EP3090765B1 - Systems for subcutaneous administration of reduced pressure employing reconfigurable lumens - Google Patents
Systems for subcutaneous administration of reduced pressure employing reconfigurable lumens Download PDFInfo
- Publication number
- EP3090765B1 EP3090765B1 EP16164752.4A EP16164752A EP3090765B1 EP 3090765 B1 EP3090765 B1 EP 3090765B1 EP 16164752 A EP16164752 A EP 16164752A EP 3090765 B1 EP3090765 B1 EP 3090765B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- lumen
- applicator
- apertures
- tissue site
- frangible
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000007920 subcutaneous administration Methods 0.000 title description 2
- 230000004913 activation Effects 0.000 claims description 67
- 239000012530 fluid Substances 0.000 claims description 60
- 239000007788 liquid Substances 0.000 claims description 19
- 229920000954 Polyglycolide Polymers 0.000 claims description 4
- 239000004633 polyglycolic acid Substances 0.000 claims description 4
- 239000001828 Gelatine Substances 0.000 claims description 2
- 229920000159 gelatin Polymers 0.000 claims description 2
- 235000019322 gelatine Nutrition 0.000 claims description 2
- 239000000017 hydrogel Substances 0.000 claims description 2
- 239000004626 polylactic acid Substances 0.000 claims description 2
- 238000010926 purge Methods 0.000 description 39
- 206010033675 panniculitis Diseases 0.000 description 27
- 210000004304 subcutaneous tissue Anatomy 0.000 description 27
- 210000001519 tissue Anatomy 0.000 description 27
- 238000000034 method Methods 0.000 description 20
- 230000008569 process Effects 0.000 description 14
- 239000000463 material Substances 0.000 description 9
- 230000008901 benefit Effects 0.000 description 5
- 206010052428 Wound Diseases 0.000 description 4
- 210000000988 bone and bone Anatomy 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 208000027418 Wounds and injury Diseases 0.000 description 3
- 238000004891 communication Methods 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 206010003445 Ascites Diseases 0.000 description 1
- 206010063560 Excessive granulation tissue Diseases 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000005465 channeling Effects 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 210000004207 dermis Anatomy 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 210000000416 exudates and transudate Anatomy 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 210000001126 granulation tissue Anatomy 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 230000002706 hydrostatic effect Effects 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 210000003041 ligament Anatomy 0.000 description 1
- 238000002324 minimally invasive surgery Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 238000009581 negative-pressure wound therapy Methods 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/71—Suction drainage systems
- A61M1/74—Suction control
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/71—Suction drainage systems
- A61M1/74—Suction control
- A61M1/743—Suction control by changing the cross-section of the line, e.g. flow regulating valves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/84—Drainage tubes; Aspiration tips
- A61M1/85—Drainage tubes; Aspiration tips with gas or fluid supply means, e.g. for supplying rinsing fluids or anticoagulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/91—Suction aspects of the dressing
- A61M1/916—Suction aspects of the dressing specially adapted for deep wounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/92—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing with liquid supply means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/96—Suction control thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/84—Drainage tubes; Aspiration tips
- A61M1/87—Details of the aspiration tip, not otherwise provided for
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M2005/1401—Functional features
- A61M2005/1403—Flushing or purging
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/18—General characteristics of the apparatus with alarm
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/33—Controlling, regulating or measuring
- A61M2205/3331—Pressure; Flow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2209/00—Ancillary equipment
- A61M2209/10—Equipment for cleaning
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/02—Bones
Definitions
- the present disclosure relates generally to medical treatment systems and, more particularly, but not by way of limitation, to systems, methods, and devices for the subcutaneous administration of reduced pressure that include reconfigurable lumens.
- a manifold for providing reduced pressure to a subcutaneous tissue includes a longitudinal manifold body formed with at least one purging lumen and a reduced-pressure lumen.
- a multi-lumen applicator for delivering reduced pressure to a tissue site and receiving fluids
- the multi-lumen applicator comprising an applicator body having a distal end and a proximal end and formed with a first plurality of apertures proximate to the distal end for receiving fluid and a second plurality of apertures proximate the distal end for receiving fluid from the tissue site and for delivering reduced pressure; a first lumen fluidly coupled to the first plurality of apertures, a second lumen fluid coupled to the second plurality of apertures, and a first plurality of activation members coupled to the second plurality of apertures, wherein the first plurality of activation members is operable to move from a closed position to an open position when activated to allow flow.
- a multi-lumen applicator for delivering reduced pressure to a tissue site and receiving fluids
- the multi-lumen applicator comprising, an applicator body having a distal end and a proximal end and formed with a first plurality of apertures for receiving fluid from the tissue site and a second plurality of apertures for receiving fluid from the tissue site and for delivering reduced pressure, a first lumen fluidly coupled to the first plurality of apertures, a second lumen fluidly coupled to the second plurality of apertures, wherein at least one of the second plurality of apertures comprises a first activation member configured to close the aperture in a first configuration and open the aperture in a second configuration.
- Providing reduced pressure to a subcutaneous tissue site may assist with removing fluids, e.g., ascites or exudates, or enhance tissue growth as an aspect of reduced pressure therapy.
- fluids e.g., ascites or exudates
- enhance tissue growth as an aspect of reduced pressure therapy.
- "or" does not require mutual exclusivity.
- a multi-lumen applicator is used.
- blockage of lumens may occur and pose a problem to the ongoing treatment.
- the lumens may be reconfigured relative to the blockage in order to restore flow of reduced pressure to the subcutaneous tissue site or to remove the blockage.
- the blockage may be removed using a blockage-removal device.
- the subcutaneous tissue site 102 may be, for example, a defect 104 in or on a bone 106 (e.g., a fractured bone).
- the subcutaneous tissue site 102 may be any site that may benefit from treatment with reduced pressure to remove fluids or as an aspect of reduced pressure therapy.
- the subcutaneous tissue site 102 may be the bodily tissue of any human, animal, or other organism, including bone tissue, adipose tissue, muscle tissue, vascular tissue, connective tissue, cartilage, tendons, ligaments, or any other tissue.
- the system 100 includes a multi-lumen applicator 108 that is inserted into the patient 110 and placed proximate to the subcutaneous tissue site 102.
- the multi-lumen applicator 108 is shown having been inserted through epidermis 112, dermis 114, and into subcutaneous tissue 116.
- the multi-lumen applicator 108 is positioned proximate to the subcutaneous tissue site 102.
- the multi-lumen applicator 108 is fluidly coupled to a reduced-pressure delivery conduit 118, which may be a multi-lumen conduit that has lumens coordinated and fluidly coupled to the multiple lumens of the multi-lumen applicator 108.
- the reduced-pressure delivery conduit 118 may be fluidly coupled to a connector 120 that may facilitate connecting multiple lumens to the multiple lumens of the reduced-pressure delivery conduit 118.
- a reduced-pressure source 122 is fluidly coupled by a conduit 124 to the connector 120 to provide reduced pressure thereto.
- the reduced-pressure source 122 may include a reduced-pressure supply portion 126 and a fluid reservoir 128.
- the reduced-pressure supply portion 126 may be a vacuum pump, wall suction, or any other source of reduced pressure.
- the fluid reservoir 128 may provide a place to receive and retain fluids delivered from the patient 110.
- Reduced pressure is typically a pressure less than the ambient pressure at a tissue site that is being subjected to treatment. In most cases, this reduced pressure will be less than the atmospheric pressure at which the patient is located. Alternatively, the reduced pressure may be less than a hydrostatic pressure at the tissue site. Unless otherwise indicated, quantitative values of pressure stated herein are gauge pressures.
- the reduced pressure delivered may be constant or varied (patterned or random) and may be delivered continuously or intermittently. Although the terms "vacuum” and “negative pressure” may be used to describe the pressure applied to the tissue site, the actual pressure applied to the tissue site may be more than the pressure normally associated with a complete vacuum. Consistent with the use herein, an increase in reduced pressure or vacuum pressure typically refers to a relative reduction in absolute pressure. For example, going from -50 mm Hg to -100 mm Hg may be referred to as an increase in reduced pressure, but on an absolute pressure scale it is a decrease in pressure.
- a purging unit 130 may be fluidly coupled by a conduit 132 to the connector 120.
- the purging unit 130 may provide atmospheric air or another purging gas or pressurized gas to the multi-lumen applicator 108 in order to avoid or remove blockages therein.
- the purge gas provided by the purging unit 130 may be at an elevated pressure with respect to atmosphere or relative to the operational pressure of the system 100.
- a liquid source 134 may be fluidly coupled by a conduit 136 to the connector 120.
- the liquid source 134 may be used to provide a liquid purge to the multi-lumen applicator 108 or may be used to provide a treatment liquid, or therapeutic liquid, to the multi-lumen applicator 108 and ultimately to the subcutaneous tissue site 102.
- a controller 138 may be coupled by coupling lines 140, 142 and 144 to the reduced-pressure source 122, purging unit 130, and liquid source 134, respectively.
- the controller 138 may include a microprocessor, memory, and other components for providing control to the reduced-pressure source 122, purging unit 130, and liquid supply 134.
- the controller 138 may also be coupled to the connector 120 to control valves within the connector 120 as shown in the illustrative embodiment of FIGURE 11 .
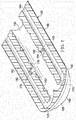
- the multi-lumen applicator 108 is formed with an applicator body 146, which has a first side 148 and a second, tissue-facing side 150.
- the multi-lumen applicator 108 may be formed by injection molding or other techniques.
- the multi-lumen applicator 108 may also be extruded into parts and then bonded or otherwise coupled to form an integral unit.
- the multi-lumen applicator 108 may be extruded and then undergo a secondary controller melt "tipping" process to form an integral unit.
- the multi-lumen applicator 108 may be made from a flexible or semi-rigid material.
- the multi-lumen applicator 108 may be made from any medical-grade polymer, such as polyurethane.
- the multi-lumen applicator 108 is made from a material with a stiffness of approximately 80 Shore A, but other stiffnesses may be used.
- a coating may be added to the multi-lumen applicator 108 to avoid material buildup on the multi-lumen applicator 108.
- a plurality of apertures 152 are formed on the second, tissue-facing side 150 of the applicator body 146 for providing reduced pressure to the subcutaneous tissue site 102. While the apertures 152 are shown in a symmetrically spaced pattern, it should be understood that the apertures 152 may be formed with any pattern or with a random placement.
- a plurality of manifold surface features 154 may be formed on the second, tissue-facing side 150. The plurality of manifold surface features 154 may include a plurality of standoffs or offsets 156. The plurality of offsets 156 may be formed integrally with or coupled to the second, tissue-facing side 150 of the applicator body 146.
- the offsets 156 may be any surface feature creating effective flow channels between the second, tissue-facing side 150 and the tissue site.
- the manifold surface features 154 may detach from the applicator body 146 when the multi-lumen applicator 108 is percutaneously removed, and the manifold surface features 154 may be bioresorbable.
- the plurality of apertures 152 are fluidly coupled to the first lumen 158 that is formed in the applicator body 146.
- the first lumen 158 may be fluidly coupled to the apertures 152 by a plurality of conduits 160.
- the first lumen 158 extends the longitudinal length of the applicator body 146.
- the first lumen 158 may initially be used as an evacuation lumen to deliver reduced pressure to the plurality of apertures 152 and to receive and transport fluids from the subcutaneous tissue site 102.
- the applicator body 146 is also formed with a second lumen 162 and may have a third lumen 164 or even more lumens.
- the second lumen 162 and third lumen 164 also extend the longitudinal length of the applicator body 146.
- the second lumen 162 and third lumen 164 may initially be used as purge lumens, or vent lumens. While this illustrative embodiment shows two purge lumens, it should be understood that any number of purge lumens may be used.
- the second lumen 162 and third lumen 164 are shown symmetrically spaced about the first lumen 158, and while the symmetric orientation may enhance performance, other orientations may be used.
- Additional lumens such as a pressure sensing lumen (not explicitly shown), may be included within the applicator body 146.
- the purge lumens may also serve as pressure sensing lumens. It should be noted that although a slightly elliptical or triangular shape is presented, the cross sectional shape of the applicator body 146 may be any of those previously mentioned or even irregular or other shapes.
- an end cap 168 is formed or coupled.
- the end cap 168 is formed with a header space 170 that allows the second lumen 162 and the third lumen 164 (and any additional lumens) to be fluidly coupled to the first lumen 158.
- the end cap 168 is formed integrally to or as part of the applicator body 146 and, thus, avoids the risk of the end cap 168 becoming dislodged during removal from the patient 110.
- a proximal end 172 FIG.
- a connecting element, or connector 174 may be coupled to provide easy connection with the reduced-pressure delivery conduit 118, which in turn is fluidly coupled to the reduced-pressure source 122 and also to the purging unit 130 or liquid source 134.
- the multi-lumen applicator 108 has provisions to reconfigure lumens, e.g., the first lumen 158, second lumen 162, and third lumen 164, in order to restore flow through the apertures 152.
- a first port 176 is formed between the first lumen 158 and the second lumen 162.
- Port typically refers to an open flow path either between two lumens or a lumen and an exterior of the multi-lumen applicator 108.
- the first port 176 fluidly couples the first lumen 158 and the second lumen 162 when a first activation member 178 is in an open, or activated, position.
- the first activation member 178 may be any member that provides a closed position in one state and an open position in another state that allows flow.
- the first activation member 180 may be a first frangible member 180 as shown in FIGURE 3 in the closed position.
- frangible is used generally to indicate a material that fails, ruptures, tears, or dissolves in a predictable manner. The frangible material fails, ruptures, tears, or dissolves in a repeatable manner between devices.
- the first frangible member 180 may be a frangible disc or frangible port cover, which may be a piece of material covering a port that is designed to rupture or open at a first threshold pressure differential or to dissolve and thereby open after being exposed to a liquid for at least a threshold time.
- the activation member 178 may be formed from a polylactic acid (PLA), polyglycolic acid (PGA), polyactic co-glycolic acid (PLGA), hydrogel, or cross-linked or hardened gelatine, or other suitable material.
- the first activation member 178 may be a valve with a remote attachment, e.g., a line, that can be pulled to open the first activation member 178.
- the first activation member 178 may be a plug in a port that under pressure is released from the port and is removed.
- a second port 182 is formed between the first lumen 158 and the third lumen 164.
- the second port 182 fluidly couples the first lumen 158 and the third lumen 164 when a second activation member 184 is in an open position.
- the second activation member 184 may be a second frangible member 186 or other device analogous to those mentioned for first activation member 180.
- the multi-lumen applicator 108 may be inserted surgically or using minimally invasive surgery into the patient.
- the multi-lumen applicator 108 is removed percutaneously after use or in one embodiment may be bio-absorbable and left in place to absorb.
- the multi-lumen applicator 108 addresses blocks or the possibility of blocks by reconfiguring lumens during use.
- flow may continue for a first period of time.
- the first activation member 178 may be activated to open a flow path that reconfigures flow by reconfiguring the lumens 158, 162, or 164.
- the reconfiguration increases the likelihood that the system 100 will continue to operate with flow for the desired time duration.
- a number of illustrative, non-limiting examples of how lumens may be reconfigured or how blockages may be removed will be presented.
- FIGURES 5A - 5C an illustrative embodiment of a multi-lumen applicator 108 is presented to show how lumens, e.g., lumens 158 and 162, may be reconfigured according to one illustrative embodiment.
- the multi-lumen applicator 108 is shown with only two lumens: a first lumen 158 and a second lumen 162. It should be understood that other lumens or additional lumens may be involved.
- the multi-lumen applicator 108 in FIGURE 5A shows an initial state in which reduced pressure is delivered to the first lumen 158 thereby causing a flow 188 of fluid in an ante grade direction.
- the first lumen 158 in this initial condition serves as an evacuation lumen.
- the multi-lumen applicator 108 also includes the second lumen 162 that initially serves as a purge lumen providing a purge fluid, such as air, to the first lumen 158 to inhibit blocking or eliminate blocking.
- the purge fluid may be provided on a periodic basis. It should be appreciated that the second lumen 162 provides a purging fluid that travels through a head space 170 at a distal end 166 to avoid blockages.
- reduced pressure is distributed to a plurality of apertures 152 in an applicator body 146.
- Fluid e.g., wound effluent
- tissue site e.g., the subcutaneous tissue site 102 in FIGURE 1
- fluid reservoir e.g., the fluid reservoir 128 in FIGURE 1 .
- Normal operation may involve, for example, and not by way of limitation, a reduced pressure in the range of -100 mm Hg (-13.3 kPa) to -200 mm Hg (-26.6 kPa).
- a first port 176 may be formed as an aspect of a wall 159 between the first lumen 158 and second lumen 162.
- the first port 176 is controlled by a first activation member 178 that has a closed position as shown in FIGURES 5A-B and an open position as shown in FIGURE 5C .
- the first activation member 178 assumes the open position when activated.
- the first activation member 178 may be, for example, a first frangible member 180.
- a blockage 190 may result within the first lumen 158.
- the blockage 190 may inhibit or completely stop flow within the first lumen 158 and thereby inhibit or stop flow of fluids from the tissue site through the apertures 152.
- a controller or detection device or an operator determines that a blockage, e.g., the blockage 190, has occurred, a increased pressure, e.g., -300 mm Hg (-39.9 kPa) or -350 mm Hg (-46.6 kPa) may be applied to activate the first activation member 178 and in this embodiment to rupture the first frangible member 180.
- the lumens are reconfigured with respect to flow in portions and flow may begin to occur as shown in FIGURE 5C .
- the first activation member 178 may also be activated by exposure to liquid or by removal of a remote line (not shown) that activates a valve or opens the activation member 178.
- the first activation member 178 which in this embodiment is a first frangible member 180, has been activated such that the first port 176 is in an open position.
- fluid flows through the aperture 152 from the tissue site, traverses the head space 170, and flows through a portion of the second lumen 162, through the first port 176 as shown, and then continues through the first lumen 158 where flow may be received by a fluid reservoir, such as the fluid reservoir 128 in FIGURE 1 .
- a fluid reservoir such as the fluid reservoir 128 in FIGURE 1 .
- FIGURES 5A-5C While only one port 176 with a first activation member 178 is shown in FIGURES 5A-5C , it should be understood that multiple ports and activation members may be provided along the length of the multi-lumen applicator 108.
- a first port 176 is covered by a first activation member 178, such as a first frangible member 180 and a second port 192 is shown with a second activation member 194.
- the first frangible member 180 is disposed between the first lumen 158 and the second lumen 162.
- the first frangible member 180 is configured to rupture when exposed to a pressure greater than the first threshold pressure differential, whereby at least a portion of the second lumen 162 and a portion of the first lumen 158 are fluidly coupled.
- the second port 192 is shown with the activation member 194, such as an additional frangible member 196.
- Still another port 198 is shown in an open position.
- an initial flow is established through the port 198 until a blockage occurs, and then a first threshold pressure differential, e.g., -300 mm Hg, is used on the first activation member 178 to move the first activation member 178 to an open position. Activating the first activation member 178 causes flow to go through the first port 176. Later, if another blockage occurs, a second threshold pressure differential, e.g., -350 mm Hg, may be used to activate the additional activation member 196 and thereby open the additional port 192 to provide another reconfigured flow path through the lumens. As before, the activation of the activation members 178 and 196 may also be initiated based on elapsed time.
- a first threshold pressure differential e.g., -300 mm Hg
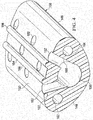
- the multi-lumen applicator 108 has a distal end 166 and a proximal end 172.
- a plurality of apertures 152 may be formed near the distal end 166 for providing reduced pressure to a tissue site, e.g., subcutaneous tissue site 102 in FIGURE 1 .
- a connector 174 may be used to connect a reduced-pressure delivery conduit 118 to the multi-lumen applicator 108.
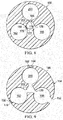
- three lumens which are shown in cross section in FIGURE 8 , e.g., a first lumen 158, a second lumen 162, and a third lumen 200, may be placed around a fourth lumen 202.
- a first lumen 158 in the initial state, only the first lumen 158 is active, or open, for removing fluids from the tissue site and is in fluid communication with the fourth lumen 202.
- the fourth lumen 202 is initially a purging lumen.
- the first lumen 158 has a first port 176, which is discrete, that fluidly couples the first lumen to the fourth lumen 202.
- the second lumen 162 has a second port 177, which is discrete, that fluidly couples the second lumen to the fourth lumen 202.
- the third lumen 200 has a third port 179, which is discrete, that fluidly couples the third lumen 200 to the fourth lumen 202.
- the first port 176 is initially in an open position.
- the second port 177 is initially closed by a first activation member 178, e.g., a first frangible member 180.
- the third port 179 is initially closed by a second activation member 184, e.g., a second frangible member.
- a pressure is applied to the lumens 158, 162, 200 that creates a pressure differential that surpasses a first threshold pressure differential whereby the first activation member 178 may be activated.
- this activating pressure can be applied to lumen 202 to create an activation pressure differential.
- the first frangible member 180 may be subjected to a pressure differential greater than the first threshold pressure differential, e.g., -300 mm Hg, such that the first frangible member 180 ruptures.
- the ruptured first frangible member 180 provides fluid communication between the second lumen 162 and the fourth lumen 202. At this point, fluids from the tissue site flow from the apertures 152 through the second lumen 162 while the fourth lumen 202 acts as a purging lumen.
- the second activation member 184 may be activated to allow fluid communication between the third lumen 200 and the fourth lumen 202. In this way, additional flow may go from the apertures 152 through the third lumen 200 to a fluid reservoir.
- the use of the activation members 178, 184 in FIGURE 8 may simplify the connector and reduce-pressure source design since the inactive lumens (e.g., initially lumens 162, 200) can be exposed to the reduced pressure.
- FIGURE 9 another illustrative embodiment of a portion of a multi-lumen applicator 108 is presented.
- the multi-lumen applicator 108 of FIGURE 9 is analogous in most respects to the multi-lumen applicator 108 of FIGURE 7 , and accordingly, some parts are labeled the same but not further described here. It should be noted, however, that while the section line 9-9 is shown in FIGURE 7 , this embodiment is nonetheless distinct from that described above in connection with FIGURES 7 and 8 in a number of respects.
- the multi-lumen applicator 108 is formed with an applicator body 146 having a first lumen 158, second lumen 162, third lumen 200, and a fourth lumen 202.
- the lumens 158, 162, 200 are positioned around the fourth lumen 202 and each lumen has at least one aperture or port of a plurality of apertures 152 that provides access to an exterior of the multi-lumen applicator 108.
- a first port 176 is formed between the first lumen 158 and the fourth lumen 202.
- pressure i.e., the pressure differential
- the second lumen 162 begins to serve as an evacuation lumen for liquids from the tissue site.
- the pressure may be increased to activate a second activation member 184 or plurality of first activation members that cover a portion of apertures 152 associated with the third lumen 200.
- the third lumen 200 begins to serve as an evacuation lumen for liquids from the tissue site.
- the fourth lumen 202 serves as a purge lumen for each of the lumens 158, 162, 200.
- activation members are referenced and typically discussed in the context of frangible members. It should be understood that the activation members may be activated by pressure exceeding a threshold pressure differential or by mere passage of time with the activation members exposed to a fluid. Thus, for example, after a first threshold time period, the first activation member may dissolve to the point that the first activation member ruptures or otherwise allows fluid flow. In another illustrative embodiment, the activation members may be activated by pulling a line that removes a plug or opens a valve, or any other technique to open the port in situ.
- the activation members may be frangible members in some embodiments.
- the frangible members may be controller with respect to when they open or rupture by controlling a number of variables.
- the material of the frangible member may be thin, strong, or stretchy, consistent, or scored to create a location for the failure.
- rupture of the frangible members may be controller by thickness of various portions and may have an adhesive for controlling aspects of the frangible members.
- the multi-lumen applicator 308 includes an applicator body 346 formed with a plurality of apertures 352.
- the multi-lumen applicator 308 includes a first lumen 358, a second lumen 362, a third lumen 364, and a fourth lumen 301.
- the multi-lumen applicator 308 is shown in the initial state in which the first lumen 358 serves as an evacuation lumen and the second lumen 362 serves as a vent or purge lumen.
- fluids from the tissue site are drawn through at least a portion of the plurality of apertures 352, into the first lumen 358, and moved from the distal end 366 to the proximal end 372 where the fluids are delivered into a fluid reservoir.
- the third lumen 364 and fourth lumen 301 are initially filled by filaments 365, 399.
- the third lumen 364 is initially filled by the first filament 365
- the fourth lumen 301 is filled by a second filament 399.
- Each filament 365, 399 may be a nylon monofilament or wire that substantially fills the space of the noted lumens to prevent flow therein.
- the first lumen 358 is initially used to deliver reduced pressure and remove fluids through at least a portion of the apertures 352.
- the first filament 365 may be removed from the third lumen by pulling the first filament 365 out from the proximal end 372. Removing the first filament 365 opens the third lumen 364-including a port to the second lumen 362 allowing removal of fluids from apertures 352.
- the second filament 399 may be removed from the fourth lumen 301 to provide flow through the fourth lumen 301.
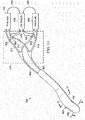
- the system 400 includes a multi-lumen applicator 408.
- the multi-lumen applicator 408 is formed with an applicator body 446 that includes a plurality of apertures 452.
- the multi-lumen applicator 408 includes at least a first lumen 458 and a second lumen 462.
- the first lumen 458 may be selectively, fluidly coupled by a connector 420 to a reduced-pressure source 422 or a purge unit 430.
- the connector 420 is only one example, of how the lumens 458, 462 may be reconfigured.
- the second lumen 462 may be selectively, fluidly coupled to the purge unit 430 or the reduced-pressure source 422.
- a controller 438 may be coupled by coupling lines 440, 442 to the reduced-pressure source 422 and the purge unit 430, respectively.
- the controller 438 may be coupled by additional coupling lines to a first valve 502, second valve 504, third valve 506, and fourth valve 508 in the connector 420.
- the controller 438 may control the reduced-pressure source 422, purge unit 430, and valves.
- the connector 420 may be under the control of the controller 438 and function to switch the functionality of the first lumen 458 and the second lumen 462.
- the lumen 458 may divide into a first sub-lumen 510 and a second sub-lumen 512.
- the first sub-lumen 510 couples the first lumen 458 to the reduced-pressure source 422.
- the first valve 502 is located on the first sub-lumen 510.
- the second sub-lumen 512 couples the first lumen 458 to the purge unit 430.
- the second sub-lumen 512 includes the third valve 506. In the initial state, the third valve 506 is closed and the first valve 502 is opened such that reduced pressure is supplied to the first lumen 458.
- the second lumen 462 is divided within the connector 420 between a third sub-lumen 514 and a fourth sub-lumen 516.
- the third sub-lumen 514 fluidly couples the second lumen 462 to the purge unit 430.
- the fourth sub-lumen 516 fluidly couples the second lumen 462 to the reduced-pressure source 422.
- the fourth valve 508 is located on the third sub-lumen 514 and selectively controls fluid flow therein, and the second valve 504 is located in the fourth sub-lumen 516 and selectively controls flow therein.
- the second lumen 462 serves as a purge lumen, and thus, the second valve 504 on the fourth sub-lumen 516 is closed and the fourth valve 508 is open on the third sub-lumen 514.
- the controller 438 will reconfigure the valves 502, 504, 506, 508 in order to reconfigure the functionality of the lumens 458, 462.
- the first valve 502 on first sub-lumen 510 is closed and the third valve 506 on the second sub-lumen is opened.
- the second valve 504 on the fourth sub-lumen 516 is opened and the fourth valve 508 on the third sub-lumen 514 is closed.
- the second lumen 462 becomes the evacuation lumen and the first lumen 458 becomes the purge lumen. This condition may be maintained or may only be temporarily assumed in order to remove the blockage in the first lumen 458.
- an incompressible purging fluid e.g., sterile saline
- reconfiguring the lumens 458, 462 may allow the blockage in the lumen to be removed more easily since the change causes flow in a retrograde direction. Once the blockage is removed, the original direction may be restored or operation may continue as configured.
- the pressure differentials and forces which are limited in the ante grade direction, may be increased in the retro grade direction since greater pressure forces are more tolerable in the retrograde direction.
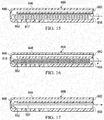
- FIGURES 11 and 14 one illustrative, non-limiting logic flow for the controller 438 in controlling the functionality of the lumens 458, 462 is presented.
- the process begins at 520, with the first lumen in use for fluid evacuation, and goes to a first interrogation box 522 where the question is asked, "Is the first lumen blocked?" If the answered is in the negative, the process returns again to the first interrogation box 522. If the answer is in the affirmative, the process continues to process box 524 and instructions are provided for the second lumen 462 to be coupled to the reduced-pressure source 422 and the first lumen 458 to be coupled to the purge unit 430. This may be accomplished with the specific instructions sent to the valves, e.g., first valve 502 closed, second valve 504 open, third valve 506 open, and fourth valve 508 closed.
- the next (second) interrogation box 526 is reached and the question is asked, "Is the second lumen blocked?" If the answer is in the negative, the process returns again to the second interrogation box 526. If in the affirmative, the process box 528 is reached.
- the process block 528 provides instructions for the first lumen 458 to be coupled to the reduced-pressure source 422 and the second lumen 462 to be coupled to the purge unit 430. In other words, the flow returns to the initial state which may now flow again since operation in the second state may remove blockages.
- the third interrogation box 530 is reached and asks the question, "Is the first lumen blocked?" If the answer is negative, the process continues to the first interrogation box 522. If the answer to the third interrogation box 530 is in the affirmative, an alarm is activated at process block 531, and the process ends at step 532.
- This process is only one illustrative way of programming the controller 438.
- a blockage may be managed by removing the blockage.
- FIGURES 15-19 a number of techniques for removing blockages from within the lumens in order to provide continued flow are presented.
- a portion of a multi-lumen applicator 608 is presented.
- a plurality of apertures 652 are formed on the distal end 666 of an applicator body 646.
- a first lumen 658 is formed within the applicator body 646 as well as at least a second lumen 662.
- a blockage-removal device e.g., a blockage-removal member 617, an elongated brush member 61.9, a fluid jet 621, or a purging element 623, is inserted into the multi-lumen applicator 608 and, the blockage-removal device is activated, which means the blockage-removal device may be removed, rotated, energized, or otherwise enabled to provide a blockage removing force within the lumen.
- the blockage-removal member 617 such as an auger, Archimedes screw, or tanglement wire, is inserted into the first lumen 658 and rotated.
- the blockage-removal member 617 may be rotated within the first lumen 658 to break a blockage free and help move any material with the flow toward the proximal end.
- the rotation may be at various speeds, e.g., slow rotation of 1 to 20 rpm.
- the blockage-removal member 617 remains within the first lumen 658 as the blockage-removal member 617 is rotated or may be slowly removed.
- the elongated brush member 619 disposed within the first lumen 658 is shown.
- the elongated brush member 619 may be rotated to remove items causing a block or inhibit flow.
- the elongated brush member 619 may be rotated at, for example, a relatively higher RPM.
- the fluid jet 621 is disposed within the first lumen 658 and removed from the first lumen 658 at the proximal end. As the fluid jet 621 is removed, water jets, which are facing the proximal end, remove any blockage. The volume of water or other purging liquid (e.g., saline) placed into the lumen will be matched with the evacuation capacity of the system in order to avoid fluid infusion into the patient.
- purging liquid e.g., saline
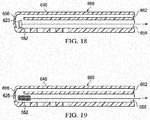
- the purging element 623 may be pulled from a distal end of the first lumen 658 to a proximal end to remove any blockages in the first lumen 658.
- the purging element 623 or device 623 may be an inflatable member that after being located at the distal end may be inflated.
- the purging element 623 may be analogous to a Fogerty catheter style device.
- a cytology brush 625 may be pulled from the first lumen 658 to remove any blockages therein.
- FIGURES 15-19 may optionally incorporate combined aspects of rotation and axial translation as part of the activation.
- the blockage-removal device may be in place when the applicator body 646 is placed into the wound.
- the blockage-removal device may be a single use item as it is removed from the applicator body 646.
- ports opening at different times are given by way of example of the general principle that ports may be defined to open sequentially dependent on a range of parameters, including pressure or time.
- ports may be defined to open at different pressures, or ports may be defined to dissolve after different lengths of exposure.
Landscapes
- Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Vascular Medicine (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Public Health (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Surgery (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Pulmonology (AREA)
- External Artificial Organs (AREA)
- Surgical Instruments (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Description
- The present disclosure relates generally to medical treatment systems and, more particularly, but not by way of limitation, to systems, methods, and devices for the subcutaneous administration of reduced pressure that include reconfigurable lumens.
- Clinical studies and practice have shown that providing a reduced pressure in proximity to a tissue site augments and accelerates the growth of new tissue at the tissue site. The applications of this phenomenon are numerous, but application of reduced pressure has been particularly successful in treating wounds. This treatment (frequently referred to in the medical community as "negative pressure wound therapy," "reduced pressure therapy", or "vacuum therapy") provides a number of benefits, which may include faster healing and increased formulation of granulation tissue. Typically, when applied to open wounds, reduced pressure is applied to tissue through a porous pad or other manifold device. The porous pad contains cells or pores that are capable of distributing reduced pressure to the tissue and channeling fluids that are drawn from the tissue. When applied subcutaneously, often the reduced pressure is delivered through a manifold that includes channels and openings in a reduced-pressure delivery apparatus.
WO 2010/080667 A1 discloses systems, methods, and apparatus for delivering reduced pressure to a subcutaneous tissue site, such as a bone tissue site. In one instance, a manifold for providing reduced pressure to a subcutaneous tissue includes a longitudinal manifold body formed with at least one purging lumen and a reduced-pressure lumen. - The invention is defined by the appended claims. There is provided a multi-lumen applicator for delivering reduced pressure to a tissue site and receiving fluids, the multi-lumen applicator comprising an applicator body having a distal end and a proximal end and formed with a first plurality of apertures proximate to the distal end for receiving fluid and a second plurality of apertures proximate the distal end for receiving fluid from the tissue site and for delivering reduced pressure; a first lumen fluidly coupled to the first plurality of apertures, a second lumen fluid coupled to the second plurality of apertures, and a first plurality of activation members coupled to the second plurality of apertures, wherein the first plurality of activation members is operable to move from a closed position to an open position when activated to allow flow.
- There is also provided a multi-lumen applicator for delivering reduced pressure to a tissue site and receiving fluids, the multi-lumen applicator comprising, an applicator body having a distal end and a proximal end and formed with a first plurality of apertures for receiving fluid from the tissue site and a second plurality of apertures for receiving fluid from the tissue site and for delivering reduced pressure, a first lumen fluidly coupled to the first plurality of apertures, a second lumen fluidly coupled to the second plurality of apertures, wherein at least one of the second plurality of apertures comprises a first activation member configured to close the aperture in a first configuration and open the aperture in a second configuration.
- A selection of optional features is set out in the dependent claims.
-
-
FIGURE 1 is a schematic diagram, with a portion shown in cross section, of an illustrative embodiment of a system for providing reduced pressure to a subcutaneous tissue site and for removing fluids from the subcutaneous tissue site; -
FIGURE 2 is a schematic, perspective view of an illustrative embodiment of a multi-lumen applicator; -
FIGURE 3 is a longitudinal cross section of the multi-lumen applicator ofFIGURE 2 showing a distal end; -
FIGURE 4 is a lateral cross section of the multi-lumen applicator ofFIGURE 2 taken along line 4-4; -
FIGURES 5A-5C are schematic cross sections of an illustrative embodiment of a multi-lumen applicator that includes a first activation member shown in different states; -
FIGURE 6 is a schematic longitudinal cross section of two lumens that may be included in an illustrative embodiment of a multi-lumen applicator; -
FIGURE 7 is a schematic, plan view of an illustrative embodiment of a multi- lumen applicator for use as part of a system for providing reduced pressure to subcutaneous
tissue site and for removing fluids from the subcutaneous tissue site; -
FIGURE 8 is a schematic lateral cross section taken along line 8-8 of the multi- lumen applicator ofFIGURE 7 ; -
FIGURE 9 is a schematic, lateral cross section taken along line 9-9 inFIGURE 7 of the illustrative, non-limiting multi-lumen applicator; -
FIGURE 10 is a schematic, perspective view (with a portion shown in cross section) of another illustrative embodiment of a multi-lumen applicator for distributing reduced pressure that may be used as an aspect of a system for providing reduced pressure to a subcutaneous tissue site and for removing fluids from the subcutaneous tissue site; -
FIGURE 11 is a schematic diagram, with a portion shown in perspective view, of an illustrative embodiment of a system for delivering reduced pressure to a subcutaneous tissue site; -
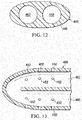
FIGURE 12 is a schematic, lateral cross section of the multi-lumen applicator ofFIGURE 11 taken along line 12-12; -
FIGURE 13 is a longitudinal cross section taken in part along line 13-13 of the multi-lumen applicator ofFIGURE 11 ; -
FIGURE 14 is a schematic diagram showing an illustrative embodiment of a process for a controller used as part of a system for delivering reduced pressure to a subcutaneous tissue site; -
FIGURE 15 is a schematic, longitudinal cross section of a distal portion of an illustrative embodiment of a multi-lumen applicator shown with a wire cleaning element; -
FIGURE 16 is a schematic, longitudinal cross section of a distal portion of an illustrative embodiment of a multi-lumen applicator shown with a elongated brush; -
FIGURE 17 is a schematic, longitudinal cross section of a distal portion of an illustrative embodiment of a multi-lumen applicator shown with a removable water jet; -
FIGURE 18 is a schematic, longitudinal cross section of a distal portion of an illustrative embodiment of a multi-lumen applicator shown with a purging implement; and -
FIGURE 19 is a schematic, longitudinal cross section of a distal portion of an illustrative embodiment of a multi-lumen applicator shown with a cytology brush. - In the following detailed description of the illustrative, non-limiting embodiments, reference is made to the accompanying drawings that form a part hereof. These embodiments are described in sufficient detail to enable those skilled in the art to practice the invention, and it is understood that other embodiments may be utilized and that logical structural, mechanical, electrical, and chemical changes may be made without departing from the spirit or scope of the invention. To avoid detail not necessary to enable those skilled in the art to practice the embodiments described herein, the description may omit certain information known to those skilled in the art. The following detailed description is, therefore, not to be taken in a limiting sense, and the scope of the illustrative embodiments are defined only by the appended claims.
- Providing reduced pressure to a subcutaneous tissue site may assist with removing fluids, e.g., ascites or exudates, or enhance tissue growth as an aspect of reduced pressure therapy. As used throughout this document, "or" does not require mutual exclusivity. In applying reduced pressure to a subcutaneous tissue site often a multi-lumen applicator is used. At times, blockage of lumens may occur and pose a problem to the ongoing treatment. According to an illustrative embodiment, the lumens may be reconfigured relative to the blockage in order to restore flow of reduced pressure to the subcutaneous tissue site or to remove the blockage. In other illustrative embodiments, the blockage may be removed using a blockage-removal device.
- Referring now to the drawings and initially to
FIGURE 1 , an illustrative embodiment of asystem 100 for providing reduced pressure to asubcutaneous tissue site 102 is presented. Thesubcutaneous tissue site 102 may be, for example, adefect 104 in or on a bone 106 (e.g., a fractured bone). Thesubcutaneous tissue site 102 may be any site that may benefit from treatment with reduced pressure to remove fluids or as an aspect of reduced pressure therapy. Thesubcutaneous tissue site 102 may be the bodily tissue of any human, animal, or other organism, including bone tissue, adipose tissue, muscle tissue, vascular tissue, connective tissue, cartilage, tendons, ligaments, or any other tissue. - The
system 100 includes amulti-lumen applicator 108 that is inserted into thepatient 110 and placed proximate to thesubcutaneous tissue site 102. In the illustrative non-limiting embodiment, themulti-lumen applicator 108 is shown having been inserted throughepidermis 112,dermis 114, and intosubcutaneous tissue 116. Themulti-lumen applicator 108 is positioned proximate to thesubcutaneous tissue site 102. - The
multi-lumen applicator 108 is fluidly coupled to a reduced-pressure delivery conduit 118, which may be a multi-lumen conduit that has lumens coordinated and fluidly coupled to the multiple lumens of themulti-lumen applicator 108. The reduced-pressure delivery conduit 118 may be fluidly coupled to aconnector 120 that may facilitate connecting multiple lumens to the multiple lumens of the reduced-pressure delivery conduit 118. - A reduced-
pressure source 122 is fluidly coupled by aconduit 124 to theconnector 120 to provide reduced pressure thereto. The reduced-pressure source 122 may include a reduced-pressure supply portion 126 and afluid reservoir 128. The reduced-pressure supply portion 126 may be a vacuum pump, wall suction, or any other source of reduced pressure. Thefluid reservoir 128 may provide a place to receive and retain fluids delivered from thepatient 110. - Reduced pressure is typically a pressure less than the ambient pressure at a tissue site that is being subjected to treatment. In most cases, this reduced pressure will be less than the atmospheric pressure at which the patient is located. Alternatively, the reduced pressure may be less than a hydrostatic pressure at the tissue site. Unless otherwise indicated, quantitative values of pressure stated herein are gauge pressures. The reduced pressure delivered may be constant or varied (patterned or random) and may be delivered continuously or intermittently. Although the terms "vacuum" and "negative pressure" may be used to describe the pressure applied to the tissue site, the actual pressure applied to the tissue site may be more than the pressure normally associated with a complete vacuum. Consistent with the use herein, an increase in reduced pressure or vacuum pressure typically refers to a relative reduction in absolute pressure. For example, going from -50 mm Hg to -100 mm Hg may be referred to as an increase in reduced pressure, but on an absolute pressure scale it is a decrease in pressure.
- A
purging unit 130 may be fluidly coupled by aconduit 132 to theconnector 120. Thepurging unit 130 may provide atmospheric air or another purging gas or pressurized gas to themulti-lumen applicator 108 in order to avoid or remove blockages therein. The purge gas provided by thepurging unit 130 may be at an elevated pressure with respect to atmosphere or relative to the operational pressure of thesystem 100. - A
liquid source 134 may be fluidly coupled by aconduit 136 to theconnector 120. Theliquid source 134 may be used to provide a liquid purge to themulti-lumen applicator 108 or may be used to provide a treatment liquid, or therapeutic liquid, to themulti-lumen applicator 108 and ultimately to thesubcutaneous tissue site 102. - A
controller 138 may be coupled by couplinglines pressure source 122, purgingunit 130, andliquid source 134, respectively. Thecontroller 138 may include a microprocessor, memory, and other components for providing control to the reduced-pressure source 122, purgingunit 130, andliquid supply 134. Thecontroller 138 may also be coupled to theconnector 120 to control valves within theconnector 120 as shown in the illustrative embodiment ofFIGURE 11 . - Referring now primarily to
FIGURES 2-4 , an illustrative embodiment of amulti-lumen applicator 108 is presented. Themulti-lumen applicator 108, or manifold, is formed with anapplicator body 146, which has afirst side 148 and a second, tissue-facingside 150. Themulti-lumen applicator 108 may be formed by injection molding or other techniques. Themulti-lumen applicator 108 may also be extruded into parts and then bonded or otherwise coupled to form an integral unit. Alternatively, themulti-lumen applicator 108 may be extruded and then undergo a secondary controller melt "tipping" process to form an integral unit. Themulti-lumen applicator 108 may be made from a flexible or semi-rigid material. For example, themulti-lumen applicator 108 may be made from any medical-grade polymer, such as polyurethane. In one embodiment, themulti-lumen applicator 108 is made from a material with a stiffness of approximately 80 Shore A, but other stiffnesses may be used. A coating may be added to themulti-lumen applicator 108 to avoid material buildup on themulti-lumen applicator 108. - A plurality of
apertures 152 are formed on the second, tissue-facingside 150 of theapplicator body 146 for providing reduced pressure to thesubcutaneous tissue site 102. While theapertures 152 are shown in a symmetrically spaced pattern, it should be understood that theapertures 152 may be formed with any pattern or with a random placement. A plurality of manifold surface features 154 may be formed on the second, tissue-facingside 150. The plurality of manifold surface features 154 may include a plurality of standoffs or offsets 156. The plurality ofoffsets 156 may be formed integrally with or coupled to the second, tissue-facingside 150 of theapplicator body 146. Theoffsets 156 may be any surface feature creating effective flow channels between the second, tissue-facingside 150 and the tissue site. The manifold surface features 154 may detach from theapplicator body 146 when themulti-lumen applicator 108 is percutaneously removed, and the manifold surface features 154 may be bioresorbable. - The plurality of
apertures 152 are fluidly coupled to thefirst lumen 158 that is formed in theapplicator body 146. Thefirst lumen 158 may be fluidly coupled to theapertures 152 by a plurality ofconduits 160. Thefirst lumen 158 extends the longitudinal length of theapplicator body 146. Thefirst lumen 158 may initially be used as an evacuation lumen to deliver reduced pressure to the plurality ofapertures 152 and to receive and transport fluids from thesubcutaneous tissue site 102. - The
applicator body 146 is also formed with asecond lumen 162 and may have athird lumen 164 or even more lumens. Thesecond lumen 162 andthird lumen 164 also extend the longitudinal length of theapplicator body 146. Thesecond lumen 162 andthird lumen 164 may initially be used as purge lumens, or vent lumens. While this illustrative embodiment shows two purge lumens, it should be understood that any number of purge lumens may be used. Additionally, thesecond lumen 162 andthird lumen 164 are shown symmetrically spaced about thefirst lumen 158, and while the symmetric orientation may enhance performance, other orientations may be used. Additional lumens, such as a pressure sensing lumen (not explicitly shown), may be included within theapplicator body 146. The purge lumens may also serve as pressure sensing lumens. It should be noted that although a slightly elliptical or triangular shape is presented, the cross sectional shape of theapplicator body 146 may be any of those previously mentioned or even irregular or other shapes. - On the
distal end 166 of theapplicator body 146, anend cap 168 is formed or coupled. Theend cap 168 is formed with aheader space 170 that allows thesecond lumen 162 and the third lumen 164 (and any additional lumens) to be fluidly coupled to thefirst lumen 158. Theend cap 168 is formed integrally to or as part of theapplicator body 146 and, thus, avoids the risk of theend cap 168 becoming dislodged during removal from thepatient 110. At a proximal end 172 (FIG. 2 ) of theapplicator body 146, a connecting element, orconnector 174, may be coupled to provide easy connection with the reduced-pressure delivery conduit 118, which in turn is fluidly coupled to the reduced-pressure source 122 and also to thepurging unit 130 orliquid source 134. - Referring now to
FIGURES 2-5C , themulti-lumen applicator 108 has provisions to reconfigure lumens, e.g., thefirst lumen 158,second lumen 162, andthird lumen 164, in order to restore flow through theapertures 152. In this illustrative embodiment, afirst port 176 is formed between thefirst lumen 158 and thesecond lumen 162. Port typically refers to an open flow path either between two lumens or a lumen and an exterior of themulti-lumen applicator 108. Thefirst port 176 fluidly couples thefirst lumen 158 and thesecond lumen 162 when afirst activation member 178 is in an open, or activated, position. Thefirst activation member 178 may be any member that provides a closed position in one state and an open position in another state that allows flow. For example, thefirst activation member 180 may be a firstfrangible member 180 as shown inFIGURE 3 in the closed position. The term "frangible" is used generally to indicate a material that fails, ruptures, tears, or dissolves in a predictable manner. The frangible material fails, ruptures, tears, or dissolves in a repeatable manner between devices. - The first frangible member 180 (and other frangible members) may be a frangible disc or frangible port cover, which may be a piece of material covering a port that is designed to rupture or open at a first threshold pressure differential or to dissolve and thereby open after being exposed to a liquid for at least a threshold time. In the embodiment where the
activation member 178 dissolves in the presence of a liquid (body fluids or a supplied liquid), theactivation member 178 may be formed from a polylactic acid (PLA), polyglycolic acid (PGA), polyactic co-glycolic acid (PLGA), hydrogel, or cross-linked or hardened gelatine, or other suitable material. In other embodiments, thefirst activation member 178 may be a valve with a remote attachment, e.g., a line, that can be pulled to open thefirst activation member 178. In another illustrative embodiment, thefirst activation member 178 may be a plug in a port that under pressure is released from the port and is removed. - A
second port 182 is formed between thefirst lumen 158 and thethird lumen 164. Thesecond port 182 fluidly couples thefirst lumen 158 and thethird lumen 164 when asecond activation member 184 is in an open position. Thesecond activation member 184 may be a secondfrangible member 186 or other device analogous to those mentioned forfirst activation member 180. - In operation, the
multi-lumen applicator 108 may be inserted surgically or using minimally invasive surgery into the patient. Typically, themulti-lumen applicator 108 is removed percutaneously after use or in one embodiment may be bio-absorbable and left in place to absorb. In one illustrative embodiment in which it is desirable to provide reduced-pressure treatment with themulti-lumen applicator 108 for an extended period of time, e.g., 24 hours, themulti-lumen applicator 108 addresses blocks or the possibility of blocks by reconfiguring lumens during use. Thus, after themulti-lumen applicator 108 is inserted, and reduced pressure is supplied to thesubcutaneous tissue site 102, flow may continue for a first period of time. After this first period of time, which may be a preset time or may be when a blockage occurs, thefirst activation member 178 may be activated to open a flow path that reconfigures flow by reconfiguring thelumens system 100 will continue to operate with flow for the desired time duration. A number of illustrative, non-limiting examples of how lumens may be reconfigured or how blockages may be removed will be presented. - Referring now primarily to
FIGURES 5A - 5C , an illustrative embodiment of amulti-lumen applicator 108 is presented to show how lumens, e.g.,lumens multi-lumen applicator 108 is shown with only two lumens: afirst lumen 158 and asecond lumen 162. It should be understood that other lumens or additional lumens may be involved. - The
multi-lumen applicator 108 inFIGURE 5A shows an initial state in which reduced pressure is delivered to thefirst lumen 158 thereby causing aflow 188 of fluid in an ante grade direction. Thefirst lumen 158 in this initial condition serves as an evacuation lumen. Themulti-lumen applicator 108 also includes thesecond lumen 162 that initially serves as a purge lumen providing a purge fluid, such as air, to thefirst lumen 158 to inhibit blocking or eliminate blocking. The purge fluid may be provided on a periodic basis. It should be appreciated that thesecond lumen 162 provides a purging fluid that travels through ahead space 170 at adistal end 166 to avoid blockages. In this initial state, reduced pressure is distributed to a plurality ofapertures 152 in anapplicator body 146. Fluid, e.g., wound effluent, is pulled from the tissue site (not shown), e.g., thesubcutaneous tissue site 102 inFIGURE 1 , into theapertures 152 and along thefirst lumen 158 to a fluid reservoir (not shown), e.g., thefluid reservoir 128 inFIGURE 1 . Normal operation may involve, for example, and not by way of limitation, a reduced pressure in the range of -100 mm Hg (-13.3 kPa) to -200 mm Hg (-26.6 kPa). - A
first port 176 may be formed as an aspect of awall 159 between thefirst lumen 158 andsecond lumen 162. Thefirst port 176 is controlled by afirst activation member 178 that has a closed position as shown inFIGURES 5A-B and an open position as shown inFIGURE 5C . Thefirst activation member 178 assumes the open position when activated. Thefirst activation member 178 may be, for example, a firstfrangible member 180. - Referring now primarily to
FIGURE 5B , after sufficient time, ablockage 190 may result within thefirst lumen 158. Theblockage 190 may inhibit or completely stop flow within thefirst lumen 158 and thereby inhibit or stop flow of fluids from the tissue site through theapertures 152. When a controller or detection device or an operator determines that a blockage, e.g., theblockage 190, has occurred, a increased pressure, e.g., -300 mm Hg (-39.9 kPa) or -350 mm Hg (-46.6 kPa) may be applied to activate thefirst activation member 178 and in this embodiment to rupture the firstfrangible member 180. After thefirst activation member 178 is activated, the lumens are reconfigured with respect to flow in portions and flow may begin to occur as shown inFIGURE 5C . Thefirst activation member 178 may also be activated by exposure to liquid or by removal of a remote line (not shown) that activates a valve or opens theactivation member 178. - Referring to
FIGURES 5A-5C and primarily toFIGURE 5C , thefirst activation member 178, which in this embodiment is a firstfrangible member 180, has been activated such that thefirst port 176 is in an open position. Thus, as reduced pressure is applied to thefirst lumen 158, fluid flows through theaperture 152 from the tissue site, traverses thehead space 170, and flows through a portion of thesecond lumen 162, through thefirst port 176 as shown, and then continues through thefirst lumen 158 where flow may be received by a fluid reservoir, such as thefluid reservoir 128 inFIGURE 1 . Thus, the reconfiguring of at least a portion of thefirst lumen 158 andsecond lumen 162 allows flow to continue notwithstanding theblockage 190. - While only one
port 176 with afirst activation member 178 is shown inFIGURES 5A-5C , it should be understood that multiple ports and activation members may be provided along the length of themulti-lumen applicator 108. For example, as shown inFIGURE 6 , afirst port 176 is covered by afirst activation member 178, such as a firstfrangible member 180 and asecond port 192 is shown with asecond activation member 194. The firstfrangible member 180 is disposed between thefirst lumen 158 and thesecond lumen 162. The firstfrangible member 180 is configured to rupture when exposed to a pressure greater than the first threshold pressure differential, whereby at least a portion of thesecond lumen 162 and a portion of thefirst lumen 158 are fluidly coupled. In addition, thesecond port 192 is shown with theactivation member 194, such as an additionalfrangible member 196. Still anotherport 198 is shown in an open position. - In operation of the illustrative embodiment of
FIGURE 6 , an initial flow is established through theport 198 until a blockage occurs, and then a first threshold pressure differential, e.g., -300 mm Hg, is used on thefirst activation member 178 to move thefirst activation member 178 to an open position. Activating thefirst activation member 178 causes flow to go through thefirst port 176. Later, if another blockage occurs, a second threshold pressure differential, e.g., -350 mm Hg, may be used to activate theadditional activation member 196 and thereby open theadditional port 192 to provide another reconfigured flow path through the lumens. As before, the activation of theactivation members - Referring now primarily to
FIGURES 7 - 8 , another illustrative embodiment of amulti-lumen applicator 108 is presented. Themulti-lumen applicator 108 has adistal end 166 and aproximal end 172. A plurality ofapertures 152 may be formed near thedistal end 166 for providing reduced pressure to a tissue site, e.g.,subcutaneous tissue site 102 inFIGURE 1 . Aconnector 174 may be used to connect a reduced-pressure delivery conduit 118 to themulti-lumen applicator 108. In one illustrative embodiment, three lumens, which are shown in cross section inFIGURE 8 , e.g., afirst lumen 158, asecond lumen 162, and athird lumen 200, may be placed around afourth lumen 202. In this illustrative embodiment, in the initial state, only thefirst lumen 158 is active, or open, for removing fluids from the tissue site and is in fluid communication with thefourth lumen 202. Thefourth lumen 202 is initially a purging lumen. - The
first lumen 158 has afirst port 176, which is discrete, that fluidly couples the first lumen to thefourth lumen 202. Thesecond lumen 162 has asecond port 177, which is discrete, that fluidly couples the second lumen to thefourth lumen 202. Thethird lumen 200 has athird port 179, which is discrete, that fluidly couples thethird lumen 200 to thefourth lumen 202. In this illustrative embodiment, thefirst port 176 is initially in an open position. Thesecond port 177 is initially closed by afirst activation member 178, e.g., a firstfrangible member 180. Thethird port 179 is initially closed by asecond activation member 184, e.g., a second frangible member. - After a specified period of time or when it is determined that a blockage exists, a pressure is applied to the
lumens first activation member 178 may be activated. Alternately, this activating pressure can be applied tolumen 202 to create an activation pressure differential. For example, if thefirst activation member 178 is the firstfrangible member 180, the firstfrangible member 180 may be subjected to a pressure differential greater than the first threshold pressure differential, e.g., -300 mm Hg, such that the firstfrangible member 180 ruptures. The ruptured firstfrangible member 180 provides fluid communication between thesecond lumen 162 and thefourth lumen 202. At this point, fluids from the tissue site flow from theapertures 152 through thesecond lumen 162 while thefourth lumen 202 acts as a purging lumen. - When sufficient time has passed or when a blockage exists in the
second lumen 162, thesecond activation member 184 may be activated to allow fluid communication between thethird lumen 200 and thefourth lumen 202. In this way, additional flow may go from theapertures 152 through thethird lumen 200 to a fluid reservoir. The use of theactivation members FIGURE 8 may simplify the connector and reduce-pressure source design since the inactive lumens (e.g., initiallylumens 162, 200) can be exposed to the reduced pressure. - Referring now primarily to
FIGURE 9 , another illustrative embodiment of a portion of amulti-lumen applicator 108 is presented. Themulti-lumen applicator 108 ofFIGURE 9 is analogous in most respects to themulti-lumen applicator 108 ofFIGURE 7 , and accordingly, some parts are labeled the same but not further described here. It should be noted, however, that while the section line 9-9 is shown inFIGURE 7 , this embodiment is nonetheless distinct from that described above in connection withFIGURES 7 and8 in a number of respects. In this embodiment, themulti-lumen applicator 108 is formed with anapplicator body 146 having afirst lumen 158,second lumen 162,third lumen 200, and afourth lumen 202. Thelumens fourth lumen 202 and each lumen has at least one aperture or port of a plurality ofapertures 152 that provides access to an exterior of themulti-lumen applicator 108. For example, in this illustrative embodiment, afirst port 176 is formed between thefirst lumen 158 and thefourth lumen 202. In this instance, in the initial state, fluids are drawn through the portion ofapertures 152 associated with thefirst lumen 158 until a blockage occurs. Then pressure, i.e., the pressure differential, may be increased to activate afirst activation member 178 or plurality of first activation members that cover a portion ofapertures 152 associated with thesecond lumen 162. Thus, thesecond lumen 162 begins to serve as an evacuation lumen for liquids from the tissue site. - When the
second lumen 162 becomes blocked, the pressure may be increased to activate asecond activation member 184 or plurality of first activation members that cover a portion ofapertures 152 associated with thethird lumen 200. Thus, thethird lumen 200 begins to serve as an evacuation lumen for liquids from the tissue site. Thefourth lumen 202 serves as a purge lumen for each of thelumens - Throughout this document, activation members are referenced and typically discussed in the context of frangible members. It should be understood that the activation members may be activated by pressure exceeding a threshold pressure differential or by mere passage of time with the activation members exposed to a fluid. Thus, for example, after a first threshold time period, the first activation member may dissolve to the point that the first activation member ruptures or otherwise allows fluid flow. In another illustrative embodiment, the activation members may be activated by pulling a line that removes a plug or opens a valve, or any other technique to open the port in situ.
- As previously noted, the activation members, e.g.,
activation members - Referring now primarily to
FIGURE 10 , another illustrative embodiment of a portion of amulti-lumen applicator 308 is presented. Themulti-lumen applicator 308 includes anapplicator body 346 formed with a plurality ofapertures 352. Themulti-lumen applicator 308 includes afirst lumen 358, asecond lumen 362, athird lumen 364, and afourth lumen 301. Themulti-lumen applicator 308 is shown in the initial state in which thefirst lumen 358 serves as an evacuation lumen and thesecond lumen 362 serves as a vent or purge lumen. Thus, fluids from the tissue site are drawn through at least a portion of the plurality ofapertures 352, into thefirst lumen 358, and moved from thedistal end 366 to theproximal end 372 where the fluids are delivered into a fluid reservoir. - The
third lumen 364 andfourth lumen 301 are initially filled byfilaments third lumen 364 is initially filled by thefirst filament 365, and thefourth lumen 301 is filled by asecond filament 399. Eachfilament - In operation, the
first lumen 358 is initially used to deliver reduced pressure and remove fluids through at least a portion of theapertures 352. When thefirst lumen 358 is blocked or sufficient time has passed, thefirst filament 365 may be removed from the third lumen by pulling thefirst filament 365 out from theproximal end 372. Removing thefirst filament 365 opens the third lumen 364-including a port to thesecond lumen 362 allowing removal of fluids fromapertures 352. Similarly, when thethird lumen 364 is blocked or a sufficient amount of time has passed, thesecond filament 399 may be removed from thefourth lumen 301 to provide flow through thefourth lumen 301. - Referring now primarily to
FIGURES 11-13 , another illustrative embodiment of asystem 400 for providing reduced pressure to a subcutaneous tissue site (and removing fluids therefrom) is presented. Thesystem 400 includes amulti-lumen applicator 408. Themulti-lumen applicator 408 is formed with anapplicator body 446 that includes a plurality ofapertures 452. Themulti-lumen applicator 408 includes at least afirst lumen 458 and asecond lumen 462. Thefirst lumen 458 may be selectively, fluidly coupled by aconnector 420 to a reduced-pressure source 422 or apurge unit 430. Theconnector 420 is only one example, of how thelumens second lumen 462 may be selectively, fluidly coupled to thepurge unit 430 or the reduced-pressure source 422. - A
controller 438 may be coupled by couplinglines pressure source 422 and thepurge unit 430, respectively. Thecontroller 438 may be coupled by additional coupling lines to afirst valve 502,second valve 504,third valve 506, andfourth valve 508 in theconnector 420. Thecontroller 438 may control the reduced-pressure source 422,purge unit 430, and valves. Theconnector 420 may be under the control of thecontroller 438 and function to switch the functionality of thefirst lumen 458 and thesecond lumen 462. - After the
first lumen 458 enters theconnector 420 from themulti-lumen applicator 408, thelumen 458 may divide into a first sub-lumen 510 and asecond sub-lumen 512. The first sub-lumen 510 couples thefirst lumen 458 to the reduced-pressure source 422. Thefirst valve 502 is located on thefirst sub-lumen 510. The second sub-lumen 512 couples thefirst lumen 458 to thepurge unit 430. Thesecond sub-lumen 512 includes thethird valve 506. In the initial state, thethird valve 506 is closed and thefirst valve 502 is opened such that reduced pressure is supplied to thefirst lumen 458. - In a similar fashion, the
second lumen 462 is divided within theconnector 420 between a third sub-lumen 514 and afourth sub-lumen 516. The third sub-lumen 514 fluidly couples thesecond lumen 462 to thepurge unit 430. The fourth sub-lumen 516 fluidly couples thesecond lumen 462 to the reduced-pressure source 422. Thefourth valve 508 is located on the third sub-lumen 514 and selectively controls fluid flow therein, and thesecond valve 504 is located in thefourth sub-lumen 516 and selectively controls flow therein. - In the initial state, the
second lumen 462 serves as a purge lumen, and thus, thesecond valve 504 on the fourth sub-lumen 516 is closed and thefourth valve 508 is open on thethird sub-lumen 514. When a blockage is determined bycontroller 438, a specified time has passed, or upon receiving manual instructions, thecontroller 438 will reconfigure thevalves lumens first valve 502 on first sub-lumen 510 is closed and thethird valve 506 on the second sub-lumen is opened. Additionally, thesecond valve 504 on the fourth sub-lumen 516 is opened and thefourth valve 508 on the third sub-lumen 514 is closed. Thus, in reconfigured position, thesecond lumen 462 becomes the evacuation lumen and thefirst lumen 458 becomes the purge lumen. This condition may be maintained or may only be temporarily assumed in order to remove the blockage in thefirst lumen 458. - Often reversing the flow for a period of time removes the blockage. Thus, it may be possible to return to the initial state and have the lumens function in that state again. Alternatively or in addition, an incompressible purging fluid, e.g., sterile saline, may be used in the retrograde direction of the
first lumen 458 to remove the blockage. With respect toFIGURE 11 , reconfiguring thelumens lumens - Referring now to
FIGURES 11 and14 and primarily toFIGURE 14 , one illustrative, non-limiting logic flow for thecontroller 438 in controlling the functionality of thelumens first interrogation box 522 where the question is asked, "Is the first lumen blocked?" If the answered is in the negative, the process returns again to thefirst interrogation box 522. If the answer is in the affirmative, the process continues to processbox 524 and instructions are provided for thesecond lumen 462 to be coupled to the reduced-pressure source 422 and thefirst lumen 458 to be coupled to thepurge unit 430. This may be accomplished with the specific instructions sent to the valves, e.g.,first valve 502 closed,second valve 504 open,third valve 506 open, andfourth valve 508 closed. - After resetting the
valves lumens interrogation box 526 is reached and the question is asked, "Is the second lumen blocked?" If the answer is in the negative, the process returns again to thesecond interrogation box 526. If in the affirmative, theprocess box 528 is reached. Theprocess block 528 provides instructions for thefirst lumen 458 to be coupled to the reduced-pressure source 422 and thesecond lumen 462 to be coupled to thepurge unit 430. In other words, the flow returns to the initial state which may now flow again since operation in the second state may remove blockages. After theprocess block 528, thethird interrogation box 530 is reached and asks the question, "Is the first lumen blocked?" If the answer is negative, the process continues to thefirst interrogation box 522. If the answer to thethird interrogation box 530 is in the affirmative, an alarm is activated atprocess block 531, and the process ends atstep 532. This process is only one illustrative way of programming thecontroller 438. - In addition or in lieu of reconfiguring the flow of lumens, a blockage may be managed by removing the blockage. Referring now to
FIGURES 15-19 , a number of techniques for removing blockages from within the lumens in order to provide continued flow are presented. In these figures, a portion of amulti-lumen applicator 608 is presented. A plurality ofapertures 652 are formed on thedistal end 666 of anapplicator body 646. Afirst lumen 658 is formed within theapplicator body 646 as well as at least asecond lumen 662. When a blockage exists, is suspected, or after set time period elapses, a blockage-removal device, e.g., a blockage-removal member 617, an elongated brush member 61.9, afluid jet 621, or apurging element 623, is inserted into themulti-lumen applicator 608 and, the blockage-removal device is activated, which means the blockage-removal device may be removed, rotated, energized, or otherwise enabled to provide a blockage removing force within the lumen. - Referring now primarily to
FIGURE 15 , the blockage-removal member 617, such as an auger, Archimedes screw, or tanglement wire, is inserted into thefirst lumen 658 and rotated. For example, the blockage-removal member 617 may be rotated within thefirst lumen 658 to break a blockage free and help move any material with the flow toward the proximal end. The rotation may be at various speeds, e.g., slow rotation of 1 to 20 rpm. The blockage-removal member 617 remains within thefirst lumen 658 as the blockage-removal member 617 is rotated or may be slowly removed. - Similarly, referring primarily to
FIGURE 16 , theelongated brush member 619 disposed within thefirst lumen 658 is shown. Theelongated brush member 619 may be rotated to remove items causing a block or inhibit flow. Theelongated brush member 619 may be rotated at, for example, a relatively higher RPM. - Referring now primarily to
FIGURE 17 , thefluid jet 621 is disposed within thefirst lumen 658 and removed from thefirst lumen 658 at the proximal end. As thefluid jet 621 is removed, water jets, which are facing the proximal end, remove any blockage. The volume of water or other purging liquid (e.g., saline) placed into the lumen will be matched with the evacuation capacity of the system in order to avoid fluid infusion into the patient. - Referring now primarily to
FIGURE 18 , the purgingelement 623 may be pulled from a distal end of thefirst lumen 658 to a proximal end to remove any blockages in thefirst lumen 658. The purgingelement 623 ordevice 623 may be an inflatable member that after being located at the distal end may be inflated. For example, the purgingelement 623 may be analogous to a Fogerty catheter style device. Similarly, with reference primarily toFIGURE 19 , acytology brush 625 may be pulled from thefirst lumen 658 to remove any blockages therein. - The embodiments of
FIGURES 15-19 may optionally incorporate combined aspects of rotation and axial translation as part of the activation. The blockage-removal device may be in place when theapplicator body 646 is placed into the wound. The blockage-removal device may be a single use item as it is removed from theapplicator body 646. - Although the present invention and its advantages have been disclosed in the context of certain illustrative, non-limiting embodiments, it should be understood that various changes, substitutions, permutations, and alterations can be made without departing from the scope of the invention as defined by the appended claims. It will be appreciated that any feature that is described in connection to any one embodiment may also be applicable to any other embodiment.
- As will be appreciated, the various examples of ports opening at different times are given by way of example of the general principle that ports may be defined to open sequentially dependent on a range of parameters, including pressure or time. For example, ports may be defined to open at different pressures, or ports may be defined to dissolve after different lengths of exposure.
- It will be understood that the benefits and advantages described above may relate to one embodiment or may relate to several embodiments. It will further be understood that reference to "an" item refers to one or more of those items.
- The steps of the methods described herein may be carried out in any suitable order, or simultaneously where appropriate.
- Where appropriate, aspects of any of the embodiments described above may be combined with aspects of any of the other embodiments described to form further examples having comparable or different properties and addressing the same or different problems.
- It will be understood that the above description of preferred embodiments is given by way of example only and that various modifications may be made by those skilled in the art. The above specification, examples and data provide a complete description of the structure and use of exemplary embodiments of the invention. Although various embodiments of the invention have been described above with a certain degree of particularity, or with reference to one or more individual embodiments, those skilled in the art could make numerous alterations to the disclosed embodiments without departing from the scope of the claims.
Claims (15)
- A multi-lumen applicator (108) for delivering reduced pressure to a tissue site (102) and receiving fluids, characterised by
the multi-lumen applicator (108)comprising:an applicator body (146) having a distal end (166) and a proximal end (172) and formed with a first plurality of apertures (152) proximate to the distal end (166) for receiving fluid and a second plurality of apertures (176) proximate the distal end (166) for receiving fluid from the tissue site (102) and for delivering reduced pressure;a first lumen (158) fluidly coupled to the first plurality of apertures (152);a second lumen (162) fluidly coupled to the second plurality of apertures (176); anda first plurality of activation members (178) coupled to the second plurality of apertures (176), wherein the first plurality of activation members (178) is operable to move from a closed position to an open position when activated to allow flow. - The multi-lumen applicator (108) of claim 1, wherein the first plurality of activation members (178) comprise a plurality of frangible members (180) and wherein the frangible members (180) rupture when exposed to a pressure differential greater than a threshold pressure differential, whereby the second plurality of apertures (176) are operable to receive liquids.
- The multi-lumen applicator (108) of claim 1, wherein the first plurality of activation members (178) comprise a plurality of frangible members (180) and wherein the frangible members (180) rupture when exposed to a fluid greater than a threshold time, whereby the second plurality of apertures (176) are operable to receive fluids from the tissue site (102).
- The multi-lumen applicator (108) of claim 1, wherein the first plurality of activation members (178) comprise a plurality of frangible members (180) and wherein the frangible members (180) rupture when exposed to a liquid greater than a threshold time, whereby the second plurality of apertures (176) are operable to receive fluids from the tissue site (102).
- A multi-lumen applicator (308) for delivering reduced pressure to a tissue site (102) and receiving fluids, characterised by
the multi-lumen applicator (308) comprising:an applicator body (346) having a distal end (366) and a proximal end (372) and formed with a first plurality of apertures (352) for receiving fluid from the tissue site (102) and a second plurality of apertures for receiving fluid from the tissue site (102) and for delivering reduced pressure;a first lumen (358) fluidly coupled to the first plurality of apertures (352);a second lumen (362) fluidly coupled to the second plurality of apertures;wherein at least one of the second plurality of apertures comprises a first activation member (178) configured to close the aperture in a first configuration and open the aperture in a second configuration. - The multi-lumen applicator (308) of claim 5, further comprising a third lumen (364) fluidly coupled to a third plurality of apertures in the applicator body (346) for receiving fluid from the tissue site (102), at least one of the third plurality of apertures comprises a second activation member (184) configured to close the aperture in a first configuration and open the aperture in a second configuration.
- The multi-lumen applicator (308) of claim 5, wherein the first activation member (178) comprises a first frangible member (180) that ruptures when activated, the first configuration being with the member (180) intact, the second configuration being with the member (180) ruptured.
- The multi-lumen applicator (308) of claim 6, wherein the second activation member (184) comprises a second frangible member (186) that ruptures when activated, the first configuration being with the member (186) intact, the second configuration being with the member (186) ruptured.
- The multi-lumen applicator (308) of claim 7, wherein the first frangible member (180) is ruptured when a pressure differential between first and second sides of the first frangible member (180) exceeds a first threshold pressure differential.
- The multi-lumen applicator (308) of claim 8 or 9, wherein the second frangible member (186) is ruptured when a pressure differential between first and second sides of the second frangible member (186) exceeds a second threshold pressure differential.
- The multi-lumen applicator (308) of claim 10 wherein the second threshold pressure differential is greater than the first threshold pressure differential.
- The multi-lumen applicator (308) of claim 5 or 8 wherein the first activation member (178) is converted from the first configuration to the second configuration when exposed to liquid for greater than a first threshold time.
- The multi-lumen applicator (308)of claim 5 or 12 wherein the second activation member (184) is converted from the first configuration to the second configuration when exposed to liquid for greater than a second threshold time.
- The multi-lumen application (308) of claim 13 wherein the second threshold time is greater than the first threshold time.
- The multi-lumen applicator (308) of claim 12 or 13, wherein the first (178) and/or second (184) activation member is formed of a polylactic acid (PLA), polyglycolic acid (PGA), polyactic co-glycolic acid (PLGA), hydrogel, or cross-linked or hardened gelatine.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41471110P | 2010-11-17 | 2010-11-17 | |
| EP11784905.9A EP2640437B1 (en) | 2010-11-17 | 2011-11-09 | Systems for subcutaneous administration of reduced pressure employing reconfigurable lumens |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP11784905.9A Division EP2640437B1 (en) | 2010-11-17 | 2011-11-09 | Systems for subcutaneous administration of reduced pressure employing reconfigurable lumens |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3090765A1 EP3090765A1 (en) | 2016-11-09 |
| EP3090765B1 true EP3090765B1 (en) | 2018-02-28 |
Family
ID=44993952
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP11784905.9A Active EP2640437B1 (en) | 2010-11-17 | 2011-11-09 | Systems for subcutaneous administration of reduced pressure employing reconfigurable lumens |
| EP16164752.4A Active EP3090765B1 (en) | 2010-11-17 | 2011-11-09 | Systems for subcutaneous administration of reduced pressure employing reconfigurable lumens |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP11784905.9A Active EP2640437B1 (en) | 2010-11-17 | 2011-11-09 | Systems for subcutaneous administration of reduced pressure employing reconfigurable lumens |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US9180233B2 (en) |
| EP (2) | EP2640437B1 (en) |
| JP (1) | JP6162605B2 (en) |
| CN (1) | CN103189081B (en) |
| AU (1) | AU2011329278B2 (en) |
| CA (1) | CA2814867C (en) |
| TW (1) | TW201223568A (en) |
| WO (1) | WO2012067921A2 (en) |
Families Citing this family (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2814657A1 (en) | 2010-10-12 | 2012-04-19 | Kevin J. Tanis | Medical device |
| AU2013264938B2 (en) | 2012-05-22 | 2017-11-23 | Smith & Nephew Plc | Apparatuses and methods for wound therapy |
| EP2928515B1 (en) | 2012-12-06 | 2018-07-18 | IC Surgical, Inc. | Adaptable wound drainage system |
| US9737649B2 (en) | 2013-03-14 | 2017-08-22 | Smith & Nephew, Inc. | Systems and methods for applying reduced pressure therapy |
| USD764654S1 (en) | 2014-03-13 | 2016-08-23 | Smith & Nephew, Inc. | Canister for collecting wound exudate |
| CA2902634C (en) | 2013-03-14 | 2023-01-10 | Smith & Nephew Inc. | Systems and methods for applying reduced pressure therapy |
| PT2968731T (en) * | 2013-03-15 | 2019-01-31 | Childrens Medical Center | Shunt flusher |
| US10155070B2 (en) | 2013-08-13 | 2018-12-18 | Smith & Nephew, Inc. | Systems and methods for applying reduced pressure therapy |
| USD764047S1 (en) | 2014-05-28 | 2016-08-16 | Smith & Nephew, Inc. | Therapy unit assembly |
| USD764653S1 (en) | 2014-05-28 | 2016-08-23 | Smith & Nephew, Inc. | Canister for collecting wound exudate |
| USD764048S1 (en) | 2014-05-28 | 2016-08-16 | Smith & Nephew, Inc. | Device for applying negative pressure to a wound |
| USD765830S1 (en) | 2014-06-02 | 2016-09-06 | Smith & Nephew, Inc. | Therapy unit assembly |
| USD770173S1 (en) | 2014-06-02 | 2016-11-01 | Smith & Nephew, Inc. | Bag |
| CA3179001A1 (en) | 2014-07-31 | 2016-02-04 | Smith & Nephew, Inc. | Systems and methods for applying reduced pressure therapy |
| US10549016B2 (en) | 2014-12-30 | 2020-02-04 | Smith & Nephew, Inc. | Blockage detection in reduced pressure therapy |
| JP6942698B2 (en) | 2015-10-07 | 2021-09-29 | スミス アンド ネフュー インコーポレイテッド | Systems and methods for performing decompression therapy |
| CA3014354A1 (en) | 2016-02-12 | 2017-08-17 | Smith & Nephew, Inc. | Systems and methods for detecting operational conditions of reduced pressure therapy |
| CN109069713A (en) | 2016-05-13 | 2018-12-21 | 史密夫和内修有限公司 | Automatic wound in negative pressure wound treating system couples detection |
| JP7063887B2 (en) | 2016-09-29 | 2022-05-09 | スミス アンド ネフュー インコーポレイテッド | Construction and protection of components in negative pressure wound healing systems |
| USD835648S1 (en) | 2016-10-27 | 2018-12-11 | Smith & Nephew, Inc. | Display screen or portion thereof with a graphical user interface for a therapy device |
| WO2018165049A1 (en) | 2017-03-07 | 2018-09-13 | Smith & Nephew, Inc. | Reduced pressure therapy systems and methods including an antenna |
| US10918769B2 (en) * | 2017-04-03 | 2021-02-16 | Boehringer Technologie, LP | Medical drainage device with squeegee-based lumen cleaner and method of draining a biological fluid from the body of a patient |
| WO2018195101A1 (en) | 2017-04-19 | 2018-10-25 | Smith & Nephew, Inc. | Negative pressure wound therapy canisters |
| US11712508B2 (en) | 2017-07-10 | 2023-08-01 | Smith & Nephew, Inc. | Systems and methods for directly interacting with communications module of wound therapy apparatus |
| CN112040915B (en) | 2018-03-26 | 2022-04-01 | 帝皇工业有限公司 | Multi-lumen bridge for negative pressure wound therapy system |
| EP3873551A1 (en) * | 2018-11-02 | 2021-09-08 | KCI Licensing, Inc. | Wound therapy tubeset system for wound volume estimation |
| GB201820668D0 (en) | 2018-12-19 | 2019-01-30 | Smith & Nephew Inc | Systems and methods for delivering prescribed wound therapy |
| CN113286624A (en) * | 2019-01-25 | 2021-08-20 | 凯希特许有限公司 | Systems and methods for drip purging |
| US11504457B2 (en) * | 2020-01-29 | 2022-11-22 | Travis L. Perry | Wound irrigation system |
| US20220218962A1 (en) * | 2021-01-13 | 2022-07-14 | NoviRad, Inc. | Systems and methods for percutaneous drainage |
| US20220379004A1 (en) * | 2021-05-26 | 2022-12-01 | Tennessee Technological University | Drug assisted wound drainage line |
Family Cites Families (143)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1355846A (en) | 1920-02-06 | 1920-10-19 | David A Rannells | Medical appliance |
| GB511928A (en) * | 1939-03-13 | 1939-08-25 | Derek Richard Barker | Improvements in or relating to propellor fans |
| US2547758A (en) | 1949-01-05 | 1951-04-03 | Wilmer B Keeling | Instrument for treating the male urethra |
| US2632443A (en) | 1949-04-18 | 1953-03-24 | Eleanor P Lesher | Surgical dressing |
| GB692578A (en) | 1949-09-13 | 1953-06-10 | Minnesota Mining & Mfg | Improvements in or relating to drape sheets for surgical use |
| US2682873A (en) | 1952-07-30 | 1954-07-06 | Johnson & Johnson | General purpose protective dressing |
| NL189176B (en) | 1956-07-13 | 1900-01-01 | Hisamitsu Pharmaceutical Co | PLASTER BASED ON A SYNTHETIC RUBBER. |
| US2969057A (en) | 1957-11-04 | 1961-01-24 | Brady Co W H | Nematodic swab |
| US3066672A (en) | 1960-09-27 | 1962-12-04 | Jr William H Crosby | Method and apparatus for serial sampling of intestinal juice |
| US3367332A (en) | 1965-08-27 | 1968-02-06 | Gen Electric | Product and process for establishing a sterile area of skin |
| US3520300A (en) | 1967-03-15 | 1970-07-14 | Amp Inc | Surgical sponge and suction device |
| US3568675A (en) | 1968-08-30 | 1971-03-09 | Clyde B Harvey | Fistula and penetrating wound dressing |
| US3682180A (en) | 1970-06-08 | 1972-08-08 | Coilform Co Inc | Drain clip for surgical drain |
| BE789293Q (en) | 1970-12-07 | 1973-01-15 | Parke Davis & Co | MEDICO-SURGICAL DRESSING FOR BURNS AND SIMILAR LESIONS |
| US3826254A (en) | 1973-02-26 | 1974-07-30 | Verco Ind | Needle or catheter retaining appliance |
| US3948255A (en) * | 1974-06-06 | 1976-04-06 | Davidson Kenneth L | Apparatus for endotracheal and esophageal intubation |
| DE2527706A1 (en) | 1975-06-21 | 1976-12-30 | Hanfried Dr Med Weigand | DEVICE FOR THE INTRODUCTION OF CONTRAST AGENTS INTO AN ARTIFICIAL INTESTINAL OUTLET |
| DE2640413C3 (en) | 1976-09-08 | 1980-03-27 | Richard Wolf Gmbh, 7134 Knittlingen | Catheter monitor |
| NL7710909A (en) | 1976-10-08 | 1978-04-11 | Smith & Nephew | COMPOSITE STRAPS. |
| GB1562244A (en) | 1976-11-11 | 1980-03-05 | Lock P M | Wound dressing materials |
| US4080970A (en) | 1976-11-17 | 1978-03-28 | Miller Thomas J | Post-operative combination dressing and internal drain tube with external shield and tube connector |
| US4139004A (en) | 1977-02-17 | 1979-02-13 | Gonzalez Jr Harry | Bandage apparatus for treating burns |
| US4184510A (en) | 1977-03-15 | 1980-01-22 | Fibra-Sonics, Inc. | Valued device for controlling vacuum in surgery |
| US4165748A (en) | 1977-11-07 | 1979-08-28 | Johnson Melissa C | Catheter tube holder |
| US4256109A (en) | 1978-07-10 | 1981-03-17 | Nichols Robert L | Shut off valve for medical suction apparatus |
| SE414994B (en) | 1978-11-28 | 1980-09-01 | Landstingens Inkopscentral | VENKATETERFORBAND |
| GB2047543B (en) | 1978-12-06 | 1983-04-20 | Svedman Paul | Device for treating tissues for example skin |
| US4266545A (en) | 1979-04-06 | 1981-05-12 | Moss James P | Portable suction device for collecting fluids from a closed wound |
| US4284079A (en) | 1979-06-28 | 1981-08-18 | Adair Edwin Lloyd | Method for applying a male incontinence device |
| US4261363A (en) | 1979-11-09 | 1981-04-14 | C. R. Bard, Inc. | Retention clips for body fluid drains |
| US4569348A (en) | 1980-02-22 | 1986-02-11 | Velcro Usa Inc. | Catheter tube holder strap |
| ATE14835T1 (en) | 1980-03-11 | 1985-08-15 | Schmid Eduard | SKIN GRAFT PRESSURE BANDAGE. |
| US4297995A (en) | 1980-06-03 | 1981-11-03 | Key Pharmaceuticals, Inc. | Bandage containing attachment post |
| US4333468A (en) | 1980-08-18 | 1982-06-08 | Geist Robert W | Mesentery tube holder apparatus |
| US4465485A (en) | 1981-03-06 | 1984-08-14 | Becton, Dickinson And Company | Suction canister with unitary shut-off valve and filter features |
| US4392853A (en) | 1981-03-16 | 1983-07-12 | Rudolph Muto | Sterile assembly for protecting and fastening an indwelling device |
| US4373519A (en) | 1981-06-26 | 1983-02-15 | Minnesota Mining And Manufacturing Company | Composite wound dressing |
| US4392858A (en) | 1981-07-16 | 1983-07-12 | Sherwood Medical Company | Wound drainage device |
| US4419097A (en) | 1981-07-31 | 1983-12-06 | Rexar Industries, Inc. | Attachment for catheter tube |
| AU550575B2 (en) | 1981-08-07 | 1986-03-27 | Richard Christian Wright | Wound drainage device |
| SE429197B (en) | 1981-10-14 | 1983-08-22 | Frese Nielsen | SAR TREATMENT DEVICE |
| DE3146266A1 (en) | 1981-11-21 | 1983-06-01 | B. Braun Melsungen Ag, 3508 Melsungen | COMBINED DEVICE FOR A MEDICAL SUCTION DRAINAGE |
| US4551139A (en) | 1982-02-08 | 1985-11-05 | Marion Laboratories, Inc. | Method and apparatus for burn wound treatment |
| US4475909A (en) | 1982-05-06 | 1984-10-09 | Eisenberg Melvin I | Male urinary device and method for applying the device |
| EP0100148B1 (en) | 1982-07-06 | 1986-01-08 | Dow Corning Limited | Medical-surgical dressing and a process for the production thereof |
| NZ206837A (en) | 1983-01-27 | 1986-08-08 | Johnson & Johnson Prod Inc | Thin film adhesive dressing:backing material in three sections |
| US4548202A (en) | 1983-06-20 | 1985-10-22 | Ethicon, Inc. | Mesh tissue fasteners |
| US4540412A (en) | 1983-07-14 | 1985-09-10 | The Kendall Company | Device for moist heat therapy |
| US4543100A (en) | 1983-11-01 | 1985-09-24 | Brodsky Stuart A | Catheter and drain tube retainer |
| US4525374A (en) | 1984-02-27 | 1985-06-25 | Manresa, Inc. | Treating hydrophobic filters to render them hydrophilic |
| GB2157958A (en) | 1984-05-03 | 1985-11-06 | Ernest Edward Austen Bedding | Ball game net support |
| US4897081A (en) | 1984-05-25 | 1990-01-30 | Thermedics Inc. | Percutaneous access device |
| US5215522A (en) | 1984-07-23 | 1993-06-01 | Ballard Medical Products | Single use medical aspirating device and method |
| GB8419745D0 (en) | 1984-08-02 | 1984-09-05 | Smith & Nephew Ass | Wound dressing |
| US4872450A (en) | 1984-08-17 | 1989-10-10 | Austad Eric D | Wound dressing and method of forming same |
| US4826494A (en) | 1984-11-09 | 1989-05-02 | Stryker Corporation | Vacuum wound drainage system |
| US4655754A (en) | 1984-11-09 | 1987-04-07 | Stryker Corporation | Vacuum wound drainage system and lipids baffle therefor |
| US4605399A (en) | 1984-12-04 | 1986-08-12 | Complex, Inc. | Transdermal infusion device |
| US5037397A (en) | 1985-05-03 | 1991-08-06 | Medical Distributors, Inc. | Universal clamp |
| US4640688A (en) | 1985-08-23 | 1987-02-03 | Mentor Corporation | Urine collection catheter |
| US4710165A (en) | 1985-09-16 | 1987-12-01 | Mcneil Charles B | Wearable, variable rate suction/collection device |
| US4758220A (en) | 1985-09-26 | 1988-07-19 | Alcon Laboratories, Inc. | Surgical cassette proximity sensing and latching apparatus |
| US4733659A (en) | 1986-01-17 | 1988-03-29 | Seton Company | Foam bandage |
| WO1987004626A1 (en) | 1986-01-31 | 1987-08-13 | Osmond, Roger, L., W. | Suction system for wound and gastro-intestinal drainage |
| US4838883A (en) | 1986-03-07 | 1989-06-13 | Nissho Corporation | Urine-collecting device |
| JPS62281965A (en) | 1986-05-29 | 1987-12-07 | テルモ株式会社 | Catheter and catheter fixing member |
| GB8621884D0 (en) | 1986-09-11 | 1986-10-15 | Bard Ltd | Catheter applicator |
| GB2195255B (en) | 1986-09-30 | 1991-05-01 | Vacutec Uk Limited | Apparatus for vacuum treatment of an epidermal surface |
| US4743232A (en) | 1986-10-06 | 1988-05-10 | The Clinipad Corporation | Package assembly for plastic film bandage |
| DE3634569A1 (en) | 1986-10-10 | 1988-04-21 | Sachse Hans E | CONDOM CATHETER, A URINE TUBE CATHETER FOR PREVENTING RISING INFECTIONS |
| JPS63135179A (en) | 1986-11-26 | 1988-06-07 | 立花 俊郎 | Subcataneous drug administration set |
| GB8628564D0 (en) | 1986-11-28 | 1987-01-07 | Smiths Industries Plc | Anti-foaming agent suction apparatus |
| GB8706116D0 (en) | 1987-03-14 | 1987-04-15 | Smith & Nephew Ass | Adhesive dressings |
| US4787888A (en) | 1987-06-01 | 1988-11-29 | University Of Connecticut | Disposable piezoelectric polymer bandage for percutaneous delivery of drugs and method for such percutaneous delivery (a) |
| US4863449A (en) | 1987-07-06 | 1989-09-05 | Hollister Incorporated | Adhesive-lined elastic condom cathether |
| US5176663A (en) | 1987-12-02 | 1993-01-05 | Pal Svedman | Dressing having pad with compressibility limiting elements |
| US4906240A (en) | 1988-02-01 | 1990-03-06 | Matrix Medica, Inc. | Adhesive-faced porous absorbent sheet and method of making same |
| US4985019A (en) | 1988-03-11 | 1991-01-15 | Michelson Gary K | X-ray marker |
| GB8812803D0 (en) | 1988-05-28 | 1988-06-29 | Smiths Industries Plc | Medico-surgical containers |
| US4919654A (en) | 1988-08-03 | 1990-04-24 | Kalt Medical Corporation | IV clamp with membrane |
| US5000741A (en) | 1988-08-22 | 1991-03-19 | Kalt Medical Corporation | Transparent tracheostomy tube dressing |
| DE69017479T2 (en) | 1989-01-16 | 1995-07-13 | Roussel Uclaf | Azabicyclohepten derivatives and their salts, processes for their preparation, their use as medicaments and preparations containing them. |
| GB8906100D0 (en) | 1989-03-16 | 1989-04-26 | Smith & Nephew | Laminates |
| US5100396A (en) | 1989-04-03 | 1992-03-31 | Zamierowski David S | Fluidic connection system and method |
| US5261893A (en) | 1989-04-03 | 1993-11-16 | Zamierowski David S | Fastening system and method |
| US4969880A (en) | 1989-04-03 | 1990-11-13 | Zamierowski David S | Wound dressing and treatment method |
| US5527293A (en) | 1989-04-03 | 1996-06-18 | Kinetic Concepts, Inc. | Fastening system and method |
| JPH0329665A (en) * | 1989-06-27 | 1991-02-07 | ▲えび▼名 勉 | Drain tube with variable discharge ports for brains |
| US5358494A (en) | 1989-07-11 | 1994-10-25 | Svedman Paul | Irrigation dressing |
| JP2719671B2 (en) | 1989-07-11 | 1998-02-25 | 日本ゼオン株式会社 | Wound dressing |
| US5232453A (en) | 1989-07-14 | 1993-08-03 | E. R. Squibb & Sons, Inc. | Catheter holder |
| GB2235877A (en) | 1989-09-18 | 1991-03-20 | Antonio Talluri | Closed wound suction apparatus |
| US5134994A (en) | 1990-02-12 | 1992-08-04 | Say Sam L | Field aspirator in a soft pack with externally mounted container |
| US5092858A (en) | 1990-03-20 | 1992-03-03 | Becton, Dickinson And Company | Liquid gelling agent distributor device |
| US5197949A (en) * | 1991-01-22 | 1993-03-30 | Kraivit Angsupanich | Suction irrigation device with a scraper |
| US5149331A (en) | 1991-05-03 | 1992-09-22 | Ariel Ferdman | Method and device for wound closure |
| US5147332A (en) * | 1991-05-17 | 1992-09-15 | C.R. Bard, Inc. | Multi-valve catheter for improved reliability |
| JP2713515B2 (en) * | 1991-09-17 | 1998-02-16 | 禎祐 山内 | Catheter device |
| US5278100A (en) | 1991-11-08 | 1994-01-11 | Micron Technology, Inc. | Chemical vapor deposition technique for depositing titanium silicide on semiconductor wafers |
| US5645081A (en) | 1991-11-14 | 1997-07-08 | Wake Forest University | Method of treating tissue damage and apparatus for same |
| US5636643A (en) | 1991-11-14 | 1997-06-10 | Wake Forest University | Wound treatment employing reduced pressure |
| US5279550A (en) | 1991-12-19 | 1994-01-18 | Gish Biomedical, Inc. | Orthopedic autotransfusion system |
| US5167613A (en) | 1992-03-23 | 1992-12-01 | The Kendall Company | Composite vented wound dressing |
| FR2690617B1 (en) | 1992-04-29 | 1994-06-24 | Cbh Textile | TRANSPARENT ADHESIVE DRESSING. |
| DE4306478A1 (en) | 1993-03-02 | 1994-09-08 | Wolfgang Dr Wagner | Drainage device, in particular pleural drainage device, and drainage method |
| US6241747B1 (en) | 1993-05-03 | 2001-06-05 | Quill Medical, Inc. | Barbed Bodily tissue connector |
| US5342376A (en) | 1993-05-03 | 1994-08-30 | Dermagraphics, Inc. | Inserting device for a barbed tissue connector |
| US5344415A (en) | 1993-06-15 | 1994-09-06 | Deroyal Industries, Inc. | Sterile system for dressing vascular access site |
| US5437651A (en) | 1993-09-01 | 1995-08-01 | Research Medical, Inc. | Medical suction apparatus |
| US5549584A (en) | 1994-02-14 | 1996-08-27 | The Kendall Company | Apparatus for removing fluid from a wound |
| JP3009108U (en) * | 1994-06-13 | 1995-03-28 | 清重 乾 | Drain with blockage release function |
| US5556375A (en) | 1994-06-16 | 1996-09-17 | Hercules Incorporated | Wound dressing having a fenestrated base layer |
| US5607388A (en) | 1994-06-16 | 1997-03-04 | Hercules Incorporated | Multi-purpose wound dressing |
| US5664270A (en) | 1994-07-19 | 1997-09-09 | Kinetic Concepts, Inc. | Patient interface system |
| PT853950E (en) | 1994-08-22 | 2003-03-31 | Kinetic Concepts Inc | WASTE DRAIN BOX |
| DE29504378U1 (en) | 1995-03-15 | 1995-09-14 | MTG Medizinisch, technische Gerätebau GmbH, 66299 Friedrichsthal | Electronically controlled low-vacuum pump for chest and wound drainage |
| JPH0910315A (en) * | 1995-06-30 | 1997-01-14 | Ado Polymer:Kk | Medical catheter |
| GB9523253D0 (en) | 1995-11-14 | 1996-01-17 | Mediscus Prod Ltd | Portable wound treatment apparatus |
| US6135116A (en) | 1997-07-28 | 2000-10-24 | Kci Licensing, Inc. | Therapeutic method for treating ulcers |
| US5968013A (en) * | 1997-08-21 | 1999-10-19 | Scimed Life Systems, Inc. | Multi-function dilatation catheter |
| GB9719520D0 (en) | 1997-09-12 | 1997-11-19 | Kci Medical Ltd | Surgical drape and suction heads for wound treatment |
| AU755496B2 (en) | 1997-09-12 | 2002-12-12 | Kci Licensing, Inc. | Surgical drape and suction head for wound treatment |
| US6071267A (en) | 1998-02-06 | 2000-06-06 | Kinetic Concepts, Inc. | Medical patient fluid management interface system and method |
| CN1240630A (en) * | 1998-07-06 | 2000-01-12 | 王明忠 | Pressure reducer for percutaneous treating osteoendohypotension and osteoischemic necrosis |
| US6488643B1 (en) | 1998-10-08 | 2002-12-03 | Kci Licensing, Inc. | Wound healing foot wrap |
| US6129701A (en) * | 1998-11-19 | 2000-10-10 | Sound Surgical Technologies, Llc | Vented aspirator and method |
| US6725492B2 (en) * | 1998-11-25 | 2004-04-27 | Neosci Medical, Inc. | Cleaning brush for medical devices |
| US6287316B1 (en) | 1999-03-26 | 2001-09-11 | Ethicon, Inc. | Knitted surgical mesh |
| US7799004B2 (en) | 2001-03-05 | 2010-09-21 | Kci Licensing, Inc. | Negative pressure wound treatment apparatus and infection identification system and method |
| US6856821B2 (en) | 2000-05-26 | 2005-02-15 | Kci Licensing, Inc. | System for combined transcutaneous blood gas monitoring and vacuum assisted wound closure |
| US6991643B2 (en) | 2000-12-20 | 2006-01-31 | Usgi Medical Inc. | Multi-barbed device for retaining tissue in apposition and methods of use |
| JP2001029434A (en) * | 1999-07-16 | 2001-02-06 | Create Medic Co Ltd | Catheter for transstomach and transintestine fistula |
| AU4176101A (en) | 2000-02-24 | 2001-09-03 | Venetec Int Inc | Universal catheter anchoring system |
| GB2359755A (en) * | 2000-03-03 | 2001-09-05 | Mediplus Ltd | Apparatus for assisting wound healing |
| US6540705B2 (en) | 2001-02-22 | 2003-04-01 | Core Products International, Inc. | Ankle brace providing upper and lower ankle adjustment |
| US7004915B2 (en) * | 2001-08-24 | 2006-02-28 | Kci Licensing, Inc. | Negative pressure assisted tissue treatment system |
| JP2003260127A (en) * | 2002-03-10 | 2003-09-16 | Nippon Clean Engine Lab Co Ltd | Suction method by pressurized reverse jet supply of fluid and apparatus therefor |
| US7226441B2 (en) * | 2003-06-23 | 2007-06-05 | Codman & Shurtleff, Inc. | Catheter with block-overriding system |
| GB2427142B (en) * | 2005-06-13 | 2010-10-20 | Single Use Surgical Ltd | Multi lumen suction irrigator |
| US7798999B2 (en) * | 2007-06-05 | 2010-09-21 | Cook Incorporated | Adjustable length catheter |
| US20090157002A1 (en) * | 2007-12-14 | 2009-06-18 | Csa Medical, Inc. | Catheter having communicating lumens |
| RU2011107118A (en) * | 2008-09-18 | 2012-10-27 | КейСиАй Лайсензинг, Инк. (US) | SYSTEMS AND METHOD OF DELIVERING REDUCED PRESSURE TO THE SUBCUTANEOUS TISSUE |
| KR20110102931A (en) | 2008-12-31 | 2011-09-19 | 케이씨아이 라이센싱 인코포레이티드 | Manifolds, systems, and methods for administering reduced pressure to a subcutaneous tissue site |
-
2011
- 2011-11-09 US US13/292,880 patent/US9180233B2/en active Active
- 2011-11-09 JP JP2013539905A patent/JP6162605B2/en active Active
- 2011-11-09 EP EP11784905.9A patent/EP2640437B1/en active Active
- 2011-11-09 CN CN201180052764.XA patent/CN103189081B/en active Active
- 2011-11-09 AU AU2011329278A patent/AU2011329278B2/en not_active Ceased
- 2011-11-09 EP EP16164752.4A patent/EP3090765B1/en active Active
- 2011-11-09 WO PCT/US2011/060040 patent/WO2012067921A2/en active Application Filing
- 2011-11-09 CA CA2814867A patent/CA2814867C/en not_active Expired - Fee Related
- 2011-11-17 TW TW100142141A patent/TW201223568A/en unknown
-
2015
- 2015-10-09 US US14/879,990 patent/US10195315B2/en active Active
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201223568A (en) | 2012-06-16 |
| EP2640437B1 (en) | 2016-05-11 |
| CN103189081B (en) | 2015-09-09 |
| US20120123323A1 (en) | 2012-05-17 |
| WO2012067921A3 (en) | 2012-07-12 |
| EP2640437A2 (en) | 2013-09-25 |
| EP3090765A1 (en) | 2016-11-09 |
| JP6162605B2 (en) | 2017-07-12 |
| CA2814867A1 (en) | 2012-05-24 |
| CA2814867C (en) | 2018-09-18 |
| US10195315B2 (en) | 2019-02-05 |
| AU2011329278B2 (en) | 2016-06-09 |
| CN103189081A (en) | 2013-07-03 |
| JP2014501562A (en) | 2014-01-23 |
| US20160030645A1 (en) | 2016-02-04 |
| AU2011329278A1 (en) | 2013-04-18 |
| US9180233B2 (en) | 2015-11-10 |
| WO2012067921A2 (en) | 2012-05-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10195315B2 (en) | Systems and methods for subcutaneous administration of reduced pressure employing reconfigurable lumens | |
| JP5823571B2 (en) | Manifold, system and method for applying reduced pressure to a subcutaneous tissue site | |
| JP5290405B2 (en) | Catheter / filament type device and method for treating wounds under the skin surface | |
| CA2644898C (en) | System for percutaneously administering reduced pressure treatment using balloon dissection | |
| CA2726997C (en) | Absorbable, reduced-pressure manifolds and systems | |
| AU2007225050B2 (en) | System and method for purging a reduced pressure apparatus during the administration of reduced pressure treatment | |
| EP3590554B1 (en) | Sleeves, manifolds, and systems for applying reduced pressure to a subcutaneous tissue site | |
| EP2081483B1 (en) | Anti-extravasation catheter | |
| US6692459B2 (en) | Anti-occlusion catheter | |
| CN101431945A (en) | Mechanical barrier in wound healing | |
| CN211356991U (en) | Negative pressure drainage and cleaning system for suture-free closed skin wound | |
| CN111939333A (en) | Negative pressure drainage and cleaning system for suture-free closed skin wound | |
| BRPI0709297A2 (en) | reduced pressure delivery system for applying a reduced pressure tissue treatment to a tissue site and method for promoting tissue growth in a tissue site | |
| BRPI0709293A2 (en) | method of administering a reduced pressure tissue treatment to a tissue site and method of preparing a tissue site to receive a reduced pressure tissue treatment |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2640437 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: KCI LICENSING, INC. |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: KCI LICENSING, INC. |
|
| 17P | Request for examination filed |
Effective date: 20170427 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: KCI LICENSING, INC. |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61M 1/00 20060101AFI20171026BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20171128 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2640437 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 973448 Country of ref document: AT Kind code of ref document: T Effective date: 20180315 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602011046184 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20180228 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 602011046184 Country of ref document: DE Representative=s name: SIMMONS & SIMMONS LLP, DE |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 973448 Country of ref document: AT Kind code of ref document: T Effective date: 20180228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180528 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180528 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180529 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602011046184 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20181129 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181109 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20181130 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181109 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181109 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20191022 Year of fee payment: 9 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20111109 Ref country code: MK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180628 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 602011046184 Country of ref document: DE Owner name: SOLVENTUM INTELLECTUAL PROPERTIES CO. (N.D.GES, US Free format text: FORMER OWNER: KCI LICENSING, INC., SAN ANTONIO, TEX., US Ref country code: DE Ref legal event code: R082 Ref document number: 602011046184 Country of ref document: DE Representative=s name: SIMMONS & SIMMONS LLP, DE Ref country code: DE Ref legal event code: R081 Ref document number: 602011046184 Country of ref document: DE Owner name: 3M INNOVATIVE PROPERTIES COMPANY, ST. PAUL, US Free format text: FORMER OWNER: KCI LICENSING, INC., SAN ANTONIO, TEX., US |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20201109 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201109 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20231019 Year of fee payment: 13 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 602011046184 Country of ref document: DE Owner name: SOLVENTUM INTELLECTUAL PROPERTIES CO. (N.D.GES, US Free format text: FORMER OWNER: 3M INNOVATIVE PROPERTIES COMPANY, ST. PAUL, MN, US |