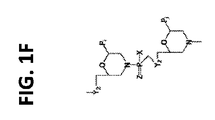
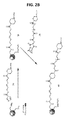
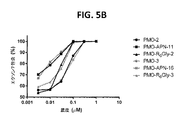
JP6987041B2 - 脊髄性筋萎縮症におけるエクソン包含のための修飾アンチセンスオリゴマー - Google Patents
脊髄性筋萎縮症におけるエクソン包含のための修飾アンチセンスオリゴマー Download PDFInfo
- Publication number
- JP6987041B2 JP6987041B2 JP2018510718A JP2018510718A JP6987041B2 JP 6987041 B2 JP6987041 B2 JP 6987041B2 JP 2018510718 A JP2018510718 A JP 2018510718A JP 2018510718 A JP2018510718 A JP 2018510718A JP 6987041 B2 JP6987041 B2 JP 6987041B2
- Authority
- JP
- Japan
- Prior art keywords
- formula
- alkyl
- cpp
- seq
- modified antisense
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 0 *CCOC(N(CC1)CCN1P(O*)=O)=O Chemical compound *CCOC(N(CC1)CCN1P(O*)=O)=O 0.000 description 37
- WNSVBRGSXQQTDL-UHFFFAOYSA-N IN1CCNCC1 Chemical compound IN1CCNCC1 WNSVBRGSXQQTDL-UHFFFAOYSA-N 0.000 description 2
- DKCHXFGPRHASQK-UHFFFAOYSA-N C=CN(CC1)CCC1N Chemical compound C=CN(CC1)CCC1N DKCHXFGPRHASQK-UHFFFAOYSA-N 0.000 description 1
- URFVWTZCSJOSSD-UHFFFAOYSA-N C=CN(CC1)CCC1N=C Chemical compound C=CN(CC1)CCC1N=C URFVWTZCSJOSSD-UHFFFAOYSA-N 0.000 description 1
- UFETTXCVHFVMPU-UHFFFAOYSA-N CCN(CC1)CCC1N Chemical compound CCN(CC1)CCC1N UFETTXCVHFVMPU-UHFFFAOYSA-N 0.000 description 1
- LPAIQFANJHTSPM-UHFFFAOYSA-N CCN(CC1)CCC1NCC=C(C)C Chemical compound CCN(CC1)CCC1NCC=C(C)C LPAIQFANJHTSPM-UHFFFAOYSA-N 0.000 description 1
- ALOCUZOKRULSAA-UHFFFAOYSA-N CN(CC1)CCC1N Chemical compound CN(CC1)CCC1N ALOCUZOKRULSAA-UHFFFAOYSA-N 0.000 description 1
- PVOAHINGSUIXLS-UHFFFAOYSA-N CN1CCNCC1 Chemical compound CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 1
- MPNITAZSUCHPAN-UHFFFAOYSA-N COCCOC(N(CC1)CCN1P(N)(OI)=O)=O Chemical compound COCCOC(N(CC1)CCN1P(N)(OI)=O)=O MPNITAZSUCHPAN-UHFFFAOYSA-N 0.000 description 1
- YHRLSHKBKMOFJW-UHFFFAOYSA-N C[IH]CCCCCCCC[U]C(N1CCC(CCC(CN(C2)C3(C4C3C4)C(CC3)CCC3N)[U]C2C=C)CC1)=[Cl+] Chemical compound C[IH]CCCCCCCC[U]C(N1CCC(CCC(CN(C2)C3(C4C3C4)C(CC3)CCC3N)[U]C2C=C)CC1)=[Cl+] YHRLSHKBKMOFJW-UHFFFAOYSA-N 0.000 description 1
- ZBRIUEJNNCNBPQ-UHFFFAOYSA-N ICN1CCNCC1 Chemical compound ICN1CCNCC1 ZBRIUEJNNCNBPQ-UHFFFAOYSA-N 0.000 description 1
- WCVZGRYRRAEJHV-UHFFFAOYSA-N NC(CC1)CCN1I Chemical compound NC(CC1)CCN1I WCVZGRYRRAEJHV-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/712—Nucleic acids or oligonucleotides having modified sugars, i.e. other than ribose or 2'-deoxyribose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7125—Nucleic acids or oligonucleotides having modified internucleoside linkage, i.e. other than 3'-5' phosphodiesters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/314—Phosphoramidates
- C12N2310/3145—Phosphoramidates with the nitrogen in 3' or 5'-position
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/323—Chemical structure of the sugar modified ring structure
- C12N2310/3233—Morpholino-type ring
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biotechnology (AREA)
- Neurology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Zoology (AREA)
- Epidemiology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Neurosurgery (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Psychology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562211678P | 2015-08-28 | 2015-08-28 | |
| US62/211,678 | 2015-08-28 | ||
| US201662379696P | 2016-08-25 | 2016-08-25 | |
| US62/379,696 | 2016-08-25 | ||
| PCT/US2016/048965 WO2017040271A1 (en) | 2015-08-28 | 2016-08-26 | Modified antisense oligomers for exon inclusion in spinal muscular atrophy |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020216610A Division JP2021048877A (ja) | 2015-08-28 | 2020-12-25 | 脊髄性筋萎縮症におけるエクソン包含のための修飾アンチセンスオリゴマー |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018525015A JP2018525015A (ja) | 2018-09-06 |
| JP2018525015A5 JP2018525015A5 (OSRAM) | 2019-10-03 |
| JP6987041B2 true JP6987041B2 (ja) | 2021-12-22 |
Family
ID=56883867
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018510718A Active JP6987041B2 (ja) | 2015-08-28 | 2016-08-26 | 脊髄性筋萎縮症におけるエクソン包含のための修飾アンチセンスオリゴマー |
| JP2020216610A Withdrawn JP2021048877A (ja) | 2015-08-28 | 2020-12-25 | 脊髄性筋萎縮症におけるエクソン包含のための修飾アンチセンスオリゴマー |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020216610A Withdrawn JP2021048877A (ja) | 2015-08-28 | 2020-12-25 | 脊髄性筋萎縮症におけるエクソン包含のための修飾アンチセンスオリゴマー |
Country Status (8)
| Country | Link |
|---|---|
| US (3) | US10905709B2 (OSRAM) |
| EP (1) | EP3341480A1 (OSRAM) |
| JP (2) | JP6987041B2 (OSRAM) |
| AU (1) | AU2016317667A1 (OSRAM) |
| CA (1) | CA2996164A1 (OSRAM) |
| HK (1) | HK1257498A1 (OSRAM) |
| MA (1) | MA42695A (OSRAM) |
| WO (1) | WO2017040271A1 (OSRAM) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016040748A1 (en) | 2014-09-12 | 2016-03-17 | Ionis Pharmaceuticals, Inc. | Compositions and methods for detection of smn protein in a subject and treatment of a subject |
| WO2017040271A1 (en) | 2015-08-28 | 2017-03-09 | Sarepta Therapeutics, Inc. | Modified antisense oligomers for exon inclusion in spinal muscular atrophy |
| JP7394753B2 (ja) * | 2017-10-18 | 2023-12-08 | サレプタ セラピューティクス, インコーポレイテッド | アンチセンスオリゴマー化合物 |
| US12215382B2 (en) | 2019-03-01 | 2025-02-04 | The General Hospital Corporation | Liver protective MARC variants and uses thereof |
| AU2020368539A1 (en) | 2019-10-16 | 2022-04-28 | Massachusetts Institute Of Technology | Engineered muscle targeting compositions |
| BR112022016238A2 (pt) | 2020-02-28 | 2022-10-11 | Ionis Pharmaceuticals Inc | Compostos e métodos para modular smn2 |
Family Cites Families (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2802206B1 (fr) | 1999-12-14 | 2005-04-22 | Sod Conseils Rech Applic | Derives de 4-aminopiperidine et leur utilisation en tant que medicament |
| KR20020097241A (ko) | 2000-05-04 | 2002-12-31 | 에이브이아이 바이오파마 인코포레이티드 | 스플라이스-영역 안티센스 조성물 및 방법 |
| DK1910395T3 (da) * | 2005-06-23 | 2013-02-04 | Isis Pharmaceuticals Inc | Sammensætninger og fremgangsmåder til modulation af SMN2-splejsning |
| US8067571B2 (en) | 2005-07-13 | 2011-11-29 | Avi Biopharma, Inc. | Antibacterial antisense oligonucleotide and method |
| US7943762B2 (en) | 2006-05-10 | 2011-05-17 | Avi Biopharma, Inc. | Oligonucleotide analogs having cationic intersubunit linkages |
| ES2373246T3 (es) | 2006-08-11 | 2012-02-01 | Prosensa Technologies B.V. | Oligonucleótidos monocatenarios complementarios de elementos repetitivos para el tratamiento de trastornos genéticos asociados a la inestabilidad de repeticiones de adn. |
| US20100016215A1 (en) | 2007-06-29 | 2010-01-21 | Avi Biopharma, Inc. | Compound and method for treating myotonic dystrophy |
| WO2010120820A1 (en) * | 2009-04-13 | 2010-10-21 | Isis Pharmaceuticals, Inc. | Compositions and methods for modulation of smn2 splicing |
| SI3305302T1 (sl) * | 2009-06-17 | 2018-12-31 | Biogen Ma Inc. | Sestave in metode za modulacijo združevanja smn2 pri subjektu |
| US8802642B2 (en) | 2010-04-28 | 2014-08-12 | Iowa State University Research Foundation, Inc. | Spinal muscular atrophy treatment via targeting SMN2 catalytic core |
| JP5585822B2 (ja) | 2010-05-11 | 2014-09-10 | 東レ・ファインケミカル株式会社 | 光学活性ニペコチン酸誘導体の製造方法 |
| CA2799501C (en) | 2010-05-28 | 2022-02-15 | Sarepta Therapeutics, Inc. | Oligonucleotide analogues having modified intersubunit linkages and/or terminal groups |
| EP2582397A4 (en) | 2010-06-15 | 2014-10-29 | Isis Pharmaceuticals Inc | COMPOUNDS AND METHOD FOR MODULATING INTERACTION BETWEEN PROTEINS AND TARGET NUCLEIC ACIDS |
| US9161948B2 (en) | 2011-05-05 | 2015-10-20 | Sarepta Therapeutics, Inc. | Peptide oligonucleotide conjugates |
| KR102229650B1 (ko) * | 2011-05-05 | 2021-03-19 | 사렙타 쎄러퓨틱스, 인코퍼레이티드 | 펩타이드 올리고뉴클레오타이드 접합체 |
| WO2012178122A2 (en) | 2011-06-23 | 2012-12-27 | Cold Spring Harbor Laboratory | Phenocopy model of disease |
| ES2727481T3 (es) * | 2011-11-30 | 2019-10-16 | Sarepta Therapeutics Inc | Inclusión inducida de exón en atrofia muscular espinal |
| JP6317675B2 (ja) | 2011-11-30 | 2018-04-25 | サレプタ セラピューティクス, インコーポレイテッド | 延長リピート病を処置するためのオリゴヌクレオチド |
| US9725716B2 (en) * | 2011-12-06 | 2017-08-08 | Ohio State Innovation Foundation and Research Institute at Nationwide Children's Hospital | Non-ionic, low osmolar contrast agents for delivery of antisense oligonucleotides and treatment of disease |
| EP2828395B1 (en) * | 2012-03-20 | 2018-10-24 | Sarepta Therapeutics, Inc. | Boronic acid conjugates of oligonucleotide analogues |
| DK2850186T3 (en) * | 2012-05-16 | 2019-04-08 | Translate Bio Ma Inc | COMPOSITIONS AND PROCEDURES FOR MODULATING SMN GENFAMILY EXPRESSION |
| WO2013173789A2 (en) | 2012-05-17 | 2013-11-21 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide compositions |
| EP2943225A4 (en) * | 2013-01-09 | 2016-07-13 | Ionis Pharmaceuticals Inc | COMPOSITIONS AND METHODS FOR MODULATING SMN2 DISTRIBUTION IN THE BODY OF A PATIENT |
| US9856474B2 (en) * | 2013-01-16 | 2018-01-02 | Iowa State University Research Foundation, Inc. | Deep intronic target for splicing correction on spinal muscular atrophy gene |
| US9885040B2 (en) * | 2013-04-12 | 2018-02-06 | The Curators Of The University Of Missouri | SMN2 element 1 antisense compositions and methods and uses thereof |
| US9988626B2 (en) * | 2013-07-29 | 2018-06-05 | Universität Zu Köln | Neurocalcin delta inhibitors and therapeutic and non-therapeutic uses thereof |
| EP3044317A1 (en) * | 2013-09-13 | 2016-07-20 | The University Of Western Australia | Antisense oligomers and methods for treating smn-related pathologies |
| US9845469B2 (en) * | 2014-02-10 | 2017-12-19 | Ohio State Innovation Foundation | Antisense oligonucleotides for treatment of spinal muscular atrophy |
| WO2017040271A1 (en) | 2015-08-28 | 2017-03-09 | Sarepta Therapeutics, Inc. | Modified antisense oligomers for exon inclusion in spinal muscular atrophy |
-
2016
- 2016-08-26 WO PCT/US2016/048965 patent/WO2017040271A1/en not_active Ceased
- 2016-08-26 JP JP2018510718A patent/JP6987041B2/ja active Active
- 2016-08-26 HK HK18116288.7A patent/HK1257498A1/zh unknown
- 2016-08-26 CA CA2996164A patent/CA2996164A1/en not_active Abandoned
- 2016-08-26 MA MA042695A patent/MA42695A/fr unknown
- 2016-08-26 US US15/754,782 patent/US10905709B2/en active Active
- 2016-08-26 AU AU2016317667A patent/AU2016317667A1/en not_active Abandoned
- 2016-08-26 EP EP16762935.1A patent/EP3341480A1/en not_active Withdrawn
-
2020
- 2020-11-23 US US16/949,980 patent/US12121532B2/en active Active
- 2020-12-25 JP JP2020216610A patent/JP2021048877A/ja not_active Withdrawn
-
2024
- 2024-08-23 US US18/813,696 patent/US20250241940A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| AU2016317667A1 (en) | 2018-03-22 |
| US12121532B2 (en) | 2024-10-22 |
| EP3341480A1 (en) | 2018-07-04 |
| MA42695A (fr) | 2018-07-04 |
| HK1257498A1 (zh) | 2019-10-25 |
| JP2021048877A (ja) | 2021-04-01 |
| WO2017040271A1 (en) | 2017-03-09 |
| US20190015440A1 (en) | 2019-01-17 |
| CA2996164A1 (en) | 2017-03-09 |
| US10905709B2 (en) | 2021-02-02 |
| JP2018525015A (ja) | 2018-09-06 |
| US20250241940A1 (en) | 2025-07-31 |
| US20210169918A1 (en) | 2021-06-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7057385B2 (ja) | 延長リピート病を処置するためのオリゴヌクレオチド | |
| JP6865871B2 (ja) | 酸性α−グルコシダーゼにおけるアンチセンス誘導エクソン2包含 | |
| JP2022017594A (ja) | デュシェンヌ型筋ジストロフィーおよび関連障害の処置のための組成物および方法 | |
| US20250241940A1 (en) | Modified antisense oligomers for exon inclusion in spinal muscular atrophy | |
| JP2019059769A (ja) | 脊髄性筋萎縮症における誘発されたエクソン包含 | |
| US20220090084A1 (en) | Antisense-induced exon exclusion in myostatin | |
| US20240415857A1 (en) | Antisense-induced exon exclusion in type vii collagen | |
| JP2021500016A (ja) | アンチセンスオリゴマー化合物 | |
| KR20250130411A (ko) | 근 이영양증을 위한 엑손 스키핑 올리고머 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190822 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20190822 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200928 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201225 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20210426 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210826 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20210826 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20210826 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20210930 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20211001 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20211102 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20211130 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6987041 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |