JP2005298621A - Method of using surfactant - Google Patents
Method of using surfactant Download PDFInfo
- Publication number
- JP2005298621A JP2005298621A JP2004115083A JP2004115083A JP2005298621A JP 2005298621 A JP2005298621 A JP 2005298621A JP 2004115083 A JP2004115083 A JP 2004115083A JP 2004115083 A JP2004115083 A JP 2004115083A JP 2005298621 A JP2005298621 A JP 2005298621A
- Authority
- JP
- Japan
- Prior art keywords
- water
- surfactant
- concentration
- polyvalent cation
- polyvalent cations
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000004094 surface-active agent Substances 0.000 title claims abstract description 30
- 238000000034 method Methods 0.000 title claims abstract description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 40
- 150000001768 cations Chemical class 0.000 claims abstract description 23
- 150000003839 salts Chemical class 0.000 abstract description 10
- 229910052751 metal Inorganic materials 0.000 abstract description 7
- 239000002184 metal Substances 0.000 abstract description 7
- 239000012528 membrane Substances 0.000 abstract description 4
- 239000003673 groundwater Substances 0.000 abstract description 3
- 239000008235 industrial water Substances 0.000 abstract description 3
- 239000008399 tap water Substances 0.000 abstract description 3
- 235000020679 tap water Nutrition 0.000 abstract description 3
- 238000004821 distillation Methods 0.000 abstract description 2
- 238000000909 electrodialysis Methods 0.000 abstract description 2
- 238000005342 ion exchange Methods 0.000 abstract description 2
- 238000005374 membrane filtration Methods 0.000 abstract description 2
- 238000001223 reverse osmosis Methods 0.000 abstract description 2
- -1 iron ions Chemical class 0.000 description 16
- 238000004140 cleaning Methods 0.000 description 11
- 238000005406 washing Methods 0.000 description 9
- 239000007788 liquid Substances 0.000 description 7
- 125000000129 anionic group Chemical group 0.000 description 5
- 239000002244 precipitate Substances 0.000 description 5
- 239000000271 synthetic detergent Substances 0.000 description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 125000000524 functional group Chemical group 0.000 description 3
- 239000000344 soap Substances 0.000 description 3
- 125000000542 sulfonic acid group Chemical group 0.000 description 3
- 241000238876 Acari Species 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 2
- 206010012438 Dermatitis atopic Diseases 0.000 description 2
- 206010016952 Food poisoning Diseases 0.000 description 2
- 208000019331 Foodborne disease Diseases 0.000 description 2
- JLVVSXFLKOJNIY-UHFFFAOYSA-N Magnesium ion Chemical compound [Mg+2] JLVVSXFLKOJNIY-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical group OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- 201000008937 atopic dermatitis Diseases 0.000 description 2
- 229910001424 calcium ion Inorganic materials 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 229910001425 magnesium ion Inorganic materials 0.000 description 2
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- 239000004711 α-olefin Substances 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- NPYPAHLBTDXSSS-UHFFFAOYSA-N Potassium ion Chemical compound [K+] NPYPAHLBTDXSSS-UHFFFAOYSA-N 0.000 description 1
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000004996 alkyl benzenes Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 238000004851 dishwashing Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 235000019387 fatty acid methyl ester Nutrition 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229910001414 potassium ion Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 125000001273 sulfonato group Chemical class [O-]S(*)(=O)=O 0.000 description 1
Images
Landscapes
- Cleaning By Liquid Or Steam (AREA)
- Detergent Compositions (AREA)
Abstract
Description
本発明は、界面活性剤の使用方法、特に、界面活性剤を水に溶解して使用するための方法に関する。 The present invention relates to a method for using a surfactant, and more particularly to a method for using a surfactant dissolved in water.
石けんや陰イオン系合成洗剤などの界面活性剤は、アルキル基等の有機鎖に結合したカルボキシル基、スルホン酸基若しくはリン酸基等の官能基部分がナトリウムやカリウムと塩を形成したものであり、水中で溶解して洗浄作用を示す。 Surfactants such as soaps and anionic synthetic detergents are those in which functional groups such as carboxyl groups, sulfonic acid groups or phosphoric acid groups bonded to organic chains such as alkyl groups form salts with sodium or potassium. It dissolves in water and exhibits a cleaning action.
ところで、水道水、工業用水および地下水などの水は、カルシウムイオン、マグネシウムイオン、鉄イオン等の多価陽イオン、特に、二価の陽イオンを含んでいる。このため、水中に添加された界面活性剤は、上記官能基部分が多価陽イオンと結合し、金属塩を形成する。この金属塩は、水に難溶性であり、水中における解離状態の界面活性剤濃度を減少させることになるため、界面活性剤本来の洗浄作用を阻害する。また、この金属塩は、家庭の台所、食器、洗濯物、浴室およびトイレ等の水まわりに残留してカビ、ダニおよび細菌類の繁殖の温床になる可能性があるといった衛生上の問題があり、食中毒や各種のアレルギー性疾患(例えば、アトピー性皮膚炎)を引き起こす原因になることが懸念される。 By the way, water such as tap water, industrial water, and groundwater contains polyvalent cations such as calcium ions, magnesium ions, and iron ions, in particular, divalent cations. For this reason, in the surfactant added in water, the functional group part is combined with a polyvalent cation to form a metal salt. This metal salt is sparingly soluble in water and decreases the concentration of the surfactant in the dissociated state in water, thus inhibiting the original cleaning action of the surfactant. In addition, this metal salt has a hygienic problem that it can remain in water around household kitchens, tableware, laundry, bathrooms, toilets, etc., and become a hotbed for the growth of mold, mites and bacteria. There is a concern that it may cause food poisoning and various allergic diseases (for example, atopic dermatitis).
本発明の目的は、界面活性剤を水に溶解して使用するに当り、水に難溶性の金属塩が生成しにくいようにすることにある。 An object of the present invention is to make it difficult to form a metal salt that is hardly soluble in water when a surfactant is dissolved in water.
本発明に係る界面活性剤の使用方法は、多価陽イオンを除去処理した処理水を用意する工程と、界面活性剤を当該処理水に溶解する工程とを含んでいる。 The method for using the surfactant according to the present invention includes a step of preparing treated water from which polyvalent cations have been removed, and a step of dissolving the surfactant in the treated water.
本発明に係る界面活性剤の使用方法は、多価陽イオンを除去処理した処理水に界面活性剤を溶解しているため、水に難溶性の金属塩が生成しにくくなる。 In the method of using the surfactant according to the present invention, since the surfactant is dissolved in the treated water from which the polyvalent cation has been removed, it is difficult to form a metal salt that is hardly soluble in water.
本発明に係る界面活性剤の使用方法では、先ず、多価陽イオンを除去処理した処理水を用意する。処理水は、例えば、水道水、工業用水および地下水などを原水とし、これに対してイオン交換処理、逆浸透膜(RO膜)による膜濾過処理、電気透析若しくは蒸留等の処理を施すことにより、原水に含まれる多価陽イオンを除去するか若しくは多価陽イオン濃度を原水よりも減少させたものであってもよいし、純水であってもよい。 In the method of using the surfactant according to the present invention, first, treated water from which polyvalent cations have been removed is prepared. The treated water is, for example, tap water, industrial water, groundwater, etc., and subjected to treatment such as ion exchange treatment, membrane filtration treatment by reverse osmosis membrane (RO membrane), electrodialysis or distillation, The polyvalent cation contained in the raw water may be removed, or the polyvalent cation concentration may be reduced from that of the raw water, or pure water may be used.
次に、界面活性剤を上述の処理水に溶解し、洗浄液を得る。ここで用いられる界面活性剤は、通常、石けんや陰イオン系(アニオン系)の合成洗剤である。石けんは、高級脂肪酸のナトリウム塩やカリウム塩であり、種類が特に限定されるものではなく、各種のものを用いることができる。また、合成洗剤としては、例えば、α−スルホ脂肪酸メチルエステル塩等の脂肪酸系、直鎖アルキルベンゼンスルホン酸塩等の直鎖アルキルベンゼン系、アルキル硫酸エステル塩、アルキルエーテル硫酸エステル塩および(モノ)アルキルリン酸エステル塩等の高級アルコール系、α−オレフィンスルホン酸塩等のα−オレフィン系並びにアルカンスルホン酸塩等のノルマルパラフィン系のものなどを用いることができる。これらの合成洗剤を形成する塩は、通常、ナトリウム塩若しくはカリウム塩が好ましい。 Next, the surfactant is dissolved in the above treated water to obtain a cleaning liquid. The surfactant used here is usually a soap or an anionic (anionic) synthetic detergent. The soap is a sodium salt or potassium salt of a higher fatty acid, and the type is not particularly limited, and various types can be used. Synthetic detergents include, for example, fatty acid series such as α-sulfo fatty acid methyl ester salt, linear alkylbenzene series such as linear alkylbenzene sulfonate, alkyl sulfate ester salt, alkyl ether sulfate ester salt and (mono) alkyl phosphorus. Higher alcohols such as acid ester salts, α-olefins such as α-olefin sulfonates, and normal paraffins such as alkane sulfonates can be used. The salt forming these synthetic detergents is usually preferably a sodium salt or a potassium salt.
この工程において得られる洗浄液において、洗浄液に含まれる界面活性剤は、解離し、有機鎖に結合したカルボキシル基やスルホン酸基に由来の陰イオン成分と、ナトリウムイオンやカリウムイオンのような陽イオン成分とを洗浄液中で生成する。この結果、洗浄液は、界面活性剤による洗浄作用を発揮し、界面活性剤の種類に応じ、手洗い、洗顔、洗髪、身体の洗浄、食器洗い、洗濯、洗車およびその他の洗浄に用いることができる。 In the cleaning liquid obtained in this step, the surfactant contained in the cleaning liquid is dissociated and an anionic component derived from a carboxyl group or a sulfonic acid group bonded to an organic chain, and a cationic component such as sodium ion or potassium ion In the cleaning solution. As a result, the cleaning liquid exhibits a cleaning action by the surfactant and can be used for hand washing, face washing, hair washing, body washing, dish washing, washing, car washing and other washing depending on the type of the surfactant.
ここで、洗浄液は、多価陽イオンを除去処理した処理水を用いたものであり、実質的に多価陽イオンを含まないか、多価陽イオン濃度が原水に比べて大幅に低下しているため、界面活性剤のカルボキシル基、スルホン酸基若しくはリン酸基等の官能基部分と多価陽イオンとの結合による金属塩、すなわち、水に難溶性の多価陽イオン塩が生成しにくい。このため、洗浄液は、上述の陰イオン成分の濃度低下が生じにくく、処理水に溶解した界面活性剤量に応じた洗浄作用を発揮することができる。したがって、この使用方法は、界面活性剤の使用量を抑制することができ、環境保全に貢献することができる。また、洗浄液による洗浄後、身体、食器、洗濯物およびその他の被洗浄体および浴室や流し台などの水周りには、カビ、ダニおよび細菌類の繁殖の温床になる可能性のある水に難溶性の多価陽イオン塩が残留しにくい。このため、この界面活性剤の使用方法によれば、衛生上の問題、例えば食中毒や各種のアレルギー性疾患、特にアトピー性皮膚炎の発生を効果的に抑制できる可能性がある。 Here, the cleaning liquid uses treated water from which polyvalent cations have been removed, and is substantially free of polyvalent cations or has a significantly reduced polyvalent cation concentration compared to the raw water. Therefore, it is difficult to form a metal salt due to a bond between a functional group such as a carboxyl group, sulfonic acid group or phosphoric acid group of a surfactant and a polyvalent cation, that is, a polyvalent cation salt that is hardly soluble in water. . For this reason, the cleaning liquid is less likely to cause a decrease in the concentration of the anionic component described above, and can exhibit a cleaning action according to the amount of the surfactant dissolved in the treated water. Therefore, this usage method can suppress the usage-amount of surfactant, and can contribute to environmental conservation. In addition, after washing with the washing liquid, the body, tableware, laundry and other objects to be washed and the surrounding water such as bathrooms and sinks are sparingly soluble in water that may become a hotbed for the growth of mold, mites and bacteria. The polyvalent cation salt hardly remains. For this reason, according to the method of using this surfactant, there is a possibility that hygiene problems such as food poisoning and various allergic diseases, particularly atopic dermatitis, can be effectively suppressed.
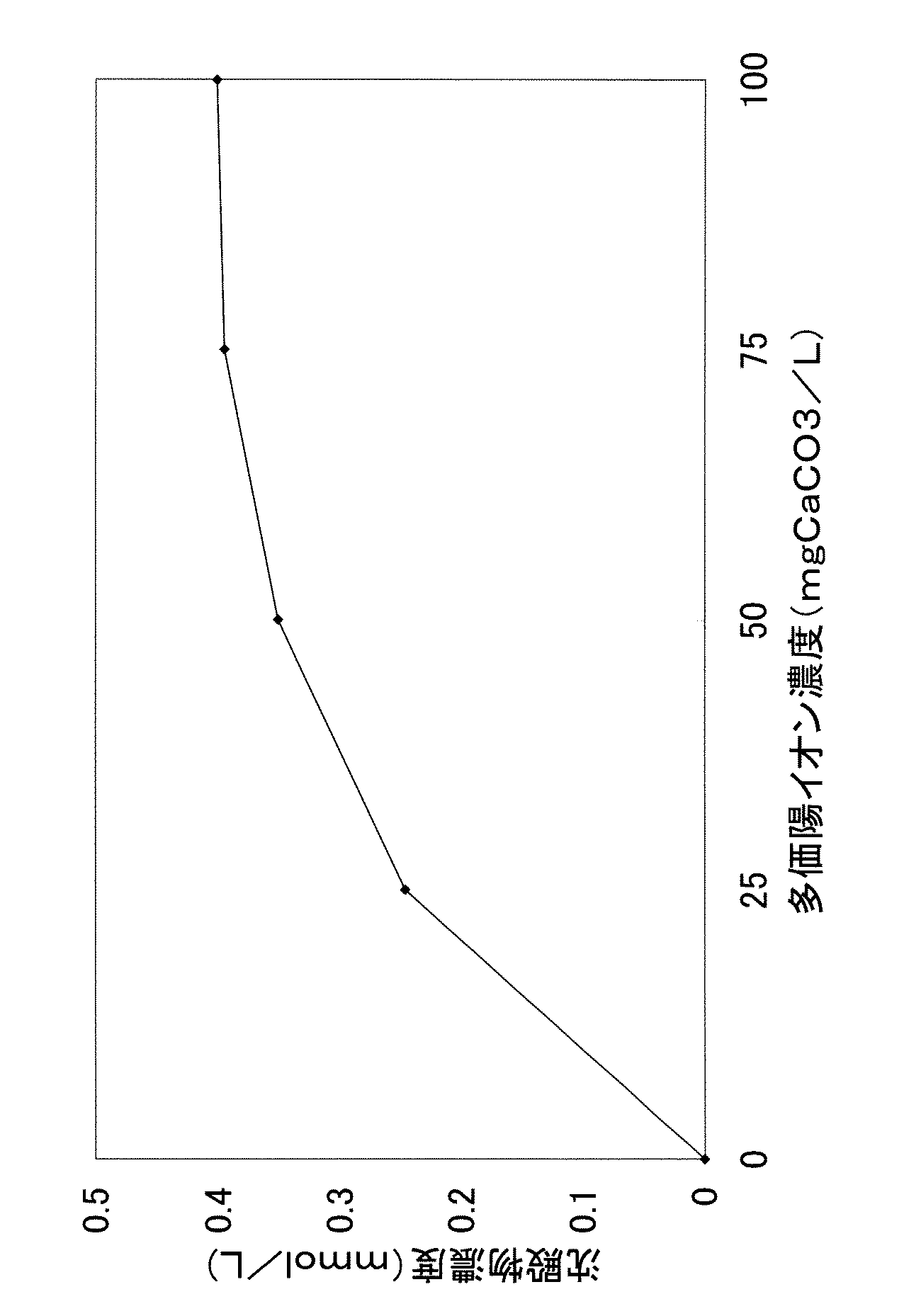
多価陽イオン濃度の異なる水に直鎖アルキルベンゼンスルホン酸ナトリウム塩(界面活性剤)を濃度が1mmol/リットルになるよう添加して溶解し、十分に撹拌した。そして、12時間後、水中に生成した沈殿物の濃度を測定した。結果を表1および図1に示す。図1は、表1をグラフ化したものである。 Linear alkylbenzene sulfonic acid sodium salt (surfactant) was added and dissolved in water having different polyvalent cation concentrations so as to have a concentration of 1 mmol / liter, followed by thorough stirring. Then, after 12 hours, the concentration of the precipitate formed in water was measured. The results are shown in Table 1 and FIG. FIG. 1 is a graph of Table 1.
表1および図1において、多価陽イオン濃度は、カルシウムイオン、マグネシウムイオンおよび鉄イオン等の各種の多価イオンを炭酸カルシウム濃度に換算した濃度(mgCaCO3/リットル)として表示している。また、沈殿物の濃度は、ろ過により水中から沈殿物を分離し、得られたろ液中の多価陽イオン濃度を測定した後、予め測定しておいた、界面活性剤の添加前の水中の多価陽イオン濃度から、ろ液中の多価陽イオン濃度を差し引いて求めた。因みに、ここで生成した沈殿物は、直鎖アルキルベンゼンスルホン酸の多価陽イオン塩の混合物である。 In Table 1 and FIG. 1, the polyvalent cation concentration is expressed as a concentration (mgCaCO 3 / liter) obtained by converting various polyvalent ions such as calcium ion, magnesium ion and iron ion into calcium carbonate concentration. The concentration of the precipitate is determined by separating the precipitate from the water by filtration, measuring the polyvalent cation concentration in the obtained filtrate, and then measuring the concentration in the water before the addition of the surfactant. It was determined by subtracting the polyvalent cation concentration in the filtrate from the polyvalent cation concentration. Incidentally, the precipitate produced here is a mixture of polyvalent cation salts of linear alkylbenzene sulfonic acids.
表1および図1によると、多価陽イオン濃度が高まるのに従い、沈殿物の濃度、すなわち多価陽イオン塩濃度が顕著に増加する傾向がある。換言すると、界面活性剤を溶解する水の多価陽イオン濃度が低い程、多価陽イオン塩が生成しにくくなる。 According to Table 1 and FIG. 1, as the polyvalent cation concentration increases, the concentration of the precipitate, that is, the polyvalent cation salt concentration tends to increase significantly. In other words, the lower the polyvalent cation concentration of water in which the surfactant is dissolved, the more difficult it is to produce the polyvalent cation salt.
Claims (1)
界面活性剤を前記処理水に溶解する工程と、
を含む界面活性剤の使用方法。 A step of preparing treated water from which polyvalent cations have been removed;
Dissolving a surfactant in the treated water;
A method of using a surfactant comprising:
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2004115083A JP2005298621A (en) | 2004-04-09 | 2004-04-09 | Method of using surfactant |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2004115083A JP2005298621A (en) | 2004-04-09 | 2004-04-09 | Method of using surfactant |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2005298621A true JP2005298621A (en) | 2005-10-27 |
Family
ID=35330559
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004115083A Pending JP2005298621A (en) | 2004-04-09 | 2004-04-09 | Method of using surfactant |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2005298621A (en) |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01186812A (en) * | 1988-01-14 | 1989-07-26 | Kao Corp | Detergent composition |
| JPH01287017A (en) * | 1988-05-11 | 1989-11-17 | Kao Corp | Low-irritant washing agent composition |
| JPH09208997A (en) * | 1996-02-05 | 1997-08-12 | Kaoru Asada | Washing method |
| JP2002338993A (en) * | 2001-05-16 | 2002-11-27 | Kawaken Fine Chem Co Ltd | Liquid detergent composition |
| JP2002348595A (en) * | 2001-05-29 | 2002-12-04 | Rausu Kaiyo Shinsosui:Kk | Soap containing ocean deep water and method for producing the same |
-
2004
- 2004-04-09 JP JP2004115083A patent/JP2005298621A/en active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01186812A (en) * | 1988-01-14 | 1989-07-26 | Kao Corp | Detergent composition |
| JPH01287017A (en) * | 1988-05-11 | 1989-11-17 | Kao Corp | Low-irritant washing agent composition |
| JPH09208997A (en) * | 1996-02-05 | 1997-08-12 | Kaoru Asada | Washing method |
| JP2002338993A (en) * | 2001-05-16 | 2002-11-27 | Kawaken Fine Chem Co Ltd | Liquid detergent composition |
| JP2002348595A (en) * | 2001-05-29 | 2002-12-04 | Rausu Kaiyo Shinsosui:Kk | Soap containing ocean deep water and method for producing the same |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20080274939A1 (en) | Water treatment system and downstream cleaning methods | |
| CN103215144B (en) | Acidic lasting wall built-up type toilet bowl cleaner and preparation method thereof | |
| JP2004536197A5 (en) | ||
| IE872539L (en) | Detergent compositions. | |
| NZ589510A (en) | Light duty liquid cleaning compositions and methods of manufacture and use thereof | |
| EP2519623B2 (en) | Phosphate substitutes for membrane-compatible cleaning and/or detergent compositions | |
| WO2014139984A1 (en) | Cleaners for hard surfaces comprising phosphoric acid esters of a polyether-modified alkyl alcohol | |
| WO2015093164A1 (en) | Bactericidal cleaning composition for hard surfaces | |
| MX2007014165A (en) | Liquid acidic hard surface cleaning composition. | |
| JP5567330B2 (en) | Composition with unexpected cleaning performance comprising a biodegradable chelating agent | |
| PT666303E (en) | COMPOSITIONS FOR REMOVAL OF CALCARY SCREW | |
| JP6093280B2 (en) | Liquid detergent composition for hard surfaces | |
| JP2005298621A (en) | Method of using surfactant | |
| EP3383986A1 (en) | Hard surface cleaning composition | |
| JP2022511731A (en) | How to treat fabric with variable PH profile during wash and rinse cycle | |
| JP4794844B2 (en) | Pipe cleaning method | |
| WO2021250599A1 (en) | Cleaning product and related synthesis process | |
| DE1767683A1 (en) | Detergents, washing auxiliaries and cleaning agents containing antimicrobial agents | |
| WO2015171090A1 (en) | Use of oxidized humic acid its salts and derivatives in cleaning compositions | |
| JP5798961B2 (en) | Liquid cleaner for bathroom | |
| EP2764076B1 (en) | Improved treatment of hard surfaces | |
| DK142791B (en) | DETERGENT | |
| JP2008081428A (en) | Disinfectant | |
| DK152586B (en) | DIFFICULTY CLEANING OBJECTIVE | |
| JPS59221392A (en) | Phosphorus-free detergent composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Effective date: 20061020 Free format text: JAPANESE INTERMEDIATE CODE: A621 |
|
| A977 | Report on retrieval |
Effective date: 20090417 Free format text: JAPANESE INTERMEDIATE CODE: A971007 |
|
| A131 | Notification of reasons for refusal |
Effective date: 20090519 Free format text: JAPANESE INTERMEDIATE CODE: A131 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A712 Effective date: 20090624 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090721 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20091201 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100330 |