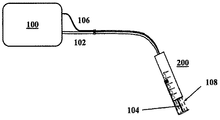
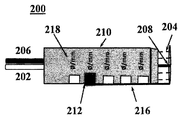
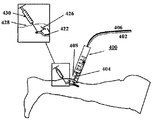
JP5759367B2 - 経皮的な血管治療方法及び装置 - Google Patents
経皮的な血管治療方法及び装置 Download PDFInfo
- Publication number
- JP5759367B2 JP5759367B2 JP2011509490A JP2011509490A JP5759367B2 JP 5759367 B2 JP5759367 B2 JP 5759367B2 JP 2011509490 A JP2011509490 A JP 2011509490A JP 2011509490 A JP2011509490 A JP 2011509490A JP 5759367 B2 JP5759367 B2 JP 5759367B2
- Authority
- JP
- Japan
- Prior art keywords
- radiation
- treated
- treatment
- laser
- handpiece
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000011282 treatment Methods 0.000 title claims description 76
- 238000000034 method Methods 0.000 title description 54
- 230000002792 vascular Effects 0.000 title description 3
- 230000005855 radiation Effects 0.000 claims description 79
- 239000000126 substance Substances 0.000 claims description 58
- 239000000203 mixture Substances 0.000 claims description 45
- 238000001816 cooling Methods 0.000 claims description 35
- 239000000243 solution Substances 0.000 claims description 24
- 239000000819 hypertonic solution Substances 0.000 claims description 20
- 229940021223 hypertonic solution Drugs 0.000 claims description 19
- 239000013307 optical fiber Substances 0.000 claims description 10
- 208000037997 venous disease Diseases 0.000 claims description 8
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 claims description 6
- 229960004194 lidocaine Drugs 0.000 claims description 6
- 230000003287 optical effect Effects 0.000 claims description 6
- 239000012809 cooling fluid Substances 0.000 claims description 5
- 238000012544 monitoring process Methods 0.000 claims description 2
- 239000000463 material Substances 0.000 claims 1
- 206010046996 Varicose vein Diseases 0.000 description 63
- 208000027185 varicose disease Diseases 0.000 description 41
- 210000003462 vein Anatomy 0.000 description 38
- 210000003491 skin Anatomy 0.000 description 23
- 239000012530 fluid Substances 0.000 description 19
- 208000009056 telangiectasis Diseases 0.000 description 19
- 210000004204 blood vessel Anatomy 0.000 description 16
- 239000000499 gel Substances 0.000 description 13
- 206010043189 Telangiectasia Diseases 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- 239000007924 injection Substances 0.000 description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- 208000002193 Pain Diseases 0.000 description 9
- 230000006378 damage Effects 0.000 description 9
- 238000001356 surgical procedure Methods 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 239000002826 coolant Substances 0.000 description 8
- 230000036407 pain Effects 0.000 description 8
- 239000003229 sclerosing agent Substances 0.000 description 7
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 6
- 230000003204 osmotic effect Effects 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 5
- 239000000460 chlorine Substances 0.000 description 5
- 229910052801 chlorine Inorganic materials 0.000 description 5
- 210000003989 endothelium vascular Anatomy 0.000 description 5
- 206010020751 Hypersensitivity Diseases 0.000 description 4
- 208000012641 Pigmentation disease Diseases 0.000 description 4
- 206010042674 Swelling Diseases 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 239000013543 active substance Substances 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 210000002683 foot Anatomy 0.000 description 4
- 238000007632 sclerotherapy Methods 0.000 description 4
- 230000008961 swelling Effects 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 206010014080 Ecchymosis Diseases 0.000 description 3
- 206010018852 Haematoma Diseases 0.000 description 3
- 208000032843 Hemorrhage Diseases 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 230000000740 bleeding effect Effects 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 230000005670 electromagnetic radiation Effects 0.000 description 3
- 230000003176 fibrotic effect Effects 0.000 description 3
- 230000001678 irradiating effect Effects 0.000 description 3
- 238000002647 laser therapy Methods 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 238000002604 ultrasonography Methods 0.000 description 3
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 208000003251 Pruritus Diseases 0.000 description 2
- 208000025865 Ulcer Diseases 0.000 description 2
- 206010047141 Vasodilatation Diseases 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 238000002430 laser surgery Methods 0.000 description 2
- 229910001507 metal halide Inorganic materials 0.000 description 2
- 150000005309 metal halides Chemical class 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 239000003504 photosensitizing agent Substances 0.000 description 2
- 239000004848 polyfunctional curative Substances 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 231100000245 skin permeability Toxicity 0.000 description 2
- 239000013076 target substance Substances 0.000 description 2
- 231100000397 ulcer Toxicity 0.000 description 2
- 230000006439 vascular pathology Effects 0.000 description 2
- 229910052724 xenon Inorganic materials 0.000 description 2
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 206010002329 Aneurysm Diseases 0.000 description 1
- 241000239290 Araneae Species 0.000 description 1
- 206010053567 Coagulopathies Diseases 0.000 description 1
- 208000034656 Contusions Diseases 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 208000007101 Muscle Cramp Diseases 0.000 description 1
- 208000028389 Nerve injury Diseases 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- 206010047249 Venous thrombosis Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 208000030961 allergic reaction Diseases 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 210000003423 ankle Anatomy 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 239000012459 cleaning agent Substances 0.000 description 1
- 230000035602 clotting Effects 0.000 description 1
- 230000001427 coherent effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000002692 epidural anesthesia Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 230000005496 eutectics Effects 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000002695 general anesthesia Methods 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000005414 inactive ingredient Substances 0.000 description 1
- MOFVSTNWEDAEEK-UHFFFAOYSA-M indocyanine green Chemical compound [Na+].[O-]S(=O)(=O)CCCCN1C2=CC=C3C=CC=CC3=C2C(C)(C)C1=CC=CC=CC=CC1=[N+](CCCCS([O-])(=O)=O)C2=CC=C(C=CC=C3)C3=C2C1(C)C MOFVSTNWEDAEEK-UHFFFAOYSA-M 0.000 description 1
- 229960004657 indocyanine green Drugs 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 230000007803 itching Effects 0.000 description 1
- 210000000629 knee joint Anatomy 0.000 description 1
- 238000013532 laser treatment Methods 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 238000002690 local anesthesia Methods 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 230000008764 nerve damage Effects 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 208000001297 phlebitis Diseases 0.000 description 1
- 230000019612 pigmentation Effects 0.000 description 1
- 230000000541 pulsatile effect Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000006748 scratching Methods 0.000 description 1
- 230000002393 scratching effect Effects 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 210000000434 stratum corneum Anatomy 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- ANRHNWWPFJCPAZ-UHFFFAOYSA-M thionine Chemical compound [Cl-].C1=CC(N)=CC2=[S+]C3=CC(N)=CC=C3N=C21 ANRHNWWPFJCPAZ-UHFFFAOYSA-M 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 230000024883 vasodilation Effects 0.000 description 1
- 210000002073 venous valve Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/20—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser
- A61B18/203—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser applying laser energy to the outside of the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/20—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser
- A61B18/22—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser the beam being directed along or through a flexible conduit, e.g. an optical fibre; Couplings or hand-pieces therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N5/0613—Apparatus adapted for a specific treatment
- A61N5/062—Photodynamic therapy, i.e. excitation of an agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N5/067—Radiation therapy using light using laser light
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/20—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00743—Type of operation; Specification of treatment sites
- A61B2017/00747—Dermatology
- A61B2017/00765—Decreasing the barrier function of skin tissue by radiated energy, e.g. using ultrasound, using laser for skin perforation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00005—Cooling or heating of the probe or tissue immediately surrounding the probe
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00345—Vascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00452—Skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00452—Skin
- A61B2018/00458—Deeper parts of the skin, e.g. treatment of vascular disorders or port wine stains
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/20—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser
- A61B2018/206—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser the laser light passing along a liquid-filled conduit
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/20—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser
- A61B2018/2065—Multiwave; Wavelength mixing, e.g. using four or more wavelengths
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/20—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser
- A61B18/22—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser the beam being directed along or through a flexible conduit, e.g. an optical fibre; Couplings or hand-pieces therefor
- A61B2018/225—Features of hand-pieces
- A61B2018/2253—Features of hand-pieces characterised by additional functions, e.g. surface cooling or detecting pathological tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N2005/002—Cooling systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N2005/002—Cooling systems
- A61N2005/005—Cooling systems for cooling the radiator
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N2005/002—Cooling systems
- A61N2005/007—Cooling systems for cooling the patient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N2005/065—Light sources therefor
- A61N2005/0651—Diodes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N2005/0658—Radiation therapy using light characterised by the wavelength of light used
- A61N2005/0659—Radiation therapy using light characterised by the wavelength of light used infrared
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Physics & Mathematics (AREA)
- Surgery (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Optics & Photonics (AREA)
- Otolaryngology (AREA)
- Electromagnetism (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Radiology & Medical Imaging (AREA)
- Pathology (AREA)
- Biophysics (AREA)
- Laser Surgery Devices (AREA)
- Radiation-Therapy Devices (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12791808P | 2008-05-15 | 2008-05-15 | |
| US61/127,918 | 2008-05-15 | ||
| PCT/US2009/003029 WO2009139900A1 (en) | 2008-05-15 | 2009-05-15 | Method/device for transdermal vascular treatment |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2011520508A JP2011520508A (ja) | 2011-07-21 |
| JP2011520508A5 JP2011520508A5 (enExample) | 2012-07-05 |
| JP5759367B2 true JP5759367B2 (ja) | 2015-08-05 |
Family
ID=41318984
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011509490A Expired - Fee Related JP5759367B2 (ja) | 2008-05-15 | 2009-05-15 | 経皮的な血管治療方法及び装置 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8906079B2 (enExample) |
| EP (1) | EP2288307B1 (enExample) |
| JP (1) | JP5759367B2 (enExample) |
| KR (1) | KR20110016940A (enExample) |
| WO (1) | WO2009139900A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2691114A1 (en) * | 2011-03-29 | 2014-02-05 | Principium Europe S.r.l. | Delivery of large molecular weight biologically active substances |
| KR101322658B1 (ko) * | 2011-11-24 | 2013-10-30 | 주식회사 루트로닉 | 의료용 레이저 치료장치 |
| CA2920835A1 (en) | 2013-08-20 | 2015-02-26 | Anutra Medical, Inc. | Syringe fill system and method |
| USD750768S1 (en) | 2014-06-06 | 2016-03-01 | Anutra Medical, Inc. | Fluid administration syringe |
| USD763433S1 (en) | 2014-06-06 | 2016-08-09 | Anutra Medical, Inc. | Delivery system cassette |
| USD774182S1 (en) | 2014-06-06 | 2016-12-13 | Anutra Medical, Inc. | Anesthetic delivery device |
| WO2016035082A1 (en) * | 2014-09-04 | 2016-03-10 | Dr. Eyal Bressler Ltd. | Assembly for photodynamic therapy |
| GB202005780D0 (en) * | 2020-04-21 | 2020-06-03 | Whiteley Clinic | Vein Ablation |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6251100B1 (en) * | 1993-09-24 | 2001-06-26 | Transmedica International, Inc. | Laser assisted topical anesthetic permeation |
| EP1131099A2 (en) * | 1999-01-15 | 2001-09-12 | Light Sciences Corporation | Noninvasive vascular therapy |
| DE19954710C1 (de) * | 1999-11-17 | 2001-03-15 | Pulsion Medical Sys Ag | Vorrichtung zur Behandlung von wachsenden, erweiterten oder mißgebildeten Blutgefäßen |
| US20020091377A1 (en) * | 2000-01-25 | 2002-07-11 | Anderson R. Rox | Method and apparatus for medical treatment utilizing long duration electromagnetic radiation |
| WO2001074265A1 (en) * | 2000-03-30 | 2001-10-11 | Coherent, Inc. | Dual-wavelength laser-treatment of vascular disorders |
| EP1455671B1 (en) * | 2001-12-10 | 2007-06-13 | Inolase 2002 Ltd. | Method and apparatus for improving safety during exposure to a monochromatic light source |
| EP1627662B1 (en) * | 2004-06-10 | 2011-03-02 | Candela Corporation | Apparatus for vacuum-assisted light-based treatments of the skin |
| US7153298B1 (en) * | 2003-03-28 | 2006-12-26 | Vandolay, Inc. | Vascular occlusion systems and methods |
| US9149322B2 (en) * | 2003-03-31 | 2015-10-06 | Edward Wells Knowlton | Method for treatment of tissue |
| US8257411B2 (en) * | 2003-09-30 | 2012-09-04 | Biolitec Pharma Marketing Ltd | Method for treatment of varices |
| EP1799138A4 (en) * | 2004-06-21 | 2008-05-07 | Alex Rapoport | WÄRMEENERGIEAPPLIKATOR |
| JP2006289098A (ja) * | 2005-04-12 | 2006-10-26 | Inolase 2002 Ltd | 皮膚の真空援用式光ベース治療用の装置 |
| US20070255355A1 (en) * | 2006-04-06 | 2007-11-01 | Palomar Medical Technologies, Inc. | Apparatus and method for skin treatment with compression and decompression |
| US20070260229A1 (en) * | 2006-05-05 | 2007-11-08 | Luis Navarro | Method and kit for treatment of varicose veins and other superficial venous pathology |
| US8246611B2 (en) * | 2006-06-14 | 2012-08-21 | Candela Corporation | Treatment of skin by spatial modulation of thermal heating |
| WO2010102099A1 (en) * | 2009-03-04 | 2010-09-10 | Gradiant Research, Llc | Method and apparatus for cancer therapy |
-
2009
- 2009-05-15 KR KR1020107028093A patent/KR20110016940A/ko not_active Ceased
- 2009-05-15 US US12/992,533 patent/US8906079B2/en not_active Expired - Fee Related
- 2009-05-15 EP EP09746987.8A patent/EP2288307B1/en not_active Not-in-force
- 2009-05-15 WO PCT/US2009/003029 patent/WO2009139900A1/en not_active Ceased
- 2009-05-15 JP JP2011509490A patent/JP5759367B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| KR20110016940A (ko) | 2011-02-18 |
| EP2288307A4 (en) | 2011-06-15 |
| EP2288307A1 (en) | 2011-03-02 |
| EP2288307B1 (en) | 2013-09-25 |
| US8906079B2 (en) | 2014-12-09 |
| JP2011520508A (ja) | 2011-07-21 |
| US20110275979A1 (en) | 2011-11-10 |
| WO2009139900A1 (en) | 2009-11-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7691457B2 (ja) | 血管腔内治療装置 | |
| JP5759367B2 (ja) | 経皮的な血管治療方法及び装置 | |
| US20080269735A1 (en) | Optical array for treating biological tissue | |
| US20150005751A1 (en) | Cosmetic Laser Treatment Device and Method for Loacalized Lipodystrophies and Flaccidity | |
| Sadick et al. | Combined endovascular laser with ambulatory phlebectomy for the treatment of superficial venous incompetence: a 2‐year perspective | |
| US20050010271A1 (en) | Method of using radiation to treat cutaneous and sub-cutaneous conditions | |
| US20130138033A1 (en) | Laser assisted sclerotherapy method for treating varicose veins | |
| US20250161707A1 (en) | Negative pressure laser handpiece | |
| RU2400261C1 (ru) | Способ катетерной склерооблитерации магистральных подкожных вен нижних конечностей | |
| US20050256204A1 (en) | Topical phenyl-epinephrine Rosacea treatment | |
| US8709004B2 (en) | Method and device for vascular treatment | |
| RU2800323C1 (ru) | Способ лечения посттравматических рубцов | |
| RU2653639C1 (ru) | Способ эндовазальной лазерной облитерации несостоятельной большой подкожной вены при посттромбофлебитических изменениях и анатомически сложном строении ее устья | |
| Minaev | Laser apparatus for surgery and force therapy based on high-power semiconductor and fibre lasers | |
| US11903909B2 (en) | Combined liposuction method | |
| RU2212917C2 (ru) | Способ облитерации варикозно-расширенных вен | |
| Eyuboglu et al. | Laser-assisted vascular malformation resection: A novel surgical approach | |
| Saxena | Laser Techniques for Advanced Medical Research | |
| Hsu | 5 An Overview of Therapy for Leg Veins | |
| Roenigk et al. | Treatment of Veins and Varicosities/Robert A. Weiss | |
| Hartwig | Lasers in dermatology | |
| Sadick | Sclerotherapy and Alternatives | |
| Weiss et al. | Treatment of Varicose and Telangiectatic Veins: Sclerotherapy, Ambulatory Phlebectomy, and Laser | |
| US20100234832A1 (en) | Method for vascular treatment | |
| Quaba | Telangiectasia: Is Treatment Worthwhile and Who Should Pay? |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120515 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120515 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20121001 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130827 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131127 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131204 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131226 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140109 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140124 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140131 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140225 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140805 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141104 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20141111 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141204 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20141211 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141226 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150205 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150507 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150605 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5759367 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| LAPS | Cancellation because of no payment of annual fees |