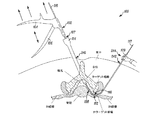
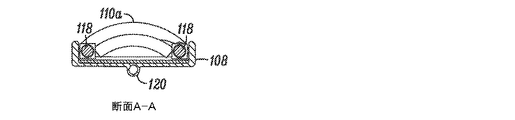
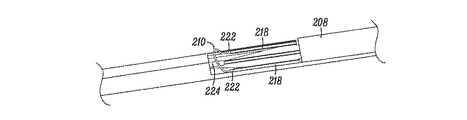
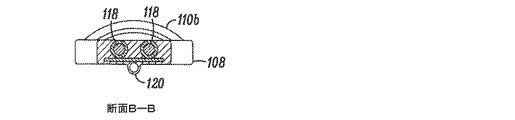JP5175264B2 - 組織を修正する装置及び方法 - Google Patents
組織を修正する装置及び方法 Download PDFInfo
- Publication number
- JP5175264B2 JP5175264B2 JP2009500564A JP2009500564A JP5175264B2 JP 5175264 B2 JP5175264 B2 JP 5175264B2 JP 2009500564 A JP2009500564 A JP 2009500564A JP 2009500564 A JP2009500564 A JP 2009500564A JP 5175264 B2 JP5175264 B2 JP 5175264B2
- Authority
- JP
- Japan
- Prior art keywords
- tissue
- patient
- distal
- proximal
- target
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034 method Methods 0.000 title claims description 96
- 230000004048 modification Effects 0.000 claims description 242
- 238000012986 modification Methods 0.000 claims description 242
- 238000005498 polishing Methods 0.000 claims description 50
- 230000001681 protective effect Effects 0.000 claims description 48
- 238000005520 cutting process Methods 0.000 claims description 27
- 230000006378 damage Effects 0.000 claims description 20
- 208000005198 spinal stenosis Diseases 0.000 claims description 17
- 210000001519 tissue Anatomy 0.000 description 753
- 230000004888 barrier function Effects 0.000 description 132
- 238000012937 correction Methods 0.000 description 62
- 239000000463 material Substances 0.000 description 48
- 210000005036 nerve Anatomy 0.000 description 31
- 238000012800 visualization Methods 0.000 description 23
- 230000007246 mechanism Effects 0.000 description 22
- 239000000523 sample Substances 0.000 description 21
- 229910052751 metal Inorganic materials 0.000 description 18
- 239000002184 metal Substances 0.000 description 18
- 210000000988 bone and bone Anatomy 0.000 description 17
- 210000000278 spinal cord Anatomy 0.000 description 15
- 210000003041 ligament Anatomy 0.000 description 14
- 229920000642 polymer Polymers 0.000 description 14
- 230000001012 protector Effects 0.000 description 13
- -1 Darcon® Polymers 0.000 description 12
- 229920001652 poly(etherketoneketone) Polymers 0.000 description 12
- 210000004872 soft tissue Anatomy 0.000 description 12
- 239000012530 fluid Substances 0.000 description 11
- 238000011282 treatment Methods 0.000 description 11
- 239000004696 Poly ether ether ketone Substances 0.000 description 10
- 238000004140 cleaning Methods 0.000 description 10
- 230000033001 locomotion Effects 0.000 description 10
- 239000013307 optical fiber Substances 0.000 description 10
- 229920002530 polyetherether ketone Polymers 0.000 description 10
- 239000004677 Nylon Substances 0.000 description 8
- 230000006870 function Effects 0.000 description 8
- 229920001778 nylon Polymers 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- 239000000017 hydrogel Substances 0.000 description 7
- 230000001537 neural effect Effects 0.000 description 7
- 230000005540 biological transmission Effects 0.000 description 6
- 210000000845 cartilage Anatomy 0.000 description 6
- 239000000835 fiber Substances 0.000 description 6
- 238000005286 illumination Methods 0.000 description 6
- 210000004749 ligamentum flavum Anatomy 0.000 description 6
- 230000036961 partial effect Effects 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 238000001356 surgical procedure Methods 0.000 description 6
- 239000000919 ceramic Substances 0.000 description 5
- 239000013078 crystal Substances 0.000 description 5
- 150000002739 metals Chemical class 0.000 description 5
- 229910001000 nickel titanium Inorganic materials 0.000 description 5
- 239000004417 polycarbonate Substances 0.000 description 5
- 229920000515 polycarbonate Polymers 0.000 description 5
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 5
- 239000004810 polytetrafluoroethylene Substances 0.000 description 5
- 239000004698 Polyethylene Substances 0.000 description 4
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 238000002567 electromyography Methods 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 239000004744 fabric Substances 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- 229920001903 high density polyethylene Polymers 0.000 description 4
- 239000004700 high-density polyethylene Substances 0.000 description 4
- 238000001746 injection moulding Methods 0.000 description 4
- 238000009434 installation Methods 0.000 description 4
- 239000012212 insulator Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 229920001684 low density polyethylene Polymers 0.000 description 4
- 239000004702 low-density polyethylene Substances 0.000 description 4
- 230000003287 optical effect Effects 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000002633 protecting effect Effects 0.000 description 4
- 229910001220 stainless steel Inorganic materials 0.000 description 4
- 239000010935 stainless steel Substances 0.000 description 4
- 230000002792 vascular Effects 0.000 description 4
- 210000002517 zygapophyseal joint Anatomy 0.000 description 4
- 229920004943 Delrin® Polymers 0.000 description 3
- 241000195955 Equisetum hyemale Species 0.000 description 3
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 208000008558 Osteophyte Diseases 0.000 description 3
- 239000004699 Ultra-high molecular weight polyethylene Substances 0.000 description 3
- WAIPAZQMEIHHTJ-UHFFFAOYSA-N [Cr].[Co] Chemical class [Cr].[Co] WAIPAZQMEIHHTJ-UHFFFAOYSA-N 0.000 description 3
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 3
- 125000002777 acetyl group Chemical class [H]C([H])([H])C(*)=O 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 230000002457 bidirectional effect Effects 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 210000004204 blood vessel Anatomy 0.000 description 3
- 230000008468 bone growth Effects 0.000 description 3
- 230000006835 compression Effects 0.000 description 3
- 238000007906 compression Methods 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 229910000701 elgiloys (Co-Cr-Ni Alloy) Inorganic materials 0.000 description 3
- 230000003628 erosive effect Effects 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 230000002262 irrigation Effects 0.000 description 3
- 238000003973 irrigation Methods 0.000 description 3
- 210000001503 joint Anatomy 0.000 description 3
- 238000002684 laminectomy Methods 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 238000012806 monitoring device Methods 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 230000002669 organ and tissue protective effect Effects 0.000 description 3
- 230000008520 organization Effects 0.000 description 3
- 229920000728 polyester Polymers 0.000 description 3
- 229920001296 polysiloxane Polymers 0.000 description 3
- 229920002635 polyurethane Polymers 0.000 description 3
- 239000004814 polyurethane Substances 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 210000002435 tendon Anatomy 0.000 description 3
- 230000008719 thickening Effects 0.000 description 3
- 229920000785 ultra high molecular weight polyethylene Polymers 0.000 description 3
- 238000003466 welding Methods 0.000 description 3
- 206010019909 Hernia Diseases 0.000 description 2
- 229920002614 Polyether block amide Polymers 0.000 description 2
- DYAHQFWOVKZOOW-UHFFFAOYSA-N Sarin Chemical compound CC(C)OP(C)(F)=O DYAHQFWOVKZOOW-UHFFFAOYSA-N 0.000 description 2
- 208000005400 Synovial Cyst Diseases 0.000 description 2
- HZEWFHLRYVTOIW-UHFFFAOYSA-N [Ti].[Ni] Chemical compound [Ti].[Ni] HZEWFHLRYVTOIW-UHFFFAOYSA-N 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 238000005452 bending Methods 0.000 description 2
- 238000001574 biopsy Methods 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 238000002591 computed tomography Methods 0.000 description 2
- 239000004020 conductor Substances 0.000 description 2
- 208000031513 cyst Diseases 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000009760 electrical discharge machining Methods 0.000 description 2
- 230000000763 evoking effect Effects 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 238000007373 indentation Methods 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000002350 laparotomy Methods 0.000 description 2
- 239000010410 layer Substances 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 238000003754 machining Methods 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 210000000944 nerve tissue Anatomy 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 231100000241 scar Toxicity 0.000 description 2
- 210000003594 spinal ganglia Anatomy 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 230000017423 tissue regeneration Effects 0.000 description 2
- UONOETXJSWQNOL-UHFFFAOYSA-N tungsten carbide Chemical compound [W+]#[C-] UONOETXJSWQNOL-UHFFFAOYSA-N 0.000 description 2
- SUXAHTCWWUUZNF-ARJAWSKDSA-N CC/C=C\C1NC1 Chemical compound CC/C=C\C1NC1 SUXAHTCWWUUZNF-ARJAWSKDSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 208000032544 Cicatrix Diseases 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 241001653121 Glenoides Species 0.000 description 1
- 206010018852 Haematoma Diseases 0.000 description 1
- 229920000271 Kevlar® Polymers 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 241000446313 Lamella Species 0.000 description 1
- 239000004962 Polyamide-imide Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 239000012891 Ringer solution Substances 0.000 description 1
- 208000007103 Spondylolisthesis Diseases 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 229910001080 W alloy Inorganic materials 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 238000004873 anchoring Methods 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 230000036770 blood supply Effects 0.000 description 1
- 239000007767 bonding agent Substances 0.000 description 1
- 238000005219 brazing Methods 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 210000003164 cauda equina Anatomy 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000001804 debridement Methods 0.000 description 1
- 238000002059 diagnostic imaging Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000002783 friction material Substances 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 210000000609 ganglia Anatomy 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000023597 hemostasis Effects 0.000 description 1
- 230000001969 hypertrophic effect Effects 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 210000002414 leg Anatomy 0.000 description 1
- 210000004705 lumbosacral region Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 230000003387 muscular Effects 0.000 description 1
- 230000007830 nerve conduction Effects 0.000 description 1
- 210000000276 neural tube Anatomy 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000000088 plastic resin Substances 0.000 description 1
- 229920002312 polyamide-imide Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 239000003223 protective agent Substances 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000000452 restraining effect Effects 0.000 description 1
- 230000037387 scars Effects 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 239000012781 shape memory material Substances 0.000 description 1
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 238000005476 soldering Methods 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000003238 somatosensory effect Effects 0.000 description 1
- 206010062261 spinal cord neoplasm Diseases 0.000 description 1
- 210000001032 spinal nerve Anatomy 0.000 description 1
- 210000000273 spinal nerve root Anatomy 0.000 description 1
- 229910001256 stainless steel alloy Inorganic materials 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 238000003325 tomography Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 238000007514 turning Methods 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 238000012285 ultrasound imaging Methods 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/16—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans
- A61B17/1659—Surgical rasps, files, planes, or scrapers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/00234—Surgical instruments, devices or methods for minimally invasive surgery
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/02—Surgical instruments, devices or methods for holding wounds open, e.g. retractors; Tractors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/16—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans
- A61B17/1613—Component parts
- A61B17/1626—Control means; Display units
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/16—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans
- A61B17/1662—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans for particular parts of the body
- A61B17/1671—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans for particular parts of the body for the spine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/16—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans
- A61B17/17—Guides or aligning means for drills, mills, pins or wires
- A61B17/1739—Guides or aligning means for drills, mills, pins or wires specially adapted for particular parts of the body
- A61B17/1757—Guides or aligning means for drills, mills, pins or wires specially adapted for particular parts of the body for the spine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/34—Trocars; Puncturing needles
- A61B17/3417—Details of tips or shafts, e.g. grooves, expandable, bendable; Multiple coaxial sliding cannulas, e.g. for dilating
- A61B17/3421—Cannulas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws or setting implements
- A61B17/88—Osteosynthesis instruments; Methods or means for implanting or extracting internal or external fixation devices
- A61B17/8861—Apparatus for manipulating flexible wires or straps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/02—Surgical instruments, devices or methods for holding wounds open, e.g. retractors; Tractors
- A61B17/0218—Surgical instruments, devices or methods for holding wounds open, e.g. retractors; Tractors for minimally invasive surgery
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B17/320016—Endoscopic cutting instruments, e.g. arthroscopes, resectoscopes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B17/320016—Endoscopic cutting instruments, e.g. arthroscopes, resectoscopes
- A61B17/32002—Endoscopic cutting instruments, e.g. arthroscopes, resectoscopes with continuously rotating, oscillating or reciprocating cutting instruments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws or setting implements
- A61B17/88—Osteosynthesis instruments; Methods or means for implanting or extracting internal or external fixation devices
- A61B17/8897—Guide wires or guide pins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/00234—Surgical instruments, devices or methods for minimally invasive surgery
- A61B2017/00238—Type of minimally invasive operation
- A61B2017/00261—Discectomy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A61B2017/00867—Material properties shape memory effect
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B2017/32006—Surgical cutting instruments with a cutting strip, band or chain, e.g. like a chainsaw
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/34—Trocars; Puncturing needles
- A61B2017/348—Means for supporting the trocar against the body or retaining the trocar inside the body
- A61B2017/3482—Means for supporting the trocar against the body or retaining the trocar inside the body inside
- A61B2017/3484—Anchoring means, e.g. spreading-out umbrella-like structure
- A61B2017/3488—Fixation to inner organ or inner body tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/08—Accessories or related features not otherwise provided for
- A61B2090/0801—Prevention of accidental cutting or pricking
- A61B2090/08021—Prevention of accidental cutting or pricking of the patient or his organs
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medical Informatics (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Dentistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Pathology (AREA)
- Surgical Instruments (AREA)
Applications Claiming Priority (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/375,265 | 2006-03-13 | ||
| US11/375,265 US7887538B2 (en) | 2005-10-15 | 2006-03-13 | Methods and apparatus for tissue modification |
| US11/405,859 US20070213734A1 (en) | 2006-03-13 | 2006-04-17 | Tissue modification barrier devices and methods |
| US11/405,859 | 2006-04-17 | ||
| US11/405,848 US8430881B2 (en) | 2004-10-15 | 2006-04-17 | Mechanical tissue modification devices and methods |
| US11/405,848 | 2006-04-17 | ||
| US11/406,486 US7938830B2 (en) | 2004-10-15 | 2006-04-17 | Powered tissue modification devices and methods |
| US11/406,486 | 2006-04-17 | ||
| US11/429,377 | 2006-05-04 | ||
| US11/429,377 US8048080B2 (en) | 2004-10-15 | 2006-05-04 | Flexible tissue rasp |
| PCT/US2007/063679 WO2007106740A2 (en) | 2006-03-13 | 2007-03-09 | Methods and apparatus for tissue modification |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009529981A JP2009529981A (ja) | 2009-08-27 |
| JP2009529981A5 JP2009529981A5 (enExample) | 2010-04-22 |
| JP5175264B2 true JP5175264B2 (ja) | 2013-04-03 |
Family
ID=38510184
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009500564A Expired - Fee Related JP5175264B2 (ja) | 2006-03-13 | 2007-03-09 | 組織を修正する装置及び方法 |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP2019635A2 (enExample) |
| JP (1) | JP5175264B2 (enExample) |
| AU (1) | AU2007226692B2 (enExample) |
| CA (1) | CA2646251A1 (enExample) |
| WO (1) | WO2007106740A2 (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100022824A1 (en) * | 2008-07-22 | 2010-01-28 | Cybulski James S | Tissue modification devices and methods of using the same |
| KR100946270B1 (ko) | 2008-08-12 | 2010-03-09 | 주식회사 메가젠임플란트 | 연조직 절단 치과용 공구 |
| US20100121139A1 (en) | 2008-11-12 | 2010-05-13 | Ouyang Xiaolong | Minimally Invasive Imaging Systems |
| DE202011103583U1 (de) | 2011-07-22 | 2011-10-28 | Martin Fähndrich | Instrumentarium zum Behandeln von Spinalkanalstenosen |
| EP3035870A1 (en) * | 2013-08-19 | 2016-06-29 | Smith & Nephew, Inc. | Bone removal under direct visualization |
| JP6524071B2 (ja) | 2013-09-30 | 2019-06-05 | コーディーナミックス, インコーポレイテッド | 心臓拡張を補助するための方法および装置 |
| US10772678B2 (en) | 2013-09-30 | 2020-09-15 | Michael D. Laufer | Methods and devices for diastolic assist |
| US10342579B2 (en) | 2014-01-13 | 2019-07-09 | Trice Medical, Inc. | Fully integrated, disposable tissue visualization device |
| US11547446B2 (en) | 2014-01-13 | 2023-01-10 | Trice Medical, Inc. | Fully integrated, disposable tissue visualization device |
| US9370295B2 (en) | 2014-01-13 | 2016-06-21 | Trice Medical, Inc. | Fully integrated, disposable tissue visualization device |
| US20170042408A1 (en) | 2015-08-11 | 2017-02-16 | Trice Medical, Inc. | Fully integrated, disposable tissue visualization device |
| US10335225B2 (en) | 2016-11-21 | 2019-07-02 | Arthrex, Inc. | Electrosurgical medical device handpiece with insulated aspiration system |
| WO2019191705A1 (en) | 2018-03-29 | 2019-10-03 | Trice Medical, Inc. | Fully integrated endoscope with biopsy capabilities and methods of use |
| WO2020213934A1 (ko) * | 2019-04-16 | 2020-10-22 | 랩앤피플주식회사 | 조직 제거장치 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE395001T1 (de) * | 1997-02-11 | 2008-05-15 | Warsaw Orthopedic Inc | Platte für die vordere halswirbelsäule mit fixierungssystem für eine schraube |
| US6440147B1 (en) * | 1998-09-03 | 2002-08-27 | Rubicor Medical, Inc. | Excisional biopsy devices and methods |
| ATE306213T1 (de) * | 1998-12-23 | 2005-10-15 | Nuvasive Inc | Vorrichtungen zur kannulation und zur nervenüberwachung |
| IL131197A (en) * | 1999-08-01 | 2009-12-24 | Assaf Dekel | Apparatus for spinal procedures |
| US20030191474A1 (en) * | 2000-02-16 | 2003-10-09 | Cragg Andrew H. | Apparatus for performing a discectomy through a trans-sacral axial bore within the vertebrae of the spine |
| CA2500973C (en) * | 2002-09-27 | 2012-05-15 | Surgifile, Inc. | Shielded reciprocating surgical file |
| WO2005007202A2 (en) * | 2003-07-08 | 2005-01-27 | Board Of Regents, The University Of Texas System | Surgical cutting tools and related methods |
| US8328810B2 (en) * | 2004-06-17 | 2012-12-11 | Boston Scientific Scimed, Inc. | Slidable sheaths for tissue removal devices |
| US7896879B2 (en) * | 2004-07-29 | 2011-03-01 | Vertos Medical, Inc. | Spinal ligament modification |
-
2007
- 2007-03-09 CA CA002646251A patent/CA2646251A1/en not_active Abandoned
- 2007-03-09 EP EP07758250A patent/EP2019635A2/en not_active Withdrawn
- 2007-03-09 WO PCT/US2007/063679 patent/WO2007106740A2/en not_active Ceased
- 2007-03-09 JP JP2009500564A patent/JP5175264B2/ja not_active Expired - Fee Related
- 2007-03-09 AU AU2007226692A patent/AU2007226692B2/en not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| CA2646251A1 (en) | 2007-09-20 |
| AU2007226692A1 (en) | 2007-09-20 |
| EP2019635A2 (en) | 2009-02-04 |
| JP2009529981A (ja) | 2009-08-27 |
| WO2007106740A3 (en) | 2008-11-06 |
| WO2007106740A2 (en) | 2007-09-20 |
| AU2007226692B2 (en) | 2013-01-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5175264B2 (ja) | 組織を修正する装置及び方法 | |
| US9345491B2 (en) | Flexible tissue rasp | |
| US7938830B2 (en) | Powered tissue modification devices and methods | |
| US7887538B2 (en) | Methods and apparatus for tissue modification | |
| US8430881B2 (en) | Mechanical tissue modification devices and methods | |
| US9456829B2 (en) | Powered tissue modification devices and methods | |
| US7578819B2 (en) | Spinal access and neural localization | |
| US8617163B2 (en) | Methods, systems and devices for carpal tunnel release | |
| US9463041B2 (en) | Devices and methods for tissue access | |
| JP5213138B2 (ja) | 組織除去器具及び方法 | |
| US20080103504A1 (en) | Percutaneous spinal stenosis treatment | |
| US20080051812A1 (en) | Multi-Wire Tissue Cutter | |
| US20080033465A1 (en) | Multi-Wire Tissue Cutter | |
| US20060089633A1 (en) | Devices and methods for tissue access | |
| AU2012201909B2 (en) | Methods and apparatus for tissue modification | |
| AU2007272427B2 (en) | Spinal access and neural localization |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20100227 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100308 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100308 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100610 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120308 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120313 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120612 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20121211 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130104 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5175264 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |