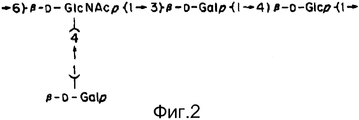RU2549438C2 - Комбинации, включающие сахарид пневмококка серотипа 14 - Google Patents
Комбинации, включающие сахарид пневмококка серотипа 14 Download PDFInfo
- Publication number
- RU2549438C2 RU2549438C2 RU2011142747/15A RU2011142747A RU2549438C2 RU 2549438 C2 RU2549438 C2 RU 2549438C2 RU 2011142747/15 A RU2011142747/15 A RU 2011142747/15A RU 2011142747 A RU2011142747 A RU 2011142747A RU 2549438 C2 RU2549438 C2 RU 2549438C2
- Authority
- RU
- Russia
- Prior art keywords
- seq
- contain
- los
- composition
- amino acids
- Prior art date
Links
- 150000001720 carbohydrates Chemical class 0.000 title claims abstract description 99
- 239000000203 mixture Substances 0.000 claims abstract description 129
- 230000002163 immunogen Effects 0.000 claims abstract description 25
- 238000000034 method Methods 0.000 claims abstract description 25
- 150000004044 tetrasaccharides Chemical class 0.000 claims abstract description 23
- 239000002671 adjuvant Substances 0.000 claims abstract description 19
- 230000028993 immune response Effects 0.000 claims abstract description 14
- 241000124008 Mammalia Species 0.000 claims abstract description 8
- 230000001939 inductive effect Effects 0.000 claims abstract description 5
- 108090000623 proteins and genes Proteins 0.000 claims description 67
- 102000004169 proteins and genes Human genes 0.000 claims description 58
- 102000014914 Carrier Proteins Human genes 0.000 claims description 16
- 239000012528 membrane Substances 0.000 claims description 16
- 108010078791 Carrier Proteins Proteins 0.000 claims description 15
- 230000021615 conjugation Effects 0.000 claims description 7
- 229920001542 oligosaccharide Polymers 0.000 claims description 7
- 108010031133 Transferrin-Binding Protein A Proteins 0.000 claims description 6
- GZQKNULLWNGMCW-PWQABINMSA-N lipid A (E. coli) Chemical compound O1[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 GZQKNULLWNGMCW-PWQABINMSA-N 0.000 claims description 6
- STCOOQWBFONSKY-UHFFFAOYSA-N tributyl phosphate Chemical compound CCCCOP(=O)(OCCCC)OCCCC STCOOQWBFONSKY-UHFFFAOYSA-N 0.000 claims description 6
- 108010071134 CRM197 (non-toxic variant of diphtheria toxin) Proteins 0.000 claims description 5
- 102100037840 Dehydrogenase/reductase SDR family member 2, mitochondrial Human genes 0.000 claims description 5
- 241000606768 Haemophilus influenzae Species 0.000 claims description 5
- 101710188053 Protein D Proteins 0.000 claims description 5
- 101710132893 Resolvase Proteins 0.000 claims description 5
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 claims description 5
- 230000002255 enzymatic effect Effects 0.000 claims description 5
- 229960003983 diphtheria toxoid Drugs 0.000 claims description 4
- 229960000814 tetanus toxoid Drugs 0.000 claims description 4
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical group O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 claims description 2
- 150000004043 trisaccharides Chemical class 0.000 claims description 2
- IEQCXFNWPAHHQR-YKLSGRGUSA-N beta-D-Gal-(1->4)-beta-D-GlcNAc-(1->3)-beta-D-Gal-(1->4)-D-Glc Chemical compound O([C@H]1[C@H](O)[C@H]([C@@H](O[C@@H]1CO)O[C@@H]1[C@H]([C@H](O[C@@H]2[C@H](OC(O)[C@H](O)[C@H]2O)CO)O[C@H](CO)[C@@H]1O)O)NC(=O)C)[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O IEQCXFNWPAHHQR-YKLSGRGUSA-N 0.000 abstract description 32
- IEQCXFNWPAHHQR-UHFFFAOYSA-N lacto-N-neotetraose Natural products OCC1OC(OC2C(C(OC3C(OC(O)C(O)C3O)CO)OC(CO)C2O)O)C(NC(=O)C)C(O)C1OC1OC(CO)C(O)C(O)C1O IEQCXFNWPAHHQR-UHFFFAOYSA-N 0.000 abstract description 32
- 239000003814 drug Substances 0.000 abstract description 7
- 230000000694 effects Effects 0.000 abstract description 3
- 239000000126 substance Substances 0.000 abstract description 3
- 239000012634 fragment Substances 0.000 description 158
- 108091007433 antigens Proteins 0.000 description 131
- 102000036639 antigens Human genes 0.000 description 131
- 239000000427 antigen Substances 0.000 description 129
- 229940024606 amino acid Drugs 0.000 description 116
- 235000001014 amino acid Nutrition 0.000 description 116
- 150000001413 amino acids Chemical class 0.000 description 115
- 125000003275 alpha amino acid group Chemical group 0.000 description 97
- 108090000765 processed proteins & peptides Proteins 0.000 description 95
- 102000004196 processed proteins & peptides Human genes 0.000 description 91
- 229920001184 polypeptide Polymers 0.000 description 89
- 235000018102 proteins Nutrition 0.000 description 57
- 239000000839 emulsion Substances 0.000 description 37
- 229960005486 vaccine Drugs 0.000 description 35
- 229940062780 lacto-n-neotetraose Drugs 0.000 description 31
- 108020001580 protein domains Proteins 0.000 description 26
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N dodecahydrosqualene Natural products CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 25
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 23
- 229920000053 polysorbate 80 Polymers 0.000 description 23
- -1 glucosamine phospholipid Chemical class 0.000 description 19
- 230000003053 immunization Effects 0.000 description 19
- 238000002649 immunization Methods 0.000 description 19
- 239000003921 oil Substances 0.000 description 17
- 235000019198 oils Nutrition 0.000 description 17
- YYGNTYWPHWGJRM-UHFFFAOYSA-N (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene Chemical compound CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C YYGNTYWPHWGJRM-UHFFFAOYSA-N 0.000 description 15
- BHEOSNUKNHRBNM-UHFFFAOYSA-N Tetramethylsqualene Natural products CC(=C)C(C)CCC(=C)C(C)CCC(C)=CCCC=C(C)CCC(C)C(=C)CCC(C)C(C)=C BHEOSNUKNHRBNM-UHFFFAOYSA-N 0.000 description 15
- 229940031439 squalene Drugs 0.000 description 15
- TUHBEKDERLKLEC-UHFFFAOYSA-N squalene Natural products CC(=CCCC(=CCCC(=CCCC=C(/C)CCC=C(/C)CC=C(C)C)C)C)C TUHBEKDERLKLEC-UHFFFAOYSA-N 0.000 description 15
- 108010076504 Protein Sorting Signals Proteins 0.000 description 14
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 14
- 230000000844 anti-bacterial effect Effects 0.000 description 13
- 239000004094 surface-active agent Substances 0.000 description 13
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 13
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 12
- 239000011732 tocopherol Substances 0.000 description 12
- 229930003799 tocopherol Natural products 0.000 description 12
- 101000874355 Escherichia coli (strain K12) Outer membrane protein assembly factor BamA Proteins 0.000 description 11
- 239000003599 detergent Substances 0.000 description 11
- 229940068968 polysorbate 80 Drugs 0.000 description 10
- 235000010384 tocopherol Nutrition 0.000 description 10
- 229960001295 tocopherol Drugs 0.000 description 10
- 241000588653 Neisseria Species 0.000 description 9
- 101710099976 Photosystem I P700 chlorophyll a apoprotein A1 Proteins 0.000 description 8
- 229920000136 polysorbate Polymers 0.000 description 8
- 239000002243 precursor Substances 0.000 description 8
- 238000000746 purification Methods 0.000 description 8
- 241000894006 Bacteria Species 0.000 description 7
- 108010052285 Membrane Proteins Proteins 0.000 description 7
- PRXRUNOAOLTIEF-ADSICKODSA-N Sorbitan trioleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](OC(=O)CCCCCCC\C=C/CCCCCCCC)[C@H]1OC[C@H](O)[C@H]1OC(=O)CCCCCCC\C=C/CCCCCCCC PRXRUNOAOLTIEF-ADSICKODSA-N 0.000 description 7
- 229920004890 Triton X-100 Polymers 0.000 description 7
- 239000013504 Triton X-100 Substances 0.000 description 7
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 7
- 150000003839 salts Chemical class 0.000 description 7
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 6
- 102000004190 Enzymes Human genes 0.000 description 6
- 108090000790 Enzymes Proteins 0.000 description 6
- 102000018697 Membrane Proteins Human genes 0.000 description 6
- 201000005702 Pertussis Diseases 0.000 description 6
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 6
- 101100289918 Streptococcus pneumoniae (strain ATCC BAA-255 / R6) lytB gene Proteins 0.000 description 6
- 101100312899 Streptococcus pneumoniae (strain ATCC BAA-255 / R6) ply gene Proteins 0.000 description 6
- 238000006640 acetylation reaction Methods 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 206010013023 diphtheria Diseases 0.000 description 6
- 229940088598 enzyme Drugs 0.000 description 6
- 230000035772 mutation Effects 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000004044 response Effects 0.000 description 6
- 235000015112 vegetable and seed oil Nutrition 0.000 description 6
- 201000009906 Meningitis Diseases 0.000 description 5
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 5
- 101150002054 galE gene Proteins 0.000 description 5
- 229930182830 galactose Natural products 0.000 description 5
- 230000005847 immunogenicity Effects 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- JXTPJDDICSTXJX-UHFFFAOYSA-N n-Triacontane Natural products CCCCCCCCCCCCCCCCCCCCCCCCCCCCCC JXTPJDDICSTXJX-UHFFFAOYSA-N 0.000 description 5
- 239000008363 phosphate buffer Substances 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 229940032094 squalane Drugs 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- 101710183389 Pneumolysin Proteins 0.000 description 4
- 206010043376 Tetanus Diseases 0.000 description 4
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical compound OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 4
- 230000000890 antigenic effect Effects 0.000 description 4
- 230000001580 bacterial effect Effects 0.000 description 4
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 4
- 238000010367 cloning Methods 0.000 description 4
- 238000012217 deletion Methods 0.000 description 4
- 230000037430 deletion Effects 0.000 description 4
- KXGVEGMKQFWNSR-LLQZFEROSA-N deoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 KXGVEGMKQFWNSR-LLQZFEROSA-N 0.000 description 4
- 150000002191 fatty alcohols Chemical class 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 108700039582 histidine triad Proteins 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 235000010445 lecithin Nutrition 0.000 description 4
- 239000000787 lecithin Substances 0.000 description 4
- 229940067606 lecithin Drugs 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 230000000717 retained effect Effects 0.000 description 4
- SQVRNKJHWKZAKO-OQPLDHBCSA-N sialic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)OC1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-OQPLDHBCSA-N 0.000 description 4
- 229960000984 tocofersolan Drugs 0.000 description 4
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 3
- 108010060123 Conjugate Vaccines Proteins 0.000 description 3
- 235000001815 DL-alpha-tocopherol Nutrition 0.000 description 3
- 239000011627 DL-alpha-tocopherol Substances 0.000 description 3
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 3
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 3
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 3
- 241000588650 Neisseria meningitidis Species 0.000 description 3
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 3
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 3
- 210000001744 T-lymphocyte Anatomy 0.000 description 3
- 108010031127 Transferrin-Binding Protein B Proteins 0.000 description 3
- 239000001361 adipic acid Substances 0.000 description 3
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical group OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 3
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 3
- 229920001400 block copolymer Polymers 0.000 description 3
- 210000004899 c-terminal region Anatomy 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- 235000013339 cereals Nutrition 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 229940031670 conjugate vaccine Drugs 0.000 description 3
- 235000014113 dietary fatty acids Nutrition 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 239000000194 fatty acid Substances 0.000 description 3
- 229930195729 fatty acid Natural products 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 239000008103 glucose Substances 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 238000010255 intramuscular injection Methods 0.000 description 3
- 239000007927 intramuscular injection Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 3
- 239000002736 nonionic surfactant Substances 0.000 description 3
- 229920002113 octoxynol Polymers 0.000 description 3
- 230000001717 pathogenic effect Effects 0.000 description 3
- 150000003904 phospholipids Chemical class 0.000 description 3
- 230000000069 prophylactic effect Effects 0.000 description 3
- 238000004904 shortening Methods 0.000 description 3
- 125000005629 sialic acid group Chemical group 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 238000011282 treatment Methods 0.000 description 3
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 3
- QMXCRMQIVATQMR-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-pyridin-2-ylsulfanylpropanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCSC1=CC=CC=N1 QMXCRMQIVATQMR-UHFFFAOYSA-N 0.000 description 2
- RUDATBOHQWOJDD-UHFFFAOYSA-N (3beta,5beta,7alpha)-3,7-Dihydroxycholan-24-oic acid Chemical class OC1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)CC2 RUDATBOHQWOJDD-UHFFFAOYSA-N 0.000 description 2
- HNLXNOZHXNSSPN-UHFFFAOYSA-N 2-[2-[2-[2-[2-[2-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol Chemical compound CC(C)(C)CC(C)(C)C1=CC=C(OCCOCCOCCOCCOCCOCCOCCO)C=C1 HNLXNOZHXNSSPN-UHFFFAOYSA-N 0.000 description 2
- 241000251468 Actinopterygii Species 0.000 description 2
- 231100000699 Bacterial toxin Toxicity 0.000 description 2
- 241000588832 Bordetella pertussis Species 0.000 description 2
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 2
- 241000193163 Clostridioides difficile Species 0.000 description 2
- 102000016550 Complement Factor H Human genes 0.000 description 2
- 108010053085 Complement Factor H Proteins 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- IELOKBJPULMYRW-NJQVLOCASA-N D-alpha-Tocopheryl Acid Succinate Chemical compound OC(=O)CCC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C IELOKBJPULMYRW-NJQVLOCASA-N 0.000 description 2
- 108010016626 Dipeptides Proteins 0.000 description 2
- 101710186862 Factor H binding protein Proteins 0.000 description 2
- 102000001554 Hemoglobins Human genes 0.000 description 2
- 108010054147 Hemoglobins Proteins 0.000 description 2
- 102000008072 Lymphokines Human genes 0.000 description 2
- 108010074338 Lymphokines Proteins 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 2
- 108700020354 N-acetylmuramyl-threonyl-isoglutamine Proteins 0.000 description 2
- 241001644525 Nastus productus Species 0.000 description 2
- 241000040340 Oat mosaic virus Species 0.000 description 2
- 108010038807 Oligopeptides Proteins 0.000 description 2
- 102000015636 Oligopeptides Human genes 0.000 description 2
- 229940124950 Prevnar 13 Drugs 0.000 description 2
- 101710084578 Short neurotoxin 1 Proteins 0.000 description 2
- NWGKJDSIEKMTRX-AAZCQSIUSA-N Sorbitan monooleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O NWGKJDSIEKMTRX-AAZCQSIUSA-N 0.000 description 2
- 108010055044 Tetanus Toxin Proteins 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 101710182223 Toxin B Proteins 0.000 description 2
- 101710182532 Toxin a Proteins 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 230000021736 acetylation Effects 0.000 description 2
- 235000011037 adipic acid Nutrition 0.000 description 2
- 125000003172 aldehyde group Chemical group 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 238000000889 atomisation Methods 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 239000000688 bacterial toxin Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 235000012000 cholesterol Nutrition 0.000 description 2
- 229940001442 combination vaccine Drugs 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 101150117300 ctrC gene Proteins 0.000 description 2
- 229940099418 d- alpha-tocopherol succinate Drugs 0.000 description 2
- 229940009976 deoxycholate Drugs 0.000 description 2
- 229960003964 deoxycholic acid Drugs 0.000 description 2
- KXGVEGMKQFWNSR-UHFFFAOYSA-N deoxycholic acid Chemical class C1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)C(O)C2 KXGVEGMKQFWNSR-UHFFFAOYSA-N 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 229940013317 fish oils Drugs 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 150000002386 heptoses Chemical class 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000003308 immunostimulating effect Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 229940066429 octoxynol Drugs 0.000 description 2
- 150000002482 oligosaccharides Chemical class 0.000 description 2
- 230000000625 opsonophagocytic effect Effects 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 235000021400 peanut butter Nutrition 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 2
- 229920001983 poloxamer Polymers 0.000 description 2
- 108010094020 polyglycine Proteins 0.000 description 2
- 229920000232 polyglycine polymer Polymers 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 229950008882 polysorbate Drugs 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000002335 preservative effect Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 235000004252 protein component Nutrition 0.000 description 2
- 239000001397 quillaja saponaria molina bark Substances 0.000 description 2
- 238000006268 reductive amination reaction Methods 0.000 description 2
- 229930182490 saponin Natural products 0.000 description 2
- 150000007949 saponins Chemical class 0.000 description 2
- 238000010845 search algorithm Methods 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 239000010686 shark liver oil Substances 0.000 description 2
- 229940069764 shark liver oil Drugs 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 2
- 150000003505 terpenes Chemical class 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 125000002640 tocopherol group Chemical class 0.000 description 2
- 235000019149 tocopherols Nutrition 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 238000000108 ultra-filtration Methods 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 235000004835 α-tocopherol Nutrition 0.000 description 2
- PZFZLRNAOHUQPH-DJBVYZKNSA-N (2r)-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-2-(hexadecanoylamino)propanoic acid Chemical compound CCCCCCCCCCCCCCCC(=O)N[C@H](C(O)=O)CSC[C@H](OC(=O)CCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCC PZFZLRNAOHUQPH-DJBVYZKNSA-N 0.000 description 1
- WCDDVEOXEIYWFB-VXORFPGASA-N (2s,3s,4r,5r,6r)-3-[(2s,3r,5s,6r)-3-acetamido-5-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,5,6-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@@H]1C[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O)[C@H](O)[C@H]1O WCDDVEOXEIYWFB-VXORFPGASA-N 0.000 description 1
- BHQCQFFYRZLCQQ-UHFFFAOYSA-N (3alpha,5alpha,7alpha,12alpha)-3,7,12-trihydroxy-cholan-24-oic acid Chemical class OC1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)C(O)C2 BHQCQFFYRZLCQQ-UHFFFAOYSA-N 0.000 description 1
- UGXDVELKRYZPDM-XLXQKPBQSA-N (4r)-4-[[(2s,3r)-2-[[(2r)-2-[(2r,3r,4r,5r)-2-acetamido-4,5,6-trihydroxy-1-oxohexan-3-yl]oxypropanoyl]amino]-3-hydroxybutanoyl]amino]-5-amino-5-oxopentanoic acid Chemical compound OC(=O)CC[C@H](C(N)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](C)O[C@@H]([C@H](O)[C@H](O)CO)[C@@H](NC(C)=O)C=O UGXDVELKRYZPDM-XLXQKPBQSA-N 0.000 description 1
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 1
- PORPENFLTBBHSG-MGBGTMOVSA-N 1,2-dihexadecanoyl-sn-glycerol-3-phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCC PORPENFLTBBHSG-MGBGTMOVSA-N 0.000 description 1
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 1
- 229940031767 13-valent pneumococcal conjugate vaccine Drugs 0.000 description 1
- PXFBZOLANLWPMH-UHFFFAOYSA-N 16-Epiaffinine Natural products C1C(C2=CC=CC=C2N2)=C2C(=O)CC2C(=CC)CN(C)C1C2CO PXFBZOLANLWPMH-UHFFFAOYSA-N 0.000 description 1
- MSWZFWKMSRAUBD-CBPJZXOFSA-N 2-amino-2-deoxy-D-mannopyranose Chemical group N[C@@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-CBPJZXOFSA-N 0.000 description 1
- QCDWFXQBSFUVSP-UHFFFAOYSA-N 2-phenoxyethanol Chemical compound OCCOC1=CC=CC=C1 QCDWFXQBSFUVSP-UHFFFAOYSA-N 0.000 description 1
- LYFYWXLKKQIOKO-UHFFFAOYSA-N 3,3-diaminopentan-1-ol Chemical compound CCC(N)(N)CCO LYFYWXLKKQIOKO-UHFFFAOYSA-N 0.000 description 1
- KYQCXUMVJGMDNG-UHFFFAOYSA-N 4,5,6,7,8-pentahydroxy-2-oxooctanoic acid Chemical compound OCC(O)C(O)C(O)C(O)CC(=O)C(O)=O KYQCXUMVJGMDNG-UHFFFAOYSA-N 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- XZIIFPSPUDAGJM-UHFFFAOYSA-N 6-chloro-2-n,2-n-diethylpyrimidine-2,4-diamine Chemical compound CCN(CC)C1=NC(N)=CC(Cl)=N1 XZIIFPSPUDAGJM-UHFFFAOYSA-N 0.000 description 1
- 102000005416 ATP-Binding Cassette Transporters Human genes 0.000 description 1
- 108010006533 ATP-Binding Cassette Transporters Proteins 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 108010042708 Acetylmuramyl-Alanyl-Isoglutamine Proteins 0.000 description 1
- 101800000713 Adhesion and penetration protein Proteins 0.000 description 1
- 235000007319 Avena orientalis Nutrition 0.000 description 1
- 244000075850 Avena orientalis Species 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- YDNKGFDKKRUKPY-JHOUSYSJSA-N C16 ceramide Natural products CCCCCCCCCCCCCCCC(=O)N[C@@H](CO)[C@H](O)C=CCCCCCCCCCCCCC YDNKGFDKKRUKPY-JHOUSYSJSA-N 0.000 description 1
- 101150095624 CPX gene Proteins 0.000 description 1
- 239000004380 Cholic acid Chemical class 0.000 description 1
- 101710164918 Choline-binding protein Proteins 0.000 description 1
- 208000011038 Cold agglutinin disease Diseases 0.000 description 1
- 206010009868 Cold type haemolytic anaemia Diseases 0.000 description 1
- 102100035149 Cytosolic endo-beta-N-acetylglucosaminidase Human genes 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 101100041687 Drosophila melanogaster san gene Proteins 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 101710144190 Endo-beta-N-acetylglucosaminidase Proteins 0.000 description 1
- 241000991587 Enterovirus C Species 0.000 description 1
- 101710204837 Envelope small membrane protein Proteins 0.000 description 1
- 244000140063 Eragrostis abyssinica Species 0.000 description 1
- 235000014966 Eragrostis abyssinica Nutrition 0.000 description 1
- 101710154643 Filamentous hemagglutinin Proteins 0.000 description 1
- 241001076388 Fimbria Species 0.000 description 1
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 1
- BCCRXDTUTZHDEU-VKHMYHEASA-N Gly-Ser Chemical compound NCC(=O)N[C@@H](CO)C(O)=O BCCRXDTUTZHDEU-VKHMYHEASA-N 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 241000700721 Hepatitis B virus Species 0.000 description 1
- 101100005713 Homo sapiens CD4 gene Proteins 0.000 description 1
- 101000957351 Homo sapiens Myc-associated zinc finger protein Proteins 0.000 description 1
- 229920002884 Laureth 4 Polymers 0.000 description 1
- NFNVDJGXRFEYTK-YUMQZZPRSA-N Leu-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(O)=O)CCC(O)=O NFNVDJGXRFEYTK-YUMQZZPRSA-N 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- SMEROWZSTRWXGI-UHFFFAOYSA-N Lithocholsaeure Natural products C1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)CC2 SMEROWZSTRWXGI-UHFFFAOYSA-N 0.000 description 1
- 102000004317 Lyases Human genes 0.000 description 1
- 108090000856 Lyases Proteins 0.000 description 1
- 102000008826 LysM domains Human genes 0.000 description 1
- 108050000721 LysM domains Proteins 0.000 description 1
- 101710145006 Lysis protein Proteins 0.000 description 1
- 108050000633 Lysozyme C Proteins 0.000 description 1
- 108010090665 Mannosyl-Glycoprotein Endo-beta-N-Acetylglucosaminidase Proteins 0.000 description 1
- 229940124904 Menactra Drugs 0.000 description 1
- 208000034762 Meningococcal Infections Diseases 0.000 description 1
- WXHHTBVYQOSYSL-FXQIFTODSA-N Met-Ala-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O WXHHTBVYQOSYSL-FXQIFTODSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 101100368144 Mus musculus Synb gene Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 102100038750 Myc-associated zinc finger protein Human genes 0.000 description 1
- OVRNDRQMDRJTHS-UHFFFAOYSA-N N-acelyl-D-glucosamine Natural products CC(=O)NC1C(O)OC(CO)C(O)C1O OVRNDRQMDRJTHS-UHFFFAOYSA-N 0.000 description 1
- OVRNDRQMDRJTHS-RTRLPJTCSA-N N-acetyl-D-glucosamine Chemical group CC(=O)N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-RTRLPJTCSA-N 0.000 description 1
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 1
- SQVRNKJHWKZAKO-PFQGKNLYSA-N N-acetyl-beta-neuraminic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)O[C@H]1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-PFQGKNLYSA-N 0.000 description 1
- MBLBDJOUHNCFQT-LXGUWJNJSA-N N-acetylglucosamine Natural products CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO MBLBDJOUHNCFQT-LXGUWJNJSA-N 0.000 description 1
- 102100030397 N-acetylmuramoyl-L-alanine amidase Human genes 0.000 description 1
- CRJGESKKUOMBCT-VQTJNVASSA-N N-acetylsphinganine Chemical compound CCCCCCCCCCCCCCC[C@@H](O)[C@H](CO)NC(C)=O CRJGESKKUOMBCT-VQTJNVASSA-N 0.000 description 1
- 101100165129 Neisseria meningitidis serogroup B (strain MC58) bamA gene Proteins 0.000 description 1
- 101100446533 Neisseria meningitidis serogroup B (strain MC58) fhbP gene Proteins 0.000 description 1
- 101100026367 Neisseria meningitidis serogroup B (strain MC58) nhhA gene Proteins 0.000 description 1
- 108700018753 Neisseria porin Proteins 0.000 description 1
- 101710171321 Neuraminyllactose-binding hemagglutinin Proteins 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 101710116435 Outer membrane protein Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 101150093941 PORA gene Proteins 0.000 description 1
- 244000046052 Phaseolus vulgaris Species 0.000 description 1
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 1
- 208000035109 Pneumococcal Infections Diseases 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 1
- 101710138270 PspA protein Proteins 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- 241000209056 Secale Species 0.000 description 1
- 235000007238 Secale cereale Nutrition 0.000 description 1
- 244000044822 Simmondsia californica Species 0.000 description 1
- 235000004433 Simmondsia californica Nutrition 0.000 description 1
- 239000004147 Sorbitan trioleate Substances 0.000 description 1
- 241001136276 Sphingobacterium multivorum Species 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- 241000193998 Streptococcus pneumoniae Species 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- 102000019197 Superoxide Dismutase Human genes 0.000 description 1
- 108010012715 Superoxide dismutase Proteins 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- WPMWEFXCIYCJSA-UHFFFAOYSA-N Tetraethylene glycol monododecyl ether Chemical compound CCCCCCCCCCCCOCCOCCOCCOCCO WPMWEFXCIYCJSA-UHFFFAOYSA-N 0.000 description 1
- 108090000992 Transferases Proteins 0.000 description 1
- 235000019714 Triticale Nutrition 0.000 description 1
- 235000021307 Triticum Nutrition 0.000 description 1
- 244000098338 Triticum aestivum Species 0.000 description 1
- ZBNRGEMZNWHCGA-PDKVEDEMSA-N [(2r)-2-[(2r,3r,4s)-3,4-bis[[(z)-octadec-9-enoyl]oxy]oxolan-2-yl]-2-hydroxyethyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](OC(=O)CCCCCCC\C=C/CCCCCCCC)[C@H]1OC(=O)CCCCCCC\C=C/CCCCCCCC ZBNRGEMZNWHCGA-PDKVEDEMSA-N 0.000 description 1
- NKVLDFAVEWLOCX-GUSKIFEASA-N [(2s,3r,4s,5r,6r)-3-[(2s,3r,4s,5r,6s)-5-[(2s,3r,4s,5r)-4-[(2s,3r,4r)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy-3,5-dihydroxyoxan-2-yl]oxy-3,4-dihydroxy-6-methyloxan-2-yl]oxy-4,5-dihydroxy-6-methyloxan-2-yl] (4ar,5r,6as,6br,9s,10s,12ar)-10-[(2r,3r,4s, Chemical compound O([C@H]1[C@H](O)CO[C@H]([C@@H]1O)O[C@H]1[C@H](C)O[C@H]([C@@H]([C@@H]1O)O)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](C)O[C@H]1OC(=O)[C@]12CCC(C)(C)CC1C1=CCC3[C@@]([C@@]1(C[C@H]2O)C)(C)CCC1[C@]3(C)CC[C@@H]([C@@]1(C)C=O)O[C@@H]1O[C@@H]([C@H]([C@H](O[C@H]2[C@@H]([C@@H](O)[C@H](O)CO2)O)[C@H]1O[C@H]1[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O1)O)O)C(=O)NCCCCCCCCCCCC)[C@@H]1OC[C@](O)(CO)[C@H]1O NKVLDFAVEWLOCX-GUSKIFEASA-N 0.000 description 1
- FZLJPEPAYPUMMR-ZTVVOAFPSA-N [(3s,4r,5s,6r)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen phosphate Chemical class CC(=O)N[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)OC1OP(O)(O)=O FZLJPEPAYPUMMR-ZTVVOAFPSA-N 0.000 description 1
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 238000005903 acid hydrolysis reaction Methods 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- IBVAQQYNSHJXBV-UHFFFAOYSA-N adipic acid dihydrazide Chemical compound NNC(=O)CCCCC(=O)NN IBVAQQYNSHJXBV-UHFFFAOYSA-N 0.000 description 1
- 230000000240 adjuvant effect Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 238000005576 amination reaction Methods 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000010775 animal oil Substances 0.000 description 1
- 230000001727 anti-capsular Effects 0.000 description 1
- 230000001948 anti-meningococcal effect Effects 0.000 description 1
- 238000010913 antigen-directed enzyme pro-drug therapy Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 238000013528 artificial neural network Methods 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 108010085377 beta-N-Acetylhexosaminidases Proteins 0.000 description 1
- 102000007478 beta-N-Acetylhexosaminidases Human genes 0.000 description 1
- 239000003833 bile salt Substances 0.000 description 1
- 229940093761 bile salts Drugs 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000001851 biosynthetic effect Effects 0.000 description 1
- 208000002352 blister Diseases 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 150000001721 carbon Chemical class 0.000 description 1
- 229940106189 ceramide Drugs 0.000 description 1
- ZVEQCJWYRWKARO-UHFFFAOYSA-N ceramide Natural products CCCCCCCCCCCCCCC(O)C(=O)NC(CO)C(O)C=CCCC=C(C)CCCCCCCCC ZVEQCJWYRWKARO-UHFFFAOYSA-N 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- RUDATBOHQWOJDD-BSWAIDMHSA-N chenodeoxycholic acid Chemical class C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)CC1 RUDATBOHQWOJDD-BSWAIDMHSA-N 0.000 description 1
- 229960001091 chenodeoxycholic acid Drugs 0.000 description 1
- BHQCQFFYRZLCQQ-OELDTZBJSA-N cholic acid Chemical class C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 BHQCQFFYRZLCQQ-OELDTZBJSA-N 0.000 description 1
- 235000019416 cholic acid Nutrition 0.000 description 1
- 229960002471 cholic acid Drugs 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 235000012716 cod liver oil Nutrition 0.000 description 1
- 239000003026 cod liver oil Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 239000002577 cryoprotective agent Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001784 detoxification Methods 0.000 description 1
- RAABOESOVLLHRU-UHFFFAOYSA-N diazene Chemical compound N=N RAABOESOVLLHRU-UHFFFAOYSA-N 0.000 description 1
- 229910000071 diazene Inorganic materials 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 125000000600 disaccharide group Chemical group 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- ZGSPNIOCEDOHGS-UHFFFAOYSA-L disodium [3-[2,3-di(octadeca-9,12-dienoyloxy)propoxy-oxidophosphoryl]oxy-2-hydroxypropyl] 2,3-di(octadeca-9,12-dienoyloxy)propyl phosphate Chemical compound [Na+].[Na+].CCCCCC=CCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COP([O-])(=O)OCC(O)COP([O-])(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COC(=O)CCCCCCCC=CCC=CCCCCC ZGSPNIOCEDOHGS-UHFFFAOYSA-L 0.000 description 1
- NLEBIOOXCVAHBD-QKMCSOCLSA-N dodecyl beta-D-maltoside Chemical compound O[C@@H]1[C@@H](O)[C@H](OCCCCCCCCCCCC)O[C@H](CO)[C@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 NLEBIOOXCVAHBD-QKMCSOCLSA-N 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 150000002194 fatty esters Chemical class 0.000 description 1
- 101150046339 fur gene Proteins 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 238000003209 gene knockout Methods 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 150000002339 glycosphingolipids Chemical class 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 244000144993 groups of animals Species 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 229940047650 haemophilus influenzae Drugs 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 108010037896 heparin-binding hemagglutinin Proteins 0.000 description 1
- 238000000703 high-speed centrifugation Methods 0.000 description 1
- 229930186900 holotoxin Natural products 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 235000020256 human milk Nutrition 0.000 description 1
- 210000004251 human milk Anatomy 0.000 description 1
- 229940014041 hyaluronate Drugs 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 239000000568 immunological adjuvant Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 229940119170 jojoba wax Drugs 0.000 description 1
- 108010011519 keratan-sulfate endo-1,4-beta-galactosidase Proteins 0.000 description 1
- 229940062711 laureth-9 Drugs 0.000 description 1
- 101150018810 lgtB gene Proteins 0.000 description 1
- 101150071729 lgtE gene Proteins 0.000 description 1
- 229940059904 light mineral oil Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- SMEROWZSTRWXGI-HVATVPOCSA-N lithocholic acid Chemical class C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)CC1 SMEROWZSTRWXGI-HVATVPOCSA-N 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 229960005037 meningococcal vaccines Drugs 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 108010016686 methionyl-alanyl-serine Proteins 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229940035032 monophosphoryl lipid a Drugs 0.000 description 1
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 1
- 238000000569 multi-angle light scattering Methods 0.000 description 1
- RYRIMPODRHVEIW-UHFFFAOYSA-N n-(2-phenylethyl)nitramide Chemical compound [O-][N+](=O)NCCC1=CC=CC=C1 RYRIMPODRHVEIW-UHFFFAOYSA-N 0.000 description 1
- 229950006780 n-acetylglucosamine Drugs 0.000 description 1
- VVGIYYKRAMHVLU-UHFFFAOYSA-N newbouldiamide Natural products CCCCCCCCCCCCCCCCCCCC(O)C(O)C(O)C(CO)NC(=O)CCCCCCCCCCCCCCCCC VVGIYYKRAMHVLU-UHFFFAOYSA-N 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 235000019488 nut oil Nutrition 0.000 description 1
- 235000014571 nuts Nutrition 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- 229920002114 octoxynol-9 Polymers 0.000 description 1
- 229940098514 octoxynol-9 Drugs 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 125000000913 palmityl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- KHIWWQKSHDUIBK-UHFFFAOYSA-N periodic acid Chemical compound OI(=O)(=O)=O KHIWWQKSHDUIBK-UHFFFAOYSA-N 0.000 description 1
- 108010021711 pertactin Proteins 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 229960005323 phenoxyethanol Drugs 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 150000003905 phosphatidylinositols Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229940031999 pneumococcal conjugate vaccine Drugs 0.000 description 1
- 229960001973 pneumococcal vaccines Drugs 0.000 description 1
- ONJQDTZCDSESIW-UHFFFAOYSA-N polidocanol Chemical compound CCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO ONJQDTZCDSESIW-UHFFFAOYSA-N 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 229960000856 protein c Drugs 0.000 description 1
- 239000003725 proteoliposome Substances 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 101150024840 rmpM gene Proteins 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 238000007127 saponification reaction Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 229960001790 sodium citrate Drugs 0.000 description 1
- 235000011083 sodium citrates Nutrition 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 229940035044 sorbitan monolaurate Drugs 0.000 description 1
- 235000019337 sorbitan trioleate Nutrition 0.000 description 1
- 229960000391 sorbitan trioleate Drugs 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 229940084106 spermaceti Drugs 0.000 description 1
- 239000012177 spermaceti Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 150000003421 squalenes Chemical class 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 229940031000 streptococcus pneumoniae Drugs 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229960004906 thiomersal Drugs 0.000 description 1
- 150000003611 tocopherol derivatives Chemical class 0.000 description 1
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 1
- 238000005199 ultracentrifugation Methods 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- BHQCQFFYRZLCQQ-UTLSPDKDSA-N ursocholic acid Chemical class C([C@H]1C[C@@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 BHQCQFFYRZLCQQ-UTLSPDKDSA-N 0.000 description 1
- RUDATBOHQWOJDD-UZVSRGJWSA-N ursodeoxycholic acid Chemical class C([C@H]1C[C@@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)CC1 RUDATBOHQWOJDD-UZVSRGJWSA-N 0.000 description 1
- 229960001661 ursodiol Drugs 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 239000010698 whale oil Substances 0.000 description 1
- 241000228158 x Triticosecale Species 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000002076 α-tocopherol Substances 0.000 description 1
- 150000003772 α-tocopherols Chemical class 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/195—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria
- C07K14/315—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Streptococcus (G), e.g. Enterococci
- C07K14/3156—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Streptococcus (G), e.g. Enterococci from Streptococcus pneumoniae (Pneumococcus)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/095—Neisseria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Mycology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Microbiology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Epidemiology (AREA)
- Biochemistry (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16299609P | 2009-03-24 | 2009-03-24 | |
| US61/162,996 | 2009-03-24 | ||
| PCT/IB2010/000735 WO2010109325A2 (en) | 2009-03-24 | 2010-03-24 | Combinations including pneumococcal serotype 14 saccharide |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2014122163/15A Division RU2014122163A (ru) | 2009-03-24 | 2014-05-30 | Комбинации, включающие сахарид пневмококка серотипа 14 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2011142747A RU2011142747A (ru) | 2013-04-27 |
| RU2549438C2 true RU2549438C2 (ru) | 2015-04-27 |
Family
ID=42352288
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2011142747/15A RU2549438C2 (ru) | 2009-03-24 | 2010-03-24 | Комбинации, включающие сахарид пневмококка серотипа 14 |
| RU2014122163/15A RU2014122163A (ru) | 2009-03-24 | 2014-05-30 | Комбинации, включающие сахарид пневмококка серотипа 14 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2014122163/15A RU2014122163A (ru) | 2009-03-24 | 2014-05-30 | Комбинации, включающие сахарид пневмококка серотипа 14 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20120064104A1 (enExample) |
| EP (1) | EP2411044A2 (enExample) |
| JP (2) | JP5806204B2 (enExample) |
| CN (1) | CN102421449A (enExample) |
| AU (1) | AU2010227221B2 (enExample) |
| CA (1) | CA2756398A1 (enExample) |
| NZ (1) | NZ595223A (enExample) |
| RU (2) | RU2549438C2 (enExample) |
| WO (1) | WO2010109325A2 (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2258851A1 (en) | 1999-05-19 | 2010-12-08 | Novartis Vaccines and Diagnostics S.r.l. | Combination neisserial compositions |
| GB0316560D0 (en) | 2003-07-15 | 2003-08-20 | Chiron Srl | Vesicle filtration |
| AR086405A1 (es) | 2011-05-17 | 2013-12-11 | Glaxosmithkline Biolog Sa | Vacuna de streptococcus pneumoniae |
| US9427476B2 (en) | 2012-05-24 | 2016-08-30 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Multivalent meningococcal conjugates and methods for preparing conjugates |
| NZ630133A (en) * | 2012-06-14 | 2016-10-28 | Novartis Ag | Vaccines for serogroup x meningococcus |
| CA2875391A1 (en) | 2012-07-27 | 2014-01-30 | Institut National De La Sante Et De La Recherche Medicale | Cd147 as receptor for pilus-mediated adhesion of meningococci to vascular endothelia |
| CA2937186C (en) * | 2014-01-21 | 2022-11-01 | Pfizer Inc. | Immunogenic compositions comprising conjugated capsular saccharide antigens and uses thereof |
| WO2015183676A1 (en) * | 2014-05-29 | 2015-12-03 | Kapre Subhash V | Synthetic peptides as carriers for conjugation with polysaccharides |
| US9107906B1 (en) | 2014-10-28 | 2015-08-18 | Adma Biologics, Inc. | Compositions and methods for the treatment of immunodeficiency |
| US10738338B2 (en) | 2016-10-18 | 2020-08-11 | The Research Foundation for the State University | Method and composition for biocatalytic protein-oligonucleotide conjugation and protein-oligonucleotide conjugate |
| US10259865B2 (en) | 2017-03-15 | 2019-04-16 | Adma Biologics, Inc. | Anti-pneumococcal hyperimmune globulin for the treatment and prevention of pneumococcal infection |
| WO2023132795A2 (en) * | 2022-01-05 | 2023-07-13 | National University Of Singapore | Modified bacterial glycans and conjugates thereof |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NZ587398A (en) * | 2002-08-02 | 2012-03-30 | Glaxosmithkline Biolog Sa | Neisserial bleb preparations and vaccines comprising them |
| HUE032903T2 (hu) * | 2005-12-22 | 2017-11-28 | Glaxosmithkline Biologicals Sa | Streptococcus pneumoniae kapszulárispoliszacharid-konjugátumok |
-
2010
- 2010-03-24 EP EP10714681A patent/EP2411044A2/en not_active Withdrawn
- 2010-03-24 RU RU2011142747/15A patent/RU2549438C2/ru not_active IP Right Cessation
- 2010-03-24 AU AU2010227221A patent/AU2010227221B2/en not_active Ceased
- 2010-03-24 JP JP2012501410A patent/JP5806204B2/ja not_active Expired - Fee Related
- 2010-03-24 WO PCT/IB2010/000735 patent/WO2010109325A2/en not_active Ceased
- 2010-03-24 NZ NZ595223A patent/NZ595223A/en not_active IP Right Cessation
- 2010-03-24 CN CN2010800212325A patent/CN102421449A/zh active Pending
- 2010-03-24 CA CA2756398A patent/CA2756398A1/en not_active Abandoned
- 2010-03-24 US US13/260,550 patent/US20120064104A1/en not_active Abandoned
-
2014
- 2014-05-30 RU RU2014122163/15A patent/RU2014122163A/ru not_active Application Discontinuation
-
2015
- 2015-06-17 JP JP2015121897A patent/JP2015163651A/ja not_active Ceased
Non-Patent Citations (2)
| Title |
|---|
| VAN DEN DOBBELSTEEN GP., et al., Immunogenicity of a combination vaccine containing pneumococcal conjugates and meningococcal PorA OMVs. Vaccine. 2007 Mar 22;25(13):2491-6. Epub 2006 Sep 20. TSAI CM., Molecular mimicry of host structures by lipooligosaccharides of Neisseria meningitidis: characterization of sialylated and nonsialylated lacto-N-neotetraose (Galbeta1-4GlcNAcbeta1-3Galbeta1-4Glc) structures in lipooligosaccharides using monoclonal antibodies and specific lectins. Adv Exp Med Biol. 2001;491:525-42 * |
| WESSELS M.R., et al., Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J Exp Med. 1989 Jun 1;169(6):2121-31. * |
Also Published As
| Publication number | Publication date |
|---|---|
| RU2011142747A (ru) | 2013-04-27 |
| JP5806204B2 (ja) | 2015-11-10 |
| US20120064104A1 (en) | 2012-03-15 |
| EP2411044A2 (en) | 2012-02-01 |
| JP2015163651A (ja) | 2015-09-10 |
| JP2012521404A (ja) | 2012-09-13 |
| AU2010227221A1 (en) | 2011-10-13 |
| AU2010227221B2 (en) | 2015-01-22 |
| WO2010109325A2 (en) | 2010-09-30 |
| CN102421449A (zh) | 2012-04-18 |
| NZ595223A (en) | 2013-12-20 |
| CA2756398A1 (en) | 2010-09-30 |
| RU2014122163A (ru) | 2015-12-10 |
| WO2010109325A3 (en) | 2011-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2549438C2 (ru) | Комбинации, включающие сахарид пневмококка серотипа 14 | |
| RU2555757C2 (ru) | Комбинации менингококкового фактор н-связывающего белка и пневмококковых сахаридных конъюгатов | |
| RU2498815C2 (ru) | НАБОР ДЛЯ ПОЛУЧЕНИЯ ИММУНОГЕННОЙ КОМПОЗИЦИИ ПРОТИВ Neisseria meningitidis СЕРОЛОГИЧЕСКОЙ ГРУППЫ В | |
| JP7074793B2 (ja) | 髄膜炎菌ワクチン | |
| JP2012512240A (ja) | ヘモグロビン受容体を含む髄膜炎菌ワクチン | |
| CN102724988B (zh) | 脑膜炎球菌fHBP多肽的表达 | |
| US20130071422A1 (en) | Adjuvanted vaccines for serogroup b meningococcus | |
| AU2013204315B2 (en) | Meningococcal vaccine formulations |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20170325 |