EP3581614A1 - Asphaltic sheet materials including expandable graphite - Google Patents
Asphaltic sheet materials including expandable graphite Download PDFInfo
- Publication number
- EP3581614A1 EP3581614A1 EP19182058.8A EP19182058A EP3581614A1 EP 3581614 A1 EP3581614 A1 EP 3581614A1 EP 19182058 A EP19182058 A EP 19182058A EP 3581614 A1 EP3581614 A1 EP 3581614A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- asphaltic
- sheet
- expandable graphite
- asphalt
- styrene
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 title claims abstract description 180
- 239000010439 graphite Substances 0.000 title claims abstract description 176
- 229910002804 graphite Inorganic materials 0.000 title claims abstract description 176
- 239000000463 material Substances 0.000 title description 48
- 239000010426 asphalt Substances 0.000 claims abstract description 144
- 239000011230 binding agent Substances 0.000 claims abstract description 64
- 239000003607 modifier Substances 0.000 claims description 36
- 239000003063 flame retardant Substances 0.000 claims description 34
- 238000000034 method Methods 0.000 claims description 27
- RNFJDJUURJAICM-UHFFFAOYSA-N 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyclohexa-1,3,5-triene Chemical compound N=1P(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP=1(OC=1C=CC=CC=1)OC1=CC=CC=C1 RNFJDJUURJAICM-UHFFFAOYSA-N 0.000 claims description 22
- 229920000468 styrene butadiene styrene block copolymer Polymers 0.000 claims description 19
- 229920001400 block copolymer Polymers 0.000 claims description 15
- -1 polypropylene Polymers 0.000 claims description 13
- 239000004744 fabric Substances 0.000 claims description 9
- 239000004743 Polypropylene Substances 0.000 claims description 8
- RTACIUYXLGWTAE-UHFFFAOYSA-N buta-1,3-diene;2-methylbuta-1,3-diene;styrene Chemical compound C=CC=C.CC(=C)C=C.C=CC1=CC=CC=C1 RTACIUYXLGWTAE-UHFFFAOYSA-N 0.000 claims description 8
- 229910021540 colemanite Inorganic materials 0.000 claims description 8
- 229920001155 polypropylene Polymers 0.000 claims description 8
- 229920000346 polystyrene-polyisoprene block-polystyrene Polymers 0.000 claims description 8
- 229920003048 styrene butadiene rubber Polymers 0.000 claims description 8
- ROGIWVXWXZRRMZ-UHFFFAOYSA-N 2-methylbuta-1,3-diene;styrene Chemical compound CC(=C)C=C.C=CC1=CC=CC=C1 ROGIWVXWXZRRMZ-UHFFFAOYSA-N 0.000 claims description 4
- 239000005062 Polybutadiene Substances 0.000 claims description 4
- 230000001070 adhesive effect Effects 0.000 claims description 4
- 229920001084 poly(chloroprene) Polymers 0.000 claims description 4
- 229920002857 polybutadiene Polymers 0.000 claims description 4
- 229920001195 polyisoprene Polymers 0.000 claims description 4
- 239000002131 composite material Substances 0.000 claims description 3
- 229920000098 polyolefin Polymers 0.000 claims description 2
- 238000010030 laminating Methods 0.000 claims 3
- 239000010410 layer Substances 0.000 description 69
- 229910052799 carbon Inorganic materials 0.000 description 45
- 239000000203 mixture Substances 0.000 description 43
- 229920005989 resin Polymers 0.000 description 42
- 239000011347 resin Substances 0.000 description 42
- 239000002245 particle Substances 0.000 description 29
- 239000012528 membrane Substances 0.000 description 26
- 230000004888 barrier function Effects 0.000 description 19
- 229920000642 polymer Polymers 0.000 description 18
- 230000003014 reinforcing effect Effects 0.000 description 17
- 230000000295 complement effect Effects 0.000 description 16
- 238000009413 insulation Methods 0.000 description 16
- 229910052751 metal Inorganic materials 0.000 description 16
- 239000002184 metal Substances 0.000 description 16
- 239000003921 oil Substances 0.000 description 15
- 235000019198 oils Nutrition 0.000 description 15
- 238000012360 testing method Methods 0.000 description 15
- 239000000945 filler Substances 0.000 description 11
- 239000004114 Ammonium polyphosphate Substances 0.000 description 10
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 10
- 235000019826 ammonium polyphosphate Nutrition 0.000 description 10
- 229920001276 ammonium polyphosphate Polymers 0.000 description 10
- 239000007787 solid Substances 0.000 description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- 239000000470 constituent Substances 0.000 description 9
- 238000001816 cooling Methods 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 239000003208 petroleum Substances 0.000 description 8
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 7
- 229920006272 aromatic hydrocarbon resin Polymers 0.000 description 7
- 239000004615 ingredient Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 239000011521 glass Substances 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 239000011159 matrix material Substances 0.000 description 6
- 239000005011 phenolic resin Substances 0.000 description 6
- 229920001083 polybutene Polymers 0.000 description 6
- 239000004753 textile Substances 0.000 description 6
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 5
- 125000003118 aryl group Chemical group 0.000 description 5
- 229930195733 hydrocarbon Natural products 0.000 description 5
- 150000002430 hydrocarbons Chemical class 0.000 description 5
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 5
- 239000000347 magnesium hydroxide Substances 0.000 description 5
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- 229910052717 sulfur Inorganic materials 0.000 description 5
- 239000011593 sulfur Substances 0.000 description 5
- 229920002633 Kraton (polymer) Polymers 0.000 description 4
- 229920006271 aliphatic hydrocarbon resin Polymers 0.000 description 4
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 4
- 238000003490 calendering Methods 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 238000005253 cladding Methods 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 230000002939 deleterious effect Effects 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 239000011152 fibreglass Substances 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 229920001568 phenolic resin Polymers 0.000 description 4
- 229920000728 polyester Polymers 0.000 description 4
- 230000002787 reinforcement Effects 0.000 description 4
- BIKXLKXABVUSMH-UHFFFAOYSA-N trizinc;diborate Chemical compound [Zn+2].[Zn+2].[Zn+2].[O-]B([O-])[O-].[O-]B([O-])[O-] BIKXLKXABVUSMH-UHFFFAOYSA-N 0.000 description 4
- WSSSPWUEQFSQQG-UHFFFAOYSA-N 4-methyl-1-pentene Chemical compound CC(C)CC=C WSSSPWUEQFSQQG-UHFFFAOYSA-N 0.000 description 3
- 239000004606 Fillers/Extenders Substances 0.000 description 3
- 235000019738 Limestone Nutrition 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- 239000006028 limestone Substances 0.000 description 3
- 239000000155 melt Substances 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 239000004576 sand Substances 0.000 description 3
- 238000009738 saturating Methods 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 229920001059 synthetic polymer Polymers 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 229920001169 thermoplastic Polymers 0.000 description 3
- 239000002023 wood Substances 0.000 description 3
- KXGFMDJXCMQABM-UHFFFAOYSA-N 2-methoxy-6-methylphenol Chemical compound [CH]OC1=CC=CC([CH])=C1O KXGFMDJXCMQABM-UHFFFAOYSA-N 0.000 description 2
- RSWGJHLUYNHPMX-UHFFFAOYSA-N Abietic-Saeure Natural products C12CCC(C(C)C)=CC2=CCC2C1(C)CCCC2(C)C(O)=O RSWGJHLUYNHPMX-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 239000013032 Hydrocarbon resin Substances 0.000 description 2
- 229920000877 Melamine resin Polymers 0.000 description 2
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 229920000388 Polyphosphate Polymers 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- KHPCPRHQVVSZAH-HUOMCSJISA-N Rosin Natural products O(C/C=C/c1ccccc1)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 KHPCPRHQVVSZAH-HUOMCSJISA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- RREGISFBPQOLTM-UHFFFAOYSA-N alumane;trihydrate Chemical compound O.O.O.[AlH3] RREGISFBPQOLTM-UHFFFAOYSA-N 0.000 description 2
- 229920013640 amorphous poly alpha olefin Polymers 0.000 description 2
- GHPGOEFPKIHBNM-UHFFFAOYSA-N antimony(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Sb+3].[Sb+3] GHPGOEFPKIHBNM-UHFFFAOYSA-N 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 238000000113 differential scanning calorimetry Methods 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- 238000005227 gel permeation chromatography Methods 0.000 description 2
- 230000009477 glass transition Effects 0.000 description 2
- 229920006270 hydrocarbon resin Polymers 0.000 description 2
- 230000002687 intercalation Effects 0.000 description 2
- 238000009830 intercalation Methods 0.000 description 2
- JDSHMPZPIAZGSV-UHFFFAOYSA-N melamine Chemical compound NC1=NC(N)=NC(N)=N1 JDSHMPZPIAZGSV-UHFFFAOYSA-N 0.000 description 2
- 239000004745 nonwoven fabric Substances 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- 239000013047 polymeric layer Substances 0.000 description 2
- 239000001205 polyphosphate Substances 0.000 description 2
- 235000011176 polyphosphates Nutrition 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 150000003097 polyterpenes Chemical class 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 229920001187 thermosetting polymer Polymers 0.000 description 2
- KHPCPRHQVVSZAH-UHFFFAOYSA-N trans-cinnamyl beta-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OCC=CC1=CC=CC=C1 KHPCPRHQVVSZAH-UHFFFAOYSA-N 0.000 description 2
- VLCLHFYFMCKBRP-UHFFFAOYSA-N tricalcium;diborate Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]B([O-])[O-].[O-]B([O-])[O-] VLCLHFYFMCKBRP-UHFFFAOYSA-N 0.000 description 2
- 239000002759 woven fabric Substances 0.000 description 2
- KPAPHODVWOVUJL-UHFFFAOYSA-N 1-benzofuran;1h-indene Chemical compound C1=CC=C2CC=CC2=C1.C1=CC=C2OC=CC2=C1 KPAPHODVWOVUJL-UHFFFAOYSA-N 0.000 description 1
- CBXRMKZFYQISIV-UHFFFAOYSA-N 1-n,1-n,1-n',1-n',2-n,2-n,2-n',2-n'-octamethylethene-1,1,2,2-tetramine Chemical compound CN(C)C(N(C)C)=C(N(C)C)N(C)C CBXRMKZFYQISIV-UHFFFAOYSA-N 0.000 description 1
- 239000004594 Masterbatch (MB) Substances 0.000 description 1
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 229920006372 Soltex Polymers 0.000 description 1
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 238000004026 adhesive bonding Methods 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910021502 aluminium hydroxide Inorganic materials 0.000 description 1
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical class Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000010692 aromatic oil Substances 0.000 description 1
- 229910052785 arsenic Inorganic materials 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229910001730 borate mineral Inorganic materials 0.000 description 1
- 239000010429 borate mineral Substances 0.000 description 1
- 239000011449 brick Substances 0.000 description 1
- 239000004566 building material Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- QGJOPFRUJISHPQ-NJFSPNSNSA-N carbon disulfide-14c Chemical compound S=[14C]=S QGJOPFRUJISHPQ-NJFSPNSNSA-N 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 150000008280 chlorinated hydrocarbons Chemical class 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 239000004567 concrete Substances 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000007766 curtain coating Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 230000000447 dimerizing effect Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000009970 fire resistant effect Effects 0.000 description 1
- 230000009969 flowable effect Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 229910001679 gibbsite Inorganic materials 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000010440 gypsum Substances 0.000 description 1
- 229910052602 gypsum Inorganic materials 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 239000011256 inorganic filler Substances 0.000 description 1
- 229910003475 inorganic filler Inorganic materials 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910001507 metal halide Inorganic materials 0.000 description 1
- 150000005309 metal halides Chemical class 0.000 description 1
- QLTDWCHQCKHGGO-UHFFFAOYSA-N methane;sulfuric acid Chemical compound C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.C.OS(O)(=O)=O.OS(O)(=O)=O.OS(O)(=O)=O.OS(O)(=O)=O.OS(O)(=O)=O.OS(O)(=O)=O.OS(O)(=O)=O QLTDWCHQCKHGGO-UHFFFAOYSA-N 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 239000000025 natural resin Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- CLNYHERYALISIR-UHFFFAOYSA-N nona-1,3-diene Chemical compound CCCCCC=CC=C CLNYHERYALISIR-UHFFFAOYSA-N 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 238000002103 osmometry Methods 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 239000010690 paraffinic oil Substances 0.000 description 1
- 238000004525 petroleum distillation Methods 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 239000011120 plywood Substances 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920000582 polyisocyanurate Polymers 0.000 description 1
- 239000011495 polyisocyanurate Substances 0.000 description 1
- 230000000379 polymerizing effect Effects 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 229920005604 random copolymer Polymers 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 229920003987 resole Polymers 0.000 description 1
- 239000011435 rock Substances 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 125000003011 styrenyl group Chemical group [H]\C(*)=C(/[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 239000000057 synthetic resin Substances 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 235000007586 terpenes Nutrition 0.000 description 1
- 229910052716 thallium Inorganic materials 0.000 description 1
- 238000005979 thermal decomposition reaction Methods 0.000 description 1
- 229920002725 thermoplastic elastomer Polymers 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 description 1
- 239000012808 vapor phase Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 238000004078 waterproofing Methods 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B11/00—Layered products comprising a layer of bituminous or tarry substances
- B32B11/04—Layered products comprising a layer of bituminous or tarry substances comprising such bituminous or tarry substance as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B11/046—Layered products comprising a layer of bituminous or tarry substances comprising such bituminous or tarry substance as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B11/00—Layered products comprising a layer of bituminous or tarry substances
- B32B11/04—Layered products comprising a layer of bituminous or tarry substances comprising such bituminous or tarry substance as the main or only constituent of a layer, which is next to another layer of the same or of a different material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B11/00—Layered products comprising a layer of bituminous or tarry substances
- B32B11/02—Layered products comprising a layer of bituminous or tarry substances with fibres or particles being present as additives in the layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B11/00—Layered products comprising a layer of bituminous or tarry substances
- B32B11/10—Layered products comprising a layer of bituminous or tarry substances next to a fibrous or filamentary layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B11/00—Layered products comprising a layer of bituminous or tarry substances
- B32B11/12—Layered products comprising a layer of bituminous or tarry substances next to a particulate layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/16—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by features of a layer formed of particles, e.g. chips, powder or granules
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/04—Reinforcing macromolecular compounds with loose or coherent fibrous material
- C08J5/0405—Reinforcing macromolecular compounds with loose or coherent fibrous material with inorganic fibres
- C08J5/043—Reinforcing macromolecular compounds with loose or coherent fibrous material with inorganic fibres with glass fibres
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/18—Manufacture of films or sheets
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/043—Improving the adhesiveness of the coatings per se, e.g. forming primers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/048—Forming gas barrier coatings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/05—Forming flame retardant coatings or fire resistant coatings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/06—Coating with compositions not containing macromolecular substances
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/38—Boron-containing compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L95/00—Compositions of bituminous materials, e.g. asphalt, tar, pitch
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D195/00—Coating compositions based on bituminous materials, e.g. asphalt, tar, pitch
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K21/00—Fireproofing materials
-
- E—FIXED CONSTRUCTIONS
- E04—BUILDING
- E04D—ROOF COVERINGS; SKY-LIGHTS; GUTTERS; ROOF-WORKING TOOLS
- E04D12/00—Non-structural supports for roofing materials, e.g. battens, boards
- E04D12/002—Sheets of flexible material, e.g. roofing tile underlay
-
- E—FIXED CONSTRUCTIONS
- E04—BUILDING
- E04D—ROOF COVERINGS; SKY-LIGHTS; GUTTERS; ROOF-WORKING TOOLS
- E04D3/00—Roof covering by making use of flat or curved slabs or stiff sheets
- E04D3/02—Roof covering by making use of flat or curved slabs or stiff sheets of plane slabs, slates, or sheets, or in which the cross-section is unimportant
- E04D3/18—Roof covering by making use of flat or curved slabs or stiff sheets of plane slabs, slates, or sheets, or in which the cross-section is unimportant of specified materials, or of combinations of materials, not covered by any of groups E04D3/04, E04D3/06 or E04D3/16
-
- E—FIXED CONSTRUCTIONS
- E04—BUILDING
- E04D—ROOF COVERINGS; SKY-LIGHTS; GUTTERS; ROOF-WORKING TOOLS
- E04D5/00—Roof covering by making use of flexible material, e.g. supplied in roll form
-
- E—FIXED CONSTRUCTIONS
- E04—BUILDING
- E04D—ROOF COVERINGS; SKY-LIGHTS; GUTTERS; ROOF-WORKING TOOLS
- E04D5/00—Roof covering by making use of flexible material, e.g. supplied in roll form
- E04D5/10—Roof covering by making use of flexible material, e.g. supplied in roll form by making use of compounded or laminated materials, e.g. metal foils or plastic films coated with bitumen
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/02—Synthetic macromolecular fibres
- B32B2262/0261—Polyamide fibres
- B32B2262/0269—Aromatic polyamide fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/02—Synthetic macromolecular fibres
- B32B2262/0276—Polyester fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/10—Inorganic fibres
- B32B2262/101—Glass fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/30—Properties of the layers or laminate having particular thermal properties
- B32B2307/306—Resistant to heat
- B32B2307/3065—Flame resistant or retardant, fire resistant or retardant
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2419/00—Buildings or parts thereof
- B32B2419/06—Roofs, roof membranes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2395/00—Bituminous materials, e.g. asphalt, tar or pitch
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2453/00—Characterised by the use of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Derivatives of such polymers
- C08J2453/02—Characterised by the use of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Derivatives of such polymers of vinyl aromatic monomers and conjugated dienes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/38—Boron-containing compounds
- C08K2003/387—Borates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2555/00—Characteristics of bituminous mixtures
- C08L2555/40—Mixtures based upon bitumen or asphalt containing functional additives
- C08L2555/50—Inorganic non-macromolecular ingredients
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2555/00—Characteristics of bituminous mixtures
- C08L2555/40—Mixtures based upon bitumen or asphalt containing functional additives
- C08L2555/80—Macromolecular constituents
- C08L2555/84—Polymers comprising styrene, e.g., polystyrene, styrene-diene copolymers or styrene-butadiene-styrene copolymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2666/00—Composition of polymers characterized by a further compound in the blend, being organic macromolecular compounds, natural resins, waxes or and bituminous materials, non-macromolecular organic substances, inorganic substances or characterized by their function in the composition
- C08L2666/54—Inorganic substances
- C08L2666/55—Carbon
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31815—Of bituminous or tarry residue
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T442/00—Fabric [woven, knitted, or nonwoven textile or cloth, etc.]
- Y10T442/20—Coated or impregnated woven, knit, or nonwoven fabric which is not [a] associated with another preformed layer or fiber layer or, [b] with respect to woven and knit, characterized, respectively, by a particular or differential weave or knit, wherein the coating or impregnation is neither a foamed material nor a free metal or alloy layer
- Y10T442/2213—Coating or impregnation is specified as weather proof, water vapor resistant, or moisture resistant
Definitions
- Embodiments of the present invention are directed toward asphaltic sheet materials that include expandable graphite. These sheet materials are useful as roofing underlayment, as roofing membranes, and as barrier materials such as air, vapor, and/or moisture barriers.
- Asphaltic sheet materials are widely used in the construction industry.
- polymer-modified asphaltic sheet material is used as membrane for waterproofing flat or low-sloped roofs.
- these roofing systems may include multiple layers of asphaltic sheet including base sheets and cap sheets.
- barriers materials such air, vapor, or moisture barriers. These materials are typically used on roofs or vertical surfaces such as walls to provide the desired air, vapor and/or moisture resistance to a structure.
- Still other examples include underlayments, which are used in the roofing industry to provide an extra layer of protection to the roof. This additional protection may provide, among other benefits, water, moisture, thermal, and/or fire resistance.
- underlayment is typically positioned below the external or primary roofing protection, which may include shingles, membranes such as polymeric or asphaltic membranes, roofing tiles, and metal panels or cladding.
- felt paper that is saturated with asphalt has historically been used as underlayment to provide additional water and/or moisture resistance to the roof.
- Other forms of underlayment include synthetic materials such as thermoplastic or thermoset materials formed into sheets.
- fire or flame resistant underlayment may be employed.
- These underlayment may include textiles, including woven and non-woven fabrics, made of fire resistant materials such as fiberglass. These fabrics may include a coating, such as a mineral coating, that further enhances the flame or fire resistance of the underlayment.
- underlayment such as with metal roofing systems
- underlayment such as with metal roofing systems
- first underlayment may be used to provide moisture or water resistance
- second underlayment may be used to provide flame or fire resistance.
- This technique suffers from several drawbacks including the added difficulty of installing multiple underlayments.
- One or more embodiments of the present invention provide an asphaltic sheet comprising an asphaltic component including an asphalt binder and expandable graphite.
- Still other embodiments of the present invention provide a roof system comprising a roof deck; an underlayment; and one or more metal panels or roofing tilescovering the underlayment, where the underlayment includes an asphaltic component including an asphalt binder and expandable graphite dispersed within the asphalt binder.
- Still other embodiments of the present invention provide a method for producing an asphaltic sheet, the method comprising preparing a molten asphaltic composition by introducing an expandable graphite to an asphalt binder at a temperature below that which will cause deleterious expansion of the expandable graphite, and fabricating an asphaltic sheet with the molten asphaltic composition.
- Still other embodiments of the present invention provide an asphaltic sheet comprising an asphaltic component including an asphalt binder and a layer including expandable graphite, where said layer including expandable graphite is adjacent to said asphaltic component.
- Still other embodiments of the present invention provide a method for producing an asphaltic sheet, the method comprising providing an asphaltic sheet, and applying expandable graphite particles to the asphaltic sheet.
- Embodiments of the present invention are based, at least in part, on the discovery of an asphaltic sheet having an asphaltic component with expandable graphite.
- the asphaltic sheet includes an asphalt component with the expandable graphite dispersed within the asphalt component.
- the asphaltic sheet includes a layer of expandable graphite adjacent to the asphalt component.
- the asphaltic sheet is advantageously resistant to water, moisture, an/or air due to the asphaltic nature of the sheet, and it is also flame resistant due to the presence of the expandable graphite. It is believed that one or more advantages of the present invention derive from the presence of the expandable graphite within or adjacent to the asphaltic component of the sheet.
- the prior art contemplates the use of expandable graphite as a flame retardant
- the prior art does not contemplate incorporating the expandable graphite into the asphaltic component of a sheet material or placing a layer of the expandable graphite adjacent to the asphaltic component.
- Embodiments of the present invention provide a method for incorporating the expandable graphite into the asphaltic component of the sheet without deleteriously expanding the graphite (i.e. the graphite is not expanded to a deleterious degree).
- the asphaltic component of the sheet material further includes a complementary flame retardant material that is believed to synergistically interact with the expandable graphite to provide unexpected flame resistance.
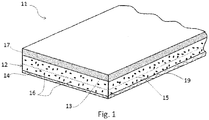
- the asphaltic sheet is or includes a planar body of asphalt material, which may also be referred to as the asphalt component of the sheet or asphalt layer 12.
- asphaltic sheet 11 includes asphalt component 12 having a first planar surface 13 and second planer surface 14.
- Sheet 11 may include an optional textile fabric 15 embedded or impregnated within asphaltic component 12.
- the sheet is devoid of a scrim or fabric.
- Asphaltic component 12 as will be described in greater detail below, may include various constituents such as polymeric modifiers and fillers, as well as expandable graphite 16 and optional complementary flame retardants (not shown) according to the present invention.
- sheet 11 may further include one or more polymeric layers 17 laminated to asphalt component 12 of sheet 11.
- asphaltic sheet 11 may include an asphaltic component 12 laminated to a polypropylene sheet.
- layer 17 may include a layer of release agents, such as silica, sand or talc.
- a release film 19 may be removably secured to at least one of the exposed planar surfaces 13 or 14.
- optional textile fabric 15, which may also be referred to as fabric reinforcement 15, reinforcing member 15, or simply reinforcement 15, may include woven and/or non-woven fabrics.
- fabric reinforcement 15 may be fabricated from fiberglass and/or synthetic yards or filaments.
- Exemplary synthetic yarns include those prepared from polyesters or polyimides.
- the thickness of asphaltic sheet 11 may be at least 10, in other embodiments at least 20, and in other embodiments at least 30 mils. In these or other embodiments, the thickness of asphaltic sheet 11 may be at most 120, in other embodiments at most 100, in other embodiments at most 90, and in other embodiments at most 80 mils. In one or more embodiments, the thickness of asphaltic sheet 11 may be from about 10 to about 100, in other embodiments from about 20 to about 90, and in other embodiments from about 30 to about 80 mils. In other embodiments, especially where the asphaltic sheet is used in a vertical application, the thickness of the asphaltic sheet may be substantially thinner.
- the thickness of the sheet may be less than 20, in other embodiments less than 15, and in other embodiments less than 10 mils, with the thickness ranging from 2 to 20 mils, in other embodiments from 3 to 15 mils, and in other embodiments from 5 to 10 mils.
- the weight of the asphaltic sheet may be at least 5, in other embodiments at least 10 and in other embodiments at least 15 pounds per hundred square feet. In these or other embodiments, the weight of the asphaltic sheet may be at most 90, in other embodiments at most 70, and in other embodiments at most 50 pounds per hundred square feet. In these or other embodiments, the weight of the asphaltic sheet may be from 5 to 100, in other embodiments from 10 to 80, and in other embodiments from 15 to 50 pounds per hundred square feet. In other embodiments, especially where the asphaltic sheet is used in a vertical application, the weight of the asphaltic sheet may be substantially lighter.
- the weight of the sheet may be less than 60, in other embodiments less than 50, and in other embodiments less than 40 pounds per hundred square feet, with the weight ranging from 5 to 60, in other embodiments from 10 to 50, and in other embodiments from 15 to 40 pounds per hundred square feet.
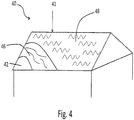
- asphaltic sheet 11 includes asphaltic component 12 having first planer surface 13 and second planar surface 14. Adjacent to asphaltic component 12 is a layer 18 including expandable graphite 16. Layer 18 may be adjacent to asphaltic component 12 as a result of the manner in which asphaltic sheet 11 is fabricated, as will be described in greater detail below.
- Asphaltic sheet 11 may carry optional polymeric layer 17, which may also be referred to as polymeric liner 17.
- layer 17 may include a layer of release agents, such as silica, sand or talc.
- layer 17 may be a glass scrim, polyester mat, metal foil (e.g., aluminum foil), fabric, elastomeric layer, and the like.
- layer 18 includes one or more layers of particles of expandable graphite 16. These particles may be held in place by a matrix of asphalt material present within at least a portion of layer 18. In these or other embodiments, the expandable graphite 16 is held in place by being adhered to the surface of the asphalt.
- asphaltic component 12 may also include expandable graphite dispersed therein. In other words, asphaltic sheet may include expandable graphite dispersed throughout the asphaltic component and layer 18 of expandable graphite adjacent to component 12.
- layer 18 may include a planar region within sheet 11 that includes a higher concentration of expandable graphite relative to any other region of sheet 11.
- layer 18 may include a continuous layer of expandable graphite having a variable or relatively constant thickness across sheet 11.
- the expandable graphite may be discontinuous throughout the region so long as the concentration of expandable graphite within the region is higher than in other areas or regions of sheet 11.
- the discontinuity of the expandable graphite within the layer 18 may result from the asphaltic material which may form a matrix in which the expandable graphite is at least partially dispersed within this region or layer. It should also be appreciated that the concentration of the expandable graphite may not be constant within this layer.
- a concentration gradient may exist whereby the concentration of the expandable graphite moves from a region of maximum concentration to a region of decreased concentration.
- the concentration of expandable graphite 16 furthest from planar surface 13 within layer 18 is the highest, which corresponds to a minimum in asphalt concentration.
- the concentration of expandable graphite 16 proximate to planar surface 13 is a minimum relative to the concentration of expandable graphite within layer 18.
- an additional layer of asphalt 12' is adjacent expandable graphite layer 18 opposite asphaltic component or layer 12.
- Layer 12' may also be referred to as a skin layer 12', and aspects of this embodiment may be described as layer or region 18 being embedded between layers of asphaltic binder or material. Consistent with the other embodiments described above, this layer 12' may include expandable graphite 16 at a concentration lower than layer 18. Nonetheless, in one or more embodiments, layer 12' may include expandable graphite dispersed therein, although the concentration is lower than the concentration of the expandable graphite within region 18. In fact, in one or more embodiments, a concentration gradient may exist between layers 12' and 18 in a similar fashion to the concentration gradient described above.
- the sheet can include multiple discreet regions of the expandable graphite, such as may exist in a pattern where the expandable graphite is applied on the top of the asphaltic sheet in rows or strips in the machine direction of the sheet. This may be advantageous where greater adhesion to a top sheet (e.g. sheet 17) is desired.
- the thickness of layer 18 may be at least 10 ⁇ m, in other embodiments at least 20 ⁇ m, in other embodiments at least 30 ⁇ m, in other embodiments at least 75 ⁇ m, and in other embodiments at least 100 ⁇ m. In these or other embodiments, the thickness of layer 18 may be at most 3 mm, in other embodiments at most 2 mm, and in other embodiments at most 1 mm. In one or more embodiments, the thickness of layer 18 may be from about 10 ⁇ m to about 3 mm, in other embodiments from about 75 ⁇ m to about 2 mm, and in other embodiments from about 100 ⁇ m to about 1 mm.
- the thickness of layer 12' may be at least 2 ⁇ m, in other embodiments at least 5 ⁇ m, and in other embodiments at least 20 ⁇ m. In these or other embodiments, the thickness of layer 12' may be at most 1 mm, in other embodiments at most 0.5 mm, in other embodiments at most 0.25 mm, in other embodiments at most 0.1 mm, and in other embodiments at most 0.050 mm. In one or more embodiments, the thickness of layer 12' may be from about 1 ⁇ m to about 3 mm, in other embodiments from about 2 ⁇ m to about 0.5 mm, and in other embodiments from about 5 ⁇ m to about 0.050 mm.
- the asphaltic sheet of one or more embodiments of the present invention includes an asphaltic component.
- the asphaltic component includes an asphalt binder and expandable graphite dispersed within the binder.
- the asphaltic component may also include, dispersed within the binder, polymeric modifiers, fillers, tackifiers, complementary flame retardants, and other constituents conventionally used in asphaltic-based building materials.
- asphalt binder is used as understood by those skilled in the art and is consistent with the meaning provided by AASHTO M320. As used within this specification, the terms “asphalt” and “asphalt binder” may be used synonymously.
- the asphalt binder material may be derived from any asphalt source, such as natural asphalt, rock asphalt, produced from tar sands, or petroleum asphalt obtained in the process of refining petroleum.
- asphalt binders may include a blend of various asphalts not meeting any specific grade definition. This includes airblown asphalt, vacuum-distilled asphalt, steam-distilled asphalt, cutback asphalt or roofing asphalt. Alternatively, gilsonite, natural or synthetic, used alone or mixed with petroleum asphalt, may be selected.
- asphalt includes petroleum derived asphalt and asphaltic residual. These compositions may include asphaltenes, resins, cyclics, and saturates. The percentage of these constituents in the overall asphalt binder composition may vary based on the source of the asphalt.
- Asphaltenes include black amorphous solids containing, in addition to carbon and hydrogen, some nitrogen, sulfur, and oxygen. Trace elements such as nickel and vanadium may also be present. Asphaltenes are generally considered as highly polar aromatic materials of a number average molecular weight of about 2000 to about 5000 g/mol, and may constitute about 5 to about 25% of the weight of asphalt.
- Resins include dark-colored, solid and semi-solid, very adhesive fractions of relatively high molecular weight present in the maltenes. They may include the dispersing agents of peptizers for the asphaltenes, and the proportion of resins to asphaltenes governs, to a degree, the sol-or gel-type character of asphalts. Resins separated from bitumens may have a number average molecular weight of about 0.8 to about 2 kg/mol but there is a wide molecular distribution. This component may constitute about 15 to about 25% of the weight of asphalts.
- Cyclics include the compounds of lowest molecular weight in bitumens and represent the major portion of the dispersion medium for the peptized asphaltenes. They may constitute about 45 to about 60% by weight of the total asphalt binder, and may be dark viscous liquids. They may include compounds with aromatic and naphthenic aromatic nuclei with side chain constituents and may have molecular weights of 0.5 to about 9 kg/mol.
- Saturates include predominantly the straight-and branched-chain aliphatic hydrocarbons present in bitumens, together with alkyl naphthenes and some alkyl aromatics.
- the average molecular weight range may be approximately similar to that of the cyclics, and the components may include the waxy and non-waxy saturates. This fraction may from about 5 to about 20% of the weight of asphalts.
- asphalt binders may include bitumens that occur in nature or may be obtained in petroleum processing.
- Asphalts may contain very high molecular weight hydrocarbons called asphaltenes, which may be soluble in carbon disulfide, pyridine, aromatic hydrocarbons, chlorinated hydrocarbons, and THF.
- Asphalts or bituminous materials may be solids, semi-solids or liquids.
- the asphalt binder includes AC-5, AC-10 and AC-15. These asphalts typically contain about 40 to about 52 parts by weight of aromatic hydrocarbons, about 20 to about 44 parts by weight of a polar organic compound, about 10 to about 15 parts by weight of asphaltene, about 6 to about 8 parts by weight of saturates, and about 4 to about 5 parts by weight of sulfur. Nevertheless, practice of the present invention is not limited by selection of any particular asphalt.
- the molecular weight of the aromatic hydrocarbons present in asphalt may range between about 300 and 2000, while the polar organic compounds, which generally include hydroxylated, carboxylated and heterocyclic compounds, may have a weight average molecular weight of about 500 to 50,000.
- Asphaltenes which are generally known as heavy hydrocarbons, are typically of a high molecular weight and are heptane insoluble. Saturates generally include paraffinic and cycloaliphatic hydrocarbons having about 300 to 2000 molecular weight.
- bitumens may be used.
- Bitumens are naturally occurring solidified hydrocarbons, typically collected as a residue of petroleum distillation. Gilsonite is believed to be the purest naturally formed bitumen, typically having a molecular weight of about 3,000 with about 3 parts by weight complexed nitrogen.
- Expandable graphite may also be referred to as expandable flake graphite, intumescent flake graphite, or expandable flake; and, for the purposes herein, these terms may be used interchangeably.
- expandable graphite includes intercalated graphite in which an intercallant material is included between the graphite layers of graphite crystal or particle.
- intercallant materials include halogens, alkali metals, sulfates, nitrates, various organic acids, aluminum chlorides, ferric chlorides, other metal halides, arsenic sulfides, and thallium sulfides.
- the expandable graphite includes non-halogenated intercallant materials.
- the expandable graphite includes sulfate intercallants, also referred to as graphite bisulfate. As is known in the art, bisulfate intercalation is achieved by treating highly crystalline natural flake graphite with a mixture of sulfuric acid and other oxidizing agents which act to catalyze the sulfate intercalation.
- expandable graphite examples include HPMS Expandable Graphite (HP Materials Solutions, Inc., Woodland Hills, CA) and Expandable Graphite Grades 1721 (Asbury Carbons, Asbury, NJ).
- HPMS Expandable Graphite HP Materials Solutions, Inc., Woodland Hills, CA
- Expandable Graphite Grades 1721 (Asbury Carbons, Asbury, NJ).
- Other commercial grades contemplated as useful in the present invention include 1722, 3393, 3577, 3626, and 1722HT (Asbury Carbons, Asbury, NJ).
- the expandable graphite may be characterized as having a mean or average size in the range from about 30 ⁇ m to about 1.5 mm, in other embodiments from about 50 ⁇ m to about 1.0 mm, and in other embodiments from about 180 to about 850 ⁇ m. In certain embodiments, the expandable graphite may be characterized as having a mean or average size of at least 30 ⁇ m, in other embodiments at least 44 ⁇ m, in other embodiments at least 180 ⁇ m, and in other embodiments at least 300 ⁇ m.
- expandable graphite may be characterized as having a mean or average size of at most 1.5 mm, in other embodiments at most 1.0 mm, in other embodiments at most 850 ⁇ m, in other embodiments at most 600 ⁇ m, in yet other embodiments at most 500 ⁇ m, and in still other embodiments at most 400 ⁇ m.
- Useful expandable graphite includes Graphite Grade #1721 (Asbury Carbons), which has a nominal size of greater than 300 ⁇ m.
- the expandable graphite may be characterized as having a median size in the range from about 30 ⁇ m to about 1.5 mm, in other embodiments from about 50 ⁇ m to about 1.0 mm, and in other embodiments from about 180 to about 850 ⁇ m. In certain embodiments, the expandable graphite may be characterized as having a median size of at least 30 ⁇ m, in other embodiments at least 44 ⁇ m, in other embodiments at least 180 ⁇ m, and in other embodiments at least 300 ⁇ m.
- expandable graphite may be characterized as having a median size of at most 1.5 mm, in other embodiments at most 1.0 mm, in other embodiments at most 850 ⁇ m, in other embodiments at most 600 ⁇ m, in yet other embodiments at most 500 ⁇ m, and in still other embodiments at most 400 ⁇ m.
- the expandable graphite may be characterized as having a nominal particle size of 20x50 (US sieve). US sieve 20 has an opening equivalent to 0.841 mm and US sieve 50 has an opening equivalent to 0.297 mm. Therefore, a nominal particle size of 20x50 indicates the graphite particles are at least 0.297 mm and at most 0.841 mm.
- the expandable graphite may be characterized as having a carbon content in the range from about 80% to about 99%. In certain embodiments, the expandable graphite may be characterized as having a carbon content of at least 80%, in other embodiments at least 85%, in other embodiments at least 90%, in yet other embodiments at least 95%, in other embodiments at least 98%, and in still other embodiments at least 99% carbon.
- the expandable graphite may be characterized as having a sulfur content in the range from about 0% to about 8%, in other embodiments from about 2.6% to about 5.0%, and in other embodiments from about 3.0% to about 3.5%. In certain embodiments, the expandable graphite may be characterized as having a sulfur content of at least 0%, in other embodiments at least 2.6%, in other embodiments at least 2.9%, in other embodiments at least 3.2%, and in other embodiments 3.5%. In certain embodiments, the expandable graphite may be characterized as having a sulfur content of at most 8%, in other embodiments at most 5%, in other embodiments at most 3.5%.
- the expandable graphite may be characterized as having an expansion ratio (cc/g) in the range from about 10:1 to about 500:1, in other embodiments at least 20:1 to about 450:1, in other embodiments at least 30:1 to about 400:1, in other embodiments from about 50:1 to about 350:1.
- cc/g expansion ratio
- the expandable graphite may be characterized as having an expansion ratio (cc/g) of at least 10:1, in other embodiments at least 20:1, in other embodiments at least 30:1, in other embodiments at least 40:1, in other embodiments at least 50:1, in other embodiments at least 60:1, in other embodiments at least 90:1, in other embodiments at least 160:1, in other embodiments at least 210:1, in other embodiments at least 220:1, in other embodiments at least 230:1, in other embodiments at least 270:1, in other embodiments at least 290:1, and in yet other embodiments at least 300:1.
- the expandable graphite may be characterized as having an expansion ratio (cc/g) of at most 350:1, and in yet other embodiments at most 300:1.
- the expandable graphite as it exists with the asphaltic component of the asphaltic sheet of the present invention, is partially expanded. In one or more embodiments, the expandable graphite is not expanded, however, to a deleterious degree, which includes that amount or more of expansion that will deleteriously the ability to form the sheet product and the ability of the graphite to serve as flame retardant at desirable levels, which include those levels that allow proper formation of the sheet. In one or more embodiments, the expandable graphite is expanded to at most 60%, in other embodiments at most 50%, in other embodiments at most 40%, in other embodiments at most 30%, in other embodiments at most 20%, and in other embodiments at most 10% beyond its original unexpanded size.
- the expandable graphite may be characterized as having a pH in the range from about 1 to about 10; in other embodiments from about 1 to about 6; and in yet other embodiments from about 5 to about 10. In certain embodiments, the expandable graphite may be characterized as having a pH in the range from about 4 to about 7. In one or more embodiments, the expandable graphite may be characterized as having a pH of at least 1, in other embodiments at least 4, and in other embodiments at least 5. In certain embodiments, the expandable graphite may be characterized as having a pH of at most 10, in other embodiments at most 7, and in other embodiments at most 6.
- the expandable graphite may be characterized by an onset temperature ranging from about 100 oC to about 250 oC; in other embodiments from about 160 oC to about 225 oC; and in other embodiments from about 180 oC to about 200 oC. In one or more embodiments, the expandable graphite may be characterized by an onset temperature of at least 100 oC, in other embodiments at least 130 oC, in other embodiments at least 160 oC, and in other embodiments at least 180 oC. In one or more embodiments, the expandable graphite may be characterized by an onset temperature of at most 250 oC, in other embodiments at most 225 oC, and in other embodiments at most 200 oC. Onset temperature may also be interchangeably referred to as expansion temperature and also alternatively referred to as the temperature at which expansion of the graphite starts.
- the polymeric modifier which may simply be referred to as polymer, includes thermoplastic polymers, thermosetting elastomers, thermoplastic elastomers, and/or mixtures thereof. Each of these polymers have been used, either alone or in combination with each other to modify asphalt binders, and practice of the present invention is not necessarily limited by the selection of any particular polymeric modifier.
- the polymers may be characterized by a glass transition temperature (Tg), as measured by DSC analysis, of less than 150°C, in other embodiment less than 125°C, in other embodiment less than 100°C, in other embodimetns less than 20°C, in other embodiments less than 0°C, in other embodiments less than -20°C, in other embodiments less than -35°C, and in other embodiments from about -90°C to about -20°C.
- Tg glass transition temperature
- the polymers may be characterized by a glass transition temperature (Tg), as measured by DSC analysis, of more than -20°C, in other embodiments more than 0°C, in other embodiments more than 20°C, in other embodiments more than 50°C, and in other embodiments more than 100°C.
- Tg glass transition temperature
- the polymeric modifier may be characterized by a melt index (ASTM D-1238;2.16 kg load @ 190°C) of less than 1,000 dg/min, in other embodiments less than 500 dg/min, in other embodiments less than 50 dg/min, in other embodiments less than 20 dg/min, in other embodiments less than 10 dg/min, and in other embodiments less than 1 dg/min.
- the unsaturated polymers may have a melt index of between 3 and 15 dg/min, and other embodiments between 4 and 12 dg/min.
- the polymeric modifier may be characterized by a number average molecular weight (M n ) of from about 10 to about 1,000 kg/mol, in other embodiments from about 40 to about 500 kg/mol, and in other embodiments from about 80 to about 200 kg/mol.
- M n number average molecular weight
- the polymeric modifier may also be characterized by a weight average molecular weight (M w ) of from about 10 to about 4,000 kg/mol, in other embodiments from about 40 to about 2,000 kg/mol, and in other embodiments from about 80 to about 800 kg/mol.
- the polymeric modifier may be characterized by a molecular weight distribution of from about 1.1 to about 5, in other embodiments from about 1.5 to about 4.5, and in other embodiments from about 1.8 to about 4.0.
- Molecular weight can be determined by gel permeation chromatography (GPC) calibrated with polystyrene standards and adjusted for the Mark-Houwink constants for the polymer in question.
- the polymeric modifier may be linear, branched, or coupled polymers.
- Types of polymers may include both natural and synthetic polymers.
- Useful synthetic polymers may include polydienes or polydiene copolymers with non-diene comonomer (e.g., styrene).
- the copolymers may include block and random copolymers.
- the coupled polymers may include linearly coupled polymers (e.g. di-coupled polymers) or raidally coupled polymers (e.g. tri-coupled or, tetra-coupled penta-coupled, hexa-coupled etc.).
- Exemplary polydienes include polybutadiene and polyisoprene.
- Exemplary copolymers may include random styrene-butadiene rubber, styrene-butadiene block copolymer, styrene-butadiene-styrene block copolymer, random styrene-isoprene, styrene-isoprene block copolymer, styrene-isoprene-butadiene block copolymer, random styrene-isoprene-butadiene, styrene-isoprene-styrene block copolymer, and chloroprene rubber.
- the polymeric modifier include linear or radial block copolymers wherein the block copolymers include terminal styrene blocks.
- the styrene content of these block copolymers may be from 10% to 50% by weight, in other embodiments from 15% to 45% by weight, and in other embodiments from 20% to 40% by weight.
- the polymeric modifier is an SBS block copolymer (i.e. poly(styrene-b-butadiene-b-styrene).
- SBS block copolymer i.e. poly(styrene-b-butadiene-b-styrene).
- these block copolymers may be characterized by a weight average molecular weight of from about 90,000 to about 750,000, or in other embodiments from about 150,000 to about 250,000.
- these polymers may be characterized by a polydispersity of up to about 1.1 or in other embodiments up to about 1.05.
- the SBS block copolymers have from about 27 to about 43 parts by weight of styrene.
- SBS block copolymer useful for practice of the present invention is that sold under the tradename Kraton D (Kraton Polymer Group), including, for example, D1118, D1101, and D1184. Included among these polymers are SBS block linear and radial block copolymers. In particular embodiments, two block copolymers, linear and radial, can be mixed to achieve the desired results. In certain embodiments, the weight ratio of linear to radial SBS copolymers may be from about 0 to about 7 parts by weight of radial and from about 7 to about 15 parts by weight of linear SBS block copolymer.
- useful thermoplastic polymers that may used as the polymeric modifier include polyolefins.
- polypropylenes include polypropylenes.
- polypropylenes may have a weight average molecular weight of from about 50,000 to about 250,000, or in other embodiments from about 150,000 to about 170,000.
- the polydispersity may be from about 2.5 to about 3.5.
- the polypropylene may be further characterized by a melt temperature of from about 160° C to about 175 ° C, and may have a cold crystallization temperature above 120° C.
- the polymeric modifier is isotactic polypropylene (IPP).
- IPP isotactic polypropylene
- the IPP has at least 45 percent by weight crystallinity, or in other embodiments from about 46 to about 50 percent by weight crystallinity.
- Blends of atactic polypropylene and isotactic polypropylene may be used.
- atactic polyalpha olefins APAOs
- the expandable graphite may be used in conjunction with a complementary flame retardant.
- Flame retardants may include any compound that increases the burn resistivity, particularly flame spread such as tested by UL 94 and/or UL 790, in the polymeric compositions of the present invention.
- useful flame retardants include those that operate by forming a char-layer across the surface of a specimen when exposed to a flame.
- Other flame retardants include those that operate by releasing water upon thermal decomposition of the flame retardant compound.
- Useful flame retardants may also be categorized as halogenated flame retardants or non-halogenated flame retardants.
- Exemplary non-halogenated flame retardants include magnesium hydroxide, aluminum trihydrate, zinc borate, ammonium polyphosphate, melamine polyphosphate, and antimony oxide (Sb 2 O 3 ).
- Magnesium hydroxide (Mg(OH) 2 ) is commercially available under the tradename VertexTM 60
- ammonium polyphosphate is commercially available under the tradename ExoliteTM AP 760 (Clarian), which is sold together as a polyol masterbatch
- melamine polyphosphate is available under the tradename BuditTM 3141 (Budenheim)
- antimony oxide (Sb 2 O 3 ) is commercially available under the tradename FireshieldTM.
- the complementary flame retardant includes colemanite, which is a borate mineral that is believed to include about 50-80% calcium borate.
- the asphaltic component may include tackifier resins. These resins include, but are not limited to, petroleum resins, synthetic polyterpenes, resin esters and natural terpenes, and combinations thereof.
- the resin modifiers soften or become liquid at temperatures of about 40o C to about 150o C.
- the resin modifiers have number average molecular weights, as measured by vapor phase osmometry, below that of the polymeric material included in the polymeric film. In certain embodiments, the number average molecular weights of the resin modifiers are less than about 5,000. In other embodiments, the number average molecular weights of the resin modifiers are less than about 1,000. In additional embodiments, the number average molecular weights of the resin modifiers are from about 500 to about 1000.
- the resin modifiers have ring and ball softening point of about 20o C to about 160o C. In additional embodiments, resin modifiers have ring and ball softening points of about 40o C to about 160o C. In still other embodiments, resin modifiers have ring and ball softening points of about 50o C to about 160o C.
- Suitable resins include, but are not limited to, natural rosins and rosin esters, hydrogenated rosins and hydrogenated rosin esters, coumarone-indene resins, petroleum resins, polyterpene resins, and terpene-phenolic resins.
- suitable petroleum resins include, but are not limited to, aliphatic hydrocarbon resins, hydrogenated aliphatic hydrocarbon resins, mixed aliphatic and aromatic hydrocarbon resins, hydrogenated mixed aliphatic and aromatic hydrocarbon resins, cycloaliphatic hydrocarbon resins, hydrogenated cycloaliphatic resins, mixed cycloaliphatic and aromatic hydrocarbon resins, hydrogenated mixed cycloaliphatic and aromatic hydrocarbon resins, aromatic hydrocarbon resins, substituted aromatic hydrocarbons, and hydrogenated aromatic hydrocarbon resins.
- hydrogenated includes fully, substantially and at least partially hydrogenated resins.
- Suitable aromatic resins include aromatic modified aliphatic resins, aromatic modified cycloaliphatic resin, and hydrogenated aromatic hydrocarbon resins.
- any of the above resins may be grafted with an unsaturated ester or anhydride to provide enhanced properties to the resin.
- resin modifiers e.g., Hydrocarbon Resins, Kirk-Othmer, Encyclopedia of Chemical Technology, 4th Ed. v.13, pp. 717-743 (J. Wiley & Sons, 1995 ).
- the tackifier resins include phenol-based resins. Included among the phenol-based resins are phenolic resins. These resins may include reactive phenol resins (also referred to as functionalized phenol resins), as well as unreactive resins.
- the phenolic resin is a resole resin, which can be made by the condensation of alkyl, substituted phenols, or unsubstituted phenols with aldehydes such as formaldehyde in an alkaline medium or by condensation of bi-functional phenoldialcohols. In one or more embodiments, this condensation reaction occurs in the excess or molar equivalent of formaldehyde. In other embodiments, the phenolic resin may be formed by an acid-catalyzed reaction.
- the tackifier resin is a polybutene polymer or oligomer.
- polybutene oils are employed.
- Useful polybutene oils include high-viscosity oils that may be characterized by a viscosity at 100 °C of at least 80 cst, in other embodiments at least 100 cst, or in other embodiments at least 120 cst up to, for example, about 700 or 800 cst.
- the high viscosity polybutene oils may be characterized by a molecular weight of at least 1000 g/mole, in other embodiments at least 1200 g/mole, or in other embodiments at least 1300 g/mole up to, for example, 1400 or 1500 g/mole.
- An exemplary high-viscosity polybutene oil is available under the tradename Indapol H300 (Ineos) or PB32 (Soltex).
- the asphaltic component may include oil, which may also be referred to as processing oil or extender oil.
- these extenders may include high-boiling hydrocarbons.
- these oils include paraffinic oils, aromatic oils, naphthenic oils, vegetable oils, and low PCA oils including MES, TDAE, and SRAE, and heavy naphthenic oils, and various synthetic oils such as, but not limited, polybutene oils.
- the oil employed is selected based upon its compatibility with the rubber, as well as its ability to provide advantageous properties to the final composition (e.g., green strength or tack).
- the asphaltic component may also include fillers, extenders, antioxidants, waxes, antiozonants, and the like.
- Useful fillers include, but are not limited to, inorganic fillers such as calcium carbonate (i.e. limestone) and glass, such as glass beads.
- the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3 and in other embodiments at least 5 parts by weight expandable graphite per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight expandable graphite per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight expandable graphite per 100 parts by weight asphalt binder.
- the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3, and in other embodiments at least 5 parts by weight polymeric modifier per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight polymeric modifier per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight polymeric modifier per 100 parts by weight asphalt binder.
- the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3, and in other embodiments at least 5 parts by weight complementary flame retardant per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight complementary flame retardant per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight complementary flame retardant per 100 parts by weight asphalt binder.
- the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3, and in other embodiments at least 5 parts by weight tackifier resin per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight tackifier resin per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight tackifier resin per 100 parts by weight asphalt binder.
- the asphaltic component of the asphaltic sheet of the present invention includes at least 0, in other embodiments at least 5, in other embodiments at least 10, and in other embodiments at least 20 parts by weight filler other than flame retardant material per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 350, in other embodiments at most 100, in other embodiments at least 70, in other embodiments at least 50, and in other embodiments at most 40 parts by weight filler other than flame retardant material per 100 parts by weight asphalt binder.
- the asphaltic component of the asphaltic sheet of the present invention includes from 0 to 350, in other embodiments from 1 to 100, and in other embodiments from 5 to 45 parts by weight filler other than flame retardant material per 100 parts by weight asphalt binder.
- the expandable graphite, complementary flame retardant, and filler may be referred to as dispersed solids within a matrix of the asphalt material (which for purposes of this definition includes the asphalt material plus other non-solid constituents such as the polymer modifier and/or tackifier).
- the asphaltic binder therefore, is the continuous phase in which the solids are dispersed.
- the asphaltic binder is a major volume fraction of the particle/matrix system. In these or other embodiments, the asphaltic binder is a major weight fraction of the particle/matrix system.
- the weight ratio of asphaltic binder to solids dispersed in the binder is at least 0.4:1, in other embodiments at least 0.6:1, in other embodiments at least 0.7:1 in other embodiments at least 0.8:1, in other embodiments at least 1:1, in other embodiments at least 1.2:1, in other embodiments at least 1.5:1, in other embodiments at least 2:1, in other embodiments at least 2.5:1, and in other embodiments at least 3:1.
- the asphaltic sheet of the present invention may generally be prepared by using conventional techniques for forming asphaltic sheet.
- the technique may include, in certain embodiments, saturating a reinforcing textile with a molten asphalt composition.
- the step of saturating the sheet may include submerging the reinforcing sheet into a bath of molten asphalt.
- the step of saturating the sheet may include spraying, roll coating, or otherwise applying a molten asphalt composition to a reinforcing sheet. Where a reinforcing sheet is not employed, a molten asphalt material can be applied to release paper or film and then processed into a sheet that is devoid of reinforcing scrim.
- the molten asphalt composition is prepared by introducing the expandable graphite to a molten asphalt composition.
- the temperature of the molten asphalt composition at the time of introduction of the expandable graphite is less than 270 °C, in other embodiments is less than 230 °C, in other embodiments less than 220 °C, in other embodiments less than 210 °C, in other embodiments less than 200 °C, in other embodiments less than 190 °C, in other embodiments less than 185 °C, in other embodiments less than 175 °C, in other embodiments less than 165 °C, in other embodiments less than 155 °C, and in other embodiments less than 145 °C.
- the temperature of the molten asphalt composition at the time of introduction of the expandable graphite is at least 125 °C, in other embodiments at least 140 °C, in other embodiments at least 150 °C, in other embodiments at least 160 °C, and in other embodiments at least 170 °C. In one or more embodiments, these temperatures are maintained during mixing and processing in the presence of the expandable graphite.
- the molten asphalt composition is prepared in a multi-stage process whereby the asphalt binder and optionally one or more additional ingredients (e.g. polymer modifier), other than the expandable graphite, are mixed at elevated temperatures up to, for example, 250 °C, in other embodiments up to 240 °C, in other embodiments up to 230°C, in other embodiments up to 220 °C, and in other embodiments up to 210 °C.
- additional ingredients e.g. polymer modifier
- the asphaltic composition is cooled below 200 °C, in other embodiments below 190 °C, and in other embodiments below than 185 °C, in other embodiments below 175 °C, in other embodiments below 165 °C, in other embodiments below 155 °C, and in other embodiments below 145 °C.
- cooling of the asphalt may take place my adding additional asphalt to the mixture, where the temperature of the added asphalt is cooler then the asphalt heated and mixed in the first step.
- asphalts are typically flowable at temperatures as low as about 130 °C to about 150 °C. Accordingly, asphalt having a temperature of about130 °C to about 150 °C can be added to the asphalt mixture prepared in the first mixing step to quickly and homogeneously cool the asphalt.
- the expandable graphite is introduced to the molten asphalt composition and mixed at temperatures of less than 200 °C, in other embodiments less than 190 °C, in other embodiments less than 185 °C, in other embodiments less than 175 °C, in other embodiments less than 165 °C, in other embodiments less than 155 °C, and in other embodiments less than 145 °C.
- a reinforcing sheet which may be in the form of a planar sheet and may be provided in the form of a roll, is provided.
- reinforcing sheet may be a scrim, or fiberglass mesh sheet, as is known in the art.
- Useful scrims include those that are commercially available.
- fiberglass scrims are available under the trade name STYLETM 930120 (Milliken & Co.; Spartanburg, South Carolina) and also available from J. P. Stevens (Greenville, South Carolina).
- reinforcing sheet may be an organic felt or a combination polyester and glass mat. Useful polyester mats are available from Freudenberg & Co. of Germany.
- the asphalt coater may be a reservoir of hot liquid asphalt.
- the asphalt coater may include spraying apparatus to coat the reinforcing sheet with liquid asphalt.
- reinforcing sheet may be coated with hot liquid asphalt by any alternative methods known to persons having ordinary skill in the art.
- the reinforcing sheet is drawn through an asphalt coater, which applies hot liquid (i.e. molten asphalt) to the reinforcing sheet to create a sheet that is saturated with asphalt.
- hot liquid i.e. molten asphalt
- the asphalt composition includes may include polymeric modifiers, fillers, and other ingredients conventionally employed with asphalt compositions.
- the asphalt composition may include the expandable graphite according to the practice of this invention.
- the expandable graphite is added to the molten asphalt composition prior to the introduction of the reinforcing sheet to the molten asphalt.
- Also included within the molten asphalt may be complementary flame retardant and other ingredients mentioned above.
- the molten asphalt composition is devoid or substantially devoid of expandable graphite.
- the expandable graphite is incorporated into the asphaltic sheet downstream of the bath or reservoir of molten asphalt.
- the expandable graphite can be dropped onto the sheet after leaving the coater.
- the expandable graphite is included in the molten asphalt composition prior to the introduction of the reinforcing sheet to the molten asphalt and the expandable graphite is incorporated into the asphaltic sheet downstream of the bath or reservoir of molten asphalt (e.g. by dropping).
- expandable graphite particles are dropped on to a sheet that has been coated with molten asphalt, wherein the asphalt may or may not include expandable graphite. These particles are dropped at a rate and amount to create at least a partial layer of expandable graphite particles adjacent to the asphalt of the coated asphalt sheet.
- the act of dropping the expandable graphite particles on to a coated sheet may at least partially embed some of the graphite particles in to the asphalt such that the asphalt serves as a binder to hold the graphite particles in place.
- one or more of the plurality of expandable graphite particles are adhered to the surface of the coated asphalt sheet by way of the adhesive properties of the asphalt material.
- the step of dropping the expandable graphite creates a concentration gradient of the expandable graphite and the asphalt.
- the process of dropping expandable graphite particles on to an asphaltic sheet takes place after the asphaltic sheet is prepared from a molten asphalt composition and prior to a substantial cooling of the asphalt material so as to take advantage of the adhesive properties of the asphalt.
- at least a portion of the expandable graphite particles are dropped on or otherwise applied to the coated asphalt sheet within 15 seconds, in other embodiments within 10 seconds, and in other embodiments within 5 seconds of the asphaltic sheet being prepared (e.g., removal of the asphaltic sheet from a molten bath in which the asphaltic sheet is prepared).
- the expandable graphite is dropped on the asphaltic sheet prior to solidification of the asphalt material (e.g. prior to the asphaltic sheet cooling to a temperature below about 85 ° C.
- the expandable graphite particles are applied to the surface of an asphaltic sheet using a multi-stage process.
- a multi-stage process may include multiple drops of graphite particles.
- the various stages or drops can be configured to achieve certain characteristics.
- different sized expandable graphite particles can be dropped at different stages in order to achieve desirable coverage of the surface of the asphaltic sheet.
- additional asphaltic material applied to the sheet after application of the expandable graphite e.g. after dropping the expandable graphite onto the sheet, which can form the layer of expandable graphite or concentrated region of expandable graphite. This may take place by using curtain coating or roll coating techniques.
- the expandable graphite is dropped onto the hot asphaltic sheet prior to the sheet being calendered or sized within a nip roll. As a result, then the sheet is calendered or sized within a nip roll, the excess asphaltic material at the nip roll will serve to form a layer (or skin) of asphaltic material over the layer of expandable graphite.
- a polymeric layer is applied to the asphaltic sheet after application of the expandable graphite particles.
- a polymeric film may be applied over the expandable graphite particles. In one or more embodiments, this may facilitate subsequent calendaring of the asphaltic sheet carrying the expandable graphite particles.
- the layer of expandable graphite particles may be modified by the application of a release agent, such as sand, silica, or talc, over the expandable graphite particles. The presence of release agents may, like the polymeric film, facilitate subsequent calendaring of the asphaltic sheet.
- the asphaltic sheet may be drawn through a cooling station to cool the hot asphalt and create a more stable substrate for the application of granules.
- the cooling station may include a water reservoir through which the asphaltic sheet is drawn.
- the asphaltic sheet may float across a water reservoir to cool the sheet while allowing the top surface to retain a higher temperature than the bottom surface.
- the cooling station may include other cooling mechanisms known to those skilled in the art.
- the asphaltic sheet of the present invention may be used as an underlayment.
- the sheet may be employed as an underlayment within a metal roofing system.
- the metal roofing system may include a roof deck, an optional insulation layer, the underlayment of the present invention, and metal panels, which may also be referred to as metal cladding.
- the asphaltic sheet of the present invention may be employed as an underlayment within a tile roofing system.
- the tile roofing system may include a roof deck, an optional insulation layer, the underlayment of the present invention, and roofing tiles.
- the roof deck may include a flat roof, a low-slope roof, or a high-slope roof.
- the deck may be fabricated from wood, metal, concrete, or any material useful for fabricating a roof deck.
- the insulation layer may include insulation boards such as polyisocyanurate or polystyrene insulation boards. These insulation boards may be secured to the roof deck using known techniques, such as adhesive bonding or mechanical fastening.
- the underlayment may be applied directly to the roof deck and the insulation boards can be applied over the underlayment. In other embodiments, the underlayment may be applied over the optional insulation layer. Where an insulation layer is not present, the underlayment may be applied directly to the deck.
- the metal panels or tile are then secured to the roof on top of the underlayment.
- the metal panels or tiles may be secured over the insulation layer using known techniques.
- Fig. 4 shows a building structure 40 having a roof system 41 including a roof deck 42, an asphaltic sheet 46 according to one or more embodiments of the present invention, and a weather-resistant covering 48, which may include metal cladding or tile.
- An optional insulation layer may be positioned above or below asphaltic sheet 46.
- asphaltic sheet 46 may operate as an underlayment in roofing system 41.
- the asphaltic sheet of the present invention may be used as a barrier sheet, which may also be referred to as a material.
- barrier materials may include air barriers, which are employed to prevent or reduce the flow of oxygen and nitrogen into and/or out of a building structure.
- these barrier materials may include vapor barriers, which are employed to prevent or reduce the flow of water vapor into and/or out of a building structure.
- these barrier materials include moisture barriers, which are employed to prevent or reduce the flow of moisture (i.e. liquid water) into and/or out of a building structure.
- Fig. 5 shows a building structure 50 having a flat roof system 51 including roof deck 52, asphaltic sheets 58 according to embodiments of the present invention, insulation layer 54, and weather-resistant layer 56.
- Asphaltic sheet 58 which may operate as a vapor barrier, may be above or below insulation layer 54.
- the barrier sheet may be applied to a vertical surface (such as a wall) of a building structure.
- the barrier may be applied to the exterior surface of a building structure prior to application of the external sheathing, such as metal wall panels, brick, composite siding, wood siding, or vinyl siding.
- FIG. 6 shows a building structure 60 having a wall system 62 including structural supports 63, insulation layer 64, asphaltic sheet 66 according to embodiments of the present invention, and exterior cladding 68.
- Asphaltic sheet 66 which may operate as a vapor barrier in the wall system, may be positioned on the interior or exterior of insulation layer 64.
- the asphaltic sheet may be used as a roofing membrane.
- the asphaltic sheet may be used as a base sheet or cap sheet in an asphaltic roofing membrane system.
- these asphaltic membranes are modified asphaltic membranes of the type known in the art. Examples of these membranes, albeit without the expandable graphite, are shown in U.S. Pat. No., 6,492,439 , 6,486,236 , 4,835,199 , 7,442,270 , 7,146,771 , 7,070,843 , 4,992,315 , and 6,924,015 , which are incorporated herein by reference.
- the present invention provides a metal roofing system or tile roofing system wherein the asphaltic sheet of one or more embodiments of the present invention is employed as an underlayment, and the roofing system is characterized as a Class A roofing system pursuant to UL and/or ASTM classifications; this is in fact true where a single layer of the asphaltic sheet employed within the roofing system as the sole fire barrier.
- the asphaltic sheets of the present invention can be used in a roofing system that can meet the performance standards of the Burning Brand test of UL 790.
- the roofing system can include a single sheet of the asphaltic sheets of the present invention as the sole fire barrier and can meet the performance standards of the Burning Brand test of UL 790.
- the asphaltic sheet may be used in a wall system to meet the performance standards of NFPA-285.
- the asphaltic sheet of the present invention is advantageously moisture resistant and can meet the requirements of ASTM D1970-09.
- the asphaltic sheets are characterized by a water vapor transmission, as defined by ASTM E96, of less than 1.0, in other embodiments less than 0.5, in other embodiments less than 0.1, and in other embodiments less than 0.08 g/hr-m 2 .
- the asphaltic sheets of the present invention are advantageously self-adhering (e.g., can be self-adhered to a substrate), which properties derive, at least in part, from the asphaltic nature of the matrix in which the other constituents are dispersed.
- this advantage is also believed to derive, at least in part, from the amount of asphalt material present in the sheet relative to the other constituents of the sheet (e.g., the solid such as filler and flame retardants).
- the asphaltic sheet of certain embodiments is advantageously self-sealing; in other words, the sheet can form a water-tight seal around nail holes and the like. Again, the advantage is believed to derive from the level of asphaltic material present in the sheet of certain embodiments.
- the membranes were formed by first preparing an asphaltic mixture and then fabricating a membrane from the mixture.
- the fabrication technique included embedding a glass scrim into the membrane.
- the asphaltic composition was prepared by heating the asphalt to about 300 °F and then adding the SBS copolymers to the molten asphalt under medium to high shear through the use of a conventional mixing apparatus. Mixing continued for close to 20 minutes until the mixture appeared to be smooth and uniform, which was indicative of the fact that the polymers dissolved. The temperature of the mixture during the process peaked at around 340-380 °F.
- the other fillers excluding expandable graphite
- tackifiers were added under similar shear while generally maintaining the same temperature.
- the membrane was fabricated.
- the membrane was generally fabricated by pouring the mixture on to a release paper on which the glass scrim was positioned. Spacers were placed at the edges of the release paper to control the membrane thickness, and a release paper was then applied over the top of the mixture and pressed to form a flat membrane. Once the membrane was cooled for sufficient handling, the test sample was prepared by stapling the membrane to a 4" x 5" plywood or OSB board. A thermocouple was sandwiched between the membrane sample and the wood. The test samples were then suspended at a 5/12 slope.
- the asphalt was obtained under the tradename AC-5 (Marathon); SBS I was obtained under the tradename D1184 (Kraton); SBS II was obtained under the tradename D1118 (Kraton); the tackifier resin was obtained under the tradename H300 (Ineos); and the expandable graphite was obtained under the tradename 1721 (Asberry).
- Sample 4 was prepared without expandable graphite. When subjected to the burn test, this sample began to burn rapidly and the asphaltic material began to flow rapidly. As a result, the test had to be terminated within the first minute for safety reasons. In other words, the sample without expandable graphite unequivocally failed. In contradistinction, Samples 1-3 show that the presence of as little as 3 weight percent graphite withstood the burn test by forming a thick char, which resulted in little flow of the asphaltic material and ultimately the fire was self-extinguished within less than 2 minutes of removal of the flame.
- each formulation included 81 percent by weight asphalt, 7 percent by weight SBS copolymer I, 7 percent by weight limestone, and 5 percent by weight of the flame retardant identified in Table III. Table III also provides the results of the burn test performed on each sample. Table III Sample No. Ingredients Char (Yes/No) Flow (Yes/No) Dripping (Yes/No) Max Temp (°F) Time (s) Total Time (min) Self Ext.
- ammonium polyphosphate I was obtained under the tradename AP 760 (Clariant); the ammonium polyphosphate II was obtained under the tradename CROS 486 (Budenheim); the ammonium polyphosphate III was obtained under the tradename CROS 484 (Budenheim); the ammonium polyphosphate IV was obtained under the tradename CROS C30 (Budenheim); and the zinc borate was obtained under the tradename FireBrake ZB.
- the membrane prepared using expandable graphite was the only sample that could withstand the burn test. Indeed, the test was extended for the full four minute length. All other samples had to be extinguished within one minute for safety reasons.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Civil Engineering (AREA)
- Structural Engineering (AREA)
- Architecture (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Inorganic Chemistry (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Laminated Bodies (AREA)
- Road Paving Structures (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Synthetic Leather, Interior Materials Or Flexible Sheet Materials (AREA)
Abstract
An asphaltic sheet comprising an asphaltic component including an asphalt binder and expandable graphite.
Description
- This application claims the benefit of
U.S. Non-Provisional Application Serial No. 13/804,202, filed March 14, 2013 U.S. Provisional Application Serial Nos. 61/694,435, filed on August 29, 2012 61/684,180, filed on August 17, 2012 61/670,864, filed on July 12, 2012 - Embodiments of the present invention are directed toward asphaltic sheet materials that include expandable graphite. These sheet materials are useful as roofing underlayment, as roofing membranes, and as barrier materials such as air, vapor, and/or moisture barriers.
- Asphaltic sheet materials are widely used in the construction industry. For example, polymer-modified asphaltic sheet material is used as membrane for waterproofing flat or low-sloped roofs. As is known in the art, these roofing systems may include multiple layers of asphaltic sheet including base sheets and cap sheets. Other examples include barriers materials such air, vapor, or moisture barriers. These materials are typically used on roofs or vertical surfaces such as walls to provide the desired air, vapor and/or moisture resistance to a structure. Still other examples include underlayments, which are used in the roofing industry to provide an extra layer of protection to the roof. This additional protection may provide, among other benefits, water, moisture, thermal, and/or fire resistance. As the name implies, underlayment is typically positioned below the external or primary roofing protection, which may include shingles, membranes such as polymeric or asphaltic membranes, roofing tiles, and metal panels or cladding.
- With regard to underlayments, felt paper that is saturated with asphalt has historically been used as underlayment to provide additional water and/or moisture resistance to the roof. Other forms of underlayment include synthetic materials such as thermoplastic or thermoset materials formed into sheets. Composites, such as laminates of asphalt and synthetic polymer, have also been employed as underlayment.
- In order to meet certain fire resistance properties, which may be required by code or classification, fire or flame resistant underlayment may be employed. These underlayment may include textiles, including woven and non-woven fabrics, made of fire resistant materials such as fiberglass. These fabrics may include a coating, such as a mineral coating, that further enhances the flame or fire resistance of the underlayment.
- Where there is a desire to achieve both moisture resistance and flame or fire resistance through the use of underlayment, such as with metal roofing systems, multiple underlayments are used. For example, a first underlayment may be used to provide moisture or water resistance, and a second underlayment may be used to provide flame or fire resistance. This technique, however, suffers from several drawbacks including the added difficulty of installing multiple underlayments.
-
-
Fig. 1 is a perspective view of a cross section of an asphaltic sheet according to one or more embodiments of the present invention. -
Fig. 2 is a cross-sectional view of an asphaltic sheet according to one or more embodiments of the present invention. -
Fig. 3 is a cross-sectional view of an asphaltic sheet according to one or more embodiments of the present invention. -
Fig. 4 is a perspective view of a building structure having a metal or tile roofing system including an asphaltic sheet according to embodiments of the present invention. -
Fig. 5 is a perspective view of a building structure having a flat roofing system including an asphaltic sheet according to embodiments of the present invention. -
Fig. 6 is a perspective view of a building structure having a wall system including an asphaltic sheet according to embodiments of the present invention. - One or more embodiments of the present invention provide an asphaltic sheet comprising an asphaltic component including an asphalt binder and expandable graphite.
- Still other embodiments of the present invention provide a roof system comprising a roof deck; an underlayment; and one or more metal panels or roofing tilescovering the underlayment, where the underlayment includes an asphaltic component including an asphalt binder and expandable graphite dispersed within the asphalt binder.
- Still other embodiments of the present invention provide a method for producing an asphaltic sheet, the method comprising preparing a molten asphaltic composition by introducing an expandable graphite to an asphalt binder at a temperature below that which will cause deleterious expansion of the expandable graphite, and fabricating an asphaltic sheet with the molten asphaltic composition.
- Still other embodiments of the present invention provide an asphaltic sheet comprising an asphaltic component including an asphalt binder and a layer including expandable graphite, where said layer including expandable graphite is adjacent to said asphaltic component.
- Still other embodiments of the present invention provide a method for producing an asphaltic sheet, the method comprising providing an asphaltic sheet, and applying expandable graphite particles to the asphaltic sheet.
- Embodiments of the present invention are based, at least in part, on the discovery of an asphaltic sheet having an asphaltic component with expandable graphite. In certain embodiments, the asphaltic sheet includes an asphalt component with the expandable graphite dispersed within the asphalt component. In these or other embodiments, the asphaltic sheet includes a layer of expandable graphite adjacent to the asphalt component. In one or more embodiments, the asphaltic sheet is advantageously resistant to water, moisture, an/or air due to the asphaltic nature of the sheet, and it is also flame resistant due to the presence of the expandable graphite. It is believed that one or more advantages of the present invention derive from the presence of the expandable graphite within or adjacent to the asphaltic component of the sheet. While the prior art contemplates the use of expandable graphite as a flame retardant, the prior art does not contemplate incorporating the expandable graphite into the asphaltic component of a sheet material or placing a layer of the expandable graphite adjacent to the asphaltic component. Embodiments of the present invention provide a method for incorporating the expandable graphite into the asphaltic component of the sheet without deleteriously expanding the graphite (i.e. the graphite is not expanded to a deleterious degree). And, in certain embodiments, the asphaltic component of the sheet material further includes a complementary flame retardant material that is believed to synergistically interact with the expandable graphite to provide unexpected flame resistance.
- In one or more embodiments, the asphaltic sheet is or includes a planar body of asphalt material, which may also be referred to as the asphalt component of the sheet or
asphalt layer 12. For example, as shown inFig. 1 , asphaltic sheet 11 includesasphalt component 12 having a firstplanar surface 13 andsecond planer surface 14. Sheet 11 may include anoptional textile fabric 15 embedded or impregnated withinasphaltic component 12. In certain embodiments, the sheet is devoid of a scrim or fabric.Asphaltic component 12, as will be described in greater detail below, may include various constituents such as polymeric modifiers and fillers, as well asexpandable graphite 16 and optional complementary flame retardants (not shown) according to the present invention. In one or more embodiments, sheet 11 may further include one or morepolymeric layers 17 laminated toasphalt component 12 of sheet 11. For example, asphaltic sheet 11 may include anasphaltic component 12 laminated to a polypropylene sheet. In other embodiments,layer 17 may include a layer of release agents, such as silica, sand or talc. Additionally, arelease film 19 may be removably secured to at least one of the exposedplanar surfaces - In one or more embodiments,
optional textile fabric 15, which may also be referred to asfabric reinforcement 15, reinforcingmember 15, or simplyreinforcement 15, may include woven and/or non-woven fabrics. Various fabric reinforcements are known in the art, and practice of the present invention is not necessarily limited by the selection of a particular fabric. In one or more embodiments,reinforcement 15 may be fabricated from fiberglass and/or synthetic yards or filaments. Exemplary synthetic yarns include those prepared from polyesters or polyimides. - In one or more embodiments, the thickness of asphaltic sheet 11 may be at least 10, in other embodiments at least 20, and in other embodiments at least 30 mils. In these or other embodiments, the thickness of asphaltic sheet 11 may be at most 120, in other embodiments at most 100, in other embodiments at most 90, and in other embodiments at most 80 mils. In one or more embodiments, the thickness of asphaltic sheet 11 may be from about 10 to about 100, in other embodiments from about 20 to about 90, and in other embodiments from about 30 to about 80 mils. In other embodiments, especially where the asphaltic sheet is used in a vertical application, the thickness of the asphaltic sheet may be substantially thinner. For example, the thickness of the sheet may be less than 20, in other embodiments less than 15, and in other embodiments less than 10 mils, with the thickness ranging from 2 to 20 mils, in other embodiments from 3 to 15 mils, and in other embodiments from 5 to 10 mils.
- In one or more embodiments, the weight of the asphaltic sheet may be at least 5, in other embodiments at least 10 and in other embodiments at least 15 pounds per hundred square feet. In these or other embodiments, the weight of the asphaltic sheet may be at most 90, in other embodiments at most 70, and in other embodiments at most 50 pounds per hundred square feet. In these or other embodiments, the weight of the asphaltic sheet may be from 5 to 100, in other embodiments from 10 to 80, and in other embodiments from 15 to 50 pounds per hundred square feet. In other embodiments, especially where the asphaltic sheet is used in a vertical application, the weight of the asphaltic sheet may be substantially lighter. For example, the weight of the sheet may be less than 60, in other embodiments less than 50, and in other embodiments less than 40 pounds per hundred square feet, with the weight ranging from 5 to 60, in other embodiments from 10 to 50, and in other embodiments from 15 to 40 pounds per hundred square feet.
- In other embodiments, as shown, for example, in
Fig. 2 , asphaltic sheet 11 includesasphaltic component 12 havingfirst planer surface 13 and secondplanar surface 14. Adjacent toasphaltic component 12 is a layer 18 includingexpandable graphite 16. Layer 18 may be adjacent toasphaltic component 12 as a result of the manner in which asphaltic sheet 11 is fabricated, as will be described in greater detail below. Asphaltic sheet 11 may carryoptional polymeric layer 17, which may also be referred to aspolymeric liner 17. In other embodiments,layer 17 may include a layer of release agents, such as silica, sand or talc. In yet other embodiments,layer 17 may be a glass scrim, polyester mat, metal foil (e.g., aluminum foil), fabric, elastomeric layer, and the like. - In one or more embodiments, layer 18 includes one or more layers of particles of
expandable graphite 16. These particles may be held in place by a matrix of asphalt material present within at least a portion of layer 18. In these or other embodiments, theexpandable graphite 16 is held in place by being adhered to the surface of the asphalt. In one or more embodiments,asphaltic component 12 may also include expandable graphite dispersed therein. In other words, asphaltic sheet may include expandable graphite dispersed throughout the asphaltic component and layer 18 of expandable graphite adjacent tocomponent 12. - In one or more embodiments, layer 18 may include a planar region within sheet 11 that includes a higher concentration of expandable graphite relative to any other region of sheet 11. Thus, layer 18 may include a continuous layer of expandable graphite having a variable or relatively constant thickness across sheet 11. Or, in other embodiments, the expandable graphite may be discontinuous throughout the region so long as the concentration of expandable graphite within the region is higher than in other areas or regions of sheet 11. In one or more embodiments, the discontinuity of the expandable graphite within the layer 18 may result from the asphaltic material which may form a matrix in which the expandable graphite is at least partially dispersed within this region or layer. It should also be appreciated that the concentration of the expandable graphite may not be constant within this layer. Indeed, as will be appreciated from the description of how to fabricate the sheets of this embodiment, a concentration gradient may exist whereby the concentration of the expandable graphite moves from a region of maximum concentration to a region of decreased concentration. As shown in expanded view in
Fig. 2 , the concentration ofexpandable graphite 16 furthest fromplanar surface 13 within layer 18 is the highest, which corresponds to a minimum in asphalt concentration. On the other hand, the concentration ofexpandable graphite 16 proximate toplanar surface 13 is a minimum relative to the concentration of expandable graphite within layer 18. - In yet other embodiments, which may be described with reference to
Fig. 3 , an additional layer of asphalt 12' is adjacent expandable graphite layer 18 opposite asphaltic component orlayer 12. Layer 12' may also be referred to as a skin layer 12', and aspects of this embodiment may be described as layer or region 18 being embedded between layers of asphaltic binder or material. Consistent with the other embodiments described above, this layer 12' may includeexpandable graphite 16 at a concentration lower than layer 18. Nonetheless, in one or more embodiments, layer 12' may include expandable graphite dispersed therein, although the concentration is lower than the concentration of the expandable graphite within region 18. In fact, in one or more embodiments, a concentration gradient may exist between layers 12' and 18 in a similar fashion to the concentration gradient described above. - While a continuous layer or region (e.g. region 18) is believed to be advantageous, it is also contemplated that the sheet can include multiple discreet regions of the expandable graphite, such as may exist in a pattern where the expandable graphite is applied on the top of the asphaltic sheet in rows or strips in the machine direction of the sheet. This may be advantageous where greater adhesion to a top sheet (e.g. sheet 17) is desired.
- In one or more embodiments, the thickness of layer 18 may be at least 10 µm, in other embodiments at least 20 µm, in other embodiments at least 30 µm, in other embodiments at least 75 µm, and in other embodiments at least 100 µm. In these or other embodiments, the thickness of layer 18 may be at most 3 mm, in other embodiments at most 2 mm, and in other embodiments at most 1 mm. In one or more embodiments, the thickness of layer 18 may be from about 10 µm to about 3 mm, in other embodiments from about 75 µm to about 2 mm, and in other embodiments from about 100 µm to about 1 mm.
- In one or more embodiments, the thickness of layer 12' may be at least 2 µm, in other embodiments at least 5 µm, and in other embodiments at least 20 µm. In these or other embodiments, the thickness of layer 12' may be at most 1 mm, in other embodiments at most 0.5 mm, in other embodiments at most 0.25 mm, in other embodiments at most 0.1 mm, and in other embodiments at most 0.050 mm. In one or more embodiments, the thickness of layer 12' may be from about 1 µm to about 3 mm, in other embodiments from about 2 µm to about 0.5 mm, and in other embodiments from about 5 µm to about 0.050 mm.
- As noted above, the asphaltic sheet of one or more embodiments of the present invention includes an asphaltic component. The asphaltic component includes an asphalt binder and expandable graphite dispersed within the binder. The asphaltic component may also include, dispersed within the binder, polymeric modifiers, fillers, tackifiers, complementary flame retardants, and other constituents conventionally used in asphaltic-based building materials.
- The term "asphalt binder" is used as understood by those skilled in the art and is consistent with the meaning provided by AASHTO M320. As used within this specification, the terms "asphalt" and "asphalt binder" may be used synonymously. The asphalt binder material may be derived from any asphalt source, such as natural asphalt, rock asphalt, produced from tar sands, or petroleum asphalt obtained in the process of refining petroleum. In other embodiments, asphalt binders may include a blend of various asphalts not meeting any specific grade definition. This includes airblown asphalt, vacuum-distilled asphalt, steam-distilled asphalt, cutback asphalt or roofing asphalt. Alternatively, gilsonite, natural or synthetic, used alone or mixed with petroleum asphalt, may be selected. Synthetic asphalt mixtures suitable for use in the present invention are described, for example, in
U.S. Pat. No. 4,437,896 . In one or more embodiments, asphalt includes petroleum derived asphalt and asphaltic residual. These compositions may include asphaltenes, resins, cyclics, and saturates. The percentage of these constituents in the overall asphalt binder composition may vary based on the source of the asphalt. - Asphaltenes include black amorphous solids containing, in addition to carbon and hydrogen, some nitrogen, sulfur, and oxygen. Trace elements such as nickel and vanadium may also be present. Asphaltenes are generally considered as highly polar aromatic materials of a number average molecular weight of about 2000 to about 5000 g/mol, and may constitute about 5 to about 25% of the weight of asphalt.
- Resins (polar aromatics) include dark-colored, solid and semi-solid, very adhesive fractions of relatively high molecular weight present in the maltenes. They may include the dispersing agents of peptizers for the asphaltenes, and the proportion of resins to asphaltenes governs, to a degree, the sol-or gel-type character of asphalts. Resins separated from bitumens may have a number average molecular weight of about 0.8 to about 2 kg/mol but there is a wide molecular distribution. This component may constitute about 15 to about 25% of the weight of asphalts.
- Cyclics (naphthene aromatics) include the compounds of lowest molecular weight in bitumens and represent the major portion of the dispersion medium for the peptized asphaltenes. They may constitute about 45 to about 60% by weight of the total asphalt binder, and may be dark viscous liquids. They may include compounds with aromatic and naphthenic aromatic nuclei with side chain constituents and may have molecular weights of 0.5 to about 9 kg/mol.
- Saturates include predominantly the straight-and branched-chain aliphatic hydrocarbons present in bitumens, together with alkyl naphthenes and some alkyl aromatics. The average molecular weight range may be approximately similar to that of the cyclics, and the components may include the waxy and non-waxy saturates. This fraction may from about 5 to about 20% of the weight of asphalts.
- In these or other embodiments, asphalt binders may include bitumens that occur in nature or may be obtained in petroleum processing. Asphalts may contain very high molecular weight hydrocarbons called asphaltenes, which may be soluble in carbon disulfide, pyridine, aromatic hydrocarbons, chlorinated hydrocarbons, and THF. Asphalts or bituminous materials may be solids, semi-solids or liquids.
- In one or more embodiments, the asphalt binder includes AC-5, AC-10 and AC-15. These asphalts typically contain about 40 to about 52 parts by weight of aromatic hydrocarbons, about 20 to about 44 parts by weight of a polar organic compound, about 10 to about 15 parts by weight of asphaltene, about 6 to about 8 parts by weight of saturates, and about 4 to about 5 parts by weight of sulfur. Nevertheless, practice of the present invention is not limited by selection of any particular asphalt.
- In one or more embodiments, the molecular weight of the aromatic hydrocarbons present in asphalt may range between about 300 and 2000, while the polar organic compounds, which generally include hydroxylated, carboxylated and heterocyclic compounds, may have a weight average molecular weight of about 500 to 50,000. Asphaltenes, which are generally known as heavy hydrocarbons, are typically of a high molecular weight and are heptane insoluble. Saturates generally include paraffinic and cycloaliphatic hydrocarbons having about 300 to 2000 molecular weight.
- In one or more embodiments, bitumens may be used. Bitumens are naturally occurring solidified hydrocarbons, typically collected as a residue of petroleum distillation. Gilsonite is believed to be the purest naturally formed bitumen, typically having a molecular weight of about 3,000 with about 3 parts by weight complexed nitrogen.
- Expandable graphite may also be referred to as expandable flake graphite, intumescent flake graphite, or expandable flake; and, for the purposes herein, these terms may be used interchangeably.
- In one or more embodiments, expandable graphite includes intercalated graphite in which an intercallant material is included between the graphite layers of graphite crystal or particle. Examples of intercallant materials include halogens, alkali metals, sulfates, nitrates, various organic acids, aluminum chlorides, ferric chlorides, other metal halides, arsenic sulfides, and thallium sulfides. In certain embodiments of the present invention, the expandable graphite includes non-halogenated intercallant materials. In certain embodiments, the expandable graphite includes sulfate intercallants, also referred to as graphite bisulfate. As is known in the art, bisulfate intercalation is achieved by treating highly crystalline natural flake graphite with a mixture of sulfuric acid and other oxidizing agents which act to catalyze the sulfate intercalation.
- Commercially available examples of expandable graphite include HPMS Expandable Graphite (HP Materials Solutions, Inc., Woodland Hills, CA) and Expandable Graphite Grades 1721 (Asbury Carbons, Asbury, NJ). Other commercial grades contemplated as useful in the present invention include 1722, 3393, 3577, 3626, and 1722HT (Asbury Carbons, Asbury, NJ).
- In one or more embodiments, the expandable graphite may be characterized as having a mean or average size in the range from about 30 µm to about 1.5 mm, in other embodiments from about 50 µm to about 1.0 mm, and in other embodiments from about 180 to about 850 µm. In certain embodiments, the expandable graphite may be characterized as having a mean or average size of at least 30 µm, in other embodiments at least 44 µm, in other embodiments at least 180 µm, and in other embodiments at least 300 µm. In one or more embodiments, expandable graphite may be characterized as having a mean or average size of at most 1.5 mm, in other embodiments at most 1.0 mm, in other embodiments at most 850 µm, in other embodiments at most 600 µm, in yet other embodiments at most 500 µm, and in still other embodiments at most 400 µm. Useful expandable graphite includes Graphite Grade #1721 (Asbury Carbons), which has a nominal size of greater than 300 µm.
- In one or more embodiments, the expandable graphite may be characterized as having a median size in the range from about 30 µm to about 1.5 mm, in other embodiments from about 50 µm to about 1.0 mm, and in other embodiments from about 180 to about 850 µm. In certain embodiments, the expandable graphite may be characterized as having a median size of at least 30 µm, in other embodiments at least 44 µm, in other embodiments at least 180 µm, and in other embodiments at least 300 µm. In one or more embodiments, expandable graphite may be characterized as having a median size of at most 1.5 mm, in other embodiments at most 1.0 mm, in other embodiments at most 850 µm, in other embodiments at most 600 µm, in yet other embodiments at most 500 µm, and in still other embodiments at most 400 µm.
- In one or more embodiments of the present invention, the expandable graphite may be characterized as having a nominal particle size of 20x50 (US sieve). US sieve 20 has an opening equivalent to 0.841 mm and
US sieve 50 has an opening equivalent to 0.297 mm. Therefore, a nominal particle size of 20x50 indicates the graphite particles are at least 0.297 mm and at most 0.841 mm. - In one or more embodiments, the expandable graphite may be characterized as having a carbon content in the range from about 80% to about 99%. In certain embodiments, the expandable graphite may be characterized as having a carbon content of at least 80%, in other embodiments at least 85%, in other embodiments at least 90%, in yet other embodiments at least 95%, in other embodiments at least 98%, and in still other embodiments at least 99% carbon.
- In one or more embodiments, the expandable graphite may be characterized as having a sulfur content in the range from about 0% to about 8%, in other embodiments from about 2.6% to about 5.0%, and in other embodiments from about 3.0% to about 3.5%. In certain embodiments, the expandable graphite may be characterized as having a sulfur content of at least 0%, in other embodiments at least 2.6%, in other embodiments at least 2.9%, in other embodiments at least 3.2%, and in other embodiments 3.5%. In certain embodiments, the expandable graphite may be characterized as having a sulfur content of at most 8%, in other embodiments at most 5%, in other embodiments at most 3.5%.
- In one or more embodiments, the expandable graphite may be characterized as having an expansion ratio (cc/g) in the range from about 10:1 to about 500:1, in other embodiments at least 20:1 to about 450:1, in other embodiments at least 30:1 to about 400:1, in other embodiments from about 50:1 to about 350:1. In certain embodiments, the expandable graphite may be characterized as having an expansion ratio (cc/g) of at least 10:1, in other embodiments at least 20:1, in other embodiments at least 30:1, in other embodiments at least 40:1, in other embodiments at least 50:1, in other embodiments at least 60:1, in other embodiments at least 90:1, in other embodiments at least 160:1, in other embodiments at least 210:1, in other embodiments at least 220:1, in other embodiments at least 230:1, in other embodiments at least 270:1, in other embodiments at least 290:1, and in yet other embodiments at least 300:1. In certain embodiments, the expandable graphite may be characterized as having an expansion ratio (cc/g) of at most 350:1, and in yet other embodiments at most 300:1.
- In one or more embodiments, the expandable graphite, as it exists with the asphaltic component of the asphaltic sheet of the present invention, is partially expanded. In one or more embodiments, the expandable graphite is not expanded, however, to a deleterious degree, which includes that amount or more of expansion that will deleteriously the ability to form the sheet product and the ability of the graphite to serve as flame retardant at desirable levels, which include those levels that allow proper formation of the sheet. In one or more embodiments, the expandable graphite is expanded to at most 60%, in other embodiments at most 50%, in other embodiments at most 40%, in other embodiments at most 30%, in other embodiments at most 20%, and in other embodiments at most 10% beyond its original unexpanded size.
- In one or more embodiments, the expandable graphite may be characterized as having a pH in the range from about 1 to about 10; in other embodiments from about 1 to about 6; and in yet other embodiments from about 5 to about 10. In certain embodiments, the expandable graphite may be characterized as having a pH in the range from about 4 to about 7. In one or more embodiments, the expandable graphite may be characterized as having a pH of at least 1, in other embodiments at least 4, and in other embodiments at least 5. In certain embodiments, the expandable graphite may be characterized as having a pH of at most 10, in other embodiments at most 7, and in other embodiments at most 6.
- In one or more embodiments, the expandable graphite may be characterized by an onset temperature ranging from about 100 ºC to about 250 ºC; in other embodiments from about 160 ºC to about 225 ºC; and in other embodiments from about 180 ºC to about 200 ºC. In one or more embodiments, the expandable graphite may be characterized by an onset temperature of at least 100 ºC, in other embodiments at least 130 ºC, in other embodiments at least 160 ºC, and in other embodiments at least 180 ºC. In one or more embodiments, the expandable graphite may be characterized by an onset temperature of at most 250 ºC, in other embodiments at most 225 ºC, and in other embodiments at most 200 ºC. Onset temperature may also be interchangeably referred to as expansion temperature and also alternatively referred to as the temperature at which expansion of the graphite starts.
- In one or more embodiments, the polymeric modifier, which may simply be referred to as polymer, includes thermoplastic polymers, thermosetting elastomers, thermoplastic elastomers, and/or mixtures thereof. Each of these polymers have been used, either alone or in combination with each other to modify asphalt binders, and practice of the present invention is not necessarily limited by the selection of any particular polymeric modifier.
- In one or more embodiments, the polymers may be characterized by a glass transition temperature (Tg), as measured by DSC analysis, of less than 150°C, in other embodiment less than 125°C, in other embodiment less than 100°C, in other embodimetns less than 20°C, in other embodiments less than 0°C, in other embodiments less than -20°C, in other embodiments less than -35°C, and in other embodiments from about -90°C to about -20°C. In these or other embodiments, the polymers may be characterized by a glass transition temperature (Tg), as measured by DSC analysis, of more than -20°C, in other embodiments more than 0°C, in other embodiments more than 20°C, in other embodiments more than 50°C, and in other embodiments more than 100°C.
- In one or more embodiments, the polymeric modifier may be characterized by a melt index (ASTM D-1238;2.16 kg load @ 190°C) of less than 1,000 dg/min, in other embodiments less than 500 dg/min, in other embodiments less than 50 dg/min, in other embodiments less than 20 dg/min, in other embodiments less than 10 dg/min, and in other embodiments less than 1 dg/min. In these or other embodiments, the unsaturated polymers may have a melt index of between 3 and 15 dg/min, and other embodiments between 4 and 12 dg/min.
- In one or more embodiments, the polymeric modifier may be characterized by a number average molecular weight (Mn) of from about 10 to about 1,000 kg/mol, in other embodiments from about 40 to about 500 kg/mol, and in other embodiments from about 80 to about 200 kg/mol. In these or other embodiments, the polymeric modifier may also be characterized by a weight average molecular weight (Mw) of from about 10 to about 4,000 kg/mol, in other embodiments from about 40 to about 2,000 kg/mol, and in other embodiments from about 80 to about 800 kg/mol. In one or more embodiments, the polymeric modifier may be characterized by a molecular weight distribution of from about 1.1 to about 5, in other embodiments from about 1.5 to about 4.5, and in other embodiments from about 1.8 to about 4.0. Molecular weight can be determined by gel permeation chromatography (GPC) calibrated with polystyrene standards and adjusted for the Mark-Houwink constants for the polymer in question.
- The polymeric modifier may be linear, branched, or coupled polymers. Types of polymers may include both natural and synthetic polymers. Useful synthetic polymers may include polydienes or polydiene copolymers with non-diene comonomer (e.g., styrene). The copolymers may include block and random copolymers. The coupled polymers may include linearly coupled polymers (e.g. di-coupled polymers) or raidally coupled polymers (e.g. tri-coupled or, tetra-coupled penta-coupled, hexa-coupled etc.). Exemplary polydienes include polybutadiene and polyisoprene. Exemplary copolymers may include random styrene-butadiene rubber, styrene-butadiene block copolymer, styrene-butadiene-styrene block copolymer, random styrene-isoprene, styrene-isoprene block copolymer, styrene-isoprene-butadiene block copolymer, random styrene-isoprene-butadiene, styrene-isoprene-styrene block copolymer, and chloroprene rubber. In one or more embodiments, the polymeric modifier include linear or radial block copolymers wherein the block copolymers include terminal styrene blocks. In these or other embodiments, the styrene content of these block copolymers may be from 10% to 50% by weight, in other embodiments from 15% to 45% by weight, and in other embodiments from 20% to 40% by weight.
- In one or more embodiments, the polymeric modifier is an SBS block copolymer (i.e. poly(styrene-b-butadiene-b-styrene). In one or more embodiments, these block copolymers may be characterized by a weight average molecular weight of from about 90,000 to about 750,000, or in other embodiments from about 150,000 to about 250,000. In these or other embodiments, these polymers may be characterized by a polydispersity of up to about 1.1 or in other embodiments up to about 1.05. In particular embodiments, the SBS block copolymers have from about 27 to about 43 parts by weight of styrene.
- An example of an SBS block copolymer useful for practice of the present invention is that sold under the tradename Kraton D (Kraton Polymer Group), including, for example, D1118, D1101, and D1184. Included among these polymers are SBS block linear and radial block copolymers. In particular embodiments, two block copolymers, linear and radial, can be mixed to achieve the desired results. In certain embodiments, the weight ratio of linear to radial SBS copolymers may be from about 0 to about 7 parts by weight of radial and from about 7 to about 15 parts by weight of linear SBS block copolymer.
- In one or more embodiments, useful thermoplastic polymers that may used as the polymeric modifier include polyolefins. For example, several derivatives of polypropylene are useful including those prepared by first dimerizing propylene to give 4-methyl-1-pentene and subsequently polymerizing this dimer to give poly-4-methyl-1-pentene. These polypropylenes may have a weight average molecular weight of from about 50,000 to about 250,000, or in other embodiments from about 150,000 to about 170,000. In one or more embodiments, the polydispersity may be from about 2.5 to about 3.5. The polypropylene may be further characterized by a melt temperature of from about 160° C to about 175 ° C, and may have a cold crystallization temperature above 120° C.
- In one or more embodiments, the polymeric modifier is isotactic polypropylene (IPP). In one or more embodiments, the IPP has at least 45 percent by weight crystallinity, or in other embodiments from about 46 to about 50 percent by weight crystallinity. Blends of atactic polypropylene and isotactic polypropylene may be used. In yet other embodiments, atactic polyalpha olefins (APAOs) may be used.
- As mentioned above, the expandable graphite may be used in conjunction with a complementary flame retardant. Flame retardants may include any compound that increases the burn resistivity, particularly flame spread such as tested by UL 94 and/or UL 790, in the polymeric compositions of the present invention. Generally, useful flame retardants include those that operate by forming a char-layer across the surface of a specimen when exposed to a flame. Other flame retardants include those that operate by releasing water upon thermal decomposition of the flame retardant compound. Useful flame retardants may also be categorized as halogenated flame retardants or non-halogenated flame retardants.
- Exemplary non-halogenated flame retardants include magnesium hydroxide, aluminum trihydrate, zinc borate, ammonium polyphosphate, melamine polyphosphate, and antimony oxide (Sb2O3). Magnesium hydroxide (Mg(OH)2) is commercially available under the
tradename Vertex™ 60, ammonium polyphosphate is commercially available under the tradename Exolite™ AP 760 (Clarian), which is sold together as a polyol masterbatch, melamine polyphosphate is available under the tradename Budit™ 3141 (Budenheim), and antimony oxide (Sb2O3) is commercially available under the tradename Fireshield™. - Examples of other complementary calcium borate, magnesium hydroxide, basic magnesium carbonate, aluminum trihydrate, zinc borate, gypsum, and mixtures thereof. In these or other embodiments, the complementary flame retardant includes colemanite, which is a borate mineral that is believed to include about 50-80% calcium borate.
- In one or more embodiments, the asphaltic component may include tackifier resins. These resins include, but are not limited to, petroleum resins, synthetic polyterpenes, resin esters and natural terpenes, and combinations thereof. In certain embodiments, the resin modifiers soften or become liquid at temperatures of about 40º C to about 150º C. In certain embodiments, the resin modifiers have number average molecular weights, as measured by vapor phase osmometry, below that of the polymeric material included in the polymeric film. In certain embodiments, the number average molecular weights of the resin modifiers are less than about 5,000. In other embodiments, the number average molecular weights of the resin modifiers are less than about 1,000. In additional embodiments, the number average molecular weights of the resin modifiers are from about 500 to about 1000.
- In certain embodiments, the resin modifiers have ring and ball softening point of about 20º C to about 160º C. In additional embodiments, resin modifiers have ring and ball softening points of about 40º C to about 160º C. In still other embodiments, resin modifiers have ring and ball softening points of about 50º C to about 160º C.
- Various types of natural and synthetic resins, alone or in admixture with each other, may be used be selected as the resin modifier. Suitable resins include, but are not limited to, natural rosins and rosin esters, hydrogenated rosins and hydrogenated rosin esters, coumarone-indene resins, petroleum resins, polyterpene resins, and terpene-phenolic resins. Specific examples of suitable petroleum resins include, but are not limited to, aliphatic hydrocarbon resins, hydrogenated aliphatic hydrocarbon resins, mixed aliphatic and aromatic hydrocarbon resins, hydrogenated mixed aliphatic and aromatic hydrocarbon resins, cycloaliphatic hydrocarbon resins, hydrogenated cycloaliphatic resins, mixed cycloaliphatic and aromatic hydrocarbon resins, hydrogenated mixed cycloaliphatic and aromatic hydrocarbon resins, aromatic hydrocarbon resins, substituted aromatic hydrocarbons, and hydrogenated aromatic hydrocarbon resins. As used herein, "hydrogenated" includes fully, substantially and at least partially hydrogenated resins. Suitable aromatic resins include aromatic modified aliphatic resins, aromatic modified cycloaliphatic resin, and hydrogenated aromatic hydrocarbon resins. Any of the above resins may be grafted with an unsaturated ester or anhydride to provide enhanced properties to the resin. For additional description of resin modifiers, reference can be made to technical literature, e.g., Hydrocarbon Resins, Kirk-Othmer, Encyclopedia of Chemical Technology, 4th Ed. v.13, pp. 717-743 (J. Wiley & Sons, 1995).
- In one or more embodiments, the tackifier resins include phenol-based resins. Included among the phenol-based resins are phenolic resins. These resins may include reactive phenol resins (also referred to as functionalized phenol resins), as well as unreactive resins. In one or more embodiments, the phenolic resin is a resole resin, which can be made by the condensation of alkyl, substituted phenols, or unsubstituted phenols with aldehydes such as formaldehyde in an alkaline medium or by condensation of bi-functional phenoldialcohols. In one or more embodiments, this condensation reaction occurs in the excess or molar equivalent of formaldehyde. In other embodiments, the phenolic resin may be formed by an acid-catalyzed reaction.
- In one or more embodiments, the tackifier resin is a polybutene polymer or oligomer. In particular embodiments, polybutene oils are employed. Useful polybutene oils include high-viscosity oils that may be characterized by a viscosity at 100 °C of at least 80 cst, in other embodiments at least 100 cst, or in other embodiments at least 120 cst up to, for example, about 700 or 800 cst. In these or other embodiments, the high viscosity polybutene oils may be characterized by a molecular weight of at least 1000 g/mole, in other embodiments at least 1200 g/mole, or in other embodiments at least 1300 g/mole up to, for example, 1400 or 1500 g/mole. An exemplary high-viscosity polybutene oil is available under the tradename Indapol H300 (Ineos) or PB32 (Soltex).
- In one or more embodiments, the asphaltic component may include oil, which may also be referred to as processing oil or extender oil. These extenders may include high-boiling hydrocarbons. Examples of these oils include paraffinic oils, aromatic oils, naphthenic oils, vegetable oils, and low PCA oils including MES, TDAE, and SRAE, and heavy naphthenic oils, and various synthetic oils such as, but not limited, polybutene oils. In one or more embodiments, the oil employed is selected based upon its compatibility with the rubber, as well as its ability to provide advantageous properties to the final composition (e.g., green strength or tack). In these or other embodiments, the asphaltic component may also include fillers, extenders, antioxidants, waxes, antiozonants, and the like. Useful fillers include, but are not limited to, inorganic fillers such as calcium carbonate (i.e. limestone) and glass, such as glass beads.
- In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3 and in other embodiments at least 5 parts by weight expandable graphite per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight expandable graphite per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight expandable graphite per 100 parts by weight asphalt binder.
- In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3, and in other embodiments at least 5 parts by weight polymeric modifier per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight polymeric modifier per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight polymeric modifier per 100 parts by weight asphalt binder.
- In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3, and in other embodiments at least 5 parts by weight complementary flame retardant per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight complementary flame retardant per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight complementary flame retardant per 100 parts by weight asphalt binder.
- In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at least 0.5, in other embodiments at least 1, in other embodiments at least 3, and in other embodiments at least 5 parts by weight tackifier resin per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 40, in other embodiments at most 30, and in other embodiments at most 20 parts by weight tackifier resin per 100 parts by weight asphalt binder. In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from about 0.5 to about 40, in other embodiments from about 1 to about 30, and in other embodiments from about 3 to about 20 parts by weight tackifier resin per 100 parts by weight asphalt binder.
- In one or more embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at least 0, in other embodiments at least 5, in other embodiments at least 10, and in other embodiments at least 20 parts by weight filler other than flame retardant material per 100 parts by weight asphalt binder. In these or other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes at most 350, in other embodiments at most 100, in other embodiments at least 70, in other embodiments at least 50, and in other embodiments at most 40 parts by weight filler other than flame retardant material per 100 parts by weight asphalt binder. In still other embodiments, the asphaltic component of the asphaltic sheet of the present invention includes from 0 to 350, in other embodiments from 1 to 100, and in other embodiments from 5 to 45 parts by weight filler other than flame retardant material per 100 parts by weight asphalt binder.
- In one or more embodiments, the expandable graphite, complementary flame retardant, and filler may be referred to as dispersed solids within a matrix of the asphalt material (which for purposes of this definition includes the asphalt material plus other non-solid constituents such as the polymer modifier and/or tackifier). The asphaltic binder, therefore, is the continuous phase in which the solids are dispersed. In one or more embodiments, the asphaltic binder is a major volume fraction of the particle/matrix system. In these or other embodiments, the asphaltic binder is a major weight fraction of the particle/matrix system. In one or more embodiments, the weight ratio of asphaltic binder to solids dispersed in the binder is at least 0.4:1, in other embodiments at least 0.6:1, in other embodiments at least 0.7:1 in other embodiments at least 0.8:1, in other embodiments at least 1:1, in other embodiments at least 1.2:1, in other embodiments at least 1.5:1, in other embodiments at least 2:1, in other embodiments at least 2.5:1, and in other embodiments at least 3:1.
- In one or more embodiments, the asphaltic sheet of the present invention may generally be prepared by using conventional techniques for forming asphaltic sheet. For example, the technique may include, in certain embodiments, saturating a reinforcing textile with a molten asphalt composition. The step of saturating the sheet may include submerging the reinforcing sheet into a bath of molten asphalt. In other embodiments, the step of saturating the sheet may include spraying, roll coating, or otherwise applying a molten asphalt composition to a reinforcing sheet. Where a reinforcing sheet is not employed, a molten asphalt material can be applied to release paper or film and then processed into a sheet that is devoid of reinforcing scrim.
- In certain embodiments, the molten asphalt composition is prepared by introducing the expandable graphite to a molten asphalt composition. In one or more embodiments, the temperature of the molten asphalt composition at the time of introduction of the expandable graphite is less than 270 °C, in other embodiments is less than 230 °C, in other embodiments less than 220 °C, in other embodiments less than 210 °C, in other embodiments less than 200 °C, in other embodiments less than 190 °C, in other embodiments less than 185 °C, in other embodiments less than 175 °C, in other embodiments less than 165 °C, in other embodiments less than 155 °C, and in other embodiments less than 145 °C. In these or other embodiments, the temperature of the molten asphalt composition at the time of introduction of the expandable graphite is at least 125 °C, in other embodiments at least 140 °C, in other embodiments at least 150 °C, in other embodiments at least 160 °C, and in other embodiments at least 170 °C. In one or more embodiments, these temperatures are maintained during mixing and processing in the presence of the expandable graphite.
- In one or more embodiments, the molten asphalt composition is prepared in a multi-stage process whereby the asphalt binder and optionally one or more additional ingredients (e.g. polymer modifier), other than the expandable graphite, are mixed at elevated temperatures up to, for example, 250 °C, in other embodiments up to 240 °C, in other embodiments up to 230°C, in other embodiments up to 220 °C, and in other embodiments up to 210 °C. Following this initial mix, the asphaltic composition is cooled below 200 °C, in other embodiments below 190 °C, and in other embodiments below than 185 °C, in other embodiments below 175 °C, in other embodiments below 165 °C, in other embodiments below 155 °C, and in other embodiments below 145 °C.
- In one more embodiments, cooling of the asphalt may take place my adding additional asphalt to the mixture, where the temperature of the added asphalt is cooler then the asphalt heated and mixed in the first step. For example, asphalts are typically flowable at temperatures as low as about 130 °C to about 150 °C. Accordingly, asphalt having a temperature of about130 °C to about 150 °C can be added to the asphalt mixture prepared in the first mixing step to quickly and homogeneously cool the asphalt.
- Following the cooling step, the expandable graphite is introduced to the molten asphalt composition and mixed at temperatures of less than 200 °C, in other embodiments less than 190 °C, in other embodiments less than 185 °C, in other embodiments less than 175 °C, in other embodiments less than 165 °C, in other embodiments less than 155 °C, and in other embodiments less than 145 °C.
- In an exemplary process, a reinforcing sheet, which may be in the form of a planar sheet and may be provided in the form of a roll, is provided. In one or more embodiments, reinforcing sheet may be a scrim, or fiberglass mesh sheet, as is known in the art. Useful scrims include those that are commercially available. For example, fiberglass scrims are available under the trade name STYLE™ 930120 (Milliken & Co.; Spartanburg, South Carolina) and also available from J. P. Stevens (Greenville, South Carolina). In other embodiments, reinforcing sheet may be an organic felt or a combination polyester and glass mat. Useful polyester mats are available from Freudenberg & Co. of Germany. In one or more embodiments, the asphalt coater may be a reservoir of hot liquid asphalt. In other embodiments, the asphalt coater may include spraying apparatus to coat the reinforcing sheet with liquid asphalt. In yet other embodiments, reinforcing sheet may be coated with hot liquid asphalt by any alternative methods known to persons having ordinary skill in the art.
- In one or more embodiments, the reinforcing sheet is drawn through an asphalt coater, which applies hot liquid (i.e. molten asphalt) to the reinforcing sheet to create a sheet that is saturated with asphalt. As noted above, the asphalt composition includes may include polymeric modifiers, fillers, and other ingredients conventionally employed with asphalt compositions.
- In one or more embodiments, the asphalt composition may include the expandable graphite according to the practice of this invention. In other words, the expandable graphite is added to the molten asphalt composition prior to the introduction of the reinforcing sheet to the molten asphalt. Also included within the molten asphalt may be complementary flame retardant and other ingredients mentioned above.
- In other embodiments, the molten asphalt composition is devoid or substantially devoid of expandable graphite. In these embodiments, the expandable graphite is incorporated into the asphaltic sheet downstream of the bath or reservoir of molten asphalt. For example, the expandable graphite can be dropped onto the sheet after leaving the coater.
- In yet other embodiments, the expandable graphite is included in the molten asphalt composition prior to the introduction of the reinforcing sheet to the molten asphalt and the expandable graphite is incorporated into the asphaltic sheet downstream of the bath or reservoir of molten asphalt (e.g. by dropping).
- In one or more embodiments, expandable graphite particles are dropped on to a sheet that has been coated with molten asphalt, wherein the asphalt may or may not include expandable graphite. These particles are dropped at a rate and amount to create at least a partial layer of expandable graphite particles adjacent to the asphalt of the coated asphalt sheet. In one or more embodiments, the act of dropping the expandable graphite particles on to a coated sheet may at least partially embed some of the graphite particles in to the asphalt such that the asphalt serves as a binder to hold the graphite particles in place. In these or other embodiments, one or more of the plurality of expandable graphite particles are adhered to the surface of the coated asphalt sheet by way of the adhesive properties of the asphalt material. In one or more embodiments, the step of dropping the expandable graphite creates a concentration gradient of the expandable graphite and the asphalt.
- In one or more embodiments, the process of dropping expandable graphite particles on to an asphaltic sheet takes place after the asphaltic sheet is prepared from a molten asphalt composition and prior to a substantial cooling of the asphalt material so as to take advantage of the adhesive properties of the asphalt. In one or more embodiments, at least a portion of the expandable graphite particles are dropped on or otherwise applied to the coated asphalt sheet within 15 seconds, in other embodiments within 10 seconds, and in other embodiments within 5 seconds of the asphaltic sheet being prepared (e.g., removal of the asphaltic sheet from a molten bath in which the asphaltic sheet is prepared). In one or more embodiments, the expandable graphite is dropped on the asphaltic sheet prior to solidification of the asphalt material (e.g. prior to the asphaltic sheet cooling to a temperature below about 85 ° C.
- In one or more embodiments, the expandable graphite particles are applied to the surface of an asphaltic sheet using a multi-stage process. For example, a multi-stage process may include multiple drops of graphite particles. In certain embodiments, the various stages or drops can be configured to achieve certain characteristics. For example, different sized expandable graphite particles can be dropped at different stages in order to achieve desirable coverage of the surface of the asphaltic sheet.
- In one or more embodiments, additional asphaltic material applied to the sheet after application of the expandable graphite (e.g. after dropping the expandable graphite onto the sheet, which can form the layer of expandable graphite or concentrated region of expandable graphite). This may take place by using curtain coating or roll coating techniques. In other embodiments, the expandable graphite is dropped onto the hot asphaltic sheet prior to the sheet being calendered or sized within a nip roll. As a result, then the sheet is calendered or sized within a nip roll, the excess asphaltic material at the nip roll will serve to form a layer (or skin) of asphaltic material over the layer of expandable graphite.
- In certain embodiments, a polymeric layer is applied to the asphaltic sheet after application of the expandable graphite particles. For example, following one or more drops or applications of the expandable graphite particles to a surface of the asphaltic sheet, a polymeric film may be applied over the expandable graphite particles. In one or more embodiments, this may facilitate subsequent calendaring of the asphaltic sheet carrying the expandable graphite particles. In other embodiments, the layer of expandable graphite particles may be modified by the application of a release agent, such as sand, silica, or talc, over the expandable graphite particles. The presence of release agents may, like the polymeric film, facilitate subsequent calendaring of the asphaltic sheet.
- In one or more embodiments, the asphaltic sheet may be drawn through a cooling station to cool the hot asphalt and create a more stable substrate for the application of granules. In one or more embodiments, the cooling station may include a water reservoir through which the asphaltic sheet is drawn. In certain embodiments, the asphaltic sheet may float across a water reservoir to cool the sheet while allowing the top surface to retain a higher temperature than the bottom surface. In other embodiments, the cooling station may include other cooling mechanisms known to those skilled in the art.
- In one or more embodiments, the asphaltic sheet of the present invention may be used as an underlayment. For example, the sheet may be employed as an underlayment within a metal roofing system. In one or more embodiments, the metal roofing system may include a roof deck, an optional insulation layer, the underlayment of the present invention, and metal panels, which may also be referred to as metal cladding. In other embodiments, the asphaltic sheet of the present invention may be employed as an underlayment within a tile roofing system. In one or more embodiments, the tile roofing system may include a roof deck, an optional insulation layer, the underlayment of the present invention, and roofing tiles.
- Practice of the present invention is not necessarily limited by the type of roof deck. For example, the roof deck may include a flat roof, a low-slope roof, or a high-slope roof. The deck may be fabricated from wood, metal, concrete, or any material useful for fabricating a roof deck.
- As is known in the art, the insulation layer may include insulation boards such as polyisocyanurate or polystyrene insulation boards. These insulation boards may be secured to the roof deck using known techniques, such as adhesive bonding or mechanical fastening.
- In one or more embodiments, the underlayment may be applied directly to the roof deck and the insulation boards can be applied over the underlayment. In other embodiments, the underlayment may be applied over the optional insulation layer. Where an insulation layer is not present, the underlayment may be applied directly to the deck.
- In one or more embodiments, the metal panels or tile are then secured to the roof on top of the underlayment. Where the insulation board is applied over the underlayment, the metal panels or tiles may be secured over the insulation layer using known techniques.
- For example,
Fig. 4 shows abuilding structure 40 having aroof system 41 including aroof deck 42, anasphaltic sheet 46 according to one or more embodiments of the present invention, and a weather-resistant covering 48, which may include metal cladding or tile. An optional insulation layer may be positioned above or belowasphaltic sheet 46. In one or more of the embodiments,asphaltic sheet 46 may operate as an underlayment inroofing system 41. - In other embodiments, the asphaltic sheet of the present invention may be used as a barrier sheet, which may also be referred to as a material. These barrier materials may include air barriers, which are employed to prevent or reduce the flow of oxygen and nitrogen into and/or out of a building structure. In other embodiments, these barrier materials may include vapor barriers, which are employed to prevent or reduce the flow of water vapor into and/or out of a building structure. In yet other embodiments, these barrier materials include moisture barriers, which are employed to prevent or reduce the flow of moisture (i.e. liquid water) into and/or out of a building structure.
- For example,
Fig. 5 shows abuilding structure 50 having aflat roof system 51 includingroof deck 52,asphaltic sheets 58 according to embodiments of the present invention,insulation layer 54, and weather-resistant layer 56.Asphaltic sheet 58, which may operate as a vapor barrier, may be above or belowinsulation layer 54. - In one or more embodiments, the barrier sheet may be applied to a vertical surface (such as a wall) of a building structure. Using conventional techniques, the barrier may be applied to the exterior surface of a building structure prior to application of the external sheathing, such as metal wall panels, brick, composite siding, wood siding, or vinyl siding.
- For example,
Fig. 6 shows abuilding structure 60 having awall system 62 including structural supports 63,insulation layer 64, asphaltic sheet 66 according to embodiments of the present invention, and exterior cladding 68. Asphaltic sheet 66, which may operate as a vapor barrier in the wall system, may be positioned on the interior or exterior ofinsulation layer 64. - In still other embodiments, the asphaltic sheet may be used as a roofing membrane. For example, the asphaltic sheet may be used as a base sheet or cap sheet in an asphaltic roofing membrane system. In one or more embodiments, these asphaltic membranes are modified asphaltic membranes of the type known in the art. Examples of these membranes, albeit without the expandable graphite, are shown in
U.S. Pat. No., 6,492,439 ,6,486,236 ,4,835,199 ,7,442,270 ,7,146,771 ,7,070,843 ,4,992,315 , and6,924,015 , which are incorporated herein by reference. - In one or more embodiments, the present invention provides a metal roofing system or tile roofing system wherein the asphaltic sheet of one or more embodiments of the present invention is employed as an underlayment, and the roofing system is characterized as a Class A roofing system pursuant to UL and/or ASTM classifications; this is in fact true where a single layer of the asphaltic sheet employed within the roofing system as the sole fire barrier. In one or more embodiments of the present invention, the asphaltic sheets of the present invention can be used in a roofing system that can meet the performance standards of the Burning Brand test of UL 790. In fact, the roofing system can include a single sheet of the asphaltic sheets of the present invention as the sole fire barrier and can meet the performance standards of the Burning Brand test of UL 790. In other embodiments, the asphaltic sheet may be used in a wall system to meet the performance standards of NFPA-285.
- In one or more embodiments, the asphaltic sheet of the present invention is advantageously moisture resistant and can meet the requirements of ASTM D1970-09. In these or other embodiments, the asphaltic sheets are characterized by a water vapor transmission, as defined by ASTM E96, of less than 1.0, in other embodiments less than 0.5, in other embodiments less than 0.1, and in other embodiments less than 0.08 g/hr-m2.
- In one or more embodiments, the asphaltic sheets of the present invention are advantageously self-adhering (e.g., can be self-adhered to a substrate), which properties derive, at least in part, from the asphaltic nature of the matrix in which the other constituents are dispersed. In certain embodiments, this advantage is also believed to derive, at least in part, from the amount of asphalt material present in the sheet relative to the other constituents of the sheet (e.g., the solid such as filler and flame retardants). Additionally, the asphaltic sheet of certain embodiments is advantageously self-sealing; in other words, the sheet can form a water-tight seal around nail holes and the like. Again, the advantage is believed to derive from the level of asphaltic material present in the sheet of certain embodiments.
- Further embodiments of the present invention are the following:
- 1. An asphaltic sheet comprising:
an asphaltic component including an asphalt binder and expandable graphite. - 2. The asphaltic sheet of clause 1, where the expandable graphite is dispersed throughout the asphalt binder.
- 3. The asphaltic sheet of clause 1, where the expandable graphite is located within one or more highly concentrated regions of the sheet.
- 4. The asphaltic sheet of clause 1, where the expandable graphite is located within one or more highly concentrated regions of the sheet and where the expandable graphite is dispersed throughout the asphalt binder.
- 5. The asphaltic sheet of clause 3, where the one or more highly concentrated regions of the sheet is embedded between layers of asphalt binder.
- 6. The asphaltic sheet of clause 1, where the asphaltic component further includes a polymeric modifier dispersed within the asphalt binder.
- 7. The asphaltic sheet of clause 1, where the polymeric modifier is selected from the group consisting of polybutadiene, polyisoprene, styrene-butadiene rubber, styrene-butadiene block copolymer, styrene-butadiene-styrene block copolymer, random styrene-isoprene, styrene-isoprene block copolymer, styrene-isoprene-butadiene block copolymer, random styrene-isoprene-butadiene, styrene-isoprene-styrene block copolymer, and chloroprene rubber.
- 8. The asphaltic sheet of clause 1, where the asphaltic component further includes a complementary flame retardant dispersed within the asphalt binder.
- 9. The asphaltic sheet of clause 1, where the complementary flame retardant is colemanite.
- 10. The asphaltic sheet of clause 1, where the asphaltic component includes from about 0.5 to about 40 parts by weight expandable graphite per 100 parts by weight asphaltic binder.
- 11. The asphaltic sheet of clause 1, where the asphaltic sheet includes a reinforcing textile.
- 12. The use of the asphaltic sheet of clause 1 as one or more layers of a roofing membrane.
- 13. The use of the asphaltic sheet of clause 1 as a roofing underlayment.
- 14. The use of the asphaltic sheet of clause 1 as a barrier sheet.
- 15. A roof system comprising:
- a. a roof deck;
- b. an underlayment; and
- c. one or more metal panels covering the underlayment, where the underlayment an asphaltic component including an asphalt binder and expandable graphite dispersed within the asphalt binder.
- 16. A method for producing an asphaltic sheet, the method comprising:
- a. preparing a molten asphaltic composition by introducing an expandable graphite to an asphalt binder at a temperature below that which will cause deleterious expansion of the expandable graphite; and
- b. fabricating an asphaltic sheet with the molten asphaltic composition.
- 17. An asphaltic sheet comprising:
- a. an asphaltic component including an asphalt binder; and
- b. a layer including expandable graphite, where said layer is adjacent to said asphaltic component.
- 18. The asphaltic sheet of
clause 17, where the asphaltic component further includes a polymeric modifier dispersed within the asphalt binder. - 19. The asphaltic sheet of
clause 17, where the polymeric modifier is selected from the group consisting of polybutadiene, polyisoprene, styrene-butadiene rubber, styrene-butadiene block copolymer, styrene-butadiene-styrene block copolymer, random styrene-isoprene, styrene-isoprene block copolymer, styrene-isoprene-butadiene block copolymer, random styrene-isoprene-butadiene, styrene-isoprene-styrene block copolymer, and chloroprene rubber. - 20. The asphaltic sheet of
clause 17, where the asphaltic component further includes a complementary flame retardant dispersed within the asphalt binder. - 21. The asphaltic sheet of
clause 17, where the complementary flame retardant is colemanite. - 22. The asphaltic sheet of
clause 17, where the asphaltic component includes from about 0.5 to about 40 parts by weight expandable graphite per 100 parts by weight asphaltic binder. - 23. The asphaltic sheet of
clause 17, where the asphaltic sheet includes a reinforcing textile. - 24. The use of the asphaltic sheet of
clause 17 as one or more layers of a roofing membrane. - 25. The use of the asphaltic sheet of
clause 17 as a roofing underlayment. - 26. The use of the asphaltic sheet of
clause 17 as a barrier sheet. - 27. A method for producing an asphaltic sheet, the method comprising:
- a. providing an asphaltic sheet; and
- b. applying expandable graphite particles to the asphaltic sheet.
- 28. The method of clause 27, where the asphaltic sheet includes an asphaltic component wherein the asphaltic component is characterized by a degree of tack that allows the expandable graphite particles to adhere to the asphaltic component.
- In order to demonstrate the practice of the present invention, the following examples have been prepared and tested. The examples should not, however, be viewed as limiting the scope of the invention. The claims will serve to define the invention.
- Laboratory-scale asphaltic membranes were prepared by employing the ingredients set forth in Table I, which shows the ingredients in percent by weight. The membranes were then subjected to burn tests, and the results of those tests are likewise provided in Table I.
- The membranes were formed by first preparing an asphaltic mixture and then fabricating a membrane from the mixture. The fabrication technique included embedding a glass scrim into the membrane. Specifically, the asphaltic composition was prepared by heating the asphalt to about 300 °F and then adding the SBS copolymers to the molten asphalt under medium to high shear through the use of a conventional mixing apparatus. Mixing continued for close to 20 minutes until the mixture appeared to be smooth and uniform, which was indicative of the fact that the polymers dissolved. The temperature of the mixture during the process peaked at around 340-380 °F. Following the addition of the SBS copolymers, the other fillers (excluding expandable graphite) and tackifiers were added under similar shear while generally maintaining the same temperature. The mixture was then allowed to cool to 300-350 °F, and then the expandable graphite was added while mixing continued for about 2 minutes. Once it was determined that the graphite was evenly distributed, the membrane was fabricated. The membrane was generally fabricated by pouring the mixture on to a release paper on which the glass scrim was positioned. Spacers were placed at the edges of the release paper to control the membrane thickness, and a release paper was then applied over the top of the mixture and pressed to form a flat membrane. Once the membrane was cooled for sufficient handling, the test sample was prepared by stapling the membrane to a 4" x 5" plywood or OSB board. A thermocouple was sandwiched between the membrane sample and the wood. The test samples were then suspended at a 5/12 slope. A propane torch having a 4" long flame was positioned so that the tip of the flame was close to the sample without touching the sample.
Table I Samples 1 2 3 4 Ingredients Asphalt 83.80 81.80 76.10 83.80 Expandable Graphite 3.00 5.00 7.10 0 SBS Copolymer I 3.40 3.40 4.20 3.4 SBS Copolymer II 3.50 3.50 4.30 3.5 Tackifier 6.30 6.30 8.30 6.3 Burn Test Data Highest Temp. (° F) 577.00 576.00 467.00 -- Time @ highest Temp. (Min.) 4:00 5:00 5:00 -- Flow Slow flow, thick char Very slow flow, thick char No flow, thick char -- Self-extinguishing (Fire out @ Sec) 105 5 45 -- Exposure of Glass Scrim No No No -- - The asphalt was obtained under the tradename AC-5 (Marathon); SBS I was obtained under the tradename D1184 (Kraton); SBS II was obtained under the tradename D1118 (Kraton); the tackifier resin was obtained under the tradename H300 (Ineos); and the expandable graphite was obtained under the tradename 1721 (Asberry).
- As suggested in Table I, Sample 4 was prepared without expandable graphite. When subjected to the burn test, this sample began to burn rapidly and the asphaltic material began to flow rapidly. As a result, the test had to be terminated within the first minute for safety reasons. In other words, the sample without expandable graphite unequivocally failed. In contradistinction, Samples 1-3 show that the presence of as little as 3 weight percent graphite withstood the burn test by forming a thick char, which resulted in little flow of the asphaltic material and ultimately the fire was self-extinguished within less than 2 minutes of removal of the flame.
- Using procedures similar to those described for samples 1-4, 28 additional samples were prepared wherein colmanite was also added to the asphaltic mixture, together with expandable graphite. The specific recipes for each asphaltic membrane is set forth at Table II, together with the results of the burn tests.
Table II Sample Asphalt Expandable Graphite colemanite SBS I SBS II Tackifier Flow 5 65.49 4.00 19.50 3.38 3.25 4.39 Very slow flow, thick char 6 75.19 2.60 11.01 3.15 2.56 5.50 little or no flow, thick char (initial heavy flow) 7 76.85 4.00 5.15 4.00 4.00 6.00 Little or no flow, thick char 8 66.00 4.00 20.00 4.00 4.00 2.00 Very slow flow, thick char 9 67.94 4.00 15.56 2.50 4.00 6.00 Very slow flow, thick char 10 80.76 4.00 5.00 2.50 2.54 5.20 Very slow flow, thick char 11 64.37 3.13 20.00 4.00 2.50 6.00 Little or no flow, thick char 12 69.73 2.42 20.00 2.50 3.35 2.00 Slow flow, thick char 13 78.72 4.00 8.73 3.06 3.50 2.00 Slow flow, thick char 14 83.13 3.38 5.00 4.00 2.50 2.00 Very slow flow, thick char 15 67.94 4.00 15.56 2.50 4.00 6.00 Slow flow, thick char 16 70.87 4.00 18.13 2.50 2.50 2.00 Very slow flow, thick char 17 64.37 3.13 20.00 4.00 2.50 6.00 Little or no flow, thick char 18 73.99 2.21 12.59 4.00 3.22 3.99 Slow flow, thick char 19 81.27 2.82 5.00 2.91 4.00 4.00 Slow flow, char 20 82.28 1.00 9.27 2.50 2.95 2.00 Heavy flow, drip 21 64.82 1.63 20.00 3.56 4.00 6.00 medium flow, char 22 82.00 1.00 5.00 4.00 2.50 5.50 Heavy flow, drip 23 69.07 1.00 20.00 2.50 2.50 4.93 Heavy flow, drip 24 75.65 1.00 13.46 2.50 4.00 3.39 Heavy flow, drip 25 84.36 1.00 5.00 3.73 3.91 2.00 Heavy flow, drip 26 71.90 1.00 15.98 2.63 2.50 6.00 medium flow, dripped some 27 73.09 1.00 18.02 3.39 2.50 2.00 Heavy flow, drip 28 87.00 1.00 5.00 2.50 2.50 2.00 Heavy flow, drip 29 84.36 1.00 5.00 3.73 3.91 2.00 Heavy flow, drip 30 79.00 1.00 7.17 2.83 4.00 6.00 Heavy flow, drip 31 82.00 1.00 5.00 4.00 2.50 5.50 Heavy, drip 32 82.20 1.00 5.00 2.50 3.30 6.00 Heavy flow, drip - As should be evident from the data presented in Table II, the addition of colemanite had negligible impact on the burn resistivity of the membranes. Instead, and quite unexpectedly, burn resistivity, as highlighted by the flow of the material during the burn test, was advantageously impacted by the expandable graphite.
- In an effort to compare the performance of expandable graphite in the practice of the present invention with other intumescent materials, 11 additional samples were prepared and tested. Specifically, each formulation included 81 percent by weight asphalt, 7 percent by weight SBS copolymer I, 7 percent by weight limestone, and 5 percent by weight of the flame retardant identified in Table III. Table III also provides the results of the burn test performed on each sample.
Table III Sample No. Ingredients Char (Yes/No) Flow (Yes/No) Dripping (Yes/No) Max Temp (°F) Time (s) Total Time (min) Self Ext. (Yes/No) Flame Retardant 33 None N Y Y 950 42 1 Y 34 Expandable Graphite Y N N 1150 240 4 Y 35 Ammonium Polyphosphate I N Y Y 1227 44 1 No 36 Ammonium Polyphosphate II N Y Y 1131 60 1 Y 37 Ammonium Polyphosphate III N Y Y 1385 60 1 Y 38 Ammonium Polyphosphate IV N Y Y 1857 60 1 Y 39 Limestone N Y Y 1880 37 1 Y 40 Colemanite N Y Y 1990 38 1 Y 41 Al(OH)3 N Y Y 1858 27 1 Y 42 Mg(OH)2 N Y Y 1365 60 1 No 43 Zinc Borate N Y Y 1512 60 1 No - The ammonium polyphosphate I was obtained under the tradename AP 760 (Clariant); the ammonium polyphosphate II was obtained under the tradename CROS 486 (Budenheim); the ammonium polyphosphate III was obtained under the tradename CROS 484 (Budenheim); the ammonium polyphosphate IV was obtained under the tradename CROS C30 (Budenheim); and the zinc borate was obtained under the tradename FireBrake ZB.
- As should be appreciated from the data within Table III, the membrane prepared using expandable graphite was the only sample that could withstand the burn test. Indeed, the test was extended for the full four minute length. All other samples had to be extinguished within one minute for safety reasons.
- Various modifications and alterations that do not depart from the scope and spirit of this invention will become apparent to those skilled in the art. This invention is not to be duly limited to the illustrative embodiments set forth herein.
Claims (15)
- An asphaltic sheet comprising:(i) an asphaltic component including an asphalt binder, said asphaltic component including first and second planar surfaces;(ii) a release film removably secured to said first planar surface;(iii) a polymeric sheet adhesively secured to said second planar surface; and(iv) a concentrated region of expandable graphite at or near at least one of said first and second planar surfaces, where said concentrated region is in the form of a concentration gradient, where the concentration of expandable graphite within the concentration gradient changes from a plane of maximum concentration to a plane of minimum concentration.
- The asphaltic sheet of claim 1, where said concentrated region of expandable graphite is at or near said second planar surface and is sandwiched between said asphaltic component and said polymeric sheet.
- The asphaltic sheet of any of the preceding claims, where said asphaltic component includes from about 0.5 to about 40 parts by weight of a flame retardant, other than expandable graphite, per 100 parts by weight asphalt binder.
- The asphaltic sheet of any of the preceding claims, where said asphaltic component includes from about 3 to about 20 parts by weight of a flame retardant, other than expandable graphite, per 100 parts by weight asphalt binder.
- The asphaltic sheet of any of the preceding claims, where the asphaltic component further includes a polymeric modifier dispersed within the asphalt binder.
- The asphaltic sheet of any of the preceding claims, where the polymeric modifier is selected from the group consisting of polybutadiene, polyisoprene, styrene-butadiene rubber, styrene-butadiene block copolymer, styrene-butadiene-styrene block copolymer, random styrene-isoprene, styrene-isoprene block copolymer, styrene-isoprene-butadiene block copolymer, random styrene-isoprene-butadiene, styrene-isoprene-styrene block copolymer, and chloroprene rubber.
- The asphaltic sheet of any of the preceding claims, where the polymeric sheet is a polypropylene sheet.
- The asphaltic sheet of any of the preceding claims, where said concentrated region of expandable graphite is formed by dropping expandable graphite onto an asphaltic sheet.
- The asphaltic sheet of any of the preceding claims where said flame retardant, other than expandable graphite, is colemanite.
- The asphaltic sheet of any of the preceding claims, where said polymeric sheet is a polyolefin sheet.
- A method forming an asphaltic composite, the method comprising:(i) providing an asphaltic sheet, where the asphaltic sheet includes an asphaltic binder, and where said asphaltic sheet includes first and second planar surfaces;(ii) dropping expandable graphite on to the first planar surface of the asphaltic sheet to form a concentration gradient of the expandable graphite, wherein the concentration of the expandable graphite within the concentration gradient changes from a plane of maximum concentration to a plane of minimum concentration;(iii) laminating a polymeric sheet to the first planar surface of the asphaltic sheet; and(iv) removably securing a release film to the second planar surface of the asphaltic sheet.
- The method of claim 11, where said step of providing an asphaltic sheet includes submerging a fabric into molten asphalt binder.
- The method of claim 11, where said step of laminating a polymeric sheet includes contacting a polymeric sheet to the first planar surface and allowing the adhesive properties of the asphaltic sheet to mate the asphaltic sheet and the polymeric sheet.
- The method of claim 13, where said step of laminating sandwiches at least a portion of the expandable graphite between the polymeric sheet and the asphaltic binder.
- The method of claim 11, where the asphaltic sheet further includes colemanite dispersed within said asphaltic binder.
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261670864P | 2012-07-12 | 2012-07-12 | |
| US201261684180P | 2012-08-17 | 2012-08-17 | |
| US201261694435P | 2012-08-29 | 2012-08-29 | |
| US13/804,202 US9441140B2 (en) | 2012-07-12 | 2013-03-14 | Asphaltic sheet materials including expandable graphite |
| PCT/US2013/050251 WO2014011977A1 (en) | 2012-07-12 | 2013-07-12 | Asphaltic sheet materials including expandable graphite |
| EP13742557.5A EP2872553B1 (en) | 2012-07-12 | 2013-07-12 | Asphaltic sheet materials including expandable graphite |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13742557.5A Division EP2872553B1 (en) | 2012-07-12 | 2013-07-12 | Asphaltic sheet materials including expandable graphite |
| EP13742557.5A Division-Into EP2872553B1 (en) | 2012-07-12 | 2013-07-12 | Asphaltic sheet materials including expandable graphite |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP3581614A1 true EP3581614A1 (en) | 2019-12-18 |
Family
ID=49912721
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13742557.5A Active EP2872553B1 (en) | 2012-07-12 | 2013-07-12 | Asphaltic sheet materials including expandable graphite |
| EP19182058.8A Withdrawn EP3581614A1 (en) | 2012-07-12 | 2013-07-12 | Asphaltic sheet materials including expandable graphite |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13742557.5A Active EP2872553B1 (en) | 2012-07-12 | 2013-07-12 | Asphaltic sheet materials including expandable graphite |
Country Status (11)
| Country | Link |
|---|---|
| US (5) | US9441140B2 (en) |
| EP (2) | EP2872553B1 (en) |
| JP (2) | JP6209606B2 (en) |
| CN (1) | CN104583289A (en) |
| AU (2) | AU2013290057B2 (en) |
| BR (1) | BR112015000706A2 (en) |
| CA (1) | CA2879016C (en) |
| IN (1) | IN2014DN10909A (en) |
| MX (1) | MX2014015388A (en) |
| RU (1) | RU2656504C2 (en) |
| WO (1) | WO2014011977A1 (en) |
Families Citing this family (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2012362118B2 (en) | 2011-12-29 | 2016-11-17 | Firestone Building Products Co., LLC | Roofing membranes with expandable graphite as flame retardant |
| US9441140B2 (en) | 2012-07-12 | 2016-09-13 | Firestone Building Products Co., LLC | Asphaltic sheet materials including expandable graphite |
| US10508193B2 (en) * | 2012-11-07 | 2019-12-17 | Firestone Building Products Co., LLC | Pressure-sensitive adhesives including expandable graphite |
| US8968853B2 (en) | 2012-11-07 | 2015-03-03 | Firestone Building Products Company, Llc | Pressure-sensitive adhesives including expandable graphite |
| US9045904B2 (en) * | 2012-11-16 | 2015-06-02 | Firestone Building Products Co., LLC | Thermoplastic membranes containing expandable graphite |
| US20140205789A1 (en) | 2013-01-23 | 2014-07-24 | Firestone Building Products Co., LLC | Coated fabrics including expandable graphite |
| US9523203B2 (en) | 2013-01-23 | 2016-12-20 | Firestone Building Products Co., LLC | Fire-resistant roof system and membrane composite |
| US10017943B1 (en) | 2013-02-14 | 2018-07-10 | Firestone Building Products Co., LLC | Liquid coatings including expandable graphite |
| US9296007B2 (en) * | 2013-07-12 | 2016-03-29 | The Boeing Company | Methods and apparatuses for forming large-area carbon coatings |
| CA2911276A1 (en) * | 2013-07-23 | 2015-01-29 | Firestone Building Products Co., LLC | Method for manufacturing asphaltic sheet materials including expandable graphite |
| FR3016903B1 (en) * | 2014-01-28 | 2019-04-05 | Onduline | METHOD OF MAKING IMPROVED BITUMEN IMPREGNATED FIBER COVERING ELEMENT WITH IMPROVED FIRE BEHAVIOR, DEVICE AND COMPOSITION THEREFOR. |
| US10415249B2 (en) | 2014-07-03 | 2019-09-17 | Firestone Building Products Co., LLC | EPDM roofing membranes with expandable graphite as flame retardant |
| US10240076B2 (en) * | 2014-07-23 | 2019-03-26 | Tecnofilm S.P.A. | Self-adhesive bituminous sheath for building and bitumen modifier for self-adhesive bituminous sheath |
| US10308839B2 (en) * | 2014-11-12 | 2019-06-04 | Dongre Laboratory Services, Inc. | Asphalt additive compositions and methods of making and using thereof |
| US10011992B2 (en) * | 2015-02-07 | 2018-07-03 | Colorado Roofing Products, LLC | Polymeric foam product |
| WO2016130636A1 (en) * | 2015-02-10 | 2016-08-18 | Firestone Building Products Co., LLC | Peel and stick roofing membranes with cured pressure-sensitive adhesives and expandable graphite |
| KR102507982B1 (en) * | 2015-11-11 | 2023-03-09 | 한화 아즈델 인코포레이티드 | Acoustic prepregs, cores and composite articles and methods of use thereof |
| US11192328B2 (en) * | 2016-05-11 | 2021-12-07 | Firestone Building Products Company, Llc | Fire-resistant thermoplastic membrane composite and method of manufacturing the same |
| US20190219455A1 (en) * | 2016-09-01 | 2019-07-18 | Bridgestone Americas Tire Operations, Llc | Tire temperature indicator |
| US11111979B2 (en) * | 2016-09-17 | 2021-09-07 | Firestone Industrial Products Company, Llc | Elastomeric articles with improved fire protection properties |
| US10604929B2 (en) | 2016-11-01 | 2020-03-31 | United States Gypsum Company | Fire resistant gypsum board comprising expandable graphite and related methods and slurries |
| US20190063067A1 (en) * | 2017-08-28 | 2019-02-28 | TopFiberRoof, LLC | Flexible Elastomer And Fiberglass Layered Building Element |
| WO2019055964A1 (en) | 2017-09-18 | 2019-03-21 | Bridgestone Americas Tire Operations, Llc | Rubber articles with improved fire properties |
| US10443190B2 (en) * | 2017-11-09 | 2019-10-15 | Milliken & Company | Fire resistant composite roofing membrane |
| US11078669B2 (en) | 2018-03-05 | 2021-08-03 | Tamko Building Products Llc | Applying a release material to a shingle during manufacturing |
| US10982441B2 (en) | 2018-03-09 | 2021-04-20 | Tamko Building Products, Llc | Multiple layer substrate for roofing materials |
| US10982446B2 (en) | 2018-04-06 | 2021-04-20 | Tamko Building Products, Llc | Heavy glass mat impact resistant roofing |
| CN111117499A (en) * | 2018-10-31 | 2020-05-08 | 山东清池防水材料股份有限公司 | Elastomer modified asphalt waterproof coiled material and preparation method thereof |
| US12110688B2 (en) * | 2019-07-03 | 2024-10-08 | Holcim Technology Ltd | Flame-resistant composites for roofing underlayment |
| RU2745089C1 (en) * | 2020-03-10 | 2021-03-19 | Александр Александрович Ненашев | Sealant for passage of engineering utilities in roof |
| MX2023005046A (en) * | 2020-10-30 | 2023-06-29 | Bmic Llc | Self-adhered roofing systems and methods. |
| US11865821B2 (en) | 2021-03-24 | 2024-01-09 | Ft Synthetics Inc. | Fire-resistant multi-layer membrane |
| US11987985B2 (en) | 2021-04-20 | 2024-05-21 | Milliken & Company | Metal roofing system |
| EP4367339A2 (en) * | 2021-07-09 | 2024-05-15 | Bmic Llc | Coatings for roofing materials and related methods |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3574644A (en) * | 1965-03-22 | 1971-04-13 | Dow Chemical Co | Method of rendering normally flamable materials flame resistant |
| US4437896A (en) | 1982-09-30 | 1984-03-20 | Partanen John F | Synthetic asphalt mixtures and processes for making them |
| US4835199A (en) | 1987-04-10 | 1989-05-30 | The Firestone Tire & Rubber Company | Bituminous composition comprising a blend of bitumen and a thermoplastic elastomer |
| US4992315A (en) | 1989-11-13 | 1991-02-12 | Gaf Buildinhg Materials Corp. | Roofing membrane and method |
| US6486236B2 (en) | 2000-12-28 | 2002-11-26 | Firestone Polymers, Llc | Asphalt composition comprising bismaleimides |
| US6492439B2 (en) | 2000-12-28 | 2002-12-10 | Firestone Polymers, Llc | Asphalt compositions |
| US20050145139A1 (en) * | 2003-12-31 | 2005-07-07 | Amir Khan | Intumescent reflective coating |
| US6924015B2 (en) | 2002-05-21 | 2005-08-02 | Polyglass, U.S.A. | Modified bitumen roofing membrane with enhanced sealability |
| US7070843B2 (en) | 2003-09-10 | 2006-07-04 | Johns Manville | Highly reflective asphalt-based roofing membrane |
| US20060273290A1 (en) * | 2005-06-01 | 2006-12-07 | Building Materials Investment Corporation | Fire retardant composition |
| US7146771B2 (en) | 2003-03-04 | 2006-12-12 | Johns Manville | Cap sheet, roofing installation, and method |
| US7442270B2 (en) | 2003-09-10 | 2008-10-28 | Johns Manville | Highly reflective asphalt-based roofing membrane |
| KR20110087720A (en) * | 2010-01-27 | 2011-08-03 | 한국석유공업 주식회사 | Nonflammable asphalt composition and waterproofing sheet using the same |
| EP2617894A1 (en) * | 2012-01-20 | 2013-07-24 | Onduline | Fire-resistant bituminous cellulose cover plate and manufacturing method |
Family Cites Families (74)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3808275A1 (en) * | 1988-03-12 | 1989-09-21 | Bayer Ag | FIRE PROTECTION ELEMENTS |
| US5397643A (en) * | 1990-04-03 | 1995-03-14 | Bayer Aktiengesellschaft | Lightweight shaped articles containing expandable graphite, their production and their use |
| RU2002914C1 (en) * | 1991-10-08 | 1993-11-15 | Стеканов Дмитрий Ильич; Рудой Александр Феодосьевич; Марковский Александр Иванович. Полевой Владимир Михайлович | Method for production of three-layer bituminous roofing material |
| JPH0752316A (en) * | 1993-08-17 | 1995-02-28 | Nippon Steel Chem Co Ltd | Inorganic composite molded material for building material and its manufacture |
| US5516817A (en) | 1995-04-27 | 1996-05-14 | Bridgestone/Firestone, Inc. | Flame retardant modified asphalt-based material and products therefrom |
| US5849338A (en) | 1996-04-10 | 1998-12-15 | Chronorx Llc | Unit dosage forms for treatment of vasoconstriction and related conditions |
| RU2131964C1 (en) * | 1997-04-16 | 1999-06-20 | Левичев Александр Николаевич | Roof hydraulic seal of low fire hazard |
| DE29709804U1 (en) * | 1997-05-30 | 1997-08-14 | Hoechst Trevira GmbH & Co KG, 65929 Frankfurt | Flame retardant shingle |
| DE19825497C1 (en) * | 1998-06-08 | 1999-06-24 | Hoechst Trevira Gmbh & Co Kg | Fire=resistant supporting interlayer, e.g. for bitumen roofing strip |
| US5968669A (en) | 1998-06-23 | 1999-10-19 | J. M. Huber Corporation | Fire retardant intumescent coating for lignocellulosic materials |
| US6207085B1 (en) | 1999-03-31 | 2001-03-27 | The Rectorseal Corporation | Heat expandable compositions |
| JP4566328B2 (en) * | 2000-04-28 | 2010-10-20 | ロンシール工業株式会社 | Waterproof sheet and construction method |
| US6544596B2 (en) | 2000-11-29 | 2003-04-08 | Pacific Northwest Coatings | Method of coating a substrate using a thermosetting basecoat composition and a thermoplastic top coat composition |
| JP3718639B2 (en) * | 2001-03-12 | 2005-11-24 | 筒中シート防水株式会社 | Seat waterproof structure with fire resistance |
| US6706793B2 (en) | 2002-01-23 | 2004-03-16 | Delphi Technologies, Inc. | Intumescent fire retardant composition and method of manufacture thereof |
| US6809129B2 (en) | 2002-01-23 | 2004-10-26 | Delphi Technologies, Inc. | Elastomeric intumescent material |
| US6696125B2 (en) * | 2002-04-25 | 2004-02-24 | Polyglass, U.S.A. | Self-adhered modified bitumen roofing material |
| CN2573591Y (en) * | 2002-08-26 | 2003-09-17 | 沈阳蓝光新型防水材料有限公司 | Rootproof and waterproof modified asphalt roll |
| US20040121152A1 (en) | 2002-12-19 | 2004-06-24 | Certainteed Corporation | Flame-resistant insulation |
| US7605188B2 (en) | 2004-12-31 | 2009-10-20 | Owens Corning Intellectual Capital, Llc | Polymer foams containing multi-functional layered nano-graphite |
| JP2005187522A (en) * | 2003-12-24 | 2005-07-14 | Nippon Zeon Co Ltd | Halogen-containing rubber composition |
| US20050139126A1 (en) | 2003-12-31 | 2005-06-30 | Building Materials Investment Corporation | Intumescent coating |
| US20050257875A1 (en) | 2004-05-21 | 2005-11-24 | Building Materials Investment Corporation | Process for coating modified bitumen membranes using reflective laminate coatings |
| US20050288394A1 (en) | 2004-06-29 | 2005-12-29 | John Rothman | Insulative, emissive and reflective coating |
| EP1791919A1 (en) | 2004-09-20 | 2007-06-06 | 3M Innovative Properties Company | Surface support method |
| US8277882B2 (en) * | 2004-09-29 | 2012-10-02 | Garland Industries, Inc. | Roofing and/or siding material and a method of forming thereof |
| US20060144012A1 (en) | 2004-12-01 | 2006-07-06 | Norman Manning | Recycled energy absorbing underlayment and moisture barrier for hard flooring system |
| US20070039268A1 (en) | 2004-12-01 | 2007-02-22 | L&P Property Management Company | Energy Absorptive/Moisture Resistive Underlayment Formed using Recycled Materials and a Hard Flooring System Incorporating the Same |
| US20060160978A1 (en) | 2005-01-20 | 2006-07-20 | Gupta Laxmi C | Flame retardant systems, and related methods and uses |
| CN104453081A (en) | 2005-02-25 | 2015-03-25 | 诺瓦化学品公司 | Composite pre-formed building panels, a building and a framing stud |
| EP1861559B1 (en) | 2005-03-22 | 2011-07-06 | Nova Chemicals Inc. | Lightweight concrete compositions |
| US20080275149A1 (en) | 2007-05-04 | 2008-11-06 | Nova Chemicals Inc. | Durable concrete compositions |
| US20060217451A1 (en) | 2005-03-28 | 2006-09-28 | Vittorio Bonapersona | Polyurethane or polyisocyanurate foam composition |
| US7878301B2 (en) | 2005-04-01 | 2011-02-01 | Buckeye Technologies Inc. | Fire retardant nonwoven material and process for manufacture |
| ITVI20050160A1 (en) | 2005-05-27 | 2006-11-28 | Giampaolo Benussi | INTUMESCENT GASKET |
| US7833575B2 (en) | 2005-11-08 | 2010-11-16 | Gupta Laxmi C | Methods for applying fire retardant systems, compositions and uses |
| US20110313084A1 (en) | 2006-07-27 | 2011-12-22 | Ppg Industries Ohio, Inc. | Coating compositions comprising polyurea and graphite |
| JP5057506B2 (en) * | 2006-08-18 | 2012-10-24 | 住江織物株式会社 | Non-halogen vehicle waterproofing floor sheet |
| US20080102243A1 (en) | 2006-10-11 | 2008-05-01 | Gupta Laxmi C | Laminate fire retardant systems and uses |
| WO2008076264A2 (en) | 2006-12-13 | 2008-06-26 | Gupta Laxmi C | Fire retardant body and methods of use |
| US7677009B2 (en) | 2007-02-02 | 2010-03-16 | Nova Chemicals Inc. | Roof truss system |
| US20080184642A1 (en) | 2007-02-05 | 2008-08-07 | Laura Sebastian | Latex foam insulation and method of making and using same |
| US7681365B2 (en) | 2007-10-04 | 2010-03-23 | James Alan Klein | Head-of-wall fireblock systems and related wall assemblies |
| US20110002998A1 (en) | 2007-12-21 | 2011-01-06 | Basf Se | Process for the production of insecticide-modified bead material composed of expandable polystyrene and insecticide-modified moldings obtainable therefrom |
| US8312963B2 (en) | 2008-02-14 | 2012-11-20 | Nagoya Oilchemical Co., Ltd | Sound absorbing skin material and sound absorbing material utilizing the same |
| US8932497B2 (en) | 2008-03-13 | 2015-01-13 | Laxmi C. Gupta | Fire retardant coatings and bodies, and methods of use |
| WO2009138379A2 (en) | 2008-05-13 | 2009-11-19 | Basf Se | Method for producing polyol dispersions |
| US20100167013A1 (en) | 2008-12-30 | 2010-07-01 | Cruz Carlos A | Thermoplastic roofing membranes |
| CA2737162C (en) | 2008-09-15 | 2015-06-16 | Preferred Solutions, Inc. | Polyurethane foam compositions and process for making same |
| JP2010070983A (en) * | 2008-09-19 | 2010-04-02 | Ube Ind Ltd | Waterproof sheet material |
| US20100080920A1 (en) | 2008-09-26 | 2010-04-01 | Tony Lagrange | Flame retardant coating |
| US8491992B2 (en) | 2008-12-17 | 2013-07-23 | Basf Se | Laminar component made from composite material |
| GB0908154D0 (en) | 2009-05-12 | 2009-06-24 | Nullifire Ltd | Intumescent composition |
| US8178449B2 (en) | 2009-07-17 | 2012-05-15 | Building Materials Investment Corp. | Fire resistant slipsheet |
| DE102009033710A1 (en) | 2009-07-18 | 2011-01-20 | Evonik Goldschmidt Gmbh | Use of metal salts of a carboxylic acid in the production of polyurethane systems |
| ITMI20091776A1 (en) | 2009-10-15 | 2011-04-16 | Dow Global Technologies Inc | POLYESOCYANURATE-BASED ADHESIVE WITH TWO COMPONENTS AND INSULATING PANELS PREPARED WITH ITS USE |
| US8468759B2 (en) | 2010-01-29 | 2013-06-25 | Blazeframe Industries Ltd. | Fire retardant cover for fluted roof deck |
| EP2563878B1 (en) | 2010-04-26 | 2019-06-26 | Gala Industries, Inc. | Pelletizing asphalt |
| CA2743359C (en) | 2010-06-18 | 2018-09-11 | Basf Se | Polyurethane foam article and method of forming same |
| EP2591034A1 (en) | 2010-07-08 | 2013-05-15 | Basf Se | Rigid polyurethane foam |
| US20120100289A1 (en) | 2010-09-29 | 2012-04-26 | Basf Se | Insulating compositions comprising expanded particles and methods for application and use |
| JP5879047B2 (en) * | 2010-10-05 | 2016-03-08 | 積水化学工業株式会社 | Drainage pipe fitting |
| US20120142240A1 (en) | 2010-12-07 | 2012-06-07 | Basf Se | Polyurethane composite material |
| CN102173631B (en) * | 2011-02-21 | 2012-04-18 | 山西省交通科学研究院 | Flame retardant for tunnel asphalt pavement |
| DE102011007468A1 (en) | 2011-04-15 | 2012-10-18 | Evonik Goldschmidt Gmbh | Composition containing specific carbamate-type compounds suitable for the preparation of polyurethane foams |
| CA2772874A1 (en) | 2011-04-21 | 2012-10-21 | Certainteed Corporation | System, method and apparatus for thermal energy management in a roof |
| RU2460834C1 (en) | 2011-06-06 | 2012-09-10 | Закрытое акционерное общество "ТехноНИКОЛЬ" | Multi-layer bitumen-polymer material and method of its production |
| AU2012362118B2 (en) | 2011-12-29 | 2016-11-17 | Firestone Building Products Co., LLC | Roofing membranes with expandable graphite as flame retardant |
| US9441140B2 (en) | 2012-07-12 | 2016-09-13 | Firestone Building Products Co., LLC | Asphaltic sheet materials including expandable graphite |
| US8968853B2 (en) | 2012-11-07 | 2015-03-03 | Firestone Building Products Company, Llc | Pressure-sensitive adhesives including expandable graphite |
| US9045904B2 (en) | 2012-11-16 | 2015-06-02 | Firestone Building Products Co., LLC | Thermoplastic membranes containing expandable graphite |
| US9523203B2 (en) | 2013-01-23 | 2016-12-20 | Firestone Building Products Co., LLC | Fire-resistant roof system and membrane composite |
| US20140205789A1 (en) | 2013-01-23 | 2014-07-24 | Firestone Building Products Co., LLC | Coated fabrics including expandable graphite |
| CA2911276A1 (en) | 2013-07-23 | 2015-01-29 | Firestone Building Products Co., LLC | Method for manufacturing asphaltic sheet materials including expandable graphite |
-
2013
- 2013-03-14 US US13/804,202 patent/US9441140B2/en active Active
- 2013-07-12 EP EP13742557.5A patent/EP2872553B1/en active Active
- 2013-07-12 CN CN201380037169.8A patent/CN104583289A/en active Pending
- 2013-07-12 EP EP19182058.8A patent/EP3581614A1/en not_active Withdrawn
- 2013-07-12 RU RU2015104641A patent/RU2656504C2/en not_active IP Right Cessation
- 2013-07-12 WO PCT/US2013/050251 patent/WO2014011977A1/en active Application Filing
- 2013-07-12 BR BR112015000706A patent/BR112015000706A2/en not_active Application Discontinuation
- 2013-07-12 IN IN10909DEN2014 patent/IN2014DN10909A/en unknown
- 2013-07-12 AU AU2013290057A patent/AU2013290057B2/en not_active Ceased
- 2013-07-12 MX MX2014015388A patent/MX2014015388A/en active IP Right Grant
- 2013-07-12 CA CA2879016A patent/CA2879016C/en active Active
- 2013-07-12 JP JP2015521837A patent/JP6209606B2/en not_active Expired - Fee Related
-
2016
- 2016-08-12 US US15/235,530 patent/US10011092B2/en active Active
-
2017
- 2017-04-11 AU AU2017202392A patent/AU2017202392B2/en not_active Ceased
- 2017-04-27 US US15/499,597 patent/US11065841B2/en active Active
- 2017-07-03 JP JP2017130510A patent/JP6370447B2/en not_active Expired - Fee Related
-
2018
- 2018-06-19 US US16/011,868 patent/US11230085B2/en active Active
-
2022
- 2022-01-10 US US17/571,637 patent/US20220126547A1/en active Pending
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3574644A (en) * | 1965-03-22 | 1971-04-13 | Dow Chemical Co | Method of rendering normally flamable materials flame resistant |
| US4437896A (en) | 1982-09-30 | 1984-03-20 | Partanen John F | Synthetic asphalt mixtures and processes for making them |
| US4835199A (en) | 1987-04-10 | 1989-05-30 | The Firestone Tire & Rubber Company | Bituminous composition comprising a blend of bitumen and a thermoplastic elastomer |
| US4992315A (en) | 1989-11-13 | 1991-02-12 | Gaf Buildinhg Materials Corp. | Roofing membrane and method |
| US6486236B2 (en) | 2000-12-28 | 2002-11-26 | Firestone Polymers, Llc | Asphalt composition comprising bismaleimides |
| US6492439B2 (en) | 2000-12-28 | 2002-12-10 | Firestone Polymers, Llc | Asphalt compositions |
| US6924015B2 (en) | 2002-05-21 | 2005-08-02 | Polyglass, U.S.A. | Modified bitumen roofing membrane with enhanced sealability |
| US7146771B2 (en) | 2003-03-04 | 2006-12-12 | Johns Manville | Cap sheet, roofing installation, and method |
| US7070843B2 (en) | 2003-09-10 | 2006-07-04 | Johns Manville | Highly reflective asphalt-based roofing membrane |
| US7442270B2 (en) | 2003-09-10 | 2008-10-28 | Johns Manville | Highly reflective asphalt-based roofing membrane |
| US20050145139A1 (en) * | 2003-12-31 | 2005-07-07 | Amir Khan | Intumescent reflective coating |
| US20060273290A1 (en) * | 2005-06-01 | 2006-12-07 | Building Materials Investment Corporation | Fire retardant composition |
| KR20110087720A (en) * | 2010-01-27 | 2011-08-03 | 한국석유공업 주식회사 | Nonflammable asphalt composition and waterproofing sheet using the same |
| EP2617894A1 (en) * | 2012-01-20 | 2013-07-24 | Onduline | Fire-resistant bituminous cellulose cover plate and manufacturing method |
Non-Patent Citations (1)
| Title |
|---|
| HYDROCARBON RESINSKIRK-OTHMER: "Encyclopedia of Chemical Technology", 1995, J. WILEY & SONS, pages: 717 - 743 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN104583289A (en) | 2015-04-29 |
| CA2879016A1 (en) | 2014-01-16 |
| US10011092B2 (en) | 2018-07-03 |
| US20220126547A1 (en) | 2022-04-28 |
| JP2017198066A (en) | 2017-11-02 |
| US11065841B2 (en) | 2021-07-20 |
| EP2872553A1 (en) | 2015-05-20 |
| AU2017202392A1 (en) | 2017-04-27 |
| RU2015104641A (en) | 2016-08-27 |
| JP2015529756A (en) | 2015-10-08 |
| US20140013693A1 (en) | 2014-01-16 |
| IN2014DN10909A (en) | 2015-09-11 |
| AU2017202392B2 (en) | 2018-08-16 |
| US20170298201A1 (en) | 2017-10-19 |
| US20180326702A1 (en) | 2018-11-15 |
| MX2014015388A (en) | 2015-03-05 |
| WO2014011977A1 (en) | 2014-01-16 |
| US20160347033A1 (en) | 2016-12-01 |
| US9441140B2 (en) | 2016-09-13 |
| JP6370447B2 (en) | 2018-08-08 |
| AU2013290057A1 (en) | 2015-02-05 |
| BR112015000706A2 (en) | 2017-06-27 |
| US11230085B2 (en) | 2022-01-25 |
| JP6209606B2 (en) | 2017-10-04 |
| RU2656504C2 (en) | 2018-06-05 |
| EP2872553B1 (en) | 2019-08-21 |
| AU2013290057B2 (en) | 2017-02-02 |
| CA2879016C (en) | 2020-10-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11065841B2 (en) | Asphaltic sheet materials including expandable graphite | |
| US10138375B2 (en) | Method for manufacturing asphaltic sheet materials including expandable graphite | |
| US12110688B2 (en) | Flame-resistant composites for roofing underlayment | |
| US9688887B2 (en) | Pressure-sensitive adhesives including expandable graphite | |
| US20150197884A1 (en) | Asphaltic sheet materials including expandable graphite | |
| US20150040503A1 (en) | Roofing system and method for preparing the same | |
| WO2016145188A1 (en) | Asphalt-based roofing membrane composite |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2872553 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 20200619 |