JP6571985B2 - 金属マグネシウムの製造方法とその製造装置 - Google Patents
金属マグネシウムの製造方法とその製造装置 Download PDFInfo
- Publication number
- JP6571985B2 JP6571985B2 JP2015102618A JP2015102618A JP6571985B2 JP 6571985 B2 JP6571985 B2 JP 6571985B2 JP 2015102618 A JP2015102618 A JP 2015102618A JP 2015102618 A JP2015102618 A JP 2015102618A JP 6571985 B2 JP6571985 B2 JP 6571985B2
- Authority
- JP
- Japan
- Prior art keywords
- magnesium
- plasma
- container
- anhydrous magnesium
- halide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Landscapes
- Manufacture And Refinement Of Metals (AREA)
Description
安価で大量の金属マグネシウムを得ることができる。原料の無水ハロゲン化マグネシウムも入手が容易であるので、金属マグネシウムの製造が容易でコスト低減となる。また、製造装置も構成が簡単であって、量産に適しており、製造コストが低減される。これからのエネルギーの可搬性や財蓄性に優れた製環境負荷の少ないエネルギー資源として、各方面に需要が拡大すると見込まれる金属マグネシウムを安価に入手し、国際競争力を高めることができると言う優れた効果を奏するものである。
尚、前記マイクロ波表面波プラズマの場合においても、適切に反応速度を高める上で、前記加熱手段を使用するようにしても良い。
この還元反応は、
MgCl2+H2(活性種としての励起水素)→Mg+2HClである(発熱反応、マグネシウム,塩酸,マグネシウムイオン,塩酸イオン,塩素,塩素イオンなど)。
1.蒸着物の金属光沢を目視で確認、
2.テスターでの導通を確認(3cm離れた場所で5オーム以下)、
3.SEM(走査型電子顕微鏡)によるEDS(エネルギー分散形分光器)解析で製膜部でのマグネシウムの検出、
4.プラズマ分光分析にて約525nm近辺のマグネシウム発光(青)あり、
5.金属光沢部に水滴をたらして放置すると、水滴部は透明化する。
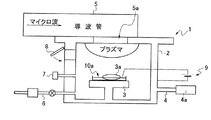
2 容器、
3 載置台、 3a 原料、
4 真空装置、 4a 真空ポンプ、
5 プラズマ装置、 5a 窓、
6 還元ガス供給装置、
7 真空圧計器、
8 原料取出し口、
9 電源、
10a 電極(タングステン等)、
10b ヒータ(フィラメント等)、
11 光源。
Claims (6)
- 大気圧以下の減圧下で、無水ハロゲン化マグネシウムを水素ガス雰囲気中でプラズマに晒すとともに、前記無水ハロゲン化マグネシウムを活性種によって加熱し、前記無水ハロゲン化マグネシウムを還元させて、金属マグネシウムを得ること、
を特徴とする金属マグネシウムの製造方法。 - 無水ハロゲン化マグネシウムが、塩化マグネシウムであること、
を特徴とする請求項1に記載の金属マグネシウムの製造方法。 - プラズマが、マイクロ波プラズマであること、
を特徴とする請求項1又は2に記載の金属マグネシウムの製造方法。 - プラズマを照射中に、原料である無水ハロゲン化マグネシウムをプラズマ雰囲気下で昇華開始温度まで加熱する加熱手段で加熱すること、
を特徴とする請求項1乃至3のいずれか1項に記載の金属マグネシウムの製造方法。 - 容器と、
前記容器の中を減圧する真空装置と、
前記容器の中に水素ガスを供給するガス供給装置と、
前記容器の中の無水ハロゲン化マグネシウムを加熱して金属マグネシウムに還元するプラズマを照射するプラズマ装置と、
を備えること、
を特徴とする金属マグネシウムの製造装置。 - 前記無水塩化マグネシウムをプラズマ雰囲気下で昇華開始温度まで加熱する加熱手段を有していること、
を特徴とする請求項5に記載の金属マグネシウムの製造装置。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015102618A JP6571985B2 (ja) | 2015-05-20 | 2015-05-20 | 金属マグネシウムの製造方法とその製造装置 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015102618A JP6571985B2 (ja) | 2015-05-20 | 2015-05-20 | 金属マグネシウムの製造方法とその製造装置 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018045672A Division JP6487087B2 (ja) | 2018-03-13 | 2018-03-13 | 金属マグネシウムの製造方法とその製造装置 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2016216780A JP2016216780A (ja) | 2016-12-22 |
| JP6571985B2 true JP6571985B2 (ja) | 2019-09-04 |
Family
ID=57579983
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015102618A Active JP6571985B2 (ja) | 2015-05-20 | 2015-05-20 | 金属マグネシウムの製造方法とその製造装置 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP6571985B2 (ja) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY176543A (en) | 2017-06-02 | 2020-08-15 | Se Corp | Method for producing magnesium hydride,power generation system using magnesium hydride, and apparatus for producing magnesium hydride |
| JP6471211B2 (ja) * | 2017-06-02 | 2019-02-13 | 株式会社エスイー | 水素化マグネシウム等の製造方法、水素化マグネシウムを用いた発電方法及び水素化マグネシウム等の製造装置 |
| WO2019230184A1 (ja) * | 2018-05-29 | 2019-12-05 | 株式会社エスイー | 原料をマイクロ波表面波プラズマで処理して原料と異なる生成物を得る製造装置及び製造方法 |
| SG11202101203QA (en) * | 2018-11-26 | 2021-03-30 | Se Corp | Hydrogen generation system, power generation system, hydrogen generation method and power generation method |
| JP7210824B2 (ja) * | 2018-11-27 | 2023-01-24 | 株式会社エスイー | プラズマを用いた処理装置及びその処理装置を用いて水素発生材料を製造する製造方法 |
| JP7152638B2 (ja) * | 2018-11-28 | 2022-10-13 | 株式会社エスイー | 水素化マグネシウムの生成反応の向上を図った水素化マグネシウムを含む水素発生材料を製造する材料製造方法、及び、その材料製造方法で製造された水素化マグネシウムを含む水素発生材料を用いた水素製造方法 |
| JP2023103699A (ja) * | 2022-01-14 | 2023-07-27 | アンヴァール株式会社 | マグネシウム化合物の還元装置 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| LU81469A1 (fr) * | 1979-07-05 | 1981-02-03 | Luniversite Libre Bruxelles | Procede et installation pour la production de metaux reactifs par reduction de leurs halogenures |
| JPS58176126A (ja) * | 1982-04-07 | 1983-10-15 | Inoue Japax Res Inc | 希土類精鉱の処理方法 |
| JPH10258262A (ja) * | 1997-03-19 | 1998-09-29 | Toshiba Corp | 焼却灰処理装置 |
| JP2001040428A (ja) * | 1999-07-29 | 2001-02-13 | Daido Steel Co Ltd | 廃棄煉瓦の処理方法 |
| US20090107290A1 (en) * | 2007-10-25 | 2009-04-30 | Los Alamos National Security, Llc | Plasma-based reduction of titanium oxides |
| EP2766502B1 (en) * | 2011-10-11 | 2016-08-17 | The South African Nuclear Energy Corporation Limited | Treatment of chemical feedstocks |
| US20130209308A1 (en) * | 2012-02-15 | 2013-08-15 | Baker Hughes Incorporated | Method of making a metallic powder and powder compact and powder and powder compact made thereby |
| JP2013185582A (ja) * | 2012-03-09 | 2013-09-19 | Techno Bank:Kk | 波力電気変換装置および海洋の資源の生産方法 |
| JP6100098B2 (ja) * | 2013-05-29 | 2017-03-22 | 住友重機械工業株式会社 | 還元装置及び還元方法 |
-
2015
- 2015-05-20 JP JP2015102618A patent/JP6571985B2/ja active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2016216780A (ja) | 2016-12-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6571985B2 (ja) | 金属マグネシウムの製造方法とその製造装置 | |
| US11685664B2 (en) | Method for producing tetrahydroborate and tetrahydroborate | |
| CN102264927B (zh) | 激光提炼装置及激光提炼方法 | |
| JP6510155B1 (ja) | 金属原子を含む原料をプラズマで処理して原料と異なる生成物を得る製造装置、及び、製造方法 | |
| Steill et al. | Structure of the observable histidine radical cation in the gas phase: a captodative α‐radical ion | |
| SG177478A1 (en) | Methods and apparatus for protecting plasma chamber surfaces | |
| JP6487087B2 (ja) | 金属マグネシウムの製造方法とその製造装置 | |
| US11058984B2 (en) | Method for treating sulfur hexafluoride using radiation and apparatus for collecting and treating by-products | |
| Stratton et al. | In situ diagnostics for nanomaterial synthesis in carbon arc plasma | |
| Sarkas et al. | Photoelectron spectroscopy of lithium hydride anion | |
| Nevar et al. | Synthesis of silicon nanocrystals in electrical discharge in liquid with spectroscopic plasma characterization | |
| Licht et al. | Intense, self-induced sustainable microwave plasma using carbon nanotubes made from CO 2 | |
| CN112655056A (zh) | 核反应堆结构元件的去污方法 | |
| Kawano et al. | Experimental methods and techniques for negative-ion production by surface ionization. Part II. Instrumentation and operation | |
| CN101563182B (zh) | 产生热能的方法 | |
| Kondo et al. | Direct recycling of anode active material from Li-ion batteries using TiNb2O7 anode | |
| TW200305919A (en) | Decomposing apparatus for perfluorinated compounds and processing system for perfluorinated compounds | |
| CN112969659B (zh) | 四氢硼酸盐的制造装置以及四氢硼酸盐的制造方法 | |
| Hirooka et al. | Aerosol formation and hydrogen co-deposition by colliding ablation plasma plumes of lithium and lead | |
| Song et al. | Flash recovery of lithium from spent anode graphite by carbothermal shock and water leaching | |
| Rutberg et al. | Possibilities of application of plasma technologies to recycle organic-containing substances: Particularities of high current free burning arcs | |
| Baranov et al. | High-power, high-pressure pulsed CO2 lasers and their applications | |
| Lobanov | Production of intensive negative lithium beam with caesium sputter-type ion source | |
| JP2005062025A (ja) | 核種変換装置からの核種変換量を増大させる方法及び核種変換装置 | |
| Kentsch et al. | Observation of 3 d metal ion charge state distributions in the Dresden EBIT |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180507 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20190228 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190326 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190517 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20190801 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20190809 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6571985 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |