JP2013533305A - 治療 - Google Patents
治療 Download PDFInfo
- Publication number
- JP2013533305A JP2013533305A JP2013523660A JP2013523660A JP2013533305A JP 2013533305 A JP2013533305 A JP 2013533305A JP 2013523660 A JP2013523660 A JP 2013523660A JP 2013523660 A JP2013523660 A JP 2013523660A JP 2013533305 A JP2013533305 A JP 2013533305A
- Authority
- JP
- Japan
- Prior art keywords
- adra2a
- antagonist
- liver
- individual
- activity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000011282 treatment Methods 0.000 title claims description 62
- 239000005557 antagonist Substances 0.000 claims abstract description 155
- 238000000034 method Methods 0.000 claims abstract description 79
- 208000019423 liver disease Diseases 0.000 claims abstract description 61
- 230000001965 increasing effect Effects 0.000 claims abstract description 36
- 108020004101 alpha-2 Adrenergic Receptor Proteins 0.000 claims abstract description 24
- 230000003247 decreasing effect Effects 0.000 claims abstract description 23
- 230000000694 effects Effects 0.000 claims description 117
- 239000003814 drug Substances 0.000 claims description 60
- 210000004185 liver Anatomy 0.000 claims description 60
- 238000012360 testing method Methods 0.000 claims description 57
- 230000014509 gene expression Effects 0.000 claims description 56
- 210000004027 cell Anatomy 0.000 claims description 55
- 229940079593 drug Drugs 0.000 claims description 55
- 230000007882 cirrhosis Effects 0.000 claims description 47
- 208000019425 cirrhosis of liver Diseases 0.000 claims description 47
- SGOFAUSEYBZKDQ-UHFFFAOYSA-N brl-44408 Chemical compound C1C2=CC=CC=C2C(C)N1CC1=NCCN1 SGOFAUSEYBZKDQ-UHFFFAOYSA-N 0.000 claims description 44
- 206010016654 Fibrosis Diseases 0.000 claims description 36
- 210000001519 tissue Anatomy 0.000 claims description 36
- 230000007423 decrease Effects 0.000 claims description 29
- 208000007232 portal hypertension Diseases 0.000 claims description 22
- 230000017531 blood circulation Effects 0.000 claims description 19
- 230000002829 reductive effect Effects 0.000 claims description 19
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 claims description 18
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 claims description 18
- 210000002216 heart Anatomy 0.000 claims description 14
- 210000004556 brain Anatomy 0.000 claims description 13
- 210000000013 bile duct Anatomy 0.000 claims description 12
- 210000003734 kidney Anatomy 0.000 claims description 12
- 230000011664 signaling Effects 0.000 claims description 12
- 206010019663 Hepatic failure Diseases 0.000 claims description 11
- 210000002767 hepatic artery Anatomy 0.000 claims description 11
- 208000007903 liver failure Diseases 0.000 claims description 11
- 231100000835 liver failure Toxicity 0.000 claims description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 11
- 230000000747 cardiac effect Effects 0.000 claims description 10
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 claims description 10
- 229910021529 ammonia Inorganic materials 0.000 claims description 9
- 150000001875 compounds Chemical class 0.000 claims description 9
- 229940109239 creatinine Drugs 0.000 claims description 9
- 238000010171 animal model Methods 0.000 claims description 8
- 230000004872 arterial blood pressure Effects 0.000 claims description 8
- 230000002440 hepatic effect Effects 0.000 claims description 8
- 210000002569 neuron Anatomy 0.000 claims description 8
- 230000024883 vasodilation Effects 0.000 claims description 8
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 claims description 6
- 206010061218 Inflammation Diseases 0.000 claims description 5
- 208000006454 hepatitis Diseases 0.000 claims description 5
- 230000004054 inflammatory process Effects 0.000 claims description 5
- 239000004310 lactic acid Substances 0.000 claims description 5
- 235000014655 lactic acid Nutrition 0.000 claims description 5
- 230000003907 kidney function Effects 0.000 claims description 4
- 230000009278 visceral effect Effects 0.000 claims description 4
