JP5118475B2 - Softener composition - Google Patents
Softener composition Download PDFInfo
- Publication number
- JP5118475B2 JP5118475B2 JP2007336578A JP2007336578A JP5118475B2 JP 5118475 B2 JP5118475 B2 JP 5118475B2 JP 2007336578 A JP2007336578 A JP 2007336578A JP 2007336578 A JP2007336578 A JP 2007336578A JP 5118475 B2 JP5118475 B2 JP 5118475B2
- Authority
- JP
- Japan
- Prior art keywords
- component
- carbon atoms
- mass
- group
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 0 *C(*)=C(*)*N(*)* Chemical compound *C(*)=C(*)*N(*)* 0.000 description 1
Description
本発明は柔軟剤組成物に関する。 The present invention relates to a softener composition.
衣料等の繊維製品を対象とした柔軟剤には一般に香料が用いられている。従来、洗濯処理を行った衣料の香りは、処理直後の香りが強い、心地よいなどが求められていた。しかし、近年、香りに対する関心が高まっており、洗濯された衣料の保存後、着用開始時、さらには着用中においても香りを楽しむ生活習慣が生まれてきた。そのような変化に対応して、柔軟処理後の衣料に香りを残すいわゆる残香性を付与する技術が開発されている(例えば特許文献1、2)。 A fragrance is generally used as a softening agent for textile products such as clothing. Conventionally, the scent of clothes that have been subjected to a washing process has been demanded to have a strong scent immediately after the process and a pleasant scent. However, in recent years, interest in fragrance has increased, and after storing washed clothes, a lifestyle habit of enjoying the fragrance has been born at the start of wearing and even during wearing. Corresponding to such changes, techniques for imparting a so-called residual fragrance that leaves a scent on the garment after flexible processing have been developed (for example, Patent Documents 1 and 2).
長時間に渡り心地よい香りが持続する高残香性能のためには、洗濯浴中で香料を衣料上に多く残すことが効果的である。しかし、通常の洗濯工程において、柔軟処理を行う場合、香料は洗浄水とともに排出されてしまい、香りが残りにくいという問題点がある。そのため、高い残香性能を発現させるためには、香料を多量に配合することが必要となり、経済性、製品安定性が悪くなる。また、これまでの技術では、高い残香性を発現するため、および、審美的外観の観点から柔軟剤自身の色合いを損なわないために、香料種が限定された中から調合しないといけないといった問題点もあり(特許文献1、2)、香りのバリエーションが限定されてきた。
したがって、本発明が解決しようとする課題は、柔軟性能に優れ、洗濯後の繊維製品に心地よい、さまざまな香りを付与でき、長期間保管後においても長く心地よい香りが残る柔軟剤組成物を提供することである。 Therefore, the problem to be solved by the present invention is to provide a softener composition that is excellent in softness performance, can impart various scents that are comfortable to textile products after washing, and remains scented for a long time even after long-term storage. That is.
本発明は、下記(a)成分、(b)成分及び(c)成分を含有する柔軟剤組成物に関する。
<(a)成分>
下記一般式(1)で示される化合物、又はその酸塩もしくは4級塩に由来するモノマー単位(A)及び、下記一般式(2)で示される化合物に由来するモノマー単位(B)を含有し、モノマー単位(A)とモノマー単位(B)の合計中、モノマー単位(A)の割合が(A)/〔(A)+(B)〕=20〜100モル%である、高分子化合物。
The present invention relates to a softener composition comprising the following component (a), component (b) and component (c).
<(A) component>
It contains a monomer unit (A) derived from a compound represented by the following general formula (1), or an acid salt or quaternary salt thereof, and a monomer unit (B) derived from a compound represented by the following general formula (2). The high molecular compound whose ratio of a monomer unit (A) is (A) / [(A) + (B)] = 20-100 mol% in the sum total of a monomer unit (A) and a monomer unit (B).
〔式中、R1、R2は、それぞれ独立に水素原子、又はメチル基を示し、R3は−COOM(Mは水素原子、又はアルカリ金属原子)、又は水素原子を示す。Xは−COO−R6−、−CONR7−R8−、又は−CH2−を示す。R4はXが−CH2−の場合には一般式(3) [Wherein, R 1, R 2 each independently represent a hydrogen atom or a methyl group, R 3 is -COOM (M represents a hydrogen atom, or an alkali metal atom) is shown, or hydrogen atom. X represents —COO—R 6 —, —CONR 7 —R 8 —, or —CH 2 —. R 4 has the general formula (3) when X is —CH 2 —.
で表される基を示し、Xがそれ以外の場合は炭素数1〜3のアルキル基、又は炭素数1〜3のヒドロキシアルキル基を示す。R5は炭素数1〜3のアルキル基、炭素数1〜3のヒドロキシアルキル基、又は水素原子を示す。R6、R8は、それぞれ独立に炭素数2〜3のアルキレン基、R7は水素原子、又は炭素数1〜3のアルキル基を示す。〕 When X is other than that, it represents an alkyl group having 1 to 3 carbon atoms or a hydroxyalkyl group having 1 to 3 carbon atoms. R 5 represents an alkyl group having 1 to 3 carbon atoms, a hydroxyalkyl group having 1 to 3 carbon atoms, or a hydrogen atom. R 6 and R 8 each independently represent an alkylene group having 2 to 3 carbon atoms, and R 7 represents a hydrogen atom or an alkyl group having 1 to 3 carbon atoms. ]
(式中、R11、R12は、それぞれ独立に水素原子、又は炭素数1〜3のアルキル基を示し、Yはアリール基、−O−CO−R13、−COO−R14、又は−CONR15−R16を示す。R13、R14、R16は、それぞれ独立に炭素数1〜5の直鎖状、分岐鎖状、もしくは環状のアルキル基もしくはアルケニル基を示し、R15は水素原子、又は炭素数1〜3のアルキル基を示す。)
<(b)成分>
アミド基で分断されている総炭素数12〜29の炭化水素基を1〜3個有する3級アミン、該アミンの4級化物、及び前記アミンの酸塩から選ばれる1種以上の化合物。
<(c)成分>
ClogPが4を超える香料化合物。
(In the formula, R 11 and R 12 each independently represent a hydrogen atom or an alkyl group having 1 to 3 carbon atoms, and Y represents an aryl group, —O—CO—R 13 , —COO—R 14 , or — CONR 15 -R 16 R 13 , R 14 , and R 16 each independently represent a linear, branched, or cyclic alkyl or alkenyl group having 1 to 5 carbon atoms, and R 15 represents hydrogen. An atom or a C1-C3 alkyl group is shown.)
<(B) component>
One or more compounds selected from tertiary amines having 1 to 3 hydrocarbon groups having 12 to 29 carbon atoms separated by amide groups, quaternized products of the amines, and acid salts of the amines.
<(C) component>
A perfume compound having a ClogP of more than 4.
本発明によれば、ClogPが4を超える香料を効率よく繊維製品に付与でき、繊維処理直後、および長期保管後においても長く香りが残る効果がある柔軟剤組成物が提供される。 ADVANTAGE OF THE INVENTION According to this invention, the softener composition which can provide the fragrance | flavor in which ClogP exceeds 4 to a fiber product efficiently, and has an effect with which a fragrance remains for a long time just after fiber processing and after long-term storage is provided.
<(a)成分>
モノマー単位(A)の由来となる、一般式(1)で表される化合物のうち、Xは−COO−R6−、−CONR7−R8−(R6、R7、R8は前記の意味を表す)が好ましく、R4、R5はメチル基、又はエチル基が好ましい。また、好ましい具体的な化合物としてXが−COO−R6−である化合物としては、アクリル酸N,N−ジメチルアミノエチル、アクリル酸N,N−ジメチルアミノメチル、アクリル酸N,N−ジメチルアミノブチル、アクリル酸N,N−ジメチルアミノプロピル、メタクリル酸N,N−ジメチルアミノエチル、メタクリル酸N,N−ジメチルアミノメチル、メタクリル酸N,N−ジメチルアミノブチル、メタクリル酸N,N−ジメチルアミノプロピル、アクリル酸N,N−ジエチルアミノエチル、アクリル酸N,N−ジエチルアミノメチル、アクリル酸N,N−ジエチルアミノブチル、アクリル酸N,N−ジエチルアミノプロピル、メタクリル酸N,N−ジエチルアミノエチル、メタクリル酸N,N−ジエチルアミノメチル、メタクリル酸N,N−ジエチルアミノブチル、メタクリル酸N,N−ジエチルアミノプロピル等が挙げられる。さらに好ましい化合物としては、アクリル酸N,N−ジメチルアミノエチル、アクリル酸N,N−ジメチルアミノメチル、アクリル酸N,N−ジメチルアミノプロピル、メタクリル酸N,N−ジメチルアミノエチル、メタクリル酸N,N−ジメチルアミノメチル、メタクリル酸N,N−ジメチルアミノプロピル、アクリル酸N,N−ジエチルアミノエチル、アクリル酸N,N−ジエチルアミノメチル、アクリル酸N,N−ジエチルアミノプロピル、メタクリル酸N,N−ジエチルアミノエチル、メタクリル酸N,N−ジエチルアミノメチル、メタクリル酸N,N−ジエチルアミノプロピルが挙げられる。
<(A) component>
Among the compounds represented by the general formula (1) that are derived from the monomer unit (A), X represents —COO—R 6 —, —CONR 7 —R 8 — (R 6 , R 7 , R 8 are the same as those described above. R 4 and R 5 are preferably a methyl group or an ethyl group. As preferred specific compounds, compounds in which X is —COO—R 6 — include N, N-dimethylaminoethyl acrylate, N, N-dimethylaminomethyl acrylate, and N, N-dimethylamino acrylate. Butyl, N, N-dimethylaminopropyl acrylate, N, N-dimethylaminoethyl methacrylate, N, N-dimethylaminomethyl methacrylate, N, N-dimethylaminobutyl methacrylate, N, N-dimethylamino methacrylate Propyl, N, N-diethylaminoethyl acrylate, N, N-diethylaminomethyl acrylate, N, N-diethylaminobutyl acrylate, N, N-diethylaminopropyl acrylate, N, N-diethylaminoethyl methacrylate, N methacrylate , N-diethylaminomethyl, methacrylic acid , N- diethylamino-butyl methacrylate N, N- diethylamino propyl and the like. More preferred compounds include N, N-dimethylaminoethyl acrylate, N, N-dimethylaminomethyl acrylate, N, N-dimethylaminopropyl acrylate, N, N-dimethylaminoethyl methacrylate, N, methacrylate, N-dimethylaminomethyl, N, N-dimethylaminopropyl methacrylate, N, N-diethylaminoethyl acrylate, N, N-diethylaminomethyl acrylate, N, N-diethylaminopropyl acrylate, N, N-diethylamino methacrylate Examples thereof include ethyl, N, N-diethylaminomethyl methacrylate, and N, N-diethylaminopropyl methacrylate.
特に好ましくは、アクリル酸N,N−ジメチルアミノエチル、アクリル酸N,N−ジメチルアミノメチル、メタクリル酸N,N−ジメチルアミノエチル、メタクリル酸N,N−ジメチルアミノメチル、アクリル酸N,N−ジエチルアミノエチル、アクリル酸N,N−ジエチルアミノメチル、メタクリル酸N,N−ジエチルアミノエチル、メタクリル酸N,N−ジエチルアミノメチルが挙げられる。 Particularly preferred are N, N-dimethylaminoethyl acrylate, N, N-dimethylaminomethyl acrylate, N, N-dimethylaminoethyl methacrylate, N, N-dimethylaminomethyl methacrylate, N, N-acrylate. Examples include diethylaminoethyl, N, N-diethylaminomethyl acrylate, N, N-diethylaminoethyl methacrylate, and N, N-diethylaminomethyl methacrylate.
また、一般式(1)で表される化合物のうち、一般式(1)中のXが−CONR7−R8−である化合物としては、N,N−ジメチルアミノプロピルアクリル酸(またはメタクリル酸)アミド、N,N−ジメチルアミノメチルアクリル酸(またはメタクリル酸)アミド、N,N−ジメチルアミノエチルアクリル酸(またはメタクリル酸)アミド、N,N−ジメチルアミノブチルアクリル酸(またはメタクリル酸)アミド等が挙げられる。好ましくはN,N−ジメチルアミノプロピルアクリル酸(またはメタクリル酸)アミド、N,N−ジメチルアミノメチルアクリル酸(またはメタクリル酸)アミド、N,N−ジメチルアミノエチルアクリル酸(またはメタクリル酸)アミドである。 In addition, among the compounds represented by the general formula (1), as the compound in which X in the general formula (1) is —CONR 7 —R 8 —, N, N-dimethylaminopropylacrylic acid (or methacrylic acid) ) Amide, N, N-dimethylaminomethylacrylic acid (or methacrylic acid) amide, N, N-dimethylaminoethylacrylic acid (or methacrylic acid) amide, N, N-dimethylaminobutylacrylic acid (or methacrylic acid) amide Etc. Preferably, N, N-dimethylaminopropylacrylic acid (or methacrylic acid) amide, N, N-dimethylaminomethylacrylic acid (or methacrylic acid) amide, N, N-dimethylaminoethylacrylic acid (or methacrylic acid) amide is there.
また、一般式(1)中のXが−CH2−の場合、R4は前記一般式(3)で表される基である。かかる化合物としては、ジアリルアミン、ジアリルメチルアミン等が挙げられる。 Further, X in the formula (1) is -CH 2 - For, R 4 is a group represented by the general formula (3). Examples of such a compound include diallylamine and diallylmethylamine.
一般式(1)で示される化合物は、その酸塩又は4級塩を用いることができる。酸塩としては、例えば、1級、2級、3級アミンの塩酸塩、硫酸塩などの無機塩の中和塩や各種有機酸の中和塩が挙げられ、4級塩としては炭素数1〜3のハロゲン化アルキル、炭素数1〜3のアルキル硫酸等によって4級化された化合物が挙げられる。4級塩としてはN,N,N−トリメチル−N−(2−メタクリロイルオキシエチル)アンモニウムクロライド、N,N−ジメチル−N−エチル−N−(2−メタクリロイルオキシエチル)アンモニウムエチルサルフェート、ジアリルジメチルアンモニウムクロリドが挙げられる。これらの化合物は、例えばMRCユニテック(株)からQDMやMOEDESという商品名で販売されている。 As the compound represented by the general formula (1), an acid salt or a quaternary salt thereof can be used. Examples of the acid salt include neutral salts of inorganic salts such as primary, secondary, tertiary amine hydrochlorides and sulfates, and neutral salts of various organic acids. And a compound quaternized with an alkyl halide of ˜3, an alkyl sulfuric acid having 1 to 3 carbon atoms, and the like. Quaternary salts include N, N, N-trimethyl-N- (2-methacryloyloxyethyl) ammonium chloride, N, N-dimethyl-N-ethyl-N- (2-methacryloyloxyethyl) ammonium ethyl sulfate, diallyldimethyl Ammonium chloride is mentioned. These compounds are sold, for example, under the trade names QDM and MOEDES by MRC Unitech Co., Ltd.
(a)成分が、モノマー単位(A)のみからなる高分子化合物である場合には、モノマー単位(A)は、一般式(1)で示される化合物、又はその酸塩から構成されることが好ましい。 When the component (a) is a polymer compound composed only of the monomer unit (A), the monomer unit (A) may be composed of the compound represented by the general formula (1) or an acid salt thereof. preferable.
また、モノマー単位(B)の由来となる、一般式(2)で表される化合物としては、ブチルアクリレート等のアクリル酸アルキル(炭素数1〜5)エステル、ブチルメタクリレート、メチルメタクリレート、ブチルメタクリレート等のメタクリル酸アルキル(炭素数1〜5)エステル等が挙げられる。 Moreover, as a compound represented by General formula (2) from which a monomer unit (B) originates, alkyl acrylate (C1-C5) ester, such as butyl acrylate, butyl methacrylate, methyl methacrylate, butyl methacrylate, etc. And alkyl methacrylate (having 1 to 5 carbon atoms).
(a)成分は、モノマー単位(A)、(B)以外のモノマー単位として、共重合可能な不飽和結合含有モノマー〔モノマー(C)〕に由来するモノマー単位〔モノマー単位(C)〕を本発明の効果を損なわない範囲で有しても良い。かかるモノマー(C)としては、例えば、アクリルアミド、ビニルアルコール;ヒドロキシエチル(メタ)アクリレート、ヒドロキシプロピル(メタ)アクリルアミド等の炭素数1〜22のヒドロキシアルキル基を有する(メタ)アクリル酸エステル又は(メタ)アクリルアミド;ポリエチレングリコール(メタ)アクリレート、メトキシポリエチレングリコール(メタ)アクリレート、ラウロキシポリエチレングリコール(メタ)アクリレート(エチレングリコールの重合度が1〜100)、ポリプロピレングリコール(メタ)アクリレート(プロピレングリコールの重合度が1〜50)、ポリブチレングリコール(メタ)アクリレート(ブチレングリコールの重合度が1〜50)等のポリアルキレン(アルキレン基の炭素数1〜8;直鎖もしくは分岐鎖)オキシド鎖を有する(メタ)アクリル酸エステル;グリセリン(メタ)アクリレート等の多価アルコールの(メタ)アクリル酸エステル;ジアセトン(メタ)アクリルアミド;N−ビニルピロリドン等のN−ビニル環状アミド;N−(メタ)アクロイルモルホリン;塩化ビニル;アクリロニトリル;(メタ)アクリル酸、マレイン酸、イタコン酸、スチレンカルボン酸等のカルボキシル基を有するビニル化合物;2−アクリルアミド−2−メチルプロパンスルホン酸、スチレンスルホン酸等のスルホン酸基を有するビニル化合物等が例示される。これらのモノマー(C)の共重合量は、モノマー全量に対して80質量%以下、好ましくは50質量%以下、さらに好ましくは30質量%以下である。 Component (a) is a monomer unit derived from a copolymerizable unsaturated bond-containing monomer [monomer (C)] [monomer unit (C)] as a monomer unit other than the monomer units (A) and (B). You may have in the range which does not impair the effect of invention. Examples of the monomer (C) include acrylamide, vinyl alcohol; (meth) acrylic acid ester having a hydroxyalkyl group having 1 to 22 carbon atoms such as hydroxyethyl (meth) acrylate, hydroxypropyl (meth) acrylamide, or (meta ) Acrylamide; polyethylene glycol (meth) acrylate, methoxypolyethylene glycol (meth) acrylate, lauroxy polyethylene glycol (meth) acrylate (ethylene glycol polymerization degree 1 to 100), polypropylene glycol (meth) acrylate (propylene glycol polymerization degree) 1-50), polyalkylenes such as polybutylene glycol (meth) acrylate (the degree of polymerization of butylene glycol is 1-50) (alkylene having 1 to 8 carbon atoms; linear (Branch) (meth) acrylic acid ester having an oxide chain; (meth) acrylic acid ester of polyhydric alcohol such as glycerin (meth) acrylate; diacetone (meth) acrylamide; N-vinyl cyclic such as N-vinylpyrrolidone Amide; N- (meth) acryloylmorpholine; Vinyl chloride; Acrylonitrile; Vinyl compounds having a carboxyl group such as (meth) acrylic acid, maleic acid, itaconic acid, styrene carboxylic acid; 2-acrylamido-2-methylpropanesulfonic acid And vinyl compounds having a sulfonic acid group such as styrene sulfonic acid. The copolymerization amount of these monomers (C) is 80% by mass or less, preferably 50% by mass or less, more preferably 30% by mass or less, based on the total amount of monomers.
(a)成分は、モノマー単位(A)及びモノマー単位(B)を、モノマー単位(A)とモノマー単位(B)の合計中、モノマー単位(A)の割合が(A)/〔(A)+(B)〕=20〜100モル%の割合で含有し、柔軟効果の点から好ましくは、50〜95モル%、より好ましくは70〜95モル%で含有する。また、特にlogPが5以上の香料を効率よく繊維製品に付与する場合には、モノマー単位(A)のみからなる高分子化合物が好ましい。 The component (a) includes the monomer unit (A) and the monomer unit (B), and the ratio of the monomer unit (A) in the total of the monomer unit (A) and the monomer unit (B) is (A) / [(A) + (B)] = 20 to 100 mol%, preferably from 50 to 95 mol%, more preferably from 70 to 95 mol% from the viewpoint of the softening effect. In particular, when a perfume having a log P of 5 or more is efficiently applied to a fiber product, a polymer compound consisting only of the monomer unit (A) is preferred.
また、(a)成分の重量平均分子量(Mw)は、2,000〜200,000、更に3,000〜150,000、特に5,000〜100,000、とりわけ5,000〜75,000、最も5,000〜60,000が好ましい。 The weight average molecular weight (Mw) of the component (a) is 2,000 to 200,000, further 3,000 to 150,000, particularly 5,000 to 100,000, especially 5,000 to 75,000, Most preferred is 5,000-60,000.
尚、本発明の(a)成分のMwは、ゲル・パーミエーション・クロマトグラフィー(GPC)測定による値を使用する。溶離液としては、水、アルコール、クロロホルム、ジメチルホルムアミド、テトラヒドロフラン、アセトニトリル及びこれらの溶媒を組み合わせた液の何れかを使用し、ポリエチレンオキシド又はポリスチレン換算の分子量とする。 In addition, Mw of (a) component of this invention uses the value by a gel permeation chromatography (GPC) measurement. As an eluent, any one of water, alcohol, chloroform, dimethylformamide, tetrahydrofuran, acetonitrile and a combination of these solvents is used, and the molecular weight is converted to polyethylene oxide or polystyrene.
その際、測定対象のポリマーが、モノマー単位(A)の割合が大きく比較的親水性であると考えられる場合は、(1%酢酸/エタノール):水=3:7(質量比)の混合溶媒で調製したLiBrの50mmol/L溶液を溶媒として、極性溶媒用GPCカラム「α−M(東ソー(株)製)」を2本直列して用い、ポリエチレングリコール換算の分子量により算出する(測定法A)。一方、モノマー単位(B)の割合が大きく、ポリマーが比較的疎水性であると考えられる場合は、ファーミンDM20(花王(株)製)の1mmol/L−CHCl3溶液にて、有機溶媒用GPCカラム「K−804(昭和電工(株)製)」を2本直列して用い、ポリスチレン換算の分子量により算出する(測定法B)。 At that time, when the polymer to be measured is considered to be relatively hydrophilic with a large proportion of monomer units (A), a mixed solvent of (1% acetic acid / ethanol): water = 3: 7 (mass ratio) Using a LiBr 50 mmol / L solution prepared in step 1 as a solvent, two GPC columns “α-M (manufactured by Tosoh Corp.)” for polar solvents are used in series, and the molecular weight in terms of polyethylene glycol is calculated (measurement method A). ). On the other hand, when the proportion of the monomer unit (B) is large and the polymer is considered to be relatively hydrophobic, GPC for organic solvents is used in a 1 mmol / L-CHCl 3 solution of Farmin DM20 (manufactured by Kao Corporation). Two columns “K-804 (manufactured by Showa Denko KK)” are used in series, and the molecular weight in terms of polystyrene is calculated (measurement method B).
<(b)成分>
本発明の(b)成分は、アミド基で分断されている総炭素数12〜29の炭化水素基を1〜3個有する3級アミン、該アミンの4級化物、及び前記アミンの酸塩から選ばれる1種以上の化合物である。
<(B) component>
The component (b) of the present invention includes a tertiary amine having 1 to 3 hydrocarbon groups having 12 to 29 carbon atoms separated by an amide group, a quaternized product of the amine, and an acid salt of the amine. One or more selected compounds.
例えば、(b)成分としては、
(1)窒素原子に結合する基のうち、1〜3個がアミド基で分断されている総炭素数12〜29の炭化水素基である3級アミン、
(2)窒素原子に結合する基のうち、1〜3個がアミド基で分断されている総炭素数12〜29の炭化水素基である3級アミンの酸塩、
(3)窒素原子に結合する基のうち、1〜3個がアミド基で分断されている総炭素数12〜29の炭化水素基である4級アンモニウム塩、
が挙げられる。
For example, as component (b),
(1) A tertiary amine which is a hydrocarbon group having a total carbon number of 12 to 29, wherein 1 to 3 of groups bonded to a nitrogen atom are separated by an amide group,
(2) A tertiary amine acid salt which is a hydrocarbon group having 12 to 29 carbon atoms in total, wherein 1 to 3 of the groups bonded to the nitrogen atom are separated by an amide group,
(3) A quaternary ammonium salt which is a hydrocarbon group having 12 to 29 carbon atoms in which 1 to 3 of the groups bonded to the nitrogen atom are separated by an amide group,
Is mentioned.
好ましい化合物としては分子内にアミド基で分断されている総炭素数12〜29の炭化水素基を1〜2個有する3級アミン、該アミンの4級化物、及び前記アミンの酸塩から選ばれる1種以上の化合物である。この化合物は、更にエステル基で分断されている総炭素数12〜29の炭化水素基を1〜2個(ただし、アミド基で分断されている総炭素数12〜29の炭化水素基との合計は3個)有することが好ましい。 Preferred compounds are selected from tertiary amines having 1 to 2 hydrocarbon groups having 12 to 29 total carbon atoms separated by amide groups in the molecule, quaternized products of the amines, and acid salts of the amines. One or more compounds. This compound further has 1 to 2 hydrocarbon groups having 12 to 29 carbon atoms separated by ester groups (provided that the total number of hydrocarbon groups having 12 to 29 carbon atoms separated by amide groups). Is preferably 3).
(b)成分の具体的に好ましい化合物としては下記一般式(b1)の化合物及び下記一般式(b2)の化合物から選ばれる1種以上が好適である。 As the specifically preferred compound of component (b), one or more selected from compounds of the following general formula (b1) and compounds of the following general formula (b2) are preferred.
〔R1a、R2aは、それぞれ炭素数11〜23、好ましくは13〜23、より好ましくは15〜19のアルキル基又はアルケニル基であり、R1b、R2bは、それぞれ炭素数1〜5、好ましくは2又は3のアルキレン基であり、R1c、R1dは、それぞれ炭素数1〜3のアルキル基、炭素数1〜3のヒドロキシアルキル基、R1a−A−R1b−、又はR1a−D−R1b−であり、R2c、R2dは、それぞれ炭素数1〜3のアルキル基、炭素数1〜3のヒドロキシアルキル基、R2a−B−R2b−、又はR2a−E−R2b−である。R2eは水素原子、炭素数1〜3のアルキル基、又は炭素数1〜3のヒドロキシアルキル基である。A、Bは、それぞれ−CONH−、−NHCO−から選ばれる基であり、好ましくは−CONH−であり、D、Eは、それぞれ−COO−、−OCO−、−O−から選ばれる基であり、好ましくは−COO−である。Z-は有機、又は無機の陰イオンであり、好ましくは塩素イオン、炭素数1〜3のアルキル硫酸エステルイオン、炭素数1〜18の脂肪酸イオン、炭素数1〜3のアルキル基が1〜3個置換していてもよいベンゼンスルホン酸イオンである。〕 [R 1a and R 2a are each an alkyl group or alkenyl group having 11 to 23 carbon atoms, preferably 13 to 23, more preferably 15 to 19 carbon atoms, and R 1b and R 2b are respectively 1 to 5 carbon atoms, Preferably it is a 2 or 3 alkylene group, R <1c> , R <1d> is respectively a C1-C3 alkyl group, a C1-C3 hydroxyalkyl group, R <1a > -A-R <1b> -, or R <1a>. -D-R 1b - a and, R 2c, R 2d are each an alkyl group having 1 to 3 carbon atoms, hydroxyalkyl group having 1 to 3 carbon atoms, R 2a -B-R 2b - , or R 2a -E -R 2b - it is. R 2e is a hydrogen atom, an alkyl group having 1 to 3 carbon atoms, or a hydroxyalkyl group having 1 to 3 carbon atoms. A and B are groups selected from —CONH— and —NHCO—, respectively, preferably —CONH—, and D and E are groups selected from —COO—, —OCO—, and —O—, respectively. Yes, preferably -COO-. Z − is an organic or inorganic anion, preferably a chlorine ion, an alkyl sulfate ion having 1 to 3 carbon atoms, a fatty acid ion having 1 to 18 carbon atoms, or an alkyl group having 1 to 3 carbon atoms. This is a benzenesulfonate ion which may be substituted. ]
<(c)成分>
本発明の柔軟剤組成物は、ClogPが4を超える香料化合物を含有する。ここで、logPとは、有機化合物の水と1−オクタノールに対する親和性を示す係数である。1−オクタノール/水分配係数Pは、1−オクタノールと水の2液相の溶媒に微量の化合物が溶質として溶け込んだときの分配平衡で、それぞれの溶媒中における化合物の平衡濃度の比であり、底10に対するそれらの対数logPの形で示すのが一般的である。
<(C) component>
The softener composition of this invention contains the fragrance | flavor compound in which ClogP exceeds four. Here, log P is a coefficient indicating the affinity of an organic compound for water and 1-octanol. 1-octanol / water partition coefficient P is a distribution equilibrium when a trace amount of compound is dissolved as a solute in a two-liquid solvent of 1-octanol and water, and is a ratio of the equilibrium concentration of the compound in each solvent. It is common to show them in the form of their logarithm logP relative to the base 10.
多くの化合物のlogP値が報告され、Daylight Chemical Information Systems, Inc. (Daylight CIS)などから入手しうるデータベースには多くの値が掲載されているので参照できる。実測のLogP値がない場合には、Daylight CISから入手できるプログラム“CLOGP"で計算すると最も便利である。このプログラムは、実測のlogP値がある場合にはそれと伴に、Hansch, Leoのフラグメントアプローチにより算出される“計算logP(ClogP)”の値を出力する。 Log P values of many compounds are reported, and many values are listed in a database available from Daylight Chemical Information Systems, Inc. (Daylight CIS), and can be referred to. When there is no measured LogP value, it is most convenient to calculate with the program “CLOGP” available from Daylight CIS. This program outputs the value of “calculated logP (ClogP)” calculated by the fragment approach of Hansch and Leo along with the measured logP value, if any.
フラグメントアプローチは化合物の化学構造に基づいており、原子の数及び化学結合のタイプを考慮している(cf. A. Leo, Comprehensive Medicinal Chemistry, Vol.4, C. Hansch, P.G. Sammens, J.B. Taylor and C.A. Ramsden, Eds., p.295, Pergamon Press, 1990)。このClogP値は現在最も汎用的で信頼できる推定値であるので、化合物の選択に際して実測のlogP値の代わりに用いることができる。本発明では、logPの実測値があればそれを、無い場合はプログラムCLOGP v4.01により計算したClogP値を用いる。 The fragment approach is based on the chemical structure of the compound and takes into account the number of atoms and the type of chemical bond (cf. A. Leo, Comprehensive Medicinal Chemistry, Vol. 4, C. Hansch, PG Sammens, JB Taylor and CA Ramsden, Eds., P.295, Pergamon Press, 1990). Since this ClogP value is currently the most general and reliable estimate, it can be used in place of the actual logP value when selecting a compound. In the present invention, if there is a measured value of logP, the ClogP value calculated by the program CLOGP v4.01 is used.
ClogPが4を超える香料化合物としては、例えば、下記の香料化合物が挙げられる。
p-t-B.C.H.A、BENZYL SALICYLATE、DAMASCENONE、LIMONENE、TERPINOLENE、ALDEHYDE C-11、HEXYL CINNAMIC ALDEHYDE、PATCHOULI ALCOHOL、AMBROXAN、PEARLIDE、PENTALIDE
As a fragrance | flavor compound in which ClogP exceeds 4, the following fragrance | flavor compound is mentioned, for example.
ptB.CHA, BENZYL SALICYLATE, DAMASCENONE, LIMONENE, TERPINOLENE, ALDEHYDE C-11, HEXYL CINNAMIC ALDEHYDE, PATCHOULI ALCOHOL, AMBROXAN, PEARLIDE, PENTALIDE
本発明の香料化合物吸着量向上効果の発現機構は必ずしも全てが解明された訳ではないが、本発明の組成物を用いて繊維製品を処理した時、特定の構造を有する(a)成分及び(b)成分が繊維表面に吸着し、(c)成分であるClogPが4を超える比較的疎水性の香料化合物を繊維製品表面に選択的に捕捉、吸着させることにより(c)成分の吸着率を向上させているものと考えられる。 The expression mechanism of the effect of improving the adsorption amount of the fragrance compound of the present invention is not necessarily completely elucidated, but when the fiber product is treated with the composition of the present invention, the component (a) having a specific structure and ( b) component is adsorbed on the fiber surface, and the relatively hydrophobic fragrance compound with (C) component ClogP exceeding 4 is selectively captured and adsorbed on the fiber product surface, thereby increasing the adsorption rate of component (c). It is thought that it is improving.
<柔軟剤組成物>
本発明の柔軟剤組成物は、(a)成分を0.005〜10質量%、更に0.05〜5質量%、特に0.5〜3質量%含有することが好ましい。また、(b)成分を3〜30質量%、更に5〜25質量%、特に10〜20質量%含有することが好ましい。また、(c)成分を0.05〜5質量%、更に0.3〜3質量%、特に0.5〜2質量%含有することが好ましい。なお、本発明の柔軟剤組成物が液体の場合、残部は全部又は主に水である。
<Softener composition>
The softener composition of the present invention preferably contains (a) component in an amount of 0.005 to 10% by mass, further 0.05 to 5% by mass, and particularly 0.5 to 3% by mass. Moreover, it is preferable to contain (b) component 3-30 mass%, 5-25 mass%, especially 10-20 mass%. Moreover, it is preferable to contain (c) component 0.05-5 mass%, 0.3-3 mass%, especially 0.5-2 mass%. In addition, when the softening agent composition of this invention is a liquid, the remainder is all or mainly water.
また、本発明の柔軟剤組成物では、(a)成分、(b)成分及び(c)成分の含有量の合計中、(c)成分の含有量の割合が、(c)成分/〔(a)成分+(b)成分+(c)成分〕×100で、0.05〜30質量%、更に0.5〜20質量%、特に1〜10質量%であることが、残香性および保存安定性の観点から好ましい。 Moreover, in the softening agent composition of this invention, in the sum total of content of (a) component, (b) component, and (c) component, the ratio of content of (c) component is (c) component / [(( a) component + (b) component + (c) component] × 100, 0.05 to 30% by mass, more preferably 0.5 to 20% by mass, and particularly 1 to 10% by mass. From the viewpoint of
また、本発明の柔軟剤組成物では、(a)成分、(b)成分及び(c)成分の含有量の合計中、(a)成分の含有量の割合が、(a)成分/〔(a)成分+(b)成分+(c)成分〕×100で、0.01〜30質量%、好ましくは0.1〜20質量%、より好ましくは1〜20質量%、特に好ましくは5〜20質量%、9〜20質量%であり、最も好ましくは12〜20質量%であることが、香料の吸着性、残香性、柔軟性、および保存安定性の観点から好ましい。また、経済性の観点から2〜10質量%が好ましい。 Moreover, in the softening agent composition of this invention, in the sum total of content of (a) component, (b) component, and (c) component, the ratio of content of (a) component is (a) component / [(( a) component + (b) component + (c) component] × 100, 0.01 to 30% by mass, preferably 0.1 to 20% by mass, more preferably 1 to 20% by mass, particularly preferably 5 to 5%. It is 20 mass% and 9-20 mass%, Most preferably, it is 12-20 mass% from a viewpoint of the adsorptivity of a fragrance | flavor, a residual fragrance property, a softness | flexibility, and storage stability. Moreover, 2-10 mass% is preferable from a viewpoint of economical efficiency.
なお、本発明の柔軟剤組成物は、本発明の(a)、(b)、(c)成分以外に、抗菌剤、安定化剤、増粘剤、染料、アニオン性界面活性剤等を含有することができる。本発明の柔軟剤組成物は、衣料等の繊維製品用として好適である。 The softener composition of the present invention contains antibacterial agents, stabilizers, thickeners, dyes, anionic surfactants and the like in addition to the components (a), (b) and (c) of the present invention. can do. The softener composition of the present invention is suitable for textile products such as clothing.
以下に、(a)成分(ポリマー1〜5)の合成例を示す。
(合成例1)
メタクリル酸N,N−ジメチルアミノエチル(分子量:157.21)36.0g、ブチルメタクリレート(分子量:142.2)14.0g、エタノール180.0gを均一に混合し、内容量300mLのガラス製セパラブルフラスコに入れ、窒素雰囲気下で一定時間攪拌した。そこに2,2’−アゾビス(2,4−ジメチルバレロニトリル)(V−65;和光純薬工業(株)製)1.41gをエタノール20.0gに溶解した溶液を添加し、60℃付近まで昇温した。60〜70℃付近で合計8時間保持することで重合・熟成した。そこにエタノール100.0gを加えて希釈した後、室温まで降温した。この反応溶液をイオン交換水4000.0g中に滴下して再沈殿精製し、沈殿物を乾燥してポリマー1を得た。ポリマーのMwは12800であった(水/エタノール=7/3系、ポリエチレンオキシド換算)。また1H−NMRにより分析したポリマーの組成は仕込みモノマー組成どおり(DMAEMA/BMA=70/30(モル比))であった。
Below, the synthesis example of (a) component (polymers 1-5) is shown.
(Synthesis Example 1)
36.0 g of N, N-dimethylaminoethyl methacrylate (molecular weight: 157.21), 14.0 g of butyl methacrylate (molecular weight: 142.2) and 180.0 g of ethanol are uniformly mixed, and placed in a glass separable flask having an internal volume of 300 mL. The mixture was stirred for a certain time under a nitrogen atmosphere. Thereto was added a solution prepared by dissolving 1.41 g of 2,2′-azobis (2,4-dimethylvaleronitrile) (V-65; manufactured by Wako Pure Chemical Industries, Ltd.) in 20.0 g of ethanol, and around 60 ° C. The temperature was raised to. Polymerization and aging were carried out by maintaining the temperature at around 60 to 70 ° C. for a total of 8 hours. 100.0 g of ethanol was added and diluted there, and then the temperature was lowered to room temperature. This reaction solution was dropped into 4000.0 g of ion-exchanged water and purified by reprecipitation, and the precipitate was dried to obtain polymer 1. The polymer Mw was 12800 (water / ethanol = 7/3 system, in terms of polyethylene oxide). The composition of the polymer analyzed by 1 H-NMR was the same as the charged monomer composition (DMAEMA / BMA = 70/30 (molar ratio)).
(合成例2)以降は、(合成例1)と同様の方法にて使用するモノマーを変更することにより、下記組成(モル比)、分子量のポリマーを得た。
(合成例2)
ポリマー2:DMAEMA/BMA=65/35、Mw11400
(合成例3)
ポリマー3:DMAEMA/BMA=55/45、Mw10200
(合成例4)
ポリマー4:DMAEMA/BMA=40/60、Mw6500
(Synthesis Example 2) After that, a polymer having the following composition (molar ratio) and molecular weight was obtained by changing the monomer used in the same manner as in (Synthesis Example 1).
(Synthesis Example 2)
Polymer 2: DMAEMA / BMA = 65/35, Mw11400
(Synthesis Example 3)
Polymer 3: DMAEMA / BMA = 55/45, Mw10200
(Synthesis Example 4)
Polymer 4: DMAEMA / BMA = 40/60, Mw6500
(合成例5)
内容量1Lのガラス製セパラブルフラスコを一定時間窒素置換した。そこにエタノール46.8gを添加し、撹拌しながら内温が78〜80℃になるまで加熱し、保持した。ジメチルアミノエチルメタクリレート300.00g、2,2’−アゾビス(2,4−ジメチルバレロニトリル)(V−65B;和光純薬工業(株)製)7.11g、エタノール114.3gを予め均一に混合し、この溶液を上記フラスコ中に3時間かけて一定速度で滴下した。次に、V−65B 11.85gをエタノール47.4gに溶解した溶液を上記フラスコ中に4時間かけて一定速度で滴下した。滴下終了後、80℃付近で2時間保持することでポリジメチルアミノエチルメタクリレート(ポリマー5)のエタノール溶液を得た。ポリマーのMwは11200であった。また1H−NMRにより分析したポリマーの組成は仕込みモノマー組成どおりであった。
(Synthesis Example 5)
A glass separable flask having an internal volume of 1 L was purged with nitrogen for a certain period of time. Ethanol 46.8g was added there, and it heated and maintained until internal temperature became 78-80 degreeC, stirring. 300.00 g of dimethylaminoethyl methacrylate, 7.11 g of 2,2′-azobis (2,4-dimethylvaleronitrile) (V-65B; manufactured by Wako Pure Chemical Industries, Ltd.) and 114.3 g of ethanol are mixed uniformly in advance. Then, this solution was dropped into the flask at a constant rate over 3 hours. Next, a solution obtained by dissolving 11.85 g of V-65B in 47.4 g of ethanol was dropped into the flask at a constant rate over 4 hours. After completion of dropping, the solution was kept at around 80 ° C. for 2 hours to obtain an ethanol solution of polydimethylaminoethyl methacrylate (polymer 5). The Mw of the polymer was 11200. The composition of the polymer analyzed by 1 H-NMR was the same as the charged monomer composition.
また、ポリマー6として、以下のものを用いた。
・ポリマー6:ポリジアリルジメチルアンモニウムクロライド、Poly(diallyldimethylammonium chloride), low molecular weight(MW:100,000〜200,000、アルドリッチ製)
Moreover, the following were used as the polymer 6.
-Polymer 6: Polydiallyldimethylammonium chloride, Poly (diallyldimethylammonium chloride), low molecular weight (MW: 100,000-200,000, manufactured by Aldrich)
上記略号は以下の意味を示す。また、ポリマーの分子量Mwは前述の測定法Aにより測定したものである。
・DMAEMA:メタクリル酸N,N−ジメチルアミノエチル
・DADMAC:ジアリルジメチルアンモニウムクロライド
・BMA:ブチルメタクリレート
The above abbreviations have the following meanings. Further, the molecular weight Mw of the polymer is measured by the above-described measuring method A.
DMAEMA: N, N-dimethylaminoethyl methacrylate DADMAC: diallyldimethylammonium chloride BMA: butyl methacrylate
以下に、各実施例、比較例で用いた(b)成分〔(b−1)〕の合成例を示す。 Below, the synthesis example of (b) component [(b-1)] used by each Example and the comparative example is shown.
(合成例)
N−(3−アミノプロピル)−N−(2−ヒドロキシエチル)−N−メチルアミンと脂肪酸組成物(ステアリン酸とパルミチン酸を質量比で6/4の割合で混合した脂肪酸)とを1/1.9のモル比で公知の方法に従って脱水縮合させた。反応物中の脂肪酸含量が5%になった時点で反応を終了させた。この反応生成物を(b−1)として用いた。反応生成物(b−1)中、(b)成分に相当する化合物の含有量は95質量%であった。
(Synthesis example)
N- (3-aminopropyl) -N- (2-hydroxyethyl) -N-methylamine and a fatty acid composition (fatty acid obtained by mixing stearic acid and palmitic acid at a mass ratio of 6/4) It was dehydrated and condensed according to a known method at a molar ratio of 1.9. The reaction was terminated when the fatty acid content in the reaction product reached 5%. This reaction product was used as (b-1). In the reaction product (b-1), the content of the compound corresponding to the component (b) was 95% by mass.
<実施例1及び比較例1>
(1)前処理方法
あらかじめ、市販の弱アルカリ性洗剤(花王(株)アタック)を用いて、木綿メリヤス24枚、木綿タオル24枚をそれぞれ日立全自動洗濯機NW-6CYで5回洗浄を繰り返し、室内乾燥することによって、過分の薬剤を除去した(洗剤濃度0.0667質量%、水道水47L使用、水温20℃、洗浄10分、ため濯ぎ2回)。
<Example 1 and Comparative Example 1>
(1) Pretreatment method In advance, using a commercially available weak alkaline detergent (attack from Kao Corporation), 24 cotton knitted fabrics and 24 cotton towels were each washed 5 times with Hitachi fully automatic washing machine NW-6CY. Excess chemicals were removed by drying in the room (detergent concentration: 0.0667% by mass, use of 47 L of tap water, water temperature of 20 ° C., washing for 10 minutes, twice for rinsing).
(2)柔軟剤組成物及び試験用処理液の調製方法
はじめに、(a)成分1.9%(質量%、以下特記しない限り同様)、(b)成分17.0%、(c)成分1.5%になるようそれぞれを水と混合し、塩酸にてpH=3にして60℃に加熱し撹拌した。その後、室温まで冷却し柔軟剤組成物を調製した。これを水で3700倍希釈し、(a)成分5ppm、(b)成分46ppm、(c)成分4ppmの試験用処理液を得た。
(2) Preparation method of softener composition and test treatment liquid First, (a) component 1.9% (mass%, the same unless otherwise specified), (b) component 17.0%, (c) component 1 Each was mixed with water so as to be 5%, adjusted to pH = 3 with hydrochloric acid, heated to 60 ° C. and stirred. Then, it cooled to room temperature and prepared the softening agent composition. This was diluted 3700 times with water to obtain a test treatment liquid of (a) component 5 ppm, (b) component 46 ppm, and (c) component 4 ppm.
(3)吸着率の測定方法
上記の方法で調製した試験用処理液(52.5g)をNo.11の規格瓶に入れた。さらに、前処理を行った木綿メリヤス布(10cm×10cm、2.1g)を入れ、マグネティックスターラー(スターラーピースの長さ4.5cm×径1cm、回転数200rpm)で5分間攪拌するモデル柔軟処理を行った。処理前の試験用処理液中の香料化合物含有量(x)と処理後の香料化合物含有量(y)の差分〔(x)−(y)〕をタオルに吸着している量として、処理前の香料化合物含有量(x)に対する割合(百分率)、すなわち、〔(x)−(y)〕/(x)×100を香料化合物の吸着率(%)とする。その結果を表1に示す。なお、処理前後の試験用処理液中の香料化合物の含有量は、下記の液体クロマトグラフィー装置を用いて測定した。
液体クロマトグラフィー装置:HITACHI L−6000
カラム:Lichrospher 100 RP−18(e) 5μm 125mm×4φ
カラム温度:40℃
溶離剤:アセトニトリル/水=7/3(質量比)の混合溶液
流速:1.0mL/min
検出器:UV(220nm)
(3) Measurement method of adsorption rate The test treatment solution (52.5 g) prepared by the above method was No. 11 standard bottles. Furthermore, pre-treated cotton knitted fabric (10 cm x 10 cm, 2.1 g) is put, and the model is softly stirred for 5 minutes with a magnetic stirrer (stirrer piece length 4.5 cm x diameter 1 cm, rotation speed 200 rpm). went. The difference [(x)-(y)] between the fragrance compound content (x) in the test treatment liquid before treatment and the fragrance compound content (y) after treatment is the amount adsorbed on the towel before treatment. The ratio (percentage) to the fragrance compound content (x), that is, [(x)-(y)] / (x) × 100 is defined as the fragrance compound adsorption rate (%). The results are shown in Table 1. In addition, content of the fragrance | flavor compound in the test processing liquid before and behind a process was measured using the following liquid chromatography apparatus.
Liquid chromatography device: HITACHI L-6000
Column: Lichlorosphere 100 RP-18 (e) 5 μm 125 mm × 4φ
Column temperature: 40 ° C
Eluent: A mixed solution of acetonitrile / water = 7/3 (mass ratio) Flow rate: 1.0 mL / min
Detector: UV (220 nm)
表中、吸着率の対ブランク相対値は、ブランクを1.0とする相対値である(以下同様)。 In the table, the relative value of the adsorption rate with respect to the blank is a relative value where the blank is 1.0 (the same applies hereinafter).
次に、実施例1−1とブランク(ポリマーなし)の条件での処理布を、25℃、40%RHにて24時間乾燥させ、残香性の比較官能評価を、さらに、同条件で処理した木綿タオルを25℃、40%RHにて24時間乾燥させ柔軟性の比較官能評価を、それぞれパネラー10人に対して行った。結果を表2に示す。 Next, the treated cloth under the conditions of Example 1-1 and a blank (no polymer) was dried at 25 ° C. and 40% RH for 24 hours, and a comparative sensory evaluation of residual fragrance was further treated under the same conditions. The cotton towel was dried at 25 ° C. and 40% RH for 24 hours, and a comparative sensory evaluation of flexibility was performed on 10 panelists. The results are shown in Table 2.
これより、本発明の柔軟剤組成物では、残香性がよく、更に柔軟性も向上していることがわかる。 From this, it can be seen that the softener composition of the present invention has good residual fragrance and further improved flexibility.
<実施例2及び比較例2>
(a)成分として合成例1の方法で調製したポリマー1、(b)成分として前記(b−1)、及び(c)成分としてClogPの異なる各種香料化合物、を用いて、上述した調製方法と同様に試験用処理液を調製し、実施例1と同様に処理を行い、吸着率を求めた。また、(a)成分を配合しない試験用処理液〔(a)成分の配合量の分を水に置き換える〕についても吸着率を求めた。その結果を表3に示した。
<Example 2 and Comparative Example 2>
(A) Polymer 1 prepared by the method of Synthesis Example 1 as component, (b) component (b-1) as component, and (c) various fragrance compounds having different ClogP as component, Similarly, a test treatment solution was prepared and treated in the same manner as in Example 1 to determine the adsorption rate. Further, the adsorption rate was also determined for the test treatment liquid in which the component (a) was not blended [replace the blended amount of the component (a) with water]. The results are shown in Table 3.
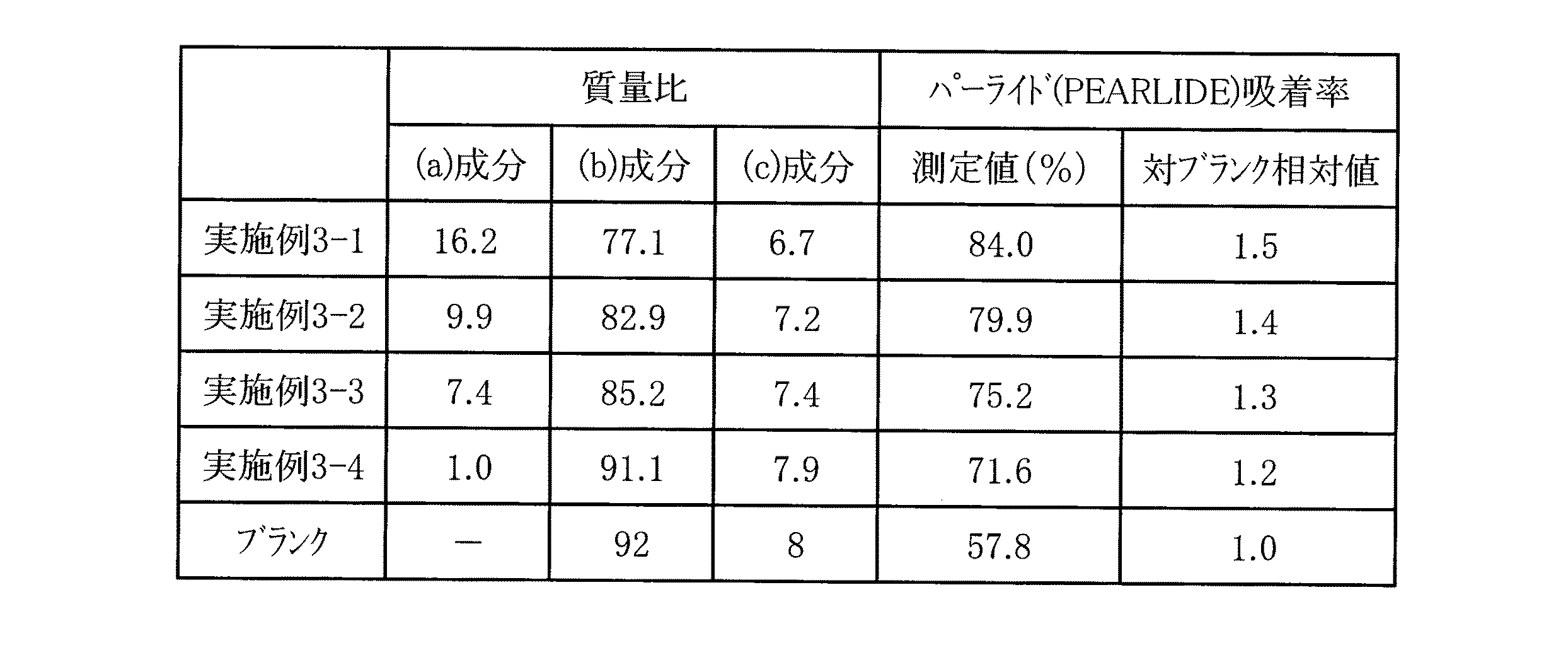
<実施例3及び比較例3>
合成例1の方法で調製したポリマー1〔(a)成分〕、前記(b−1)〔(b)成分〕、及び実施例2において効果の高かったパーライド〔(c)成分〕を用いて、(a)成分0〜3.7%、(b)成分17.0%、(c)成分1.5%になるようそれぞれを混合し、塩酸にてpH=2にして60℃に加熱し撹拌した。なお、(a)〜(c)成分の三者の質量比は、表4の数値となるように調整した。その後、室温まで冷却し柔軟剤組成物を調製した。これを水で3700倍希釈し、(a)成分0〜10ppm、(b)成分46ppm、(c)成分4ppmの試験用処理液を得た。この試験用処理液を用いて実施例1と同様に処理を行い香料の吸着率を測定した。その結果を表4に示す。ブランクは、(a)成分0ppm〔(a)成分なし〕、(b)成分46ppm、(c)成分4ppmの組成物である。
<Example 3 and Comparative Example 3>
Using the polymer 1 [(a) component] prepared by the method of Synthesis Example 1, the above (b-1) [(b) component], and the paride [(c) component] which was highly effective in Example 2, (A) Components 0 to 3.7%, (b) Component 17.0%, (c) Components 1.5% are mixed, heated to 60 ° C. with hydrochloric acid to pH = 2, and stirred. did. The mass ratio of the three components (a) to (c) was adjusted to the numerical values shown in Table 4. Then, it cooled to room temperature and prepared the softening agent composition. This was diluted 3700 times with water to obtain a test treatment liquid of (a) component 0 to 10 ppm, (b) component 46 ppm, and (c) component 4 ppm. Using this test treatment solution, the same treatment as in Example 1 was performed, and the perfume adsorption rate was measured. The results are shown in Table 4. The blank is a composition of (a) component 0 ppm [no (a) component], (b) component 46 ppm, and (c) component 4 ppm.
表1〜4の結果より、(a)、(b)、(c)成分を含有する本発明の柔軟剤組成物で処理された繊維製品は、香料を効率的に繊維製品に吸着させ、残香性および柔軟性を向上させることが出来ることがわかった。 From the results of Tables 1 to 4, the fiber product treated with the softener composition of the present invention containing the components (a), (b), and (c) efficiently adsorbs the fragrance to the fiber product, It was found that the property and flexibility can be improved.
Claims (4)
<(a)成分>
下記一般式(1)で示される化合物、又はその酸塩に由来するモノマー単位(A)のみからなる、高分子化合物。
〔式中、R1、R2は、それぞれ独立に水素原子、又はメチル基を示し、R3 は水素原子を示す。Xは−COO−R6−、又は−CONR7−R8 −を示す。R4 は炭素数1〜3のアルキル基、又は炭素数1〜3のヒドロキシアルキル基を示す。R5は炭素数1〜3のアルキル基、炭素数1〜3のヒドロキシアルキル基、又は水素原子を示す。R6、R8は、それぞれ独立に炭素数2〜3のアルキレン基、R7は水素原子、又は炭素数1〜3のアルキル基を示す。〕
<(b)成分>
アミド基で分断されている総炭素数12〜29の炭化水素基を1〜3個有する3級アミン、該アミンの4級化物、及び前記アミンの酸塩から選ばれる1種以上の化合物。
<(c)成分>
ClogPが4を超える香料化合物。 The softening agent composition containing the following (a) component, (b) component, and (c) component.
<(A) component>
The high molecular compound which consists only of the monomer unit (A) derived from the compound shown by following General formula (1), or its acid salt .
[Wherein, R 1, R 2 each independently represent a hydrogen atom or a methyl group, R 3 denotes a water atom. X represents —COO—R 6 — or —CONR 7 —R 8 — . R 4 represents an alkyl group, or hydroxyalkyl group having 1 to 3 carbon atoms having a carbon number of 1-3. R 5 represents an alkyl group having 1 to 3 carbon atoms, a hydroxyalkyl group having 1 to 3 carbon atoms, or a hydrogen atom. R 6 and R 8 each independently represent an alkylene group having 2 to 3 carbon atoms, and R 7 represents a hydrogen atom or an alkyl group having 1 to 3 carbon atoms. ]
<(B) component>
One or more compounds selected from tertiary amines having 1 to 3 hydrocarbon groups having 12 to 29 carbon atoms separated by amide groups, quaternized products of the amines, and acid salts of the amines.
<(C) component>
A perfume compound having a ClogP of more than 4.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007336578A JP5118475B2 (en) | 2007-12-27 | 2007-12-27 | Softener composition |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007336578A JP5118475B2 (en) | 2007-12-27 | 2007-12-27 | Softener composition |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009155766A JP2009155766A (en) | 2009-07-16 |
| JP5118475B2 true JP5118475B2 (en) | 2013-01-16 |
Family
ID=40960066
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007336578A Expired - Fee Related JP5118475B2 (en) | 2007-12-27 | 2007-12-27 | Softener composition |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP5118475B2 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5883569B2 (en) * | 2011-03-23 | 2016-03-15 | 花王株式会社 | Liquid softener composition |
| JP6820719B2 (en) * | 2016-11-07 | 2021-01-27 | 花王株式会社 | Liquid fabric softener composition |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002285474A (en) * | 2001-03-28 | 2002-10-03 | Kao Corp | Softening agent composition |
| JP4387149B2 (en) * | 2003-09-09 | 2009-12-16 | 花王株式会社 | Softener composition |
| JP2005105508A (en) * | 2003-10-01 | 2005-04-21 | Rohm & Haas Co | Polymer and process for controlling rheology of aqueous composition |
| JP5250860B2 (en) * | 2007-08-31 | 2013-07-31 | ライオン株式会社 | Liquid softener composition |
-
2007
- 2007-12-27 JP JP2007336578A patent/JP5118475B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009155766A (en) | 2009-07-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN104854227B (en) | Fabric conditioner containing amine function silicone | |
| US20100035791A1 (en) | Treatment composition for textile products | |
| EP2751244B1 (en) | Cleaning compositions and soil capture agent for cleaning objects | |
| AU2013382220B2 (en) | Fabric conditioner | |
| JP4387149B2 (en) | Softener composition | |
| JP5118475B2 (en) | Softener composition | |
| TWI417438B (en) | Fiber treatment agent | |
| EP2083114B1 (en) | Fiber product-treating agent | |
| JP4944756B2 (en) | Textile treatment agent | |
| JP5118476B2 (en) | Softener composition | |
| JP4926681B2 (en) | Transparent or translucent liquid softener composition | |
| JP2008144316A (en) | Liquid softener composition | |
| JPH0215665B2 (en) | ||
| JP4997180B2 (en) | Textile processing method | |
| JP4944757B2 (en) | Textile treatment agent | |
| JP7051202B2 (en) | Finishing agent composition for clothing | |
| JP2018512461A (en) | Amino-modified hydrocarbon | |
| JP7254424B2 (en) | Garment finish composition | |
| JP6803718B2 (en) | Textile softener | |
| US8426351B2 (en) | Liquid softener composition or transparent or semitransparent liquid softener composition | |
| JP4877967B2 (en) | Textile treatment agent | |
| JP2014177727A (en) | Softening agent for fiber product | |
| JP2006176641A (en) | Quick drying property-imparting agent, and detergent composition for clothes and finishing agent composition for clothes using this | |
| JP2021107598A (en) | Durability and antistaticity agent for synthetic fiber, durable and antistatic fiber product, and method for producing durable and antistatic fiber product | |
| JP5000271B2 (en) | Textile treatment agent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100928 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120222 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120228 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120426 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20121016 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20121019 |
|
| R151 | Written notification of patent or utility model registration |
Ref document number: 5118475 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R151 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151026 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |