TW202237726A - Stabilizer composition for silyl-modified polymer sealants - Google Patents
Stabilizer composition for silyl-modified polymer sealants Download PDFInfo
- Publication number
- TW202237726A TW202237726A TW110140946A TW110140946A TW202237726A TW 202237726 A TW202237726 A TW 202237726A TW 110140946 A TW110140946 A TW 110140946A TW 110140946 A TW110140946 A TW 110140946A TW 202237726 A TW202237726 A TW 202237726A
- Authority
- TW
- Taiwan
- Prior art keywords
- weight
- carbon atoms
- stabilizer
- composition
- stabilizer composition
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 313
- 239000003381 stabilizer Substances 0.000 title claims abstract description 231
- 239000000565 sealant Substances 0.000 title abstract description 23
- 239000003707 silyl modified polymer Substances 0.000 title abstract 5
- 229920000642 polymer Polymers 0.000 claims abstract description 144
- 239000007788 liquid Substances 0.000 claims abstract description 125
- 239000006096 absorbing agent Substances 0.000 claims abstract description 117
- 150000001412 amines Chemical class 0.000 claims abstract description 96
- 239000004611 light stabiliser Substances 0.000 claims abstract description 88
- 239000003085 diluting agent Substances 0.000 claims abstract description 70
- 239000002530 phenolic antioxidant Substances 0.000 claims abstract description 48
- FTWUXYZHDFCGSV-UHFFFAOYSA-N n,n'-diphenyloxamide Chemical compound C=1C=CC=CC=1NC(=O)C(=O)NC1=CC=CC=C1 FTWUXYZHDFCGSV-UHFFFAOYSA-N 0.000 claims abstract description 27
- 125000004432 carbon atom Chemical group C* 0.000 claims description 164
- -1 2-hydroxyphenyl Chemical group 0.000 claims description 99
- 125000000217 alkyl group Chemical group 0.000 claims description 80
- 229910052739 hydrogen Inorganic materials 0.000 claims description 58
- 239000001257 hydrogen Substances 0.000 claims description 58
- 150000001875 compounds Chemical class 0.000 claims description 55
- 239000000654 additive Substances 0.000 claims description 52
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 49
- 150000002431 hydrogen Chemical class 0.000 claims description 43
- 230000000996 additive effect Effects 0.000 claims description 40
- 229910052710 silicon Inorganic materials 0.000 claims description 37
- 239000010703 silicon Substances 0.000 claims description 37
- 239000003054 catalyst Substances 0.000 claims description 34
- 229920000570 polyether Polymers 0.000 claims description 31
- 125000003545 alkoxy group Chemical group 0.000 claims description 28
- 239000004014 plasticizer Substances 0.000 claims description 28
- 239000002318 adhesion promoter Substances 0.000 claims description 22
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 21
- 239000000945 filler Substances 0.000 claims description 20
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 20
- 239000003963 antioxidant agent Substances 0.000 claims description 19
- 239000002904 solvent Substances 0.000 claims description 18
- 230000003078 antioxidant effect Effects 0.000 claims description 16
- 125000003118 aryl group Chemical group 0.000 claims description 16
- 229910052736 halogen Inorganic materials 0.000 claims description 15
- 150000002367 halogens Chemical class 0.000 claims description 15
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 14
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 13
- 125000000304 alkynyl group Chemical group 0.000 claims description 12
- 150000002989 phenols Chemical class 0.000 claims description 12
- 125000000000 cycloalkoxy group Chemical group 0.000 claims description 11
- 238000000034 method Methods 0.000 claims description 10
- UHOVQNZJYSORNB-UHFFFAOYSA-N monobenzene Natural products C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 claims description 10
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 claims description 7
- 125000003342 alkenyl group Chemical group 0.000 claims description 7
- 125000004104 aryloxy group Chemical group 0.000 claims description 7
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 7
- 125000002252 acyl group Chemical group 0.000 claims description 5
- 125000002102 aryl alkyloxo group Chemical group 0.000 claims description 5
- SFHYNDMGZXWXBU-LIMNOBDPSA-N 6-amino-2-[[(e)-(3-formylphenyl)methylideneamino]carbamoylamino]-1,3-dioxobenzo[de]isoquinoline-5,8-disulfonic acid Chemical compound O=C1C(C2=3)=CC(S(O)(=O)=O)=CC=3C(N)=C(S(O)(=O)=O)C=C2C(=O)N1NC(=O)N\N=C\C1=CC=CC(C=O)=C1 SFHYNDMGZXWXBU-LIMNOBDPSA-N 0.000 claims description 3
- 125000004356 hydroxy functional group Chemical group O* 0.000 claims description 2
- 125000002071 phenylalkoxy group Chemical group 0.000 claims description 2
- ZLFHNCHMEGLFKL-UHFFFAOYSA-N 3,3-bis(3-tert-butyl-4-hydroxyphenyl)butanoic acid Chemical compound C1=C(O)C(C(C)(C)C)=CC(C(C)(CC(O)=O)C=2C=C(C(O)=CC=2)C(C)(C)C)=C1 ZLFHNCHMEGLFKL-UHFFFAOYSA-N 0.000 claims 1
- NMUHVOOZCRVPLA-UHFFFAOYSA-N C(C)(C)(C)C=1C=C(C=CC1O)C(C(=O)OCCO)CC Chemical compound C(C)(C)(C)C=1C=C(C=CC1O)C(C(=O)OCCO)CC NMUHVOOZCRVPLA-UHFFFAOYSA-N 0.000 claims 1
- NAHBVNMACPIHAH-HLICZWCASA-N p-ii Chemical compound C([C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H]2CSSC[C@H](NC(=O)[C@H](CC=3C=CC=CC=3)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC2=O)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)=O)C(C)C)C1=CC=CC=C1 NAHBVNMACPIHAH-HLICZWCASA-N 0.000 claims 1
- 239000000853 adhesive Substances 0.000 abstract description 14
- 230000001070 adhesive effect Effects 0.000 abstract description 14
- HWRLEEPNFJNTOP-UHFFFAOYSA-N 2-(1,3,5-triazin-2-yl)phenol Chemical compound OC1=CC=CC=C1C1=NC=NC=N1 HWRLEEPNFJNTOP-UHFFFAOYSA-N 0.000 abstract 2
- 125000001424 substituent group Chemical group 0.000 description 29
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 23
- NKSJNEHGWDZZQF-UHFFFAOYSA-N ethenyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)C=C NKSJNEHGWDZZQF-UHFFFAOYSA-N 0.000 description 22
- 239000004721 Polyphenylene oxide Substances 0.000 description 20
- 239000002516 radical scavenger Substances 0.000 description 20
- 238000012360 testing method Methods 0.000 description 20
- 150000004756 silanes Chemical class 0.000 description 19
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 15
- 239000000126 substance Substances 0.000 description 15
- FWDBOZPQNFPOLF-UHFFFAOYSA-N ethenyl(triethoxy)silane Chemical compound CCO[Si](OCC)(OCC)C=C FWDBOZPQNFPOLF-UHFFFAOYSA-N 0.000 description 13
- 238000002360 preparation method Methods 0.000 description 13
- 238000002156 mixing Methods 0.000 description 12
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 12
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 11
- 239000007795 chemical reaction product Substances 0.000 description 11
- 239000008096 xylene Substances 0.000 description 11
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 10
- 239000002585 base Substances 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 235000014113 dietary fatty acids Nutrition 0.000 description 9
- 229930195729 fatty acid Natural products 0.000 description 9
- 239000000194 fatty acid Substances 0.000 description 9
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 9
- SJECZPVISLOESU-UHFFFAOYSA-N 3-trimethoxysilylpropan-1-amine Chemical compound CO[Si](OC)(OC)CCCN SJECZPVISLOESU-UHFFFAOYSA-N 0.000 description 8
- BRLQWZUYTZBJKN-UHFFFAOYSA-N Epichlorohydrin Chemical compound ClCC1CO1 BRLQWZUYTZBJKN-UHFFFAOYSA-N 0.000 description 8
- 230000033228 biological regulation Effects 0.000 description 8
- 235000010216 calcium carbonate Nutrition 0.000 description 8
- 238000009833 condensation Methods 0.000 description 8
- 230000005494 condensation Effects 0.000 description 8
- 101000648528 Homo sapiens Transmembrane protein 50A Proteins 0.000 description 7
- 102100028770 Transmembrane protein 50A Human genes 0.000 description 7
- 229910000019 calcium carbonate Inorganic materials 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 7
- 239000003960 organic solvent Substances 0.000 description 7
- 239000003495 polar organic solvent Substances 0.000 description 7
- 229940088417 precipitated calcium carbonate Drugs 0.000 description 7
- ARXJGSRGQADJSQ-UHFFFAOYSA-N 1-methoxypropan-2-ol Chemical compound COCC(C)O ARXJGSRGQADJSQ-UHFFFAOYSA-N 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- 239000004593 Epoxy Substances 0.000 description 6
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 6
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 6
- 150000004665 fatty acids Chemical class 0.000 description 6
- 238000002372 labelling Methods 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 238000004806 packaging method and process Methods 0.000 description 6
- 239000003208 petroleum Substances 0.000 description 6
- 239000003444 phase transfer catalyst Substances 0.000 description 6
- 239000004814 polyurethane Substances 0.000 description 6
- 229920002635 polyurethane Polymers 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- SCPYDCQAZCOKTP-UHFFFAOYSA-N silanol Chemical compound [SiH3]O SCPYDCQAZCOKTP-UHFFFAOYSA-N 0.000 description 6
- JOWXNCPELQZFHF-UHFFFAOYSA-N 2-[3,3-bis(3-tert-butyl-4-hydroxyphenyl)butanoyloxy]ethyl 3,3-bis(3-tert-butyl-4-hydroxyphenyl)butanoate Chemical compound C1=C(O)C(C(C)(C)C)=CC(C(C)(CC(=O)OCCOC(=O)CC(C)(C=2C=C(C(O)=CC=2)C(C)(C)C)C=2C=C(C(O)=CC=2)C(C)(C)C)C=2C=C(C(O)=CC=2)C(C)(C)C)=C1 JOWXNCPELQZFHF-UHFFFAOYSA-N 0.000 description 5
- XITRBUPOXXBIJN-UHFFFAOYSA-N bis(2,2,6,6-tetramethylpiperidin-4-yl) decanedioate Chemical compound C1C(C)(C)NC(C)(C)CC1OC(=O)CCCCCCCCC(=O)OC1CC(C)(C)NC(C)(C)C1 XITRBUPOXXBIJN-UHFFFAOYSA-N 0.000 description 5
- HGQSXVKHVMGQRG-UHFFFAOYSA-N dioctyltin Chemical compound CCCCCCCC[Sn]CCCCCCCC HGQSXVKHVMGQRG-UHFFFAOYSA-N 0.000 description 5
- 239000008393 encapsulating agent Substances 0.000 description 5
- 239000012442 inert solvent Substances 0.000 description 5
- AUHZEENZYGFFBQ-UHFFFAOYSA-N mesitylene Substances CC1=CC(C)=CC(C)=C1 AUHZEENZYGFFBQ-UHFFFAOYSA-N 0.000 description 5
- 125000001827 mesitylenyl group Chemical group [H]C1=C(C(*)=C(C([H])=C1C([H])([H])[H])C([H])([H])[H])C([H])([H])[H] 0.000 description 5
- YIMHRDBSVCPJOV-UHFFFAOYSA-N n'-(2-ethoxyphenyl)-n-(2-ethylphenyl)oxamide Chemical compound CCOC1=CC=CC=C1NC(=O)C(=O)NC1=CC=CC=C1CC YIMHRDBSVCPJOV-UHFFFAOYSA-N 0.000 description 5
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- WYTZZXDRDKSJID-UHFFFAOYSA-N (3-aminopropyl)triethoxysilane Chemical compound CCO[Si](OCC)(OCC)CCCN WYTZZXDRDKSJID-UHFFFAOYSA-N 0.000 description 4
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 4
- NIQCNGHVCWTJSM-UHFFFAOYSA-N Dimethyl phthalate Chemical compound COC(=O)C1=CC=CC=C1C(=O)OC NIQCNGHVCWTJSM-UHFFFAOYSA-N 0.000 description 4
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 125000003277 amino group Chemical group 0.000 description 4
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 4
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 4
- 239000012964 benzotriazole Substances 0.000 description 4
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 4
- HQABUPZFAYXKJW-UHFFFAOYSA-N butan-1-amine Chemical compound CCCCN HQABUPZFAYXKJW-UHFFFAOYSA-N 0.000 description 4
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- PAFZNILMFXTMIY-UHFFFAOYSA-N cyclohexylamine Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 4
- DOIRQSBPFJWKBE-UHFFFAOYSA-N dibutyl phthalate Chemical compound CCCCOC(=O)C1=CC=CC=C1C(=O)OCCCC DOIRQSBPFJWKBE-UHFFFAOYSA-N 0.000 description 4
- JQVDAXLFBXTEQA-UHFFFAOYSA-N dibutylamine Chemical compound CCCCNCCCC JQVDAXLFBXTEQA-UHFFFAOYSA-N 0.000 description 4
- AYOHIQLKSOJJQH-UHFFFAOYSA-N dibutyltin Chemical compound CCCC[Sn]CCCC AYOHIQLKSOJJQH-UHFFFAOYSA-N 0.000 description 4
- FLKPEMZONWLCSK-UHFFFAOYSA-N diethyl phthalate Chemical compound CCOC(=O)C1=CC=CC=C1C(=O)OCC FLKPEMZONWLCSK-UHFFFAOYSA-N 0.000 description 4
- 239000006185 dispersion Substances 0.000 description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 4
- 231100001261 hazardous Toxicity 0.000 description 4
- UDKSLGIUCGAZTK-UHFFFAOYSA-N phenyl pentadecane-1-sulfonate Chemical compound CCCCCCCCCCCCCCCS(=O)(=O)OC1=CC=CC=C1 UDKSLGIUCGAZTK-UHFFFAOYSA-N 0.000 description 4
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 4
- 239000011541 reaction mixture Substances 0.000 description 4
- CXMXRPHRNRROMY-UHFFFAOYSA-N sebacic acid Chemical compound OC(=O)CCCCCCCCC(O)=O CXMXRPHRNRROMY-UHFFFAOYSA-N 0.000 description 4
- 230000002195 synergetic effect Effects 0.000 description 4
- 238000010998 test method Methods 0.000 description 4
- YUYCVXFAYWRXLS-UHFFFAOYSA-N trimethoxysilane Chemical compound CO[SiH](OC)OC YUYCVXFAYWRXLS-UHFFFAOYSA-N 0.000 description 4
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 4
- 229920002554 vinyl polymer Polymers 0.000 description 4
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 3
- IRIAEXORFWYRCZ-UHFFFAOYSA-N Butylbenzyl phthalate Chemical compound CCCCOC(=O)C1=CC=CC=C1C(=O)OCC1=CC=CC=C1 IRIAEXORFWYRCZ-UHFFFAOYSA-N 0.000 description 3
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N N-phenyl amine Natural products NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- 239000004793 Polystyrene Substances 0.000 description 3
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 3
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000003463 adsorbent Substances 0.000 description 3
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 3
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 239000006229 carbon black Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000010276 construction Methods 0.000 description 3
- JQCXWCOOWVGKMT-UHFFFAOYSA-N diheptyl phthalate Chemical compound CCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCC JQCXWCOOWVGKMT-UHFFFAOYSA-N 0.000 description 3
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 3
- 238000000227 grinding Methods 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 3
- MQWFLKHKWJMCEN-UHFFFAOYSA-N n'-[3-[dimethoxy(methyl)silyl]propyl]ethane-1,2-diamine Chemical compound CO[Si](C)(OC)CCCNCCN MQWFLKHKWJMCEN-UHFFFAOYSA-N 0.000 description 3
- 239000002798 polar solvent Substances 0.000 description 3
- 229920002223 polystyrene Polymers 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000005855 radiation Effects 0.000 description 3
- 229910000077 silane Inorganic materials 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 235000012239 silicon dioxide Nutrition 0.000 description 3
- 125000003396 thiol group Chemical group [H]S* 0.000 description 3
- 150000003606 tin compounds Chemical class 0.000 description 3
- 229910052719 titanium Inorganic materials 0.000 description 3
- 239000010936 titanium Substances 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 238000004383 yellowing Methods 0.000 description 3
- LHENQXAPVKABON-UHFFFAOYSA-N 1-methoxypropan-1-ol Chemical compound CCC(O)OC LHENQXAPVKABON-UHFFFAOYSA-N 0.000 description 2
- VILCJCGEZXAXTO-UHFFFAOYSA-N 2,2,2-tetramine Chemical compound NCCNCCNCCN VILCJCGEZXAXTO-UHFFFAOYSA-N 0.000 description 2
- AHDSRXYHVZECER-UHFFFAOYSA-N 2,4,6-tris[(dimethylamino)methyl]phenol Chemical compound CN(C)CC1=CC(CN(C)C)=C(O)C(CN(C)C)=C1 AHDSRXYHVZECER-UHFFFAOYSA-N 0.000 description 2
- MCRZWYDXIGCFKO-UHFFFAOYSA-N 2-butylpropanedioic acid Chemical compound CCCCC(C(O)=O)C(O)=O MCRZWYDXIGCFKO-UHFFFAOYSA-N 0.000 description 2
- REWYMBAFAAYCAR-UHFFFAOYSA-N 3-[bis(2-methoxyethoxy)-phenylsilyl]propanoic acid Chemical compound COCCO[Si](CCC(O)=O)(OCCOC)C1=CC=CC=C1 REWYMBAFAAYCAR-UHFFFAOYSA-N 0.000 description 2
- OZFSDOFRXYCNKS-UHFFFAOYSA-N 3-[diethoxy(1-phenylpropan-2-yloxy)silyl]-n-ethenylpropan-1-amine Chemical compound C=CNCCC[Si](OCC)(OCC)OC(C)CC1=CC=CC=C1 OZFSDOFRXYCNKS-UHFFFAOYSA-N 0.000 description 2
- HXLAEGYMDGUSBD-UHFFFAOYSA-N 3-[diethoxy(methyl)silyl]propan-1-amine Chemical compound CCO[Si](C)(OCC)CCCN HXLAEGYMDGUSBD-UHFFFAOYSA-N 0.000 description 2
- MBNRBJNIYVXSQV-UHFFFAOYSA-N 3-[diethoxy(methyl)silyl]propane-1-thiol Chemical compound CCO[Si](C)(OCC)CCCS MBNRBJNIYVXSQV-UHFFFAOYSA-N 0.000 description 2
- ZYAASQNKCWTPKI-UHFFFAOYSA-N 3-[dimethoxy(methyl)silyl]propan-1-amine Chemical compound CO[Si](C)(OC)CCCN ZYAASQNKCWTPKI-UHFFFAOYSA-N 0.000 description 2
- IKYAJDOSWUATPI-UHFFFAOYSA-N 3-[dimethoxy(methyl)silyl]propane-1-thiol Chemical compound CO[Si](C)(OC)CCCS IKYAJDOSWUATPI-UHFFFAOYSA-N 0.000 description 2
- LZMNXXQIQIHFGC-UHFFFAOYSA-N 3-[dimethoxy(methyl)silyl]propyl 2-methylprop-2-enoate Chemical compound CO[Si](C)(OC)CCCOC(=O)C(C)=C LZMNXXQIQIHFGC-UHFFFAOYSA-N 0.000 description 2
- YBXSYMIDZDBSFD-UHFFFAOYSA-L 3-acetyl-2,4-dioxopentanoate;dibutyltin(2+) Chemical compound CCCC[Sn+2]CCCC.CC(=O)C(C(C)=O)C(=O)C([O-])=O.CC(=O)C(C(C)=O)C(=O)C([O-])=O YBXSYMIDZDBSFD-UHFFFAOYSA-L 0.000 description 2
- OXYZDRAJMHGSMW-UHFFFAOYSA-N 3-chloropropyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)CCCCl OXYZDRAJMHGSMW-UHFFFAOYSA-N 0.000 description 2
- FMGBDYLOANULLW-UHFFFAOYSA-N 3-isocyanatopropyl(trimethoxy)silane Chemical group CO[Si](OC)(OC)CCCN=C=O FMGBDYLOANULLW-UHFFFAOYSA-N 0.000 description 2
- NNTRMVRTACZZIO-UHFFFAOYSA-N 3-isocyanatopropyl-dimethoxy-methylsilane Chemical group CO[Si](C)(OC)CCCN=C=O NNTRMVRTACZZIO-UHFFFAOYSA-N 0.000 description 2
- DCQBZYNUSLHVJC-UHFFFAOYSA-N 3-triethoxysilylpropane-1-thiol Chemical compound CCO[Si](OCC)(OCC)CCCS DCQBZYNUSLHVJC-UHFFFAOYSA-N 0.000 description 2
- AXEFESAPMLYPEF-UHFFFAOYSA-N 3-triethoxysilylpropanoic acid Chemical compound CCO[Si](OCC)(OCC)CCC(O)=O AXEFESAPMLYPEF-UHFFFAOYSA-N 0.000 description 2
- UUEWCQRISZBELL-UHFFFAOYSA-N 3-trimethoxysilylpropane-1-thiol Chemical compound CO[Si](OC)(OC)CCCS UUEWCQRISZBELL-UHFFFAOYSA-N 0.000 description 2
- LVACOMKKELLCHJ-UHFFFAOYSA-N 3-trimethoxysilylpropylurea Chemical compound CO[Si](OC)(OC)CCCNC(N)=O LVACOMKKELLCHJ-UHFFFAOYSA-N 0.000 description 2
- QGIPACNZBRJHAV-UHFFFAOYSA-N 4-[diethoxy(methyl)silyl]oxypentyl prop-2-enoate Chemical compound CCO[Si](C)(OCC)OC(C)CCCOC(=O)C=C QGIPACNZBRJHAV-UHFFFAOYSA-N 0.000 description 2
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 2
- MQIUGAXCHLFZKX-UHFFFAOYSA-N Di-n-octyl phthalate Natural products CCCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCCC MQIUGAXCHLFZKX-UHFFFAOYSA-N 0.000 description 2
- QSJXEFYPDANLFS-UHFFFAOYSA-N Diacetyl Chemical group CC(=O)C(C)=O QSJXEFYPDANLFS-UHFFFAOYSA-N 0.000 description 2
- VOWAEIGWURALJQ-UHFFFAOYSA-N Dicyclohexyl phthalate Chemical compound C=1C=CC=C(C(=O)OC2CCCCC2)C=1C(=O)OC1CCCCC1 VOWAEIGWURALJQ-UHFFFAOYSA-N 0.000 description 2
- RPNUMPOLZDHAAY-UHFFFAOYSA-N Diethylenetriamine Chemical compound NCCNCCN RPNUMPOLZDHAAY-UHFFFAOYSA-N 0.000 description 2
- ZVFDTKUVRCTHQE-UHFFFAOYSA-N Diisodecyl phthalate Chemical compound CC(C)CCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCCC(C)C ZVFDTKUVRCTHQE-UHFFFAOYSA-N 0.000 description 2
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical compound NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 2
- 239000004609 Impact Modifier Substances 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 235000019738 Limestone Nutrition 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 239000012963 UV stabilizer Substances 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- GKXVJHDEWHKBFH-UHFFFAOYSA-N [2-(aminomethyl)phenyl]methanamine Chemical compound NCC1=CC=CC=C1CN GKXVJHDEWHKBFH-UHFFFAOYSA-N 0.000 description 2
- BGYHLZZASRKEJE-UHFFFAOYSA-N [3-[3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoyloxy]-2,2-bis[3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoyloxymethyl]propyl] 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate Chemical compound CC(C)(C)C1=C(O)C(C(C)(C)C)=CC(CCC(=O)OCC(COC(=O)CCC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)(COC(=O)CCC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)COC(=O)CCC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)=C1 BGYHLZZASRKEJE-UHFFFAOYSA-N 0.000 description 2
- ISKQADXMHQSTHK-UHFFFAOYSA-N [4-(aminomethyl)phenyl]methanamine Chemical compound NCC1=CC=C(CN)C=C1 ISKQADXMHQSTHK-UHFFFAOYSA-N 0.000 description 2
- NOKSMMGULAYSTD-UHFFFAOYSA-N [SiH4].N=C=O Chemical class [SiH4].N=C=O NOKSMMGULAYSTD-UHFFFAOYSA-N 0.000 description 2
- UKLDJPRMSDWDSL-UHFFFAOYSA-L [dibutyl(dodecanoyloxy)stannyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)O[Sn](CCCC)(CCCC)OC(=O)CCCCCCCCCCC UKLDJPRMSDWDSL-UHFFFAOYSA-L 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 239000002216 antistatic agent Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- BPSLVNCMKDXZPC-UHFFFAOYSA-N benzyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC1=CC=CC=C1 BPSLVNCMKDXZPC-UHFFFAOYSA-N 0.000 description 2
- 239000003139 biocide Substances 0.000 description 2
- BJQHLKABXJIVAM-UHFFFAOYSA-N bis(2-ethylhexyl) phthalate Chemical compound CCCCC(CC)COC(=O)C1=CC=CC=C1C(=O)OCC(CC)CCCC BJQHLKABXJIVAM-UHFFFAOYSA-N 0.000 description 2
- LGBAGUMSAPUZPU-UHFFFAOYSA-N bis(9-methyldecyl) benzene-1,2-dicarboxylate Chemical compound CC(C)CCCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCCCC(C)C LGBAGUMSAPUZPU-UHFFFAOYSA-N 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Chemical compound BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- OCWYEMOEOGEQAN-UHFFFAOYSA-N bumetrizole Chemical compound CC(C)(C)C1=CC(C)=CC(N2N=C3C=C(Cl)C=CC3=N2)=C1O OCWYEMOEOGEQAN-UHFFFAOYSA-N 0.000 description 2
- YHWCPXVTRSHPNY-UHFFFAOYSA-N butan-1-olate;titanium(4+) Chemical compound [Ti+4].CCCC[O-].CCCC[O-].CCCC[O-].CCCC[O-] YHWCPXVTRSHPNY-UHFFFAOYSA-N 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 239000004927 clay Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 229960002380 dibutyl phthalate Drugs 0.000 description 2
- 239000012975 dibutyltin dilaurate Substances 0.000 description 2
- PUFGCEQWYLJYNJ-UHFFFAOYSA-N didodecyl benzene-1,2-dicarboxylate Chemical compound CCCCCCCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCCCCCCC PUFGCEQWYLJYNJ-UHFFFAOYSA-N 0.000 description 2
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 2
- PJIFJEUHCQYNHO-UHFFFAOYSA-N diethoxy-(3-isocyanatopropyl)-methylsilane Chemical group CCO[Si](C)(OCC)CCCN=C=O PJIFJEUHCQYNHO-UHFFFAOYSA-N 0.000 description 2
- HBGGXOJOCNVPFY-UHFFFAOYSA-N diisononyl phthalate Chemical compound CC(C)CCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCC(C)C HBGGXOJOCNVPFY-UHFFFAOYSA-N 0.000 description 2
- WHGNXNCOTZPEEK-UHFFFAOYSA-N dimethoxy-methyl-[3-(oxiran-2-ylmethoxy)propyl]silane Chemical compound CO[Si](C)(OC)CCCOCC1CO1 WHGNXNCOTZPEEK-UHFFFAOYSA-N 0.000 description 2
- FBSAITBEAPNWJG-UHFFFAOYSA-N dimethyl phthalate Natural products CC(=O)OC1=CC=CC=C1OC(C)=O FBSAITBEAPNWJG-UHFFFAOYSA-N 0.000 description 2
- 229960001826 dimethylphthalate Drugs 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- JRBPAEWTRLWTQC-UHFFFAOYSA-N dodecylamine Chemical compound CCCCCCCCCCCCN JRBPAEWTRLWTQC-UHFFFAOYSA-N 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 125000003700 epoxy group Chemical group 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 229910021485 fumed silica Inorganic materials 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 230000000855 fungicidal effect Effects 0.000 description 2
- 239000000417 fungicide Substances 0.000 description 2
- 238000005227 gel permeation chromatography Methods 0.000 description 2
- 239000012760 heat stabilizer Substances 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 239000012948 isocyanate Substances 0.000 description 2
- 125000001261 isocyanato group Chemical group *N=C=O 0.000 description 2
- 239000006028 limestone Substances 0.000 description 2
- 239000000944 linseed oil Substances 0.000 description 2
- 235000021388 linseed oil Nutrition 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000004579 marble Substances 0.000 description 2
- 239000006078 metal deactivator Substances 0.000 description 2
- 150000004702 methyl esters Chemical class 0.000 description 2
- PHQOGHDTIVQXHL-UHFFFAOYSA-N n'-(3-trimethoxysilylpropyl)ethane-1,2-diamine Chemical compound CO[Si](OC)(OC)CCCNCCN PHQOGHDTIVQXHL-UHFFFAOYSA-N 0.000 description 2
- YLBPOJLDZXHVRR-UHFFFAOYSA-N n'-[3-[diethoxy(methyl)silyl]propyl]ethane-1,2-diamine Chemical compound CCO[Si](C)(OCC)CCCNCCN YLBPOJLDZXHVRR-UHFFFAOYSA-N 0.000 description 2
- CLYWMXVFAMGARU-UHFFFAOYSA-N n-benzyl-3-trimethoxysilylpropan-1-amine Chemical compound CO[Si](OC)(OC)CCCNCC1=CC=CC=C1 CLYWMXVFAMGARU-UHFFFAOYSA-N 0.000 description 2
- IOQPZZOEVPZRBK-UHFFFAOYSA-N octan-1-amine Chemical compound CCCCCCCCN IOQPZZOEVPZRBK-UHFFFAOYSA-N 0.000 description 2
- 238000006384 oligomerization reaction Methods 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 150000002902 organometallic compounds Chemical class 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical class OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 2
- 239000000049 pigment Substances 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 229920000768 polyamine Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920001228 polyisocyanate Polymers 0.000 description 2
- 239000005056 polyisocyanate Substances 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- HKJYVRJHDIPMQB-UHFFFAOYSA-N propan-1-olate;titanium(4+) Chemical compound CCCO[Ti](OCCC)(OCCC)OCCC HKJYVRJHDIPMQB-UHFFFAOYSA-N 0.000 description 2
- JTQPTNQXCUMDRK-UHFFFAOYSA-N propan-2-olate;titanium(2+) Chemical compound CC(C)O[Ti]OC(C)C JTQPTNQXCUMDRK-UHFFFAOYSA-N 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 229910052702 rhenium Inorganic materials 0.000 description 2
- FZHAPNGMFPVSLP-UHFFFAOYSA-N silanamine Chemical class [SiH3]N FZHAPNGMFPVSLP-UHFFFAOYSA-N 0.000 description 2
- ACECBHHKGNTVPB-UHFFFAOYSA-N silylformic acid Chemical class OC([SiH3])=O ACECBHHKGNTVPB-UHFFFAOYSA-N 0.000 description 2
- 239000003549 soybean oil Substances 0.000 description 2
- 235000012424 soybean oil Nutrition 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000011885 synergistic combination Substances 0.000 description 2
- KSBAEPSJVUENNK-UHFFFAOYSA-L tin(ii) 2-ethylhexanoate Chemical compound [Sn+2].CCCCC(CC)C([O-])=O.CCCCC(CC)C([O-])=O KSBAEPSJVUENNK-UHFFFAOYSA-L 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- FRGPKMWIYVTFIQ-UHFFFAOYSA-N triethoxy(3-isocyanatopropyl)silane Chemical group CCO[Si](OCC)(OCC)CCCN=C=O FRGPKMWIYVTFIQ-UHFFFAOYSA-N 0.000 description 2
- UDUKMRHNZZLJRB-UHFFFAOYSA-N triethoxy-[2-(7-oxabicyclo[4.1.0]heptan-4-yl)ethyl]silane Chemical compound C1C(CC[Si](OCC)(OCC)OCC)CCC2OC21 UDUKMRHNZZLJRB-UHFFFAOYSA-N 0.000 description 2
- JXUKBNICSRJFAP-UHFFFAOYSA-N triethoxy-[3-(oxiran-2-ylmethoxy)propyl]silane Chemical compound CCO[Si](OCC)(OCC)CCCOCC1CO1 JXUKBNICSRJFAP-UHFFFAOYSA-N 0.000 description 2
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 2
- HQYALQRYBUJWDH-UHFFFAOYSA-N trimethoxy(propyl)silane Chemical compound CCC[Si](OC)(OC)OC HQYALQRYBUJWDH-UHFFFAOYSA-N 0.000 description 2
- DQZNLOXENNXVAD-UHFFFAOYSA-N trimethoxy-[2-(7-oxabicyclo[4.1.0]heptan-4-yl)ethyl]silane Chemical compound C1C(CC[Si](OC)(OC)OC)CCC2OC21 DQZNLOXENNXVAD-UHFFFAOYSA-N 0.000 description 2
- BPSIOYPQMFLKFR-UHFFFAOYSA-N trimethoxy-[3-(oxiran-2-ylmethoxy)propyl]silane Chemical compound CO[Si](OC)(OC)CCCOCC1CO1 BPSIOYPQMFLKFR-UHFFFAOYSA-N 0.000 description 2
- 238000009827 uniform distribution Methods 0.000 description 2
- UKRDPEFKFJNXQM-UHFFFAOYSA-N vinylsilane Chemical class [SiH3]C=C UKRDPEFKFJNXQM-UHFFFAOYSA-N 0.000 description 2
- 238000011179 visual inspection Methods 0.000 description 2
- HJIAMFHSAAEUKR-UHFFFAOYSA-N (2-hydroxyphenyl)-phenylmethanone Chemical class OC1=CC=CC=C1C(=O)C1=CC=CC=C1 HJIAMFHSAAEUKR-UHFFFAOYSA-N 0.000 description 1
- ZBBLRPRYYSJUCZ-GRHBHMESSA-L (z)-but-2-enedioate;dibutyltin(2+) Chemical compound [O-]C(=O)\C=C/C([O-])=O.CCCC[Sn+2]CCCC ZBBLRPRYYSJUCZ-GRHBHMESSA-L 0.000 description 1
- QGLWBTPVKHMVHM-KTKRTIGZSA-N (z)-octadec-9-en-1-amine Chemical compound CCCCCCCC\C=C/CCCCCCCCN QGLWBTPVKHMVHM-KTKRTIGZSA-N 0.000 description 1
- OWRCNXZUPFZXOS-UHFFFAOYSA-N 1,3-diphenylguanidine Chemical compound C=1C=CC=CC=1NC(=N)NC1=CC=CC=C1 OWRCNXZUPFZXOS-UHFFFAOYSA-N 0.000 description 1
- PBHKYFKEAWZKKM-UHFFFAOYSA-N 1-isocyanato-1,3,5-triazinane-2,4,6-trione Chemical group O=C=NN1C(=O)NC(=O)NC1=O PBHKYFKEAWZKKM-UHFFFAOYSA-N 0.000 description 1
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- PSMYELRXRQIDAX-UHFFFAOYSA-N 2-[4,6-bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl]-5-(2-hydroxy-3-tridecoxypropoxy)phenol Chemical compound OC1=CC(OCC(O)COCCCCCCCCCCCCC)=CC=C1C1=NC(C=2C(=CC(C)=CC=2)C)=NC(C=2C(=CC(C)=CC=2)C)=N1 PSMYELRXRQIDAX-UHFFFAOYSA-N 0.000 description 1
- SITYOOWCYAYOKL-UHFFFAOYSA-N 2-[4,6-bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl]-5-(3-dodecoxy-2-hydroxypropoxy)phenol Chemical compound OC1=CC(OCC(O)COCCCCCCCCCCCC)=CC=C1C1=NC(C=2C(=CC(C)=CC=2)C)=NC(C=2C(=CC(C)=CC=2)C)=N1 SITYOOWCYAYOKL-UHFFFAOYSA-N 0.000 description 1
- 125000004918 2-methyl-2-pentyl group Chemical group CC(C)(CCC)* 0.000 description 1
- 125000004922 2-methyl-3-pentyl group Chemical group CC(C)C(CC)* 0.000 description 1
- VYVFQBFOMKEKBG-UHFFFAOYSA-L 3,3-dibutyl-2,4,3-benzodioxastannepine-1,5-dione Chemical compound O=C1O[Sn](CCCC)(CCCC)OC(=O)C2=CC=CC=C21 VYVFQBFOMKEKBG-UHFFFAOYSA-L 0.000 description 1
- FBIXXCXCZOZFCO-UHFFFAOYSA-N 3-dodecyl-1-(2,2,6,6-tetramethylpiperidin-4-yl)pyrrolidine-2,5-dione Chemical group O=C1C(CCCCCCCCCCCC)CC(=O)N1C1CC(C)(C)NC(C)(C)C1 FBIXXCXCZOZFCO-UHFFFAOYSA-N 0.000 description 1
- 125000004919 3-methyl-2-pentyl group Chemical group CC(C(C)*)CC 0.000 description 1
- 125000004921 3-methyl-3-pentyl group Chemical group CC(CC)(CC)* 0.000 description 1
- UPMLOUAZCHDJJD-UHFFFAOYSA-N 4,4'-Diphenylmethane Diisocyanate Chemical compound C1=CC(N=C=O)=CC=C1CC1=CC=C(N=C=O)C=C1 UPMLOUAZCHDJJD-UHFFFAOYSA-N 0.000 description 1
- 125000004920 4-methyl-2-pentyl group Chemical group CC(CC(C)*)C 0.000 description 1
- HQQTZCPKNZVLFF-UHFFFAOYSA-N 4h-1,2-benzoxazin-3-one Chemical class C1=CC=C2ONC(=O)CC2=C1 HQQTZCPKNZVLFF-UHFFFAOYSA-N 0.000 description 1
- ULKLGIFJWFIQFF-UHFFFAOYSA-N 5K8XI641G3 Chemical compound CCC1=NC=C(C)N1 ULKLGIFJWFIQFF-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- NNRVHEGGJZTRKR-WTUPQPTJSA-L C(CCC)/C(/C(=O)[O-])=C/C(=O)[O-].C(CCC)[Sn+2]CCCC Chemical compound C(CCC)/C(/C(=O)[O-])=C/C(=O)[O-].C(CCC)[Sn+2]CCCC NNRVHEGGJZTRKR-WTUPQPTJSA-L 0.000 description 1
- MFSOEVGLMJDXTD-GDNGEXCGSA-L C(CCC)[Sn+2]CCCC.C/C(/C(=O)[O-])=C/C(=O)[O-] Chemical compound C(CCC)[Sn+2]CCCC.C/C(/C(=O)[O-])=C/C(=O)[O-] MFSOEVGLMJDXTD-GDNGEXCGSA-L 0.000 description 1
- BWDCHQNZGUCXLM-UHFFFAOYSA-N C(CCCCCCCC)C=1C(=C(C=CC1)O)CCCCCCCCC.C(CCC)[Sn]CCCC Chemical compound C(CCCCCCCC)C=1C(=C(C=CC1)O)CCCCCCCCC.C(CCC)[Sn]CCCC BWDCHQNZGUCXLM-UHFFFAOYSA-N 0.000 description 1
- SDEJOFWFZCMBFX-UHFFFAOYSA-N C(CCCCCCCCCCC)(=O)OCCCCCCCC.C(CCCCCCCCCCC)(=O)OCCCCCCCC.[Sn] Chemical compound C(CCCCCCCCCCC)(=O)OCCCCCCCC.C(CCCCCCCCCCC)(=O)OCCCCCCCC.[Sn] SDEJOFWFZCMBFX-UHFFFAOYSA-N 0.000 description 1
- BPNQYNRPXZDFLQ-UHFFFAOYSA-N CC(C)(C)C(C=C1)=CC(C(C)(C)C)=C1C1=C(C2=C(C(C)(C)C)C=C(C(C)(C)C)C=C2)C2=C(C3=C(C(C)(C)C)C=C(C(C)(C)C)C=C3)C(C3=C(C(C)(C)C)C=C(C(C)(C)C)C=C3)=C1C(C=C1)=CC=C1OPOPO2 Chemical compound CC(C)(C)C(C=C1)=CC(C(C)(C)C)=C1C1=C(C2=C(C(C)(C)C)C=C(C(C)(C)C)C=C2)C2=C(C3=C(C(C)(C)C)C=C(C(C)(C)C)C=C3)C(C3=C(C(C)(C)C)C=C(C(C)(C)C)C=C3)=C1C(C=C1)=CC=C1OPOPO2 BPNQYNRPXZDFLQ-UHFFFAOYSA-N 0.000 description 1
- KHWHYENEFTZPPK-UHFFFAOYSA-N CCNCC.NCCCN Chemical compound CCNCC.NCCCN KHWHYENEFTZPPK-UHFFFAOYSA-N 0.000 description 1
- 229920000049 Carbon (fiber) Polymers 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- IEPRKVQEAMIZSS-UHFFFAOYSA-N Di-Et ester-Fumaric acid Natural products CCOC(=O)C=CC(=O)OCC IEPRKVQEAMIZSS-UHFFFAOYSA-N 0.000 description 1
- IEPRKVQEAMIZSS-WAYWQWQTSA-N Diethyl maleate Chemical compound CCOC(=O)\C=C/C(=O)OCC IEPRKVQEAMIZSS-WAYWQWQTSA-N 0.000 description 1
- 239000004262 Ethyl gallate Substances 0.000 description 1
- 239000005057 Hexamethylene diisocyanate Substances 0.000 description 1
- 239000005058 Isophorone diisocyanate Substances 0.000 description 1
- 240000007049 Juglans regia Species 0.000 description 1
- 235000009496 Juglans regia Nutrition 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 1
- 229920000459 Nitrile rubber Polymers 0.000 description 1
- QFKZKLVFOGGPJK-UHFFFAOYSA-N ON1NC=CC(=N1)C1=CC=CC=C1 Chemical class ON1NC=CC(=N1)C1=CC=CC=C1 QFKZKLVFOGGPJK-UHFFFAOYSA-N 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000005062 Polybutadiene Substances 0.000 description 1
- 239000004650 Polymer ST Substances 0.000 description 1
- 229920001131 Pulp (paper) Polymers 0.000 description 1
- 239000006087 Silane Coupling Agent Substances 0.000 description 1
- 229920002323 Silicone foam Polymers 0.000 description 1
- 229910010413 TiO 2 Inorganic materials 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- NIMXEFLZWRGNFN-UHFFFAOYSA-K [Al+3].CC(=O)C(C(C)=O)(C(C)=O)C(=O)C([O-])=O.CC(=O)C(C(C)=O)(C(C)=O)C(=O)C([O-])=O.CC(=O)C(C(C)=O)(C(C)=O)C(=O)C([O-])=O Chemical compound [Al+3].CC(=O)C(C(C)=O)(C(C)=O)C(=O)C([O-])=O.CC(=O)C(C(C)=O)(C(C)=O)C(=O)C([O-])=O.CC(=O)C(C(C)=O)(C(C)=O)C(=O)C([O-])=O NIMXEFLZWRGNFN-UHFFFAOYSA-K 0.000 description 1
- CQQXCSFSYHAZOO-UHFFFAOYSA-L [acetyloxy(dioctyl)stannyl] acetate Chemical compound CCCCCCCC[Sn](OC(C)=O)(OC(C)=O)CCCCCCCC CQQXCSFSYHAZOO-UHFFFAOYSA-L 0.000 description 1
- NBJODVYWAQLZOC-UHFFFAOYSA-L [dibutyl(octanoyloxy)stannyl] octanoate Chemical compound CCCCCCCC(=O)O[Sn](CCCC)(CCCC)OC(=O)CCCCCCC NBJODVYWAQLZOC-UHFFFAOYSA-L 0.000 description 1
- UZFVQGTYOXJWTF-UHFFFAOYSA-L [octadecanoyloxy(dioctyl)stannyl] octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Sn](CCCCCCCC)(CCCCCCCC)OC(=O)CCCCCCCCCCCCCCCCC UZFVQGTYOXJWTF-UHFFFAOYSA-L 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 125000003668 acetyloxy group Chemical group [H]C([H])([H])C(=O)O[*] 0.000 description 1
- 239000003377 acid catalyst Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- 125000002344 aminooxy group Chemical group [H]N([H])O[*] 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000003849 aromatic solvent Substances 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- NTXGQCSETZTARF-UHFFFAOYSA-N buta-1,3-diene;prop-2-enenitrile Chemical compound C=CC=C.C=CC#N NTXGQCSETZTARF-UHFFFAOYSA-N 0.000 description 1
- LUZSPGQEISANPO-UHFFFAOYSA-N butyltin Chemical compound CCCC[Sn] LUZSPGQEISANPO-UHFFFAOYSA-N 0.000 description 1
- QXJJQWWVWRCVQT-UHFFFAOYSA-K calcium;sodium;phosphate Chemical compound [Na+].[Ca+2].[O-]P([O-])([O-])=O QXJJQWWVWRCVQT-UHFFFAOYSA-K 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 235000019241 carbon black Nutrition 0.000 description 1
- 239000004917 carbon fiber Substances 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000013043 chemical agent Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 150000001913 cyanates Chemical class 0.000 description 1
- 125000002933 cyclohexyloxy group Chemical group C1(CCCCC1)O* 0.000 description 1
- WPCPXPTZTOMGRF-UHFFFAOYSA-K di(butanoyloxy)alumanyl butanoate Chemical compound [Al+3].CCCC([O-])=O.CCCC([O-])=O.CCCC([O-])=O WPCPXPTZTOMGRF-UHFFFAOYSA-K 0.000 description 1
- XFTXLPHTDSLKPF-FGSKAQBVSA-L dibutyltin(2+);(z)-2-ethylbut-2-enedioate Chemical compound CCCC[Sn+2]CCCC.CC\C(C([O-])=O)=C\C([O-])=O XFTXLPHTDSLKPF-FGSKAQBVSA-L 0.000 description 1
- JFHKCZFPXJVELF-UHFFFAOYSA-L dibutyltin(2+);2,2-diethylhexanoate Chemical compound CCCC[Sn+2]CCCC.CCCCC(CC)(CC)C([O-])=O.CCCCC(CC)(CC)C([O-])=O JFHKCZFPXJVELF-UHFFFAOYSA-L 0.000 description 1
- ZXDVQYBUEVYUCG-UHFFFAOYSA-N dibutyltin(2+);methanolate Chemical compound CCCC[Sn](OC)(OC)CCCC ZXDVQYBUEVYUCG-UHFFFAOYSA-N 0.000 description 1
- 125000005594 diketone group Chemical group 0.000 description 1
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- FPAFDBFIGPHWGO-UHFFFAOYSA-N dioxosilane;oxomagnesium;hydrate Chemical compound O.[Mg]=O.[Mg]=O.[Mg]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O FPAFDBFIGPHWGO-UHFFFAOYSA-N 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 238000010410 dusting Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- NTKDCNGQGOYAOO-UHFFFAOYSA-N ethyl acetate propan-2-ol Chemical compound CC(C)O.CC(C)O.CCOC(C)=O NTKDCNGQGOYAOO-UHFFFAOYSA-N 0.000 description 1
- 239000003063 flame retardant Substances 0.000 description 1
- 235000013312 flour Nutrition 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 238000007306 functionalization reaction Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 239000010439 graphite Substances 0.000 description 1
- 229910002804 graphite Inorganic materials 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- RRAMGCGOFNQTLD-UHFFFAOYSA-N hexamethylene diisocyanate Chemical compound O=C=NCCCCCCN=C=O RRAMGCGOFNQTLD-UHFFFAOYSA-N 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate Chemical compound [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 description 1
- 150000002513 isocyanates Chemical class 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- NIMLQBUJDJZYEJ-UHFFFAOYSA-N isophorone diisocyanate Chemical compound CC1(C)CC(N=C=O)CC(C)(CN=C=O)C1 NIMLQBUJDJZYEJ-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- YMQPOZUUTMLSEK-UHFFFAOYSA-L lead(2+);octanoate Chemical compound [Pb+2].CCCCCCCC([O-])=O.CCCCCCCC([O-])=O YMQPOZUUTMLSEK-UHFFFAOYSA-L 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- ARYZCSRUUPFYMY-UHFFFAOYSA-N methoxysilane Chemical compound CO[SiH3] ARYZCSRUUPFYMY-UHFFFAOYSA-N 0.000 description 1
- XKBGEWXEAPTVCK-UHFFFAOYSA-M methyltrioctylammonium chloride Chemical compound [Cl-].CCCCCCCC[N+](C)(CCCCCCCC)CCCCCCCC XKBGEWXEAPTVCK-UHFFFAOYSA-M 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000005609 naphthenate group Chemical group 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 239000002667 nucleating agent Substances 0.000 description 1
- CYCFYXLDTSNTGP-UHFFFAOYSA-L octadecanoate;tin(2+) Chemical compound [Sn+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CYCFYXLDTSNTGP-UHFFFAOYSA-L 0.000 description 1
- 125000005447 octyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- ZMHZSHHZIKJFIR-UHFFFAOYSA-N octyltin Chemical compound CCCCCCCC[Sn] ZMHZSHHZIKJFIR-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 150000004980 phosphorus peroxides Chemical class 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920001084 poly(chloroprene) Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920006122 polyamide resin Polymers 0.000 description 1
- 229920002857 polybutadiene Polymers 0.000 description 1
- 229920001083 polybutene Polymers 0.000 description 1
- 229920001748 polybutylene Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229940113115 polyethylene glycol 200 Drugs 0.000 description 1
- 229920001195 polyisoprene Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001955 polyphenylene ether Polymers 0.000 description 1
- 150000007519 polyprotic acids Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- LVTJOONKWUXEFR-FZRMHRINSA-N protoneodioscin Natural products O(C[C@@H](CC[C@]1(O)[C@H](C)[C@@H]2[C@]3(C)[C@H]([C@H]4[C@@H]([C@]5(C)C(=CC4)C[C@@H](O[C@@H]4[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@@H](O)[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@H](CO)O4)CC5)CC3)C[C@@H]2O1)C)[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](CO)O1 LVTJOONKWUXEFR-FZRMHRINSA-N 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229940116351 sebacate Drugs 0.000 description 1
- 125000003548 sec-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000004432 silane-modified polyurethane Substances 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 239000003017 thermal stabilizer Substances 0.000 description 1
- 230000008646 thermal stress Effects 0.000 description 1
- 239000013008 thixotropic agent Substances 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical class O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- DVKJHBMWWAPEIU-UHFFFAOYSA-N toluene 2,4-diisocyanate Chemical compound CC1=CC=C(N=C=O)C=C1N=C=O DVKJHBMWWAPEIU-UHFFFAOYSA-N 0.000 description 1
- 150000003918 triazines Chemical class 0.000 description 1
- 150000003673 urethanes Chemical class 0.000 description 1
- 235000020234 walnut Nutrition 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/20—Carboxylic acid amides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/13—Phenols; Phenolates
- C08K5/134—Phenols containing ester groups
- C08K5/1345—Carboxylic esters of phenolcarboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3467—Heterocyclic compounds having nitrogen in the ring having more than two nitrogen atoms in the ring
- C08K5/3477—Six-membered rings
- C08K5/3492—Triazines
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/35—Heterocyclic compounds having nitrogen in the ring having also oxygen in the ring
- C08K5/353—Five-membered rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/36—Sulfur-, selenium-, or tellurium-containing compounds
- C08K5/41—Compounds containing sulfur bound to oxygen
- C08K5/42—Sulfonic acids; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K9/00—Use of pretreated ingredients
- C08K9/04—Ingredients treated with organic substances
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L101/00—Compositions of unspecified macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L101/00—Compositions of unspecified macromolecular compounds
- C08L101/02—Compositions of unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups
- C08L101/10—Compositions of unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups containing hydrolysable silane groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L71/00—Compositions of polyethers obtained by reactions forming an ether link in the main chain; Compositions of derivatives of such polymers
- C08L71/02—Polyalkylene oxides
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J11/00—Features of adhesives not provided for in group C09J9/00, e.g. additives
- C09J11/02—Non-macromolecular additives
- C09J11/06—Non-macromolecular additives organic
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J201/00—Adhesives based on unspecified macromolecular compounds
- C09J201/02—Adhesives based on unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups
- C09J201/10—Adhesives based on unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups containing hydrolysable silane groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/24—Acids; Salts thereof
- C08K3/26—Carbonates; Bicarbonates
- C08K2003/265—Calcium, strontium or barium carbonate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0016—Plasticisers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/005—Stabilisers against oxidation, heat, light, ozone
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2201/00—Properties
- C08L2201/08—Stabilised against heat, light or radiation or oxydation
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Sealing Material Composition (AREA)
- Anti-Oxidant Or Stabilizer Compositions (AREA)
Abstract
Description
本發明關於穩定劑組成物,其包含至少一種草醯苯胺UV吸收劑及/或至少一種(2-羥基苯基)-對稱-三嗪UV吸收劑,以及至少一種寡聚受阻胺光穩定劑(hindered amine light stabilizer) (HALS),隨意地另包含至少一種酚類抗氧化劑、至少一種液體稀釋劑及/或其他組分。該穩定劑組成物較佳地用於基於矽基改質聚合物(SMP)之密封劑或黏著劑。本發明也涉及基於矽基改質聚合物(SMP)之聚合物組成物,其包含草醯苯胺UV吸收劑及/或(2-羥基苯基)-對稱-三嗪UV吸收劑以及寡聚受阻胺光穩定劑(HALS),隨意地另包含酚類抗氧化劑及液體稀釋劑及/或其他組分之新穎穩定劑組合。The present invention relates to a stabilizer composition comprising at least one oxanilide UV absorber and/or at least one (2-hydroxyphenyl)-symmetric-triazine UV absorber, and at least one oligomeric hindered amine light stabilizer ( hindered amine light stabilizer) (HALS), optionally further comprising at least one phenolic antioxidant, at least one liquid diluent and/or other components. The stabilizer composition is preferably used in a silicon-modified polymer (SMP)-based sealant or adhesive. The present invention also relates to polymer compositions based on silicon-modified polymers (SMP) comprising oxanilide UV absorbers and/or (2-hydroxyphenyl)-symmetric-triazine UV absorbers and oligomerization hindered Amine Light Stabilizers (HALS), optionally additionally comprising novel stabilizer combinations of phenolic antioxidants and liquid diluents and/or other components.
再者,本發明關於一種製備穩定劑組成物之方法及這些穩定劑組成物在SMP密封劑和黏合劑中作為熱及/或UV穩定劑之用途。Furthermore, the present invention relates to a method for preparing stabilizer compositions and the use of these stabilizer compositions as thermal and/or UV stabilizers in SMP sealants and adhesives.
矽基改質聚合物(SMP),如矽基改質聚醚(也稱為MS聚合物)、矽基改質聚胺酯(也稱為SPUR聚合物)及矽基改質丙烯酸聚合物,以及其作為密封劑和黏合劑,例如在建築業中,的用途已為人所知多年,參見例如US 2002/198308、US 6,077,896、EP-A 1 288 247及US 2003/ 0105261。市售SMP產品的實例是來自Evonik的Kaneka MS Polymers ®(如MS Polymer ®S203H、MS Polymer ®S303H)或Polymers ST。有多種MS Polymer ®等級皆為市售可得,其官能化程度(連接到骨幹之基團的數量及性質)和骨幹結構在寬廣之黏度範圍內都不同。 Silicon-modified polymers (SMPs), such as silicon-modified polyethers (also known as MS polymers), silicon-based modified polyurethanes (also known as SPUR polymers), and silicon-based modified acrylic polymers, as well as other The use as sealants and adhesives, eg in the construction industry, has been known for many years, see eg US 2002/198308, US 6,077,896, EP-A 1 288 247 and US 2003/0105261. Examples of commercially available SMP products are Kaneka MS Polymers ® (eg MS Polymer ® S203H, MS Polymer ® S303H) or Polymers ST from Evonik. Various MS Polymer ® grades are commercially available, varying in degree of functionalization (number and nature of groups attached to the backbone) and backbone structure over a wide range of viscosities.
此矽基改質聚合物可作為水分可固化之1K或2K密封劑或黏合劑組成物的基礎,其中此組成物可以實質上無水分的狀態儲存,且當暴露於大氣條件時,從表面快速固化。典型地,SMP密封劑從液體或凝膠狀態固化為彈性固體,其中固化意味著藉由矽基之水解及交聯反應使聚合物鏈發生交聯。This silicon-modified polymer can be used as the basis for a moisture-curable 1K or 2K sealant or adhesive composition, where the composition can be stored in a substantially moisture-free state and rapidly decomposes from the surface when exposed to atmospheric conditions. solidify. Typically, SMP sealants cure from a liquid or gel state to an elastic solid, where curing means cross-linking the polymer chains through hydrolysis and cross-linking reactions of the silicon groups.
矽基改質聚合物(SMPs)是例如多年來,在建築業、航空航天、汽車、船舶及其他相關行業中用作密封劑及黏合劑。這些產品典型地不含(異)氰酸酯及溶劑並顯現良好特性,如在各種基材上之黏著力、在有限的表面處理情況下具有優異之黏著持久性及良好之溫度和UV穩定性。典型地,SMP密封劑之主要特點為其非常彈性且可上漆。Silicon-modified polymers (SMPs) are, for example, used for many years as sealants and adhesives in the construction, aerospace, automotive, marine and other related industries. These products are typically free of (iso)cyanates and solvents and exhibit good properties such as adhesion on various substrates, excellent adhesion durability with limited surface preparation and good temperature and UV stability . Typically, the main features of SMP sealants are that they are very elastic and paintable.
WO 2016/081823描述用於保護有機材料免受UV光及熱降解影響之穩定劑組成物。該穩定劑組成物包含選自鄰羥基苯基三嗪化合物、鄰羥基二苯甲酮化合物、鄰羥基苯基苯并三嗪酮化合物及苯并噁嗪酮化合物之UV吸收劑;共活性劑(co-active agent) (例如選自C 12-C 60醇、脂肪酸酯及其烷氧基化衍生物)及受阻胺光穩定劑(HALS)。 WO 2016/081823 describes stabilizer compositions for protecting organic materials from UV light and thermal degradation. The stabilizer composition comprises UV absorbers selected from o-hydroxyphenyl triazine compounds, o-hydroxybenzophenone compounds, o-hydroxyphenyl benzotriazinone compounds and benzoxazinone compounds; co-active agents ( co-active agent) (for example selected from C 12 -C 60 alcohols, fatty acid esters and alkoxylated derivatives thereof) and hindered amine light stabilizers (HALS).
文件US 2010/0087576描述包含草醯苯胺UV穩定劑之水分可固化性有機矽組成物。再者,矽基改質聚合物密封劑中之UV吸收劑和受阻胺光穩定劑的組合係例如描述於JP 2010/248408及CN 107892900中。Document US 2010/0087576 describes moisture-curable silicone compositions comprising oxanilide UV stabilizers. Furthermore, the combination of UV absorber and hindered amine light stabilizer in silicon-based modified polymer sealant is described in JP 2010/248408 and CN 107892900, for example.
文件CN 106833479揭示汽車用矽烷聚醚密封劑,其包含熱穩定劑、UV吸收劑、受阻胺光穩定劑及抗氧化劑。文件JP 2010/270241描述含有穩定劑之矽酮泡沫,其包含受阻酚抗氧化劑、受阻胺光穩定劑及UV吸收劑。Document CN 106833479 discloses a silane polyether sealant for automobiles, which includes heat stabilizers, UV absorbers, hindered amine light stabilizers and antioxidants. Document JP 2010/270241 describes stabilizer-containing silicone foams comprising hindered phenol antioxidants, hindered amine light stabilizers and UV absorbers.
WO 2012/010570教導一種密封劑(例如基於矽烷封端之聚醚的密封劑),其中該添加物組合包含至少兩種不同立體受阻胺,例如Tinuvin ®622及Tinuvin ®144,及UV吸收劑,例如Tinuvin ®312,的添加物組合。該文件揭示一種添加物組合,其包含至少兩種立體受阻胺、至少另一種穩定劑、分散劑(分散劑聚合物)及塑化劑。 WO 2012/010570 teaches a sealant (e.g. a sealant based on silane-terminated polyether), wherein the additive combination comprises at least two different sterically hindered amines, such as Tinuvin ® 622 and Tinuvin ® 144, and a UV absorber, Such as Tinuvin ® 312, additive combination. This document discloses an additive combination comprising at least two sterically hindered amines, at least one other stabilizer, a dispersant (dispersant polymer) and a plasticizer.
目前,受阻胺光穩定劑及苯并三唑型UV吸收劑之組合常用在光熱穩定化的SMP密封劑行業。通常HALS癸二酸雙(2,2,6,6-四甲基-4-六氫吡啶基酯) (CAS-No. 52829-07-9)及UV吸收劑2-(2’-羥基-3’-三級丁基-5’-甲基苯基)-5-氯苯并三唑(CAS-No. 3896-11-5)之組合係用以提供光熱穩定化。然而,苯并三唑型UV吸收劑具有SMP密封劑及黏著劑在應用之前和過程中會變黃而導致最終產品著色之缺點,這在許多應用中是不可接受的。At present, the combination of hindered amine light stabilizer and benzotriazole type UV absorber is commonly used in the light and heat stabilized SMP sealant industry. Usually HALS sebacic acid bis(2,2,6,6-tetramethyl-4-hexahydropyridyl ester) (CAS-No. 52829-07-9) and UV absorber 2-(2'-hydroxy- A combination of 3'-tert-butyl-5'-methylphenyl)-5-chlorobenzotriazole (CAS-No. 3896-11-5) was used to provide photothermal stabilization. However, benzotriazole-based UV absorbers have the disadvantage of SMP sealants and adhesives turning yellow before and during application, resulting in coloring of the final product, which is unacceptable in many applications.
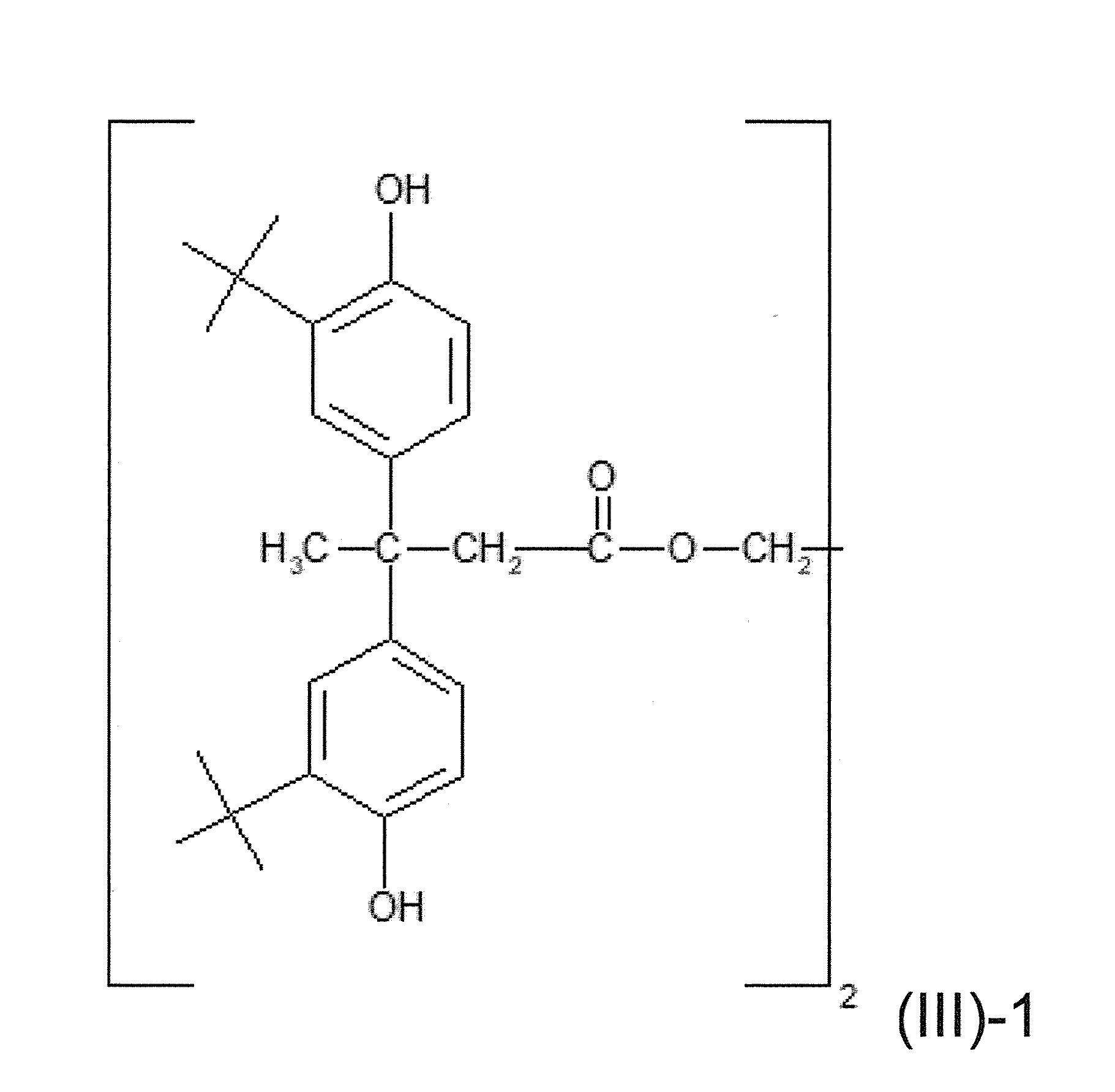
此外,穩定劑Tinuvin ®5866是草醯苯胺UV吸收劑和鹼性HALS穩定劑之混合物,常用在SMP組成物中。有一個缺點,這些穩定劑根據當前之歐盟化學物質分類、標籤及包裝法規被歸類為危險品。此外,這些穩定劑典型為固體並採粉末形式提供。應用粉狀穩定劑具有配料更困難,特別是少量,且粉狀穩定劑在測量、混合及/或應用過程中容易起粉之缺點。再者,粉狀穩定劑團塊之形成阻礙了穩定劑在客戶最終產品中的均勻分佈,並典型地需要對粉狀穩定劑成分進行昂貴的加工(例如研磨)。 In addition, the stabilizer Tinuvin ® 5866 is a mixture of oxaniline UV absorber and alkaline HALS stabilizer, which is commonly used in SMP compositions. One disadvantage is that these stabilizers are classified as hazardous under the current EU regulations on classification, labeling and packaging of chemicals. Furthermore, these stabilizers are typically solid and supplied in powder form. The use of powdered stabilizers has the disadvantages of more difficult ingredients, especially in small amounts, and the powdered stabilizers are prone to dusting during measurement, mixing and/or application. Furthermore, the formation of powdered stabilizer clumps prevents uniform distribution of the stabilizer in the customer's final product and typically requires costly processing (eg, grinding) of the powdered stabilizer ingredients.
有需要提供用於SMP密封劑之新穎並經改進的穩定劑組合,其具有改進之耐熱性及耐候性,不會引起初始黃化(這意指在固化步驟之前與聚合物混合後(例如於筒中)),不含有害化合物,不會在熱處理後造成穩定化SMP組成物之色彩受損,且終究不會損及SMP密封劑之性能(例如機械性質、耐化學藥品性、黏著力及流變性質)。此外,期望在配料及應用方面具有改進之適用性的穩定劑組合。有鑑於此,許多應用需要液體穩定劑組成物。There is a need to provide new and improved stabilizer combinations for SMP encapsulants with improved heat and weather resistance that do not cause incipient yellowing (this means after mixing with the polymer prior to the curing step (such as in barrel)), does not contain harmful compounds, will not cause color damage to the stabilized SMP composition after heat treatment, and will not damage the performance of the SMP sealant (such as mechanical properties, chemical resistance, adhesion and flow) variable nature). Furthermore, stabilizer combinations with improved applicability in terms of formulation and application are desired. For this reason, many applications require liquid stabilizer compositions.
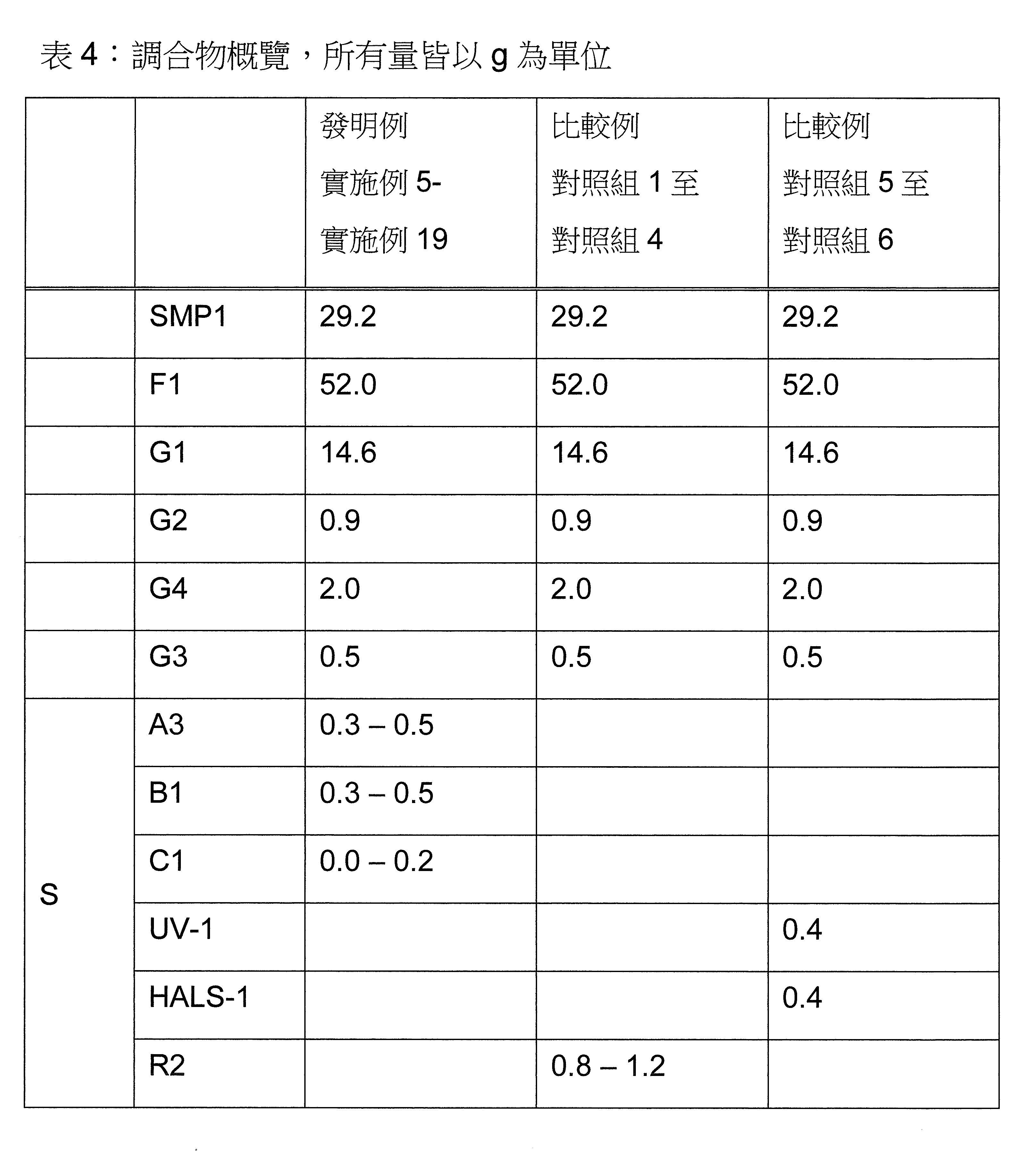
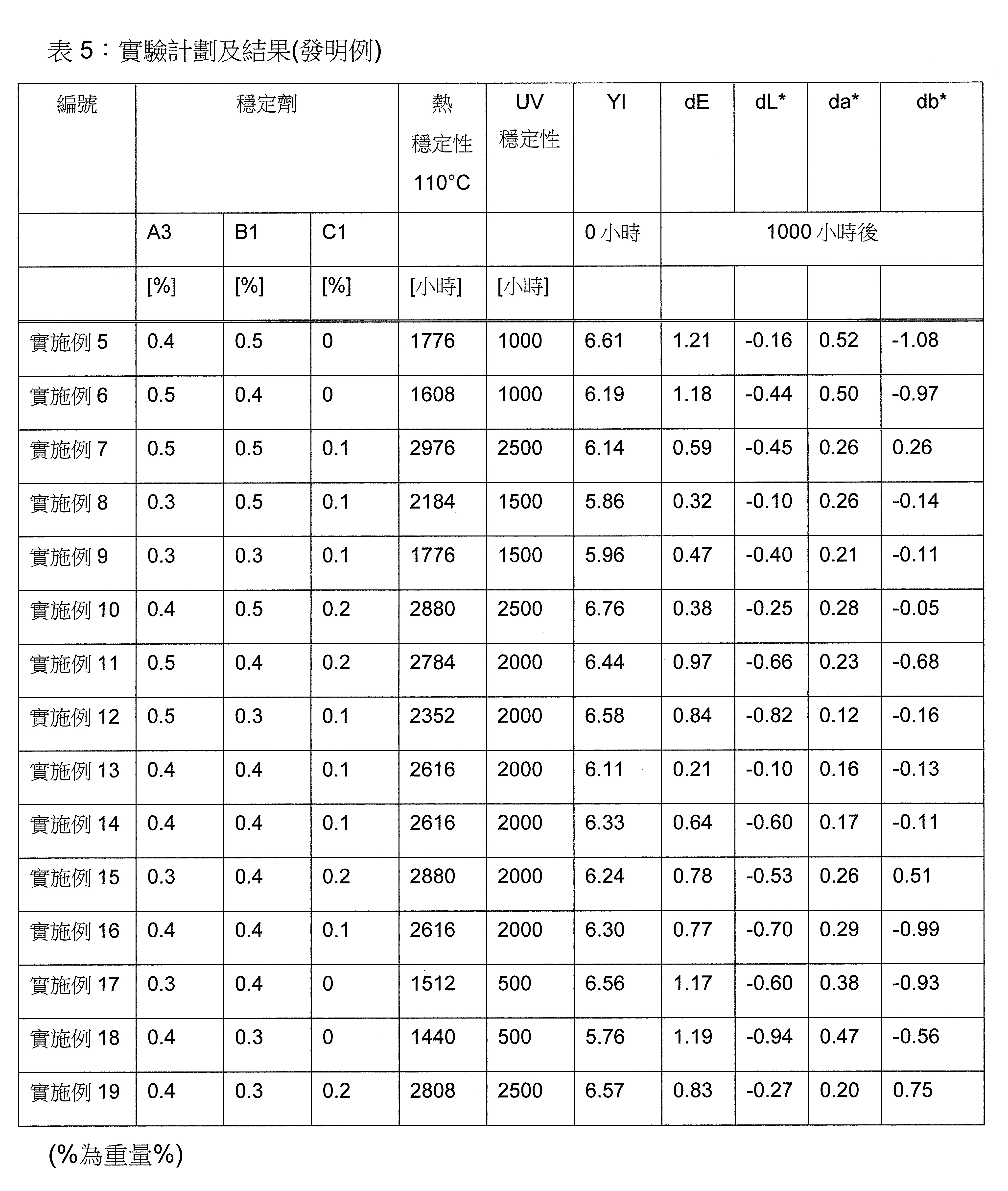
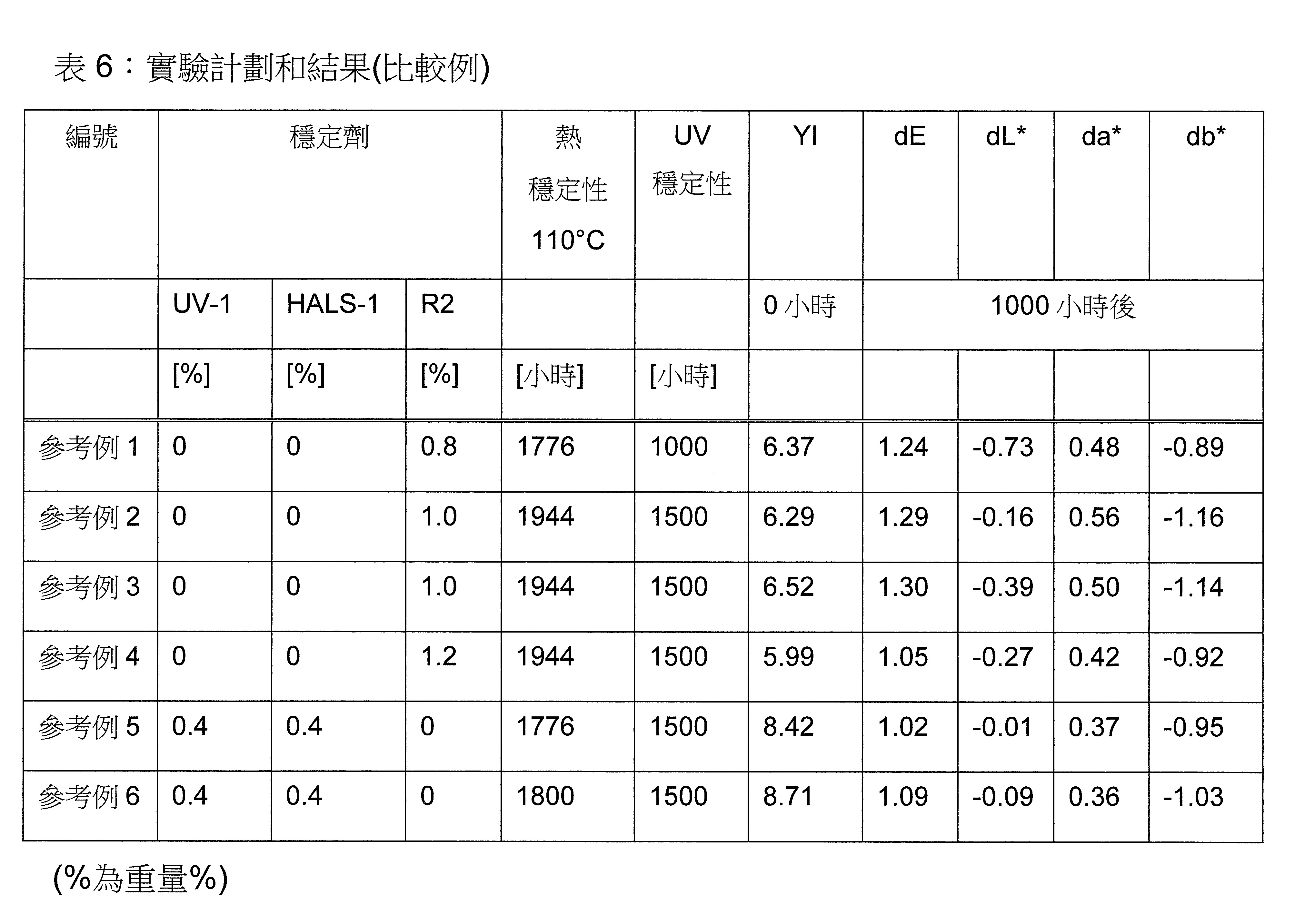
頃令人驚訝地發現,包含至少一種草醯苯胺UV吸收劑及/或至少一種(2-羥基苯基)-對稱-三嗪UV吸收劑和至少一種選定之受阻胺穩定劑(HALS),並隨意地包含至少一種選定之酚類抗氧化劑和至少一種液體稀釋劑,的穩定劑組合,特別是協同穩定劑組合,顯著改善了光和熱穩定化而不影響SMP密封劑固有之有利性質。流體稀釋劑藉由將穩定劑組合簡單快速地提供並均勻分佈於最終SMP密封劑中進一步改善穩定劑組合之適用性。It has surprisingly been found that, comprising at least one oxanilide UV absorber and/or at least one (2-hydroxyphenyl)-symmetric-triazine UV absorber and at least one selected hindered amine stabilizer (HALS), and Stabilizer combinations, especially synergistic stabilizer combinations, optionally including at least one selected phenolic antioxidant and at least one liquid diluent, significantly improve light and heat stabilization without affecting the inherently beneficial properties of SMP encapsulants. The fluid diluent further improves the applicability of the stabilizer combination by providing it simply, quickly, and evenly distributing it in the final SMP encapsulant.
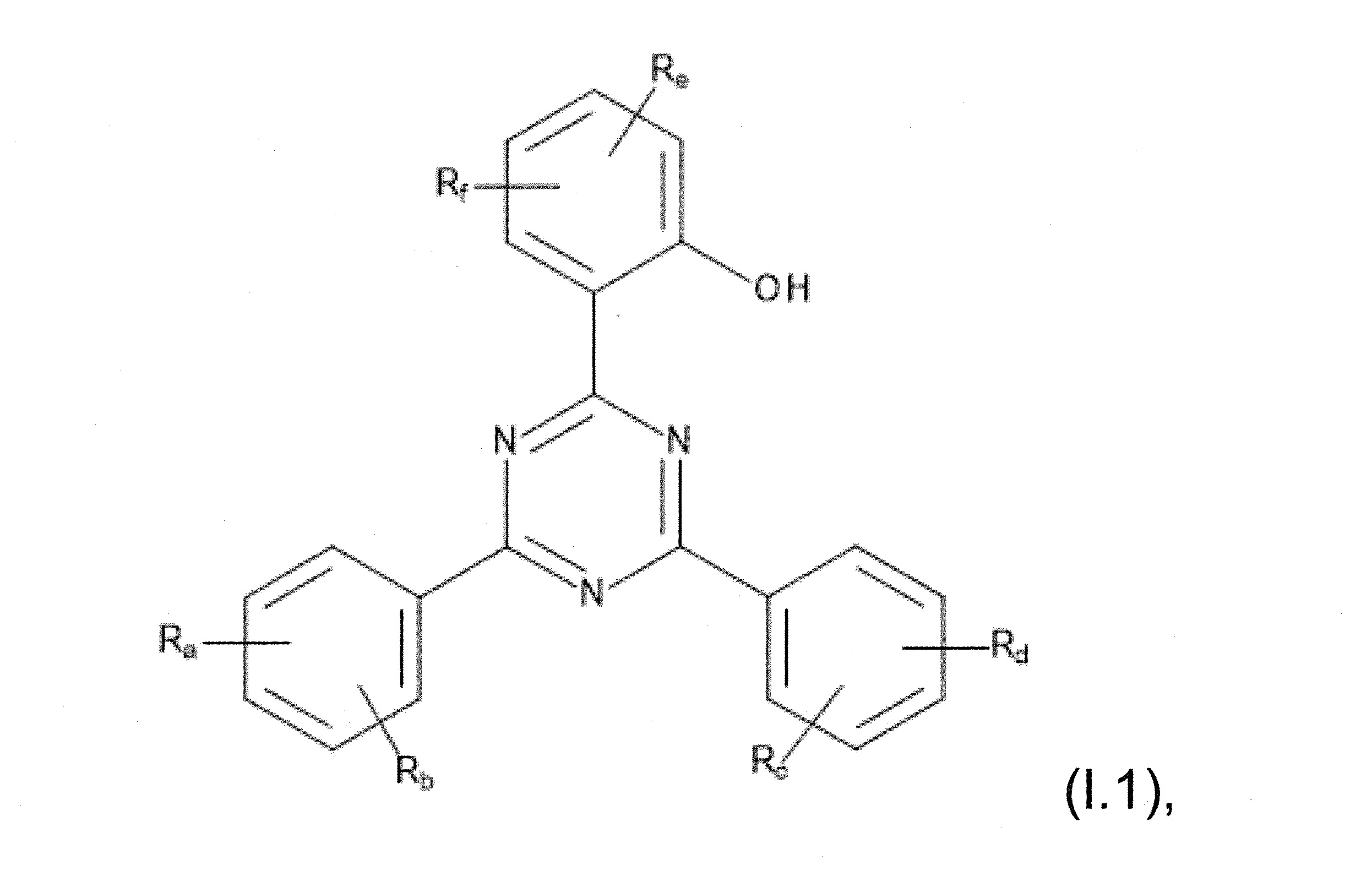
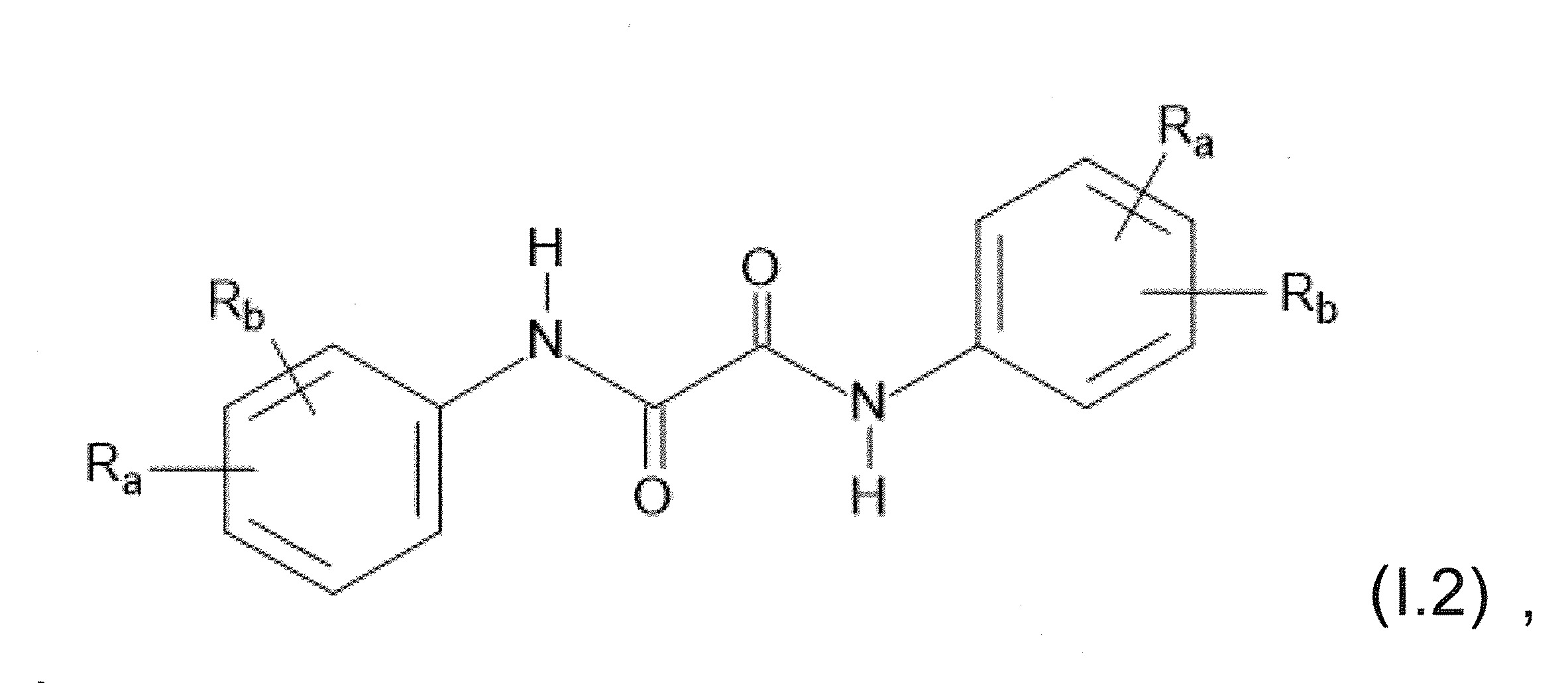
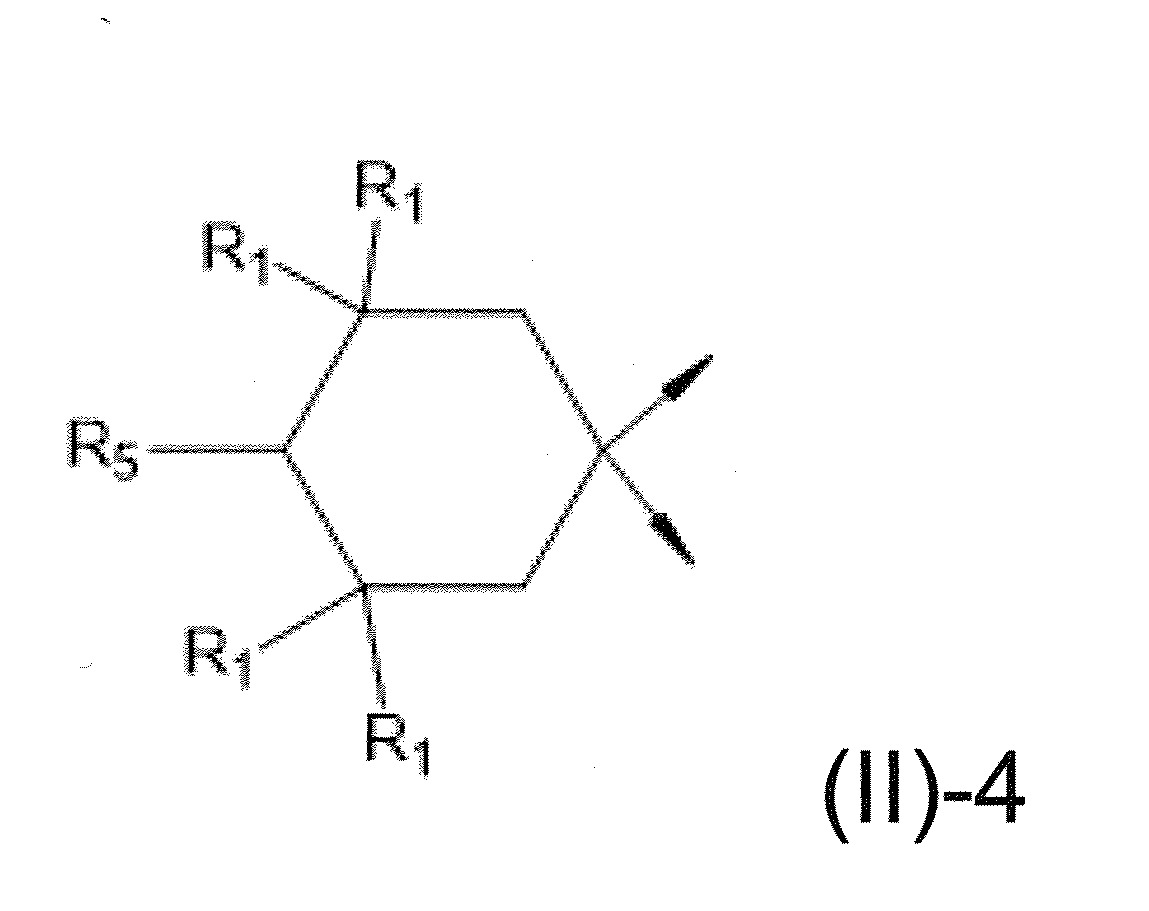
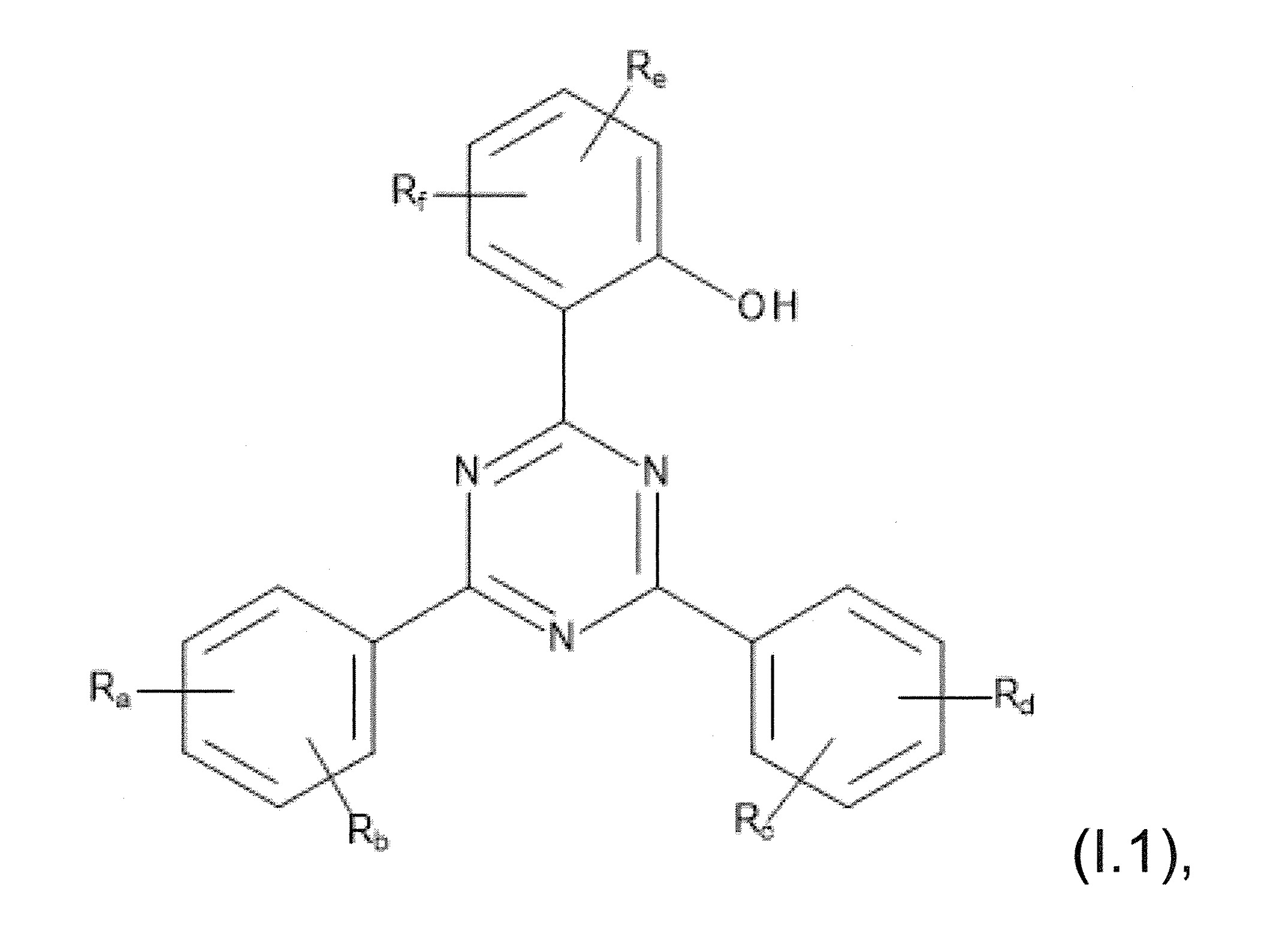
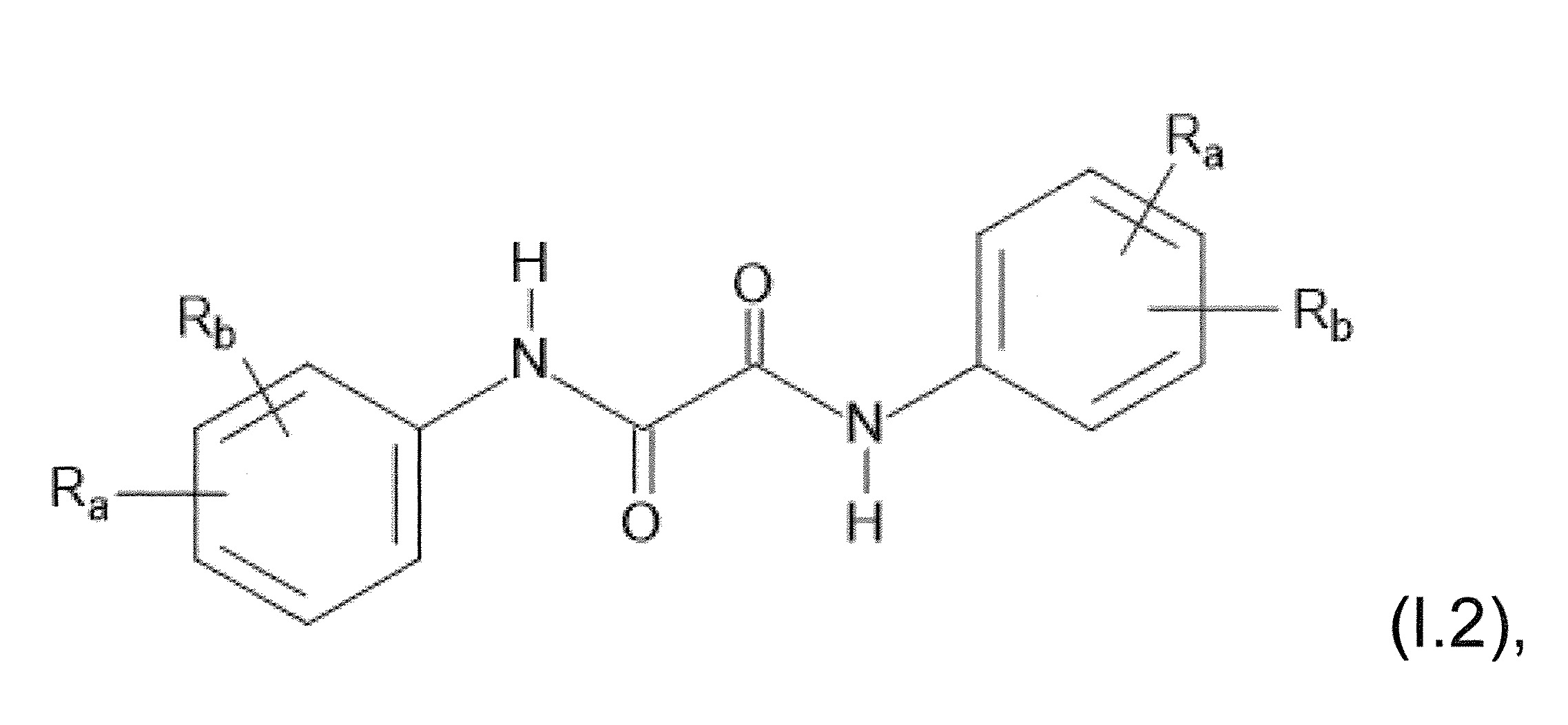
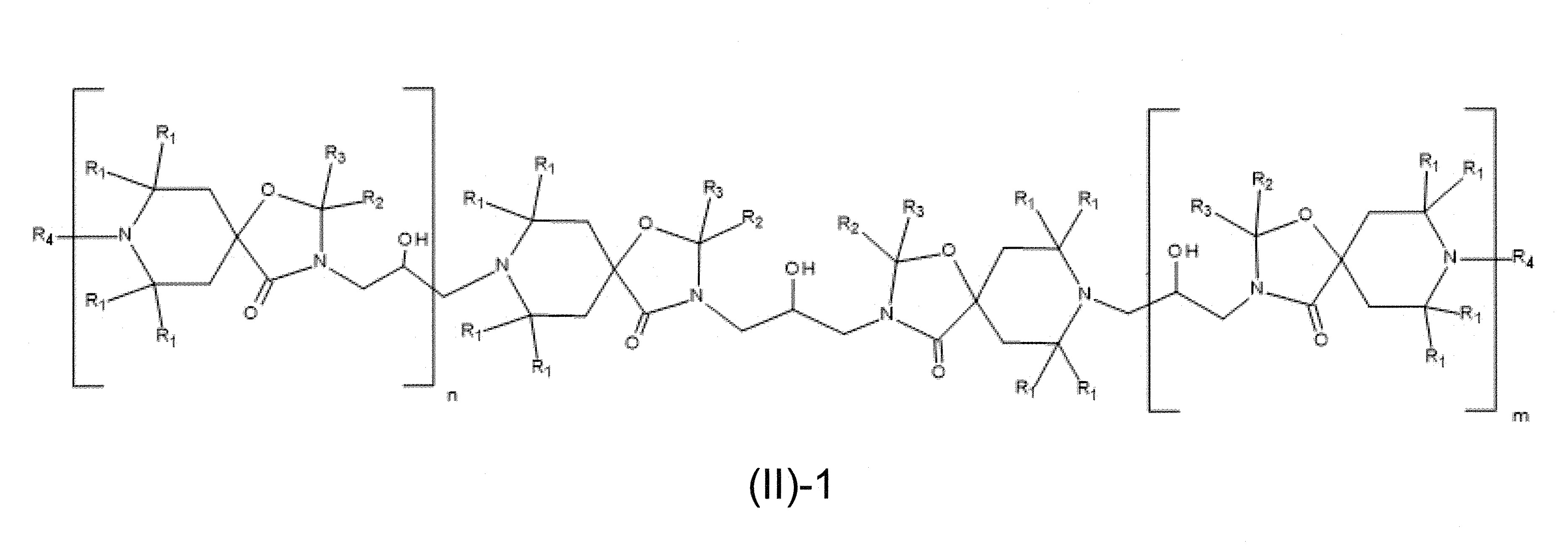
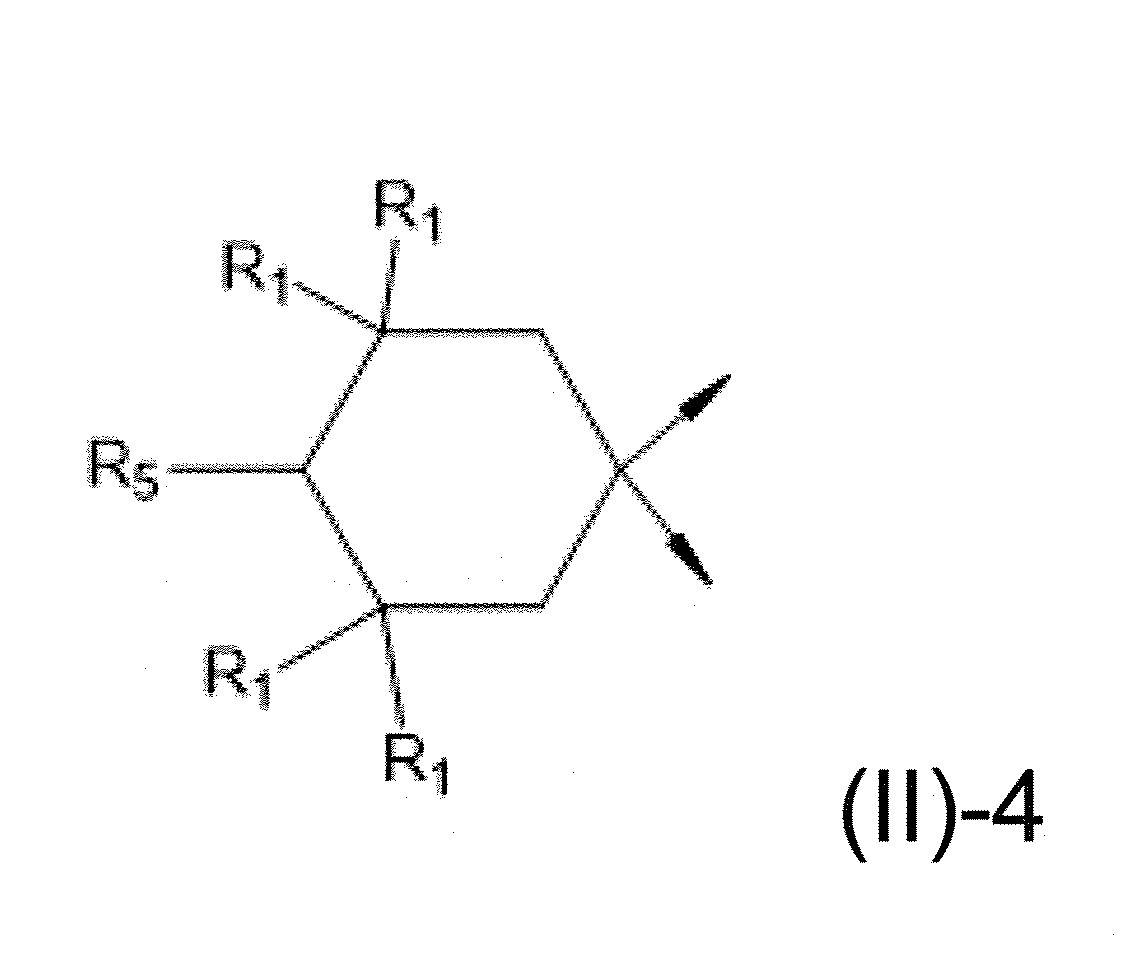
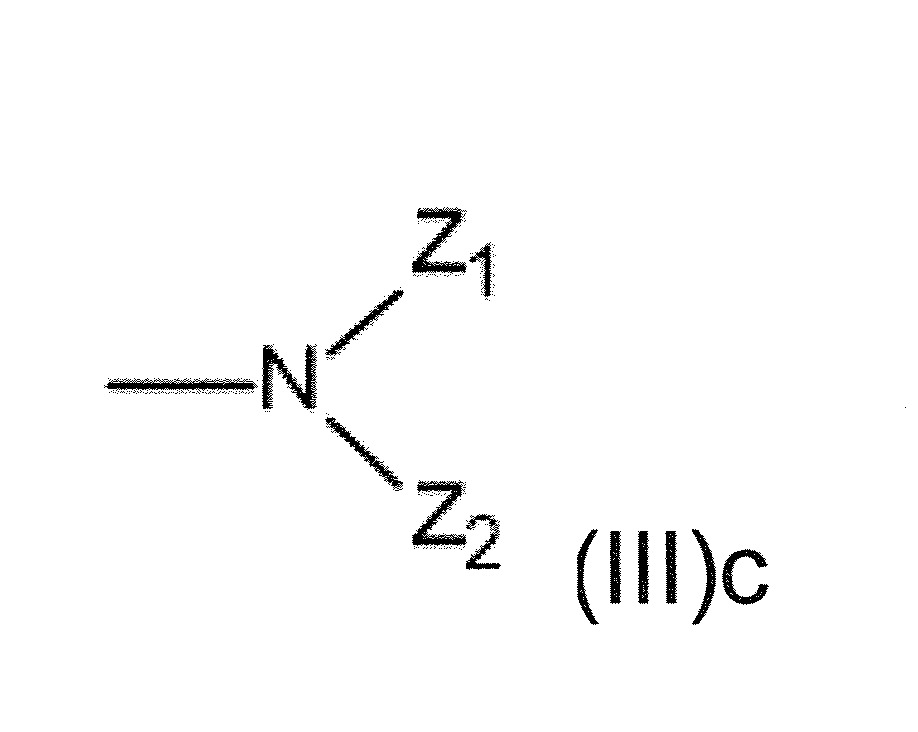
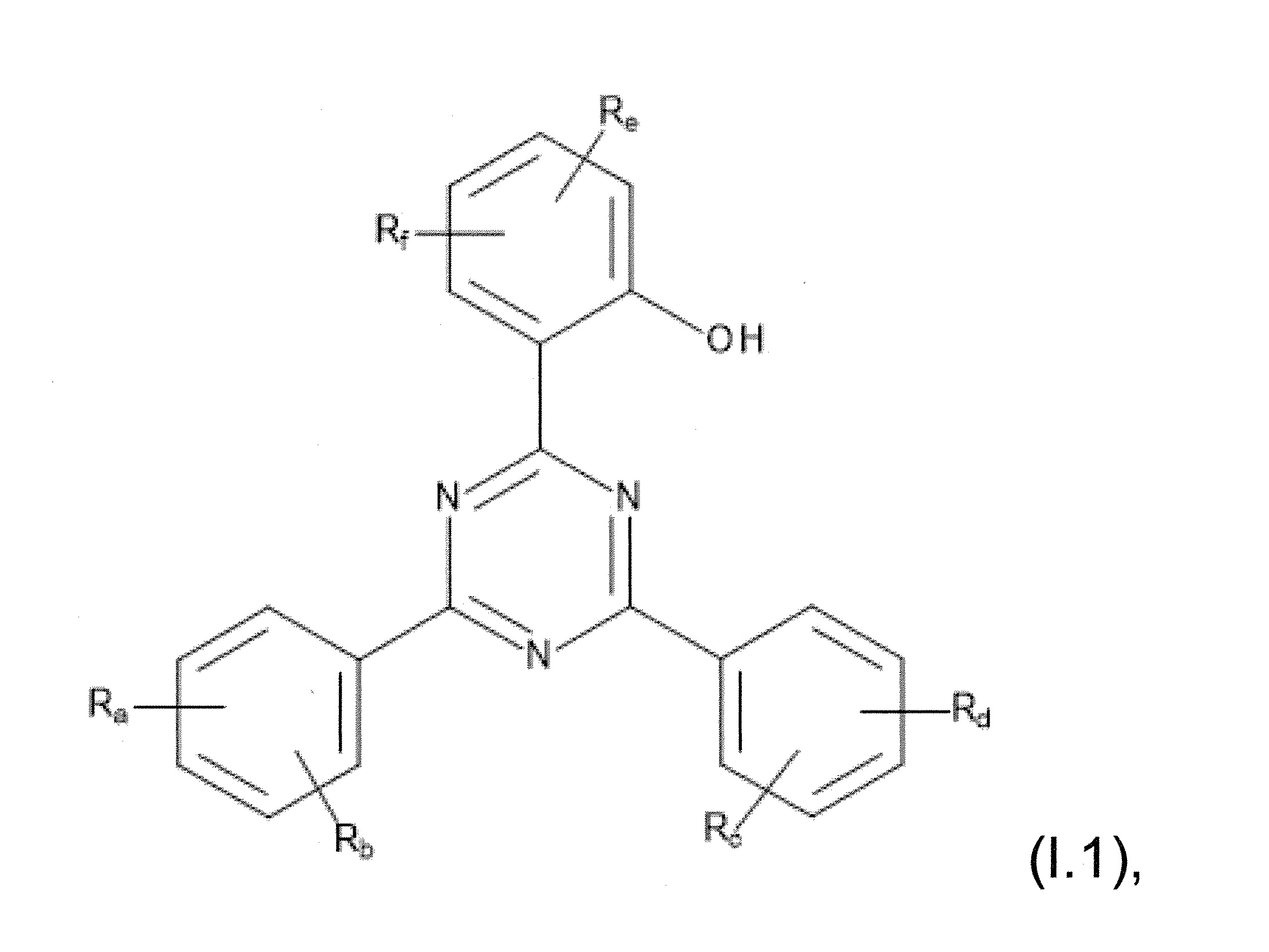
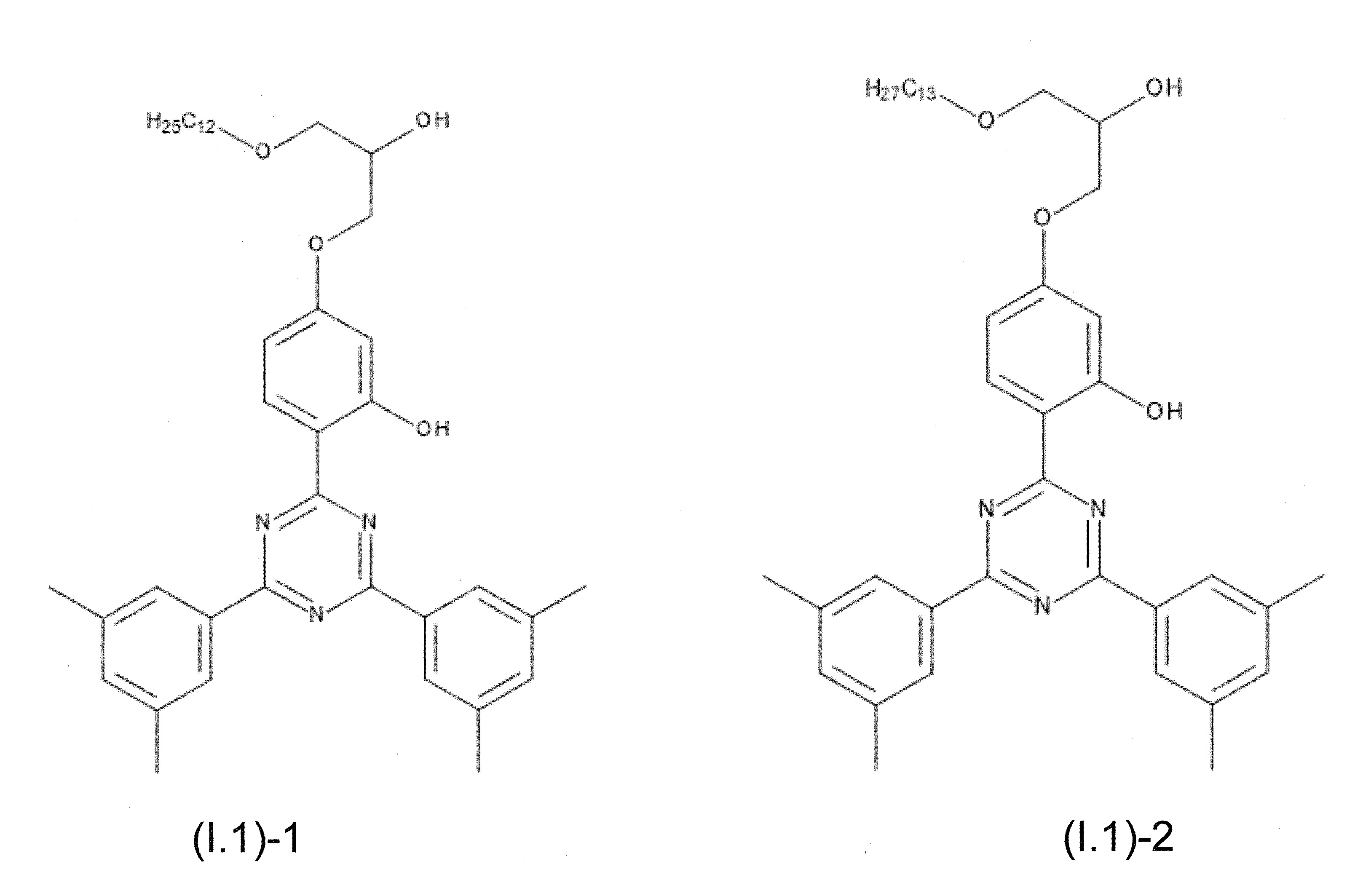
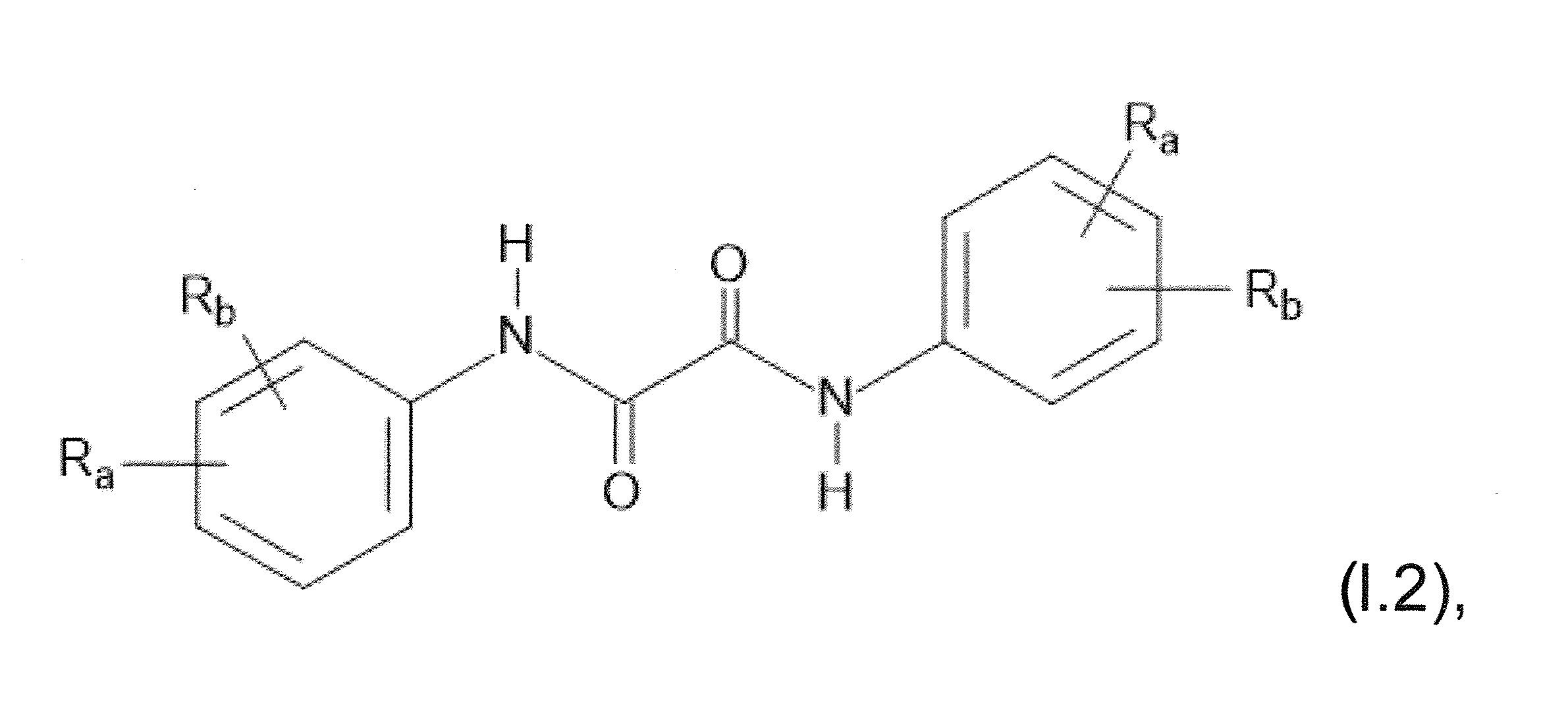
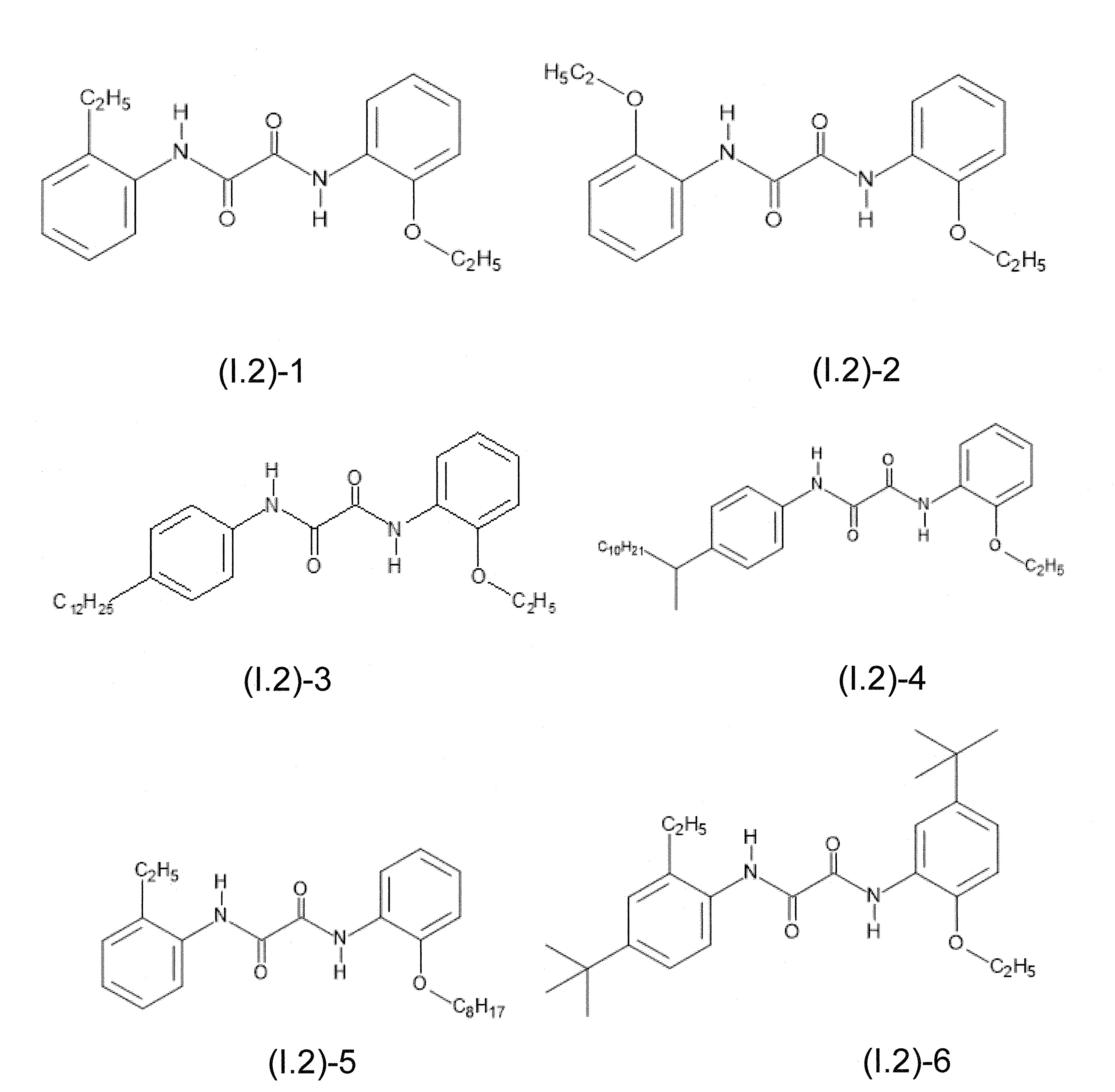
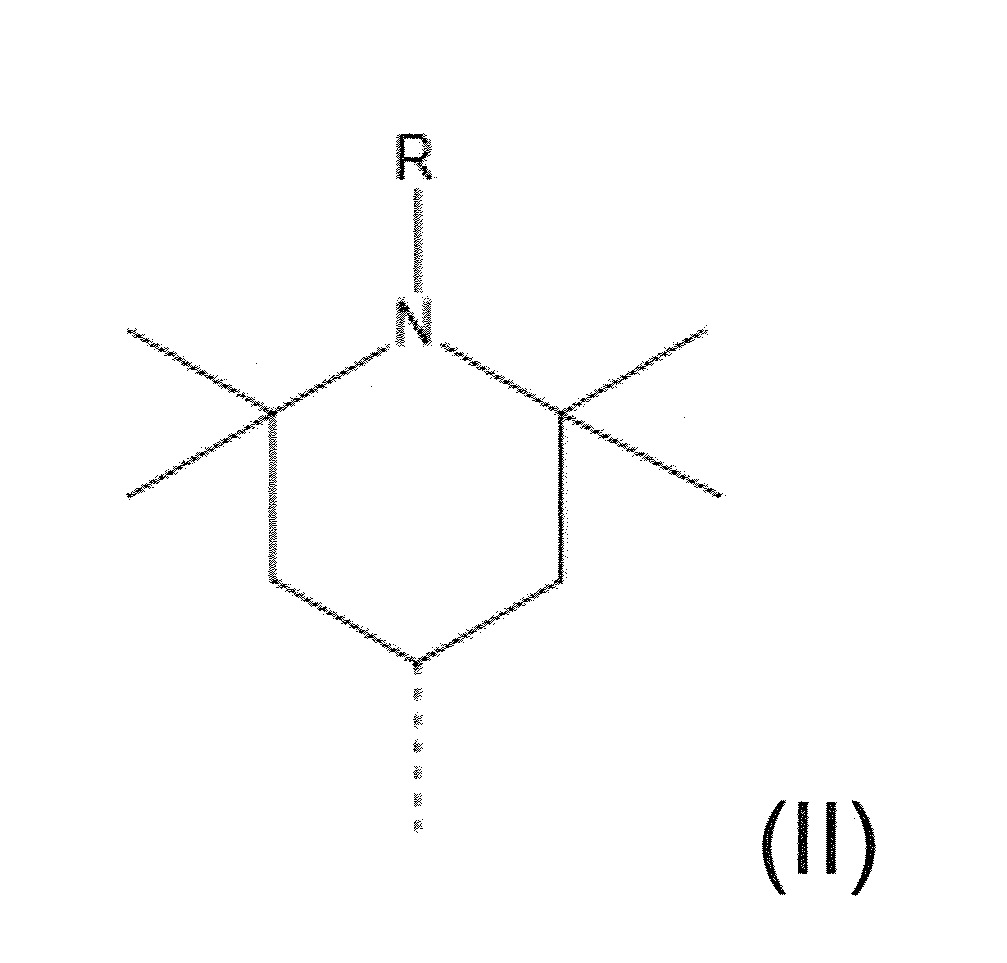
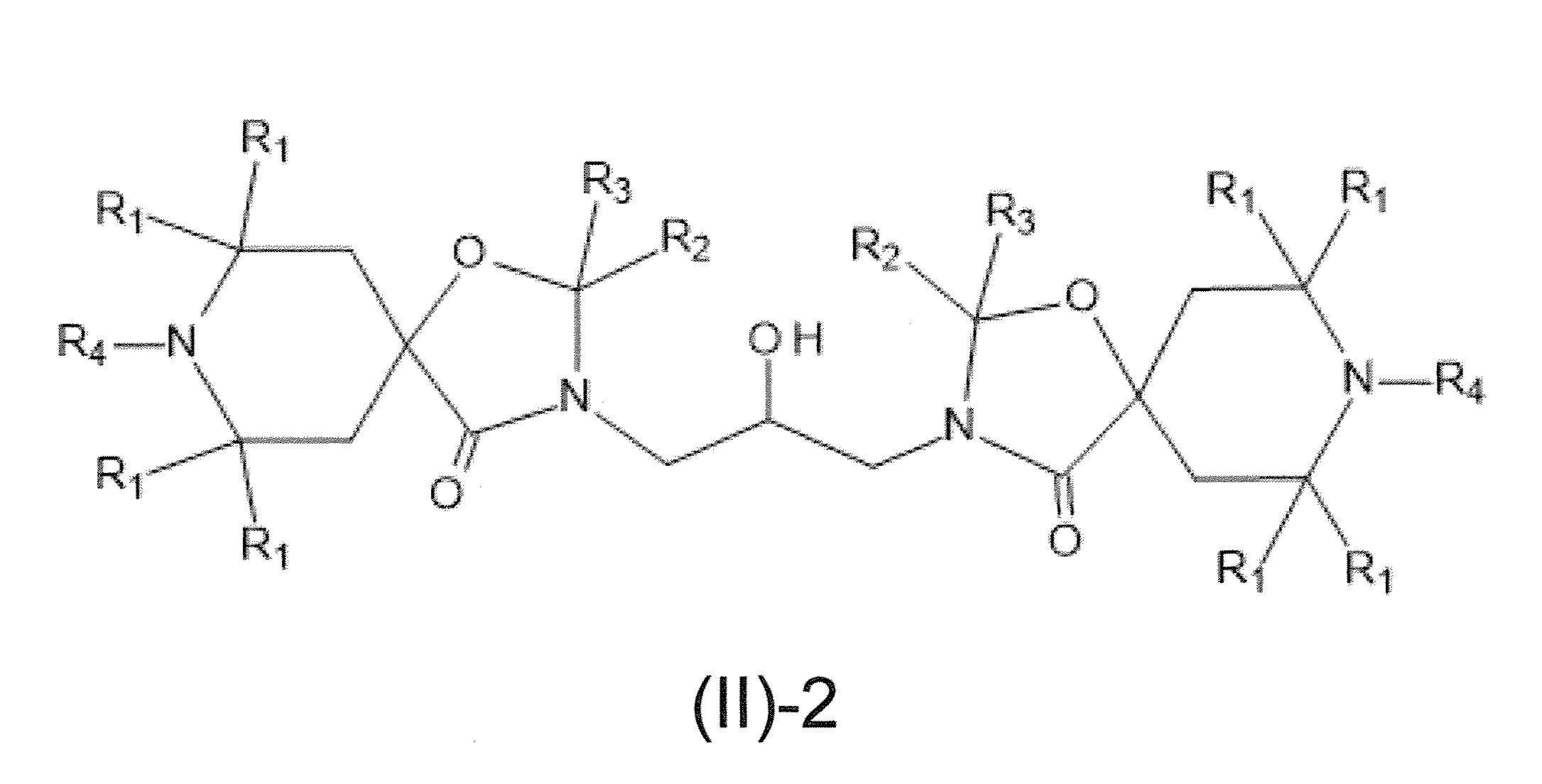
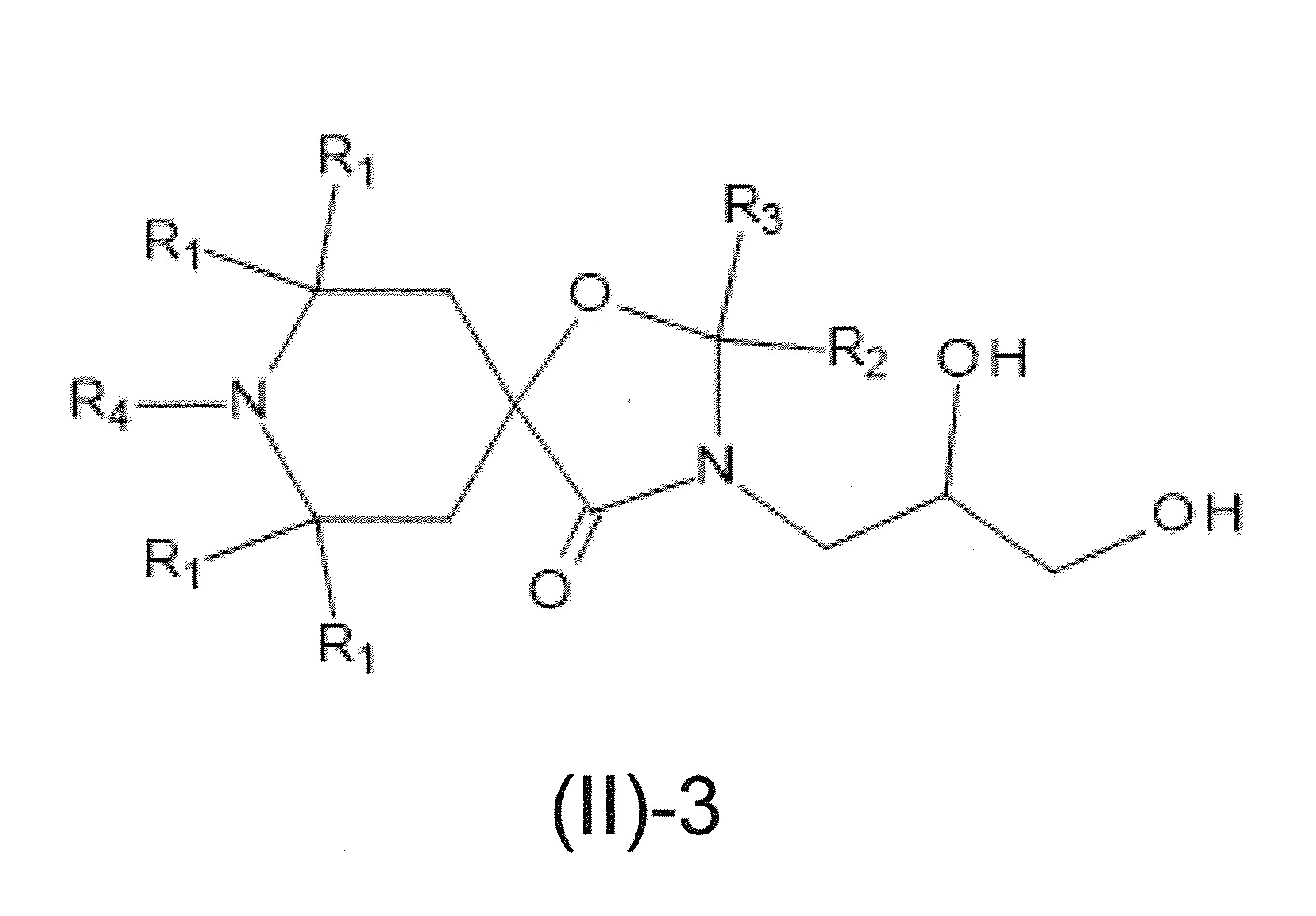
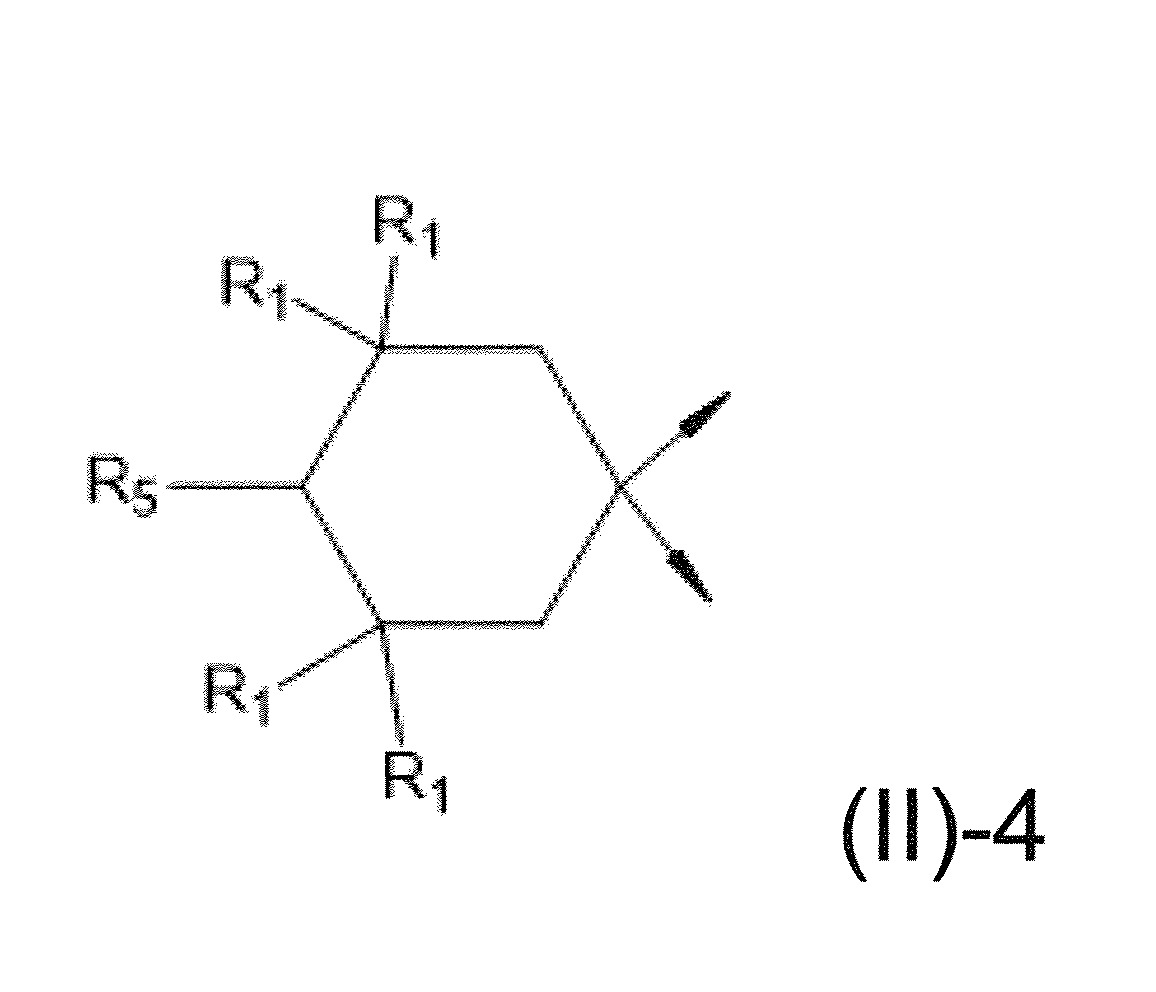
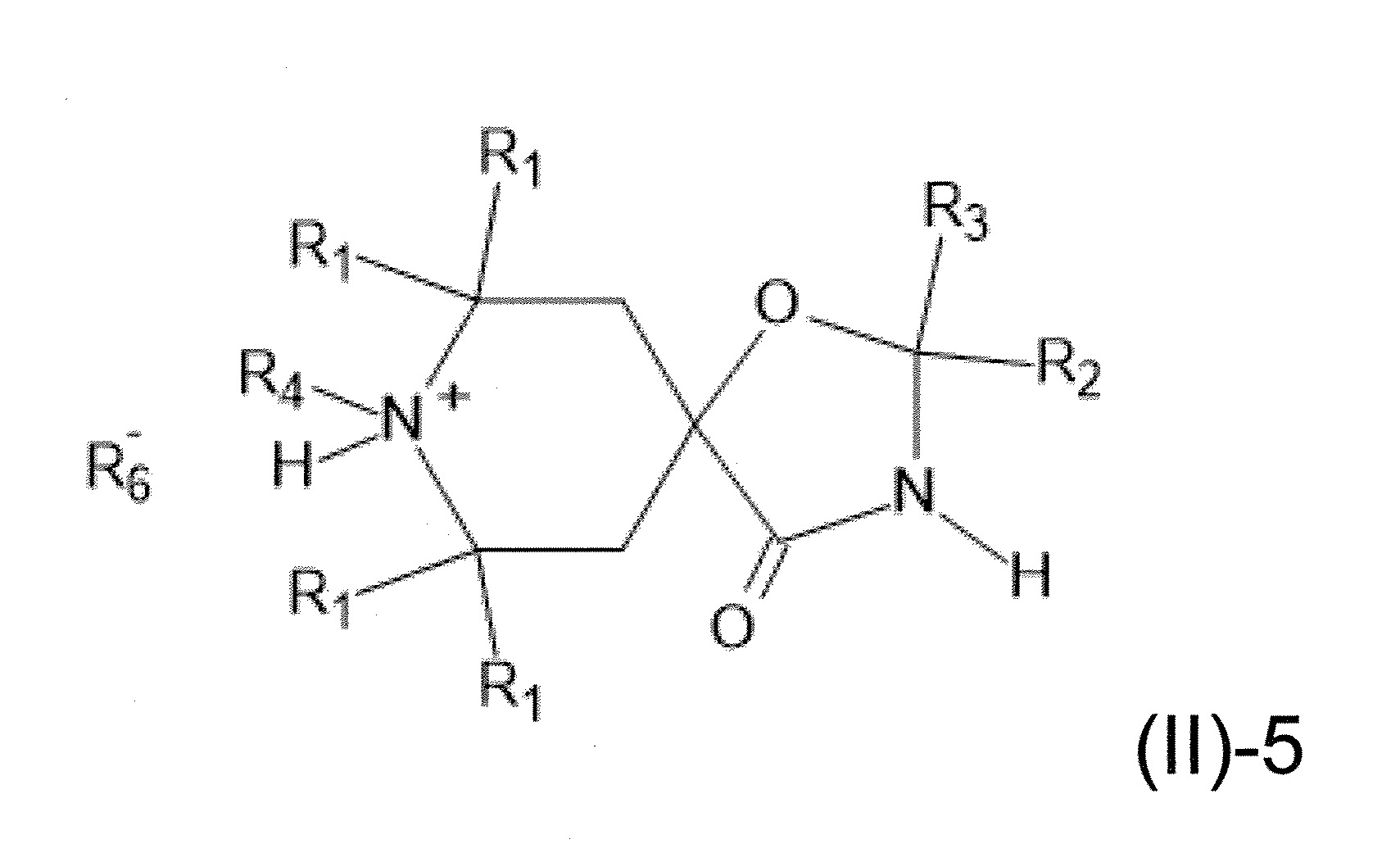
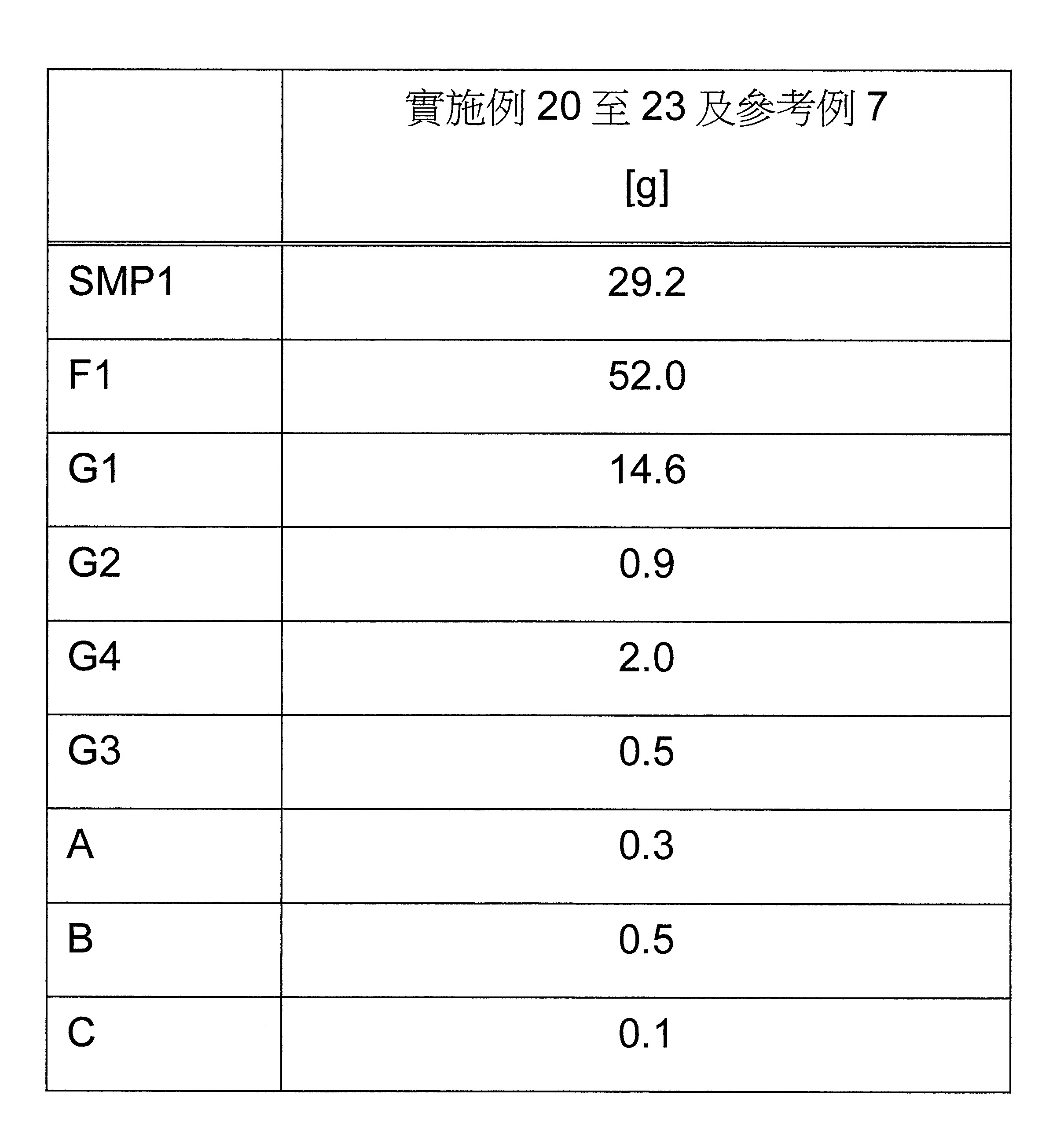
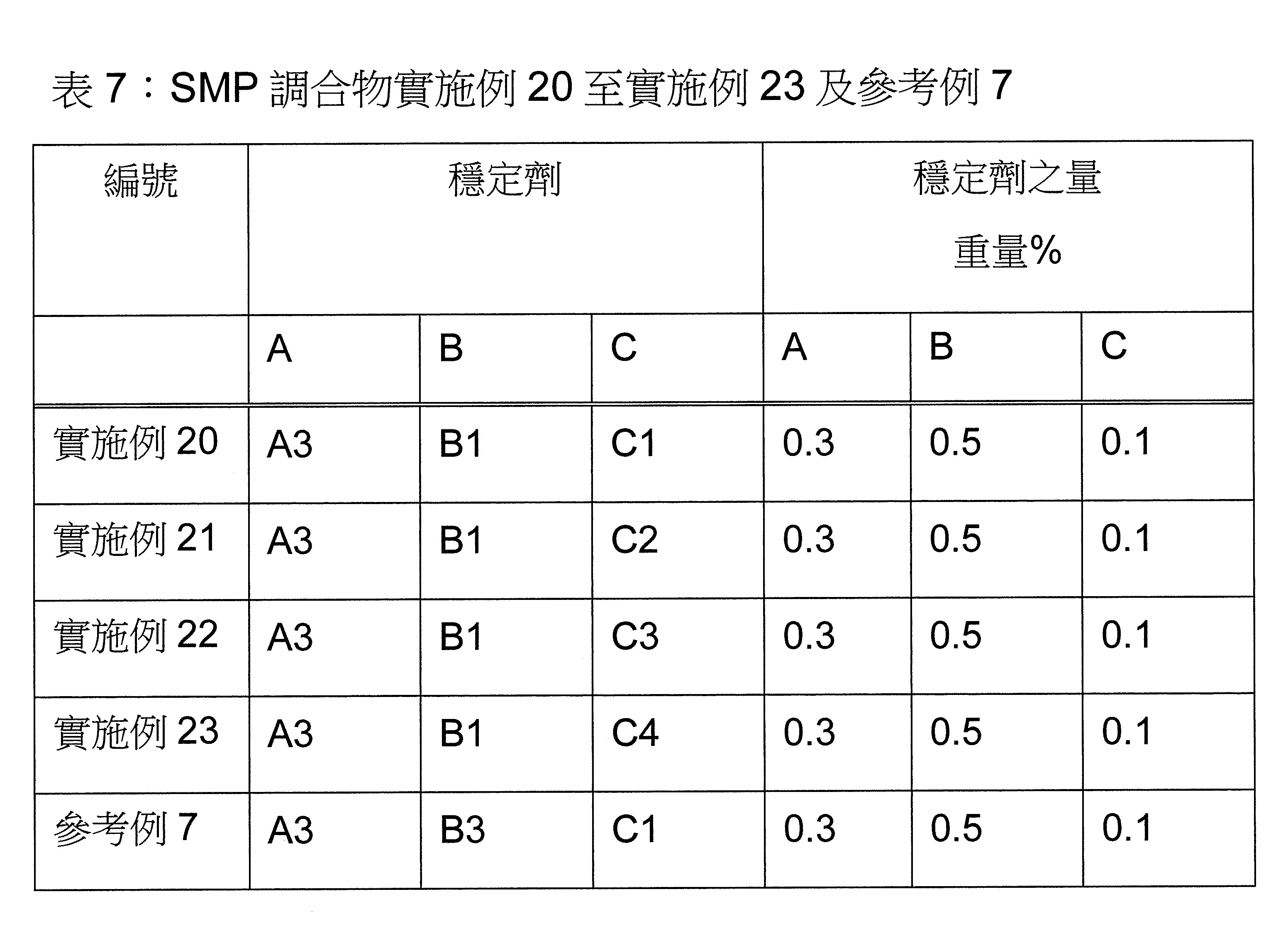
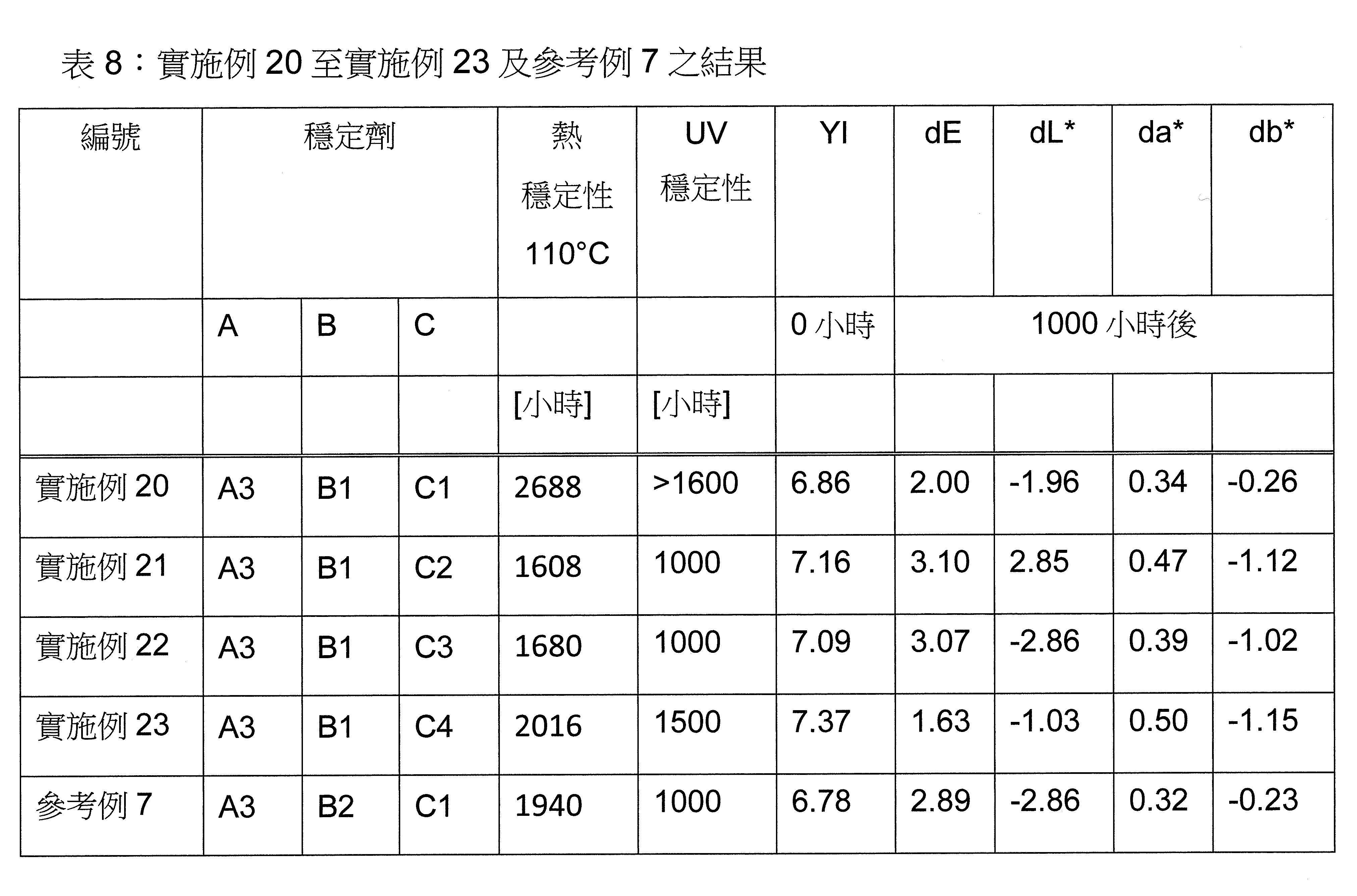
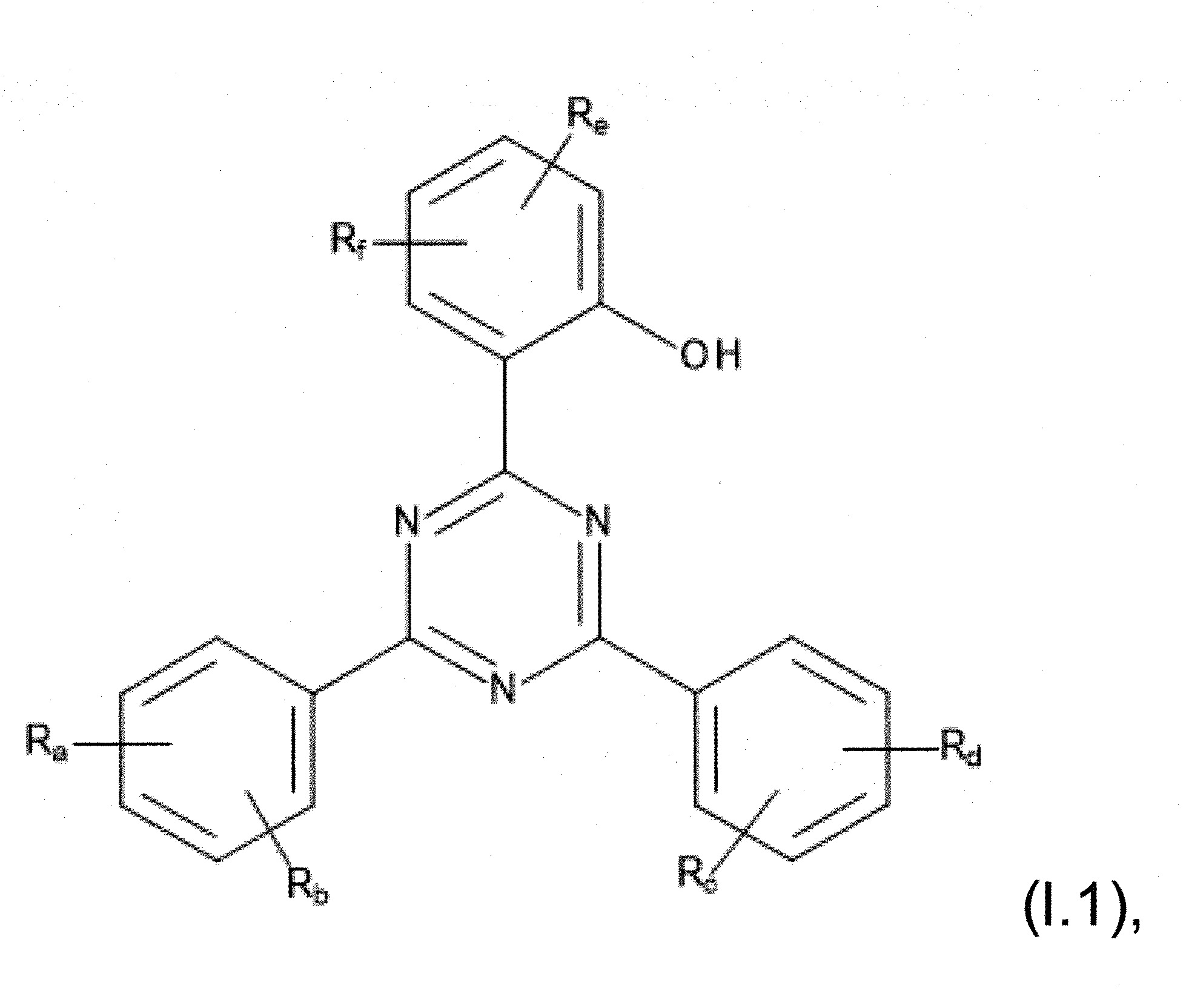
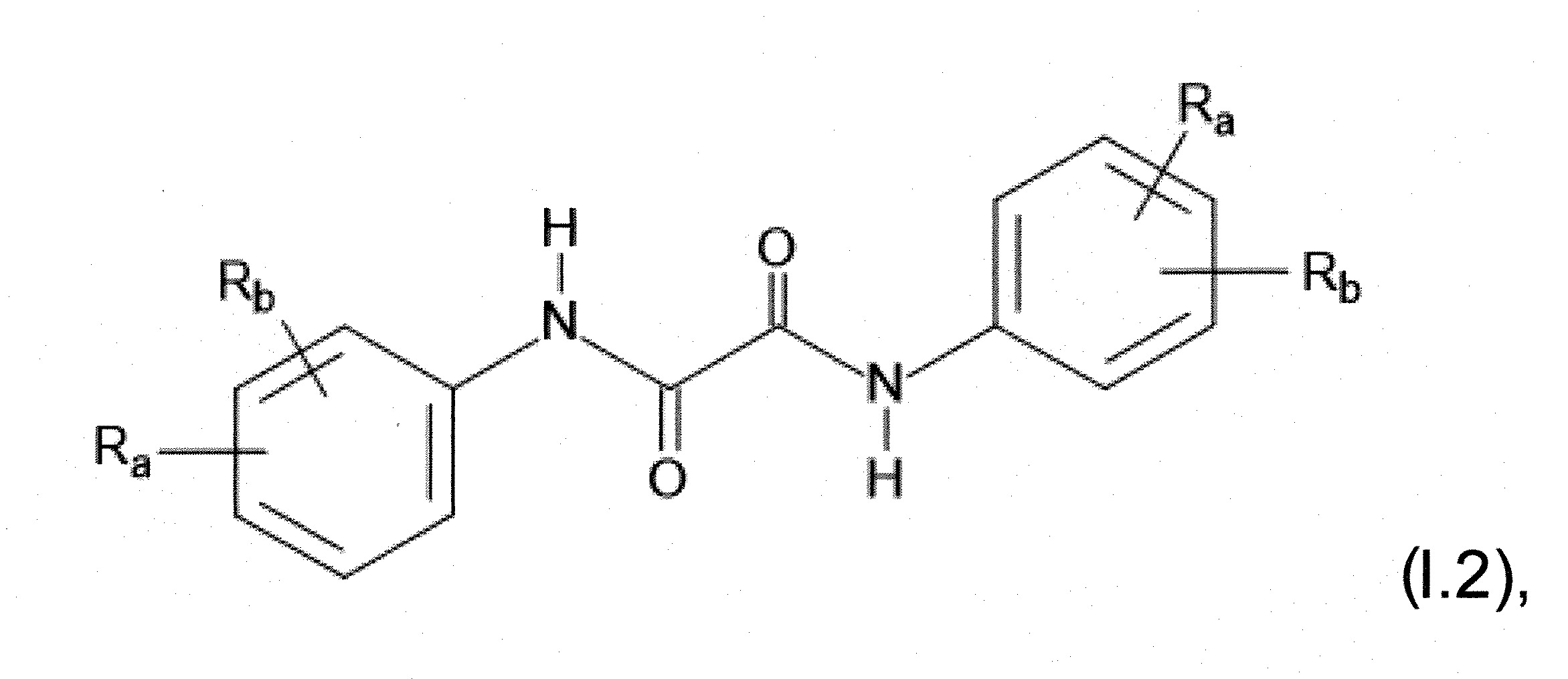
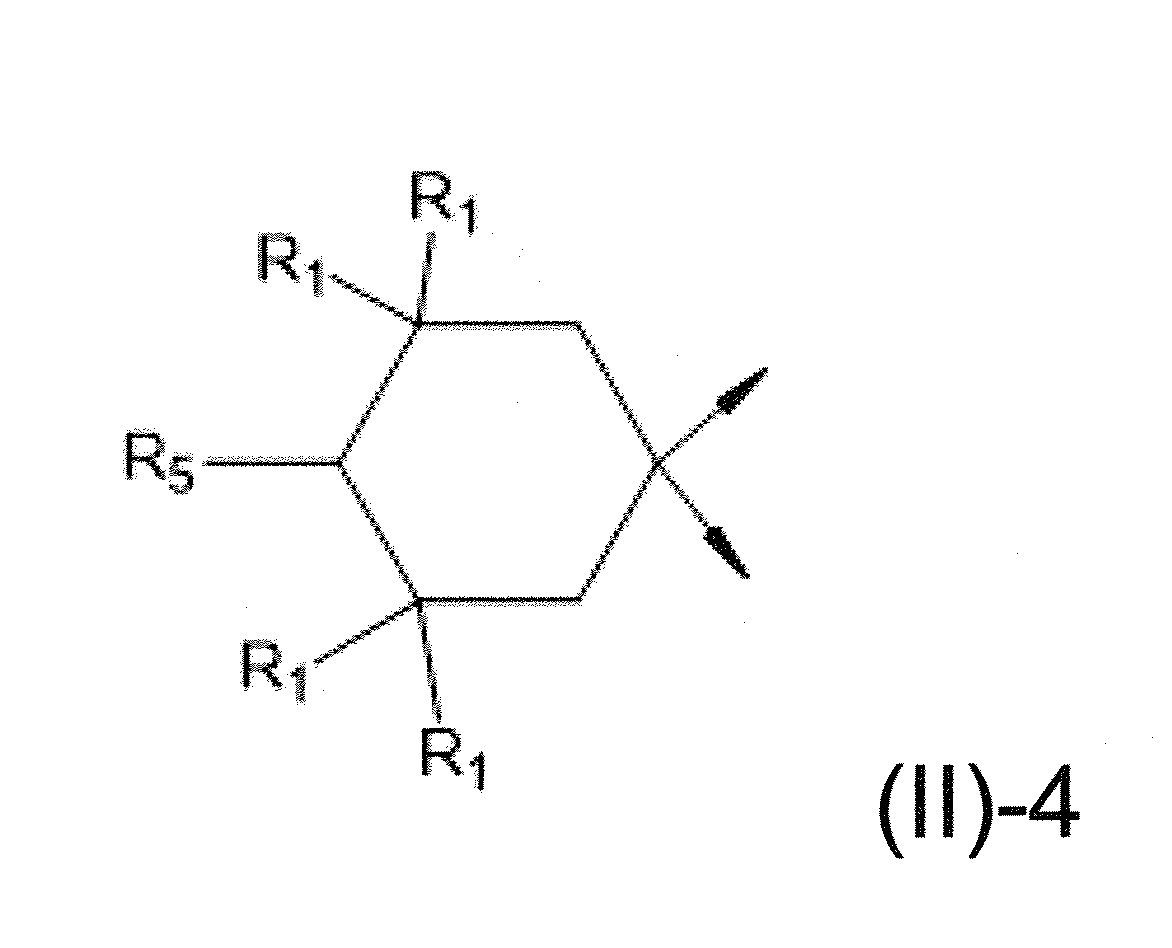
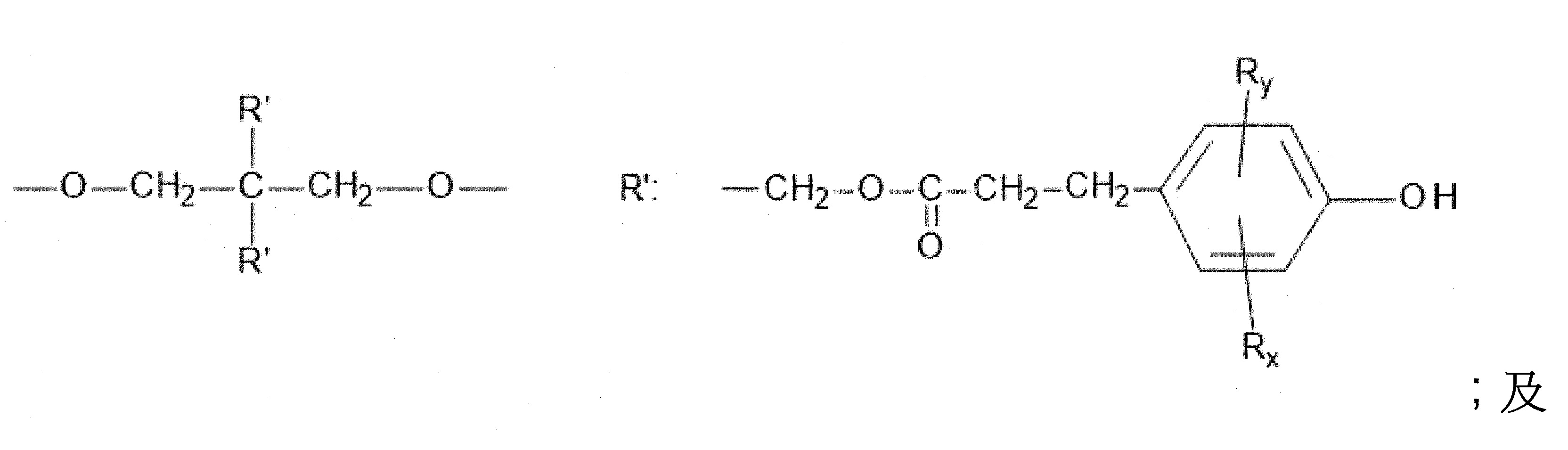
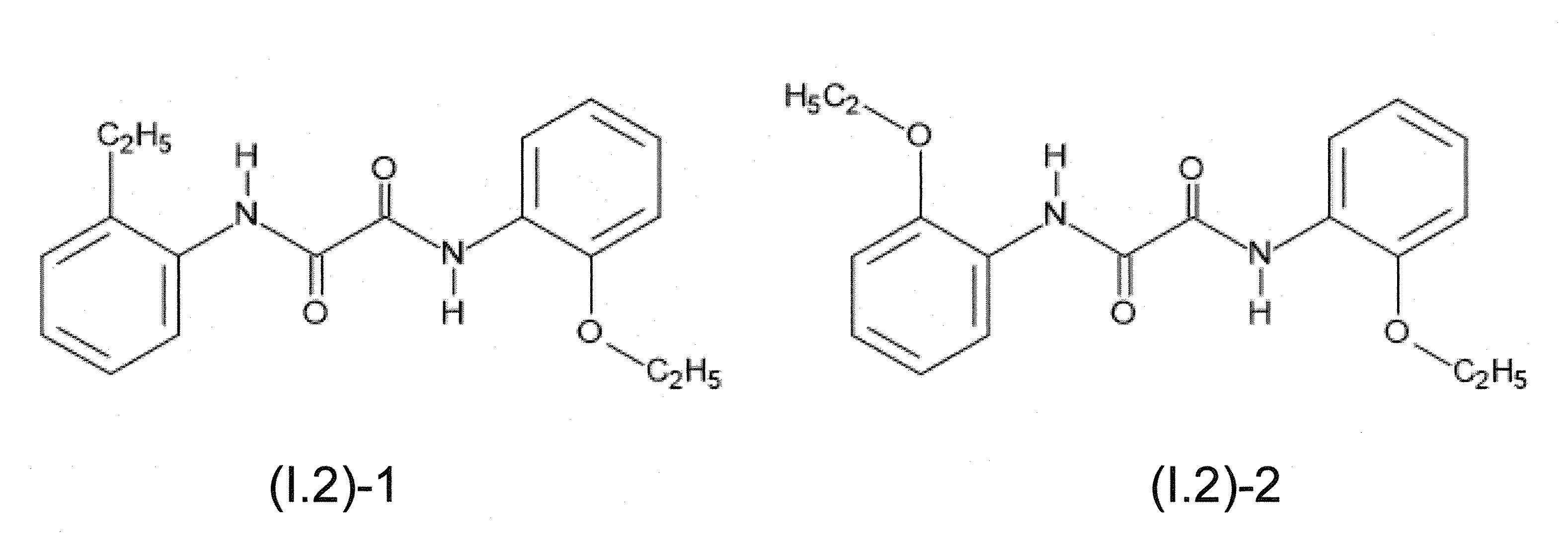
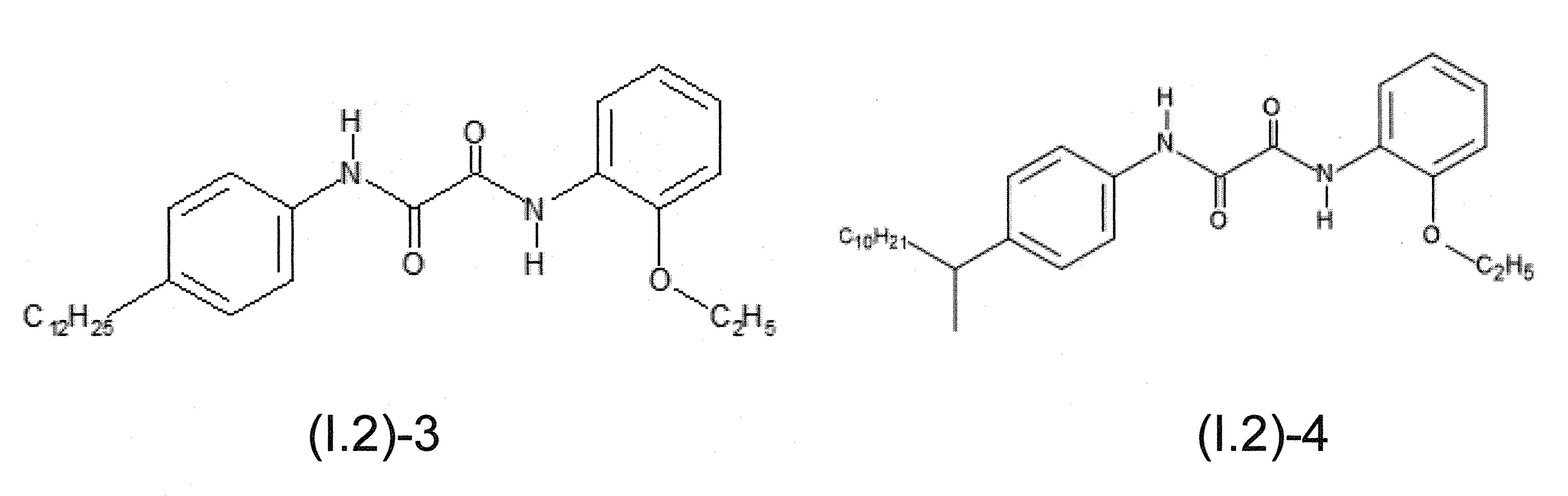
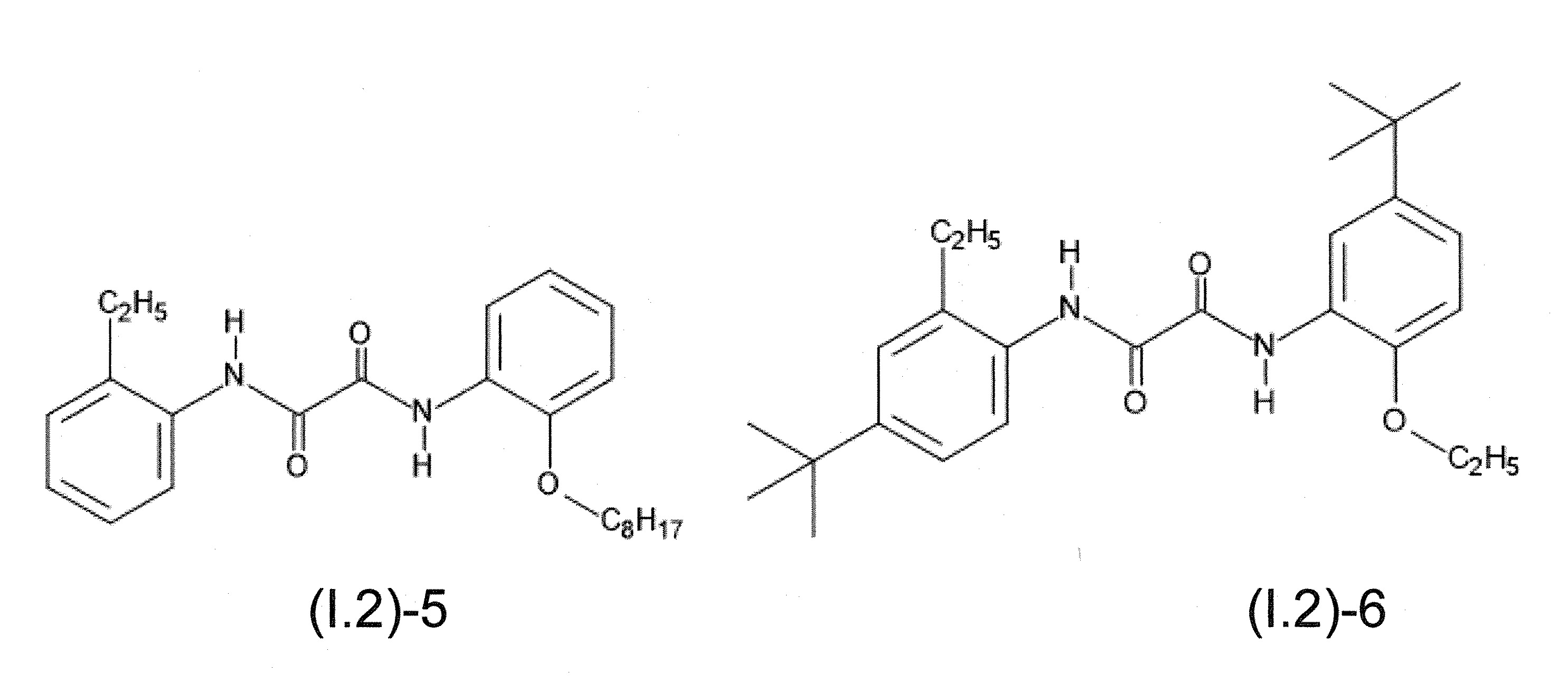
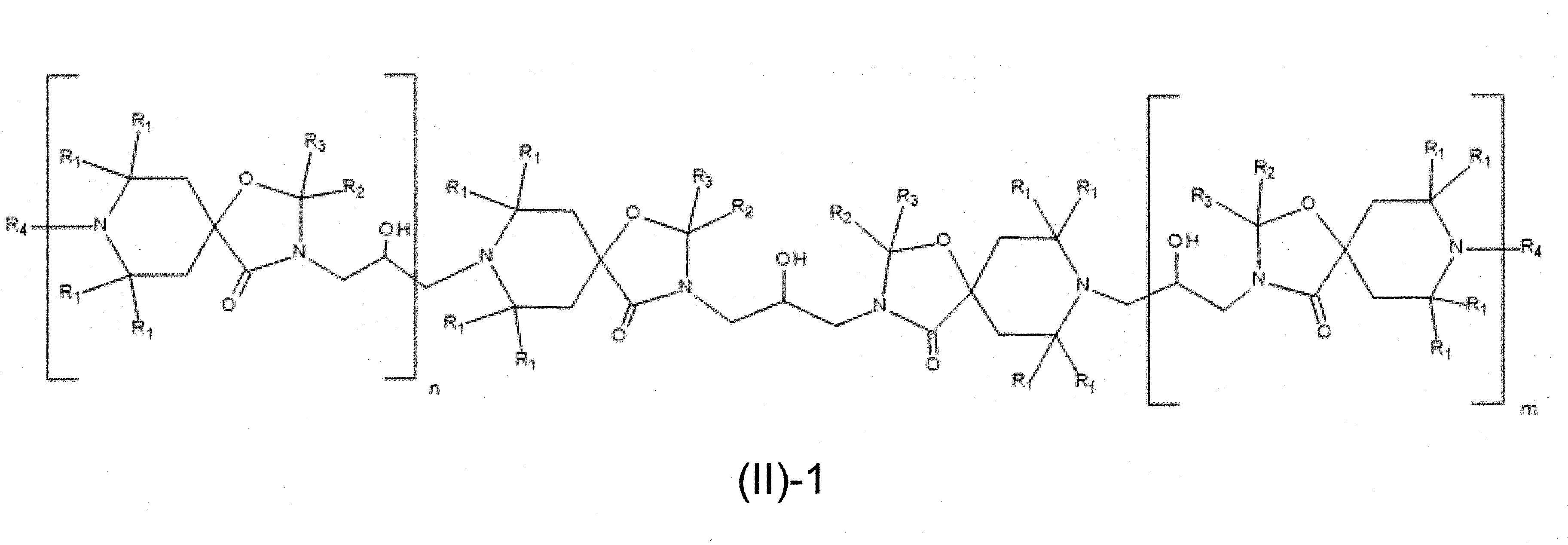
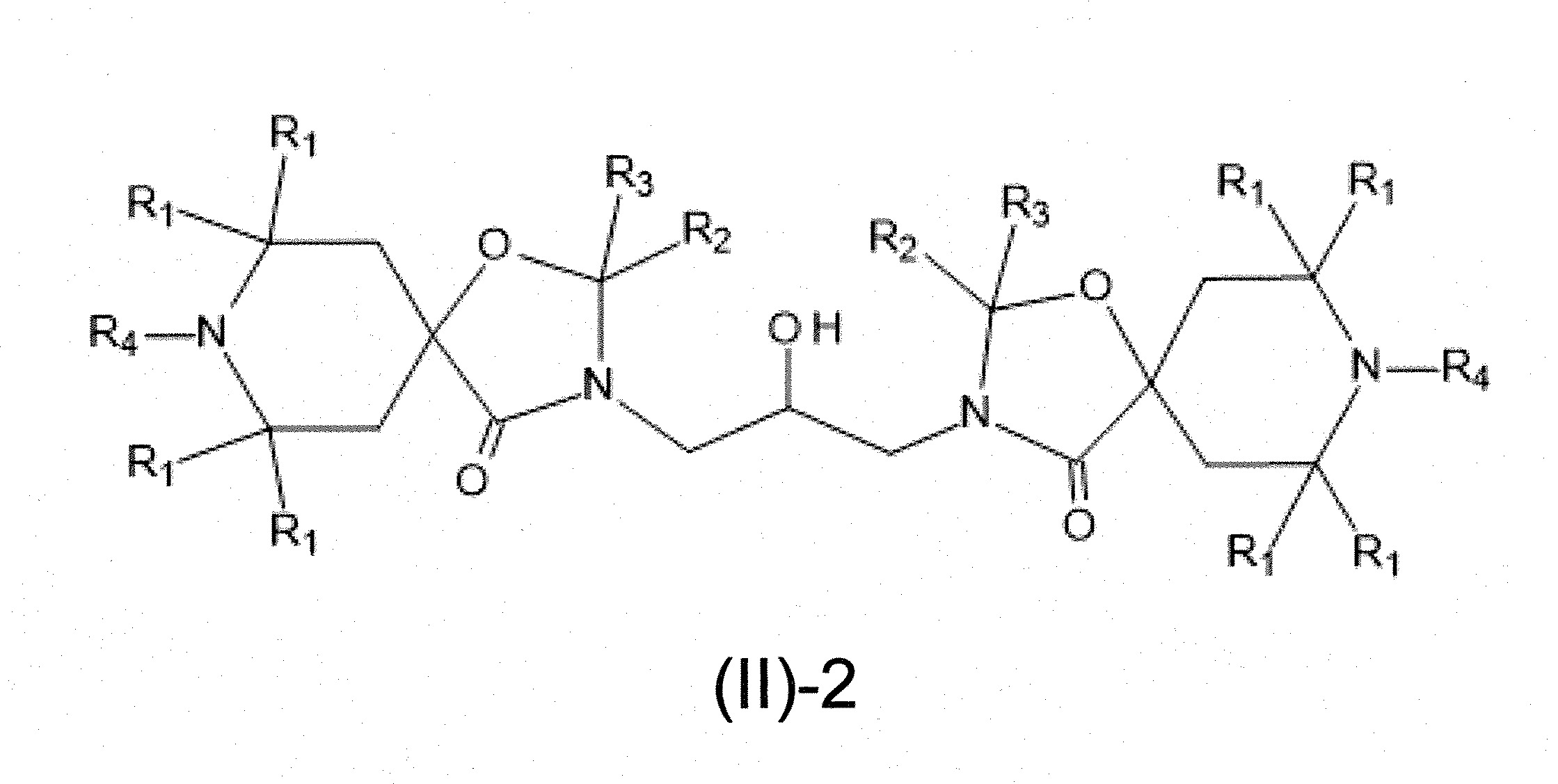
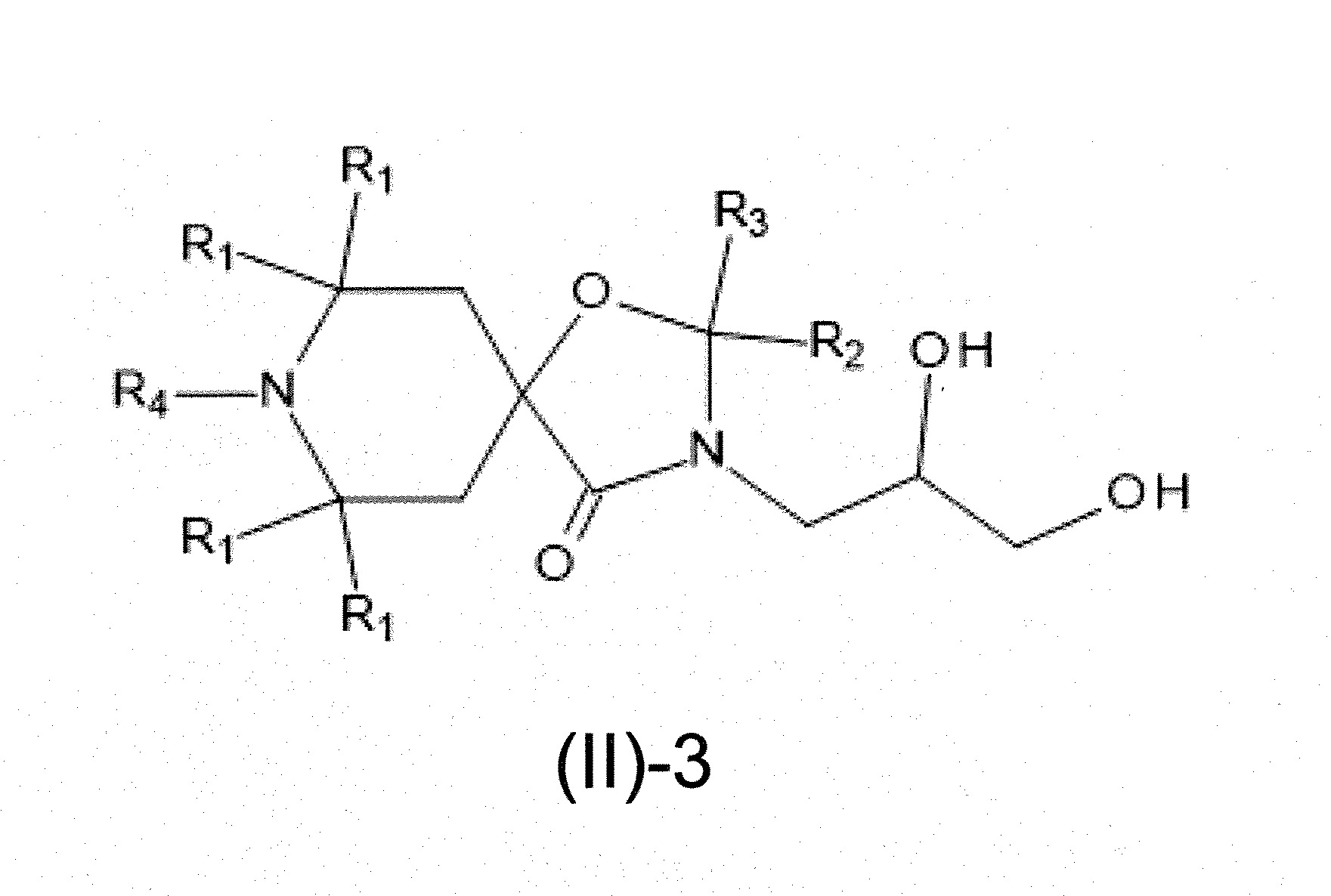
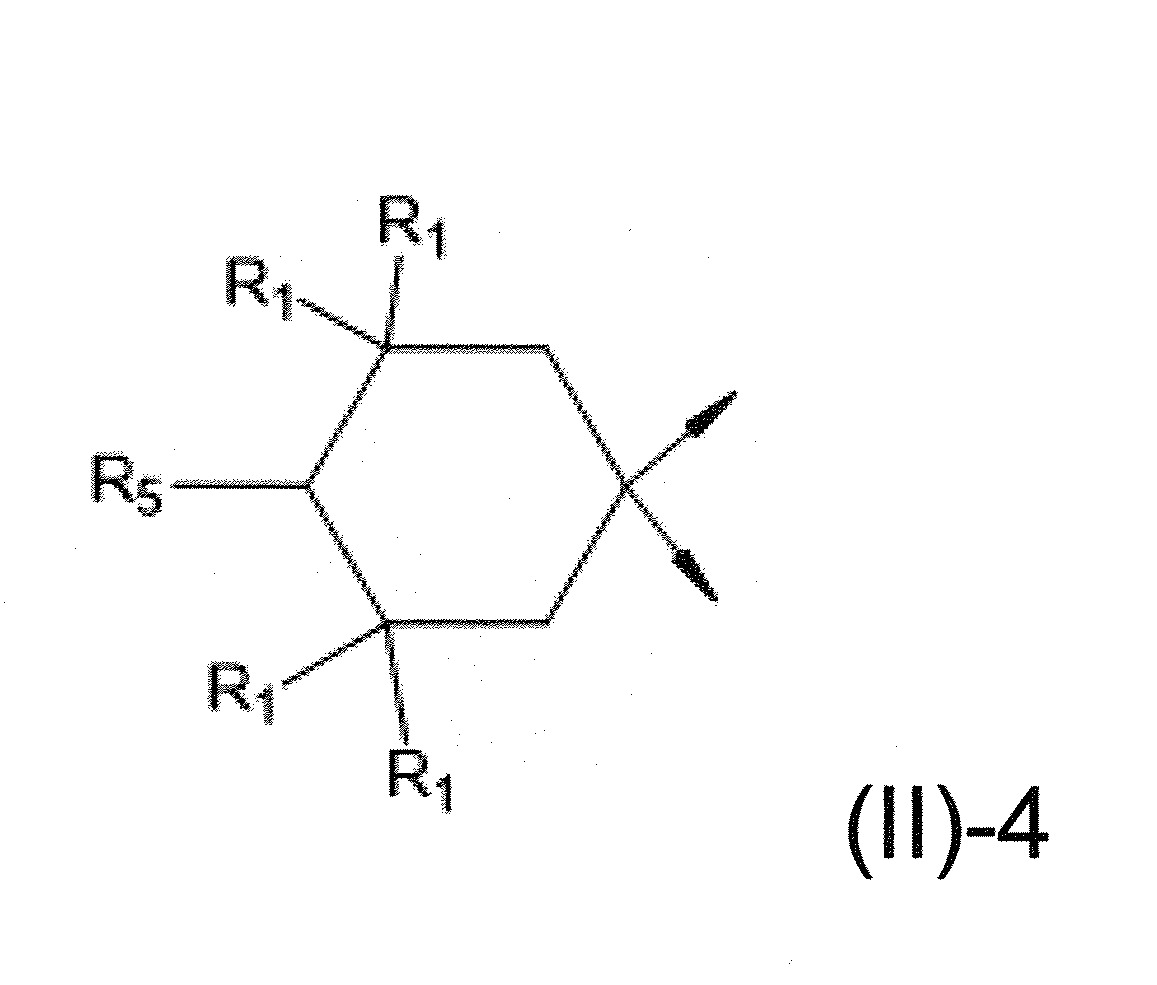
本發明關於一種穩定劑組成物,其包含: (A) 至少一種UV吸收劑作為組分A,其中該UV吸收劑A包含: (A1) 一或多種根據式(I.1)之(2-羥基苯基)-對稱-三嗪化合物作為組分A1: 其中 R a、R b、R c、R d、R e及R f係彼此獨立地選自氫、具有1至20個碳原子的烷基、具有1至20個碳原子和1至4個氧原子的烷氧基; 及/或 (A2) 一或多種根據式(I.2)之草醯苯胺化合物作為組分A2: 其中 R a及R b係彼此獨立地選自氫、具有1至20個,較佳地2至12個,碳原子的烷基、具有1至20個,較佳地2至12個,碳原子和1至4個,較佳地1至2個,氧原子(烷氧基之氧原子)的烷氧基, 及 (B) 至少一種受阻胺光穩定劑作為組分B,其包含至少一種根據式(II)-1的寡聚受阻胺光穩定劑B1: 其中 n及m彼此獨立地為0至100,較佳地0至10,更佳地0至5的數字,其先決條件為n和m不皆為0; R 1為氫、具有5至7個碳原子的環烷基、或具有1至12個碳原子的烷基; R 2及R 3係彼此獨立地選自氫、具有1至18個碳原子的烷基,或與彼等所連接的碳原子一起為5至13員環烷基環,或與彼等所連接的碳原子一起為式(II)-4的基團: 其中R 1係如上所定義;及 R 4及R 5係彼此獨立地選自氫、具有1至22個碳原子的烷基、氧基O*、-OH、-NO、-CH 2CN、苯甲基、烯丙基、具有1至30個碳原子的烷氧基、具有5至12個碳原子的環烷氧基、具有6至10個碳原子的芳氧基,其中該芳基可另外經取代、具有7至20個碳原子的芳烷氧基,其中該芳基可另外經取代、具有3至10個碳原子的烯基、具有3至6個碳原子的炔基、具有1至10個碳原子的醯基、鹵素、未經取代之苯基或經C 1-C 4-烷基取代之苯基。 The present invention relates to a stabilizer composition comprising: (A) at least one UV absorber as component A, wherein the UV absorber A comprises: (A1) one or more (2- Hydroxyphenyl)-symmetric-triazine compounds as component A1: wherein R a , R b , R c , R d , Re and R f are independently selected from hydrogen, an alkyl group having 1 to 20 carbon atoms, an alkyl group having 1 to 20 carbon atoms and 1 to 4 oxygen atom; and/or (A2) one or more oxanilide compounds according to formula (I.2) as component A2: wherein R a and R b are independently selected from hydrogen, having 1 to 20, preferably 2 to 12, alkyl groups of carbon atoms, having 1 to 20, preferably 2 to 12, carbon atoms and 1 to 4, preferably 1 to 2, alkoxy groups of oxygen atoms (oxygen atoms of alkoxy groups), and (B) at least one hindered amine light stabilizer as component B comprising at least one Oligomeric hindered amine light stabilizer B1 of formula (II)-1: Wherein n and m are 0 to 100 independently of each other, preferably 0 to 10, more preferably 0 to 5 figures, and its prerequisite is that n and m are not all 0; R 1 is hydrogen, has 5 to 7 A cycloalkyl group of carbon atoms, or an alkyl group having 1 to 12 carbon atoms; R 2 and R 3 are independently selected from hydrogen, an alkyl group having 1 to 18 carbon atoms, or an alkyl group connected to them The carbon atoms together are a 5 to 13 membered cycloalkyl ring, or together with the carbon atoms to which they are attached a group of formula (II)-4: wherein R 1 is as defined above; and R 4 and R 5 are independently selected from hydrogen, alkyl having 1 to 22 carbon atoms, oxy O*, -OH, -NO, -CH 2 CN, benzene Methyl, allyl, alkoxy having 1 to 30 carbon atoms, cycloalkoxy having 5 to 12 carbon atoms, aryloxy having 6 to 10 carbon atoms, wherein the aryl can be additionally Substituted, aralkyloxy groups having 7 to 20 carbon atoms, wherein the aryl group may be additionally substituted, alkenyl groups having 3 to 10 carbon atoms, alkynyl groups having 3 to 6 carbon atoms, alkynyl groups having 1 to 6 carbon atoms Acyl of 10 carbon atoms, halogen, unsubstituted phenyl or phenyl substituted by C 1 -C 4 -alkyl.
該穩定劑組成物可隨意地另包含以下組分C、D及/或E中之至少一者: (C) 至少一種酚類抗氧化劑作為組分C; (D) 至少一種液體稀釋劑作為組分D;及 (E) 至少一種其他添加物作為組分E。 The stabilizer composition may optionally further comprise at least one of the following components C, D and/or E: (C) at least one phenolic antioxidant as component C; (D) at least one liquid diluent as component D; and (E) At least one other additive as component E.
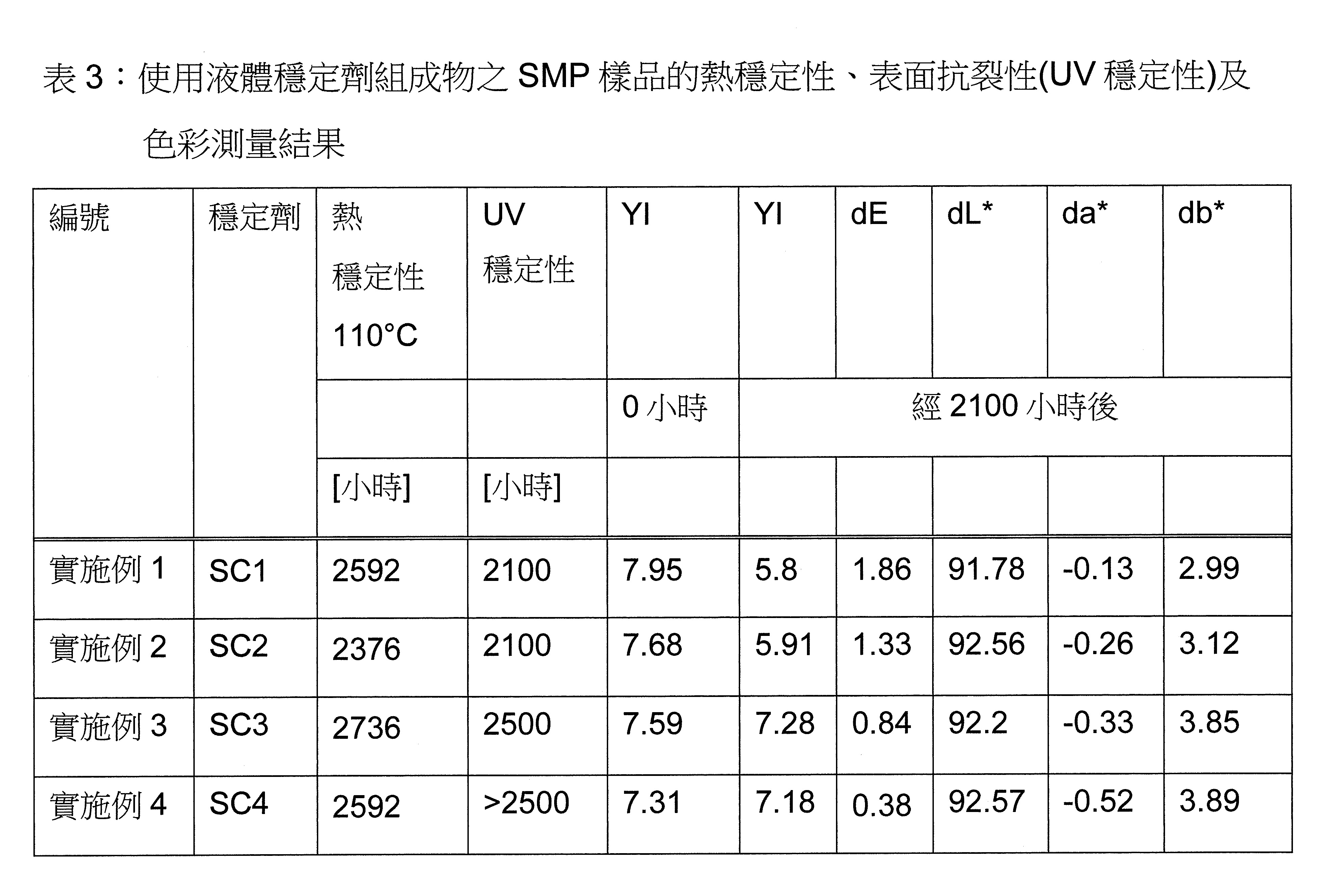
本發明之穩定劑組成物可有利地用於基於矽基改質聚合物之密封劑及黏合劑,如矽基改質聚醚。與標準穩定劑(例如Tinuvin® 326)相比,穩定劑組成物導致改善之UV及熱穩定性,並防止SMP密封劑之初始黃化。再者,本發明之穩定劑組成物在穩定化的SMP組成物熱處理後不會造成色彩受損(例如基於CIE色彩系統)。較佳地,穩定化的SMP組成物之機械性質相同或得到改善。The stabilizer composition of the present invention can be advantageously used in sealants and adhesives based on silicon-based modified polymers, such as silicon-based modified polyethers. The stabilizer composition results in improved UV and thermal stability compared to standard stabilizers (eg Tinuvin® 326) and prevents incipient yellowing of SMP encapsulants. Furthermore, the stabilizer composition of the present invention does not cause color damage (eg, based on the CIE color system) after heat treatment of the stabilized SMP composition. Preferably, the mechanical properties of the stabilized SMP composition are the same or improved.
較佳地,本發明之穩定劑組成物要求不得根據現行的歐盟化學物質分類、標籤和包裝法規(CLP)被歸類為危險性。特別是,該法規適用於歐洲議會和理事會2008年12月16日關於物質和混合物之分類、標籤及包裝的法規(EC)第1272/2008號、修訂和廢除指令67/548/EEC和1999/45/EC、及修訂法規(EC)第1907/2006號中所述者。Preferably, the stabilizer composition of the present invention is required not to be classified as hazardous according to the current European Union Regulation on Classification, Labeling and Packaging of Chemical Substances (CLP). In particular, this Regulation applies to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on the classification, labeling and packaging of substances and mixtures, amending and repealing Directive 67/548/EEC and 1999 /45/EC, and as amended by Regulation (EC) No 1907/2006.
本發明之穩定劑組成物較佳為實質上液體組成物的形式,如溶液、分散體、乳液、懸浮液或糊劑,較佳地溶液或懸浮液,特別是溶液。特別是,穩定劑組成物由於液體稀釋劑D而為實質上液體形式。此外,液體組分A、B、C及/或E中之一或多者可為液體形式。The stabilizer composition of the present invention is preferably in the form of a substantially liquid composition, such as a solution, dispersion, emulsion, suspension or paste, preferably a solution or a suspension, especially a solution. In particular, the stabilizer composition is in substantially liquid form due to the liquid diluent D. Furthermore, one or more of the liquid components A, B, C and/or E may be in liquid form.
為了本發明之目的,措辭“實質上液體”意指組成物以及主要液體部分可另包含一定部分的固體成分,實例為未溶解的成分A、B及隨意的C及/或E。For the purposes of the present invention, the expression "substantially liquid" means that the composition and the main liquid part may additionally contain a certain part of solid components, examples being undissolved components A, B and optionally C and/or E.
為了本發明之目的,措辭“液體”意指組成物至少於室溫下具有低流動性,較佳地至少於10℃至30℃的範圍內,更佳地至少於0℃至40℃的範圍內。For the purposes of the present invention, the expression "liquid" means that the composition has low fluidity at least at room temperature, preferably at least in the range of less than 10°C to 30°C, more preferably at least in the range of less than 0°C to 40°C Inside.
為了本發明之目的,措辭“烷基”包括線性直鏈或支鏈烷基。較佳為直鏈C 1-C 20烷基,特別是直鏈C 1-C 18烷基,較佳地直鏈C 1-C 12烷基及支鏈C 3-C 20烷基,特別是支鏈C 3-C 18烷基,較佳地支鏈C 3-C 12烷基。烷基之實例為,特別是,甲基、乙基、正丙基、異丙基、正丁基、異丁基、二級丁基、三級丁基、正戊基、異戊基、1-甲基丁基、三級戊基、新戊基、正己基、3-己基、2-甲基-1-戊基、3-甲基-1-戊基、4-甲基-1-戊基、2-甲基-2-戊基、3-甲基-2-戊基、4-甲基-2-戊基、2-甲基-3-戊基、3-甲基-3-戊基、2,2-二甲基-1-丁基、2,3-二甲基-1-丁基、3,3-二甲基-1-丁基、2-乙基-1-丁基、2,3-二甲基-2-丁基、3,3-二甲基-2-丁基、正庚基、正辛基、1-甲基庚基、2-乙基己基、2,4,4-三甲基-戊基、1,1,3,3-四甲基丁基、正壬基、正癸基、正十一烷基、正十二烷基、正十三烷基、正十四烷基、正十五烷基、正十六烷基、正十七烷基、正十八烷基及正二十烷基。 For the purposes of the present invention, the expression "alkyl" includes linear straight or branched chain alkyl groups. Preferably straight chain C 1 -C 20 alkyl, especially straight chain C 1 -C 18 alkyl, preferably straight chain C 1 -C 12 alkyl and branched C 3 -C 20 alkyl, especially Branched C 3 -C 18 alkyl, preferably branched C 3 -C 12 alkyl. Examples of alkyl groups are, in particular, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, secondary butyl, tertiary butyl, n-pentyl, isopentyl, 1 -Methylbutyl, tertiary pentyl, neopentyl, n-hexyl, 3-hexyl, 2-methyl-1-pentyl, 3-methyl-1-pentyl, 4-methyl-1-pentyl Base, 2-methyl-2-pentyl, 3-methyl-2-pentyl, 4-methyl-2-pentyl, 2-methyl-3-pentyl, 3-methyl-3-pentyl base, 2,2-dimethyl-1-butyl, 2,3-dimethyl-1-butyl, 3,3-dimethyl-1-butyl, 2-ethyl-1-butyl , 2,3-dimethyl-2-butyl, 3,3-dimethyl-2-butyl, n-heptyl, n-octyl, 1-methylheptyl, 2-ethylhexyl, 2, 4,4-Trimethyl-pentyl, 1,1,3,3-tetramethylbutyl, n-nonyl, n-decyl, n-undecyl, n-dodecyl, n-tridecyl , n-tetradecyl, n-pentadecyl, n-hexadecyl, n-heptadecyl, n-octadecyl and n-eicosyl.
為了本發明之目的,措辭“烷氧基”為經由氧原子結合之烷基,其中烷基之碳鏈可能被一或多個,較佳地1至3個不相鄰之雜原子-O-中斷。較佳為上述烷基。烷氧基之實例為甲氧基、乙氧基、正丙氧基、1-甲基乙氧基、丁氧基、1-甲基丙氧基、2甲基丙氧基、1,1-二甲基乙氧基、正戊氧基、1-甲基丁氧基、2-甲基丁氧基、3-甲基丁氧基、1,1-二甲基丙氧基、1,2-二甲基丙氧基、2,2-二甲基丙氧基、1-乙基丙氧基、己氧基、1-甲基戊氧基、2-甲基戊氧基、3-甲基戊氧基、4-甲基戊氧基、1,1-二甲基丁氧基、1,2-二甲基丁氧基、1,3-二甲基丁氧基、2,2二甲基丁氧基、2,3-二甲基丁氧基、3,3-二甲基丁氧基、1-乙基丁氧基、2-乙基丁氧基、1,1,2-三甲基丙氧基、1,2,2-三甲基丙氧基、1-乙基-1-甲基丙氧基或1-乙基-2-甲基丙氧基、己氧基、基團-(OC 2H 4) u-(OC 3H 6) v-H,其中u及v彼此獨立地為0至19之數字,較佳地0至12,先決條件為u+v為1至20,較佳地2至12。 For the purposes of the present invention, the expression "alkoxy" is an alkyl group bonded via an oxygen atom, wherein the carbon chain of the alkyl group may be interrupted by one or more, preferably 1 to 3 non-adjacent heteroatoms -O- interruption. Preferred are the above-mentioned alkyl groups. Examples of alkoxy are methoxy, ethoxy, n-propoxy, 1-methylethoxy, butoxy, 1-methylpropoxy, 2-methylpropoxy, 1,1- Dimethylethoxy, n-pentyloxy, 1-methylbutoxy, 2-methylbutoxy, 3-methylbutoxy, 1,1-dimethylpropoxy, 1,2 -Dimethylpropoxy, 2,2-dimethylpropoxy, 1-ethylpropoxy, hexyloxy, 1-methylpentyloxy, 2-methylpentyloxy, 3-methoxy pentyloxy, 4-methylpentyloxy, 1,1-dimethylbutoxy, 1,2-dimethylbutoxy, 1,3-dimethylbutoxy, 2,2 di Methylbutoxy, 2,3-dimethylbutoxy, 3,3-dimethylbutoxy, 1-ethylbutoxy, 2-ethylbutoxy, 1,1,2- Trimethylpropoxy, 1,2,2-trimethylpropoxy, 1-ethyl-1-methylpropoxy or 1-ethyl-2-methylpropoxy, hexyloxy, Group -(OC 2 H 4 ) u -(OC 3 H 6 ) v -H, wherein u and v are independently numbers from 0 to 19, preferably from 0 to 12, with the prerequisite that u+v is 1 to 20, preferably 2 to 12.
穩定劑組成物stabilizer composition
本發明之穩定劑組成物至少包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A,其中該UV吸收劑A包含(或由以下組成): (A1) 一或多種根據式(I.1)之(2-羥基苯基)-對稱-三嗪化合物作為組分A1: 其中 R a、R b、R c、R d、R e及R f係彼此獨立地選自氫、具有1至20個碳原子的烷基、具有1至20個碳原子和1至4個氧原子的烷氧基; 及/或 (A2) 一或多種根據式(I.2)之草醯苯胺化合物作為組分A2: 其中 R a及R b係彼此獨立地選自氫、具有1至20個碳原子的烷基、具有1至20個碳原子和1至4個氧原子的烷氧基, 及 (B) 至少一種受阻胺光穩定劑作為組分B,其包含(或由以下組成)至少一種根據式(II)-1的寡聚受阻胺光穩定劑B1: 其中 n及m彼此獨立地為0至100,較佳地0至10,更佳地0至5的數字,其先決條件為n和m不皆為0; R 1為氫、具有5至7個碳原子的環烷基、或具有1至12個碳原子的烷基; R 2及R 3係彼此獨立地選自氫、具有1至18個碳原子的烷基,或與彼等所連接的碳原子一起為5至13員環烷基環,或與彼等所連接的碳原子一起為式(II)-4的基團: 其中R 1係如上所定義;及 R 4及R 5係彼此獨立地選自氫、具有1至22個碳原子的烷基、氧基O*、-OH、-NO、-CH 2CN、苯甲基、烯丙基、具有1至30個碳原子的烷氧基、具有5至12個碳原子的環烷氧基、具有6至10個碳原子的芳氧基,其中該芳基可另外經取代、具有7至20個碳原子的芳烷氧基,其中該芳基可另外經取代、具有3至10個碳原子的烯基、具有3至6個碳原子的炔基、具有1至10個碳原子的醯基、鹵素、未經取代之苯基或經C 1-C 4-烷基取代之苯基。 The stabilizer composition of the present invention at least comprises (or consists of): (A) at least one UV absorber as component A, wherein the UV absorber A comprises (or consists of): (A1) one or more (2-Hydroxyphenyl)-symmetric-triazine compounds of the formula (I.1) as component A1: wherein R a , R b , R c , R d , Re and R f are independently selected from hydrogen, an alkyl group having 1 to 20 carbon atoms, an alkyl group having 1 to 20 carbon atoms and 1 to 4 oxygen atom; and/or (A2) one or more oxanilide compounds according to formula (I.2) as component A2: wherein R a and R b are independently selected from hydrogen, alkyl having 1 to 20 carbon atoms, alkoxy having 1 to 20 carbon atoms and 1 to 4 oxygen atoms, and (B) at least one A hindered amine light stabilizer as component B comprising (or consisting of) at least one oligomeric hindered amine light stabilizer B1 according to formula (II)-1: Wherein n and m are 0 to 100 independently of each other, preferably 0 to 10, more preferably 0 to 5 figures, and its prerequisite is that n and m are not all 0; R 1 is hydrogen, has 5 to 7 A cycloalkyl group of carbon atoms, or an alkyl group having 1 to 12 carbon atoms; R 2 and R 3 are independently selected from hydrogen, an alkyl group having 1 to 18 carbon atoms, or an alkyl group connected to them The carbon atoms together are a 5 to 13 membered cycloalkyl ring, or together with the carbon atoms to which they are attached a group of formula (II)-4: wherein R 1 is as defined above; and R 4 and R 5 are independently selected from hydrogen, alkyl having 1 to 22 carbon atoms, oxy O*, -OH, -NO, -CH 2 CN, benzene Methyl, allyl, alkoxy having 1 to 30 carbon atoms, cycloalkoxy having 5 to 12 carbon atoms, aryloxy having 6 to 10 carbon atoms, wherein the aryl can be additionally Substituted, aralkyloxy groups having 7 to 20 carbon atoms, wherein the aryl group may be additionally substituted, alkenyl groups having 3 to 10 carbon atoms, alkynyl groups having 3 to 6 carbon atoms, alkynyl groups having 1 to 6 carbon atoms Acyl of 10 carbon atoms, halogen, unsubstituted phenyl or C 1 -C 4 -alkyl substituted phenyl.
除UV吸收劑A及受阻胺光穩定劑B之外,該穩定劑組成物另包含以下組分C、D及/或E中之一或多者: (C) 至少一種酚類抗氧化劑作為組分C; (D) 至少一種液體稀釋劑作為組分D;及/或 (E) 至少一種其他添加物作為組分E。 In addition to the UV absorber A and the hindered amine light stabilizer B, the stabilizer composition further comprises one or more of the following components C, D and/or E: (C) at least one phenolic antioxidant as component C; (D) at least one liquid diluent as component D; and/or (E) At least one other additive as component E.
在本發明之一具體實例中,本發明之穩定劑組成物至少包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A,其中該UV吸收劑A包含(或由以下組成):一或多種根據式(I.1)之(2-羥基苯基)-對稱-三嗪化合物作為組分A1; 及 (B) 至少一種受阻胺光穩定劑作為組分B,其包含(或由以下組成):至少一種寡聚受阻胺光穩定劑B1。 In a specific example of the present invention, the stabilizer composition of the present invention at least includes (or consists of): (A) At least one UV absorber as component A, wherein the UV absorber A comprises (or consists of): one or more (2-hydroxyphenyl)-symmetric-triazines according to formula (I.1) Compound as component A1; and (B) At least one hindered amine light stabilizer as component B, which comprises (or consists of): at least one oligomeric hindered amine light stabilizer B1.
在本發明之替代具體實例中,本發明之穩定劑組成物至少包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A,其中該UV吸收劑A包含(或由以下組成):一或多種根據式(I.2)之草醯苯胺化合物作為組分A2; 及 (B) 至少一種受阻胺光穩定劑作為組分B,其包含(或由以下組成):至少一種寡聚受阻胺光穩定劑B1。 In an alternative embodiment of the present invention, the stabilizer composition of the present invention at least comprises (or consists of): (A) at least one UV absorber as component A, wherein the UV absorber A comprises (or consists of): one or more oxanilide compounds according to formula (I.2) as component A2; and (B) At least one hindered amine light stabilizer as component B, which comprises (or consists of): at least one oligomeric hindered amine light stabilizer B1.
在本發明之另一個替代具體實例中,本發明之穩定劑組成物至少包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A,其中該UV吸收劑A包含(或由以下組成): (A1) 一或多種根據式(I.1)之(2-羥基苯基)-對稱-三嗪化合物作為組分A1; 及 (A2) 一或多種根據式(I.2)之草醯苯胺化合物作為組分A2; 及 (B) 至少一種受阻胺光穩定劑作為組分B,其包含(或由以下組成):至少一種寡聚受阻胺光穩定劑B1。 In another alternative embodiment of the present invention, the stabilizer composition of the present invention at least includes (or consists of): (A) at least one UV absorber as component A, wherein the UV absorber A comprises (or consists of): (A1) one or more (2-hydroxyphenyl)-symmetric-triazine compounds according to formula (I.1) as component A1; and (A2) one or more oxanilide compounds according to formula (I.2) as component A2; and (B) At least one hindered amine light stabilizer as component B, which comprises (or consists of): at least one oligomeric hindered amine light stabilizer B1.
在本發明之較佳具體實例中,穩定劑組成物係採液體形式。這可藉由使用於室溫下,較佳地至少於10℃至30℃的範圍內,更佳地至少於0℃至40℃的範圍內為液體的UV吸附劑A及/或受阻胺光穩定劑B達成。In a preferred embodiment of the present invention, the stabilizer composition is in liquid form. This can be achieved by using UV absorber A and/or hindered amine light which are liquid at room temperature, preferably at least in the range of less than 10°C to 30°C, more preferably at least in the range of at least 0°C to 40°C. Stabilizer B is achieved.
在替代具體實例中,穩定劑組成物另包含至少一種液體稀釋劑D以提供液體形式之穩定劑組成物。液體稀釋劑D可選自合適之液體稀釋劑,如合適之溶劑或液體助劑或上述者的組合。因此,在本發明之一個具體實例中,穩定劑組成物包含(或由以下組成): (A) 至少一種UV吸收劑A; (B) 至少一種受阻胺光穩定劑B;及 (D) 至少一種液體稀釋劑作為組分D。 In an alternative embodiment, the stabilizer composition further comprises at least one liquid diluent D to provide the stabilizer composition in liquid form. The liquid diluent D can be selected from a suitable liquid diluent, such as a suitable solvent or a liquid adjuvant or a combination of the above. Therefore, in a specific example of the present invention, the stabilizer composition comprises (or consists of the following): (A) at least one UV absorber A; (B) at least one hindered amine light stabilizer B; and (D) At least one liquid diluent as component D.
在本發明之一個具體實例中,穩定劑組成物包含(或由以下組成): (A) 至少一種UV吸收劑A; (B) 至少一種受阻胺光穩定劑B;及 (C) 至少一種酚類抗氧化劑作為組分C。 In a specific example of the present invention, the stabilizer composition comprises (or consists of): (A) at least one UV absorber A; (B) at least one hindered amine light stabilizer B; and (C) At least one phenolic antioxidant as component C.
頃發現添加至少一種酚類抗氧化劑作為組分C進一步改善包含組分A、B及C之穩定劑組合的SMP組成物之熱穩定性及UV穩定性。It has been found that the addition of at least one phenolic antioxidant as component C further improves the thermal and UV stability of SMP compositions comprising stabilizer combinations of components A, B and C.
在本發明之另一個較佳具體實例中,穩定劑組成物包含(或由以下組成): (A) 至少一種UV吸收劑A; (B) 至少一種受阻胺光穩定劑B;及 (C) 至少一種酚類抗氧化劑作為組分C,其中該抗氧化劑C由一或多種根據式(III)a及/或(III)b之酚類化合物構成: 其中 R x及R y係彼此獨立地選自氫、鹵素或具有1至10個碳原子的烷基; R z係選自氫或具有1至10個碳原子的烷基; R w係選自羥基、具有1至18個碳原子的烷氧基、具有1至4個烷基碳原子的苯基烷氧基、具有5至8個碳原子的環烷氧基或式(III)c之基團 其中Z 1和Z 2係彼此獨立地選自氫、具有1至18個碳原子的烷基、苯基及具有5至8個碳原子的環烷基; X 係選自-O-(CH 2) q-O-;-(OCH 2-CH 2) q-O-; -O-(CH 2) q-S-(CH 2) q-O;-NH-(CH 2) q-NH-;-NH-NH-,其中q為1至12之整數,或以下基團: p 係0或1至6,較佳地1至4之整數。 In another preferred embodiment of the present invention, the stabilizer composition comprises (or consists of): (A) at least one UV absorber A; (B) at least one hindered amine light stabilizer B; and (C) At least one phenolic antioxidant as component C, wherein the antioxidant C consists of one or more phenolic compounds according to formula (III)a and/or (III)b: wherein R x and R y are independently selected from hydrogen, halogen or an alkyl group having 1 to 10 carbon atoms; R z is selected from hydrogen or an alkyl group having 1 to 10 carbon atoms; R w is selected from Hydroxy, alkoxy having 1 to 18 carbon atoms, phenylalkoxy having 1 to 4 alkyl carbon atoms, cycloalkoxy having 5 to 8 carbon atoms or a group of formula (III)c group wherein Z 1 and Z 2 are independently selected from hydrogen, alkyl having 1 to 18 carbon atoms, phenyl and cycloalkyl having 5 to 8 carbon atoms; X is selected from -O-(CH 2 ) q -O-; -(OCH 2 -CH 2 ) q -O-; -O-(CH 2 ) q -S-(CH 2 ) q -O; -NH-(CH 2 ) q -NH-; -NH-NH-, wherein q is an integer from 1 to 12, or the following groups: p is 0 or an integer of 1-6, preferably 1-4.
在本發明之另一個具體實例中,穩定劑組成物係採實質上液體的形式提供,其中組分A、B及/或隨意地C之至少一者係採液體的形式提供。In another embodiment of the present invention, the stabilizer composition is provided in a substantially liquid form, wherein at least one of components A, B and/or optionally C is provided in a liquid form.
在替代具體實例中,穩定劑組成物另包含至少一種液體稀釋劑D以提供液體形式之穩定劑組成物,該穩定劑組成物包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A; (B) 至少一種受阻胺光穩定劑作為組分B; (C) 至少一種酚類抗氧化劑作為組分C;及 (D) 至少一種液體稀釋劑作為組分D。 In an alternative embodiment, the stabilizer composition further comprises at least one liquid diluent D to provide a stabilizer composition in liquid form, the stabilizer composition comprising (or consisting of): (A) at least one UV absorber as component A; (B) at least one hindered amine light stabilizer as component B; (C) at least one phenolic antioxidant as component C; and (D) At least one liquid diluent as component D.
在本發明之另一個具體實例中,穩定劑組成物可另包含至少一種其他添加物作為組分E。因此本發明也關於一種穩定劑組成物,其包含: (A) 至少一種UV吸收劑作為組分A; (B) 至少一種受阻胺光穩定劑作為組分B;及 (E) 至少一種其他添加物作為組分E。 In another embodiment of the present invention, the stabilizer composition may further include at least one other additive as component E. Therefore the present invention also relates to a stabilizer composition comprising: (A) at least one UV absorber as component A; (B) at least one hindered amine light stabilizer as component B; and (E) At least one other additive as component E.
在本發明之一個具體實例中,穩定劑組成物包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A; (B) 至少一種受阻胺光穩定劑作為組分B; (C) 至少一種酚類抗氧化劑作為組分C;及 (E) 至少一種其他添加物作為組分E。 In a specific example of the present invention, the stabilizer composition comprises (or consists of): (A) at least one UV absorber as component A; (B) at least one hindered amine light stabilizer as component B; (C) at least one phenolic antioxidant as component C; and (E) At least one other additive as component E.
在本發明之另一個具體實例中,穩定劑組成物採實質上液體的形式提供,其包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A; (B) 至少一種受阻胺光穩定劑作為組分B; (C) 至少一種酚類抗氧化劑作為組分C;及 (E) 至少一種其他添加物作為組分E; 其中組分A、B、C及/或E之至少一者係採液體的形式提供。 In another embodiment of the present invention, the stabilizer composition is provided in a substantially liquid form, which comprises (or consists of): (A) at least one UV absorber as component A; (B) at least one hindered amine light stabilizer as component B; (C) at least one phenolic antioxidant as component C; and (E) at least one other additive as component E; Wherein at least one of components A, B, C and/or E is provided in a liquid form.
在本發明之替代具體實例中,穩定劑組成物採實質上液體的形式提供,其包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A; (B) 至少一種受阻胺光穩定劑作為組分B; (C) 至少一種酚類抗氧化劑作為組分C; (D) 至少一種液體稀釋劑作為組分D;及 (E) 至少一種其他添加物作為組分E; 其中至少組分D及隨意地組分A、B、C及/或E之至少一者係採液體的形式提供。 In an alternative embodiment of the invention, the stabilizer composition is provided in a substantially liquid form comprising (or consisting of): (A) at least one UV absorber as component A; (B) at least one hindered amine light stabilizer as component B; (C) at least one phenolic antioxidant as component C; (D) at least one liquid diluent as component D; and (E) at least one other additive as component E; Wherein at least component D and optionally at least one of components A, B, C and/or E are provided in liquid form.
在本發明之一個具體實例中,穩定劑組成物包含(或由以下組成): (A) 至少一種UV吸收劑作為組分A; (B) 至少一種受阻胺光穩定劑作為組分B; (C) 至少一種酚類抗氧化劑作為組分C; (D) 至少一種液體稀釋劑作為組分D;及 (E) 隨意地至少一種其他添加物作為組分E。 In a specific example of the present invention, the stabilizer composition comprises (or consists of): (A) at least one UV absorber as component A; (B) at least one hindered amine light stabilizer as component B; (C) at least one phenolic antioxidant as component C; (D) at least one liquid diluent as component D; and (E) optionally at least one other additive as component E.
在較佳具體實例中,穩定劑組成物包含(或由以下組成): (A) 以總穩定劑組成物為基準,20至60重量%,較佳地25至50重量%,更佳地30至45重量%的至少一種UV吸收劑A;及 (B) 以總穩定劑組成物為基準,40至80重量%,較佳地50至75重量%,更佳地55至70重量%的至少一種受阻胺光穩定劑B; 其中組分A及B的量總和為該穩定劑組成物的100重量%。 In a preferred embodiment, the stabilizer composition comprises (or consists of): (A) 20 to 60% by weight, preferably 25 to 50% by weight, more preferably 30 to 45% by weight of at least one UV absorber A, based on the total stabilizer composition; and (B) based on the total stabilizer composition, 40 to 80% by weight, preferably 50 to 75% by weight, more preferably 55 to 70% by weight of at least one hindered amine light stabilizer B; Wherein the sum of the amounts of components A and B is 100% by weight of the stabilizer composition.
在替代方案中,穩定劑組成物包含(或由以下組成): (A) 以總穩定劑組成物為基準,15至49重量%,較佳地20至44重量%,更佳地25至39重量%的至少一種UV吸收劑A; (B) 以總穩定劑組成物為基準,20至69重量%,較佳地30至64重量%,更佳地40至59重量%的至少一種受阻胺光穩定劑B;及 (C) 以總穩定劑組成物為基準,1至50重量%,較佳地1至30重量%,更佳地1至20重量%的至少一種酚類抗氧化劑C; 其中組分A、B及C的量總和為該穩定劑組成物的100重量%。 In the alternative, the stabilizer composition comprises (or consists of): (A) 15 to 49% by weight, preferably 20 to 44% by weight, more preferably 25 to 39% by weight of at least one UV absorber A, based on the total stabilizer composition; (B) 20 to 69% by weight, preferably 30 to 64% by weight, more preferably 40 to 59% by weight of at least one hindered amine light stabilizer B, based on the total stabilizer composition; and (C) based on the total stabilizer composition, 1 to 50% by weight, preferably 1 to 30% by weight, more preferably 1 to 20% by weight of at least one phenolic antioxidant C; Wherein the sum of the amounts of components A, B and C is 100% by weight of the stabilizer composition.
在替代方案中,穩定劑組成物包含(或由以下組成): (A) 以總穩定劑組成物為基準,5至49重量%,較佳地8至39重量%,更佳地10至29重量%的至少一種UV吸收劑A; (B) 以總穩定劑組成物為基準,10至59重量%,較佳地12至49重量%,更佳地15至39重量%的至少一種受阻胺光穩定劑B; (C) 以總穩定劑組成物為基準,1至50重量%,較佳地1至20重量%,更佳地1至15重量%的至少一種酚類抗氧化劑C; (D) 以總穩定劑組成物為基準,30至79重量%,較佳地35至69重量%,更佳地40至64重量%的至少一種液體稀釋劑D;及 (E) 以總穩定劑組成物為基準,0至50重量%,較佳地0至20重量%,更佳地0至10重量%的至少一種其他添加物E; 其中組分A、B、C、D及E的量總和為該穩定劑組成物的100重量%。 In the alternative, the stabilizer composition comprises (or consists of): (A) 5 to 49% by weight, preferably 8 to 39% by weight, more preferably 10 to 29% by weight of at least one UV absorber A, based on the total stabilizer composition; (B) based on the total stabilizer composition, 10 to 59% by weight, preferably 12 to 49% by weight, more preferably 15 to 39% by weight of at least one hindered amine light stabilizer B; (C) based on the total stabilizer composition, 1 to 50% by weight, preferably 1 to 20% by weight, more preferably 1 to 15% by weight of at least one phenolic antioxidant C; (D) 30 to 79% by weight, preferably 35 to 69% by weight, more preferably 40 to 64% by weight of at least one liquid diluent D, based on the total stabilizer composition; and (E) based on the total stabilizer composition, 0 to 50% by weight, preferably 0 to 20% by weight, more preferably 0 to 10% by weight of at least one other additive E; Wherein the sum of the amounts of components A, B, C, D and E is 100% by weight of the stabilizer composition.
在存有隨意之組分E的情況下可調整組分A之量,其中量之總和為或不超過100重量%。The amount of component A can be adjusted in the presence of optional components E, wherein the sum of the amounts is or does not exceed 100% by weight.
較佳地,UV吸附劑A及受阻胺光穩定劑B以在10:1至1:10,較佳地5:1至1:5,更佳地2:1至1:2,特別是1:1至1:2的範圍內之A:B重量比存在於本發明的穩定劑組成物中。Preferably, UV adsorbent A and hindered amine light stabilizer B are in a ratio of 10:1 to 1:10, preferably 5:1 to 1:5, more preferably 2:1 to 1:2, especially 1 A: B weight ratio within the range of 1 to 1:2 exists in the stabilizer composition of the present invention.
較佳地,UV吸收劑A及抗氧化劑C-若存有抗氧化劑C-以在10:1至1:5,較佳地8:1至1:1,更佳地5:1至1.5:1的範圍內之A:C重量比存在於本發明的穩定劑組成物中。Preferably, UV absorber A and antioxidant C - if present - are present in a ratio of 10:1 to 1:5, preferably 8:1 to 1:1, more preferably 5:1 to 1.5: The A:C weight ratio within the range of 1 exists in the stabilizer composition of the present invention.
在較佳具體實例中,穩定劑組成物包含(或較佳地由以下組成): (A) 以總穩定劑組成物為基準,8至39重量%,較佳地10至29重量%,更佳地10至20重量%的至少一種UV吸收劑A; (B) 以總穩定劑組成物為基準,12至49重量%,較佳地15至39重量%,更佳地20至35重量%的至少一種受阻胺光穩定劑B; (C) 以總穩定劑組成物為基準,1至20重量%,較佳地1至15重量%,更佳地2至10重量%的至少一種酚類抗氧化劑C;及 (D) 以總穩定劑組成物為基準,35至69重量%,較佳地40至64重量%,更佳地40至55重量%的至少一種液體稀釋劑D; 其中組分A、B、C及D的量總和為該穩定劑組成物的100重量%。 In a preferred embodiment, the stabilizer composition comprises (or preferably consists of): (A) 8 to 39% by weight, preferably 10 to 29% by weight, more preferably 10 to 20% by weight of at least one UV absorber A, based on the total stabilizer composition; (B) based on the total stabilizer composition, 12 to 49% by weight, preferably 15 to 39% by weight, more preferably 20 to 35% by weight of at least one hindered amine light stabilizer B; (C) 1 to 20% by weight, preferably 1 to 15% by weight, more preferably 2 to 10% by weight of at least one phenolic antioxidant C, based on the total stabilizer composition; and (D) 35 to 69% by weight, preferably 40 to 64% by weight, more preferably 40 to 55% by weight of at least one liquid diluent D, based on the total stabilizer composition; Wherein the sum of the amounts of components A, B, C and D is 100% by weight of the stabilizer composition.
在較佳具體實例中,本發明之穩定劑組成物包含(或較佳地由以下組成): (A) 以總穩定劑組成物為基準,10至29重量%,較佳地12至25重量%,更佳地13至20重量%的至少一種UV吸收劑A; (B) 以總穩定劑組成物為基準,15至39重量%,較佳地20至35重量%,更佳地22至33重量%的至少一種受阻胺光穩定劑B; (C) 以總穩定劑組成物為基準,1至15重量%,較佳地2至10重量%,更佳地3至9重量%的至少一種酚類抗氧化劑C; (D) 以總穩定劑組成物為基準,40至64重量%,較佳地40至55重量%,更佳地43至53重量%的至少一種液體稀釋劑D,其較佳地包含至少一種液體水分清除劑(liquid moisture scavenger)及/或至少一種溶劑; 其中組分A、B、C及D的量總和為該穩定劑組成物的100重量%。 In a preferred embodiment, the stabilizer composition of the present invention comprises (or preferably consists of): (A) 10 to 29% by weight, preferably 12 to 25% by weight, more preferably 13 to 20% by weight of at least one UV absorber A, based on the total stabilizer composition; (B) based on the total stabilizer composition, 15 to 39% by weight, preferably 20 to 35% by weight, more preferably 22 to 33% by weight of at least one hindered amine light stabilizer B; (C) 1 to 15% by weight, preferably 2 to 10% by weight, more preferably 3 to 9% by weight of at least one phenolic antioxidant C, based on the total stabilizer composition; (D) 40 to 64% by weight, preferably 40 to 55% by weight, more preferably 43 to 53% by weight, based on the total stabilizer composition, of at least one liquid diluent D, preferably comprising at least one a liquid moisture scavenger and/or at least one solvent; Wherein the sum of the amounts of components A, B, C and D is 100% by weight of the stabilizer composition.
在另一個較佳具體實例中,本發明之穩定劑組成物包含(或較佳地由以下組成): (A) 以總穩定劑組成物為基準,10至29重量%的至少一種UV吸收劑A; (B) 以總穩定劑組成物為基準,15至39重量%的至少一種受阻胺光穩定劑B; (C) 以總穩定劑組成物為基準,1至15重量%的至少一種酚類抗氧化劑C; (D) 以總穩定劑組成物為基準,40至64重量%的至少一種液體稀釋劑D; (E) 以總穩定劑組成物為基準,0.01至20重量%的至少一種其他添加物E; 其中組分A、B、C、D及E的量總和為該穩定劑組成物的100重量%。 UV吸收劑(組分A) In another preferred embodiment, the stabilizer composition of the present invention comprises (or preferably consists of): (A) 10 to 29% by weight of at least one UV absorber A, based on the total stabilizer composition; (B) 15 to 39% by weight of at least one hindered amine light stabilizer B, based on the total stabilizer composition; (C) 1 to 15% by weight of at least one phenolic antioxidant C, based on the total stabilizer composition; (D) 40 to 64% by weight of at least one liquid diluent D, based on the total stabilizer composition; (E) 0.01 to 20% by weight of at least one other additive E, based on the total stabilizer composition; Wherein the sum of the amounts of components A, B, C, D and E is 100% by weight of the stabilizer composition. UV absorber (component A)
本發明之穩定劑組成物包含UV吸收劑作為組分A,其中UV吸收劑係選自:一或多種草醯苯胺化合物,其係草醯苯胺(N,N’-二苯基草酸二醯胺,CAS-No. 620-81-5)或其衍生物;及/或一或多種(2-羥基苯基)-對稱-三嗪化合物。較佳地,UV吸收劑組分A不包含經羥基取代之草醯苯胺化合物。The stabilizer composition of the present invention comprises a UV absorber as component A, wherein the UV absorber is selected from: one or more oxalyl anilide compounds, which are oxalyl anilide (N,N'-diphenyl oxalic acid diamide , CAS-No. 620-81-5) or derivatives thereof; and/or one or more (2-hydroxyphenyl)-symmetry-triazine compounds. Preferably, the UV absorber component A does not contain hydroxy-substituted oxanilide compounds.
令人驚訝地發現,與習用穩定劑組成物,特別是包含苯并三唑型UV吸收劑之穩定劑組成物相比,UV吸收劑A與寡聚受阻胺光穩定劑B1之組合導致穩定器組合的改進,特別是在色彩方面。It was surprisingly found that the combination of UV absorber A and oligomeric hindered amine light stabilizer B1 results in a stabilizer Composition improvements, especially in color.
較佳地,以穩定劑組成物中之組分A和B的總量為基準,UV吸收劑A之存在量為20至60重量%,較佳地25至50重量%,更佳地30至45重量%。在較佳具體實例中,以穩定劑組成物中之組分A、B、C、D和E的總量為基準,UV吸附劑A之存在量為5至49重量%,較佳地8至39重量%,更佳地10至29重量%,也較佳地12至25重量%,特別是13至20重量%。 組分A1 Preferably, the UV absorber A is present in an amount of 20 to 60% by weight, preferably 25 to 50% by weight, more preferably 30 to 50% by weight, based on the total amount of components A and B in the stabilizer composition. 45% by weight. In a preferred embodiment, based on the total amount of components A, B, C, D and E in the stabilizer composition, the UV adsorbent A is present in an amount of 5 to 49% by weight, preferably 8 to 49% by weight. 39% by weight, more preferably 10 to 29% by weight, also preferably 12 to 25% by weight, especially 13 to 20% by weight. Component A1
在一個具體實例中,UV吸收劑A由一或多種根據式(I.1)之(2-羥基苯基)-對稱-三嗪化合物A1構成: 其中 R a、R b、R c、R d、R e及R f係彼此獨立地選自氫、具有1至20個碳原子的烷基、具有1至20個碳原子和1至4個氧原子的烷氧基。 In a specific example, the UV absorber A consists of one or more (2-hydroxyphenyl)-symmetric-triazine compounds A1 according to the formula (I.1): wherein R a , R b , R c , R d , Re and R f are independently selected from hydrogen, an alkyl group having 1 to 20 carbon atoms, an alkyl group having 1 to 20 carbon atoms and 1 to 4 oxygen Atom alkoxy.
取代基R a、R b、R c及/或R d較佳為位於各環上之間位。較佳地,各環上之至少一個取代基R a、R b、R c及/或R d不是氫。更佳地,R a、R b、R c及R d係位於各環上之間位且不是氫,即R a、R b、R c及R d係選自具有1至20個碳原子之烷基、具有1至20個碳原子及1至4個氧原子之烷氧基。 The substituents R a , R b , R c and/or R d are preferably located at intermediate positions on each ring. Preferably, at least one substituent R a , R b , R c and/or R d on each ring is not hydrogen. More preferably, R a , R b , R c and R d are located between each ring and are not hydrogen, that is, R a , R b , R c and R d are selected from the group having 1 to 20 carbon atoms Alkyl, alkoxy having 1 to 20 carbon atoms and 1 to 4 oxygen atoms.
在較佳之具體實例中,取代基R a、R b、R c及R d代表具有1至20個,較佳地1至12個,更佳地1至4個碳原子的烷基。更佳地,取代基R a、R b、R c及R d係選自甲基、乙基或丙基,更佳地甲基,並存在位於各環上的間位。 In a preferred embodiment, the substituents R a , R b , R c and R d represent an alkyl group having 1 to 20, preferably 1 to 12, more preferably 1 to 4 carbon atoms. More preferably, the substituents R a , R b , R c and R d are selected from methyl, ethyl or propyl, more preferably methyl, and there is a meta position on each ring.
取代基R e及/或R f較佳為於鄰位及/或對位。較佳地,環上之一個取代基R e或R f是氫。較佳地,環上之一個取代基R e或R f是氫且不是氫之取代基R e或R f係於環上之對位。 The substituents R e and/or R f are preferably at the ortho and/or para positions. Preferably, one of the substituents R e or R f on the ring is hydrogen. Preferably, one of the substituents Re or Rf on the ring is hydrogen and the substituent Re or Rf which is not hydrogen is in the para position on the ring.
在較佳之具體實例中,環上之一個取代基R e或R f是氫,且環上之一個取代基R e或R f係於對位並代表具有1至20個,較佳地5至18個,更佳地10至17個碳原子及1至4個,較佳地2至4個氧原子的烷氧基。更佳地環上之一個取代基R e或R f是氫,且在另一個環上之對位的一個取代基R e或R f是C 10-C 17烷氧基,更佳地下式(I.1)-a的取代基: 。 In a preferred embodiment, one substituent R e or R f on the ring is hydrogen, and one substituent R e or R f on the ring is at the para position and represents 1 to 20, preferably 5 to An alkoxy group of 18, more preferably 10 to 17 carbon atoms and 1 to 4, preferably 2 to 4 oxygen atoms. More preferably one substituent R e or R f on the ring is hydrogen, and a substituent R e or R f at the para-position on the other ring is C 10 -C 17 alkoxy, more preferably the following formula ( I.1) Substituents of-a: .
在另一個較佳具體實例中,UV吸收劑A包含或由一或多種根據式(I.1’)之(2-羥基苯基)-對稱-三嗪化合物A1組成: 其中 R a、R b、R c及R d係係如上所定義,且 R g係選自具有7至15個碳原子之烷基。 In another preferred embodiment, the UV absorber A comprises or consists of one or more (2-hydroxyphenyl)-symmetric-triazine compounds A1 according to the formula (I.1'): wherein R a , R b , R c and R d are as defined above, and R g is selected from alkyl groups having 7 to 15 carbon atoms.
較佳之UV吸收劑A包含或由一或多種選自下式(I.1)-1及(I.1)-2之化合物的(2‑羥基苯基)-對稱-三嗪化合物A1組成: Preferred UV absorbers A comprise or consist of one or more (2-hydroxyphenyl)-symmetric-triazine compounds A1 selected from compounds of the following formulas (I.1)-1 and (I.1)-2:
在本發明之較佳具體實例中,較佳之UV吸收劑A包含或由選自上式(I.1)-1及(I.1)-2之化合物的(2-羥基苯基)-對稱-三嗪化合物A1之混合物組成。較佳地,UV吸收劑A包含選自採化合物(I.1)-1對化合物(I.1)-2之比率約1:5至5:1,更佳地1:2至2:1,特別是1:1.2至1.2:1的化合物(I.1)-1和(I.1)-2之(2-羥基苯基)-對稱-三嗪化合物的混合物。 組分A2 In a preferred embodiment of the present invention, the preferred UV absorber A comprises or consists of (2-hydroxyphenyl)-symmetric - Composition of mixtures of triazine compounds A1. Preferably, the UV absorber A comprises compounds selected from the group consisting of compound (I.1)-1 to compound (I.1)-2 in a ratio of about 1:5 to 5:1, more preferably 1:2 to 2:1 , especially mixtures of (2-hydroxyphenyl)-symmetric-triazine compounds of compounds (I.1)-1 and (I.1)-2 from 1:1.2 to 1.2:1. Component A2
在替代具體實例中,穩定劑組成物包含UV吸收劑A,該UV吸收劑A包含或由一或多種根據式(I.2)之草醯苯胺化合物A2構成: 其中 R a及R b係彼此獨立地選自氫、具有1至20個,較佳地2至12個碳原子的烷基、具有1至20個,較佳地2至12個碳原子及1至4個,較佳地1至2個氧原子(即,烷氧基氧原子)的烷氧基。 In an alternative embodiment, the stabilizer composition comprises a UV absorber A comprising or consisting of one or more oxanilide compounds A2 according to formula (I.2): wherein R a and R b are independently selected from hydrogen, an alkyl group having 1 to 20, preferably 2 to 12 carbon atoms, an alkyl group having 1 to 20, preferably 2 to 12 carbon atoms and 1 An alkoxy group of up to 4, preferably 1 to 2 oxygen atoms (ie, alkoxy oxygen atoms).
取代基R a及/或R b較佳為於鄰位及/或對位。較佳地,各環上之一個取代基R a或R b不是氫。較佳地,各環上之一個取代基R a或R b是氫且不是氫之取代基R a或R b係位位於各環上之鄰位。 The substituents R a and/or R b are preferably at the ortho and/or para positions. Preferably, one of the substituents R a or R b on each ring is not hydrogen. Preferably, one substituent R a or R b on each ring is hydrogen and the substituent R a or R b that is not hydrogen is located in an ortho position on each ring.
在較佳之具體實例中,於一個環上之取代基R a或R b係具有1至20個,較佳地2至12個,更佳地2至4個碳原子的烷基,且於另一個環上之取代基R a或R b係具有1至20個,較佳地2至12個碳原子及1至4個,較佳地1至2個氧原子的烷氧基。更佳地,於一個環上之取代基R a或R b係甲基、乙基或丙基,更佳地乙基,且於另一個環上之取代基R a或R b係C 2-C 4烷氧基,更佳地-OCH 2CH 3。 In a preferred embodiment, the substituent R a or R b on one ring is an alkyl group having 1 to 20, preferably 2 to 12, more preferably 2 to 4 carbon atoms, and in another The substituent R a or R b on one ring is an alkoxy group having 1 to 20, preferably 2 to 12 carbon atoms and 1 to 4, preferably 1 to 2 oxygen atoms. More preferably, the substituent R a or R b on one ring is methyl, ethyl or propyl, more preferably ethyl, and the substituent R a or R b on the other ring is C 2 - C 4 alkoxy, more preferably -OCH 2 CH 3 .
較佳之UV吸收劑A包含或由一或多種選自下列式(I.2)-1至(I.2)-7的化合物之草醯苯胺化合物A2組成: Preferred UV absorbers A comprise or consist of one or more oxanilide compounds A2 selected from compounds of the following formulas (I.2)-1 to (I.2)-7:
在較佳之具體實例中,UV吸收劑A由上述草醯苯胺化合物其中之一者構成。In a preferred embodiment, the UV absorber A is composed of one of the above-mentioned oxanilide compounds.
較佳地,UV吸收劑A包含(或由以下組成)如上所述之根據式(I.2)-1的草醯苯胺化合物A2 (N-(2-乙氧基苯基)-N’-(2-乙基苯基)-乙二醯胺;2-乙基-2’-乙氧基-草醯苯胺,CAS-No. 23949-66-8)。Preferably, the UV absorber A comprises (or consists of) the oxanilide compound A2 (N-(2-ethoxyphenyl)-N'- (2-Ethylphenyl)-oxalamide; 2-ethyl-2'-ethoxy-oxanilide, CAS-No. 23949-66-8).
適合作為UV吸收劑組分A之組成成分的較佳草醯苯胺化合物係描述於US 5,969,014及/或US 6,916,867中。在另一個較佳具體實例中,UV吸收劑A由一或多種如上所定義之根據式(I.2)的(2-羥基苯基)-對稱-三嗪化合物A1、一或多種如上所定義之根據式(I.1)的草醯苯胺化合物A2、或包含一或多種如上所定義之根據式(I.1)的(2-羥基苯基)-對稱-三嗪化合物A1及一或多種如上所定義之根據式(I.2)的草醯苯胺化合物A2之混合物。較佳地,式(I.1)之化合物與式(I.2)之化合物於其混合物中的比率可介於約1:5至5:1,更佳地1:2至2:1。Preferred oxanilide compounds suitable as constituents of UV absorber component A are described in US 5,969,014 and/or US 6,916,867. In another preferred embodiment, the UV absorber A is composed of one or more (2-hydroxyphenyl)-symmetric-triazine compounds A1 according to formula (I.2) as defined above, one or more Oxanilide compounds A2 according to formula (I.1), or comprising one or more (2-hydroxyphenyl)-symmetric-triazine compounds A1 and one or more as defined above according to formula (I.1) Mixtures of oxanilide compounds A2 according to formula (I.2) as defined above. Preferably, the ratio of the compound of formula (I.1) to the compound of formula (I.2) in the mixture thereof may be about 1:5 to 5:1, more preferably 1:2 to 2:1.
在另一個較佳具體實例中,UV吸收劑A包含一或多種如上所定義之根據式(I.1)的(2-羥基苯基)-對稱-三嗪化合物A1,該化合物A1隨意地存於包含一或多種如上所定義之根據式(I.2)的草醯苯胺化合物A2之混合物中。較佳地,UV吸收劑A包含以總UV吸收劑A為基準計為至少30重量%,更佳地至少40重量%,特別是至少50重量%的一或多種如上所定義之根據式(I.1)的(2-羥基苯基)-對稱-三嗪化合物A1、及隨意地以總UV吸收劑A為基準計為至高70重量%,更佳地至高60重量%,特別是至高50重量%的一種或多種如上所定義之根據式(I.2)的草醯苯胺化合物A2。頃發現對於包含穩定劑組成物之矽基改質聚合物,在矽基改質聚合物(SMP)的UV穩定性(抗裂性)方面獲得了協同效應(synergistic effect),該穩定劑組成物包含如上所定義之(2-羥基苯基)-對稱-三嗪UV吸收劑A1、隨意地如上所定義之草醯苯胺UV吸收劑A2作為UV吸收劑A、及寡聚受阻胺光穩定劑(HALS) B1、隨意地酚類抗氧化劑C及隨意地液體稀釋劑D的組合,其中(2-羥基苯基)-對稱-三嗪UV吸收劑A1佔UV吸收劑A之總量的至少50重量%。In another preferred embodiment, the UV absorber A comprises one or more (2-hydroxyphenyl)-symmetric-triazine compounds A1 as defined above according to formula (I.1), optionally present In a mixture comprising one or more oxanilide compounds A2 according to formula (I.2) as defined above. Preferably, the UV absorber A comprises at least 30% by weight, more preferably at least 40% by weight, especially at least 50% by weight, based on the total UV absorber A, of one or more compounds according to formula (I) as defined above .1) of (2-hydroxyphenyl)-symmetric-triazine compounds A1, and optionally up to 70% by weight, more preferably up to 60% by weight, in particular up to 50% by weight, based on the total UV absorber A % of one or more oxanilide compounds A2 according to formula (I.2) as defined above. It has been found that a synergistic effect is obtained on the UV stability (crack resistance) of silicon-based modified polymers (SMP) for silicon-based modified polymers comprising a stabilizer composition Comprising (2-hydroxyphenyl)-symmetric-triazine UV absorber A1 as defined above, optionally oxanilide UV absorber A2 as defined above as UV absorber A, and oligomeric hindered amine light stabilizer ( A combination of HALS) B1, optionally a phenolic antioxidant C and optionally a liquid diluent D, wherein the (2-hydroxyphenyl)-symmetric-triazine UV absorber A1 accounts for at least 50% by weight of the total amount of UV absorber A %.
較佳地,UV吸收劑A採實質上液體之形式使用。UV吸收劑A本身可為液體,或可與液體稀釋劑D,較佳地至少一種惰性有機溶劑,組合使用。合適之溶劑係選自此領域常用的惰性極性或惰性非極性有機溶劑。該至少一種惰性有機溶劑構成根據本發明之液體稀釋劑D。對於本發明之目的,惰性溶劑係於室溫下,較佳地至少於10℃至30℃的範圍內,更佳地至少於0℃至40℃的範圍內,對任何組分A、B、C、D及/或E呈化學惰性之溶劑,即於室溫下,較佳地至少於10℃至30℃的範圍內,更佳地至少於0℃至40℃的範圍內,不會與任何組分A、B、C、D及/或E發生化學反應之溶劑。Preferably, UV absorber A is used in a substantially liquid form. The UV absorber A can be liquid by itself, or can be used in combination with a liquid diluent D, preferably at least one inert organic solvent. Suitable solvents are selected from inert polar or inert non-polar organic solvents commonly used in this field. The at least one inert organic solvent constitutes the liquid diluent D according to the invention. For the purposes of the present invention, an inert solvent is at room temperature, preferably at least less than 10°C to 30°C, more preferably at least less than 0°C to 40°C, for any components A, B, C, D and/or E are chemically inert solvents, that is, at room temperature, preferably at least within the range of 10°C to 30°C, more preferably at least within the range of 0°C to 40°C, will not interact with Solvent for any chemical reaction of components A, B, C, D and/or E.
在一個具體實例中,UV吸附劑A與至少一種惰性溶劑,特別是至少一種惰性極性有機溶劑組合應用。合適之極性溶劑包含醚、二醇醚、及特別是甲氧基丙醇。該至少一種惰性極性有機溶劑構成根據本發明之液體稀釋劑D。In a specific example, the UV adsorbent A is used in combination with at least one inert solvent, especially at least one inert polar organic solvent. Suitable polar solvents include ethers, glycol ethers, and especially methoxypropanol. The at least one inert polar organic solvent constitutes the liquid diluent D according to the invention.
在替代具體實例中,UV吸數劑A與,例如,至少一種惰性非極性有機溶劑,較佳地脂族烴或芳族烴,如石油醚、己烷、庚烷、石油餾分、甲苯、環己烷、均三甲苯或二甲苯組合使用。該至少一種惰性非極性有機溶劑構成根據本發明之液體稀釋劑D。In an alternative embodiment, the UV absorber A is mixed with, for example, at least one inert non-polar organic solvent, preferably an aliphatic or aromatic hydrocarbon, such as petroleum ether, hexane, heptane, petroleum fractions, toluene, cyclo Combination of hexane, mesitylene or xylene. The at least one inert non-polar organic solvent constitutes the liquid diluent D according to the invention.
合適之UV吸收劑A在市面上可自Clariant以Hostavin® 3206 LIQ及Hostavin® 3400 LIQ購得。 受阻胺光穩定劑(組分B) Suitable UV absorbers A are commercially available from Clariant as Hostavin® 3206 LIQ and Hostavin® 3400 LIQ. HALS (Component B)
令人驚訝地發現到UV吸收劑A與寡聚受阻胺光穩定劑B1之組合導致具有獨特的性質分佈,特別是在熱穩定性、抗UV/耐候性及色彩方面,之穩定劑組合。因此,本發明之穩定劑組成物至少包含含至少一種如上所定義之根據式(II)-1的寡聚受阻胺光穩定劑B1之受阻胺光穩定劑(HALS)作為組分B。It was surprisingly found that the combination of UV absorber A and oligomeric hindered amine light stabilizer B1 leads to a stabilizer combination with a unique property profile, especially in terms of thermal stability, UV/weather resistance and colour. Accordingly, the stabilizer composition of the present invention comprises at least as component B a hindered amine light stabilizer (HALS) comprising at least one oligomeric hindered amine light stabilizer B1 according to formula (II)-1 as defined above.
較佳地,受阻胺光穩定劑由一或多種包括根據式(II)之四甲基六氫吡啶基的化合物構成: 其中R係選自氫、具有1至20個,較佳地2至12個碳原子之烷基、具有1至20個,較佳地2至12個碳原子及1至4個,較佳地1至2個氧原子(即烷氧基氧原子)之烷氧基。 Preferably, the hindered amine light stabilizer consists of one or more compounds comprising a tetramethylhexahydropyridyl group according to formula (II): Wherein R is selected from hydrogen, alkyl having 1 to 20, preferably 2 to 12 carbon atoms, alkyl having 1 to 20, preferably 2 to 12 carbon atoms and 1 to 4, preferably An alkoxy group of 1 to 2 oxygen atoms (ie alkoxy oxygen atoms).
較佳地,用作活性B之受阻胺光穩定劑(HALS)不需要根據當前的歐盟法規分類、標籤和包裝化學物質(CLP)分類為危險性。Preferably, the hindered amine light stabilizers (HALS) used as active B do not need to be classified as hazardous according to the current European Union regulations on Classification, Labeling and Packaging Chemical Substances (CLP).
較佳地,受阻胺光穩定劑B之存在量以穩定劑組成物中的組分A和B之總量為基準計為40至80重量%,較佳地50至75重量%,更佳地55至70重量%。在較佳具體實例中,受阻胺光穩定劑B之存在量以穩定劑組成物中的組分A、B、C、D和E之總量為基準計為10至59重量%,較佳地12至49重量%,更佳地15至39重量%,也較佳地20至35重量%,特別是22至33重量%。Preferably, the hindered amine light stabilizer B is present in an amount of 40 to 80% by weight based on the total amount of components A and B in the stabilizer composition, preferably 50 to 75% by weight, more preferably 55 to 70% by weight. In a preferred embodiment, the hindered amine light stabilizer B is present in an amount of 10 to 59% by weight based on the total amount of components A, B, C, D and E in the stabilizer composition, preferably 12 to 49% by weight, more preferably 15 to 39% by weight, also preferably 20 to 35% by weight, especially 22 to 33% by weight.
較佳地,受阻胺光穩定劑(HALS)組分B包含至多100重量%之至少一種寡聚受阻胺光穩定劑B1,更佳地至多95重量%。除了寡聚受阻胺光穩定劑B1之外,阻分B可包含其他受阻胺光穩定劑。合適之受阻胺光穩定劑之實例為2-十二烷基-N-(2,2,6,6-四甲基-4-六氫吡啶基)丁二醯亞胺(CAS-No 79720-19-7);癸二酸雙(2,2,6,6-四甲基-4-六氫吡啶基酯) (CAS-No 52829-07-9);癸二酸雙(1,2,2,6,6-五甲基-4-六氫吡啶基酯) (CAS-No 41556-26-7);癸二酸雙(1-辛氧基-2,2,6,6-四甲基-4-六氫吡啶基酯) (CAS-No 129757-67-1);2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)-二螺環-(5.1.11.2)-二十一烷-21-酮(CAS-No. 64338-16-5)及其混合物。Preferably, the hindered amine light stabilizer (HALS) component B comprises at most 100% by weight of at least one oligomeric hindered amine light stabilizer B1, more preferably at most 95% by weight. In addition to the oligomeric hindered amine light stabilizer B1, block B may contain other hindered amine light stabilizers. An example of a suitable hindered amine light stabilizer is 2-dodecyl-N-(2,2,6,6-tetramethyl-4-hexahydropyridyl)succinimide (CAS-No 79720- 19-7); bis(2,2,6,6-tetramethyl-4-hexahydropyridyl) sebacate (CAS-No 52829-07-9); bis(1,2, 2,6,6-Pentamethyl-4-hexahydropyridyl ester) (CAS-No 41556-26-7); bis(1-octyloxy-2,2,6,6-tetramethyl sebacate yl-4-hexahydropyridyl ester) (CAS-No 129757-67-1); 2,2,4,4-tetramethyl-7-oxa-3,20-diazabispiro-20 -(2,3-Epoxy-propyl)-bispiro-(5.1.11.2)-eicodecan-21-one (CAS-No. 64338-16-5) and mixtures thereof.
合適之寡聚受阻胺光穩定劑B1的實例包括2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)二螺環-(5.1.11.2)-二十一烷-21-酮和環氧氯丙烷的反應產物(來自Clariant之Hostavin® N30)。Examples of suitable oligomeric hindered amine light stabilizers B1 include 2,2,4,4-tetramethyl-7-oxa-3,20-diazabispiro-20-(2,3-epoxy Reaction product of -propyl)bispiro-(5.1.11.2)-eicodecan-21-one and epichlorohydrin (Hostavin® N30 from Clariant).
受阻胺光穩定劑B包含至少一種如上所定義之根據式(II)-1的寡聚受阻胺光穩定劑B1。對於本發明之目的,寡聚受阻胺光穩定劑B1由至少2個重複單元,較佳地≥ 3個重複單元構成。較佳地,寡聚受阻胺光穩定劑B1由≤ 300個重複單元,更佳地≤ 202個重複單元,又更佳地≤ 22個重複單元,特別是≤ 12個重複單元構成。HALS B comprises at least one oligomeric hindered amine light stabilizer B1 according to formula (II)-1 as defined above. For the purposes of the present invention, the oligomeric hindered amine light stabilizer B1 consists of at least 2 repeating units, preferably ≧3 repeating units. Preferably, the oligomeric hindered amine light stabilizer B1 consists of ≤ 300 repeating units, more preferably ≤ 202 repeating units, more preferably ≤ 22 repeating units, especially ≤ 12 repeating units.
更佳地,受阻胺光穩定劑B由如下所定義之根據式(II)-1和(II)-2及隨意的(II)-3的噁二氮雜-螺癸烷化合物之混合物構成。More preferably, the hindered amine light stabilizer B consists of a mixture of oxadiaza-spirodecane compounds according to formulas (II)-1 and (II)-2 and optionally (II)-3 as defined below.
在較佳之具體實例中,受阻胺光穩定劑B由以組分B為基準計為65至95重量%,較佳地75至94重量%,更佳地85至95重量%的至少一種式(II)-1化合物、以組分B為基準計為5至35重量%,較佳地5至20重量%,更佳地5至12重量%的至少一種式(II)-2化合物及以組分B為基準計為0至10重量%,較佳地1至5重量%,更佳地1至3重量%的至少一種式(II)-3化合物構成: 其中 n及m彼此獨立地為0至100,較佳地0至10,更佳地0至5的數字,其先決條件為n和m不皆為0; R 1係氫、具有5至7個碳原子的環烷基、或具有1至12個碳原子的烷基; R 2及R 3係彼此獨立地選自氫、具有1至18個碳原子的烷基、或與彼等所連接的碳原子一起為5至13員環烷基環或與彼等所連接的碳原子一起為式(II)-4的基團: 其中R 1係如上所定義;及 R 4及R 5係彼此獨立地選自氫、具有1至22個碳原子的烷基、氧基O*、-OH、-NO、-CH 2CN、苯甲基、烯丙基、具有1至30個碳原子的烷氧基、具有5至12個碳原子的環烷氧基、具有6至10個碳原子的芳氧基,其中該芳基可另外經取代、具有7至20個碳原子的芳烷氧基,其中該芳基可另外經取代、具有3至10個碳原子的烯基、具有3至6個碳原子的炔基、具有1至10個碳原子的醯基、鹵素、未經取代之苯基或經C 1-C 4-烷基取代之苯基。 In a preferred embodiment, the hindered amine light stabilizer B is at least one formula ( II)-1 compound, 5 to 35% by weight, preferably 5 to 20% by weight, more preferably 5 to 12% by weight, based on component B, of at least one compound of formula (II)-2 and 0 to 10% by weight, preferably 1 to 5% by weight, more preferably 1 to 3% by weight of at least one compound of formula (II)-3 based on part B constitutes: Wherein n and m are independently 0 to 100, preferably 0 to 10, more preferably 0 to 5 numbers, the prerequisite for which is that n and m are not all 0; R 1 is hydrogen, with 5 to 7 A cycloalkyl group of carbon atoms, or an alkyl group with 1 to 12 carbon atoms; R 2 and R 3 are independently selected from hydrogen, an alkyl group with 1 to 18 carbon atoms, or an alkyl group connected to them The carbon atoms together are a 5 to 13 membered cycloalkyl ring or together with the carbon atoms to which they are attached a group of formula (II)-4: wherein R 1 is as defined above; and R 4 and R 5 are independently selected from hydrogen, alkyl having 1 to 22 carbon atoms, oxy O*, -OH, -NO, -CH 2 CN, benzene Methyl, allyl, alkoxy having 1 to 30 carbon atoms, cycloalkoxy having 5 to 12 carbon atoms, aryloxy having 6 to 10 carbon atoms, wherein the aryl can be additionally Substituted, aralkyloxy groups having 7 to 20 carbon atoms, wherein the aryl group may be additionally substituted, alkenyl groups having 3 to 10 carbon atoms, alkynyl groups having 3 to 6 carbon atoms, alkynyl groups having 1 to 6 carbon atoms Acyl of 10 carbon atoms, halogen, unsubstituted phenyl or C 1 -C 4 -alkyl substituted phenyl.
較佳地,本發明之穩定劑組成物包含如上所示之受阻胺光穩定劑B,該受阻胺光穩定劑B由以組分B為基準計為65至95重量%,較佳地75至94重量%,更佳地85至95重量%的至少一種式(II)-1化合物、以組分B為基準計為5至35重量%,較佳地5至20重量%,更佳地5至12重量%的至少一種式(II)-2化合物及以組分B為基準計為0至10重量%,較佳地1至5重量%,更佳地1至3重量%的至少一種式(II)-3化合物構成, 其中 n及m彼此獨立地為0至100,較佳地0至10,更佳地0至5的數字,其先決條件為n和m不皆為0; R 1係氫、具有5至7個,較佳地6個碳原子的環烷基、或具有1至12個,較佳地1至6個碳原子的烷基; R 2及R 3係彼此獨立地選自氫、具有1至18個碳原子的烷基、或與彼等所連接的碳原子一起為5至13員環烷基環或與彼等所連接的碳原子一起為如上所定義之式(II)-4的基團;及 R 4及R 5係彼此獨立地選自氫、具有1至22個碳原子的烷基、氧基O*、-OH、-NO、-CH 2CN、苯甲基、烯丙基、具有1至30個碳原子的烷氧基、具有5至12個碳原子的環烷氧基、具有6至10個碳原子的芳氧基,其中該芳基可另外經取代、具有7至20個碳原子的芳烷氧基,其中該芳基可另外經取代、具有3至10個碳原子的烯基、具有3至6個碳原子的炔基、具有1至10個碳原子的醯基、鹵素、未經取代之苯基或經C 1-C 4-烷基取代之苯基。 Preferably, the stabilizer composition of the present invention comprises the hindered amine light stabilizer B shown above, and the hindered amine light stabilizer B is calculated from 65 to 95% by weight based on component B, preferably 75 to 95% by weight. 94% by weight, preferably 85 to 95% by weight of at least one compound of formula (II)-1, based on component B, 5 to 35% by weight, preferably 5 to 20% by weight, more preferably 5% by weight to 12% by weight of at least one compound of formula (II)-2 and 0 to 10% by weight, preferably 1 to 5% by weight, more preferably 1 to 3% by weight of at least one compound of formula (II)-2 based on component B (II)-3 compounds constitute, wherein n and m are independently 0 to 100, preferably 0 to 10, more preferably 0 to 5 numbers, and the prerequisite is that n and m are not all 0; R 1 is hydrogen, cycloalkyl having 5 to 7, preferably 6 carbon atoms, or alkyl having 1 to 12, preferably 1 to 6 carbon atoms; R 2 and R 3 are independently of each other selected from hydrogen, alkyl groups having 1 to 18 carbon atoms, or together with the carbon atoms to which they are attached 5 to 13 membered cycloalkyl rings or together with the carbon atoms to which they are attached are of the formula as defined above (II) a group of -4; and R 4 and R 5 are independently selected from hydrogen, an alkyl group having 1 to 22 carbon atoms, an oxygen group O*, -OH, -NO, -CH 2 CN, Benzyl, allyl, alkoxy having 1 to 30 carbon atoms, cycloalkoxy having 5 to 12 carbon atoms, aryloxy having 6 to 10 carbon atoms, wherein the aryl can Additionally substituted, aralkoxy having 7 to 20 carbon atoms, wherein the aryl group may be additionally substituted, alkenyl having 3 to 10 carbon atoms, alkynyl having 3 to 6 carbon atoms, having 1 Acyl group of up to 10 carbon atoms, halogen, unsubstituted phenyl or phenyl substituted by C 1 -C 4 -alkyl.
穩定劑組分B之較佳具體實例為根據式(II)-1、(II)-2及隨意地(II)-3的化合物之混合物,例如US 6,174,940及/或US 2005/0228086中描述的。式(II)-1至(II)-4之取代基R 1至R 5及下標n和m是指US 6,174,940中定義之相應取代基及下標。 Preferred embodiments of stabilizer component B are mixtures of compounds according to formula (II)-1, (II)-2 and optionally (II)-3, such as described in US 6,174,940 and/or US 2005/0228086 . The substituents R 1 to R 5 and subscripts n and m of formulas (II)-1 to (II)-4 refer to the corresponding substituents and subscripts defined in US 6,174,940.
較佳地,HALS組分B由根據式(II)-1及(II)-2及隨意地(II)-3的化合物構成,其中取代基R 1至R 5具有相同定義。 Preferably, HALS component B consists of compounds according to formulas (II)-1 and (II)-2 and optionally (II)-3, wherein the substituents R 1 to R 5 have the same definition.
較佳地,HALS組分B由根據式(II)-1及(II)-2及隨意地(II)-3的化合物構成, 其中 n及m彼此獨立地為0至10,更佳地0至5的數字,其先決條件為n和m不皆為0; R 1為氫、具有6個碳原子的環烷基、或具有1至4個碳原子的烷基; R 2及R 3係彼此獨立地選自氫、具有1至6個碳原子的烷基、或與彼等所連接的碳原子一起為6至12員環烷基環、或與彼等所連接的碳原子一起為式(II)-4的基團;及 R 4及R 5係彼此獨立地選自氫、具有1至5個碳原子的烷基、氧基O*、-OH、-NO、-CH 2CN、苯甲基、烯丙基、具有1至10個碳原子的烷氧基、具有5至6個碳原子的環烷氧基、具有6至7個碳原子的芳氧基,其中該芳基可另外經取代、具有7至10個碳原子的芳烷氧基,其中該芳基可另外經取代、具有3至6個碳原子的烯基、具有3至6個碳原子的炔基、具有1至4個碳原子的醯基、鹵素、未經取代之苯基或經C 1-C 2-烷基取代之苯基。 Preferably, HALS component B consists of compounds according to formulas (II)-1 and (II)-2 and optionally (II)-3, wherein n and m are independently of each other from 0 to 10, more preferably 0 The number to 5, its prerequisite is that n and m are not all 0; R 1 is hydrogen, a cycloalkyl group with 6 carbon atoms, or an alkyl group with 1 to 4 carbon atoms; R 2 and R 3 are each independently selected from hydrogen, an alkyl group having 1 to 6 carbon atoms, or a 6 to 12 membered cycloalkyl ring together with the carbon atoms to which they are attached, or together with the carbon atoms to which they are attached is of the formula (II) a group of -4; and R 4 and R 5 are independently selected from hydrogen, an alkyl group having 1 to 5 carbon atoms, an oxygen group O*, -OH, -NO, -CH 2 CN, Benzyl, allyl, alkoxy having 1 to 10 carbon atoms, cycloalkoxy having 5 to 6 carbon atoms, aryloxy having 6 to 7 carbon atoms, wherein the aryl can Additionally substituted, aralkoxy having 7 to 10 carbon atoms, wherein the aryl group may be additionally substituted, alkenyl having 3 to 6 carbon atoms, alkynyl having 3 to 6 carbon atoms, having 1 Acyl of up to 4 carbon atoms, halogen, unsubstituted phenyl or C 1 -C 2 -alkyl substituted phenyl.
更佳地,HALS組分B由根據式(II)-1及(II)-2及隨意地(II)-3的化合物構成, 其中 n及m彼此獨立地為0至5的數字,其先決條件為n和m不皆為0; R 1為具有1至4個碳原子的烷基,更佳地甲基; R 2及R 3與彼等所連接的碳原子一起為6至12員環烷基環,較佳地12員環烷基環,或與彼等所連接的碳原子一起為式(II)-4的基團; R 4及R 5係彼此獨立地選自氫、具有1至4個碳原子的烷基、具有1至6個碳原子的烷氧基、具有5至6個碳原子的環烷氧基及具有1至4個碳原子的醯基。 More preferably, HALS component B consists of compounds according to formulas (II)-1 and (II)-2 and optionally (II)-3, wherein n and m are independently of each other a number from 0 to 5, which presupposes with the proviso that n and m are not both 0; R is an alkyl group having 1 to 4 carbon atoms, more preferably methyl; R and R together with the carbon atoms to which they are attached are a 6 to 12 membered ring An alkyl ring, preferably a 12-membered cycloalkyl ring, or a group of formula (II)-4 together with the carbon atoms to which they are attached; R 4 and R 5 are independently selected from hydrogen, having 1 An alkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 6 carbon atoms, a cycloalkoxy group having 5 to 6 carbon atoms, and an acyl group having 1 to 4 carbon atoms.
較佳地,式(II)-1至(II)-4中之取代基R 1彼此獨立地為氫或具有1至4個碳原子的烷基,更佳地所有取代基R 1皆為甲基。 Preferably, the substituents R 1 in formulas (II)-1 to (II)-4 are independently hydrogen or an alkyl group having 1 to 4 carbon atoms, more preferably all substituents R 1 are methyl base.
較佳地,式(II)-1至(II)-3中之取代基R 2及R 3與彼等所連接的碳原子一起為12員環烷基環。 Preferably, the substituents R 2 and R 3 in formulas (II)-1 to (II)-3 together with the carbon atoms to which they are attached are 12-membered cycloalkyl rings.
較佳地,式(II)-1至(II)-4中之取代基R 4及R 5係彼此獨立地選自氫、甲基、乙醯基、辛氧基或環己氧基。 Preferably, the substituents R 4 and R 5 in formulas (II)-1 to (II)-4 are independently selected from hydrogen, methyl, acetyl, octyloxy or cyclohexyloxy.
在較佳之具體實例中,HALS組分B由根據式(II)-1及(II)-2及隨意地(II)-3的化合物構成, 其中 n及m彼此獨立地為0至10,更佳地0至5的數字,其先決條件為n和m不皆為0; R 1為甲基; R 2及R 3與彼等所連接的碳原子一起為12員環烷基環,及 R 4為氫。 In a preferred embodiment, HALS component B consists of compounds according to formulas (II)-1 and (II)-2 and optionally (II)-3, wherein n and m are independently of each other from 0 to 10, more Preferably a number from 0 to 5, provided that n and m are not both 0; R 1 is methyl; R 2 and R 3 together with the carbon atoms to which they are attached are a 12-membered cycloalkyl ring, and R 4 is hydrogen.
在更佳之具體實例中,受阻胺光穩定劑B由以組分B為基準計為85至95重量%的至少一種式(II)-1之化合物、以組分B為基準計為5至12重量%的至少一種式(II)-2之化合物及以組分B為基準計為1至3重量%的至少一種式(II)-3之化合物構成, 其中 n及m彼此獨立地為0至10,更佳地0至5的數字,其先決條件為n和m不皆為0; R 1為甲基; R 2及R 3與彼等所連接的碳原子一起為12員環烷基環,及 R 4為氫。 In a more preferred embodiment, the hindered amine light stabilizer B consists of 85 to 95% by weight of at least one compound of formula (II)-1 based on component B, and 5 to 12% based on component B. % by weight of at least one compound of formula (II)-2 and 1 to 3% by weight of at least one compound of formula (II)-3 based on component B, wherein n and m are independently of each other from 0 to 10, more preferably a number from 0 to 5, provided that n and m are not both 0; R 1 is methyl; R 2 and R 3 together with the carbon atoms to which they are attached are 12-membered cycloalkyl rings , and R 4 is hydrogen.
典型地,寡聚HALS組分B1具有大於540 g/mol,較佳地大於1,000 g/mol,特別是大於1,500 g/mol之(重量平均)分子量。較佳地,寡聚HALS組分B1之(重量平均)分子量為≤ 20.000 g/mol,較佳地≤ 18.000 g/mol,更佳地≤ 17.000 g/mol。寡聚HALS組分B1之(重量平均)分子量藉由凝膠滲透層析法(GPC)使用四氫呋喃和乙醇胺之混合物作為溶劑及分子量為17.670 g/mol之聚苯乙烯作為標準物測定。Typically, the oligomeric HALS component B1 has a (weight average) molecular weight of greater than 540 g/mol, preferably greater than 1,000 g/mol, especially greater than 1,500 g/mol. Preferably, the (weight average) molecular weight of the oligomeric HALS component B1 is ≤ 20.000 g/mol, preferably ≤ 18.000 g/mol, more preferably ≤ 17.000 g/mol. The (weight average) molecular weight of the oligomeric HALS fraction B1 was determined by gel permeation chromatography (GPC) using a mixture of tetrahydrofuran and ethanolamine as solvent and polystyrene with a molecular weight of 17.670 g/mol as standard.
穩定劑組分B為如上所定義之根據式(II)-1及(II)-2及隨意地(II)-3的噁二氮雜螺癸烷化合物之混合物,該穩定劑組分B較佳為藉由使聚烷基-1-噁二氮雜螺癸烷與表鹵代醇(epihalogenohydrin),特別是表氯醇,反應獲得。合適之穩定劑B的製法係例如描述於US4,340,534及/或US2005/228086中。Stabilizer component B is a mixture of oxadiazaspirodecane compounds according to formulas (II)-1 and (II)-2 and optionally (II)-3 as defined above, which stabilizer component B is It is preferably obtained by reacting polyalkyl-1-oxadiazaspirodecane with epihalogenohydrin, especially epichlorohydrin. The preparation of suitable stabilizers B is described, for example, in US 4,340,534 and/or US 2005/228086.
特別是,根據(II)1、(II)-2及(II)-3之噁二氮雜-螺癸烷化合物的混合物係藉由使通式(II)-5之化合物與式(II)-6之表鹵代醇反應獲得: 其中基團R 1、R 2、R 3及R 4係如上所定義,且R 6為主族(V)、(VI)或(VII)元素之質子酸的陰離子,特別是氯陰離子; 其中Hal是鹵素,被理解為意指氯、溴或碘原子,較佳地氯。 In particular, the mixture of oxadiaza-spirodecane compounds according to (II)1, (II)-2 and (II)-3 is obtained by combining a compound of general formula (II)-5 with formula (II) The epihalohydrin reaction of -6 is obtained: wherein the groups R 1 , R 2 , R 3 and R 4 are as defined above, and R 6 is an anion of a protonic acid of an element of group (V), (VI) or (VII), especially a chloride anion; Where Hal is halogen, it is understood to mean chlorine, bromine or iodine atom, preferably chlorine.
典型地,於第一步中構建式(II)-3之化合物,其後加熱反應混合物導致形成式(II)-1及(II)-2之化合物。較佳地,化合物(II)-5及(II)-6以1:1至1:2.9之莫耳比反應;較佳地1:1至1:2.7,特別是1:2至1:2.5。特別是,反應於惰性有機溶劑中在鹼金屬氫氧化物之莫耳量相對於式(II)-5化合物之莫耳量為4至20倍存在的情況下進行。典型地,反應溫度位於20至220℃,較佳地40至120℃,特別是60至90℃之範圍內。Typically, compounds of formula (II)-3 are constructed in a first step, followed by heating of the reaction mixture resulting in the formation of compounds of formula (II)-1 and (II)-2. Preferably, compounds (II)-5 and (II)-6 react at a molar ratio of 1:1 to 1:2.9; preferably 1:1 to 1:2.7, especially 1:2 to 1:2.5 . In particular, the reaction is carried out in an inert organic solvent in the presence of 4 to 20 times the molar amount of alkali metal hydroxide relative to the molar amount of the compound of formula (II)-5. Typically, the reaction temperature is in the range of 20 to 220°C, preferably 40 to 120°C, especially 60 to 90°C.
較佳之惰性有機溶劑係脂族或芳族烴,例如石油醚、己烷、庚烷、石油餾分、甲苯、環己烷、均三甲苯或二甲苯。特佳為芳烴,尤其是二甲苯。相對於化合物(II)-5,惰性有機溶劑較佳地以2:1至1:5,更佳地2:1至1:3,特別是2:1至1:2之重量比使用。Preferred inert organic solvents are aliphatic or aromatic hydrocarbons, such as petroleum ether, hexane, heptane, petroleum fractions, toluene, cyclohexane, mesitylene or xylene. Particularly preferred are aromatic hydrocarbons, especially xylene. Relative to compound (II)-5, the inert organic solvent is preferably used in a weight ratio of 2:1 to 1:5, more preferably 2:1 to 1:3, especially 2:1 to 1:2.
典型地,在反應中使用相轉移觸媒(phase transfer catalyst)。例如,所使用之相轉移觸媒(phase transfer catalyst)係相對於式(II)-5之化合物的量,定量比例為1.5至10重量%,較佳地3至7重量%,特別是4至6重量%的聚乙二醇,較佳地具有一定平均寡聚合度之聚乙二醇,特別是聚乙二醇200。例如,所使用之相轉移觸媒可為季銨鹵化物,如氯化三辛基甲基銨,特別是相對於化合物(II)-5之定量比例為0.1至5重量%。Typically, a phase transfer catalyst is used in the reaction. For example, the used phase transfer catalyst (phase transfer catalyst) is relative to the amount of the compound of formula (II)-5, the quantitative ratio is 1.5 to 10% by weight, preferably 3 to 7% by weight, especially 4 to 6% by weight of polyethylene glycol, preferably polyethylene glycol with a certain average degree of oligomerization, especially polyethylene glycol 200. For example, the phase transfer catalyst used may be a quaternary ammonium halide, such as trioctylmethylammonium chloride, especially in a quantitative proportion of 0.1 to 5% by weight relative to compound (II)-5.
化合物(II)-5及(II)-6之反應一般在30至60分鐘後結束。緊接著該反應之後,自反應混合物中除去過量之表鹵代醇,較佳地藉由蒸餾除去。分離有機相及水相;有機相用水清洗並除去惰性有機溶劑,較佳地藉由蒸餾除去。所獲得之混合物可在無需進一步純化步驟的情況下,藉由於100至240℃,較佳地於120至220℃,特別是於150至200℃下,較佳地在減壓之下加熱轉化為所需的式(II)-1、(II)-2及(II)-3之混合物。The reaction of compounds (II)-5 and (II)-6 is generally completed after 30 to 60 minutes. Immediately after the reaction, excess epihalohydrin is removed from the reaction mixture, preferably by distillation. The organic and aqueous phases are separated; the organic phase is washed with water and freed of the inert organic solvent, preferably by distillation. The mixture obtained can be converted, without further purification steps, by heating at 100 to 240° C., preferably at 120 to 220° C., especially at 150 to 200° C., preferably under reduced pressure, into Desired mixtures of formulas (II)-1, (II)-2 and (II)-3.
藉由改變式(II)-5之化合物的用量、表鹵代醇(II)-6的量、鹼金屬氫氧化物的用量及相轉移觸媒的用量,可調整組分(II)-1、(II)-2及(II)-3之混合物的組成。組分(II)-1、(II)-2及(II)-3之混合物的組成可藉由習用光譜法(IR及 13C-NMR光譜術)顯示。 By changing the amount of compound of formula (II)-5, the amount of epihalohydrin (II)-6, the amount of alkali metal hydroxide and the amount of phase transfer catalyst, component (II)-1 can be adjusted , (II)-2 and the composition of the mixture of (II)-3. The composition of the mixture of components (II)-1, (II)-2 and (II)-3 can be revealed by customary spectroscopic methods (IR and 13 C-NMR spectroscopy).
在特佳之具體實例中,HALS組分B包含(或較佳地由以下組成:) 2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)雙螺環-(5.1.11.2)-二十一烷-21-酮及環氧氯丙烷的反應產物。前述反應產物典型地如上所述獲得。較佳地,前述反應產物係藉由使2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)二螺環-(5.1.11.2)-二十一烷-21-酮與環氧氯丙烷以1:2至1:2.5之莫耳比在鹼金屬氫氧化物之莫耳量相對於2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)二螺環-(5.1.11.2)-二十一烷-21-酮之莫耳量為4至20倍存在的情況下,於惰性有機溶劑中使用相轉移觸媒反應獲得。 酚類抗氧化劑(組分C) In a particularly preferred embodiment, HALS component B comprises (or preferably consists of:) 2,2,4,4-tetramethyl-7-oxa-3,20-diazabispiro- The reaction product of 20-(2,3-epoxy-propyl)bispiro-(5.1.11.2)-21-21-one and epichlorohydrin. The aforementioned reaction products are typically obtained as described above. Preferably, the aforementioned reaction product is prepared by making 2,2,4,4-tetramethyl-7-oxa-3,20-diazabispiro-20-(2,3-epoxy-propane Base) dispiro-(5.1.11.2)-21-21-one and epichlorohydrin at a molar ratio of 1:2 to 1:2.5 in the molar amount of alkali metal hydroxide relative to 2 ,2,4,4-Tetramethyl-7-oxa-3,20-diazabispiro-20-(2,3-epoxy-propyl)bispiro-(5.1.11.2)- Hecodecan-21-one is obtained by using a phase transfer catalyst reaction in an inert organic solvent in the presence of 4 to 20 times the molar amount. Phenolic Antioxidants (Component C)
令人驚訝地發現到添加酚類抗氧化劑與UV吸收劑A和受阻胺光穩定劑B之組合導致穩定劑組合的進一步協同改善作用,特別是以熱穩定性、UV/耐候性及色彩之觀點來看。It was surprisingly found that the addition of phenolic antioxidants in combination with UV absorber A and HALS B leads to a further synergistic improvement of the stabilizer combination, especially from the standpoint of thermal stability, UV/weather resistance and color come and see.
較佳地,抗氧化劑C之存在量以包含組分A、B及C之總穩定劑組成物為基準計為1至50重量%,較佳地1至30重量%,更佳地1至25重量%,也較佳地2至17重量%,特別是5至16重量%。若穩定劑組成物中存有隨意的組分D及E,抗氧化劑C之存在量較佳地以包含組分A、B、C、D及E之總穩定劑組成物為基準計為1至50重量%,較佳地1至20重量%,更佳地1至15重量%,也較佳地2至10重量%,特別是3至9重量%。Preferably, antioxidant C is present in an amount of 1 to 50% by weight, preferably 1 to 30% by weight, more preferably 1 to 25% by weight, based on the total stabilizer composition comprising components A, B and C % by weight, also preferably 2 to 17% by weight, especially 5 to 16% by weight. If there are optional components D and E in the stabilizer composition, the presence of antioxidant C is preferably based on the total stabilizer composition comprising components A, B, C, D and E, ranging from 1 to 50% by weight, preferably 1 to 20% by weight, more preferably 1 to 15% by weight, also preferably 2 to 10% by weight, especially 3 to 9% by weight.
本發明之穩定劑組成物包含抗氧化劑作為組分C,其中該抗氧化劑由一或多種根據如上所示之式(III)a及/或(III)b的酚類化合物構成, 其中 R x及R y係彼此獨立地選自氫、鹵素或具有1至10個,較佳地1至6個,更佳地1至4個碳原子的烷基; R z係選自氫或具有1至10個碳原子,較佳地1至6個,更佳地1至4個碳原子的烷基; R w係選自羥基、具有1至18個碳原子的烷氧基、具有1至4個烷基碳原子的苯基烷氧基、具有5至8個碳原子的環烷氧基或式(III)c之基團 其中Z 1和Z 2係彼此獨立地選自氫、具有1至18個,較佳地1至10個碳原子的烷基、苯基及具有5至8個碳原子的環烷基; X 係選自-O-(CH 2) q-O-;-(OCH 2-CH 2) q-O-; -O-(CH 2) q-S-(CH 2) q-O;-NH-(CH 2) q-NH-;-NH-NH-,其中q為1至12,較佳地1至6之整數,或以下基團: p 係0或1至6,較佳地1至4之整數。 The stabilizer composition of the present invention comprises an antioxidant as component C, wherein the antioxidant consists of one or more phenolic compounds according to formula (III)a and/or (III)b as shown above, wherein R x and R y is independently selected from hydrogen, halogen or an alkyl group having 1 to 10, preferably 1 to 6, more preferably 1 to 4 carbon atoms; R z is selected from hydrogen or has 1 to 10 carbon atoms, preferably 1 to 6, more preferably an alkyl group of 1 to 4 carbon atoms; R is selected from hydroxyl, alkoxy having 1 to 18 carbon atoms, and alkyl having 1 to 4 A phenylalkoxy radical with carbon atoms, a cycloalkoxy radical with 5 to 8 carbon atoms or a group of formula (III)c wherein Z and Z are independently selected from hydrogen , alkyl having 1 to 18, preferably 1 to 10 carbon atoms, phenyl and cycloalkyl having 5 to 8 carbon atoms; X is -O-(CH 2 ) q -O-; -(OCH 2 -CH 2 ) q -O-; -O-(CH 2 ) q -S-(CH 2 ) q -O; -NH-( CH 2 ) q -NH-; -NH-NH-, wherein q is an integer of 1 to 12, preferably 1 to 6, or the following groups: p is 0 or an integer of 1-6, preferably 1-4.
在本發明之穩定劑組成物中所用的酚類抗氧化劑化合物C之較佳具體實例中不包括胺基。Preferred embodiments of the phenolic antioxidant compound C used in the stabilizer composition of the present invention do not include amine groups.
較佳地,R x及R y係彼此獨立地選自具有1至10個,較佳地1至6個,更佳地1至4個碳原子之分支烷基。更佳地,R x及R y之一或兩者為三級丁基。 Preferably, R x and R y are independently selected from branched alkyl groups having 1 to 10, preferably 1 to 6, more preferably 1 to 4 carbon atoms. More preferably, one or both of R x and R y is tertiary butyl.
較佳地,X係來自-O-(CH 2) q-O-之基團,其中q係1至12的整數,較佳地2至6,更佳地2至4。 Preferably, X is a group derived from -O-(CH 2 ) q -O-, wherein q is an integer of 1 to 12, preferably 2 to 6, more preferably 2 to 4.
在較佳之具體實例中,抗氧化劑C由一或多種根據式(III)a’及/或(III)b’的酚類化合物構成: 其中取代基及下標係如上文對式(III)a及(III)b所述。 In a preferred embodiment, antioxidant C consists of one or more phenolic compounds according to formula (III)a' and/or (III)b': Wherein the substituents and subscripts are as described above for formulas (III)a and (III)b.
在較佳之具體實例中,抗氧化劑C包含(或較佳地由以下組成:)根據式(III)b或較佳地(III)b’的酚類化合物, 其中 R x及R y係彼此獨立地選自具有1至6個,更佳地1至4個碳原子的分支烷基,更佳地R x及R y之一或兩者為三級丁基; R z係選自氫或具有1至4個碳原子之烷基; X 係選自-O-(CH 2) q-O-,其中q為2至4之整數:且 p 係1至4之整數。 In a preferred embodiment, the antioxidant C comprises (or preferably consists of:) a phenolic compound according to formula (III)b or preferably (III)b', wherein R x and R y are independent of each other is selected from branched alkyl groups having 1 to 6, more preferably 1 to 4 carbon atoms, more preferably one or both of R x and R y is a tertiary butyl group; R z is selected from hydrogen or having An alkyl group of 1 to 4 carbon atoms; X is selected from -O-(CH 2 ) q -O-, wherein q is an integer of 2 to 4; and p is an integer of 1 to 4.
在較佳之具體實例中,抗氧化劑C包含(或較佳地由以下組成:)雙-(3,3-雙-(4’-羥基-3’-三級丁基-苯基)丁酸)-甘醇酯(CAS編號32509-66-3)。In a preferred embodiment, the antioxidant C comprises (or preferably consists of:) bis-(3,3-bis-(4'-hydroxy-3'-tertiary butyl-phenyl)butanoic acid) - Glycol esters (CAS No. 32509-66-3).
在較佳之具體實例中,抗氧化劑C包含(或較佳地由以下組成:)根據式(III)-1之酚類化合物;雙-(3,3-雙-(4’-羥基-3’-三級丁基-苯基)丁酸)-乙二醇酯(CAS編號32509-66-3)。 In a preferred embodiment, the antioxidant C comprises (or preferably consists of:) a phenolic compound according to formula (III)-1; bis-(3,3-bis-(4'-hydroxyl-3' - tertiary butyl-phenyl)butanoic acid)-ethylene glycol ester (CAS No. 32509-66-3).
抗氧化劑組分C之較佳具體實例也描述於US 6,270,692及/或GB1440391中。Preferred specific examples of antioxidant component C are also described in US 6,270,692 and/or GB1440391.
在本發明之穩定劑組成物的較佳具體實例中,UV吸收劑A包含(或較佳地由以下組成:)式(I.1)-1及/或(1.1)-2的(2-羥基苯基)-對稱-三嗪化合物及/或根據式(I.2)-1的草醯苯胺化合物,且抗氧化劑C包含(或較佳地由以下組成:)酚類化合物雙-(3,3-雙-(4’-羥基-3’-三級丁基-苯基)丁酸)-乙二醇酯。In a preferred embodiment of the stabilizer composition of the present invention, the UV absorber A comprises (or preferably consists of:) (2- Hydroxyphenyl)-symmetry-triazine compound and/or the oxanilide compound according to formula (I.2)-1, and antioxidant C comprises (or preferably is made up of following:) phenolic compound bis-(3 , 3-bis-(4'-hydroxy-3'-tertiary butyl-phenyl)butanoic acid)-ethylene glycol ester.
在本發明之穩定劑組成物的更佳具體實例中,UV吸收劑A包含(或較佳地由以下組成:)根據式(I.2)-1的草醯苯胺化合物及/或根據式(I.1)-1及/或(I.1)-2的(2-羥基苯基)-對稱-三嗪化合物,受阻胺光穩定劑B包含(或較佳地由以下組成:) 2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)二螺環-(5.1.11.2)-二十一烷-21-酮與環氧氯丙烷之反應產物,且抗氧化劑C包含(或較佳地由以下組成:) 酚類化合物雙-(3,3-雙-(4’-羥基-3’-三級丁基-苯基)丁酸)-乙二醇酯。 液體稀釋劑(組分D) In a more preferred embodiment of the stabilizer composition of the present invention, the UV absorber A comprises (or preferably consists of:) the oxalanilide compound according to the formula (I.2)-1 and/or according to the formula ( The (2-hydroxyphenyl)-symmetric-triazine compound of I.1)-1 and/or (I.1)-2, the hindered amine light stabilizer B comprises (or preferably consists of:) 2, 2,4,4-Tetramethyl-7-oxa-3,20-diazabispiro-20-(2,3-epoxy-propyl)bispiro-(5.1.11.2)-di The reaction product of undecane-21-one and epichlorohydrin, and the antioxidant C comprises (or preferably consists of:) phenolic compound bis-(3,3-bis-(4'-hydroxyl-3 '-tertiary butyl-phenyl)butanoic acid)-ethylene glycol ester. Liquid Diluent (Component D)
為了以較佳液體形式提供本發明之穩定劑組成物,可使用液體組分A及/或B及隨意的C。然而,藉由添加液體稀釋劑作為組分D來提供液體穩定劑組成物可能更為方便,該組分D較佳地對穩定劑組成物之組分,特別是組分A、B及隨意的C為化學惰性的。因此,本發明之穩定劑組成物可包含一或多種液體稀釋劑作為組分D。較佳地,至少一種液體稀釋劑D之存在量以包含組分A、B、隨意的C、E及D之總穩定劑組成物為基準計為30至79重量%,較佳地35至69重量%,更佳地,40至64重量%,也較佳地40至55重量%,特別是43至53重量%。適切地選擇該用量以採溶液、分散體、乳液、懸浮液或糊劑,較佳地懸浮液之形式提供實質上液體的穩定劑組成物。To provide the stabilizer composition of the present invention in preferably liquid form, liquid components A and/or B and optionally C may be used. However, it may be more convenient to provide a liquid stabilizer composition by adding a liquid diluent as Component D, which preferably acts on the components of the stabilizer composition, particularly components A, B and optionally C is chemically inert. Accordingly, the stabilizer composition of the present invention may comprise as component D one or more liquid diluents. Preferably, at least one liquid diluent D is present in an amount of 30 to 79% by weight, preferably 35 to 69% by weight, based on the total stabilizer composition comprising components A, B, optionally C, E and D % by weight, more preferably 40 to 64% by weight, also preferably 40 to 55% by weight, especially 43 to 53% by weight. The amount is suitably selected to provide a substantially liquid stabilizer composition in the form of a solution, dispersion, emulsion, suspension or paste, preferably a suspension.
較佳地,液體稀釋劑D可選自合適之液體稀釋劑,如合適之溶劑、液體助劑或其混合物。較佳地,至少一種液體稀釋劑D係選自化學惰性溶劑及液體助劑,其可隨意地存於(或典型地存於)基於矽基改質聚合物(SMP)之聚合物組成物中。Preferably, the liquid diluent D can be selected from a suitable liquid diluent, such as a suitable solvent, a liquid adjuvant or a mixture thereof. Preferably, at least one liquid diluent D is selected from chemically inert solvents and liquid additives, which can be optionally present (or typically present) in the polymer composition based on silicon-modified polymer (SMP) .
在較佳之具體實例中,穩定劑組成物包含以總穩定劑組成物為基準計為35至69重量%,較佳地40至64重量%,更佳地40至55重量%,特別是43至53重量%之至少一種選自作為液體稀釋劑D的液體助劑及/或液體溶劑之液體稀釋劑D。In a preferred embodiment, the stabilizer composition comprises 35 to 69% by weight, preferably 40 to 64% by weight, more preferably 40 to 55% by weight, especially 43 to 55% by weight, based on the total stabilizer composition. 53% by weight of at least one liquid diluent D selected from liquid auxiliaries and/or liquid solvents of the liquid diluent D.
在較佳之具體實例中,穩定劑組成物包含至少一種可存於基於矽基改質聚合物(SMP)之聚合物組成物中作為液體稀釋劑D的液體助劑及/或液體溶劑,較佳地包含至少一種用作基於矽基改質聚合物(SMP)之聚合物組成物中的助劑之液體稀釋劑D。In a preferred embodiment, the stabilizer composition comprises at least one liquid additive and/or liquid solvent that can be present in a silicon-based modified polymer (SMP)-based polymer composition as a liquid diluent D, preferably Contains at least one liquid diluent D used as an auxiliary in polymer compositions based on silicon-modified polymers (SMP).
更佳地,穩定劑組成物包含至少一種選自液體塑化劑D1、液體黏著促進劑D2、液體觸媒D3、水分清除劑D4及液體溶劑D5之液體稀釋劑D。More preferably, the stabilizer composition comprises at least one liquid diluent D selected from liquid plasticizer D1, liquid adhesion promoter D2, liquid catalyst D3, moisture scavenger D4 and liquid solvent D5.
較佳之液體塑化劑D1係選自鄰苯二甲酸酯塑化劑,如鄰苯二甲酸二甲酯、鄰苯二甲酸二乙酯、鄰苯二甲酸二丁酯、鄰苯二甲酸二庚酯、鄰苯二甲酸二辛酯、鄰苯二甲酸二異壬酯、鄰苯二甲酸二異癸酯、鄰苯二甲酸二異十一烷酯、鄰苯二甲酸丁基苯甲酯、鄰苯二甲酸二月桂酯及鄰苯二甲酸二環己酯;環氧化塑化劑,如環氧化大豆油、環氧化亞麻籽油及環氧硬脂酸苯甲酯;脂肪酸酯,如C 4-C 28脂肪酸之烷基酯和苯基酯、氯化石蠟等。 The preferred liquid plasticizer D1 is selected from phthalate plasticizers, such as dimethyl phthalate, diethyl phthalate, dibutyl phthalate, diphthalate Heptyl phthalate, Dioctyl phthalate, Diisononyl phthalate, Diisodecyl phthalate, Diisoundecyl phthalate, Butylbenzyl phthalate, Dilauryl phthalate and dicyclohexyl phthalate; epoxidized plasticizers, such as epoxidized soybean oil, epoxidized linseed oil, and epoxidized benzyl stearate; fatty acid esters, such as C Alkyl and phenyl esters of 4 -C 28 fatty acids, chlorinated paraffin, etc.
塑化劑D1可為上述塑化劑中之一種或二或更多種的組合。The plasticizer D1 can be one or a combination of two or more of the above plasticizers.
較佳地,塑化劑D1不含鄰苯二甲酸酯化合物。較佳地,塑化劑D1包含(或較佳地由以下構成:)至少一種C 10-C 21烷磺酸苯酯(例如可從Lanxess以商品名Mesamoll ®購得)。 Preferably, the plasticizer D1 does not contain phthalate compounds. Preferably, the plasticizer D1 comprises (or preferably consists of) at least one phenyl C 10 -C 21 alkanesulfonate (commercially available for example under the trade name Mesamoll ® from Lanxess).
較佳地,至少一種黏著促進劑D2係選自具有至少一個附加官能基之矽烷化合物,例如選自胺基、巰基、環氧基、羧基、乙烯基、異氰酸基、異三聚異氰酸基、鹵素等。典型地,黏著促進劑D2係選自環氧矽烷及胺基矽烷。Preferably, at least one adhesion promoter D2 is selected from silane compounds having at least one additional functional group, such as amino, mercapto, epoxy, carboxyl, vinyl, isocyanato, isocyanuric Acid groups, halogens, etc. Typically, the adhesion promoter D2 is selected from epoxy silanes and amino silanes.
例如,黏著促進劑D2可選自: 含異氰酸基之矽烷如γ-異氰酸基丙基三甲氧基矽烷、γ-異氰酸基丙基三乙氧基矽烷、γ-異氰酸基丙基甲基二乙氧基矽烷及γ-異氰酸基丙基甲基二甲氧基矽烷; 含胺基之矽烷,例如選自γ-胺丙基三甲氧基矽烷、γ-胺丙基三乙氧基矽烷、γ-胺丙基甲基二甲氧基矽烷、γ-胺丙基甲基二乙氧基矽烷、γ-(2-胺乙基)胺丙基三甲氧基矽烷、γ-(2-胺乙基)胺丙基甲基二甲氧基矽烷、γ-(2-胺乙基)胺丙基三乙氧基矽烷、γ-(2-胺乙基)胺丙基甲基二乙氧基矽烷、γ-脲基丙基三甲氧基矽烷、N-苯基-γ-胺丙基三甲氧基矽烷、N-苯甲基-γ-胺丙基三甲氧基矽烷及N-乙烯基苯甲基-γ-胺丙基三乙氧基矽烷; 含巰基之矽烷,如γ-巰基丙基三甲氧基矽烷、γ-巰基丙基三乙氧基矽烷、γ-巰基丙基甲基二甲氧基矽烷及γ-巰基丙基甲基二乙氧基矽烷; 含環氧基之矽烷,如γ-環氧丙氧基丙基三甲氧基矽烷、γ-環氧丙氧基丙基三乙氧基矽烷、γ-環氧丙氧基丙基甲基二甲氧基矽烷、β-(3,4-環氧環己基)乙基三甲氧基矽烷及β-(3,4-環氧環己基)乙基三乙氧基矽烷; 羧基矽烷,如β-羧乙基三乙氧基矽烷、β-羧乙基苯基雙-(2-甲氧基乙氧基)矽烷及N-β-(羧甲基)胺基乙基-γ-胺基丙基三甲氧基矽烷; 含鹵素之矽烷,如γ-氯丙基三甲氧基矽烷;及 異三聚異氰酸酯矽烷如異三聚異氰酸三(三甲氧基矽烷酯)。 For example, the adhesion promoter D2 can be selected from: Silanes containing isocyanato groups such as γ-isocyanatopropyltrimethoxysilane, γ-isocyanatopropyltriethoxysilane, γ-isocyanatopropylmethyldiethoxysilane and γ-isocyanatopropylmethyldimethoxysilane; Amino-containing silanes, for example selected from γ-aminopropyltrimethoxysilane, γ-aminopropyltriethoxysilane, γ-aminopropylmethyldimethoxysilane, γ-aminopropylmethyl Diethoxysilane, γ-(2-aminoethyl)aminopropyltrimethoxysilane, γ-(2-aminoethyl)aminopropylmethyldimethoxysilane, γ-(2-aminoethyl) base) aminopropyltriethoxysilane, γ-(2-aminoethyl)aminopropylmethyldiethoxysilane, γ-ureidopropyltrimethoxysilane, N-phenyl-γ-amine Propyltrimethoxysilane, N-benzyl-γ-aminopropyltrimethoxysilane and N-vinylbenzyl-γ-aminopropyltriethoxysilane; Silanes containing mercapto groups, such as γ-mercaptopropyltrimethoxysilane, γ-mercaptopropyltriethoxysilane, γ-mercaptopropylmethyldimethoxysilane and γ-mercaptopropylmethyldiethoxysilane base silane; Silanes containing epoxy groups, such as γ-glycidoxypropyltrimethoxysilane, γ-glycidoxypropyltriethoxysilane, γ-glycidoxypropylmethyl dimethyl Oxysilane, β-(3,4-epoxycyclohexyl)ethyltrimethoxysilane and β-(3,4-epoxycyclohexyl)ethyltriethoxysilane; Carboxysilanes, such as β-carboxyethyltriethoxysilane, β-carboxyethylphenylbis-(2-methoxyethoxy)silane and N-β-(carboxymethyl)aminoethyl- γ-Aminopropyltrimethoxysilane; Halogen-containing silanes, such as γ-chloropropyltrimethoxysilane; and Isotrimeric isocyanate silanes such as tris(trimethoxysilyl isocyanurate).
在較佳之具體實例中,該至少一種黏著促進劑D2係選自含胺基之矽烷,特別是含胺基之三甲氧基矽烷。較佳地,該至少一種黏著促進劑D2包含至少一種含胺基之三甲氧基矽烷,較佳地3-胺基丙基三甲氧基矽烷(例如來自Evonik的Dynasylan® AMMO)。In a preferred embodiment, the at least one adhesion promoter D2 is selected from amine-containing silanes, especially amine-containing trimethoxysilane. Preferably, the at least one adhesion promoter D2 comprises at least one amino-containing trimethoxysilane, preferably 3-aminopropyltrimethoxysilane (eg Dynasylan® AMMO from Evonik).
在較佳之具體實例中,該至少一種D3係選自公知的矽烷醇縮合觸媒。此縮合觸媒可為例如四價錫化合物如二月桂酸二丁基錫、二乙酸二丁基錫、二丁基錫;二價錫化合物如辛酸亞錫;鈦酸酯如鈦酸四丁酯及鈦酸四丙酯;胺化合物如丁胺、辛胺、月桂胺、二丁胺、單乙醇胺、二乙醇胺、三乙醇胺、二伸乙基三胺、三伸乙基四胺、環己胺、苯甲胺、二乙基胺基丙基胺、伸二甲苯二胺(xylylenediamine)、2,4,6-三(二甲基胺基甲基)酚、嗎啉、N-甲基嗎啉及1,8-二氮雜雙環[5.4.0]十一碳烯-7 (DBU);含胺基之矽烷偶合劑如γ-胺基丙基三甲氧基矽烷及N-(β-胺基乙基)胺基丙基甲基-二甲氧基矽烷;等矽烷醇縮合觸媒,再者,其他已知之矽烷醇縮合觸媒如酸性觸媒及鹼性觸媒。In a preferred embodiment, the at least one D3 is selected from known silanol condensation catalysts. The condensation catalyst can be, for example, tetravalent tin compounds such as dibutyltin dilaurate, dibutyltin diacetate, dibutyltin; divalent tin compounds such as stannous octoate; titanates such as tetrabutyl titanate and tetrapropyl titanate ; Amine compounds such as butylamine, octylamine, laurylamine, dibutylamine, monoethanolamine, diethanolamine, triethanolamine, diethylenetriamine, triethylenetetramine, cyclohexylamine, benzylamine, diethylamine Aminopropylamine, xylylenediamine, 2,4,6-tris(dimethylaminomethyl)phenol, morpholine, N-methylmorpholine and 1,8-diazepine Bicyclo[5.4.0]undecene-7 (DBU); amino-containing silane coupling agents such as γ-aminopropyltrimethoxysilane and N-(β-aminoethyl)aminopropylmethyl base-dimethoxysilane; other silanol condensation catalysts, and other known silanol condensation catalysts such as acidic catalysts and basic catalysts.
觸媒D3可為上述觸媒中之一種或二或更多種的組合。The catalyst D3 can be one or a combination of two or more of the above catalysts.
較佳地,該至少一種觸媒D3係選自基於四價鈦之金屬有機化合物(如雙乙醯丙酮酸二異丙氧基鈦)及/或基於四價錫之有機化合物(如雙乙醯丙酮酸二烷基錫化合物及鄰苯二甲酸酯二烷基錫)。此觸媒例如可自TIB Chemicals購得。較佳地,該觸媒D3包含至少一種有機錫化合物,更較佳雙乙醯丙酮酸二丁基錫及/或雙乙醯丙酮酸二辛基錫(例如來自TIB Chemicals的TIB KAT® 223,二酮酸二辛基錫)。Preferably, the at least one catalyst D3 is selected from metal organic compounds based on tetravalent titanium (such as diisopropoxytitanium bisacetylacetonate) and/or organic compounds based on tetravalent tin (such as diacetyl dialkyltin pyruvate and dialkyltin phthalate). Such catalysts are commercially available, for example, from TIB Chemicals. Preferably, the catalyst D3 comprises at least one organotin compound, more preferably dibutyltin bisacetylpyruvate and/or dioctyltin bisacetylpyruvate (eg TIB KAT® 223 from TIB Chemicals, diketone dioctyltin acid).
較佳地,水分清除劑D4係選自液態乙烯基矽烷,特別是含乙烯基型不飽和基團的矽烷,如乙烯基三甲氧基矽烷(VTMO)、乙烯基三乙氧基矽烷(VTEO)、γ-甲基丙烯醯氧基丙基-甲基二甲氧基矽烷及γ-丙烯醯氧基丙基甲基三乙氧基矽烷。例如,水分清除劑D4係乙烯基三甲氧基矽烷(VTMO) (例如來自Evonik之Dynasylan ®VTMO)及/或乙烯基三乙氧基矽烷(VTEO) (例如來自Evonik之Dynasylan ®VTEO)。在本發明之特佳具體實例中,液體稀釋劑D包含(或由以下組成:)至少一種水分清除劑D4,更佳地乙烯基三甲氧基矽烷(VTMO) (例如來自Evonik之Dynasylan ®VTMO)及/或乙烯基三乙氧基矽烷(VTEO) (例如來自Evonik之Dynasylan ®VTEO),最佳地乙烯基三甲氧基矽烷(VTMO)。 Preferably, the moisture scavenger D4 is selected from liquid vinyl silanes, especially silanes containing vinyl unsaturated groups, such as vinyl trimethoxysilane (VTMO), vinyl triethoxysilane (VTEO) , γ-methacryloxypropyl-methyldimethoxysilane and γ-acryloxypropylmethyltriethoxysilane. For example, the moisture scavenger D4 is vinyltrimethoxysilane (VTMO) (eg Dynasylan ® VTMO from Evonik) and/or vinyltriethoxysilane (VTEO) (eg Dynasylan ® VTEO from Evonik). In a particularly preferred embodiment of the invention, the liquid diluent D comprises (or consists of:) at least one moisture scavenger D4, more preferably vinyltrimethoxysilane (VTMO) (eg Dynasylan ® VTMO from Evonik) And/or vinyltriethoxysilane (VTEO) (eg Dynasylan ® VTEO from Evonik), most preferably vinyltrimethoxysilane (VTMO).
較佳地,溶劑D5係選自本領域中常用之惰性極性的或惰性非極性的有機溶劑。Preferably, the solvent D5 is selected from inert polar or inert non-polar organic solvents commonly used in the art.
在一個具體實例中,穩定劑組成物包含至少一種惰性溶劑,特別是至少一種惰性極性溶劑。合適之極性溶劑包含醚、二醇醚,特別是甲氧基丙醇。In a specific example, the stabilizer composition comprises at least one inert solvent, especially at least one inert polar solvent. Suitable polar solvents include ethers, glycol ethers, especially methoxypropanol.
在替代具體實例中,穩定劑組成物可包含惰性非極性有機溶劑,較佳地脂族或芳族烴,例如石油醚、己烷、庚烷、石油餾分、甲苯、環己烷、均三甲苯或二甲苯。In alternative embodiments, the stabilizer composition may comprise an inert non-polar organic solvent, preferably an aliphatic or aromatic hydrocarbon such as petroleum ether, hexane, heptane, petroleum fractions, toluene, cyclohexane, mesitylene or xylene.
在本發明之一個具體實例中,穩定劑組成物可不含可能對健康及/或環境有害之實質量的溶劑。特別是,穩定劑組成物之分類要求可不含實質量的溶劑,根據現行之歐盟化學物質分類、標籤及包裝法規(CLP),這些溶劑必需歸類為危險性。根據本發明之一個態樣,穩定劑組成物可不含實質量的芳烴溶劑,如甲苯、均三甲苯或二甲苯。在本發明之一個具體實例中,穩定劑組成物不含實質量的甲苯。在本發明之一個具體實例中,穩定劑組成物不含實質量的均三甲苯。在本發明的一個具體實例中,穩定劑組成物不含實質量的二甲苯。為了本發明之目的,措辭“實質量”應理解為以總穩定劑組成物為基準計為≥1重量%的量。In one embodiment of the invention, the stabilizer composition may be free of substantial amounts of solvents that may be harmful to health and/or the environment. In particular, the classification requirements for stabilizer compositions may not contain substantial amounts of solvents, which must be classified as hazardous according to the current European Union Regulation on Classification, Labeling and Packaging of Chemical Substances (CLP). According to an aspect of the present invention, the stabilizer composition may not contain substantial amounts of aromatic solvents, such as toluene, mesitylene or xylene. In one embodiment of the invention, the stabilizer composition does not contain a substantial amount of toluene. In one embodiment of the present invention, the stabilizer composition does not contain a substantial amount of mesitylene. In one embodiment of the invention, the stabilizer composition does not contain a substantial amount of xylene. For the purposes of the present invention, the expression "substantial amount" is to be understood as an amount of ≧1% by weight, based on the total stabilizer composition.
在本發明之較佳具體實例中,液體稀釋劑D包含(或由以下組成:)選自至少一種液體塑化劑D1、至少一種液體水分清除劑D4、至少一種液體溶劑D5及上述化合物的混合物中之至少一種組分。In a preferred embodiment of the present invention, the liquid diluent D comprises (or consists of:) a mixture selected from at least one liquid plasticizer D1, at least one liquid moisture scavenger D4, at least one liquid solvent D5 and the above-mentioned compounds at least one of the components.
在另一個較佳具體實例中,液體稀釋劑D包含(或由以下組成:)選自至少一種液體水分清除劑D4,特別是乙烯基三甲氧基矽烷;至少一種液體溶劑D5,特別是甲氧基丙醇及/或二甲苯;及上述化合物的混合物中之至少一種組分。 其他添加物(組分E) In another preferred embodiment, the liquid diluent D comprises (or consists of:) selected from at least one liquid moisture scavenger D4, especially vinyltrimethoxysilane; at least one liquid solvent D5, especially methoxysilane; propanol and/or xylene; and at least one component of a mixture of the aforementioned compounds. Other additives (component E)
本發明之穩定劑組成物可另包含一或多種其他添加物E作為隨意組分,如用於穩定劑組成物及SMP密封劑和黏著劑的常用添加物。The stabilizer composition of the present invention may further comprise one or more other additives E as optional components, such as commonly used additives for stabilizer compositions and SMP sealants and adhesives.
較佳地,至少一種添加物E之存在量可以總穩定劑組成物為基準計為0至50重量%,較佳地0至20重量%,更佳地0.01至20重量%,也較佳地0.01至10重量%,也較佳地0.1至1重量%。Preferably, the at least one additive E is present in an amount of 0 to 50% by weight, preferably 0 to 20% by weight, more preferably 0.01 to 20% by weight, also preferably 0.01 to 10% by weight, preferably 0.1 to 1% by weight.
其他添加物E可選自常見之添加物,如抗靜電劑、防火劑(flame proofing agent)、軟化劑、成核劑、金屬去活化劑(metal deactivator)、殺生物劑、耐衝擊改質劑(impact modifier)、填料、顏料及殺黴菌劑(fungicide)、殺生物劑。再者,本發明之穩定劑組成物中可使用一或多種上述添加物作為以下添加物G,其先決條件為添加物E與液體稀釋劑D不同。 用於製備穩定劑組成物之方法 Other additives E can be selected from common additives, such as antistatic agents, flame proofing agents, softeners, nucleating agents, metal deactivators, biocides, impact modifiers (impact modifier), filler, pigment and fungicide (fungicide), biocide. Furthermore, one or more of the above-mentioned additives can be used as the following additive G in the stabilizer composition of the present invention, and the prerequisite is that the additive E is different from the liquid diluent D. Method for preparing stabilizer composition
本發明也關於一種用於製備本發明之穩定劑組成物的方法,其中組分A、B、及隨意地C、D及/或E混合在一起。特別是,混合步驟可藉由採液體形式,特別是採溶液、分散體、乳液、懸浮液或糊劑,較佳地懸浮液或溶液,特別是溶液的形式混合組分A、B、及隨意地C、D及/或E進行。The invention also relates to a process for the preparation of the stabilizer composition of the invention, wherein components A, B, and optionally C, D and/or E are mixed together. In particular, the mixing step can be achieved by mixing components A, B, and optionally Proceed with C, D and/or E.
較佳地,製備本發明之穩定劑組成物的方法可包含研磨組分A、B、及隨意地C、D及/或E中之一或多者的步驟。其後,可將組分A、B、及隨意地C、D及/或E混合在一起。較佳地,穩定劑組成物可,例如藉由研磨步驟(例如微粒化(micronisation)),採包含粒徑d 50於10至1000μm範圍內之固體顆粒的懸浮液形式獲得。研磨步驟可打碎聚集物並改善非液體組分A、B及隨意之C和E的均勻分佈。 Preferably, the method for preparing the stabilizer composition of the present invention may comprise the step of grinding one or more of components A, B, and optionally C, D and/or E. Thereafter, components A, B, and optionally C, D and/or E may be mixed together. Preferably, the stabilizer composition is obtainable, eg by a grinding step (eg micronisation), in the form of a suspension comprising solid particles with a particle size d50 in the range of 10 to 1000 μm. The milling step breaks up aggregates and improves the uniform distribution of the non-liquid components A, B and optionally C and E.
在另一個具體實例中,製備本發明之穩定劑組成物的方法包含將至少一種選自組分A、B及隨意的C及/或E之固體組分溶於液體中的步驟,其中該液體可為該至少一種液體稀釋劑D或採液體形式之組分A、B、C或E中之一或更多者。例如,該組分A、B、C或E中之一或更多者可採分散或溶解的形式提供,例如與液體稀釋劑D結合,其後與穩定劑組成物之其他組分混合。In another embodiment, the method for preparing the stabilizer composition of the present invention comprises the step of dissolving at least one solid component selected from components A, B and optional C and/or E in a liquid, wherein the liquid It may be the at least one liquid diluent D or one or more of components A, B, C or E in liquid form. For example, one or more of the components A, B, C or E may be provided in dispersed or dissolved form, for example in combination with the liquid diluent D, and thereafter mixed with the other components of the stabilizer composition.
再者,本發明關於本發明之穩定劑組成物作為基於矽基改質聚合物的密封劑或黏著劑中之穩定劑的用途。特別是,本發明之穩定劑組成物係用以改善基於矽基改質聚合物之密封劑或黏著劑的UV和熱穩定性,即抵抗暴露於UV輻射及/或熱之下的機械及/或光學表面性質之降低。 聚合物組成物 Furthermore, the present invention relates to the use of the stabilizer composition of the present invention as a stabilizer in a silicon-based modified polymer-based sealant or adhesive. In particular, the stabilizer compositions of the present invention are used to improve the UV and thermal stability of sealants or adhesives based on silicon-modified polymers, i.e. against mechanical and/or thermal stresses from exposure to UV radiation and/or heat. or degradation of optical surface properties. polymer composition
再者,本發明關於一種基於矽基改質聚合物(SMP)之聚合物組成物,其包含前述UV吸收劑A、受阻胺光穩定劑B及酚類抗氧化劑C的穩定劑組合。本發明關於一種聚合物組成物,其包含: (P) 至少一種選自矽基改質聚合物的聚合物P; (S) 由以下組分構成的穩定劑組合S: 至少一種UV吸收劑A; 至少一種受阻胺光穩定劑B; 隨意地至少一種酚類抗氧化劑C; 隨意地至少一種液體稀釋劑D;及 隨意地至少一種其他添加物E; 其中該組分A、B、C、D及E與對於本發明之穩定劑組成物的定義相同; (F) 隨意地至少一種填料F; (G) 隨意地至少一種其他添加物G,其較佳為選自塑化劑G1、黏著促進劑G2、觸媒G3及水分清除劑G4。 Furthermore, the present invention relates to a silicon-based modified polymer (SMP)-based polymer composition, which includes the stabilizer combination of the aforementioned UV absorber A, hindered amine light stabilizer B and phenolic antioxidant C. The present invention relates to a polymer composition, which comprises: (P) at least one polymer P selected from silicon-based modified polymers; (S) A stabilizer combination S consisting of: at least one UV absorber A; at least one hindered amine light stabilizer B; Optionally at least one phenolic antioxidant C; optionally at least one liquid diluent D; and Optionally at least one other additive E; Wherein the components A, B, C, D and E are the same as those defined for the stabilizer composition of the present invention; (F) optionally at least one filler F; (G) optionally at least one other additive G, preferably selected from plasticizer G1, adhesion promoter G2, catalyst G3 and moisture scavenger G4.
在較佳之具體實例中,本發明關於一種聚合物組成物,其包含(或較佳地由以下組成): (P) 以總聚合物組成物為基準,5至99重量%,較佳地10至80重量%,更佳地15至40重量%之至少一種選自矽基改質聚合物的聚合物P; (S) 由以下組分構成的穩定劑組合S: 以總聚合物組成物為基準,0.001至3重量%,較佳地0.01至1重量%,更佳地0.1至0.8重量%的至少一種UV吸收劑A; 以總聚合物組成物為基準,0.001至3重量%,較佳地0.01至1重量%,更佳地0.1至0.8重量%的至少一種受阻胺光穩定劑B; 以總聚合物組成物為基準,0至3重量%,較佳地0.01至1重量%,更佳地0.05至0.3重量%的至少一種酚類抗氧化劑C; 以總聚合物組成物為基準,0至3重量%,較佳地0.01至2重量%,更佳地0.1至1.6重量%的至少一種液體稀釋劑D;及 以總聚合物組成物為基準,0至3重量%,較佳地0.01至1重量%,更佳地0.05至0.3重量%的至少一種其他添加物E; (F) 以總聚合物組成物為基準,0至85重量%,較佳地0至80重量%,更佳地1至70重量%的至少一種填料F,其較佳為選自碳酸鈣; (G) 以總聚合物組成物為基準,0至35重量%,較佳地0至33重量%,更佳地1至30重量%的至少一種其他添加物G,其較佳為選自塑化劑G1、黏著促進劑G2、觸媒G3及水分清除劑G4。 In a preferred embodiment, the present invention relates to a polymer composition comprising (or preferably consisting of): (P) Based on the total polymer composition, 5 to 99% by weight, preferably 10 to 80% by weight, more preferably 15 to 40% by weight of at least one polymer P selected from silicon-based modified polymers ; (S) A stabilizer combination S consisting of: 0.001 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.1 to 0.8% by weight of at least one UV absorber A, based on the total polymer composition; 0.001 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.1 to 0.8% by weight of at least one hindered amine light stabilizer B, based on the total polymer composition; 0 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.05 to 0.3% by weight of at least one phenolic antioxidant C, based on the total polymer composition; 0 to 3% by weight, preferably 0.01 to 2% by weight, more preferably 0.1 to 1.6% by weight of at least one liquid diluent D, based on the total polymer composition; and 0 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.05 to 0.3% by weight of at least one other additive E, based on the total polymer composition; (F) 0 to 85% by weight, preferably 0 to 80% by weight, more preferably 1 to 70% by weight, based on the total polymer composition, of at least one filler F, preferably selected from calcium carbonate; (G) Based on the total polymer composition, 0 to 35% by weight, preferably 0 to 33% by weight, more preferably 1 to 30% by weight of at least one other additive G, preferably selected from plastic Chemical agent G1, adhesion promoter G2, catalyst G3 and moisture scavenger G4.
在存有隨意之組分F及/或G的情況下,可調整組分P之量使量的總和為或不超過100重量%。若液體稀釋劑D包含也可作為添加物G之活性成分的液體稀釋劑D,則可使G之量相應減少以保持上述範圍內的添加物之總量。Where optional components F and/or G are present, the amount of component P can be adjusted so that the sum of the amounts is or does not exceed 100% by weight. If the liquid diluent D comprises a liquid diluent D which is also the active ingredient of the additive G, the amount of G can be reduced accordingly to maintain the total amount of the additive within the above range.
上述組分A、B、C及D之較佳具體實例也適用於本發明之聚合物組成物。The preferred embodiments of the above components A, B, C and D are also applicable to the polymer composition of the present invention.
在較佳之具體實例中,該聚合物組成物包含(或較佳地由以下組成): (P) 以總聚合物組成物為基準,7.5至95重量%,較佳地12.5至72重量%,更佳地17至57重量%之至少一種選自矽基改質聚合物的聚合物P; (S) 由以下組分構成的穩定劑組合S: 以總聚合物組成物為基準,0.001至3重量%,較佳地0.01至1重量%,更佳地0.1至0.8重量%的至少一種UV吸收劑A; 以總聚合物組成物為基準,0.001至3重量%,較佳地0.01至1重量%,更佳地0.1至0.8重量%的至少一種受阻胺光穩定劑B; 以總聚合物組成物為基準,0至3重量%,較佳地0.01至1重量%,更佳地0.05至0.3重量%的至少一種酚類抗氧化劑C; 以總聚合物組成物為基準,0至3重量%,較佳地0.01至2重量%,更佳地0.1至1.6重量%的至少一種液體稀釋劑D;及 以總聚合物組成物為基準,0至3重量%,較佳地0.01至1重量%,更佳地0.05至0.3重量%的至少一種其他添加物E; (F) 以總聚合物組成物為基準,1至90重量%,較佳地20至80重量%,更佳地30至70重量%的至少一種填料F; (G1) 以總聚合物組成物為基準,1至25重量%,較佳地5至25重量%,更佳地10至20重量%的至少一種塑化劑作為其他添加物G1; (G2) 以總聚合物組成物為基準,0.5至5重量%,較佳地0.7至3重量%,更佳地0.8至2重量%的至少一種黏著促進劑作為其他添加物G2; (G3) 以總聚合物組成物為基準,0.01至3重量%,較佳地0.1至2重量%,更佳地0.2至1重量%的至少一種觸媒其他添加物G3; (G4) 0.5至5重量%,較佳地0.7至5重量%,更佳地0.8至3重量%的至少一種水分清除劑G4; (G) 以總聚合物組成物為基準,0至10重量%的至少一種與G1至G4不同之其他添加物G。 In a preferred embodiment, the polymer composition comprises (or preferably consists of): (P) Based on the total polymer composition, 7.5 to 95% by weight, preferably 12.5 to 72% by weight, more preferably 17 to 57% by weight of at least one polymer P selected from silicon-based modified polymers ; (S) A stabilizer combination S consisting of: 0.001 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.1 to 0.8% by weight of at least one UV absorber A, based on the total polymer composition; 0.001 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.1 to 0.8% by weight of at least one hindered amine light stabilizer B, based on the total polymer composition; 0 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.05 to 0.3% by weight of at least one phenolic antioxidant C, based on the total polymer composition; 0 to 3% by weight, preferably 0.01 to 2% by weight, more preferably 0.1 to 1.6% by weight of at least one liquid diluent D, based on the total polymer composition; and 0 to 3% by weight, preferably 0.01 to 1% by weight, more preferably 0.05 to 0.3% by weight of at least one other additive E, based on the total polymer composition; (F) 1 to 90% by weight, preferably 20 to 80% by weight, more preferably 30 to 70% by weight of at least one filler F, based on the total polymer composition; (G1) based on the total polymer composition, 1 to 25% by weight, preferably 5 to 25% by weight, more preferably 10 to 20% by weight of at least one plasticizer as other additive G1; (G2) 0.5 to 5% by weight, preferably 0.7 to 3% by weight, more preferably 0.8 to 2% by weight, based on the total polymer composition, of at least one adhesion promoter as further additive G2; (G3) based on the total polymer composition, 0.01 to 3% by weight, preferably 0.1 to 2% by weight, more preferably 0.2 to 1% by weight of at least one catalytic further additive G3; (G4) 0.5 to 5% by weight, preferably 0.7 to 5% by weight, more preferably 0.8 to 3% by weight of at least one moisture scavenger G4; (G) Based on the total polymer composition, 0 to 10% by weight of at least one other additive G different from G1 to G4.
在較佳之具體實例中,組分P、S、F及G總和為100重量%,其中特別是組分P及/或F的量可以被調整。In a preferred embodiment, the sum of components P, S, F and G is 100% by weight, wherein especially the amount of components P and/or F can be adjusted.
特別是,本發明之聚合物組成物可使用上述本發明的穩定劑組成物獲得。特別是,聚合物組成物包含以總聚合物組成物為基準計為0.01至10重量%,較佳地0.1至6重量%,更佳地0.2至3重量%,特別是1.6至2.4重量%的本發明之穩定劑組成物。 矽基改質聚合物P In particular, the polymer composition of the present invention can be obtained using the stabilizer composition of the present invention described above. In particular, the polymer composition comprises 0.01 to 10% by weight, preferably 0.1 to 6% by weight, more preferably 0.2 to 3% by weight, especially 1.6 to 2.4% by weight, based on the total polymer composition The stabilizer composition of the present invention. Silicon modified polymer P
典型地,矽基改質聚合物P係選自具有至少一個,較佳地兩個末端可交聯的可水解矽基之有機聚合物,特別是以聚醚、聚胺酯及/或丙烯酸聚合物為主。較佳地,末端可交聯的可水解矽基係矽基改質聚合物P中唯一的可交聯的可水解矽基。Typically, the silicon-based modified polymer P is selected from organic polymers having at least one, preferably two, cross-linkable hydrolyzable silicon groups, especially polyether, polyurethane and/or acrylic polymers. host. Preferably, the terminal crosslinkable hydrolyzable silicon group is the only crosslinkable hydrolyzable silicon group in the silicon-based modified polymer P.
例如合適之矽基改質聚合物P係來自Evonik的商業產品Kaneka MS Polymers ®(如MS Polymer ®S203H、MS Polymer ®S303H)或Polymer ST,其係包括聚醚和聚胺酯嵌段之矽基改質聚合物;或可自Dow Chemical公司以商品名VORASIL ®獲得的聚合物,其係具有聚胺酯聚合物骨幹之矽基改質聚合物。 Examples of suitable silicon-modified polymers P are the commercial products Kaneka MS Polymers ® (eg MS Polymer ® S203H, MS Polymer ® S303H) or Polymer ST from Evonik, which are silicon-based modifications comprising polyether and polyurethane blocks. polymer; or polymers available from Dow Chemical Company under the trade name VORASIL ® , which are silicon-based modified polymers with a polyurethane polymer backbone.
製備矽基改質聚合物之聚合方法係本領域公知的且例如描述於US3,971,751及EP-A 1 288 247中。Polymerization processes for the preparation of silicon-based modified polymers are well known in the art and are described, for example, in US 3,971,751 and EP-A 1 288 247 .
特別是,可交聯的可水解矽基可藉由下式(P-I)描述: 其中 R Q係選自具有1至20個碳原子的烷基、具有6至20個碳原子的芳基及具有7至20個碳原子的芳烷基, Y 係羥基或可水解基團; a 係1、2或3,且若存有二或更多個基團R Q或Y,其可為相同或不同。 In particular, crosslinkable hydrolyzable silicon groups can be described by the following formula (PI): Wherein R Q is selected from an alkyl group having 1 to 20 carbon atoms, an aryl group having 6 to 20 carbon atoms and an aralkyl group having 7 to 20 carbon atoms, Y is a hydroxyl group or a hydrolyzable group; a is 1, 2 or 3, and if there are two or more groups R Q or Y, they may be the same or different.
例如,可水解基團Y可選自氫、鹵素原子、具有1至20個碳原子之烷氧基,較佳地甲氧基或乙氧基、醯氧基、酮肟基、胺基、醯胺基、醯胺基、胺基氧基、巰基。較佳地,可水解基團Y係選自烷氧基,特別是甲氧基及乙氧基。For example, the hydrolyzable group Y may be selected from hydrogen, a halogen atom, an alkoxy group having 1 to 20 carbon atoms, preferably a methoxy or ethoxy group, an acyloxy group, a ketoxime group, an amino group, an acyl group Amino group, amido group, aminooxy group, mercapto group. Preferably, the hydrolyzable group Y is selected from alkoxy groups, especially methoxy and ethoxy.
至少一種矽基改質聚合物P可包含含至少一個可交聯的可水解矽基之經一或多個矽基改質之聚胺酯及/或矽基改質聚胺酯/聚醚共聚物。特別是,矽基改質胺基甲酸酯聚合物可例如衍生自芳族聚異氰酸酯,如甲苯二異氰酸酯、二苯基甲烷二異氰酸酯或二甲苯二異氰酸酯或脂族聚異氰酸酯(例如異佛爾酮二異氰酸酯或六亞甲基二異氰酸酯)與多元醇的反應。The at least one silicon-modified polymer P may comprise one or more silicon-modified polyurethanes and/or silicon-modified polyurethane/polyether copolymers containing at least one crosslinkable hydrolyzable silicon group. In particular, silicon-modified urethane polymers may, for example, be derived from aromatic polyisocyanates such as toluene diisocyanate, diphenylmethane diisocyanate or xylene diisocyanate or from aliphatic polyisocyanates such as isophorone diisocyanate or hexamethylene diisocyanate) with polyols.
在較佳之具體實例中,至少一種矽基改質聚合物P至少包含(或較佳地由以下組成:)矽基改質聚醚。典型地,矽基改質聚醚包含被至少一個可交聯的可水解矽基改質的,更佳地被兩個末端可交聯的可水解矽基改質的聚醚主鏈。例如,聚醚主鏈可包括選自聚環氧乙烷、聚環氧丙烷、聚環氧丁烷及/或聚苯醚之重複單元。再者,聚醚可於主鏈中含有胺基甲酸酯鍵或脲鍵。較佳地,矽基改質聚醚於聚醚主鏈中包含聚環氧乙烷重複單元,其中較佳地至少50重量%,較佳至少70重量%,更佳至少90重量%之重複單元為聚環氧乙烷重複單元。較佳之具有兩個末端可交聯的可水解矽基之矽基改質聚醚係例如描述於US 3,971,751及US 2002/198308中。In a preferred embodiment, at least one silicon-based modified polymer P at least includes (or preferably consists of:) silicon-based modified polyether. Typically, the silicon-modified polyether comprises a polyether backbone modified with at least one crosslinkable hydrolyzable silicon group, more preferably two terminal crosslinkable hydrolyzable silicon groups. For example, the polyether backbone may comprise repeating units selected from polyethylene oxide, polypropylene oxide, polybutylene oxide and/or polyphenylene ether. Furthermore, the polyether may contain a urethane bond or a urea bond in the main chain. Preferably, the silicon-based modified polyether comprises polyethylene oxide repeating units in the polyether main chain, wherein preferably at least 50% by weight, preferably at least 70% by weight, more preferably at least 90% by weight of repeating units It is a repeating unit of polyethylene oxide. Preferred silicon-modified polyethers having two terminally crosslinkable hydrolyzable silicon groups are described, for example, in US 3,971,751 and US 2002/198308.
在較佳之具體實例中,矽基改質聚合物P係選自根據下式(P-II)的矽基改質聚醚: 其中 Q 1、Q 2及Q 3係彼此獨立地選自具有1至40個,較佳地1至20個,更佳地1至4個碳原子烷基、具有1至10個,較佳地1至4個碳原子的烷氧基,如甲氧基或乙氧基、及乙醯氧基,其先決條件為Q 1、Q 2及Q 3中的至少一者為可交聯的可水解矽基,及 n 1及n 2彼此獨立地為0至1000,較佳地0至500,更佳地0至300的整數,其先決條件為n 1+n 2為至少50,較佳地至少100。 In a preferred embodiment, the silicon-based modified polymer P is selected from silicon-based modified polyethers according to the following formula (P-II): Wherein Q 1 , Q 2 and Q 3 are independently selected from alkyl groups having 1 to 40, preferably 1 to 20, more preferably 1 to 4 carbon atoms, alkyl groups having 1 to 10, preferably Alkoxy groups of 1 to 4 carbon atoms, such as methoxy or ethoxy, and acetyloxy, the prerequisite for which is that at least one of Q 1 , Q 2 and Q 3 is crosslinkable and hydrolyzable Silicon base, and n 1 and n 2 are independently of each other an integer of 0 to 1000, preferably 0 to 500, more preferably 0 to 300, with the prerequisite that n 1 +n 2 is at least 50, preferably at least 100.
在更佳之具體實例中,矽基改質聚合物P係選自根據下式(P-III)及(P-IV)的矽基改質聚醚: 其中n 3係5至1000,較佳地10至500,更佳地20至500的整數,也較佳地50至300。 In a more preferred embodiment, the silicon-based modified polymer P is selected from silicon-based modified polyethers according to the following formulas (P-III) and (P-IV): Where n 3 is an integer from 5 to 1000, preferably from 10 to 500, more preferably from 20 to 500, and also preferably from 50 to 300.
較佳地,矽基改質聚醚之聚醚主鏈,例如如式(P-I)至(P-III)所述,的分子量係於500至30,000 g/mol,較佳地1,000至15,000 g/mol,更佳地3,000至12,000 g/mol之範圍內。Preferably, the polyether backbone of the silicon-based modified polyether, for example as described in formulas (P-I) to (P-III), has a molecular weight of 500 to 30,000 g/mol, preferably 1,000 to 15,000 g/mol mol, more preferably in the range of 3,000 to 12,000 g/mol.
較佳地,以一或多種二甲氧基矽基封端聚醚(DMS MS)及/或三甲氧基矽基封端聚醚(TMS MS)用作矽基改質聚合物P。更佳地,以一或多種二甲氧基矽基封端聚醚(DMS MS)用作聚合物P。更特別的是,使用甲基二甲氧基矽基封端聚醚。Preferably, one or more dimethoxysilyl-terminated polyethers (DMS MS) and/or trimethoxysilyl-terminated polyethers (TMS MS) are used as the silicon-based modified polymer P. More preferably, one or more dimethoxysilyl-terminated polyethers (DMS MS) are used as polymer P. More particularly, methyldimethoxysilyl-terminated polyethers are used.
典型地,用作聚合物P之矽基改質聚醚,較佳地具有兩個末端可交聯的可水解矽基,具有於約1,000至50,000 g/mol,較佳地約5,000至約40,000 g/mol,更佳地約8,000至約35,000 g/mol,更佳地約10,000至約30,000 g/mol之範圍內的分子量(特別是數量平均分子量)。Typically, the silicon-modified polyether used as polymer P preferably has two terminally cross-linkable hydrolyzable silicon groups with a concentration of about 1,000 to 50,000 g/mol, preferably about 5,000 to about 40,000 g/mol A molecular weight (especially a number average molecular weight) in the range of g/mol, more preferably about 8,000 to about 35,000 g/mol, more preferably about 10,000 to about 30,000 g/mol.
典型地,用作聚合物P之矽基改質聚醚,較佳地具有兩個末端可交聯的可水解矽基,顯現於約1至約50 Pa s,較佳地約3至約40 Pa s,更佳地約5至約30 Pa s,更佳地約7至約20 Pa s之範圍內的黏度。典型地,使用布氏黏度計(Brookfield viscosimeter)測量黏度。例如,布氏黏度(以mPa・s為單位)係藉由Brookfield DV III Ultra黏度計於24℃±3℃及100 rpm下使用適合待測量黏度範圍之合適轉子測量,例如選自Brookfield RV轉子組(通常為7號轉子)。典型地,一旦將轉子插入樣品中,便以100 rpm之恆定轉速開始測量。報告之布氏黏度值為測量開始後60秒顯示的值。在較佳之具體實例中,矽基改質聚合物P包含(或較佳地由以下組成)至少一種具有兩個末端甲基二甲氧基矽基之矽基改質聚醚(例如Kaneka MS polymer ®S203H及/或Kaneka MS polymer ®S303H)。 填料F Typically, the silicon-modified polyether used as polymer P, preferably having two terminally cross-linkable hydrolyzable silicon groups, exhibits at about 1 to about 50 Pa s, preferably about 3 to about 40 Pa s Pa s, more preferably a viscosity in the range of about 5 to about 30 Pa s, more preferably about 7 to about 20 Pa s. Typically, viscosity is measured using a Brookfield viscosimeter. For example, the Brookfield viscosity (in mPa・s) is measured by a Brookfield DV III Ultra viscometer at 24°C±3°C and 100 rpm using a suitable spindle suitable for the viscosity range to be measured, such as selected from the Brookfield RV spindle set (Usually rotor #7). Typically, once the rotor is inserted into the sample, the measurement is started at a constant rotational speed of 100 rpm. The reported Brookfield viscosity value is the value displayed 60 seconds after the start of the measurement. In a preferred embodiment, the silicon-based modified polymer P comprises (or preferably consists of) at least one silicon-based modified polyether having two terminal methyldimethoxysilyl groups (such as Kaneka MS polymer ® S203H and/or Kaneka MS polymer ® S303H). Filler F
較佳地,本發明之聚合物組成物包含至少一種填料F,該至少一種填料F之量以總聚合物組成物為基準計為1至90重量%,較佳地20至80重量%,更佳地30至70重量%。Preferably, the polymer composition of the invention comprises at least one filler F in an amount of 1 to 90% by weight, preferably 20 to 80% by weight, more preferably 20 to 80% by weight, based on the total polymer composition Preferably 30 to 70% by weight.
較佳地,該至少一種填料F係選自木粉、核桃殼粉、稻殼粉、紙漿、棉片、雲母、石墨、矽藻土、陶土、高嶺土、黏土、滑石粉、二氧化矽、發煙二氧化矽、沉澱二氧化矽、矽酸酐、石英粉、玻璃珠、碳酸鈣(例如白堊)、碳酸鎂、氧化鈦、氧化鐵、氧化鋅、碳黑、玻璃球、玻璃纖維及碳纖維。填料F可為上述填料中之一種或二或更多種的組合。較佳地,該至少一種填料係選自碳酸鈣、二氧化矽、碳黑及其組合。合適之填料係亦描述於US 6,077,896及EP1288247中。Preferably, the at least one filler F is selected from wood flour, walnut shell powder, rice hull powder, paper pulp, cotton sheet, mica, graphite, diatomaceous earth, pottery clay, kaolin, clay, talcum powder, silicon dioxide, hair Fumed silica, precipitated silica, silicic anhydride, quartz powder, glass beads, calcium carbonate (such as chalk), magnesium carbonate, titanium oxide, iron oxide, zinc oxide, carbon black, glass spheres, glass fibers and carbon fibers. Filler F can be one or a combination of two or more of the above fillers. Preferably, the at least one filler is selected from calcium carbonate, silicon dioxide, carbon black and combinations thereof. Suitable filler systems are also described in US 6,077,896 and EP1288247.
較佳地,該至少一種填料F為碳酸鈣,特別是選自白堊、大理石、石灰石、沉澱碳酸鈣(PCC)、經塗覆之沉澱碳酸鈣、磨細之碳酸鈣(GCC) (特別是以白堊、大理石及/或石灰石為基礎之GCC)及經塗覆之碳酸鈣中的一或多種碳酸鈣。例如,塗覆脂肪酸之PCC及/或GCC可用作填料F。Preferably, the at least one filler F is calcium carbonate, in particular selected from chalk, marble, limestone, precipitated calcium carbonate (PCC), coated precipitated calcium carbonate, ground calcium carbonate (GCC) (in particular in the form of One or more calcium carbonates in chalk, marble and/or limestone based GCC) and coated calcium carbonate. For example, fatty acid coated PCC and/or GCC can be used as filler F.
較佳地,填料F包含(或較佳地由以下構成:)至少一種碳酸鈣,特別是至少一種塗覆脂肪酸之沉澱碳酸鈣。 添加物G Preferably, the filler F comprises (or preferably consists of): at least one calcium carbonate, in particular at least one precipitated calcium carbonate coated with a fatty acid. Additive G
在較佳之具體實例中,本發明之聚合物組成物包含至少一種選自塑化劑G1、黏著促進劑G2、觸媒G3、水分清除劑G4及其他用於SMP密封劑之公知的添加物之添加物G。較佳地,本發明之聚合物組成物包含至少一種塑化劑G1、至少一種黏著促進劑G2、至少一種觸媒G3及至少一種水分清除劑G4作為添加物G。In a preferred embodiment, the polymer composition of the present invention comprises at least one selected from plasticizer G1, adhesion promoter G2, catalyst G3, moisture scavenger G4 and other known additives used in SMP sealants Addition G. Preferably, the polymer composition according to the invention comprises, as additives G, at least one plasticizer G1, at least one adhesion promoter G2, at least one catalyst G3 and at least one moisture scavenger G4.
這種用於SMP密封劑及黏著劑之典型添加物係例如描述於US 6,077,896、EP-A 1288247及US 2003/ 105261中。Such typical additives for SMP sealants and adhesives are described, for example, in US 6,077,896, EP-A 1288247 and US 2003/105261.
較佳地,本發明之聚合物組成物包含至少一種塑化劑G1,該至少一種塑化劑G1之量以總聚合物組成物為基準計為1至25重量%,較佳地5至25重量%,更佳地10至20重量%。Preferably, the polymer composition of the invention comprises at least one plasticizer G1 in an amount of 1 to 25% by weight, preferably 5 to 25% by weight, based on the total polymer composition. % by weight, more preferably 10 to 20% by weight.
較佳地,該至少一種塑化劑G1係選自鄰苯二甲酸酯塑化劑,如鄰苯二甲酸二甲酯、鄰苯二甲酸二乙酯、鄰苯二甲酸二丁酯、鄰苯二甲酸二庚酯、鄰苯二甲酸二辛酯、鄰苯二甲酸二異壬酯、鄰苯二甲酸二異癸酯、鄰苯二甲酸二異十一烷酯、鄰苯二甲酸丁基苯甲酯、鄰苯二甲酸二月桂酯及鄰苯二甲酸二環己酯;環氧化塑化劑,如環氧化大豆油、環氧化亞麻油、環氧硬脂酸苯甲酯;脂肪酸酯,如C 4-C 28脂肪酸之烷基酯和苯基酯;衍生自二元酸和二元醇之聚酯塑化劑;聚醚,如聚丙二醇及其衍生物;聚苯乙烯,如聚-α-甲基苯乙烯和聚苯乙烯;聚丁二烯、丁二烯-丙烯腈共聚物、聚氯丁二烯、聚異戊二烯、聚丁烯、氯化石蠟等。 Preferably, the at least one plasticizer G1 is selected from phthalate plasticizers, such as dimethyl phthalate, diethyl phthalate, dibutyl phthalate, ortho Diheptyl phthalate, Dioctyl phthalate, Diisononyl phthalate, Diisodecyl phthalate, Diisoundecyl phthalate, Butyl phthalate Benzyl esters, dilauryl phthalate, and dicyclohexyl phthalate; epoxidized plasticizers, such as epoxidized soybean oil, epoxidized linseed oil, epoxidized benzyl stearate; fatty acid esters , such as alkyl and phenyl esters of C 4 -C 28 fatty acids; polyester plasticizers derived from dibasic acids and diols; polyethers, such as polypropylene glycol and its derivatives; polystyrene, such as poly - α-methylstyrene and polystyrene; polybutadiene, butadiene-acrylonitrile copolymer, polychloroprene, polyisoprene, polybutene, chlorinated paraffin, etc.
塑化劑G1可為上述塑化劑中之一種或二或更多種的組合。The plasticizer G1 can be one or a combination of two or more of the above plasticizers.
較佳地,塑化劑G1不含鄰苯二甲酸酯化合物。較佳地,塑化劑G1包含(或較佳地由以下組成:)至少一種C 10-C 21烷磺酸苯基酯(例如可自Lanxess以Mesamoll ®購得)。 Preferably, the plasticizer G1 does not contain phthalate compounds. Preferably, the plasticizer G1 comprises (or preferably consists of): at least one C 10 -C 21 phenyl sulfonate (commercially available as Mesamoll ® from Lanxess, for example).
較佳地,本發明之聚合物組成物包含至少一種黏著促進劑G2,該至少一種黏著促進劑G2之量以總聚合物組成物為基準計為0.5至5重量%,較佳地0.7至3重量%,更佳地0.8至2重量%。Preferably, the polymer composition according to the invention comprises at least one adhesion promoter G2 in an amount of 0.5 to 5% by weight, preferably 0.7 to 3% by weight, based on the total polymer composition. % by weight, more preferably 0.8 to 2% by weight.
較佳地,該至少一種黏著促進劑G2係選自具有至少一種附加官能基之矽烷化合物,例如選自胺基、巰基、環氧基、羧基、乙烯基、異氰酸基、異三聚異氰酸基、鹵素等。典型地,該黏著促進劑G2係選自環氧矽烷及胺基矽烷。Preferably, the at least one adhesion promoter G2 is selected from silane compounds having at least one additional functional group, such as amino, mercapto, epoxy, carboxyl, vinyl, isocyanate, isotrimeric iso Cyanate, halogen, etc. Typically, the adhesion promoter G2 is selected from epoxy silanes and amino silanes.
例如,黏著促進劑G2可選自: 含異氰酸基之矽烷如γ-異氰酸基丙基三甲氧基矽烷、γ-異氰酸基丙基三乙氧基矽烷、γ-異氰酸基丙基甲基二乙氧基矽烷及γ-異氰酸基丙基甲基二甲氧基矽烷; 含胺基之矽烷,例如選自γ-胺丙基三甲氧基矽烷、γ-胺丙基三乙氧基矽烷、γ-胺丙基甲基二甲氧基矽烷、γ-胺丙基甲基二乙氧基矽烷、γ-(2-胺乙基)胺丙基三甲氧基矽烷、γ-(2-胺乙基)胺丙基甲基二甲氧基矽烷、γ-(2-胺乙基)胺丙基三乙氧基矽烷、γ-(2-胺乙基)胺丙基甲基二乙氧基矽烷、γ-脲基丙基三甲氧基矽烷、N-苯基-γ-胺丙基三甲氧基矽烷、N-苯甲基-γ-胺丙基三甲氧基矽烷及N-乙烯基苯甲基-γ-胺丙基三乙氧基矽烷; 含巰基之矽烷,如γ-巰基丙基三甲氧基矽烷、γ-巰基丙基三乙氧基矽烷、γ-巰基丙基甲基二甲氧基矽烷及γ-巰基丙基甲基二乙氧基矽烷; 含環氧基之矽烷,如γ-環氧丙氧基丙基三甲氧基矽烷、γ-環氧丙氧基丙基三乙氧基矽烷、γ-環氧丙氧基丙基甲基二甲氧基矽烷、β-(3,4-環氧環己基)乙基三甲氧基矽烷及β-(3,4-環氧環己基)乙基三乙氧基矽烷; 羧基矽烷,如β-羧乙基三乙氧基矽烷、β-羧乙基苯基雙-(2-甲氧基乙氧基)矽烷及N-β-(羧甲基)胺基乙基-γ-胺基丙基三甲氧基矽烷; 含鹵素之矽烷,如γ-氯丙基三甲氧基矽烷;及 異三聚異氰酸酯矽烷如異三聚異氰酸三(三甲氧基矽烷酯)。 For example, the adhesion promoter G2 can be selected from: Silanes containing isocyanato groups such as γ-isocyanatopropyltrimethoxysilane, γ-isocyanatopropyltriethoxysilane, γ-isocyanatopropylmethyldiethoxysilane and γ-isocyanatopropylmethyldimethoxysilane; Amino-containing silanes, for example selected from γ-aminopropyltrimethoxysilane, γ-aminopropyltriethoxysilane, γ-aminopropylmethyldimethoxysilane, γ-aminopropylmethyl Diethoxysilane, γ-(2-aminoethyl)aminopropyltrimethoxysilane, γ-(2-aminoethyl)aminopropylmethyldimethoxysilane, γ-(2-aminoethyl) base) aminopropyltriethoxysilane, γ-(2-aminoethyl)aminopropylmethyldiethoxysilane, γ-ureidopropyltrimethoxysilane, N-phenyl-γ-amine Propyltrimethoxysilane, N-benzyl-γ-aminopropyltrimethoxysilane and N-vinylbenzyl-γ-aminopropyltriethoxysilane; Silanes containing mercapto groups, such as γ-mercaptopropyltrimethoxysilane, γ-mercaptopropyltriethoxysilane, γ-mercaptopropylmethyldimethoxysilane and γ-mercaptopropylmethyldiethoxysilane base silane; Silanes containing epoxy groups, such as γ-glycidoxypropyltrimethoxysilane, γ-glycidoxypropyltriethoxysilane, γ-glycidoxypropylmethyl dimethyl Oxysilane, β-(3,4-epoxycyclohexyl)ethyltrimethoxysilane and β-(3,4-epoxycyclohexyl)ethyltriethoxysilane; Carboxysilanes, such as β-carboxyethyltriethoxysilane, β-carboxyethylphenylbis-(2-methoxyethoxy)silane and N-β-(carboxymethyl)aminoethyl- γ-Aminopropyltrimethoxysilane; Halogen-containing silanes, such as γ-chloropropyltrimethoxysilane; and Isotrimeric isocyanate silanes such as tris(trimethoxysilyl isocyanurate).
在較佳之具體實例中,至少一種黏著促進劑G2係選自含胺基之矽烷,特別是含胺基之三甲氧基矽烷。較佳地,至少一種黏著促進劑G2包含至少一種含胺基之三甲氧基矽烷,較佳地3-胺基丙基三甲氧基矽烷(例如來自Evonik的Dynasylan ®AMMO)。 In a preferred embodiment, at least one adhesion promoter G2 is selected from amine-containing silanes, especially amine-containing trimethoxysilane. Preferably, at least one adhesion promoter G2 comprises at least one amino-containing trimethoxysilane, preferably 3-aminopropyltrimethoxysilane (eg Dynasylan ® AMMO from Evonik).
較佳地,以總聚合物組成物為基準計,本發明之聚合物組成物包含至少一種觸媒G3,該至少一種觸媒G3之量為0.01至3重量%,較佳地0.1至2重量%,更佳地0.2至1重量%。Preferably, based on the total polymer composition, the polymer composition of the present invention comprises at least one catalyst G3 in an amount of 0.01 to 3% by weight, preferably 0.1 to 2% by weight %, more preferably 0.2 to 1% by weight.
典型地,該至少一種觸媒G3係選自公知的矽烷醇縮合觸媒。合適之觸媒G3係例如描述於US6,077,896及EP1288247中。Typically, the at least one catalyst G3 is selected from known silanol condensation catalysts. Suitable catalysts G3 are described, for example, in US6,077,896 and EP1288247.
此縮合觸媒可為例如四價錫化合物如二月桂酸二丁基錫、鄰苯二甲酸二丁基錫、二乙醯基丙酮酸二丁基錫、二乙酸二丁基錫、二乙基己酸二丁基錫、二辛酸二丁基錫、二(馬來酸甲酯)二丁基錫、二(馬來酸乙酯)二丁基錫、二(馬來酸丁酯)二丁基錫、二(馬來酸異辛酯)二丁基錫、二(馬來酸十三烷酯)二丁基錫、二(馬來酸苯甲酯)二丁基錫、馬來酸二丁基錫、二乙酸二辛基錫、二硬脂酸二辛基錫、二月桂酸二辛基錫、二(馬來酸乙酯)二辛基錫、二(馬來酸異辛酯)二辛基錫、二甲氧基二丁基錫、二壬酚二丁基錫及氧化二丁烯基錫;二價錫化合物如辛酸亞錫、環烷酸亞錫(stannous naphthenate)及硬脂酸亞錫;鈦酸酯如鈦酸四丁酯及鈦酸四丙酯;有機鋁化合物如三乙醯丙酮酸鋁、三(乙醯乙酸乙酯)鋁及乙醯乙酸乙酯二異丙氧基鋁;螯合化合物如四乙醯丙酮酸鋯及四乙醯丙酮酸鈦;辛酸鉛;胺化合物如丁胺、辛胺、月桂胺、二丁胺、單乙醇胺、二乙醇胺、三乙醇胺、二伸乙基三胺、三伸乙基四胺、油胺、環己胺、苯甲胺、二乙基胺基丙基胺、伸二甲苯二胺、三伸乙基二胺、胍、二苯基胍、2,4,6-三(二甲基胺基甲基)酚、嗎啉、N-甲基嗎啉、2-乙基-4-甲基咪唑及1,8-二氮雜雙環[5.4.0]十一烯-7 (DBU)或這些胺化合物與羧酸之鹽類;胺化合物-有機錫化合物反應產物及混合物,例如月桂胺-辛酸亞錫反應產物或混合物;可自過量聚胺和多元酸獲得之低分子量聚醯胺樹脂;過量聚胺和環氧基化合物之反應產物;含胺基之矽烷偶合劑如γ-胺基丙基三甲氧基矽烷及N-(β-胺基乙基)胺基丙基甲基-二甲氧基矽烷;等矽烷醇縮合觸媒,再者,其他已知之矽烷醇縮合觸媒如酸性觸媒及鹼性觸媒。This condensation catalyst can be, for example, a tetravalent tin compound such as dibutyltin dilaurate, dibutyltin phthalate, dibutyltin diacetylacetonate, dibutyltin diacetate, dibutyltin diethylhexanoate, dibutyltin dioctoate Butyl tin, two (methyl maleate) dibutyl tin, two (ethyl maleate) dibutyl tin, two (butyl maleate) dibutyl tin, two (isooctyl maleate) dibutyl tin, two (maleic acid) dibutyl tin Tridecylmaleate) dibutyltin, di(benzyl maleate) dibutyltin, dibutyltin maleate, dioctyltin diacetate, dioctyltin distearate, dioctyl dilaurate Tin, di(ethyl maleate) dioctyl tin, bis(isooctyl maleate) dioctyl tin, dimethoxy dibutyl tin, dinonyl phenol dibutyl tin and dibutenyl tin oxide; Sn compounds such as stannous octoate, stannous naphthenate and stannous stearate; titanates such as tetrabutyl titanate and tetrapropyl titanate; organoaluminum compounds such as aluminum triacetylpyruvate , tri(ethyl acetate) aluminum and ethyl acetate diisopropoxide; chelating compounds such as zirconium tetraacetylpyruvate and titanium tetraacetylpyruvate; lead octanoate; amine compounds such as butylamine, Octylamine, Laurylamine, Dibutylamine, Monoethanolamine, Diethanolamine, Triethanolamine, Diethylenetriamine, Triethylenetetramine, Oleylamine, Cyclohexylamine, Benzylamine, Diethylaminopropyl Baseamine, xylylenediamine, triethylenediamine, guanidine, diphenylguanidine, 2,4,6-tris(dimethylaminomethyl)phenol, morpholine, N-methylmorpholine, 2-Ethyl-4-methylimidazole and 1,8-diazabicyclo[5.4.0]undecene-7 (DBU) or salts of these amine compounds and carboxylic acids; amine compound-organotin compound reaction Products and mixtures, for example laurylamine-stannous octoate reaction products or mixtures; low molecular weight polyamide resins obtainable from excess polyamines and polybasic acids; reaction products of excess polyamines and epoxy compounds; silanes containing amine groups Coupling agents such as γ-aminopropyltrimethoxysilane and N-(β-aminoethyl)aminopropylmethyl-dimethoxysilane; silanol condensation catalysts, and other known Silanol condensation catalysts such as acid catalysts and alkaline catalysts.
觸媒G3可為上述觸媒中之一種或二或更多種的組合。The catalyst G3 can be one or a combination of two or more of the above catalysts.
較佳地,該至少一種觸媒G3係選自基於四價鈦之金屬有機化合物(如二乙醯丙酮酸二異丙氧基鈦)及/或基於四價錫之有機化合物(如二乙醯丙酮酸二烷基錫化合物及鄰苯二甲酸酯二烷基錫)。此觸媒例如可自TIB Chemicals購得。較佳地,觸媒G3包含至少一種有機錫化合物,更佳地二乙醯丙酮酸二丁基錫及/或二乙醯丙酮酸二辛基錫(例如TIB Chemicals之TIB KAT® 223,二酮酸二辛基錫)。Preferably, the at least one catalyst G3 is selected from metal organic compounds based on tetravalent titanium (such as diisopropoxytitanium diacetylacetonate) and/or organic compounds based on tetravalent tin (such as diacetyl dialkyltin pyruvate and dialkyltin phthalate). Such catalysts are commercially available, for example, from TIB Chemicals. Preferably, catalyst G3 comprises at least one organotin compound, more preferably dibutyltin diacetylpyruvate and/or dioctyltin diacetylpyruvate (such as TIB KAT® 223 of TIB Chemicals, diketoacid di octyl tin).
較佳地,本發明之聚合物組成物包含至少一種水分清除劑D,以總聚合物組成物為基準,該至少一種水分清除劑D之量為0.5至5重量%,較佳地0.7至5重量%,更佳地0.8至3重量%。Preferably, the polymer composition of the present invention comprises at least one moisture scavenger D, based on the total polymer composition, the amount of the at least one moisture scavenger D is 0.5 to 5% by weight, preferably 0.7 to 5% by weight % by weight, more preferably 0.8 to 3% by weight.
較佳地,水分清除劑G4係選自乙烯基矽烷,特別是含乙烯基型不飽和基團的矽烷,如乙烯基三甲氧基矽烷(VTMO)、乙烯基三乙氧基矽烷(VTEO)、γ-甲基丙烯醯氧基丙基-甲基二甲氧基矽烷及γ-丙烯醯氧基丙基甲基三乙氧基矽烷。例如,水分清除劑G4係乙烯基三甲氧基矽烷(VTMO) (例如來自Evonik之Dynasylan ®VTMO)及/或乙烯基三乙氧基矽烷(VTEO) (例如來自Evonik之Dynasylan ®VTEO)。 Preferably, the moisture scavenger G4 is selected from vinyl silanes, especially silanes containing vinyl unsaturated groups, such as vinyl trimethoxy silane (VTMO), vinyl triethoxy silane (VTEO), γ-methacryloxypropyl-methyldimethoxysilane and γ-acryloxypropylmethyltriethoxysilane. For example, the moisture scavenger G4 is vinyltrimethoxysilane (VTMO) (eg Dynasylan ® VTMO from Evonik) and/or vinyltriethoxysilane (VTEO) (eg Dynasylan ® VTEO from Evonik).
再者,聚合物組成物可包含各自適量之0至10重量%的至少其他添加物G,不同於G1、G2、G3和G4以及不同於A、B、C和C,如增容劑(compatibilizer)、增黏劑、物理性質改質劑、儲存穩定性改進劑、金屬去活化劑、抗臭氧劑、光穩定劑、熱穩定劑、含磷過氧化物分解劑、潤滑劑、顏料(如TiO 2和碳黑)、觸變劑(如聚醯胺蠟、發煙二氧化矽、氫化蓖麻油)、發泡劑、阻燃劑及抗靜電劑。 Furthermore, the polymer composition may contain a respective appropriate amount of 0 to 10% by weight of at least other additives G, different from G1, G2, G3 and G4 and different from A, B, C and C, such as compatibilizers ), tackifier, physical property modifier, storage stability improver, metal deactivator, antiozonant, light stabilizer, heat stabilizer, phosphorus peroxide decomposer, lubricant, pigment (such as TiO 2 and carbon black), thixotropic agents (such as polyamide wax, fumed silica, hydrogenated castor oil), foaming agents, flame retardants and antistatic agents.
再者,本發明也包含一種製備本發明之聚合物組成物的方法,其中該方法包含混合組分P和S及隨意地F及/或G。Furthermore, the present invention also includes a process for preparing the polymer composition of the present invention, wherein the process comprises mixing components P and S and optionally F and/or G.
在現有技術中已知並描述了幾種製備SMP密封劑之方法。典型地,該製備方法包含採任意順序混合上述組分並於常溫下捏合所得之混合物或使用混合器、輥、捏合機等加熱。該組成物可例如藉由將化合物A、B、C、D及隨意地F和G加於矽基改質聚合物P並實現均勻分散和溶解,若有需要,調節例如加熱和攪拌條件而製備。典型地,組分之混合係於已知設備,如捏合機(行星式混合機)、配備真空加熱系統的多軸混合機中進行。再者,該製備方法可包含使用適當份數之適當溶劑溶解一或多種組分,然後混合溶液。若有需要,可使用分散改進劑(dispersion improving agent)。Several methods of preparing SMP encapsulants are known and described in the prior art. Typically, the production method involves mixing the above-mentioned components in an arbitrary order and kneading the resulting mixture at normal temperature or heating using a mixer, a roller, a kneader or the like. The composition can be prepared, for example, by adding compounds A, B, C, D and optionally F and G to the silicon-based modified polymer P and achieving uniform dispersion and dissolution, and adjusting conditions such as heating and stirring if necessary . Typically, the mixing of the components is carried out in known equipment, such as kneaders (planetary mixers), multishaft mixers equipped with a vacuum heating system. Furthermore, the preparation method may comprise dissolving one or more components using appropriate parts of an appropriate solvent and then mixing the solutions. A dispersion improving agent may be used if desired.
典型地,SMP聚合物組成物係於沒有空氣和濕氣之情況下製備並於製備後直接填充於可封閉之容器如藥筒中。Typically, SMP polymer compositions are prepared in the absence of air and moisture and filled directly after preparation into closeable containers such as cartridges.
以上述方式獲得的本發明之聚合物組成物可作為單組分可固化組成物應用。此可固化組成物可藉由將本發明之聚合物組成物製備成實質上無水分的狀態獲得。當採密閉狀態儲存時,此組成物可經受長期儲存,且當暴露於大氣條件下時,其會從表面迅速固化。The polymer composition of the present invention obtained in the above manner can be used as a one-component curable composition. The curable composition can be obtained by preparing the polymer composition of the present invention in a substantially moisture-free state. This composition withstands long-term storage when stored in an airtight state, and it solidifies rapidly from the surface when exposed to atmospheric conditions.
本發明之可固化聚合物組成物可用作建築工程領域及工業應用中的彈性密封劑或黏著劑。也可用作油漆、黏著劑、澆注填料、塗料等。The curable polymer composition of the present invention can be used as an elastic sealant or adhesive in the field of construction engineering and industrial applications. It can also be used as paint, adhesive, pouring filler, coating, etc.
除非另行指明,否則以%提供之所有值皆指重量%,且所有提供之比率皆基於重量比。除非另行指明,否則措辭ppm意指mg/kg。All values provided in % refer to % by weight and all ratios provided are by weight unless otherwise indicated. The word ppm means mg/kg unless otherwise indicated.
本發明藉由以下實施例及申請專利範圍進一步說明。 實施例 The present invention is further illustrated by the following examples and claims. Example
製備包含本發明之液體穩定劑組成物、本發明之粉狀穩定劑組成物及對照組穩定劑組成物的實施例和比較例。 1. 液體穩定劑組成物SC之製備 Examples and comparative examples including the liquid stabilizer composition of the present invention, the powder stabilizer composition of the present invention and the control stabilizer composition were prepared. 1. Preparation of liquid stabilizer composition SC
藉由將組分A1、A2、B1和C1與液體稀釋劑D4混合以製備以下穩定劑組成物。將穩定劑組分A1、A2、B1、C1和D4定義如下。分別地提供組分A1和A2作為包含構成液體稀釋劑D的總量之溶劑D5.1 (1-甲氧基-2-丙醇)和D5.2 (二甲苯)的組成。表1及2列出之所有量皆參照活性成分之量以重量%表示。稀釋劑D之合併量及稀釋劑D4、D5.1和D5.2之單獨量以各自穩定劑組成物總重量為基準計以重量%表示。 The following stabilizer composition was prepared by mixing components A1, A2, B1 and C1 with liquid diluent D4. The stabilizer components A1, A2, B1, C1 and D4 are defined as follows. Components A1 and A2 are respectively provided as compositions comprising solvents D5.1 (1-methoxy-2-propanol) and D5.2 (xylene) constituting the total amount of liquid diluent D. All amounts listed in Tables 1 and 2 are expressed in % by weight with reference to the amount of active ingredient. The combined amount of diluent D and the individual amounts of diluents D4, D5.1 and D5.2 are expressed in % by weight based on the total weight of the respective stabilizer compositions.
組分: A1:UV吸收劑組成物,其包含85重量%之2-[-4-[(2-羥基-3-十二烷氧基丙基)氧基]-2-羥基苯基]-4,6-雙(2,4-二甲基苯基)-1,3,5-三嗪和2-[4-[(2-羥基-3-十三烷氧基丙基)氧基]-2-羥基苯基]-4,6-雙(2,4-二甲基苯基)-1,3,5-三嗪作為活性成分的混合物及15重量%之1-甲氧基-2-丙醇作為液體稀釋劑D5.1 (來自法蘭克福,Clariant的Hostavin® 3400 LIQ); A2:UV吸收劑組成物,其包含80重量%之2-乙基-2’-乙氧基-草醯苯胺(CAS-No.23949-66-8)作為活性成分及20重量%之二甲苯作為液體稀釋劑D5.2 (來自Clariant的 Hostavin 3206 LIQ); B1:HALS,2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧丙基)二螺環-(5.1.11.2)-二十一烷-21-酮與環氧氯丙烷之反應產物,(來自法蘭克福,Clariant 的 Hostavin® N30); C1:酚類抗氧化劑,雙-(3,3-雙-(4’-羥基-3’-三級丁基-苯基)丁酸)-甘醇酯(CAS編號32509-66-3); D:液體稀釋劑,特別是D4、D5.1和D5.2; D4:液體稀釋劑,乙烯基三甲氧基矽烷(Dynasylan ®VTMO,Evonik); D5.1:液體稀釋劑,1-甲氧基-2-丙醇; D5.2:液體稀釋劑,二甲苯。 Components: A1: UV absorber composition comprising 85% by weight of 2-[-4-[(2-hydroxy-3-dodecyloxypropyl)oxy]-2-hydroxyphenyl]- 4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine and 2-[4-[(2-hydroxy-3-tridecyloxypropyl)oxy] -2-hydroxyphenyl]-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine as a mixture of active ingredients and 15% by weight of 1-methoxy-2 - propanol as liquid diluent D5.1 (Hostavin® 3400 LIQ from Clariant, Frankfurt); A2: UV absorber composition comprising 80% by weight of 2-ethyl-2'-ethoxy-oxalyl Aniline (CAS-No. 23949-66-8) as active ingredient and 20% by weight xylene as liquid diluent D5.2 (Hostavin 3206 LIQ from Clariant); B1: HALS, 2,2,4,4- Tetramethyl-7-oxa-3,20-diazabispiro-20-(2,3-epoxypropyl)bispiro-(5.1.11.2)-eicosan-21-one Reaction product with epichlorohydrin, (Hostavin® N30 from Clariant, Frankfurt); C1: phenolic antioxidant, bis-(3,3-bis-(4'-hydroxy-3'-tertiary butyl- Phenyl)butyrate)-glycol ester (CAS No. 32509-66-3); D: liquid diluent, especially D4, D5.1 and D5.2; D4: liquid diluent, vinyltrimethoxysilane (Dynasylan ® VTMO, Evonik); D5.1: Liquid diluent, 1-methoxy-2-propanol; D5.2: Liquid diluent, xylene.
液體穩定劑組成物SC1、SC2、SC3及SC4係藉由於室溫下以表1所列之量混合組分而獲得。獲得之穩定劑組成物以透明、穩定之液體溶液形式獲得。穩定劑組成物SC1、SC2、SC3及SC4係用於下述SMP組成物之製備。 使用液體穩定劑組成物製備SMP測試樣品 The liquid stabilizer compositions SC1, SC2, SC3 and SC4 were obtained by mixing the components in the amounts listed in Table 1 at room temperature. The obtained stabilizer composition is obtained in the form of a transparent, stable liquid solution. Stabilizer compositions SC1, SC2, SC3 and SC4 are used in the preparation of the following SMP compositions. Preparation of SMP Test Samples Using Liquid Stabilizer Composition
使用SMP1 (Kaneka ®S303H,矽基封端聚醚)及液體穩定劑組成物SC1、SC2、SC3或SC4中之一者製備以下SMP組成物,其中組分SMP1、F1、G1、G2、G3及G4如下所述。 Use SMP1 (Kaneka ® S303H, silicon-terminated polyether) and one of the liquid stabilizer compositions SC1, SC2, SC3 or SC4 to prepare the following SMP composition, wherein the components SMP1, F1, G1, G2, G3 and G4 is described below.
使用以下組分: 組分P: SMP1:Kaneka MS Polymer ®S303H,Kaneka,矽基末端聚醚 填料組分F: F1:Hakuenka ®CCR-S10,Omya,塗覆脂肪酸之沉澱碳酸鈣 其他組分G: G1:塑化劑,Mesamoll ®,Lanxess,C 10-C 21烷磺酸苯基酯, G2:黏著促進劑,3-胺基丙基三甲氧基矽烷(Dynasylan ®AMMO,Evonik); G3:觸媒TIB KAT ®223,TIB Chemicals,二酮酸二辛基錫, G4:水分清除劑,乙烯基三甲氧基矽烷(Dynasylan ®VTMO,Evonik); 穩定劑組分: 根據表1,分別使用液體穩定劑組成物SC1、SC2、SC3及SC4製備本發明之實施例(實施例1至實施例4)。 The following components were used: Component P: SMP1: Kaneka MS Polymer ® S303H, Kaneka, silicon-terminated polyether filler Component F: F1: Hakuenka ® CCR-S10, Omya, fatty acid-coated precipitated calcium carbonate Other Component G : G1: plasticizer, Mesamoll ® , Lanxess, C 10 -C 21 phenyl alkanesulfonate, G2: adhesion promoter, 3-aminopropyltrimethoxysilane (Dynasylan ® AMMO, Evonik); G3: Catalyst TIB KAT ® 223, TIB Chemicals, dioctyltin diketoate, G4: moisture scavenger, vinyltrimethoxysilane (Dynasylan ® VTMO, Evonik); The stabilizer compositions SC1, SC2, SC3 and SC4 were used to prepare the examples (Example 1 to Example 4) of the present invention.
將製備之SMP組成物總結於表2。 SMP測試樣品之製備 The prepared SMP compositions are summarized in Table 2. Preparation of SMP test samples
於150 ml高速混合杯(PP 250 ml)中,將52.0 g之填料F1加於29.2 g之聚合物SMP1及14.6 g之塑化劑G1中。將混合物在SpeedMixer DAC 600.1 FVZ中以 2300 rpm於周遭溫度下攪拌30秒。將根據表2之量的穩定劑組成物SC1、SC2、SC3或SC3加於所得混合物中並於2300 rpm下再攪拌60秒。最後,將0.9 g之黏著促進劑G2、1.0 g之水分清除劑G4及0.5 g之觸媒G3加於所得混合物並以2300 rpm再攪拌30秒。In a 150 ml high-speed mixing cup (PP 250 ml), 52.0 g of filler F1 was added to 29.2 g of polymer SMP1 and 14.6 g of plasticizer G1. The mixture was stirred in a SpeedMixer DAC 600.1 FVZ at 2300 rpm for 30 seconds at ambient temperature. The stabilizer composition SC1, SC2, SC3 or SC3 in the amount according to Table 2 was added to the resulting mixture and stirred for a further 60 seconds at 2300 rpm. Finally, 0.9 g of adhesion promoter G2, 1.0 g of moisture scavenger G4 and 0.5 g of catalyst G3 were added to the resulting mixture and stirred at 2300 rpm for another 30 seconds.
液體穩定劑組成物SC1、SC2、SC3及SC4可輕易準確地投料,並於製備SMP測試樣品期間不顯示任何粉塵形成。 SMP測試樣品之測試 The liquid stabilizer compositions SC1 , SC2, SC3 and SC4 were easily and accurately dosed and did not show any dust formation during the preparation of the SMP test samples. SMP test sample test
使用下文第4節所述之測試方法測定各樣品的熱穩定性、表面抗裂性及色彩。將測試結果彙總於表3。 The thermal stability, surface crack resistance, and color of each sample were determined using the test methods described in Section 4 below. The test results are summarized in Table 3.
結果證實使用本發明之UV吸收劑A1及/或A2、受阻胺光穩定劑B1、酚類抗氧化劑C1和液體稀釋劑D的液體組合之SMP測試樣品滿足對熱穩定性和表面抗裂性的高要求(參見實施例1 - 實施例4)。對於使用本發明之穩定劑組成物的SMP測試樣品實現了特別高之UV穩定性,其中UV吸收劑A1與UV吸收劑A2之比率高於1:1,即當UV吸收劑A1佔UV吸收劑總量之多於50重量% (參見實施例3及實施例4)。另一方面,對於包含至少一種UV吸收劑A2之穩定劑組成物,觀察到更好的熱穩定性(參見實施例3與實施例4)。這也說明了降低穩定劑組成物的成本,因為與(2-羥基苯基)-對稱-三嗪UV吸收劑如UV吸收劑A1相比,草醯苯胺UV吸收劑如UV吸收劑A2更便宜。初始黃化指數(0小時時之 YI)於樣品之間並沒有顯著變化。 2. 具有粉狀穩定劑組成物之SMP測試樣品的製備及測試 The results confirm that the SMP test sample using the liquid combination of UV absorber A1 and/or A2 of the present invention, hindered amine light stabilizer B1, phenolic antioxidant C1 and liquid diluent D meets the requirements for thermal stability and surface crack resistance High requirements (see Example 1 - Example 4). A particularly high UV stability was achieved for the SMP test samples using the stabilizer composition of the invention, wherein the ratio of UV absorber A1 to UV absorber A2 was higher than 1:1, i.e. when UV absorber A1 accounted for UV absorber The total amount is more than 50% by weight (referring to embodiment 3 and embodiment 4). On the other hand, better thermal stability is observed for stabilizer compositions comprising at least one UV absorber A2 (see Example 3 and Example 4). This also accounts for the reduced cost of the stabilizer composition, since oxanilide UV absorbers such as UV absorber A2 are cheaper compared to (2-hydroxyphenyl)-symmetric-triazine UV absorbers such as UV absorber A1 . The initial yellowness index (YI at 0 hours) did not vary significantly between samples. 2. Preparation and testing of SMP test samples with powder stabilizer composition
在不含液體稀釋劑D的情況下使用包含組分A、B和隨意地C之粉狀穩定劑組合製備了幾種SMP測試樣品。再者,對照組實施例係藉由使用習用穩定劑組合製備SMP測試樣品製成。Several SMP test samples were prepared using a powdered stabilizer combination comprising components A, B and optionally C without liquid diluent D. Furthermore, control examples were made by preparing SMP test samples using conventional stabilizer combinations.
使用以下組分: 組分P: SMP1:Kaneka ®S303H,矽基末端聚醚 穩定劑S: A3:UV吸收劑,不含液體稀釋劑之2-乙基-2’-乙氧基-草醯苯胺(CAS-No. 23949-66-8); B1:HALS,2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)二螺環-(5.1.11.2)-二十一烷-21-酮和環氧氯丙烷的反應產物(來自Clariant之Hostavin ®N30); C1:酚類抗氧化劑,雙-(3,3-雙-(4’-羥基-3’-三級丁基苯基)丁酸)-甘醇酯(CAS-No. 32509-66-3); R1:對照組1係以下組合 UV-1 UV吸收劑,2-(2’-羥基-3’-三級丁基-5’-甲基苯基)-5-氯苯并三唑(CAS-No. 3896-11-5),及 HALS-1 癸二酸雙(2,2,6,6-四甲基-4-六氫吡啶基酯) (CAS-No. 52829-07-9) R2:對照組2為Tinuvin ®5866 (來自BASF),其係UV吸收劑和鹼性HALS之混合物,其中包括HALS組分丁基丙二酸雙(1,2,2,6,6-五甲基-4-氮雜環己基)[[3,5-雙(1,1-二甲基乙基)-4-羥基苯基]甲基酯]。 填料組分F: F1:Hakuenka ®CCR-S10,塗覆脂肪酸之沉澱碳酸鈣 其他組分G: G1:塑化劑,Mesamoll ®,C 10-C 21烷磺酸苯基酯, G2:黏著促進劑,3-胺基丙基三甲氧基矽烷(Dynasylan ®AMMO,Degussa Evonik); G3:觸媒TIB KAT 223,二酮酸二辛基錫, G4:水分清除劑,乙烯基三甲氧基矽烷(Dynasylan ®VTMO,Degussa Evonik)。 The following components were used: Component P: SMP1: Kaneka ® S303H, silicon-terminated polyether stabilizer S: A3: UV absorber, 2-ethyl-2'-ethoxy-oxalyl without liquid diluent Aniline (CAS-No. 23949-66-8); B1: HALS, 2,2,4,4-tetramethyl-7-oxa-3,20-diazabispiro-20-(2, Reaction product of 3-epoxy-propyl)bispiro-(5.1.11.2)-eicosan-21-one and epichlorohydrin (Hostavin ® N30 from Clariant); C1: phenolic antioxidant, Bis-(3,3-bis-(4'-hydroxy-3'-tertiary butylphenyl)butanoic acid)-glycol ester (CAS-No. 32509-66-3); R1: control group 1 The following combination UV-1 UV absorber, 2-(2'-hydroxy-3'-tertiary butyl-5'-methylphenyl)-5-chlorobenzotriazole (CAS-No. 3896-11- 5), and HALS-1 sebacic acid bis(2,2,6,6-tetramethyl-4-hexahydropyridyl ester) (CAS-No. 52829-07-9) R2: Control group 2 is Tinuvin ® 5866 (from BASF), which is a mixture of UV absorbers and basic HALS, including the HALS component butylmalonate bis(1,2,2,6,6-pentamethyl-4-azacyclo hexyl)[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]methyl ester]. Filler component F: F1: Hakuenka ® CCR-S10, precipitated calcium carbonate coated with fatty acid Other components G: G1: Plasticizer, Mesamoll ® , C 10 -C 21 phenyl alkanesulfonate, G2: Adhesion promoter agent, 3-aminopropyltrimethoxysilane (Dynasylan ® AMMO, Degussa Evonik); G3: catalyst TIB KAT 223, dioctyltin diketoate, G4: moisture scavenger, vinyltrimethoxysilane ( Dynasylan® VTMO , Degussa Evonik).
於150 ml高速混合杯(PP 250 ml)中,將52.0 g之填料F1加於29.2 g之聚合物SMP1及14.6 g之塑化劑G1中。將混合物在SpeedMixer DAC 600.1 FVZ中以2300 rpm於周遭溫度下攪拌30秒。將根據以下表4、5及6之量的組分A3、B1及C1加於所得混合物中並於2300 rpm下再攪拌60秒。最後,將0.9 g之黏著促進劑G2、2.0 g之水分清除劑G4及0.5 g之觸媒G3加於所得混合物並以2300 rpm再攪拌30秒。相應地使用對照組穩定劑R1和R2製備對照組樣品對照組1至對照組6。In a 150 ml high-speed mixing cup (PP 250 ml), 52.0 g of filler F1 was added to 29.2 g of polymer SMP1 and 14.6 g of plasticizer G1. The mixture was stirred in a SpeedMixer DAC 600.1 FVZ at 2300 rpm for 30 seconds at ambient temperature. Components A3, B1 and C1 in amounts according to the following Tables 4, 5 and 6 were added to the resulting mixture and stirred for a further 60 seconds at 2300 rpm. Finally, 0.9 g of adhesion promoter G2, 2.0 g of moisture scavenger G4 and 0.5 g of catalyst G3 were added to the resulting mixture and stirred at 2300 rpm for another 30 seconds. Control samples Control 1 to Control 6 were prepared using control stabilizers R1 and R2 accordingly.
使用下文第4節所述之測試方法測定各樣品的熱穩定性、表面抗裂性及色彩。The thermal stability, surface crack resistance, and color of each sample were determined using the test methods described in Section 4 below.
使用如以下表4所述之鹼性調合物: Use an alkaline blend as described in Table 4 below:
將測試結果彙總於以下表5及6。The test results are summarized in Tables 5 and 6 below.
結果證實使用本發明之UV吸收劑A3、HALS B1及酚類抗氧化劑C1的組合滿足或改進了對照組材料之熱穩定性和表面抗裂性(對照組1-對照組6)。通常,與對照組相比,本發明之穩定劑組合之熱穩定性提高了44%且表面抗裂性提高了67%。The results confirmed that the combination of UV absorber A3, HALS B1 and phenolic antioxidant C1 of the present invention satisfied or improved the thermal stability and surface crack resistance of the control group (control group 1-control group 6). Typically, the stabilizer combination of the present invention had a 44% increase in thermal stability and a 67% increase in surface crack resistance compared to the control.
再者,實驗數據顯示儘管本發明之UV吸收劑A3和受阻胺光穩定劑B1的組合在沒有酚類抗氧化劑C1之情況下為SMP測試樣品提供了熱穩定性和UV穩定性的組合,這足以滿足眾多應用(熱穩定性> 1000小時,UV穩定性≥ 500小時,實施例5、6、17、18),但是酚類之添加卻導致熱穩定性和表面抗裂性的顯著協同改善作用(實施例7至實施例16、實施例19)。Furthermore, the experimental data show that although the combination of UV absorber A3 and HALS B1 of the present invention provided a combination of thermal stability and UV stability for SMP test samples in the absence of phenolic antioxidant C1, this Sufficient for many applications (thermal stability > 1000 hours, UV stability ≥ 500 hours, examples 5, 6, 17, 18), but the addition of phenols leads to a significant synergistic improvement in thermal stability and surface crack resistance (embodiment 7 to embodiment 16, embodiment 19).
初始黃化指數(0小時時的YI)於根據本發明之樣品中並沒有顯著變化。6.29之平均YI與對照組1至對照組4的結果一致,且比對照組5至對照組6 (包含習用之受阻胺光穩定劑及苯并三唑型UV吸收劑的組合)的結果低。 The initial yellowness index (YI at 0 hours) did not change significantly in the samples according to the invention. The average YI of 6.29 is consistent with the results of control group 1 to control group 4, and lower than the results of control group 5 to control group 6 (combining conventional hindered amine light stabilizers and benzotriazole UV absorbers).
再者,令人驚訝地發現酚類抗氧化劑之化學性質很重要,且使用酚類抗氧化劑C1與本發明之穩定劑組分A和B組合實現了有利的協同組合。 Furthermore, it was surprisingly found that the chemical nature of the phenolic antioxidants is important and that using the phenolic antioxidant C1 in combination with the stabilizer components A and B according to the invention an advantageous synergistic combination is achieved.
對照組1至對照組4證實本發明之UV吸收劑 A3、受阻胺光穩定劑B1及酚類抗氧化劑C1的粉狀穩定劑組合經常表現優於習用之現有技術組合。儘管穩定劑組合對照組1至對照組4根據現行歐盟化學物質分類、標籤及包裝法規被歸類為危險品,但是實施例5至19之粉狀穩定劑組合則不需要此標籤。此外,對照組1至對照組4證實本發明之UV吸收劑A1及/或A2、受阻胺光穩定劑B1、酚類抗氧化劑C1及液化稀釋劑D的液體穩定劑組合表現優於習用之現有技術粉狀穩定劑組合(實施例1至實施例4)。根據本發明之液體穩定劑組成物另具有可輕易地計量並均勻地摻入SMP聚合物組成物中的優點。Controls 1 to 4 demonstrate that the inventive powdered stabilizer combination of UV absorber A3, hindered amine light stabilizer B1 and phenolic antioxidant C1 often outperforms conventional prior art combinations. Although the stabilizer combination control group 1 to control group 4 are classified as dangerous goods according to the current EU regulations on the classification of chemical substances, labeling and packaging, the powdered stabilizer combinations of examples 5 to 19 do not require this label. In addition, control group 1 to control group 4 prove that the liquid stabilizer combination of UV absorber A1 and/or A2, hindered amine light stabilizer B1, phenolic antioxidant C1 and liquefied diluent D of the present invention performs better than the conventional existing Technical Powdered Stabilizer Combinations (Example 1 to Example 4). The liquid stabilizer composition according to the invention also has the advantage that it can be easily metered and uniformly incorporated into the SMP polymer composition.
其他對照組5及對照組6證實受阻胺光穩定劑及苯并三唑型UV吸附劑之習用穩定劑組合在初始黃化指數(0小時時的YI)方面特別差。 3. SMP測試樣品之製備及測試 The other control groups 5 and 6 demonstrate that the conventional stabilizer combination of hindered amine light stabilizer and benzotriazole type UV absorber is particularly poor in terms of initial yellowness index (YI at 0 hours). 3. Preparation and testing of SMP test samples
SMP測試樣品係根據第2節中描述之組分和方法製備。使用第4節中描述之測試方法測定各樣品之熱穩定性、表面抗裂性及色彩。調合物係根據以上表5中之實施例8並列舉如下(所有量皆以g為單位): SMP test samples were prepared according to the components and methods described in Section 2. The thermal stability, surface crack resistance, and color of each sample were determined using the test methods described in Section 4. The blend is based on Example 8 in Table 5 above and is listed below (all amounts are in g):
組分SMP1、F1、G1、G2、G4及G3如前所述。使用以下穩定劑A、B及C: A3:UV吸收劑,不含液體稀釋劑之2-乙基-2’-乙氧基-草醯苯胺(CAS-No. 23949-66-8); B1:HALS,2,2,4,4-四甲基-7-氧雜-3,20-二氮雜二螺環-20-(2,3-環氧-丙基)二螺環-(5.1.11.2)-二十一烷-21-酮和環氧氯丙烷的反應產物(來自Clariant之Hostavin ®N30); B2:Tinuvin® 622 (BASF SE),聚(4-羥基-2,2,6,6-四甲基-1-六氫吡啶乙醇-交替共聚-1,4-丁二酸) (CAS-No. 65447-77-0); C1:酚類抗氧化劑,雙-(3,3-雙-(4’-羥基-3’-三級丁基苯基)丁酸)-甘醇酯(CAS-No. 32509-66-3); C2:來自Clariant之Hostanox ®P-EPQ,以四(2,4-二(三級丁基)苯基)-1,1-聯苯-4,4’-二基二亞膦酸酯(CAS 38613-77-3)為主組分之多組分系統; C3:Irganox ®1010,季戊四醇四(3-(3,5-二(三級丁基)-4-羥基苯基)丙酸酯),CAS-No. 6683-19-8; C4:Tinuvin ®144 (BASF SE),丁基丙二酸雙(1,2,2,6,6-五甲基-4-六氫吡啶基)-[[3,5-雙(1,1-二甲基乙基)-4-羥基苯基]甲基酯] (CAS-No. 63843-89-0)。 Components SMP1, F1, G1, G2, G4 and G3 are as previously described. The following stabilizers A, B and C were used: A3: UV absorber, 2-ethyl-2'-ethoxy-oxanilide without liquid diluent (CAS-No. 23949-66-8); B1 : HALS, 2,2,4,4-Tetramethyl-7-oxa-3,20-diazabispiro-20-(2,3-epoxy-propyl)bispiro-(5.1 .11.2) Reaction product of -eicosan -21-one and epichlorohydrin (Hostavin® N30 from Clariant); B2: Tinuvin® 622 (BASF SE), poly(4-hydroxy-2,2,6 ,6-tetramethyl-1-hexahydropyridine ethanol-alternating copolymerization-1,4-butanedioic acid) (CAS-No. 65447-77-0); C1: phenolic antioxidant, bis-(3,3 -bis-(4'-hydroxy-3'-tertiary butylphenyl)butanoic acid)-glycol ester (CAS-No. 32509-66-3); C2: Hostanox ® P-EPQ from Clariant, with Tetrakis(2,4-bis(tertiary butyl)phenyl)-1,1-biphenyl-4,4'-diyl diphosphonite (CAS 38613-77-3) is the main component Component system; C3: Irganox ® 1010, pentaerythritol tetrakis(3-(3,5-bis(tertiary butyl)-4-hydroxyphenyl)propionate), CAS-No. 6683-19-8; C4 : Tinuvin ® 144 (BASF SE), butylmalonate bis(1,2,2,6,6-pentamethyl-4-hexahydropyridyl)-[[3,5-bis(1,1- Dimethylethyl)-4-hydroxyphenyl]methyl ester] (CAS-No. 63843-89-0).
將SMP調合物及測試結果彙總於以下表7及8。 The SMP formulations and test results are summarized in Tables 7 and 8 below.
上述實施例證實與習用寡聚合HALS如Tinuvin® 622相比,包含根據式(II)-1之寡聚受阻胺光穩定劑的本發明之穩定劑組分A及B1的組合導致優異之熱穩定性及UV穩定性(參見實施例20及對照組7)。The above examples demonstrate that the combination of stabilizer components A and B1 according to the invention comprising an oligomeric hindered amine light stabilizer according to formula (II)-1 leads to an excellent thermal stability compared to conventional oligomeric HALS such as Tinuvin® 622 Sexuality and UV stability (see embodiment 20 and control group 7).
此外,酚類抗氧化劑之化學性質的重要影響已藉由上述實施例得到證實。據發現使用酚類抗氧化劑C1與本發明之穩定劑組分A和B,特別是B1,的組合實現了有利之協同作用組合。使用另一種抗氧化劑(例如比較組抗氧化劑C2、C3或C4)並未實現關於改進之熱穩定性和改進之表面抗裂性(UV穩定性)的有利效果。 4. 測試方法 熱安定性 Furthermore, the important influence of the chemical nature of the phenolic antioxidants has been demonstrated by the above examples. It was found that the use of the phenolic antioxidant C1 in combination with the stabilizer components A and B according to the invention, especially B1, achieves an advantageous synergistic combination. The beneficial effects regarding improved thermal stability and improved surface crack resistance (UV stability) were not achieved using another antioxidant (eg comparative antioxidants C2, C3 or C4). 4. Test method thermal stability
如上所述製備之SMP樣品的熱穩定性係藉由以下程序進行測試。自固化膜切下25x25x2 mm之樣片並置於硬紙板上,以確保均勻的溫度。將硬紙板置於烘箱之中央段,於110℃之設定溫度下預熱(Memmert UF30通風烘箱)。烘箱在實驗室環境中,於23±1℃之溫度控制及50±5%之濕度控制下操作。定期檢查樣品。於110℃下觀察之間的最大間隔為100小時。樣品在最初開裂跡象時被歸類為“不合格”,並自烘箱中取出。熱穩定性以小時為單位直至失效為止,如表3所記載。 表面抗裂性 The thermal stability of the SMP samples prepared as described above was tested by the following procedure. Samples of 25x25x2 mm were cut from the cured film and placed on cardboard to ensure uniform temperature. The cardboard was placed in the central section of the oven and preheated at a set temperature of 110°C (Memmert UF30 ventilated oven). The oven is operated in a laboratory environment with a temperature control of 23±1°C and a humidity control of 50±5%. Samples are checked regularly. The maximum interval between observations at 110°C was 100 hours. Samples were classified as "Fail" at the first sign of cracking and were removed from the oven. Thermal stability is measured in hours until failure, as reported in Table 3. surface crack resistance
根據ISO4892-2 E2013濕/乾,使用耐候試驗機(weather-o-meter) (WoM)測定上述製備之SMP樣品的UV穩定性(表面抗裂性)。將固化樣品暴露於UV輻射下經歷102分鐘之乾燥週期之後緊接著用新鮮去礦物質水噴水18分鐘數個循環。輻射強度為60 W/m 2(300至400 nm)。測試係根據ISO 4892-2 E2013於50%相對濕度和65℃+/- 3℃ (黑色標準)之溫度下進行。 The UV stability (surface crack resistance) of the SMP samples prepared above was measured using a weather-o-meter (WoM) according to ISO4892-2 E2013 wet/dry. The cured samples were exposed to UV radiation for a drying cycle of 102 minutes followed by several cycles of water spraying with fresh demineralized water for 18 minutes. The radiation intensity is 60 W/m 2 (300 to 400 nm). The test is carried out according to ISO 4892-2 E2013 at 50% relative humidity and a temperature of 65°C +/- 3°C (black standard).
樣品每500小時檢查一次直至2000小時為止,包括表面裂紋之目測檢查及色彩測量。自2000小時開始樣品每100小時檢查一次,包括表面裂紋之目測檢查及色彩測量。當表面裂紋可見時將樣品記錄為“不合格”。表面抗裂性以小時為單位列出直至失效為止。許多應用將≥500小時之初始失效(特別是結合其他優異性質,例如≥ 1000小時之熱穩定性及良好之初始黃化指數)視為足夠,但是較佳為≥ 1000小時之初始失效。表面抗裂性以小時為單位列出直至失效為止,如表3記載的。 黃化 Samples are inspected every 500 hours until 2000 hours, including visual inspection of surface cracks and color measurement. Samples are inspected every 100 hours since 2000 hours, including visual inspection of surface cracks and color measurement. Samples were recorded as "Fail" when surface cracks were visible. Surface crack resistance is listed in hours until failure. An initial failure of ≥ 500 hours is considered sufficient for many applications, especially in combination with other excellent properties such as thermal stability of ≥ 1000 hours and a good initial yellowness index, but an initial failure of ≥ 1000 hours is preferred. Surface crack resistance is listed in hours until failure as reported in Table 3. yellowing
根據ASTM E313在沒有UV輻射暴露之情況下測量測試樣品的黃化指數(YI)。再者,根據CIE色彩系統之色彩測量係於通風烘箱中於110℃熱暴露1000小時前後進行。使用利用零盒(Zero box)及白色參考板校準之分光光度計Minolta CM-3600d測定CIE LAB值L*、a*和b*以及dE,其中L*定義亮度,a*表示紅/綠值,且b*表示黃/藍值。將UV輻射暴露前後之CIE LAB值的差異列於表3。The yellowness index (YI) of the test samples was measured according to ASTM E313 without UV radiation exposure. Furthermore, the color measurement according to the CIE color system was carried out before and after heat exposure at 110° C. for 1000 hours in a ventilated oven. Use a spectrophotometer Minolta CM-3600d calibrated with a zero box (Zero box) and a white reference plate to measure the CIE LAB values L*, a*, b* and dE, where L* defines the brightness, a* represents the red/green value, And b* represents the yellow/blue value. Table 3 lists the differences in CIE LAB values before and after UV radiation exposure.
Claims (19)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP21151071.4 | 2021-01-12 | ||
| EP21151071 | 2021-01-12 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| TW202237726A true TW202237726A (en) | 2022-10-01 |
Family
ID=74175618
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW110140946A TW202237726A (en) | 2021-01-12 | 2021-11-03 | Stabilizer composition for silyl-modified polymer sealants |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US20240110043A1 (en) |
| EP (1) | EP4277947A1 (en) |
| JP (1) | JP2024502483A (en) |
| KR (1) | KR20230132507A (en) |
| CN (1) | CN116917396A (en) |
| AU (1) | AU2022209196A1 (en) |
| BR (1) | BR112023013796A2 (en) |
| CA (1) | CA3204633A1 (en) |
| CL (1) | CL2023002019A1 (en) |
| MX (1) | MX2023008144A (en) |
| TW (1) | TW202237726A (en) |
| WO (1) | WO2022152668A1 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2025082760A1 (en) | 2023-10-19 | 2025-04-24 | Clariant International Ltd | Stabilizer composition for silyl-modified polymer sealants |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2228448C2 (en) | 1972-06-10 | 1984-05-24 | Hoechst Ag, 6230 Frankfurt | Hypolipidemic agents |
| US3971751A (en) | 1975-06-09 | 1976-07-27 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Vulcanizable silylether terminated polymer |
| DE2941004A1 (en) | 1979-10-10 | 1981-04-23 | Hoechst Ag, 6000 Frankfurt | ETHER ON POLYALKYL-1-OXA-DIAZASPIRODECAN BASE |
| DE19523115A1 (en) | 1995-06-26 | 1997-01-02 | Hoechst Ag | Dispersion powder with increased autoignition temperature |
| DE19735255B4 (en) | 1997-08-14 | 2007-08-23 | Clariant Produkte (Deutschland) Gmbh | Synergistic stabilizer mixture based on polyalkyl-1-oxa-diazaspirodecane compounds and its use |
| JP3913859B2 (en) | 1997-09-10 | 2007-05-09 | 株式会社カネカ | Curable composition |
| US5969014A (en) | 1997-09-23 | 1999-10-19 | Clariant Finance (Bvi) Limited | Synergistic polyamide stabilization method |
| GB2361005B (en) | 2000-04-04 | 2002-08-14 | Ciba Sc Holding Ag | Synergistic mixtures of uv-absorbers in polyolefins |
| DE60134851D1 (en) | 2000-05-24 | 2008-08-28 | Kaneka Corp | HARDENABLE COMPOSITIONS AND COMPATIBILITY AGENTS |
| US6664323B2 (en) | 2001-02-02 | 2003-12-16 | General Electric Company | Moisture curable sealants |
| US6642309B2 (en) | 2001-08-14 | 2003-11-04 | Kaneka Corporation | Curable resin composition |
| DE10204690A1 (en) | 2002-02-06 | 2003-08-07 | Clariant Gmbh | Process for the preparation of synergistic stabilizer mixtures |
| JP5288520B2 (en) * | 2003-10-10 | 2013-09-11 | セメダイン株式会社 | Curable composition with excellent weather resistance |
| MY139524A (en) * | 2004-06-30 | 2009-10-30 | Ciba Holding Inc | Stabilization of polyether polyol, polyester polyol or polyurethane compositions |
| DE102008042632A1 (en) | 2008-10-06 | 2010-04-08 | Wacker Chemie Ag | Crosslinkable compositions based on organosilicon compounds |
| JP2010248408A (en) | 2009-04-17 | 2010-11-04 | Kaneka Corp | Curable composition |
| JP5539671B2 (en) | 2009-05-22 | 2014-07-02 | 株式会社カネカ | Modified silicone resin foam and bedding comprising the modified silicone resin foam |
| US9051478B2 (en) | 2010-07-22 | 2015-06-09 | Basf Se | Additive combination for sealants applications |
| HUE048473T2 (en) | 2014-11-20 | 2020-07-28 | Cytec Ind Inc | Stabilizer compositions and methods for using same for protecting organic materials from uv light and thermal degradation |
| CN106833479A (en) | 2017-01-05 | 2017-06-13 | 河南瑞朗达新材料有限公司 | Vehicle environment protection type single-component silylated polyether fluid sealant and preparation method thereof |
| CN107892900B (en) | 2017-12-01 | 2021-07-02 | 北京东方雨虹防水技术股份有限公司 | Low-modulus high-resilience single-component silane modified polyether sealant and preparation method thereof |
| US20220259410A1 (en) * | 2019-07-10 | 2022-08-18 | Clariant International Ltd | Stabilizer composition for silyl-modified polymer sealants |
-
2021
- 2021-11-03 TW TW110140946A patent/TW202237726A/en unknown
-
2022
- 2022-01-11 CA CA3204633A patent/CA3204633A1/en active Pending
- 2022-01-11 JP JP2023541886A patent/JP2024502483A/en active Pending
- 2022-01-11 US US18/261,065 patent/US20240110043A1/en active Pending
- 2022-01-11 AU AU2022209196A patent/AU2022209196A1/en not_active Abandoned
- 2022-01-11 BR BR112023013796A patent/BR112023013796A2/en not_active Application Discontinuation
- 2022-01-11 MX MX2023008144A patent/MX2023008144A/en unknown
- 2022-01-11 WO PCT/EP2022/050390 patent/WO2022152668A1/en not_active Ceased
- 2022-01-11 KR KR1020237027088A patent/KR20230132507A/en active Pending
- 2022-01-11 EP EP22700754.9A patent/EP4277947A1/en active Pending
- 2022-01-11 CN CN202280014596.3A patent/CN116917396A/en active Pending
-
2023
- 2023-07-11 CL CL2023002019A patent/CL2023002019A1/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CN116917396A (en) | 2023-10-20 |
| BR112023013796A2 (en) | 2023-10-03 |
| MX2023008144A (en) | 2023-10-04 |
| AU2022209196A1 (en) | 2023-07-27 |
| JP2024502483A (en) | 2024-01-19 |
| CA3204633A1 (en) | 2022-07-21 |
| EP4277947A1 (en) | 2023-11-22 |
| WO2022152668A1 (en) | 2022-07-21 |
| CL2023002019A1 (en) | 2023-12-29 |
| US20240110043A1 (en) | 2024-04-04 |
| AU2022209196A9 (en) | 2025-03-06 |
| KR20230132507A (en) | 2023-09-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6162480B2 (en) | Curable composition | |
| CN106414537B (en) | Tin and phthalate free sealants based on silane terminated polymers | |
| US11643557B2 (en) | Coating composition for sealing surfaces | |
| CN108368334B (en) | Curable composition and cured product thereof | |
| US20220213272A1 (en) | One-Component Moisture-Curable Silicone Compositions | |
| EP4174050B1 (en) | Curing catalyst used for curing of polymer, method for producing same, moisture-curable composition, and method for producing cured product | |
| JP2010248408A (en) | Curable composition | |
| EP3392036A1 (en) | Method for producing laminate, and laminate | |
| KR102634515B1 (en) | Stabilizer composition for silyl modified polymer sealants | |
| RU2743538C2 (en) | Liquid composition for waterproof membrane | |
| TW202237726A (en) | Stabilizer composition for silyl-modified polymer sealants | |
| EP3156430A1 (en) | Urethane adhesive composition | |
| CN102782046A (en) | Curable composition | |
| WO2025082760A1 (en) | Stabilizer composition for silyl-modified polymer sealants | |
| KR20130045837A (en) | Curable composition | |
| WO2021002171A1 (en) | Adhesive composition containing organic silicon compound | |
| WO2025206194A1 (en) | Method for producing polymer having carbon-carbon unsaturated bond or reactive silicon group |