KR20150080555A - Plla로부터 제조된 풍선 팽창성 스텐트 - Google Patents
Plla로부터 제조된 풍선 팽창성 스텐트 Download PDFInfo
- Publication number
- KR20150080555A KR20150080555A KR1020157013706A KR20157013706A KR20150080555A KR 20150080555 A KR20150080555 A KR 20150080555A KR 1020157013706 A KR1020157013706 A KR 1020157013706A KR 20157013706 A KR20157013706 A KR 20157013706A KR 20150080555 A KR20150080555 A KR 20150080555A
- Authority
- KR
- South Korea
- Prior art keywords
- scaffold
- diameter
- stiffness
- radial
- days
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 229920000642 polymer Polymers 0.000 claims abstract description 64
- 210000005259 peripheral blood Anatomy 0.000 claims abstract description 11
- 239000011886 peripheral blood Substances 0.000 claims abstract description 11
- 238000000034 method Methods 0.000 claims description 76
- 238000002513 implantation Methods 0.000 claims description 67
- 239000000463 material Substances 0.000 claims description 56
- 238000012360 testing method Methods 0.000 claims description 51
- 238000002788 crimping Methods 0.000 claims description 37
- JVTAAEKCZFNVCJ-REOHCLBHSA-N L-lactic acid Chemical compound C[C@H](O)C(O)=O JVTAAEKCZFNVCJ-REOHCLBHSA-N 0.000 claims description 27
- 230000008859 change Effects 0.000 claims description 27
- 229920001432 poly(L-lactide) Polymers 0.000 claims description 24
- 238000004519 manufacturing process Methods 0.000 claims description 21
- 230000001965 increasing effect Effects 0.000 claims description 20
- 230000002792 vascular Effects 0.000 claims description 20
- 239000007943 implant Substances 0.000 claims description 19
- 230000007423 decrease Effects 0.000 claims description 18
- 238000011084 recovery Methods 0.000 claims description 17
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 claims description 12
- 238000000338 in vitro Methods 0.000 claims description 11
- 239000002243 precursor Substances 0.000 claims description 9
- 210000000078 claw Anatomy 0.000 claims description 7
- 239000000203 mixture Substances 0.000 claims description 6
- 239000013078 crystal Substances 0.000 claims description 3
- 230000000737 periodic effect Effects 0.000 claims description 3
- 238000002560 therapeutic procedure Methods 0.000 claims description 3
- 230000005540 biological transmission Effects 0.000 claims description 2
- 230000003247 decreasing effect Effects 0.000 claims description 2
- 238000013461 design Methods 0.000 description 58
- 210000004204 blood vessel Anatomy 0.000 description 36
- 230000002093 peripheral effect Effects 0.000 description 33
- 210000004027 cell Anatomy 0.000 description 30
- 230000006870 function Effects 0.000 description 19
- 238000002054 transplantation Methods 0.000 description 19
- 230000006835 compression Effects 0.000 description 17
- 238000007906 compression Methods 0.000 description 17
- 230000009467 reduction Effects 0.000 description 17
- 230000002829 reductive effect Effects 0.000 description 16
- 210000001519 tissue Anatomy 0.000 description 16
- 238000005452 bending Methods 0.000 description 13
- 238000005259 measurement Methods 0.000 description 13
- 239000002184 metal Substances 0.000 description 13
- 229910052751 metal Inorganic materials 0.000 description 13
- 230000008569 process Effects 0.000 description 13
- 230000001154 acute effect Effects 0.000 description 11
- 230000010261 cell growth Effects 0.000 description 11
- 230000000694 effects Effects 0.000 description 10
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N Lactic Acid Natural products CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 9
- 208000031481 Pathologic Constriction Diseases 0.000 description 9
- 238000002399 angioplasty Methods 0.000 description 9
- 239000000306 component Substances 0.000 description 9
- 238000001727 in vivo Methods 0.000 description 9
- 230000036262 stenosis Effects 0.000 description 9
- 208000037804 stenosis Diseases 0.000 description 9
- 230000006378 damage Effects 0.000 description 8
- 230000033001 locomotion Effects 0.000 description 8
- 238000012545 processing Methods 0.000 description 8
- 210000001105 femoral artery Anatomy 0.000 description 7
- 230000035876 healing Effects 0.000 description 7
- 210000001367 artery Anatomy 0.000 description 6
- 230000006399 behavior Effects 0.000 description 6
- 230000015556 catabolic process Effects 0.000 description 6
- 230000001413 cellular effect Effects 0.000 description 6
- 210000004351 coronary vessel Anatomy 0.000 description 6
- 230000003511 endothelial effect Effects 0.000 description 6
- 230000006698 induction Effects 0.000 description 6
- 230000003902 lesion Effects 0.000 description 6
- 230000004044 response Effects 0.000 description 6
- 208000037803 restenosis Diseases 0.000 description 6
- 230000021164 cell adhesion Effects 0.000 description 5
- 238000009661 fatigue test Methods 0.000 description 5
- 230000009477 glass transition Effects 0.000 description 5
- 235000014655 lactic acid Nutrition 0.000 description 5
- 239000004310 lactic acid Substances 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 238000010171 animal model Methods 0.000 description 4
- 239000006227 byproduct Substances 0.000 description 4
- 238000000354 decomposition reaction Methods 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 230000001954 sterilising effect Effects 0.000 description 4
- 238000004659 sterilization and disinfection Methods 0.000 description 4
- 238000012384 transportation and delivery Methods 0.000 description 4
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 229920013641 bioerodible polymer Polymers 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- 239000008358 core component Substances 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- 230000007547 defect Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 239000007769 metal material Substances 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 230000005855 radiation Effects 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- 230000003068 static effect Effects 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 230000008093 supporting effect Effects 0.000 description 3
- 230000036962 time dependent Effects 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 238000000692 Student's t-test Methods 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 230000001174 ascending effect Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 230000002301 combined effect Effects 0.000 description 2
- 230000003750 conditioning effect Effects 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000011038 discontinuous diafiltration by volume reduction Methods 0.000 description 2
- 238000006073 displacement reaction Methods 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 210000002889 endothelial cell Anatomy 0.000 description 2
- 238000001125 extrusion Methods 0.000 description 2
- 230000000004 hemodynamic effect Effects 0.000 description 2
- 230000036571 hydration Effects 0.000 description 2
- 238000006703 hydration reaction Methods 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 210000002414 leg Anatomy 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 2
- 229910001000 nickel titanium Inorganic materials 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 238000013146 percutaneous coronary intervention Methods 0.000 description 2
- 229920001434 poly(D-lactide) Polymers 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 238000012353 t test Methods 0.000 description 2
- 230000004580 weight loss Effects 0.000 description 2
- RBMHUYBJIYNRLY-UHFFFAOYSA-N 2-[(1-carboxy-1-hydroxyethyl)-hydroxyphosphoryl]-2-hydroxypropanoic acid Chemical compound OC(=O)C(O)(C)P(O)(=O)C(C)(O)C(O)=O RBMHUYBJIYNRLY-UHFFFAOYSA-N 0.000 description 1
- 208000000884 Airway Obstruction Diseases 0.000 description 1
- 229930182843 D-Lactic acid Natural products 0.000 description 1
- JVTAAEKCZFNVCJ-UWTATZPHSA-N D-lactic acid Chemical compound C[C@@H](O)C(O)=O JVTAAEKCZFNVCJ-UWTATZPHSA-N 0.000 description 1
- 101100346656 Drosophila melanogaster strat gene Proteins 0.000 description 1
- 201000005538 Exotropia Diseases 0.000 description 1
- 206010016717 Fistula Diseases 0.000 description 1
- 229920001963 Synthetic biodegradable polymer Polymers 0.000 description 1
- 102100029469 WD repeat and HMG-box DNA-binding protein 1 Human genes 0.000 description 1
- 101710097421 WD repeat and HMG-box DNA-binding protein 1 Proteins 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000000964 angiostatic effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 230000003143 atherosclerotic effect Effects 0.000 description 1
- 238000000418 atomic force spectrum Methods 0.000 description 1
- 210000000013 bile duct Anatomy 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 238000002144 chemical decomposition reaction Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000002826 coolant Substances 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 229940022769 d- lactic acid Drugs 0.000 description 1
- 238000013016 damping Methods 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000010102 embolization Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 230000003890 fistula Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000005755 formation reaction Methods 0.000 description 1
- 230000008571 general function Effects 0.000 description 1
- 210000003709 heart valve Anatomy 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 238000005286 illumination Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 238000003698 laser cutting Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- 230000001338 necrotic effect Effects 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 230000003534 oscillatory effect Effects 0.000 description 1
- 230000003094 perturbing effect Effects 0.000 description 1
- 238000013310 pig model Methods 0.000 description 1
- 238000013166 platelet test Methods 0.000 description 1
- 238000013001 point bending Methods 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 1
- 229920002959 polymer blend Polymers 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000000250 revascularization Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 230000004218 vascular function Effects 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 238000002629 virtual reality therapy Methods 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
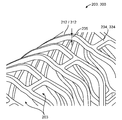
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/958—Inflatable balloons for placing stents or stent-grafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/04—Macromolecular materials
- A61L31/06—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C55/00—Shaping by stretching, e.g. drawing through a die; Apparatus therefor
- B29C55/22—Shaping by stretching, e.g. drawing through a die; Apparatus therefor of tubes
- B29C55/26—Shaping by stretching, e.g. drawing through a die; Apparatus therefor of tubes biaxial
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C69/00—Combinations of shaping techniques not provided for in a single one of main groups B29C39/00 - B29C67/00, e.g. associations of moulding and joining techniques; Apparatus therefore
- B29C69/001—Combinations of shaping techniques not provided for in a single one of main groups B29C39/00 - B29C67/00, e.g. associations of moulding and joining techniques; Apparatus therefore a shaping technique combined with cutting, e.g. in parts or slices combined with rearranging and joining the cut parts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
- A61F2002/91575—Adjacent bands being connected to each other connected peak to trough
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0004—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof bioabsorbable
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0028—Shapes in the form of latin or greek characters
- A61F2230/0054—V-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2240/00—Manufacturing or designing of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2240/00—Manufacturing or designing of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2240/001—Designing or manufacturing processes
- A61F2240/008—Means for testing implantable prostheses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/0071—Additional features; Implant or prostheses properties not otherwise provided for breakable or frangible
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C55/00—Shaping by stretching, e.g. drawing through a die; Apparatus therefor
- B29C55/22—Shaping by stretching, e.g. drawing through a die; Apparatus therefor of tubes
- B29C55/24—Shaping by stretching, e.g. drawing through a die; Apparatus therefor of tubes radial
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2031/00—Other particular articles
- B29L2031/753—Medical equipment; Accessories therefor
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/49—Method of mechanical manufacture
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/49—Method of mechanical manufacture
- Y10T29/49826—Assembling or joining
- Y10T29/49908—Joining by deforming
- Y10T29/49925—Inward deformation of aperture or hollow body wall
- Y10T29/49927—Hollow body is axially joined cup or tube
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Cardiology (AREA)
- Mechanical Engineering (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Surgery (AREA)
- Epidemiology (AREA)
- Prostheses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Materials For Medical Uses (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261717613P | 2012-10-23 | 2012-10-23 | |
| US61/717,613 | 2012-10-23 | ||
| US13/842,547 US9956097B2 (en) | 2012-10-23 | 2013-03-15 | Methods for vascular restoration therapy |
| US13/842,432 US9517150B2 (en) | 2012-10-23 | 2013-03-15 | Time-dependent polymer scaffolds |
| US13/842,547 | 2013-03-15 | ||
| US13/842,432 | 2013-03-15 | ||
| PCT/US2013/036434 WO2014065885A1 (en) | 2012-10-23 | 2013-04-12 | A balloon expandable stent made from plla |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150080555A true KR20150080555A (ko) | 2015-07-09 |
Family
ID=50486030
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157013706A Withdrawn KR20150080555A (ko) | 2012-10-23 | 2013-04-12 | Plla로부터 제조된 풍선 팽창성 스텐트 |
Country Status (7)
| Country | Link |
|---|---|
| US (3) | US9517150B2 (enExample) |
| EP (1) | EP2911619B1 (enExample) |
| JP (1) | JP6258340B2 (enExample) |
| KR (1) | KR20150080555A (enExample) |
| CN (1) | CN104736108A (enExample) |
| HK (1) | HK1212195A1 (enExample) |
| WO (1) | WO2014065885A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102783996B1 (ko) * | 2023-09-27 | 2025-03-19 | 주식회사 도터 | 생분해성 고분자 스텐트 및 이를 포함하는 카테터 시스템 |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8814930B2 (en) | 2007-01-19 | 2014-08-26 | Elixir Medical Corporation | Biodegradable endoprosthesis and methods for their fabrication |
| US9517150B2 (en) | 2012-10-23 | 2016-12-13 | Abbott Cardiovascular Systems Inc. | Time-dependent polymer scaffolds |
| US10335300B2 (en) * | 2013-01-18 | 2019-07-02 | Memory Effect Medical, LLC | System for deploying a capacitive shape memory catheterization device and methods for use therewith |
| US9700244B2 (en) * | 2013-01-18 | 2017-07-11 | Memory Effect Medical, LLC | Wireless degradation data generator for use with a therapeutic scaffold and methods for use therewith |
| US9662231B2 (en) * | 2013-03-15 | 2017-05-30 | Abbott Cardiovascular Systems Inc. | Polymer scaffolds having enhanced axial fatigue properties |
| US10098771B2 (en) | 2013-09-25 | 2018-10-16 | Abbott Cardiovascular Systems Inc. | Clip sheath for a polymer scaffold |
| US10022906B2 (en) | 2014-07-30 | 2018-07-17 | Abbott Cardiovascular Systems Inc. | Methods for solid phase processing of tubes and medical devices made from the processed tubes |
| US9855156B2 (en) * | 2014-08-15 | 2018-01-02 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9730819B2 (en) | 2014-08-15 | 2017-08-15 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9931787B2 (en) | 2014-09-18 | 2018-04-03 | Abbott Cardiovascular Systems Inc. | Crimping polymer scaffolds |
| WO2016044788A2 (en) | 2014-09-18 | 2016-03-24 | Abbott Cardiovascular Systems Inc. | Thermal processing of polymer scaffolds |
| US10010653B2 (en) | 2016-02-05 | 2018-07-03 | Abbott Cardiovascular Systems Inc. | Methods for increasing coating strength to improve scaffold crimping yield |
| US11622872B2 (en) | 2016-05-16 | 2023-04-11 | Elixir Medical Corporation | Uncaging stent |
| CN109561955B (zh) | 2016-05-16 | 2021-04-16 | 万能医药公司 | 撑开支架 |
| US11628077B2 (en) * | 2016-10-31 | 2023-04-18 | Razmodics Llc | Post deployment radial force recovery of biodegradable scaffolds |
| US10709589B2 (en) * | 2017-05-09 | 2020-07-14 | Abbott Cardiovascular Systems Inc. | Accelerated aging for polymeric scaffolds |
| PL3700475T3 (pl) * | 2018-03-29 | 2022-01-03 | Sahajanand Medical Technologies Limited | Stent |
| JP1651803S (ja) * | 2019-04-15 | 2020-02-03 | 注射器用キャップ | |
| US11517457B2 (en) * | 2019-07-03 | 2022-12-06 | Abbott Cardiovascular Systems Inc. | Intravascular stent |
Family Cites Families (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2380683C (en) | 1991-10-28 | 2006-08-08 | Advanced Cardiovascular Systems, Inc. | Expandable stents and method for making same |
| US5951540A (en) * | 1998-10-22 | 1999-09-14 | Medtronic, Inc. | Device and method for mounting stents |
| US20030050692A1 (en) | 2000-12-22 | 2003-03-13 | Avantec Vascular Corporation | Delivery of therapeutic capable agents |
| AU2003228890A1 (en) * | 2002-05-08 | 2003-11-11 | Abbott Laboratories | Endoprosthesis having foot extensions |
| US7122049B2 (en) * | 2003-03-19 | 2006-10-17 | Advanced Bio Prosthetic Surfaces, Ltd. | Endoluminal stent having mid-strut interconnecting members |
| US20060020330A1 (en) * | 2004-07-26 | 2006-01-26 | Bin Huang | Method of fabricating an implantable medical device with biaxially oriented polymers |
| US20110066222A1 (en) | 2009-09-11 | 2011-03-17 | Yunbing Wang | Polymeric Stent and Method of Making Same |
| US7875233B2 (en) * | 2004-09-30 | 2011-01-25 | Advanced Cardiovascular Systems, Inc. | Method of fabricating a biaxially oriented implantable medical device |
| US7404823B2 (en) * | 2005-10-31 | 2008-07-29 | Boston Scientific Scimed, Inc. | Stent configurations |
| US20100010622A1 (en) | 2006-03-13 | 2010-01-14 | Abbott Laboratories | Hybrid segmented endoprosthesis |
| US8882826B2 (en) * | 2006-08-22 | 2014-11-11 | Abbott Cardiovascular Systems Inc. | Intravascular stent |
| JP4871692B2 (ja) * | 2006-09-29 | 2012-02-08 | テルモ株式会社 | 生体内留置用ステントおよび生体器官拡張器具 |
| US8512392B2 (en) * | 2007-03-09 | 2013-08-20 | Boston Scientific Scimed, Inc. | Stent design with struts of various angles and stiffness |
| US8002817B2 (en) * | 2007-05-04 | 2011-08-23 | Abbott Cardiovascular Systems Inc. | Stents with high radial strength and methods of manufacturing same |
| US8388673B2 (en) | 2008-05-02 | 2013-03-05 | Abbott Cardiovascular Systems Inc. | Polymeric stent |
| DE102007034041A1 (de) | 2007-07-20 | 2009-01-22 | Biotronik Vi Patent Ag | Medikamentendepots für medizinische Implantate |
| US20100217377A1 (en) * | 2007-10-10 | 2010-08-26 | Miv Therapeutics, Inc. | Calcium phosphate coated stents comprising cobalt chromium alloy |
| US8206635B2 (en) * | 2008-06-20 | 2012-06-26 | Amaranth Medical Pte. | Stent fabrication via tubular casting processes |
| US9572692B2 (en) | 2009-02-02 | 2017-02-21 | Abbott Cardiovascular Systems Inc. | Bioabsorbable stent that modulates plaque geometric morphology and chemical composition |
| RU2566225C2 (ru) | 2009-02-09 | 2015-10-20 | ЭВИЗИО МЕДИКАЛ ДЕВАЙСИЗ ЮЭлСи | Стент |
| EP3241530B1 (en) * | 2009-04-10 | 2024-01-17 | Covidien LP | Implants having high fatigue resistance and implant delivery systems |
| US20110066223A1 (en) | 2009-09-14 | 2011-03-17 | Hossainy Syed F A | Bioabsorbable Stent With Time Dependent Structure And Properties |
| US8425587B2 (en) | 2009-09-17 | 2013-04-23 | Abbott Cardiovascular Systems Inc. | Method of treatment with a bioabsorbable stent with time dependent structure and properties and regio-selective degradation |
| US8808353B2 (en) * | 2010-01-30 | 2014-08-19 | Abbott Cardiovascular Systems Inc. | Crush recoverable polymer scaffolds having a low crossing profile |
| US8568471B2 (en) | 2010-01-30 | 2013-10-29 | Abbott Cardiovascular Systems Inc. | Crush recoverable polymer scaffolds |
| US20120029618A1 (en) | 2010-07-28 | 2012-02-02 | Boston Scientific Scimed, Inc. | Stent Connector Bump Design |
| US9345602B2 (en) | 2010-09-23 | 2016-05-24 | Abbott Cardiovascular Systems Inc. | Processes for making crush recoverable polymer scaffolds |
| US8414528B2 (en) | 2011-05-27 | 2013-04-09 | Abbott Cardiovascular Systems Inc. | Polymer scaffold sheaths |
| US8726483B2 (en) | 2011-07-29 | 2014-05-20 | Abbott Cardiovascular Systems Inc. | Methods for uniform crimping and deployment of a polymer scaffold |
| US20130085564A1 (en) | 2011-10-03 | 2013-04-04 | Abbott Cardiovascular Systems Inc. | Modified scaffolds for peripheral applications |
| US20130123905A1 (en) * | 2011-11-15 | 2013-05-16 | Abbott Cardiovascular Systems Inc. | Offset peak-to-peak stent pattern |
| US20130338762A1 (en) | 2012-06-15 | 2013-12-19 | Abbott Cardiovascular Systems Inc. | Bioresorbable polymer peripheral scaffolds made from block copolymers of poly(l-lactide) and hydrophilic polymers |
| US9498359B2 (en) | 2012-07-13 | 2016-11-22 | Abbott Cardiovascular Systems Inc. | Polymer scaffolds for peripheral vessels |
| US9724219B2 (en) | 2012-10-04 | 2017-08-08 | Abbott Cardiovascular Systems Inc. | Method of uniform crimping and expansion of medical devices |
| US9517150B2 (en) | 2012-10-23 | 2016-12-13 | Abbott Cardiovascular Systems Inc. | Time-dependent polymer scaffolds |
| US9662231B2 (en) | 2013-03-15 | 2017-05-30 | Abbott Cardiovascular Systems Inc. | Polymer scaffolds having enhanced axial fatigue properties |
-
2013
- 2013-03-15 US US13/842,432 patent/US9517150B2/en active Active
- 2013-03-15 US US13/842,547 patent/US9956097B2/en active Active
- 2013-04-12 KR KR1020157013706A patent/KR20150080555A/ko not_active Withdrawn
- 2013-04-12 JP JP2015539578A patent/JP6258340B2/ja active Active
- 2013-04-12 HK HK16100214.2A patent/HK1212195A1/xx unknown
- 2013-04-12 EP EP13721148.8A patent/EP2911619B1/en not_active Not-in-force
- 2013-04-12 CN CN201380055308.XA patent/CN104736108A/zh active Pending
- 2013-04-12 WO PCT/US2013/036434 patent/WO2014065885A1/en not_active Ceased
-
2017
- 2017-10-16 US US15/785,368 patent/US11135074B2/en active Active
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102783996B1 (ko) * | 2023-09-27 | 2025-03-19 | 주식회사 도터 | 생분해성 고분자 스텐트 및 이를 포함하는 카테터 시스템 |
Also Published As
| Publication number | Publication date |
|---|---|
| HK1212195A1 (en) | 2016-06-10 |
| US9517150B2 (en) | 2016-12-13 |
| US20140114398A1 (en) | 2014-04-24 |
| JP2015533578A (ja) | 2015-11-26 |
| CN104736108A (zh) | 2015-06-24 |
| US20180036154A1 (en) | 2018-02-08 |
| US9956097B2 (en) | 2018-05-01 |
| US11135074B2 (en) | 2021-10-05 |
| WO2014065885A1 (en) | 2014-05-01 |
| EP2911619A1 (en) | 2015-09-02 |
| US20140114399A1 (en) | 2014-04-24 |
| EP2911619B1 (en) | 2018-10-31 |
| JP6258340B2 (ja) | 2018-01-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11135074B2 (en) | Methods for vascular restoration therapy | |
| CN105188618B (zh) | 具有增强的轴向疲劳特性的聚合物支架 | |
| JP5175277B2 (ja) | 遅発型炎症反応の確率を低減する植込型医療装置の製作方法 | |
| US9498359B2 (en) | Polymer scaffolds for peripheral vessels | |
| EP2763630B1 (en) | Modified scaffolds for peripheral applications | |
| JP6317346B2 (ja) | 形状記憶生体再吸収性ポリマーの末梢スキャフォールド | |
| US9907685B2 (en) | Crush recoverable polymer scaffolds | |
| US20110190872A1 (en) | Crush Recoverable Polymer Scaffolds Having a Low Crossing Profile | |
| US20130245746A1 (en) | Bioabsorbable stent with time dependent structure and properties and regio-selective degradation | |
| US20070253996A1 (en) | Method of fabricating an implantable medical device by controlling crystalline structure | |
| RS55103B1 (sr) | Polimerni stentovi koji se mogu obnoviti posle gnječenja | |
| KR20150141956A (ko) | 말초-이식되는 스캐폴드에서 리코일의 감소 | |
| US20110066223A1 (en) | Bioabsorbable Stent With Time Dependent Structure And Properties | |
| HK1195239B (en) | Modified scaffolds for peripheral applications |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150522 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |