EP4259756B1 - Verwendung eines reinigungsmittelzusatzes - Google Patents
Verwendung eines reinigungsmittelzusatzes Download PDFInfo
- Publication number
- EP4259756B1 EP4259756B1 EP21819525.3A EP21819525A EP4259756B1 EP 4259756 B1 EP4259756 B1 EP 4259756B1 EP 21819525 A EP21819525 A EP 21819525A EP 4259756 B1 EP4259756 B1 EP 4259756B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fuel
- week
- none
- gtl
- yes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/238—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds
- C10L1/2383—Polyamines or polyimines, or derivatives thereof (poly)amines and imines; derivatives thereof (substituted by a macromolecular group containing 30C)
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/192—Macromolecular compounds
- C10L1/198—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds homo- or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon to carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/222—Organic compounds containing nitrogen containing at least one carbon-to-nitrogen single bond
- C10L1/2222—(cyclo)aliphatic amines; polyamines (no macromolecular substituent 30C); quaternair ammonium compounds; carbamates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/238—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/04—Use of additives to fuels or fires for particular purposes for minimising corrosion or incrustation
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/18—Use of additives to fuels or fires for particular purposes use of detergents or dispersants for purposes not provided for in groups C10L10/02 - C10L10/16
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2200/00—Components of fuel compositions
- C10L2200/04—Organic compounds
- C10L2200/0461—Fractions defined by their origin
- C10L2200/0469—Renewables or materials of biological origin
- C10L2200/0476—Biodiesel, i.e. defined lower alkyl esters of fatty acids first generation biodiesel
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2200/00—Components of fuel compositions
- C10L2200/04—Organic compounds
- C10L2200/0461—Fractions defined by their origin
- C10L2200/0469—Renewables or materials of biological origin
- C10L2200/0492—Fischer-Tropsch products
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2230/00—Function and purpose of a components of a fuel or the composition as a whole
- C10L2230/08—Inhibitors
- C10L2230/083—Disinfectants, biocides, anti-microbials
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2250/00—Structural features of fuel components or fuel compositions, either in solid, liquid or gaseous state
- C10L2250/02—Microbial additives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/02—Specifically adapted fuels for internal combustion engines
- C10L2270/026—Specifically adapted fuels for internal combustion engines for diesel engines, e.g. automobiles, stationary, marine
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/04—Specifically adapted fuels for turbines, planes, power generation
Definitions
- the present invention relates to the use of a detergent additive in a fuel composition for reducing microbial growth.
- Fuel tanks and distribution systems provide an environment where microorganisms can flourish.
- microorganisms There are three main types of spoilage and corrosive hydrocarbon-utilising microorganisms in fuel systems, namely bacteria, yeasts and moulds, the latter two often named as fungi.
- Microbes are ubiquitous and can infect fuel at any point in the distribution chain, surviving and proliferating in water/aqueous phase associated with the fuel and drawing their nutrients across the fuel/water interface (most nutrients diffuse into the cell in aqueous solution).
- Microbes require elements such as carbon, hydrogen, sulphur, nitrogen and phosphorus in substantial amounts, trace amounts of other elements and some form of oxygen.
- the rate and type of microbial proliferation is dependent on factors such as water phase pH, oxygen availability, ambient temperature, nutrient availability, etc.
- Optimum growth is achieved in the temperature range 15°C to 40°C and a neutral/slightly acidic pH. Most of these conditions are met in fuel tanks and distribution systems thereby providing an ideal environment for microbial growth. Water as one key contributor exacerbates the situation and can never be completely eradicated due to condensation effects, leaking tank roofs, poor housekeeping, etc. A fuel storage system is therefore never sterile and at best, the aim is to restrict microbial growth to an acceptable level by the adoption of good housekeeping practices.
- MIC Microbially Induced Corrosion
- Microbial cells can exist in planktonic (single cells floating/swimming in the fuel) or sessile forms (attached to a surface).
- a biofilm forms when a collection of microorganisms (mainly bacteria) adhere irreversibly to a surface and begin to excrete slimy, extracellular biopolymers. Biofilms enhance microbial interactions, giving more access to nutrients, environmental stability, protection from viruses and biocides. These microbial communities can attach to the sidewalls and bottoms of fuel storage tanks causing Microbially Induced Corrosion (MIC).
- MIC Microbially Induced Corrosion
- MIC is one of the most serious consequences of microbial growth. Aerobes produce organic acids and deplete oxygen supplies, creating an oxygen-deficient zone around them. Oxygen gradients develop causing the formation of anodic corrosion pits. Sulphate Reducing Bacteria (SRB) can increase this corrosion by producing H 2 S, HS and S 2 , all of which are very aggressive to steel.
- SRB Sulphate Reducing Bacteria
- US 5 314 510 A and US 2005/262759 A1 relate to the reduction of microbial growth in fuels.
- Biofuels can play a role in fossil fuel replacement to meet reduction targets of greenhouse gas emissions.
- FAMEs can play a role in fossil fuel replacement to meet reduction targets of greenhouse gas emissions.
- B30 (30% biofuel), which is well above the current EU mandated levels of B7 (7% biofuel).
- biodiesel and its blends, particularly at the EU mandated levels are more susceptible to microbial growth than conventional hydrocarbon diesel.
- FAME is easier for microorganisms to digest and more hydroscopic than conventional diesel leading to greater free water entrainment.
- Gas-to-liquid (GTL) technology converts CH 4 /methane currently from natural gas - the cleanest-burning fossil fuel - into high-quality liquid fuel products that would otherwise be made from crude oil.
- GTL fuel can help to reduce local emissions in conventional diesel vehicles.
- GTL fuel also has other applications, e.g. as a synthetic marine fuel in inland water way vessels in conventional marine diesel engines. It is known that GTL fuel is more biodegradable than conventional diesel due to its negligible aromatic content and simple structure (it is fully saturated) but may be more susceptible to microbial growth than conventional diesel. Fuel handling and storage conditions with respect to microbial growth could be considered as even more severe for inland water way vessels than for road applications due to the potential presence of water inside the tank, during fuelling and on board bunker ships.
- a detergent additive in a fuel composition for reducing microbial growth, wherein the detergent additive is a polyolefin substituted succinimide, wherein the fuel composition is a diesel fuel composition and wherein the diesel fuel composition comprises a paraffinic base fuel selected from hydrotreated vegetable oil, Fischer-Tropsch derived base fuels, and mixtures thereof.
- the term "reducing microbial growth” embraces any degree of reduction in microbial growth. Microbial growth may be measured by any suitable method such as the biomass method described in the Examples below.
- the reduction in microbial growth may be of the order of 10% or more, preferably 20% or more, more preferably 50% or more, and especially 70% or more compared to the microbial growth in an analogous fuel composition which does not contain a detergent additive, in particular during the time period where the fuel composition is stored in a fuel tank, for example a time period of up to 12 weeks.
- the term "reducing microbial growth” also encompasses the prevention of microbes growing in the first place.
- the present invention is relevant for a wide range of fuel applications such as diesel fuel compositions and wherein the diesel fuel composition comprises a paraffinic base fuel selected from hydrotreated vegetable oil, Fischer-Tropsch derived base fuels, and mixtures thereof.
- a paraffinic base fuel selected from hydrotreated vegetable oil, Fischer-Tropsch derived base fuels, and mixtures thereof.
- a first essential component herein is a detergent additive, by which is meant an agent (suitably a surfactant) which can act to remove, and/or to prevent the build-up of, combustion related deposits within an engine, in particular in the fuel injection system such as in the injector nozzles.
- agent suitably a surfactant
- Such materials are sometimes referred to as dispersant additives.
- Detergent-containing diesel fuel additives are known and commercially available.
- suitable detergent additives include, but are not necessarily limited to, polyolefin substituted succinimides or succinimides of polyamines, aliphatic amines, Mannich bases or amines, polyolefin maleic anhydrides, and quaternary ammonium salts, and mixtures thereof.
- the detergent additive for use herein is a polyolefin substituted succinimide, such as a polyisobutylene succinimide.
- nitrogen-containing detergents are nitrogen-containing detergents. With nitrogen being an essential nutrient for microbes, it is particularly surprising that the nitrogen-containing detergents mentioned above have been found to reduce microbial growth when included in a fuel composition.
- Fuel additives can encourage water entrainment in fuel due to their surfactant nature, particularly at higher treat rates, and also act as food sources; additives containing the essential nutrient nitrogen are particularly important. Therefore, it would be reasonable to assume that fuels blended with performance additives, especially nitrogen-containing performance additives, such as nitrogen-containing detergents, are more conducive to microbial attack. It has been surprisingly found by the present inventors that detergent additives, such as nitrogen-containing detergents, have surprisingly been found to inhibit microbial growth in a fuel composition. In particular, it has been found that detergent additives, such as nitrogen-containing detergents, have surprisingly been found to inhibit microbial growth in a fuel composition, wherein the microbes are fungal species. This is particularly advantageous as filamentous fungi are implicated in filter blocking problems.
- the detergent additive is preferably present in the fuel composition at a level from 5 ppmw to 10000ppmw, preferably 5 ppmw to 1000ppmw, more preferably in the range 5 to 500 ppmw, even more preferably in the range from 10 to 200 ppmw active matter detergent based on the overall fuel composition.
- the detergent additive is a component of a detergent additive package together with one or more other additive components.
- suitable additive components are provided in more detail hereinbelow.
- the diesel fuel compositions described herein may comprise a diesel base fuel in addition to the detergent additive.
- the diesel base fuel may comprise any petroleum derived diesel suitable for use in an internal combustion engine, such as a petroleum derived low sulphur diesel comprising ⁇ 50 ppm of sulphur, for example, an ultra-low sulphur diesel (ULSD) or a zero sulphur diesel (ZSD).
- a petroleum derived low sulphur diesel comprising ⁇ 50 ppm of sulphur
- ULSD ultra-low sulphur diesel
- ZSD zero sulphur diesel
- the low sulphur diesel comprises ⁇ 10 ppm of sulphur.
- the petroleum derived low sulphur diesel preferred for use in the present invention meets the EN590 specification. It will typically have a density from 0.81 to 0.865, preferably 0.82 to 0.85, more preferably 0.825 to 0.845 g/cm 3 at 15°C; a cetane number (ASTM D613) of at least 51; and a kinematic viscosity (ASTM D445) from 1.5 to 4.5, preferably 2.0 to 4.0, more preferably from 2.2 to 3.7 mm 2 /s at 40°C.
- the diesel base fuel comprises a conventional petroleum-derived diesel.
- a further preferred component of the fuel composition herein comprises a biodiesel fuel.
- Biodiesel fuels are fuels which derive from biological materials.
- the biodiesel component is preferably present in the fuel composition herein at a level of 5% v/v or greater up to a level of 50% v/v, preferably from 5% v/v to 30% v/v, for example at levels of 7%v/v, 10%v/v, 20%v/v and 30%v/v.
- the fuel composition herein is free of biodiesel component (i.e. a so-called 'B0' fuel).
- a preferred biodiesel component for use herein is a fatty acid alkyl ester (FAAE). It is known to include fatty acid alkyl esters (FAAEs), in particular fatty acid methyl esters (FAMEs), in diesel fuel compositions. Examples of suitable FAAEs include rapeseed methyl ester (RME), palm oil methyl ester (POME), and soy methyl ester (SME). FAAEs are typically derivable from biological sources and are typically included to reduce the environmental impact of the fuel production and consumption process or to improve lubricity.
- FAAEs of which the most commonly used in the context of diesel fuels are the methyl esters, are already known as renewable diesel fuels (so-called 'biodiesel' fuels). They contain long chain carboxylic acid molecules (generally from 10 to 22 carbon atoms long), each having an alcohol molecule attached to one end.
- Organically derived oils such as vegetable oils (including recycled vegetable oils) and animal fats (including fish oils) can be subjected to a transesterification process with an alcohol (typically a C 1 to C 5 alcohol) to form the corresponding fatty esters, typically mono-alkylated.
- This process which is suitably either acid- or base-catalysed, such as with the base KOH, converts the triglycerides contained in the oils into fatty acid components of the oils from their glycerol backbone.
- FAAEs can also be prepared from used cooking oils, and can be prepared by standard esterification from fatty acids.
- the FAAE may be any alkylated fatty acid or mixture of fatty acids.
- Its fatty acid component(s) are preferably derived from a biological source, more preferably a vegetable source. They may be saturated or unsaturated; if the latter, they may have one or more, preferably up to 6, double bonds. They may be linear or branched, cyclic or polycyclic. Suitably, they will have from 6 to 30, preferably 10 to 30, more suitably from 10 to 22 or from 12 to 24 or from 16 to 18, carbon atoms including the acid group(s) -CO 2 H.
- a FAAE will typically comprise a mixture of different fatty acid esters of different fatty acid esters of different chain lengths, depending on its source.
- a preferred FAAE for use in the present invention is selected from a natural fatty oil, for instance tall oil, rapeseed oil, palm oil or soy oil.
- the FAAE is preferably a C 1 to C 5 alkyl ester, more preferably a methyl, ethyl, propyl, (suitably iso-propyl) or butyl ester, yet more preferably a methyl or ethyl ester and in particular a methyl ester.
- the FAAE is selected from methyl ester of palm oil (POME) and methyl ester of rapeseed oil (RME, and mixtures thereof.
- the FAAE may contain impurities or by-products as a result of the manufacturing process.
- the FAAE suitably complies with specifications applying to the rest of the fuel composition, and/or to the base fuel to which it is added, bearing in mind the intended use to which the composition is to be put (for example, in which geographical area and at what time of year).
- the FAAE preferably has a flash point (IP 34) of greater than 101°C; a kinematic viscosity at 40°C (IP 71) of 1.9 to 6.0 mm 2 /s, preferably 3.5 to 5.0 mm 2 /s; a density of 845 to 910 kg/m 3 , preferably from 860 to 900 kg/m 3 , at 15°C (IP 365, EN ISO 12185 or EN ISO 3675); a water content (IP 386) of less than 500 ppm; a T95 (the temperature at which 95% of the fuel has evaporated, measured according to IP 123) of less than 360°C; an acid number (IP 139) of less than 0.8mgKOG/g, preferably less than 0.5mgK
- It also preferably contains (e.g. by gas chromatography (GC)) less than 0.2% w/w of free methanol, less than 0.02% w/w of free glycerol and greater than 96.5% w/w esters.
- GC gas chromatography
- the FAAE to conform to the European specification EN14214 for fatty methyl esters for use as diesel fuels.
- Two or more FAAEs may be added to the fuel composition in accordance with the present invention, either separately or as a pre-prepared blend.
- the FAAE can be incorporated into the fuel composition typically as a blend (i.e. a physical mixture) and optionally with one or more other fuel components (such as diesel base fuels) and optionally with one or more fuel additives.
- the FAAE is conveniently incorporated into the fuel composition before the composition is introduced into the engine which is to be run on the fuel composition.
- the fuel composition herein comprises a paraffinic gasoil, in addition to or instead of the diesel base fuel mentioned above.
- Suitable paraffinic gasoils include Fischer-Tropsch derived gasoils, and gasoils derived from hydrogenated vegetable oil (HVO), and mixtures thereof.
- a preferred paraffinic gasoil for use herein is a Fischer-Tropsch derived gasoil fuel.
- the paraffinic nature of Fischer-Tropsch derived gasoil means that diesel fuel compositions containing it will have high cetane numbers compared to conventional diesel.
- paraffinic gasoil While Fischer-Tropsch derived gasoil is a preferred paraffinic gasoil for use herein, the term "paraffinic gasoil” as used herein also includes those paraffinic gasoils derived from the hydrotreating of vegetable oils (HVO).
- HVO hydrotreating of vegetable oils
- the HVO process is based on an oil refining technology. In the process, hydrogen is used to remove oxygen from the triglyceride vegetable oil molecules and to split the triglyceride into three separate chains thus creating paraffinic hydrocarbons.
- the paraffinic gasoil i.e. the Fischer-Tropsch derived gasoil, the hydrogenated vegetable oil derived gasoil
- the paraffinic gasoil will preferably consist of at least 95% w/w, more preferably at least 98% w/w, even more preferably at least 99.5% w/w, and most preferably up to 100% w/w of paraffinic components, preferably iso- and normal paraffins, preferably comprising 80% w/w or greater of iso-paraffins.
- Fischer-Tropsch derived is meant that a fuel or base oil is, or derives from, a synthesis product of a Fischer-Tropsch condensation process.
- non-Fischer-Tropsch derived may be interpreted accordingly.
- a Fischer-Tropsch derived fuel may also be referred to as a GTL (gas-to-liquid) fuel.
- the carbon monoxide and hydrogen may themselves be derived from organic or inorganic, natural or synthetic sources, typically either from natural gas or from organically derived methane. More recently techniques to derive carbon monoxide and hydrogen from other sources, including more sustainable ones are being explored and used. For example, starting with carbon dioxide and water, the water can be electrolysed to give free hydrogen, typically using electricity from a sustainable source. This hydrogen can react with the carbon dioxide in the 'reverse water shift reaction' to give a source of carbon monoxide. This carbon monoxide can then be reacted with the remaining hydrogen in the typical Fischer-Tropsch synthesis process. Because of the use of electrolysis, some of these production processes are referred to as 'Power-to-liquids'
- Gas oil, kerosene fuel and base oil products may be obtained directly from the Fischer-Tropsch reaction, or indirectly for instance by fractionation of Fischer-Tropsch synthesis products or from hydrotreated Fischer-Tropsch synthesis products.
- Hydrotreatment can involve hydrocracking to adjust the boiling range (see, e. g. GB2077289 and EP0147873 ) and/or hydroisomerisation which can improve cold flow properties by increasing the proportion of branched paraffins.
- EP0583836 describes a two-step hydrotreatment process in which a Fischer-Tropsch synthesis product is firstly subjected to hydroconversion under conditions such that it undergoes substantially no isomerisation or hydrocracking (this hydrogenates the olefinic and oxygen-containing components), and then at least part of the resultant product is hydroconverted under conditions such that hydrocracking and isomerisation occur to yield a substantially paraffinic hydrocarbon fuel or oil. Desired diesel fuel fraction(s) may subsequently be isolated for instance by distillation.
- Typical catalysts for the Fischer-Tropsch synthesis of paraffinic hydrocarbons comprise, as the catalytically active component, a metal from Group VIII of the periodic table, in particular ruthenium, iron, cobalt or nickel. Suitable such catalysts are described for instance in EP0583836 .
- Fischer-Tropsch based process is the SMDS (Shell Middle Distillate Synthesis) described in " The Shell Middle Distillate Synthesis Process", van der Burgt et al (vide supra).
- This process also sometimes referred to as the Shell “Gas-to-Liquids” or “GTL” technology) produces diesel range products by conversion of a natural gas (primarily methane) derived synthesis gas into a heavy long-chain hydrocarbon (paraffin) wax which can then be hydroconverted and fractionated to produce liquid transport fuels such as gasoils and kerosene.
- a natural gas primarily methane
- paraffin paraffin
- a Fischer-Tropsch derived gasoil has essentially no, or undetectable levels of, sulphur and nitrogen. Compounds containing these heteroatoms tend to act as poisons for Fischer-Tropsch catalysts and are therefore removed from the synthesis gas feed. Further, the process as usually operated produces no or virtually no aromatic components.
- the aromatics content of a Fischer-Tropsch gasoil will typically be below 1% w/w, preferably below 0.5% w/w and more preferably below 0.1% w/w.
- Fischer-Tropsch derived fuels have relatively low levels of polar components, in particular polar surfactants, for instance compared to petroleum derived fuels. It is believed that this can contribute to improved antifoaming and dehazing performance.
- polar components may include for example oxygenates, and sulphur and nitrogen containing compounds.
- a low level of sulphur in a Fischer-Tropsch derived fuel is generally indicative of low levels of both oxygenates and nitrogen-containing compounds, since all are removed by the same treatment processes.
- a preferred Fischer-Tropsch derived gasoil fuel for use herein is a liquid hydrocarbon middle distillate fuel with a distillation range similar to that of a petroleum derived diesel, that is typically within the 160°C to 400°C range, preferably with a T95 of 360°C or less.
- Fischer-Tropsch derived fuels tend to be low in undesirable fuel components such as sulphur, nitrogen and aromatics.
- a preferred Fischer-Tropsch derived gasoil for use herein meets the EN15940 specification.
- a preferred Fischer-Tropsch derived gasoil fuel will typically have a density (as measured by EN ISO 12185) of from 0.76 to 0.80, preferably from 0.77 to 0.79, more preferably from 0.775 to 0.785 g/cm 3 at 15°C.
- a preferred Fischer-Tropsch derived gasoil fuel for use herein has a cetane number (ASTM D613) of greater than 70, suitably from 70 to 85, most suitably from 70 to 77.
- a preferred Fischer-Tropsch derived gasoil fuel for use herein has a kinematic viscosity at 40°C (as measured according to ASTM D445) in the range from 2.0 mm 2 /s to 5.0 mm 2 /s, preferably from 2.5 mm 2 /s to 4.0 mm 2 /s.
- a preferred Fischer-Tropsch derived gasoil for use herein has a sulphur content (ASTM D2622) of 5 ppmw (parts per million by weight) or less, preferably of 2 ppmw or less.
- a preferred Fischer-Tropsch derived gasoil fuel for use in the present invention is that produced as a distinct finished product, that is suitable for sale and used in applications that require the particular characteristics of a gasoil fuel. In particular, it exhibits a distillation range falling within the range normally relating to Fischer-Tropsch derived gasoil fuels, as set out above.
- a fuel composition used in the present invention may include a mixture of two or more paraffinic gasoils, such as two or more Fisher-Tropsch derived gasoil fuels.
- the Fischer-Tropsch derived components used herein i.e. the Fischer-Tropsch derived gasoil
- the Fischer-Tropsch derived components used herein i.e. the Fischer-Tropsch derived gasoil
- the Fischer-Tropsch derived components used herein i.e. the Fischer-Tropsch derived gasoil
- the Fischer-Tropsch derived component preferably comprise no more than 1% w/w, more preferably no more than 0.5% w/w, of olefins, by weight of the Fischer-Tropsch derived component.
- the fuel compositions described herein for use in the present invention are particularly suitable for use as a diesel fuel, in which case the fuel composition is a diesel fuel composition, and can be used for arctic applications, as winter grade diesel fuel due to the excellent cold flow properties.
- a cloud point of -10°C or lower (EN 23015) or a cold filter plugging point (CFPP) of -20°C or lower (as measured by EN 116) may be possible with fuel compositions herein.
- CFPP cold filter plugging point
- the fuel composition may be additivated with fuel additives, in addition to the detergent additive already mentioned.
- the (active matter) concentration of each such additive in a fuel composition is preferably up to 10000 ppmw, more preferably in the range from 1 to 1000 ppmw, advantageously from 1 to 400 ppmw, such as from 1 to 200 ppmw.
- additives may be added at various stages during the production of a fuel composition; those added to a base fuel at the refinery for example might be selected from anti-static agents, pipeline drag reducers, middle distillate flow improvers (MDFI) (e.g., ethylene/vinyl acetate copolymers or acrylate/maleic anhydride copolymers), lubricity enhancers, anti-oxidants and wax anti-settling agents.
- MDFI middle distillate flow improvers
- lubricity enhancers include lubricity enhancers; dehazers, e.g. alkoxylated phenol formaldehyde polymers; anti-foaming agents (e.g. commercially available polyether-modified polysiloxanes); ignition improvers (cetane improvers) (e.g. 2-ethylhexyl nitrate (EHN), cyclohexyl nitrate, di-tert-butyl peroxide and those disclosed in US4208190 at column 2, line 27 to column 3, line 21); anti-rust agents (e.g.
- a propane-1,2-diol semi-ester of tetrapropenyl succinic acid, or polyhydric alcohol esters of a succinic acid derivative the succinic acid derivative having on at least one of its alpha-carbon atoms an unsubstituted or substituted aliphatic hydrocarbon group containing from 20 to 500 carbon atoms, e.g. the pentaerythritol diester of polyisobutylene-substituted succinic acid); corrosion inhibitors; reodorants; anti-wear additives; anti-oxidants (e.g.
- phenolics such as 2,6-di-tert-butylphenol, or phenylenediamines such as N,N'-di-sec-butyl-p-phenylenediamine); metal deactivators; static dissipator additives; and mixtures thereof.
- the other additive components in the detergent additive package are selected from anti-corrosion additives, metal passivators, antioxidants, metal deactivators, reodorants, and the like, and mixtures thereof.
- the present invention may in particular be applicable where the fuel composition is used or intended to be used in a direct injection diesel engine, for example of the rotary pump, in-line pump, unit pump, electronic unit injector or common rail type, or in an indirect injection diesel engine, or in an HCCI engine.
- the fuel composition may be suitable for use in heavy- and/or light-duty diesel engines, and in engines designed for on-road use or off-road use.
- the final fuel composition is a diesel fuel composition, preferably which meets the EN590 specification (October 2017).
- the final fuel composition fuel meets the EN15940 specification (2019).
- the present invention may also be applicable where the fuel composition is used or intended to be used in a stationary application such as a heating oil system, heating oil burner, and/or stationary power generators.
- a suitable heating oil composition for use herein has characteristics according to standard DIN 51603 (2020).
- Table 1 Fuel No. Fuel Type Performance Additive Package 1 2 (ppm) 1 GTL Fuel 1 0 2 GTL Fuel 1 500 3 B7 (93% EN590 diesel base fuel, 7% FAME) 0 4 B7 (93% EN590 diesel base fuel, 7% FAME) 500 1.
- a defined inoculum with known hydrocarbon degrading capacity was used, ex. contaminated field diesel sample. 1ml was withdrawn from the contaminated field diesel sample and used to inoculate an aqueous medium/fuel mixture in the ratio 70:30ml. This was done for each of the fuels in Table 1 above and allowed to grow for 8 days to form cultures or microbial communities. Incubation was carried out in the dark at 25°C. 100 ⁇ l from the aqueous phase of each microbial community was then used to inoculate the microcosms.
- a stainless steel coupon (to promote biofilm growth) was inserted into a vial.
- 5ml of Bushnell Haas nutrient medium (aqueous phase) was decanted in and overlaid with 5ml of fuel.
- Microbial communities were then inoculated with either the GTL fuel or B7 cultures. Therefore, two inocula were derived from one field sample.
- Example 1 Microbial growth and diversity was assessed over 1 month by dry biomass weight to determine the amount of growth. This is simple technique designed to give a direct measurement of the total microbial burden at the end of the experiment. Growth visible at the interface was captured, solvent washed to remove fuel residues, dried in an oven, cooled and weighed. The results of Example 1 are shown in Figure 1 .
- the key to the fuel samples is as follows:
- Example 2 The fuels used in Example 2 are set out in Table 5 below. Each fuel contained an EN590 base fuel or a GTL EN15940 base fuel, either with or without FAME, and either with or without a performance additive package/detergent additive as indicated.
- the physicochemical properties of the EN590 base fuel used in this example are set out in Table 3 below.
- the physicochemical properties of the GTL fuel used in this example are set out in Table 4 below.
- the FAME in the B7 fuels (containing 7% biofuel) was derived from SME/RME.
- the FAME used in the B30 fuels (containing 30% of biofuel, respectively) was derived from SME/RME and POME.
- the FAME used in the B50 fuels (containing 50% of biofuel) was derived from POME.
- the testing protocol was the same as that in Example 1 with assessment of the observed microbial growth in all tested fuel types over a period of 3 months (2 time points of 4 weeks and 12 weeks) based on total biomass dry weight. Three replicate microcosms were set up for each time point. One microbial community was used as inoculum.
- Figure 2 is a graphical representation of the data shown in Table 5 , and in particular shows the average dry biomass weight (g) after 4 weeks and after 12 weeks of all fuels tested in Example 2, with and without additive.
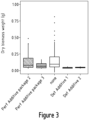
- Figure 3 is a graphical representation of the data shown in Table 5, and in particular shows the average dry biomass weight (g) for all fuel formulations tested with and without Performance Additive Package 2, Performance Additive Package 3, Detergent Additive 1 and Detergent Additive 2.
- the block entitled 'Perf Additive package 2' represents the average dry biomass weight (g) for all formulations shown in Table 5 containing Performance Additive Package 2
- the block entitled 'Perf Additive package 3' represents the average dry biomass weight (g) for all formulations shown in Table 5 containing Performance Additive Package 3
- the block entitled 'none' represents the average dry biomass weight (g) for all formulations shown in Table 5 containing no performance additive package and no detergent additive
- the block entitled 'Det Additive 1' represents the average dry biomass weight (g) for all formulations shown in Table 5 containing Detergent Additive 1
- the block entitled 'Det Additive 2' represents the average dry biomass weight (g) for all formulations shown in Table 5 containing De
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Combustion & Propulsion (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Liquid Carbonaceous Fuels (AREA)
Claims (6)
- Verwendung eines Detergenszusatzes in einer Kraftstoffzusammensetzung zum Verringern von mikrobiellem Wachstum, wobei der Detergenszusatz ein polyolefinsubstituiertes Succinimid ist, wobei die Kraftstoffzusammensetzung eine Dieselkraftstoffzusammensetzung ist und wobei die Dieselkraftstoffzusammensetzung einen paraffinischen Basiskraftstoff umfasst, der aus mit Wasserstoff behandeltem Pflanzenöl, aus Fischer-Tropsch gewonnenen Basiskraftstoffen und Mischungen davon ausgewählt ist.
- Verwendung nach Anspruch 1, wobei der Detergenszusatz ein Polysobutylensuccinimid ist.
- Verwendung nach Anspruch 1 oder 2, wobei der Detergenszusatz in der Kraftstoffzusammensetzung in einer Konzentration von 5 ppmw bis 10.000 ppmw vorhanden ist.
- Verwendung nach einem der Ansprüche 1 bis 3, wobei der Detergenszusatz eine Komponente eines Leistungszusatzpakets ist.
- Verwendung nach einem der Ansprüche 1 bis 4, wobei die Dieselkraftstoffzusammensetzung einen aus Mineralöl gewonnenen Dieselbasiskraftstoff umfasst.
- Verwendung nach Anspruch 5, wobei die Dieselkraftstoffzusammensetzung eine Biodieselkomponente, vorzugsweise einen Fettsäurealkylester, umfasst.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP20213495 | 2020-12-11 | ||
| PCT/EP2021/084936 WO2022122888A1 (en) | 2020-12-11 | 2021-12-09 | Use of a detergent additive |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP4259756A1 EP4259756A1 (de) | 2023-10-18 |
| EP4259756B1 true EP4259756B1 (de) | 2025-04-23 |
| EP4259756C0 EP4259756C0 (de) | 2025-04-23 |
Family
ID=73834328
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP21819525.3A Active EP4259756B1 (de) | 2020-12-11 | 2021-12-09 | Verwendung eines reinigungsmittelzusatzes |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP4259756B1 (de) |
| JP (1) | JP2023552631A (de) |
| CA (1) | CA3203137A1 (de) |
| ES (1) | ES3031976T3 (de) |
| WO (1) | WO2022122888A1 (de) |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3560507A (en) * | 1968-02-27 | 1971-02-02 | Millmaster Onyx Corp | Quaternary ammonium alkenyl succinates |
| FR2362208A1 (fr) | 1976-08-17 | 1978-03-17 | Inst Francais Du Petrole | Procede de valorisation d'effluents obtenus dans des syntheses de type fischer-tropsch |
| US4208190A (en) | 1979-02-09 | 1980-06-17 | Ethyl Corporation | Diesel fuels having anti-wear properties |
| NL8003313A (nl) | 1980-06-06 | 1982-01-04 | Shell Int Research | Werkwijze voor de bereiding van middeldestillaten. |
| US4478955A (en) | 1981-12-21 | 1984-10-23 | The Standard Oil Company | Upgrading synthesis gas |
| IN161735B (de) | 1983-09-12 | 1988-01-30 | Shell Int Research | |
| US5314510A (en) * | 1988-06-29 | 1994-05-24 | Bp Chemicals (Additives) Limited | Method for preventing the growth of aerobic fungi in aqueous hydrocarbons |
| EP0583836B2 (de) | 1992-08-18 | 2002-02-13 | Shell Internationale Researchmaatschappij B.V. | Verfahren zur Herstellung von Kohlenwasserstoffbrennstoffen |
| US6800101B2 (en) * | 2001-10-18 | 2004-10-05 | Chevron U.S.A. Inc. | Deactivatable biocides for hydrocarbonaceous products |
| FR2842820B1 (fr) * | 2002-07-26 | 2005-06-17 | Totalfinaelf France | Combustible emulsionne eau/hydrocarbures, sa preparation et ses utilisations |
| WO2009085552A2 (en) * | 2007-12-20 | 2009-07-09 | Dow Global Technologies Inc. | Improved corrosion and microbial control in hydrocarbonaceous compositions |
| GB0913644D0 (en) * | 2009-08-05 | 2009-09-16 | Palox Offshore S A L | Compositions for preparing emulsions |
| CN101857501B (zh) * | 2010-06-21 | 2013-02-20 | 万光存 | 一种高吸水保水性微生物肥料菌剂及其制备方法 |
| CA2969936A1 (en) * | 2017-06-07 | 2018-12-07 | David D. L. Leung | A novel non-volatile diesel additive exhibits micro-emulsification and induces micro-explosion, suitable for compression internal combustion engines, direct combustion devices andjet propulsion engines |
-
2021
- 2021-12-09 EP EP21819525.3A patent/EP4259756B1/de active Active
- 2021-12-09 CA CA3203137A patent/CA3203137A1/en active Pending
- 2021-12-09 WO PCT/EP2021/084936 patent/WO2022122888A1/en not_active Ceased
- 2021-12-09 ES ES21819525T patent/ES3031976T3/es active Active
- 2021-12-09 JP JP2023535656A patent/JP2023552631A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP2023552631A (ja) | 2023-12-18 |
| ES3031976T3 (en) | 2025-07-14 |
| CA3203137A1 (en) | 2022-06-16 |
| WO2022122888A1 (en) | 2022-06-16 |
| EP4259756A1 (de) | 2023-10-18 |
| EP4259756C0 (de) | 2025-04-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7189269B2 (en) | Fuel composition comprising a base fuel, a fischer tropsch derived gas oil, and an oxygenate | |
| EP2371931B1 (de) | Kraftstoffzusammensetzungen enthaltend Biodiesel und Fischer-Tropsch-Diesel | |
| CN101517044B (zh) | 燃料组合物 | |
| US20080282603A1 (en) | Process to prepare an aviation fuel | |
| EP4259759A1 (de) | Verwendung eines paraffinischen gasöls | |
| US11634652B2 (en) | Use of a paraffinic gasoil | |
| EP4259756B1 (de) | Verwendung eines reinigungsmittelzusatzes | |
| EP2370553B1 (de) | KRAFTSTOFFZUSAMMENSETZUNGEN enthaltend Tetrahydroquinolin | |
| AU2021367047B2 (en) | Use of a diesel fuel composition | |
| EP2935530B1 (de) | Fischer-tropsch-basierte kraftstoffzusammensetzungen | |
| US10407637B2 (en) | Fuel composition | |
| US20120090224A1 (en) | Fuel compositions | |
| AU2006298850A1 (en) | Process to blend a mineral derived hydrocarbon product and a Fisher-Tropsch derived hydrocarbon product | |
| EP2078744A1 (de) | Kraftstoffzusammensetzungen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20230606 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20250124 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| U01 | Request for unitary effect filed |
Effective date: 20250423 |
|
| U07 | Unitary effect registered |
Designated state(s): AT BE BG DE DK EE FI FR IT LT LU LV MT NL PT RO SE SI Effective date: 20250428 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 3031976 Country of ref document: ES Kind code of ref document: T3 Effective date: 20250714 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250723 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250724 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250423 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250423 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250723 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250823 |
|
| U20 | Renewal fee for the european patent with unitary effect paid |
Year of fee payment: 5 Effective date: 20251110 |