EP0932166B1 - Polymeric PTC composition and circuit protection device made therefrom - Google Patents
Polymeric PTC composition and circuit protection device made therefrom Download PDFInfo
- Publication number
- EP0932166B1 EP0932166B1 EP19980120252 EP98120252A EP0932166B1 EP 0932166 B1 EP0932166 B1 EP 0932166B1 EP 19980120252 EP19980120252 EP 19980120252 EP 98120252 A EP98120252 A EP 98120252A EP 0932166 B1 EP0932166 B1 EP 0932166B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- ptc
- conductive particles
- composition
- metal
- particles
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01C—RESISTORS
- H01C7/00—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material
- H01C7/02—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having positive temperature coefficient
- H01C7/027—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having positive temperature coefficient consisting of conducting or semi-conducting material dispersed in a non-conductive organic material
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01C—RESISTORS
- H01C7/00—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material
- H01C7/13—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material current responsive
Definitions
- the present invention relates to an electric material, in particular it relates to a material composition having a positive temperature coefficient (PTC) of resistivity, which undergoes a rapid and sharp increase in resistance over a relatively narrow temperature range as temperature increases, i.e. a polymeric PTC composition, and to a circuit protection device employing the same, which is used for a breaker and the like.
- PTC positive temperature coefficient
- the PTC composition having the above-mentioned PTC characteristics has been generally used for a circuit protection element and the like which limits the current-flow in the circuit including a heater, a positive character thermistor, a heat sensor, a battery and the like, under short-circuit condition, and resets the circuit when the cause of the short-circuit is removed.
- the PTC composition include a circuit protection device incorporated in the circuit which comprises a PTC element made of the PTC composition and at least two electrodes electrically connected thereto, for use in protecting against over-voltage or over-temperature by the temperature self controlling function of the PTC element.
- the PTC element i.e., the PTC composition needs to have such current limiting performance that can be exhibited repeatedly even under high voltage. Also a sufficiently lowered initial resistivity ( ⁇ L ) and an effective PTC characteristic (a large ⁇ H / ⁇ L ) will improve the current limiting performance of the PTC element.
- ⁇ H refers to the peak resistivity which is given by a PTC curve at a high temperature.
- the PTC composition Various materials have been developed as the PTC composition, and one of the conventionally known compositions comprises BaTiO 3 and an oxide of a monovalent or trivalent metal added thereto.
- This material has a problem that it exhibits NTC (Negative Temperature Coefficient) characteristics immediately after the PTC characteristics are exhibited, thus the current starts to flow again within 1 msec or less.
- NTC Negative Temperature Coefficient
- PTC compositions which comprise an organic polymer such as polyethylene (abbreviated as PE), polypropylene and ethylene-acrylic acid copolymer, and conductive particles such as carbon black (abbreviated as CB), carbon fiber, graphite or finely divided metal particles, dispersed therein.
- PE polyethylene
- CB carbon black
- PTC compositions are generally produced by adding, followed by kneading, conductive particles of a necessary amount to one or more kinds of resins which are used as the organic polymer.
- ⁇ L of the resulting PTC element cannot be lowered to 0.1 ⁇ cm or less, even when the organic polymer is loaded with these conductive particles by closest packing, and when the ⁇ L of the PTC element is decreased to the minimum value as low as 0.1 ⁇ cm, ⁇ H / ⁇ L is decreased as well to be around 100 or so. Accordingly, the current limiting performance cannot be improved sufficiently.
- the resistivity of the metal particles per se is of the order of 10 -6 ⁇ cm, and it is much lower than 0.05 ⁇ cm that is the resistivity of CB per se. Accordingly, the ⁇ L of the resulting PTC device is expected to be lowered by the use of metal particles such as Cu and Ni, and yet those metal particles have not been used as the conductive particles for PTC compositions in comparison with CB.
- metal particles such as Cu and Ni
- those metal particles have not been used as the conductive particles for PTC compositions in comparison with CB.
- One of the biggest reasons for that is that the PTC compositions containing the conventionally known metal particles, used under large current and high voltage, cause internal arc phenomenon (micro arc is generated between conductive particles) and the composition undergoes electrical breakdown.
- the metal particles in the PTC composition are molten and the molten metal particles are bonded together to locally form a conductive circuit and the large current is concentrated on a part of the element and the element is destroyed. Discharge is also easily caused in a micro space between the composition and the electrode interface, the resin on the discharged part is degraded, and decomposed, thus the deterioration is accelerated disadvantageously. This inconveniency has been remarkable under an electric voltage of some 10 volts or higher. Accordingly, this type of composition has not been used for a self-reset type over-current protection element.
- EP-A-0 758 131 dicloses an organic PTC composition based on conductive substances having a temperature of fusion greater than 2000°C. Further, a coupling agent is added to the polymer before mixing with the conductive particles.
- the metal particles have a very low resistivity compared to that of CB, thus when metal particles are used as conductive particles in the PTC composition, the resistivity ( ⁇ L ) of the PTC element at an ordinary room temperature is decreased, and the PTC element is naturally expected to show good conductivity, but the conventionally known PTC composition containing the metal particles causes internal arc phenomenon when used under large current and high voltage, and the metal particles are melted and a conductive circuit is locally formed, resulting in the destruction of the composition as well the PTC element. Therefore, the conventionally known PTC composition containing the metal particles has a drawback that it lacks in safety and reliability and cannot protect the circuit repeatedly against the over-current.
- an object of the present invention is to provide a PTC composition having a low resistance and good conductivity under normal operating conditions, which does not locally form a conductive circuit under large current and high voltage but exhibits PTC characteristics to increase the resistivity of the PTC element, and protects the circuit against over-current. That means, an object of the present invention is to provide a PTC composition having excellent current limiting performance, high safety, and high reliability and which can be used favorably, for example, for a self-reset type over-current protection element.
- Another object of the present invention is to provide a circuit protection device of high safety and high reliability which has good conductivity under normal operating conditions, which shows excellent current limiting performance even under large current and high voltage and which works with high repeat stability.
- the polymeric PTC composition according to the present invention is a polymeric PTC composition which comprises an organic polymer and conductive particles having a melting point of not less than 2000 °C which are dispersed in the organic polymer, and the conductive particles are treated by a coupling agent before the organic polymer and the conductive particles are mixed.
- the conductive particles have an average particle size of 0.01 - 50 microns.
- the conductive particles are contained in an amount of 50 - 99 % by weight based on the composition.
- the conductive particles are particles containing at least one kind of substance selected from a metal, metal carbide, metal boride, metal siliside and metal nitride.
- tungsten is used as the metal.
- the coupling agent is contained in an amount of 0.05 - 10 % by weight based on the conductive particles and the coupling agent is an aluminium type or a titanium type coupling agent.
- the circuit protection device is defined by claim 10 and comprises a PTC element in which conductive particles having a melting point of not less than 2000 °C which are treated by a coupling agent before being dispersed in an organic polymer, and at least two electrodes which are electrically connected to the PTC element.
- the polymeric PTC composition according to the present invention comprises an organic polymer and conductive particles having a melting point of not less than 2000 °C that are dispersed in the organic polymer, and the conductive particles have been preliminarily treated with a coupling agent before the organic polymer and the conductive particles are mixed with each other.
- the conductive particles having a melting point as high as not less than 2000 °C are not melted and do not form a local conductive circuit in the PTC composition, or in the element, when it is used under large current and high voltage, and even an internal arc is generated, unlike the PTC composition containing the conventional metal particles, therefore the PTC composition, or the PTC element of the present invention is not electrically destroyed. Also, when large current flows, the temperature of the PTC element increases and the resistance increases as well, therefore the circuit can be protected against the over-current.
- the resistivity of the PTC composition according to the present invention at an ordinary room temperature ( ⁇ L ) can be sufficiently decreased so that good conductivity is exhibited under normal operating conditions and the peak resistance ( ⁇ H ) at an elevated temperature can be increased, that means ⁇ H / ⁇ L can be increased, therefore the flow of current can be securely cut-off when large current flows through the PTC composition.
- ⁇ L room temperature
- ⁇ H peak resistance
- the circuit protection device employing a PTC element comprising this PTC composition functions well repeatedly as a self-reset type over-current protection element.
- CB is a sublimating substance having no melting point and is not included in the category of the conductive particles according to the present invention.
- the average particle size of the conductive particle is preferably 0.01 - 50 ⁇ m, more preferably it is 0.1 - 30 ⁇ m.
- the reason is as follows: when the organic polymer is loaded with the conductive particles, particles having a small average particle size - having a narrow particle size distribution, and being bulky - cannot be loaded in a large amount and the resistivity of the PTC element at an ordinary room temperature is increased. On the other hand, the particles having a large average particle size result in the increase of the resistivity of the PTC element at an ordinary room temperature when the same amount of the particles are loaded in the polymer.
- FIG. 1 is a characteristic diagram showing the relationship between the particle size of tungsten contained in the PTC element, and the resistivity of the PTC element at an ordinary room temperature; wherein ⁇ (black circle) represents the case wherein the tungsten was loaded in an amount of 90 % by weight, and O (white circle) represents the case wherein the tungsten was loaded in an amount of 95 % by weight. It is shown that the resistivity of the PTC element at an ordinary room temperature increases with increase in average particle size.
- the conductive particles having the above-mentioned average particle size a PTC composition having a low resistivity at an ordinary room temperature can be obtained.
- the conductive particles having various particle sizes can be appropriately selected according to the application and the desired characteristics of the PTC composition.
- the content of the conductive particles is preferably 50 - 99 % by weight, more preferably it is 70 - 97 % by weight based on the PTC composition.
- the resistivity at an ordinary room temperature is increased.
- the content of the conductive particles is increased, the kneading torque during the kneading of the organic polymer with the conductive particles becomes high, and the kneading becomes difficult to carry out and the resulting PTC element shows low elasticity and a weak impact resistance.
- FIG. 2 is a characteristic diagram showing the relationship between the amount of tungsten loaded and the resistivity of the PTC element at an ordinary room temperature, and it is shown that the resistivity of the PTC element at an ordinary room temperature increases with decrease in amount of tungsten loaded.
- FIG. 3 is a characteristic diagram showing the relationship between the amount of tungsten loaded and the torque during the kneading, and it is shown that the torque during the kneading increases with increase in amount of tungsten loaded. The measurement was carried out by Laboplastomill equipment under the kneading condition of 200 °C and 50 rpm.
- any particle can be used as far as it has a melting point of not less than 2000 °C, has such electric conductivity, heat conductivity and fusion resistance to micro arc that are good enough for a PTC composition, and provides excellent PTC characteristics.
- Particles of a metal, metal carbide, metal boride, metal siliside and metal nitride are used as the conductive particles. These can be used alone or in admixture of two or more kinds, and appropriately selected according to the application and the desired characteristics of the PTC composition.
- An example of the metal particles includes tungsten (W).
- the metal carbide include TiC, ZrC, VC, NbC, TaC, Mo 2 C, and WC.
- the metal nitride include TiN, ZrN, VN, NbN, TaN, and Cr 2 N.
- the metal siliside include TaSi 2 , MoSi 2 , and WSi 2 .
- the metal boride include TiB 2 , ZrB 2 , NbB 2 , TaB 2 , CrB, MoB, and WB. (Ti: titanium, Zr: zirconium, V: vanadium, Nb: niobium, Ta: tantalum, Mo: molybdenum, and Cr: chromium.)
- Tungsten is a metal having the highest melting point (3410 °C) among metal particles, besides tungsten and a tungsten compound of a desired particle size are easily available as they are supplied steadily.
- organic polymer polyethylene, polyethylene oxide, polybutadiene, polyethylene acrylate, ethylene-ethyl acrylate copolymer, ethylene-acrylic acid copolymer, polyester, polyamide, polyether, polycaprolactam, fluorinated ethylene-propylene copolymer, chlorinated polyethylene, chlorosulphonated ethylene, ethylene-vinyl acetate copolymer, polypropylene, polystyrene, styrene-acrylonitrile copolymer, polyvinyl chloride, polycarbonate, polyacetal, polyalkylene oxide, polyphenylene oxide, polysulphone and a fluororesin are used according to the present invention and these can be used alone or two or more kinds of the compounds selected from these are used in admixture as a blended polymer.
- the kind, and the composition ratio of the organic polymer can be appropriately selected according to the desired property, and application.
- the PTC composition is prepared by mixing the organic polymer, conductive particles preliminarily treated with a coupling agent and other additives at a desired ratio followed by kneading.
- the conducive particles preliminarily treated with a coupling agent can be added to the organic polymer, then kneaded, or both materials can be simultaneously mixed and kneaded.
- the blending ratio of the organic polymer and the conductive particles can be appropriately selected according to the content of the conductive particles in the desired composition, the kind of the organic polymer, and the kind of the kneaders such as Banbury type mixer, pressure kneader and roll mill, but the amount of the conductive particles loaded shall be within the range of from 50 to 99 % by weight based on the PTC composition.
- a titanate type coupling agent and an aluminium type coupling agent can be used.
- the titanate type coupling agent include monoalkoxy type such as isopropyltriisostearoyl titanate, isopropyltrioctanoyl titanate, isopropyldiisostearoylcumylphenyl titanate, isopropyldistearoylmethacryl titanate, isopropyltri(dioctylpyrophosphate) titanate, or tetraisopropyldi(dilaurylphosphite) titanate, isostearoyloxy acetate, isostearoylacryloxy acetate, distearoylethylene titanate, and dimethacrylethylene titanate.
- an aluminium coupling agent any agent which is effective for improving the adhesion between the metal and the plastic such as acetoalkoxyaluminium diisopropylate can be used.
- the amount of the above-mentioned coupling agent is 0.05 - 10 % by weight based on the conductive particles in order to improve the environmental resistance properties.
- additives can be mixed, if necessary, with the above-mentioned organic polymer, conductive particles and the coupling agent.
- the additive include an antioxidant, a stabilizer, and a flame-retardant such an antimony compound, phosphorus compound, chlorine compound and bromine compound.
- the PTC composition of the present invention can be used for various uses.
- the PTC composition can be molded into, illustratively, a film form and metal foil electrodes are bonded on the front and the back surfaces of the film by thermocompression bonding to form a laminate, then the laminate is cut to a desired size and lead wires are welded on the electrode surface by soldering, brazing, or spot welding and the like to provide a PTC element.
- the polymeric PTC composition of the present invention shows a low resistance and good conductivity under normal operating conditions, and even under large current and high voltage, the conductive particles are not melted to locally form a conductive circuit, but the resistance is increased due to the PTC characteristics to protect the circuit against the over-current, by dispersing the conductive particles having a melting point of not less than 2000 °C in an organic polymer and after having treated the conductive particles with a coupling agent.
- a polymeric PTC composition having excellent PTC characteristics, and current limiting performance, high safety and long-term reliability (environmental resistance properties) can be obtained.
- a polymeric PTC composition having a low resistivity at an ordinary room temperature can be obtained by the use of conductive particles having an average particle size of 0.01 - 50 ⁇ m in the first constitution.
- the third embodiment of the polymeric PTC composition of the present invention there is an advantage that a polymeric PTC composition having a low resistivity at an ordinary room temperature which is more suited for practical use can be obtained by incorporating the conductive particles in the composition in an amount of 50 - 99 % by weight in the first or second constitution.
- a polymeric PTC composition having excellent PTC characteristics and current limiting performance can be obtained by employing particles containing at least one kind of a metal, metal carbide, metal boride, metal siliside and metal nitride as conductive particles in the first, second or third constitution.
- the fifth embodiment of the polymeric PTC composition of the present invention there is an advantage that a polymeric PTC composition having higher safety and reliability, excellent PTC characteristics and current limiting performance can be obtained by employing tungsten as the metal in the fourth constitution.
- the sixth embodiment of the polymeric PTC composition of the present invention there is an advantage that a polymeric PTC composition having reliable environmental resistance properties, excellent PTC characteristics and current limiting performance can be obtained by incorporating the coupling agent in an amount of 0.05 - 10 % by weight based on the conductive particles and by employing an aluminium type or titanate type coupling agent as the coupling agent.
- the circuit protection device comprises a PTC element wherein conductive particles having a melting point of not less than 2000 °C, which are treated with a coupling agent are dispersed in an organic polymer, and at least two electrodes which are electrically connected to the PTC element, and it is advantageous since the circuit protection device provides excellent current limiting performance.
- tungsten having an average particle size of 0.88 ⁇ m, available from Nippon Shinkinzoku Co., Ltd. under the trade name of W-1
- a titanate type coupling agent available from Ajinomoto Co., Ltd., under the trade name of KR TTS
- the isopropyl alcohol was removed by filtration and the composition left on the filter paper was dried under vacuum for 24 hours.
- the produced PTC composition was hot-pressed to provide a plate of 40 x 60 x 1 mm.
- a polyethylene frame was produced by injection molding on the periphery of the plate for 20 mm to carry out insulation at the cut-off. Then the plate of the PTC composition with the frame was sandwiched between electrodes to provide a PTC element.
- the characteristic diagram of FIG. 4 shows the PTC curve representing the relationship between the temperature and the resistivity of the PTC element.
- the resistivity at an ordinary room temperature ( ⁇ L ) was 0.02 ⁇ cm
- the peak resistivity ( ⁇ H ) was 10 5 ⁇ cm
- ⁇ H / ⁇ L was 10 7 .
- the current limiting peak value (the peak current at the cut-off of the over-current: I P ) for the over-current of 50 kA at 300 V was 7.5 kA.
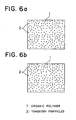
- FIG. 6 is a schematic illustration of an optical microscope photograph showing the dispersion condition of tungsten particles 2, which are the conductive particles of the PTC composition;
- FIG. 6 (a) shows the condition before the cut-off (current limiting) test, and
- FIG. 6 (b) shows the condition after the cut-off test.
- the FIGs. show that there was no change between the conditions before and after the cut-off test, and that tungsten particles 2 were similarly and homogeneously dispersed in the organic polymer 1.
- the PTC element having an initial resistivity of 0.02 ⁇ cm was subjected to an environmental test (under high temperature, high humidity of 85 °C and 85 %) and it showed a resistivity of 0.1 ⁇ cm after 1000 hours.
- the resistivity of a PTC element which had not subjected to the coupling treatment was very much increased from its initial value of 0.02 ⁇ cm to 960 ⁇ cm after 1000 hours.
- the resistivity of a PTC element which had not been subjected to the coupling treatment was very much increased from its initial value of 0.02 ⁇ cm to 100 ⁇ cm after 300 cycles.
- tungsten carbide having an average particle size of 0.7 ⁇ m, available from Nippon Shinkinzoku Co., Ltd. under the trade name of WC-10) were added to a solution comprising 1 part by weight of an aluminium type coupling agent (available from Ajinomoto Co., Ltd., under the trade name of AL-M) dissolved in 28 parts by weight of isopropyl alcohol, and mixed for 10 minutes.
- AL-M aluminium type coupling agent
- the dried composition (tungsten carbide having subjected to coupling treatment), 10 parts by weight of HDPE (available from Mitsubishi Chemical Co., Ltd., under the trade name of HJ560), and 2 parts by weight of a phenol type antioxidant (available from Ciba-Geigy Ltd., under the trade name of Irganox 1010) were kneaded by Laboplastomill equipment (manufactured by Toyo Seiki Co., Ltd.) at 200 °C for 15 minutes.
- the produced PTC composition was hot-pressed to provide a plate of 40 x 60 x 1 mm.
- a polyethylene frame was produced by injection molding on the periphery of the plate for 20 mm to carry out insulation at the cut-off.
- the plate of the PTC composition with the frame was sandwiched between electrodes to provide a PTC element.
- the resistivity at an ordinary room temperature ( ⁇ L ) was 0.02 ⁇ cm
- the peak resistivity ( ⁇ H ) was 10 5 ⁇ cm
- ⁇ H / ⁇ L was 10 7 .
- the resistance of the PTC element at a room temperature was 1.5 m ⁇
- the current limiting peak value for the over-current of 50 kA at 300 V was 8 kA.
- a PTC element having an initial resistivity of 0.02 ⁇ cm was subjected to an environmental test (under high temperature, high humidity of 85 °C and 85 %), and it showed a resistivity of 0.03 ⁇ cm after 1000 hours.
- the resistivity of a PTC element which had not been subjected to the coupling treatment was very much increased from its initial value of 0.02 ⁇ cm to 115 ⁇ cm after 1000 hours.
- a PTC element having an initial resistivity of 0.02 ⁇ cm was subjected to an environmental test (heat shock cycle test : -25 °C for 30 minutes 85 °C for 30 minutes) and it showed a resistivity of 0.15 ⁇ cm after 300 cycles.
- the resistivity of a PTC element which had not been subjected to the coupling treatment was very much increased from its initial value of 0.02 ⁇ cm to 1.6 ⁇ cm after 300 cycles.
- Example 1 The results of current limiting peak value and environmental tests obtained for Examples 1 and 2 and the results of current limiting peak value and environmental tests obtained when the kinds of the polymers, fillers and coupling agents in the PTC compositions are changed (Examples 3 - 27) are summarized in Table 1. It is shown that the coupling treatment does not affect the current limiting peak value but provides good results for the environmental tests (high temperature, high humidity of 85 °C and 85 %, heat shock: -25 °C for 30 minutes 85 °C for 30 minutes). Table 1 Example No.
- the plate of PTC composition with the frame was sandwiched between electrodes to provide a PTC element.
- the PTC element having a resistance at a room temperature of 1 m ⁇ , could not cut off the flow of current when large current of 50 kA flowed at high voltage of 300 V.
- the PTC composition of the Comparative Example was loaded with silver particles having a low melting point, caused internal arc phenomenon (micro arc was generated among the conductive particles) under large current and high voltage, and the PTC composition was electrically destroyed. It is deemed that once the internal arc phenomenon was caused, the heat thereof melted the silver particles in the PTC composition, then the silver particles were bonded together and large current flowed through the bonded part and the composition underwent the electrical breakdown.
- the PTC element having a resistance at a room temperature of 1 m ⁇ could not cut off the flow of current when large current of 50 kA flowed at high voltage of 300 V. It is deemed that this was because nickel particles in the PTC composition were melted to locally form a conductive circuit as is the case with Comparative Examples 1 and 2.
- FIG. 7 is a schematic illustration of an optical microscope photograph showing the dispersion condition of nickel particles 3 in the PTC composition
- FIG. 7 (a) illustrates the condition prior to the cut-off (current limiting) test
- FIG. 7 (b) illustrates the condition after the cut-off test in which the device was destroyed.
- the nickel particles 3 Prior to the cut-off test, the nickel particles 3 were homogeneously dispersed in the organic polymer 1, but after the cut-off test, the nickel particles 3 were melted and bonded together to form the bonded part of nickel particles 3a. It is deemed that since the nickel particles 3 in the PTC composition were melted to form the bonded part of nickel particles 3a (i.e. a conductive circuit was formed), the over-current could not be cut off as is the case with Comparative Examples 1 and 2, and the element was destroyed.
Landscapes
- Engineering & Computer Science (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Physics & Mathematics (AREA)
- Electromagnetism (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Ceramic Engineering (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Thermistors And Varistors (AREA)
Description
- The present invention relates to an electric material, in particular it relates to a material composition having a positive temperature coefficient (PTC) of resistivity, which undergoes a rapid and sharp increase in resistance over a relatively narrow temperature range as temperature increases, i.e. a polymeric PTC composition, and to a circuit protection device employing the same, which is used for a breaker and the like.
- The PTC composition having the above-mentioned PTC characteristics has been generally used for a circuit protection element and the like which limits the current-flow in the circuit including a heater, a positive character thermistor, a heat sensor, a battery and the like, under short-circuit condition, and resets the circuit when the cause of the short-circuit is removed.
- Further applications of the PTC composition include a circuit protection device incorporated in the circuit which comprises a PTC element made of the PTC composition and at least two electrodes electrically connected thereto, for use in protecting against over-voltage or over-temperature by the temperature self controlling function of the PTC element.
- Now, the protection mechanism obtained with the PTC element against over-current will be described. As the resistivity (ρL) of a PTC composition at an ordinary room temperature is sufficiently low, normally current flows through the circuit. But, if large current flows through the circuit by short-circuit accident and the like, Joule heat is generated in the PTC element due to the large current, and the temperature of the element rises, thus the resistivity increases (exhibition of PTC behavior), so that the current does not flow through the element and the circuit can be protected (this is referred to as current limiting performance).
- The PTC element, i.e., the PTC composition needs to have such current limiting performance that can be exhibited repeatedly even under high voltage. Also a sufficiently lowered initial resistivity (ρL) and an effective PTC characteristic (a large ρH/ρL) will improve the current limiting performance of the PTC element. ρH refers to the peak resistivity which is given by a PTC curve at a high temperature.
- Various materials have been developed as the PTC composition, and one of the conventionally known compositions comprises BaTiO3 and an oxide of a monovalent or trivalent metal added thereto. This material, however, has a problem that it exhibits NTC (Negative Temperature Coefficient) characteristics immediately after the PTC characteristics are exhibited, thus the current starts to flow again within 1 msec or less.
- To cope with this problem, PTC compositions have been developed which comprise an organic polymer such as polyethylene (abbreviated as PE), polypropylene and ethylene-acrylic acid copolymer, and conductive particles such as carbon black (abbreviated as CB), carbon fiber, graphite or finely divided metal particles, dispersed therein. Theses PTC compositions are generally produced by adding, followed by kneading, conductive particles of a necessary amount to one or more kinds of resins which are used as the organic polymer.
- If CB, carbon fiber or graphite is used as conductive particles, ρL of the resulting PTC element cannot be lowered to 0.1 Ωcm or less, even when the organic polymer is loaded with these conductive particles by closest packing, and when the ρL of the PTC element is decreased to the minimum value as low as 0.1 Ωcm, ρH/ρL is decreased as well to be around 100 or so. Accordingly, the current limiting performance cannot be improved sufficiently.
- On the other hand, the resistivity of the metal particles per se is of the order of 10-6 Ωcm, and it is much lower than 0.05 Ωcm that is the resistivity of CB per se. Accordingly, the ρL of the resulting PTC device is expected to be lowered by the use of metal particles such as Cu and Ni, and yet those metal particles have not been used as the conductive particles for PTC compositions in comparison with CB. One of the biggest reasons for that is that the PTC compositions containing the conventionally known metal particles, used under large current and high voltage, cause internal arc phenomenon (micro arc is generated between conductive particles) and the composition undergoes electrical breakdown. When the internal arc phenomenon is caused, the metal particles in the PTC composition are molten and the molten metal particles are bonded together to locally form a conductive circuit and the large current is concentrated on a part of the element and the element is destroyed. Discharge is also easily caused in a micro space between the composition and the electrode interface, the resin on the discharged part is degraded, and decomposed, thus the deterioration is accelerated disadvantageously. This inconveniency has been remarkable under an electric voltage of some 10 volts or higher. Accordingly, this type of composition has not been used for a self-reset type over-current protection element.
- A PTC composition containing both CB and metal particles as conductive particles has been disclosed in the publication of Japanese Patent Laid-Open NO. 64-53503. There, the metal particles are contained in order to improve the heat-conductivity of the PTC composition. EP-A-0 758 131 dicloses an organic PTC composition based on conductive substances having a temperature of fusion greater than 2000°C. Further, a coupling agent is added to the polymer before mixing with the conductive particles.
- As described above, the metal particles have a very low resistivity compared to that of CB, thus when metal particles are used as conductive particles in the PTC composition, the resistivity (ρL) of the PTC element at an ordinary room temperature is decreased, and the PTC element is naturally expected to show good conductivity, but the conventionally known PTC composition containing the metal particles causes internal arc phenomenon when used under large current and high voltage, and the metal particles are melted and a conductive circuit is locally formed, resulting in the destruction of the composition as well the PTC element. Therefore, the conventionally known PTC composition containing the metal particles has a drawback that it lacks in safety and reliability and cannot protect the circuit repeatedly against the over-current.
- The present invention has been achieved in order to solve the above-mentioned problems, and an object of the present invention is to provide a PTC composition having a low resistance and good conductivity under normal operating conditions, which does not locally form a conductive circuit under large current and high voltage but exhibits PTC characteristics to increase the resistivity of the PTC element, and protects the circuit against over-current. That means, an object of the present invention is to provide a PTC composition having excellent current limiting performance, high safety, and high reliability and which can be used favorably, for example, for a self-reset type over-current protection element.
- Another object of the present invention is to provide a circuit protection device of high safety and high reliability which has good conductivity under normal operating conditions, which shows excellent current limiting performance even under large current and high voltage and which works with high repeat stability.
- The polymeric PTC composition according to the present invention is a polymeric PTC composition which comprises an organic polymer and conductive particles having a melting point of not less than 2000 °C which are dispersed in the organic polymer, and the conductive particles are treated by a coupling agent before the organic polymer and the conductive particles are mixed.
- In a second embodiment of the present invention, the conductive particles have an average particle size of 0.01 - 50 microns.
- In a third embodiment of the present invention, the conductive particles are contained in an amount of 50 - 99 % by weight based on the composition.
- In a fourth embodiment of the present invention, the conductive particles are particles containing at least one kind of substance selected from a metal, metal carbide, metal boride, metal siliside and metal nitride.
- In a fifth embodiment of the present invention, tungsten is used as the metal.
- In a sixth embodiment of the present invention, the coupling agent is contained in an amount of 0.05 - 10 % by weight based on the conductive particles and the coupling agent is an aluminium type or a titanium type coupling agent.
- The circuit protection device according to the present invention is defined by
claim 10 and comprises a PTC element in which conductive particles having a melting point of not less than 2000 °C which are treated by a coupling agent before being dispersed in an organic polymer, and at least two electrodes which are electrically connected to the PTC element. -
- FIG. 1 is a characteristic diagram showing the relationship between the particle size of the conductive particles (tungsten) according to the present invention and the resistivity of the PTC element at an ordinary room temperature;
- FIG. 2 is a characteristic diagram showing the relationship between the amount of the conductive particles (tungsten) loaded according to the present invention and the resistivity of the PTC element at an ordinary room temperature;
- FIG. 3 is a characteristic diagram showing the relationship between the amount of the conductive particles (tungsten) loaded according to the present invention and the torque during the kneading;
- FIG. 4 is a characteristic diagram showing the PTC curve representing the relationship between the temperature and the resistivity of the PTC element according to Example 1 of the present invention;
- FIG. 5 is a characteristic diagram showing the relationship between the resistivity of the PTC element according to Example 1 and current limiting peak value (the peak current at the cut-off of the over-current: IP).
- FIG. 6 is a schematic illustration of an optical microscope photograph taken before and after current limiting test, showing the dispersion condition of tungsten particles, which are the conductive particles of the PTC composition according to Example 1 of the present invention; and
- FIG. 7 is a schematic illustration of an optical microscope photograph taken before and after current limiting test, showing the dispersion condition of nickel particles, which are the conductive particles of the PTC element according to Comparative Example 3 of the present invention.
- The polymeric PTC composition according to the present invention comprises an organic polymer and conductive particles having a melting point of not less than 2000 °C that are dispersed in the organic polymer, and the conductive particles have been preliminarily treated with a coupling agent before the organic polymer and the conductive particles are mixed with each other.
- With the polymeric PTC composition according to the present invention, the conductive particles having a melting point as high as not less than 2000 °C are not melted and do not form a local conductive circuit in the PTC composition, or in the element, when it is used under large current and high voltage, and even an internal arc is generated, unlike the PTC composition containing the conventional metal particles, therefore the PTC composition, or the PTC element of the present invention is not electrically destroyed. Also, when large current flows, the temperature of the PTC element increases and the resistance increases as well, therefore the circuit can be protected against the over-current.
- In addition, the resistivity of the PTC composition according to the present invention at an ordinary room temperature (ρL) can be sufficiently decreased so that good conductivity is exhibited under normal operating conditions and the peak resistance (ρH) at an elevated temperature can be increased, that means ρH/ρL can be increased, therefore the flow of current can be securely cut-off when large current flows through the PTC composition. Thus a PTC composition having an excellent current limiting performance, high safety and high reliability can be obtained, and the circuit protection device employing a PTC element comprising this PTC composition functions well repeatedly as a self-reset type over-current protection element.
- CB is a sublimating substance having no melting point and is not included in the category of the conductive particles according to the present invention.
- The average particle size of the conductive particle is preferably 0.01 - 50 µm, more preferably it is 0.1 - 30 µm. The reason is as follows: when the organic polymer is loaded with the conductive particles, particles having a small average particle size - having a narrow particle size distribution, and being bulky - cannot be loaded in a large amount and the resistivity of the PTC element at an ordinary room temperature is increased. On the other hand, the particles having a large average particle size result in the increase of the resistivity of the PTC element at an ordinary room temperature when the same amount of the particles are loaded in the polymer. FIG. 1 is a characteristic diagram showing the relationship between the particle size of tungsten contained in the PTC element, and the resistivity of the PTC element at an ordinary room temperature; wherein ● (black circle) represents the case wherein the tungsten was loaded in an amount of 90 % by weight, and O (white circle) represents the case wherein the tungsten was loaded in an amount of 95 % by weight. It is shown that the resistivity of the PTC element at an ordinary room temperature increases with increase in average particle size. By the use of the conductive particles having the above-mentioned average particle size, a PTC composition having a low resistivity at an ordinary room temperature can be obtained. The conductive particles having various particle sizes can be appropriately selected according to the application and the desired characteristics of the PTC composition.
- The content of the conductive particles is preferably 50 - 99 % by weight, more preferably it is 70 - 97 % by weight based on the PTC composition. With low content of the conductive particles, the resistivity at an ordinary room temperature is increased. When the content of the conductive particles is increased, the kneading torque during the kneading of the organic polymer with the conductive particles becomes high, and the kneading becomes difficult to carry out and the resulting PTC element shows low elasticity and a weak impact resistance. FIG. 2 is a characteristic diagram showing the relationship between the amount of tungsten loaded and the resistivity of the PTC element at an ordinary room temperature, and it is shown that the resistivity of the PTC element at an ordinary room temperature increases with decrease in amount of tungsten loaded. FIG. 3 is a characteristic diagram showing the relationship between the amount of tungsten loaded and the torque during the kneading, and it is shown that the torque during the kneading increases with increase in amount of tungsten loaded. The measurement was carried out by Laboplastomill equipment under the kneading condition of 200 °C and 50 rpm.
- As the conductive particles, any particle can be used as far as it has a melting point of not less than 2000 °C, has such electric conductivity, heat conductivity and fusion resistance to micro arc that are good enough for a PTC composition, and provides excellent PTC characteristics. Particles of a metal, metal carbide, metal boride, metal siliside and metal nitride are used as the conductive particles. These can be used alone or in admixture of two or more kinds, and appropriately selected according to the application and the desired characteristics of the PTC composition.
- An example of the metal particles includes tungsten (W). Examples of the metal carbide include TiC, ZrC, VC, NbC, TaC, Mo2C, and WC. Examples of the metal nitride include TiN, ZrN, VN, NbN, TaN, and Cr2N. Examples of the metal siliside include TaSi2, MoSi2, and WSi2. Examples of the metal boride include TiB2, ZrB2, NbB2, TaB2, CrB, MoB, and WB. (Ti: titanium, Zr: zirconium, V: vanadium, Nb: niobium, Ta: tantalum, Mo: molybdenum, and Cr: chromium.)
- In particular, it is preferable to use particles of tungsten, and the carbide, boride, siliside and nitride thereof. Tungsten is a metal having the highest melting point (3410 °C) among metal particles, besides tungsten and a tungsten compound of a desired particle size are easily available as they are supplied steadily.
- As the organic polymer, polyethylene, polyethylene oxide, polybutadiene, polyethylene acrylate, ethylene-ethyl acrylate copolymer, ethylene-acrylic acid copolymer, polyester, polyamide, polyether, polycaprolactam, fluorinated ethylene-propylene copolymer, chlorinated polyethylene, chlorosulphonated ethylene, ethylene-vinyl acetate copolymer, polypropylene, polystyrene, styrene-acrylonitrile copolymer, polyvinyl chloride, polycarbonate, polyacetal, polyalkylene oxide, polyphenylene oxide, polysulphone and a fluororesin are used according to the present invention and these can be used alone or two or more kinds of the compounds selected from these are used in admixture as a blended polymer. The kind, and the composition ratio of the organic polymer can be appropriately selected according to the desired property, and application.
- The PTC composition is prepared by mixing the organic polymer, conductive particles preliminarily treated with a coupling agent and other additives at a desired ratio followed by kneading. The conducive particles preliminarily treated with a coupling agent can be added to the organic polymer, then kneaded, or both materials can be simultaneously mixed and kneaded. The blending ratio of the organic polymer and the conductive particles can be appropriately selected according to the content of the conductive particles in the desired composition, the kind of the organic polymer, and the kind of the kneaders such as Banbury type mixer, pressure kneader and roll mill, but the amount of the conductive particles loaded shall be within the range of from 50 to 99 % by weight based on the PTC composition.
- In the preparation of the above-mentioned PTC composition, use of conductive particles which have been preliminary subjected to coupling treatment improves the environmental resistance properties such as high temperature, high humidity resistance or heat shock resistance.
- As a coupling agent, a titanate type coupling agent and an aluminium type coupling agent can be used. Examples of the titanate type coupling agent include monoalkoxy type such as isopropyltriisostearoyl titanate, isopropyltrioctanoyl titanate, isopropyldiisostearoylcumylphenyl titanate, isopropyldistearoylmethacryl titanate, isopropyltri(dioctylpyrophosphate) titanate, or tetraisopropyldi(dilaurylphosphite) titanate, isostearoyloxy acetate, isostearoylacryloxy acetate, distearoylethylene titanate, and dimethacrylethylene titanate. As an aluminium coupling agent, any agent which is effective for improving the adhesion between the metal and the plastic such as acetoalkoxyaluminium diisopropylate can be used.
- The amount of the above-mentioned coupling agent is 0.05 - 10 % by weight based on the conductive particles in order to improve the environmental resistance properties.
- For preparation of the PTC composition, various additives can be mixed, if necessary, with the above-mentioned organic polymer, conductive particles and the coupling agent. Examples of the additive include an antioxidant, a stabilizer, and a flame-retardant such an antimony compound, phosphorus compound, chlorine compound and bromine compound.
- The PTC composition of the present invention can be used for various uses. When it is used as a PTC element, the PTC composition can be molded into, illustratively, a film form and metal foil electrodes are bonded on the front and the back surfaces of the film by thermocompression bonding to form a laminate, then the laminate is cut to a desired size and lead wires are welded on the electrode surface by soldering, brazing, or spot welding and the like to provide a PTC element.
- According to the polymeric PTC composition of the present invention, there is an advantage that the polymeric PTC composition shows a low resistance and good conductivity under normal operating conditions, and even under large current and high voltage, the conductive particles are not melted to locally form a conductive circuit, but the resistance is increased due to the PTC characteristics to protect the circuit against the over-current, by dispersing the conductive particles having a melting point of not less than 2000 °C in an organic polymer and after having treated the conductive particles with a coupling agent. There is also an advantage that a polymeric PTC composition having excellent PTC characteristics, and current limiting performance, high safety and long-term reliability (environmental resistance properties) can be obtained.
- According to the second embodiment of the polymeric PTC composition of the present invention, there is an advantage that a polymeric PTC composition having a low resistivity at an ordinary room temperature can be obtained by the use of conductive particles having an average particle size of 0.01 - 50 µm in the first constitution.
- According to the third embodiment of the polymeric PTC composition of the present invention, there is an advantage that a polymeric PTC composition having a low resistivity at an ordinary room temperature which is more suited for practical use can be obtained by incorporating the conductive particles in the composition in an amount of 50 - 99 % by weight in the first or second constitution.
- According to the fourth embodiment of the polymeric PTC composition of the present invention, there is an advantage that a polymeric PTC composition having excellent PTC characteristics and current limiting performance can be obtained by employing particles containing at least one kind of a metal, metal carbide, metal boride, metal siliside and metal nitride as conductive particles in the first, second or third constitution.
- According to the fifth embodiment of the polymeric PTC composition of the present invention, there is an advantage that a polymeric PTC composition having higher safety and reliability, excellent PTC characteristics and current limiting performance can be obtained by employing tungsten as the metal in the fourth constitution.
- According to the sixth embodiment of the polymeric PTC composition of the present invention, there is an advantage that a polymeric PTC composition having reliable environmental resistance properties, excellent PTC characteristics and current limiting performance can be obtained by incorporating the coupling agent in an amount of 0.05 - 10 % by weight based on the conductive particles and by employing an aluminium type or titanate type coupling agent as the coupling agent.
- The circuit protection device according to the present invention comprises a PTC element wherein conductive particles having a melting point of not less than 2000 °C, which are treated with a coupling agent are dispersed in an organic polymer, and at least two electrodes which are electrically connected to the PTC element, and it is advantageous since the circuit protection device provides excellent current limiting performance.
- To further illustrate this invention, and not by way of limitation, the following examples are given.
- 100 parts by weight of tungsten (having an average particle size of 0.88 µm, available from Nippon Shinkinzoku Co., Ltd. under the trade name of W-1) were added to a solution comprising 1 part by weight of a titanate type coupling agent (available from Ajinomoto Co., Ltd., under the trade name of KR TTS) dissolved in 28 parts by weight of isopropyl alcohol, and mixed for 10 minutes. The isopropyl alcohol was removed by filtration and the composition left on the filter paper was dried under vacuum for 24 hours. 90 parts by weight of the dried composition (tungsten having subjected to coupling treatment), 10 parts by weight of HDPE (available from Mitsubishi Chemical Co., Ltd., under the trade name of HJ560), and 2 parts by weight of a phenol type antioxidant (available from Ciba-Geigy Ltd., under the trade name of Irganox 1010) were kneaded by Laboplastomill equipment (manufactured by Toyo Seiki Co., Ltd.) at 200 °C for 15 minutes.
- The produced PTC composition was hot-pressed to provide a plate of 40 x 60 x 1 mm. A polyethylene frame was produced by injection molding on the periphery of the plate for 20 mm to carry out insulation at the cut-off. Then the plate of the PTC composition with the frame was sandwiched between electrodes to provide a PTC element. The characteristic diagram of FIG. 4 shows the PTC curve representing the relationship between the temperature and the resistivity of the PTC element. The resistivity at an ordinary room temperature (ρL) was 0.02 Ωcm, the peak resistivity (ρH) was 105 Ωcm, ρH/ρL was 107. When the resistance of the PTC element at a room temperature was 1.2 mΩ, the current limiting peak value (the peak current at the cut-off of the over-current: IP) for the over-current of 50 kA at 300 V was 7.5 kA.
- Using PTC elements having different sizes and different resistance, the relationship between the resistance of the PTC element comprising the PTC composition and the current limiting peak value was examined. The results are shown by the characteristic diagram of FIG. 5.
- FIG. 6 is a schematic illustration of an optical microscope photograph showing the dispersion condition of
tungsten particles 2, which are the conductive particles of the PTC composition; FIG. 6 (a) shows the condition before the cut-off (current limiting) test, and FIG. 6 (b) shows the condition after the cut-off test. The FIGs. show that there was no change between the conditions before and after the cut-off test, and thattungsten particles 2 were similarly and homogeneously dispersed in theorganic polymer 1. - The PTC element having an initial resistivity of 0.02 Ωcm was subjected to an environmental test (under high temperature, high humidity of 85 °C and 85 %) and it showed a resistivity of 0.1 Ωcm after 1000 hours. On the other hand, the resistivity of a PTC element which had not subjected to the coupling treatment was very much increased from its initial value of 0.02 Ωcm to 960 Ωcm after 1000 hours.
- A PTC element having an initial resistivity of 0.02 Qcm was subjected to an environmental test (heat shock cycle test : -25 °C for 30 minutes <=> 85 °C for 30 minutes) then it showed a resistivity of 0.2 Ωcm after 300 cycles. On the other hand, the resistivity of a PTC element which had not been subjected to the coupling treatment was very much increased from its initial value of 0.02 Ωcm to 100 Ωcm after 300 cycles.
- 100 parts by weight of tungsten carbide (having an average particle size of 0.7 µm, available from Nippon Shinkinzoku Co., Ltd. under the trade name of WC-10) were added to a solution comprising 1 part by weight of an aluminium type coupling agent (available from Ajinomoto Co., Ltd., under the trade name of AL-M) dissolved in 28 parts by weight of isopropyl alcohol, and mixed for 10 minutes. The isopropyl alcohol was removed by filtration and the composition left on the filter paper was dried under vacuum for 24 hours.
- 90 parts by weight of the dried composition (tungsten carbide having subjected to coupling treatment), 10 parts by weight of HDPE (available from Mitsubishi Chemical Co., Ltd., under the trade name of HJ560), and 2 parts by weight of a phenol type antioxidant (available from Ciba-Geigy Ltd., under the trade name of Irganox 1010) were kneaded by Laboplastomill equipment (manufactured by Toyo Seiki Co., Ltd.) at 200 °C for 15 minutes. The produced PTC composition was hot-pressed to provide a plate of 40 x 60 x 1 mm. A polyethylene frame was produced by injection molding on the periphery of the plate for 20 mm to carry out insulation at the cut-off. Then the plate of the PTC composition with the frame was sandwiched between electrodes to provide a PTC element. The resistivity at an ordinary room temperature (ρL) was 0.02 Ωcm, the peak resistivity (ρH) was 105 Ωcm, ρH/ρL was 107. When the resistance of the PTC element at a room temperature was 1.5 mΩ, the current limiting peak value for the over-current of 50 kA at 300 V was 8 kA.
- A PTC element having an initial resistivity of 0.02 Ωcm was subjected to an environmental test (under high temperature, high humidity of 85 °C and 85 %), and it showed a resistivity of 0.03 Ωcm after 1000 hours. On the other hand, the resistivity of a PTC element which had not been subjected to the coupling treatment was very much increased from its initial value of 0.02 Ωcm to 115 Ωcm after 1000 hours.
- A PTC element having an initial resistivity of 0.02 Ωcm was subjected to an environmental test (heat shock cycle test : -25 °C for 30
minutes 85 °C for 30 minutes) and it showed a resistivity of 0.15 Ωcm after 300 cycles. On the other hand, the resistivity of a PTC element which had not been subjected to the coupling treatment was very much increased from its initial value of 0.02 Ω cm to 1.6 Ωcm after 300 cycles. - The results of current limiting peak value and environmental tests obtained for Examples 1 and 2 and the results of current limiting peak value and environmental tests obtained when the kinds of the polymers, fillers and coupling agents in the PTC compositions are changed (Examples 3 - 27) are summarized in Table 1. It is shown that the coupling treatment does not affect the current limiting peak value but provides good results for the environmental tests (high temperature, high humidity of 85 °C and 85 %, heat shock: -25 °C for 30
minutes 85 °C for 30 minutes).Table 1 Example No. Polymer (parts by weight) Filler (parts by weight) Coupling agent (parts by weight) Current limiting peak value at 300 V, 50 KA (KA) Initial resistivity (Ωcm) High temperature high humidity: 85°C 85% (Ωcm) Heat shock: after 300 cycles (Ωcm) 1 HDPE(10) W(90) KRTTS(0.27) 7.5 0.02 0.1 0.2 2 HDPE(10) WC(90) AL-M(0.27) 8 0.02 0.03 0.15 3 HDPE(10) W(90) KR138S(0.27) 7.5 0.03 0.1 0.2 4 HDPE(10) W(90) KR9SA(0.27) 7.5 0.02 0.1 0.2 5 HDPE(10) W(90) KR55(0.27) 8 0.02 0.3 0.5 6 HDPE(10) W(90) KR41B(0.27) 7.7 0.02 0.4 0.5 7 HDPE(10) W(90) KR38S(0.27) 7.8 0.02 0.3 0.5 8 BDPE(10) W(90) KR46B(0.27) 8 0.02 0.4 0.6 9 HDPE(10) W(90) KR238S(0.27) 7.7 0.02 0.4 0.4 10 HDPE(10) W(90) 338X(0.27) 7.9 0.02 0.3 0.5 11 HDPE(10) W(90) KR44(0.27) 8.1 0.02 0.3 0.5 12 HDPE(10) WC(90) KRTTS(0.27) 9 0.02 0.1 0.2 13 HDPE(10) WC(90) KR138S(0.27) 9 0.02 0.1 0.2 14 HDPE(10) WC(90) KR9SA(0.27) 9 0.02 0.1 0.2 15 HDPE(10) WC(90) KR55(0.27) 9 0.02 0.4 0.5 16 HDPE (10) WC(90) KR41B(0.27) 9 0.02 0.4 0.5 17 HDPE(10) WC(90) KR38S(0.27) 9 0.02 0.4 0.5 18 HDPE(10) WC(90) KR46B(0.27) 9 0.02 0.4 0.5 19 HDPE(10) WC(90) KR238S(0.27) 9 0.02 0.4 0.5 20 HDPE(10) WC(90) 338X(0.27) 9 0.02 0.3 0.5 21 HDPE(10) WC(90) KR44(0.27) 9 0.02 0.3 0.5 22 PP(10) W(90) KRTTS(0.27) 10 0.02 0.1 0.2 23 PP(10) WC(90) AL-M(0.27) 10 0.02 0.03 0.15 24 PS(10) WSi2(90) KRTTS(0.27) 12 0.02 0.03 0.15 25 PS(10) WB(90) AL-M(0.27) 12 0.02 0.03 0.15 26 PS(10) TiC(90) KRTTS(0.27) 15 0.02 0.03 0.15 27 PA(10) TiN(90) AL-M(0.27) 15 0.02 0.03 0.15 * HDPE: High density polyethylene (HJ560 from Mitsubishi Chemical Co., Ltd.), PP: polypropylene (WAO3 from Mitsubishi Chemical Co., Ltd.), PS: Polystyrene (S-PS from Idemitsu Chemical Co., Ltd.), and PA: Polyamide (1012c2 from Mitsubishi Empura).
* All of the coupling agents are manufactured by Ajinomoto Co., Ltd. - In the above-mentioned Examples, only one kind of metal or metal composite was used as the conductive particles, however, two or more kinds can be appropriately combined and used.
- 90 parts by weight of silver particles (having a melting point of 960.5 °C, available from Novamet Co.) as conductive particles, 10 parts by weight of HDPE and 2 parts by weight of a phenol type antioxidant (available from Ciba-Geigy Ltd., under the trade name of Irganox 1010) were kneaded by Laboplastomill equipment (manufactured by Toyo Seiki Co., Ltd.) at 200 °C for 15 minutes. The produced PTC composition was hot-pressed to provide a plate of 40 x 60 x 1 mm. A polyethylene frame was produced by injection molding on the periphery of the plate for 20 mm to carry out insulation at the cut-off. Then the plate of PTC composition with the frame was sandwiched between electrodes to provide a PTC element. The PTC element having a resistance at a room temperature of 1 mΩ, could not cut off the flow of current when large current of 50 kA flowed at high voltage of 300 V. We understand that this was because the PTC composition of the Comparative Example was loaded with silver particles having a low melting point, caused internal arc phenomenon (micro arc was generated among the conductive particles) under large current and high voltage, and the PTC composition was electrically destroyed. It is deemed that once the internal arc phenomenon was caused, the heat thereof melted the silver particles in the PTC composition, then the silver particles were bonded together and large current flowed through the bonded part and the composition underwent the electrical breakdown.
- 85 parts by weight of copper particles (having a melting point of 1083 °C, an average particle size of 1.0 µm, available from Fukuda Kinzokuhaku Kogyo Co., Ltd.) as conductive particles, 15 parts by weight of HDPE and 2 parts by weight of a phenol type antioxidant (available from Ciba-Geigy Ltd., under the trade name of Irganox 1010) were kneaded by Laboplastomill equipment (manufactured by Toyo Seiki Co., Ltd.) at 200 °C for 15 minutes. A polyethylene frame was produced by injection molding on the periphery of the plate for 20 mm to carry out insulation at the cut-off. Then the plate of PTC composition with the frame was sandwiched between electrodes to provide a PTC element. The PTC element having a resistance at a room temperature of 3 mΩ could not cut off the flow of current when large current of 50 kA flowed at high voltage of 300 V. It is deemed that this was caused because copper particles having a low melting point were melted in the PTC composition to locally form a conductive circuit as is the case with Comparative Example 1.
- 85 parts by weight of nickel particles (having a melting point of 1452 °C, available from Novamet Co.) as conductive particles, 15 parts by weight of HDPE and 2 parts by weight of a phenol type antioxidant (available from Ciba-Geigy Ltd., under the trade name of Irganox 1010) were kneaded by Laboplastomill equipment (manufactured by Toyo Seiki Co., Ltd.) at 200 °C for 15 minutes. A polyethylene frame was produced by injection molding on the periphery of the plate for 20 mm to carry out insulation at the cut-off. Then the plate of PTC composition with the frame was sandwiched between electrodes to provide a PTC element. The PTC element having a resistance at a room temperature of 1 mΩ, could not cut off the flow of current when large current of 50 kA flowed at high voltage of 300 V. It is deemed that this was because nickel particles in the PTC composition were melted to locally form a conductive circuit as is the case with Comparative Examples 1 and 2.
- FIG. 7 is a schematic illustration of an optical microscope photograph showing the dispersion condition of
nickel particles 3 in the PTC composition, and FIG. 7 (a) illustrates the condition prior to the cut-off (current limiting) test, and FIG. 7 (b) illustrates the condition after the cut-off test in which the device was destroyed. Prior to the cut-off test, thenickel particles 3 were homogeneously dispersed in theorganic polymer 1, but after the cut-off test, thenickel particles 3 were melted and bonded together to form the bonded part ofnickel particles 3a. It is deemed that since thenickel particles 3 in the PTC composition were melted to form the bonded part ofnickel particles 3a (i.e. a conductive circuit was formed), the over-current could not be cut off as is the case with Comparative Examples 1 and 2, and the element was destroyed.
Claims (10)
- A polymeric PTC composition comprising an organic polymer and conductive particles having a melting point of not less than 2000 °C dispersed therein,
characterized in that the conductive particles have been preliminarily treated with a coupling agent before the organic polymer and the conductive particles are mixed with each other. - The composition according to claim 1, characterized in that
the average particle size of the conductive particles is 0.01 µm to 50 µm. - The composition according to claim 1 or 2, characterized in that
the average particle size of the conductive particles is 0.1 µm to 30 µm. - The composition according to any of claims 1 to 3, characterized in that
the conductive particles are contained in an amount of 50 to 99 % by weight based on the composition. - The composition according to any of claims 1 to 4, characterized in that
the conductive particles are contained in an amount of 70 to 97 % by weight based on the composition. - The composition according to any of claims 1 to 5, characterized in that
the conductive particles are particles selected from the group consisting of a metal, a metal carbide, a metal boride, a metal silicide and a metal nitride. - The composition according to claim 6, characterized in that
the metal is tungsten. - The composition according to any of claims 1 to 7, characterized in that
the coupling agent is contained in an amount of 0.05 to 10 % by weight based on the conductive particles,
and wherein the coupling agent is an aluminium type or a titanate type coupling agent. - The composition according to any of claims 1 to 8, characterized in that
the coupling treatment uses a solution comprising the coupling agent dissolved in isopropyl alcohol. - A circuit protection device having a PTC element, comprising- the polymeric PTC composition according to any of claims 1 to 9, and- at least two electrodes which are pressure welded to the PTC element and electrically connected thereto.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP1051398 | 1998-01-22 | ||
| JP1051398A JP3168262B2 (en) | 1997-02-28 | 1998-01-22 | Circuit protection device |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0932166A1 EP0932166A1 (en) | 1999-07-28 |
| EP0932166B1 true EP0932166B1 (en) | 2007-03-21 |
Family
ID=11752312
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19980120252 Expired - Lifetime EP0932166B1 (en) | 1998-01-22 | 1998-10-26 | Polymeric PTC composition and circuit protection device made therefrom |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0932166B1 (en) |
| DE (1) | DE69837378T2 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2578624A1 (en) | 2011-10-06 | 2013-04-10 | Henkel Italia S.p.A. | Polymeric PTC thermistors |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4884163A (en) * | 1985-03-14 | 1989-11-28 | Raychem Corporation | Conductive polymer devices |
| DE4427161A1 (en) * | 1994-08-01 | 1996-02-08 | Abb Research Ltd | Process for the manufacture of a PTC resistor and resistor produced thereafter |
| US5793276A (en) * | 1995-07-25 | 1998-08-11 | Tdk Corporation | Organic PTC thermistor |
| DE19548741A1 (en) * | 1995-12-23 | 1997-06-26 | Abb Research Ltd | Process for the production of a material for PTC resistors |
| TW344828B (en) * | 1997-02-28 | 1998-11-11 | Mitsubishi Electric Corp | Organic positive temperature coefficient composition and a circuit protection device using such composition |
-
1998
- 1998-10-26 DE DE69837378T patent/DE69837378T2/en not_active Expired - Lifetime
- 1998-10-26 EP EP19980120252 patent/EP0932166B1/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| DE69837378T2 (en) | 2007-11-29 |
| EP0932166A1 (en) | 1999-07-28 |
| DE69837378D1 (en) | 2007-05-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0815569B1 (en) | Conductive polymer composition and device | |
| US6358438B1 (en) | Electrically conductive polymer composition | |
| US7001538B2 (en) | PTC composition and PTC device comprising the same | |
| US20020137831A1 (en) | Polymeric PTC composition and circuit protection device made therefrom | |
| US6090313A (en) | High temperature PTC device and conductive polymer composition | |
| JPH0777161B2 (en) | PTC composition, method for producing the same and PTC element | |
| US20140327513A1 (en) | Macromolecule-based conductive composite material and PTC element | |
| US6074576A (en) | Conductive polymer materials for high voltage PTC devices | |
| US5817423A (en) | PTC element and process for producing the same | |
| JP2003163104A (en) | Organic ptc composition | |
| US20040232387A1 (en) | Conductive polymer having positive temperature coefficient, method of controlling positive temperature coefficient property of the same and electrical device using the same | |
| EP0866471B1 (en) | Polymeric PTC composition and circuit protection device made from the same | |
| EP0932166B1 (en) | Polymeric PTC composition and circuit protection device made therefrom | |
| JP3168262B2 (en) | Circuit protection device | |
| US6197220B1 (en) | Conductive polymer compositions containing fibrillated fibers and devices | |
| JP2007036230A (en) | Overcurrent protection element | |
| US20020128333A1 (en) | Low switching temperature polymer positive temperature coefficient device | |
| US20020161090A1 (en) | PTC conductive polymer compositions | |
| JP2000331804A (en) | Ptc composition | |
| JP3609573B2 (en) | Organic PTC composition | |
| KR20090103491A (en) | low resistor polymer PTC thermistor and thereof. | |
| JPH10241907A (en) | Circuit protector | |
| JPH1140402A (en) | Organic ptc compound | |
| JPH11329675A (en) | Ptc composition | |
| JP2000021605A (en) | Ptc composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE FR GB IT |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| 17P | Request for examination filed |
Effective date: 19990913 |
|
| AKX | Designation fees paid |
Free format text: DE FR GB IT |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: MITSUBISHI DENKI KABUSHIKI KAISHA |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: THE INVENTORS HAVE AGREED TO WAIVE THEIR ENTITLEME |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: ISHIKAWA, MASAHIRO Inventor name: SOGABE, MANABU Inventor name: NISHINA, KENICHI Inventor name: MURATA, SHIRO Inventor name: TAKAHASHI, CHIE Inventor name: HAYASHI, TATSUYA Inventor name: MORI, TEIJIRO Inventor name: HIROI, OSAMU Inventor name: NISHIYAMA, ITSUO Inventor name: HORIBE, HIDEO |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB IT |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 69837378 Country of ref document: DE Date of ref document: 20070503 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 727 |
|
| EN | Fr: translation not filed | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 727A |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20071227 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070321 Ref country code: FR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071123 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070321 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20101020 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20101020 Year of fee payment: 13 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20111026 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120501 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 69837378 Country of ref document: DE Effective date: 20120501 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111026 |