US20100000033A1 - Basic Bisazo Compounds - Google Patents
Basic Bisazo Compounds Download PDFInfo
- Publication number
- US20100000033A1 US20100000033A1 US12/375,375 US37537507A US2010000033A1 US 20100000033 A1 US20100000033 A1 US 20100000033A1 US 37537507 A US37537507 A US 37537507A US 2010000033 A1 US2010000033 A1 US 2010000033A1
- Authority
- US
- United States
- Prior art keywords
- alkyl group
- group
- substituted
- unsubstituted
- formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N NC1=CC=CC=C1 Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 38
- 0 *C1=C([1*])C(=O)N(*)C(O)=C1/N=N/C1=CC=CC=C1.*C1=C([1*])C(=O)N([2*])C(O)=C1/N=N/C1=CC=CC=C1.[3*]C.[3*]C.[4*]C.[4*]C.[5*]C([6*])(C)C Chemical compound *C1=C([1*])C(=O)N(*)C(O)=C1/N=N/C1=CC=CC=C1.*C1=C([1*])C(=O)N([2*])C(O)=C1/N=N/C1=CC=CC=C1.[3*]C.[3*]C.[4*]C.[4*]C.[5*]C([6*])(C)C 0.000 description 17
- VMPITZXILSNTON-UHFFFAOYSA-N COC1=C(N)C=CC=C1 Chemical compound COC1=C(N)C=CC=C1 VMPITZXILSNTON-UHFFFAOYSA-N 0.000 description 8
- RNVCVTLRINQCPJ-UHFFFAOYSA-N CC1=C(N)C=CC=C1 Chemical compound CC1=C(N)C=CC=C1 RNVCVTLRINQCPJ-UHFFFAOYSA-N 0.000 description 7
- UFFBMTHBGFGIHF-UHFFFAOYSA-N CC1=CC=CC(C)=C1N Chemical compound CC1=CC=CC(C)=C1N UFFBMTHBGFGIHF-UHFFFAOYSA-N 0.000 description 6
- ULHFFAFDSSHFDA-UHFFFAOYSA-N CCOC1=C(N)C=CC=C1 Chemical compound CCOC1=C(N)C=CC=C1 ULHFFAFDSSHFDA-UHFFFAOYSA-N 0.000 description 5
- NAZDVUBIEPVUKE-UHFFFAOYSA-N COC1=CC(N)=C(OC)C=C1 Chemical compound COC1=CC(N)=C(OC)C=C1 NAZDVUBIEPVUKE-UHFFFAOYSA-N 0.000 description 4
- XITGBAPJMIIYQP-UHFFFAOYSA-N CC1=C([N+]2=CC=CC=C2)C(=O)N(CCCN(C)C)C([O-])=C1 Chemical compound CC1=C([N+]2=CC=CC=C2)C(=O)N(CCCN(C)C)C([O-])=C1 XITGBAPJMIIYQP-UHFFFAOYSA-N 0.000 description 3
- FANQUUGICQRGJV-UHFFFAOYSA-N CC1=CC(C(C2=CC=CC=C2)C2=CC(C)=C(N)C=C2)=CC=C1N Chemical compound CC1=CC(C(C2=CC=CC=C2)C2=CC(C)=C(N)C=C2)=CC=C1N FANQUUGICQRGJV-UHFFFAOYSA-N 0.000 description 3
- DXMPETNNWLQTEJ-UHFFFAOYSA-N CCC(CC)(C1=CC(C)=C(N)C(C)=C1)C1=CC(C)=C(N)C(C)=C1 Chemical compound CCC(CC)(C1=CC(C)=C(N)C(C)=C1)C1=CC(C)=C(N)C(C)=C1 DXMPETNNWLQTEJ-UHFFFAOYSA-N 0.000 description 3
- HGACYDJLFXNRIZ-UHFFFAOYSA-N CCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 HGACYDJLFXNRIZ-UHFFFAOYSA-N 0.000 description 3
- JZCGQHHSEAXNCV-UHFFFAOYSA-N CCC(CC)C(C1=CC(C)=C(N)C(C)=C1)C1=CC(C)=C(N)C(C)=C1 Chemical compound CCC(CC)C(C1=CC(C)=C(N)C(C)=C1)C1=CC(C)=C(N)C(C)=C1 JZCGQHHSEAXNCV-UHFFFAOYSA-N 0.000 description 3
- MKDNQLOZVPBAKL-UHFFFAOYSA-N CCCCC(CC)(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 Chemical compound CCCCC(CC)(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 MKDNQLOZVPBAKL-UHFFFAOYSA-N 0.000 description 3
- YPIAOYDWAGQTPI-UHFFFAOYSA-N CC(C)CC(CC(C)C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CC(C)CC(CC(C)C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 YPIAOYDWAGQTPI-UHFFFAOYSA-N 0.000 description 2
- BQWPGJOJHBFSTF-UHFFFAOYSA-N CC(C)CCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CC(C)CCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 BQWPGJOJHBFSTF-UHFFFAOYSA-N 0.000 description 2
- XAQNIOKJLXBIOI-UHFFFAOYSA-N CC(CC1=CC=CC=C1)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CC(CC1=CC=CC=C1)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 XAQNIOKJLXBIOI-UHFFFAOYSA-N 0.000 description 2
- XITGBAPJMIIYQP-UHFFFAOYSA-O CC1=C([N+]2=CC=CC=C2)C(=O)N(CCCN(C)C)C(O)=C1.[Cl-] Chemical compound CC1=C([N+]2=CC=CC=C2)C(=O)N(CCCN(C)C)C(O)=C1.[Cl-] XITGBAPJMIIYQP-UHFFFAOYSA-O 0.000 description 2
- MADRVTFUZLHYOU-UHFFFAOYSA-N CC1=CC(C(C2=CC=CC=C2)C2=CC(C)=C(N)C(C)=C2)=CC(C)=C1N Chemical compound CC1=CC(C(C2=CC=CC=C2)C2=CC(C)=C(N)C(C)=C2)=CC(C)=C1N MADRVTFUZLHYOU-UHFFFAOYSA-N 0.000 description 2
- BTOBTMVKHWWKBU-UHFFFAOYSA-N CC1=CC=C(C(C2=CC=C(N)C(C)=C2)C2=CC(C)=C(N)C=C2)C=C1 Chemical compound CC1=CC=C(C(C2=CC=C(N)C(C)=C2)C2=CC(C)=C(N)C=C2)C=C1 BTOBTMVKHWWKBU-UHFFFAOYSA-N 0.000 description 2
- JBZNNQQKRRSWHI-UHFFFAOYSA-N CC1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 Chemical compound CC1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 JBZNNQQKRRSWHI-UHFFFAOYSA-N 0.000 description 2
- AEIZJCFUJGDAFI-UHFFFAOYSA-N CCC(C)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCC(C)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 AEIZJCFUJGDAFI-UHFFFAOYSA-N 0.000 description 2
- VIFHHCWFCOEORZ-UHFFFAOYSA-N CCC(CC)(C1=CC=C(N)C(C)=C1)C1=CC(C)=C(N)C=C1 Chemical compound CCC(CC)(C1=CC=C(N)C(C)=C1)C1=CC(C)=C(N)C=C1 VIFHHCWFCOEORZ-UHFFFAOYSA-N 0.000 description 2
- BVPLBJPSCKVLBL-UHFFFAOYSA-N CCC(CC)(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 Chemical compound CCC(CC)(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 BVPLBJPSCKVLBL-UHFFFAOYSA-N 0.000 description 2
- AVPKNPZUAHPCJD-UHFFFAOYSA-N CCC(CC)C(C1=CC=C(N)C(C)=C1)C1=CC(C)=C(N)C=C1 Chemical compound CCC(CC)C(C1=CC=C(N)C(C)=C1)C1=CC(C)=C(N)C=C1 AVPKNPZUAHPCJD-UHFFFAOYSA-N 0.000 description 2
- VYYVPQOKEMLHRY-UHFFFAOYSA-N CCC(CC)C(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 Chemical compound CCC(CC)C(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 VYYVPQOKEMLHRY-UHFFFAOYSA-N 0.000 description 2
- ZGWCHIAFDJSPKU-UHFFFAOYSA-N CCC(CC)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCC(CC)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 ZGWCHIAFDJSPKU-UHFFFAOYSA-N 0.000 description 2
- AZKWKIYQFQKBKR-UHFFFAOYSA-N CCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 AZKWKIYQFQKBKR-UHFFFAOYSA-N 0.000 description 2
- ZMSOXEYVMBEMPJ-UHFFFAOYSA-N CCCC(C)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCC(C)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 ZMSOXEYVMBEMPJ-UHFFFAOYSA-N 0.000 description 2
- NUASBYYUVXSQNU-UHFFFAOYSA-N CCCC(CC)(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 Chemical compound CCCC(CC)(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 NUASBYYUVXSQNU-UHFFFAOYSA-N 0.000 description 2
- FUTSJNDYAXBQFK-UHFFFAOYSA-N CCCCC(CC)(C1=CC(C)=C(N)C(C)=C1)C1=CC(C)=C(N)C(C)=C1 Chemical compound CCCCC(CC)(C1=CC(C)=C(N)C(C)=C1)C1=CC(C)=C(N)C(C)=C1 FUTSJNDYAXBQFK-UHFFFAOYSA-N 0.000 description 2
- GRSLCUYSZOSVGN-UHFFFAOYSA-N CCCCC(CC)(C1=CC=C(N)C(C)=C1)C1=CC(C)=C(N)C=C1 Chemical compound CCCCC(CC)(C1=CC=C(N)C(C)=C1)C1=CC(C)=C(N)C=C1 GRSLCUYSZOSVGN-UHFFFAOYSA-N 0.000 description 2
- FAUUEAGCUYEKNL-UHFFFAOYSA-N CCCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 FAUUEAGCUYEKNL-UHFFFAOYSA-N 0.000 description 2
- GVAJGFIURUODAH-UHFFFAOYSA-N CCCCC(CC)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCC(CC)C(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 GVAJGFIURUODAH-UHFFFAOYSA-N 0.000 description 2
- DPUCTIGJEDRZKF-UHFFFAOYSA-N CCCCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 DPUCTIGJEDRZKF-UHFFFAOYSA-N 0.000 description 2
- KMQWDNGGCPTACV-UHFFFAOYSA-N CCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 KMQWDNGGCPTACV-UHFFFAOYSA-N 0.000 description 2
- QGGBXVBTXFPQKC-UHFFFAOYSA-N CCCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 QGGBXVBTXFPQKC-UHFFFAOYSA-N 0.000 description 2
- XVMMFGYHASEGAO-UHFFFAOYSA-N CCOC1=CC(C(C2=CC(OCC)=C(N)C=C2)C(CC)CC)=CC=C1N Chemical compound CCOC1=CC(C(C2=CC(OCC)=C(N)C=C2)C(CC)CC)=CC=C1N XVMMFGYHASEGAO-UHFFFAOYSA-N 0.000 description 2
- NZBXKLIGKFCNNJ-UHFFFAOYSA-N CCOC1=CC(C(C2=CC=CC=C2)C2=CC(OCC)=C(N)C=C2)=CC=C1N Chemical compound CCOC1=CC(C(C2=CC=CC=C2)C2=CC(OCC)=C(N)C=C2)=CC=C1N NZBXKLIGKFCNNJ-UHFFFAOYSA-N 0.000 description 2
- BQMQNEHLCBIPEI-UHFFFAOYSA-N COC1=CC(C(C2=CC=C(C)C=C2)C2=CC(OC)=C(N)C=C2)=CC=C1N Chemical compound COC1=CC(C(C2=CC=C(C)C=C2)C2=CC(OC)=C(N)C=C2)=CC=C1N BQMQNEHLCBIPEI-UHFFFAOYSA-N 0.000 description 2
- MLYNBXBOZUQBFW-UHFFFAOYSA-N COC1=CC(C(C2=CC=CC=C2)C2=CC(OC)=C(N)C=C2)=CC=C1N Chemical compound COC1=CC(C(C2=CC=CC=C2)C2=CC(OC)=C(N)C=C2)=CC=C1N MLYNBXBOZUQBFW-UHFFFAOYSA-N 0.000 description 2
- RYPSJWNWIYAKTK-UHFFFAOYSA-N COC1=CC=C(C(C2=CC=C(N)C(C)=C2)C2=CC(C)=C(N)C=C2)C=C1 Chemical compound COC1=CC=C(C(C2=CC=C(N)C(C)=C2)C2=CC(C)=C(N)C=C2)C=C1 RYPSJWNWIYAKTK-UHFFFAOYSA-N 0.000 description 2
- VZOJJJQYMCJZCB-UHFFFAOYSA-N COC1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 Chemical compound COC1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 VZOJJJQYMCJZCB-UHFFFAOYSA-N 0.000 description 2
- NQTPPFMDQSYGGJ-UHFFFAOYSA-N CC(C)(C)C1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 Chemical compound CC(C)(C)C1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 NQTPPFMDQSYGGJ-UHFFFAOYSA-N 0.000 description 1
- JSZFNNMVDOIPTE-UHFFFAOYSA-N CC(C)C(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CC(C)C(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 JSZFNNMVDOIPTE-UHFFFAOYSA-N 0.000 description 1
- HLAJYKSYBRUSCN-UHFFFAOYSA-N CC(C)C(C1=CC=C(N)C=C1)(C1=CC=C(N)C=C1)C(C)C Chemical compound CC(C)C(C1=CC=C(N)C=C1)(C1=CC=C(N)C=C1)C(C)C HLAJYKSYBRUSCN-UHFFFAOYSA-N 0.000 description 1
- NMBIXTPVIDNDRJ-UHFFFAOYSA-N CC(C)C1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 Chemical compound CC(C)C1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 NMBIXTPVIDNDRJ-UHFFFAOYSA-N 0.000 description 1
- PYVGYSQEQJITES-UHFFFAOYSA-N CC(C)CC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CC(C)CC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 PYVGYSQEQJITES-UHFFFAOYSA-N 0.000 description 1
- HDLMDZCRUGTIJI-UHFFFAOYSA-N CC(C)CC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CC(C)CC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 HDLMDZCRUGTIJI-UHFFFAOYSA-N 0.000 description 1
- SRZQRPMIAPLPFJ-UHFFFAOYSA-N CC(CCC1=CC=CC=C1)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CC(CCC1=CC=CC=C1)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 SRZQRPMIAPLPFJ-UHFFFAOYSA-N 0.000 description 1
- PRAFLFRRTJUEKH-UHFFFAOYSA-N CC1=CC(C(CC(C)C)C2=CC(C)=C(N)C=C2)=CC=C1N Chemical compound CC1=CC(C(CC(C)C)C2=CC(C)=C(N)C=C2)=CC=C1N PRAFLFRRTJUEKH-UHFFFAOYSA-N 0.000 description 1
- HAPZLJSYCBILMD-UHFFFAOYSA-N CC1=CC=C(C(C2=CC(C)=C(N)C(C)=C2)C2=CC(C)=C(N)C(C)=C2)C=C1 Chemical compound CC1=CC=C(C(C2=CC(C)=C(N)C(C)=C2)C2=CC(C)=C(N)C(C)=C2)C=C1 HAPZLJSYCBILMD-UHFFFAOYSA-N 0.000 description 1
- PCXDOGSTKDYHIP-UHFFFAOYSA-N CC1=CC=[N+](C2=C(C)C=C([O-])N(CCCN(C)C)C2=O)C=C1 Chemical compound CC1=CC=[N+](C2=C(C)C=C([O-])N(CCCN(C)C)C2=O)C=C1 PCXDOGSTKDYHIP-UHFFFAOYSA-N 0.000 description 1
- MWUVTHFCIONEHF-UHFFFAOYSA-N CCC(C)CC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCC(C)CC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 MWUVTHFCIONEHF-UHFFFAOYSA-N 0.000 description 1
- LXZJUKVPNWHTPG-UHFFFAOYSA-N CCC(CC)C(C1=CC(OC)=C(N)C=C1OC)C1=C(OC)C=C(N)C(OC)=C1 Chemical compound CCC(CC)C(C1=CC(OC)=C(N)C=C1OC)C1=C(OC)C=C(N)C(OC)=C1 LXZJUKVPNWHTPG-UHFFFAOYSA-N 0.000 description 1
- XCHJVAIPNTUAMG-UHFFFAOYSA-N CCC(CC)C(C1=CC=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C(OC)=C1)C1=CC(OC)=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C=C1 Chemical compound CCC(CC)C(C1=CC=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C(OC)=C1)C1=CC(OC)=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C=C1 XCHJVAIPNTUAMG-UHFFFAOYSA-N 0.000 description 1
- CQUKIOWKPBBEEM-UHFFFAOYSA-N CCC(CC)C(C1=CC=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C=C1)C1=CC=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C=C1 Chemical compound CCC(CC)C(C1=CC=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C=C1)C1=CC=C(/N=N/C2=C(C)C([N+]3=CC=CC=C3)=C([O-])N(CCCN(C)C)C2=O)C=C1 CQUKIOWKPBBEEM-UHFFFAOYSA-N 0.000 description 1
- MPSVLQHJYZLIMT-UHFFFAOYSA-N CCC(CCC(C)C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCC(CCC(C)C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 MPSVLQHJYZLIMT-UHFFFAOYSA-N 0.000 description 1
- HSZDSPUROYDCKG-UHFFFAOYSA-N CCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 HSZDSPUROYDCKG-UHFFFAOYSA-N 0.000 description 1
- ULDSADCHIQKXEN-UHFFFAOYSA-N CCCC(CCC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCC(CCC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 ULDSADCHIQKXEN-UHFFFAOYSA-N 0.000 description 1
- DZXSOGZLDYHFFW-UHFFFAOYSA-N CCCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 DZXSOGZLDYHFFW-UHFFFAOYSA-N 0.000 description 1
- NUOJREFBUXCQAQ-UHFFFAOYSA-N CCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 NUOJREFBUXCQAQ-UHFFFAOYSA-N 0.000 description 1
- IADHRKWHCWWFNZ-UHFFFAOYSA-N CCCCC(CC)C(C1=CC(OC)=C(N)C=C1OC)C1=C(OC)C=C(N)C(OC)=C1 Chemical compound CCCCC(CC)C(C1=CC(OC)=C(N)C=C1OC)C1=C(OC)C=C(N)C(OC)=C1 IADHRKWHCWWFNZ-UHFFFAOYSA-N 0.000 description 1
- NHCCTYVOVRFVHP-UHFFFAOYSA-N CCCCC(CC)C(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 Chemical compound CCCCC(CC)C(C1=CC=C(N)C(OC)=C1)C1=CC(OC)=C(N)C=C1 NHCCTYVOVRFVHP-UHFFFAOYSA-N 0.000 description 1
- WDQBOVPSWPRQNK-UHFFFAOYSA-N CCCCC(CC)C(C1=CC=C(N)C(OCC)=C1)C1=CC(OCC)=C(N)C=C1 Chemical compound CCCCC(CC)C(C1=CC=C(N)C(OCC)=C1)C1=CC(OCC)=C(N)C=C1 WDQBOVPSWPRQNK-UHFFFAOYSA-N 0.000 description 1
- IOTLEGLFLBSZCA-UHFFFAOYSA-N CCCCC(CCCC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCC(CCCC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 IOTLEGLFLBSZCA-UHFFFAOYSA-N 0.000 description 1
- SBAINVNGZDWHJO-UHFFFAOYSA-N CCCCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 SBAINVNGZDWHJO-UHFFFAOYSA-N 0.000 description 1
- XPLAZHDCTNEPMK-UHFFFAOYSA-N CCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 XPLAZHDCTNEPMK-UHFFFAOYSA-N 0.000 description 1
- RMIJJNLNVQSDFR-UHFFFAOYSA-N CCCCCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCCC(C)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 RMIJJNLNVQSDFR-UHFFFAOYSA-N 0.000 description 1
- ZRNVZSWTKKAUTN-UHFFFAOYSA-N CCCCCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCCC(CC)(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 ZRNVZSWTKKAUTN-UHFFFAOYSA-N 0.000 description 1
- SGFCOVSYMHQLGF-UHFFFAOYSA-N CCCCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 SGFCOVSYMHQLGF-UHFFFAOYSA-N 0.000 description 1
- DODZITHJXOKQAB-UHFFFAOYSA-N CCCCCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 Chemical compound CCCCCCCCCC(C1=CC=C(N)C=C1)C1=CC=C(N)C=C1 DODZITHJXOKQAB-UHFFFAOYSA-N 0.000 description 1
- XXLWNNVSLNYWFH-UHFFFAOYSA-N CCOC1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 Chemical compound CCOC1=CC=C(C(C2=CC=C(N)C=C2)C2=CC=C(N)C=C2)C=C1 XXLWNNVSLNYWFH-UHFFFAOYSA-N 0.000 description 1
- JYOITEXTJRPMCC-UHFFFAOYSA-N COC1=CC(C(C)(CC(C)C)C2=CC(OC)=C(N)C=C2)=CC=C1N Chemical compound COC1=CC(C(C)(CC(C)C)C2=CC(OC)=C(N)C=C2)=CC=C1N JYOITEXTJRPMCC-UHFFFAOYSA-N 0.000 description 1
- BFQYXOJWQIXEAM-UHFFFAOYSA-N COC1=CC(C(C2=CC=CC=C2)C2=CC(OC)=C(N)C=C2OC)=C(OC)C=C1N Chemical compound COC1=CC(C(C2=CC=CC=C2)C2=CC(OC)=C(N)C=C2OC)=C(OC)C=C1N BFQYXOJWQIXEAM-UHFFFAOYSA-N 0.000 description 1
- QUYSXDHTSYOUGU-UHFFFAOYSA-N COC1=CC=C(C(C2=CC(C)=C(N)C(C)=C2)C2=CC(C)=C(N)C(C)=C2)C=C1 Chemical compound COC1=CC=C(C(C2=CC(C)=C(N)C(C)=C2)C2=CC(C)=C(N)C(C)=C2)C=C1 QUYSXDHTSYOUGU-UHFFFAOYSA-N 0.000 description 1
- QOUBPUNCGSGOPB-UHFFFAOYSA-N COC1=CC=C(C(C2=CC=C(N)C(OC)=C2)C2=CC(OC)=C(N)C=C2)C=C1 Chemical compound COC1=CC=C(C(C2=CC=C(N)C(OC)=C2)C2=CC(OC)=C(N)C=C2)C=C1 QOUBPUNCGSGOPB-UHFFFAOYSA-N 0.000 description 1
- XECVXFWNYNXCBN-UHFFFAOYSA-N NC1=CC=C(C(C2=CC=CC=C2)C2=CC=C(N)C=C2)C=C1 Chemical compound NC1=CC=C(C(C2=CC=CC=C2)C2=CC=C(N)C=C2)C=C1 XECVXFWNYNXCBN-UHFFFAOYSA-N 0.000 description 1
- OORVACRDWMZTOP-UHFFFAOYSA-N NC1=CC=C(C(CC2=CC=CC=C2)C2=CC=C(N)C=C2)C=C1 Chemical compound NC1=CC=C(C(CC2=CC=CC=C2)C2=CC=C(N)C=C2)C=C1 OORVACRDWMZTOP-UHFFFAOYSA-N 0.000 description 1
- ZOKPVTGSQSVRHH-UHFFFAOYSA-N NC1=CC=[I]C=C1 Chemical compound NC1=CC=[I]C=C1 ZOKPVTGSQSVRHH-UHFFFAOYSA-N 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-O [NH3+]c1ccccc1 Chemical compound [NH3+]c1ccccc1 PAYRUJLWNCNPSJ-UHFFFAOYSA-O 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H21/00—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties
- D21H21/14—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties characterised by function or properties in or on the paper
- D21H21/28—Colorants ; Pigments or opacifying agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/72—Nitrogen atoms
- C07D213/76—Nitrogen atoms to which a second hetero atom is attached
- C07D213/77—Hydrazine radicals
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B35/00—Disazo and polyazo dyes of the type A<-D->B prepared by diazotising and coupling
- C09B35/02—Disazo dyes
- C09B35/021—Disazo dyes characterised by two coupling components of the same type
- C09B35/03—Disazo dyes characterised by two coupling components of the same type in which the coupling component is a heterocyclic compound
- C09B35/031—Disazo dyes characterised by two coupling components of the same type in which the coupling component is a heterocyclic compound containing a six membered ring with one nitrogen atom as the only ring hetero atom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B35/00—Disazo and polyazo dyes of the type A<-D->B prepared by diazotising and coupling
- C09B35/02—Disazo dyes
- C09B35/039—Disazo dyes characterised by the tetrazo component
- C09B35/08—Disazo dyes characterised by the tetrazo component the tetrazo component being a derivative of biphenyl
- C09B35/10—Disazo dyes characterised by the tetrazo component the tetrazo component being a derivative of biphenyl from two coupling components of the same type
- C09B35/12—Disazo dyes characterised by the tetrazo component the tetrazo component being a derivative of biphenyl from two coupling components of the same type from amines
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B35/00—Disazo and polyazo dyes of the type A<-D->B prepared by diazotising and coupling
- C09B35/02—Disazo dyes
- C09B35/039—Disazo dyes characterised by the tetrazo component
- C09B35/205—Disazo dyes characterised by the tetrazo component the tetrazo component being a derivative of a diaryl- or triaryl- alkane or-alkene
- C09B35/21—Disazo dyes characterised by the tetrazo component the tetrazo component being a derivative of a diaryl- or triaryl- alkane or-alkene of diarylmethane or triarylmethane
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B67/00—Influencing the physical, e.g. the dyeing or printing properties of dyestuffs without chemical reactions, e.g. by treating with solvents grinding or grinding assistants, coating of pigments or dyes; Process features in the making of dyestuff preparations; Dyestuff preparations of a special physical nature, e.g. tablets, films
- C09B67/0033—Blends of pigments; Mixtured crystals; Solid solutions
- C09B67/0046—Mixtures of two or more azo dyes
- C09B67/0055—Mixtures of two or more disazo dyes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/02—Printing inks
- C09D11/03—Printing inks characterised by features other than the chemical nature of the binder
- C09D11/037—Printing inks characterised by features other than the chemical nature of the binder characterised by the pigment
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/30—Inkjet printing inks
- C09D11/32—Inkjet printing inks characterised by colouring agents
- C09D11/328—Inkjet printing inks characterised by colouring agents characterised by dyes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S8/00—Bleaching and dyeing; fluid treatment and chemical modification of textiles and fibers
- Y10S8/916—Natural fiber dyeing
- Y10S8/919—Paper
Definitions
- the invention relates to basic bisazo compounds, salts thereof and mixtures of these compounds, which may be in internal or external, salt form. They are suitable for use as dyestuffs.
- GB 1296857 or GB2173210 disclose basic metal-free or metallised disazo pyridone dyes free from sulphonic acid groups, are useful for dyeing paper, textiles and leather.
- the sum of carbon atoms of R 5 and R 6 together is at least 4 carbon atoms, more preferred R 5 and R 6 have together at least 5 carbon atoms. Even more preferred, the sum of carbon atoms of R 5 and R 6 together is 5 or 6 or 7 or 8 or 9 carbon atoms.
- the substituent R 6 signifies H the substituent R 6 signifies by preference H.
- R 1 signifies
- R 13 signifies H, a substituted C 1 to C 4 alkyl group or an unsubstituted C 1 to C 4 alkyl group, a substituted C 1 to C 4 alkoxy group or an unsubstituted C 1 to C 4 alkoxy group.
- the preferred group R 13 signifies H or Methyl.
- the preferred group R 13 is attached in the para-position to the nitrogen. Preferably the substituent is attached in para position to the nitrogen atom.
- R 2 or R 2′ signifly a group with the formula
- R 8 , R 8′ , R 9 , R 9′ , R 10 or R 10′ signify independently H, a substituted C 1 to C 4 alkyl group or an unsubstituted C 1 to C 4 alkyl group; and R 12 or R 12′ signifies independently H, a substituted C 1 to C 4 alkyl group or an unsubstituted C 1 to C 4 alkyl group and, even more preferred R 8 , R 8′ , R 9 , R 9′ , R 10 or R 10′ signify independently H and R 12 or R 12′ signify independently from each other H, methyl or ethyl, more preferred methyl.
- R 2 and R 2′ have the same meaning.
- Aryl means phenyl or naphtyl, by preference phenyl, Substituted aryl means aryl groups substituted by —COOH, —OH, C 1-4 alkyl groups or C 1-4 alkoxy groups.
- alkyl or alkoxy groups are by preference C 1-4 alkyl groups or C 1-4 alkoxy groups; C 1-4 alkyl groups or C 1-4 alkoxy which may be further substituted by C 1-4 alkyl, —COOH, —OH.
- Preferred alkyl groups are methyl or ethyl.
- Preferred substituents of the alky groups or or alkoxy groups is OH.
- R 8 ′, R 8 ′′ R 9 ′; R 9 ′′ are substituted alkyl groups, the preferred substituent is —OH.
- Preferred alkoxy groups are methoxy or ethoxy.
- the alkyl groups and the alkoxy groups are branched or linear.
- the more preferred alkyl or alkoxy groups for R 5 and R 6 signify a substituted C 1 to C 9 alkyl group or an unsubstituted C 1 to C 9 alkyl group and are branched or linear and the substituents may be selected from the group of —COOH, —OH.
- the most preferred alkyl groups are methyl, ethyl, propyl, iso-proply, butyl, iso-butyl, pentyl, hexyl, heptyl, octyl, or nonyl.
- the present invention further provides a process for the preparation of compounds of formula (I) comprising reacting the bis-diazonium salt of a di-amine of formula (II),
- R 0 , R 1 , R 2 , R 2′ , R 3 , R 4 , R 5 and R 6 are defined as above defined.
- Diazotisation and coupling may be effected in accordance with conventional methods.
- the coupling reaction advantageously is carried out in an aqueous reaction medium in a temperature range of from 0-60° C., preferably at 0-40° C., more preferred at 0-10° C., even more preferred at 0-5° C. and in a pH range of from 2 to 9, preferably at pH 3 to 6. All temperatures are given in degrees Celsius.
- reaction mixtures comprising compounds of formula (I) thus obtained may be converted into stable liquid formulations with improved long term stability by desalting by ultra filtration.
- the compounds of formula (I) containing free basic groups may be converted wholly or in part into water-soluble salts by reacting with any inorganic or organic acids for example with lactic acid, or acetic acid, or formic acid, or with hydrochloric acid, or with sulfuric acid.
- the compounds of formula (I) containing free basic groups of different salts by applying a mixture of inorganic or organic acids, for examples mixtures of the two or more acids selected from lactic acid, acetic acid, formic acid, hydrochloric acid, and sulfuric acid.
- a mixture of inorganic or organic acids for examples mixtures of the two or more acids selected from lactic acid, acetic acid, formic acid, hydrochloric acid, and sulfuric acid.
- the compounds of formula (I) containing free basic groups may after the treatment with lactic acid and hydrochloric acids consist of a mixed salt with chloride and lactate anions or the compounds of formula (I) containing free basic groups may after the treatment with acetic acid and hydrochloric acids consist of a mixed salt with chloride and acetate anions.
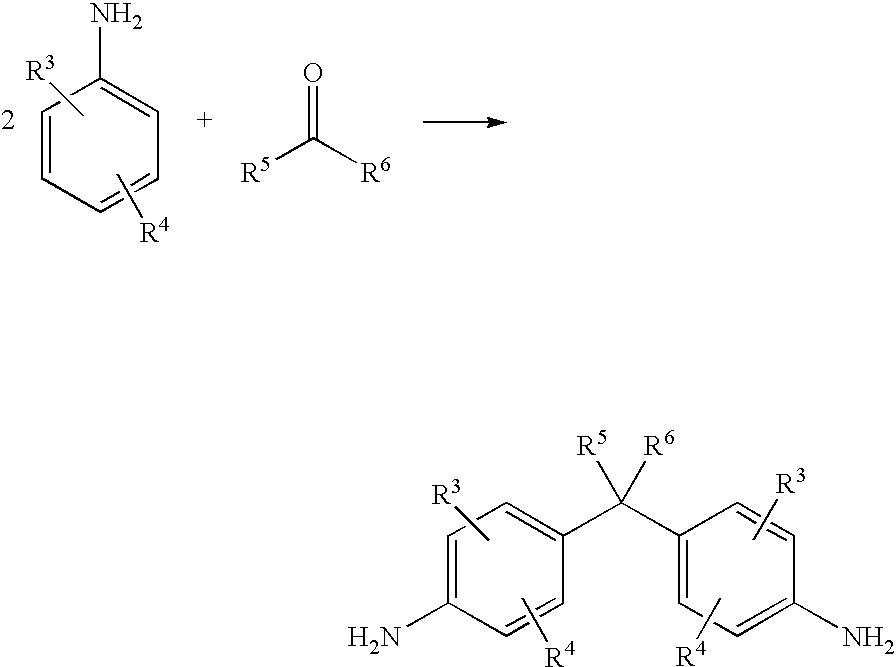
- the starting compounds, the amines of formula (II) and of compounds of formula (III), are either known or may be prepared in accordance with known methods from available starting materials. Suitabel methods are described e.g. in DE399149; DE505475; DE1220863; DE1793020 (GB1129306), DE3226889, DE4014847.
- novel amines according to the formula (II) may be prepared according the methods disclosed in DE399149; DE505475; DE1220863; DE1793020 (GB 1129306), DE3226889, DE4014847, thus more precisely either starting form aldehydes (when R 5 is H and R 6 is different from H) or from ketones (when both R 5 and R 6 are different from H) of the formula
- the reaction mixture is heated in a closed autoclave at 120° C.-250° C., preferably 140° C.-200° C., more preferably 140° C. to 150° C. the reaction mixture is kept at this temperature for 3-8 hours, preferably for 4-5 hours.
- the elevated temperature leads in this closed autoclave to the elevated pressure.
- the synthesis may be performed in the melt of the aminocompound-hydrochloride adding the ketocompound at elevated temperature 200 to 250° C. and the pressure is atmospheric pressure.
- the compounds according to the invention in acid addition salt form or quaternary ammonium salt form, may be used for dyeing cationic dyeable materials such as: homo- or mixed-polymers of acrylonitrile, acid modified polyester or polyamide; wool; leather including low affinity vegetable-tanned leather; cotton; bast fibers such as hemp, flax, sisal, jute, coir and straw; regenerated cellulose fibers, glass or glass products comprising glass fibers; and substrates comprising cellulose for example paper and cotton. They may also be used for printing fibers, filaments and textiles comprising any of the above mentioned materials in accordance with known methods.
- Printing may be effected by impregnation of the material to be printed with a suitable printing paste comprising one or more compounds of the present invention.
- the type of printing paste employed may vary depending on the material to be printed. Choice of a suitable commercially available printing paste or production of a suitable paste, is routine for one skilled in the art.
- the compounds of the present invention may be used in the preparation of inks suitable for example for jet printing, in accordance with conventional methods.
- the dyestuffs are used for dyeing or printing of paper e.g., sized or unsized, wood-free or wood-containing paper or paper-based products such as cardboard. They may be used in continuous dyeing in the stock, dyeing in the size press, in a conventional dipping or surface coloring process. The dyeing and printing of paper is effected by known methods.
- the dyeings and prints and particularly those obtained on paper show good fastness properties.
- waste water values are very good when dyed or printed paper or paper-based products are produced in medium or deep shades.
- the compounds of formula (I) may be converted into dyeing preparations. Processing into stable liquid, preferably aqueous, or solid (granulated or powder form) dyeing preparations may take place in a generally known manner.
- suitable liquid dyeing preparations may be made by dissolving the dyestuff in suitable solvents or in a mixture of suitable solvents such as mineral acids or organic acids, e.g., hydrochloric acid, sulphuric acid, phosphoric acid, formic acid, acetic acid, lactic acid, glycolic acid, citric acid and methanesulphonic acid.
- formamide, dimethylformamide, urea, glycols and ethers thereof, dextrin or addition products of boric acid with sorbit may be used together with water, optionally adding an assistant, e.g. a stabilizer.
- an assistant e.g. a stabilizer.
- Such preparations may be obtained, for example, as described in FR1572030 (U.S. Pat. No. 4,023,924).
- the compounds of formula (I) (in the corresponding salt form) have good solubility especially in cold water. Owing to their high substantivity the compounds of the present invention exhaust practically quantitatively and show a good build-up power. They can be added to the stock directly, i.e. without previously dissolving, as either a dry powder or granulate, without reducing the brilliance or the yield of color. They can also be used in soft water without loss of yield. They do not mottle when applied on paper, are not inclined to give two-sided dyeing on paper and are practically insensitive to filler or pH variations. They operate over a broad pH range, in the range of from pH 3 to 10. When producing sized or unsized paper, the wastewater is essentially colorless. This feature, which is extremely important from an environmental viewpoint, when compared with similar known dyes, shows a marked improvement. A sized paper dyeing when compared with the corresponding unsized paper dyeing does not show any decrease in strength.
- the paper dyeings or printings made with the compounds according to the invention are clear and brilliant and have good light fastness.
- the shade of the dyeing fades tone in tone. They show very good wet fastness properties; being fast to water, milk, fruit juice, sweetened mineral water, tonic water, soap and sodium chloride solution, urine etc. Furthermore, they have good alcohol fastness properties.
- the wet fastness properties are improved compared to known dyes showing otherwise similar properties. They do not exhibit a tendency towards two-sidedness.
- Paper dyed or printed with the compounds of the present invention can be bleached either oxidatively or reductively, a feature, which is important for the recycling of waste paper and old paper products.
- the compounds of the present invention may also be used to dye paper containing wood-pulp where even dyeings, having good fastness properties are obtained. Furthermore, they may be used for the production of coated paper in accordance with known methods. Preferably when coating, a suitable filler, for example kaolin, is employed in order to give a one-side coated paper.
- a suitable filler for example kaolin
- the compounds of the present invention are also suitable for dyeing in combination with other dyes for example other cationic or anionic dyes.
- the compatibility of the compounds of the present invention when used as a dye in mixtures with other commercially available dyes, may be determined according to conventional methods. The thus obtained dyeings have good fastness properties.
- the invention yet further provides use of a compound of the present invention for dyeing or printing any of the abovementioned substrates.
- the invention further provides a substrate, which has been dyed or printed with a compound of the present invention.
- the substrate may be selected from any of the above mentioned substrates.
- a preferred substrate is a substrate comprising cellulose such as cotton or paper or paper based product.
- the dye preparations of the present invention can also be used for dyeing and tinting wood.
- the wood can be in the form of articles, such as bowls, dishes, toys, but also solid slats and beams, and also in the form of shavings, chips or chipboard. Parts of buildings can similarly be treated with the dye preparations of the present invention, as can furniture.
- the application of the liquid dye preparations of the present invention can be utilized for equalizing colour differences in the wood or in a veneer, but also for completely changing the colour of the wood or of a veneer.
- the liquid dye preparations of the present invention can be utilized as an aqueous stain (in which case water is the main solvent), as an alcoholic-aqueous stain (i.e. the solvent is an alcohol-water mixture) or as stains involving organic solvents (about 30-95% of organic solvents; such stains may also possibly be water thinnable).
- the temperature of the melt falls from initially ca. 200° C. to 185° C. because of the reflux. The temperature is kept for one hour at 185° C. and the hot melt is poured on a mixture of 1.6 kg ice and 1.05 kg of sodium hydroxide solution (30%).
- the organic layer is separated and washed free from salt with demineralised water.
- the excess of aniline is extracted by water steam distillation.
- the temperature of the melt falls from initially ca. 200° C. to 185° C. because of the reflux.
- the temperature is kept for one hour at 185° C. and the hot melt is poured on a mixture of 1.6 kg ice and 1.05 kg of sodium hydroxide solution (30%).
- the organic layer is separated and washed free from salt with demineralised water.
- the aniline excess is extracted by water steam distillation.
- the dyestuff can be isolated by concentration under vacuum or by precipitation in aceton/alcohol.
- the reaction mixture however can be used directly for dyeing without isolation the product.
- the dyestuff of formula (5) has surprisingly very high solubility in water and gives yellow dyeings with very good fastness properties.
- the dyestuff can be isolated by concentration under vacuum or by precipitation in aceton/alcohol.
- the reaction mixture howevercan be used directly for dyeing without isolation the product.
- the dyestuff of formula (6) has very high solubility in water and gives yellow dyeings with surprisingly very good fastness properties.
- An absorbent length of unsized paper is drawn at 40-50° C. through a dyestuff solution having the following composition:
- the excess dyestuff solution is squeezed out through two rollers.
- the dried length of paper is dyed a yellow shade.
- the dyestuffs of Examples 69 to 159 may also be used for dyeing by a method analogous to that of Application Examples A to C.
- the paper dyeings obtained show good fastness properties.
- the dyestuffs according to Examples 69-159 may be used for dyeing cotton.
- 100 parts freshly tanned and neutralized chrome leather are agitated for 30 minutes in a vessel with a liquor consisting of 250 parts of water at 55° C. and 0.5 parts of the dyestuff of Example 68 in acid addition salt form, and then treated in the same bath for 30 minutes with 2 parts of an anionic fatty liquor based on sulphonated train oil.
- the leather is then dried and prepared in the normal way, giving a leather evenly dyed in a yellow shade.
- the dyestuffs according to Examples 69-159 may be used for dyeing leather.
- Water is added to a dry pulp in Hollander consisting of 60% (by weight) of mechanical wood pulp and 40% (by weight) of unbleached sulphite cellulose, and the slurry is beaten in order to obtain a dry content slightly exceeding 2.5% and having a beating degree of 40° SR (degrees Schopper-Riegler).
- the slurry is then exactly adjusted to a high density dry content of 2.5% by adding water.
- 5 Parts of a 2.5% aqueous solution of the dyestuff according to Example 68 are added to 200 parts of the above resulting slurry.
- the mixture is stirred for about 5 minutes and, after the addition of 2% (by weight) resin size and then 4% (by weight) alum (based on the dry weight) is further stirred for a few minutes until homogeneous.
- the resulting pulp is diluted with about 500 parts water to a volume of 700 parts and then used for the production of paper sheets by suction on a sheet former.
- the resulting paper sheets are yellow.
- any one of the dyestuffs of Examples 69-159 may be used instead of that of Example 68. In all cases, the waste paper exhibits a substantially low residual dye concentration.
- Water is added to a dry pulp in a Hollander consisting of 50% (by weight) of chemically bleached sulphite cellulose obtained from pinewood and 50% (by weight) of chemically bleached sulphite cellulose obtained from birchwood, and the slurry is ground until a degree of grinding of 35° SR is reached.
- the slurry is then adjusted to a high density dry content of 2.5% by adding water, and the pH of this suspension is adjusted to 7.10 Parts of a 0.5% aqueous solution of the dyestuff according to Example 68 are added to 200 parts of the above resulting slurry, and the mixture is stirred for 5 minutes.
- the resulting pulp is diluted with 500 parts water and then used for the production of sheets by suction on a sheet former.
- the paper sheets thus obtained have a yellow shade.
- dye mixtures consisting of any one of the dyestuffs of Examples 69-159. In all cases, paper sheets are formed having a yellow shade.
- a roof batten composed of Norway spruce and a roof batten composed of beechwood are sawn into pieces 5 cm in length and one piece of the sprucewood roof batten and one piece of the beechwood roof batten are dipped into a dilute solution of the reaction solution according to Example 68 (30 parts by weight of water and 1 part by weight of reaction solution, thus without isolating the dye stuff). Yellowish roof batten pieces are obtained on drying.
- any one of the dyestuffs of Examples 69-159 may be used.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Coloring (AREA)
- Inks, Pencil-Leads, Or Crayons (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Paper (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Pyridine Compounds (AREA)
Abstract
Description
- The invention relates to basic bisazo compounds, salts thereof and mixtures of these compounds, which may be in internal or external, salt form. They are suitable for use as dyestuffs.
- GB 1296857 or GB2173210 disclose basic metal-free or metallised disazo pyridone dyes free from sulphonic acid groups, are useful for dyeing paper, textiles and leather.
- However there is still a need to produce dyes having improved properties. Surprisingly, it was found that dyes according to formula (I) as shown below of the present application have those desired properties.
- According to the invention there are provided compounds of formula (I)
- R0 signifies a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group,
- R1 signifies H, N(R7′R7″), a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group or CN,
- R2 or R2′ signify H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group, or a group with the formula
-
—[(CR8R8′)—(CR9′R9′)m—(CR10R10′)n—(CR11R11′)o]—NR12R12′ -
- where m, n and o have the meaning of 1 or 0, and R8, R8′, R9, R9′, R10, R10′, R11 or R11′ signify independently H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group; and R12 or R12′ signify independently H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group.
- R3 signify H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group, a substituted C1 to C4 alkoxy group or an unsubstituted C1 to C4 alkoxy group,
- R4 signifies H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group, a substituted C1 to C4 alkoxy group or an unsubstituted C1 to C4 alkoxy group,
- R5 signifies substituted H, C1 to C9 alkyl group or an unsubstituted C1 to C9 alkyl group,
- R6 signifies a substituted C1 to C9 alkyl group or an unsubstituted C1 to C9 alkyl group, an unsubstituted aryl group or a substituted aryl group,
- R7′ or R7″ signify independently a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group, or R7′ and R7″ form together with the nitrogen atom a five or six membered aromatic or a five or six membered cyclo alipahatic, wherein the five or six membered rings are substituted by a C1 to C4 alkyl group or the five or six membered rings are not further substituted,
- By preference, the sum of carbon atoms of R5 and R6 together is at least 4 carbon atoms, more preferred R5 and R6 have together at least 5 carbon atoms. Even more preferred, the sum of carbon atoms of R5 and R6 together is 5 or 6 or 7 or 8 or 9 carbon atoms. When the substituent R6 signifies H the substituent R6 signifies by preference H.
- In preferred compounds of formula (I)
- R0 signifies a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group,
- R1 signifies N(R7′R7″), a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group,
- R2 or R2′ a group with the formula
-
—[(CR8R8′)—(CR9′R9′)m—(CR10R10′)n—(CR11R11′)o]—NR12R12′ -
- where m, n and o have the meaning of 1 or 0, and R8, R8′, R9, R9′, R10, R10′, R11 or R11′ signify independently H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group; and R12 or R12′ signify independently H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group,
- R3 signifies H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group, a substituted C1 to C4 alkoxy group or an unsubstituted C1 to C4 alkoxy group,
- R4 signifies H, C1 to C4 alkyl group, C1 to C4 alkoxy group
- R5 signifies substituted H, C1 to C9 alkyl group or an unsubstituted C1 to C9 alkyl group,
- R6 signifies a substituted C1 to C9 alkyl group or an unsubstituted C1 to C9 alkyl group, an unsubstituted aryl group or a substituted aryl group,
- R7′ and R7″ form together with the nitrogen atom a five or six membered aromatic,
- wherein the five or six membered rings is substituted by a C1 to C4 alkyl group or the five or six membered ring is not further substituted,
- By preference R1 signifies
- wherein the * shows the point of attachment to the rest of the molecule and wherein R13 signifies H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group, a substituted C1 to C4 alkoxy group or an unsubstituted C1 to C4 alkoxy group. The preferred group R13 signifies H or Methyl. The preferred group R13 is attached in the para-position to the nitrogen. Preferably the substituent is attached in para position to the nitrogen atom.
- By preference R2 or R2′ signifly a group with the formula
-
—[(CR8R8′)—(CR9′R9′)—(CR10R10′)]—NR12R12′ - where R8, R8′, R9, R9′, R10 or R10′ signify independently H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group; and R12 or R12′ signifies independently H, a substituted C1 to C4 alkyl group or an unsubstituted C1 to C4 alkyl group and, even more preferred R8, R8′, R9, R9′, R10 or R10′ signify independently H and R12 or R12′ signify independently from each other H, methyl or ethyl, more preferred methyl. In very preferred compounds R2 and R2′ have the same meaning.
- Aryl means phenyl or naphtyl, by preference phenyl, Substituted aryl means aryl groups substituted by —COOH, —OH, C1-4alkyl groups or C1-4alkoxy groups.
- Generally, alkyl or alkoxy groups are by preference C1-4alkyl groups or C1-4alkoxy groups; C1-4alkyl groups or C1-4alkoxy which may be further substituted by C1-4alkyl, —COOH, —OH. Preferred alkyl groups are methyl or ethyl. Preferred substituents of the alky groups or or alkoxy groups is OH. When R8′, R8″ R9′; R9″ are substituted alkyl groups, the preferred substituent is —OH. Preferred alkoxy groups are methoxy or ethoxy. The alkyl groups and the alkoxy groups are branched or linear.
- However, the more preferred alkyl or alkoxy groups for R5 and R6 signify a substituted C1 to C9 alkyl group or an unsubstituted C1 to C9 alkyl group and are branched or linear and the substituents may be selected from the group of —COOH, —OH. The most preferred alkyl groups are methyl, ethyl, propyl, iso-proply, butyl, iso-butyl, pentyl, hexyl, heptyl, octyl, or nonyl.
- The present invention further provides a process for the preparation of compounds of formula (I) comprising reacting the bis-diazonium salt of a di-amine of formula (II),
- with one equivalent compound of formula (III) and one equivalent compound of formula (III′)
- in which R0, R1, R2, R2′, R3, R4, R5 and R6 are defined as above defined.
- Diazotisation and coupling may be effected in accordance with conventional methods. The coupling reaction advantageously is carried out in an aqueous reaction medium in a temperature range of from 0-60° C., preferably at 0-40° C., more preferred at 0-10° C., even more preferred at 0-5° C. and in a pH range of from 2 to 9, preferably at pH 3 to 6. All temperatures are given in degrees Celsius.
- The reaction mixtures comprising compounds of formula (I) thus obtained may be converted into stable liquid formulations with improved long term stability by desalting by ultra filtration.
- The compounds of formula (I) thus obtained may be isolated in accordance with known methods.
- The compounds of formula (I) containing free basic groups may be converted wholly or in part into water-soluble salts by reacting with any inorganic or organic acids for example with lactic acid, or acetic acid, or formic acid, or with hydrochloric acid, or with sulfuric acid.
- Further more it is also possible to convert the compounds of formula (I) containing free basic groups of different salts by applying a mixture of inorganic or organic acids, for examples mixtures of the two or more acids selected from lactic acid, acetic acid, formic acid, hydrochloric acid, and sulfuric acid. Thus the compounds of formula (I) containing free basic groups may after the treatment with lactic acid and hydrochloric acids consist of a mixed salt with chloride and lactate anions or the compounds of formula (I) containing free basic groups may after the treatment with acetic acid and hydrochloric acids consist of a mixed salt with chloride and acetate anions.
- The starting compounds, the amines of formula (II) and of compounds of formula (III), are either known or may be prepared in accordance with known methods from available starting materials. Suitabel methods are described e.g. in DE399149; DE505475; DE1220863; DE1793020 (GB1129306), DE3226889, DE4014847.
- However, novel amines according to the formula (II) may be prepared according the methods disclosed in DE399149; DE505475; DE1220863; DE1793020 (GB 1129306), DE3226889, DE4014847, thus more precisely either starting form aldehydes (when R5 is H and R6 is different from H) or from ketones (when both R5 and R6 are different from H) of the formula
- by reacting at elevated temperature and elevated pressure under acidic condition with two equivalents of an aromatic amine of the formula
- forming the diamine of the formula (III)
- The reaction mixture is heated in a closed autoclave at 120° C.-250° C., preferably 140° C.-200° C., more preferably 140° C. to 150° C. the reaction mixture is kept at this temperature for 3-8 hours, preferably for 4-5 hours. The elevated temperature leads in this closed autoclave to the elevated pressure. Alternatively the synthesis may be performed in the melt of the aminocompound-hydrochloride adding the ketocompound at elevated temperature 200 to 250° C. and the pressure is atmospheric pressure.
- The compounds according to the invention, in acid addition salt form or quaternary ammonium salt form, may be used for dyeing cationic dyeable materials such as: homo- or mixed-polymers of acrylonitrile, acid modified polyester or polyamide; wool; leather including low affinity vegetable-tanned leather; cotton; bast fibers such as hemp, flax, sisal, jute, coir and straw; regenerated cellulose fibers, glass or glass products comprising glass fibers; and substrates comprising cellulose for example paper and cotton. They may also be used for printing fibers, filaments and textiles comprising any of the above mentioned materials in accordance with known methods. Printing may be effected by impregnation of the material to be printed with a suitable printing paste comprising one or more compounds of the present invention. The type of printing paste employed, may vary depending on the material to be printed. Choice of a suitable commercially available printing paste or production of a suitable paste, is routine for one skilled in the art. Alternatively the compounds of the present invention may be used in the preparation of inks suitable for example for jet printing, in accordance with conventional methods.
- Most preferably, the dyestuffs are used for dyeing or printing of paper e.g., sized or unsized, wood-free or wood-containing paper or paper-based products such as cardboard. They may be used in continuous dyeing in the stock, dyeing in the size press, in a conventional dipping or surface coloring process. The dyeing and printing of paper is effected by known methods.
- The dyeings and prints and particularly those obtained on paper, show good fastness properties.
- Especially the waste water values are very good when dyed or printed paper or paper-based products are produced in medium or deep shades.
- The compounds of formula (I) may be converted into dyeing preparations. Processing into stable liquid, preferably aqueous, or solid (granulated or powder form) dyeing preparations may take place in a generally known manner. Advantageously suitable liquid dyeing preparations may be made by dissolving the dyestuff in suitable solvents or in a mixture of suitable solvents such as mineral acids or organic acids, e.g., hydrochloric acid, sulphuric acid, phosphoric acid, formic acid, acetic acid, lactic acid, glycolic acid, citric acid and methanesulphonic acid. Furthermore formamide, dimethylformamide, urea, glycols and ethers thereof, dextrin or addition products of boric acid with sorbit may be used together with water, optionally adding an assistant, e.g. a stabilizer. Such preparations may be obtained, for example, as described in FR1572030 (U.S. Pat. No. 4,023,924).
- The compounds of formula (I) (in the corresponding salt form) have good solubility especially in cold water. Owing to their high substantivity the compounds of the present invention exhaust practically quantitatively and show a good build-up power. They can be added to the stock directly, i.e. without previously dissolving, as either a dry powder or granulate, without reducing the brilliance or the yield of color. They can also be used in soft water without loss of yield. They do not mottle when applied on paper, are not inclined to give two-sided dyeing on paper and are practically insensitive to filler or pH variations. They operate over a broad pH range, in the range of from pH 3 to 10. When producing sized or unsized paper, the wastewater is essentially colorless. This feature, which is extremely important from an environmental viewpoint, when compared with similar known dyes, shows a marked improvement. A sized paper dyeing when compared with the corresponding unsized paper dyeing does not show any decrease in strength.
- The paper dyeings or printings made with the compounds according to the invention are clear and brilliant and have good light fastness. On exposure to light for a long time, the shade of the dyeing fades tone in tone. They show very good wet fastness properties; being fast to water, milk, fruit juice, sweetened mineral water, tonic water, soap and sodium chloride solution, urine etc. Furthermore, they have good alcohol fastness properties. The wet fastness properties are improved compared to known dyes showing otherwise similar properties. They do not exhibit a tendency towards two-sidedness.
- Paper dyed or printed with the compounds of the present invention can be bleached either oxidatively or reductively, a feature, which is important for the recycling of waste paper and old paper products.
- The compounds of the present invention may also be used to dye paper containing wood-pulp where even dyeings, having good fastness properties are obtained. Furthermore, they may be used for the production of coated paper in accordance with known methods. Preferably when coating, a suitable filler, for example kaolin, is employed in order to give a one-side coated paper.
- The compounds of the present invention are also suitable for dyeing in combination with other dyes for example other cationic or anionic dyes. The compatibility of the compounds of the present invention when used as a dye in mixtures with other commercially available dyes, may be determined according to conventional methods. The thus obtained dyeings have good fastness properties.
- The invention yet further provides use of a compound of the present invention for dyeing or printing any of the abovementioned substrates.
- The invention further provides a substrate, which has been dyed or printed with a compound of the present invention. The substrate may be selected from any of the above mentioned substrates. A preferred substrate is a substrate comprising cellulose such as cotton or paper or paper based product.
- The dye preparations of the present invention can also be used for dyeing and tinting wood. The wood can be in the form of articles, such as bowls, dishes, toys, but also solid slats and beams, and also in the form of shavings, chips or chipboard. Parts of buildings can similarly be treated with the dye preparations of the present invention, as can furniture. The application of the liquid dye preparations of the present invention can be utilized for equalizing colour differences in the wood or in a veneer, but also for completely changing the colour of the wood or of a veneer. The liquid dye preparations of the present invention can be utilized as an aqueous stain (in which case water is the main solvent), as an alcoholic-aqueous stain (i.e. the solvent is an alcohol-water mixture) or as stains involving organic solvents (about 30-95% of organic solvents; such stains may also possibly be water thinnable).
- The following examples further serve to illustrate the invention. In the Examples all parts and all percentages are by weight or volume, and the temperatures given are in degrees Celsius, unless indicated to the contrary.
- The invention will now be illustrated by the following Examples in which all parts are by weight and all temperatures are in degrees Celsius.
- 106 g benzaldehyde, 400 g o-anisidine, 450 g hydrochloric acid (ca. 30%) and 800 ml water were heated in an autoclave at 140° C. for 6 hours.
- The reaction mixture was poured on 1 kg ice and 500 g sodium hydroxide solution (30%). The organic layer was separated and the excess of o-anisidine separated with toluene. The residue was re-crystallized from toluene and the press cake washed with cold alcohol. A compound of the formula (I) was obtained); Yield: 41%
- 780 g (6 mol) of aniline hydrochloride are melted in a 1.5-1 reaction vessel under nitrogen at 220° C. and 100 g (1 mol) of 2-Ethylbutyraldehyde is slowly added thereto while stirring over a period of 4 hour.
- The temperature of the melt falls from initially ca. 200° C. to 185° C. because of the reflux. The temperature is kept for one hour at 185° C. and the hot melt is poured on a mixture of 1.6 kg ice and 1.05 kg of sodium hydroxide solution (30%).
- The organic layer is separated and washed free from salt with demineralised water. The excess of aniline is extracted by water steam distillation.
- The residue, ca. 180 g was re-crystallized from toluene and the press cake is washed with cold ethanol. A compound of the formula (2) was obtained); Yield: 48%
-
TABLE 1 Synthesis of the di amines starting with aldehydes Nr. R5 R6 3 H CH2CH2CH2CH3 4 H CH2CH(CH3)2 5 H CH(CH3)CH2CH3 6 H CH(CH3)CH2CH2CH3 7 H (CH2)5CH3 8 H (CH2)4CH3 9 H CH(CH2CH3)(CH2)3CH3 10 H (CH2)6CH3 11 H (CH2)7CH3 12 H (CH2)8CH3 13 H CH2-Ph 14 H Ph 15 H 4-Ph-CH3 16 H 4-Ph-CH(CH3)2 17 H 4-Ph-t-Bu 18 H 4-Ph-OCH3 19 H 4-Ph-OCH2CH3 20 H CH2CH(CH3)2 21 H CH(CH2CH3)2 22 H Phenyl 23 H 4-Ph-CH3 24 H 4-Ph-OCH3 25 H CH(CH2CH3)2 26 H Phenyl 27 H 4-Ph-CH3 28 H 4-Ph-OCH3 29 H CH(CH2CH3)2 30 H CH(CH2CH3)(CH2)3CH3 31 H 4-Ph-CH3 32 H 4-Ph-OCH3 33 H CH(CH2CH3)2 34 H CH(CH2CH3)(CH2)3CH3 35 H Phenyl 36 H CH(CH2CH3)2 37 H CH(CH2CH3)(CH2)3CH3 38 H Phenyl 39 H 4-Ph-OCH3 - 101 g Ethyl-propylketone, 500 g o-anisidine, 500 g hydrochloric acid (a. 30%) and 1000 ml water were heated in an autoclave at 140° C. for 6 hours.
- The reaction mixture was poured on 1 kg ice and 600 g sodium hydroxide solution (30%). The organic layer was separated and the excess of o-anisidine extracted with toluene. The residue was re-crystallized from toluene and the press cake washed with cold alcohol. A compound of the formula (3) was obtained; Yield: 33%
- 780 g (6 mol) of aniline hydrochloride are melted in a 1-1 reaction vessel under nitrogen at 220° C. and 86 g (1 mol) of 3-Pentanone is slowly added thereto while stirring over a period of 3-4 hour.
- The temperature of the melt falls from initially ca. 200° C. to 185° C. because of the reflux.
- The temperature is kept for one hour at 185° C. and the hot melt is poured on a mixture of 1.6 kg ice and 1.05 kg of sodium hydroxide solution (30%).
- The organic layer is separated and washed free from salt with demineralised water. The aniline excess is extracted by water steam distillation.
- The residue, ca. 160 g was re-crystallized from toluene and the press cake is washed with cold ethanol. A compound of the formula (4) was obtained; Yield: 52%
-
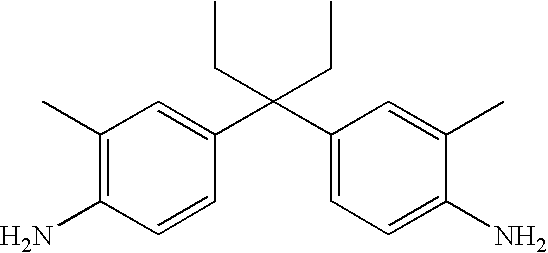
TABLE 2 Synthesis of the di-amines starting with ketones Nr. R5 R6 42 CH3 CH2CH2CH3 43 CH3 CH(CH3)2 44 CH3 CH2CH2CH2CH3 45 CH3 CH2CH(CH3)2 46 CH3 CH2CH2CH2CH2CH3 47 CH3 CH2CH2CH(CH3)2 48 CH3 CH2CH2CH2CH2CH2CH3 49 CH3 CH2Ph 50 CH3 CH2CH2Ph 51 CH2CH3 CH2CH2CH3 52 CH2CH3 CH2CH2CH2CH3 53 CH2CH3 CH2CH2CH2CH2CH3 54 CH2CH3 CH2CH2CH2CH2CH2CH3 55 CH2CH3 CH2CH2CH(CH3)2 56 CH2CH3 CH2CH(CH3)CH2CH3 57 CH2CH2CH3 CH2CH2CH3 58 CH(CH3)2 CH(CH3)2 59 CH2CH2CH2CH3 CH2CH2CH2CH3 60 CHCH2(CH3)2 CHCH2(CH3)2 61 CH2CH3 CH2CH3 62 CH2CH3 CH2CH2CH2CH3 63 CH2CH3 CH2CH3 64 CH2CH3 CH2CH2CH2CH3 65 CH3 CH2CH(CH3)2 66 CH2CH3 CH2CH3 67 CH2CH3 CH2CH2CH2CH3 - 26.8 Parts (0.1 mol) of 1,1-bis-(4-aminophenyl)-2-ethyl-butane (bridge-example 2) are tetrazotised according to known methods with 13.8 parts (0.2 mol) of sodium nitrite at 0-5° C. in 200 parts of water and 60 parts of hydrochloric acid (ca. 30%). 64.4 parts (0.2 mol) of a compound of the formula
- dissolved in 250 parts of water are added over 30 minutes to the ice cold tetrazotised solution. By the addition of 30% NaOH solution the pH is brought to 3-4.5 yielding a dyestuff of formula (5) and the dyestuff is in solution. λmax=459 nm.
- The dyestuff can be isolated by concentration under vacuum or by precipitation in aceton/alcohol.
- The reaction mixture however can be used directly for dyeing without isolation the product. The dyestuff of formula (5) has surprisingly very high solubility in water and gives yellow dyeings with very good fastness properties.
- 33.4 Parts (0.1 mol) of Bis-(3-methoxy-4-aminophenyl)-phenylmethane (bridge-example 1) are tetrazotised according to known methods with 13.8 parts (0.2 mol) of sodium nitrite at 0-5° C. in 200 parts of water and 60 parts of hydrochloric acid (ca. 30%).
- 64.4 parts (0.2 mol) of a compound of the formula
- dissolved in 250 parts of water are added over 30 minutes to the ice cold tetrazotised solution. By the addition of 30% NaOH solution the pH is brought to 3-4.5 yielding a dyestuff of formula (6) and the dyestuff is in solution. λmax 475 nm.
- The dyestuff can be isolated by concentration under vacuum or by precipitation in aceton/alcohol.
- The reaction mixture howevercan be used directly for dyeing without isolation the product. The dyestuff of formula (6) has very high solubility in water and gives yellow dyeings with surprisingly very good fastness properties.
-
TABLE 3 synthesis of the dyesstuff with the diamines from Table 1 The following compounds shown in the table 3a were synthesized according to the example 68 or 69 using the diamine diazo component and reacted with coupling component λ max (lambda max) is indicated in nm (nano meters; measured in 1% acetic acid solution). Dye- Bridge- stuff-nr. Diamine nr. λ max 70 3 445 71 4 447 72 5 450 73 6 448 74 7 449 75 8 446 76 9 452 77 10 449 78 11 449 79 12 451 80 13 451 81 14 448 82 15 458 83 16 454 84 17 456 85 18 449 86 19 453 87 20 458 88 21 456 89 22 459 90 23 455 91 24 458 92 25 444 93 26 445 94 27 446 95 28 445 96 29 476 97 30 475 98 31 472 99 32 474 100 33 485 101 34 479 102 35 480 103 36 500 104 37 501 105 38 498 -
TABLE 4 synthesis of the dyesstuff with the diamines from Table 1 The following compounds shown in the table 4 were synthesized according to the example 68 or 69 using the diamine diazo component and reacted with coupling component λ max (lambda max) is indicated in nm (nano meters; measured in 1% acetic acid solution). Dye- stuff-nr. Diamine Bridge-nr. λ max 106 2 457 107 1 472 108 5 446 109 6 447 110 7 446 111 9 449 112 15 452 113 18 450 114 21 458 115 22 459 116 23 461 117 24 460 118 25 443 119 26 442 120 29 475 121 33 476 122 35 472 -
TABLE 5 synthesis of the dyesstuff with the diamines from Table 2 The following compounds shown in the table 5 were synthesized according to the example 68 or 69 using the diamine diazo component and reacted with coupling component λ max (lambda max) is indicated in nm (nano meters; measured in 1% acetic acid solution). Dye- stuff- Bridge- nr. Diamine nr. λ max 123 41 459 124 42 450 125 43 448 126 44 441 127 45 443 128 46 447 129 47 444 130 48 445 131 49 445 132 50 446 133 51 440 134 52 440 135 53 439 136 54 438 137 55 434 138 56 440 139 57 443 140 58 441 141 59 439 142 60 442 143 61 453 144 62 452 145 63 454 146 64 452 147 65 473 148 66 475 149 40 472 150 67 475 -
TABLE 6 synthesis of the dyesstuff with the diamines from Table 2 The following compounds shown in the table 6 were synthesized according to the example 68 or 69 using the diamine diazo component and reacted with coupling component λ max (lambda max) is indicated in nm (nano meters; measured in 1% acetic acid solution). Dye- stuff- Bridge- nr. Diamine nr. λ max 151 41 446 152 42 441 153 47 446 154 49 445 155 52 441 156 61 460 157 63 455 158 66 472 159 67 475 - 70 parts chemically bleached sulphite cellulose obtained from pinewood and 30 parts chemically bleached cellulose obtained from birchwood are beaten in 2000 parts water in a Hollander. 0.2 parts of the dyestuff of Example 68 are sprinkled into this pulp. After mixing for 10 min, paper is produced from this pulp. The absorbent paper obtained in this way is dyed yellow. The wastewater is colorless.
- 0.2 parts of the dyestuff powder according to Example 68, were dissolved in 100 parts hot water and cooled to room temperature. The solution is added to 100 parts chemically bleached sulphite cellulose which have been ground with 2000 parts water in a Hollander. After 15 minutes thorough mixing resin size and aluminium sulphate are added thereto. Paper produced in this way has a yellow nuance and exhibits perfect light and wet fastness.
- An absorbent length of unsized paper is drawn at 40-50° C. through a dyestuff solution having the following composition:
-
0.3 parts of the dyestuff according to Example 68 0.5 parts of starch and 99.0 parts of water. - The excess dyestuff solution is squeezed out through two rollers. The dried length of paper is dyed a yellow shade.
- The dyestuffs of Examples 69 to 159 may also be used for dyeing by a method analogous to that of Application Examples A to C. The paper dyeings obtained show good fastness properties.
- 0.2 Parts of the dyestuff of Example 68 in acid addition salt form are dissolved in 4000 part of demineralised water at 40° C. 100 Parts of a pre-wetted cotton textile substrate are added, and the bath is raised to the boiling point over 30 minutes and held at the boil for one hour. Any water, which evaporates during dyeing, is replaced continuously. The dyed substrate is removed form the bath, and after rinsing and drying, a yellow dyeing is obtained having good light- and wet-fastness properties. The dyestuff exhausts practically totally onto the fiber, and the wastewater is almost colorless.
- In a similar manner as described in Application Example D the dyestuffs according to Examples 69-159 may be used for dyeing cotton.
- 100 parts freshly tanned and neutralized chrome leather are agitated for 30 minutes in a vessel with a liquor consisting of 250 parts of water at 55° C. and 0.5 parts of the dyestuff of Example 68 in acid addition salt form, and then treated in the same bath for 30 minutes with 2 parts of an anionic fatty liquor based on sulphonated train oil. The leather is then dried and prepared in the normal way, giving a leather evenly dyed in a yellow shade.
- In a similar manner as described in Application Example E the dyestuffs according to Examples 69-159 may be used for dyeing leather.
- Further vegetable-tanned leathers of low affinity may be dyed using the dyestuffs as described herein in accordance with known methods.
- Water is added to a dry pulp in Hollander consisting of 60% (by weight) of mechanical wood pulp and 40% (by weight) of unbleached sulphite cellulose, and the slurry is beaten in order to obtain a dry content slightly exceeding 2.5% and having a beating degree of 40° SR (degrees Schopper-Riegler). The slurry is then exactly adjusted to a high density dry content of 2.5% by adding water. 5 Parts of a 2.5% aqueous solution of the dyestuff according to Example 68 are added to 200 parts of the above resulting slurry. The mixture is stirred for about 5 minutes and, after the addition of 2% (by weight) resin size and then 4% (by weight) alum (based on the dry weight) is further stirred for a few minutes until homogeneous. The resulting pulp is diluted with about 500 parts water to a volume of 700 parts and then used for the production of paper sheets by suction on a sheet former. The resulting paper sheets are yellow. By a method analogous to that described in Application Example F any one of the dyestuffs of Examples 69-159 may be used instead of that of Example 68. In all cases, the waste paper exhibits a substantially low residual dye concentration.
- Water is added to a dry pulp in a Hollander consisting of 50% (by weight) of chemically bleached sulphite cellulose obtained from pinewood and 50% (by weight) of chemically bleached sulphite cellulose obtained from birchwood, and the slurry is ground until a degree of grinding of 35° SR is reached. The slurry is then adjusted to a high density dry content of 2.5% by adding water, and the pH of this suspension is adjusted to 7.10 Parts of a 0.5% aqueous solution of the dyestuff according to Example 68 are added to 200 parts of the above resulting slurry, and the mixture is stirred for 5 minutes. The resulting pulp is diluted with 500 parts water and then used for the production of sheets by suction on a sheet former. The paper sheets thus obtained have a yellow shade.
- By a method analogous to that described in Application Example G further dye mixtures may be used consisting of any one of the dyestuffs of Examples 69-159. In all cases, paper sheets are formed having a yellow shade.
- 12.6 parts dyestuff of Example 68 are added dropwise at room temperature to a stirred mixture of 20.0 parts diethyleneglycole and 67.4 parts of demineralized water. The resulting ink exhibits good light- and waterfastness properties. In a similar manner as described in Application Example H any one of the dyestuffs of Examples 69-159 may be used.
- A roof batten composed of Norway spruce and a roof batten composed of beechwood are sawn into pieces 5 cm in length and one piece of the sprucewood roof batten and one piece of the beechwood roof batten are dipped into a dilute solution of the reaction solution according to Example 68 (30 parts by weight of water and 1 part by weight of reaction solution, thus without isolating the dye stuff). Yellowish roof batten pieces are obtained on drying. In a similar manner as described in Application Example I any one of the dyestuffs of Examples 69-159 may be used.
Claims (10)
—[(CR8R8′)—(CR9′R9′)m—(CR10R10′)n—(CR11R11′)o]—NR12R12′
—[(CR8R8′)—(CR9′R9′)m—(CR10R10′)n—(CR11R11′)o]—NR12R12′
—[(CR8R8′)—(CR9′R9′)—(CR10R10′)]—NR12R12′
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06118116.0 | 2006-07-28 | ||
| EP06118116 | 2006-07-28 | ||
| EP06118116 | 2006-07-28 | ||
| PCT/EP2007/057652 WO2008012322A1 (en) | 2006-07-28 | 2007-07-25 | Basic bisazo compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20100000033A1 true US20100000033A1 (en) | 2010-01-07 |
| US8921564B2 US8921564B2 (en) | 2014-12-30 |
Family
ID=37665740
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/375,375 Active 2029-02-28 US8921564B2 (en) | 2006-07-28 | 2007-07-25 | Basic bisazo compounds |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US8921564B2 (en) |
| EP (1) | EP2049599B1 (en) |
| JP (1) | JP2009544788A (en) |
| KR (1) | KR101457233B1 (en) |
| CN (1) | CN101495578B (en) |
| BR (1) | BRPI0715213B1 (en) |
| ES (1) | ES2625119T3 (en) |
| HK (1) | HK1136001A1 (en) |
| NO (1) | NO20090382L (en) |
| TW (1) | TWI415902B (en) |
| WO (1) | WO2008012322A1 (en) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI461408B (en) * | 2008-07-02 | 2014-11-21 | Clariant Finance Bvi Ltd | Acid dyes |
| PT2258685E (en) * | 2009-05-14 | 2013-12-10 | Clariant Finance Bv Ltd | Bisazo compounds |
| EP2251385A1 (en) * | 2009-05-14 | 2010-11-17 | Clariant International Ltd. | Pyrazolone bisazo dyes |
| EP2251325A1 (en) * | 2009-05-14 | 2010-11-17 | Clariant International Ltd. | Bisazo compounds |
| EP2251386A1 (en) * | 2009-05-14 | 2010-11-17 | Clariant International Ltd. | Pyrazolone bisazo dyes |
| EP2258683A1 (en) * | 2009-05-14 | 2010-12-08 | Clariant International Ltd. | Bisazo compounds |
| JP2013512971A (en) | 2009-12-02 | 2013-04-18 | クラリアント・ファイナンス・(ビーブイアイ)・リミテッド | Concentrated storage-stable fluorescent brightener aqueous solution |
| CN105985285A (en) * | 2015-02-02 | 2016-10-05 | 上海申伦科技发展有限公司 | Pyridone azo compound and preparation method thereof |
| CN105985286A (en) * | 2015-02-02 | 2016-10-05 | 上海申伦科技发展有限公司 | Pyridone azo compound, N-substituted pyridone-based pyridine quaternary ammonium salt intermediate thereof, and preparation methods thereof |
| CN108530304B (en) * | 2018-04-24 | 2021-02-19 | 武汉理工大学 | Aromatic diamine and polyimide containing tolyl and non-coplanar structure and preparation method thereof |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4023924A (en) * | 1967-07-13 | 1977-05-17 | Sandoz Ltd. | Concentrated aqueous dye compositions containing a low molecular weight amide and their use for dyeing paper |
| US4068085A (en) * | 1974-12-06 | 1978-01-10 | Ciba-Geigy Corporation | 3-Nitro-5-azopyridine dyestuffs |
| US4149851A (en) * | 1967-07-13 | 1979-04-17 | Fidelity Union Trust Company, Executive Trustee Under Sandoz Trust | Concentrated aqueous dye compositions containing a low molecular weight amide |
| US4739042A (en) * | 1985-08-08 | 1988-04-19 | Bayer Aktiengesellschaft | Halftone gravure printing inks containing pyridone azo dyestuffs |
| US5037964A (en) * | 1984-11-08 | 1991-08-06 | Sandoz Ltd. | Metal-free sulfo group-free basis disazo and trisazo compounds containing two different 6-hydroxypyrid-2-one coupling component radicals |
| US5352334A (en) * | 1985-03-30 | 1994-10-04 | Sandoz Ltd. | The use of metal-free sulfo group free basic disazo compounds containing two identical 6-hydroxypyrid-2-one coupling component radicals for producing colored paper |
| US6140478A (en) * | 1995-07-21 | 2000-10-31 | Clariant Finance (Bvi) Limited | Basic azo compounds, their production and use |
| US7132516B2 (en) * | 2002-01-31 | 2006-11-07 | Clariant Finance (Bvi) Limited | Mono-or bisazo copper complex dyestuffs |
| US7183409B2 (en) * | 2001-06-01 | 2007-02-27 | Clariant Finance (Bvi) Limited | Basic mono- and bisazo compounds |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE399149C (en) | 1922-09-30 | 1924-07-23 | Hoechst Ag | Process for the preparation of diaminodiaryldialkylmethanes |
| DE505475C (en) | 1927-09-23 | 1930-08-18 | I G Farbenindustrie Akt Ges | Process for the separation of condensation products from aromatic bases and hydroaromatic ring ketones of the cyclohexanone series |
| DE1220863B (en) | 1964-11-04 | 1966-07-14 | Bayer Ag | Process for the preparation of 4,4'-diaminodiarylalkanes |
| CH480422A (en) | 1967-07-13 | 1969-10-31 | Sandoz Ag | Concentrated, liquid preparation |
| FR1555580A (en) | 1967-07-24 | 1969-01-31 | ||
| CH552661A (en) | 1968-11-12 | 1974-08-15 | Ciba Geigy Ag | PROCESS FOR PRODUCING NEW AZO DYES. |
| DE3226889A1 (en) | 1982-07-17 | 1984-01-19 | Basf Ag, 6700 Ludwigshafen | METHOD FOR PRODUCING BIS (AMINOCYCLOHEXYL) DIALKYLMETHANES |
| DE3538517A1 (en) | 1984-11-08 | 1986-05-15 | Sandoz-Patent-GmbH, 7850 Lörrach | Basic azo compounds, their preparation and use |
| DE3609590A1 (en) | 1985-03-30 | 1986-10-02 | Sandoz-Patent-GmbH, 7850 Lörrach | Basic azo compounds which are free of sulpho groups |
| CH667464A5 (en) * | 1985-03-30 | 1988-10-14 | Sandoz Ag | SULPHONIC ACID-FREE BASIC AZO COMPOUNDS. |
| DE4014847A1 (en) | 1990-05-09 | 1991-11-14 | Bayer Ag | New 1,1-bis-aminophenyl-cyclo:alkane(s) - useful as starting materials for prodn. of polyamide(s), polyurea(s), polyurethane-urea(s), epoxide-di:amine addn. prods. etc. |
| JP4402917B2 (en) | 2002-08-06 | 2010-01-20 | 富士フイルム株式会社 | Ink, inkjet recording method, and bisazo compound |
-
2007
- 2007-07-25 BR BRPI0715213A patent/BRPI0715213B1/en active IP Right Grant
- 2007-07-25 ES ES07787882.5T patent/ES2625119T3/en active Active
- 2007-07-25 WO PCT/EP2007/057652 patent/WO2008012322A1/en active Application Filing
- 2007-07-25 US US12/375,375 patent/US8921564B2/en active Active
- 2007-07-25 JP JP2009521254A patent/JP2009544788A/en not_active Ceased
- 2007-07-25 EP EP07787882.5A patent/EP2049599B1/en active Active
- 2007-07-25 CN CN2007800286365A patent/CN101495578B/en active Active
- 2007-07-25 KR KR1020097001785A patent/KR101457233B1/en active IP Right Grant
- 2007-07-26 TW TW096127299A patent/TWI415902B/en active
-
2009
- 2009-01-26 NO NO20090382A patent/NO20090382L/en not_active Application Discontinuation
-
2010
- 2010-01-13 HK HK10100350.2A patent/HK1136001A1/en not_active IP Right Cessation
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4023924A (en) * | 1967-07-13 | 1977-05-17 | Sandoz Ltd. | Concentrated aqueous dye compositions containing a low molecular weight amide and their use for dyeing paper |
| US4149851A (en) * | 1967-07-13 | 1979-04-17 | Fidelity Union Trust Company, Executive Trustee Under Sandoz Trust | Concentrated aqueous dye compositions containing a low molecular weight amide |
| US4068085A (en) * | 1974-12-06 | 1978-01-10 | Ciba-Geigy Corporation | 3-Nitro-5-azopyridine dyestuffs |
| US5037964A (en) * | 1984-11-08 | 1991-08-06 | Sandoz Ltd. | Metal-free sulfo group-free basis disazo and trisazo compounds containing two different 6-hydroxypyrid-2-one coupling component radicals |
| US5352334A (en) * | 1985-03-30 | 1994-10-04 | Sandoz Ltd. | The use of metal-free sulfo group free basic disazo compounds containing two identical 6-hydroxypyrid-2-one coupling component radicals for producing colored paper |
| US4739042A (en) * | 1985-08-08 | 1988-04-19 | Bayer Aktiengesellschaft | Halftone gravure printing inks containing pyridone azo dyestuffs |
| US6140478A (en) * | 1995-07-21 | 2000-10-31 | Clariant Finance (Bvi) Limited | Basic azo compounds, their production and use |
| US7183409B2 (en) * | 2001-06-01 | 2007-02-27 | Clariant Finance (Bvi) Limited | Basic mono- and bisazo compounds |
| US7132516B2 (en) * | 2002-01-31 | 2006-11-07 | Clariant Finance (Bvi) Limited | Mono-or bisazo copper complex dyestuffs |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2008012322A1 (en) | 2008-01-31 |
| ES2625119T3 (en) | 2017-07-18 |
| TWI415902B (en) | 2013-11-21 |
| KR101457233B1 (en) | 2014-11-03 |
| BRPI0715213A2 (en) | 2013-06-18 |
| NO20090382L (en) | 2009-04-20 |
| JP2009544788A (en) | 2009-12-17 |
| HK1136001A1 (en) | 2010-06-18 |
| CN101495578B (en) | 2013-06-12 |
| TW200813165A (en) | 2008-03-16 |
| EP2049599B1 (en) | 2017-02-22 |
| US8921564B2 (en) | 2014-12-30 |
| CN101495578A (en) | 2009-07-29 |
| BRPI0715213B1 (en) | 2017-04-04 |
| KR20090045207A (en) | 2009-05-07 |
| EP2049599A1 (en) | 2009-04-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8921564B2 (en) | Basic bisazo compounds | |
| US8734532B2 (en) | Azo dyes | |
| US5929215A (en) | Basic monoazo compounds | |
| JP3902672B2 (en) | Basic disazo compounds containing sulfonic acid groups | |
| US5424404A (en) | Biscationic azo dyes and intermediates therefor | |
| EP2078737A1 (en) | Basic monoazo compounds | |
| EP2098573A2 (en) | Basic monoazo compounds | |
| EP2085429A1 (en) | Basic bisazo compounds | |
| EP2085428A1 (en) | Basic bisazo compounds | |
| US8734534B2 (en) | Azo dyes | |
| US6844428B2 (en) | Use of disazo compounds | |
| US6302923B1 (en) | Triphendioxazine compounds | |
| US7183409B2 (en) | Basic mono- and bisazo compounds | |
| EP2103656A2 (en) | Basic monoazo compounds | |
| EP0891394B1 (en) | Basic monoazo compounds | |
| US9023118B2 (en) | Azo dyes | |
| US5545724A (en) | Cationically bridged tetrakisazo dyestuffs with variable couplers, their production and use | |
| US6406525B1 (en) | Organic compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: CLARIANT FINANCE (BVI) LTD., VIRGIN ISLANDS, BRITI Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:HASEMANN, LUDWIG;LEHR, FRIEDRICH;OBERHOLZER, MARTIN;AND OTHERS;REEL/FRAME:022165/0046 Effective date: 20081014 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| AS | Assignment |
Owner name: ARCHROMA IP GMBH, SWITZERLAND Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:CLARIANT FINANCE (BVI) LIMITED;REEL/FRAME:039103/0455 Effective date: 20160623 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 4TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1551) Year of fee payment: 4 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 8TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1552); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Year of fee payment: 8 |