KR20130114961A - Method for preparing azodicarbonamide - Google Patents
Method for preparing azodicarbonamide Download PDFInfo
- Publication number
- KR20130114961A KR20130114961A KR1020120037432A KR20120037432A KR20130114961A KR 20130114961 A KR20130114961 A KR 20130114961A KR 1020120037432 A KR1020120037432 A KR 1020120037432A KR 20120037432 A KR20120037432 A KR 20120037432A KR 20130114961 A KR20130114961 A KR 20130114961A
- Authority
- KR
- South Korea
- Prior art keywords
- reaction
- azodicarbonamide
- ozone
- hydrazodicarbonamide
- acid
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C281/00—Derivatives of carbonic acid containing functional groups covered by groups C07C269/00 - C07C279/00 in which at least one nitrogen atom of these functional groups is further bound to another nitrogen atom not being part of a nitro or nitroso group
- C07C281/20—Derivatives of carbonic acid containing functional groups covered by groups C07C269/00 - C07C279/00 in which at least one nitrogen atom of these functional groups is further bound to another nitrogen atom not being part of a nitro or nitroso group the two nitrogen atoms of the functional groups being doubly-bound to each other, e.g. azoformamide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C245/00—Compounds containing chains of at least two nitrogen atoms with at least one nitrogen-to-nitrogen multiple bond
- C07C245/02—Azo compounds, i.e. compounds having the free valencies of —N=N— groups attached to different atoms, e.g. diazohydroxides
- C07C245/04—Azo compounds, i.e. compounds having the free valencies of —N=N— groups attached to different atoms, e.g. diazohydroxides with nitrogen atoms of azo groups bound to acyclic carbon atoms or to carbon atoms of rings other than six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J9/00—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof
- C08J9/04—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof using blowing gases generated by a previously added blowing agent
- C08J9/06—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof using blowing gases generated by a previously added blowing agent by a chemical blowing agent
- C08J9/10—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof using blowing gases generated by a previously added blowing agent by a chemical blowing agent developing nitrogen, the blowing agent being a compound containing a nitrogen-to-nitrogen bond
- C08J9/102—Azo-compounds
- C08J9/103—Azodicarbonamide
Abstract
Description
본 발명은 아조디카본아미드의 제조방법에 관한 것으로서, 더욱 상세하게는, 히드라조디카본아미드의 산화반응을 통한 아조디카본아미드 제조 시, 공정이 단순하고, 유해한 물질이 생성되지 않아 친환경적인 아조디카본아미드의 제조방법에 관한 것이다.
The present invention relates to a method for producing azodicarbonamide, and more particularly, in the preparation of azodicarbonamide through the oxidation reaction of hydrazodicarbonamide, the process is simple and does not produce harmful substances, which is environmentally friendly azodica It relates to a method for producing the present amide.
발포제는 폴리머와 배합되어 다공성의 발포체를 제조하기 위한 첨가제로서, 아조디카본아미드(azodicarbonamide: ADCA)는 가열에 의한 질소 가스의 발생이 빠르게 진행되고, 분해 생성물이 불연성이며, 독성이 없는 특성으로 인하여 발포제로서 널리 사용되고 있다. 상기 아조디카본아미드는 통상적으로 히드라조디카본아미드(hydrazodicarbonamide: HDCA)의 산화반응을 통하여 제조되며, 예를 들면, 산화제로서, 염소 또는 과산화수소를 사용한 산화반응을 통하여 제조되고 있다.
The blowing agent is an additive for blending with the polymer to produce a porous foam. The azodicarbonamide (ADCA) has a rapid progress of generation of nitrogen gas by heating, and the decomposition product is nonflammable and has no toxicity. It is widely used as a blowing agent. The azodicarbonamide is typically prepared through the oxidation of hydrazodicarbonamide (HDCA), for example, through the oxidation reaction using chlorine or hydrogen peroxide as the oxidizing agent.
염소를 산화제로 사용하여 아조디카본아미드를 제조하는 방법은 히드라조디카본아미드와 물로 구성된 반응계에 염소를 연속적으로 투입하는 공정으로 구성되어 있으나, 반응 중 염산(HCl)이 생성되어 통상 1 내지 2의 낮은 pH의 폐수가 다량 발생하는 문제점이 있다.The method for preparing azodicarbonamide using chlorine as an oxidizing agent consists of continuously adding chlorine to a reaction system composed of hydrazodicarbonamide and water. There is a problem that a large amount of low pH wastewater occurs.
또한, 산화제로서 과산화수소를 사용하는 아조디카본아미드의 제조방법으로는 브롬산 또는 브롬산염 촉매 하에서 반응시키는 방법과 요오드(iodine) 촉매 하에서 반응하는 방법 등을 예시할 수 있다. 그러나, 상기 브롬산 또는 브롬산염 촉매를 사용하는 방법은 과량의 산(황산 등)을 사용함으로써 낮은 pH의 폐수가 다량 발생하는 문제점이 있고, 요오드 촉매를 사용하는 방법은 제조 시, pH 1 내지 5(바람직하게는 2 내지 4)를 유지하기 위해 황산과 같은 산을 지속적으로 투입하여야 하며, 반응온도를 60 내지 90℃(바람직하게는 75 내지 85℃)로 유지하여야 하므로 다량의 에너지 비용이 발생하는 문제점이 있다.
Moreover, as a manufacturing method of the azodicarbonamide which uses hydrogen peroxide as an oxidizing agent, the method of making it react on a bromic acid or bromate catalyst, the method of making it react on an iodine catalyst, etc. are mentioned. However, the method of using the bromic acid or bromate catalyst has a problem that a large amount of waste water of low pH occurs by using an excess of acid (sulfuric acid, etc.), the method of using the iodine catalyst is pH 1 to 5 ( Preferably, an acid such as sulfuric acid should be continuously added in order to maintain 2 to 4), and a large amount of energy cost is generated because the reaction temperature should be maintained at 60 to 90 ° C (preferably 75 to 85 ° C). There is this.
따라서, 본 발명의 목적은, 히드라조디카본아미드의 산화반응을 통한 아조디카본아미드 제조 시, 낮은 pH(예를 들면, pH 1 내지 5)의 폐수 등이 발생하지 않고 높은 반응온도(예를 들면, 60℃ 이상)를 요구하지 않는, 공정이 단순하고 친환경적인 아조디카본아미드의 제조방법을 제공하는 것이다.
Accordingly, it is an object of the present invention to produce azodicarbonamide through the oxidation of hydrazodicarbonamide, in which a low pH (eg, pH 1 to 5) wastewater does not occur and a high reaction temperature (eg, It is to provide a process for producing azodicarbonamide which is simple and environmentally friendly, which does not require more than 60 ° C).
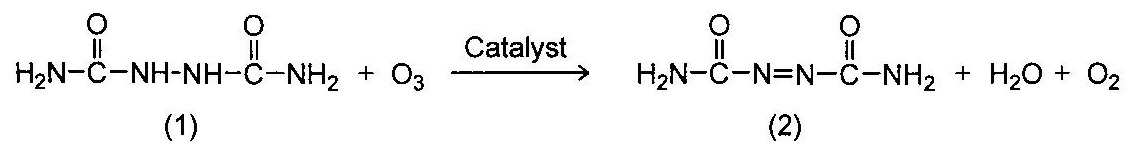
상기 목적을 달성하기 위하여, 본 발명은 하기 반응식 1과 같이, 히드라조디카본아미드(1)를, 불소, 염소, 브롬 또는 요오드를 포함하는 물질로서, 반응 중 불소 이온, 염소 이온, 브롬 이온 또는 요오드 이온을 발생시키는 촉매의 존재 하에, 오존(O3)과 반응시켜서 아조디카본아미드(2)를 제조하는 것을 특징으로 하는, 아조디카본아미드의 제조방법을 제공한다.In order to achieve the above object, the present invention is a material containing hydrazodicarbonamide (1), fluorine, chlorine, bromine or iodine, as shown in Scheme 1 below, during the reaction fluorine ion, chlorine ion, bromine ion or iodine Azodicarbonamide (2) is produced by reacting with ozone (O 3 ) in the presence of a catalyst for generating ions, thereby producing azodicarbonamide.
[반응식 1][Reaction Scheme 1]
본 발명에 따른 아조디카본아미드(azodicarbonamide: ADCA)의 제조방법은, 히드라조디카본아미드의 산화반응을 통한 아조디카본아마이드 제조 시, 산화제로서 오존을 사용하는 것으로서, 염소 또는 과산화수소를 산화제로 사용한 반응 시 발생되는 낮은 pH(예를 들면, pH 1 내지 5)의 폐수 등이 발생하지 않고 높은 반응온도(예를 들면, 60℃ 이상)를 요구하지 않으므로, 친환경적이고, 폐수 제거 등의 공정이 필요하지 않아 공정이 단순하다.
The method for preparing azodicarbonamide (ADCA) according to the present invention uses ozone as an oxidizing agent when preparing azodicarbonamide through oxidation of hydrazodicarbonamide, and uses chlorine or hydrogen peroxide as an oxidizing agent. Waste water of low pH (e.g., pH 1-5) does not occur and does not require a high reaction temperature (e.g., 60 ° C or higher), so it is environmentally friendly and does not require processes such as wastewater removal. The process is simple.
이하, 본 발명을 상세히 설명하면 다음과 같다.Hereinafter, the present invention will be described in detail.
본 발명에 따른 아조디카본아미드(azodicarbonamide: ADCA)의 제조방법은 산화제로서 오존(O3)을 사용하여 히드라조디카본아미드(hydrazodicarbonamide: HDCA)를 산화시키는 방법으로서, 하기 반응식 1과 같이, 히드라조디카본아미드(1)를, 불소, 염소, 브롬 또는 요오드를 포함하는 물질로서, 반응 중 불소 이온, 염소 이온, 브롬 이온 또는 요오드 이온을 발생시키는 촉매의 존재 하에, 오존(O3)과 반응시켜서 아조디카본아미드(2)를 제조하는 것을 특징으로 한다.A method for preparing azodicarbonamide (ADCA) according to the present invention is a method of oxidizing hydrazodicarbonamide (HDCA) using ozone (O 3 ) as an oxidizing agent, as shown in Scheme 1 below. The carbonamide (1) is a substance containing fluorine, chlorine, bromine or iodine, and reacted with ozone (O 3 ) in the presence of a catalyst which generates fluorine ions, chlorine ions, bromine ions or iodine ions during the reaction. It is characterized by producing dicarbonamide (2).
[반응식 1][Reaction Scheme 1]
본 발명에 사용되는 히드라조디카본아미드는, 세계적으로 가장 널리 사용되는 발포제 중 하나인 아조디카본아미드의 원료물질로서, 통상적인 히드라조디카본아미드를 사용할 수 있으며, 예를 들면, 상용화된 제품을 사용하거나, 하이드라진 하이드레이트(hydrazine hydrate)법, 요소(urea)법, 세미카바자이드(semicarbazide)법, 뷰렛(biuret)법 등을 통해 얻을 수 있다.
Hydrazodicarbonamide used in the present invention is a raw material of azodicarbonamide, which is one of the most widely used blowing agents in the world, and may use a conventional hydrazodicarbonamide, for example, a commercially available product. Or, it can be obtained through the hydrazine hydrate (hydrazine hydrate) method, urea method, semicarbazide method, biuret method and the like.
본 발명에 사용되는 오존(O3)은 산화제로서, 산소 또는 공기를 오존 발생기에 투입하여 얻을 수 있으며, 단위 투입 산소량(또는 공기량) 대비 높은 농도의 오존을 생성하는 오존 발생기를 사용할 경우 공정 시간이 단축되는 장점이 있다. 상기 오존의 사용량은 상기 히드라조디카본아미드 100중량부에 대하여, 10 내지 100중량부, 바람직하게는 30 내지 50중량부이다. 상기 오존의 사용량이 상기 히드라조디카본아미드 100중량부에 대하여, 10중량부 미만이면, 반응 수율이 낮아질 우려가 있고, 100중량부를 초과하면, 오존이 반응에 사용되지 않고 소모될 우려가 있다.
Ozone (O 3 ) used in the present invention can be obtained by adding oxygen or air to an ozone generator as an oxidant, and when using an ozone generator that generates ozone at a high concentration relative to the unit amount of oxygen (or air), the process time is long. It has the advantage of being shortened. The use amount of the ozone is 10 to 100 parts by weight, preferably 30 to 50 parts by weight with respect to 100 parts by weight of the hydrazodicarbonamide. When the amount of the ozone used is less than 10 parts by weight with respect to 100 parts by weight of the hydrazodicarbonamide, the reaction yield may be lowered. When the amount of the ozone used exceeds 100 parts by weight, ozone may be consumed without being used for the reaction.
본 발명에 사용되는 촉매는 불소, 염소, 브롬 또는 요오드를 포함하는 물질로서, 반응 중 불소 이온, 염소 이온, 브롬 이온 또는 요오드 이온을 발생시킬 수 있는 불소계, 염소계, 브롬계 또는 요오드계 촉매로서, 예를 들면, 불산(HF), 불산염(LiF, NaF, KF 등), 염산(HCl), 염산염(LiCl, NaCl, KCl 등), 브롬산(HBr), 브롬산염(LiBr, NaBr, KBr 등), 요오드산(HI), 요오드염(LiI, NaI, KI 등), 반응 조건에서 불소 이온, 염소 이온, 브롬 이온 또는 요오드 이온을 생성하는 화합물 등을 사용할 수 있다. 상기 촉매의 사용량은 상기 히드라조디카본아미드 100중량부에 대하여, 0.01 내지 10중량부, 바람직하게는 0.2 내지 5.0중량부이다. 상기 촉매의 사용량이 상기 히드라조디카본아미드 100중량부에 대하여, 0.01중량부 미만이면, 반응시간이 길어질 우려가 있고, 10중량부를 초과하면, 경제성이 떨어질 우려가 있다.
The catalyst used in the present invention is a material containing fluorine, chlorine, bromine or iodine, and is a fluorine, chlorine, bromine or iodine catalyst capable of generating fluorine ions, chlorine ions, bromine ions or iodine ions during the reaction. For example, hydrofluoric acid (HF), hydrochloride (LiF, NaF, KF, etc.), hydrochloric acid (HCl), hydrochloride (LiCl, NaCl, KCl, etc.), bromic acid (HBr), bromate (LiBr, NaBr, KBr, etc.) , Iodic acid (HI), iodine salts (LiI, NaI, KI, etc.), compounds that generate fluorine ions, chlorine ions, bromine ions or iodine ions under the reaction conditions. The amount of the catalyst used is 0.01 to 10 parts by weight, preferably 0.2 to 5.0 parts by weight based on 100 parts by weight of the hydrazodicarbonamide. When the amount of the catalyst used is less than 0.01 part by weight with respect to 100 parts by weight of the hydrazodicarbonamide, the reaction time may be long, and when it is more than 10 parts by weight, the economy may be deteriorated.
본 발명에 따른 아조디카본아미드의 제조방법은, 히드라조디카본아미드, 물, 촉매로 구성된 반응계에 오존 가스를 투입하여 반응을 진행하는 슬러리 수용액 반응이거나, 습윤 케이크(wet cake) 상 반응이다. 즉, 본 발명의 아조디카본아미드 제조방법에서, 히드라조디카본아미드와 물의 비율은 반응에 큰 영향을 주지 않으며, 사용되는 촉매의 종류에 따라 사용량이 변화할 수 있다. 상기 물의 사용량은 크게 한정되지는 않으나, 예를 들면, 상기 히드라조디카본아미드 100중량부에 대하여, 1 내지 500중량부, 바람직하게는 10 내지 300중량부를 사용할 수 있으며, 촉매의 종류에 따라 적절한 양의 물을 사용하는 것이 바람직하다.
The method for producing azodicarbonamide according to the present invention is a slurry aqueous solution reaction in which ozone gas is introduced into a reaction system composed of hydrazodicarbonamide, water, and a catalyst, or a wet cake phase reaction. That is, in the azodicarbonamide production method of the present invention, the ratio of hydrazodicarbonamide and water does not significantly affect the reaction, and the amount of use may vary depending on the type of catalyst used. The amount of the water used is not particularly limited, but, for example, 1 to 500 parts by weight, preferably 10 to 300 parts by weight, based on 100 parts by weight of the hydrazodicarbonamide, may be used in an appropriate amount depending on the type of catalyst. It is preferable to use water.
본 발명에 따른 아조디카본아미드의 제조방법에 있어서, 상기 반응(반응식 1)의 반응온도는 예를 들면, 5 내지 90℃, 바람직하게는 10 내지 40℃이다. 상기 반응온도가 5℃ 미만이며, 반응 시간이 길어질 우려가 있고, 90℃를 초과하면, 반응속도는 빨라지나, 아조디카본아미드의 분해 반응이 일어날 우려가 있다.
In the method for producing azodicarbonamide according to the present invention, the reaction temperature of the reaction (Scheme 1) is, for example, 5 to 90 ° C, preferably 10 to 40 ° C. When the said reaction temperature is less than 5 degreeC, reaction time may become long, and when it exceeds 90 degreeC, reaction speed will become high but there exists a possibility that the decomposition reaction of azodicarbonamide may occur.
또한, 상기 반응은 pH 1 내지 13의 범위의 반응계에서 수행될 수 있으나, 반응 후 생성되는 폐수의 pH 조절 공정을 생략하기 위해서는 pH 6 내지 8의 범위의 반응계에서 반응시키는 것이 바람직하다. 히드라조디카본아미드와 오존 반응의 경우 반응계 내의 pH 변화가 없으므로, 반응 도중 또는 반응 종료 후의 pH에 변화가 없으며, 반응 종료 후 pH 조정을 위한 추가적인 조치가 필요하지 않다.
In addition, the reaction may be carried out in the reaction system in the range of pH 1 to 13, but in order to omit the pH control process of the wastewater generated after the reaction, it is preferable to react in the reaction system in the range of pH 6 to 8. In the case of hydrazodicarbonamide and ozone reaction, there is no change in pH in the reaction system, so there is no change in pH during or after the reaction, and no further measures for pH adjustment after the reaction are completed.
또한, 본 발명에 따른 아조디카본아미드의 제조방법은 산화제로서 오존을 투입하는 것과 함께, 필요에 따라, 염소 투입, 과산화수소 투입, UV 조사 등의 산화방법을 하나 이상 병용할 수도 있다.
In addition, in the method for producing azodicarbonamide according to the present invention, in addition to adding ozone as an oxidizing agent, one or more oxidation methods such as chlorine addition, hydrogen peroxide addition and UV irradiation may be used in combination.
이하, 구체적인 실시예를 통하여 본 발명을 더욱 상세히 설명한다. 하기 실시예는 본 발명을 예시하기 위한 것으로서, 본 발명이 하기 실시예에 의해 한정되는 것은 아니다.
Hereinafter, the present invention will be described in more detail with reference to specific examples. The following examples illustrate the present invention and are not intended to limit the scope of the present invention.
[실시예 1] 아조디카본아마이드의 제조 Example 1 Preparation of Azodicarbonamide
히드라조디카본아미드 100g, 브롬화 나트륨(NaBr) 2.0g, 물 20g을 잘 혼합하여 유리 반응기에 넣고, 온도를 30℃로 유지하며, 오존(오존 6중량%, 산소 94중량%로 구성)을 3 리터(L)/분의 유량으로 8시간 동안 투입하여 히드라조디카본아미드와 오존을 반응시켰다. 반응 종료 후, 탈수 및 건조 과정을 거쳐 아조디카본아미드를 얻었으며, 이때의 반응 수율은 98.2%였다.
100 g of hydrazodicarbonamide, 2.0 g of sodium bromide (NaBr) and 20 g of water are mixed well, placed in a glass reactor, the temperature is maintained at 30 ° C., and 3 liters of ozone (consisting of 6% by weight of ozone and 94% by weight of oxygen) 8 hours at a flow rate of (L) / min was reacted with hydrazodicarbonamide and ozone. After the reaction was completed, azodicarbonamide was obtained through dehydration and drying, and the reaction yield was 98.2%.
[실시예 2] 아조디카본아마이드의 제조 Example 2 Preparation of Azodicarbonamide
히드라조디카본아미드 100g, 요오드화 칼륨(KI) 1.8g, 물 200g을 잘 혼합하여 유리 반응기에 넣고, 온도를 20℃로 유지하며, 오존(오존 6중량%, 산소 94중량%로 구성)을 3 리터(L)/분의 유량으로 6시간 동안 투입하여 히드라조디카본아미드와 오존을 반응시켰다. 반응 종료 후, 별도의 추가 과정 없이 아조디카본아미드를 얻었으며, 이때의 반응 수율은 98.5%였다.
100 g of hydrazodicarbonamide, 1.8 g of potassium iodide (KI), and 200 g of water are mixed well, placed in a glass reactor, the temperature is maintained at 20 ° C, and 3 liters of ozone (consisting of 6% by weight of ozone and 94% by weight of oxygen) 6 hours at a flow rate of (L) / min was reacted with hydrazodicarbonamide and ozone. After the completion of the reaction, azodicarbonamide was obtained without additional processing, and the reaction yield was 98.5%.
[실시예 3] 아조디카본아마이드의 제조 Example 3 Preparation of Azodicarbonamide
히드라조디카본아미드 100g, 요오드화 칼륨(KI) 3.4g, 물 20g을 혼합하여 유리 반응기에 넣고, 온도를 25℃로 유지하며, 오존(오존 6중량%, 산소 94중량%로 구성)을 3 리터(L)/분의 유량으로 7시간 동안 투입하여 히드라조디카본아미드와 오존을 반응시켰다. 반응 종료 후, 탈수 및 건조 과정을 거쳐 아조디카본아미드를 얻었으며, 이때의 반응 수율은 98.4%였다.
100 g of hydrazodicarbonamide, 3.4 g of potassium iodide (KI), and 20 g of water are mixed and placed in a glass reactor, the temperature is maintained at 25 ° C, and 3 liters of ozone (consisting of 6 wt% ozone and 94 wt% oxygen) 7 hours at a flow rate of L) / min was reacted with hydrazodicarbonamide and ozone. After the reaction was completed, azodicarbonamide was obtained through dehydration and drying, and the reaction yield was 98.4%.
상기 결과로부터, 히드라조디카본아미드를 오존으로 산화시켜 아조디카본아미드를 제조할 경우(실시예 1~3), 낮은 pH(예를 들면, pH 1 내지 5)의 폐수 등이 발생하지 않고, 높은 반응온도(예를 들면, 60℃ 이상)를 요구하지 않으므로, 친환경적이고 공정이 단순함을 알 수 있다.From the above results, when hydrazodicarbonamide is oxidized with ozone to produce azodicarbonamide (Examples 1 to 3), waste water at a low pH (for example, pH 1 to 5) does not occur, and high Since it does not require a reaction temperature (for example, 60 ℃ or more), it can be seen that the environment is simple and the process is simple.
Claims (6)
[반응식 1]
A method for producing azodicarbonamide, wherein the azodicarbonamide (2) is produced by reacting hydrazodicarbonamide (1) with ozone (O 3 ) in the presence of a catalyst as in Scheme 1 below.
[Reaction Scheme 1]
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020120037432A KR20130114961A (en) | 2012-04-10 | 2012-04-10 | Method for preparing azodicarbonamide |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020120037432A KR20130114961A (en) | 2012-04-10 | 2012-04-10 | Method for preparing azodicarbonamide |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20130114961A true KR20130114961A (en) | 2013-10-21 |
Family
ID=49634754
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020120037432A KR20130114961A (en) | 2012-04-10 | 2012-04-10 | Method for preparing azodicarbonamide |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR20130114961A (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104478763A (en) * | 2014-11-19 | 2015-04-01 | 杭州海虹精细化工有限公司 | Method for synthesizing ADC foaming agent through two-section type complex oxidization |
| KR20160058578A (en) * | 2014-11-17 | 2016-05-25 | 주식회사 동진쎄미켐 | A azo-based foaming agent, method for preparing the same and method for foaming resin using the same |
| WO2022039525A1 (en) * | 2020-08-19 | 2022-02-24 | 주식회사 동진쎄미켐 | Method for preparing azo compound |
| WO2022039527A1 (en) * | 2020-08-19 | 2022-02-24 | 주식회사 동진쎄미켐 | Device for producing azo compound |
-
2012
- 2012-04-10 KR KR1020120037432A patent/KR20130114961A/en not_active Application Discontinuation
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20160058578A (en) * | 2014-11-17 | 2016-05-25 | 주식회사 동진쎄미켐 | A azo-based foaming agent, method for preparing the same and method for foaming resin using the same |
| CN104478763A (en) * | 2014-11-19 | 2015-04-01 | 杭州海虹精细化工有限公司 | Method for synthesizing ADC foaming agent through two-section type complex oxidization |
| WO2022039525A1 (en) * | 2020-08-19 | 2022-02-24 | 주식회사 동진쎄미켐 | Method for preparing azo compound |
| WO2022039527A1 (en) * | 2020-08-19 | 2022-02-24 | 주식회사 동진쎄미켐 | Device for producing azo compound |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20130114961A (en) | Method for preparing azodicarbonamide | |
| JPH11503995A (en) | Production method of disinfectant containing chlorine dioxide for water treatment | |
| US8911612B2 (en) | Method of operating metal-bromine cells | |
| CN107986248A (en) | A kind of preparation method of double fluorine sulfimides | |
| CN105621764B (en) | A kind for the treatment of process of epoxychloropropane production waste water | |
| JP2015507089A (en) | In situ production of biocidal bromine species by electrolysis | |
| US3876622A (en) | Process for the preparation of azodicarbonamides modified with metallic compounds | |
| JP6484460B2 (en) | Separation membrane operation method and separation membrane modification method | |
| Serikawa et al. | Wet electrolytic oxidation of organic pollutants in wastewater treatment | |
| CA2333247C (en) | A method of improving yield of chlorine dioxide generation processes | |
| CN103360316A (en) | Preparation method of fipronil | |
| JP2011080079A5 (en) | ||
| JP2016155067A (en) | Modification method for reverse osmosis membrane, reverse osmosis membrane, and processing method for water containing boron | |
| CN110437169B (en) | Preparation method of sodium dichloroisocyanurate | |
| US2335808A (en) | Method for producing chlorine dioxide | |
| CN102675158B (en) | Method for producing ADC foaming agent by using chlorine gas-oxidized HDCA (biurea) in saturated hydrochloric acid solution | |
| CN106748878B (en) | A kind of synthetic method of butanone azine | |
| US20070012570A1 (en) | Electrochemical methods for making highly soluble oxidizing agents | |
| US11850572B2 (en) | Activated carbon catalyst for hydrogen peroxide decomposition, method for producing same, and method for decomposing hydrogen peroxide by using same | |
| US3649484A (en) | Electrolytic process for the manufacture of azo compounds | |
| RU2471718C1 (en) | Method of removing nitrite ions from water solutions | |
| CN105329949B (en) | Method for in-situ preparation of ferrate by means of singlet oxygen | |
| CN104627964B (en) | Method for preparing bromine by using brine | |
| JP6165349B2 (en) | New production method of azodicarbonamide | |
| CN104528673B (en) | A kind of method preparing hydrazine hydrate |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WITN | Withdrawal due to no request for examination |