JP2004217436A - 水硬性硬化体およびその製造方法 - Google Patents
水硬性硬化体およびその製造方法 Download PDFInfo
- Publication number
- JP2004217436A JP2004217436A JP2003003521A JP2003003521A JP2004217436A JP 2004217436 A JP2004217436 A JP 2004217436A JP 2003003521 A JP2003003521 A JP 2003003521A JP 2003003521 A JP2003003521 A JP 2003003521A JP 2004217436 A JP2004217436 A JP 2004217436A
- Authority
- JP
- Japan
- Prior art keywords
- weight
- compound
- pulp sludge
- alkali metal
- hydraulic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000463 material Substances 0.000 title claims abstract description 47
- 238000004519 manufacturing process Methods 0.000 title abstract description 8
- 239000010802 sludge Substances 0.000 claims abstract description 43
- 150000001339 alkali metal compounds Chemical class 0.000 claims abstract description 27
- 239000002002 slurry Substances 0.000 claims abstract description 14
- 239000002657 fibrous material Substances 0.000 claims abstract description 8
- 239000004606 Fillers/Extenders Substances 0.000 claims abstract description 3
- 239000002245 particle Substances 0.000 claims description 29
- -1 potassium hydroxide compound Chemical class 0.000 claims description 23
- 239000000203 mixture Substances 0.000 claims description 20
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 20
- 238000000034 method Methods 0.000 claims description 17
- KWYUFKZDYYNOTN-UHFFFAOYSA-M potassium hydroxide Inorganic materials [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 claims description 14
- HEMHJVSKTPXQMS-UHFFFAOYSA-M sodium hydroxide Inorganic materials [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 11
- 230000001186 cumulative effect Effects 0.000 claims description 10
- 239000011734 sodium Substances 0.000 claims description 9
- 230000008569 process Effects 0.000 claims description 8
- 239000000945 filler Substances 0.000 claims description 6
- 229910052744 lithium Inorganic materials 0.000 claims description 5
- 229910052700 potassium Inorganic materials 0.000 claims description 5
- 229910002651 NO3 Inorganic materials 0.000 claims description 4
- 229910018068 Li 2 O Inorganic materials 0.000 claims description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 claims description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 2
- 239000011591 potassium Substances 0.000 claims description 2
- 239000000470 constituent Substances 0.000 claims 1
- 239000004566 building material Substances 0.000 abstract description 10
- 239000002956 ash Substances 0.000 description 52
- 230000000052 comparative effect Effects 0.000 description 30
- 239000004568 cement Substances 0.000 description 26
- 238000005452 bending Methods 0.000 description 22
- 230000005484 gravity Effects 0.000 description 12
- 238000010298 pulverizing process Methods 0.000 description 12
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 10
- 239000002994 raw material Substances 0.000 description 10
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 9
- 230000000694 effects Effects 0.000 description 6
- 238000004898 kneading Methods 0.000 description 6
- 150000003839 salts Chemical class 0.000 description 6
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 6
- 229910000019 calcium carbonate Inorganic materials 0.000 description 5
- 230000018044 dehydration Effects 0.000 description 5
- 238000006297 dehydration reaction Methods 0.000 description 5
- 239000000835 fiber Substances 0.000 description 5
- 238000006703 hydration reaction Methods 0.000 description 5
- 229910052708 sodium Inorganic materials 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 229910052783 alkali metal Inorganic materials 0.000 description 4
- 150000001340 alkali metals Chemical class 0.000 description 4
- 239000011575 calcium Substances 0.000 description 4
- 235000012241 calcium silicate Nutrition 0.000 description 4
- 229910052918 calcium silicate Inorganic materials 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 230000036571 hydration Effects 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 239000000123 paper Substances 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- FGIUAXJPYTZDNR-UHFFFAOYSA-N potassium nitrate Chemical compound [K+].[O-][N+]([O-])=O FGIUAXJPYTZDNR-UHFFFAOYSA-N 0.000 description 4
- 230000009257 reactivity Effects 0.000 description 4
- 230000000996 additive effect Effects 0.000 description 3
- 239000003513 alkali Substances 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- 239000000378 calcium silicate Substances 0.000 description 3
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 3
- 238000011049 filling Methods 0.000 description 3
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 3
- 239000007791 liquid phase Substances 0.000 description 3
- 238000000465 moulding Methods 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000004317 sodium nitrate Substances 0.000 description 3
- 235000010344 sodium nitrate Nutrition 0.000 description 3
- 239000005995 Aluminium silicate Substances 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- YKTSYUJCYHOUJP-UHFFFAOYSA-N [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] Chemical compound [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] YKTSYUJCYHOUJP-UHFFFAOYSA-N 0.000 description 2
- 235000012211 aluminium silicate Nutrition 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 230000006866 deterioration Effects 0.000 description 2
- 238000006253 efflorescence Methods 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 239000010440 gypsum Substances 0.000 description 2
- 229910052602 gypsum Inorganic materials 0.000 description 2
- 239000002440 industrial waste Substances 0.000 description 2
- XGZVUEUWXADBQD-UHFFFAOYSA-L lithium carbonate Chemical compound [Li+].[Li+].[O-]C([O-])=O XGZVUEUWXADBQD-UHFFFAOYSA-L 0.000 description 2
- 229910052808 lithium carbonate Inorganic materials 0.000 description 2
- 239000011087 paperboard Substances 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 239000004323 potassium nitrate Substances 0.000 description 2
- 235000010333 potassium nitrate Nutrition 0.000 description 2
- 206010037844 rash Diseases 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 239000011343 solid material Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000002699 waste material Substances 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920001131 Pulp (paper) Polymers 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000004760 aramid Substances 0.000 description 1
- 229920003235 aromatic polyamide Polymers 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- JHLNERQLKQQLRZ-UHFFFAOYSA-N calcium silicate Chemical compound [Ca+2].[Ca+2].[O-][Si]([O-])([O-])[O-] JHLNERQLKQQLRZ-UHFFFAOYSA-N 0.000 description 1
- JLDKGEDPBONMDR-UHFFFAOYSA-N calcium;dioxido(oxo)silane;hydrate Chemical compound O.[Ca+2].[O-][Si]([O-])=O JLDKGEDPBONMDR-UHFFFAOYSA-N 0.000 description 1
- XFWJKVMFIVXPKK-UHFFFAOYSA-N calcium;oxido(oxo)alumane Chemical compound [Ca+2].[O-][Al]=O.[O-][Al]=O XFWJKVMFIVXPKK-UHFFFAOYSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000002734 clay mineral Substances 0.000 description 1
- 239000011362 coarse particle Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 239000004567 concrete Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- BCAARMUWIRURQS-UHFFFAOYSA-N dicalcium;oxocalcium;silicate Chemical compound [Ca+2].[Ca+2].[Ca]=O.[O-][Si]([O-])([O-])[O-] BCAARMUWIRURQS-UHFFFAOYSA-N 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000010881 fly ash Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000011490 mineral wool Substances 0.000 description 1
- 239000012778 molding material Substances 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 238000011056 performance test Methods 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000012209 synthetic fiber Substances 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 235000019976 tricalcium silicate Nutrition 0.000 description 1
- 229910021534 tricalcium silicate Inorganic materials 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
Images
Landscapes
- Producing Shaped Articles From Materials (AREA)
- Curing Cements, Concrete, And Artificial Stone (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003003521A JP2004217436A (ja) | 2003-01-09 | 2003-01-09 | 水硬性硬化体およびその製造方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003003521A JP2004217436A (ja) | 2003-01-09 | 2003-01-09 | 水硬性硬化体およびその製造方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004217436A true JP2004217436A (ja) | 2004-08-05 |
| JP2004217436A5 JP2004217436A5 (enExample) | 2005-09-22 |
Family
ID=32894764
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003003521A Pending JP2004217436A (ja) | 2003-01-09 | 2003-01-09 | 水硬性硬化体およびその製造方法 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2004217436A (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009203101A (ja) * | 2008-02-27 | 2009-09-10 | Nagoya Institute Of Technology | セラミックス粉体の固化方法 |
| JP2018065731A (ja) * | 2016-10-21 | 2018-04-26 | 株式会社東芝 | ジオポリマー成型体製造方法およびジオポリマー成型体製造システム |
| JP2021155311A (ja) * | 2020-03-30 | 2021-10-07 | 株式会社フジタ | コンクリートとその作製方法 |
-
2003
- 2003-01-09 JP JP2003003521A patent/JP2004217436A/ja active Pending
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009203101A (ja) * | 2008-02-27 | 2009-09-10 | Nagoya Institute Of Technology | セラミックス粉体の固化方法 |
| JP2018065731A (ja) * | 2016-10-21 | 2018-04-26 | 株式会社東芝 | ジオポリマー成型体製造方法およびジオポリマー成型体製造システム |
| JP2021155311A (ja) * | 2020-03-30 | 2021-10-07 | 株式会社フジタ | コンクリートとその作製方法 |
| JP7546371B2 (ja) | 2020-03-30 | 2024-09-06 | 株式会社フジタ | コンクリートとその作製方法 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN106810169A (zh) | 一种含铝托勃莫来石渣土蒸压加气混凝土砌块及生产方法 | |
| EP2514727B1 (en) | An alkali activated limestone concrete composition and use of composition in concrete casting | |
| KR101410056B1 (ko) | 바텀애시를 포함하는 결합재에 의한 무시멘트 콘크리트 | |
| JP2014210709A (ja) | セメント系配合物 | |
| EP4155278A1 (en) | Improving reactivity of carbonated recycled concrete fines | |
| KR101018009B1 (ko) | 결합재로 폐유리 미분말과 플라이애쉬를 이용한 무시멘트 콘크리트의 제조방법 | |
| EP2878585B1 (en) | Method for the manufacturing of cementitious C-S-H seeds | |
| CN115536350A (zh) | 一种利用多种固废协同的道路基层铺设材料及其制备方法 | |
| CN109704617A (zh) | 一种建筑垃圾墙板抗裂改性剂及其制备方法 | |
| CN115466094B (zh) | 一种工业固废基胶结注浆充填材料、制备方法及应用 | |
| KR101275435B1 (ko) | 에어젯 밀을 이용한 고미분말 고로수쇄슬래그 혼합시멘트의 제조방법 | |
| CN115427372B (zh) | 使用溶解度高于石英的二氧化硅原料生产蒸压加气混凝土的方法 | |
| RU2448929C1 (ru) | Сырьевая смесь и способ ее получения для наноструктурированного автоклавного газобетона | |
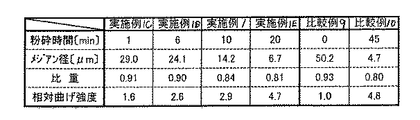
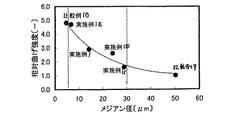
| JP2004217436A (ja) | 水硬性硬化体およびその製造方法 | |
| KR100653311B1 (ko) | 중유회를 함유하는 경량기포 콘크리트 제조용 조성물, 이를이용한 alc의 제조방법 | |
| JP2004210584A (ja) | 水硬性硬化体およびその製造方法 | |
| CN118561561A (zh) | 一种利用电厂固废制备的实心砖及其制备方法 | |
| KR101262447B1 (ko) | 인조석 제조용 페이스트 조성물, 이를 이용한 인조석 제조 방법 및 그 방법으로 제조된 무기 바인더계 인조석 | |
| JP2002114562A (ja) | 水熱硬化体およびその製造方法 | |
| CN1257845A (zh) | 湿磨废渣水泥的制备方法 | |
| KR100212032B1 (ko) | 암석미분 슬러지를 이용한 경량기포콘크리트용 조성물 및 경량기포콘크리트의 제조방법 | |
| EP4269370A1 (en) | Process for the production of alkali-activated materials (aams), products obtainable with said process and heat insulating materials comprising said aam products | |
| JP2004018353A (ja) | 低比重珪酸カルシウム硬化体およびその製造方法 | |
| JPH08198648A (ja) | 石炭灰質固化物の製造方法 | |
| RU2392253C1 (ru) | Смесь для пенобетона |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050413 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050413 |
|
| A977 | Report on retrieval |
Effective date: 20080417 Free format text: JAPANESE INTERMEDIATE CODE: A971007 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080422 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20080815 |