US8093432B2 - Processes for epimerizing cyclohexenyl ketones with subsequent aldol condensation to produce fragrance compounds - Google Patents
Processes for epimerizing cyclohexenyl ketones with subsequent aldol condensation to produce fragrance compounds Download PDFInfo
- Publication number
- US8093432B2 US8093432B2 US12/641,786 US64178609A US8093432B2 US 8093432 B2 US8093432 B2 US 8093432B2 US 64178609 A US64178609 A US 64178609A US 8093432 B2 US8093432 B2 US 8093432B2
- Authority
- US
- United States
- Prior art keywords
- isomer
- alkyl
- cyclohexen
- alkanone
- trans
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034 method Methods 0.000 title claims abstract description 115
- 230000008569 process Effects 0.000 title claims abstract description 100
- 150000001875 compounds Chemical class 0.000 title claims abstract description 22
- 238000005882 aldol condensation reaction Methods 0.000 title claims description 97
- 239000003205 fragrance Substances 0.000 title description 17
- NOFSNYQYGQRNMS-UHFFFAOYSA-N di(cyclohexen-1-yl)methanone Chemical class C=1CCCCC=1C(=O)C1=CCCCC1 NOFSNYQYGQRNMS-UHFFFAOYSA-N 0.000 title 1
- 238000006345 epimerization reaction Methods 0.000 claims abstract description 47
- HGCIXCUEYOPUTN-UHFFFAOYSA-N cyclohexene Chemical compound C1CCC=CC1 HGCIXCUEYOPUTN-UHFFFAOYSA-N 0.000 claims abstract description 33
- 150000004703 alkoxides Chemical class 0.000 claims abstract description 28
- 229910052751 metal Inorganic materials 0.000 claims abstract description 28
- 239000002184 metal Substances 0.000 claims abstract description 28
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims abstract description 14
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 10
- 239000000203 mixture Substances 0.000 claims description 67
- 239000007859 condensation product Substances 0.000 claims description 64
- 238000006243 chemical reaction Methods 0.000 claims description 57
- 239000000047 product Substances 0.000 claims description 55
- 150000001299 aldehydes Chemical class 0.000 claims description 39
- 238000003379 elimination reaction Methods 0.000 claims description 29
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 claims description 28
- 150000002576 ketones Chemical class 0.000 claims description 27
- WLTIDHLMFJRJHE-PSASIEDQSA-N 1-[(1s,2r)-2,6,6-trimethylcyclohex-3-en-1-yl]ethanone Chemical group C[C@@H]1C=CCC(C)(C)[C@H]1C(C)=O WLTIDHLMFJRJHE-PSASIEDQSA-N 0.000 claims description 26
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 26
- 230000008030 elimination Effects 0.000 claims description 25
- DYLIWHYUXAJDOJ-OWOJBTEDSA-N (e)-4-(6-aminopurin-9-yl)but-2-en-1-ol Chemical compound NC1=NC=NC2=C1N=CN2C\C=C\CO DYLIWHYUXAJDOJ-OWOJBTEDSA-N 0.000 claims description 20
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical group CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 claims description 20
- 238000007115 1,4-cycloaddition reaction Methods 0.000 claims description 17
- 238000004821 distillation Methods 0.000 claims description 13
- VXUYXOFXAQZZMF-UHFFFAOYSA-N titanium(IV) isopropoxide Chemical compound CC(C)O[Ti](OC(C)C)(OC(C)C)OC(C)C VXUYXOFXAQZZMF-UHFFFAOYSA-N 0.000 claims description 12
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 239000003960 organic solvent Substances 0.000 claims description 8
- YHWCPXVTRSHPNY-UHFFFAOYSA-N butan-1-olate;titanium(4+) Chemical compound [Ti+4].CCCC[O-].CCCC[O-].CCCC[O-].CCCC[O-] YHWCPXVTRSHPNY-UHFFFAOYSA-N 0.000 claims description 6
- 239000002841 Lewis acid Substances 0.000 claims description 5
- 150000007517 lewis acids Chemical class 0.000 claims description 5
- LZWQNOHZMQIFBX-UHFFFAOYSA-N lithium;2-methylpropan-2-olate Chemical compound [Li+].CC(C)(C)[O-] LZWQNOHZMQIFBX-UHFFFAOYSA-N 0.000 claims description 5
- 239000002608 ionic liquid Substances 0.000 claims description 3
- 239000002585 base Substances 0.000 description 37
- -1 cyclic organic compounds Chemical class 0.000 description 34
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 24
- 239000002904 solvent Substances 0.000 description 18
- 239000000543 intermediate Substances 0.000 description 17
- 238000000746 purification Methods 0.000 description 16
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 15
- 239000002304 perfume Substances 0.000 description 15
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 14
- 238000002955 isolation Methods 0.000 description 14
- 238000010626 work up procedure Methods 0.000 description 14
- 125000004429 atom Chemical group 0.000 description 13
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 12
- 0 [1*]C(=O)C1([H])C([2*])([H])c([3*])c([4*])C([5*])([6*])C1([7*])[8*] Chemical compound [1*]C(=O)C1([H])C([2*])([H])c([3*])c([4*])C([5*])([6*])C1([7*])[8*] 0.000 description 11
- 238000006117 Diels-Alder cycloaddition reaction Methods 0.000 description 10
- 239000003153 chemical reaction reagent Substances 0.000 description 10
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 9
- 238000000605 extraction Methods 0.000 description 9
- WLTIDHLMFJRJHE-UHFFFAOYSA-N 1-(2,6,6-trimethylcyclohex-3-en-1-yl)ethanone Chemical compound CC1C=CCC(C)(C)C1C(C)=O WLTIDHLMFJRJHE-UHFFFAOYSA-N 0.000 description 8
- 238000005698 Diels-Alder reaction Methods 0.000 description 8
- 125000001424 substituent group Chemical group 0.000 description 8
- XEJGJTYRUWUFFD-FNORWQNLSA-N (e)-1-(2,6,6-trimethyl-1-cyclohex-3-enyl)but-2-en-1-one Chemical compound C\C=C\C(=O)C1C(C)C=CCC1(C)C XEJGJTYRUWUFFD-FNORWQNLSA-N 0.000 description 7
- 238000002425 crystallisation Methods 0.000 description 7
- 230000008025 crystallization Effects 0.000 description 7
- 239000007858 starting material Substances 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- HSJKGGMUJITCBW-UHFFFAOYSA-N 3-hydroxybutanal Chemical compound CC(O)CC=O HSJKGGMUJITCBW-UHFFFAOYSA-N 0.000 description 6
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000006227 byproduct Substances 0.000 description 6
- 125000002837 carbocyclic group Chemical group 0.000 description 6
- 150000001728 carbonyl compounds Chemical class 0.000 description 6
- 238000004587 chromatography analysis Methods 0.000 description 6
- 238000004508 fractional distillation Methods 0.000 description 6
- 238000000926 separation method Methods 0.000 description 6
- 239000010936 titanium Substances 0.000 description 6
- LLVWLCAZSOLOTF-UHFFFAOYSA-N 1-methyl-4-[1,4,4-tris(4-methylphenyl)buta-1,3-dienyl]benzene Chemical group C1=CC(C)=CC=C1C(C=1C=CC(C)=CC=1)=CC=C(C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 LLVWLCAZSOLOTF-UHFFFAOYSA-N 0.000 description 5
- BGTBFNDXYDYBEY-FNORWQNLSA-N 4-(2,6,6-Trimethylcyclohex-1-enyl)but-2-en-4-one Chemical class C\C=C\C(=O)C1=C(C)CCCC1(C)C BGTBFNDXYDYBEY-FNORWQNLSA-N 0.000 description 5
- 238000006668 aldol addition reaction Methods 0.000 description 5
- 125000004122 cyclic group Chemical group 0.000 description 5
- 239000003599 detergent Substances 0.000 description 5
- 238000001914 filtration Methods 0.000 description 5
- 238000006317 isomerization reaction Methods 0.000 description 5
- 239000000376 reactant Substances 0.000 description 5
- 238000004064 recycling Methods 0.000 description 5
- PMJHHCWVYXUKFD-SNAWJCMRSA-N (E)-1,3-pentadiene Chemical compound C\C=C\C=C PMJHHCWVYXUKFD-SNAWJCMRSA-N 0.000 description 4
- XEJGJTYRUWUFFD-NPNFKUNOSA-N (e)-1-[(1s,2r)-2,6,6-trimethylcyclohex-3-en-1-yl]but-2-en-1-one Chemical compound C\C=C\C(=O)[C@H]1[C@H](C)C=CCC1(C)C XEJGJTYRUWUFFD-NPNFKUNOSA-N 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 4
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 4
- 239000011831 acidic ionic liquid Substances 0.000 description 4
- 238000009833 condensation Methods 0.000 description 4
- 230000005494 condensation Effects 0.000 description 4
- 238000006297 dehydration reaction Methods 0.000 description 4
- 150000001993 dienes Chemical class 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- AZVCGYPLLBEUNV-UHFFFAOYSA-N lithium;ethanolate Chemical compound [Li+].CC[O-] AZVCGYPLLBEUNV-UHFFFAOYSA-N 0.000 description 4
- SHOJXDKTYKFBRD-UHFFFAOYSA-N mesityl oxide Natural products CC(C)=CC(C)=O SHOJXDKTYKFBRD-UHFFFAOYSA-N 0.000 description 4
- PMJHHCWVYXUKFD-UHFFFAOYSA-N piperylene Natural products CC=CC=C PMJHHCWVYXUKFD-UHFFFAOYSA-N 0.000 description 4
- RPDAUEIUDPHABB-UHFFFAOYSA-N potassium ethoxide Chemical compound [K+].CC[O-] RPDAUEIUDPHABB-UHFFFAOYSA-N 0.000 description 4
- BDAWXSQJJCIFIK-UHFFFAOYSA-N potassium methoxide Chemical compound [K+].[O-]C BDAWXSQJJCIFIK-UHFFFAOYSA-N 0.000 description 4
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 230000009466 transformation Effects 0.000 description 4
- OZXIZRZFGJZWBF-UHFFFAOYSA-N 1,3,5-trimethyl-2-(2,4,6-trimethylphenoxy)benzene Chemical compound CC1=CC(C)=CC(C)=C1OC1=C(C)C=C(C)C=C1C OZXIZRZFGJZWBF-UHFFFAOYSA-N 0.000 description 3
- GQRHHNHXCPWOGY-ZYHUDNBSSA-N 1-[(1s,2r)-2,6,6-trimethylcyclohex-3-en-1-yl]but-3-en-1-one Chemical compound C[C@@H]1C=CCC(C)(C)[C@H]1C(=O)CC=C GQRHHNHXCPWOGY-ZYHUDNBSSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 239000002386 air freshener Substances 0.000 description 3
- 238000005575 aldol reaction Methods 0.000 description 3
- 229910052783 alkali metal Inorganic materials 0.000 description 3
- 235000019568 aromas Nutrition 0.000 description 3
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 230000018044 dehydration Effects 0.000 description 3
- 235000013305 food Nutrition 0.000 description 3
- 238000010791 quenching Methods 0.000 description 3
- 230000000171 quenching effect Effects 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 238000000859 sublimation Methods 0.000 description 3
- 230000008022 sublimation Effects 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 238000000844 transformation Methods 0.000 description 3
- 238000001665 trituration Methods 0.000 description 3
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 238000000023 Kugelrohr distillation Methods 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 238000010533 azeotropic distillation Methods 0.000 description 2
- BTANRVKWQNVYAZ-UHFFFAOYSA-N butan-2-ol Chemical compound CCC(C)O BTANRVKWQNVYAZ-UHFFFAOYSA-N 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 150000001934 cyclohexanes Chemical class 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000010537 deprotonation reaction Methods 0.000 description 2
- VILAVOFMIJHSJA-UHFFFAOYSA-N dicarbon monoxide Chemical compound [C]=C=O VILAVOFMIJHSJA-UHFFFAOYSA-N 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 229910052736 halogen Inorganic materials 0.000 description 2
- 150000002367 halogens Chemical class 0.000 description 2
- 125000000623 heterocyclic group Chemical group 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 229910001867 inorganic solvent Inorganic materials 0.000 description 2
- 239000003049 inorganic solvent Substances 0.000 description 2
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-N isobutanol Chemical compound CC(C)CO ZXEKIIBDNHEJCQ-UHFFFAOYSA-N 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- AFRJJFRNGGLMDW-UHFFFAOYSA-N lithium amide Chemical compound [Li+].[NH2-] AFRJJFRNGGLMDW-UHFFFAOYSA-N 0.000 description 2
- JILPJDVXYVTZDQ-UHFFFAOYSA-N lithium methoxide Chemical compound [Li+].[O-]C JILPJDVXYVTZDQ-UHFFFAOYSA-N 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 150000002736 metal compounds Chemical class 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 238000006053 organic reaction Methods 0.000 description 2
- 238000005191 phase separation Methods 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 2
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 2
- 239000011736 potassium bicarbonate Substances 0.000 description 2
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 2
- CBMSDILKECEMOT-UHFFFAOYSA-N potassium;2-methylpropan-1-olate Chemical compound [K+].CC(C)C[O-] CBMSDILKECEMOT-UHFFFAOYSA-N 0.000 description 2
- WQKGAJDYBZOFSR-UHFFFAOYSA-N potassium;propan-2-olate Chemical compound [K+].CC(C)[O-] WQKGAJDYBZOFSR-UHFFFAOYSA-N 0.000 description 2
- 230000035484 reaction time Effects 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- 238000007086 side reaction Methods 0.000 description 2
- 125000004469 siloxy group Chemical group [SiH3]O* 0.000 description 2
- 238000001577 simple distillation Methods 0.000 description 2
- ODZPKZBBUMBTMG-UHFFFAOYSA-N sodium amide Chemical compound [NH2-].[Na+] ODZPKZBBUMBTMG-UHFFFAOYSA-N 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- WBQTXTBONIWRGK-UHFFFAOYSA-N sodium;propan-2-olate Chemical compound [Na+].CC(C)[O-] WBQTXTBONIWRGK-UHFFFAOYSA-N 0.000 description 2
- 238000001256 steam distillation Methods 0.000 description 2
- 230000002194 synthesizing effect Effects 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 239000002699 waste material Substances 0.000 description 2
- 239000001674 (E)-1-(2,6,6-trimethyl-1-cyclohexenyl)but-2-en-1-one Substances 0.000 description 1
- CRIGTVCBMUKRSL-FNORWQNLSA-N 1-(2,6,6-trimethylcyclohex-2-en-1-yl)but-2-enone Chemical compound C\C=C\C(=O)C1C(C)=CCCC1(C)C CRIGTVCBMUKRSL-FNORWQNLSA-N 0.000 description 1
- BGTBFNDXYDYBEY-UHFFFAOYSA-N 1-(2,6,6-trimethylcyclohexen-1-yl)but-2-en-1-one Chemical compound CC=CC(=O)C1=C(C)CCCC1(C)C BGTBFNDXYDYBEY-UHFFFAOYSA-N 0.000 description 1
- LTYLUDGDHUEBGX-UHFFFAOYSA-N 1-(cyclohexen-1-yl)ethanone Chemical compound CC(=O)C1=CCCCC1 LTYLUDGDHUEBGX-UHFFFAOYSA-N 0.000 description 1
- JKCCWRSADCYENS-DFERZTECSA-N C/C=C/C(=O)C1=C(C)CCCC1(C)C.C/C=C/C(=O)C1C(C)=CCCC1(C)C.C/C=C/C(=O)[C@H]1[C@H](C)C=CCC1(C)C Chemical compound C/C=C/C(=O)C1=C(C)CCCC1(C)C.C/C=C/C(=O)C1C(C)=CCCC1(C)C.C/C=C/C(=O)[C@H]1[C@H](C)C=CCC1(C)C JKCCWRSADCYENS-DFERZTECSA-N 0.000 description 1
- DMHGJJWKPYMCAS-IRHIUKMXSA-N C/C=C/C(=O)[C@H]1[C@H](C)C=CCC1(C)C.C=CCC(=O)[C@H]1[C@H](C)C=CCC1(C)C Chemical compound C/C=C/C(=O)[C@H]1[C@H](C)C=CCC1(C)C.C=CCC(=O)[C@H]1[C@H](C)C=CCC1(C)C DMHGJJWKPYMCAS-IRHIUKMXSA-N 0.000 description 1
- 238000003820 Medium-pressure liquid chromatography Methods 0.000 description 1
- 229910018954 NaNH2 Inorganic materials 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- NBXMJDVWESETMK-UHFFFAOYSA-N acetaldehyde Chemical compound CC=O.CC=O NBXMJDVWESETMK-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 238000007171 acid catalysis Methods 0.000 description 1
- 239000003377 acid catalyst Substances 0.000 description 1
- 150000001260 acyclic compounds Chemical class 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000005456 alcohol based solvent Substances 0.000 description 1
- 229910000288 alkali metal carbonate Inorganic materials 0.000 description 1
- 150000008041 alkali metal carbonates Chemical class 0.000 description 1
- CRIGTVCBMUKRSL-UHFFFAOYSA-N alpha-Damascone Natural products CC=CC(=O)C1C(C)=CCCC1(C)C CRIGTVCBMUKRSL-UHFFFAOYSA-N 0.000 description 1
- JZQOJFLIJNRDHK-CMDGGOBGSA-N alpha-irone Chemical class CC1CC=C(C)C(\C=C\C(C)=O)C1(C)C JZQOJFLIJNRDHK-CMDGGOBGSA-N 0.000 description 1
- 239000003849 aromatic solvent Substances 0.000 description 1
- 150000001555 benzenes Chemical class 0.000 description 1
- POIARNZEYGURDG-UHFFFAOYSA-N beta-damascenone Natural products CC=CC(=O)C1=C(C)C=CCC1(C)C POIARNZEYGURDG-UHFFFAOYSA-N 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 230000002153 concerted effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 150000001923 cyclic compounds Chemical class 0.000 description 1
- 238000006352 cycloaddition reaction Methods 0.000 description 1
- 230000005595 deprotonation Effects 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000003818 flash chromatography Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229930002839 ionone Natural products 0.000 description 1
- 150000002499 ionone derivatives Chemical class 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 238000005580 one pot reaction Methods 0.000 description 1
- UCMSRHPIFRZHDO-UHFFFAOYSA-N penta-1,3-diene Chemical compound CC=CC=C.CC=CC=C UCMSRHPIFRZHDO-UHFFFAOYSA-N 0.000 description 1
- 235000011056 potassium acetate Nutrition 0.000 description 1
- 235000015497 potassium bicarbonate Nutrition 0.000 description 1
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 1
- 229940086066 potassium hydrogencarbonate Drugs 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000012429 reaction media Substances 0.000 description 1
- 239000013557 residual solvent Substances 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 238000004809 thin layer chromatography Methods 0.000 description 1
- 235000019505 tobacco product Nutrition 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C45/00—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds
- C07C45/61—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups
- C07C45/67—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups by isomerisation; by change of size of the carbon skeleton
- C07C45/68—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups by isomerisation; by change of size of the carbon skeleton by increase in the number of carbon atoms
- C07C45/69—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups by isomerisation; by change of size of the carbon skeleton by increase in the number of carbon atoms by addition to carbon-to-carbon double or triple bonds
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/582—Recycling of unreacted starting or intermediate materials
Definitions
- the present invention is related to single reactor processes for synthesizing substituted cyclohexanes useful as fragrance compounds utilizing an epimerization and aldol condensation of cyclic organic compounds.
- Perfume and aroma enhancing compounds are widely used as additives in the detergent and food industries. These compounds are used, for example, to augment or enhance the aromas of certain detergent compositions and perfumes, or to enhance the aroma and flavor characteristics of certain food or tobacco products among other products. Compounds with floral, fruity, woody, or other pleasing aroma are particularly desirable.
- fragrance components may include a substituted cyclohexene structure. These structures include, for example, various ionone isomers, damascone isomers, cyclogeranate isomers, and irone isomers. Other cyclic fragrance compounds are also known.
- the damascones including ⁇ -damascone, ⁇ -damascone, and ⁇ -damascone are examples of compounds having pleasing floral, fruity aromas used in the perfumery art.
- the damascone isomers differ in the position of the ring double bond as shown in Scheme 1.
- trans,trans- ⁇ -damascone is one of the most widely used fragrance additives in the detergent and food industries. Therefore, the industrial scale production of ⁇ -damascone and other related compounds is of great interest.
- damascones typically involves a three-step process: (1) a Diels-Alder cycloaddition of a diene and a dienophile to produce a mixture of cis- and trans-cycloadducts; (2) epimerization of the cis-cycloadduct to the trans-cycloadduct; and (3) an aldol condensation of the trans-cycloadduct using an aldehyde followed by the elimination of water to generate a damascone. These reactions are performed in three separate reaction processes. A process for synthesizing damascone is described in Ayyer et al., Journal of the Chemical Society Perkin Trans., 1975, 1, 1727-1736.
- 1-(2,6,6-Trimethylcyclohex-3-en-1-yl)-ethanone is a useful compound as an intermediate for the synthesis of the damascones.
- 1-(2,6,6-Trimethylcyclohex-3-en-1-yl)-ethanone can be readily synthesized by a Diels-Alder reaction between 1,3-pentadiene (piperylene) and mesityl oxide to produce a mixture of cis- and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)ethanone.
- the cycloadduct from the Diels-Alder cycloaddition is a mixture of cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)ethanone and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)ethanone.
- further synthetic transformations are necessary to convert the Diels-Alder cycloadduct to ⁇ -damascone.
- damascone isomers and other substituted cyclohexene based perfume components is necessary.
- the present disclosure provides processes for converting cyclohexene compounds into compounds suitable for use as fragrance and perfume components.
- processes for the production of ⁇ -damascone from 1-(2,6,6-trimethylcyclohex-3-en-1-yl)ethanone in a single reactor procedure are disclosed.
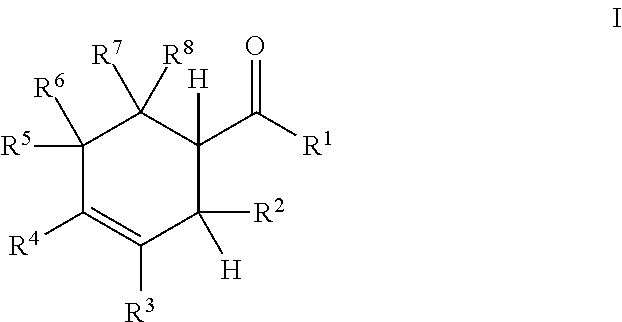
- the present disclosure provides a process for the epimerization of a substituted cyclohexene comprising the steps of providing to a reactor a first isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone compound according to Formula I:
- R 1 and R 2 are each independently C 1 -C 4 alkyl
- R 3 , R 4 , R 5 , R 6 , R 7 , and R 8 are each independently selected from the group consisting of H and C 1 -C 4 alkyl.
- the present disclosure provides a process for producing an aldol condensation product comprising the steps of epimerizing a first cis/trans isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone according to Formula I:
- R 1 and R 2 are each independently C 1 -C 4 alkyl
- R 3 , R 4 , R 5 , R 6 , R 7 , and R 8 are each independently selected from the group consisting of H and C 1 -C 4 alkyl.
- the present disclosure provides for a single reactor process for the production of a cyclohexene product comprising the steps of epimerizing a 1,2-cis-isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with potassium tert-butoxide to form a 1,2-trans-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone; and condensing the 1,2-trans-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with an aldehyde followed by elimination of water to form a first aldol condensation product, wherein the epimerizing step and the condensing step are performed in a single reactor.
- the present disclosure provides for a perfume composition
- a perfume composition comprising ( ⁇ )- ⁇ -damascone having greater than 82% by weight of the trans-ring isomer.
- the ( ⁇ )- ⁇ -damascone perfume composition may be incorporated into a detergent composition, a fine fragrance composition, or an air freshener composition.
- FIG. 1 illustrates a Diels-Alder cycloaddition and a single reactor approach involving an epimerization and aldol condensation to synthesize ⁇ -damascone from the Diels-Alder cycloadduct.
- the term “comprising” means various components conjointly employed in the preparation of the compositions of the present disclosure. Accordingly, the terms “consisting essentially of” and “consisting of” are embodied in the term “comprising”.
- the term “plurality” means more than one.
- Diels-Alder cycloaddition describes a [4+2] cycloaddition between a diene component having a conjugated 1,3-diene functionality and a dienophile component having a reactive double or triple bond. Reactions via step-wise or concerted mechanisms are included within this term.
- Diels-Alder cycloadduct and “Diels-Alder adduct” describe the cyclohexene product resulting from a Diels-Alder cycloaddition.
- epimerization describes the transformation of a first epimer to a second epimer. Epimerization may be accomplished by a deprotonation-deprotonation approach.
- aldol reaction includes a reaction of the enolate of a carbonyl compound, such as a ketone or an aldehyde, with a second carbonyl compound, such as a ketone or an aldehyde, to form the aldol addition product (a ⁇ -hydroxycarbonyl compound).
- aldol condensation describes the reaction of the enolate of a carbonyl compound, such as a ketone or an aldehyde, with a second carbonyl compound, such as a ketone or an aldehyde, to form the aldol addition product (a ⁇ -hydroxycarbonyl compound) followed by the elimination of water to form an unsaturated carbonyl compound.
- a carbonyl compound such as a ketone or an aldehyde
- aldol condensation product describes the product resulting from an aldol condensation reaction.
- the term “elimination of water” includes the loss of a hydroxy moiety from a first atom and a hydrogen atom from an adjacent atom, combined with the formation of a pi-bond between the first atom and the adjacent atom.
- the “elimination of water” includes the elimination of the elements of water (i.e., the elimination of hydroxy and a hydrogen) where the elements of water do not necessarily combine to form a molecule of water during the elimination.
- the phrase “performed in a single reactor” means that the two or more reaction processes are performed in one or more reactors without an intermediate isolation step. In certain examples, the two or more reaction processes may also be performed without an intermediate workup or quenching step.
- component or composition levels are in reference to the active portion of that component or composition, and are exclusive of impurities, for example, residual solvents or by-products, which may be present in commercially available sources of such components or compositions.
- the present disclosure is related to processes for producing substituted cyclohexanes from cyclic organic compounds useful in a variety of applications, such as fragrance components, by novel epimerization and aldol condensation processes.
- a first isomer of a cyclohexenyl alkyl ketone may be epimerized to a second isomer by reaction with a metal alkoxide base; and the second isomer may be condensed with a carbonyl compound, such as an aldehyde or ketone, followed by the elimination of water to form a first aldol condensation product.
- the processes disclosed herein may have certain advantages over conventional approaches, including, but not limited to, capability of being performed in a single reactor and/or without an intermediate isolation or purification step, does not produce dangerous products (i.e., H 2 (g)) on a large scale, demonstrates better atom efficiency, reduced side reactions, simplified workup conditions, better yields, recycling of reaction components, and fewer waste products.
- dangerous products i.e., H 2 (g)
- the starting materials, intermediates, and products of the various embodiments of the present disclosure may contain one or more chiral centers and/or double bonds and therefore, may exist as stereoisomers, such as double-bond isomers (i.e., geometric isomers), enantiomers, or diastereomers.
- the starting materials, intermediates, and products may also exist as epimers when diastereomers have the opposite stereochemical configuration at only one of two or more stereogenic centers. Accordingly, compounds within the scope of the present disclosure encompass all possible enantiomers and stereoisomers of the illustrated compounds, including the stereoisomerically pure form (e.g., geometrically pure, enantiomerically pure, or diastereomerically pure) and enantiomeric and stereoisomeric mixtures.
- Enantiomeric and stereoisomeric mixtures may be resolved into their component enantiomers or stereoisomers, respectively, using separation techniques or chiral synthesis techniques known to the skilled artisan.
- the starting materials, intermediates, and products of the present disclosure may exist as geometric isomers.
- the present disclosure encompasses the various geometric isomers and mixtures thereof resulting from the position of a double bond, the arrangement of substituents around a carbon-carbon double bond, or arrangement of substituents around a carbocyclic ring.
- Non-hydrogen substituents around a carbon-carbon double bond may be referred to as having a “cis” or “trans” configuration, where “cis” represents substituents on the same side of the double bond and “trans” represents substituents on opposite sides of the double bond.
- the arrangements of substituents around a carbocyclic ring may also be designated as “cis” or “trans”, where the term “cis” represents substituents on the same face of the carbocyclic ring and the term “trans” represents substituents on the opposite face of the carbocyclic ring.
- the terms “cis” and “trans” are common terms for representing isomeric differences in organic compounds and will be readily understood by one having ordinary skill in the art. For a more detailed discussion of ring substitution, see, Eliel and Wilen, “Sterochemistry of Organic Compounds”, Wiley-Interscience, New York, 1994, pp 726-727.
- the present disclosure provides for processes for converting a first isomer of a cyclic organic compound to a second isomer by one or more chemical transformations for use in the synthesis of aroma or perfume components; in particular, the epimerization of a first isomer of a cyclohexenyl alkyl ketone to a second isomer. Subsequent to the epimerization, the second isomer of the cyclohexenyl alkyl ketone may be converted to a component for a fragrance or perfume composition.
- the chemical transformations may be performed in situ (i.e., without isolation or purification), or with isolation of intermediate compounds. In certain embodiments, the chemical transformations may be performed in a single reactor.
- the chemical transformations may be performed in one step or in multiple steps.
- the chemical transformations may include reacting the cyclic organic compounds or intermediates with additional reagents using the epimerization/aldol condensation process described herein.
- Intermediates may be used with or without purification by techniques known to those skilled in the art, e.g., filtration, distillation, sublimation, crystallization, trituration, extraction, chromatography, and any combinations thereof.
- the present disclosure provides a process for the epimerization of a substituted cyclohexene.
- the process may comprise the steps of providing to a reactor a first isomer of a cyclohexenyl alkyl ketone compound according to Formula I
- epimerizing the first isomer includes deprotonating the hydrogen at the C1 carbon in the first isomer of the cyclohexenyl alkyl ketone and reprotonating the C1 carbon on the opposite face of the ring to form the second isomer of the cyclohexenyl alkyl ketone.
- the cyclohexenyl alkyl ketone is a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone according to Formula I wherein R 2 may be an alkyl (such as C 1 -C 4 alkyl).
- R 2 may be selected from non-alkyl substituents, such as, for example, alkoxy (such as C 1 -C 4 alkoxy), siloxy (—OSi(alkyl) 3 ), aromatic (e.g., phenyl) or halogen.
- each of R 1 , R 3 , R 4 , R 5 , R 6 , R 7 , and R 8 may be independently selected from hydrogen, alkyl (such as C 1 -C 4 alkyl), alkoxy (such as C 1 -C 4 alkoxy), siloxy (—OSi(alkyl) 3 ), aromatic (e.g., phenyl) or halogen.
- alkyl such as C 1 -C 4 alkyl
- alkoxy such as C 1 -C 4 alkoxy
- siloxy —OSi(alkyl) 3
- aromatic e.g., phenyl
- two substituents may come together to form a carbocyclic or heterocyclic 5-, 6-, or 7-membered ring.
- any of R 1 and R 2 , R 1 and R 7 or R 8 , R 5 or R 6 and R 7 or R 8 , R 4 and R 5 or R 6 , R 4 and R 3 , or R 3 and R 2 may come together to form a carbocyclic or heterocyclic 5-, 6-, or 7-membered ring.
- R 1 and R 2 may be each independently C 1 -C 4 alkyl
- R 3 , R 4 , R 5 , R 6 , R 7 , and R 8 may be each independently selected from the group consisting of H and C 1 -C 4 alkyl.
- R 1 , R 2 , R 7 , and R 8 are methyl and R 3 , R 4 , R 5 , and R 6 are hydrogen (i.e., the structure is 1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone).
- Other cyclic structures corresponding to the general structure of Formula I are within the subject matter of the present application, such as, for example, but not limited to, cyclohexanyl alkyl ketones (for example, where the cyclohexenyl double bond has been reduced, such as, by a hydrogenation reaction).
- the dotted bond between C3 and C4 carbons indicates that structure encompasses compounds having either a single or double bond between C3 and C4.
- the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be a product of a Diels-Alder-type [4+2] cycloaddition reaction.
- a Diels-Alder-type [4+2] cycloaddition reaction describes a [4+2] cycloaddition between a diene component having a conjugated 1,3-diene functionality and a dienophile component having a reactive double bond.
- the diene may be any suitable cyclic and acyclic compounds having a conjugated 1,3-diene moiety that may adopt an s-cis conformation, for example, but not limited to, 1,3-pentadiene (piperylene).
- the dienophile may be any suitable compounds having a double carbon-carbon bond that may be reactive with a 1,3-diene, for example, in a Diels-Alder cycloaddition, such as, but not limited to, 4-methyl-3-penten-2-one (mesityl oxide).
- the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be a product of a Diels-Alder-type [4+2] cycloaddition reaction obtained via standard, well-known synthetic methodologies, see e.g.
- the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be a product of a Diels-Alder-type [4+2] cycloaddition reaction conducted in a Lewis acidic ionic liquid.
- Diels-Alder-type [4+2] cycloaddition reactions conducted in a Lewis acidic ionic liquid are described in detail in co-pending U.S.
- a Diels-Alder cycloaddition of piperylene and mesityl oxide may produce 1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone (as a mixture of cis and trans ring isomers).
- the first isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be the kinetically favored isomer in the Diels-Alder-type [4+2] cycloaddition reaction and the second isomer may be the thermodynamically favored isomer in the Diels-Alder-type [4+2] cycloaddition reaction.
- the term “kinetically favored isomer” means the product isomer that is produced at a faster rate than the other isomer(s).
- the term “thermodynamically favored isomer” means the product isomer that is more thermodynamically stable than the other isomer(s).
- the kinetically favored isomer may be a cis-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone, such as, for example, cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone and the thermodynamically favored isomer may be a trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone, such as, for example, trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone.
- the process for the epimerization of a substituted cyclohexene comprises the steps of providing to a reactor a first isomer that is a cis-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone according to Formula II and the process comprises epimerizing the cis-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to the second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone, where the second isomer is a trans-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone according to Formula III, wherein R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , and R 8 are described herein.
- R 2 and the carbonyl substituent are on the same side (i.e., cis) or opposite side (i.e., trans) of the plane of the cyclohexene ring, respectively.
- the first isomer may be cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone and the second isomer may be trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone.
- Other cyclic structures corresponding to the general structure of Formulas II and/or III are within the subject matter of the present application.
- the process for the epimerization of a substituted cyclohexene comprises the steps of providing to a reactor a mixture of substituted cyclohexene isomers, such as, a mixture of stereoisomers, enantiomers, diastereomers, and/or epimers having a general structure according to Formula I.
- the mixture of substituted cyclohexene isomers comprises a mixture of cis-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone and trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone.
- the mixture of substituted cyclohexene isomers comprises more cis-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone than trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone.
- the cis:trans ratio of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be greater than 10:1.
- epimerizing the first isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to the second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone results in conversion of the cis isomer in the mixture of stereoisomers into the trans isomer of 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone.
- a majority, and in certain embodiments, substantially all of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone in the product is trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone.
- the term “majority” means greater than 50% and the term “substantially all” means greater than 90%.
- the epimerizing the first isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may comprise contacting the cyclohexenyl alkyl ketone with a base, such as basic alkali and/or alkaline metal compounds, for example, an alkali metal amide (for example, but not limited to, sodium amide (NaNH 2 ) or lithium amide (LiNH 2 )), a metal alkoxide (for example, but not limited to, sodium methoxide (NaOMe), lithium methoxide (LiOMe), potassium methoxide (KOMe), sodium ethoxide (NaOEt), lithium ethoxide (LiOEt), potassium ethoxide (KOEt), lithium tert-butoxide (LiOt-Bu), potassium tert-butoxide (KOt-Bu), potassium isobutoxide (KOi-Bu), sodium isopropoxide (NaOi-
- the first isomer may be epimerized with a metal alkoxide base such as a metal alkoxide selected from the group consisting of potassium tert-butoxide, lithium tert-butoxide, sodium methoxide, titanium tetra-n-butoxide, titanium tetraisopropoxide, and mixtures of any thereof.
- a metal alkoxide base such as a metal alkoxide selected from the group consisting of potassium tert-butoxide, lithium tert-butoxide, sodium methoxide, titanium tetra-n-butoxide, titanium tetraisopropoxide, and mixtures of any thereof.
- the metal alkoxide base may be potassium tert-butoxide.
- Other bases may also be suitable for epimerizing the cis- to the trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)ethanone and are within the scope of this disclosure.
- epimerizing the first isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with a base to for the second isomer may comprise epimerizing the kinetically favored isomer from a Diels-Alder cycloaddition reaction to a thermodynamically favored isomer.
- the molar ratio of substituted cyclohexene to metal alkoxide base may range from about 0.25:1.0 to about 1.50:1.0, and in other embodiments from about 0.50:1.0 to about 1.10:1.0, and in still other embodiments from about 0.55:1.0 to about 0.75:1.0.
- the molar ratio of the mixture of cis/trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone isomers to KOt-Bu may range from about 0.25:1.0 to about 1.50:1.0, and in other embodiments from about 0.50:1.0 to about 1.10:1.0, and in still other embodiments from about 0.55:1.0 to about 0.75:1.0.
- the epimerization of a cyclohexene of the present disclosure comprises the step of contacting the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with a mixture of metal alkoxide bases.
- the mixture of metal alkoxide bases may comprise potassium tert-butoxide and titanium tetra-n-butoxide in a ratio ranging from 0.25:1.0 to 2:1, in other embodiments from 0.5:1.0 to 2:1 and in specific embodiments from 1:1 to 2:1.
- each metal alkoxide base may have an independent molar ratio of substituted cyclohexene to metal alkoxide base ranging from about 0.25:1.0 to about 1.50:1.0, and in other embodiments from about 0.50:1.0 to about 1.10:1.0, and in still other embodiments from about 0.55:1.0 to about 0.75:1.0.
- a mixture of metal alkoxide bases comprising KOt-Bu and Ti(On-Bu) 4 may be used to epimerize a first isomer of 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to a second isomer in which the molar ratio of the mixture of cis/trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone isomers to KOt-Bu may range from about 0.25:1.0 to about 1.50:1.0, and in other embodiments from about 0.50:1.0 to about 1.10:1.0, and in still other embodiments from about 0.55:1.0 to about 0.75:1.0, and the molar ratio of the mixture of cis/trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone isomers to Ti(On-Bu) 4 may range from about 0.25:1.0 to about 1.50:1.0, and in other embodiments from about 0.50:1.0 to about 1.10:1.0,
- the atom efficiency, atom utilization, or atom economy evaluates the amount of waste generated by alternative routes to a specific product.
- the atom efficiency may be calculated by dividing the molecular weight of the desired product by the sum total of the molecular weights of all chemical components produced in the stoichiometric equation for the reactions involved. The comparison is made on a theoretical basis, i.e., 100% chemical yield. Therefore, in certain embodiments, the atom efficiency of the epimerization process using one metal alkoxide base may be greater than the atom efficiency of the epimerization process using more than one metal alkoxide base.
- Epimerizing the first (cis) isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to the second (trans) isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be done in any suitable inert organic solvent, such as, for example, diethyl ether, tetrahydrofuran, and alcohol solvents, such as methanol, ethanol, and n-, sec-, iso- or tert-butanol.
- Epimerizing may comprise contacting the first isomer with a metal alkoxide base, such as, for example, potassium tert-butoxide in an organic solvent at a temperature ranging from 0° C.
- the epimerized product i.e., the trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone
- the epimerized product may be directly submitted to an aldol condensation process, for example, in a single reactor process.
- the aldol condensation reaction is the acid- or base-catalyzed condensation of an aldehyde or ketone with a second aldehyde or ketone.
- the enolate of an aldehyde or a ketone adds to the carbonyl carbon of another aldehyde or ketone to form an aldol addition product.
- the aldol addition product may undergo further transformations, e.g., dehydration to from an aldol condensation product. In certain embodiments, the dehydration occurs spontaneously or may be performed in another step.
- the second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be converted to a composition suitable for use as a fragrance enhancement compound.
- the second isomer may be converted by an aldol condensation process with an aldehyde or ketone to produce a fragrance component.
- the second isomer may be converted to 6-damascone via an aldol condensation.
- the process for epimerization of a cyclohexene may further comprise condensing the second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with an aldehyde followed by elimination of water to form a first aldol condensation product.
- the condensing reaction is an aldol condensation reaction.
- the first aldol condensation product may be an unsaturated ketone, such as an ⁇ , ⁇ -unsaturated ketone.
- condensing the second isomer with an aldehyde may comprise condensing trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone with an aldehyde followed by an elimination of water to form an aldol condensation product.
- the aldehyde may be acetaldehyde (ethanal) and the aldol condensation product may be ( ⁇ )- ⁇ -damascone.
- the aldol condensation may be performed in the same reactor (i.e., single reactor process) as the epimerization step.
- the epimerization product i.e., trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone
- the epimerization process of the present disclosure utilizes a metal alkoxide base to catalyze the epimerization, that base may also be used to deprotonate the trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to form an enolate of the ketone.
- additional base may be added to the reaction medium. Addition of a second aldehyde or ketone may then result in an aldol condensation between the enolate of the trans-1-(2-alkyl-3-cyclohexen-1-yl)-alkanone and the second aldehyde or ketone as shown in equation 1.
- the epimerization product may be isolated (i.e., worked up) prior to deprotonation with a base (i.e., a metal alkoxide base) and condensation with an aldehyde or ketone in an aldol condensation.
- the elimination of water during the aldol condensation forms an unsaturated ketone, as discussed herein.
- the dehydration step can occur spontaneously during the aldol reaction or in a separate step. In certain embodiments, the elimination of water may occur under the reaction conditions to produce the unsaturated ketone. In other embodiments, the dehydration reaction can be conducted by exposing the reactants to air or vacuum at a temperature ranging from about 10° C. to about 30° C. In still other embodiments, the elimination of water may occur under acid catalysis. Suitable acid catalysts include, but are not limited to, organic acids, Lewis acids, and inorganic acids.
- Organic acids include, carboxylic acids (such as, for examples, acetic acid) and sulfonic acids (such as, for example, p-toluenesulfonic acid).
- Lewis acids include, but are not limited to, many common Lewis acids and may also include Lewis acidic ionic liquids.
- Inorganic acids include, but are not limited to, hydrochloric acid, sulfuric acid, nitric acid and the like
- the elimination of water may produce a mixture of a first aldol condensation product and a second aldol condensation product.
- the first aldol condensation product may be an ⁇ , ⁇ -unsaturated ketone and the second aldol condensation product may be a constitutional isomer of the first aldol condensation product, for example, an unconjugated unsaturated ketone, such as a ⁇ , ⁇ -unsaturated ketone.
- the process for epimerization of the cyclohexene may further comprise isomerizing the second aldol condensation product to the first aldol condensation product.
- the process may further comprise isomerizing the ⁇ , ⁇ -unsaturated ketone to the ⁇ , ⁇ -unsaturated ketone.
- the isomerization may occur spontaneously during the quenching and/or workup conditions.
- the aldol condensation reaction may be quenched or worked up with a mild acid which may also catalyze the isomerization of the second aldol condensation product to the first aldol condensation product.
- the isomerization may require a separate chemical step, such as treating the second aldol condensation product with an isomerization catalyst to isomerize it to the first aldol condensation product.
- the second aldol condensation product may be trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)but-3-en-1-one and the first aldol condensation product may be (E)-trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)but-2-en-1-one (i.e., ( ⁇ )- ⁇ -damascone).
- the present disclosure also relates to processes for converting a first isomer of an 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to a first aldol condensation product by a sequence of chemical transformations, and in particular, the epimerization of the first isomer of 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to the second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone (for example, with a metal alkoxide base) and the aldol condensation of a second isomer of a cyclohexenyl methyl ketone to an aldol condensation product.
- the chemical transformations may be performed in situ, or with isolation of intermediate compounds.
- the chemical transformations may be performed in a single reactor. That is, the epimerizing step and the condensing step (i.e., the aldol condensation) may be performed in a single reactor.
- the chemical transformations may be performed in one step, such as by the sequential addition of reagents to the reactor, or in multiple steps (i.e., with isolation or work-up steps between chemical transformations).
- Intermediates may be used with or without purification by techniques known to those in the art, e.g., filtration, distillation, sublimation, crystallization, trituration, extraction, and chromatography.
- elimination or work-up or isolation steps may increase the overall efficiency and yield of a chemical process, for example, by reducing material lost as a result of a work-up, isolation, or purification procedure, and may generally result in greater economic efficiency, for example, by decreased process time, decreased reactor requirements (one reactor instead of multiple reactors), eliminated work-up chemical expenses (such as, but not limited to, solvent and workup reagent purchase costs and disposal/recycling costs) and eliminated purification requirements (time, purchase costs and disposal/recycling costs).
- the present disclosure relates to processes for converting a first isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone to an aldol condensation product for use as aroma or perfume ingredients.
- the present disclosure provides a process for producing an aldol condensation product comprising the steps of epimerizing a first isomer of a cyclohexenyl alkyl ketone according to Formula I:
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , and R 8 are described herein, with a base to form a second isomer of an alkyl cyclohexenyl ketone; and condensing the second isomer with an aldehyde followed by elimination of water to form a first aldol condensation product.
- the present disclosure provides for a process for producing an aldol condensation product comprising the steps of epimerizing a first cis/trans isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone according to Formula I, wherein R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , and R 8 are described herein, with a metal alkoxide base, such as potassium tert-butoxide, to form a second cis/trans isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone; and condensing the second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with an aldehyde followed by elimination of water to form a first aldol condensation product.
- a metal alkoxide base such as potassium tert-butoxide
- the first cis/trans isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone is a 1,2-cis ring isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone and the second cis/trans isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone is a 1,2-trans ring isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone.
- first cis/trans isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may be a product of a Diels-Alder-type [4+2] cycloaddition reaction.
- the Diels-Alder-type [4+2] cycloaddition reaction may be between the dienophile, mesityl oxide, and the 1,3-diene, piperylene, that produces a mixture of the first cis/trans isomer cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone and the second cis/trans isomer is trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone.
- the Diels-Alder-type cycloaddition may produce a mixture of cis- and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone, wherein the cis isomer predominates.
- the base comprises a metal alkoxide base, such as basic alkali and alkaline metal compounds.
- the metal alkoxide base comprises an alkali metal alkoxide, such as, but not limited to, sodium methoxide (NaOMe), lithium methoxide (LiOMe), potassium methoxide (KOMe), sodium ethoxide (NaOEt), lithium ethoxide (LiOEt), potassium ethoxide (KOEt), lithium tert-butoxide (LiOt-Bu), potassium tert-butoxide (KOt-Bu), potassium isobutoxide (KOi-Bu), sodium isopropoxide (NaOi-Pr), potassium isopropoxide (KOi-Pr), titanium tetra-n-butoxide (Ti(On-Bu) 4 ), titanium tetraisopropoxide (Ti(OiPr) 4 ) or any mixtures thereof.
- the metal alkoxide base may be selected from the group consisting of potassium tert-butoxide, lithium tert-butoxide, sodium methoxide, titanium tetra-n-butoxide, titanium tetra-iso-propoxide, and mixtures of any thereof and in particular embodiments; the metal alkoxide base is potassium tert-butoxide.
- Suitable solvents for the aldol condensation step of the process may be the same as those inert organic solvents discussed herein for the epimerization step, for example, ethereal solvents (diethyl ether, and tetrahydrofuran).
- the aldehyde may be selected from linear, branched, or cyclic C 2 -C 12 aliphatic aldehydes.
- Other aldehydes and ketones known in the art may also be suitable for adding to the carbonyl carbon of the cyclohexenyl alkyl ketones described herein to form an aldol addition product and are within the scope of this disclosure.
- the aldehyde is ethanal (acetaldehyde).
- the aldehyde may be acetaldehyde.
- the first aldol condensation product is ( ⁇ )- ⁇ -damascone.
- the epimerizing step and the condensing step may be performed in a single reactor, for example, without an intermediate work-up, isolation, or purification step between the epimerization step and the condensation step.
- the condensing step for the trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)ethanone or other 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may also be performed with another aldehyde or ketone reagent to form a condensation product that may be useful as a fragrance component.
- the condensation products i.e., aldol reaction and, optionally the elimination reaction
- the processes may further comprise purifying the condensation product, for example, by a purification method selected from crystallization, distillation or chromatography. Suitable examples of these purification methods are described herein in reference to purifying the product of the epimerization process.
- Equation 1 Certain embodiments of the aldol condensation process according to the present disclosure are illustrated in equation 1.
- a metal alkoxide base for example, potassium tert-butoxide and an aldehyde comprising acetaldehyde (about 1 to 10 equivalents) in an inert organic solvent, such as tetrahydrofuran.
- the reaction vessel may be held at a reaction temperature ranging from about 0° C. to 50° C., and in certain embodiments from 10° C. to 30° C. or even from 15° C. to about 20° C.
- the product such as ( ⁇ )- ⁇ -damascone
- the reagents i.e., base and aldehyde
- the addition rate may vary according to reaction size.
- the epimerization and aldol condensation processes described herein may be performed in a single reactor.
- single reactor means that two or more chemical transformations are performed in one or more reactors without intermediate isolation, workup, or quenching steps.
- the mixture or cis- and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone may be submitted to epimerization conditions and once the epimerization is substantially complete, the reaction solution comprising substantially all trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone may be subjected directly to the aldol condensation conditions by adding the aldehyde to the epimerization product.
- the present disclosure provides for a single reactor process for the production of a cyclohexene product comprising the steps of epimerizing a 1,2-cis-isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with a metal alkoxide base, such as, potassium tert-butoxide, to form a 1,2-trans-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone; and condensing the 1,2-trans-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with an aldehyde followed by elimination of water to form a first aldol condensation product, wherein the epimerizing step and the condensing step are performed in a single reactor.
- a metal alkoxide base such as, potassium tert-butoxide
- the reactant for the epimerization step may be a mixture of the 1,2-cis-isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone and the 1,2-trans-isomer of a 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone.
- the 1,2-cis-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone is cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone
- the 1,2-trans-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone is trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone.
- the aldehyde in the aldol condensation process may be acetaldehyde such that the product of the single reactor process is (E)-trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)but-2-en-1-one (i.e., ( ⁇ )- ⁇ -damascone).
- the aldol condensation reaction may result in a mixture of a first aldol condensation product and a second aldol condensation product, as described in detail herein, wherein the process may further comprise isomerizing the second aldol condensation product to the first aldol condensation product.
- the 1,2-cis-isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone may the product of a Lewis acid catalyzed Diels-Alder-type [4+2] cycloaddition reaction in a Lewis acidic ionic liquid, such as those reactions and processes described in co-pending U.S.
- the single reactor process may further comprise purifying the first aldol condensation product.
- the isolation and purification of the first aldol condensation product may be effected, if desired, by any suitable separation or purification procedure, such as those known in the art, for example, distillation, filtration, extraction, crystallization, and chromatography, or a combination of these procedures.
- the first aldol condensation product may be purified by distillation, such as, but not limited to, simple distillation, fractional distillation, reduced pressure distillation, vacuum distillation, steam distillation, azeotropic distillation, Kugelrohr distillation, or other distillation technique. Other equivalent separation or isolation procedures known in the art may also be used.
- the first aldol condensation product may have use as a component of scents, perfumes, fragrances, and air fresheners.
- the first aldol condensation product may be ( ⁇ )- ⁇ -damascone, and in other embodiments, the first aldol condensation product is ( ⁇ )- ⁇ -damascone having greater than 82% by weight of the trans-ring isomer. In other embodiments the first aldol condensation product is ( ⁇ )- ⁇ -damascone having greater than 85% by weight of the trans-ring isomer, or in certain embodiments greater than 90% by weight of the trans-ring isomer, or even greater than 95% by weight of the trans-ring isomer.
- Performing the epimerization and aldol condensation processes in a single reactor may provide certain advantages over other synthetic approaches to these compounds, including, but not limited to, increased atom efficiency; eliminating additional process steps, such as extraction steps after the epimerization process; eliminating drying steps during the aldol condensation process; reducing the volumes of organic solvents, reagents, and water necessary for the transformations; eliminating the formation of solid potassium hydroxide or other dangerous side products; and improved opportunities for recycling starting materials, products and by-products from the reaction mixture, such as solvents, reactants, and by-products by separation techniques, for example, distillation.
- the processes described herein may comprise a single reactor epimerization/aldol condensation-elimination protocol according to the present disclosure.
- a single extraction protocol after the aldol condensation-elimination step may comprise the steps of subjecting the organic phase from the extraction to fractional distillation to provide purified ( ⁇ )- ⁇ -damascone.
- the extraction step may be eliminated and a simple phase separation protocol may be used instead.
- the organic phase from the phase separation may be subjected to fractional distillation to provide purified ( ⁇ )- ⁇ -damascone.
- the aqueous work-up after the aldol condensation-elimination step may be eliminated and the product mixture may be subjected directly to fractional distillation to provide purified ( ⁇ )- ⁇ -damascone.
- Certain embodiments of the single reactor process may provide advantages over the prior art three separate reaction approach since at least one and in certain embodiments, numerous work-up and/or purification steps are eliminated, thereby reducing processing steps, infrastructure requirements, byproduct production and disposal requirements and overall increased yields of product.
- the various embodiments of the epimerization and aldol condensation processes of the present disclosure may be used to provide useful industrial products, such as scents, fragrance components, and perfumes.
- the epimerization and aldol condensation process of the present disclosure may be readily scaled-up for industrial applications to be carried out in a plant that includes reactor types known in the art. Examples of such reactors include, but are not limited to, batch reactors, semi-batch reactors, and continuous reactors.
- the plant may include, in combination, a) at least one stirred reactor system, b) at least one inlet line into a first reactor system for the substituted cyclohexene and the catalyst system, and c) at least one separator to separate the desired aldol condensation products, which may optionally include a recycle loop for solvents and/or reactants and/or products.
- a) at least one stirred reactor system b) at least one inlet line into a first reactor system for the substituted cyclohexene and the catalyst system
- at least one separator to separate the desired aldol condensation products, which may optionally include a recycle loop for solvents and/or reactants and/or products.
- reaction times and conditions are intended to be approximate, e.g., taking place at about atmospheric pressure within a temperature range of about ⁇ 10° C. to about 110° C. over a period of about 1 to about 24 hours; reactions left to run overnight average a period of about 16 hours.
- the processes of the present disclosure may be carried out at within a temperature range from ⁇ 10° C. to 200° C., and in certain embodiments in the range of 15° C. to 150° C., and in other embodiments in the range of 25° C. to 66° C.
- solvents may be suitable for use in the processes described herein, however, the solvents used in the reaction may affect the yield of the reaction and stereoselectivity of the reaction products. Typically, solvents may comprise from about 50% to about 95% by weight of the reaction mixture.
- Suitable solvents include ionic, polar or non-polar; organic or inorganic solvents, for example, but not limited to, aromatic solvents, such as, but not limited to toluene and benzene (including halogenated benzene solvents); ethereal solvents, such as, but not limited to, diethyl ether and tetrahydrofuran (THF); halogenated solvents, such as, but not limited to, dichloromethane; and water.
- aromatic solvents such as, but not limited to to toluene and benzene (including halogenated benzene solvents)
- ethereal solvents such as, but not limited to, diethyl ether and tetrahydrofuran (THF)
- halogenated solvents such as, but not limited to, dichloromethane
- the reactions may be performed neat.
- the individual reagents described herein may be combined simultaneously or sequentially in any order, and in the presence or absence of a solvent.
- the components of the catalysts may also be added separately and at different temperatures to allow for selective and controlled contact between the components.
- the reagents, solvents, or mixtures thereof may be introduced into the processes in a continuous or batch fashion.
- Isolation and purification of the reagents, intermediates and products described herein may be effected, if desired, by any suitable separation or purification procedure such as, for example, filtration, distillation, sublimation, crystallization, trituration, extraction, and chromatography, or a combination of these procedures.
- any suitable separation or purification procedure such as, for example, filtration, distillation, sublimation, crystallization, trituration, extraction, and chromatography, or a combination of these procedures.
- any conventional method of purification may be used.
- the product is a solid, it may be purified, for example, by crystallization or recrystallization. Crystallization or recrystallization using a single solvent or mixed solvent system are contemplated.
- the product may be purified using a distillation method, such as, but not limited to simple distillation, steam distillation, fractional distillation, azeotropic distillation, spinning band distillation, vacuum or reduced pressure distillation, and combination of these methods (for example, vacuum fractional distillation).
- a distillation method such as, but not limited to simple distillation, steam distillation, fractional distillation, azeotropic distillation, spinning band distillation, vacuum or reduced pressure distillation, and combination of these methods (for example, vacuum fractional distillation).
- the product may be purified by chromatographic methods, such as, but not limited to column chromatography, liquid chromatography, flash chromatography, medium pressure liquid chromatography, high performance liquid chromatography (HPLC), thin layer chromatography, reverse-phase chromatography, and combinations of these methods.
- the product may be purified by using a combination of any of the above referenced purification methods.
- Any starting material, intermediate, or product may occur as a stereoisomer mixture having at least about 60% (e.g., at least about 70%, at least about 75%, at least about 80%, at least about 85%, at least about 90%, at least about 95%, at least about 99%, greater than about 99%) of one of the two possible stereoisomers (cis/trans isomers).
- the starting materials, intermediates, and products may be substantially free of their enantiomers and the other possible stereoisomers as well as other non-stereoisomer-related materials, e.g., solvents, reagents, reaction by-products, and the like.
- the various embodiments of the processes of the present disclosure may be utilized to synthesize ( ⁇ )- ⁇ -damascone having a high degree of purity (i.e., greater than 82% by weight of the trans-ring isomer).
- Various embodiments of the present disclosure provide for perfume compositions comprising ( ⁇ )- ⁇ -damascone produced by the processes described herein.
- the present disclosure provides for a perfume composition comprising ( ⁇ )- ⁇ -damascone having greater than 82% by weight of the trans-ring isomer.
- the perfume compositions comprising the ( ⁇ )- ⁇ -damascone produced by the present processes may be utilized in a variety of commercial products.
- One embodiment provides for a detergent composition comprising a perfume composition comprising ( ⁇ )- ⁇ -damascone produced by the processes described herein.
- Another embodiment provides for a fine fragrance composition comprising the ( ⁇ )- ⁇ -damascone produced by the processes described herein.
- Still another embodiment provides for an air freshener composition comprising the ( ⁇ )- ⁇ -damascone produced by the processes described herein.
- Other commercial products requiring a specific fragrance composition that may be synthesized according to the processes described herein are also contemplated.
- a mixture consisting of cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone to trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone is epimerized to give increased content of the trans isomer.
- the base is varied to determine the optimum reaction conditions.
- Sample Procedure The mixture consisting of cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone produced by a Diels-Alder cycloaddition (1 eq., 10 mmol, 1.67 g) is added under N 2 atmosphere to a suspension of base KOt-Bu (2 eq., 20 mmol, 2.24 g) and/or Ti(On-Bu) 4 (2 eq., 20 mmol, 2.84 g) in 10 mL tetrahydrofuran (THF) at room temperature (16° C. to 20° C.).
- base KOt-Bu 2 eq., 20 mmol, 2.24 g
- Ti(On-Bu) 4 2 eq., 20 mmol, 2.84 g
- the mixture is heated to 60° C. to 66° C. and stirred for 1.5 to 5 h to epimerize the cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone to trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone (as the enolate).
- a base mixture in THF according to Table 1 is prepared and the isomeric mixture of cis- and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone is added to the suspension. Upon workup, the trans-cis isomeric ration is determined.
- Table 1 A summary of the molar ratio of certain components of the epimerization process for the epimerization of 1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone according to certain embodiments of the present disclosure as described herein is provided in Table 1.
- a substituted cyclohexene product comprising predominately ( ⁇ )- ⁇ -damascone is formed.
- a mixture consisting of cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone is epimerized and then subjected to aldol condensation conditions with acetaldehyde to produce the ( ⁇ )- ⁇ -damascone.
- the mixture consisting of cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone and trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone produced by a Diels-Alder cycloaddition (1 eq., 10 mmol, 1.67 g) is added under N 2 atmosphere to a suspension of KOt-Bu (2 eq., 20 mmol, 2.24 g) in 10 mL tetrahydrofuran (THF) at room temperature (16° C. to 20° C.). The mixture is heated to 60° C. to 66° C.
- the formed potassium acetate precipitate is removed by filtration, and the remaining liquid is evaporated to remove the THF solvent and the formed tert-butanol.
- the residue is vacuum distilled (0.066-0.27 mbar, 55° C. to 80° C. using a Kugelrohr distillation apparatus) to give the desired ( ⁇ )- ⁇ -damascone in 65.3% (calibrated GC yield after recycling of the unreacted starting material).
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
wherein R1 ad R2 are each independently C1-C4 alkyl, and R3, R4, R5, R6, R7, and R8 are each independently selected from the group consisting of H and C1-C4 alkyl; and epimerizing the first isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with a metal alkoxide base to form a second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone.
Description
For example, trans,trans-δ-damascone is one of the most widely used fragrance additives in the detergent and food industries. Therefore, the industrial scale production of δ-damascone and other related compounds is of great interest.
and epimerizing the first isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with a metal alkoxide base to form a second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone. According to Formula I, R1 and R2 are each independently C1-C4 alkyl, and R3, R4, R5, R6, R7, and R8 are each independently selected from the group consisting of H and C1-C4 alkyl.
with potassium tert-butoxide to form a second cis/trans isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone; and condensing the second isomer of the 1-(2-alkyl-3-cyclohexen-1-yl)-alkanone with an aldehyde followed by elimination of water to form a first aldol condensation product. According to Formula I, R1 and R2 are each independently C1-C4 alkyl, and R3, R4, R5, R6, R7, and R8 are each independently selected from the group consisting of H and C1-C4 alkyl.
wherein R1, R2, R3, R4, R5, R6, R7, and R8 are described herein; and epimerizing the first isomer of the cyclohexenyl alkyl ketone with a base to form a second isomer of the cyclohexenyl alkyl ketone. According to these embodiments, epimerizing the first isomer includes deprotonating the hydrogen at the C1 carbon in the first isomer of the cyclohexenyl alkyl ketone and reprotonating the C1 carbon on the opposite face of the ring to form the second isomer of the cyclohexenyl alkyl ketone.
Referring to Formulas II and III, R2 and the carbonyl substituent are on the same side (i.e., cis) or opposite side (i.e., trans) of the plane of the cyclohexene ring, respectively. In specific embodiments, the first isomer may be cis-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone and the second isomer may be trans-1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone. Other cyclic structures corresponding to the general structure of Formulas II and/or III are within the subject matter of the present application.
wherein R1, R2, R3, R4, R5, R6, R7, and R8 are described herein, with a base to form a second isomer of an alkyl cyclohexenyl ketone; and condensing the second isomer with an aldehyde followed by elimination of water to form a first aldol condensation product.
| TABLE 1 |
| Epimerization of 1-(2,6,6-trimethylcyclohex-3-en-1-yl)-ethanone |
| DA: | DA: | KOt-Bu: | trans:cis | % trans | |
| Entry | KOt-Bu | Ti(On-Bu)4 | Ti(On-Bu)4 | in product | product |
| 1 | 1.10:1.00 | 1.10:1.00 | 1.00:1.00 | 0.82:1.00 | 45 |
| 2 | 0.77:1.00 | 0.77:1.00 | 1.00:1.00 | 5.84:1.00 | 85 |
| 3 | 0.55:1.00 | 0.55:1.00 | 1.00:1.00 | 8.82:1.00 | 90 |
| 4 | 0.73:1.00 | — | — | 13.69:1.00 | 93 |
| 5 | 0.55:1.00 | — | — | 18.56:1.00 | 95 |
| 6 | 0.73:1.00 | 1.10:1.00 | 1.50:1.00 | 25.42:1.00 | 96 |
| 7 | 0.55:1.00 | 1.10:1.00 | 2.00:1.00 | 28.14:1.00 | 97 |
| 8 | 0.55:1.00 | — | — | 2.07:1.00 | 67 |
| (NaOMe) | |||||
| DA = Diels-Alder cycloadduct | |||||
Claims (21)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/641,786 US8093432B2 (en) | 2009-12-18 | 2009-12-18 | Processes for epimerizing cyclohexenyl ketones with subsequent aldol condensation to produce fragrance compounds |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/641,786 US8093432B2 (en) | 2009-12-18 | 2009-12-18 | Processes for epimerizing cyclohexenyl ketones with subsequent aldol condensation to produce fragrance compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20110152575A1 US20110152575A1 (en) | 2011-06-23 |
| US8093432B2 true US8093432B2 (en) | 2012-01-10 |
Family
ID=44152015
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/641,786 Expired - Fee Related US8093432B2 (en) | 2009-12-18 | 2009-12-18 | Processes for epimerizing cyclohexenyl ketones with subsequent aldol condensation to produce fragrance compounds |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US8093432B2 (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4198309A (en) | 1977-11-15 | 1980-04-15 | International Flavors & Fragrances Inc. | Use of trans, trans-Δ-damascone and mixtures containing 80% or more of trans, trans-Δ-damascone in augmenting or enhancing the aroma of a detergent |
| US4334098A (en) | 1978-07-27 | 1982-06-08 | International Flavors & Fragrances Inc. | Trans,trans-Δ-damascone, mixtures containing major proportions of same, processes for preparing same and organoleptic uses thereof |
| US6573405B1 (en) | 1999-03-24 | 2003-06-03 | Scionix Limited | Ionic liquids |
-
2009
- 2009-12-18 US US12/641,786 patent/US8093432B2/en not_active Expired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4198309A (en) | 1977-11-15 | 1980-04-15 | International Flavors & Fragrances Inc. | Use of trans, trans-Δ-damascone and mixtures containing 80% or more of trans, trans-Δ-damascone in augmenting or enhancing the aroma of a detergent |
| US4334098A (en) | 1978-07-27 | 1982-06-08 | International Flavors & Fragrances Inc. | Trans,trans-Δ-damascone, mixtures containing major proportions of same, processes for preparing same and organoleptic uses thereof |
| US6573405B1 (en) | 1999-03-24 | 2003-06-03 | Scionix Limited | Ionic liquids |
Non-Patent Citations (8)
| Title |
|---|
| Abbott, Andrew P., et al., "Quaternary ammonium zinc- or tin-containing ionic liquids: water insensitive, recyclable catalysts for Diels-Alder reactions," Green Chemistry, vol. 4, pp. 24-26., 2002. |
| Ayyar, et al., Synthesis of delta-Damascone [trans-1-(2,6,6-Trimethylclohex-3-enyl) but-2-en-1-one] and beta-Damascenone [trans-1-(2,6,6-Trimethylcyclohexa-1,3-dienyl) but-2-en-1-one] Journal of the Chemical Society Perkin Trans., 1975, 1, pp. 1727-1736. |
| Ayyar, et al., Synthesis of δ—Damascone [trans—1-(2,6,6—Trimethylclohex-3-enyl) but-2-en-1-one] and β-Damascenone [trans-1-(2,6,6—Trimethylcyclohexa-1,3-dienyl) but-2-en-1-one] Journal of the Chemical Society Perkin Trans., 1975, 1, pp. 1727-1736. |
| Dyson, Paul J., et al., "Effect of Lewis acis on the Diels-Alder reaction in ionic liquids with different activation modes," Journal of Physical Organic Chemistry, vol. 21, pp. 264-270, 2008. |
| Kumar, Anil, et al., "Converting exo-Selective Diels-Alder Reaction to endo-Selective in Chloroaluminate Ionic Liquids," The Journal of Organic Chemistry, 2004, 4, pp. 1419-1420. |
| Lee, C. W.a, "Diels-Alder Reactions in Chloroaluminate Ionic Liquids: Acceleration and Selectivity Enhancement," Tetrahedron Letters, Elsevier, Amsterdam, vol. 40, pp. 2461-2464, 1999. |
| PCT International Search Report, date mailed Mar. 4, 2010, 14 pages. |
| U.S. Appl. No. 12/641,818, filed Dec. 18, 2009, David Martin Bourgeois, et al. |
Also Published As
| Publication number | Publication date |
|---|---|
| US20110152575A1 (en) | 2011-06-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101087746A (en) | Method for the production of isopulegol | |
| JP4886283B2 (en) | Method for producing α, β-unsaturated aldehyde compound | |
| KR101851126B1 (en) | Novel alicyclic alcohol | |
| EP2373606B1 (en) | Processes for epimerizing cyclohexenyl ketones with subsequent aldol condensation to produce fragrance compounds | |
| EP3089956B1 (en) | Process | |
| JP2010506861A (en) | 2,2,3-Trimethylcyclopent-3-enecarbaldehyde derivatives useful as odor substances | |
| KR101873838B1 (en) | Novel alicyclic alcohol | |
| CN105384615A (en) | Process for the preparation of (e, e)-farnesyl acetone | |
| US6822121B2 (en) | Production process of cyclohexenyl ketones | |
| US8093432B2 (en) | Processes for epimerizing cyclohexenyl ketones with subsequent aldol condensation to produce fragrance compounds | |
| US8067644B2 (en) | Process for conducting an organic reaction in ionic liquids | |
| US4970345A (en) | Process for preparing oxocyclopentene derivatives | |
| US4347388A (en) | 3,6-Dimethyl-3-hydroxy-oct-1-ynes and -oct-1-enes, derivatives of these, and their use as scents, and in the preparation of 3,6-dimethyl-3-hydroxy-octane | |
| JP2602811B2 (en) | Trimethylcyclopentene derivative, process for producing the same, and aroma composition | |
| EP0373556B1 (en) | Sandalwood odorants | |
| US6770618B2 (en) | (1S,6R)-2,2,6-Trimethylcyclohexyl methyl ketone and/or (1R,6S)-2,2,6-trimethylcyclohexyl methyl ketone, process for producing the same, and perfume composition containing the same | |
| Giersch et al. | Methyl homologues of methyl jasmonate and methyl dihydrojasmonate (Hedione®) from sorbyl alcohol | |
| WO2010080505A2 (en) | Process for conducting an organic reaction in ionic liquids | |
| EP0985651A1 (en) | Process for obtaining mixtures of isomeric acyloctahydronaphthalenes | |
| JP4030623B2 (en) | 4-alkoxymethylcyclohexylmethanol and process for producing the same | |
| CN112010826A (en) | Stereoselective synthesis of perhydro-3, 6-dialkyl-2-benzo [ B ] furanones | |
| US4181683A (en) | 1,7-Octadien-3-one and process for preparing the same | |
| JP7708485B1 (en) | Method for producing β-irone | |
| EP4149913B1 (en) | Process for preparing indene acryladehyde derivatives | |
| EP4267547B1 (en) | Process of making organic compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: THE PROCTER & GAMBLE COMPANY, OHIO Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:BOURGEOIS, DANIEL MARTIN;MIRACLE, GREGORY SCOT;PORTER, PHILLIP JOHN;AND OTHERS;SIGNING DATES FROM 20090109 TO 20090227;REEL/FRAME:024966/0842 |
|
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| FEPP | Fee payment procedure |
Free format text: MAINTENANCE FEE REMINDER MAILED (ORIGINAL EVENT CODE: REM.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| LAPS | Lapse for failure to pay maintenance fees |
Free format text: PATENT EXPIRED FOR FAILURE TO PAY MAINTENANCE FEES (ORIGINAL EVENT CODE: EXP.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| STCH | Information on status: patent discontinuation |
Free format text: PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 |
|
| FP | Lapsed due to failure to pay maintenance fee |
Effective date: 20200110 |