JP3627552B2 - Method for producing reversible cross-linkable molded article - Google Patents
Method for producing reversible cross-linkable molded article Download PDFInfo
- Publication number
- JP3627552B2 JP3627552B2 JP00316999A JP316999A JP3627552B2 JP 3627552 B2 JP3627552 B2 JP 3627552B2 JP 00316999 A JP00316999 A JP 00316999A JP 316999 A JP316999 A JP 316999A JP 3627552 B2 JP3627552 B2 JP 3627552B2
- Authority
- JP
- Japan
- Prior art keywords
- component
- carboxylic acid
- molded article
- crosslinkable
- olefin polymer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 230000002441 reversible effect Effects 0.000 title claims description 35
- 238000004519 manufacturing process Methods 0.000 title claims description 27
- 238000004132 cross linking Methods 0.000 claims description 57
- 150000001244 carboxylic acid anhydrides Chemical group 0.000 claims description 54
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 51
- 238000000465 moulding Methods 0.000 claims description 46
- 239000000203 mixture Substances 0.000 claims description 44
- 229920000098 polyolefin Polymers 0.000 claims description 42
- 238000010494 dissociation reaction Methods 0.000 claims description 24
- 230000005593 dissociations Effects 0.000 claims description 23
- 229920000642 polymer Polymers 0.000 claims description 21
- 238000001125 extrusion Methods 0.000 claims description 18
- 229920001577 copolymer Polymers 0.000 claims description 17
- 239000012943 hotmelt Substances 0.000 claims description 14
- 125000003262 carboxylic acid ester group Chemical class [H]C([H])([*:2])OC(=O)C([H])([H])[*:1] 0.000 claims description 12
- 230000009477 glass transition Effects 0.000 claims description 6
- -1 polyethylene Polymers 0.000 description 25
- 230000000052 comparative effect Effects 0.000 description 23
- 238000000034 method Methods 0.000 description 20
- 150000001733 carboxylic acid esters Chemical class 0.000 description 14
- 239000008188 pellet Substances 0.000 description 14
- 239000004711 α-olefin Substances 0.000 description 13
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 12
- 229920005989 resin Polymers 0.000 description 12
- 239000011347 resin Substances 0.000 description 12
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 11
- 238000002156 mixing Methods 0.000 description 11
- 238000012545 processing Methods 0.000 description 11
- 229910052751 metal Inorganic materials 0.000 description 10
- 239000002184 metal Substances 0.000 description 10
- 150000003839 salts Chemical class 0.000 description 10
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 238000005755 formation reaction Methods 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 229920001897 terpolymer Polymers 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 6
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 5
- 239000005977 Ethylene Substances 0.000 description 5
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 5
- 239000000155 melt Substances 0.000 description 5
- 229920005862 polyol Polymers 0.000 description 5
- 150000003077 polyols Chemical class 0.000 description 5
- LIKMAJRDDDTEIG-UHFFFAOYSA-N 1-hexene Chemical compound CCCCC=C LIKMAJRDDDTEIG-UHFFFAOYSA-N 0.000 description 4
- WSSSPWUEQFSQQG-UHFFFAOYSA-N 4-methyl-1-pentene Chemical compound CC(C)CC=C WSSSPWUEQFSQQG-UHFFFAOYSA-N 0.000 description 4
- 239000004698 Polyethylene Substances 0.000 description 4
- 238000000862 absorption spectrum Methods 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 230000004927 fusion Effects 0.000 description 4
- 238000005227 gel permeation chromatography Methods 0.000 description 4
- 238000001746 injection moulding Methods 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- 229920005672 polyolefin resin Polymers 0.000 description 4
- AFFLGGQVNFXPEV-UHFFFAOYSA-N 1-decene Chemical compound CCCCCCCCC=C AFFLGGQVNFXPEV-UHFFFAOYSA-N 0.000 description 3
- YHQXBTXEYZIYOV-UHFFFAOYSA-N 3-methylbut-1-ene Chemical compound CC(C)C=C YHQXBTXEYZIYOV-UHFFFAOYSA-N 0.000 description 3
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical group CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 125000004018 acid anhydride group Chemical group 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000000748 compression moulding Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 235000012438 extruded product Nutrition 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 229920000058 polyacrylate Polymers 0.000 description 3
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 3
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 3
- 238000001175 rotational moulding Methods 0.000 description 3
- JTHZUSWLNCPZLX-UHFFFAOYSA-N 6-fluoro-3-methyl-2h-indazole Chemical compound FC1=CC=C2C(C)=NNC2=C1 JTHZUSWLNCPZLX-UHFFFAOYSA-N 0.000 description 2
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 2
- OWYWGLHRNBIFJP-UHFFFAOYSA-N Ipazine Chemical compound CCN(CC)C1=NC(Cl)=NC(NC(C)C)=N1 OWYWGLHRNBIFJP-UHFFFAOYSA-N 0.000 description 2
- RRHGJUQNOFWUDK-UHFFFAOYSA-N Isoprene Chemical compound CC(=C)C=C RRHGJUQNOFWUDK-UHFFFAOYSA-N 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- UKLDJPRMSDWDSL-UHFFFAOYSA-L [dibutyl(dodecanoyloxy)stannyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)O[Sn](CCCC)(CCCC)OC(=O)CCCCCCCCCCC UKLDJPRMSDWDSL-UHFFFAOYSA-L 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 2
- 150000007942 carboxylates Chemical class 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 150000001993 dienes Chemical class 0.000 description 2
- NKSJNEHGWDZZQF-UHFFFAOYSA-N ethenyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)C=C NKSJNEHGWDZZQF-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 229920001684 low density polyethylene Polymers 0.000 description 2
- 239000004702 low-density polyethylene Substances 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 239000011976 maleic acid Substances 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 238000005453 pelletization Methods 0.000 description 2
- 229920001281 polyalkylene Polymers 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 238000010298 pulverizing process Methods 0.000 description 2
- 239000011342 resin composition Substances 0.000 description 2
- 238000007493 shaping process Methods 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 229920001169 thermoplastic Polymers 0.000 description 2
- 229920005992 thermoplastic resin Polymers 0.000 description 2
- 239000004416 thermosoftening plastic Substances 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- QQYNRBAAQFZCLF-FBXFSONDSA-N (3ar,4s,7r,7as)-rel-3a,4,7,7a-tetrahydro-4,7-epoxyisobenzofuran-1,3-dione Chemical compound O1[C@@H]2[C@@H]3C(=O)OC(=O)[C@@H]3[C@H]1C=C2 QQYNRBAAQFZCLF-FBXFSONDSA-N 0.000 description 1
- PRBHEGAFLDMLAL-GQCTYLIASA-N (4e)-hexa-1,4-diene Chemical compound C\C=C\CC=C PRBHEGAFLDMLAL-GQCTYLIASA-N 0.000 description 1
- PMJHHCWVYXUKFD-SNAWJCMRSA-N (E)-1,3-pentadiene Chemical compound C\C=C\C=C PMJHHCWVYXUKFD-SNAWJCMRSA-N 0.000 description 1
- KMOUUZVZFBCRAM-UHFFFAOYSA-N 1,2,3,6-tetrahydrophthalic anhydride Chemical compound C1C=CCC2C(=O)OC(=O)C21 KMOUUZVZFBCRAM-UHFFFAOYSA-N 0.000 description 1
- PRBHEGAFLDMLAL-UHFFFAOYSA-N 1,5-Hexadiene Natural products CC=CCC=C PRBHEGAFLDMLAL-UHFFFAOYSA-N 0.000 description 1
- RNFJDJUURJAICM-UHFFFAOYSA-N 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyclohexa-1,3,5-triene Chemical compound N=1P(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP=1(OC=1C=CC=CC=1)OC1=CC=CC=C1 RNFJDJUURJAICM-UHFFFAOYSA-N 0.000 description 1
- MFGALGYVFGDXIX-UHFFFAOYSA-N 2,3-Dimethylmaleic anhydride Chemical compound CC1=C(C)C(=O)OC1=O MFGALGYVFGDXIX-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- XMNIXWIUMCBBBL-UHFFFAOYSA-N 2-(2-phenylpropan-2-ylperoxy)propan-2-ylbenzene Chemical compound C=1C=CC=CC=1C(C)(C)OOC(C)(C)C1=CC=CC=C1 XMNIXWIUMCBBBL-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- AGULWIQIYWWFBJ-UHFFFAOYSA-N 3,4-dichlorofuran-2,5-dione Chemical compound ClC1=C(Cl)C(=O)OC1=O AGULWIQIYWWFBJ-UHFFFAOYSA-N 0.000 description 1
- UYCICMIUKYEYEU-ZHACJKMWSA-N 3-[(e)-dodec-2-enyl]oxolane-2,5-dione Chemical compound CCCCCCCCC\C=C\CC1CC(=O)OC1=O UYCICMIUKYEYEU-ZHACJKMWSA-N 0.000 description 1
- YPRMWCKXOZFJGF-UHFFFAOYSA-N 3-bromofuran-2,5-dione Chemical compound BrC1=CC(=O)OC1=O YPRMWCKXOZFJGF-UHFFFAOYSA-N 0.000 description 1
- AYKYXWQEBUNJCN-UHFFFAOYSA-N 3-methylfuran-2,5-dione Chemical compound CC1=CC(=O)OC1=O AYKYXWQEBUNJCN-UHFFFAOYSA-N 0.000 description 1
- OFNISBHGPNMTMS-UHFFFAOYSA-N 3-methylideneoxolane-2,5-dione Chemical compound C=C1CC(=O)OC1=O OFNISBHGPNMTMS-UHFFFAOYSA-N 0.000 description 1
- FLISWPFVWWWNNP-UHFFFAOYSA-N 3-oct-1-enyloxolane-2,5-dione Chemical compound CCCCCCC=CC1CC(=O)OC1=O FLISWPFVWWWNNP-UHFFFAOYSA-N 0.000 description 1
- KAYAKFYASWYOEB-UHFFFAOYSA-N 3-octadec-1-enyloxolane-2,5-dione Chemical compound CCCCCCCCCCCCCCCCC=CC1CC(=O)OC1=O KAYAKFYASWYOEB-UHFFFAOYSA-N 0.000 description 1
- HMMBJOWWRLZEMI-UHFFFAOYSA-N 4,5,6,7-tetrahydro-2-benzofuran-1,3-dione Chemical compound C1CCCC2=C1C(=O)OC2=O HMMBJOWWRLZEMI-UHFFFAOYSA-N 0.000 description 1
- KNDQHSIWLOJIGP-UHFFFAOYSA-N 826-62-0 Chemical compound C1C2C3C(=O)OC(=O)C3C1C=C2 KNDQHSIWLOJIGP-UHFFFAOYSA-N 0.000 description 1
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 description 1
- FJNYGTDUQBOBHS-UHFFFAOYSA-N C12=C(CCC1)C(=O)OC2=O.C=CC2CC(=O)OC2=O Chemical compound C12=C(CCC1)C(=O)OC2=O.C=CC2CC(=O)OC2=O FJNYGTDUQBOBHS-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- 239000004594 Masterbatch (MB) Substances 0.000 description 1
- JKIJEFPNVSHHEI-UHFFFAOYSA-N Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) Chemical compound CC(C)(C)C1=CC(C(C)(C)C)=CC=C1OP(OC=1C(=CC(=CC=1)C(C)(C)C)C(C)(C)C)OC1=CC=C(C(C)(C)C)C=C1C(C)(C)C JKIJEFPNVSHHEI-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 239000005062 Polybutadiene Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 1
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 1
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 1
- BGYHLZZASRKEJE-UHFFFAOYSA-N [3-[3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoyloxy]-2,2-bis[3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoyloxymethyl]propyl] 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate Chemical compound CC(C)(C)C1=C(O)C(C(C)(C)C)=CC(CCC(=O)OCC(COC(=O)CCC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)(COC(=O)CCC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)COC(=O)CCC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)=C1 BGYHLZZASRKEJE-UHFFFAOYSA-N 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 150000008065 acid anhydrides Chemical class 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical class OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 150000008064 anhydrides Chemical group 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- CQEYYJKEWSMYFG-UHFFFAOYSA-N butyl acrylate Chemical compound CCCCOC(=O)C=C CQEYYJKEWSMYFG-UHFFFAOYSA-N 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 125000002843 carboxylic acid group Chemical group 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000011247 coating layer Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- NLDGJRWPPOSWLC-UHFFFAOYSA-N deca-1,9-diene Chemical compound C=CCCCCCCC=C NLDGJRWPPOSWLC-UHFFFAOYSA-N 0.000 description 1
- 239000012975 dibutyltin dilaurate Substances 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- GPLRAVKSCUXZTP-UHFFFAOYSA-N diglycerol Chemical compound OCC(O)COCC(O)CO GPLRAVKSCUXZTP-UHFFFAOYSA-N 0.000 description 1
- LDCRTTXIJACKKU-ARJAWSKDSA-N dimethyl maleate Chemical compound COC(=O)\C=C/C(=O)OC LDCRTTXIJACKKU-ARJAWSKDSA-N 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 208000018459 dissociative disease Diseases 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- HDERJYVLTPVNRI-UHFFFAOYSA-N ethene;ethenyl acetate Chemical group C=C.CC(=O)OC=C HDERJYVLTPVNRI-UHFFFAOYSA-N 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000003063 flame retardant Substances 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 229920000578 graft copolymer Polymers 0.000 description 1
- PYGSKMBEVAICCR-UHFFFAOYSA-N hexa-1,5-diene Chemical compound C=CCCC=C PYGSKMBEVAICCR-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229920001903 high density polyethylene Polymers 0.000 description 1
- 239000004700 high-density polyethylene Substances 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 229920001179 medium density polyethylene Polymers 0.000 description 1
- 239000004701 medium-density polyethylene Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- KNRCVAANTQNTPT-UHFFFAOYSA-N methyl-5-norbornene-2,3-dicarboxylic anhydride Chemical compound O=C1OC(=O)C2C1C1(C)C=CC2C1 KNRCVAANTQNTPT-UHFFFAOYSA-N 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 239000002667 nucleating agent Substances 0.000 description 1
- FSAJWMJJORKPKS-UHFFFAOYSA-N octadecyl prop-2-enoate Chemical group CCCCCCCCCCCCCCCCCCOC(=O)C=C FSAJWMJJORKPKS-UHFFFAOYSA-N 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 150000001451 organic peroxides Chemical class 0.000 description 1
- 238000007500 overflow downdraw method Methods 0.000 description 1
- 239000002530 phenolic antioxidant Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- PMJHHCWVYXUKFD-UHFFFAOYSA-N piperylene Natural products CC=CC=C PMJHHCWVYXUKFD-UHFFFAOYSA-N 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920002857 polybutadiene Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 229920000223 polyglycerol Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229920001384 propylene homopolymer Polymers 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000001226 reprecipitation Methods 0.000 description 1
- 229910000077 silane Inorganic materials 0.000 description 1
- SCPYDCQAZCOKTP-UHFFFAOYSA-N silanol Chemical compound [SiH3]O SCPYDCQAZCOKTP-UHFFFAOYSA-N 0.000 description 1
- 239000001587 sorbitan monostearate Substances 0.000 description 1
- 229940035048 sorbitan monostearate Drugs 0.000 description 1
- 235000011076 sorbitan monostearate Nutrition 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 238000009864 tensile test Methods 0.000 description 1
- 229920006027 ternary co-polymer Polymers 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- 229920001567 vinyl ester resin Polymers 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- YZYKBQUWMPUVEN-UHFFFAOYSA-N zafuleptine Chemical compound OC(=O)CCCCCC(C(C)C)NCC1=CC=C(F)C=C1 YZYKBQUWMPUVEN-UHFFFAOYSA-N 0.000 description 1
Landscapes
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Treatments Of Macromolecular Shaped Articles (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Description
【0001】
【発明の属する技術分野】
本発明は、成形体の製造方法に関し、詳しくは、低温下での架橋の形成と高温下での架橋の解離を繰り返し得る、いわゆる熱可逆架橋性の成形体の製造方法に関する。
特に、本発明は、成形性、加工安定性に優れ、且つ簡便な架橋処理が可能で、かつ、再利用も可能な熱可逆架橋性成形体の製造方法に関するものである。
【0002】
【従来の技術】
ポリエチレンやポリプロピレン等のオレフィン系樹脂は、成形性、機械的強度、透明性、耐薬品性等に優れ、押出成形、射出成形、中空成形、圧縮成形、回転成形等の各種成形法により溶融状態で所望の形状に賦形されて各種分野で汎用されており、又、耐熱性を付与し高温時の機械的強度等を改良すべく、有機過酸化物の配合、放射線の照射、或いはシラノール縮合反応の利用等により架橋処理を施した架橋体としても多用されている。
【0003】
一方、環境保護や省資源等の立場から、使用済の樹脂の再利用が益々要求される状況となっているが、この、架橋処理を施して架橋体とされた樹脂は、もはや熱可塑性を有さず溶融成形による再利用は不可能であって、この架橋体への熱可塑性の付与が強く求められている。
【0004】
この解決のため、低温下では架橋を形成し、高温下ではその架橋を解離させ熱可塑性を有せしめる可逆架橋方法がいくつか提案されている。例えば、架橋形成反応速度と架橋解離反応速度が高く優れた熱可逆架橋性を有するオレフィン系樹脂組成物として、特開平6−57062号公報、及び同7−94029号公報においては、不飽和カルボン酸無水物変性オレフィン系樹脂と、分子内に少なくとも2個の水酸基を有する多価アルコール化合物、例えば、エチレングリコール等のグリコール類、1,4−ブタンジオール等のアルコール類、ソルビトール等の糖類、トリメチロールプロパン等のポリオキシアルキレン化合物類、ジグリセリンモノステアレート等のポリグリセリンアルキルエステル類、ソルビタンモノステアレート等のソルビタンアルキルエステル類、エチレン−ヒドロキシエチルアクリレート共重合体等の分子内に複数個の水酸基を有する重合体等と、カルボン酸の金属塩等の反応促進剤とからなるオレフィン系樹脂組成物が開示されている。
【0005】
この種の、カルボン酸無水物基と水酸基との反応に基づく熱可逆架橋性組成物においては、本発明者等の検討によると、基本的には、1分子のカルボン酸無水物基と1分子の水酸基が反応してカルボン酸モノエステルを生成する反応と、生成したカルボン酸モノエステル1分子と1分子の水酸基がさらに反応してカルボン酸ジエステルを生成する反応の二つの反応が起こり、前者のカルボン酸モノエステル生成反応は熱可逆性が良好であるが、後者のカルボン酸ジエステル生成反応は熱可逆性が不良であること、そして、更に、前述の従来技術においては、カルボン酸金属塩の促進効果により初期の架橋度は高いが、酸無水物基が有機カルボン酸金属塩と反応して金属塩を生成してしまうため、その後の架橋の形成が著しく遅くなるとともに、この反応が起こることによってエステルの生成が減少するために全体として耐熱性のある架橋の程度が低下してしまい、結果として成形品の耐熱性が劣ること、及び、カルボン酸の金属塩は熱可逆性の劣るジエステルの生成も促進するために、架橋の解離性が悪化してしまう、等の問題があることが判明した。
一方、このようなカルボン酸金属塩を用いずに熱可逆架橋性の成形品を得ようとすると、成形条件によっては、外観の良好な成形体が得られないことがあった。
【0006】
【発明が解決しようとする課題】
本発明者は、前述の従来技術に関する検討結果を踏まえ、有機カルボン酸の金属塩を実質的に用いずに成形性、加工安定性が良好で、かつ架橋形成性と架橋解離性に優れた熱可逆架橋性成形体の製造方法について鋭意検討した結果、本発明に到達したものである。即ち、本発明は、成形性、加工安定性、及び架橋の形成/解離性に優れた品質良好な熱可逆架橋性成形体の製造方法を提供することを目的としている。
【0007】
【課題を解決するための手段】
本発明者は、不飽和カルボン酸無水物と不飽和カルボン酸エステルとによって変性された特定の変性オレフィン系重合体に特定の水酸基含有重合体を特定量配合した混合物を特定の条件下で熱溶融成形することによって前述の目的を達成できることを見出し、本発明を完成した。
【0008】
即ち、本発明の要旨は、下記の(A)成分及び(B)成分からなり、かつ(A)成分のカルボン酸無水物基数に対する(B)成分の水酸基数の比が0.1〜5である架橋性混合物を、その架橋解離温度以上の温度で熱溶融成形することを特徴とする可逆架橋性成形体の製造方法、に存している。
【0009】
(A)不飽和カルボン酸無水物と不飽和カルボン酸エステルとによって変性された変性オレフィン系重合体であって、1分子当たりのカルボン酸無水物基の平均結合数が1個以上で、かつ、該変性オレフィン系重合体中のカルボン酸無水物基数に対するカルボン酸エステル基数の比が0.5〜20である変性オレフィン系重合体
(B)1分子当たりの水酸基の平均結合数が1個以上の水酸基含有重合体
【0010】
本発明の要旨は、(A)成分の変性オレフィン系重合体が、エチレン−マレイン酸無水物−(メタ)アクリル酸アルキルエステル共重合体である上記の可逆架橋性成形体の製造方法、及び(A)成分の変性オレフィン系重合体の1分子当たりのカルボン酸無水物基の平均結合数が1.5個以上で、かつ、(B)成分の水酸基含有重合体の1分子当たりの水酸基の平均結合数が1.5個以上である上記の可逆架橋性成形体の製造方法にも存しており、また本発明の別の要旨は、前記いずれかの熱溶融成形された成形体を、そのガラス転移温度以上で、かつ該成形体の架橋解離温度未満の温度で架橋処理を行なう可逆架橋性成形体の製造方法、熱溶融成形が押出成形である前記いずれかの可逆架橋性成形体の製造方法、にも存しており、更に本発明の他の要旨は、前記いずれかの製造方法によって得られる可逆架橋性成形体、及び前記の成形体のスクラップを該成形体の架橋解離温度以上の成形温度で熱溶融成形することを特徴とする可逆架橋性成形体、にも存している。
【0011】
更に、本発明のもう一つの要旨は、下記(A)成分及び(B)成分とからなり、かつ(A)成分のカルボン酸無水物基数に対する(B)成分の水酸基数の比が0.1〜5である架橋性混合物と、請求項6に記載の成形体のスクラップとを、その両者の架橋解離温度のうち、より高い温度以上の温度で熱溶融成形することを特徴とする可逆架橋性成形体の製造方法、にも存している。
【0012】
(A)不飽和カルボン酸無水物と不飽和カルボン酸エステルとによって変性された変性オレフィン系重合体であって、1分子当たりのカルボン酸無水物基の平均結合数が1個以上で、かつ、該変性オレフィン系重合体中のカルボン酸無水物基数に対するカルボン酸エステル基数の比が0.5〜20である変性オレフィン系重合体
(B)1分子当たりの水酸基の平均結合数が1個以上の水酸基含有重合体
【0013】
【発明の実施の形態】
本発明における(A)成分の不飽和カルボン酸無水物と不飽和カルボン酸エステルとによって変性された変性オレフィン系重合体としては、基本的には、α−オレフィンとエチレン性不飽和カルボン酸無水物とエチレン性不飽和カルボン酸エステルとの三元共重合体、及び、α−オレフィンとエチレン性不飽和カルボン酸無水物との二元共重合体のエチレン性不飽和カルボン酸エステルによるグラフト体、α−オレフィンとエチレン性不飽和カルボン酸エステルとの二元共重合体のエチレン性不飽和カルボン酸無水物によるグラフト体、α−オレフィン系重合体のエチレン性不飽和カルボン酸無水物とエチレン性不飽和カルボン酸エステルとによるグラフト体を用いるのがよい。
【0014】
前者の三元共重合体におけるα−オレフィンとしては、例えば、エチレン、プロピレン、ブテン−1,3−メチルブテン−1、ペンテン−1,3−メチルペンテン−1,4−メチルペンテン−1、ヘキセン−1、オクテン−1、デセン−1等が挙げられる。
また、エチレン性不飽和カルボン酸無水物としては、例えば、コハク酸2−オクテン−1−イル無水物、コハク酸2−ドデセン−1−イル無水物、コハク酸2−オクタデセン−1−イル無水物、マレイン酸無水物、2,3−ジメチルマレイン酸無水物、ブロモマレイン酸無水物、ジクロロマレイン酸無水物、シトラコン酸無水物、イタコン酸無水物、1−ブテン−3,4−ジカルボン酸無水物、1−シクロペンテン−1,2−ジカルボン酸無水物、1,2,3,6−テトラヒドロフタル酸無水物、3,4,5,6−テトラヒドロフタル酸無水物、exo−3,6−エポキシ−1,2,3,6−テトラヒドロフタル酸無水物、5−ノルボルネン−2,3−ジカルボン酸無水物、メチル−5−ノルボルネン−2,3−ジカルボン酸無水物、endo−ビシクロ[2.2.2]オクト−5−エン−2,3−ジカルボン酸無水物、ビシクロ[2.2.2]オクト−7−エン−2,3,5,6−テトラカルボン酸無水物等が挙げられる。
【0015】
エチレン性不飽和カルボン酸エステルとしては、炭素原子数1〜20程度のアルキル基のエステルが好ましく、例えば、(メタ)アクリル酸メチル、(メタ)アクリル酸エチル、(メタ)アクリル酸プロピル、(メタ)アクリル酸ブチル、(メタ)アクリル酸ヘキシル、(メタ)アクリル酸オクチル、(メタ)アクリル酸2−エチルヘキシル、(メタ)アクリル酸ラウリル、(メタ)アクリル酸ステアリル、マレイン酸ジメチル等が挙げられる。なお、ここで、「(メタ)アクリル酸」とは、アクリル酸及びメタクリル酸を言うものとする。
【0016】
前者の三元共重合体としては、前記α−オレフィンと前記エチレン性不飽和カルボン酸無水物と前記エチレン性不飽和カルボン酸エステルとの三元共重合体の他、さらに、(メタ)アクリル酸、マレイン酸等のエチレン性不飽和カルボン酸化合物、酢酸ビニル等のエチレン性不飽和エステル化合物、(メタ)アクリルアミド、N−メチル(メタ)アクリルアミド等のエチレン性不飽和アミド化合物、スチレン、(メタ)アクリロニトリル等のその他のエチレン性不飽和化合物等を共重合した四元以上の多元共重合体であってもよい。
【0017】
これらの共重合体は、塊状、溶液、懸濁等の重合法により製造することができる。
後者のグラフト体におけるα−オレフィンとエチレン性不飽和カルボン酸無水物との二元共重合体、及び、α−オレフィンとエチレン性不飽和カルボン酸エステルとの二元共重合体としては、前者の三元共重合体において挙げたと同様のα−オレフィン、エチレン性不飽和カルボン酸無水物、及び、エチレン性不飽和カルボン酸エステルが挙げられ、又、後者のグラフト体におけるα−オレフィン系重合体としては、例えば、低密度・中密度・高密度ポリエチレン等(分岐状又は直鎖状)のエチレンの単独重合体、エチレンと、プロピレン、ブテン−1、3−メチルブテン−1、ペンテン−1,3−メチルペンテン−1,4−メチルペンテン−1、ヘキセン−1、オクテン−1、デセン−1等のα−オレフィンとの共重合体、エチレンと、酢酸ビニル等のビニルエステル、(メタ)アクリル酸又はそれらのエステル等の他単量体との共重合体等のエチレン系樹脂、プロピレンの単独重合体、プロピレンと、エチレン、ブテン−1,3−メチルブテン−1、ペンテン−1,3−メチルペンテン−1,4−メチルペンテン−1、ヘキセン−1、オクテン−1、デセン−1等のα−オレフィンとの共重合体、プロピレンと、イソプレン、1,3−ブタジエン、1,3−ペンタジエン、1,4−ヘキサジエン、1,5−ヘキサジエン、1,9−デカジエン等のジエン化合物等の他単量体との共重合体等のプロピレン系樹脂、その他ブテン−1,4−メチルペンテン−1、ヘキセン−1等のα−オレフィンの単独重合体や共重合体等が挙げられる。
グラフトされるエチレン性不飽和カルボン酸エステル、エチレン性不飽和カルボン酸無水物としては、前記三元共重合体において挙げたと同様のものが挙げられる。
【0018】
これらのグラフト体は、溶融混練、溶液、懸濁等のグラフト化法により製造することができる。
本発明における(A)成分の前記変性オレフィン系重合体としては、エチレンと、マレイン酸無水物と、(メタ)アクリル酸アルキルエステルの三元共重合体、及びα−オレフィン系重合体の、マレイン酸無水物と、(メタ)アクリル酸アルキルエステルとによるグラフト体が好ましく、特にはエチレンと、マレイン酸無水物と、(メタ)アクリル酸メチル又はエチルとの三元共重合体が好ましい。
【0019】
本発明において、(A)成分である前記変性オレフィン系重合体は、前記不飽和カルボン酸無水物単位の含有量が、0.1重量%以上、特には0.5重量%以上であるのが好ましく、変性オレフィン系重合体の数平均分子量とこの含有量との乗数に基づいて求められる、変性オレフィン系重合体1分子当たりのカルボン酸無水物基としての平均結合数が、1個以上であることが必須であり、1.5個以上であることが好ましい。この平均結合数が1個未満では、組成物としての架橋形成性が劣ることとなる。
【0020】
また、本発明において、(A)成分の前記変性オレフィン系重合体は、前記不飽和カルボン酸無水物に由来するカルボン酸無水物基数に対する前記不飽和カルボン酸エステルに由来するカルボン酸エステル基数の比が0.5〜20であることが必須であり、0.5〜15であるのが好ましい。この比が前記範囲未満では成形体としての架橋解離性が劣り、一方、前記範囲超過では架橋形成性が劣ることとなる。
【0021】
なお、本発明における(A)成分の変性オレフィン系重合体としては、1分子当たりのカルボン酸無水物基の平均結合数、及び、カルボン酸無水物基数に対するカルボン酸エステル基数の比が、前記範囲を満足する限り、変性オレフィン系重合体を未変性オレフィン系重合体で希釈したものであってもよい。
【0022】
本発明において、(A)成分の前記不飽和カルボン酸無水物及び不飽和カルボン酸エステル変性オレフィン系重合体のカルボン酸無水物基と結合して架橋を形成せしめる(B)成分の水酸基含有重合体としては、例えば、エチレン−(メタ)アクリル酸2−ヒドロキシエチル共重合体、(メタ)アクリル酸2−ヒドロキシエチルグラフトポリエチレン、エチレン−酢酸ビニル共重合体鹸化物、ポリビニルアルコール、低分子量ポリオレフィンポリオール類、ポリアルキレンエーテルグリコール類、ポリオキシアルキレンポリオール類、水酸基末端ジエンポリマー及びその水素添加物或いはそのアジペート類、水酸基末端ポリカプロラクトン類等が挙げられ、これらは、数平均分子量が500〜10000であるのが好ましい。中でも、低分子量ポリオレフィンポリオール類、ポリアルキレンエーテルグリコール類、ポリオキシアルキレンポリオール類、水酸基末端ジエンポリマー及びその水素添加誘導体などが、成形品に柔軟性が求められる場合などに賞用される。
【0023】
本発明において、(B)成分の前記水酸基含有重合体は、水酸基含有重合体の数平均分子量と水酸基の含有量との乗数に基づいて求められる、水酸基含有重合体1分子当たりの水酸基の平均結合数が1個以上であることが必須であり、1.5個以上であることが好ましい。1分子当たりの水酸基が1個未満の場合は、架橋性混合物や成形体の架橋形成性が劣ることとなる。
【0024】
なお、本発明における(B)成分の水酸基含有重合体としては、1分子当たりの水酸基の平均結合数が前記範囲を満足する限り、水酸基を含有しない重合体で希釈したものであってもよい。
本発明の可逆架橋性成形体の製造に用いられる(A)成分の前記変性オレフィン系重合体と(B)成分の前記水酸基含有重合体との組成比としては、(A)成分のカルボン酸無水物基数に対する(B)成分の水酸基数の比が0.1〜5であることが必須であり、0.1〜3であることが好ましい。ここで、カルボン酸無水物基数に対する水酸基数の比が前記範囲未満では、架橋性混合物や成形体の架橋形成性が劣ることとなり、一方、前記範囲超過では、成形体の架橋解離性が劣ることとなり、いずれの場合も本発明の目的を達成することができない。
【0025】
本発明において「可逆架橋性」とは、架橋形成性、即ち架橋時の加熱変形率が35%以下で、かつ架橋解離性、即ち架橋解離処理後の加熱変形率が65%以上となる性質を言う。
これらの値が上記の範囲を外れると、架橋の進行が遅くなったり、或は一旦架橋した後は、その解離が起きにくくなったりする等の問題が生じる。
【0026】
架橋形成性のより好ましい範囲は30%以下、更に好ましい範囲は20%以下であり、架橋解離性のより好ましい範囲は80%以上、更に好ましい範囲は90%以上である。
本発明の可逆架橋性成形体の製造に用いる架橋性混合物は、基本的には前記(A)成分と(B)成分からなるが、本発明の効果を損なわない範囲で、前記(A)、(B)成分以外の成分を含有していてもよく、具体的には、例えば、通常用いられる各種の添加剤、例えば、酸化防止剤、紫外線吸収剤、造核剤、中和剤、滑剤、ブロッキング防止剤、分散剤、流動性改良剤、離型剤、難燃剤、着色剤、充填剤等を添加することができる。
【0027】
一方、例えばカルボン酸の金属塩のような従来「反応促進剤」として用いられていたものは、前述の通り、本発明においては可逆架橋を阻害する傾向となるので、添加しない方がよい。
本発明の可逆架橋性成形体の製造方法としては、前記(A)成分と(B)成分とを必須成分とし、必要に応じその他の任意成分を加えて、各成分をヘンシェルミキサー、リボンブレンダー、V型ブレンダー等により均一に混合した後、熱溶融成形する方法、またはこれらの方法により均一に混合した後、一軸又は多軸押出機、ロール、バンバリーミキサー、ニーダー、ブラベンダー等により溶融混練し、可逆架橋性組成物を得た後、必要に応じてペレット化して熱溶融成形する方法等が挙げられる。
【0028】
また、これらの方法によって得られた成形体又は架橋成形体の用済後等のスクラップを必要に応じて切削・粉砕等の前処理を行い、更に要すれば前述の架橋性混合物と混合した上で、熱溶融成形する方法がある。
ここで用いられる具体的な成形方法としては、射出成形、押出成形、中空成形、圧縮成形、回転成形等の各種成形方法により溶融状態で所望の形状に賦形して成形体を挙げることができる。使用済成形体の再利用時等においても、同様の成形法により溶融状態で所望の形状に再度賦形して架橋成形体とすることができる。
【0029】
上記の可逆架橋性混合物及び成形体は、高温での架橋解離、低温での架橋形成という性質を有し、更に高温下では架橋が進行しない、という性質も有している。
本発明の可逆架橋性成形体の製造方法においては、前述の(A)成分の組成、(B)成分の組成、(A)成分と(B)成分の組成比により応じて定まる架橋解離温度以上で成形することが必要である。一般的にこの架橋解離温度はポリオレフィンの成形温度に比して高く、通常230〜300℃の範囲が用いられる。
【0030】
この熱溶融成形温度が該可逆架橋性成形体の架橋解離温度以下で、特に、成形時の滞留時間が長かったり、成形機内の樹脂滞留量が多い場合には架橋が速かに進行してしまい、或は一旦架橋した可逆架橋性押出成形体を再度成形しようとする場合には架橋の解離が不十分となるため、成形体の外観が悪化し、また架橋の進行による、樹脂の溶融粘度の上昇が起こって、成形機の負荷も高くなるという問題等も発生し、本発明の目的を十分達成する事ができない。
【0031】
なお、このようにして得られた可逆架橋性成形体を架橋させるためには、実質的に架橋が進行する温度条件として、該可逆架橋性成形体のガラス転移温度以上、該可逆架橋性成形体の架橋解離温度未満を用いる。この温度範囲としては、通常0〜200℃で、該成形体の融点以下の温度を用いるのがよい。
また架橋処理温度がこの成形体のガラス転移温度未満の場合には、該可逆架橋性成形体の分子運動が停止しているか、または著しく遅いため架橋が実質的に進行せず、またたとえ進行したとしてもその架橋速度は著しく遅い。一方、架橋処理温度が架橋解離温度以上では、該可逆架橋性成形体の分子運動は活発であるが、架橋解離温度を超えているために架橋反応が進行せず、たとえ進行したとしてもその架橋の程度は著しく低いので、いずれの場合も本発明の目的を達成することが困難である。
【0032】
以上のようにして製造された可逆架橋性成形体は、成形性、加工安定性に優れ、かつ、簡便な架橋処理により架橋させる事ができ、更には該成形体の架橋体や使用済み成形体も同様の成形法によって溶融成形を行うことにより、再び架橋性成形体を得ることができる。
また、本発明においては可逆架橋性混合物及び成形体の製造に際しては、実質的に架橋していない状態で成形する事が可能であるので、前述の通り熱可塑性樹脂において通常用いられる各種の成形法、即ち、射出成形、押出成形、中空成形、圧縮成形、回転成形等を適用することができる。中でも、押出成形法を用いるのが架橋制御等の点で有利であり、好適である。
【0033】
【実施例】
以下、本発明を実施例を用いて更に具体的に説明するが、本発明はその要旨を超えない限り、以下の実施例によって限定されるものではない。
尚、実施例及び比較例で用いた(A)成分の変性オレフィン系重合体、(B)成分の水酸基含有重合体、有機カルボン酸の金属塩((C)成分)、水架橋性樹脂((D)成分)、安定剤((E)成分)は、以下に示すものである。
【0034】
(A)成分
A−1;エチレン−マレイン酸無水物−アクリル酸エチル三元共重合体(赤外吸収スペクトルにより測定したマレイン酸無水物単位含有量2.4重量%、アクリル酸エチル単位含有量7.5重量%、カルボン酸無水物基数に対するカルボン酸エステル基数の比3.1、ゲルパーミエーションクロマトグラフィーにより測定した数平均分子量19300、数平均分子量とマレイン酸無水物単位含有量の乗数に基づいて求めた変性オレフィン系重合体1分子当たりのカルボン酸無水物基の平均結合数4.7個、住友化学工業社製、「ボンダインLX4110」)
【0035】
A−2;エチレン−マレイン酸無水物−アクリル酸エチル三元共重合体(赤外吸収スペクトルにより測定したマレイン酸無水物単位含有量2.5重量%、アクリル酸エチル単位含有量12.5重量%、カルボン酸無水物基数に対するカルボン酸エステル基数の比4.9、ゲルパーミエーションクロマトグラフィーにより測定した数平均分子量19800、数平均分子量とマレイン酸無水物単位含有量の乗数に基づいて求めた変性オレフィン系重合体1分子当たりのカルボン酸無水物基の平均結合数5.0個、住友化学工業社製、「ボンダインTX8030」)
【0036】
A−3;エチレン−ブテン−1共重合体(メルトフローレート9g/10分、密度0.925g/cm3 、日本ポリケム(株)製「Z−50MG」)100重量部に対し、マレイン酸無水物0.85重量部、アクリル酸ステアリル3.0重量部、2,5−ジメチル−2,5−ビス(ターシャリーブチルパーオキシ)ヘキサン0.1重量部を添加してヘンシェルミキサーにて均一に混合後、二軸押出機((株)池貝製、PCM−30、D=30mm、L/D=32)にて溶融グラフト反応を行い、エチレン−ブテン−1共重合体のマレイン酸無水物とアクリル酸ステアリルとによる変性オレフィン系重合体を得た。なお、押出機はC1 :150℃、C2 :190℃、C3 〜D:230℃、Ns:200rpm、Q:10kg/hrの条件にて運転した。得られた変性オレフィン系重合体は、メルトフローレート4.2g/10分、再沈精製処理を行い未グラフト物を除いた後に赤外吸収スペクトルにより測定したマレイン酸無水物単位含有量0.64重量%、アクリル酸ステアリル単位含有量1.6重量%、カルボン酸無水物基数に対するカルボン酸エステル基数の比0.76、ゲルパーミエーションクロマトグラフィーにより測定した数平均分子量25700、数平均分子量とマレイン酸無水物単位含有量の乗数に基づいて求めた変性オレフィン系重合体1分子当たりのカルボン酸無水物基の平均結合数1.7個であった。
【0037】
A−4(比較例用);エチレン−マレイン酸無水物−アクリル酸エチル三元共重合体(赤外吸収スペクトルにより測定したマレイン酸無水物単位含有量0.8重量%、アクリル酸エチル単位含有量30.0重量%、カルボン酸無水物基数に対するカルボン酸エステル基数の比36.7、ゲルパーミエーションクロマトグラフィーにより測定した数平均分子量18700、数平均分子量とマレイン酸無水物単位含有量の乗数に基づいて求めた変性オレフィン系重合体1分子当たりのカルボン酸無水物基の平均結合数1.5個、住友化学(株)製、「ボンダインAX8390」)
【0038】
(B)成分
B−1;水酸基末端ポリブタジエンの水素添加物(水酸基含有量2.0重量%、数平均分子量1000、数平均分子量と水酸基含有量の乗数に基づいて求めた水酸基含有重合体1分子当たりの水酸基の平均結合数1.6個、日本曹達社製「ニッソーPB GI−1000」)
【0039】
B−2;低分子量ポリオレフィンポリオール(水酸基含有量1.3重量%、数平均分子量1500、数平均分子量と水酸基含有量の乗数に基づいて求めた水酸基含有重合体1分子当たりの水酸基の平均結合数1.1個、三菱化学社製「ポリテールH」)
【0040】
(C)成分(比較例用;カルボン酸金属塩類)
C−1;酢酸亜鉛二水和物
C−2;ステアリン酸カルシウム
C−3;ジブチル錫ジラウレート1重量部を含有するマスターバッチ
【0041】
(D)成分(比較例用)
低密度ポリエチレン(メルトフローレート2g/10分、密度0.919g/cm3 、日本ポリケム(株)製「EH−30」)100重量部、ビニルトリメトキシシラン2.0重量部、ジクミルパーオキサイド0.1重量部をヘンシェルミキサーにて均一に混合後、単軸押出機(D=40mm、L/D=28)にてシラングラフトポリエチレンを得た。なお、押出機はC1 :120℃、C2 :160℃、C3 〜D:200℃、Ns:70rpm、Q:7kg/hrの条件にて運転した。得られたシラングラフトポリエチレンは、メルトフローレート1.3g/10分、蛍光X線により測定したビニルトリメトキシシラン単位含有量0.7重量%であった。
【0042】
(E)成分(安定剤)
E−1;IRGANOX1010(チバガイギー社製、フェノール系酸化防止剤)
E−2;Irgafos168(チバガイギー社製、リン系酸化防止剤)
実施例及び比較例において用いた評価方法を以下に記載する。
【0043】
加工安定性
単軸押出機(D=40mm、L/D=28)を用いて48時間の連続押出を行い、押出量の経時変化を測定した。尚、押出機条件はC1 :170℃、C2 :210℃、C3 〜D:250℃、Ns:70rpmに設定し、諸条件は押出開始から終了までの間、一定とした。
但し、(D)成分を用いた場合に限り、押出機条件の温度設定をC1 :150℃、C2 :170℃、C3 〜D:190℃に変更した。
【0044】
シート成形
シート成形機(D=20mm、L/D=20)を用いて、1mm厚のシート成形を行った。成形条件は、C1 :170℃、C2 :210℃、C3 〜D:250℃、Ns:50rpmとした。但し、(D)成分を用いた場合に限り、成形条件の温度設定をC1 :150℃、C2 :170℃、C3 〜D:190℃に変更した。
【0045】
電線成形
電線被覆装置(D=50mm、L/D=25)を用いて、1mm径の銅線7本を撚り合わせた公称断面積5.5mm2 の導体上に厚み4mmの被覆層を成形した。成形条件は、C1 :170℃、C2 :210℃、C3 〜D:250℃、押出線速5m/分とした。但し、(D)成分を用いた場合は、成形条件の温度設定をC1 :150℃、C2 :170℃、C3 〜D:190℃に変更した。
【0046】
パイプ成形
パイプ成形機(D=60mm、L/D=24)を用いて外径34mm、肉厚5mmのパイプを成形した。
成形条件は、C1 :170℃、C2 :210℃、C3 〜D:250℃、Ns:50rpmとした。但し、(D)成分を用いた場合は、成形条件の温度設定をC1 :150℃、C2 :170℃、C3 〜D:190℃に変更した。
【0047】
架橋処理
内温を80℃に保ったオーブン中にて、可逆架橋性成形体を所定時間加温することにより行った。但し、(D)成分を用いた場合は、成形体を80℃の温水中に所定時間浸漬する方法に変更した。
【0048】
加熱変形
JIS C−3005に基づいて測定した。
熱間内圧クリープ試験
JIS K−6762に基づいて測定した。
【0049】
熱融着試験
パッド融着法により、加熱温度200℃で1分間融着接合し、1分間放冷したのち融着治具を取り外した。該パイプより、接合部が中央になるように、JISK−6301に基づく2号ダンベルを作成し、引張速度50mm/分で引張試験を行い、引張強度、引張伸びを求めた。
【0050】
架橋形成性
実施例及び比較例で得た可逆架橋性組成物のペレットを230℃で、5分間予熱した後、100kg/cm2 の加圧下で5分間加熱し、120kg/cm2 の加圧下で冷却することにより作製した厚さ1mmのプレス成形試験片を、80℃で24時間加熱処理して架橋させた後、JIS C3005(加熱変形)に準拠して、140℃、1kgfの条件で加熱変形率を測定した。
【0051】
架橋解離性
上と同じペレットを230℃で、5分間予熱した後、100kg/cm2 の加圧下で5分間加熱し、120kg/cm2 の加圧下で冷却することにより作製した厚さ1mmのプレス成形試験片を、80℃で24時間加熱処理して架橋させた後、再度、230℃で、5分間予熱した後、100kg/cm2 の加圧下で5分間加熱し、120kg/cm2 の加圧下で冷却した後、JIS C3005(加熱変形)に準拠して、140℃、1kgfの条件で加熱変形率を測定した。
【0052】
ガラス転移温度(Tg)
上で得られたペレット又は成形体を230℃で5分間予熱した後、100kg/cm2 の加圧下で5分間加熱し、120kg/cm2 の加圧下で冷却して厚さ2mm、幅12.7mm、長さ63mmのプレス成形試験片を作成した。この試験片を用いて、レオメトリックサイエンティフィック・エフ・イー(株)製メカニカルスペクトロメータ(RMS 605型)にて、温度−150℃〜0℃、歪み0.1%、周波数6.28ラジアン/秒の条件で、損失弾性率G″の温度依存性を測定し、そのピークを示す温度としてガラス転移温度(Tg)を求めた。
【0053】
<実施例1>
(A)成分としてA−1と、(E)成分としてE−1及びE−2とをヘンシェルミキサーにて均一に混合して得た混合物を二軸混練機((株)池貝製、PCM−45、D=45mm、L/D=34)のホッパー口より供給し、予め約65℃に加熱し粘度を下げておいたB−1をB成分として、ギアポンプにより混練機途中から供給し、可逆架橋性組成物のペレットを得た。
【0054】
各成分の組成比はA−1:82.8重量%、B−1:17.2重量%とし、その合計100重量部に対しE−1:0.1重量部、E−2:0.1重量部になるように調整した。(カルボン酸無水物基数に対する水酸基数の比:1.00)
また、押出機の条件はC1 :50℃、C2 :150℃:C3 〜D:230℃、Ns:200rpm、Q:20kg/hrに設定し、(B)成分はC3 ゾーンから供給した。
得られた可逆架橋性組成物のペレットを用い、加工安定性を評価した。結果を表1に示す。
【0055】
<実施例2>
(A)成分としてA−3:93.5重量%と(B)成分としてB−2:6.5重量%(カルボン酸無水物基数に対する水酸基数の比:0.81)の配合比とし、その合計100重量部に対して(E)成分としてE−1:0.1重量部、E−2:0.1重量部を用い、この各成分をヘンシェルミキサーで混合して得た混合物を使用して加工安定性を評価した。結果を表1に示す。
<比較例1>
(C)成分としてC−1を加えたこと以外は実施例1と同様にして、可逆架橋性組成物のペレットを得た。このペレットを用いて加工安定性を評価した。結果を表1に示す。
なお、各成分の組成比はA−1:82.8重量%、B−1:17.2重量%の合計100重量部に対しC−1:0.06重量部、E−1:0.1重量部、E−2:0.1重量部になるように調整した。(カルボン酸無水物基数に対する水酸基数の比:1.00)
【0056】
<比較例2>
(D)成分としてD−1を100重量部用い、これに対して(C)成分としてC−3を5重量部、(E)成分としてE−1:0.1重量部、E−2:0.1重量部をそれぞれ加えてヘンシェルミキサーにて均一に混合した組成物を用いて加工安定性を評価した。結果を表1に示す。
【0057】
<実施例3>
(A)成分としてA−1を、(E)成分としてE−1及びE−2を用い、これらをヘンシェルミキサーで均一に混合して得た混合物を二軸混練機((株)池貝、PCM−45、D=45mm、L/D=34)のホッパー口より供給し、予め約65℃に加熱し粘度を下げておいたB−1をB成分としてギアポンプにより混練機途中から供給し、可逆架橋性組成物のペレットを得た。
【0058】
各成分の組成比はA−1:92.3重量%、B−1:7.7重量%とし、その合計100重量部に対しE−1:0.1重量部、E−2:0.1重量部になるように調整した。(カルボン酸無水物基数に対する水酸基数の比:0.40)
又、押出機条件はC1 :50℃、C2 :150℃、C3 〜D:230℃、Ns:200rpm、Q:20kg/hrに設定し、(B)成分はC3 ゾーンから供給した。
【0059】
得られた可逆架橋性組成物のペレットを用い、シート押出を行い、得られた押出シートを3時間又は8時間かけて架橋処理を行い加熱変形率を測定した。
8時間架橋処理を行った押出シートについてはシートペレタイザーにてペレット化した後、再度シート押出を行い、得られた押出シートについて再び3時間、8時間の架橋処理を行い加熱変形率を測定した。結果を表2に示す。
【0060】
<実施例4>
(A)成分としてA−2:88.2重量%、(B)成分としてB−2:11.8重量%(カルボン酸無水物基数に対する水酸基数の比:0.40)の配合比とし、その合計100重量部に対して、(E)成分としてE−1:0.1重量部、E−2:0.1重量部を用い、これらをヘンシェルミキサーにて均一に混合して得た混合物を用いたこと以外は実施例3と同様にしてペレットの製造及びシート押出を行い押出シートを作成した。この押出シートについて実施例3と同様に架橋処理及び再シート化・再架橋処理を行いそれぞれ加熱変形を測定した。結果を表2に示す。
【0061】
<比較例3>
(A)成分としてA−4を、(B)成分としてB−1を用い、A−4:93.5重量%、B−1:6.5重量%の組成比になるように調整(カルボン酸無水物基数に対する水酸基数の比:1.00)したこと以外は実施例3と同様に操作してシート成形及び架橋処理等を行った。結果を表2に示す。
【0062】
<比較例4>
(A)成分と(B)成分の合計100重量部に対して(C)成分としてC−1:0.06重量部を用いたこと以外は実施例3と同様にして押出シートの作成及び架橋性の評価を行った。結果を表2に示す。
【0063】
<比較例5>
実施例4の各成分に加え、更に(A)成分、(B)成分の合計100重量部に対し(C)成分としてC−2:2.0重量部を用いたこと以外は実施例4と同様に操作して押出シートの作成及び架橋性の評価を行った。結果を表2に示す。
【0064】
<比較例6>
(A)成分、(B)成分に代えて(D)成分としてD−1:100重量部を用い、これに対し(C)成分としてC−3:5重量部、更に(E)成分としてE−1:0.1重量部及びE−2:0.1重量部を用いたこと以外は実施例4と同様に操作した。結果を表2に示す。
なお、架橋押出シートの再成形は押出負荷が非常に高く成形する事ができなかった。
【0065】
<実施例5>
(A)成分としてA−1:88.7重量%、(B)成分としてB−2:11.3重量%の合計100重量部に対して(E)成分としてE−1:0.1重量部、E−2:0.1重量部を用い、これらをヘンシェルミキサーにて均一に混合して得た混合物を二軸混練機にて溶融混練し、可逆架橋性組成物のペレットを得た。
【0066】
押出機条件はC1 :50℃、C2 :150℃、C3 〜D:230℃、Ns:200rpm、Q:20kg/hrに設定した。
得られた可逆架橋性組成物のペレットを用い、プレス成形した後8時間の架橋処理を行った上でJIS K−6911に基づき体積固有抵抗の測定を行った。また、該ペレットを用いて電線成形を行いこれを8時間架橋処理した後の加熱変形を測定した。結果を表3に示す。
8時間架橋処理を行った被覆電線から剥離して得た樹脂部分を粉砕した後、再度溶融してシート成形を行った。得られたシートを再び8時間架橋処理して、加熱変形を測定した。結果を表3に示す。
【0067】
<実施例6>
(A)成分としてA−2を、(B)成分としてB−1を用い、A−2:88.2重量%、B−1:11.8重量%の組成比になるように調整(カルボン酸無水物基数に対する水酸基数の比:0.40)したこと以外は実施例1と同様にして可逆架橋性組成物のペレットを製造し、更にこれを用いて実施例5と同様にしてプレス成形、電線成形を行った。
これらの試料について上記実施例5と同様に評価を行った。結果を表3に示す。
【0068】
<比較例7>
(A)成分と(B)成分の合計100重量部に対して(C)成分としてC−1:0.06重量部を更に加えたこと以外は実施例5と同様に成形・評価を実施した。結果を表3に示す。
【0069】
<比較例8>
(A)成分、(B)成分に代えて(D)成分としてD−1:100重量部を用い、これに対し(C)成分としてC−3:5重量部、更に(E)成分としてE−1:0.1重量部及びE−2:0.1重量部を用い、これらをロールミルで温度120℃にて7分間混合した後、プレス成形を行った。得られたシートを8時間架橋処理を行った後にJIS K−6911に従って体積固有抵抗を測定した。結果を表3に示す。又同じ組成・配合で電線成形を行い、同様に8時間架橋処理した後の加熱変形を測定した。更に前記実施例5と同様にして被覆部分の再使用を試みたが、押出負荷が非常に高く成形することができなかった。結果を表3に示す。
【0070】
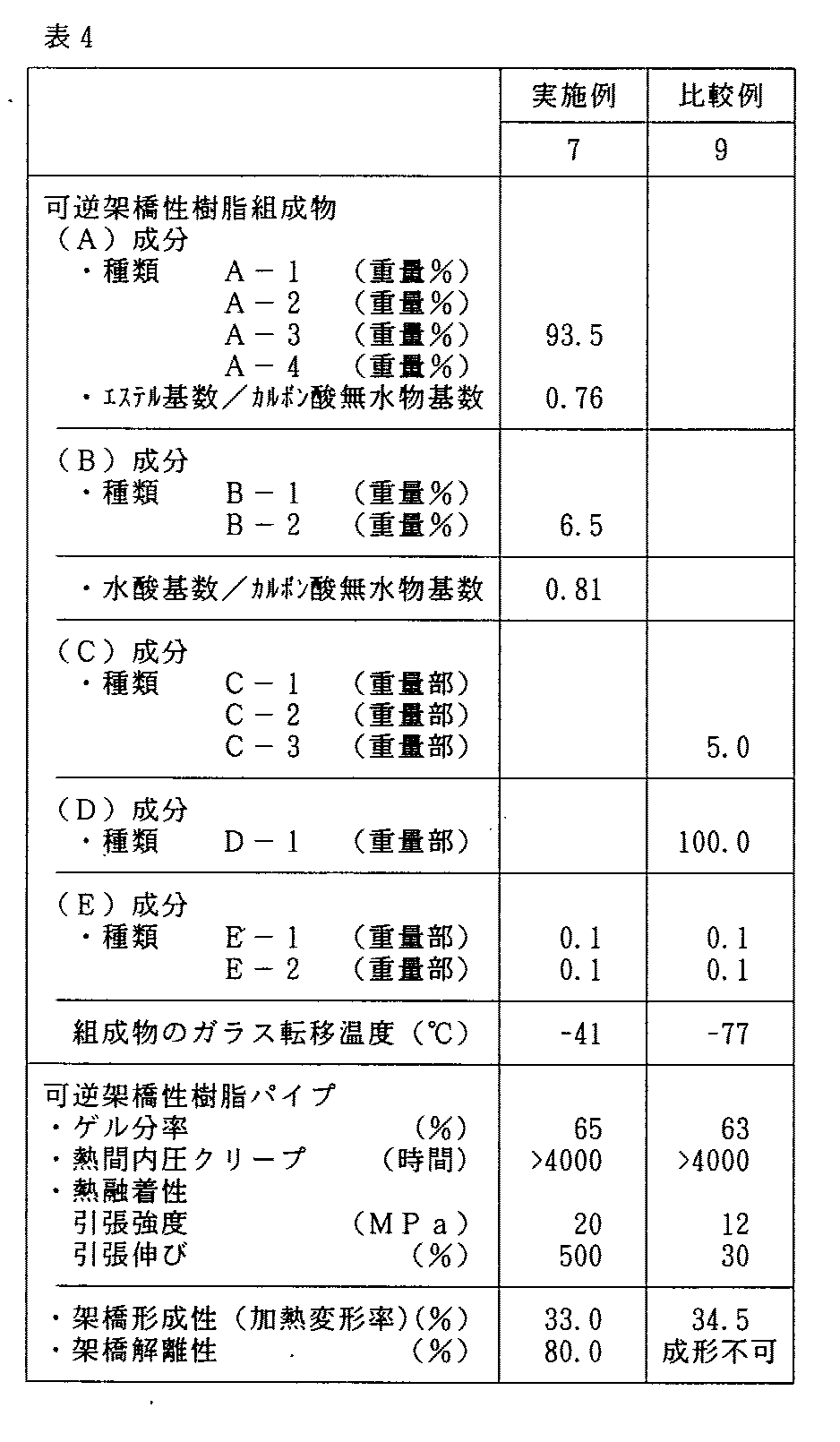
<実施例7>
実施例2と同じ組成の配合物をヘンシェルミキサーにて均一に混合して得た架橋性混合物を用いてパイプ成形を行い、得られたパイプを8時間架橋処理した。
この架橋パイプを用い、熱間内圧クリープ試験、熱融着試験を行った。結果を表4に示す。
【0071】
<比較例9>
(A)成分、(B)成分に代えて(D)成分としてD−1:100重量部を用い、これに対し(C)成分としてC−3:5重量部、更に(E)成分としてE−1:0.1重量部及びE−2:0.1重量部を用い、これらをヘンシェルミキサーにて均一に混合して得た混合物を用いたこと以外は実施例7と同様にしてパイプ成形を行い、評価を行った。結果を表4に示す。
【0072】
<結果の評価>
上記の実施例、比較例から、以下の諸点が判明した。
(1)表1において(C)成分を含み、可逆架橋性のない比較例1及びベース樹脂そのものが本発明の範囲外の比較例2では、実施例と比べて、押出量の経時的な低下が見られ、加工の安定性が乏しい。
(2)表2において、エステル基とカルボン酸基数の比が本発明の範囲外である比較例3は、加熱変形率が大きく、架橋性が劣っている。また、可逆架橋性のない比較例2、3では、再成形時の架橋性が不十分である。樹脂が本発明の範囲外である比較例6の場合は、架橋後の再成形が不可能であった。
(3)表3においても、上記と同様、可逆架橋性のない比較例7は再成形時に外観不良を起こし、また樹脂が範囲外の比較例8は、再成形できなかった。
(4)表4においても、本発明の範囲外の比較例9は、熱融着性が著しく劣っていた。
【0073】
【表1】
【0074】
【表2】
【0075】
【表3】
【0076】
【表4】
【0077】
【発明の効果】
本発明は、押出成形性、加工安定性に優れ、且つ簡便な架橋処理、再利用可能な熱可逆架橋性押出成形体の製造方法を提供する事ができる。従って、本発明の熱可逆架橋性押出成形体は、熱可塑性樹脂において通常用いられる成形法により溶融状態で所望の形状に賦形する事によって得られ、又長時間の成形においても成形機内での架橋は実質的に進行しない為、スコーチを招く事はごくまれである。
又、使用済成形体の再利用等においても、同様の成形法により溶融状態で所望の形状に再度賦形して再度架橋成形体とする事ができる為に、環境保護や省資源等の立場からも有用な押出成形体となる。
更には、このような特徴を活かして、架橋フィルム、架橋チューブ、或いはブロー成形、射出成形等に応用する事も可能である。[0001]
BACKGROUND OF THE INVENTION
The present invention relates to a method for producing a molded body, and more particularly, to a method for producing a so-called thermoreversible crosslinkable molded body that can repeatedly form crosslinks at low temperatures and dissociate crosslinks at high temperatures.
In particular, the present invention relates to a method for producing a thermoreversible crosslinkable molded article that is excellent in moldability and processing stability, can be easily crosslinked, and can be reused.
[0002]
[Prior art]
Olefin resins such as polyethylene and polypropylene are excellent in moldability, mechanical strength, transparency, chemical resistance, etc., and in a molten state by various molding methods such as extrusion molding, injection molding, hollow molding, compression molding, and rotational molding. It is shaped into the desired shape and is widely used in various fields. Also, to improve heat resistance and improve mechanical strength at high temperatures, blend organic peroxide, irradiate with radiation, or silanol condensation reaction. It is also frequently used as a cross-linked product subjected to a cross-linking treatment by use of the above.
[0003]
On the other hand, from the standpoints of environmental protection and resource saving, the reuse of used resin is increasingly required. However, this crosslinked resin that has undergone crosslinking treatment is no longer thermoplastic. Reuse by melt molding is impossible, and there is a strong demand for imparting thermoplasticity to this crosslinked product.
[0004]
In order to solve this problem, several reversible cross-linking methods have been proposed in which a cross-link is formed at a low temperature and the cross-link is dissociated at a high temperature to make it thermoplastic. For example, as an olefin resin composition having a high crosslink formation reaction rate and a high crosslink dissociation reaction rate and having excellent thermoreversible crosslinkability, JP-A-6-57062 and 7-94029 disclose unsaturated carboxylic acids. Anhydride-modified olefin resin, polyhydric alcohol compound having at least two hydroxyl groups in the molecule, for example, glycols such as ethylene glycol, alcohols such as 1,4-butanediol, saccharides such as sorbitol, trimethylol Polyoxyalkylene compounds such as propane, polyglycerol alkyl esters such as diglycerol monostearate, sorbitan alkyl esters such as sorbitan monostearate, and a plurality of hydroxyl groups in the molecule such as ethylene-hydroxyethyl acrylate copolymer A polymer having a carboxylic acid Olefin resin composition comprising a reaction accelerator such as a metal salt are disclosed.
[0005]
In this type of thermoreversible crosslinkable composition based on the reaction between a carboxylic anhydride group and a hydroxyl group, according to the study by the present inventors, basically, one molecule of carboxylic anhydride group and one molecule The reaction of producing the carboxylic acid monoester by the reaction of the hydroxyl group of 1 and the reaction of producing a carboxylic acid diester by further reacting one molecule of the produced carboxylic acid monoester with one molecule of hydroxyl group, Carboxylic acid monoester formation reaction has good thermoreversibility, but the latter carboxylic acid diester formation reaction has poor thermoreversibility, and further, in the above-mentioned prior art, the carboxylic acid metal salt is promoted. Although the initial degree of cross-linking is high due to the effect, the acid anhydride group reacts with the organic carboxylic acid metal salt to form a metal salt. As a result of this reaction, the formation of esters is reduced, so that the degree of crosslinking having heat resistance as a whole is lowered. As a result, the heat resistance of the molded product is inferior, and the metal salt of the carboxylic acid is thermally reversible. It has been found that there is a problem that the dissociation property of the cross-linking is deteriorated in order to promote the formation of a diester having poor properties.
On the other hand, when trying to obtain a thermoreversible crosslinkable molded product without using such a carboxylic acid metal salt, a molded article having a good appearance may not be obtained depending on molding conditions.
[0006]
[Problems to be solved by the invention]
Based on the results of the above-mentioned studies on the prior art, the present inventor has excellent heat moldability and processing stability without substantially using a metal salt of an organic carboxylic acid, and excellent crosslinkability and crosslinkability. As a result of intensive studies on a method for producing a reversible crosslinkable molded article, the present invention has been achieved. That is, an object of the present invention is to provide a method for producing a thermoreversible crosslinkable molded article having good quality and excellent moldability, processing stability, and crosslink formation / dissociation properties.
[0007]
[Means for Solving the Problems]
The inventor of the present invention melts a mixture obtained by blending a specific amount of a specific hydroxyl group-containing polymer with a specific modified olefin polymer modified with an unsaturated carboxylic acid anhydride and an unsaturated carboxylic acid ester under specific conditions. The inventors have found that the above-described object can be achieved by molding, and completed the present invention.
[0008]
That is, the gist of the present invention consists of the following components (A) and (B), and the ratio of the number of hydroxyl groups in component (B) to the number of carboxylic anhydride groups in component (A) is 0.1 to 5. The present invention resides in a method for producing a reversible crosslinkable molded article, which comprises hot melt molding a crosslinkable mixture at a temperature equal to or higher than the crosslinking dissociation temperature.
[0009]
(A) A modified olefin polymer modified with an unsaturated carboxylic acid anhydride and an unsaturated carboxylic acid ester, wherein the average number of bonds of carboxylic acid anhydride groups per molecule is one or more, and Modified olefin polymer in which the ratio of the number of carboxylic acid ester groups to the number of carboxylic anhydride groups in the modified olefin polymer is 0.5 to 20
(B) A hydroxyl group-containing polymer having an average number of hydroxyl groups per molecule of 1 or more.
[0010]
The gist of the present invention is that the modified olefin polymer of component (A) is an ethylene-maleic anhydride- (meth) acrylic acid alkyl ester copolymer, The average number of bonds of carboxylic acid anhydride groups per molecule of the component (A) modified olefin polymer is 1.5 or more, and the average number of hydroxyl groups per molecule of the component (B) hydroxyl group-containing polymer The present invention also exists in the above-described method for producing a reversibly crosslinkable molded article having a bond number of 1.5 or more, and another gist of the present invention is that any one of the above hot melt molded articles is obtained. A method for producing a reversible cross-linkable molded body in which a crosslinking treatment is performed at a temperature not lower than the glass transition temperature and lower than the cross-linking dissociation temperature of the molded body, or the production of any one of the above reversible cross-linkable molded bodies in which hot melt molding is extrusion Also exists in the method. Another gist of the invention is characterized in that the reversibly crosslinkable molded product obtained by any one of the above production methods, and scrap of the molded product are hot melt molded at a molding temperature equal to or higher than the crosslinking dissociation temperature of the molded product. It also exists in reversible crosslinkable shaped bodies.
[0011]
Furthermore, another gist of the present invention consists of the following components (A) and (B), and the ratio of the number of hydroxyl groups in component (B) to the number of carboxylic anhydride groups in component (A) is 0.1. Reversible crosslinkability, characterized in that the crosslinkable mixture of -5 and the scrap of the molded article according to claim 6 are hot melt molded at a temperature higher than the higher of the crosslink dissociation temperatures of both. It also exists in the manufacturing method of a molded object.
[0012]
(A) A modified olefin polymer modified with an unsaturated carboxylic acid anhydride and an unsaturated carboxylic acid ester, wherein the average number of bonds of carboxylic acid anhydride groups per molecule is one or more, and Modified olefin polymer in which the ratio of the number of carboxylic acid ester groups to the number of carboxylic anhydride groups in the modified olefin polymer is 0.5 to 20
(B) A hydroxyl group-containing polymer having an average number of hydroxyl groups per molecule of 1 or more.
[0013]
DETAILED DESCRIPTION OF THE INVENTION
As the modified olefin polymer modified with the unsaturated carboxylic acid anhydride and unsaturated carboxylic acid ester of the component (A) in the present invention, basically an α-olefin and an ethylenically unsaturated carboxylic acid anhydride are used. A graft copolymer of a terpolymer of ethylenically unsaturated carboxylic acid ester and a binary copolymer of α-olefin and ethylenically unsaturated carboxylic acid anhydride with an ethylenically unsaturated carboxylic acid ester, α -Graft of binary copolymer of olefin and ethylenically unsaturated carboxylic acid ester with ethylenically unsaturated carboxylic acid anhydride, ethylenically unsaturated carboxylic acid anhydride and ethylenically unsaturated of α-olefin polymer It is preferable to use a graft product with a carboxylic acid ester.
[0014]
Examples of the α-olefin in the former terpolymer include ethylene, propylene, butene-1,3-methylbutene-1, pentene-1,3-methylpentene-1,4-methylpentene-1, and hexene. 1, octene-1, decene-1, and the like.
Examples of the ethylenically unsaturated carboxylic acid anhydride include 2-octen-1-yl succinic anhydride, 2-dodecen-1-yl succinic anhydride, and 2-octadecene-1-yl succinic anhydride. , Maleic anhydride, 2,3-dimethylmaleic anhydride, bromomaleic anhydride, dichloromaleic anhydride, citraconic anhydride, itaconic anhydride, 1-butene-3,4-dicarboxylic anhydride 1-cyclopentene-1,2-dicarboxylic anhydride, 1,2,3,6-tetrahydrophthalic anhydride, 3,4,5,6-tetrahydrophthalic anhydride, exo-3,6-epoxy- 1,2,3,6-tetrahydrophthalic anhydride, 5-norbornene-2,3-dicarboxylic anhydride, methyl-5-norbornene-2,3-dicarboxylic anhydride, end -Bicyclo [2.2.2] oct-5-ene-2,3-dicarboxylic anhydride, bicyclo [2.2.2] oct-7-ene-2,3,5,6-tetracarboxylic anhydride Thing etc. are mentioned.
[0015]
The ethylenically unsaturated carboxylic acid ester is preferably an ester of an alkyl group having about 1 to 20 carbon atoms, such as methyl (meth) acrylate, ethyl (meth) acrylate, propyl (meth) acrylate, (meth ) Butyl acrylate, hexyl (meth) acrylate, octyl (meth) acrylate, 2-ethylhexyl (meth) acrylate, lauryl (meth) acrylate, stearyl (meth) acrylate, dimethyl maleate and the like. Here, “(meth) acrylic acid” refers to acrylic acid and methacrylic acid.
[0016]
As the former terpolymer, in addition to the terpolymer of the α-olefin, the ethylenically unsaturated carboxylic acid anhydride and the ethylenically unsaturated carboxylic acid ester, (meth) acrylic acid , Ethylenically unsaturated carboxylic acid compounds such as maleic acid, ethylenically unsaturated ester compounds such as vinyl acetate, (meth) acrylamide, ethylenically unsaturated amide compounds such as N-methyl (meth) acrylamide, styrene, (meth) It may be a quaternary or higher multi-component copolymer obtained by copolymerizing other ethylenically unsaturated compounds such as acrylonitrile.
[0017]
These copolymers can be produced by polymerization methods such as bulk, solution, suspension and the like.
As the binary copolymer of the α-olefin and the ethylenically unsaturated carboxylic acid anhydride and the binary copolymer of the α-olefin and the ethylenically unsaturated carboxylic acid ester in the latter graft, The same α-olefin, ethylenically unsaturated carboxylic acid anhydride, and ethylenically unsaturated carboxylic acid ester as mentioned in the ternary copolymer may be mentioned, and as the α-olefin polymer in the latter graft Is, for example, a homopolymer of ethylene such as low density, medium density, and high density polyethylene (branched or linear), ethylene, propylene, butene-1,3-methylbutene-1, pentene-1,3- Copolymers with α-olefins such as methylpentene-1,4-methylpentene-1, hexene-1, octene-1 and decene-1, ethylene and vinyl acetate Ethylene resins such as vinyl esters such as Nyl, copolymers with other monomers such as (meth) acrylic acid or their esters, propylene homopolymers, propylene and ethylene, butene-1,3-methylbutene -1, copolymers of α-olefins such as pentene-1,3-methylpentene-1,4-methylpentene-1, hexene-1, octene-1, decene-1, propylene, isoprene, Propylene resins such as copolymers with other monomers such as diene compounds such as 3-butadiene, 1,3-pentadiene, 1,4-hexadiene, 1,5-hexadiene, 1,9-decadiene, and other butenes Examples include homopolymers and copolymers of α-olefins such as -1,4-methylpentene-1 and hexene-1.
Examples of the ethylenically unsaturated carboxylic acid ester and ethylenically unsaturated carboxylic acid anhydride to be grafted include the same as those mentioned for the terpolymer.
[0018]
These graft bodies can be produced by grafting methods such as melt-kneading, solution and suspension.
As the modified olefin polymer of the component (A) in the present invention, a terpolymer of ethylene, maleic anhydride, (meth) acrylic acid alkyl ester, and a maleated α-olefin polymer. A graft product of an acid anhydride and a (meth) acrylic acid alkyl ester is preferable, and a terpolymer of ethylene, maleic anhydride, and methyl or ethyl (meth) acrylate is particularly preferable.
[0019]
In the present invention, the modified olefin polymer as the component (A) has a content of the unsaturated carboxylic acid anhydride unit of 0.1% by weight or more, particularly 0.5% by weight or more. Preferably, the average number of bonds as carboxylic anhydride groups per molecule of the modified olefin polymer, which is determined based on the multiplier of the number average molecular weight of the modified olefin polymer and the content thereof, is 1 or more. It is essential that the number is 1.5 or more. When the average number of bonds is less than 1, the crosslinkability as a composition is inferior.
[0020]
In the present invention, the modified olefin polymer of component (A) is a ratio of the number of carboxylic acid ester groups derived from the unsaturated carboxylic acid ester to the number of carboxylic acid anhydride groups derived from the unsaturated carboxylic acid anhydride. Is required to be 0.5 to 20, preferably 0.5 to 15. If this ratio is less than the above range, the crosslinking dissociation property as a molded article is inferior, while if it exceeds the above range, the crosslinking formability is inferior.
[0021]
As the modified olefin polymer of the component (A) in the present invention, the average number of carboxylic acid anhydride groups per molecule and the ratio of the number of carboxylic acid ester groups to the number of carboxylic acid anhydride groups are within the above range. As long as the above is satisfied, the modified olefin polymer may be diluted with an unmodified olefin polymer.
[0022]
In the present invention, the hydroxyl group-containing polymer of the component (B) which forms a crosslink by binding to the carboxylic anhydride group of the unsaturated carboxylic acid anhydride and the unsaturated carboxylate ester-modified olefin polymer of the component (A) in the present invention. As, for example, ethylene- (meth) acrylic acid 2-hydroxyethyl copolymer, (meth) acrylic acid 2-hydroxyethyl graft polyethylene, saponified ethylene-vinyl acetate copolymer, polyvinyl alcohol, low molecular weight polyolefin polyols , Polyalkylene ether glycols, polyoxyalkylene polyols, hydroxyl-terminated diene polymers and hydrogenated products or adipates thereof, hydroxyl-terminated polycaprolactones, etc., and these have a number average molecular weight of 500 to 10,000. Is preferred. Among them, low-molecular-weight polyolefin polyols, polyalkylene ether glycols, polyoxyalkylene polyols, hydroxyl-terminated diene polymers, hydrogenated derivatives thereof, and the like are used for cases where flexibility is required for molded products.
[0023]
In the present invention, the hydroxyl group-containing polymer of the component (B) is an average bond of hydroxyl groups per molecule of the hydroxyl group-containing polymer, which is determined based on a multiplier between the number average molecular weight of the hydroxyl group-containing polymer and the hydroxyl group content. The number is essential to be 1 or more, and preferably 1.5 or more. When the number of hydroxyl groups per molecule is less than 1, the crosslinkability of the crosslinkable mixture or the molded product is inferior.
[0024]
The hydroxyl group-containing polymer of the component (B) in the present invention may be a polymer diluted with a polymer not containing a hydroxyl group as long as the average number of hydroxyl groups per molecule satisfies the above range.
The composition ratio of the modified olefin polymer of the component (A) and the hydroxyl group-containing polymer of the component (B) used for the production of the reversible crosslinkable molded product of the present invention is the carboxylic acid anhydride of the component (A). It is essential that the ratio of the number of hydroxyl groups of the component (B) to the number of physical groups is 0.1 to 5, and preferably 0.1 to 3. Here, when the ratio of the number of hydroxyl groups to the number of carboxylic acid anhydride groups is less than the above range, the crosslinkability of the crosslinkable mixture or the molded product is inferior. On the other hand, when the ratio exceeds the above range, the crosslinkability of the molded product is inferior. In either case, the object of the present invention cannot be achieved.
[0025]
In the present invention, “reversible crosslinkability” means crosslinkability, that is, a heat deformation rate during crosslinking of 35% or less and a crosslink dissociation property, that is, a heat deformation rate after crosslink dissociation treatment of 65% or more. say.
If these values are out of the above ranges, problems such as slow progress of cross-linking or difficulty of dissociation once cross-linking occurs.
[0026]
A more preferable range of crosslinkability is 30% or less, a further preferable range is 20% or less, a more preferable range of crosslinkability is 80% or more, and a further preferable range is 90% or more.
The crosslinkable mixture used for the production of the reversible crosslinkable molded article of the present invention basically comprises the component (A) and the component (B), but within the range not impairing the effects of the present invention, the (A), (B) Components other than the component may be contained. Specifically, for example, various commonly used additives such as antioxidants, ultraviolet absorbers, nucleating agents, neutralizing agents, lubricants, An antiblocking agent, a dispersant, a fluidity improver, a release agent, a flame retardant, a colorant, a filler, and the like can be added.
[0027]
On the other hand, what has been conventionally used as a “reaction accelerator” such as a metal salt of a carboxylic acid tends to inhibit reversible crosslinking in the present invention as described above, so it is better not to add it.
As a manufacturing method of the reversible crosslinkable molded article of the present invention, the component (A) and the component (B) are essential components, and other optional components are added as necessary, and each component is added to a Henschel mixer, a ribbon blender, After uniformly mixing with a V-type blender or the like, hot melt molding, or after uniformly mixing with these methods, melt and knead with a single or multi-screw extruder, roll, Banbury mixer, kneader, Brabender, etc. After obtaining a reversible crosslinkable composition, a method of pelletizing and hot-melt molding as necessary may be mentioned.
[0028]
Further, scraps after use of the molded body or cross-linked molded body obtained by these methods are subjected to pretreatment such as cutting and pulverization, if necessary, and further mixed with the above-mentioned crosslinkable mixture if necessary. There is a method of hot melt molding.
As a specific molding method used here, a molded body can be formed by shaping into a desired shape in a molten state by various molding methods such as injection molding, extrusion molding, hollow molding, compression molding, and rotational molding. . Even when the used molded body is reused, it can be reshaped into a desired shape in a molten state by the same molding method to obtain a crosslinked molded body.
[0029]
The reversible crosslinkable mixture and the molded product have properties such as crosslinking dissociation at high temperature and crosslinking formation at low temperature, and further, crosslinking does not proceed at high temperature.
In the method for producing a reversible crosslinkable molded article of the present invention, the composition of the component (A), the composition of the component (B), the crosslinking dissociation temperature determined according to the composition ratio of the components (A) and (B) It is necessary to mold with. Generally, this crosslinking dissociation temperature is higher than the molding temperature of polyolefin, and a range of 230 to 300 ° C. is usually used.
[0030]
This hot melt molding temperature is below the cross-linking dissociation temperature of the reversibly crosslinkable molded body, and in particular, when the residence time during molding is long or the resin retention amount in the molding machine is large, the crosslinking proceeds quickly. Alternatively, when trying to re-form a reversible cross-linkable extruded product once cross-linked, the dissociation of the cross-linking becomes insufficient, so that the appearance of the molded product is deteriorated and the melt viscosity of the resin due to the progress of cross-linking is reduced. As a result of the increase, the load of the molding machine also increases, and the object of the present invention cannot be sufficiently achieved.
[0031]
In order to crosslink the reversibly crosslinkable molded article thus obtained, the reversibly crosslinkable molded body has a glass transition temperature higher than the reversible crosslinkable molded body as a temperature condition at which crosslinking proceeds substantially. Less than the crosslinking dissociation temperature. As this temperature range, it is preferable to use a temperature of usually 0 to 200 ° C. or less and the melting point of the molded body.
Further, when the crosslinking treatment temperature is lower than the glass transition temperature of the molded product, the molecular motion of the reversible crosslinkable molded product is stopped or extremely slow, so that the crosslinking does not proceed substantially, even if it proceeds. However, the crosslinking rate is extremely slow. On the other hand, when the crosslinking treatment temperature is equal to or higher than the crosslinking dissociation temperature, the molecular motion of the reversible crosslinkable molded product is active, but since the crosslinking dissociation temperature is exceeded, the crosslinking reaction does not proceed. In any case, it is difficult to achieve the object of the present invention.
[0032]
The reversible crosslinkable molded article produced as described above is excellent in moldability and processing stability, and can be crosslinked by a simple crosslinking treatment. Furthermore, the molded article can be crosslinked or used molded article. Can be obtained again by performing melt molding by the same molding method.
In the present invention, in the production of the reversible crosslinkable mixture and the molded body, it is possible to mold in a substantially uncrosslinked state, so that various molding methods usually used in thermoplastic resins as described above. That is, injection molding, extrusion molding, hollow molding, compression molding, rotational molding, and the like can be applied. Of these, the use of an extrusion molding method is advantageous and preferable in terms of crosslinking control and the like.
[0033]
【Example】
EXAMPLES Hereinafter, although this invention is demonstrated more concretely using an Example, this invention is not limited by a following example, unless the summary is exceeded.
The modified olefin polymer of component (A), hydroxyl group-containing polymer of component (B), metal salt of organic carboxylic acid (component (C)), water-crosslinkable resin (( The component D) and the stabilizer (component (E)) are shown below.
[0034]
(A) component
A-1; Ethylene-maleic anhydride-ethyl acrylate terpolymer (maleic anhydride unit content 2.4% by weight, measured by infrared absorption spectrum, ethyl acrylate unit content 7.5% %, The ratio of the number of carboxylic acid ester groups to the number of carboxylic anhydride groups, 3.1, the number average molecular weight measured by gel permeation chromatography 19300, the number average molecular weight and the modification determined based on the multiplier of maleic anhydride unit content (Average bond number of carboxylic anhydride group per molecule of olefin polymer is 4.7, manufactured by Sumitomo Chemical Co., Ltd., “Bondaine LX4110”)
[0035]
A-2; ethylene-maleic anhydride-ethyl acrylate terpolymer (maleic anhydride unit content 2.5% by weight measured by infrared absorption spectrum, ethyl acrylate unit content 12.5% %, The ratio of the number of carboxylic acid ester groups to the number of carboxylic acid anhydride groups, 4.9, the number average molecular weight measured by gel permeation chromatography 19800, the modification determined based on the multiplier of the number average molecular weight and maleic anhydride unit content The average number of carboxylic anhydride groups bonded to one molecule of olefin polymer is 5.0, manufactured by Sumitomo Chemical Co., Ltd., “Bondaine TX8030”)
[0036]
A-3; ethylene-butene-1 copolymer (melt flow rate 9 g / 10 min, density 0.925 g / cm3, Nippon Polychem Co., Ltd. “Z-50MG”) 100 parts by weight, maleic anhydride 0.85 parts by weight, stearyl acrylate 3.0 parts by weight, 2,5-dimethyl-2,5-bis ( (Tertiary butyl peroxy) 0.1 parts by weight of hexane was added and mixed uniformly with a Henschel mixer, and then a twin screw extruder (manufactured by Ikekai, PCM-30, D = 30 mm, L / D = 32) A melt grafting reaction was carried out to obtain a modified olefin polymer of maleic anhydride of ethylene-butene-1 copolymer and stearyl acrylate. The extruder is C1: 150 ° C, C2: 190 ° C, C3-D: It operated on condition of 230 degreeC, Ns: 200rpm, Q: 10kg / hr. The resulting modified olefin polymer had a melt flow rate of 4.2 g / 10 min, was subjected to reprecipitation purification treatment to remove the ungrafted product, and then the maleic anhydride unit content 0.64 measured by infrared absorption spectrum. % By weight, stearyl acrylate unit content 1.6% by weight, ratio of carboxylic acid ester group number to carboxylic anhydride group number 0.76, number average molecular weight 25700 measured by gel permeation chromatography, number average molecular weight and maleic acid The average number of bonds of carboxylic anhydride groups per molecule of the modified olefin polymer determined based on the multiplier of the anhydride unit content was 1.7.
[0037]
A-4 (for comparative example); ethylene-maleic anhydride-ethyl acrylate terpolymer (maleic anhydride unit content 0.8% by weight measured by infrared absorption spectrum, ethyl acrylate unit contained) 30.0% by weight, the ratio of the number of carboxylic acid ester groups to the number of carboxylic acid anhydride groups 36.7, the number average molecular weight 18700 measured by gel permeation chromatography, the multiplier of the number average molecular weight and maleic anhydride unit content The average number of bonds of carboxylic anhydride groups per molecule of the modified olefin polymer determined based on 1.5, manufactured by Sumitomo Chemical Co., Ltd., “Bondaine AX8390”)
[0038]
(B) component
B-1: Hydrogenated hydroxyl group-terminated polybutadiene (hydroxyl group content 2.0% by weight, number average molecular weight 1000, number of hydroxyl groups per molecule of hydroxyl group-containing polymer determined based on the multiplier of number average molecular weight and hydroxyl group content) 1.6 average bond number, “Nisso PB GI-1000” manufactured by Nippon Soda Co., Ltd.)
[0039]
B-2: Low molecular weight polyolefin polyol (hydroxyl group content 1.3% by weight, number average molecular weight 1500, average number of hydroxyl group bonds per molecule of hydroxyl group-containing polymer determined based on the multiplier of number average molecular weight and hydroxyl group content 1.1, "Polytail H" manufactured by Mitsubishi Chemical Corporation)
[0040]
Component (C) (for comparative example; carboxylate metal salt)
C-1: Zinc acetate dihydrate
C-2; calcium stearate
C-3: Masterbatch containing 1 part by weight of dibutyltin dilaurate
[0041]
Component (D) (for comparative example)
Low density polyethylene (melt flow rate 2 g / 10 min, density 0.919 g / cm3100 parts by weight of Nippon Polychem Co., Ltd. “EH-30”), 2.0 parts by weight of vinyltrimethoxysilane, and 0.1 parts by weight of dicumyl peroxide were uniformly mixed with a Henschel mixer, and then a single screw extruder. Silane grafted polyethylene was obtained at (D = 40 mm, L / D = 28). The extruder is C1: 120 ° C, C2: 160 ° C, C3-D: It operated on the conditions of 200 degreeC, Ns: 70rpm, Q: 7kg / hr. The obtained silane-grafted polyethylene had a melt flow rate of 1.3 g / 10 min and a vinyltrimethoxysilane unit content of 0.7% by weight measured by fluorescent X-ray.
[0042]
(E) component (stabilizer)
E-1; IRGANOX 1010 (manufactured by Ciba Geigy, phenolic antioxidant)
E-2; Irgafos 168 (manufactured by Ciba Geigy, phosphorus antioxidant)
The evaluation methods used in the examples and comparative examples are described below.
[0043]
Processing stability
Continuous extrusion for 48 hours was performed using a single screw extruder (D = 40 mm, L / D = 28), and the change over time in the amount of extrusion was measured. Extruder conditions are C1: 170 ° C, C2: 210 ° C, C3˜D: 250 ° C., Ns: 70 rpm, and various conditions were constant from the start to the end of extrusion.
However, only when the component (D) is used, the temperature setting of the extruder conditions is C.1: 150 ° C, C2: 170 ° C, C3-D: It changed into 190 degreeC.
[0044]
Sheet forming
Using a sheet molding machine (D = 20 mm, L / D = 20), 1 mm thick sheet was formed. Molding conditions are C1: 170 ° C, C2: 210 ° C, C3~ D: 250 ° C, Ns: 50 rpm. However, only when the component (D) is used, the temperature setting of the molding conditions is C1: 150 ° C, C2: 170 ° C, C3-D: It changed into 190 degreeC.
[0045]
Electric wire forming
Using a wire coating device (D = 50 mm, L / D = 25), a nominal cross-sectional area of 5.5 mm obtained by twisting seven 1 mm diameter copper wires together2A coating layer having a thickness of 4 mm was formed on the conductor. Molding conditions are C1: 170 ° C, C2: 210 ° C, C3-D: 250 degreeC and the extrusion linear velocity were 5 m / min. However, when the component (D) is used, the temperature setting of the molding conditions is C1: 150 ° C, C2: 170 ° C, C3-D: It changed into 190 degreeC.
[0046]
Pipe forming
A pipe having an outer diameter of 34 mm and a wall thickness of 5 mm was formed using a pipe forming machine (D = 60 mm, L / D = 24).
Molding conditions are C1: 170 ° C, C2: 210 ° C, C3~ D: 250 ° C, Ns: 50 rpm. However, when the component (D) is used, the temperature setting of the molding conditions is C1: 150 ° C, C2: 170 ° C, C3-D: It changed into 190 degreeC.
[0047]
Cross-linking treatment
This was carried out by heating the reversible cross-linkable molded product for a predetermined time in an oven maintained at an internal temperature of 80 ° C. However, when (D) component was used, it changed into the method of immersing a molded object in 80 degreeC warm water for a predetermined time.
[0048]
Heat deformation
It measured based on JIS C-3005.
Hot internal pressure creep test
It measured based on JIS K-6762.
[0049]
Thermal fusion test
By the pad fusion method, fusion bonding was performed at a heating temperature of 200 ° C. for 1 minute, allowed to cool for 1 minute, and then the fusion jig was removed. A No. 2 dumbbell based on JISK-6301 was prepared from the pipe so that the joint was in the center, and a tensile test was performed at a tensile speed of 50 mm / min to obtain tensile strength and tensile elongation.
[0050]
Crosslinkability
The pellets of the reversibly crosslinkable compositions obtained in Examples and Comparative Examples were preheated at 230 ° C. for 5 minutes, and then 100 kg / cm.2And heated for 5 minutes under pressure of 120 kg / cm2A 1 mm thick press-molded test piece produced by cooling under pressure was subjected to heat treatment at 80 ° C. for 24 hours to crosslink, and then in accordance with JIS C3005 (heat deformation), 140 ° C. and 1 kgf. The heating deformation rate was measured under the conditions.
[0051]
Crosslink dissociation
After preheating the same pellet at 230 ° C. for 5 minutes, 100 kg / cm2And heated for 5 minutes under pressure of 120 kg / cm2A 1 mm-thick press-molded test piece produced by cooling under pressure was crosslinked by heat treatment at 80 ° C. for 24 hours, and then preheated again at 230 ° C. for 5 minutes, and then 100 kg / cm.2And heated for 5 minutes under pressure of 120 kg / cm2After cooling under pressure, the heat deformation rate was measured under the conditions of 140 ° C. and 1 kgf in accordance with JIS C3005 (heat deformation).
[0052]
Glass transition temperature (Tg)
After preheating the pellets or compacts obtained above at 230 ° C. for 5 minutes, 100 kg / cm2And heated for 5 minutes under pressure of 120 kg / cm2A press-molded test piece having a thickness of 2 mm, a width of 12.7 mm, and a length of 63 mm was produced by cooling under pressure. Using this test piece, a mechanical spectrometer (RMS 605 type) manufactured by Rheometric Scientific F.E. Ltd., temperature -150 ° C. to 0 ° C., strain 0.1%, frequency 6.28 radians The temperature dependence of the loss modulus G ″ was measured under the conditions of / sec, and the glass transition temperature (Tg) was determined as the temperature showing the peak.
[0053]
<Example 1>
A mixture obtained by uniformly mixing A-1 as component (A) and E-1 and E-2 as components (E) with a Henschel mixer is a biaxial kneader (Ikegai, PCM- 45, D = 45 mm, L / D = 34), B-1 that has been heated to about 65 ° C. and lowered in viscosity in advance as B component, is supplied from the middle of the kneader by a gear pump, and is reversible. A pellet of the crosslinkable composition was obtained.
[0054]
The composition ratio of each component is A-1: 82.8% by weight, B-1: 17.2% by weight, E-1: 0.1 parts by weight, E-2: 0.0. It adjusted so that it might become 1 weight part. (Ratio of the number of hydroxyl groups to the number of carboxylic anhydride groups: 1.00)
The extruder conditions are C1: 50 ° C, C2: 150 ° C: C3~ D: 230 ° C, Ns: 200rpm, Q: 20kg / hr, (B) component is C3Feeded from the zone.
Processing stability was evaluated using the pellets of the obtained reversibly crosslinkable composition. The results are shown in Table 1.
[0055]
<Example 2>
(A) Component A-3: 93.5 wt% and (B) component B-2: 6.5 wt% (ratio of hydroxyl group number to carboxylic anhydride group number: 0.81) E-1: 0.1 part by weight and E-2: 0.1 part by weight are used as component (E) for a total of 100 parts by weight, and a mixture obtained by mixing these components with a Henschel mixer is used. Then, the processing stability was evaluated. The results are shown in Table 1.
<Comparative Example 1>
A pellet of a reversible crosslinkable composition was obtained in the same manner as in Example 1 except that C-1 was added as the component (C). Processing stability was evaluated using this pellet. The results are shown in Table 1.
The composition ratio of each component was C-1: 0.06 parts by weight, E-1: 0.04 parts by weight with respect to a total of 100 parts by weight of A-1: 82.8% and B-1: 17.2% by weight. It adjusted so that it might become 1 weight part and E-2: 0.1 weight part. (Ratio of the number of hydroxyl groups to the number of carboxylic anhydride groups: 1.00)
[0056]
<Comparative example 2>
As component (D), 100 parts by weight of D-1 is used. On the other hand, C-3 as component (C) is 5 parts by weight, as component (E): E-1: 0.1 part by weight, E-2: Processing stability was evaluated using the composition which added 0.1 weight part and mixed uniformly with the Henschel mixer, respectively. The results are shown in Table 1.
[0057]
<Example 3>
(A) A-1 was used as the component, E-1 and E-2 were used as the component (E), and the mixture obtained by uniformly mixing them with a Henschel mixer was mixed with a biaxial kneader (Ikegai, PCM Co., Ltd.). -45, D = 45 mm, L / D = 34), and B-1 which has been heated to about 65 ° C. and lowered in viscosity in advance is supplied as a B component from the middle of the kneader by means of a gear pump. A pellet of the crosslinkable composition was obtained.
[0058]
The composition ratio of each component is A-1: 92.3% by weight, B-1: 7.7% by weight, and E-1: 0.1 part by weight, E-2: 0. It adjusted so that it might become 1 weight part. (Ratio of the number of hydroxyl groups to the number of carboxylic anhydride groups: 0.40)
Extruder conditions are C1: 50 ° C, C2: 150 ° C, C3~ D: 230 ° C, Ns: 200rpm, Q: 20kg / hr, (B) component is C3Feeded from the zone.
[0059]
Sheet extrusion was performed using the pellets of the obtained reversible crosslinkable composition, and the obtained extruded sheet was subjected to crosslinking treatment for 3 hours or 8 hours, and the heat deformation rate was measured.
About the extrusion sheet | seat which performed the crosslinking process for 8 hours, after pelletizing with the sheet | seat pelletizer, sheet | seat extrusion was performed again, the crosslinking process of the obtained extruded sheet was again performed for 3 hours and 8 hours, and the heat deformation rate was measured. The results are shown in Table 2.
[0060]
<Example 4>
(A) Component A-2: 88.2% by weight, (B) Component B-2: 11.8% by weight (ratio of hydroxyl group number to carboxylic anhydride group number: 0.40) E-1: 0.1 part by weight and E-2: 0.1 part by weight as component (E) with respect to a total of 100 parts by weight, and a mixture obtained by uniformly mixing them with a Henschel mixer Except that was used, pellet production and sheet extrusion were performed in the same manner as in Example 3 to produce an extruded sheet. The extruded sheet was subjected to crosslinking treatment and re-sheeting / re-crosslinking treatment in the same manner as in Example 3 to measure heating deformation. The results are shown in Table 2.
[0061]
<Comparative Example 3>
Using A-4 as the component (A) and B-1 as the component (B), the composition ratio was adjusted to A-4: 93.5 wt% and B-1: 6.5 wt% (carbon Sheet molding and crosslinking treatment were carried out in the same manner as in Example 3 except that the ratio of the number of hydroxyl groups to the number of acid anhydride groups was 1.00). The results are shown in Table 2.
[0062]
<Comparative example 4>
Preparation and cross-linking of extruded sheet in the same manner as in Example 3 except that C-1: 0.06 parts by weight was used as component (C) for 100 parts by weight of component (A) and component (B). Sexuality was evaluated. The results are shown in Table 2.
[0063]
<Comparative Example 5>
In addition to the components of Example 4, Example 2 was used except that C-2: 2.0 parts by weight was used as the component (C) with respect to a total of 100 parts by weight of the components (A) and (B). The same operation was performed to prepare an extruded sheet and to evaluate the crosslinkability. The results are shown in Table 2.
[0064]
<Comparative Example 6>
Instead of component (A) and component (B), D-1: 100 parts by weight is used as component (D), whereas C-3: 5 parts by weight as component (C) and E as component (E). -1: 0.1 parts by weight and E-2: The same operation as in Example 4 was performed except that 0.1 parts by weight was used. The results are shown in Table 2.
Incidentally, the re-formation of the cross-linked extruded sheet has a very high extrusion load and cannot be molded.
[0065]
<Example 5>
As component (A), A-1: 88.7% by weight; as component (B), B-2: 11.3% by weight, with respect to a total of 100 parts by weight, as component (E), E-1: 0.1% by weight Parts, E-2: 0.1 parts by weight, and a mixture obtained by uniformly mixing them with a Henschel mixer was melt-kneaded with a biaxial kneader to obtain pellets of a reversible crosslinkable composition.
[0066]
Extruder conditions are C1: 50 ° C, C2: 150 ° C, C3~ D: 230 ° C, Ns: 200 rpm, Q: 20 kg / hr.
The pellets of the reversible crosslinkable composition thus obtained were subjected to press molding and subjected to a crosslinking treatment for 8 hours, and then volume resistivity was measured based on JIS K-6911. Moreover, electric deformation was performed using the pellets, and the heat deformation after crosslinking for 8 hours was measured. The results are shown in Table 3.
After pulverizing the resin portion obtained by peeling from the covered electric wire that had been subjected to the crosslinking treatment for 8 hours, it was melted again to form a sheet. The obtained sheet was cross-linked again for 8 hours, and the heat deformation was measured. The results are shown in Table 3.
[0067]
<Example 6>
A-2 was used as the component (A), B-1 was used as the component (B), and the composition ratios of A-2: 88.2% by weight and B-1: 11.8% by weight were adjusted. Except for the ratio of the number of hydroxyl groups to the number of acid anhydride groups: 0.40), pellets of the reversibly crosslinkable composition were produced in the same manner as in Example 1 and further press-molded in the same manner as in Example 5 using this. Then, the wire was formed.
These samples were evaluated in the same manner as in Example 5 above. The results are shown in Table 3.
[0068]
<Comparative Example 7>
Molding / evaluation was carried out in the same manner as in Example 5 except that C-1: 0.06 parts by weight as component (C) was further added to 100 parts by weight of component (A) and component (B). . The results are shown in Table 3.
[0069]
<Comparative Example 8>
Instead of component (A) and component (B), D-1: 100 parts by weight is used as component (D), whereas C-3: 5 parts by weight as component (C) and E as component (E). -1: 0.1 part by weight and E-2: 0.1 part by weight were mixed with a roll mill at a temperature of 120 ° C for 7 minutes, and then press-molded. The resulting sheet was subjected to a crosslinking treatment for 8 hours, and then the volume resistivity was measured according to JIS K-6911. The results are shown in Table 3. Further, the wire was molded with the same composition and blending, and the heat deformation after the cross-linking treatment for 8 hours was measured. Further, the coating portion was tried to be reused in the same manner as in Example 5, but the extrusion load was very high and molding could not be performed. The results are shown in Table 3.
[0070]
<Example 7>
Pipe forming was performed using a crosslinkable mixture obtained by uniformly mixing a composition having the same composition as in Example 2 with a Henschel mixer, and the obtained pipe was subjected to a crosslinking treatment for 8 hours.
Using this cross-linked pipe, a hot internal pressure creep test and a heat fusion test were conducted. The results are shown in Table 4.
[0071]
<Comparative Example 9>
Instead of component (A) and component (B), D-1: 100 parts by weight is used as component (D), whereas C-3: 5 parts by weight as component (C) and E as component (E). -1: 0.1 parts by weight and E-2: 0.1 parts by weight, and pipe forming in the same manner as in Example 7 except that a mixture obtained by uniformly mixing them with a Henschel mixer was used. And evaluated. The results are shown in Table 4.
[0072]
<Evaluation of results>
From the examples and comparative examples described above, the following points were found.
(1) In Table 1, the comparative example 1 containing the component (C) and having no reversible crosslinkability and the comparative resin 2 in which the base resin itself is outside the scope of the present invention are reduced in the amount of extrusion over time as compared with the examples. Is seen and processing stability is poor.
(2) In Table 2, Comparative Example 3 in which the ratio of the number of ester groups to the number of carboxylic acid groups is outside the scope of the present invention has a large heat deformation rate and poor crosslinkability. In Comparative Examples 2 and 3 having no reversible crosslinkability, the crosslinkability at the time of remolding is insufficient. In the case of Comparative Example 6 in which the resin was outside the scope of the present invention, re-molding after crosslinking was impossible.
(3) In Table 3, as in the above, Comparative Example 7 without reversible cross-linking caused poor appearance during re-molding, and Comparative Example 8 out of the range of resin could not be re-molded.
(4) Also in Table 4, Comparative Example 9 outside the scope of the present invention was extremely inferior in heat-fusibility.
[0073]
[Table 1]
[0074]
[Table 2]
[0075]
[Table 3]
[0076]
[Table 4]
[0077]
【The invention's effect】
INDUSTRIAL APPLICABILITY The present invention can provide a method for producing a thermoreversible crosslinkable extrudate that is excellent in extrusion moldability and processing stability, and that can be easily crosslinked and reused. Therefore, the thermoreversible cross-linkable extruded product of the present invention can be obtained by shaping into a desired shape in a molten state by a molding method usually used for thermoplastic resins, and also in a molding machine even for long-time molding. Since cross-linking does not proceed substantially, it is rare to cause scorch.
In reuse of used moldings, etc., it can be re-shaped into a desired shape in the molten state by the same molding method, so that it can be made into a crosslinked molded body again. Therefore, it becomes a useful extruded product.
Furthermore, taking advantage of such characteristics, it can be applied to a crosslinked film, a crosslinked tube, blow molding, injection molding or the like.
Claims (8)
(A)不飽和カルボン酸無水物と不飽和カルボン酸エステルとによって変性された変性オレフィン系重合体であって、1分子当たりのカルボン酸無水物基の平均結合数が1個以上で、かつ、該変性オレフィン系重合体中のカルボン酸無水物基数に対するカルボン酸エステル基数の比が0.5〜20である変性オレフィン系重合体
(B)1分子当たりの水酸基の平均結合数が1個以上の水酸基含有重合体A crosslinkable mixture comprising the following component (A) and component (B), wherein the ratio of the number of hydroxyl groups in component (B) to the number of carboxylic anhydride groups in component (A) is 0.1 to 5, A method for producing a reversibly crosslinkable molded article, characterized by hot-melt molding at a temperature higher than the dissociation temperature.
(A) A modified olefin polymer modified with an unsaturated carboxylic acid anhydride and an unsaturated carboxylic acid ester, wherein the average number of bonds of carboxylic acid anhydride groups per molecule is one or more, and The modified olefin polymer (B) in which the ratio of the number of carboxylic acid ester groups to the number of carboxylic acid anhydride groups in the modified olefin polymer is 0.5 to 20 has an average number of hydroxyl groups per molecule of 1 or more Hydroxyl-containing polymer
(A)不飽和カルボン酸無水物と不飽和カルボン酸エステルとによって変性された変性オレフィン系重合体であって、1分子当たりのカルボン酸無水物基の平均結合数が1個以上で、かつ、該変性オレフィン系重合体中のカルボン酸無水物基数に対するカルボン酸エステル基数の比が0.5〜20である変性オレフィン系重合体
(B)1分子当たりの水酸基の平均結合数が1個以上の水酸基含有重合体A crosslinkable mixture comprising the following component (A) and component (B), wherein the ratio of the number of hydroxyl groups in component (B) to the number of carboxylic anhydride groups in component (A) is 0.1 to 5; 6. A method for producing a reversible crosslinkable molded article, characterized in that the scrap of the molded article according to item 6 is hot melt molded at a temperature higher than a higher temperature among the crosslinking dissociation temperatures of the two.
(A) A modified olefin polymer modified with an unsaturated carboxylic acid anhydride and an unsaturated carboxylic acid ester, wherein the average number of bonds of carboxylic acid anhydride groups per molecule is one or more, and The modified olefin polymer (B) in which the ratio of the number of carboxylic acid ester groups to the number of carboxylic acid anhydride groups in the modified olefin polymer is 0.5 to 20 has an average number of hydroxyl groups per molecule of 1 or more Hydroxyl-containing polymer
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP00316999A JP3627552B2 (en) | 1999-01-08 | 1999-01-08 | Method for producing reversible cross-linkable molded article |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP00316999A JP3627552B2 (en) | 1999-01-08 | 1999-01-08 | Method for producing reversible cross-linkable molded article |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2000204204A JP2000204204A (en) | 2000-07-25 |
| JP3627552B2 true JP3627552B2 (en) | 2005-03-09 |
Family
ID=11549887
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP00316999A Expired - Fee Related JP3627552B2 (en) | 1999-01-08 | 1999-01-08 | Method for producing reversible cross-linkable molded article |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP3627552B2 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002105442A (en) * | 2000-10-02 | 2002-04-10 | Mitsubishi Cable Ind Ltd | Thermal storage material composition |
| JP2006066238A (en) * | 2004-08-27 | 2006-03-09 | Yazaki Corp | Electric wire / cable |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1163805A (en) * | 1997-08-11 | 1999-03-05 | Nakano Refrigerators Co Ltd | Open show case |
| JPH1183298A (en) * | 1997-08-29 | 1999-03-26 | Nakano Refrigerators Co Ltd | Flat type open showcase |
-
1999
- 1999-01-08 JP JP00316999A patent/JP3627552B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2000204204A (en) | 2000-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2283726C (en) | Elastomer compositions having improved abrasion resistance, coefficient of friction and hot green strength | |
| EP0336780B1 (en) | Thermoplastic resin or elastomer composition having excellent paint adhesion and laminate comprising layer of said thermoplastic elastomer and polyurethane layer | |
| EP1789489B1 (en) | Thermoplastic polyolefin composition | |
| CN101525471A (en) | Crosslinkable compositions, thermoplastic elastomers obtainable therefrom and their use | |
| JPH0781041B2 (en) | Thermoplastic composition of crystalline polyolefin and ethylene-containing copolymer | |
| JPS59135242A (en) | Thermoplastic blend of elastomer and ethylene copolymer composed of polyolefin or isobutylene as main chain | |
| CA2019164A1 (en) | Method for improving the processing characteristics of polyethylene blends | |
| EP1474476B1 (en) | Engineered polyolefin materials with enhanced surface durability and methods of making same | |
| CN115210316A (en) | High melt strength polypropylene and preparation method thereof | |
| CN1472244A (en) | Preparation method of low-smoke halogen-free flame-retardant polyolefin material | |
| JP3627552B2 (en) | Method for producing reversible cross-linkable molded article | |
| JPS63305148A (en) | Glass fiber-reinforced polyamide composition | |
| JP2000034376A (en) | Olefin polymer composition | |
| JP2004210868A (en) | Automotive molding | |
| CN118525052A (en) | Articles containing polyvinyl butyral and polyolefin elastomer | |
| JP2585699B2 (en) | Partially crosslinked thermoplastic elastomer composition for bonding polyurethane | |
| JP4705711B2 (en) | Ionomer composition and use thereof | |
| JP3895857B2 (en) | Thermoformable thermoplastic polymer alloy composition | |
| JP3669235B2 (en) | Olefin resin composition | |
| JP7749961B2 (en) | Silane-modified polypropylene, silane-crosslinked polypropylene, silane-modified polypropylene composition, and molded article, crosslinked molded article, and three-dimensional network fiber assembly using these | |
| JPS63258941A (en) | Polymer composition and encapsulated window using same | |
| JP3274181B2 (en) | Polymer composition and method for producing the same | |
| JPH02272032A (en) | Produciton of crosslinked film | |
| EP0710701A1 (en) | Thermoplastic compositions containing ground vulcanized rubber and polyolefin resin | |
| JPH0564662B2 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20041105 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20041116 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20041129 |
|
| R151 | Written notification of patent or utility model registration |
Ref document number: 3627552 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R151 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20081217 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20091217 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20091217 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20101217 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20101217 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20111217 Year of fee payment: 7 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20121217 Year of fee payment: 8 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131217 Year of fee payment: 9 |
|
| LAPS | Cancellation because of no payment of annual fees |