JP6461306B2 - インサイチュで自己組織化した、毒性のあるrna阻害剤 - Google Patents
インサイチュで自己組織化した、毒性のあるrna阻害剤 Download PDFInfo
- Publication number
- JP6461306B2 JP6461306B2 JP2017502695A JP2017502695A JP6461306B2 JP 6461306 B2 JP6461306 B2 JP 6461306B2 JP 2017502695 A JP2017502695 A JP 2017502695A JP 2017502695 A JP2017502695 A JP 2017502695A JP 6461306 B2 JP6461306 B2 JP 6461306B2
- Authority
- JP
- Japan
- Prior art keywords
- rna
- dmf
- k4nmes
- mmol
- cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 231100000331 toxic Toxicity 0.000 title claims description 25
- 230000002588 toxic effect Effects 0.000 title claims description 25
- 238000011065 in-situ storage Methods 0.000 title claims description 9
- 239000003112 inhibitor Substances 0.000 title description 9
- 108091032973 (ribonucleotides)n+m Proteins 0.000 claims description 134
- 201000009340 myotonic dystrophy type 1 Diseases 0.000 claims description 50
- 230000027455 binding Effects 0.000 claims description 38
- 150000001345 alkine derivatives Chemical class 0.000 claims description 21
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 21
- IVRMZWNICZWHMI-UHFFFAOYSA-N azide group Chemical group [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 claims description 20
- 201000010099 disease Diseases 0.000 claims description 16
- 150000001540 azides Chemical class 0.000 claims description 13
- 238000003776 cleavage reaction Methods 0.000 claims description 11
- 230000007017 scission Effects 0.000 claims description 11
- 238000006352 cycloaddition reaction Methods 0.000 claims description 9
- 108020004707 nucleic acids Proteins 0.000 claims description 9
- 102000039446 nucleic acids Human genes 0.000 claims description 9
- 150000007523 nucleic acids Chemical class 0.000 claims description 9
- 230000035699 permeability Effects 0.000 claims description 9
- 208000035955 Proximal myotonic myopathy Diseases 0.000 claims description 8
- 201000008709 myotonic dystrophy type 2 Diseases 0.000 claims description 8
- 208000037140 Steinert myotonic dystrophy Diseases 0.000 claims description 6
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical class N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 claims description 6
- 238000006482 condensation reaction Methods 0.000 claims description 5
- 230000001939 inductive effect Effects 0.000 claims description 4
- 108091081062 Repeated sequence (DNA) Proteins 0.000 claims description 2
- 239000008194 pharmaceutical composition Substances 0.000 claims 9
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 250
- 210000004027 cell Anatomy 0.000 description 123
- 150000001875 compounds Chemical class 0.000 description 98
- 239000000243 solution Substances 0.000 description 87
- 239000011347 resin Substances 0.000 description 66
- 229920005989 resin Polymers 0.000 description 66
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 58
- 239000011616 biotin Substances 0.000 description 47
- 229960002685 biotin Drugs 0.000 description 47
- 239000000539 dimer Substances 0.000 description 45
- 230000015572 biosynthetic process Effects 0.000 description 44
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 39
- 238000006243 chemical reaction Methods 0.000 description 38
- 238000003786 synthesis reaction Methods 0.000 description 36
- ZNXRHVBJGULNNR-GQYFIECQSA-N N-[4-[(2-amino-2-oxoethyl)-[(2S)-2-[methyl-[(2S)-2-[methyl-[(2S)-2-[methyl-[(2S)-2-[methyl-[4-[3-[6-[5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazol-2-yl]phenoxy]butanoyl]amino]propanoyl]amino]propanoyl]amino]propanoyl]amino]propanoyl]amino]butyl]-N-methyl-4-[3-[6-[5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazol-2-yl]phenoxy]butanamide Chemical compound C[C@H](N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](C)N(C)C(=O)CCCOc1cccc(c1)-c1nc2ccc(cc2[nH]1)-c1nc2cc(ccc2[nH]1)N1CCN(C)CC1)C(=O)N(CCCCN(C)C(=O)CCCOc1cccc(c1)-c1nc2ccc(cc2[nH]1)-c1nc2cc(ccc2[nH]1)N1CCN(C)CC1)CC(N)=O ZNXRHVBJGULNNR-GQYFIECQSA-N 0.000 description 35
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 33
- 108020004999 messenger RNA Proteins 0.000 description 33
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 32
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 30
- 239000000047 product Substances 0.000 description 25
- 150000003384 small molecules Chemical class 0.000 description 25
- 238000004458 analytical method Methods 0.000 description 24
- 102100021970 Myc box-dependent-interacting protein 1 Human genes 0.000 description 22
- 101710146921 Myc box-dependent-interacting protein 1 Proteins 0.000 description 22
- 238000012650 click reaction Methods 0.000 description 22
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 21
- FPIRBHDGWMWJEP-UHFFFAOYSA-N 1-hydroxy-7-azabenzotriazole Chemical compound C1=CN=C2N(O)N=NC2=C1 FPIRBHDGWMWJEP-UHFFFAOYSA-N 0.000 description 20
- 125000002355 alkine group Chemical group 0.000 description 20
- 235000020958 biotin Nutrition 0.000 description 18
- 238000004007 reversed phase HPLC Methods 0.000 description 18
- 108010052185 Myotonin-Protein Kinase Proteins 0.000 description 17
- 102100022437 Myotonin-protein kinase Human genes 0.000 description 17
- 238000013459 approach Methods 0.000 description 17
- 238000000034 method Methods 0.000 description 17
- 239000001963 growth medium Substances 0.000 description 16
- 239000000203 mixture Substances 0.000 description 16
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 16
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 15
- 238000000338 in vitro Methods 0.000 description 15
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 14
- 239000011324 bead Substances 0.000 description 14
- 230000007547 defect Effects 0.000 description 14
- 210000002950 fibroblast Anatomy 0.000 description 14
- 230000006870 function Effects 0.000 description 14
- 239000000523 sample Substances 0.000 description 14
- 230000000694 effects Effects 0.000 description 13
- WBJINCZRORDGAQ-UHFFFAOYSA-N ethyl formate Chemical compound CCOC=O WBJINCZRORDGAQ-UHFFFAOYSA-N 0.000 description 13
- 230000007812 deficiency Effects 0.000 description 12
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 12
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 11
- 238000011529 RT qPCR Methods 0.000 description 11
- 238000000692 Student's t-test Methods 0.000 description 11
- 238000004949 mass spectrometry Methods 0.000 description 11
- CMWYAOXYQATXSI-UHFFFAOYSA-N n,n-dimethylformamide;piperidine Chemical compound CN(C)C=O.C1CCNCC1 CMWYAOXYQATXSI-UHFFFAOYSA-N 0.000 description 11
- 238000006384 oligomerization reaction Methods 0.000 description 11
- 150000007942 carboxylates Chemical class 0.000 description 10
- 229920002678 cellulose Polymers 0.000 description 10
- 239000001913 cellulose Substances 0.000 description 10
- 238000011156 evaluation Methods 0.000 description 10
- 239000000178 monomer Substances 0.000 description 10
- 230000003389 potentiating effect Effects 0.000 description 10
- 239000011541 reaction mixture Substances 0.000 description 10
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- 238000005481 NMR spectroscopy Methods 0.000 description 9
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 9
- 239000007795 chemical reaction product Substances 0.000 description 9
- 108090000623 proteins and genes Proteins 0.000 description 9
- 102000004169 proteins and genes Human genes 0.000 description 9
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 8
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 8
- 239000000499 gel Substances 0.000 description 8
- DCKVNWZUADLDEH-UHFFFAOYSA-N sec-butyl acetate Chemical compound CCC(C)OC(C)=O DCKVNWZUADLDEH-UHFFFAOYSA-N 0.000 description 8
- 238000004088 simulation Methods 0.000 description 8
- OZFAFGSSMRRTDW-UHFFFAOYSA-N (2,4-dichlorophenyl) benzenesulfonate Chemical compound ClC1=CC(Cl)=CC=C1OS(=O)(=O)C1=CC=CC=C1 OZFAFGSSMRRTDW-UHFFFAOYSA-N 0.000 description 7
- -1 -N-acylated kanamycin A compound Chemical class 0.000 description 7
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 7
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 7
- 238000005516 engineering process Methods 0.000 description 7
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical class O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 7
- 238000001890 transfection Methods 0.000 description 7
- JOFHWKQIQLPZTC-LBPRGKRZSA-N (2s)-2-[9h-fluoren-9-ylmethoxycarbonyl(methyl)amino]propanoic acid Chemical compound C1=CC=C2C(COC(=O)N(C)[C@@H](C)C(O)=O)C3=CC=CC=C3C2=C1 JOFHWKQIQLPZTC-LBPRGKRZSA-N 0.000 description 6
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 6
- FZTIWOBQQYPTCJ-UHFFFAOYSA-N 4-[4-(4-carboxyphenyl)phenyl]benzoic acid Chemical compound C1=CC(C(=O)O)=CC=C1C1=CC=C(C=2C=CC(=CC=2)C(O)=O)C=C1 FZTIWOBQQYPTCJ-UHFFFAOYSA-N 0.000 description 6
- 208000023514 Barrett esophagus Diseases 0.000 description 6
- 108010090804 Streptavidin Proteins 0.000 description 6
- 230000001857 anti-mycotic effect Effects 0.000 description 6
- 239000002543 antimycotic Substances 0.000 description 6
- 230000003115 biocidal effect Effects 0.000 description 6
- 239000003054 catalyst Substances 0.000 description 6
- 238000010586 diagram Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 235000019253 formic acid Nutrition 0.000 description 6
- 230000001976 improved effect Effects 0.000 description 6
- 238000002955 isolation Methods 0.000 description 6
- 238000010172 mouse model Methods 0.000 description 6
- 238000003753 real-time PCR Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 5
- 102100034343 Integrase Human genes 0.000 description 5
- 229960001561 bleomycin Drugs 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 238000010382 chemical cross-linking Methods 0.000 description 5
- 208000035475 disorder Diseases 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 239000003814 drug Substances 0.000 description 5
- 230000004064 dysfunction Effects 0.000 description 5
- 239000003797 essential amino acid Substances 0.000 description 5
- 235000020776 essential amino acid Nutrition 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 230000006872 improvement Effects 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 239000007758 minimum essential medium Substances 0.000 description 5
- 238000000329 molecular dynamics simulation Methods 0.000 description 5
- 238000011002 quantification Methods 0.000 description 5
- 230000003252 repetitive effect Effects 0.000 description 5
- 238000003757 reverse transcription PCR Methods 0.000 description 5
- 230000002441 reversible effect Effects 0.000 description 5
- 108010051423 streptavidin-agarose Proteins 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 238000013519 translation Methods 0.000 description 5
- 239000013638 trimer Substances 0.000 description 5
- 108020005345 3' Untranslated Regions Proteins 0.000 description 4
- 108010006654 Bleomycin Proteins 0.000 description 4
- 101710203526 Integrase Proteins 0.000 description 4
- 239000012097 Lipofectamine 2000 Substances 0.000 description 4
- 206010068871 Myotonic dystrophy Diseases 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 4
- 229910052786 argon Inorganic materials 0.000 description 4
- 230000004071 biological effect Effects 0.000 description 4
- 239000013592 cell lysate Substances 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 230000004069 differentiation Effects 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 210000001589 microsome Anatomy 0.000 description 4
- 239000013612 plasmid Substances 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 239000003643 water by type Substances 0.000 description 4
- OYBOVXXFJYJYPC-UHFFFAOYSA-N 3-azidopropan-1-amine Chemical compound NCCCN=[N+]=[N-] OYBOVXXFJYJYPC-UHFFFAOYSA-N 0.000 description 3
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- 108091092878 Microsatellite Proteins 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- 108091034117 Oligonucleotide Proteins 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 3
- 125000000852 azido group Chemical group *N=[N+]=[N-] 0.000 description 3
- 238000010805 cDNA synthesis kit Methods 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 230000002860 competitive effect Effects 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 231100000676 disease causative agent Toxicity 0.000 description 3
- 238000004896 high resolution mass spectrometry Methods 0.000 description 3
- 238000007901 in situ hybridization Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 238000004811 liquid chromatography Methods 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 239000012139 lysis buffer Substances 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- INAAIJLSXJJHOZ-UHFFFAOYSA-N pibenzimol Chemical compound C1CN(C)CCN1C1=CC=C(N=C(N2)C=3C=C4NC(=NC4=CC=3)C=3C=CC(O)=CC=3)C2=C1 INAAIJLSXJJHOZ-UHFFFAOYSA-N 0.000 description 3
- 238000006116 polymerization reaction Methods 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 239000002356 single layer Substances 0.000 description 3
- 238000010183 spectrum analysis Methods 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 150000003852 triazoles Chemical class 0.000 description 3
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 2
- BDNKZNFMNDZQMI-UHFFFAOYSA-N 1,3-diisopropylcarbodiimide Chemical compound CC(C)N=C=NC(C)C BDNKZNFMNDZQMI-UHFFFAOYSA-N 0.000 description 2
- LINBWYYLPWJQHE-UHFFFAOYSA-N 3-(9h-fluoren-9-ylmethoxycarbonylamino)propanoic acid Chemical compound C1=CC=C2C(COC(=O)NCCC(=O)O)C3=CC=CC=C3C2=C1 LINBWYYLPWJQHE-UHFFFAOYSA-N 0.000 description 2
- YMZMTOFQCVHHFB-UHFFFAOYSA-N 5-carboxytetramethylrhodamine Chemical compound C=12C=CC(N(C)C)=CC2=[O+]C2=CC(N(C)C)=CC=C2C=1C1=CC=C(C(O)=O)C=C1C([O-])=O YMZMTOFQCVHHFB-UHFFFAOYSA-N 0.000 description 2
- HJCMDXDYPOUFDY-WHFBIAKZSA-N Ala-Gln Chemical compound C[C@H](N)C(=O)N[C@H](C(O)=O)CCC(N)=O HJCMDXDYPOUFDY-WHFBIAKZSA-N 0.000 description 2
- 0 C*(CC1)CC*1C1C=C(*=C(*2=C)c3ccc(C)c(C)c3)C2=CC1 Chemical compound C*(CC1)CC*1C1C=C(*=C(*2=C)c3ccc(C)c(C)c3)C2=CC1 0.000 description 2
- 102100033849 CCHC-type zinc finger nucleic acid binding protein Human genes 0.000 description 2
- 101710116319 CCHC-type zinc finger nucleic acid binding protein Proteins 0.000 description 2
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 230000004570 RNA-binding Effects 0.000 description 2
- 108091036066 Three prime untranslated region Proteins 0.000 description 2
- 102100025342 Voltage-dependent N-type calcium channel subunit alpha-1B Human genes 0.000 description 2
- 101710088658 Voltage-dependent N-type calcium channel subunit alpha-1B Proteins 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 239000000074 antisense oligonucleotide Substances 0.000 description 2
- 238000012230 antisense oligonucleotides Methods 0.000 description 2
- PFYXSUNOLOJMDX-UHFFFAOYSA-N bis(2,5-dioxopyrrolidin-1-yl) carbonate Chemical compound O=C1CCC(=O)N1OC(=O)ON1C(=O)CCC1=O PFYXSUNOLOJMDX-UHFFFAOYSA-N 0.000 description 2
- 108700004675 bleomycetin Proteins 0.000 description 2
- QYOAUOAXCQAEMW-UTXKDXHTSA-N bleomycin A5 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCCNCCCCN)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C QYOAUOAXCQAEMW-UTXKDXHTSA-N 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 2
- 229960004630 chlorambucil Drugs 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 230000000875 corresponding effect Effects 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 239000013613 expression plasmid Substances 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 125000001841 imino group Chemical group [H]N=* 0.000 description 2
- 239000003999 initiator Substances 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000000074 matrix-assisted laser desorption--ionisation tandem time-of-flight detection Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- UORVCLMRJXCDCP-UHFFFAOYSA-N propynoic acid Chemical compound OC(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-N 0.000 description 2
- 230000009919 sequestration Effects 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 230000002194 synthesizing effect Effects 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- WGXJEEHMLCFJFB-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) prop-2-ynoate Chemical compound C#CC(=O)ON1C(=O)CCC1=O WGXJEEHMLCFJFB-UHFFFAOYSA-N 0.000 description 1
- HBZBAMXERPYTFS-SECBINFHSA-N (4S)-2-(6,7-dihydro-5H-pyrrolo[3,2-f][1,3]benzothiazol-2-yl)-4,5-dihydro-1,3-thiazole-4-carboxylic acid Chemical compound OC(=O)[C@H]1CSC(=N1)c1nc2cc3CCNc3cc2s1 HBZBAMXERPYTFS-SECBINFHSA-N 0.000 description 1
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- 108020004463 18S ribosomal RNA Proteins 0.000 description 1
- VCFCFPNRQDANPN-UHFFFAOYSA-N 2-(9h-fluoren-9-ylmethoxycarbonylamino)hexanoic acid Chemical compound C1=CC=C2C(COC(=O)NC(CCCC)C(O)=O)C3=CC=CC=C3C2=C1 VCFCFPNRQDANPN-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- OSBLTNPMIGYQGY-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetic acid;boric acid Chemical compound OB(O)O.OCC(N)(CO)CO.OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O OSBLTNPMIGYQGY-UHFFFAOYSA-N 0.000 description 1
- UNBAJBIGCBVWJU-UHFFFAOYSA-N 3',6'-dihydroxy-3-oxo-n-prop-2-ynylspiro[2-benzofuran-1,9'-xanthene]-5-carboxamide Chemical compound O1C(=O)C2=CC(C(=O)NCC#C)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 UNBAJBIGCBVWJU-UHFFFAOYSA-N 0.000 description 1
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 1
- NSPMIYGKQJPBQR-UHFFFAOYSA-N 4H-1,2,4-triazole Chemical compound C=1N=CNN=1 NSPMIYGKQJPBQR-UHFFFAOYSA-N 0.000 description 1
- FPCPONSZWYDXRD-UHFFFAOYSA-N 6-(9h-fluoren-9-ylmethoxycarbonylamino)hexanoic acid Chemical compound C1=CC=C2C(COC(=O)NCCCCCC(=O)O)C3=CC=CC=C3C2=C1 FPCPONSZWYDXRD-UHFFFAOYSA-N 0.000 description 1
- 108010022752 Acetylcholinesterase Proteins 0.000 description 1
- 102000012440 Acetylcholinesterase Human genes 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 206010001497 Agitation Diseases 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 108010063104 Apoptosis Regulatory Proteins Proteins 0.000 description 1
- 102000010565 Apoptosis Regulatory Proteins Human genes 0.000 description 1
- 101150086792 CLCN1 gene Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 108010062745 Chloride Channels Proteins 0.000 description 1
- 102000011045 Chloride Channels Human genes 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 235000000638 D-biotin Nutrition 0.000 description 1
- 239000011665 D-biotin Substances 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 102000016911 Deoxyribonucleases Human genes 0.000 description 1
- 108010053770 Deoxyribonucleases Proteins 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 101001039207 Homo sapiens Low-density lipoprotein receptor-related protein 8 Proteins 0.000 description 1
- 101001018147 Homo sapiens Mitogen-activated protein kinase kinase kinase 4 Proteins 0.000 description 1
- 101000652343 Homo sapiens Transcription factor SPT20 homolog-like 1 Proteins 0.000 description 1
- 102100040705 Low-density lipoprotein receptor-related protein 8 Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- WLLGXSLBOPFWQV-UHFFFAOYSA-N MGK 264 Chemical compound C1=CC2CC1C1C2C(=O)N(CC(CC)CCCC)C1=O WLLGXSLBOPFWQV-UHFFFAOYSA-N 0.000 description 1
- 108091027974 Mature messenger RNA Proteins 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 108700011259 MicroRNAs Proteins 0.000 description 1
- 102100033060 Mitogen-activated protein kinase kinase kinase 4 Human genes 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 108010043958 Peptoids Proteins 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 230000006819 RNA synthesis Effects 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- CGNLCCVKSWNSDG-UHFFFAOYSA-N SYBR Green I Chemical compound CN(C)CCCN(CCC)C1=CC(C=C2N(C3=CC=CC=C3S2)C)=C2C=CC=CC2=[N+]1C1=CC=CC=C1 CGNLCCVKSWNSDG-UHFFFAOYSA-N 0.000 description 1
- 229940124639 Selective inhibitor Drugs 0.000 description 1
- 102100036049 T-complex protein 1 subunit gamma Human genes 0.000 description 1
- 239000008049 TAE buffer Substances 0.000 description 1
- 239000008051 TBE buffer Substances 0.000 description 1
- 102100030261 Transcription factor SPT20 homolog-like 1 Human genes 0.000 description 1
- 108091023045 Untranslated Region Proteins 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- HGEVZDLYZYVYHD-UHFFFAOYSA-N acetic acid;2-amino-2-(hydroxymethyl)propane-1,3-diol;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetic acid Chemical compound CC(O)=O.OCC(N)(CO)CO.OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O HGEVZDLYZYVYHD-UHFFFAOYSA-N 0.000 description 1
- 229940022698 acetylcholinesterase Drugs 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 239000011543 agarose gel Substances 0.000 description 1
- 229960003767 alanine Drugs 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- DEDGUGJNLNLJSR-UHFFFAOYSA-N alpha-hydroxycinnamic acid Natural products OC(=O)C(O)=CC1=CC=CC=C1 DEDGUGJNLNLJSR-UHFFFAOYSA-N 0.000 description 1
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000001668 ameliorated effect Effects 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- LSTJLLHJASXKIV-UHFFFAOYSA-N amino hexanoate Chemical compound CCCCCC(=O)ON LSTJLLHJASXKIV-UHFFFAOYSA-N 0.000 description 1
- GWCQSPUAEOPDKF-UHFFFAOYSA-N amino octanoate Chemical compound CCCCCCCC(=O)ON GWCQSPUAEOPDKF-UHFFFAOYSA-N 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 238000010256 biochemical assay Methods 0.000 description 1
- 230000008499 blood brain barrier function Effects 0.000 description 1
- 210000001218 blood-brain barrier Anatomy 0.000 description 1
- 230000005978 brain dysfunction Effects 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- KDPAWGWELVVRCH-UHFFFAOYSA-N bromoacetic acid Chemical compound OC(=O)CBr KDPAWGWELVVRCH-UHFFFAOYSA-N 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 101150062912 cct3 gene Proteins 0.000 description 1
- 238000000423 cell based assay Methods 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 230000030570 cellular localization Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- SUCQPHMWFOCTTR-UHFFFAOYSA-L dichlororuthenium;triphenylphosphane Chemical compound Cl[Ru]Cl.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 SUCQPHMWFOCTTR-UHFFFAOYSA-L 0.000 description 1
- 235000021186 dishes Nutrition 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000008482 dysregulation Effects 0.000 description 1
- 108010048367 enhanced green fluorescent protein Proteins 0.000 description 1
- DEDGUGJNLNLJSR-VURMDHGXSA-N enol-phenylpyruvate Chemical compound OC(=O)C(\O)=C\C1=CC=CC=C1 DEDGUGJNLNLJSR-VURMDHGXSA-N 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 238000012744 immunostaining Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000003468 luciferase reporter gene assay Methods 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- MGJXBDMLVWIYOQ-UHFFFAOYSA-N methylazanide Chemical compound [NH-]C MGJXBDMLVWIYOQ-UHFFFAOYSA-N 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 210000003757 neuroblast Anatomy 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- UORVCLMRJXCDCP-UHFFFAOYSA-M propynoate Chemical compound [O-]C(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-M 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 230000002040 relaxant effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 108020004418 ribosomal RNA Proteins 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 229940126586 small molecule drug Drugs 0.000 description 1
- YZHUMGUJCQRKBT-UHFFFAOYSA-M sodium chlorate Chemical group [Na+].[O-]Cl(=O)=O YZHUMGUJCQRKBT-UHFFFAOYSA-M 0.000 description 1
- JUJBNYBVVQSIOU-UHFFFAOYSA-M sodium;4-[2-(4-iodophenyl)-3-(4-nitrophenyl)tetrazol-2-ium-5-yl]benzene-1,3-disulfonate Chemical compound [Na+].C1=CC([N+](=O)[O-])=CC=C1N1[N+](C=2C=CC(I)=CC=2)=NC(C=2C(=CC(=CC=2)S([O-])(=O)=O)S([O-])(=O)=O)=N1 JUJBNYBVVQSIOU-UHFFFAOYSA-M 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000003883 substance clean up Methods 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- CIZQQISFIQZTDF-UHFFFAOYSA-N tert-butyl n-(4-aminobutyl)-n-methylcarbamate Chemical compound CC(C)(C)OC(=O)N(C)CCCCN CIZQQISFIQZTDF-UHFFFAOYSA-N 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000013334 tissue model Methods 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/702—Oligosaccharides, i.e. having three to five saccharide radicals attached to each other by glycosidic linkages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/07—Tetrapeptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/549—Sugars, nucleosides, nucleotides or nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/55—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound the modifying agent being also a pharmacologically or therapeutically active agent, i.e. the entire conjugate being a codrug, i.e. a dimer, oligomer or polymer of pharmacologically or therapeutically active compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/555—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound pre-targeting systems involving an organic compound, other than a peptide, protein or antibody, for targeting specific cells
- A61K47/557—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound pre-targeting systems involving an organic compound, other than a peptide, protein or antibody, for targeting specific cells the modifying agent being biotin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Neurology (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Pain & Pain Management (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Saccharide Compounds (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Description
本願は、2014年7月18日出願の米国特許仮出願第62/026,266号の優先権を主張し、その開示は参照によりその全文が本明細書に援用される。
[1]M.L.Yeung,Y.Bennasser,K.T.Jeang,Curr.Med.Chem.2007,14,191−197。
コンピュータ解析のための方法
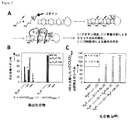
本発明者らは以前、2K−4、すなわちペプトイドによって連結された2つのK RNA−結合モジュールと、2つの5’CCUG/3’GUCCループを含有するRNAとの結合のモデルを公開した[1]。ペプトイドを除去し、リンカーを含めるためにK RNA−結合モジュールを次のように編集した:(i)1つのKの環IのC6’には、ヘキサ−5−インアミドかまたはN−(2−アミノ−2−オキソエチル)プロピオルアミドのいずれかを含めた;そして(ii)アジド基をもう一方のKの環IIIのC6”に付加した。リンカーの立体構造の走査を実施して、2つのKの反応性の終端が互いのすぐ近くにあるかどうかを調べた。非冗長性の立体構造の徹底的走査を、MacroModel(Schrodinger、LLC)でOPLS_2005力場を使用して実施した[2]。結果は、複数の立体構造が2つの反応性基を非常に近接させていて、エチニルCとアジドNとの最も短い距離は、ヘキサ−5−インアミドおよびN−(2−アミノ−2−オキソエチル)プロピオルアミドリンカーにおいて、それぞれ2.61Åおよび2.37Åであることを示した(図1、BI)。
略語。DIC、N,N’−ジイソプロピルカルボジイミド;DIEA、N,N−ジイソプロピルエチルアミン;DMF、N,N−ジメチルホルムアミド;HPLC、高速液体クロマトグラフィー;HRMS、高解像度質量分析;LC−MS、液体クロマトグラフィー−質量分析;MeOH、メタノール;MALDI ToF/ToF、マトリックス支援レーザー脱離イオン化飛行時間型/飛行時間型;MS、質量分析;NBD、7−ニトロベンズ−2−オキサ−1,3−ジアゾール−4−イル;TFA、トリフルオロ酢酸
合成。Fmoc−Rinkアミド樹脂(0.59ミリモル/g)は、Advanced ChemTechより購入した。N,N−ジメチルホルムアミド(DMF、無水)は、EMDより購入し、さらなる精製を行わずに使用した。ピペリジン、トリフルオロ酢酸(TFA)、N,N−ジイソプロピルエチルアミン(DIEA)、および2−ブロモ酢酸は、シグマ・アルドリッチより購入した。N,N’−ジイソプロピルカルボジイミド(DIC)、1−1−ヒドロキシ−7−アザベンゾトリアゾール(HOAt)、およびFmoc−β−アラニンは、Advanced ChemTechより購入した。Fmoc−N−メチル−L−アラニンおよびN−(4−アミノブチル)−N−メチルカルバミン酸tert−ブチルエステルは、Combi−Blocksより購入した。N−(4−アミノエチル)−N−メチルカルバミン酸tert−ブチルエステルは、Oakwood Productsより購入した。クロラムブシルは、MP Biomedicalsより購入した。ブレオマイシンA5は、LKT Laboratoriesより購入した。ヘキストカルボン酸塩(Pushechnikov A,Lee MM,Childs−Disney JL,Sobczak K,French JM,Thornton CA,Disney MD.J Am Chem Soc.2009 Jul 22;131(28):9767−79)、2H−K4NMe(Rzuczek SG,Gao Y,Tang ZZ,Thornton CA,Kodadek T,Disney MD.ACS Chem Biol.2013 Oct 18;8(10):2312−21)、およびビオチンアミン(Yamada,M.;Harada,K.;Maeda,Y.;Hasegawa,T.New Journal of Chemistry 2013,37,3762)は、これまでに報告されたように合成した。N−メチルペプチドは、Biotage Initiator+SP Waveマイクロ波を用いて合成した。
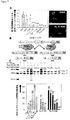
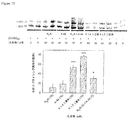
液体クロマトグラフィー・質量分析(LC−MS)によるインビトロクリック反応の評価。DM2を引き起こす反復RNAが、HDCRによるオリゴマー化の鋳型となるかどうかを判定するために、アルキンを含有する化合物およびアジドを含有する化合物を、12のCCUGリピートを含むRNAとともにインキュベートした。rCCUG12(最終濃度50μM)は、5分間60℃の1×フォールディングバッファー(8mM Na2HPO4、pH7.0、185mM NaCl、および1mM EDTA)中で折り畳まれた。室温に冷却した後、N3−KおよびK−Ak(各々の最終濃度500μM)を添加し、反応混合物を37℃で48時間インキュベートした。各々のサンプルを、Thermo Scientific LTQ−ETD質量分析計を用いるLC−MSによって分析した。10分にわたる、0.1%ギ酸を加えた水中0〜100%アセトニトリルの勾配を分析に使用した。各成分の全イオン数を、N3−K、K−Ak、およびK1,4二量体の等モル混合物を含む制御注入を使用することにより測定される、各々の成分のイオン化率に正規化した。r(CUG)12、r(AUUCU)12、r(CAG)12、r(CGG)12、完全に塩基対形成したステムを含むRNAヘアピン、およびビール酵母tRNA(ロシュ)を使用する制御反応も、同様に評価した。
[1]J.L.Childs−Disney,I.Yildirim,H.Park,J.R.Lohman,L.Guan,T.Tran,P.Sarkar,G.C.Schatz,M.D.Disney,ACS Chem.Biol.2014,9,538−550。
[項目1]
毒性のある疾患誘導性RNAの機能のモジュレーターであって、前記RNAが伸長したリピート配列を含み、前記モジュレーターが、一つの細胞透過性RNA伸長リピート配列結合モジュールであって、アルキンとアジドの両方の部分を含む、細胞透過性RNA伸長リピート配列結合モジュールの縮合反応から、または、一対の細胞透過性RNA伸長リピート配列結合モジュールであって、第1のモジュールがアルキン部分を含み、第2のモジュールがアジド部分を含む、一対の細胞透過性RNA伸長リピート配列結合モジュールの縮合から、生細胞内でインサイチュで形成されるRNAに結合するオリゴマーであり、前記縮合反応が、前記RNA伸長リピート結合モジュール間で前記アルキン部分とアジド部分との間の1,3ヒュスゲン双極子付加環化反応によって起こる、RNAに結合するオリゴマーを含む、モジュレーター。
[項目2]
前記毒性のあるRNAが、RNA伸長リピート配列r(CCUG)expによって形成される隣接する内部ループを含む、項目1に記載のモジュレーター。
[項目3]
各々の細胞透過性モジュールが、修飾されたカナマイシン部分を含む、項目2に記載のモジュレーター。
[項目4]
前記細胞透過性モジュールがN3−K−Ak、またはN3−K−AaKであるか、あるいは、一緒に使用される一対の細胞透過性モジュールがN3−KとK−Akである、項目3に記載のモジュレーター。
[項目5]
筋緊張性ジストロフィー2型(DM2)の処置のための項目2〜4のいずれか1項に記載のモジュレーター。
[項目6]
前記毒性のあるRNAと結合した項目2に記載のモジュレーターを形成する方法であって、一つの細胞透過性RNA伸長リピート配列結合モジュールであって、前記細胞透過性モジュールがアルキン部分とアジド部分の両方を含む、細胞透過性RNA伸長リピート配列結合モジュールに、または、一対の細胞透過性RNA伸長リピート配列結合モジュールであって、第1のモジュールがアルキン部分を含み、第2のモジュールがアジド部分を含む、一対の細胞透過性RNA伸長リピート配列結合モジュールに、前記毒性のあるRNAを含む前記生細胞を曝露する工程を含む、方法。
[項目10]
前記毒性のあるRNAが、RNA伸長リピート配列r(CUG)expによって形成される隣接する内部ループを含む、項目1に記載のモジュレーター。
[項目11]
各々の細胞透過性モジュールが、ビス−ヘテロアリール部分、好ましくはビス−ベンズイミダゾール部分を含む、項目10に記載のモジュレーター。
[項目12]
前記細胞透過性モジュールが、N3−2H−K4NMeS−Aakである、項目11に記載のモジュレーター。
[項目13]
筋緊張性ジストロフィー1型(DM1)の処置のための、項目10〜12のいずれか一項に記載のモジュレーター。
[項目14]
前記毒性のあるRNAと結合した項目11に記載のモジュレーターを形成する方法であって、一つの細胞透過性RNA伸長リピート配列結合モジュールであって、前記細胞透過性モジュールがアルキン部分とアジド部分の両方を含む、細胞透過性RNA伸長リピート配列結合モジュールに、または、一対の細胞透過性RNA伸長リピート配列結合モジュールであって、第1のモジュールがアルキン部分を含み、第2のモジュールがアジド部分を含む、一対の細胞透過性RNA伸長リピート配列結合モジュールに、前記毒性のあるRNAを含む前記生細胞を曝露する工程を含む、方法。
[項目15]
毒性のある疾患誘導性RNAを切断する方法であって、前記RNAが伸長リピート配列を含み、前記モジュレーターが、核酸切断部分をさらに含む、項目1〜4または10〜12のいずれか1項に記載のモジュレーターを含む、方法。
[項目16]
前記核酸切断部分がブレオマイシンの誘導体である、項目15に記載の方法。
[項目17]
患者において疾患を処置する方法であって、前記疾患が伸長リピート配列を有する毒性のあるRNAの存在下で誘導され、前記患者の細胞の中に項目1に記載のモジュレーターを形成することを含む、方法。
[項目18]
前記疾患が筋緊張性ジストロフィー2型(DM2)であり、前記毒性のあるRNAがr(CCUG)exp伸長リピート配列を含み、前記モジュレーターが、アルキンおよびアジド基を有するカナマイシン誘導体を含む細胞透過性モジュール、または、各々がカナマイシン誘導体を含む第1および第2のモジュールであって、アルキン基を有する第1のモジュール、およびアジド基を有する第2のモジュールを含む一対の細胞透過性モジュールからインサイチュで形成される、項目17に記載の方法。
[項目19]
前記疾患が筋緊張性ジストロフィー1型(DM1)であり、前記毒性のあるRNAがまたはr(CUG)exp伸長リピート配列を含み、前記モジュレーターが、アルキンおよびアジド基を有する1つの細胞透過性モジュール、または、各々がビス−ベンズイミダゾール誘導体を含む第1および第2のモジュールであって、アルキン基を有する第1のモジュール、およびアジド基を有する第2のモジュールを含む一対の細胞透過性モジュールからインサイチュで形成される、項目17に記載の方法。
[項目20]
前記モジュレーターが核酸切断部分をさらに含む、項目18または19に記載の方法。
Claims (6)
- 一つの細胞透過性RNA伸長リピート配列結合モジュールまたは一対の細胞透過性RNA伸長リピート配列結合モジュールを含む医薬組成物であって、
前記モジュールは患者の細胞中で、毒性のある疾患誘導性RNAの機能のモジュレーターを形成し、
前記疾患が、筋緊張性ジストロフィー2型(DM2)または筋緊張性ジストロフィー1型(DM1)であり、
前記RNAが、各々、RNA伸長リピート配列r(CCUG)expまたはRNA伸長リピート配列r(CUG)expによって形成される隣接する内部ループを含む、伸長したリピート配列を含み、
前記モジュレーターが、前記一つの細胞透過性RNA伸長リピート配列結合モジュールであって、アルキンとアジドの両方の部分を含む、細胞透過性RNA伸長リピート配列結合モジュールの縮合反応から、または、前記一対の細胞透過性RNA伸長リピート配列結合モジュールであって、第1のモジュールがアルキン部分を含み、第2のモジュールがアジド部分を含む、一対の細胞透過性RNA伸長リピート配列結合モジュールの縮合反応から、生細胞内でインサイチュで形成されるRNAに結合するオリゴマーを含み、
前記縮合反応が、前記RNA伸長リピートに結合したモジュール間で前記アルキン部分とアジド部分との間の1,3ヒュスゲン双極子付加環化反応によって起こり、
前記細胞透過性モジュールが下記式で表されるN3−K−Ak、
または下記式で表されるN3−K−AaK
であるか、
あるいは、一緒に使用される一対の細胞透過性モジュールが下記式で表されるN3−K
と下記式で表されるK−Ak
である、前記医薬組成物。 - 請求項1に記載の医薬組成物であって、
請求項1に記載の前記一つの細胞透過性RNA伸長リピート配列結合モジュールに、または、請求項1に記載の前記一対の細胞透過性RNA伸長リピート配列結合モジュールに、前記毒性のあるRNAを含む前記生細胞を曝露することにより、前記毒性のあるRNAと結合した請求項1に記載の前記モジュレーターを形成するための前記医薬組成物。 - 毒性のある疾患誘導性RNAを切断するための請求項1に記載の医薬組成物であって、
前記RNAが伸長リピート配列を含み、
前記モジュレーターが、核酸切断部分をさらに含む、前記医薬組成物。 - 前記核酸切断部分がブレオマイシンの誘導体である、請求項3に記載の医薬組成物。
- r(CCUG)exp伸長リピート配列を含む毒性のあるRNAの存在下で誘導される筋緊張性ジストロフィー2型(DM2)、または、r(CUG)exp伸長リピート配列を含む毒性のあるRNAの存在下で誘導される筋緊張性ジストロフィー1型(DM1)を患者において処置するための、請求項1に記載の医薬組成物。
- 前記モジュレーターが核酸切断部分をさらに含む、請求項5に記載の医薬組成物。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462026266P | 2014-07-18 | 2014-07-18 | |
| US62/026,266 | 2014-07-18 | ||
| PCT/US2015/040902 WO2016011348A1 (en) | 2014-07-18 | 2015-07-17 | Toxic rna inhibitors self-assembled in situ |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018241478A Division JP2019068831A (ja) | 2014-07-18 | 2018-12-25 | インサイチュで自己組織化した、毒性のあるrna阻害剤 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2017522323A JP2017522323A (ja) | 2017-08-10 |
| JP2017522323A5 JP2017522323A5 (ja) | 2018-08-30 |
| JP6461306B2 true JP6461306B2 (ja) | 2019-01-30 |
Family
ID=55079084
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017502695A Active JP6461306B2 (ja) | 2014-07-18 | 2015-07-17 | インサイチュで自己組織化した、毒性のあるrna阻害剤 |
| JP2018241478A Pending JP2019068831A (ja) | 2014-07-18 | 2018-12-25 | インサイチュで自己組織化した、毒性のあるrna阻害剤 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018241478A Pending JP2019068831A (ja) | 2014-07-18 | 2018-12-25 | インサイチュで自己組織化した、毒性のあるrna阻害剤 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US10220031B2 (ja) |
| EP (1) | EP3169404A4 (ja) |
| JP (2) | JP6461306B2 (ja) |
| CN (1) | CN106714908A (ja) |
| AU (1) | AU2015289524A1 (ja) |
| CA (1) | CA2955428A1 (ja) |
| IL (1) | IL250113A0 (ja) |
| SG (1) | SG11201700387QA (ja) |
| WO (1) | WO2016011348A1 (ja) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3169404A4 (en) * | 2014-07-18 | 2018-02-07 | The Scripps Research Institute | Toxic rna inhibitors self-assembled in situ |
| WO2018098297A1 (en) * | 2016-11-28 | 2018-05-31 | The Scripps Research Institute | Precise small molecule recognition of a toxic cug rna repeat expansion |
| MX2020003695A (es) | 2017-11-30 | 2020-08-03 | Arrakis Therapeutics Inc | Fotosondas de union a acido nucleico y usos de las mismas. |
| CN116601307A (zh) * | 2020-09-02 | 2023-08-15 | 普罗美加公司 | 核酸修饰试剂和其用途 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK2591795T3 (en) * | 2007-02-23 | 2018-05-07 | Univ New York State Res Found | RNA TARGETING COMPOUNDS AND PROCEDURES FOR PREPARING AND USING THE SAME |
| WO2012050896A2 (en) * | 2010-09-29 | 2012-04-19 | Kansas State University Research Foundation | Protease selective supramolecular assemblies |
| WO2012092367A1 (en) * | 2010-12-28 | 2012-07-05 | University Of Rochester | Nucleic acid binding compounds, methods of making, and use thereof |
| CA2907072A1 (en) * | 2012-03-16 | 2013-09-19 | Valerion Therapeutics, Llc | Antisense conjugates for decreasing expression of dmpk |
| US9550769B2 (en) * | 2012-08-30 | 2017-01-24 | The Scripps Research Institute | Small molecules targeting repeat r(CGG) sequences |
| EP3169404A4 (en) * | 2014-07-18 | 2018-02-07 | The Scripps Research Institute | Toxic rna inhibitors self-assembled in situ |
-
2015
- 2015-07-17 EP EP15822000.4A patent/EP3169404A4/en not_active Withdrawn
- 2015-07-17 CA CA2955428A patent/CA2955428A1/en not_active Abandoned
- 2015-07-17 WO PCT/US2015/040902 patent/WO2016011348A1/en active Application Filing
- 2015-07-17 CN CN201580050560.0A patent/CN106714908A/zh active Pending
- 2015-07-17 JP JP2017502695A patent/JP6461306B2/ja active Active
- 2015-07-17 AU AU2015289524A patent/AU2015289524A1/en not_active Abandoned
- 2015-07-17 SG SG11201700387QA patent/SG11201700387QA/en unknown
- 2015-07-17 US US15/327,117 patent/US10220031B2/en active Active
-
2017
- 2017-01-15 IL IL250113A patent/IL250113A0/en unknown
-
2018
- 2018-12-25 JP JP2018241478A patent/JP2019068831A/ja active Pending
-
2019
- 2019-02-04 US US16/266,832 patent/US10471057B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US20190151310A1 (en) | 2019-05-23 |
| US10220031B2 (en) | 2019-03-05 |
| IL250113A0 (en) | 2017-03-30 |
| EP3169404A4 (en) | 2018-02-07 |
| EP3169404A1 (en) | 2017-05-24 |
| JP2017522323A (ja) | 2017-08-10 |
| CN106714908A (zh) | 2017-05-24 |
| SG11201700387QA (en) | 2017-02-27 |
| US10471057B2 (en) | 2019-11-12 |
| JP2019068831A (ja) | 2019-05-09 |
| AU2015289524A1 (en) | 2017-02-02 |
| CA2955428A1 (en) | 2016-01-21 |
| US20170143703A1 (en) | 2017-05-25 |
| WO2016011348A1 (en) | 2016-01-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10471057B2 (en) | Toxic RNA inhibitors self-assembled in situ | |
| US7399583B2 (en) | Method for the identification of inhibitors of the binding of ARE-containing mRNA and a HuR protein | |
| EP3022219B1 (en) | Specific targeting of rna expanded repeat sequences | |
| EP3038637B1 (en) | Modularly assembled small molecules for the treatment of myotonic dystrophy type 1 | |
| JP5721433B2 (ja) | 核酸と直接的または間接的に会合するタンパク質因子の単離 | |
| Xu et al. | A chemical probe targets DNA 5-formylcytosine sites and inhibits TDG excision, polymerases bypass, and gene expression | |
| US10233442B2 (en) | Method for affinity purification | |
| EP3380841B1 (en) | Autonomous sensing molecules (asm) | |
| JP2019106996A (ja) | タンパク質の安定性を測定する方法およびその使用 | |
| CN104774923B (zh) | 一种测定转录调控复合体的方法 | |
| US20020103349A1 (en) | Drug-oligonucleotides chimeric molecules | |
| Peralta et al. | Molecular recognition of HIV-1 RNAs with branched peptides | |
| Englert et al. | Aptamer-based proximity labeling guides covalent RNA modification | |
| JP2011024434A (ja) | オリゴヌクレオチドのスクリーニング方法及びオリゴヌクレオチドライブラリー | |
| KR20080077450A (ko) | 표적 rna에 특이적으로 결합하는 아크리딘-알파 나선형펩티드 결합체 | |
| Bargiela et al. | Cpy (a/t)(q/w) e D-Hexapeptides Bind CUG Repeats and Rescue Phenotypes of Myotonic Dystrophy Myoblasts and A Drosophila Model of the Disease | |
| Phillips | Biological Activity of a Py-Im Polyamide Androgen Receptor Antagonist | |
| KR20200016853A (ko) | 신속하게 발달하는 생물학적 엔티티의 치료 | |
| KR20020014283A (ko) | C형 간염 바이러스 증식조절단백질에 특이적으로결합하는 rna 및 이를 이용한 hcv 감염 진단방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180713 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180713 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20180713 |
|
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20180724 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20180814 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20181019 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20181204 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20181225 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6461306 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |