JP6435183B2 - Spring member - Google Patents
Spring member Download PDFInfo
- Publication number
- JP6435183B2 JP6435183B2 JP2014252501A JP2014252501A JP6435183B2 JP 6435183 B2 JP6435183 B2 JP 6435183B2 JP 2014252501 A JP2014252501 A JP 2014252501A JP 2014252501 A JP2014252501 A JP 2014252501A JP 6435183 B2 JP6435183 B2 JP 6435183B2
- Authority
- JP
- Japan
- Prior art keywords
- zinc
- powder
- coating film
- spring body
- resin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000576 coating method Methods 0.000 claims description 160
- 239000011248 coating agent Substances 0.000 claims description 145
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims description 98
- 239000000843 powder Substances 0.000 claims description 98
- 239000008199 coating composition Substances 0.000 claims description 90
- 239000011701 zinc Substances 0.000 claims description 51
- 229910052725 zinc Inorganic materials 0.000 claims description 50
- 239000003822 epoxy resin Substances 0.000 claims description 42
- 229920000647 polyepoxide Polymers 0.000 claims description 42
- 229920005989 resin Polymers 0.000 claims description 42
- 239000011347 resin Substances 0.000 claims description 42
- 229920001225 polyester resin Polymers 0.000 claims description 32
- 239000004645 polyester resin Substances 0.000 claims description 32
- 238000010438 heat treatment Methods 0.000 claims description 25
- 238000004519 manufacturing process Methods 0.000 claims description 14
- 239000002253 acid Substances 0.000 claims description 4
- 238000000151 deposition Methods 0.000 claims description 4
- 230000008021 deposition Effects 0.000 claims description 2
- 239000010410 layer Substances 0.000 description 74
- 238000000034 method Methods 0.000 description 29
- 239000002245 particle Substances 0.000 description 19
- 238000005260 corrosion Methods 0.000 description 15
- 230000007797 corrosion Effects 0.000 description 14
- 239000000049 pigment Substances 0.000 description 12
- 238000011156 evaluation Methods 0.000 description 11
- 229910052751 metal Inorganic materials 0.000 description 11
- 239000002184 metal Substances 0.000 description 11
- 239000003973 paint Substances 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 8
- 229910019142 PO4 Inorganic materials 0.000 description 8
- 230000000052 comparative effect Effects 0.000 description 8
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 8
- 239000010452 phosphate Substances 0.000 description 8
- 239000000463 material Substances 0.000 description 7
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 6
- 238000001879 gelation Methods 0.000 description 6
- 239000000654 additive Substances 0.000 description 5
- 238000007591 painting process Methods 0.000 description 5
- 230000003449 preventive effect Effects 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- LRXTYHSAJDENHV-UHFFFAOYSA-H zinc phosphate Chemical compound [Zn+2].[Zn+2].[Zn+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O LRXTYHSAJDENHV-UHFFFAOYSA-H 0.000 description 5
- 229910000165 zinc phosphate Inorganic materials 0.000 description 5
- 229910052782 aluminium Inorganic materials 0.000 description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 4
- 239000010953 base metal Substances 0.000 description 4
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 4
- PXKLMJQFEQBVLD-UHFFFAOYSA-N bisphenol F Chemical compound C1=CC(O)=CC=C1CC1=CC=C(O)C=C1 PXKLMJQFEQBVLD-UHFFFAOYSA-N 0.000 description 4
- 239000003518 caustics Substances 0.000 description 4
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 4
- 229910052742 iron Inorganic materials 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 229920003002 synthetic resin Polymers 0.000 description 4
- 239000000057 synthetic resin Substances 0.000 description 4
- 239000004593 Epoxy Substances 0.000 description 3
- 229910000639 Spring steel Inorganic materials 0.000 description 3
- 230000005856 abnormality Effects 0.000 description 3
- XBJJRSFLZVLCSE-UHFFFAOYSA-N barium(2+);diborate Chemical compound [Ba+2].[Ba+2].[Ba+2].[O-]B([O-])[O-].[O-]B([O-])[O-] XBJJRSFLZVLCSE-UHFFFAOYSA-N 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000004898 kneading Methods 0.000 description 3
- 230000009993 protective function Effects 0.000 description 3
- 238000010298 pulverizing process Methods 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- 239000002344 surface layer Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- VOAMNTKOJLSJCP-UHFFFAOYSA-N [Ca].[Zn].NC#N Chemical compound [Ca].[Zn].NC#N VOAMNTKOJLSJCP-UHFFFAOYSA-N 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 2
- -1 and for example Chemical compound 0.000 description 2
- 239000004305 biphenyl Substances 0.000 description 2
- 235000010290 biphenyl Nutrition 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 230000032798 delamination Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 238000007598 dipping method Methods 0.000 description 2
- YMNMFUIJDSASQW-UHFFFAOYSA-N distrontium;oxygen(2-);vanadium Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[O-2].[O-2].[V].[V].[Sr+2].[Sr+2] YMNMFUIJDSASQW-UHFFFAOYSA-N 0.000 description 2
- 230000005684 electric field Effects 0.000 description 2
- 238000009503 electrostatic coating Methods 0.000 description 2
- 238000007654 immersion Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 229920003986 novolac Polymers 0.000 description 2
- 238000010422 painting Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 239000013034 phenoxy resin Substances 0.000 description 2
- 229920006287 phenoxy resin Polymers 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 238000005480 shot peening Methods 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- XAEWLETZEZXLHR-UHFFFAOYSA-N zinc;dioxido(dioxo)molybdenum Chemical compound [Zn+2].[O-][Mo]([O-])(=O)=O XAEWLETZEZXLHR-UHFFFAOYSA-N 0.000 description 2
- FVCSARBUZVPSQF-UHFFFAOYSA-N 5-(2,4-dioxooxolan-3-yl)-7-methyl-3a,4,5,7a-tetrahydro-2-benzofuran-1,3-dione Chemical compound C1C(C(OC2=O)=O)C2C(C)=CC1C1C(=O)COC1=O FVCSARBUZVPSQF-UHFFFAOYSA-N 0.000 description 1
- 229910000975 Carbon steel Inorganic materials 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 229940049676 bismuth hydroxide Drugs 0.000 description 1
- TZSXPYWRDWEXHG-UHFFFAOYSA-K bismuth;trihydroxide Chemical compound [OH-].[OH-].[OH-].[Bi+3] TZSXPYWRDWEXHG-UHFFFAOYSA-K 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 150000001642 boronic acid derivatives Chemical class 0.000 description 1
- 239000010962 carbon steel Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 230000005489 elastic deformation Effects 0.000 description 1
- 238000004453 electron probe microanalysis Methods 0.000 description 1
- 238000007610 electrostatic coating method Methods 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910000398 iron phosphate Inorganic materials 0.000 description 1
- WBJZTOZJJYAKHQ-UHFFFAOYSA-K iron(3+) phosphate Chemical compound [Fe+3].[O-]P([O-])([O-])=O WBJZTOZJJYAKHQ-UHFFFAOYSA-K 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 239000003595 mist Substances 0.000 description 1
- MEFBJEMVZONFCJ-UHFFFAOYSA-N molybdate Chemical class [O-][Mo]([O-])(=O)=O MEFBJEMVZONFCJ-UHFFFAOYSA-N 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- NJPPVKZQTLUDBO-UHFFFAOYSA-N novaluron Chemical compound C1=C(Cl)C(OC(F)(F)C(OC(F)(F)F)F)=CC=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F NJPPVKZQTLUDBO-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 229940105570 ornex Drugs 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000010970 precious metal Substances 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 239000011435 rock Substances 0.000 description 1
- 102200082816 rs34868397 Human genes 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 235000019832 sodium triphosphate Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 230000003746 surface roughness Effects 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- UNXRWKVEANCORM-UHFFFAOYSA-I triphosphate(5-) Chemical compound [O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O UNXRWKVEANCORM-UHFFFAOYSA-I 0.000 description 1
- LSGOVYNHVSXFFJ-UHFFFAOYSA-N vanadate(3-) Chemical class [O-][V]([O-])([O-])=O LSGOVYNHVSXFFJ-UHFFFAOYSA-N 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000004018 waxing Methods 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/18—Layered products comprising a layer of synthetic resin characterised by the use of special additives
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/36—Layered products comprising a layer of synthetic resin comprising polyesters
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/38—Layered products comprising a layer of synthetic resin comprising epoxy resins
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D163/00—Coating compositions based on epoxy resins; Coating compositions based on derivatives of epoxy resins
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D167/00—Coating compositions based on polyesters obtained by reactions forming a carboxylic ester link in the main chain; Coating compositions based on derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/03—Powdery paints
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C26/00—Coating not provided for in groups C23C2/00 - C23C24/00
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16F—SPRINGS; SHOCK-ABSORBERS; MEANS FOR DAMPING VIBRATION
- F16F1/00—Springs
- F16F1/02—Springs made of steel or other material having low internal friction; Wound, torsion, leaf, cup, ring or the like springs, the material of the spring not being relevant
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16F—SPRINGS; SHOCK-ABSORBERS; MEANS FOR DAMPING VIBRATION
- F16F1/00—Springs
- F16F1/02—Springs made of steel or other material having low internal friction; Wound, torsion, leaf, cup, ring or the like springs, the material of the spring not being relevant
- F16F1/04—Wound springs
- F16F1/12—Attachments or mountings
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16F—SPRINGS; SHOCK-ABSORBERS; MEANS FOR DAMPING VIBRATION
- F16F1/00—Springs
- F16F1/02—Springs made of steel or other material having low internal friction; Wound, torsion, leaf, cup, ring or the like springs, the material of the spring not being relevant
- F16F1/18—Leaf springs
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16F—SPRINGS; SHOCK-ABSORBERS; MEANS FOR DAMPING VIBRATION
- F16F1/00—Springs
- F16F1/02—Springs made of steel or other material having low internal friction; Wound, torsion, leaf, cup, ring or the like springs, the material of the spring not being relevant
- F16F1/18—Leaf springs
- F16F1/20—Leaf springs with layers, e.g. anti-friction layers, or with rollers between the leaves
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- General Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Metallurgy (AREA)
- Springs (AREA)
- Paints Or Removers (AREA)
- Application Of Or Painting With Fluid Materials (AREA)
- Laminated Bodies (AREA)
- Other Surface Treatments For Metallic Materials (AREA)
Description
本発明は、表面に防食性塗膜が形成されたばね部材に関する。 The present invention relates to a spring member having a corrosion-resistant coating film formed on the surface.
金属材料からなるばね部材は、使用環境により腐食を受け、特性変化および強度劣化などが生じる。このような腐食を防止するために、金属部材の表面にはめっきによる被膜や塗料による塗膜などの保護膜を設けている。 The spring member made of a metal material is corroded depending on the use environment, and changes in characteristics and deterioration of strength occur. In order to prevent such corrosion, a protective film such as a coating film by plating or a coating film by paint is provided on the surface of the metal member.
腐食は、金属部材表面の金属原子がイオン化して部材表面から脱離することによって生じる。金属部材の表面に部材を構成する金属よりもイオン化傾向の大きい金属(卑の金属)が存在すると、腐食環境下では金属部材を構成する金属(貴の金属)よりも先にイオン化傾向が大きい卑の金属がイオン化して金属部材の腐食を防止できる。このような防食は犠牲防食と呼ばれ、鉄部材の場合には犠牲材として主に亜鉛を用いる。 Corrosion occurs when metal atoms on the surface of the metal member are ionized and desorbed from the surface of the member. If there is a metal (base metal) that has a higher ionization tendency than the metal constituting the member on the surface of the metal member, the base ion has a higher ionization tendency than the metal (precious metal) that constitutes the metal part in a corrosive environment. The metal of this can be ionized to prevent corrosion of the metal member. Such anticorrosion is called sacrificial anticorrosion. In the case of an iron member, zinc is mainly used as a sacrificial material.
亜鉛による犠牲防食を利用した保護膜としては、亜鉛粉末を比較的高濃度で含む粉体塗料を塗布し、焼き付けによって硬化して得られる塗膜がある。 As a protective film using sacrificial anticorrosion by zinc, there is a coating film obtained by applying a powder paint containing a relatively high concentration of zinc powder and curing it by baking.
このような亜鉛粉末を高濃度で含む塗料による塗膜は、塗装時に発生する気孔に起因するクラックが生じ、腐食性物質がクラックに侵入して塗膜の保護機能が低下してしまう場合がある。 A coating film made of a paint containing such zinc powder at a high concentration may cause cracks due to pores generated during coating, and corrosive substances may enter the cracks, resulting in a decrease in the protective function of the coating film. .
特にばね部材は、弾性変形を繰り返すことになるので、使用時間の経過によって塗膜に生じた亀裂が伸展しやすい。また、車両などの懸架用ばねとして用いられる場合、車輪で跳ね上げられた小石や砂利が懸架用ばね表面に衝突し、塗膜に生じた亀裂が伸展しやすい。 In particular, since the spring member repeatedly undergoes elastic deformation, cracks generated in the coating film with the passage of time of use tend to extend. In addition, when used as a suspension spring for a vehicle or the like, pebbles and gravel jumped up by a wheel collide with the surface of the suspension spring, and a crack generated in the coating film easily extends.
塗膜に生じた亀裂が伸展すると、腐食性物質が侵入しやすくなり、防食性が大きく低下する。 When the crack generated in the coating film extends, the corrosive substance easily enters and the anticorrosion property is greatly reduced.
特許文献1には、ばね板の引張力作用側の表面にエポキシ系合成樹脂をバインダとする高濃度亜鉛粉末塗料を塗布して第1塗膜とし、第1塗膜の上に同系統のエポキシ系合成樹脂塗料を塗布して第2塗膜を形成するさび止め方法が記載されている。特許文献2には、エポキシ樹脂、硬化剤、粒度が異なる2種の亜鉛粉末、防錆顔料を含む粉体塗料が記載されている。 In Patent Document 1, a high-concentration zinc powder coating material using an epoxy-based synthetic resin as a binder is applied to the surface of the spring plate on the tensile force acting side to form a first coating film, and the same series of epoxy is coated on the first coating film. A rust prevention method is described in which a synthetic resin coating is applied to form a second coating film. Patent Document 2 describes a powder coating containing an epoxy resin, a curing agent, two types of zinc powders having different particle sizes, and a rust preventive pigment.
特許文献1記載のさび止め方法は、高濃度亜鉛粉末塗料を塗布した第1塗膜の上に第2塗膜を形成することで、腐食性物質の侵入を防止して塗膜による保護機能を向上させようとしている。しかしながら、この第2塗膜を設けることで生じる問題がある。 The rust prevention method described in Patent Document 1 forms a second coating film on a first coating film coated with a high-concentration zinc powder paint, thereby preventing the invasion of corrosive substances and providing a protective function by the coating film. Trying to improve. However, there is a problem caused by providing the second coating film.
ばね部材は、特許文献1に記載されているように、ボルトなどの締結部材によってフレームなどに固定して用いられることが多い。特許文献1記載の第2塗膜として用いているエポキシ系合成樹脂塗料などの樹脂塗膜は、締結部材が接触している部分において、経時的に厚みの変動、多くは厚みが薄くなる現象が生じる。これは、いわゆる塗膜のへたりと呼ばれる現象で、ボルトの座面部分の塗膜が薄くなると、ボルトの締結軸力が低下し、ボルトが緩んでしまう。特許文献1には、第2塗膜のへたりにより締め付け具が緩みやすくなるのを防止するために、締め付け具の部分には第2塗膜を形成しないことが記載されている(段落[0006])。 As described in Patent Document 1, the spring member is often used by being fixed to a frame or the like by a fastening member such as a bolt. The resin coating film such as an epoxy-based synthetic resin paint used as the second coating film described in Patent Document 1 has a variation in thickness over time at the part where the fastening member is in contact, and the phenomenon that the thickness is often reduced over time. Arise. This is a phenomenon called so-called sag of the coating film. When the coating film on the bearing surface portion of the bolt becomes thin, the fastening axial force of the bolt decreases and the bolt loosens. Patent Document 1 describes that the second coating film is not formed on the portion of the fastening tool in order to prevent the fastening tool from becoming loose easily due to the sag of the second coating film (paragraph [0006]. ]).
特許文献2記載の粉体塗料による塗膜は、亜鉛粉末を含む1層からなる塗膜であるので、塗膜のへたりはほとんど発生しないが、腐食性物質がクラックに侵入することによる保護機能の低下を十分防止できず、防食性に劣る。 Since the coating film by the powder coating described in Patent Document 2 is a single-layer coating film containing zinc powder, there is almost no sag of the coating film, but the protective function by corrosive substances entering the cracks Can not be sufficiently prevented, and the corrosion resistance is poor.
本発明の目的は、高い防食性を維持するとともに耐へたり性も向上したばね部材を提供することである。 An object of the present invention is to provide a spring member that maintains high anticorrosion properties and also has improved sag resistance.
本発明は、ばね本体と、
前記ばね本体の表面に、エポキシ樹脂、酸末端ポリエステル樹脂および亜鉛粉末を含む粉体塗料組成物を、未硬化での厚さが60〜100μmとなるように塗装して形成される塗膜であって、前記ばね本体の表面側の、前記エポキシ樹脂および前記酸末端ポリエステル樹脂よりも前記亜鉛粉末を多く含む亜鉛含有層、ならびに前記亜鉛含有層上の、前記亜鉛粉末よりも前記エポキシ樹脂および前記酸末端ポリエステル樹脂を多く含む樹脂含有層を有する塗膜と、を含み、
前記粉体塗料組成物は、
前記エポキシ樹脂の含有量と前記酸末端ポリエステル樹脂の含有量との和を100質量部としたとき、前記エポキシ樹脂の含有量が10〜80質量部であり、前記酸末端ポリエステル樹脂の含有量が20〜90質量部であり、
前記亜鉛粉末の含有量が、粉体塗料組成物全体に対して55〜80質量%であることを特徴とするばね部材である。
The present invention includes a spring body,
It is a coating film formed by coating the surface of the spring body with a powder coating composition containing an epoxy resin, an acid-terminated polyester resin and zinc powder so that the uncured thickness becomes 60 to 100 μm. A zinc-containing layer containing more zinc powder than the epoxy resin and the acid-terminated polyester resin on the surface side of the spring body, and the epoxy resin and the acid rather than the zinc powder on the zinc-containing layer. a coating film having a resin-containing layer having a lot of terminal polyester resin, only including,
The powder coating composition is
When the sum of the content of the epoxy resin and the content of the acid-terminated polyester resin is 100 parts by mass, the content of the epoxy resin is 10 to 80 parts by mass, and the content of the acid-terminated polyester resin is 20 to 90 parts by mass,
The spring member is characterized in that the content of the zinc powder is 55 to 80 mass% with respect to the whole powder coating composition .
また本発明は、請求項1記載のばね部材を製造するばね部材の製造方法であって、
1種の粉体塗料組成物を1度塗装することで、前記ばね本体表面に、前記亜鉛含有層および前記樹脂含有層を有するように前記塗膜を形成することを特徴とするばね部材の製造方法である。
Moreover, this invention is a manufacturing method of the spring member which manufactures the spring member of Claim 1, Comprising:
One of the powder coating composition by coating once, the the spring body surface characteristics and to Luba ne member to form the coating film so as to have the zinc-containing layer and the resin-containing layer It is a manufacturing method .
また本発明は、予め加熱された前記ばね本体に、前記粉体塗料組成物を付着させる前加熱によって、前記亜鉛含有層および前記樹脂含有層を有するように前記塗膜を形成するか、または前記粉体塗料組成物を前記ばね本体に付着させた後に、該ばね本体を加熱する後加熱によって、前記亜鉛含有層および前記樹脂含有層を有するように前記塗膜を形成することを特徴とする。 The present invention, in the spring body that is heated pre Me, by heating before the deposition of the powder coating composition, or forming the coating film so as to have the zinc-containing layer and the resin-containing layer, or the powder coating composition after depositing on the spring body, by heating after heating the spring body, and forming the coating film so as to have the zinc-containing layer and the resin-containing layer .
本発明によれば、ばね部材は、ばね本体と、このばね本体の表面に形成される塗膜とを含む。塗膜は、エポキシ樹脂、酸末端ポリエステル樹脂および亜鉛粉末を含む粉体塗料組成物を、未硬化での厚さが60〜100μmとなるように塗装して形成され、亜鉛粉末を多く含む亜鉛含有層と樹脂を多く含む樹脂含有層の二層構造を有する。亜鉛含有層が、ばね本体の表面側にあり、樹脂含有層はその上にある。 According to the present invention, the spring member includes a spring body and a coating film formed on the surface of the spring body. The coating film is formed by coating a powder coating composition containing an epoxy resin, an acid-terminated polyester resin and zinc powder so that the uncured thickness becomes 60 to 100 μm, and contains zinc containing a large amount of zinc powder. It has a two-layer structure of a layer and a resin-containing layer containing a large amount of resin. A zinc-containing layer is on the surface side of the spring body, and a resin-containing layer is on it.
塗膜中で亜鉛がばね本体の表面側に偏在するので、従来と比較して亜鉛の含有量が少なくても、ばね本体と亜鉛とが十分に接触し、より高い防食性を発揮する。塗膜中で樹脂成分が塗膜の表層側に偏在するので、外部からの衝撃を緩和し、塗膜に生じる亀裂を防止する。樹脂含有層は、層厚みが比較的薄く形成されるので、層厚みの変動が小さくへたりが生じることがなく耐へたり性が向上する。そして、層厚みが薄くても亜鉛含有層とは一体的に形成されるため、層間に明確な界面が存在せず、層間剥離なども発生しない。 Since zinc is unevenly distributed on the surface side of the spring main body in the coating film, even if the zinc content is small compared to the conventional case, the spring main body and zinc are sufficiently in contact with each other, and higher corrosion resistance is exhibited. Since the resin component is unevenly distributed on the surface side of the coating film in the coating film, the impact from the outside is alleviated and cracks occurring in the coating film are prevented. Since the resin-containing layer is formed with a relatively thin layer thickness, the variation in the layer thickness is small and the sag resistance is improved without causing sag. And even if the layer thickness is thin, it is formed integrally with the zinc-containing layer, so there is no clear interface between layers, and no delamination occurs.
このように本発明の塗膜は、亜鉛含有層と樹脂含有層とによって高い防食性を維持するとともに耐へたり性も向上したばね部材を実現できる。 Thus, the coating film of this invention can implement | achieve the spring member which improved high sag-proof property while maintaining high corrosion resistance with a zinc content layer and a resin content layer.
また本発明によれば、粉体塗料組成物は、前記エポキシ樹脂の含有量と前記酸末端ポリエステル樹脂の含有量との和を100質量部としたとき、前記エポキシ樹脂の含有量を10〜80質量部とし、前記酸末端ポリエステル樹脂の含有量を20〜90質量部とし、前記亜鉛粉末の含有量を、粉体塗料組成物全体に対して55〜80質量%とすることで、1コートで亜鉛含有層と樹脂含有層とを含む塗膜を形成することができ、さらに防食性を向上することができる。 According to the invention, the powder coating composition has a content of the epoxy resin of 10 to 80 when the sum of the content of the epoxy resin and the content of the acid-terminated polyester resin is 100 parts by mass. In one coat, the content of the acid-terminated polyester resin is 20 to 90 parts by mass, and the content of the zinc powder is 55 to 80% by mass with respect to the entire powder coating composition. A coating film containing a zinc-containing layer and a resin-containing layer can be formed , and the corrosion resistance can be further improved.
また本発明によれば、亜鉛含有層と樹脂含有層の2つの層は、1種の粉体塗料組成物を1度塗装するだけでばね本体表面に形成することができるので、各層を個別に塗装する必要がなく、高機能な塗膜でありながら、工程数を短縮することができ、ばね部材を容易に製造することができる。 Further, according to the present invention, the two layers, the zinc-containing layer and the resin-containing layer, can be formed on the surface of the spring main body by simply applying one kind of powder coating composition once. There is no need for painting, and the number of steps can be reduced while the coating film is highly functional, and the spring member can be easily manufactured.
また本発明によれば、粉体塗料組成物を付着させる前に予めばね本体を加熱しておく前加熱でも、粉体塗料組成物を付着させた後にばね本体を加熱する後加熱でも、亜鉛含有層および樹脂含有層を有する高機能な塗膜を形成することができる。 In addition, according to the present invention, either the pre-heating in which the spring body is heated in advance before the powder coating composition is attached, or the post-heating in which the spring body is heated after the powder coating composition is attached is contained in zinc. A highly functional coating film having a layer and a resin-containing layer can be formed.
本発明のばね部材の種類は特に限定されない。たとえば、コイルスプリング、リーフスプリング、トーションバー、スタビライザなどが挙げられる。本発明のばね部材は、ばね本体と、ばね本体の表面に、エポキシ樹脂、酸末端ポリエステル樹脂および亜鉛粉末を含む粉体塗料組成物を用いて形成される塗膜と、を有する。 The kind of the spring member of the present invention is not particularly limited. For example, a coil spring, a leaf spring, a torsion bar, a stabilizer and the like can be mentioned. The spring member of this invention has a spring main body and the coating film formed on the surface of a spring main body using the powder coating composition containing an epoxy resin, an acid terminal polyester resin, and zinc powder.
<ばね本体>
ばね本体の材質は、亜鉛によって防食効果が発揮される金属であれば特に限定されない。本発明の塗膜による防食効果は、いわゆる犠牲防食によるものであり、素地金属よりもイオン化傾向が大きい卑の金属を用いて素地金属の腐食を防止する。本発明の塗膜は、亜鉛粉末を含むので、ばね本体の材質としては、亜鉛よりもイオン化傾向が小さい貴の金属であればよく、たとえば鉄、ニッケル、銅などを用いることができる。
<Spring body>
The material of the spring body is not particularly limited as long as it is a metal that exhibits an anticorrosive effect by zinc. The anticorrosive effect by the coating film of the present invention is due to so-called sacrificial anticorrosion, and the base metal is prevented from being corroded using a base metal having a higher ionization tendency than the base metal. Since the coating film of the present invention contains zinc powder, the spring body may be made of a noble metal having a smaller ionization tendency than zinc, and for example, iron, nickel, copper and the like can be used.
具体的には、一般にばね本体の材料として用いられるJIS規定のばね鋼などが好適である。ばね本体については、たとえば、ばね鋼などを熱間または冷間成形した後、ショットピーニングなどにより表面硬化処理を施したり、表面粗さを調整しておくとよい。また、ばね本体の素地表面には、リン酸亜鉛、リン酸鉄などのリン酸塩の皮膜が形成されることが望ましい。リン酸塩皮膜を形成することにより、ばね部材の耐食性および塗膜の密着性が向上する。この場合、リン酸塩皮膜は、ばね本体の塗装面の面積の80%以上を覆っていると効果的である。特に、リン酸塩がリン酸亜鉛の場合には、耐食性がより向上する。リン酸塩皮膜の形成は、公知の形成方法を用いることができる。たとえば、リン酸塩の溶液槽にばね本体を浸漬する浸漬法、リン酸塩の溶液をスプレーガンなどでばね本体に吹き付けるスプレー法などを用いる。 Specifically, JIS-regulated spring steel that is generally used as a material for the spring body is suitable. For the spring main body, for example, after hot or cold forming of spring steel or the like, surface hardening treatment may be performed by shot peening or the like, or the surface roughness may be adjusted. Moreover, it is desirable that a coating of a phosphate salt such as zinc phosphate or iron phosphate is formed on the surface of the spring body. By forming the phosphate film, the corrosion resistance of the spring member and the adhesion of the coating film are improved. In this case, it is effective that the phosphate coating covers 80% or more of the area of the painted surface of the spring body. In particular, when the phosphate is zinc phosphate, the corrosion resistance is further improved. For forming the phosphate film, a known forming method can be used. For example, a dipping method in which the spring body is immersed in a phosphate solution tank, or a spray method in which a phosphate solution is sprayed onto the spring body with a spray gun or the like is used.
<塗膜>
塗膜は、ばね本体の表面に、エポキシ樹脂、酸末端ポリエステル樹脂および亜鉛粉末を含む粉体塗料組成物を塗装し焼き付けによって硬化させることで得られる。この塗膜は、エポキシ樹脂および酸末端ポリエステル樹脂よりも亜鉛粉末を多く含む亜鉛含有層と、亜鉛粉末よりもエポキシ樹脂および酸末端ポリエステル樹脂を多く含む樹脂含有層とを有する。亜鉛含有層はばね本体の表面側に形成され、樹脂含有層は亜鉛含有層上に形成される。塗膜は、ばね本体表面の全体に形成されていてもよく、一部のみに形成されていてもよい。
<Coating film>
The coating film is obtained by applying a powder coating composition containing an epoxy resin, an acid-terminated polyester resin and zinc powder on the surface of the spring body and curing it by baking. This coating film has a zinc-containing layer containing more zinc powder than the epoxy resin and acid-terminated polyester resin, and a resin-containing layer containing more epoxy resin and acid-terminated polyester resin than the zinc powder. The zinc-containing layer is formed on the surface side of the spring body, and the resin-containing layer is formed on the zinc-containing layer. The coating film may be formed on the entire surface of the spring body, or may be formed only on a part thereof.
以下では、本発明の塗膜を形成するための粉体塗料組成物について説明する。
・粉体塗料組成物
本発明の塗膜を形成するための粉体塗料組成物は、上記のようにエポキシ樹脂、酸末端ポリエステル樹脂および亜鉛粉末を含むものである。
Below, the powder coating composition for forming the coating film of this invention is demonstrated.
-Powder coating composition The powder coating composition for forming the coating film of this invention contains an epoxy resin, an acid terminal polyester resin, and zinc powder as mentioned above.
粉体塗料組成物における各成分の含有量については、エポキシ樹脂の含有量と酸末端ポリエステル樹脂の含有量との和を100質量部としたとき、エポキシ樹脂の含有量を10〜80質量部とし、酸末端ポリエステル樹脂の含有量を20〜90質量部とし、亜鉛粉末の含有量を、粉体塗料組成物全体に対して30〜80質量%とすることが好ましい。 Regarding the content of each component in the powder coating composition, when the sum of the content of the epoxy resin and the content of the acid-terminated polyester resin is 100 parts by mass, the content of the epoxy resin is 10 to 80 parts by mass. The content of the acid-terminated polyester resin is preferably 20 to 90 parts by mass, and the content of the zinc powder is preferably 30 to 80% by mass with respect to the entire powder coating composition.
粉体塗料組成物は、ばね本体の表面に塗布する以前の塗料組成物の状態では、樹脂成分と亜鉛粉末とは偏ることなく均一に分散しており、粉体塗料組成物としては従来の粉体塗料組成物と同様に取り扱うことができる。また、塗装方法も従来と同様に静電塗装方法を用いることができ、従来と同様の取り扱いが可能である。すなわち、粉体塗料組成物としては、従来のものと何ら変わることなく、これまでと同様に取り扱うことができる。 In the state of the coating composition before being applied to the surface of the spring body, the powder coating composition is uniformly distributed without any bias between the resin component and the zinc powder. It can be handled in the same manner as the body coating composition. Moreover, the electrostatic coating method can be used similarly to the conventional coating method, and the same handling as the conventional method is possible. That is, the powder coating composition can be handled in the same manner as before without any change from the conventional one.
その一方で、防食性を付与したいばね本体の表面に粉体塗料組成物を塗装し、ばね本体の表面に塗膜が形成されると、その塗膜では、ばね本体の表面側に亜鉛粉末が偏り、表層側に樹脂成分が偏り、特性の異なる2つの層が形成される。 On the other hand, when a powder coating composition is applied to the surface of the spring body to which corrosion protection is to be imparted and a coating film is formed on the surface of the spring body, zinc powder is formed on the surface side of the spring body. Bias, the resin component is biased on the surface layer side, and two layers having different characteristics are formed.
亜鉛粉末を相対的に多く含む亜鉛含有層は、ばね本体の表面を亜鉛粉末で被覆し、鉄材料に対する亜鉛の犠牲防食効果が発揮される。亜鉛粉末が亜鉛含有層に濃縮されるので、粉体塗料組成物全体に対する亜鉛粉末の含有量を比較的少なくしても十分に防食性を発揮する。亜鉛粉末の含有量を少なくできるので、従来の過剰な亜鉛量を含む粉体塗料組成物よりも取り扱いが簡単で、塗装作業性が良好である。塗膜表層側には樹脂成分が偏在し、亜鉛粉末が存在しないので、表面の平滑性が容易に得られる。また粉体塗料組成物に顔料などの着色剤を含む場合には、顔料も樹脂含有層側に多く含まれることになり、樹脂含有層には亜鉛粉末がほとんど存在しないので、塗膜表層での発色性が向上する。亜鉛含有層と樹脂含有層とは、1コートで一体的に形成され、亜鉛含有層と樹脂含有層との間には明確な界面が形成されないので、これらの層間で剥離することもない。 The zinc-containing layer containing a relatively large amount of zinc powder covers the surface of the spring body with zinc powder, and exhibits the sacrificial anticorrosive effect of zinc on the iron material. Since the zinc powder is concentrated in the zinc-containing layer, even if the content of the zinc powder with respect to the whole powder coating composition is relatively small, the anticorrosion property is sufficiently exhibited. Since the content of zinc powder can be reduced, handling is easier and coating workability is better than conventional powder coating compositions containing an excessive amount of zinc. Since the resin component is unevenly distributed on the surface side of the coating film and no zinc powder is present, surface smoothness can be easily obtained. In addition, when the powder coating composition contains a colorant such as a pigment, a large amount of pigment is also contained on the resin-containing layer side, and since there is almost no zinc powder in the resin-containing layer, Color development is improved. The zinc-containing layer and the resin-containing layer are integrally formed with one coat, and a clear interface is not formed between the zinc-containing layer and the resin-containing layer, so that there is no separation between these layers.
(エポキシ樹脂)
塗料組成物が含有するエポキシ樹脂としては、主として常温で固形のビスフェノールA型エポキシ樹脂、ビスフェノールF型エポキシ樹脂、ノボラック型エポキシ樹脂、ビフェニル型エポキシ樹脂、高分子型エポキシ樹脂(フェノキシ樹脂)およびこれらの樹脂に水素添加した水添エポキシ樹脂を例示することができる。軟化点が60〜120℃好ましくは60〜100℃であることが好適である。軟化点が60℃未満であると、粉体塗料組成物のブロッキングが発生するために好ましくなく、120℃を超えると溶融混練を行う際に、粘度が高すぎて分散が十分に行われず、塗膜性能が発揮されない。またエポキシ樹脂の当量は400〜3000g/当量、好ましくは600〜2500g/当量であることが好適である。ビスフェノールA型エポキシ樹脂、ビスフェノールF型エポキシ樹脂を具体的に例示すると三菱化学株式会社製jER−1001、1002、1003、1004、1055、1007、1003F、1004F、1005F、1009F、1004FS、1006FS、1007FS、4005P、4007P、新日鐵化学株式会社製エポトートYD−011、012、013、014、017、902、903N、904、907、2004、2005RL、ダウケミカル社製DER−662E、663U、664U、666E、667E等が挙げられる。
(Epoxy resin)
Examples of the epoxy resin contained in the coating composition include bisphenol A type epoxy resin, bisphenol F type epoxy resin, novolac type epoxy resin, biphenyl type epoxy resin, polymer type epoxy resin (phenoxy resin), which are solid at room temperature, and these A hydrogenated epoxy resin obtained by hydrogenating a resin can be exemplified. It is preferable that the softening point is 60 to 120 ° C, preferably 60 to 100 ° C. When the softening point is less than 60 ° C., blocking of the powder coating composition occurs, which is not preferable. When the softening point exceeds 120 ° C., when the melt kneading is performed, the viscosity is too high and the dispersion is not sufficiently performed. Film performance is not demonstrated. The equivalent of the epoxy resin is 400 to 3000 g / equivalent, preferably 600 to 2500 g / equivalent. Specific examples of bisphenol A type epoxy resin and bisphenol F type epoxy resin include jER-1001, 1002, 1003, 1004, 1055, 1007, 1003F, 1004F, 1005F, 1009F, 1004FS, 1006FS, 1007FS, manufactured by Mitsubishi Chemical Corporation. 4005P, 4007P, Nippon Steel Chemical Co., Ltd. Epototo YD-011, 012, 013, 014, 017, 902, 903N, 904, 907, 2004, 2005RL, Dow Chemical Co., Ltd. DER-662E, 663U, 664U, 666E, 667E etc. are mentioned.
ノボラック型エポキシ樹脂を具体的に例示するとDIC株式会社製EPICLON N−660、N−665、N−670、N−673、N−680、N−695、N−770、N−775、新日鐵化学株式会社製エポトートYDCN−700−5、700−7、700−10などが挙げられる。ビフェニル型エポキシ樹脂、高分子型エポキシ樹脂(フェノキシ樹脂)を具体的に例示すると三菱化学株式会社製JER−YX4000、YX4000H、YL612H、YX7399、1256、4250、4275などが挙げられる。 Specific examples of novolak type epoxy resins include EPICLON N-660, N-665, N-670, N-673, N-680, N-695, N-770, N-775 manufactured by DIC Corporation, Nippon Steel Examples include Epototo YDCN-700-5, 700-7, and 700-10 manufactured by Chemical Co., Ltd. Specific examples of biphenyl type epoxy resin and polymer type epoxy resin (phenoxy resin) include JER-YX4000, YX4000H, YL612H, YX7399, 1256, 4250, 4275, etc. manufactured by Mitsubishi Chemical Corporation.
粉体塗料組成物におけるエポキシ樹脂の含有量は、エポキシ樹脂の含有量と酸末端ポリエステル樹脂の含有量との和を100質量部としたときに、10〜80質量部とすることが好ましい。 The content of the epoxy resin in the powder coating composition is preferably 10 to 80 parts by mass when the sum of the content of the epoxy resin and the content of the acid-terminated polyester resin is 100 parts by mass.
(酸末端ポリエステル樹脂)
塗料組成物が含有する酸末端ポリエステル樹脂は、通常のエポキシ樹脂含有粉体塗料組成物において使用されるものであれば、特に制限はない。酸末端ポリエステル樹脂は、ポリエステル樹脂の末端に、たとえば、カルボキシル基などの官能基を有するもの、ポリエステル樹脂の末端に、りん酸化合物をエステル結合させたものなどを用いることができる。カルボキシル基末端ポリエステル樹脂を具体的に例示するとダイセル・オルネクス株式会社製CRYLCOAT(CC)1683−0、2621−1、2670などが挙げられる。
(Acid-terminated polyester resin)
The acid-terminated polyester resin contained in the coating composition is not particularly limited as long as it is used in an ordinary epoxy resin-containing powder coating composition. Examples of the acid-terminated polyester resin include those having a functional group such as a carboxyl group at the end of the polyester resin, and those having a phosphate compound ester-bonded to the end of the polyester resin. Specific examples of the carboxyl group-terminated polyester resin include CRYLCOAT (CC) 1683-0, 2621-1, and 2670 manufactured by Daicel Ornex Co., Ltd.
粉体塗料組成物における酸末端ポリエステル樹脂の含有量は、エポキシ樹脂の含有量と酸末端ポリエステル樹脂の含有量との和を100質量部としたときに、20〜90質量部とすることが好ましい。 The content of the acid-terminated polyester resin in the powder coating composition is preferably 20 to 90 parts by mass when the sum of the content of the epoxy resin and the content of the acid-terminated polyester resin is 100 parts by mass. .
(亜鉛粉末)
塗料組成物が含有する亜鉛粉末としては、通常の粉体塗料組成物において使用されるものであれば、特に制限はない。たとえば、中位粒度1〜30μm、好ましくは4〜15μmの亜鉛粉末を用いることができる。このような亜鉛粉末としては、本荘ケミカル株式会社製亜鉛末F−500(中位粒度7.5μm)、F−1000(中位粒度4.9μm)、F−3000(中位粒度3.7μm)、堺化学工業株式会社製亜鉛末#1(中位粒度5.0μm)、#3(中位粒度4.0μm)、#40(中位粒度約50.0μm)、エカルト社製Zink Flake GTT(中位粒度13μm)、AT(中位粒度20μm)などが挙げられる。亜鉛粉末は、中位粒度が異なる複数種類を混合して用いてもよい。なお、亜鉛粉末の中位粒度とは、日機装株式会社製マイクロトラック等のレーザー式粒度分布測定機などを用いて測定した粒度分布における、積算値50%での粒径を意味する。
(Zinc powder)
The zinc powder contained in the coating composition is not particularly limited as long as it is used in an ordinary powder coating composition. For example, a zinc powder having a median particle size of 1 to 30 μm, preferably 4 to 15 μm can be used. As such zinc powder, zinc powder F-500 (medium particle size 7.5 μm), F-1000 (medium particle size 4.9 μm), F-3000 (medium particle size 3.7 μm) manufactured by Honjo Chemical Co., Ltd. Sakai Chemical Industry Co., Ltd. zinc powder # 1 (median particle size 5. 0 .mu.m), # 3 (median particle size 4.0 .mu.m), # 40 (median particle size of about 50.0 micrometers), Ekaruto Co. Zink Flake GTT (
粉体塗料組成物における亜鉛粉末の含有量は、粉体塗料組成物全体に対して30〜80質量%であり、好ましくは55〜80質量%であり、最も好ましくは60〜70質量%である。亜鉛粉末として上記のように粒径が異なる亜鉛粉末を混合して用いる場合は、その合計量が上記の範囲内であればよい。 The content of zinc powder in the powder coating composition is 30 to 80% by mass, preferably 55 to 80% by mass, and most preferably 60 to 70% by mass with respect to the entire powder coating composition. . When zinc powders having different particle sizes are mixed and used as the zinc powder as described above, the total amount may be within the above range.
粉体塗料組成物における亜鉛粉末の含有量が、粉体塗料組成物全体に対して30〜80質量%であると、塗膜が亜鉛含有層と樹脂含有層とを有するように層分離する。さらに含有量が、55〜80質量%であれば、防錆顔料を含まなくとも粉体塗料組成物が十分な防食性を発揮する。 When the content of the zinc powder in the powder coating composition is 30 to 80% by mass with respect to the whole powder coating composition, layers are separated so that the coating film has a zinc-containing layer and a resin-containing layer. Furthermore, if content is 55-80 mass%, even if it does not contain a rust preventive pigment, a powder coating composition will exhibit sufficient corrosion resistance.
(防錆顔料)
粉体塗料組成物は、上記各成分を必須構成として含んでいれば所望の塗膜を得ることができるが、さらに防錆顔料を含んでいてもよい。防錆顔料としては、リン酸アルミニウム等のリン酸塩誘導体、モリブデン酸亜鉛、リンモリブデン酸アルミニウムなどのモリブデン酸誘導体、シアナミドカルシウム亜鉛、硼酸バリウムなどの硼酸塩誘導体、バナジン酸ストロンチウムなどのバナジン酸塩誘導体、水酸化ビスマス等が挙げられ、好ましくはリン酸アルミニウム、リン酸亜鉛、モリブデン酸亜鉛、リンモリブデン酸アルミニウム、シアナミドカルシウム亜鉛、硼酸バリウム、バナジン酸ストロンチウムが挙げられる。このような防錆顔料としては、PM−300(リンモリブデン酸アルミニウム、キクチカラー株式会社製)、LFボウセイCP−Z(リン酸亜鉛、キクチカラー株式会社製)、K−WHITE(トリポリリン酸アルミニウム、テイカ株式会社製)、ビューサン11M−1(硼酸バリウム、堺化学工業株式会社)などが挙げられる。
(Anti-rust pigment)
The powder coating composition can obtain a desired coating film as long as it contains the above-described components as essential components, but may further contain a rust preventive pigment. Antirust pigments include phosphate derivatives such as aluminum phosphate, molybdate derivatives such as zinc molybdate and aluminum phosphomolybdate, borate derivatives such as cyanamide calcium zinc and barium borate, and vanadates such as strontium vanadate. Derivatives, bismuth hydroxide and the like can be mentioned, and preferably aluminum phosphate, zinc phosphate, zinc molybdate, aluminum phosphomolybdate, zinc cyanamide calcium, barium borate, strontium vanadate. As such rust preventive pigments, PM-300 (aluminum phosphomolybdate, manufactured by Kikuchi Color Co., Ltd.), LF Bowsei CP-Z (zinc phosphate, manufactured by Kikuchi Color Co., Ltd.), K-WHITE (aluminum tripolyphosphate, Manufactured by Teika Corporation), View Sun 11M-1 (barium borate, Sakai Chemical Industry Co., Ltd.), and the like.
粉体塗料組成物における防錆顔料の含有量は、粉体塗料組成物全体に対して0〜70質量%であり、好ましくは10〜50質量%である。 Content of the antirust pigment in a powder coating composition is 0-70 mass% with respect to the whole powder coating composition, Preferably it is 10-50 mass%.
(その他の添加剤)
粉体塗料組成物は、上記各成分に加えてさらに通常の粉体塗料組成物に用いられる着色顔料、体質顔料、流動性調整剤、ブロッキング防止剤、表面調整剤、ワキ防止剤、帯電制御剤、硬化促進剤等のその他の添加剤を含有してもよい。
(Other additives)
In addition to the above-mentioned components, the powder coating composition further includes a coloring pigment, an extender pigment, a fluidity adjusting agent, an antiblocking agent, a surface adjusting agent, an anti-waxing agent, and a charge control agent that are used in ordinary powder coating compositions. In addition, other additives such as a curing accelerator may be contained.
(粉体塗料組成物の製造方法)
粉体塗料組成物の製造方法は、たとえば、粉砕法などの公知の製造方法を用いることができる。粉砕法では、エポキシ樹脂、酸末端ポリエステル樹脂、亜鉛粉末および必要により防錆顔料、その他添加剤などの混合物を、タンブラーミキサ、ヘンシェルミキサなどの混合機で乾式混合し、混練機によって溶融混練する。混練機としては、たとえば、1軸または2軸のエクストルーダ、三本ロール、ラボブラストミルなどの一般的な混練機を使用できる。
(Method for producing powder coating composition)
As a method for producing the powder coating composition, for example, a known production method such as a pulverization method can be used. In the pulverization method, a mixture of an epoxy resin, an acid-terminated polyester resin, zinc powder and, if necessary, a rust preventive pigment and other additives is dry-mixed with a mixer such as a tumbler mixer or a Henschel mixer, and melt-kneaded with a kneader. As a kneading machine, for example, a general kneading machine such as a monoaxial or biaxial extruder, a triple roll, a laboratory blast mill can be used.
混練物を冷却固化し、固化物を粗粉砕および微粉砕して粉砕物を得る。粉砕機としては、たとえば、超音速ジェット気流を利用して粉砕するジェット式粉砕機、および高速で回転する回転子(ロータ)と固定子(ライナ)との間に形成される空間に固化物を導入して粉砕する衝撃式粉砕機が挙げられる。また必要により粉砕物に後添加剤(外添剤)を添加してもよい。 The kneaded product is cooled and solidified, and the solidified product is coarsely and finely pulverized to obtain a pulverized product. As the pulverizer, for example, a jet type pulverizer that pulverizes using a supersonic jet stream, and a solidified material in a space formed between a rotor (rotor) and a stator (liner) that rotate at high speed. An impact pulverizer that introduces and pulverizes can be used. If necessary, a post additive (external additive) may be added to the pulverized product.
粉砕物を分級して粉体を所望の粒子径および所望の粒径分布に調整して粉体塗料組成物を得る。分級には、遠心力および風力による分級により過粉砕トナー母粒子を除去できる公知の分級機を使用でき、たとえば、旋回式風力分級機(ロータリー式風力分級機)などを使用できる。 The pulverized product is classified to adjust the powder to a desired particle size and a desired particle size distribution to obtain a powder coating composition. For classification, a known classifier capable of removing excessively pulverized toner base particles by classification with centrifugal force and wind force can be used. For example, a swirl type wind classifier (rotary wind classifier) can be used.
なお、粉体塗料組成物の製造方法は上記の粉砕法に限らず、各成分が均一に分散した粉体塗料組成物を得ることができる製造方法であればよい。 In addition, the manufacturing method of a powder coating composition is not restricted to said grinding | pulverization method, What is necessary is just a manufacturing method which can obtain the powder coating composition in which each component was disperse | distributed uniformly.
<粉体塗料組成物の塗装方法>
上記のようにして得られた粉体塗料組成物を用いてばね本体の表面に塗膜を形成する。粉体塗料組成物を塗装する塗装方法は、少なくとも粉体塗料組成物をばね本体表面の塗膜形成面に塗布する塗布工程と、塗布した粉体塗料組成物を焼き付けて硬化させる焼き付け工程とを含む。
<Method of coating powder coating composition>
A coating film is formed on the surface of the spring body using the powder coating composition obtained as described above. The coating method for applying the powder coating composition includes at least a coating process for applying the powder coating composition to the coating film forming surface of the spring body surface, and a baking process for baking and curing the applied powder coating composition. Including.
[前加熱]
第1の塗装方法として、予め加熱されたばね本体に、粉体塗料組成物を付着させる実施形態を説明する。第一の塗装方法は、ばね本体を加熱する加熱工程と、該ばね本体の表面温度T(℃)がTf−20≦T<Tf+20(Tf:粉体塗料組成物の硬化完了点温度(℃))の状態において、該粉体塗料組成物を該ばね本体の表面に付着させる塗布工程と、付着した粉体塗料組成物を硬化させる硬化工程と、を有する。
[Preheating]
As a first coating method, an embodiment in which a powder coating composition is attached to a preheated spring body will be described. The first coating method is a heating step for heating the spring body, and the surface temperature T (° C.) of the spring body is Tf−20 ≦ T <Tf + 20 (Tf: the temperature at which the powder coating composition is cured (° C.)) ), A coating process for attaching the powder coating composition to the surface of the spring body, and a curing process for curing the attached powder coating composition.
加熱工程において、ばね本体の加熱方法は、特に限定されない。例えば、ばね本体を熱風炉、遠赤外線炉等に収容して加熱すればよい。また、ばね本体を通電加熱、あるいは誘導加熱してもよい。なかでも、通電加熱は、熱効率が高く、ばね本体の形状を問わず加熱できる等の理由から好適である。なお、加熱工程、塗装工程および硬化工程において、ばね本体の表面温度は、例えば、サーモグラフ等の非接触式温度計を用いて測定すればよい。 In the heating step, the method for heating the spring body is not particularly limited. For example, the spring body may be housed in a hot air furnace, a far infrared furnace, or the like and heated. Further, the spring body may be energized or induction heated. Among these, current heating is preferable because it has high thermal efficiency and can be heated regardless of the shape of the spring body. In addition, what is necessary is just to measure the surface temperature of a spring main body using non-contact-type thermometers, such as a thermograph, in a heating process, a painting process, and a hardening process.
加熱工程において、ばね本体の表面温度T(℃)が、Tf−20≦T<Tf+20に達したら、加熱を停止する。そして、続く塗装工程において、粉体塗料組成物をばね本体の表面に付着させる。なお、粉体塗料組成物の発熱ピークの始点と終点とから、予め粉体塗料組成物の硬化開始点温度(Ts)と、硬化完了点温度(Tf)とを求めておく。 In the heating process, when the surface temperature T (° C.) of the spring body reaches Tf−20 ≦ T <Tf + 20, the heating is stopped. In the subsequent coating process, the powder coating composition is adhered to the surface of the spring body. Note that the curing start point temperature (Ts) and the curing completion point temperature (Tf) of the powder coating composition are determined in advance from the start point and end point of the exothermic peak of the powder coating composition.
塗布工程では、上記の粉体塗料組成物を、加熱工程で予め加熱されたばね本体表面に塗布する。ばね本体に粉体塗料組成物を塗布する方法としては、公知の静電粉体塗装方法を用いることができる。たとえば、コロナ帯電方式、摩擦帯電方式などを用いることができる。 In the application step, the powder coating composition is applied to the surface of the spring body that has been heated in advance in the heating step. As a method for applying the powder coating composition to the spring body, a known electrostatic powder coating method can be used. For example, a corona charging method, a friction charging method, or the like can be used.
コロナ帯電方式、摩擦帯電方式いずれの方式でも先端筒状のガンユニットを用いて塗装を行う。コロナ帯電方式の場合、ガンユニットの先端に配置したコロナ電極に高電圧を印加してコロナ放電を起こし、発生したイオンでコロナ電極近傍の塗料粉体を帯電させる。ばね本体を接地電位としてコロナ電極とばね本体との間に電界を形成し、帯電した塗料粉体を電界によってばね本体に付着させる。 Both the corona charging method and the friction charging method are used for coating using a gun unit with a cylindrical tip. In the case of the corona charging method, a high voltage is applied to the corona electrode arranged at the tip of the gun unit to cause corona discharge, and the generated powder charges the paint powder near the corona electrode. An electric field is formed between the corona electrode and the spring body with the spring body as a ground potential, and the charged paint powder is adhered to the spring body by the electric field.
摩擦帯電方式の場合、ガンユニットの内部を移動する塗料粉体をガンユニットの内面で摩擦させて帯電させる。ばね本体を接地電位とすることで、帯電した塗料粉体をガンユニットから射出してばね本体に付着させる。 In the case of the friction charging method, the coating powder moving inside the gun unit is charged by being rubbed on the inner surface of the gun unit. By setting the spring body to the ground potential, the charged paint powder is injected from the gun unit and attached to the spring body.
コロナ帯電方式での放電による荷電量および摩擦帯電方式の射出量は、それぞれ粉体塗料組成物を塗布しようとするばね本体に応じて適宜設定すればよい。 What is necessary is just to set suitably the charge amount by the discharge by a corona charge system, and the injection amount of a friction charge system according to the spring main body which is going to apply | coat a powder coating composition, respectively.
硬化工程においては、原則放冷した状態で、つまり、ばね本体の余熱により、粉体塗料組成物を硬化させる。ここで、硬化を充分に行うためには、硬化完了時のばね本体の表面温度T(℃)は、Ts+30≦T(Ts:粉体塗料組成物の硬化開始点温度(℃))であることが望ましい。ばね本体の表面温度が、Ts+30℃未満になると、硬化が進行しにくくなるからである。したがって、硬化が完了する以前に、ばね本体の表面温度がTs+30℃未満になる場合には、再度加熱を行い、ばね本体の表面温度を上昇させるこが望ましい。すなわち、硬化工程において、さらに加熱することにより、粉体塗料組成物を硬化させることが望ましい。 In the curing step, the powder coating composition is cured in a cooled state, that is, by the residual heat of the spring body. Here, in order to perform sufficient curing, the surface temperature T (° C.) of the spring body at the completion of curing is Ts + 30 ≦ T (Ts: the temperature at which the powder coating composition is cured (° C.)). Is desirable. This is because when the surface temperature of the spring main body is less than Ts + 30 ° C., curing is difficult to proceed. Therefore, when the surface temperature of the spring body becomes less than Ts + 30 ° C. before the curing is completed, it is desirable to heat again to increase the surface temperature of the spring body. That is, it is desirable to cure the powder coating composition by further heating in the curing step.
以上のように第1の塗装方法では、ばね本体を加熱して、ばね本体の表面温度T(℃)がTf−20≦T<Tf+20の範囲にある間に、粉体塗料組成物を付着させる。その後、放冷により、ばね本体の表面温度は時間の経過と共に低下する。硬化は、ばね本体の表面温度がTs+30℃以上である間に完了することが望ましい。塗布工程開始時のばね本体の表面温度や、塗膜の厚さ等にもよるが、例えば、塗布工程開始から180秒後のばね本体の表面温度がTs+0℃以上であれば、充分に硬化させることができる。 As described above, in the first coating method, the spring body is heated, and the powder coating composition is adhered while the surface temperature T (° C.) of the spring body is in the range of Tf−20 ≦ T <Tf + 20. . Thereafter, the surface temperature of the spring body decreases with time due to cooling. Curing is desirably completed while the surface temperature of the spring body is Ts + 30 ° C. or higher. Although it depends on the surface temperature of the spring body at the start of the coating process, the thickness of the coating film, etc., for example, if the surface temperature of the spring body 180 seconds after the start of the coating process is Ts + 0 ° C. or more, it is sufficiently cured. be able to.
硬化工程における硬化の程度は、塗膜のゲル化率を測定することにより確認することができる。ゲル化率は、アセトンやキシレン等の溶剤に対する不溶分の質量分率である。例えば、塗膜の一部(試料)を溶剤に所定時間浸漬した後、乾燥させて質量を測定する。そして、次式(I)によりゲル化率を算出する。
ゲル化率(%)=(溶剤浸漬後の試料の乾燥質量/溶剤浸漬前の試料の質量)
×100・・・(I)
硬化が進行している程、ゲル化率は高くなる。例えば、ゲル化率が90%以上であれば、硬化が充分に進行していると判断することができる。
The degree of curing in the curing step can be confirmed by measuring the gelation rate of the coating film. The gelation rate is a mass fraction of insoluble matter in a solvent such as acetone or xylene. For example, after immersing a part (sample) of the coating film in a solvent for a predetermined time, it is dried and the mass is measured. Then, the gelation rate is calculated by the following formula (I).
Gelation rate (%) = (dry mass of sample after solvent immersion / mass of sample before solvent immersion)
× 100 ... (I)
As the curing proceeds, the gelation rate increases. For example, if the gelation rate is 90% or more, it can be determined that curing is sufficiently advanced.
粉体塗料組成物の硬化が完了した後は、塗膜表面の品質を保持しつつハンドリングを容易にするために、塗膜の温度を粉体塗料組成物の溶融温度未満に急冷することが望ましい。塗膜の急冷は、衝風、ミスト、シャワー、ディッピング等により行えばよい。 After the powder coating composition is cured, it is desirable to rapidly cool the coating temperature below the melting temperature of the powder coating composition in order to facilitate handling while maintaining the quality of the coating surface. . The coating film may be rapidly cooled by blast, mist, shower, dipping, or the like.
[後加熱]
第2の塗装方法として、粉体塗料組成物をばね本体に付着させた後に、ばね本体を加熱する実施形態を説明する。第2の塗装方法は、ばね本体の表面に粉体塗料組成物を塗布して、該ばね本体において、未硬化の塗膜を有する塗装部と、該塗装部を挟んで配置され未硬化の塗膜を有しない2つの非塗装部と、を形成する塗布工程と、2つの非塗装部それぞれに電極を接続する電極接続工程と、該電極間に電圧を印加してばね本体を通電加熱することにより、2つの非塗装部間に配置される塗装部の未硬化の塗膜を硬化する硬化工程と、を有する。
[Post-heating]
As a second coating method, an embodiment will be described in which a powder coating composition is attached to a spring body and then the spring body is heated. In the second coating method, a powder coating composition is applied to the surface of the spring body, and in the spring body, a coating portion having an uncured coating film and an uncured coating disposed between the coating portions are arranged. An application process for forming two non-coating parts having no film, an electrode connection process for connecting electrodes to each of the two non-coating parts, and applying a voltage between the electrodes to energize and heat the spring body And a curing step of curing the uncured coating film of the painted part disposed between the two non-painted parts.
なお、上記の各工程に加えて、ばね本体表面に前述のリン酸塩皮膜を形成する前処理工程を行ってもよい。 In addition to the above steps, a pretreatment step of forming the above-described phosphate film on the surface of the spring body may be performed.
塗布工程および焼き付け工程を経て、ばね本体の表面に塗膜が形成されたばね部材が得られる。ばね本体の表面に形成された塗膜は、亜鉛粉末を相対的に多く含む亜鉛含有層が、ばね本体の表面側に形成されており、エポキシ樹脂および酸末端ポリエステル樹脂の樹脂成分を相対的に多く含む樹脂含有層が、亜鉛含有層上に形成される。 A spring member having a coating film formed on the surface of the spring main body is obtained through the application process and the baking process. The coating film formed on the surface of the spring body has a zinc-containing layer containing a relatively large amount of zinc powder formed on the surface side of the spring body, and the resin components of the epoxy resin and acid-terminated polyester resin are relatively A resin-containing layer containing a large amount is formed on the zinc-containing layer.
塗膜中では、亜鉛粉末がばね本体の表面側に偏在するので、従来の塗膜と比較して亜鉛の含有量が少なくても、ばね本体と亜鉛とが十分に接触し、より高い防食性を発揮する。また、塗膜中で樹脂成分が塗膜の表層側に偏在するので、外部からの衝撃を緩和し、塗膜に生じた亀裂の伸展を防止する。樹脂含有層は、層厚みが比較的薄く形成されるので、層厚みの変動が小さくへたりが生じることがなく耐へたり性が向上する。そして、樹脂含有層の層厚みが薄くても亜鉛含有層とは一体的に形成されるため、層間に明確な界面が存在せず、層間剥離なども発生しない。 In the coating film, zinc powder is unevenly distributed on the surface side of the spring body, so even if the zinc content is small compared to the conventional coating film, the spring body and zinc are fully in contact with each other, resulting in higher anticorrosion properties. Demonstrate. Further, since the resin component is unevenly distributed on the surface layer side of the coating film, the impact from the outside is alleviated and the extension of cracks generated in the coating film is prevented. Since the resin-containing layer is formed with a relatively thin layer thickness, the variation in the layer thickness is small and the sag resistance is improved without causing sag. And even if the layer thickness of the resin-containing layer is thin, it is formed integrally with the zinc-containing layer, so there is no clear interface between layers, and no delamination occurs.
このように本発明の塗膜は、亜鉛含有層と樹脂含有層とによって高い防食性を維持するとともに耐へたり性も向上したばね部材を実現できる。さらにこれら2つの層は、各層を個別に塗装する必要がなく、1種の粉体塗料組成物を1度塗装するだけでばね本体表面に形成することができる。 Thus, the coating film of this invention can implement | achieve the spring member which improved high sag-proof property while maintaining high corrosion resistance with a zinc content layer and a resin content layer. Further, these two layers do not need to be individually applied to each layer, and can be formed on the surface of the spring body by applying only one powder coating composition once.
したがって、高機能な塗膜でありながら、1コートで済むので、ばね部材の製造工程数を短縮することができ、ばね部材を容易に製造することができる。 Therefore, since it is a highly functional coating film, only one coat is required, so the number of manufacturing steps of the spring member can be reduced, and the spring member can be easily manufactured.
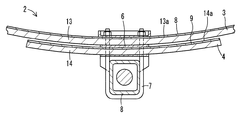
図1は、本発明の第1実施形態であるばね板1を示す図である。本実施形態では、ばね部材の一例として1枚のばね板1からなる板ばねについて示すが、本発明はこれに限定されるものではない。 FIG. 1 is a view showing a spring plate 1 according to the first embodiment of the present invention. In the present embodiment, a leaf spring including one spring plate 1 is shown as an example of a spring member, but the present invention is not limited to this.
ばね板1は、ばね本体10と塗膜11とを備えている。ばね本体10は、ばね鋼などの鉄系材料からなり、図1に示すように中央部分が湾曲した形状である。ばね本体10の引張力の作用する上側面10aに塗膜11が形成されている。塗膜11は、エポキシ樹脂、酸末端ポリエステル樹脂および亜鉛粉末を含む粉体塗料組成物から形成されている。前述のように亜鉛粉末を相対的に多く含む亜鉛含有層がばね本体10の表面側に形成され、樹脂成分を相対的に多く含む樹脂含有層が亜鉛含有層上に形成される。
The spring plate 1 includes a
図2は、本発明の第2実施形態である重ねばね板2を示す図である。重ねばね板2は、たとえば、車台と車軸とをつなぐリーフ式サスペンションに用いられる。重ねばね板2は、親板3と子板4とインタリーフ6とUボルト7とを含む。親板3の下面に子板4を重ねて2本のUボルト7により相手部材5に締め付けた重ね板ばね2であって、親板3のばね本体13と子板4のばね本体14の引張力の作用面13a、14aには、それぞれ塗膜8および塗膜9が形成される。塗膜8および塗膜9は、いずれも上記のように亜鉛含有層と樹脂含有層とが形成され、ばね本体13,14側に亜鉛含有層が位置し、表層側に樹脂含有層が位置する。親板3と子板4との間にはUボルト7の締め付けによる摩耗を防止するために亜鉛板または合成樹脂製板などからなるインタリーフ6がUボルト7の外側まで延長して挟着されている。
FIG. 2 is a view showing a lap spring plate 2 according to the second embodiment of the present invention. The lap spring plate 2 is used, for example, in a leaf type suspension that connects a chassis and an axle. The lap spring plate 2 includes a
次に、本実施形態のばね板1の塗装方法について説明する。本実施形態のばね板1の塗装方法は、塗装工程と、電極接続工程と、硬化工程と、部分塗装工程と、を有している。 Next, the coating method of the spring plate 1 of this embodiment is demonstrated. The method for painting the spring plate 1 of the present embodiment includes a painting process, an electrode connection process, a curing process, and a partial painting process.
塗装工程においては、ばね本体10の表面に上記の粉体塗料組成物を塗装して、ばね本体10において、塗装部と両端の2つの非塗装部とを形成する。ばね本体10の表面には、予めショットピーニング処理が施された後、リン酸亜鉛皮膜が形成されている。
In the painting process, the powder coating composition is coated on the surface of the
次に、ばね本体10の表面全体に、コロナ帯電塗装ガンを用いて粉体塗料組成物を付着させ、当該粉体塗料組成物からなる未硬化の塗膜を形成する。未硬化の塗膜の厚さは、60〜100μmとする。それから、ばね本体10の左右方向両端部に形成された未硬化の塗膜を、エアガンによりエアを吹き付けて除去する。これにより、ばね本体10の左右方向両端部は、ばね本体10が露出する。このようにして、ばね本体10および未硬化の塗膜からなる塗装部と、露出したばね本体10のみからなる2つの非塗装部と、を形成する。
Next, the powder coating composition is adhered to the entire surface of the
電極接続工程においては、ばね本体10に形成された2つの非塗装部に、各々、電極を接続する。電極は、配線によって電源と接続されている。電源が備える制御部により、電圧のオン/オフや、印加する電圧値等が制御される。
In the electrode connection step, electrodes are connected to the two non-painted portions formed on the
硬化工程においては、電源を動作させて電極間に電圧を印加し、ばね本体10を通電加熱することにより、塗装部の未硬化の塗膜を硬化させる。まず、電源をオンにして、電圧を印加すると、塗装部のばね本体10に、電流が流れ、ジュール熱が発生する。これにより、ばね本体10が加熱され、ばね本体10表面の未硬化の塗膜が加熱される。そして、塗装部の温度が所定の温度に達したら、通電加熱を止めて放冷することにより、未硬化の塗膜を硬化させる。なお、放冷時に塗装部の温度が低下し過ぎて、塗膜の硬化が充分に進行しない場合には、再度通電加熱を行い、塗装部の温度を、所定の温度まで上昇させることが望ましい。塗装部の温度は、例えば、サーモグラフ等の非接触式温度計を用いて測定すればよい。硬化によって塗膜11を形成した後、2つの非塗装部から電極を取り外す。
In the curing process, the power source is operated to apply a voltage between the electrodes, and the
部分塗装工程においては、2つの非塗装部を塗装する。まず、2つの非塗装部に、塗装工程において使用したのと同じ粉体塗料組成物を、コロナ帯電塗装ガンを用いて付着させ、当該粉体塗料組成物からなる未硬化の塗膜を形成する。次に、未硬化の塗膜を、誘導加熱装置を用いて加熱して硬化させることにより、塗膜11を形成する。このようにしてばね本体10の表面全体に塗膜11を形成し、ばね板1を製造する。
In the partial painting process, two non-painted parts are painted. First, the same powder coating composition used in the coating process is attached to two non-coating portions using a corona charging coating gun to form an uncured coating film composed of the powder coating composition. . Next, the
次に、本発明について実施例および比較例を挙げてさらに詳細に説明するが、本発明はこれらに限定されるものではない。なお、表中の粉体塗料組成物についての配合量は特別な記載がない限り、質量部を表す。 Next, although an Example and a comparative example are given and this invention is demonstrated further in detail, this invention is not limited to these. In addition, the compounding quantity about the powder coating composition in a table | surface represents a mass part unless there is special description.
表1に示す配合の粉体塗料組成物を、前記の製造方法で製造し、被塗物としてワッシャ(外径40mm、内径21mm、厚み2.9mm、炭素鋼(S45C)製)を用い、塗装膜厚が50μmになるようにワッシャの片面に静電塗装し、170℃、20分間の焼き付け(後加熱)によって実施例1,2の試験片を作製した。さらに、加熱条件を前加熱としたこと以外は実施例1と同様にして、実施例3の試験片を作製した。前加熱では、粉体塗料組成物を試験片に塗装する直前の試験片の表面温度が180℃となるように、予め試験片を加熱した。 A powder coating composition having the composition shown in Table 1 was manufactured by the above-described manufacturing method, and washer (outer diameter 40 mm, inner diameter 21 mm, thickness 2.9 mm, made of carbon steel (S45C)) was used as an object to be coated. The test piece of Examples 1 and 2 was produced by electrostatic coating on one side of the washer so that the film thickness was 50 μm, and baking (post-heating) at 170 ° C. for 20 minutes. Furthermore, a test piece of Example 3 was produced in the same manner as Example 1 except that the heating condition was preheating. In the preheating, the test piece was heated in advance so that the surface temperature of the test piece immediately before the powder coating composition was applied to the test piece was 180 ° C.
比較例1として、特許文献1(特開平11−188309号公報)の記載に準じて、第1塗膜と第2塗膜とからなる塗膜を実施例と同様にワッシャの片面に設け、比較例用の試験片を作製した。第1塗膜は、神東塗料株式会社製の板ばねZEプライマー焼き付け用を用い、第2塗膜は、ロックペイント株式会社製のタフコートシャーシーブラックを用いた。 As Comparative Example 1, according to the description in Patent Document 1 (Japanese Patent Laid-Open No. 11-188309), a coating film composed of a first coating film and a second coating film is provided on one side of a washer in the same manner as in the example, and compared. Example test specimens were prepared. The first coating film was used for baking a leaf spring ZE primer manufactured by Shinto Paint Co., Ltd., and the tough coating chassis black manufactured by Rock Paint Co., Ltd. was used for the second coating film.
比較例2として、特許文献2(特開2013−119582号公報)の実施例3の記載に準じて、粉体塗料組成物を製造し、実施例と同様にワッシャの片面に静電塗装して塗膜を設け、比較例用の試験片を作製した。 As Comparative Example 2, a powder coating composition was produced according to the description in Example 3 of Patent Document 2 (Japanese Patent Laid-Open No. 2013-119582), and electrostatic coating was performed on one side of the washer in the same manner as in Example. The coating film was provided and the test piece for a comparative example was produced.
次に、実施例用の試験片および比較例用の試験片を用いて性能評価した。
<評価方法>
・樹脂含有層の形成
塗膜に層分離が生じているかどうかを評価した。塗膜を切断して切断面を光学顕微鏡および電子顕微鏡(SEM)にて確認、またEPMA(Znマッピング)にて亜鉛の密度を目視で観察し、樹脂成分を相対的に多く含む樹脂含有層が形成されていれば評価を「あり」とし、樹脂含有層が形成されていなければ評価を「なし」とした。
Next, performance evaluation was performed using the test piece for an Example and the test piece for a comparative example.
<Evaluation method>
-Formation of resin-containing layer It was evaluated whether or not layer separation occurred in the coating film. The coating film is cut and the cut surface is confirmed with an optical microscope and an electron microscope (SEM), and the density of zinc is visually observed with EPMA (Zn mapping). If formed, the evaluation was “Yes”, and if the resin-containing layer was not formed, the evaluation was “None”.
・耐へたり性
図3は、耐へたり性を測定するための測定方法を説明するための模式図である。耐へたり性については、実施例ごと、比較例ごとに4つのワッシャ100を準備し、塗膜が形成された面同士を重ね、一対の押さえ冶具102によって4つのワッシャ100を上下から挟み、スラスト軸受103を介して、一対の押さえ冶具102と4つのワッシャ100とを締め付けボルト104で台座101に固定する。締め付けボルト104の塔頂部に歪ゲージ(株式会社共和電業製、ボルト軸力測定用ゲージ KFG−3−120−C20−11)を埋め込み、軸力を測定可能としている。
-Sag resistance FIG. 3: is a schematic diagram for demonstrating the measuring method for measuring sag resistance. For sag resistance, four
図3に示すように、台座101にワッシャ100を締め付けボルト104で固定した状態で恒温槽に投入し、60℃で48時間静置した。恒温槽への投入前の軸力と、48時間後の軸力とを測定し、へたり率(%)=(試験前後で低下した軸力/試験前の軸力)×100を算出した。へたり率が小さいほど耐へたり性に優れていると評価することができる。具体的には、単位膜厚あたりのへたり率で評価し、塗膜の膜厚が10μmあたりのへたり率が1%未満であれば、評価が良好であり、1%以上であれば評価が不良と判断する。本実施例では、塗膜の膜厚が50μmであるので、へたり率が5%未満であれば評価を良好「○」とし、へたり率が5%以上であれば評価を不良「×」とした。
As shown in FIG. 3, the
・防食性
防食性については、JIS K5600−7−1に準拠する試験方法(液温35℃の5%塩化ナトリウム水溶液を840時間噴霧)により耐中性塩水噴霧性を評価した。噴霧後の塗膜に、剥離や膨れなどの異常があるかどうかを目視にて確認した。塗膜に異常が無ければ評価を良好「○」とし、塗膜に異常があれば評価を「×」とした。
-Corrosion-proof property About anti-corrosion property, the neutral salt-water spray property was evaluated by the test method (spraying 5% sodium chloride aqueous solution of 35 degreeC of liquid temperature for 840 hours) based on JISK5600-7-1. It was visually confirmed whether or not the sprayed coating film had an abnormality such as peeling or swelling. If there was no abnormality in the coating film, the evaluation was “good”, and if there was an abnormality in the coating film, the evaluation was “x”.
実施例1〜3は、塗膜に亜鉛含有層と樹脂含有層とが形成され、評価結果が全て良好であった。比較例1は、第2塗膜が樹脂塗膜であるために耐へたり性に劣り、ボルトなどの締結部材によってフレームなどに固定して用いられることが多い板ばねなどのばね部材の塗膜としては適用できない。比較例2は、亜鉛粉末を含む1層からなる塗膜であるので、耐へたり性は良好であるが、防食性に劣り、ばね部材の塗膜としては適用できない。 In Examples 1 to 3, a zinc-containing layer and a resin-containing layer were formed on the coating film, and the evaluation results were all good. In Comparative Example 1, since the second coating film is a resin coating film, it is inferior in sag resistance, and is often used by being fixed to a frame or the like by a fastening member such as a bolt. Is not applicable. Since the comparative example 2 is a coating film composed of one layer containing zinc powder, the sag resistance is good, but the corrosion resistance is inferior and cannot be applied as a coating film for a spring member.
1 ばね板
2 重ねばね板
3 親板
4 子板
5 相手部材
6 インタリーフ
7 Uボルト
8 塗膜
9 塗膜
10 ばね本体
10a 上側面
11 塗膜
13 ばね本体
13a 作用面
14 ばね本体
100 ワッシャ
101 台座
102 冶具
103 スラスト軸受
104 ボルト
DESCRIPTION OF SYMBOLS 1 Spring board 2
Claims (3)
前記ばね本体の表面に、エポキシ樹脂、酸末端ポリエステル樹脂および亜鉛粉末を含む粉体塗料組成物を、未硬化での厚さが60〜100μmとなるように塗装して形成される塗膜であって、前記ばね本体の表面側の、前記エポキシ樹脂および前記酸末端ポリエステル樹脂よりも前記亜鉛粉末を多く含む亜鉛含有層、ならびに前記亜鉛含有層上の、前記亜鉛粉末よりも前記エポキシ樹脂および前記酸末端ポリエステル樹脂を多く含む樹脂含有層を有する塗膜と、を含み、
前記粉体塗料組成物は、
前記エポキシ樹脂の含有量と前記酸末端ポリエステル樹脂の含有量との和を100質量部としたとき、前記エポキシ樹脂の含有量が10〜80質量部であり、前記酸末端ポリエステル樹脂の含有量が20〜90質量部であり、
前記亜鉛粉末の含有量が、粉体塗料組成物全体に対して55〜80質量%であることを特徴とするばね部材。 A spring body;
It is a coating film formed by coating the surface of the spring body with a powder coating composition containing an epoxy resin, an acid-terminated polyester resin and zinc powder so that the uncured thickness becomes 60 to 100 μm. A zinc-containing layer containing more zinc powder than the epoxy resin and the acid-terminated polyester resin on the surface side of the spring body, and the epoxy resin and the acid rather than the zinc powder on the zinc-containing layer. a coating film having a resin-containing layer having a lot of terminal polyester resin, only including,
The powder coating composition is
When the sum of the content of the epoxy resin and the content of the acid-terminated polyester resin is 100 parts by mass, the content of the epoxy resin is 10 to 80 parts by mass, and the content of the acid-terminated polyester resin is 20 to 90 parts by mass,
The spring member, wherein the content of the zinc powder is 55 to 80% by mass with respect to the whole powder coating composition .
1種の粉体塗料組成物を1度塗装することで、前記ばね本体表面に、前記亜鉛含有層および前記樹脂含有層を有するように前記塗膜を形成することを特徴とするばね部材の製造方法。 A spring member manufacturing method for manufacturing the spring member according to claim 1,
One of the powder coating composition by coating once, the the spring body surface characteristics and to Luba ne member to form the coating film so as to have the zinc-containing layer and the resin-containing layer Manufacturing method .
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014252501A JP6435183B2 (en) | 2014-12-12 | 2014-12-12 | Spring member |
| PCT/JP2015/071892 WO2016092899A1 (en) | 2014-12-12 | 2015-07-31 | Spring member |
| CN201580067514.1A CN107002793B (en) | 2014-12-12 | 2015-07-31 | Spring members |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014252501A JP6435183B2 (en) | 2014-12-12 | 2014-12-12 | Spring member |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2016114137A JP2016114137A (en) | 2016-06-23 |
| JP2016114137A5 JP2016114137A5 (en) | 2017-11-09 |
| JP6435183B2 true JP6435183B2 (en) | 2018-12-05 |
Family
ID=56107098
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014252501A Active JP6435183B2 (en) | 2014-12-12 | 2014-12-12 | Spring member |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP6435183B2 (en) |
| CN (1) | CN107002793B (en) |
| WO (1) | WO2016092899A1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016113526A (en) * | 2014-12-12 | 2016-06-23 | 神東塗料株式会社 | Powder coating composition |
| WO2017163877A1 (en) * | 2016-03-25 | 2017-09-28 | 中央発條株式会社 | Highly durable spring and method for coating same |
| JP6772996B2 (en) * | 2017-09-28 | 2020-10-21 | 新東工業株式会社 | Anti-corrosion treatment method |
| WO2019137069A1 (en) * | 2018-01-12 | 2019-07-18 | 太仓卡兰平汽车零部件有限公司 | Spring surface flocking method |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2148133T1 (en) * | 1998-10-15 | 2000-10-16 | Morton Int Inc | CORROSION AND FRAGMENTATION RESISTANT COATINGS FOR HIGH TRACTION STEELS. |
| US6663968B2 (en) * | 2000-11-01 | 2003-12-16 | Rohm And Haas Company | Corrosion-and chip-resistant coatings for high tensile steel |
| JP4723390B2 (en) * | 2006-01-26 | 2011-07-13 | 中央発條株式会社 | High durability spring and its coating method |
| JP2007308067A (en) * | 2006-05-19 | 2007-11-29 | Toyota Motor Corp | Suspension spring |
| EP2236563A3 (en) * | 2009-04-03 | 2010-12-08 | Rohm and Haas Company | Powder corrosion and chip-resistant coating |
| JP2013119582A (en) * | 2011-12-07 | 2013-06-17 | Shinto Paint Co Ltd | Metal zinc-containing powder coating material composition |
| JP2014018727A (en) * | 2012-07-17 | 2014-02-03 | Chuo Spring Co Ltd | Spring member |
-
2014
- 2014-12-12 JP JP2014252501A patent/JP6435183B2/en active Active
-
2015
- 2015-07-31 WO PCT/JP2015/071892 patent/WO2016092899A1/en active Application Filing
- 2015-07-31 CN CN201580067514.1A patent/CN107002793B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| WO2016092899A1 (en) | 2016-06-16 |
| CN107002793B (en) | 2019-11-08 |
| CN107002793A (en) | 2017-08-01 |
| JP2016114137A (en) | 2016-06-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6435183B2 (en) | Spring member | |
| JP6817289B2 (en) | High durability spring and its painting method | |
| JP6567783B1 (en) | Powder coating composition and coating film forming method | |
| JP4907054B2 (en) | High durability spring and its coating method | |
| DE102008051883A1 (en) | Coating for cathodic corrosion protection of metal, method for producing the coating and use of the coating. | |
| KR101722793B1 (en) | Conductive metallic coating material, method of corrosion prevention with conductive metallic coating material, and method of corrosion-preventive repair therewith | |
| JP5606807B2 (en) | Powder coating method | |
| JP2009078263A (en) | Metallic member being subjected to rust-preventive treatment and coating paint | |
| WO2014013827A1 (en) | Spring member | |
| JP2003083376A (en) | Plate spring and manufacturing method thereof | |
| JP2017171705A (en) | Powder coating composition | |
| JP5981636B2 (en) | Zinc-based composite materials and use thereof | |
| JP7040529B2 (en) | Compositions for powder coatings and coated articles | |
| JP4208050B2 (en) | Powder coating composition, method of coating anticorrosive coating, steel for automobile | |
| JP2012213709A (en) | Method of forming powder coating film | |
| CN108138882B (en) | Coated spring | |
| JP6814857B1 (en) | Powder coating composition, manufacturing method thereof, and coating film forming method using the same | |
| JP2016113526A (en) | Powder coating composition | |
| TWI731335B (en) | Coating with oxidation resistance at high temperature and method for coating surface of carbon steel | |
| JP4309017B2 (en) | Coating method | |
| JP2013119582A (en) | Metal zinc-containing powder coating material composition | |
| WO2013099513A1 (en) | Method for coating spring member | |
| JP2013014701A (en) | Powder coating and powder coating method | |
| JP4197164B2 (en) | Thermosetting powder paint, painted iron-based material, and method for producing painted iron-based material | |
| EP3972744A1 (en) | Coated surface, use and method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170926 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20170926 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20180703 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180829 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20181030 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20181112 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6435183 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |