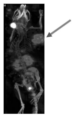
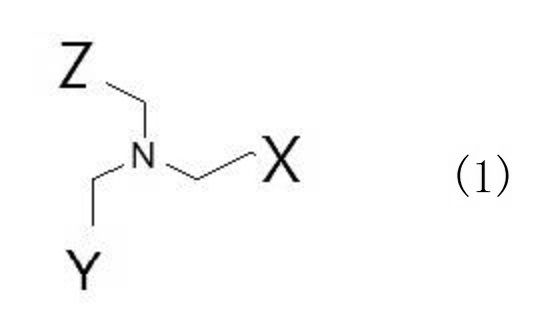
JP7239662B2 - 複合体、造影剤、及びcxcr4関連疾患を治療又は診断するための医薬品 - Google Patents
複合体、造影剤、及びcxcr4関連疾患を治療又は診断するための医薬品 Download PDFInfo
- Publication number
- JP7239662B2 JP7239662B2 JP2021164751A JP2021164751A JP7239662B2 JP 7239662 B2 JP7239662 B2 JP 7239662B2 JP 2021164751 A JP2021164751 A JP 2021164751A JP 2021164751 A JP2021164751 A JP 2021164751A JP 7239662 B2 JP7239662 B2 JP 7239662B2
- Authority
- JP
- Japan
- Prior art keywords
- cancer
- cxcr4
- metal
- diagnosing
- complex according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/041—Heterocyclic compounds
- A61K51/044—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins
- A61K51/0455—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/0497—Organic compounds conjugates with a carrier being an organic compounds
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G15/00—Compounds of gallium, indium or thallium
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nuclear Medicine (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Description
Claims (6)
- 請求項1に記載の複合体に、放射性金属核種又は非放射性金属が標識されていることを特徴とする、金属含有複合体。
- 前記放射性金属は、インジウム-111、ルテチウム-177、ガリウム-68、ガリウム-67、イットリウム-90、又は銅-64であることを特徴とする、請求項2に記載の金属含有複合体。
- 前記放射性金属は、インジウム-111であることを特徴とする、請求項3に記載の金属含有複合体。
- 請求項1に記載の複合体又は請求項2~4のいずれか一項に記載の金属含有複合体と、造影賦形剤と、を含むことを特徴とする、造影剤。
- 請求項1に記載の複合体又は請求項2~4のいずれか一項に記載の金属含有複合体を含む、CXCR4関連疾患を治療又は診断するための医薬品であって、前記CXCR4関連疾患は、悪性リンパ腫、多発性骨髄腫、精巣腫瘍、甲状腺がん、前立腺がん、咽頭がん、子宮頸がん、上咽頭がん、乳がん、大腸がん、膵臓がん、胃がん、頭頸部がん、食道がん、直腸がん、膀胱がん、腎臓がん、肺がん、肝臓がん、脳腫瘍、悪性黒色腫、及び皮膚がんからなる群から選ばれるがんであることを特徴とする、CXCR4関連疾患を治療又は診断するための医薬品。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TW109137179A TWI781469B (zh) | 2020-10-27 | 2020-10-27 | 複合物、造影劑及治療與cxcr4接受體相關疾病的用途 |
| TW109137179 | 2020-10-27 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2022070814A JP2022070814A (ja) | 2022-05-13 |
| JP7239662B2 true JP7239662B2 (ja) | 2023-03-14 |
Family
ID=78371780
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2021164751A Active JP7239662B2 (ja) | 2020-10-27 | 2021-10-06 | 複合体、造影剤、及びcxcr4関連疾患を治療又は診断するための医薬品 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US11951091B2 (ja) |
| EP (1) | EP3991756B1 (ja) |
| JP (1) | JP7239662B2 (ja) |
| TW (1) | TWI781469B (ja) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2024022291A (ja) | 2022-08-05 | 2024-02-16 | 日亜化学工業株式会社 | 半導体レーザ素子 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009504788A (ja) | 2005-08-19 | 2009-02-05 | ジェンザイム・コーポレーション | 化学療法の強化方法 |
| US20190031640A1 (en) | 2017-07-03 | 2019-01-31 | The Board Of Regents Of The University Of Texas System | Zinc sensors for in vivo imaging of beta-cell function by mri |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7208140B2 (en) * | 2003-02-19 | 2007-04-24 | Schering Aktiengesellschaft | Trimeric macrocyclic substituted benzene derivatives |
| EP3727381A4 (en) * | 2017-12-19 | 2022-01-19 | X4 Pharmaceuticals, Inc. | Acyclic cxcr4 inhibitors and uses thereof |
-
2020
- 2020-10-27 TW TW109137179A patent/TWI781469B/zh active
- 2020-12-18 US US17/126,364 patent/US11951091B2/en active Active
-
2021
- 2021-10-06 JP JP2021164751A patent/JP7239662B2/ja active Active
- 2021-10-08 EP EP21201791.7A patent/EP3991756B1/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009504788A (ja) | 2005-08-19 | 2009-02-05 | ジェンザイム・コーポレーション | 化学療法の強化方法 |
| US20190031640A1 (en) | 2017-07-03 | 2019-01-31 | The Board Of Regents Of The University Of Texas System | Zinc sensors for in vivo imaging of beta-cell function by mri |
Non-Patent Citations (1)
| Title |
|---|
| Bioorganic & Medicinal Chemistry,2019年,27(6),P.1130-1138 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20220125761A1 (en) | 2022-04-28 |
| JP2022070814A (ja) | 2022-05-13 |
| EP3991756A1 (en) | 2022-05-04 |
| TW202216216A (zh) | 2022-05-01 |
| EP3991756B1 (en) | 2025-10-29 |
| US11951091B2 (en) | 2024-04-09 |
| TWI781469B (zh) | 2022-10-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20250057993A1 (en) | Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of psma-expressing cancers | |
| JP7630149B2 (ja) | Psmaを標的としたイメージング及び放射線治療のための金属/放射性金属標識psma阻害剤 | |
| JP6661010B2 (ja) | ペプチドチオ尿素誘導体、これを含有する放射線同位体標識化合物、およびこれを活性成分として含有する前立腺癌を処置または診断するための医薬組成物 | |
| JP2022523727A (ja) | Psma結合デュアルモード放射性トレーサーおよび療法 | |
| JPH075527B2 (ja) | 二官能性dtpa型リガンド | |
| CN118267495A (zh) | 一种靶向HER2的rk多肽放射性药物及其制备方法 | |
| JP7239662B2 (ja) | 複合体、造影剤、及びcxcr4関連疾患を治療又は診断するための医薬品 | |
| WO2024193724A1 (zh) | 用于诊断或治疗表达前列腺特异性膜抗原癌症的新型标记靶向剂 | |
| JP7805170B2 (ja) | 前立腺特異的膜抗原(psma)リガンド及びその使用 | |
| Salouti et al. | Preparation and biological evaluation of 177Lu conjugated PR81 for radioimmunotherapy of breast cancer | |
| CN119874628B (zh) | 一种含氮化合物及其制备方法与用途 | |
| JP2005047821A (ja) | シクロペンタジエニルカルボニル基を含む放射性化合物を用いた診断及び治療用薬剤 | |
| JPH10307138A (ja) | 腫瘍診断剤および治療剤 | |
| JP2024533992A (ja) | ジホスフィン化合物および錯体 | |
| JP2024124308A (ja) | 放射性同位体標識診療薬剤 | |
| JP4339846B2 (ja) | 腫瘍治療剤 | |
| HK40120870A (en) | Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of psma-expressing cancers | |
| JPWO2011096374A1 (ja) | 新規ヨードベンジル−ブレオマイシン化合物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20211006 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20220908 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20221011 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20221221 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20230221 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20230302 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7239662 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |