KR20120099703A - 불화수소-HFC-254eb 공비혼합물 및 그의 용도 - Google Patents
불화수소-HFC-254eb 공비혼합물 및 그의 용도 Download PDFInfo
- Publication number
- KR20120099703A KR20120099703A KR1020127013791A KR20127013791A KR20120099703A KR 20120099703 A KR20120099703 A KR 20120099703A KR 1020127013791 A KR1020127013791 A KR 1020127013791A KR 20127013791 A KR20127013791 A KR 20127013791A KR 20120099703 A KR20120099703 A KR 20120099703A
- Authority
- KR
- South Korea
- Prior art keywords
- tetrafluoropropane
- composition
- hydrogen fluoride
- azeotropic
- pentafluoropropane
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000001257 hydrogen Substances 0.000 title description 13
- 229910052739 hydrogen Inorganic materials 0.000 title description 13
- 125000004435 hydrogen atom Chemical class [H]* 0.000 title 1
- 239000000203 mixture Substances 0.000 claims abstract description 194
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 claims abstract description 147
- 229910000040 hydrogen fluoride Inorganic materials 0.000 claims abstract description 144
- INEMUVRCEAELBK-UHFFFAOYSA-N 1,1,1,2-tetrafluoropropane Chemical compound CC(F)C(F)(F)F INEMUVRCEAELBK-UHFFFAOYSA-N 0.000 claims abstract description 100
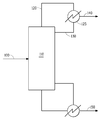
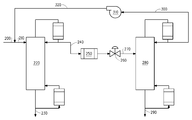
- 238000004821 distillation Methods 0.000 claims abstract description 73
- 238000000034 method Methods 0.000 claims abstract description 49
- ZDCWZRQSHBQRGN-UHFFFAOYSA-N 1,1,1,2,3-pentafluoropropane Chemical compound FCC(F)C(F)(F)F ZDCWZRQSHBQRGN-UHFFFAOYSA-N 0.000 claims abstract description 40
- 239000007788 liquid Substances 0.000 claims abstract description 35
- 230000008569 process Effects 0.000 claims description 16
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical group CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 claims 2
- IATQMZSYBZMIPZ-UHFFFAOYSA-N 1,1,1,2,3-pentafluoropropane 1,1,1,2-tetrafluoropropane Chemical compound CC(F)C(F)(F)F.FCC(F)C(F)(F)F IATQMZSYBZMIPZ-UHFFFAOYSA-N 0.000 claims 1
- 239000001294 propane Substances 0.000 claims 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 20
- 238000009835 boiling Methods 0.000 description 20
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 11
- 238000006243 chemical reaction Methods 0.000 description 11
- 239000000047 product Substances 0.000 description 11
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 10
- 229910052757 nitrogen Inorganic materials 0.000 description 10
- 239000003054 catalyst Substances 0.000 description 9
- 238000005984 hydrogenation reaction Methods 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 238000010992 reflux Methods 0.000 description 9
- 239000012071 phase Substances 0.000 description 8
- 230000008901 benefit Effects 0.000 description 7
- WFHFXEYKXJKYMG-UHFFFAOYSA-N 1,1,2-trichloro-1,3,3,3-tetrafluoropropane Chemical compound FC(F)(F)C(Cl)C(F)(Cl)Cl WFHFXEYKXJKYMG-UHFFFAOYSA-N 0.000 description 5
- DMUPYMORYHFFCT-UHFFFAOYSA-N 1,2,3,3,3-pentafluoroprop-1-ene Chemical compound FC=C(F)C(F)(F)F DMUPYMORYHFFCT-UHFFFAOYSA-N 0.000 description 5
- 238000001704 evaporation Methods 0.000 description 5
- 230000008020 evaporation Effects 0.000 description 5
- 239000007791 liquid phase Substances 0.000 description 5
- 238000000926 separation method Methods 0.000 description 5
- BIPNYHXPHOUMCL-UHFFFAOYSA-N 1,1,2-trichloro-1,2,3,3,3-pentafluoropropane Chemical group FC(F)(F)C(F)(Cl)C(F)(Cl)Cl BIPNYHXPHOUMCL-UHFFFAOYSA-N 0.000 description 4
- FXRLMCRCYDHQFW-UHFFFAOYSA-N 2,3,3,3-tetrafluoropropene Chemical compound FC(=C)C(F)(F)F FXRLMCRCYDHQFW-UHFFFAOYSA-N 0.000 description 4
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 4
- 239000006227 byproduct Substances 0.000 description 4
- 238000012423 maintenance Methods 0.000 description 4
- 229910052763 palladium Inorganic materials 0.000 description 4
- 239000003507 refrigerant Substances 0.000 description 4
- 238000010792 warming Methods 0.000 description 4
- 238000013459 approach Methods 0.000 description 3
- 238000010533 azeotropic distillation Methods 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- QQONPFPTGQHPMA-UHFFFAOYSA-N Propene Chemical compound CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 238000005796 dehydrofluorination reaction Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 2
- 150000008282 halocarbons Chemical class 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000010926 purge Methods 0.000 description 2
- 238000005201 scrubbing Methods 0.000 description 2
- -1 1,1,1,2,3-pentafluoropropane Fluoropropane forms Chemical group 0.000 description 1
- LVGUZGTVOIAKKC-UHFFFAOYSA-N 1,1,1,2-tetrafluoroethane Chemical compound FCC(F)(F)F LVGUZGTVOIAKKC-UHFFFAOYSA-N 0.000 description 1
- NDMMKOCNFSTXRU-UHFFFAOYSA-N 1,1,2,3,3-pentafluoroprop-1-ene Chemical compound FC(F)C(F)=C(F)F NDMMKOCNFSTXRU-UHFFFAOYSA-N 0.000 description 1
- GRSQYISVQKPZCW-UHFFFAOYSA-N 1,1,2-trichloropropane Chemical compound CC(Cl)C(Cl)Cl GRSQYISVQKPZCW-UHFFFAOYSA-N 0.000 description 1
- LDMYCECIGXPKDO-UHFFFAOYSA-N 2-chloro-1,1,1,2,3-pentafluoropropane Chemical compound FCC(F)(Cl)C(F)(F)F LDMYCECIGXPKDO-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 208000033962 Fontaine progeroid syndrome Diseases 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 230000000779 depleting effect Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000004508 fractional distillation Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000014509 gene expression Effects 0.000 description 1
- 229910000856 hastalloy Inorganic materials 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 229910001026 inconel Inorganic materials 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000005057 refrigeration Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000002463 transducing effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 239000012808 vapor phase Substances 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B7/00—Halogens; Halogen acids
- C01B7/19—Fluorine; Hydrogen fluoride
- C01B7/191—Hydrogen fluoride
- C01B7/195—Separation; Purification
- C01B7/196—Separation; Purification by distillation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C17/00—Preparation of halogenated hydrocarbons
- C07C17/38—Separation; Purification; Stabilisation; Use of additives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C17/00—Preparation of halogenated hydrocarbons
- C07C17/38—Separation; Purification; Stabilisation; Use of additives
- C07C17/383—Separation; Purification; Stabilisation; Use of additives by distillation
- C07C17/386—Separation; Purification; Stabilisation; Use of additives by distillation with auxiliary compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C19/00—Acyclic saturated compounds containing halogen atoms
- C07C19/08—Acyclic saturated compounds containing halogen atoms containing fluorine
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US25634009P | 2009-10-30 | 2009-10-30 | |
| US61/256,340 | 2009-10-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20120099703A true KR20120099703A (ko) | 2012-09-11 |
Family
ID=43447920
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127013791A Withdrawn KR20120099703A (ko) | 2009-10-30 | 2010-10-12 | 불화수소-HFC-254eb 공비혼합물 및 그의 용도 |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US8486293B2 (enExample) |
| EP (1) | EP2493836B1 (enExample) |
| JP (1) | JP5706909B2 (enExample) |
| KR (1) | KR20120099703A (enExample) |
| CN (1) | CN102666453B (enExample) |
| BR (1) | BR112012009821A2 (enExample) |
| CA (1) | CA2778250A1 (enExample) |
| ES (1) | ES2545740T3 (enExample) |
| HU (1) | HUP1200449A2 (enExample) |
| IN (1) | IN2012DN03132A (enExample) |
| MX (1) | MX2012004921A (enExample) |
| RU (1) | RU2012122225A (enExample) |
| TW (1) | TW201119978A (enExample) |
| WO (1) | WO2011053449A1 (enExample) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5393454B2 (ja) * | 2006-06-27 | 2014-01-22 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | 1,2,3,3,3−ペンタフルオロプロペンの製造方法 |
| US8513474B2 (en) | 2010-06-24 | 2013-08-20 | Honeywell International Inc. | Process for the manufacture of fluorinated olefins |
| BR112014021670B1 (pt) * | 2012-03-02 | 2020-09-29 | Arkema Inc | Métodos para a remoção de contaminante de uma hidroclorofluorolefina por destilação extrativa, trans-1,1,1-trifluoro-3-cloro-2-propeno (1233zd(e)) purificado e processo de destilação |
| CA3040740A1 (en) * | 2016-12-21 | 2018-06-28 | Arkema Inc. | Processes and systems for recovering r1233zd in purified form |
| JP6485493B2 (ja) * | 2017-06-16 | 2019-03-20 | ダイキン工業株式会社 | ペンタフルオロプロパンと水とを含む共沸又は共沸様組成物、並びにペンタフルオロプロパンの製造方法 |
| CN110945099B (zh) | 2017-07-27 | 2024-07-05 | 科慕埃弗西有限公司 | 包含氟化氢和氟碳化合物的共沸组合物 |
| CN111094503B (zh) * | 2017-09-11 | 2022-04-01 | 科慕埃弗西有限公司 | 包含氟化氢和含氟烃的共沸组合物 |
| JP7670992B2 (ja) * | 2020-03-19 | 2025-05-01 | セントラル硝子株式会社 | (ハイドロ)ハロカーボンの製造方法 |
| EP4235702A4 (en) * | 2020-10-22 | 2024-07-24 | Agc Inc. | ELECTRICAL EQUIPMENT |
| CN116802170A (zh) * | 2021-01-29 | 2023-09-22 | Agc株式会社 | 3-氯-1,1,2,2-四氟丙烷的制造方法和1-氯-2,3,3-三氟丙烯的制造方法 |
| CN113624570B (zh) * | 2021-08-06 | 2023-04-28 | 清华大学 | 汽液相平衡装置及氟代烃中的hf的分析方法 |
| CN114350321B (zh) * | 2021-12-03 | 2024-02-09 | 湖北瑞能华辉能源管理有限公司 | 一种节能环保型热泵工质及其应用 |
| JP2025536644A (ja) | 2022-11-18 | 2025-11-07 | ザ ケマーズ カンパニー エフシー リミテッド ライアビリティ カンパニー | 3-クロロ-3,3-ジフルオロ-1-プロペン、3,3,3-トリフルオロプロペン、及びフッ化水素の共沸性混合物 |
| JP2025536643A (ja) | 2022-11-18 | 2025-11-07 | ザ ケマーズ カンパニー エフシー リミテッド ライアビリティ カンパニー | 1,1,1,3-テトラフルオロプロパン、3,3,3-トリフルオロプロペン、及びフッ化水素を含む混合物並びにそれらの共沸混合物の分離のためのプロセス |
| FR3151590A1 (fr) | 2023-07-27 | 2025-01-31 | Dehon | Procede et installation pour la separation d’un melange comprenant au moins un fluide fluore et un ou plusieurs contaminants |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4944846A (en) | 1988-08-01 | 1990-07-31 | E. I. Dupont De Nemours And Company | Process for the separation of HF via Azeotropic distillation |
| US5396000A (en) | 1993-05-24 | 1995-03-07 | E. I. Du Pont De Nemours And Company | Process for the manufacture of 1,1,1,2,3,-pentafluoropropane |
| EP0713485B1 (de) * | 1993-08-11 | 1998-04-15 | Bayer Ag | Thiocarbamoylverbindungen als mikrobizide |
| US5918481A (en) * | 1997-11-20 | 1999-07-06 | Alliedsignal Inc. | Process for separating hydrogen fluoride from fluorocarbons |
| US7897823B2 (en) * | 2004-10-29 | 2011-03-01 | E. I. Du Pont De Nemours And Company | Process for production of azeotrope compositions comprising hydrofluoroolefin and hydrogen fluoride and uses of said azeotrope compositions in separation processes |
| US7476771B2 (en) | 2005-11-01 | 2009-01-13 | E.I. Du Pont De Nemours + Company | Azeotrope compositions comprising 2,3,3,3-tetrafluoropropene and hydrogen fluoride and uses thereof |
| US7888539B2 (en) | 2007-02-23 | 2011-02-15 | E.I. Du Pont De Nemours And Company | Azeotrope compositions of octafluorocyclobutane and uses thereof |
| GB0801209D0 (en) | 2008-01-23 | 2008-02-27 | Ineos Fluor Holdings Ltd | Process |
| KR102035526B1 (ko) * | 2008-02-21 | 2019-10-24 | 더 케무어스 컴퍼니 에프씨, 엘엘씨 | 3,3,3-트라이플루오로프로펜 및 플루오르화수소를 포함하는 공비 조성물 및 이의 분리방법 |
| EP2098499B2 (en) | 2008-03-06 | 2017-11-22 | Honeywell International Inc. | Azeotrope-like composition of 2-chloro-3,3,3-trifluoropropene (HCFC-1233xf) and hydrogen fluoride (HF) |
| US8845921B2 (en) | 2008-04-09 | 2014-09-30 | Honeywell International Inc. | Separation of close boiling compounds by addition of a third compound |
-
2010
- 2010-10-06 US US12/898,983 patent/US8486293B2/en active Active
- 2010-10-12 RU RU2012122225/04A patent/RU2012122225A/ru unknown
- 2010-10-12 WO PCT/US2010/052237 patent/WO2011053449A1/en not_active Ceased
- 2010-10-12 ES ES10768151.2T patent/ES2545740T3/es active Active
- 2010-10-12 MX MX2012004921A patent/MX2012004921A/es active IP Right Grant
- 2010-10-12 BR BR112012009821A patent/BR112012009821A2/pt not_active IP Right Cessation
- 2010-10-12 CA CA2778250A patent/CA2778250A1/en not_active Abandoned
- 2010-10-12 JP JP2012536843A patent/JP5706909B2/ja active Active
- 2010-10-12 KR KR1020127013791A patent/KR20120099703A/ko not_active Withdrawn
- 2010-10-12 EP EP10768151.2A patent/EP2493836B1/en active Active
- 2010-10-12 CN CN201080048381.0A patent/CN102666453B/zh active Active
- 2010-10-12 HU HU1200449A patent/HUP1200449A2/hu unknown
- 2010-10-18 TW TW099135476A patent/TW201119978A/zh unknown
-
2012
- 2012-04-11 IN IN3132DEN2012 patent/IN2012DN03132A/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| JP2013509409A (ja) | 2013-03-14 |
| RU2012122225A (ru) | 2013-12-10 |
| HUP1200449A2 (en) | 2012-11-28 |
| TW201119978A (en) | 2011-06-16 |
| CN102666453A (zh) | 2012-09-12 |
| IN2012DN03132A (enExample) | 2015-09-18 |
| US20110101264A1 (en) | 2011-05-05 |
| EP2493836A1 (en) | 2012-09-05 |
| US8486293B2 (en) | 2013-07-16 |
| WO2011053449A1 (en) | 2011-05-05 |
| JP5706909B2 (ja) | 2015-04-22 |
| CN102666453B (zh) | 2015-11-25 |
| CA2778250A1 (en) | 2011-05-05 |
| EP2493836B1 (en) | 2015-07-08 |
| ES2545740T3 (es) | 2015-09-15 |
| MX2012004921A (es) | 2012-05-22 |
| BR112012009821A2 (pt) | 2016-10-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5706909B2 (ja) | フッ化水素−HFC−254eb共沸物およびその使用 | |
| ES2483995T3 (es) | Procesos para la separación de 1,3,3,3-tetrafluoropropeno del fluoruro de hidrógeno por medio de destilación azeotrópica | |
| CN101952230B (zh) | 通过共沸蒸馏使2,3,3,3-四氟丙烯与氟化氢分离的方法 | |
| KR101438240B1 (ko) | E-1,3,3,3-테트라플루오로프로펜 및 불화수소를 포함하는공비 조성물 및 그의 용도 | |
| JP2019196393A (ja) | 第3の化合物を加えることによる沸点の近い化合物の分離 | |
| CA2717216A1 (en) | Azeotrope compositions comprising 3,3,3-trifluoropropene and hydrogen fluoride and processes for separation thereof | |
| US20100203167A1 (en) | Azeotrope compositions comprising decafluoropentane and hydrogen fluoride and uses thereof | |
| EP1474370A1 (en) | Processes for purification and production of fluorocarbons | |
| WO2008030438A2 (en) | A process and methods of purification for the manufacture fluorocarbons | |
| US20180056210A1 (en) | SEPARATION OF (Z)-1-CHLORO-3,3,3-TRIFLUOROPROPENE (HCFO-1233zd(Z)) AND 1-CHLORO-1,3,3,3-TETRAFLUOROPROPANE (HCFC-244fa) BY ADDING A THIRD COMPONENT | |
| JP2025536644A (ja) | 3-クロロ-3,3-ジフルオロ-1-プロペン、3,3,3-トリフルオロプロペン、及びフッ化水素の共沸性混合物 | |
| JP2025536643A (ja) | 1,1,1,3-テトラフルオロプロパン、3,3,3-トリフルオロプロペン、及びフッ化水素を含む混合物並びにそれらの共沸混合物の分離のためのプロセス | |
| HK1153188A (en) | Processes for separation of 1,3,3,3-tetrafluoropropene from hydrogen fluoride by azeotropic distillation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20120529 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |