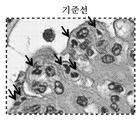
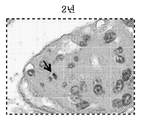
KR20200059279A - 막증식성 사구체신염을 갖는 환자의 치료를 위한 항-c5 항체의 투여량 및 투여 - Google Patents
막증식성 사구체신염을 갖는 환자의 치료를 위한 항-c5 항체의 투여량 및 투여 Download PDFInfo
- Publication number
- KR20200059279A KR20200059279A KR1020207012177A KR20207012177A KR20200059279A KR 20200059279 A KR20200059279 A KR 20200059279A KR 1020207012177 A KR1020207012177 A KR 1020207012177A KR 20207012177 A KR20207012177 A KR 20207012177A KR 20200059279 A KR20200059279 A KR 20200059279A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- binding fragment
- week
- patients
- ser
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000011282 treatment Methods 0.000 title claims abstract description 153
- 201000008350 membranous glomerulonephritis Diseases 0.000 title claims description 3
- 230000027455 binding Effects 0.000 claims abstract description 180
- 102000036639 antigens Human genes 0.000 claims abstract description 179
- 108091007433 antigens Proteins 0.000 claims abstract description 179
- 239000000427 antigen Substances 0.000 claims abstract description 176
- 239000012634 fragment Substances 0.000 claims abstract description 172
- 238000000034 method Methods 0.000 claims abstract description 162
- 201000008171 proliferative glomerulonephritis Diseases 0.000 claims abstract description 7
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 claims description 166
- 208000011511 primary membranoproliferative glomerulonephritis Diseases 0.000 claims description 130
- 208000004451 Membranoproliferative Glomerulonephritis Diseases 0.000 claims description 127
- 229940109239 creatinine Drugs 0.000 claims description 84
- 210000002700 urine Anatomy 0.000 claims description 79
- 102000009027 Albumins Human genes 0.000 claims description 73
- 108010088751 Albumins Proteins 0.000 claims description 73
- 201000001474 proteinuria Diseases 0.000 claims description 72
- 235000018102 proteins Nutrition 0.000 claims description 67
- 108090000623 proteins and genes Proteins 0.000 claims description 67
- 102000004169 proteins and genes Human genes 0.000 claims description 67
- 238000012423 maintenance Methods 0.000 claims description 63
- 210000002966 serum Anatomy 0.000 claims description 62
- 230000006698 induction Effects 0.000 claims description 60
- 238000011156 evaluation Methods 0.000 claims description 52
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 42
- 238000001802 infusion Methods 0.000 claims description 42
- 230000008021 deposition Effects 0.000 claims description 40
- HUOOMAOYXQFIDQ-UHFFFAOYSA-N isoginkgetin Chemical compound C1=CC(OC)=CC=C1C1=CC(=O)C2=C(O)C=C(O)C(C=3C(=CC=C(C=3)C=3OC4=CC(O)=CC(O)=C4C(=O)C=3)OC)=C2O1 HUOOMAOYXQFIDQ-UHFFFAOYSA-N 0.000 claims description 40
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 33
- 229910052708 sodium Inorganic materials 0.000 claims description 33
- 239000011734 sodium Substances 0.000 claims description 33
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 30
- 238000001990 intravenous administration Methods 0.000 claims description 29
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 27
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 26
- 229910052700 potassium Inorganic materials 0.000 claims description 26
- 239000011591 potassium Substances 0.000 claims description 26
- 201000010099 disease Diseases 0.000 claims description 24
- 230000036961 partial effect Effects 0.000 claims description 24
- 210000002381 plasma Anatomy 0.000 claims description 24
- 230000037396 body weight Effects 0.000 claims description 23
- 230000024924 glomerular filtration Effects 0.000 claims description 22
- 230000003907 kidney function Effects 0.000 claims description 22
- 235000012000 cholesterol Nutrition 0.000 claims description 20
- NTHXOOBQLCIOLC-UHFFFAOYSA-N iohexol Chemical compound OCC(O)CN(C(=O)C)C1=C(I)C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C1I NTHXOOBQLCIOLC-UHFFFAOYSA-N 0.000 claims description 20
- 229960001025 iohexol Drugs 0.000 claims description 20
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 20
- 229920001184 polypeptide Polymers 0.000 claims description 19
- 210000003743 erythrocyte Anatomy 0.000 claims description 18
- 230000001404 mediated effect Effects 0.000 claims description 18
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 16
- 238000005303 weighing Methods 0.000 claims description 14
- 102000001554 Hemoglobins Human genes 0.000 claims description 12
- 108010054147 Hemoglobins Proteins 0.000 claims description 12
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 claims description 10
- 238000002604 ultrasonography Methods 0.000 claims description 10
- 208000029574 C3 glomerulopathy Diseases 0.000 claims description 9
- 208000027134 non-immunoglobulin-mediated membranoproliferative glomerulonephritis Diseases 0.000 claims description 9
- 102000004506 Blood Proteins Human genes 0.000 claims description 8
- 108010017384 Blood Proteins Proteins 0.000 claims description 8
- 238000005259 measurement Methods 0.000 claims description 8
- 208000016323 C3 glomerulonephritis Diseases 0.000 claims description 6
- 238000006467 substitution reaction Methods 0.000 claims description 4
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 claims description 3
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 claims description 3
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 claims description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 claims description 3
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 3
- 229960001230 asparagine Drugs 0.000 claims description 3
- 235000009582 asparagine Nutrition 0.000 claims description 3
- 229930182817 methionine Natural products 0.000 claims description 3
- 108010068617 neonatal Fc receptor Proteins 0.000 claims description 3
- 239000012528 membrane Substances 0.000 abstract description 9
- 229960002224 eculizumab Drugs 0.000 description 121
- 238000011084 recovery Methods 0.000 description 48
- 230000029142 excretion Effects 0.000 description 35
- 230000009467 reduction Effects 0.000 description 30
- 206010029164 Nephrotic syndrome Diseases 0.000 description 28
- 230000024203 complement activation Effects 0.000 description 28
- 230000002829 reductive effect Effects 0.000 description 26
- 238000002560 therapeutic procedure Methods 0.000 description 26
- 239000008280 blood Substances 0.000 description 24
- 230000001434 glomerular Effects 0.000 description 24
- 210000004369 blood Anatomy 0.000 description 23
- 229910052698 phosphorus Inorganic materials 0.000 description 23
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 22
- 230000000694 effects Effects 0.000 description 21
- 102100022133 Complement C3 Human genes 0.000 description 20
- 238000010186 staining Methods 0.000 description 19
- 239000004202 carbamide Substances 0.000 description 17
- 239000000203 mixture Substances 0.000 description 17
- 239000003814 drug Substances 0.000 description 16
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 15
- 210000003734 kidney Anatomy 0.000 description 15
- 241000880493 Leptailurus serval Species 0.000 description 14
- 108010087924 alanylproline Proteins 0.000 description 14
- 230000000295 complement effect Effects 0.000 description 14
- 230000003247 decreasing effect Effects 0.000 description 14
- 230000006872 improvement Effects 0.000 description 14
- 238000011862 kidney biopsy Methods 0.000 description 14
- 230000036470 plasma concentration Effects 0.000 description 14
- 108010031719 prolyl-serine Proteins 0.000 description 14
- 238000005353 urine analysis Methods 0.000 description 14
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 13
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 13
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 13
- 238000001574 biopsy Methods 0.000 description 13
- 239000000523 sample Substances 0.000 description 13
- 239000000243 solution Substances 0.000 description 13
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 12
- 108060003951 Immunoglobulin Proteins 0.000 description 12
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 12
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 12
- 108010071390 Serum Albumin Proteins 0.000 description 12
- 102000007562 Serum Albumin Human genes 0.000 description 12
- 230000008859 change Effects 0.000 description 12
- 229940079593 drug Drugs 0.000 description 12
- 239000008103 glucose Substances 0.000 description 12
- 102000018358 immunoglobulin Human genes 0.000 description 12
- 238000008214 LDL Cholesterol Methods 0.000 description 11
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 11
- 239000011574 phosphorus Substances 0.000 description 11
- 208000024891 symptom Diseases 0.000 description 11
- 108010010234 HDL Lipoproteins Proteins 0.000 description 10
- 102000015779 HDL Lipoproteins Human genes 0.000 description 10
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 10
- 108010007622 LDL Lipoproteins Proteins 0.000 description 10
- 102000007330 LDL Lipoproteins Human genes 0.000 description 10
- 238000004458 analytical method Methods 0.000 description 10
- 238000009533 lab test Methods 0.000 description 10
- 150000003626 triacylglycerols Chemical class 0.000 description 10
- 108010044292 tryptophyltyrosine Proteins 0.000 description 10
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 9
- 230000008901 benefit Effects 0.000 description 9
- 208000020832 chronic kidney disease Diseases 0.000 description 9
- 208000006750 hematuria Diseases 0.000 description 9
- 108010057821 leucylproline Proteins 0.000 description 9
- 210000000440 neutrophil Anatomy 0.000 description 9
- 230000035755 proliferation Effects 0.000 description 9
- 230000004044 response Effects 0.000 description 9
- 230000008961 swelling Effects 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 8
- 108010079364 N-glycylalanine Proteins 0.000 description 8
- YMIZSYUAZJSOFL-SRVKXCTJSA-N Phe-Ser-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O YMIZSYUAZJSOFL-SRVKXCTJSA-N 0.000 description 8
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 8
- 230000035487 diastolic blood pressure Effects 0.000 description 8
- 210000000585 glomerular basement membrane Anatomy 0.000 description 8
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 8
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 8
- 208000015181 infectious disease Diseases 0.000 description 8
- 238000007619 statistical method Methods 0.000 description 8
- 238000007920 subcutaneous administration Methods 0.000 description 8
- 230000035488 systolic blood pressure Effects 0.000 description 8
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 7
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 7
- YJHKTAMKPGFJCT-NRPADANISA-N Ala-Val-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O YJHKTAMKPGFJCT-NRPADANISA-N 0.000 description 7
- ZUVMUOOHJYNJPP-XIRDDKMYSA-N Arg-Trp-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZUVMUOOHJYNJPP-XIRDDKMYSA-N 0.000 description 7
- WONGRTVAMHFGBE-WDSKDSINSA-N Asn-Gly-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N WONGRTVAMHFGBE-WDSKDSINSA-N 0.000 description 7
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 7
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 7
- 108010028778 Complement C4 Proteins 0.000 description 7
- OHLLDUNVMPPUMD-DCAQKATOSA-N Cys-Leu-Val Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N OHLLDUNVMPPUMD-DCAQKATOSA-N 0.000 description 7
- SMEYEQDCCBHTEF-FXQIFTODSA-N Cys-Pro-Ala Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O SMEYEQDCCBHTEF-FXQIFTODSA-N 0.000 description 7
- KSMSFCBQBQPFAD-GUBZILKMSA-N Cys-Pro-Pro Chemical compound SC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 KSMSFCBQBQPFAD-GUBZILKMSA-N 0.000 description 7
- VIOQRFNAZDMVLO-NRPADANISA-N Cys-Val-Glu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O VIOQRFNAZDMVLO-NRPADANISA-N 0.000 description 7
- 102000008857 Ferritin Human genes 0.000 description 7
- 108050000784 Ferritin Proteins 0.000 description 7
- 238000008416 Ferritin Methods 0.000 description 7
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 7
- CKOFNWCLWRYUHK-XHNCKOQMSA-N Glu-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O CKOFNWCLWRYUHK-XHNCKOQMSA-N 0.000 description 7
- RFDHKPSHTXZKLL-IHRRRGAJSA-N Glu-Gln-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)N RFDHKPSHTXZKLL-IHRRRGAJSA-N 0.000 description 7
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 7
- CQMFNTVQVLQRLT-JHEQGTHGSA-N Gly-Thr-Gln Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O CQMFNTVQVLQRLT-JHEQGTHGSA-N 0.000 description 7
- TTYKEFZRLKQTHH-MELADBBJSA-N His-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC2=CN=CN2)N)C(=O)O TTYKEFZRLKQTHH-MELADBBJSA-N 0.000 description 7
- CSTDQOOBZBAJKE-BWAGICSOSA-N His-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CN=CN2)N)O CSTDQOOBZBAJKE-BWAGICSOSA-N 0.000 description 7
- VGSPNSSCMOHRRR-BJDJZHNGSA-N Ile-Ser-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N VGSPNSSCMOHRRR-BJDJZHNGSA-N 0.000 description 7
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 7
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 7
- NTBFKPBULZGXQL-KKUMJFAQSA-N Lys-Asp-Tyr Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NTBFKPBULZGXQL-KKUMJFAQSA-N 0.000 description 7
- MWVUEPNEPWMFBD-SRVKXCTJSA-N Lys-Cys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCCN MWVUEPNEPWMFBD-SRVKXCTJSA-N 0.000 description 7
- GQFDWEDHOQRNLC-QWRGUYRKSA-N Lys-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN GQFDWEDHOQRNLC-QWRGUYRKSA-N 0.000 description 7
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 7
- YKBSXQFZWFXFIB-VOAKCMCISA-N Lys-Thr-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O YKBSXQFZWFXFIB-VOAKCMCISA-N 0.000 description 7
- QLFAPXUXEBAWEK-NHCYSSNCSA-N Lys-Val-Asp Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O QLFAPXUXEBAWEK-NHCYSSNCSA-N 0.000 description 7
- IHRFZLQEQVHXFA-RHYQMDGZSA-N Met-Thr-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCCN IHRFZLQEQVHXFA-RHYQMDGZSA-N 0.000 description 7
- JOXIIFVCSATTDH-IHPCNDPISA-N Phe-Asn-Trp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N JOXIIFVCSATTDH-IHPCNDPISA-N 0.000 description 7
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 7
- QARPMYDMYVLFMW-KKUMJFAQSA-N Phe-Pro-Glu Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(O)=O)C1=CC=CC=C1 QARPMYDMYVLFMW-KKUMJFAQSA-N 0.000 description 7
- ZJPGOXWRFNKIQL-JYJNAYRXSA-N Phe-Pro-Pro Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 ZJPGOXWRFNKIQL-JYJNAYRXSA-N 0.000 description 7
- SJRQWEDYTKYHHL-SLFFLAALSA-N Phe-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=CC=C3)N)C(=O)O SJRQWEDYTKYHHL-SLFFLAALSA-N 0.000 description 7
- HQVPQXMCQKXARZ-FXQIFTODSA-N Pro-Cys-Ser Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O HQVPQXMCQKXARZ-FXQIFTODSA-N 0.000 description 7
- UAYHMOIGIQZLFR-NHCYSSNCSA-N Pro-Gln-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UAYHMOIGIQZLFR-NHCYSSNCSA-N 0.000 description 7
- KIPIKSXPPLABPN-CIUDSAMLSA-N Pro-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 KIPIKSXPPLABPN-CIUDSAMLSA-N 0.000 description 7
- FMLRRBDLBJLJIK-DCAQKATOSA-N Pro-Leu-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 FMLRRBDLBJLJIK-DCAQKATOSA-N 0.000 description 7
- YDTUEBLEAVANFH-RCWTZXSCSA-N Pro-Val-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 YDTUEBLEAVANFH-RCWTZXSCSA-N 0.000 description 7
- KAAPNMOKUUPKOE-SRVKXCTJSA-N Ser-Asn-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KAAPNMOKUUPKOE-SRVKXCTJSA-N 0.000 description 7
- UGJRQLURDVGULT-LKXGYXEUSA-N Ser-Asn-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UGJRQLURDVGULT-LKXGYXEUSA-N 0.000 description 7
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 7
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 7
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 7
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 7
- UDCHKDYNMRJYMI-QEJZJMRPSA-N Trp-Glu-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O UDCHKDYNMRJYMI-QEJZJMRPSA-N 0.000 description 7
- PWKMJDQXKCENMF-MEYUZBJRSA-N Tyr-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O PWKMJDQXKCENMF-MEYUZBJRSA-N 0.000 description 7
- SQUMHUZLJDUROQ-YDHLFZDLSA-N Tyr-Val-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O SQUMHUZLJDUROQ-YDHLFZDLSA-N 0.000 description 7
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 7
- HJSLDXZAZGFPDK-ULQDDVLXSA-N Val-Phe-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N HJSLDXZAZGFPDK-ULQDDVLXSA-N 0.000 description 7
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 7
- SSYBNWFXCFNRFN-GUBZILKMSA-N Val-Pro-Ser Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SSYBNWFXCFNRFN-GUBZILKMSA-N 0.000 description 7
- KSFXWENSJABBFI-ZKWXMUAHSA-N Val-Ser-Asn Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O KSFXWENSJABBFI-ZKWXMUAHSA-N 0.000 description 7
- SDHZOOIGIUEPDY-JYJNAYRXSA-N Val-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 SDHZOOIGIUEPDY-JYJNAYRXSA-N 0.000 description 7
- BZDGLJPROOOUOZ-XGEHTFHBSA-N Val-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N)O BZDGLJPROOOUOZ-XGEHTFHBSA-N 0.000 description 7
- JVGDAEKKZKKZFO-RCWTZXSCSA-N Val-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)N)O JVGDAEKKZKKZFO-RCWTZXSCSA-N 0.000 description 7
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 7
- 108010047857 aspartylglycine Proteins 0.000 description 7
- 108010092854 aspartyllysine Proteins 0.000 description 7
- 239000000090 biomarker Substances 0.000 description 7
- 210000001772 blood platelet Anatomy 0.000 description 7
- 230000007423 decrease Effects 0.000 description 7
- 238000001962 electrophoresis Methods 0.000 description 7
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 7
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 7
- 230000002757 inflammatory effect Effects 0.000 description 7
- 235000013902 inosinic acid Nutrition 0.000 description 7
- 208000017169 kidney disease Diseases 0.000 description 7
- 210000000265 leukocyte Anatomy 0.000 description 7
- 108010003700 lysyl aspartic acid Proteins 0.000 description 7
- 108010017391 lysylvaline Proteins 0.000 description 7
- 239000008194 pharmaceutical composition Substances 0.000 description 7
- 108010084525 phenylalanyl-phenylalanyl-glycine Proteins 0.000 description 7
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 7
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 7
- 108010073969 valyllysine Proteins 0.000 description 7
- WYPUMLRSQMKIJU-BPNCWPANSA-N Ala-Arg-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O WYPUMLRSQMKIJU-BPNCWPANSA-N 0.000 description 6
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 6
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 6
- ISJWBVIYRBAXEB-CIUDSAMLSA-N Arg-Ser-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O ISJWBVIYRBAXEB-CIUDSAMLSA-N 0.000 description 6
- LGCVSPFCFXWUEY-IHPCNDPISA-N Asn-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC(=O)N)N LGCVSPFCFXWUEY-IHPCNDPISA-N 0.000 description 6
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 6
- 108010074051 C-Reactive Protein Proteins 0.000 description 6
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 6
- 108010028780 Complement C3 Proteins 0.000 description 6
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 6
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 6
- 206010018364 Glomerulonephritis Diseases 0.000 description 6
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 6
- STVHDEHTKFXBJQ-LAEOZQHASA-N Gly-Glu-Ile Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O STVHDEHTKFXBJQ-LAEOZQHASA-N 0.000 description 6
- 108010023302 HDL Cholesterol Proteins 0.000 description 6
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 6
- LZDNBBYBDGBADK-UHFFFAOYSA-N L-valyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C(C)C)C(O)=O)=CNC2=C1 LZDNBBYBDGBADK-UHFFFAOYSA-N 0.000 description 6
- 108010028554 LDL Cholesterol Proteins 0.000 description 6
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 6
- UCBPDSYUVAAHCD-UWVGGRQHSA-N Leu-Pro-Gly Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O UCBPDSYUVAAHCD-UWVGGRQHSA-N 0.000 description 6
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 6
- UWKNTTJNVSYXPC-CIUDSAMLSA-N Lys-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN UWKNTTJNVSYXPC-CIUDSAMLSA-N 0.000 description 6
- FLCMXEFCTLXBTL-DCAQKATOSA-N Lys-Asp-Arg Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N FLCMXEFCTLXBTL-DCAQKATOSA-N 0.000 description 6
- SJDQOYTYNGZZJX-SRVKXCTJSA-N Met-Glu-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O SJDQOYTYNGZZJX-SRVKXCTJSA-N 0.000 description 6
- 108010066427 N-valyltryptophan Proteins 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 6
- FRPVPGRXUKFEQE-YDHLFZDLSA-N Phe-Asp-Val Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O FRPVPGRXUKFEQE-YDHLFZDLSA-N 0.000 description 6
- IWZRODDWOSIXPZ-IRXDYDNUSA-N Phe-Phe-Gly Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)NCC(O)=O)C1=CC=CC=C1 IWZRODDWOSIXPZ-IRXDYDNUSA-N 0.000 description 6
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 6
- BMKNXTJLHFIAAH-CIUDSAMLSA-N Ser-Ser-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O BMKNXTJLHFIAAH-CIUDSAMLSA-N 0.000 description 6
- BDMWLJLPPUCLNV-XGEHTFHBSA-N Ser-Thr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BDMWLJLPPUCLNV-XGEHTFHBSA-N 0.000 description 6
- LGIMRDKGABDMBN-DCAQKATOSA-N Ser-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N LGIMRDKGABDMBN-DCAQKATOSA-N 0.000 description 6
- GLQFKOVWXPPFTP-VEVYYDQMSA-N Thr-Arg-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O GLQFKOVWXPPFTP-VEVYYDQMSA-N 0.000 description 6
- CQNFRKAKGDSJFR-NUMRIWBASA-N Thr-Glu-Asn Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)O CQNFRKAKGDSJFR-NUMRIWBASA-N 0.000 description 6
- LKEKWDJCJSPXNI-IRIUXVKKSA-N Thr-Glu-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 LKEKWDJCJSPXNI-IRIUXVKKSA-N 0.000 description 6
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 6
- JLNMFGCJODTXDH-WEDXCCLWSA-N Thr-Lys-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O JLNMFGCJODTXDH-WEDXCCLWSA-N 0.000 description 6
- RVMNUBQWPVOUKH-HEIBUPTGSA-N Thr-Ser-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O RVMNUBQWPVOUKH-HEIBUPTGSA-N 0.000 description 6
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 6
- OCCYDHCUKXRPSJ-SXNHZJKMSA-N Trp-Ile-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O OCCYDHCUKXRPSJ-SXNHZJKMSA-N 0.000 description 6
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 6
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 description 6
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 description 6
- JQTYTBPCSOAZHI-FXQIFTODSA-N Val-Ser-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N JQTYTBPCSOAZHI-FXQIFTODSA-N 0.000 description 6
- USXYVSTVPHELAF-RCWTZXSCSA-N Val-Thr-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](C(C)C)N)O USXYVSTVPHELAF-RCWTZXSCSA-N 0.000 description 6
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 6
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 6
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 6
- 108010008355 arginyl-glutamine Proteins 0.000 description 6
- 210000003651 basophil Anatomy 0.000 description 6
- 229910052791 calcium Inorganic materials 0.000 description 6
- 239000011575 calcium Substances 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 230000001684 chronic effect Effects 0.000 description 6
- 238000001493 electron microscopy Methods 0.000 description 6
- 210000003979 eosinophil Anatomy 0.000 description 6
- 239000012530 fluid Substances 0.000 description 6
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 6
- 108010089804 glycyl-threonine Proteins 0.000 description 6
- 108010015792 glycyllysine Proteins 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 210000004698 lymphocyte Anatomy 0.000 description 6
- 239000011159 matrix material Substances 0.000 description 6
- 210000001616 monocyte Anatomy 0.000 description 6
- 230000010412 perfusion Effects 0.000 description 6
- 230000002085 persistent effect Effects 0.000 description 6
- 238000011160 research Methods 0.000 description 6
- 229940116269 uric acid Drugs 0.000 description 6
- 238000002255 vaccination Methods 0.000 description 6
- SUHLZMHFRALVSY-YUMQZZPRSA-N Ala-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)NCC(O)=O SUHLZMHFRALVSY-YUMQZZPRSA-N 0.000 description 5
- 208000023275 Autoimmune disease Diseases 0.000 description 5
- 102100032752 C-reactive protein Human genes 0.000 description 5
- SNLOOPZHAQDMJG-CIUDSAMLSA-N Gln-Glu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SNLOOPZHAQDMJG-CIUDSAMLSA-N 0.000 description 5
- UHVIQGKBMXEVGN-WDSKDSINSA-N Glu-Gly-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O UHVIQGKBMXEVGN-WDSKDSINSA-N 0.000 description 5
- 206010061218 Inflammation Diseases 0.000 description 5
- BTNXKBVLWJBTNR-SRVKXCTJSA-N Leu-His-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O BTNXKBVLWJBTNR-SRVKXCTJSA-N 0.000 description 5
- BNFVPSRLHHPQKS-WHFBIAKZSA-N Ser-Asp-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O BNFVPSRLHHPQKS-WHFBIAKZSA-N 0.000 description 5
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 5
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 5
- RRVFEDGUXSYWOW-BZSNNMDCSA-N Ser-Phe-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O RRVFEDGUXSYWOW-BZSNNMDCSA-N 0.000 description 5
- YEDSOSIKVUMIJE-DCAQKATOSA-N Ser-Val-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O YEDSOSIKVUMIJE-DCAQKATOSA-N 0.000 description 5
- XKWABWFMQXMUMT-HJGDQZAQSA-N Thr-Pro-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XKWABWFMQXMUMT-HJGDQZAQSA-N 0.000 description 5
- RYHUIHUOYRNNIE-NRPADANISA-N Val-Ser-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N RYHUIHUOYRNNIE-NRPADANISA-N 0.000 description 5
- 230000002411 adverse Effects 0.000 description 5
- 239000003242 anti bacterial agent Substances 0.000 description 5
- 201000000523 end stage renal failure Diseases 0.000 description 5
- 230000007717 exclusion Effects 0.000 description 5
- 210000005086 glomerual capillary Anatomy 0.000 description 5
- 108010049041 glutamylalanine Proteins 0.000 description 5
- 238000010820 immunofluorescence microscopy Methods 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 150000002632 lipids Chemical class 0.000 description 5
- 210000004379 membrane Anatomy 0.000 description 5
- 230000006996 mental state Effects 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- 238000010606 normalization Methods 0.000 description 5
- 238000003860 storage Methods 0.000 description 5
- 230000003442 weekly effect Effects 0.000 description 5
- MQIGTEQXYCRLGK-BQBZGAKWSA-N Ala-Gly-Pro Chemical compound C[C@H](N)C(=O)NCC(=O)N1CCC[C@H]1C(O)=O MQIGTEQXYCRLGK-BQBZGAKWSA-N 0.000 description 4
- LRPZJPMQGKGHSG-XGEHTFHBSA-N Arg-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N)O LRPZJPMQGKGHSG-XGEHTFHBSA-N 0.000 description 4
- SRUUBQBAVNQZGJ-LAEOZQHASA-N Asn-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC(=O)N)N SRUUBQBAVNQZGJ-LAEOZQHASA-N 0.000 description 4
- HNXWVVHIGTZTBO-LKXGYXEUSA-N Asn-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O HNXWVVHIGTZTBO-LKXGYXEUSA-N 0.000 description 4
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 4
- 102000016574 Complement C3-C5 Convertases Human genes 0.000 description 4
- 108010067641 Complement C3-C5 Convertases Proteins 0.000 description 4
- NDUSUIGBMZCOIL-ZKWXMUAHSA-N Cys-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CS)N NDUSUIGBMZCOIL-ZKWXMUAHSA-N 0.000 description 4
- NDNZRWUDUMTITL-FXQIFTODSA-N Cys-Ser-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NDNZRWUDUMTITL-FXQIFTODSA-N 0.000 description 4
- 208000032928 Dyslipidaemia Diseases 0.000 description 4
- OJGLIOXAKGFFDW-SRVKXCTJSA-N Glu-Arg-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)O)N OJGLIOXAKGFFDW-SRVKXCTJSA-N 0.000 description 4
- HHSOPSCKAZKQHQ-PEXQALLHSA-N Gly-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)CN HHSOPSCKAZKQHQ-PEXQALLHSA-N 0.000 description 4
- GMTXWRIDLGTVFC-IUCAKERBSA-N Gly-Lys-Glu Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMTXWRIDLGTVFC-IUCAKERBSA-N 0.000 description 4
- KOYUSMBPJOVSOO-XEGUGMAKSA-N Gly-Tyr-Ile Chemical compound [H]NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O KOYUSMBPJOVSOO-XEGUGMAKSA-N 0.000 description 4
- FYVHHKMHFPMBBG-GUBZILKMSA-N His-Gln-Asp Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N FYVHHKMHFPMBBG-GUBZILKMSA-N 0.000 description 4
- ALPXXNRQBMRCPZ-MEYUZBJRSA-N His-Thr-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ALPXXNRQBMRCPZ-MEYUZBJRSA-N 0.000 description 4
- DFJJAVZIHDFOGQ-MNXVOIDGSA-N Ile-Glu-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N DFJJAVZIHDFOGQ-MNXVOIDGSA-N 0.000 description 4
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 4
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 4
- 208000017170 Lipid metabolism disease Diseases 0.000 description 4
- VHTOGMKQXXJOHG-RHYQMDGZSA-N Lys-Thr-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O VHTOGMKQXXJOHG-RHYQMDGZSA-N 0.000 description 4
- 101100462505 Mus musculus Pik3c2a gene Proteins 0.000 description 4
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 4
- KBUAPZAZPWNYSW-SRVKXCTJSA-N Pro-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 KBUAPZAZPWNYSW-SRVKXCTJSA-N 0.000 description 4
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 4
- VQBCMLMPEWPUTB-ACZMJKKPSA-N Ser-Glu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O VQBCMLMPEWPUTB-ACZMJKKPSA-N 0.000 description 4
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 4
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 4
- ZESGVALRVJIVLZ-VFCFLDTKSA-N Thr-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)O)N)O ZESGVALRVJIVLZ-VFCFLDTKSA-N 0.000 description 4
- LVRFMARKDGGZMX-IZPVPAKOSA-N Thr-Tyr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)O)C(O)=O)CC1=CC=C(O)C=C1 LVRFMARKDGGZMX-IZPVPAKOSA-N 0.000 description 4
- XGFGVFMXDXALEV-XIRDDKMYSA-N Trp-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N XGFGVFMXDXALEV-XIRDDKMYSA-N 0.000 description 4
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 4
- OACSGBOREVRSME-NHCYSSNCSA-N Val-His-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(O)=O OACSGBOREVRSME-NHCYSSNCSA-N 0.000 description 4
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 4
- 230000001154 acute effect Effects 0.000 description 4
- 229940088710 antibiotic agent Drugs 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 238000004820 blood count Methods 0.000 description 4
- 238000004140 cleaning Methods 0.000 description 4
- 230000004154 complement system Effects 0.000 description 4
- 230000009850 completed effect Effects 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 208000028208 end stage renal disease Diseases 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 206010020718 hyperplasia Diseases 0.000 description 4
- 230000002055 immunohistochemical effect Effects 0.000 description 4
- 229940090044 injection Drugs 0.000 description 4
- 229940124731 meningococcal vaccine Drugs 0.000 description 4
- 108010077112 prolyl-proline Proteins 0.000 description 4
- 235000002639 sodium chloride Nutrition 0.000 description 4
- 150000003431 steroids Chemical class 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 239000002699 waste material Substances 0.000 description 4
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 3
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 3
- 206010001580 Albuminuria Diseases 0.000 description 3
- JEOCWTUOMKEEMF-RHYQMDGZSA-N Arg-Leu-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JEOCWTUOMKEEMF-RHYQMDGZSA-N 0.000 description 3
- GIMTZGADWZTZGV-DCAQKATOSA-N Arg-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N GIMTZGADWZTZGV-DCAQKATOSA-N 0.000 description 3
- JZLFYAAGGYMRIK-BYULHYEWSA-N Asn-Val-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O JZLFYAAGGYMRIK-BYULHYEWSA-N 0.000 description 3
- DPNWSMBUYCLEDG-CIUDSAMLSA-N Asp-Lys-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O DPNWSMBUYCLEDG-CIUDSAMLSA-N 0.000 description 3
- 241000283707 Capra Species 0.000 description 3
- 102000016550 Complement Factor H Human genes 0.000 description 3
- 108010053085 Complement Factor H Proteins 0.000 description 3
- 238000002965 ELISA Methods 0.000 description 3
- DHNWZLGBTPUTQQ-QEJZJMRPSA-N Gln-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N DHNWZLGBTPUTQQ-QEJZJMRPSA-N 0.000 description 3
- FITIQFSXXBKFFM-NRPADANISA-N Gln-Val-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FITIQFSXXBKFFM-NRPADANISA-N 0.000 description 3
- QDMVXRNLOPTPIE-WDCWCFNPSA-N Glu-Lys-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QDMVXRNLOPTPIE-WDCWCFNPSA-N 0.000 description 3
- JWNZHMSRZXXGTM-XKBZYTNZSA-N Glu-Ser-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JWNZHMSRZXXGTM-XKBZYTNZSA-N 0.000 description 3
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 3
- SYOJVRNQCXYEOV-XVKPBYJWSA-N Gly-Val-Glu Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SYOJVRNQCXYEOV-XVKPBYJWSA-N 0.000 description 3
- FULZDMOZUZKGQU-ONGXEEELSA-N Gly-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN FULZDMOZUZKGQU-ONGXEEELSA-N 0.000 description 3
- 206010019233 Headaches Diseases 0.000 description 3
- MAABHGXCIBEYQR-XVYDVKMFSA-N His-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N MAABHGXCIBEYQR-XVYDVKMFSA-N 0.000 description 3
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 3
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 3
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 3
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 3
- ODUQLUADRKMHOZ-JYJNAYRXSA-N Lys-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)N)O ODUQLUADRKMHOZ-JYJNAYRXSA-N 0.000 description 3
- RFQATBGBLDAKGI-VHSXEESVSA-N Lys-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CCCCN)N)C(=O)O RFQATBGBLDAKGI-VHSXEESVSA-N 0.000 description 3
- RKIIYGUHIQJCBW-SRVKXCTJSA-N Met-His-Glu Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O RKIIYGUHIQJCBW-SRVKXCTJSA-N 0.000 description 3
- FWAHLGXNBLWIKB-NAKRPEOUSA-N Met-Ile-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCSC FWAHLGXNBLWIKB-NAKRPEOUSA-N 0.000 description 3
- 241000283973 Oryctolagus cuniculus Species 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 3
- XDKKMRPRRCOELJ-GUBZILKMSA-N Pro-Val-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 XDKKMRPRRCOELJ-GUBZILKMSA-N 0.000 description 3
- KHRLUIPIMIQFGT-AVGNSLFASA-N Pro-Val-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHRLUIPIMIQFGT-AVGNSLFASA-N 0.000 description 3
- IXCHOHLPHNGFTJ-YUMQZZPRSA-N Ser-Gly-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N IXCHOHLPHNGFTJ-YUMQZZPRSA-N 0.000 description 3
- XNCUYZKGQOCOQH-YUMQZZPRSA-N Ser-Leu-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O XNCUYZKGQOCOQH-YUMQZZPRSA-N 0.000 description 3
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 3
- JMBRNXUOLJFURW-BEAPCOKYSA-N Thr-Phe-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N)O JMBRNXUOLJFURW-BEAPCOKYSA-N 0.000 description 3
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 3
- KPMIQCXJDVKWKO-IFFSRLJSSA-N Thr-Val-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPMIQCXJDVKWKO-IFFSRLJSSA-N 0.000 description 3
- BKVICMPZWRNWOC-RHYQMDGZSA-N Thr-Val-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)[C@@H](C)O BKVICMPZWRNWOC-RHYQMDGZSA-N 0.000 description 3
- QYSBJAUCUKHSLU-JYJNAYRXSA-N Tyr-Arg-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O QYSBJAUCUKHSLU-JYJNAYRXSA-N 0.000 description 3
- CNLKDWSAORJEMW-KWQFWETISA-N Tyr-Gly-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](C)C(O)=O CNLKDWSAORJEMW-KWQFWETISA-N 0.000 description 3
- QFHRUCJIRVILCK-YJRXYDGGSA-N Tyr-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O QFHRUCJIRVILCK-YJRXYDGGSA-N 0.000 description 3
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 3
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 3
- 230000005856 abnormality Effects 0.000 description 3
- 108010047495 alanylglycine Proteins 0.000 description 3
- 230000037007 arousal Effects 0.000 description 3
- 230000036772 blood pressure Effects 0.000 description 3
- 230000001364 causal effect Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 108010004073 cysteinylcysteine Proteins 0.000 description 3
- 238000003745 diagnosis Methods 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 3
- 231100000869 headache Toxicity 0.000 description 3
- 230000000004 hemodynamic effect Effects 0.000 description 3
- 230000028993 immune response Effects 0.000 description 3
- 238000003125 immunofluorescent labeling Methods 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 230000008595 infiltration Effects 0.000 description 3
- 238000001764 infiltration Methods 0.000 description 3
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 3
- 108010091871 leucylmethionine Proteins 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 201000008383 nephritis Diseases 0.000 description 3
- 230000008506 pathogenesis Effects 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 230000035935 pregnancy Effects 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 230000000069 prophylactic effect Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 230000002269 spontaneous effect Effects 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 230000002459 sustained effect Effects 0.000 description 3
- 230000001052 transient effect Effects 0.000 description 3
- 108010051110 tyrosyl-lysine Proteins 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- KIUYPHAMDKDICO-WHFBIAKZSA-N Ala-Asp-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O KIUYPHAMDKDICO-WHFBIAKZSA-N 0.000 description 2
- ARHJJAAWNWOACN-FXQIFTODSA-N Ala-Ser-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O ARHJJAAWNWOACN-FXQIFTODSA-N 0.000 description 2
- CREYEAPXISDKSB-FQPOAREZSA-N Ala-Thr-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CREYEAPXISDKSB-FQPOAREZSA-N 0.000 description 2
- PNQWAUXQDBIJDY-GUBZILKMSA-N Arg-Glu-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PNQWAUXQDBIJDY-GUBZILKMSA-N 0.000 description 2
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 2
- WUQXMTITJLFXAU-JIOCBJNQSA-N Asn-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)N)N)O WUQXMTITJLFXAU-JIOCBJNQSA-N 0.000 description 2
- CBHVAFXKOYAHOY-NHCYSSNCSA-N Asn-Val-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O CBHVAFXKOYAHOY-NHCYSSNCSA-N 0.000 description 2
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 2
- SNDBKTFJWVEVPO-WHFBIAKZSA-N Asp-Gly-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SNDBKTFJWVEVPO-WHFBIAKZSA-N 0.000 description 2
- QNFRBNZGVVKBNJ-PEFMBERDSA-N Asp-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N QNFRBNZGVVKBNJ-PEFMBERDSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 101100512078 Caenorhabditis elegans lys-1 gene Proteins 0.000 description 2
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 2
- 102000000989 Complement System Proteins Human genes 0.000 description 2
- 108010069112 Complement System Proteins Proteins 0.000 description 2
- 208000032170 Congenital Abnormalities Diseases 0.000 description 2
- CVLIHKBUPSFRQP-WHFBIAKZSA-N Cys-Gly-Ala Chemical compound [H]N[C@@H](CS)C(=O)NCC(=O)N[C@@H](C)C(O)=O CVLIHKBUPSFRQP-WHFBIAKZSA-N 0.000 description 2
- 102000012192 Cystatin C Human genes 0.000 description 2
- 108010061642 Cystatin C Proteins 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 108060006698 EGF receptor Proteins 0.000 description 2
- XKBASPWPBXNVLQ-WDSKDSINSA-N Gln-Gly-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O XKBASPWPBXNVLQ-WDSKDSINSA-N 0.000 description 2
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 2
- AFODTOLGSZQDSL-PEFMBERDSA-N Glu-Asn-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)O)N AFODTOLGSZQDSL-PEFMBERDSA-N 0.000 description 2
- SBYVDRJAXWSXQL-AVGNSLFASA-N Glu-Asn-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SBYVDRJAXWSXQL-AVGNSLFASA-N 0.000 description 2
- PAQUJCSYVIBPLC-AVGNSLFASA-N Glu-Asp-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PAQUJCSYVIBPLC-AVGNSLFASA-N 0.000 description 2
- QXDXIXFSFHUYAX-MNXVOIDGSA-N Glu-Ile-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCC(O)=O QXDXIXFSFHUYAX-MNXVOIDGSA-N 0.000 description 2
- DXVOKNVIKORTHQ-GUBZILKMSA-N Glu-Pro-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O DXVOKNVIKORTHQ-GUBZILKMSA-N 0.000 description 2
- BKMOHWJHXQLFEX-IRIUXVKKSA-N Glu-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CCC(=O)O)N)O BKMOHWJHXQLFEX-IRIUXVKKSA-N 0.000 description 2
- LJPIRKICOISLKN-WHFBIAKZSA-N Gly-Ala-Ser Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O LJPIRKICOISLKN-WHFBIAKZSA-N 0.000 description 2
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 2
- 241000606768 Haemophilus influenzae Species 0.000 description 2
- 208000032843 Hemorrhage Diseases 0.000 description 2
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 2
- PRTZQMBYUZFSFA-XEGUGMAKSA-N Ile-Tyr-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)NCC(=O)O)N PRTZQMBYUZFSFA-XEGUGMAKSA-N 0.000 description 2
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 2
- USTCFDAQCLDPBD-XIRDDKMYSA-N Leu-Asn-Trp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N USTCFDAQCLDPBD-XIRDDKMYSA-N 0.000 description 2
- PVMPDMIKUVNOBD-CIUDSAMLSA-N Leu-Asp-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O PVMPDMIKUVNOBD-CIUDSAMLSA-N 0.000 description 2
- CQGSYZCULZMEDE-UHFFFAOYSA-N Leu-Gln-Pro Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)N1CCCC1C(O)=O CQGSYZCULZMEDE-UHFFFAOYSA-N 0.000 description 2
- APFJUBGRZGMQFF-QWRGUYRKSA-N Leu-Gly-Lys Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCCN APFJUBGRZGMQFF-QWRGUYRKSA-N 0.000 description 2
- OYQUOLRTJHWVSQ-SRVKXCTJSA-N Leu-His-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(O)=O OYQUOLRTJHWVSQ-SRVKXCTJSA-N 0.000 description 2
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 2
- IXHKPDJKKCUKHS-GARJFASQSA-N Lys-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N IXHKPDJKKCUKHS-GARJFASQSA-N 0.000 description 2
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 2
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 2
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 2
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 2
- 208000031481 Pathologic Constriction Diseases 0.000 description 2
- 208000037581 Persistent Infection Diseases 0.000 description 2
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 2
- DXTOOBDIIAJZBJ-BQBZGAKWSA-N Pro-Gly-Ser Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CO)C(O)=O DXTOOBDIIAJZBJ-BQBZGAKWSA-N 0.000 description 2
- 208000035977 Rare disease Diseases 0.000 description 2
- 208000034189 Sclerosis Diseases 0.000 description 2
- SQBLRDDJTUJDMV-ACZMJKKPSA-N Ser-Glu-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O SQBLRDDJTUJDMV-ACZMJKKPSA-N 0.000 description 2
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 2
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 2
- JCLAFVNDBJMLBC-JBDRJPRFSA-N Ser-Ser-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JCLAFVNDBJMLBC-JBDRJPRFSA-N 0.000 description 2
- ZKOKTQPHFMRSJP-YJRXYDGGSA-N Ser-Thr-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZKOKTQPHFMRSJP-YJRXYDGGSA-N 0.000 description 2
- RCOUFINCYASMDN-GUBZILKMSA-N Ser-Val-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O RCOUFINCYASMDN-GUBZILKMSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- SKHPKKYKDYULDH-HJGDQZAQSA-N Thr-Asn-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O SKHPKKYKDYULDH-HJGDQZAQSA-N 0.000 description 2
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 2
- GXUWHVZYDAHFSV-FLBSBUHZSA-N Thr-Ile-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GXUWHVZYDAHFSV-FLBSBUHZSA-N 0.000 description 2
- FIFDDJFLNVAVMS-RHYQMDGZSA-N Thr-Leu-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O FIFDDJFLNVAVMS-RHYQMDGZSA-N 0.000 description 2
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 2
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 2
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 2
- RWOKVQUCENPXGE-IHRRRGAJSA-N Tyr-Ser-Arg Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RWOKVQUCENPXGE-IHRRRGAJSA-N 0.000 description 2
- KUXCBJFJURINGF-PXDAIIFMSA-N Tyr-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CC3=CC=C(C=C3)O)N KUXCBJFJURINGF-PXDAIIFMSA-N 0.000 description 2
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 2
- XTDDIVQWDXMRJL-IHRRRGAJSA-N Val-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N XTDDIVQWDXMRJL-IHRRRGAJSA-N 0.000 description 2
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 2
- OFTXTCGQJXTNQS-XGEHTFHBSA-N Val-Thr-Ser Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](C(C)C)N)O OFTXTCGQJXTNQS-XGEHTFHBSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 125000000539 amino acid group Chemical group 0.000 description 2
- 230000001948 anti-meningococcal effect Effects 0.000 description 2
- 230000001597 anti-proteinuria Effects 0.000 description 2
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 210000002469 basement membrane Anatomy 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000002146 bilateral effect Effects 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 210000001736 capillary Anatomy 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000000546 chi-square test Methods 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- -1 coatings Substances 0.000 description 2
- 230000000875 corresponding effect Effects 0.000 description 2
- 239000003246 corticosteroid Substances 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- 230000006866 deterioration Effects 0.000 description 2
- 230000001627 detrimental effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- AAOVKJBEBIDNHE-UHFFFAOYSA-N diazepam Chemical compound N=1CC(=O)N(C)C2=CC=C(Cl)C=C2C=1C1=CC=CC=C1 AAOVKJBEBIDNHE-UHFFFAOYSA-N 0.000 description 2
- 235000005911 diet Nutrition 0.000 description 2
- 230000037213 diet Effects 0.000 description 2
- 230000009266 disease activity Effects 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 230000008482 dysregulation Effects 0.000 description 2
- 206010014599 encephalitis Diseases 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 206010061989 glomerulosclerosis Diseases 0.000 description 2
- 108010010147 glycylglutamine Proteins 0.000 description 2
- 231100001261 hazardous Toxicity 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- 239000003018 immunosuppressive agent Substances 0.000 description 2
- 238000002650 immunosuppressive therapy Methods 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 238000007917 intracranial administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 239000007928 intraperitoneal injection Substances 0.000 description 2
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 2
- 108010053037 kyotorphin Proteins 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000013507 mapping Methods 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 201000011475 meningoencephalitis Diseases 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 201000003045 paroxysmal nocturnal hemoglobinuria Diseases 0.000 description 2
- 230000007310 pathophysiology Effects 0.000 description 2
- 238000002203 pretreatment Methods 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 108010090894 prolylleucine Proteins 0.000 description 2
- 230000007420 reactivation Effects 0.000 description 2
- 229960004641 rituximab Drugs 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 239000001488 sodium phosphate Substances 0.000 description 2
- 229910000162 sodium phosphate Inorganic materials 0.000 description 2
- 230000036262 stenosis Effects 0.000 description 2
- 208000037804 stenosis Diseases 0.000 description 2
- 238000013517 stratification Methods 0.000 description 2
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 2
- 108010003137 tyrosyltyrosine Proteins 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 108010027345 wheylin-1 peptide Proteins 0.000 description 2
- HSTOKWSFWGCZMH-UHFFFAOYSA-N 3,3'-diaminobenzidine Chemical compound C1=C(N)C(N)=CC=C1C1=CC=C(N)C(N)=C1 HSTOKWSFWGCZMH-UHFFFAOYSA-N 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- WCBVQNZTOKJWJS-ACZMJKKPSA-N Ala-Cys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O WCBVQNZTOKJWJS-ACZMJKKPSA-N 0.000 description 1
- WQLDNOCHHRISMS-NAKRPEOUSA-N Ala-Pro-Ile Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WQLDNOCHHRISMS-NAKRPEOUSA-N 0.000 description 1
- 102000005862 Angiotensin II Human genes 0.000 description 1
- 101800000733 Angiotensin-2 Proteins 0.000 description 1
- PNHQRQTVBRDIEF-CIUDSAMLSA-N Asn-Leu-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)N)N PNHQRQTVBRDIEF-CIUDSAMLSA-N 0.000 description 1
- RBOBTTLFPRSXKZ-BZSNNMDCSA-N Asn-Phe-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RBOBTTLFPRSXKZ-BZSNNMDCSA-N 0.000 description 1
- YNQIDCRRTWGHJD-ZLUOBGJFSA-N Asp-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(O)=O YNQIDCRRTWGHJD-ZLUOBGJFSA-N 0.000 description 1
- HSWYMWGDMPLTTH-FXQIFTODSA-N Asp-Glu-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HSWYMWGDMPLTTH-FXQIFTODSA-N 0.000 description 1
- VNXQRBXEQXLERQ-CIUDSAMLSA-N Asp-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N VNXQRBXEQXLERQ-CIUDSAMLSA-N 0.000 description 1
- YIDFBWRHIYOYAA-LKXGYXEUSA-N Asp-Ser-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YIDFBWRHIYOYAA-LKXGYXEUSA-N 0.000 description 1
- 206010003694 Atrophy Diseases 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000003950 B-cell lymphoma Diseases 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 102100021277 Beta-secretase 2 Human genes 0.000 description 1
- 101710150190 Beta-secretase 2 Proteins 0.000 description 1
- 101150071258 C3 gene Proteins 0.000 description 1
- 102100032957 C5a anaphylatoxin chemotactic receptor 1 Human genes 0.000 description 1
- 101710098483 C5a anaphylatoxin chemotactic receptor 1 Proteins 0.000 description 1
- 206010008479 Chest Pain Diseases 0.000 description 1
- 108090000056 Complement factor B Proteins 0.000 description 1
- 102000003712 Complement factor B Human genes 0.000 description 1
- 102100035432 Complement factor H Human genes 0.000 description 1
- 102220592819 Complement factor H_R78G_mutation Human genes 0.000 description 1
- 229940124073 Complement inhibitor Drugs 0.000 description 1
- 102100032768 Complement receptor type 2 Human genes 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 208000028399 Critical Illness Diseases 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical group [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 108010067770 Endopeptidase K Proteins 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 238000000729 Fisher's exact test Methods 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- LMPBBFWHCRURJD-LAEOZQHASA-N Gln-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)N)N LMPBBFWHCRURJD-LAEOZQHASA-N 0.000 description 1
- FGYPOQPQTUNESW-IUCAKERBSA-N Gln-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)N)N FGYPOQPQTUNESW-IUCAKERBSA-N 0.000 description 1
- 208000022461 Glomerular disease Diseases 0.000 description 1
- 206010018367 Glomerulonephritis chronic Diseases 0.000 description 1
- 206010018370 Glomerulonephritis membranoproliferative Diseases 0.000 description 1
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 1
- QSDKBRMVXSWAQE-BFHQHQDPSA-N Gly-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN QSDKBRMVXSWAQE-BFHQHQDPSA-N 0.000 description 1
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 1
- BIRKKBCSAIHDDF-WDSKDSINSA-N Gly-Glu-Cys Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(O)=O BIRKKBCSAIHDDF-WDSKDSINSA-N 0.000 description 1
- QSVMIMFAAZPCAQ-PMVVWTBXSA-N Gly-His-Thr Chemical compound [H]NCC(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QSVMIMFAAZPCAQ-PMVVWTBXSA-N 0.000 description 1
- SJLKKOZFHSJJAW-YUMQZZPRSA-N Gly-Met-Glu Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)CN SJLKKOZFHSJJAW-YUMQZZPRSA-N 0.000 description 1
- WCORRBXVISTKQL-WHFBIAKZSA-N Gly-Ser-Ser Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WCORRBXVISTKQL-WHFBIAKZSA-N 0.000 description 1
- LCRDMSSAKLTKBU-ZDLURKLDSA-N Gly-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN LCRDMSSAKLTKBU-ZDLURKLDSA-N 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 206010061190 Haemophilus infection Diseases 0.000 description 1
- 241000711549 Hepacivirus C Species 0.000 description 1
- 208000005176 Hepatitis C Diseases 0.000 description 1
- TVMNTHXFRSXZGR-IHRRRGAJSA-N His-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O TVMNTHXFRSXZGR-IHRRRGAJSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101100440311 Homo sapiens C5 gene Proteins 0.000 description 1
- 101000941929 Homo sapiens Complement receptor type 2 Proteins 0.000 description 1
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 208000019758 Hypergammaglobulinemia Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 208000003623 Hypoalbuminemia Diseases 0.000 description 1
- CZGUSIXMZVURDU-JZXHSEFVSA-N Ile(5)-angiotensin II Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C([O-])=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@@H]([NH3+])CC([O-])=O)C(C)C)C1=CC=C(O)C=C1 CZGUSIXMZVURDU-JZXHSEFVSA-N 0.000 description 1
- NZGTYCMLUGYMCV-XUXIUFHCSA-N Ile-Lys-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N NZGTYCMLUGYMCV-XUXIUFHCSA-N 0.000 description 1
- PZWBBXHHUSIGKH-OSUNSFLBSA-N Ile-Thr-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PZWBBXHHUSIGKH-OSUNSFLBSA-N 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 238000012313 Kruskal-Wallis test Methods 0.000 description 1
- LHSGPCFBGJHPCY-UHFFFAOYSA-N L-leucine-L-tyrosine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 LHSGPCFBGJHPCY-UHFFFAOYSA-N 0.000 description 1
- FIJMQLGQLBLBOL-HJGDQZAQSA-N Leu-Asn-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O FIJMQLGQLBLBOL-HJGDQZAQSA-N 0.000 description 1
- GPICTNQYKHHHTH-GUBZILKMSA-N Leu-Gln-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GPICTNQYKHHHTH-GUBZILKMSA-N 0.000 description 1
- KXODZBLFVFSLAI-AVGNSLFASA-N Leu-His-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)CC1=CN=CN1 KXODZBLFVFSLAI-AVGNSLFASA-N 0.000 description 1
- IAJFFZORSWOZPQ-SRVKXCTJSA-N Leu-Leu-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O IAJFFZORSWOZPQ-SRVKXCTJSA-N 0.000 description 1
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- MPGHETGWWWUHPY-CIUDSAMLSA-N Lys-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN MPGHETGWWWUHPY-CIUDSAMLSA-N 0.000 description 1
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 1
- XABXVVSWUVCZST-GVXVVHGQSA-N Lys-Val-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN XABXVVSWUVCZST-GVXVVHGQSA-N 0.000 description 1
- OOSPRDCGTLQLBP-NHCYSSNCSA-N Met-Glu-Val Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O OOSPRDCGTLQLBP-NHCYSSNCSA-N 0.000 description 1
- UROWNMBTQGGTHB-DCAQKATOSA-N Met-Leu-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O UROWNMBTQGGTHB-DCAQKATOSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 208000019695 Migraine disease Diseases 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 241000588650 Neisseria meningitidis Species 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 102000043276 Oncogene Human genes 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 208000030852 Parasitic disease Diseases 0.000 description 1
- 102000002508 Peptide Elongation Factors Human genes 0.000 description 1
- 108010068204 Peptide Elongation Factors Proteins 0.000 description 1
- 108010067902 Peptide Library Proteins 0.000 description 1
- LJUUGSWZPQOJKD-JYJNAYRXSA-N Phe-Arg-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccccc1)C(O)=O LJUUGSWZPQOJKD-JYJNAYRXSA-N 0.000 description 1
- HXSUFWQYLPKEHF-IHRRRGAJSA-N Phe-Asn-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N HXSUFWQYLPKEHF-IHRRRGAJSA-N 0.000 description 1
- BWTKUQPNOMMKMA-FIRPJDEBSA-N Phe-Ile-Phe Chemical compound C([C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 BWTKUQPNOMMKMA-FIRPJDEBSA-N 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 208000035109 Pneumococcal Infections Diseases 0.000 description 1
- 208000009362 Pneumococcal Pneumonia Diseases 0.000 description 1
- 206010035728 Pneumonia pneumococcal Diseases 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 1
- RETPETNFPLNLRV-JYJNAYRXSA-N Pro-Asn-Trp Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O RETPETNFPLNLRV-JYJNAYRXSA-N 0.000 description 1
- UIMCLYYSUCIUJM-UWVGGRQHSA-N Pro-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 UIMCLYYSUCIUJM-UWVGGRQHSA-N 0.000 description 1
- VTFXTWDFPTWNJY-RHYQMDGZSA-N Pro-Leu-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VTFXTWDFPTWNJY-RHYQMDGZSA-N 0.000 description 1
- RPLMFKUKFZOTER-AVGNSLFASA-N Pro-Met-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1 RPLMFKUKFZOTER-AVGNSLFASA-N 0.000 description 1
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 1
- 229940078123 Ras inhibitor Drugs 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 206010062237 Renal impairment Diseases 0.000 description 1
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 1
- NADLKBTYNKUJEP-KATARQTJSA-N Ser-Thr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O NADLKBTYNKUJEP-KATARQTJSA-N 0.000 description 1
- DYEGLQRVMBWQLD-IXOXFDKPSA-N Ser-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CO)N)O DYEGLQRVMBWQLD-IXOXFDKPSA-N 0.000 description 1
- 238000011869 Shapiro-Wilk test Methods 0.000 description 1
- 208000021386 Sjogren Syndrome Diseases 0.000 description 1
- 208000007271 Substance Withdrawal Syndrome Diseases 0.000 description 1
- DWYAUVCQDTZIJI-VZFHVOOUSA-N Thr-Ala-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O DWYAUVCQDTZIJI-VZFHVOOUSA-N 0.000 description 1
- GKWNLDNXMMLRMC-GLLZPBPUSA-N Thr-Glu-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O GKWNLDNXMMLRMC-GLLZPBPUSA-N 0.000 description 1
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 1
- IJVNLNRVDUTWDD-MEYUZBJRSA-N Thr-Leu-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IJVNLNRVDUTWDD-MEYUZBJRSA-N 0.000 description 1
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 1
- VYVBSMCZNHOZGD-RCWTZXSCSA-N Thr-Val-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O VYVBSMCZNHOZGD-RCWTZXSCSA-N 0.000 description 1
- NLWCSMOXNKBRLC-WDSOQIARSA-N Trp-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NLWCSMOXNKBRLC-WDSOQIARSA-N 0.000 description 1
- SLCSPPCQWUHPPO-JYJNAYRXSA-N Tyr-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SLCSPPCQWUHPPO-JYJNAYRXSA-N 0.000 description 1
- KEANSLVUGJADPN-LKTVYLICSA-N Tyr-His-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC2=CC=C(C=C2)O)N KEANSLVUGJADPN-LKTVYLICSA-N 0.000 description 1
- BXPOOVDVGWEXDU-WZLNRYEVSA-N Tyr-Ile-Thr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BXPOOVDVGWEXDU-WZLNRYEVSA-N 0.000 description 1
- LMKKMCGTDANZTR-BZSNNMDCSA-N Tyr-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 LMKKMCGTDANZTR-BZSNNMDCSA-N 0.000 description 1
- JXGUUJMPCRXMSO-HJOGWXRNSA-N Tyr-Phe-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 JXGUUJMPCRXMSO-HJOGWXRNSA-N 0.000 description 1
- MQGGXGKQSVEQHR-KKUMJFAQSA-N Tyr-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 MQGGXGKQSVEQHR-KKUMJFAQSA-N 0.000 description 1
- TVGWMCTYUFBXAP-QTKMDUPCSA-N Val-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N)O TVGWMCTYUFBXAP-QTKMDUPCSA-N 0.000 description 1
- GVNLOVJNNDZUHS-RHYQMDGZSA-N Val-Thr-Lys Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O GVNLOVJNNDZUHS-RHYQMDGZSA-N 0.000 description 1
- ZHWZDZFWBXWPDW-GUBZILKMSA-N Val-Val-Cys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(O)=O ZHWZDZFWBXWPDW-GUBZILKMSA-N 0.000 description 1
- SSKKGOWRPNIVDW-AVGNSLFASA-N Val-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N SSKKGOWRPNIVDW-AVGNSLFASA-N 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 239000012080 ambient air Substances 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 230000002491 angiogenic effect Effects 0.000 description 1
- 229950006323 angiotensin ii Drugs 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 238000011203 antimicrobial therapy Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 108010013835 arginine glutamate Proteins 0.000 description 1
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000037444 atrophy Effects 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 230000007698 birth defect Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 239000008366 buffered solution Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 230000007211 cardiovascular event Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000004074 complement inhibitor Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 229940124301 concurrent medication Drugs 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 208000018631 connective tissue disease Diseases 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 238000007728 cost analysis Methods 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 229940080205 eculizumab injection Drugs 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 230000003631 expected effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 229940125397 factor b inhibitor Drugs 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 238000010448 genetic screening Methods 0.000 description 1
- 231100000852 glomerular disease Toxicity 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 208000002672 hepatitis B Diseases 0.000 description 1
- 208000010710 hepatitis C virus infection Diseases 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 230000002962 histologic effect Effects 0.000 description 1
- 230000036732 histological change Effects 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 230000037417 hyperactivation Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 208000026278 immune system disease Diseases 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 229960003444 immunosuppressant agent Drugs 0.000 description 1
- 229940124589 immunosuppressive drug Drugs 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 239000003978 infusion fluid Substances 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 238000011545 laboratory measurement Methods 0.000 description 1
- 230000006651 lactation Effects 0.000 description 1
- 108010012058 leucyltyrosine Proteins 0.000 description 1
- 201000002364 leukopenia Diseases 0.000 description 1
- 231100001022 leukopenia Toxicity 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000008297 liquid dosage form Substances 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 206010027599 migraine Diseases 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229940031346 monovalent vaccine Drugs 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- RTGDFNSFWBGLEC-SYZQJQIISA-N mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1C\C=C(/C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-SYZQJQIISA-N 0.000 description 1
- 229960004866 mycophenolate mofetil Drugs 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 231100000590 oncogenic Toxicity 0.000 description 1
- 230000002246 oncogenic effect Effects 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 230000014207 opsonization Effects 0.000 description 1
- 229910052762 osmium Inorganic materials 0.000 description 1
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 1
- 238000007427 paired t-test Methods 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 239000013618 particulate matter Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 239000003186 pharmaceutical solution Substances 0.000 description 1
- 108010018625 phenylalanylarginine Proteins 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 238000002616 plasmapheresis Methods 0.000 description 1
- 210000000557 podocyte Anatomy 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000009597 pregnancy test Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 208000037920 primary disease Diseases 0.000 description 1
- 230000002250 progressing effect Effects 0.000 description 1
- 230000007425 progressive decline Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 201000008158 rapidly progressive glomerulonephritis Diseases 0.000 description 1
- 238000011867 re-evaluation Methods 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 210000005084 renal tissue Anatomy 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 239000008299 semisolid dosage form Substances 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 1
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229940105067 sodium chloride 9 mg/ml Drugs 0.000 description 1
- 239000008354 sodium chloride injection Substances 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 229940055944 soliris Drugs 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 208000022218 streptococcal pneumonia Diseases 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000002889 sympathetic effect Effects 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 229940031351 tetravalent vaccine Drugs 0.000 description 1
- 238000011285 therapeutic regimen Methods 0.000 description 1
- 229960000103 thrombolytic agent Drugs 0.000 description 1
- 230000002537 thrombolytic effect Effects 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 238000012384 transportation and delivery Methods 0.000 description 1
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/55—Medicinal preparations containing antigens or antibodies characterised by the host/recipient, e.g. newborn with maternal antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Immunology (AREA)
- General Chemical & Material Sciences (AREA)
- Biophysics (AREA)
- Urology & Nephrology (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762568060P | 2017-10-04 | 2017-10-04 | |
| US62/568,060 | 2017-10-04 | ||
| US201862652615P | 2018-04-04 | 2018-04-04 | |
| US62/652,615 | 2018-04-04 | ||
| PCT/US2018/053976 WO2019070714A1 (en) | 2017-10-04 | 2018-10-02 | ASSAY AND ADMINISTRATION OF ANTI-C5 ANTIBODIES FOR THE TREATMENT OF PATIENTS WITH MEMBRANOPROLIFERATIVE GLOMERULONEPHRIPT |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200059279A true KR20200059279A (ko) | 2020-05-28 |
Family
ID=64051671
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207012177A Withdrawn KR20200059279A (ko) | 2017-10-04 | 2018-10-02 | 막증식성 사구체신염을 갖는 환자의 치료를 위한 항-c5 항체의 투여량 및 투여 |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US20200262897A1 (enExample) |
| EP (2) | EP3692064A1 (enExample) |
| JP (2) | JP2020536097A (enExample) |
| KR (1) | KR20200059279A (enExample) |
| CN (1) | CN111278857A (enExample) |
| AU (1) | AU2018345625A1 (enExample) |
| BR (1) | BR112020006692A2 (enExample) |
| CA (1) | CA3078362A1 (enExample) |
| CO (1) | CO2020004831A2 (enExample) |
| MX (1) | MX2020003619A (enExample) |
| WO (1) | WO2019070714A1 (enExample) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017035401A1 (en) | 2015-08-26 | 2017-03-02 | Achillion Pharmaceuticals, Inc. | Amide compounds for treatment of immune and inflammatory disorders |
| AR106018A1 (es) | 2015-08-26 | 2017-12-06 | Achillion Pharmaceuticals Inc | Compuestos de arilo, heteroarilo y heterocíclicos para el tratamiento de trastornos médicos |
| EP3340983B1 (en) | 2015-08-26 | 2023-10-04 | Achillion Pharmaceuticals, Inc. | Aryl, heteroaryl, and heterocyclic compounds for treatment of immune and inflammatory disorders |
| WO2017035405A1 (en) | 2015-08-26 | 2017-03-02 | Achillion Pharmaceuticals, Inc. | Amino compounds for treatment of immune and inflammatory disorders |
| EP3589287B1 (en) | 2017-03-01 | 2022-09-14 | Achillion Pharmaceuticals, Inc. | Macrocyclic compounds for treatment of medical disorders |
| WO2018160891A1 (en) | 2017-03-01 | 2018-09-07 | Achillion Pharmaceutical, Inc. | Pharmaceutical compounds for treatment of medical disorders |
| CN110603252A (zh) | 2017-03-01 | 2019-12-20 | 艾其林医药公司 | 用于治疗医学障碍的芳基、杂芳基和杂环药物化合物 |
| EP3661493A4 (en) | 2017-08-02 | 2021-04-14 | Achillion Pharmaceuticals, Inc. | TREATMENT DIET FOR THE TREATMENT OF PAROXYSTIC NOCTURAL HEMOGLOBINURIA |
| WO2019227102A1 (en) * | 2018-05-25 | 2019-11-28 | Achillion Pharmaceuticals, Inc. | Complement alternative pathway-associated nephropathy biomarkers |
| US11814391B2 (en) | 2018-09-06 | 2023-11-14 | Achillion Pharmaceuticals, Inc. | Macrocyclic compounds for the treatment of medical disorders |
| MX2021002640A (es) | 2018-09-06 | 2021-07-16 | Achillion Pharmaceuticals Inc | Formas morficas de los inhibidores del factor d del complemento. |
| EP3856164B1 (en) | 2018-09-25 | 2024-08-07 | Achillion Pharmaceuticals, Inc. | Morphic forms of complement factor d inhibitors |
| US12239645B2 (en) | 2018-12-17 | 2025-03-04 | Achillion Pharmaceuticals, Inc. | Targeted dosing for the treatment of complement mediated disorders |
| JP7635138B2 (ja) | 2019-03-22 | 2025-02-25 | アキリオン ファーマシューティカルズ, インコーポレーテッド | 補体媒介性障害の治療のための医薬化合物 |
| JP2022542430A (ja) * | 2019-08-01 | 2022-10-03 | ヤンセン バイオテツク,インコーポレーテツド | Fcrn抗体およびその使用の方法 |
| CN116712390B (zh) * | 2023-08-04 | 2023-11-14 | 上海览屹医药科技有限公司 | 一种高浓度高稳定性的抗体制剂及其制备方法 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6074642A (en) | 1994-05-02 | 2000-06-13 | Alexion Pharmaceuticals, Inc. | Use of antibodies specific to human complement component C5 for the treatment of glomerulonephritis |
| RS64039B1 (sr) * | 2008-11-10 | 2023-04-28 | Alexion Pharma Inc | Metode i kompozicije za lečenje poremećaja povezanih sa komplementom |
| NZ631007A (en) * | 2014-03-07 | 2015-10-30 | Alexion Pharma Inc | Anti-c5 antibodies having improved pharmacokinetics |
| US20180142010A1 (en) * | 2015-06-26 | 2018-05-24 | Alexion Pharmaceuticals, Inc. | Method for treating a patient in compliance with vaccination with eculizumab or an eculizumab variant |
| PE20240825A1 (es) * | 2016-06-17 | 2024-04-18 | Chugai Pharmaceutical Co Ltd | Anticuerpos anti-c5 y metodos de uso |
-
2018
- 2018-10-02 EP EP18796165.1A patent/EP3692064A1/en not_active Withdrawn
- 2018-10-02 JP JP2020519138A patent/JP2020536097A/ja active Pending
- 2018-10-02 CN CN201880065034.5A patent/CN111278857A/zh active Pending
- 2018-10-02 EP EP24174774.0A patent/EP4424324A3/en active Pending
- 2018-10-02 US US16/651,520 patent/US20200262897A1/en not_active Abandoned
- 2018-10-02 BR BR112020006692-3A patent/BR112020006692A2/pt not_active IP Right Cessation
- 2018-10-02 MX MX2020003619A patent/MX2020003619A/es unknown
- 2018-10-02 WO PCT/US2018/053976 patent/WO2019070714A1/en not_active Ceased
- 2018-10-02 CA CA3078362A patent/CA3078362A1/en not_active Abandoned
- 2018-10-02 AU AU2018345625A patent/AU2018345625A1/en not_active Abandoned
- 2018-10-02 KR KR1020207012177A patent/KR20200059279A/ko not_active Withdrawn
-
2020
- 2020-04-20 CO CONC2020/0004831A patent/CO2020004831A2/es unknown
-
2023
- 2023-02-28 US US18/115,226 patent/US20240025975A1/en active Pending
- 2023-05-31 JP JP2023089908A patent/JP2023101717A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| AU2018345625A1 (en) | 2020-04-16 |
| US20200262897A1 (en) | 2020-08-20 |
| WO2019070714A1 (en) | 2019-04-11 |
| JP2020536097A (ja) | 2020-12-10 |
| JP2023101717A (ja) | 2023-07-21 |
| EP3692064A1 (en) | 2020-08-12 |
| US20240025975A1 (en) | 2024-01-25 |
| CN111278857A (zh) | 2020-06-12 |
| EP4424324A2 (en) | 2024-09-04 |
| CO2020004831A2 (es) | 2020-05-29 |
| BR112020006692A2 (pt) | 2020-10-06 |
| CA3078362A1 (en) | 2019-04-11 |
| EP4424324A3 (en) | 2024-11-06 |
| MX2020003619A (es) | 2020-10-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240025975A1 (en) | Administration of anti-c5 antibodies for treatment of patients with membranoproliferative glomerulonephritis | |
| JP7577542B2 (ja) | 小児患者における非典型溶血性尿毒症症候群(aHUS)の治療のための抗C5抗体の用量および投与 | |
| JP2022141713A (ja) | 炎症性腸疾患の治療を目的とする三剤併用療法 | |
| US20250026858A1 (en) | Methods of treating chronic spontaneous urticaria using ligelizumab | |
| US20240092881A1 (en) | Methods of treating complement mediated thrombotic microangiopathy using an anti-c5 antibody | |
| US20230009657A1 (en) | Methods of treating lupus nephritis using interleukin-17 (il-17) antagonists | |
| CN111886249A (zh) | 用于鉴定和治疗适合长期抗-ccr5剂治疗的hiv-1感染患者亚群的筛选方法 | |
| CN116249550A (zh) | 用于治疗造血干细胞移植相关性血栓性微血管病(hsct-tma)的抗c5抗体的剂量和施用 | |
| US20230348579A1 (en) | Dosage and administration of anti-c5 antibodies for treating c5-mediated glomerular nephritis (gn), including lupus nephritis (ln) and/or iga nephropathy (igan) | |
| JP2022537134A (ja) | Cd38抗体を使用する併用療法 | |
| HK40110798A (en) | Methods of treating complement mediated thrombotic microangiopathy using an anti-c5 antibody | |
| WO2025166118A1 (en) | Dosage and administration of anti-c5 antibodies for treating iga nephropathy (igan) | |
| JP2023507852A (ja) | 抗c5抗体を使用して妊娠関連非典型溶血性尿毒症症候群を治療する方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20200427 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210924 Comment text: Request for Examination of Application |
|
| PC1202 | Submission of document of withdrawal before decision of registration |
Comment text: [Withdrawal of Procedure relating to Patent, etc.] Withdrawal (Abandonment) Patent event code: PC12021R01D Patent event date: 20220803 |
|
| WITB | Written withdrawal of application |