04-23 CA CA002445294A patent/CA2445294A1/en not_active Abandoned
- 2002-04-23 WO PCT/US2002/012853 patent/WO2002087569A1/en active Application Filing
- 2002-04-23 JP JP2002584915A patent/JP2004532229A/en active Pending
- 2002-04-23 US US10/128,480 patent/US6562846B2/en not_active Ceased
- 2002-04-23 EP EP02728952A patent/EP1389107A4/en not_active Withdrawn
- 2002-04-23 MX MXPA03009850A patent/MXPA03009850A/en active IP Right Grant
-
2004
- 2004-07-01 US US10/883,195 patent/USRE39634E1/en not_active Expired - Lifetime
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5648373A (en) | 1992-02-11 | 1997-07-15 | Smithkline Beecham Corporation | CoA-IT and PAF inhibitors |
Non-Patent Citations (13)
Also Published As
| Publication number | Publication date |
|---|---|
| EP1389107A1 (en) | 2004-02-18 |
| EP1389107A4 (en) | 2005-10-12 |
| JP2004532229A (en) | 2004-10-21 |
| CA2445294A1 (en) | 2002-11-07 |
| MXPA03009850A (en) | 2004-02-12 |
| US20020188009A1 (en) | 2002-12-12 |
| IL158300A0 (en) | 2004-05-12 |
| US6562846B2 (en) | 2003-05-13 |
| WO2002087569A1 (en) | 2002-11-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| USRE39634E1 (en) | Bisarylimidazoly fatty acid amide hydrolase inhibitors | |
| US6949574B2 (en) | (Oxime)carbamoyl fatty acid amide hydrolase inhibitors | |
| US6906080B1 (en) | Pyrazolecarboxylic acid tricyclic derivatives, preparation and pharmaceutical compositions containing same | |
| US7687665B2 (en) | 2-methylprop anamides and their use as pharmaceuticals | |
| US7041682B2 (en) | NK1 antagonists | |
| US11724989B2 (en) | MCT4 inhibitors for treating disease | |
| US8299085B2 (en) | Quinazoline derivatives | |
| US7507742B2 (en) | Spirocyclic derivatives | |
| US5990126A (en) | Quinolinic sulfide derivatives acting as NMDA receptor antagonists and process for preparation thereof | |
| US8962659B2 (en) | Phenoxyethyl piperidine compounds | |
| US20080009506A1 (en) | Imidazole Compound | |
| US20070185078A1 (en) | Substituted triazole derivatives as oxytocin antagonists | |
| US20090170895A1 (en) | S1p3 receptor antagonist | |
| CN103237788A (en) | Phenalkylamine derivatives, pharmaceutical compositions containing them, and their use in therapy | |
| JP6148400B2 (en) | Phenoxyethyldihydro-1H-isoquinoline compound | |
| HU206195B (en) | Process for producing benzocycloalkane derivatives and pharmaceutical compositions comprising same | |
| US9975873B2 (en) | Isoindoline derivatives | |
| JP5868994B2 (en) | KATII inhibitor | |
| MXPA02003673A (en) | Use of carbonylamino derivatives against cns disorders. | |
| EP2346847B1 (en) | Aryl sulfonamide amine compounds and their use as 5-ht6 ligands | |
| AU2002258973A1 (en) | Bisarylimidazolyl fatty acid amide hydrolase inhibitors | |
| JP3930081B2 (en) | 2-Ureido-benzamide derivatives | |
| KR19990038717A (en) | 3- (substituted-phenylthio) -4-hydroxyquinolin-2 (1H) -one derivatives and methods for their preparation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| FPAY | Fee payment |
Year of fee payment: 12 |