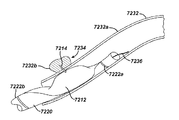
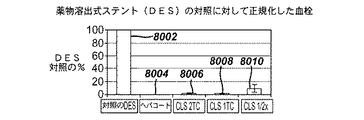
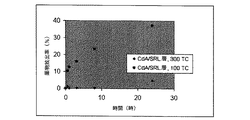
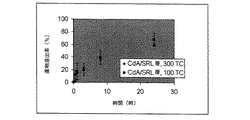
JP5138303B2 - 治療剤の溶出調整方法 - Google Patents
治療剤の溶出調整方法 Download PDFInfo
- Publication number
- JP5138303B2 JP5138303B2 JP2007193386A JP2007193386A JP5138303B2 JP 5138303 B2 JP5138303 B2 JP 5138303B2 JP 2007193386 A JP2007193386 A JP 2007193386A JP 2007193386 A JP2007193386 A JP 2007193386A JP 5138303 B2 JP5138303 B2 JP 5138303B2
- Authority
- JP
- Japan
- Prior art keywords
- rapamycin
- stent
- coating
- drug
- drugs
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
- A61L31/10—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/16—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A61F2/07—Stent-grafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/954—Instruments specially adapted for placement or removal of stents or stent-grafts for placing stents or stent-grafts in a bifurcation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A61F2002/065—Y-shaped blood vessels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A61F2/07—Stent-grafts
- A61F2002/075—Stent-grafts the stent being loosely attached to the graft material, e.g. by stitching
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/416—Anti-neoplastic or anti-proliferative or anti-restenosis or anti-angiogenic agents, e.g. paclitaxel, sirolimus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/606—Coatings
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Surgery (AREA)
- Vascular Medicine (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Materials For Medical Uses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Prostheses (AREA)
- Surgical Instruments (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Hydrogenated Pyridines (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/493,324 US8506984B2 (en) | 2006-07-26 | 2006-07-26 | Therapeutic agent elution control process |
| US11/493,324 | 2006-07-26 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008068067A JP2008068067A (ja) | 2008-03-27 |
| JP2008068067A5 JP2008068067A5 (enExample) | 2009-04-09 |
| JP5138303B2 true JP5138303B2 (ja) | 2013-02-06 |
Family
ID=38668800
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007193386A Expired - Fee Related JP5138303B2 (ja) | 2006-07-26 | 2007-07-25 | 治療剤の溶出調整方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US8506984B2 (enExample) |
| EP (1) | EP1891991B1 (enExample) |
| JP (1) | JP5138303B2 (enExample) |
| AT (1) | ATE495767T1 (enExample) |
| CA (1) | CA2594906C (enExample) |
| DE (1) | DE602007012024D1 (enExample) |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2594259A1 (en) * | 2004-08-04 | 2013-05-22 | Brookwood Pharmaceuticals, Inc. | Methods for manufacturing delivery devices and devices thereof |
| US11207457B2 (en) * | 2004-08-27 | 2021-12-28 | Edwards Lifesciences Corporation | Device and method for establishing an artificial arterio-venous fistula |
| US8066757B2 (en) * | 2007-10-17 | 2011-11-29 | Mindframe, Inc. | Blood flow restoration and thrombus management methods |
| US8124601B2 (en) * | 2007-11-21 | 2012-02-28 | Bristol-Myers Squibb Company | Compounds for the treatment of Hepatitis C |
| US8728528B2 (en) | 2007-12-20 | 2014-05-20 | Evonik Corporation | Process for preparing microparticles having a low residual solvent volume |
| US20100101295A1 (en) * | 2008-10-28 | 2010-04-29 | Warsaw Orthopedic, Inc. | Isulated sheath for bending polymer-based rod |
| AU2010239605B2 (en) * | 2009-04-20 | 2014-06-26 | Rox Medical, Inc. | Device and method for establishing an artificial arterio-venous fistula |
| US20100280600A1 (en) * | 2009-04-30 | 2010-11-04 | Vipul Bhupendra Dave | Dual drug stent |
| US20110098797A1 (en) * | 2009-10-23 | 2011-04-28 | Cleek Robert L | Drug eluting composite |
| US9320890B2 (en) * | 2009-11-09 | 2016-04-26 | W. L. Gore & Associates, Inc. | Drug eluting composite |
| US9504771B2 (en) | 2009-11-09 | 2016-11-29 | W. L. Gore & Associates, Inc. | Drug eluting composite |
| KR101140002B1 (ko) * | 2010-02-11 | 2012-05-02 | 이화여자대학교 산학협력단 | 약물 코팅 스텐트 제조방법 및 스텐트 |
| US20120180802A1 (en) * | 2010-06-17 | 2012-07-19 | The Board Of Regents Of The University Of Texas System | Shape memory devices and their use in controlling device-environment interactions |
| WO2012118978A1 (en) * | 2011-03-03 | 2012-09-07 | The Regents Of The University Of Colorado, A Body Corporate | Methods for treating oncovirus positive cancers |
| KR101370607B1 (ko) * | 2011-07-27 | 2014-03-07 | 재단법인 유타 인하 디디에스 및 신의료기술개발 공동연구소 | 전기방사를 이용한 비혈관용 약물 방출 스텐트 멤브레인 및 이의 제조방법 |
| WO2014182542A1 (en) | 2013-05-06 | 2014-11-13 | Abbott Cardiovascular Systems Inc. | A hollow stent filled with a therapeutic agent formulation |
| US10727122B2 (en) | 2014-12-08 | 2020-07-28 | International Business Machines Corporation | Self-aligned via interconnect structures |
| CA2998170C (en) | 2015-09-15 | 2023-10-03 | Savage Medical, Inc. | Devices and methods for anchoring a sheath in a tissue cavity |
| US11058804B2 (en) * | 2017-06-13 | 2021-07-13 | Ethicon Llc | Surgical fastener device for the prevention of ECM degradation |
| CN114568015A (zh) | 2019-08-22 | 2022-05-31 | 爱德华兹生命科学公司 | 穿刺针 |
| AU2020383327A1 (en) | 2019-11-14 | 2022-06-02 | Edwards Lifesciences Corporation | Transcatheter medical implant delivery |
Family Cites Families (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3379803A (en) * | 1964-05-04 | 1968-04-23 | Union Carbide Corp | Coating method and apparatus for deposition of polymer-forming vapor under vacuum |
| ZA737247B (en) | 1972-09-29 | 1975-04-30 | Ayerst Mckenna & Harrison | Rapamycin and process of preparation |
| US3959078A (en) | 1973-05-18 | 1976-05-25 | Midwest Research Institute | Enzyme immobilization with a thermochemical-photochemical bifunctional agent |
| US4127124A (en) * | 1977-05-12 | 1978-11-28 | W. R. Grace & Co. | Urethane bioimplant membrane |
| SU1088712A1 (ru) | 1979-11-14 | 1984-04-30 | Всесоюзный научно-исследовательский и испытательный институт медицинской техники | Аппарат дл циркул рного сшивани кровеносных сосудов |
| JPS5776256A (en) * | 1980-10-30 | 1982-05-13 | Toyota Motor Corp | Heating device for suction air |
| US4366819A (en) | 1980-11-17 | 1983-01-04 | Kaster Robert L | Anastomotic fitting |
| US4368736A (en) | 1980-11-17 | 1983-01-18 | Kaster Robert L | Anastomotic fitting |
| SE431609B (sv) | 1982-06-24 | 1984-02-20 | Unilink Ab | Kirurgiskt instrument for astadkommande av anastomos och festelement herfor |
| US4917091A (en) | 1982-06-24 | 1990-04-17 | Unilink Ab | Annular fastening means |
| US4722906A (en) | 1982-09-29 | 1988-02-02 | Bio-Metric Systems, Inc. | Binding reagents and methods |
| US4733665C2 (en) | 1985-11-07 | 2002-01-29 | Expandable Grafts Partnership | Expandable intraluminal graft and method and apparatus for implanting an expandable intraluminal graft |
| US5234447A (en) | 1990-08-28 | 1993-08-10 | Robert L. Kaster | Side-to-end vascular anastomotic staple apparatus |
| US5308641A (en) | 1993-01-19 | 1994-05-03 | Medtronic, Inc. | Biocompatibility of solid surfaces |
| US5229172A (en) | 1993-01-19 | 1993-07-20 | Medtronic, Inc. | Modification of polymeric surface by graft polymerization |
| US5350800A (en) | 1993-01-19 | 1994-09-27 | Medtronic, Inc. | Method for improving the biocompatibility of solid surfaces |
| US6120536A (en) * | 1995-04-19 | 2000-09-19 | Schneider (Usa) Inc. | Medical devices with long term non-thrombogenic coatings |
| US5837313A (en) | 1995-04-19 | 1998-11-17 | Schneider (Usa) Inc | Drug release stent coating process |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| US6245026B1 (en) | 1996-07-29 | 2001-06-12 | Farallon Medsystems, Inc. | Thermography catheter |
| US5924997A (en) | 1996-07-29 | 1999-07-20 | Campbell; Thomas Henderson | Catheter and method for the thermal mapping of hot spots in vascular lesions of the human body |
| WO1998033443A1 (en) | 1997-02-03 | 1998-08-06 | Angioguard, Inc. | Vascular filter |
| US6120636A (en) | 1998-01-26 | 2000-09-19 | Reflexite Corporation | Apparatus and method for producing retroreflective material having printed patterns thereon |
| US6425898B1 (en) | 1998-03-13 | 2002-07-30 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US6245898B1 (en) * | 1998-06-15 | 2001-06-12 | The Research Foundation Of State University Of New York | Monoclonal antibodies that recognize antigens associated with tumor metastasis |
| US6153252A (en) | 1998-06-30 | 2000-11-28 | Ethicon, Inc. | Process for coating stents |
| US7682647B2 (en) * | 1999-09-03 | 2010-03-23 | Advanced Cardiovascular Systems, Inc. | Thermal treatment of a drug eluting implantable medical device |
| US7807211B2 (en) * | 1999-09-03 | 2010-10-05 | Advanced Cardiovascular Systems, Inc. | Thermal treatment of an implantable medical device |
| US20020111590A1 (en) * | 2000-09-29 | 2002-08-15 | Davila Luis A. | Medical devices, drug coatings and methods for maintaining the drug coatings thereon |
| US20020136893A1 (en) * | 2000-11-27 | 2002-09-26 | Schlesinger Todd Evan | Musical instrument strings with polymer treated surface |
| WO2002098476A1 (en) | 2001-06-01 | 2002-12-12 | Ams Research Corporation | Bioresorbable medical devices |
| US20030050687A1 (en) | 2001-07-03 | 2003-03-13 | Schwade Nathan D. | Biocompatible stents and method of deployment |
| US7449307B2 (en) * | 2002-10-28 | 2008-11-11 | Transform Pharmaceuticals, Inc. | Raised surface assay plate |
| US6932930B2 (en) * | 2003-03-10 | 2005-08-23 | Synecor, Llc | Intraluminal prostheses having polymeric material with selectively modified crystallinity and methods of making same |
| US20050037052A1 (en) | 2003-08-13 | 2005-02-17 | Medtronic Vascular, Inc. | Stent coating with gradient porosity |
| CA2539751C (en) * | 2003-09-05 | 2016-04-26 | Norian Corporation | Bone cement compositions having fiber-reinforcement and/or increased flowability |
| US7303758B2 (en) * | 2004-01-20 | 2007-12-04 | Cordis Corporation | Local vascular delivery of mycophenolic acid in combination with rapamycin to prevent restenosis following vascular injury |
| US8591568B2 (en) * | 2004-03-02 | 2013-11-26 | Boston Scientific Scimed, Inc. | Medical devices including metallic films and methods for making same |
| US7846940B2 (en) | 2004-03-31 | 2010-12-07 | Cordis Corporation | Solution formulations of sirolimus and its analogs for CAD treatment |
| US7699832B2 (en) | 2004-05-27 | 2010-04-20 | Medtronic, Inc. | Medical device having a surface including a biologically active agent therein, and methods |
| US20060129225A1 (en) * | 2004-12-15 | 2006-06-15 | Kopia Gregory A | Device for the delivery of a cardioprotective agent to ischemic reperfused myocardium |
-
2006
- 2006-07-26 US US11/493,324 patent/US8506984B2/en not_active Expired - Fee Related
-
2007
- 2007-07-25 AT AT07252946T patent/ATE495767T1/de not_active IP Right Cessation
- 2007-07-25 DE DE602007012024T patent/DE602007012024D1/de active Active
- 2007-07-25 JP JP2007193386A patent/JP5138303B2/ja not_active Expired - Fee Related
- 2007-07-25 CA CA2594906A patent/CA2594906C/en active Active
- 2007-07-25 EP EP07252946A patent/EP1891991B1/en not_active Not-in-force
-
2013
- 2013-06-12 US US13/916,468 patent/US20140004254A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20140004254A1 (en) | 2014-01-02 |
| EP1891991B1 (en) | 2011-01-19 |
| US20080026034A1 (en) | 2008-01-31 |
| ATE495767T1 (de) | 2011-02-15 |
| US8506984B2 (en) | 2013-08-13 |
| EP1891991A2 (en) | 2008-02-27 |
| EP1891991A3 (en) | 2008-08-27 |
| DE602007012024D1 (de) | 2011-03-03 |
| CA2594906C (en) | 2014-10-07 |
| CA2594906A1 (en) | 2008-01-26 |
| JP2008068067A (ja) | 2008-03-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5138303B2 (ja) | 治療剤の溶出調整方法 | |
| JP5225556B2 (ja) | 治療剤との組み合わせにおける内腔内医療装置 | |
| JP4990583B2 (ja) | 薬物溶出用医療装置のための抗血栓性被膜 | |
| JP5172130B2 (ja) | 薄膜ニチノール基材型の薬物溶出式ステント | |
| JP5550823B2 (ja) | ペルオキシソーム増殖因子活性化受容体刺激剤と組み合わせたmTOR抑制剤の局所脈管送達 | |
| JP5398996B2 (ja) | 再狭窄、不安定プラーク、腹部大動脈瘤(aaa)、および発作を治療するための、プロブコールの単独またはシロリムスとの組み合わせでの局所脈管送達 | |
| JP5362172B2 (ja) | 虚血性の再灌流されている心筋層への心臓保護薬の配給用の装置 | |
| JP4987241B2 (ja) | Cad治療のためのシロリムスおよびその類似体の溶液配合物 | |
| JP4832787B2 (ja) | 薬物溶出式の医療装置における酸化を防ぎ薬物の劣化を減少するための酸化防止剤の使用 | |
| JP5774268B2 (ja) | 医療装置における薬物デポー剤担体または被膜としての多層ステレオコンプレックス型ポリマー | |
| JP5160066B2 (ja) | 脈管の病気の治療のためのラパマイシンおよびシロスタゾールの組み合わせ物の局所的投与 | |
| JP5052757B2 (ja) | 脈管の傷害後の再狭窄を防ぐためのラパマイシンとの組み合わせにおけるパンゼム(登録商標)の局所的脈管配給 | |
| JP2008068067A5 (enExample) | ||
| JP2006341101A (ja) | 被覆型動脈瘤修復装置 | |
| JP2005305154A (ja) | 脆弱性プラークの治療のためのラパマイシンおよび17ベータ−エストラジオールの組み合わせ物の局所的投与 | |
| JP2007105466A (ja) | 動脈瘤の病気を治療するための内腔内装置および治療剤の組み合わせ物 | |
| JP2006006938A (ja) | 調整された薬物放出のためのヘパリン・バリア被膜 | |
| JP2007037998A (ja) | 動脈瘤の病気を治療するためのシステム | |
| JP2005288170A (ja) | 薬物配給装置 | |
| JP5042460B2 (ja) | 脈管の傷害後の再狭窄を防ぐためのラパマイシンとの組み合わせにおけるエトポシドの局所的脈管配給 | |
| JP5047593B2 (ja) | 脈管の傷害後の再狭窄を防ぐための、pi3キナーゼ抑制因子の、単独の、あるいは、シロリムスとの組み合わせにおける、局所脈管送達 | |
| JP5042458B2 (ja) | 脈管の傷害後の再狭窄を防ぐためのラパマイシンとの組み合わせにおけるトポテカンの局所的脈管配給 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20080109 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20081110 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090225 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100428 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120127 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120207 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120507 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20121030 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20121114 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5138303 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151122 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R371 | Transfer withdrawn |
Free format text: JAPANESE INTERMEDIATE CODE: R371 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |