JP6883090B2 - A composition for skin whitening containing 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide. - Google Patents
A composition for skin whitening containing 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide. Download PDFInfo
- Publication number
- JP6883090B2 JP6883090B2 JP2019506499A JP2019506499A JP6883090B2 JP 6883090 B2 JP6883090 B2 JP 6883090B2 JP 2019506499 A JP2019506499 A JP 2019506499A JP 2019506499 A JP2019506499 A JP 2019506499A JP 6883090 B2 JP6883090 B2 JP 6883090B2
- Authority
- JP
- Japan
- Prior art keywords
- composition
- trans
- pyridinyl
- methylamino
- carboxamide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title claims description 47
- 230000002087 whitening effect Effects 0.000 title claims description 26
- VFSUUTYAEQOIMW-YHBQERECSA-N 3-chloro-N-[trans-4-(methylamino)cyclohexyl]-N-[3-(pyridin-4-yl)benzyl]-1-benzothiophene-2-carboxamide Chemical compound C1C[C@@H](NC)CC[C@@H]1N(C(=O)C1=C(C2=CC=CC=C2S1)Cl)CC1=CC=CC(C=2C=CN=CC=2)=C1 VFSUUTYAEQOIMW-YHBQERECSA-N 0.000 title claims description 19
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 claims description 41
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 claims description 30
- 125000000250 methylamino group Chemical group [H]N(*)C([H])([H])[H] 0.000 claims description 30
- -1 4-pyridinyl Chemical group 0.000 claims description 26
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 20
- 150000003839 salts Chemical class 0.000 claims description 20
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 19
- GYSCBCSGKXNZRH-UHFFFAOYSA-N 1-benzothiophene-2-carboxamide Chemical compound C1=CC=C2SC(C(=O)N)=CC2=C1 GYSCBCSGKXNZRH-UHFFFAOYSA-N 0.000 claims description 17
- 239000012453 solvate Substances 0.000 claims description 14
- 208000012641 Pigmentation disease Diseases 0.000 claims description 11
- 239000004480 active ingredient Substances 0.000 claims description 11
- 230000003287 optical effect Effects 0.000 claims description 10
- 230000019612 pigmentation Effects 0.000 claims description 10
- 102000003425 Tyrosinase Human genes 0.000 claims description 9
- 108060008724 Tyrosinase Proteins 0.000 claims description 9
- 239000002537 cosmetic Substances 0.000 claims description 9
- 201000001441 melanoma Diseases 0.000 claims description 8
- 239000008194 pharmaceutical composition Substances 0.000 claims description 6
- 208000003351 Melanosis Diseases 0.000 claims description 5
- 229940079593 drug Drugs 0.000 claims description 5
- 239000003814 drug Substances 0.000 claims description 5
- 201000004624 Dermatitis Diseases 0.000 claims description 3
- 206010036229 Post inflammatory pigmentation change Diseases 0.000 claims description 2
- 208000000069 hyperpigmentation Diseases 0.000 claims description 2
- 230000003810 hyperpigmentation Effects 0.000 claims description 2
- DENPQNAWGQXKCU-UHFFFAOYSA-N thiophene-2-carboxamide Chemical compound NC(=O)C1=CC=CS1 DENPQNAWGQXKCU-UHFFFAOYSA-N 0.000 claims description 2
- 206010008570 Chloasma Diseases 0.000 claims 1
- 210000003491 skin Anatomy 0.000 description 47
- 210000004027 cell Anatomy 0.000 description 20
- 239000002552 dosage form Substances 0.000 description 19
- 150000001875 compounds Chemical class 0.000 description 16
- 239000000126 substance Substances 0.000 description 12
- 239000006210 lotion Substances 0.000 description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 11
- 206010027145 Melanocytic naevus Diseases 0.000 description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 9
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 238000004519 manufacturing process Methods 0.000 description 9
- 239000003795 chemical substances by application Substances 0.000 description 8
- BJRNKVDFDLYUGJ-RMPHRYRLSA-N hydroquinone O-beta-D-glucopyranoside Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=CC=C(O)C=C1 BJRNKVDFDLYUGJ-RMPHRYRLSA-N 0.000 description 8
- 239000006071 cream Substances 0.000 description 7
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 6
- 208000007256 Nevus Diseases 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- 238000000034 method Methods 0.000 description 6
- 239000008213 purified water Substances 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerol Natural products OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 229920002472 Starch Polymers 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 235000014113 dietary fatty acids Nutrition 0.000 description 5
- 239000000194 fatty acid Substances 0.000 description 5
- 229930195729 fatty acid Natural products 0.000 description 5
- 239000008187 granular material Substances 0.000 description 5
- 235000013402 health food Nutrition 0.000 description 5
- 210000002752 melanocyte Anatomy 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 235000018102 proteins Nutrition 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 239000008107 starch Substances 0.000 description 5
- 235000019698 starch Nutrition 0.000 description 5
- 239000003826 tablet Substances 0.000 description 5
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 4
- 229960000271 arbutin Drugs 0.000 description 4
- 125000005605 benzo group Chemical group 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- BJRNKVDFDLYUGJ-UHFFFAOYSA-N p-hydroxyphenyl beta-D-alloside Natural products OC1C(O)C(O)C(CO)OC1OC1=CC=C(O)C=C1 BJRNKVDFDLYUGJ-UHFFFAOYSA-N 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 150000003384 small molecules Chemical class 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 229940058015 1,3-butylene glycol Drugs 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 206010014970 Ephelides Diseases 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 108090000385 Fibroblast growth factor 7 Proteins 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 239000000637 Melanocyte-Stimulating Hormone Substances 0.000 description 3
- 108010007013 Melanocyte-Stimulating Hormones Proteins 0.000 description 3
- 102000013380 Smoothened Receptor Human genes 0.000 description 3
- 108010090739 Smoothened Receptor Proteins 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- 235000019437 butane-1,3-diol Nutrition 0.000 description 3
- 235000015165 citric acid Nutrition 0.000 description 3
- 230000008021 deposition Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 210000004209 hair Anatomy 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000008099 melanin synthesis Effects 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 235000016709 nutrition Nutrition 0.000 description 3
- 230000035764 nutrition Effects 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 239000000049 pigment Substances 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 239000007901 soft capsule Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 239000011782 vitamin Substances 0.000 description 3
- 229940088594 vitamin Drugs 0.000 description 3
- 229930003231 vitamin Natural products 0.000 description 3
- 235000013343 vitamin Nutrition 0.000 description 3
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 2
- XMIIGOLPHOKFCH-UHFFFAOYSA-N 3-phenylpropionic acid Chemical compound OC(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 206010010356 Congenital anomaly Diseases 0.000 description 2
- 206010011732 Cyst Diseases 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- 102000003972 Fibroblast growth factor 7 Human genes 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 108010010995 MART-1 Antigen Proteins 0.000 description 2
- 102100028389 Melanoma antigen recognized by T-cells 1 Human genes 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 208000000453 Skin Neoplasms Diseases 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 208000001119 benign fibrous histiocytoma Diseases 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- 235000013361 beverage Nutrition 0.000 description 2
- 230000002146 bilateral effect Effects 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 208000031513 cyst Diseases 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 210000001508 eye Anatomy 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 239000012091 fetal bovine serum Substances 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 239000013538 functional additive Substances 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 2
- BEJNERDRQOWKJM-UHFFFAOYSA-N kojic acid Chemical compound OCC1=CC(=O)C(O)=CO1 BEJNERDRQOWKJM-UHFFFAOYSA-N 0.000 description 2
- 229960004705 kojic acid Drugs 0.000 description 2
- WZNJWVWKTVETCG-UHFFFAOYSA-N kojic acid Natural products OC(=O)C(N)CN1C=CC(=O)C(O)=C1 WZNJWVWKTVETCG-UHFFFAOYSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 230000036564 melanin content Effects 0.000 description 2
- 230000000684 melanotic effect Effects 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- 230000002335 preservative effect Effects 0.000 description 2
- 239000001294 propane Substances 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 230000009759 skin aging Effects 0.000 description 2
- 201000000849 skin cancer Diseases 0.000 description 2
- 230000008410 smoothened signaling pathway Effects 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 239000001993 wax Substances 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- QGVLYPPODPLXMB-UBTYZVCOSA-N (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b,9,9a-tetrahydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-1,1a,1b,4,4a,7a,7b,8,9,9a-decahydro-5H-cyclopropa[3,4]benzo[1,2-e]azulen-5-one Chemical compound C1=C(CO)C[C@]2(O)C(=O)C(C)=C[C@H]2[C@@]2(O)[C@H](C)[C@@H](O)[C@@]3(O)C(C)(C)[C@H]3[C@@H]21 QGVLYPPODPLXMB-UBTYZVCOSA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- BWSWZBCSFZAYOB-CABCVRRESA-N (2s,4r)-1-dodecanoyl-4-hydroxypyrrolidine-2-carboxylic acid Chemical compound CCCCCCCCCCCC(=O)N1C[C@H](O)C[C@H]1C(O)=O BWSWZBCSFZAYOB-CABCVRRESA-N 0.000 description 1
- YYGNTYWPHWGJRM-UHFFFAOYSA-N (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene Chemical compound CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C YYGNTYWPHWGJRM-UHFFFAOYSA-N 0.000 description 1
- PHIQHXFUZVPYII-ZCFIWIBFSA-N (R)-carnitine Chemical compound C[N+](C)(C)C[C@H](O)CC([O-])=O PHIQHXFUZVPYII-ZCFIWIBFSA-N 0.000 description 1
- MIOPJNTWMNEORI-GMSGAONNSA-N (S)-camphorsulfonic acid Chemical compound C1C[C@@]2(CS(O)(=O)=O)C(=O)C[C@@H]1C2(C)C MIOPJNTWMNEORI-GMSGAONNSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- AMMPLVWPWSYRDR-UHFFFAOYSA-N 1-methylbicyclo[2.2.2]oct-2-ene-4-carboxylic acid Chemical compound C1CC2(C(O)=O)CCC1(C)C=C2 AMMPLVWPWSYRDR-UHFFFAOYSA-N 0.000 description 1
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical class CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- RFVNOJDQRGSOEL-UHFFFAOYSA-N 2-hydroxyethyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCO RFVNOJDQRGSOEL-UHFFFAOYSA-N 0.000 description 1
- UPHOPMSGKZNELG-UHFFFAOYSA-N 2-hydroxynaphthalene-1-carboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=C(O)C=CC2=C1 UPHOPMSGKZNELG-UHFFFAOYSA-N 0.000 description 1
- MLMQPDHYNJCQAO-UHFFFAOYSA-N 3,3-dimethylbutyric acid Chemical compound CC(C)(C)CC(O)=O MLMQPDHYNJCQAO-UHFFFAOYSA-N 0.000 description 1
- XLZYKTYMLBOINK-UHFFFAOYSA-N 3-(4-hydroxybenzoyl)benzoic acid Chemical compound OC(=O)C1=CC=CC(C(=O)C=2C=CC(O)=CC=2)=C1 XLZYKTYMLBOINK-UHFFFAOYSA-N 0.000 description 1
- KIBJSFKLSFCWSF-UHFFFAOYSA-N 3-(cyclopenten-1-yl)propanoic acid Chemical compound OC(=O)CCC1=CCCC1 KIBJSFKLSFCWSF-UHFFFAOYSA-N 0.000 description 1
- LKKMLIBUAXYLOY-UHFFFAOYSA-N 3-Amino-1-methyl-5H-pyrido[4,3-b]indole Chemical compound N1C2=CC=CC=C2C2=C1C=C(N)N=C2C LKKMLIBUAXYLOY-UHFFFAOYSA-N 0.000 description 1
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- RJWBTWIBUIGANW-UHFFFAOYSA-N 4-chlorobenzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=C(Cl)C=C1 RJWBTWIBUIGANW-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 208000023268 Becker naevus Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 241001474374 Blennius Species 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- 206010067248 Congenital naevus Diseases 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 208000000471 Dysplastic Nevus Syndrome Diseases 0.000 description 1
- 206010062805 Dysplastic naevus Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 101150056978 HMGS gene Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 206010023256 Juvenile melanoma benign Diseases 0.000 description 1
- 206010023347 Keratoacanthoma Diseases 0.000 description 1
- 102100031413 L-dopachrome tautomerase Human genes 0.000 description 1
- 101710093778 L-dopachrome tautomerase Proteins 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- 239000012741 Laemmli sample buffer Substances 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 244000141359 Malus pumila Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 102000013760 Microphthalmia-Associated Transcription Factor Human genes 0.000 description 1
- 108010050345 Microphthalmia-Associated Transcription Factor Proteins 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 201000009139 Mongolian Spot Diseases 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 240000008790 Musa x paradisiaca Species 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 208000032452 Nevus, Epithelioid and Spindle Cell Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 206010029488 Nodular melanoma Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 235000019482 Palm oil Nutrition 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 101100011891 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) ERG13 gene Proteins 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- ULUAUXLGCMPNKK-UHFFFAOYSA-N Sulfobutanedioic acid Chemical compound OC(=O)CC(C(O)=O)S(O)(=O)=O ULUAUXLGCMPNKK-UHFFFAOYSA-N 0.000 description 1
- 206010042553 Superficial spreading melanoma stage unspecified Diseases 0.000 description 1
- 239000006180 TBST buffer Substances 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- BHEOSNUKNHRBNM-UHFFFAOYSA-N Tetramethylsqualene Natural products CC(=C)C(C)CCC(=C)C(C)CCC(C)=CCCC=C(C)CCC(C)C(=C)CCC(C)C(C)=C BHEOSNUKNHRBNM-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 206010000583 acral lentiginous melanoma Diseases 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 230000001668 ameliorated effect Effects 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000021016 apples Nutrition 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 235000021015 bananas Nutrition 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 208000007047 blue nevus Diseases 0.000 description 1
- UDSAIICHUKSCKT-UHFFFAOYSA-N bromophenol blue Chemical compound C1=C(Br)C(O)=C(Br)C=C1C1(C=2C=C(Br)C(O)=C(Br)C=2)C2=CC=CC=C2S(=O)(=O)O1 UDSAIICHUKSCKT-UHFFFAOYSA-N 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 150000005827 chlorofluoro hydrocarbons Chemical class 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 210000000736 corneocyte Anatomy 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N dodecahydrosqualene Natural products CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 229940043264 dodecyl sulfate Drugs 0.000 description 1
- 230000035622 drinking Effects 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 230000000481 effect on pigmentation Effects 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-N ethanedisulfonic acid Chemical compound OS(=O)(=O)CCS(O)(=O)=O AFAXGSQYZLGZPG-UHFFFAOYSA-N 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 150000002191 fatty alcohols Chemical class 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 235000021255 galacto-oligosaccharides Nutrition 0.000 description 1
- 150000003271 galactooligosaccharides Chemical class 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 230000009459 hedgehog signaling Effects 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 201000010118 hypomelanosis of Ito Diseases 0.000 description 1
- 150000004693 imidazolium salts Chemical class 0.000 description 1
- 210000004263 induced pluripotent stem cell Anatomy 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 208000007098 intradermal nevus Diseases 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 229940069445 licorice extract Drugs 0.000 description 1
- 208000022742 linear and whorled nevoid hypermelanosis Diseases 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000003101 melanogenic effect Effects 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 208000004649 neutrophil actin dysfunction Diseases 0.000 description 1
- 208000024645 nevus of Ito Diseases 0.000 description 1
- 208000004942 nevus of Ota Diseases 0.000 description 1
- 201000000032 nodular malignant melanoma Diseases 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 239000012860 organic pigment Substances 0.000 description 1
- 238000012261 overproduction Methods 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 239000002540 palm oil Substances 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- QGVLYPPODPLXMB-QXYKVGAMSA-N phorbol Natural products C[C@@H]1[C@@H](O)[C@]2(O)[C@H]([C@H]3C=C(CO)C[C@@]4(O)[C@H](C=C(C)C4=O)[C@@]13O)C2(C)C QGVLYPPODPLXMB-QXYKVGAMSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 201000000734 pigmented basal cell carcinoma Diseases 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 1
- 239000000419 plant extract Substances 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- WSHYKIAQCMIPTB-UHFFFAOYSA-M potassium;2-oxo-3-(3-oxo-1-phenylbutyl)chromen-4-olate Chemical compound [K+].[O-]C=1C2=CC=CC=C2OC(=O)C=1C(CC(=O)C)C1=CC=CC=C1 WSHYKIAQCMIPTB-UHFFFAOYSA-M 0.000 description 1
- 235000012015 potatoes Nutrition 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 238000002331 protein detection Methods 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000017403 regulation of melanin biosynthetic process Effects 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 229940071089 sarcosinate Drugs 0.000 description 1
- 210000004761 scalp Anatomy 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 235000019615 sensations Nutrition 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 235000020183 skimmed milk Nutrition 0.000 description 1
- 208000030099 skin pigmented basal cell carcinoma Diseases 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- ZUFONQSOSYEWCN-UHFFFAOYSA-M sodium;2-(methylamino)acetate Chemical compound [Na+].CNCC([O-])=O ZUFONQSOSYEWCN-UHFFFAOYSA-M 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 150000003408 sphingolipids Chemical class 0.000 description 1
- 208000011584 spitz nevus Diseases 0.000 description 1
- 229940031439 squalene Drugs 0.000 description 1
- TUHBEKDERLKLEC-UHFFFAOYSA-N squalene Natural products CC(=CCCC(=CCCC(=CCCC=C(/C)CCC=C(/C)CC=C(C)C)C)C)C TUHBEKDERLKLEC-UHFFFAOYSA-N 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010972 statistical evaluation Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 208000030457 superficial spreading melanoma Diseases 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 239000006211 transdermal dosage form Substances 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 230000007279 water homeostasis Effects 0.000 description 1
- 239000004563 wettable powder Substances 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/38—Heterocyclic compounds having sulfur as a ring hetero atom
- A61K31/381—Heterocyclic compounds having sulfur as a ring hetero atom having five-membered rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4418—Non condensed pyridines; Hydrogenated derivatives thereof having a carbocyclic group directly attached to the heterocyclic ring, e.g. cyproheptadine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4906—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with one nitrogen as the only hetero atom
- A61K8/4926—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with one nitrogen as the only hetero atom having six membered rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4986—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with sulfur as the only hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/02—Preparations for care of the skin for chemically bleaching or whitening the skin
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Birds (AREA)
- Dermatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
- Non-Alcoholic Beverages (AREA)
- Cosmetics (AREA)
Description
本明細書は、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドを含む皮膚美白用組成物に関するものであって、具体的に、メラニンの生成及び沈着を調節して皮膚美白効能を発揮する物質を含む組成物に関するものである。 This specification describes skin containing 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide. It relates to a composition for whitening, and specifically, it relates to a composition containing a substance that regulates the production and deposition of melanin and exerts a skin whitening effect.
メラニン(melanin)は、黒い色素とタンパク質との複合体形態を持つフェノール類の生体高分子物質であって、リンゴ、じゃがいも、バナナの切断された表面が空気中に露出したときに発生する褐変又は動物の外皮毛、皮膚、毛髪、眼部などで観察される。メラニンが過剰生成して皮膚に沈着すると、しみやそばかすなどができるため皮膚美白などと直結し、メラニンによって皮膚老化も促進され、皮膚癌を誘発することもある。 Melanin is a phenolic biopolymer in the form of a complex of black pigment and protein that causes browning or browning that occurs when the cut surface of apples, potatoes and bananas is exposed to the air. It is observed in the outer skin, skin, hair, and eyes of animals. When melanin is overproduced and deposited on the skin, stains and freckles are formed, which is directly linked to skin whitening, and melanin also promotes skin aging and may induce skin cancer.
紫外線、炎症、ホルモンなどによってメラニン細胞刺激ホルモン(melanocyte stimulating hormone、MSH)が分泌され、該MSHは受容体と反応してメラニン形成細胞内においてcAMPを向上させてメラニンを合成し、合成されたメラニンは、メラニン形成細胞の外部へ分泌され、紫外線などから皮膚を保護する役割をするようになる。メラニンの合成は、主にα−MSHによって調節され、メラニンの合成に関与するタンパク質としては、MITF、TYR、TRP1、TRP2などが知られている。 Melanocyte stimulating hormone (MSH) is secreted by ultraviolet rays, inflammation, hormones, etc., and the MSH reacts with the receptor to improve cAMP in melanin-forming cells to synthesize melanin, and the synthesized melanin Is secreted to the outside of melanin-forming cells and plays a role in protecting the skin from ultraviolet rays and the like. The synthesis of melanin is mainly regulated by α-MSH, and MITF, TYR, TRP1, TRP2 and the like are known as proteins involved in the synthesis of melanin.
本発明は、一側面において、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物を有効成分として含む皮膚美白用組成物を提供して、皮膚美白関連分野の発展を図り、且つ皮膚美白関連消費者の需要を満たすことをその目的とする。 The present invention, in one aspect, is 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide. To provide a composition for skin whitening containing, an optical or stereoisomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof as an active ingredient, to develop a field related to skin whitening, and to develop skin. Its purpose is to meet the demands of whitening-related consumers.
本発明は、一側面において、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物を有効成分として含む皮膚美白用組成物を提供する。 The present invention, in one aspect, is 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide. , An optical or stereoisomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof as an active ingredient.
本発明の他の側面において、前記3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドは、メラニンの生成及び沈着を抑制させるものであってよい。 In another aspect of the present invention, the 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2- Carboxamide may be one that suppresses the production and deposition of melanin.
また、本発明の他の側面において、前記組成物は薬学組成物又は化粧料組成物であってよい。 Further, in another aspect of the present invention, the composition may be a pharmaceutical composition or a cosmetic composition.
本発明は、一側面において、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド(3−Chloro−N−[trans−4−(methylamino)cyclohexyl]−N−[[3−(4−pyridinyl)phenyl]methyl]benzo[b]thiophene−2−carboxamide)、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物を個体に投与することを含む皮膚美白方法を提供する。 The present invention, in one aspect, is 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide. (3-Chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide), its optical or stereoisomer , A skin whitening method comprising administering to an individual an acceptable salt thereof, a hydrate thereof, or a solvate thereof.
また、本発明は、他の側面において、皮膚美白用途に用いるための有効成分として、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物を提供する。また、皮膚美白のための有効成分として、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物の非治療的化粧用途を提供する。 In addition, in another aspect, the present invention comprises 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) as an active ingredient for use in skin whitening applications. ) Phenyl] methyl] benzo [b] thiophen-2-carboxamide, an optical or stereoisomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof. In addition, as an active ingredient for skin whitening, 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene- Provided are non-therapeutic cosmetic uses of 2-carboxamide, an optical or stereoisomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof.
また、本発明は、他の側面において、皮膚美白用組成物の製造に用いるための3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物の用途を提供する。 In addition, in another aspect, the present invention comprises 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) for use in the production of skin whitening compositions. ) Phenyl] methyl] benzo [b] thiophen-2-carboxamide, an optical or stereoisomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof.
本発明は、一側面において、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物を有効成分として含む新しい皮膚美白用組成物を発掘及び提供することで、皮膚美白に関する新しい分野の開拓及び市場の拡張に寄与することができる。 The present invention, in one aspect, is 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide. Opening up new fields related to skin whitening by discovering and providing new skin whitening compositions containing, its optical or stereoisomer, its acceptable salt, its hydrate, or its solvate as an active ingredient. And can contribute to the expansion of the market.
本明細書において「皮膚」とは、動物の体表を覆う組織のことを意味し、顔又はボディーなどの体表を覆う組織だけではなく、頭皮や毛髪を含む最広義の概念である。 As used herein, the term "skin" means a tissue covering the body surface of an animal, and is a broadest concept including not only the tissue covering the body surface such as the face or the body but also the scalp and hair.
本明細書において、「3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド」は、Smoアゴニスト、Smoothened agonist、Smo agonist、又はSAGと表されることがある。前記3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドは、ヘッジホッグシグナル伝達経路(hedgehog signaling pathway)で主な役割を担うSmoothenedタンパク質の発現を誘導する物質を含むものであってよい。 In the present specification, "3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide" is used. , Smo agonist, Smoothered agent, Smo agent, or SAG. The 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide is a Hedgehog signaling pathway. It may contain a substance that induces the expression of a Smoothened protein that plays a major role in (hedgehog signaling pathway).
本明細書において「異性体」は、特に光学異性体(optical isomers)(例えば、本質的に純粋なエナンチオマー(essentially pure enantiomers)、本質的に純粋な立体異性体又はそれらの混合物、本質的に純粋なジアステレオマー(essentially pure diastereomers)だけでなく、立体異性体、配座異性体(conformation isomers)(すなわち、1つ以上の化学結合のその角度のみ異なる異性体)、位置異性体(position isomers)(特に、互変異性体(tautomers))、又は幾何異性体(geometric isomers)(例えば、シス−トランス異性体)を含む。 As used herein, "isomers" are particularly pure optical isomers (eg, essentially pure isomers), essentially pure steric isomers or mixtures thereof, essentially pure. Essentially pure diastereomers, as well as steric isomers, conformation isomers (ie, isomers that differ only in their angle of one or more chemical bonds), position isomers. (In particular, tautomers), or geometric isomers (eg, cis-trans isomers).
本明細書において「本質的に純粋な(essentially pure)」とは、例えば、エナンチオマー、立体異性体、又はジアステレオマーと関連して用いた場合、エナンチオマー、立体異性体、又はジアステレオマーを例として挙げることのできる具体的な化合物が約90%以上、好ましくは約95%以上、より好ましくは約97%以上、又は約98%以上、さらに好ましくは約99%以上、最も好ましくは約99.5%以上(w/w)存在することを意味する。 As used herein, "essentially pure" is, for example, an enantiomer, a stereoisomer, or a diastereomer when used in connection with an enantiomer, a stereoisomer, or a diastereomer. About 90% or more, preferably about 95% or more, more preferably about 97% or more, or about 98% or more, still more preferably about 99% or more, most preferably about 99% or more. It means that it is present at 5% or more (w / w).
本明細書において「許容可能」とは、通常的又は医薬学的服用量(dosage)で利用する際に相当な毒性を避けることにより、動物、より具体的には、ヒトに使用することができるという政府またはこれに準ずる規制機構の承認を受けることができ、又は承認を受け、又は食品基準(Food code)、健康機能食品基準、又は一般的な薬局方に列挙され、又はその他一般的な文献に記載されたものと認定されることを意味する。 As used herein, "acceptable" can be used in animals, more specifically in humans, by avoiding significant toxicity when used in normal or pharmaceutical doses. Can be approved or approved by the government or an equivalent regulatory body, or listed in the Food Code, Health Function Food Standards, or General Pharmacopoeia, or other general literature. It means that it is certified as described in.
本明細書において「許容可能な塩」とは、通常的に又は医薬学的に許容可能であり、親化合物(parent compound)の好ましい活性を有する本発明の一側面に係る塩を意味する。前記塩は、(1)塩酸、臭化水素酸、硫酸、硝酸、リン酸等といった無機酸から形成されるか;又は、酢酸、プロピオン酸、ヘキサン酸、シクロペンテンプロピオン酸、グリコール酸、ピルビン酸、乳酸、マロン酸、コハク酸、リンゴ酸、マレイン酸、フマル酸、酒石酸、クエン酸、安息香酸、3−(4−ヒドロキシベンゾイル)安息香酸、桂皮酸、マンデル酸、メタンスルホン酸、エタンスルホン酸、1,2−エタン−ジスルホン酸、2−ヒドロキシエタンスルホン酸、ベンゼンスルホン酸、4−クロロベンゼンスルホン酸、2−ナフタレンスルホン酸、4−トルエンスルホン酸、カンファースルホン酸、4−メチルビシクロ[2,2,2]−oct−2−エン−1-カルボン酸、グルコヘプトン酸、3−フェニルプロピオン酸、トリメチル酢酸、tert−ブチル酢酸、ラウリル硫酸、グルコン酸、グルタミン酸、ヒドロキシナフトエ酸、サリチル酸、ステアリン酸、ムコン酸といった有機酸から形成される酸付加塩(acid addition salt);又は、(2)親化合物に存在する酸性プロトンが置換されるときに形成される塩を含んでよい。 As used herein, the term "acceptable salt" means a salt according to one aspect of the invention that is usually or pharmaceutically acceptable and has the preferred activity of the parent compound. Is the salt formed from (1) inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitrate, phosphoric acid and the like; or acetic acid, propionic acid, hexanic acid, cyclopentenepropionic acid, glycolic acid, pyruvate, Lactic acid, malonic acid, succinic acid, malic acid, maleic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, 3- (4-hydroxybenzoyl) benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-ethane-disulfonic acid, 2-hydroxyethanesulfonic acid, benzenesulfonic acid, 4-chlorobenzenesulfonic acid, 2-naphthalenesulfonic acid, 4-toluenesulfonic acid, camphorsulfonic acid, 4-methylbicyclo [2,2 , 2] -oct-2-ene-1-carboxylic acid, glucoheptonic acid, 3-phenylpropionic acid, trimethylacetic acid, tert-butylacetic acid, laurylsulfate, gluconic acid, glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid, mucon It may include an acid addition salt formed from an organic acid such as an acid; or (2) a salt formed when the acidic protons present in the parent compound are replaced.
本明細書において「水和物(hydrate)」とは、水が結合している化合物を意味し、水と混合物との間に化学的な結合力のない内包化合物を含む広範囲な概念である。 As used herein, the term "hydrate" means a compound to which water is bound, and is a broad concept including an inclusion compound having no chemical binding force between water and a mixture.
本明細書において「溶媒和物」とは、溶質の分子やイオンと溶媒の分子やイオンとの間に生じた高次の化合物を意味する。 As used herein, the term "solvate" means a higher-order compound formed between a molecule or ion of a solute and a molecule or ion of a solvent.
本発明は、一側面において、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その光学又は立体異性体、その許容可能な塩、その水和物、又はその溶媒和物を有効成分として含む皮膚美白用組成物を提供する。 The present invention, in one aspect, is 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide. , An optical or stereoisomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof as an active ingredient.
一具現例として、前記3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド(3−Chloro−N−[trans−4−(methylamino)cyclohexyl]−N−[[3−(4−pyridinyl)phenyl]methyl]benzo[b]thiophene−2−carboxamide)は、クロロベンゾチオフェン系化合物であって、C28H28ClN3OSを含むものであってよく、下記の化学式で示されるものであってよい。 As an embodiment, the 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide (3) -Chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide) is a chlorobenzothiophene compound. It may contain C 28 H 28 ClN 3 OS, and may be represented by the following chemical formula.
他の具現例として、前記化合物は合成により得たものであってよく、他の物質を加工して得たものであってもよく、生物体や微生物などに由来のものであってもよい。 As another embodiment, the compound may be obtained by synthesis, may be obtained by processing another substance, or may be derived from an organism, a microorganism, or the like.
他の具現例として、前記3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドは、メラニンの生成又は沈着を抑制するものであってよい。 As another embodiment, the 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide , It may suppress the production or deposition of melanin.
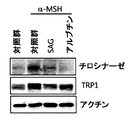
本発明の他の側面によると、前記3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドは、チロシナーゼ(tyrosinase)又はTRP1(Tyrosinase−Related protein 1)の発現を増進させるものであってよい。前記化合物の処理後にチロシナーゼ又はTRP1の発現が前記化合物の処理前よりも少なくなるため、これを通じて前記化合物を皮膚美白用組成物の有効成分として用い得ることが分かる。また、前記化合物を処理した場合、チロシナーゼ又はTRP1が発現された細胞数を減少させたりもし、これを通じて前記化合物を皮膚美白用組成物の有効成分として用い得ることが分かる。 According to another aspect of the present invention, the 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2 -Carboxamide may enhance the expression of tyrosinase or TRP1 (Tyrosinase-Related productin 1). Since the expression of tyrosinase or TRP1 after the treatment of the compound is lower than that before the treatment of the compound, it can be seen that the compound can be used as an active ingredient of the skin whitening composition. In addition, when the compound is treated, the number of cells expressing tyrosinase or TRP1 may be reduced, and it can be seen that the compound can be used as an active ingredient of a skin whitening composition.
メラニン(melanin)は動物の外皮毛、皮膚、頭部、眼部などから観察され、メラニンが過剰産生されると、皮膚に沈着してしみ、そばかすなどを形成したり、皮膚老化も促進したり、皮膚癌も誘発したりすることがある。前記メラニン過多生成による疾患又は症状は、しみ、そばかす、老人性しみ、細かいほこり、表皮メラニン細胞性病変(Epidermal melanocytic lesion)、カフェオレ斑(Cafe's au lait macules)、母斑、ベッカー母斑(Becker's Nevus)、扁平母斑(Nevus Spilus)、黒子(Lentigines)、黒色点、真皮メラニン細胞性病変(Dermal melanocytic lesions)、蒙古斑(Mongolian spot)、太田母斑(Nevus of Ota)、遅発性両側性太田母斑様色素斑(Acquired bilateral nevus of Ota−like macules)、伊藤母斑(Nevus of Ito)、青色母斑(Blue nevus)、メラニン形成細胞性母斑(Melanocytic nevus)、境界性母斑(Junctional nevus)、複合性母斑(Compound nevus)、真皮内母斑(Intradermal nevus)、暈状母斑(Halo nevus)、先天性メラニン細胞性母斑(Congenital nevocytic nevus)、スピッツ母斑(Spitz nevus)、異形成母斑(Dysplastic nevus)、黒色腫(Melanoma)、悪性黒字型黒色腫(Lentigo maligna melanoma)、表在拡大型黒色腫(Superficial spreading melanoma)、末端性黒子性黒色腫(Acral lentiginous melanoma)、結節型黒色腫(Nodular melanoma)、色素性基底細胞癌(pigment basal cell carcinoma)、色素性皮膚線維腫(dermatofibromas)、色素性類皮嚢胞(dermoid cyst)、色素性ケロイド(keloid)、紫外線による色素沈着、薬物による色素沈着、炎症後色素沈着、及び皮膚炎から発生する色素沈着及び色素性角化棘細胞腫(keratoacanthomas)からなる群より選ばれる1以上であってよい。 Melanin is observed from the outer skin hair, skin, head, eyes, etc. of animals, and when melanin is overproduced, it deposits on the skin and forms spots, freckles, etc., and also accelerates skin aging. , May also induce skin cancer. Diseases or symptoms due to overproduction of melanin include stains, moles, senile stains, fine dust, epidermal melanotic lesions, cafe's au light scales, moles, and moles of Becker. (Becker's Nevus), Mole (Nevus Spils), Mole (Lentiines), Black spots, Dermal melanotic lesions, Mongolian spot, Ota mother's spot (Nevus) Two-sided bilateral Ota mole-like pigmented mole (Acquired bilateral nevus of Ota-like moles), Ito mole (Nevus of Ito), blue mole (Blue nevus), melanogenic cell mole (Melanocytic) Junctional nevus, Compound nevus, Intradermal nevus, Mole nevus, Congenital melanin cell spicy nevus, Congenital nevocatic nevus Spots (Spitz nevus), dysplastic nevus, melanoma (Melanoma), malignant melanoma, superficial spreading melanoma, terminal melanoma (Acral lentiginous melanoma), nodular melanoma, pigmented basal cell carcinoma, pigmented dermatofibromas, pigmented mole cyst, pigmented skin cyst (dermatofibromas) It may be one or more selected from the group consisting of moleid), ultraviolet pigmentation, drug pigmentation, post-inflammatory pigmentation, and pigmentation and pigmented keratoacanthomas resulting from dermatitis.
本発明の一具現例として、前記3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドは、メラニンの生成及び蓄積を抑制するため、しみ、そばかす、黒色点、母斑、黒色腫、紫外線による色素沈着、薬物による色素沈着、炎症後色素沈着、及び皮膚炎から発生する色素沈着からなる群より選ばれる一つ以上を予防、改善又は治療するものであってよい。 As an embodiment of the present invention, the 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2- Carboxamide suppresses the production and accumulation of melanin from spots, freckles, black spots, nevus, melanoma, UV pigmentation, drug-induced hyperpigmentation, post-inflammatory hyperpigmentation, and pigmentation resulting from dermatitis. One or more selected from the group may be prevented, ameliorated or treated.
本発明は、一側面において、メラニン形成細胞にメラニン形成を誘導する物質を処理してメラニンを形成する過程が、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドによって抑制されることを確認した。 In one aspect of the present invention, the process of treating melanin-forming cells with a substance that induces melanin formation to form melanin is 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [. It was confirmed that it was suppressed by [3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide.
このような結果から、本発明者らは、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドを皮膚美白用物質に用い得ることを見出した。このような3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドの効能を用いてメラニンに係る前記疾患や症状に対して予防、改善、及び治療などを図ることもできる。 Based on these results, the present inventors have identified 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene. It has been found that -2-carboxamide can be used as a skin whitening substance. Using the efficacy of 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide. It is also possible to prevent, improve, and treat the above-mentioned diseases and symptoms related to melanin.
本発明のまた他の側面によると、前記組成物中の3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その異性体、その許容可能な塩、その水和物、又はその溶媒和物の含量は、前記組成物の総重量に対し、0.0001質量%〜20質量%の範囲であってよい。一具現例において、前記含量は、前記組成物の総重量に対し、0.0001質量%以上、0.0005質量%以上、0.001質量%以上、0.005質量%以上、0.01質量%以上、0.05質量%以上、0.1質量%以上、0.3質量%以上、0.5質量%以上、0.8質量%以上、1質量%以上、3質量%以上、5質量%以上、8質量%以上、10質量%以上、12質量%以上、15質量%以上、又は18質量%以上であってよい。また、前記含量は、前記組成物の総重量に対し、20質量%以下、18質量%以下、15質量%以下、12質量%以下、10質量%以下、8質量%以下、5質量%以下、3質量%以下、1質量%以下、0.8質量%以下、0.5質量%以下、0.3質量%以下、0.1質量%以下、0.05質量%以下、0.01質量%以下、0.005質量%以下、0.001質量%以下、又は0.0005質量%以下であってよい。 According to yet another aspect of the invention, 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [in the composition. b] The content of thiophen-2-carboxamide, an isomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof is 0.0001% by mass to 20% by mass based on the total weight of the composition. It may be in the range of. In one embodiment, the content is 0.0001% by mass or more, 0.0005% by mass or more, 0.001% by mass or more, 0.005% by mass or more, 0.01% by mass with respect to the total weight of the composition. % Or more, 0.05% by mass or more, 0.1% by mass or more, 0.3% by mass or more, 0.5% by mass or more, 0.8% by mass or more, 1% by mass or more, 3% by mass or more, 5% by mass % Or more, 8% by mass or more, 10% by mass or more, 12% by mass or more, 15% by mass or more, or 18% by mass or more. The content is 20% by mass or less, 18% by mass or less, 15% by mass or less, 12% by mass or less, 10% by mass or less, 8% by mass or less, 5% by mass or less, based on the total weight of the composition. 3% by mass or less, 1% by mass or less, 0.8% by mass or less, 0.5% by mass or less, 0.3% by mass or less, 0.1% by mass or less, 0.05% by mass or less, 0.01% by mass Hereinafter, it may be 0.005% by mass or less, 0.001% by mass or less, or 0.0005% by mass or less.
本発明のまた他の側面によると、前記3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド、その異性体、その許容可能な塩、その水和物、又はその溶媒和物の投与量は、0.0001mg/kg/日〜20mg/kg/日の範囲であってよい。一具現例において、前記投与量は、0.0001mg/kg/日以上、0.0005mg/kg/日以上、0.001mg/kg/日以上、0.005mg/kg/日以上、0.01mg/kg/日以上、0.05mg/kg/日以上、0.1mg/kg/日以上、0.5mg/kg/日以上、0.8mg/kg/日以上、1mg/kg/日以上、2mg/kg/日以上、3mg/kg/日以上、5mg/kg/日以上、8mg/kg/日以上、10mg/kg/日以上、12mg/kg/日以上、15mg/kg/日以上、又は18mg/kg/日以上であってよい。また、前記投与量は、20mg/kg/日以下、18mg/kg/日以下、15mg/kg/日以下、12mg/kg/日以下、10mg/kg/日以下、8mg/kg/日以下、5mg/kg/日以下、3mg/kg/日以下、2mg/kg/日以下、1mg/kg/日以下、0.8mg/kg/日以下、0.5mg/kg/日以下、0.1mg/kg/日以下、0.05mg/kg/日以下、0.01mg/kg/日以下、0.005mg/kg/日以下、0.001mg/kg/日以下、又は0.0005mg/kg/日以下であってよい。 According to yet another aspect of the present invention, the 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene- The dose of 2-carboxamide, an isomer thereof, an acceptable salt thereof, a hydrate thereof, or a solvate thereof may be in the range of 0.0001 mg / kg / day to 20 mg / kg / day. In one embodiment, the doses are 0.0001 mg / kg / day or higher, 0.0005 mg / kg / day or higher, 0.001 mg / kg / day or higher, 0.005 mg / kg / day or higher, 0.01 mg / day or higher. kg / day or more, 0.05 mg / kg / day or more, 0.1 mg / kg / day or more, 0.5 mg / kg / day or more, 0.8 mg / kg / day or more, 1 mg / kg / day or more, 2 mg / day or more kg / day or more, 3 mg / kg / day or more, 5 mg / kg / day or more, 8 mg / kg / day or more, 10 mg / kg / day or more, 12 mg / kg / day or more, 15 mg / kg / day or more, or 18 mg / day It may be kg / day or more. The doses are 20 mg / kg / day or less, 18 mg / kg / day or less, 15 mg / kg / day or less, 12 mg / kg / day or less, 10 mg / kg / day or less, 8 mg / kg / day or less, 5 mg. / Kg / day or less, 3 mg / kg / day or less, 2 mg / kg / day or less, 1 mg / kg / day or less, 0.8 mg / kg / day or less, 0.5 mg / kg / day or less, 0.1 mg / kg / Day or less, 0.05 mg / kg / day or less, 0.01 mg / kg / day or less, 0.005 mg / kg / day or less, 0.001 mg / kg / day or less, or 0.0005 mg / kg / day or less It may be there.
本発明の一側面によると、前記組成物が薬学組成物である皮膚美白用組成物であってよい。 According to one aspect of the present invention, the composition may be a skin whitening composition which is a pharmaceutical composition.
前記薬学組成物は、保存剤、防腐剤、安定化剤、水和剤又は乳化促進剤、浸透圧調節のための塩及び/又は緩衝剤などの薬剤学的補助剤、並びにその他治療的に有用な物質をさらに含有していてよく、通常的な方法に従い多様な経口投与剤又は非経口投与剤の形態で剤形化していてよい。 The pharmaceutical composition is a preservative, a preservative, a stabilizer, a wettable powder or an emulsion promoter, a pharmacological aid such as a salt and / or a buffer for osmoregulation, and other therapeutically useful. Substances may be further contained and may be emulsified in the form of various oral or parenteral administrations according to conventional methods.
前記経口投与剤は、例えば、錠剤、丸剤、硬質及び軟質カプセル剤、液剤、懸濁剤、乳化剤、シロップ剤、粉剤、散剤、細粒剤、顆粒剤、ペレット剤などがあり、これらの剤形は、有効成分の他、界面活性剤、希釈剤(例:ラクトース、デキストロース、スクロース、マンニトール、ソルビトール、セルロース及びグリシン)、滑沢剤(例:シリカ、タルク、ステアリン酸及びそのマグネシウム又はカルシウム塩、並びにポリエチレングリコール)を含有していてよい。錠剤は、さらに、マグネシウムアルミニウムシリケート、澱粉ペースト、ゼラチン、トラガカンス、メチルセルロース、ナトリウムカルボキシメチルセルロース及びポリビニルピロリジンといった結合剤を含有していてよく、場合によって、澱粉、寒天、アルギン酸又はそのナトリウム塩といった崩解剤、吸収剤、着色剤、香味剤、及び甘味剤などの薬剤学的添加剤を含有していてよい。前記錠剤は、通常の混合、顆粒化又はコーティング方法によって製造されていてよい。 Examples of the oral administration agent include tablets, pills, hard and soft capsules, liquids, suspending agents, emulsifiers, syrups, powders, powders, fine granules, granules, pellets and the like. In addition to the active ingredient, the form is a surfactant, a diluent (eg lactose, dextrose, syrup, mannitol, sorbitol, cellulose and glycine), a lubricant (eg silica, talc, stearic acid and its magnesium or calcium salt). , And polyethylene glycol). The tablets may further contain binders such as magnesium aluminum silicate, starch paste, gelatin, tragacanth, methyl cellulose, sodium carboxymethyl cellulose and polyvinylpyrrolidin, and optionally a disrupting agent such as starch, agar, alginic acid or a sodium salt thereof. , Absorbents, colorants, flavors, and pharmaceutical additives such as sweeteners. The tablets may be produced by conventional mixing, granulation or coating methods.
また、前記非経口投与の形態としては、経皮投与型剤形であってよく、例えば、注射剤、点滴剤、軟膏、ローション、ゲル、クリーム、スプレー、懸濁剤、乳剤、坐剤、パッチなどの剤形であってよいが、これらに制限されるものではない。 The form of parenteral administration may be a transdermal dosage form, for example, an injection, an infusion, an ointment, a lotion, a gel, a cream, a spray, a suspension, an emulsion, a suppository, or a patch. However, the dosage form is not limited to these.
前記薬学組成物は非経口、直腸、局所、経皮、皮下などに投与されてよい。 The pharmaceutical composition may be administered parenterally, rectal, topically, transdermally, subcutaneously or the like.
前記有効成分の投与量の決定は、通常の技術者の水準内にあり、薬物の1日投与量は、投与しようとする対象の進行程度、発病時期、年齢、健康状態、合併症などの多様な要因に応じて異なり得る。 The determination of the dose of the active ingredient is within the standard of a normal technician, and the daily dose of the drug varies depending on the degree of progression of the subject to be administered, the time of onset, age, health condition, complications, etc. It can vary depending on various factors.
前記薬学組成物は皮膚外用剤であってよく、前記皮膚外用剤は、外皮に塗布されるものであればいずれも含み得る組成物の総称であって、多様な剤形の医薬品又は医薬外品がこれに含まれ得る。 The pharmaceutical composition may be an external preparation for skin, and the external preparation for skin is a general term for compositions that can contain any of those applied to the outer skin, and is a pharmaceutical product or quasi-drug in various dosage forms. Can be included in this.
本発明のまた他の側面によると、前記組成物が化粧料組成物である皮膚美白用組成物であってよい。 According to yet another aspect of the present invention, the composition may be a skin whitening composition which is a cosmetic composition.
前記化粧料組成物には、機能性添加物及び一般的な化粧料組成物に含まれる成分がさらに含まれていてよい。前記機能性添加物としては、水溶性ビタミン、油溶性ビタミン、高分子ペプチド、高分子多糖、スフィンゴ脂質及び海草エキスからなる群より選ばれた成分を含んでいてよい。その他含まれる配合成分としては、油脂成分、保湿剤、エモリエント剤、界面活性剤、有機及び無機顔料、有機粉体、紫外線吸収剤、防腐剤、殺菌剤、酸化防止剤、植物抽出物、pH調整剤、アルコール、色素、香料、血行促進剤、冷感剤、制汗剤、精製水などが挙げられる。 The cosmetic composition may further contain functional additives and ingredients contained in general cosmetic compositions. The functional additive may contain a component selected from the group consisting of water-soluble vitamins, oil-soluble vitamins, high molecular weight peptides, high molecular weight polysaccharides, sphingolipids and seaweed extracts. Other ingredients included include fats and oils, moisturizers, emollients, surfactants, organic and inorganic pigments, organic powders, UV absorbers, preservatives, disinfectants, antioxidants, plant extracts, pH adjustments. Examples include agents, alcohols, pigments, fragrances, blood circulation promoters, cold sensation agents, antiseptic agents, purified water and the like.
前記化粧料組成物は、その剤形が特に限定されず、目的とするところに応じて適宜選択すればよい。例えば、スキンローション、スキンソフナー、スキントナー、アストリンゼント、ローション、ミルクローション、モイスチャーローション、栄養ローション、マッサージクリーム、栄養クリーム、モイスチャークリーム、ハンドクリーム、ファウンデーション、エッセンス、栄養エッセンス、パック、石鹸、クレンジングフォーム、クレンジングローション、クレンジングクリーム、ボディーローション、及びボディークレンザーからなる群より選ばれたいずれか一つ以上の剤形で製造されていてよいが、これらに制限されるものではない。 The dosage form of the cosmetic composition is not particularly limited, and the cosmetic composition may be appropriately selected depending on the intended purpose. For example, Skin Lotion, Skin Softener, Skin Toner, Astringent, Lotion, Milk Lotion, Moisture Lotion, Nutrition Lotion, Massage Cream, Nutrition Cream, Moisture Cream, Hand Cream, Foundation, Essence, Nutrition Essence, Pack, Soap, Cleansing Foam, It may be produced in any one or more dosage forms selected from the group consisting of cleansing lotions, cleansing creams, body lotions, and body cleansers, but is not limited thereto.
本発明の一側面に係る剤形がペースト、クリーム、又はゲルの場合は、担体成分として、動物繊維、植物繊維、ワックス、パラフィン、澱粉、トラガカント、セルロース誘導体、ポリエチレングリコール、シリコン、ベントナイト、シリカ、タルク、又は酸化亜鉛などが用いられていてよい。 When the dosage form according to one aspect of the present invention is a paste, cream, or gel, the carrier components include animal fiber, plant fiber, wax, paraffin, starch, tragacanth, cellulose derivative, polyethylene glycol, silicon, bentonite, silica, and the like. Starch, zinc oxide, or the like may be used.
本発明の一側面に係る剤形がパウダー又はスプレーの場合は、担体成分として、ラクトース、タルク、シリカ、水酸化アルミニウム、ケイ酸カルシウム、又はポリアミドパウダーが用いられていてよく、特にスプレーである場合は、さらに、クロロフルオロハイドロカーボン、プロパン/ブタン、又はジメチルエーテルのような推進剤を含んでいてよい。 When the dosage form according to one aspect of the present invention is powder or spray, propane, talc, silica, aluminum hydroxide, calcium silicate, or polyamide powder may be used as the carrier component, and particularly when it is a spray. May further include propellants such as chlorofluorohydrocarbon, propane / butane, or dimethyl ether.
本発明の一側面に係る剤形が溶液又は乳濁液である場合は、担体成分として、溶媒、溶媒和剤、又は乳濁化剤が用いられ、例えば、水、エタノール、イソプロパノール、エチルカーボネート、エチルアセテート、ベンジルアルコール、安息香酸ベンジル、プロピレングリコール、1,3−ブチルグリコールオイル、グリセロール脂肪族エステル、ポリエチレングリコール、又はソルビタンの脂肪酸エステルがある。 When the dosage form according to one aspect of the present invention is a solution or an emulsion, a solvent, a solvent admixture, or an emulsion agent is used as a carrier component, for example, water, ethanol, isopropanol, ethyl carbonate, etc. There are ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butyl glycol oil, glycerol aliphatic esters, polyethylene glycol, or fatty acid esters of sorbitan.
本発明の一側面に係る剤形が懸濁液である場合は、担体成分として、水、エタノール、又はプロピレングリコールのような液状希釈剤、エトキシル化イソステアリルアルコール、ポリオキシエチレンソルビトールエステル、及びポリオキシエチレンソルビタンエステルのような懸濁剤、微結晶性セルロース、アルミニウムメタヒドロキシド、ベントナイト、寒天、又はトラガカントなどが用いられていてよい。 When the dosage form according to one aspect of the present invention is a suspension, the carrier components include water, ethanol, or a liquid diluent such as propylene glycol, ethoxylated isostearyl alcohol, polyoxyethylene sorbitol ester, and poly. Suspensions such as oxyethylene sorbitan ester, microcrystalline cellulose, aluminum metahydroxylide, bentonite, agar, tragacant and the like may be used.
本発明の一側面に係る剤形が界面活性剤含有クレンジングである場合は、担体成分として、脂肪族アルコールスルフェート、脂肪族アルコールエーテルスルフェート、スルホサクシネートモノエステル、イセチオン酸、イミダゾリウム誘導体、メチルタウレート、サルコシネート、脂肪酸アミドエーテルスルフェート、アルキルアミドベタイン、脂肪族アルコール、脂肪酸グリセリド、脂肪酸ジエタノールアミド、植物性油、ラノリン誘導体、又はエトキシル化グリセロール脂肪酸エステルなどが用いられていてよい。 When the dosage form according to one aspect of the present invention is a surfactant-containing cleansing, as a carrier component, an aliphatic alcohol sulfate, an aliphatic alcohol ether sulfate, a sulfosuccinate monoester, an acetylonic acid, an imidazolium derivative, Methyl tauret, sarcosinate, fatty acid amide ether sulfate, alkylamide betaine, fatty alcohol, fatty acid glyceride, fatty acid diethanolamide, vegetable oil, lanolin derivative, ethoxylated glycerol fatty acid ester and the like may be used.
以下、実施例、実験例を通じて、本発明の一側面の構成及び効果をより具体的に説明する。なお、下記の例は本発明に関する理解を助けるための目的から例示したものに過ぎず、本発明の範疇及び範囲がそれらによって制限されるものではない。 Hereinafter, the configuration and effect of one aspect of the present invention will be described more specifically through Examples and Experimental Examples. It should be noted that the following examples are merely exemplified for the purpose of assisting the understanding of the present invention, and the scope and scope of the present invention are not limited by them.
[実施例1]
細胞及び皮膚モデルの準備
正常ヒト表皮メラニン形成細胞(Normal human epidermal melanocytes、NHEMs、Cascade Biologics、Portland、OR、USA)を入手し、C57BL/6 J(black、a/a)マウス由来のメラニン形成細胞であるメラン−A(Melan−A)細胞株をDorothy C. Bennett博士(St. George’s Hospital Medical School、London、UK)から入手した。B16F1マウス黒色腫(melanoma)細胞株はATCC(Manassas、VA、USA)から入手した。
[Example 1]
Preparation of cell and skin model Normal human epidermal melanocytes (Normal human epidermal melanocytes, NHEMs, Cascade Biologics, Portland, OR, USA) were obtained and melanocytes derived from C57BL / 6J (black, a / a) mice. A melan-A cell line was obtained from Dr. Dorothy C. Bennett (St. George's Hospital Medical School, London, UK). The B16F1 mouse melanoma cell line was obtained from ATCC (Manassas, VA, USA).
正常ヒト表皮メラニン形成細胞は、M−254培地でヒトメラニン形成細胞増殖サプリメント(Human Melanocyte Growth Supplements、HMGS)(Cascade Biologics、Inc.、Mansfield、UK)下で維持させた。Melan−A細胞はRPMI 1640培地で10%(v/v)ウシ胎児血清、1%(v/v)ペニシリン−ストレプトマイシン、及び0.2μM ホルボール12−ミリスタート13−アセタート(phorbol 12−myristate 13−acetate)下で維持させた。B16F1マウス黒色腫細胞株は10%(v/v)ウシ胎児血清及び1%(v/v)ペニシリン−ストレプトマイシンを添加したDMEMで培養した。 Normal human epidermal melanocytes were maintained in M-254 medium under Human Melanocyte Growth Supplements, HMGS (Cascade Biologics, Inc., Mansfield, UK). Melan-A cells were found in RPMI 1640 medium with 10% (v / v) fetal bovine serum, 1% (v / v) penicillin-streptomycin, and 0.2 μM phorbol 12-mylstart 13-acetylate 13-. It was maintained under accept). B16F1 mouse melanoma cell lines were cultured in DMEM supplemented with 10% (v / v) fetal bovine serum and 1% (v / v) penicillin-streptomycin.
三次元ヒト皮膚代替材(Three−dimensional human skin substitute、MelanoDerm(商標)、MEL−312−B、MatTek社、Seoul、Korea)は入手した後、KGF(Keratinocyte Growth Factor)、b−FGF(Fibroblast Growth Factor)、及びα−MSH(alpha−melanocyte stimulating hormones)に最適化したEPI−100−NMM−113培地で製造者の指示に従って維持した。 After obtaining the three-dimensional human skin substitute material (Thre-dimensional human skin substitut, MelanoDerm ™, MEL-312-B, MatTek, Seoul, Korea), KGF (Keratinocyte Growth Factor (Keratinocyte Growth Factor) It was maintained in EPI-100-NMM-113 medium optimized for Factor) and α-MSH (alpha-melanocyte stimulating homones) according to the manufacturer's instructions.
前記皮膚モデルの明るさの変化(ΔL)指数は、色度計(colorimeter、CR−300、Konica Minolta社、Tokyo、Japan)で測定されたL指数(L value、lightness index)から計算し、ΔL=13日目又は20日目のL(処置皮膚)−L(対照群皮膚)で計算した。ΔL指数の増加は、皮膚において化合物誘導による色素の過少沈着を示す(Lee et al., 2013)。 The change in brightness (ΔL) index of the skin model is calculated from the L index (L value, lightness index) measured by a chromaticity meter (colorimeter, CR-300, Konica Minolta, Tokyo, Japan), and ΔL. = Calculated as L (treated skin) -L (control group skin) on day 13 or 20. Increased ΔL index indicates compound-induced pigment under-deposition in the skin (Lee et al., 2013).
試薬
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドである「SAG」は、Calbiochem(San Diego、CA、USA)から入手した。α−MSH、アルブチン(arbutin)、及びコウジ酸(kojic acid)はSigma−Aldrich社(St. Louis、MO、USA)から入手した。
Reagent 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide "SAG" Obtained from Calbiochem (San Diego, CA, USA). α-MSH, arbutin, and kojic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA).
メラニンアッセイ
細胞をトリプシン/EDTAで処理後、1000xgで5分間遠心分離し、PBSで2回洗浄した。チューブ内の最終細胞ペレットを写真に撮り、細胞ペレットは1N NaOHに溶解させた。均質化させた細胞抽出物を96−ウェル(well)プレートに移載し、ELISAプレートリーダーを利用して吸光度405nmにおける相対的メラニン量を測定した。
Melanin assay cells were treated with trypsin / EDTA, centrifuged at 1000 xg for 5 minutes and washed twice with PBS. The final cell pellet in the tube was photographed and the cell pellet was dissolved in 1N NaOH. The homogenized cell extract was transferred to a 96-well plate and the relative melanin content at absorbance 405 nm was measured using an ELISA plate reader.
ウエスタンブロット分析
すべての溶解物は2×Laemmliサンプルバッファー(2×Laemmli sample buffer、62.5mM Tris−HCl、pH6.8、25%[v/v]glycerol、2%[w/v]SDS、5%[v/v]β−mercaptoethanol、及び0.01%[w/v]bromophenol blue)(Bio−Rad、Hercules社、CA、USA)を用いて準備した。すべての細胞タンパク質はBradford solution(Bio−Rad)を用いて測定した。その後、前記試料はSDS−PAGEで分離し、PVDFメンブレイン(Bio−Rad)に移転した。TBST(25mM Tris、140mM NaCl、及び0.05%[v/v]Tween(登録商標)20)中の4%(w/v)スキムミルク(skim milk)でブロッキングした後、メンブレインを特定の1次抗体とともにオーバーナイト(overnight)培養させた。
Western blot analysis All lysates were 2 x Laemmli sample buffer, 62.5 mM Tris-HCl, pH 6.8, 25% [v / v] glycerol, 2% [w / v] SDS, 5 Prepared using% [v / v] β-mercaptoethanolol and 0.01% [w / v] bromophenol blue) (Bio-Rad, Hercules, CA, USA). All cellular proteins were measured using Bradford solution (Bio-Rad). The sample was then separated by SDS-PAGE and transferred to PVDF membrane (Bio-Rad). After blocking with 4% (w / v) skim milk in TBST (25 mM Tris, 140 mM NaCl, and 0.05% [v / v] Tween® 20), the membrane is identified as 1 It was cultured overnight with the next antibody.
抗アクチン抗体(anti−actin antibody、MAB1501、1:10,000)はMillipore社(Temecula、CA、USA)から入手し、抗αPEP7(チロシナーゼ)抗体(anti−αPEP7(tyrosinase)antibody、1:1000)と抗αPEP1(TRP1)抗体(anti−αPEP1(TRP1)antibody、1:1000)は、V. J. Hearing(NIH、Bethesda、MD)から入手した。タンパク質の検出のために、前記メンブレインはHRP−コンジュゲーティッド2次抗体を用いて培養し、シグナルはSuperSignal(登録商標) West Dura HRP Detection Kit(Pierce社、Rockford、IL、USA)を利用して測定した。 Anti-actin antibody (anti-actin antibody, MAB1501, 1: 10,000) was obtained from Millipore (Temecula, CA, USA) and anti-αPEP7 (tyrosinase) antibody (anti-αPEP7 (tyrosinase) antibody, 1: 1000). And anti-αPEP1 (TRP1) antibody (anti-αPEP1 (TRP1) antibody, 1: 1000) was obtained from VJ Hairing (NIH, Bethesda, MD). For protein detection, the membrane was cultured with HRP-conjugated secondary antibody and the signal was signaled using SuperSignal® West Dura HRP Detection Kit (Pierce, Rockford, IL, USA). Was measured.
統計分析
各データは少なくとも3回の独立した実験を施して得たものとし、平均±SE(標準誤差)で表した。結果の統計的評価は一元分散分析(1−way ANOVA)を用いて行った(*:p<0.05、**:p<0.01)。
Statistical analysis Each data was obtained by performing at least 3 independent experiments and was expressed as mean ± SE (standard error). Statistical evaluation of the results was performed using one-way analysis of variance (1-way ANOVA) (*: p <0.05, **: p <0.01).
[実験例1]メラニン形成調節
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミドは、下記の化学式1で示された物質である。
[Experimental Example 1] Regulation of melanin formation 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide Is the substance represented by the following chemical formula 1.
B16F1細胞をα−MSH(1μM)で事前処理し、24時間後に、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド(5μM)又はアルブチン(arbutin、500μM)を処理した。24時間後に、細胞メラニンの含量とチロシナーゼ、TRP1タンパク質の発現水準をウエスタンブロットで分析した。その結果、前記化合物は、α−MSHを処理し培養したB16F1細胞においてメラニンの生成を抑制させた(図1)。前記化合物によってチロシナーゼ、TRP1タンパク質の発現水準も減少した(図2)。 B16F1 cells were pretreated with α-MSH (1 μM) and 24 hours later, 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl. ] Benzo [b] thiophene-2-carboxamide (5 μM) or arbutin (500 μM) was treated. After 24 hours, the cell melanin content and the expression levels of tyrosinase and TRP1 protein were analyzed by Western blotting. As a result, the compound suppressed the production of melanin in B16F1 cells treated and cultured with α-MSH (Fig. 1). The compound also reduced the expression levels of tyrosinase and TRP1 protein (Fig. 2).
[実験例2]人工皮膚実験
三次元ヒト皮膚代替材(正常ヒト表皮角質細胞及びNHEMsを含む組換え表皮モデル)を用いてメラニンの生成の調節を実験した。
[Experimental Example 2] Artificial skin experiment A three-dimensional human skin substitute material (recombinant epidermal model containing normal human epidermal corneocytes and NHEMs) was used to experiment with the regulation of melanin production.
具体的に、3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド(5μM)、コウジ酸(kojic acid、KA、5mM)を、三次元ヒト皮膚代替材に3日に一回ずつ13日間及び20日間投与して色素沈着に及ぼす影響を評価した。色素沈着は色度計(colorimeter)で評価し、図4中、ΔLは対照群と物質処理群間の明るさの差を計算した値である。 Specifically, 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N-[[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide (5 μM), Kojic acid (KA, 5 mM) was administered to a three-dimensional human skin substitute once every three days for 13 and 20 days to evaluate its effect on pigmentation. The pigmentation was evaluated by a colorimeter, and in FIG. 4, ΔL is a value obtained by calculating the difference in brightness between the control group and the substance treatment group.
その結果、前記化合物によって皮膚色素沈着が減少した(図3及び図4)。これにより、前記化合物が三次元ヒト皮膚代替材でもメラニン形成を調節し且つ色素沈着を調節することを確認した。 As a result, the compound reduced skin pigmentation (FIGS. 3 and 4). From this, it was confirmed that the compound regulates melanin formation and pigmentation even in a three-dimensional human skin substitute.
以下、本発明の一側面に係る組成物の剤形例について説明するが、他の様々な剤形への応用も可能であり、且つ、これらは本発明を限定するためのものではない、単に具体的に説明するためのものである。 Hereinafter, examples of dosage forms of the composition according to one aspect of the present invention will be described, but application to various other dosage forms is also possible, and these are not intended to limit the present invention, simply. This is for a specific explanation.
[剤形例1]軟質カプセル剤
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド20mg、L−カルニチン80〜140mg、大豆油180mg、パーム油2mg、植物性硬化油8mg、黄蝋4mg及びレシチン6mgを混合し、通常の方法に従い1カプセルに充填して軟質カプセル剤を製造した。
[Dosage Form Example 1] Soft Capsules 3-Chloro-N- [Trans-4- (Methylamino) Cyclohexyl] -N- [[3- (4-Pyridinyl) Phenyl] Methyl] Benzo [b] Thiophen-2- 20 mg of carboxamide, 80 to 140 mg of L-carnitine, 180 mg of soybean oil, 2 mg of palm oil, 8 mg of hydrogenated vegetable oil, 4 mg of yellow wax and 6 mg of lecithin were mixed and filled into one capsule according to a usual method to prepare a soft capsule. ..
[剤形例2]錠剤
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド20mg、ガラクトオリゴ糖200mg、乳糖60mg及び麦芽糖140mgを混合して流動層乾燥機を利用して顆粒した後、糖エステル(sugar ester)6mgを添加して、打錠機で打錠して錠剤を製造した。
[Dosage Form Example 2] Tablets 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide 20 mg , 200 mg of galactooligosaccharide, 60 mg of lactose and 140 mg of maltose were mixed and granulated using a fluidized layer dryer, and then 6 mg of sugar ester was added and tableted with a tableting machine to produce tablets. ..
[剤形例3]顆粒剤
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド20mg、無水結晶ブドウ糖250mg及び澱粉550mgを混合し、流動層造粒機を使用して顆粒に成形した後、包に充填して顆粒剤を製造した。
[Dosage Form Example 3] Granules 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide 20 mg, 250 mg of anhydrous crystalline glucose and 550 mg of starch were mixed, formed into granules using a fluidized bed granulator, and then packed in a packet to produce granules.
[剤形例4]ドリンク剤
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド20mg、ブドウ糖10g、クエン酸0.6g、及び液状オリゴ糖25gを混合した後、精製水300mlを加えて各瓶に200mlずつ充填する。瓶に充填した後、130℃で4〜5秒間殺菌してドリンク剤を製造した。
[Formation Example 4] Drinking agent 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide After mixing 20 mg, 10 g of glucose, 0.6 g of citric acid, and 25 g of liquid oligosaccharide, 300 ml of purified water is added, and each bottle is filled with 200 ml. After filling the bottle, it was sterilized at 130 ° C. for 4 to 5 seconds to produce a drink.
[剤形例5]注射剤
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド50mg、適量の注射用滅菌蒸留水、適量のpH調節剤を用いて通常の方法に従い注射剤を製造した。
[Dosage Form Example 5] Injection 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide An injection was prepared according to a usual method using 50 mg, an appropriate amount of sterile distilled water for injection, and an appropriate amount of a pH adjuster.
[剤形例6]柔軟化粧水(スキンローション)
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド3.0質量%、L−アスコルビン酸−2−リン酸マグネシウム塩1.00質量%、水溶性コラーゲン(1%水溶液)1.00質量%、クエン酸ナトリウム0.10質量%、クエン酸0.05質量%、甘草エキス0.20質量%、1,3−ブチレングリコール3.00質量%、残量として精製水を用いて柔軟化粧水(スキンローション)を製造した。
[Dosage form example 6] Soft lotion (skin lotion)
3-Chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide 3.0% by mass, L- Ascorbic acid-2-magnesium phosphate salt 1.00% by mass, water-soluble collagen (1% aqueous solution) 1.00% by mass, sodium citrate 0.10% by mass, citric acid 0.05% by mass, licorice extract 0. A soft cosmetic solution (skin lotion) was produced using 20% by mass, 1,3-butylene glycol 3.00% by mass, and purified water as the remaining amount.
[剤形例7]クリーム
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド3.00質量%、ポリエチレングリコールモノステアレート2.00質量%、自己乳化型モノステアリン酸グリセリン5.00質量%、プロピレングリコール4.00質量%、スクアレン6.00質量%、トリ2−エチルヘキサングリセリル6.00質量%、スフィンゴ糖脂質1.00質量%、1,3−ブチレングリコール7.00質量%、蜜蝋5.00質量%、残量として精製水を用いてクリーム状製剤を製造した。
[Dosage Form Example 7] Cream 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide 3 .00% by mass, polyethylene glycol monostearate 2.00% by mass, self-emulsifying glycerin monostearate 5.00% by mass, propylene glycol 4.00% by mass, squalene 6.00% by mass, tri2-ethylhexaneglyceryl A creamy preparation was produced using 6.00% by mass, 1.00% by mass of sphingo glycolipid, 7.00% by mass of 1,3-butylene glycol, 5.00% by mass of beeswax, and purified water as the remaining amount.
[剤形例8]パック
3−クロロ−N−[トランス−4−(メチルアミノ)シクロヘキシル]−N−[[3−(4−ピリジニル)フェニル]メチル]ベンゾ[b]チオフェン−2−カルボキサミド3.00質量%、ポリビニルアルコール13.00質量%、L−アスコルビン酸−2−リン酸マグネシウム塩1.00質量%、ラウロイルヒドロキシプロリン1.00質量%、水溶性コラーゲン(1%水溶液)2.00質量%、1,3−ブチレングリコール3.00質量%、エタノール5.00質量%、残量として精製水を用いてパックを製造した。
[Formation Example 8] Pack 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophen-2-carboxamide 3 0.00% by mass, polyvinyl alcohol 13.00% by mass, L-ascorbic acid-2-maganoate phosphate 1.00% by mass, lauroyl hydroxyproline 1.00% by mass, water-soluble collagen (1% aqueous solution) 2.00 A pack was produced using purified water in an amount of% by mass, 1,3-butylene glycol 3.00% by mass, ethanol 5.00% by mass, and the remaining amount.
[剤形例9]健康食品
下記の表に記載された組成にて通常の方法に従い健康食品を製造した。
[Dosage Form Example 9] Health food A health food was produced according to a usual method with the composition shown in the table below.
前記ビタミン及び無機質混合物の組成比は、比較的に健康食品に適合した成分を例にして混合組成したが、その配合比を任意に変形実施しても構わなく、通常の健康食品の製造方法に従い前記成分を混合した後、通常の方法に従い健康食品の組成物の製造に用いてもよい。 The composition ratio of the vitamin and the inorganic mixture is a mixture of ingredients that are relatively suitable for health foods as an example, but the composition ratio may be arbitrarily modified and according to a normal health food production method. After mixing the above components, it may be used in the production of a composition of a health food according to a usual method.
[剤形例10]健康飲料 [Dosage form example 10] Health drink
前記表2のように総体積900mlになるように残量の精製水を添加して通常の健康飲料の製造方法に従い前記成分を混合してから約1時間85℃で撹拌加熱し、これにより調製された溶液をろ過して得られたろ液を滅菌された2リットルの容器に入れて密封滅菌した後に冷蔵保管して健康飲料を製造した。 As shown in Table 2, the remaining amount of purified water is added so as to have a total volume of 900 ml, the components are mixed according to a normal method for producing a healthy beverage, and then stirred and heated at 85 ° C. for about 1 hour to prepare the mixture. The filtrate obtained by filtering the resulting solution was placed in a sterilized 2 liter container, sterilized by sealing, and then refrigerated to produce a healthy beverage.
以上、本発明内容の特定の部分を詳しく記述したが、当業界の通常の知識を有する者にとってこのような具体的な記述は単に好適な実施態様であるに過ぎず、これによって本発明の範囲が制限されるものではないことは明白であろう。したがって、本発明の実質的な範囲は添付の請求項とそれらの等価物によって定義されるといえよう。 Although a specific part of the content of the present invention has been described in detail above, such a specific description is merely a preferred embodiment for a person having ordinary knowledge in the art, and thereby the scope of the present invention. It will be clear that is not limited. Therefore, it can be said that the substantial scope of the present invention is defined by the appended claims and their equivalents.
Claims (8)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020160107873A KR102610930B1 (en) | 2016-08-24 | 2016-08-24 | COMPOSITION FOR SKIN-WHITENING COMPRISING 3-CHLORO-N-[TRANS-4-(METHYLAMINO)CYCLOHEXYL]-N-[[3-(4-PYRIDINYL)PHENYL]METHYL]BENZO[b]THIOPHENE-2-CARBOXAMIDE |
| KR10-2016-0107873 | 2016-08-24 | ||
| PCT/KR2017/009033 WO2018038465A1 (en) | 2016-08-24 | 2017-08-18 | Skin-whitening composition containing 3-chloro-n-[trans-4-(methylamino)cyclohexyl]-n-[[3-(4-pyridinyl)phenyl]methyl]benzo[b]thiophene-2-carboxamide |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2019526545A JP2019526545A (en) | 2019-09-19 |
| JP2019526545A5 JP2019526545A5 (en) | 2020-07-16 |
| JP6883090B2 true JP6883090B2 (en) | 2021-06-09 |
Family
ID=61246138
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019506499A Active JP6883090B2 (en) | 2016-08-24 | 2017-08-18 | A composition for skin whitening containing 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide. |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP6883090B2 (en) |
| KR (1) | KR102610930B1 (en) |
| CN (1) | CN109890383B (en) |
| WO (1) | WO2018038465A1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7088937B2 (en) | 2016-12-30 | 2022-06-21 | イノビュージョン インコーポレイテッド | Multi-wavelength rider design |
| KR102511934B1 (en) * | 2021-04-27 | 2023-03-21 | 재단법인 아산사회복지재단 | Composition for preventing, improving or treating skin pigmentation |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN100422290C (en) * | 2003-09-10 | 2008-10-01 | 株式会社资生堂 | Antioxidant, whitening agent and skin preparation for external use containing the same |
| CN101597280B (en) * | 2008-08-19 | 2013-06-05 | 北京大学深圳研究生院 | Compound used as seven-pass transmembrane protein and based on sag structure |
| WO2011122840A2 (en) * | 2010-03-31 | 2011-10-06 | (주)아모레퍼시픽 | Inhibitor for melanin, and cosmetic composition containing same |
| CN101993415B (en) * | 2010-09-15 | 2013-08-14 | 北京韩美药品有限公司 | Compound as Hedgehog path inhibitor, medicine composition containing same and application thereof |
| CN102731373B (en) * | 2012-07-19 | 2013-11-27 | 南京药石药物研发有限公司 | Preparation method of intermediate of antitumor drug GDC-0449 (vismodegib) |
| WO2014084085A1 (en) * | 2012-11-28 | 2014-06-05 | 国立大学法人名古屋大学 | Prophylactic/therapeutic agent for hearing disorder or cerebellar ataxia |
| KR101480600B1 (en) * | 2013-01-22 | 2015-01-08 | 경북대학교 산학협력단 | A use for skin whitening of resveratrol derivatives |
| KR101628282B1 (en) * | 2013-07-12 | 2016-06-08 | 주식회사 바이오랜드 | Composition for skin-whitening comprising Arbutin derivative |
| GB2519344A (en) * | 2013-10-18 | 2015-04-22 | Redx Pharma Ltd | Compounds |
-
2016
- 2016-08-24 KR KR1020160107873A patent/KR102610930B1/en active IP Right Grant
-
2017
- 2017-08-18 JP JP2019506499A patent/JP6883090B2/en active Active
- 2017-08-18 WO PCT/KR2017/009033 patent/WO2018038465A1/en active Application Filing
- 2017-08-18 CN CN201780066049.9A patent/CN109890383B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019526545A (en) | 2019-09-19 |
| CN109890383B (en) | 2022-06-07 |
| WO2018038465A1 (en) | 2018-03-01 |
| KR102610930B1 (en) | 2023-12-08 |
| CN109890383A (en) | 2019-06-14 |
| KR20180022439A (en) | 2018-03-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101839484B1 (en) | Composition for improving skin wrinkle and whitening | |
| CN103547250A (en) | Novel use of flavone-based compound | |
| JP6883090B2 (en) | A composition for skin whitening containing 3-chloro-N- [trans-4- (methylamino) cyclohexyl] -N- [[3- (4-pyridinyl) phenyl] methyl] benzo [b] thiophene-2-carboxamide. | |
| JP7038700B2 (en) | A skin whitening composition containing Cytochalasin D | |
| KR101951559B1 (en) | Composition comprising Sinapinic acid or as active ingredients for anti-wrinkle, skin moisturizing, improving skin elasticity, exfoliating, inhibiting erythema or improving skin photo-aging | |
| KR101777992B1 (en) | Composition comprising Jasmone or as active ingredients for anti-wrinkle, skin moisturizing, improving skin elasticity, exfoliating, inhibiting erythema or improving skin photo-aging | |
| KR101787319B1 (en) | Composition comprising Cinchonine or as active ingredients for anti-wrinkle, skin moisturizing, improving skin elasticity, exfoliating, inhibiting erythema or improving skin photo-aging | |
| JP7121757B2 (en) | Whitening composition containing novel quercetin compound | |
| JP6636467B2 (en) | Composition containing extract of autumnal soybean leaves and method for producing the same | |
| US20200345694A1 (en) | Antiaging composition comprising cytochalasin d or sag, and method for screening antiaging substance | |
| KR101617198B1 (en) | Skin whitening composition containing keratin peptide | |
| JP6952104B2 (en) | Composition for enhancing melanin containing 2,4-dichloro-a- (3,4-dihydro-4-oxo-2 (1H) -quinazolinylidene) -β-oxo-benzenepropanenitrile | |
| KR20210050191A (en) | Composition for skin whitening | |
| US20240156717A1 (en) | Composition for enhancing skin whitening effect according to autophagy activity with nypa fruticans extract as an active ingredient | |
| JP7121758B2 (en) | Whitening composition containing novel kaempferol compound derived from post-fermented tea | |
| JP7030692B2 (en) | Moisturizing composition containing 3-O-galloyl-3,3', 5,5', 7-pentahydroxyflavon | |
| KR20190018892A (en) | Composition comprising Guaiyl acetate or as active ingredients for anti-wrinkle, skin moisturizing, improving skin elasticity, exfoliating, inhibiting erythema or improving skin photo-aging | |
| KR102672903B1 (en) | Cosmetic composition comprising theasinensin A | |
| KR101793652B1 (en) | Cosmetics or pharmaceutical composition containing malaxinic acid for skin whitening | |
| JP2022164564A (en) | Skin-whitening composition and skin-whitening method utilizing the same | |
| KR101947914B1 (en) | Composition comprising DL-menthol or as active ingredients for anti-wrinkle, skin moisturizing, improving skin elasticity, exfoliating, inhibiting erythema or improving skin photo-aging | |
| JP6238394B2 (en) | Ceramide production promoter | |
| KR20170099699A (en) | Composition for improving skin conditions comprising Demethoxycurcumin |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200604 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200604 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210427 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210507 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6883090 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |