EP2801424A1 - Magnetic component, soft magnetic metal powder used in same, and method for producing same - Google Patents
Magnetic component, soft magnetic metal powder used in same, and method for producing same Download PDFInfo
- Publication number
- EP2801424A1 EP2801424A1 EP13848104.9A EP13848104A EP2801424A1 EP 2801424 A1 EP2801424 A1 EP 2801424A1 EP 13848104 A EP13848104 A EP 13848104A EP 2801424 A1 EP2801424 A1 EP 2801424A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- metal powder
- soft magnetic
- magnetic metal
- precursor
- iron
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 230000005291 magnetic effect Effects 0.000 title claims abstract description 130
- 239000000843 powder Substances 0.000 title claims abstract description 122
- 229910052751 metal Inorganic materials 0.000 title claims abstract description 112
- 239000002184 metal Substances 0.000 title claims abstract description 112
- 238000004519 manufacturing process Methods 0.000 title claims description 18
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims abstract description 79
- 229910052742 iron Inorganic materials 0.000 claims abstract description 41
- 239000002245 particle Substances 0.000 claims abstract description 39
- 238000000034 method Methods 0.000 claims abstract description 23
- 230000035699 permeability Effects 0.000 claims abstract description 11
- 230000005415 magnetization Effects 0.000 claims abstract description 9
- 239000000523 sample Substances 0.000 claims abstract description 6
- 239000002075 main ingredient Substances 0.000 claims abstract description 3
- 239000002243 precursor Substances 0.000 claims description 50
- 239000003822 epoxy resin Substances 0.000 claims description 26
- 229920000647 polyepoxide Polymers 0.000 claims description 26
- 230000009467 reduction Effects 0.000 claims description 21
- 229910052782 aluminium Inorganic materials 0.000 claims description 14
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 14
- 239000001301 oxygen Substances 0.000 claims description 14
- 229910052760 oxygen Inorganic materials 0.000 claims description 14
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 12
- 239000007789 gas Substances 0.000 claims description 12
- 239000000243 solution Substances 0.000 claims description 12
- 239000007864 aqueous solution Substances 0.000 claims description 11
- 229910017052 cobalt Inorganic materials 0.000 claims description 11
- 239000010941 cobalt Substances 0.000 claims description 11
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 11
- 239000011777 magnesium Substances 0.000 claims description 10
- 229910052749 magnesium Inorganic materials 0.000 claims description 9
- 229910052710 silicon Inorganic materials 0.000 claims description 9
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims description 8
- 229910052761 rare earth metal Inorganic materials 0.000 claims description 8
- 239000010703 silicon Substances 0.000 claims description 8
- QVYYOKWPCQYKEY-UHFFFAOYSA-N [Fe].[Co] Chemical compound [Fe].[Co] QVYYOKWPCQYKEY-UHFFFAOYSA-N 0.000 claims description 6
- 150000002506 iron compounds Chemical class 0.000 claims description 6
- 229910000531 Co alloy Inorganic materials 0.000 claims description 4
- 238000007664 blowing Methods 0.000 claims description 3
- 150000001869 cobalt compounds Chemical class 0.000 claims description 3
- 239000011261 inert gas Substances 0.000 claims description 3
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 2
- 239000002131 composite material Substances 0.000 claims description 2
- 239000013078 crystal Substances 0.000 claims description 2
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 2
- 239000006247 magnetic powder Substances 0.000 abstract description 6
- 238000000465 moulding Methods 0.000 abstract 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 16
- 229910000361 cobalt sulfate Inorganic materials 0.000 description 15
- 229940044175 cobalt sulfate Drugs 0.000 description 15
- KTVIXTQDYHMGHF-UHFFFAOYSA-L cobalt(2+) sulfate Chemical compound [Co+2].[O-]S([O-])(=O)=O KTVIXTQDYHMGHF-UHFFFAOYSA-L 0.000 description 15
- 239000000203 mixture Substances 0.000 description 13
- 238000007254 oxidation reaction Methods 0.000 description 13
- VTLYFUHAOXGGBS-UHFFFAOYSA-N Fe3+ Chemical compound [Fe+3] VTLYFUHAOXGGBS-UHFFFAOYSA-N 0.000 description 12
- 230000000704 physical effect Effects 0.000 description 12
- 229920005989 resin Polymers 0.000 description 12
- 239000011347 resin Substances 0.000 description 12
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 11
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 10
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical compound [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 description 10
- -1 iron ion Chemical class 0.000 description 10
- 239000000696 magnetic material Substances 0.000 description 10
- 239000003153 chemical reaction reagent Substances 0.000 description 9
- 230000000052 comparative effect Effects 0.000 description 8
- 239000006249 magnetic particle Substances 0.000 description 7
- 238000004804 winding Methods 0.000 description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 229920003986 novolac Polymers 0.000 description 6
- 230000003647 oxidation Effects 0.000 description 6
- 239000002994 raw material Substances 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 239000004593 Epoxy Substances 0.000 description 5
- PXKLMJQFEQBVLD-UHFFFAOYSA-N bisphenol F Chemical compound C1=CC(O)=CC=C1CC1=CC=C(O)C=C1 PXKLMJQFEQBVLD-UHFFFAOYSA-N 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- BAUYGSIQEAFULO-UHFFFAOYSA-L iron(2+) sulfate (anhydrous) Chemical compound [Fe+2].[O-]S([O-])(=O)=O BAUYGSIQEAFULO-UHFFFAOYSA-L 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 229930185605 Bisphenol Natural products 0.000 description 4
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 4
- 229910045601 alloy Inorganic materials 0.000 description 4
- 239000000956 alloy Substances 0.000 description 4
- 239000004020 conductor Substances 0.000 description 4
- 239000010410 layer Substances 0.000 description 4
- 230000005381 magnetic domain Effects 0.000 description 4
- 230000001590 oxidative effect Effects 0.000 description 4
- 150000002989 phenols Chemical class 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000005855 radiation Effects 0.000 description 4
- 238000004833 X-ray photoelectron spectroscopy Methods 0.000 description 3
- DIZPMCHEQGEION-UHFFFAOYSA-H aluminium sulfate (anhydrous) Chemical compound [Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O DIZPMCHEQGEION-UHFFFAOYSA-H 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 239000011790 ferrous sulphate Substances 0.000 description 3
- 235000003891 ferrous sulphate Nutrition 0.000 description 3
- 229910000359 iron(II) sulfate Inorganic materials 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 3
- 238000004321 preservation Methods 0.000 description 3
- 238000005245 sintering Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- CWLKGDAVCFYWJK-UHFFFAOYSA-N 3-aminophenol Chemical compound NC1=CC=CC(O)=C1 CWLKGDAVCFYWJK-UHFFFAOYSA-N 0.000 description 2
- VPWNQTHUCYMVMZ-UHFFFAOYSA-N 4,4'-sulfonyldiphenol Chemical class C1=CC(O)=CC=C1S(=O)(=O)C1=CC=C(O)C=C1 VPWNQTHUCYMVMZ-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 description 2
- 230000001133 acceleration Effects 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 229910000358 iron sulfate Inorganic materials 0.000 description 2
- RUTXIHLAWFEWGM-UHFFFAOYSA-H iron(3+) sulfate Chemical compound [Fe+3].[Fe+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O RUTXIHLAWFEWGM-UHFFFAOYSA-H 0.000 description 2
- 229910000360 iron(III) sulfate Inorganic materials 0.000 description 2
- 239000007800 oxidant agent Substances 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 229920001187 thermosetting polymer Polymers 0.000 description 2
- 229910052727 yttrium Inorganic materials 0.000 description 2
- 229910000859 α-Fe Inorganic materials 0.000 description 2
- OUPZKGBUJRBPGC-UHFFFAOYSA-N 1,3,5-tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione Chemical compound O=C1N(CC2OC2)C(=O)N(CC2OC2)C(=O)N1CC1CO1 OUPZKGBUJRBPGC-UHFFFAOYSA-N 0.000 description 1
- STMDPCBYJCIZOD-UHFFFAOYSA-N 2-(2,4-dinitroanilino)-4-methylpentanoic acid Chemical compound CC(C)CC(C(O)=O)NC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O STMDPCBYJCIZOD-UHFFFAOYSA-N 0.000 description 1
- YSUQLAYJZDEMOT-UHFFFAOYSA-N 2-(butoxymethyl)oxirane Chemical compound CCCCOCC1CO1 YSUQLAYJZDEMOT-UHFFFAOYSA-N 0.000 description 1
- JPEGUDKOYOIOOP-UHFFFAOYSA-N 2-(hexoxymethyl)oxirane Chemical compound CCCCCCOCC1CO1 JPEGUDKOYOIOOP-UHFFFAOYSA-N 0.000 description 1
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 1
- VEORPZCZECFIRK-UHFFFAOYSA-N 3,3',5,5'-tetrabromobisphenol A Chemical compound C=1C(Br)=C(O)C(Br)=CC=1C(C)(C)C1=CC(Br)=C(O)C(Br)=C1 VEORPZCZECFIRK-UHFFFAOYSA-N 0.000 description 1
- ALKYHXVLJMQRLQ-UHFFFAOYSA-N 3-Hydroxy-2-naphthoate Chemical compound C1=CC=C2C=C(O)C(C(=O)O)=CC2=C1 ALKYHXVLJMQRLQ-UHFFFAOYSA-N 0.000 description 1
- 229940018563 3-aminophenol Drugs 0.000 description 1
- YBRVSVVVWCFQMG-UHFFFAOYSA-N 4,4'-diaminodiphenylmethane Chemical compound C1=CC(N)=CC=C1CC1=CC=C(N)C=C1 YBRVSVVVWCFQMG-UHFFFAOYSA-N 0.000 description 1
- ODJUOZPKKHIEOZ-UHFFFAOYSA-N 4-[2-(4-hydroxy-3,5-dimethylphenyl)propan-2-yl]-2,6-dimethylphenol Chemical compound CC1=C(O)C(C)=CC(C(C)(C)C=2C=C(C)C(O)=C(C)C=2)=C1 ODJUOZPKKHIEOZ-UHFFFAOYSA-N 0.000 description 1
- WFCQTAXSWSWIHS-UHFFFAOYSA-N 4-[bis(4-hydroxyphenyl)methyl]phenol Chemical compound C1=CC(O)=CC=C1C(C=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 WFCQTAXSWSWIHS-UHFFFAOYSA-N 0.000 description 1
- YXALYBMHAYZKAP-UHFFFAOYSA-N 7-oxabicyclo[4.1.0]heptan-4-ylmethyl 7-oxabicyclo[4.1.0]heptane-4-carboxylate Chemical compound C1CC2OC2CC1C(=O)OCC1CC2OC2CC1 YXALYBMHAYZKAP-UHFFFAOYSA-N 0.000 description 1
- YWFPGFJLYRKYJZ-UHFFFAOYSA-N 9,9-bis(4-hydroxyphenyl)fluorene Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)C2=CC=CC=C2C2=CC=CC=C21 YWFPGFJLYRKYJZ-UHFFFAOYSA-N 0.000 description 1
- 238000004438 BET method Methods 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 229910019819 Cr—Si Inorganic materials 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 229910002546 FeCo Inorganic materials 0.000 description 1
- 206010065042 Immune reconstitution inflammatory syndrome Diseases 0.000 description 1
- 229920000877 Melamine resin Polymers 0.000 description 1
- 239000004640 Melamine resin Substances 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- FQYUMYWMJTYZTK-UHFFFAOYSA-N Phenyl glycidyl ether Chemical compound C1OC1COC1=CC=CC=C1 FQYUMYWMJTYZTK-UHFFFAOYSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 1
- 238000003917 TEM image Methods 0.000 description 1
- KYPYTERUKNKOLP-UHFFFAOYSA-N Tetrachlorobisphenol A Chemical compound C=1C(Cl)=C(O)C(Cl)=CC=1C(C)(C)C1=CC(Cl)=C(O)C(Cl)=C1 KYPYTERUKNKOLP-UHFFFAOYSA-N 0.000 description 1
- 229920001807 Urea-formaldehyde Polymers 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 238000005275 alloying Methods 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- GVPFVAHMJGGAJG-UHFFFAOYSA-L cobalt dichloride Chemical compound [Cl-].[Cl-].[Co+2] GVPFVAHMJGGAJG-UHFFFAOYSA-L 0.000 description 1
- UFMZWBIQTDUYBN-UHFFFAOYSA-N cobalt dinitrate Chemical compound [Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O UFMZWBIQTDUYBN-UHFFFAOYSA-N 0.000 description 1
- 229910001981 cobalt nitrate Inorganic materials 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 238000000748 compression moulding Methods 0.000 description 1
- 239000007771 core particle Substances 0.000 description 1
- 229930003836 cresol Natural products 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 230000002542 deteriorative effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- GYZLOYUZLJXAJU-UHFFFAOYSA-N diglycidyl ether Chemical compound C1OC1COCC1CO1 GYZLOYUZLJXAJU-UHFFFAOYSA-N 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 230000005294 ferromagnetic effect Effects 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 235000000396 iron Nutrition 0.000 description 1
- FBAFATDZDUQKNH-UHFFFAOYSA-M iron chloride Chemical compound [Cl-].[Fe] FBAFATDZDUQKNH-UHFFFAOYSA-M 0.000 description 1
- MVFCKEFYUDZOCX-UHFFFAOYSA-N iron(2+);dinitrate Chemical compound [Fe+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O MVFCKEFYUDZOCX-UHFFFAOYSA-N 0.000 description 1
- 239000012948 isocyanate Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- NXPPAOGUKPJVDI-UHFFFAOYSA-N naphthalene-1,2-diol Chemical compound C1=CC=CC2=C(O)C(O)=CC=C21 NXPPAOGUKPJVDI-UHFFFAOYSA-N 0.000 description 1
- 229940110728 nitrogen / oxygen Drugs 0.000 description 1
- JKXONPYJVWEAEL-UHFFFAOYSA-N oxiran-2-ylmethyl acetate Chemical compound CC(=O)OCC1CO1 JKXONPYJVWEAEL-UHFFFAOYSA-N 0.000 description 1
- XRQKARZTFMEBBY-UHFFFAOYSA-N oxiran-2-ylmethyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1CO1 XRQKARZTFMEBBY-UHFFFAOYSA-N 0.000 description 1
- YLNSNVGRSIOCEU-UHFFFAOYSA-N oxiran-2-ylmethyl butanoate Chemical compound CCCC(=O)OCC1CO1 YLNSNVGRSIOCEU-UHFFFAOYSA-N 0.000 description 1
- 239000005011 phenolic resin Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000010420 shell particle Substances 0.000 description 1
- 239000011863 silicon-based powder Substances 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 1
- 239000012798 spherical particle Substances 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000004627 transmission electron microscopy Methods 0.000 description 1
- 229920006337 unsaturated polyester resin Polymers 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/12—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials
- H01F1/33—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials mixtures of metallic and non-metallic particles; metallic particles having oxide skin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/16—Metallic particles coated with a non-metal
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01Q—ANTENNAS, i.e. RADIO AERIALS
- H01Q17/00—Devices for absorbing waves radiated from an antenna; Combinations of such devices with active antenna elements or systems
- H01Q17/005—Devices for absorbing waves radiated from an antenna; Combinations of such devices with active antenna elements or systems using woven or wound filaments; impregnated nets or clothes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
- B22F2998/10—Processes characterised by the sequence of their steps
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2999/00—Aspects linked to processes or compositions used in powder metallurgy
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C2202/00—Physical properties
- C22C2202/02—Magnetic
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/12—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials
- H01F1/14—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys
- H01F1/20—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys in the form of particles, e.g. powder
- H01F1/22—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys in the form of particles, e.g. powder pressed, sintered, or bound together
- H01F1/24—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys in the form of particles, e.g. powder pressed, sintered, or bound together the particles being insulated
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F41/00—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties
- H01F41/02—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties for manufacturing cores, coils, or magnets
- H01F41/0206—Manufacturing of magnetic cores by mechanical means
- H01F41/0246—Manufacturing of magnetic circuits by moulding or by pressing powder
Definitions
- the present invention relates to a magnetic component used in a high frequency band, and a soft magnetic metal powder constituting the magnetic component and a manufacturing method of the soft magnetic metal powder.
- a passive element has the properties determined on the basis of the physical properties of the used material in many cases. Accordingly, the properties thereof in a high frequency band are not easily improved.
- the product properties of magnetic components such as inductors and antennas may be determined by physical properties including dielectric permittivity and magnetic permeability.
- An inductor is a component that utilizes magnetic flux flowing in the body of the component. In order to obtain an inductor that can be utilized in a high frequency range, a magnetic material that not only maintains the magnetic permeability in a high frequency range but also has a reduced loss even in a high frequency range needs to be developed.
- an antenna As a communication scheme or technology progresses, an antenna corresponding to a plurality of frequency bands is becoming necessary to be installed. Furthermore, it is desired that the occupied area of an antenna in an electronic device be as small as possible. It is known that the length of an antenna when receiving a certain frequency may be a length inversely proportional to the 1/2 power of the product of the real part of magnetic permeability and the real part of dielectric permittivity. In brief, in order to obtain a short antenna length, a magnetic material having high magnetic permeability in the frequency range used needs to be developed. Furthermore, since it is most important for an antenna to have a small loss, a magnetic body having a small loss in a high frequency range is needed.
- a magnetic iron oxide represented by ferrite, a metal magnetic material which mainly includes iron, and an alloy thereof, (hereafter referred to as a "conventional magnetic material") is currently used.
- the magnetic materials cannot be suitably used, because a loss attributable to these magnetic materials increases in a high frequency range of not lower than several hundred MHz. It is considered that this is because the particle diameter larger than the magnetic domain size causes movement of a magnetic domain wall when magnetization is reversed, resulting in generation of a large hysteresis loss. Another possible cause is that the particle diameter equal to or larger than the skin size causes generation of a large eddy current loss.
- nano-order plate-like particles are proposed as metal magnetic particles used in an antenna in Patent Literature 1.
- Patent Literature 1 Japanese Patent Application Laid-Open No. 2010-103427
- Nano-order magnetic particles are used in a magnetic component used in a high frequency band, so that magnetization is reversed in a unit smaller than that for forming a magnetic domain wall, thereby to reduce a hysteresis loss associated with movement of a magnetic domain wall. Furthermore, the magnetic particles have a size of not larger than a skin depth, so that an eddy current loss is also reduced. In brief, it is considered that the nano-order magnetic particles can be used to obtain a magnetic component having a low loss in a high frequency band.
- an object of the present invention is to provide a magnetic component having a sufficiently low loss, and a nano-order soft magnetic metal powder used for obtaining the magnetic component and a manufacturing method thereof.
- the above-described problem can be solved by forming a magnetic component with a soft magnetic metal powder having a specific structure.
- the soft magnetic metal powder has the following properties, wherein iron is a main ingredient, the average particle diameter thereof is not larger than 300 nm, the coercive force (Hc) thereof is 16 to 119 kA/m (200 to 1500 Oe), the saturation magnetization thereof is not less than 90 Am 2 /kg, and the volume resistivity thereof is not less than 1.0 ⁇ 10 1 ⁇ cm where the volume resistivity is measured by a four probe method in a state of vertically pressurizing 1.0 g of the soft magnetic metal powder at 64 MPa (20 kN).
- the above-described soft magnetic metal powder has a core/shell structure, in which the core contains iron or an iron-cobalt alloy while the shell is a composite oxide containing at least one of iron, cobalt, aluminum, silicon, a rare-earth element (including Y), and magnesium.
- the present invention provides an inductor and an antenna in which the above-described soft magnetic metal powder is used.
- a method of manufacturing a soft magnetic metal powder according to the present invention includes:
- the solution containing an iron ion is an aqueous solution of an iron compound and a cobalt compound.
- the precursor obtained in the precursor forming step shows a spinel-type crystal structure by a powder X-ray diffraction method.
- the precursor reducing step includes exposing the precursor to a reduction gas at a temperature of 250°C to 650°C.
- the slow-oxidizing step is a step of exposing the metal powder to an inert gas containing oxygen at a temperature of 20°C to 150°C.
- a magnetic component having a low loss in which the real part ⁇ ' of magnetic permeability at 1 GHz is not less than 1.5 and the loss factor is not more than 0.15, can be obtained.
- a magnetic component, and a soft magnetic metal powder used in the magnetic component and a manufacturing method thereof according to the present invention will be described below.
- this embodiment is intended to exemplify one embodiment of the present invention, and the contents described below can be modified as long as the gist of the present invention is not deviated.
- the magnetic component according to the present invention is composed of a molded body obtained by compression molding the soft magnetic metal powder according to the present invention.
- an antenna and a coil component will be exemplified.
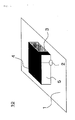
- Fig. 1 is a view illustrating one example of an antenna to which a magnetic material for high frequency is applied.
- An antenna 10 illustrated in the drawing is configured to include a conductor plate 1, a radiation plate 4 disposed on the conductor plate 1, a power supply point 2 and a short circuit plate 3 for supplying power to the radiation plate 4, and a molded body 5.
- the molded body 5 made of the soft magnetic metal powder is held between the conductor plate 1 and the radiation plate 4.
- FIG. 2 is a view illustrating one example of a coil component in which a magnetic material for high frequency is used.
- a coil component 12 illustrated in Fig. 2 is configured to include an electrode 6, a flange 7, a winding wire 8 and a winding core 9.
- the winding core 9 that is a molded body of the soft magnetic metal powder is shaped as an elongated columnar rectangular parallelepiped, and the cross section in the minor axis direction of the rectangular parallelepiped has a rectangular shape.
- the flange 7 has a rectangular cross section larger than the rectangular cross section of the winding core 9, and is configured to be a rectangular parallelepiped having a thinner thickness in the major axis direction of the winding core 9.
- the flange 7 may also be formed with the molded body of the soft magnetic metal powder.
- the soft magnetic metal powder according to the present invention contains Fe (iron), or Fe and Co (cobalt), and at least one of A1 (aluminum), Si (silicon), a rare-earth element (including Y (yttrium)) and Mg (magnesium) (hereinafter referred to as "Al and the like") in Fe, or Fe and Co.
- A1 aluminum
- Si silicon
- a rare-earth element including Y (yttrium)
- Mg manganesium
- the contained amount of A1 and the like to the total sum of Fe and Co is not more than 20 at%.
- A1 or the like is solid-dissolved in Fe, or Fe and Co.
- the precursor is reduced to produce a metal powder.
- Fe and Co which are easily reduced, exist inside the particle in a large amount, while aluminum oxide or the like, which is not reduced, exists on the surface of the particle in a large amount.
- the surface of the metal powder is oxidized to form an insulating film containing A1 and the like.
- the electric resistance of the particle constituting the metal powder becomes high. Accordingly, in a magnetic component formed from the metal powder, a loss based on an eddy current loss and the like is improved. Furthermore, when the contained amount of Al is increased, an oxidized film containing a large amount of A1 on the surface layer can be formed. Accordingly, the electric resistance of the particle becomes high, and the eddy current loss can be reduced. Thus, tan ⁇ becomes smaller.
- other than Al or the like, Fe, or Fe and Co can be remained on the surface.
- Co/Fe atomic ratio When Co is contained, the contained amount of Co to Fe in terms of atomic ratio (hereinafter referred to as a "Co/Fe atomic ratio") is 0 to 60 at%.
- the Co/Fe atomic ratio is more preferably 5 to 55 at%, and further preferably 10 to 50 at%.
- the soft magnetic metal powder is likely to have high saturation magnetization and stable magnetic properties.
- Al and the like also have a sintering prevention effect, preventing the particle from becoming large due to sintering during heat processing.
- A1 and the like are considered to be one of "sintering prevention elements".
- Al and the like are non-magnetic components. Therefore, it is not preferable that an excessively large amount of Al and the like be contained, because magnetic properties are diluted.
- the contained amount of Al and the like to the total sum of Fe and Co is preferably 1 at% to 20 at%, more preferably 3 at% to 18 at%, and further preferably 5 at% to 15 at%.
- the method of manufacturing a soft magnetic metal powder according to the present invention includes a precursor forming step of forming a precursor, and a precursor reducing step of reducing the obtained precursor into a soft magnetic metal powder.
- the manufacturing method may further include a slow-oxidizing step of, following to the precursor reducing step, slightly forming an oxidized film on the surface of the soft magnetic metal powder so that handling of the powder becomes easy.
- the precursor forming step is a wet process, while the precursor reducing step and the slow-oxidizing step are dry processes.
- an aqueous solution containing an element (s) as a raw material is oxidized to cause an oxidation reaction, resulting in obtaining particles (a precursor) composed of the element(s) as a raw material.
- the precursor reducing step the precursor is reduced to remove oxygen contained in the precursor forming step, to obtain a soft magnetic metal powder composed of the element(s) as a raw material.
- the slow-oxidizing step an oxidized film is slightly formed on the surface of the obtained soft magnetic metal powder.
- the nano-order (soft magnetic) metal powder has high activity and is easily oxidized even at normal temperature. By forming the oxidized film on the surface, the powder can stably exist even in air.
- a water-soluble iron compound is suitably used as a raw material.
- the water-soluble iron compound iron sulfate, iron nitrate and iron chloride may be preferably used, and iron sulfate may be further preferably used.
- the reaction is performed by allowing a gas containing oxygen to flow through an aqueous solution of the iron compound, or by adding an aqueous solution of an oxidizing agent such as hydrogen peroxide, so that iron oxide is formed.
- the oxidation reaction may be performed in an environment in which divalent iron (Fe 2+ ) and trivalent iron (Fe 3+ ) coexist.
- divalent iron Fe 2+
- trivalent iron Fe 3+
- an existence ratio between the divalent iron and the trivalent iron is important for controlling the final particle size of the precursor.
- the existence ratio by mol of Fe 2+ /Fe 3 + may be 1 to 300, preferably 10 to 150, and further preferably 15 to 100.
- Trivalent iron may be provided by adding a trivalent iron compound, or oxidizing divalent iron to produce trivalent iron.
- cobalt may be added to iron.
- a water-soluble cobalt compound may be used. This is because the reaction is based on wet system.
- water-soluble cobalt cobalt sulfate, cobalt nitrate, cobalt chloride and the like may be preferably used, and cobalt sulfate may be further preferably used.
- Cobalt may be added preferably before a nucleus is formed, and more preferably together with an iron material. Cobalt can also be added after the end of the oxidation reaction for deposition.
- the oxidation for growing a nucleus is preferably performed by blowing air or oxygen into the aqueous solution. This is because the flow rate and the flow velocity can be easily controlled, and a uniform oxidation reaction can be caused in the solution even when a larger manufacturing apparatus is used, by additionally providing an air outlet to the apparatus. Oxidation can also be caused by a method of adding an oxidizing agent.
- an element such as aluminum, silicon, a rare-earth element (including Y) and magnesium may be added as a raw material.
- water-soluble compounds thereof may also be preferably used. These elements may be added after iron or iron and cobalt are added in a reaction vessel, during the oxidation reaction so as to be solid dissolved in the precursor, or after the end of the oxidation reaction for deposition. These elements may be added at once or continuously.
- the precursor obtained through the wet process described above is continued to be subjected to the treatment in a dry process.
- the precursor is subjected to a thermal reduction treatment by exposing the precursor to a reducing gas such as carbon monoxide, acetylene or hydrogen at a temperature of 250°C to 650°C.
- a reducing gas such as carbon monoxide, acetylene or hydrogen
- the multi-stage reduction means that a reduction treatment of retaining a treatment object at a predetermined temperature for a predetermined time is performed a plurality of times while changing the temperature.
- the properties of the resultant metal magnetic powder can be controlled.
- water vapor may be preferably added in a reducing gas.
- An alloy magnetic particle powder is obtained after the thermal reduction.
- the alloy magnetic particle powder When the alloy magnetic particle powder is handled as it is in the air, it may be rapidly oxidized. Therefore, an oxide layer is formed in the following slow-oxidizing step.
- the obtained powder is treated while gradually increasing the amount of an oxidizing gas in an inert gas, at a temperature of 20 to 300°C for a predetermined time, so as to produce an oxide layer on the surface of the particle.
- the reduced powder be cooled to a temperature at which the slow-oxidizing step is performed, and slow oxidation be performed at that temperature.
- the oxide layer may be formed on the surface of the particle with a weak oxidizing gas at this temperature for performing a stabilization treatment.
- water vapor may also be added in the weak oxidizing gas for the slow-oxidizing process. By adding water vapor, a more dense film can be formed.
- the soft magnetic metal powder which has been subjected to the slow-oxidizing step and obtained as described above was studied on the powder properties and composition by the method shown below.
- the average particle diameter was determined as follows: photographing an image of the metal magnetic powder observed in the bright field (for example, at a magnification of 58000) using a transmission electron microscopy (JEM-100CX Mark-II type manufactured by JEOL Ltd.) with an acceleration voltage of 100 kV; enlarging the photographed image (for example, 9 times in vertical and horizontal magnifications), randomly selecting 300 monodispersed particles from a plurality of photographs, measuring the particle diameter for each particle, and calculating an average of the measured particle diameters.
- the BET specific surface area was determined by the BET method using 4 Sorb US manufactured by Yuasa Ionics Inc.
- an index ( ⁇ s) for evaluating weather resistance of the soft magnetic metal powder was determined by retaining the soft magnetic metal powder in a constant temperature and humidity container at a set temperature of 60°C and a relative humidity of 90% for one week, measuring the saturation magnetizations ⁇ s before and after retaining the soft magnetic metal powder under the constant temperature and humidity, and calculating pre - preservation ⁇ s - post - preservation ⁇ s / pre - preservation ⁇ s ⁇ 100 % .
- the composition of the soft magnetic metal powder was determined by performing mass analysis of the whole particle containing a metal magnetic phase and an oxidized film.
- Co, Al, Y, Mg, and Si were quantified using a high frequency induction plasma emission spectrometer ICP (IRIS/AP) manufactured by Nippon Jarrell-Ash Co. Ltd.
- Fe was quantified using Hiranuma Automatic Titrator (COMTIME-980) manufactured by Hiranuma Sangyo Co., Ltd.
- Oxygen was quantified using NITROGEN/OXYGEN DETERMETER (TC-436 type) manufactured by LECO Corporation. These quantified results were provided based on mass%. Therefore, the results were appropriately converted to atom% (at%), to calculate a Co/Fe atomic ratio and an Al/(Fe + Co) atomic ratio.
- the volume resistivity of the soft magnetic metal powder was determined by measuring 1.0 g of the powder in a state of being vertically pressurized at 64 MPa (20 kN) by the four probe method, using a powder resistance measuring unit (MCP-PD51) manufactured by Mitsubishi Chemical Analytech Co., Ltd. and a low-resistance powder measuring system software (MCP-PDLGPWIN) manufactured by Mitsubishi Chemical Analytech Co., Ltd.
- MCP-PD51 powder resistance measuring unit
- MCP-PDLGPWIN low-resistance powder measuring system software manufactured by Mitsubishi Chemical Analytech Co., Ltd.
- thermosetting resin any publicly known thermosetting resin can be used.
- thermosetting resin can be selected from a phenol resin, an epoxy resin, an unsaturated polyester resin, an isocyanate compound, a melamine resin, a urea resin, a silicone resin and the like.
- epoxy resin one or a mixture of a mono-epoxy compound and a polyvalent epoxy compound is used.
- examples of the mono-epoxy compound may include butyl glycidyl ether, hexyl glycidyl ether, phenyl glycidyl ether, allyl glycidyl ether, para-tert-butylphenyl glycidyl ether, ethylene oxide, propylene oxide, paraxylyl glycidyl ether, glycidyl acetate, glycidyl butyrate, glycidyl hexoate, and glycidyl benzoate.
- Examples of the polyvalent epoxy compound may include a bisphenol-type epoxy resin obtained by glycidylating bisphenols such as bisphenol A, bisphenol F, bisphenol AD, bisphenol S, tetramethyl bisphenol A, tetramethyl bisphenol F, tetramethyl bisphenol AD, tetramethyl bisphenol S, tetrabromo bisphenol A, tetrachloro bisphenol A, and tetrafluoro bisphenol A; an epoxy resin obtained by glycidylating other divalent phenols such as biphenol, dihydroxy naphthalene, and 9,9-bis(4-hydroxyphenyl)fluorene; an epoxy resin obtained by glycidylating trisphenols such as 1,1,1-tris(4-hydroxyphenyl)methane and 4,4-(1-(4-(1-(4-hydroxy phenyl)-1-methyl ethyl)phenyl)ethylidene)bisphenol; an epoxy resin obtained by g
- the polyvalent epoxy resin is preferable from the viewpoint of improving storage stability.
- a glycidyl-type epoxy resin is preferable since productivity is remarkably high, and the epoxy resin obtained by glycidylating polyvalent phenols is more preferable since a cured product has excellent adhesion and heat resistance.
- the bisphenol-type epoxy resin is further preferable, and an epoxy resin obtained by glycidylating bisphenol A and an epoxy resin obtained by glycidylating bisphenol F are particularly preferable.
- the resin is preferably in a liquid form.
- the epoxy equivalent is preferably not less than 300 in order to maintain the composition in a solid form.
- the mixed ratio of the soft magnetic metal powder and the epoxy resin in terms of metal/resin is preferably 30/70 to 99/1, more preferably 50/50 to 95/5, and further preferably 70/30 to 90/10 at a mass ratio. This is because too little resin does not form a molded body, while too much resin does not provide desired magnetic properties.
- the soft magnetic metal powder according to the present invention can be compression molded into a predetermined shape.
- the molded article to be supplied has a shape exemplified in Fig. 1 and Fig. 2 .
- the soft magnetic metal powder was molded into a doughnut shape in the example below, and the molded doughnut-shaped article was evaluated for properties as a magnetic component.
- the soft magnetic metal powder and the epoxy resin were weighted at a weight ratio of 80:20, and the soft magnetic metal powder was dispersed in the epoxy resin using a vacuum agitation defoaming mixer (V-mini 300) manufactured by EME Co., Ltd. to prepare a paste.
- the paste was dried on a hot plate at 60°C for 2 hours to obtain a soft magnetic metal powder-resin complex.
- the complex was pulverized to prepare a powder of the complex. Then, 0.2 g of the complex powder was put in a doughnut-shaped container, and subjected to a load of 1 t by a hand press machine. Thus, a toroidal-shaped molded body having an outer diameter of 7 mm and an inner diameter of 3 mm was obtained.
- a real part ( ⁇ ') of magnetic permeability, an imaginary part ( ⁇ ") of magnetic permeability, and tan ⁇ representing a loss factor were measured at 0.5 to 3 GHz, using a network analyzer (E8362C) manufactured by Agilent Technologies Inc. and a coaxial-type S-parameter method sample holder kit (product model No. : CSH2-APC7, sample dimension: 7.0 mm to 3.04 mm in diameter ⁇ 5 mm) manufactured by Kanto Electronic Application and Development Inc.
- the precursor reducing step was performed.
- the precursor powder in which Al was solid-dissolved in Fe was put in a basket having a ventilation function, and the basket was placed in a through-type reducing furnace.
- a reduction treatment was conducted while allowing a hydrogen gas to flow through at 500°C for 60 minutes. After the reduction treatment was finished, a powder of metal iron (a soft magnetic metal powder) was obtained.
- the atmosphere in the furnace was changed from hydrogen to nitrogen, and the temperature in the furnace was reduced to 80°C at a temperature drop rate of 20°C/min while allowing nitrogen to flow.
- a gas with a mixed ratio of air to N 2 of 1/125 was added in the furnace in order to inhibit the metal iron powder from being rapidly oxidized, to form an oxidized film in the mixed atmosphere of oxygen and nitrogen. Then a supply of air was gradually increased to raise the oxygen concentration in the atmosphere.
- the flow rate of added air to be finally supplied was 1/25 relative to N 2 .
- a total amount of the gas to be introduced in the furnace was maintained constant by adjusting the flow rate of nitrogen.
- This slow-oxidizing treatment was conducted in the atmosphere of maintaining the temperature at approximately 80°C.
- a final soft magnetic metal powder (having a surface oxidized film) was obtained.
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- the composition, and the conditions of the precursor reducing step and the slow-oxidizing step are shown in Table 1, including other examples.
- Example 2 After that, the same procedure as in Example 1 was repeated to obtain a soft magnetic metal powder (having a surface oxidized film in which Fe is partly replaced with Co).
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- Example 2 The same procedure as in Example 2 was repeated, except that the mixed ratio between 1 mol/L ferrous sulfate (special grade reagent) aqueous solution and a 1 mol/L cobalt sulfate (special grade reagent) solution in Example 2 was changed to be 8:5.
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- an increase of ⁇ ' usually shifts the resonance frequency to the lower frequency side, so that tan ⁇ tends to deteriorate (become larger).
- an increase of a Co amount for raising ⁇ ' did not cause tan ⁇ to deteriorate (become larger). It is considered that this is because inclusion of Co enabled formation of a more dense oxidized film, so that the volume resistivity of the powder increased, resulting in a reduced eddy current loss. That is, it was found that there is an effect that inclusion of Co improves ⁇ ' without deteriorating (increasing) tan ⁇ .
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- Example 5 the same procedure as in Example 4 was repeated, except that the amount of aluminum sulfate in Example 4 was changed to the amount described in Table 1.
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- FIG. 3 A TEM photograph of the particles obtained in Example 7 is shown in Fig. 3 .
- This TEM image was photographed with an acceleration voltage of 100 kV applied, and the contrast was adjusted so that the core part appears black.
- Fig. 3 shown as an observed example there is a spherical portion which appears dark in the center of a spherical particle, and a thin portion which appears substantially transparent around the spherical portion.
- the soft magnetic metal powder obtained in the present invention includes a core portion formed from metal and a shell portion formed from an oxidized film.
- the composition of the core/shell particle may be analyzed by a method such as ICP emission analysis, ESCA, TEM-EDX, XPS, and SIMS.
- ESCA the transition of the composition from the particle surface in the depth direction can be observed. Therefore, the core portion formed from metal and the shell portion formed from an oxide can be recognized.
- TEM-EDX the particle is irradiated with EDX-rays with the beam focused, and is semi-quantified, so that the composition of the particle can be roughly observed. Therefore, the core portion formed from metal and the shell portion formed from an oxide can be recognized (for example, see paragraph [0078] of Japanese Patent Application Laid-Open No. 2006-128535 ).
- Example 9 the same procedure as in Example 8 was repeated, except that the reduction temperature in Example 8 was changed to the temperature described in Table 1.
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- the reduction temperature differs among Examples 7, 9, and 10. It was found that the example at a higher temperature has a higher ⁇ ' value. It is considered that this is because an increase of the reduction temperature promotes the reduction and the alloying of Fe and Co.
- Example 2 The same procedure as in Example 2 was repeated, except that the reduction temperature by the hydrogen gas in the through-type reducing furnace in Example 2 was changed to 600°C.
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- the physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, the high-frequency properties of the molded body produced with the powder are shown in Table 3, and a TEM photograph of the obtained powder is shown in Fig. 4 . This photograph also shows that the soft magnetic metal powder obtained in the present invention includes the core portion formed from metal and the shell portion formed from an oxidized film.
- a soft magnetic metal powder of Comparative Example 1 As a soft magnetic metal powder of Comparative Example 1, a commercially available Mn-Zn based ferrite powder was used. The physical properties and bulk properties of this soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- a soft magnetic metal powder of Comparative Example 2 As a soft magnetic metal powder of Comparative Example 2, a commercially available Fe-Cr-Si powder was used. The physical properties and bulk properties of this soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- the soft magnetic metal powder according to the present invention can be used not only in inductors and antennas but also in soft magnetic applications such as magnetic heads, lower layer members of magnetic recording media, iron cores of electromagnets, transformer cores, antennas, electromagnetic shield members, and wave absorbers.
Landscapes
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Power Engineering (AREA)
- Powder Metallurgy (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Soft Magnetic Materials (AREA)
Abstract
Description
- The present invention relates to a magnetic component used in a high frequency band, and a soft magnetic metal powder constituting the magnetic component and a manufacturing method of the soft magnetic metal powder.
- The frequency of signals used in an electronic device such as a cellular phone, a laptop computer and a liquid crystal television device is recently being increased. Signals in the GHz band are already in practical use. It is expected that a frequency band exceeding 10 GHz will be in use in the future. As the frequency of such a device is increased, it is required that individual components such as an electronic circuit and other passive elements also have improved performance in a high frequency range.
- In addition, these devices are being decreased in size and power consumption so as to be used as a mobile device. Therefore, the individual components are required to have high performance in a high frequency band, and a lowered loss. However, among the components constituting the device, a passive element has the properties determined on the basis of the physical properties of the used material in many cases. Accordingly, the properties thereof in a high frequency band are not easily improved.
- For example, the product properties of magnetic components such as inductors and antennas may be determined by physical properties including dielectric permittivity and magnetic permeability. An inductor is a component that utilizes magnetic flux flowing in the body of the component. In order to obtain an inductor that can be utilized in a high frequency range, a magnetic material that not only maintains the magnetic permeability in a high frequency range but also has a reduced loss even in a high frequency range needs to be developed.
- Regarding an antenna, as a communication scheme or technology progresses, an antenna corresponding to a plurality of frequency bands is becoming necessary to be installed. Furthermore, it is desired that the occupied area of an antenna in an electronic device be as small as possible. It is known that the length of an antenna when receiving a certain frequency may be a length inversely proportional to the 1/2 power of the product of the real part of magnetic permeability and the real part of dielectric permittivity. In brief, in order to obtain a short antenna length, a magnetic material having high magnetic permeability in the frequency range used needs to be developed. Furthermore, since it is most important for an antenna to have a small loss, a magnetic body having a small loss in a high frequency range is needed.
- As a magnetic material used in the inductor and the antenna described above, a magnetic iron oxide represented by ferrite, a metal magnetic material which mainly includes iron, and an alloy thereof, (hereafter referred to as a "conventional magnetic material") is currently used. However, there is a problem in that the magnetic materials cannot be suitably used, because a loss attributable to these magnetic materials increases in a high frequency range of not lower than several hundred MHz. It is considered that this is because the particle diameter larger than the magnetic domain size causes movement of a magnetic domain wall when magnetization is reversed, resulting in generation of a large hysteresis loss. Another possible cause is that the particle diameter equal to or larger than the skin size causes generation of a large eddy current loss.
- In such circumstances, nano-order plate-like particles are proposed as metal magnetic particles used in an antenna in Patent Literature 1.
- Patent Literature 1: Japanese Patent Application Laid-Open No.
2010-103427 - Nano-order magnetic particles are used in a magnetic component used in a high frequency band, so that magnetization is reversed in a unit smaller than that for forming a magnetic domain wall, thereby to reduce a hysteresis loss associated with movement of a magnetic domain wall. Furthermore, the magnetic particles have a size of not larger than a skin depth, so that an eddy current loss is also reduced. In brief, it is considered that the nano-order magnetic particles can be used to obtain a magnetic component having a low loss in a high frequency band.
- However, although the magnetic component in which the metal magnetic particles are used, disclosed in Patent Document 1, has a loss smaller than that of the conventional magnetic material, the value of tanδ indicating a loss at 1 GHz is 0.18 according to the description in Paragraph 0104. Therefore, a substance having a further low loss is desired.
- Thus, an object of the present invention is to provide a magnetic component having a sufficiently low loss, and a nano-order soft magnetic metal powder used for obtaining the magnetic component and a manufacturing method thereof.
- The above-described problem can be solved by forming a magnetic component with a soft magnetic metal powder having a specific structure.
- More specifically, the soft magnetic metal powder has the following properties, wherein
iron is a main ingredient,
the average particle diameter thereof is not larger than 300 nm,
the coercive force (Hc) thereof is 16 to 119 kA/m (200 to 1500 Oe),
the saturation magnetization thereof is not less than 90 Am2/kg, and
the volume resistivity thereof is not less than 1.0 × 101 Ω·cm where the volume resistivity is measured by a four probe method in a state of vertically pressurizing 1.0 g of the soft magnetic metal powder at 64 MPa (20 kN). - Furthermore, the above-described soft magnetic metal powder has a core/shell structure, in which the core contains iron or an iron-cobalt alloy while the shell is a composite oxide containing at least one of iron, cobalt, aluminum, silicon, a rare-earth element (including Y), and magnesium.
- In the above-described soft magnetic metal powder, the iron-cobalt ratio in the iron-cobalt alloy is Co/Fe = 0.0 to 0.6 in terms of atomic ratio.
- The above-described soft magnetic metal powder contains aluminum, and the atomic ratio of aluminum to a total sum of Fe and Co is Al/(total of Fe and Co) = 0.01 to 0.30.
- When the above-described soft magnetic metal powder and an epoxy resin are mixed in a ratio by mass of 80:20 and molded under pressure,
it is satisfied that µ' > 1.5 and µ" < 0.5, and tanδ < 0.15 at a frequency of 1 GHz, as well as µ' > 1.5 and µ" < 1.5, and tanδ < 0.5 at a frequency of 2 GHz, where a real part of complex magnetic permeability thereof is µ', an imaginary part thereof is µ", and a loss factor thereof is tan δ (= µ"/µ'). - Furthermore, the present invention provides an inductor and an antenna in which the above-described soft magnetic metal powder is used.
- A method of manufacturing a soft magnetic metal powder according to the present invention includes:
- a precursor forming step of adding an aqueous solution of at least one of aluminum, silicon, a rare-earth element (including Y), and magnesium into a solution containing an iron ion while blowing a gas containing oxygen thereinto, to form a precursor containing at least one of aluminum, silicon, a rare-earth element (including Y), and magnesium;
- a precursor reducing step of reducing the precursor to obtain a metal powder; and
- a slow-oxidizing step of further reacting the metal powder obtained in the precursor reducing step with oxygen to form an oxidized film on the surface of the metal powder.
- In the above-described manufacturing method, the solution containing an iron ion is an aqueous solution of an iron compound and a cobalt compound.
- Furthermore, in the above-described manufacturing method, the precursor obtained in the precursor forming step shows a spinel-type crystal structure by a powder X-ray diffraction method.
- Also, in the above-described manufacturing method, the precursor reducing step includes exposing the precursor to a reduction gas at a temperature of 250°C to 650°C.
- Also, in the above-described manufacturing method, the slow-oxidizing step is a step of exposing the metal powder to an inert gas containing oxygen at a temperature of 20°C to 150°C.
- According to the soft magnetic metal powder of the present invention, a magnetic component having a low loss, in which the real part µ' of magnetic permeability at 1 GHz is not less than 1.5 and the loss factor is not more than 0.15, can be obtained.
-
-
Fig. 1 is a diagram illustrating a structure of an antenna that is the magnetic component according to the present invention. -
Fig. 2 is a diagram illustrating a structure of a coil component that is the magnetic component according to the present invention. -
Fig. 3 is a TEM photograph of the soft magnetic powder according to the present invention. -
Fig. 4 is a TEM photograph of the soft magnetic powder according to the present invention. - A magnetic component, and a soft magnetic metal powder used in the magnetic component and a manufacturing method thereof according to the present invention will be described below. However, this embodiment is intended to exemplify one embodiment of the present invention, and the contents described below can be modified as long as the gist of the present invention is not deviated.
- The magnetic component according to the present invention is composed of a molded body obtained by compression molding the soft magnetic metal powder according to the present invention. As a particular example of the magnetic component, an antenna and a coil component will be exemplified.
-
Fig. 1 is a view illustrating one example of an antenna to which a magnetic material for high frequency is applied. Anantenna 10 illustrated in the drawing is configured to include a conductor plate 1, aradiation plate 4 disposed on the conductor plate 1, apower supply point 2 and ashort circuit plate 3 for supplying power to theradiation plate 4, and a molded body 5. The molded body 5 made of the soft magnetic metal powder is held between the conductor plate 1 and theradiation plate 4. Such a structure enables the wavelength to be shortened, and can realize reduction in size of theantenna 10. -
Fig. 2 is a view illustrating one example of a coil component in which a magnetic material for high frequency is used. A coil component 12 illustrated inFig. 2 is configured to include anelectrode 6, aflange 7, a windingwire 8 and a windingcore 9. The windingcore 9 that is a molded body of the soft magnetic metal powder is shaped as an elongated columnar rectangular parallelepiped, and the cross section in the minor axis direction of the rectangular parallelepiped has a rectangular shape. Theflange 7 has a rectangular cross section larger than the rectangular cross section of the windingcore 9, and is configured to be a rectangular parallelepiped having a thinner thickness in the major axis direction of the windingcore 9. Theflange 7 may also be formed with the molded body of the soft magnetic metal powder. - Next, the soft magnetic metal powder according to the present invention will be described in detail.
- The soft magnetic metal powder according to the present invention contains Fe (iron), or Fe and Co (cobalt), and at least one of A1 (aluminum), Si (silicon), a rare-earth element (including Y (yttrium)) and Mg (magnesium) (hereinafter referred to as "Al and the like") in Fe, or Fe and Co.
- The contained amount of A1 and the like to the total sum of Fe and Co is not more than 20 at%. In the state of a precursor before being subjected to a reduction treatment, A1 or the like is solid-dissolved in Fe, or Fe and Co. Then, the precursor is reduced to produce a metal powder. In the reduced metal powder, Fe and Co, which are easily reduced, exist inside the particle in a large amount, while aluminum oxide or the like, which is not reduced, exists on the surface of the particle in a large amount.
- Thereafter, the surface of the metal powder is oxidized to form an insulating film containing A1 and the like. Thus, the electric resistance of the particle constituting the metal powder becomes high. Accordingly, in a magnetic component formed from the metal powder, a loss based on an eddy current loss and the like is improved. Furthermore, when the contained amount of Al is increased, an oxidized film containing a large amount of A1 on the surface layer can be formed. Accordingly, the electric resistance of the particle becomes high, and the eddy current loss can be reduced. Thus, tanδ becomes smaller. Here, other than Al or the like, Fe, or Fe and Co can be remained on the surface.
- When Co is contained, the contained amount of Co to Fe in terms of atomic ratio (hereinafter referred to as a "Co/Fe atomic ratio") is 0 to 60 at%. The Co/Fe atomic ratio is more preferably 5 to 55 at%, and further preferably 10 to 50 at%. When the Co/Fe atomic ratio falls within this range, the soft magnetic metal powder is likely to have high saturation magnetization and stable magnetic properties.
- Al and the like also have a sintering prevention effect, preventing the particle from becoming large due to sintering during heat processing. As described herein, A1 and the like are considered to be one of "sintering prevention elements". However, Al and the like are non-magnetic components. Therefore, it is not preferable that an excessively large amount of Al and the like be contained, because magnetic properties are diluted. The contained amount of Al and the like to the total sum of Fe and Co is preferably 1 at% to 20 at%, more preferably 3 at% to 18 at%, and further preferably 5 at% to 15 at%.
- The method of manufacturing a soft magnetic metal powder according to the present invention includes a precursor forming step of forming a precursor, and a precursor reducing step of reducing the obtained precursor into a soft magnetic metal powder. The manufacturing method may further include a slow-oxidizing step of, following to the precursor reducing step, slightly forming an oxidized film on the surface of the soft magnetic metal powder so that handling of the powder becomes easy. The precursor forming step is a wet process, while the precursor reducing step and the slow-oxidizing step are dry processes.
- In the precursor forming step, an aqueous solution containing an element (s) as a raw material is oxidized to cause an oxidation reaction, resulting in obtaining particles (a precursor) composed of the element(s) as a raw material.
- In the precursor reducing step, the precursor is reduced to remove oxygen contained in the precursor forming step, to obtain a soft magnetic metal powder composed of the element(s) as a raw material. In the slow-oxidizing step, an oxidized film is slightly formed on the surface of the obtained soft magnetic metal powder. The nano-order (soft magnetic) metal powder has high activity and is easily oxidized even at normal temperature. By forming the oxidized film on the surface, the powder can stably exist even in air. Each step will be described in detail below.
- In the precursor forming step, a water-soluble iron compound is suitably used as a raw material. As the water-soluble iron compound, iron sulfate, iron nitrate and iron chloride may be preferably used, and iron sulfate may be further preferably used. The reaction is performed by allowing a gas containing oxygen to flow through an aqueous solution of the iron compound, or by adding an aqueous solution of an oxidizing agent such as hydrogen peroxide, so that iron oxide is formed.
- The oxidation reaction may be performed in an environment in which divalent iron (Fe2+) and trivalent iron (Fe3+) coexist. Such coexistence of irons having different valences enables a nucleus to be easily formed, so that particles having an appropriate size can be obtained. Here, an existence ratio between the divalent iron and the trivalent iron is important for controlling the final particle size of the precursor. The existence ratio by mol of Fe2+/Fe3+may be 1 to 300, preferably 10 to 150, and further preferably 15 to 100.
- Fe2+/Fe3+ exceeding 300 causes deterioration of particle size distribution, and therefore is not preferred. A larger ratio of Fe2+/Fe3+ causes a decrease of the number of nuclei and the number of particles. Therefore, the particle size becomes larger. Conversely, a smaller ratio of Fe2+/Fe3+ causes an increase of the number of nuclei and the number of particles. Therefore, the particle size becomes smaller. Trivalent iron may be provided by adding a trivalent iron compound, or oxidizing divalent iron to produce trivalent iron.
- As a raw material, cobalt may be added to iron. As a cobalt material, a water-soluble cobalt compound may be used. This is because the reaction is based on wet system. As the water-soluble cobalt, cobalt sulfate, cobalt nitrate, cobalt chloride and the like may be preferably used, and cobalt sulfate may be further preferably used.
- Cobalt may be added preferably before a nucleus is formed, and more preferably together with an iron material. Cobalt can also be added after the end of the oxidation reaction for deposition.
- The oxidation for growing a nucleus is preferably performed by blowing air or oxygen into the aqueous solution. This is because the flow rate and the flow velocity can be easily controlled, and a uniform oxidation reaction can be caused in the solution even when a larger manufacturing apparatus is used, by additionally providing an air outlet to the apparatus. Oxidation can also be caused by a method of adding an oxidizing agent.
- In addition to iron and cobalt, an element such as aluminum, silicon, a rare-earth element (including Y) and magnesium may be added as a raw material. In order to add these elements, water-soluble compounds thereof may also be preferably used. These elements may be added after iron or iron and cobalt are added in a reaction vessel, during the oxidation reaction so as to be solid dissolved in the precursor, or after the end of the oxidation reaction for deposition. These elements may be added at once or continuously.
- The precursor obtained through the wet process described above is continued to be subjected to the treatment in a dry process.
- In the precursor reducing step, the precursor is subjected to a thermal reduction treatment by exposing the precursor to a reducing gas such as carbon monoxide, acetylene or hydrogen at a temperature of 250°C to 650°C. At this time, a multi-stage reduction may be performed. The multi-stage reduction means that a reduction treatment of retaining a treatment object at a predetermined temperature for a predetermined time is performed a plurality of times while changing the temperature. By properly controlling the temperature and time for retaining the treatment object, the properties of the resultant metal magnetic powder can be controlled. As an atmosphere for the reduction treatment, water vapor may be preferably added in a reducing gas.
- An alloy magnetic particle powder is obtained after the thermal reduction. When the alloy magnetic particle powder is handled as it is in the air, it may be rapidly oxidized. Therefore, an oxide layer is formed in the following slow-oxidizing step. In the slow-oxidizing step, the obtained powder is treated while gradually increasing the amount of an oxidizing gas in an inert gas, at a temperature of 20 to 300°C for a predetermined time, so as to produce an oxide layer on the surface of the particle. Actually, it is preferable that the reduced powder be cooled to a temperature at which the slow-oxidizing step is performed, and slow oxidation be performed at that temperature. Also, the oxide layer may be formed on the surface of the particle with a weak oxidizing gas at this temperature for performing a stabilization treatment. In this process, water vapor may also be added in the weak oxidizing gas for the slow-oxidizing process. By adding water vapor, a more dense film can be formed.
- The soft magnetic metal powder which has been subjected to the slow-oxidizing step and obtained as described above was studied on the powder properties and composition by the method shown below.
- The average particle diameter was determined as follows: photographing an image of the metal magnetic powder observed in the bright field (for example, at a magnification of 58000) using a transmission electron microscopy (JEM-100CX Mark-II type manufactured by JEOL Ltd.) with an acceleration voltage of 100 kV; enlarging the photographed image (for example, 9 times in vertical and horizontal magnifications), randomly selecting 300 monodispersed particles from a plurality of photographs, measuring the particle diameter for each particle, and calculating an average of the measured particle diameters.
- The BET specific surface area was determined by the BET method using 4 Sorb US manufactured by Yuasa Ionics Inc.
- As magnetic properties (bulk properties) of the obtained soft magnetic metal powder, a coercive force Hc (Oe and kA/m), a saturation magnetization σs (Am2/kg) and a squareness ratio SQ were measured using a VSM apparatus (VSM-7P) manufactured by Toei Industry Co. , Ltd. with an external magnetic field of 10 kOe (795.8 kA/m). Also, an index (Δσs) for evaluating weather resistance of the soft magnetic metal powder was determined by retaining the soft magnetic metal powder in a constant temperature and humidity container at a set temperature of 60°C and a relative humidity of 90% for one week, measuring the saturation magnetizations σs before and after retaining the soft magnetic metal powder under the constant temperature and humidity, and calculating
- The composition of the soft magnetic metal powder was determined by performing mass analysis of the whole particle containing a metal magnetic phase and an oxidized film. Co, Al, Y, Mg, and Si were quantified using a high frequency induction plasma emission spectrometer ICP (IRIS/AP) manufactured by Nippon Jarrell-Ash Co. Ltd. Fe was quantified using Hiranuma Automatic Titrator (COMTIME-980) manufactured by Hiranuma Sangyo Co., Ltd. Oxygen was quantified using NITROGEN/OXYGEN DETERMETER (TC-436 type) manufactured by LECO Corporation. These quantified results were provided based on mass%. Therefore, the results were appropriately converted to atom% (at%), to calculate a Co/Fe atomic ratio and an Al/(Fe + Co) atomic ratio.
- The volume resistivity of the soft magnetic metal powder was determined by measuring 1.0 g of the powder in a state of being vertically pressurized at 64 MPa (20 kN) by the four probe method, using a powder resistance measuring unit (MCP-PD51) manufactured by Mitsubishi Chemical Analytech Co., Ltd. and a low-resistance powder measuring system software (MCP-PDLGPWIN) manufactured by Mitsubishi Chemical Analytech Co., Ltd.
- The obtained soft magnetic metal powder is kneaded together with a resin into a molded body. As a resin used at this time, any publicly known thermosetting resin can be used. An example of the thermosetting resin can be selected from a phenol resin, an epoxy resin, an unsaturated polyester resin, an isocyanate compound, a melamine resin, a urea resin, a silicone resin and the like. As the epoxy resin, one or a mixture of a mono-epoxy compound and a polyvalent epoxy compound is used.
- Here, examples of the mono-epoxy compound may include butyl glycidyl ether, hexyl glycidyl ether, phenyl glycidyl ether, allyl glycidyl ether, para-tert-butylphenyl glycidyl ether, ethylene oxide, propylene oxide, paraxylyl glycidyl ether, glycidyl acetate, glycidyl butyrate, glycidyl hexoate, and glycidyl benzoate.
- Examples of the polyvalent epoxy compound may include a bisphenol-type epoxy resin obtained by glycidylating bisphenols such as bisphenol A, bisphenol F, bisphenol AD, bisphenol S, tetramethyl bisphenol A, tetramethyl bisphenol F, tetramethyl bisphenol AD, tetramethyl bisphenol S, tetrabromo bisphenol A, tetrachloro bisphenol A, and tetrafluoro bisphenol A; an epoxy resin obtained by glycidylating other divalent phenols such as biphenol, dihydroxy naphthalene, and 9,9-bis(4-hydroxyphenyl)fluorene; an epoxy resin obtained by glycidylating trisphenols such as 1,1,1-tris(4-hydroxyphenyl)methane and 4,4-(1-(4-(1-(4-hydroxy phenyl)-1-methyl ethyl)phenyl)ethylidene)bisphenol; an epoxy resin obtained by glycidylating tetrakisphenols such as 1,1,2,2,-tetrakis(4-hydroxyphenyl)ethane; a novolak-type epoxy resin and the like obtained by glycidylating novolaks such as phenol novolak, cresol novolak, bisphenol A novolak, brominated phenol novolak, and brominated bisphenol A novolak; an epoxy resin obtained by glycidylating polyvalent phenols, an aliphatic ether-type epoxy resin obtained by glycidylating polyvalent alcohols such as glycerin and polyethylene glycol; an ether ester-type epoxy resin obtained by glycidylating a hydroxycarboxylic acid such as p-oxybenzoic acid and β-oxynaphthoic acid; an ester-type epoxy resin obtained by glycidylating a polycarboxylic acid like a phthalic acid and a terephthalic acid; a glycidyl-type epoxy resin such as an amine-type epoxy resin such as a product obtained by glycidylating an amine compound such as 4,4-diaminodiphenylmethane and m-aminophenol, and triglycidyl isocyanurate, and alicyclic epoxide and the like such as 3,4-epoxycyclohexylmethyl-3',4'-epoxycyclohexanecarboxylate.
- Among the above-described epoxy resins, the polyvalent epoxy resin is preferable from the viewpoint of improving storage stability. Among the polyvalent epoxy resins, a glycidyl-type epoxy resin is preferable since productivity is remarkably high, and the epoxy resin obtained by glycidylating polyvalent phenols is more preferable since a cured product has excellent adhesion and heat resistance. The bisphenol-type epoxy resin is further preferable, and an epoxy resin obtained by glycidylating bisphenol A and an epoxy resin obtained by glycidylating bisphenol F are particularly preferable.
- The resin is preferably in a liquid form. The epoxy equivalent is preferably not less than 300 in order to maintain the composition in a solid form.
- The mixed ratio of the soft magnetic metal powder and the epoxy resin in terms of metal/resin is preferably 30/70 to 99/1, more preferably 50/50 to 95/5, and further preferably 70/30 to 90/10 at a mass ratio. This is because too little resin does not form a molded body, while too much resin does not provide desired magnetic properties.
- The soft magnetic metal powder according to the present invention can be compression molded into a predetermined shape. In a practical use, the molded article to be supplied has a shape exemplified in
Fig. 1 andFig. 2 . However, the soft magnetic metal powder was molded into a doughnut shape in the example below, and the molded doughnut-shaped article was evaluated for properties as a magnetic component. - The soft magnetic metal powder and the epoxy resin were weighted at a weight ratio of 80:20, and the soft magnetic metal powder was dispersed in the epoxy resin using a vacuum agitation defoaming mixer (V-mini 300) manufactured by EME Co., Ltd. to prepare a paste. The paste was dried on a hot plate at 60°C for 2 hours to obtain a soft magnetic metal powder-resin complex. The complex was pulverized to prepare a powder of the complex. Then, 0.2 g of the complex powder was put in a doughnut-shaped container, and subjected to a load of 1 t by a hand press machine. Thus, a toroidal-shaped molded body having an outer diameter of 7 mm and an inner diameter of 3 mm was obtained.
- As high-frequency properties of the molded body of the obtained soft magnetic metal powder-resin complex, a real part (µ') of magnetic permeability, an imaginary part (µ") of magnetic permeability, and tanδ representing a loss factor were measured at 0.5 to 3 GHz, using a network analyzer (E8362C) manufactured by Agilent Technologies Inc. and a coaxial-type S-parameter method sample holder kit (product model No. : CSH2-APC7, sample dimension: 7.0 mm to 3.04 mm in diameter × 5 mm) manufactured by Kanto Electronic Application and Development Inc.
- The precursor forming step was performed as follows. In a 5000 mL beaker, 3000 mL of pure water and 100 ml of 12 mol/L sodium hydroxide were put, and then stirred while maintaining the temperature at 40°C with a temperature controller. To the mixture, 900 mL of a solution in which a 2 mol/L ferric sulfate (special grade reagent) solution and a 1 mol/L ferrous sulfate (special grade reagent) aqueous solution were mixed at a mixed ratio of Fe2+/Fe3+ = 20 was added.
- Thereafter, the temperature was increased to 90°C, and furthermore, air was flown through at 200 mL/min. Thus, oxidation was continued for 40 minutes. Air was switched to nitrogen, and then aging was conducted for 10 minutes. Thereafter, 80 ml of 0.3 mol/L aluminum sulfate (special grade reagent) was added, and air was flown through at 200 mL/min. Thus, oxidation was continued for 50 minutes to complete the oxidation. In this manner, particles of a precursor in which Al was solid-dissolved in Fe were obtained. The precursor was filtrated and washed with water by an ordinary method, and then dried at 110°C. Thus, a dried solid matter of the precursor (also referred to as a precursor powder) was obtained.
- Next, the precursor reducing step was performed. The precursor powder in which Al was solid-dissolved in Fe was put in a basket having a ventilation function, and the basket was placed in a through-type reducing furnace. A reduction treatment was conducted while allowing a hydrogen gas to flow through at 500°C for 60 minutes. After the reduction treatment was finished, a powder of metal iron (a soft magnetic metal powder) was obtained.
- Thereafter, in order to proceed to the slow-oxidizing step, the atmosphere in the furnace was changed from hydrogen to nitrogen, and the temperature in the furnace was reduced to 80°C at a temperature drop rate of 20°C/min while allowing nitrogen to flow. In the initial stage of oxidized film formation of the slow-oxidizing step, a gas with a mixed ratio of air to N2 of 1/125 was added in the furnace in order to inhibit the metal iron powder from being rapidly oxidized, to form an oxidized film in the mixed atmosphere of oxygen and nitrogen. Then a supply of air was gradually increased to raise the oxygen concentration in the atmosphere.
- The flow rate of added air to be finally supplied was 1/25 relative to N2. At that time, a total amount of the gas to be introduced in the furnace was maintained constant by adjusting the flow rate of nitrogen. This slow-oxidizing treatment was conducted in the atmosphere of maintaining the temperature at approximately 80°C.
- Thus, a final soft magnetic metal powder (having a surface oxidized film) was obtained. The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3. The composition, and the conditions of the precursor reducing step and the slow-oxidizing step are shown in Table 1, including other examples.
- In a 5000 mL beaker, 3000 mL of pure water and 100 ml of 12 mol/L sodium hydroxide were put, and then stirred while maintaining the temperature at 40°C with a temperature controller. To the mixture, 900 mL of a solution in which a 1 mol/L ferrous sulfate (special grade reagent) aqueous solution and a 1 mol/L cobalt sulfate (special grade reagent) solution were mixed at a mixed ratio of 4:1, and a 2 mol/L ferric sulfate (special grade reagent) solution in an amount of satisfying (Fe2+ + Co2+) /Fe3+ = 20 were simultaneously added. After that, the same procedure as in Example 1 was repeated to obtain a soft magnetic metal powder (having a surface oxidized film in which Fe is partly replaced with Co). The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- The same procedure as in Example 2 was repeated, except that the mixed ratio between 1 mol/L ferrous sulfate (special grade reagent) aqueous solution and a 1 mol/L cobalt sulfate (special grade reagent) solution in Example 2 was changed to be 8:5. The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- Here, Examples 1 to 3 will be discussed. The results show that µ' was higher in Examples 2 and 3 in which Co was included than in Example 1 in which Co was not included. It is considered that this is because inclusion of Co for providing a FeCo alloy increased the magnetic moment, resulting in higher saturation magnetization, so that the magnetic permeability increased. That is, there is an effect that inclusion of Co increases µ'.
- Also, an increase of µ' usually shifts the resonance frequency to the lower frequency side, so that tanδ tends to deteriorate (become larger). However, in the examples, an increase of a Co amount for raising µ' did not cause tanδ to deteriorate (become larger). It is considered that this is because inclusion of Co enabled formation of a more dense oxidized film, so that the volume resistivity of the powder increased, resulting in a reduced eddy current loss. That is, it was found that there is an effect that inclusion of Co improves µ' without deteriorating (increasing) tanδ.
- The same procedure as in Example 2 was repeated, except that the amount of Fe3+ for forming a nucleus was changed to (Fe2+ + Co2+)/Fe3+= 33, and the amount of 0. 3 mol/L aluminum sulfate (special grade reagent) to be added on the way of the oxidation reaction in Example 2 was changed to 45 ml. The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- In Examples 5 to 8, the same procedure as in Example 4 was repeated, except that the amount of aluminum sulfate in Example 4 was changed to the amount described in Table 1. The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- Here, Examples 4 to 8 will be discussed. It was found that there is an effect that an increase of the amount of Al contained reduces tanδ. In the present invention, when the precursor containing Fe, Co and Al is reduced, Fe and Co which are easily reduced exist inside the particle, while aluminum oxide which is not easily reduced exists on the surface of the particle. Accordingly, an oxidized film containing Al on the surface of the particle is formed. For this reason, when the amount of Al is increased, an oxidized film containing more aluminum oxide on the surface of the particle is formed. It is considered that this is the reason why the volume resistivity of the particle was increased, the eddy current loss was decreased, and tanδ was reduced.
- A TEM photograph of the particles obtained in Example 7 is shown in
Fig. 3 . This TEM image was photographed with an acceleration voltage of 100 kV applied, and the contrast was adjusted so that the core part appears black. As a result, inFig. 3 shown as an observed example, there is a spherical portion which appears dark in the center of a spherical particle, and a thin portion which appears substantially transparent around the spherical portion. As shown in this photograph, the soft magnetic metal powder obtained in the present invention includes a core portion formed from metal and a shell portion formed from an oxidized film. - The composition of the core/shell particle may be analyzed by a method such as ICP emission analysis, ESCA, TEM-EDX, XPS, and SIMS. Especially, when ESCA is employed, the transition of the composition from the particle surface in the depth direction can be observed. Therefore, the core portion formed from metal and the shell portion formed from an oxide can be recognized. When TEM-EDX is employed, the particle is irradiated with EDX-rays with the beam focused, and is semi-quantified, so that the composition of the particle can be roughly observed. Therefore, the core portion formed from metal and the shell portion formed from an oxide can be recognized (for example, see paragraph [0078] of Japanese Patent Application Laid-Open No.
2006-128535 - In Examples 9 to 10, the same procedure as in Example 8 was repeated, except that the reduction temperature in Example 8 was changed to the temperature described in Table 1. The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- Here, the reduction temperature differs among Examples 7, 9, and 10. It was found that the example at a higher temperature has a higher µ' value. It is considered that this is because an increase of the reduction temperature promotes the reduction and the alloying of Fe and Co.
- The same procedure as in Example 2 was repeated, except that the reduction temperature by the hydrogen gas in the through-type reducing furnace in Example 2 was changed to 600°C. The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- The same procedure as in Example 11 was repeated, except that the amount of Fe3+ for forming a nucleus in Example 11 was changed to (Fe2+ + Co2+)/Fe3+ = 85. The physical properties and bulk properties of the obtained soft magnetic metal powder are shown in Table 2, the high-frequency properties of the molded body produced with the powder are shown in Table 3, and a TEM photograph of the obtained powder is shown in
Fig. 4 . This photograph also shows that the soft magnetic metal powder obtained in the present invention includes the core portion formed from metal and the shell portion formed from an oxidized film. - As a soft magnetic metal powder of Comparative Example 1, a commercially available Mn-Zn based ferrite powder was used. The physical properties and bulk properties of this soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
- As a soft magnetic metal powder of Comparative Example 2, a commercially available Fe-Cr-Si powder was used. The physical properties and bulk properties of this soft magnetic metal powder are shown in Table 2, and the high-frequency properties of the molded body produced with the powder are shown in Table 3.
[Table 1] Ferromagnetic metal species Non-magnetic component Reduction conditions Stabilizing (slow-oxidizing) conditions Fe2+/Fe3+ Cobalt Additives Reduction temperature Reduction time Reduction species Temperature Initial oxygen existence ratio Final oxygen existence ratio Type Co/Fe ratio Type Concentration Added amount Adding method (Atomic ratio) (Atomic ratio) mol/L ml °C Minutes °C Volume ratio Volume ratio Example 1 20 None Al 0.3 80 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 2 20 Cobalt sulfate 1/4 Al 0.3 80 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 3 20 Cobalt sulfate 8/5 Al 0.3 80 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 4 33 Cobalt sulfate 1/4 Al 0.3 45 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 5 33 Cobalt sulfate 1/4 Al 0.3 80 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 6 33 Cobalt sulfate 1/4 Al 0.3 150 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 7 33 Cobalt sulfate 1/4 Al 0.3 220 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 8 33 Cobalt sulfate 1/4 Al 0.3 290 Doping 500 60 Hydrogen gas 80 1/125 1/25 Example 9 33 Cobalt sulfate 1/4 Al 0.3 290 Doping 400 60 Hydrogen gas 80 1/125 1/25 Example 10 33 Cobalt sulfate 1/4 Al 0.3 290 Doping 600 60 Hydrogen gas 80 1/125 1/25 Example 11 20 Cobalt sulfate 1/4 Al 0.3 80 Doping 600 60 Hydrogen gas 80 1/125 1/25 Example 12 85 Cobalt sulfate 1/4 Al 0.3 80 Doping 600 60 Hydrogen gas 80 1/125 1/25 [Table 2] Composition Particle size Bulk properties Volume resistivity Co/Fe (at%) Al/(Fe+Co) (at%) (nm) BET TAP He σs SQ b.SFD Δσs Ω·cm Example 1 0.00 4.15 45.3 21.7 1.17 457 137 0.22 3.62 8.5 1.8E+02 Example 2 19.63 5.45 42.1 29.4 1.28 487 179 0.24 3.88 9.1 9.1E+03 Example 3 49.24 5.15 44.3 31.5 1.35 533 176 0.25 3.77 9.2 1.2E+04 Example 4 20.43 3.64 59.1 21.5 1.26 450 189 0.18 4.10 7.6 5.2E+01 Example 5 19.82 6.02 59.9 28.1 1.31 397 183 0.15 5.13 4.9 3.5E+04 Example 6 20.97 10.19 58.9 38.3 1.18 427 164 0.18 4.66 4.9 1.1E+05 Example 7 20.48 13.01 58.2 41.0 1.10 474 148 0.21 4.23 4.8 1.2E+06 Example 8 19.10 17.08 59.5 43.1 1.07 514 132 0.25 3.59 4.9 1.8E+06 Example 9 20.48 13.01 68.4 34.5 1.16 718 98 0.30 3.53 4.3 7.2E+04 Example 10 20.48 13.01 60.5 35.8 1.16 413 171 0.16 5.01 4.0 1.0E+08 Example 11 19.63 5.45 44.6 23.0 1.27 490 182 0.24 3.85 5.8 6.3E+03 Example 12 20.17 5.90 212.6 27.1 1.56 288 185 0.08 12.21 5.9 7.7E+02 Comparative Example 1 - - 5000.0 0.5 2.85 24 85 0.02 39.56 0.4 2.5E+02 Comparative Example 2 - - 10000.0 0.1 4.02 16 167 0.01 79.88 0.9 2.9E+02 [Table 3] Ratio of magnetic powder Ratio of resin 1.0 GHz 2.0 GHz 3.0 GHz wt% wt% µ' µ" tanδ µ' µ" tan δ µ' µ" tan δ Example 1 80 20 2.65 0.169 0.064 2.51 0.690 0.275 2.12 0.777 0.366 Example 2 80 20 3.47 0.3011 0.087 3.17 1.025 0.323 2.63 1.112 0.424 Example 3 80 20 3.33 0.213 0.064 3.27 0.795 0.243 2.83 1.002 0.354 Example 4 80 20 3.57 0.405 0.114 2.95 1.243 0.421 2.30 1.038 0.451 Example 5 80 20 3.61 0.436 0.121 2.95 1.367 0.463 2.20 1.088 0.494 Example 6 80 20 2.99 0.228 0.076 2.77 0.979 0.354 2.10 0.943 0.449 Example 7 80 20 2.42 0.119 0.049 2.44 0.552 0.226 2.00 0.708 0.354 Example 8 80 20 2.05 0.089 0.043 2.10 0.314 0.150 1.86 0.477 0.256 Example 9 80 20 1.60 0.024 0.015 1.61 0.038 0.024 1.64 0.069 0.042 Example 10 80 20 3.39 0.378 0.112 2.77 1.265 0.456 2.03 1.045 0.515 Example 11 80 20 3.68 0.412 0.112 3.18 1.156 0.363 2.62 1.166 0.445 Example 12 80 20 3.09 0.336 0.109 2.89 0.526 0.182 2.81 0.619 0.221 Comparative Example 1 80 20 3.56 2.122 0.596 2.24 2.087 0.933 1.56 1.779 1.139 Comparative Example 2 80 20 4.17 1.792 0.430 3.24 1.890 0.583 2.68 1.893 0.706 - The soft magnetic metal powder according to the present invention can be used not only in inductors and antennas but also in soft magnetic applications such as magnetic heads, lower layer members of magnetic recording media, iron cores of electromagnets, transformer cores, antennas, electromagnetic shield members, and wave absorbers.
-
- 1
- Conductor plate
- 2
- Power supply point
- 3
- Short circuit plate
- 4
- Radiation plate
- 5
- Molded body
- 6
- Electrode
- 7
- Flange
- 8
- Winding wire
- 9
- Winding core
- 10
- Antenna
- 11
- Coil
- 12
- Coil component
Claims (12)
- A soft magnetic metal powder comprising iron as a main ingredient, wherein
an average particle diameter thereof is not larger than 300 nm,
a coercive force (Hc) thereof is 16 to 119 kA/m (200 to 1500 Oe),
a saturation magnetization thereof is not less than 90 Am2/kg, and
a volume resistivity thereof is not less than 1.0 × 101 Ω·cm where the volume resistivity is measured by a four probe method in a state of vertically pressurizing 1.0 g of the soft magnetic metal powder at 64 MPa (20 kN). - The soft magnetic metal powder according to claim 1, having a core/shell structure, in which the core contains iron or an iron-cobalt alloy and the shell is a composite oxide containing at least one of iron, cobalt, aluminum, silicon, a rare-earth element (including Y), and magnesium.
- The soft magnetic metal powder according to claim 2, wherein an iron-cobalt ratio in the iron-cobalt alloy is Co/Fe = 0.0 to 0.6 in terms of atomic ratio.
- The soft magnetic metal powder according to any of claims 1 to 3, comprising aluminum, and wherein an atomic ratio of aluminum to a total sum of Fe and Co is Al/(total of Fe and Co) = 0.01 to 0.30.
- The soft magnetic metal powder according to any of claims 1 to 4, wherein, when the soft magnetic metal powder and an epoxy resin are mixed in a ratio by mass of 80:20 and molded under pressure,
it is satisfied that µ' > 1.5 and µ" < 0.5, and tanδ < 0.15 at a frequency of 1 GHz, and that µ' > 1.5 and µ" < 1.5, and tanδ < 0.5 at a frequency of 2 GHz, where a real part of complex magnetic permeability thereof is µ', an imaginary part thereof is µ", and a loss factor thereof is tan δ (= µ"/µ'). - An inductor formed by using the soft magnetic metal powder according to any of claims 1 to 5.
- An antenna formed by using the soft magnetic metal powder according to any of claims 1 to 5.
- A method of manufacturing a soft magnetic metal powder, comprising:a precursor forming step of adding an aqueous solution of at least one of aluminum, silicon, a rare-earth element (including Y), and magnesium into a solution containing an iron ion while blowing a gas containing oxygen thereinto, to form a precursor containing at least one of aluminum, silicon, a rare-earth element (including Y), and magnesium;a precursor reducing step of reducing the precursor to obtain a metal powder; anda slow-oxidizing step of further reacting the metal powder obtained in the precursor reducing step with oxygen to form an oxidized film on the surface of the metal powder.
- The method of manufacturing a soft magnetic metal powder according to claim 8, wherein the solution containing an iron ion is an aqueous solution of an iron compound and a cobalt compound.
- The method of manufacturing a soft magnetic metal powder according to claim 8 or 9, wherein the precursor obtained in the precursor forming step shows a spinel-type crystal structure by a powder X-ray diffraction method.
- The method of manufacturing a soft magnetic metal powder according to any of claims 8 to 10, wherein the precursor reducing step includes exposing the precursor to a reduction gas at a temperature of 250°C to 650°C.
- The method of manufacturing a soft magnetic metal powder according to any of claims 8 to 11, wherein the slow-oxidizing step is a step of exposing the metal powder to an inert gas containing oxygen at a temperature of 20°C to 150°C.
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/JP2013/001638 WO2014141318A1 (en) | 2013-03-13 | 2013-03-13 | Magnetic component, soft magnetic metal powder used in same, and method for producing same |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2801424A1 true EP2801424A1 (en) | 2014-11-12 |
| EP2801424A4 EP2801424A4 (en) | 2015-03-25 |
| EP2801424B1 EP2801424B1 (en) | 2017-03-08 |
Family
ID=51311856
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13848104.9A Not-in-force EP2801424B1 (en) | 2013-03-13 | 2013-03-13 | Magnetic component, soft magnetic metal powder used in same, and method for producing same |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP2801424B1 (en) |
| KR (1) | KR101496626B1 (en) |
| CN (1) | CN103999170B (en) |
| TW (1) | TWI471876B (en) |
| WO (1) | WO2014141318A1 (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE112016001291B4 (en) * | 2015-03-19 | 2023-06-15 | Rogers Corporation | MAGNETODIELECTRIC SUBSTRATE AND PRODUCTION THEREOF, CIRCUIT MATERIAL AND PRODUCTION THEREOF AND AN ARRANGEMENT WITH THE CIRCUIT MATERIAL AND CIRCUIT AND PRODUCTION OF THE CIRCUIT, ANTENNA AND RF COMPONENT |
| JP6963950B2 (en) * | 2017-09-22 | 2021-11-10 | Dowaエレクトロニクス株式会社 | Iron powder and its manufacturing method, inductor moldings and inductors |
| JP7097702B2 (en) * | 2018-01-17 | 2022-07-08 | Dowaエレクトロニクス株式会社 | Fe-Co alloy powder and inductor moldings and inductors using it |
| JP7201417B2 (en) * | 2018-01-17 | 2023-01-10 | Dowaエレクトロニクス株式会社 | SILICON OXIDE-COATED IRON POWDER AND ITS MANUFACTURING METHOD AND INDUCTOR MOLDED BODY AND INDUCTOR USING THE SAME |
| CN110181036A (en) * | 2019-05-14 | 2019-08-30 | 合肥博微田村电气有限公司 | A kind of composite soft-magnetic metal powder, preparation method and integrated inductance |
| JP2022175110A (en) * | 2021-05-12 | 2022-11-25 | セイコーエプソン株式会社 | Soft magnetic powder, powder magnetic core, magnetic element, electronic device, and mobile body |
| CN116825468B (en) * | 2023-08-04 | 2024-01-12 | 广东泛瑞新材料有限公司 | Iron-cobalt magnetic core and preparation method and application thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006128535A (en) * | 2004-11-01 | 2006-05-18 | Dowa Mining Co Ltd | Metal magnetism powder and magnetic recording medium using it |
| US20100035087A1 (en) * | 2008-08-08 | 2010-02-11 | Toda Kogyo Corporation | Process for producing ferromagnetic metal particles and magnetic recording medium |
| US20100060539A1 (en) * | 2008-09-08 | 2010-03-11 | Tomohiro Suetsuna | Core-shell magnetic material, method of manufacturing core-shell magnetic material, device, and antenna device |
| JP2011162882A (en) * | 2011-03-11 | 2011-08-25 | Dowa Holdings Co Ltd | Ferromagnetic metal powder, and magnetic recording medium using the same |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5556756B2 (en) * | 2004-02-27 | 2014-07-23 | 日立金属株式会社 | Iron-based nano-sized particles and method for producing the same |
| JP4677734B2 (en) * | 2004-04-19 | 2011-04-27 | Dowaエレクトロニクス株式会社 | Magnetic powder for magnetic recording media |
| JP4431769B2 (en) * | 2005-09-15 | 2010-03-17 | Dowaエレクトロニクス株式会社 | Ferromagnetic powder and paint and magnetic recording medium using the same |
| CN1971718B (en) * | 2005-11-23 | 2011-04-27 | 同和电子科技有限公司 | Ferromagnetic powder used for magnetic recording medium, preparing method of the same and magnetic recording medium using the same |
| JP5418754B2 (en) * | 2008-10-15 | 2014-02-19 | 戸田工業株式会社 | Ferromagnetic metal particle powder, method for producing the same, and magnetic recording medium |
| JP5177542B2 (en) | 2008-10-27 | 2013-04-03 | 国立大学法人東北大学 | Composite magnetic body, circuit board using the same, and electronic component using the same |
-
2013
- 2013-03-13 CN CN201380003390.1A patent/CN103999170B/en not_active Expired - Fee Related
- 2013-03-13 KR KR1020147008494A patent/KR101496626B1/en active IP Right Grant
- 2013-03-13 WO PCT/JP2013/001638 patent/WO2014141318A1/en active Application Filing
- 2013-03-13 EP EP13848104.9A patent/EP2801424B1/en not_active Not-in-force
- 2013-04-09 TW TW102112474A patent/TWI471876B/en not_active IP Right Cessation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006128535A (en) * | 2004-11-01 | 2006-05-18 | Dowa Mining Co Ltd | Metal magnetism powder and magnetic recording medium using it |
| US20100035087A1 (en) * | 2008-08-08 | 2010-02-11 | Toda Kogyo Corporation | Process for producing ferromagnetic metal particles and magnetic recording medium |
| US20100060539A1 (en) * | 2008-09-08 | 2010-03-11 | Tomohiro Suetsuna | Core-shell magnetic material, method of manufacturing core-shell magnetic material, device, and antenna device |
| JP2011162882A (en) * | 2011-03-11 | 2011-08-25 | Dowa Holdings Co Ltd | Ferromagnetic metal powder, and magnetic recording medium using the same |
Non-Patent Citations (1)
| Title |
|---|
| See also references of WO2014141318A1 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103999170B (en) | 2016-06-15 |
| WO2014141318A1 (en) | 2014-09-18 |
| TWI471876B (en) | 2015-02-01 |
| EP2801424A4 (en) | 2015-03-25 |
| CN103999170A (en) | 2014-08-20 |
| TW201426773A (en) | 2014-07-01 |
| KR101496626B1 (en) | 2015-02-26 |
| EP2801424B1 (en) | 2017-03-08 |
| KR20140121810A (en) | 2014-10-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2851910B1 (en) | Metal powder and its use | |
| EP2801424B1 (en) | Magnetic component, soft magnetic metal powder used in same, and method for producing same | |
| JP2013236021A5 (en) | ||
| JP5373127B2 (en) | Magnetic component, soft magnetic metal powder used therefor, and method for producing the same | |
| US20160001371A1 (en) | Magnetic component, and soft magnetic metal powder used therein and manufacturing method thereof | |
| JP6632702B2 (en) | Method for producing Fe-Co alloy powder | |
| CN109215922B (en) | Composite magnetic material and magnetic core | |
| US20160086705A1 (en) | Magnetic material and device | |
| JP6607751B2 (en) | Fe-Co alloy powder, manufacturing method thereof, antenna, inductor, and EMI filter | |
| US11424059B2 (en) | Composite magnetic body | |
| US11456097B2 (en) | Composite magnetic body | |
| JP7069949B2 (en) | Composite magnetic material | |
| CN111599567B (en) | Composite magnetic material, magnetic core, and electronic component |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20140417 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20150223 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: H01F 1/33 20060101ALI20150217BHEP Ipc: H01F 1/24 20060101ALI20150217BHEP Ipc: B22F 1/00 20060101AFI20150217BHEP Ipc: H01Q 17/00 20060101ALI20150217BHEP |
|
| DAX | Request for extension of the european patent (deleted) | ||
| 17Q | First examination report despatched |
Effective date: 20160204 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ref document number: 602013018434 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: B22F0001000000 Ipc: H01F0041020000 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: H01F 41/02 20060101AFI20160927BHEP Ipc: B22F 1/00 20060101ALI20160927BHEP Ipc: H01Q 17/00 20060101ALI20160927BHEP Ipc: H01F 1/24 20060101ALI20160927BHEP Ipc: H01F 1/33 20060101ALI20160927BHEP |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20161111 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 874200 Country of ref document: AT Kind code of ref document: T Effective date: 20170315 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602013018434 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20170308 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170609 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170608 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 874200 Country of ref document: AT Kind code of ref document: T Effective date: 20170308 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170608 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170710 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170708 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602013018434 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170313 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20171229 |
|
| 26N | No opposition filed |
Effective date: 20171211 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20170608 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170331 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170331 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170313 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20170331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170608 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170331 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170509 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170313 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20130313 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170308 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20210302 Year of fee payment: 9 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602013018434 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221001 |