JP2012167167A - Curable resin composition, cured product thereof, resin composition for printed wiring board, printed wiring board, resin composition for flexible wiring board, resin composition for semiconductor sealing material and resin composition for interlayer insulation material for build-up substrate - Google Patents
Curable resin composition, cured product thereof, resin composition for printed wiring board, printed wiring board, resin composition for flexible wiring board, resin composition for semiconductor sealing material and resin composition for interlayer insulation material for build-up substrate Download PDFInfo
- Publication number
- JP2012167167A JP2012167167A JP2011028490A JP2011028490A JP2012167167A JP 2012167167 A JP2012167167 A JP 2012167167A JP 2011028490 A JP2011028490 A JP 2011028490A JP 2011028490 A JP2011028490 A JP 2011028490A JP 2012167167 A JP2012167167 A JP 2012167167A
- Authority
- JP
- Japan
- Prior art keywords
- resin composition
- wiring board
- curable resin
- printed wiring
- mass
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000011342 resin composition Substances 0.000 title claims abstract description 97
- 239000004065 semiconductor Substances 0.000 title claims abstract description 15
- 239000000758 substrate Substances 0.000 title claims abstract description 13
- 239000003566 sealing material Substances 0.000 title claims abstract description 12
- 239000011229 interlayer Substances 0.000 title claims abstract description 10
- 239000012774 insulation material Substances 0.000 title claims abstract description 5
- 239000003822 epoxy resin Substances 0.000 claims abstract description 61
- 229920000647 polyepoxide Polymers 0.000 claims abstract description 61
- 229910052698 phosphorus Inorganic materials 0.000 claims abstract description 25
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 23
- 125000004437 phosphorous atom Chemical group 0.000 claims abstract description 20
- 150000001875 compounds Chemical class 0.000 claims abstract description 19
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims abstract description 14
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims abstract description 8
- 239000000203 mixture Substances 0.000 claims description 28
- 239000000463 material Substances 0.000 claims description 13
- 239000011521 glass Substances 0.000 claims description 12
- 239000003960 organic solvent Substances 0.000 claims description 9
- 239000011256 inorganic filler Substances 0.000 claims description 7
- 229910003475 inorganic filler Inorganic materials 0.000 claims description 7
- 238000002156 mixing Methods 0.000 claims description 7
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 3
- 125000004018 acid anhydride group Chemical group 0.000 claims description 3
- 229910052739 hydrogen Inorganic materials 0.000 claims description 3
- 239000001257 hydrogen Substances 0.000 claims description 3
- 125000003700 epoxy group Chemical group 0.000 claims description 2
- -1 2,7-diglycidyloxy-1-naphthyl Chemical group 0.000 description 32
- 238000001723 curing Methods 0.000 description 27
- 239000003063 flame retardant Substances 0.000 description 27
- 229920005989 resin Polymers 0.000 description 26
- 239000011347 resin Substances 0.000 description 26
- 238000000034 method Methods 0.000 description 25
- 229920003986 novolac Polymers 0.000 description 23
- 239000000047 product Substances 0.000 description 21
- RNFJDJUURJAICM-UHFFFAOYSA-N 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyclohexa-1,3,5-triene Chemical compound N=1P(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP=1(OC=1C=CC=CC=1)OC1=CC=CC=C1 RNFJDJUURJAICM-UHFFFAOYSA-N 0.000 description 17
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 15
- 229910052751 metal Inorganic materials 0.000 description 14
- 239000010410 layer Substances 0.000 description 13
- 239000002184 metal Substances 0.000 description 13
- 239000005011 phenolic resin Substances 0.000 description 13
- 150000002989 phenols Chemical class 0.000 description 13
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 12
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 11
- 239000002313 adhesive film Substances 0.000 description 11
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 10
- 239000000853 adhesive Substances 0.000 description 10
- 230000001070 adhesive effect Effects 0.000 description 10
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 9
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 9
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 8
- 229910052736 halogen Inorganic materials 0.000 description 8
- 150000002367 halogens Chemical class 0.000 description 8
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 7
- 125000003118 aryl group Chemical group 0.000 description 7
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 7
- 239000011889 copper foil Substances 0.000 description 7
- 239000000945 filler Substances 0.000 description 7
- 239000011810 insulating material Substances 0.000 description 7
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 6
- 238000010030 laminating Methods 0.000 description 6
- 229910052757 nitrogen Inorganic materials 0.000 description 6
- 230000001681 protective effect Effects 0.000 description 6
- 150000003839 salts Chemical class 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 5
- 239000000654 additive Substances 0.000 description 5
- 229930003836 cresol Natural products 0.000 description 5
- 239000011888 foil Substances 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 239000011574 phosphorus Substances 0.000 description 5
- 229920001296 polysiloxane Polymers 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- XCZKKZXWDBOGPA-UHFFFAOYSA-N 2-phenylbenzene-1,4-diol Chemical compound OC1=CC=C(O)C(C=2C=CC=CC=2)=C1 XCZKKZXWDBOGPA-UHFFFAOYSA-N 0.000 description 4
- 239000004593 Epoxy Substances 0.000 description 4
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 4
- 125000003545 alkoxy group Chemical group 0.000 description 4
- PXKLMJQFEQBVLD-UHFFFAOYSA-N bisphenol F Chemical compound C1=CC(O)=CC=C1CC1=CC=C(O)C=C1 PXKLMJQFEQBVLD-UHFFFAOYSA-N 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 239000004020 conductor Substances 0.000 description 4
- 150000007973 cyanuric acids Chemical class 0.000 description 4
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 4
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 4
- 239000004744 fabric Substances 0.000 description 4
- 238000011049 filling Methods 0.000 description 4
- 239000005350 fused silica glass Substances 0.000 description 4
- 239000012796 inorganic flame retardant Substances 0.000 description 4
- 238000003475 lamination Methods 0.000 description 4
- JDSHMPZPIAZGSV-UHFFFAOYSA-N melamine Chemical compound NC1=NC(N)=NC(N)=N1 JDSHMPZPIAZGSV-UHFFFAOYSA-N 0.000 description 4
- AZQWKYJCGOJGHM-UHFFFAOYSA-N para-benzoquinone Natural products O=C1C=CC(=O)C=C1 AZQWKYJCGOJGHM-UHFFFAOYSA-N 0.000 description 4
- FAIAAWCVCHQXDN-UHFFFAOYSA-N phosphorus trichloride Chemical compound ClP(Cl)Cl FAIAAWCVCHQXDN-UHFFFAOYSA-N 0.000 description 4
- KJCVRFUGPWSIIH-UHFFFAOYSA-N 1-naphthol Chemical compound C1=CC=C2C(O)=CC=CC2=C1 KJCVRFUGPWSIIH-UHFFFAOYSA-N 0.000 description 3
- GSKNLOOGBYYDHV-UHFFFAOYSA-N 2-methylphenol;naphthalen-1-ol Chemical compound CC1=CC=CC=C1O.C1=CC=C2C(O)=CC=CC2=C1 GSKNLOOGBYYDHV-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 229920000877 Melamine resin Polymers 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 239000007809 chemical reaction catalyst Substances 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 238000013329 compounding Methods 0.000 description 3
- 229910000000 metal hydroxide Inorganic materials 0.000 description 3
- 150000004692 metal hydroxides Chemical class 0.000 description 3
- VSWALKINGSNVAR-UHFFFAOYSA-N naphthalen-1-ol;phenol Chemical compound OC1=CC=CC=C1.C1=CC=C2C(O)=CC=CC2=C1 VSWALKINGSNVAR-UHFFFAOYSA-N 0.000 description 3
- 239000002994 raw material Substances 0.000 description 3
- 230000003014 reinforcing effect Effects 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- ARXJGSRGQADJSQ-UHFFFAOYSA-N 1-methoxypropan-2-ol Chemical compound COCC(C)O ARXJGSRGQADJSQ-UHFFFAOYSA-N 0.000 description 2
- HECLRDQVFMWTQS-RGOKHQFPSA-N 1755-01-7 Chemical compound C1[C@H]2[C@@H]3CC=C[C@@H]3[C@@H]1C=C2 HECLRDQVFMWTQS-RGOKHQFPSA-N 0.000 description 2
- BSYJHYLAMMJNRC-UHFFFAOYSA-N 2,4,4-trimethylpentan-2-ol Chemical compound CC(C)(C)CC(C)(C)O BSYJHYLAMMJNRC-UHFFFAOYSA-N 0.000 description 2
- FPZWZCWUIYYYBU-UHFFFAOYSA-N 2-(2-ethoxyethoxy)ethyl acetate Chemical compound CCOCCOCCOC(C)=O FPZWZCWUIYYYBU-UHFFFAOYSA-N 0.000 description 2
- KXGFMDJXCMQABM-UHFFFAOYSA-N 2-methoxy-6-methylphenol Chemical compound [CH]OC1=CC=CC([CH])=C1O KXGFMDJXCMQABM-UHFFFAOYSA-N 0.000 description 2
- YZEZMSPGIPTEBA-UHFFFAOYSA-N 2-n-(4,6-diamino-1,3,5-triazin-2-yl)-1,3,5-triazine-2,4,6-triamine Chemical compound NC1=NC(N)=NC(NC=2N=C(N)N=C(N)N=2)=N1 YZEZMSPGIPTEBA-UHFFFAOYSA-N 0.000 description 2
- FVCSARBUZVPSQF-UHFFFAOYSA-N 5-(2,4-dioxooxolan-3-yl)-7-methyl-3a,4,5,7a-tetrahydro-2-benzofuran-1,3-dione Chemical compound C1C(C(OC2=O)=O)C2C(C)=CC1C1C(=O)COC1=O FVCSARBUZVPSQF-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 229940058905 antimony compound for treatment of leishmaniasis and trypanosomiasis Drugs 0.000 description 2
- 150000001463 antimony compounds Chemical class 0.000 description 2
- 239000004760 aramid Substances 0.000 description 2
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 2
- 229920003235 aromatic polyamide Polymers 0.000 description 2
- 239000004305 biphenyl Substances 0.000 description 2
- 235000010290 biphenyl Nutrition 0.000 description 2
- 150000001639 boron compounds Chemical class 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- PPQREHKVAOVYBT-UHFFFAOYSA-H dialuminum;tricarbonate Chemical compound [Al+3].[Al+3].[O-]C([O-])=O.[O-]C([O-])=O.[O-]C([O-])=O PPQREHKVAOVYBT-UHFFFAOYSA-H 0.000 description 2
- 239000000428 dust Substances 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- AMWRITDGCCNYAT-UHFFFAOYSA-L hydroxy(oxo)manganese;manganese Chemical compound [Mn].O[Mn]=O.O[Mn]=O AMWRITDGCCNYAT-UHFFFAOYSA-L 0.000 description 2
- ZFSLODLOARCGLH-UHFFFAOYSA-N isocyanuric acid Chemical compound OC1=NC(O)=NC(O)=N1 ZFSLODLOARCGLH-UHFFFAOYSA-N 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 2
- 239000001095 magnesium carbonate Substances 0.000 description 2
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 239000005078 molybdenum compound Substances 0.000 description 2
- 150000002752 molybdenum compounds Chemical class 0.000 description 2
- JKQOBWVOAYFWKG-UHFFFAOYSA-N molybdenum trioxide Chemical compound O=[Mo](=O)=O JKQOBWVOAYFWKG-UHFFFAOYSA-N 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 150000004780 naphthols Chemical class 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 239000004843 novolac epoxy resin Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 150000003018 phosphorus compounds Chemical class 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 238000007747 plating Methods 0.000 description 2
- 229920000139 polyethylene terephthalate Polymers 0.000 description 2
- 239000005020 polyethylene terephthalate Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- LLHKCFNBLRBOGN-UHFFFAOYSA-N propylene glycol methyl ether acetate Chemical compound COCC(C)OC(C)=O LLHKCFNBLRBOGN-UHFFFAOYSA-N 0.000 description 2
- 238000007788 roughening Methods 0.000 description 2
- 229910000679 solder Inorganic materials 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 150000003918 triazines Chemical class 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 2
- LTVUCOSIZFEASK-MPXCPUAZSA-N (3ar,4s,7r,7as)-3a-methyl-3a,4,7,7a-tetrahydro-4,7-methano-2-benzofuran-1,3-dione Chemical compound C([C@H]1C=C2)[C@H]2[C@H]2[C@]1(C)C(=O)OC2=O LTVUCOSIZFEASK-MPXCPUAZSA-N 0.000 description 1
- MUTGBJKUEZFXGO-OLQVQODUSA-N (3as,7ar)-3a,4,5,6,7,7a-hexahydro-2-benzofuran-1,3-dione Chemical compound C1CCC[C@@H]2C(=O)OC(=O)[C@@H]21 MUTGBJKUEZFXGO-OLQVQODUSA-N 0.000 description 1
- KMOUUZVZFBCRAM-OLQVQODUSA-N (3as,7ar)-3a,4,7,7a-tetrahydro-2-benzofuran-1,3-dione Chemical compound C1C=CC[C@@H]2C(=O)OC(=O)[C@@H]21 KMOUUZVZFBCRAM-OLQVQODUSA-N 0.000 description 1
- 0 *P1(Oc(cccc2)c2-c2c1cccc2)=O Chemical compound *P1(Oc(cccc2)c2-c2c1cccc2)=O 0.000 description 1
- KGSFMPRFQVLGTJ-UHFFFAOYSA-N 1,1,2-triphenylethylbenzene Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C=1C=CC=CC=1)CC1=CC=CC=C1 KGSFMPRFQVLGTJ-UHFFFAOYSA-N 0.000 description 1
- WBODDOZXDKQEFS-UHFFFAOYSA-N 1,2,3,4-tetramethyl-5-phenylbenzene Chemical group CC1=C(C)C(C)=CC(C=2C=CC=CC=2)=C1C WBODDOZXDKQEFS-UHFFFAOYSA-N 0.000 description 1
- IVORCBKUUYGUOL-UHFFFAOYSA-N 1-ethynyl-2,4-dimethoxybenzene Chemical compound COC1=CC=C(C#C)C(OC)=C1 IVORCBKUUYGUOL-UHFFFAOYSA-N 0.000 description 1
- LHENQXAPVKABON-UHFFFAOYSA-N 1-methoxypropan-1-ol Chemical compound CCC(O)OC LHENQXAPVKABON-UHFFFAOYSA-N 0.000 description 1
- VILCJCGEZXAXTO-UHFFFAOYSA-N 2,2,2-tetramine Chemical compound NCCNCCNCCN VILCJCGEZXAXTO-UHFFFAOYSA-N 0.000 description 1
- OAYXUHPQHDHDDZ-UHFFFAOYSA-N 2-(2-butoxyethoxy)ethanol Chemical compound CCCCOCCOCCO OAYXUHPQHDHDDZ-UHFFFAOYSA-N 0.000 description 1
- QLVPICNVQBBOQP-UHFFFAOYSA-N 2-(4,6-diamino-1,3,5-triazin-2-yl)guanidine Chemical compound NC(N)=NC1=NC(N)=NC(N)=N1 QLVPICNVQBBOQP-UHFFFAOYSA-N 0.000 description 1
- SFRDXVJWXWOTEW-UHFFFAOYSA-N 2-(hydroxymethyl)propane-1,3-diol Chemical compound OCC(CO)CO SFRDXVJWXWOTEW-UHFFFAOYSA-N 0.000 description 1
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 1
- OGRULRAOMCDCBO-UHFFFAOYSA-N 2-[[1-(oxiran-2-ylmethoxy)naphthalen-2-yl]oxymethyl]oxirane Chemical compound C1OC1COC1=CC=C2C=CC=CC2=C1OCC1CO1 OGRULRAOMCDCBO-UHFFFAOYSA-N 0.000 description 1
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 description 1
- SVONRAPFKPVNKG-UHFFFAOYSA-N 2-ethoxyethyl acetate Chemical compound CCOCCOC(C)=O SVONRAPFKPVNKG-UHFFFAOYSA-N 0.000 description 1
- RNLHGQLZWXBQNY-UHFFFAOYSA-N 3-(aminomethyl)-3,5,5-trimethylcyclohexan-1-amine Chemical compound CC1(C)CC(N)CC(C)(CN)C1 RNLHGQLZWXBQNY-UHFFFAOYSA-N 0.000 description 1
- ULKLGIFJWFIQFF-UHFFFAOYSA-N 5K8XI641G3 Chemical compound CCC1=NC=C(C)N1 ULKLGIFJWFIQFF-UHFFFAOYSA-N 0.000 description 1
- ZUHMEUFBTDOKPX-UHFFFAOYSA-N 6-[2-(4,6-diamino-1,3,5-triazin-2-yl)ethyl]-1,3,5-triazine-2,4-diamine Chemical compound NC1=NC(N)=NC(CCC=2N=C(N)N=C(N)N=2)=N1 ZUHMEUFBTDOKPX-UHFFFAOYSA-N 0.000 description 1
- MWSKJDNQKGCKPA-UHFFFAOYSA-N 6-methyl-3a,4,5,7a-tetrahydro-2-benzofuran-1,3-dione Chemical compound C1CC(C)=CC2C(=O)OC(=O)C12 MWSKJDNQKGCKPA-UHFFFAOYSA-N 0.000 description 1
- GZVHEAJQGPRDLQ-UHFFFAOYSA-N 6-phenyl-1,3,5-triazine-2,4-diamine Chemical compound NC1=NC(N)=NC(C=2C=CC=CC=2)=N1 GZVHEAJQGPRDLQ-UHFFFAOYSA-N 0.000 description 1
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- 229930185605 Bisphenol Natural products 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- DKPFZGUDAPQIHT-UHFFFAOYSA-N Butyl acetate Natural products CCCCOC(C)=O DKPFZGUDAPQIHT-UHFFFAOYSA-N 0.000 description 1
- 206010007269 Carcinogenicity Diseases 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- QPLDLSVMHZLSFG-UHFFFAOYSA-N Copper oxide Chemical compound [Cu]=O QPLDLSVMHZLSFG-UHFFFAOYSA-N 0.000 description 1
- 239000005751 Copper oxide Substances 0.000 description 1
- 244000241257 Cucumis melo Species 0.000 description 1
- 235000015510 Cucumis melo subsp melo Nutrition 0.000 description 1
- MQJKPEGWNLWLTK-UHFFFAOYSA-N Dapsone Chemical compound C1=CC(N)=CC=C1S(=O)(=O)C1=CC=C(N)C=C1 MQJKPEGWNLWLTK-UHFFFAOYSA-N 0.000 description 1
- RPNUMPOLZDHAAY-UHFFFAOYSA-N Diethylenetriamine Chemical compound NCCNCCN RPNUMPOLZDHAAY-UHFFFAOYSA-N 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 239000002841 Lewis acid Substances 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- LGRFSURHDFAFJT-UHFFFAOYSA-N Phthalic anhydride Natural products C1=CC=C2C(=O)OC(=O)C2=C1 LGRFSURHDFAFJT-UHFFFAOYSA-N 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229910052581 Si3N4 Inorganic materials 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 239000006087 Silane Coupling Agent Substances 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical group [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- WGLPBDUCMAPZCE-UHFFFAOYSA-N Trioxochromium Chemical compound O=[Cr](=O)=O WGLPBDUCMAPZCE-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- FMRLDPWIRHBCCC-UHFFFAOYSA-L Zinc carbonate Chemical compound [Zn+2].[O-]C([O-])=O FMRLDPWIRHBCCC-UHFFFAOYSA-L 0.000 description 1
- FJJCIZWZNKZHII-UHFFFAOYSA-N [4,6-bis(cyanoamino)-1,3,5-triazin-2-yl]cyanamide Chemical compound N#CNC1=NC(NC#N)=NC(NC#N)=N1 FJJCIZWZNKZHII-UHFFFAOYSA-N 0.000 description 1
- NJYZCEFQAIUHSD-UHFFFAOYSA-N acetoguanamine Chemical compound CC1=NC(N)=NC(N)=N1 NJYZCEFQAIUHSD-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 238000007259 addition reaction Methods 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- 229940118662 aluminum carbonate Drugs 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 150000003934 aromatic aldehydes Chemical class 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- QBLDFAIABQKINO-UHFFFAOYSA-N barium borate Chemical compound [Ba+2].[O-]B=O.[O-]B=O QBLDFAIABQKINO-UHFFFAOYSA-N 0.000 description 1
- RQPZNWPYLFFXCP-UHFFFAOYSA-L barium dihydroxide Chemical compound [OH-].[OH-].[Ba+2] RQPZNWPYLFFXCP-UHFFFAOYSA-L 0.000 description 1
- 229910001863 barium hydroxide Inorganic materials 0.000 description 1
- AYJRCSIUFZENHW-DEQYMQKBSA-L barium(2+);oxomethanediolate Chemical compound [Ba+2].[O-][14C]([O-])=O AYJRCSIUFZENHW-DEQYMQKBSA-L 0.000 description 1
- VCCBEIPGXKNHFW-UHFFFAOYSA-N biphenyl-4,4'-diol Chemical group C1=CC(O)=CC=C1C1=CC=C(O)C=C1 VCCBEIPGXKNHFW-UHFFFAOYSA-N 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 229910000416 bismuth oxide Inorganic materials 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229910021538 borax Inorganic materials 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- JHIWVOJDXOSYLW-UHFFFAOYSA-N butyl 2,2-difluorocyclopropane-1-carboxylate Chemical compound CCCCOC(=O)C1CC1(F)F JHIWVOJDXOSYLW-UHFFFAOYSA-N 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 239000003990 capacitor Substances 0.000 description 1
- 150000001728 carbonyl compounds Chemical class 0.000 description 1
- 230000007670 carcinogenicity Effects 0.000 description 1
- 231100000260 carcinogenicity Toxicity 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 229910000423 chromium oxide Inorganic materials 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 229910021446 cobalt carbonate Inorganic materials 0.000 description 1
- 229910000428 cobalt oxide Inorganic materials 0.000 description 1
- ZOTKGJBKKKVBJZ-UHFFFAOYSA-L cobalt(2+);carbonate Chemical compound [Co+2].[O-]C([O-])=O ZOTKGJBKKKVBJZ-UHFFFAOYSA-L 0.000 description 1
- IVMYJDGYRUAWML-UHFFFAOYSA-N cobalt(ii) oxide Chemical compound [Co]=O IVMYJDGYRUAWML-UHFFFAOYSA-N 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 238000011437 continuous method Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910000431 copper oxide Inorganic materials 0.000 description 1
- 238000003851 corona treatment Methods 0.000 description 1
- 229910002026 crystalline silica Inorganic materials 0.000 description 1
- 238000007766 curtain coating Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- GDVKFRBCXAPAQJ-UHFFFAOYSA-A dialuminum;hexamagnesium;carbonate;hexadecahydroxide Chemical compound [OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Al+3].[Al+3].[O-]C([O-])=O GDVKFRBCXAPAQJ-UHFFFAOYSA-A 0.000 description 1
- TYIXMATWDRGMPF-UHFFFAOYSA-N dibismuth;oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Bi+3].[Bi+3] TYIXMATWDRGMPF-UHFFFAOYSA-N 0.000 description 1
- QGBSISYHAICWAH-UHFFFAOYSA-N dicyandiamide Chemical compound NC(N)=NC#N QGBSISYHAICWAH-UHFFFAOYSA-N 0.000 description 1
- IMHDGJOMLMDPJN-UHFFFAOYSA-N dihydroxybiphenyl Natural products OC1=CC=CC=C1C1=CC=CC=C1O IMHDGJOMLMDPJN-UHFFFAOYSA-N 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 150000002013 dioxins Chemical class 0.000 description 1
- ZZTCPWRAHWXWCH-UHFFFAOYSA-N diphenylmethanediamine Chemical compound C=1C=CC=CC=1C(N)(N)C1=CC=CC=C1 ZZTCPWRAHWXWCH-UHFFFAOYSA-N 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229910000514 dolomite Inorganic materials 0.000 description 1
- 239000010459 dolomite Substances 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000007772 electroless plating Methods 0.000 description 1
- 238000009713 electroplating Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000008393 encapsulating agent Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- WLPKFQRBARNCNR-UHFFFAOYSA-N ethene 1,3,5-triazine-2,4,6-triamine Chemical compound C=C.NC1=NC(N)=NC(N)=N1.NC1=NC(N)=NC(N)=N1 WLPKFQRBARNCNR-UHFFFAOYSA-N 0.000 description 1
- KTWOOEGAPBSYNW-UHFFFAOYSA-N ferrocene Chemical compound [Fe+2].C=1C=C[CH-]C=1.C=1C=C[CH-]C=1 KTWOOEGAPBSYNW-UHFFFAOYSA-N 0.000 description 1
- RAQDACVRFCEPDA-UHFFFAOYSA-L ferrous carbonate Chemical compound [Fe+2].[O-]C([O-])=O RAQDACVRFCEPDA-UHFFFAOYSA-L 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- ANSXAPJVJOKRDJ-UHFFFAOYSA-N furo[3,4-f][2]benzofuran-1,3,5,7-tetrone Chemical compound C1=C2C(=O)OC(=O)C2=CC2=C1C(=O)OC2=O ANSXAPJVJOKRDJ-UHFFFAOYSA-N 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 150000002357 guanidines Chemical class 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 238000013007 heat curing Methods 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-M hexanoate Chemical compound CCCCCC([O-])=O FUZZWVXGSFPDMH-UHFFFAOYSA-M 0.000 description 1
- 229910001701 hydrotalcite Inorganic materials 0.000 description 1
- 229960001545 hydrotalcite Drugs 0.000 description 1
- 150000002460 imidazoles Chemical class 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 150000007517 lewis acids Chemical class 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 1
- 239000000944 linseed oil Substances 0.000 description 1
- 235000021388 linseed oil Nutrition 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- YSRVJVDFHZYRPA-UHFFFAOYSA-N melem Chemical compound NC1=NC(N23)=NC(N)=NC2=NC(N)=NC3=N1 YSRVJVDFHZYRPA-UHFFFAOYSA-N 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- VYKXQOYUCMREIS-UHFFFAOYSA-N methylhexahydrophthalic anhydride Chemical compound C1CCCC2C(=O)OC(=O)C21C VYKXQOYUCMREIS-UHFFFAOYSA-N 0.000 description 1
- 239000006082 mold release agent Substances 0.000 description 1
- 239000012778 molding material Substances 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229910000476 molybdenum oxide Inorganic materials 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910000480 nickel oxide Inorganic materials 0.000 description 1
- QGLKJKCYBOYXKC-UHFFFAOYSA-N nonaoxidotritungsten Chemical compound O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1 QGLKJKCYBOYXKC-UHFFFAOYSA-N 0.000 description 1
- 239000004745 nonwoven fabric Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 150000002894 organic compounds Chemical group 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- PQQKPALAQIIWST-UHFFFAOYSA-N oxomolybdenum Chemical compound [Mo]=O PQQKPALAQIIWST-UHFFFAOYSA-N 0.000 description 1
- GNRSAWUEBMWBQH-UHFFFAOYSA-N oxonickel Chemical compound [Ni]=O GNRSAWUEBMWBQH-UHFFFAOYSA-N 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 229920001568 phenolic resin Polymers 0.000 description 1
- 150000002990 phenothiazines Chemical class 0.000 description 1
- 229920002120 photoresistant polymer Polymers 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920003207 poly(ethylene-2,6-naphthalate) Polymers 0.000 description 1
- 229920006122 polyamide resin Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000011112 polyethylene naphthalate Substances 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 235000011176 polyphosphates Nutrition 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 150000004053 quinones Chemical class 0.000 description 1
- 238000010125 resin casting Methods 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 description 1
- 229920002545 silicone oil Polymers 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 239000004328 sodium tetraborate Substances 0.000 description 1
- 235000010339 sodium tetraborate Nutrition 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- KCNSDMPZCKLTQP-UHFFFAOYSA-N tetraphenylen-1-ol Chemical compound C12=CC=CC=C2C2=CC=CC=C2C2=CC=CC=C2C2=C1C=CC=C2O KCNSDMPZCKLTQP-UHFFFAOYSA-N 0.000 description 1
- 229920001187 thermosetting polymer Polymers 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 239000011135 tin Substances 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- FOZHTJJTSSSURD-UHFFFAOYSA-J titanium(4+);dicarbonate Chemical compound [Ti+4].[O-]C([O-])=O.[O-]C([O-])=O FOZHTJJTSSSURD-UHFFFAOYSA-J 0.000 description 1
- 238000001721 transfer moulding Methods 0.000 description 1
- SRPWOOOHEPICQU-UHFFFAOYSA-N trimellitic anhydride Chemical compound OC(=O)C1=CC=C2C(=O)OC(=O)C2=C1 SRPWOOOHEPICQU-UHFFFAOYSA-N 0.000 description 1
- AAAQKTZKLRYKHR-UHFFFAOYSA-N triphenylmethane Chemical compound C1=CC=CC=C1C(C=1C=CC=CC=1)C1=CC=CC=C1 AAAQKTZKLRYKHR-UHFFFAOYSA-N 0.000 description 1
- BIKXLKXABVUSMH-UHFFFAOYSA-N trizinc;diborate Chemical compound [Zn+2].[Zn+2].[Zn+2].[O-]B([O-])[O-].[O-]B([O-])[O-] BIKXLKXABVUSMH-UHFFFAOYSA-N 0.000 description 1
- 239000002383 tung oil Substances 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 229910001930 tungsten oxide Inorganic materials 0.000 description 1
- 239000002966 varnish Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 239000011667 zinc carbonate Substances 0.000 description 1
- 235000004416 zinc carbonate Nutrition 0.000 description 1
- 229910000010 zinc carbonate Inorganic materials 0.000 description 1
- 239000011592 zinc chloride Substances 0.000 description 1
- 235000005074 zinc chloride Nutrition 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- XAEWLETZEZXLHR-UHFFFAOYSA-N zinc;dioxido(dioxo)molybdenum Chemical compound [Zn+2].[O-][Mo]([O-])(=O)=O XAEWLETZEZXLHR-UHFFFAOYSA-N 0.000 description 1
- BNEMLSQAJOPTGK-UHFFFAOYSA-N zinc;dioxido(oxo)tin Chemical compound [Zn+2].[O-][Sn]([O-])=O BNEMLSQAJOPTGK-UHFFFAOYSA-N 0.000 description 1
- PZRXQXJGIQEYOG-UHFFFAOYSA-N zinc;oxido(oxo)borane Chemical compound [Zn+2].[O-]B=O.[O-]B=O PZRXQXJGIQEYOG-UHFFFAOYSA-N 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Landscapes
- Structures Or Materials For Encapsulating Or Coating Semiconductor Devices Or Solid State Devices (AREA)
- Fireproofing Substances (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Epoxy Resins (AREA)
Abstract
Description
本発明は、難燃性と耐熱性とに優れる硬化性樹脂組成物、その硬化物、並びに前記硬化性樹脂組成物を用いたプリント配線基板用樹脂組成物、プリント配線基板、フレキシブル配線基板用樹脂組成物、半導体封止材料用樹脂組成物、及びビルドアップ基板用層間絶縁材料用樹脂組成物に関する。 The present invention relates to a curable resin composition excellent in flame retardancy and heat resistance, a cured product thereof, a printed wiring board resin composition using the curable resin composition, a printed wiring board, and a flexible wiring board resin. The present invention relates to a composition, a resin composition for a semiconductor sealing material, and a resin composition for an interlayer insulating material for a build-up substrate.
エポキシ樹脂及びその硬化剤を必須成分とするエポキシ樹脂組成物は、高耐熱性、耐湿性等の諸物性に優れる点から半導体封止材やプリント回路基板等の電子部品分野、導電ペースト等の導電性接着剤、その他接着剤、複合材料用マトリックス、塗料、フォトレジスト材料、顕色材料等で広く用いられている。 Epoxy resin compositions containing an epoxy resin and its curing agent as essential components are excellent in various physical properties such as high heat resistance and moisture resistance, and are used in the field of electronic components such as semiconductor sealing materials and printed circuit boards, and conductive materials such as conductive pastes. Widely used in adhesives, other adhesives, matrix for composite materials, paints, photoresist materials, developer materials, etc.
近年、プリント配線板材料や半導体封止材などの電子部品や導電性接着剤などの分野では、難燃性を付与するために臭素等のハロゲン系難燃剤がアンチモン化合物とともに配合されている。しかしながら、近年の環境・安全への取り組みのなかで、ダイオキシン発生が懸念されるハロゲン系難燃剤を用いず、且つ発ガン性が疑われているアンチモン化合物を用いない環境・安全対応型の難燃化方法の開発が強く要求されている。また、プリント配線板材料の分野ではハロゲン系難燃剤の使用が高温放置信頼性を損なう要因となっていることから非ハロゲン化への期待が高い。 In recent years, in the fields of electronic parts such as printed wiring board materials and semiconductor encapsulants and conductive adhesives, halogen-based flame retardants such as bromine are blended together with antimony compounds in order to impart flame retardancy. However, in recent environmental and safety initiatives, environmentally and flame-resistant flame retardants that do not use halogen-based flame retardants that may cause dioxins and do not use antimony compounds that are suspected of carcinogenicity. There is a strong demand for the development of a conversion method. In the field of printed wiring board materials, the use of halogenated flame retardants is a factor that impairs reliability at high temperatures.
このような要求特性に応え、難燃性と高耐熱性とを兼備したエポキシ樹脂組成物として、例えば、下記特許文献1には、9,10−ジヒドロ−9−オキサ−10−ホスファフェナントレン−10−オキサイド(以下、「HCA」と略記する。)を配合させてなる技術が開示されている(下記特許文献1参照)。 As an epoxy resin composition that meets such required characteristics and has both flame retardancy and high heat resistance, for example, Patent Document 1 listed below includes 9,10-dihydro-9-oxa-10-phosphaphenanthrene- A technique in which 10-oxide (hereinafter abbreviated as “HCA”) is blended is disclosed (see Patent Document 1 below).
しかしながら、前記した電子部品や導電性接着剤の分野では、鉛フリー半田への対応によりリフロー処理温度が高温化する等、これまでに増して耐熱性に優れた材料が求められているところ、かかるHCAを配合してなるエポキシ樹脂組成物は、難燃性は良好なものとなるものの、その硬化物における耐熱性が十分なものでなく、とりわけ、プリント配線板材料の分野における高温放置信頼性を損なうものであった。 However, in the field of electronic components and conductive adhesives described above, there is a demand for materials with higher heat resistance than ever, such as higher reflow processing temperatures due to the compatibility with lead-free solder. The epoxy resin composition containing HCA has good flame retardancy, but its heat resistance in the cured product is not sufficient, and in particular, it has high temperature storage reliability in the field of printed wiring board materials. It was a loss.
従って、本発明が解決しようとする課題は、硬化物における難燃性、耐熱性に優れる硬化性樹脂組成物、その硬化物、及び、該硬化性樹脂組成物を用いたプリント配線基板用樹脂組成物、プリント配線基板、フレキシブル配線基板用樹脂組成物、半導体封止材料用樹脂組成物、及びビルドアップ基板用層間絶縁材料用樹脂組成物を提供することにある。 Accordingly, the problems to be solved by the present invention are a curable resin composition excellent in flame retardancy and heat resistance in a cured product, the cured product, and a resin composition for a printed wiring board using the curable resin composition. It is providing the resin composition for printed circuit boards, the resin composition for flexible wiring boards, the resin composition for semiconductor sealing materials, and the interlayer insulation material for buildup boards.
本発明者らは、上記課題を解決するため、鋭意検討した結果、芳香核を有するリン原子含有化合物の該芳香核上の置換基として水酸基を有するものをエポキシ樹脂組成物における添加系難燃剤として用いた場合に、その硬化物において難燃性と優れた耐熱性が得られることを見出し、本発明を完成するに至った。 As a result of intensive studies to solve the above problems, the present inventors have determined that a phosphorus atom-containing compound having an aromatic nucleus has a hydroxyl group as a substituent on the aromatic nucleus as an additive flame retardant in the epoxy resin composition. When used, the cured product was found to have flame retardancy and excellent heat resistance, and the present invention was completed.
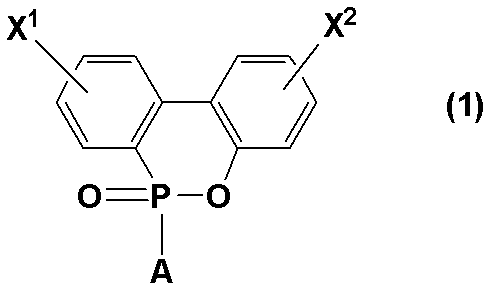
即ち、本発明は、エポキシ樹脂(A)、硬化剤(B)、及び、下記構造式(1) That is, the present invention includes an epoxy resin (A), a curing agent (B), and the following structural formula (1).
(式中、X1及びX2は、それぞれ独立的に水素原子又は水酸基であり、Aは水素原子又は水酸基である。)で表されるリン原子含有化合物(C)を必須成分とすることを特徴とする硬化性樹脂組成物に関する。
(Wherein, X 1 and X 2 are each independently a hydrogen atom or a hydroxyl group, and A is a hydrogen atom or a hydroxyl group). The phosphorus atom-containing compound (C) represented by The present invention relates to a curable resin composition.
本発明は、更に、前記硬化性樹脂組成物を硬化させてなる硬化物に関する。 The present invention further relates to a cured product obtained by curing the curable resin composition.
本発明は、更に、前記硬化性樹脂組成物からなるプリント配線基板用樹脂組成物に関する。 The present invention further relates to a printed wiring board resin composition comprising the curable resin composition.
本発明は、更に、前記硬化性樹脂組成物からなるフレキシブル配線基板用樹脂組成物に関する。 The present invention further relates to a resin composition for a flexible wiring board comprising the curable resin composition.
本発明は、更に、前記硬化性樹脂組成物をガラス基材に含浸、次いで硬化させてなるプリント配線基板に関する。 The present invention further relates to a printed wiring board obtained by impregnating a glass substrate with the curable resin composition and then curing the glass substrate.
本発明は、更に、前記硬化性樹脂組成物に加え、更に無機充填剤を含有する半導体封止材料用樹脂組成物に関する。 The present invention further relates to a resin composition for a semiconductor sealing material further containing an inorganic filler in addition to the curable resin composition.
本発明は、更に、前記硬化性樹脂組成物からなるビルドアップ基板用層間絶縁材料用樹脂組成物に関する。 The present invention further relates to a resin composition for an interlayer insulating material for build-up substrates, comprising the curable resin composition.
本発明によれば、硬化物における難燃性、耐熱性に優れる硬化性樹脂組成物、その硬化物、及び、該硬化性樹脂組成物を用いたプリント配線基板用樹脂組成物、プリント配線基板、フレキシブル配線基板用樹脂組成物、半導体封止材料用樹脂組成物、及びビルドアップ基板用層間絶縁材料用樹脂組成物を提供できる。 According to the present invention, a curable resin composition excellent in flame retardancy and heat resistance in a cured product, the cured product, and a resin composition for a printed wiring board using the curable resin composition, a printed wiring board, The resin composition for flexible wiring boards, the resin composition for semiconductor sealing materials, and the resin composition for interlayer insulation materials for buildup boards can be provided.
以下、本発明を詳細に説明する。 Hereinafter, the present invention will be described in detail.
本発明の硬化性樹脂組成物に用いるリン原子含有化合物(C)は、前記した通り、下記構造式(1) As described above, the phosphorus atom-containing compound (C) used in the curable resin composition of the present invention has the following structural formula (1).
(式中、X1及びX2は、それぞれ独立的に水素原子又は水酸基であり、Aは水素原子又は水酸基である。但し、X1及びX2の少なくとも一方は水酸基である。)で表されるものであり、具体的には、下記構造式a1〜a6のものが挙げられる。
(Wherein, X 1 and X 2 are each independently a hydrogen atom or a hydroxyl group, and A is a hydrogen atom or a hydroxyl group, provided that at least one of X 1 and X 2 is a hydroxyl group). Specifically, those of the following structural formulas a1 to a6 can be mentioned.
本発明では、これらの中でも特に溶剤溶解性に優れ、かつ硬化性樹脂組成物の硬化物における耐熱性がより顕著に優れたものとなる点から構造式a1、a2、又はa3で表されるものが好ましい。 In the present invention, among these, those represented by the structural formula a1, a2, or a3 are particularly excellent in solvent solubility and more remarkably excellent in heat resistance in the cured product of the curable resin composition. Is preferred.
上記したリン原子含有化合物(C)は、例えば、以下の方法で製造することができる。
例えば、前記化合物a1を製造する場合には、フェニルハイドロキノンを原料とし、三塩化リンを反応触媒として下記反応方法1に従って製造することができる。また、前記化合物a2を製造する場合には、ジヒドロキシビフェニルを原料とし、三塩化リンを反応触媒として下記反応方法2に従って製造することができ、同様に、前記化合物a3を製造する場合には、ヒドロキシフェニルハイドロキノンを原料とし、三塩化リンを反応触媒として下記反応方法3に従って製造することができる。
The phosphorus atom-containing compound (C) described above can be produced, for example, by the following method.
For example, when the compound a1 is produced, it can be produced according to the following reaction method 1 using phenylhydroquinone as a raw material and phosphorus trichloride as a reaction catalyst. Further, when the compound a2 is produced, it can be produced according to the following reaction method 2 using dihydroxybiphenyl as a raw material and phosphorus trichloride as a reaction catalyst. Similarly, when producing the compound a3, It can be produced according to the following reaction method 3 using phenylhydroquinone as a raw material and phosphorus trichloride as a reaction catalyst.
また、本発明では、リン原子含有化合物(C)の配合割合は、(A)〜(C)の合計質量を基準に5〜30質量%の範囲であることが好ましいが、特に、(A)〜(C)の合計質量を基準にして、リン原子の質量割合(リン含有量)が1.0〜4.0質量%となる割合であることが硬化物の難燃性に優れる点から好ましく、中でも、1.0〜3.0質量%となる割合であることが耐熱性に優れる点から好ましい。ここでリン原子の質量割合は、具体的には、「JIS K0102−46」に準拠して、リン原子含有化合物(C)中の燐含有率を測定し、(A)〜(C)の合計質量中のリン原子の質量を算出した値である。 In the present invention, the proportion of the phosphorus atom-containing compound (C) is preferably in the range of 5 to 30% by mass based on the total mass of (A) to (C). It is preferable from the point which is excellent in the flame retardance of hardened | cured material that it is a ratio from which the mass ratio (phosphorus content) of a phosphorus atom will be 1.0-4.0 mass% on the basis of the total mass of-(C). Of these, a ratio of 1.0 to 3.0% by mass is preferable from the viewpoint of excellent heat resistance. Here, the mass proportion of the phosphorus atom is specifically determined based on “JIS K0102-46” by measuring the phosphorus content in the phosphorus atom-containing compound (C), and the total of (A) to (C). It is the value which calculated the mass of the phosphorus atom in mass.
次に、本発明の硬化性樹脂組成物において用いるエポキシ樹脂(A)は、種々のエポキシ樹脂を用いることができるが、例えば、ビスフェノールA型エポキシ樹脂、ビスフェノールF型エポキシ樹脂等のビスフェノール型エポキシ樹脂;ビフェニル型エポキシ樹脂、テトラメチルビフェニル型エポキシ樹脂等のビフェニル型エポキシ樹脂;フェノールノボラック型エポキシ樹脂、クレゾールノボラック型エポキシ樹脂、ビスフェノールAノボラック型エポキシ樹脂、フェノール化合物とフェノール性水酸基を有する芳香族アルデヒドとの縮合物のエポキシ化物、ビフェノールノボラック型エポキシ樹脂等のノボラック型エポキシ樹脂;トリフェニルメタン型エポキシ樹脂;テトラフェニルエタン型エポキシ樹脂;ジシクロペンタジエン−フェノール付加反応型エポキシ樹脂;フェノールアラルキル型エポキシ樹脂;ナフトールノボラック型エポキシ樹脂、ナフトールアラルキル型エポキシ樹脂、ナフトール−フェノール共縮ノボラック型エポキシ樹脂、ナフトール−クレゾール共縮ノボラック型エポキシ樹脂、ジグリシジルオキシナフタレン、1,1−ビス(2,7−ジグリシジルオキシ−1−ナフチル)アルカン等の分子構造中にナフタレン骨格を有するエポキシ樹脂;リン原子含有エポキシ樹脂等が挙げられる。また、これらのエポキシ樹脂は単独で用いてもよく、2種以上を混合してもよい。 Next, as the epoxy resin (A) used in the curable resin composition of the present invention, various epoxy resins can be used. For example, bisphenol type epoxy resins such as bisphenol A type epoxy resin and bisphenol F type epoxy resin. Biphenyl type epoxy resins such as biphenyl type epoxy resins and tetramethyl biphenyl type epoxy resins; phenol novolac type epoxy resins, cresol novolac type epoxy resins, bisphenol A novolac type epoxy resins, phenolic compounds and aromatic aldehydes having phenolic hydroxyl groups; Epoxy products of condensates, novolac epoxy resins such as biphenol novolac epoxy resins; triphenylmethane epoxy resins; tetraphenylethane epoxy resins; dicyclopentadiene-fe Addition reaction type epoxy resin; phenol aralkyl type epoxy resin; naphthol novolak type epoxy resin, naphthol aralkyl type epoxy resin, naphthol-phenol co-condensed novolac type epoxy resin, naphthol-cresol co-condensed novolac type epoxy resin, diglycidyloxynaphthalene An epoxy resin having a naphthalene skeleton in a molecular structure such as 1,1-bis (2,7-diglycidyloxy-1-naphthyl) alkane; a phosphorus atom-containing epoxy resin, and the like. Moreover, these epoxy resins may be used independently and may mix 2 or more types.
ここで、リン原子含有エポキシ樹脂としては、9,10−ジヒドロ−9−オキサ−10−ホスファフェナントレン−10−オキサイド(以下、「HCA」と略記する。)のエポキシ化物、HCAとキノン化合物とを反応させて得られるフェノール樹脂のエポキシ化物、フェノールノボラック型エポキシ樹脂をHCAで変性したエポキシ樹脂、クレゾールノボラック型エポキシ樹脂をHCAで変性したエポキシ樹脂、また、ビスフェノールA型エポキシ樹脂を、HCAとキノン化合物とを反応させて得られるフェノール樹脂で変成して得られるエポキシ樹脂、及びビスフェノールF型エポキシ樹脂を、HCAとキノン類とを反応させて得られるフェノール樹脂で変成して得られるエポキシ樹脂等が挙げられる。 Here, as the phosphorus atom-containing epoxy resin, an epoxidized product of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (hereinafter abbreviated as “HCA”), HCA and a quinone compound Epoxy product of phenol resin obtained by reacting phenolic resin, epoxy resin obtained by modifying phenol novolac type epoxy resin with HCA, epoxy resin obtained by modifying cresol novolac type epoxy resin with HCA, and bisphenol A type epoxy resin using HCA and quinone An epoxy resin obtained by modifying with a phenol resin obtained by reacting with a compound, an epoxy resin obtained by modifying a bisphenol F type epoxy resin with a phenol resin obtained by reacting HCA and quinones, and the like Can be mentioned.
上記したエポキシ樹脂(A)のなかでも、特に耐熱性の点から、分子構造中にノボラック型エポキシ樹脂、ナフタレン骨格を有するエポキシ樹脂が好ましく、また、溶剤溶解性の点からビスフェノール型エポキシ樹脂、ノボラック型エポキシ樹脂が好ましい。 Among the above-mentioned epoxy resins (A), novolak-type epoxy resins and epoxy resins having a naphthalene skeleton are preferable in the molecular structure particularly from the viewpoint of heat resistance, and bisphenol-type epoxy resins and novolaks from the viewpoint of solvent solubility. Type epoxy resin is preferred.
本発明の硬化性樹脂組成物において用いる前記硬化剤(B)は、アミン系化合物、アミド系化合物、酸無水物系化合物、フェノ−ル系化合物などが挙げられる。具体的には、アミン系化合物としてはジアミノジフェニルメタン、ジエチレントリアミン、トリエチレンテトラミン、ジアミノジフェニルスルホン、イソホロンジアミン、イミダゾ−ル、BF3−アミン錯体、グアニジン誘導体等が挙げられ、アミド系化合物としては、ジシアンジアミド、リノレン酸の2量体とエチレンジアミンとより合成されるポリアミド樹脂等が挙げられ、酸無水物系化合物としては、無水フタル酸、無水トリメリット酸、無水ピロメリット酸、無水マレイン酸、テトラヒドロ無水フタル酸、メチルテトラヒドロ無水フタル酸、無水メチルナジック酸、ヘキサヒドロ無水フタル酸、メチルヘキサヒドロ無水フタル酸等が挙げられ、フェノール系化合物としては、フェノールノボラック樹脂、クレゾールノボラック樹脂、芳香族炭化水素ホルムアルデヒド樹脂変性フェノール樹脂、ジシクロペンタジエンフェノール付加型樹脂、フェノールアラルキル樹脂(ザイロック樹脂)、ナフトールアラルキル樹脂、トリメチロールメタン樹脂、テトラフェニロールエタン樹脂、ナフトールノボラック樹脂、ナフトール−フェノール共縮ノボラック樹脂、ナフトール−クレゾール共縮ノボラック樹脂、ビフェニル変性フェノール樹脂(ビスメチレン基でフェノール核が連結された多価フェノール化合物)、ビフェニル変性ナフトール樹脂(ビスメチレン基でフェノール核が連結された多価ナフトール化合物)、アルコキシ基含有芳香環変性ノボラック樹脂(ホルムアルデヒドでフェノール核及びアルコキシ基含有芳香環が連結された多価フェノール化合物)等の多価フェノール化合物が挙げられる。 Examples of the curing agent (B) used in the curable resin composition of the present invention include amine compounds, amide compounds, acid anhydride compounds, phenol compounds, and the like. Specifically, examples of the amine compound include diaminodiphenylmethane, diethylenetriamine, triethylenetetramine, diaminodiphenylsulfone, isophoronediamine, imidazole, BF 3 -amine complex, and guanidine derivative. Examples of the amide compound include dicyandiamide. And polyamide resins synthesized from dimer of linolenic acid and ethylenediamine. Examples of acid anhydride compounds include phthalic anhydride, trimellitic anhydride, pyromellitic anhydride, maleic anhydride, and tetrahydrophthalic anhydride. Acid, methyltetrahydrophthalic anhydride, methyl nadic anhydride, hexahydrophthalic anhydride, methylhexahydrophthalic anhydride, etc., and phenolic compounds include phenol novolac resin, cresol novolac resin Aromatic hydrocarbon formaldehyde resin modified phenolic resin, dicyclopentadiene phenol addition type resin, phenol aralkyl resin (Zyloc resin), naphthol aralkyl resin, trimethylol methane resin, tetraphenylol ethane resin, naphthol novolak resin, naphthol-phenol co-condensation Novolac resin, naphthol-cresol co-condensed novolak resin, biphenyl-modified phenol resin (polyhydric phenol compound with phenol nucleus linked by bismethylene group), biphenyl-modified naphthol resin (polyvalent naphthol compound with phenol nucleus linked by bismethylene group) , Polyhydric phenols such as alkoxy group-containing aromatic ring-modified novolak resins (polyhydric phenol compounds in which a phenol nucleus and an alkoxy group-containing aromatic ring are linked with formaldehyde) Compounds.
これらの中でも、特に芳香族骨格を分子構造内に多く含むものが低熱膨張性の点から好ましく、具体的には、フェノールノボラック樹脂、クレゾールノボラック樹脂、芳香族炭化水素ホルムアルデヒド樹脂変性フェノール樹脂、フェノールアラルキル樹脂、ナフトールアラルキル樹脂、ナフトールノボラック樹脂、ナフトール−フェノール共縮ノボラック樹脂、ナフトール−クレゾール共縮ノボラック樹脂、ビフェニル変性フェノール樹脂、ビフェニル変性ナフトール樹脂、アルコキシ基含有芳香環変性ノボラック樹脂(ホルムアルデヒドでフェノール核及びアルコキシ基含有芳香環が連結された多価フェノール化合物)が低熱膨張性に優れることから好ましい。 Among these, those containing a large amount of an aromatic skeleton in the molecular structure are preferred from the viewpoint of low thermal expansion, and specifically, phenol novolac resins, cresol novolac resins, aromatic hydrocarbon formaldehyde resin-modified phenol resins, phenol aralkyls. Resin, naphthol aralkyl resin, naphthol novolak resin, naphthol-phenol co-condensed novolak resin, naphthol-cresol co-condensed novolak resin, biphenyl-modified phenol resin, biphenyl-modified naphthol resin, alkoxy group-containing aromatic ring-modified novolak resin (formaldehyde with phenol nucleus and A polyhydric phenol compound in which an alkoxy group-containing aromatic ring is linked is preferred because of its low thermal expansion.
本発明の硬化性樹脂組成物におけるエポキシ樹脂(A)と硬化剤(B)の配合割合は、前記したとおり、エポキシ樹脂(A)のエポキシ基の合計1当量に対して、硬化剤(B)中の活性水素又は酸無水物基が0.7〜1.5当量となる割合であることが好ましい。 The blending ratio of the epoxy resin (A) and the curing agent (B) in the curable resin composition of the present invention is as described above, with respect to a total of 1 equivalent of the epoxy groups of the epoxy resin (A), the curing agent (B). It is preferable that the ratio of the active hydrogen or acid anhydride group is 0.7 to 1.5 equivalents.
また必要に応じて本発明の硬化性樹脂組成物に硬化促進剤(D)を適宜併用することもできる。前記硬化促進剤(D)としては種々のものが使用できるが、例えば、リン系化合物、第3級アミン、イミダゾール、有機酸金属塩、ルイス酸、アミン錯塩等が挙げられる。特に半導体封止材料用途として使用する場合には、硬化性、耐熱性、電気特性、耐湿信頼性等に優れる点から、リン系化合物ではトリフェニルフォスフィン、アミン系化合物では2−エチル4−メチルイミダゾールが好ましい。 Moreover, a hardening accelerator (D) can also be suitably used together with the curable resin composition of this invention as needed. Various curing accelerators (D) can be used, and examples thereof include phosphorus compounds, tertiary amines, imidazoles, organic acid metal salts, Lewis acids, and amine complex salts. In particular, when used as a semiconductor sealing material, it is excellent in curability, heat resistance, electrical characteristics, moisture resistance reliability, etc., so that triphenylphosphine is used for phosphorus compounds and 2-ethyl 4-methyl is used for amine compounds. Imidazole is preferred.
以上詳述した本発明の硬化性樹脂組成物は、上記各成分の他に有機溶剤(E)を配合することが好ましい。ここで使用し得る前記有機溶剤(E)としては、メチルエチルケトン、アセトン、ジメチルホルムアミド、メチルイソブチルケトン、メトキシプロパノール、シクロヘキサノン、メチルセロソルブ、エチルジグリコールアセテート、プロピレングリコールモノメチルエーテルアセテート等が挙げられ、その選択や適正な使用量は用途によって適宜選択し得るが、例えば、プリント配線板用途では、メチルエチルケトン、アセトン、1−メトキシ−2−プロパノール等の沸点が160℃以下の極性溶剤であることが好ましく、また、不揮発分40〜80質量%となる割合で使用することが好ましい。一方、ビルドアップ用接着フィルム用途では、有機溶剤(E)として、例えば、アセトン、メチルエチルケトン、シクロヘキサノン等のケトン類、酢酸エチル、酢酸ブチル、セロソルブアセテート、プロピレングリコールモノメチルエーテルアセテート、カルビトールアセテート等の酢酸エステル類、セロソルブ、ブチルカルビトール等のカルビトール類、トルエン、キシレン等の芳香族炭化水素類、ジメチルホルムアミド、ジメチルアセトアミド、N−メチルピロリドン等を用いることが好ましく、また、不揮発分30〜60質量%となる割合で使用することが好ましい。 The curable resin composition of the present invention described in detail above preferably contains an organic solvent (E) in addition to the above components. Examples of the organic solvent (E) that can be used here include methyl ethyl ketone, acetone, dimethylformamide, methyl isobutyl ketone, methoxypropanol, cyclohexanone, methyl cellosolve, ethyl diglycol acetate, propylene glycol monomethyl ether acetate, etc. The proper amount used can be appropriately selected depending on the application, but for example, in a printed wiring board application, it is preferable to use a polar solvent having a boiling point of 160 ° C. or lower, such as methyl ethyl ketone, acetone, 1-methoxy-2-propanol, etc. The non-volatile content is preferably 40 to 80% by mass. On the other hand, in build-up adhesive film applications, examples of organic solvents (E) include ketones such as acetone, methyl ethyl ketone, and cyclohexanone, acetic acid such as ethyl acetate, butyl acetate, cellosolve acetate, propylene glycol monomethyl ether acetate, and carbitol acetate. Esters, carbitols such as cellosolve and butyl carbitol, aromatic hydrocarbons such as toluene and xylene, dimethylformamide, dimethylacetamide, N-methylpyrrolidone, etc. are preferably used, and non-volatile content is 30 to 60 mass. It is preferable to use it in the ratio which becomes%.
また、上記熱硬化性樹脂組成物は、難燃性を発揮させるために、例えばプリント配線板の分野においては、信頼性を低下させない範囲で、実質的にハロゲン原子を含有しない非ハロゲン系難燃剤を配合してもよい。 The thermosetting resin composition is a non-halogen flame retardant that substantially does not contain a halogen atom in order to exert flame retardancy, for example, in the field of printed wiring boards, as long as the reliability is not lowered. May be blended.
前記非ハロゲン系難燃剤としては、例えば、窒素系難燃剤、シリコーン系難燃剤、無機系難燃剤、有機金属塩系難燃剤等が挙げられ、それらの使用に際しても何等制限されるものではなく、単独で使用しても、同一系の難燃剤を複数用いても良く、また、異なる系の難燃剤を組み合わせて用いることも可能である。 Examples of the non-halogen flame retardants include nitrogen flame retardants, silicone flame retardants, inorganic flame retardants, organometallic salt flame retardants, and the like, and are not limited in any way. Even if it uses independently, multiple flame retardants of the same system may be used, and it is also possible to use a combination of flame retardants of different systems.
前記窒素系難燃剤としては、例えば、トリアジン化合物、シアヌル酸化合物、イソシアヌル酸化合物、フェノチアジン等が挙げられ、トリアジン化合物、シアヌル酸化合物、イソシアヌル酸化合物が好ましい。 Examples of the nitrogen-based flame retardant include triazine compounds, cyanuric acid compounds, isocyanuric acid compounds, phenothiazines, and the like, and triazine compounds, cyanuric acid compounds, and isocyanuric acid compounds are preferable.
前記トリアジン化合物としては、例えば、メラミン、アセトグアナミン、ベンゾグアナミン、メロン、メラム、サクシノグアナミン、エチレンジメラミン、ポリリン酸メラミン、トリグアナミン等の他、例えば、硫酸グアニルメラミン、硫酸メレム、硫酸メラムなどの硫酸アミノトリアジン化合物、前記アミノトリアジン変性フェノール樹脂、及び該アミノトリアジン変性フェノール樹脂を更に桐油、異性化アマニ油等で変性したもの等が挙げられる。 Examples of the triazine compound include melamine, acetoguanamine, benzoguanamine, melon, melam, succinoguanamine, ethylene dimelamine, melamine polyphosphate, triguanamine, and the like, for example, guanylmelamine sulfate, melem sulfate, melam sulfate, etc. Examples thereof include an aminotriazine sulfate compound, aminotriazine-modified phenol resin, and aminotriazine-modified phenol resin further modified with tung oil, isomerized linseed oil, and the like.
前記シアヌル酸化合物の具体例としては、例えば、シアヌル酸、シアヌル酸メラミン等を挙げることができる。 Specific examples of the cyanuric acid compound include cyanuric acid and cyanuric acid melamine.
前記窒素系難燃剤の配合量としては、窒素系難燃剤の種類、硬化性樹脂組成物の他の成分、所望の難燃性の程度によって適宜選択されるものであるが、例えば、エポキシ樹脂、硬化剤、非ハロゲン系難燃剤及びその他の充填材や添加剤等全てを配合した硬化性樹脂組成物100質量部中、0.05〜10質量部の範囲で配合することが好ましく、特に0.1〜5質量部の範囲で配合することが好ましい。 The compounding amount of the nitrogen-based flame retardant is appropriately selected according to the type of the nitrogen-based flame retardant, the other components of the curable resin composition, and the desired degree of flame retardancy. For example, an epoxy resin, It is preferable to mix in the range of 0.05 to 10 parts by mass in 100 parts by mass of the curable resin composition containing all of the curing agent, non-halogen flame retardant and other fillers and additives. It is preferable to mix | blend in the range of 1-5 mass parts.
また前記窒素系難燃剤を使用する際、金属水酸化物、モリブデン化合物等を併用してもよい。 Moreover, when using the said nitrogen-type flame retardant, you may use together a metal hydroxide, a molybdenum compound, etc.
前記シリコーン系難燃剤としては、ケイ素原子を含有する有機化合物であれば特に制限がなく使用でき、例えば、シリコーンオイル、シリコーンゴム、シリコーン樹脂等が挙げられる。 The silicone flame retardant is not particularly limited as long as it is an organic compound containing a silicon atom, and examples thereof include silicone oil, silicone rubber, and silicone resin.
前記シリコーン系難燃剤の配合量としては、シリコーン系難燃剤の種類、硬化性樹脂組成物の他の成分、所望の難燃性の程度によって適宜選択されるものであるが、例えば、エポキシ樹脂、硬化剤、非ハロゲン系難燃剤及びその他の充填材や添加剤等全てを配合した硬化性樹脂組成物100質量部中、0.05〜20質量部の範囲で配合することが好ましい。また前記シリコーン系難燃剤を使用する際、モリブデン化合物、アルミナ等を併用してもよい。 The amount of the silicone-based flame retardant is appropriately selected depending on the type of the silicone-based flame retardant, the other components of the curable resin composition, and the desired degree of flame retardancy. For example, an epoxy resin, It is preferable to mix in the range of 0.05 to 20 parts by mass in 100 parts by mass of the curable resin composition containing all of the curing agent, non-halogen flame retardant and other fillers and additives. Moreover, when using the said silicone type flame retardant, you may use a molybdenum compound, an alumina, etc. together.
前記無機系難燃剤としては、例えば、金属水酸化物、金属酸化物、金属炭酸塩化合物、金属粉、ホウ素化合物、低融点ガラス等が挙げられる。 Examples of the inorganic flame retardant include metal hydroxide, metal oxide, metal carbonate compound, metal powder, boron compound, and low melting point glass.
前記金属水酸化物の具体例としては、例えば、水酸化アルミニウム、水酸化マグネシウム、ドロマイト、ハイドロタルサイト、水酸化カルシウム、水酸化バリウム、水酸化ジルコニウム等を挙げることができる。 Specific examples of the metal hydroxide include aluminum hydroxide, magnesium hydroxide, dolomite, hydrotalcite, calcium hydroxide, barium hydroxide, zirconium hydroxide and the like.
前記金属酸化物の具体例としては、例えば、モリブデン酸亜鉛、三酸化モリブデン、スズ酸亜鉛、酸化スズ、酸化アルミニウム、酸化鉄、酸化チタン、酸化マンガン、酸化ジルコニウム、酸化亜鉛、酸化モリブデン、酸化コバルト、酸化ビスマス、酸化クロム、酸化ニッケル、酸化銅、酸化タングステン等を挙げることができる。 Specific examples of the metal oxide include, for example, zinc molybdate, molybdenum trioxide, zinc stannate, tin oxide, aluminum oxide, iron oxide, titanium oxide, manganese oxide, zirconium oxide, zinc oxide, molybdenum oxide, and cobalt oxide. Bismuth oxide, chromium oxide, nickel oxide, copper oxide, tungsten oxide and the like.
前記金属炭酸塩化合物の具体例としては、例えば、炭酸亜鉛、炭酸マグネシウム、炭酸カルシウム、炭酸バリウム、塩基性炭酸マグネシウム、炭酸アルミニウム、炭酸鉄、炭酸コバルト、炭酸チタン等を挙げることができる。 Specific examples of the metal carbonate compound include zinc carbonate, magnesium carbonate, calcium carbonate, barium carbonate, basic magnesium carbonate, aluminum carbonate, iron carbonate, cobalt carbonate, and titanium carbonate.
前記金属粉の具体例としては、例えば、アルミニウム、鉄、チタン、マンガン、亜鉛、モリブデン、コバルト、ビスマス、クロム、ニッケル、銅、タングステン、スズ等を挙げることができる。 Specific examples of the metal powder include aluminum, iron, titanium, manganese, zinc, molybdenum, cobalt, bismuth, chromium, nickel, copper, tungsten, and tin.
前記ホウ素化合物の具体例としては、例えば、ホウ酸亜鉛、メタホウ酸亜鉛、メタホウ酸バリウム、ホウ酸、ホウ砂等を挙げることができる。 Specific examples of the boron compound include zinc borate, zinc metaborate, barium metaborate, boric acid, and borax.
前記低融点ガラスの具体例としては、例えば、シープリー(ボクスイ・ブラウン社)、水和ガラスSiO2−MgO−H2O、PbO−B2O3系、ZnO−P2O5−MgO系、P2O5−B2O3−PbO−MgO系、P−Sn−O−F系、PbO−V2O5−TeO2系、Al2O3−H2O系、ホウ珪酸鉛系等のガラス状化合物を挙げることができる。 Specific examples of the low-melting-point glass include, for example, Ceeley (Bokusui Brown), hydrated glass SiO 2 —MgO—H 2 O, PbO—B 2 O 3 system, ZnO—P 2 O 5 —MgO system, P 2 O 5 —B 2 O 3 —PbO—MgO, P—Sn—O—F, PbO—V 2 O 5 —TeO 2 , Al 2 O 3 —H 2 O, lead borosilicate, etc. The glassy compound can be mentioned.
前記無機系難燃剤の配合量としては、無機系難燃剤の種類、硬化性樹脂組成物の他の成分、所望の難燃性の程度によって適宜選択されるものであるが、例えば、エポキシ樹脂、硬化剤、非ハロゲン系難燃剤及びその他の充填材や添加剤等全てを配合した硬化性樹脂組成物100質量部中、0.05〜20質量部の範囲で配合することが好ましく、特に0.5〜15質量部の範囲で配合することが好ましい。 The amount of the inorganic flame retardant is appropriately selected depending on the type of the inorganic flame retardant, the other components of the curable resin composition, and the desired degree of flame retardancy. For example, an epoxy resin, It is preferable to mix in the range of 0.05 to 20 parts by mass in 100 parts by mass of the curable resin composition containing all of the curing agent, non-halogen flame retardant and other fillers and additives. It is preferable to mix | blend in 5-15 mass parts.
前記有機金属塩系難燃剤としては、例えば、フェロセン、アセチルアセトナート金属錯体、有機金属カルボニル化合物、有機コバルト塩化合物、有機スルホン酸金属塩、金属原子と芳香族化合物又は複素環化合物がイオン結合又は配位結合した化合物等が挙げられる。 Examples of the organic metal salt flame retardant include ferrocene, acetylacetonate metal complex, organic metal carbonyl compound, organic cobalt salt compound, organic sulfonic acid metal salt, metal atom and aromatic compound or heterocyclic compound or an ionic bond or Examples thereof include a coordinated compound.
前記有機金属塩系難燃剤の配合量としては、有機金属塩系難燃剤の種類、硬化性樹脂組成物の他の成分、所望の難燃性の程度によって適宜選択されるものであるが、例えば、エポキシ樹脂、硬化剤、非ハロゲン系難燃剤及びその他の充填材や添加剤等全てを配合した硬化性樹脂組成物100質量部中、0.005〜10質量部の範囲で配合することが好ましい。 The amount of the organic metal salt flame retardant is appropriately selected depending on the type of the organic metal salt flame retardant, the other components of the curable resin composition, and the desired degree of flame retardancy. In 100 parts by mass of the curable resin composition in which all of epoxy resin, curing agent, non-halogen flame retardant and other fillers and additives are blended, it is preferably blended in the range of 0.005 to 10 parts by mass. .
本発明の硬化性樹脂組成物には、必要に応じて無機質充填材を配合することができる。前記無機質充填材としては、例えば、溶融シリカ、結晶シリカ、アルミナ、窒化珪素、水酸化アルミ等が挙げられる。前記無機充填材の配合量を特に大きくする場合は溶融シリカを用いることが好ましい。前記溶融シリカは破砕状、球状のいずれでも使用可能であるが、溶融シリカの配合量を高め且つ成形材料の溶融粘度の上昇を抑制するためには、球状のものを主に用いる方が好ましい。更に球状シリカの配合量を高めるためには、球状シリカの粒度分布を適当に調整することが好ましい。その充填率は難燃性を考慮して、高い方が好ましく、硬化性樹脂組成物の全体量に対して20質量%以上が特に好ましい。また導電ペーストなどの用途に使用する場合は、銀粉や銅粉等の導電性充填剤を用いることができる。 An inorganic filler can be mix | blended with the curable resin composition of this invention as needed. Examples of the inorganic filler include fused silica, crystalline silica, alumina, silicon nitride, and aluminum hydroxide. When particularly increasing the blending amount of the inorganic filler, it is preferable to use fused silica. The fused silica can be used in either a crushed shape or a spherical shape. However, in order to increase the blending amount of the fused silica and suppress an increase in the melt viscosity of the molding material, it is preferable to mainly use a spherical shape. In order to further increase the blending amount of the spherical silica, it is preferable to appropriately adjust the particle size distribution of the spherical silica. The filling rate is preferably higher in consideration of flame retardancy, and particularly preferably 20% by mass or more with respect to the total amount of the curable resin composition. Moreover, when using for uses, such as an electrically conductive paste, electroconductive fillers, such as silver powder and copper powder, can be used.
本発明の硬化性樹脂組成物は、必要に応じて、シランカップリング剤、離型剤、顔料、乳化剤等の種々の配合剤を添加することができる。 Various compounding agents, such as a silane coupling agent, a mold release agent, a pigment, an emulsifier, can be added to the curable resin composition of this invention as needed.
本発明の硬化性樹脂組成物は、上記した各成分を均一に混合することにより得られる。本発明のエポキシ樹脂、硬化剤、更に必要により硬化促進剤の配合された本発明の硬化性樹脂組成物は従来知られている方法と同様の方法で容易に硬化物とすることができる。該硬化物としては積層物、注型物、接着層、塗膜、フィルム等の成形硬化物が挙げられる。 The curable resin composition of the present invention can be obtained by uniformly mixing the above-described components. The curable resin composition of the present invention in which the epoxy resin of the present invention, a curing agent, and further, if necessary, a curing accelerator are blended can be easily made into a cured product by a method similar to a conventionally known method. Examples of the cured product include molded cured products such as laminates, cast products, adhesive layers, coating films, and films.
本発明の硬化性樹脂組成物が用いられる用途としては、プリント配線板材料、フレキシルブル配線基板用樹脂組成物、ビルドアップ基板用層間絶縁材料、半導体封止材料、導電ペースト、ビルドアップ用接着フィルム、樹脂注型材料、接着剤、等が挙げられる。また、これら各種用途のうち、プリント配線板や電子回路基板用絶縁材料、ビルドアップ用接着フィルム用途では、コンデンサ等の受動部品やICチップ等の能動部品を基板内に埋め込んだ所謂電子部品内蔵用基板用の絶縁材料として用いることができる。これらの中でも、高難燃性、高耐熱性、低熱膨張性、及び溶剤溶解性といった特性からフレキシルブル配線基板用樹脂組成物、ビルドアップ基板用層間絶縁材料、半導体封止材料に用いることが好ましい。 Applications for use of the curable resin composition of the present invention include printed wiring board materials, resin compositions for flexible wiring boards, interlayer insulating materials for build-up boards, semiconductor sealing materials, conductive pastes, and adhesive films for build-ups Resin casting materials, adhesives, and the like. Among these various applications, in printed circuit boards, insulating materials for electronic circuit boards, and adhesive films for build-up, passive parts such as capacitors and active parts such as IC chips are embedded in so-called electronic parts. It can be used as an insulating material for a substrate. Among these, from the characteristics such as high flame retardancy, high heat resistance, low thermal expansibility, and solvent solubility, it is preferably used for a resin composition for a flexible wiring board, an interlayer insulating material for a build-up board, and a semiconductor sealing material. .
ここで、本発明の硬化性樹脂組成物からプリント回路基板を製造するには、前記有機溶剤(E)を含むワニス状の硬化性樹脂組成物を、更に有機溶剤(E)を配合してワニス化した樹脂組成物を、補強基材に含浸し銅箔を重ねて加熱圧着させる方法が挙げられる。ここで使用し得る補強基材は、紙、ガラス布、ガラス不織布、アラミド紙、アラミド布、ガラスマット、ガラスロービング布などが挙げられる。かかる方法を更に詳述すれば、先ず、前記したワニス状の硬化性樹脂組成物を、用いた溶剤種に応じた加熱温度、好ましくは50〜170℃で加熱することによって、硬化物であるプリプレグを得る。この時用いる樹脂組成物と補強基材の質量割合としては、特に限定されないが、通常、プリプレグ中の樹脂分が20〜60質量%となるように調製することが好ましい。次いで、上記のようにして得られたプリプレグを、常法により積層し、適宜銅箔を重ねて、1〜10MPaの加圧下に170〜250℃で10分〜3時間、加熱圧着させることにより、目的とするプリント回路基板を得ることができる。 Here, in order to produce a printed circuit board from the curable resin composition of the present invention, the varnish-like curable resin composition containing the organic solvent (E) is further blended with the organic solvent (E) to obtain a varnish. A method of impregnating a reinforced resin composition into a reinforcing base material and stacking a copper foil to heat-press is mentioned. Examples of the reinforcing substrate that can be used here include paper, glass cloth, glass nonwoven fabric, aramid paper, aramid cloth, glass mat, and glass roving cloth. If this method is described in further detail, first, the varnish-like curable resin composition is heated at a heating temperature corresponding to the solvent type used, preferably 50 to 170 ° C., thereby being a prepreg which is a cured product. Get. The mass ratio of the resin composition and the reinforcing substrate used at this time is not particularly limited, but it is usually preferable that the resin content in the prepreg is 20 to 60% by mass. Next, the prepreg obtained as described above is laminated by a conventional method, and a copper foil is appropriately stacked, and then subjected to thermocompression bonding at a pressure of 1 to 10 MPa at 170 to 250 ° C. for 10 minutes to 3 hours, A desired printed circuit board can be obtained.
本発明の硬化性樹脂組成物からフレキシルブル配線基板を製造するには、前記エポキシ樹脂(A)、硬化剤(B)、リン原子含有化合物(C)、硬化促進剤(D)、及び有機溶剤(E)を配合して、リバースロールコータ、コンマコータ等の塗布機を用いて、電気絶縁性フィルムに塗布する。次いで、加熱機を用いて60〜170℃で1〜15分間加熱し、溶媒を揮発させて、接着剤組成物をB−ステージ化する。次いで、加熱ロール等を用いて、接着剤に金属箔を熱圧着する。その際の圧着圧力は2〜200N/cm、圧着温度は40〜200℃が好ましい。それで十分な接着性能が得られれば、ここで終えても構わないが、完全硬化が必要な場合は、さらに100〜200℃で1〜24時間の条件で後硬化させることが好ましい。最終的に硬化させた後の接着剤組成物膜の厚みは、5〜100μmの範囲が好ましい。 In order to produce a flexible wiring board from the curable resin composition of the present invention, the epoxy resin (A), the curing agent (B), the phosphorus atom-containing compound (C), the curing accelerator (D), and an organic solvent are used. (E) is blended and applied to the electrically insulating film using a coating machine such as a reverse roll coater or a comma coater. Subsequently, it heats at 60-170 degreeC for 1 to 15 minutes using a heating machine, volatilizes a solvent, and B-stages an adhesive composition. Next, the metal foil is thermocompression bonded to the adhesive using a heating roll or the like. At that time, the pressure is preferably 2 to 200 N / cm and the pressure is preferably 40 to 200 ° C. If sufficient adhesive performance can be obtained, the process may be completed here. However, when complete curing is required, it is preferably post-cured at 100 to 200 ° C. for 1 to 24 hours. The thickness of the adhesive composition film after finally curing is preferably in the range of 5 to 100 μm.
本発明の硬化性樹脂組成物から半導体封止材料を調整するには、半導体封止材用に調製されたエポキシ樹脂組成物を作製するためには、前記エポキシ樹脂(A)、硬化剤(B)、リン原子含有化合物(C)、硬化促進剤(D)、及び無機充填剤等の配合剤を必要に応じて押出機、ニ−ダ、ロ−ル等を用いて均一になるまで充分に溶融混合して得ることができる。その際、無機充填剤としては、通常シリカが用いられるが、その充填率はエポキシ樹脂組成物100質量部当たり、充填剤を30〜95質量%の範囲が用いることが好ましく、中でも、難燃性や耐湿性や耐ハンダクラック性の向上、線膨張係数の低下を図るためには、70質量部以上が特に好ましく、それらの効果を格段に上げるためには、80質量部以上が一層その効果を高めることができる。半導体パッケージ成形としては、該組成物を注型、或いはトランスファー成形機、射出成形機などを用いて成形し、さらに50〜200℃で2〜10時間に加熱することにより成形物である半導体装置を得る方法がある In order to adjust the semiconductor sealing material from the curable resin composition of the present invention, the epoxy resin (A), the curing agent (B ), Phosphorus atom-containing compound (C), curing accelerator (D), and compounding agents such as inorganic fillers, if necessary, using an extruder, kneader, roll, etc., until they are uniform. It can be obtained by melt mixing. At that time, silica is usually used as the inorganic filler, and the filling rate is preferably in the range of 30 to 95% by mass of the filler per 100 parts by mass of the epoxy resin composition. 70 parts by mass or more is particularly preferable in order to improve the moisture resistance and solder crack resistance and decrease the linear expansion coefficient, and 80 parts by mass or more is more effective in order to significantly increase these effects. Can be increased. For semiconductor package molding, the composition is molded by casting, using a transfer molding machine, an injection molding machine or the like, and further heated at 50 to 200 ° C. for 2 to 10 hours to form a semiconductor device which is a molded product. There is a way to get
本発明の硬化性樹脂組成物からビルドアップ基板用層間絶縁材料を得る方法としては例えば、ゴム、フィラーなどを適宜配合した当該硬化性樹脂組成物を、回路を形成した配線基板にスプレーコーティング法、カーテンコーティング法等を用いて塗布した後、硬化させる。その後、必要に応じて所定のスルーホール部等の穴あけを行った後、粗化剤により処理し、その表面を湯洗することによって、凹凸を形成させ、銅などの金属をめっき処理する。前記めっき方法としては、無電解めっき、電解めっき処理が好ましく、また前記粗化剤としては酸化剤、アルカリ、有機溶剤等が挙げられる。このような操作を所望に応じて順次繰り返し、樹脂絶縁層及び所定の回路パターンの導体層を交互にビルドアップして形成することにより、ビルドアップ基盤を得ることができる。但し、スルーホール部の穴あけは、最外層の樹脂絶縁層の形成後に行う。また、銅箔上で当該樹脂組成物を半硬化させた樹脂付き銅箔を、回路を形成した配線基板上に、170〜250℃で加熱圧着することで、粗化面を形成、メッキ処理の工程を省き、ビルドアップ基板を作製することも可能である。 As a method for obtaining an interlayer insulating material for a build-up substrate from the curable resin composition of the present invention, for example, the curable resin composition appropriately blended with rubber, filler, etc., spray coating method on a wiring board on which a circuit is formed, After applying using a curtain coating method or the like, it is cured. Then, after drilling a predetermined through-hole part etc. as needed, it treats with a roughening agent, forms the unevenness | corrugation by washing the surface with hot water, and metal-treats, such as copper. As the plating method, electroless plating or electrolytic plating treatment is preferable, and examples of the roughening agent include an oxidizing agent, an alkali, and an organic solvent. Such operations are sequentially repeated as desired, and a build-up base can be obtained by alternately building up and forming the resin insulating layer and the conductor layer having a predetermined circuit pattern. However, the through-hole portion is formed after the outermost resin insulating layer is formed. In addition, a resin-coated copper foil obtained by semi-curing the resin composition on the copper foil is thermocompression-bonded at 170 to 250 ° C. on a circuit board on which a circuit is formed, thereby forming a roughened surface and plating treatment. It is also possible to produce a build-up substrate by omitting the process.
本発明の硬化性樹脂組成物からビルドアップ用接着フィルムを製造する方法は、例えば、本発明の硬化性樹脂組成物を、支持フィルム上に塗布し樹脂組成物層を形成させて多層プリント配線板用の接着フィルムとする方法が挙げられる。 The method for producing an adhesive film for buildup from the curable resin composition of the present invention is, for example, a multilayer printed wiring board in which the curable resin composition of the present invention is applied on a support film to form a resin composition layer. And an adhesive film for use.
本発明の硬化性樹脂組成物をビルドアップ用接着フィルムに用いる場合、該接着フィルムは、真空ラミネート法におけるラミネートの温度条件(通常70℃〜140℃)で軟化し、回路基板のラミネートと同時に、回路基板に存在するビアホール或いはスルーホール内の樹脂充填が可能な流動性(樹脂流れ)を示すことが肝要であり、このような特性を発現するよう上記各成分を配合することが好ましい。 When the curable resin composition of the present invention is used for a build-up adhesive film, the adhesive film is softened under the temperature condition of the laminate in the vacuum laminating method (usually 70 ° C. to 140 ° C.), and simultaneously with the lamination of the circuit board, It is important to show fluidity (resin flow) that allows resin filling in via holes or through holes present in a circuit board, and it is preferable to blend the above-described components so as to exhibit such characteristics.
ここで、多層プリント配線板のスルーホールの直径は通常0.1〜0.5mm、深さは通常0.1〜1.2mmであり、通常この範囲で樹脂充填を可能とするのが好ましい。なお回路基板の両面をラミネートする場合はスルーホールの1/2程度充填されることが望ましい。 Here, the diameter of the through hole of the multilayer printed wiring board is usually 0.1 to 0.5 mm, and the depth is usually 0.1 to 1.2 mm. It is usually preferable to allow resin filling in this range. When laminating both surfaces of the circuit board, it is desirable to fill about 1/2 of the through hole.
上記した接着フィルムを製造する方法は、具体的には、ワニス状の本発明の硬化性樹脂組成物を調製した後、支持フィルムの表面に、このワニス状の組成物を塗布し、更に加熱、あるいは熱風吹きつけ等により有機溶剤を乾燥させて硬化性樹脂組成物の層(α)を形成させることにより製造することができる。 Specifically, the method for producing the adhesive film described above is, after preparing the varnish-like curable resin composition of the present invention, coating the varnish-like composition on the surface of the support film, further heating, Or it can manufacture by drying an organic solvent by hot air spraying etc. and forming the layer ((alpha)) of a curable resin composition.
形成される層(α)の厚さは、通常、導体層の厚さ以上とする。回路基板が有する導体層の厚さは通常5〜70μmの範囲であるので、樹脂組成物層の厚さは10〜100μmの厚みを有するのが好ましい。 The thickness of the formed layer (α) is usually not less than the thickness of the conductor layer. Since the thickness of the conductor layer of the circuit board is usually in the range of 5 to 70 μm, the thickness of the resin composition layer is preferably 10 to 100 μm.
なお、前記層(α)は、後述する保護フィルムで保護されていてもよい。保護フィルムで保護することにより、樹脂組成物層表面へのゴミ等の付着やキズを防止することができる。 In addition, the said layer ((alpha)) may be protected with the protective film mentioned later. By protecting with a protective film, it is possible to prevent dust and the like from being attached to the surface of the resin composition layer and scratches.
前記した支持フィルム及び保護フィルムは、ポリエチレン、ポリプロピレン、ポリ塩化ビニル等のポリオレフィン、ポリエチレンテレフタレート(以下「PET」と略称することがある。)、ポリエチレンナフタレート等のポリエステル、ポリカーボネート、ポリイミド、更には離型紙や銅箔、アルミニウム箔等の金属箔などを挙げることができる。なお、支持フィルム及び保護フィルムはマッド処理、コロナ処理の他、離型処理を施してあってもよい。 The above-mentioned support film and protective film are made of polyolefin such as polyethylene, polypropylene and polyvinyl chloride, polyethylene terephthalate (hereinafter sometimes abbreviated as “PET”), polyester such as polyethylene naphthalate, polycarbonate, polyimide, and further. Examples thereof include metal foil such as pattern paper, copper foil, and aluminum foil. In addition, the support film and the protective film may be subjected to a release treatment in addition to the mud treatment and the corona treatment.
支持フィルムの厚さは特に限定されないが、通常10〜150μmであり、好ましくは25〜50μmの範囲で用いられる。また保護フィルムの厚さは1〜40μmとするのが好ましい。 Although the thickness of a support film is not specifically limited, Usually, it is 10-150 micrometers, Preferably it is used in 25-50 micrometers. Moreover, it is preferable that the thickness of a protective film shall be 1-40 micrometers.
上記した支持フィルムは、回路基板にラミネートした後に、或いは加熱硬化することにより絶縁層を形成した後に、剥離される。接着フィルムを加熱硬化した後に支持フィルムを剥離すれば、硬化工程でのゴミ等の付着を防ぐことができる。硬化後に剥離する場合、通常、支持フィルムには予め離型処理が施される。 The above support film is peeled off after being laminated on a circuit board or after forming an insulating layer by heat curing. If the support film is peeled after the adhesive film is heat-cured, adhesion of dust and the like in the curing process can be prevented. In the case of peeling after curing, the support film is usually subjected to a release treatment in advance.
次に、上記のようして得られた接着フィルムを用いて多層プリント配線板を製造する方法は、例えば、層(α)が保護フィルムで保護されている場合はこれらを剥離した後、層(α)を回路基板に直接接するように、回路基板の片面又は両面に、例えば真空ラミネート法によりラミネートする。ラミネートの方法はバッチ式であってもロールでの連続式であってもよい。またラミネートを行う前に接着フィルム及び回路基板を必要により加熱(プレヒート)しておいてもよい。 Next, the method for producing a multilayer printed wiring board using the adhesive film obtained as described above is, for example, when the layer (α) is protected with a protective film, Lamination is performed on one or both sides of the circuit board by, for example, vacuum laminating so that α) is in direct contact with the circuit board. The laminating method may be a batch method or a continuous method using a roll. Further, the adhesive film and the circuit board may be heated (preheated) as necessary before lamination.
ラミネートの条件は、圧着温度(ラミネート温度)を好ましくは70〜140℃、圧着圧力を好ましくは1〜11kgf/cm2(9.8×104〜107.9×104N/m2)とし、空気圧20mmHg(26.7hPa)以下の減圧下でラミネートすることが好ましい。 The laminating conditions are preferably a pressure bonding temperature (laminating temperature) of 70 to 140 ° C., a pressure bonding pressure of preferably 1 to 11 kgf / cm 2 (9.8 × 10 4 to 107.9 × 10 4 N / m 2), Lamination is preferably performed under reduced pressure with an air pressure of 20 mmHg (26.7 hPa) or less.
本発明の硬化性樹脂組成物を導電ペーストとして使用する場合には、例えば、微細導電性粒子を該硬化性樹脂組成物中に分散させ異方性導電膜用組成物とする方法、室温で液状である回路接続用ペースト樹脂組成物や異方性導電接着剤とする方法が挙げられる。 When the curable resin composition of the present invention is used as a conductive paste, for example, a method of dispersing fine conductive particles in the curable resin composition to obtain a composition for anisotropic conductive film, liquid at room temperature And a paste resin composition for circuit connection and an anisotropic conductive adhesive.
本発明の硬化物を得る方法としては、一般的な硬化性樹脂組成物の硬化方法に準拠すればよいが、例えば加熱温度条件は、組み合わせる硬化剤の種類や用途等によって、適宜選択すればよいが、上記方法によって得られた組成物を、室温〜250℃程度の温度範囲で加熱すればよい。 The method for obtaining the cured product of the present invention may be based on a general curing method for a curable resin composition, but for example, the heating temperature condition may be appropriately selected depending on the kind of curing agent to be combined and the use. However, what is necessary is just to heat the composition obtained by the said method in the temperature range of about room temperature-250 degreeC.
従って、該フェノール樹脂を用いることによって、従来のリンで変性したフェノール樹脂に比べ溶剤溶解性が飛躍的に向上し、さらに硬化物とした際、難燃性と耐熱性及び耐熱信頼性が発現でき、最先端のプリント配線板材料に適用できる。また、該フェノール樹脂は、本発明の製造方法にて容易に効率よく製造する事が出来、目的とする前述の性能のレベルに応じた分子設計が可能となる。 Therefore, by using this phenol resin, the solvent solubility is dramatically improved compared to the conventional phenol resin modified with phosphorus, and when it is made into a cured product, flame retardancy, heat resistance and heat reliability can be expressed. Applicable to the most advanced printed wiring board materials. In addition, the phenol resin can be easily and efficiently produced by the production method of the present invention, and a molecular design corresponding to the target level of performance described above becomes possible.
次に本発明を実施例、比較例により具体的に説明する。 Next, the present invention will be specifically described with reference to examples and comparative examples.
合成例1(リン原子含有化合物(P1)の合成)
コンデンサー、窒素導入管つきの乾燥したフラスコに液状の三塩化燐78mlとベンゾキノン80g(0.43モル)を仕込み、反応させた。発生した塩酸ガスはNaOH水溶液でトラップした。得られた懸濁液を30分間で100℃に昇温し、HClガスが発生しなくなるまで反応させた。続いて、塩化亜鉛(ZnCl2)3gを添加し、1時間で140℃に昇温した。この時点で、HClガスが再び発生していることが確認された。1時間後、この混合物を160℃で撹拌し、全ての揮発成分を除去した。4時間後、温度を50℃に下げ、水30mlを慎重に添加し更に撹拌した。続いて、トルエン20mlと水100mlを添加し、0℃に冷却してろ過した後100mlの冷水で洗浄して98.8gの中間物を得た。この固体は分析により目的物が加水分解した構造であることが確認された(0.395ミリモル、収率92質量%)。
この固体を窒素還流させたフラスコ中で加熱溶解し、140℃1時間撹拌して発生した水分を除去し、目的の燐化合物(P1)2−ヒドロキシ−6H−ジベンゾ[c,e][1,2]オキサフォスフィネン6−オキサイドを得た(収率85質量%)。得られた燐化合物(P2)のリン原子含有量は13.4質量%であった。燐化合物(P1)のNMR分析結果を下記に示す。
Synthesis Example 1 (Synthesis of phosphorus atom-containing compound (P1))
A dry flask equipped with a condenser and a nitrogen introducing tube was charged with 78 ml of liquid phosphorus trichloride and 80 g (0.43 mol) of benzoquinone and reacted. The generated hydrochloric acid gas was trapped with an aqueous NaOH solution. The resulting suspension was heated to 100 ° C. over 30 minutes and reacted until no HCl gas was generated. Subsequently, 3 g of zinc chloride (ZnCl 2 ) was added, and the temperature was raised to 140 ° C. over 1 hour. At this point, it was confirmed that HCl gas was generated again. After 1 hour, the mixture was stirred at 160 ° C. to remove all volatile components. After 4 hours, the temperature was lowered to 50 ° C., 30 ml of water was carefully added and further stirred. Subsequently, 20 ml of toluene and 100 ml of water were added, cooled to 0 ° C., filtered, and washed with 100 ml of cold water to obtain 98.8 g of an intermediate. This solid was confirmed by analysis to have a structure in which the target product was hydrolyzed (0.395 mmol, yield 92 mass%).
This solid was heated and dissolved in a flask refluxed with nitrogen, and the generated water was removed by stirring at 140 ° C. for 1 hour. 2] Oxaphosphinene 6-oxide was obtained (yield 85% by mass). The phosphorus atom content of the obtained phosphorus compound (P2) was 13.4% by mass. The NMR analysis result of the phosphorus compound (P1) is shown below.
NMR(DMSO):
δ(1H)= 8.0 (d, 1H, 606Hz) , 8.03-8.1 (m, 1H) , 7.87-8.0 (m, 1H) , 7.78-7.85 (m, 1H) , 7.58-7.66 (m, 1H) , 7.44-7.47 (m, 1H) , 7.15-7.22 (m, 1H) , 6.88-6.95 (m, 1H);δ(31P)=15.6 ppm,
NMR (DMSO):
δ (1H) = 8.0 (d, 1H, 606Hz), 8.03-8.1 (m, 1H), 7.87-8.0 (m, 1H), 7.78-7.85 (m, 1H), 7.58-7.66 (m, 1H), 7.44-7.47 (m, 1H), 7.15-7.22 (m, 1H), 6.88-6.95 (m, 1H); δ (31P) = 15.6 ppm,
実施例1、2、及び比較例1、2
表1に示した配合に従い、下記の方法でエポキシ樹脂組成物を調整し、次いで、下記の条件で硬化させて、積層板を試作し、各種評価を行った。結果を表2に示す。
Examples 1 and 2 and Comparative Examples 1 and 2
According to the formulation shown in Table 1, the epoxy resin composition was prepared by the following method, and then cured under the following conditions to produce a laminate and various evaluations were performed. The results are shown in Table 2.
[エポキシ樹脂組成物の調整]
下記表2記載の組成に従い、エポキシ樹脂、硬化剤及びその他の各成分を配合した後、最終的に組成物の不揮発分(N.V.)を58質量%となるように調整した。
[Preparation of epoxy resin composition]
According to the composition shown in Table 2 below, an epoxy resin, a curing agent and other components were blended, and the nonvolatile content (NV) of the composition was finally adjusted to 58% by mass.
[積層板作成条件]
基材:100μm;日東紡績株式会社製ガラスクロス「#2116」
プライ数:6
プリプレグ化条件:160℃/2分
銅箔:18μm;日鉱金属株式会社製 JTC箔
硬化条件:200℃、40kg/cm2で1.5時間
成型後板厚:0.8mm
[Laminate creation conditions]
Base material: 100 μm; glass cloth “# 2116” manufactured by Nitto Boseki Co., Ltd.
Number of plies: 6
Pre-pregation condition: 160 ° C / 2 weight foil: 18 µm; Nikko Metal Co., Ltd. JTC foil curing condition: 200 ° C, 40 kg / cm 2 after 1.5 hours molding Plate thickness: 0.8 mm
[物性試験条件]
ガラス転移温度:エッチング処理を施し銅箔除去した後、TMA法(圧縮荷重法)にて測定。昇温スピード10℃/分。
[Physical property test conditions]
Glass transition temperature: measured by TMA method (compression load method) after removing copper foil by etching. Temperature rising speed 10 ° C / min.
燃焼試験:試験方法はUL−94垂直試験に準拠。 Combustion test: Test method conforms to UL-94 vertical test.
耐熱剥離性試験(T288試験):IPC TM650に準拠し、288℃における耐熱剥離性評価(銅箔付)を行った。 Heat-resistant peelability test (T288 test): In accordance with IPC TM650, heat-resistant peelability evaluation (with copper foil) at 288 ° C was performed.
表中の略号は以下の通りである。
N−690:クレゾールノボラック型エポキシ樹脂(DIC(株)製「エピクロンN−690」、エポキシ当量:214g/eq.)
HP−7200H:ジシクロペンタジエン型エポキシ樹脂(DIC(株)製「エピクロンHP−7200H」、エポキシ当量:279g/eq.)
P1:合成例1で得られたリン原子含有化合物(P1)
HCA:9,10−ジヒドロ−9−オキサ−10−ホスファフェナントレン−10−オキサイド
TD−2090:フェノールノボラック型フェノール樹脂(DIC(株)製「TD−2090」、水酸基当量105g/eq)
2E4MZ:2−エチル−4−メチルイミダゾール
Abbreviations in the table are as follows.
N-690: Cresol novolac type epoxy resin (“Epiclon N-690” manufactured by DIC Corporation, epoxy equivalent: 214 g / eq.)
HP-7200H: dicyclopentadiene type epoxy resin (“Epiclon HP-7200H” manufactured by DIC Corporation, epoxy equivalent: 279 g / eq.)
P1: Phosphorus atom-containing compound (P1) obtained in Synthesis Example 1
HCA: 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide TD-2090: phenol novolac type phenol resin (“TD-2090” manufactured by DIC Corporation, hydroxyl group equivalent 105 g / eq)
2E4MZ: 2-ethyl-4-methylimidazole
Claims (11)
(式中、X1及びX2は、それぞれ独立的に水素原子又は水酸基であり、Aは水素原子、又は水酸基である。但し、X1及びX2の少なくとも一方は水酸基である。)で表されるリン原子含有化合物(C)を必須成分とすることを特徴とする硬化性樹脂組成物。 Epoxy resin (A), curing agent (B), and the following structural formula (1)
(Wherein, X 1 and X 2 are each independently a hydrogen atom or a hydroxyl group, and A is a hydrogen atom or a hydroxyl group, provided that at least one of X 1 and X 2 is a hydroxyl group). A curable resin composition comprising a phosphorus atom-containing compound (C) as an essential component.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011028490A JP5598361B2 (en) | 2011-02-14 | 2011-02-14 | Curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build-up board interlayer insulation material resin composition object |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011028490A JP5598361B2 (en) | 2011-02-14 | 2011-02-14 | Curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build-up board interlayer insulation material resin composition object |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012167167A true JP2012167167A (en) | 2012-09-06 |
| JP5598361B2 JP5598361B2 (en) | 2014-10-01 |
Family
ID=46971621
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011028490A Active JP5598361B2 (en) | 2011-02-14 | 2011-02-14 | Curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build-up board interlayer insulation material resin composition object |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP5598361B2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112442258A (en) * | 2019-09-05 | 2021-03-05 | Dic株式会社 | Thermosetting composition, semi-cured product or cured product, semiconductor sealing material, semiconductor device, insulating material, and printed wiring board |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS57205418A (en) * | 1981-05-19 | 1982-12-16 | Hitachi Chem Co Ltd | Epoxy resin composition |
| JP2005097352A (en) * | 2003-09-22 | 2005-04-14 | Dainippon Ink & Chem Inc | Epoxy resin composition, semiconductor sealing material, and semiconductor device |
| JP2010037443A (en) * | 2008-08-06 | 2010-02-18 | Toyo Ink Mfg Co Ltd | Flame retardant and flame-retardant resin composition |
| JP2010184963A (en) * | 2009-02-10 | 2010-08-26 | Dic Corp | Epoxy resin composition and cured product of the same, epoxy resin and method for producing the same, sealing material for semiconductor, and semiconductor device |
| JP2011017026A (en) * | 2010-10-15 | 2011-01-27 | Hitachi Chem Co Ltd | Resin composition, use of the same, and method for preparing the same |
| JP2011026385A (en) * | 2009-07-22 | 2011-02-10 | Dic Corp | Epoxy resin composition, cured product thereof, semiconductor sealing material, semiconductor device and epoxy resin |
-
2011
- 2011-02-14 JP JP2011028490A patent/JP5598361B2/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS57205418A (en) * | 1981-05-19 | 1982-12-16 | Hitachi Chem Co Ltd | Epoxy resin composition |
| JP2005097352A (en) * | 2003-09-22 | 2005-04-14 | Dainippon Ink & Chem Inc | Epoxy resin composition, semiconductor sealing material, and semiconductor device |
| JP2010037443A (en) * | 2008-08-06 | 2010-02-18 | Toyo Ink Mfg Co Ltd | Flame retardant and flame-retardant resin composition |
| JP2010184963A (en) * | 2009-02-10 | 2010-08-26 | Dic Corp | Epoxy resin composition and cured product of the same, epoxy resin and method for producing the same, sealing material for semiconductor, and semiconductor device |
| JP2011026385A (en) * | 2009-07-22 | 2011-02-10 | Dic Corp | Epoxy resin composition, cured product thereof, semiconductor sealing material, semiconductor device and epoxy resin |
| JP2011017026A (en) * | 2010-10-15 | 2011-01-27 | Hitachi Chem Co Ltd | Resin composition, use of the same, and method for preparing the same |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112442258A (en) * | 2019-09-05 | 2021-03-05 | Dic株式会社 | Thermosetting composition, semi-cured product or cured product, semiconductor sealing material, semiconductor device, insulating material, and printed wiring board |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5598361B2 (en) | 2014-10-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4953039B2 (en) | Phosphorus atom-containing oligomer, production method thereof, curable resin composition, cured product thereof, and printed wiring board | |
| JP4930656B2 (en) | Phenol resin composition, production method thereof, curable resin composition, cured product thereof, and printed wiring board | |
| JP5557033B2 (en) | Phosphorus atom-containing oligomer, production method thereof, curable resin composition, cured product thereof, and printed wiring board | |
| JP5458916B2 (en) | Method for producing phosphorus atom-containing phenols, phosphorus atom-containing phenols, curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, for semiconductor sealing material Resin composition and resin composition for interlayer insulation material for build-up substrate | |
| JP5146793B2 (en) | Phosphorus atom-containing oligomer composition, curable resin composition, cured product thereof, and printed wiring board | |
| JP5747725B2 (en) | Novel phosphorus atom-containing epoxy resin, production method thereof, curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, and semiconductor sealing material resin composition | |
| JP5402091B2 (en) | Curable resin composition, cured product thereof, printed wiring board, novel phenol resin, and production method thereof | |
| JP2013067697A (en) | Naphthol resin, curable resin composition, its cured product, and printed circuit board | |
| JP2012201798A (en) | Curable resin composition, cured product thereof, printed wiring board, and naphthol resin | |
| JP5640588B2 (en) | Method for producing phosphorus atom-containing epoxy resin, curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build Resin composition for interlayer insulation material for up-substrate | |
| JP5454009B2 (en) | Curable resin composition, cured product thereof, and printed wiring board | |
| JP5402761B2 (en) | Curable resin composition, cured product thereof, method for producing phosphorus atom-containing phenols, resin composition for printed wiring board, printed wiring board, resin composition for flexible wiring board, resin composition for semiconductor sealing material, and build Resin composition for interlayer insulation material for up-substrate | |
| JP5532307B2 (en) | Method for producing phosphorus atom-containing polyfunctional phenol, phosphorus atom-containing polyfunctional phenol, curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor encapsulation A resin composition for a material and a resin composition for an interlayer insulating material for a build-up substrate. | |
| JP5637368B2 (en) | Curable resin composition, cured product thereof, method for producing phosphorus atom-containing phenol compound, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build Resin composition for interlayer insulation material for up-substrate | |
| JP5516980B2 (en) | Novel phosphorus atom-containing phenol resin, production method thereof, curable resin composition, cured product thereof, resin composition for printed wiring board, printed wiring board, resin composition for flexible wiring board, resin composition for semiconductor sealing material, And resin composition for interlayer insulation material for build-up substrate | |
| JP5598361B2 (en) | Curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build-up board interlayer insulation material resin composition object | |
| JP6257020B2 (en) | Phenylphenol-naphthol resin, curable resin composition, cured product thereof, and printed wiring board | |
| JP5590405B2 (en) | Novel phosphorus atom-containing epoxy resin, production method thereof, curable resin composition, cured product thereof, resin composition for printed wiring board, printed wiring board, resin composition for flexible wiring board, resin composition for semiconductor sealing material, And resin composition for interlayer insulation material for build-up substrate | |
| JP5637367B2 (en) | Curable resin composition, cured product thereof, method for producing phosphorus atom-containing phenol resin, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build Resin composition for interlayer insulation material for up-substrate | |
| JP5713045B2 (en) | Epoxy resin composition, cured product thereof, and printed wiring board | |
| JP5516979B2 (en) | Novel phosphorus atom-containing phenol resin, production method thereof, curable resin composition, cured product thereof, resin composition for printed wiring board, printed wiring board, resin composition for flexible wiring board, resin composition for semiconductor sealing material, And resin composition for interlayer insulation material for build-up substrate | |
| JP5598373B2 (en) | Curable resin composition, cured product thereof, printed wiring board resin composition, printed wiring board, flexible wiring board resin composition, semiconductor sealing material resin composition, and build-up board interlayer insulation material resin composition object | |
| JP2011105910A (en) | Epoxy resin composition, cured product of the same, and printed wiring board | |
| JP5505703B2 (en) | Curable resin composition, cured product thereof, printed wiring board, novolac type epoxy resin, and production method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130902 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20131226 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140107 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140110 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140715 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140728 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5598361 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |