US20110098280A1 - 2,4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders - Google Patents
2,4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders Download PDFInfo
- Publication number
- US20110098280A1 US20110098280A1 US12/984,519 US98451911A US2011098280A1 US 20110098280 A1 US20110098280 A1 US 20110098280A1 US 98451911 A US98451911 A US 98451911A US 2011098280 A1 US2011098280 A1 US 2011098280A1
- Authority
- US
- United States
- Prior art keywords
- methyl
- amino
- sulfamoyl
- ethyl
- dmso
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
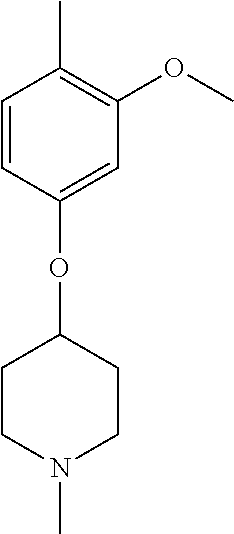
- KAUYJLLBKNEBDW-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCOCC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCOCC2)=C1 KAUYJLLBKNEBDW-UHFFFAOYSA-N 0.000 description 25
- 0 C*S(c(cccc1)c1Nc1nc(Nc(c(OC)c2)ccc2O[C@]2CN(C)CC2)ncc1*)(=Cl)=Cl Chemical compound C*S(c(cccc1)c1Nc1nc(Nc(c(OC)c2)ccc2O[C@]2CN(C)CC2)ncc1*)(=Cl)=Cl 0.000 description 22
- RWUBMNRQJITULS-UHFFFAOYSA-N COC1=C(C)C=CC(OC2CCN(C)CC2)=C1 Chemical compound COC1=C(C)C=CC(OC2CCN(C)CC2)=C1 RWUBMNRQJITULS-UHFFFAOYSA-N 0.000 description 14
- MAACBJMQAURITE-UHFFFAOYSA-N COC1=CC(N2CCN(C(C)=O)CC2)=CC=C1C Chemical compound COC1=CC(N2CCN(C(C)=O)CC2)=CC=C1C MAACBJMQAURITE-UHFFFAOYSA-N 0.000 description 12
- ZNZVSZJBZHQEPT-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(N3CCCCC3)CC2)=C1 ZNZVSZJBZHQEPT-UHFFFAOYSA-N 0.000 description 10
- FMPNUMXHDIPFNE-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCCC(C(N)=O)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCCC(C(N)=O)C2)=C1 FMPNUMXHDIPFNE-UHFFFAOYSA-N 0.000 description 10
- DSMLGLOTXAJZGR-UHFFFAOYSA-N COC1=CC(N2CCN(C)CC2)=CC=C1C Chemical compound COC1=CC(N2CCN(C)CC2)=CC=C1C DSMLGLOTXAJZGR-UHFFFAOYSA-N 0.000 description 10
- CXTIUQIZWCABPV-UHFFFAOYSA-N COC1=C(C)C=CC(F)=C1 Chemical compound COC1=C(C)C=CC(F)=C1 CXTIUQIZWCABPV-UHFFFAOYSA-N 0.000 description 8
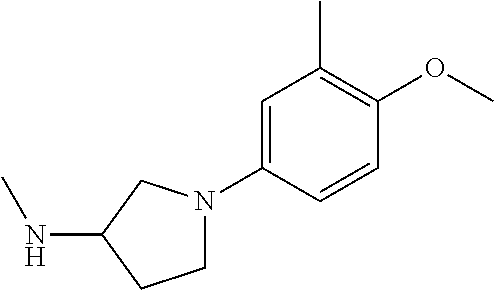
- YQUYKPJJPYACJR-UHFFFAOYSA-N CNC1CCN(C2=CC(OC)=C(C)C=C2)C1 Chemical compound CNC1CCN(C2=CC(OC)=C(C)C=C2)C1 YQUYKPJJPYACJR-UHFFFAOYSA-N 0.000 description 7
- ASRDZYDQDGUYRP-UHFFFAOYSA-N COC1=C(C)C=C(N2CCC(N3CCCCC3)CC2)C=C1 Chemical compound COC1=C(C)C=C(N2CCC(N3CCCCC3)CC2)C=C1 ASRDZYDQDGUYRP-UHFFFAOYSA-N 0.000 description 7
- GQJHUPMAMPQYCZ-UHFFFAOYSA-N COC1=C(C)C=C(N2CCOCC2)C=C1 Chemical compound COC1=C(C)C=C(N2CCOCC2)C=C1 GQJHUPMAMPQYCZ-UHFFFAOYSA-N 0.000 description 7
- FMPNUMXHDIPFNE-LLVKDONJSA-N COC1=C(C)C=CC(N2CCC[C@@H](C(N)=O)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC[C@@H](C(N)=O)C2)=C1 FMPNUMXHDIPFNE-LLVKDONJSA-N 0.000 description 7
- HMWUXWBIDIFRQQ-UHFFFAOYSA-N COC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1C Chemical compound COC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1C HMWUXWBIDIFRQQ-UHFFFAOYSA-N 0.000 description 7
- LUCHLDHKERKBCZ-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(N3CCCC3)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(N3CCCC3)CC2)=C1 LUCHLDHKERKBCZ-UHFFFAOYSA-N 0.000 description 5
- SSVDASSLXVQLPY-UHFFFAOYSA-N Cc(c(OC)c1)ccc1N(CC1)CC1N(C)C Chemical compound Cc(c(OC)c1)ccc1N(CC1)CC1N(C)C SSVDASSLXVQLPY-UHFFFAOYSA-N 0.000 description 5
- YQYZZTOIMUEGDJ-UHFFFAOYSA-N CC1=CC=CC2=C1C=CN2C Chemical compound CC1=CC=CC2=C1C=CN2C YQYZZTOIMUEGDJ-UHFFFAOYSA-N 0.000 description 4
- JAEDWIGUUKLIPP-MRXNPFEDSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 JAEDWIGUUKLIPP-MRXNPFEDSA-N 0.000 description 4
- QXOBYWRKNIDHJG-UHFFFAOYSA-N COC1=C(C)C=C(F)C=C1 Chemical compound COC1=C(C)C=C(F)C=C1 QXOBYWRKNIDHJG-UHFFFAOYSA-N 0.000 description 4
- QJKKHVDHXKQPBD-UHFFFAOYSA-N COC1=C(C)C=C(N2CCN(C(C)=O)CC2)C=C1 Chemical compound COC1=C(C)C=C(N2CCN(C(C)=O)CC2)C=C1 QJKKHVDHXKQPBD-UHFFFAOYSA-N 0.000 description 4
- AFZNYMBOKBQAKA-UHFFFAOYSA-N COC1=C(C)C=C(N2CCN(C)CC2)C=C1 Chemical compound COC1=C(C)C=C(N2CCN(C)CC2)C=C1 AFZNYMBOKBQAKA-UHFFFAOYSA-N 0.000 description 4
- ZKHUDIDPKPFFFT-UHFFFAOYSA-N COC1=C(C)C=CC(N2C=NC=N2)=C1 Chemical compound COC1=C(C)C=CC(N2C=NC=N2)=C1 ZKHUDIDPKPFFFT-UHFFFAOYSA-N 0.000 description 4
- KKEGIRRJODJKHQ-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(O)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(O)CC2)=C1 KKEGIRRJODJKHQ-UHFFFAOYSA-N 0.000 description 4
- FMPNUMXHDIPFNE-NSHDSACASA-N COC1=C(C)C=CC(N2CCC[C@H](C(N)=O)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC[C@H](C(N)=O)C2)=C1 FMPNUMXHDIPFNE-NSHDSACASA-N 0.000 description 4
- HRNZGACPSMLHAK-UHFFFAOYSA-N COC1=C(C)C=CC(OC2CCN(C)C2)=C1 Chemical compound COC1=C(C)C=CC(OC2CCN(C)C2)=C1 HRNZGACPSMLHAK-UHFFFAOYSA-N 0.000 description 4
- USJNNFPVGWDRND-UHFFFAOYSA-N COC1=C(C)C=CC(OCCN2CCOCC2)=C1 Chemical compound COC1=C(C)C=CC(OCCN2CCOCC2)=C1 USJNNFPVGWDRND-UHFFFAOYSA-N 0.000 description 4
- DQBFSAOZWSXGPM-UHFFFAOYSA-N COC1=CC(N2CCC(C(N)=O)CC2)=CC=C1C Chemical compound COC1=CC(N2CCC(C(N)=O)CC2)=CC=C1C DQBFSAOZWSXGPM-UHFFFAOYSA-N 0.000 description 4
- HMZHOMYDLCHOBQ-UHFFFAOYSA-N COC1=CC(N2CCNCC2)=CC=C1C Chemical compound COC1=CC(N2CCNCC2)=CC=C1C HMZHOMYDLCHOBQ-UHFFFAOYSA-N 0.000 description 4
- WKUFGZSXYMXLPA-UHFFFAOYSA-N CC1=C(C)C=C(N2CCOCC2)C=C1 Chemical compound CC1=C(C)C=C(N2CCOCC2)C=C1 WKUFGZSXYMXLPA-UHFFFAOYSA-N 0.000 description 3
- PZOUSPYUWWUPPK-UHFFFAOYSA-N CC1=C2C=CNC2=CC=C1 Chemical compound CC1=C2C=CNC2=CC=C1 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 3
- MXLDKRRQOFSNOX-UHFFFAOYSA-N CC1=CC=CC2=C1C=CN2CCCN1CCOCC1 Chemical compound CC1=CC=CC2=C1C=CN2CCCN1CCOCC1 MXLDKRRQOFSNOX-UHFFFAOYSA-N 0.000 description 3
- RVNZVWJCTGZHIF-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(C(=O)N4CCC(N5CCCCC5)CC4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(C(=O)N4CCC(N5CCCCC5)CC4)C=C3OC)=N2)C=CC=C1 RVNZVWJCTGZHIF-UHFFFAOYSA-N 0.000 description 3
- STHWCLWDEICBOH-UHFFFAOYSA-N COC1=C(C)C=C(N2CCC(N(C)C)C2)C=C1 Chemical compound COC1=C(C)C=C(N2CCC(N(C)C)C2)C=C1 STHWCLWDEICBOH-UHFFFAOYSA-N 0.000 description 3
- AJDZCWUSACTDFL-UHFFFAOYSA-N COC1=C(C)C=C(N2CCC(N3CCN(C)CC3)CC2)C=C1 Chemical compound COC1=C(C)C=C(N2CCC(N3CCN(C)CC3)CC2)C=C1 AJDZCWUSACTDFL-UHFFFAOYSA-N 0.000 description 3
- XKWBILZDOQVJBC-UHFFFAOYSA-N COC1=C(C)C=CC(OCCN2CCN(C)CC2)=C1 Chemical compound COC1=C(C)C=CC(OCCN2CCN(C)CC2)=C1 XKWBILZDOQVJBC-UHFFFAOYSA-N 0.000 description 3
- IIZDGGYJQYBIMK-UHFFFAOYSA-N COC1=CC(N2CCC(NC(C)=O)C2)=CC=C1C Chemical compound COC1=CC(N2CCC(NC(C)=O)C2)=CC=C1C IIZDGGYJQYBIMK-UHFFFAOYSA-N 0.000 description 3
- KDORZHCDYBEDTG-CQSZACIVSA-N [H][C@@]12COCCN1CCN(C1=CC=C(C)C(OC)=C1)C2 Chemical compound [H][C@@]12COCCN1CCN(C1=CC=C(C)C(OC)=C1)C2 KDORZHCDYBEDTG-CQSZACIVSA-N 0.000 description 3
- MAZHGVFUZMIQBL-UHFFFAOYSA-N CC(C)OC1=C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)C=CC(N2CCC(N3CCCC3=O)CC2)=C1 Chemical compound CC(C)OC1=C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)C=CC(N2CCC(N3CCCC3=O)CC2)=C1 MAZHGVFUZMIQBL-UHFFFAOYSA-N 0.000 description 2
- WFGGUGXMJJKKGM-UHFFFAOYSA-N CC(C)OC1=C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)C=CC(N2CCN(CCN(C)C)CC2)=C1 Chemical compound CC(C)OC1=C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)C=CC(N2CCN(CCN(C)C)CC2)=C1 WFGGUGXMJJKKGM-UHFFFAOYSA-N 0.000 description 2
- CSARJIQZOSVYHA-UHFFFAOYSA-N CC1=C(Cl)C=C(F)C=C1 Chemical compound CC1=C(Cl)C=C(F)C=C1 CSARJIQZOSVYHA-UHFFFAOYSA-N 0.000 description 2
- NVOXXNCQSYTUMU-UHFFFAOYSA-N CC1=C(F)C=C(OCCN2CCOCC2)C=C1 Chemical compound CC1=C(F)C=C(OCCN2CCOCC2)C=C1 NVOXXNCQSYTUMU-UHFFFAOYSA-N 0.000 description 2
- SNDMSKBSLVZQAR-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 SNDMSKBSLVZQAR-UHFFFAOYSA-N 0.000 description 2
- GWHJZXXIDMPWGX-UHFFFAOYSA-N CC1=CC(C)=C(C)C=C1 Chemical compound CC1=CC(C)=C(C)C=C1 GWHJZXXIDMPWGX-UHFFFAOYSA-N 0.000 description 2
- DOXALDISRDZGNV-UHFFFAOYSA-N CC1=CC=CC2=C1C(=O)CC2 Chemical compound CC1=CC=CC2=C1C(=O)CC2 DOXALDISRDZGNV-UHFFFAOYSA-N 0.000 description 2
- PPSGCZYRZSGAKE-SFHVURJKSA-N CC1=NC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 PPSGCZYRZSGAKE-SFHVURJKSA-N 0.000 description 2
- JUMVWNXYBLBYSL-UHFFFAOYSA-N CCCNC1=CC(OCCC)=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=C1 Chemical compound CCCNC1=CC(OCCC)=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=C1 JUMVWNXYBLBYSL-UHFFFAOYSA-N 0.000 description 2
- MBGIICQBIQMKAA-UHFFFAOYSA-N CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCCCC2)=C1 Chemical compound CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCCCC2)=C1 MBGIICQBIQMKAA-UHFFFAOYSA-N 0.000 description 2
- PTFGJOQQWACJOI-UHFFFAOYSA-N CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCOCC2)=C1 PTFGJOQQWACJOI-UHFFFAOYSA-N 0.000 description 2
- LTGMWNGHBPKESO-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCCC4)C=C3OCC)=N2)C=CC=C1 LTGMWNGHBPKESO-UHFFFAOYSA-N 0.000 description 2
- HOHWNGBOWQEEHQ-UHFFFAOYSA-N CCCS(=O)(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(C(N)=O)CC3)C=C2C)=N1 Chemical compound CCCS(=O)(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(C(N)=O)CC3)C=C2C)=N1 HOHWNGBOWQEEHQ-UHFFFAOYSA-N 0.000 description 2
- RMAJTUMZUXFAAP-UHFFFAOYSA-N CCN(C)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)=C1 Chemical compound CCN(C)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)=C1 RMAJTUMZUXFAAP-UHFFFAOYSA-N 0.000 description 2
- ZYEHBTFVJWNMFX-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 ZYEHBTFVJWNMFX-UHFFFAOYSA-N 0.000 description 2
- YKIFNPUDZFQMNC-UHFFFAOYSA-N CCNC1CCN(C2=CC(OC)=C(C)C=C2)C1 Chemical compound CCNC1CCN(C2=CC(OC)=C(C)C=C2)C1 YKIFNPUDZFQMNC-UHFFFAOYSA-N 0.000 description 2
- LUQIIGFONLNOOL-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 LUQIIGFONLNOOL-UHFFFAOYSA-N 0.000 description 2
- SWOCXCXSALQXPU-NRFANRHFSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 SWOCXCXSALQXPU-NRFANRHFSA-N 0.000 description 2
- FQCROQUOIDKVCX-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 FQCROQUOIDKVCX-UHFFFAOYSA-N 0.000 description 2
- MMEVYMSHMYTLFT-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 MMEVYMSHMYTLFT-UHFFFAOYSA-N 0.000 description 2
- XWQYAYAURQFGHH-NRFANRHFSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 XWQYAYAURQFGHH-NRFANRHFSA-N 0.000 description 2
- MVWODPVZFLEDIN-BGERDNNASA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CC[C@H](N(C)C)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CC[C@H](N(C)C)C2)=C1 MVWODPVZFLEDIN-BGERDNNASA-N 0.000 description 2
- UVCAOWFTHZZKKA-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 UVCAOWFTHZZKKA-UHFFFAOYSA-N 0.000 description 2
- KEMUZIZZYWGHFQ-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 KEMUZIZZYWGHFQ-UHFFFAOYSA-N 0.000 description 2
- WWKYCJQJJIMFFZ-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 WWKYCJQJJIMFFZ-UHFFFAOYSA-N 0.000 description 2
- UCEYELFZFSJVFD-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 UCEYELFZFSJVFD-UHFFFAOYSA-N 0.000 description 2
- VYGDZXFAMXGDBM-UHFFFAOYSA-N CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 VYGDZXFAMXGDBM-UHFFFAOYSA-N 0.000 description 2
- ZYKBOAOPPMOANM-UHFFFAOYSA-N CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 ZYKBOAOPPMOANM-UHFFFAOYSA-N 0.000 description 2
- GVDMSADBJPPLJD-UHFFFAOYSA-N CCOC1=CC(N2CCN(CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCN(CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 GVDMSADBJPPLJD-UHFFFAOYSA-N 0.000 description 2
- YPFFSUYCJLJKNM-UHFFFAOYSA-N CCOC1=CC(N2CCN(CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 Chemical compound CCOC1=CC(N2CCN(CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 YPFFSUYCJLJKNM-UHFFFAOYSA-N 0.000 description 2
- MTDXJDAOWHZROR-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Br)C=N1 MTDXJDAOWHZROR-UHFFFAOYSA-N 0.000 description 2
- JSUWIRUNLOCPIF-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CC)C=CC=C2)=C(F)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CC)C=CC=C2)=C(F)C=N1 JSUWIRUNLOCPIF-UHFFFAOYSA-N 0.000 description 2
- ZNOYMAUISBRYOL-QGZVFWFLSA-N CCOC1=CC(N2CC[C@@H](NC)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@@H](NC)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 ZNOYMAUISBRYOL-QGZVFWFLSA-N 0.000 description 2
- VQRCAUGSNVHTRC-SFHVURJKSA-N CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 VQRCAUGSNVHTRC-SFHVURJKSA-N 0.000 description 2
- REMMUCNJDVZPGN-UHFFFAOYSA-N CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 REMMUCNJDVZPGN-UHFFFAOYSA-N 0.000 description 2
- IASQFFDCBHCNOD-LJQANCHMSA-N CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 IASQFFDCBHCNOD-LJQANCHMSA-N 0.000 description 2
- IASQFFDCBHCNOD-IBGZPJMESA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 IASQFFDCBHCNOD-IBGZPJMESA-N 0.000 description 2
- YVRUWEWBQBNQLG-SFHVURJKSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 YVRUWEWBQBNQLG-SFHVURJKSA-N 0.000 description 2
- JKNUAMWKSRVARZ-FQEVSTJZSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 JKNUAMWKSRVARZ-FQEVSTJZSA-N 0.000 description 2
- LZNKQRLLXOCSJT-UHFFFAOYSA-N CNC1=C(Cl)C=NC(NC2=CC=C(N3CCOCC3)C=C2OC)=N1 Chemical compound CNC1=C(Cl)C=NC(NC2=CC=C(N3CCOCC3)C=C2OC)=N1 LZNKQRLLXOCSJT-UHFFFAOYSA-N 0.000 description 2
- LKLQGAOBKWYJFL-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)CC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)CC(C)C)C=CC=C2)=C(Cl)C=N1 LKLQGAOBKWYJFL-UHFFFAOYSA-N 0.000 description 2
- XVDPOMKTJLMHIQ-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 XVDPOMKTJLMHIQ-UHFFFAOYSA-N 0.000 description 2
- JXXVFEWKGGSEHP-UHFFFAOYSA-N CNC1CCN(C2=CC(C)=C(OC)C=C2)C1 Chemical compound CNC1CCN(C2=CC(C)=C(OC)C=C2)C1 JXXVFEWKGGSEHP-UHFFFAOYSA-N 0.000 description 2
- OPPXVVYDGBULFD-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCC4)=CC=C3OC)=N2)C=CC=C1 OPPXVVYDGBULFD-UHFFFAOYSA-N 0.000 description 2
- SJXJUAGEVNRISQ-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCC(N5CCCCC5)CC4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCC(N5CCCCC5)CC4)C=C3OC)=N2)C=CC=C1 SJXJUAGEVNRISQ-UHFFFAOYSA-N 0.000 description 2
- ZFDPCOAAZFBTRF-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCN(C)CC4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCN(C)CC4)C=C3OC)=N2)C=CC=C1 ZFDPCOAAZFBTRF-UHFFFAOYSA-N 0.000 description 2
- LDXWTISJDMRVQR-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=NC(NC3=CC(OCCN4CCOCC4)=CC=C3OC)=NC=C2Br)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=NC(NC3=CC(OCCN4CCOCC4)=CC=C3OC)=NC=C2Br)C=CC=C1 LDXWTISJDMRVQR-UHFFFAOYSA-N 0.000 description 2
- NHIFURJSFQMRDX-UHFFFAOYSA-N CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=CC(C)=NN2C)=NC=C1Cl Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=CC(C)=NN2C)=NC=C1Cl NHIFURJSFQMRDX-UHFFFAOYSA-N 0.000 description 2
- SSABYSNJIDUESA-UHFFFAOYSA-N COC1=C(C)C=C(N2CCC(O)CC2)C=C1 Chemical compound COC1=C(C)C=C(N2CCC(O)CC2)C=C1 SSABYSNJIDUESA-UHFFFAOYSA-N 0.000 description 2
- ZAQZURDXIQYTML-UHFFFAOYSA-N COC1=C(C)C=C(OCCN2CCOCC2)C=C1 Chemical compound COC1=C(C)C=C(OCCN2CCOCC2)C=C1 ZAQZURDXIQYTML-UHFFFAOYSA-N 0.000 description 2
- MYOURQYSJHUELV-UHFFFAOYSA-N COC1=C(C)C=CC(CN2CCN(C)CC2)=C1 Chemical compound COC1=C(C)C=CC(CN2CCN(C)CC2)=C1 MYOURQYSJHUELV-UHFFFAOYSA-N 0.000 description 2
- KBXDMZZDPOXQBP-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(N3CCOCC3)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(N3CCOCC3)CC2)=C1 KBXDMZZDPOXQBP-UHFFFAOYSA-N 0.000 description 2
- JZXYOJZLXOENQL-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(OC)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(OC)CC2)=C1 JZXYOJZLXOENQL-UHFFFAOYSA-N 0.000 description 2
- YTGILVNWYHNEJI-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCN(C(C)C)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCN(C(C)C)CC2)=C1 YTGILVNWYHNEJI-UHFFFAOYSA-N 0.000 description 2
- GUANEGGXUMYWDF-UHFFFAOYSA-N COC1=C(C)C=CC(OC2CCN(C)CC2)=C1F Chemical compound COC1=C(C)C=CC(OC2CCN(C)CC2)=C1F GUANEGGXUMYWDF-UHFFFAOYSA-N 0.000 description 2
- DJNGSZPCMSSVOT-UHFFFAOYSA-N COC1=C(C)C=CC(OCC2CCCN(C)C2)=C1 Chemical compound COC1=C(C)C=CC(OCC2CCCN(C)C2)=C1 DJNGSZPCMSSVOT-UHFFFAOYSA-N 0.000 description 2
- DDVXQMXTCWGCQZ-UHFFFAOYSA-N COC1=C(C)C=CC(OCC2CCN(C)CC2)=C1 Chemical compound COC1=C(C)C=CC(OCC2CCN(C)CC2)=C1 DDVXQMXTCWGCQZ-UHFFFAOYSA-N 0.000 description 2
- ZWTDWXDVSLFTTB-UHFFFAOYSA-N COC1=C(C)C=CC(OCCCN2CCOCC2)=C1 Chemical compound COC1=C(C)C=CC(OCCCN2CCOCC2)=C1 ZWTDWXDVSLFTTB-UHFFFAOYSA-N 0.000 description 2
- JYMBPJZOJBTNEB-UHFFFAOYSA-N COC1=C(F)C(N2CCOCC2)=CC=C1C Chemical compound COC1=C(F)C(N2CCOCC2)=CC=C1C JYMBPJZOJBTNEB-UHFFFAOYSA-N 0.000 description 2
- KXMILIKHAOQNQU-UHFFFAOYSA-N COC1=C(NC2=NC=C(Cl)C(NC3=C(S(=O)(=O)NC(C)C)C=CC=C3)=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound COC1=C(NC2=NC=C(Cl)C(NC3=C(S(=O)(=O)NC(C)C)C=CC=C3)=N2)C=CC(OC2CCN(C)C2)=C1 KXMILIKHAOQNQU-UHFFFAOYSA-N 0.000 description 2
- ZWMDEVPYVGQJHU-UHFFFAOYSA-N COC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 Chemical compound COC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 ZWMDEVPYVGQJHU-UHFFFAOYSA-N 0.000 description 2
- UJEUDJUGBOMEOQ-UHFFFAOYSA-N COC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C3C(=O)N(C)CC3=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C3C(=O)N(C)CC3=CC=C2)=C(Cl)C=N1 UJEUDJUGBOMEOQ-UHFFFAOYSA-N 0.000 description 2
- CEROJJHQGYIYQH-KRWDZBQOSA-N COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 CEROJJHQGYIYQH-KRWDZBQOSA-N 0.000 description 2
- JVXVCEFIYQQHJR-FQEVSTJZSA-N COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 JVXVCEFIYQQHJR-FQEVSTJZSA-N 0.000 description 2
- HADXGOYZAMVEGY-UHFFFAOYSA-N COC1=CC=C(N(C)C)C=C1[N+](=O)[O-] Chemical compound COC1=CC=C(N(C)C)C=C1[N+](=O)[O-] HADXGOYZAMVEGY-UHFFFAOYSA-N 0.000 description 2
- KRFLXEYSMNRRAI-UHFFFAOYSA-N COCCOC1=CC=C(OC)C=C1N Chemical compound COCCOC1=CC=C(OC)C=C1N KRFLXEYSMNRRAI-UHFFFAOYSA-N 0.000 description 2
- RSPMENKJHMCJSO-UHFFFAOYSA-N Cc(c(OC)c1)ccc1N(CC1)CCC1NC Chemical compound Cc(c(OC)c1)ccc1N(CC1)CCC1NC RSPMENKJHMCJSO-UHFFFAOYSA-N 0.000 description 2
- UHHZGSLXPQGPJL-UHFFFAOYSA-N NC1=C2OCCC2=CC=C1 Chemical compound NC1=C2OCCC2=CC=C1 UHHZGSLXPQGPJL-UHFFFAOYSA-N 0.000 description 2
- UZMXDPPOKPYMPR-UHFFFAOYSA-N [H]C(F)(F)OC1=C(C)C=CC=C1 Chemical compound [H]C(F)(F)OC1=C(C)C=CC=C1 UZMXDPPOKPYMPR-UHFFFAOYSA-N 0.000 description 2
- PZTUXPLCHMPLOE-UHFFFAOYSA-N [H]N(CC)C(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCN(C)CC4)C=C3OC)=N2)C=CC=C1 Chemical compound [H]N(CC)C(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCN(C)CC4)C=C3OC)=N2)C=CC=C1 PZTUXPLCHMPLOE-UHFFFAOYSA-N 0.000 description 2
- NNPPMTNAJDCUHE-UHFFFAOYSA-N CC(C)C Chemical compound CC(C)C NNPPMTNAJDCUHE-UHFFFAOYSA-N 0.000 description 1
- XARPGHCEBSQZHU-UHFFFAOYSA-N CC(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CC(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 XARPGHCEBSQZHU-UHFFFAOYSA-N 0.000 description 1
- XVFJHYDFNUTKFM-UHFFFAOYSA-N CC(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CC(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 XVFJHYDFNUTKFM-UHFFFAOYSA-N 0.000 description 1
- FEJHHJBEUKMMLL-UHFFFAOYSA-N CC(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)C)C=C(N4CCC(N5CCOCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CC(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)C)C=C(N4CCC(N5CCOCC5)CC4)C=C3)=N2)C=CC=C1 FEJHHJBEUKMMLL-UHFFFAOYSA-N 0.000 description 1
- CUQUXMNGFJAYBN-UHFFFAOYSA-N CC(C)CNS(c(cccc1)c1Nc(nc(Nc(ccc(N1CCOCC1)c1)c1OC)nc1)c1Br)(=O)=O Chemical compound CC(C)CNS(c(cccc1)c1Nc(nc(Nc(ccc(N1CCOCC1)c1)c1OC)nc1)c1Br)(=O)=O CUQUXMNGFJAYBN-UHFFFAOYSA-N 0.000 description 1
- GULQUZWANGQYLW-UHFFFAOYSA-N CC(C)CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 Chemical compound CC(C)CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 GULQUZWANGQYLW-UHFFFAOYSA-N 0.000 description 1
- VOYUZPQCJSWDNC-QHCPKHFHSA-N CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 Chemical compound CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 VOYUZPQCJSWDNC-QHCPKHFHSA-N 0.000 description 1
- NFAIYMDVSUZAKY-UHFFFAOYSA-N CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(NCCCN(C)C)C=C3)=N2)C=CC=C1 Chemical compound CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(NCCCN(C)C)C=C3)=N2)C=CC=C1 NFAIYMDVSUZAKY-UHFFFAOYSA-N 0.000 description 1
- BTGBUNQQKMKQPG-UHFFFAOYSA-N CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(OC4CCN(C)C4)C=C3)=N2)C=CC=C1 Chemical compound CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(OC4CCN(C)C4)C=C3)=N2)C=CC=C1 BTGBUNQQKMKQPG-UHFFFAOYSA-N 0.000 description 1
- DLNMMDNSXZEUGB-UHFFFAOYSA-N CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 Chemical compound CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 DLNMMDNSXZEUGB-UHFFFAOYSA-N 0.000 description 1
- BTGBUNQQKMKQPG-JOCHJYFZSA-N CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(O[C@@H]4CCN(C)C4)C=C3)=N2)C=CC=C1 Chemical compound CC(C)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(O[C@@H]4CCN(C)C4)C=C3)=N2)C=CC=C1 BTGBUNQQKMKQPG-JOCHJYFZSA-N 0.000 description 1
- RCGKLPKGBXKKJK-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 RCGKLPKGBXKKJK-UHFFFAOYSA-N 0.000 description 1
- DHJUPHFMLOXFRR-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 DHJUPHFMLOXFRR-UHFFFAOYSA-N 0.000 description 1
- PPBKWNOGTAKLLS-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 PPBKWNOGTAKLLS-UHFFFAOYSA-N 0.000 description 1
- MGGMEXBIZBYPJG-QHCPKHFHSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 MGGMEXBIZBYPJG-QHCPKHFHSA-N 0.000 description 1
- PXGQBJWGGDNODZ-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 PXGQBJWGGDNODZ-UHFFFAOYSA-N 0.000 description 1
- RHLLGVPFCLPRMT-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 RHLLGVPFCLPRMT-UHFFFAOYSA-N 0.000 description 1
- PXGQBJWGGDNODZ-JOCHJYFZSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 PXGQBJWGGDNODZ-JOCHJYFZSA-N 0.000 description 1
- FCEVVZVMJAECLN-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 FCEVVZVMJAECLN-UHFFFAOYSA-N 0.000 description 1
- CCZFVJZRRUFCEW-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 CCZFVJZRRUFCEW-UHFFFAOYSA-N 0.000 description 1
- IUCCELVOFRXUFN-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 IUCCELVOFRXUFN-UHFFFAOYSA-N 0.000 description 1
- DDZBQANNFLGQQD-QFIPXVFZSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 DDZBQANNFLGQQD-QFIPXVFZSA-N 0.000 description 1
- DEDDDEALRUSXOQ-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 DEDDDEALRUSXOQ-UHFFFAOYSA-N 0.000 description 1
- MWDLYBYMLVEYNT-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 MWDLYBYMLVEYNT-UHFFFAOYSA-N 0.000 description 1
- DEDDDEALRUSXOQ-OAQYLSRUSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 DEDDDEALRUSXOQ-OAQYLSRUSA-N 0.000 description 1
- YLZSODVFACQTBW-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 YLZSODVFACQTBW-UHFFFAOYSA-N 0.000 description 1
- QXLGPZQJKAEGRL-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 QXLGPZQJKAEGRL-UHFFFAOYSA-N 0.000 description 1
- FQFYNVSAGRZFSN-DEOSSOPVSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 FQFYNVSAGRZFSN-DEOSSOPVSA-N 0.000 description 1
- CHRVVLDUAMEWJD-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 CHRVVLDUAMEWJD-UHFFFAOYSA-N 0.000 description 1
- DFSUYAZLCHMAEZ-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 DFSUYAZLCHMAEZ-UHFFFAOYSA-N 0.000 description 1
- CHRVVLDUAMEWJD-HSZRJFAPSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 CHRVVLDUAMEWJD-HSZRJFAPSA-N 0.000 description 1
- FFPKBJBNUWMROA-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 FFPKBJBNUWMROA-UHFFFAOYSA-N 0.000 description 1
- JXHIIZFMEGWKGK-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 JXHIIZFMEGWKGK-UHFFFAOYSA-N 0.000 description 1
- QOQMKUHXZYINIQ-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 QOQMKUHXZYINIQ-UHFFFAOYSA-N 0.000 description 1
- JPWCJZISWADYIZ-DEOSSOPVSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 JPWCJZISWADYIZ-DEOSSOPVSA-N 0.000 description 1
- UIAGATFJIGLDTL-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 UIAGATFJIGLDTL-UHFFFAOYSA-N 0.000 description 1
- KLKKBDVUERHRLT-UHFFFAOYSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 KLKKBDVUERHRLT-UHFFFAOYSA-N 0.000 description 1
- UIAGATFJIGLDTL-HSZRJFAPSA-N CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 Chemical compound CC(C)OC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(O[C@@H]2CCN(C)C2)=C1 UIAGATFJIGLDTL-HSZRJFAPSA-N 0.000 description 1
- MFROSDHIJVOTKH-UHFFFAOYSA-N CC(C)OC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 Chemical compound CC(C)OC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 MFROSDHIJVOTKH-UHFFFAOYSA-N 0.000 description 1
- HLKUMYOLIRDYPH-UHFFFAOYSA-N CC(C)OC1=C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)C=CC(N2CCC3(CC2)OCCO3)=C1 Chemical compound CC(C)OC1=C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)C=CC(N2CCC3(CC2)OCCO3)=C1 HLKUMYOLIRDYPH-UHFFFAOYSA-N 0.000 description 1
- XTNZLWZJMFGFDC-UHFFFAOYSA-N CC(C)OC1=CC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 XTNZLWZJMFGFDC-UHFFFAOYSA-N 0.000 description 1
- GTVDJRZLNOANRW-UHFFFAOYSA-N CC(C)OC1=CC(N2CCC(N3CCCC3=O)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCC(N3CCCC3=O)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 GTVDJRZLNOANRW-UHFFFAOYSA-N 0.000 description 1
- ILCOZGZVOJQZAG-UHFFFAOYSA-N CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(C(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(C(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 ILCOZGZVOJQZAG-UHFFFAOYSA-N 0.000 description 1
- BIPABLMPFDAJOW-UHFFFAOYSA-N CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 BIPABLMPFDAJOW-UHFFFAOYSA-N 0.000 description 1
- ATAYJGHGXZOVGP-UHFFFAOYSA-N CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 ATAYJGHGXZOVGP-UHFFFAOYSA-N 0.000 description 1
- NDJLYXPRMAWPTK-UHFFFAOYSA-N CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCC(N3CCN(C)CC3)CC2)=CC=C1CC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 NDJLYXPRMAWPTK-UHFFFAOYSA-N 0.000 description 1
- BYAJTBDTZBNORX-UHFFFAOYSA-N CC(C)OC1=CC(N2CCCC(C(N)=O)C2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCCC(C(N)=O)C2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 BYAJTBDTZBNORX-UHFFFAOYSA-N 0.000 description 1
- OTTLYYDHCPWJGJ-UHFFFAOYSA-N CC(C)OC1=CC(N2CCCC(C(N)=O)C2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCCC(C(N)=O)C2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 OTTLYYDHCPWJGJ-UHFFFAOYSA-N 0.000 description 1
- LFWLFXYQGHXOEZ-UHFFFAOYSA-N CC(C)OC1=CC(N2CCN(CCN(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC(C)OC1=CC(N2CCN(CCN(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 LFWLFXYQGHXOEZ-UHFFFAOYSA-N 0.000 description 1
- AOPMOFVHLVVNCS-UHFFFAOYSA-N CC(C)OC1=NC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC(C)OC1=NC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 AOPMOFVHLVVNCS-UHFFFAOYSA-N 0.000 description 1
- GBCUOXFYZCFJBP-UHFFFAOYSA-N CC(C)Oc(cc(cc1)N(CC2)CCC2C(N)=O)c1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Cl Chemical compound CC(C)Oc(cc(cc1)N(CC2)CCC2C(N)=O)c1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Cl GBCUOXFYZCFJBP-UHFFFAOYSA-N 0.000 description 1
- MHZJXPHCDVKBBS-FQEVSTJZSA-N CC(C)Oc(cc(cc1)N(CC2)C[C@H]2N(C)C)c1Nc(nc1Nc2ccccc2S(N(C)C)(=O)=O)ncc1Cl Chemical compound CC(C)Oc(cc(cc1)N(CC2)C[C@H]2N(C)C)c1Nc(nc1Nc2ccccc2S(N(C)C)(=O)=O)ncc1Cl MHZJXPHCDVKBBS-FQEVSTJZSA-N 0.000 description 1
- IMTAIWOVJVFZIX-IBGZPJMESA-N CC(C)Oc(cc(cc1)N(CC2)C[C@H]2N(C)C)c1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Br Chemical compound CC(C)Oc(cc(cc1)N(CC2)C[C@H]2N(C)C)c1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Br IMTAIWOVJVFZIX-IBGZPJMESA-N 0.000 description 1
- CTPFJUDLHHSMBQ-LJQANCHMSA-N CC(C)Oc(cc(cc1)O[C@H]2CN(C)CC2)c1Nc(nc1)nc(Nc2ccccc2S(N(C)C)(=O)=O)c1Cl Chemical compound CC(C)Oc(cc(cc1)O[C@H]2CN(C)CC2)c1Nc(nc1)nc(Nc2ccccc2S(N(C)C)(=O)=O)c1Cl CTPFJUDLHHSMBQ-LJQANCHMSA-N 0.000 description 1
- HBDUCHKWWJTXRF-UHFFFAOYSA-N CC(C)Oc1cc(OC2CCN(C)CC2)ccc1Nc(nc1Nc2ccccc2S(NC2CCC2)(=O)=O)ncc1Cl Chemical compound CC(C)Oc1cc(OC2CCN(C)CC2)ccc1Nc(nc1Nc2ccccc2S(NC2CCC2)(=O)=O)ncc1Cl HBDUCHKWWJTXRF-UHFFFAOYSA-N 0.000 description 1
- BUNIJWIIEFFXPA-UHFFFAOYSA-N CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3C(F)(F)F)=N2)C=CC=C1 Chemical compound CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3C(F)(F)F)=N2)C=CC=C1 BUNIJWIIEFFXPA-UHFFFAOYSA-N 0.000 description 1
- OMWBYARJNOGYLG-UHFFFAOYSA-N CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC(F)(F)F)=N2)C=CC=C1 Chemical compound CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC(F)(F)F)=N2)C=CC=C1 OMWBYARJNOGYLG-UHFFFAOYSA-N 0.000 description 1
- ICZJJGCRJCFHJW-DEOSSOPVSA-N CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C#N)=N2)C=CC=C1 Chemical compound CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C#N)=N2)C=CC=C1 ICZJJGCRJCFHJW-DEOSSOPVSA-N 0.000 description 1
- XEXBRDUIDMIFGA-QFIPXVFZSA-N CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C(F)(F)F)=N2)C=CC=C1 Chemical compound CC(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C(F)(F)F)=N2)C=CC=C1 XEXBRDUIDMIFGA-QFIPXVFZSA-N 0.000 description 1
- HGMUDMYTTMFRPV-UHFFFAOYSA-N CC(C)S(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 Chemical compound CC(C)S(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 HGMUDMYTTMFRPV-UHFFFAOYSA-N 0.000 description 1
- SHBXCQHHTQLACL-UHFFFAOYSA-N CC(C)S(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCOCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 Chemical compound CC(C)S(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCOCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 SHBXCQHHTQLACL-UHFFFAOYSA-N 0.000 description 1
- KROVSJZXBOZJJJ-CUQPBNISSA-N CC(C)S(c(cccc1)c1Nc1nc(Nc(c(OC)c2)ccc2O[C@H]2CN(C)CC2)ncc1Cl)(=O)=[ClH] Chemical compound CC(C)S(c(cccc1)c1Nc1nc(Nc(c(OC)c2)ccc2O[C@H]2CN(C)CC2)ncc1Cl)(=O)=[ClH] KROVSJZXBOZJJJ-CUQPBNISSA-N 0.000 description 1
- PVXZKFCIINNQFU-UHFFFAOYSA-N CC(Oc(cc1S(O)=O)ccc1OC)=O Chemical compound CC(Oc(cc1S(O)=O)ccc1OC)=O PVXZKFCIINNQFU-UHFFFAOYSA-N 0.000 description 1
- PEQWRCVMFAWSGO-UHFFFAOYSA-N CC.CC.CCC1=NC(NC2=CC=CC=C2)=CC=N1 Chemical compound CC.CC.CCC1=NC(NC2=CC=CC=C2)=CC=N1 PEQWRCVMFAWSGO-UHFFFAOYSA-N 0.000 description 1
- GTGXDFSCNTVGNZ-UHFFFAOYSA-N CC1=C(C)C=C(C(=O)N2CCNCC2)C=C1 Chemical compound CC1=C(C)C=C(C(=O)N2CCNCC2)C=C1 GTGXDFSCNTVGNZ-UHFFFAOYSA-N 0.000 description 1
- IPHMFPONENJIJB-UHFFFAOYSA-N CC1=C(C)C=C(C(=O)N2CCOCC2)C=C1 Chemical compound CC1=C(C)C=C(C(=O)N2CCOCC2)C=C1 IPHMFPONENJIJB-UHFFFAOYSA-N 0.000 description 1
- CRVWBTCVHJBKKG-UHFFFAOYSA-N CC1=C(C)C=C(C(=O)NC2CCCCC2)C=C1 Chemical compound CC1=C(C)C=C(C(=O)NC2CCCCC2)C=C1 CRVWBTCVHJBKKG-UHFFFAOYSA-N 0.000 description 1
- MGMSKQZIAGFMRU-UHFFFAOYSA-N CC1=C(C)C=C(C(C)C)C=C1 Chemical compound CC1=C(C)C=C(C(C)C)C=C1 MGMSKQZIAGFMRU-UHFFFAOYSA-N 0.000 description 1
- YLOYUTXRSFXMIT-UHFFFAOYSA-N CC1=C(C)C=C(N2CCCCC2)C=C1 Chemical compound CC1=C(C)C=C(N2CCCCC2)C=C1 YLOYUTXRSFXMIT-UHFFFAOYSA-N 0.000 description 1
- DUVKEQFLSFTVFO-UHFFFAOYSA-N CC1=C(C)C=C(OCCCN2CCOCC2)C=C1 Chemical compound CC1=C(C)C=C(OCCCN2CCOCC2)C=C1 DUVKEQFLSFTVFO-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N CC1=C(C)C=CC=C1 Chemical compound CC1=C(C)C=CC=C1 CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- NIOGDCDTKPQEAT-UHFFFAOYSA-N CC1=C(Cl)C=CC(F)=C1 Chemical compound CC1=C(Cl)C=CC(F)=C1 NIOGDCDTKPQEAT-UHFFFAOYSA-N 0.000 description 1
- MPXDAIBTYWGBSL-UHFFFAOYSA-N CC1=C(F)C=C(F)C=C1 Chemical compound CC1=C(F)C=C(F)C=C1 MPXDAIBTYWGBSL-UHFFFAOYSA-N 0.000 description 1
- OPBPXBXEYVEKEH-UHFFFAOYSA-N CC1=C(F)C=C(N2CCOCC2)C=C1 Chemical compound CC1=C(F)C=C(N2CCOCC2)C=C1 OPBPXBXEYVEKEH-UHFFFAOYSA-N 0.000 description 1
- WXRCBVHACYPTBU-UHFFFAOYSA-N CC1=C(F)C=C(OC2CCN(C)CC2)C=C1 Chemical compound CC1=C(F)C=C(OC2CCN(C)CC2)C=C1 WXRCBVHACYPTBU-UHFFFAOYSA-N 0.000 description 1
- YSNVKDGEALPJGC-UHFFFAOYSA-N CC1=C(F)C=CC(F)=C1 Chemical compound CC1=C(F)C=CC(F)=C1 YSNVKDGEALPJGC-UHFFFAOYSA-N 0.000 description 1
- QYPPUFQYPQBDPR-UHFFFAOYSA-N CC1=C(N(C)C)C=CC=C1N Chemical compound CC1=C(N(C)C)C=CC=C1N QYPPUFQYPQBDPR-UHFFFAOYSA-N 0.000 description 1
- KPUSVKGMJNXHTC-UHFFFAOYSA-N CC1=C(N(C)C)C=CC=C1[N+](=O)[O-] Chemical compound CC1=C(N(C)C)C=CC=C1[N+](=O)[O-] KPUSVKGMJNXHTC-UHFFFAOYSA-N 0.000 description 1
- LIDMYQRDZLWVQQ-UHFFFAOYSA-N CC1=C(N)C=C(C(=O)N2CCN(C(=O)OC(C)(C)C)CC2)C=C1 Chemical compound CC1=C(N)C=C(C(=O)N2CCN(C(=O)OC(C)(C)C)CC2)C=C1 LIDMYQRDZLWVQQ-UHFFFAOYSA-N 0.000 description 1
- IUAGWHGZKMGPOR-UHFFFAOYSA-N CC1=C(N)C=C(N2CCOCC2)C=C1 Chemical compound CC1=C(N)C=C(N2CCOCC2)C=C1 IUAGWHGZKMGPOR-UHFFFAOYSA-N 0.000 description 1
- SPAAJONMYHHEBD-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCCCN(C)C)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCCCN(C)C)=C1 SPAAJONMYHHEBD-UHFFFAOYSA-N 0.000 description 1
- CVJKUYYSUCIEKW-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 CVJKUYYSUCIEKW-UHFFFAOYSA-N 0.000 description 1
- LQNGGMUBFOVRJT-JOCHJYFZSA-N CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@@H](N(C)C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@@H](N(C)C)C2)=C1 LQNGGMUBFOVRJT-JOCHJYFZSA-N 0.000 description 1
- LQNGGMUBFOVRJT-QFIPXVFZSA-N CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 LQNGGMUBFOVRJT-QFIPXVFZSA-N 0.000 description 1
- SVDWXKLXSGRTBZ-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 SVDWXKLXSGRTBZ-UHFFFAOYSA-N 0.000 description 1
- VKQHGVXBOCQLDE-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(CCCCN(C)C)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(CCCCN(C)C)=C1 VKQHGVXBOCQLDE-UHFFFAOYSA-N 0.000 description 1
- NCWGRJYORQKDJL-RUZDIDTESA-N CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@@H](N(C)C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@@H](N(C)C)C2)=C1 NCWGRJYORQKDJL-RUZDIDTESA-N 0.000 description 1
- NCWGRJYORQKDJL-VWLOTQADSA-N CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 NCWGRJYORQKDJL-VWLOTQADSA-N 0.000 description 1
- FHSNGKISRZGIQO-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 FHSNGKISRZGIQO-UHFFFAOYSA-N 0.000 description 1
- CHTMFPOXBGKOBJ-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 CHTMFPOXBGKOBJ-UHFFFAOYSA-N 0.000 description 1
- DMDLGVDPNPMCCC-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C(C)C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C(C)C)C2)=C1 DMDLGVDPNPMCCC-UHFFFAOYSA-N 0.000 description 1
- XDHGRUQJMLTYGP-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 XDHGRUQJMLTYGP-UHFFFAOYSA-N 0.000 description 1
- FLQPQVOTNMWQJD-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCCCN(C)C)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCCCN(C)C)=C1 FLQPQVOTNMWQJD-UHFFFAOYSA-N 0.000 description 1
- GELKUHSVWUSFGV-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 GELKUHSVWUSFGV-UHFFFAOYSA-N 0.000 description 1
- GELCVSAOOAPRSL-QFIPXVFZSA-N CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CC[C@H](N(C)C)C2)=C1 GELCVSAOOAPRSL-QFIPXVFZSA-N 0.000 description 1
- SVJWPXDRTHJVGT-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CNC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 SVJWPXDRTHJVGT-UHFFFAOYSA-N 0.000 description 1
- KWFNGYXFHMESPO-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Br)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Br)C=N2)C=CC(OC2CCN(C)CC2)=C1 KWFNGYXFHMESPO-UHFFFAOYSA-N 0.000 description 1
- FOGBVHLQESILGE-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 FOGBVHLQESILGE-UHFFFAOYSA-N 0.000 description 1
- DVUCJMUMNASTNP-UHFFFAOYSA-N CC1=C(NC2=NC(NC3=C(CNCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CC1=C(NC2=NC(NC3=C(CNCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 DVUCJMUMNASTNP-UHFFFAOYSA-N 0.000 description 1
- YFKPBFKOUVIQTN-UHFFFAOYSA-N CC1=C(OC(F)(F)F)C=CC=C1 Chemical compound CC1=C(OC(F)(F)F)C=CC=C1 YFKPBFKOUVIQTN-UHFFFAOYSA-N 0.000 description 1
- UJHQTIAOYSYPHZ-UHFFFAOYSA-N CC1=C(S(=O)(=O)C2=CC=CC=C2)C=CC=C1 Chemical compound CC1=C(S(=O)(=O)C2=CC=CC=C2)C=CC=C1 UJHQTIAOYSYPHZ-UHFFFAOYSA-N 0.000 description 1
- NVBDXRAGYUMMCC-UHFFFAOYSA-N CC1=C(S(=O)(=O)C2CCCCC2)C=CC=C1 Chemical compound CC1=C(S(=O)(=O)C2CCCCC2)C=CC=C1 NVBDXRAGYUMMCC-UHFFFAOYSA-N 0.000 description 1
- BJYFWJNJRKCIDJ-UHFFFAOYSA-N CC1=C(S(=O)(=O)CC2=CC=CC=C2)C=CC=C1 Chemical compound CC1=C(S(=O)(=O)CC2=CC=CC=C2)C=CC=C1 BJYFWJNJRKCIDJ-UHFFFAOYSA-N 0.000 description 1
- HJULVGRSYIMFRZ-UHFFFAOYSA-N CC1=C(S(=O)(=O)CCC(C)C)C=CC=C1 Chemical compound CC1=C(S(=O)(=O)CCC(C)C)C=CC=C1 HJULVGRSYIMFRZ-UHFFFAOYSA-N 0.000 description 1
- YQDRMSMOVHLZDG-UHFFFAOYSA-N CC1=C(S(=O)(=O)N2CCOCC2)C=CC=C1 Chemical compound CC1=C(S(=O)(=O)N2CCOCC2)C=CC=C1 YQDRMSMOVHLZDG-UHFFFAOYSA-N 0.000 description 1
- LNNSODHYZXCEJP-UHFFFAOYSA-N CC1=C2CCCC2=CC=C1 Chemical compound CC1=C2CCCC2=CC=C1 LNNSODHYZXCEJP-UHFFFAOYSA-N 0.000 description 1
- YXOVIGZJPGLNGM-UHFFFAOYSA-N CC1=C2CCCCC2=CC=C1 Chemical compound CC1=C2CCCCC2=CC=C1 YXOVIGZJPGLNGM-UHFFFAOYSA-N 0.000 description 1
- QPPDZXVWPHKPKT-UHFFFAOYSA-N CC1=C2CN=CC2=CC=C1 Chemical compound CC1=C2CN=CC2=CC=C1 QPPDZXVWPHKPKT-UHFFFAOYSA-N 0.000 description 1
- XQGXAMYQIOWWOV-UHFFFAOYSA-N CC1=C2OC(F)(F)OC2=CC=C1 Chemical compound CC1=C2OC(F)(F)OC2=CC=C1 XQGXAMYQIOWWOV-UHFFFAOYSA-N 0.000 description 1
- KVZJDSFEPWQXTF-UHFFFAOYSA-N CC1=C2OCCC2=CC=C1 Chemical compound CC1=C2OCCC2=CC=C1 KVZJDSFEPWQXTF-UHFFFAOYSA-N 0.000 description 1
- BFIMMTCNYPIMRN-UHFFFAOYSA-N CC1=CC(C)=C(C)C(C)=C1 Chemical compound CC1=CC(C)=C(C)C(C)=C1 BFIMMTCNYPIMRN-UHFFFAOYSA-N 0.000 description 1
- WJAVYWPXOXAOBS-UHFFFAOYSA-N CC1=CC(F)=C(C)C=C1 Chemical compound CC1=CC(F)=C(C)C=C1 WJAVYWPXOXAOBS-UHFFFAOYSA-N 0.000 description 1
- MHWFYZQXHZKMJO-UHFFFAOYSA-N CC1=CC(N2CCC(N3CCCCC3)CC2)=NC=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)N(C)C)=N1 Chemical compound CC1=CC(N2CCC(N3CCCCC3)CC2)=NC=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)N(C)C)=N1 MHWFYZQXHZKMJO-UHFFFAOYSA-N 0.000 description 1
- IOOYJZRDODJMJC-UHFFFAOYSA-N CC1=CC(N2CCOCC2)=NC=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)N(C)C)=N1 Chemical compound CC1=CC(N2CCOCC2)=NC=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)N(C)C)=N1 IOOYJZRDODJMJC-UHFFFAOYSA-N 0.000 description 1
- BMLWGFRPPLURHG-UHFFFAOYSA-N CC1=CC=C(C2=CC(C)=C(C)C=C2)C=C1 Chemical compound CC1=CC=C(C2=CC(C)=C(C)C=C2)C=C1 BMLWGFRPPLURHG-UHFFFAOYSA-N 0.000 description 1
- BVZYMQZFHWEQDB-UHFFFAOYSA-N CC1=CC=C(C2=CC=NC=C2)C=C1C Chemical compound CC1=CC=C(C2=CC=NC=C2)C=C1C BVZYMQZFHWEQDB-UHFFFAOYSA-N 0.000 description 1
- OSCQHDWIGRRRNY-UHFFFAOYSA-N CC1=CC=C(C2=CC=NC=C2)C=C1N Chemical compound CC1=CC=C(C2=CC=NC=C2)C=C1N OSCQHDWIGRRRNY-UHFFFAOYSA-N 0.000 description 1
- RBQAQISFFINFTM-UHFFFAOYSA-N CC1=CC=C(C2=CC=NC=C2)C=C1[N+](=O)[O-] Chemical compound CC1=CC=C(C2=CC=NC=C2)C=C1[N+](=O)[O-] RBQAQISFFINFTM-UHFFFAOYSA-N 0.000 description 1
- QLJPQQBLMHJENH-UHFFFAOYSA-N CC1=CC=C(N2CCOCC2)C2=C1C=CN2C Chemical compound CC1=CC=C(N2CCOCC2)C2=C1C=CN2C QLJPQQBLMHJENH-UHFFFAOYSA-N 0.000 description 1
- HYXIJVZYRWWFOO-UHFFFAOYSA-N CC1=CC=CC(N(C)C)=C1C Chemical compound CC1=CC=CC(N(C)C)=C1C HYXIJVZYRWWFOO-UHFFFAOYSA-N 0.000 description 1
- XOMZAAUDUJJMIA-UHFFFAOYSA-N CC1=CC=CC2=C1C=CN2CCCN1CCN(C)CC1 Chemical compound CC1=CC=CC2=C1C=CN2CCCN1CCN(C)CC1 XOMZAAUDUJJMIA-UHFFFAOYSA-N 0.000 description 1
- LVALCNRQGAWRFC-UHFFFAOYSA-N CC1=CC=CC=C1C(=O)N(C)C Chemical compound CC1=CC=CC=C1C(=O)N(C)C LVALCNRQGAWRFC-UHFFFAOYSA-N 0.000 description 1
- BYKLMXBCNKNGLB-UHFFFAOYSA-N CC1=CC=CC=C1C(=O)N1CCCC1 Chemical compound CC1=CC=CC=C1C(=O)N1CCCC1 BYKLMXBCNKNGLB-UHFFFAOYSA-N 0.000 description 1
- QEMAILGKGBKPKQ-UHFFFAOYSA-N CC1=CC=CC=C1C(=O)NC(C)C Chemical compound CC1=CC=CC=C1C(=O)NC(C)C QEMAILGKGBKPKQ-UHFFFAOYSA-N 0.000 description 1
- JTAYQEOIFHUQQF-UHFFFAOYSA-N CC1=NC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 JTAYQEOIFHUQQF-UHFFFAOYSA-N 0.000 description 1
- WWKFWYPTQWSEGQ-UHFFFAOYSA-N CC1=NC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CCC(N(C)C)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 WWKFWYPTQWSEGQ-UHFFFAOYSA-N 0.000 description 1
- ACUPMRPXNPVBHT-UHFFFAOYSA-N CC1=NC(N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 ACUPMRPXNPVBHT-UHFFFAOYSA-N 0.000 description 1
- CPEYCWZHMNEOLW-UHFFFAOYSA-N CC1=NC(N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 CPEYCWZHMNEOLW-UHFFFAOYSA-N 0.000 description 1
- BXVIPXFLPNRKAI-UHFFFAOYSA-N CC1=NC(N2CCOCC2)=CC=C1NC1=NC(NC2=CC=CC=C2C(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CCOCC2)=CC=C1NC1=NC(NC2=CC=CC=C2C(=O)N(C)C)=C(Cl)C=N1 BXVIPXFLPNRKAI-UHFFFAOYSA-N 0.000 description 1
- RCOPBJOQDVYTFN-UHFFFAOYSA-N CC1=NC(N2CCOCC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CCOCC2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 RCOPBJOQDVYTFN-UHFFFAOYSA-N 0.000 description 1
- PPSGCZYRZSGAKE-GOSISDBHSA-N CC1=NC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)C(C)C)=C(Cl)C=N1 PPSGCZYRZSGAKE-GOSISDBHSA-N 0.000 description 1
- KPPNAPXPRQMZQU-QGZVFWFLSA-N CC1=NC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 KPPNAPXPRQMZQU-QGZVFWFLSA-N 0.000 description 1
- KPPNAPXPRQMZQU-KRWDZBQOSA-N CC1=NC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 Chemical compound CC1=NC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=CC=CC=C2S(=O)(=O)N(C)C)=C(Cl)C=N1 KPPNAPXPRQMZQU-KRWDZBQOSA-N 0.000 description 1
- QHRPLLMCINHLBM-UHFFFAOYSA-N CC1=NN(C)C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)=C1 Chemical compound CC1=NN(C)C(NC2=NC=C(Cl)C(NC3=CC=CC=C3S(=O)(=O)C(C)C)=N2)=C1 QHRPLLMCINHLBM-UHFFFAOYSA-N 0.000 description 1
- VNXBKJFUJUWOCW-UHFFFAOYSA-N CC1CC1 Chemical compound CC1CC1 VNXBKJFUJUWOCW-UHFFFAOYSA-N 0.000 description 1
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N CCC Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 1
- ZSVWURPADHMOFA-UHFFFAOYSA-N CCC(=O)N1CCN(C2=CC(OC)=C(C)C=C2)CC1 Chemical compound CCC(=O)N1CCN(C2=CC(OC)=C(C)C=C2)CC1 ZSVWURPADHMOFA-UHFFFAOYSA-N 0.000 description 1
- NSGZGJKPTMTLAV-UHFFFAOYSA-N CCC(=O)N1CCN(C2=CC(OC)=C(N)C=C2)CC1 Chemical compound CCC(=O)N1CCN(C2=CC(OC)=C(N)C=C2)CC1 NSGZGJKPTMTLAV-UHFFFAOYSA-N 0.000 description 1
- XQADIHOCUQQAAN-UHFFFAOYSA-N CCC(=O)N1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 Chemical compound CCC(=O)N1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 XQADIHOCUQQAAN-UHFFFAOYSA-N 0.000 description 1
- WGECXQBGLLYSFP-UHFFFAOYSA-N CCC(C)C(C)C Chemical compound CCC(C)C(C)C WGECXQBGLLYSFP-UHFFFAOYSA-N 0.000 description 1
- PFEOZHBOMNWTJB-UHFFFAOYSA-N CCC(C)CC Chemical compound CCC(C)CC PFEOZHBOMNWTJB-UHFFFAOYSA-N 0.000 description 1
- OGTAAZDJDUUWDZ-UHFFFAOYSA-N CCC(C)CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CCC(C)CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 OGTAAZDJDUUWDZ-UHFFFAOYSA-N 0.000 description 1
- DUPUVYJQZSLSJB-UHFFFAOYSA-N CCC(CC)C(C)C Chemical compound CCC(CC)C(C)C DUPUVYJQZSLSJB-UHFFFAOYSA-N 0.000 description 1
- ZFDCEWKDOADJNM-UHFFFAOYSA-N CCC(CC)CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CCC(CC)CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 ZFDCEWKDOADJNM-UHFFFAOYSA-N 0.000 description 1
- LJRQSGSCHQMNFU-UHFFFAOYSA-N CCC(CC)NCC1=C(NC2=C(Cl)C=NC(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCC(CC)NCC1=C(NC2=C(Cl)C=NC(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 LJRQSGSCHQMNFU-UHFFFAOYSA-N 0.000 description 1
- SHYYQWYOUYSVLM-UHFFFAOYSA-N CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN(CC)CC)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN(CC)CC)=CC=C3OC)=N2)C=CC=C1 SHYYQWYOUYSVLM-UHFFFAOYSA-N 0.000 description 1
- NWXABQFMKNJPSA-UHFFFAOYSA-N CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCC4)=CC=C3OC)=N2)C=CC=C1 NWXABQFMKNJPSA-UHFFFAOYSA-N 0.000 description 1
- UOUMNCDDFNFBNG-HXUWFJFHSA-N CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 Chemical compound CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 UOUMNCDDFNFBNG-HXUWFJFHSA-N 0.000 description 1
- UOUMNCDDFNFBNG-FQEVSTJZSA-N CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 Chemical compound CCC(CC)NS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 UOUMNCDDFNFBNG-FQEVSTJZSA-N 0.000 description 1
- MGMLXHFJSDRVGT-UHFFFAOYSA-N CCC(CC)S(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CCC(CC)S(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 MGMLXHFJSDRVGT-UHFFFAOYSA-N 0.000 description 1
- HYFLWBNQFMXCPA-UHFFFAOYSA-N CCC1=C(C)C=CC=C1 Chemical compound CCC1=C(C)C=CC=C1 HYFLWBNQFMXCPA-UHFFFAOYSA-N 0.000 description 1
- LXQCYKMUNZAQOJ-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 LXQCYKMUNZAQOJ-UHFFFAOYSA-N 0.000 description 1
- VGYDZCLUDRFYMS-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 VGYDZCLUDRFYMS-UHFFFAOYSA-N 0.000 description 1
- ZLOHAVVDKLVBQA-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC(CC)CC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC(CC)CC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 ZLOHAVVDKLVBQA-UHFFFAOYSA-N 0.000 description 1
- KQSIVAIXBPUZLL-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCOCC2)=C1 KQSIVAIXBPUZLL-UHFFFAOYSA-N 0.000 description 1
- VJTOMILZBUBKIF-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 VJTOMILZBUBKIF-UHFFFAOYSA-N 0.000 description 1
- VYUOCCZEYTVXIC-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 VYUOCCZEYTVXIC-UHFFFAOYSA-N 0.000 description 1
- YEEIMXFEMHBIST-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 YEEIMXFEMHBIST-UHFFFAOYSA-N 0.000 description 1
- DEXBMIOTBOXSSL-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 DEXBMIOTBOXSSL-UHFFFAOYSA-N 0.000 description 1
- XPRFVLDYJJTJJI-UHFFFAOYSA-N CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 Chemical compound CCC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCN(C)CC2)=C1 XPRFVLDYJJTJJI-UHFFFAOYSA-N 0.000 description 1
- GEQCGDVMKZVNRI-UHFFFAOYSA-N CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3C(F)(F)F)=N2)C=CC=C1 Chemical compound CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3C(F)(F)F)=N2)C=CC=C1 GEQCGDVMKZVNRI-UHFFFAOYSA-N 0.000 description 1
- YCNKGQYDQCZDKB-UHFFFAOYSA-N CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC(F)(F)F)=N2)C=CC=C1 Chemical compound CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC(F)(F)F)=N2)C=CC=C1 YCNKGQYDQCZDKB-UHFFFAOYSA-N 0.000 description 1
- JAUZOQZRZBSQES-DEOSSOPVSA-N CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C#N)=N2)C=CC=C1 Chemical compound CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C#N)=N2)C=CC=C1 JAUZOQZRZBSQES-DEOSSOPVSA-N 0.000 description 1
- LYDSDYKODIXKHZ-QFIPXVFZSA-N CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C(F)(F)F)=N2)C=CC=C1 Chemical compound CCCC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CC[C@H](N(C)C)C4)C=C3C(F)(F)F)=N2)C=CC=C1 LYDSDYKODIXKHZ-QFIPXVFZSA-N 0.000 description 1
- AMEHDEOGAKICMI-UHFFFAOYSA-N CCCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCOCC2)=C1 AMEHDEOGAKICMI-UHFFFAOYSA-N 0.000 description 1
- QTLKDWBGPQPFDO-UHFFFAOYSA-N CCCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCCC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 QTLKDWBGPQPFDO-UHFFFAOYSA-N 0.000 description 1
- NUJILYKLNKQOOX-UHFFFAOYSA-N CCCCC1=C(C)C=CC=C1 Chemical compound CCCCC1=C(C)C=CC=C1 NUJILYKLNKQOOX-UHFFFAOYSA-N 0.000 description 1
- HDDUSOQGURGKGC-UHFFFAOYSA-N CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OC)C=CC(OCCN4CCOCC4)=C3)=N2)C=CC=C1 Chemical compound CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OC)C=CC(OCCN4CCOCC4)=C3)=N2)C=CC=C1 HDDUSOQGURGKGC-UHFFFAOYSA-N 0.000 description 1
- VPHDZRWDKLDHIR-UHFFFAOYSA-N CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 VPHDZRWDKLDHIR-UHFFFAOYSA-N 0.000 description 1
- FYXNNFLSNVUYTF-UHFFFAOYSA-N CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CCOCC4)C=C3)=N2)C=CC=C1 Chemical compound CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CCOCC4)C=C3)=N2)C=CC=C1 FYXNNFLSNVUYTF-UHFFFAOYSA-N 0.000 description 1
- LSDUTQFUBMVXHO-UHFFFAOYSA-N CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCCCC1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 LSDUTQFUBMVXHO-UHFFFAOYSA-N 0.000 description 1
- MMMSBPHKGINBEE-UHFFFAOYSA-N CCCCCS(=O)(=O)C1=C(C)C=CC=C1 Chemical compound CCCCCS(=O)(=O)C1=C(C)C=CC=C1 MMMSBPHKGINBEE-UHFFFAOYSA-N 0.000 description 1
- LQGYHUITDSQCOX-UHFFFAOYSA-N CCCCS(=O)(=O)C1=C(C)C=CC=C1 Chemical compound CCCCS(=O)(=O)C1=C(C)C=CC=C1 LQGYHUITDSQCOX-UHFFFAOYSA-N 0.000 description 1
- WAHRQVFCESPVQR-UHFFFAOYSA-N CCCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 Chemical compound CCCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 WAHRQVFCESPVQR-UHFFFAOYSA-N 0.000 description 1
- MDCVJZSTZBXGDN-UHFFFAOYSA-N CCCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 MDCVJZSTZBXGDN-UHFFFAOYSA-N 0.000 description 1
- PJUIKSUEJNOHRO-UHFFFAOYSA-N CCCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 PJUIKSUEJNOHRO-UHFFFAOYSA-N 0.000 description 1
- VAQHRGUTPGIUEF-UHFFFAOYSA-N CCCN(CCO)C1=CC(C)=C(C)C=C1 Chemical compound CCCN(CCO)C1=CC(C)=C(C)C=C1 VAQHRGUTPGIUEF-UHFFFAOYSA-N 0.000 description 1
- WJIIDMSHZCIREQ-UHFFFAOYSA-N CCCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 WJIIDMSHZCIREQ-UHFFFAOYSA-N 0.000 description 1
- JSCGIIVTQOZHJQ-UHFFFAOYSA-N CCCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 JSCGIIVTQOZHJQ-UHFFFAOYSA-N 0.000 description 1
- JPWSSWIWOOLTLD-UHFFFAOYSA-N CCCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 JPWSSWIWOOLTLD-UHFFFAOYSA-N 0.000 description 1
- RPCHUVCYXCRTEA-UHFFFAOYSA-N CCCNS(=O)(=O)C1=CC=CC(CC2=C(Br)C=NC(CC3=CC=C(N4CCOCC4)C=C3OCC)=N2)=C1 Chemical compound CCCNS(=O)(=O)C1=CC=CC(CC2=C(Br)C=NC(CC3=CC=C(N4CCOCC4)C=C3OCC)=N2)=C1 RPCHUVCYXCRTEA-UHFFFAOYSA-N 0.000 description 1
- KWIDJPBQRLHBKJ-UHFFFAOYSA-N CCCNS(c(cccc1)c1Nc(nc(Nc(c(OCC)c1)cc(OCC)c1N1CCOCC1)nc1)c1Br)(=O)=O Chemical compound CCCNS(c(cccc1)c1Nc(nc(Nc(c(OCC)c1)cc(OCC)c1N1CCOCC1)nc1)c1Br)(=O)=O KWIDJPBQRLHBKJ-UHFFFAOYSA-N 0.000 description 1
- YCRBGYHILMNRHD-UHFFFAOYSA-N CCCOC1=C(C)C=CC(N2CCN(C)CC2)=C1 Chemical compound CCCOC1=C(C)C=CC(N2CCN(C)CC2)=C1 YCRBGYHILMNRHD-UHFFFAOYSA-N 0.000 description 1
- LPXNIFIAUGSOPS-UHFFFAOYSA-N CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 LPXNIFIAUGSOPS-UHFFFAOYSA-N 0.000 description 1
- QCYNELZTJJYZPU-UHFFFAOYSA-N CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 QCYNELZTJJYZPU-UHFFFAOYSA-N 0.000 description 1
- AFWLMHYRYFPXDN-UHFFFAOYSA-N CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCOCC2)=C1 AFWLMHYRYFPXDN-UHFFFAOYSA-N 0.000 description 1
- OMZCVDXBUBFNFY-UHFFFAOYSA-N CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 OMZCVDXBUBFNFY-UHFFFAOYSA-N 0.000 description 1
- MADLROAYXJGWDI-UHFFFAOYSA-N CCCOC1=C(N)C=CC(N2CCN(C)CC2)=C1 Chemical compound CCCOC1=C(N)C=CC(N2CCN(C)CC2)=C1 MADLROAYXJGWDI-UHFFFAOYSA-N 0.000 description 1
- JDYSUDFBCKHQCA-UHFFFAOYSA-N CCCOC1=C([N+](=O)[O-])C=CC(N2CCN(C)CC2)=C1 Chemical compound CCCOC1=C([N+](=O)[O-])C=CC(N2CCN(C)CC2)=C1 JDYSUDFBCKHQCA-UHFFFAOYSA-N 0.000 description 1
- VYBQDFOQCDZBBV-UHFFFAOYSA-N CCCOC1=CC=C(OC)C=C1C Chemical compound CCCOC1=CC=C(OC)C=C1C VYBQDFOQCDZBBV-UHFFFAOYSA-N 0.000 description 1
- NYOFHPHWNWJULD-UHFFFAOYSA-N CCCOC1=CC=C(OC)C=C1N Chemical compound CCCOC1=CC=C(OC)C=C1N NYOFHPHWNWJULD-UHFFFAOYSA-N 0.000 description 1
- GXWXLELTQSINRD-UHFFFAOYSA-N CCCOC1=CC=C(OC)C=C1[N+](=O)[O-] Chemical compound CCCOC1=CC=C(OC)C=C1[N+](=O)[O-] GXWXLELTQSINRD-UHFFFAOYSA-N 0.000 description 1
- BNWVZZRTXGCCSA-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 BNWVZZRTXGCCSA-UHFFFAOYSA-N 0.000 description 1
- XNJKYVTYEVWAIW-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 XNJKYVTYEVWAIW-UHFFFAOYSA-N 0.000 description 1
- YZBZKERQPIXJQO-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 YZBZKERQPIXJQO-UHFFFAOYSA-N 0.000 description 1
- YCJIFXBTXIKKJV-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Cl)C=N(C)C(NC3=C(OC)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Cl)C=N(C)C(NC3=C(OC)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 YCJIFXBTXIKKJV-UHFFFAOYSA-N 0.000 description 1
- ROWNOQSMBAURLR-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 ROWNOQSMBAURLR-UHFFFAOYSA-N 0.000 description 1
- LJLGKHUPWZMUFS-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 LJLGKHUPWZMUFS-UHFFFAOYSA-N 0.000 description 1
- FAKVVNZBPAFOCM-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 FAKVVNZBPAFOCM-UHFFFAOYSA-N 0.000 description 1
- WLGBXGJEBRKUKG-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCOCC4)C=C3OC)=N2)C=CC=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCOCC4)C=C3OC)=N2)C=CC=C1 WLGBXGJEBRKUKG-UHFFFAOYSA-N 0.000 description 1
- CUOOCKAPVMBPTM-UHFFFAOYSA-N CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(F)C=C3OC)=N2)C=CC(N2CCN(C)CC2)=C1 Chemical compound CCCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(F)C=C3OC)=N2)C=CC(N2CCN(C)CC2)=C1 CUOOCKAPVMBPTM-UHFFFAOYSA-N 0.000 description 1
- ZWJALBNEKPZZJC-UHFFFAOYSA-N CCCS(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(F)C=C3OC)=N2)C=CC=C1 Chemical compound CCCS(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(F)C=C3OC)=N2)C=CC=C1 ZWJALBNEKPZZJC-UHFFFAOYSA-N 0.000 description 1
- ROPWFBRSGAXBKU-UHFFFAOYSA-N CCCS(c(cccc1)c1Nc(nc(Nc(c(OCC)c1)ccc1N1CCOCC1)nc1)c1Cl)(=O)=O Chemical compound CCCS(c(cccc1)c1Nc(nc(Nc(c(OCC)c1)ccc1N1CCOCC1)nc1)c1Cl)(=O)=O ROPWFBRSGAXBKU-UHFFFAOYSA-N 0.000 description 1
- SCNUGLCBAKORRC-UHFFFAOYSA-N CCCS(c(cccc1)c1Nc1nc(Nc(ccc(N(CC2)CCC2C(N)=O)c2)c2OC)ncc1Cl)(=O)=O Chemical compound CCCS(c(cccc1)c1Nc1nc(Nc(ccc(N(CC2)CCC2C(N)=O)c2)c2OC)ncc1Cl)(=O)=O SCNUGLCBAKORRC-UHFFFAOYSA-N 0.000 description 1
- UKLOXTBYNBDFDV-UHFFFAOYSA-N CCN(C)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)=C1 Chemical compound CCN(C)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)=C1 UKLOXTBYNBDFDV-UHFFFAOYSA-N 0.000 description 1
- PRRATRVRMKPPMV-UHFFFAOYSA-N CCN(CC)C(=O)C1CCCN(C2=CC=C(C)C(OC)=C2)C1 Chemical compound CCN(CC)C(=O)C1CCCN(C2=CC=C(C)C(OC)=C2)C1 PRRATRVRMKPPMV-UHFFFAOYSA-N 0.000 description 1
- UVZWPMSKQJQOSK-UHFFFAOYSA-N CCN(CC)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)=C1 Chemical compound CCN(CC)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)=C1 UVZWPMSKQJQOSK-UHFFFAOYSA-N 0.000 description 1
- MSBMCWZWHQWXCU-UHFFFAOYSA-N CCN(CC)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)=C1 Chemical compound CCN(CC)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)=C1 MSBMCWZWHQWXCU-UHFFFAOYSA-N 0.000 description 1
- SRGVRVXMPWIBJY-UHFFFAOYSA-N CCN(CC)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC4CCCC4)C=CC=C3)=C(Cl)C=N2)=C1 Chemical compound CCN(CC)CCOC1=CC=C(OC)C(NC2=NC(NC3=C(S(=O)(=O)NC4CCCC4)C=CC=C3)=C(Cl)C=N2)=C1 SRGVRVXMPWIBJY-UHFFFAOYSA-N 0.000 description 1
- QYGXWSNKCLNKBS-UHFFFAOYSA-N CCN1CC2=CC=CC(NC3=C(Cl)C=NC(NC)=N3)=C2C1=O Chemical compound CCN1CC2=CC=CC(NC3=C(Cl)C=NC(NC)=N3)=C2C1=O QYGXWSNKCLNKBS-UHFFFAOYSA-N 0.000 description 1
- RAWNFHZQDWQMMV-UHFFFAOYSA-N CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 RAWNFHZQDWQMMV-UHFFFAOYSA-N 0.000 description 1
- WLQZWUGNPJDSGM-UHFFFAOYSA-N CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CC5CCCCC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CC5CCCCC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 WLQZWUGNPJDSGM-UHFFFAOYSA-N 0.000 description 1
- SZFGMJSFSZCYRE-UHFFFAOYSA-N CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CN(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CN(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 SZFGMJSFSZCYRE-UHFFFAOYSA-N 0.000 description 1
- ZUZRTSZDXPAEIN-UHFFFAOYSA-N CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CNC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CNC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 ZUZRTSZDXPAEIN-UHFFFAOYSA-N 0.000 description 1
- FVUPVRYSLDESPM-UHFFFAOYSA-N CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Br)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Br)C=N3)C=C2)C1 FVUPVRYSLDESPM-UHFFFAOYSA-N 0.000 description 1
- WRMOVDCRQQTSEC-UHFFFAOYSA-N CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(C)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 WRMOVDCRQQTSEC-UHFFFAOYSA-N 0.000 description 1
- KVMDHTMNEMYZKK-UHFFFAOYSA-N CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)N(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)N(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 KVMDHTMNEMYZKK-UHFFFAOYSA-N 0.000 description 1
- WWCSTWUFZUECJD-UHFFFAOYSA-N CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 WWCSTWUFZUECJD-UHFFFAOYSA-N 0.000 description 1
- XRMMNSCIFSAZNR-UHFFFAOYSA-N CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 XRMMNSCIFSAZNR-UHFFFAOYSA-N 0.000 description 1
- NAUNTVANGAPVHW-UHFFFAOYSA-N CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NCC5CC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(OC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NCC5CC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 NAUNTVANGAPVHW-UHFFFAOYSA-N 0.000 description 1
- HNGLIFSITNPVCS-UHFFFAOYSA-N CCN1CCC(OC2=CC(OC)=C(C)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(OC)=C(C)C=C2)C1 HNGLIFSITNPVCS-UHFFFAOYSA-N 0.000 description 1
- GYGFURJSDQYDGS-UHFFFAOYSA-N CCN1CCC(OC2=CC(OCC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN1CCC(OC2=CC(OCC(C)C)=C(CC3=NC(NC4=C(S(=O)(=O)NC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 GYGFURJSDQYDGS-UHFFFAOYSA-N 0.000 description 1
- YLMZUEGPDIEZFH-UHFFFAOYSA-N CCN1CCN(C2=CC(OC)=C(C)C=C2)CC1 Chemical compound CCN1CCN(C2=CC(OC)=C(C)C=C2)CC1 YLMZUEGPDIEZFH-UHFFFAOYSA-N 0.000 description 1
- HMHILCRWXQZODU-UHFFFAOYSA-N CCN1CCN(C2=CC(OC)=C(N)C=C2)CC1 Chemical compound CCN1CCN(C2=CC(OC)=C(N)C=C2)CC1 HMHILCRWXQZODU-UHFFFAOYSA-N 0.000 description 1
- TZTBDDXLEXKVET-UHFFFAOYSA-N CCN1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 Chemical compound CCN1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 TZTBDDXLEXKVET-UHFFFAOYSA-N 0.000 description 1
- CFEVDLPAOXRAEB-UHFFFAOYSA-N CCN1N=CC=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)NC)=N1 Chemical compound CCN1N=CC=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)NC)=N1 CFEVDLPAOXRAEB-UHFFFAOYSA-N 0.000 description 1
- YCFFGLJHFGMZDH-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 YCFFGLJHFGMZDH-UHFFFAOYSA-N 0.000 description 1
- PLOKSCKFQBPWER-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 PLOKSCKFQBPWER-UHFFFAOYSA-N 0.000 description 1
- KKFULZMHGGUKDY-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 KKFULZMHGGUKDY-UHFFFAOYSA-N 0.000 description 1
- RJENGVJNHCRTFN-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCCN4CCOCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCCN4CCOCC4)=CC=C3OC)=N2)C=CC=C1 RJENGVJNHCRTFN-UHFFFAOYSA-N 0.000 description 1
- ZGAHBZQUOVXZJS-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 ZGAHBZQUOVXZJS-UHFFFAOYSA-N 0.000 description 1
- RTPLFXKIUAKWCM-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 RTPLFXKIUAKWCM-UHFFFAOYSA-N 0.000 description 1
- JIIPIJFBTKGBCZ-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 JIIPIJFBTKGBCZ-UHFFFAOYSA-N 0.000 description 1
- XKRAMFMPBCLQAK-UHFFFAOYSA-N CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCCN4CCOCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCCN4CCOCC4)=CC=C3OC)=N2)C=CC=C1 XKRAMFMPBCLQAK-UHFFFAOYSA-N 0.000 description 1
- VRKYQHKBSNPATO-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=N(C)C(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=N(C)C(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 VRKYQHKBSNPATO-UHFFFAOYSA-N 0.000 description 1
- DUJIBEWUZYZTOG-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 DUJIBEWUZYZTOG-UHFFFAOYSA-N 0.000 description 1
- ALNPXJKDQKRBSU-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 ALNPXJKDQKRBSU-UHFFFAOYSA-N 0.000 description 1
- MJEWTXYQUJPASO-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 MJEWTXYQUJPASO-UHFFFAOYSA-N 0.000 description 1
- ZJWMFDQAKISTGR-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C(=O)NC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C(=O)NC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 ZJWMFDQAKISTGR-UHFFFAOYSA-N 0.000 description 1
- GRQWKONCSHYFIC-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(C(=O)N4CCOCC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(C(=O)N4CCOCC4)C=C3)=N2)C=CC=C1 GRQWKONCSHYFIC-UHFFFAOYSA-N 0.000 description 1
- WVKONMJTTPROTE-IBGZPJMESA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 WVKONMJTTPROTE-IBGZPJMESA-N 0.000 description 1
- ATZXLQPVTJCQOM-KRWDZBQOSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CC[C@H](NC)C4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CC[C@H](NC)C4)C=C3)=N2)C=CC=C1 ATZXLQPVTJCQOM-KRWDZBQOSA-N 0.000 description 1
- PCAJDDCUGVVAEE-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 PCAJDDCUGVVAEE-UHFFFAOYSA-N 0.000 description 1
- GXJHLUMIRCZKGN-SFHVURJKSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(O[C@H]4CCN(C)C4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(O[C@H]4CCN(C)C4)C=C3)=N2)C=CC=C1 GXJHLUMIRCZKGN-SFHVURJKSA-N 0.000 description 1
- XDTLDCSPZJSQRI-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 XDTLDCSPZJSQRI-UHFFFAOYSA-N 0.000 description 1
- OXYIYESFGMSZST-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 OXYIYESFGMSZST-UHFFFAOYSA-N 0.000 description 1
- YAQFCMPMFSYYND-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)C)C=C(N4CCC(N5CCOCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)C)C=C(N4CCC(N5CCOCC5)CC4)C=C3)=N2)C=CC=C1 YAQFCMPMFSYYND-UHFFFAOYSA-N 0.000 description 1
- BGTRVWFYIWYEPD-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 BGTRVWFYIWYEPD-UHFFFAOYSA-N 0.000 description 1
- RFJGXEBZNXCOPB-GOSISDBHSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CC[C@@H](NCC)C4)C=C3)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=C(OCC)C=C(N4CC[C@@H](NCC)C4)C=C3)=N2)C=CC=C1 RFJGXEBZNXCOPB-GOSISDBHSA-N 0.000 description 1
- SLJFYIXPHZWRLY-UHFFFAOYSA-N CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCOCC4)C=C3OC)=N2)C=CC=C1 Chemical compound CCNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(C(=O)N4CCOCC4)C=C3OC)=N2)C=CC=C1 SLJFYIXPHZWRLY-UHFFFAOYSA-N 0.000 description 1
- TYWWTSULIDYOOY-UHFFFAOYSA-N CCNC(=O)C1=CC(F)=CC=C1C Chemical compound CCNC(=O)C1=CC(F)=CC=C1C TYWWTSULIDYOOY-UHFFFAOYSA-N 0.000 description 1
- NDUSDDNZVDNMFG-UHFFFAOYSA-N CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N(C)C)CC3)C=C2OC(C)C)=N1 Chemical compound CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N(C)C)CC3)C=C2OC(C)C)=N1 NDUSDDNZVDNMFG-UHFFFAOYSA-N 0.000 description 1
- ZLBNNCWCDYYQMR-UHFFFAOYSA-N CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N(C)C)CC3)N=C2C)=N1 Chemical compound CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N(C)C)CC3)N=C2C)=N1 ZLBNNCWCDYYQMR-UHFFFAOYSA-N 0.000 description 1
- RNLAICFPOAQZAQ-UHFFFAOYSA-N CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N4CCCC4=O)CC3)C=C2OC(C)C)=N1 Chemical compound CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N4CCCC4=O)CC3)C=C2OC(C)C)=N1 RNLAICFPOAQZAQ-UHFFFAOYSA-N 0.000 description 1
- VAIAQPYEPJBPQB-UHFFFAOYSA-N CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N4CCCCC4)CC3)N=C2C)=N1 Chemical compound CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N4CCCCC4)CC3)N=C2C)=N1 VAIAQPYEPJBPQB-UHFFFAOYSA-N 0.000 description 1
- XFKZPVXVEVHSDA-UHFFFAOYSA-N CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCOCC3)N=C2C)=N1 Chemical compound CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCOCC3)N=C2C)=N1 XFKZPVXVEVHSDA-UHFFFAOYSA-N 0.000 description 1
- JZWONNKYLLLMFO-QGZVFWFLSA-N CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CC[C@@H](N(C)C)C3)N=C2C)=N1 Chemical compound CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CC[C@@H](N(C)C)C3)N=C2C)=N1 JZWONNKYLLLMFO-QGZVFWFLSA-N 0.000 description 1
- JZWONNKYLLLMFO-KRWDZBQOSA-N CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CC[C@H](N(C)C)C3)N=C2C)=N1 Chemical compound CCNC(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CC[C@H](N(C)C)C3)N=C2C)=N1 JZWONNKYLLLMFO-KRWDZBQOSA-N 0.000 description 1
- DAMCNVWLPZVCEK-IBGZPJMESA-N CCNC(c(cccc1)c1Nc(nc(Nc(ccc(N(CC1)C[C@H]1N(C)C)c1)c1C#N)nc1)c1Cl)=O Chemical compound CCNC(c(cccc1)c1Nc(nc(Nc(ccc(N(CC1)C[C@H]1N(C)C)c1)c1C#N)nc1)c1Cl)=O DAMCNVWLPZVCEK-IBGZPJMESA-N 0.000 description 1
- YOBMWQNLBVUXTD-UHFFFAOYSA-N CCNC1CCN(C2=CC(OC)=C(N)C=C2)C1 Chemical compound CCNC1CCN(C2=CC(OC)=C(N)C=C2)C1 YOBMWQNLBVUXTD-UHFFFAOYSA-N 0.000 description 1
- FMBNITWPGFAQOW-UHFFFAOYSA-N CCNC1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)C1 Chemical compound CCNC1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)C1 FMBNITWPGFAQOW-UHFFFAOYSA-N 0.000 description 1
- QTGZQOSGZHNDAF-UHFFFAOYSA-N CCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 QTGZQOSGZHNDAF-UHFFFAOYSA-N 0.000 description 1
- PFUMSZFWGDSCOH-UHFFFAOYSA-N CCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC=CC=C3OC3=CC=CC=C3)=N2)C=CC=C1 Chemical compound CCNS(=O)(=O)C1=C(CC2=C(Br)C=NC(CC3=CC=CC=C3OC3=CC=CC=C3)=N2)C=CC=C1 PFUMSZFWGDSCOH-UHFFFAOYSA-N 0.000 description 1
- YFHJHSGKDZVCDA-UHFFFAOYSA-N CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(C)=CC=C3OC)=N2)C=CC=C1 YFHJHSGKDZVCDA-UHFFFAOYSA-N 0.000 description 1
- YYMIDBUGANYBJW-UHFFFAOYSA-N CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OC)=CC=C3OC)=N2)C=CC=C1 YYMIDBUGANYBJW-UHFFFAOYSA-N 0.000 description 1
- GGALWCJTLBYXTH-UHFFFAOYSA-N CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 Chemical compound CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCC)=C(N4CCOCC4)C=C3OCC)=N2)C=CC=C1 GGALWCJTLBYXTH-UHFFFAOYSA-N 0.000 description 1
- MQQLIICRHGVVHG-UHFFFAOYSA-N CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCCN4CCOCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CCNS(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC(OCCN4CCOCC4)=CC=C3OC)=N2)C=CC=C1 MQQLIICRHGVVHG-UHFFFAOYSA-N 0.000 description 1
- KKFWMSMMDDWFSZ-UHFFFAOYSA-N CCNS(=O)(=O)C1=CC=CC(CC2=C(Br)C=NC(CC3=CC=C(N4CCOCC4)C=C3OCC)=N2)=C1 Chemical compound CCNS(=O)(=O)C1=CC=CC(CC2=C(Br)C=NC(CC3=CC=C(N4CCOCC4)C=C3OCC)=N2)=C1 KKFWMSMMDDWFSZ-UHFFFAOYSA-N 0.000 description 1
- PKENWTKIFVYKSJ-UHFFFAOYSA-N CCNS(c(cccc1)c1Nc(nc(Nc(cc(c(N1CCOCC1)c1)OCC)c1OCC)nc1)c1Cl)(=O)=O Chemical compound CCNS(c(cccc1)c1Nc(nc(Nc(cc(c(N1CCOCC1)c1)OCC)c1OCC)nc1)c1Cl)(=O)=O PKENWTKIFVYKSJ-UHFFFAOYSA-N 0.000 description 1
- MYSTYKZOSCIGFH-UHFFFAOYSA-N CCNS(c(cccc1)c1Nc1nc(Nc(c(OC)c2)ccc2N2CCOCC2)ncc1Br)(=O)=O Chemical compound CCNS(c(cccc1)c1Nc1nc(Nc(c(OC)c2)ccc2N2CCOCC2)ncc1Br)(=O)=O MYSTYKZOSCIGFH-UHFFFAOYSA-N 0.000 description 1
- LEMOJBKIECNQJX-UHFFFAOYSA-N CCNS(c(cccc1)c1Nc1nc(Nc(c(OCC)c2)ccc2N2CCOCC2)ncc1Br)(=O)=O Chemical compound CCNS(c(cccc1)c1Nc1nc(Nc(c(OCC)c2)ccc2N2CCOCC2)ncc1Br)(=O)=O LEMOJBKIECNQJX-UHFFFAOYSA-N 0.000 description 1
- RAUCTMKPTDANIK-OAQYLSRUSA-N CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 RAUCTMKPTDANIK-OAQYLSRUSA-N 0.000 description 1
- GHKAPLUWPKKCQN-HXUWFJFHSA-N CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CN(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CN(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 GHKAPLUWPKKCQN-HXUWFJFHSA-N 0.000 description 1
- GFTXPUWYABBZSP-OAQYLSRUSA-N CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC(C)C)C=CC=C4)=C(Cl)C=N3)C=C2)C1 GFTXPUWYABBZSP-OAQYLSRUSA-N 0.000 description 1
- ZUCRTLMWKCLYNX-LJQANCHMSA-N CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Br)C=N3)C=C2)C1 Chemical compound CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Br)C=N3)C=C2)C1 ZUCRTLMWKCLYNX-LJQANCHMSA-N 0.000 description 1
- YEGURPGKKPBZGQ-LJQANCHMSA-N CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC)C=CC=C4)=C(Cl)C=N3)C=C2)C1 YEGURPGKKPBZGQ-LJQANCHMSA-N 0.000 description 1
- KOMJTOBHIZUQEI-JOCHJYFZSA-N CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC5CCC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNC5CCC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 KOMJTOBHIZUQEI-JOCHJYFZSA-N 0.000 description 1
- MZNFPNOEVWBBDD-JOCHJYFZSA-N CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNCC5CC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 Chemical compound CCN[C@@H]1CCN(C2=CC(OCC)=C(NC3=NC(NC4=C(CNCC5CC5)C=CC=C4)=C(Cl)C=N3)C=C2)C1 MZNFPNOEVWBBDD-JOCHJYFZSA-N 0.000 description 1
- VDBUOILEDLYEJJ-UHFFFAOYSA-N CCOC1=C(C)C=C(C2=CC=C(OC)C=C2)C=C1 Chemical compound CCOC1=C(C)C=C(C2=CC=C(OC)C=C2)C=C1 VDBUOILEDLYEJJ-UHFFFAOYSA-N 0.000 description 1
- HCTQXTYOWLRJSQ-UHFFFAOYSA-N CCOC1=C(C)C=C(C2=CC=CC=C2)C=C1 Chemical compound CCOC1=C(C)C=C(C2=CC=CC=C2)C=C1 HCTQXTYOWLRJSQ-UHFFFAOYSA-N 0.000 description 1
- PGHJRRILOYJGFS-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(C(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(C(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 PGHJRRILOYJGFS-UHFFFAOYSA-N 0.000 description 1
- AWBLXGCUKYOZJP-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 AWBLXGCUKYOZJP-UHFFFAOYSA-N 0.000 description 1
- LZXWDOOVKLATKF-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCC3)CC2)=C1 LZXWDOOVKLATKF-UHFFFAOYSA-N 0.000 description 1
- ZJSRJYYRPIUUJK-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 ZJSRJYYRPIUUJK-UHFFFAOYSA-N 0.000 description 1
- ZEOXZPVRKBUZJT-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 ZEOXZPVRKBUZJT-UHFFFAOYSA-N 0.000 description 1
- APNYVVHBRUQEPK-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 APNYVVHBRUQEPK-UHFFFAOYSA-N 0.000 description 1
- SMZHAJQVIHUZSO-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCC3)CC2)=C1 SMZHAJQVIHUZSO-UHFFFAOYSA-N 0.000 description 1
- DMOOPBNIFDTWNS-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCOCC3)CC2)=C1 DMOOPBNIFDTWNS-UHFFFAOYSA-N 0.000 description 1
- KUABIGIFVZYNDL-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 KUABIGIFVZYNDL-UHFFFAOYSA-N 0.000 description 1
- MFFFXCVDZUGXQY-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 MFFFXCVDZUGXQY-UHFFFAOYSA-N 0.000 description 1
- BCATYCPCUDKIOG-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(C#N)C=N2)C=CC(N2CCOCC2)=C1 BCATYCPCUDKIOG-UHFFFAOYSA-N 0.000 description 1
- GYRCZXCLVQMIQJ-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 GYRCZXCLVQMIQJ-UHFFFAOYSA-N 0.000 description 1
- AAOBYCHVVFNIAV-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=CC=N2)C=CC(N2CCOCC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC)C=CC=C3)=CC=N2)C=CC(N2CCOCC2)=C1 AAOBYCHVVFNIAV-UHFFFAOYSA-N 0.000 description 1
- WKMOWBRVCAMMAS-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 WKMOWBRVCAMMAS-UHFFFAOYSA-N 0.000 description 1
- YVWPBEKTIHSEDM-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCC3)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCC3)CC2)=C1 YVWPBEKTIHSEDM-UHFFFAOYSA-N 0.000 description 1
- JUEYCHCJXYZXCB-UHFFFAOYSA-N CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 Chemical compound CCOC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(CCN2CCN(C)CC2)=C1 JUEYCHCJXYZXCB-UHFFFAOYSA-N 0.000 description 1
- CWHUSPWDRIOJMA-DEOSSOPVSA-N CCOC1=C(NC2=NC(NC3=C(C(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(C(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 CWHUSPWDRIOJMA-DEOSSOPVSA-N 0.000 description 1
- FOSNSBRBYDFKSG-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 FOSNSBRBYDFKSG-UHFFFAOYSA-N 0.000 description 1
- SYYCDCCJZDLHJY-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CC4CCCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 SYYCDCCJZDLHJY-UHFFFAOYSA-N 0.000 description 1
- AEZVKVYDTKIXHS-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 AEZVKVYDTKIXHS-UHFFFAOYSA-N 0.000 description 1
- HOQXHPUXFIMDCW-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 HOQXHPUXFIMDCW-UHFFFAOYSA-N 0.000 description 1
- YXGRYAIXXVNFPQ-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC(CC)CC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC(CC)CC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 YXGRYAIXXVNFPQ-UHFFFAOYSA-N 0.000 description 1
- RCXYOSAIMLKOCP-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC(CC)CC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC(CC)CC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 RCXYOSAIMLKOCP-UHFFFAOYSA-N 0.000 description 1
- BJFSMWRMLMCWOQ-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC)C=CC=C3)=C(Br)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 BJFSMWRMLMCWOQ-UHFFFAOYSA-N 0.000 description 1
- XBGOMARTWWWMLE-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 XBGOMARTWWWMLE-UHFFFAOYSA-N 0.000 description 1
- HJWCSAJNXAWYAI-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 HJWCSAJNXAWYAI-UHFFFAOYSA-N 0.000 description 1
- UUJYFZXYRHPCRF-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 UUJYFZXYRHPCRF-UHFFFAOYSA-N 0.000 description 1
- SIZFLGCKAOGRCR-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 SIZFLGCKAOGRCR-UHFFFAOYSA-N 0.000 description 1
- QCWAQBPEIOKVKQ-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Br)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Br)C=N2)C=CC(OC2CCN(CC)C2)=C1 QCWAQBPEIOKVKQ-UHFFFAOYSA-N 0.000 description 1
- KMIDMTREYWOOMN-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(CNCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(CNCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(C(N)=O)CC2)=C1 KMIDMTREYWOOMN-UHFFFAOYSA-N 0.000 description 1
- JJNYHVIJDJLUKI-QHCPKHFHSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 JJNYHVIJDJLUKI-QHCPKHFHSA-N 0.000 description 1
- CAIKSXJZGSRMMI-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)CC)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 CAIKSXJZGSRMMI-UHFFFAOYSA-N 0.000 description 1
- VKVREBKLKRZVTR-QFIPXVFZSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 VKVREBKLKRZVTR-QFIPXVFZSA-N 0.000 description 1
- BJZRVMQDRMFGOT-DEOSSOPVSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC[C@H]2CN2CCCC2)=C1 BJZRVMQDRMFGOT-DEOSSOPVSA-N 0.000 description 1
- JKNUAMWKSRVARZ-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 JKNUAMWKSRVARZ-UHFFFAOYSA-N 0.000 description 1
- JZAGUOJTYCMNIY-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 JZAGUOJTYCMNIY-UHFFFAOYSA-N 0.000 description 1
- VFNFBPICQIQOCV-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 VFNFBPICQIQOCV-UHFFFAOYSA-N 0.000 description 1
- JQDMFNREMMQLLX-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)C2)=C1 JQDMFNREMMQLLX-UHFFFAOYSA-N 0.000 description 1
- MIIIVLLXSVWNNM-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(C)CC2)=C1 MIIIVLLXSVWNNM-UHFFFAOYSA-N 0.000 description 1
- SEDMRANOOVXNEW-UHFFFAOYSA-N CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 Chemical compound CCOC1=C(NC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=CC(OC2CCN(CC)C2)=C1 SEDMRANOOVXNEW-UHFFFAOYSA-N 0.000 description 1
- RECFPCZNHGHKAU-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCC(N(C)CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCC(N(C)CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 RECFPCZNHGHKAU-UHFFFAOYSA-N 0.000 description 1
- UBYALEHFGYUHNA-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCC(N3CCCCC3)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 UBYALEHFGYUHNA-UHFFFAOYSA-N 0.000 description 1
- LQWZCZPVEPUJAP-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 LQWZCZPVEPUJAP-UHFFFAOYSA-N 0.000 description 1
- PFTGSOUFJVJZRE-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 PFTGSOUFJVJZRE-UHFFFAOYSA-N 0.000 description 1
- XBSFAMAHMDVCTF-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 XBSFAMAHMDVCTF-UHFFFAOYSA-N 0.000 description 1
- LISRJYJYZXZFCL-UHFFFAOYSA-N CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 LISRJYJYZXZFCL-UHFFFAOYSA-N 0.000 description 1
- QGPUBTOWISAKPN-UHFFFAOYSA-N CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 QGPUBTOWISAKPN-UHFFFAOYSA-N 0.000 description 1
- OLQPUWKAURXAPV-UHFFFAOYSA-N CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 OLQPUWKAURXAPV-UHFFFAOYSA-N 0.000 description 1
- QGXAVIMUGLBRDG-UHFFFAOYSA-N CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 Chemical compound CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 QGXAVIMUGLBRDG-UHFFFAOYSA-N 0.000 description 1
- NDOQQAXBTRYJSG-UHFFFAOYSA-N CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 NDOQQAXBTRYJSG-UHFFFAOYSA-N 0.000 description 1
- URTBTGUFIYPOCQ-UHFFFAOYSA-N CCOC1=CC(N2CCCC(C(N)=O)C2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCCC(C(N)=O)C2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 URTBTGUFIYPOCQ-UHFFFAOYSA-N 0.000 description 1
- KLCMXXXXZIUKMQ-UHFFFAOYSA-N CCOC1=CC(N2CCCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(C)C)C=CC=C2)=C(Cl)C=N1 KLCMXXXXZIUKMQ-UHFFFAOYSA-N 0.000 description 1
- XSBSVUDJQSCMQE-UHFFFAOYSA-N CCOC1=CC(N2CCCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 XSBSVUDJQSCMQE-UHFFFAOYSA-N 0.000 description 1
- ZGGYEEGTCXMKKQ-UHFFFAOYSA-N CCOC1=CC(N2CCN(C(C)=O)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCN(C(C)=O)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 ZGGYEEGTCXMKKQ-UHFFFAOYSA-N 0.000 description 1
- USIDIMIZKWIROU-UHFFFAOYSA-N CCOC1=CC(N2CCN(C(C)=O)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCN(C(C)=O)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 USIDIMIZKWIROU-UHFFFAOYSA-N 0.000 description 1
- VGGQLWNZWZHPHN-UHFFFAOYSA-N CCOC1=CC(N2CCN(C(C)=O)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 Chemical compound CCOC1=CC(N2CCN(C(C)=O)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 VGGQLWNZWZHPHN-UHFFFAOYSA-N 0.000 description 1
- JKZAUJMXKHOGGC-UHFFFAOYSA-N CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 JKZAUJMXKHOGGC-UHFFFAOYSA-N 0.000 description 1
- WDGYRAJYBZYCIU-UHFFFAOYSA-N CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 Chemical compound CCOC1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 WDGYRAJYBZYCIU-UHFFFAOYSA-N 0.000 description 1
- BPUCHTKTPGFFHN-UHFFFAOYSA-N CCOC1=CC(N2CCN(CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCN(CC)CC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 BPUCHTKTPGFFHN-UHFFFAOYSA-N 0.000 description 1
- KYPGZHKFXISXES-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=C(C)C=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=C(C)C=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 KYPGZHKFXISXES-UHFFFAOYSA-N 0.000 description 1
- OOFQXWQRJUTPHH-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=C(C)C=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(C)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=C(C)C=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(C)C=N1 OOFQXWQRJUTPHH-UHFFFAOYSA-N 0.000 description 1
- JNUWFGMTEPUTAE-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=C(C)C=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=C(C)C=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 JNUWFGMTEPUTAE-UHFFFAOYSA-N 0.000 description 1
- CSJGJNHGRKXFNV-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=C(OCC)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=C(OCC)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 CSJGJNHGRKXFNV-UHFFFAOYSA-N 0.000 description 1
- PLNDKXJHSLXDSO-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=C(OCC)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=C(OCC)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Br)C=N1 PLNDKXJHSLXDSO-UHFFFAOYSA-N 0.000 description 1
- IOYCNPFIDXBXQG-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=C(OCC)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=C(OCC)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 IOYCNPFIDXBXQG-UHFFFAOYSA-N 0.000 description 1
- FTWXAHJADSBEQC-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1C Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1C FTWXAHJADSBEQC-UHFFFAOYSA-N 0.000 description 1
- UVGBWTNYYFHFNB-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1C.CCOC1=CC=C(N2CCOCC2)C=C1C.CCOC1=CC=C(OCC)C(C)=C1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1C.CCOC1=CC=C(N2CCOCC2)C=C1C.CCOC1=CC=C(OCC)C(C)=C1 UVGBWTNYYFHFNB-UHFFFAOYSA-N 0.000 description 1
- RCFXUNGIJHLHEY-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(NS(C)(=O)=O)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(NS(C)(=O)=O)C=CC=C2)=C(Br)C=N1 RCFXUNGIJHLHEY-UHFFFAOYSA-N 0.000 description 1
- AHINUBRXIXXMOJ-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 AHINUBRXIXXMOJ-UHFFFAOYSA-N 0.000 description 1
- QKXCSBVHQHPUBW-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Br)C=N1 QKXCSBVHQHPUBW-UHFFFAOYSA-N 0.000 description 1
- WAKYQLCORNCGEC-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Br)C=N1 WAKYQLCORNCGEC-UHFFFAOYSA-N 0.000 description 1
- WXNYKVVBIKSVDB-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 WXNYKVVBIKSVDB-UHFFFAOYSA-N 0.000 description 1
- IMEKWBYKVSJQSS-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=CC(S(=O)(=O)NC)=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=CC(S(=O)(=O)NC)=CC=C2)=C(Br)C=N1 IMEKWBYKVSJQSS-UHFFFAOYSA-N 0.000 description 1
- HGNBRSCCHPCHOB-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC=C(Br)C(CC2=C(Br)C=CC=C2)=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC=C(Br)C(CC2=C(Br)C=CC=C2)=N1 HGNBRSCCHPCHOB-UHFFFAOYSA-N 0.000 description 1
- SYBFWKHWTDTBDT-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC=C(Br)C(CC2=CC(Br)=CC=C2)=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1CC1=NC=C(Br)C(CC2=CC(Br)=CC=C2)=N1 SYBFWKHWTDTBDT-UHFFFAOYSA-N 0.000 description 1
- KGYSUUTWBWYMPY-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1N Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1N KGYSUUTWBWYMPY-UHFFFAOYSA-N 0.000 description 1
- QFMRTEMCCBYFOQ-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CC)C=CC=C2)=C(Br)C=N1 QFMRTEMCCBYFOQ-UHFFFAOYSA-N 0.000 description 1
- ZJFSNQIYZIYZKB-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CC)C=CC=C2)=C(Cl)C=N1 ZJFSNQIYZIYZKB-UHFFFAOYSA-N 0.000 description 1
- XPJPHQASENFHGZ-UHFFFAOYSA-N CCOC1=CC(N2CCOCC2)=CC=C1[N+](=O)[O-] Chemical compound CCOC1=CC(N2CCOCC2)=CC=C1[N+](=O)[O-] XPJPHQASENFHGZ-UHFFFAOYSA-N 0.000 description 1
- DPTYYMFFISGYBY-GOSISDBHSA-N CCOC1=CC(N2CC[C@@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 DPTYYMFFISGYBY-GOSISDBHSA-N 0.000 description 1
- AJPIDHFXAABXDV-GOSISDBHSA-N CCOC1=CC(N2CC[C@@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 AJPIDHFXAABXDV-GOSISDBHSA-N 0.000 description 1
- UHMHEHNGYBNRAA-GOSISDBHSA-N CCOC1=CC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 UHMHEHNGYBNRAA-GOSISDBHSA-N 0.000 description 1
- VQRCAUGSNVHTRC-GOSISDBHSA-N CCOC1=CC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 VQRCAUGSNVHTRC-GOSISDBHSA-N 0.000 description 1
- OQVMWOLAKDKAPN-OAHLLOKOSA-N CCOC1=CC(N2CC[C@@H](N)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@@H](N)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 OQVMWOLAKDKAPN-OAHLLOKOSA-N 0.000 description 1
- DPTYYMFFISGYBY-SFHVURJKSA-N CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 DPTYYMFFISGYBY-SFHVURJKSA-N 0.000 description 1
- NMHNYAZTEJQECB-SFHVURJKSA-N CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(C)C)C=CC=C2)=C(Cl)C=N1 NMHNYAZTEJQECB-SFHVURJKSA-N 0.000 description 1
- CVESRMDKKTYQDQ-SFHVURJKSA-N CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 CVESRMDKKTYQDQ-SFHVURJKSA-N 0.000 description 1
- AJPIDHFXAABXDV-SFHVURJKSA-N CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 AJPIDHFXAABXDV-SFHVURJKSA-N 0.000 description 1
- CVAGIQYSSHBLOZ-QFIPXVFZSA-N CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 CVAGIQYSSHBLOZ-QFIPXVFZSA-N 0.000 description 1
- UHMHEHNGYBNRAA-SFHVURJKSA-N CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 UHMHEHNGYBNRAA-SFHVURJKSA-N 0.000 description 1
- PVMSCHTZGYKSAN-SFHVURJKSA-N CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 PVMSCHTZGYKSAN-SFHVURJKSA-N 0.000 description 1
- HUZXXXGCIFRYFW-QFIPXVFZSA-N CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 HUZXXXGCIFRYFW-QFIPXVFZSA-N 0.000 description 1
- LORPAHRPEGOUQK-NRFANRHFSA-N CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](N(C)C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 LORPAHRPEGOUQK-NRFANRHFSA-N 0.000 description 1
- ZNOYMAUISBRYOL-KRWDZBQOSA-N CCOC1=CC(N2CC[C@H](NC)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](NC)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 ZNOYMAUISBRYOL-KRWDZBQOSA-N 0.000 description 1
- OCXGPSBKMWFEAD-INIZCTEOSA-N CCOC1=CC(N2CC[C@H](NC)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(N2CC[C@H](NC)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 OCXGPSBKMWFEAD-INIZCTEOSA-N 0.000 description 1
- BTXCAXBACOHZKX-UHFFFAOYSA-N CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 BTXCAXBACOHZKX-UHFFFAOYSA-N 0.000 description 1
- SFZZTFBAMOSYLV-UHFFFAOYSA-N CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 SFZZTFBAMOSYLV-UHFFFAOYSA-N 0.000 description 1
- BDBFVCHZQYJNNG-UHFFFAOYSA-N CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 Chemical compound CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(F)C=N1 BDBFVCHZQYJNNG-UHFFFAOYSA-N 0.000 description 1
- FJYWXSPPDFIDEF-UHFFFAOYSA-N CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCC(C)(C)CN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 FJYWXSPPDFIDEF-UHFFFAOYSA-N 0.000 description 1
- YPCCCZAFXYHWEY-UHFFFAOYSA-N CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 YPCCCZAFXYHWEY-UHFFFAOYSA-N 0.000 description 1
- MZRIVRBDOIAKTD-UHFFFAOYSA-N CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 MZRIVRBDOIAKTD-UHFFFAOYSA-N 0.000 description 1
- WAGHIKDWWYINDC-UHFFFAOYSA-N CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 WAGHIKDWWYINDC-UHFFFAOYSA-N 0.000 description 1
- IGNBMGVKUBEPJW-UHFFFAOYSA-N CCOC1=CC(NCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 IGNBMGVKUBEPJW-UHFFFAOYSA-N 0.000 description 1
- CGFIAXAFIPPMJM-UHFFFAOYSA-N CCOC1=CC(NCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(NCCN(C)C)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 CGFIAXAFIPPMJM-UHFFFAOYSA-N 0.000 description 1
- BTKWYYOSUMLQOQ-OAQYLSRUSA-N CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 BTKWYYOSUMLQOQ-OAQYLSRUSA-N 0.000 description 1
- YVRUWEWBQBNQLG-GOSISDBHSA-N CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 YVRUWEWBQBNQLG-GOSISDBHSA-N 0.000 description 1
- YOYCVJKZYMLVQC-OAQYLSRUSA-N CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 YOYCVJKZYMLVQC-OAQYLSRUSA-N 0.000 description 1
- JKNUAMWKSRVARZ-HXUWFJFHSA-N CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 JKNUAMWKSRVARZ-HXUWFJFHSA-N 0.000 description 1
- NLGVFVMNYZVFJK-OAQYLSRUSA-N CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 NLGVFVMNYZVFJK-OAQYLSRUSA-N 0.000 description 1
- JQDMFNREMMQLLX-HXUWFJFHSA-N CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 JQDMFNREMMQLLX-HXUWFJFHSA-N 0.000 description 1
- BTKWYYOSUMLQOQ-NRFANRHFSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 BTKWYYOSUMLQOQ-NRFANRHFSA-N 0.000 description 1
- XWZDGGDSFVKQGT-IBGZPJMESA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(C)C)C=CC=C2)=C(Cl)C=N1 XWZDGGDSFVKQGT-IBGZPJMESA-N 0.000 description 1
- YOYCVJKZYMLVQC-NRFANRHFSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC(CC)CC)C=CC=C2)=C(Cl)C=N1 YOYCVJKZYMLVQC-NRFANRHFSA-N 0.000 description 1
- SPQWOWVUXDLRDL-KRWDZBQOSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Br)C=N1 SPQWOWVUXDLRDL-KRWDZBQOSA-N 0.000 description 1
- KROIGLIAHHJUNF-KRWDZBQOSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC)C=CC=C2)=C(Cl)C=N1 KROIGLIAHHJUNF-KRWDZBQOSA-N 0.000 description 1
- NLGVFVMNYZVFJK-NRFANRHFSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 NLGVFVMNYZVFJK-NRFANRHFSA-N 0.000 description 1
- JQDMFNREMMQLLX-FQEVSTJZSA-N CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CCOC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 JQDMFNREMMQLLX-FQEVSTJZSA-N 0.000 description 1
- MQHVIPCGOFIBCW-UHFFFAOYSA-N CCOC1=CC=C(N2CCOCC2)C=C1C Chemical compound CCOC1=CC=C(N2CCOCC2)C=C1C MQHVIPCGOFIBCW-UHFFFAOYSA-N 0.000 description 1
- XMAUADUSJJMFED-UHFFFAOYSA-N CCOC1=CC=C(N2CCOCC2)C=C1N Chemical compound CCOC1=CC=C(N2CCOCC2)C=C1N XMAUADUSJJMFED-UHFFFAOYSA-N 0.000 description 1
- ACPSRGSNNINXMS-UHFFFAOYSA-N CCOC1=CC=C(N2CCOCC2)C=C1[N+](=O)[O-] Chemical compound CCOC1=CC=C(N2CCOCC2)C=C1[N+](=O)[O-] ACPSRGSNNINXMS-UHFFFAOYSA-N 0.000 description 1
- JCCSSGCBRVDZJX-UHFFFAOYSA-N CCOC1=CC=C(OC)C=C1C Chemical compound CCOC1=CC=C(OC)C=C1C JCCSSGCBRVDZJX-UHFFFAOYSA-N 0.000 description 1
- IRRIDSMXMHAYOV-UHFFFAOYSA-N CCOC1=CC=C(OC)C=C1N Chemical compound CCOC1=CC=C(OC)C=C1N IRRIDSMXMHAYOV-UHFFFAOYSA-N 0.000 description 1
- ZPGYSLMPUPBSDK-UHFFFAOYSA-N CCOC1=CC=C(OC)C=C1[N+](=O)[O-] Chemical compound CCOC1=CC=C(OC)C=C1[N+](=O)[O-] ZPGYSLMPUPBSDK-UHFFFAOYSA-N 0.000 description 1
- YIFVHORAWWWSQT-UHFFFAOYSA-N CCOC1=CC=C(OCC)C(C)=C1 Chemical compound CCOC1=CC=C(OCC)C(C)=C1 YIFVHORAWWWSQT-UHFFFAOYSA-N 0.000 description 1
- UBMAQSNTAJDCBF-UHFFFAOYSA-N CCOC1=NC=C(C(=O)N2CCOCC2)C=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)N(C)C)=N1 Chemical compound CCOC1=NC=C(C(=O)N2CCOCC2)C=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)N(C)C)=N1 UBMAQSNTAJDCBF-UHFFFAOYSA-N 0.000 description 1
- SMUDATNLZVHJGF-UHFFFAOYSA-N CCOC1=NC=NC(C)=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)NC)=N1 Chemical compound CCOC1=NC=NC(C)=C1NC1=NC=C(Cl)C(NC2=CC=CC=C2S(=O)(=O)NC)=N1 SMUDATNLZVHJGF-UHFFFAOYSA-N 0.000 description 1
- VVEPSXXQNLXMPA-UHFFFAOYSA-N CCOCCOC1CCN(C2=CC(OC)=C(C)C=C2)CC1 Chemical compound CCOCCOC1CCN(C2=CC(OC)=C(C)C=C2)CC1 VVEPSXXQNLXMPA-UHFFFAOYSA-N 0.000 description 1
- LGLISYPJDNQVTE-UHFFFAOYSA-N CCOCCOC1CCN(C2=CC=C(N)C(OC)=C2)CC1 Chemical compound CCOCCOC1CCN(C2=CC=C(N)C(OC)=C2)CC1 LGLISYPJDNQVTE-UHFFFAOYSA-N 0.000 description 1
- KMQIBKWSOIKCTB-UHFFFAOYSA-N CCOCCOC1CCN(C2=CC=C([NH2+]C(=O)[O-])C(OC)=C2)CC1 Chemical compound CCOCCOC1CCN(C2=CC=C([NH2+]C(=O)[O-])C(OC)=C2)CC1 KMQIBKWSOIKCTB-UHFFFAOYSA-N 0.000 description 1
- FJPMVTDPAKPRMY-UHFFFAOYSA-N CCOc(c(Nc1ncc(C)c(Nc(cccc2)c2S(NC)(=O)=O)n1)c1)cc(N2CCOCC2)c1OCC Chemical compound CCOc(c(Nc1ncc(C)c(Nc(cccc2)c2S(NC)(=O)=O)n1)c1)cc(N2CCOCC2)c1OCC FJPMVTDPAKPRMY-UHFFFAOYSA-N 0.000 description 1
- IGFYDOPRGAKWCV-UHFFFAOYSA-N CCOc(cc(cc1)N(CC2)CCC2C(N)=O)c1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Br Chemical compound CCOc(cc(cc1)N(CC2)CCC2C(N)=O)c1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Br IGFYDOPRGAKWCV-UHFFFAOYSA-N 0.000 description 1
- GAZYYMOSNVEUMW-UHFFFAOYSA-N CCOc(cc(cc1)N(CC2)CCC2N2CCCCC2)c1Nc(nc1)nc(Nc(cccc2)c2S(NC2CCC2)(=O)=O)c1Cl Chemical compound CCOc(cc(cc1)N(CC2)CCC2N2CCCCC2)c1Nc(nc1)nc(Nc(cccc2)c2S(NC2CCC2)(=O)=O)c1Cl GAZYYMOSNVEUMW-UHFFFAOYSA-N 0.000 description 1
- ZQLOODDJVJYMLM-UHFFFAOYSA-N CCOc(cc(cc1)N(CC2)CCC2N2CCCCC2)c1Nc(nc1)nc(Nc(cccc2)c2S(NCC2CC2)(=O)=O)c1Cl Chemical compound CCOc(cc(cc1)N(CC2)CCC2N2CCCCC2)c1Nc(nc1)nc(Nc(cccc2)c2S(NCC2CC2)(=O)=O)c1Cl ZQLOODDJVJYMLM-UHFFFAOYSA-N 0.000 description 1
- SJXLTWYDEAIKFI-JOCHJYFZSA-N CCOc(cc(cc1)N(CC2)C[C@@H]2N(C)C)c1Nc(nc1)nc(Nc(cccc2)c2S(C2CCCCC2)(=O)=O)c1Cl Chemical compound CCOc(cc(cc1)N(CC2)C[C@@H]2N(C)C)c1Nc(nc1)nc(Nc(cccc2)c2S(C2CCCCC2)(=O)=O)c1Cl SJXLTWYDEAIKFI-JOCHJYFZSA-N 0.000 description 1
- PIKBRRCIFHEOKB-FQEVSTJZSA-N CCOc(cc(cc1)N(CC2)C[C@H]2N(C)C)c1Nc(nc1)nc(Nc(cccc2)c2S(C(C)C)(=O)=O)c1Cl Chemical compound CCOc(cc(cc1)N(CC2)C[C@H]2N(C)C)c1Nc(nc1)nc(Nc(cccc2)c2S(C(C)C)(=O)=O)c1Cl PIKBRRCIFHEOKB-FQEVSTJZSA-N 0.000 description 1
- FULYHPMNRTZYOR-UHFFFAOYSA-N CCOc(cc(cc1)N2CCOCC2)c1Nc(nc1)nc(Nc(cccc2)c2S(NC)(=O)=O)c1Br Chemical compound CCOc(cc(cc1)N2CCOCC2)c1Nc(nc1)nc(Nc(cccc2)c2S(NC)(=O)=O)c1Br FULYHPMNRTZYOR-UHFFFAOYSA-N 0.000 description 1
- DFGRPFSAGANGTC-UHFFFAOYSA-N CCOc(cc(cc1)OC2CCN(C)CC2)c1Nc(nc1Nc(cccc2)c2S(NC)(=O)=O)ncc1Cl Chemical compound CCOc(cc(cc1)OC2CCN(C)CC2)c1Nc(nc1Nc(cccc2)c2S(NC)(=O)=O)ncc1Cl DFGRPFSAGANGTC-UHFFFAOYSA-N 0.000 description 1
- QVECVDYLPBHGQG-UHFFFAOYSA-N CCOc1cc(N(CC2)CCC2N2CCN(C)CC2)ccc1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Br Chemical compound CCOc1cc(N(CC2)CCC2N2CCN(C)CC2)ccc1Nc(nc1Nc2ccccc2S(NC)(=O)=O)ncc1Br QVECVDYLPBHGQG-UHFFFAOYSA-N 0.000 description 1
- NRRSOZPNWBVFAO-UHFFFAOYSA-N CCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC(F)=C1 Chemical compound CCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC(F)=C1 NRRSOZPNWBVFAO-UHFFFAOYSA-N 0.000 description 1
- NAQPEHULFLUFIH-UHFFFAOYSA-N CCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 Chemical compound CCS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 NAQPEHULFLUFIH-UHFFFAOYSA-N 0.000 description 1
- ZBIGXRRIWFOTQE-UHFFFAOYSA-N CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 Chemical compound CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 ZBIGXRRIWFOTQE-UHFFFAOYSA-N 0.000 description 1
- HOMDQXILZNSCFW-UHFFFAOYSA-N CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 HOMDQXILZNSCFW-UHFFFAOYSA-N 0.000 description 1
- HCOFPVUXRXERKJ-UHFFFAOYSA-N CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 HCOFPVUXRXERKJ-UHFFFAOYSA-N 0.000 description 1
- VXQQZLKVSVQXKJ-UHFFFAOYSA-N CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 Chemical compound CCS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 VXQQZLKVSVQXKJ-UHFFFAOYSA-N 0.000 description 1
- TXXUNFWPHZVDGT-UHFFFAOYSA-N CCS(=O)(=O)C1=C(NC2=NC(Cl)=NC=C2Br)C=CC=C1.CCS(=O)(=O)C1=C(NC2=NC(NC3=C(OC)C=C(OC)C=C3)=NC=C2Br)C=CC=C1 Chemical compound CCS(=O)(=O)C1=C(NC2=NC(Cl)=NC=C2Br)C=CC=C1.CCS(=O)(=O)C1=C(NC2=NC(NC3=C(OC)C=C(OC)C=C3)=NC=C2Br)C=CC=C1 TXXUNFWPHZVDGT-UHFFFAOYSA-N 0.000 description 1
- CYIJQEUFACHWQJ-UHFFFAOYSA-N CCc(cc(cc1)N2CCOCC2)c1Nc(nc1Nc2ccccc2S(NC2CCCC2)(=O)=O)ncc1Cl Chemical compound CCc(cc(cc1)N2CCOCC2)c1Nc(nc1Nc2ccccc2S(NC2CCCC2)(=O)=O)ncc1Cl CYIJQEUFACHWQJ-UHFFFAOYSA-N 0.000 description 1
- RJRFGEJABIHUQL-UHFFFAOYSA-N CN(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CN(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 RJRFGEJABIHUQL-UHFFFAOYSA-N 0.000 description 1
- ISXBYTUJHIQRJN-UHFFFAOYSA-N CN(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CN(C)CC1=C(NC2=C(Cl)C=NC(NC3=C(OC(C)(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 ISXBYTUJHIQRJN-UHFFFAOYSA-N 0.000 description 1
- LWPNREIDLSOBKQ-UHFFFAOYSA-N CN(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3C(F)(F)F)=N2)C=CC=C1 Chemical compound CN(C)S(=O)(=O)C1=C(CC2=C(Cl)C=NC(CC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3C(F)(F)F)=N2)C=CC=C1 LWPNREIDLSOBKQ-UHFFFAOYSA-N 0.000 description 1
- OHLLDOZZCXSJEK-QHCPKHFHSA-N CN(C)[C@H]1CCN(C2=CC=C(CC3=NC(CC4=C(S(=O)(=O)N(C)C)C=CC=C4)=C(Cl)C=N3)C(C#N)=C2)C1 Chemical compound CN(C)[C@H]1CCN(C2=CC=C(CC3=NC(CC4=C(S(=O)(=O)N(C)C)C=CC=C4)=C(Cl)C=N3)C(C#N)=C2)C1 OHLLDOZZCXSJEK-QHCPKHFHSA-N 0.000 description 1
- MGIBJLGALTUIIY-NRFANRHFSA-N CN(C)[C@H]1CCN(C2=CC=C(CC3=NC(CC4=C(S(=O)(=O)N(C)C)C=CC=C4)=C(Cl)C=N3)C(C(F)(F)F)=C2)C1 Chemical compound CN(C)[C@H]1CCN(C2=CC=C(CC3=NC(CC4=C(S(=O)(=O)N(C)C)C=CC=C4)=C(Cl)C=N3)C(C(F)(F)F)=C2)C1 MGIBJLGALTUIIY-NRFANRHFSA-N 0.000 description 1
- UUOMUKUKENQLGL-UHFFFAOYSA-N CN.CNC(=O)C1=C(N)C=CC=C1.O=C1CC2=CC=CC=C2C(=O)O1.OC1CCCO1 Chemical compound CN.CNC(=O)C1=C(N)C=CC=C1.O=C1CC2=CC=CC=C2C(=O)O1.OC1CCCO1 UUOMUKUKENQLGL-UHFFFAOYSA-N 0.000 description 1
- TXFMIQKWNDSWAQ-UHFFFAOYSA-N CN1CC2=CC=CC(N)=C2C1=O.CN1CC2=CC=CC(NC3=C(Cl)C=NC(Cl)=N3)=C2C1=O.CN1CC2=CC=CC([N+](=O)[O-])=C2C1=O.COC(=O)C1=C([N+](=O)[O-])C=CC=C1CBr Chemical compound CN1CC2=CC=CC(N)=C2C1=O.CN1CC2=CC=CC(NC3=C(Cl)C=NC(Cl)=N3)=C2C1=O.CN1CC2=CC=CC([N+](=O)[O-])=C2C1=O.COC(=O)C1=C([N+](=O)[O-])C=CC=C1CBr TXFMIQKWNDSWAQ-UHFFFAOYSA-N 0.000 description 1
- GPASDLXNNAPVRX-UHFFFAOYSA-N CN1CCC(N2CCCCC2)CC1 Chemical compound CN1CCC(N2CCCCC2)CC1 GPASDLXNNAPVRX-UHFFFAOYSA-N 0.000 description 1
- UUXDUMJQYYVOOD-UHFFFAOYSA-N CN1CCC(O)CC1.COC1=C([N+](=O)[O-])C=CC(F)=C1.COC1=C([N+](=O)[O-])C=CC(OC2CCN(C)CC2)=C1 Chemical compound CN1CCC(O)CC1.COC1=C([N+](=O)[O-])C=CC(F)=C1.COC1=C([N+](=O)[O-])C=CC(OC2CCN(C)CC2)=C1 UUXDUMJQYYVOOD-UHFFFAOYSA-N 0.000 description 1
- RXYPXQSKLGGKOL-UHFFFAOYSA-N CN1CCN(C)CC1 Chemical compound CN1CCN(C)CC1 RXYPXQSKLGGKOL-UHFFFAOYSA-N 0.000 description 1
- SJRJJKPEHAURKC-UHFFFAOYSA-N CN1CCOCC1 Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 1
- PMRUFZSRMCUNMG-UHFFFAOYSA-N CNC(=O)C1=C(N)C=CC=C1.CNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.ClC1=NC(Cl)=C(Cl)C=N1 Chemical compound CNC(=O)C1=C(N)C=CC=C1.CNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.ClC1=NC(Cl)=C(Cl)C=N1 PMRUFZSRMCUNMG-UHFFFAOYSA-N 0.000 description 1
- FUDRAYXWDWGURS-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Br)C=NC(Cl)=N2)C=CC=C1 Chemical compound CNC(=O)C1=C(NC2=C(Br)C=NC(Cl)=N2)C=CC=C1 FUDRAYXWDWGURS-UHFFFAOYSA-N 0.000 description 1
- NUDXETSCRLQHKB-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Br)C=NC(NC)=N2)C=CC=C1 Chemical compound CNC(=O)C1=C(NC2=C(Br)C=NC(NC)=N2)C=CC=C1 NUDXETSCRLQHKB-UHFFFAOYSA-N 0.000 description 1
- RSWXNYLWAQFEAV-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.CNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1.COC1=C(N)C=CC(N2CCOCC2)=C1 Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.CNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1.COC1=C(N)C=CC(N2CCOCC2)=C1 RSWXNYLWAQFEAV-UHFFFAOYSA-N 0.000 description 1
- QFWNQBDIOVRQIY-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.COC1=C(N)C=CC(N2CCOCC2)=C1.COC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(C(=O)O)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1.COC1=C(N)C=CC(N2CCOCC2)=C1.COC1=CC(N2CCOCC2)=CC=C1NC1=NC(NC2=C(C(=O)O)C=CC=C2)=C(Cl)C=N1 QFWNQBDIOVRQIY-UHFFFAOYSA-N 0.000 description 1
- LSEQGQXEAWCOTK-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC(F)=C1 Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC(F)=C1 LSEQGQXEAWCOTK-UHFFFAOYSA-N 0.000 description 1
- NOIIJRPPFFYVMX-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC(N2CCCC2)=C1 Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC(N2CCCC2)=C1 NOIIJRPPFFYVMX-UHFFFAOYSA-N 0.000 description 1
- XLWDGSBZNVBLKP-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC(N2CCOCC2)=C1 Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC(N2CCOCC2)=C1 XLWDGSBZNVBLKP-UHFFFAOYSA-N 0.000 description 1
- YLLFOVCKELSSSJ-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1 YLLFOVCKELSSSJ-UHFFFAOYSA-N 0.000 description 1
- CQRPRFQGLZOIGP-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1C Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(NC)=N2)C=CC=C1C CQRPRFQGLZOIGP-UHFFFAOYSA-N 0.000 description 1
- YMOFNLTXTIHXNL-UHFFFAOYSA-N CNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(F)C=C3OC)=N2)C=CC(C)=C1 Chemical compound CNC(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(F)C=C3OC)=N2)C=CC(C)=C1 YMOFNLTXTIHXNL-UHFFFAOYSA-N 0.000 description 1
- UHVQUSZUAHKJJU-UHFFFAOYSA-N CNC(=O)C1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CNC(=O)C1=C(NC2=NC(NC3=C(CC(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 UHVQUSZUAHKJJU-UHFFFAOYSA-N 0.000 description 1
- APAPCSDBUFHSRA-UHFFFAOYSA-N CNC(=O)C1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound CNC(=O)C1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 APAPCSDBUFHSRA-UHFFFAOYSA-N 0.000 description 1
- JAIPCBJEQDCXHX-UHFFFAOYSA-N CNC(=O)C1=CC(F)=C(F)C=C1C Chemical compound CNC(=O)C1=CC(F)=C(F)C=C1C JAIPCBJEQDCXHX-UHFFFAOYSA-N 0.000 description 1
- BFVXXBKDASYYKF-UHFFFAOYSA-N CNC(=O)C1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC)=NC=C1Cl Chemical compound CNC(=O)C1=CC(N2CCN(C)CC2)=CC=C1NC1=NC(NC)=NC=C1Cl BFVXXBKDASYYKF-UHFFFAOYSA-N 0.000 description 1
- ZESUBTJUTLCKRD-UHFFFAOYSA-N CNC(=O)C1CCN(C2=CC(OC)=C(C)C=C2)CC1 Chemical compound CNC(=O)C1CCN(C2=CC(OC)=C(C)C=C2)CC1 ZESUBTJUTLCKRD-UHFFFAOYSA-N 0.000 description 1
- NJIFTTMFAIBXIO-UHFFFAOYSA-N CNC(=O)C1CCN(C2=CC(OC)=C(N)C=C2)CC1 Chemical compound CNC(=O)C1CCN(C2=CC(OC)=C(N)C=C2)CC1 NJIFTTMFAIBXIO-UHFFFAOYSA-N 0.000 description 1
- WAQKSHWNDWJNJN-UHFFFAOYSA-N CNC(=O)C1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 Chemical compound CNC(=O)C1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 WAQKSHWNDWJNJN-UHFFFAOYSA-N 0.000 description 1
- NZHRQPHBIHJYBX-UHFFFAOYSA-N CNC(=O)N1CCN(C2=CC(OC)=C(C)C=C2)CC1 Chemical compound CNC(=O)N1CCN(C2=CC(OC)=C(C)C=C2)CC1 NZHRQPHBIHJYBX-UHFFFAOYSA-N 0.000 description 1
- CZCUSEXWTVLUCJ-UHFFFAOYSA-N CNC(=O)N1CCN(C2=CC(OC)=C(N)C=C2)CC1 Chemical compound CNC(=O)N1CCN(C2=CC(OC)=C(N)C=C2)CC1 CZCUSEXWTVLUCJ-UHFFFAOYSA-N 0.000 description 1
- RHSRRTXTOAAHKK-UHFFFAOYSA-N CNC(=O)N1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 Chemical compound CNC(=O)N1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 RHSRRTXTOAAHKK-UHFFFAOYSA-N 0.000 description 1
- RMQGDQCMOBFTOR-UHFFFAOYSA-N CNC(c(cc(cc1)N(CC2)CCC2N2CCCCC2)c1Nc(nc1)nc(Nc(cccc2)c2SN(C)C)c1Cl)=O Chemical compound CNC(c(cc(cc1)N(CC2)CCC2N2CCCCC2)c1Nc(nc1)nc(Nc(cccc2)c2SN(C)C)c1Cl)=O RMQGDQCMOBFTOR-UHFFFAOYSA-N 0.000 description 1
- ZSYZYSTURIOXLI-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 ZSYZYSTURIOXLI-UHFFFAOYSA-N 0.000 description 1
- HLIWZYXZFHGZJM-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)C3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)C3CC3)C=CC=C2)=C(Cl)C=N1 HLIWZYXZFHGZJM-UHFFFAOYSA-N 0.000 description 1
- HXDVQZNGXGQFOA-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)C3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)C3CCCC3)C=CC=C2)=C(Cl)C=N1 HXDVQZNGXGQFOA-UHFFFAOYSA-N 0.000 description 1
- JIJUALBXNUSXEB-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)CC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)CC3CCC3)C=CC=C2)=C(Cl)C=N1 JIJUALBXNUSXEB-UHFFFAOYSA-N 0.000 description 1
- ZWCQLPZKXLGHGT-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)CC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)CC3CCCC3)C=CC=C2)=C(Cl)C=N1 ZWCQLPZKXLGHGT-UHFFFAOYSA-N 0.000 description 1
- VFCBQXJJQFNRFM-UHFFFAOYSA-N CNC1=NC(NC2=C(S(=O)(=O)CCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(=O)(=O)CCC(C)C)C=CC=C2)=C(Cl)C=N1 VFCBQXJJQFNRFM-UHFFFAOYSA-N 0.000 description 1
- JPJMLYNQUJDKGW-UHFFFAOYSA-N CNC1=NC(NC2=C(S(C)(=O)=O)C=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C(S(C)(=O)=O)C=CC=C2)=C(Cl)C=N1 JPJMLYNQUJDKGW-UHFFFAOYSA-N 0.000 description 1
- WWMRORBOHFBFEA-UHFFFAOYSA-N CNC1=NC(NC2=C3C(=O)N(C)CC3=CC=C2)=C(Cl)C=N1 Chemical compound CNC1=NC(NC2=C3C(=O)N(C)CC3=CC=C2)=C(Cl)C=N1 WWMRORBOHFBFEA-UHFFFAOYSA-N 0.000 description 1
- NXBYXSBRRYPRCB-UHFFFAOYSA-N CNC1CCN(C2=CC(OC)=C(N)C=C2)C1 Chemical compound CNC1CCN(C2=CC(OC)=C(N)C=C2)C1 NXBYXSBRRYPRCB-UHFFFAOYSA-N 0.000 description 1
- JKORNSZKWXJSPS-UHFFFAOYSA-N CNC1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)C1 Chemical compound CNC1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)C1 JKORNSZKWXJSPS-UHFFFAOYSA-N 0.000 description 1
- ATWKUBNPICAIKR-UHFFFAOYSA-N CNC1CCN(C2=CC=C(C)C(OC)=C2F)C1 Chemical compound CNC1CCN(C2=CC=C(C)C(OC)=C2F)C1 ATWKUBNPICAIKR-UHFFFAOYSA-N 0.000 description 1
- TTZKDEYUQOSMJM-UHFFFAOYSA-N CNCC1=C(NC2=C(Br)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Br)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 TTZKDEYUQOSMJM-UHFFFAOYSA-N 0.000 description 1
- SIOVFXVSHXTWPU-UHFFFAOYSA-N CNCC1=C(NC2=C(Br)C=NC(NC3=C(C)C=C(OC4CCN(C(C)C)C4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Br)C=NC(NC3=C(C)C=C(OC4CCN(C(C)C)C4)C=C3)=N2)C=CC=C1 SIOVFXVSHXTWPU-UHFFFAOYSA-N 0.000 description 1
- GKFQTTNJWQVJMX-UHFFFAOYSA-N CNCC1=C(NC2=C(Br)C=NC(NC3=C(C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Br)C=NC(NC3=C(C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 GKFQTTNJWQVJMX-UHFFFAOYSA-N 0.000 description 1
- HJLBCBHWJDFPGG-UHFFFAOYSA-N CNCC1=C(NC2=C(Br)C=NC(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Br)C=NC(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 HJLBCBHWJDFPGG-UHFFFAOYSA-N 0.000 description 1
- PLUSWYOUVDJAJW-UHFFFAOYSA-N CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 PLUSWYOUVDJAJW-UHFFFAOYSA-N 0.000 description 1
- NIZZXCVNTAGHRS-UHFFFAOYSA-N CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 NIZZXCVNTAGHRS-UHFFFAOYSA-N 0.000 description 1
- XHUFLKXWRGDEQB-UHFFFAOYSA-N CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(OC4CCN(C(C)C)C4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(OC4CCN(C(C)C)C4)C=C3)=N2)C=CC=C1 XHUFLKXWRGDEQB-UHFFFAOYSA-N 0.000 description 1
- YTNRRIMNUWJNRA-UHFFFAOYSA-N CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Cl)C=NC(NC3=C(C)C=C(OC4CCN(C)CC4)C=C3)=N2)C=CC=C1 YTNRRIMNUWJNRA-UHFFFAOYSA-N 0.000 description 1
- QBOVTZJSZJZEBY-UHFFFAOYSA-N CNCC1=C(NC2=C(Cl)C=NC(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNCC1=C(NC2=C(Cl)C=NC(NC3=C(OC)C=C(N4CCC(N5CCCCC5)CC4)C=C3)=N2)C=CC=C1 QBOVTZJSZJZEBY-UHFFFAOYSA-N 0.000 description 1
- JSXITDJRMUJGNK-NRFANRHFSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(CC3=C(OC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(CC3=C(OC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 JSXITDJRMUJGNK-NRFANRHFSA-N 0.000 description 1
- HCPLJTFWHJREKI-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(Cl)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(Cl)=N2)C=CC=C1 HCPLJTFWHJREKI-UHFFFAOYSA-N 0.000 description 1
- GGMNSZGMQUWEAW-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(C(C)(C)C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(C(C)(C)C)=CC=C3OC)=N2)C=CC=C1 GGMNSZGMQUWEAW-UHFFFAOYSA-N 0.000 description 1
- BPYUKUPARFNIGG-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(C)=CC=C3OC)=N2)C=CC=C1 BPYUKUPARFNIGG-UHFFFAOYSA-N 0.000 description 1
- GFPHILWUWDROAO-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(OCCN4CCCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(OCCN4CCCC4)=CC=C3OC)=N2)C=CC=C1 GFPHILWUWDROAO-UHFFFAOYSA-N 0.000 description 1
- JJEBJAFGKXTQHS-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(OCCN4CCCCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(OCCN4CCCCC4)=CC=C3OC)=N2)C=CC=C1 JJEBJAFGKXTQHS-UHFFFAOYSA-N 0.000 description 1
- IMRNQLWZPDMKQN-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(OCCN4CCCCCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC(OCCN4CCCCCC4)=CC=C3OC)=N2)C=CC=C1 IMRNQLWZPDMKQN-UHFFFAOYSA-N 0.000 description 1
- XYSDRLNRGRPZNR-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(C(=O)N4CCN(C)CC4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(C(=O)N4CCN(C)CC4)C=C3OC)=N2)C=CC=C1 XYSDRLNRGRPZNR-UHFFFAOYSA-N 0.000 description 1
- WEXHNOBKFGXGHB-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 WEXHNOBKFGXGHB-UHFFFAOYSA-N 0.000 description 1
- FEBOEFTVPRRXTN-MRXNPFEDSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(O[C@@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(O[C@@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 FEBOEFTVPRRXTN-MRXNPFEDSA-N 0.000 description 1
- FEBOEFTVPRRXTN-INIZCTEOSA-N CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(O[C@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=C(O[C@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 FEBOEFTVPRRXTN-INIZCTEOSA-N 0.000 description 1
- IHKJNQADFPTQHO-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(C#N)C=NC(CC3=C(OC)C=C(N4CCOCC4)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(C#N)C=NC(CC3=C(OC)C=C(N4CCOCC4)C=C3)=N2)C=CC=C1 IHKJNQADFPTQHO-UHFFFAOYSA-N 0.000 description 1
- LAEPXVJJRZVZKA-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(C(N)=O)CC4)C=C3)=N2)C=CC=C1 LAEPXVJJRZVZKA-UHFFFAOYSA-N 0.000 description 1
- JUGLQKUVVHWSAO-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CCC(N5CCN(C)CC5)CC4)C=C3)=N2)C=CC=C1 JUGLQKUVVHWSAO-UHFFFAOYSA-N 0.000 description 1
- GPFVYOHMNNOSJU-NRFANRHFSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 GPFVYOHMNNOSJU-NRFANRHFSA-N 0.000 description 1
- ARDSFWKAJKEAHG-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(NCCCN(C)C)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OC(C)C)C=C(NCCCN(C)C)C=C3)=N2)C=CC=C1 ARDSFWKAJKEAHG-UHFFFAOYSA-N 0.000 description 1
- LRSWIVSWDWIPKF-QFIPXVFZSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OCC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OCC(C)C)C=C(N4CC[C@H](N(C)C)C4)C=C3)=N2)C=CC=C1 LRSWIVSWDWIPKF-QFIPXVFZSA-N 0.000 description 1
- AMPLDOWLUPBQMQ-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OCC(C)C)C=C(NCCCN(C)C)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(CC3=C(OCC(C)C)C=C(NCCCN(C)C)C=C3)=N2)C=CC=C1 AMPLDOWLUPBQMQ-UHFFFAOYSA-N 0.000 description 1
- CUBHDXYOAVGZNN-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 CUBHDXYOAVGZNN-UHFFFAOYSA-N 0.000 description 1
- UDOMUJXTUBZNNM-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(C)=CC=C3OC)=N2)C=CC=C1 UDOMUJXTUBZNNM-UHFFFAOYSA-N 0.000 description 1
- ADKALOHGJDJRPW-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCCC4)=CC=C3OC)=N2)C=CC=C1 ADKALOHGJDJRPW-UHFFFAOYSA-N 0.000 description 1
- CDWQWCZTLMJWMA-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCCCC4)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC(OCCN4CCCCCC4)=CC=C3OC)=N2)C=CC=C1 CDWQWCZTLMJWMA-UHFFFAOYSA-N 0.000 description 1
- CTPIWWBBOZVNCS-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCCCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 CTPIWWBBOZVNCS-UHFFFAOYSA-N 0.000 description 1
- GNLVASXFGMRKGP-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCOCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(N4CCC(N5CCOCC5)CC4)C=C3OCC3CC3)=N2)C=CC=C1 GNLVASXFGMRKGP-UHFFFAOYSA-N 0.000 description 1
- JAEDWIGUUKLIPP-INIZCTEOSA-N CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(Cl)C=NC(NC3=CC=C(O[C@H]4CCN(C)C4)C=C3OC)=N2)C=CC=C1 JAEDWIGUUKLIPP-INIZCTEOSA-N 0.000 description 1
- SDMRBNHRWQFYQX-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(F)C=NC(NC3=CC(C)=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(F)C=NC(NC3=CC(C)=C(N4CCOCC4)C=C3OC)=N2)C=CC=C1 SDMRBNHRWQFYQX-UHFFFAOYSA-N 0.000 description 1
- MKHIUOGDUFRZLH-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C(F)C=NC(NC3=CC(C)=CC=C3OC)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C(F)C=NC(NC3=CC(C)=CC=C3OC)=N2)C=CC=C1 MKHIUOGDUFRZLH-UHFFFAOYSA-N 0.000 description 1
- LUVNLXYMPRWSHP-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=C([N+](=O)[O-])C=NC(Cl)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=C([N+](=O)[O-])C=NC(Cl)=N2)C=CC=C1 LUVNLXYMPRWSHP-UHFFFAOYSA-N 0.000 description 1
- LXHXJEIIXXRFQR-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=CC=NC(CC3=C(OC)C=C(N4CCOCC4)C=C3)=N2)C=CC=C1 Chemical compound CNS(=O)(=O)C1=C(NC2=CC=NC(CC3=C(OC)C=C(N4CCOCC4)C=C3)=N2)C=CC=C1 LXHXJEIIXXRFQR-UHFFFAOYSA-N 0.000 description 1
- DNAWJRSDBZNEGM-UHFFFAOYSA-N CNS(=O)(=O)C1=C(NC2=NC(Cl)=NC=C2[N+](=O)[O-])C=CC=C1.CNS(=O)(=O)C1=C(NC2=NC(NC3=CC(OC)=CC=C3OC)=NC=C2[N+](=O)[O-])C=CC=C1.[HH].[HH] Chemical compound CNS(=O)(=O)C1=C(NC2=NC(Cl)=NC=C2[N+](=O)[O-])C=CC=C1.CNS(=O)(=O)C1=C(NC2=NC(NC3=CC(OC)=CC=C3OC)=NC=C2[N+](=O)[O-])C=CC=C1.[HH].[HH] DNAWJRSDBZNEGM-UHFFFAOYSA-N 0.000 description 1
- DTKKLLDNPPLKAH-UHFFFAOYSA-N CNS(=O)(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N4CCCCC4)CC3)C3=C2OC(C)(C)C3)=N1 Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=C(Cl)C=NC(NC2=CC=C(N3CCC(N4CCCCC4)CC3)C3=C2OC(C)(C)C3)=N1 DTKKLLDNPPLKAH-UHFFFAOYSA-N 0.000 description 1
- YTPJYOFGBIOQDQ-UHFFFAOYSA-N CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=C(C)N=C(C)C=C2)=NC=C1Cl Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=C(C)N=C(C)C=C2)=NC=C1Cl YTPJYOFGBIOQDQ-UHFFFAOYSA-N 0.000 description 1
- ANRVFDDMSMNQEF-UHFFFAOYSA-N CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=C(OC(C)C)C=C(N3CCC(N(C)C)CC3)C=C2)=NC=C1Cl Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=C(OC(C)C)C=C(N3CCC(N(C)C)CC3)C=C2)=NC=C1Cl ANRVFDDMSMNQEF-UHFFFAOYSA-N 0.000 description 1
- SRCREZSUAAVJBX-UHFFFAOYSA-N CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=C(OC(C)C)C=C(N3CCN(CCN(C)C)CC3)C=C2)=NC=C1Cl Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=C(OC(C)C)C=C(N3CCN(CCN(C)C)CC3)C=C2)=NC=C1Cl SRCREZSUAAVJBX-UHFFFAOYSA-N 0.000 description 1
- OJHROYVVLWVTIX-UHFFFAOYSA-N CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=CC=CN=C2OC)=NC=C1Cl Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=CC=CN=C2OC)=NC=C1Cl OJHROYVVLWVTIX-UHFFFAOYSA-N 0.000 description 1
- YOMHVSVKOIYDPL-UHFFFAOYSA-N CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=CC=NN2C)=NC=C1Cl Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=NC(NC2=CC=NN2C)=NC=C1Cl YOMHVSVKOIYDPL-UHFFFAOYSA-N 0.000 description 1
- LCGLNKUTAGEVQW-UHFFFAOYSA-N COC Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 1
- FHNQDTFFVMRZBH-UHFFFAOYSA-N COC1=C(C)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound COC1=C(C)C=C(C(=O)N2CCN(C)CC2)C=C1 FHNQDTFFVMRZBH-UHFFFAOYSA-N 0.000 description 1
- UJCFZCTTZWHRNL-UHFFFAOYSA-N COC1=C(C)C=C(C)C=C1 Chemical compound COC1=C(C)C=C(C)C=C1 UJCFZCTTZWHRNL-UHFFFAOYSA-N 0.000 description 1
- OFRLFBNTGXOFPC-UHFFFAOYSA-N COC1=C(C)C=C(C2=CC=CC=C2)C=C1 Chemical compound COC1=C(C)C=C(C2=CC=CC=C2)C=C1 OFRLFBNTGXOFPC-UHFFFAOYSA-N 0.000 description 1
- VDYDAUQHTVCCBX-UHFFFAOYSA-N COC1=C(C)C=C(Cl)C=C1 Chemical compound COC1=C(C)C=C(Cl)C=C1 VDYDAUQHTVCCBX-UHFFFAOYSA-N 0.000 description 1
- MQAUPOIDTDCYHZ-UHFFFAOYSA-N COC1=C(C)C=C(N2CCC(N3CCOCC3)CC2)C=C1 Chemical compound COC1=C(C)C=C(N2CCC(N3CCOCC3)CC2)C=C1 MQAUPOIDTDCYHZ-UHFFFAOYSA-N 0.000 description 1
- ZXAILIWZQPOJDF-UHFFFAOYSA-N COC1=C(C)C=C(N2CCCC(C(N)=O)C2)C=C1 Chemical compound COC1=C(C)C=C(N2CCCC(C(N)=O)C2)C=C1 ZXAILIWZQPOJDF-UHFFFAOYSA-N 0.000 description 1
- WDACEDHGXYVEHH-UHFFFAOYSA-N COC1=C(C)C=C(OC2CCN(C(C)=O)CC2)C=C1 Chemical compound COC1=C(C)C=C(OC2CCN(C(C)=O)CC2)C=C1 WDACEDHGXYVEHH-UHFFFAOYSA-N 0.000 description 1
- NNCOOMYLTSCTRT-UHFFFAOYSA-N COC1=C(C)C=C(OC2CCN(C)CC2)C=C1 Chemical compound COC1=C(C)C=C(OC2CCN(C)CC2)C=C1 NNCOOMYLTSCTRT-UHFFFAOYSA-N 0.000 description 1
- SMYRBVVGVQPBNM-UHFFFAOYSA-N COC1=C(C)C=C(OC2CCNCC2)C=C1 Chemical compound COC1=C(C)C=C(OC2CCNCC2)C=C1 SMYRBVVGVQPBNM-UHFFFAOYSA-N 0.000 description 1
- RMVRYAQNKAFDTM-UHFFFAOYSA-N COC1=C(C)C=C(OCC2CCN(C)CC2)C=C1 Chemical compound COC1=C(C)C=C(OCC2CCN(C)CC2)C=C1 RMVRYAQNKAFDTM-UHFFFAOYSA-N 0.000 description 1
- FJSXDYIVOJJMSF-UHFFFAOYSA-N COC1=C(C)C=C(OCCCN2CCOCC2)C=C1 Chemical compound COC1=C(C)C=C(OCCCN2CCOCC2)C=C1 FJSXDYIVOJJMSF-UHFFFAOYSA-N 0.000 description 1
- BOQVENNRBPQPOA-UHFFFAOYSA-N COC1=C(C)C=C(OCCN2CCN(C(C)=O)CC2)C=C1 Chemical compound COC1=C(C)C=C(OCCN2CCN(C(C)=O)CC2)C=C1 BOQVENNRBPQPOA-UHFFFAOYSA-N 0.000 description 1
- YZBKLYBZIVISKE-UHFFFAOYSA-N COC1=C(C)C=C(OCCN2CCN(C)CC2)C=C1 Chemical compound COC1=C(C)C=C(OCCN2CCN(C)CC2)C=C1 YZBKLYBZIVISKE-UHFFFAOYSA-N 0.000 description 1
- JFWFKSFYDXXLLT-UHFFFAOYSA-N COC1=C(C)C=CC(C(=O)N2CCN(C(C)=O)CC2)=C1 Chemical compound COC1=C(C)C=CC(C(=O)N2CCN(C(C)=O)CC2)=C1 JFWFKSFYDXXLLT-UHFFFAOYSA-N 0.000 description 1
- SBPPRCPYQOQXNQ-UHFFFAOYSA-N COC1=C(C)C=CC(C(=O)N2CCOCC2)=C1 Chemical compound COC1=C(C)C=CC(C(=O)N2CCOCC2)=C1 SBPPRCPYQOQXNQ-UHFFFAOYSA-N 0.000 description 1
- SJZAUIVYZWPNAS-UHFFFAOYSA-N COC1=C(C)C=CC(C)=C1 Chemical compound COC1=C(C)C=CC(C)=C1 SJZAUIVYZWPNAS-UHFFFAOYSA-N 0.000 description 1
- DQUGDVIOZGPYKD-UHFFFAOYSA-N COC1=C(C)C=CC(N2C=CN=C2)=C1 Chemical compound COC1=C(C)C=CC(N2C=CN=C2)=C1 DQUGDVIOZGPYKD-UHFFFAOYSA-N 0.000 description 1
- VOXMAOQSQHRJTC-UHFFFAOYSA-N COC1=C(C)C=CC(N2C=CN=C2C)=C1 Chemical compound COC1=C(C)C=CC(N2C=CN=C2C)=C1 VOXMAOQSQHRJTC-UHFFFAOYSA-N 0.000 description 1
- LFLHRXRZQMBJQB-UHFFFAOYSA-N COC1=C(C)C=CC(N2CC(C)N(C(C)=O)C(C)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CC(C)N(C(C)=O)C(C)C2)=C1 LFLHRXRZQMBJQB-UHFFFAOYSA-N 0.000 description 1
- WSHJCQJGPHTLHR-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(CO)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(CO)CC2)=C1 WSHJCQJGPHTLHR-UHFFFAOYSA-N 0.000 description 1
- UDJDFSAGBKJSPQ-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(N3CCNCC3)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(N3CCNCC3)CC2)=C1 UDJDFSAGBKJSPQ-UHFFFAOYSA-N 0.000 description 1
- LJXJLTHJIGZIGB-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(O)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(O)C2)=C1 LJXJLTHJIGZIGB-UHFFFAOYSA-N 0.000 description 1
- XPQGPNGZBQQFSL-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCC(OC)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCC(OC)C2)=C1 XPQGPNGZBQQFSL-UHFFFAOYSA-N 0.000 description 1
- WCFDYHLPCTXJEW-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCN(C(=O)C(C)C)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCN(C(=O)C(C)C)CC2)=C1 WCFDYHLPCTXJEW-UHFFFAOYSA-N 0.000 description 1
- BQDFHVKJZVVITN-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCN(C(C)=O)C(C)(C)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCN(C(C)=O)C(C)(C)C2)=C1 BQDFHVKJZVVITN-UHFFFAOYSA-N 0.000 description 1
- SDIWAGSLCNXEKZ-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCN(C)C(=O)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCN(C)C(=O)C2)=C1 SDIWAGSLCNXEKZ-UHFFFAOYSA-N 0.000 description 1
- QRGYHRRUJOWPJL-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCN(C3CCN(C)CC3)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCN(C3CCN(C)CC3)CC2)=C1 QRGYHRRUJOWPJL-UHFFFAOYSA-N 0.000 description 1
- WJQPCSULHZJIGR-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCN(CC(=O)NC(C)C)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCN(CC(=O)NC(C)C)CC2)=C1 WJQPCSULHZJIGR-UHFFFAOYSA-N 0.000 description 1
- WIMTVJLGYWZKAL-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCN(CCO)CC2)=C1 Chemical compound COC1=C(C)C=CC(N2CCN(CCO)CC2)=C1 WIMTVJLGYWZKAL-UHFFFAOYSA-N 0.000 description 1
- OJNDYYVKKYYFBP-UHFFFAOYSA-N COC1=C(C)C=CC(N2CCNC(=O)C2)=C1 Chemical compound COC1=C(C)C=CC(N2CCNC(=O)C2)=C1 OJNDYYVKKYYFBP-UHFFFAOYSA-N 0.000 description 1
- MKBFOXWSWRVCTP-UHFFFAOYSA-N COC1=C(C)C=CC(NC(C)(C)CO)=C1 Chemical compound COC1=C(C)C=CC(NC(C)(C)CO)=C1 MKBFOXWSWRVCTP-UHFFFAOYSA-N 0.000 description 1
- OWQVXDRJQIMZQM-UHFFFAOYSA-N COC1=C(C)C=CC(NC2CCN(C)CC2)=C1 Chemical compound COC1=C(C)C=CC(NC2CCN(C)CC2)=C1 OWQVXDRJQIMZQM-UHFFFAOYSA-N 0.000 description 1
- NLJABLDNWZHHRS-UHFFFAOYSA-N COC1=C(C)C=CC(NCC(C)(C)CO)=C1 Chemical compound COC1=C(C)C=CC(NCC(C)(C)CO)=C1 NLJABLDNWZHHRS-UHFFFAOYSA-N 0.000 description 1
- YSLMFXBLZYQCMI-UHFFFAOYSA-N COC1=C(C)C=CC(NCCN2CCOCC2)=C1 Chemical compound COC1=C(C)C=CC(NCCN2CCOCC2)=C1 YSLMFXBLZYQCMI-UHFFFAOYSA-N 0.000 description 1
- QWIQPWUHQWOSLK-UHFFFAOYSA-N COC1=C(C)C=CC(OC2CCN(C(C)=O)CC2)=C1 Chemical compound COC1=C(C)C=CC(OC2CCN(C(C)=O)CC2)=C1 QWIQPWUHQWOSLK-UHFFFAOYSA-N 0.000 description 1
- JIXOKWMVCMKJKD-UHFFFAOYSA-N COC1=C(C)C=CC(OC2CCN(C(C)C)C2)=C1 Chemical compound COC1=C(C)C=CC(OC2CCN(C(C)C)C2)=C1 JIXOKWMVCMKJKD-UHFFFAOYSA-N 0.000 description 1
- JLFZSDAILPCDNJ-UHFFFAOYSA-N COC1=C(C)C=CC(OC2CCNCC2)=C1 Chemical compound COC1=C(C)C=CC(OC2CCNCC2)=C1 JLFZSDAILPCDNJ-UHFFFAOYSA-N 0.000 description 1
- LFLTWFOJTYMIAF-UHFFFAOYSA-N COC1=C(C)C=CC(OCC(C)(C)CN(C)C)=C1 Chemical compound COC1=C(C)C=CC(OCC(C)(C)CN(C)C)=C1 LFLTWFOJTYMIAF-UHFFFAOYSA-N 0.000 description 1
- YYZDOVHUDZSRHF-UHFFFAOYSA-N COC1=C(C)C=CC(OCCCN2CCN(C)CC2)=C1 Chemical compound COC1=C(C)C=CC(OCCCN2CCN(C)CC2)=C1 YYZDOVHUDZSRHF-UHFFFAOYSA-N 0.000 description 1
- QXWOZGVQRHPBSR-UHFFFAOYSA-N COC1=C(C)C=CC(OCCN2CCCC2=O)=C1 Chemical compound COC1=C(C)C=CC(OCCN2CCCC2=O)=C1 QXWOZGVQRHPBSR-UHFFFAOYSA-N 0.000 description 1
- WVQGZNRUEVFXKR-UHFFFAOYSA-N COC1=C(C)C=CC([N+](=O)[O-])=C1 Chemical compound COC1=C(C)C=CC([N+](=O)[O-])=C1 WVQGZNRUEVFXKR-UHFFFAOYSA-N 0.000 description 1
- DTFKRVXLBCAIOZ-UHFFFAOYSA-N COC1=C(C)C=CC=C1 Chemical compound COC1=C(C)C=CC=C1 DTFKRVXLBCAIOZ-UHFFFAOYSA-N 0.000 description 1
- ILHRYKXZYUAGSG-UHFFFAOYSA-N COC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound COC1=C(CC2=NC(NC3=C(S(=O)(=O)C(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 ILHRYKXZYUAGSG-UHFFFAOYSA-N 0.000 description 1
- DXLKCXDBNDTAQT-UHFFFAOYSA-N COC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound COC1=C(CC2=NC(NC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 DXLKCXDBNDTAQT-UHFFFAOYSA-N 0.000 description 1
- XKJSKDFTXSEGMH-UHFFFAOYSA-N COC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound COC1=C(CC2=NC(NC3=C(S(=O)(=O)NC4CCC4)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 XKJSKDFTXSEGMH-UHFFFAOYSA-N 0.000 description 1
- NEIDXHRXHBJITP-UHFFFAOYSA-N COC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound COC1=C(CC2=NC(NC3=C(S(=O)(=O)NCC4CC4)C=CC=C3)=C(Cl)C=N2)C=C(C(=O)N2CCN(C)CC2)C=C1 NEIDXHRXHBJITP-UHFFFAOYSA-N 0.000 description 1
- VYZUBHRSGQAROM-UHFFFAOYSA-N COC1=C(N)C=C(F)C=C1 Chemical compound COC1=C(N)C=C(F)C=C1 VYZUBHRSGQAROM-UHFFFAOYSA-N 0.000 description 1
- SFTODGRDKBRQMU-UHFFFAOYSA-N COC1=C(N)C=C(N2CCC(N3CCOCC3)CC2)C=C1 Chemical compound COC1=C(N)C=C(N2CCC(N3CCOCC3)CC2)C=C1 SFTODGRDKBRQMU-UHFFFAOYSA-N 0.000 description 1
- VWXDBOITRNAMCN-UHFFFAOYSA-N COC1=C(N)C=C(N2CCN(C(C)=O)CC2)C=C1 Chemical compound COC1=C(N)C=C(N2CCN(C(C)=O)CC2)C=C1 VWXDBOITRNAMCN-UHFFFAOYSA-N 0.000 description 1
- BZBMDGSUYMJTBZ-UHFFFAOYSA-N COC1=C(N)C=C(OCCN2CCOCC2)C=C1 Chemical compound COC1=C(N)C=C(OCCN2CCOCC2)C=C1 BZBMDGSUYMJTBZ-UHFFFAOYSA-N 0.000 description 1
- IXLZFVBZAUWBFD-UHFFFAOYSA-N COC1=C(N)C=CC(C(=O)N2CCN(C(C)=O)CC2)=C1 Chemical compound COC1=C(N)C=CC(C(=O)N2CCN(C(C)=O)CC2)=C1 IXLZFVBZAUWBFD-UHFFFAOYSA-N 0.000 description 1
- GPRUBQPFDXFIJN-UHFFFAOYSA-N COC1=C(N)C=CC(C(=O)N2CCOCC2)=C1 Chemical compound COC1=C(N)C=CC(C(=O)N2CCOCC2)=C1 GPRUBQPFDXFIJN-UHFFFAOYSA-N 0.000 description 1
- OYUZZUIRBLEIRK-UHFFFAOYSA-N COC1=C(N)C=CC(N2C=NC=N2)=C1 Chemical compound COC1=C(N)C=CC(N2C=NC=N2)=C1 OYUZZUIRBLEIRK-UHFFFAOYSA-N 0.000 description 1
- CVXCDXZIKZUASA-UHFFFAOYSA-N COC1=C(N)C=CC(N2CC(C)N(C(C)=O)C(C)C2)=C1 Chemical compound COC1=C(N)C=CC(N2CC(C)N(C(C)=O)C(C)C2)=C1 CVXCDXZIKZUASA-UHFFFAOYSA-N 0.000 description 1
- ISEWBTUTEFXAIX-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(C(N)=O)CC2)=C1 ISEWBTUTEFXAIX-UHFFFAOYSA-N 0.000 description 1
- NDZHINADVXBXJL-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(CO)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(CO)CC2)=C1 NDZHINADVXBXJL-UHFFFAOYSA-N 0.000 description 1
- STOCRCLYMPKTEC-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(N(C)C)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(N(C)C)CC2)=C1 STOCRCLYMPKTEC-UHFFFAOYSA-N 0.000 description 1
- YZDLCNZESODCNS-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(N3CCCCC3)CC2)=C1 YZDLCNZESODCNS-UHFFFAOYSA-N 0.000 description 1
- FEBQMZWXOBGBAY-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(N3CCN(C(C)=O)CC3)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(N3CCN(C(C)=O)CC3)CC2)=C1 FEBQMZWXOBGBAY-UHFFFAOYSA-N 0.000 description 1
- WDQZQPCYVLHWRA-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 WDQZQPCYVLHWRA-UHFFFAOYSA-N 0.000 description 1
- VOGIRDWDQHZNNW-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(N3CCOCC3)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(N3CCOCC3)CC2)=C1 VOGIRDWDQHZNNW-UHFFFAOYSA-N 0.000 description 1
- ZRACRPDLDZUFIO-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(O)C2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(O)C2)=C1 ZRACRPDLDZUFIO-UHFFFAOYSA-N 0.000 description 1
- PLRKSIZPNZPJOS-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(O)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(O)CC2)=C1 PLRKSIZPNZPJOS-UHFFFAOYSA-N 0.000 description 1
- JVSPAGCGSNXLKA-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCC(OC)C2)=C1 Chemical compound COC1=C(N)C=CC(N2CCC(OC)C2)=C1 JVSPAGCGSNXLKA-UHFFFAOYSA-N 0.000 description 1
- KJEDBCRHXGHTHF-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCCC(C(N)=O)C2)=C1 Chemical compound COC1=C(N)C=CC(N2CCCC(C(N)=O)C2)=C1 KJEDBCRHXGHTHF-UHFFFAOYSA-N 0.000 description 1
- JGIQKERUAPRAAI-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCN(C(=O)C(C)C)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCN(C(=O)C(C)C)CC2)=C1 JGIQKERUAPRAAI-UHFFFAOYSA-N 0.000 description 1
- PJDBCOPPXHDQNP-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCN(C(C)=O)C(C)(C)C2)=C1 Chemical compound COC1=C(N)C=CC(N2CCN(C(C)=O)C(C)(C)C2)=C1 PJDBCOPPXHDQNP-UHFFFAOYSA-N 0.000 description 1
- IYFHZZYIEKQGQT-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCN(C(C)C)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCN(C(C)C)CC2)=C1 IYFHZZYIEKQGQT-UHFFFAOYSA-N 0.000 description 1
- XVSISYHQUMYJNF-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCN(C)C(=O)C2)=C1 Chemical compound COC1=C(N)C=CC(N2CCN(C)C(=O)C2)=C1 XVSISYHQUMYJNF-UHFFFAOYSA-N 0.000 description 1
- LUYPGLRYFIVYTH-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCN(C)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCN(C)CC2)=C1 LUYPGLRYFIVYTH-UHFFFAOYSA-N 0.000 description 1
- YHBKRSOZTBGAIC-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCN(CC(=O)NC(C)C)CC2)=C1 Chemical compound COC1=C(N)C=CC(N2CCN(CC(=O)NC(C)C)CC2)=C1 YHBKRSOZTBGAIC-UHFFFAOYSA-N 0.000 description 1
- JUAIJTBNLKFRNN-UHFFFAOYSA-N COC1=C(N)C=CC(N2CCNC(=O)C2)=C1 Chemical compound COC1=C(N)C=CC(N2CCNC(=O)C2)=C1 JUAIJTBNLKFRNN-UHFFFAOYSA-N 0.000 description 1
- ACKAWQHBZXMXNX-UHFFFAOYSA-N COC1=C(N)C=CC(NCCN2CCOCC2)=C1 Chemical compound COC1=C(N)C=CC(NCCN2CCOCC2)=C1 ACKAWQHBZXMXNX-UHFFFAOYSA-N 0.000 description 1
- BRJYBWYRDYPPKM-UHFFFAOYSA-N COC1=C(N)C=CC(OC2CCN(C)CC2)=C1.COC1=C([N+](=O)[O-])C=CC(OC2CCN(C)CC2)=C1 Chemical compound COC1=C(N)C=CC(OC2CCN(C)CC2)=C1.COC1=C([N+](=O)[O-])C=CC(OC2CCN(C)CC2)=C1 BRJYBWYRDYPPKM-UHFFFAOYSA-N 0.000 description 1
- QCVOJTJHLFDBRR-UHFFFAOYSA-N COC1=C(N)C=CC(OCC2CCCN(C)C2)=C1 Chemical compound COC1=C(N)C=CC(OCC2CCCN(C)C2)=C1 QCVOJTJHLFDBRR-UHFFFAOYSA-N 0.000 description 1
- KGXAABXRXDBUFB-UHFFFAOYSA-N COC1=C(N)C=CC(OCCN2CCCC2=O)=C1 Chemical compound COC1=C(N)C=CC(OCCN2CCCC2=O)=C1 KGXAABXRXDBUFB-UHFFFAOYSA-N 0.000 description 1
- GUWNFKANOMGPMK-UHFFFAOYSA-N COC1=C(N)C=CC(OCCN2CCOCC2)=C1 Chemical compound COC1=C(N)C=CC(OCCN2CCOCC2)=C1 GUWNFKANOMGPMK-UHFFFAOYSA-N 0.000 description 1
- YBXNOQVTGQDQNQ-UHFFFAOYSA-N COC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound COC1=C(NC2=NC(NC3=C(CN(C)C)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 YBXNOQVTGQDQNQ-UHFFFAOYSA-N 0.000 description 1
- OIUDETKMSFZBKQ-UHFFFAOYSA-N COC1=C(NC2=NC(NC3=C(CNC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound COC1=C(NC2=NC(NC3=C(CNC4CCCC4)C=CC=C3)=C(Cl)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 OIUDETKMSFZBKQ-UHFFFAOYSA-N 0.000 description 1
- DNDWLQYYDCFOHI-UHFFFAOYSA-N COC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Br)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound COC1=C(NC2=NC(NC3=C(CNCC(C)C)C=CC=C3)=C(Br)C=N2)C=CC(N2CCC(N3CCCCC3)CC2)=C1 DNDWLQYYDCFOHI-UHFFFAOYSA-N 0.000 description 1
- KXMILIKHAOQNQU-SFHVURJKSA-N COC1=C(NC2=NC=C(Cl)C(NC3=C(S(=O)(=O)NC(C)C)C=CC=C3)=N2)C=CC(O[C@H]2CCN(C)C2)=C1 Chemical compound COC1=C(NC2=NC=C(Cl)C(NC3=C(S(=O)(=O)NC(C)C)C=CC=C3)=N2)C=CC(O[C@H]2CCN(C)C2)=C1 KXMILIKHAOQNQU-SFHVURJKSA-N 0.000 description 1
- LQVRZWFRAHZYNP-UHFFFAOYSA-N COC1=C([N+](=O)O)C=C(N2CCN(C(C)=O)CC2)C=C1 Chemical compound COC1=C([N+](=O)O)C=C(N2CCN(C(C)=O)CC2)C=C1 LQVRZWFRAHZYNP-UHFFFAOYSA-N 0.000 description 1
- SEBCMXBMRAVLDL-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=C(C(=O)N2CCN(C)CC2)C=C1 Chemical compound COC1=C([N+](=O)[O-])C=C(C(=O)N2CCN(C)CC2)C=C1 SEBCMXBMRAVLDL-UHFFFAOYSA-N 0.000 description 1
- KFAXRRBQIRLFOK-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=C(N2CCC(N3CCOCC3)CC2)C=C1 Chemical compound COC1=C([N+](=O)[O-])C=C(N2CCC(N3CCOCC3)CC2)C=C1 KFAXRRBQIRLFOK-UHFFFAOYSA-N 0.000 description 1
- JAFFUHCAQBFINM-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=C(O)C=C1.COC1=C([N+](=O)[O-])C=C(OC(C)=O)C=C1 Chemical compound COC1=C([N+](=O)[O-])C=C(O)C=C1.COC1=C([N+](=O)[O-])C=C(OC(C)=O)C=C1 JAFFUHCAQBFINM-UHFFFAOYSA-N 0.000 description 1
- BRULBBDHHQFOIJ-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=C(O)C=C1.COC1=C([N+](=O)[O-])C=C(OCCN2CCOCC2)C=C1 Chemical compound COC1=C([N+](=O)[O-])C=C(O)C=C1.COC1=C([N+](=O)[O-])C=C(OCCN2CCOCC2)C=C1 BRULBBDHHQFOIJ-UHFFFAOYSA-N 0.000 description 1
- KHGDOSRRJCDOFD-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=C(OC(C)=O)C=C1.COC1=CC=C(O)C=C1.COC1=CC=C(OC(C)=O)C=C1 Chemical compound COC1=C([N+](=O)[O-])C=C(OC(C)=O)C=C1.COC1=CC=C(O)C=C1.COC1=CC=C(OC(C)=O)C=C1 KHGDOSRRJCDOFD-UHFFFAOYSA-N 0.000 description 1
- ODCQLDDADGQFJE-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(C(=O)N2CCN(C(C)=O)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(C(=O)N2CCN(C(C)=O)CC2)=C1 ODCQLDDADGQFJE-UHFFFAOYSA-N 0.000 description 1
- KRZHNWCGVABBQS-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(C(=O)N2CCOCC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(C(=O)N2CCOCC2)=C1 KRZHNWCGVABBQS-UHFFFAOYSA-N 0.000 description 1
- GQDYOFSFOYOSSZ-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2C=CN=C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2C=CN=C2)=C1 GQDYOFSFOYOSSZ-UHFFFAOYSA-N 0.000 description 1
- OXNJXEKFMUFVCZ-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2C=NC=N2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2C=NC=N2)=C1 OXNJXEKFMUFVCZ-UHFFFAOYSA-N 0.000 description 1
- ADNFOMUPGAJSBT-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CC(C)N(C(C)=O)C(C)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CC(C)N(C(C)=O)C(C)C2)=C1 ADNFOMUPGAJSBT-UHFFFAOYSA-N 0.000 description 1
- PYAPEERBRXYYPN-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CC(C)NC(C)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CC(C)NC(C)C2)=C1 PYAPEERBRXYYPN-UHFFFAOYSA-N 0.000 description 1
- VWVGKNTWOBCSTI-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(=O)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(=O)CC2)=C1 VWVGKNTWOBCSTI-UHFFFAOYSA-N 0.000 description 1
- VLPZFFCCBLZJRH-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(C(N)=O)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(C(N)=O)CC2)=C1 VLPZFFCCBLZJRH-UHFFFAOYSA-N 0.000 description 1
- LHOUEXISFKGKEG-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(CO)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(CO)CC2)=C1 LHOUEXISFKGKEG-UHFFFAOYSA-N 0.000 description 1
- MOPOHRMOFIJXCP-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(N(C)C)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(N(C)C)C2)=C1 MOPOHRMOFIJXCP-UHFFFAOYSA-N 0.000 description 1
- PZVSRJZDEMTYLK-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(N(C)C)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(N(C)C)CC2)=C1 PZVSRJZDEMTYLK-UHFFFAOYSA-N 0.000 description 1
- OANMQYNIWUUWMA-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCCCC3)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCCCC3)CC2)=C1 OANMQYNIWUUWMA-UHFFFAOYSA-N 0.000 description 1
- CCVLIOWBKCEQKD-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCN(C(C)=O)CC3)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCN(C(C)=O)CC3)CC2)=C1 CCVLIOWBKCEQKD-UHFFFAOYSA-N 0.000 description 1
- HNYSXOZEEKWPNM-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCN(C)CC3)CC2)=C1 HNYSXOZEEKWPNM-UHFFFAOYSA-N 0.000 description 1
- ZAGSOGWOHGTLIX-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCOCC3)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(N3CCOCC3)CC2)=C1 ZAGSOGWOHGTLIX-UHFFFAOYSA-N 0.000 description 1
- IRPVQBHMMZEOMC-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(O)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(O)C2)=C1 IRPVQBHMMZEOMC-UHFFFAOYSA-N 0.000 description 1
- SNYOQELAUCYAKA-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(O)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(O)CC2)=C1 SNYOQELAUCYAKA-UHFFFAOYSA-N 0.000 description 1
- LHGCCUZCEIPSBQ-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCC(OC)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCC(OC)C2)=C1 LHGCCUZCEIPSBQ-UHFFFAOYSA-N 0.000 description 1
- JXWCLVKJJFFJMF-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCN(C(=O)C(C)C)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCN(C(=O)C(C)C)CC2)=C1 JXWCLVKJJFFJMF-UHFFFAOYSA-N 0.000 description 1
- YMAPYJVFNHLOND-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCN(C(C)=O)C(C)(C)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCN(C(C)=O)C(C)(C)C2)=C1 YMAPYJVFNHLOND-UHFFFAOYSA-N 0.000 description 1
- PZASQQVFJKITCK-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCN(C(C)C)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCN(C(C)C)CC2)=C1 PZASQQVFJKITCK-UHFFFAOYSA-N 0.000 description 1
- IBLGHEFOUXFKIO-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCN(C)C(=O)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCN(C)C(=O)C2)=C1 IBLGHEFOUXFKIO-UHFFFAOYSA-N 0.000 description 1
- MKTFAOFIZMORTQ-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCN(C)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCN(C)CC2)=C1 MKTFAOFIZMORTQ-UHFFFAOYSA-N 0.000 description 1
- DAKBEORPSFARMX-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCN(CC(=O)NC(C)C)CC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCN(CC(=O)NC(C)C)CC2)=C1 DAKBEORPSFARMX-UHFFFAOYSA-N 0.000 description 1
- WMUOOUCXHUBRAA-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCNC(=O)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCNC(=O)C2)=C1 WMUOOUCXHUBRAA-UHFFFAOYSA-N 0.000 description 1
- DJKFKHDMPIGVCK-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(N2CCNC(C)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(N2CCNC(C)C2)=C1 DJKFKHDMPIGVCK-UHFFFAOYSA-N 0.000 description 1
- LXSLNDIHUHAXOU-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(NCCN2CCOCC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(NCCN2CCOCC2)=C1 LXSLNDIHUHAXOU-UHFFFAOYSA-N 0.000 description 1
- RBBFTNJDSZFHGC-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(O)=C1.COC1=C([N+](=O)[O-])C=CC(OCCN2CCOCC2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(O)=C1.COC1=C([N+](=O)[O-])C=CC(OCCN2CCOCC2)=C1 RBBFTNJDSZFHGC-UHFFFAOYSA-N 0.000 description 1
- YMHOUADYBWDKNB-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(O)=C1.O=[N+]([O-])C1=C(F)C=C(O)C=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(O)=C1.O=[N+]([O-])C1=C(F)C=C(O)C=C1 YMHOUADYBWDKNB-UHFFFAOYSA-N 0.000 description 1
- IQXLYADAWVRVCP-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(OCC2CCCN(C)C2)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(OCC2CCCN(C)C2)=C1 IQXLYADAWVRVCP-UHFFFAOYSA-N 0.000 description 1
- JZVXMZTUUPPVFG-UHFFFAOYSA-N COC1=C([N+](=O)[O-])C=CC(OCCN2CCCC2=O)=C1 Chemical compound COC1=C([N+](=O)[O-])C=CC(OCCN2CCCC2=O)=C1 JZVXMZTUUPPVFG-UHFFFAOYSA-N 0.000 description 1
- NDEIOBKHXCPGLB-UHFFFAOYSA-N COC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 NDEIOBKHXCPGLB-UHFFFAOYSA-N 0.000 description 1
- JTIURBFYRIKRGK-UHFFFAOYSA-N COC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 JTIURBFYRIKRGK-UHFFFAOYSA-N 0.000 description 1
- YKPATXXNIXWMNF-UHFFFAOYSA-N COC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(C(=O)N2CCOCC2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 YKPATXXNIXWMNF-UHFFFAOYSA-N 0.000 description 1
- UNEZMMWAAMQSBE-UHFFFAOYSA-N COC1=CC(C)=C(C)C=C1C Chemical compound COC1=CC(C)=C(C)C=C1C UNEZMMWAAMQSBE-UHFFFAOYSA-N 0.000 description 1
- VCFFLBDRLPCAPF-UHFFFAOYSA-N COC1=CC(C)=C(C)C=C1OC Chemical compound COC1=CC(C)=C(C)C=C1OC VCFFLBDRLPCAPF-UHFFFAOYSA-N 0.000 description 1
- JKHZHBRJEXMITJ-UHFFFAOYSA-N COC1=CC(C)=C(OC)C=C1N1CCN(C)CC1 Chemical compound COC1=CC(C)=C(OC)C=C1N1CCN(C)CC1 JKHZHBRJEXMITJ-UHFFFAOYSA-N 0.000 description 1
- CJUWYBPEYDQEEX-UHFFFAOYSA-N COC1=CC(C)=C(OC)C=C1N1CCOCC1 Chemical compound COC1=CC(C)=C(OC)C=C1N1CCOCC1 CJUWYBPEYDQEEX-UHFFFAOYSA-N 0.000 description 1
- UUJBBNSZJILFNT-UHFFFAOYSA-N COC1=CC(C)=C(OC)C=C1OC Chemical compound COC1=CC(C)=C(OC)C=C1OC UUJBBNSZJILFNT-UHFFFAOYSA-N 0.000 description 1
- XNMKDLBXKCDHQB-UHFFFAOYSA-N COC1=CC(C2=CC(C)=C(C)C=C2)=CC=C1 Chemical compound COC1=CC(C2=CC(C)=C(C)C=C2)=CC=C1 XNMKDLBXKCDHQB-UHFFFAOYSA-N 0.000 description 1
- YFIRCQYWRVJXBL-UHFFFAOYSA-N COC1=CC(F)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CCC(F)(F)F)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(F)=CC=C1NC1=NC(NC2=C(S(=O)(=O)CCC(F)(F)F)C=CC=C2)=C(Cl)C=N1 YFIRCQYWRVJXBL-UHFFFAOYSA-N 0.000 description 1
- HJUAQLHCCJGGFK-UHFFFAOYSA-N COC1=CC(F)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N3CCOCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(F)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N3CCOCC3)C=CC=C2)=C(Cl)C=N1 HJUAQLHCCJGGFK-UHFFFAOYSA-N 0.000 description 1
- XTAKHOLATHBLNP-UHFFFAOYSA-N COC1=CC(N)=C(OC)C=C1N1CCN(C)CC1 Chemical compound COC1=CC(N)=C(OC)C=C1N1CCN(C)CC1 XTAKHOLATHBLNP-UHFFFAOYSA-N 0.000 description 1
- UPKAFSNUROXYDY-UHFFFAOYSA-N COC1=CC(N)=C(OC)C=C1N1CCOCC1 Chemical compound COC1=CC(N)=C(OC)C=C1N1CCOCC1 UPKAFSNUROXYDY-UHFFFAOYSA-N 0.000 description 1
- SXVWPPJHFOCGAD-UHFFFAOYSA-N COC1=CC(N)=C(OC)C=C1OC Chemical compound COC1=CC(N)=C(OC)C=C1OC SXVWPPJHFOCGAD-UHFFFAOYSA-N 0.000 description 1
- QVPCJUDPXYQOGR-UHFFFAOYSA-N COC1=CC(N2CCC(C#N)CC2)=CC=C1C Chemical compound COC1=CC(N2CCC(C#N)CC2)=CC=C1C QVPCJUDPXYQOGR-UHFFFAOYSA-N 0.000 description 1
- JBLKSBPLSTVGJW-UHFFFAOYSA-N COC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1C Chemical compound COC1=CC(N2CCC(N(C)C(C)=O)C2)=CC=C1C JBLKSBPLSTVGJW-UHFFFAOYSA-N 0.000 description 1
- DTZQHVIYPYAMKA-UHFFFAOYSA-N COC1=CC(N2CCC(N(C)C)C2)=CC=C1N Chemical compound COC1=CC(N2CCC(N(C)C)C2)=CC=C1N DTZQHVIYPYAMKA-UHFFFAOYSA-N 0.000 description 1
- HCHALWUUMIGDGM-UHFFFAOYSA-N COC1=CC(N2CCC(N3CCCC3)CC2)=CC=C1N Chemical compound COC1=CC(N2CCC(N3CCCC3)CC2)=CC=C1N HCHALWUUMIGDGM-UHFFFAOYSA-N 0.000 description 1
- NIOXJCJXTQMUOD-UHFFFAOYSA-N COC1=CC(N2CCC(N3CCCC3)CC2)=CC=C1[N+](=O)[O-] Chemical compound COC1=CC(N2CCC(N3CCCC3)CC2)=CC=C1[N+](=O)[O-] NIOXJCJXTQMUOD-UHFFFAOYSA-N 0.000 description 1
- ODLLBNYGOFKFCQ-UHFFFAOYSA-N COC1=CC(N2CCCC(C(N)=O)C2)=CC=C1[N+](=O)[O-] Chemical compound COC1=CC(N2CCCC(C(N)=O)C2)=CC=C1[N+](=O)[O-] ODLLBNYGOFKFCQ-UHFFFAOYSA-N 0.000 description 1
- XRKSFOVBQIIAPC-INIZCTEOSA-N COC1=CC(N2CCC[C@H]2CN2CCCC2)=CC=C1C Chemical compound COC1=CC(N2CCC[C@H]2CN2CCCC2)=CC=C1C XRKSFOVBQIIAPC-INIZCTEOSA-N 0.000 description 1
- PGMXELISHVKXPN-UHFFFAOYSA-N COC1=CC(N2CCN(CCO)CC2)=CC=C1N Chemical compound COC1=CC(N2CCN(CCO)CC2)=CC=C1N PGMXELISHVKXPN-UHFFFAOYSA-N 0.000 description 1
- DTWUAOIUBIYGMC-UHFFFAOYSA-N COC1=CC(N2CCN(CCO)CC2)=CC=C1[N+](=O)[O-] Chemical compound COC1=CC(N2CCN(CCO)CC2)=CC=C1[N+](=O)[O-] DTWUAOIUBIYGMC-UHFFFAOYSA-N 0.000 description 1
- NFHAMCQDJRDIAZ-UHFFFAOYSA-N COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 Chemical compound COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 NFHAMCQDJRDIAZ-UHFFFAOYSA-N 0.000 description 1
- WOTYRTHBTGEEBL-UHFFFAOYSA-N COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 WOTYRTHBTGEEBL-UHFFFAOYSA-N 0.000 description 1
- AHFVJFHYFOQRHF-UHFFFAOYSA-N COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Br)C=N1 Chemical compound COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Br)C=N1 AHFVJFHYFOQRHF-UHFFFAOYSA-N 0.000 description 1
- BOKKFBXRPCHEIE-UHFFFAOYSA-N COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(N2CCOCC2)=CC=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 BOKKFBXRPCHEIE-UHFFFAOYSA-N 0.000 description 1
- XWNUCZHCWPCFRC-UHFFFAOYSA-N COC1=CC(N2CCS(=O)(=O)CC2)=CC=C1C Chemical compound COC1=CC(N2CCS(=O)(=O)CC2)=CC=C1C XWNUCZHCWPCFRC-UHFFFAOYSA-N 0.000 description 1
- LJKTXIZEEZCKGH-UHFFFAOYSA-N COC1=CC(N2CCSCC2)=CC=C1C Chemical compound COC1=CC(N2CCSCC2)=CC=C1C LJKTXIZEEZCKGH-UHFFFAOYSA-N 0.000 description 1
- NTMSVSPOVCBPOY-UHFFFAOYSA-N COC1=CC(NCC(C)(C)CN(C)C)=CC=C1C Chemical compound COC1=CC(NCC(C)(C)CN(C)C)=CC=C1C NTMSVSPOVCBPOY-UHFFFAOYSA-N 0.000 description 1
- SXVFZZZETDRISD-UHFFFAOYSA-N COC1=CC(OC)=C([N+](=O)[O-])C=C1OC Chemical compound COC1=CC(OC)=C([N+](=O)[O-])C=C1OC SXVFZZZETDRISD-UHFFFAOYSA-N 0.000 description 1
- UPPZCKBNQCZMJI-UHFFFAOYSA-N COC1=CC(OCC2CCCCN2C)=CC=C1C Chemical compound COC1=CC(OCC2CCCCN2C)=CC=C1C UPPZCKBNQCZMJI-UHFFFAOYSA-N 0.000 description 1
- HFDLJSFNMLGZNM-GOSISDBHSA-N COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 HFDLJSFNMLGZNM-GOSISDBHSA-N 0.000 description 1
- IQOVKKNJOSUWBS-HXUWFJFHSA-N COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 IQOVKKNJOSUWBS-HXUWFJFHSA-N 0.000 description 1
- CEROJJHQGYIYQH-QGZVFWFLSA-N COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 CEROJJHQGYIYQH-QGZVFWFLSA-N 0.000 description 1
- XJOLFRKCWDVGQY-LJQANCHMSA-N COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCC3)C=CC=C2)=C(Cl)C=N1 XJOLFRKCWDVGQY-LJQANCHMSA-N 0.000 description 1
- JVXVCEFIYQQHJR-HXUWFJFHSA-N COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 JVXVCEFIYQQHJR-HXUWFJFHSA-N 0.000 description 1
- NWEYERDVINHCRN-LJQANCHMSA-N COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 NWEYERDVINHCRN-LJQANCHMSA-N 0.000 description 1
- HFDLJSFNMLGZNM-SFHVURJKSA-N COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C(C)C)C=CC=C2)=C(Cl)C=N1 HFDLJSFNMLGZNM-SFHVURJKSA-N 0.000 description 1
- IQOVKKNJOSUWBS-FQEVSTJZSA-N COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)C3CCCCC3)C=CC=C2)=C(Cl)C=N1 IQOVKKNJOSUWBS-FQEVSTJZSA-N 0.000 description 1
- NWEYERDVINHCRN-IBGZPJMESA-N COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC(O[C@H]2CCN(C)C2)=CC=C1NC1=NC(NC2=C(S(=O)(=O)NCC3CC3)C=CC=C2)=C(Cl)C=N1 NWEYERDVINHCRN-IBGZPJMESA-N 0.000 description 1
- PUNXUNOLLYACNX-UHFFFAOYSA-N COC1=CC([N+](=O)[O-])=C(OC)C=C1N1CCN(C)CC1 Chemical compound COC1=CC([N+](=O)[O-])=C(OC)C=C1N1CCN(C)CC1 PUNXUNOLLYACNX-UHFFFAOYSA-N 0.000 description 1
- IMMNPCLCFUZRHP-UHFFFAOYSA-N COC1=CC([N+](=O)[O-])=C(OC)C=C1N1CCOCC1 Chemical compound COC1=CC([N+](=O)[O-])=C(OC)C=C1N1CCOCC1 IMMNPCLCFUZRHP-UHFFFAOYSA-N 0.000 description 1
- RMLZGAIONDKCCF-UHFFFAOYSA-N COC1=CC=C(C)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 Chemical compound COC1=CC=C(C)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 RMLZGAIONDKCCF-UHFFFAOYSA-N 0.000 description 1
- ULTQQNVVKXTNTH-UHFFFAOYSA-N COC1=CC=C(C)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC=C(C)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 ULTQQNVVKXTNTH-UHFFFAOYSA-N 0.000 description 1
- AOAXFCRGDVFXAC-UHFFFAOYSA-N COC1=CC=C(C)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC=C(C)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 AOAXFCRGDVFXAC-UHFFFAOYSA-N 0.000 description 1
- AIROINZDCGLJBZ-UHFFFAOYSA-N COC1=CC=C(C2=CC(N)=C(C)C=C2)C=C1 Chemical compound COC1=CC=C(C2=CC(N)=C(C)C=C2)C=C1 AIROINZDCGLJBZ-UHFFFAOYSA-N 0.000 description 1
- LUJRXBPJCKLPLU-UHFFFAOYSA-N COC1=CC=C(C2=CC([N+](=O)[O-])=C(C)C=C2)C=C1 Chemical compound COC1=CC=C(C2=CC([N+](=O)[O-])=C(C)C=C2)C=C1 LUJRXBPJCKLPLU-UHFFFAOYSA-N 0.000 description 1
- LSUGZJBCDMLHSV-UHFFFAOYSA-N COC1=CC=C(C2=CC=C(OC)C(C)=C2)C=C1 Chemical compound COC1=CC=C(C2=CC=C(OC)C(C)=C2)C=C1 LSUGZJBCDMLHSV-UHFFFAOYSA-N 0.000 description 1
- NFJPORSGUNOHIG-UHFFFAOYSA-N COC1=CC=C(C2=CC=C(OC)C(N)=C2)C=C1 Chemical compound COC1=CC=C(C2=CC=C(OC)C(N)=C2)C=C1 NFJPORSGUNOHIG-UHFFFAOYSA-N 0.000 description 1
- AQYOLTOCAOEGDY-UHFFFAOYSA-N COC1=CC=C(C2=CC=C(OC)C([N+](=O)[O-])=C2)C=C1 Chemical compound COC1=CC=C(C2=CC=C(OC)C([N+](=O)[O-])=C2)C=C1 AQYOLTOCAOEGDY-UHFFFAOYSA-N 0.000 description 1
- JAIFJSLVGSXSGJ-UHFFFAOYSA-N COC1=CC=C(C2=CC=CC=N2)C=C1C Chemical compound COC1=CC=C(C2=CC=CC=N2)C=C1C JAIFJSLVGSXSGJ-UHFFFAOYSA-N 0.000 description 1
- HAADUUDUGSTQQY-UHFFFAOYSA-N COC1=CC=C(C2=CC=CC=N2)C=C1N Chemical compound COC1=CC=C(C2=CC=CC=N2)C=C1N HAADUUDUGSTQQY-UHFFFAOYSA-N 0.000 description 1
- YHDWWNMOSUHEIM-UHFFFAOYSA-N COC1=CC=C(C2=CC=CC=N2)C=C1[N+](=O)[O-] Chemical compound COC1=CC=C(C2=CC=CC=N2)C=C1[N+](=O)[O-] YHDWWNMOSUHEIM-UHFFFAOYSA-N 0.000 description 1
- BSBBCAIINMTYHE-UHFFFAOYSA-N COC1=CC=C(C2=CC=CN=C2)C=C1C Chemical compound COC1=CC=C(C2=CC=CN=C2)C=C1C BSBBCAIINMTYHE-UHFFFAOYSA-N 0.000 description 1
- HBXIIOCOQXRVLM-UHFFFAOYSA-N COC1=CC=C(C2=CC=CN=C2)C=C1N Chemical compound COC1=CC=C(C2=CC=CN=C2)C=C1N HBXIIOCOQXRVLM-UHFFFAOYSA-N 0.000 description 1
- BRFJEYNZENTOPT-UHFFFAOYSA-N COC1=CC=C(C2=CC=CN=C2)C=C1[N+](=O)[O-] Chemical compound COC1=CC=C(C2=CC=CN=C2)C=C1[N+](=O)[O-] BRFJEYNZENTOPT-UHFFFAOYSA-N 0.000 description 1
- GHPMUGSRJXRPOV-UHFFFAOYSA-N COC1=CC=C(C2=CC=NC=C2)C=C1C Chemical compound COC1=CC=C(C2=CC=NC=C2)C=C1C GHPMUGSRJXRPOV-UHFFFAOYSA-N 0.000 description 1
- FBAWXJMSEFDNOH-UHFFFAOYSA-N COC1=CC=C(C2=CC=NC=C2)C=C1N Chemical compound COC1=CC=C(C2=CC=NC=C2)C=C1N FBAWXJMSEFDNOH-UHFFFAOYSA-N 0.000 description 1
- JAQDWEQNWOQEKQ-UHFFFAOYSA-N COC1=CC=C(C2=CC=NC=C2)C=C1[N+](=O)[O-] Chemical compound COC1=CC=C(C2=CC=NC=C2)C=C1[N+](=O)[O-] JAQDWEQNWOQEKQ-UHFFFAOYSA-N 0.000 description 1
- KJEQPCUWVXOYHC-UHFFFAOYSA-N COC1=CC=C(N(C)C)C=C1C Chemical compound COC1=CC=C(N(C)C)C=C1C KJEQPCUWVXOYHC-UHFFFAOYSA-N 0.000 description 1
- KSJMQUBTZAZILT-UHFFFAOYSA-N COC1=CC=C(N(C)C)C=C1N Chemical compound COC1=CC=C(N(C)C)C=C1N KSJMQUBTZAZILT-UHFFFAOYSA-N 0.000 description 1
- XRJCCHMXYCMCFG-UHFFFAOYSA-N COC1=CC=C(OC(C)C)C(C)=C1 Chemical compound COC1=CC=C(OC(C)C)C(C)=C1 XRJCCHMXYCMCFG-UHFFFAOYSA-N 0.000 description 1
- PRBGVIKREXVYLU-UHFFFAOYSA-N COC1=CC=C(OC(C)C)C(N)=C1 Chemical compound COC1=CC=C(OC(C)C)C(N)=C1 PRBGVIKREXVYLU-UHFFFAOYSA-N 0.000 description 1
- KTKMQHLMVIAMGZ-UHFFFAOYSA-N COC1=CC=C(OC(C)C)C([N+](=O)[O-])=C1 Chemical compound COC1=CC=C(OC(C)C)C([N+](=O)[O-])=C1 KTKMQHLMVIAMGZ-UHFFFAOYSA-N 0.000 description 1
- FUFSCVSNCACEPB-UHFFFAOYSA-N COC1=CC=C(OC)C(CC2=NC(CC3=C(C(=O)OC(C)C)C=CC=C3)=C(Br)C=N2)=C1 Chemical compound COC1=CC=C(OC)C(CC2=NC(CC3=C(C(=O)OC(C)C)C=CC=C3)=C(Br)C=N2)=C1 FUFSCVSNCACEPB-UHFFFAOYSA-N 0.000 description 1
- UBFVRVBQJVCXKJ-UHFFFAOYSA-N COC1=CC=C(OC)C(CC2=NC(CC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Br)C=N2)=C1 Chemical compound COC1=CC=C(OC)C(CC2=NC(CC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Br)C=N2)=C1 UBFVRVBQJVCXKJ-UHFFFAOYSA-N 0.000 description 1
- YLVGJAQWUOINAH-UHFFFAOYSA-N COC1=CC=C(OC)C(CC2=NC(CC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)=C1 Chemical compound COC1=CC=C(OC)C(CC2=NC(CC3=C(S(=O)(=O)N(C)C)C=CC=C3)=C(Cl)C=N2)=C1 YLVGJAQWUOINAH-UHFFFAOYSA-N 0.000 description 1
- MLPLJLCVYCBHAV-UHFFFAOYSA-N COC1=CC=C(OC)C(CC2=NC(CC3=C(S(=O)(=O)NCC(C)C)C=CC=C3)=C(Cl)C=N2)=C1 Chemical compound COC1=CC=C(OC)C(CC2=NC(CC3=C(S(=O)(=O)NCC(C)C)C=CC=C3)=C(Cl)C=N2)=C1 MLPLJLCVYCBHAV-UHFFFAOYSA-N 0.000 description 1
- QGOSFLMTROAPNU-UHFFFAOYSA-N COC1=CC=C(OC2=CC=CC=C2)C=C1C Chemical compound COC1=CC=C(OC2=CC=CC=C2)C=C1C QGOSFLMTROAPNU-UHFFFAOYSA-N 0.000 description 1
- BIYOFLKPGVGSDF-UHFFFAOYSA-N COC1=CC=C(OCCN2CCCC2)C=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC=C(OCCN2CCCC2)C=C1NC1=NC(NC2=C(S(=O)(=O)NC3CCCC3)C=CC=C2)=C(Cl)C=N1 BIYOFLKPGVGSDF-UHFFFAOYSA-N 0.000 description 1
- VPGSULGWWPWFLI-UHFFFAOYSA-N COC1=CC=C(OCCN2CCOCC2)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 Chemical compound COC1=CC=C(OCCN2CCOCC2)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Br)C=N1 VPGSULGWWPWFLI-UHFFFAOYSA-N 0.000 description 1
- DVHOHFJOXOBQJI-UHFFFAOYSA-N COC1=CC=C(OCCN2CCOCC2)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC=C(OCCN2CCOCC2)C=C1CC1=NC(CC2=C(S(=O)(=O)N(C)C)C=CC=C2)=C(Cl)C=N1 DVHOHFJOXOBQJI-UHFFFAOYSA-N 0.000 description 1
- DCMVGZFEQYCNEU-UHFFFAOYSA-N COC1=CC=C(OCCN2CCOCC2)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 Chemical compound COC1=CC=C(OCCN2CCOCC2)C=C1CC1=NC(CC2=C(S(=O)(=O)NCC(C)C)C=CC=C2)=C(Cl)C=N1 DCMVGZFEQYCNEU-UHFFFAOYSA-N 0.000 description 1
- UAIGOQVLBORIRB-UHFFFAOYSA-N COC1=CC=C(OCCO)C(C)=C1 Chemical compound COC1=CC=C(OCCO)C(C)=C1 UAIGOQVLBORIRB-UHFFFAOYSA-N 0.000 description 1
- JXNXXWTVMSWDCW-UHFFFAOYSA-N COC1=CC=C(OCCO)C(N)=C1 Chemical compound COC1=CC=C(OCCO)C(N)=C1 JXNXXWTVMSWDCW-UHFFFAOYSA-N 0.000 description 1
- WLAVZKYXYUGWHW-UHFFFAOYSA-N COC1=CC=C(OCCO)C([N+](=O)[O-])=C1 Chemical compound COC1=CC=C(OCCO)C([N+](=O)[O-])=C1 WLAVZKYXYUGWHW-UHFFFAOYSA-N 0.000 description 1
- WMXFNCKPYCAIQW-UHFFFAOYSA-N COC1=CC=CC(C)=C1OC Chemical compound COC1=CC=CC(C)=C1OC WMXFNCKPYCAIQW-UHFFFAOYSA-N 0.000 description 1
- VWESGTZRVFVUFH-UHFFFAOYSA-N COC1=CC=CC=C1C1=CC(C)=C(C)C=C1 Chemical compound COC1=CC=CC=C1C1=CC(C)=C(C)C=C1 VWESGTZRVFVUFH-UHFFFAOYSA-N 0.000 description 1
- BDEUBOJWIKLFSM-UHFFFAOYSA-N COC1=NC(OC)=C(C)C=C1 Chemical compound COC1=NC(OC)=C(C)C=C1 BDEUBOJWIKLFSM-UHFFFAOYSA-N 0.000 description 1
- IGQDYHCAFLITPL-UHFFFAOYSA-N COCC1CCN(C2=CC(OC)=C(C)C=C2)CC1 Chemical compound COCC1CCN(C2=CC(OC)=C(C)C=C2)CC1 IGQDYHCAFLITPL-UHFFFAOYSA-N 0.000 description 1
- QQZNDVJGSVYEEO-UHFFFAOYSA-N COCC1CCN(C2=CC(OC)=C(N)C=C2)CC1 Chemical compound COCC1CCN(C2=CC(OC)=C(N)C=C2)CC1 QQZNDVJGSVYEEO-UHFFFAOYSA-N 0.000 description 1
- BTDAWPWGFITZHE-UHFFFAOYSA-N COCC1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 Chemical compound COCC1CCN(C2=CC(OC)=C([N+](=O)[O-])C=C2)CC1 BTDAWPWGFITZHE-UHFFFAOYSA-N 0.000 description 1
- CSKIEQKLEBRXIJ-UHFFFAOYSA-N COCCN(CCOC)C1=CC=C(C)C(OC)=C1 Chemical compound COCCN(CCOC)C1=CC=C(C)C(OC)=C1 CSKIEQKLEBRXIJ-UHFFFAOYSA-N 0.000 description 1
- SEVZBOJYKZUQEK-UHFFFAOYSA-N COCCOC1=CC=C(OC)C=C1C Chemical compound COCCOC1=CC=C(OC)C=C1C SEVZBOJYKZUQEK-UHFFFAOYSA-N 0.000 description 1
- WJTHAZBXYONIPL-UHFFFAOYSA-N COCCOC1=CC=C(OC)C=C1[N+](=O)[O-] Chemical compound COCCOC1=CC=C(OC)C=C1[N+](=O)[O-] WJTHAZBXYONIPL-UHFFFAOYSA-N 0.000 description 1
- AKUSOKZVYAWHOH-UHFFFAOYSA-N COCCOC1CCN(C2=CC(OC)=C(C)C=C2)CC1 Chemical compound COCCOC1CCN(C2=CC(OC)=C(C)C=C2)CC1 AKUSOKZVYAWHOH-UHFFFAOYSA-N 0.000 description 1
- HTQAWMGZQSBCBS-UHFFFAOYSA-N COCCOC1CCN(C2=CC=C(N)C(OC)=C2)CC1 Chemical compound COCCOC1CCN(C2=CC=C(N)C(OC)=C2)CC1 HTQAWMGZQSBCBS-UHFFFAOYSA-N 0.000 description 1
- ZZDMRKSFGGRJHT-UHFFFAOYSA-N COCCOC1CCN(C2=CC=C([N+](=O)[O-])C(OC)=C2)CC1 Chemical compound COCCOC1CCN(C2=CC=C([N+](=O)[O-])C(OC)=C2)CC1 ZZDMRKSFGGRJHT-UHFFFAOYSA-N 0.000 description 1
- KJGJIVLKUPKZIH-UHFFFAOYSA-N CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 Chemical compound CS(=O)(=O)C1=C(NC2=C(Cl)C=NC(Cl)=N2)C=CC=C1 KJGJIVLKUPKZIH-UHFFFAOYSA-N 0.000 description 1
- JWWGCIVBADCZQT-UHFFFAOYSA-N Cc(c([ClH]C)c1)ccc1N(CC1)CC1NC Chemical compound Cc(c([ClH]C)c1)ccc1N(CC1)CC1NC JWWGCIVBADCZQT-UHFFFAOYSA-N 0.000 description 1
- YIVVBOHKFNPJIN-UHFFFAOYSA-N NS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=CC4=C3OC(F)(F)O4)=N2)C=CC=C1 Chemical compound NS(=O)(=O)C1=C(NC2=C(Br)C=NC(NC3=CC=CC4=C3OC(F)(F)O4)=N2)C=CC=C1 YIVVBOHKFNPJIN-UHFFFAOYSA-N 0.000 description 1
- SYVWXWNXSOOJCK-AWEZNQCLSA-N [H][C@@]12CCOCN1CCN(C1=CC(OC)=C(C)C=C1)C2 Chemical compound [H][C@@]12CCOCN1CCN(C1=CC(OC)=C(C)C=C1)C2 SYVWXWNXSOOJCK-AWEZNQCLSA-N 0.000 description 1
- YVJFBLDWJNKPRY-GFCCVEGCSA-N [H][C@@]12COCCN1CCN(C1=CC=C(N)C(OC)=C1)C2 Chemical compound [H][C@@]12COCCN1CCN(C1=CC=C(N)C(OC)=C1)C2 YVJFBLDWJNKPRY-GFCCVEGCSA-N 0.000 description 1
- ZQLGHXAHGKDNRY-GFCCVEGCSA-N [H][C@@]12COCCN1CCN(C1=CC=C([N+](=O)[O-])C(OC)=C1)C2 Chemical compound [H][C@@]12COCCN1CCN(C1=CC=C([N+](=O)[O-])C(OC)=C1)C2 ZQLGHXAHGKDNRY-GFCCVEGCSA-N 0.000 description 1
- KDORZHCDYBEDTG-AWEZNQCLSA-N [H][C@]12COCCN1CCN(C1=CC=C(C)C(OC)=C1)C2 Chemical compound [H][C@]12COCCN1CCN(C1=CC=C(C)C(OC)=C1)C2 KDORZHCDYBEDTG-AWEZNQCLSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/48—Two nitrogen atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/04—Antipruritics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/626—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B
- C04B35/63—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B using additives specially adapted for forming the products, e.g.. binder binders
- C04B35/632—Organic additives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
Abstract
Description
- This application is a continuation of U.S. application Ser. No. 10/568,367, filed Aug. 18, 2006, which is a 371 U.S. national phase application of international application number PCT/EP2004/009099 filed Aug. 13, 2004, which application claims priority to GB application no. 0322370.8 filed Sep. 24, 2003 and GB application no. 0319227.5 filed Aug. 15, 2003, each of which is incorporated herein by reference in its entirety and for all purposes.
- The present invention relates to the use novel pyrimidine derivatives, the certain novel pyrimidine derivatives, to processes for their production, their use as pharmaceuticals and to pharmaceutical compositions comprising them.
- More particularly the present invention provides in a first aspect, the use of a compound of formula I
- wherein
- R is selected from C6-10aryl, C5-10heteroaryl, C3-12cycloalkyl and C3-10heterocycloalkyl;
- each of R0, R1, R2, and R3 independently is hydrogen, C1-C8alkyl, C2-C8alkenyl, C2-C8alkinyl, C3-C8cycloalkyl, C3-C8cycloalkylC1-C8alkyl, C5-C10arylC1-C8alkyl, hydroxyC1-C8alkyl, C1-C8alkoxyC1-C8alkyl, aminoC1-C8alkyl, haloC1-C8alkyl, unsubstituted or substituted C5-C10aryl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1, 2 or 3 hetero atoms selected from N, O and S, hydroxy, C1-C8alkoxy, hydroxyC1-C8alkoxy, C1-C8alkoxyC1-C8alkoxy, haloC1-C8alkoxy, unsubstituted or substituted C5-C10arylC1-C8alkoxy, unsubstituted or substituted heterocyclyloxy, or unsubstituted or substituted heterocyclylC1-C8alkoxy, unsubstituted or substituted amino, C1-C8alkylthio, C1-C8alkylsulfinyl, C1-C8alkylsulfonyl, C5-C10arylsulfonyl, halogen, carboxy, C1-C8alkoxycarbonyl, unsubstituted or substituted carbamoyl, unsubstituted or substituted sulfamoyl, cyano, nitro, —S(O)0-2NR12R13, —S(O)0-2R13, —NR12S(O)0-2R13, —C(O)NR12R13, —C(O)R13 and —C(O)OR13; wherein R12 is selected from hydrogen and C1-6alkyl; and R13 is selected from hydrogen, C1-6alkyl and C3-12cycloalkyl; or R0 and R1, R1 and R2, and/or R2 and R3 form, together with the carbon atoms to which they are attached, a 5 or 6 membered carbocyclic or heterocyclic ring comprising 0, 1, 2 or 3 heteroatoms selected from N, O and S;
- R4 is hydrogen or C1-C8alkyl;
- each of R5 and R6 independently is hydrogen, C1-C8alkyl, C1-C8alkoxyC1-C8alkyl, haloC1-C8alkyl, C1-C8alkoxy, halogen, carboxy, C1-C8alkoxycarbonyl, unsubstituted or substituted carbamoyl, cyano, or nitro;
- R is unsubstituted or substituted by R7, R8, R9, R10, and R′10;
- R7, R3, R9, R10, or R′10 is a substituent independently selected from hydrogen, C1-C8alkyl, C2-C8alkenyl, C2-C8alkinyl, C3-C8cycloalkyl, C3-C8cycloalkylC1-C8alkyl, C5-C10arylC1-C8alkyl, hydroxyC1-C8alkyl, C1-C8alkoxyC1-C8alkyl, aminoC1-C8alkyl, haloC1-C8alkyl, unsubstituted or substituted C5-C10aryl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1, 2 or 3 hetero atoms selected from N, O and S, hydroxy, C1-C8alkoxy, hydroxyC1-C8alkoxy, C1-C8alkoxyC1-C8alkoxy, haloC1-C8alkoxy, unsubstituted or substituted aminoC1-C8alkoxy, unsubstituted or substituted C5-C10arylC1-C8alkoxy, unsubstituted or substituted heterocyclyloxy, or unsubstituted or substituted heterocyclylC1-C8alkyl, unsubstituted or substituted heterocyclylC1-C8alkoxy, unsubstituted or substituted amino, C1-C8alkylthio, C1-C8alkylsulfinyl, C1-C8alkylsulfonyl, C5-C10arylsulfonyl, heterocyclosulfonyl, halogen, carboxy, C1-C8alkylcarbonyl, C1-C8alkoxycarbonyl, unsubstituted or substituted carbamoyl, unsubstituted or substituted sulfamoyl, cyano, nitro, —S(O)0-2NR12R13, —S(O)0-2R12, —C(O)R11, —OXR11, —NR12XR11, —NR12XNR12R13, —OXNR12R13, —OXR12 and —XR11;
- or two adjacent substituents on R may form together with the carbon atoms to which they are attached, a unsubstituted or substituted 5 or 6 membered carbocyclic or heterocyclic ring comprising 0, 1, 2 or 3 heteroatoms selected from N, O and S;
- X is a bond or C1-6alkylene; and
- R11 is independently selected from C6-10aryl, C5-10heteroaryl, C3-12cycloalkyl and C3-10heterocycloalkyl;
- and any aryl, heteroaryl, cycloalkyl or heterocycloalkyl of R11 is optionally substituted by 1 to 3 radicals independently selected from C1-6alkyl, C3-10heterocycloalkyl-C0-4alkyl optionally substituted with C1-6alkyl, —C(O)R12, —C(O)NR12R13, —XNR12R13, —NR12XNR12R13 and —NR12C(O)R13; wherein X is a bond or C1-6alkylene; R12 and R13 are independently selected from hydrogen and C1-6alkyl;
and salts thereof in the treatment of a disease associated to tyrosine kinase activity of anaplastic lymphoma kinase (ALK) or for the manufacture of pharmaceutical compositions for use in the treatment of said diseases, as well as use in the treatment of said diseases, to methods of use of such pyrimidine derivatives in the treatment of said diseases, and to pharmaceutical compositions comprising such pyrimidine derivatives for the treatment of said diseases. - The general terms used hereinbefore and hereinafter preferably have within the context of this disclosure the following meanings, unless otherwise indicated:
- Where the plural form is used for compounds, salts, and the like, this is taken to mean also a single compound, salt, or the like.
- Any asymmetric carbon atoms may be present in the (R)-, (S)- or (R,S)-configuration, preferably in the (R)- or (S)-configuration. The compounds may thus be present as mixtures of isomers or as pure isomers, preferably as enantiomer-pure diastereomers.
- The invention relates also to possible tautomers of the compounds of formula I.
- C1-C8alkyl denotes a an alkyl radical having from 1 up to 8, especially up to 4 carbon atoms, the radicals in question being either linear or branched with single or multiple branching; preferably, C1-C8alkyl is butyl, such as n-butyl, sec-butyl, isobutyl, tert-butyl, propyl, such as n-propyl or isopropyl, ethyl or methyl; especially methyl, propyl or tert-butyl.
- C2-C8alkenyl denotes a an alkenyl radical having from 2 up to 8, especially up to 5 carbon atoms, the radicals in question being either linear or branched with single or multiple branching; preferably, C2-C8alkenyl is pentenyl, such as 3-methyl-2-buten-2-yl, butenyl, such as 1- or 2-butenyl or 2-buten-2-yl, propenyl, such as 1-propenyl or allyl, or vinyl.
- C2-C8alkinyl denotes a an alkinyl radical having from 2 up to 8, especially up to 5 carbon atoms, the radicals in question being either linear or branched; preferably, C2-C8alkinyl is propinyl, such as 1-propinyl or propargyl, or acetylenyl.
- C3-C8cycloalkyl denotes a cycloalkyl radical having from 3 up to 8 carbon atoms, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl or cyclooctyl, preferably cyclopropyl, cyclopentyl or cyclohexyl.
- C1-C8alkoxy is especially methoxy, ethoxy, isopropyloxy, or tert-butoxy.
- HydroxyC1-C8alkyl is especially hydroxymethyl, 2-hydroxyethyl or 2-hydroxy-2-propyl.
- HydroxyC1-C8alkoxy is especially 2-hydroxyethoxy or 3-hydroxypropoxy.
- C1-C8alkoxyC1-C8alkoxy is especially 2-methoxyethoxy.
- C1-C8alkoxyC1-C8alkyl is especially methoxymethyl, 2-methoxyethyl or 2-ethoxyethyl.
- Halogen is preferably fluorine, chlorine, bromine, or iodine, especially fluorine, chlorine, or bromine.
- HaloC1-C8alkyl is preferably chloroC1-C8alkyl or fluoroC1-C8alkyl, especially trifluoromethyl or pentafluoroethyl.
- HaloC1-C8alkoxy is preferably chloroC1-C8alkoxy or fluoroC1-C8alkoxy, especially trifluoromethoxy.
- C1-C8alkoxycarbonyl is especially tert-butoxycarbonyl, iso-propoxycarbonyl, methoxycarbonyl or ethoxycarbonyl.
- Unsubstituted or substituted carbamoyl is carbamoyl substituted by one or two substituents selected from hydrogen, C1-C8alkyl, C2-C8alkenyl, C2-C8alkinyl, C3-C8cycloalkyl, C3-C8cycloalkylC1-C8alkyl, C5-C10arylC1-C8alkyl, hydroxyC1-C8alkyl, C1-C8alkoxyC1-C8alkyl, haloC1-C8alkyl, unsubstituted or substituted C5-C10aryl, or aminoC1-C8alkyl, or carbamoyl wherein the substituents and the nitrogen atom of the carbamoyl group represent a 5 or 6 membered heterocyclyl further comprising 0, 1 or 2 hetero atoms selected from N, O and S; and is preferably carbamoyl, methylcarbamoyl, dimethylcarbamoyl, propylcarbamoyl, hydroxyethyl-methyl-carbamoyl, di(hydroxyethyl)carbamoyl, dimethylaminoethylcarbamoyl, or pyrrolidinocarbonyl, piperidinocarbonyl, N-methylpiperazinocarbonyl or morpholinocarbonyl, especially carbamoyl or dimethylcarbamoyl.
- Unsubstituted or substituted sulfamoyl is sulfamoyl substituted by one or two substituents selected from hydrogen, C1-C8alkyl, C2-C8alkenyl, C2-C8alkinyl, C3-C8cycloalkyl, C3-C8cycloalkylC1-C8alkyl, C5-C10arylC1-C8alkyl, hydroxyC1-C8alkyl, C1-C8alkoxyC1-C8alkyl, haloC1-C8alkyl, unsubstituted or substituted C5-C10aryl, or aminoC1-C8alkyl, or sulfamoyl wherein the substituents and the nitrogen atom of the sulfamoyl group represent a 5 or 6 membered heterocyclyl further comprising 0, 1 or 2 hetero atoms selected from N, O and S; and is preferably sulfamoyl, methylsulfamoyl, propylsulfamoyl, cyclopropylmethyl-sulfamoyl, 2,2,2-trifluoroethylsulfamoyl, dimethylaminoethylsulfamoyl, dimethylsulfamoyl, hydroxyethyl-methyl-sulfamoyl, di(hydroxyethyl)sulfamoyl, or pyrrolidinosulfonyl, piperidinosulfonyl, N-methylpiperazinosulfonyl or morpholinosulfonyl, especially sulfamoyl or methylsulfamoyl.
- Unsubstituted or substituted amino is amino substituted by one or two substituents selected from hydrogen, C1-C8alkyl, C2-C8alkenyl, C2-C8alkinyl, C3-C8cycloalkyl, C3-C8cycloalkylC1-C8alkyl, C5-C10arylC1-C8alkyl, hydroxyC1-C8alkyl, C1-C8alkoxyC1-C8alkyl, haloC1-C8alkyl, unsubstituted or substituted C5-C10aryl, aminoC1-C8alkyl, acyl, e.g. formyl, C1-C8alkylcarbonyl, C5-C10arylcarbonyl, C1-C8alkylsulfonyl or C5-C10arylsulfonyl, and is preferably amino, methylamino, dimethylamino, propylamino, benzylamino, hydroxyethyl-methyl-amino, di(hydroxyethyl)amino, dimethylaminoethylamino, acetylamino, acetyl-methyl-amino, benzoylamino, methylsulfonylamino or phenylsulfonylamino, especially amino or dimethylamino.
- AminoC1-C8alkyl is especially aminoethyl, methylaminoethyl, dimethylaminoethyl or dimethylaminopropyl.
- Unsubstituted or substituted C5-C10aryl is, for example, phenyl, indenyl, indanyl, naphthyl, or 1,2,3,4-tetrahydronaphthalenyl, optionally substituted by C1-C8alkyl, C1-C8alkoxyC1-C8alkyl, haloC1-C8alkyl, hydroxy, C1-C8alkoxy, methylenedioxy, amino, substituted amino, halogen, carboxy, C1-C8alkoxycarbonyl, carbamoyl, sulfamoyl, cyano or nitro; preferably phenyl, tolyl, trifluoromethylphenyl, methoxyphenyl, dimethoxyphenyl, methylenedioxyphenyl, chlorophenyl or bromophenyl, whereby the substituents may be in ortho, meta or para position, preferably meta or para.
- C5-C10aryloxy is especially phenoxy or methoxyphenoxy, e.g. p-methoxyphenoxy.
- C5-C10arylC1-C8alkyl is especially benzyl or 2-phenylethyl.
- C5-C10arylC1-C8alkoxy is especially benzyloxy or 2-phenylethoxy.
- Unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1, 2 or 3 hetero atoms selected from N, O and S may be unsaturated, partially unsaturated or saturated, and further condensed to a benzo group or a 5 or 6 membered heterocyclyl group, and may be bound through a hetero or a carbon atom, and is, for example, pyrrolyl, indolyl, pyrrolidinyl, imidazolyl, benzimidazolyl, pyrazolyl, triazolyl, benzotriazolyl, tetrazolyl, pyridyl, quinolinyl, isoquinolinyl, 1,2,3,4-tetrahydroquinolinyl, piperidyl, pyrimidinyl, pyrazinyl, piperazinyl, purinyl, tetrazinyl, oxazolyl, isoxalyl, morpholinyl, thiazolyl, benzothiazolyl, oxadiazolyl, and benzoxadiazolyl. Substituents considered are C1-C8alkyl, hydroxyC1-C8alkyl, C1-C8alkoxyC1-C8alkyl, C1-C8alkoxyC1-C8alkoxy, haloC1-C8alkyl, hydroxy, amino, substituted amino, C1-C8alkoxy, halogen, carboxy, C1-C8alkylcarbonyl, C1-C8alkoxycarbonyl, carbamoyl, C1-C8alkylcarbamoyl, cyano, oxo, or unsubstituted or substituted 5 or 6 membered heterocyclyl as defined in this paragraph. 5 or 6 membered heterocyclyl preferably comprises 1 or 2 hetero atoms selected from N, O and S, and is especially indolyl, pyrrolidinyl, pyrrolidonyl, imidazolyl, N-methylimidazolyl, benzimidazolyl, S,S-dioxoisothiazolidinyl, piperidyl, 4-acetylaminopiperidyl, 4-methylcarbamoylpiperidyl, 4-piperidinopiperidyl, 4-cyanopiperidyl, piperazinyl, N-methylpiperazinyl, N-(2-hydroxyethyl)piperazinyl, morpholinyl, 1-aza-2,2-dioxo-2-thiacyclohexyl, or sulfolanyl.
- In unsubstituted or substituted heterocyclyloxy, heterocyclyl has the meaning as defined above, and is especially N-methyl-4-piperidyloxy. In unsubstituted or substituted heterocyclylC1-C8alkoxy, heterocyclyl has the meaning as defined above, and is especially 2-pyrrolidinoethoxy, 2-morpholinoethoxy, 3-morpholinopropoxy, 1-methyl-piperidin-3-ylmethoxy, 3-(N-methylpiperazino)propoxy or 2-(1-imidazolyl)ethoxy.
- In a 5 or 6 membered carbocyclic or heterocyclic ring comprising 0, 1, 2 or 3 heteroatoms selected from N, O and S, and formed by two adjacent substituents together with the benzene ring, the ring may be further substituted, e.g. by C1-C8alkyl, C1-C8alkoxy, haloC1-C8alkyl, hydroxy, amino, substituted amino, C1-C8alkoxy, halogen, carboxy, C1-C8alkoxycarbonyl, carbamoyl, cyano, or oxo. The two adjacent substituents forming such a ring are preferably propylene, butylene, 1-aza-2-propylidene, 3-aza-1-propylidene, 1,2-diaza-2-propylidene, 2,3-diaza-1-propylidene, 1-oxapropylene, 1-oxapropylidene, methylenedioxy, difluoromethylene-dioxy, 2-aza-1-oxopropylene, 2-aza-2-methyl-1-oxopropylene, 1-aza-2-oxopropylene, 2-aza-1,1-dioxo-1-thiapropylene or the corresponding butylene derivatives forming a 6 membered ring.
- Salts are especially the pharmaceutically acceptable salts of compounds of formula I.
- Such salts are formed, for example, as acid addition salts, preferably with organic or inorganic acids, from compounds of formula I with a basic nitrogen atom, especially the pharmaceutically acceptable salts. Suitable inorganic acids are, for example, halogen acids, such as hydrochloric acid, sulfuric acid, or phosphoric acid. Suitable organic acids are, for example, carboxylic, phosphonic, sulfonic or sulfamic acids, for example acetic acid, propionic acid, octanoic acid, decanoic acid, dodecanoic acid, glycolic acid, lactic acid, fumaric acid, succinic acid, adipic acid, pimelic acid, suberic acid, azelaic acid, malic acid, tartaric acid, citric acid, amino acids, such as glutamic acid or aspartic acid, maleic acid, hydroxymaleic acid, methylmaleic acid, cyclohexanecarboxylic acid, adamantanecarboxylic acid, benzoic acid, salicylic acid, 4-aminosalicylic acid, phthalic acid, phenylacetic acid, mandelic acid, cinnamic acid, methane- or ethane-sulfonic acid, 2-hydroxyethanesulfonic acid, ethane-1,2-disulfonic acid, benzenesulfonic acid, 2-naphthalenesulfonic acid, 1,5-naphthalene-disulfonic acid, 2-, 3- or 4-methylbenzenesulfonic acid, methylsulfuric acid, ethylsulfuric acid, dodecylsulfuric acid, N-cyclohexylsulfamic acid, N-methyl-, N-ethyl- or N-propyl-sulfamic acid, or other organic protonic acids, such as ascorbic acid.
- For isolation or purification purposes it is also possible to use pharmaceutically unacceptable salts, for example picrates or perchlorates. For therapeutic use, only pharmaceutically acceptable salts or free compounds are employed (where applicable in the form of pharmaceutical preparations), and these are therefore preferred.
- In view of the close relationship between the novel compounds in free form and those in the form of their salts, including those salts that can be used as intermediates, for example in the purification or identification of the novel compounds, any reference to the free compounds hereinbefore and hereinafter is to be understood as referring also to the corresponding salts, as appropriate and expedient.
- The compounds of formula I have valuable pharmacological properties, as described hereinbefore and hereinafter.
- In formula I the following significances are preferred independently, collectively or in any combination or sub-combination. R is C6-10aryl, C5-10heteroaryl, C3-12cycloalkyl or C3-10heterocycloalkyl, preferably R is
- wherein R7, R8, R9, R10, or R′10 are as defined above;
- In each of the following significances A, D or E is C or N but A, D and E may not all be N, preferably A, D or E is C:
- (a) each of R0 or R2 independently is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, hydroxyC1-C8alkyl, e.g. hydroxyethyl or hydroxybutyl, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted C5-C10aryl, e.g. phenyl or methoxyphenyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, haloC1-C8alkoxy, e.g. trifluoromethoxy, C5-C10aryloxy, e.g. phenoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino, dimethylamino or acetylamino, C1-C8alkylsulfonyl, e.g. methylsulfonyl, halogen, e.g. fluoro or chloro, unsubstituted or substituted carbamoyl, e.g. cyclohexylcarbamoyl, piperidinocarbonyl, piperazinocarbonyl, N-methylpiperazinocarbonyl or morpholinocarbonyl, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl or dimethylsulfamoyl; preferably hydrogen, piperazino, N-methylpiperazino or 1-methyl-4-piperidyloxy, —S(O)0-2NR12R13, —S(O)0-2R13, —NR12S(O)0-2R13, —C(O)NR12R13, and —C(O)OR13 in particular hydrogen;
- (b) R1 is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, hydroxyC1-C8alkyl, e.g. hydroxyethyl or hydroxybutyl, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted C5-C10aryl, e.g. phenyl or methoxyphenyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, haloC1-C8alkoxy, e.g. trifluoromethoxy, C5-C10aryloxy, e.g. phenoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino, dimethylamino or acetylamino, C1-C8alkylsulfonyl, e.g. methylsulfonyl, halogen, e.g. fluoro or chloro, unsubstituted or substituted carbamoyl, e.g. cyclohexylcarbamoyl, piperidinocarbonyl, piperazinocarbonyl, N-methylpiperazinocarbonyl or morpholinocarbonyl, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl or dimethylsulfamoyl; preferably hydrogen, piperazino, N-methylpiperazino, morpholino, 1-methyl-4-piperidinyloxy, 3-morpholinopropoxy or 2-morpholinoethoxy, in particular hydrogen;
- (c) R3 is hydrogen, C1-C8alkyl, e.g. methyl or ethyl, hydroxyC1-C8alkyl, e.g. hydroxyethyl or hydroxybutyl, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 heteroatoms selected from N, O and S, e.g. 2-pyrrolidonyl or S,S-dioxoisothiazolidinyl, C1-C8alkoxy, e.g. methoxy, substituted amino, e.g. acetylamino, acetyl-methyl-amino, benzoylamino, methylsulfonylamino or phenylsulfonylamino, C1-C8alkylsulfonyl, e.g. methylsulfonyl, propyl-sulfonyl, cyclohexyl-sulfonyl, isopropyl-sulfonyl, C5-C10arylsulfonyl, e.g. phenylsulfonyl, halogen, e.g. fluoro or chloro, carboxy, substituted or unsubstituted carbamoyl, e.g. carbamoyl, methylcarbamoyl, ethyl-amino-carbonyl or dimethylcarbamoyl, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl, propylsulfamoyl, isopropylsulfamoyl, isobutylsulfamoyl, cyclopropylmethyl-sulfamoyl, 2,2,2-trifluoroethylsulfamoyl, dimethylsulfamoyl or morpholinosulfonyl dimethyl-sulfamoyl, ethyl-sulfamoyl, 1-ethyl-propyl-sulfamoyl, cyclopentyl-sulfamoyl, cyclobutyl-sulfamoyl; preferably sulfamoyl, methylsulfamoyl or propylsulfamoyl;
- (d) each pair of adjacent substituents R0 and R1, or R1 and R2, or R2 and R3 are —CH2—NH—CO—, —CH2—CH2—NH—CO—, —CH2—CO—NH—, —CH2—CH2—CO—NH—, —CH2—NH—SO2—, —CH2—CH2—NH—SO2—, —CH2—SO2—NH—, —CH2—CH2—SO2—NH—, —CH2—CH2—SO2—, —CH2—CH2—CH2—SO2—, —O—CH2—O—, or —O—CF2—O—, and such pairs wherein hydrogen in NH is replaced by C1-C8alkyl; preferably the pair of adjacent substituents R0 and R1, or R1 and R2 being —O—CH2—O—, and the pair of adjacent substituents R2 and R3 being —CH2—NH—CO— or —CH2—NH—SO2—.
- (e) R4 is hydrogen or C1-C8alkyl, e.g. methyl; preferably hydrogen;
- (f) R5 is hydrogen; C1-C8alkyl, e.g. methyl or ethyl, halogen, e.g. chloro or bromo, haloC1-C8alkyl, e.g. trifluoromethyl, cyano or nitro; preferably hydrogen, methyl, ethyl, chloro, bromo, trifluoromethyl or nitro; in particular chloro or bromo;
- (g) R6 is hydrogen;
- (h) each of R7 and R9 independently is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, hydroxyC1-C8alkyl, e.g. hydroxyethyl or hydroxybutyl, C1-C8alkylcarbonyl, e.g methyl carbonyl, aminoalkoxy, e.g diethylaminoethoxy, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted C5-C10aryl, e.g. phenyl or methoxyphenyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, haloC1-C8alkoxy, e.g. trifluoromethoxy, C5-C10aryloxy, e.g. phenoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino, dimethylamino or acetylamino, C1-C8alkylsulfonyl, e.g. methylsulfonyl, heterocyclosulfonyl, e.g piperazinylsulfonyl, heterocyclocarbonyl, e.g. methylpiperazinylcarbonyl, cyano, halogen, e.g. fluoro or chloro, unsubstituted or substituted carbamoyl, e.g. cyclohexylcarbamoyl, piperidinocarbonyl, piperazinocarbonyl, N-methylpiperazinocarbonyl or morpholinocarbonyl, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl or dimethylsulfamoyl; preferably hydrogen, methyl, isopropyl, trifluoromethyl, phenyl, methoxyphenyl, piperidino, piperazino, N-methylpiperazino, morpholino, methoxy, ethoxy, isopropoxy, phenoxy, 3-morpholinopropoxy, 2-morpholinoethoxy, 2-(1-imidazolyl)ethoxy, dimethylamino, fluoro, morpholinocarbonyl, piperidinocarbonyl, piperazinocarbonyl or cyclohexylcarbamoyl;
- (i) R8 is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, hydroxyC1-C8alkyl, e.g. hydroxyethyl or hydroxybutyl, haloC1-C8alkyl, e.g. trifluoromethyl, C5-C10aryl, e.g. phenyl or methoxyphenyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, heterocyclylalkyl, e.g. methylpiperazinoethyl, heterocyclylcarbonyl, e.g. piperazinocarbonyl, heterocyclyl C1-C8alkylamino, e.g. pyridylethyl(methyl)amino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, haloC1-C8alkoxy, e.g. trifluoromethoxy, C5-C10aryloxy, e.g. phenoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino or dimethylamino, C1-C8alkylamino-C1-C8alkylamino, e.g. dimethylamino-propylamino, C1-C8alkylsulfonyl, e.g. methylsulfonyl, halogen, e.g. fluoro or chloro, unsubstituted or substituted carbamoyl, e.g. cyclohexylcarbamoyl, piperidinocarbonyl, piperazinocarbonyl, N-methylpiperazinocarbonyl or morpholinocarbonyl, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl or dimethylsulfamoyl, cyano, or nitro; preferably hydrogen, methyl, piperidino, piperazino, N-methylpiperazino, morpholino, methoxy, ethoxy, trifluoromethoxy, phenoxy, 1-methyl-4-piperidyloxy, 3-morpholinopropoxy, 2-morpholinoethoxy, 3-(N-methylpiperazino)-propoxy, methylamino, fluoro, chloro, sulfamoyl or nitro;
- (j) R10 is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or butyl, hydroxy, cyano, hydroxyC1-C8alkyl, e.g. hydroxyethyl or hydroxybutyl, haloC1-C8alkyl, e.g. trifluoromethyl, C1-C8alkoxy, e.g. methoxy or ethoxy, cycloalkylalkoxy, aryloxy, haloC1-C8alkoxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, unsubstituted or substituted amino, e.g. methylamino or dimethylamino, halogen, e.g. fluoro or chloro; carboxy, carbamoyl, or unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl or dimethylsulfamoyl; preferably methyl, butyl, methoxy, ethoxy, 2-(1-imidazolyl)ethoxy, methylamino, dimethylamino or fluoro; and
- (k) each pair of adjacent substituents R7 and R8, or R8 and R9 or R9 and R10, are —NH—CH═CH—, —CH═CH—NH—, —NH—N═CH—, —CH═N—NH—, —CH2—CH2—CH2—, —CH2—CH2—CH2—CH2—, —CH2—CH2—O—, —CH2C(CH3)2O—, —CH═C(CH3)O—, —OCH2CH2O—, -(Morpholinopropyl)N—CH═CH—, —CH═CH—O—, —O—CH2—O—, or —O—CF2—O—; preferably the pair of adjacent substituents R7 and R8 or R8 and R9 being —O—CH2—O— or the pair of adjacent substituents R9 and R10 being —NH—CH═CH—, —CH═N—NH—, —CH2—CH2—CH2—, —CH2—CH2—CH2—CH2— or —O—CF2—O—.
- (l) or R7, R8, R9, R10 and R′10 are ethoxy, ethyl, propyl, methyl, t-butyl, trifluoromethyl, nitrile, cyclobutyloxy, 2,2,2-trifluoroethoxy, methoxy, isobutyloxy, t-butyloxy, isopropyloxy, methyl-amino-carbonyl, cyclopropyl-methoxy, dimethylamino-propyl-amino, methoxy-ethoxy, —XR11, —C(O)R11 and —OXR11; wherein X is a bond, methylene or ethylene; R11 is selected from piperazinyl, piperidinyl, pyrrolidinyl, morpholino, azepanyl and 1,4-dioxa-8-aza-spiro[4.5]dec-8-yl; wherein R11 is optionally substituted by 1 to 3 radicals independently selected from methyl, isopropyl, acetyl, acetyl-methyl-amino, 3-dimethylamino-2,2-dimethyl-propylamino, ethyl-methyl-amino-ethoxy, diethyl-amino-ethoxy, amino-carbonyl, ethyl, 2-oxo-pyrrolidin-1-yl, pyrrolidinyl, pyrrolidinyl-methyl, piperidinyl optionally substituted with methyl or ethyl, morpholino, dimethylamino, dimethylamino-propyl-amino, methyl-amino and ethyl-amino.
- More preferred are the following meanings, independently, collectively or in any combination or sub-combination:
- (a′) each of R0 or R2 independently is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino, dimethylamino or acetylamino, halogen, e.g. fluoro or chloro; preferably hydrogen, piperazino, N-methylpiperazino or 1-methyl-4-piperidyloxy, in particular hydrogen;
- (b′) R1 is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino, dimethylamino or acetylamino, halogen, e.g. fluoro or chloro; preferably hydrogen, piperazino, N-methylpiperazino, morpholino, 1-methyl-4-piperidinyloxy, 3-morpholinopropoxy or 2-morpholinoethoxy, in particular hydrogen;
- (c′) R3 is hydrogen, C1-C8alkyl, e.g. methyl or ethyl, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 heteroatoms selected from N, O and S, e.g. 2-pyrrolidonyl or S,S-dioxoisothiazolidinyl, C1-C8alkoxy, e.g. methoxy, substituted amino, e.g. acetylamino, acetyl-methyl-amino, benzoylamino, methylsulfonylamino or phenylsulfonylamino, C1-C8alkylsulfonyl, e.g. methylsulfonyl, C5-C10arylsulfonyl, e.g. phenylsulfonyl, halogen, e.g. fluoro or chloro, carboxy, substituted or unsubstituted carbamoyl, e.g. carbamoyl, methylcarbamoyl or dimethylcarbamoyl, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl, propylsulfamoyl, isopropylsulfamoyl, isobutylsulfamoyl, cyclopropylmethyl-sulfamoyl, 2,2,2-trifluoroethylsulfamoyl, dimethylsulfamoyl or morpholinosulfonyl; preferably sulfamoyl, methylsulfamoyl or propylsulfamoyl;
- (d′) each pair of adjacent substituents R0 and R1, or R1 and R2, or R2 and R3 are —CH2—NH—CO—, —CH2—NH—SO2—, —CH2—CH2—SO2—, —O—CH2—O—, or —O—CF2—O—, and such pairs wherein hydrogen in NH is replaced by C1-C8alkyl; preferably the pair of adjacent substituents R0 and R1, or R1 and R2 being —O—CH2—O—, and the pair of adjacent substituents R2 and R3 being —CH2—NH—CO— or —CH2—NH—SO2—.
- (e′) R4 is hydrogen;
- (f′) R5 is hydrogen, halogen, e.g. chloro or bromo, haloC1-C8alkyl, e.g. trifluoromethyl, or nitro; preferably hydrogen, chloro, bromo, trifluoromethyl or nitro; in particular chloro or bromo;
- (g′) R6 is hydrogen;
- (h′) each of R7 and R9 independently is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, haloC1-C8alkyl, e.g. trifluoromethyl, unsubstituted or substituted C5-C10aryl, e.g. phenyl or methoxyphenyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino, dimethylamino or acetylamino, halogen, e.g. fluoro or chloro, unsubstituted or substituted carbamoyl, e.g. cyclohexylcarbamoyl, piperidinocarbonyl, piperazinocarbonyl, N-methylpiperazinocarbonyl or morpholinocarbonyl, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl or dimethylsulfamoyl; preferably hydrogen, methyl, isopropyl, trifluoromethyl, phenyl, o-, m- or p-methoxyphenyl, piperidino, piperazino, N-methylpiperazino, morpholino, methoxy, ethoxy, isopropoxy, phenoxy, 3-morpholinopropoxy, 2-morpholinoethoxy, 2-(1-imidazolyl)ethoxy, dimethylamino, fluoro, morpholinocarbonyl, piperidinocarbonyl, piperazinocarbonyl or cyclohexylcarbamoyl;
- (i′) R8 is hydrogen, C1-C8alkyl, e.g. methyl, ethyl or isopropyl, haloC1-C8alkyl, e.g. trifluoromethyl, C5-C10aryl, e.g. phenyl or methoxyphenyl, unsubstituted or substituted 5 or 6 membered heterocyclyl comprising 1 or 2 hetero atoms selected from N, O and S, e.g. morpholino, piperidino, piperazino or N-methylpiperazino, C1-C8alkoxy, e.g. methoxy, ethoxy or isopropoxy, haloC1-C8alkoxy, e.g. trifluoromethoxy, C5-C10aryloxy, e.g. phenoxy, unsubstituted or substituted heterocyclyloxy, e.g. 1-methyl-4-piperidyloxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, 3-morpholinopropoxy or 2-morpholinoethoxy, unsubstituted or substituted amino, e.g. methylamino or dimethylamino, halogen, e.g. fluoro or chloro, unsubstituted or substituted sulfamoyl, e.g. sulfamoyl, methylsulfamoyl or dimethylsulfamoyl, or nitro; preferably hydrogen, methyl, piperidino, piperazino, N-methylpiperazino, morpholino, methoxy, ethoxy, trifluoromethoxy, phenoxy, 1-methyl-4-piperidyloxy, 3-morpholinopropoxy, 2-morpholinoethoxy, 3-(N-methylpiperazino)-propoxy, methylamino, fluoro, chloro, sulfamoyl or nitro;
- (j′) R10 is C1-C8alkyl, e.g. methyl, ethyl or butyl, haloC1-C8alkyl, e.g. trifluoromethyl, C1-C8alkoxy, e.g. methoxy or ethoxy, unsubstituted or substituted heterocyclylC1-C8alkoxy, e.g. 2-(1-imidazolyl)ethoxy, unsubstituted or substituted amino, e.g. methylamino or dimethylamino, halogen, e.g. fluoro or chloro; preferably methyl, butyl, methoxy, ethoxy, 2-(1-imidazolyl)ethoxy, methylamino, dimethylamino or fluoro; and
- (k′) each pair of adjacent substituents R7 and R8, or R8 and R9 or R9 and R10, are —NH—CH═CH—, —CH═CH—NH—, —NH—N═CH—, —CH═N—NH—, —CH2—CH2—CH2—, —CH2—CH2—CH2—CH2—, —O—CH2—O—, or —O—CF2—O—; preferably the pair of adjacent substituents R7 and R8 or R8 and R9 being —O—CH2—O— or the pair of adjacent substituents R9 and R10 being —NH—CH═CH—, —CH═N—NH—, —CH2—CH2—CH2—, —CH2—CH2—CH2—CH2— or —O—CF2—O—.
- Most preferred as compounds of the formula I are those wherein the substituents have the meaning given in the Examples.
- In another embodiment of the invention the invention provides a compound of formula I′ with the proviso that this does not include any of the compounds of examples 1 to 52 inclusive.
-
- in which:
- n′ is selected from 1, 2 and 3;
- R′1 is selected from C6-10aryl, C5-10heteroaryl, C3-12cycloalkyl and C3-10heterocycloalkyl;
- wherein any aryl, heteroaryl, cycloalkyl or heterocycloalkyl of R′1 is optionally substituted by 1 to 3 radicals independently selected from C1-6alkyl, C1-6alkoxy, alkoxy-substituted-C1-6alkyl, halo-substituted-C1-6alkyl, halo-substituted-C1-6alkoxy, —C(O)NR′5R′6, —S(O)0-2NR′5R′6, —S(O)0-2R′5, —C(O)R′4, —OXR′4, —NR′5XNR′5R′, —OXNR′5R′6, —OXOR′5 and —XR′4;
- wherein X′ is a bond or C1-6alkylene; R′5 is selected from hydrogen and C1-6alkyl; R′6 is selected from hydrogen, C1-6alkyl and C3-12cycloalkyl-C1-4alkyl; and R′4 is independently selected from C6-10aryl, C5-10heteroaryl, C3-12cycloalkyl and C3-10heterocycloalkyl;
- and any aryl, heteroaryl, cycloalkyl or heterocycloalkyl of R′4 is optionally substituted by 1 to 3 radicals independently selected from C1-6alkyl, C3-10heterocycloalkyl-C0-4alkyl optionally substituted with C1-6alkyl, —C(O)NR′5R′6, —XNR′5R′6, —NR′5XNR′5R′6 and —NR′5C(O)R′6; wherein X is a bond or C1-6alkylene; R′5 and R′6 are independently selected from hydrogen and C1-6alkyl;
- R′2 is selected from hydrogen and halo, cyano, C1-6alkyl, halo-substituted-C1-6alkyl;
- R′3 is selected from halo, —S(O)0-2NR′5R′6, —S(O)0-2R′6, —NR′5S(O)0-2R′6, —C(O)NR′5R′6, —C(O)R′6 and —C(O)OR′6; wherein R′5 is selected from hydrogen and C1-6alkyl; and R′6 is selected from hydrogen, C1-6alkyl and C3-12cycloalkyl;
- and the pharmaceutically acceptable salts, hydrates, solvates, isomers and prodrugs thereof.
- Preferably a compound of formula I′ in which:
-
- n′ is selected from 1 and 2;
- R′1 is selected from C6-10aryl and C5-10heteroaryl; wherein any aryl or heteroaryl of R1 is optionally substituted by 1 to 3 radicals independently selected from C1-6alkyl, C1-6alkoxy, —C(O)NR′5R′6, —OX′R′4, —C(O)R′4, —NR′5X′NR′5R′6, —OX′NR′5R′6, —OX′OR′5 and —X′R′4; wherein X′ is a bond or C1-6alkylene; R′5 is selected from hydrogen and C1-6alkyl; R′6 is selected from hydrogen, C1-6alkyl and C3-12cycloalkyl-C1-4alkyl; and R′4 is C3-10heterocycloalkyl optionally substituted by 1 to 3 radicals independently selected from C1-6alkyl, halo-substituted-C1-6alkyl, C3-10heterocycloalkyl-C0-4alkyl optionally substituted with C1-6alkyl, —C(O)NR′5R′6, —X′NR′5R′6, —NR′5X′NR′5R′6 and —NR′5C(O)R′6; wherein X′ is a bond or C1-6alkylene; R′5 and R′6 are independently selected from hydrogen and C1-6alkyl;
- R′2 is selected from hydrogen and halo;
- R′3 is selected from halo, —S(O)0-2NR′5R′6, —S(O)0-2R′6, —NR′5S(O)0-2R′6, —C(O)NR′5R′6 and —C(O)OR′6; wherein R′5 is selected from hydrogen and C1-6alkyl; and R′6 is selected from hydrogen, C1-6alkyl and C3-12cycloalkyl.
- more preferably a compound of formula I′ in which R′1 is selected from phenyl, pyridinyl, pyrazolyl and pyrimidinyl; wherein any aryl or heteroaryl of R′1 is optionally substituted by 1 to 3 radicals independently selected from ethoxy, ethyl, propyl, methyl, t-butyl, trifluoromethyl, nitrile, cyclobutyloxy, 2,2,2-trifluoroethoxy, methoxy, isobutyloxy, t-butyloxy, isopropyloxy, methyl-amino-carbonyl, cyclopropyl-methoxy, dimethylamino-propyl-amino, methoxy-ethoxy, —X′R′4, —C(O)R′4 and —OX′R′4; wherein X′ is a bond, methylene or ethylene; R′4 is selected from piperazinyl, piperidinyl, pyrrolidinyl, morpholino, azepanyl and 1,4-dioxa-8-aza-spiro[4.5]dec-8-yl; wherein R′4 is optionally substituted by 1 to 3 radicals independently selected from methyl, isopropyl, acetyl, acetyl-methyl-amino, 3-dimethylamino-2,2-dimethyl-propylamino, ethyl-methyl-amino-ethoxy, diethyl-amino-ethoxy, amino-carbonyl, ethyl, 2-oxo-pyrrolidin-1-yl, pyrrolidinyl, pyrrolidinyl-methyl, piperidinyl optionally substituted with methyl or ethyl, morpholino, dimethylamino, dimethylamino-propyl-amino, methyl-amino and ethyl-amino.
- Even more preferably a compound of formula I′ in which R′2 is selected from hydrogen and halo; and R′3 is selected from halo, dimethyl-sulfamoyl, isobutyl-sulfamoyl, methyl-sulfamoyl, ethyl-sulfamoyl, propyl-sulfonyl, ethyl-amino-carbonyl, 1-ethyl-propyl-sulfamoyl, cyclopentyl-sulfamoyl, isopropyl-sulfamoyl, cyclohexyl-sulfonyl, cyclopropyl-methyl-sulfamoyl, cyclobutyl-sulfamoyl, isopropyl-sulfonyl,
- Most preferably a compound of example 53
- In a yet further embodiment of the invention the present invention also provides a process for the production of a compound of formula I, comprising reacting a compound of formula II
- wherein R0, R1, R2, R3, R4, R5, and R6 are as defined above, and Y is a leaving group, preferably halogen such as bromide, iodine, or in particular chloride;
with a compound of formula III - wherein R7, R8, R9 and R10 are as defined above; and, if desired, converting a compound of formula I, wherein the substituents have the meaning as defined above, into another compound of formula I as defined;
and recovering the resulting compound of formula I in free from or as a salt, and, when required, converting the compound of formula I obtained in free form into the desired salt, or an obtained salt into the free form. - The reaction can be carried out in a manner known per se, the reaction conditions being dependent especially on the reactivity of the leaving group Y and the reactivity of the amino group in the aniline of formula III, usually in the presence of a suitable solvent or diluent or of a mixture thereof and, if necessary, in the presence of an acid or a base, with cooling or, preferably, with heating, for example in a temperature range from approximately −30° C. to approximately +150° C., especially approximately from 0° C. to +100° C., preferably from room temperature (approx. +20° C.) to +80° C., in an open or closed reaction vessel and/or in the atmosphere of an inert gas, for example nitrogen. Alternatively, the reaction can proceed in the presence of a suitable catalyst (for example, palladium di-benzyl-acetone), in the presence of a base (for example, caesium carbonate) and in the presence of a suitable reaction facilitator (for example, xanthphos).
- If one or more other functional groups, for example carboxy, hydroxy or amino, are or need to be protected in a compound of formula II or III, because they should not take part in the reaction, these are such groups as are usually used in the synthesis of peptide compounds, cephalosporins and penicillins, as well as nucleic acid derivatives and sugars.
- The protecting groups may already be present in precursors and should protect the functional groups concerned against unwanted secondary reactions, such as substitution reaction or solvolysis. It is a characteristic of protecting groups that they lend themselves readily, i.e. without undesired secondary reactions, to removal, typically by solvolysis, reduction, photolysis or also by enzyme activity, for example under conditions analogous to physiological conditions, and that they are not present in the end-products. The specialist knows, or can easily establish, which protecting groups are suitable with the reactions mentioned hereinabove.
- Salts of a compound of formula I with a salt-forming group may be prepared in a manner known per se. Acid addition salts of compounds of formula I may thus be obtained by treatment with an acid or with a suitable anion exchange reagent.
- Salts can usually be converted to compounds in free form, e.g. by treating with suitable basic agents, for example with alkali metal carbonates, alkali metal hydrogencarbonates, or alkali metal hydroxides, typically potassium carbonate or sodium hydroxide.
- Stereoisomeric mixtures, e.g. mixtures of diastereomers, can be separated into their corresponding isomers in a manner known per se by means of suitable separation methods. Diastereomeric mixtures for example may be separated into their individual diastereomers by means of fractionated crystallization, chromatography, solvent distribution, and similar procedures. This separation may take place either at the level of a starting compound or in a compound of formula I itself. Enantiomers may be separated through the formation of diastereomeric salts, for example by salt formation with an enantiomer-pure chiral acid, or by means of chromatography, for example by HPLC, using chromatographic substrates with chiral ligands.
- It should be emphasized that reactions analogous to the conversions mentioned in this chapter may also take place at the level of appropriate intermediates.
- The compounds of formula I, including their salts, are also obtainable in the form of hydrates, or their crystals can include for example the solvent used for crystallization (present as solvates).
- The compound of formula II used as starting materials may be obtained by reacting a compound of formula IV
- with a compound of formula V
- wherein R1, R2, R3, R4, R5 and R6 are as defined above, and Y1 and Y2 are identical or different leaving groups as defined above for Y. The reaction conditions are those mentioned above for the reaction of a compound of formula II with a compound of formula III.
- The compounds of formula IV and V are known or may be produced in accordance with known procedures.
- The compounds of formula I and their pharmaceutically acceptable salts exhibit valuable pharmacological properties when tested in vitro in cell-free kinase assays and in cellular assays, and are therefore useful as pharmaceuticals. In particular, the compounds of the invention are inhibitors of Focal Adhesion Kinase, and are useful as pharmaceuticals to treat conditions caused by a malfunction of signal cascades connected with Focal Adhesion Kinase, in particular tumors as described hereinbelow.
- Focal Adhesion Kinase (FAK) is a key enzyme in the integrin-mediated outside-in signal cascade (D. Schlaepfer et al., Prog Biophys Mol Biol 1999, 71, 435-478). Interaction between cells and extracellular matrix (ECM) proteins is transduced as intracellular signals important for growth, survival and migration through cell surface receptors, integrins. FAK plays an essential role in these integrin-mediated outside-in signal cascades. The trigger in the signal transduction cascade is the autophosphorylation of Y397. Phosphorylated Y397 is a SH2 docking site for Src family tyrosine kinases. The bound c-Src kinase phosphorylates other tyrosine residues in FAK. Among them, phosphorylated Y925 becomes a binding site for the SH2 site of Grb2 small adaptor protein. This direct binding of Grb2 to FAK is one of the key steps for the activation of down stream targets such as the Ras-ERK2/MAP kinase cascade.
- The inhibition of endogenous FAK signalling results in reduced motility and in some cases induces cell death. On the other hand, enhancing FAK signalling by exogenous expression increases cell motility and transmitting a cell survival signal from ECM. In addition FAK is overexpressed in invasive and metastatic epithelial, mesenchymal, thyroid and prostate cancers. Consequently, an inhibitor of FAK is likely to be a drug for anti-tumor growth and metastasis. The compounds of the invention are thus indicated, for example, to prevent and/or treat a vertebrate and more particularly a mammal, affected by a neoplastic disease, in particular breast tumor, cancer of the bowel (colon and rectum), stomach cancer and cancer of the ovary and prostate, non-small cell lung cancer, small cell lung cancer, cancer of liver, melanoma, bladder tumor and cancer of head and neck.
- The relation between FAK inhibition and immuno-system is described e.g. in G. A. van Seventer et al., Eur. J. Immunol. 2001, 31, 1417-1427. Therefore, the compounds of the invention are, for example, useful to prevent and/or treat a vertebrate and more particularly a mammal, affected by immune system disorders, diseases or disorders mediated by T lymphocytes, B lymphocytes, mast cells and/or eosinophils e.g. acute or chronic rejection of organ or tissue allo- or xenografts, atherosclerosis, vascular occlusion due to vascular injury such as angioplasty, restenosis, hypertension, heart failure, chronic obstructive pulmonary disease, CNS disease such as Alzheimer disease or amyotrophic lateral sclerosis, cancer, infectious disease such as AIDS, septic shock or adult respiratory distress syndrome, ischemia/reperfusion injury e.g. myocardial infarction, stroke, gut ischemia, renal failure or hemorrhage shock, or traumatic shock. The agent of the invention are also useful in the treatment and/or prevention of acute or chronic inflammatory diseases or disorders or autoimmune diseases e.g. rheumatoid arthritis, osteoarthritis, systemic lupus erythematosus, Hashimoto's thyroiditis, multiple sclerosis, myasthenia gravis, diabetes (type I and II) and the disorders associated with therewith, respiratory diseases such as asthma or inflammatory liver injury, inflammatory glomerular injury, cutaneous manifestations of immunologically-mediated disorders or illnesses, inflammatory and hyperproliferative skin diseases (such as psoriasis, atopic dermatitis, allergic contact dermatitis, irritant contact dermatitis and further eczematous dermatitises, seborrhoeic dermatitis), inflammatory eye diseases, e.g. Sjoegren's syndrome, keratoconjunctivitis or uveitis, inflammatory bowel disease, Crohn's disease or ulcerative colitis.
- Compounds of the invention are active in a FAK assay system as described in the Examples, and show an inhibition IC50 in the range of 1 nM to 100 nM. Particularly active are the compounds Example No. 3-12 and No. 3-17 described hereinbelow showing IC50 vales in the range of 1 to 5 nM.
- Some of the compounds of the invention exhibit also ZAP-70 (zeta chain-associated protein of 70 kD) protein tyrosine kinase inhibiting activity. ZAP-70 protein tyrosine kinase interaction of the agents of the invention may be demonstrated by their ability to prevent phosphorylation of e.g. LAT-11 (linker for activation of T cell) by human ZAP-70 protein tyrosine kinase in aqueous solution, as described in the Examples. The compounds of the invention are thus also indicated for the prevention or treatment of disorders or diseases where ZAP-70 inhibition play a role.
- Compounds of the invention are active in a ZAP-70 assay system as described in the Examples, and show an inhibition IC50 in the range of 1 μM to 10 μM, e.g. the compounds Example No. 2 and No. 3-2 described hereinbelow.
- Compounds of the present invention are also good inhibitors of the IGF-IR (insulin like growth factor receptor 1) and are therefore useful in the treatment of IGF-1R mediated diseases for example such diseases include proliferative diseases, such as tumours, like for example breast, renal, prostate, colorectal, thyroid, ovarian, pancreas, neuronal, lung, uterine and gastro-intestinal tumours as well as osteosarcomas and melanomas. The efficacy of the compounds of the invention as inhibitors of IGF-IR tyrosine kinase activity can be demonstrated using a cellular “Capture ELISA”. In this assay the activity of the compounds of the invention against Insulin-like growth factor I (IGF-I) induced autophosphorylation of the IGF-IR is determined.
- The compounds of formula I and their pharmaceutically acceptable salts exhibit valuable pharmacological properties when tested in vitro in cell-free kinase assays and in cellular assays, and are therefore useful as pharmaceuticals. In particular, the compounds of the invention are inhibitors of Anaplastic Lymphoma Kinase (ALK), and are useful as pharmaceuticals to treat conditions caused by a malfunction of signal cascades connected with Anaplastic Lymphoma Kinase, in particular tumors as described hereinbelow.
- ALK-mediated signaling could play a role in the development and/or progression of a number of common solid tumors (Pulford, K., et al., J. Cell. Physiol. 2004 June; 199(3):330-58). The compounds of the present invention also exhibit powerful inhibition of the tyrosine kinase activity of anaplastic lymphoma kinase (ALK) and its fusion proteins, particularly the fusion protein of NPM-ALK. This protein tyrosine kinase results from a gene fusion of nucleophosmin (NPM) and the anaplastic lymphoma kinase (ALK), rendering the protein tyrosine kinase activity of ALK ligand-independent. NPM-ALK plays a key role in signal transmission in a number of hematopoetic and other human cells leading to hematological and neoplastic diseases, for example in anaplastic large-cell lymphoma (ALCL) and non-Hodgkin's lymphomas (NHL), specifically in ALK+NHL or Alkomas, in inflammatory myofibroblastic tumors (IMT) and neuroblastomas. (Duyster J et al. 2001 Oncogene 20, 5623-5637). NPM-ALK has been shown to be a potent oncogene in vitro, being able to transform various cell lines and primary hematopoetic cells. Furthermore, NPM-ALK transduced bone marrow cells are able to induce a lymphoma-like disease after transplantation into irradiated recipient mice. Signaling pathways activated by NPM-ALK include ras, PLC and PI3K pathways and, in addition, STAT5 has been shown to be phosphorylated by NPM-ALK. In addition to NPM-ALK, other gene fusions have been identified in human hematological and neoplastic diseases; mainly TPM3-ALK (a fusion of nonmuscle tropomyosin 3 with ALK). Further, the ALK fusion protein CLTC-ALK, is associated with diseases that include classical T cell or null ALCL, ALK+ DLBCL and inflammatory myofibroblastic tumors. CLTCL-ALK is also thought to play a role in the pathogenesis of large B-cell lymphomas.
- Further, the ALK fusion protein CLTC-ALK is associated with diseases that include classical T cell or null ALCL, ALK+ DLBCL and inflammatory myofibroblastic tumors. CLTCL-ALK is also thought to play a role in the pathogenesis of large B-cell lymphomas.
- Aberrant activity of ALK is involved in the development of brain tumors and overexpression of ALK has been reported in neuroblastomas and several cell lines derived from neural tissue. ALK-mediated signaling could play a role in the development and/or progression of a number of common solid tumors (Pulford, K., et al., J. Cell. Physiol. 2004 June; 199(3):330-58).
- The inhibition of ALK tyrosine kinase activity can be demonstrated using known methods, for example using the recombinant kinase domain of the ALK in analogy to the VEGF-R kinase assay described in J. Wood et al. Cancer Res. 60, 2178-2189 (2000). In vitro enzyme assays using GST-ALK protein tyrosine kinase are performed in 96-well plates as a filter binding assay in 20 mM Tris.HCl, pH=7.5, 3 mM MgCl2, 10 mM MnCl2, 1 mM DTT, 0.1 μCi/assay (=30 μl) [γ-33P]-ATP, 2 μM ATP, 3 μg/ml poly(Glu, Tyr 4:1) Poly-EY (Sigma P-0275), 1% DMSO, 25 ng ALK enzyme. Assays are incubated for 10 min at ambient temperature. Reactions are terminated by adding 50 μl of 125 mM EDTA, and the reaction mixture is transferred onto a MAIP Multiscreen plate (Millipore, Bedford, Mass., USA), previously wet with methanol, and rehydrated for 5 min with H2O. Following washing (0.5% H3PO4), plates are counted in a liquid scintillation counter. IC50 values are calculated by linear regression analysis of the percentage inhibition. Compared with the control without inhibitor, the compounds of formula I inhibit the enzyme activity by 50% (IC50), for example in a concentration of from 0.001 to 0.5 μM, especially from 0.01 to 0.1 μM.
- The compounds of formula I potently inhibit the growth of human NPM-ALK overexpressing murine BaF3 cells (DSMZ Deutsche Sammlung von Mikroorganismen and Zellkulturen GmbH, Braunschweig, Germany). The expression of NPM-ALK is achieved by transfecting the BaF3 cell line with an expression vector pCIneo™ (Promega Corp., Madison Wis., USA) coding for NPM-ALK and subsequent selection of G418 resistant cells. Non-transfected BaF3 cells depend on IL-3 for cell survival. In contrast NPM-ALK expressing BaF3 cells (named BaF3-NPM-ALK hereinafter) can proliferate in the absence of IL-3 because they obtain proliferative signal through NPM-ALK kinase. Putative inhibitors of the NPM-ALK kinase therefore abolish the growth signal and result in antiproliferative activity. The antiproliferative activity of putative inhibitors of the NPM-ALK kinase can however be overcome by addition of IL-3 which provides growth signals through an NPM-ALK independent mechanism. [For an analogous cell system using FLT3 kinase see E Weisberg et al. Cancer Cell; 1, 433-443 (2002)]. The inhibitory activity of the compounds of formula I is determined, briefly, as follows: BaF3-NPM-ALK cells (15,000/microtitre plate well) are transferred to 96-well microtitre plates. The test compounds [dissolved in dimethyl sulfoxide (DMSO)] are added in a series of concentrations (dilution series) in such a manner that the final concentration of DMSO is not greater than 1% (v/v). After the addition, the plates are incubated for two days during which the control cultures without test compound are able to undergo two cell-division cycles. The growth of the BaF3-NPM-ALK cells is measured by means of Yopro™ staining [T Idziorek et al. J. Immunol. Methods; 185: 249-258 (1995)]: 25 μl of lysis buffer consisting of 20 mM sodium citrate, pH 4.0, 26.8 mM sodium chloride, 0.4% NP40, 20 mM EDTA and 20 mM is added to each well. Cell lysis is completed within 60 min at room temperature and total amount of Yopro bound to DNA is determined by measurement using the Cytofluor II 96-well reader (PerSeptive Biosystems) with the following settings: Excitation (nm) 485/20 and Emission (nm) 530/25.
- IC50 values are determined by a computer-aided system using the formula:
-
IC50=[(ABStest−ABSstart)/(ABScontrol−ABSstart)]×100. (ABS=absorption) - The IC50 value in those experiments is given as that concentration of the test compound in question that results in a cell count that is 50% lower than that obtained using the control without inhibitor. The compounds of formula I exhibit inhibitory activity with an IC50 in the range from approximately 0.01 to 1 μM.
- The antiproliferative action of the compounds of formula I can also be determined in the human KARPAS-299 lymphoma cell line (DSMZ Deutsche Sammlung von Mikroorganismen and Zellkulturen GmbH, Braunschweig, Germany) [described in W G Dirks et al. Int. J. Cancer 100, 49-56 (2002)] using the same methodology described above for the BaF3-NPM-ALK cell line. The compounds of formula I exhibit inhibitory activity with an IC50 in the range from approximately 0.01 to 1 μM.
- The action of the compounds of formula I on autophosphorylation of the ALK can be determined in the human KARPAS-299 lymphoma cell line by means of an immunoblot as described in W G Dirks et al. Int. J. Cancer 100, 49-56 (2002). In that test the compounds of formula I exhibit an IC50 of approximately from 0.001 to 1 μM.
- Among the compounds of formula I, 2-[5-chloro-2-(2-methoxy-4-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzamide is an especially potent ALK inhibitor, in that this compound inhibits the growth of the BaF3-NPM-ALK cells with an IC50 of 97 nM. Further specifically preferred compounds that inhibit the tyrosine kinase activity of anaplastic lymphoma kinase (ALK) are the compounds described hereinafter in the examples 7A and 7B, as well as 7-2, 7-15, 19-5, 21-1, 26-3 and 28-5, respectively, all of which are having an IC50 within the range from <0.5 to 200 nM.
- For the above uses in the treatment of neoplastic diseases and immune system disorders the required dosage will of course vary depending on the mode of administration, the particular condition to be treated and the effect desired. In general, satisfactory results are indicated to be obtained systemically at daily dosages of from about 0.1 to about 100 mg/kg body weight. An indicated daily dosage in the larger mammal, e.g. humans, is in the range from about 0.5 mg to about 2000 mg, conveniently administered, for example, in divided doses up to four times a day or in retard form.
- The compounds of the invention may be administered by any conventional route, in particular parenterally, for example in the form of injectable solutions or suspensions, enterally, preferably orally, for example in the form of tablets or capsules, topically, e.g. in the form of lotions, gels, ointments or creams, or in a nasal or a suppository form. Pharmaceutical compositions comprising a compound of the invention in association with at least one pharmaceutical acceptable carrier or diluent may be manufactured in conventional manner by mixing with a pharmaceutically acceptable carrier or diluent. Unit dosage forms for oral administration contain, for example, from about 0.1 mg to about 500 mg of active substance. Topical administration is e.g. to the skin. A further form of topical administration is to the eye.
- The pharmaceutical compositions of the present invention are prepared in a manner known per se, for example by means of conventional mixing, granulating, coating, dissolving or lyophilizing processes.
- Preference is given to the use of solutions of the active ingredient, and also suspensions or dispersions, especially isotonic aqueous solutions, dispersions or suspensions which, for example in the case of lyophilized compositions comprising the active ingredient alone or together with a carrier, for example mannitol, can be made up before use. The pharmaceutical compositions may be sterilized and/or may comprise excipients, for example preservatives, stabilizers, wetting agents and/or emulsifiers, solubilizers, salts for regulating osmotic pressure and/or buffers and are prepared in a manner known per se, for example by means of conventional dissolving and lyophilizing processes. The said solutions or suspensions may comprise viscosity-increasing agents, typically sodium carboxymethylcellulose, carboxymethylcellulose, dextran, polyvinylpyrrolidone, or gelatins, or also solubilizers, e.g. Tween 80® (polyoxyethylene(20)sorbitan mono-oleate).
- Suspensions in oil comprise as the oil component the vegetable, synthetic, or semi-synthetic oils customary for injection purposes. In respect of such, special mention may be made of liquid fatty acid esters that contain as the acid component a long-chained fatty acid having from 8 to 22, especially from 12 to 22, carbon atoms, for example lauric acid, tridecylic acid, myristic acid, pentadecylic acid, palmitic acid, margaric acid, stearic acid, arachidic acid, behenic acid or corresponding unsaturated acids, for example oleic acid, elaidic acid, erucic acid, brassidic acid or linoleic acid, if desired with the addition of antioxidants, for example vitamin E, β-carotene or 3,5-di-tert-butyl-4-hydroxytoluene. The alcohol component of these fatty acid esters has a maximum of 6 carbon atoms and is a monovalent or polyvalent, for example a mono-, di- or trivalent, alcohol, for example methanol, ethanol, propanol, butanol or pentanol or the isomers thereof, but especially glycol and glycerol. As fatty acid esters, therefore, the following are mentioned: ethyl oleate, isopropyl myristate, isopropyl palmitate, “Labrafil M 2375” (polyoxyethylene glycerol), “Labrafil M 1944 CS” (unsaturated polyglycolized glycerides prepared by alcoholysis of apricot kernel oil and consisting of glycerides and polyethylene glycol ester), “Labrasol” (saturated polyglycolized glycerides prepared by alcoholysis of TCM and consisting of glycerides and polyethylene glycol ester; all available from Gattefossé, France), and/or “Miglyol 812” (triglyceride of saturated fatty acids of chain length C8 to C12 from Hüls AG, Germany), but especially vegetable oils such as cottonseed oil, almond oil, olive oil, castor oil, sesame oil, soybean oil and more especially groundnut oil.
- The manufacture of injectable preparations is usually carried out under sterile conditions, as is the filling, for example, into ampoules or vials, and the sealing of the containers.
- Pharmaceutical compositions for oral administration can be obtained, for example, by combining the active ingredient with one or more solid carriers, if desired granulating a resulting mixture, and processing the mixture or granules, if desired or necessary, by the inclusion of additional excipients, to form tablets or tablet cores.
- Suitable carriers are especially fillers, such as sugars, for example lactose, saccharose, mannitol or sorbitol, cellulose preparations, and/or calcium phosphates, for example tricalcium phosphate or calcium hydrogen phosphate, and also binders, such as starches, for example corn, wheat, rice or potato starch, methylcellulose, hydroxypropyl methylcellulose, sodium carboxymethylcellulose, and/or polyvinylpyrrolidone, and/or, if desired, disintegrators, such as the above-mentioned starches, also carboxymethyl starch, crosslinked polyvinylpyrrolidone, alginic acid or a salt thereof, such as sodium alginate. Additional excipients are especially flow conditioners and lubricants, for example silicic acid, talc, stearic acid or salts thereof, such as magnesium or calcium stearate, and/or polyethylene glycol, or derivatives thereof.
- Tablet cores can be provided with suitable, optionally enteric, coatings through the use of, inter alia, concentrated sugar solutions which may comprise gum arabic, talc, polyvinylpyrrolidone, polyethylene glycol and/or titanium dioxide, or coating solutions in suitable organic solvents or solvent mixtures, or, for the preparation of enteric coatings, solutions of suitable cellulose preparations, such as acetylcellulose phthalate or hydroxypropylmethylcellulose phthalate. Dyes or pigments may be added to the tablets or tablet coatings, for example for identification purposes or to indicate different doses of active ingredient.
- Pharmaceutical compositions for oral administration also include hard capsules consisting of gelatin, and also soft, sealed capsules consisting of gelatin and a plasticizer, such as glycerol or sorbitol. The hard capsules may contain the active ingredient in the form of granules, for example in admixture with fillers, such as corn starch, binders, and/or glidants, such as talc or magnesium stearate, and optionally stabilizers. In soft capsules, the active ingredient is preferably dissolved or suspended in suitable liquid excipients, such as fatty oils, paraffin oil or liquid polyethylene glycols or fatty acid esters of ethylene or propylene glycol, to which stabilizers and detergents, for example of the polyoxyethylene sorbitan fatty acid ester type, may also be added.
- Pharmaceutical compositions suitable for rectal administration are, for example, suppositories that consist of a combination of the active ingredient and a suppository base. Suitable suppository bases are, for example, natural or synthetic triglycerides, paraffin hydrocarbons, polyethylene glycols or higher alkanols.
- For parenteral administration, aqueous solutions of an active ingredient in water-soluble form, for example of a water-soluble salt, or aqueous injection suspensions that contain viscosity-increasing substances, for example sodium carboxymethylcellulose, sorbitol and/or dextran, and, if desired, stabilizers, are especially suitable. The active ingredient, optionally together with excipients, can also be in the form of a lyophilizate and can be made into a solution before parenteral administration by the addition of suitable solvents.
- Solutions such as are used, for example, for parenteral administration can also be employed as infusion solutions.
- Preferred preservatives are, for example, antioxidants, such as ascorbic acid, or microbicides, such as sorbic acid or benzoic acid.
- The compounds of the invention may be administered as the sole active ingredient or together with other drugs useful against neoplastic diseases or useful in immunomodulating regimens. For example, the agents of the invention may be used in accordance with the invention in combination with pharmaceutical compositions effective in various diseases as described above, e.g. with cyclophosphamide, 5-fluorouracil, fludarabine, gemcitabine, cisplatinum, carboplatin, vincristine, vinblastine, etoposide, irinotecan, paclitaxel, docetaxel, rituxan, doxorubicine, gefitinib, or imatinib; or also with cyclosporins, rapamycins, ascomycins or their immunosuppressive analogs, e.g. cyclosporin A, cyclosporin G, FK-506, sirolimus or everolimus, corticosteroids, e.g. prednisone, cyclophosphamide, azathioprene, methotrexate, gold salts, sulfasalazine, antimalarials, brequinar, leflunomide, mizoribine, mycophenolic acid, mycophenolate, mofetil, 15-deoxyspergualine, immuno-suppressive monoclonal antibodies, e.g. monoclonal antibodies to leukocyte receptors, e.g. MHC, CD2, CD3, CD4, CD7, CD25, CD28, CD40, CD45, CD58, CD80, CD86, CD152, CD137, CD154, ICOS, LFA-1, VLA-4 or their ligands, or other immunomodulatory compounds, e.g. CTLA4Ig.
- In accordance with the foregoing, the present invention also provides:
- (1) A compound of the invention for use as a pharmaceutical;
(2) a compound of the invention for use as a 5-Chloro-N*2*-{2-methoxy-4-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-phenyl}-N*4*-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine, for example for use in any of the particular indications hereinbefore set forth;
(3) a pharmaceutical composition, e.g. for use in any of the indications herein before set forth, comprising a compound of the invention as active ingredient together with one or more pharmaceutically acceptable diluents or carriers;
(4) a method for the treatment of any particular indication set forth hereinbefore in a subject in need thereof which comprises administering an effective amount of a compound of the invention or a pharmaceutical composition comprising same;
(5) the use of a compound of the invention for the manufacture of a medicament for the treatment or prevention of a disease or condition in which FAK and/or ALK and/or ZAP-70 and/or IGF-I activation plays a role or is implicated, preferably ALK;
(6) the method as defined above under (4) comprising co-administration, e.g. concomitantly or in sequence, of a therapeutically effective amount of a compound of the invention and one or more further drug substances, said further drug substance being useful in any of the particular indications set forth hereinbefore;
(7) a combination comprising a therapeutically effective amount of a compound of the invention and one or more further drug substances, said further drug substance being useful in any of the particular indications set forth hereinbefore;
(8) use of a compound of the invention for the manufacture of a medicament for the treatment or prevention of a disease which responds to inhibition of the anaplastic lymphoma kinase;
(9) the use according to (8), wherein the disease to be treated is selected from lymphoma, anaplastic large-cell lymphoma, non-Hodgkin's lymphomas, inflammatory myofibroblastic tumors and neuroblastomas; (10) the use according to (8) or (9), wherein the compound is 2-[5-chloro-2-(2-methoxy-4-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzamide or 5-Chloro-N*2*-{2-methoxy-4-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-phenyl}-N*4*-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine or a pharmaceutically acceptable salt thereof, or any of the compounds described hereinafter in the examples or a pharmaceutically acceptable salt of any one of these;
(11) a method for the treatment of a disease which responds to inhibition of the anaplastic lymphoma kinase, especially a disease selected from anaplastic large-cell lymphoma, non-Hodgkin's lymphomas, inflammatory myofibroblastic tumors and neuroblastomas, comprising administering an effective amount of a compound of the invention, especially 2-[5-chloro-2-(2-methoxy-4-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzamide or 5-Chloro-N*2*-{2-methoxy-4-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-phenyl}-N*4*-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine or a pharmaceutically acceptable salt thereof. - Additionally preferred a compound according to the present invention that is useful as herein before described is a compound specifically mentioned in the examples.
- Additional specifically preferred compounds according to the present invention that are useful either as FAK inhibitor, as ALK inhibitor or for inhibition of both and which may be prepared essentially according to the methods described hereinbefore are the following:
- 2-[5-chloro-2-(2-methoxy-4-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzamide,
- N2-(4-[1,4]Bipiperidinyl-1′-yl-2-methoxy-phenyl)-5-chloro-N4-[2-(propane-1-sulfonyl)-phenyl]-pyrimidine-2,4-diamine,
- 2-{5-Chloro-2-[2-methoxy-4-(4-methyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-ylamino}-N-isopropyl-benzenesulfonamide,
- 2-[5-Bromo-2-(2-methoxy-5-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzenesulfonamide
- 2-{2-[5-(1-Acetyl-piperidin-4-yloxy)-2-methoxy-phenylamino]-5-bromo-pyrimidin-4-ylamino}-N-methyl-benzenesulfonamide,
- N-[5-Bromo-2-(2,5-dimethoxy-phenylamino)-pyrimidin-4-yl]-N-(4-morpholin-4-yl-phenyl)-methanesulfonamide,
- 5-Bromo-N-4-(4-fluoro-phenyl)-N*2*-(2-methoxy-4-morpholin-4-yl-phenyl)-pyrimidine-2,4-diamine,
- 2-[5-Chloro-2-(2-methoxy-4-piperazin-1-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzenesulfonamide,
- 2-[5-Bromo-2-(5-fluoro-2-methoxy-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzenesulfonamide,
- 2-[5-Chloro-2-(5-fluoro-2-methoxy-phenylamino)-pyrimidin-4-ylamino]-N-isobutyl-benzenesulfonamide, and
- 2-{5-Chloro-2-[2-methoxy-5-(4-methyl-piperazin-1-ylmethyl)-phenylamino]-pyrimidin-4-ylamino}-N-methyl-benzenesulfonamide,
- 5-Chloro-N*2*-{2-methoxy-4-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-phenyl}-N*4*-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine.
- The invention also provides a compound of formula 2-{5-Chloro-2-[4-(3-methylamino-pyrrolidin-1-yl)-phenylamino]-pyrimidin-4-ylamino}-N-isopropyl-benzenesulfonamide
- The invention also provides a compound of formula 5-Chloro-N*2*-{2-methoxy-4-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-phenyl}-N*4*-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine
- The following Examples serve to illustrate the invention without limiting the invention in its scope.
- AcOH=acetic acid, ALK=anaplastic lymphoma kinase, ATP=adenosine 5′-triphosphate, brine=saturated sodium chloride solution, BSA=bovine serum albumin, DIAD=diisopropyl azodicarboxylate, DIPCDI=N,N′-diisopropylcarbodiimid, DMAP=4-dimethylaminopyridine, DMF=N,N-dimethylformamide, DTT=1,4-dithio-D,L-threitol, EDTA=ethylene diamine tetraacetic acid, Et=ethyl, EtOAc=ethyl acetate, EtOH=ethanol, Eu-PT66=LANCE™ europium-W1024-labelled anti-phosphotyrosine antibody (Perkin Elmer), FAK=Focal Adhesion Kinase, FRET=fluorescence resonance energy transfer, HEPES=N-2-hydroxyethyl-piperazine-N′-2-ethanesulfonic acid, HOAt=1-hydroxy-7-azabenzotriazole, Me=methyl, RT-PCR=reverse transcription polymerase chain reaction, SA-(SL)APC=Streptavidin conjugated to SuperLight™ allophycocyanin (Perkin Elmer), subst.=substituted, TBTU=O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethylammonium tetrafluoroborate, THF=tetrahydrofuran.
-
- To a solution of 2-(2-chloro-5-nitro-pyrimidin-4-ylamino)-N-methyl-benzenesulfonamide (100 mg, 0.29 mmol) in EtOH (3 mL), 2,5-dimethoxyaniline (49 mg, 0.32 mmol) is added at room temperature. The mixture is heated at 78° C. for 5 h. The solvent is evaporated, and the mixture is purified by reverse phase HPLC to give the title product in.
- Rf=0.47 (n-hexane:ethyl acetate=1:1). 1H-NMR (400 MHz, CDCl3), δ (ppm): 2.36 (d, 3H), 3.57 (s, 3H), 3.73 (s, 3H), 6.72 (d, 1H), 6.99 (d, 1H), 7.17 (s, 1H), 7.35 (t, 1H), 7.4-7.6 (m, 1H), 7.63 (d, 1H), 7.81 (d, 1H), 8.0-8.2 (m, 1H), 9.13 (s, 1H), 9.41 (br.s, 1H), 11.0 (s, 1H).
- 2,4-Dichloro-5-nitro-pyrimidine (1.94 g, 10 mmol) and 2-amino-N-methyl-benzenesulfonamide (1.86 g, 10 mmol) are dissolved in CHCl3 (30 mL). The reaction mixture is heated at 61° C. for 2 h. The solvent is evaporated and the residue is washed with ether to give the title product.
- Rf=0.5 (n-hexane:ethyl acetate=1:1). 1H-NMR (400 MHz, CDCl3), δ (ppm): 2.67 (d, 3H), 4.6-4.7 (m, 2H), 7.41 (dd, 1H), 7.7 (dd, 1H), 8.04 (d, 1H), 8.15 (d, 1H), 9.21 (s, 1H), 11.2 (s, 1H).
-
- To a solution of 2-(5-bromo-2-chloro-pyrimidin-4-ylamino)-N-methyl-benzenesulfonamide (300 mg, 0.79 mmol), 2,4-dimethoxyaniline (181.5 mg, 1.18 mmol) in ethanol (3 mL), 1 N hydrochloric acid (0.03 mL) is added and stirred under reflux condition for 5 hours. The reaction mixture is cooled to room temperature, poured into water and extracted twice with ethyl acetate. The organic layer is successively washed with water and brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1 to 1:1) to afford the title compound.
- 1H-NMR (CDCl3), δ (ppm): 8.95 (s, 1H), 8.44 (d, 1H), 8.20 (s, 1H), 7.98 (dd, 1H), 7.58 (ddd, 1H), 7.22-7.32 (m, 1H), 6.51 (d, 1H), 6.40 (d, 1H), 4.56-4.48 (m, 1H), 3.86 (s, 3H), 3.81 (s, 3H), 2.64 (d, 3H). Rf (n-hexane:ethyl acetate=1:1): 0.31.
- A solution of 5-bromo-2,4-dichloropyrimidine (684 mg, 3.0 mmol) and 2-amino-N-methyl-benzenesulfonamide (559 mg, 3.0 mmol) in N,N-dimethylformamide (10 mL) containing potassium carbonate (830 mg, 6.0 mmol) is stirred at room temperature for 23 hours. Saturated aqueous ammonium chloride is added and the mixture is poured into water and extracted twice with ethyl acetate. The organic layer is washed with brine, dried over sodium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane-ethyl acetate gradient) to afford the title compound as a slightly yellow solid.
- 1H-NMR (CDCl3), δ (ppm): 2.67 (d, 3H), 4.79 (q, 1H), 7.26 (s, 1H), 7.29 (ddd, 1H), 7.66 (ddd, 1H), 7.95 (dd, 1H), 8.37 (s, 1H), 8.48 (d, 1H), 9.52 (s, 1H). Rf (n-hexane:ethyl acetate=10:3): 0.33.
- The following 2-[5-bromo-2-(subst. phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzene-sulfonamides are prepared from 2-(5-bromo-2-chloro-pyrimidin-4-ylamino)-N-methyl-benzenesulfonamide and the corresponding aniline following the procedure of Example 2:
-
Rf (solvent) ExplNo. Rx or MS 1H-NMR (400 MHz), δ (ppm) 3-1 0.48 (n-hexane: AcOEt = 1:1) CDCl3: 2.64 (d, 3H), 4.48-4.40 (m, 1H), 6.78 (d, 1H), 6.87 (bs, 1H), 6.99 (dd, 1H), 6.82 (s, 1H), 7.54 (ddd, 1H), 7.79 (d, 1H), 7.97 (dd, 1H), 8.28 (s, 1H), 8.32 (dd, 1H), 9.07 (s, 1H) 3-2 0.58 (n-hexane: AcOEt = 1:1) CDCl3: 2.25 (s, 3H), 2.33 (s, 3H), 2.63 (d, 3H), 4.53- 4.45 (m, 1H), 6.61 (bs, 1H), 6.99 (dd, 1H), 7.04 (s, 1H), 7.18 (ddd, 1H), 7.43 (ddd, 1H), 7.56 (d, 1H), 7.92 (dd, 1H), 8.19 (s, 1H), 8.41 (dd, 1H), 9.08 (s, 1H) 3-3 0.36 (n-hexane: AcOEt = 1:1) CDCl3: 2.23 (s, 3H), 2.62 (d, 3H), 3.69 (s, 3H), 4.53- 4.44 (m, 1H), 6.62 (dd, 1H), 6.69 (bs, 1H), 7.10 (d, 1H), 7.19 (dd, 1H), 7.48 (d, 1H), 7.51 (dd, 1H), 7.93 (dd, 1H), 8.22 (s, 1H), 8.44 (dd, 1H), 9.09 (s1, 1H) 3-4 0.41 (n-hexane: AcOEt = 1:1) CDCl3: 2.32 (s, 3H), 2.63 (d, 3H), 4.45-4.44 (m, 1H), 6.85 (d, 1H), 6.91 (d, 1H), 7.00 (bs, 1H), 7.28-7.24 (m, 1H), 7.57 (dd, 1H), 7.99 (dd, 1H), 8.25 (s, 1H), 8.39 (d, 1H), 9.00 (bs, 1H) 3-5 0.39 (n-hexane: AcOEt = 1:1) CDCl3: 2.33 (s, 3H), 2.63 (d, 3H), 3.87 (s, 3H), 4.46- 4.44 (m, 1H), 6.66 (d, 1H), 6.71 (s, 1H), 7.48 (bs, 1H), 7.63-7.59 (m, 1H), 7.97 (dd, 1H), 8.05 (d, 1H), 8.23 (s, 1H), 8.44 (d, 1H), 8.92 (bs, 1H) 3-6 0.27 (n-hexane: AcOEt = 3:1) CDCl3: 2.63 (d, 3H), 3.90 (s, 3H), 4.45-4.40 (m, 1H), 6.90-6.86 (m, 2H), 7.00-6.96 (m, 1H), 7.23-7.17 (m, 3H), 7.45 (dd, 1H), 7.50-7.60 (m, 2H), 7.97 (dd, 1H), 8.22 (d, 1H), 8.26 (s, 1H), 8.43 (d, 1H), 8.94 (bs, 1H) 3-7 0.34 (n-hexane: AcOEt = 3:1) CDCl3: 2.30 (s, 3H), 2.63 (d, 3H), 4.44-4.43 (m, 1H), 6.68 (bs, 1H), 7.00-6.68 (m, 1H), 7.23-7.17 (m, 2H), 7.46-7.43 (m, 1H), 7.76 (d, 1H), 7.93 (dd, 1H), 8.22 (s, 1H), 8.40 (d, 1H), 9.01 (bs, 1H) 3-8 0.12 (n-hexane: AcOEt = 3:1) CDCl3: 2.62 (d, 3H), 2.81 (s, 3H), 4.07-3.98 (m, 1H), 4.52-4.45 (m, 1H), 6.37 (bs, 1H), 6.77-6.73 (m, 2H), 7.12 (dd, 1H), 7.24-7.20 (m, 1H), 7.30-7.27 (m, 1H), 7.35 (dd, 1H), 7.88 (dd, 1H), 8.18 (s, 1H), 8.41 (d, 1H), 9.19 (bs, 1H) 3-9 0.28 (n-hexane: AcOEt = 3:1) CDCl3: 2.62 (d, 3H), 3.94 (s, 3H), 4.49-4.43 (m, 1H), 6.99-6.90 (m, 3H), 7.18-7.23 (m, 1H), 7.31-7.24 (m, 3H), 7.63 (bs, 1H), 7.93-7.86 (m, 1H), 8.28-8.23 (m, 1H), 8.28 (s, 1H), 8.45 (bs, 1H), 8.89 (bs, 1H) 3-10 0.23 (n-hexane: AcOEt = 3:1) CDCl3: 0.91 (t, 3H), 1.37 (dd, 2H), 1.64-1.55 (m, 2H), 2.64-2.60 (m, 2H), 4.45-4.40 (m, 1H), 6.69 (bs, 1H), 7.23-7.10 (m, 1H), 7.46-7.38 (m, 1H), 7.73 (d 1H), 7.92 (d, 1H), 8.21 (s, 1H), 8.38-8.46 (m, 1H), 9.09 (bs, 1H) 3-11 0.12 (n-hexane: AcOEt = 3:1) CDCl3: 2.63 (d, 3H), 4.15-4.10 (m, 1H), 6.58 (bs, 1H), 7.31-7.10 (m, 4H), 7.53-7.49 (m, 1H), 7.71 (d 1H), 7.95 (d, 1H), 8.30-8.23 (m, 1H), 8.26 (s, 1H), 8.45 (d, 1H), 9.03 (bs, 1H) 3-12 0.4 (n-hexane: AcOEt = 3:1) CDCl3: 2.09 (dd, 2H), 2.63 (d, 3H), 2.85 (t, 2H), 2.96 (t, 2H), 4.46-4.43 (m, 2H), 6.73 (bs, 1H), 6.99 (d, 1H), 7.09 (dd, 1H), 7.25-7.20 (m, 1H), 7.52 (dd, 1H), 7.74 (d 1H), 7.92 (dd, 1H), 8.22 (s, 1H), 8.42 (d, 1H), 9.02 (bs, 1H) 3-13 0.33 (AcOEt) CDCl3: 2.63 (d, 3H), 4.63-4.64 (m, 1H), 7.11 (d, 2H), 7.18 (dd, 1H), 7.42-7.34 (m, 1H), 7.58-7.55 (m, 1H), 7.96 (d, 1H), 8.07 (s, 1H), 8.19-8.10 (m, 1H), 8.24 (s, 1H), 9.15 (s, 1H), 11.6-11.4 (m, 1H) 3-14 0.28 (n-hexane: AcOEt = 3:1) CDCl3: 2.63 (d, 3H), 3.88 (s, 3H), 3.89 (s, 3H), 4.47- 4.41 (m, 1H), 6.60 (d, 1H), 6.92 (dd,1H), 7.64 (dd, 1H), 7.66-7.61 (m, 1H), 7.89 (d, 1H), 7.98 (dd, 1H), 8.26 (s, 1H), 8.43 (d, 1H), 8.95 (s, 1H) 3-15 0.30 (n-hexane: AcOEt = 3:1) CDCl3: 2.63 (d, 3H), 3.66 (s, 3H), 3.85 (s, 3H), 4.45- 4.44 (m, 1H), 6.48 (dd, 1H), 6.79 (d, 1H), 7.64 (dd, 1H), 7.97 (dd, 2H), 8.26 (s, 1H), 8.44 (d, 1H), 8.96 (s, 1H) 3-16 0.22 (n-hexane: AcOEt = 3:1) CDCl3: 2.17 (s, 3H), 2.22 (s, 3H), 2.64 (s, 3H), 2.63 (d, 3H), 4.46-4.44 (m, 1H), 6.57 (bs, 1H), 7.00 (s, 1H), 7.17 (dd, 1H), 7.44-7.40 (m, 1H), 7.44 (s, 1H), 7.93 (dd, 1H), 8.19 (s, 1H), 8.43 (d, 1H), 9.06 (s, 1H) 3-17 0.46 (AcOEt) CDCl3: 2.22 (s, 3H), 2.63 (d, 3H), 3.68 (s, 3H), 3.89 (s, 3H), 4.52-4.47 (m, 1H), 6.51 (s, 1H), 6.74 (s, 1H), 7.12 (s, 1H), 7.16-7.12 (m, 1H), 7.40 (dd, 1H), 7.91 (dd, 1H), 8.19 (s, 1H), 8.42 (d, 1H), 9.12 (s, 1H) 3-18 0.35 (n-hexane: AcOEt = 3:1) CDCl3: 1.16 (d, 6H), 2.25 (s, 3H), 2.62 (d, 3H), 2.77 (t, 1H), 4.49-4.48 (m, 1H), 7.00 (s, 1H), 7.15 (d, 1H), 7.41- 7.37 (m, 1H), 7.49 (d, 2H), 7.54 (dd, 1H), 7.92 (dd, 1H), 8.21 (s, 1H), 8.32 (d, 1H), 9.02 (s, 1H) 3-19 0.23 (n-hexane: AcOEt = 1:1) CDCl3: 2.63 (d, 3H), 3.13-3.10 (m, 4H), 3.87 (s, 3H), 3.89-3.86 (m, 4H), 4.97-4.93 (m, 1H), 6.41 (dd, 1H), 6.52 (d, 1H), 7.24-7.22 (m, 1H), 7.32 (s, 1H), 7.57 (dd, 1H), 7.96 (dd, 1H), 8.01 (d, 1H), 8.14 (s, 1H), 8.44 (d, 1H), 8.98 (s, 1H) 3-20 0.36 (n-hexane: AcOEt = 1:1) CDCl3: 2.22 (s, 3H), 2.64 (d, 3H), 3.00-3.2.97 (m, 4H), 3.76-3.74 (m, 4H), 4.54-4.50 (m, 1H), 6.64 (d, 1H), 6.66 (dd, 1H), 7.11 (d, 1H), 7.18 (dd, 1H), 7.37 (d, 1H), 7.46 (dd, 1H), 7.93 (dd, 1H), 8.22 (s, 1H), 8.42 (d, 1H), 9.09 (s, 1H) 3-21 3-22 0.27 (AcOEt) CDCl3: 2.33 (s, 3H), 2.65 (d, 3H), 3.60-3.45 (m, 8H), 4.53-4.49 (m, 1H), 6.74 (s, 1H), 7.11 (d, 1H), 7.22- 7.18 (m, 1H), 7.58-7.54 (m 1H), 7.94 (dd, 1H), 8.00 (d, 1H), 8.22 (s, 1H), 8.37 (d, 1H), 9.13 (s, 1H) 3-23 0.38 (AcOEt) CDCl3: 1.24-1.08 (m, 2H), 1.46-1.32 (m, 2H), 1.76- 1.67 (m, 2H), 1.98-1.90 (m, 2H), 2.33 (s, 3H), 2.64 (d, 3H), 3.95-3.90 (m, 1H), 4.49-4.47 (m, 1H), 5.89- 5.80 (m, 1H), 6.66 (s, 1H), 7.15 (dd, 1H), 7.48-7.31 (m, 2H), 7.91 (dd, 1H), 8.12 (s, 1H), 8.23 (s, 1H), 8.41 (d, 1H), 9.18 (s, 1H) 3-24 0.11 (AcOEt) CDCl3: 2.35 (s, 3H), 2.71 (s, 3H), 3.07-2.73 (m, 2H), 3.86-3.31 (m, 6H), 6.85 (s, 1H), 7.10 (d, 1H), 7.24- 7.19 (m, 1H), 7.52-7.48 (m, 1H), 7.66-7.59 (m, 2H), 7.93 (d, 1H), 8.06 (s, 1H), 8.27-8.21 (m, 1H), 8.23 (s, 1H), 9.11 (s, 1H) 3-25 0.5 (n-hexane: AcOEt = 1:1) CDCl3: 2.52 (d, 3H), 2.62 (s, 3H), 4.36-4.32 (m, 1H), 6.74 (s, 1H), 6.87 (d, 2H), 7.00-6.91 (m, 2H), 7.00- 6.97 (m, 2H), 7.38 (dd, 2H), 7.86 (dd, 1H), 7.98 (s, 1H), 8.23 (s, 1H), 8.28 (d, 1H), 9.04 (s, 1H) 3-26 0.45 (n-hexane: AcOEt = 1:1) CDCl3: 1.62-1.34 (m, 6H), 2.13 (s, 3H), 2.56 (d, 3H), 3.01-2.87 (m, 4H), 4.54-4.38 (m, 1H), 6.59 (s, 1H), 6.69-6.59 (m, 1H), 7.02 (d, 1H), 7.10-7.07 (m, 1H), 7.37 (dd, 1H), 7.84 (dd, 1H), 8.15 (s, 1H), 8.34 (d, 1H), 9.01 (s, 1H) 3-27 0.45 (n-hexane: AcOEt = 1:1) CDCl3: 2.32 (s, 3H), 2.58 (d, 3H), 3.75 (s, 3H), 4.37- 4.44 (m, 1H), 6.77-6.73 (m, 1H), 6.89-6.82 (m 1H), 6.97-6.91 (m, 2H), 6.96 (d, 1H), 7.20 (dd, 1H), 7.25- 7.24 (m, 1H), 7.33-7.29 (m, 1H) 3-28 0.35 (n-hexane: AcOEt = 1:1) CDCl3: 2.34 (s, 3H), 2.64 (d, 3H), 3.81 (s, 3H), 4.57- 4.50 (m, 1H), 6.76 (bs, 1H), 6.91-6.84 (m, 41H), 7.04 (d, 1H), 7.83 (dd, 1H), 8.06 (d, 1H), 8.19 (dd, 1H), 8.23 (s, 1H), 9.00 (s, 1H) 3-29 0.45 (n-hexane: AcOEt = 1:1) CDCl3: 1.50 (t, 3H), 2.62 (d, 3H), 4.17 (dd, 2H), 4.51- 4.44 (m, 1H), 6.95-6.89 (m, 2H), 6.94 (d, 1H), 7.16 (dd, 1H), 7.31-7.23 (m, 5H), 7.67 (s, 1H), 7.11 (dd, 1H), 7.23 (d, 2H), 7.65 (s, 1H), 7.88 (dd, 1H), 8.28-8.23 (m, 1H), 8.28 (s, 1H), 8.43 (s, 1H), 8.89 (s, 1H) 3-30 0.45 (n-hexane: AcOEt = 1:1) CDCl3: 1.49 (t, 3H), 2.63 (d, 3H), 3.85 (s, 3H), 4.16 (dd, 2H), 4.55-4.48 (m, 1H), 6.81 (dd, 1H), 6.95-6.91 (m, 3H), 7.11 (dd, 1H), 7.23 (d, 2H), 7.65 (s, 1H), 7.90- 7.88 (m, 1H), 8.28-8.26 (m, 1H), 8.27 (s, 1H), 8.39 (s, 1H), 8.90 (s, 1H) 3-31 0.29 (n-hexane: AcOEt = 1:1) 1H-NMR: (CDCl3) 1.83-1.72 (4H, m), 2.63 (3H, d), 2.66-2.62 (2H, m), 2.80 (2H, t), 4.41-4.44 (1H, m), 6.64 (1H, br.s), 6.92 (1H, d), 7.09 (1H, dd), 7.18 (1H, dd), 7.45 (1H, dd), 7.59 (1H, dd), 7.92 (1H, d), 8.20 (1H, s), 8.42 (1H, d), 9.08 (1H, br.s). 3-32 0.3 (n-hexane: AcOEt = 1:1) DMSO-d6: 2.43 (s, 3H), 2.80-2.82 (m, 4H), 3.61-3.64 (m, 4H), 3.75 (s, 3H), 6.62 (dd, 1H), 6.93 (d, 1H), 7.46 (d, 1H), 7.54 (dd, 1H), 7.77 (dd, 2H), 8.14 (bs, 1H), 8.32 (s, 1H), 8.38-8.30 (m, 1H), 9.14 (bs, 1H) 3-33 0.61 (MeOH: CH2Cl2 = 1:1) DMSO-d6: 1.59-1.68 (m, 2H), 1.88-1.98 (m, 2H), 2.13- 2.25 (m, 2H), 2.19 (s, 3H), 2.43 (s, 3H), 2.60-2.70 (m, 2H), 3.75 (s, 3H), 4.32-4.40 (m, 1H), 6.51 (dd, 1H), 6.64 (d, 1H), 7.20 (dd, 1H), 7.39 (d, 1H), 7.75 (dd, 1H), 7.70-7.78 (s, 1H), 8.22 (s, 1H), 8.26 (s, 1H), 8.38- 8.41 (m, 1H), 9.22 (s, 1H) 3-34 0.17 (AcOEt) CDCl3: 2.11 (s, 3H), 2.68 (d, 3H), 2.76-2.83 (m, 2H), 2.89-2.97 (m, 2H), 3.47-3.55 (m, 2H), 3.58-3.66 (m, 2H), 3.86 (s, 3H), 4.70-4.78 (m, 1H), 6.53 (dd, 1H), 6.81 (d, 1H), 7.23 (dd, 1H), 7.54-7.62 (m, 2H), 7.97 (dd, 1H), 8.02-8.03 (m, 1H), 8.29 (s, 1H), 8.40 (d, 1H), 8.99 (bs, 1H) 3-35 0.22 (AcOEt only) DMSO-d6: 2.40-2.48 (m, 7H), 2.63 (t, 2H), 3.50-3.58 (m, 4H), 3.77 (s, 3H), 3.91 (t, 2H), 6.60 (dd, 1H), 6.93 (d, 1H), 7.28 (dd, 1H), 7.56 (d, 1H), 7.60 (dd, 1H), 7.75- 7.80 (m, 1H), 7.80 (dd, 1H), 8.10 (s, 1H), 8.35 (s, 1H), 8.40 (d, 1H), 9.21 (s, 1H) 3-36 0.4 (n-hexane: AcOEt = 1:1) DMSO-d6: 2.43 (s, 3H), 7.03-7.08 (m, 1H), 7.21- 7.23 (m, 1H), 7.25-7.36 (m, 1H), 7.47-7.57 (m, 2H), 7.74-7.77 (m, 2H), 8.28 (s, 1H), 8.35 (d, 1H), 9.09 (s, 1H), 9.24 (s, 1H) 3-37 0.4 (n-hexane: AcOEt = 1:1) CDCl3: 2.64 (d, 3H), 4.53-4.54 (m, 1H), 6.88-6.93 (m, 1H), 7.14-7.28 (m, 3H), 7.54-7.58 (m, 1H), 7.95- 7.98 (m, 1H), 8.16-8.21 (m, 1H), 8.24 (s, 1H), 8.33- 8.36 (m, 1H), 9.05 (s, 1H) 3-38 0.42 (n-hexane: AcOEt = 1:1) CDCl3: 2.64 (d, 3H), 4.46-4.47 (m, 1H), 6.63-6.68 (m, 1H), 7.30-7.32 (m, 2H), 7.55 (s, 1H), 7.64-7.68 (m, 1H), 7.97-7.99 (m, 1H), 8.20-8.39 (m, 3H), 9.03 (s, 1H) 3-39 562, 564 [M + 1]+ CDCl3: 2.37 (s, 3H), 2.58-2.64 (m, 7H), 3.15- 3.18 (m, 4H), 3.87 (s, 3H), 4.60-4.65 (m, 1H), 6.43 (dd, 1H), 6.44-6.54 (m, 1H), 7.22 (d, 1H), 7.30 (s, 1H), 7.57 (dd, 1H), 7.94-7.99 (m, 2H), 8.18 (s, 1H), 8.45 (d, 1H), 8.95 (s, 1H) 3-40 572, 574 [M + 1]+ DMSO-d6: 1.79-1.88 (m, 2H), 1.98-2.02 (m, 2H), 2.43 (s, 3H), 3.02-3.08 (m, 3H), 3.28-3.39 (m, 2H), 3.76 (s, 3H), 6.47 (dd, 1H), 6.65 (d, 1H), 7.22 (dd, 1H), 7.39 (d, 1H), 7.45-7.50 (m, 1H), 7.74-7.77 (m, 2H), 8.18 (s, 1H), 8.22 (s, 1H), 8.41-8.44 (m, 1H), 9.21 (bs, 1H) 3-41 565, 567 [M + 1]+ DMSO-d6: 2.44 (d, 3H), 2.69-2.71 (m, 4H), 3.49- 3.52 (m, 4H), 3.76 (s, 3H), 6.45 (dd, 1H), 6.62 (d, 1H), 7.23 (ddd, 1H), 7.38 (d, 1H), 7.46-7.50 (m, 1H), 7.72- 7.77 (m, 2H), 8.19 (s, 1H), 8.22 (s, 1H), 8.42-8.45 (m, 1H), 9.22 (s, 1H) 3-42 595, 597 [M + 1]+ DMSO-d6: 2.44 (s, 3H), 3.31 (s, 6H), 3.48-3.53 (m, 8H), 3.72 (s, 3H), 6.24 (dd, 1H), 6.37 (d, 1H), 7.18-7.21 (m, 2H), 7.40-7.55 (m, 1H), 7.72-7.76 (m, 2H), 8.17- 8.19 (m, 2H), 8.40-8.50 (m, 1H), 9.23 (s, 1H) 3-43 590, 592 [M + 1]+ DMSO-d6: 1.64-1.71 (m, 2H), 1.75-1.82 (m, 2H), 2.21- 2.28 (m, 1H), 2.43 (d, 3H), 2.62-2.67 (m, 2H), 3.68- 3.74 (m, 2H), 3.76 (s, 3H), 6.45 (dd, 1H), 6.63 (d, 1H), 6.75-6.81 (m, 1H), 7.20 (ddd, 1H), 7.25-7.30 (m, 1H), 7.35 (d, 1H), 7.45-7.52 (m, 1H), 7.70-7.77 (m, 2H), 8.18 (s, 1H), 8.21 (s, 1H), 8.40-8.47 (m, 1H), 9.22 (s, 1H) 3-44 597, 599 [M + 1]+ DMSO-d6: 2.44 (s, 3H), 3.12-3.17 (m, 4H), 3.68- 3.85 (m, 4H), 3.79 (s, 3H), 6.55 (dd, 1H), 6.71 (d, 1H), 7.19-7.25 (m, 1H), 7.43 (d, 1H), 7.46-7.53 (m, 1H), 7.73-7.78 (m, 2H), 8.19-8.22 (m, 1H), 8.22 (s, 1H), 8.38-8.45 (m, 1H), 9.20 (bs, 1H) 3-45 600, 602 [M + 1]+ DMSO-d6: 1.85-1.95 (m, 2H), 2.19 (t, 2H), 2.25- 2.35 (m, 4H), 2.43 (s, 3H), 3.52-3.64 (m, 4H), 4.19 (t, 2H), 6.65 (d, 1H), 7.05 (dd, 1H), 7.20 (d, 1H), 7.23 (ddd, 1H), 7.27 (d, 1H), 7.40-7.46 (m, 1H), 7.42 (d, 1H), 7.70- 7.75 (m, 1H), 7.76 (dd, 1H), 8.32 (s, 1H), 8.45 (d, 1H), 9.22 (s, 1H), 9.23 (s, 1H) 3-46 590, 592 [M + 1]+ DMSO-d6: 2.05 (s, 3H), 2.44 (s, 3H), 3.08-3.17 (m, 4H), 3.55-3.63 (m, 4H), 3.77 (s, 3H), 6.48 (dd, 1H), 6.67 (d, 1H), 7.23 (dd, 1H), 7.41 (d, 1H), 7.45-7.52 (m, 1H), 7.76 (dd, 1H), 7.72-7.78 (m, 1H), 8.19 (s, 1H), 8.22 (s, 1H), 8.40-8.47 (m, 1H), 9.22 (bs, 1H) 3-47 548, 550 [M + 1]+ DMSO-d6: 2.43 (s, 3H), 2.82-2.87 (m, 4H), 2.99- 3.15 (m, 4H), 3.76 (s, 3H), 6.43 (dd, 1H), 6.61 (d, 1H), 7.22 (dd, 1H), 7.36 (d, 1H), 7.43-7.51 (m, 1H), 7.75 (dd, 1H), 8.17 (s, 1H), 8.21 (s, 1H), 8.38-8.45 (m, 1H), 9.12- 9.28 (m, 1H) 3-48 MS 530, 532 CDCl3: 2.65 (d, 3H), 3.96 (s, 3H), 4.40-4.48 (m, 1H), 6.85-6.88 (m, 2H), 7.22 (d, 1H), 7.25-7.31 (m, 1H), 7.56-7.65 (m, 3H), 7.79 (s, 1H), 8.00 (dd, 1H), 8.29 (s, 1H), 8.39 (dd, 1H), 9.00 (s, 1H). 3-49 Rf (AcOEt: MeOH = 9:1) 0.20 CDCl3: 2.18-2.50 (m, 4H), 2.28 (s, 3H), 2.65 (d, 3H), 3.10-3.75 (m, 4H), 3.93 (s, 3H), 4.50-4.61 (m, 1H), 6.89 (d, 1H), 7.06 (dd, 1H), 7.59-7.67 (m, 2H), 7.93- 7.97 (m, 1H), 8.26 (s, 1H), 8.37-8.43 (m, 2H), 9.02 (s, 1H). 3-50 Rf 0.4 (Hexane/Ac OEt = 1/1) CDCl3: 2.63 (d, 3H), 3.90 (s, 3H), 4.00 (s, 3H), 4.39- 4.47 (m, 1H), 6.23 (d, 1H), 7.00 (s, 1H), 7.22-7.25 (m, 1H), 7.57 (dd, 1H), 7.96 (dd, 1H), 8.22 (s, 1H), 8.25 (d, 1H), 8.37 (d, 1H), 8.96 (s, 1H) 3-51 MS 535, 537 CDCl3: 1.17 (t, 3H), 1.71-1.79 (m, 1H), 2.28 (s, 3H), 2.62 (d, 3H), 3.41 (q, 2H), 3.46 (t, 2H), 3.79 (q, 2H), 4.41-4.48 (m, 1H), 6.43 (s, 1H), 6.10-6.18 (m, 2H), 7.15 (dd, 1H), 7.33 (d, 1H), 7.35-7.42 (m, 1H), 7.90 (dd, 1H), 8.16 (s, 1H), 8.45 (d, 1H), 9.07 (s, 1H). 3-52 Rf CDCl3: 2.66 (d, 3H), 3.91 (s, 3H), 4.41-4.47 (m, 1H), 6.80 (d, 1H), 6.92 (dd, 1H), 7.26-7.35 (m, 1H), 7.54 (s, 1H), 7.76 (dd, 1H), 8.00 (dd, 1H), 8.27-8.32 (m, 2H), 8.38 (dd, 1H), 8.97 (s, 1H). 3-53 MS 491, 493 CDCl3: 2.26 (s, 3H), 2.62 (d, 3H), 2.68 (s, 6H), 4.72 (q, 1H), 6.78 (s, 1H), 6.89 (d, 1H), 7.12 (d, 1H), 7.15 (d, 1H), 7.40-7.47 (m, 2H), 7.91 (dd, 1H), 8.40 (s, 1H), 8.41 (dd, 1H), 9.11 (s, 1H). 3-54 MS 525, 527 CDCl3: 2.04 (s, 3H), 2.65 (d, 3H), 4.42-4.48 (m, 1H), 6.79 (s, 1H), 6.96-7.00 (m, 2H), 7.28-7.34 (m, 4H), 7.87-7.91 (m, 1H), 8.18 (s, 1H), 8.23-8.26 (m, 2H), 8.53 (d, 2H), 9.07 (s, 1H). 3-55 Rf (Hexane: AcOEt = 3:1 0.19 CDCl3: 1.34 (t, 3H), 1.44 (t, 3H), 2.63 (d, 3H), 3.81 (q, 2H), 4.06 (q, 2H), 4.46 (q, 1H), 6.43 (dd, 1H), 6.76 (d, 1H), 7.63-7.69 (m, 2H), 7.94 (d, 1H), 7.98 (dd, 1H), 8.42 (d, 1H), 8.93 (s, 1H). 3-56 MS 570, 572 CDCl3: 2.63 (d, 3H), 3.85 (s, 3H), 3.93 (s, 3H), 4.52 (q, 1H), 6.78-6.83 (m, 2H), 6.93 (d, 2H), 6390-7.02 (m, 1H), 7.11-7.15 (m, 1H), 7.21-7.27 (m, 1H), 7.61 (s, 1H), 7.87-7.92 (m, 1H), 8.26 (s, 1H), 8.20-8.30 (m, 1H), 8.38-8.41 (m, 1H), 8.92 (s, 1H). 3-57 Rf (Hexane:Ac OEt = 3:1) 0.16 CDCl3: 1.44 (t, 3H), 2.65 (d, 3H), 2.79-2.89 (m, 4H), 3.65-3.74 (m, 4H), 4.07 (q, 2H), 4.52 (q, 4H), 6.48 (dd, 1H), 6.80 (d, 1H), 7.20-7.25 (m, 1H), 7.55-7.67 (m, 2H), 7.92-7.98 (m, 2H), 8.29 (s, 1H), 8.43 (d, 1H), 8.95 (s, 1H). 3-58 Rf 0.17 (Hexane/Ac OEt = 1/1) CDCl3: 1.46 (t, 3H), 2.63 (d, 3H), 3.08-3.13 (m, 4H), 3.83-3.90 (m, 4H), 4.09 (q, 2H), 4.46 (q, 1H), 6.39 (dd, 1H), 6.51 (d, 1H), 7.21-7.28 (m, 1H), 7.37 (s, 1H), 7.58 (dd, 1H), 7.97 (dd, 1H), 8.03 (d, 1H), 8.21 (s, 1H), 8.46 (d, 1H), 8.94 (s, 1H). 3-59 MS 538, 540 CDCl3: 2.63 (d, 3H), 3.44 (s, 3H), 3.65 (s, 3H), 3.69- 3.73 (m, 2H), 4.10-4.15 (m, 2H), 4.40 (q, 1H), 6.45 (dd, 1H), 6.85 (d, 1H), 7.19-7.25 (m, 1H), 7.61 (dd, 1H), 7.88 (s, 1H), 7.93-7.97 (m, 2H), 8.27 (s, 1H), 8.46 (d, 1H), 8.95 (s, 1H). 3-60 Rf (AcOEt) 0.54 CDCl3: 2.63 (d, 3H), 3.67 (s, 3H), 4.18 (t, 2H), 4.38- 4.49 (m, 3H), 6.46 (dd, 1H), 6.81 (d, 1H), 7.60-7.69 (m, 2H), 7.92-7.99 (m, 2H), 8.27 (s, 1H), 8.49 (d, 1H), 9.00 (s, 1H). 3-61 Rf (Hexane: AcOEt = 2:1) 0.46 CDCl3: 1.44 (t, 3H), 2.63 (d, 3H), 3.64 (s, 3H), 4.07 (q, 2H), 4.47 (q, 1H), 6.45 (dd, 1H), 6.78 (d, 1H), 7.21- 7.28 (m, 1H), 7.40-7.48 (m, 2H), 7.93-7.99 (m, 2H), 8.26 (s, 1H), 8.44 (d, 1H), 8.96 (s, 1H). 3-62 Rf (Hexane:Ac OEt = 3:1) 0.31 CDCl3: 1.36 (d, 6H), 2.63 (d, 3H), 3.63 (s, 3H), 4.41- 4.52 (m, 2H), 6.45 (dd, 1H), 6.81 (d, 1H), 7.21-7.26 (m, 1H), 7.59-7.68 (m, 2H), 7.91-7.98 (m, 2H), 8.26 (s, 1H), 8.45 (d, 1H), 8.96 (s, 1H). 3-63 Rf (Hexane: AcOEt = 3:1) 0.40 CDCl3: 1.07 (t, 3H), 1.84 (m, 2H), 6.63 (d, 3H), 3.64 (s, 3H), 3.96 (t, 2H), 4.40-4.49 (m, 1H), 6.46 (dd, 1H), 6.79 (d, 1H), 7.20-7.27 (m, 1H), 7.58-7.66 (m, 2H), 7.94-7.97 (m, 2H), 8.26 (s, 1H), 8.45 (d, 1H), 8.97 (s, 1H). 3-64 Rf (Hexane:Ac OEt = 3:1) 0.19 CDCl3: 2.62 (d, 3H), 6.68 (s, 6H), 3.84 (s, 3H), 4.41- 4.48 (m, 1H), 6.36 (dd, 1H), 6.80 (d, 1H), 7.17-7.24 (m, 1H), 7.51-7.62 (m, 2H), 7.83 (s, 1H), 7.95 (dd, 1H), 8.27 (s, 1H), 8.3*9-8.45 (m, 1H), 8.91 (s, 1H). 3-65 Rf (Hexane:Ac OEt = 1:1) 0.12 CDCl3: 2.66 (d, 3H), 3.97 (s, 3H), 4.47-4.55 (m, 1H), 6.96-7.10 (m, 3H), 7.21-7.24 (m, 1H), 7.66 (s, 1H), 7.93 (dd, 1H), 8.25 (d, 1H), 8.31 (s, 1H), 8.47 (d, 2H), 8.59 (s, 1H), 8.96 (s, 1H). 3-66 MS 541, 543 CDCl3: 2.65 (d, 3H), 3.96 (s, 3H), 4.61-4.71 (m, 1H), 6.89-7.05 (m, 3H), 7.16 (dd, 1H), 7.15-7.23 (m, 1H), 7.60 (d, 1H), 7.65 (s, 1H), 7.89 (d, 1H), 8.21 (d, 1H), 8.28 (d, 1H), 8.51 (br. s, 2H), 8.57 (s, 1H), 8.93 (s, 1H). 3-67 MS 541, 543 CDCl3: 2.65 (d, 3H), 3.96 (s, 3H), 4.51 (q, 1H), 6.90- 7.06 (m, 3H), 7.11-7.16 (m, 1H), 7.38 (d, 1H), 7.50- 7.61 (m, 2H), 7.62-7.67 (m, 1H), 7.89 (dd, 1H), 8.29 (s, 1H), 8.34 (d, 1H), 8.53 (d, 1H), 8.79 (br.s, 1H), 8.94 (s, 1H). 3-68 LC-MS 590 CDCl3: 1.45-1.59 (m, 2H), 1.70-1.78 (m, 1H), 1.82- 1.90 (m, 1H), 2.38-2.50 (m, 1H), 2.43 (s, 3H), 2.62- 2.77 (m, 2H), 3.56-3.70 (m, 2H), 3.76 (s, 3H), 6.46 (dd, 1H), 6.63 (d, 1H), 6.82-6.88 (br, 1H), 7.22 (dd, 1H), 7.31-7.40 (m, 2H), 7.43-7.51 (m, 1H), 7.50-7.80 (m, 2H), 8.14-8.20 (br, 1H), 8.21 (s, 1H), 8.39-8.48 (m, 1H), 9.16-9.26 (br, 1H) 3-69 0.34 (CH2Cl2:M eOH = 9:1) CDCl3: 1.58-1.82 (br, 7H), 1.88-2.03 (br, 3H), 2.44- 2.45 (m,5H), 3.42-3.52 (m, 3H), 3.75 (s, 3H), 6.66 (dd, 1H), 6.92 (d, 1H), 7.28 (dd, 1H), 7.44 (br, 1H), 7.51 (dd, 1H), 7.79-7.81 (m, 2H), 8.18 (s, 1H), 8.32 (s, 1H), 8.35-8.37 (m, 1H), 9.17 (s, 1H) 3-70 Ms: 607, 609 DMSO-d6: 1.84-1.92 (m, 2H), 2.34-2.41 (m, 4H), 2.41- 2.45 (m, 3H), 2.44 (t, 2H), 3.58 (t, 4H), 3.75 (s, 3H), 4.02 (t, 2H), 6.48 (dd, 1H), 6.63 (d, 1H), 7.21 (dd, 1H), 7.41 (d, 1H), 7.46 (dd, 1H), 7.72-7.78 (m, 1H), 7.76 (dd, 1H), 8.22 (s, 1H), 8.25 (s, 1H), 8.40 (d, 1H), 9.22 (s, 1H) 3-71 Ms: 591, 593 DMSO-d6: 1.84-1.92 (m, 2H), 2.14 (s, 3H), 2.35-2.4 (m, 4H), 2.43 (t, 2H), 2.44 (d, 3H), 3.58 (t, 4H), 4.01 (t, 2H), 6.77 (dd, 1H), 6.82 (d, 1H), 7.17 (dd, 1H), 7.20 (d, 1H), 7.3-7.39 (m, 1H, 7.71-7.77 (m, 2H), 8.2 (s, 1H), 8.35-8.44 (m, 1H), 8.71 (s, 1H), 9.27 (s, 1H) 3-72 Ms: 620, 622 DMSO-d6: 1.82-1.9 (m, 2H), 2.13-2.17 (m, 3H), 2.25-2.47 (m, 13H), 3.75 (s, 3H), 4.01 (t, 2H), 6.47 (dd, 1H), 6.63 (d, 1H), 7.19-7.24 (m, 1H), 7.41 (d, 1H), 7.43-7.5 (m, 1H), 7.70-7.79 (m, 2H), 8.22 (s, 1H), 8.25 (brs, 1H), 8.37-8.44 (m, 1H), 9.22 (s, 1H) 3-73 Ms: 607, 609 DMSO-d6: 1.78 (t, 2H), 2.32-2.36 (m, 4H9, 2.35-2.38 (m, 3H), 3.54-3.59 (m, 4H), 3.74 (t, 3H), 3.78 (s, 3H), 6.38-6.42 (m, 1H), 6.85 (d, 1H), 6.86-6.95 (m, 1H), 7.33-7.43 (m, 2H), 7.63-7.68 (m, 1H), 7.85-8.15 (m, 3H), 8.64-8.8 (m, 1H). 3-74 Ms: 605, 607 DMSO-d6: 1.47-1.67 (m, 2H), 1.84-2.01 (m, 2H), 2.03 (s, 3H), 2.41-2.46 (m, 3H), 3.23-3.39 (m, 2H), 3.65- 3.73 (m, 1H), 3.81 (s, 3H), 3.8-3.88 (m, 1H), 4.58-4.65 (m, 1H), 6.55 (dd, 1H), 6.68 (d, 1H), 7.2-7.26 (m, 1H), 7.43 (d, 1H), 7.42-7.51 (m, 1H), 7.7-7.8 (m, 2H), 8.23 (s, 1H), 8.26 (brs, 1H), 8.37-8.44 (m, 1H), 9.22 (brs, 1H) 3-75 Ms: 605, 607 DMSO-d6: 1.38-1.6 (m, 2H), 1.74-1.9 (m, 2H), 2.0 (s, 3H), 2.42-2.47 (m, 3H), 3.12-3.3 (m, 2H), 3.55-3.65 (m, 1H), 3.7-3.8 (m, 1H), 3.78 (s, 3H), 4.27-4.34 (m, 1H), 6.65 (dd, 1H), 6.94 (d, 1H), 7.24-7.3 (m, 1H), 7.53-7.63 (m, 2H), 7.74-7.83 (m, 2H), 8.09 (brs, 1H), 8.35 (s, 1H), 8.38 (d, 1H), 9.19 (brs, 1H) 3-76 Ms: 577, 579 DMSO-d6: 1.51-1.61 (m, 2H), 1.79-1.87 (m, 2H), 2.03-2.11 (m, 2H), 2.14 (s, 3H), 2.42-2.47 (m, 3H), 2.52-2.6 (m, 2H), 3.77 (s, 3H), 4.02-4.09 (m, 1H), 6.6 (dd, 1H), 6.92 (d, 1H), 7.24-7.3 (m, 1H), 7.52-7.6 (m, 2H), 7.74-7.82 (m, 2H), 8.08 (brs, 1H), 8.34 (s, 1H), 8.4 (d, 1H), 9.2 (brs, 1H) 3-77 Rf: 0.4 (n-hexane: AcOEt = 7:3) DMSO-d6: 2.41-2.45 (m, 3H), 6.89-6.96 (m, 1H), 6.69 (bs, 1H), 7.24-7.33 (m, 2H), 7.51-7.57 (m, 1H), 7.63-7.7 (m, 1H), 7.73-7.78 (m, 1H), 7.79 (dd, 1H), 8.37 (s, 1H), 8.41 (d, 1H), 9.21 (brs, 1H), 9.24 (brs, 1H) 3-78 Ms: 563, 565 DMSO-d6: 1.33-1.43 (m, 2H), 1.79-1.86 (m, 2H), 2.43-2.46 (m, 3H), 2.46-2.53 (m, 2H), 2.87-2.94 (m, 2H), 3.77 (s, 3H), 4.07-4.14 (m, 1H), 6.59 (dd, 1H), 6.91 (d, 1H), 7.23-7.28 (m, 1H), 7.53-7.59 (m, 2H), 7.79 (dd, 1H), 8.03 (brs, 1H), 8.32 (s, 1H), 8.38 (d, 1H), 8.7-9.5 (brs, 1H) 3-79 Ms: 563, 565 DMSO-d6: 1.41-1.51 (m, 2H), 1.88-1.95 (m, 2H), 2.41-2.45 (m, 3H), 2.54-2.63 (m, 2H), 2.92-3.0 (m, 2H), 3.75 (s, 3H), 4.35-4.43 (m, 1H), 6.50 (dd, 1H), 6.63 (d, 1H), 7.18-7.23 (m, 1H), 7.40 (d, 1H), 7.42- 7.48 (m, 1H), 7.75 (dd, 1H), 8.21 (s, 1H), 8.22-8.25 (m, 1H), 8.37-8.42 (m, 1H), 8.9-9.5 (brs, 1H) 3-80 Ms: 482, 484 DMSO-d6: 2.4-2.46 (m, 3H), 3.79 (s, 3H), 6.72 (ddd, 1H), 6.99 (dd, 1H), 7.21-7.26 (m, 1H), 7.47-7.53 (m, 1H), 7.59-7.64 (m, 1H), 7.76 (dd, 1H), 8.25 (s, 1H), 8.29-8.37 (m, 2H), 8.8-9.6 (m, 1H) 3-81 Ms: 482, 484 DMSO-d6: 2.41-2.49 (m, 3H), 3.82 (s, 3H), 6.80 (ddd, 1H), 7.01 (dd, 1H), 7.3-7.35 (m, 1H), 7.56-7.63 (m, 1H), 7.7-7.8 (m, 1H), 7.82 (dd, 1H), 7.85 (dd, 1H), 8.16 (s, 1H), 8.35 (dd, 1H), 9.18 (brs, 1H) 3-82 Ms: 563, 565 DMSO-d6: 1.73-1.82 (m, 1H), 2.23-2.34 (m, 4H), 2.34-2.42 (m, 3H), 2.42-2.46 (m, 3H), 2.59 (dd, 1H), 2.62-2.68 (m, 1H), 2.80 (dd, 1H), 3.75 (s, 1H), 4.85- 4.91 (m, 1H), 6.42 (dd, 1H), 6.57 (d, 1H), 7.19-7.24 (m, 1H), 7.41 (d, 1H), 7.43-7.51 (m, 1H), 7.68-7.79 (m, 2H), 8.22 (s, 1H), 8.23 (s, 1H), 8.37-8.43 (m, 1H), 9.21 (brs, 1H). 3-83 MS 544, 546 2.36 (s, 3H), 2.65 (d, 3H), 3.93 (s, 3H), 4.46-4.51 (m, 1H), 6.75-6.80 (m, 2H), 6.97-7.04 (m, 2h), 7.25-7.30 (m, 1H), 7.56-7.66 (m, 2H), 7.98 (dd, 1H), 8.29 (s, 1H), 8.36-8.44 (m, 2H), 9.01 (s, 1H). 3-84 MS 562, 564 CDCl3: 2.32 (s, 3H), 2.39-2.47 (m, 4H), 2.64 (d, 3H), 2.89-2.97 (m, 4H), 3.85 (s, 3H), 4.54-4.52 (m, 1H), 6.52 (dd, 1H), 6.79 (d, 1H), 7.22 (m, 1H), 7.52-7.64 (m, 2H), 7.94-7.99 (m, 2H), 8.28 (s, 1H), 8.42 (d, 1H), 8.93 (s, 1H). - These compounds are prepared in analogy to Example 2 using 2-(5-bromo-2-chloro-pyrimidin-4-ylamino)-N-propyl-benzenesulfonamide and the corresponding aniline to give compounds No. 4-1 to 4-31 having the substituent Rx as listed under Example 3 for compounds No. 3-1 to 3-31.
- To a solution of 5-bromo-2,4-dichloropyrimidine (90 μL, 0.70 mmol) and 2-amino-N-propyl-benzenesulfonamide (100 mg, 0.47 mmol), sodium hydride (54.2 mg, 0.56 mmol) in DMSO (1.0 mL) is added and the resulting solution is stirred at 80° C. for 3.0 h. The mixture is poured into water and extracted with ethyl acetate three times. The organic layer is washed with water and then brine, dried over sodium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1) to afford the title compound as a slightly yellow solid.
- 1H-NMR (δ, ppm): 0.89 (t, 3H), 1.41 (q, 2H), 3.56 (t, 2H), 4.92 (br.s, 2H), 6.71 (dd, 1H), 6.77 (dd, 1H), 7.33 (dd, 1H), 7.54 (dd, 1H), 8.79 (s, 1H)
- Rf (hexane:ethyl acetate=1:1): 0.64.
- These compounds are prepared in analogy to Example 2 using 2-(2-chloro-5-trifluoromethyl-pyrimidin-4-ylamino)-N-methyl-benzenesulfonamide and the corresponding aniline to give compounds No. 5-1 to 5-31 having the substituent Rx as listed under Example 3 for compounds No. 3-1 to 3-31.
- To a solution of 2,4-dichloro-5-trifluoromethyl-pyrimidine (386 mg, 1.79 mmol) in acetonitrile (10 mL), 2-amino-N-methyl-benzenesulfonamide (333 mg, 1.79 mmol) and 1,8-diaza[5.4.0]-bicyclo-7-undecene (280 μL, 1.88 mmol) are added successively at ambient temperature. After stirring for 15 h at room temperature, dichloromethane (30 mL) is added to the mixture, and the solution is washed with saturated aqueous sodium hydrogen carbonate and saturated aqueous sodium chloride, dried over magnesium sulfate, and evaporated in vacuo. The resulting solid is purified by flash chromatography.
- 1H NMR (CDCl3) δ: 3.73 (s, 3H), 6.67-6.69 (m, 1H), 6.72-6.73 (m, 1H), 7.27-7.31 (m, 1H), 7.78 (dd, 1H), 8.60 (s, 1H). Rf (hexane:ethyl acetate=1:1): 0.28.
-
- This compound is obtained as a side product formed by N-demethylation on reaction of 2-(5-bromo-2-chloropyrimidin-4-ylamino)-N-methyl-benzenesulfonamide with 2,3-(difluoromethylene-dioxy)aniline following the procedure of Example 2. It may also be prepared by reaction of 2-(5-bromo-2-chloropyrimidin-4-ylamino)benzenesulfonamide with 2,3-(difluoromethylenedioxy)-aniline.
- Rf (n-hexane:ethyl acetate=1:1): 0.46.
- 1H-NMR: (CDCl3) 4.83 (bs, 2H), 6.77 (dd, 1H), 6.86 (s, 1H), 6.97 (dd, 1H), 7.31-7.24 (m, 1H), 7.57 (dd, 1H), 7.81 (d, 1H), 8.02 (dd, 1H), 8.28 (d, 1H), 8.29 (s, 1H), 8.88 (s, 1H).
- Preparation of 2-(5-bromo-2-chloropyrimidin-4-ylamino)benzenesulfonamide: To a solution of 5-bromo-2,4-dichloropyrimidine (300 mg, 1.32 mmol) and 2-amino-benzenesulfonamide (340 mg, 1.97 mmol) in 2-propanol (3 mL), concentrated hydrochloric acid (0.06 mL) is added and the mixture is stirred at 90° C. for 4.5 hours. The mixture is poured into aqueous sodium hydrogen carbonate and extracted with ethyl acetate three times. The organic layer is washed with water, dried over sodium sulfate, and evaporated in vacuo. The residue is purified by column chromatography (hexane:ethyl acetate=2:1) to afford the title compound.
- Rf (hexane:ethyl acetate=1:1): 0.55. 1H-NMR (400 MHz, CDCl3) δ: 4.78 (br.s, 2H), 7.22 (dd, 1H), 7.61 (ddd, 1H), 7.95 (dd, 1H), 8.35 (s, 1H), 8.35 (d, 1H), 9.18 (s, 1H).
-
- To a suspension of 2-(2,5-dichloro-pyrimidin-4-yl-amino)-N-methyl-benzamide (5.05 g, 17.0 mmol) in 90 mL of 2-methoxyethanol are added 2-methoxy-4-morpholinoaniline dihydrochloride (4.56 g, 16.2 mmol) and 17.0 mL of 1N ethanolic solution of hydrogen chloride (17.0 mmol). After the reaction mixture is stirred at 110° C. for 4 hours and cooled to room temperature, the mixture is neutralized with 1N aqueous NaOH solution and extracted with EtOAc (100 mL×3). The organic layer is washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The resulting black solid is washed with EtOH (90 mL), then purified with silica gel column chromatography (CH2Cl2 to CH2Cl2:AcOEt=1:2) to give 2-[5-chloro-2-(2-methoxy-4-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzamide as a pale yellow solid.
- 1H-NMR (400 MHz, DMSO-d6, δ): 2.80 (d, 3H, J=4.52 Hz), 3.10-3.20 (m, 4H), 3.78 (s, 3H), 3.70-3.80 (m, 4H), 6.49 (dd, 1H, J=8.56, 2.52 Hz), 6.66 (d, 1H, J=2.52 Hz), 7.08 (dd, 1H, J=8.04, 8.04 Hz), 7.44 (d, 1H, J=8.56 Hz), 7.71 (dd, 1H, J=8.04, 1.48 Hz), 8.10 (s, 1H), 8.13 (s, 1H), 8.59 (d, 1H, J=8.04 Hz) 8.68-8.75 (m, 1H), 11.59 (S, 1H). MS m/z 469, 471 (M+1)+.
- The following 2-[5-Chloro-2-(substituted phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzamide are prepared from 2-(2,5-Dichloro-pyrimidin-4-ylamino)-N-methyl-benzamide and the corresponding aniline following the procedure of Example 7A.
-
Expl Rf (solvent) No. Rx or MS NMR (400 MHz), δ (ppm) 7-1 MS: m/z 550, 552 (M + 1) DMSO-d6: 1.44-1.33 (m, 2H), 1.64-1.45 (m, 6H), 1.73-1.89 (m, 2H), 2.34-2.44 (m, 1H), 2.43-2.55 (m, 4H), 2.65 (t, 2H), 2.80 (d, 3H), 3.75 (s, 3H), 3.72-3.75 (m, 2H), 6.48 (dd, 1H), 6.62 (d, 1H), 7.06 (dd, 1H), 7.32 (dd, 1H), 7.39 (d, 1H), 7.71 (dd, 1H), 8.09 (s, 1H), 8.60 (d, 1H), 8.70 (d, 1H), 11.58 (s, 1H) 7-2 0.3 (MeOH:AcOEt = 5:95) CDCl3: 1.70-1.97 (m, 4H), 2.62-2.79 (m, 1H), 3.04 (d, 3H), 3.02-3.18 (m, 2H), 3.23-3.33 (m, 2H), 3.88 (s, 3H), 5.39-5.47 (m, 1H), 6.15-6.24 (m, 1H), 6.55-6.62 (m, 2H), 6.74-6.82 (m, 1H), 7.09 (dd, 1H), 7.23-7.32 (m, 1H), 7.46-7.52 (m, 2H), 8.09 (s, 1H), 8.15 (d, 1H), 8.68 (d, 1H) 11.0 (bs, 1H) 7-3 MS (ESI) mz/ 482, 484 (M + 1)+ DMSO-d6: 2.24 (s, 3H), 2.45-2.55 (m, 4H), 2.80 (d, 3H, J = 4.52 Hz), 3.12-3.17 (m, 4H), 3.76 (s, 3H), 6.48 (dd, 1H, J = 8.56, 2.52 Hz), 6.63 (d, 1H, J = 2.52 Hz), 7.05-7.10 (m, 1H), 7.27-7.35 (m, 1H), 7.40 (d, 1H, J = 8.56 Hz), 7.69-7.72 (m, 1H), 8.09 (s, 1H), 8.12 (s, 1H), 8.55-8.65 (m, 1H), 8.67-8.75 (m, 1H), 11.59 (s, 1H) 7-4 0.46 (MeOH:CH2Cl2 = 1:4) DMSO-d6: 2.48-2.55 (m, 4H), 2.71 (t, 2H), 2.80 (d, 3H), 3.58-3.61 (m, 4H), 3.76 (s, 3H), 4.11 (t, 2H), 6.52 (dd, 1H), 6.66 (d, 1H), 7.06 (dd, 1H), 7.32 (dd, 1H), 7.46 (d, 1H), 7.71 (dd, 1H), 8.11 (s, 1H), 8.19 (s, 1H), 8.54-8.60 (m, 1H), 8.60-8.75 (m, 1H), 11.6 (s, 1H) 7-5 mz/ 497, 499 (M + 1)+ DMSO-d6: 1.60-1.70 (m, 2H), 1.90-1.98 (m, 2H), 2.13-2.25 (m, 2H), 2.19 (s, 3H), 2.60-2.67 (m, 2H), 2.80 (d, 3H, J = 4.52 Hz), 3.75 (s, 3H), 4.30-4.40 (m, 1H), 6.54 (dd, 1H, J = 8.56, 2.0 Hz), 6.65 (d, 1H, J = 2.0 Hz), 7.04-7.09 (m, 1H), 7.25-7.35 (m, 1H), 7.43 (d, 1H, J = 8.56 Hz), 7.68-7.73 (m, 1H), 8.10 (s, 1H), 8.18 (s, 1H) 8.52-8.59 (m, 1H), 8.68-8.75 (m, 1H), 11.57 (s, 1H) 7-6 0.25 (n-hexane:AcOEt = 1:2) CDCl3: 2.95 (m, 4H), 3.03 (d, 3H), 3.75 (m, 4H), 3.86 (s, 3H), 6.21-6.19 (br, 1H), 6.49 (dd, 1H), 6.80 (d, 1H), 7.09-7.05 (m, 1H), 7.50 (dd, 1H), 8.08 (d, 1H), 8.13 (s, 1H), 8.68 (d, 1H), 11.07 (s, 1H) 7-7 MS m/z 510 512 (M + 1) DMSO-d6: 2.06 (s, 3H), 2.80 (d, 3H), 3.11 (t, 2H), 3.16 (t, 2H), 3.60 (dd, 4H), 3.77 (s, 3H), 6.51 (dd, 1H), 6.68 (d, 1H), 7.08 (dd, 1H), 7.33 (dd, 1H), 7.46 (d, 1H), 7.71 (d, 1H), 8.10 (s, 1H), 8.12 (s, 1H), 8.59-8.61 (m, 1H), 8.70-8.71 (m, 1H), 11.59 (s, 1H) 7-8 0.48 (MeOH:AcOEt = 5:95) CDCl3: 1.46 (d, 1H), 1.68-1.82 (m, 2H), 2.02-2.09 (m, 2H), 2.83-2.96 (m, 2H), 3.03 (d, 3H), 3.44-3.53 (m, 2H), 3.82-3.92 (m, 1H), 3.87 (s, 3H), 6.15-6.23 (m, 1H), 6.51 (d, 1H), 6.56 (bs, 1H), 7.07 (dd, 1H), 7.48 (d, 2H), 8.08 (s, 1H), 8.08-8.10 (m, 1H), 8.69 (d, 1H), 11.0 (bs, 1H) 7-9 0.4 (n-hexane:AcOEt = 1:1) CDCl3: 1.22 (t, 3H), 1.73-1.85 (m, 2H), 2.00-2.09 (m, 2H), 2.81-2.90 (m, 2H), 3.03 (d, 3H), 3.41-3.56 (m, 3H), 3.56 (dd, 2H), 3.58-3.62 (m, 2H), 3.64-3.68 (m, 2H), 3.86 (s, 3H), 6.15-6.24 (m, 1H), 6.50 (dd, 1H), 6.56 (d, 1H), 7.07 (dd, 1H), 7.24-7.30 (m, 1H), 7.45-7.52 (m, 2H), 8.08 (s, 1H), 8.06-8.08 (m, 1H), 8.69 (d, 1H), 11.0 (bs, 1H) 7-10 0.4 (n-hexane:AcOEt = 1:1) CDCl3: 1.73-1.85 (m, 2H), 2.01-2.10 (m, 2H), 2.82-2.90 (m, 2H), 3.03 (d, 3H), 3.41 (s, 3H), 3.45-3.51 (m, 2H), 3.56-3.58 (m, 2H), 3.65-3.68 (m, 2H), 3.86 (s, 3H), 6.14-6.22 (m, 1H), 6.50 (dd, 1H), 6.56 (d, 1H), 7.07 (dd, 1H), 7.23-7.30 (m, 1H), 7.44-7.52 (m, 2H), 8.08 (s, 1H), 8.06-8.08 (m, 1H), 8.69 (d, 1H), 11.0 (bs, 1H) 7-11 0.54 (MeOH:CH2Cl2 = 1:4) DMSO-d6: 1.78-1.89 (m, 1H), 2.13-2.22 (m, 1H), 2.22 (s, 6H), 2.77-2.87 (m, 1H), 2.79 (d, 3H), 3.04-3.10 (m, 1H), 3.23-3.50 (m, 3H), 3.75 (s, 3H), 6.11 (dd, 1H), 6.22 (d, 1H), 7.05 (dd1H), 7.21-7.32 (m, 1H), 7.26 (d, 1H), 7.70 (d, 1H), 8.06 (s, 1H), 8.08 (s, 1H), 8.57-8.66 (m, 1H), 8.66-8.73 (m, 1H), 11.6 (s, 1H) 7-12 0.27 (MeOH:CH2Cl2 = 1:1) DMSO-d6: 1.77-1.87 (m, 1H), 2.09-2.18 (m, 1H), 2.35 (s, 3H), 2.79 (d, 1H), 3.02-3.07 (m, 1H), 3.23-3.50 (m, 4H), 3.74 (s, 3H), 6.09 (dd, 1H), 6.20 (d, 1H), 7.04 (dd, H), 7.22-7.32 (m, 1H), 7.26 (d, 1H), 7.70 (d, 1H), 8.05 (s, 1H), 8.08 (s, 1H), 8.57-8.67 (m, 1H), 8.67-8.73 (m, 1H), 11.6 (s, 1H) 7-13 0.23 (MeOH:AcOEt = 5:95) CDCl3: 1.62-1.74 (m, 3H), 1.76-1.85 (m, 2H), 2.00-2.09 (m, 2H), 2.20-2.31 (m, 1H), 2.64-2.69 (m, 2H), 2.79 (d, 3H), 3.56-4.04 (m, 2H), 4.04 (s, 3H), 6.49 (dd, 1H), 6.63 (d, 1H), 6.78 (bs, 1H), 7.07 (dd, 1H), 7.28-7.38 (m, 1H), 7.39 (d, 1H), 7.71 (d, 1H), 8.09-8.11 (m, 2H), 8.09 (s, 1H), 8.60 (d, 1H), 8.71 (d, 1H), 11.6 (bs, 1H) 7-14 0.30 (MeOH:CH2Cl2 = 4:1) DMSO-d6: 1.61-1.46 (m, 2H), 1.92-1.82 (m, 2H), 2.14 (s, 3H), 2.41-2.23 (m, 5H, 2.60-2.45 (m, 4H), 2.67 (t, 2H), 2.79 (d, 3H), 3.75 (s, 3H), 3.71-3.75 (m, 2H), 6.48 (dd, 1H), 6.63 (d, 1H), 7.10-7.03 (m, 1H), 7.34-7.27 (m, 1H), 7.43-7.35 (m, 1H), 7.71 (dd, 1H), 8.09 (s, 1H), 8.11 (bs, 1H), 8.65-8.56 (m, 1H), 8.75-8.67 (m, 1H), 11.6 (s, 1H) 7-15 MS (ESI) mz/ 524, 526 (M + 1)+ DMSO-d6: 2.19-2.37 (m, 4H), 2.65-2.85 (m, 3H), 2.80 (d, 3H, J = 4.5 Hz), 3.15-3.21 (m, 1H), 3.48-3.59 (m, 2H), 3.61-3.67 (m, 1H), 3.72-3.81 (m, 1H), 3.76 (s, 3H), 6.47 (dd, 1H, J = 8.6, 2.5 Hz), 6.65 (d, 1H, J = 2.5 Hz), 7.04-7.10 (m, 1H), 7.28-7.35 (m, 1H), 7.42 (d, 1H, J = 8.6 Hz), 7.69-7.74 (m, 1H), 8.09 (s, 1H), 8.12 (s, 1H), 8.55-8.63 (m, 1H), 8.68-8.73 (m, 1H), 11.60 (s, 1H) 7-16 MS (ESI) mz/ 524, 526 (M + 1)+ DMSO-d6: 2.19-2.37 (m, 4H), 2.65-2.85 (m, 3H), 2.80 (d, 3H, J = 4.5 Hz), 3.15-3.21 (m, 1H), 3.48-3.59 (m, 2H), 3.61-3.67 (m, 1H), 3.72-3.81 (m, 1H), 3.76 (s, 3H), 6.47 (dd, 1H, J = 8.6, 2.5 Hz), 6.65 (d, 1H, J = 2.5 Hz), 7.04-7.10 (m, 1H), 7.28-7.35 (m, 1H), 7.42 (d, 1H, J = 8.6 Hz), 7.69-7.74 (m, 1H), 8.09 (s, 1H), 8.12 (s, 1H), 8.55-8.63 (m, 1H), 8.68-8.73 (m, 1H), 11.60 (s, 1H) 7-17 MS 510 DMSO-d6: 0.98 (t, 3H), 1.81-1.71 (m, 3H), 1.95-1.84 (m, 3H), 2.68-2.63 (m, 1H), 2.80 (d, 3H), 3.12-3.08 (m, 4H), 3.28 (d, 2H), 3.76 (s, 3H), 6.50 (dd, 1H), 6.64 (d, 1H), 6.86 (bs, 1H), 7.07 (dd, 1H), 7.46-7.19 (m, 3H), 7.71 (d, 1H), 8.09 (s, 1H), 8.15-8.10 (m, 1H), 8.66-8.58 (m, 1H), 8.77-8.70 (m, 1H), 11.6 (s, 1H) 7-18 MS 510 DMSO-d6: 0.98 (t, 3H), 1.81-1.71 (m, 3H), 1.95-1.84 (m, 3H), 2.68-2.63 (m, 1H), 2.80 (d, 3H), 3.12-3.08 (m, 4H), 3.28 (d, 2H), 3.76 (s, 3H), 6.50 (dd, 1H), 6.64 (d, 1H), 6.86 (bs, 1H), 7.07 (dd, 1H), 7.46-7.19 (m, 3H), 7.71 (d, 1H), 8.09 (s, 1H), 8.15-8.10 (m, 1H), 8.66-8.58 (m, 1H), 8.77-8.70 (m, 1H), 11.6 (s, 1H) 7-19 0.16 (CH2Cl2:MeOH = 9:1) 1.40-1.53 (m, 2H), 1.72-1.80 (m, 2H), 2.18 (s, 3H), 2.19-2.44 (m, 5H), 2.80 (d, 3H), 3.46 (m, 2H), 3.74 (s, 3H), 6.65 (dd, 1H), 6.91 (d, 1H), 7.07-7.10 (m, 1H), 7.36-7.40 (m, 1H), 7.45-7.49 (m, 1H), 7.73 (dd, 1H), 8.12 (s, 1H), 8.18 (s, 1H), 8.61 (d, 1H), 8.72-8.77 (m, 1H), 11.68 (s, 1H) 7-20 Ms: 511 1.25-1.37 (m, 2H), 1.62-1.79 (m, 3H), 1.81-1.9 (m, 2H), 2.16 (s, 3H), 2.75-2.85 (m, 5H), 3.76 (s, 3H), 3.8-3.88 (m, 2H), 6.45-6.55 (m, 1H), 6.6-6.67 (m, 1H), 7.02-7.12 (m, 1H), 7.25-7.35 (m, 1H), 7.4-7.5 (m, 1H), 7.67-7.78 (m, 1H), 8.1 (s, 1H), 8.19 (brs, 1H) 8.5-8.62 (m, 1H), 8.66-8.8 (m, 1H), 11.6 (s, 1H) 7-21 Ms: 526 2.17 (s, 3H), 2.29-2.39 (m, 3H), 2.45-2.56 (m, 4H), 2.7 (t, 2H), 3.76 (s, 3H), 4.09 (t, 2H), 6.52 (dd, 1H), 6.66 (d, 1H), 7.06 (dd, 1H), 7.31 (dd, 1H), 7.45 (d, 1H), 7.71 (dd, 1H), 8.1 (s, 1H), 8.19 (s, 1H), 8.5-8.6 (m, 1H), 8.67-8.75 (m, 1H), 11.6 (s, 1H) 7-22 Ms: 482 2.24 (s, 3H), 2.42-2.5 (m, 4H), 2.8 (d, 3H), 2.94-3.0 (m, 4H), 3.74 (s, 3H), 6.65 (dd, 1H), 6.93 (d, 1H), 7.07-7.14 (m, 1H), 7.34-7.4 (m, 1H), 7.45 (d, 1H), 7.73 (dd, 1H), 8.14 (s, 1H), 8.18 (s, 1H), 8.61 (dd, 1H), 8.7-8.77 (m, 1H), 11.7 (s, 1H) 7-23 Ms: 482 1.67-1.76 (m, 1H), 2.0-2.1 (m, 1H), 2.25-2.31 (m, 3H), 2.8 (d, 3H), 2.85-2.91 (m, 1H), 3.04-3.12 (m, 1H), 3.14-3.3 (m, 3H), 3.7 (s, 3H), 6.26 (dd, 1H), 6.91 (d, 1H), 7.01-7.04 (m, 1H), 7.07 (dd, 1H), 7.32 (dd, 1H), 7.72 (d, 1H), 8.14 (s, 1H), 8.17 (s, 1H), 8.63 (d, 1H), 8.7-8.78 (m, 1H), 11.6 (s, 1H) 7-24 Ms: 550 1.35-1.57 (m, 8H), 1.7-1.78 (m, 2H), 2.81 (d, 3H), 3.46-3.52 (m, 2H), 3.74 (s, 3H), 6.65 (dd, 1H), 6.91 (d, 1H), 7.05-7.12 (m, 1H), 7.34-7.42 (m, 1H), 7.46 (d, 1H), 7.73 (dd, 1H), 8.11 (s, 1H), 8.18 (s, 1H), 8.62 (dd, 1H), 8.71-8.78 (m, 1H), 11.7 (s, 1H) 7-25 536 [M + 1]+ DMSO-d6: 1.48-1.58 (m, 2H), 1.65-1.72 (m, 4H), 1.90-1.97 (m, 2H), 2.07-2.14 (m, 1H), 2.49-2.55 (m, 4H), 2.70-2.77 (m, 2H), 2.79 (d, 3H), 3.60-3.65 (m, 2H), 3.75 (s, 3H), 6.48 (dd, 1H), 6.63 (d, 1H), 7.03-7.09 (m, 1H), 7.28-7.34 (m, 1H), 7.39 (d, 1H), 7.71 (dd, 1H), 8.09 (s, 1H), 8.11 (s, 1H), 8.55-8.65 (m, 1H), 8.69-8.73 (m, 1H), 11.59 (s, 1H) 7-26 468 [M + 1]+ DMSO-d6: 2.80 (d, 3H), 2.84-2.89 (m, 4H), 3.04-3.08 (m, 4H), 3.76 (s, 3H), 6.47 (dd, 1H), 6.62 (dd, 1H), 7.04-7.10 (m, 1H), 7.28-7.35 (m, 1H), 7.40 (d, 1H), 7.69-7.73 (m, 1H), 8.09 (s, 1H), 8.12 (s, 1H), 8.55-8.63 (m, 1H), 8.68-8.73 (m, 1H), 11.59 (s, 1H) (an aliphatic NH is hidden) 7-27 393 [M + 1]+ DMSO-d6: 2.80 (d, 3H), 6.64-6.67 (m, 1H), 7.01-7.08 (m, 2H), 7.15 (d, 1H), 7.24-7.29 (m, 2H), 7.44 (d, 1H), 7.69-7.73 (m, 1H), 8.20 (s, 1H), 8.65-8.73 (m, 2H), 9.15 (s, 1H), 11.06 (s, 1H), 11.63 (s, 1H) 7-28 407 [M + 1]+ DMSO-d6: 2.81 (d, 3H), 3.79 (s, 3H), 6.67 (d, 1H), 7.05-7.10 (m, 1H), 7.12 (d, 1H), 7.17 (d, 1H), 7.23 (d, 1H), 7.25-7.30 (m, 1H), 7.50 (d, 1H), 7.70-7.73 (m, 1H), 8.20 (s, 1H), 8.67 (d, 1H), 8.70-8.75 (m, 1H), 9.17 (s, 1H), 11.64 (s, 1H) 7-29 492 [M + 1]+ DMSO-d6: 2.80 (d, 3H), 2.91-2.99 (m, 4H), 3.65-3.81 (m, 2H), 3.82-3.95 (m, 2H), 4.12 (s, 3H), 6.58 (d, 1H), 6.90 (d, 1H), 7.05-7.09 (m, 1H), 7.14 (d, 1H), 7.22-7.28 (m, 1H), 7.30 (d, 1H), 7.70 (dd, 1H), 8.16 (s, 1H), 8.63-8.67 (m, 1H), 8.68-8.72 (m, 1H), 9.06 (s, 1H), 11.64 (s, 1H) 7-30 MS m/z 510 DMSO-d6: 2.02 (s, 3H), 2.80 (d, 3H), 2.82-2.92 (m, 2H), 2.92-3.01 (m, 2H), 3.44-3.53 (m, 4H), 3.76 (s, 3H), 6.68 (dd, 1H), 6.95 (d, 1H), 7.09 (dd, 1H), 7.35-7.40 (m, 1H), 7.50 (brs, 1H), 7.73 (d, 1H), 8.15 (s, 1H), 8.19 (s, 1H), 8.59 (d, 1H), 8.69-8.76 (m, 1H), 11.66 (s, 1H). - The following 2-[5-Bromo-2-(substituted phenylamino)-pyrimidin-4-ylamino]-N-ethyl-benzamide are prepared from 2-(5-bromo-2-chloro-pyrimidin-4-ylamino)-N-ethyl-benzamide and the corresponding aniline following the procedure of Example 7A
-
Expl Rf (solvent) No. Rx or MS NMR 8-1 0.27 (n-hexane:AcOEt = 1:2) DMSO-d6: 2.80 (d, 3H), 2.88 (t, 4H), 3.65 (m, 4H), 3.75 (s, 3H), 6.64 (dd, 1H), 6.94 (d, 1H), 7.11-7.08 (m, 1H), 7.38-7.34 (m, 1H), 7.47-7.46 (m, 1H), 7.70 (dd, 1H), 8.11 (s, 1H), 8.26 (s, 1H), 8.51-8.49 (m, 1H), 8.72-8.71 (m, 1H), 11.41 (s, 1H) 8-2 mz/ 513, 515 (M + 1) DMSO-d6: 2.79 (d, 3H, J = 4.04 Hz), 3.10-3.20 (m, 4H), 3.77 (s, 3H), 3.70-3.80 (m, 4H), 6.45-6.55 (m, 1H), 6.63-6.69 (m, 1H), 7.05-7.10 (m, 1H), 7.28-7.34 (m, 1H), 7.40-7.45 (m, 1H), 7.65-7.70 (m, 1H), 8.13 (s, 1H), 8.16 (s, 1H), 8.50-8.56 (m, 1H) 8.65-8.72 (m, 1H), 11.40 (s, 1H) 8-3 0.48 (n-Hexane:AcOEt = 4:1) DMSO-d6: 2.80 (d, 3H), 3.83 (s, 3H), 4.11 (t, 2H), 6.82 (ddd, 1H), 7.03 (dd, 1H), 7.15 (dd, 1H), 7.44 (dd, 1H), 7.73 (d, 1H), 7.93 (dd, 1H), 8.13 (s, 1H), 8.33 (s, 1H), 8.50 (d, 1H), 8.70-8.77 (m, 1H), 11.3 (s, 1H). 8-4 MS 446, 448 2.79 (d, 3H), 3.79 (s, 3H), 6.75 (ddd, 1H), 7.0 (dd, 1H), 7.05-7.12 (m, 1H), 7.3-7.36 (m, 1H), 7.62 (dd, 1H), 7.69 (dd, 1H), 8.2 (s, 1H), 8.29 (s, 1H), 8.45 (d, 1H), 8.66-8.73 (m, 1H), 11.4 (brs, 1H). - The following 2-[5-Chloro-2-(substituted phenylamino)-pyrimidin-4-ylamino]-N-ethyl-benzamide are prepared from 2-(2,5-Dichloro-pyrimidin-4-ylamino)-N-ethyl-benzamide and the corresponding aniline following the procedure of Example 7A
-
Expl Rf (solvent) No. Rx or MS NMR (400 MHz), δ (ppm) 9-1 0.35 (n-hexane:AcOEt = 1:2) CDCl3: 1.27 (t, 3H), 3.10-3.15 (m, 4H), 3.47-3.58 (m, 2H), 3.85-3.93 (m, 4H), 3.89 (s, 3H), 6.08-6.17 (m, 1H), 6.48 (dd, 1H), 6.53 (d, 1H), 7.05-7.11 (m, 1H), 7.42-7.53 (m, 2H), 8.08 (s, 1H), 8.12 (d, 1H), 8.67 (d, 1H), 10.94 (brs, 1H). 9-2 MS (ESI) mz/ 497, 499 (M + 1)+ CDCl3: 1.26 (t, 3H, J = 7.56 Hz), 2.37 (s, 3H), 2.57-2.62 (m, 4H), 3.15-3.20 (m, 4H), 3.49 (dq, 2H, J = 7.56, 1.52 Hz), 3.87 (s, 3H), 6.11-6.16 (m, 1H), 6.49 (dd, 1H, J = 8.56, 2.52 Hz), 6.55 (d, 1H, J = 2.52 Hz), 7.05-7.10 (m, 1H), 7.23 (s, 1H), 7.41-7.50 (m, 2H), 8.07 (s, 1H), 8.08 (d, 1H, J = 8.56 Hz), 8.65-8.69 (m, 1H), 10.93 (s, 1H) 9-3 mz/ 564, 566 (M + 1)+ DMSO-d6: 1.26 (t, 3H, J = 7.56 Hz), 1.40-1.50 (m, 2H), 1.56-1.64 (m, 4H), 1.67-1.82 (m, 2H), 1.88-1.97 (m, 2H), 2.33-2.44 (m, 1H), 2.52-2.57 (m, 4H), 2.63-2.73 (m, 2H), 3.51 (dq, 2H, J = 7.56, 1.52 Hz), 3.62-3.69 (m, 2H), 3.86 (s, 3H), 6.10-6.15 (m, 1H), 6.49 (dd, 1H, J = 8.56, 2.52 Hz), 6.55 (d, 1H, J = 2.52 Hz), 7.05-7.10 (m, 1H), 7.23 (s, 1H), 7.43-7.50 (m, 2H) 8.05-8.11 (m, 1H), 8.07 (s, 1H), 8.65-8.69 (m, 1H), 10.91 (s, 1H) 9-4 0.39 (MeOH:CH2Cl2 = 1:4) DMSO-d6: 1.19 (t, 3H), 1.52-1.68 (m, 2H), 1.71-1.79 (m, 4H), 1.92-2.05 (m, 2H), 2.12-2.23 (m, 1H), 2.76-2.85 (m, 2H), 3.65-3.73 (m, 2H), 3.82 (s, 3H), 6.54 (dd, 1H), 6.69 (d, 1H), 7.13 (m, 1H), 7.45 (d, 1H), 7.79 (dd, 1H), 8.15 (s, 1H), 8.15-8.18 (m, 1H), 8.60-8.68 (m, 1H), 8.74-8.83 (m, 1H). 9-5 Rf (Hexane:AcOEt = 1:2): 0.30 CDCl3: 1.27 (t, 3H), 3.08-3.14 (m, 4H), 3.52 (q, 2H), 3.71-3.90 (m, 7H), 6.05-6.18 (m, 1H), 6.47 (dd, 1H), 6.53 (dd, 1H), 7.08 (dd, 1H), 7.41-7.53 (m, 2H), 8.08 (s, 1H), 8.12 (d, 1H), 8.67 (d, 1H), 10.94 (s, 1H). 9-6 Rf (AcOEt:MeOH = 4:1) 0.050 DMSO: 1.11 (t, 3H), 1.60-1.69 (m, 1H), 1.88-1.96 (m, 2H), 2.19 (s, 3H), 2.55-2.68 (m, 2H), 3.30-3.45 (m, 2H), 3.75 (s, 3H), 4.33-4.43 (m, 1H), 6.54 (dd, 1H), 6.65 (d, 1H), 7.07 (dd, 1H), 7.30 (dd, 1H), 7.43 (d, 1H), 7.71 (dd, 1H), 8.11 (s, 1H), 8.20 (s, 1H), 8.54 (br.d, 1H), 8.75 (dd, 1H), 11.49 (s, 1H). 9-7 0.44 (CH2Cl2:MeOH = 8:2) CDCl3: 1.34 (t, 3H), 1.62-1.68 (m, 2H), 1.93-2.18 (m, 8H), 2.37-2.40 (br, 2H), 2.74-2.86 (br, 3H), 3.20-3.23 (m, 2H), 3.34 (br, 2H), 3.53 (q, 2H), 3.85 (s, 3H), 6.47 (dd, 1H), 6.76 (d, 1H), 7.04-7.08 (m, 1H), 7.30 (dd, 1H), 7.53 (s, 1H), 8.00 (d, 1H), 8.13-8.17 (m, 1H), 8.22 (d, 1H), 8.42-8.53 (br, 1H), 10.91 (s, 1H), 11.59-11.75 (br, 1H) - The following 2-[5-Chloro-2-(substituted phenylamino)-pyrimidin-4-ylamino]-6,N-dimethyl-benzamide are prepared from 2-(2,5-Dichloro-pyrimidin-4-ylamino)-6,N-dimethyl-benzamide and the corresponding aniline following the procedure of Example 7A
-
Expl No. Rx Identification 10-1 NMR (400 MHz, DMSO-d6, δ): 1.58-1.68(m, 2H), 1.87-1.96(m, 2H), 2.13-2.22(m, 2H), 2.18(s, 3H), 2.18(s, 3H), 2.29(s, 3H), 2.57- 2.65(m, 2H), 2.76(d, 3H), 3.75(s, 3H), 4.29-4.37(m, 1H), 6.45(dd, 1H), 6.61(d, 1H), 6.98(d, 1H), 7.18(dd, 1H), 7.47(d, 1H), 7.89(d, 1H), 8.02(s, 1H), 8.07(s, 1H), 8.37- 8.43(m, 1H), 8.49(s, 1H). Rf: 0.39 (MeOH: CH2Cl2 = 1:4). 10-2 NMR (400MHz, DMSO-d6, δ): 1.35-1.42 (m, 2H), 1.45-1.60 (m, 6H), 1.75-1.85 (m, 2H), 2.29 (s, 3H), 2.30-2.35 (m, 1H), 2.43-2.50 (m, 4H), 2.57-2.66 (m, 2H), 2.76 (d, 3H, J = 5.0 Hz), 3.65-3.74 (m, 2H), 3.76 (s, 3H), 6.40 (dd, 1H, J = 9.0, 2.0 Hz), 6.59 (d, 1H, J = 2.0 Hz), 6.98 (d, 1H, J = 7.6 Hz), 7.20 (dd, 1H, J = 7.6, 7.6 Hz), 7.43 (d, 1H, J = 9.0 Hz), 7.91-7.94 (m, 1H), 7.93 (s, 1H), 8.06 (s, 1H), 8.36-8.42 (m, 1H) 8.47 (s, 1H). MS (ESI) m/z 564, 566 (M + 1)+ 10-3 DMSO-d6: 2.29(s, 3H), 2.77(d, 3H), 3.07-3.11(m, 4H), 3.73- 3.76(m, 4H), 3.77(s, 3H), 6.41(dd, 1H), 6.63(d, 1H), 7.00(d, 1H), 7.21(dd, 1H), 7.49(d, 1H), 7.93(d, 1H), 7.96(s, 1H), 8.07(s, 1H), 8.37-8.42(m, 1H), 8.49(s, 1H). MS m/z 483 [M + 1]+ - The following 2-[5-Chloro-2-(substituted phenylamino)-pyrimidin-4-ylamino]-5-fluoro-N-methyl-benzamide are prepared from 2-(2,5-Dichloro-pyrimidin-4-ylamino)-5-fluoro-N-methyl-benzamide and the corresponding aniline following the procedure of Example 7A
-
Expl No. Rx Identification 11-1 NMR (400 MHz, DMSO-d6, δ): 2.79 (d, 3 H), 3.10-3.15 (m, 4 H), 3.74-3.78 (m, 7 H), 6.50 (dd, 1 H), 6.66 (d, 1 H), 7.13-7.20 (m, 1 H), 7.41 (d, 1 H), 7.57 (dd, 1 H), 8.09 (s, 1 H), 8.14 (s, 1 H), 8.55-8.65 (m, 1 H), 8.75-8.82 (m, 1 H), 11.39 (s, 1 H). MS (ESI): m/z 487, 489 (M + 1). 11-2 NMR (400 MHz, DMSO-d6, δ): 1.68-1.33 (m, 8 H), 1.93-1.73 (m, 2 H), 2.35-2.60 (m, 1 H), 2.62-2.74 (m, 2 H), 2.67 (t, 2 H), 2.74 (d, 3 H), 3.25-3.38 (m, 4 H), 3.76 (s, 3 H), 3.83-3.71 (m, 2 H), 6.48 (dd, 1 H), 6.49 (dd, 1 H), 6.63 (d, 1 H), 7.15 (dd, 1 H), 7.36 (d, 1 H), 7.57 (dd, 1 H), 8.09 (s, 1 H), 8.12 (s, 1 H), 8.65- 8.55 (m, 1 H), 8.78 (d, 1 H), 11.39 (s, 1 H) MS (ESI): m/z 568, 570 (M + 1) 11-3 DMSO-d6: 2.80 (d, 3 H), 3.79 (s, 3 H), 6.64 (d, 1 H), 7.05-7.20 (m, 3 H), 7.23 (d, 1 H), 7.42-7.49 (d, 1 H), 7.57 (dd, 1 H), 8.20 (s, 1 H), 8.62-8.69 (m, 1 H), 8.75-8.82 (m, 1 H), 9.17 (s, 1 H), 11.43 (s, 1 H). MS m/z 425 [M + 1]+ 11-4 DMSO-d6: 2.06 (s, 3 H), 2.79 (d, 3 H), 3.10-3.14 (m, 2 H), 3.15- 3.19 (m, 2 H), 3.55-3.62 (m, 4 H), 3.77 (s, 3 H), 6.52 (dd, 1 H), 6.69 (d, 1 H), 7.15-7.23 (m, 1 H), 7.43 (d, 1 H), 7.58 (dd, 1 H), 8.10 (s, 1 H), 8.14 (s, 1 H), 8.56-8.65 (m, 1 H), 8.75-8.81 (m, 1 H), 11.39 (s, 1 H). MS m/z 528 [M + 1]+ - N-Methyl-7-nitro-2,3-dihydroisoindole-1-one. At room temperature, a solution of methyl 2-bromomethyl-6-nitrobenzoate (1.26 g, 4.63 mmol) in THF (13 mL) is treated with 2M soln. of methylamine in THF (14 mL), stirred for 5 h, diluted with EtOAc (100 mL), washed with sat. aqueous solution of NaHCO3 (15 mL) and brine (15 mL), dried (MgSO4), and evaporated. A flash chromatography (30 g of silica gel; CH2Cl2/EtOAc 1:1) gives N-Methyl-7-nirto-2,3-dihydroisoindole-1-one (0.561 g, 2.92 mmol) in 63%. Yellow solid. Rf (CH2Cl2/EtOAc 1:1) 0.46.
- 1H-NMR (400 MHz, CDCl3) 3.21 (s), 4.44 (s), 7.63-7.69 (m, 2H), 7.70-7.75 (m, 1H).
- 7-Amino-N-methyl-2,3-dihydroisoindole-1-one. At room temperature, a solution of N-Methyl-7-nirto-2,3-dihydroisoindole-1-one (561.0 mg, 2.92 mmol) in EtOAc (8.4 mL) is treated with SnCl2.2H2O (2.68 g), stirred at 80° C. under reflux for 5 h, and treated with 30 mL of 5N NaOH at 0° C. After the both layers are separated, the aqueous layer is extracted with EtOAc (2×8 mL), the combined extracts are washed with brine (5 mL), dried (MgSO4), and evaporated to give 7-Amino-N-methyl-2,3-dihydroisoindole-1-one (455.9 g, 2.81 mmol) in 96%. Yellow solid.
- Rf (CH2Cl2/EtOAc 1:1) 0.53. 1H-NMR (400 MHz, CDCl3) 3.12 (s), 4.28 (s), 5.20 (br. s), 6.56 (d, J=8.0), 6.68 (d, J=8.0), 7.21 (dd, J=8.0, 8.0).
- 7-(4-Amino-2,5-dichloropyrimidin-4-yl)amino-N-methyl-2,3-dihydroisoindole-1-one. At 0° C., a solution of 7-Amino-N-methyl-2,3-dihydroisoindole-1-one (232.6 mg, 1.43 mmol) in DMF (2.0 mL) is treated with 60% NaH (89.8 mg), stirred at the same temperature for 1.5 h, treated with a solution of 2,4,5-trichlropyrimidine (0.557 g) in DMF (3.5 mL), stirred for 1 h, and warmed to room temperature. After furthermore stirring for 13 h, the mixture is treated with sat. aqueous NH4Cl (6 mL), and the resulting brown precipitates are collected by a filtration, followed by washing with H2O, hexane, and CH3CN to give 7-(4-Amino-2,5-dichloropyrimidin-4-yl)amino-N-methyl-2,3-dihydroisoindole-1-one (130.2 g, 0.416 mmol) in 26%. Brown solid. Rf (CH2Cl2/EtOAc 1:1) 0.50. 1H-NMR (400 MHz, CDCl3): 3.22 (s), 4.43 (s), 7.15 (d, J=8.0), 7.59 (dd, J=8.0, 8.0), 8.24 (s), 8.71 (d, J=8.0), 11.05 (br. s).
- The following 7-[5-Chloro-2-(substituted phenylamino)-pyrimidin-4-ylamino]-2-methyl-2,3-dihydro-isoindol-1-one are prepared from 7-(2,5-Dichloro-pyrimidin-4-ylamino)-2-methyl-2,3-dihydro-isoindol-1-one and the corresponding aniline following the procedure of Example 7A.
-
- 1H-NMR (400 MHz, DMSO-d6, δ): 3.07 (s, 3H), 3.13-3.17 (m, 4H), 3.75 (s, 3H), 3.34-3.78 (m, 4H), 4.46 (s, 2H), 6.54 (dd, 1H, J=8.6, 2.5 Hz), 6.67 (d, 1H, J=2.5 Hz), 7.15 (d, 1H, J=7.6 Hz), 7.25-7.34 (m, 1H) 7.36 (d, 1H, J=8.6 Hz), 8.13 (s, 1H), 8.36 (s, 1H), 8.37-8.50 (m, 1H) 10.57 (s, 1H). MS (ESI) m/z 481. 483 (M+1)+
- The following 7-(5-Chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino)-2-methyl-2,3-dihydro-isoindol-1-ones are prepared from 7-(2,5-Dichloro-pyrimidin-4-ylamino)-2-methyl-2,3-dihydro-isoindol-1-one and the corresponding aniline following the procedure of Example 2:
-
Expl Mass No. Rx (m/z) NMR (400 MHz) δ (ppm) 12-2 494 [M + 1]+ DMSO-d6: 2.24 (s, 3 H), 2.45-2.50 (m, 4 H), 3.07 (s, 3 H), 3.15-3.19 (m, 4 H), 3.74 (s, 3 H), 4.46 (s, 2 H), 6.52 (dd, 1 H), 6.66 (d, 1 H), 7.15 (d, 1 H), 7.25-7.36 (m, 2 H), 8.12 (s, 1 H), 8.35 (s, 1 H), 8.35-8.45 (m, 1 H), 10.57 (s, 1 H) 12-3 495 [M + 1]+ DMSO-d6: 1.48-1.57 (m, 2 H), 1.83-1.88 (m, 2 H), 2.83- 2.90 (m, 2 H), 3.07 (s, 3 H), 3.51-3.60 (m, 2 H), 3.61- 3.70 (m, 2 H), 3.73 (s, 3 H), 4.46 (s, 2 H), 4.69 (d, 1 H), 6.52 (dd, 1 H), 6.64 (d, 1 H), 7.14 (d, 1 H), 7.25-7.35 (m, 2 H), 8.12 (s, 1 H), 8.33 (s, 1 H), 8.35-8.45 (m, 1 H), 10.57 (s, 1 H) 12-4 577 [M + 1]+ DMSO-d6: 1.48-1.59 (m, 2 H), 1.83-1.88 (m, 2 H), 2.14 (s, 3 H), 2.25-2.39 (m, 4 H), 2.42-2.60 (m, 5 H), 2.66- 2.73 (m, 2 H), 3.07 (s, 3 H), 3.73-3.77 (m, 2 H), 3.74 (s, 3 H), 4.46 (s, 2 H), 6.52 (dd, 1 H), 6.64 (d, 1 H), 7.14 (d, 1 H), 7.25-7.34 (m, 2 H), 8.12 (s, 1 H), 8.34 (s, 1 H), 8.35- 8.45 (m, 1 H), 10.57 (s, 1 H) 12-5 562 [M + 1]+ DMSO-d6: 1.35-1.65 (m, 8 H), 1.73-1.85 (m, 2 H), 2.40- 2.59 (m, 7 H), 3.08 (s, 3 H), 3.52-3.61 (m, 2 H), 3.73 (s, 3 H), 4.47 (s, 2 H), 6.72 (dd, 1 H), 6.94 (d, 1 H), 7.17 (d, 1 H), 7.34-7.39 (m, 2 H), 8.21 (s, 1 H), 8.37 (s, 1 H), 8.45- 8.53 (m, 1 H), 10.64 (s, 1 H) 12-6 MS m/z 536 DMSO-d6: 2.19-2.42 (m, 4 H), 2.65-2.89 (m, 3 H), 3.07 (s, 3 H), 3.11-3.30 (m, 1 H), 3.48-3.61 (m, 2 H), 3.62- 3.71 (m, 1 H), 3.75 (s, 3 H), 3.75-3.83 (m, 2 H), 4.47 (s, 2 H), 6.48-6.52 (m, 1 H), 6.66 (d, 1 H), 7.15 (d, 1 H), 7.26-7.37 (m, 2 H), 8.13 (s, 1 H), 8.35 (s, 1 H), 8.42 (brs, 1 H), 10.57 (s, 1 H). - The following 7-(5-Chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino)-2-ethyl-2,3-dihydroisoindol-1-ones are prepared from 7-(2,5-Dichloro-pyrimidin-4-ylamino)-2-ethyl-2,3-dihydro-isoindol-1-one and the corresponding aniline following the procedure of Example 2:
-
Expl Mass No. Rx (m/z) NMR (400 MHz) δ (ppm) 13-1 508 [M + 1]+ DMSO-d6: 1.19 (t, 3 H), 2.24 (s, 3 H), 2.47-2.51 (m, 4 H), 3.15-3.21 (m, 4 H), 3.54 (q, 2 H), 3.74 (s, 3 H), 4.48 (s, 2 H), 6.54 (dd, 1 H), 6.65 (d, 1 H), 7.15 (d, 1 H), 7.26- 7.36 (m, 2 H), 8.12 (s, 1 H), 8.34 (s, 1 H), 8.37-8.48 (m, 1 H), 10.58 (s, 1 H) - To a suspension of 2-[5-chloro-2-(2-methoxy-4-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-benzoic acid (5.5 g, 12.1 mmol) in 100 mL of THF are added Et3N (2.06 mL, 14.8 mmol) and isobutyl chloroformate (1.7 mL, 12.8 mmol) at −5° C. After stirring at the same temperature for 30 min, the reaction mixture is further stirred at room temperature for 1 hour and then H2O is added to the reaction mixture. The resulting precipitate is collected by filtration, washed with H2O, and dried under reduced pressure to give an intermediate (4.80 g) (10.96 mmol, 91%) as yellow solid.
- NMR (400 MHz, DMSO-d6, δ): 3.10-3.20 (m, 4H), 3.70-3.80 (m, 4H), 3.93 (s, 3H), 6.53 (dd, 1H, J=9.08, 2.0 Hz), 6.70 (d, 1H, J=2.0 Hz), 7.49-7.54 (m, 1H), 7.67 (d, 1H, J=8.56 Hz), 7.89 (s, 1H), 7.85-7.95 (m, 1H), 8.23 (d, 1H, J=9.08 Hz), 8.26 (d, 1H, J=8.56 Hz), 12.60 (s, 1H).
- To a 1M solution of methylamine in THF (560 μL, 0.56 mmol) is added 82 mg of the obtained intermediate (0.187 mmol) followed by 1M solution of NaHMDS in THF (560 μl, 0.56 mmol) dropwise. After the reaction mixture is stirred for 10 minutes, 5 mL of H2O is added and extraction is performed with AcOEt. The organic layer is washed with brine, dried over Na2SO4, concentrated under reduced pressure, and purified by silica gel column chromatography (Hexane:AcOEt=1:1 to AcOEt) to give the title compound as a pale yellow solid. Data are given in Example 7A.
- By repeating the procedures described above using appropriate starting materials and conditions the following compounds are obtained as identified below.
-
Expl Rf (solvent) No. Ry or MS NMR (400 MHz), δ (ppm) 14-1 0.10 (n-hexane: AcOEt = 1:1) CDCl3: 3.02-3.19 (m, 10 H), 3.83-3.91 (m, 4 H), 3.87 (s, 3 H), 6.45 (dd, 1 H), 6.52 (d, 1 H), 7.09-7.14 (m, 1 H), 7.29 (m, 1 H), 7.31 (dd, 1 H), 7.38-7.45 (m, 1 H), 8.06 (s, 1 H), 8.14 (d, 1 H), 8.39 (d, 1 H), 8.97 (s, 1 H). 14-2 0.36 (n-hexane: AcOEt = 1:2) CDCl3: 1.27 (d, 6 H), 3.09-3.16 (m, 4 H), 3.81-3.92 (m, 4 H), 3.89 (s, 3 H), 4.26-4.37 (m, 1 H), 5.93-5.98 (m, 1 H), 6.48 (dd, 1 H), 6.53 (d, 1 H), 7.05-7.11 (m, 1 H), 7.42-7.49 (m, 2 H), 8.08 (s, 1 H), 8.12 (d, 1 H), 8.65 (d, 1 H), 10.88 (br. s, 1 H). 14-3 505 [M + 1]+ DMSO-d6: 2.79 (d, 3 H), 3.09-3.14 (m, 4 H), 3.74- 3.77 (m, 4 H), 3.75 (s, 3 H), 6.49 (dd, 1 H), 6.65 (d, 1 H), 7.30 (d, 1 H), 7.84 (dd, 1 H), 8.12 (s, 1 H), 8.40 (s, 1 H), 8.65-8.79 (m, 2 H), 11.39 (s, 1 H) 14-4 466 [M + 1]+ DMS0-d6: 2.70-2.75 (m, 2 H), 3.04-3.09 (m, 2 H), 3.12- 3.18 (m, 4 H), 3.74-3.80 (m, 4 H), 3.75 (s, 3 H), 6.54 (dd, 1 H), 6.67 (d, 1 H), 7.14 (d, 1 H), 7.34 (d, 1 H), 7.37- 7.44 (m, 1 H), 8.17 (s, 1 H), 8.35-8.50 (m, 1 H), 8.44 (s, 1 H), 10.59 (s, 1 H) 14-5 Rf (Hexane: AcOEt = 1:2): 0.31 DMSO: 1.18 (t, 3 H), 3.11-3.21 (4, 4 H), 3.30-3.60 (m, 2 H), 3.71-3.85 (m, 7 H), 6.50-6.58 (m, 1 H), 6.71 (d, 1 H), 7.17-7.26 (m, 1 H), 7.46 (d, 1 H), 7.64 (dd, 1 H), 8.14 (s, 1 H), 8.19 (s, 1 H), 8.57-8.68 (m, 1 H), 8.80- 8.87 (m, 1 H), 11.36 (s, 1 H). 14-6 Rf (Hexane: AcOEt = 1:1): 0.051 DMSO: 1.71-1.92 (m, 2 H), 1.92-2.06 (m, 2 H), 3.08- 3.14 (m, 4 H), 3.48-3.57 (m, 2 H), 3.63-3.75 (m, 2 H), 3.84-3.90 (m, 7 H), 6.47 (dd, 1 H), 6.53 (d, 1 H), 7.09 (ddd, 1 H), 7.25-7.29 (m, 1 H), 7.38-7.44 (m, 1 H), 8.06 (s, 1 H), 8.15 (d, 1 H), 8.45 (dd, 1 H), 9.60 (s, 1 H). 14-7 1H-NMR (400MHz, δ ppm, CDCl3): 3.04-3.10 (m, 4 H), 3.10-3.16 (m, 4 H), 3.63-3.68 (m, 4 H), 3.85-3.90 (m, 7 H), 6.46 (dd, 1 H), 6.53 (d, 1 H), 7.20-7.25 (m, 1 H), 7.33 (brs, 1 H), 7.56-7.62 (m, 1 H), 7.85 (dd, 1 H), 8.03 (d, 1 H), 8.12 (s, 1 H), 8.57-8.61 (m, 1 H), 9.30 (s, 1 H). - The following 2-(5-Chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino)-N-methyl-5-pyrrolidin-1-yl-benzamides are prepared from 2-(5-Chloro-2-methyl-pyrimidin-4-ylamino)-N-methyl-5-pyrrolidin-1-yl-benzamide and the corresponding aniline following the procedure of Example 2:
-
Expl Mass No. Rx (m/z) NMR (400 MHz) δ (ppm) 15-1 551 [M + 1]+ DMSO-d6: 1.94-1.99 (m, 4 H), 2.23 (s, 3 H), 2.43- 2.48 (m, 4 H), 2.78 (d, 3 H), 3.11-3.17 (m, 4 H), 3.22- 3.29 (m, 4 H), 3.76 (s, 3 H), 6.46 (dd, 1 H), 6.48-6.53 (m, 1 H), 6.63 (d, 1 H), 6.79 (d, 1 H), 7.44 (d, 1 H), 7.89 (s, 1 H), 7.99 (s, 1 H), 8.24 (d, 1 H), 8.60 (d, 1 H), 10.88 (s, 1 H) 15-2 566 [M + 1]+ DMSO-d6: 1.60-1.70 (m, 2 H), 1.90-2.00 (m, 6 H), 2.12- 2.20 (m, 2 H), 2.18 (s, 3 H), 2.60-2.65 (m, 2 H), 2.78 (d, 3 H), 3.22-3.28 (m, 4 H), 3.75 (s, 3 H), 4.25-4.37 (m, 1 H), 6.49-6.55 (m, 2 H), 6.62 (d, 1 H), 6.80 (d, 1 H), 7.53 (d, 1 H), 7.90 (s, 1 H), 8.00 (s, 1 H), 8.24 (d, 1 H), 8.58- 8.63 (m, 1 H), 10.88 (s, 1 H) 15-3 538 [M + 1]+ DMSO-d6: 1.94-1.99 (m, 4 H), 2.78 (d, 3 H), 3.09- 3.15 (m, 4 H), 3.22-3.27 (m, 4 H), 3.73-3.77 (m, 4 H), 3.76 (s, 3 H), 6.47 (dd, 1 H), 6.47-6.53 (m, 1 H), 6.65 (d, 1 H), 6.79 (d, 1 H), 7.47 (d, 1 H), 7.90 (s, 1 H), 7.99 (s, 1 H), 8.24 (d, 1 H), 8.60 (d, 1 H), 10.88 (s, 1 H) - The following 2-[5-Chloro-2-(4-fluoro-2-methoxy-phenylamino)-pyrimidin-4-ylamino]-5-subst.-N-methyl-benzamide are prepared from the corresponding aniline following the procedure of Example 2
-
Expl Mass No. Ry (m/z) NMR (400 MHz) δ (ppm) 16-1 487 [M + 1]+ DMSO-d6: 2.79 (d, 3 H), 3.11-3.15 (m, 4 H), 3.74-3.81 (m, 4 H), 3.81 (s, 3 H), 6.76 (ddd, 1 H), 6.95-7.05 (m, 2 H), 7.21 (d, 1 H), 7.72 (dd, 1 H), 8.08 (s, 1 H), 8.09 (s, 1 H), 8.33 (d, 1 H), 8.63-8.73 (m, 1 H), 11.17 (s, 1 H) 16-2 500 [M + 1]+ DMSO-d6: 2.24 (s, 3 H), 2.45-2.52 (m, 4 H), 2.79 (d, 3 H), 3.13-3.18 (m, 4 H), 3.81 (s, 3 H), 6.75 (ddd, 1 H), 6.94- 7.02 (m, 2 H), 7.20 (d, 1 H), 7.73 (dd, 1 H), 8.03-8.11 (m, 2 H), 8.30 (d, 1 H), 8.60-8.70 (m, 1 H), 11.14 (s, 1 H) 16-3 432 [M + 1]+ DMSO-d6: 2.79 (d, 3 H), 3.80-3.81 (m, 6 H), 6.75 (ddd, 1 H), 6.90-7.02 (m, 2 H), 7.27 (d, 1 H), 7.67 (dd, 1 H), 8.10 (s, 1 H), 8.16 (s, 1 H), 8.39 (d, 1 H), 8.70-8.76 (m, 1 H), 11.20 (s, 1 H) 16-4 568 [M + 1]+ DMSO-d6: 1.35-1.62 (m, 8 H), 1.78-1.85 (m, 2 H), 2.30-2.40 (m, 1 H), 2.41-2.52 (m, 4 H), 2.60-2.70 (m, 2 H), 2.78 (d, 3 H), 3.70- 3.80 (m, 2 H), 3.81 (s, 3 H), 6.75 (ddd, 1 H), 6.95-7.02 (m, 2 H), 7.20 (d, 1 H), 7.72 (dd, 1 H), 8.05-8.08 (m, 2 H), 8.28 (d, 1 H), 8.63-8.69 (m, 1 H), 11.12 (s, 1 H) -
- CDCl3: 3.01-3.10 (m, 4H), 3.63-3.68 (m, 4H), 3.89 (s, 3H), 6.59 (ddd, 1H), 6.66 (dd, 1H), 7.20-7.26 (m, 1H), 7.36 (s, 1H), 7.57-7.63 (m, 1H), 7.84 (dd, 1H), 8.09-8.14 (m, 1H), 8.14 (s, 1H), 8.53 (d, 1H), 9.30 (s, 1H).
-
- CDCl3: 3.56-3.65 (m, 2H), 3.88 (s, 3H), 5.11-5.19 (m, 1H), 6.50-6.56 (m, 1H), 6.61-6.66 (m, 1H), 7.25-7.29 (m, 1H), 7.38 (brs, 1H), 7.58-7.62 (m, 1H), 7.97 (dd, 1H), 8.02-8.10 (m, 1H), 8.15 (s, 1H), 8.41 (dd, 1H), 8.81 (s, 1H).
- The following 2-(5-Chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino)-5-fluoro-N-methyl-benzamide are prepared from 2-(2,5-Dichloro-pyrimidin-4-ylamino)-5-fluoro-N-methyl-benzamide and the corresponding aniline following the procedure of Example 2:
-
Expl Mass No. Rx (m/z) NMR (400 MHz) δ (ppm) 18-1 595 [M + 1]+ DMSO-d6: 2.06 (s, 3 H), 2.78 (d, 3 H), 3.05-3.18 (m, 8 H), 3.53-3.64 (m, 4 H), 3.68-3.77 (m, 4 H), 3.77 (s, 3 H), 6.51 (dd, 1 H), 6.69 (d, 1 H), 6.88 (br.d, 1 H), 7.20 (d, 1 H), 7.43 (d, 1 H), 7.99-8.03 (m, 2 H), 8.34 (br.d, 1 H), 8.63-8.71 (m, 1 H), 11.15 (s, 1 H). - The following 2-[5-chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino]-N-isopropyl-benzene-sulfonamides are prepared from 2-(5-chloro-2-chloro-pyrimidin-4-ylamino)-N-isopropyl-benzenesulfonamide and the corresponding aniline following the procedure of Example 7A
-
Expl Rf (solvent) No. Rx Or MS NMR (400 MHz), δ (ppm) 19-1 0.39 (MeOH: CH2Cl2 = 1:4) DMSO-d6: 0.94 (d, 6 H), 1.75-1.84 (m, 1 H), 2.07- 2.16 (m, 1 H), 2.33 (s, 3 H), 2.98-3.04 (m, 1 H), 3.22- 3.36 (m, 5 H), 3.42-3.47 (m, 1 H), 3.74 (s, 3 H), 6.05 (dd, 1 H), 6.18 (d, 1 H), 7.18 (dd, 1 H), 7.25 (d, 1 H), 7.35- 7.45 (m, 1 H), 7.77-7.82 (m, 1 H), 7.70-8.10 (m, 1 H), 8.09-8.17 (m, 2 H), 8.45-8.63 (m, 1 H), 9.34 (s, 1 H) 19-2 0.40 (n-hexane: AcOEt = 1:1) CDCl3: 1.00 (d, 6 H), 1.13 (t, 3 H), 1.83-1.92 (m, 1 H), 2.23-2.30 (m, 1 H), 2.70-2.78 (m, 2 H), 3.08-3.13 (m, 1 H), 3.27-3.54 (m, 5 H), 3.85 (s, 3 H), 4.33 (d, 1 H), 6.05 (d, 1 H), 6.13 (s, 1 H), 7.13 (bs, 1 H), 7.18-7.22 (m, 1 H), 7.52-7.56 (m, 1 H), 7.83-7.86 (m, 1 H), 7.95-7.98 (m, 1 H), 8.09 (s, 1 H), 8.47-8.49 (m, 1 H), 8.89 (s, 1 H) 19-3 0.30 (n-hexane: AcOEt = 1:1) CDCl3: 0.93 (d, 6 H), 1.05-1.09 (m, 1 H), 1.48-1.99 (m, 6 H), 2.16 (s, 3 H), 2.61-2.67 (m, 1 H), 2.80-2.83 (m, 1 H), 3.75 (s, 3 H), 3.80-3.89 (m, 2 H), 6.44-6.47 (m, 1 H), 6.62-6.63 (m, 1 H), 7.18-7.22 (m, 1 H), 7.42-7.46 (m, 1 H), 7.80-7.89 (m, 2 H), 8.17 (s, 1 H), 8.23 (s, 1 H), 8.42-8.44 (m, 1 H), 8.89 (s, 1 H) 19-4 0.69 (MeOH: CH2Cl2 = 1:3) DMSO-d6: 0.94 (d, 6 H), 1.45-1.57 (m, 2 H), 1.80- 1.88 (m, 2 H), 2.14 (s, 3 H), 2.25-2.35 (m, 4 H), 2.45- 2.55 (m, 4 H), 2.62-2.70 (m, 2 H), 3.28-3.37 (m, 1 H), 3.68-3.74 (m, 2 H), 3.75 (s, 3 H), 6.44 (dd, 1 H, J = 8.82, 2.0 Hz), 6.61 (d, 1 H, J = 2.0 Hz), 7.21 (dd, 1 H), 7.37 (d, 1 H), 7.45 (dd, 1 H), 7.81 (dd, 1 H, J = 1.82, 1.52 Hz), 7.84- 7.92 (m, 1 H), 8.12-8.20 (m, 1 H), 8.16 (s, 1 H), 8.43- 8.51 (m, 1 H), 9.31 (s, 1 H) 19-5 0.35 (n-hexane: AcOEt = 1:1) CDCl3: 0.93 (d, 6 H), 2.23 (s, 3 H), 2.45-2.48 (m, 4 H), 3.12-3.15 (m, 4 H), 3.75 (s, 3 H), 6.42-6.45 (m, 1 H), 6.63 (s, 1 H), 7.19-7.23 (m, 1 H), 7.38-7.47 (m, 2 H), 7.80- 7.89 (m, 2 H), 8.16 (s, 1 H), 8.46-8.48 (m, 1 H), 9.34 (s, 1 H) 19-6 0.45 (n-hexane: AcOEt = 1:1) CDCl3: 0.99 (d, 6 H), 3.40-3.49 (m, 1 H), 3.88 (s, 3 H), 4.29-4.31 (d, 1 H), 6.51-6.56 (m, 1 H), 6.62-6.65 (m, 1 H), 7.24-7.28 (m, 1 H), 7.37 (s, 1 H), 7.56-7.60 (m, 1 H), 7.98-8.15 (m, 3 H), 8.34-8.37 (m, 1 H), 8.89 (s, 1 H) 19-7 0.28 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.93 (d, 6 H), 1.59-1.67 (m, 2 H), 1.90- 1.93 (m, 2 H), 2.10-2.24 (m, 5 H), 2.60-2.67 (m, 2 H), 3.74 (s, 3 H), 4.33-4.37 (m, 1 H), 6.47-6.50 (m, 1 H), 6.63 (d, 1 H), 7.18-7.22 (m, 1 H), 7.41-7.45 (m, 2 H), 7.79-7.87 (m, 2 H), 8.16 (s, 1 H), 8.21 (s, 1 H), 8.41- 8.43 (m, 1 H), 9.29 (s, 1 H) 19-8 0.25 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.93 (d, 6 H), 3.09-3.12 (m, 4 H), 3.74- 3.76 (m, 7 H), 6.43-6.46 (m, 1 H), 6.64 (s, 1 H), 7.19- 7.23 (m, 1 H), 7.41-7.48 (m, 2 H), 7.80 (d, 1 H), 7.82 (d, 1 H), 8.17 (s, 1 H), 8.46-8.48 (m, 1 H), 9.31 (s, 1 H) 19-9 0.56 (MeOH: CH2Cl2 = 1:4) DMSO-d6: 0.93 (d, 6 H), 1.89-1.90 (m, 1 H), 2.30 (bs, 6 H), 3.13-3.50 (m, 6 H), 3.74 (s, 3 H), 6.10 (d, 1 H), 6.22 (s, 1 H), 7.16-7.20 (m, 1 H), 7.25-7.27 (m, 1 H), 7.40 (bs, 1 H), 7.79-7.81 (m, 1 H), 7.86-7.88 (m, 1 H), 8.12 (s, 1 H), 8.15 (s, 1 H), 8.51 (s, 1 H), 9.34 (s, 1 H) 19-10 0.45 (MeOH: CH2Cl2 = 1:4) CDCl3: 0.99 (d, 12 H), 2.27 (s, 2 H), 2.31 (s, 6 H), 2.96 (s, 2 H), 3.39-3.48 (m, 1 H), 3.83 (s, 3 H), 4.30 (d, 1 H), 6.09- 6.12 (m, 1 H), 6.19 (d, 1 H), 7.11 (s, 1 H), 7.19-7.23 (m, 1 H), 7.51-7.57 (m, 1 H), 7.76-7.79 (m, 1 H), 7.95 (d, 1 H), 8.09 (s, 1 H), 8.46-8.49 (m, 1 H), 8.88 (s, 1 H) 19-11 0.30 (n-hexane: AcOEt = 1:1) DMS0-d6: 0.93 (d, 6 H), 2.96-2.99 (m, 4 H), 3.74- 3.76 (m, 7 H), 6.67-6.72 (m, 1 H), 7.21-7.25 (m, 1 H), 7.31-7.34 (m, 1 H), 7.44-7.48 (m, 1 H), 7.80-7.83 (m, 1 H), 7.88 (d, 1 H), 8.21 (s, 1 H), 8.42 (d, 1 H), 8.58 (s, 1 H), 9.30 (s, 1 H) 19-12 0.42 (MeOH: CH2Cl2 = 1:4) DMSO-d6: 0.94 (d, 6 H), 1.68-1.76 (m, 1 H), 1.99- 2.07 (m, 1 H), 2.29 (s, 3 H), 3.05-3.49 (m, 6 H), 3.75 (s, 3 H), 6.36-6.40 (m, 1 H), 7.10-7.37 (m, 3 H), 7.70- 7.80 (m, 1 H), 8.08-8.39 (m, 3 H), 9.24 (s, 1 H) 19-13 0.50 (MeOH: CH2Cl2 = 1:4) CDCl3: 1.01 (d, 6 H), 1.94-1.96 (m, 1 H), 2.01 (s, 3 H), 2.29-2.37 (m, 1 H), 3.19-3.58 (m, 5 H), 3.86 (s, 3 H), 4.42 (d, 1 H), 4.59-4.63 (m, 1 H), 5.70 (d, 1 H), 6.05- 6.08 (m, 1 H), 6.15-6.16 (m, 1 H), 7.17-7.24 (m, 2 H), 7.53-7.57 (m, 1 H), 7.90 (d, 1 H), 7.91-7.98 (m, 1 H), 8.09 (s, 1 H), 8.47 (d, 1 H), 8.91 (s, 1 H) 19-14 0.53 (MeOH: CH2Cl2 = 1:4) CDCl3: 1.00 (d, 6 H), 2.04 (s, 3 H), 2.05-2.29 (m, 2 H), 2.96 (s, 3 H), 3.19-3.54 (m, 5 H), 3.86 (s, 3 H), 4.57- 4.63 (m, 1 H), 5.39-5.46 (m, 1 H), 6.07-6.09 (m, 1 H), 6.16 (d, 1 H), 7.18-7.26 (m, 2 H), 7.53-7.57 (m, 1 H), 7.89-7.98 (m, 2 H), 8.08 (s, 1 H), 8.47 (d, 1 H), 8.94 (d, 1 H) 19-15 0.56 (MeOH: CH2Cl2 = 1:4) DMSO-d6: 0.93 (d, 6 H), 1.48-1.56 (m, 2 H), 1.65- 1.75 (m, 4 H), 1.90-1.93 (m, 2 H), 2.05-2.15 (m, 1 H), 2.45-2.55 (m, 5 H), 2.69-2.75 (m, 2 H), 3.61 (d, 2 H), 3.74 (s, 1 H), 6.42-6.51 (m, 1 H), 6.61 (d, 1 H), 7.18- 7.22 (m, 1 H), 7.37 (d, 1 H), 7.43-7.47 (m, 1 H), 7.80 (d, 1 H), 7.81-7.89 (m, 1 H), 8.16 (d, 1 H), 8.46-8.48 (m, 1 H), 9.31 (s, 1 H) 19-16 0.56 (MeOH: CH2Cl2 = 1:4) DMSO-d6: 0.92 (d, 6 H), 1.65-1.75 (m, 4 H), 1.88- 2.00 (m, 4 H), 2.39-2.43 (m, 2 H), 2.60-2.65 (m, 2 H), 3.03-3.07 (m, 1 H), 3.03-3.40 (m, 2 H), 3.70 (s, 3 H), 3.77-3.78 (m, 1 H), 6.09 (d, 1 H), 6.23 (s, 1 H), 7.13- 7.17 (m, 1 H), 7.23-7.25 (m, 1 H), 7.30-7.42 (m, 1 H), 7.78 (d, 1 H), 7.86 (d, 1 H), 8.10 (s, 1 H), 8.13 (s, 1 H), 8.40-8.50 (m, 1 H), 9.31 (s, 1 H) 19-17 0.23 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.93 (d, 6 H), 1.24-1.57 (m, 4 H), 1.69- 1.78 (m, 2 H), 1.98-2.04 (m, 1 H), 2.15-2.33 (m, 5 H), 2.70-2.80 (m, 1 H), 3.74 (s, 3 H), 3.91-3.94 (m, 1 H), 4.05-4.09 (m, 1 H), 6.46-6.49 (m, 1 H), 6.63 (d, 1 H), 7.18-7.22 (m, 1 H), 7.42-7.46 (m, 2 H), 7.80 (d, 1 H), 7.89 (d, 1 H), 8.17 (s, 1 H), 8.25 (s, 1 H), 8.42-8.44 (m, 1 H), 9.31 (s, 1 H) 19-18 0.48 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.93 (d, 6 H), 1.03 (t, 3 H), 1.13 (t, 3 H), 1.42- 1.81 (m, 4 H), 2.57-2.83 (m, 4 H), 3.17-3.41 (m, 4 H), 3.65-3.75 (m, 1 H), 3.80 (s, 3 H), 4.21 (bs, 1 H), 6.42- 6.47 (m, 2 H), 6.51 (d, 1 H), 6.63 (d, 1 H), 7.18-7.22 (m, 1 H), 7.38-7.47 (m, 2 H), 7.80-7.82 (m, 1 H), 7.89 (d, 1 H), 8.16 (s, 1 H), 8.47-8.49 (m, 1 H), 9.31 (s, 1 H) 19-19 0.44 (CH2Cl2: MeOH = 9:1) CDCl3: 1.45-1.62 (m, 2 H), 1.72-1.78 (m, 1 H), 1.82- 1.90 (m, 1 H), 2.40-2.46 (m, 1 H), 2.61-2.75 (m, 2 H), 3.75-3.70 (m, 2 H), 3.76 (s, 3 H), 6.45 (dd, 1 H), 6.62 (d, 1 H), 6.85 (s, 1 H), 7.19-7.23 (m, 1 H), 7.36-7.48 (m, 3 H), 7.80-7.82 (m, 1 H), 7.85-7.93 (br, 1 H), 8.16 (s, 2 H), 8.43-8.52 (m, 1 H), 9.31 (s, 1 H) 19-20 Ms: 547 DMSO-d6: 0.94 (d, 6 H), 1.73-1.82 (m, 1 H), 2.23-2.33 (m, 4 H), 2.34-2.41 (m, 1 H), 2.54-2.62 (m, 1 H), 2.62- 2.69 (m, 1 H), 2.77-2.82 (m, 1 H), 3.25-3.35 (m, 1 H), 3.74 (s, 3 H), 4.85-4.92 (m, 1 H), 6.4 (dd, 1 H), 6.57 (d, 1 H), 7.16-7.24 (m, 1 H), 7.38-7.51 (m, 1 H), 7.81 (d, 1 H), 7.82-7.94 (m, 1 H), 8.16 (s, 1 H), 8.22 (brs, 1 H), 8.38-8.48 (m, 1 H), 9.3 (brs, 1 H) 19-21 Ms: 579 DMSO-d6: 0.92 (d, 6 H), 1.61-1.71 (m, 2 H), 1.86-1.96 (m, 2 H), 2.12-2.22 (m, 5 H), 2.57-2.64 (m, 2 H), 3.2-3.4 (m, 1 H), 3.77 (s, 3 H), 4.27-4.35 (m, 1 H), 6.86 (dd, 1 H), 7.19-7.27 (m, 1 H), 7.39-7.46 (m, 1 H), 7.81 (dd, 1 H), 7.84-7.92 (m, 1 H), 8.21 (s, 1 H), 8.36-8.42 (m, 1 H), 8.62 (s, 1 H), 9.28 (s, 1 H) 19-22 Ms: 549 DMS0-d6: 0.90 (s, 6 H), 0.94 (d, 6 H), 2.9 (d, 2 H), 3.24 (d, 2 H), 3.25-3.35 (m, 1 H), 3.27-3.36 (m, 1 H), 3.68 (s, 3 H), 4.58 (t, 1 H), 5.3 (t, 1 H), 6.16 (dd, 1 H), 6.39 (d, 1 H), 7.13 (d, 1 H), 7.15-7.21 (m, 1 H), 7.35- 7.45 (m, 1 H), 7.8 (dd, 1 H), 7.83-7.92 (m, 1 H), 8.09 (s, 1 H), 8.11 (s, 1 H), 8.45-8.57 (m, 1 H), 9.33 (s, 1 H) 19-23 Rf: 0.51 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.94 (d, 6 H), 1.22 (s, 6 H), 3.25-3.35 (m, 1 H), 3.36 (d, 2 H), 3.68 (s, 3 H), 4.73-4.79 (brs, 1 H), 4.81 (t, 1 H), 6.29 (dd, 1 H), 6.44 (d, 1 H), 7.14-7.22 (m, 2 H), 7.38-7.46 (m, 1 H), 7.8 (dd, 1 H), 7.85-7.9 (m, 1 H), 8.1 (s, 1 H), 8.13 (s, 1 H), 8.45-8.55 (m, 1 H), 9.32 (s, 1 H) 19-24 Ms: 577 DMSO-d6: 0.93 (d, 6 H), 0.96 (s, 6 H), 2.22 (s, 6 H), 3.25-3.35 (m, 1 H), 3.7 (s, 3 H), 3.75 (s, 3 H), 6.46 (dd, 1 H), 6.62 (d, 1 H), 7.16-7.23 (m, 1 H), 7.38-7.47 (m, 1 H), 7.81 (dd, 1 H), 7.85-7.9 (m, 1 H), 8.17 (s, 1 H), 8.23 (s, 1 H), 8.38-8.48 (m, 1 H), 9.31 (s, 1 H) 19-25 Ms: 521 DMSO-d6: 0.94 (d, 6 H), 3.12 (t, 4 H), 3.25-3.35 (m, 1 H), 3.75 (t, 4 H), 6.73 (dd, 1 H), 6.85 (dd, 1 H), 7.16- 7.24 (m, 1 H), 7.25-7.32 (m, 1 H), 7.38-7.47 (m, 1 H), 7.8 (dd, 1 H), 7.88 (d, 1 H), 8.18 (s, 1 H), 8.42-8.52 (m, 1 H), 8.86 (s, 1 H), 9.36 (s, 1 H) 19-26 Ms: 565 DMSO-d6: 0.93 (d, 6 H), 2.4-2.56 (m, 4 H), 2.69 (t, 2 H), 3.25-3.38 (m, 1 H), 3.59 (t, 4 H), 4.11 (t, 1 H), 6.75 (dd, 1 H), 6.93 (dd, 1 H), 7.16-7.23 (m, 1 H), 7.3-7.4 (m, 1 H), 7.4-7.38 (m, 1 H), 7.8 (dd, 1 H), 7.88 (d, 1 H), 8.19 (s, 1 H), 8.36-8.5 (m, 1 H), 8.92 (s, 1 H), 9.34 (s, 1 H) 19-27 Ms: 614 DMSO-d6: 0.93 (d, 6 H), 1.3-1.62 (m, 8 H), 1.75-1.85 (m, sH), 2.26-2.4 (m, 1 H), 2.4-2.58 (m, 4 H), 3.28-3.38 (m, 1 H), 3.68-3.78 (m, 5 H), 6.42 (dd, 1 H), 6.64 (d, 1 H), 7.18-7.24 (m, 1 H), 7.42-7.5 (m, 2 H), 7.77 (d, 1 H), 7.82 (dd, 1 H), 8.13 (s, 1 H), 8.17 (s, 1 H), 8.4-8.5 (m, 1 H), 9.36 (s, 1 H) 19-28 Rf: 0.5 (MeOH: CH2Cl2 = 3:7) DMSO-d6: 0.93 (d, 6 H), 1.6-1.7 (m, 2 H), 1.88-1.98 (m, 2 H), 2.17-2.35 (m, 5 H), 2.6-2.73 (m, 2 H), 3.25-3.4 (m, 1 H), 4.34-4.44 (m, 1 H), 6.75 (dd, 1 H), 6.93 (dd, 1 H), 7.16-7.23 (m, 1 H), 7.29-7.36 (m, 1 H), 7.37-7.47 (m, 1 H), 7.8 (dd, 1 H), 7.89 (d, 1 H), 8.19 (s, 1 H), 8.36- 8.46 (m, 1 H), 8.92 (s, 1 H), 9.31 (s, 1 H) 19-29 Ms: 577 DMSO-d6: 0.93 (d, 6 H), 2.45-2.55 (m, 4 H), 2.7 (t, 2 H), 3.25-3.35 (m, 1 H), 3.59 (t, 3 H), 3.76 (s, 3 H), 4.1 (t, 1 H), 6.48 (dd, 1 H), 6.65 (d, 1 H), 7.18-7.24 (m, 1 H), 7.4-7.5 (m, 2 H), 7.82 (dd, 1 H), 7.88 (d, 1 H), 8.17 (s, 1 H), 8.24 (s, 1 H), 8.4-8.48 (m, 1 H), 9.31 (s, 1 H) 19-30 Ms: 590 DMSO-d6: 0.93 (d, 6 H), 2.15 (s, 3 H), 2.2-2.4 (m, 4 H), 2.4-2.6 (m, 4 H), 2.69 (t, 2 H), 3.25-3.35 (m, 1 H), 3.75 (s, 3 H), 4.08 (t, 2 H), 6.47 (dd, 1 H), 6.64 (d, 1 H), 7.18- 7.24 (m, 1 H), 7.41-7.49 (m, 2 H), 7.81 (dd, 1 H), 7.86- 7.91 (m, 1 H), 8.17 (s, 1 H), 8.24 (s, 1 H), 8.39-8.46 (m, 1 H), 9.31 (s, 1 H) 19-31 Ms: 588 DMSO-d6: 0.94 (d, 6 H), 2.19-2.36 (m, 4 H), 2.66-2.85 (m, 3 H), 3.15-3.21 (m, 1 H), 3.73-3.8 (m, 5 H), 6.43 (dd, 1 H), 6.63 (d, 1 H), 7.18-7.25 (m, 1 H), 7.4 (d, 1 H), 7.43-7.5 (m, 1 H), 7.81 (dd, 1 H), 7.89 (d, 1 H), 8.16 (s, 1 H), 8.17 (s, 1 H), 8.42-8.52 (m, 1 H), 9.32 (s, 1 H) 19-32 Ms: 560 CDCl3: 1.01 (s, 6 H), 1.45-1.56 (m, 2 H), 2.03-2.11 (m, 2 H), 2.11-2.2 (m, 2 H), 2.31 (s, 3 H), 2.78-2.87 (m, 2 H), 3.22-3.31 (m, 1 H), 3.39-3.5 (m, 1 H), 3.82 (s, 3 H), 4.5- 4.6 (m, 1 H), 6.13 (dd, 1 H), 6.21 (d, 1 H), 7.16 (s, 1 H), 7.18-7.24 (m, 1 H), 7.5-7.57 (m, 1 H), 7.82 (d, 1 H), 7.97 (dd, 1 H), 8.16 (s, 1 H), 8.08 (s, 1 H), 8.46 (d, 1 H), 8.92 (s, 1 H) - The following 2-[5-chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzene-sulfonamides are prepared from 2-(5-chloro-2-chloro-pyrimidin-4-ylamino)-N-methyl-benzenesulfonamide and the corresponding aniline following the procedure of Example A
-
Rf (solvent) ExplNo. Rx or MS NMR (400 MHz), δ (ppm) 20-1 0.50 (AcOEt) CDCl3: 2.63(d, 3H), 3.14(t, 4H), 3.87-3.90(m, 7H), 4.64(m,1H), 6.45(dd, 1H), 6.55(d, 1H), 7.23-7.26(m, 1H), 7.51-7.55(m, 1H), 7.91(d, 1H), 7.95(dd, 1H), 8.06(s, 1H), 8.47(d, 1H), 9.26(s, 1H) 20-2 m/z 546, 548 (M + 1) DMSO-d6: 2.06 (s, 3H), 2.43 (s, 3H), 3.10 (m, 2H), 3.16 (m, 2H), 3.59-3.62 (m, 4H), 3.77 (s, 3H), 6.49 (dd, 1H), 6.68 (d, 1H), 7.21-7.25 (m, 1H), 7.42 (d, 1H), 7.49 (dd, 1H), 7.75-7.77 (m, 1H), 7.78(s, 1H), 8.16(s, 1H), 8.21 (s, 1H), 8.50(d, 1H), 9.35 (s, 1H) 20-3 0.27 (n-hexane: AcOEt = 3:1) CDCl3: 2.65(d, 3H), 4.45-4.49(m, 1H), 6.99-7.04(m, 1H), 7.17-7.28(m, 4H), 7.56-7.60(m, 1H), 7.96- 7.98(m, 1H), 8.18(s, 1H), 8.31-8.34(m, 1H), 8.41- 8.44(m, 1H), 9.14(s, 1H) 20-4 0.27 (n-hexane: AcOEt = 3:1) CDCl3: 2.65(d, 3H), 4.54-4.58(m, 1H), 6.53(dd, 1H), 6.98-7.02(m, 1H), 7.11 -7.15(m, 2H), 7.24-7.28(m, 1H), 7.35(bs, 1H), 7.57-7.61(m, 1H), 7.95-7.98(m, 1H), 8.16(s, 1H), 8.29-8.32(m, 1H), 8.42-8.46(m, 1H), 9.14(s, 1H) 20-5 0.46 (MeOH: CH2Cl2 = 1:4) CDCl3: 1.95-2.00(m, 5H), 2.29-2.37(m, 1H), 2.62(d, 3H), 3.20-3.78(m, 4H), 3.86(s, 3H), 4.60-4.64(m, 2H), 5.68-5.69(m, 1H), 6.09-6.16(m, 2H), 7.15(bs, 1H), 7.19-7.23(m, 1H), 7.54-7.58(m, 1H), 7.88-7.95(m, 2H), 8.06(s, 1H), 8.55-8.57(m, 1H), 9.08(s, 1H) 20-6 518 [M + 1]+ DMSO-d6: 2.23(s, 3H), 2.43(s, 3H), 2.45-2.50(m, 4H), 3.12-3.17(m, 4H), 3.76(s, 3H), 6.45(dd, 1H), 6.63(d, 1H), 7.22(dd, 1H), 7.37(d, 1H), 7.45-7.50(m, 1H), 7.74-7.78(m, 1H), 7.76(d, 1H), 8.15(s, 1H), 8.19(s, 1H), 8.46-8.53(m, 1H), 9.35(bs, 1H) 20-7 504 [M + 1]+ DMSO-d6: 2.43(s, 3H), 2.80-2.89(m, 4H), 2.99- 3.07(m, 4H), 3.76(s, 3H), 6.44(dd, 1H), 6.61(d, 1H), 7.18-7.24(m, 1H), 7.37(d, 1H), 7.44-7.50(m, 1H), 7.76(dd, 1H), 8.15(s, 1H), 8.18(s, 1H), 8.45-8.55(m, 1H), 9.20-9.45(m, 1H) 20-8 586 [M + 1]+ DMSO-d6: 1.35-1.43(m, 2H), 1.45-1.61(m, 6H), 1.75- 1.85(m, 2H), 2.30-2.40(m, 1H), 2.43(d, 3H), 2.42- 2.55(m, 4H), 2.60-2.70(m, 2H), 3.68-3.77(m, 2H), 3.75(s, 3H), 6.45(dd, 1H), 6.62(d, 1H), 7.21(dd, 1H), 7.36(d, 1H), 7.43-7.51(m, 1H), 7.73-7.81(m, 1H), 7.75(dd, 1H), 8.15(s, 1H), 8.17(s, 1H), 8.45-8.52(m, 1H), 9.34(bs, 1H) 20-9 569 [M + 1]+ DMSO-d6: 1.85-1.95(m, 2H), 2.15(s, 3H), 2.18(t, 2H), 2.22-2.40(m, 8H), 2.43(s, 3H), 4.17(t, 2H), 6.65(d, 1H), 7.06(dd, 1H), 7.20(d, 1H), 7.22(ddd, 1H), 7.25(d, 1H), 7.39-7.47(m, 2H), 7.72-7.82(m, 1H), 7.77(dd, 1H), 8.26(s, 1H), 8.52(d, 1H), 9.22(s, 1H), 9.36(s, 1H) 20-10 556 [M + 1]+ DMSO-d6: 1.85-1.95(m, 2H), 2.19(t, 2H), 2.25- 2.35(m, 4H), 2.43(s, 3H), 3.55-3.60(m, 4H), 4.19(t, 2H), 6.66(d, 1H), 7.06(dd, 1H), 7.17-7.24(m, 1H), 7.21(d, 1H), 7.27(d, 1H), 7.39-7.45(m, 1H), 7.44(d, 1H), 7.70-7.80(m, 1H), 7.76(dd, 1H), 8.26(s, 1H), 8.52(d, 1H), 9.21(s, 1H), 9.36(s, 1H) 20-11 Rf (Hexane: AcOEt = 1:1) 0.29 DMSO-d6: 2.64 (d, 3H), 2.87-2.96 (m, 4H), 3.65-3.74 (m, 4H), 3.86 (s, 3H), 4.41-4.51 (m, 1H), 6.50 (dd, 1H), 6.81 (d, 1H), 7.55-7.64 (m, 2H), 7.96 (d, 1H), 8.01 (s, 1H), 8.19 (s, 1H), 8.49 (d, 1H), 9.07 (s, 1H). 20-12 MS 535 DMSO-d6: 2.64 (d, 3H), 3.05 (bs, 4H), 3.59 (bs, 3H), 3.87(bs, 3H), 3.89 (bs, 4H), 4.52-4.48 (m, 1H), 6.57(bs, 1H), 7.25-7.20(m, 1H), 7.44-7.32 (m, 1H), 7.63-7.52 (m, 1H), 7.94(bs, 1H), 8.06 (d, 1H), 8.25(s, 1H), 8.48(d, 1H), 9.06(bs, 1H) 20-13 MS 548 DMSO-d6: 2.17 (bs, 3H), 2.63 (d, 3H), 2.68 (bs, 4H), 3.10(bs, 4H), 3.57 (s, 3H), 4.54-4.46 (m, 1H), 6.59(bs, 1H), 7.27-7.18(m, 1H), 7.37 (bs, 1H), 7.62- 7.55 (m, 1H), 7.94(bs, 1H), 7.95 (d, 1H), 8.16(s, 1H), 8.48(d, 1H), 9.04(bs, 1H) 20-14 MS 546 DMSO-d6: 1.06 (t, 3H), 1.86 (dd, 2H), 2.37 (s, 3H), 2.62-2.59 (m, 4H), 2.64(d, 3H), 4.00-3.97 (m, 4H), 4.62-4.54 (m, 1H), 6.44 (dd, 1H), 6.54(d, 1H), 7.27- 7.22(m, 1H), 7.34(bs, 1H), 7.58-7.54(m, 1H), 7.95(dd, 1H), 8.02(d, 1H), 8.11(s, 1H), 8.53(d, 1H), 9.07(bs, 1H) 20-15 LC-MS 545 DMSO-d6: 1.46-1.62 (m, 2H), 1.72-1.79 (m, 1H), 1.82-1.90 (m, 1H), 2.38-2.46 (m, 1H), 2.43 (s, 3H), 2.62-2.76 (m, 2H), 3.59-3.69 (m, 2H), 3.43 (s, 3H), 6.47 (dd, 1H), 6.63 (d, 1H), 6.82-6.89 (br, 1H), 7.21 (dd, 1H), 7.32-7.41 (m, 2H), 7.44-7.52 (m, 1H), 7.71- 7.82 (m, 2H), 8.15 (s, 1H), 8.15-8.20 (br, 1H), 8.44- 8.53 (m, 1H), 9.28-9.38 (m, 1H) 20-16 0.24 (CH2Cl2: MeOH = 8:2) DMSO-d6: 1.47-1.55 (m, 2H), 1.80-1.91 (m, 2H), 2.16 (s, 3H), 2.25-2.41 (m, 5H), 2.42-2.48 (m, 3H), 2.61- 2.73 (m, 2H), 3.68-3.79 (m, 5H), 6.45 (dd, 1H), 6.62 (d, 1H), 7.21 (dd, 1H), 7.34 (d, 1H), 7.45-7.49 (m, 1H), 7.73-7.80 (m, 2H), 8.15 (s, 1H), 8.20 (s, 1H), 8.45-8.54 (m, 1H), 9.34 (s, 1H) 20-17 LC-MS 518 DMSO-d6: 1.76-1.84 (m, 1H), 2.08-2.16 (m, 1H), 2.33 (s, 3H), 2.42 (s, 3H), 3.00-3.03 (m, 1H), 3.23-3.27 (m, 3H), 3.42-3.46 (m, 1H), 3.74 (s, 3H), 6.06 (dd, 1H), 6.18- 6.20 (m, 1H), 7.17-7.23 (m, 1H), 7.38-7.48 (br, 1H), 7.72-7.77 (m, 1H), 8.12 (s, 1H), 8.17-8.21 (br, 1H), 8.46-8.58 (br, 1H), 9.30-9.40 (br, 1H) 20-18 LC-MS 601 DMSO-d6: 1.36-1.49 (m, 2H), 1.69-1.76 (m, 2H), 2.13 (s, 3H), 2.15-2.23 (m, 1H), 2.24-2.36 (br, 4H), 2.39- 2.48 (m, 5H), 2.43 (s, 3H), 3.27-3.40 (m, 2H), 3.74 (s, 3H), 6.62 (dd, 1H), 6.90 (d, 1H), 7.22-7.26 (m, 1H), 7.41-7.46 (m, 1H), 7.49-7.53 (m, 1H), 7.55-7.86 (br, 1H), 7.77 (dd, 1H), 8.16 (s, 1H), 8.25 (s, 1H), 8.42 (d, 1H), 9.28 (s, 1H) 20-19 LC-MS 519 DMSO-d6: 1.37-1.46 (m, 2H), 1.69-1.75 (m, 2H), 2.43 (s, 3H), 2.53-2.61 (m, 2H), 3.18-3.26 (m, 2H), 3.40- 3.74 (m, 2H), 4.62 (d, 1H), 6.62 (dd, 1H), 6.90 (d, 1H), 7.22-7.26 (m, 1H), 7.42-7.46 (br, 1H), 7.48-7.55 (m, 1H), 7.77-7.80 (m, 2H), 8.13-8.18 (br, 1H), 8.25 (s, 1H), 8.40-8.45 (m, 1H), 9.25-9.30 (m, 1H) 20-20 LC-MS 532 DMSO-d6: 1.66-1.76 (m, 1H), 2.00-2.07 (m, 1H), 2.14 (s, 6H), 2.43 (s, 3H), 2.68-2.76 (m, 1H), 2.87-2.91 (m, 1H), 2.99-3.10 (m, 2H), 3.24-3.28 (m, 1H), 3.71 (s, 3H), 6.25 (dd, 1H), 6.90 (d, 1H), 7.00-7.03 (m, 1H), 7.21-7.24 (m, 1H), 7.40-7.45 (m, 1H), 7.78-7.83 (m, 2H), 8.19 (s, 1H), 8.24 (s, 1H), 8.46 (d, 1H), 9.27-9.36 (br, 1H) 20-21 MS: 549 DMSO-d6: 2.37-2.47 (m, 4H), 2.48-2.53 (m, 3H), 2.64 (t, 2H), 3.57 (t, 3H), 3.77 (s, 3H), 3.92 (t, 2H), 6.61 (dd, 1H), 6.93 (d, 1H), 7.28 (dd, 1H), 7.56-7.63 (m, 2H), 7.75-7.85 (m, 2H), 7.74-7.84 (m, 2H), 8.14 (s, 1H), 8.29 (s, 1H) 8.46 (d, 1H), 9.33(s, 1H) 20-22 Ms: 562 DMSO-d6: 2.20 (s, 3H), 2.3-2.5 (m, 11H), 2.64 (t, 2H), 3.77 (s, 3H), 3.91 (t, 2H), 6.61(dd, 1H), 6.94 (d, 1H), 7.25-7.31(m, 1H), 7.57 (d, 1H), 7.58-7.64 (m, 1H), 7.74-7.84 (m, 2H), 8.12 (brs, 1H), 8.28 (s, 1H) 8.46 (d, 1H), 9.33(brs, 1H) 20-23 Ms: 438 DMSO-d6: 2.42-2.45 (m, 3H), 3.83 (s, 2H), 6.8 (ddd, 1H), 7.02 (dd, 1H), 7.3-7.36 (m, 1H), 7.58-7.64 (m, 1H), 7.74-7.8 (m, 1H), 7.82 (dd, 1H), 7.85 (dd, 1H), 8.18 (brs, 1H), 8.31 (s, 1H), 8.41 (d, 1H), 9.3 (brs, 1H) 20-24 Ms: 438 DMSO-d6: 2.41-2.45 (m, 3H), 3.79 (s, 2H), 6.74 (ddd, 1H), 7.0 (dd, 1H), 7.22-7.28 (m, 1H), 7.49-7.55 (m, 1H), 7.6 (dd, 1H), 7.75-7.8 (m, 2H), 8.21 (s, 1H), 8.37 (brs, 1H), 8.39-8.45 (m, 1H), 9.34 (brs, 1H) 20-25 Ms: 547 DMSO-d6: 1.24-1.38 (m, 2H), 1.64-1.8 (m, 3H), 1.83- 1.92 (m, 2H), 2.16 (s, 3H), 2.41-2.45(m, 3H), 2.76- 2.83 (m, 2H), 3.75 (s, 3H), 3.84 (d, 2H), 6.48 (dd, 1H), 6.64 (d, 1H), 7.2-7.25 (m, 1H), 7.41 (d, 1H), 7.43-7.5 (m, 1H), 7.74-7.8 (m, 2H), 8.16 (s, 1H), 8.26 (brs, 1H) 8.44-8.5 (m, 1H), 9.34 (brs, 1H) 20-26 Ms: 547 DMSO-d6: 1.18-1.3 (m, 2H), 1.56-1.7 (m, 3H), 1.8- 1.88 (m, 2H), 2.15 (s, 3H), 2.41-2.45(m, 3H), 2.73-2.8 (m, 2H), 3.75 (s, 3H), 3.65 (d, 2H), 3.77 (s, 3H), 6.57 (dd, 1H), 6.93 (d, 1H), 7.25 (dd, 1H), 7.51 -7.6 (m, 2H), 7.7-7.9 (m, 2H), 8.09 (brs, 1H), 8.28 (s, 1H), 8.45 (d, 1H), 9.31 (brs, 1H) 20-27 Ms: 533 DMSO-d6: 1.62-1.72 (m, 2H), 1.9-1.99 (m, 2H), 2.3- 2.35 (m, 5H), 2.41-2.45(m, 3H), 2.64-2.74 (m, 2H), 3.75 (s, 3H), 4.35-4.43 (m, 1H), 6.52 (dd, 1H), 6.65 (d, 1H), 7.19-7.25 (m, 1H), 7.41 (d, 1H), 7.43-7.49 (m, 1H), 7.74-7.8 (m, 2H), 8.16 (s, 1H), 8.27 (brs, 1H), 8.42-8.5 (m, 1H), 9.34 (brs, 1H) 20-28 Ms: 547 DMSO-d6: 0.96-1.2 (m, 2H), 1.75-1.9 (m, 1H), 2.2-2.3 (m, 1H), 2.35-2.45 (m, 1H), 2.41-2.45(m, 2H), 2.43(d, 3H), 2.6-3.0 (m, 3H), 3.76 (s, 3H), 4.85-5.0 (m, 1H), 6.43-6.49 (m, 1H), 6.57-6.64 (m, 1H), 7.18-7.25 (m, 1H), 7.39-7.52 (m, 2H), 7.73-7.83 (m, 2H), 8.17 (s, 1H), 8.27 (brs, 1H), 8.44-8.51 (m, 1H), 9.35 (brs, 1H) 20-29 Ms: 519 DMSO-d6: 1.74-1.83 (m, 1H), 2.23-2.31 (m, 1H), 2.28 (s, 3H), 2.35-2.4 (m, 1H), 2.41-2.45(m, 3H), 2.58-2.63 (m, 1H), 2.63-2.7 (m, 1H), 2.78-2.83 (m, 1H), 3.75 (s, 3H), 4.86-4.92 (m, 1H), 6.43 (dd, 1H), 6.58 (d, 1H), 7.19-7.25 (m, 1H), 7.41 (d, 1H), 7.44-7.51 (m, 1H), 7.73-7.83 (m, 2H), 8.16 (s, 1H), 8.26 (brs, 1H), 8.43- 8.52 (m, 1H), 9.34 (brs, 1H) 20-30 Ms: 533 DMSO-d6: 1.04 (t, 3H), 1.74-1.82 (m, 1H), 2.23-2.33 (m, 1H), 2.47-2.5(m, 6H), 2.62-2.72 (m, 2H), 2.8-2.87 (m, 1H), 3.75 (s, 3H), 4.86-4.92 (m, 1H), 6.44 (dd, 1H), 6.59 (d, 1H), 7.19-7.25 (m, 1H), 7.41 (d, 1H), 7.44-7.51 (m, 1H), 7.73-7.8 (m, 2H), 8.16 (s, 1H), 8.26 (brs, 1H), 8.44-8.51 (m, 1H), 9.34 (brs, 1H) 20-31 Ms: 518 DMSO-d6: 2.23(s, 3H), 2.38-2.47 (m, 7H), 2.87-2.93 (m, 4H), 3.75 (s, 3H), 6.63 (dd, 1H), 6.93 (d, 1H), 7.22-7.28 (m, 1H), 7.42 (d, 1H), 7.48-7.54 (m, 1H), 7.76-7.84 (m, 1H), 8.2 (s, 1H), 8.25(s, 1H), 8.43 (dd, 1H) 9.29 (s, 1H) 20-32 Ms: 586 DMSO-d6: 1.35-1.55 (m, 8H), 1.66-1.75 (m, 2H), 2.23(s, 3H), 2.41-2.45 (m, 3H), 3.74 (s, 3H), 6.63 (dd, 1H), 6.91 (d, 1H), 7.21-7.28 (m, 1H), 7.44 (d, 1H), 7.48-7.54 (m, 1H), 7.76-7.87 (m, 1H), 8.16 (s, 1H), 8.25 (s, 1H), 8.43 (dd, 1H) 9.29 (s, 1H) 20-33 Ms: 518 DMSO-d6: 1.62-1.71 (m, 1H), 1.95-2.04 (m, 1H), 2.23-2.27 (m, 3H), 2.39-2.43 (m, 3H), 2.93-3.1 (m, 2H), 3.13-3.26 (m, 2H), 3.71 (s, 3H), 6.19 (dd, 1H), 6.88 (d, 1H), 7.07-7.13 (m, 1H), 7.13-7.2 (m, 1H), 7.4- 7.48 (m, 1H), 7.75 (dd, 1H), 8.06 (brs, 1H), 8.18 (s, 1H), 8.4 (d, 1H) 20-34 Ms: 546 DMSO-d6: 2.02 (m, 1H), 2.42-2.46 (m, 3H), 2.71-2.91 (m, 4H), 3.44-3.51 (m, 4H), 3.76 (s, 3H), 6.66 (dd, 1H), 6.94 (d, 1H), 7.21-7.27 (m, 1H), 7.75-7.85 (m, 2H), 8.19 (s, 1H), 8.26 (s, 1H), 8.41 (d, 1H), 9.28 (brs, 1H). 20-35 MS (ESI) 464 (M + H) HPLC Retention time (min) 2.68 - The following 2-[5-chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino]-N-sec-butyl-benzene-sulfonamides are prepared from 2-(5-chloro-2-chloro-pyrimidin-4-ylamino)-N-sec-butyl-benzenesulfonamide and the corresponding aniline following the procedure of Example 7A
-
Rf (solvent) ExplNo. Rx or MS NMR (400 MHz), δ (ppm) 21-1 0.35 (n-hexane: AcOEt = 1:1) CDCl3: 0.62(t, 3H), 0.88(d, 3H), 1.22-1.29(m, 2H), 2.23(s,3H), 2.45-2.47(m, 4H), 3.05-3.14(m, 5H), 3.75 (s, 3H), 6.40-6.43(m, 1H), 6.62(s, 1H), 7.18-7.22(m, 1H), 7.39-7.47(m, 2H), 7.80-7.82(m, 1H), 8.15- 8.16(m, 2H), 8.44-8.46(m, 1H), 9.32(s, 1H) 21-2 0.30 (n-hexane: AcOEt = 3:1) DMSO-d6: 0.62(t, 3H), 0.87(d, 3H), 1.17-1.26(m, 2H), 3.03-3.10(m, 1H), 3.79(s, 3H), 6.66-6.71(m, 1H), 6.96-7.00(m, 1H), 7.21-7.25(m, 1H), 7.47-7.51(m, 1H), 7.60-7.64(m, 1H), 7.79-7.83(m, 2H), 8.21(s, 1H), 8.31(s, 1H), 8.35-8.37(m, 1H), 9.29(s, 1H) 21-3 0.30 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.61(t, 3H), 0.87(d, 3H), 1.21-1.29(m, 2H), 1.58-1.67(m, 2H), 1.86-1.93(m, 2H), 2.14-2.20(m, 5H), 2.59-2.67(m, 2H), 3.06-3.08(m, 1H), 3.74(s, 3H), 4.32-4.36(m, 1H), 6.46-6.48(m, 1H), 6.63(d, 1H), 7.17-7.21(m, 1H), 7.40-7.50(m, 2H), 7.79-7.81(m, 2H), 8.16(s, 1H), 8.21(bs, 1H), 8.35-8.42(m, 1H), 9.29(s, 1H) 21-4 0.30 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.61(t, 3H), 0.87(d, 3H), 1.22-1.29(m, 2H), 2.43-2.47(m, 2H), 2.61-2.63(m, 1H), 2.68-2.70(m, 2H), 3.04-3.11(m, 1H), 3.56-3.60(m, 5H), 3.75(s, 3H), 3.93-3.96(m, 1H), 4.08-4.11(m, 2H), 6.45-6.47(m, 1H), 6.64(d, 1H), 7.18-7.22(m, 1H), 7.43-7.46(m, 2H), 7.80-7.82(m, 2H), 8.17(s, 1H), 8.21(s, 1H), 8.42- 8.44(m, 1H), 9.31(s, 1H) - The following 2-[5-chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino]-N-iso-butyl-benzene-sulfonamides are prepared from 2-(5-chloro-2-chloro-pyrimidin-4-ylamino)-N-sec-butyl-benzenesulfonamide and the corresponding aniline following the procedure of Example 7A
-
Rf (solvent) ExplNo. Rx or MS NMR (400 MHz), δ (ppm) 22-1 0.30 (n-hexane: AcOEt = 3:1) DMSO-d6: 0.69(d, 6H), 1.52-1.59(m, 1H), 2.57- 2.58(m, 2H), 3.82(s, 3H), 6.75-6.80(m, 1H), 6.99- 7.02(m, 1H), 7.29-7.33(m, 1H), 7.56-7.60(m, 1H), 7.82-7.93(m, 3H), 8.14(bs, 1H), 8.31(s, 1H), 8.33(s, 1H), 9.23(s, 1 H) 22-2 0.30 (n-hexane: AcOEt = 3:1) CDCl3: 0.74(d, 6H), 1.57-1.64(m, 1 H), 2.72- 2.76(m, 2H), 3.88(s, 3H), 4.55-4.56(m, 1H), 6.52-6.57 (m, 1H), 6.62-6.65(m, 1H), 7.24-7.28(m, 2H), 7.36(bs, 1H), 7.56-7.60(m, 1H), 7.95-8.08(m, 1H), 8.10- 8.14(m, 2H), 8.36-8.39(m, 1H), 8.98(bs, 1H) 22-3 0.54 (AcOEt) DMSO-d6: 0.73(d, 6H), 1.55-1.62(m, 1H), 2.56- 2.59(m, 2H), 3.10-3.12(m, 4H), 3.74-3.76(m, 7H), 6.43-6.46(m, 1H), 6.65(d, 1H), 7.20-7.24(m, 1H), 7.40-7.48(m, 2H), 7.76-7.78(m, 1H), 7.90-7.95(m, 1H), 8.16(s, 1H), 8.17(s, 1H), 8.43-8.45(m, 1H), 9.32(s, 1H) - The following 2-[5-chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino]-N-(1-ethyl-propyl)-benzenesulfonamides are prepared from 2-(5-chloro-2-chloro-pyrimidin-4-ylamino)-N-(1-ethyl-propyl)-benzenesulfonamide and the corresponding aniline following the procedure of Example 7A
-
Rf (solvent) ExplNo. Rx or MS NMR (400 MHz), δ (ppm) 23-1 0.46 (MeOH: CH2Cl2 = 3:7) DMSO-d6: 0.58(t, 6H), 1.14-1.34(m, 4H), 1.58- 1.68(m, 2H), 1.87-1.96(m, 2H), 2.12-2.22(m, 2H), 2.18(s, 3H), 2.57-2.65(m, 2H), 2.86-2.96(m, 1H), 3.75(s, 3H), 4.30-4.39(m, 1H), 6.46(dd, 1H), 6.63(d, 1H), 7.19(dd, 1H), 7.39-7.48(m, 2H), 7.75-7.84(m, 2H), 8.18(s, 1H), 8.20 (s, 1H), 8.39(m, 1H), 9.33(bs, 1H) 23-2 0.35 (n-hexane: AcOEt = 1:1) CDCl3: 0.59(t, 6H), 1.14-1.34(m, 4H), 2.23(s, 3H), 2.45-2.47(m, 4H), 2.90-2.95(m, 1H), 3.11-3.14(m, 4H), 3.76 (s, 3H), 6.39-6.42(m, 1H), 6.62(s, 1H), 7.18- 7.22(m, 1H), 7.41-7.46(m, 2H), 7.76-7.82(m, 2H), 8.12(s, 1H), 8.16(s, 1H), 8.43-8.44(m, 1H), 9.35(s, 1H) 23-3 0.41 (MeOH: CH2Cl2 = 1:4) DMSO-d6: 0.59(t, 6H), 1.16-1.35(m, 4H), 1.75- 1.89(m, 1H), 2.08-2.15(m, 1H), 2.32(s, 3H), 2.90- 3.02(m, 2H), 3.21-3.45(m, 4H), 3.73(s, 3H), 6.02(dd, 1H), 6.18(d, 1H), 7.16(dd, 1H), 7.27(d, 1H), 7.35- 7.45(m, 1H), 7.77-7.82(m, 2H), 8.10 (s, 1H) 8.12 (s, 1H), 8.45-8.55(m, 1H), 9.38(s, 1H) 23-4 0.41 (MeOH: CH2Cl2 = 1:4) DMSO-d6: 0.60(t, 6H), 1.04(t, 3H), 1.17-1.35(m, 4H), 1.76-1.83(m, 1H), 2.10-2.15(m, 1H), 2.56-2.64(m, 2H), 2.91-3.01(m, 2H), 3.21-3.47(m, 4H), 3.74(s, 3H), 6.02(dd, 1H), 6.18(d, 1H), 7.14-7.17(m, 1H), 7.28(d, 1H), 7.35-7.45(m, 1H), 7.77-7.82(m, 2H), 8.11 (s, 1H) 8.12 (s, 1H), 8.45-8.55(m, 1H), 9.38(s, 1H) 23-5 0.25 (n-hexane: AcOEt = 1:1) DMSO-d6: 0.58(t, 6H), 1.06-2.16(m, 11H), 2.16(s, 3H), 2.62-2.67(m, 1H), 2.81-2.94(m, 2H), 3.75(s, 3H), 3.80-3.89(m, 2H), 6.41-6.44(m, 1H), 6.62(d, 1H), 7.17-7.21(m, 1H), 7.42-7.47(m, 2H), 7.77-7.82(m, 2H), 8.18(s, 1H), 8.19(s, 1H), 8.35-8.42(m, 1H), 9.35(s, 1H) 23-6 Ms: 561 DMSO-d6: 0.59 (t, 6H), 1.14-1.38 (m, 4H), 2.87-2.98 (m, 1H), 3.1 (t, 4H), 3.72-3.79 (m, 7H), 6.42 (dd, 1H), 6.64 (d, 1H), 7.18-7.24 (m, 1H), 7.42-7.5 (m, 2H), 7.77 (d, 1H), 7.81 (dd, 1H), 8.13 (s, 1H), 8.17 (s, 1H), 8.4-8.5 (m, 1H), 9.36 (s, 1H) 23-7 MS: 575 DMSO-d6: 0.58 (t, 6H), 1.13-1.37 (m, 4H), 1.72-1.82 (m, 1H), 2.21-2.31 (m, 4H), 2.32-2.4 (m, 1H), 2.54- 2.61 (m, 1H), 2.62-2.68 (m, 1H), 2.75-2.82 (m, 1H), 2.87-2.97 (m, 1H), 3.75 (s, 3H), 4.84-4.91 (m, 1H), 6.37 (dd, 1H), 6.56 (d, 1H), 7.14-7.24 (m, 1H), 7.38- 7.52 (m, 2H), 7.72-7.86 (m, 1H), 8.12-8.25 (m, 2H), 8.34-8.45 (m, 1H), 9.33 (brs, 1H) 23-8 Ms: 605 DMSO-d6: 0.58 (t, 6H), 1.14-1.36 (m, 4H), 2.43-2.53 (m, 4H), 2.69 (t, 2H), 2.89-2.95 (m, 1H), 3.59 (t, 4H), 3.76 (s, 3H), 4.09 (t, 1H), 6.45 (dd, 1H), 6.64 (d, 1H), 7.17-7.23 (m, 1H), 7.41-7.52 (m, 2H), 7.78 (d, 1H), 7.81 (dd, 1H), 8.18 (s, 1H), 8.19 (s, 1H), 8.36-8.46 (m, 1H), 9.35 (s, 1H) 23-9 Ms: 618 DMSO-d6: 0.58 (t, 6H), 1.14-1.37 (m, 4H), 2.15 (s, 1H), 2.25-2.4 (m, 4H), 2.45-2.55 (m, 4H), 2.68 (t, 2H), 2.88-2.97 (m, 1H), 3.76 (s, 3H), 4.07 (t, 1H), 6.44 (dd, 1H), 6.64 (d, 1H), 7.15-7.23 (m, 1H), 7.41-7.51 (m, 2H), 7.7-7.84 (m, 2H), 8.12-8.22 (m, 1H), 8.34-8.44 (m, 1H), 9.34 (s, 1H) - The following 2-[5-chloro-2-(subst. phenylamino)-pyrimidin-4-ylamino]-N-iso-butyl-benzene-sulfonamides are prepared from 2-(5-chloro-2-chloro-pyrimidin-4-ylamino)-N-cyclobutyl-benzenesulfonamide and the corresponding aniline following the procedure of Example 7A
-
Rf (solvent) ExplNo. Rx or MS NMR (400 MHz), δ (ppm) 24-1 0.35 (n-hexane: AcOEt = 1:1) DMSO-d6: 1.37-1.48(m, 2H), 1.69-1.91(m, 4H), 3.09- 3.12(m, 4H), 3.63-3.74(m, 1H), 3.76(s, 3H), 6.43- 6.45(m, 1H), 6.63(d, 1H), 7.18-7.22(m, 1H), 7.41- 7.47(m, 2H), 7.76-7.78(m, 1H), 8.17-8.24(m, 3H), 8.46(d, 1H), 9.33(s, 1H) 24-2 0.46 (MeOH: CH2Cl2 = 3:7) DMSO-d6: 1.37-1.93(m, 10H), 2.18(s, 3H), 2.59- 2.62(m, 1H), 3.60-3.74(m, 1H), 3.77(s, 3H), 4.32- 4.36(m, 1H), 6.46-6.49(m, 1H), 6.62(d, 1H), 7.16- 7.20(m, 1H), 7.41-7.44(m, 2H), 7.75-7.77(m, 1H), 8.16(s, 1H), 8.22(bs, 1H), 8.40-8.42(m, 1H), 9.30(bs, 1H) 24-3 0.46 (MeOH: CH2Cl2 = 1:4) CDCl3: 1.45-1.75(m, 5H), 1.94-2.06(m, 6H), 2.29- 2.37(m, 1H), 3.21-3.56(m, 4H), 3.72-3.81(m, 1H), 3.86(s, 3H), 4.55-4.65(m, 1H), 4.90(d, 1H), 5.72(d, 1H), 6.07(bs, 1H), 6.15(bs, 1H), 7.18-7.22(m, 2H), 7.52-7.56(m, 1H), 7.89-7.94(m, 2H), 8.08(s, 1H), 8.50(d, 1H), 9.00(s, 1H) -
Expl No. Rx Ms NMR (400 MHz), δ (ppm) 25-1 Ms: 559 DMSO-d6: 1.2-1.38 (m, 4H), 1.4-1.65 (m, 4H), 3.11 (t, 4H), 3.42-3.5 (m, 1H), 3.7-3.8 (m, 7H), 6.44 (dd, 1H), 6.64 (d, 1H), 7.18-7.26 (m, 1H), 7.38-7.5 (m, 2H), 7.81 (d, 1H), 7.88-7.96 (m, 1H), 8.16 (s, 1H), 8.17 (s, 1H), 8.4-8.5 (m, 1H), 9.34 (s, 1H) 25-2 Ms: 587 DMSO-d6: 1.2-1.38 (m, 4H), 1.42-1.6 (m, 6H), 1.88- 1.98 (m, 2H), 2.1-2.25 (m, 5H), 2.55-2.65 (m, 2H), 3.4-3.5 (m, 1H), 3.74 (s, 3H), 4.3-4.4 (m, 1H), 6.48 (dd, 1H), 6.63 (d, 1H), 7.18- 7.24 (m, 1H), 7.38-7.47 (m, 1H), 7.77-7.82 (m, 1H), 7.88-7.96 (m, 1H), 8.17 (s, 1H), 8.22 (s, 1H), 8.36-8.46 (m, 1H), 9.31 (s, 1H) - The following 5-Chloro-N2-(substituted phenyl)-N4-[2-(propane-1-sulfonyl)-phenyl]-pyrimidine-2,4-diamine are prepared from (2,5-Dichloro-pyrimidin-4-yl)-[2-(propane-1-sulfonyl)-phenyl]-amine and the corresponding aniline following the procedure of Example 7A
-
Rf (solvent), ExplNo. Rx MS or Mp NMR (400 MHz), δ (ppm) 26-1 0.58 (AcOEt) CDCl3: 0.97(t, 3H), 1.72-1.82(m, 2H), 3.08-3.14(m, 6H), 3.87-3.89(m, 7H), 6.46(dd, 1H), 6.53(d, 1H), 7.24- 7.28(m, 1H), 7.30(s, 1H), 7.60-7.64(m, 1H), 7.94(dd, 1H), 8.05(d, 1H), 8.15(s, 1H), 8.59(d, 1H), 9.40(s, 1H) 26-2 0.57 (MeOH: AcOEt = 1:4) CDCl3: 0.98(t, 3H), 1.85-1.68(m, 2H), 2.15(s, 3H), 3.16-3.07(m, 6H), 3.67-3.62(m, 2H), 3.81-3.78(m, 2H), 3.89(s, 3H), 6.47(d, 1H), 6.55(d, 1H), 7.36- 7.33(m, 1H), 7.62(dd, 1H), 7.95(dd, 1H), 8.08(d, 1H), 8.15(s, 1H), 8.58(d, 1H), 9.41(s, 1H) 26-3 0.13 (MeOH: AcOEt = 1:4) CDCl3: 0.97(t, 3H), 1.43-1.52(m, 2H), 1.52-1.67(m, 4H), 1.69-1.72(m, 4H), 1.90-1.98(m, 2H), 2.34- 2.46(m, 1H), 2.51-2.59(m, 4H), 2.64-2.74(m, 2H), 3.11(dd, 2H), 3.64-3.73(m, 2H), 3.87(s, 3H), 6.47(dd, 1H), 6.56(d, 1H), 7.24-7.33(m, 1H), 7.62(dd, 1H), 7.94(dd, 1H), 8.00(d, 1H), 8.14(s, 1H), 8.59(d, 1H), 9.39(bs, 1H). 26-4 0.22 (AcOEt) CDCl3: 0.97(t, 3H), 1.45(d, 1H), 1.68-1.82(m, 4H), 2.0- 2.1(m, 2H), 2.91(ddd, 2H), 3.10(ddd, 2H), 3.46-3.51 (m, 2H), 3.84-3.92(m, 1H), 3.88 (s, 1H), 6.48(dd, 1H), 6.57(d, 1H), 7.23-7.32 (m, 1 H), 7.62(dd, 1H), 7.94(dd, 1H), 8.02 (dd, 1H), 8.14(s, 1H), 8.59(d, 1H), 9.39(bs, 1H) 26-5 0.1 (AcOEt) CDCl3: 0.97(t, 3H), 1.71-1.82(m, 2H), 1.86-1.98(m, 2H), 2.01-2.08(m, 2H), 2.25-2.37(m, 1H), 2.75(ddd, 2H), 3.10(ddd, 2H), 3.63-3.66(m, 2H), 3.88(s, 3H), 5.25-5.40(m, 1H), 5.40-5.58 (m, 1H), 6.48(dd, 1H), 6.57(d, 1H), 7.22-7.34 (m, 1H), 7.62(ddd, 1H), 7.93 (d, 1H), 7.94 (dd, 1H), 8.02 (d, 1H), 8.14(s, 1H), 8.59(d, 1H), 9.40(m, 1H) 26-6 MS 587 CDCl3: 0.97 (t, 3H), 1.77 (ddd, 2H), 2.00-1.85 (m, 4H), 2.27-2.18(m, 1H), 2.72(ddd, 2H) 3.12-3.08 (m, 2H), 3.69-3.61 (m, 2H), 3.58-3.46(m, 1H), 3.64 (t, 2H), 3.80(t, 2H), 3.88(s, 3H), 5.56-5.46 (m, 1H), 6.47(dd, 1H), 6.55(d, 1H), 7.32-7.23 (m, 1H), 7.30(bs, 1H), 7.64-7.60(m, 1H), 7.94(dd, 1H), 8.02(d, 1H), 8.14(s, 1H), 8.59(d, 1H), 9.40(s, 1H) 26-7 MS 587 CDCl3: 0.98 (t, 3H), 1.46(bs, 6H) 1.82-1.73 (m, 2H), 2.17(s, 3H), 3.58-3.46(m, 1H), 2.95-2.84 (m, 2H), 3.12-3.08 (m, 2H), 3.90(s, 3H), 6.48(dd, 1H), 6.52(d, 1H), 7.30-7.22 (m, 1H), 7.31(bs, 1H), 7.66-7.60(m, 1H), 7.95(dd, 1H), 8.06(d, 1H), 8.15(s, 1H), 8.59(d, 1H), 9.43(s, 1H) 26-8 MS 573 CDCl3: 0.97 (t, 3H), 1.19(t, 3H), 1.77 (ddd, 2H), 2.41(m, 2H), 3.18-3.09(m, 6H), 3.68-3.64 (m, 2H), 3.85-3.78 (m, 2H), 3.89(s, 3H), 6.47(dd, 1H), 6.55(d, 1H), 7.29-7.25 (m, 1H), 7.34(bs, 1H), 7.64-7.60(m, 1H), 7.95(dd, 1H), 8.07(d, 1H), 8.15(s, 1H), 8.58(d, 1H), 9.41(s, 1H) 26-9 MS 587 CDCl3: 0.97 (t, 3H), 1.17(d, 3H) 1.76 (ddd, 2H), 2.88- 2.81(m, 2H), 3.18-3.05(m, 6H), 3.74-3.67 (m, 2H), 3.86-3.78 (m, 2H), 3.89(s, 3H), 6.47(dd, 1H), 6.55(d, 1H), 7.29-7.20(m, 1H), 7.34(bs, 1H), 7.64-7.60(m, 1H), 7.95(dd, 1H), 8.07(d, 1H), 8.15(s, 1H), 8.58(d, 1H), 9.41(s, 1H) 26-10 MS 517 CDCl3: 0.97 (t, 3H), 1.76 (ddd, 2H), 2.86(d, 3H), 3.14- 3.08(m, 2H), 3.13(t, 4H), 3.55(t, 4H), 3.89(s, 3H), 4.48-4.39 (m, 1H), 6.46(dd, 1H), 6.55(d, 1H), 7.29- 7.21 (m, 1H), 7.34(bs, 1H), 7.64-7.60(m, 1H), 7.95(dd, 1H), 8.06(d, 1H), 8.15(s, 1H), 8.58(d, 1H), 9.41(s, 1H) 26-11 MS 587 CDCl3: 0.98 (t, 3H), 1.51 (s, 6H), 1.82-1.72 (m, 1H), 2.13 (s, 3H), 3.12-3.08 (m, 2H), 3.26(s, 2H), 3.44 (t, 2H), 3.74(t, 2H), 3.88(s, 3H), 5.56-5.46 (m, 1H), 6.45(dd, 1H), 6.51(d, 1H), 7.00(bs, 1H), 7.62-7.58 (m, 1H), 7.64-7.60(m, 1H), 7.93(d, 1H), 7.96(dd, 1H), 8.13(s, 1H), 8.62(d, 1H), 9.42(s, 1H) 26-12 MS 559 CDCl3: 0.98(t, 3H), 1.81-1.71(m, 3H), 1.95-1.84(m, 3H), 2.68-2.63(m, 1H), 3.12-3.08(m, 4H), 3.28(d, 2H), 3.89(s, 3H), 5.45-5.38(m, 1H), 6.53(dd, 1H), 6.59(d, 1H), 6.71-6.62(m, 1H), 7.28-7.21(m, 1H), 7.35(bs, 1H), 7.65-7.61(m, 1H), 7.95(dd, 1H), 8.08(d, 1H), 8.15(s, 1H), 8.58(d, 1H), 9.41(s, 1H) 26-13 MS 559 CDCl3: 0.98 (t, 3H), 1.81-1.71 (m, 3H), 1.95-1.84 (m, 3H), 2.68-2.63(m, 1H), 3.12-3.08 (m, 4H), 3.28(d, 2H), 3.89(s, 3H), 5.45-5.38 (m, 1H), 6.53(dd, 1H), 6.59(d, 1H), 6.71-6.62 (m, 1H), 7.28-7.21 (m, 1H), 7.35(bs, 1H), 7.65-7.61(m, 1H), 7.95(dd, 1H), 8.08(d, 1H), 8.15(s, 1H), 8.58(d, 1H), 9.41(s, 1H) 26-14 MS 559 CDCl3: 0.98 (t, 3H), 1.85-1.74 (m, 3H), 2.00-1.86 (m, 3H), 2.70-2.51(m, 1H), 3.13-3.08 (m, 4H), 3.29-3.27 (m, 2H), 3.89(s, 3H), 5.46-5.37 (m, 1H), 6.53(dd, 1H), 6.59(d, 1H), 6.69-6.56 (m, 1H), 7.29-7.19 (m, 1H), 7.34(bs, 1H), 7.65-7.61(m, 1H), 7.95(dd, 1H), 8.08(d, 1H), 8.15(s, 1H), 8.58(d, 1H), 9.41(s, 1H) 26-15 MS 559 CDCl3: 0.99(t, 3H), 1.79-1.72 (m, 2H), 2.92 (t, 2H), 2.98(t, 2H), 3.16-3.12 (m, 2H), 3.53(t, 2H), 3.67(t, 2H), 3.87(s, 3H), 6.54(dd, 1H), 6.82 (d, 1H), 7.29-7.19 (m, 1H), 7.56(bs, 1H), 7.67-7.62(m, 1H), 7.96(dd, 1H), 8.07(d, 1H), 8.21(s, 1H), 8.59(dd, 1H), 9.46(s, 1H) 26-16 MS 561 CDCl3: 0.98 (t, 3H), 1.81-1.72 (m, 2H), 2.49 (t, 4H), 2.66 (t, 2H), 3.12-3.08(m, 2H), 3.18 (t, 2H), 3.74(t, 4H), 3.86(s, 3H), 6.53(dd, 1H), 6.20(dd, 1H), 6.26 (d, 1H), 7.13(bs, 1H), 7.25-7.21 (m, 1H), 7.62-7.57(m, 1H), 7.87(dd, 1H), 7.93(dd, 1H), 8.12(s, 1H), 8.62(d, 1H), 9.40(s, 1H) 26-17 MS 518 CDCl3: 0.98 (t, 3H), 1.78-1.73 (m, 2H), 2.49 (t, 4H), 2.66 (t, 2H), 2.94-2.92 (m, 4H), 3.15-3.11(m, 2H), 3.76-3.73(m, 4H), 3.88(s, 3H), 6.52(dd, 1H), 6.82(d, 1H), 7.28-7.24 (m, 1H), 7.57(bs, 1H), 7.25-7.21 (m, 1H), 7.68-7.63(m, 1H), 7.95(dd, 1H), 8.02(d, 1H), 8.20(s, 1H), 8.56(d, 1H), 9.41(s, 1H) 26-18 MS 559 CDCl3: 0.97 (t, 3H), 1.81-1.72 (m, 2H), 2.08-2.00 (m, 2H), 2.49 (t, 4H), 2.66 (t, 2H), 2.40 (t, 2H), 3.59 (t, 2H), 3.69(t, 2H), 3.87(s, 3H), 6.41 (dd, 1H), 6.51(d, 1H), 7.29-7.25 (m, 2H), 7.65-7.60(m, 1H), 7.95(dd, 1H), 8.05(d, 1H), 8.15(s, 1H), 8.56(d, 1H), 9.41(s, 1H) 26-19 MS 587 CDCl3: 0.98 (t, 3H), 1.82-1.73 (m, 2H), 2.14 (s, 3H), 3.12-3.08 (m, 2H), 3.55-3.45(m, 2H), 3.66-3.56 (m, 4H), 3.79-3.68 (m, 2H), 3.95(s, 3H), 6.95(dd, 1H), 7.03 (d, 1H), 7.32-7.28(m, 1H), 7.69-7.64 (m, 1H), 7.71(s, 1H), 7.97(dd, 1H), 8.22(s, 1H), 8.39(d, 1H), 8.52(d, 1H), 9.46(s, 1H) 26-20 MS 546 CDCl3: 0.97 (t, 3H), 1.82-1.73 (m, 2H), 3.12-3.08 (m, 2H), 3.80-3.58(m, 8H), 3.94(s, 3H), 6.94(dd, 1H), 7.02 (d, 1H), 7.32-7.28(m, 1H), 7.69-7.64 (m, 1H), 7.32- 7.28(m, 1H), 7.97(dd, 1H), 8.21 (s, 1H), 8.34(d, 1H), 8.52(d, 1H), 9.45(s, 1H) 26-21 MS 615 CDCl3: 0.97 (t, 3H), 1.82-1.72 (m, 2H), 2.71 (t, 3H), 3.05 (s, 2H), 3.10 (m, 2H), 3.18 (t, 4H), 3.88(s, 3H), 4.17-4.08 (m, 1H), 6.47 (dd, 1H), 6.54 (d, 1H), 6.99- 6.89(m, 1H), 7.28-7.24 (m, 1H), 7.31(bs, 1H), 7.65- 7.60 (m, 1H), 7.32-7.28(m, 1H), 7.95(dd, 1H), 8.05 (d, 1H), 8.15(s, 1H), 8.59(d, 1H), 9.41(s, 1H) 26-22 MS 530 CDCl3: 0.98 (t, 3H), 1.80-1.74 (m, 2H), 3.12-3.08 (m, 2H), 3.45-3.42 (m, 2H), 3.55-3.53 (m, 2H), 3.87 (s, 2H), 3.89(s,3H), 5.98-5.89 (m, 1H), 6.44 (dd, 1H), 6.50(d, 1H), 7.35-7.19 (m, 2H), 7.62-7.58(m, 1H), 7.95(dd, 1H), 8.09 (d, 1H), 8.15(s, 1H), 8.57(d, 1H), 9.43(s, 1H) 26-23 MS 558 CDCl3: 0.97 (t, 3H), 1.10 (s, 3H), 1.12 (s, 3H), 1.80- 1.74(m, 2H), 2.80-2.63 (m, 5H), 3.12-3.08 (m, 2H), 3.19-3.17 (m, 4H), 3.87(s, 3H), 6.48 (dd, 1H), 6.56(d, 1H), 7.30-7.23 (m, 2H), 7.62-7.58(m, 1H), 7.94(dd, 1H), 8.00 (d, 1H), 8.14(s, 1H), 8.59(d, 1H), 9.40(s, 1H) 26-24 MS 544 CDCl3: 0.98 (t, 3H), 1.81-1.72 (m, 2H), 2.03-1.91 (m, 1H), 2.28-2.19 (m, 1H), 2.33 (s, 6H), 2.92-2.84 (m, 1H), 3.12-3.08 (m, 2H), 3.17(t, 1H), 3.35(ddd, 1H), 3.51-3.42 (m, 2H), 3.87 (s, 3H), 6.11 (dd, 1H), 6.14 (d, 1H), 7.09 (s, 1H), 7.26-7.20(m, 1H), 7.60-7.56 (m, 1H), 7.85(d, 1H), 7.92(dd, 1H), 8.11(s, 1H), 8.38(d, 1H), 9.41(s, 1 H) 26-25 MS 530 CDCl3: 0.98 (t, 3H), 1.82-1.71 (m, 2H), 1.96-1.86 (m, 1H), 2.33-2.20 (m, 1H), 2.51 (s, 1H), 3.17-3.08 (m, 3H), 3.35-3.30 (m, 1H), 3.54-3.30 (m, 3H), 3.87 (s, 3H), 6.12 (dd, 1H), 6.16 (d, 1H), 7.09 (s, 1H), 7.32- 7.21(m, 1H), 7.58 (dd, 1H), 7.85(d, 1H), 7.92(dd, 1H), 8.11(s, 1H), 8.64(d, 1H), 9.40(s, 1H) 26-26 MS 546 CDCl3: 0.98 (t, 3H), 1.83-1.71 (m, 2H), 1.98-1.81 (m, 2H), 2.16-2.02(m, 2H), 2.53-2.28 (m, 5H), 2.87-2.72 (m, 2H), 3.12-3.08 (m, 2H), 3.88 (s, 3H), 4.32 (bs, 3H), 6.44 (dd, 1H), 6.53(d, 1H), 7.32-7.25(m, 2H), 7.63-7.59 (m, 2H), 7.94(dd, 1H), 8.04 (d, 1H), 8.15(s, 1H), 8.57(d, 1H), 9.42(s, 1H) 26-27 MS 545 CDCl3: 0.97 (t, 3H), 1.38-1.30 (m, 1H), 1.49-1.40 (m, 2H), 1.70-1.62 (m, 1H), 1.83-1.72 (m, 2H), 1.89 (d, 2H), 2.74-2.10 (m, 2H), 3.12-3.08 (m, 2H), 3.57 (d, 2H), 3.63 (d, 2H), 3.90 (s, 3H), 6.50 (d, 1H), 6.58 (s, 1H), 7.34-7.24 (m, 2H), 7.64-7.60(m, 1H), 7.94(dd, 1H), 8.02 (d, 1H), 8.14(s, 1H), 8.60(dd, 1H), 9.40(s, 1H) 26-28 MS 517 CDCl3: 0.97 (t, 3H), 1.88-1.65 (m, 3H), 2.05-1.97 (m, 2H), 2.21-2.08 (m, 1H), 2.67-2.55 (m, 4H), 2.78- 2.71(m, 2H), 3.12-3.08 (m, 2H), 3.61 (d, 2H), 3.87 (s, 3H), 6.47 (dd, 1H), 6.56 (d, 1H), 7.28-7.23 (m, 2H), 7.64-7.60(m, 1H), 7.94(dd, 1H), 7.99 (d, 1H), 8.13(s, 1H), 8.60(dd, 1H), 9.39(s, 1H) 26-29 MS 585 CDCl3: 0.97 (t, 3H), 1.89-1.65 (m, 8H), 2.03 (d, 2H), 2.20-2.10 (m, 1H), 2.68-2.58 (m, 4H), 2.78-2.72 (m, 2H), 3.12-3.08 (m, 2H), 3.61(d, 2H), 3.87(s, 3H), 6.47 (dd, 1H), 6.56(d, 1H), 7.30-7.23 (m, 2H), 7.64-7.60(m, 1H), 7.94(dd, 1H), 7.99 (dd, 1H), 8.13(s, 1H), 8.60(dd, 1H), 9.39 (s, 1H) 26-30 MS 451, 453 0.97 (t, 3H), 1.71-1.82 (m, 2H), 3.06-3.14 (m, 2H), 3.89 (s, 1H), 6.60 (ddd, 1H), 6.66 (dd, 1H), 7.25-7.30 (m, 1H), 7.35 (br.s, 1H), 7.63 (dd, 1H), 7.95 (dd, 1H), 8.09-8.18 (m, 1H), 8.17 (s, 1H), 8.52 (dd, 1H), 9.42 (s, 1H). 26-31 MS 647, 649 0.98 (t, 3H), 1.71-1.83 (m, 2H), 2.18 (s, 3H), 2.47-2.64 (m, 4H), 2.72-2.84 (m, 2H), 3.08-3.15 (m, 2H), 3.42- 3.54 (m, 2H), 3.58-3.69 (m, 2H), 3.84 (s, 3H), 3.94- 4.03 (m, 2H), 6.45-6.51 (m, 1H), 6.78 (d, 1H), 7.22- 7.27 (m, 1H), 7.60 (s, 1H), 7.67-7.74 (m, 1H), 7.93- 7.97 (m, 1H), 7.73 (d, 1H), 8.02 (s, 1H), 8.54 (d, 1H), 9.33 (s, 1H). 26-32 m.p. 139.4 400 MHz, CDCl3, δ (ppm): 0.98(t; 3H), 1.55-1.90 (m; 6H), 2.38 (s; 3H), 2.45-2.80 (m; 6H), 3.13 (m; 2H), 3.47 (m; 2H), 3.84 (s; 3H), 6.54 (dd; 1H), 6.79 (d; 1H), 7.23 (dd; 1H); 7.51 (s; 1H), 7.64 (dd: 1H), 7.92 (d; 1H), 8.00 (s; 1H), 8.19 (s; 1H), 8.57 (d; 1H), 9.41 (s; 1H). 26-33 m.p. 163.4 400 MHz, CDCl3, δ (ppm): 0.98 (t; 3H), 1.50-1.90 (m; 6H), 2.24 (bs; 1H), 2.45-2.65 (m; 6H), 3.12 (m; 2H), 3.45 (m; 2H), 3.77 (m; 4H9, 3.85(s; 3H), 6.55(dd; 1H); 6.79 (d; 1H), 7.24 (dd; 1H). 7.52 (s; 1H), 7.64 (dd; 1H), 7.93 (d; 1H), 8.01 (s; 1H), 8.20 (s; 1H9, 9.42 (s; 1H). 26-34 m.p. 232.9 400 MHz, CDCl3, δ (ppm): 1.00 (s; 3H), 1.78 (m; 2H), 2.83 (s; 3H), 3.03 (m; 2H), 3.12 (m; 2H), 3.38-3.60 (m; 8H), 3.88 (s; 3H), 6.56 (m; 1H), 6.82 (d; 1H), 7.29 (m; 1H), 7.60 (s; 1H), 7.64 (m; 1H), 7.95 (d; 1H), 8.12 (s; 1H), 8.20 (s; 1H), 8.59 (d; 1H), 9.50 (s; 1H). 26-35 m.p. 197.3 400 MHz, CDCl3, δ (ppm): 0.99 (t; 3H), 1.43 (m; 1H), 1.63 (m; 2H), 1.77 (m; 2H), 1.90 (m; 2H), 2.70 (m; 2H), 3.13 (m; 2H), 3.28 (m; 2H), 3.75 (s; 1H), 3.84 (s; 3H), 6.55 (m; 1H), 6.80 (d; 1H), 7.24 (m; 1H), 7.53 (s; 1H), 7.64 (s; 1H), 7.93 (d; 1H), 8.02 (s; 1H), 8.20 (s; 1H), 8.58 (d; 1H), 9.41 (s; 1H). 26-36 m.p. 147.6 400 MHz, CDCl3, δ (ppm): 1.00 (t; 3H), 1.78 (m; 2H), 3.12 (m; 2H), 3.56 (m; 1H), 3.87 (s; 3H), 6.53 (dd; 1H), 6.80 (d; 1H), 7.30 (dd; 1H), 7.52 (s; 1H), 7.64 (m; 1H), 7.95 (dd; 1H), 8.08 (s; 1H), 8.20 (s; 1H), 8.60 (d; 1H), 9.48(s; 1H). 26-37 m.p. 143.2 500 MHz, CDCl3, δ (ppm): 0.96 (t; 3H), 1.70 (m; 2H), 2.11 (m; 1H), 2.39 (m; 1H), 2.75 (s; 3H), 3.02 (m; 1H), 3.22 (m; 2H), 3.43 (d; 2H), 3.82 (s; 3H), 3.86 (m; 1H), 6.40 (dd; 1H), 6.94 (d; 1H), 7.34 (ddd; 1H), 7.47 (s; 1H), 7.63 (ddd; 1H), 7.93 (dd; 1H), 8.18 (s; 1H), 8.51 (d; 1H). 26-38 m.p. 133.5 400 MHz, CDCl3, δ (ppm): 1.00 (t; 3H), 1.70-1.95 (m; 6H), 2.63 (s; 1H), 2.92 (s; 1H), 3.00-3.25 (m; 5H), 3.89 (s; 3H), 5.42 (s; 1H), 6.70 (s; 1H), 6.83 (m; 2H), 7.25 (m; 1H), 7.55 (s; 1H), 7.63 (m; 1H9, 8.95 (m; 1H), 8.15 (s; 1H), 8.23 (s; 1H), 8.54 (d; 1H), 9.45 (s; 1H). 26-39 m.p. 188.8 400 MHz, CDCl3, δ (ppm): 0.99 (t; 3H), 1.70-1.90 (m; 3H), 2.08 (m; 1H), 2.28 (s; 6H9, 2.83 (s; 1H), 3.00- 3.23 (m; 4H9, 3.37 (m; 1H), 3.83 (s; 3H), 6.19 (dd; 1H), 6.83 (d; 1H), 7.23 (dd; 1H), 7.50 (s; 1H), 7.59 (m; 2H), 7.93 (d; 1H), 8.19 (s; 1H), 8.60 (d; 1H), 9.42 (s; 1H). - The following 5-Chloro-N2-(substituted phenyl)-N4-[2-ethanesulfonyl-phenyl]-pyrimidine-2,4-diamine are prepared from (2,5-Dichloro-pyrimidin-4-yl)-[2-ethanesulfonyl-phenyl]-amine and the corresponding aniline following the procedure of Example 7A
-
Expl Rf (solvent) NMR (400 MHz), δ (ppm) or No. Rx or MS Retention time min. (HPLC) 27-1 0.53 (AcOEt) CDCl3: 1.28(t, 3H), 3.12-3.19(m, 6H), 3.87-3.89(m, 7H), 6.45(dd, 1H), 6.53(d, 1H), 7.24-7.28(m, 1H), 7.31(s, 1H), 7.60-7.64(m, 1H), 7.95(dd, 1H), 8.04(d, 1H), 8.14(s, 1H), 8.58(d, 1H), 9.39(s, 1H) 27-2 585 (M + H) 2.38 27-3 486 (M + H) 3.07 27-4 587 (M + H) 2.29 27-5 545 (M + H) 2.59 27-6 545 (M + H) 2.45 27-7 531 (M + H) 2.25 27-8 545 (M + H) 2.45 27-9 600 (M + H) 2.17 HPLC condition Column: YMC CombiScreen ODS-A (5 um, 12 nm), 50 x 4.6 mm I.D. Flow rate: 2.0 ml/min Eluent: A) TFA/water (0.1/100), B) TFA/acetonitrile (0.1/100) Gradient: 5-100% B (0-5 min) Detection: UV at 215 nm - The following 5-Chloro-N2-(substituted phenyl)-N4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine are prepared from (2,5-Dichloro-pyrimidin-4-yl)-[2-(propane-2-sulfonyl)-phenyl]-amine and the corresponding aniline following the procedure of Example 7A
-
Expl Rf (solvent) NMR (400 MHz), δ (ppm) or No. Rx or MS Retention time min. (HPLC) 28-1 0.2 (AcOEt) CDCl3: 1.31 (d, 6H), 1.85-1.73 (m, 1H), 1.86-1.98 (m, 3H), 2.62-2.70 (m, 1H), 3.11-3.13 (m, 2H), 3.21-8.28 (m, 1H), 3.28 (m, 2H), 3.88 8 s, 3H), 5.41 (brs, 1H), 6.53 (d, 1H), 6.59 (d, 1H), 6.64 (brs, 1H), 7.28-7.34 (m, 1H), 7.34 (s, 1H), 7.60-7.67 (m, 1H), 7.91 (dd, 1H), 8.08 (d, 1H), 8.13 (s, 1H), 8.60 (d, 1H), 9.55 (s, 1H). 28-2 MS m/z 561, 563 (M + 1). CDCl3: 1.31 (d, 6H), 2.64 (t, 2H), 2.68-2.77 (m, 4H), 3.19 (t, 4H), 3.17-3.28 (m, 1H), 3.68 (t, 2H), 3.88 (s, 3H), 6.48 (dd, 1H), 6.55 (d, 1H), 7.23-7.32 (m, 1H), 7.62 (ddd, 1H), 7.91 (dd, 1H), 8.04 (dd, 1H), 8.12 (s, 1H), 8.60 (d, 1H), 9.54 (bs, 1H) 28-3 0.55 (AcOEt) CDCl3: 1.31 (d, 6H), 3.12-3.14 (m, 4H), 3.21-3.27 (m, 1H), 3.87-3.89 (m, 7H), 6.46 (dd, 1H), 6.53 (d, 1H), 7.23- 7.27 (m, 1H), 7.30 (s, 1H), 7.59-7.64 (m, 1H), 7.91 (dd, 1H), 8.05 (d, 1H), 8.14 (s, 1H), 8.60 (d, 1H), 9.55 (s, 1H) 28-4 0.37 (AcOEt) CDCl3: 1.32 (d, 6H), 3.21-3.27 (m, 1H), 4.00 (s, 1H), 7.11 (dd, 1H), 7.26-7.27 (m, 1H), 7.29-7.33 (m, 1H), 7.64 (s, 1H), 7.66-7.71 (m, 1H), 7.95 (dd, 1H), 8.10 (s, 1H), 8.21 (s, 1H), 8.46 (d, 1H), 8.50 (s, 1H), 8.54 (d, H), 9.59 (s, 1H) 28-5 0.03 (AcOEt) CDCl3: 1.31 (d, 6H), 1.67-1.77 (m, 2H), 1.95-2.05 (m, 2H), 2.39-2.48 (m, 1H), 2.48-2.61 (m, 2H), 2.63- 2.78 (m, 8H), 3.24 (sept, 1H), 3.71-3.63 (m, 2H), 3.87 (s, 3H), 6.47 (dd, 1H), 6.55 (d, 1H), 7.21-7.28 (m, 1H), 7.61 (ddd, 1H), 7.91 (dd, 1H), 8.00 (dd, 1H), 8.12 (s, 1H), 8.60 (d, 1H), 9.53 (bs, 1H) 28-6 502 (M + H) 2.84 28-7 478 (M + H) 4.53 28-8 MS 599 CDCl3: 1.31 (d, 6H), 1.51-1.42 (m, 2H), 1.67-1.53 (m, 4H), 1.81-1.68 (m, 2H), 1.96-1.89 (m, 2H), 2.47- 2.36 (m, 1H), 2.57-2.54 (m, 4H), 2.69 (dd, 2H) 3.24 (sept, 1H), 3.67 (d, 1H), 3.87 (s, 1H), 6.48 (dd, 1H), 6.56 (d, 1H), 7.31-7.21 (m, 1H), 7.63-7.59 (m, 1H), 8.00 (d, 1H), 8.12 (s, 1H), 8.60 (d, 1H), 9.55 (s, 1H) 28-9 MS 585 CDCl3: 1.26 (t, 3H), 1.31 (d, 6H), 1.74-1.68 (m, 2H), 1.85-1.76 (m, 4H), 2.08-1.98 (m, 2H), 2.19-2.10 (m, 2H), 2.67-2.58 (m, 4H), 2.79-2.72 (m, 2H), 3.24 (sept, 1H) 3.61 (d, 2H), 3.87 (s, 3H), 6.48 (dd, 1H), 6.56 (d, 1H), 7.29-7.22 (m, 1H), 7.62 (dd, 1H), 7.90 (dd, 1H), 7.99 (d, 1H), 8.12 (s, 1H), 8.60 (d, 1H), 9.53 (s, 1H) 28-10 MS 559 CDCl3: 1.31 (d, 6H), 1.59-1.37 (m, 2H), 1.81-1.69 (m, 1H), 1.87 (d, 2H), 2.73-2.67 (m, 2H), 3.28-3.21 (m, 1H), 3.37 (s, 3H), 3.61 (d, 1H), 3.87 (s, 3H), 6.49 (dd, 1H), 6.57 (s, 1H), 7.31-7.21 (m, 1H), 7.64-7.60 (m, 1H), 7.91 (dd, 1H), 8.00 (d, 1H), 8.60 (d, 1H) 9.53 (s, 1H) 28-11 MS 558 CDCl3: 1.31 (d, 6H), 2.15 (s, 3H), 3.12 (ddd, 4H), 3.24 (sept, 1H), 3.64 (t, 2H), 3.80 (t, 2H), 3.89 (s, 3H), 6.47 (dd, 1H), 6.55 (d, 1H), 7.29-7.24 (m, 1H), 7.33 (bs, 1H), 7.62 (m, 1H), 7.92 (dd, 1H), 8.08 (d, 1H), 8.14 (s, 1H), 8.60 (d, 1H) 9.55 (s, 1H) 28-12 MS 544 CDCl3: 1.16, (t, 3H), 1.31 (d, 6H), 2.56-2.44 (b, 2H), 2.71-2.60 (m, 4H), 3.28-3.17 (m, 5H), 3.88 (s, 3H), 6.48 (dd, 1H), 6.58 (d, 1H), 7.30-7.22 (m, 1H), 7.63- 7.58 (m, 1H), 7.90 (dd, 1H), 8.01 (d, 1H), 8.12 (s, 1H), 8.60 (d, 1H) 9.54 (s, 1H) 28-13 MS 601 CDCl3: 1.31 (d, 6H, J = 6.55), 1.75-1.63 (m, 2H), 2.00- 1.91 (m, 2H), 2.37-2.27 (m, 1H), 2.60 (t, 4H, J = 4.79), 2.74-2.59 (m, 2H), 3.24 (sept, 1H), 3.66 (d, 2H, J = 12.1), 3.75 (t, 4H, J = 4.53), 3.88 (s, 3H), 6.48 (dd, 1H, J = 2.52, 8.56), 6.56 (d, 1H, J = 2.52), 7.33-7.22 (m, 1H), 7.64-7.59 (m, 1H), 7.91 (dd, 1H, J = 8.05, 1.51), 8.01 (d, 1H, J = 8.56), 8.12 (s, 1H), 8.61 (d, 1H, J = 7.55) 9.54 (s, 1H) 28-14 MS 559 CDCl3: 1.11 (d, 6H, J = 6.55), 1.31 (d, 6H, J = 7.05), 2.82-2.68 (m, 5H), 3.20-3.17 (m, 4H), 3.28-3.17 (m, 1H), 3.87 (s, 3H), 6.48 (dd, 1H, J = 2.52, 8.56), 6.56 (d, 1H, J = 2.52), 7.33-7.24 (m, 1H), 7.62-7.58 (m, 1H), 7.90 (dd, 1H, J = ), 8.01 (d, 1H, J = 8.56), 8.12 (s, 1H), 8.60 (d, 1H, J = 8.56) 9.54 (s, 1H) 28-15 MS 559 CDCl3: 1.31 (d, 6H, J = 7.05), 1.97-1.85 (m, 2H), 2.17- 1.98 (m, 2H), 2.35-2.25 (m, 1H), 2.75 (m, 2H), 3.24 (sept, 1H), 3.65 (d, 2H), 3.88 (s, 3H), 5.30 (bs, 1H), 5.48 (bs, 1H), 6.48 (dd, 1H, J = 2.51, 8.56), 6.56 (d, 1H, J = 2.52), 7.33-7.21 (m, 1H), 7.62 (m, 1H), 7.91 (dd, 1H, J = 1.51, 8.06), 8.03 (dd, 1H, J = 3.02, 8.56), 8.13 (s, 1H), 8.60 (d, 1H, J = 8.57), 9.54 (s, 1H) 28-16 MS 532 CDCl3: 1.31 (d, 6H, J = 7.06), 1.46-1.43 (m, 1H), 1.79- 1.68 (m, 2H), 2.08-1.99 (m, 2H), 2.99-2.88 (m, 2H), 3.24 (sept, 1H), 3.51-3.45 (m, 2H), 3.91-3.80 (m, 1H), 3.88 (s, 3H), 6.49 (dd, 1H, J = 2.52, 8.56), 6.57 (d, 1H, J = 2.52), 7.34-7.23 (m, 1H), 7.64-7.60 (m, 1H), 7.91 (dd, 1H, J = 1.51, 8.06), 8.02 (dd, 1H, J = 3.02, 9.06), 8.13 (s, 1H), 8.60 (d, 1H, J = 8.06) 9.53 (s, 1H) 28-17 MS 532 CDCl3: 1.31 (d, 6H, J = 6.96), 2.18-2.12 (m, 2H), 3.24 (sept, 1H), 3.37-3.32 (m, 2H), 3.39 (s, 3H), 3.43 (d, 1H, J = 8.56), 3.51 (dd, 1H, J = 5.04, 10.6), 3.87 (s, 3H), 4.17-4.09 (m, 1H) 6.13 (dd, 1H, J = 2.51, 8.56), 6.16 (d, 1H, J = 2.52), 7.09 (bs, 1H), 7.31-7.21 (m, 1H), 7.60-7.56 (m, 1H), 7.85 (d, 1H, J = 8.56), 7.89 (dd, 1H, J = 1.51, 8.06), 8.10 (s, 1H), 8.65 (d, 1H, J = 9.06) 9.54 (s, 1H) 28-18 MS 546 CDCl3: 1.31 (d, 6H, J = 7.05), 1.82-1.70 (m, 2H), 2.08- 1.99 (m, 2H), 2.96-2.87 (m, 2H), 3.24 (sept, 1H), 3.41- 3.33 (m, 1H), 3.40 (s, 3H), 3.51-3.42 (m, 2H), 3.87 (s, 3H), 6.49 (dd, 1H, J = 2.52, 9.07), 6.57 (d, 1H, J = 2.52), 7.32-7.22 (m, 1H), 7.64-7.60 (m, 1H), 7.91 (dd, 1H,), 8.00 (dd, 1H, J = 3.02, 9.06), 8.12 (s, 1H), 8.60 (d, 1H, J = 8.56) 9.53 (s, 1H) 28-19 0.33 (AcOEt) CDCl3: 1.31 (d, 6H, J = 7.05), 1.82-1.70 (m, 2H), 2.08- 1.99 (m, 2H), 2.96-2.87 (m, 2H), 3.24 (sept, 1H), 3.41- 3.33 (m, 1H), 3.40 (s, 3H), 3.51-3.42 (m, 2H), 3.87 (s, 3H), 6.49 (dd, 1H, J = 2.52, 9.07), 6.57 (d, 1H, J = 2.52), 7.32-7.22 (m, 1H), 7.62 (m, 1H), 7.91 (dd, 1H,), 8.00 (dd, 1H, J = 3.02, 9.06), 8.12 (s, 1H), 8.60 (d, 1H, J = 8.56) 9.53 (s, 1H) 28-20 MS 544 CDCl3: 1.31 (d, 6H), 1.66-1.53 (m, 2H), 2.10-2.01 (m, 2H), 2.51 (s, 3H), 2.70-2.13 (m, 1H), 2.83-2.74 (m, 2H), 3.24 (Sept, 1H), 3.63-3.55 (m, 2H), 3.87 (s, 3H), 4.34- 4.25 (m, 1H), 6.48 (dd, 1H), 6.56 (d, 1H), 7.34-7.24 (m, 1H), 7.64-7.60 (m, 1H), 7.90 (dd, 1H), 8.00 (d, 1H), 8.12 (s, 1H), 8.60 (dd, 1H), 9.53 (s, 1H) 28-21 MS 531 CDCl3: 1.30 (s, 3H), 1.32 (s, 3H), 2.33-2.22 (m, 1H), 2.54 (s, 3H), 3.37-3.20 (m, 3H), 3.57-3.44 (m, 3H), 3.86 (s, 3H), 6.12 (dd, 1H), 6.16 (d, 1H), 7.14-7.08 (m, 1H), 7.30-7.20 (m, 1H), 7.65-7.58 (m, 1H), 7.93-7.87 (m, 1H,), 8.10 (s, 1H), 8.64 (d, 1H) 9.54 (s, 1H) 28-22 MS 545 CDCl3: 1.30 (s, 3H), 1.32 (s, 3H), 2.03-1.89 (m, 1H), 2.30-2.18 (m, 1H), 2.34 (s, 6H), 2.96-2.83 (m, 1H), 3.29-3.16 (m, 2H), 3.40-3.34 (m, 1H), 3.53-3.43 (m, 2H), 3.87 (s, 3H), 6.11 (dd, 1H,) 6.13 (dd, 1H), 7.08 (bs, 1H), 7.31-7.21 (m, 1H), 7.60-7.56 (m, 1H), 7.85 (d, 1H), 7.89 (dd, 1H), 8.10 (s, 1H), 8.66 (d, 1H) 9.54 (s, 1H) 28-23 MS 545 CDCl3: 1.31 (s, 3H), 1.32 (s, 3H), 3.05 (s, 3H), 3.24 (sept, 1H), 3.50-3.43 (m, 4H), 3.85 (s, 2H), 3.89 (s, 3H), 6.11 (dd, 1H,) 6.43 (dd, 1H), 6.50 (d, 1H), 7.31- 7.28 (m, 1H), 7.64-7.60 (m, 1H), 7.92 (dd, 1H), 8.09 (d, 1H), 8.13 (s, 1H), 8.58 (d, 1H) 9.55 (s, 1H) 28-24 0.05 (AcOEt/ MeOH = 4/1) CDCl3: 1.30 (s, 3H), 1.32 (s, 3H), 1.92-1.83 (m, 1H), 2.17-1.95 (m, 1H), 2.43-2.27 (m, 2H), 2.79-2.71 (m, 4H), 3.15-2.97 (m, 4H), 3.23-3.16 (m, 4H), 3.24 (sept, 1H), 3.87 (s, 3H), 6.11 (dd, 1H,) 6.47 (dd, 1H), 6.55 (d, 1H), 7.33-7.23 (m, 1H), 7.63-7.59 (m, 1H), 7.95 (dd, 1H), 8.01 (dd, 1H), 8.12 (s, 1H), 8.60 (d, 1H) 9.54 (s, 1H) 28-25 MS 600 CDCl3: 1.30 (s, 3H), 1.32 (s, 3H), 1.80-1.70 (m, 2H), 2.01-1.93 (m, 2H), 2.49-2.28 (m, 12H), 2.76- 2.62 (m, 4H), 3.04-2.96 (m, 4H), 3.16-3.05 (m, 2H), 3.24 (sept, 1H), 3.72-3.63 (m, 2H), 3.87 (s, 3H), 6.48 (dd, 1H), 6.55 (d, 1H), 7.31-7.23 (m, 1H), 7.66- 7.589 (m, 1H), 7.91 (dd, 1H), 8.01 (d, 1H), 8.12 (s, 1H), 8.60 (d, 1H) 9.53 (s, 1H) 28-26 MS 573 CDCl3: 1.30 (s, 3H), 1.32 (s, 3H), 2.59-2.43 (m, 4H), 2.78-2.73 (m, 1H), 3.00-2.86 (m, 2H), 3.38-3.20 (m, 3H), 3.54-2.45 (m, 1H), 3.73 (dd, 1H), 3.84-3.77 (m, 1H), 3.94-3.87 (m, 1H), 3.88 (s, 3H), 6.46 (dd, 1H), 6.53 (d, 1H), 7.32-7.23 (m, 1H), 7.31 (bs, 1H), 7.63-7.52 (m, 1H), 7.91 (dd, 1H), 8.04 (d, 1H), 8.13 (s, 1H), 8.60 (d, 1H) 9.54 (s, 1H) 28-27 MS 559 CDCl3: 1.30 (s, 3H), 1.32 (s, 3H), 1.82-1.73 (m, 1H), 1.97-1.84 (m, 3H), 2.73-2.51 (m, 1H), 3.12 (t, 2H), 3.31-3.20 (m, 3H), 3.90 (s, 3H), 5.46-5.37 (m, 1H), 6.53 (dd, 1H), 6.59 (d, 1H), 6.68-6.62 (m, 1H), 7.28- 7.21 (m, 1H), 7.33 (bs, 1H), 7.65-7.61 (m, 1H), 7.92 (dd, 1H), 8.08 (d, 1H), 8.14 (s, 1H), 8.60 (d, 1H), 9.55 (s, 1H) 28-28 MS 559 CDCl3: 1.30 (s, 3H), 1.32 (s, 3H), 1.82-1.73 (m, 1H), 1.97-1.84 (m, 3H), 2.73-2.51 (m, 1H), 3.12 (t, 2H), 3.31-3.20 (m, 3H), 3.90 (s, 3H), 5.46-5.37 (m, 1H), 6.53 (dd, 1H), 6.59 (d, 1H), 6.68-6.62 (m, 1H), 7.28- 7.21 (m, 1H), 7.33 (bs, 1H), 7.65-7.61 (m, 1H), 7.92 (dd, 1H), 8.08 (d, 1H), 8.14 (s, 1H), 8.60 (d, 1H), 9.55 (s, 1H) 28-29 MS 413 CDCl3: 1.31 (s, 3H), 1.33 (s, 3H), 2.92 (t, 4H), 3.28 (sept, 1H) 3.73 (t, 4H), 3.87 (s, 3H), 6.51 (dd, 1H), 6.82 (d, 1H), 7.32-7.23 (m, 1H), 7.57 (bs, 1H), 7.70- 7.64 (m, 1H), 7.92 (dd, 1H), 8.01 (bs, 1H), 8.12 (s, 1H), 8.60 (d, 1H), 9.53 (s, 1H) 28-30 MS 493 CDCl3: 1.30 (s, 3H), 1.33 (s, 3H), 3.25 (sept, 1H) 3.60 (bs, 3H), 3.89 (s, 3H), 6.59 (s, 1H), 7.27-7.18 (m, 1H), 7.61 (dd, 1H), 7.83 (bs, 1H), 7.90 (dd, 1H), 8.15 (s, 1H), 8.55 (d, 1H), 9.55 (s, 1H) 28-31 MS 445 CDCl3: 1.31 (d, 6H), 1.59-1.37 (m, 2H), 1.81-1.69 (m, 1H), 1.87 (d, 2H), 2.73-2.67 (m, 2H), 3.28-3.21 (m, 1H), 3.37 (s, 3H), 3.61 (d, 1H), 3.87 (s, 3H), 6.49 (dd, 1H), 7.025 (bs, 1H), 7.28-7.23 (m, 1H), 7.64-7.59 (m, 1H), 7.93-7.89 (m, 2H), 8.15 (s, 1H), 8.57 (dd, 1H) 9.56 (s, 1H) -
Expl No. Rx MS NMR (400 MHz) in CDCl3, δ (ppm) 32-1 585.3 1.03 (s, 3H), 1.04 (s, 3H), 2.15 (s, 3H), 2.32 (sept, 1H) 3.00 (d, 2H) 3.10 (t, 2H), 3.13 (t, 2H), 3.64 (t, 2H), 3.79 (t, 2H), 3.89 (s, 3H), 6.45 (dd, 1H), 6.55 (d, 1H), 7.34-7.26 (m, 1H), 7.52 (bs, 1H), 7.64-7.60 (m, 1H), 7.97 (dd, 1H), 8.07 (d, 1H), 8.15 (s, 1H), 8.54 (d, 1H), 9.32 (s, 1H) 32-2 532 (M + H) 3.17 -
Expl No. Rx MS NMR (400 MHz) in CDCl3, δ (ppm) 33-1 585.3 1.66-1.52 (m, 2H), 1.92-1.73 (m, 4H), 2.12-2.03 (m, 2H), 2.15 (s, 3H), 3.00 (d, 2H) 3.11 (t, 2H), 3.14 (t, 2H), 3.58-3.46 (m, 1H), 3.64 (t, 2H), 3.80 (t, 2H), 3.89 (s, 3H), 6.48 (dd, 1H), 6.55 (d, 1H), 7.30-7.24 (m, 1H), 7.52 (bs, 1H), 7.63-7.58 (m, 1H), 7.94 (dd, 1H), 8.08 (d, 1H), 8.14 (s, 1H), 8.60 (d, 1H), 9.54 (s, 1H) 33-2 544 (M + H) 3.15 -
HPLC Expl Retention Mass (ESI) No. Rx time (min) m/z 34-1 3.15 532 (M + H) 34-2 3.34 558 (M + H) 34-3 3.35 546 (M + H) 34-4 3.32 546 (M + H) 34-5 3.09 566 (M + H) 34-6 2.87 552 (M + H) Ex No MS NMR (400 MHz), CDCl3, δ ppm 34-7 MS 435, 436 1.05 (t, 3H), 1.69-1.78 (m, 2H), 2.86-2.95 (m, 1H), 3.16-3.25 (m, 1H), 6.57-6.68 (m, 2H), 7.17 (dd, 1H), 7.35-7.39 (m, 1H), 7.50 (dd, 1H), 8.13 (s, 1H), 8.16-8.21 (m, 1H), 8.48 (d, 1H), 10.14 (s, 1H) 34-8 MS 549, 551 0.94 (t, 3H), 1.69-1.80 (m, 2H), 2.38 (s, 3H), 2.55-2.64 (m, 4H), 3.02-3.08 (m, 2H), 3.22-3.29 (m, 4H), 3.88 (s, 3H), 6.55 (ddd, 1H), 6.60-6.66 (m, 1H), 7.13-7.18 (m, 1H), 7.34 (br.s, 1H), 7.44 (d, 1H), 8.10 (s, 1H), 8.10-8.23 (m, 2H), 8.88 (s, 1H). -
35-1 567 [M + 1]+ DMSO-d6: 2.24 (s, 3H), 2.45-2.50 (m, 4H), 2.78 (d, 3H), 3.10-3.17 (m, 8H), 3.74-3.79 (m, 7H), 6.49 (dd, 1H), 6.66 (d, 1H), 6.85-6.89 (m, 1H), 7.18 (d, 1H), 7.40 (d, 1H), 7.98-8.02 (m, 2H), 8.29 (br.d, 1H), 8.60-8.66 (m, 1H), 11.17 (s, 1H). 35-2 505 [M + 1]+ DMSO-d6: 2.24 (s, 3H), 2.46-2.50 (m, 4H), 2.79 (d, 3H), 3.13-3.17 (m, 4H), 3.78 (s, 3H), 6.69 (d, 1H), 6.87 (dd, 1H), 7.07-7.17 (m, 2H), 7.19-7.23 (m, 2H), 7.54 (d, 1H), 8.13 (s, 1H), 8.45 (s, 1H), 8.65-8.75 (m, 1H), 9.04 (s, 1H), 11.19 (s, 1H) -
- To a suspension of 16.3 g (100 mmol) of isatoic anhydride in 100 mL of H2O is added portionwise 100 mL of 2N methylamine-tetrahydrofuran solution (200 mmol) at room temperature. The reaction mixture is stirred for 1 hour and then extracted with AcOEt. The organic layer is washed with H2O and brine, dried over Na2SO4, and concentrated under reduced pressure to give 13.79 g of desired product, 2-amino-N-methyl-benzamide (92 mmol, 92%) as colorless solid.
- NMR (400 MHz, CDCl3, δ): 2.97 (d, 3H, J=4.52 Hz), 5.49 (bs, 1H), 6.07 (bs, 1H), 6.64 (ddd, 1H, J=8.04, 7.56, 1.0 Hz), 6.68 (dd, 1H, J=8.32, 1.0 Hz), 7.20 (ddd, 1H, J=8.32, 7.56, 1.52 Hz), 7.29 (dd, 1H, J=8.04, 1.52 Hz).
-
- To a solution of 15.0 g (99.8 mmol) of 2-amino-N-methyl-benzamide in DMF (300 mL) are added 2,4,5-trichloropyrimidine (23.8 g, 130 mmol) and potassium carbonate (17.9 g, 130 mmol). The reaction mixture is stirred at 75° C. for 5 hours, cooled to room temperature, and then poured into H2O (600 mL). The resulting precipitate is collected by a filtration followed by washing with 50% aqueous CH3CN (200 mL) and dried under reduced pressure (40° C., 10 hours) to give desired 2-(2,5-dichloro-pyrimidin-4-yl-amino)-N-methyl-benzamide as ivory solid (26.4 g, 88.9 mmol, 89%).
- NMR (400 MHz, DMSO-d6, δ): 2.81 (d, 3H, J=4.52 Hz), 7.22 (dd, 1H, J=8.56, 8.04 Hz), 7.60 (ddd, 1H, J=8.56, 8.56, 1.0 Hz), 7.81 (dd, 1H, J=8.04, 1.0 Hz), 8.48 (s, 1H), 8.52 (d, 1H, J=8.56 Hz) 8.80-8.90 (m, 1H), 12.18 (s, 1H).
- According the manner described above, the following compounds are prepared.
-
- NMR (400 MHz, DMSO-d6, δ): 2.81 (d, 3H), 7.23 (ddd, 1H, J=7.54, 7.54, 1.0 Hz), 7.59 (ddd, 1H, J=7.93, 8.06, 1.52 Hz), 7.79 (dd, 1H, J=7.8, 1.52 Hz), 8.47 (dd, 1H J=8.06, 1.0 Hz), 8.55 (s, 1H), 8.81-8.87 (m, 1H), 12.0 (brs, 1H). Rf: 0.46 (n-Hexane:AcOEt=7:3).
-
- NMR (400 MHz, CDCl3, δ): 1.28 (t, d=7.04, 3H), 3.48-3.57 (m, 2H), 6.22 (br. s, 1H), 7.11-7.17 (m, 1H), 7.51 (dd, J=1.0, 8.04, 1H), 7.53-7.61 (m, 1H), 8.22 (s, 1H), 8.69-8.74 (m, 1H), 11.66 (br. s, 1H). Rf: 0.60 (Hexane:AcOEt=1:1).
-
- A suspension of 5-bromo-2,4-dichloropyrimidine (684 mg, 3.0 mmol) and 2-amino-N-methyl-benzenesulfonamide (559 mg, 3.0 mmol) in N,N-dimethylformamide (10 mL) containing potassium carbonate (830 mg, 6.0 mmol) is stirred at room temperature for 23 hours. Saturated aqueous ammonium chloride is added and the mixture is poured into water and extracted twice with ethyl acetate. The organic layer is washed with brine, dried over sodium sulfate, and evaporated in vacuo. The residue is purified by silica gel column chromatography (n-hexane-ethyl acetate gradient) to afford the title compound as a slightly yellow solid.
- 1H-NMR (CDCl3), δ (ppm): 2.67 (d, 3H), 4.79 (q, 1H), 7.26 (s, 1H), 7.29 (ddd, 1H), 7.66 (ddd, 1H), 7.95 (dd, 1H), 8.37 (s, 1H), 8.48 (d, 1H), 9.52 (s, 1H). Rf (n-hexane:ethyl acetate=10:3): 0.33.
- According to the manner described above, the following compound is prepared.
-
- 1H-NMR (400 MHz, CDCl3, δ); 2.67 (d, 3H), 4.97-5.04 (m, 1H), 7.29 (ddd, 1H, J=7.54, 7.54, 1.0 Hz), 7.66 (ddd, 1H, J=7.93, 8.08, 1.48 Hz), 7.94 (dd, 1H, J=8.04, 1.52 Hz), 8.24 (s, 1H), 8.51 (dd, 1H J=8.06, 1.0 Hz), 9.64 (brs, 1H). Rf: 0.45 (n-Hexane:AcOEt=4:1).
-
- To a solution of 2-amino-N-isopropyl-benzenesulfonamide (16.1 g, 75.1 mmol) in DMI (150 mL) is added sodium hydride (6.6 g, 165.3 mmol) portionwise at 0° C. After the mixture is stirred at room temperature for one hour, 2,4,5-trichloropyrimidine (20.7 g, 112.7 mmol) is added at 0° C. After further stirring at room temperature for 5 hrs, water is added and the mixture is extracted with AcOEt three times. Organic layer is washed with brine, dried over sodium sulfate and evaporated under reduced pressure. The residue is purified by silica gel column chromatography (Hexane to Hexane:AcOEt=4:1) to afford the title compound as pale brown solid (10.2 g, 38%).
- 1H-NMR (400 MHz, CDCl3, δ); 1.06 (d, 6H), 3.43-3.53 (m, 1H), 4.38 (d, 1H), 7.29 (dd, 1H), 7.66 (dd, 1H), 7.98 (d, 1H), 8.29 (s, 1H), 8.51 (d, 1H), 9.51 (brs, 1H). Rf: 0.45 (n-Hexane:AcOEt=4:1)
- The following compounds are prepared in the same manner described above.
-
Expl Rf (solvent) No. Rz or MS NMR (400 MHz), δ (ppm) 36-8 0.45 (n-Hexane: AcOEt = 4:1) DMSO-d6; 0.63 (t, 6H), 0.86 (d, 3H), 1.21-1.31 (m, 2H), 3.02-3.12 (m, 1H), 7.37 (dd, 1H), 7.71 (d, 1H), 7.85 (d, 1H), 7.89 (d, 1H), 8.20 (d, 1H), 8.56 (s, 1H), 9.51 (brs, 1H) 36-9 0.46 (n-Hexane: AcOEt = 7:3) CDCl3; 0.70 (t, 6H), 1.23-1.45 (m, 4H), 3.03-3.13 (m, 1H), 4.27 (d, 1H), 7.27 (dd, 1H), 7.65 (dd, 1H), 7.98 (d, 1H), 8.29 (s, 1H), 8.52 (d, 1H), 9.59 (brs, 1H) -
- 2,4-Dichloro-5-nitro-pyrimidine (1.94 g, 10 mmol) and 2-amino-N-methyl-benzenesulfonamide (1.86 g, 10 mmol) are dissolved in CHCl3 (30 mL). The reaction mixture is heated at 61° C. for 2 h. The solvent is evaporated and the residue is washed with ether to give the title product.
- Rf=0.5 (n-hexane:ethyl acetate=1:1). 1H-NMR (400 MHz, CDCl3), δ (ppm): 2.67 (d, 3H), 4.6-4.7 (m, 2H), 7.41 (t, 1H), 7.7 (t, 1H), 8.04 (d, 1H), 8.15 (d, 1H), 9.21 (s, 1H), 11.2 (s, 1H).
-
- To a solution of 2-(Propane-1-sulfonyl)-phenylamine (3.69 g, 18.5 mmol) of N,N-dimethylformamide (40 mL), sodium hydride (1.48 g, 37 mmol) is added portionwise at 0° C. After stirring, 2,4,5-trichloropyrimidine (2.1 mL, 18.5 mmol) is added. The mixture is stirred at 0° C. for 30 minutes and is further stirred at room temperature for 7 hrs. After adding saturated aqueous ammonium chloride, the mixture is poured into water and extracted twice with ethyl acetate. The organic layer is washed with brine, dried over sodium sulfate, and evaporated in vacuo. The residue is purified by silica gel column chromatography (n-hexane-ethyl acetate gradient) to afford the title compound as colorless solids.
- 1H-NMR (CDCl3), δ (ppm): 0.99 (t, 3H), 1.77 (d, 2H), 3.07-3.11 (m, 2H), 7.26 (s, 1H), 7.32 (ddd, 1H), 7.73 (ddd, 1H), 7.95 (dd, 1H), 8.31 (s, 1H), 8.61 (dd, 1H), 9.94 (bs, 1H). Rf (n-hexane:ethyl acetate=3:1): 0.63
- According to the manner described above, the following compounds are prepared.
-
Expl No. Rx Identification 36-12 1H-NMR (CDCl3), δ (ppm): 1.35 (d, 6H), 3.18-3.24 (m, 1H), 7.30-7.34 (m, 1H), 7.70-7.75 (m, 1H), 7.92 (dd, 1H), 8.30 (s, 1H), 8.63 (d, 1H), 10.06 (s, 1H). Rf 0.70: (AcOEt) 36-13 NMR (400 MHz) in CDCl3, δ (ppm): 1.29 (t, 3H), 3.15 (q, 1H), 7.31-7.35 (m, 1H), 7.71-7.75 (m, 1H), 7.96 (dd, 1H), 8.31 (s, 1H), 8.60 (d, 1H), 9.92 (s, 1H). Rf: 0.67 (AcOEt). 36-14 1.01-1.06 (m, 2H), 1.32-1.37 (m, 2H), 2.49-2.55 (m, 1H), 7.29-7.33 (m, 1H), 7.69-7.73 (m, 1H), 7.91 (dd, 1H), 8.31 (s, 1H), 8.58 (d, 1H), 9.90 (s, 1H). Rf 0.69 (AcOEt) 36-15 0.99 (t, 6H), 1.72-1.90 (m, 4H), 2.76-2.82 (m, 1H), 7.26-7.34 (m, 1H), 7.69-7.74 (m, 1H), 7.92 (dd, 1H), 8.30 (s, 1H), 8.62 (d, 1H), 10.02 (s, 1H). Rf: 0.73 (AcOEt) - To a solution of 4-methoxyphenyl-boronic acid (500 mg, 3.29 mmol) in toluene (5.2 mL) and ethanol (1.3 mL), potassium carbonate (910 mg, 6.58 mmol), tetrakis(triphenylphosphine)-palladium (228.1 mg, 0.099 mmol) and 4-bromo-1-methyl-2-nitrobenzene (711 mg, 3.29 mmol) are added and stirred at 100° C. for 7 hours. The mixture is poured into water and extracted with ethyl acetate two times. The organic layer is washed with water and then brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1) to afford the 4′-methoxy-4-methyl-3-nitro-biphenyl as a yellow solid.
- 1H-NMR (δ, ppm): 2.62 (s, 3H), 3.86 (s, 3H), 7.02-6.98 (m, 2H), 7.37 (d, 1H), 7.54 (dd, 2H), 7.68 (dd, 1H), 8.18 (d, 1H). Rf (hexane:ethyl acetate=3:1): 0.40.
- A suspension of 4′-methoxy-4-methyl-3-nitrobiphenyl (630 mg, 2.95 mmol) and 10% palladium on charcoal (63 mg, 0.059 mmol) in methanol (6 mL) is stirred under hydrogen atmosphere for 12 hours. Palladium catalyst is removed by filtration and the resulting solution is evaporated in vacuo to afford the title compound.
- 1H-NMR (δ, ppm): 2.20 (s, 3H), 3.84 (s, 3H), 6.87 (d, 1H), 6.89 (dd, 1H), 6.95 (d, 2H), 7.09 (d, 1H), 7.48 (d, 2H). Rf (n-hexane:ethyl acetate=1:1): 0.50.
- To a solution of 4-methyl-3-nitro-benzoic acid (300 mg, 2.76 mmol), N-butoxycarbonyl-piperazine (340 mg, 1.83 mmol) in DMF (3.0 mL), triethylamine (300 μL, 3.59 mmol), TBTU (800 mg, 2.49 mmol) and HOAt (270.5 mg, 1.99 mmol) are added and stirred at room temperature for 24 hours. The mixture is poured into water and extracted twice with ethyl acetate. The organic layer is washed with water and then brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1) to afford 4-(4-methyl-3-nitrobenzoyl)-piperazine-1-carboxylic acid tert-butyl ester as a colorless solid.
- 1H-NMR (δ, ppm): 1.47 (s, 9H), 2.64 (s, 3H), 3.28-3.88 (m, 8H), 7.42 (d, 1H), 7.56 (dd, 1H), 8.03 (d, 1H). Rf (hexane:ethyl acetate=10:1): 0.13.
- The title compound is obtained by reduction with hydrogen over 10% palladium on charcoal in methanol solution.
- To a solution of 4-bromo-1-methyl-2-nitrobenzene (225 mg, 1.04 mmol), morpholine (125 μL, 1.25 mmol), and cesium carbonate (474.4 mg, 1.46 mmol) in toluene, palladium diacetate (31.2 mg, 0.139 mmol) and 2-(di-t-butylphosphino)biphenyl (125 mg, 0.403 mmol) are added and stirred at 100° C. for 5 hours. After cooling, the mixture is filtered to remove insoluble material. The filtrate is poured into water and extracted with ethyl acetate twice. The organic layer is washed with water and then brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1) to afford 4-(4-methyl-3-nitrophenyl)-morpholine as a yellow solid.
- 1H-NMR (δ, ppm): 2.50 (s, 3H), 3.17-3.19 (m, 4H), 3.86-3.88 (m, 4H), 7.04 (dd, 1H), 7.21 (d, 1H), 7.47 (d, 1H). Rf (hexane:ethyl acetate=5:1): 0.20.
- The title compound is obtained by reduction with hydrogen over 10% palladium on charcoal in methanol solution.
-
- To a suspension of piperidin-4-ol (2.79 g, 28 mmol) and potassium carbonate (3.88 g, 28 mmol) in N,N-dimethylformamide (40 mL), 4-Fluoro-2-methoxy-1-nitro-benzene (4.0 g, 23 mmol) is added and stirred at room temperature for 24 hours. The mixture is poured into water and the precipitate is collected by a filtration. The resulting solid is dried in vacuo at 50° C. to afford 1-(3-methoxy-4-nitro-phenyl)-piperidin-4-ol (5.23 g) as yellow solids in 89% yield.
- 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.54 (d, 1H), 1.62-1.71 (m, 2H), 1.98-2.04 (m, 2H), 3.22 (ddd, 4H), 3.73-3.80 (m, 2H), 3.95 (s, 3H), 3.98-4.02 (m, 1H), 6.33 (d, 1H), 6.43 (dd, 1H), 8.00 (d, 1H).
- By repeating the procedures described above using appropriate starting materials and conditions the following compounds are obtained.
-
Ex-No Rx Identification 37-2 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.53-1.72 (m, 2H), 1.80-1.83 (m, 4H), 1.99-2.04 (m, 2H), 2.24-2.31 (m, 1H), 2.54-2.67 (m, 4H), 3.03 (dt, 2H), 3.84-3.89 (m, 2H), 3.95 (s, 3H), 6.31 (d, 1H), 6.42 (dd, 1H), 8.01 (d, 1H). Rf 0.54 (AcOEt) 37-3 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.81-1.91 (m, 2H), 1.99-2.04 (m, 2H), 2.38-2.48 (m, 1H), 3.03 (ddd, 2H), 3.91-3.96 (m, 2H), 3.95 (s, 3H), 5.22-5.41 (m, 1H), 5.40-5.53 (m, 1H), 6.36 (d, 1H), 6.43 (dd, 1H), 8.00 (d, 1H). Rf 0.15 (AcOEt) 37-4 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.15 (t, 3H), 1.88-1.96 (m, 1H), 2.22-2.30 (m, 1H), 2.68-2.77 (m, 2H), 3.15-3.18 (m, 1H), 3.38-3.44 (m, 1H), 3.52-3.62 (m, 2H), 3.93 (s, 3H), 5.92 (d, 1H), 6.07-6.10 (m, 1H), 8.00-8.02 (m, 1H). Rf 0.65 (n- hexane:AcOEt = 1:1). 37-5 1H-NMR (400 MHz, CDCl3, δ, ppm): 2.36 (s, 3H), 2.52-2.57 (m, 4H), 3.40-3.43 (m, 4H), 3.95 (s, 3H), 6.32 (d, 1H, J = 2.52 Hz), 6.43 (dd, 1H, J = 9.56, 2.52 Hz), 7.99 (d, 1H, J = 9.08 Hz). Rf 0.60 (MeOH:CH2Cl2 = 4:1). 37-6 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.10-1.19 (m, 1H), 1.59-2.18 (m, 6H), 2.28 (s, 3H), 2.71-2.74 (m, 1H), 2.88-2.91 (m, 1H), 3.86-3.95 (m, 5H), 6.47-6.52 (m, 2H), 7.97-8.00 (m, 1H). Rf 0.65 (n- hexane:AcOEt = 1:1) 37-7 1H-NMR (400 MHz, CDCl3, δ, ppm): 4.08 (s, 3H), 7.30 (dd, 1H), 7.58 (d, 1H), 8.05 (d, 1H), 8.15 (s, 1H), 8.67 (s, 1H). Rf: 0.42 (AcOEt) 37-8 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.40-1.50 (m, 2H), 1.55-1.69 (m, 6H), 1.90-1.96 (m, 2H), 2.45-2.53 (m, 5H), 2.90-2.99 (m, 2H), 3.90-4.00 (m, 2H), 3.94 (s, 3H), 6.30 (d, 1H, J =, 2.5 Hz), 6.41 (dd, 1H, J = 9.0, 2.5 Hz), 7.99 (d, 1H, J = 9.0 Hz) 37-9 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 1.95-1.82 (m, 2H), 2.15-2.06 (m, 1H), 2.30 (s, 3H), 3.17 (dd, 1H), 3.32-3.23 (m, 1H), 3.56-3.34 (m, 3H), 3.96 (s, 1H), 6.09 (d, 1H), 6.21 (dd, 1H), 7.91 (d, 1H) 37-10 1H-NMR (400 MHz, CDCl3, δ, ppm): 2.30-2.48 (m, 3H), 2.59-2.66 (m, 1H), 2.70-2.76 (m, 1H), 2.85-2.92 (m, 1H), 3.09-3.17 (m, 1H), 3.30-3.34 (m, 1H), 3.52-3.58 (m, 1H), 3.68-3.84 (m, 3H), 3.87-3.91 (m, 1H), 3.96 (s, 3H), 6.32 (d, 1H, J = 2.5 Hz), 6.42 (dd, 1H, J = 9.6, 2.5 Hz), 8.00 (d, 1H, J = 9.6 Hz) 37-11 1H-NMR (400 MHz, DMSO-d6, CDCl3, δ, ppm): 1.90-1.79 (m, 1H), 2.25-2.15 (m, 1H), 2.21 (s, 3H), 2.87-2.77 (m, 1H), 3.16 (dd, 1H), 3.42-3.32 (m, 1H), 3.59-3.52 (m, 1H), 3.67-3.61 (m, 1H), 3.91 (s, 3H), 6.13 (d, 1H), 6.24 (dd, 1H) ), 7.91 (dd, 1H) 37-12 1H-NMR (400 MHz, CDCl3): 1.43-1.00 (m, 2H), 1.95-1.81 (m, 2H), 2.94-2.17 (m, 2H), 2.96 (s, 3H), 3.27 (d, 2H), 3.35 (s, 3H), 3.97-3.90 (m, 2H), 3.95 (s, 3H), 6.30 (d, 1H), 6.42 (dd, 1H) 8.00 (d, 1H). Rf: 0.25 (AcOEt) 37-13 1H-NMR (400 MHz, CDCl3): 1.14 (t, 3H), 2.48 (dd, 2H), 2.59 (t, 4H), 3.42 (t, 4H), 3.95 (s, 3H), 6.32 (d, 1H), 6.43 (dd, 1H) 8.01 (d, 1H). Rf 0.15 (AcOEt) 37-14 1H-NMR (400 MHz, CDCl3): 1.02-0.89 (m, 2H), 2.01-1.94 (m, 2H), 2.52-2.38 (m, 1H), 2.65-2.53 (m, 4H), 3.04-2.94 (m, 2H), 3.79-3.69 (m, 4H), 3.97-3.88 (m, 2H), 3.95 (s, 3H), 6.32 (d, 1H), 6.42 (dd, 1H), 8.00 (d, 1H). . Rf 0.10 (AcOEt) 37-15 1H-NMR (400 MHz, CDCl3): 1.08 (s, 3H), 1.09 (s, 3H), 2.66 (t, 4H), 2.74 (sept, 1H), 3.41 (t, 4H), 3.95 (s, 3H), 6.32 (d, 1H), 6.42 (dd, 1H) 8.00 (d, 1H). Rf 0.15 (AcOEt) 37-16 1H-NMR (400 MHz, CDCl3): 1.91-1.81 (m, 2H), 2.06-1.97 (m, 2H), 2.48-2.40 (m, 1H), 3.07-2.98 (m, 2H), 3.97-3.93 (m, 2H), 3.93 (s, 3H), 5.37-5.30 (m, 1H), 5.55-5.43 (m, 1H), 6.33 (d, 1H), 6.43 (dd, 1H) 8.00 (d, 1H). Rf 0.10 (AcOEt) 37-17 1H-NMR (400 MHz, CDCl3): 2.18-2.07 (m, 1H), 2.30-2.22 (m, 1H), 3.38 (s, 3H), 3.56-3.44 (m, 4H), 3.95 (s, 3H), 4.13 (ddd, 1H), 5.96 (d, 1H), 6.12 (dd, 1H) 8.03 (d, 1H). Rf 0.30 (AcOEt) 37-18 1H-NMR (400 MHz, CDCl3): 1.46 (s, 9H), 1.81-1.68 (m, 4H), 2.73 (bs, 3H), 3.07-2.97 (m, 2H), 3.95 (s, 3H), 4.03-3.94 (m, 2H), 6.32 (d, 1H), 6.43 (dd, 1H) 8.00 (d, 1H). Rf 0.55 (Hexane:AcOEt) 37-19 1H-NMR (400 MHz, CDCl3): 3.60-3.57 (m, 2H), 3.68-3.65 (m, 2H), 3.97 (s, 3H), 4.07 (s, 2H), 6.17 (bs, 1H), 6.26 (d, 1H), 6.39 (dd, 1H), 8.04 (d, 1H). Rf 0.85 (AcOEt) 37-20 1H-NMR (400 MHz, CDCl3): 3.08 (s, 3H), 3.54 (dd, 2H), 3.67 (dd, 2H), 3.96 (s, 3H), 4.05 (s, 2H), 6.25 (d, 1H), 6.38 (dd, 1H) 8.03 (d, 1H). Rf 0.30 (AcOEt) 37-21 1H-NMR (400 MHz, CDCl3): 1.73-1.55 (m, 2H), 1.99-1.91 (m, 2H), 2.09 (s, 3H), 2.61-2.49 (m, 5H), 3.47 (t, 2H), 3.63 (t, 2H), 3.99-3.89 (m, 3H), 3.95 (s, 3H), 6.32 (d, 1H), 6.42 (dd, 1H) 8.01 (d, 1H). Rf 0.10 (AcOEt:MeOH = 4:1) 37-22 1H-NMR (400 MHz, CDCl3): 3.90 (s, 3H), 3.98 (s, 3H), 3.98 (s, 3H), 6.56 (s, 1H), 7.59 (s, 1H). Rf 0.605 (AcOEt) 37-23 1H-NMR (400 MHz, CDCl3): 3.25-3.22 (m, 4H), 3.90-3.87 (m, 4H), 3.95 (s, 3H), 6.48 (s, 1H), 7.57 (s, 1H). Rf 0.060 (Hexane:AcOEt = 5:1) 37-24 1H-NMR (400 MHz, CDCl3): 2.37 (s, 3H), 2.61 (bs, 4H), 3.27 (bs, 4H), 3.88 (s, 3H), 3.95 (s, 3H), 6.48 (s, 1H), 7.56 (s, 1H). Rf 0.10 (AcOEt:MeOH = 5:1) 37-25 1H-NMR (400 MHz, CDCl3): 1.09 (t, 3H), 1.89 (dd, 2H), 2.36 (s, 3H), 2.55 (t, 4H), 3.39 (t, 4H), 4.03 (t, 2H), 6.32 (d, 1H), 6.42 (dd, 1H), 7.98 (d, 1H). Rf 0.12 (AcOEt:MeOH = 9:1) 37-26 1H-NMR (400 MHz, CDCl3): 1.36 (s, 3H), 1.38 (s, 3H), 2.10 (s, 2H), 2.17 (s, 3H), 3.27-2.96 (m, 2H), 3.71 (d, 2H), 3.96 (s, 3H), 6.33 (d, 1H), 6.43 (dd, 1H), 8.02 (d, 1H). Rf 0.10 (AcOEt) 37-27 1H-NMR (400 MHz, CDCl3): 1.16 (s, 3H), 1.18 (s, 3H), 2.50 (dd, 2H), 3.02-2.47 (m, 2H), 3.69 (dd, 2H), 3.96 (s, 3H), 6.31 (d, 1H), 6.43 (dd, 1H), 8.00 (d, 1H). Rf 0.070 (AcOEt) 37-28 1H-NMR (400 MHz, CDCl3): 1.16 (d, 3H), 2.57 (dd, 1H), 3.00-2.89 (m, 4H), 3.18-3.11 (m, 1H), 3.75-3.68 (m, 2H), 3.96 (s, 3H), 6.31 (d, 1H), 6.43 (dd, 1H), 8.01 (d, 1H). Rf 0.070 (AcOEt) 37-29 1H-NMR (400 MHz, CDCl3): 1.18 (t, 3H), 2.40 (dd, 2H), 3.47-3.38 (m, 4H), 3.71-3.63 (m, 2H), 3.85-3.79 (m, 2H), 3.96 (s, 3H), 6.32 (d, 1H), 6.42 (dd, 1H), 8.01 (d, 1H). Rf 0.20 (AcOEt) 37-30 1H-NMR (400 MHz, CDCl3): 1.16 (s, 3H), 1.18 (s, 3H), 2.82 (sept, 1H), 3.50-3.37 (m, 4H), 3.77-3.65 (m, 2H), 3.86-3.78 (m, 2H), 3.96 (s, 3H), 6.33 (d, 1H), 6.43 (dd, 1H), 8.01 (d, 1H). Rf 0.48 (AcOEt) 37-31 1H-NMR (400 MHz, CDCl3): 2.86 (d, 3H), 3.48-3.45 (m, 4H), 3.61-3.58 (m, 4H), 3.96 (s, 3H), 4.48-4.37 (m, 1H), 6.29 (d, 1H), 6.40 (dd, 1H), 8.01 (d, 1H). Rf 0.20 (AcOEt) 37-32 1H-NMR (400 MHz, CDCl3): 1.72-1.60 (m, 2H), 2.06-1.97 (m, 2H), 3.25-3.17 (d, 3H), 3.78-3.70 (m, 2H), 3.95 (s, 3H), 4.04-3.99 (m, 1H), 6.33 (d, 1H), 6.43 (dd, 1H), 8.00 (d, 1H). Rf 0.20 (AcOEt) 37-33 1H-NMR (400 MHz, CDCl3): 1.53 (s, 6H), 2.14 (s, 3H), 3.50 (s, 2H), 3.61-3.58 (m, 2H), 3.97-3.81 (m, 2H), 3.97 (s, 3H), 6.10 (d, 1H), 6.26 (dd, 1H), 8.05 (d, 1H). Rf 0.030 (AcOEt) 37-34 1H-NMR (400 MHz, CDCl3): 2.54-2.23 (m, 4H), 2.67 (t, 2H), 3.29-3.23 (m, 2H), 3.74 (t, 4H), 3.94 (s, 3H), 6.07 (d, 1H), 6.16 (dd, 1H), 8.00 (d, 1H). Rf 0.15 (AcOEt) 37-35 1H-NMR (400 MHz, CDCl3): 2.10-2.02 (m, 2H), 2.41 (t, 2H), 3.56 (dd, 2H), 3.71 (t, 2H), 3.95 (s, 3H), 4.19 (t, 2H), 6.49 (dd, 1H), 6.55 (d, 1H), 7.99 (d, 1H). Rf 0.10 (AcOEt) 37-36 1H-NMR (400 MHz, CDCl3): 2.14 (s, 3H), 3.87-3.34 (m, 8H), 3.99 (s, 3H), 7.01 (dd, 1H), 7.16 (d, 1H), 7.88 (d, 1H). Rf 0.25 (AcOEt) 37-37 1H-NMR (400 MHz, CDCl3): 3.49-3.37 (m, 2H), 3.88-3.55 (m, 6H), 3.99 (s, 3H), 7.00 (dd, 1H), 7.16 (d, 1H), 7.87 (d, 1H). Rf 0.50 (AcOEt) 37-38 1H-NMR (400 MHz, CDCl3): 1.17 (s, 3H), 1.19 (s, 3H), 2.69 (t, 4H), 3.06 (s, 2H), 3.42 (t, 4H), 3.96 (s, 3H), 4.13 (sept, 1H), 6.34 (d, 1H), 6.44 (dd, 1H), 6.90-6.79 (m, 1H), 8.00 (d, 1H). Rf 0.20 (AcOEt) 37-39 1H-NMR (400 MHz, CDCl3): 1.44-1.34 (m, 2H), 1.84-1.77 (m, 1H), 1.94-1.85 (m, 2H), 3.04-2.94 (m, 2H), 3.55 (t, 2H), 3.96-3.57 (m, 2H), 3.95 (s, 3H), 6.31 (d, 1H), 6.42 (dd, 1H), 8.00 (d, 1H). Rf 0.30 (AcOEt) 37-40 1H-NMR (400 MHz, CDCl3): 1.44-1.34 (m, 2H), 1.84-1.77 (m, 1H), 1.94-1.85 (m, 2H), 3.04-2.94 (m, 2H), 3.55 (t, 2H), 3.96-3.57 (m, 2H), 3.95 (s, 3H), 6.31 (d, 1H), 6.42 (dd, 1H), 8.04 (d, 1H). Rf 0.45 (AcOEt) 37-41 1H-NMR (400 MHz, CDCl3): 4.05 (s, 3H), 7.07 (d, 1H), 7.08 (d, 1H), 7.27-7.26 (m, 1H), 7.33 (t, 1H), 7.92 (s, 1H), 8.04 (d, 1H). Rf: 0.20 (AcOEt) 37-42 1H-NMR (400 MHz, CDCl3): 2.34 (s, 3H), 2.55-2.37 (m, 4H), 3.86-3.38 (m, 4H), 4.00 (s, 3H), 7.13 (d, 1H). 7.66 (dd, 1H). 7.93 (d, 1H). Rf: 0.30 (AcOEt:MeOH = 4:1) 37-43 1H-NMR (400 MHz, CDCl3): 2.43 (s, 3H), 2.74 (s, 6H), 7.91 (dd, 1H), 7.23 (d, 1H), 7.24 (d, 1H), 7.46 (dd, 1H). Rf: 0.70 (Hexane:AcOEt = 5:1) 37-44 1H-NMR (400 MHz, CDCl3): 2.15 (s, 3H), 3.80-3.48 (m, 2H), 6.87 (dd, 1H), 6.92 (dd, 1H), 7.09 (d, 1H), 7.40 (dd, 2H), 8.54 (dd, 2H). 37-45 1H-NMR (400 MHz, CDCl3): 3.86 (s, 3H), 4.00 (s, 3H), 6.78 (d, 1H), 6.99 (dd, 2H), 7.14 (d, 1H), 7.48 (dd, 2H), 7.71 (dd, 1H), 8.03 (d, 1H). Rf: 0.30 (Hexane:AcOEt = 3:1) 37-46 1H-NMR (400 MHz, CDCl3): 1.44 (t, 3H), 3.10 (t, 4H), 3.86 (t, 4H), 4.13 (q, 2H), 7.01 (dd, 1H), 7.08 (dd, 1H), 7.35 (d, 1H). Rf: 0.25 (Hexane:AcOEt = 3:1) 37-47 1H-NMR (400 MHz, CDCl3): 1.26 (t, 3H), 3.32 (t, 4H), 3.85 (t, 4H), 4.15 (q, 2H), 6.34 (d, 1H), 6.42 (dd, 1H), 7.98 (d, 1H). Rf: 0.45 (Hexane:AcOEt = 5:1) 37-48 1H-NMR (400 MHz, CDCl3): 3.45 (s, 3H), 3.77 (dd, 2H), 3.81 (s, 3H), 4.06 (t, 2H), 7.08-7.08 (m, 2H), 7.37 (t, 1H). Rf: 0.45 (Hexane:AcOEt = 3:1) 37-49 1H-NMR (400 MHz, CDCl3): 2.44 (t, 1H), 3.83 (s, 3H), 3.96 (ddd, 2H), 4.20 (t, 2H), 7.06 (d, 1H), 7.12 (dd, 1H), 7.40 (d, 1H). Rf: 0.10 (Hexane:AcOEt = 3:1) 37-50 1H-NMR (400 MHz, CDCl3): 1.45 (t, 3H), 3.81 (s, 3H), 4.13 (q, 2H), 7.01 (d, 1H) 7.08 (dd, 1H), 7.36 (d, 1H). Rf: 0.20 (Hexane:AcOEt = 3:1) 37-51 1H-NMR (400 MHz, CDCl3): 1.35 (s, 3H), 1.36 (s, 3H), 3.81 (s, 3H), 4.52 (sept, 1H), 7.08-7.01 (m, 2H), 7.31 (d, 1H). Rf: 0.30 (Hexane:AcOEt = 3:1) 37-52 1H-NMR (400 MHz, CDCl3): 1.05 (t, 3H), 1.83 (ddd, 2H), 3.81 (s, 3H), 4.01 (t, 2H), 7.01 (d, 1H), 7.08 (dd, 1H), 7.36 (d, 1H). Rf: 0.35 (Hexane:AcOEt = 3:1) 37-53 1H-NMR (400 MHz, CDCl3): 3.86 (s, 6H), 3.79 (s, 3H), 6.91 (dd, 1H), 7.00 (d, 1H), 7.18 (d, 1H). Rf: 0.5 (Hexane:AcOEt = 9:1) 37-54 1H-NMR (400 MHz, CDCl3): 4.04 (s, 3H), 7.22 (d, 1H), 7.48 (dd, 2H), 7.83 (dd, 1H), 8.16 (d, 1H), 8.69 (dd, 2H). Rf: 0.12 (Hexane:AcOEt = 1:1) 37-55 1H-NMR (400 MHz, CDCl3): 4.02 (s, 3H), 7.22 (d, 1H), 7.39 (ddd, 1H), 7.77 (dd, 1H), 7.85 (ddd, 1H), 8.08 (d, 1H), 8.63 (dd, 1H), 8.83 (d, 1H). Rf: 0.55 (Hexane:AcOEt = 2:1) 37-56 1H-NMR (400 MHz, CDCl3): 4.03 (s, 3H), 7.19 (d, 1H), 7.28-7.24 (m, 1H), 7.72 (dd, 1H), 7.80-7.76 (m, 1H), 8.25 (dd, 1H), 8.52 (d, 1H), 8.69 (ddd, 1H). Rf: 0.55 (Hexane:AcOEt = 2:1) 37-57 mp 90.7° C.; 1H-NMR (400 MHz, CDCl3) δ (ppm): 1.68 (m; 2H), 2.00 (m; 2H), 2.36 (s; 1H), 2.62 (bs; 4H), 2.72 (m; 2H), 3.62 (m; 2H), 3.78 (bs; 4H), 3.90 (s; 3H), 6.99 (d; 1H); 7.13 (dd; 1H), 7.26 (s; 1H); 7.40 (s; 1H). -
- To a solution of 5-bromo-1-methoxy-2-nitrobenzene (300 mg, 1.29 mmol) in dioxane, 1-acetyl piperazine (400 mg, 3.12 mmol), cesium carbonate (1.0 g, 3.07 mmol), palladium diacetate (29.0 mg, 0.129 mmol) and 2-(di-t-butylphosphino)biphenyl (77 mg, 0.258 mmol) are added and stirred at 100° C. for 8 hours. After cooling, the mixture is filtered to remove insoluble material. The filtrate is poured into water and extracted with ethyl acetate twice. The organic layer is washed with water and then brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified by silica gel column chromatography (n-hexane:ethyl acetate gradient) to afford 1-[4-(4-Methoxy-3-nitro-phenyl)-piperazin-1-yl]ethanone (319 mg, 44%) as yellow solids.
- 1H-NMR (400 MHz, CDCl3, δ, ppm): 2.14 (s, 3H), 3.63 (ddd, 4H), 3.63 (t, 2H), 3.78 (t, 2H), 3.92 (s, 3H), 7.03 (d, 1H), 7.12 (d, 1H), 7.41 (d, 1H). Rf (ethyl acetate): 0.18
-
- To a solution of 4-piperidone hydrochloride monohydrate (10.0 g, 0.065 mol) in DMF (80 mL) are added 4-Fluoro-2-methoxy-1-nitro-benzene (10.0 g, 0.058 mol) and potassium carbonate (20.2 g), and the mixture is stirred at 70° C. for 20 h. After a filtration, the filtrate is poured into H2O (ca. 300 mL), and the resulting precipitates are collected by a filtration followed by washing with H2O for several times to give title compound (8.98 g) in 61% yield. Orange solid. 1H-NMR (400 MHz, CDCl3, δ): 2.65-2.62 (4H, m), 3.81-3.78 (4H, m), 3.98 (3H, s), 6.34 (1H, d), 6.45 (1H, dd), 8.05 (1H, d).
-
- To a solution of 1-(3-Methoxy-4-nitro-phenyl)-piperidin-4-one (4.96 g, 0.020 mol) in dichloroethane (50 ml) is added N-methylpiperazine (2.7 ml, 0.024 mol) at 0° C. and the mixture is stirred at room temperature. After 4 h, sodium triacetoxy-borohydride (5.04 g, 0.024 mol) is added and the mixture is further stirred at room temperature for 24 h. After addition of 1N sodium hydroxide at 0° C., the mixture is poured into water and extracted three times with dichloromethane. The organic layer is combined and extracted three times with 1N hydrochloride. The water layer is basified with 2N sodium hydroxide and extracted three times with dichloromethane. The organic layer is washed with brine, dried over sodium sulfate, and evaporated in vacuo to give the title compound as yellow solids (6.04 g) in 91% yield.
- 1H-NMR (400 MHz, CDCl3, δ): 1.70-1.57 (2H, m), 2.03-1.93 (2H, m), 2.29 (3H, s), 2.55-2.38 (5H, m), 2.70-2.56 (4H, m), 2.97 (2H, ddd), 3.97-3.92 (2H, m), 3.95 (3H, s), 6.31 (1H, d,), 6.42 (1H, dd), 8.00 (1H, d).
-
- To a solution of 4-methoxyphenyl-boronic acid (500 mg, 3.29 mmol) in toluene (5.2 mL) and ethanol (1.3 mL), potassium carbonate (910 mg, 6.58 mmol), tetrakis(triphenylphosphine)-palladium (228.1 mg, 0.099 mmol) and 4-bromo-1-methyl-2-nitrobenzene (711 mg, 3.29 mmol) are added and stirred at 100° C. for 7 hours. The mixture is poured into water and extracted with ethyl acetate two times. The organic layer is washed with water and then brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1) to afford the 4′-methoxy-4-methyl-3-nitro-biphenyl (630 mg, 79%) as a yellow solid.
- 1H-NMR (400 MHz, CDCl3, δ, ppm): 2.62 (s, 3H), 3.86 (s, 3H), 7.02-6.98 (m, 2H), 7.37 (d, 1H), 7.54 (dd, 2H), 7.68 (dd, 1H), 8.18 (d, 1H). Rf (hexane:ethyl acetate=3:1): 0.40.
-
- To a solution of 1-(3-Methoxy-4-nitro-phenyl)-piperidin-4-ol (300 mg, 1.2 mmol) in N,N-dimethylformamide (3.0 mL), sodium hydride (1.52 g, 3.8 mmol) is added. After stirring, 2-bromoethyl methyl ether (150 μl, 1.6 mmol) is added and the mixture is further stirred at 70° C. for 15 hours. After addition of saturated aqueous ammonium chloride, the mixture is poured into water and extracted twice with ethyl acetate. The organic layer is washed with brine, dried over sodium sulfate, and evaporated in vacuo. The residue is purified by silica gel column chromatography (n-hexane-ethyl acetate gradient) to afford 4-(2-Methoxy-ethoxy)-1-(3-methoxy-4-nitro-phenyl)-piperidine (111 mg, 29%) as a yellow oil.
- 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.52 (t, 3H), 1.95-2.00 (m, 2H), 1.70-1.79 (m, 2H), 3.23 (ddd, 2H), 3.58-3.64 (m, 2H), 3.65-3.68 (m, 2H), 3.64-3.72 (m, 2H), 3.95 (s, 3H), 6.31 (d, 1H), 6.42 (dd, 1H), 8.00 (d, 1H). Rf 0.53 (n-hexane:AcOEt=1:1).
- According the procedure described above using appropriate alkyl halides, the following compounds are prepared.
-
Ex- No. Rx Identification 42-1 1H-NMR (400 MHz, CDCl3, δ, ppm): 2.04-2.21 (m, 1H), 2.63 (t, 2H), 2.68 (t, 2H), 3.42 (t, 4H), 3.87 (t, 4H), 3.96 (s, 3H), 6.33 (d, 1H), 6.44 (dd, 1H), 8.02 (d, 1H). Rf 0.09 (AcOEt). 42-2 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.71-1.79 (m, 2H), 1.95- 2.02 (m, 2H), 3.22 (ddd, 2H), 3.40 (s, 3H), 3.55-3.57 (m, 2H), 3.59-3.73 (m, 3H), 3.65-3.67 (m, 2H), 3.95 (s, 3H), 6.31 (d, 1H), 6.42 (dd, 1H), 8.00 (d, 1H). Rf 0.35 (n-hexane: AcOEt = 1:1) -
- To a solution of 4-Fluoro-2-methoxy-1-nitro-benzene (10.3 g, 60 mmol) in toluene (50 mL) and 25% KOH aq. (50 mL), 4-hydroxy-1-methylpiperidine (13.8 g, 120 mmol) and tetra-n-butyl ammonium bromide (3.87 g, 12 mmol) are added at room temperature. The mixture is heated at 60° C. for 1 day. The reaction mixture is cooled to room temperature, poured into ice water and extracted twice with ethyl acetate. The organic layer is successively washed with dil.HCl and brine, dried over sodium sulfate, and evaporated in vacuo to afford the crude compound in quantitative yield (13.4 g).
- Rf=0.22 (methanol:dichloromethane=1:4). 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.84-1.92 (m, 2H), 2.0-2.1 (m, 2H), 2.3-2.4 (m, 2H), 2.33 (s, 3H), 2.65-2.75 (m, 2H), 3.94 (s, 3H), 4.39-4.46 (m, 1H), 6.49 (dd, 1H), 6.99 (d, 1H), 6.54 (d, 1H), 7.99 (d, 1H).
-
- To a solution of 3-Fluoro-4-nitro-phenol (15.7 g, 100 mmol) in THF (300 mL), 30% KOMe in Methanol (49 mL, 210 mmol) is added at 0° C. The mixture is heated to gentle reflux for 18 hours.
-
- To a solution of 3-Methoxy-4-nitro-phenol (1.69 g, 10 mmol) in DMF (25 mL), 4-(2-Chloroethyl)morpholine hydrochloride (2.05 g, 11 mmol), K2CO3 (1.52 g, 11 mmol), KI (332 mg, 2 mmol) are added at room temperature. The mixture is heated to gentle reflux for 4 hours. The reaction mixture is cooled to room temperature and quenched with water. The resulting mixture is extracted twice with ethyl acetate and then the organic layer is successively washed with water and brine, dried over sodium sulfate, filtered and evaporated in vacuo to afford the crude compound in 90% yield (2.55 g).
- Rf=0.11 (AcOEt only). 1H-NMR (400 MHz, CDCl3), δ (ppm): 2.56-2.61 (m, 4H), 2.83 (t, The reaction mixture is cooled to room temperature and quenched slowly with 1 NHCl aq at 0° C. The resulting mixture is extracted twice with ethyl acetate and then the organic layer is successively washed with brine, dried over sodium sulfate, filtered and evaporated in vacuo to afford the crude compound in 94% yield (15.9 g).
- Rf=0.22 (methanol:dichloromethane=1:4). 1H-NMR (400 MHz, CDCl3), δ (ppm): 3.95 (s, 3H), 5.49 (s, 1H), 6.44 (dd, 1H, J=8.8, 2.52 Hz), 6.54 (d, 1H, J=2.52 Hz), 7.96 (d, 1H J=8.6 Hz). 3.72-3.76 (m, 4H), 3.94 (s, 3H), 4.18 (t, 2H), 6.51 (dd, 1H, J=9.08, 2.52 Hz), 6.56 (d, 1H, J=2.48 Hz), 8.00 (d, 1H J=9.08 Hz). 2H),
-
- To a solution of 4-Methoxyphenol (12.4 g, 100 mmol) in AcOH (50 mL), Ac2O (50 mL) is added at room temperature. The mixture is heated to gentle reflux for 1.5 hour. The reaction mixture is cooled to room temperature and c.HNO3 (d=1.38, 10 mL) is added slowly at 0° C. The mixture is heated to 55° C. for 1.5 h. The reaction mixture is cooled to room temperature and quenched with water at 0° C. The resulting solid is filtered on Buchner funnel to afford the crude compound in 76% yield (16.0 g).
- Rf=0.59 (AcOEt:n-Hexane=3:7). 1H-NMR (400 MHz, CDCl3), δ (ppm): 2.31 (s, 3H), 3.96 (s, 3H), 7.08 (d, 1H, J=9.04 Hz), 7.31 (dd, 1H, J=9.04, 3.04 Hz), 7.96 (d, 1H J=3.04 Hz).
-
- To a solution of Acetic acid 4-methoxy-3-nitro-phenyl ester (1.06 g, 5 mmol) in EtOH (20 mL), 1N NaOH aq (5.5 mL) is added at 0° C. The mixture is stirred at room temperature for 2 hours. The reaction mixture is quenched with AcOH and extracted twice with ethyl acetate. The organic layer is successively washed with water and brine, dried over sodium sulfate, filtered and evaporated in vacuo to afford the crude compound in quantitative yield (840 mg).
- Rf=0.59 (AcOEt:n-Hexane=3:7). 1H-NMR (400 MHz, CDCl3), δ (ppm): 3.91 (s, 3H), 6.99 (d, 1H, J=9.04 Hz), 7.17 (dd, 1H, J=9.04, 3.00 Hz), 7.38 (d, 1H J=3.04 Hz).
-
- To a solution of 4-Methoxy-3-nitro-phenol (1.01 g, 6 mmol) in DMF (15 mL), 4-(2-Chloroethyl)morpholine hydrochloride (1.34 g, 7.2 mmol), K2CO3 (2.49 g, 18 mmol), KI (2.99 g, 18 mmol) are added at room temperature. The mixture is heated to 80° C. for 4 hours. The reaction mixture is cooled to room temperature and quenched with saturated NH4Cl solution in water. The resulting mixture is extracted twice with ethyl acetate and then the organic layer is successively washed with water and brine, dried over sodium sulfate, filtered and evaporated in vacuo to afford the crude compound in quantitative yield (1.70 g). Rf=0.14 (AcOEt only). 1H-NMR (400 MHz, DMSO, δ, ppm): 2.36-2.51 (m, 4H), 2.67 (t, J=5.5, 2H), 3.52-3.60 (m, 4H), 3.86 (s, 3H), 4.11 (t, J=6.0, 2H), 7.25-7.29 (m, 2H), 7.46-7.49 (m, 1H).
-
- To a solution of 4-(3-Methoxy-4-nitro-phenoxy)-1-methyl-piperidine (3.0 g, 11.3 mmol) in ethanol (50 mL), 5% palladium on carbon (300 mg) is added under a nitrogen atmosphere. The reaction vessel is fitted with a balloon adapter and charged with hydrogen and evacuated three times until the reaction is under a hydrogen atmosphere. The reaction is allowed to stir overnight. The reaction mixture is filtered through a pad of Celite and washed with methanol. The filtrate is concentrated in vacuo to afford 2-Methoxy-4-(1-methyl-piperidin-4-yloxy)-phenylamine in quantitative yield (2.7 g).
- Rf=0.41 (methanol:dichloromethane=1:1). 1H-NMR (400 MHz, CDCl3), δ (ppm): 1.75-1.86 (m, 2H), 1.92-2.05 (m, 2H), 2.2-2.32 (m, 2H), 2.30 (s, 3H), 3.4-3.7 (brs, 2H), 3.82 (s, 3H), 4.1-4.2 (m, 1H), 6.37 (dd, 1H), 6.46 (d, 1H), 6.61 (d, 1H).
- By repeating the procedures described above using appropriate starting materials and conditions the following compounds are obtained.
-
Ex- No. Rx Identification 46-1 1H-NMR (400 MHz, CDCl3, δ, ppm): 3.92 (s, 3H), 3.97 (br, 2H), 6.75 (d, 1H), 7.00 (dd, 1H), 7.12 (d, 1H), 8.06 (s, 1H), 8.41 (s, 1H). Rf 0.32 (AcOEt) 46-2 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.13 (t, 3H), 1.77-1.86 (m, 1H), 2.19-2.27 (m, 1H), 2.67-2.75 (m, 2H), 3.01-3.06 (m, 1H), 3.20-3.26 (m, 1H), 3.33-3.38 (m, 1H), 3.42-3.49 (m, 2H), 3.84 (s, 3H), 6.04-6.07 (m, 1H), 6.14-6.15 (m, 1H), 6.64-6.66 (m, 1H). Rf 0.2 (AcOEt only) 46-3 1H-NMR (400 MHz, CDCl3, δ, ppm): 2.44 (s, 3H), 2.70-2.73 (m, 4H), 3.13-3.17 (m, 4H), 3.48 (brs, 2H), 3.84 (s, 3H), 6.41 (dd, 1H, J = 8.5, 2.52 Hz), 6.51 (d, 1H, J = 2.52 Hz), 6.64 (d, 1H, J = 8.5 Hz). Rf 0.2 (AcOEt only). 46-4 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.01-1.12 (m, 1H), 1.57- 2.13 (m, 6H), 2.26 (s,3H), 2.74-2.77 (m, 1H), 2.93-2.96 (m, 1H), 3.47 (bs, 2H), 3.70-3.80 (m, 2H), 3.82 (s, 3H), 6.31-6.34 (m, 1H), 6.44-6.45 (m, 1H), 6.60-6.62 (m, 1H). Rf 0.2 (AcOEt only) 46-5 1H-NMR (400 MHz, CDCl3) 1.80-1.67 (2H, m), 1.99-1.90 (2H, m), 2.42-2.27 (1H, m), 2.56-2.43 (4H, m), 2.68-2.58 (2H, m), 2.76-2.58 (4H, m), 3.57-3.48 (2H, m), 3.83 (3H. s), 6.41 (1H, dd), 6.52 (1H, d), 6.63 (1H, d). Rf (hexane/acetone 1:1) 0.44. 46-6 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.83-1.95 (m, 2H), 1.97- 2.08 (m, 2H), 2.20-2.31 (m, 1H), 2.60-2.72 (m, 2H), 3.46-3.53 (m, 2H), 3.84 (s, 3H), 5.42-5.60 (m, 1H), 6.43 (dd, 1H), 6.53 (d, 1H), 6.64 (d, 1H). 46-7 1H-NMR (400 MHz, CDCl3, δ, ppm) : 2.13 (s, 3H), 3.01-3.05 (m, 4H), 3.59 (t, 2H), 3.75 (t, 2H), 3.81 (s, 3H), 6.30 (dd, 1H), 6.39 (bs, 1H), 6.71 (d, 1H). 46-8 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.84-1.97 (m, 2H), 1.98- 2.07 (m, 2H), 2.20-2.32 (m, 1H), 2.61-2.72 (m, 2H), 3.47-3.55 (m, 2H), 3.95 (s, 3H), 5.20-5.38 (m, 1H), 5.40-5.56 (m, 2H), 6.43 (d, 1H), 6.53 (bs, 1H), 6.64 (d, 1H). 46-9 1H-NMR (400 MHz, CDCl3, δ, ppm) : 2.59-2.67 (m, 2H), 2.77- 2.68 (m, 4H), 3.08-3.15 (m, 4H), 3.49-3.56 (m, 1H), 3.67-3.77 (m, 2H), 3.98 (s, 3H), 6.41-6.43 (m, 1H), 6.52 (bs, 1H), 6.65 (d, 1H). 46-10 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.72-1.96 (m, 2H), 1.98- 2.10 (m, 2H), 2.63 (s, 3H), 2.73-2.84 (m, 2H), 3.40 (s, 3H), 3.34-3.42 (m, 2H), 3.44-3.49 (m, 1H), 3.55-3.57 (m, 2H), 3.64- 3.66 (m, 2H), 3.83 (s, 3H), 6.41-6.43 (m, 1H), 6.53 (bs, 1H), 6.63 (d, 1H). 46-11 1H-NMR (400 MHz, CDCl3, δ, ppm) : 1.22 (t, 3H), 1.72-1.84 (m, 2H), 2.00-2.10 (m, 2H), 2.72-2.82 (m, 2H), 3.33-3.38 (m, 2H), 3.43-3.49 (m, 1H), 3.55 (q, 2H), 3.58-3.61 (m, 2H), 3.64-3.66 (m, 2H), 3.83 (s, 3H), 6.41-6.43 (m, 1H), 6.53 (bs, 1H), 6.63 (d, 1H). 46-12 1H-NMR (400 MHz, CDCl3, δ, ppm) : 2.20 (s, 3H), 3.84 (s, 3H), 6.87 (d, 1H), 6.89 (dd, 1H), 6.95 (d, 2H), 7.09 (d, 1H), 7.48 (d, 2H). Rf (n-hexane:ethyl acetate = 1:1): 0.50. 46-13 1H- NMR (400 MHz, CDCl3, δ, ppm): 1.49 - 1.59 (m, 3H), 1.70-1.95 (m, 6H), 2.00-2.20 (m, 2H), 2.60-2.90 (m, 7H), 3.50-3.60 (m, 3H), 3.83 (s, 3H), 3.85-3.91 (m, 1H), 6.41 (dd, 1H, J = 8.0, 2.5 Hz), 6.50 (d, 1H, J = 2.5 Hz), 6.63 (d, 1H, J = 8.0 Hz ) 46-14 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 1.87-1.79(m, 1H), 2.22 (ddd, 1H), 2.48 (s, 3H), 3.05 (dd, 1H), 3.28-3.21 (m, 1H), 3.40-3.32 (m, 2H), 3.45 (dd, 1H), 3.84 (s, 3H), 6.06 (dd, 1H), 6.15 (d, 1H)), 6.66 (d, 1H) 46-15 1H-NMR (400 MHz, CDCl3, δ, ppm): 2.35-2.73 (m, 4H), 2.68- 2.75 (m, 1H), 2.82-2.93 (m, 2H), 3.14-3.19 (m, 1H), 3.29- 3.40 (m, 2H), 3.50-3.60 (bs, 2H), 3.69-3.78 (m, 2H), 3.84 (s, 3H), 3.85-3.91 (m, 1H), 6.40 (dd, 1H, J = 8.0, 2.5 Hz), 6.50 (d, 1H, J = 2.5 Hz), 6.64 (d, 1H, J = 8.0 Hz) 46-16 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 1.95-1.85 (m, 1H), 2.22-2.14 (m, 1H), 2.31 (s, 3H), 2.89-2.79 (m, 1H), 3.10 (t, 1H), 3.39-3.25 (m, 3H), 3.42 (t, 1H), 3.85 (s, 3H), 6.05 (dd, 1H), 6.14 (d, 1H), 6.67 (d, 1H) 46-17 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.68-1.81 (m, 2H), 1.97- 2.09 (m, 2H), 2.74-2.87 (m, 2H), 3.31-3.41 (m, 2H), 3.77-3.88 (m, 1H), 3.84 (s, 3H), 6.40-6.48 (m, 1H), 6.65 (bs, 1H), 6.64 (d, 1H). 46-18 1H-NMR (400 MHz, CDCl3), δ (ppm): 2.55-2.61 (m, 4H), 2.80 (t, 2H), 3.72-3.77 (m, 4H), 3.81 (s, 3H), 4.05 (t, 2H), 6.24 (dd, 1H, J = 8.56, 2.52 Hz), 6.34 (d, 1H, J = 2.52 Hz), 6.68 (d, 1H J = 8.56 Hz). Rf = 0.31 (methanol:dichloromethane = 1:9). 46-19 1H-NMR (400 MHz, CDCl3), δ (ppm): 2.55-2.61 (m, 4H), 2.78 (t, 2H), 3.72-3.77 (m, 4H), 3.82 (s, 3H), 4.05 (t, 2H), 6.35 (dd, 1H, J = 8.56, 2.52 Hz), 6.47 (d, 1H, J = 2.52 Hz), 6.63 (d, 1H J = 8.56 Hz). Rf = 0.61 (methanol:dichloromethane = 1:4). 46-20 1H-NMR (DMSO), δ (ppm): 3.84 (s, 3H), 6.95-7.00 (m, 1H), 7.08-7.12 (m, 2H). 46-21 1H-NMR (400 MHz, CDCl3): 1.47-1.34 (m, 2H), 1.75-1.63 (m, 1H), 1.86-1.79 (m, 2H), 2.64-2.58 (m, 2H), 3.28 (d, 2H), 3.61 (d, 3H), 3.87 (s, 3H), 3.36 (s, 1H), 3.49-3.45 (m, 2H), 3.84 (s,3H), 6.43 (dd, 1H), 6.53 (d, 1H) 6.64 (d, 1H) 46-22 1H-NMR (400 MHz, CDCl3): 1.13 (t, 3H), 2.49 (dd, 2H), 2.68-2.59 (m, 4H), 3.10 (t, 4H), 3.84 (s,3H), 6.43 (dd, 1H), 6.53 (d, 1H) 6.65 (d, 1H) 46-23 1H-NMR (400 MHz, CDCl3): 1.78-1.68 (m, 2H), 1.99-1.89 (m, 2H), 2.36-2.20 (m, 1H), 2.67-2.50 (m, 6H), 3.56-3.48 (m, 2H), 3.79-3.69 (m, 4H), 3.84 (s, 3H), 6.42 (dd, 1H), 6.52 (d, 1H) 6.64 (d, 1H) 46-24 1H-NMR (400 MHz, CDCl3): 1.08 (s, 3H), 1.10 (s, 3H), 2.69 (t, 4H), 2.72-2.68 (m, 1H), 3.08 (t, 4H), 3.83 (s, 3H), 6.42 (dd, 1H), 6.53 (d, 1H) 6.64 (d, 1H) 46-25 1H-NMR (400 MHz, CDCl3): 1.96-1.84 (m, 2H), 2.07-1.99 (m, 2H), 2.32-2.28 (m, 1H), 2.70-2.60 (m, 2H), 3.54-3.47 (m, 2H), 3.84 (s, 3H), 5.35-5.24 (m,1H), 5.50-5.45 (m, 1H), 6.42 (dd, 1H), 6.52 (d, 1H) 6.64 (d, 1H) 46-26 1H-NMR (400 MHz, CDCl3): 2.18-2.03 (m, 2H), 3.28-3.19 (m, 2H), 3.39-3.31 (m, 1H), 3.36 (s, 3H), 3.49-3.42 (m, 1H), 3.85 (s, 3H), 6.07 (dd, 1H), 6.16 (d, 1H), 6.66 (d, 1H) 46-27 1H-NMR (400 MHz, CDCl3): 1.48 (s, 9H), 1.88-1.71 (m, 2H), 1.97-1.82 (m, 2H), 2.78 (s, 3H), 2.84-2.64 (m, 2H), 3.55- 3.48 (m, 2H), 3.95 (s, 3H), 3.84 (s, 3H), 6.43 (d, 1H), 6.52 (bs, 1H), 6.64 (d, 1H) 46-28 1H-NMR (400 MHz, CDCl3): 3.02 (s, 3H), 3.33 (dd, 2H), 3.44 (t, 2H), 3.74 (s, 2H), 3.83(s, 3H), 6.38 (dd, 1H), 6.47 (d 1H), 6.66 (d, 1H) 46-29 1H-NMR (400 MHz, CDCl3): 1.78-1.38 (m, 2H), 1.96-1.89 (m, 2H), 2.30 (s, 3H), 2.39-2.31 (m, 1H), 2.55-2.42 (m, 4H), 2.71- 2.56 (m, 6H), 3.35-3.49 (m, 2H), 3.83 (s, 3H), 6.41 (dd, 1H), 6.52 (d, 1H) 6.63 (d, 1H) 46-30 1H-NMR (400 MHz, CDCl3): 3.80 (s, 3H), 3.82 (s, 3H), 3.82 (s, 3H), 6.40 (s, 1H), 6.54 (s, 1H) 46-31 1H-NMR (400 MHz, CDCl3): 3.20 (t, 2H), 4.57 (t, 2H), 6.55 (dd, 1H), 6.70-6.65 (m, 1H), 6.68 (d, 1H). Rf 040 (AcOEt) 46-32 1H-NMR (400 MHz, CDCl3): 2.98 (t, 4H), 3.62 (bs, 2H), 3.79 (s, 3H), 3.81 (s, 3H), 3.87 (t, 4H), 6.36 (s, 1H), 6.53 (s, 1H) 46-33 1H-NMR (400 MHz, CDCl3): 2.37 (s, 3H), 2.61 (t, 4H), 3.27 (t, 4H), 3.88 (s, 3H), 3.95 (s, 3H), 6.48 (s, 1H), 7.56 (s, 1H) 46-34 1H-NMR (400 MHz, CDCl3): 1.05 (t, 3H), 1.83 (ddd, 2H), 2.35 (s, 3H), 2.58 (t, 4H), 3.07 (t, 4H), 3.94 (t, 2H), 6.41 (dd, 1H), 6.51 (d, 1H), 6.65 (d, 1H) 46-35 1H-NMR (400 MHz, CDCl3): 1.28 (s, 3H), 1.30 (s, 3H), 2.04 (s, 2H), 2.17 (s, 3H), 2.84-2.72 (m, 2H), 3.20 (d, 2H), 3.86 (s, 3H), 6.41 (d, 1H), 6.46 (dd, 1H), 6.66 (d, 1H), 46-36 1H-NMR (400 MHz, CDCl3): 1.18 (t, 3H), 2.39 (dd, 2H), 3.07- 2.98 (m, 4H), 3.61 (t, 2H), 3.78 (t, 2H), 3.88 (s, 3H), 6.41 (dd, 1H), 6.51 (d, 1H), 6.65 (d, 1H) 46-37 1H-NMR (400 MHz, CDCl3): 1.15 (s, 3H), 1.16 (s, 3H), 2.83 (sept, 1H), 3.07-2.98 (m, 4H), 3.73-3.64 (m, 2H), 3.83-3.76 (m, 2H), 3.84 (s, 3H), 6.41 (dd, 1H), 6.51 (d, 1H), 6.65 (d, 1H) 46-38 1H-NMR (400 MHz, CDCl3): 2.84 (d, 3H), 3.02 (t, 4H), 3.51 (t, 4H), 3.84 (s, 3H), 4.48-4.38 (m, 1H), 6.41 (dd, 1H), 6.51 (d, 1H), 6.65 (d, 1H) 46-39 1H-NMR (400 MHz, CDCl3): 1.99-1.81 (m, 2H), 2.23-2.12 (m, 2H), 2.69-2.58 (m, 2H), 2.84 (d, 3H), 3.54-3.45 (m, 2H), 3.84 (s, 3H), 5.55-5.45 (m, 1H), 6.42 (dd, 1H), 6.52 (d, 1H), 6.64 (d, 1H) 46-40 1H-NMR (400 MHz, CDCl3): 1.53 (s, 6H), 2.11 (s, 3H), 3.05 (s, 2H), 3.28 (t, 2H), 3.64 (t, 2H), 3.86 (s, 3H), 6.26 (dd, 1H), 6.33 (d, 1H), 6.67 (d, 1H) 46-41 1H-NMR (400 MHz, CDCl3): 2.55-2.41 (m, 4H), 2.63 (t, 2H), 3.13 (t, 2H), 3.77-3.68 (m, 4H), 3.83 (s, 3H), 6.15 (dd, 1H), 6.25 (d, 1H), 6.62 (d, 1H) 46-42 1H-NMR (400 MHz, CDCl3): 2.05-2.00 (m, 2H), 2.39 (t, 2H), 3.57 (t, 2H), 3.64 (t, 2H), 3.83 (s, 3H), 4.04 (t, 2H), 6.32 (dd, 1H), 6.44 (d, 1H), 6.63 (d, 1H) 46-43 1H-NMR (400 MHz, CDCl3): 2.13 (s, 3H), 3.53-3.46 (m, 2H), 3.65-3.55 (m, 4H), 3.71-3.66 (m, 2H), 3.88 (s, 3H), 6.67 (d, 1H), 6.87 (dd, 1H), 6.95 (d, 1H) 46-44 1H-NMR (400 MHz, CDCl3): 3.73-3.61 (m, 8H), 3.87 (s, 3H), 6.65 (d, 1H), 6.86 (dd, 1H), 6.95 (d, 1H) 46-46 1H-NMR (400 MHz, CDCl3): 1.17 (s, 3H), 1.19 (s, 3H), 2.69 (t, 4H), 3.04 (s, 2H), 3.08 (t, 4H), 4.15-4.07 (m, 1H), 6.41 (dd, 1H), 6.51 (d, 1H), 6.65 (d, 1H), 7.01-6.94 (m 1H) 46-47 1H-NMR (400 MHz, CDCl3): 3.35-3.28 (m, 2H), 3.53-3.46 (m, 2H), 3.76 (s, 2H), 3.84 (s, 3H), 5.92-5.83 (m, 1H), 6.40 (dd, 1H), 6.48 (d, 1H), 6.67 (d,1H) 46-48 1H-NMR (400 MHz, CDCl3): 2.09-2.00 (m, 2H), 2.25-2.15 (m, 2H), 3.29-3.20 (m, 2H), 3.51-3.40 (m, 4H), 3.85 (s, 3H), 4.62- 4.55 (m, 1H), 6.08 (d, 1H), 6.18 (d, 1H), 6.67 (d, 1H) 46-49 1H-NMR (400 MHz, CDCl3): 1.52-1.40 (m, 2H), 1.90-1.84 (m, 2H), 2.68-2.59 (m, 2H), 3.51-3.45 (m, 2H), 3.84 (s, 3H), 6.44 (dd, 1H), 6.54 (d, 1H), 6.64 (d, 1H) 46-50 1H-NMR (400 MHz, CDCl3): 2.14 (s, 3H), 2.66 (s, 6H), 6.44 (d, 1H), 6.54 (d, 1H), 6.98 (t, 1H). 46-51 1H-NMR (400 MHz, CDCl3): 2.63 (s, 3H), 7.49-7.45 (m, 1H), 7.74-7.62 (m, 2H), 7.76 (dd, 1H), 8.24 (d, 1H), 8.77-8.64 (m, 2H). 46-52 1H-NMR (400 MHz, CDCl3): 3.84 (s, 3H), 3.88 (s, 3H), 6.78 (d, 1H), 6.83 (d, 1H), 7.00-6.89 (m, 3H), 7.45 (d, 1H). 46-53 1H-NMR (400 MHz, CDCl3): 1.40 (t, 3H), 3.03 (t, 4H), 3.84 (t, 4H), 4.00 (q, 2H), 6.27 (dd, 1H), 6.38 (d, 1H), 6.71 (dd, 1H), 46-54 1H-NMR (400 MHz, CDCl3): 1.26 (t, 3H), 3.02 (t, 4H), 3.85 (t, 4H), 4.05 (q, 2H), 6.40 (dd, 1H), 6.49 (d, 1H), 6.66 (d, 1H), 46-55 1H-NMR (400 MHz, CDCl3): 3.44 (s, 3H), 3.73 (s, 3H), 3.74- 3.68 (m, 2H), 3.95-3.85 (m, 2H), 4.10-4.05 (m, 2H), 6.21 (dd, 1H), 6.32 (d, 1H), 6.75 (d, 1H). 46-56 1H-NMR (400 MHz, CDCl3): 2.35-2.26 (m, 1H), 3.74 (s, 3H), 3.93-3.86 (m, 2H), 4.09-4.07 (m, 2H), 6.25 (dd, 1H), 6.34 (d, 1H), 6.76 (d, 1H). 46-57 1H-NMR (400 MHz, CDCl3): 1.40 (t, 3H), 3.71 (s, 3H), 4.00 (q, 2H), 6.22 (dd, 1H), 6.33 (d, 1H), 6.69 (d, 1H). 46-58 1H-NMR (400 MHz, CDCl3): 1.32 (d, 6H), 3.73 (s, 3H), 3.85-3.71 (m, 2H), 4.37 (sept, 1H), 6.22 (dd, 1H), 6.32 (d, 1H), 6.72 (d, 1H). 46-59 1H-NMR (400 MHz, CDCl3): 1.04 (t, 3H), 1.80 (ddd, 2H), 3.72 (s, 3H), 3.85-3.75 (m, 2H), 3.90 (t, 2H), 6.22 (dd, 1H), 6.33 (d, 1H), 6.69 (d, 1H). 46-60 1H-NMR (400 MHz, CDCl3): 2.94 (s, 6H), 3.89 (s, 3H), 6.16 (dd, 1H), 6.25 (d, 1H), 6.72 (d, 1H). 46-61 1H-NMR (400 MHz, CDCl3): 3.91 (s, 3H), 6.87 (d, 1H), 7.02 (dd, 1H), 7.05 (d, 1H), 7.44 (dd, 2H), 8.59 (dd, 2H). 46-62 1H-NMR (400 MHz, CDCl3): 3.91 (s, 3H), 6.88 (d, 1H), 6.96- 6.93 (m, 1H), 7.31 (ddd, 1H), 7.83-7.80 (m, 1H), 8.51 (dd, 1H), 8.78 (dd, 1H). 46-63 1H-NMR (400 MHz, CDCl3): 3.91 (s, 3H), 6.87 (dd, 1H), 7.16 (ddd, 1H), 7.34 (dd, 1H), 7.43 (d, 1H), 7.72-7.64 (m, 2H), 8.63-8.61 (m, 1H). 46-64 mp 148.6° C.; 1H-NMR (500 MHz, CDCl3) δ (ppm): 1.63 (m; 2H), 1.99 (m; 2H), 2.27 (m; 1H), 2.60 (m; 6H), 3.52 (m; 2H), 3.71 (m; 4H), 3.78 (s; 3H), 6.36 (dd; 1H); 6.52 (d; 1H), 6.73 (d; 1H). 46-65 -
- To a solution of 4-methyl-3-nitro-benzoic acid (300 mg, 2.76 mmol), N-butoxycarbonyl-piperazine (340 mg, 1.83 mmol) in DMF (3.0 mL), triethylamine (300 μL, 3.59 mmol), TBTU (800 mg, 2.49 mmol) and HOAt (270.5 mg, 1.99 mmol) are added and stirred at room temperature for 24 hours. The mixture is poured into water and extracted twice with ethyl acetate. The organic layer is washed with water and then brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1) to afford 4-(4-methyl-3-nitrobenzoyl)-piperazine-1-carboxylic acid tert-butyl ester as a colorless solid.
- 1H-NMR (δ, ppm): 1.47 (s, 9H), 2.64 (s, 3H), 3.88-3.28 (m, 8H), 7.42 (d, 1H), 7.56 (dd, 1H), 8.03 (d, 1H). Rf (hexane:ethyl acetate=10:1): 0.13.
- The title compound is obtained by reduction with hydrogen over 10% palladium on charcoal in methanol solution.
-
- To a suspension of 4-bromo-1-methyl-2-nitrobenzene (225 mg, 1.04 mmol), morpholine (125 μL, 1.25 mmol), and cesium carbonate (474.4 mg, 1.46 mmol) in toluene, palladium diacetate (31.2 mg, 0.139 mmol) and 2-(di-t-butylphosphino)biphenyl (125 mg, 0.403 mmol) are added and stirred at 100° C. for 5 hours. After cooling, the mixture is filtered to remove insoluble material. The filtrate is poured into water and extracted with ethyl acetate twice. The organic layer is washed with water and then brine, dried over magnesium sulfate, and evaporated in vacuo. The residue is purified with silica gel column chromatography (n-hexane:ethyl acetate=5:1) to afford 4-(4-methyl-3-nitrophenyl)-morpholine as a yellow solid.
- 1H-NMR (δ, ppm): 2.50 (s, 3H), 3.19-3.17 (m, 4H), 3.88-3.86 (m, 4H), 7.04 (dd, 1H), 7.21 (d, 1H), 7.47 (d, 1H). Rf (hexane:ethyl acetate=5:1): 0.20.
- The title compound is obtained by reduction with hydrogen over 10% palladium on charcoal in methanol solution.
-
- To a solution of 1.0 g (3.37 mmol) of 2-(2,5-dichloro-pyrimidin-4-ylamino)-N-methyl-benzamide in 15 mL of acetic acid are added 2-methoxy-4-morpholinoaniline dihydrochloride (1.9 g, 6.73 mmol) and 6.0 mL of 1N ethanolic solution of hydrogen chloride (6.0 mmol). After the reaction mixture is stirred at 120° C. for 16 hours and cooled to room temperature, aqueous NaHCO3 solution is added to adjust the acidity between pH 5 and pH 6. The resulting precipitate is collected by a filtration and dried under reduced pressure to give 2-[5-chloro-2-(2-methoxy-4-morpholin-4-yl-phenyl-amino)-pyrimidin-4-ylamino]-benzoic acid (970 mg, 2.12 mmol, 63%) as ivory solid.
- NMR (400 MHz, DMSO-d6, δ): 3.10-3.20 (m, 4H), 3.78 (s, 3H), 3.70-3.80 (m, 4H), 6.52 (dd, 1H, J=8.56, 2.52 Hz), 6.67 (d, 1H, J=2.52 Hz), 7.08 (dd, 1H, J=8.04, 8.04 Hz), 7.39 (d, 1H, J=8.56 Hz), 7.35-7.45 (m, 1H), 7.99 (dd, 1H, J=8.04, 1.52 Hz), 8.14 (s, 1H), 8.28 (s, 1H) 8.70-8.80 (m, 1H).
- To a solution of 2-amino-5-chloro-4-methyl-benzenesulfonic acid (3.0 g, 1.35 mmol) in dichloroethane (10 mL) is added sulfuryl chloride (4.4 mL, 3.83 mmol) and stirred at 60° C. After one hour, thionyl chloride (1.3 mL) is added and the mixture is further stirred at 100° C. for 7.0 hours. The mixture is poured into iced water and extracted with ether three times. The organic layer is washed with water and then brine, dried over sodium sulfate, and evaporated in vacuo.
- 1H-NMR (δ, ppm): 2.35 (s, 3H), 6.68 (s, 1H), 7.75 (s, 1H).
- This substituted sulfonyl chloride is reacted with a suitable amine. On reaction e.g. with methylamine, 2-amino-5-chloro-4,N-dimethylbenzenesulfonamide is formed.
-
- To a solution of 2-[5-Bromo-2-(2-methoxy-4-morpholin-4-yl-phenylamino)-pyrimidin-4-ylamino]-N-methyl-benzenesulfonamide (Ex3-19) (1.0 g, 1.82 mmol) in DMF (10 mL), potassium carbonate (300 mg, 2.17 mmol) and iodomethane (116 μl, 1.86 mmol) are added. The resulting suspension is stirred at 50° C. for 1 h. To the reaction mixture, water is added and extracted with ethyl acetate three times. The organic layer is washed with water, dried over sodium sulfate, and concentrated in vacuo. The residue is purified by aluminum oxide column chromatography (AcOEt) to afford the title compound (728 mg, 71% yield).
- NMR (400 MHz, CDCl3, δ): 2.74 ((s, 6H), 3.05-3.18 (m, 4H), 3.84-3.93 (m, 4H), 3.88 (s, 3H), 6.43 (dd, 1H), 6.53 (d, 1H), 7.24 (m, 1H), 7.31 (s, 1H), 7.56 (m, 1H), 7.87 (dd, 1H), 8.05 (d, 1H), 8.21 (s, 1H), 8.49 (d, 1H), 8.49 (d, 1H), 9.27 (s, 1H). Rf: 0.23 (AcOEt:Hexane=1:1).
-
- To a solution of chlorosulfonylisocyanate (1.2 mL, 13.5 mmol) in nitroethane (10 mL), 4-fluoroaniline (1.0 g, 8.97 mmol) is added dropwise at 0° C. and the reaction mixture is stirred for 30 min. To the solution, aluminum chloride (1.3 g, 9.87 mmol) is added at 0° C. and the mixture is stirred at 100° C. for 1 hour. After cooling to room temperature, water is added and the mixture is extracted with ethyl acetate twice. The organic layer is washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The resulting solids are collected by a filtration and washed with ether to give slightly gray solids (803.9 mg, 41%).
- NMR (400 MHz, DMSO-d6, δ): 7.22-7.28 (m, 1H), 7.45-7.57 (m, 1H), 7.60 (m, 1H), 11.15-11.30 (m, 1H). Rf: 0.43 (MeOH:AcOEt=1:5).
- To a solution of 7-Fluoro-1,1-dioxo-1,4-dihydro-2H-1-λ6-benzo[1,2,4]thiadiazin-3-one (5.19 g, 24.0 mmol) in DMF (50 mL), sodium hydride (1.04 g, 26.0 mmol) and iodomethane (1.5 mL, 24.0 mmol) are added successively and the mixture is stirred for 1 hour at 70° C. After cooling to room temperature, the mixture is poured into water and the precipitate is collected by a filtration and washed with water and hexane, successively, to give slightly gray solids (5.38 g, 94%).
- NMR (400 MHz, DMSO-d6, δ): 3.32 (s, 3H), 7.44 (dd, 1H), 7.75 (ddd, 1H), 7.94 (dd, 1H).
- Rf (MeOH:AcOEt=1:5): 0.21. Rf: 0.39 (Hexane:AcOEt=1:1).
- 6.79 g of 7-Fluoro-2-methyl-1,1-dioxo-1,4-dihydro-2H-1 λ6-benzo[1,2,4]thiadiazin-3-one (29.5 mmol) is dissolved in 20% aq. sodium hydroxide and the resulting solution is stirred at 100° C. for 13.5 hours. The mixture is cooled to room temperature and poured into water. 78 mL of 5M HCl aq. is added and the precipitate is collected by a filtration and washed with water to afford slightly purple solids (3.96 g, 65%).
- NMR (400 MHz, CDCl3, • δ): 2.60 (d, 3H), 4.55-4.82 (m, 3H), 6.74 (dd, 1H), 7.05-7.12 (m, 1H), 7.45 (dd, 1H). Rf: 0.41 (Hexane:AcOEt=1:1).
- The reaction of pyrimidine with 2-Amino-5-fluoro-N-methyl-benzenesulfonamide is performed in the same manner described in example B.
- NMR (400 MHz, CDCl3, δ): 2.67 (d, 3H), 4.56 (m, 1H), 7.36-7.45 (m, 1H), 7.68 (dd, 1H), 8.39 (s, 1H), 8.42 (dd, 1H), 9.26 (s, 1H). Rf 0.59 (Hexane:AcOEt=1:1).
- The introduction of substituted aniline is performed according to the manner described in Example A.
- NMR (400 MHz, CDCl3, δ): 2.65 (d, 3H), 3.09-3.16 (m, 4H), 3.87 (s, 3H), 4.50 (q, 1H), 6.41 (dd, 1H), 6.52 (d, 1H), 7.25-7.33 (m, 2H), 7.69 (dd, 1H), 7.95 (d, 1H), 8.20 (s, 1H), 8.37 (dd, 1H), 8.70 (s, 1H). Rf 0.30 (Hexane:AcOEt=1:1)
-
-
Physical Data Compound 1H NMR 400 MHz Number Structure (DMSO-d6) and/or MS (m/z ) 5 1H NMR 400 MHz (CDCl3) δ 12.5 (s, br, 1H), 10.3 (s, 1H), 8.39 (d, 1H, J = 8.38 Hz), 7.97 (s, 1H), 7.87 (dd, 1H, J = 1.63, 7.55 Hz), 7.49 (d, 1H, J = 8.69 Hz), 6.54 (d, 1H, J = 2.49 Hz), 6.43 (dd, 1H, J = 2.51, 8.73 Hz), 4.70 (s, 1H), 4.04 (q, 2H, J = 6.98 Hz), 3.51 (m, 2H), 3.29 (d, 1H, J = 19.2 Hz), 3.18 (m, 2H), 2.89 (d, 3H, J = 3.26 Hz), 2.77 (s, 6H), 2.39 (m, 2H), 2.22 (m, 2H), 1.40 (t, 3H, J = 6.97 Hz). MS m/z 561.4 (M + 1) 6 MS m/z 589.4 (M + 1) 7 MS m/z 633.4/635.4 (M + 1). 8 MS m/z 591.3/593.3 (M + 1). 9 MS m/z 547.4 (M + 1). 10 1H NMR 400 MHz (acetone- d6) δ 9.45 (br, 1H), 8.88 (br, 1H), 8.23 (m, 1H), 8.07 (s, 1H), 7.83 (m, 1H), 7.72 (dd, 1H, J = 1.25, 7.96 Hz), 7.45 (m, 1H), 7.14 (m, 2H), 6.86 (m, 1H), 6.79 (br, 1H), 6.62 (m, 1H), 6.13 (m, 1H), 3.97 (t, 2H, J = 6.98 Hz), 3.63 (m, 2H), 3.21 (m, 2H), 2.44 (m, 1H), 2.34 (s, 3H), 1.99 (m, 4H), 1.17 (t, 3H, J = 6.98 Hz). MS m/z 560.4 (M + 1). 11 MS m/z 561.4 (M + 1). 12 MS m/z 591.3/593.3 (M + 1). 13 MS m/z 547.4 (M + 1). 14 MS m/z 559.4 (M + 1). 15 MS m/z 564.5 (M + 1). 16 MS m/z 575.4 (M + 1). 17 MS m/z 605.3/607.3 (M + 1). 18 MS m/z 561.4 (M + 1). 19 MS m/z 532.4 (M + 1). 20 1H NMR 400 MHz (acetone- d6) δ 8.60 (d, = 1H, J = 8.27 Hz), 8.40 (s, 1H), 8.00 (dd, J 1.55, 7.95 Hz), 7.76 (m, 2H), 7.49 (m, 1H), 7.08 (d, J = 8.94 Hz, 1H), 6.84 (dd, J = 3.00, 8.91 Hz, 1H), 4.50 (t, J = 4.99 Hz, 2H), 4.08 (m, 2H), 4.00 (m, 2H), 3.90 (s, 3H), 3.78 (m, 4H), 3.37 (m, 4H), 1.76 (m, 2H), 0.99 (t, J = 7.44 Hz, 3H). MS m/z 562.4 (M + 1). 21 MS m/z 560.4 (M + 1). 22 1H NMR 600 MHz (acetone- d6) δ 11.20 (br, 1H), 9.75 (d, J = 15.25 Hz, 1H), 8.46 (m, 2H), 8.04 (dd, J = 1.12, 7.93 Hz, 1H), 7.71 (m, 1H), 7.69 (m, 1H), 7.47 (t, J = 7.63 Hz, 1H), 7.06 (m, 1H), 7.02 (m, 1H), 4.07 (m, 2H), 3.99 (s, 3H), 3.77 (m, 2H), 3.67 (m, 1H), 3.27 (m, 2H), 3.13 (t, J = 12.2 Hz, 2H), 2.85 (m, 2H), 2.46 (m, 2H), 2.26 (m, 3H), 2.08 (m, 3H), 1.95 (m, 1H), 1.75 (m, 1H), 1.66 (m, 1H), 0.89 (d, J = 6.65 Hz, 6H). MS m/z 672.4/674.4 (M + 1). 23 MS m/z 600.4 (M + 1). 24 MS m/z 586.4 (M + 1). 25 MS m/z 642.5 (M + 1). 26 MS m/z 640.5 (M + 1). 27 MS m/z 630.3/632.3 (M + 1). 28 MS m/z 633.4/635.3 (M + 1). 29 MS m/z 603.4 (M + 1). 30 MS m/z 601.4 (M + 1). 31 MS m/z 603.4 (M + 1). 32 MS m/z 601.4 (M + 1). 33 MS m/z 575.4 (M + 1). 34 MS m/z 561.4 (M + 1). 35 1H NMR 600 MHz (acetone- d6) δ 8.47(d, J = 7.13, 1H), 8.43 (s, 1H), 8.09 (d, J = 7.85 Hz, 1H), 7.83 (m, 1H), 7.73 (t, J = 7.67 Hz, 1H), 7.50 (m, 1H), 6.87 (m, 1H), 6.71 (m, 1H), 5.46 (d, J = 31.2 Hz, 1H), 4.22 (m, 3H), 3.55 (m, 5H), 2.51 (m, 1H), 2.49 (m, 1H), 2.21 (m, 1H), 1.50 (m, 6H), 1.10 (d, J = 6.56 Hz, 6H) .MS m/z 575.4 (M + 1). 36 MS m/z 574.4 (M + 1). 37 MS m/z 562.4 (M + 1). 38 MS m/z 560.4 (M + 1). 39 MS m/z 546.4 (M + 1). 40 MS m/z 560.4 (M + 1). 41 MS m/z 559.4 (M + 1). 42 MS m/z 547.4 (M + 1). 43 MS m/z 559.4 (M + 1). 44 MS m/z 600.4 (M + 1). 45 MS m/z 586.4 (M + 1). 46 MS m/z 600.4 (M + 1). 47 MS m/z 599.4 (M + 1). 48 MS m/z 587.4 (M + 1). 49 1H NMR 600 MHz (acetone- d6) δ 10.35 (m, 1H), 8.72 (s, br, 1H), 8.42 (s, 1H), 8.00 (d, J = 7.93 Hz, 1H), 7.73 (m, 1H), 7.64 (m, 1H), 7.50 (t, J = 7.77 Hz, 1H), 6.55 (s, 1H), 6.43 (d, J = 8.51 Hz, 1H), 4.31 (m, 1H), 4.22 (q, J = 6.93 Hz, 2H), 3.97 (m, 2H), 3.80 (t, J = 8.45 Hz, 1H), 3.58 (q, J = 8.38 Hz, 1H), 3.36 (m, 1H), 3.17 (s, 1H), 2.78 (m, 1H), 2.66 (m, 1H), 2.15 (m, 2H), 1.73 (d, J = 12.9 Hz, 2H), 1.59 (d, J = 12.9 Hz, 1H), 1.55 (m, 2H), 1.43 (m, 3H), 1.35 (m, 2H), 1.25 (m, 1H). MS m/z 599.4 (M + 1). 50 MS m/z 562.4 (M + 1). 51 MS m/z 614.5 (M + 1). 52 MS m/z 640.5 (M + 1). 53 MS m/z 640.5 (M + 1). 54 MS m/z 628.5 (M + 1). 55 MS m/z 600.4 (M + 1). 56 MS m/z 644.4/646.4 (M + 1). 57 MS m/z 613.5 (M + 1). 58 MS m/z 578.5 (M + 1). 59 MS m/z 560.4 (M + 1). 60 MS m/z 586.4 (M + 1). 61 MS m/z 586.4 (M + 1). 62 MS m/z 574.4 (M + 1). 63 MS m/z 546.4 (M + 1). 64 MS m/z 590.4/592.4 (M + 1). 65 MS m/z 559.4 (M + 1). 66 1H NMR 600 MHz (CD3OD) δ 8.65 (br, 1H), 8.03 (br, 1H), 7.78 (d, J = 7.76 Hz, 1H), 7.49 (br, 1H), 7.30 (s, 1H), 6.38 (m, 1H) 6.33 (dd, J = 1.69, 8.64 Hz, 1H), 4.12 (q, J = 6.96 Hz, 2H), 4.06 (m, 1H), 3.71 (dd, J = 6.68, 10.74 Hz, 1H), 3.66 (m, 1H), 3.56 (dd, J = 4.71, 10.76 Hz, 1H), 3.42 (m, 3H), 3.22 (q, J = 7.28 Hz, 2H), 2.57 (m, 1H), 2.26 (m, 1H), 1.39 (t, J = 7.28 Hz, 3H), 1.34 (t, J = 6.96 Hz, 3H). MS m/z 524.4 (M + 1). 67 MS m/z 574.4 (M + 1). 68 1H NMR 600 MHz (CD3OD) δ 8.23 (s, 1H), 8.18 (d, J = 8.16 Hz, 1H), 7.98 (d, J = 8.44 Hz, 2H), 7.68 (m, 1H), 7.43 (m, 1H), 7.22 (d, J = 2.29 Hz, 1H), 6.97 (dd, J = 2.30, 8.81 Hz, 1H), 4.20 (q, J = 6.99 Hz, 2H), 3.77 (m, 2H), 3.58 (m, 2H), 2.75 (d, J = 7.03 Hz, 2H), 2.72 (m, 1H), 2.18 (m, 4H), 1.47 (t, J = 6.97 Hz, 3H), 0.75 (m, 1H), 0.30 (m, 2H), 0.00 (m, 2H). MS m/z 600.4 (M + 1). 69 MS m/z 600.4 (M + 1). 70 MS m/z 588.4 (M + 1). 71 MS m/z 560.4 (M + 1). 72 MS m/z 604.3/606.3 (M + 1). 73 MS m/z 573.4 (M + 1). 74 MS m/z 538.4 (M + 1). 75 MS m/z 573.4 (M + 1). 76 MS m/z 578.5 (M + 1). 77 MS m/z 613.5 (M + 1). 78 MS m/z 511.4 (M + 1). 79 MS m/z 525.5 (M + 1). 80 MS m/z 524.5 (M + 1). 81 MS m/z 510.5 (M + 1). 82 MS m/z 525.4 (M + 1). 83 MS m/z 627.5 (M + 1). 84 1H NMR 600 MHz (CD3OD) δ 9.18 (m, 2H), 8.05 (d, J = 7.93 Hz, 1H), 7.93 (t, J = 7.64 Hz, 1H), 7.78 (m, 1H), 7.68 (m, 2H), 7.58 (t, J = 7.54 Hz, 1H), 4.72 (s, 2H), 4.07 (d, J = 12.4 Hz, 2H), 3.34 (br, 4H), 2.96 (t, J = 12.3 Hz, 2H), 2.87 (s, 6H), m, 2H), 2.28 (s, 1H), 2.00 (br, 7H), 1.81 (br, 2H). MS m/z 627.5 (M + 1). 85 MS m/z 591.5 (M + 1). 86 MS m/z 601.5 (M + 1). 87 MS m/z 602.4 (M + 1). 88 MS m/z 566.5 (M + 1). 89 MS m/z 656.5 (M + 1). 90 1H NMR 600 MHz (CD3OD) δ 8.16 (d, J = 8.16 Hz, 1H), 8.23 (s, 1H), 7.94 (dd, J = 1.42, 7.98 Hz, 1H), 7.79 (d, J = 9.24 Hz, 1H), 7.72 (m, 1H), 7.48 (m, 1H), 7.15 (d, J = 2.53 Hz, 1H), 7.03 (dd, J = 2.58, 8.91 Hz, 1H), 4.00 (s, 1H), 3.82 (d, J = 12.63 Hz, 2H), 3.50 (s, br, 4H), 3.29 (m, 5H), 3.19 (m, 1H), 2.96 (s, 3H), 2.73 (s, 6H), 2.25 (d, J = 12.40 Hz, 2H), 2.03 (m, 2H), 1.40 (s, 9H). MS m/z 657.5 (M + 1). 91 MS m/z 621.6 (M + 1). 92 1H NMR 600 MHz (CD3OD) δ 8.31 (d, J = 7.65 Hz, 1H), 8.19 (s, 1H), 7.94 (dd, J = 1.33, 7.96 Hz, 1H), 7.67 (t, J = 7.70 Hz, 1H), 7.49 (t, J = 7.68 Hz, 1H), 7.44 (d, J = 8.56 Hz, 1H), 6.83 (d, J = 1.37 Hz, 1H), 6.65 (d, J = 8.66 Hz, 1H), 4.69 (m, 1H), 4.11 (br, 2H), 3.91 (d, J = 12.68 Hz, 2H), 3.83 (br, 2H), 3.58 (br, 2H), 3.46 (m, 1H), 3.23 (br, 2H), 3.00 (t, J = 12.19 Hz, 2H), 2.73 (s, 6H), 2.33 (d, J = 12.14 Hz, 2H), 1.97 (m, 2H), 1.30 (d, J = 6.04 Hz, 6H). MS m/z 630.5 (M + 1). 93 MS m/z 629.5 (M + 1). 94 MS m/z 594.5 (M + 1). 95 MS m/z 471.1 (M + 1). 96 1H NMR 600 MHz (DMSO-d6) δ 11.7 (bs, 1H), 11.14 (bs, 1H), 9.50 (b, 2H), 7.89 (d, 1H, J = 9.1 Hz), 7.72 ( m, 2H), 7.55 (m, 2H), 7.18 (m, 1H), 6.62 (s, 1H), 6.46 (m, 1H), 4.06 (q, 2H, J = 6.6 Hz), 3.73 (t, 4H, J = 4 .8 Hz), 3.11 (m, 4H), 2.40 (m, 3H), 1.24 (m, 3H); MS m/z 485.1 (M + 1). 97 MS m/z 577.1 (M + 1). 98 1H NMR 600 MHz (DMSO-d6) δ 9.97 (bs, 1H), 9.34 (s, 1H), 8.48 (bs, 1H), 8.34 (bs, 1H), 8.28 (s, 1H), 7.80 (q, 1H, J = 4.8 Hz), 7.77 (d, 1H, J = 7.8 Hz), 7.48 (m, 1H), 7.39 (d, 1H, J = 8.4 Hz,), 7.25 (t, 1H, J = 7.2 Hz), 6.71 (s, 1H), 6.49 (d, 1H, J = 8.4 Hz), 3.93 (t, 2H, J = 6.0 Hz), 3.85 (d, 2H, J = 12 Hz), 3.54 (d, 2H, J = 12 Hz), 3.18 (q, 2H, J = 9.0 Hz), 2.94 (t, 2H, J = 11 Hz), 2.88 (s, 3H), 2.42 (d, 3H, J = 4.8 Hz), 1.64 (m, 2H), 0.85 (t, 3H, J = 7.2 Hz); MS m/z 590.1 (M + 1). 99 MS m/z 578.1 (M + 1). 100 MS m/z 593.1 (M + 1). 101 1H NMR 600 MHz (DMSO- d6) δ 10.0 (bs, 1H), 9.34 (s, 1H), 8.47 (bs, 1H), 8.33 (bs, 1H), 8.30 (s, 1H), 7.79 (s, 1H), 7.78 (d, 1H, J = 7.2 Hz), 7.50 (m, 2H), 7.27 (t, 1H, J = 7.8 Hz,), 6.75 (s, 1H), 6.53 (d, 1H, J = 7.8 Hz), 4.12 (t, 2H, J = 4.8 Hz), 3.84 (d, 2H, J = 12 Hz), 3.59 (t, 2H, J = 4.8 Hz), 3.53 (d, 2H, J = 12 Hz), 3.23 (s, 3H), 3.17 (q, 2H, J = 9.0 Hz), 2.94 (t, 2H, J = 11 Hz), 2.88 (s, 3H), 2.43 (d, 3H, J = 4.8 Hz); MS m/z 606.1 (M + 1). 102 MS m/z 594.1 (M + 1). 103 MS m/z 547.1 (M + 1). 104 MS m/z 533.1 (M + 1). 105 MS m/z 496.4 (M + 1). 106 1H NMR 400 MHz (DMSO- d6) δ 9.35 (s, 1H), 9.02 (s, 1H), 8.52 (s, 1H), 8.17 (bs, 1H), 7.80 (m, 2H), 7.45 (bs, 1H), 7.29 (m, 2H), 6.66 (s, 1H), 6.47 (d, 1H, J = 8.0 Hz), 4.02 (q, 2H, J = 7.2 Hz), 3.76 (t, 4H, J = 4.8 Hz), 3.14 (t, 4H, J = 4.8 Hz), 2.41 (d, 3H, J = 4.0 Hz), 1.23 (t, 3H, J = 7.2 Hz); MS m/z 510.4 (M + 1). 107 MS m/z 524.5 (M + 1). 108 1H NMR 600 MHz (DMSO- d6) δ 9.61 (s, 1H), 9.10 (bs, 1H), 8.35 (bs, 1H), 8.18 (s, 1H), 7.77 (m, 2H), 7.41 (bs, 1H), 7.26 (t, 1H, J = 7.2 Hz), 7.15 (d, 1H, J = 7.8 Hz), 6.89 (s, 1H), 6.85 (d, 1H, J = 9.6 Hz), 3.77 (t, 4H, J = 4.8 Hz), 3.15 (t, 4H, J = 4.8 Hz), 2.52 (q, 2H, J = 7.2 Hz), 2.41 (d, 3H, J = 4.8 Hz), 1.06 (t, 3H, J = 7.2 Hz); MS m/z 503.2 (M + 1). 109 MS m/z 547.2 (M + 1). 110 MS m/z 494.2 (M + 1). 111 MS m/z 557.3 (M + 1). 112 MS m/z 559.3 (M + 1). 113 MS m/z 561.2 (M + 1). 114 MS m/z 508.2 (M + 1). 115 MS m/z 529.2 (M + 1). 116 1H NMR 400 MHz (DMSO- d6) δ 9.91 (bs, 1H), 9.50 (s, 1H), 8.94 (s, 1H), 8.49 (bs, 1H), 8.17 (s, 1H), 7.74 (d, 1H, J = 7.6 Hz), 7.41 (bs, 1H), 7.23 (t, 1H, J = 7.2 Hz), 7.18 (d, 1H, J = 8.0 Hz), 6.89 (m, 2H), 3.84 (d, 2H, J = 11.4 Hz), 3.54 (d, 2H, J = 11.4 Hz), 3.18 (q, 2H, J = 8.0 Hz), 2.95 (t, 2H, J = 11.4 Hz), 2.88 (s, 3H), 2.64 (s, 6H), 2.53 (m, 2H), 1.06 (t, 3H, J = 8.0 Hz); MS m/z 530.2 (M + 1). 117 MS m/z 556.2 (M + 1). 118 MS m/z 556.2 (M + 1). 119 MS m/z 548.2 (M + 1). 120 1H NMR 400 MHz (DMSO- d6) δ 10.24 (bs, 1H), 9.54 (s, 1H), 8.50 (bs, 1H), 8.41 (d, 1H, J = 7.2 Hz), 8.21 (s, 1H), 7.80 (m, 2H), 7.50 (t, 1H, J = 7.2 Hz), 7.32 (d, 1H, J = 8.4 Hz), 7.28 (t, 1H, J = 8.0 Hz), 6.32 (d, 1H, J = 2.4 Hz), 6.17 (dd, 1H, J1 = 2.4 Hz, J2 = 8.4 Hz), 4.61 (m, 2H), 4.01 (m, 1H), 3.60 (m, 1H), 3.48 (m, 2H), 3.25 (q, 1H, J = 8.8 Hz), 2.88 (s, 6H), 2.42 (d, 3H, J = 4.8 Hz), 2.19 (m, 1H), 1.20 (d, 6H, J = 4.8 Hz); MS m/z 560.2 (M + 1). 121 MS m/z 600.2 (M + 1). 122 MS m/z 562.2 (M + 1). 123 MS m/z 574.2 (M + 1). 124 MS m/z 575.2 (M + 1). 125 MS m/z 600.2 (M + 1). 126 MS m/z 574.2 (M + 1). 127 MS m/z 573.2 (M + 1). 128 MS m/z 604.1 (M + 1). 129 MS m/z 576.2 (M + 1). 130 MS m/z 588.2 (M + 1). 131 MS m/z 561.2 (M + 1). 132 MS m/z 561.1 (M + 1). 133 MS m/z 589.2 (M + 1). 134 MS m/z 575.2 (M + 1). 135 MS m/z 574.2 (M + 1). 136 MS m/z 601.2 (M + 1). 137 MS m/z 560.2 (M + 1). 138 MS m/z 575.2 (M + 1). 139 1H NMR 600 MHz (DMSO- d6) δ 10.35 (bs, 1H), 9.45 (s, 1H), 8.47 (d, 1H, J = 7.2 Hz), 8.33 (s, 1H), 8.23 (s, 1H), 7.79 (d, 1H, J = 8.4 Hz), 7.57 (t, 1H, J = 7.2 Hz), 7.52 (d, 1H, J = 9.6 Hz), 7.23 (t, 1H, J = 7.2 Hz), 6.68 (s, 0.4H), 6.64 (s, 0.6H), 6.50 (s, 0.6H), 6.49 (s, 0.4H), 5.19 (s, 0.6H), 5.15 (s, 0.4H), 4.58 (m, 1H), 3.99 (m, 0.4H), 3.73 (m, 1.6H), 3.36 (m, 0.6H), 3.26 (m, 0.8H), 3.15 (m, 0.6H), 2.96 (s, 1.2H), 2.89 (s, 1.8H), 2.64 (s, 6H), 2.60 (m, 1H), 2.24 (m, 0.6H), 2.07 (m, 0.4H), 1.20 (d, 6H, J = 6.0 Hz); MS m/z 561.2 (M + 1). 140 MS m/z 587.2 (M + 1). 141 MS m/z 587.2 (M + 1). 142 MS m/z 560.2 (M + 1). 143 MS m/z 575.2 (M + 1). 144 MS m/z 561.2 (M + 1). 145 MS m/z 587.2 (M + 1). 146 MS m/z 587.2 (M + 1). 147 1H NMR 600 MHz (DMSO- d6) δ 9.81 (bs, 0.4H), 9.74 (bs, 0.6H), 9.59 (bs, 1H), 8.48 (bs, 1H), 8.34 (s, 0.4H), 8.33 (s, 0.6H), 8.232 (s, 0.6H), 8.228 (s, 0.4H), 7.82 (d, 1H, J = 7.8 Hz), 7.59 (m, 1H), 7.49 (d, 0.6H, J = 8.4 Hz), 7.47 (d, 0.4H, J = 8.4 Hz), 7.34 (q, 1H, J = 7.2 Hz), 6.75 (s, 1H), 6.68 (s, 1H), 6.57 (dd, 0.6H, J1 = 2.4 Hz, J2 8.4 Hz), 6.54 (dd, 0.4H, J1 = 2.4 Hz, J2 = 8.4 Hz), 4.73 (m, 0.6H), 4.58 (m, 1H), 4.52 (m, 0.4H), 3.52 (m, 1H), 3.43 (m, 1H), 3.35 (m, 0.4H), 3.33 (m, 0.6H), 3.21 (m, 1H), 3.11 (m, 1H), 2.85 (d, 1.8H, J = 4.8 Hz), 2.82 (d, 1.2H, J = 3.0 Hz), 2.25 (m, 1H), 2.07 (m, 0.4H), 2.05 (m, 0.6H), 1.98 (m, 1H), 1.76 (m, 1H), 1.20 (d, 3.6H, J = 6.0 Hz), 1.18 (d, 2.4H, J = 6.0 Hz), 1.15 (d, 6H, J = 7.2 Hz); MS m/z 574.2 (M + 1). 148 MS m/z 589.3 (M + 1). 149 MS m/z 575.2 (M + 1). 150 MS m/z 601.3 (M + 1). 151 MS m/z 601.3 (M + 1). 152 MS m/z 559.1 (M + 1). 153 1H NMR 400 MHz (DMSO- d6) δ 10.07 (bs, 1H), 9.44 (s, 1H), 8.54 (s, 1H), 8.52 (bs, 1H), 8.29 (s, 1H), 7.91 (s, 1H), 7.79 (d, 1H, J = 8.0 Hz), 7.61 (t, 1H, J = 7.6 Hz), 7.32 (t, 1H, J = 7.6 Hz), 7.22 (d, 1H, J = 8.8 Hz), 7.14 (d, 1H, J = 8.8 Hz), 4.10 (m, 2H), 3.86 (s, 3H), 3.46 (m, 2H), 3.22 (m, 2H), 3.08 (m, 2H), 2.83 (s, 3H), 2.66 (s, 6H); MS m/z 560.1 (M + 1). 154 MS m/z 586.1 (M + 1). 155 MS m/z 586.1 (M + 1). 156 MS m/z 587.2 (M + 1). 157 1H NMR 400 MHz (DMSO- d6) δ 10.05 (bs, 1H), 9.42 (s, 1H), 8.43 (d, 1H, J = 7.6 Hz), 8.35 (s, 1H), 8.31 (s, 1H), 7.91 (s, 1H), 7.79 (dd, 1H, J1 = 1.6 Hz, J2 = 8.0 Hz), 7.58 (t, 1H, J = 8.0 Hz), 7.32 (t, 1H, J = 8.4 Hz), 7.15 (m, 2H), 4.70 (m, 1H), 4.07 (m, 2H), 3.45 (m, 2H), 3.21 (m, 2H), 3.06 (m, 2H), 2.83 (s, 3H), 2.65 (s, 6H), 1.27 (d, 6H, J = 6.0 Hz); MS m/z 588.2 (M + 1). 158 MS m/z 614.2 (M + 1). 159 MS m/z 573.2 (M + 1). 160 1H NMR 600 MHz (DMSO- d6) δ 9.45 (s, 1H), 8.44 (d, 1H, J = 7.8 Hz), 8.38 (s, 1H), 8.27 (s, 1H), 7.81 (d, 1H, J = 7.8 Hz), 7.66 (d, 1H, J = 8.4 Hz), 7.64 (t, 1H, J = 7.2 Hz), 7.35 (t, 1H, J = 7.2 Hz), 6.97 (s, 1H), 6.76 (d, 1H, J = 8.4 Hz), 4.06 (q, 2H, J = 7.2 Hz), 3.59 (m, 6H), 3.29 (m, 4H), 2.92 (m, 2H), 2.86 (s, 3H), 2.64 (s, 6H), 1.31 (t, 3H, J = 6.6 Hz); MS m/z 574.2 (M + 1). 161 MS m/z 600.2 (M + 1). 162 MS m/z 600.2 (M + 1). 163 MS m/z 627.5 (M + 1). 164 MS m/z 628.5 (M + 1). 165 MS m/z 654.5 (M + 1). 166 MS m/z 654.5 (M + 1). 167 MS m/z 592.3 (M + 1). 168 MS m/z 588.2 (M + 1). 169 MS m/z 614.2 (M + 1). 170 MS m/z 574.2 (M + 1). 171 1H NMR 600 MHz (DMSO- d6) δ 9.66 (s, 1H), 8.48 (bs, 1H), 8.40 (d, 1H, J = 8.8 Hz), 8.30 (s, 1H), 7.66 (dd, 1H, J = 1.6 Hz, J2 = 7.6 Hz), 7.71 (t, 1H, J = 8.4 Hz), 7.46 (s, 1H), 7.41 (t, 1H, J = 8.0 Hz), 7.17 (bs, 1H), 6.96 (s, 1H), 6.93 (bs, 1H), 4.67 (m, 1H), 4.02 (q, 1H, J = 7.2 Hz), 3.65 (m, 2H), 3.46 (m, 1H), 3.34 (m, 1H), 2.54 (m, 1H), 1.94 (m, 4H), 1.26 (d, 6H, J = 6.0 Hz), 1.15 (d, 6H, J = 6.0 Hz); MS m/z 587.2 (M + 1). 172 1H NMR 600 MHz (DMSO- d6) δ 9.63 (bs, 1H), 8.50 (bs, 1H), 8.46 (bs, 1H), 8.29 (s, 1H), 7.82 (d, 1H, J = 7.2 Hz), 7.61 (t, 1H, J = 7.2 Hz), 7.43 (d, 1H, J = 8.4 Hz), 7.34 (t, 1H, J = 7.2 Hz), 6.75 (s, 1H), 6.58 (d, 1H, J = 6.6 Hz), 4.04 (q, 2H, J = 7.2 Hz), 3.82 (m, 2H), 3.76 (m, 5H), 3.44 (m, 1H), 3.22 (m, 4H), 2.85 (s, 3H), 2.82 (m, 2H), 2.09 (m, 2H), 1.71 (m, 2H), 1.25 (t, 3H, J = 7.2 Hz), 1.16 (d, 6H, J = 7.2 Hz); MS m/z 628.2 (M + 1). 173 MS m/z 615.2 (M + 1). 174 MS m/z 659.1 (M + 1). 175 1H NMR 600 MHz (DMSO- d6) δ 9.43 (s, 1H), 8.45 (bs, 1H), 8.38 (bs, 1H), 8.24 (s, 1H), 7.91 (d, 1H, J = 8.4 Hz), 7.84 (d, 1H, J = 8.4 Hz), 7.51 (t, 1H, J = 7.2 Hz), 7.48 (d, 1H, J = 8.4 Hz), 7.26 (t, 1H, J = 7.2 Hz), 6.76 (s, 1H), 6.55 (d, 1H, J = 7.2 Hz), 4.05 (q, 2H, J = 6.6 Hz), 3.82 (m, 2H), 3.59 (m, 5H), 3.30 (m, 1H), 3.25 (m, 4H), 2.85 (s, 3H), 2.82 (m, 2H), 2.09 (m, 2H), 1.71 (m, 2H), 1.26 (t, 3H, J = 6.6 Hz), 0.92 (d, 6H, J = 7.2 Hz); MS m/z 643.2 (M + 1). 176 MS m/z 642.2 (M + 1). 177 MS m/z 643.3 (M + 1). 178 MS m/z 669.3 (M + 1). 179 MS m/z 633.3 (M + 1). 180 MS m/z 629.2 (M + 1). 181 MS m/z 669.2 (M + 1). 182 MS m/z 642.2 (M + 1). 183 MS m/z 615.3 (M + 1). 184 1H NMR 400 MHz (DMSO- d6) δ 9.94 (bs, 1H), 9.53 (s, 1H), 8.49 (m, 2H), 8.23 (s, 1H), 7.79 (dd, 1H, J1 = 2.0 Hz, J2 = 8.0 Hz), 7.58 (t, 1H, J = 7.6 Hz), 7.38 (d, 1H, J = 9.2 Hz), 7.32 (t, 1H, J = 8.0 Hz), 6.68 (d, 1H, J = 2.0 Hz), 6.51 (dd, 1H, J1 = 2.0 Hz, J2 = 8.4 Hz), 4.21 (m, 2H), 4.04 (q, 2H, J = 6.8 Hz), 3.86 (d, 2H, J = 12 Hz), 3.68 (t, 2H, J = 12 Hz), 3.51 (d, 2H, J = 11.2 Hz), 3.38 (t, 1H, J = 12 Hz), 3.13 (m, 2H), 2.73 (t, 2H, J = 11.2 Hz); 2.64 (s, 6H), 2.16 (d, 2H, J = 11.2 Hz), 1.73 (m, 2H), 1.24 (t, 3H, J = 7.2 Hz); MS m/z 616.3 (M + 1). 185 MS m/z 642.3 (M + 1). 186 MS m/z 606.7 (M + 1). 187 MS m/z 599.3 (M + 1). 188 MS m/z 600.3 (M + 1). 189 MS m/z 626.3 (M + 1). 190 MS m/z 590.3 (M + 1). 191 MS m/z 599.3 (M + 1). 192 1H NMR 400 MHz (DMSO- d6) δ 9.86 (bs, 1H), 9.56 (s, 1H), 8.59 (bs, 1H), 8.47 (bs, 1H), 8.24 (s, 1H), 7.79 (dd, 1H, J1 = 1.6 Hz, J2 = 8.0 Hz), 7.58 (t, 1H, J = 8.0 Hz), 7.38 (d, 1H, J = 8.4 Hz), 7.33 (t, 1H, J = 7.2 Hz), 6.70 (d, 1H, J = 2.4 Hz), 6.51 (dd, 1H, J1 = 2.4 Hz, J2 = 8.4 Hz), 4.03 (q, 2H, J = 6.8 Hz), 3.81 (d, 2H, J = 12.4 Hz), 3.58 (m, 2H), 3.28 (m, 1H), 3.11 (m, 2H), 2.74 (t, 2H, J = 12 Hz), 2.64 (s, 6H), 2.15 (d, 2H, J = 11.6 Hz), 2.03 (m, 2H), 1.86 (m, 2H), 1.73 (m, 2H), 1.24 (t, 3H, J = 6.8 Hz); MS m/z 600.2 (M + 1). 193 MS m/z 626.2 (M + 1). 194 1H NMR 400 MHz (CD3OD) δ 8.29 (s, br, 1H), 8.07 (s, br, 1H), 7.96 (d, 1H, J = 7.8 Hz), 7.60 (m, 1H), 7.39 (t, 1H, J = 7.2 Hz), 7.22 (s, br, 1H), 6.31 (s, 1H), 6.22 (s, br, 1H), 4.08 (q, 1H, J = 6.6 Hz), 3.95 (p, 1H, J = 6.0 Hz), 3.61 (m, 2H), 3.53 (dd, 1H, J = 4.2, 11.4 Hz), 3.36 (m, 2H), 2.78 (s, 3H), 2.52 (m, 1H), 2.22 (m, 1H), 1.31 (t, 3H, J = 6.6 Hz), 0.98 (d, 6H, J = 6.6 Hz); MS m/z 560.40 (M + 1). 195 1H NMR 400 MHz (CD3OD) δ 8.30 (s, br, 1H), 8.05 (s, br, 1H), 7.87 (d, 1H, J = 7.8 Hz), 7.60 (m, 1H), 7.42 (m, 1H), 7.17 (s, br, 1H), 6.28 (s, 1H), 6.19 (s, br, 1H), 4.04 (q, 2H, J = 6.6 Hz), 3.91 (p, 1H, J = 5.4 Hz), 3.60 (m, 2H), 3.50 (dd, 1H , J = 3.6 10.8 Hz), 3.33 (m, 1H), 2.75 (s, 3H), 2.66 (s, 6H), 2.48 (m, 1H), 2.20 (m, 1H), 1.27 (t, 3H, J = 6.6 Hz); MS m/z 546.40 (M + 1). 196 MS m/z 572.40 (M + 1). 197 MS m/z 545.40 (M + 1). 198 MS m/z 545.40 (M + 1). 199 1H NMR 400 MHz (CD3OD) δ 8.37 (s, br 1H), 8.11(s, br, 1H), 8.00 (d, 1H, J = 7.8 Hz,), 7.65 (s, br, 1H), 7.43 (t, 1H, J = 7.2 Hz), 7.25 (s, br, 1H), 6.34 (s, 1H), 6.25 (s, 1H), 4.11 (q, 2H, J = 7.2 Hz), 3.99 (m, 1H), 3.65 (m, 2H), 3.57 (dd, 1H, J = 3.6, 10.8 Hz), 3.41 (m, 2H), 2.82 (s, 3H), 2.56 (m, 1H), 2.28 (m, 1H), 1.35 (t, 3H, J = 6.6 Hz), 1.01 (d, 6H, J = 6.6 Hz); MS m/z 560.40 (M + 1). 200 1H NMR 400 MHz (CD3OD) δ 8.30 (s, br, 1H), 8.10 (s, br, 1H), 7.91 (d, 1H, J = 7.8 Hz), 7.57 (s, br, 1H), 7.38 (t, 1H, J = 7.2 Hz), 7.21 (s, 1H), 6.30 (s, 1H), 6.21 (s, 1H), 4.08 (q, 2H, J = 7.2 Hz), 3.95 (m, 1H), 3.68 (m, 1H), 3.62 (m, 2H), 3.53 (dd, 1H, J = 4.2, 10.8 Hz), 3.36 (m, 1H), 2.78 (s, 3H), 2.50 (m, 1H), 2.25 (m, 1H), 1.94 (m, 2H), 1.75 (m, 2H), 1.50 (m, 2H), 1.31 (t, 3H, J = 7.2 Hz); MS m/z 572.40 (M + 1). 201 MS m/z 532.40 (M + 1). 202 MS m/z 546.40 (M + 1). 203 MS m/z 545.40 (M + 1). 204 MS m/z 532.40 (M + 1). 205 1H NMR 400 MHz (CD3OD) δ 8.27 (s, br, 1H), 8.07 (m,1H), 7.94 (d, 1H, J = 7.8 Hz), 7.61 (s, br, 1H), 7.40 (t, 1H, J = 7.2 Hz), 7.20 (s, 1H), 6.30 (s, 1H), 6.20 (s, 1H), 4.08 (q, 2H, J = 7.2 Hz), 3.95 (m, 1H), 3.60 (m, 2H), 3.53 (dd, 1H, J = 4.2, 10.8 Hz), 3.35 (m, 1H), 2.78 (s, 3H), 2.73 (d, 1H, J = 6.6 Hz), 2.51 (m, 1H), 2.23 (m, 1H), 1.31 (t, 3H, J = 7.2 Hz), 0.742 (s, br 1H), 0.32 (s, 2H), 0.00 (s, 2H); MS m/z 572.40 (M + 1). 206 1H NMR 400 MHz (CD3OD) δ 8.24 (s, br, 1H), 8.07 (s, br, 1H), 7.93 (d, 1H, J = 8.4 7.57 (s, 1H), 7.38 (t, 1H, J = 7.2 Hz), 7.20 (s, 1H), 6.29 (s, 1H), 6.19 (s, br, 1H), 4.06 (q, 2H, J = 7.2 Hz), 3.93 (m, 1H), 3.60 (m, 2H), 3.50 (dd, 1H, J = 6.0, 10.8 Hz), 3.35 (m, 1H), 2.76 (s, 3H), 2.72 (d, 1H, J = 6.6 Hz), 2.50 (m, 1H), 2.22 (m, 1H), 1.30 (t, 3H, J = 7.2 Hz), 0.73 (m, 1H), 0.31 (m, 2H), 0.01(m, 2H); MS m/z 572.40 (M + 1). 207 MS m/z 545.40 (M + 1). 208 MS m/z 549.10 (M + 1). 209 MS m/z 532.40 (M + 1). 210 1H NMR 400 MHz (CD3OD) δ 8.22 (d, 1H, J = 7.8 Hz), 8.20 (s, 1H), 7.92 (dd, 1H, J = 1.2, 7.8 Hz), 7.58 (t, 1H, J = 6 Hz), 7.48 (d, 1H, J = 9.1 Hz), 7.38 (t, 1H, J = 7.8 Hz), 6.65 (s, 1H), 6.46 (d, 1H, J = 7.8 Hz), 5.20 (s, 1H), 4.06 (q, 2H, J = 7.2 Hz), 3.86 (m, 1H), 3.38 (m, 1H),, 3.05 (m, 3H), 2.64 (m, 1H), 2.50 (s, 3H), 2.30 (m, 1H), 1.34 (t, 3H, J = 7.2 Hz); MS m/z 577.30 (M + 1). 211 MS m/z 533.30 (M + 1). 212 1H NMR 400 MHz (CD3OD) δ 8.30 (d, 1H, J = 7.8 Hz), 8.11 (s, 1H), 7.91 (d, 1H, J = 7.8 Hz), 7.58) t, 1H, J = 7.8 Hz), 7.55 (d, 1H, J = 8.4 Hz), 7.36 (t, 1H, J = 7.8 Hz), 6.65 (s, 1H), 6.47 (d, 1H, J = 8.4 Hz), 5.20 (s, 1H), 4.07 (q, 2H, J = 7.2 Hz), 3.87 (m, 1H), 3.38 (m, 1H), 3.23 (m, 1H), 3.00 (m, 2H), 2.64 (m, 1H), 2.50 (s, 3H), 2.38 (m, 1H), 1.35 (t, 3H, J = 7.2 Hz); MS m/z 533.30 (M + 1). 213 MS m/z 533.30 (M + 1). 214 MS m/z 519.30 (M + 1). 215 MS m/z 563.30 (M + 1). 216 MS m/z 505.10 (M + 1). 217 MS m/z 563.30 (M + 1). 218 MS m/z 563.10 (M + 1). 219 MS m/z 561.40 (M + 1). 220 MS m/z 577.30 (M + 1). 221 MS m/z 524.45 (M + 1). 222 MS m/z 511.20 (M + 1). 223 MS m/z 573.40 (M + 1). 224 MS m/z 547.40 (M + 1). 225 MS m/z 547.40 (M + 1). 226 MS m/z 546.40 (M + 1). 227 MS m/z 546.40 (M + 1). 228 MS m/z 559.40 (M + 1). 229 MS m/z 559.40 (M + 1). 230 MS m/z 559.40 (M + 1). 231 MS m/z 519.35 (M + 1). 232 MS m/z 532.35 (M + 1). 233 1H NMR 400 MHz (CD3OD) δ 8.06 (d, 1H, J = 8.0 Hz), 7.92 (s, 1H), 7.73 (dd, 1H, J = 1.6, 8.0 Hz), 7.40 (t, 1H, J = 7.6 Hz), 7.22 (m, 1H), 7.10 (d, 1H, J = 8.8 Hz), 6.50 (d, 1H, J = 2.4 Hz), 6.38 (dd, 1H, J = 2.0, 8.8 Hz), 3.67 (m, 4H), 3.35 (m, 2H), 3.15 (m, 1H), 2.84 (m, 2H), 2.69 (m, 2H), 2.30 (s, 3H), 2.01 (m, 2H), 1.69 (m, 7H), 1.27 (m, 1H), 0.95 (m, 1H), 0.33 (m, 2H), 0.05 (m, 2H); MS m/z 626.5 (M + 1). 234 MS m/z 561.4 (M + 1). 235 MS m/z 547.25 (M + 1). 236 1H NMR 400 MHz (CD3OD) δ 8.23 (d, 1H, J = 7.6 Hz), 8.15 (s, 1H), 7.90 (dd, 1H, J = 1.6, 8.0 Hz), 7.56 (t, 1H, J = 7.2 Hz), 7.36 (t, 1H, J = 7.6 Hz), 7.31 (d, 1H, J = 8.8 Hz), 6.63 (d, 1H, J = 2.4 Hz), 6.51 (m, 1H), 4.10 (m, 2H), 3.83 (m, 6H), 3.34 (m, 2H), 2.82 (m, 2H), 2.48 (s, 3H), 2.23 (m, 2H), 1.78 (m, 2H), 1.14 (m, 1H), 0.51 (m, 2H), 0.23 (m, 2H); MS m/z 672.4 (M + 1). 237 MS m/z 532.4 (M + 1). 238 MS m/z 546.4 (M + 1). 239 MS m/z 576.3 (M + 1). 240 MS m/z 590.4 (M + 1). 241 MS m/z 533.3 (M + 1). 242 1H NMR 400 MHz (CD3OD) δ 8.26 (s, 1H), 8.10 (d, 1H, J = 8.0 Hz), 7.90 (dd, 1H, J = 1.2, 8.0 Hz), 7.80 (d, 1H, J = 8.4 Hz), 7.62 (m, 1H), 7.38 (t, 1H, J = 8.0 Hz), 7.05 (d, 1H, J = 1.6 Hz), 6.83 (dd, 1H, J = 1.6, 8.4 Hz), 3.86 (s, 3H), 3.43 (m, 4H), 3.00(m, 4H), 2.10 (m, 2H), 1.95 (m, 2H), 1.72 (m, 6H), 1.45 (m, 2H); MS m/z 658.4 (M + 1). 243 MS m/z 546.3 (M + 1). 244 MS m/z 591.2 (M + 1). 245 MS m/z 547.3 (M + 1). 246 MS m/z. 591.2 (M + 1). 247 MS m/z. 546.1 (M + 1). 248 MS m/z. 560.2 (M + 1). 249 MS m/z. 604.3 (M + 1). 250 MS m/z. 559.4 (M + 1). 251 MS m/z. 573.4 (M + 1). 252 MS m/z. 573.4 (M + 1). 253 MS m/z. 573.4 (M + 1). 254 MS m/z. 519.1 (M + 1). 255 MS m/z. 563.1 (M + 1). 256 MS m/z. 577.1 (M + 1). 257 MS m/z. 530.4 (M + 1). 258 MS m/z. 590.4 (M + 1). 259 MS m/z. 588.2 (M + 1). 260 MS m/z. 546.4 (M + 1). 261 MS m/z. 520.4 (M + 1). 262 MS m/z. 534.4 (M + 1). 263 MS m/z. 544.2 (M + 1). 264 MS m/z. 516.4 (M + 1). 265 MS m/z. 574.2 (M + 1). 266 MS m/z. 577.1 (M + 1). 267 MS m/z. 579.3 (M + 1) 268 MS m/z. 614.5 (M + 1) 269 MS m/z. 658.4 (M + 1) 270 MS m/z. 590.3 (M + 1) 271 MS m/z. 546.4 (M + 1) 272 MS m/z. 524.4 (M + 1) 273 MS m/z. 614.5 (M + 1) 274 1H NMR 400 MHz (CD3OD) δ 8.17 9s, 1H0, 8.15 (d, 1H, J = 8.0 Hz), 7.89 9dd, 1H, J = 1.2, 8.0 Hz), 7.86 (d, 1H, J = 8.4hz), 7.61 (m, 1H), 7.36 (m, 1H), 7.01 (d, 1H, J = 1.6 Hz), 6.81(dd, 1H, J = 1.6, 8.0 Hz), 4.10 (q, 2H, J = 7.2 Hz), 3.45 (m, 3H), 2.94 9m, 4H), 2.44 (s, 3H), 2.08 ( m, 2H), 1.94 (m, 2H), 1.75 (m, 6H), 1.49 (m, 1H), 1.37 (t, 3H, J = 6.8 Hz); MS m/z. 628.5 (M + 1) 275 1H NMR 400 MHz (CD3OD) δ 8.17 (d, 1H, J = 8.0 Hz), 7.93 (s, 1H), 7.74 (dd, 1H, J = 1.6, 8.0 Hz), 7.47 (t, 1H, J = 8.0 Hz), 7.25 (m, 1H), 7.13 (d, 1H, 8.8 Hz), 6.48 (d, 1H, J = 2.4 Hz), 6.37 (dd, 1H, J = 2.4, 8.8 Hz), 3.66 (m, 4H), 3.34 (m, 2H), 3.14 (m, 4H), 2.81 (m, 2H), 2.65 (m, 2H), 2.98 (d, 2H, J = 12 Hz), 1.68 (m, 7H), 1.30 (m, 1H), 1.02 (d, 6H, J = 6.8 Hz), 0.99 (m, 1H), 0.32 (m, 2H), 0.04 (m, 2H); MS m/z. 639.5 (M + 1) 276 1H NMR 400 MHz (CD3OD) δ 8.32 (d, 1H, J = 8.0 Hz), 8.11 (s, 1H), 7.92 (dd, 1H, J = 1.6, 8.0 Hz), 7.65 (t, 1H, J = 7.6 Hz), 7.43 (t, 1H, J = 7.6 Hz), 7.28 (d, 1H, J = 8.4 Hz), 6.67 (d, 1H, J = 2.4 Hz), 6.56 (dd, 1H, J = 2.4, 8.8 Hz), 4.03 (m, 2H), 3.86 (m, 4H), 3.73 (m, 2H), 3.36 (m, 2H), 3.35 (m, 2H), 3.23 (m, 2H), 2.85 (m, 2H), 2.23 (m, 2H), 1.83 (m, 2H), 1.19 (d, 6H), 1.13 (m, 1H), 0.48 (m, 2H), 0.21 (m, 2H); MS m/z. 641.5 (M + 1) 277 1H NMR 400 MHz (CD3OD) δ 8.06 (d, 1H, J = 7.6 Hz), 7.91 (S, 1H), 7.72 (dd, 1H), J = 1.6, 8.0 Hz), 7.39 (t, 1H, J = 7.6 Hz), 7.20 (m, 1H), 7.10 (d, 1H, J = 4.8 Hz), 6.48 (d, 1H, 2.4 Hz), 6.36 (dd, 1H, J = 2.4, 8.8 Hz), 3.85 (m, 2H), 3.66 (m, 4H), 3.56 (m, 2H), 3.18 (m, 2H), 3.16 (m, 1H), 2.98 (m, 2H), 2.65 (t, 2H, J = 12 Hz), 2.29 (s, 3H), 2.05 (m, 2H), 1.64 (m, 2H), 0.95 (m, 1H), 0.32 (m, 2H), 0.03 (m, 2H); MS m/z. 628.5 (M + 1) 278 1H NMR 400 MHz (CD3OD) δ 8.18 (d, 1H, J = 8.0 Hz), 8.15 (s, 1H), 7.89 (dd, 1H, J = 1.6, 8.0 Hz), 7.56 (t, 1H, J = 7.2 Hz), 7.38 (m, 1H), 7.25 (d, 1H, J = 8.8 Hz), 6.65 (d, 1H, J = 2.4 Hz), 6.53 (dd, 1H, J = 2.4, 8.8 Hz), 3.82 (m, 4H), 3.49 (m, 2H), 3.31 (m, 1H), 2.95 (m, 2H), 2.83 (m, 2H), 2.47 (s, 3H), 2.17 (m, 2H), 1.86 (m, 7H), 1.52 (m, 1H), 1.15 (m, 1H), 0.49 (m, 2H), 0.21 (m, 2H); MS m/z. 670.4 (m + 1) 279 MS m/z. 641.5 (M + 1) 280 1H NMR 400 MHz (CD3OD) δ 8.13 (s, 1H), 8.10 (d, 1H, J = 8.0 Hz), 7.85 (t, 1H, J = 1.6 Hz), 7.83 (t, 1H, J = 1.6 Hz), 7.57 (m, 1H) 7.32 (m, 1H), 7.02 (d, 1H, J = 1.2 Hz), 6.81 (dd, 1H, J = 1.6, 8.4 Hz), 4.05 (q, 2H, J = 6.8 Hz), 3.36 (m, 4H), 3.08 (m, 2H), 2.84 (s, 3H), 2.40 (s, 3H), 1.33 (t, 3H, J = 6.8 Hz); MS m/z. 560.4 (M + 1) 281 MMS m/z. 600.5 (M + 1) 282 NMR 400 MHz (CD3OD) δ 8.30 (d, 1H, J = 8.0 Hz), 8.21 (s, 1H), 8.00 (d, 1H, J = 8.4 Hz), 7.91 (dd, 1H, J = 1.63, 8.0 Hz), 7.71 (m, 1H), 7.42 (m, 1H), 7.08 (d, 1H, J = 2.0 Hz), 6.91 (dd, 1H, J = 1.6, 8.4 Hz), 4.30 (m, 2H), 4.13 (q, 2H, J = 7.2 Hz), 3.60 (m, 4H), 3.16 (m, 2H), 1.41 (t, 3H, J = 7.2 Hz), 1.90 9d, 6H, J = 6.8 Hz); MS m/z. 573.40 (M + 1) 283 MS m/z. 681.5 (M + 1) 284 MS m/z. 559.4 (M + 1) 285 MS m/z. 577.3 (M + 1) 286 NMR 400 MHz (CD3OD) δ 8.28 (s, br, 1H), 8..11 (s, br, 1H), 7.89 (d, 1H, J = 8.4 Hz), 7.53 (s, br, 1H), 7.35 (t, 1H, J = 6.0 Hz), 7.29 (m, 1H), 6.30 (s, 1H), 6.20 (d, 1H, J = 7.8 Hz), 4.07 (q, 2H, J = 7.2 Hz), 4.02 (m, 1H), 3.68 (m, 1H), 3.60 (m, 1H), 3.54 (m, 1H), 3.34 (m, 1H), 2.95 (s, 6H), 2.57 (m, 1H), 2.51 (s, 3H), 2.25 (m, 1H), 1.30 (t, 3H, J = 7.2 Hz); MS m/z. 590.3 (M + 1) 287 MS m/z. 533.3 (M + 1) 288 MS m/z. 565.3 (M + 1) 289 MS m/z. 546.4 (M + 1) 290 NMR 400 MHz (CD3OD) δ 8.29 (d, 1H, J = 8.4 Hz), 8.24 (s, 1H), 7.91 (dd, 1H, J = 1.2, 7.8 Hz), 7.57 (m, 2H), 7.35 (t, 1H, J = 7.2 Hz), 6.98 (d, 1H, J = 9.0 Hz), 6.77 (dd, 1H, J = 3.0, 9.0 Hz), 4.18 (t, 2H, J = 4.8 Hz), 3.82 (s, 3H), 3.56 (m, 2H), 3.17 (t, 2H, J = 4.8 Hz), 3.3 (s, 1H), 3.01 (m, 2H), 2.53 (s, 3H), 1.92 (m, 2H), 1.79 (m, 3H), 1.53 (m, 1H); MS m/z. 591.3 (M + 1) 291 MS m/z. 521.4 (M + 1) 292 NMR 400 MHz (CD3OD) δ 8.28 (d, 1H, J = 8.4 Hz), 8.15 (s, 1H), 7.89 (dd, 1H, J = 2.4, 7.8 Hz), 7.55 (t, 1H, J = 7.2 Hz), 7.44 (d, 1H, J = 3.0 Hz), 7.33 (t, 1H, J = 7.2 Hz), 6.99 (dd, 1H, J = 3.6, 9.0 Hz), 6.80 (dd, 1H, J = 3.0, 9.0 Hz), 4.18 (t, 2H, J = 4.8 Hz), 3.80 (s, 3H), 3.51 (t, 2H, J = 4.8 Hz), 3.29 (m, 4H), 2.50 (s, 3H), 1.30 (m, 6H); MS m/z. 535.4 (M + 1) 293 NMR 400 MHz (CD3OD) δ 8.31 (d, 1H, J = 8.4 Hz), 8.14 (s, 1H), 7.90 (dd, 1H, J = 1.2, 7.8 Hz), 7.59 (m, 1H), 7.53 (d, 1H, J = 3.0 Hz), 7.33 (t, 1H, J = 7.8 Hz), 6.96 (d, 1H, J = 9.0 Hz), 6.76 (dd, 1H, J = 3.0, 8.4 Hz), 4.18 (t, 2H, J = 4.2 Hz), 3.80 (s, 3H), 3.55 (m, 2H), 3.46 (t, 2H, J = 4.8 Hz), 3.00 (t, 2H, J = 12.0 Hz), 2.50 (s, 3H), 1.89 (m, 2H), 1.77 (m, 3H), 1.52 (m, 1H); MS m/z. 547.4 (M + 1) 294 MS m/z. 605.3 (M + 1) 295 NMR 400 MHz (CD3OD) δ .8.33 (d, 1H, J = 8.4 Hz), 8.14 )s, 1H), 7.90 (dd, 1H, J = 1.2, 7.8 Hz), 7.57 (m, 1H), 7.53 (d, 1H, J = 3.0 Hz), 7.33 (t, 1H, J = 7.2 Hz), 6.95 (d, 1H, J = 9.0 Hz), 6.74 (dd, 1H, J = 3.0, 9.0 Hz), 4.17 (t, 1H, J = 4.8 Hz), 3.80 (s, 3H), 3.51 (m, 4H), 3.27 (m, 2H), 2.50 (s, 3H), 1.85 (m, 4H0, 1.70 (m, 4H); m/z. 561.4 (M + 1) 296 MS m/z. 587.4 (M + 1) 297 MS m/z. 589.4 (M + 1) 298 MS m/z. 590.4 (M + 1) 299 MS m/z. 589.4 (M + 1) 300 MS m/z. 591.4 (M + 1) 301 MS m/z. 606.4 (M + 1) 302 MS m/z. 607.1 (M + 1) 303 MS m/z. 574.4 (M + 1) 304 MS m/z. 618.2 (M + 1) 305 MS m/z. 588.2 (M + 1) 306 MS m/z. 562.4 (M + 1) 307 MS m/z. 530.4 (M + 1) 308 MS m/z. 562.4 (M + 1) 309 MS m/z. 576.2 (M + 1) 310 MS m/z. 546.5 (M + 1) 311 MS m/z. 558.2 (M + 1) 312 MS m/z. 604.5 (M + 1) 313 MS m/z. 616.5 (M + 1) 314 NMR 400 MHz (CD3OD) δ 8.17 (d, 1H, J = 8.0 Hz), 7.88 (m, 2H), 7.56 (t, 1H, J = 8.0 Hz), 7.37 (m, 1H), 7.17 (d, 1H, J = 8.8 Hz), 6.61 (d, 1H, J = 2.4 Hz), 6.47 9dd, 1H, J = 2.4, 8.4 Hz), 3.75(m, 7H), 3.12 (m, 4H), 2.42 (s, 3H); MS m/z. 489.1 (M + 1) 315 MS m/z. 546.4 (M + 1) 316 NMR 400 MHz (CD3OD) δ 8.22 (d, 1H, J = 8.4 Hz), 7.90 (d, 1H, J = 4.4 Hz), 7.86 (dd, 1H, J = 1.6, 8.0 Hz), 7.55 (m, 1H), 7.34 (m, 1H), 7.27 (d, 1H, J = 8.8 Hz), 6.59 (d, 1H, J = 2.4 Hz), 6.45 (dd, 1H, J = 1.6, 8.8 H), 4.00 (q. 2H, J = 6.8 Hz), 3.75 (t, 4H, J = 4.8 Hz), 3.10 (m, 4H), 2.42 (s, 3H), 1.94 (m, 6H), 1.25 (t, 3H, J = 6.8 Hz); MS m/z. 503.2 (M + 1) 317 MS m/z. 575.4 (M + 1) 318 NMR 400 MHz (CD3OD) δ 8.24 9d, 1H, J = 7.6 Hz), 8.17 9s, 1H), 7.94 9dd, 1H, J = 1.6, 8.0 Hz), 7.60 (t, 1H, J = 7.6 Hz), 7.44 (m, 1H), 7.39 (t, 1H, J = 7.23 Hz), 6.68 (s, 1H), 6.50 (m, 1H), 4.10 (q, 2H, J = 7.2 Hz), 3.84 (m, 4H), 3.16 (m, 4H), 2.83 (t, 2H, J = 7.2 Hz), 1.37 (m, 6H), 0.75 (t, 3H, J = 7.6 hz); MS m/z. 591.1 (M + 1) 319 MS m/z. 573.4 (M + 1) 320 MS m/z. 586.1 (M + 1) 321 MS m/z. 575.4 (M + 1) 322 MS m/z. 572.4 (M + 1) 323 MS m/z. 573.4 (M + 1) 324 MS m/z. 572.4 (M + 1) 325 MS m/z. 589.4 (M + 1) 326 MS m/z. 589.4 (M + 1) 327 MS m/z. 587.4 (M + 1) 328 MS m/z. 587.4 (M + 1) 329 MS m/z. 586.4 (M + 1) 330 MS m/z. 587.1 (M + 1) 331 MS m/z. 586.4 (M + 1) 332 MS m/z. 600.2 (M + 1) 333 MS m/z. 434.2 (M + 1) 334 MS m/z. 520.0 (M + 1) 335 MS m/z. 478.0 (M + 1) 336 MS m/z. 543.2 (M + 1) 337 MS m/z. 563.2 (M + 1) 338 NMR 400 MHz (CD3OD) δ 8.18 (d, 1H, J = 7.6 Hz), 7.98 (d, 1H, J = 4.4 Hz), 7.86 (dd, 1H, J = 1.6, 8.0 Hz), 7.56 (m, 1H), 7.46 (s, 1H), 7.30 (m, 1H), 6.83 (m, 2H), 3.75 (s, 3H), 2.40 (s, 3H), 2.08 (s, 3H); MS m/z. 418.1 (M + 1) 339 NMR 400 MHz (CD3OD) δ 8.17 (d, 1H, J = 8.0 Hz), 8.00 (d, 1H, J = 4.4 Hz), 7.86 (dd, 1H, J = 1.6, 8.0 Hz), 7.53 (m, 1H), 7.43 (s, 1H), 7.32 (m, 1H), 6.79 (s, 1H), 4.00 (q, 2H, J = 6.8 Hz), 3.83 9m, 4H), 3.76 (m, 2H), 2.44 (s, 3H), 1.29 (t, 3H, J = 7.2 Hz), 1.24 (t, 3H, J = 7.2 Hz); MS m/z. 547.2 (M + 1) 340 MS m/z. 588.4 (M + 1) 341 MS m/z. 600.4 (M + 1) 342 MS m/z. 602.4 (M + 1) 343 MS m/z 593.00/595.00 (M + 1) 344 MS m/z 621.20/623.20 (M + 1) 345 1H NMR 400 MHz (DMSO- d6) δ 8.95 (s, br, 1H), 8.49 (s, br, 2H), 7.80 (dd, J = 7.54, 2.13 Hz, 1H), 7.34 (t, J = 8.36, 2H), 7.14 (m, 3 H), 6.93 (m, 3 H); MS m/z 540.10 / 542.10 (M + 1) 346 MS m/z 506.10/508.10 (M + 1) 347 MS m/z 522.10/524.10 (M + 1) 348 MS m/z 635.20/637.20 (M + 1) 349 MS m/z 456.10 /458.10 (M + 1) 350 MS m/z 472.10/474.10 (M + 1) 351 MS m/z 585.20/587.20 (M + 1) 352 MS m/z 571.20/573.10 (M + 1) 353 M S m/z 448.10/450.10 (M + 1) 354 MS m/z 464.10/466.10 (M + 1) 355 1H NMR 400 MHz (DMSO- d6) δ 9.54 (s, br, 1H), 8.76 (s, br, 1H), 8.31 (s, 1), 8.28 (d, J = 8.14 Hz, 1H), 7.95 (t, J = 5.64 Hz, 7.85 (dd, J = 7.95, 1.48 Hz, 1H), 7.51 (t, J = 7.53 Hz, 1H), 7.33 (m, 2H), 6.73 (s, 1H), 4.05 (q, J = 6.92, 2H), 3.98 (m, 4H), 3.71 (m, 2H), 3.12 (m, 4H), 2.82 (p, J = 5.77 356 MS m/z 563.20/565.20 (M + 1) 357 MS m/z 412.10/414.10 (M + 1) 358 MS m/z 428.10/430.10 (M + 1) 359 MS m/z 541.20/543.20 (M + 1) 360 MS m/z 527.20/529.20 (M + 1) 361 MS m/z 492.10/494.10 (M + 1) 362 MS m/z 508.00/510.05 (M + 1) 363 MS m/z 607.10/609.10 (M + 1) 364 MS m/z 563.10/565.10 (M + 1) 365 MS m/z 487.10 /489.10 (M + 1) 366 MS m/z 649.20/651.20 (M + 1) 367 MS m/z 591.20/593.20 (M + 1) 368 MS m/z 605.20/607.20 (M + 1) 369 MS m/z 448.10/450.10 (M + 1) 370 1H NMR 600 MHz (DMSO- d6) δ 9.46 (s, 1H), 8.46 (s, 1H), 8.37 (d, J = 8.12 Hz, 1H), 8.25 s, (1H), 7.75 (dd, 7.87, 1.40 Hz, 1H), 7.55 (t, J = 7.50 Hz, 1H), 7.37 (s, 1H), 7.30 (dt, J = 8.05, 0.8 Hz, 1H), 6.91 (d, J = 8.92 Hz, 1H), 6.58 (dd, J = 8.88, 3.04 Hz, 1H), 3.70 (s, 3H), 3.55 (s, 3H), 2.58 (s, 371 MS m/z 577.20/579.20 (M + 1) 372 MS m/z 563.20/565.20 (M + 1) 373 MS m/z 519.20/521.20 (M + 1) 374 MS m/z 533.20/535.20 (M + 1) 375 MS m/z 476.20/478.20 (M + 1) 376 1H NMR 600 MHz (DMSO- d6) δ 9.39 (s, 1H), 8.37 (s, 1H), 8.27 (s, 1H), 8.25 (s, 1H), 7.88 (t, J = 6.02 Hz, 1H), 7.76 (dd, J = 7.94, 1.35 Hz, 1H), 7.50 (dt, J = 8.24, 1.89 Hz, 1H), 7.40 (s, 1H), 7.25 (t, J = 7.39 Hz, 1H), 6.90 (d, J = 8.92 Hz, 1H) 5.65 (dd, J = 8.87, 3.03 Hz, 1H)., 3.70 (s, 3H), 3.54 (s, 377 MS m/z 605.30/607.20 (M + 1) 378 MS m/z 591.20/593.20 (M + 1) 379 MS m/z 547.20/549.20 (M + 1) 380 MS m/z 561.20/563.20 (M + 1) 381 MS m/z 548.20/550.20/552.20 ( M + 1) 382 MS m/z 548.20/550.20/552.20 (M + 1) 383 MS m/z 563.30/565.30 (M + 1) 384 MS m/z 577.30/579.30 (M + 1) 385 MS m/z 591.40/593.30 (M + 1) 386 MS m/z 605.40/607.40 (M + 1) 387 MS m/z 617.40/619.40 (M + 1) 388 MS m/z 619.40/621.40 (M + 1) 389 MS m/z 576.30/578.30 (M + 1) 390 MS m/z 563.30/565.30 (M + 1) 391 MS m/z 560.40/562.40 (M + 1) 392 MS m/z 574.40/576.50 (M + 1) 393 1H NMR 400 MHz (DMSO- d6) δ 9.47 (s, 1H), 8.45 (d, J = 7.37 Hz, 1H), 8.36 (s, 1H), 8.20 (s, 1H), 7.83 (d, J = 7.93 Hz, 1H), 7.59 (t, J = 8.07 Hz, 1H), 7.47 (d, J = 8.69 Hz, 1H), 7.32 (t, J = 7.54 Hz, 1H), 6.63 (dd, J = 12.67, 2.42 Hz, 1H), 6.49 (m, 1H), 5.16 (m, 1H), 4.01- 3.68 (m, 3H), 3.48- 394 MS m/z 587.40/589.40 (M + 1) 395 MS m/z 573.40/575.40 (M + 1) 396 MS m/z 587.40/589.40 (M + 1) 397 MS m/z 586.40/588.40 (M + 1) 398 MS m/z 586.40/588.40 (M + 1) 399 MS m/z 560.40/562.40 (M + 1) 400 1H NMR 400 MHz (DMSO- d6) δ 10.30 (s, br, 1H), 9.53 (s, 1H), 8.53 (s, 1H), 8.41 (d, J = 7.90 Hz, 1H), 8.26 (s, 1H), 7.90 (dd, J = 7.91,1.25 Hz, 1H), 7.64 (t, J = 7.93 Hz, 1H), 7.46 (d, J = 8.70 Hz, 1H), 7.38 (t, J = 7.48 Hz, 1H), 6.67 (dd, J = 13.68, 2.49 Hz, 1H), 6.46 (m, 1H), 5.19 (m, 1H), 4.04 (m, 401 MS m/z 573.20/575.20 (M + 1) 402 MS m/z 573.20/575.20 (M + 1) 403 MS m/z 587.40/589.40 (M + 1) 404 MS m/z 573.40/575.40 (M + 1) 405 MS m/z 587.40/589.40 (M + 1) 406 MS m/z 586.40/588.40 (M + 1) 407 MS m/z 586.40/588.40 (M + 1) 408 1H NMR 600 MHz (CD3OD) δ 8.18 (d, J = 7.80 Hz, 1H), 8.03 (s, 1H), 7.87 (dd, J = 7.96, 1.21 Hz, 1H), 7.47 (m, 2H), 7.25 (t, J = 7.82 Hz, 1H), 6.59 (s, 1H), 6.40 (d, J = 8.56 Hz, 1H), 5.13 (s, 1H), 3.78 (m, 4H), 3.30 (m, 2H), 3.28 (s, 3H), 2.92 (m, 2H), 2.56 (m, 1H), 2.30 (m, 2H), 0.89 (d, J = 409 MS m/z 547.40/549.40 (M + 1) 410 MS m/z 547.40/549.40 (M + 1) 411 MS m/z 587.50/589.40 (M + 1) 412 MS m/z 588.50/590.40 (M + 1) 413 MS m/z 637.50/639.50 (M + 1) 414 MS m/z 638.50/640.50 (M + 1) 415 MS m/z 602.50/604.50 (M + 1) 416 MS m/z 583.40/585.40 (M + 1) 417 MS m/z 584.40/586.40 (M + 1) 418 MS m/z 548.40/550.40 (M + 1) 419 MS m/z 540.40/542.40 (M + 1) 420 MS m/z 541.40 /543.40 (M + 1) 421 MS m/z 505.40 /507.40 (M + 1) 422 MS m/z 667.30 /669.30 (M + 1) 423 MS m/z 632.30 /634.30 (M + 1) 424 MS m/z 573.40 /575.40 (M + 1) 425 1H NMR 400 MHz (CD3OD) δ 8.34 (d, J = 8.4 Hz, 1H), 8.13 (s, 1H), 7.96 (dd, J = 1.2, 7.8 Hz, 1H), 7.63 (t, J = 7.8 Hz, 1H), 7.43 (m, 2H), 6.72 (d, J = 2.4 Hz, 1H), 6.57 (m, 1H), 4.07 (m, 1H), 3.40 (m, 1H), 3.02 (t, J = 10.6 Hz, 1H), 2.80 (t, J = 11.8, 1H), 2.65 (m, 4H), 2.54, (s, 3H), 2.26 (s, 6H), 1.56 (m, 4H), 1.36 (d, J = 7.2 Hz, 6H). MS m/z 574.20 (M + 1). 426 MS m/z 603.20 (M + 1). 427 MS m/z 504.20 (M + 1). 428 MS m/z 585.30 (M + 1). 429 MS m/z 627.20 (M + 1). 430 MS m/z 616.20 (M + 1). 431 MS m/z 602.20 (M + 1). 432 MS m/z 562.10 (M + 1). 433 MS m/z 408.1 (M + 1). 434 MS m/z 421.1 (M + 1). 435 MS m/z 421.1 (M + 1). 436 MS m/z 480.10 (M + 1). 437 MS m/z 394.10 (M + 1). 438 MS m/z 408.10 (M + 1). 439 1H NMR 400 MHz (CD3OD) δ 8.34 (d, J = 8.4 Hz, 1H), 8.13 (s, 1H), 7.96 (dd, J = 1.3, 7.9 Hz, 1H), 7.64 (t, J = 7.9 Hz, 1H), 7.43 (m, 1H), 7.13 (d, J = 2.8 Hz, 1H), 6.24 (d, J = 2.8 Hz, 1H), 4.08 (m, 1H), 3.41 (m, 1H), 2.58 (s, 6H), 2.54 (s, 3H). MS m/z 574.20 (M + 1). 440 MS m/z 468.20 (M + 1). 441 MS m/z 552.30 (M + 1). 442 MS m/z 592.30 (M + 1) 443 MS m/z 504.20 (M + 1) 444 MS m/z 588.30 (M + 1) 445 MS m/z 628.30 (M + 1) 446 MS m/z 617.20 (M + 1) 447 MS m/z 503.20 (M + 1) 448 MS m/z 587.20 (M + 1) 449 MS m/z 627.30 (M + 1) 450 MS m/z 616.30 (M + 1) 451 MS m/z 549.30 (M + 1) 452 MS m/z 509.30 (M + 1) 453 MS m/z 495.20 (M + 1) 454 MS m/z 495.20 (M + 1) 455 MS m/z 585.20 (M + 1) 456 MS m/z 545.20 (M + 1) 457 MS m/z 531.20 (M + 1) 458 MS m/z 531.20 (M + 1) 459 MS m/z 584.30 (M + 1) 460 MS m/z 544.20 (M + 1) 461 MS m/z 530.20 (M + 1) 462 MS m/z 530.20 (M + 1) 463 MS m/z 601.30 (M + 1) 464 MS m/z 626.30 (M + 1) - The ZAP-70 kinase assay is based on time-resolved fluorescence resonance energy transfer (FRET). 80 nM ZAP-70 are incubated with 80 nM Lck (lymphoid T-cell protein tyrosine kinase) and 4 μM ATP in ZAP-70 kinase buffer (20 mM Tris, pH 7.5, 10 μM Na3VO4, 1 mM DTT, 1 mM MnCl2, 0.01% BSA, 0.05% Tween-20) for 1 hour at room temperature in a siliconized polypropylene tube. Then, the selective Lck inhibitor PP2 (1-tert-butyl-3-(4-chloro-phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-ylamine; Alexis Biochemicals) is added (final concentration 1.2 μM) and incubated for further 10 min. 10 μL of this solution is mixed with the 10 μL biotinylated peptide LAT-11 (1 μM) as substrate and 20 μL of serial dilutions of inhibitors and incubated for 4 hours at room temperature. The kinase reaction is terminated with 10 μL of a 10 mM EDTA solution in detection buffer (20 mM Tris, pH 7.5, 0.01% BSA, 0.05% Tween-20). 50 μL europium-labelled anti-phosphotyrosine antibody (Eu-PT66; final concentration 0.125 nM); and 50 μL streptavidin-allophycocyanine (SA-APC; final concentration 40 nM) in detection buffer are added. After 1 hour incubation at room temperature fluorescence is measured on the Victor2 Multilabel Counter (Wallac) at 665 nm. Background values (low control) are obtained in the absence of test samples and ATP and are subtracted from all values. Signals obtained in the absence of test samples are taken as 100% (high control). The inhibition obtained in the presence of test compounds is calculated as percent inhibition of the high control. The concentration of test compounds resulting in 50% inhibition (IC50) is determined from the dose-response curves. In this assay, the agents of the invention have IC50 values in the range of 10 nM to 2 μM, preferably from 10 nM to 100 nM.
- Recombinant ZAP-70 kinase is obtained as follows: A nucleic acid encoding full-length human ZAP-70 (GenBank #L05148) is amplified from a Jurkat cDNA library by RT-PCR and cloned into the pBluescript KS vector (Stratagene, Calif., USA). The authenticity of the ZAP-70 cDNA insert is validated by complete sequence analysis. This donor plasmid is then used to construct a recombinant baculovirus transfer vector based on the plasmid pVL1392 (Pharmingen, Calif., USA) featuring in addition an N-terminal hexahistidine tag. Following co-transfection with AcNPV viral DNA, 10 independent viral isolates are derived via plaque-purification, amplified on small scale and subsequently analyzed for recombinant ZAP-70 expression by Western Blot using a commercially available anti-ZAP-70 antibody (Clone 2F3.1, Upstate Biotechnology, Lake Placid, N.Y., USA). Upon further amplification of one positive recombinant plaque, titrated virus stocks are prepared and used for infection of Sf9 cells grown in serum-free SF900 II medium (Life Technologies, Basel, Switzerland) under defined, optimized conditions. ZAP-70 protein is isolated from the lysate of infected Sf9 cells by affinity chromatography on a Ni-NTAcolumn (Qiagen, Basel, Switzerland).
- Recombinant His-tagged ZAP-70 is also available from PanVera LLC, Madison, Wis., USA.
- LAT-11 (linker for activation of T cell): The biotinylated peptide LAT-11 (Biotin-EEGAPDYENLQELN) used as a substrate in the ZAP-70 kinase assay is prepared in analogy to known methods of peptide synthesis. The N-α Fmoc group of Fmoc-Asn(Trt)-oxymethyl-4-phenoxymethyl-co(polystyrene-1%-divinyl-benzene), content of Asn approx. 0.5 mmol/g, is cleaved using piperidine, 20% in DMF. Four equivalents per amino-group of Fmoc-amino acid protected in their side chains [Asp(OtBu), Glu(OtBu), Asn(Trt), Gln(Trt) and Tyr(tBu)] are coupled using DIPCDI and HOBt in DMF. After complete assembly of the peptide chain the terminal Fmoc-protecting group is removed with piperidine in DMF as before. L(+)-biotinyl-aminohexanoic acid is then coupled to the terminal amino group using DIPCDI and HOBt in DMF using four equivalents of the reagents for four days at RT. The peptide is cleaved from the resin support and all side-chain protecting groups are simultaneously removed by using a reagent consisting of 5% dodecylmethylsulfide and 5% water in TFA for two hours at RT. Resin particles are filtered off, washed with TFA and the product is precipitated from the combined filtrates by the addition of 10 to 20 volumes of diethyl ether, washed with ether and dried. The product is purified by chromatography on a C-18 wide-pore silica column using a gradient of acetonitrile in 2% aqueous phosphoric acid. Fractions containing the pure compound are collected, filtered through an anion-exchange resin (Biorad, AG4-X4 acetate form) and lyophilized to give the title compound. MS: 1958.0 (M-H)−1
- Mouse mammary carcinoma 4T1 cells (5×103) are plated in 96-well Ultra low Attachment plates (#3474, Corning Inc.) in 100 μL of Dulbecco's modified eagle medium containing 10% FBS. Cells are cultured for 2 h and inhibitors are added at various concentrations in a final concentration of 0.1% DMSO. After 48 h, cell growth is assayed with the cell counting kit-8 (Wako Pure Chemical), which uses a water soluble tetrazolium salt WST8. Twenty μL of the reagent is added into each well and cells are further cultured for 2 h. The optical density is measured at 450 nm. The concentration of compound causing 50% inhibition of growth is determined.
- female or male BALB/c nude mice (5-8 weeks old, Charles River Japan, Inc., Yokohama, Japan) are kept under sterile conditions with water and feed ad libitum. Tumours are induced by subcutaneous injection of tumour cells (human epithelial cell line MIA PaCa-2; European Collection of Cell Cultures (ECACC), Salisbury, Wiltshire, UK, Catalogue Number 85062806; cell line from a 65 year old Caucasian male; undifferentiated human pancreatic carcinoma cell line) into left or right flank of mice under Forene® anaesthesia (Abbott Japan Co., Ltd., Tokyo, Japan). Treatment with the test compound is started when the mean tumor volumes reached approximately 100 mm3. Tumour growth is measured two times per week and 1 day after the last treatment by determining the length of two perpendicular axis. The tumour volumes are calculated in accordance with published methods (see Evans et al., Brit. J. Cancer 45, 466-8, 1982). The anti-tumour efficacy is determined as the mean increase in tumour volume of the treated animals divided by the mean increase in tumour volume of the untreated animals (controls) and, after multiplication by 100, is expressed as delta T/C [%]. Tumour regression is reported as the mean changes of tumor volume of the treated animals divided by the mean tumor volume at start of treatment and, after multiplication by 100, is expressed as regression [%]. The test compound is orally administered daily with or without drug holidays.
- As an alternative to cell line MIA PaCa-2, another cell line may also be used in the same manner, for example:
-
- the 4T1 breast carcinoma cell line (ATCC Number CRL-2539; see also Cancer. 88(12 Supple), 2979-2988, 2000) with female BALB/c mice (injection into mammary fat pad).
- On the basis of these studies, a compound of formula I according to the invention shows therapeutic efficacy especially against proliferative diseases responsive to an inhibition of a tyrosine kinase.
- Tablets comprising 50 mg of active ingredient, for example one of the compounds of formula I described in Examples 1 to 131, and having the following composition are prepared in customary manner:
-
Composition: active ingredient 50 mg wheat starch 150 mg lactose 125 mg colloidal silicic acid 12.5 mg talc 22.5 mg magnesium stearate 2.5 mg Total: 362.5 mg - Preparation: The active ingredient is mixed with a portion of the wheat starch, with the lactose and the colloidal silicic acid and the mixture is forced through a sieve. A further portion of the wheat starch is made into a paste, on a water bath, with five times the amount of water and the powder mixture is kneaded with the paste until a slightly plastic mass is obtained.
- The plastic mass is pressed through a sieve of about 3 mm mesh size and dried, and the resulting dry granules are again forced through a sieve. Then the remainder of the wheat starch, the talc and the magnesium stearate are mixed in and the mixture is compressed to form tablets weighing 145 mg and having a breaking notch.
- 5000 soft gelatin capsules comprising each 50 mg of active ingredient, for example one of the compounds of formula I described in Examples 1 to 131, are prepared in customary manner:
-
Composition: active ingredient 250 g Lauroglykol 2 litres - Preparation: The pulverized active ingredient is suspended in Lauroglykol® (propylene glycol laurate, Gattefossé S.A., Saint Priest, France) and ground in a wet pulverizer to a particle size of approx. 1 to 3 μm. 0.419 g portions of the mixture are then dispensed into soft gelatin capsules using a capsule-filling machine.
-
Biological results: FAK Phos Growth T Cell IGF- IC50 IC50 IC50 Migration 1R IC50 Example (nM) (μM) (μM) IC50 (μM) (μM) 1.00 140 0.7 >10 2.00 13 1.2 3.01 44 0.34 >10 3.02 36 0.85 4 3.03 9.1 0.14 0.8 3.04 32 0.53 2 3.05 21 0.17 2 >10 3.06 13 0.11 2 3.07 16 0.45 2 3.08 74 0.3 6 3.09 48 0.5 0.7 3.10 52 0.95 >10 3.11 9 0.04 0.3 0.2 3.12 5.4 0.01 1 3.13 58 1.7 0.6 0.74 3.14 54 0.4 5 3.15 7 0.02 0.8 0.94 3.16 48 1.1 3 3.17 2.8 0.03 0.2 <0.08 3.18 130 1.5 9 3.19 6.8 0.35 0.8 0.1 3.20 16 0.22 0.3 3.22 120 0.9 2 3.23 38 0.39 0.5 3.24 64 3.5 5 3.25 22 0.3 0.3 0.81 3.26 50 0.79 2 3.28 43 0.71 0.7 3.29 89 0.6 >10 3.30 69 0.6 3 3.31 13 1.1 5 3.32 14 0.18 0.49 0.28 0.12 3.33 2.9 0.03 0.05 0.09 0.13 3.34 7 0.1 0.24 0.13 <0.08 3.35 13 0.02 0.17 0.8 3.55 3.36 43 1.8 2.8 3.37 39 1.1 2.6 3.38 64 1.7 3.8 3.39 2 0.02 0.03 1 0.09 3.40 9 >10 0.9 3.41 22 >10 0.43 3.42 29 0.35 0.3 3.43 5.6 0.2 0.11 0.27 3.44 11 0.05 0.09 0.09 3.45 0.9 0.02 0.02 3.46 4 0.1 0.18 0.3 3.47 1 0.1 0.06 3.48 7 0.07 0.3 0.21 3.49 39 10 0.39 3.50 13 0.12 1 1.19 3.51 29 0.2 0.4 0.41 3.52 29 0.42 2 3.53 6 0.07 0.21 3.54 0.9 0.01 0.07 <0.08 3.55 34 >10 3 3.56 28 0.53 0.15 3.57 28 0.61 3 3.58 21 0.08 0.3 0.14 3.59 95 1.2 >10 3.60 90 0.93 2 3.61 12 10 >10 3.62 63 >10 >10 3.63 27 >10 >10 3.64 5 0.13 0.7 0.21 3.65 8 0.08 0.1 0.15 3.66 1 0.08 0.07 0.25 3.67 6 0.38 0.39 3.68 5.5 0.2 0.63 1 3.69 4 0.2 0.11 0.58 3.70 3.5 0.02 0.13 3.71 11 0.05 0.08 3.72 2.1 0.11 0.06 3.73 11 0.03 0.29 1.63 3.74 15 0.1 0.15 3.75 72 0.5 1.3 3.76 15 0.29 1.3 0.7 3.77 65 >10 3 3.78 10 >10 0.22 3.79 5 1.3 0.12 3.80 12 0.22 0.45 5 3.81 21 0.52 0.98 >10 3.82 4.8 0.2 0.07 3.83 20 0.08 0.32 0.68 3.84 10 1 0.08 6.00 110 0.35 5 7.00 5.3 0.21 0.47 0.04 0.19 7.01 4.7 0.6 0.54 0.19 7.02 7.5 0.1 0.36 0.77 7.03 2.9 0.3 0.39 0.27 7.04 5.2 1 0.29 7.05 6.2 0.3 0.2 0.25 7.06 17 0.8 1.09 0.25 7.07 4.1 0.9 0.18 7.08 8.7 0.8 1 7.09 8.2 1 0.85 7.10 6.6 1 0.98 7.11 2.5 0.6 1.2 0.77 7.12 1.9 0.9 1 0.31 0.62 7.13 5.5 0.8 1.22 7.14 7.6 0.3 0.36 0.33 7.15 4.5 0.06 0.19 0.26 7.16 6.4 0.2 0.42 7.17 4.3 0.7 0.69 7.18 6.2 0.5 0.7 7.19 13 0.33 7.20 2.5 >10 0.11 7.21 3.3 >10 0.46 7.22 25 0.48 7.23 1.4 0.25 7.24 5.1 0.09 7.25 13 0.2 0.73 7.25 2 >10 0.57 7.26 4.1 0.15 7.27 21 0.5 0.22 7.28 34 1 0.15 7.29 57 2 0.48 7.30 2.1 0.3 1 8.01 6.6 0.6 0.33 8.02 2.4 0.5 0.99 8.03 13 0.22 1 >10 8.04 8 >10 1.1 9.01 22 0.36 1 0.6 9.02 15 0.5 0.81 9.03 18 0.1 0.37 9.04 13 0.2 0.73 9.05 22 0.36 1.6 0.6 9.06 23 3 0.4 0.3 9.07 17 >10 0.26 10.01 39 1 0.44 10.02 26 0.9 1.06 10.03 23 0.9 2.4 11.01 9 0.7 0.85 11.02 4.1 0.8 0.69 11.03 26 0.41 0.1 11.04 4.3 >10 3.2 12.01 2.5 0.09 0.4 0.22 12.02 1.6 0.05 12.03 2.3 0.25 12.04 1.1 0.14 12.06 2.6 13.01 65 0.81 14.01 19 0.2 1.47 0.28 14.02 190 2 1.1 1 14.03 30 10 1.01 14.04 18 0.54 14.05 37 >10 1 14.06 63 10 1.11 14.07 7.5 0.2 1.4 15.01 15 10 0.47 15.02 21 >10 0.66 15.03 44 2 1.67 16.01 44 >10 4 16.02 6 >10 0.6 16.03 21 3 >10 16.04 9.5 >10 0.92 16B 11 3 7 16.C 28 0.9 >10 18.01 19 >10 1.29 19.01 <1 0.2 0.3 0.29 1.41 19.02 1.6 0.13 0.38 0.91 19.03 <1 0.3 0.09 0.64 19.04 1.6 0.2 0.34 0.14 19.05 1.8 0.2 0.67 0.07 0.47 19.06 5 1 0.7 19.07 2.1 0.3 0.11 19.08 3.2 0.03 0.4 0.29 0.13 19.09 1.3 0.17 0.39 0.3 0.48 19.10 1.3 0.06 0.56 1.02 19.11 38 >10 2 19.12 9 >10 0.7 0.63 19.13 2.5 0.3 1.1 19.14 2.6 0.4 1.13 0.44 19.15 3.1 0.5 0.36 19.16 2.3 0.7 1.1 19.17 1 >10 0.17 19.18 7 0.13 0.87 19.19 5.7 0.4 19.20 1.6 0.03 0.07 0.23 19.21 84 >10 1.71 19.22 3.4 0.12 0.51 19.23 6.4 0.7 0.71 19.24 1.8 0.05 0.12 19.25 7.2 1 0.49 0.24 19.26 6.1 0.1 0.3 19.27 1.5 0.3 0.4 19.28 4.8 0.1 0.12 0.3 0.46 19.29 1.9 19.30 <1 0.06 0.1 19.31 1.8 0.4 0.38 19.32 1.4 0.2 0.31 20.01 10 0.3 0.18 0.25 0.7 20.02 9 0.12 0.17 0.75 0.52 20.03 42 0.4 2.5 2.78 20.04 23 0.58 1.9 20.05 6.8 0.87 1.46 20.06 5 0.36 0.14 49 20.07 3 0.1 0.05 0.38 20.08 6.8 0.17 0.05 0.29 20.09 2 0.3 0.01 20.10 2 0.1 0.02 20.11 26 2 0.4 20.12 9.5 20.13 6.3 0.04 20.14 33 0.32 20.15 14 0.4 0.97 0.3 20.16 7.5 0.06 20.17 2 0.14 20.18 15 0.81 20.19 28 0.21 20.20 3.12 0.1 20.21 26 3 0.68 20.22 8 >10 0.19 20.23 30 0.49 3 20.24 19 0.48 2 20.25 6.2 0.21 0.06 20.26 5.3 0.76 0.27 20.27 12 0.85 0.05 0.29 20.28 9.2 0.17 0.08 0.42 20.29 6.1 0.2 0.05 0.31 20.30 7.6 0.3 0.08 0.67 20.31 39 0.5 20.32 13 0.11 20.33 2.5 0.38 20.34 13 1 0.12 20.35 8.7 0.09 0.09 0.15 21.01 1 0.07 0.19 0.47 21.02 8.5 0.33 >10 21.03 1.7 0.3 0.3 21.04 1.8 0.05 0.3 22.01 43 >10 >10 22.02 26 1 3 22.03 6.6 0.09 0.15 0.26 23.01 3.4 0.6 0.2 0.63 0.53 23.02 1.5 0.2 0.4 0.8 23.03 1.7 1 1.12 0.82 23.04 1.2 0.9 1.07 0.6 23.05 1.9 >10 0.59 23.06 16 1 0.57 23.07 2.1 3 0.84 23.08 6.7 0.3 0.49 23.09 2.1 0.2 0.28 24.01 3.6 0.11 0.44 0.05 24.02 2.1 0.5 0.11 0.39 24.03 1 0.3 1.08 25.01 8.5 3 1 25.02 3 0.4 0.13 0.64 26.01 4.4 0.05 0.35 0.29 26.02 1.9 0.03 0.12 0.09 0.39 26.03 1.4 0.1 0.13 0.23 26.04 4.9 0.05 0.43 0.29 1.16 26.05 2.1 0.09 0.23 1.5 26.06 4.4 0.1 0.35 26.07 11 0.5 0.95 26.08 2.9 0.01 0.18 26.09 2.3 0.04 0.22 26.10 2 0.01 0.14 26.11 4.4 0.4 0.78 0.5 26.12 3.7 0.2 0.19 26.13 1.6 0.2 0.44 26.14 5 0.19 26.15 6.9 1.2 0.08 0.07 26.16 9 0.32 2 26.17 17 0.3 0.1 0.26 26.18 1.3 6 1.17 26.19 9.2 0.43 0.79 26.20 10 0.14 0.22 0.6 0.49 26.21 1.1 0.1 0.49 26.22 <1 0.1 0.28 26.23 1.4 0.3 0.09 0.3 0.18 26.24 1 0.5 0.48 0.9 26.25 <1 0.6 0.73 0.3 26.26 1.9 0.2 0.07 0.34 26.27 4.8 0.6 1.49 26.28 2.1 0.5 1.52 26.29 <1 0.31 0.26 26.30 4.4 1 0.76 26.31 2 0.3 0.16 26.32 1.6 0.05 0.6 26.33 4 0.06 0.23 26.34 7 0.1 0.25 26.35 4.5 0.05 0.3 26.36 1.9 0.07 0.09 26.37 <1 26.38 <1 26.39 3.1 27.01 14 0.06 0.47 27.02 5.1 0.5 1.1 27.03 6.3 >10 0.56 27.04 11 0.1 0.27 27.05 8.2 0.04 0.3 27.06 1 0.08 0.31 27.07 5.5 2 0.57 27.08 9.3 0.6 0.75 27.09 4.2 0.5 0.36 28.01 12 0.3 0.46 0.3 28.02 1.9 0.08 0.44 3.71 28.03 7.4 0.07 0.29 28.04 7.5 0.3 0.3 28.05 6.7 0.1 0.12 1.39 28.06 17 0.6 0.56 28.07 47 3 >10 28.08 4.6 0.4 0.37 28.09 3.1 0.5 0.36 28.10 20 3 1.85 28.11 4.2 0.5 0.63 28.12 3.2 0.3 0.43 0.1 28.13 7.8 0.1 0.55 0.29 28.14 3 0.1 1.44 28.15 10 0.5 0.69 28.16 11 0.11 1 0.6 28.17 15 0.16 1.9 28.18 9.1 >10 2.03 28.19 3.7 0.5 0.14 28.20 4.4 2 0.4 28.21 1.3 0.1 0.23 28.22 1.3 0.1 0.3 28.23 5.9 0.5 0.28 28.24 2.9 0.2 0.09 2.57 28.25 3.9 0.04 0.13 28.26 6.6 0.2 0.57 28.27 2.4 0.3 0.42 0.5 28.28 5.2 0.4 0.52 1 28.29 11 0.4 0.36 28.30 2.3 0.9 0.11 28.31 7.4 0.06 1.06 29.01 13 0.7 2.2 0.09 29.02 3.3 0.7 1.1 29.03 5.6 0.1 0.99 30.01 22 0.2 0.89 30.02 12 0.2 0.47 30.03 19 0.5 0.68 30.04 25 0.3 0.99 30.05 8.5 2 0.29 30.06 15 1 1.03 30.07 8.8 0.6 0.47 31.01 30 >10 1.6 31.02 31 0.28 0.29 0.42 32.01 4.1 0.1 0.29 32.02 5.9 0.05 0.37 0.12 33.01 2.5 0.08 0.25 33.02 5.2 0.06 0.25 0.1 34.01 8 0.1 0.37 0.28 34.02 11 0.08 1.17 34.03 33 0.19 2.25 34.04 13 >10 1.22 34.05 51 0.36 5.1 34.06 14 >10 3 34.07 27 >10 2.7 34.08 8.7 >10 1.9 35.01 6.8 >10 1.43 35.02 6.1 0.7 0.23 51.00 8.1 0.013 0.19 0.2 52.00 13 0.2 0.41 <0.08
Claims (9)
1. A compound of formula I
wherein
each of R0, R1, and R2 independently is hydrogen, —S(O)0-2NR12R13, —S(O)0-2R13, —NR12S(O)0-2R13 or —C(O)NR12R13;
R3 is —S(O)0-2NR12R13 or —NR12S(O)0-2R13;
wherein R12 is selected from hydrogen and C1-6alkyl; and R13 is selected from hydrogen, C1-6alkyl and C3-12cycloalkyl;
R4 is hydrogen;
each of R5 and R6 independently is hydrogen or halogen;
each of R7, R8, R9, and R10 independently is ethoxy, ethyl, propyl, t-butyl, trifluoromethyl, nitrile, cyclobutyloxy, 2,2,2-trifluoroethoxy, isobutyloxy, t-butyloxy, isopropyloxy, methyl-amino-carbonyl, cyclopropyl-methoxy, dimethylamino-propyl-amino, methoxy-ethoxy, —XR11, —C(O)R11 or —OXR11; wherein X is a bond, methylene or ethylene; R11 is selected from piperazinyl, piperidinyl, pyrrolidinyl, morpholino, azepanyl and 1,4-dioxa-8-aza-spiro[4.5]dec-8-yl; wherein R11 is optionally substituted by 1 to 3 radicals independently selected from methyl, isopropyl, acetyl, acetyl-methyl-amino, 3-dimethylamino-2,2-dimethyl-propylamino, ethyl-methyl-amino-ethoxy, diethyl-amino-ethoxy, amino-carbonyl, ethyl, 2-oxo-pyrrolidin-1-yl, pyrrolidinyl, pyrrolidinyl-methyl, piperidinyl optionally substituted with methyl or ethyl, morpholino, dimethylamino, dimethylamino-propyl-amino, methyl-amino and ethyl-amino;
wherein one of R7, R8 and R9 independently of each other can also be hydrogen; and
A is C; or
salts thereof;
with the proviso that said compound of Formula (I) is not
2. The compound of claim 1 , wherein
each of R0 or R2 independently is hydrogen;
R1 is hydrogen; and
R3 is selected from dimethyl-sulfamoyl, isobutyl-sulfamoyl, methyl-sulfamoyl, ethyl-sulfamoyl, 1-ethyl-propyl-sulfamoyl, cyclopentyl-sulfamoyl, isopropyl-sulfamoyl, cyclopropyl-methyl-sulfamoyl or cyclobutyl-sulfamoyl.
3. A pharmaceutical composition comprising a compound according to claim 1 , as active ingredient together with one or more pharmaceutically acceptable diluents or carriers.
4. A combination comprising a therapeutically effective amount of a compound according to claim 1 and one or more further known drug substances, said further drug substance being useful in the treatment of neoplastic diseases or immune system disorders.
5. A method for the treatment of breast tumor in a subject in need thereof which comprises administering an effective amount of a compound according to claim 1 .
6. A compound of Formula I′
in which:
n′ is selected from 1 and 2;
R′1 is selected from pyridinyl, pyrazolyl and pyrimidinyl; each of which is substituted by 1 to 3 radicals independently selected from ethoxy, ethyl, propyl, methyl, t-butyl, trifluoromethyl, nitrile, cyclobutyloxy, 2,2,2-trifluoroethoxy, isobutyloxy, t-butyloxy, isopropyloxy, methyl-amino-carbonyl, cyclopropyl-methoxy, dimethylamino-propyl-amino, methoxy-ethoxy, —X′R′4, —C(O)R′4 and —OX′R′4; wherein X′ is a bond, methylene or ethylene; R′4 is selected from piperazinyl, piperidinyl, pyrrolidinyl, morpholino, azepanyl and 1,4-dioxa-8-aza-spiro[4.5]dec-8-yl; wherein R′4 is optionally substituted by 1 to 3 radicals independently selected from methyl, isopropyl, acetyl, acetyl-methyl-amino, 3-dimethylamino-2,2-dimethyl-propylamino, ethyl-methyl-amino-ethoxy, diethyl-amino-ethoxy, amino-carbonyl, ethyl, 2-oxo-pyrrolidin-1-yl, pyrrolidinyl, pyrrolidinyl-methyl, piperidinyl optionally substituted with methyl or ethyl, morpholino, dimethylamino, dimethylamino-propyl-amino, methyl-amino and ethyl-amino.
R′2 is selected from hydrogen and halo; and
R3 is selected from dimethyl-sulfamoyl, isobutyl-sulfamoyl, methyl-sulfamoyl, ethyl-sulfamoyl, 1-ethyl-propyl-sulfamoyl, cyclopentyl-sulfamoyl, isopropyl-sulfamoyl, cyclopropyl-methyl-sulfamoyl or cyclobutyl-sulfamoyl.
7. A pharmaceutical composition comprising a compound according to claim 6 , as active ingredient together with one or more pharmaceutically acceptable diluents or carriers.
8. A combination comprising a therapeutically effective amount of a compound according to claim 6 and one or more further known drug substances, said further drug substance being useful in the treatment of neoplastic diseases or immune system disorders.
9. A method for the treatment of breast tumor in a subject in need thereof which comprises administering an effective amount of a compound according to claim 6 .
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/984,519 US20110098280A1 (en) | 2003-08-15 | 2011-01-04 | 2,4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0319227.5 | 2003-08-15 | ||
| GB0319227A GB0319227D0 (en) | 2003-08-15 | 2003-08-15 | Organic compounds |
| GB0322370A GB0322370D0 (en) | 2003-09-24 | 2003-09-24 | Organic compounds |
| GB0322370.8 | 2003-09-24 | ||
| PCT/EP2004/009099 WO2005016894A1 (en) | 2003-08-15 | 2004-08-13 | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| US56836706A | 2006-08-18 | 2006-08-18 | |
| US12/984,519 US20110098280A1 (en) | 2003-08-15 | 2011-01-04 | 2,4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2004/009099 Continuation WO2005016894A1 (en) | 2003-08-15 | 2004-08-13 | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| US56836706A Continuation | 2003-08-15 | 2006-08-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20110098280A1 true US20110098280A1 (en) | 2011-04-28 |
Family
ID=34196260
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/568,367 Active 2026-04-25 US7893074B2 (en) | 2003-08-15 | 2004-08-13 | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| US12/984,519 Abandoned US20110098280A1 (en) | 2003-08-15 | 2011-01-04 | 2,4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/568,367 Active 2026-04-25 US7893074B2 (en) | 2003-08-15 | 2004-08-13 | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
Country Status (32)
| Country | Link |
|---|---|
| US (2) | US7893074B2 (en) |
| EP (2) | EP1660458B1 (en) |
| JP (2) | JP4607879B2 (en) |
| KR (1) | KR100904570B1 (en) |
| AR (1) | AR045458A1 (en) |
| AT (1) | ATE542801T1 (en) |
| AU (2) | AU2004264382A1 (en) |
| BR (2) | BR122019017579B8 (en) |
| CA (1) | CA2533320A1 (en) |
| CO (1) | CO5680434A2 (en) |
| CY (2) | CY1112571T1 (en) |
| DK (2) | DK1660458T3 (en) |
| EC (1) | ECSP066371A (en) |
| ES (2) | ES2380206T3 (en) |
| HK (1) | HK1091813A1 (en) |
| HR (2) | HRP20120335T1 (en) |
| IL (1) | IL173129A0 (en) |
| IS (1) | IS2873B (en) |
| MA (1) | MA27994A1 (en) |
| MX (1) | MXPA06001759A (en) |
| MY (1) | MY147449A (en) |
| NO (1) | NO333306B1 (en) |
| NZ (1) | NZ585188A (en) |
| PL (2) | PL1660458T3 (en) |
| PT (2) | PT2287156E (en) |
| RU (1) | RU2395500C2 (en) |
| SG (1) | SG145749A1 (en) |
| SI (2) | SI1660458T1 (en) |
| TN (1) | TNSN06052A1 (en) |
| TW (1) | TWI378923B (en) |
| WO (1) | WO2005016894A1 (en) |
| ZA (1) | ZA200600464B (en) |
Cited By (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8927547B2 (en) | 2010-05-21 | 2015-01-06 | Noviga Research Ab | Pyrimidine derivatives |
| US9006241B2 (en) | 2011-03-24 | 2015-04-14 | Noviga Research Ab | Pyrimidine derivatives |
| US9012462B2 (en) | 2008-05-21 | 2015-04-21 | Ariad Pharmaceuticals, Inc. | Phosphorous derivatives as kinase inhibitors |
| US9145387B2 (en) | 2013-02-08 | 2015-09-29 | Celgene Avilomics Research, Inc. | ERK inhibitors and uses thereof |
| WO2015164604A1 (en) * | 2014-04-23 | 2015-10-29 | Dana-Farber Cancer Institute, Inc. | Hydrophobically tagged janus kinase inhibitors and uses thereof |
| WO2015164614A1 (en) * | 2014-04-23 | 2015-10-29 | Dana-Farber Cancer Institute, Inc. | Janus kinase inhibitors and uses thereof |
| US9273077B2 (en) | 2008-05-21 | 2016-03-01 | Ariad Pharmaceuticals, Inc. | Phosphorus derivatives as kinase inhibitors |
| WO2016029002A3 (en) * | 2014-08-22 | 2016-04-14 | Clovis Oncology, Inc. | Growth factor receptor inhibitors |
| US9505784B2 (en) | 2009-06-12 | 2016-11-29 | Dana-Farber Cancer Institute, Inc. | Fused 2-aminothiazole compounds |
| US9611283B1 (en) | 2013-04-10 | 2017-04-04 | Ariad Pharmaceuticals, Inc. | Methods for inhibiting cell proliferation in ALK-driven cancers |
| US9758522B2 (en) | 2012-10-19 | 2017-09-12 | Dana-Farber Cancer Institute, Inc. | Hydrophobically tagged small molecules as inducers of protein degradation |
| US9834518B2 (en) | 2011-05-04 | 2017-12-05 | Ariad Pharmaceuticals, Inc. | Compounds for inhibiting cell proliferation in EGFR-driven cancers |
| US9834571B2 (en) | 2012-05-05 | 2017-12-05 | Ariad Pharmaceuticals, Inc. | Compounds for inhibiting cell proliferation in EGFR-driven cancers |
| WO2018088749A1 (en) * | 2016-11-08 | 2018-05-17 | 한국화학연구원 | Novel pyrimidine compound, method for preparing same, and pharmaceutical composition containing same as active ingredient for preventing or treating cancer and inflammatory diseases |
| US10000483B2 (en) | 2012-10-19 | 2018-06-19 | Dana-Farber Cancer Institute, Inc. | Bone marrow on X chromosome kinase (BMX) inhibitors and uses thereof |
| US10005760B2 (en) | 2014-08-13 | 2018-06-26 | Celgene Car Llc | Forms and compositions of an ERK inhibitor |
| US10112927B2 (en) | 2012-10-18 | 2018-10-30 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinase 7 (CDK7) |
| US20180338973A1 (en) * | 2015-11-05 | 2018-11-29 | Hubei Bio-Pharmacutical Industrial Technological Institute Inc. | Pyrimidine derivative and use thereof |
| US10144730B2 (en) | 2011-11-17 | 2018-12-04 | Dana-Farber Cancer Institute, Inc. | Inhibitors of c-Jun-N-terminal kinase (JNK) |
| WO2018230934A1 (en) * | 2017-06-13 | 2018-12-20 | 한국화학연구원 | N2,n4-diphenylpyrimidine-2,4-diamine derivative, method for preparing same, and pharmaceutical composition containing same as active ingredient for prevention or treatment of cancer |
| RU2675850C2 (en) * | 2012-11-06 | 2018-12-25 | Шанхай Фокон Фармасьютикал Ко Лтд | Alk kinase inhibitors |
| US10550121B2 (en) | 2015-03-27 | 2020-02-04 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinases |
| US10702527B2 (en) | 2015-06-12 | 2020-07-07 | Dana-Farber Cancer Institute, Inc. | Combination therapy of transcription inhibitors and kinase inhibitors |
| US10870651B2 (en) | 2014-12-23 | 2020-12-22 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinase 7 (CDK7) |
| US10906889B2 (en) | 2013-10-18 | 2021-02-02 | Dana-Farber Cancer Institute, Inc. | Polycyclic inhibitors of cyclin-dependent kinase 7 (CDK7) |
| US11013741B1 (en) | 2018-04-05 | 2021-05-25 | Sumitomo Dainippon Pharma Oncology, Inc. | AXL kinase inhibitors and use of the same |
| US11040957B2 (en) | 2013-10-18 | 2021-06-22 | Dana-Farber Cancer Institute, Inc. | Heteroaromatic compounds useful for the treatment of proliferative diseases |
| US11142507B2 (en) | 2015-09-09 | 2021-10-12 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinases |
| US11529350B2 (en) | 2019-07-03 | 2022-12-20 | Sumitomo Pharma Oncology, Inc. | Tyrosine kinase non-receptor 1 (TNK1) inhibitors and uses thereof |
Families Citing this family (155)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI329105B (en) | 2002-02-01 | 2010-08-21 | Rigel Pharmaceuticals Inc | 2,4-pyrimidinediamine compounds and their uses |
| GB0206215D0 (en) * | 2002-03-15 | 2002-05-01 | Novartis Ag | Organic compounds |
| US7517886B2 (en) | 2002-07-29 | 2009-04-14 | Rigel Pharmaceuticals, Inc. | Methods of treating or preventing autoimmune diseases with 2,4-pyrimidinediamine compounds |
| EP1599468B1 (en) | 2003-01-14 | 2007-10-03 | Arena Pharmaceuticals, Inc. | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| GB0305929D0 (en) * | 2003-03-14 | 2003-04-23 | Novartis Ag | Organic compounds |
| JP2004313181A (en) * | 2003-04-02 | 2004-11-11 | Canon Inc | Probe for detecting infection-inducing microbe, probe set, carrier and method for testing gene |
| CN102358738A (en) | 2003-07-30 | 2012-02-22 | 里格尔药品股份有限公司 | 2,4-pyrimidinediamine compounds and uses of treating or preventing autoimmune diseases thereof |
| PL1660458T3 (en) * | 2003-08-15 | 2012-07-31 | Novartis Ag | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| US8131475B2 (en) | 2003-09-03 | 2012-03-06 | The United States Of America As Represented By The Secretary, Department Of Health And Human Services | Methods for identifying, diagnosing, and predicting survival of lymphomas |
| AU2005231507B2 (en) | 2004-04-08 | 2012-03-01 | Targegen, Inc. | Benzotriazine inhibitors of kinases |
| EP1598343A1 (en) * | 2004-05-19 | 2005-11-23 | Boehringer Ingelheim International GmbH | 2-Arylaminopyrimidine derivatives as PLK inhibitors |
| US7521457B2 (en) * | 2004-08-20 | 2009-04-21 | Boehringer Ingelheim International Gmbh | Pyrimidines as PLK inhibitors |
| JP5275628B2 (en) | 2004-08-25 | 2013-08-28 | ターゲジェン インコーポレーティッド | Heterocyclic compounds and methods of use |
| GB0419161D0 (en) * | 2004-08-27 | 2004-09-29 | Novartis Ag | Organic compounds |
| DE602006010979D1 (en) | 2005-01-19 | 2010-01-21 | Rigel Pharmaceuticals Inc | PRODRUGS FROM 2,4-PYRIMIDIN DIAMIN COMPOUNDS AND THEIR USES |
| US20070203161A1 (en) | 2006-02-24 | 2007-08-30 | Rigel Pharmaceuticals, Inc. | Compositions and methods for inhibition of the jak pathway |
| WO2006133426A2 (en) * | 2005-06-08 | 2006-12-14 | Rigel Pharmaceuticals, Inc. | Compositions and methods for inhibition of the jak pathway |
| WO2007028445A1 (en) * | 2005-07-15 | 2007-03-15 | Glaxo Group Limited | 6-indolyl-4-yl-amino-5-halogeno-2-pyrimidinyl-amino derivatives |
| PE20070362A1 (en) * | 2005-07-15 | 2007-04-23 | Glaxo Group Ltd | COMPOUNDS DERIVED FROM INDAZOLE-4-IL-2,4-PYRIMIDINDIAMINE AS INHIBITORS OF TYROSINE KINASE (KINASE Syk) |
| GB0517329D0 (en) * | 2005-08-25 | 2005-10-05 | Merck Sharp & Dohme | Stimulation of neurogenesis |
| US8604042B2 (en) | 2005-11-01 | 2013-12-10 | Targegen, Inc. | Bi-aryl meta-pyrimidine inhibitors of kinases |
| DK1951684T3 (en) * | 2005-11-01 | 2016-10-24 | Targegen Inc | BIARYLMETAPYRIMIDIN kinase inhibitors |
| US8133900B2 (en) | 2005-11-01 | 2012-03-13 | Targegen, Inc. | Use of bi-aryl meta-pyrimidine inhibitors of kinases |
| TW200736232A (en) * | 2006-01-26 | 2007-10-01 | Astrazeneca Ab | Pyrimidine derivatives |
| WO2007085540A1 (en) * | 2006-01-27 | 2007-08-02 | Glaxo Group Limited | 1h-indaz0l-4-yl-2 , 4-pyrimidinediamine derivatives |
| PT1984357E (en) | 2006-02-17 | 2013-12-23 | Rigel Pharmaceuticals Inc | 2,4-pyrimidinediamine compounds for treating or preventing autoimmune diseases |
| CA2642229C (en) | 2006-02-24 | 2015-05-12 | Rigel Pharmaceuticals, Inc. | Compositions and methods for inhibition of the jak pathway |
| US8168383B2 (en) | 2006-04-14 | 2012-05-01 | Cell Signaling Technology, Inc. | Gene defects and mutant ALK kinase in human solid tumors |
| EP2447360A1 (en) | 2006-04-14 | 2012-05-02 | Cell Signaling Technology, Inc. | Gene defects and mutant ALK kinase in human solid tumors |
| EP1914240B1 (en) | 2006-10-11 | 2009-12-02 | Astellas Pharma Inc. | EML4-ALK fusion gene |
| CA2598893C (en) | 2006-10-11 | 2012-04-10 | Astellas Pharma Inc. | Eml4-alk fusion gene |
| TWI432427B (en) | 2006-10-23 | 2014-04-01 | Cephalon Inc | Fused bicyclic derivatives of 2,4-diaminopyrimidine as alk and c-met inhibitors |
| AU2007333394C1 (en) * | 2006-12-08 | 2011-08-18 | Novartis Ag | Compounds and compositions as protein kinase inhibitors |
| EP3012249A1 (en) * | 2006-12-08 | 2016-04-27 | Novartis AG | Compounds and composition as protein kinase inhibitors |
| WO2008079719A1 (en) * | 2006-12-19 | 2008-07-03 | Genentech, Inc. | Pyrimidine kinase inhibitors |
| TW200902010A (en) | 2007-01-26 | 2009-01-16 | Smithkline Beecham Corp | Anthranilamide inhibitors of aurora kinase |
| MX2009011090A (en) | 2007-04-18 | 2009-11-02 | Pfizer Prod Inc | Sulfonyl amide derivatives for the treatment of abnormal cell growth. |
| TWI389893B (en) | 2007-07-06 | 2013-03-21 | Astellas Pharma Inc | Di (arylamino) ary1 compound |
| CN101796046A (en) | 2007-07-16 | 2010-08-04 | 阿斯利康(瑞典)有限公司 | Pyrimidine derivatives 934 |
| JP5298128B2 (en) * | 2007-08-08 | 2013-09-25 | グラクソスミスクライン エルエルシー | 2-[(2- {Phenylamino} -1H-pyrrolo [2,3-d] pyrimidin-4-yl) amino] benzamide derivatives as IGF-1R inhibitors for the treatment of cancer |
| WO2009032668A2 (en) * | 2007-08-28 | 2009-03-12 | Irm Llc | 2 -biphenylamino-4 -aminopyrimidine derivatives as kinase inhibitors |
| WO2009032694A1 (en) * | 2007-08-28 | 2009-03-12 | Dana Farber Cancer Institute | Amino substituted pyrimidine, pyrollopyridine and pyrazolopyrimidine derivatives useful as kinase inhibitors and in treating proliferative disorders and diseases associated with angiogenesis |
| WO2009080638A2 (en) | 2007-12-20 | 2009-07-02 | Cellzome Limited | Sulfamides as zap-70 inhibitors |
| EP2249650A4 (en) * | 2008-02-19 | 2012-01-11 | Glaxosmithkline Llc | Anilinopyridines as inhibitors of fak |
| MX2010010968A (en) | 2008-04-07 | 2010-10-26 | Irm Llc | Compounds and compositions as protein kinase inhibitors. |
| US8138339B2 (en) | 2008-04-16 | 2012-03-20 | Portola Pharmaceuticals, Inc. | Inhibitors of protein kinases |
| NZ589315A (en) | 2008-04-16 | 2012-11-30 | Portola Pharm Inc | 2,6-diamino-pyrimidin-5-yl-carboxamides as Spleen tryosine kinase (syk) or Janus kinase (JAK) inhibitors |
| KR20170051521A (en) | 2008-04-16 | 2017-05-11 | 포톨라 파마슈티컬스, 인코포레이티드 | 2,6-diamino-pyrimidin-5-yl-carboxamides as syk or jak kinases inhibitors |
| EP2271631B1 (en) | 2008-04-22 | 2018-07-04 | Portola Pharmaceuticals, Inc. | Inhibitors of protein kinases |
| JP5551689B2 (en) * | 2008-06-17 | 2014-07-16 | アストラゼネカ アクチボラグ | Pyridine compounds |
| US8445505B2 (en) | 2008-06-25 | 2013-05-21 | Irm Llc | Pyrimidine derivatives as kinase inhibitors |
| UY31929A (en) | 2008-06-25 | 2010-01-05 | Irm Llc | COMPOUNDS AND COMPOSITIONS AS CINASE INHIBITORS |
| BRPI0914682B8 (en) | 2008-06-27 | 2021-05-25 | Avila Therapeutics Inc | heteroaryl compounds and compositions comprising said compounds |
| US8338439B2 (en) | 2008-06-27 | 2012-12-25 | Celgene Avilomics Research, Inc. | 2,4-disubstituted pyrimidines useful as kinase inhibitors |
| US11351168B1 (en) | 2008-06-27 | 2022-06-07 | Celgene Car Llc | 2,4-disubstituted pyrimidines useful as kinase inhibitors |
| JO3067B1 (en) * | 2008-10-27 | 2017-03-15 | Glaxosmithkline Llc | Pyrazolylaminopyridines as inhibitors of fak |
| TWI491605B (en) | 2008-11-24 | 2015-07-11 | Boehringer Ingelheim Int | New compounds |
| AR074209A1 (en) | 2008-11-24 | 2010-12-29 | Boehringer Ingelheim Int | USEFUL PYRIMIDINE DERIVATIVES FOR CANCER TREATMENT |
| EP2376491B1 (en) | 2008-12-19 | 2015-03-04 | Cephalon, Inc. | Pyrrolotriazines as alk and jak2 inhibitors |
| JP5802136B2 (en) | 2009-01-23 | 2015-10-28 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Compositions and methods for inhibition of the JAK pathway |
| US20120270237A9 (en) | 2009-04-03 | 2012-10-25 | Cellzone AG | Methods for the identification of kinase interacting molecules and for the purification of kinase proteins |
| EP2419423A1 (en) | 2009-04-14 | 2012-02-22 | Cellzome Limited | Fluoro substituted pyrimidine compounds as jak3 inhibitors |
| CN102482277B (en) | 2009-05-05 | 2017-09-19 | 达纳-法伯癌症研究所有限公司 | Epidermal growth factor receptor inhibitor and the method for treating obstacle |
| TW201100441A (en) | 2009-06-01 | 2011-01-01 | Osi Pharm Inc | Amino pyrimidine anticancer compounds |
| US20120142667A1 (en) * | 2009-06-10 | 2012-06-07 | Nigel Ramsden | Pyrimidine derivatives as zap-70 inhibitors |
| EP2440548A1 (en) * | 2009-06-10 | 2012-04-18 | Abbott Laboratories | 2- ( lh-pyrazol-4 -ylamino ) -pyrimidine as kinase inhibitors |
| US20120172384A1 (en) * | 2009-06-18 | 2012-07-05 | Mihiro Sunose | Heterocyclylaminopyrimidines as kinase inhibitors |
| WO2010146132A1 (en) * | 2009-06-18 | 2010-12-23 | Cellzome Limited | Sulfonamides and sulfamides as zap-70 inhibitors |
| CA2771675A1 (en) * | 2009-09-11 | 2011-03-17 | Cellzome Limited | Ortho substituted pyrimidine compounds as jak inhibitors |
| US20130137709A1 (en) * | 2010-05-05 | 2013-05-30 | Nathanael S. Gray | Compounds that modulate EGFR activity and methods for treating or preventing conditions therewith |
| US20120101108A1 (en) | 2010-08-06 | 2012-04-26 | Cell Signaling Technology, Inc. | Anaplastic Lymphoma Kinase In Kidney Cancer |
| KR20130099040A (en) | 2010-08-10 | 2013-09-05 | 셀진 아빌로믹스 리서치, 인코포레이티드 | Besylate salt of a btk inhibitor |
| AU2011305525B2 (en) | 2010-09-22 | 2016-08-18 | Arena Pharmaceuticals, Inc. | Modulators of the GPR119 receptor and the treatment of disorders related thereto |
| EP2635557A2 (en) | 2010-11-01 | 2013-09-11 | Portola Pharmaceuticals, Inc. | Nicotinamides as jak kinase modulators |
| US8846928B2 (en) | 2010-11-01 | 2014-09-30 | Portola Pharmaceuticals, Inc. | Benzamides and nicotinamides as Syk modulators |
| BR112013010564B1 (en) | 2010-11-01 | 2021-09-21 | Celgene Car Llc | HETEROCYCLIC COMPOUNDS AND COMPOSITIONS COMPRISING THE SAME |
| JP5956999B2 (en) | 2010-11-01 | 2016-07-27 | セルジーン アヴィロミクス リサーチ, インコーポレイテッド | Heteroaryl compounds and uses thereof |
| US20130317029A1 (en) | 2010-11-01 | 2013-11-28 | Portola Pharmaceuticals, Inc. | Oxypyrimidines as syk modulators |
| WO2012060847A1 (en) | 2010-11-07 | 2012-05-10 | Targegen, Inc. | Compositions and methods for treating myelofibrosis |
| ES2665013T3 (en) | 2010-11-10 | 2018-04-24 | Celgene Car Llc | EGFR selective mutant inhibitors and uses thereof |
| PT2646448T (en) | 2010-11-29 | 2017-10-04 | Osi Pharmaceuticals Llc | Macrocyclic kinase inhibitors |
| EP3453708B8 (en) * | 2010-12-17 | 2022-03-16 | Novartis AG | Process for the preparation of 5-chloro-n2-(2-isopropoxy-5-methyl-4-piperidin-4-yl-phenyl)-n4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2, 4-diamine di-hydrochloride |
| UY33817A (en) | 2010-12-21 | 2012-07-31 | Boehringer Ingelheim Int | ? NEW BENCYLIC OXINDOLPIRIMIDINES ?. |
| JP2012153674A (en) | 2011-01-28 | 2012-08-16 | Astellas Pharma Inc | Method for producing di(arylamino)aryl compound and synthetic intermediate for the method |
| CN103458881B (en) * | 2011-02-02 | 2015-08-12 | Irm责任有限公司 | The using method of ALK inhibitor |
| CA2827171C (en) | 2011-02-17 | 2019-04-09 | Cancer Therapeutics Crc Pty Limited | Fak inhibitors |
| EP2675794B1 (en) | 2011-02-17 | 2019-02-13 | Cancer Therapeutics Crc Pty Limited | Selective fak inhibitors |
| US9249124B2 (en) | 2011-03-30 | 2016-02-02 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Aurora kinase inhibitors and methods of making and using thereof |
| TWI681952B (en) | 2011-04-22 | 2020-01-11 | 美商標誌製藥公司 | Substituted diaminocarboxamide and diaminocarbonitrile pyrimidines, compositions thereof, and methods of treatment therewith |
| US9364476B2 (en) | 2011-10-28 | 2016-06-14 | Celgene Avilomics Research, Inc. | Methods of treating a Bruton's Tyrosine Kinase disease or disorder |
| IN2014CN04065A (en) | 2011-11-23 | 2015-09-04 | Portola Pharm Inc | |
| SG10201507865QA (en) | 2012-03-06 | 2015-10-29 | Cephalon Inc | Fused bicyclic 2,4-diaminopyrimidine derivative as a dual alk and fak inhibitor |
| BR112014022789B1 (en) | 2012-03-15 | 2022-04-19 | Celgene Car Llc | Solid forms of an epidermal growth factor receptor kinase inhibitor, pharmaceutical composition and uses thereof |
| EP2825042B1 (en) | 2012-03-15 | 2018-08-01 | Celgene CAR LLC | Salts of an epidermal growth factor receptor kinase inhibitor |
| KR101582852B1 (en) | 2012-05-24 | 2016-01-07 | 서울대학교 산학협력단 | Therapeutics for the treatment of neurodegenerative diseases mediated by Tau proteins |
| KR101446742B1 (en) * | 2012-08-10 | 2014-10-01 | 한국화학연구원 | N2,N4-bis(4-(piperazin-1-yl)phenyl)pyrimidine-2,4-diamine derivatives or pharmaceutically acceptable salt thereof, and pharmaceutical composition for the prevention or treatment of cancer containing the same as an active ingredient |
| WO2014058921A2 (en) | 2012-10-08 | 2014-04-17 | Portola Pharmaceuticals, Inc. | Substituted pyrimidinyl kinase inhibitors |
| AU2013337264B2 (en) | 2012-11-05 | 2018-03-08 | Foundation Medicine, Inc. | Novel fusion molecules and uses thereof |
| CN103804299A (en) * | 2012-11-14 | 2014-05-21 | 韩冰 | Compound with neuroprotective effect and use thereof |
| WO2014100748A1 (en) | 2012-12-21 | 2014-06-26 | Celgene Avilomics Research, Inc. | Heteroaryl compounds and uses thereof |
| CA3150658A1 (en) | 2013-01-18 | 2014-07-24 | Foundation Medicine, Inc. | Methods of treating cholangiocarcinoma |
| JP2016509045A (en) | 2013-02-22 | 2016-03-24 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | How to treat cancer and prevent drug resistance |
| MX2015016332A (en) | 2013-05-29 | 2016-07-20 | Cephalon Inc | Pyrrolotriazines as alk inhibitors. |
| JP2016522247A (en) | 2013-06-18 | 2016-07-28 | ノバルティス アーゲー | Combination medicine |
| CA2917735C (en) | 2013-07-11 | 2017-04-18 | Betta Pharmaceuticals Co., Ltd | Protein tyrosine kinase modulators and methods of use |
| US9492471B2 (en) | 2013-08-27 | 2016-11-15 | Celgene Avilomics Research, Inc. | Methods of treating a disease or disorder associated with Bruton'S Tyrosine Kinase |
| WO2015038868A1 (en) * | 2013-09-13 | 2015-03-19 | Cephalon, Inc. | Fused bicyclic 2,4-diaminopyrimidine derivatives |
| RU2550346C2 (en) | 2013-09-26 | 2015-05-10 | Общество с ограниченной ответственностью "Отечественные Фармацевтические Технологии" ООО"ФармТех" | New chemical compounds (versions) and using them for treating oncological diseases |
| EP3066215B1 (en) | 2013-11-06 | 2019-04-24 | The United States of America, represented by the Secretary, Department of Health and Human Services | Method for subtyping lymphoma types by means of expression profiling |
| US9415049B2 (en) | 2013-12-20 | 2016-08-16 | Celgene Avilomics Research, Inc. | Heteroaryl compounds and uses thereof |
| NZ715903A (en) | 2014-01-30 | 2017-06-30 | Signal Pharm Llc | Solid forms of 2-(tert-butylamino)-4-((1r,3r,4r)-3-hydroxy-4-methylcyclohexylamino)-pyrimidine-5-carboxamide, compositions thereof and methods of their use |
| TN2017000157A1 (en) | 2014-10-21 | 2018-10-19 | Ariad Pharma Inc | Crystalline forms of 5-chloro-n4-[-2-(dimethylphosphoryl) phenyl]-n2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl) piperidin-1-yl] pyrimidine-2,4-diamine |
| US9796685B2 (en) | 2014-12-16 | 2017-10-24 | Signal Pharmaceuticals, Llc | Formulations of 2-(tert-butylamino)-4-((1R,3R,4R)-3-hydroxy-4-Methylcyclohexylamino)-pyrimidine-5-carboxamide |
| ES2882954T3 (en) | 2014-12-16 | 2021-12-03 | Signal Pharm Llc | Medical uses comprising methods for the measurement of inhibition of c-Jun N-terminal kinase in the skin |
| CN116850181A (en) | 2015-01-06 | 2023-10-10 | 艾尼纳制药公司 | Treatment and S1P 1 Methods of receptor-related disorders |
| US20180022710A1 (en) | 2015-01-29 | 2018-01-25 | Signal Pharmaceuticals, Llc | Isotopologues of 2-(tert-butylamino)-4-((1r,3r,4r)-3-hydroxy-4-methylcyclohexylamino)-pyrimidine-5-carboxamide |
| WO2016167511A2 (en) * | 2015-04-14 | 2016-10-20 | 한국화학연구원 | N2-(2-methoxyphenyl)pyrimidine derivative, method for preparing same, and pharmaceutical composition for cancer prevention or treatment containing same as active ingredient |
| ES2929526T3 (en) | 2015-06-22 | 2022-11-29 | Arena Pharm Inc | (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclo-penta[b]indol-3-yl) acid L-arginine crystal salt acetic acid for use in disorders associated with the S1P1 receptor |
| CA2993173C (en) | 2015-07-24 | 2023-10-03 | Celgene Corporation | Methods of synthesis of (1r,2r,5r)-5-amino-2-methylcyclohexanol hydrochloride and intermediates useful therein |
| CN106883213B (en) * | 2015-12-15 | 2021-04-20 | 合肥中科普瑞昇生物医药科技有限公司 | Dual inhibitor of EGFR (epidermal growth factor receptor) and ALK (anaplastic lymphoma kinase) |
| CN107540666B (en) * | 2016-06-27 | 2023-03-24 | 杭州雷索药业有限公司 | Benzofuran pyrazole amine protein kinase inhibitor |
| SG10201914030UA (en) | 2016-08-29 | 2020-03-30 | Univ Michigan Regents | Aminopyrimidines as alk inhibitors |
| MA47504A (en) | 2017-02-16 | 2019-12-25 | Arena Pharm Inc | COMPOUNDS AND TREATMENT METHODS FOR PRIMITIVE BILIARY ANGIOCHOLITIS |
| KR101923852B1 (en) | 2017-02-24 | 2018-11-29 | 재단법인 대구경북첨단의료산업진흥재단 | Pharmaceutical composition for preventing or treating brain cancer comprising compounds penetrating blood-brain barrier |
| WO2019051084A1 (en) | 2017-09-07 | 2019-03-14 | Revolution Medicines, Inc. | Shp2 inhibitor compositions and methods for treating cancer |
| KR101992621B1 (en) | 2017-12-07 | 2019-09-27 | 주식회사 온코빅스 | Novel pyrimidine derivative showing growth inhibition of cancer cell and pharmaceutical composition comprising the same |
| WO2019117813A1 (en) * | 2017-12-15 | 2019-06-20 | National University Of Singapore | Focal adhesion kinase targeted therapeutics for the treatment of glaucoma and fibrosis |
| CN109369721A (en) * | 2017-12-21 | 2019-02-22 | 深圳市塔吉瑞生物医药有限公司 | For inhibiting the aryl phosphorous oxides of kinase activity |
| EP3863636A1 (en) | 2018-10-08 | 2021-08-18 | Revolution Medicines, Inc. | Shp2 inhibitor compositions for use in treating cancer |
| CN112771032B (en) * | 2018-10-29 | 2023-01-03 | 江苏先声药业有限公司 | Pyrimidine pyrazole compounds as fourth generation EGFR inhibitors |
| US20220064196A1 (en) * | 2019-01-17 | 2022-03-03 | Betta Pharmaceuticals Co., Ltd. | EGFR Inhibitors, Compositions and Methods Thereof |
| US20230096028A1 (en) | 2019-03-01 | 2023-03-30 | Revolution Medicines, Inc. | Bicyclic heterocyclyl compounds and uses thereof |
| AU2020242287A1 (en) | 2019-03-21 | 2021-09-02 | INSERM (Institut National de la Santé et de la Recherche Médicale) | A Dbait molecule in combination with kinase inhibitor for the treatment of cancer |
| TW202132314A (en) | 2019-11-04 | 2021-09-01 | 美商銳新醫藥公司 | Ras inhibitors |
| EP4054719A1 (en) | 2019-11-04 | 2022-09-14 | Revolution Medicines, Inc. | Ras inhibitors |
| CA3159559A1 (en) | 2019-11-04 | 2021-05-14 | Revolution Medicines, Inc. | Ras inhibitors |
| US20220401436A1 (en) | 2019-11-08 | 2022-12-22 | INSERM (Institute National de la Santé et de la Recherche Médicale) | Methods for the treatment of cancers that have acquired resistance to kinase inhibitors |
| PE20230249A1 (en) | 2019-11-08 | 2023-02-07 | Revolution Medicines Inc | BICYCLIC HETEROARYL COMPOUNDS AND THEIR USES |
| WO2021111311A2 (en) * | 2019-12-03 | 2021-06-10 | 삼진제약주식회사 | Novel adamantane derivatives as inhibitors of focal adhesion kinase |
| BR112022010086A2 (en) | 2020-01-07 | 2022-09-06 | Revolution Medicines Inc | SHP2 INHIBITOR DOSAGE AND CANCER TREATMENT METHODS |
| WO2021148581A1 (en) | 2020-01-22 | 2021-07-29 | Onxeo | Novel dbait molecule and its use |
| AU2021227907A1 (en) * | 2020-02-25 | 2022-09-29 | Dana-Farber Cancer Institute, Inc. | Potent and selective degraders of ALK |
| CN115916194A (en) | 2020-06-18 | 2023-04-04 | 锐新医药公司 | Methods for delaying, preventing and treating acquired resistance to RAS inhibitors |
| AU2021344830A1 (en) | 2020-09-03 | 2023-04-06 | Revolution Medicines, Inc. | Use of SOS1 inhibitors to treat malignancies with SHP2 mutations |
| KR20230067635A (en) | 2020-09-15 | 2023-05-16 | 레볼루션 메디슨즈, 인크. | Indole derivatives as RAS inhibitors in the treatment of cancer |
| CN117396472A (en) | 2020-12-22 | 2024-01-12 | 上海齐鲁锐格医药研发有限公司 | SOS1 inhibitors and uses thereof |
| EP4320153A1 (en) | 2021-04-09 | 2024-02-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for the treatment of anaplastic large cell lymphoma |
| CN117500811A (en) | 2021-05-05 | 2024-02-02 | 锐新医药公司 | Covalent RAS inhibitors and uses thereof |
| CR20230570A (en) | 2021-05-05 | 2024-01-22 | Revolution Medicines Inc | Ras inhibitors |
| WO2022235870A1 (en) | 2021-05-05 | 2022-11-10 | Revolution Medicines, Inc. | Ras inhibitors for the treatment of cancer |
| AR127308A1 (en) | 2021-10-08 | 2024-01-10 | Revolution Medicines Inc | RAS INHIBITORS |
| TW202340214A (en) | 2021-12-17 | 2023-10-16 | 美商健臻公司 | Pyrazolopyrazine compounds as shp2 inhibitors |
| EP4227307A1 (en) | 2022-02-11 | 2023-08-16 | Genzyme Corporation | Pyrazolopyrazine compounds as shp2 inhibitors |
| WO2023172940A1 (en) | 2022-03-08 | 2023-09-14 | Revolution Medicines, Inc. | Methods for treating immune refractory lung cancer |
| WO2023240263A1 (en) | 2022-06-10 | 2023-12-14 | Revolution Medicines, Inc. | Macrocyclic ras inhibitors |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3367149A (en) * | 1966-12-15 | 1968-02-06 | Minnesota Mining & Mfg | Radiant white light source |
| US3432493A (en) * | 1966-06-27 | 1969-03-11 | Abbott Lab | Substituted sulfanilamides |
| US5958935A (en) * | 1995-11-20 | 1999-09-28 | Celltech Therapeutics Limited | Substituted 2-anilinopyrimidines useful as protein kinase inhibitors |
| US6048866A (en) * | 1997-03-14 | 2000-04-11 | Celltech Therapeutics, Limited | Substituted 2-anilinopryimidines useful as protein kinase inhibitors |
| US6093716A (en) * | 1996-09-16 | 2000-07-25 | Celltech Therapeutics, Limited | Substituted 2-pyrimidineamines and processes for their preparation |
| US6114333A (en) * | 1996-10-28 | 2000-09-05 | Celltech Therapeutics Ltd. | 2-Pyrimidineamine derivatives and processes for their preparation |
| US6593326B1 (en) * | 1998-12-24 | 2003-07-15 | Astrazeneca Ab | 2,4-diamino pyrimidine compounds having anti-cell proliferative activity |
| US20060100227A1 (en) * | 2002-03-15 | 2006-05-11 | Rolf Baenteli | Pyrimidine derivaties |
| US7514446B2 (en) * | 2003-02-20 | 2009-04-07 | Smithkline Beecham Corporation | Pyrimidine compounds |
| US7671063B2 (en) * | 2003-09-16 | 2010-03-02 | Novartis Ag | 2,4 Di (hetero) -arylamino-pyrimidine derivatives as ZAP-70 and/or syk inhibitors |
| US7893074B2 (en) * | 2003-08-15 | 2011-02-22 | Novartis Ag | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| US8263590B2 (en) * | 2003-03-14 | 2012-09-11 | Carlos Garcia-Echeverria | Pyrimidine derivatives |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL129020C (en) | 1964-12-15 | |||
| JPS5490121A (en) * | 1977-11-28 | 1979-07-17 | Boettcher Barry | Neutral copper bonded body and antiinflaming agent |
| JPS5964558A (en) * | 1982-09-30 | 1984-04-12 | 三菱電機株式会社 | Manufacture of heat-resistant soft composite body |
| AU1507199A (en) * | 1997-12-15 | 1999-07-05 | Yamanouchi Pharmaceutical Co., Ltd. | Novel pyrimidine-5-carboxamide derivatives |
| WO1999050250A1 (en) | 1998-03-27 | 1999-10-07 | Janssen Pharmaceutica N.V. | Hiv inhibiting pyrimidine derivatives |
| DE69933680T2 (en) * | 1998-08-29 | 2007-08-23 | Astrazeneca Ab | PYRIMIDINE COMPOUNDS |
| ATE288420T1 (en) | 1999-06-09 | 2005-02-15 | Yamanouchi Pharma Co Ltd | NOVEL HETEROCYCLIC CARBOXAMIDE DERIVATIVES |
| JP4622047B2 (en) * | 1999-06-09 | 2011-02-02 | アステラス製薬株式会社 | Novel heterocyclic carboxamide derivatives |
| JP2003511378A (en) | 1999-10-07 | 2003-03-25 | アムジエン・インコーポレーテツド | Triazine kinase inhibitors |
| WO2001060816A1 (en) * | 2000-02-17 | 2001-08-23 | Amgen Inc. | Kinase inhibitors |
| US6376770B1 (en) * | 2000-02-28 | 2002-04-23 | Douglas Hyde | Quick connecting universal electrical box and wiring system |
| GB0004887D0 (en) | 2000-03-01 | 2000-04-19 | Astrazeneca Uk Ltd | Chemical compounds |
| GB0004890D0 (en) * | 2000-03-01 | 2000-04-19 | Astrazeneca Uk Ltd | Chemical compounds |
| GB0004888D0 (en) | 2000-03-01 | 2000-04-19 | Astrazeneca Uk Ltd | Chemical compounds |
| US20020132823A1 (en) | 2001-01-17 | 2002-09-19 | Jiahuai Han | Assay method |
| CA2451128A1 (en) * | 2001-06-26 | 2003-01-09 | Bristol-Myers Squibb Company | N-heterocyclic inhibitors of tnf-alpha expression |
| JO3429B1 (en) * | 2001-08-13 | 2019-10-20 | Janssen Pharmaceutica Nv | Hiv inhibiting pyrimidines derivatives |
| US6939874B2 (en) * | 2001-08-22 | 2005-09-06 | Amgen Inc. | Substituted pyrimidinyl derivatives and methods of use |
| WO2003030909A1 (en) | 2001-09-25 | 2003-04-17 | Bayer Pharmaceuticals Corporation | 2- and 4-aminopyrimidines n-substtituded by a bicyclic ring for use as kinase inhibitors in the treatment of cancer |
| MXPA04004176A (en) * | 2001-11-01 | 2004-09-06 | Janssen Pharmaceutica Nv | AMINOBENZAMIDE DERIVATIVES AS GLYCOGEN SYNTHASE KINASE 3beta INHIBITORS. |
| TWI329105B (en) * | 2002-02-01 | 2010-08-21 | Rigel Pharmaceuticals Inc | 2,4-pyrimidinediamine compounds and their uses |
| AU2003209077A1 (en) | 2002-02-08 | 2003-09-02 | Smithkline Beecham Corporation | Pyrimidine compounds |
| AU2003231231A1 (en) * | 2002-05-06 | 2003-11-11 | Bayer Pharmaceuticals Corporation | Pyridinyl amino pyrimidine derivatives useful for treating hyper-proliferative disorders |
| EP1518855B1 (en) * | 2002-06-28 | 2011-10-26 | Astellas Pharma Inc. | Diaminopyrimidinecarboxa mide derivative |
| US7517886B2 (en) * | 2002-07-29 | 2009-04-14 | Rigel Pharmaceuticals, Inc. | Methods of treating or preventing autoimmune diseases with 2,4-pyrimidinediamine compounds |
| UA80767C2 (en) | 2002-12-20 | 2007-10-25 | Pfizer Prod Inc | Pyrimidine derivatives for the treatment of abnormal cell growth |
| EP1597237B1 (en) * | 2003-02-07 | 2016-07-27 | Janssen Pharmaceutica NV | Pyrimidine derivatives for the prevention of HIV infection |
| WO2005012304A2 (en) * | 2003-07-16 | 2005-02-10 | Janssen Pharmaceutica N.V. | Triazolopyrimidine derivatives as glycogen synthase kinase 3 inhibitors |
| CA2531333A1 (en) * | 2003-07-16 | 2005-02-10 | Janssen Pharmaceutica N.V. | Triazolopyrimidine derivatives as glycogen synthase kinase 3 inhibitors |
| DE602004032446D1 (en) * | 2003-08-07 | 2011-06-09 | Rigel Pharmaceuticals Inc | 2,4-PYRIMIDINDIAMIN COMPOUNDS AND USES AS ANTIPROLIFERATIVE AGENTS |
| CA2538413A1 (en) * | 2003-09-18 | 2005-03-24 | Novartis Ag | 2,4-di (phenylamino) pyrimidines useful in the treatment of proliferative disorders |
| CA2584295C (en) | 2004-11-24 | 2014-08-26 | Rigel Pharmaceuticals, Inc. | Spiro-2, 4-pyrimidinediamine compounds and their uses |
-
2004
- 2004-08-13 PL PL04764093T patent/PL1660458T3/en unknown
- 2004-08-13 PL PL10010291T patent/PL2287156T3/en unknown
- 2004-08-13 RU RU2006107785/04A patent/RU2395500C2/en active
- 2004-08-13 DK DK04764093.3T patent/DK1660458T3/en active
- 2004-08-13 BR BR122019017579A patent/BR122019017579B8/en active IP Right Grant
- 2004-08-13 CA CA002533320A patent/CA2533320A1/en not_active Abandoned
- 2004-08-13 EP EP04764093A patent/EP1660458B1/en active Active
- 2004-08-13 EP EP10010291.2A patent/EP2287156B1/en active Active
- 2004-08-13 PT PT100102912T patent/PT2287156E/en unknown
- 2004-08-13 ES ES04764093T patent/ES2380206T3/en active Active
- 2004-08-13 WO PCT/EP2004/009099 patent/WO2005016894A1/en active Application Filing
- 2004-08-13 SI SI200431849T patent/SI1660458T1/en unknown
- 2004-08-13 KR KR1020067003056A patent/KR100904570B1/en active Protection Beyond IP Right Term
- 2004-08-13 US US10/568,367 patent/US7893074B2/en active Active
- 2004-08-13 MY MYPI20043301A patent/MY147449A/en unknown
- 2004-08-13 NZ NZ585188A patent/NZ585188A/en not_active IP Right Cessation
- 2004-08-13 DK DK10010291.2T patent/DK2287156T3/en active
- 2004-08-13 AT AT04764093T patent/ATE542801T1/en active
- 2004-08-13 PT PT04764093T patent/PT1660458E/en unknown
- 2004-08-13 AU AU2004264382A patent/AU2004264382A1/en not_active Abandoned
- 2004-08-13 TW TW093124290A patent/TWI378923B/en active
- 2004-08-13 SI SI200432067T patent/SI2287156T1/en unknown
- 2004-08-13 ES ES10010291T patent/ES2424881T3/en active Active
- 2004-08-13 MX MXPA06001759A patent/MXPA06001759A/en active IP Right Grant
- 2004-08-13 SG SG200806063-4A patent/SG145749A1/en unknown
- 2004-08-13 BR BRPI0413616A patent/BRPI0413616B8/en active IP Right Grant
- 2004-08-13 JP JP2006522998A patent/JP4607879B2/en active Active
- 2004-08-17 AR ARP040102945A patent/AR045458A1/en active IP Right Grant
-
2006
- 2006-01-12 IL IL173129A patent/IL173129A0/en unknown
- 2006-01-17 ZA ZA2006/00464A patent/ZA200600464B/en unknown
- 2006-02-14 TN TNP2006000052A patent/TNSN06052A1/en unknown
- 2006-02-15 EC EC2006006371A patent/ECSP066371A/en unknown
- 2006-02-17 MA MA28815A patent/MA27994A1/en unknown
- 2006-02-20 CO CO06016492A patent/CO5680434A2/en active IP Right Grant
- 2006-03-13 IS IS8349A patent/IS2873B/en unknown
- 2006-03-15 NO NO20061214A patent/NO333306B1/en unknown
- 2006-11-07 HK HK06112236.3A patent/HK1091813A1/en unknown
-
2008
- 2008-09-29 AU AU2008229685A patent/AU2008229685B2/en active Active
-
2010
- 2010-07-16 JP JP2010162177A patent/JP2010241830A/en active Pending
-
2011
- 2011-01-04 US US12/984,519 patent/US20110098280A1/en not_active Abandoned
-
2012
- 2012-03-22 CY CY20121100300T patent/CY1112571T1/en unknown
- 2012-04-16 HR HRP20120335TT patent/HRP20120335T1/en unknown
-
2013
- 2013-08-01 HR HRP20130724TT patent/HRP20130724T1/en unknown
- 2013-08-13 CY CY20131100696T patent/CY1117015T1/en unknown
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3432493A (en) * | 1966-06-27 | 1969-03-11 | Abbott Lab | Substituted sulfanilamides |
| US3367149A (en) * | 1966-12-15 | 1968-02-06 | Minnesota Mining & Mfg | Radiant white light source |
| US6235746B1 (en) * | 1995-11-20 | 2001-05-22 | Celltech Therapeutics, Limited | Substituted 2-anilinopyrimidines useful as protein kinase inhibitors |
| US5958935A (en) * | 1995-11-20 | 1999-09-28 | Celltech Therapeutics Limited | Substituted 2-anilinopyrimidines useful as protein kinase inhibitors |
| US6093716A (en) * | 1996-09-16 | 2000-07-25 | Celltech Therapeutics, Limited | Substituted 2-pyrimidineamines and processes for their preparation |
| US6114333A (en) * | 1996-10-28 | 2000-09-05 | Celltech Therapeutics Ltd. | 2-Pyrimidineamine derivatives and processes for their preparation |
| US6048866A (en) * | 1997-03-14 | 2000-04-11 | Celltech Therapeutics, Limited | Substituted 2-anilinopryimidines useful as protein kinase inhibitors |
| US6593326B1 (en) * | 1998-12-24 | 2003-07-15 | Astrazeneca Ab | 2,4-diamino pyrimidine compounds having anti-cell proliferative activity |
| US20060100227A1 (en) * | 2002-03-15 | 2006-05-11 | Rolf Baenteli | Pyrimidine derivaties |
| US7514446B2 (en) * | 2003-02-20 | 2009-04-07 | Smithkline Beecham Corporation | Pyrimidine compounds |
| US8263590B2 (en) * | 2003-03-14 | 2012-09-11 | Carlos Garcia-Echeverria | Pyrimidine derivatives |
| US7893074B2 (en) * | 2003-08-15 | 2011-02-22 | Novartis Ag | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| US7671063B2 (en) * | 2003-09-16 | 2010-03-02 | Novartis Ag | 2,4 Di (hetero) -arylamino-pyrimidine derivatives as ZAP-70 and/or syk inhibitors |
Cited By (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9012462B2 (en) | 2008-05-21 | 2015-04-21 | Ariad Pharmaceuticals, Inc. | Phosphorous derivatives as kinase inhibitors |
| US9273077B2 (en) | 2008-05-21 | 2016-03-01 | Ariad Pharmaceuticals, Inc. | Phosphorus derivatives as kinase inhibitors |
| US9505784B2 (en) | 2009-06-12 | 2016-11-29 | Dana-Farber Cancer Institute, Inc. | Fused 2-aminothiazole compounds |
| US8927547B2 (en) | 2010-05-21 | 2015-01-06 | Noviga Research Ab | Pyrimidine derivatives |
| US9006241B2 (en) | 2011-03-24 | 2015-04-14 | Noviga Research Ab | Pyrimidine derivatives |
| US9834518B2 (en) | 2011-05-04 | 2017-12-05 | Ariad Pharmaceuticals, Inc. | Compounds for inhibiting cell proliferation in EGFR-driven cancers |
| US10981903B2 (en) | 2011-11-17 | 2021-04-20 | Dana-Farber Cancer Institute, Inc. | Inhibitors of c-Jun-N-terminal kinase (JNK) |
| US10144730B2 (en) | 2011-11-17 | 2018-12-04 | Dana-Farber Cancer Institute, Inc. | Inhibitors of c-Jun-N-terminal kinase (JNK) |
| US9834571B2 (en) | 2012-05-05 | 2017-12-05 | Ariad Pharmaceuticals, Inc. | Compounds for inhibiting cell proliferation in EGFR-driven cancers |
| US10787436B2 (en) | 2012-10-18 | 2020-09-29 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinase 7 (CDK7) |
| US10112927B2 (en) | 2012-10-18 | 2018-10-30 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinase 7 (CDK7) |
| USRE48175E1 (en) | 2012-10-19 | 2020-08-25 | Dana-Farber Cancer Institute, Inc. | Hydrophobically tagged small molecules as inducers of protein degradation |
| US10000483B2 (en) | 2012-10-19 | 2018-06-19 | Dana-Farber Cancer Institute, Inc. | Bone marrow on X chromosome kinase (BMX) inhibitors and uses thereof |
| US9758522B2 (en) | 2012-10-19 | 2017-09-12 | Dana-Farber Cancer Institute, Inc. | Hydrophobically tagged small molecules as inducers of protein degradation |
| RU2675850C2 (en) * | 2012-11-06 | 2018-12-25 | Шанхай Фокон Фармасьютикал Ко Лтд | Alk kinase inhibitors |
| US9980964B2 (en) | 2013-02-08 | 2018-05-29 | Celgene Car Llc | ERK inhibitors and uses thereof |
| US9561228B2 (en) | 2013-02-08 | 2017-02-07 | Celgene Avilomics Research, Inc. | ERK inhibitors and uses thereof |
| US9504686B2 (en) | 2013-02-08 | 2016-11-29 | Celgene Avilomics Research, Inc. | ERK inhibitors and uses thereof |
| US9145387B2 (en) | 2013-02-08 | 2015-09-29 | Celgene Avilomics Research, Inc. | ERK inhibitors and uses thereof |
| US9796700B2 (en) | 2013-02-08 | 2017-10-24 | Celgene Car Llc | ERK inhibitors and uses thereof |
| US9611283B1 (en) | 2013-04-10 | 2017-04-04 | Ariad Pharmaceuticals, Inc. | Methods for inhibiting cell proliferation in ALK-driven cancers |
| US11040957B2 (en) | 2013-10-18 | 2021-06-22 | Dana-Farber Cancer Institute, Inc. | Heteroaromatic compounds useful for the treatment of proliferative diseases |
| US10906889B2 (en) | 2013-10-18 | 2021-02-02 | Dana-Farber Cancer Institute, Inc. | Polycyclic inhibitors of cyclin-dependent kinase 7 (CDK7) |
| US9862688B2 (en) | 2014-04-23 | 2018-01-09 | Dana-Farber Cancer Institute, Inc. | Hydrophobically tagged janus kinase inhibitors and uses thereof |
| WO2015164614A1 (en) * | 2014-04-23 | 2015-10-29 | Dana-Farber Cancer Institute, Inc. | Janus kinase inhibitors and uses thereof |
| US10017477B2 (en) | 2014-04-23 | 2018-07-10 | Dana-Farber Cancer Institute, Inc. | Janus kinase inhibitors and uses thereof |
| WO2015164604A1 (en) * | 2014-04-23 | 2015-10-29 | Dana-Farber Cancer Institute, Inc. | Hydrophobically tagged janus kinase inhibitors and uses thereof |
| US10005760B2 (en) | 2014-08-13 | 2018-06-26 | Celgene Car Llc | Forms and compositions of an ERK inhibitor |
| US10202364B2 (en) | 2014-08-13 | 2019-02-12 | Celgene Car Llc | Forms and compositions of an ERK inhibitor |
| WO2016029002A3 (en) * | 2014-08-22 | 2016-04-14 | Clovis Oncology, Inc. | Growth factor receptor inhibitors |
| US10870651B2 (en) | 2014-12-23 | 2020-12-22 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinase 7 (CDK7) |
| US10550121B2 (en) | 2015-03-27 | 2020-02-04 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinases |
| US11325910B2 (en) | 2015-03-27 | 2022-05-10 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinases |
| US10702527B2 (en) | 2015-06-12 | 2020-07-07 | Dana-Farber Cancer Institute, Inc. | Combination therapy of transcription inhibitors and kinase inhibitors |
| US11142507B2 (en) | 2015-09-09 | 2021-10-12 | Dana-Farber Cancer Institute, Inc. | Inhibitors of cyclin-dependent kinases |
| US10695347B2 (en) * | 2015-11-05 | 2020-06-30 | Hubei Bio-Pharmaceutical Industrial Technological Institute, Inc. | Pyrimidine derivative and use thereof |
| US20180338973A1 (en) * | 2015-11-05 | 2018-11-29 | Hubei Bio-Pharmacutical Industrial Technological Institute Inc. | Pyrimidine derivative and use thereof |
| WO2018088749A1 (en) * | 2016-11-08 | 2018-05-17 | 한국화학연구원 | Novel pyrimidine compound, method for preparing same, and pharmaceutical composition containing same as active ingredient for preventing or treating cancer and inflammatory diseases |
| US10647686B2 (en) | 2016-11-08 | 2020-05-12 | Korea Research Institute Of Chemical Technology | Substituted pyrimidines for preventing or treating cancer and inflammatory diseases |
| WO2018230934A1 (en) * | 2017-06-13 | 2018-12-20 | 한국화학연구원 | N2,n4-diphenylpyrimidine-2,4-diamine derivative, method for preparing same, and pharmaceutical composition containing same as active ingredient for prevention or treatment of cancer |
| CN110740999A (en) * | 2017-06-13 | 2020-01-31 | 韩国化学研究院 | N2, N4-diphenylpyrimidine-2, 4-diamine derivative, process for producing the same, and pharmaceutical composition for preventing or treating cancer comprising the same as active ingredient |
| US11253516B2 (en) | 2017-06-13 | 2022-02-22 | Korea Research Institute Of Chemical Technology | N2,N4-diphenylpyrimidine-2,4-diamine derivative, method for preparing same, and pharmaceutical composition containing same as active ingredient for prevention or treatment of cancer |
| US11013741B1 (en) | 2018-04-05 | 2021-05-25 | Sumitomo Dainippon Pharma Oncology, Inc. | AXL kinase inhibitors and use of the same |
| US11400091B2 (en) | 2018-04-05 | 2022-08-02 | Sumitomo Pharma Oncology, Inc. | AXL kinase inhibitors and use of the same |
| US11529350B2 (en) | 2019-07-03 | 2022-12-20 | Sumitomo Pharma Oncology, Inc. | Tyrosine kinase non-receptor 1 (TNK1) inhibitors and uses thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7893074B2 (en) | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders | |
| US8263590B2 (en) | Pyrimidine derivatives | |
| US20090131436A1 (en) | Pyrimidine Derivatives | |
| US7910585B2 (en) | Pyrimidine derivatives | |
| ES2360502T3 (en) | 2,4-DI (PHENYLAMINE) USEFUL PYRIMIDINS FOR THE TREATMENT OF NEOPLASTIC DISEASES AND INFLAMMATORY DISORDERS AND THE IMMUNE SYSTEM. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: IRM LLC, BERMUDA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:GARCIA-ECHEVERRIA, CARLOS;KANAZAWA, TAKANORI;KAWAHARA, EIJI;AND OTHERS;SIGNING DATES FROM 20060102 TO 20060113;REEL/FRAME:029756/0525 Owner name: NOVARTIS AG, SWITZERLAND Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:GARCIA-ECHEVERRIA, CARLOS;KANAZAWA, TAKANORI;KAWAHARA, EIJI;AND OTHERS;SIGNING DATES FROM 20060102 TO 20060113;REEL/FRAME:029756/0525 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO PAY ISSUE FEE |