US20040127632A1 - Perfluorostyrene compound, and coating solution and optical waveguide device using the same - Google Patents
Perfluorostyrene compound, and coating solution and optical waveguide device using the same Download PDFInfo
- Publication number
- US20040127632A1 US20040127632A1 US10/616,889 US61688903A US2004127632A1 US 20040127632 A1 US20040127632 A1 US 20040127632A1 US 61688903 A US61688903 A US 61688903A US 2004127632 A1 US2004127632 A1 US 2004127632A1
- Authority
- US
- United States
- Prior art keywords
- group
- compound
- fluorine compound
- perfluorostyrene
- coating solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 C*(CCC1=CCC=CC(C=C)=C1)NC(*)(*)N Chemical compound C*(CCC1=CCC=CC(C=C)=C1)NC(*)(*)N 0.000 description 16
- JORIDPOXTRUINS-UHFFFAOYSA-N C.C.C=Cc1ccccc1.C=Cc1ccccc1.CCCC[Ar]CCCC.CF.CFF.FF.FFF Chemical compound C.C.C=Cc1ccccc1.C=Cc1ccccc1.CCCC[Ar]CCCC.CF.CFF.FF.FFF JORIDPOXTRUINS-UHFFFAOYSA-N 0.000 description 3
- VVIYDJCRJLPMNY-UHFFFAOYSA-N C.Cc1ccc(C(c2ccc(C)cc2)(C(F)(F)F)C(F)(F)F)cc1 Chemical compound C.Cc1ccc(C(c2ccc(C)cc2)(C(F)(F)F)C(F)(F)F)cc1 VVIYDJCRJLPMNY-UHFFFAOYSA-N 0.000 description 3
- AKYIMYSJDNBGFM-POWKINDLSA-N CC.CC.CC.CC.CC.CC.CC.CC.[2H](c1ccccc1)c1ccccc1.c1ccc(C2(c3ccccc3)c3ccccc3-c3ccccc32)cc1.c1ccccc1 Chemical compound CC.CC.CC.CC.CC.CC.CC.CC.[2H](c1ccccc1)c1ccccc1.c1ccc(C2(c3ccccc3)c3ccccc3-c3ccccc32)cc1.c1ccccc1 AKYIMYSJDNBGFM-POWKINDLSA-N 0.000 description 3
- RVMNEEPYFTYCNX-UHFFFAOYSA-N C.CCc1ccc(C(c2ccc(CC)cc2)(C(F)(F)F)C(F)(F)F)cc1 Chemical compound C.CCc1ccc(C(c2ccc(CC)cc2)(C(F)(F)F)C(F)(F)F)cc1 RVMNEEPYFTYCNX-UHFFFAOYSA-N 0.000 description 2
- YOZXBCBNMKSZHP-UHFFFAOYSA-N Cc1cc(C)cc(C)c1.Cc1ccc(C(c2ccc(C)cc2)c2ccc(C)cc2)cc1 Chemical compound Cc1cc(C)cc(C)c1.Cc1ccc(C(c2ccc(C)cc2)c2ccc(C)cc2)cc1 YOZXBCBNMKSZHP-UHFFFAOYSA-N 0.000 description 2
- JWPBZLHREPHIKE-UHFFFAOYSA-N C=CC(=O)OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)COC(=O)C=C.C=CC(=O)OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COC(=O)C=C.C=CC(=O)OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COC(=O)C=C.C=CC(=O)OCCOc1ccc(C(c2ccc(OCCOC(=O)C=C)cc2)(C(F)(F)F)C(F)(F)F)cc1.[CaH2].[Cd] Chemical compound C=CC(=O)OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)COC(=O)C=C.C=CC(=O)OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COC(=O)C=C.C=CC(=O)OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COC(=O)C=C.C=CC(=O)OCCOc1ccc(C(c2ccc(OCCOC(=O)C=C)cc2)(C(F)(F)F)C(F)(F)F)cc1.[CaH2].[Cd] JWPBZLHREPHIKE-UHFFFAOYSA-N 0.000 description 1
- SYAUAPYKYLRNIS-UHFFFAOYSA-N C=Cc1c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)COc2c(F)c(F)c(F)c(C=C)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc2c(F)c(F)c(F)c(C=C)c2F)c1F.C=Cc1c(F)c(F)c(F)c(Oc2cc(Oc3c(F)c(F)c(F)c(C=C)c3F)cc(Oc3c(F)c(F)c(F)c(C=C)c3F)c2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(C)(c3ccc(Oc4c(F)c(F)c(F)c(C=C)c4F)cc3)c3ccc(Oc4c(F)c(F)c(F)c(C=C)c4F)cc3)cc2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(c3ccc(Oc4c(F)c(F)c(F)c(C=C)c4F)cc3)(C(F)(F)F)C(F)(F)F)cc2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C3(c4ccc(Oc5c(F)c(F)c(F)c(C=C)c5F)cc4)c4ccccc4-c4ccccc43)cc2)c1F.CC=O.[BaH2] Chemical compound C=Cc1c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)COc2c(F)c(F)c(F)c(C=C)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc2c(F)c(F)c(F)c(C=C)c2F)c1F.C=Cc1c(F)c(F)c(F)c(Oc2cc(Oc3c(F)c(F)c(F)c(C=C)c3F)cc(Oc3c(F)c(F)c(F)c(C=C)c3F)c2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(C)(c3ccc(Oc4c(F)c(F)c(F)c(C=C)c4F)cc3)c3ccc(Oc4c(F)c(F)c(F)c(C=C)c4F)cc3)cc2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(c3ccc(Oc4c(F)c(F)c(F)c(C=C)c4F)cc3)(C(F)(F)F)C(F)(F)F)cc2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C3(c4ccc(Oc5c(F)c(F)c(F)c(C=C)c5F)cc4)c4ccccc4-c4ccccc43)cc2)c1F.CC=O.[BaH2] SYAUAPYKYLRNIS-UHFFFAOYSA-N 0.000 description 1
- MHVWKRCEVOCRGE-UHFFFAOYSA-N C=Cc1c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc2c(F)c(Br)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc2c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc2c(F)c(Br)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc2c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(c3ccc(Oc4c(F)c(F)c(-c5c(F)c(F)c(Oc6ccc(C(c7ccc(Oc8c(F)c(F)c(F)c(C=C)c8F)cc7)(C(F)(F)F)C(F)(F)F)cc6)c(F)c5F)c(F)c4F)cc3)(C(F)(F)F)C(F)(F)F)cc2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(c3ccc(Oc4c(F)c(F)c(F)c(Oc5ccc(C(c6ccc(Oc7c(F)c(F)c(F)c(C=C)c7F)cc6)(C(F)(F)F)C(F)(F)F)cc5)c4F)cc3)(C(F)(F)F)C(F)(F)F)cc2)c1F Chemical compound C=Cc1c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc2c(F)c(Br)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc2c(F)c(F)c(F)c(OCC(F)(F)C(F)(F)C(F)(F)C(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc2c(F)c(Br)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc2c(F)c(F)c(F)c(OCC(F)(F)OC(F)(F)C(F)(F)OC(F)(F)C(F)(F)OC(F)(F)COc3c(F)c(F)c(F)c(C=C)c3F)c2F)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(c3ccc(Oc4c(F)c(F)c(-c5c(F)c(F)c(Oc6ccc(C(c7ccc(Oc8c(F)c(F)c(F)c(C=C)c8F)cc7)(C(F)(F)F)C(F)(F)F)cc6)c(F)c5F)c(F)c4F)cc3)(C(F)(F)F)C(F)(F)F)cc2)c1F.C=Cc1c(F)c(F)c(F)c(Oc2ccc(C(c3ccc(Oc4c(F)c(F)c(F)c(Oc5ccc(C(c6ccc(Oc7c(F)c(F)c(F)c(C=C)c7F)cc6)(C(F)(F)F)C(F)(F)F)cc5)c4F)cc3)(C(F)(F)F)C(F)(F)F)cc2)c1F MHVWKRCEVOCRGE-UHFFFAOYSA-N 0.000 description 1
- OWEIAGSMFHSSES-UHFFFAOYSA-N Cc1ccc(C(c2ccc(C)cc2)(C(F)(F)F)C(F)(F)F)cc1 Chemical compound Cc1ccc(C(c2ccc(C)cc2)(C(F)(F)F)C(F)(F)F)cc1 OWEIAGSMFHSSES-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F212/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring
- C08F212/02—Monomers containing only one unsaturated aliphatic radical
- C08F212/04—Monomers containing only one unsaturated aliphatic radical containing one ring
- C08F212/14—Monomers containing only one unsaturated aliphatic radical containing one ring substituted by heteroatoms or groups containing heteroatoms
- C08F212/16—Halogens
- C08F212/20—Fluorine
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/20—Ethers having an ether-oxygen atom bound to a carbon atom of a six-membered aromatic ring
- C07C43/225—Ethers having an ether-oxygen atom bound to a carbon atom of a six-membered aromatic ring containing halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/257—Ethers having an ether-oxygen atom bound to carbon atoms both belonging to six-membered aromatic rings
- C07C43/29—Ethers having an ether-oxygen atom bound to carbon atoms both belonging to six-membered aromatic rings containing halogen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F214/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a halogen
- C08F214/18—Monomers containing fluorine
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D4/00—Coating compositions, e.g. paints, varnishes or lacquers, based on organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond ; Coating compositions, based on monomers of macromolecular compounds of groups C09D183/00 - C09D183/16
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B6/00—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings
- G02B6/10—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings of the optical waveguide type
- G02B6/12—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings of the optical waveguide type of the integrated circuit kind
- G02B6/13—Integrated optical circuits characterised by the manufacturing method
- G02B6/138—Integrated optical circuits characterised by the manufacturing method by using polymerisation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/04—Ortho- or ortho- and peri-condensed systems containing three rings
- C07C2603/06—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members
- C07C2603/10—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members containing five-membered rings
- C07C2603/12—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members containing five-membered rings only one five-membered ring
- C07C2603/18—Fluorenes; Hydrogenated fluorenes
Definitions
- the present invention relates, in general, to perfluorostyrene compounds, and coating solutions and optical waveguide devices using the same.
- the present invention is directed to a fluorine compound having perfluorostyrene moiety, and a coating solution and an optical waveguide device using the same.
- fluorinated compounds are applied for a core and a cladding material of various planar optical waveguide devices, such as optical switches, variable optical attenuators (VOA), tunable and fixed wavelength filters, arrayed waveguide grating (AWG) devices, etc.
- polymeric optical waveguide devices should be required the reliability based on Telcodia test for the optical communication network.
- the polymer material should have very high thermal stability and environmental stability. Further, there are required accurate control of a refractive index and low birefringence as well as low optical propagation loss at a telecommunication wavelength region.
- the polymer material should have excellent adhesion to any substrate.
- the optical propagation loss and the birefringence are regarded as very important characteristics.
- the optical propagation loss on a polymer thin film is mainly caused by the light absorption by a harmonic overtone vibration mode of a C—H bond in the presence of polymer.
- Such light absorption at wavelengths of near far infrared can be decreased by substituting deuterium (D) or halogen elements, such as fluorine (F), for hydrogen of C—H bond (or O—H, N—H), whereby an absorption wavelength band can be shifted to 5-25 ⁇ m. Therefore, the loss can be lowered at communication wavelengths.
- D deuterium
- F fluorine
- the birefringence of the thin film is caused by a molecular structure and a stress of a thin film-preparing process.
- UV-curable fluorinated acrylate including various compositions, which is advantageous in terms of relatively low optical loss of 0.3 dB/cm at 1.55 ⁇ m and a birefringence of 0.0008.
- fluorinated polyarylene ether having a low dielectric constant, and excellent mechanical strength and processability (U.S. Pat. No. 5,115,082), which shows the possibility as a potential optical polymer.
- the above polymer system is added with a thermally curable reactive group, to drastically increase chemical resistance, whereby such a polymer is applied for the optical waveguide device (Korean Patent No. 226,442). Consequently, fluorinated polyarylene ether based polymers have been further improved in optical loss (0.4 dB/cm) and birefringence (0.004), compared to polyamide-based polymers, but is disadvantageous of still high birefringence and high processing temperatures (280° C. or more).
- perfluorostyrene is introduced at a terminal of a compound for use in an optical device, whereby inherent light absorption caused by a higher order harmonic vibration mode of a C—H bond in the compound can be prevented in optical communication wavelength, thus realizing low optical loss, low optical birefringence, precise control of a refractive index, and a fabrication of optical devices at low temperatures in a short process time.
- Another object of the present invention is to provide a coating solution using the fluorinated compound.
- Still another object of the present invention is to provide an optical waveguide device using the fluorinated compound.
- FIG. 1 shows the refractive index according to blending ratio of fluorinated compounds of the present invention
- FIG. 2 shows the precise control of refractive index according to blending ratio of coating solutions of the present invention.
- a fluorinated compound having perfluorostyrene introduced at a terminal thereof is synthesized through a reaction of polyol and pentafluorostyrene, which is represented by the following Formula 1:
- Z is O or S; R F is an aliphatic or aromatic group; y is a natural number of 1-10; y′ is an integer of 0-1; x is an integer of 0-200; and
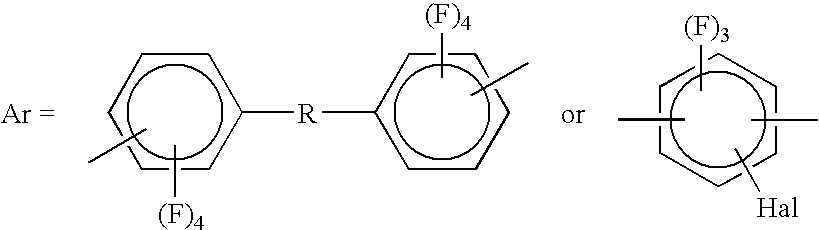
- B is a single bond or selected from the group consisting of —CO—, —SO 2 —, —S— and —O—; and Hal is selected from the group consisting of F, Cl, Br and I.
- the fluorinated polymer compound having perfluorostyrene introduced at a terminal thereof is represented by the following Formula 2 in which y and y′ are 1:
- Z is O or S, preferably O, and preferred R F is —CH 2 (CF 2 ) n CH 2 —, —CH 2 CF 2 O(CF 2 CF 2 O) n CF 2 CH 2 —, or
- x is an integer of 0-200, and preferably 2-50;
- D is selected from the group consisting of —C(CF 3 ) 2 —, —C(CH 3 ) 2 —, —CO—, —SO 2 —, —O— and —S—;
- R 1 and R 2 are independently selected from the group consisting of H, or halogen elements, such as F, Cl, Br and I; and
- m is a natural number of 1-3.
- the perfluorostyrene-introduced fluorine compound can be synthesized.
- R F is an aliphatic or aromatic compound, and y is a natural number of 1-10.
- Z is O, and R F is a substituted or unsubstituted alkyl group when y is 1, and R F is the same as R F of Formula 2 when y is 2.
- the fluorine compound having perfluorostyrene introduced at a terminal thereof can be synthesized, as represented by Formula 3 in which R F is —CH 2 (CF 2 ) n CH 2 —, —CH 2 CF 2 (OCF 2 CF 2 ) n OCF 2 CH 2 —, or
- the fluorine compound having perfluorostyrene introduced at a terminal thereof can be synthesized, as represented by Formula 3 in which when y is 3, R F is an aromatic or aliphatic group, and more preferably,
- M is selected from the group consisting of C—CH 3 , C—CF 3 , C—CCl 3 , and C—CBr 3 , or selected from the group consisting of N, P and P ⁇ O.
- the fluorine compound represented by Formula 2 can be synthesized by reaction of an aliphatic or aromatic diol and a fluorinated aromatic compound in the presence of a base such as NaOH or K 2 CO 3 in DMAc (dimetylacetamide). The reaction mixture was stirred at room temperature for ambient hours. And then to this mixture, pentafluorostyrene was added and stirred for more hours for complete reaction.
- a base such as NaOH or K 2 CO 3 in DMAc (dimetylacetamide).
- Formula 4 shows representative the polymers having perfluorostyrene introduced at a terminal thereof.
- derivatives substituted at a para-position through the above reaction may be produced with any amounts. Such derivatives are used without additional separation, to control the refractive index of an optical waveguide.
- ‘a’ as a repeat unit number is preferably 2-50.
- the compound represented by Formula 3 can be prepared by the reaction of selected from alcohol-containing R F , preferably diol or triol, and pentafluorostyrene in the presence of a base such as NaOH or K 2 CO 3 in DMAc (dimethylacetamide).
- a base such as NaOH or K 2 CO 3 in DMAc (dimethylacetamide).
- the representative fluorinated compounds having perfluorostyrene introduced at a terminal thereof are obtained, as represented by the following Formulas 5 and 6.
- Formulas 5 and 6 derivatives substituted at a para-position through the above reaction may be produced with any amounts.
- the derivatives are used without additional separation for the control of a refractive index and curing characteristics of an optical waveguide device.
- the compounds represented by Formula 5 are ones in which y is 2 in Formula 3
- the compounds represented by Formula 6 are ones in which y is 3 in Formula 3:
- a polymer material for application of optical waveguide device is used as a mixture comprising the fluorine compound having perfluorostyrene introduced at a terminal thereof, as represented by Formula 2 or 3, a photoinitiator and a reactive fluorinated acrylate compound represented by the following Formula 7, so as to control the refractive index and viscosity.
- the photoinitiator is not particularly limited so long as it can initiate a reaction of a styrene group, which is exemplified by Irgacure 184, Irgacure 651, etc., sold by CIBA GEIGY:
- A is a fluorinated aliphatic or aromatic group
- Y is H or CH 3 .
- A is —CH 2 (CF 2 ) n CH 2 —, —CH 2 CF 2 (OCF 2 CF 2 ) n OCF 2 CH 2 — or
- the compound represented by Formula 7 is obtained by the reaction of a fluorinated diol with acryloyl chloride in the presence of triethylamine.
- Synthesized acrylate compounds are represented by the following Formula 8:
- a polymer material suitable for application in the optical waveguide device can be used as a mixture of the fluorine compound represented by Formula 1, the photoinitiator and the acrylate compound represented by Formula 7 or commercially available acrylate compound, such as 1,6-hexanediol diacrylate, tris(2-hydroxy ethyl)isocyanurate triacrylate, and pentaerythrol triacrylate.
- a coating solution for use in the formation of a core layer and a cladding layer in the optical waveguide device at least one fluorine compound represented by Formula 1 is mixed with the photoinitiator and the compound of Formula 7 or the reactive compound and solvents are added as necessary.
- perfluorostyrene introduced at a terminal thereof dissolved in propylene glycol methyl ether acetate (PGMEA) or cyclohexanone, the photoinitiator and the compound (Formula 7) or commercially available reactive acrylate were blended. And then the solution was filtered with a Teflon membrane filter to remove fine particles having a size of 0.2 ⁇ m or more.
- the filtered solution is spin-coated onto various types of substrates, preferably, a silicon wafer substrate, and then subjected to a UV curing by the use of a UV irradiating apparatus in a nitrogen atmosphere, thereby obtaining a desired thin film.
- the coating mixture comprises 30-70 wt % of the fluorine compound selected from the group consisting of fluorine compounds of Formula 1, 30-70 wt % of acrylate selected from the group consisting of acrylate compounds of Formula 7 or 8, and 0.5-4 wt % of the photoinitiator.
- the optical waveguide device using the fluorine compound includes a lower cladding layer, a core layer and an upper cladding layer, laminated sequentially on a planar substrate.
- the core layer and the upper and lower cladding layers are formed of the fluorine compound.
- examples of the substrate for use in the polymer device include polymer plate, glass, silica plate and so on.
- a silicon wafer substrate is used.
- a silica layer is formed or a polymer material having a refractive index lower than that of a polymer constituting a core layer is coated on such a substrate and cured. The formation of a thin film accords to the above manner.
- An optical waveguide core material is coated on the lower cladding layer and cured, after which a photolithographic process is performed to form optical waveguide patterns.
- RIE reactive ion etching
- ICP inductive coupled plasma
- the fluorine compound having perfluorostyrene introduced at a terminal thereof has higher fluorine content, compared to acrylate compounds. Hence, inherent light absorption of the compound by vibrations of C—H bonds is prevented, thus lowering the optical loss in optical communication wavelength. In addition, the optical birefringence is very low, and thus the fabrication of the optical device with low polarization dependence becomes facile (Table 1). Further, since the inventive fluorine compound has no polar functional groups, the moisture absorption is low. Referring to FIG. 1, it can be seen that the mixture of fluorine compounds of the present invention has an influence on the control of the refractive index. By a UV curing or a thermal curing, a thin film can be easily formed, thus fabricating the optical waveguide device having excellent thermal stability and chemical resistance.
- reaction mixture was extracted with deionized water and ether.
- the extracted ether layer was dried with magnesium sulfate, and ether was evaporated by a rotary evaporator.
- the produced liquid compound with very high viscosity was dried at room temperature using a vacuum pump to remove the residual solvent.
- 1H-NMR (Acetone d 6 ): ⁇ 4.90 (m), 5.75 (d of d), 6.04 (d of d), 6.68 (d of d).
- Mn 2,560 (NMR).
- the refractive index was measured by a prism coupler, and the optical loss was determined by the incorporation of a slab waveguide using an index matching oil. The refractive index and the optical loss were measured at a wavelength of 1550 nm.
- FIG. 2 shows the relationship between the refractive index and the coating solution mixture obtained by mixing the coating solutions shown in experimental numbers 6 and 7 of Table 1 in example 14.
- a silicon wafer substrate was preferably used.
- Such a substrate was spin-coated with the filtered polymer coating solution at 500-5000 rpm, and cured under a UV light intensity of 5-200 mW/cm 2 , preferably 10-50 mW/cm 2 , using a mercury lamp in a nitrogen atmosphere for 2-30 min, and then post baked on a hot plate at 100-200° C. for 0.5-1 hour, to prepare a desired polymer thin film.
- the obtained thin film is superior in chemical resistance, thus realizing a facile fabrication of an optical device having multi-layered thin films.
- a substrate suitable for use in the fabrication of an optical device a silicon wafer was used.
- a silica layer was formed or the inventive polymer having a refractive index lower by about 0.3-1% than that of a core layer polymer was coated on the silicon wafer substrate, and then cured.
- the formation of the thin film was performed in the same manner as in example 16.
- a polymer core material was coated on the lower cladding layer and then cured, after which a photomask was aligned and a photolithographic process was performed, thereby forming optical waveguide patterns. Then, by the use of a reactive ion etching process or an inductive coupled plasma process, the core layer of the optical waveguide, were etched.
- the same polymer material as the coating solution used for the lower cladding layer was coated on the core layer and then cured, to obtain an upper cladding layer.
- a desired optical waveguide device was fabricated.
- a drive electrode forming process might be further performed for driving an optical device on the upper cladding layer.
- the fabricated optical device wafer was diced and polished by the use of a saw and a polisher, thereby forming an end face of the device for input and output of light waves.
- the present invention provides a fluorine compound having perfluorostyrene introduced at a terminal thereof, and a coating solution and an optical waveguide device using such a fluorine compound.
- the fluorine compound has high fluorine content on a molecular structure thereof, whereby inherent light absorption due to molecular vibrations can be prevented in optical communication wavelength, thus decreasing optical loss.
- the optical birefringence of the thin film which is attributed to a molecular structure of the film material, is remarkably reduced, and thus the optical device with low polarization dependence can be easily fabricated.
- the fluorine compounds are mixed together, thereby achieving precise control of the refractive index.
- the fluorine compound has no polar functional groups, resulting in low moisture absorption.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Health & Medical Sciences (AREA)
- Polymers & Plastics (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Optics & Photonics (AREA)
- General Physics & Mathematics (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Optical Integrated Circuits (AREA)
Abstract
Description
- 1. Field of the Invention
- The present invention relates, in general, to perfluorostyrene compounds, and coating solutions and optical waveguide devices using the same. In particular, the present invention is directed to a fluorine compound having perfluorostyrene moiety, and a coating solution and an optical waveguide device using the same. These fluorinated compounds are applied for a core and a cladding material of various planar optical waveguide devices, such as optical switches, variable optical attenuators (VOA), tunable and fixed wavelength filters, arrayed waveguide grating (AWG) devices, etc.
- 2. Description of the Related Art
- Generally, polymeric optical waveguide devices should be required the reliability based on Telcodia test for the optical communication network. In such a case, the polymer material should have very high thermal stability and environmental stability. Further, there are required accurate control of a refractive index and low birefringence as well as low optical propagation loss at a telecommunication wavelength region. Furthermore, in order to fabricate a desirable optical device, the polymer material should have excellent adhesion to any substrate. Of the above-mentioned requirements, the optical propagation loss and the birefringence are regarded as very important characteristics. The optical propagation loss on a polymer thin film is mainly caused by the light absorption by a harmonic overtone vibration mode of a C—H bond in the presence of polymer. Such light absorption at wavelengths of near far infrared can be decreased by substituting deuterium (D) or halogen elements, such as fluorine (F), for hydrogen of C—H bond (or O—H, N—H), whereby an absorption wavelength band can be shifted to 5-25 μm. Therefore, the loss can be lowered at communication wavelengths.
- On the other hand, the birefringence of the thin film is caused by a molecular structure and a stress of a thin film-preparing process.
- Accordingly, various polymer materials have been developed to meet all the requirements. In this regard, a fluorinated polyimide compound, which is known to have excellent heat resistance, even at about 400° C., has been continuously applied for optical waveguide devices (U.S. Pat. No. 5,598,501, Macromolecules, vol 27, pp 6665, 1994 and Electronics Letters, 29(3) 269, 1993). However, polyimide suffers from drawbacks, such as relatively high optical loss of 0.7 dB/cm or more and a high birefringence of 0.008 or more.
- As another polymer material, there is proposed UV-curable fluorinated acrylate including various compositions, which is advantageous in terms of relatively low optical loss of 0.3 dB/cm at 1.55 μm and a birefringence of 0.0008. (U.S. Pat. No. 6,306,563 B1, and IEEE Journal of selected topics in quantum electronics vol. 6, pp 54, 2000).
- As still another polymer material, there is proposed fluorinated polyarylene ether having a low dielectric constant, and excellent mechanical strength and processability (U.S. Pat. No. 5,115,082), which shows the possibility as a potential optical polymer. In addition, the above polymer system is added with a thermally curable reactive group, to drastically increase chemical resistance, whereby such a polymer is applied for the optical waveguide device (Korean Patent No. 226,442). Consequently, fluorinated polyarylene ether based polymers have been further improved in optical loss (0.4 dB/cm) and birefringence (0.004), compared to polyamide-based polymers, but is disadvantageous of still high birefringence and high processing temperatures (280° C. or more).
- To avoid the problems encountered in the related art, perfluorostyrene is introduced at a terminal of a compound for use in an optical device, whereby inherent light absorption caused by a higher order harmonic vibration mode of a C—H bond in the compound can be prevented in optical communication wavelength, thus realizing low optical loss, low optical birefringence, precise control of a refractive index, and a fabrication of optical devices at low temperatures in a short process time.
- Therefore, it is the object of the present invention to provide a fluorinated compound having perfluorostyrene introduced at a terminal thereof.
- Another object of the present invention is to provide a coating solution using the fluorinated compound.
- Still another object of the present invention is to provide an optical waveguide device using the fluorinated compound.
- The above and other objects, features and other advantages of the present invention will be better understood from the following detailed description taken in conjunction with the accompanying drawings, in which:
- FIG. 1 shows the refractive index according to blending ratio of fluorinated compounds of the present invention; and
- FIG. 2 shows the precise control of refractive index according to blending ratio of coating solutions of the present invention.
-
-
- Wherein B is a single bond or selected from the group consisting of —CO—, —SO 2—, —S— and —O—; and Hal is selected from the group consisting of F, Cl, Br and I.
-
-
- Wherein x is an integer of 0-200, and preferably 2-50; D is selected from the group consisting of —C(CF 3)2—, —C(CH3)2—, —CO—, —SO2—, —O— and —S—; R1 and R2 are independently selected from the group consisting of H, or halogen elements, such as F, Cl, Br and I; and m is a natural number of 1-3.
-
- the perfluorostyrene-introduced fluorine compound can be synthesized.
-
- Wherein R F is an aliphatic or aromatic compound, and y is a natural number of 1-10. Preferably, Z is O, and RF is a substituted or unsubstituted alkyl group when y is 1, and RF is the same as RF of Formula 2 when y is 2.
-
- when y is 2.
-
- Wherein M is selected from the group consisting of C—CH 3, C—CF3, C—CCl3, and C—CBr3, or selected from the group consisting of N, P and P═O.
- Furthermore, it is possible to synthesize the perfluorostyrene-introduced fluorine compound as represented by Formula 3 in which -Z-R F is an aromatic or aliphatic polyol when y is 4 or more.
- The fluorine compound represented by Formula 2 can be synthesized by reaction of an aliphatic or aromatic diol and a fluorinated aromatic compound in the presence of a base such as NaOH or K 2CO3 in DMAc (dimetylacetamide). The reaction mixture was stirred at room temperature for ambient hours. And then to this mixture, pentafluorostyrene was added and stirred for more hours for complete reaction.
- Below, Formula 4 shows representative the polymers having perfluorostyrene introduced at a terminal thereof. In addition to the chemical structures shown in Formula 4, derivatives substituted at a para-position through the above reaction may be produced with any amounts. Such derivatives are used without additional separation, to control the refractive index of an optical waveguide. In Formula 4, ‘a’ as a repeat unit number is preferably 2-50.
- In addition, the compound represented by Formula 3 can be prepared by the reaction of selected from alcohol-containing R F, preferably diol or triol, and pentafluorostyrene in the presence of a base such as NaOH or K2CO3 in DMAc (dimethylacetamide).
- Thereby, the representative fluorinated compounds having perfluorostyrene introduced at a terminal thereof are obtained, as represented by the following
Formulas 5 and 6. In addition to the chemical structures shown inFormulas 5 and 6, derivatives substituted at a para-position through the above reaction may be produced with any amounts. As such, the derivatives are used without additional separation for the control of a refractive index and curing characteristics of an optical waveguide device. Below, the compounds represented by Formula 5 are ones in which y is 2 in Formula 3, and the compounds represented byFormula 6 are ones in which y is 3 in Formula 3: - Meanwhile, a polymer material for application of optical waveguide device is used as a mixture comprising the fluorine compound having perfluorostyrene introduced at a terminal thereof, as represented by Formula 2 or 3, a photoinitiator and a reactive fluorinated acrylate compound represented by the following
Formula 7, so as to control the refractive index and viscosity. -
- Wherein A is a fluorinated aliphatic or aromatic group, and Y is H or CH 3.
-
-
- Further, with the aim of achieving a desired curing density, a formation of multi-layered thin films and a high adhesion to a substrate, a polymer material suitable for application in the optical waveguide device can be used as a mixture of the fluorine compound represented by
Formula 1, the photoinitiator and the acrylate compound represented byFormula 7 or commercially available acrylate compound, such as 1,6-hexanediol diacrylate, tris(2-hydroxy ethyl)isocyanurate triacrylate, and pentaerythrol triacrylate. - More particularly, as for a coating solution for use in the formation of a core layer and a cladding layer in the optical waveguide device, at least one fluorine compound represented by
Formula 1 is mixed with the photoinitiator and the compound ofFormula 7 or the reactive compound and solvents are added as necessary. To produce a coating solution, perfluorostyrene introduced at a terminal thereof dissolved in propylene glycol methyl ether acetate (PGMEA) or cyclohexanone, the photoinitiator and the compound (Formula 7) or commercially available reactive acrylate were blended. And then the solution was filtered with a Teflon membrane filter to remove fine particles having a size of 0.2 μm or more. Thereafter, the filtered solution is spin-coated onto various types of substrates, preferably, a silicon wafer substrate, and then subjected to a UV curing by the use of a UV irradiating apparatus in a nitrogen atmosphere, thereby obtaining a desired thin film. - Preferably, the coating mixture comprises 30-70 wt % of the fluorine compound selected from the group consisting of fluorine compounds of
Formula 1, 30-70 wt % of acrylate selected from the group consisting of acrylate compounds ofFormula 7 or 8, and 0.5-4 wt % of the photoinitiator. - The optical waveguide device using the fluorine compound includes a lower cladding layer, a core layer and an upper cladding layer, laminated sequentially on a planar substrate. In such a case, the core layer and the upper and lower cladding layers are formed of the fluorine compound.
- As for the fabrication of the optical waveguide device, examples of the substrate for use in the polymer device include polymer plate, glass, silica plate and so on. Preferably, a silicon wafer substrate is used. As a lower cladding, a silica layer is formed or a polymer material having a refractive index lower than that of a polymer constituting a core layer is coated on such a substrate and cured. The formation of a thin film accords to the above manner. An optical waveguide core material is coated on the lower cladding layer and cured, after which a photolithographic process is performed to form optical waveguide patterns. Using a reactive ion etching (RIE) process or an inductive coupled plasma (ICP) etching process, the core layer are etched. Finally, a polymer material for an upper cladding layer is coated on the core layer and cured. Thusly fabricated optical device is diced and polished, thus forming an end face of the device for input and output of light waves.
- The fluorine compound having perfluorostyrene introduced at a terminal thereof has higher fluorine content, compared to acrylate compounds. Hence, inherent light absorption of the compound by vibrations of C—H bonds is prevented, thus lowering the optical loss in optical communication wavelength. In addition, the optical birefringence is very low, and thus the fabrication of the optical device with low polarization dependence becomes facile (Table 1). Further, since the inventive fluorine compound has no polar functional groups, the moisture absorption is low. Referring to FIG. 1, it can be seen that the mixture of fluorine compounds of the present invention has an influence on the control of the refractive index. By a UV curing or a thermal curing, a thin film can be easily formed, thus fabricating the optical waveguide device having excellent thermal stability and chemical resistance.
- Having generally described this invention, a further understanding can be obtained by reference to specific examples which are provided herein for the purpose of illustration only and are not intended to be limiting unless otherwise specified.
- 3.0 g (16.12 mmol) of hexafluorobenzene and 5.17 g (19.70 mmol) of 2,2,3,3,4,4,5,5-octafluoro-1,6-hexanediol were placed into a 100 mL three-neck flask, to which 46 mL of a DMAc solvent was added to completely dissolve the reactants in the flask. 2.05 g of NaOH was further added into the flask, after which the resulting mixture was stirred at room temperature for 24 hours in a nitrogen atmosphere. Then, the reaction mixture was added with 1.39 g (7.16 mmol) of pentafluorostyrene and stirred for more 12 hours. Thusly obtained reaction mixture was extracted with deionized water and ether. The extracted ether layer was dried with magnesium sulfate, and ether was evaporated by a rotary evaporator. The produced liquid compound with very high viscosity was dried at room temperature using a vacuum pump to remove the residual solvent. 1H-NMR (Acetone d 6): δ 4.90 (m), 5.75 (d of d), 6.04 (d of d), 6.68 (d of d). Mn=2,560 (NMR).
- The present example was performed in the same manner as in example 1, with the exception being that 4.87 g (19.70 mmol) of bromopentafluorobenzene was used, instead of hexafluorobenzene. 1H-NMR (Acetone d 6). δ 4.92 (m), 5.73 (d of d), 6.04 (d of d), 6.67 (d of d). Mn=2,900 (NMR).
- 2.26 g (12.15 mmol) of hexafluorobenzene and 6.09 g (14.85 mmol) of perfluorotetraethylene glycol were placed into a 100 mL three-neck flask, to which 47 mL of a DMAc solvent was added to completely dissolve the reactants in the flask. 1.54 g of NaOH was further added into the flask, after which the resulting mixture was stirred at room temperature for 24 hours in a nitrogen atmosphere. Then, the reaction mixture was added with 1.05 g (5.40 mmol) of pentafluorostyrene and stirred for 12 hours. Thusly obtained reaction mixture was extracted with deionized water and ether. The extracted ether layer was dried with magnesium sulfate, and ether was evaporated by a rotary evaporator. The produced liquid compound was dried at room temperature using a vacuum pump. 1H-NMR (CDCl 3). δ 4.48(m), 5.66 (d of d), 6.03 (d of d), 6.59 (d of d). Mn=3,150 (NMR).
- The present example was performed in the same manner as in example 3, with the exception being that 3.0 g (12.15 mmol) of bromopentafluorobenzene was used, instead of hexafluorobenzene. 1H-NMR (Acetone d 6): δ 4.50 (m), 5.65 (d of d), 6.03 (d of d), 6.60 (d of d). Mn=3,470 (NMR)
- 3.0 g (16.12 mmol) of hexafluorobenzene and 6.62 g (19.70 mmol) of 2,2-bis (4-hydroxyphenyl)hexafluoropropane were placed into a 100 mL three-neck flask, to which 55 mL of a DMAc solvent was added to completely dissolve the reactants in the flask. 2.05 g of NaOH was further added into the flask, after which the resulting mixture was stirred at room temperature for 24 hours in a nitrogen atmosphere. Then, 1.39 g (7.16 mmol) of pentafluorostyrene was added to the reaction mixture, which was then stirred for 12 hours. The reaction mixture was extracted with deionized water and ether. The extracted ether layer was dehydrated with magnesium sulfate, and ether was evaporated by a rotary evaporator. The produced white solid compound was dried at 30° C. in a vacuum oven. 1H-NMR (Acetone d 6): δ 5.80 (d of d), 6.09 (d of d), 6.74 (d of d), 7.26(d), 7.43 (d). Mn=2,980 (NMR).
- In a 100 mL three-neck flask, 5.0 g (14.97 mmol) of decafluorobiphenyl and 6.15 g (18.29 mmol) of 2,2-bis(4-hydroxyphenyl)hexafluoropropane were completely dissolved in 63 mL of a DMAc solvent. Then, 1.90 g of NaOH was further added into the flask, after which the resulting mixture was stirred at room temperature for 24 hours in a nitrogen atmosphere. To this reaction 1.29 g (6.64 mmol) of pentafluorostyrene was added and stirred for ˜12 hours. The reaction mixture was extracted with deionized water and ether. The extracted ether layer was dehydrated with magnesium sulfate, and ether was evaporated by a rotary evaporator. The produced white solid compound was dried at 30° C. in a vacuum oven. 1H-NMR (Acetone d 6): δ 5.82 (d of d), 6.11 (d of d), 6.75 (d of d), 7.30 (d), 7.43 (d). Mn=3,610 (NMR).
- In a 100 mL three-neck flask, 5.0 g (13.81 mmol) of 2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro-1,8-octanediol and 5.36 g (27.62 mmol) of pentafluorostyrene were completely dissolved in 59 mL of a DMAc solvent. 1.44 g of NaOH was further added into the flask. The resulting mixture was stirred at room temperature for 10 hours in a nitrogen atmosphere. Thusly obtained reaction mixture was cooled and then extracted with deionized water and ether. The extracted ether layer was dehydrated with magnesium sulfate, and ether was evaporated by a rotary evaporator. The produced white solid compound was dried at 30° C. in a vacuum oven. 1H-NMR (CDCl 3): δ 4.48 (t, 4H), 5.67 (d of d, 2H), 6.05 (d of d, 2H), 6.61 (d of d, 2H)
- The present example was performed in the same manner as in example 7, with the exception being that 5.66 g (13.81 mmol) of perfluorotetraethylene glycol was used, instead of 2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro-1,8-octanediol. 1H-NMR (CDCl 3): δ 4.64 (t, 4H), 5.66 (d of d, 2H), 6.05 (d of d, 2H), 6.60 (d of d, 2H).
- 5.0 g (14.87 mmol) of 2,2-bis(4-hydroxyphenyl)hexafluoropropane and 5.77 g (27.74 mmol) of pentafluorostyrene were placed into a 100 mL three-neck flask, to which 61 mL of a DMAc solvent was added to completely dissolve the reactants in the flask. 1.55 g of NaOH was further added into the flask, after which the resulting mixture was stirred at room temperature for 12 hours in a nitrogen atmosphere. Then, the reaction mixture was cooled and extracted with deionized water and ether. The extracted ether layer was dehydrated with magnesium sulfate, and ether was evaporated by a rotary evaporator. Finally, the produced white solid compound was dried at 30° C. in a vacuum oven. 1H-NMR (Acetone d 6): δ 5.81 (d of d, 2H), 6.10 (d of d, 2H), 6.74 (d of d, 2H), 7.25 (d, 4H) 7.44 (d, 4H).
- 5.0 g (14.27 mmol) of 9,9-bis (4-hydroxyphenyl) fluorene and 5.54 g (28.54 mmol) of pentafluorostyrene were placed into a 100 mL three-neck flask, and then completely dissolved with 60 mL of a DMAc solvent. 1.48 g of NaOH was further added into the flask. The resulting mixture was stirred at room temperature for 8 hours in a nitrogen atmosphere. Then, the reaction mixture was cooled and extracted with deionized water and ether. The extracted ether layer was dehydrated with magnesium sulfate, and ether was evaporated by a rotary evaporator. Finally, the produced white solid compound was dried at 30° C. in a vacuum oven. 1H-NHR (Acetone d 6): δ 5.77 (d of d, 2H), 6.07 (d of d, 2H), 6.70 (d of d, 2H), 7.00 (d, 4H), 7.20 (d, 4H), 7.33 (t, 2H), 7.39 (t, 2H), 7.46(d, 2H), 7.88 (d, 2H).
- Into a 100 mL three-neck flask, 3.0 g (9.79 mmol) of 1,1,1-tris(4-hydroxyphenyl)ethane and 5.70 g (29.38 mmol) of pentafluorostyrene were placed and then completely dissolved with 49 mL of a DMAc solvent. 1.57 g of NaOH was further added into the flask. The resulting mixture was stirred at room temperature for 8 hours in a nitrogen atmosphere, after which the reaction mixture was extracted with deionized water and ether. The extracted ether layer was dehydrated with magnesium sulfate, and ether was evaporated by a rotary evaporator. The produced white solid compound was dried at 30° C. in a vacuum oven. 1H-NMR (Acetone d 6): δ 2.16 (s, 3H), 5.78 (d of d, 3H), 6.08 (d of d, 3H), 6.73 (d of d, 3H), 7.04 (d, 6H), 7.10 (d, 6H)
- The present example was performed in the same manner as in example 11, with the exception being that 1.23 g (9.79 mmol) of 1,2,4-benzenetriol was used, instead of 1,1,1-tris(4-hydroxyphenyl)ethane. 1H-NMR (Acetone d 6): δ 5.3 (d of d, 3H), 5.4 (d of d, 3H), 6.3 (s, 3H), 6.9 (d of d, 3H).
- In a 100 mL three-neck flask, 5.0 g (13.81 mmol) of 2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro-1,8-octanediol was completely dissolved in 80 mL of a DMAc solvent. 3 g of triethylamine was further added into the flask. While a reactor was maintained at 0° C. or lower in a nitrogen atmosphere, acryloyl chloride was droplets slowly added to the reaction in the reactor, after which the reaction mixture was stirred for 3 hours. The reaction mixture was filtered to remove a formed ammonium salt, and extracted with deionized water and ether. The extracted ether layer was dehydrated with magnesium sulfate, and ether was evaporated by a rotary evaporator. The produced liquid compound was vacuum distilled to produce a pure compound. 1H-NMR (CDCl 3): d 4.66 (t, 4H), 5.99 (d, 2H), 6.22 (q, 2H), 6.54 (d, 2H).
- Each fluorine compound having perfluorostyrene introduced at a terminal thereof, prepared in examples 1-12, was admixed with Irgacure 651 as a photoinitiator, and then dissolved in 10-100 wt % of PGMEA or cyclohexanone solvent, depending on viscosity. The solution was further mixed with a compound represented by Formula 5 and 10-60 wt % of reactive acrylate, to produce a coating solution, which was then filtered with a 0.2 μm Teflon filter. Thereby, a coating solution suitable for use in the core and cladding layers as thin films of an optical waveguide device was produced. The following Table 1 shows the refractive index and the optical loss of thin films formed after being cured, depending on the composition and the content of the composition.
TABLE 1 Exp. Content Refractive Light Loss No. Composition (wt %) index (dB/cm) 1 Compound (B) 70 1.4540 0.16 Photoinitiator 1 (Irgacure 651) Solvent (PGMEA) 29 2 Compound (C) 70 1.3910 0.15 Photoinitiator 1 (Irgacure 651) Solvent (PGMEA) 29 3 Compound (D) 70 1.4110 0.17 Photoinitiator 1 (Irgacure 651) Solvent (PGMEA) 29 4 Compound (F) 40 1.4930 0.21 Photoinitiator 1 (Irgacure 651) Solvent (PGMEA) 59 5 Compound (B) 40 1.4790 0.3 Compound (Ba) 15 Photoinitiator 1 (Irgacure 651) Solvent 44 (cyclohexanone) 6 Compound (B) 40 1.4450 0.34 Compound (Cb) 30 Compound (Cc) 10 Pentaerythrol 19 triacrylate Photoinitiator 1 (Irgacure 651) 7 Compound (B) 40 1.4320 0.31 Compound (Cb) 25 Compound (Cc) 25 Pentaerythrol 9 triacrylate Photoinitiator 1 (Irgacure 651) - In Table 1, the refractive index was measured by a prism coupler, and the optical loss was determined by the incorporation of a slab waveguide using an index matching oil. The refractive index and the optical loss were measured at a wavelength of 1550 nm.
- In an optical waveguide device, precise control of refractive index is needed between the core and cladding layers in order to contain the single mode condition. For this, the coating solutions, having different refractive indexes as shown in example 14, were mixed together by a weight ratio. FIG. 2 shows the relationship between the refractive index and the coating solution mixture obtained by mixing the coating solutions shown in
experimental numbers - The polymer coating solution having perfluorostyrene, prepared in example 14, was filtered with a 0.2 μm Teflon filter. Of various types of substrates, a silicon wafer substrate was preferably used. Such a substrate was spin-coated with the filtered polymer coating solution at 500-5000 rpm, and cured under a UV light intensity of 5-200 mW/cm 2, preferably 10-50 mW/cm2, using a mercury lamp in a nitrogen atmosphere for 2-30 min, and then post baked on a hot plate at 100-200° C. for 0.5-1 hour, to prepare a desired polymer thin film. The obtained thin film is superior in chemical resistance, thus realizing a facile fabrication of an optical device having multi-layered thin films.
- As a substrate suitable for use in the fabrication of an optical device, a silicon wafer was used. As a lower cladding of the optical device, a silica layer was formed or the inventive polymer having a refractive index lower by about 0.3-1% than that of a core layer polymer was coated on the silicon wafer substrate, and then cured. The formation of the thin film was performed in the same manner as in example 16. A polymer core material was coated on the lower cladding layer and then cured, after which a photomask was aligned and a photolithographic process was performed, thereby forming optical waveguide patterns. Then, by the use of a reactive ion etching process or an inductive coupled plasma process, the core layer of the optical waveguide, were etched. Finally, the same polymer material as the coating solution used for the lower cladding layer was coated on the core layer and then cured, to obtain an upper cladding layer. Thereby, a desired optical waveguide device was fabricated. As necessary, a drive electrode forming process might be further performed for driving an optical device on the upper cladding layer. The fabricated optical device wafer was diced and polished by the use of a saw and a polisher, thereby forming an end face of the device for input and output of light waves.
- As described above, the present invention provides a fluorine compound having perfluorostyrene introduced at a terminal thereof, and a coating solution and an optical waveguide device using such a fluorine compound. The fluorine compound has high fluorine content on a molecular structure thereof, whereby inherent light absorption due to molecular vibrations can be prevented in optical communication wavelength, thus decreasing optical loss.
- Further, the optical birefringence of the thin film, which is attributed to a molecular structure of the film material, is remarkably reduced, and thus the optical device with low polarization dependence can be easily fabricated. Moreover, the fluorine compounds are mixed together, thereby achieving precise control of the refractive index. In addition, the fluorine compound has no polar functional groups, resulting in low moisture absorption. By a UV curing or a thermal curing, the thin film can be readily formed, thus obtaining an optical waveguide device having excellent thermal stability and chemical resistance.
- The present invention has been described in an illustrative manner, and it should be understood that the terminology used is intended to be in the nature of description rather than of limitations. Many modifications and variations of the present invention are possible in light of the above teachings. Therefore, it should be understood that within the scope of the appended claims, the invention may be practiced otherwise than as specifically described.
Claims (15)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/034,646 US7202324B2 (en) | 2002-07-12 | 2005-01-13 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
| US11/725,398 US20070173592A1 (en) | 2002-07-12 | 2007-03-19 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR10-2002-0040901 | 2002-07-12 | ||
| KR10-2002-0040901A KR100511100B1 (en) | 2002-07-12 | 2002-07-12 | Perfluorostyrene compounds, Coating solution and Optical waveguide device using the same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/034,646 Continuation US7202324B2 (en) | 2002-07-12 | 2005-01-13 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040127632A1 true US20040127632A1 (en) | 2004-07-01 |
Family
ID=32653084
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/616,889 Abandoned US20040127632A1 (en) | 2002-07-12 | 2003-07-10 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
| US11/034,646 Expired - Lifetime US7202324B2 (en) | 2002-07-12 | 2005-01-13 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
| US11/725,398 Abandoned US20070173592A1 (en) | 2002-07-12 | 2007-03-19 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/034,646 Expired - Lifetime US7202324B2 (en) | 2002-07-12 | 2005-01-13 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
| US11/725,398 Abandoned US20070173592A1 (en) | 2002-07-12 | 2007-03-19 | Perfluorostyrene compound, and coating solution and optical waveguide device using the same |
Country Status (2)
| Country | Link |
|---|---|
| US (3) | US20040127632A1 (en) |
| KR (1) | KR100511100B1 (en) |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020057882A1 (en) * | 2000-09-28 | 2002-05-16 | Zen Photonics Co. Ltd. | Fluorinated polyethers having perfluorinated aliphatic group and optical waveguide using the same |
| JP2006045159A (en) * | 2004-08-06 | 2006-02-16 | Asahi Glass Co Ltd | Fluorine-containing ether and its use |
| JP2006076893A (en) * | 2004-09-07 | 2006-03-23 | Asahi Glass Co Ltd | New fluorine-containing ether |
| US20060068207A1 (en) * | 2004-09-28 | 2006-03-30 | Brewer Science Inc., A Missouri Corporation | Curable high refractive index resins for optoelectronic applications |
| US20080287624A1 (en) * | 2004-09-14 | 2008-11-20 | Maria Petrucci-Samija | Process for Preparing an Optical Organic Polymer |
| WO2011065312A1 (en) * | 2009-11-26 | 2011-06-03 | 旭硝子株式会社 | Ether compound, lubricant containing same, and composition for lubricant containing same |
| WO2011122392A1 (en) * | 2010-03-31 | 2011-10-06 | Dic株式会社 | Fluorine-containing styrene compound and active energy ray curable composition using same |
| CN105452209A (en) * | 2013-08-07 | 2016-03-30 | 旭硝子株式会社 | Fluorine-containing aromatic compound, method for producing same, curable material, cured product thereof, and optical member |
| US20160137809A1 (en) * | 2013-08-07 | 2016-05-19 | Asahi Glass Company, Limited | Crosslinkable fluorinated elastomer composition and crosslinked product thereof |
| US9620378B1 (en) * | 2015-12-24 | 2017-04-11 | Jsr Corporation | Composition for film formation, film, production method of patterned substrate, and compound |
| KR101907410B1 (en) * | 2017-01-17 | 2018-10-12 | 주식회사 트리엘 | NOVEL COMPOUND having VINYLPHENYLOXY moiety AND PHOTOSENSITIVE PHOTORESIST COMPOSITION INCLUDING THE SAME |
| WO2019043142A1 (en) | 2017-09-04 | 2019-03-07 | Solvay Specialty Polymers Italy S.P.A. | Fluorinated poly(arylene ether) thermoset |
| US10421857B2 (en) | 2017-04-12 | 2019-09-24 | Samsung Display Co., Ltd. | Photocurable composition and patterned body manufactured by using the composition |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100536439B1 (en) * | 2002-07-12 | 2005-12-16 | 김미화 | Perfluorophenylacetylene compounds, Coating solution and Optical waveguide device using the same |
| US8716403B2 (en) | 2008-12-10 | 2014-05-06 | Electronics And Telecommunications Research Institute | Prepolymer prepared by a condensation reaction and a polymer sheet obtained therefrom |
| TW201127822A (en) | 2009-09-02 | 2011-08-16 | Du Pont | Fluorinated 4-oxo-chroman-carboxylates |
| WO2011028771A2 (en) | 2009-09-02 | 2011-03-10 | E. I. Du Pont De Nemours And Company | Polyester films with improved oil repellency |
| WO2011028767A2 (en) | 2009-09-02 | 2011-03-10 | E. I. Du Pont De Nemours And Company | Fluoroether functionalized aromatic diesters and derivatives thereof |
| KR20120083366A (en) * | 2009-09-02 | 2012-07-25 | 이 아이 듀폰 디 네모아 앤드 캄파니 | Polyesters comprising fluorovinylether functionalized aromatic moieties |
| TW201118113A (en) * | 2009-09-02 | 2011-06-01 | Du Pont | Polyaramid comprising fluorovinylether functionalized aromatic moieties |
| US20110218319A1 (en) * | 2009-09-02 | 2011-09-08 | E. I. Du Pont De Nemours And Company | Polyaramid films comprising fluorovinylether functionalized aromatic moieties |
| KR101272381B1 (en) * | 2010-04-23 | 2013-06-07 | 경북대학교 산학협력단 | Compound for optical waveguide, copolymer thereof and optical waveguide using thereof |
| US20140029267A1 (en) * | 2012-07-25 | 2014-01-30 | Electronics And Telecommunications Research Institute | Chemical compound being used for forming a random wrinkle structure, composition containing the compound, film having the structure, method of forming the film, and oled comprising the film |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5115082A (en) * | 1990-04-17 | 1992-05-19 | Raychem Corporation | Fluorinated poly(arylene ether) |
| US5438142A (en) * | 1992-02-03 | 1995-08-01 | Hoechst Celanese Corp. | Functionalized tris(hydroxyphenyl) compounds |
| US6002828A (en) * | 1996-10-18 | 1999-12-14 | Telefonaktiebolaget Lm Ericsson | Polymer optical guide made from a monomer with at least one epoxy group and a vinyl type monomer |
| US6235353B1 (en) * | 1998-02-24 | 2001-05-22 | Alliedsignal Inc. | Low dielectric constant films with high glass transition temperatures made by electron beam curing |
| US6323301B1 (en) * | 1996-08-29 | 2001-11-27 | Xerox Corporation | High performance UV and heat crosslinked or chain extended polymers |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS59214822A (en) * | 1983-05-20 | 1984-12-04 | Hoya Corp | Oxygen-permeable contact lens |
| JP2509274B2 (en) * | 1987-02-03 | 1996-06-19 | 財団法人相模中央化学研究所 | Photocrosslinkable fluorine-containing styrene polymer |
| EP0616234B1 (en) * | 1993-03-18 | 1999-06-02 | Nippon Telegraph And Telephone Corporation | Method of manufacturing a polyimide optical waveguide |
| JPH0812669A (en) * | 1994-06-24 | 1996-01-16 | Mitsubishi Gas Chem Co Inc | Aromatic vinyl compound containing sulfur |
| US5998501A (en) * | 1997-02-07 | 1999-12-07 | Kao Corporation | Process for producing aqueous ink for inkjet printing |
| KR19990024596A (en) * | 1997-09-04 | 1999-04-06 | 윤종용 | Polyarylene ether for optical communication |
| KR100226442B1 (en) | 1997-10-24 | 1999-10-15 | 이계철 | Fluorinated poly(arylene ether) containing thermally curable ethynyl group, preparation method thereof, and optical devices using the same |
| KR100243406B1 (en) * | 1997-11-12 | 2000-02-01 | 이계철 | Perfluorinated poly(arylether) for optical material, its preparation method, and optical material for optical waveguide |
| US6306563B1 (en) * | 1999-06-21 | 2001-10-23 | Corning Inc. | Optical devices made from radiation curable fluorinated compositions |
| AU2001287147A1 (en) * | 2000-09-08 | 2002-03-22 | Shipley Company, L.L.C. | Fluorinated phenolic polymers and photoresist compositions comprising same |
| KR100350412B1 (en) * | 2000-09-28 | 2002-08-28 | (주)젠포토닉스 | Fluorinated polyethers having perfluorinated aliphatic group and optical waveguide using the same |
| WO2003037964A1 (en) * | 2001-11-02 | 2003-05-08 | Sanyo Chemical Industries, Ltd. | Composite resin particles |
-
2002
- 2002-07-12 KR KR10-2002-0040901A patent/KR100511100B1/en active IP Right Grant
-
2003
- 2003-07-10 US US10/616,889 patent/US20040127632A1/en not_active Abandoned
-
2005
- 2005-01-13 US US11/034,646 patent/US7202324B2/en not_active Expired - Lifetime
-
2007
- 2007-03-19 US US11/725,398 patent/US20070173592A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5115082A (en) * | 1990-04-17 | 1992-05-19 | Raychem Corporation | Fluorinated poly(arylene ether) |
| US5438142A (en) * | 1992-02-03 | 1995-08-01 | Hoechst Celanese Corp. | Functionalized tris(hydroxyphenyl) compounds |
| US6323301B1 (en) * | 1996-08-29 | 2001-11-27 | Xerox Corporation | High performance UV and heat crosslinked or chain extended polymers |
| US6002828A (en) * | 1996-10-18 | 1999-12-14 | Telefonaktiebolaget Lm Ericsson | Polymer optical guide made from a monomer with at least one epoxy group and a vinyl type monomer |
| US6235353B1 (en) * | 1998-02-24 | 2001-05-22 | Alliedsignal Inc. | Low dielectric constant films with high glass transition temperatures made by electron beam curing |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020057882A1 (en) * | 2000-09-28 | 2002-05-16 | Zen Photonics Co. Ltd. | Fluorinated polyethers having perfluorinated aliphatic group and optical waveguide using the same |
| US6946534B2 (en) * | 2000-09-28 | 2005-09-20 | Mi-Hwa Kim | Fluorinated polyethers having perfluorinated aliphatic group and optical waveguide using the same |
| JP2006045159A (en) * | 2004-08-06 | 2006-02-16 | Asahi Glass Co Ltd | Fluorine-containing ether and its use |
| JP2006076893A (en) * | 2004-09-07 | 2006-03-23 | Asahi Glass Co Ltd | New fluorine-containing ether |
| JP4661140B2 (en) * | 2004-09-07 | 2011-03-30 | 旭硝子株式会社 | Solution composition for water and oil repellent agent, substrate and method for producing the same |
| US20080287624A1 (en) * | 2004-09-14 | 2008-11-20 | Maria Petrucci-Samija | Process for Preparing an Optical Organic Polymer |
| US20060068207A1 (en) * | 2004-09-28 | 2006-03-30 | Brewer Science Inc., A Missouri Corporation | Curable high refractive index resins for optoelectronic applications |
| US20090087666A1 (en) * | 2004-09-28 | 2009-04-02 | Mercado Ramil-Marcelo L | Curable high refractive index resins for optoelectronic applications |
| WO2011065312A1 (en) * | 2009-11-26 | 2011-06-03 | 旭硝子株式会社 | Ether compound, lubricant containing same, and composition for lubricant containing same |
| WO2011122392A1 (en) * | 2010-03-31 | 2011-10-06 | Dic株式会社 | Fluorine-containing styrene compound and active energy ray curable composition using same |
| CN105452209A (en) * | 2013-08-07 | 2016-03-30 | 旭硝子株式会社 | Fluorine-containing aromatic compound, method for producing same, curable material, cured product thereof, and optical member |
| US20160137809A1 (en) * | 2013-08-07 | 2016-05-19 | Asahi Glass Company, Limited | Crosslinkable fluorinated elastomer composition and crosslinked product thereof |
| JPWO2015020002A1 (en) * | 2013-08-07 | 2017-03-02 | 旭硝子株式会社 | Fluorine-containing aromatic compound, production method thereof, curable material, cured product thereof, and optical member |
| EP3031793A4 (en) * | 2013-08-07 | 2017-03-15 | Asahi Glass Company, Limited | Fluorine-containing aromatic compound, method for producing same, curable material, cured product thereof, and optical member |
| TWI632184B (en) * | 2013-08-07 | 2018-08-11 | 旭硝子股份有限公司 | Fluorine-containing aromatic compound, method for producing the same, curable material, cured product thereof, and optical member |
| US10066077B2 (en) * | 2013-08-07 | 2018-09-04 | Asahi Glass Company, Limited | Crosslinkable fluorinated elastomer composition and crosslinked product thereof |
| US10087128B2 (en) | 2013-08-07 | 2018-10-02 | AGC Inc. | Fluorinated aromatic compound, method for its production, curable material, its cured product, and optical member |
| US9620378B1 (en) * | 2015-12-24 | 2017-04-11 | Jsr Corporation | Composition for film formation, film, production method of patterned substrate, and compound |
| KR101907410B1 (en) * | 2017-01-17 | 2018-10-12 | 주식회사 트리엘 | NOVEL COMPOUND having VINYLPHENYLOXY moiety AND PHOTOSENSITIVE PHOTORESIST COMPOSITION INCLUDING THE SAME |
| US10421857B2 (en) | 2017-04-12 | 2019-09-24 | Samsung Display Co., Ltd. | Photocurable composition and patterned body manufactured by using the composition |
| WO2019043142A1 (en) | 2017-09-04 | 2019-03-07 | Solvay Specialty Polymers Italy S.P.A. | Fluorinated poly(arylene ether) thermoset |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20040006591A (en) | 2004-01-24 |
| KR100511100B1 (en) | 2005-08-31 |
| US20050163451A1 (en) | 2005-07-28 |
| US20070173592A1 (en) | 2007-07-26 |
| US7202324B2 (en) | 2007-04-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7202324B2 (en) | Perfluorostyrene compound, and coating solution and optical waveguide device using the same | |
| Lee et al. | Crosslinkable fluorinated poly (arylene ethers) bearing phenyl ethynyl moiety for low‐loss polymer optical waveguide devices | |
| JP4501391B2 (en) | Crosslinkable fluorine-containing aromatic prepolymer and use thereof | |
| US20030055120A1 (en) | Halogenated optical polymer composition | |
| Lee et al. | Crosslinkable polymers for optical waveguide devices. II. Fluorinated ether ketone oligomers bearing ethynyl group at the chain end | |
| EP1600797A1 (en) | Transparent resin material | |
| JP2002533304A (en) | Polymerizable halogenated vinyl ether | |
| KR100947710B1 (en) | Halogenated Optical Polymer Composition | |
| JP2013181140A (en) | Optical resin composition, cured matter, optical part, sealant for semiconductor light-emitting device, optical lens, optical adhesive, optical sealing agent and optical waveguide | |
| Ding et al. | Fluorinated poly (arylene ether ketone) s bearing pentafluorostyrene moieties prepared by a modified polycondensation | |
| US6512076B2 (en) | Poly (arylene ether sulfide) and poly (arylene ether sulfone) for optical device and method for preparing the same | |
| US6136929A (en) | Polyarylene ether for optical communication | |
| KR100350412B1 (en) | Fluorinated polyethers having perfluorinated aliphatic group and optical waveguide using the same | |
| JPH0260933A (en) | Fluorinated polyimide and its production | |
| KR101298770B1 (en) | Photoactive fluorinated polymer and the coating solution | |
| KR100226442B1 (en) | Fluorinated poly(arylene ether) containing thermally curable ethynyl group, preparation method thereof, and optical devices using the same | |
| KR100536439B1 (en) | Perfluorophenylacetylene compounds, Coating solution and Optical waveguide device using the same | |
| KR100927593B1 (en) | A polymer comprising an imide repeating unit having a crosslinking group, a polymer film for optical waveguide, and a method of manufacturing the same | |
| JP4209171B2 (en) | Silicon-containing curable polymer composition, optical waveguide device using the same, wiring board, and method for producing silicon-containing curable polymer composition | |
| KR100509197B1 (en) | Fluorinated polyarylene ether Compound, Preparing Method of the Same and Optical Waveguide Material Using the Same | |
| KR20000033986A (en) | Fluorine substituted polyarylene ether copolymer and polymer light element using the same | |
| KR20030097532A (en) | Fluorinated acrylate derivative having carbonate groups and polymerizable composition comprising same | |
| KR100644237B1 (en) | Novel Fluorinated Polyarylene Ether Phosphine Oxide and its Preparation Method | |
| KR100533253B1 (en) | Synthesis of 1, 1-Bis(4-hydroxypheny1)-1-(3, 5-bis(trifluoromethy1)-pheny1)-ethane and poly (arylene)et-her using 1,1-Bis(4-hydroxypheny1)-1-(3,5-bis(trifluoromethy1) pyeny1)-ethane | |
| JP2020134799A (en) | Polymer optical waveguide |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: ZEN PHOTONICS CO., LTD., KOREA, REPUBLIC OF Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:KIM, JI-HYANG;KIM, JAE-II;KIM, TAE-KYUN;AND OTHERS;REEL/FRAME:014942/0027 Effective date: 20030903 |
|
| AS | Assignment |
Owner name: KIM, MI-HWA, KOREA, REPUBLIC OF Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:ZEN PHOTONICS CO., LTD.;REEL/FRAME:015659/0654 Effective date: 20050113 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |