RU2682622C2 - Комбинированное использование клинических факторов риска и молекулярных маркеров тромбоза для поддержки принятия клинических решений - Google Patents
Комбинированное использование клинических факторов риска и молекулярных маркеров тромбоза для поддержки принятия клинических решений Download PDFInfo
- Publication number
- RU2682622C2 RU2682622C2 RU2015119520A RU2015119520A RU2682622C2 RU 2682622 C2 RU2682622 C2 RU 2682622C2 RU 2015119520 A RU2015119520 A RU 2015119520A RU 2015119520 A RU2015119520 A RU 2015119520A RU 2682622 C2 RU2682622 C2 RU 2682622C2
- Authority
- RU
- Russia
- Prior art keywords
- thrombosis
- risk
- clinical
- input features
- patient
- Prior art date
Links
- 208000007536 Thrombosis Diseases 0.000 title claims abstract description 69
- 238000000034 method Methods 0.000 claims abstract description 63
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 40
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 40
- 238000004422 calculation algorithm Methods 0.000 claims abstract description 16
- 239000002773 nucleotide Substances 0.000 claims abstract description 8
- 125000003729 nucleotide group Chemical group 0.000 claims abstract description 8
- 206010047249 Venous thrombosis Diseases 0.000 claims description 26
- 238000005457 optimization Methods 0.000 claims description 23
- 238000012549 training Methods 0.000 claims description 20
- 230000006870 function Effects 0.000 claims description 18
- 239000008280 blood Substances 0.000 claims description 16
- 210000004369 blood Anatomy 0.000 claims description 16
- 238000010200 validation analysis Methods 0.000 claims description 16
- 238000012360 testing method Methods 0.000 claims description 13
- 238000010801 machine learning Methods 0.000 claims description 12
- 206010051055 Deep vein thrombosis Diseases 0.000 claims description 10
- 238000012502 risk assessment Methods 0.000 claims description 10
- 102100030951 Tissue factor pathway inhibitor Human genes 0.000 claims description 9
- 108010013555 lipoprotein-associated coagulation inhibitor Proteins 0.000 claims description 9
- 208000008589 Obesity Diseases 0.000 claims description 8
- 235000020824 obesity Nutrition 0.000 claims description 8
- 230000035935 pregnancy Effects 0.000 claims description 8
- 230000008569 process Effects 0.000 claims description 7
- 102000015081 Blood Coagulation Factors Human genes 0.000 claims description 6
- 108010039209 Blood Coagulation Factors Proteins 0.000 claims description 6
- 239000003114 blood coagulation factor Substances 0.000 claims description 6
- 229940011871 estrogen Drugs 0.000 claims description 5
- 239000000262 estrogen Substances 0.000 claims description 5
- 238000011477 surgical intervention Methods 0.000 claims description 5
- 238000011156 evaluation Methods 0.000 claims description 4
- 102000054765 polymorphisms of proteins Human genes 0.000 claims description 4
- 238000004590 computer program Methods 0.000 claims description 3
- 101000911390 Homo sapiens Coagulation factor VIII Proteins 0.000 claims 2
- 230000000694 effects Effects 0.000 abstract description 4
- 239000003814 drug Substances 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- 238000011282 treatment Methods 0.000 description 10
- 238000013459 approach Methods 0.000 description 8
- 238000013528 artificial neural network Methods 0.000 description 8
- 108010054218 Factor VIII Proteins 0.000 description 7
- 102000001690 Factor VIII Human genes 0.000 description 7
- 239000003146 anticoagulant agent Substances 0.000 description 7
- 229940127219 anticoagulant drug Drugs 0.000 description 7
- 238000004364 calculation method Methods 0.000 description 7
- 238000002790 cross-validation Methods 0.000 description 7
- 230000002068 genetic effect Effects 0.000 description 7
- 108010074864 Factor XI Proteins 0.000 description 6
- 108010049003 Fibrinogen Proteins 0.000 description 6
- 102000008946 Fibrinogen Human genes 0.000 description 6
- 229940012952 fibrinogen Drugs 0.000 description 6
- 239000004019 antithrombin Substances 0.000 description 5
- 230000006872 improvement Effects 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 230000035945 sensitivity Effects 0.000 description 5
- 238000001356 surgical procedure Methods 0.000 description 5
- 206010020608 Hypercoagulation Diseases 0.000 description 4
- 101800004937 Protein C Proteins 0.000 description 4
- 102000017975 Protein C Human genes 0.000 description 4
- 229940096437 Protein S Drugs 0.000 description 4
- 108010066124 Protein S Proteins 0.000 description 4
- 102000029301 Protein S Human genes 0.000 description 4
- 108010094028 Prothrombin Proteins 0.000 description 4
- 101800001700 Saposin-D Proteins 0.000 description 4
- 238000013103 analytical ultracentrifugation Methods 0.000 description 4
- 239000003433 contraceptive agent Substances 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
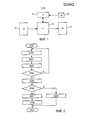
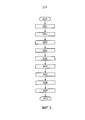
- 238000010586 diagram Methods 0.000 description 4
- 229940127234 oral contraceptive Drugs 0.000 description 4
- 239000003539 oral contraceptive agent Substances 0.000 description 4
- 229960000856 protein c Drugs 0.000 description 4
- 238000012216 screening Methods 0.000 description 4
- 201000005665 thrombophilia Diseases 0.000 description 4
- 201000011510 cancer Diseases 0.000 description 3
- 230000002254 contraceptive effect Effects 0.000 description 3
- 229960000301 factor viii Drugs 0.000 description 3
- 238000007620 mathematical function Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 230000003449 preventive effect Effects 0.000 description 3
- 230000008707 rearrangement Effects 0.000 description 3
- 238000013517 stratification Methods 0.000 description 3
- 102100035023 Carboxypeptidase B2 Human genes 0.000 description 2
- 108090000201 Carboxypeptidase B2 Proteins 0.000 description 2
- 108010023321 Factor VII Proteins 0.000 description 2
- 208000032843 Hemorrhage Diseases 0.000 description 2
- 102100024078 Plasma serine protease inhibitor Human genes 0.000 description 2
- 102100027378 Prothrombin Human genes 0.000 description 2
- 108090000190 Thrombin Proteins 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 238000003491 array Methods 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 208000034158 bleeding Diseases 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 230000023555 blood coagulation Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- AGVAZMGAQJOSFJ-WZHZPDAFSA-M cobalt(2+);[(2r,3s,4r,5s)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2r)-1-[3-[(1r,2r,3r,4z,7s,9z,12s,13s,14z,17s,18s,19r)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2 Chemical compound [Co+2].N#[C-].[N-]([C@@H]1[C@H](CC(N)=O)[C@@]2(C)CCC(=O)NC[C@@H](C)OP(O)(=O)O[C@H]3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)\C2=C(C)/C([C@H](C\2(C)C)CCC(N)=O)=N/C/2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O AGVAZMGAQJOSFJ-WZHZPDAFSA-M 0.000 description 2
- 108010091897 factor V Leiden Proteins 0.000 description 2
- 230000009395 genetic defect Effects 0.000 description 2
- 238000002657 hormone replacement therapy Methods 0.000 description 2
- 108010025221 plasma protein Z Proteins 0.000 description 2
- 239000011505 plaster Substances 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 229940039716 prothrombin Drugs 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 229960004072 thrombin Drugs 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 1
- 102100037084 C4b-binding protein alpha chain Human genes 0.000 description 1
- 101710159767 C4b-binding protein alpha chain Proteins 0.000 description 1
- 102000006912 Complement C4b-Binding Protein Human genes 0.000 description 1
- 108010047548 Complement C4b-Binding Protein Proteins 0.000 description 1
- 102100031673 Corneodesmosin Human genes 0.000 description 1
- 101710139375 Corneodesmosin Proteins 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- 206010014522 Embolism venous Diseases 0.000 description 1
- 108010076282 Factor IX Proteins 0.000 description 1
- 108010014172 Factor V Proteins 0.000 description 1
- 108010014173 Factor X Proteins 0.000 description 1
- 108010080865 Factor XII Proteins 0.000 description 1
- 108010071289 Factor XIII Proteins 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- 102100024515 GDP-L-fucose synthase Human genes 0.000 description 1
- 101710119007 GDP-L-fucose synthase Proteins 0.000 description 1
- 206010071602 Genetic polymorphism Diseases 0.000 description 1
- 108010001953 Protein C Inhibitor Proteins 0.000 description 1
- 229940122929 Protein C inhibitor Drugs 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000010100 anticoagulation Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000002146 bilateral effect Effects 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 229940105778 coagulation factor viii Drugs 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 229940124558 contraceptive agent Drugs 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000013100 final test Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000011990 functional testing Methods 0.000 description 1
- 229910052602 gypsum Inorganic materials 0.000 description 1
- 239000010440 gypsum Substances 0.000 description 1
- 238000012905 input function Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000003147 molecular marker Substances 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 238000012706 support-vector machine Methods 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000007631 vascular surgery Methods 0.000 description 1
- 208000004043 venous thromboembolism Diseases 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H50/00—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics
- G16H50/30—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics for calculating health indices; for individual health risk assessment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B10/00—Instruments for taking body samples for diagnostic purposes; Other methods or instruments for diagnosis, e.g. for vaccination diagnosis, sex determination or ovulation-period determination; Throat striking implements
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H50/00—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics
- G16H50/70—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics for mining of medical data, e.g. analysing previous cases of other patients
Landscapes
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Medical Informatics (AREA)
- Public Health (AREA)
- Data Mining & Analysis (AREA)
- Pathology (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Primary Health Care (AREA)
- Epidemiology (AREA)
- Databases & Information Systems (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Heart & Thoracic Surgery (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261718242P | 2012-10-25 | 2012-10-25 | |
| US61/718,242 | 2012-10-25 | ||
| PCT/IB2013/059424 WO2014064585A1 (en) | 2012-10-25 | 2013-10-17 | Combined use of clinical risk factors and molecular markers for thrombosis for clinical decision support |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2015119520A RU2015119520A (ru) | 2016-12-20 |
| RU2682622C2 true RU2682622C2 (ru) | 2019-03-19 |
Family
ID=49956255
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015119520A RU2682622C2 (ru) | 2012-10-25 | 2013-10-17 | Комбинированное использование клинических факторов риска и молекулярных маркеров тромбоза для поддержки принятия клинических решений |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20150278470A1 (enExample) |
| EP (1) | EP2912584B1 (enExample) |
| JP (1) | JP6335910B2 (enExample) |
| CN (1) | CN104756117B (enExample) |
| BR (1) | BR112015009056A2 (enExample) |
| RU (1) | RU2682622C2 (enExample) |
| WO (1) | WO2014064585A1 (enExample) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11568982B1 (en) | 2014-02-17 | 2023-01-31 | Health at Scale Corporation | System to improve the logistics of clinical care by selectively matching patients to providers |
| US11114204B1 (en) | 2014-04-04 | 2021-09-07 | Predictive Modeling, Inc. | System to determine inpatient or outpatient care and inform decisions about patient care |
| US10748659B2 (en) * | 2015-03-10 | 2020-08-18 | Abbott Cardiovascular Systems Inc. | Method and system for predicting risk of thrombosis |
| US20160283686A1 (en) * | 2015-03-23 | 2016-09-29 | International Business Machines Corporation | Identifying And Ranking Individual-Level Risk Factors Using Personalized Predictive Models |
| JP2019514148A (ja) * | 2016-04-07 | 2019-05-30 | ホワイト・アンヴィル・イノベーションズ,エルエルシー | デジタルデータを解析するための方法 |
| WO2018071845A1 (en) * | 2016-10-13 | 2018-04-19 | Krishnamurti Tamar Priya | A structured medical data classification system for monitoring and remediating treatment risks |
| KR101865110B1 (ko) * | 2016-11-21 | 2018-06-07 | 재단법인 아산사회복지재단 | 급성뇌경색 발생시점 추정시스템, 방법 및 프로그램 |
| US20180181718A1 (en) * | 2016-12-23 | 2018-06-28 | King Abdulaziz University | Interactive clinical decision support system |
| EP3422011A1 (en) * | 2017-06-28 | 2019-01-02 | Koninklijke Philips N.V. | Parameter value estimation in coagulation system |
| WO2020054028A1 (ja) * | 2018-09-13 | 2020-03-19 | 株式会社島津製作所 | データ解析装置 |
| CN109324188B (zh) * | 2018-10-11 | 2022-04-08 | 珠海沃姆电子有限公司 | 一种精准化动态尿液测量方法和系统 |
| US11922301B2 (en) * | 2019-04-05 | 2024-03-05 | Samsung Display Co., Ltd. | System and method for data augmentation for trace dataset |
| US11710045B2 (en) | 2019-10-01 | 2023-07-25 | Samsung Display Co., Ltd. | System and method for knowledge distillation |
| US11610679B1 (en) | 2020-04-20 | 2023-03-21 | Health at Scale Corporation | Prediction and prevention of medical events using machine-learning algorithms |
| US12094582B1 (en) | 2020-08-11 | 2024-09-17 | Health at Scale Corporation | Intelligent healthcare data fabric system |
| US12080428B1 (en) | 2020-09-10 | 2024-09-03 | Health at Scale Corporation | Machine intelligence-based prioritization of non-emergent procedures and visits |
| JP7623120B2 (ja) | 2020-10-01 | 2025-01-28 | キヤノンメディカルシステムズ株式会社 | 診療支援システム |
| CN112767350B (zh) * | 2021-01-19 | 2024-04-26 | 深圳麦科田生物医疗技术股份有限公司 | 血栓弹力图最大区间预测方法、装置、设备和存储介质 |
| CN115188476B (zh) * | 2022-07-20 | 2025-04-18 | 西安市红会医院(西安市骨科研究所) | 基于遗传学特征预测骨折合并深静脉血栓风险的方法 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030069528A1 (en) * | 2001-09-21 | 2003-04-10 | Herz Frederick Stephan Michael | Prevention and treatment of deep venous thrombosis |
| US20090089079A1 (en) * | 2004-11-09 | 2009-04-02 | The Brigham And Women's Hospital, Inc. | System and method for determining whether to issue an alert to consider prophylaxis for a risk condition |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003505678A (ja) * | 1999-07-23 | 2003-02-12 | ザ スクリップス リサーチ インスティテュート | 全血の凝固因子活性を測定する方法 |
| AUPR005600A0 (en) * | 2000-09-12 | 2000-10-05 | University Of Sydney, The | Diagnostic assay |
| US7713705B2 (en) * | 2002-12-24 | 2010-05-11 | Biosite, Inc. | Markers for differential diagnosis and methods of use thereof |
| WO2005116246A2 (en) * | 2004-05-26 | 2005-12-08 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Method and device for detection of splice form and alternative splice forms in dna or rna sequences |
| US20090298103A1 (en) | 2008-05-20 | 2009-12-03 | The University Of Vermont And State Agriculture College | Predicting hemostatic risk; dependence on plasma composition |
-
2013
- 2013-10-17 CN CN201380055556.4A patent/CN104756117B/zh active Active
- 2013-10-17 EP EP13821154.5A patent/EP2912584B1/en active Active
- 2013-10-17 BR BR112015009056A patent/BR112015009056A2/pt not_active IP Right Cessation
- 2013-10-17 RU RU2015119520A patent/RU2682622C2/ru not_active IP Right Cessation
- 2013-10-17 US US14/434,286 patent/US20150278470A1/en not_active Abandoned
- 2013-10-17 WO PCT/IB2013/059424 patent/WO2014064585A1/en not_active Ceased
- 2013-10-17 JP JP2015538597A patent/JP6335910B2/ja not_active Expired - Fee Related
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030069528A1 (en) * | 2001-09-21 | 2003-04-10 | Herz Frederick Stephan Michael | Prevention and treatment of deep venous thrombosis |
| US20090089079A1 (en) * | 2004-11-09 | 2009-04-02 | The Brigham And Women's Hospital, Inc. | System and method for determining whether to issue an alert to consider prophylaxis for a risk condition |
Non-Patent Citations (5)
| Title |
|---|
| JENKINS P.V. et al., Elevated factor VIII levels and risk of venous thrombosis, British Journal of Haematology, 25.04.2012, pp. 653-663. * |
| PENCO S. et al., Assessment of the Role of Genetic Polymorphism in Venous Thrombosis Through Artificial Neural Networks, Annals of Human Genetics, 2005, N 69, pp. 693-706. * |
| PENCO S. et al., Assessment of the Role of Genetic Polymorphism in Venous Thrombosis Through Artificial Neural Networks, Annals of Human Genetics, 2005, N 69, pp. 693-706. JENKINS P.V. et al., Elevated factor VIII levels and risk of venous thrombosis, British Journal of Haematology, 25.04.2012, pp. 653-663. МОЗГОВОЙ П.В. Тромбогеморрагические осложнения после реконструктивных операций на брюшном отделе аорты и магистральных артериях нижних конечностей (профилактика, диагностика, лечение), Автореферат диссертации на соискание ученой степени доктора медицинских наук, Волгоград, 2004, с. 19. * |
| И.М. СЕЧЕНОВА, Лабораторные Методы Исследования Системы Свертывания Крови, Методические рекомендации, Второе издание, Москва, 2011, cc. 1-15. * |
| МОЗГОВОЙ П.В. Тромбогеморрагические осложнения после реконструктивных операций на брюшном отделе аорты и магистральных артериях нижних конечностей (профилактика, диагностика, лечение), Авто диссертации на соискание ученой степени доктора медицинских наук, Волгоград, 2004, с. 19. * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2912584A1 (en) | 2015-09-02 |
| RU2015119520A (ru) | 2016-12-20 |
| EP2912584B1 (en) | 2020-10-07 |
| JP6335910B2 (ja) | 2018-05-30 |
| CN104756117A (zh) | 2015-07-01 |
| JP2016502650A (ja) | 2016-01-28 |
| US20150278470A1 (en) | 2015-10-01 |
| WO2014064585A1 (en) | 2014-05-01 |
| BR112015009056A2 (pt) | 2017-07-04 |
| CN104756117B (zh) | 2019-01-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2682622C2 (ru) | Комбинированное использование клинических факторов риска и молекулярных маркеров тромбоза для поддержки принятия клинических решений | |
| Munafò et al. | Triangulating evidence through the inclusion of genetically informed designs | |
| Spector et al. | Influence of vitamin D receptor genotype on bone mineral density in postmenopausal women: a twin study in Britain | |
| Gilpin et al. | A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center | |
| McGwin Jr et al. | Improving the ability to predict mortality among burn patients | |
| EP3701534B1 (en) | Molecular evidence platform for auditable, continuous optimization of variant interpretation in genetic and genomic testing and analysis | |
| Moenen et al. | The diagnostic accuracy of bleeding assessment tools for the identification of patients with mild bleeding disorders: a systematic review | |
| Rowe et al. | Quality of life among women undergoing hysterectomies | |
| Gates et al. | Screening for the primary prevention of fragility fractures among adults aged 40 years and older in primary care: systematic reviews of the effects and acceptability of screening and treatment, and the accuracy of risk prediction tools | |
| Card et al. | The health effects of cesarean delivery for low-risk first births | |
| Metintas et al. | Menopause Rating Scale as a screening tool in rural Turkey | |
| Dinescu et al. | Socioeconomic modifiers of genetic and environmental influences on body mass index in adult twins. | |
| Damian et al. | Association of detected depression and undetected depressive symptoms with long-term mortality in a cohort of institutionalised older people | |
| Scorza et al. | Adverse childhood experiences and perceived stress in early adulthood in the context of disadvantage | |
| Lyons et al. | Development and validation of an algorithm for identifying patients with hemophilia A in an administrative claims database | |
| Verma et al. | Physician-level variation in clinical outcomes and resource use in inpatient general internal medicine: an observational study | |
| Liu et al. | Does prenatal care benefit maternal health? A study of post-partum maternal care use | |
| Birhan et al. | Joint clinical and socio-demographic determinants of CD4 cell count and body weight in HIV/TB co-infected adult patients on HAART | |
| Hellwege et al. | Predictive models for abdominal aortic aneurysms using polygenic scores and PheWAS-derived risk factors | |
| Schneider et al. | Quality of care in for-profit and not-for-profit health plans enrolling Medicare beneficiaries | |
| Fedorowski et al. | Risk factors for syncope associated with multigenerational relatives with a history of syncope | |
| Laditka et al. | Evaluating hospital care for individuals with Alzheimer's disease using inpatient quality indicators | |
| Rahman et al. | Predictors of intentional self-harm among Medicaid mental health clinic clients in New York | |
| Phillips et al. | Post-acute care for children with special health care needs | |
| Botero et al. | Diagnostic Testing Approaches for Activated Protein C Resistance and Factor V Leiden |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20201018 |