- 231100000283 hepatitis Toxicity 0.000 claims description 3
- 229960000317 yohimbine Drugs 0.000 claims description 3
- BLGXFZZNTVWLAY-CCZXDCJGSA-N Yohimbine Natural products C1=CC=C2C(CCN3C[C@@H]4CC[C@@H](O)[C@H]([C@H]4C[C@H]33)C(=O)OC)=C3NC2=C1 BLGXFZZNTVWLAY-CCZXDCJGSA-N 0.000 claims description 2
- BLGXFZZNTVWLAY-UHFFFAOYSA-N beta-Yohimbin Natural products C1=CC=C2C(CCN3CC4CCC(O)C(C4CC33)C(=O)OC)=C3NC2=C1 BLGXFZZNTVWLAY-UHFFFAOYSA-N 0.000 claims description 2
- BLGXFZZNTVWLAY-SCYLSFHTSA-N yohimbine Chemical compound C1=CC=C2C(CCN3C[C@@H]4CC[C@H](O)[C@@H]([C@H]4C[C@H]33)C(=O)OC)=C3NC2=C1 BLGXFZZNTVWLAY-SCYLSFHTSA-N 0.000 claims description 2
- AADVZSXPNRLYLV-UHFFFAOYSA-N yohimbine carboxylic acid Natural products C1=CC=C2C(CCN3CC4CCC(C(C4CC33)C(O)=O)O)=C3NC2=C1 AADVZSXPNRLYLV-UHFFFAOYSA-N 0.000 claims description 2
- 102100022815 Alpha-2A adrenergic receptor Human genes 0.000 claims 2
- 208000010002 alcoholic liver cirrhosis Diseases 0.000 claims 2
- 102100024401 Alpha-1D adrenergic receptor Human genes 0.000 claims 1
- 206010009208 Cirrhosis alcoholic Diseases 0.000 claims 1
- 101000689696 Homo sapiens Alpha-1D adrenergic receptor Proteins 0.000 claims 1
- 230000001771 impaired effect Effects 0.000 claims 1
- 208000024891 symptom Diseases 0.000 abstract description 29
- 102000030484 alpha-2 Adrenergic Receptor Human genes 0.000 abstract description 22
- 238000001727 in vivo Methods 0.000 abstract description 11
- 241000700159 Rattus Species 0.000 description 69
- 239000003795 chemical substances by application Substances 0.000 description 37
- 108060003345 Adrenergic Receptor Proteins 0.000 description 34
- 102000017910 Adrenergic receptor Human genes 0.000 description 34
- 108090000623 proteins and genes Proteins 0.000 description 26
- 102000005962 receptors Human genes 0.000 description 24
- 108020003175 receptors Proteins 0.000 description 24
- 102000039446 nucleic acids Human genes 0.000 description 22
- 108020004707 nucleic acids Proteins 0.000 description 22
- 150000007523 nucleic acids Chemical class 0.000 description 22
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 17
- 239000011780 sodium chloride Substances 0.000 description 17
- 210000003240 portal vein Anatomy 0.000 description 16
- 239000000203 mixture Substances 0.000 description 15
- 239000000902 placebo Substances 0.000 description 14
- 229940068196 placebo Drugs 0.000 description 14
- 102000004169 proteins and genes Human genes 0.000 description 14
- SFLSHLFXELFNJZ-QMMMGPOBSA-N (-)-norepinephrine Chemical compound NC[C@H](O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-QMMMGPOBSA-N 0.000 description 13
- 229960002748 norepinephrine Drugs 0.000 description 13
- SFLSHLFXELFNJZ-UHFFFAOYSA-N norepinephrine Natural products NCC(O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-UHFFFAOYSA-N 0.000 description 13
- 230000002265 prevention Effects 0.000 description 13
- 238000012216 screening Methods 0.000 description 13
- 241001465754 Metazoa Species 0.000 description 12
- 238000001356 surgical procedure Methods 0.000 description 12
- 238000009472 formulation Methods 0.000 description 11
- 210000000056 organ Anatomy 0.000 description 11
- 235000018102 proteins Nutrition 0.000 description 10
- 108091023037 Aptamer Proteins 0.000 description 9
- 230000006870 function Effects 0.000 description 8
- 230000009467 reduction Effects 0.000 description 8
- 238000003556 assay Methods 0.000 description 7
- 230000027455 binding Effects 0.000 description 7
- 239000002773 nucleotide Substances 0.000 description 7
- 125000003729 nucleotide group Chemical group 0.000 description 7
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 6
- 108010082126 Alanine transaminase Proteins 0.000 description 6
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 6
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 6
- 108091034117 Oligonucleotide Proteins 0.000 description 6
- 102000030619 alpha-1 Adrenergic Receptor Human genes 0.000 description 6
- 108020004102 alpha-1 Adrenergic Receptor Proteins 0.000 description 6
- 238000000338 in vitro Methods 0.000 description 6
- 238000011534 incubation Methods 0.000 description 6
- 210000004969 inflammatory cell Anatomy 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 230000002889 sympathetic effect Effects 0.000 description 6
- 230000008485 antagonism Effects 0.000 description 5
- 210000001772 blood platelet Anatomy 0.000 description 5
- 239000012634 fragment Substances 0.000 description 5
- 238000009396 hybridization Methods 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 108020004999 messenger RNA Proteins 0.000 description 5
- 208000022309 Alcoholic Liver disease Diseases 0.000 description 4
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- 239000000074 antisense oligonucleotide Substances 0.000 description 4
- 238000012230 antisense oligonucleotides Methods 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 230000036772 blood pressure Effects 0.000 description 4
- 210000004204 blood vessel Anatomy 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 230000001976 improved effect Effects 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 229960004793 sucrose Drugs 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 3
- 208000007788 Acute Liver Failure Diseases 0.000 description 3
- 206010000804 Acute hepatic failure Diseases 0.000 description 3
- 208000010334 End Stage Liver Disease Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 108091006027 G proteins Proteins 0.000 description 3
- 102000030782 GTP binding Human genes 0.000 description 3
- 108091000058 GTP-Binding Proteins 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 231100000836 acute liver failure Toxicity 0.000 description 3
- 239000000674 adrenergic antagonist Substances 0.000 description 3
- 239000000427 antigen Substances 0.000 description 3
- 108091007433 antigens Proteins 0.000 description 3
- 102000036639 antigens Human genes 0.000 description 3
- 102000012740 beta Adrenergic Receptors Human genes 0.000 description 3
- 108010079452 beta Adrenergic Receptors Proteins 0.000 description 3
- 208000011444 chronic liver failure Diseases 0.000 description 3
- 235000013681 dietary sucrose Nutrition 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 210000002989 hepatic vein Anatomy 0.000 description 3
- 210000005228 liver tissue Anatomy 0.000 description 3
- 238000012423 maintenance Methods 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 230000019491 signal transduction Effects 0.000 description 3
- 230000006641 stabilisation Effects 0.000 description 3
- 238000011105 stabilization Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 210000002820 sympathetic nervous system Anatomy 0.000 description 3
- 230000001839 systemic circulation Effects 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 206010048962 Brain oedema Diseases 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 101000979342 Homo sapiens Nuclear factor NF-kappa-B p105 subunit Proteins 0.000 description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 2
- 108010057466 NF-kappa B Proteins 0.000 description 2
- 102000003945 NF-kappa B Human genes 0.000 description 2
- 102100023050 Nuclear factor NF-kappa-B p105 subunit Human genes 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 108091027967 Small hairpin RNA Proteins 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 206010046996 Varicose vein Diseases 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 235000004279 alanine Nutrition 0.000 description 2
- 230000001476 alcoholic effect Effects 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 238000000540 analysis of variance Methods 0.000 description 2
- 230000003042 antagnostic effect Effects 0.000 description 2
- 208000006752 brain edema Diseases 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 210000001715 carotid artery Anatomy 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 238000000423 cell based assay Methods 0.000 description 2
- 210000004671 cell-free system Anatomy 0.000 description 2
- 230000001447 compensatory effect Effects 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 230000008323 hepatic arterial blood flow Effects 0.000 description 2
- 238000013537 high throughput screening Methods 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 208000018191 liver inflammation Diseases 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 229930014626 natural product Natural products 0.000 description 2
- 210000005036 nerve Anatomy 0.000 description 2
- WYWIFABBXFUGLM-UHFFFAOYSA-N oxymetazoline Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C)=C1CC1=NCCN1 WYWIFABBXFUGLM-UHFFFAOYSA-N 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 230000010412 perfusion Effects 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 238000011552 rat model Methods 0.000 description 2
- 230000008085 renal dysfunction Effects 0.000 description 2
- 239000004055 small Interfering RNA Substances 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 230000008700 sympathetic activation Effects 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 230000008718 systemic inflammatory response Effects 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 210000001631 vena cava inferior Anatomy 0.000 description 2
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- OBKXEAXTFZPCHS-UHFFFAOYSA-N 4-phenylbutyric acid Chemical compound OC(=O)CCCC1=CC=CC=C1 OBKXEAXTFZPCHS-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- 208000002467 Acute-On-Chronic Liver Failure Diseases 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 208000007257 Budd-Chiari syndrome Diseases 0.000 description 1
- 108010074051 C-Reactive Protein Proteins 0.000 description 1
- 102100032752 C-reactive protein Human genes 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 1
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 102000034354 Gi proteins Human genes 0.000 description 1
- 108091006101 Gi proteins Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 206010019670 Hepatic function abnormal Diseases 0.000 description 1
- 206010019728 Hepatitis alcoholic Diseases 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 1
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000003810 Interleukin-18 Human genes 0.000 description 1
- 108090000171 Interleukin-18 Proteins 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 102000004890 Interleukin-8 Human genes 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 208000001145 Metabolic Syndrome Diseases 0.000 description 1
- 108700011259 MicroRNAs Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 101000783356 Naja sputatrix Cytotoxin Proteins 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 206010062237 Renal impairment Diseases 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 206010039163 Right ventricular failure Diseases 0.000 description 1
- 108700028909 Serum Amyloid A Proteins 0.000 description 1
- 102000054727 Serum Amyloid A Human genes 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 108090000340 Transaminases Proteins 0.000 description 1
- 102000003929 Transaminases Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 102000014384 Type C Phospholipases Human genes 0.000 description 1
- 108010079194 Type C Phospholipases Proteins 0.000 description 1
- 206010047139 Vasoconstriction Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- IPBVNPXQWQGGJP-UHFFFAOYSA-N acetic acid phenyl ester Natural products CC(=O)OC1=CC=CC=C1 IPBVNPXQWQGGJP-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- GFFGJBXGBJISGV-UHFFFAOYSA-N adenyl group Chemical group N1=CN=C2N=CNC2=C1N GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 1
- 210000002934 adrenergic neuron Anatomy 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 208000026594 alcoholic fatty liver disease Diseases 0.000 description 1
- 208000002353 alcoholic hepatitis Diseases 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- 102000016967 beta-1 Adrenergic Receptors Human genes 0.000 description 1
- 108010014494 beta-1 Adrenergic Receptors Proteins 0.000 description 1
- 102000016966 beta-2 Adrenergic Receptors Human genes 0.000 description 1
- 108010014499 beta-2 Adrenergic Receptors Proteins 0.000 description 1
- 102000016959 beta-3 Adrenergic Receptors Human genes 0.000 description 1
- 108010014502 beta-3 Adrenergic Receptors Proteins 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000012742 biochemical analysis Methods 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 229960003150 bupivacaine Drugs 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000002340 cardiotoxin Substances 0.000 description 1
- 231100000677 cardiotoxin Toxicity 0.000 description 1
- 150000003943 catecholamines Chemical class 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 230000009137 competitive binding Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 238000009109 curative therapy Methods 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 231100000517 death Toxicity 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 230000003467 diminishing effect Effects 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000007888 film coating Substances 0.000 description 1
- 238000009501 film coating Methods 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 229960005150 glycerol Drugs 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 230000000004 hemodynamic effect Effects 0.000 description 1
- 208000007386 hepatic encephalopathy Diseases 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 102000034345 heterotrimeric G proteins Human genes 0.000 description 1
- 108091006093 heterotrimeric G proteins Proteins 0.000 description 1
- 230000009097 homeostatic mechanism Effects 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 230000000544 hyperemic effect Effects 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000002055 immunohistochemical effect Effects 0.000 description 1
- 238000012151 immunohistochemical method Methods 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 238000010874 in vitro model Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 230000008991 intestinal motility Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000002350 laparotomy Methods 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 229960004393 lidocaine hydrochloride Drugs 0.000 description 1
- YECIFGHRMFEPJK-UHFFFAOYSA-N lidocaine hydrochloride monohydrate Chemical compound O.[Cl-].CC[NH+](CC)CC(=O)NC1=C(C)C=CC=C1C YECIFGHRMFEPJK-UHFFFAOYSA-N 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 230000006609 metabolic stress Effects 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000013425 morphometry Methods 0.000 description 1
- 229920001206 natural gum Polymers 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 239000002858 neurotransmitter agent Substances 0.000 description 1
- 230000003957 neurotransmitter release Effects 0.000 description 1
- 206010053219 non-alcoholic steatohepatitis Diseases 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 230000002474 noradrenergic effect Effects 0.000 description 1
- 108091008104 nucleic acid aptamers Proteins 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 210000004789 organ system Anatomy 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 229960001528 oxymetazoline Drugs 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 239000008024 pharmaceutical diluent Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940049953 phenylacetate Drugs 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- 229950009215 phenylbutanoic acid Drugs 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 231100000857 poor renal function Toxicity 0.000 description 1
- 230000001242 postsynaptic effect Effects 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000003518 presynaptic effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 210000001747 pupil Anatomy 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 230000009711 regulatory function Effects 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 201000004409 schistosomiasis Diseases 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 238000009495 sugar coating Methods 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 238000002636 symptomatic treatment Methods 0.000 description 1
- 230000005062 synaptic transmission Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 208000027185 varicose disease Diseases 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 230000025033 vasoconstriction Effects 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/417—Imidazole-alkylamines, e.g. histamine, phentolamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4178—1,3-Diazoles not condensed 1,3-diazoles and containing further heterocyclic rings, e.g. pilocarpine, nitrofurantoin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4375—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a six-membered ring having nitrogen as a ring heteroatom, e.g. quinolizines, naphthyridines, berberine, vincamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/475—Quinolines; Isoquinolines having an indole ring, e.g. yohimbine, reserpine, strychnine, vinblastine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Food Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- Pathology (AREA)
- Gastroenterology & Hepatology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
【選択図】図1A
Description
本発明は、アルファ2aアドレナリン受容体(ADRA2a)の拮抗作用に関する。ADRA2aのアンタゴニストは、ADRA2aの活性、機能又は量を阻害又は減少させるいかなる化合物又は分子でもよい。好ましくは、アンタゴニストは、患者の肝臓における等、ADRA2aを発現する細胞、組織又は臓器において機能する。アンタゴニストは、肝臓において優先的に作用し得、又は肝臓を含むいくつかの部位において作用し得る。アンタゴニストは、炎症細胞、血小板又はニューロン等、特定の細胞型において優先的に作用し得る。好ましくは、アンタゴニストは、個体の肝臓、腎臓、脳及び心臓のうち1又は複数における等、アンタゴニストが投与された個体の細胞、組織又は臓器におけるADRA2a活性、機能又は量の減少をもたらす。活性の減少は、ADRA2aを介したシグナル伝達の減少であり得る。アンタゴニストは、更に後述する通り投与において、上述の肝臓又は他の臓器、細胞若しくは組織を標的とし得る。
本発明はまた、肝疾患の治療における使用に適した薬剤の同定のための方法を提供する。例えば、本発明は、門脈圧の低下における等、肝疾患の治療における使用に適したADRA2aのアンタゴニストの同定のための方法を提供する。本方法により同定されたアンタゴニストは、前述の特徴又は効果のいずれかを有するADRA2aのアンタゴニストであり得る。本明細書に記載されている方法により同定されたアンタゴニストは、肝疾患の治療における又は本明細書に記載されている状態若しくは症状のいずれかの治療若しくは予防における使用に適切であり得る。
本明細書に記載されている適切なADRA2aアンタゴニストは、典型的には、投与のために薬学的に許容される担体又は希釈剤と共に製剤化される。アンタゴニストは、本発明のスクリーニング方法により同定されたいずれかのアンタゴニストを含む、本明細書に定義されているいかなるアンタゴニストであってもよい。よって、アンタゴニストは、製薬技術においてルーチンである標準的な薬学的に許容される担体(複数可)及び/又は添加剤(複数可)と共に医薬として製剤化し得る。製剤の正確な性質は、所望の投与経路を含む数種の因子に依存する。典型的には、アンタゴニストは、経口、静脈内、胃内、血管内又は腹腔内投与のために製剤化し得る。
本発明は、肝疾患を有する個体の治療のため、特に、肝硬変に関連する又は起因する症状及び状態の治療のための方法を提供する。従って、本発明は、肝疾患を有する個体を治療する方法であって、前記被験体にADRA2aのアンタゴニストを投与するステップを含む方法を提供する。同様に、ADRA2aのアンタゴニストは、肝疾患を有する個体を治療する方法における使用のために提供し得る。肝疾患を有する個体の治療における使用のための医薬の製造における、ADRA2aのアンタゴニストの使用も提供される。
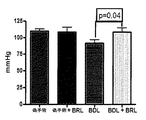
本発明は、その必要がある個体における硬変等の肝疾患の治療に関する。従って、本発明における治療対象の個体は、硬変等の肝疾患を有してよく、又は硬変等の肝疾患のリスクが増加していてもよい。例えば、被験体は硬変を有してよい。被験体は門脈圧亢進症を有してよい。門脈圧亢進症は、門脈静脈及びその分岐における血圧上昇として定義し得る。門脈静脈は、腸から肝臓へと血液を運ぶ大きな静脈である。門脈圧勾配(肝静脈又は門脈静脈の圧力(例えば、門脈静脈又は肝静脈に挿し込まれたカテーテルの測定値)と、肝静脈又は下大静脈の圧力(例えば、肝静脈又は下大静脈における自由に動く位置での圧力読み取り値)との間の差)が、5mmHg以上、好ましくは10mmHg以上である場合、門脈圧亢進症は、臨床的に有意であると定義し得る。
標準的診断基準を用いて代償性、非代償性又は慢性肝不全急性化(ACLF)として患者を分類した。ノルアドレナリン、門脈圧(HVPG)及び肝血流のレベルに関して患者を評価した。
方法
これらの実験は、硬変の確立された動物モデルである、胆管結紮(BDL)ラットを利用した。BDLラットは、当技術分野において公知の方法により作製し得る。例えば、この手順のために雄のスプラーグドーリー(Sprague-Dawley)ラット(200〜250g)を用い得る。麻酔後、正中開腹手術を行い、胆管を露出し、4.0シルク縫合糸により三重に結紮し、第二と第三の結紮の間を切断し得る。続いて吸収性縫合糸により傷を層縫合し、動物保管施設に戻すまで静音室(quiet room)で動物を回復させる。
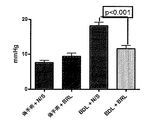
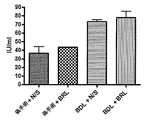
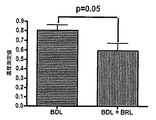
ADRA2aアンタゴニストで処置した後、プラセボ処置群と比較して、MAPの有意な増加(p<0.05)及び門脈圧の有意な低下が見られた(11.4±3.4対18.0±3.7mmHg、p<0.001)。
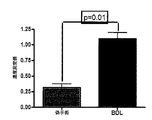
正常肝臓は、α2aアドレナリン受容体の発現が非常に限定的であることが判明したが、一方、BDLラット(硬変のモデル)の肝臓は、顕著な発現増加を示した。
- 門脈圧低下、
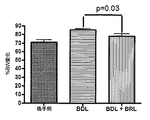
- MAP及び肝動脈血流増加、
- 肝内抵抗低下、並びに
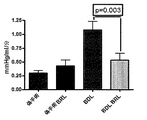
- アンモニア、脳腫脹及び腎機能障害低下
等、いくつかの効果を有した。
Claims (14)
- 肝疾患を患っている個体を治療する方法における使用のためのアルファ2aアドレナリン受容体(ADRA2a)のアンタゴニスト。
- 前記個体が慢性肝疾患を患っている、請求項1に記載の使用のためのアンタゴニスト。
- 前記個体が肝硬変を患っている、請求項1又は2に記載の使用のためのアンタゴニスト。
- 前記方法が、肝不全を治療又は予防するための方法である、請求項1〜3のいずれか一項に記載の使用のためのアンタゴニスト。
- 個体が、肝疾患を患っていない被験体と比較して、(a)内臓血管拡張及び正常、低下又は増加した心拍出量、(b)門脈圧亢進症、(c)平均動脈圧低下、(d)肝動脈血流低下、(e)肝内抵抗増加、(f)血漿アンモニア増加、(g)肝腎機能障害、(h)脳水増加、(i)血漿クレアチニン増加、(j)血漿乳酸増加、(k)アルコール性硬変及び/又は(l)非アルコール性脂肪肝疾患のうち1又は複数を患っている、又はそのリスクがある、請求項1〜4のいずれか一項に記載の使用のためのアンタゴニスト。
- 個体が活動性肝炎を患っていない、及び/又は個体が肝臓の顕著な炎症を有さない、請求項1〜5のいずれか一項に記載の使用のためのアンタゴニスト。
- (a)個体の肝臓におけるADRA2a発現の減少、及び/又は
(b)個体の肝臓におけるADRA2aレベルの減少、及び/又は
(c)個体の肝臓におけるADRA2a活性の減少、及び/又は
(d)個体の肝臓におけるADRA2aを介したシグナル伝達の減少
をもたらす、請求項1〜6のいずれか一項に記載の使用のためのアンタゴニスト。 - BRL-44408、ヨヒンビン及びラウオルシンから選択される、請求項1〜7のいずれか一項に記載の使用のためのアンタゴニスト。
- (a)ADRA2aの特異的アンタゴニストであり、
(b)ADRA2aの選択的アンタゴニストであり、
(c)ADRA2bのアンタゴニストではなく、
(d)ADRA2cのアンタゴニストではなく、
(e)ADRA1のアンタゴニストではなく、及び/又は
(f)ADRBのアンタゴニストではない、
請求項1〜8のいずれか一項に記載の使用のためのアンタゴニスト。 - その必要がある個体における肝疾患を治療する方法であって、アルファ2aアドレナリン受容体(ADRA2a)のアンタゴニストを前記個体に投与するステップを含む方法。
- 肝疾患の治療における使用に適した薬剤を同定する方法であって、被験薬が、ADRA2aの量又は活性を減少させることができるか決定するステップを含み、ADRA2aの量又は活性を減少させる能力が、化合物が肝疾患の治療における使用に適切であり得ることを示す方法。
- ADRA2aの量又は活性が、
(a)肝臓又は肝臓に由来する組織若しくは細胞、
(b)腎臓若しくは心臓又は腎臓若しくは心臓に由来する細胞、
(c)血小板又はニューロン、あるいは
(d)ADRA2aを発現する別の細胞又は組織
のうち1又は複数において評価される、請求項11に記載の方法。 - 被験薬を門脈圧亢進症又は硬変の動物モデルに投与するステップと、被験薬の存在が、ラットの肝臓におけるADRA2aの量又は活性の減少をもたらすか決定するステップとを含む、請求項11又は12に記載の方法。
- 動物モデルが、胆管結紮ラットである、請求項13に記載の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1013573.9 | 2010-08-12 | ||
| GBGB1013573.9A GB201013573D0 (en) | 2010-08-12 | 2010-08-12 | Treatment |
| PCT/GB2011/001217 WO2012020235A1 (en) | 2010-08-12 | 2011-08-12 | Treatment |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013533305A true JP2013533305A (ja) | 2013-08-22 |
| JP2013533305A5 JP2013533305A5 (ja) | 2014-09-18 |
Family
ID=42937912
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013523660A Pending JP2013533305A (ja) | 2010-08-12 | 2011-08-12 | 治療 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20130237557A1 (ja) |
| EP (1) | EP2603210A1 (ja) |
| JP (1) | JP2013533305A (ja) |
| GB (1) | GB201013573D0 (ja) |
| WO (1) | WO2012020235A1 (ja) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014120642A1 (en) * | 2013-01-30 | 2014-08-07 | The General Hospital Corporation | Diagnosis and treatment of hepatorenal syndrome |
| CA2978597A1 (en) * | 2015-03-06 | 2016-09-15 | Georgia State University Research Foundation, Inc. | Integrin-targeting protein and methods of use thereof |
| GB201804922D0 (en) * | 2018-03-27 | 2018-05-09 | Ucl Business Plc | Traatment |
| WO2020216669A1 (de) * | 2019-04-23 | 2020-10-29 | Bayer Aktiengesellschaft | Phenylsubstituierte imidazopyridinamide und ihre verwendung |
| EP4127134A4 (en) * | 2020-04-01 | 2024-07-31 | Alnylam Pharmaceuticals, Inc. | COMPOSITIONS OF ALPHA-2A ADRENERGIC RECEPTOR (ADRA2A) ARNI AGENTS AND METHODS OF USE THEREOF |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5503978A (en) | 1990-06-11 | 1996-04-02 | University Research Corporation | Method for identification of high affinity DNA ligands of HIV-1 reverse transcriptase |
| US5654151A (en) | 1990-06-11 | 1997-08-05 | Nexstar Pharmaceuticals, Inc. | High affinity HIV Nucleocapsid nucleic acid ligands |
| US5567588A (en) | 1990-06-11 | 1996-10-22 | University Research Corporation | Systematic evolution of ligands by exponential enrichment: Solution SELEX |
| US6207816B1 (en) | 1995-06-02 | 2001-03-27 | Nexstar Pharmaceuticals, Inc. | High affinity oligonucleotide ligands to growth factors |
| US7345065B2 (en) * | 2002-05-21 | 2008-03-18 | Allergan, Inc. | Methods and compositions for alleviating pain |
| WO2005025570A1 (en) * | 2003-09-15 | 2005-03-24 | Diamedica Inc. | Use of antagonists of hepatic sympathetic nerve activity |
| EP1885365A4 (en) * | 2005-05-13 | 2012-07-11 | The Feinstein Inst Medical Res | TREATMENT OF SEPSIS AND INFLAMMATION WITH ADRENEER ALPHA2A ANTAGONISTS |
-
2010
- 2010-08-12 GB GBGB1013573.9A patent/GB201013573D0/en not_active Ceased
-
2011
- 2011-08-12 WO PCT/GB2011/001217 patent/WO2012020235A1/en active Application Filing
- 2011-08-12 JP JP2013523660A patent/JP2013533305A/ja active Pending
- 2011-08-12 US US13/816,708 patent/US20130237557A1/en not_active Abandoned
- 2011-08-12 EP EP11748700.9A patent/EP2603210A1/en not_active Withdrawn
Non-Patent Citations (6)
| Title |
|---|
| JPN5013009302; ZHU J: '[EFFECT OF PHENTOLAMINE ON PORTAL PRESSURE IN CIRRHOTIC PATIENTS WITH PORTAL HYPERTENSION]' CHINESE JOURNAL OF SURGERY [ONLINE] V35 N2, 199702, P92-94 * |
| JPN5013009304; IKEDA M: 'EFFECT OF PHENOXYBENZAMINE (POB) ON PORTAL VENOUS PRESSURE IN PATIENTS WITH PORTAL HYPERTENSION' AMERICAN JOURNAL OF GASTROENTEROLOGY V71 N4, 1979, P389-394 * |
| JPN6015023081; Am J Physiol Cell Physiol Vol.293, 2007, p.C1252-C1262 * |
| JPN6015023082; Experimental Biology and Medicine Vol.232, No.10, 2007, p.1360-1367 * |
| JPN6015023083; Journal of Pharmacology and Experimental Therapeutics Vol.242, No.2, 1987, p.726-732 * |
| JPN6015023084; J Clin Gastroenterol Vol.39, Supp.2, 2005, p.S131-S137 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2603210A1 (en) | 2013-06-19 |
| WO2012020235A1 (en) | 2012-02-16 |
| US20130237557A1 (en) | 2013-09-12 |
| GB201013573D0 (en) | 2010-09-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11752196B2 (en) | Treatment of pyroptosis | |
| Yang et al. | Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells | |
| Grote Beverborg et al. | Phospholamban antisense oligonucleotides improve cardiac function in murine cardiomyopathy | |
| Lin et al. | CB1 cannabinoid receptor antagonist attenuates left ventricular hypertrophy and Akt-mediated cardiac fibrosis in experimental uremia | |
| EP2854865B1 (en) | Methods of treating a metabolic syndrome by modulating heat shock protein (hsp) 90-beta | |
| Mous et al. | Clinically relevant timing of antenatal sildenafil treatment reduces pulmonary vascular remodeling in congenital diaphragmatic hernia | |
| EP3336548B1 (en) | Method for providing information on chronic myeloid leukemia | |
| US20200323948A1 (en) | Treating Renal and Liver Dysfunction with TLR4 Antagonists | |
| BR112020007139A2 (pt) | tratamento de doença mediada por smc | |
| JP2013533305A (ja) | 治療 | |
| Yusifov et al. | Cardiac response to adrenergic stress differs by sex and across the lifespan | |
| Zhang et al. | Monoclonal antibody to marinobufagenin downregulates tgf β profibrotic signaling in left ventricle and kidney and reduces tissue remodeling in salt‐sensitive hypertension | |
| Matsiukevich et al. | Characterization of a robust mouse model of heart failure with preserved ejection fraction | |
| CN118903215A (zh) | 试剂在制备治疗或改善心力衰竭的药物中的用途及药物筛选方法 | |
| Miao et al. | ErbB3 binding protein 1 (EBP1) participates in the regulation of intestinal inflammation via mediating Akt signaling pathway | |
| US20210085668A1 (en) | Treatment | |
| WO2023070008A1 (en) | Methods and compositions for improving neuromuscular junction morphology and function | |
| US20160120938A1 (en) | P2x7 receptor agonist for use in preventing or treating kidney injury | |
| WO2014119387A1 (ja) | 拡張性心不全を治療または診断するための組成物およびその利用 | |
| Swarnkar | Mechanistic differences in mouse models of heart failure with preserved ejection fraction | |
| Ali et al. | Toll-like receptor 4 inhibition by pyridostigmine is associated with a reduction in hypertension and inflammation in rat models of preeclampsia | |
| WO2023108053A1 (en) | Compositions for and methods of treating hereditary aortopathies | |
| Garfield | Growth and differentiation factor 15 causes skeletal muscle wasting in pulmonary arterial hypertension through actions on transforming growth factor β activated kinase 1 | |
| CN119343148A (zh) | 使用angptl4拮抗剂治疗肝纤维化、炎症或相关疾病的方法 | |
| Oladipupo et al. | Characterization of a robust mouse model of heart failure with preserved ejection fraction 4 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD01 | Notification of change of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7426 Effective date: 20130909 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20130909 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140801 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20140801 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150616 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150910 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20151216 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20160517 |