KR100676996B1 - Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof - Google Patents
Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof Download PDFInfo
- Publication number
- KR100676996B1 KR100676996B1 KR1020050004752A KR20050004752A KR100676996B1 KR 100676996 B1 KR100676996 B1 KR 100676996B1 KR 1020050004752 A KR1020050004752 A KR 1020050004752A KR 20050004752 A KR20050004752 A KR 20050004752A KR 100676996 B1 KR100676996 B1 KR 100676996B1
- Authority
- KR
- South Korea
- Prior art keywords
- butadiene
- polymerization
- polymerization inhibitor
- distillation column
- extractive distillation
- Prior art date
Links
Images
Classifications
-
- E—FIXED CONSTRUCTIONS
- E02—HYDRAULIC ENGINEERING; FOUNDATIONS; SOIL SHIFTING
- E02B—HYDRAULIC ENGINEERING
- E02B3/00—Engineering works in connection with control or use of streams, rivers, coasts, or other marine sites; Sealings or joints for engineering works in general
- E02B3/04—Structures or apparatus for, or methods of, protecting banks, coasts, or harbours
- E02B3/12—Revetment of banks, dams, watercourses, or the like, e.g. the sea-floor
- E02B3/129—Polyhedrons, tetrapods or similar bodies, whether or not threaded on strings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C7/00—Purification; Separation; Use of additives
- C07C7/20—Use of additives, e.g. for stabilisation
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F36/00—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds
- C08F36/02—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds the radical having only two carbon-to-carbon double bonds
- C08F36/04—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds the radical having only two carbon-to-carbon double bonds conjugated
- C08F36/06—Butadiene
-
- E—FIXED CONSTRUCTIONS
- E02—HYDRAULIC ENGINEERING; FOUNDATIONS; SOIL SHIFTING
- E02B—HYDRAULIC ENGINEERING
- E02B3/00—Engineering works in connection with control or use of streams, rivers, coasts, or other marine sites; Sealings or joints for engineering works in general
- E02B3/04—Structures or apparatus for, or methods of, protecting banks, coasts, or harbours
- E02B3/12—Revetment of banks, dams, watercourses, or the like, e.g. the sea-floor
- E02B3/14—Preformed blocks or slabs for forming essentially continuous surfaces; Arrangements thereof
-
- E—FIXED CONSTRUCTIONS
- E02—HYDRAULIC ENGINEERING; FOUNDATIONS; SOIL SHIFTING
- E02B—HYDRAULIC ENGINEERING
- E02B3/00—Engineering works in connection with control or use of streams, rivers, coasts, or other marine sites; Sealings or joints for engineering works in general
- E02B3/16—Sealings or joints
-
- E—FIXED CONSTRUCTIONS
- E02—HYDRAULIC ENGINEERING; FOUNDATIONS; SOIL SHIFTING
- E02D—FOUNDATIONS; EXCAVATIONS; EMBANKMENTS; UNDERGROUND OR UNDERWATER STRUCTURES
- E02D17/00—Excavations; Bordering of excavations; Making embankments
- E02D17/20—Securing of slopes or inclines
- E02D17/205—Securing of slopes or inclines with modular blocks, e.g. pre-fabricated
-
- E—FIXED CONSTRUCTIONS
- E02—HYDRAULIC ENGINEERING; FOUNDATIONS; SOIL SHIFTING
- E02D—FOUNDATIONS; EXCAVATIONS; EMBANKMENTS; UNDERGROUND OR UNDERWATER STRUCTURES
- E02D2600/00—Miscellaneous
- E02D2600/20—Miscellaneous comprising details of connection between elements
Landscapes
- Engineering & Computer Science (AREA)
- General Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Structural Engineering (AREA)
- Organic Chemistry (AREA)
- Environmental & Geological Engineering (AREA)
- Ocean & Marine Engineering (AREA)
- Mechanical Engineering (AREA)
- Civil Engineering (AREA)
- Analytical Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Water Supply & Treatment (AREA)
- Crystallography & Structural Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Polymerisation Methods In General (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
Abstract
본 발명은 4-터셔리-부틸카테콜(TBC), 디에틸 히드록실아민(DEHA) 및 유기용매로 이루어진 것을 특징으로 하는 1,3-부타디엔의 중합방지제 및 이것을 투입하는 1,3-부타디엔의 중합방지방법에 관한 것이다.The present invention relates to a polymerization inhibitor of 1,3-butadiene and to 1,3-butadiene, comprising 4 tert-butyl catechol (TBC), diethyl hydroxylamine (DEHA) and an organic solvent. It relates to a polymerization prevention method.
본 발명에 의한 우수한 중합반응 억제 내지 파울링 방지 효과를 갖는 중합방지제에 의하여 기체 및 액체 상태의 광범위한 온도 범위에서 1,3-부타디엔의 중합을 억제함으로써 고순도의 1,3-부타디엔 제품을 추출 증류할 수 있고, 파울링으로 인해 야기될 수 있는 1,3-부타디엔 제조공장의 비상시 가동정지를 미연에 방지하고, 장기간 안전운전을 할 수 있다.The high-purity 1,3-butadiene product can be extracted and distilled by inhibiting the polymerization of 1,3-butadiene in a wide temperature range of gas and liquid state by the polymerization inhibitor having excellent polymerization inhibition to antifouling effect according to the present invention. It is possible to prevent emergency shutdown of the 1,3-butadiene manufacturing plant which may be caused by fouling, and to perform a safe operation for a long time.
중합방지제, 1,3-부타디엔, 디에틸 히드록실아민, 이소부틸 알콜, 이소프로필 알콜. Polymerization inhibitors, 1,3-butadiene, diethyl hydroxylamine, isobutyl alcohol, isopropyl alcohol.
Description
도 1은 본 발명의 중합방지제가 주입되는 공정을 포함한 1,3-부타디엔 생산을 위한 추출 증류 공정도이다. 1 is an extractive distillation process diagram for the production of 1,3-butadiene including a process in which the polymerization inhibitor of the present invention is injected.
※ 도면 중 주요부분에 대한 부호설명※ Explanation of Codes on Major Parts of Drawings
1: 추출 증류 컬럼(Extrative distillation column)1: extractive distillation column
1-a: 제 1 침니 트레이(First Chimney Tray)1-a: First Chimney Tray
1-b: 제 2 침니 트레이(Second Chimney Tray)1-b: Second Chimney Tray
2: 혼합 C4 유분 투입처(Mixed C4 injection point)2: In a mixed C 4 fraction processing (Mixed C 4 injection point)
3: 디메틸포름아마이드 투입처(Dimethylformamide injection point)3: Dimethylformamide injection point
4: 중합방지제 저장 탱크(Polymerization inhibitor storage tank)4: Polymerization inhibitor storage tank
5: 중합방지제 주입 펌프(Polymerization inhibitor injection pump)5: Polymerization inhibitor injection pump
6: 응축기(Condensor)6: Condensor
7: 응축드럼(Reflux drum)7: Reflux drum
8: 환류 펌프 스트레이너(Reflux pump strainer)8: Reflux pump strainer
9: 환류 펌프(Reflux pump)9: Reflux pump
10: 하부 컬럼 스트레이너(Column bottom strainer)10: column bottom strainer
11: 제 1 재비기(Reboiler)11: First Reboiler
12: 제 2 재비기(Reboiler)12: Second Reboiler
가: 1,3-부타디엔 정제 공정(1,3-Butadiene distillation unit)1,3-Butadiene distillation unit
나: 1,3-부타디엔 회수 공정(1,3-Butadiene recovery unit))B: 1,3-butadiene recovery unit
본 발명은 1,3-부타디엔의 중합방지제 및 이것을 투입하는 1,3-부타디엔의 중합방지방법에 관한 것으로서, 구체적으로는 1,3-부타디엔의 제조시 파울링의 생성을 차단, 방지하기 위한 신규한 중합방지제 및 이것을 추출 증류 컬럼에 투입하여 1,3-부타디엔의 중합방지방법에 관한 것이다. The present invention relates to a polymerization inhibitor of 1,3-butadiene and a method for preventing polymerization of 1,3-butadiene, which is added thereto. Specifically, the present invention provides a novel method for blocking and preventing the formation of fouling during the production of 1,3-butadiene. It relates to a polymerization inhibitor and a method for preventing polymerization of 1,3-butadiene by putting it in an extractive distillation column.
혼합 C4 유분(Mixed C4) 중 각종 고무 및 합성수지 원료가 되는 1,3-부타디엔(1,3-Butadiene) 제조공장 뿐만 아니라 스티렌 모노머(styrene monomer) 및 이소프렌(isoprene) 제조공장 등에서는 생산 제품이 불포화 탄화수소이기 때문에 자체가 쉽게 중합이 가능하여 정상 운전 중에도 공정 내에 유기물 파울링(fouling) 문 제와 같은 심각한 문제를 일으킨다. 예를 들면, 추출 증류 컬럼에서 생성된 파울링은 추출 증류 컬럼내의 트레이를 막히게 하여 컬럼 효율을 떨어뜨리거나, 상부 콘덴서의 유로를 폐쇄하여 전열효율을 감소시키거나 기기장치의 파열을 유발할 수 있다. In the mixed C 4 oil (Mixed C 4 ), not only 1,3-butadiene manufacturing plant, which is a raw material for rubber and synthetic resin, but also styrene monomer and isoprene manufacturing plant Because it is an unsaturated hydrocarbon, it can be easily polymerized, causing serious problems such as organic fouling problems in the process even during normal operation. For example, fouling produced in an extractive distillation column may clog the trays in the extractive distillation column, reducing column efficiency, or closing the flow path of the upper condenser to reduce heat transfer efficiency or cause rupture of the apparatus.
상기 파울링의 생성기전을 살펴보면, 일반적으로 유기물 파울링은 자유 라디칼 반응에 의해 생성되는 것이다. 자유 라디칼, 즉 전자 하나가 부족한 원자 내지 원자의 그룹은 부족한 전자를 보충하여 안정한 전자쌍을 이루려는 성질을 가지므로 자유 라디칼 반응의 반응성은 매우 크다. 자유 라디칼은 열, 과산화물 또는 산소와의 반응 또는 금속의 촉매 작용으로 생성된다. 이렇게 생성된 자유 라디칼은 불포화 탄화수소와 빠르게 반응하여 수지 또는 고무형 폴리머를 형성하게 되며, 고온이거나 체류시간이 길어져 반응이 계속 진전되며 분자랑이 커지게 된다. 이러한 자유 라디칼 중합(polymerization)은 두개의 라디칼이 충돌하거나, 자유 라디칼과 반응성이 큰 화합물과 만나면 중합 반응이 종료되며, 활성이 없는 매우 안정된 화합물을 형성하여 공정 내에서 파울링을 유발한다.Looking at the generation mechanism of the fouling, organic fouling is generally produced by a free radical reaction. Free radicals, that is, atoms or groups of atoms that lack one electron, have a property to make up a stable electron pair by supplementing the lacking electrons, so the reactivity of the free radical reaction is very high. Free radicals are produced by reaction with heat, peroxides or oxygen or catalysis of metals. The free radicals thus formed react quickly with unsaturated hydrocarbons to form resins or rubbery polymers. The free radicals are either hot or have a longer residence time, leading to continued reactions and larger molecular weights. This free radical polymerization terminates the polymerization reaction when two radicals collide or encounter a compound that is highly reactive with free radicals, forming a very stable compound with no activity, causing fouling in the process.
1,3-부타디엔 제조공장의 추출 증류 단계에서의 파울링은 상기와 같이 주로 자유 라디칼에 의해 팝콘(popcorn) 형태의 폴리머가 생성된 유기 파울링이다. The fouling in the extractive distillation step of the 1,3-butadiene manufacturing plant is an organic fouling in which a popcorn polymer is produced mainly by free radicals.
다시 말해 1,3-부타디엔 제조시 폴리머 생성반응은 자유 라디칼 반응으로써 과산화물 및 산화철 라디칼로 개시되며, 제조공정 내에 존재하는 산소, 산화철 등에 의하여 촉진되어 팝콘 형태의 폴리머가 생성된다. In other words, the polymer production reaction in the production of 1,3-butadiene is initiated as a peroxide and iron oxide radical as a free radical reaction, and is promoted by oxygen, iron oxide, etc. present in the production process to produce a popcorn type polymer.
이러한 유기 파울링의 생성문제를 해결하기 위하여, 설비의 정기 보수시에 화학 처리를 통한 잔류산소 및 금속성분을 제거한다. 또한 정상 운전시에는 4-터셔리-부틸카테콜(4-tertiary-butylcatechol, 이하 'TBC'로 칭함), 디에틸 히드록실아민(N,N-Diethyl hydroxy amine, 이하 'DEHA'로 칭함) 및 안정화된 자유 라디칼 (Stable free radical , 이하 'SFR'로 칭함)과 같은 파울링 방지제를 단독으로 사용하고 있다. In order to solve this problem of organic fouling, residual oxygen and metal components are removed through chemical treatment during regular maintenance of the equipment. In normal operation, 4-tertiary-butylcatechol (4-tertiary-butylcatechol, hereinafter referred to as 'TBC'), diethyl hydroxylamine (N, N-Diethyl hydroxy amine, hereinafter referred to as 'DEHA'), and Antifouling agents such as stabilized free radicals (hereinafter referred to as 'SFR') are used alone.
그러나 상기와 같은 단일 성분의 중합방지제는 공정 내에서 기상 및 액상 환경에서 폴리머 생성 방지 기능이 미비하고, 이미 진행된 폴리머의 제거에는 효과가 없다. 또한, 4-터셔리-부틸카테콜(TBC) 및 디에틸 히드록실아민(DEHA)은 제품 특성상 85중량%의 수용액으로 제조됨에 따라 공정내에 불필요한 수분이 도입됨으로 인해 파울링이 유발되는 부작용이 뒤따른다. However, such a single component of the polymerization inhibitor in the process in the gas phase and liquid environment is insufficient to prevent the formation of the polymer, it is not effective for the removal of the polymer already advanced. In addition, 4-tertiary-butylcatechol (TBC) and diethyl hydroxylamine (DEHA) are produced in an aqueous solution of 85% by weight due to the nature of the product is a side effect that causes fouling due to the introduction of unnecessary moisture in the process Follow.
구체적으로 상기와 같은 단일 성분의 중합방지제는 다음과 같은 문제점이 있다. Specifically, the single component polymerization inhibitor has the following problems.
대부분의 팝콘 형태의 폴리머는 기체 상태에서 발생되는데 반해 4-터셔리-부틸카테콜(TBC)은 낮은 증기압을 가지므로 TBC 단독으로는 기체상태의 공정부분에서는 중합방지 효과를 잘 발휘할 수 없다. 또한, TBC 단독으로는 이미 생성된 팝콘 형태의 폴리머의 진행을 중단시킬 수 없으며, 고상인 TBC는 15중량%의 물에 녹여서 사용되므로 제품 특성상 물에 의하여 자유 라디칼 반응이 촉진되어 폴리머가 증진되고 파울링이 오히려 더 잘 생기게 하는 단점이 있다. Most popcorn-type polymers occur in the gaseous state, whereas 4-tertiary-butylcatechol (TBC) has a low vapor pressure, so TBC alone cannot exert its anti-polymerization effect in the gaseous process part. In addition, TBC alone cannot stop the progress of the already produced popcorn-type polymer, and since the solid TBC is used by dissolving in 15% by weight of water, the free radical reaction is promoted by water due to the characteristics of the product to promote and foul the polymer. The disadvantage is that the ring is better produced.
또한, 디에틸 히드록실아민(DEHA)을 단독으로 사용하는 경우, 공정 부분내의 산소 제거가 가능해서 기체 상태의 공정 부분에서는 일정 효과가 있으나, 액체 상 태 및 기체-액체의 공정 부분에서는 그 효과가 미비하다. In addition, when diethyl hydroxylamine (DEHA) is used alone, it is possible to remove oxygen in the process portion, so that there is a certain effect in the gaseous process portion, but the effect in the liquid state and the gas-liquid process portion Incomplete
또한, 안정화된 자유 라디칼(SFR)을 단독으로 사용하는 경우, 극성의 반응 종료제이므로 TBC에 비해 액체 상태에서 더욱 좋은 효과를 보이지만, 기체 상태에서는 팝콘 형태의 폴리머 생성 방지 효과가 없으며, 고가인 단점도 있다.In addition, when stabilized free radicals (SFR) are used alone, since the polarization terminator is a good effect in the liquid state compared to TBC, in the gas state there is no effect of preventing the formation of polymers in the form of popcorn, expensive disadvantages There is also.
현재 석유화학산업은 설비, 운전 및 정비 기술의 발달로 정기 보수 주기를 장기화하여 많은 생산성 및 경제성을 도모하고 있으나, 유기 파울링을 방지하기 위한 상기와 같은 종래 기술상의 문제점을 극복하지 못하고서는 정기 보수 주기까지의 안정적인 운전을 기대할 수 없다. Currently, the petrochemical industry promotes a lot of productivity and economics by prolonging the regular maintenance cycle with the development of equipment, operation and maintenance technology.However, the petrochemical industry does not overcome the above-mentioned problems in the prior art for preventing organic fouling. Stable operation up to cycle cannot be expected.
나아가, 대량의 유기 파울링에 의한 예상치 못한 공장의 정지 등으로 많은 비용 부담이 발생할 수 있고, 설비의 폭발위험까지도 있다. 1,3-부타디엔 제조 공장의 가동 정지는 석유화학산업의 계통도상 상부와 하부 공장의 가동율에 주는 파급 효과가 대단히 크므로 1,3-부타디엔 제조 설비의 안정적인 운전을 확보하기 위하여 효과적인 중합방지제가 절실히 요구된다. Furthermore, an unexpected plant stop due to a large amount of organic fouling may cause a lot of cost and even a risk of explosion of the equipment. Since the shutdown of the 1,3-butadiene manufacturing plant has a great effect on the operation rate of the upper and lower factories in the petrochemical industry, an effective polymerization inhibitor is urgently needed to secure stable operation of the 1,3-butadiene manufacturing plant. Required.
상기와 같은 문제를 해결하기 위하여 고순도의 1,3-부타디엔을 수득하기 위한 1,3-부타디엔 제조공정의 추출 증류 단계 중 기상 및 액상의 광범위한 온도환경에서 자유 라디칼, 과산화물, 물 및 산소를 제거하여 1,3-부타디엔의 중합을 억제하고 폴리머 생성을 차단함으로써 파울링을 방지할 수 있는 중합방지제 및 이것을 투입하는 1,3-부타디엔의 중합방지방법을 제공하는 것을 목적으로 한다.
In order to solve the above problems, free radicals, peroxides, water and oxygen are removed in a wide temperature environment of gas and liquid phase during the extraction distillation step of 1,3-butadiene manufacturing process to obtain
이로써, 파울링으로 인해 야기될 수 있는 1,3-부타디엔 제조 공장의 비상시 가동정지를 미연에 방지하고, 장기간 안전운전을 가능하게 하고자 한다.
As a result, the emergency stop of the 1,3-butadiene manufacturing plant that may be caused by fouling is prevented in advance, and a long-term safe operation is possible.
상기와 같은 과제를 해결하기 위한 본 발명은 고순도의 1,3-부타디엔 제조공정 중 추출 증류 단계에서 1,3-부타디엔 화합물의 제조시 생성되는 팝콘형 폴리머 즉, 파울링을 억제하고, 이미 생성된 상기 팝콘형 폴리머의 활성을 제거하여 고순도의 1,3-부타디엔 제품을 얻기 위하여 사용되는 중합방지제를 제공한다.The present invention for solving the above problems is to suppress the popcorn-type polymer, namely, fouling produced during the production of the 1,3-butadiene compound in the extraction distillation step of the high-
또한, 본 발명은 1,3-부타디엔 제품의 제조공정 중 추출 증류 단계에서 추출 증류 컬럼에서 C4 혼합유분으로부터 1,3-부타디엔을 추출 증류함에 있어서, 4-터셔리-부틸카테콜(4-tert-butylcatechol, 이하 'TBC'로 칭함) 및 디에틸 히드록실아민(Diethyl hydroxy amine, 이하 'DEHA'로 칭함)을 유기용매와 혼합하여 조성된 일액형 중합방지제를 추출 증류탑에 투입하는 1,3-부타디엔의 중합방지방법을 제공한다. In addition, the present invention in the extraction distillation step of the manufacturing process of 1,3-butadiene product in the
이하, 본 발명을 상세하게 설명한다. EMBODIMENT OF THE INVENTION Hereinafter, this invention is demonstrated in detail.
본 발명은 4-터셔리-부틸카테콜(TBC), 디에틸 히드록실아민(DEHA) 및 유기용매로 이루어진 것을 특징으로 하는 1,3-부타디엔의 중합방지제에 관한 것이다. The present invention relates to a polymerization inhibitor of 1,3-butadiene, comprising 4-tertary-butylcatechol (TBC), diethyl hydroxylamine (DEHA), and an organic solvent.
상기와 같은 본 발명에 의한 중합방지제를 추출 증류 컬럼의 기체 및 액체가 공존하는 증류부에 투입시 증류 상부의 유체와 혼합되어 자유 라디칼, 산소와 같은 중합개시제를 효율적으로 제거하고, 이미 진전된 폴리머의 활성을 제거할 수 있도록 하기 위하여, 중합방지제의 혼합비율은 9.0 ~ 30.0중량%의 4-터셔리-부틸카테콜(TBC), 50.0 ~ 90.0중량%의 디에틸 히드록실아민(DEHA) 및 1.0 ~ 20.0중량%의 유기용매인 것이 바람직하다.When the polymerization inhibitor according to the present invention is introduced into the distillation unit where the gas and liquid of the extractive distillation column coexist, it is mixed with the fluid at the top of the distillation to efficiently remove the polymerization initiator such as free radicals and oxygen, and the polymer is already advanced. In order to be able to remove the activity of the polymerization inhibitor, the mixing ratio of the polymerization agent is 9.0 to 30.0% by weight of 4- tert-butylcatechol (TBC), 50.0 to 90.0% by weight of diethyl hydroxylamine (DEHA) and 1.0 It is preferable that it is an organic solvent of -20.0 weight%.
상기 유기용매는 중합방지 역할을 하는 4-터셔리-부틸카테콜(TBC) 및 디에틸 히드록실아민(DEHA)에 대한 우수한 용해도를 가지면서도, 최종 생성물인 1,3-부타디엔의 순도에 영향을 주지 않는 용매로부터 선택된다. 상기 유기용매는 바람직하게는 탄소수가 3 ~ 5개인 알콜류로부터 선택되고, 더욱 바람직하게는 하기 일반식 1과 같은 구조를 가지는 이소부틸 알콜(Isobutyl alcohol, 이하 'IBA'이라 칭함) 또는 하기 일반식 2와 같은 구조를 가지는 이소프로필 알콜(Isopropyl alcohol, 이하 'IPA'이라 칭함)로부터 선택된다. The organic solvent has an excellent solubility in 4-tertiary-butylcatechol (TBC) and diethyl hydroxylamine (DEHA), which acts as a polymerization inhibitor, and affects the purity of 1,3-butadiene as a final product. It is selected from solvents that do not give. The organic solvent is preferably selected from alcohols having 3 to 5 carbon atoms, and more preferably isobutyl alcohol (hereinafter referred to as 'IBA') having the same structure as the following
(일반식 1)(Formula 1)
(일반식 2)(Formula 2)
상기 이소부틸 알콜(IBA) 및 상기 이소프로필 알콜(IPA)은 4-터셔리-부틸카 테콜 및 디에틸 히드록실아민 성분을 모두 용해시킬 수 있으며 공정이나 제품에 영향이 없는 용매로서 사용된다.The isobutyl alcohol (IBA) and the isopropyl alcohol (IPA) can dissolve both 4-tertiary-butylcatechol and diethyl hydroxylamine components and are used as a solvent without affecting the process or the product.
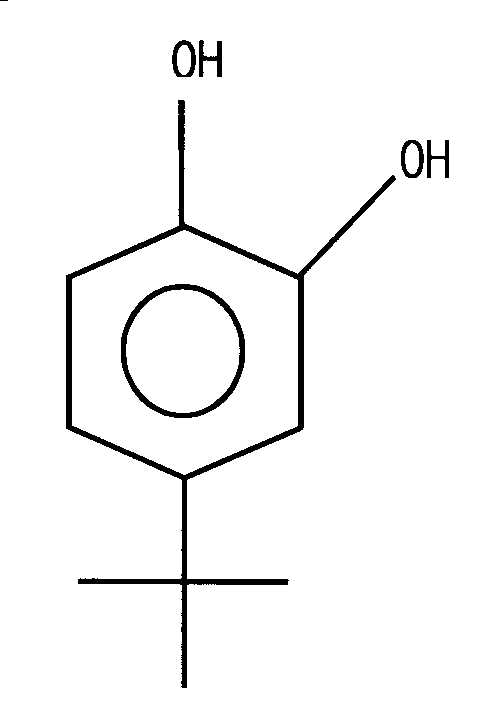
상기 4-터셔리-부틸카테콜(TBC)은 하기 일반식 3과 같은 구조를 가진다.The 4-tert-butylcatechol (TBC) has a structure as shown in the following general formula (3).
(일반식 3)(Formula 3)
상기 4-터셔리-부틸카테콜(TBC)의 분자식은 C6H3(OH)2C(CH3)
3 이고, 상태는 백색 내지 담황색의 고체로서 비점은 285℃, 융점은 53℃이며, 비중은 1,049/60℃이고, 점도는 36cps/60℃이다. 단독으로 액상 1,3-부타디엔 및 스티렌 제품의 중합방지제로서 많이 사용되는 것으로서 카테콜, 이소부틸렌, p-터셔리-부틸페놀, 염소, 가성소다를 원료로 하여 제조되는 바, 카테콜을 루이스산계 촉매의 존재 하에서 이소부틸렌으로 부틸화하여 TBC를 제조하는 카테콜법에 의하여, 혹은 p-t-부틸페놀(PTBP)을 염소화하여 2-클로로-4-터셔리-부틸페놀을 얻은 다음 가성소다를 사용하여 염소를 수산기로 치환하는 p-터셔리-부틸페놀(PTBP)법에 의하여 제조될 수 있다. The molecular formula of the 4-tert-butylcatechol (TBC) is C 6 H 3 (OH) 2 C (CH 3 ) 3 , the state is a white to pale yellow solid, the boiling point is 285 ℃, melting point is 53 ℃, Specific gravity is 1,049 / 60 ° C and viscosity is 36 cps / 60 ° C. It is widely used as a polymerization inhibitor of
4-터셔리-부틸카테콜(TBC)은 낮은 증기압을 가지므로 액체 환경의 공정부분에서 중합방지 효과를 잘 발휘한다. 4-tertiary-butylcatechol (TBC) has a low vapor pressure and thus exhibits a good anti-polymerization effect in the process part of the liquid environment.
상기 디에틸 히드록실아민(DEHA)은 하기 일반식 4와 같은 구조를 가진다. The diethyl hydroxylamine (DEHA) has the structure shown in the following general formula (4).
(일반식 4)(Formula 4)
상기 디에틸 히드록실아민(DEHA)의 분자식은 (C2H5)2NOH 이고, 상태는 액체이며, 보일러수 및 석유화학공정에서 탈산소제로 많이 사용된다. The molecular formula of the diethyl hydroxylamine (DEHA) is (C 2 H 5 ) 2 NOH, the state is a liquid, it is widely used as a deoxidant in boiler water and petrochemical processes.
디에틸 히드록실아민(DEHA)은 공정 부분내의 산소 제거가 가능하므로 기체 상태의 공정 부분에서 중합방지 효과를 발휘한다. Diethyl hydroxylamine (DEHA) is capable of removing oxygen in the process portion and thus exhibits an anti-polymerization effect in the gaseous process portion.
또한, 본 발명은 1,3-부타디엔의 제조시 추출 증류 단계 내에서 1,3-부타디엔의 폴리머 생성을 억제하여 중합을 방지하는 방법을 제공한다.The present invention also provides a method of preventing polymerization by inhibiting polymer production of 1,3-butadiene in the extraction distillation step in the preparation of 1,3-butadiene.
구체적으로, 본 발명은 기상 및 액상 환경에서 폴리머 생성을 방지하고, 나아가 이미 생성된 폴리머의 활성을 제거하는 1,3-부타디엔의 중합방지방법을 제공한다. 더욱 구체적으로, 본 발명은 추출 증류 컬럼에서 혼합 C4 유분으로부터 1,3-부타디엔을 추출 증류함에 있어서, 4-터셔리-부틸카테콜(TBC), 디에틸 히드록실아민(DEHA) 및 유기용매로 조성된 일액형 중합방지제를 상기 추출 증류 컬럼 내에 투입하는 것을 특징으로 하는 1,3-부타디엔의 중합방지방법을 제공한다. Specifically, the present invention provides a method for preventing the polymerization of 1,3-butadiene, which prevents the formation of polymers in gaseous and liquid environments and further removes the activity of polymers already produced. More specifically, the present invention is directed to extractive distillation of 1,3-butadiene from mixed C 4 fraction in an extractive distillation column, 4-tertiary-butylcatechol (TBC), diethyl hydroxylamine (DEHA) and organic solvents. It provides a method for preventing the polymerization of 1,3-butadiene, characterized in that the one-component polymerization inhibitor is added to the extraction distillation column.
상기 유기용매는 4-터셔리-부틸카테콜 및 디에틸 히드록실아민 성분을 모두 용해시킬 수 있으며 공정이나 제품에 영향이 없는 용매로서 탄소수가 3 ~ 5개인 알콜류가 사용된다. 특히, 상기 알콜류는 이소부틸 알콜(IBA) 또는 이소프로필 알콜(IPA)이 바람직하다. The organic solvent can dissolve both 4-tertiary-butylcatechol and diethyl hydroxylamine components, and alcohols having 3 to 5 carbon atoms are used as a solvent which does not affect a process or a product. In particular, the alcohols are preferably isobutyl alcohol (IBA) or isopropyl alcohol (IPA).
이하, 첨부한 도면을 통하여 본 발명을 더욱 상세하게 설명한다.Hereinafter, the present invention will be described in more detail with reference to the accompanying drawings.
도 1은 본 발명의 중합방지제가 주입되는 공정을 포함한 1,3-부타디엔 생산을 위한 추출 증류 공정도이다. 도 1과 함께 본 발명에 의한 1,3-부타디엔 제조공정 중 1,3-부타디엔의 중합방지방법을 상세히 설명하면 다음과 같다. 1 is an extractive distillation process diagram for the production of 1,3-butadiene including a process in which the polymerization inhibitor of the present invention is injected. Referring to Figure 1 in detail with respect to the polymerization prevention method of 1,3-butadiene in the 1,3-butadiene manufacturing process according to the present invention.
도 1에서 보듯이, 추출 증류 컬럼(1)은 61단의 트레이와 2단의 침니 트레이(1-a 및 1-b) 형태로 되어 있다. 상기 추출 증류 컬럼(1)에 투입되는 혼합 C4 유분은 기체상으로써 1,3-부타디엔(83 ~ 84중량%), 비닐 아세틸렌(Vinyl Actylene), 에틸 아세틸렌(Ethyl acetylene), 1,2-부타디엔(1,2-Butadiene), 메틸 아세틸렌(Methyl acetylene) 및 C5 유분으로 구성되며, 혼합 C4 유분 투입처(2)를 통해 추출 증류 컬럼의 제 1 침니 트레이(1-a) 내로 27 ~ 28톤/시간으로 투입된다. 또한, 추출 용매인 디메틸포름아마이드(Dimethylformamide)는 추출 증류 컬럼(1)의 11단 트레이에 형성된 디메틸포름아마이드 투입처(3) 내로 45 ~ 47톤/시간으로 투입된다. As shown in Fig. 1, the
본 발명에 의한 중합방지제는 중합방지제 저장 탱크(4)로부터 중합방지제 주입 펌프(5)에 의해 이송되어 추출 증류 컬럼(1)의 상부 이송관에 투입 및/또는 디메틸포름아마이드 주입관에 투입되어 추출 용매인 디메틸포름아마이드와 함께 디메틸포름아마이드 투입처(3)를 통해 추출 증류 컬럼(1)내로 투입된다. The polymerization inhibitor according to the present invention is transferred from the polymerization storage tank (4) by the polymerization injection pump (5) and introduced into the upper transfer pipe of the extraction distillation column (1) and / or into the dimethylformamide injection pipe. The dimethylformamide is introduced into the
추출 증류 컬럼(1)에 투입된 상기 혼합 C4 유분 중 추출 용매인 디메틸포름아마이드에 용해도가 낮은 1,3-부타디엔은 1차 정제되어 추출 증류 컬럼(1) 상부로 이동한다. 추출 증류 컬럼(1) 상부에서의 1,3-부타디엔의 농도는 95 ~ 98%이다. 또 추출 증류 컬럼(1) 상부의 온도와 압력은 각각 36 ~ 38.5℃, 3.10 ~ 3.15㎏/㎠G이다. 1,3-butadiene having low solubility in dimethylformamide as an extraction solvent in the mixed C 4 fraction added to the extractive distillation column (1) is first purified and moved to the upper portion of the extractive distillation column (1). The concentration of 1,3-butadiene at the top of the
반면, 혼합 C4 유분 중 1,3-부타디엔과 비점이 비슷하면서도 추출 용매(디메틸포름아마이드)에 대한 용해성이 좋은 비닐 아세틸렌, 에틸 아세틸렌, 1,2-부타디엔, 메틸 아세틸렌 및 C5 유분은 상기 디메틸포름아마이드에 용해되어 추출 증류 컬럼(1) 하부로 이동한다. 추출 증류 컬럼(1) 하부에서의 1,3-부타디엔의 농도는 5.3 ~ 5.5%이다. 또 추출 증류 컬럼(1) 하부의 온도와 압력은 130 ~ 130℃, 3.9 ~ 4.0㎏/㎠G 이다. On the other hand, vinyl acetylene, ethyl acetylene, 1,2-butadiene, methyl acetylene and C 5 fractions having similar boiling points to 1,3-butadiene in the mixed C 4 fraction and having good solubility in the extraction solvent (dimethylformamide) are the dimethyl It is dissolved in formamide and moved to the bottom of the extractive distillation column (1). The concentration of 1,3-butadiene in the bottom of the
추출 증류 컬럼(1) 상부에서 나온 정제된 C4 유분은 응축기(6)에서 액체로 상변화가 되며, 응축드럼(7)에서 일시 정지한다. 이어서 4ℓ 용량의 환류 펌프 스트레이너(8)로 이동하여 정제된 C4 유분 중에 생성된 1,3-부타디엔 중합물(파울링 물질)이 제거된다. 이어서 환류 펌프(9)에 의해 일부의 정제 C4 유분이 추출 증류 컬럼(1)내로 재투입되어 추출 증류 컬럼(1) 상부에 존재하는 혼합 C4 유분 중 1,3-부타디엔의 농도를 95 ~ 98%로 제어하고, 한편 대부분의 정제 C4 유분은 후속의 1,3-부타디엔 정제 공정(가)으로 이송된다.The purified C 4 fraction from the top of the extractive distillation column (1) is phase-changed to liquid in the condenser (6) and paused in the condensation drum (7). It is then transferred to a 4 L reflux pump strainer 8 to remove the 1,3-butadiene polymer (fouling material) generated in the purified C 4 fraction. Subsequently, a portion of the purified C 4 fraction is re-introduced into the extractive distillation column (1) by the reflux pump (9) to increase the concentration of 1,3-butadiene in the mixed C 4 fraction present on the upper portion of the extractive distillation column (1). 98%, while most of the purified C 4 fraction is passed to the subsequent 1,3-butadiene purification process.
한편, 추출 증류 컬럼(1) 하부에서 나온 디메틸포름아마이드, 1,3-부타디엔, 비닐 아세틸렌, 에틸 아세틸렌, 1,2-부타디엔, 메틸 아세틸렌 및 및 C5 유분 등의 혼합물은 탑저 스트레이너(10)로 이동하여 1,3-부타디엔 중합물(파울링 물질)이 제거된다. 이어서 추출 용매와 혼합 C4 유분의 혼합물 중 잔류한 5.3 ~ 5.5%의 1,3-부타디엔을 회수하기 위하여 후속의 1,3-부타디엔 회수 공정(나)으로 압력에 의해 이송된다. Meanwhile, a mixture of dimethylformamide, 1,3-butadiene, vinyl acetylene, ethyl acetylene, 1,2-butadiene, methyl acetylene, and C 5 fractions from the bottom of the extractive distillation column (1) is transferred to the bottom strainer (10). Migrate to remove 1,3-butadiene polymer (fouling material). It is then sent by pressure to the subsequent 1,3-butadiene recovery process (b) to recover 5.3-5.5% of 1,3-butadiene remaining in the mixture of the extraction solvent and the mixed C 4 fraction.
추출 증류 컬럼(1)의 내부 상태는 평상시 확인할 수 없으므로, 평상 운전시 개방이 가능한 환류 펌프 스트레이너(8) 및 컬럼 하부 스트레이너(10)에서 생성되는 폴리머의 상태 및 축적량이 추출 증류 컬럼의 1,3-부타디엔의 중합 및 파울링 정도를 나타내는 척도가 된다. Since the internal condition of the extractive distillation column (1) cannot be normally checked, the state and accumulation amount of the polymer produced in the reflux pump strainer (8) and the column lower strainer (10), which can be opened during normal operation, are 1,3 It is a measure of the degree of polymerization and fouling of butadiene.
만약, 1,3-부타디엔의 중합방지를 위해 적절한 조치를 취하지 않을 경우, 추출 증류 컬럼(1) 상부 또는 하부에 1,3-부타디엔의 중합으로 인해 발생한 수지형 또는 고무형의 폴리머(파울링 물질)가 추출 증류 컬럼(1)을 봉쇄함으로써 추출 증류 컬럼(1)의 폭발 또는 가동 정지, 심지어 연계 공장 가동을 정지시키게 된다. If no proper measures are taken to prevent the polymerization of 1,3-butadiene, resin or rubbery polymers (fouling materials) caused by polymerization of 1,3-butadiene on or below the extraction distillation column (1) ) Block the
본 발명의 중합방지제를 공급 수단에 의하여 추출 증류 컬럼(1)의 상부에 투입시 증류 상부의 유체(기체 및 액체)와 바람직한 조성비로 혼합되어야 추출 증류 컬럼(1) 상부에 존재하는 자유 라디칼, 산소와 같은 중합 개시제 및 진전된 폴리머의 활성을 더욱 효율적으로 제거하고, 추출 증류 컬럼(1) 하부에서 발생되는 1,3-부타디엔의 중합 및 진전된 폴리머의 활성을 제거할 수 있다. 따라서 본 발명 에 따른 1,3-부타디엔의 중합방지방법에 있어서, 혼합비율이 9.0 ~ 30.0중량%의 4-터셔리-부틸카테콜(TBC), 50.0 ~ 90.0중량%의 디에틸 히드록실아민(DEHA) 및 1.0 ~ 20.0중량%의 유기용매를 혼합한 일액형 중합방지제를 추출 증류 컬럼(1)에 투입하는 것이 바람직하다. When the polymerization inhibitor of the present invention is introduced into the upper portion of the extractive distillation column (1) by a supply means, the free radicals and oxygen present on the upper portion of the extractive distillation column (1) must be mixed with the fluid (gas and liquid) of the upper portion of the distillation at a desired composition ratio. It is possible to more efficiently remove the activity of the polymerization initiator and the advanced polymer such as, and to remove the activity of the polymerized and advanced polymer of 1,3-butadiene generated under the extraction distillation column (1). Therefore, in the method for preventing the polymerization of 1,3-butadiene according to the present invention, the mixing ratio is 9.0 to 30.0% by weight of 4- tert-butylcatechol (TBC), 50.0 to 90.0% by weight of diethyl hydroxylamine ( It is preferable to add a one-component polymerization inhibitor in which DEHA) and 1.0 to 20.0% by weight of an organic solvent are mixed into the extractive distillation column (1).
이하, 실시예를 통하여 본 발명을 더욱 구체적으로 살펴본다. 그러나 이하의 실시예는 단지 본 발명의 구체적 구현예로서 예시적인 것을 뿐이므로 본 발명의 범위를 국한시키는 것으로 이해되어서는 안 될 것이다.Hereinafter, the present invention will be described in more detail with reference to Examples. However, the following examples are merely illustrative as specific embodiments of the present invention and should not be understood as limiting the scope of the present invention.
실시예 1Example 1
15중량%의 4-터셔리-부틸카테콜(TBC), 75중량%의 디에틸 히드록실아민(DEHA) 및 10중량%의 이소부틸 알콜(IBA)을 혼합하여 이루어진 중합방지제 30ppm(혼합 C4 투입양 대비)을 중합방지제 주입 펌프를 통해 기체와 액체가 공존하는 추출 증류 컬럼의 상부로 투입하고, 축적된 폴리머의 상태 및 축적량을 주기적으로 시험하여 그 결과를 표 1에 나타내었다. 30 ppm of polymerization inhibitor (mixed C 4 ), made by mixing 15 wt% 4-tertary-butylcatechol (TBC), 75 wt% diethyl hydroxylamine (DEHA) and 10 wt% isobutyl alcohol (IBA) To the top of the extractive distillation column in which gas and liquid coexist through an anti-polymerization injection pump, and the condition and accumulation amount of the accumulated polymer are periodically tested and the results are shown in Table 1.
실시예 2Example 2
중합방지제의 조성 및 혼합비율이 15중량%의 4-터셔리-부틸카테콜(TBC), 75중량%의 디에틸 히드록실아민(DEHA) 및 10중량%의 이소프로필 알콜(IPA)인 것을 제외하고는, 실시예 1과 같은 방법으로 하여 폴리머의 상태 및 축적량을 주기적으로 시험하여 그 결과를 표 1에 나타내었다.The composition and mixing ratio of the polymerization inhibitor were 15% by weight 4-tertary-butylcatechol (TBC), 75% by weight diethyl hydroxylamine (DEHA) and 10% by weight isopropyl alcohol (IPA). In the same manner as in Example 1, the state and accumulation amount of the polymer were periodically tested, and the results are shown in Table 1.
비교 실시예 1Comparative Example 1
중합방지제를 사용하지 않는 것을 제외하고는, 실시예 1과 같은 방법으로 하여 폴리머의 상태 및 축적량을 주기적으로 시험하여 그 결과를 표 1에 나타내었다.Except not using a polymerization inhibitor, the state and accumulation amount of the polymer were periodically tested in the same manner as in Example 1, and the results are shown in Table 1.
비교 실시예 2Comparative Example 2
TBC 단독의 중합방지제 30ppm(혼합 C4 투입양 대비)을 중합방지제 주입 펌프를 통해 추출 증류탑의 상부로 주입하는 것을 제외하고는, 실시예 1과 같은 방법으로 하여 폴리머의 상태 및 축적량을 주기적으로 시험하여 그 결과를 표 1에 나타내었다. Periodically test the condition and accumulation of the polymer in the same manner as in Example 1, except that 30 ppm of the TBC-only polymerization inhibitor (compared to the mixed C 4 input amount) was injected into the top of the extraction distillation column through the polymerization injection pump. The results are shown in Table 1.
비교 실시예 3Comparative Example 3
DEHA 단독의 중합방지제 30ppm(혼합 C4 투입양 대비)을 중합방지제 주입 펌프를 통해 추출 증류탑의 상부로 투입하는 것을 제외하고는, 실시예 1과 같은 방법으로 하여 폴리머의 상태 및 축적량을 주기적으로 시험하여 그 결과를 표 1에 나타내었다.Periodically test the condition and accumulation of the polymer in the same manner as in Example 1, except that 30 ppm of the polymerization inhibitor of DEHA alone (compared to the amount of mixed C 4 ) was introduced to the top of the extraction distillation column through the polymerization injection pump. The results are shown in Table 1.
(평가 결과)(Evaluation results)
1,3-부타디엔의 추출 증류 공정에서 1,3-부타디엔의 중합반응 진행정도에 따라 폴리머(파울링 물질)의 상태는 분자량이 작은 유동성 고무형, 분자량이 중간 정도인 고무형, 분자량이 큰 수지형으로 나타난다. 생성된 폴리머(파울링 물질)의 분 자량이 작을수록 1,3-부타디엔의 중합정도가 낮은 것으로 평가된다. According to the progress of the polymerization reaction of 1,3-butadiene in the extraction distillation process of 1,3-butadiene, the state of the polymer (fouling material) is a flowable rubber type having a low molecular weight, a rubber type having a medium molecular weight, and a large molecular weight. Appear as terrain The smaller the molecular weight of the resulting polymer (fouling material), the lower the degree of polymerization of 1,3-butadiene.
비교 실시예 1에서와 같이 중합방지제를 투입하지 않은 경우, 빠른 속도의 수지형 폴리머의 생성으로 2개월 이후 추출 증류 컬럼의 압력 상승으로 인하여 공장의 비상 가동 정지 상황이 발생하였다.When the polymerization inhibitor was not added as in Comparative Example 1, an emergency shutdown situation of the plant occurred due to the increase in pressure of the extractive distillation column after two months due to the production of a high-speed resin type polymer.
비교 실시예 2에서와 같이 4-터셔리-부틸카테콜(TBC)만을 단독으로 투입한 경우, 고무형 폴리머의 생성으로 17개월 이후 추출 증류 컬럼의 압력 상승이 발생하였으며, 1,3-부타디엔 공장의 안전 운전을 위하여 비상 가동 정지하였다.When only 4-tertiary-butylcatechol (TBC) was added alone as in Comparative Example 2, the pressure rise of the extractive distillation column occurred after 17 months due to the production of a rubber polymer, and the 1,3-butadiene plant Emergency stop for safe operation.
또한 비교 실시예 3에서와 같이 디에틸 히드록실아민(DEHA)만을 단독으로 투입한 경우, 수지형 폴리머 생성으로 11개월 이후 추출 증류 컬럼의 압력 상승이 발생하여 공장의 비상 가동 정지 상황이 발생하였다.In addition, when only diethyl hydroxylamine (DEHA) alone was added as in Comparative Example 3, a pressure rise of the extractive distillation column occurred after 11 months due to the production of a resin polymer, resulting in an emergency shutdown of the plant.
한편, 실시예 1 ~ 2에서와 같이 4-터셔리-부틸카테콜(TBC), 디에틸 히드록실아민(DEHA) 및 이소프로필 알콜(IPA) 또는 이소부틸 알콜(IBA)과 같은 알콜류 유기용매로 이루어진 본 발명에 의한 중합방지제를 투입한 경우, 비교 실시예 2 및 3에서와 같은 종래의 단일 성분의 중합방지제를 사용한 경우에 비해 액상 및 기상 환경에서 동시에 탁월한 1,3-부타디엔의 중합방지효과를 나타내어, 3년 후 정기 보수를 위해 가동을 정지할 때까지 추출 증류 컬럼의 안정적 연속운전이 가능하였다.On the other hand, as in Examples 1 and 2 with an organic solvent such as 4-tertary-butylcatechol (TBC), diethyl hydroxylamine (DEHA) and isopropyl alcohol (IPA) or isobutyl alcohol (IBA) When the polymerization inhibitor according to the present invention is added, the polymerization prevention effect of 1,3-butadiene at the same time is excellent in the liquid and gaseous environment compared to the case of using the conventional single component polymerization inhibitor as in Comparative Examples 2 and 3. After three years, stable continuous operation of the extractive distillation column was possible until the operation was stopped for regular maintenance.
상기와 같이 우수한 중합반응 억제 및 파울링 방지 효과를 갖는 본 발명에 의한 중합방지제는 혼합 C4 유분 중 각종 고무 및 합성수지 원료가 되는 1,3-부타디엔을 분리 생산하기 위한 제조공정 중에 액상 및 기상의 광범위한 온도 범위에서 1,3-부타디엔의 중합을 억제함으로써 고순도의 1,3-부타디엔 제품을 추출 증류할 수 있게 한다. As described above, the polymerization inhibitor according to the present invention having the excellent polymerization inhibition and antifouling effect is a liquid and gaseous phase during the production process for separating and producing 1,3-butadiene, which is a raw material of various rubbers and synthetic resins in mixed C 4 fractions. By inhibiting the polymerization of 1,3-butadiene over a wide temperature range,
또한, 본 발명에 의한 중합 방지방법은 파울링으로 인해 야기될 수 있는 1,3-부타디엔 제조 공장의 비상시 가동정지를 미연에 방지하고, 장기간 안전운전을 가능하게 하는 장점을 가진다. In addition, the polymerization prevention method according to the present invention has the advantage of preventing the emergency stop of the 1,3-butadiene production plant that may be caused by fouling in advance, and enables a safe operation for a long time.
Claims (8)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020050004752A KR100676996B1 (en) | 2005-01-18 | 2005-01-18 | Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof |
| BRPI0606353-5A BRPI0606353A2 (en) | 2005-01-18 | 2006-01-18 | 1,3-butadiene polymerization inhibitor and method for inhibiting 1,3-butadiene polymerization by introducing it |
| PCT/KR2006/000205 WO2006078123A1 (en) | 2005-01-18 | 2006-01-18 | Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof |
| JP2007551205A JP4824038B2 (en) | 2005-01-18 | 2006-01-18 | 1,3-butadiene polymerization inhibitor and 1,3-butadiene polymerization prevention method using the same |
| CN2006800026306A CN101107274B (en) | 2005-01-18 | 2006-01-18 | Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020050004752A KR100676996B1 (en) | 2005-01-18 | 2005-01-18 | Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20060083809A KR20060083809A (en) | 2006-07-21 |
| KR100676996B1 true KR100676996B1 (en) | 2007-02-01 |
Family
ID=36692466
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020050004752A KR100676996B1 (en) | 2005-01-18 | 2005-01-18 | Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP4824038B2 (en) |
| KR (1) | KR100676996B1 (en) |
| CN (1) | CN101107274B (en) |
| BR (1) | BRPI0606353A2 (en) |
| WO (1) | WO2006078123A1 (en) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101591216B (en) * | 2009-07-10 | 2012-07-04 | 北京斯伯乐科学技术研究院 | High-efficiency multi-functional polymerization inhibitor for butadiene extraction device and using method thereof |
| JP5780072B2 (en) * | 2010-09-10 | 2015-09-16 | 三菱化学株式会社 | Method for producing conjugated diene |
| MX2014009060A (en) * | 2012-04-02 | 2014-09-25 | Borealis Ag | Ethylene polymerization process using an inhibitor. |
| FR3010717B1 (en) * | 2013-09-19 | 2015-11-06 | Rhodia Operations | COMPOSITION PREVENTING THE POLYMERIZATION OF ETHYLENE-UNATURATED MONOMERS AND ITS DISPOSAL BEFORE POLYMERIZATION |
| TWI686380B (en) | 2014-10-14 | 2020-03-01 | 美商藝康美國公司 | Reducing polymer fouling and agglomeration in acrylate/methacrylate processes |
| EP3026101A1 (en) | 2014-11-26 | 2016-06-01 | Borealis AG | Wash oil for use as an antifouling agent in gas compressors |
| CN107406388B (en) | 2015-03-18 | 2021-06-18 | 艺康美国股份有限公司 | Inhibition of polymerization of vinyl monomers using stable lipophilic hydroxylamine compounds |
| US9957209B2 (en) | 2015-03-31 | 2018-05-01 | Ecolab Usa Inc. | Use of quinone methides as antipolymerants for vinylic monomers |
| US10155705B2 (en) | 2015-04-20 | 2018-12-18 | Ecolab Usa Inc. | Sterically hindered hydroquinones as antifoulants for unsaturated monomers |
| JP6145903B1 (en) | 2016-03-11 | 2017-06-14 | 株式会社片山化学工業研究所 | Antifouling methods in petrochemical processes. |
| EP4003943B1 (en) * | 2019-07-30 | 2024-05-01 | Dow Global Technologies LLC | Methods for reducing fouling in upgrading reactors |
| CN115505055B (en) * | 2021-06-07 | 2024-03-19 | 中韩(武汉)石油化工有限公司 | Method for reducing black polymer generation in carbon nine product |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20040058112A (en) * | 2001-04-30 | 2004-07-03 | 베이커 휴지스 인코포레이티드 | Inhibition of popcorn polymer growth |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2989087B2 (en) * | 1993-04-16 | 1999-12-13 | 電気化学工業株式会社 | Method for producing chloroprene polymer |
| JP2804978B2 (en) * | 1995-03-16 | 1998-09-30 | 三洋化成工業株式会社 | Modifier for polymer, method for producing the same, and method for modifying polymer using the same |
| CN1210237C (en) * | 1998-03-03 | 2005-07-13 | 日本瑞翁公司 | Polymerization-inhibiting composition polymerization inhibitor and method for inhibiting polymerization |
| US6723255B2 (en) * | 2000-03-07 | 2004-04-20 | Atofina Chemicals, Inc. | Compositions for shortstopping free radical emulsion polymerizations and stabilizing latices made therefrom |
| WO2003035588A1 (en) * | 2001-10-19 | 2003-05-01 | Zeon Corporation | Process and apparatus for separation and purification of conjugated diene |
| CN100348561C (en) * | 2004-05-20 | 2007-11-14 | 中国石化上海石油化工股份有限公司 | Process for preventing self-polymerization or co-polymerization of C5 diolefins in separation process of petroleum C5 distillate |
| CN100503527C (en) * | 2004-05-20 | 2009-06-24 | 中国石化上海石油化工股份有限公司 | Polymerization inhibitor for preventing self-polymerization or co-polymerization of C5 diolefins |
-
2005
- 2005-01-18 KR KR1020050004752A patent/KR100676996B1/en active IP Right Grant
-
2006
- 2006-01-18 CN CN2006800026306A patent/CN101107274B/en not_active Expired - Fee Related
- 2006-01-18 JP JP2007551205A patent/JP4824038B2/en not_active Expired - Fee Related
- 2006-01-18 WO PCT/KR2006/000205 patent/WO2006078123A1/en active Application Filing
- 2006-01-18 BR BRPI0606353-5A patent/BRPI0606353A2/en not_active IP Right Cessation
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20040058112A (en) * | 2001-04-30 | 2004-07-03 | 베이커 휴지스 인코포레이티드 | Inhibition of popcorn polymer growth |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101107274A (en) | 2008-01-16 |
| KR20060083809A (en) | 2006-07-21 |
| BRPI0606353A2 (en) | 2009-11-17 |
| CN101107274B (en) | 2012-05-23 |
| JP2008527135A (en) | 2008-07-24 |
| WO2006078123A1 (en) | 2006-07-27 |
| JP4824038B2 (en) | 2011-11-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100676996B1 (en) | Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof | |
| US4061545A (en) | Polymerization inhibitor for vinyl aromatic compounds | |
| US4654451A (en) | Inhibiting polymerization of vinyl aromatic monomers | |
| KR20170138455A (en) | Hindered hydroquinone as an antifouling agent for unsaturated monomers | |
| US6686422B2 (en) | Inhibition of popcorn polymer growth | |
| US4434307A (en) | Inhibiting polymerization of vinyl aromatic monomers | |
| EP1837322B1 (en) | A method for stabilizing vinyl aromatic monomers using selected polymerization inhibitors and polymers prepared therewith | |
| KR19980080447A (en) | Method of suppressing polymerization reaction | |
| US4654450A (en) | Inhibiting polymerization of vinyl aromatic monomers | |
| US4575455A (en) | Process for removing hydrogen sulfide with reduced fouling | |
| US3793187A (en) | Process for removing carbonyl compounds from hydrocarbons | |
| CN112513005A (en) | Amine oxide and methylated quinone combination as an antifoulant for ethylene monomers | |
| EP1383722B1 (en) | Inhibition of popcorn polymer growth | |
| US4132602A (en) | Polymerization inhibitor for vinyl aromatic compounds | |
| KR20080007690A (en) | Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene | |
| CN113956127A (en) | Polymerization inhibitor with high raw material adaptability for extracting and separating carbon five by DMF (dimethyl formamide) method and application thereof | |
| US3526673A (en) | Inhibiting popcorn polymer formation with tertiary amino dialkyl phenol compound | |
| CN109422619B (en) | Method for decoloring crude styrene separated from pyrolysis gasoline | |
| CN113166020A (en) | Hydroxylated quinone anti-polymerization agents and methods of use thereof | |
| EP2995601B1 (en) | Method of inhibiting polymerization of aromatic vinyl compound | |
| US3520943A (en) | Inhibiting popcorn polymer formation with hydroxy benzene tertiary amine oxide compound | |
| JP4227099B2 (en) | Bubble reduction method in primary fractionator | |
| JP2006182718A (en) | Polymerization inhibiting method of copolymer comprising divinylbenzene and aromatic vinyl compound | |
| EP2912109B1 (en) | Quinone compounds for inhibiting monomer polymerization | |
| TW201843130A (en) | Composition for control and inhibition of polymerization of monomers, and method of use and preparation thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant | ||
| FPAY | Annual fee payment |
Payment date: 20130118 Year of fee payment: 7 |
|
| FPAY | Annual fee payment |
Payment date: 20140110 Year of fee payment: 8 |
|
| FPAY | Annual fee payment |
Payment date: 20150119 Year of fee payment: 9 |
|
| FPAY | Annual fee payment |
Payment date: 20151218 Year of fee payment: 10 |
|
| FPAY | Annual fee payment |
Payment date: 20170109 Year of fee payment: 11 |